Abstract
Background:
The increasing number of childhood cancer survivors necessitates continued follow-up to monitor for long-term complications. Inequities in loss to follow-up for patients enrolled on pediatric clinical trials have not been well studied.
Methods:
This was a retrospective study of 21,084 patients residing in the United States enrolled on phase 2/3 and phase 3 Children’s Oncology Group (COG) trials between January 1, 2000 and March 31, 2021. Rates of loss to follow-up to COG were evaluated using log-rank tests and multivariable Cox proportional hazards regression models with adjusted hazard ratios (HRs). Demographic characteristics included age at enrollment, race, ethnicity, and zip code level socioeconomic data.
Results:
Adolescent and young adult (AYA) patients 15–39 years old at diagnosis had an increased hazard of loss to follow-up compared to patients 0–14 years old (HR, 1.89; 95% confidence interval (CI), 1.76–2.02). In the overall cohort, non-Hispanic Blacks were found to have an increased hazard of loss to follow-up compared to non-Hispanic Whites (HR, 1.56; 95% CI, 1.43–1.70). Among AYAs, the highest loss to follow-up rates were among non-Hispanic Blacks (69.8% ± 3.1%), patients on germ cell tumor trials (78.2% ± 9.2%), and patients living in zip codes with a median household income ≤150% of the federal poverty line at diagnosis (66.7% ± 2.4%).
Conclusions:
AYAs, racial and ethnic minority patients, and those living in lower socioeconomic status areas had the highest rates of loss to follow-up among clinical trial participants. Targeted interventions are warranted to ensure equitable follow-up and improved assessment of long-term outcomes.
Plain Language Summary
Little is known about disparities in loss to follow-up for pediatric cancer clinical trial participants.
In this study, we found that participants who were adolescents and young adults when treated, those who identified as a racial and/or ethnic minority, or those residing in areas with lower socioeconomic status at diagnosis were associated with higher rates of loss to follow-up.
As a result, the ability to assess their long-term survival, treatment-related health conditions, and quality of life is hindered.
These findings suggest the need for targeted interventions to improve long-term follow-up among disadvantaged pediatric clinical trial participants.
Keywords: adolescent and young adults, clinical trials, long-term follow-up, loss to follow-up, pediatrics, racial and ethnic disparities, socioeconomic disparities
INTRODUCTION
Loss to follow-up after cancer therapy is a challenge in the pediatric, adolescent, and young adult (AYA) cancer populations.1 These survivors have a 14% gap in life expectancy compared to the noncancer population, and are found, on average, to have five severe, life threatening, or fatal chronic health conditions by the time they are 50 years old.2,3 One challenge in delivering appropriate long-term care to childhood and AYA cancer survivors is discontinuation of follow-up at a cancer center.4 One study found that merely 17.8% of patients received survivor-focused care and only 14.6% of those patients did so at a comprehensive cancer center.5 A majority of cancer survivors receive their health care from primary care providers, who often lack in-depth knowledge about treatment-related complications.5
AYAs, defined as individuals 15 to 39 years old, account for approximately 90,000 of the new cancer diagnoses per year in the United States.6,7 AYAs are more likely to be lost to follow-up compared with younger children.1 One study found that only approximately 50% of young adult survivors of childhood cancers return for follow-up 10 years after completion of therapy.1 Although AYAs have cancers that are treated both by pediatric and adult oncologists, they often experience challenges finding age-appropriate care given their unique psychosocial and financial needs.8–10 Inequities have been noted in long-term follow-up among AYAs, with non-Hispanic Black and uninsured patients having decreased rates of follow-up.1,5 Given AYAs’ increased therapy-related toxicities, specialized follow-up is crucial to optimize AYAs’ long-term health outcomes.11–15
Loss to follow-up can cause discrepancies in clinical trial data collection and capturing information about long-term survival, serious adverse conditions, and late relapses.16,17 Many studies in literature evaluating loss to follow-up have been single-institution studies or reports from childhood cancer survivorship cohorts, which include both clinical trial and nonclinical trial participants. There is no study that has reported on loss to follow-up with a focus on clinical trial participants. Patients enrolled in clinical trials have more stringent follow-up guidelines, with the expectation that there would be decreased loss to follow-up among these patients.
The purpose of this study was to evaluate inequities in loss to follow-up rates among children and AYAs enrolled on clinical trials sponsored by the Children’s Oncology Group (COG), the world’s largest pediatric cooperative cancer study group, and identify risk factors associated with increased loss to follow-up by using the COG trial database.
MATERIALS AND METHODS
This study included patients residing in the United States (US) whose first enrollment was on a first-line phase 2/3 or phase 3 trial and were enrolled between January 1, 2000 and March 31, 2021. Phase 2/3 and phase 3 trials require patient report of follow-up for 10 years post completion of the therapy before being designated as completed. Patients were excluded if they were enrolled on trials that were not designated as complete or those whose first enrollment was on a relapse or nontherapeutic trials were excluded.
Data collection
For each patient included in the analysis, birth sex, race, ethnicity, country of residence, diagnosis, age at diagnosis, age at enrollment, date of enrollment, protocol number, date of last follow-up, and patient status as of the last follow-up date were obtained from the COG database.
Demographic and clinical variables
Race and ethnicity were combined and recoded as non-Hispanic White, non-Hispanic Black, non-Hispanic Asian, non-Hispanic Native Hawaiian or other Pacific Islander, and Hispanic (all races). Age at enrollment was categorized as ≤14 years of age and 15–39 years of age. Year of enrollment was reclassified by 4-year intervals: 2000–2004, 2005–2009, 2010–2014, and 2015–2019.
Socioeconomic variables
Zip-code level socioeconomic status (SES) data was obtained from the 2009–2013 American Community Survey, including median household income and percentage of residents within each zip code with a bachelor’s education or higher. Median household income values were divided into tertiles of ≤150% of the federal poverty level (FPL), >150%–300% FPL, and >300% FPL. The FPL for a four-person household in the United States based on 2021 poverty guidelines from Health and Human Services was $26,500.18 The percentage of residents within each zip code with at least a bachelor’s education was also classified into tertiles: <25%, 25% to <50%, and ≥50%.
Disease categories
The protocols were classified for analysis as follows: acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), central nervous system (CNS), Ewing sarcoma (EWS), germ cell tumors (GCT), liver tumors (HEP), Hodgkin lymphoma (HOD), neuroblastoma (NBL), non-Hodgkin lymphoma (NHL), renal tumors (REN), rhabdomyosarcoma and soft tissue tumors (RST), and rare tumors (RARE), which included patients enrolled on adrenocortical tumor, nasopharyngeal carcinoma, and retinoblastoma trials.19–21 No osteosarcoma patients were included in this study because none of the osteosarcoma protocols had completed follow-up at time of analysis. The list of included trials is provided in Table S1.
Outcome variables
The primary outcome in this study was the duration of follow-up. Date of enrollment, date of last follow-up, vital status as of the last follow-up date, and whether patients were lost to follow-up were used to derive duration of follow-up for each patient. Loss to follow-up was defined per COG as patients who were not seen in follow-up despite a documented effort to contact a patient over a 12-month time-period without success. Follow-up data collection is required for patients enrolled on COG trials unless patient is taken off study.
Statistical analysis
The Kaplan–Meier method was used to estimate probability of loss to follow-up, with standard errors assessed with the Greenwood method and 95% confidence intervals (CIs) based on the complementary log-log transformation method.22 Comparisons of probability of loss to follow-up between patients with different demographic, clinical, patient or disease characteristics were conducted with log-rank test or univariate and multivariable Cox proportional hazards regression models. A patient was considered as having an event if patient was lost to follow-up. Those who were not lost to follow-up were censored at last follow-up date. Patients who died were censored at date of death. All reported p values are two-sided, and a p value of ≤.05 was considered statistically significant. Statistical analyses were performed using StataSE Version 17.0 (College Station, Texas).
RESULTS
Patient characteristics
A total of 21,084 patients were included in the final analysis. As shown in Table 1, the majority of patients included were enrolled at ≤14 years of age, with 19.1% of patients enrolled between 15–39 years of age. A total of 54.7% of pediatric patients and 57.6% of AYAs were male. In both age groups, most patients were non-Hispanic White and enrolled on ALL trials. A total of 62.3% of pediatric patients and 62.7% of AYAs lived in zip codes with a median household income between 150% and 300% FPL.
TABLE 1.
Patient characteristics.
| Characteristic | Age at enrollment, No. % |
|
|---|---|---|
| 0–14 Years (n = 17,066) | 15–39 Years (n = 4018) | |
|
| ||
| Gender | ||
| Female | 7734 (45.3) | 1704 (42.4) |
| Male | 9332 (54.7) | 2314 (57.6) |
| Race and ethnicity | ||
| Non-Hispanic White | 9804 (57.4) | 2486 (61.9) |
| Non-Hispanic Black | 1798 (10.5) | 430 (10.7) |
| Non-Hispanic Asian | 614 (3.6) | 117 (2.9) |
| Non-Hispanic Native Hawaiian or Pacific Islander | 55 (0.3) | 17 (0.4) |
| Hispanic (all races) | 3507 (20.5) | 688 (17.1) |
| Other | 1288 (7.5) | 280 (7.0) |
| Year of enrollment | ||
| 2000–2004 | 745 (4.4) | 294 (7.3) |
| 2005–2009 | 10027 (58.8) | 2021 (50.3) |
| 2010–2014 | 5408 (31.7) | 1234 (30.7) |
| 2015–2019 | 886 (5.2) | 469 (11.7) |
| Median household incomea | ||
| ≤150% FPLb | 3588 (21.0) | 769 (19.1) |
| >150%–300% FPL | 10632 (62.3) | 2518 (62.7) |
| >300% FPL | 2665 (15.6) | 652 (16.2) |
| Unknown | 181 (1.1) | 79 (2.0) |
| % zip code with at least bachelor’s educationc | ||
| <25 | 9053 (53.0) | 2100 (52.3) |
| 25 to <50 | 6029 (35.3) | 1406 (35.0) |
| ≥50 | 1815 (10.6) | 442 (11.0) |
| Unknown | 169 (1.0) | 70 (1.7) |
| Disease group | ||
| ALL | 8024 (47.0) | 1136 (28.3) |
| AML | 2267 (13.3) | 777 (19.3) |
| CNS | 846 (5.0) | 147 (3.7) |
| EWS | 522 (3.1) | 395 (9.8) |
| GCT | 220 (1.3) | 26 (0.6) |
| HEP | 206 (1.2) | 1 (0.02) |
| HOD | 828 (4.9) | 1004 (25.0) |
| NBL | 1430 (8.4) | 15 (0.4) |
| NHL | 187 (1.1) | 61 (1.5) |
| REN | 1198 (7.0) | 4 (0.1) |
| RST | 960 (5.6) | 395 (9.8) |
| RARE | 378 (2.2) | 57 (1.4) |
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CNS, central nervous system tumors; EWS, Ewing sarcoma; FPL, federal poverty level; GCT, germ cell tumors; HEP, hepatoblastoma; HOD, Hodgkin lymphoma; NBL, neuroblastoma; NHL, non-Hodgkin lymphoma; RARE, rare tumors; REN, renal tumors; RST, rhabdomyosarcoma and soft tissue sarcomas.
Median household income based on 2009–2013 American Community Survey 4-person median household income.
Federal poverty level used in analysis is $26,500, based on 2021 poverty guidelines delineated by Health and Human Services.
Data obtained from 2009 to 2013 American Community Survey.
Loss to follow-up in overall cohort
The 5- and 10-year rates (±standard error) of loss to follow-up for the entire cohort were 11.7 ± 0.2% and 37.7 ± 0.4%, respectively. AYAs had increased rates of loss to follow-up compared to the pediatric group at 5 years (22.4% ± 0.8% vs. 9.5% ± 0.2%) and 10 years (58.5% ± 1.1% vs. 33.6% ± 0.4%) (Table 2, Figure S1A). Non-Hispanic Blacks had the highest probability of loss to follow-up compared to non-Hispanic Whites (50.6 ± 1.4% vs. 35.5 ± 0.5%) (Table 2, Figure S1B). When evaluating by disease group, patients enrolled on HOD and GCT trials had the highest probabilities of loss to follow-up at 10 years (HOD, 60.6% ± 1.2%; GCT, 56.6% ± 3.4%) (Table 2, Figure S1C). Patients residing in areas where the median household income is ≤150% FPL had the highest probability of loss to follow-up compared to higher income areas (Table 2, Figure S1D). Younger AYAs 15–21 years old had a 10-year loss to follow-up rate of 57.5% ± 1.0% versus 48.5% ± 5.5% in older AYAs 22–39 years old, but this was not statistically different (Figure S2).
TABLE 2.
5- and 10-Year loss to follow-up rates and multivariable analysis of hazard of loss to follow-up for total cohort.
| Characteristic | 5-Year loss to follow-up rate ± SE | 10-Year loss to follow-up rate ± SE | HRa (95% CI) | p |
|---|---|---|---|---|
|
| ||||
| Age at enrollment, years | <.001 | |||
| ≤14 | 9.5 ± 0.2% | 33.6 ± 0.4% | [Ref] | |
| 15–39 | 22.4 ± 0.8% | 58.5 ± 1.1% | 1.89 (1.76–2.02) | |
| Gender | .06 | |||
| Male | 11.8 ± 0.3% | 38.1 ± 0.6% | [Ref] | |
| Female | 11.6 ± 0.4% | 37.2 ± 0.6% | 0.95 (0.90–1.00) | |
| Race and ethnicity | <.001 | |||
| Non-Hispanic White | 10.3 ± 0.3% | 35.5 ± 0.5% | [Ref] | |
| Non-Hispanic Black | 19.2 ± 0.9% | 50.6 ± 1.4% | 1.56 (1.43–1.70) | |
| Non-Hispanic Asian | 14.0 ± 1.4% | 37.5 ± 2.2% | 1.16 (1.00–1.35) | |
| Non-Hispanic Native Hawaiian or Pacific Islander | 10.7 ± 4.2% | 36.8 ± 7.1% | 1.09 (0.67–1.76) | |
| Hispanic (all races) | 11.1 ± 0.5% | 36.7 ± 0.9% | 1.06 (0.99–1.14) | |
| Other | 13.4 ± 1.0% | 40.6 ± 1.6% | 1.25 (1.13–1.38) | |
| Median household incomeb | <.001 | |||
| >300% FPL | 11.4 ± 0.6% | 37.8 ± 1.0% | [Ref] | |
| >150%–300% FPL | 11.0 ± 0.3% | 36.3 ± 0.5% | 0.87 (0.80–0.95) | |
| ≤150% FPLc | 14.4 ± 0.6% | 42.0 ± 0.9% | 0.99 (0.88–1.10) | |
| % zip code with at least bachelor’s educationd | <.001 | |||
| ≥50 | 10.2 ± 0.7% | 34.8 ± 1.2% | [Ref] | |
| 25 to <50 | 11.2 ± 0.4% | 36.5 ± 0.7% | 1.18 (1.06–1.31) | |
| <25 | 12.4 ± 0.3% | 39.1 ± 0.6% | 1.24 (1.11–1.39) | |
| Year of enrollment | .02 | |||
| 2000–2004 | 14.3 ± 1.2% | 44.5 ± 1.9% | [Ref] | |
| 2005–2009 | 11.8 ± 0.3% | 38.3 ± 0.5% | 1.05 (0.93–1.18) | |
| 2010–2014 | 11.3 ± 0.4% | 34.3 ± 0.8% | 0.97 (0.85–1.11) | |
| 2015–2019 | 11.4 ± 1.5% | Insufficient follow-up | 0.77 (0.58–1.03) | |
| Disease group | <.001 | |||
| ALL | 7.9 ± 0.3% | 33.5 ± 0.6% | [Ref] | |
| AML | 13.9 ± 0.8% | 42.5 ± 1.5% | 1.34 (1.22–1.48) | |
| CNS | 5.9 ± 0.9% | 24.2 ± 1.9% | 0.69 (0.58–0.81) | |
| EWS | 10.3 ± 1.3% | 39.6 ± 5.9% | 1.08 (0.88–1.33) | |
| GCT | 21.5 ± 2.8% | 56.6 ± 3.4% | 2.12 (1.76–2.55) | |
| HEP | 8.9 ± 2.2% | 23.3 ± 3.9% | 0.78 (0.55–1.12) | |
| HOD | 25.8 ± 1.1% | 60.6 ± 1.2% | 1.81 (1.66–1.97) | |
| NBL | 8.3 ± 0.9% | 22.6 ± 1.4% | 0.73 (0.63–0.84) | |
| NHL | 13.2 ± 2.3% | 37.7 ± 6.2% | 1.35 (1.02–1.78) | |
| REN | 12.6 ± 1.0% | 33.4 ± 2.7% | 1.18 (1.04–1.33) | |
| RST | 15.1 ± 1.1% | 41.8 ± 1.7% | 1.30 (1.17–1.46) | |
| RARE | 24.2 ± 2.2% | 51.6 ± 3.1% | 1.83 (1.54–2.18) | |
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CI, confidence interval; CNS, central nervous system tumors; EWS, Ewing sarcoma; FPL, federal poverty level; GCT, germ cell tumors; HEP, hepatoblastoma; HOD, Hodgkin lymphoma; HR, adjusted hazard ratio; NBL, neuroblastoma; NHL, non-Hodgkin lymphoma; RARE, rare tumors; REN, renal tumors; RST, rhabdomyosarcoma and soft tissue sarcomas; SE, standard error.
All hazard ratios were based on the multivariable Cox regression model that included all the characteristic variables listed in this table.
Median household income based on 2009–2013 American Community Survey 4-person median household income.
Federal poverty level used in analysis is $26,500, based on 2021 poverty guidelines delineated by Health and Human Services.
Data obtained from 2009–2013 American Community Survey.
As shown in Table 2, on multivariable analysis, AYAs had an increased hazard of loss to follow-up (adjusted hazard ratio [HR], 1.89; 95% CI, 1.76–2.02) compared to pediatric age group. Compared to non-Hispanic Whites, non-Hispanic Blacks had the highest hazard of loss to follow-up (HR, 1.56; 95% CI, 1.43–1.70), followed by non-Hispanic Asians (HR, 1.16; 95% CI, 1.00–1.35). When evaluating by disease group, patients with the highest hazard of loss to follow-up were those enrolled on GCT and HOD trials (HR, 2.12; 95% CI, 1.76–2.55 and HR 1.81; 95% CI, 1.66–1.97, respectively, reference = ALL).
Loss to follow-up in cohort stratified by age
The results were stratified by age into two categories: pediatric patients 0–14 years old (n = 17,066) and AYAs 15–39 years old (n = 4018). As seen in Table 3, within all patient characteristics, the AYA cohort consistently had higher rates and hazards of loss to follow-up at 10 years compared to the pediatric cohort. Figure 1 shows the probability of loss to follow-up stratified by gender, race and ethnicity, median household income, and education level within the AYA cohort. Female AYAs had a decreased rate of loss to follow-up (female: 55.9% ± 1.6% vs. male: 60.5% ± 1.4%) compared to male AYAs (Figure 1A, Table 3).
TABLE 3.
10-Year loss to follow-up rates and multivariable analysis of hazard of loss to follow-up stratified by age.
| Characteristic | Pediatric patients (0–14 years old) |
AYA patients (15–39 years old) |
||||
|---|---|---|---|---|---|---|
| 10-Year loss to follow-up rate ± SE | HRa (95% CI) | p | 10-Year loss to follow-up rate ± SE | HRa (95% CI) | p | |
|
| ||||||
| Gender | .79 | <.001 | ||||
| Male | 33.4 ± 0.6% | [Ref] | 60.5 ± 1.4% | [Ref] | ||
| Female | 33.7 ± 0.7% | 1.01 (0.95–1.07) | 55.9 ± 1.6% | 0.81 (0.73–0.91) | ||
| Race and ethnicity | <.001 | |||||
| Non-Hispanic White | 31.1 ± 0.6% | [Ref] | 55.4 ± 1.3% | [Ref] | <.001 | |
| Non-Hispanic Black | 46.7 ± 1.5% | 1.58 (1.44–1.75) | 69.8 ± 3.1% | 1.47 (1.23–1.76) | ||
| Non-Hispanic Asian | 34.0 ± 2.3% | 1.21 (1.02–1.43) | 57.4 ± 6.0% | 1.03 (0.77–1.39) | ||
| Non-Hispanic Native Hawaiian or Pacific Islander | 36.1 ± 8.0% | 1.37 (0.80–2.33) | 39.7 ± 17.5% | 0.62 (0.24–1.59) | ||
| Hispanic (all races) | 32.6 ± 1.0% | 1.02 (0.94–1.10) | 63.2 ± 2.7% | 1.22 (1.06–1.41) | ||
| Other | 37.4 ± 1.6% | 1.24 (1.11–1.39) | 61.3 ± 4.5% | 1.23 (0.98–1.55) | ||
| Median household incomeb | .003 | .01 | ||||
| >300% FPL | 33.7 ± 1.1% | [Ref] | 57.7 ± 2.6% | [Ref] | ||
| >150%–300% FPL | 32.3 ± 0.6% | 0.88 (0.79–0.98) | 56.3 ± 1.3% | 0.85 (0.72–1.01) | ||
| ≤150% FPLc | 37.5 ± 1.0% | 0.98 (0.86–1.11) | 66.7 ± 2.4% | 1.03 (0.83–1.27) | ||
| % Zip code with at least Bachelor’s educationd | .07 | .002 | ||||
| ≥50 | 31.0 ± 1.3% | [Ref] | 51.8 ± 3.1% | [Ref] | ||
| 25 to <50 | 32.8 ± 0.7% | 1.14 (1.00–1.28)1 | 56.0 ± 1.8% | 1.26 (1.04–1.53) | ||
| <25 | 34.6 ± 0.6% | 1.17 (1.02–1.34) | 61.8 ± 1.4% | 1.44 (1.17–1.78) | ||
| Year of enrollment | .38 | .02 | ||||
| 2000–2004 | 38.0 ± 2.2% | [Ref] | 59.6 ± 3.4% | [Ref] | ||
| 2005–2009 | 34.2 ± 0.5% | 1.00 (0.86–1.17) | 61.2 ± 1.3% | 1.10 (0.91–1.34) | ||
| 2010–2014 | 30.9 ± 0.9% | 0.94 (0.80–1.11) | 51.5 ± 2.2% | 0.97 (0.78–1.21) | ||
| 2015–2019 | Insufficient follow-up | 0.87 (0.60–1.26) | Insufficient follow-up | 0.63 (0.41–0.99) | ||
| Disease group | <.001 | <.001 | ||||
| ALL | 31.2 ± 0.6% | [Ref] | 53.3 ± 1.9% | [Ref] | ||
| AML | 38.0 ± 1.7% | 1.33 (1.18–1.49) | 57.5 ± 3.3% | 1.33 (1.11–1.59) | ||
| CNS | 20.4 ± 1.9% | 0.61 (0.50–0.75) | 47.9 ± 5.9% | 0.96 (0.69–1.33) | ||
| EWS | 38.3 ± 7.1% | 1.19 (0.90–1.57) | n/a | 0.93 (0.68–1.27) | ||
| GCT | 54.0 ± 3.7% | 2.10 (1.72–2.57) | 78.2 ± 9.2% | 2.45 (1.45–4.13) | ||
| HEP | 22.7 ± 3.9% | 0.78 (0.54–1.13) | n/a | 2.19 (1.79–2.68) | ||
| HOD | 54.7 ± 1.8% | 2.07 (1.86–2.32) | 65.6 ± 1.6% | 1.62 (1.41–1.85) | ||
| NBL | 22.6 ± 1.4% | 0.73 (0.63–0.85) | 10.0 ± 10.0% | 0.48 (0.06–4.00) | ||
| NHL | 35.5 ± 7.4% | 1.37 (0.97–1.92) | 43.1 ± 8.9% | 1.28 (0.79–2.05) | ||
| REN | 33.3 ± 2.7% | 1.19 (1.05–1.35) | n/a | 1.79 (0.40–7.92) | ||
| RST | 37.5 ± 1.8% | 1.31 (1.16–1.50) | 57.0 ± 3.7% | 1.25 (1.02–1.54) | ||
| RARE | 47.7 ± 3.3% | 1.97 (1.63–2.40) | 72.8 ± 7.7% | 1.36 (0.95–1.96) | ||
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CI, confidence interval; CNS, central nervous system tumors; EWS, Ewing sarcoma; GCT, germ cell tumors; HEP, hepatoblastoma; HOD, Hodgkin lymphoma; HR, adjusted hazard ratio; n/a, not available; NBL, neuroblastoma; NHL, non-Hodgkin lymphoma; RARE, rare tumors; Ref, reference; REN, renal tumors; RST, rhabdomyosarcoma and soft tissue sarcomas; SE, standard error.
All hazard ratios were based on the multivariable Cox regression model that included all the characteristic variables listed in this table.
Median household income based on 2009–2013 American Community Survey four-person median household income.
Federal poverty level used in analysis is $26,500, based on 2021 poverty guidelines delineated by Health and Human Services.
Data obtained from 2009 to 2013 American Community Survey.
FIGURE 1.
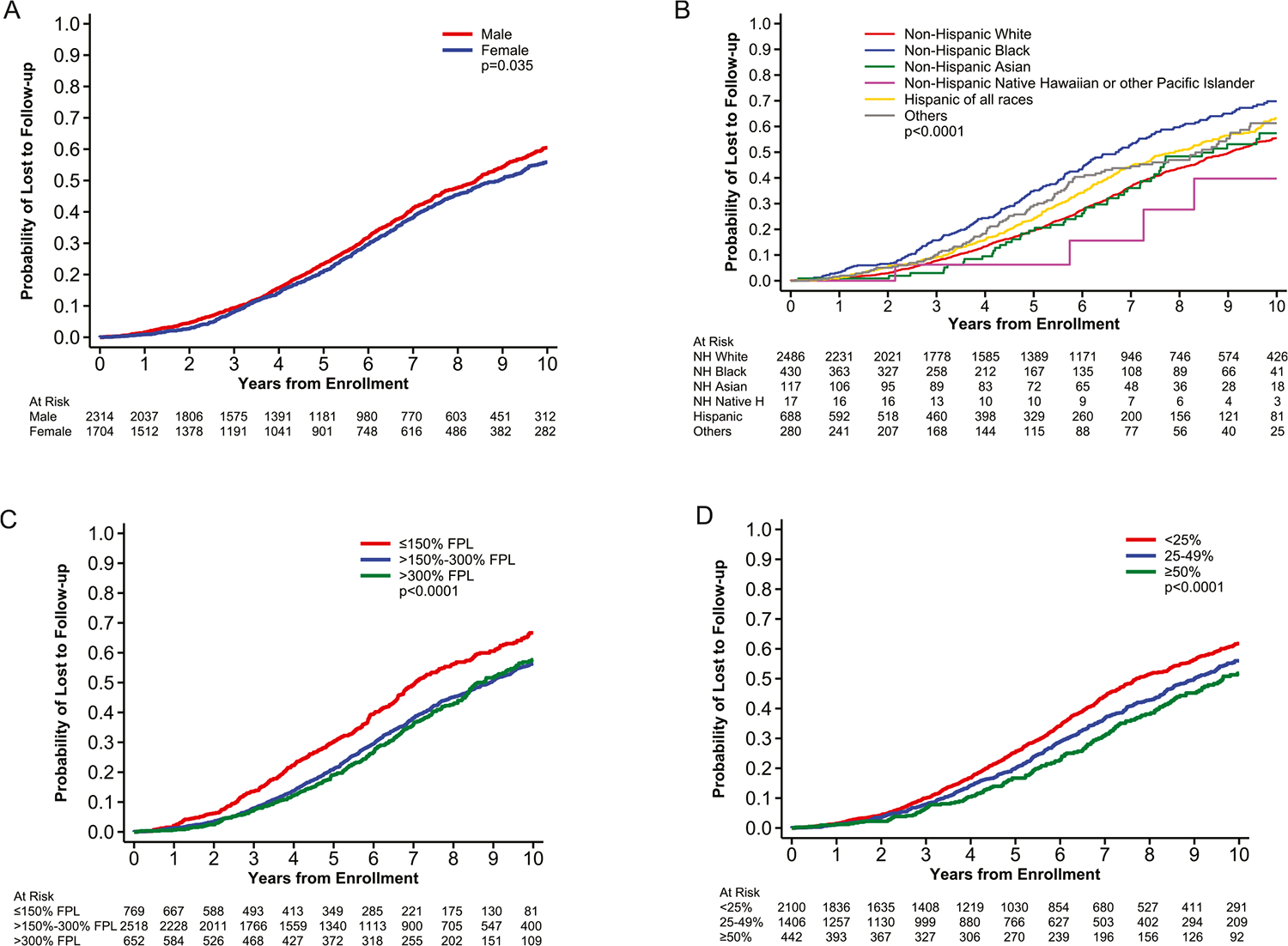
Probabilities of loss to follow-up in adolescent and young adult (AYA) patients stratified by gender, race and ethnicity, median household income, and education level. Probabilities of loss to follow-up in AYA patients stratified by (A) gender, (B) race and ethnicity, (C) median household income, and (D) education level.
Race and ethnicity and loss to follow-up
In both age cohorts, non-Hispanic Blacks had the highest 10-year loss to follow-up rates with 69.8% ± 3.1% among non-Hispanic Black AYAs (Figure 1B, Table 3) and 46.7% ± 1.5% among pediatric non-Hispanic Blacks (Table 3). Non-Hispanic Black AYAs had the highest hazard of loss to follow-up (HR, 1.47; 95% CI, 1.23–1.76, reference = non-Hispanic White). Even among non-Hispanic Whites, there was an increased rate of loss to follow-up among AYAs compared to pediatric patients (55.4% ± 1.3% vs. 31.1% ± 0.6%) (Table 3). The loss to follow-up rates of AYA non-Hispanic Asians and Hispanic patients were almost double that of their pediatric counterparts—57.4 ± 6.0% versus 34.0 ± 2.3% for non-Hispanic Asian AYAs and children and 63.2 ± 2.7% versus 32.6 ± 1.0% for Hispanic AYAs and children (Table 3).
Socioeconomic status and loss to follow-up
For both age cohorts, patients from lower income areas and areas with lower educational attainment had increased hazard of loss to follow-up (Table 3). When stratifying the data by SES indices, AYAs from areas with median household income ≤150% FPL had higher loss to follow-up rates at 10 years compared to those from areas with median household income >300% FPL (66.7 ± 2.4% vs. 57.7 ± 2.6%) (Figure 1C, Table 3). The loss to follow-up rate among AYAs from zip codes with <25% of the population having at least a bachelor’s education was higher than those from areas where ≥50% of the population had a bachelor’s education (61.8 ± 1.4% vs. 51.8 ± 3.1%) (Figure 1D, Table 3).
Disease group and loss to follow-up
The patients with the highest 10-year loss to follow-up rate in both age cohorts were those enrolled on GCT trials (Table 3), with 54.0% ± 3.7% of pediatric patients and 78.2% ± 9.2% of AYAs enrolled on GCT trials being lost to follow-up (Table 3). The disease groups with higher hazards of loss to follow-up among AYAs were GCT (HR, 2.45; 95% CI, 1.45–4.13, reference = ALL trials) and HOD (HR, 1.62; 95% CI, 1.41–1.85, reference = ALL trials) (Table 3).
DISCUSSION
AYAs, non-Hispanic Black patients, patients residing in areas with lower SES, and patients enrolled on HOD and GCT trials had the highest rate and hazard of loss to follow-up at both 5 and 10 years after therapy completion. Compared to younger patients, AYAs had an 89% increased hazard of loss to follow-up. Among AYAs, the highest 10-year loss to follow-up rates were among non-Hispanic Blacks (69.8%), patients enrolled on GCT trials (78.2%), and patients living in areas with a lower income level (66.7%). Having more than two-thirds of patients from disadvantaged populations being lost to follow-up has significant negative implications on the inequities in access to follow-up care for these patients as well as biases in centralized data collection.
AYA trial enrollment disparities on both pediatric and adult clinical trials have been noted in prior studies, which translates to fewer AYAs having access to innovative therapies and challenges in evaluating potential differences in therapy toxicities.10,15,23–31 Even among AYAs that do enroll on trials and have more structured follow-up guidelines, our study found that they have significantly higher loss to follow-up rates compared to their younger counterparts. The results of these analyses among clinical trial participants shed light on inequities in long-term follow-up and suggest that the loss to follow-up rate may be even higher in AYAs not enrolled on trials.
Potential explanations for why AYAs have increased loss to follow-up include moving away from their initial center of treatment, changes in insurance and finances, transitioning out of pediatrics care, or coping with psychosocial challenges.32 Age limits at pediatric centers is also a significant source of loss to follow-up because most centers will not see patients past the age of 21 years. Patients might be receiving appropriate follow-up care at non-COG sites, which is not always consistently captured and can affect the accuracy of COG data collection. Provider bias also can cause loss to follow-up due to difficulties managing their higher rates of nonadherence and psychosocial challenges.1,27,33 Given that AYAs are at increased risk of developing long-term physical and psychological complications from their initial therapy, it is crucial to ensure seamless transition of care between pediatric and adult providers to provide consistent survivorship care.34–36
Another factor associated with loss to follow-up was disease group. Patients enrolled on CNS and ALL trials had lower rates of loss to follow-up, whereas those enrolled on GCT and HOD trials had higher loss to follow-up rates. Both HOD and germ cell tumors predominantly affect AYAs.37,38 Given this finding, a subset analysis was completed for AYAs, and even then, those enrolled on GCT and HOD trials were more likely to be lost to follow-up. One potential explanation could be much shorter duration of therapy in these tumors compared to other malignancies as well as variability in disease surveillance recommendations between the different clinical trials. Notably, the GCT AYA cohort was small and included only 26 patients, which does limit the generalizability of these finding in GCT patients.
Race, ethnicity, and SES were significant factors affecting loss to follow-up rates in our study population. The majority of patients enrolled were non-Hispanic Whites, suggesting that there might be inequities in trial access based on race and ethnicity. Non-Hispanic Blacks and those living in zip codes with lower SES indices had higher rate of loss to follow-up compared to other groups. Health-related social risks include housing, personal safety, financial stability, education, and food security can impact therapy adherence and overall outcomes.39–41 Racial and ethnic minority patients may be more prone to loss to follow-up due to psychosocial barriers such as distrust of the health care system due to discrimination.42,43
Financial insecurity could further restrict access to care.44 The high health care costs of both active therapy and post-therapy surveillance, especially if uninsured, cause significant financial toxicity for survivors, causing them to be more likely to forego follow-up care45–47 Although the Affordable Care Act led to a decrease in the number of uninsured patients, there persists a racial and ethnic disparity in insurance coverage, with 33.4% of Hispanics and 20.7% of non-Hispanic Blacks remaining uninsured compared to 11.8% of non-Hispanic Whites.44
The study findings emphasize the urgent need to address barriers to follow-up care at the patient, provider, institution, and health care system levels. Potential approaches to increase survivor follow-up include increasing collaboration between adult and pediatric oncologists, improving primary care provider awareness, and better understanding the financial and psychosocial barriers to continuing to follow-up with their health care team.23,25,30 Other strategies include engaging AYAs through social media or other technological platforms such as telehealth to promote follow-up and improve their supportive care.48 AYAs have been found to be rapid adopters of technology, and social media is becoming an increasing cornerstone for them to exchange ideas and seek support.49,50
Limitations
There were many strengths to using the COG data set. The large sample size lends this data to be representative of United States pediatric and AYA clinical trial enrollment with the inclusion of multiple disease types and sociodemographic heterogeneity. However, the limited sample size of AYAs in some disease groups, such as GCT, does impact the generalizability of these results to those patients. In addition, the COG database only captures clinical trial patients and does not necessarily reflect the rates of loss to follow-up for patients who received standard-of-care therapies. Additionally, the SES data available within the database is limited. Historically, COG has not collected robust individual SES data when enrolling patients. Another limitation to the database is that patient migration is not consistently captured. These patients might be receiving appropriate follow-up care elsewhere, such as with a primary care provider or with adult oncologists, which is not captured consistently in this data set.
In conclusion, this study shows that there are striking inequities in receipt of long-term follow-up care in AYAs, especially racial and/or ethnic minority patients or those living in areas with lower SES indices. The results presented in this article call for a need to actively engage AYAs and address barriers to prevent them from being lost to follow-up. Specific strategies to include those patients from racial and ethnic minorities and lower SES must be used to ensure equitable follow-up care for these clinical trial participants. Increasing access to follow-up care will help mitigate disparities, improve survival, and achieve less biased data collection and understanding of long-term outcomes for childhood and AYA cancer survivors.
Supplementary Material
ACKNOWLEDGMENTS
The research in this manuscript was supported by National Cancer Institute/National Institutes of Health funding to the Children’s Oncology Group (U10CA180886 and U10CA180899) and Vidya Puthenpura (T32CA250803).
Footnotes
CONFLICT OF INTEREST STATEMENT
A. Lindsay Frazier is on the Clinical Advisory Board of Decibel Therapeutics. Farzana D. Pashankar reports funding for providing data and/or safety monitoring for Novartis. Vidya Puthenpura reports funding from the National Cancer Institute. The other authors declare no conflicts of interest.
SUPPORTING INFORMATION
Additional supporting information can be found online in the Supporting Information section at the end of this article.
REFERENCES
- 1.Rokitka DA, Curtin C, Heffler JE, Zevon MA, Attwood K, Mahoney MC. Patterns of loss to follow-up care among childhood cancer survivors. J Adolesc Young Adult Oncol. 2017;6(1):67–73. doi: 10.1089/jayao.2016.0023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yeh JM, Ward ZJ, Chaudhry A, et al. Life expectancy of adult survivors of childhood cancer over 3 decades. JAMA Oncol. 2020;6(3):350–357. doi: 10.1001/jamaoncol.2019.5582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhakta N, Liu Q, Ness KK, et al. The cumulative burden of surviving childhood cancer: an initial report from the St Jude Lifetime Cohort Study (SJLIFE). Lancet. 2017;390(10112):2569–2582. doi: 10.1016/S0140-6736(17)31610-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolfson J, Sun CL, Kang T, Wyatt L, D’Appuzzo M, Bhatia S. Impact of treatment site in adolescents and young adults with central nervous system tumors. J Natl Cancer Inst. 2014;106(8):dju166. doi: 10.1093/jnci/dju166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nathan PC, Greenberg ML, Ness KK, et al. Medical care in long-term survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2008;26(27):4401–4409. doi: 10.1200/JCO.2008.16.9607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adolescents and Young Adults with Cancer. National Cancer Institute. Updated September 24, 2020. Accessed December 12, 2021. doi:https://www.cancer.gov/types/aya [Google Scholar]
- 7.Miller KD, Fidler-Benaoudia M, Keegan TH, Hipp HS, Jemal A, Siegel RL. Cancer statistics for adolescents and young adults, 2020. CA Cancer J Clin. 2020;70(6):443–459. doi: 10.3322/caac.21637 [DOI] [PubMed] [Google Scholar]
- 8.Collaborators GBDAYAC. The global burden of adolescent and young adult cancer in 2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Oncol. 2021;23(1):27–52. doi: 10.1016/S1470-2045(21)00581-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith AW, Seibel NL, Lewis DR, et al. Next steps for adolescent and young adult oncology workshop: an update on progress and recommendations for the future. Cancer. 2016;122(7):988–999. doi: 10.1002/cncr.29870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parsons SK, Kumar AJ. Adolescent and young adult cancer care: financial hardship and continued uncertainty. Pediatr Blood Cancer. 2019;66(4):e27587. doi: 10.1002/pbc.27587 [DOI] [PubMed] [Google Scholar]
- 11.Skinner R, Wallace WH, Levitt GA, Group UKCCSGLE. Long-term follow-up of people who have survived cancer during childhood. Lancet Oncol. 2006;7(6):489–498. doi: 10.1016/S1470-2045(06)70724-0 [DOI] [PubMed] [Google Scholar]
- 12.Jacob SA, Shaw PH. No improvement in clinical trial enrollment for adolescents and young adults with cancer at a children’s hospital. Pediatr Blood Cancer. 2017;64(12):e26638. doi: 10.1002/pbc.26638 [DOI] [PubMed] [Google Scholar]
- 13.Moke DJ, Tsai K, Hamilton AS, et al. Emerging cancer survival trends, disparities, and priorities in adolescents and young adults: a California Cancer Registry-based study. JNCI Cancer Spectr. 2019;3(2):pkz031. doi: 10.1093/jncics/pkz031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu LHA, Moke D, Tsai KY, Wojcik KY, Cockburn M, Deapen D, eds. Cancer in Los Angeles County: Survival among Adolescents and Young Adults 1988–2014. Los Angeles Cancer Surveillance Program, University of Southern California; 2017. [Google Scholar]
- 15.Colon-Otero G, Smallridge RC, Solberg LA Jr., et al. Disparities in participation in cancer clinical trials in the United States: a symptom of a healthcare system in crisis. Cancer. 2008;112(3):447–454. doi: 10.1002/cncr.23201 [DOI] [PubMed] [Google Scholar]
- 16.Roth M, Beauchemin M, Kahn JM, Bleyer A. Patterns of National Cancer Institute-sponsored clinical trial enrollment in Black adolescents and young adults. Cancer Med. 2021;10(21):7620–7628. doi: 10.1002/cam4.4292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freyer DR, Seibel NL. The clinical trials gap for adolescents and young adults with cancer: recent progress and conceptual framework for continued research. Curr Pediatr Rep. 2015;3(2):137–145. doi: 10.1007/s40124-015-0075-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Annual Update of the HHS Poverty Guidelines (Federal Register); 2021. [PubMed]
- 19.Angiolillo AL, Schore RJ, Kairalla JA, et al. Excellent outcomes with reduced frequency of vincristine and dexamethasone pulses in standard-risk B-lymphoblastic leukemia: results from Children’s Oncology Group AALL0932. J Clin Oncol. 2021;39(13):1437–1447. doi: 10.1200/JCO.20.00494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ater JL, Xia C, Mazewski CM, et al. Nonrandomized comparison of neurofibromatosis type 1 and non-neurofibromatosis type 1 children who received carboplatin and vincristine for progressive low-grade glioma: a report from the Children’s Oncology Group. Cancer. 2016;122(12):1928–1936. doi: 10.1002/cncr.29987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friedman DL, Chen L, Wolden S, et al. Dose-intensive response-based chemotherapy and radiation therapy for children and adolescents with newly diagnosed intermediate-risk hodgkin lymphoma: a report from the Children’s Oncology Group Study AHOD0031. J Clin Oncol. 2014;32(32):3651–3658. doi: 10.1200/JCO.2013.52.5410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. 2nd ed. Wiley series in Probability and Statistics. J. Wiley; 2002. xiii, 439 p. [Google Scholar]
- 23.Siembida EJ, Loomans-Kropp HA, Tami-Maury I, et al. Barriers and facilitators to adolescent and young adult cancer trial enrollment: NCORP site perspectives. JNCI Cancer Spectr. 2021;5(3):pkab027. doi: 10.1093/jncics/pkab027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marshall CS, Grimes A. Pediatric physician management as a predictor of clinical trial enrollment in adolescent and young adult cancer patients. J Adolesc Young Adult Oncol. 2020;9(2):183–189. doi: 10.1089/jayao.2019.0125 [DOI] [PubMed] [Google Scholar]
- 25.Parsons HM, Penn DC, Li Q, et al. Increased clinical trial enrollment among adolescent and young adult cancer patients between 2006 and 2012–2013 in the United States. Pediatr Blood Cancer. 2019;66(1):e27426. doi: 10.1002/pbc.27426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Unger JM, Beauchemin M, Hershman DL. Adolescent and young adult enrollment to a National Cancer Institute-sponsored National Clinical Trials Network Research Group over 25 years. Cancer. 2021;127(24):4574–4584. doi: 10.1002/cncr.33855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bleyer A, Budd T, Montello M. Adolescents and young adults with cancer: the scope of the problem and criticality of clinical trials. Cancer. 2006;107(suppl 7):1645–1655. doi: 10.1002/cncr.22102 [DOI] [PubMed] [Google Scholar]
- 28.Bleyer A, Tai E, Siegel S. Role of clinical trials in survival progress of American adolescents and young adults with cancer-and lack thereof. Pediatr Blood Cancer. 2018;65(8):e27074. doi: 10.1002/pbc.27074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Rojas T, Neven A, Terada M, et al. Access to clinical trials for adolescents and young adults with cancer: a meta-research analysis. JNCI Cancer Spectr. 2019;3(4):pkz057. doi: 10.1093/jncics/pkz057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Janardan SK, Wechsler DS. Caught in the in-between: challenges in treating adolescents and young adults with cancer. JCO Oncol Pract. 2021;17(6):299–301. doi: 10.1200/OP.21.00178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hayashi RJ. Adolescent and young adult cancer survivorship: the new frontier for investigation. Cancer. 2019;125(12):1976–1978. doi: 10.1002/cncr.32064 [DOI] [PubMed] [Google Scholar]
- 32.Richter D, Koehler M, Friedrich M, Hilgendorf I, Mehnert A, Weissflog G. Psychosocial interventions for adolescents and young adult cancer patients: a systematic review and meta-analysis. Crit Rev Oncol Hematol. 2015;95(3):370–386. doi: 10.1016/j.critrevonc.2015.04.003 [DOI] [PubMed] [Google Scholar]
- 33.Kondryn HJ, Edmondson CL, Hill J, Eden TO. Treatment nonadherence in teenage and young adult patients with cancer. Lancet Oncol. 2011;12(1):100–108. doi: 10.1016/S1470-2045(10)70069-3 [DOI] [PubMed] [Google Scholar]
- 34.Armenian SH, Xu L, Cannavale KL, Wong FL, Bhatia S, Chao C. Cause-specific mortality in survivors of adolescent and young adult cancer. Cancer. 2020;126(10):2305–2316. doi: 10.1002/cncr.32775 [DOI] [PubMed] [Google Scholar]
- 35.Stoneham SJ. AYA survivorship: the next challenge. Cancer. 2020;126(10):2116–2119. doi: 10.1002/cncr.32774 [DOI] [PubMed] [Google Scholar]
- 36.Sadak KT, Gemeda M, Grafelman MC, et al. Identifying metrics of success for transitional care practices in childhood cancer survivorship: a qualitative interview study of parents. Cancer Med. 2021;10(18):6239–6248. doi: 10.1002/cam4.4164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Close AG, Dreyzin A, Miller KD, Seynnaeve BKN, Rapkin LB. Adolescent and young adult oncology-past, present, and future. CA Cancer J Clin. 2019;69(6):485–496. doi: 10.3322/caac.21585 [DOI] [PubMed] [Google Scholar]
- 38.Fonseca A, Frazier AL, Shaikh F. Germ cell tumors in adolescents and young adults. J Oncol Pract. 2019;15(8):433–441. doi: 10.1200/JOP.19.00190 [DOI] [PubMed] [Google Scholar]
- 39.Bona K, London WB, Guo D, Frank DA, Wolfe J. Trajectory of material hardship and income poverty in families of children undergoing chemotherapy: a prospective cohort study. Pediatr Blood Cancer. 2016;63(1):105–111. doi: 10.1002/pbc.25762 [DOI] [PubMed] [Google Scholar]
- 40.Bona K, Dussel V, Orellana L, et al. Economic impact of advanced pediatric cancer on families. J Pain Symptom Manag. 2014;47(3):594–603. doi: 10.1016/j.jpainsymman.2013.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bhatia S, Landier W, Hageman L, et al. 6MP adherence in a multiracial cohort of children with acute lymphoblastic leukemia: a Children’s Oncology Group study. Blood. 2014;124(15):2345–2353. doi: 10.1182/blood-2014-01-552166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berkman JM, Dallas J, Lim J, et al. Social determinants of health affecting treatment of pediatric brain tumors. J Neurosurg Pediatr. 2019;24(2):1–7. doi: 10.3171/2019.4.PEDS18594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Penner LA, Harper FWK, Dovidio JF, et al. The impact of Black cancer patients’ race-related beliefs and attitudes on racially-discordant oncology interactions: a field study. Soc Sci Med. 2017;191:99–108. doi: 10.1016/j.socscimed.2017.08.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buchmueller TC, Levinson ZM, Levy HG, Wolfe BL. Effect of the Affordable Care Act on racial and ethnic disparities in health insurance coverage. Am J Public Health. 2016;106(8):1416–1421. doi: 10.2105/AJPH.2016.303155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Landwehr MS, Watson SE, Dolphin-Krute M. Healthcare costs and access for young adult cancer survivors: a snapshot post ACA. Am J Manag Care. 2018;24(10 Spec No).:SP440–SP441. [PubMed] [Google Scholar]
- 46.Landwehr MS, Watson SE, Macpherson CF, Novak KA, Johnson RH. The cost of cancer: a retrospective analysis of the financial impact of cancer on young adults. Cancer Med. 2016;5(5):863–870. doi: 10.1002/cam4.657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weaver KE, Rowland JH, Bellizzi KM, Aziz NM. Forgoing medical care because of cost: assessing disparities in healthcare access among cancer survivors living in the United States. Cancer. 2010;116(14):3493–3504. doi: 10.1002/cncr.25209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Casillas JN, Schwartz LF, Crespi CM, et al. The use of mobile technology and peer navigation to promote adolescent and young adult (AYA) cancer survivorship care: results of a randomized controlled trial. J Cancer Surviv. 2019;13(4):580–592. doi: 10.1007/s11764-019-00777-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Puthenpura V, Marks AM. Clinical application of digital technologies in adolescent and young adult oncology supportive care. J Adolesc Young Adult Oncol. 2021;10(2):127–130. doi: 10.1089/jayao.2020.0153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beaupin LK, Pailler ME, Brewer-Spritzer E, et al. Photographs of meaning: a novel social media intervention for adolescent and young adult cancer patients. Psycho Oncol. 2019;28(1):198–200. doi: 10.1002/pon.4896 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


