Abstract
Membrane progesterone receptors (mPRs) have been detected in breast cancer cells and tissues, but their roles in cancer progression remain unclear. Here, we demonstrate the localization, signaling, and antiapoptotic actions of mPRs in two nuclear progesterone receptor (PR)-negative breast cancer cell lines, SKBR3 and MDA-MB-468 (MB468), and mPR expression in human breast tumor biopsies. mPRα, mPRβ, and mPRγ subtypes were detected in both cell lines as well as in breast tumor tissues from 13 individuals irrespective of nuclear steroid receptor expression. Competitive receptor binding studies with a selective PR ligand, R5020, and an mPR agonist, Org OD 02-0 confirmed the presence of functional mPRs on both cancer cell lines. Progesterone treatment of either cell line caused rapid activation of an inhibitory G protein, as well as activation of p42/44 MAP kinase. Treatment with progesterone or Org OD 02-0 significantly decreased cell death and apoptosis in response to serum starvation, whereas testosterone, 17β-estradiol, dexamethasone, and R5020 and RU486 were ineffective. Progesterone treatment of MB468 cells also increased mitochondrial membrane potential and Akt activity, but no decrease in caspase 3 activity was observed. Knockdown of mPRα expression in MB468 cells by siRNA transfection blocked the inhibitory effects of progesterone on cell death. The results indicate that progesterone can act through mPRs to inhibit apoptosis in breast cancer cells. The involvement of mPRs in the development or progression of breast tumor growth through inhibition of cell death is an intriguing possibility and requires further investigation.
Electronic supplementary material
The online version of this article (doi:10.1007/s12672-012-0106-x) contains supplementary material, which is available to authorized users.
Keywords: Breast cancer, Breast cancer cells, Membrane progesterone receptor, mPR, Apoptosis, Signaling
Introduction
Evidence for a direct influence of progestins on breast cancer development and growth has been demonstrated in several clinical trials, most notably the Women’s Health Initiative estrogen plus progestin hormone therapy and the Million Woman Study. These trials showed that hormone replacement therapy containing an estrogen/progesterone combination, but not estrogen alone, resulted in increased breast cancer risk characterized by increased tumor size and aggressiveness [6, 7, 10, 37, 50, 52, 53]. Progesterone causes proliferation of immortalized breast cancer cell lines expressing high levels of PR and little or no ER [25], and clinical reports of PR-positive ER-negative tumors [17, 31, 32] demonstrate that progesterone plays an important role in breast cancer biology. However, the actions of progesterone in breast tumor biology remain understudied in relation to estrogen, and multiple investigators have concluded that further studies are needed on the role of progesterone and progesterone receptors in breast cancer [23, 33, 43].
Classically, estrogen and progesterone activate nuclear steroid receptors (ligand-activated transcription factors), which modulate expression of a wide range of genes, including those regulating cell proliferation [4, 26]. In addition, progestins are able to rapidly activate growth factor signaling pathways in breast cancer cells via direct, non-nuclear-mediated actions through PR and PR/ER cross-talk [14], resulting in breast cancer cell growth and development of metastasis [9]. Progestins have also been shown to inhibit apoptosis of PR-positive human breast cancer cells [46, 63], as well as PR-negative cancer cells [45], suggesting this antiapoptotic action is mediated through both PR [46, 63] and non-nuclear progestin receptors.
One potential mediator of progesterone actions, particularly in PR-negative cells, is the novel membrane progesterone receptor (mPR). The mPR was first cloned, identified, and characterized in fish ovaries [67], and three isoforms (mPRα, mPRβ, and mPRγ) were subsequently cloned in humans [66]. The mPRs are seven—transmembrane proteins expressed on the plasma membranes of cells and bind progestins in a specific, displaceable, high affinity, limited capacity manner, characteristic of steroid membrane receptors [62, 67] and activate G proteins in several cell types [29, 61, 62]. The mPRs do not belong to the G protein coupled receptor (GPCR) superfamily but are members of the progestin and adipoQ receptor (PAQR) family [41, 60]. Several cancer cell lines express mPRs, including PR-positive MCF7 cells, and PR-negative SKBR3, MDA-MB-231, and MB468 cells [16, 49]. The observation that mPRs are expressed in both PR-positive and PR-negative cells suggests mPRs may mediate progestin’s antiapoptotic effects both in the presence and absence of PR. Indeed, we have previously shown that mPRα is involved in the inhibition of apoptosis in fish granulosa cells [15]. Therefore, the objectives of this study were to examine the expression, signaling, and biological functions of mPRs in two human breast cancer cell lines in order to assess the potential importance of mPRs in breast cancer development and growth. In addition, the expression of mPRα, mPRβ, mPRγ, PR, ERα, ERβ, and progesterone receptor membrane component 1 (PGRMC1) mRNAs were examined in paired normal and malignant human breast biopsies from 13 individuals to determine the expression patterns of mPRs in relation to those of other steroid receptors in malignancy in vivo.
Methods
Chemicals
Chemicals were purchased from Sigma Aldrich (St. Louis, MO) unless otherwise noted. Progesterone, 17β-estradiol, testosterone, and cortisol were purchased from Steraloids (Newport, RI, USA). Two selective mPR agonists [30], 10-ethenyl-19-norprogesterone (Org OD 02–0) and 19a-methylprogesterone (Org OD 13–0) were obtained from N.V. Organon (Oss, The Netherlands). R5020 and [1,2,6,7,3H ]-progesterone (100 Ci/mmol) were purchased from Perkin Elmer (Waltham, MA, USA). Polyclonal antibodies for human mPRα and mPRβ have been validated previously in human cells transfected with mPRs and in untransfected cells as well as after siRNA treatment [13, 29]. The mPRγ antibody was generated in rabbits against the peptide sequence TDIKNDSYSWPMLC. Western blotting of mPRγ-transfected MDA-MB-231 cell membranes with the mPRγ antibody shows the presence of a more prominent immunoreactive band than that observed in the untransfected controls, confirming the specificity of the immunoreaction (Suppl. Figure 1A). Additional blotting of SKBR3, Jurkat and MB468 cells shows that the mPRγ antibody does not cross-react with either mPRα or mPRβ (Suppl. Figure 1B). p44/42 MAP kinase, phospho-p44/42 MAP kinase, Akt and phospho-Akt antibodies (Cell Signaling Danvers, MA), β-actin (clone C-4, MP Biomedical Solon, OH, USA), and caspase 3 and phospho-caspase 3 (Abcam, Cambridge, MA, USA) were used. HRP-linked secondary antibodies against rabbit and mouse IgG were purchased from Abcam and Cell Signaling, respectively. Antimouse and rabbit near-infrared dye conjugated secondary antibodies were purchased from LI-COR biosciences (Lincoln, NA, USA).
Cell Culture
SKBR3 and MB468 cells were obtained from American Type Culture Collection (Manassas, VA, USA). SKBR3 cells were cultured in phenol red-free Dulbecco’s modified Eagle’s medium supplemented with 14 mM NaCO3, penicillin/streptomycin/glutamine solution, gentamicin, and 10% certified fetal bovine serum (FBS) (Gibco, Carlsbad, CA, USA). MB468 cells were cultured in Lebowitz-15 medium supplemented with 14 mM NaCO3, penicillin/streptomycin/glutamine solution, gentamicin, and 10% FBS.
Human Breast Cancer Tissues
Deidentified paired normal and malignant human breast biopsy samples were obtained from the NCI Human Tissue Network. Samples were handled in accordance with NIH guidelines approved by The University of Texas office of Research Support and Compliance. The characterizations of the carcinomas are shown in Supplementary Table 1. Samples were stored at −80°C until use.
Real-Time PCR
An approximately 50 mg tissue sample from the interior of human tumor samples was excised, minced on ice, and placed in TRI reagent (Sigma-Aldrich). The sample was agitated using a handheld homogenizer to lyse the tissue as much as possible. Alternatively, TRI reagent was added directly to cell culture dishes. RNA was isolated following the manufacturer’s instructions. Samples were DNase treated using a DNA free RNA kit (Zymo Research, Orange, CA, USA). QRT-PCR was performed on 250 ng DNA-free RNA using Brilliant II SYBR Green QRT-PCR Mastermix 1-Step (Stratagene, Cedar Creek, TX, USA) on an Eppendorf RealPlex ep2 (Hamburg, Germany) using 100 nM primers for mPRα, mPRβ, mPRγ, PR, ERα, ERβ, PGRMC1, CK19, β-actin (see Supplementary Table 2 for primer sequences), and GAPDH (RealTime Primers, Elkins Park, PA, USA). The protocol consisted of a 30-min 50°C reverse transcription incubation and a 10-min 95°C denaturation followed by a cycling profile of 30 s at 95°C, 60 s at 55°C, and 30 s at 72°C for 40 cycles. Primers for CK19, PGRMC1, and PR had been designed previously [11, 36, 59]. No template controls were performed for each sample to confirm the specificity of the reaction. Ct values were calculated using Eppendorf software, and receptor concentrations were normalized to β-actin or GAPDH expression taking into account primer efficiency as described by Fink [20].
Preparation of Plasma Membranes
Cells, grown to 70–90% confluence and serum starved overnight, were collected in ice-cold HAED (25 mM HEPES, 10 mM NaCl, 1 mM dithioerythritol, 1 mM EDTA, pH 7.6) with HALT protease inhibitor cocktail (Thermo, Rockford, IL, USA). Cells were sonicated for 10 s and centrifuged at 1,000×g for 7 min at 4°C to remove unlysed cells and nuclei. The supernatant was then centrifuged at 20,000×g at 4°C for 20 min to pellet the membrane fraction, which was resuspended with buffer for subsequent experimentation.
Western Blot Analysis
Plasma membranes for mPRα, mPRβ, mPRγ, and integrin Western blotting were isolated as described above, resuspended in PBS with HALT protease inhibitor cocktail and added in the ratio of 2:1 to 5× Lane Marker Reducing Sample Buffer (Thermo) and run on a 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gel (15 μg protein/lane). Cells were pelleted by centrifugation at 500×g for 5 min for p42/44 MAP kinase and β-actin Western blotting. Pellets were resuspended in PBS containing protease inhibitor cocktail and lysed by sonication. Crude cytosolic extracts were obtained by centrifugation for 20 min at 20,000×g and run on a SDS-PAGE gel (20 μg protein/well). Proteins were transferred onto nitrocellulose membranes and blocked in TBS-T with 5% non-fat dry milk. Membranes were incubated with primary antibodies (dilution mPR antibodies 1:2,500; dilution total and phospho-ERK1/2- antibodies 1:1,000) in PBS with 5% milk overnight at 4°C followed by incubation with appropriate HRP-linked secondary antibodies. Proteins were visualized using Supersignal WestPico (Thermo) and exposure to Hyperfilm ECL (Amersham, Piscataway, NJ, USA).
Immunocytochemistry
The immunocytochemistry was conducted as described previously with few modifications [62]. The specificity of the immunoreactions was confirmed by incubating cells with secondary antibody alone. The nucleus was counterstained DAPI (Invitrogen, Carlsbad, CA, USA), and the slides were mounted with Prolong Gold antifade reagent (Invitrogen). The presence of fluorescent-labeled mPR proteins in the cells was visualized using a Nikon Eclipse E600 fluorescent microscope.
p42/44 MAP Kinase Activation Assay
Cells, grown in six-well cell culture plates to 70% confluence and serum starved for 3 days, were incubated with 100 nM progesterone for 5–60 min prior to stopping the incubation by replacing the media with ice-cold PBS. Cells were scraped into 1× Lane Marker Reducing Sample Buffer (Thermo) and Western blotted as described above.
Plasma Membrane Progesterone Receptor Assay
Membrane progesterone receptor competition assays were conducted as described previously [62].
[35S] GTPγ-S Binding
Cells were grown to confluence and serum starved for 12 h prior to plasma membrane preparation for the [35S] GTPγ-S binding assay as described previously [62].
Immunoprecipitation of [35S] GTPγ-S with G Protein α Subunit Antibodies
Immunoprecipitation of [35S] GTPγ-S with G protein α subunit antibodies was conducted as described previously [62].
cAMP Measurement
Cells, grown to 70% confluence and serum starved for 36 h, were pretreated with serum-free media containing 10 μM IBMX dissolved in DMSO for 20–30 min prior to incubation with progesterone (100 nM) or vehicle (<0.1% media volume) for 0, 1, 5, 10, 15, or 30 min. The incubation was stopped by quickly removing the media and adding 100 μl 0.1 M HCl to each well. After 20 min HCl treatment the cells were scraped off the plates, collected, and stored at −20°C until assayed by ELISA in duplicate according to the manufacturer’s instructions (Cayman Chemical, Ann Arbor, MI, USA).
Cell Death Assay
Cell death was assayed as described previously [15] following the general protocols of earlier studies [44, 45]. Briefly, cells grown in 25-cm2 flasks until they were 100% confluent were washed and incubated for 4–7 days in serum-free media containing various steroid treatments. The incubation media were removed, and adherent cells and cells in the incubation media were harvested, washed, pelleted by centrifugation, resuspended in Hank’s saline, and incubated with filtered trypan blue stain for 5 min. Cells were loaded onto a hemocytometer, and viability was determined for 500 cells/flask by trypan blue stain exclusion. The mPRα siRNA transfections for cell death experiments were performed on MB468 cells as previously described [62], and cells were treated on the second day following transfection.
TUNEL Assay
Cells, grown in 25 cm2 flasks until they reached 100% confluence, were washed and incubated in serum-free media with various steroids for 48 h prior to harvesting in Hank’s saline as described above. TUNEL assay was performed according to the manufacturer’s instructions (Clontech, Mountain View, CA, USA) using the ApoAlert DNA Fragmentation Assay kit. Apoptotic nuclei were counted using a fluorescent microscope as a proportion of total cells in five random fields of view for each flask.
Caspase 3 Activity
Cells, treated as in the TUNEL assay, were harvested and caspase 3 activity was determined by fluorescence using caspase-3 ApoAlert Assay plate (Clontech) according to the manufacturer’s instructions. Cells were also harvested for immunoblot analysis by 30 min incubation at 4°C in RIPA buffer (50 mM Tris–HCl, pH 7.4, 150 mM NaCl, 0.25% deoxycholic acid, 1% NP-40, and 1 mM EDTA) containing HALT phosphatase inhibitors (Thermo) and protease inhibitors (EMD Chemicals, Gibbstown, NJ, USA). Protein samples (20 μg/lane) were analyzed by Western blotting using the caspase 3 and phospho-caspase 3 antibodies.
Mitochondrial Membrane Potential
Cells were cultured for 1 day in clear, black-sided (Corning, Wilkes Barre, PA, USA), 96-well plates, washed, and cultured in serum-free media containing treatments for 4 days with a change of media containing the treatments after 2 days. The cells were washed and incubated in mitochondrial membrane buffer (25 mM HEPES, 115 mM NaCl, 5 mM KCl, 1 mM KH2PO4, 1.2 mM MgSO4, 0.5 mM CaCl2, and 5 mM glucose, pH 7.4) at 37°C for 2 h prior to 20 min dye loading with 150 nM tetramethylrhodamine methyl ester (AnaSpec Inc., Fremont, CA, USA). Fluorescence was measured on a FLUOstar Omega microplate reader (excitation, 554 nm; emission, 590 nm; bottom reading with 50 flashes per well). One lane per treatment group contained 10 μM carbonyl cyanide 3-chlorophenylhydrazone (CCCP) (Thermo) as a positive control. Data were normalized to crystal violet (Thermo) as previously described [2].
Akt Activity
Cells were plated in six-well plates and treated for 4 days in serum-free medium containing 10 nM P4, R5020, or Org OD 0–02. Cells were harvested and processed as previously described for caspase 3 Western blot analysis.
Cell Proliferation Assay
Cells, incubated for 1 day in 96-well plates, were washed and incubated for 2 days in serum-free media containing treatments. Five milligrams per milliliter (3-(4,5-dimethylthiaazol-2-yl)-2,5) diphenyltetrazolium bromide (MTT) (Alfa Aesar, Ward Hill, MA, USA) was added at 1/10 total volume of medium and incubated at 37°C for 2 h. Cells were lysed in equal volume MTT lysis buffer (20% SDS in 50% N,N-dimethylformamide, 0.5% acetic acid, 0.4% 1 N HCl, pH 4.7) for 4 h and read at 570 nM.
Statistical Analyses
One-way ANOVA with either Dunnett’s or Bonferroni’s multiple comparison tests were used to determine statistical differences between control and experimental treatments using GraphPad Prism (San Diego, CA, USA). Square root transformations of the data were used as indicated in order to remove significant differences in variance.
Results
Identification of mPR and Progesterone Binding
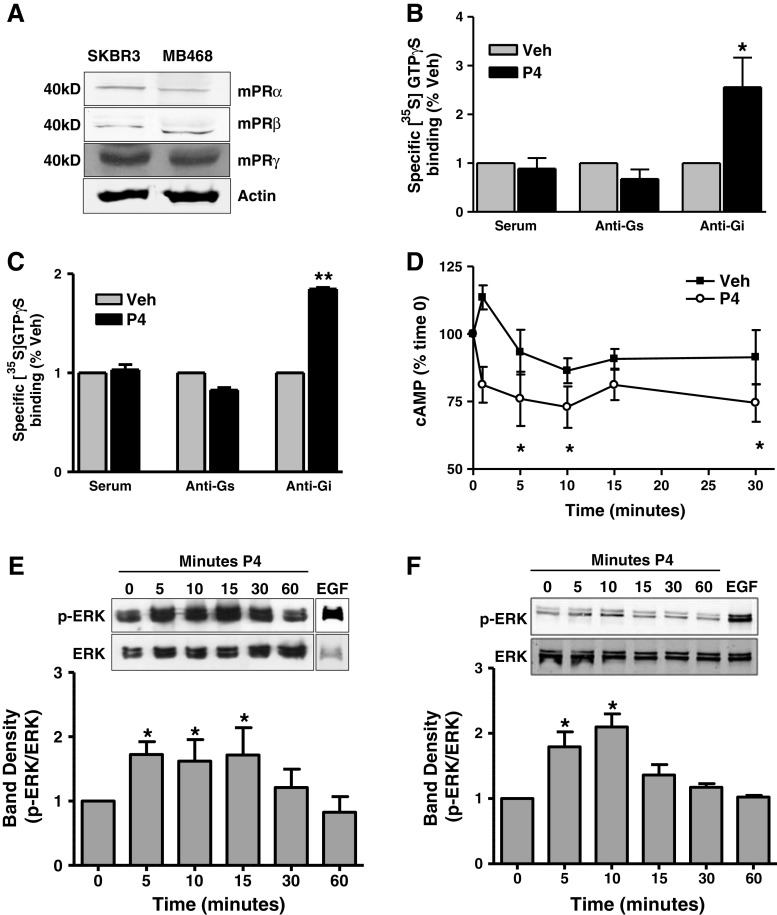
Quantitative RT-PCR detected the presence of mRNAs encoding all three mPR isoforms (α, β, and γ) in the MB468 breast cancer cell line (Suppl. Figure 2A). mPRα mRNA levels were several-fold higher than those of mPRβ or mPRγ. Full-length PR and truncated PR mRNAs were not detected in SKBR3 or MB468 cells (data not shown). Western blotting for the mPRs confirmed the presence of 40 kD mPRα, mPRβ, and mPRγ protein bands in both SKBR3 and MB468 cell membranes (Fig. 1a), although the relative protein abundance of each mPR isoform cannot be inferred from these blots due to the differences in isoform antibody affinity. Immunocytochemical analysis showed that the mPRα protein is predominantly localized on the cell membranes of both MB468 and SKBR3 cells, although some mPRα protein could also be detected intracellularly (Suppl. Figure 2B and D). No staining of the cells was observed when incubated with second antibody alone (Suppl. Figure 2C and E).
Fig. 1.
mPR protein detection, G protein activation, and second messenger signaling in SKBR3 and MB468 cells in response to progesterone treatment. a Detection of mPR proteins in plasma cell membranes by Western blot analysis. b, c Immunoprecipitation of [35S]-GTPγS in response to a 30-min 100 nM progesterone treatment by G protein antibodies in SKBR3 (b) and MB468 (c) cell membranes. Data represent mean percent of the specific [35S]-GTPγS binding of vehicle controls. n = 5, *p < 0.05, **p < 0.001 compared to vehicle (Veh) control by one-way ANOVA and Dunnett’s multiple comparison test. d. Time-course of cAMP concentrations in whole SKBR3 cells in response to 100 nM progesterone treatment. Data represent mean percent of the cAMP concentration at 0 min normalized to protein ± SEM. n = 5, *p < 0.05, compared to 0 min by one-way ANOVA and Dunnett’s multiple comparison test. e, f ERK 1/2 activation by 100 nM progesterone in SKBR3 (e) and MB468 (f) cells at 5, 10, 15, 30, and 60 min. A 10-min 20 nM EGF treatment used as positive control. Representative blot and densitometry from four separate experiments shown. Data represent mean phospho ERK 1/2 (p-ERK) band density normalized to total ERK 1/2 (ERK) band density ± SEM. *p < 0.05, n = 4
Previous saturation and Scatchard analyses have demonstrated the presence of high affinity, limited capacity, single progesterone binding sites on SKBR3 and MB468 cell membranes [16, 49]. Single point competitive binding assays showed that 50 nM progesterone displaced most of the [3H]-progesterone binding to SKBR3 plasma membranes, and the synthetic progestins, Org OD 02-0 and Org OD 13-0, which have high binding affinities for recombinant human mPRα [30], were also relatively effective competitors at this concentration (Suppl. Figure 3A). Testosterone (50 nM) caused slight displacement of [3H]-progesterone binding, whereas the nuclear progesterone receptor ligands, R5020 and RU486, cortisol and estrogen were ineffective as competitors of [3H]-progesterone binding to SKBR3 membranes (Suppl. Figure 3A). Org OD 02-0 was also an effective competitor for [3H]-progesterone binding to the receptor on MB468 cells (Suppl. Figure 3B). The specificity of binding by other steroids to the progestin receptor on MB468 cells has been reported previously and is similar to that observed with SKBR3 cells [49].
Progesterone Activation of G Proteins and Induction of Intracellular Signaling
Progesterone treatment (20,100 nM) caused significant increases in [35S]-GTPγS binding to SKBR3 cell membranes compared to vehicle controls (Suppl. Figure 3C), similar to that observed with MB468 cells after progesterone treatment [49].The amount of [35S]-GTPγ-S immunoprecipitated from solubilized SKBR3 and MB468 membranes by antibodies directed against inhibitory G proteins was significantly increased after progesterone exposure, whereas the [35S]-GTPγ-S immunoprecipitated by antibodies directed against stimulatory G proteins was unchanged (Fig. 1b, c), indicating selective progesterone activation of an inhibitory G protein.
Exposure of SKBR3 cells to 100 nM progesterone resulted in a significant reduction in intracellular cAMP levels compared to the control no treatment values at 5, 10, and 30 min (Fig. 1d). Progesterone also caused a transient, significant increase in p42/44 MAPK activation in SKBR3 and in MB468 cells between 5 and 10 min exposure, which had returned to baseline values by 60 min (Fig. 1e, f).
Progestin Inhibition of Serum Starvation-Induced Cell Death and DNA Fragmentation
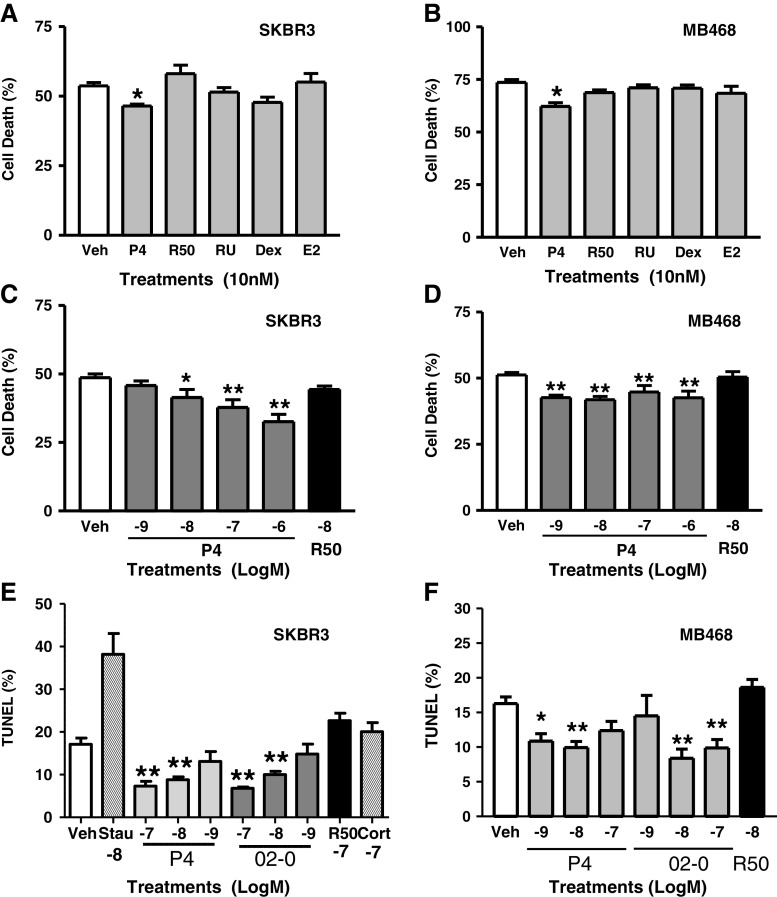
Significant decreases (approximately 14–15% ) in serum starvation-induced death assessed by trypan blue exclusion were observed in SKBR3 cells (Fig. 2a) and MB468 cells (Fig. 2b) after exposure to 10 nM progesterone, which were not replicated by the PR agonist R5020, the PR antagonist RU486, dexamethasone, or 17β-estradiol. Moreover, the progesterone-induced decrease in cell death in SKBR3 cells appeared to be concentration-dependent over the concentration range of 10–1,000 nM (Fig. 2c), whereas there were no concentration-dependent effects of progesterone on cell death in MB468 cells, in which cell death was inhibited ~20% with 1 nM progesterone, the lowest concentration investigated (Fig. 2d). Treatment of MB468 cells with 1 and 10 nM progesterone or 10 and 100 nM of the selective mPR agonist Org OD 02-0, but not with 10 nM R5020 or cortisol, resulted in 40–55% decreases (compared to ethanol controls) in DNA condensation detected by TUNEL staining (Fig. 2e, f). Treatment with 10, 50, or 100 nM progesterone or R5020 did not alter cell proliferation measured by MTT (Suppl. Figure 4). These results suggest that progesterone inhibition of serum starvation-induced cell death is due to inhibition of apoptosis through an mPR-dependent mechanism and is not associated with a proliferative effect.
Fig. 2.
Effects of steroid treatments on serum starvation-induced death of SKBR3 (a, c) and MB468 (b, d) cells and on TUNEL staining of nuclei (e, f). a, b Effects of treatment with 10 nM of various steroids on percent cell death after 5–7 days starvation/treatment. c, d Effects of treatment with different progesterone concentrations (1 nM–1 μM) on percent cell death after 4–5 days starvation/treatment. Data represent means ± SEM. Difference between vehicle control and steroid treatment determined by one way ANOVA and Dunnett’s multiple comparison.*p < 0.05, **p < 0.001, n = 6−9. Dex dexamethasone R50 R5020, RU RU486, E2 estradiol. e, f Percent TUNEL stained nuclei in SKBR3 and MB468 cells, respectively, after 2 days progestin treatment. Differences between vehicle and treatment determined by one-way ANOVA and Dunnett’s multiple comparison *p < 0.05, **p < 0.001, n = 15 Stau Staurosporine and Cort Cortisol
Effect of mPRα siRNA Treatment on Progestin-Induced Cell Death
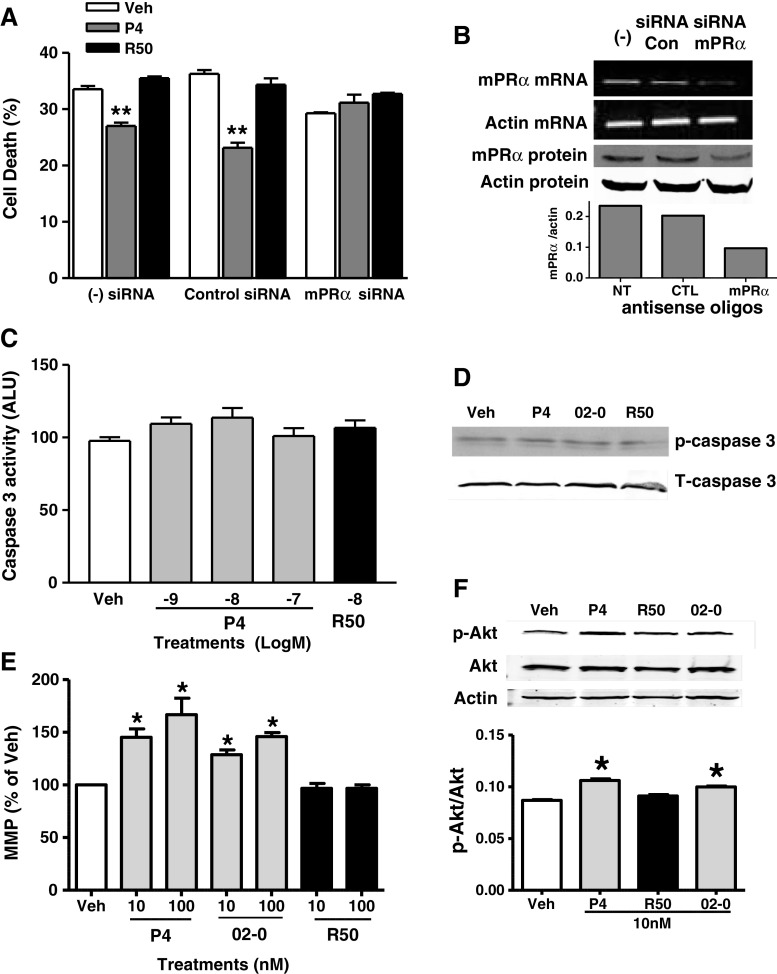
In order to further examine mPRα involvement in progestin-mediated cell survival, siRNA directed against mPRα was used to knock down mPRα expression in MB468 cells (Fig. 3b). Cells with reduced mPRα expression did not show increased survival upon progestin treatment, whereas cells with endogenous mPRα levels (control siRNA or untransfected) responded to progesterone treatment with a significant decrease in cell death (Fig. 3a). Similar to previous experiments, cells did not respond to R5020, confirming a lack of PR involvement.
Fig. 3.
Mechanism of progestin induced cell survival in MB468 cells. a MB468 cells were transfected with mPRα or control pool siRNA and treated for serum starvation induced cell death (10 nM steroid). Treatments were counted using trypan blue staining. **p < 0.001, n = 3. b RT-PCR measuring mRNA levels and immuno-blot detection of mPRα in non-transfected (−), control transfected (siRNA Con), and mPRα siRNA transfected (siRNA mPRα) MB468 cells (upper panel). Relative decrease in mPRα expressed as bar graph representing the band density ratios of mPRα/actin (lower panel). c Effect of P4 and R5020 on caspase 3 activity measured by fluorescence in MB468 cells. Data represent mean caspase 3 activity measured by arbitrary fluorescence units (ALU) normalized to protein and control treatments. n = 11. d Caspase 3 activity in MB468 cells measured by phospho-caspase 3 specific antibody in response to 10 nM P4, Org OD 02-0 (02-0) and R5020 (R50). Data representative of n = 3. e Mitochondrial membrane potential (MMP) in MB468 cells after 4 day treatment with 10 and 100 nM P4, Org OD 02-0, and R5020. Data represented as percent of vehicle for each treatment. *p < 0.05, n = 13. f p-Akt levels in MB468 cells after 4 days treatment with 10 nM P4, R5020, and Org OD 02-0. Immunoblot using phospho specific Akt (p-Akt), total Akt (Akt), and actin antibodies (top). Band density of phospho-Akt normalized to total Akt. n = 3, *p < 0.05 (bottom)
Progestin Effect on Caspase 3 Activity
Treatment of serum starved MB468 cells with 1–100 nM progesterone or 10 nM R5020 was ineffective in altering caspase 3 activity detected by either caspase 3 EIA (Fig. 3c) or phosphorylation detected by immunoblot analysis (Fig. 3d), indicating that decreases in DNA fragmentation by progestins are not associated with caspase 3 inhibition.
Progestin Effect on Mitochondrial Membrane Potential
Progesterone and Org OD 02-0 both at 10 and 100 nM significantly increased mitochondrial membrane potential (MMP) in serum starved MB468 cells, while R5020 was ineffective (Fig. 3e). Cells were also treated with CCCP, which depolarizes the mitochondrial membrane as a positive control for MMP (data not shown). This indicates that progesterone and mPR-selective ligands hyperpolarize mitochondrial membrane potential, which is associated with inhibition of apoptosis [58].
Progestin Effects on Akt Activity
PI3K/Akt pathway activation is associated with cell survival pathways and inhibition of pro-apoptotic Bcl-2 family members [1]. Therefore, we measured Akt activity via Western blot analysis using phospho-Akt antibody in serum-starved MB468 cells treated with progesterone, R5020 and Org OD 02-0. Progesterone and Org OD 02-0, but not R5020, increased phospho-Akt compared to control groups, suggesting that mPRs are mediating this response (Fig. 3f).
mPR Expression in Normal and Malignant Breast Biopsies
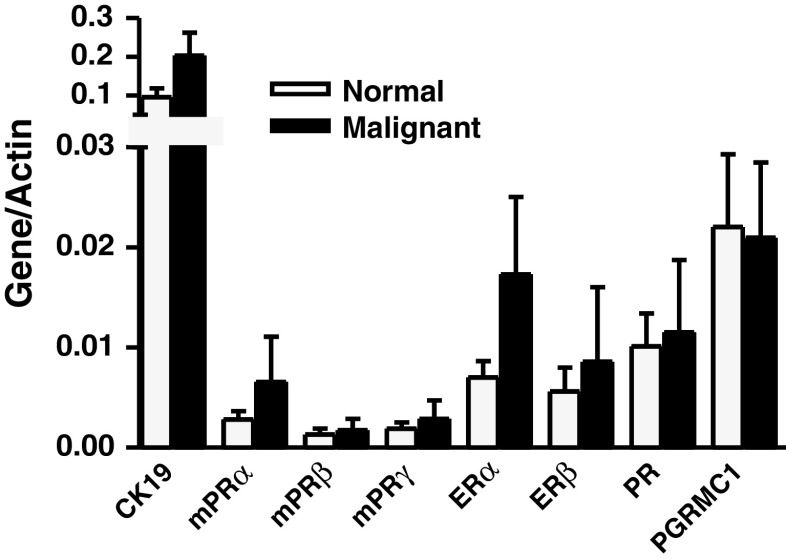
mPRα mRNA was two to three times more abundant in both normal and malignant tissues than either mPRβ or mPRγ, suggesting that mPRα is the primary mPR expressed in breast tissue. However, overall, the expression levels of the mPRs were two to five times lower than other steroid receptors measured (Fig. 4). The mPRα, mPRβ, and mPRγ mRNAs were detected in all 26 breast tissue biopsies examined, and on average, their mRNA levels were slightly upregulated in malignant tissue over normal tissue from the same breast. Ratios of malignant to normal gene expression ranged from 0.01 to over 100. In order to simplify the data, upregulation was defined as greater than a 1.2:1 ratio of expression in malignant tissue to normal tissue, which corresponds to a 20% increase in gene transcription (Table 1). This ratio was selected following a literature review [39, 40, 65]. Of the 13 paired biopsies examined, mPRα was upregulated in six cases (46%), mPRβ was upregulated in five (38%), and mPRγ was upregulated in nine (70%, Table 1). Overall mPRγ was upregulated in a higher percentage of patients with malignant breast tissue (70%), than any of the other receptors (PR, 54%; ERα, 46%; ERβ, 38%; PGRMC1, 38%). The parallel regulation of mPRα, mPRγ, and PR in breast cancer tissues suggests the possibility of an interaction between progesterone receptors, with potentially important implications in breast cancer biology. Linear regression determined a positive relationship between PR and mPRγ with a slope of 14.42 ± 3.846, p < 0.005, indicating a slope significantly different from 0 with an r 2 = 0.5609.
Fig. 4.
Average gene to actin ratio in normal and malignant human breast biopsies. Data represent average ± SEM gene to actin ratio for all measured genes grouped according to disease state. n = 13
Table 1.
Comparison of gene regulation between paired normal and malignant human breast samples: (+) denotes a ratio of malignant to normal gene expression greater than 1.2; (−) denotes a ratio of malignant to normal gene expression <0.8
| Biopsy Designation | CK19 | mPRα | mPRβ | mPRγ | ERα | ERβ | PR | PGRMC1 |
|---|---|---|---|---|---|---|---|---|
| 1 | − | + | + | + | + | − | + | − |
| 2 | + | + | − | + | − | − | + | + |
| 3 | + | + | − | + | + | − | + | − |
| 4 | + | − | − | − | − | − | − | − |
| 5 | + | + | + | + | + | − | + | − |
| 6 | + | + | − | + | + | − | + | − |
| 7 | + | − | − | − | − | − | − | − |
| 8 | + | + | + | + | + | + | + | + |
| 9 | − | − | + | − | + | − | − | + |
| 10 | + | − | − | + | − | + | − | + |
| 11 | + | − | − | + | − | − | − | + |
| 12 | + | − | + | + | − | − | + | − |
| 13 | − | − | − | − | − | − | − | − |
Discussion
The results of the present study provide the first evidence that progesterone inhibits apoptosis in PR-negative breast cancer cell lines through mPRα. PR-negative SKBR3 and MB468 cells express predominantly mPRα and lesser amounts of mPRβ and mPRγ and display the typical progestin membrane binding and signaling characteristics of mPRs. The finding that treatment with low concentrations of progesterone and the specific mPR agonist Org OD 02-0 attenuated cell death and DNA condensation induced by serum-starvation implicates mPRs in the antiapoptotic actions of progesterone in breast cancer cells. mPR agonists also activate MAPK p42/44 and Akt signaling pathways, which are both incorporated in the apoptotic signaling pathway. The demonstration that down-regulation of mPRα expression by treatment with siRNAs abrogates the protective effects of progesterone on cell death indicates that mPRα mediates at least part of this progesterone action. In addition, preliminary evidence was obtained for upregulation of the three mPRs in human breast cancer tissues. Taken together, these results suggest a role for mPRs in progestin promotion of breast cancer cell survival through inhibition of cell death.
Although mPRα, mPRβ, and mPRγ have been detected in human breast tissue [60], the expression of the three mPR subtypes in breast cancer tissues and cell lines has not been described previously. The finding that the mPRα subtype is the predominant mPR transcript in human breast cancer cell lines and normal and malignant breast tissues is in agreement with previous findings [16, 60]. PR expression is not necessary for mPR expression as both cell lines used in the present study are PR-negative [34], corroborating findings that suggest the mPRs are expressed and regulated in tissues independent of PR [29]. Several lines of evidence indicate that functional mPRs are present on SKBR3 and MB468 cell membranes. The Western blot analyses and immunocytochemistry results indicate the mPRs are localized to the plasma membranes of both cell types, consistent with their roles as membrane receptors. The binding affinities of progesterone to SKBR3 (Kd = 10.6 nM) and MB468 (Kd = 6.03 nM) cell membranes [16, 49] are within the range reported previously for recombinant mPRs [62, 66]. The progesterone binding to MB468 cells is associated with expression of both mPRα and mPRβ, because siRNA directed against either mPR results in decreased membrane [3H]-progesterone binding [49]. Steroid specificity studies also indicate the progestin receptors on these cells are mPRs. The membrane progesterone receptor on SKBR3 cells displays high steroid specificity for progesterone with low affinity for testosterone and no affinity for 17β-estradiol, cortisol, the PR agonist R5020, or the PR antagonist RU486, similar to the results with MB468 cells and with recombinant human mPRα [49, 62, 66]. Additionally, the specific human mPR agonist, Org OD 02-0 [30], shows high affinity binding to SKBR3 and MB468 cell membranes.
The current results showing that progesterone activates an inhibitory G protein in both SKBR3 and MB468 breast cancer cells is consistent with previous findings with mPRα and mPRβ in a variety of cells, including human myometrial cells [29], a human T-cell leukemia (Jurkat) cell line [13], a rodent GnRH neuronal (GT1-7) cell line [57], and teleost ovarian follicle cells and oocytes [15, 48]. As predicted, progesterone activation of an inhibitory G protein was associated with similar decreases in intracellular cAMP levels in SKBR3 cells and in MB468 cells [49] to those observed in other cells expressing mPRs [15, 47, 57]. Similarly, the progestin-induced MAPK activation in SKBR3 and MB468 cells has previously been observed in human myometrial cells [29] as well as in mammalian cells expressing teleost mPRα [24, 67] and teleost oocytes [47] and granulosa/theca cells [15]. Moreover, progestins have been shown to activate Akt in the teleost oocyte and granulosa/theca cells, which express mPRα and also in MB468 cells during EMT transition [15, 48, 68]. Thus, the current results on progestin binding and intracellular signaling initiated in these PR-negative breast cancer cell lines are consistent with mPRα activation and signaling observed previously in other vertebrate cells.
Of particular importance in the development and/or progression of breast cancer is the major finding that mPRs mediate progestin inhibition of cell death and apoptosis in human breast cancer cells. Clear evidence in support of this was obtained via trypan blue exclusion and TUNEL staining experiments showing that treatment with low nanomolar concentrations of progesterone inhibited serum starvation-induced cell death of both SKBR3 and MB468 cells, whereas R5020, RU486, 17β-estradiol, or dexamethasone were ineffective. While trypan blue staining does not distinguish between cell morbidity and cell mortality, the results from trypan blue exclusion experiments agree with the results from TUNEL staining, a more robust measure of cells undergoing a regulated cell death process, indicating that progestin acting through mPR inhibits cell death. The PR agonist, R5020, displays low binding affinity for mPRs and does not activate them at low nanomolar concentrations [29, 49, 62]. Thus, the failure of R5020 to protect against cell death further indicates that the protective effects of progesterone in these breast cancer cells are not mediated through any form of PR. On the other hand, the observation that the specific mPR agonist, Org OD 02-0, mimicked the actions of progesterone on apoptosis and mitochondrial membrane potential further indicates involvement of mPRs. Finally, the mPRα siRNA experiments showing that progesterone no longer inhibited cell death in MB468 cells in which mPRα expression was reduced provide further direct evidence that these antiapoptotic progesterone actions are mediated through mPRs. A similar degree of impairment of apoptosis after progesterone treatment has been observed in other PR-negative breast cancer cell lines [45] and in the ovarian follicle [15, 18]. There is substantial evidence that progestins inhibit apoptosis of breast cancer cells [3, 5, 44–46, 63], while other studies indicate progestins can promote apoptosis in multiple cell lines [21, 22, 27, 28]. Thus, the role of progesterone in the development and progression of breast cancer is often confusing, although progesterone and estrogen have consistently been shown to regulate the proliferation and differentiation of normal mammary tissue [19, 42, 56]. A recent study has shown that the epithelial to mesenchymal transition (EMT) of breast cancer cells, an important event in cancer metastasis, is reversed by progesterone treatment through mPRα [68]. While the magnitude of progestin inhibition of apoptosis via mPR activation is relatively small, it may be sufficient to enable cancer cells undergoing EMT/mesenchymal to epithelial transition (MET) to survive in adverse environments and establish metastatic disease. While there is no direct evidence supporting this hypothesis, our data, taken with data from other groups, suggest that mPRs are intermediaries in a variety of progestin actions in breast cancer cells that modulate cancer survival, progression, or metastasis.
The present results showing that progesterone activates MAPK and Akt are consistent with mPR’s antiapoptotic actions in these PR-negative breast cancer cell lines, as both MAPK and Akt are directly and indirectly involved in inhibiting apoptosis. Activated MAP kinases have been shown to upregulate the expression of antiapoptotic members [38] and to inactivate the proapoptotic members [55] of the Bcl-2 family of proteins. Activated Akt inhibits BAD [12], a proapoptotic member of the Bcl-2 family, and caspase 9 [8], a mediator of apoptosis. Apoptotic signaling pathways are also associated with both mitochondrial-dependent and mitochondrial-independent events, which involve activation of caspases. Caspase 3 activity is often regulated in association with DNA fragmentation [51], but in the present study, the antiapoptotic actions of progesterone are not associated with alterations of caspase 3 activity. The role of the mitochondria in cell death is highly dependent upon MMP integrity. A decrease in MMP associated with an apoptotic event leads to the rupture and subsequent release of proapoptotic proteins sequestered within the mitochondria [54]. We show that progesterone and the mPR-selective agonist Org OD 02-0 increases MMP, which correlates with increased cell survival.
The current study is also the first to describe the expression patterns of three mPR mRNA isoforms in human breast cancer biopsies. While the transcript levels of mPRs are somewhat lower compared to nuclear steroid receptors, this is not unexpected as relatively few membrane localized mPR molecules are needed to send a cell wide signal via activation of second messengers. mPRγ appears to be upregulated in 70% of the tumors examined, which is similar to the proportion of tumors defined as steroid receptor positive [64]. We show that the mPRs are widely expressed in breast tumors with at least one mPR isoform upregulated in 85% of the tumors examined. The mPRα protein has been detected in the majority of human breast cancer cell lines examined in human breast cancer biopsies [68]. These data are suggestive of a role for mPRs in breast tumor formation or progression, but due to the limited sample number, it is difficult to draw any definitive conclusions. Additional studies examining mPR protein expression on a large number of breast tumors via immunohistochemistry are warranted.
The role of progesterone in breast cancer is difficult to define [35]; the presence of mPRs in both PR-positive and PR-negative breast tumors and breast cancer cell lines may add an additional level of complexity in identifying the role of progestins in breast tumor development, progression, and metastasis. While there are currently no data on the expression patterns of the mPRs in relation to treatment response or prognosis, the activity of mPRs in breast cancer cells suggests that further study of mPRs in relation to breast cancer treatment and prognosis is warranted.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(PPTX 128 kb)
(PPTX 125 kb)
(PPTX 135 kb)
(PPTX 776 kb)
(PPTX 147 kb)
(PPTX 119 kb)
Acknowledgements
This study was funded by National Institutes of Health grant ESO 12961 to P.T. and by National Institute of Environmental Health and Safety T32ES007247 to R.A.
Conflict of Interest
The authors have no conflicts of interest to declare.
Footnotes
This is an uncopyedited author manuscript copyrighted by The Endocrine Society. This may not be duplicated or reproduced, other than for personal use or within the rule of “Fair Use of Copyrighted Materials” (section 107, Title 17, U.S. Code) without permission of the copyright owner, The Endocrine Society. From the time of acceptance following peer review, the full text of this manuscript is made freely available by The Endocrine Society at http://www.endojournals.org/. The final copy edited article can be found at http://www.endojournals.org/. The Endocrine Society disclaims any responsibility or liability for errors or omissions in this version of the manuscript or in any version derived from it by the National Institutes of Health or other parties. The citation of this article must include the following information: author(s), article title, journal title, year of publication and DOI.
References
- 1.Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281(5381):1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 2.Alyea RA, Laurence SE, Kim SH, Katzenellenbogen BS, Katzenellenbogen JA, Watson CS. The roles of membrane estrogen receptor subtypes in modulating dopamine transporters in PC-12 cells. J Neurochem. 2008;106(4):1525–1533. doi: 10.1111/j.1471-4159.2008.05491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bardon S, Vignon F, Montcourrier P, Rochefort H. Steroid receptor-mediated cytotoxicity of an antiestrogen and an antiprogestin in breast cancer cells. Cancer Res. 1987;47(5):1441–1448. [PubMed] [Google Scholar]
- 4.Basu A, Rowan BG. Genes related to estrogen action in reproduction and breast cancer. Front Biosci. 2005;10:2346–2372. doi: 10.2741/1703. [DOI] [PubMed] [Google Scholar]
- 5.Behera MA, Dai Q, Garde R, Saner C, Jungheim E, Price TM. Progesterone stimulates mitochondrial activity with subsequent inhibition of apoptosis in MCF-10A benign breast epithelial cells. Am J Physiol Endocrinol Metab. 2009;297:E1089–96. doi: 10.1152/ajpendo.00209.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beral V. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362(9382):419–427. doi: 10.1016/S0140-6736(03)14065-2. [DOI] [PubMed] [Google Scholar]
- 7.Beral V, Reeves G, Bull D, Green J. Breast cancer risk in relation to the interval between menopause and starting hormone therapy. J Natl Cancer Inst. 2011;103(4):296–305. doi: 10.1093/jnci/djq527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282(5392):1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 9.Carnevale RP, Proietti CJ, Salatino M, Urtreger A, Peluffo G, Edwards DP, Boonyaratanakornkit V, et al. Progestin effects on breast cancer cell proliferation, proteases activation, and in vivo development of metastatic phenotype all depend on progesterone receptor capacity to activate cytoplasmic signaling pathways. Mol Endocrinol. 2007;21(6):1335–1358. doi: 10.1210/me.2006-0304. [DOI] [PubMed] [Google Scholar]
- 10.Chlebowski RT, Hendrix SL, Langer RD, Stefanick ML, Gass M, Lane D, Rodabough RJ, et al. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women’s Health Initiative Randomized Trial. JAMA. 2003;289(24):3243–3253. doi: 10.1001/jama.289.24.3243. [DOI] [PubMed] [Google Scholar]
- 11.Crudden G, Loesel R, Craven RJ. Overexpression of the cytochrome p450 activator hpr6 (heme-1 domain protein/human progesterone receptor) in tumors. Tumour Biol. 2005;26(3):142–146. doi: 10.1159/000086485. [DOI] [PubMed] [Google Scholar]
- 12.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91(2):231–241. doi: 10.1016/S0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 13.Dosiou C, Hamilton AE, Pang Y, Overgaard MT, Tulac S, Dong J, Thomas P, Giudice LC. Expression of membrane progesterone receptors on human T lymphocytes and Jurkat cells and activation of G-proteins by progesterone. J Endocrinol. 2008;196(1):67–77. doi: 10.1677/JOE-07-0317. [DOI] [PubMed] [Google Scholar]
- 14.Dressing GE, Hagan CR, Knutson TP, Daniel AR, Lange CA. Progesterone receptors act as sensors for mitogenic protein kinases in breast cancer models. Endocr Relat Cancer. 2009;16(2):351–361. doi: 10.1677/ERC-08-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dressing GE, Pang Y, Dong J, Thomas P. Progestin signaling through mPRalpha in Atlantic croaker granulosa/theca cell cocultures and its involvement in progestin inhibition of apoptosis. Endocrinology. 2010;151(12):5916–5926. doi: 10.1210/en.2010-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dressing GE, Thomas P. Identification of membrane progestin receptors in human breast cancer cell lines and biopsies and their potential involvement in breast cancer. Steroids. 2007;72:111–115. doi: 10.1016/j.steroids.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 17.Dunnwald L, Rossing MA, Li CI. Hormone receptor status, tumor characteristics, and prognosis: a prospective cohort of breast cancer patients. Breast Cancer Res. 2007;9(1):R6. doi: 10.1186/bcr1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Engmann L, Losel R, Wehling M, Peluso JJ. Progesterone regulation of human granulosa/luteal cell viability by an RU486-independent mechanism. J Clin Endocrinol Metab. 2006;91(12):4962–4968. doi: 10.1210/jc.2006-1128. [DOI] [PubMed] [Google Scholar]
- 19.Feng Z, Marti A, Jehn B, Altermatt HJ, Chicaiza G, Jaggi R. Glucocorticoid and progesterone inhibit involution and programmed cell death in the mouse mammary gland. J Cell Biol. 1995;131(4):1095–1103. doi: 10.1083/jcb.131.4.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fink L, Seeger W, Ermert L, Hanze J, Stahl U, Grimminger F, Kummer W, Bohle RM. Real-time quantitative RT-PCR after laser-assisted cell picking. Nat Med. 1998;4(11):1329–1333. doi: 10.1038/3327. [DOI] [PubMed] [Google Scholar]
- 21.Formby B, Wiley TS. Progesterone inhibits growth and induces apoptosis in breast cancer cells: inverse effects on Bcl-2 and p53. Ann Clin Lab Sci. 1998;28(6):360–369. [PubMed] [Google Scholar]
- 22.Formby B, Wiley TS. Bcl-2, survivin and variant CD44 v7-v10 are downregulated and p53 is upregulated in breast cancer cells by progesterone: inhibition of cell growth and induction of apoptosis. Mol Cell Biochem. 1999;202(1–2):53–61. doi: 10.1023/A:1007081021483. [DOI] [PubMed] [Google Scholar]
- 23.Fuqua SA, Cui Y, Lee AV, Osborne CK, Horwitz KB. Insights into the role of progesterone receptors in breast cancer. J Clin Oncol. 2005;23(4):931–932. doi: 10.1200/JCO.2005.05.152. [DOI] [PubMed] [Google Scholar]
- 24.Hanna R, Pang Y, Thomas P, Zhu Y. Cell-surface expression, progestin binding, and rapid nongenomic signaling of zebrafish membrane progestin receptors alpha and beta in transfected cells. J Endocrinol. 2006;190(2):247–260. doi: 10.1677/joe.1.06694. [DOI] [PubMed] [Google Scholar]
- 25.Horwitz KB, Zava DT, Thilagar AK, Jensen EM, McGuire WL. Steroid receptor analyses of nine human breast cancer cell lines. Cancer Res. 1978;38(8):2434–2437. [PubMed] [Google Scholar]
- 26.Ikeda K, Inoue S. Estrogen receptors and their downstream targets in cancer. Arch Histol Cytol. 2004;67(5):435–442. doi: 10.1679/aohc.67.435. [DOI] [PubMed] [Google Scholar]
- 27.Kandouz M, Lombet A, Perrot JY, Jacob D, Carvajal S, Kazem A, Rostene W, Therwath A, Gompel A. Proapoptotic effects of antiestrogens, progestins and androgen in breast cancer cells. J Steroid Biochem Mol Biol. 1999;69(1–6):463–471. doi: 10.1016/S0960-0760(99)00069-2. [DOI] [PubMed] [Google Scholar]
- 28.Kandouz M, Siromachkova M, Jacob D, Chretien Marquet B, Therwath A, Gompel A. Antagonism between estradiol and progestin on Bcl-2 expression in breast-cancer cells. Int J Cancer. 1996;68(1):120–125. doi: 10.1002/(SICI)1097-0215(19960927)68:1<120::AID-IJC21>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 29.Karteris E, Zervou S, Pang Y, Dong J, Hillhouse EW, Randeva HS, Thomas P. Progesterone signaling in human myometrium through two novel membrane G protein coupled receptors: potential role in functional progesterone withdrawal at term. Mol Endocrinol. 2006;20(7):1519–1534. doi: 10.1210/me.2005-0243. [DOI] [PubMed] [Google Scholar]
- 30.Kelder J, Azevedo R, Pang Y, de Vlieg J, Dong J, Thomas P. Comparison between steroid binding to membrane progesterone receptor alpha (mPRalpha) and to nuclear progesterone receptor: correlation with physicochemical properties assessed by comparative molecular field analysis and identification of mPRalpha-specific agonists. Steroids. 2010;75(4–5):314–322. doi: 10.1016/j.steroids.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keshgegian AA. Biochemically estrogen receptor-negative, progesterone receptor-positive breast carcinoma. Immunocytochemical hormone receptors and prognostic factors. Arch Pathol Lab Med. 1994;118(3):240–244. [PubMed] [Google Scholar]
- 32.Keshgegian AA, Cnaan A. Estrogen receptor-negative, progesterone receptor-positive breast carcinoma: poor clinical outcome. Arch Pathol Lab Med. 1996;120(10):970–973. [PubMed] [Google Scholar]
- 33.Kobayashi S, Stice JP, Kazmin D, Wittmann BM, Kimbrel EA, Edwards DP, Chang CY, McDonnell DP. Mechanisms of progesterone receptor inhibition of inflammatory responses in cellular models of breast cancer. Mol Endocrinol. 2010;24(12):2292–2302. doi: 10.1210/me.2010-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lacroix M, Leclercq G. Relevance of breast cancer cell lines as models for breast tumours: an update. Breast Cancer Res Treat. 2004;83(3):249–289. doi: 10.1023/B:BREA.0000014042.54925.cc. [DOI] [PubMed] [Google Scholar]
- 35.Lange CA. Challenges to defining a role for progesterone in breast cancer. Steroids. 2008;73(9–10):914–921. doi: 10.1016/j.steroids.2007.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Latil A, Bieche I, Vidaud D, Lidereau R, Berthon P, Cussenot O, Vidaud M. Evaluation of androgen, estrogen (ER alpha and ER beta), and progesterone receptor expression in human prostate cancer by real-time quantitative reverse transcription-polymerase chain reaction assays. Cancer Res. 2001;61(5):1919–1926. [PubMed] [Google Scholar]
- 37.Li CI, Weiss NS, Stanford JL, Daling JR. Hormone replacement therapy in relation to risk of lobular and ductal breast carcinoma in middle-aged women. Cancer. 2000;88(11):2570–2577. doi: 10.1002/1097-0142(20000601)88:11<2570::AID-CNCR20>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 38.Lin H, Chen C, Li X, Chen BD. Activation of the MEK/MAPK pathway is involved in bryostatin 1-induced monocytic differentiation and up-regulation of X-linked inhibitor of apoptosis protein. Exp Cell Res. 2002;272:192–198. doi: 10.1006/excr.2001.5417. [DOI] [PubMed] [Google Scholar]
- 39.Liu CJ, Lui MT, Chen HL, Lin SC, Chang KW. MICA and MICB overexpression in oral squamous cell carcinoma. J Oral Pathol Med. 2007;36(1):43–47. doi: 10.1111/j.1600-0714.2006.00471.x. [DOI] [PubMed] [Google Scholar]
- 40.Ljuslinder I, Malmer B, Golovleva I, Thomasson M, Grankvist K, Hockenstrom T, Emdin S, Jonsson Y, Hedman H, Henriksson R. Increased copy number at 3p14 in breast cancer. Breast Cancer Res. 2005;7(5):R719–R727. doi: 10.1186/bcr1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lyons TJ, Villa NY, Regalla LM, Kupchak BR, Vagstad A, Eide DJ. Metalloregulation of yeast membrane steroid receptor homologs. Proc Natl Acad Sci U S A. 2004;101(15):5506–5511. doi: 10.1073/pnas.0306324101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Medina D. Mammary developmental fate and breast cancer risk. Endocr Relat Cancer. 2005;12:483–495. doi: 10.1677/erc.1.00804. [DOI] [PubMed] [Google Scholar]
- 43.Moore MR. A rationale for inhibiting progesterone-related pathways to combat breast cancer. Curr Cancer Drug Targets. 2004;4(2):183–189. doi: 10.2174/1568009043481515. [DOI] [PubMed] [Google Scholar]
- 44.Moore MR, Conover JL, Franks KM. Progestin effects on long-term growth, death, and Bcl-xL in breast cancer cells. Biochem Biophys Res Commun. 2000;277(3):650–654. doi: 10.1006/bbrc.2000.3728. [DOI] [PubMed] [Google Scholar]
- 45.Moore MR, Spence JB, Kiningham KK, Dillon JL. Progestin inhibition of cell death in human breast cancer cell lines. J Steroid Biochem Mol Biol. 2006;98(4–5):218–227. doi: 10.1016/j.jsbmb.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 46.Ory K, Lebeau J, Levalois C, Bishay K, Fouchet P, Allemand I, Therwath A, Chevillard S. Apoptosis inhibition mediated by medroxyprogesterone acetate treatment of breast cancer cell lines. Breast Cancer Res Treat. 2001;68(3):187–198. doi: 10.1023/A:1012288510743. [DOI] [PubMed] [Google Scholar]
- 47.Pace MC, Thomas P. Activation of a pertussis toxin-sensitive, inhibitory G-protein is necessary for steroid-mediated oocyte maturation in spotted seatrout. Dev Biol. 2005;285(1):70–79. doi: 10.1016/j.ydbio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 48.Pace MC, Thomas P. Steroid-induced oocyte maturation in Atlantic croaker (Micropogonias undulatus) is dependent on activation of the phosphatidylinositol 3-kinase/Akt signal transduction pathway. Biol Reprod. 2005;73:988–996. doi: 10.1095/biolreprod.105.041400. [DOI] [PubMed] [Google Scholar]
- 49.Pang Y, Thomas P. Progesterone signals through membrane progesterone receptors (mPRs) in MDA-MB-468 and mPR-transfected MDA-MB-231 breast cancer cells which lack full-length and N-terminally truncated isoforms of the nuclear progesterone receptor. Steroids. 2011;76:921–928. doi: 10.1016/j.steroids.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Persson I, Weiderpass E, Bergkvist L, Bergstrom R, Schairer C. Risks of breast and endometrial cancer after estrogen and estrogen-progestin replacement. Cancer Causes Control. 1999;10(4):253–260. doi: 10.1023/A:1008909128110. [DOI] [PubMed] [Google Scholar]
- 51.Porter A, Jänicke R. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6:99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- 52.Ross RK, Paganini-Hill A, Wan PC, Pike MC. Effect of hormone replacement therapy on breast cancer risk: estrogen versus estrogen plus progestin. J Natl Cancer Inst. 2000;92(4):328–332. doi: 10.1093/jnci/92.4.328. [DOI] [PubMed] [Google Scholar]
- 53.Schairer C, Lubin J, Troisi R, Sturgeon S, Brinton L, Hoover R. Estrogen-progestin replacement and risk of breast cancer. JAMA. 2000;284(6):691–694. doi: 10.1001/jama.284.6.691. [DOI] [PubMed] [Google Scholar]
- 54.Scorrano L. Opening the doors to cytochrome c: changes in mitochondrial shape and apoptosis. Int J Biochem Cell Biol. 2009;41(10):1875–1883. doi: 10.1016/j.biocel.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 55.She QB, Solit DB, Ye Q, O’Reilly KE, Lobo J, Rosen N. The BAD protein integrates survival signaling by EGFR/MAPK and PI3K/Akt kinase pathways in PTEN-deficient tumor cells. Cancer Cell. 2005;8(4):287–297. doi: 10.1016/j.ccr.2005.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shyamala G. Progesterone signaling and mammary gland morphogenesis. J Mammary Gland Biol Neoplasia. 1999;4(1):89–104. doi: 10.1023/A:1018760721173. [DOI] [PubMed] [Google Scholar]
- 57.Sleiter N, Pang Y, Park C, Horton TH, Dong J, Thomas P, Levine JE. Progesterone receptor A (PRA) and PRB-independent effects of progesterone on gonadotropin-releasing hormone release. Endocrinology. 2009;150(8):3833–3844. doi: 10.1210/en.2008-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Solaini G, Sgarbi G, Baracca A. Oxidative phosphorylation in cancer cells. Biochim Biophys Acta. 2011;1807(6):534–542. doi: 10.1016/j.bbabio.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 59.Stathopoulou A, Gizi A, Perraki M, Apostolaki S, Malamos N, Mavroudis D, Georgoulias V, Lianidou ES. Real-time quantification of CK-19 mRNA-positive cells in peripheral blood of breast cancer patients using the lightcycler system. Clin Cancer Res. 2003;9(14):5145–5151. [PubMed] [Google Scholar]
- 60.Tang YT, Hu T, Arterburn M, Boyle B, Bright JM, Emtage PC, Funk WD. PAQR proteins: a novel membrane receptor family defined by an ancient 7-transmembrane pass motif. J Mol Evol. 2005;61(3):372–380. doi: 10.1007/s00239-004-0375-2. [DOI] [PubMed] [Google Scholar]
- 61.Thomas P. Characteristics of membrane progestin receptor alpha (mPRα) and progesterone membrane receptor component one (PGRMC1) and their roles in mediating rapid progestin actions. Front Neuroendocrinol. 2008;29:292–312. doi: 10.1016/j.yfrne.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thomas P, Pang Y, Dong J, Groenen P, Kelder J, de Vlieg J, Zhu Y, Tubbs C. Steroid and G protein binding characteristics of the seatrout and human progestin membrane receptor alpha subtypes and their evolutionary origins. Endocrinology. 2007;148(2):705–718. doi: 10.1210/en.2006-0974. [DOI] [PubMed] [Google Scholar]
- 63.Vares G, Ory K, Lectard B, Levalois C, Altmeyer-Morel S, Chevillard S, Lebeau J. Progesterone prevents radiation-induced apoptosis in breast cancer cells. Oncogene. 2004;23(26):4603–4613. doi: 10.1038/sj.onc.1207601. [DOI] [PubMed] [Google Scholar]
- 64.Varghese C. The significance of oestrogen and progesterone receptors in breast cancer. J Clin Diagn Res. 2007;3:198–203. [Google Scholar]
- 65.Williams C, Mehrian Shai R, Wu Y, Hsu YH, Sitzer T, Spann B, McCleary C, Mo Y, Miller CA. Transcriptome analysis of synaptoneurosomes identifies neuroplasticity genes overexpressed in incipient Alzheimer’s disease. PLoS One. 2009;4(3):e4936. doi: 10.1371/journal.pone.0004936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhu Y, Bond J, Thomas P. Identification, classification, and partial characterization of genes in humans and other vertebrates homologous to a fish membrane progestin receptor. Proc Natl Acad Sci U S A. 2003;100(5):2237–2242. doi: 10.1073/pnas.0436133100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhu Y, Rice CD, Pang YF, Pace M, Thomas P. Cloning, expression, and characterization of a membrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes. Proc Natl Acad Sci U S A. 2003;100(5):2231–2236. doi: 10.1073/pnas.0336132100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zuo L, Li W, You S. Progesterone reverses the mesenchymal phenotypes of basal phenotype breast cancer cells via a membrane progesterone receptor mediated pathway. Breast Cancer Res. 2010;12(3):R34. doi: 10.1186/bcr2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PPTX 128 kb)
(PPTX 125 kb)
(PPTX 135 kb)
(PPTX 776 kb)
(PPTX 147 kb)
(PPTX 119 kb)