Abstract
Symptoms of depression and social anxiety elevate in late childhood. An identified cognitive risk to both depression and social anxiety is maladaptive self-schemas (or self-schematic processing). Beyond the behavioral indices of this construct, event-related potentials (ERPs) during self-schematic processing have also been observed to be associated with depression or depressive symptoms. However, no study has examined the ERPs underlying self-schematic processing in relation to social anxiety. More importantly, it was unclear to what extent behavioral and ERP indices of self-schematic processing were differentially associated with depression and social anxiety, especially in typical-risk youth with emerging symptoms. A hundred and fifteen community-dwelling children (66 girls; Mean age=10.91 years, SD=1.45) completed a self-referent encoding task (SRET) with EEG recorded. A Principal Component Analysis identified a late positive potential (LPP) component elicited in both the positive and negative SRET conditions. Multivariate multiple regression showed that in both conditions, behavioral SRET scores were associated with depressive symptoms while partialling out social anxiety symptoms, but not with social anxiety symptoms with depressive symptoms partialled out. The LPP amplitude elicited in both conditions showed marginally positive associations with social anxiety symptoms while partialling out depressive symptoms, but not with depressive symptoms while accounting for social anxiety. This study provides novel evidence concerning the ERP correlates of self-schematic processing in relation to social anxiety symptoms. More importantly, our findings for the first time speak to the differential associations between the behavioral SRET scores and SRET-elicited LPP and emerging symptoms of depression and social anxiety in late childhood.
Keywords: EEG, self-referential processing, late positive potential, pre-adolescence, depression, social anxiety, PCA
Introduction
Social anxiety and depression are common, frequently co-occurring mental health problems that emerge as early as childhood. Without effective treatments, these early conditions may persist for a lifetime and incur serious individual distress and societal costs (Graber & Sontag, 2009). Psychopathology research has identified an array of etiological factors for social anxiety and depression, including cognitive vulnerabilities (Beck, 2008; Gotlib & Joormann, 2010). One important and potentially modifiable cognitive risk for both conditions is self-schematic processing (or self-schemas), conceptualized as an early emerging, latent, trait-like cognitive construct that organizes and guides the processing of positive and negative information about oneself (Northoff et al., 2006). Maladaptive patterns of self-schematic processing indicated by behavioral measures (i.e., deeper processing of negative, and shallower processing of positive, self-descriptive information) show concurrent or prospective associations with depression (e.g., Dobson & Shaw, 1987; Kuiper & Derry, 1982; Prieto et al., 1992) and social anxiety (e.g., Dixon et al., 2022; Dozois & Dobson, 2001; Gotlib et al., 2004) in clinical and sub-clinical/non-clinical samples.
A substantial literature has applied neural measures such as event-related potentials (ERPs) in studying self-schematic processing. ERPs are quantified as voltage fluctuations of electroencephalogram (EEG) signals time-locked to “events” such as stimulus onset (Woodman, 2010; e.g., the onset of self-descriptive words in the SRET). With high temporal resolution, ERPs are particularly suitable for characterizing the individual differences during different stages of self-schematic processing. ERP studies on self-schematic processing identified altered neural activity during self-schematic processing in relation to clinical depression (Allison et al., 2021; Auerbach et al., 2015) and sub-clinical symptoms (Speed et al., 2016) in youth. Overall, the existing behavioral and ERP studies support the validity and utility of self-schematic processing, indexed by both behavioral and ERP markers, as an early etiological factor of depression and social anxiety.
However, extant studies in this field have been conducted in depression and social anxiety in parallel, although the two conditions frequently co-occur even in youth. It is unclear to what extent the observed associations between self-schematic processing and one condition are confounded by co-occurring symptoms of the other. Further, different measures of self-schematic processing (e.g., behavioral and ERP) may show differential associations with depression and social anxiety. To address these questions, we examined the unique associations between self-schematic processing (indexed by behavioral and ERP measures) and emerging symptoms of depression or social anxiety (while accounting for each other) during late childhood in a community sample. Late childhood is a critical period marked by elevated symptoms of social anxiety and depression, thus a useful time window to capture the early risk processes prior to the onset of full-blown disorders.
Behavioral studies on self-schematic processing in relation to depression and social anxiety
The very earliest studies on self-schematic processing as an etiological factor have focused on depressed adults (Derry & Kuiper, 1981; Kuiper & Derry, 1982). This literature established the self-referent encoding task (SRET; Derry & Kuiper, 1981; Kuiper & Derry, 1982), which has since been widely used in measuring self-schemas and related constructs in the broader literature. While there have been variations in the implementation of SRET, this paradigm typically consists of a self-endorsement task followed by an unexpected memory task (e.g., Gotlib et al., 2006; Hayden et al., 2013, 2014; Jacobs et al., 2008; Prieto et al., 1992). During the self-endorsement task, participants are presented with a series of positive and negative personal trait words and decide whether each word is self-descriptive (“Does this word describe you?”). The endorsement task is followed by an unexpected free recall task, when participants are asked to recall as many of the presented words as possible. Positive and negative self-schemas are typically indexed by calculating a positive and a negative SRET score as the proportion of positive and negative words both endorsed and recalled divided by the total number of words endorsed. Incorporating the performance of incidental free recall in calculating the score captures a more latent facet of the construct of self-schemas.
While the SRET was originally developed to study adults, it has become a useful tool for studying children. In the developmental psychopathology literature, the SRET has been successfully used in children as young as six years old (Goldstein et al., 2015). In performing this task, typically developing children tend to show a “self-positivity bias” manifested as highly positive self-schemas and low negative ones, i.e., they endorse (and remember) most positive words but a smaller number of negative words (Goldstein et al., 2015; Hudson et al., 2021; Liu et al., 2021; Liu, Vandemeer, et al., 2020). This self-positivity bias tends to decrease as children transition into adolescence (Crone & Fuligni, 2020), shown as reduced positive self-schemas and/or enhanced negative self-schematic processing. These developmental changes may be directly linked with elevated psychopathology risks in early adolescence, as indicated by associations between more negative and/or less positive self-schemas and depressive symptoms during late childhood or early adolescence even in typically developing youth (e.g., Connolly et al., 2016; Goldstein et al., 2015; Hayden et al., 2013, 2014).
With regard to the associations between self-schematic processing and social anxiety, less work has been done in youth and more studies have been conducted in clinically anxious adults. In an adolescent sample of ages 12–13, a smaller number of endorsed positive words in an SRET was associated with social anxiety symptoms controlling for comorbid depressive symptoms, and a larger number of self-endorsed negative words was associated with greater likelihood of lifetime social phobia controlling for other lifetime diagnoses (Alloy et al., 2012). Compared to healthy controls, adults with anxiety disorders (e.g., generalized social phobia) generally recalled a greater number of negative words and a smaller number of positive words in an SRET (Dixon et al., 2022; Dozois & Dobson, 2001; Gotlib et al., 2004; Thurston et al., 2017); in some studies, this pattern was observed in the context of anticipated social interaction (Heinrichs & Hofmann, 2001). Studies using randomized controlled designs provided additional evidence supporting the associations between self-schemas and social anxiety. For example, for individuals with social anxiety disorder, cognitive-behavioral treatment that targeted at changing maladaptive self-beliefs lead to increased positive self-schemas, decreased negative self-schemas, and decreased social anxiety symptoms. More important, the treatment effect on social anxiety symptom reduction was mediated by the increases in positive self-schemas, further supporting the etiological role of self-schemas in social anxiety (Goldin et al., 2013).
ERP substrates of self-schematic processing in relation to depression and social anxiety
Building on the behavioral literature, researchers have combined neuroscience techniques, e.g., the ERPs, with the SRET to examine the neural activity elicited by self-schematic processing in association with psychopathology. Compared to other neural measures such as magnetic resonance imaging, ERPs are low-cost and child-friendly, showing better psychometric properties even in youth (e.g., greater internal consistency and test re-test reliability; Cassidy et al., 2012; Huffmeijer et al., 2014). Other than these methodological advantages, knowledge of the neural substrates of self-schematic processing can also promote our mechanistic knowledge of the development of depression and social anxiety (Disner et al., 2011). This mechanistic knowledge, in turn, will inform early identification and prevention or intervention strategies for individuals at risk. From a developmental perspective, neural markers of cognitive risks may emerge prior to explicit manifestation of symptoms; neural markers may also tap facets of risk that cannot be captured by behavioral measures (Manoach & Agam, 2013). Indeed, previous research reported that typically developing children with elevated temperamental risk for anxiety (i.e., fearful temperament; Fu et al., 2017; Thai et al., 2016) or maternal risk for depression (Liu, Vandermeer, et al., 2020) showed altered neural patterns in processing socio-emotional information but did not differ from low-risk controls in their behavioral performance.
Despite the theoretical and clinical importance of studying the neural markers of cognitive risks, only a small number of studies has leveraged the ERPs in the SRET to characterize the neural substrates of self-schematic processing in relation to depression (Allison et al., 2021; Auerbach et al., 2015; Shestyuk & Deldin, 2010; Speed et al., 2016); no ERP study of the SRET has been done for social anxiety. The literature on depression observed altered patterns of ERPs during self-schematic processing associated with clinical depression or depression risks in youth and adults. While different ERP components have been reported in this literature, the late positive potential (LPP) component has showed the most consistent and robust associations with depression in all studies. Thus, we are focusing on the LPP in reviewing this literature.
The LPP is a late, positive deflection without a clearly defined peak, starting around 500–600 ms post stimulus and lasting for (or beyond) the duration of stimulus. In healthy individuals, LPP is typically observed in processing socio-emotionally salient cues, especially those with high arousal (Cuthbert et al., 2000). The LPP is thought to reflect more elaborative and in-depth processing that potentially involves cognitive reappraisal and effortful memory retrieval (e.g., Foti et al., 2009; Foti & Hajcak, 2008; Macnamara et al., 2009). Studies using non-referential emotion processing paradigms typically report maximized LPP in posterior channels (e.g., midline occipital, parietal, or central). In these studies, altered patterns of LPP toward emotional (versus neutral) stimuli have been observed in adolescents with clinical depression (Burkhouse et al., 2017; Grunewald et al., 2019), children with a maternal history of depression (Kujawa et al., 2012), children with greater internalizing problems (McLean et al., 2020), and adults with social anxiety disorder (Kinney et al., 2019; MacNamara et al., 2019) or elevated social anxiety symptoms (Moser et al., 2008; Schmitz et al., 2012).
More recent ERP studies using the SRET paradigm on depression have observed a similar LPP elicited by self-schematic processing, which, however, appeared to maximize over more anterior channels (e.g., midline fronto-central; Auerbach et al., 2015, 2016; Shestyuk & Deldin, 2010). During the endorsement task of the SRET (i.e., deciding whether a word is self-descriptive), healthy adolescent girls showed a potentiated LPP toward positive versus negative self-referent words (Auerbach et al., 2016), whereas depressed girls (Auerbach et al., 2015) and depressed adults (Shestyuk & Deldin, 2010) exhibited a larger LPP toward negative versus positive words. Eight-to-14-year-old girls with a maternal lifetime history of depression showed a larger LPP elicited by negative words compared to typical-risk peers (Speed et al., 2016). In our recent study in a community sample of nine-to-12-year-olds, we found that the LPP evoked by positive words predicted lower depressive symptoms beyond behavioral SRET scores (Liu & Tan, 2022). Taken together, these ERP studies suggest that modulations of the LPP in response to socio-emotional information (including self-referential information) may be a valid neural marker of depression and social anxiety.
It is also worth considering the relationship between the behavioral SRET scores and the LPP as a neural marker of self-schematic processing. Existing ERP studies of the SRET did not find associations between the two indices (Allison et al., 2021; Auerbach et al., 2015, 2016; Liu & Tan, 2022; Speed et al., 2016), suggesting that these two indices may reflect different facets of self-schematic processing. Specifically, behavioral SRET scores, the calculation of which incorporates an unexpected free recall component, may tap into a more latent aspect of self-schematic processing. On the other hand, the LPP, elicited during the endorsement phase when participants are deliberately deciding whether a personal trait describes themselves, may reflect a more deliberate aspect of self-schematic processing. It is possible that these two facets of self-schematic processing show differential associations with depression and social anxiety, which is going to be examined in the current study.
Differential associations between risk markers and depression and social anxiety
The literature reviewed so far provides evidence on the associations between maladaptive self-schematic processing and depression or social anxiety. However, these studies investigated depression and social anxiety separately, and none of them considered the common co-occurrence of the two conditions. It is therefore unclear to what extent the observed associations between maladaptive self-schematic processing and depression were confounded by co-occurring symptoms of social anxiety and vice versa. This has been an issue in other areas of psychopathology research as well. For example, early studies observed associations of heightened resting-state right frontal alpha activity (FAA), a neural marker of trait-like withdrawal tendencies, with both anxiety and depression (e.g., Davidson, 1992; Gotlib, 1998; Henriques & Davidson, 1991). However, later research that accounted for the comorbidity between anxiety and depression found associations between FFA with anxiety, but not depression (Adolph & Margraf, 2017; Blackhart et al., 2006; Mathersul et al., 2008). These studies underscore the importance of accounting for the anxiety-depression comorbidity when studying associations between risk markers and depression or anxiety.
The present study
Several gaps exist in reviewing the literature on self-schematic processing in relation to depression and social anxiety. First, no work has examined the ERP correlates of self-schematic processing in association with social anxiety symptoms. Second, none of the extant studies, behavioral or neurophysiological, accounted for co-occurring symptoms of depression or social anxiety, leaving it unclear to what extent maladaptive self-schematic processing is a unique risk marker for either condition. Finally, existing studies have focused on individuals with depressive disorders (e.g., Auerbach et al., 2015; Shestyuk & Deldin, 2010), social anxiety disorders (e.g., Dixon et al., 2022; Dozois & Dobson, 2001; Gotlib et al., 2004), or established depression risk (e.g., maternal depression, Speed et al., 2016). What remains unclear are the neural (and behavioral) correlates of self-schematic processing in typically developing youth with normative, emerging symptoms of depression and social anxiety (Costello et al., 2003; Graber & Sontag, 2009) and marked changes in self-perception (Pfeifer et al., 2016) as they transition into adolescence. To understand the shared mechanisms of typical and atypical development (Cicchetti & Toth, 2009), it is critical to examine early risk processes in “typical-risk” youth during this unique time window of late childhood or pre-adolescence.
To address these gaps, we conducted an ERP study using the SRET in a group of unselected, community-dwelling children of ages nine-12. While the rate of clinically significant depression and social anxiety remains relatively low for children of this age, many of them start to experience increased symptoms of depression and social anxiety, providing sufficient inter-individual variability to study risk processes of interest. Considering the methodological advantages of ERPs, we employed both the behavioral and ERP indices in assessing self-schematic processing. Specifically, we focused on the LPP component that has been consistently identified in relevant ERP studies of SRET and showed robust associations with depression or depression risk (Auerbach et al., 2015, 2016; Shestyuk & Deldin, 2010; Speed et al., 2016). Using multivariate multiple regression analysis, we aimed to isolate the unique variance in symptoms of depression (or social anxiety) that was explained by the behavioral SRET scores or the LPP, with comorbid social anxiety (or depression) accounted for.
Based on past work, we expected to observe high positive, and low negative, self-schemas indexed by the behavioral SRET scores, as is typical in community samples of children of this age. We also expected to identify an LPP component elicited during self-schematic processing that was similar to those found in previous studies (e.g., Auerbach et al., 2015; Liu & Tan, 2022; Speed et al., 2016). Finally, we expected that the behavioral SRET scores and the LPP may show differential associations with depressive and social anxiety symptoms. However, given the lack of relevant research in the literature, we do not have specific hypotheses concerning the patterns of the differential associations.
Method
Participants and Procedure
Data are drawn from a larger, ongoing project investigating the neurobehavioral correlates of cognitive processing risks for anxiety and depression during late childhood. Participants were recruited via a registry of local families from the Fargo-Moorhead area in North Dakota and Minnesota. Specifically, 115 nine-to-12-year-old typically developing, community-dwelling children (66 girls, 49 boys; Mean age of years = 10.91, SD = 1.45) and their caregivers were directly recruited from the community and were invited to a laboratory visit on campus. Children were not selected based on any particular criteria (e.g., whether they’ve received diagnoses of mental health disorders); none of them had any major physical diseases, serious mental illness (i.e., a mental disorder resulting in serious functional impairment and substantial interference with one or more major life activities), or neurodevelopmental disabilities (e.g., intellectual disability, developmental delay). The demographics of the sample lined up with the local demographics in the Fargo-Moorhead area (87.5% White, 3.6% Asian, 8.9% multiracial; 7.2% Hispanic or Latino; family income range: $15,000 - $350,000). A nonparametric binomial test showed that the observed proportion of girls (57%) did not different from the proportion of 50% (p = .14), suggesting a statistically equal distribution of girls and boys in our sample.
During the visit, caregiver consent and child assent were acquired first. Next, each child completed a battery of four EEG tasks (including the SRET) in a counter-balanced order and an eye-tracking task tapping different cognitive processing biases. After finishing the study visit, children also completed questionnaires on their behavioral and mental health problems via a link at home (Qualtrics, Provo, UT). Participants received monetary compensation upon completing the study visit and the questionnaires. Only data of the SRET and symptoms of social anxiety and depression were reported in this study. The study procedure was approved by the Institutional Review Board of the university.
Measures of social anxiety and depressive symptoms
Children reported on their anxiety symptoms via the child-report version of the Screen for Child Anxiety Related Disorders-Revised (SCARED; Birmaher et al., 1999). SCARED consists of 41 items assessing a range of DSM-defined anxiety disorder symptoms in eight-18-year-old youth, including social anxiety, generalized anxiety, separation anxiety, school avoidance, and panic/somatic symptoms. For each item, participants selected from a three-point scale the one that best describes themselves for the last three months (0 = Not True or Hardly Ever True, 1 = Somewhat or Sometimes True, or 2 = Very True or Often True). Given our focus on social anxiety, we calculated the total score of the subscale of social anxiety (seven items) to indicate social anxiety symptom severity (Cronbach’s α in the current sample = .86).
Children also completed a child-report version of Child Depression Inventory (CDI; Kovacs, 1978). The CDI consists of 27 items assessing the presence and severity of depressive symptoms in seven-to-17-year-old youth. Due to limited inter-individual variability, we excluded the item “I want to kill myself.” For each of the remaining 26 items, youths selected from three statements the one that best describes themselves for the past two weeks (e.g., I have fun: in many things = 0; in some things = 1; in nothing = 2). A total score (range 0–52) was calculated to indicate depressive symptom severity (Cronbach’s α in the current sample = .91).
The SRET
Following common practice in the psychopathology literature (e.g., Gotlib et al., 2006; Hayden et al., 2013, 2014; Liu et al., 2021; Prieto et al., 1992), children first watched an age-appropriate three-minute sad movie clip (The Neverending Story) to induce sad mood, which is thought necessary to activate latent cognitive vulnerability (Abela & Hankin, 2008). Children rated their mood before and after watching the clip on a five-point scale (1 = very sad, 5 = very happy). Comparing their ratings pre- (M = 3.72, SD = 0.71) and post-induction (M = 2.75, SD = 0.91) indicated that mood induction was effective, t(114) = 10.31, p < .001, Cohen’s d = 1.19.
Children next completed an SRET with EEG signals recorded. We adopted 60 personal trait words (30 positive, 30 negative) used by a previous SRET study in eight-to-14-year-old girls (Speed et al., 2016). The positive and negative words differed in valence but were matched on arousal and length based on the Affective Norms for English (Bradley & Lang, 1999). The 60 words were presented in a pseudo-random manner, such that no more than two words of the same valence were presented in a row. Each trial started with a 500 ms fixation cross, after which a positive or negative word was presented for 1000 ms, followed by another 500 ms fixation cross. Next, a question (“Does this word describe you?”) popped up on the screen until children pressed one of two buttons on a response box to indicate their answers (left button = yes, right button = no). Immediately following the endorsement task, children were unexpectedly asked to recall as many of the presented words as possible for up to two minutes.
Following standard scoring of SRET (e.g., Derry & Kuiper, 1981; Gotlib et al., 2006; Hayden et al., 2013, 2014; Kuiper & Derry, 1982), we calculated behavioral indices of self-schemas as the proportion of positive or negative words both endorsed and recalled (positive SRET score = number of positive words endorsed and recalled/total number of words endorsed; negative SRET score = number of negative words endorsed and recalled/total number of words endorsed). Consistent with previous findings in children of similar ages (Goldstein et al., 2015; Hayden et al., 2013, 2014; Mackrell et al., 2013), negative SRET score showed a positively skewed distribution (i.e., most children endorsed and recalled a small number of negative words). Thus, negative SRET score was log-transformed with base 10 to account for the non-normality. The log-transformed values were used in all subsequent statistical analyses.
EEG data acquisition and processing
Children completed the SRET in an electrically shielded chamber while continuous EEG signals were recorded via a 64-channel HydroCel GSN Electrical Geodesics Inc. (EGI) net and an EGI 200 NetAmps Amplifier. Prior to the recording session, each net was soaked in a potassium salt solution for five minutes before its application to the child. Electrode impedances was kept below 50 kΩ. EEG signals were recorded with a sampling rate of 250 Hz, referenced to the vertex electrode (Cz) during recording.
EEG data were processed using the EEGLab (Delorme & Makeig, 2004) and ERPLab (Lopez-Calderon & Luck, 2014) toolboxes operated in MATLAB 9.10.0 (Mathworks, Inc., Natick, MA). Raw EEG data were first subjected to offline filter (0.1–40Hz) and re-referenced to the average of the two mastoid electrodes. An independent component analysis approach was used to detect and remove ocular artifacts caused by eye blinks and eye movement. Next, the processed continuous EEG data were time-locked to the onset of each word and segmented into epochs from 200 ms before to 1000 ms post the word onset, with a 200 ms baseline correction. We further rejected segments with (1) voltage beyond ±100 μV, (2) a >50 μV change of voltage between timepoints, or (3) a >300 μV change of voltage between the most positive and most negative timepoints within a 200 ms moving window. Finally, average ERPs were computed for each child, time-locked to all positive or negative words, irrespective of whether the word was endorsed. Following artifact rejection, 102 children with >10 trials in each condition were retained for subsequent analyses.
Principal Component Analysis of ERP data
We examined the LPP component that has been consistently identified as a neural marker of depressive psychopathology in previous SRET studies (Allison et al., 2021; Auerbach et al., 2015; Shestyuk & Deldin, 2010; Speed et al., 2016). Many existing studies, however, quantified ERP components within an arbitrary time window across a selected number of electrodes. This approach uses a small portion of the data, yields less accurate temporo-spatial information, and cannot speak to the underlying components which may not be readily perceptible by visual inspection (Luck, 2014). To address these limitations, we used a Principal Components Analysis (PCA) to quantify the LPP in our data. PCA is a factor-analytic approach that isolates underlying ERP components by accounting for the variance across all time points and electrodes, thereby maximizing the utility of the data and generating more accurate temporo-spatial information (Dien, 2010; Dien, Beal, & Berg, 2005; Dien & Frishkoff, 2005).
We used a two-step temporo-spatial PCA approach via the ERP PCA Toolkit in MATLAB (Dien, 2010; Dien, Beal, & Berg, 2005; Dien & Frishkoff, 2005). A temporal PCA was first performed using Promax rotation to reduce the temporal dimensions of the data (Dien & Frischkoff, 2005). The temporal PCA treated time points as variables and participants, experimental conditions (positive & negative), and electrodes as observations, generating linear combinations of variables (i.e., time points) as temporal factors for each observation. In the current data, 21 temporal factors were retained based on the Scree plot (Cattell, 1966; Cattell & Jaspers, 1967). Next, a spatial PCA was performed on each of the 21 temporal factors using Infomax rotation to reduce the spatial dimensions of the data, treating electrodes as variables and participants, conditions, and temporal factors as observations. Five spatial factors were extracted for each temporal factor based on the Scree plot.
The PCA resulted in 105 temporo-spatial factors (21 temporal × five spatial). Nineteen factors, each uniquely accounting for ≥ 0.5% of the variance (Kaiser, 1960), were retained for further inspection. Among the 19 factors, the TF02SF1 factor (a fronto-central positivity peaking around 952 ms) appeared to be temporally and spatially analogous to the anterior LPP reported in previous studies using a similar SRET paradigm (Auerbach et al., 2015, 2016; Shestyuk & Deldin, 2010; Speed et al., 2016). On the other hand, the TF04SF2 factor (a parieto-occipital positivity peaking around 816 ms) was similar to the posterior LPP reported in studies using non-referential paradigms (e.g., Burkhouse et al., 2017; Kujawa et al., 2012; McLean et al., 2020). Given that the SRET literature has focused on the anterior LPP and that in our data, the TF02SF1 factor (3.303%) accounted for greater unique variance than the TF04SF2 factor (0.57%), we therefore focused on the anterior (rather than posterior) LPP as indicated by the TF02SF1 factor (Figure 1). To facilitate data interpretation, the factor scores of each temporo-spatial factor were converted into microvolt scaling, corresponding to the voltage of the original data accounted for by each factor. The peak “amplitude” of the TF02SF1 factor was extracted for each participant in each experimental condition and was subjected to subsequent statistical analyses in SPSS (version 20.0). Paired-sample t-test was run on the amplitude of LPP extracted from the PCA to test differences between the positive and negative conditions. 1
Figure 1.
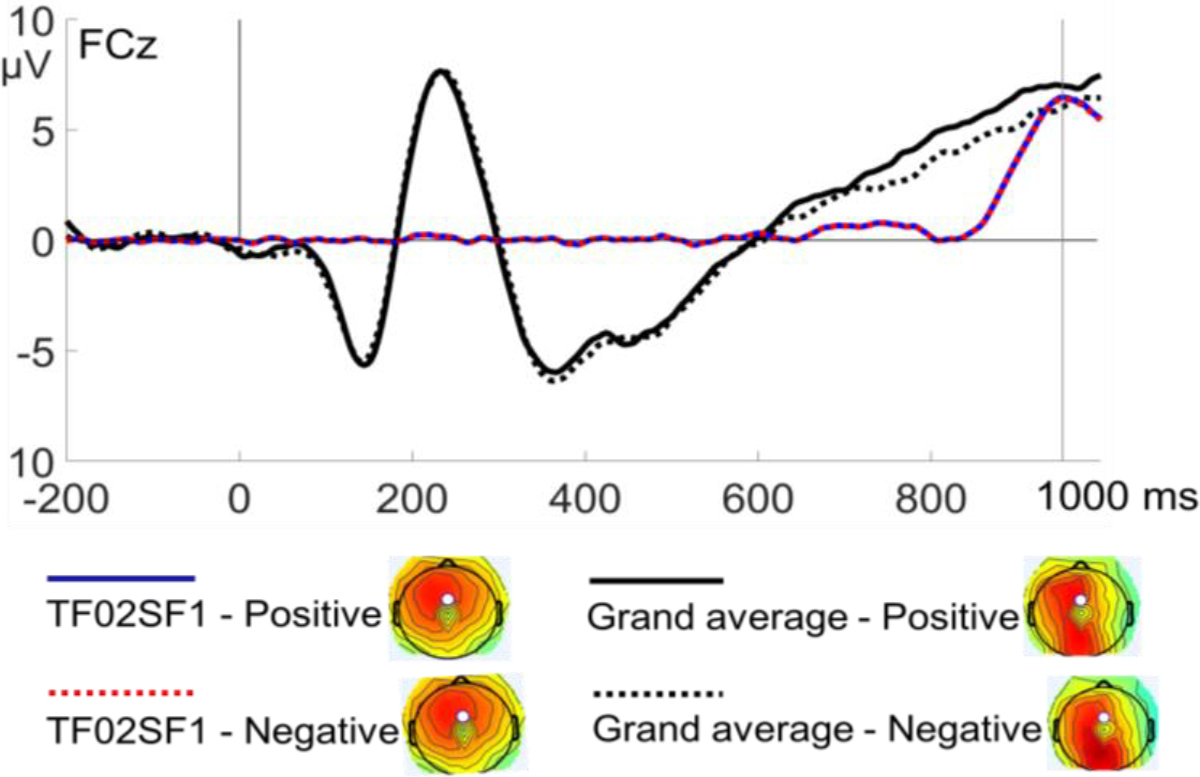
ERP waveforms and topographic maps (at 952 ms) of the TF02SF1 factor in the positive and negative conditions superimposed on the grand average of the original data.
Statistical analysis
To isolate the unique brain activity in response to the positive versus the negative condition (and vice versa), we calculated the standardized residual scores of the LPP component in each of the two conditions. Residual scores for the positive condition was calculated by regressing the LPP amplitude in the positive condition on that in the negative condition; residual scores for the negative condition was calculated by regressing the LPP amplitude in the negative condition on that in the positive condition. Similarly, given the high correlation between depressive and social anxiety symptoms (r = .56 in our data), we also calculated the standardized residual scores of depressive and social anxiety symptoms by regressing them on each other. These residual scores reflected the unique variance of social anxiety or depressive symptoms while regressing out the shared variance between depression and social anxiety. The residual scores were used as indicators of LPP and the symptoms of depression and social anxiety in subsequent analyses.
Next, we conducted a multivariate multiple regression model to examine the extent to which behavioral SRET scores and LPP were uniquely associated with depressive symptoms or social anxiety symptoms. Specifically, the behavioral SRET scores and the amplitudes of LPP in both conditions were entered as predictors, with the residual scores of depressive symptoms and social anxiety symptoms included as outcomes. Child sex and age were included as covariates.
Results
Descriptive statistics and bivariate correlations of study variables
Table 1 shows the means, standard deviations, and bivariate correlations of main study variables. As expected, social anxiety symptoms and depressive symptoms were positively correlated with each other. Children’s positive SRET score was negatively correlated with both social anxiety and depressive symptoms, whereas negative SRET score showed positive correlations with social anxiety and depressive symptoms. The amplitude of LPP (indicated by TF02SF1) in the positive condition showed a marginal positive correlation with social anxiety symptoms. The LPP was not correlated with behavioral SRET scores in either condition. Child age was correlated with higher positive SRET scores; girls were slightly older than boys. Girls also showed greater symptoms of social anxiety and depression compared to boys.
Table 1.
Mean, standard deviation, and bivariate correlations of main study variables
| Mean | SD | 1 | 2 | 3 | 4 | 5 | 6 | 7 | ||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| 1 | Sex (1=boys, 2=girls) | |||||||||
| 2 | Years of age | 10.91 | 1.45 | .20* | ||||||
| 3 | Social anxiety symptoms | 5.75 | 3.57 | .30** | .09 | |||||
| 4 | Depressive symptoms | 8.07 | 8.04 | .32** | .04 | .56** | ||||
| 5 | Positive condition - SRET score | 0.13 | 0.08 | .07 | .20* | −.26** | −.44** | |||
| 6 | Positive condition - LPP (TF02SF1) | 6.49 | 6.98 | −.06 | −.04 | .19+ | .07 | −.12 | ||
| 7 | Negative condition - SRET score† | 0.03 | 0.05 | .14 | .13 | .29** | .41** | −.13 | −.07 | |
| 8 | Negative condition - LPP (TF02SF1) | 6.56 | 6.97 | −.17 | −.08 | .15 | .03 | −.10 | .74** | −.14 |
Note. SD: standard deviation; SRET: self-referent encoding task; LPP: late positive potential;
log-transformed with base 10;
p < .01,
p < .05,
p < .10.
Results of multivariate multiple regression analysis
Figure 1 shows the waveforms and topographic maps (at the peak latency of 952 ms) of the TF02SF1 factor as an indicator of the LPP component in the two conditions, superimposed on the grand average waveforms of the original ERP data. Paired-sample t-test conducted on the amplitude of TF02SF1 showed no significant difference between the positive (Mean = 6.49, SD = 6.98) and negative (Mean = 6.56, SD = 6.97) conditions, t(101) = .13, p = .45.
Results of the multivariate multiple regression showed better overall model fit in predicting depressive symptoms (R2 = .28, F(6, 95) = 5.38, p < .01, η2 = .28) compared to predicting social anxiety symptoms (R2 = .06, F(6, 95) = 0.96, p = .46, η2 = .06). Table 2 exhibits the parameter estimates of each predictor in the model. For the behavioral measures of the SRET, both the positive and negative SRET scores were associated with lower depressive symptoms indicated by the residual scores with social anxiety symptoms partialled out. Behavioral SRET scores were not associated with social anxiety symptoms in either condition. For the ERP indices of the SRET, the residual scores of LPP amplitudes in both conditions showed marginal associations with social anxiety symptoms with depressive symptoms regressed out, but were not associated with depressive symptoms. Finally, sex marginally predicted depressive symptoms but not social anxiety symptoms, such that girls showed greater depressive symptoms than boys.
Table 2.
Results of multivariate multiple regression analyses.
| Outcomes | Predictors | B | SE | t | p | 95% CI | Effect size | Observed power |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Social anxiety symptoms (residual score) | Positive condition - SRET score | 0.06 | 0.11 | 0.54 | .59 | [−0.16, 0.27] | 0.00 | .08 |
| Positive condition - LPP (residual score) | 0.30 | 0.16 | 1.84 | .07+ | [−0.02, 0.62] | 0.04 | .44 | |
| Negative condition - SRET score | 0.05 | 0.11 | 0.47 | .64 | [−0.17, 0.28] | 0.00 | .08 | |
| Negative condition - LPP (residual score) | 0.28 | 0.16 | 1.74 | .09+ | [−0.04, 0.61] | 0.03 | .41 | |
| Sex | −0.27 | 0.23 | −1.21 | .23 | [−0.72, 0.18] | 0.02 | .22 | |
| Age | 0.06 | 0.11 | 0.51 | .61 | [−0.16, 0.27] | 0.00 | .08 | |
| Depressive symptoms (residual score) | Positive condition - SRET score | −0.38 | 0.10 | −3.84 | .00** | [−0.57, −0.18] | 0.15 | .97 |
| Positive condition - LPP (residual score) | −0.08 | 0.15 | −0.52 | .60 | [−0.37, 0.22] | 0.00 | .08 | |
| Negative condition - SRET score | 0.32 | 0.10 | 3.09 | .00** | [0.12, 0.52] | 0.10 | .86 | |
| Negative condition - LPP (residual score) | −0.03 | 0.15 | −0.21 | .84 | [−0.33, 0.26] | 0.04 | .06 | |
| Sex | −0.37 | 0.21 | −1.81 | .07+ | [−0.78, 0.04] | 0.00 | .43 | |
| Age | −0.02 | 0.10 | −0.16 | .87 | [−0.21,0.18] | 0.00 | .05 | |
Note. SE: standard error; CI: confidence interval; effect size: partial η2; observed power: 1 - β (α = .05); SRET: self-referent encoding task; LPP: late positive potential;
p < .01,
p < .05,
p < .10.
Discussion
To better understand the extent to which the behavioral and ERP indices of self-schematic processing were differentially associated with emerging symptoms of depression and social anxiety in late childhood, we examined the behavioral performance and ERP activity during an SRET in an community sample of nine-to-12-year-old children. As expected, children displayed high positive, and low negative, self-schemas, as indicated by the behavioral SRET scores. In the ERP data, the PCA analysis identified a temporo-spatial factor (TF02SF1) that uniquely accounted for 3.30% of the full variance and was temporally and spatially analogous to the anterior LPP reported in previous SRET studies (e.g., Auerbach et al., 2015; Shestyuk & Deldin, 2010; Speed et al., 2016). Further, multivariate multiple regression showed that in both the positive and negative conditions, behavioral SRET scores were associated with depressive symptoms (with the shared variance of social anxiety partialled out) but not with social anxiety symptoms. On the other hand, the amplitude of LPP in both conditions was showed a marginal positive association with social anxiety symptoms but not with depressive symptoms. Our study provides novel evidence concerning the differential associations between behavioral SRET scores and SRET-elicited LPP and emerging symptoms of depression and social anxiety in late childhood, a critical period for elevated risks of depression and social anxiety.
Associations between behavioral SRET scores and symptoms of depression and social anxiety
To examine the linkages between self-schematic processing and emerging symptoms of depression and social anxiety, we conducted bivariate correlations and multivariate multiple regression analysis. Bivariate correlations showed that both the positive and negative SRET scores were meaningfully correlated with the original scores of depressive symptoms and social anxiety symptoms: lower positive SRET scores, and higher negative SRET scores, were associated with greater symptoms of both depression and social anxiety. These patterns, however, changed in the multivariate multiple regression analysis when the residual scores of symptoms were used with comorbid symptoms partialled out. In particular, the associations between positive and negative SRET scores and depressive symptoms remained significant, whereas the associations between positive and negative SRET scores and social anxiety symptoms were not significant. These results suggest that behavioral markers of self-schemas may be uniquely associated with depressive symptoms but not social anxiety symptoms in a community sample of older children.
As discussed earlier, the behavioral SRET scores were calculated as the ratio of the number of positive (or negative) words both endorsed and recalled to the total number of words endorsed. By including the unexpected free recall component, the behavioral SRET scores may tap into a more latent aspect of self-schematic processing. Therefore, our results suggest that the more latent aspect of self-schematic processing may be distinctively associated with emerging symptoms of depression but not social anxiety. This is compatible with certain cognitive accounts of depression in the literature, which propose that negatively biased cognition portends depression by influencing automatic or implicit processes rather than explicit, conscious processes (Ingram et al., 1998; Scher et al., 2005). This proposal has also been supported by meta-analytic data showing that implicit cognition reliably predicts past, current, and prospective depression (Phillips et al., 2010).
While we did not observe an association between the SRET scores and social anxiety symptoms, a previous study on 12–13-year-old community-dwelling adolescents reported that a smaller number of self-endorsed positive words was associated with social anxiety symptoms while controlling for comorbid depressive symptoms (Alloy et al., 2012). It is important to note that this study used a different indicator of the SRET, the number of self-endorsed words, which did not incorporate performance on the unexpected free recall task and therefore may reflect a different aspect of self-referential processing. Further, the Alloy et al. study examined a more diverse sample (56% African American, 33.7% < $30,000 annual family income) than ours (87.5% White, mostly middle class), which might also have contributed to the different findings.
Associations between the SRET-elicited LPP and symptoms of depression and social anxiety
Our findings of the LPP component in association with symptoms were more equivocal. Bivariate correlations yielded a marginally significant positive correlation between the LPP in the positive condition and social anxiety symptoms. In the multivariate multiple regression analysis, a greater LPP amplitude in both the positive and negative conditions was marginally associated with greater symptoms of social anxiety, with depressive symptoms partialled out. While these marginally significant results prevented conclusive interpretations, they appeared to be consistent with some previous findings based on non-referential emotion processing paradigms. For example, a recent study in clinically anxious 9–14-year-old youth reported that higher anxiety symptoms were associated with a larger LPP amplitude elicited by both pleasant and unpleasant pictures (Bylsma et al., 2022). Four-year-old children with higher anxiety symptoms displayed an enlarged LPP toward both pleasant and unpleasant images relative to neutral images, although these effects did not survive multiple comparison correction (McLean et al., 2020). More former studies, however, reported significant results in the negative condition only. For example, increased LPPs elicited by threatening faces have been observed in children with social anxiety disorder (Kujawa et al., 2015; Schwab & Schienle, 2017, 2018). In typically developing children of ages 5–7, larger LPPs to unpleasant pictures were associated with greater anxiety symptoms and fearful behaviors (DeCicco et al., 2012).
It is noteworthy that these previous studies all used non-referential emotion processing paradigms (e.g., a passive viewing task) and many of them failed to account for comorbid depressive symptoms. In the context of self-referential processing, the LPP-social anxiety association may be more valence-general. The LPP is considered a neural marker of in-depth, elaborative, and deliberate processing of socio-emotionally salient information (e.g., Foti et al., 2009; Foti & Hajcak, 2008; Macnamara et al., 2009). In the SRET paradigm, when trying to decide whether a personal trait (positive or negative) describe themselves, some children may tend to overly deliberate on these traits or show heightened emotional reactivity regardless of the valence, reflected by a larger LPP. This pattern may turn out to be maladaptive with a particular link to social anxiety, a core feature of which is the fear of, or hypersensitivity to, social evaluation of oneself (Rapee & Heimberg, 1997). Indeed, previous research suggests that social anxiety is linked with not only fear of negative evaluation but also fear of positive evaluation (e.g., Fredrick & Luebbe, 2020; Weeks et al., 2008; Weeks & Howell, 2012).
It is unclear why the SRET-elicited LPP was not associated with depressive symptoms while partialling out social anxiety symptoms. Considering the observed unique associations between behavioral SRET scores and depressive symptom, we speculate the behavioral SRET scores and SRET-elicited LPP may reflect different facets of self-schematic processing, hence showing differential links with depressive symptoms. As discussed earlier, the behavioral SRET scores, by incorporating the incidental free recall task, may reflect a more latent aspect of self-schematic processing. On the other hand, the LPP was elicited during the endorsement phase of the SRET, when participants were deliberately making decisions on whether a given personal trait was descriptive of themselves. Compared to earlier EPR components (e.g., P1) that reflect the very initial stage of automatic information processing, the LPP, an indicator of more in-depth, elaborate processing, may reflect a more deliberate facet of self-schematic processing.
Strengths, limitations, and future directions
Our study had several strengths. We used multiple measurements including a behavioral paradigm, questionnaires, and ERPs, with ERPs tapping into the neural underpinnings of self-schematic processing. We took a dimensional approach by examining an unselected, community-dwelling youth sample characterized by emerging symptoms of depression and social anxiety, with the assumption that sub-clinical and clinical manifestations share the same mechanisms of development (Cicchetti & Toth, 2009). Our participants spanned a relatively narrow age range (nine-12 years), minimizing the potentially confounding effect of age. Considering the heterogeneity of anxiety, we focused on the specific category of social anxiety, which typically proliferates around our target ages and may be particularly tied to self-evaluative processes compared to other categories of anxiety.
However, we did not control for concurrent anxiety symptoms of other categories (e.g., generalized anxiety symptoms) that may be present in our sample. Our cross-sectional design could not establish directional associations between behavioral SRET scores, ERP markers, and symptoms. It is also worth mentioning that both the positive and negative SRET scores in our data were low (positive score range 0–0.38, negative score range 0–0.25). This may be related to the fact that we used a greater number of words (30 positive, 30 negative) compared to some previous studies (e.g., 12 positive, 12 negative, Goldstein et al., 2015; Liu et al., 2020, 2021). While using more words improved the validity of the task, it may have made the free recall task more difficult for children. However, the number of words we used was still insufficient to isolate the neural underpinnings of words being endorsed from those not endorsed. Future studies using an even greater number of words and an unexpected recognition task may be able to better tap into the individual variability in the memory component of this paradigm and in the meantime, tease apart the neural underpinnings of words endorsed versus not endorsed.
Further, our community sample showed generally low symptoms of depression and social anxiety below the clinical cut-offs (Birmaher et al., 1999; Kovacs, 1978, 2015); therefore, our results may not be readily generalizable to clinical samples with diagnosable depressive and social anxiety disorders. Finally, we conducted a negative mood induction before the SRET paradigm. Although some studies suggested that a negative mood induction was important to activate the latent construct of self-schemas (Abela & Hankin, 2008), we acknowledge that the mood induction might have impacted our results and in future research, a comparison between the SRET with and without preceding mood induction will help clarify the influences of induced mood on self-schematic processing in relation to psychopathology.
For future research, we plan to follow this cohort up into later stages of adolescence that are marked by further increases in psychopathology and greater individual variation in self-schematic processing. The psychopathology literature points to a relatively earlier onset of social anxiety disorder compared to depressive disorders in youth (Graber & Sontag, 2009); social anxiety and related impairments may even play a mediating role in tethering early risk processes and depression (Krueger et al., 2014). Therefore, longitudinal data will allow us to explore not only the directional relationships between cognitive processing risks and psychopathology, but also the directionality between social anxiety and depression. With more developed vocabulary and cognition, older youth will also be able to perform the SRET with a larger number of word stimuli, allowing for more refined measurements of self-schemas and the distinction between endorsed vs. rejected words. Finally, a larger sample size will provide greater power to account for concurrent symptoms of other categories of anxiety. By collecting longitudinal, causally informative data, we aim for a more conclusive examination of the mechanisms that underlie the development of depression and social anxiety in relation to self-schematic processing.
Conclusion
Our study provides novel information concerning the differential associations between behavioral SRET scores and SRET-elicited LPP and emerging symptoms of depression and social anxiety in late childhood, a critical developmental stage for elevated risks of depression and social anxiety. We also provided the first evidence on the ERP correlates of self-schematic processing in relation to social anxiety symptoms in youth. Our findings on the early cognitive risk processes for depression and social anxiety will add to the etiological knowledge of these conditions and also inform the early identification and prevention in youth at risk for developing depressive and social anxiety disorders.
Highlights.
Late childhood is a critical time for the development of anxiety and depression
Self-referential processing (SRP) is differentially tied to anxiety and depression
Behavioral marker of SRP is associated with depressive symptoms but not anxiety
Late positive potential during SRP is marginally associated with anxiety but not depression
Acknowledgement
This work is supported by a NIGMS Centers of Biomedical Research Excellence (COBRE P20 GM103505) pilot grant to PL. The authors want to thank the families who participated in our studies, and the many individuals who contributed to data collection. The authors report no conflicts of interest.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
To evaluate the reliability of the ERP measures, we calculated Pearson’s correlations between the amplitude of odd-numbered trials and that of even-numbered trials for LPP in each condition. Correlations in both conditions were significant with above medium effect sizes, supporting good reliability of LPP (positive condition: r = .42, p < .001; negative condition, r = .43, p < .001).
References
- Abela JRZ, & Hankin BL (2008). Cognitive vulnerability to depression in children and adolescents: A developmental psychopathology perspective. In Abela JRZ & Hankin BL (Eds.), Handbook of Depression in Children and Adolescents (pp. 35–78). The Guilford Press. [Google Scholar]
- Adolph D, & Margraf J (2017). The differential relationship between trait anxiety, depression, and resting frontal α-asymmetry. Journal of Neural Transmission, 124(3), 379–386. 10.1007/s00702-016-1664-9 [DOI] [PubMed] [Google Scholar]
- Allison GO, Benau EM, Asbaghi S, Pagliacco D, Stewart JG, & Auerbach RP (2021). Neurophysiological Markers Related to Negative Self-referential Processing Differentiate Adolescent Suicide Ideators and Attempters. Biological Psychiatry Global Open Science, 1(1), 16–27. 10.1016/j.bpsgos.2021.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach RP, Bondy E, Stanton CH, Webb CA, Shankman SA, & Pizzagalli DA (2016). Self-referential processing in adolescents: Stability of behavioral and ERP markers. Psychophysiology, 53(9), 1398–1406. 10.1111/psyp.12686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach RP, Stanton CH, Proudfit GH, & Pizzagalli DA (2015). Self-referential processing in depressed adolescents: A high-density event-related potential study. Journal of Abnormal Psychology, 124(2), 233–245. 10.1037/abn0000023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT (2008). The evolution of the cognitive model of depression and its neurobiological correlates. American Journal of Psychiatry, 165(8), 969–977. 10.1176/appi.ajp.2008.08050721 [DOI] [PubMed] [Google Scholar]
- Birmaher B, Brent DA, Chiappetta L, Bridge J, Monga S, & Baugher M (1999). Psychometric properties of the Screen for Child Anxiety Related Emotional Disorders (SCARED): a replication study. Journal of the American Academy of Child and Adolescent Psychiatry, 38(10), 1230–1236. 10.1097/00004583-199910000-00011 [DOI] [PubMed] [Google Scholar]
- Blackhart GC, Minnix JA, & Kline JP (2006). Can EEG asymmetry patterns predict future development of anxiety and depression?: A preliminary study. Biological Psychology, 72(1), 46–50. 10.1016/j.biopsycho.2005.06.010 [DOI] [PubMed] [Google Scholar]
- Bradley MM, & Lang PJ (1999). Affective Norms for English Words (ANEW): Stimuli instruction, and affective ratings (Tech. Rep. No. C-1). Gainesville, FL: University of Florida, Center for Research in Psychophysiology. [Google Scholar]
- Burkhouse KL, Owens M, Feurer C, Sosoo E, Kudinova A, & Gibb BE (2017). Increased neural and pupillary reactivity to emotional faces in adolescents with current and remitted major depressive disorder. Social Cognitive and Affective Neuroscience, 12(5), 783–792. 10.1093/scan/nsw184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bylsma LM, Tan PZ, Silk JS, Forbes EE, McMakin DL, Dahl RE, Ryan ND, & Ladouceur CD (2022). The late positive potential during affective picture processing: Associations with daily life emotional functioning among adolescents with anxiety disorders. International journal of psychophysiology : official journal of the International Organization of Psychophysiology, 182, 70–80. 10.1016/j.ijpsycho.2022.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy SM, Robertson IH, & O’Connell RG (2012). Retest reliability of event-related potentials: Evidence from a variety of paradigms. Psychophysiology, 49(5), 659–664. 10.1111/j.1469-8986.2011.01349.x [DOI] [PubMed] [Google Scholar]
- Cicchetti D, & Toth SL (2009). The past achievements and future promises of developmental psychopathology: the coming of age of a discipline. Journal of Child Psychology and Psychiatry, 50(1–2), 16–25. 10.1111/j.1469-7610.2008.01979.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello EJ, Mustillo S, Erkanli A, Keeler G, & Angold A (2003). Prevalence and Development of Psychiatric Disorders in Childhood and Adolescence. Archives of General Psychiatry, 60(8), 837–844. 10.1001/archpsyc.60.8.837 [DOI] [PubMed] [Google Scholar]
- Crone EA, & Fuligni AJ (2020). Self and Others in Adolescence. Annual Review of Psychology, 71(1), 447–469. 10.1146/annurev-psych-010419-050937 [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Schupp HT, Bradley MM, Birbaumer N, & Lang PJ (2000). Brain potentials in affective picture processing: covariation with autonomic arousal and affective report. Biological Psychology, 52(2), 95–111. 10.1016/s0301-0511(99)00044-7 [DOI] [PubMed] [Google Scholar]
- Davidson RJ (1992). Anterior cerebral asymmetry and the nature of emotion. Brain and Cognition, 20(1), 125–151. 10.1016/0278-2626(92)90065-t [DOI] [PubMed] [Google Scholar]
- DeCicco JM, O’Toole LJ, & Dennis TA (2014). The late positive potential as a neural signature for cognitive reappraisal in children. Developmental Neuropsychology, 39(7), 497–515. 10.1080/87565641.2014.959171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCicco JM, Solomon B, & Dennis TA (2012). Neural correlates of cognitive reappraisal in children: An ERP study. Developmental Cognitive Neuroscience, 2(1), 70–80. 10.1016/j.dcn.2011.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A, & Makeig S (2004). EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods, 134(1), 9–21. 10.1016/j.jneumeth.2003.10.009 [DOI] [PubMed] [Google Scholar]
- Derry PA, & Kuiper NA (1981). Schematic processing and self-reference in clinical depression. Journal of Abnormal Psychology, 90(4), 286–297. 10.1037/0021-843X.90.4.286 [DOI] [PubMed] [Google Scholar]
- Disner SG, Beevers CG, Haigh EAP, & Beck AT (2011). Neural mechanisms of the cognitive model of depression. Nature Reviews Neuroscience, 12(8), 467–477. 10.1038/nrn3027 [DOI] [PubMed] [Google Scholar]
- Dixon ML, Moodie CA, Goldin PR, Farb N, Heimberg RG, Zhang J, & Gross JJ (2022). Frontoparietal and Default Mode Network Contributions to Self-Referential Processing in Social Anxiety Disorder. Cognitive, Affective, & Behavioral Neuroscience, 22(1), 187–198. 10.3758/s13415-021-00933-6 [DOI] [PubMed] [Google Scholar]
- Dobson KS, & Shaw BF (1987). Specificity and Stability of Self-Referent Encoding in Clinical Depression. Journal of Abnormal Psychology, 96(1), 34–40. 10.1037/0021-843X.96.1.34 [DOI] [PubMed] [Google Scholar]
- Foti D, & Hajcak G (2008). Deconstructing reappraisal: descriptions preceding arousing pictures modulate the subsequent neural response. Journal of Cognitive Neuroscience, 20(6), 977–988. 10.1162/jocn.2008.20066 [DOI] [PubMed] [Google Scholar]
- Foti D, Hajcak G, & Dien J (2009). Differentiating neural responses to emotional pictures: evidence from temporal-spatial PCA. Psychophysiology, 46(3), 521–530. 10.1111/j.1469-8986.2009.00796.x [DOI] [PubMed] [Google Scholar]
- Fredrick JW, & Luebbe AM (2020). Fear of positive evaluation and social anxiety: A systematic review of trait-based findings. Journal of Affective Disorders, 265, 157–168. 10.1016/j.jad.2020.01.042 [DOI] [PubMed] [Google Scholar]
- Fu X, Taber-Thomas BC, & Pérez-Edgar K (2017). Frontolimbic functioning during threat-related attention: Relations to early behavioral inhibition and anxiety in children. Biological Psychology, 122, 98–109. 10.1016/j.biopsycho.2015.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, Jazaieri H, Ziv M, Kraemer H, Heimberg RG, & Gross JJ (2013). Changes in positive self-views mediate the effect of cognitive-behavioral therapy for social anxiety disorder. In Clinical Psychological Science (Vol. 1, Issue 3, pp. 301–310). Sage Publications. 10.1177/2167702613476867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein BL, Hayden EP, & Klein DN (2015). Stability of self-referent encoding task performance and associations with change in depressive symptoms from early to middle childhood. Cognition and Emotion, 29(8), 1445–1455. 10.1080/02699931.2014.990358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH (1998). EEG Alpha Asymmetry, Depression, and Cognitive Functioning. Cognition and Emotion, 12(3), 449–478. 10.1080/026999398379673 [DOI] [Google Scholar]
- Gotlib IH, & Joormann J (2010). Cognition and Depression: Current Status and Future Directions. Annual Review of Clinical Psychology, 6(1), 285–312. 10.1146/annurev.clinpsy.121208.131305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Joormann J, Minor KL, & Cooney RE (2006). Cognitive and Biological Functioning in Children at Risk for Depression. In Canli T (Ed.), Biology of Personality and Individual Differences (pp. 353–382). The Guilford Press. [Google Scholar]
- Graber JA, & Sontag LM (2009). Internalizing problems during adolescence. In Handbook of adolescent psychology: Individual bases of adolescent development, Vol. 1, 3rd ed. (pp. 642–682). John Wiley & Sons Inc. 10.1002/9780470479193.adlpsy001020 [DOI] [Google Scholar]
- Grunewald M, Döhnert M, Brandeis D, Klein AM, von Klitzing K, Matuschek T, & Stadelmann S (2019). Attenuated LPP to Emotional Face Stimuli Associated with Parent- and Self-Reported Depression in Children and Adolescents. Journal of Abnormal Child Psychology, 47(1), 109–118. 10.1007/s10802-018-0429-3 [DOI] [PubMed] [Google Scholar]
- Hammen C, & Zupan BA (1984). Self-schemas, depression, and the processing of personal information in children. Journal of Experimental Child Psychology, 37(3), 598–608. 10.1016/0022-0965(84)90079-1 [DOI] [PubMed] [Google Scholar]
- Harrison NR, & Chassy P (2019). Habitual use of cognitive reappraisal is associated with decreased amplitude of the late positive potential (LPP) elicited by threatening pictures. Journal of Psychophysiology, 33, 22–31. 10.1027/0269-8803/a000202 [DOI] [Google Scholar]
- Hayden EP, Hankin BL, Mackrell SVM, Sheikh HI, Jordan PL, Dozois DJA, Singh SM, Olino TM, & Badanes LS (2014). Parental depression and child cognitive vulnerability predict children’s cortisol reactivity. Development and Psychopathology, 26(4pt2), 1445–1460. 10.1017/S0954579414001138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden EP, Olino TM, Mackrell SVM, Jordan PL, Desjardins J, & Katsiroumbas P (2013). Cognitive vulnerability to depression during middle childhood: Stability and associations with maternal affective styles and parental depression. Personality and Individual Differences, 55(8), 892–897. 10.1016/j.paid.2013.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs N, & Hofmann SG (2001). Information processing in social phobia: a critical review. Clinical psychology review, 21(5), 751–770. 10.1016/s0272-7358(00)00067-2 [DOI] [PubMed] [Google Scholar]
- Henriques JB, & Davidson RJ (1991). Left frontal hypoactivation in depression. Journal of Abnormal Psychology, 100(4), 535–545. 10.1037//0021-843x.100.4.535 [DOI] [PubMed] [Google Scholar]
- Hudson A, Green ES, Wilson MJG, Itier RJ, & Henderson HA (2021). The Prominence of Self-referential Processing across ERP and Memory Consolidation in Children. Developmental Neuropsychology, 46(8), 598–615. 10.1080/87565641.2021.1991354 [DOI] [PubMed] [Google Scholar]
- Huffmeijer R, Bakermans-Kranenburg MJ, Alink LRA, & van IJzendoorn MH (2014). Reliability of event-related potentials: The influence of number of trials and electrodes. Physiology & Behavior, 130, 13–22. 10.1016/j.physbeh.2014.03.008 [DOI] [PubMed] [Google Scholar]
- Ingram RE, Miranda J, & Segal Z. v. (1998). Cognitive vulnerability to depression: Vol. null (null, Ed.). [Google Scholar]
- Jacobs RH, Reinecke MA, Gollan JK, & Kane P (2008). Empirical evidence of cognitive vulnerability for depression among children and adolescents: A cognitive science and developmental perspective. Clinical Psychology Review, 28(5), 759–782. 10.1016/j.cpr.2007.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy H, & Montreuil TC (2020). The Late Positive Potential as a Reliable Neural Marker of Cognitive Reappraisal in Children and Youth: A Brief Review of the Research Literature. Frontiers in Psychology, 11, 608522. 10.3389/fpsyg.2020.608522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney KL, Burkhouse KL, & Klumpp H (2019). Self-report and neurophysiological indicators of emotion processing and regulation in social anxiety disorder. Biological Psychology, 142, 126–131. 10.1016/j.biopsycho.2019.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs M (1978). Children’s Depression Inventory (CDI) [Database record]. APA PsycTests. 10.1037/t00788-000 [DOI] [Google Scholar]
- Kovacs M (2015). Children’s Depression Inventory (CDI and CDI 2). In The Encyclopedia of Clinical Psychology; (eds Cautin RL and Lilienfeld SO). 10.1002/9781118625392.wbecp419 [DOI] [Google Scholar]
- Krueger RF, Markon KE, Cummings CM, Caporino NE, & Kendall PC (2014). Comorbidity of anxiety and depression in children and adolescents: 20 years after. Psychological Bulletin, 2(3), 111–133. 10.1146/annurev.clinpsy.2.022305.095213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper NA, & Derry PA (1982). Depressed and nondepressed content self-reference in mild depressives. Journal of Personality, 50(1), 67–80. 10.1111/j.1467-6494.1982.tb00746.x [DOI] [PubMed] [Google Scholar]
- Kujawa A, Hajcak G, Torpey D, Kim J, & Klein DN (2012). Electrocortical reactivity to emotional faces in young children and associations with maternal and paternal depression. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 53(2), 207–215. 10.1111/j.1469-7610.2011.02461.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, MacNamara A, Fitzgerald KD, Monk CS, & Phan KL (2015). Enhanced Neural Reactivity to Threatening Faces in Anxious Youth: Evidence from Event-Related Potentials. Journal of Abnormal Child Psychology, 43(8), 1493–1501. 10.1007/s10802-015-0029-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langeslag SJE, & van Strien JW (2018). Cognitive reappraisal of snake and spider pictures: An event-related potentials study. International Journal of Psychophysiology, 130, 1–8. 10.1016/j.ijpsycho.2018.05.010 [DOI] [PubMed] [Google Scholar]
- Liu P, Hayden EP, Dougherty LR, Leung H-C, Goldstein B, & Klein DN (2021). The development of depressogenic self-schemas: Associations with children’s regional grey matter volume in ventrolateral prefrontal cortex. Development and Psychopathology, 1–11. 10.1017/S0954579421000341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, & Tan JXY (2022, August 15). Incremental validity of ERP correlates of self-referential processing in predicting emerging depressive symptoms in late childhood: Evidence from a community-dwelling sample. 10.31234/osf.io/pkt32 [DOI] [PubMed] [Google Scholar]
- Liu P, Vandemeer MRJ, Joanisse MF, Barch DM, Dozois DJA, & Hayden EP (2020). Depressogenic self-schemas are associated with smaller regional grey matter volume in never-depressed preadolescents. NeuroImage: Clinical, 28, 102422. 10.1016/j.nicl.2020.102422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Vandermeer MRJ, Joanisse MF, Barch DM, Dozois DJA, & Hayden EP (n.d.). Depressogenic self-schemas are associated with decreased grey matter volume in never-depressed preadolescents. Neuroimage: Clinical (NICL-20–744). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Vandermeer MRJ, Joanisse MF, Barch DM, Dozois DJA, & Hayden EP (2020). Neural Activity During Self-referential Processing in Children at Risk for Depression. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 5(4), 429–437. 10.1016/j.bpsc.2019.12.012 [DOI] [PubMed] [Google Scholar]
- Lopez-Calderon J, & Luck SJ (2014). ERPLAB: an open-source toolbox for the analysis of event-related potentials . In Frontiers in Human Neuroscience (Vol. 8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackrell SVM, Johnson EM, Dozois DJA, & Hayden EP (2013). Negative life events and cognitive vulnerability to depression: Informant effects and sex differences in the prediction of depressive symptoms in middle childhood. Personality and Individual Differences, 54(4), 463–468. 10.1016/j.paid.2012.09.007 [DOI] [Google Scholar]
- Macnamara A, Foti D, & Hajcak G (2009). Tell me about it: neural activity elicited by emotional pictures and preceding descriptions. Emotion (Washington, D.C.), 9(4), 531–543. 10.1037/a0016251 [DOI] [PubMed] [Google Scholar]
- MacNamara A, Jackson TB, Fitzgerald JM, Hajcak G, & Phan KL (2019). Working Memory Load and Negative Picture Processing: Neural and Behavioral Associations With Panic, Social Anxiety, and Positive Affect. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 4(2), 151–159. 10.1016/j.bpsc.2018.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoach DS, & Agam Y (2013). Neural markers of errors as endophenotypes in neuropsychiatric disorders. Frontiers in Human Neuroscience, 7. 10.3389/fnhum.2013.00350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathersul D, Williams LM, Hopkinson PJ, & Kemp AH (2008). Investigating models of affect: Relationships among EEG alpha asymmetry, depression, and anxiety. In Emotion (Vol. 8, pp. 560–572). American Psychological Association. 10.1037/a0012811 [DOI] [PubMed] [Google Scholar]
- McLean MA, Van den Bergh BRH, Baart M, Vroomen J, & van den Heuvel MI (2020). The late positive potential (LPP): A neural marker of internalizing problems in early childhood. International Journal of Psychophysiology, 155, 78–86. 10.1016/j.ijpsycho.2020.06.005 [DOI] [PubMed] [Google Scholar]
- Moser JS, Huppert JD, Duval E, & Simons RF (2008). Face processing biases in social anxiety: An electrophysiological study. Biological Psychology, 78(1), 93–103. 10.1016/j.biopsycho.2008.01.005 [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, & Panksepp J (2006). Self-referential processing in our brain-A meta-analysis of imaging studies on the self. NeuroImage, 31(1), 440–457. 10.1016/j.neuroimage.2005.12.002 [DOI] [PubMed] [Google Scholar]
- Pfeifer JH, Dapretto M, & Lieberman MD (2016). The neural foundations of evaluative self-knowledge in middle childhood, early adolescence, and adulthood. In Developmental social cognitive neuroscience (pp. 155–178). Psychology Press. [Google Scholar]
- Phillips WJ, Hine DW, & Thorsteinsson EB (2010). Implicit cognition and depression: A meta-analysis. Clinical Psychology Review, 30(6), 691–709. 10.1016/j.cpr.2010.05.002 [DOI] [PubMed] [Google Scholar]
- Prieto SL, Cole DA, & Tageson CW (1992). Depressive self-schemas in clinic and nonclinic children. Cognitive Therapy and Research, 16(5), 521–534. 10.1007/BF01175139 [DOI] [Google Scholar]
- Rapee RM, & Heimberg RG (1997). A cognitive-behavioral model of anxiety in social phobia. Behaviour Research and Therapy, 35(8), 741–756. 10.1016/S0005-7967(97)00022-3 [DOI] [PubMed] [Google Scholar]
- Scher CD, Ingram RE, & Segal Z. v. (2005). Cognitive reactivity and vulnerability: Empirical evaluation of construct activation and cognitive diatheses in unipolar depression. Clinical Psychology Review, 25(4), 487–510. 10.1016/j.cpr.2005.01.005 [DOI] [PubMed] [Google Scholar]
- Schmitz J, Scheel CN, Rigon A, Gross JJ, & Blechert J (2012). You don’t like me, do you? Enhanced ERP responses to averted eye gaze in social anxiety. Biological Psychology, 91(2), 263–269. 10.1016/j.biopsycho.2012.07.004 [DOI] [PubMed] [Google Scholar]
- Schwab D, & Schienle A (2017). Facial emotion processing in pediatric social anxiety disorder: Relevance of situational context. Journal of Anxiety Disorders, 50, 40–46. 10.1016/j.janxdis.2017.05.005 [DOI] [PubMed] [Google Scholar]
- Schwab D, & Schienle A (2018). Facial affect processing in social anxiety disorder with early onset: evidence of an intensity amplification bias. Social Neuroscience, 13(3), 318–327. 10.1080/17470919.2017.1304990 [DOI] [PubMed] [Google Scholar]
- Shestyuk AY, & Deldin PJ (2010). Automatic and strategic representation of the self in major depression: trait and state abnormalities. The American Journal of Psychiatry, 167(5), 536–544. 10.1176/appi.ajp.2009.06091444 [DOI] [PubMed] [Google Scholar]
- Speed BC, Nelson BD, Auerbach RP, Klein DN, & Hajcak G (2016). Depression Risk and Electrocortical Reactivity During Self-Referential Emotional Processing in 8 to 14 Year-Old Girls. Journal of Abnormal Psychology, 125(5), 607–619. 10.1037/abn0000173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thai N, Taber-Thomas BC, & Pérez-Edgar KE (2016). Neural correlates of attention biases, behavioral inhibition, and social anxiety in children: An ERP study. Developmental Cognitive Neuroscience, 19, 200–210. 10.1016/j.dcn.2016.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurston MD, Goldin PR, Heimberg R, & Gross JJ (2017). Self-views in social anxiety disorder: The impact of CBT versus MBSR. Journal of Anxiety Disorders, 47, 83–90. 10.1016/j.janxdis.2017.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cauwenberge V, Van Leeuwen K, Hoppenbrouwers K, & Wiersema JR (2017). Developmental changes in neural correlates of cognitive reappraisal: An ERP study using the late positive potential. Neuropsychologia, 95, 94–100. 10.1016/j.neuropsychologia.2016.12.015 [DOI] [PubMed] [Google Scholar]
- Weeks JW, Heimberg RG, Rodebaugh TL, & Norton PJ (2008). Exploring the relationship between fear of positive evaluation and social anxiety. Journal of Anxiety Disorders, 22(3), 386–400. 10.1016/j.janxdis.2007.04.009 [DOI] [PubMed] [Google Scholar]
- Weeks JW, & Howell AN (2012). The Bivalent Fear of Evaluation Model of Social Anxiety: Further Integrating Findings on Fears of Positive and Negative Evaluation. Cognitive Behaviour Therapy, 41(2), 83–95. 10.1080/16506073.2012.661452 [DOI] [PubMed] [Google Scholar]
- Weinberg A, Correa KA, Stevens ES, & Shankman SA (2021). The emotion-elicited late positive potential is stable across five testing sessions. Psychophysiology, 58(11), e13904. 10.1111/psyp.13904 [DOI] [PubMed] [Google Scholar]
- Weinberg A, Hilgard J, Bartholow BD, & Hajcak G (2012). Emotional targets: evaluative categorization as a function of context and content. International Journal of Psychophysiology : Official Journal of the International Organization of Psychophysiology, 84(2), 149–154. 10.1016/j.ijpsycho.2012.01.023 [DOI] [PubMed] [Google Scholar]
- Woodman GF (2010). A brief introduction to the use of event-related potentials in studies of perception and attention. Attention, Perception & Psychophysics, 72(8), 2031–2046. 10.3758/APP.72.8.2031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zupan BA, Hammen C, & Jaenicke C (1987). The effects of current mood and prior depressive history on self-schematic processing in children. Journal of Experimental Child Psychology, 43(1), 149–158. 10.1016/0022-0965(87)90056-7 [DOI] [PubMed] [Google Scholar]


