Abstract
Purpose
To determine the feasibility, safety and preliminary efficacy of a telehealth supervised exercise programme in patients with advanced melanoma receiving checkpoint inhibitor therapy.
Methods
A 8‐week non‐randomised feasibility pilot trial utilising a telehealth delivered multimodal exercise programme undertaken thrice weekly with assessments at baseline and post‐intervention. The study was considered feasible if there were no severe or life‐threatening adverse events as a result of exercise, and three or more of the following criteria were met: the recruitment rate was >50%, completion rate was >80%, median programme attendance was >75%, median exercise compliance >75%, and average tolerance was >70%. Preliminary efficacy was assessed for objective measures of physical function (2‐min step test, repeated chair stand test, 30‐s push‐up test, and a modified static balance test) and quality of life (QoL), fatigue and other patient‐reported outcomes were assessed using the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30.
Results
Eleven patients (32–80 years) were included in the study (6 female, 5 male). The recruitment rate was 48%, completion rate 91%, programme attendance 88%, median exercise compliance 82.1% and 84.9% for resistance and aerobic exercise, respectively, and tolerance 88%, with no severe or life‐threatening adverse events as a result of exercise. In terms of preliminary efficacy, physical function significantly improved while QoL was maintained following the intervention.
Conclusion
An 8‐week telehealth exercise intervention is feasible and safe for patients with advanced melanoma and appears to improve physical function while preserving QoL during checkpoint inhibitor therapy.
Keywords: exercise, health‐related outcomes, immunotherapy, melanoma, quality of life, telehealth
1. INTRODUCTION
The advent and widespread adoption of systemic therapies such as immunotherapy and molecular targeted therapy have substantially improved survival rates for patients with advanced melanoma. The current 5‐year survival rate of patients with advanced melanoma receiving therapies such as programmed cell death protein 1 (PD‐1)/cytotoxic T‐lymphocyte‐associated protein 4 (CTLA‐4) inhibitors and v‐Raf murine sarcoma viral oncogene homologue B (BRAF)/mitogen‐activated protein kinase kinase (MEK) inhibitors ranges from ~34% to 52%. 1 This is a significant improvement compared to a decade ago when the 5‐year survival rate was <10% for patients with advanced melanoma. 2 However, grade 3 (severe) and grade 4 (life‐threatening) adverse events are common in this population during systemic treatment, especially with combination immunotherapy. 1 Among the range of adverse events, fatigue, weakness, pneumonitis and a decreased cardiac ejection fraction have been often reported, 3 substantially affecting quality of life (QoL) and wellbeing of patients/survivors.
Exercise has been considered an important non‐pharmacological therapy for patients during and/or following cancer treatment. Current guidelines recommend using exercise and physical activity interventions for a range of cancers including breast, prostate, colorectal, lung, haematological and head and neck cancers. 4 , 5 , 6 , 7 When utilised as adjuvant therapy within these cancer populations, exercise can alleviate a range of treatment‐related side effects such as sarcopenia, lymphoedema, metabolic syndrome, myalgia and arthralgias, in addition to improvements in physical, functional and psychological distress outcomes. 4 , 5 , 6 , 7 However, for patients with melanoma, there is a paucity of evidence as to the feasibility and benefits of exercise. 8 As observed in a recent systematic review from our team, 8 most studies in patients with melanoma focused on cross‐sectional/retrospective data and only a few investigated feasibility when exercise is undertaken during treatment. 8 This indicates that the positive effects of exercise seen in other cancers are yet to be demonstrated in patients with melanoma. Concurrently, as melanoma is currently one of the few cancers for which immunotherapy is commonly prescribed, a novel opportunity exists to test exercise medicine in patients receiving this effective treatment.
The global emergence of coronavirus disease (COVID‐19) has significantly impacted traditional exercise settings and service delivery. Social distancing measures, quarantine and self‐isolation rules had been put in place to protect the population, particularly those with chronic diseases. Patients with cancer, 9 especially those of increasing age and comorbidities, 10 are at an increased risk of mortality if infected with COVID‐19. Accordingly, the utilisation of telehealth, that is, the use of telecommunication techniques for the delivery of health services and transmission of health information over a distance, 11 has been suggested as an alternative method of exercise delivery for patients with cancer. 12 , 13 Previous studies examining telehealth exercise programmes in patients with different types of cancer (primarily breast and prostate cancer) report symptom relief and no adverse events. 14 , 15 Therefore, this study aimed to determine the feasibility, safety and explore the preliminary efficacy of a telehealth supervised exercise programme in patients with melanoma receiving checkpoint inhibitor therapy. We hypothesised that an exercise intervention during treatment for melanoma is feasible and does not adversely affect patient‐reported or physiological outcomes.
2. METHODS
2.1. Study design
We undertook an 8‐week non‐randomised feasibility pilot trial with assessments at baseline and post‐intervention. Intervention sessions included resistance, aerobic and balance exercises undertaken three times per week resulting in a total of 24 exercise sessions. The research project was approved by the Edith Cowan University Human Research Ethics Committee (2019‐00795‐CROSBY) and the Sir Charles Gairdner and Osborne Park Health Care Group Human Research Ethics Committee (RGS0000004232).
2.2. Participant recruitment
Melanoma patients receiving checkpoint inhibitor therapy were identified by attending oncology services at Sir Charles Gairdner Hospital, Fiona Stanley Hospital, and Hollywood Private Hospital, who provided them with a recruitment flyer containing information about the study. Flyers were also distributed through online melanoma support groups and at community events. Participants were screened to ensure they met the inclusion criteria: 18 years or older, diagnosed with stage III‐IV melanoma and receiving or about to receive checkpoint inhibitor therapy. Participants were excluded if they: (i) had an acute illness or musculoskeletal, cardiovascular or neurological disorder that could inhibit exercise participation; (ii) had an uncontrolled medical condition (other than metastatic cancer); and (iii) presented with a cardiovascular or pulmonary contraindication to exercise listed in the American College of Sports Medicine (ACSM) guidelines. 16 Once deemed eligible, each participant was provided with an information letter with detailed experimental procedures, study details, associated risks and benefits. All participants provided written informed consent and obtained medical clearance from their general practitioner.
2.3. Exercise training programme
Exercise sessions were supervised virtually by an accredited exercise physiologist (AEP) via the online video conferencing platform Zoom (Zoom Inc). Sessions were conducted by a single exercise physiologist with up to three participants. Participants were given the option of morning or afternoon sessions at set times and could move/reschedule sessions within the same week (sessions that could not be rescheduled were recorded as missed sessions). Participants baseline exercise capability/fitness was determined based on their physical assessment scores at baseline and the AEP's clinical judgement. A Gymstick (Gymstick, Gymstick International Oy) and intervention materials (exercise information booklets and logs) were provided at no cost to participants. The exercise programme (≤60 min duration) included resistance, aerobic and balance exercises with a 5‐min warm‐up and cool‐down each session. Balance activities (i.e. side touching, heel‐toe walking and single‐leg balance), light aerobic activity and stretches were included in the warm‐up and cool‐down.
The resistance exercise component (~30 min) included a combination of exercises using bodyweight and elastic resistance (Gymstick). Based on the participants baseline exercise capability/fitness, they utilised either a black elastic band (1–20 kg) or grey elastic band (1–25 kg) on the Gymstick. Resistance exercise comprised training the major upper and lower body muscle groups with participants instructed to perform 2–3 sets of 8–12 repetitions for each exercise. A variety of exercises were utilised over the 8‐week period, for example, chest press, bent‐over row, shoulder press, biceps curl, squats, lunges and calf raise (Table S1). Six exercises were alternated weekly (two upper‐body, two lower‐body, one accessory exercise and one abdominal exercise), with autoregulation modelled on the Exercise and Sports Science Australia (ESSA) position statement for cancer and exercise to allow patients with advanced melanoma to self‐determine their intensity, frequency or duration of exercise collaboratively with the AEP. 4 Exercises were paired where appropriate, for example, upper‐body followed immediately by lower‐body, with a 1‐min rest between sets. To ensure the progressive nature of the training programme, participants were encouraged to work at an intensity where the final repetitions of each set were noticeably difficult/fatiguing. If the participant could perform more repetitions than what was pre‐determined for that session, the intensity was increased by utilising an exercise progression or altering the resistance of the Gymstick (at the AEP's discretion) for the subsequent set or training session.
The aerobic exercise component (~20 min) initially included 5 sets of 1‐min intervals of moderate‐to‐high intensity on the spot/stationary exercises such as marching, boxing and jogging on the spot (Table S2) and progressed over the 8 weeks to 10 sets of 1‐min intervals. Participants were given 30 s of rest between intervals. Target intensity for the session was set between 12 and 15 on the 6–20 point rating of perceived exertion scale (RPE). 17 Resistance and aerobic exercise progressions are shown in Table S3.
2.4. Study outcomes
Study outcomes were feasibility (i.e. recruitment and completion rates, programme attendance, exercise compliance and tolerance) and participant safety (i.e. adverse events). The intervention was considered feasible if there were no severe or life‐threatening adverse events as a result of exercise, 18 , 19 and three or more of the following criteria were met: the recruitment rate was >50%, 18 completion rate was >80%, 19 median programme attendance was >75%, 18 , 19 median exercise compliance (resistance and aerobic) was >75%, 20 , 21 and average tolerance was >70%.
2.5. Primary endpoints
Recruitment and completion rates were determined by the ratio of enrolled and referred patients, and the ratio of patients who completed and were enrolled in the exercise programme, respectively. Programme attendance was based on the number of exercise sessions attended out of the 24 scheduled sessions. Attendance outcomes were defined as missed session (i.e. missing single or two consecutive sessions), interruption (i.e. missing three or more consecutive sessions) and permanent discontinuation (i.e. loss to follow‐up). 20
Exercise compliance was assessed for resistance and aerobic exercise separately. Resistance exercise compliance is defined as the ratio of the total volume of resistance exercise (i.e. product of sessions, number of exercises, sets and repetitions) completed to that prescribed. 20 Dose modification was defined as any session requiring resistance exercise dose reduction or escalation (i.e. decrease or increase in number of exercises, sets, load and/or reps). For aerobic exercise, the overall volume was calculated as the total number of intervals completed, while aerobic exercise compliance was determined as the ratio of the total volume of aerobic exercise completed to that prescribed. 21 Participants tolerance to the intervention was determined by comparing the achieved session RPE to target session RPE.
Participant safety was based upon the number of severe (hospitalisation) or life‐threatening adverse events attributable to the exercise intervention. Adverse events (including treatment‐related side effects, exercise‐related exacerbations and additional medication prescription) were recorded in a journal/log that participants kept and updated when necessary.
2.6. Secondary endpoints
Secondary endpoints included preliminary efficacy outcomes assessed at baseline and after the 8‐week intervention. All physical assessments and exercise delivery were conducted via a telehealth video conference by an AEP, while questionnaires (paper‐based) were completed by participants at home on the day of the assessment. 22 Cardiovascular capacity was determined using the 2‐min step test (TMST). 23 The standard error of measurement for the TMST is 2.7 steps. 24 Functional performance was assessed by the repeated chair stand test. 25 The reported coefficient of variation for the repeated chair stand is 5.6%. 26 Upper body strength/endurance was measured using the 30‐s push‐up test. 27 Balance was evaluated using a modified ESSA static balance test for older adults, 25 whereby patients attempted to maintain balance for 30 s during semi‐tandem, tandem, single leg and single‐leg stance with eyes closed.
Participants' self‐reported balance confidence was determined using the Activities‐Specific Balance Confidence (ABC) Scale Questionnaire. 28 The European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQ‐C30) was used to assess cancer‐specific QoL, functional and symptom outcomes. 29 The Brief Fatigue Inventory (BFI) was used to assess fatigue levels before each intervention session. The mean of the nine BFI items was used to determine participants' global fatigue scores categorised as mild (1–3), moderate (4–6) or severe (7–10). 30
2.7. Other measures
Demographics and clinical information were collected via an online questionnaire. Self‐reported height and body weight (at baseline and post‐intervention) were used to determine participants' body mass index (BMI, expressed as kg/m2). Physical activity was assessed using the International Physical Activity Questionnaire–Short Form (IPAQ‐SF). 31 To gauge general physical activity behaviour, intervention session activity was excluded from the total. Participants were considered physically active if they met the ACSM recommendations (i.e. completing ≥150 min of moderate or 75 min of strenuous physical activity or a combination per week). 32
All patient‐reported outcome measures were scored according to their corresponding scoring manual/recommendations, ABC Scale, 28 EORTC QLQ‐C30, 33 BFI 30 and IPAQ‐SF. 34 Higher scores on the ABC Scale, EORTC QLQ‐C30 (global health status/QoL, and functional subscales), and IPAQ‐SF (physical activity subscale) indicate improvement in participant‐reported outcomes, whereas lower scores on the EORTC QLQ‐C30 (symptom subscales), BFI, and IPAQ‐SF (sedentary behaviour subscale) indicate better results.
2.8. Sample size calculation and statistical analysis
We aimed to recruit 22 diagnosed stage III/IV patients with melanoma who were receiving checkpoint inhibitor therapy as this would have enabled us to detect a statistically significant change in aerobic capacity using a two‐tailed, paired t‐test with an effect size of 0.60, α‐level of 0.05 and statistical power (1 − β) of 0.80. Study design changes due to Covid‐19 resulted in a shortened recruitment window and, as such, only 11 participants were recruited. This number of participants gives us the ability to detect an effect size of 1.0 in a number of our secondary outcome measures such as aerobic capacity.
Statistical analyses were performed using IBM SPSS Statistics software (IBM SPSS Statistics, version 27, IBM Corp). Assumptions of normality and homogeneity of variance of residuals were tested using Shapiro–Wilk and Levene test, respectively. Participant retention rate, programme attendance, exercise compliance and tolerance were described using descriptive statistics. Normally distributed data are presented as mean ± SD and/or 95% confidence intervals (95% CI), while data not normally distributed are presented as median and interquartile range (IQR). To calculate the difference between baseline and post‐assessment values, a paired t‐test or Wilcoxon signed‐rank test was undertaken, as appropriate, for continuous data. A p‐value <0.05 was considered statistically significant.
3. RESULTS
3.1. Recruitment and completion rates
Twenty‐three patients diagnosed with melanoma were assessed for eligibility between May 2021 and January 2022. The study recruitment rate was 48% as 11 participants consented and were recruited into the exercise programme. All 11 participants completed baseline testing. The recruitment process and loss to follow‐up are detailed in Figure 1. Seven patients (31%) were excluded from the study due to having progression of disease (n = 5), absence of video‐capable device (n = 1) and contraindication to exercise (n = 1). Five eligible candidates declined participation citing lack of time, poor health or preferred a clinic‐based exercise programme. Ten participants (91%) completed the 8‐week intervention, and one participant withdrew from the trial due to a severe checkpoint inhibitor treatment‐related adverse event (transient ischaemic attack).
FIGURE 1.
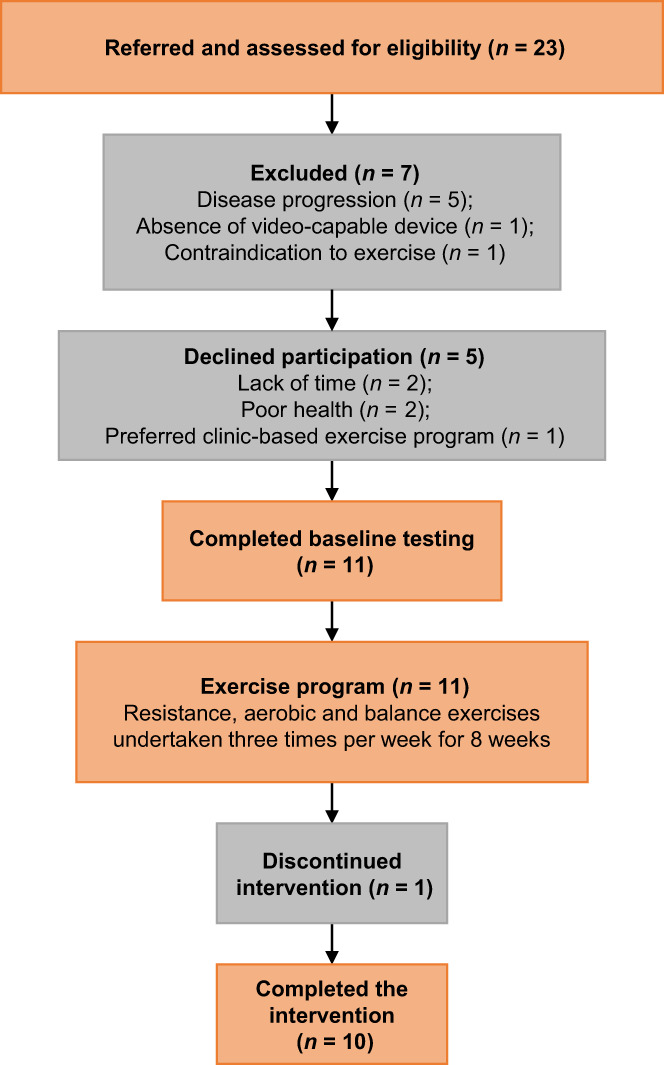
Flow chart of participant recruitment through the study.
3.2. Participant characteristics
Baseline characteristics are presented in Table 1. Six participants were female (54.5%), while the average age was 61.6 ± 13.6 years (range: 32–80 years). Three participants (27.3%) were physically active at baseline (i.e. ≥150 min per week) with most overweight or obese (63.8%). The majority of participants were diagnosed with stage IV melanoma (90.9%), with a median time since diagnosis of 11.0 months (IQR: 7.0–60.0 months). The most common previous treatment was surgery (81.8%). Nine participants were receiving monotherapy (PD‐1 or CTLA‐4) while two received combination inhibitors (PD‐1 and CTLA‐4). The median time since the beginning of checkpoint inhibitor treatment was 7.0 months (IQR: 0.0–9.0 months).
TABLE 1.
Baseline demographics and clinical characteristics.
| Characteristics | Participants (n = 11) |
|---|---|
| Demographic | |
| Age, mean ± SD, years | 61.6 ± 13.6 |
| Female, n (%) | 6 (54.5%) |
| Married, n (%) | 7 (63.6%) |
| Tertiary education, n (%) | 2 (18.2%) |
| Current employed, n (%) | 4 (36.4%) |
| Current smoker, n (%) | 0 (0%) |
| IPAQ MET‐min/week, median (IQR) a | 516 (363–1292) |
| Met PA Guidelines, n (%) | 3 (27.3%) |
| Clinical | |
| Body weight, mean ± SD, kg | 84.0 ± 18.9 |
| BMI, mean ± SD, kg.m−2 | 28.4 ± 7.6 |
| BMI categories, n (%) | |
| Normal weight (BMI < 25 kg.m−2) | 4 (36.4%) |
| Overweight (BMI ≥ 25– < 30 kg.m−2) | 5 (45.6%) |
| Obese (BMI ≥ 30 kg.m−2) | 2 (18.2%) |
| Time since diagnosis, median (IQR), months | 11 (7–60) |
| Time since treatment began, median (IQR), months | 7 (0–9) |
| Cancer stage, n (%) | |
| Stage III | 1 (9.1%) |
| Stage IV | 10 (90.9%) |
| Metastasis sites, n (%) | |
| Lung | 6 (54.5%) |
| Liver | 3 (27.3%) |
| Lymph nodes | 2 (18.2%) |
| Brain | 2 (18.2%) |
| Gastric lymphoma | 1 (9.1%) |
| Pancreas | 1 (9.1%) |
| Spine | 1 (9.1%) |
| Number of medications, median (IQR) | 1.0 (0.0–3.0) |
| Comorbidities, n (%) | |
| Hypertension | 3 (27.3%) |
| Hypercholesterolemia | 4 (36.4%) |
| Diabetes | 2 (18.2%) |
| Depression | 1 (9.1%) |
| Previous treatment, n (%) | |
| Surgery | 9 (81.8%) |
| Radiation therapy | 3 (27.3%) |
| Chemotherapy | 1 (9.1%) |
| Immunotherapy | 4 (36.4%) |
| BRAF/MEK inhibitor therapy | 1 (9.1%) |
| Checkpoint inhibitor type, n (%) | |
| PD‐1 | 7 (63.6%) |
| CTLA‐4 | 2 (18.2%) |
| PD‐1/CTLA‐4 combination | 2 (18.2%) |
Abbreviations: BMI, body mass index; BRAF/MEK, v‐Raf murine sarcoma viral oncogene homologue B/mitogen‐activated protein kinase; CTLA‐4, cytotoxic T‐lymphocyte‐associated protein 4; IPAQ, International Physical Activity Questionnaire; IQR, interquartile range; MET, metabolic equivalent minutes; PA, physical activity; PD‐1, programmed cell death protein 1; SD, standard deviation.
n = 10.
3.3. Programme attendance, compliance, tolerance, participant safety
The median programme attendance was 87.5% (IQR: 75.0–91.7%). Participants completed 226 out of the 264 exercise sessions scheduled. Seven participants (63.6%) missed 26 exercise sessions due to treatment‐related symptoms (n = 9, such as fatigue, migraine, vertigo), being unwell (n = 6), hospital admission (n = 6), psychological distress (n = 2) and impromptu hospital appointments (n = 3). One of these seven participants (participant #11; 9.1%) permanently discontinued the programme in Week 8 due to a transient ischaemic attack related to the checkpoint inhibitor treatment. Two participants (18.2%) had their exercise programme interrupted and lost six consecutive exercise sessions each due to a re‐occurrence of an axillary seroma (participant #8) and an elevated liver function test result (participant #10).
Median exercise compliance was 82.1% (IQR: 75.3–104.3%) and 84.9% (IQR: 75.8–89.0%) for resistance and aerobic exercise, respectively. The median cumulative resistance exercise dosage completed across the intervention was 4350 repetitions (IQR: 4080–5625 repetitions). For the aerobic exercise component, a total of 1716 out of 2046 aerobic exercise intervals were completed. Exercise dose had to be modified or interrupted for 17.9% of the resistance exercise sessions. All participants had their resistance exercise programme modified at some phase of the intervention, with the exercise dose reduced or escalated in 166 out of 226 sessions completed (73.5%). Participants had a median of 3 sessions (IQR: 2–7 sessions) with the resistance exercise dose reduced (59 out of 226 sessions), while the resistance exercise dose was escalated for a median of 10 resistance exercise sessions (IQR: 4–16 sessions; 107 out of 226 sessions) (Figure 2).
FIGURE 2.
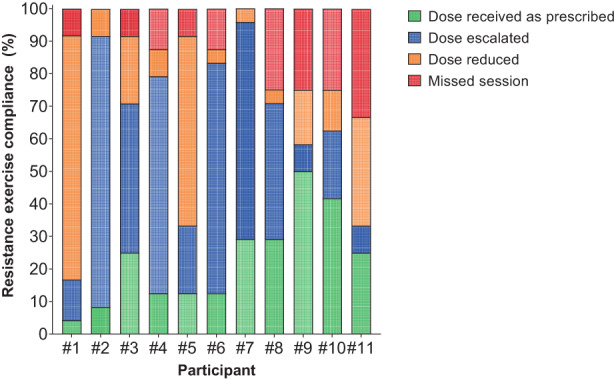
Resistance exercise compliance per participant. Data based on volume/dosage prescribed and completed.
With regard to exercise tolerance, 198 out of the 226 exercise sessions completed (87.6%) were performed at the prescribed (167 exercise sessions) or exceeded the target RPE (31 exercise sessions), while 28 exercise sessions were completed at a lower RPE than that prescribed (Figure 3).
FIGURE 3.
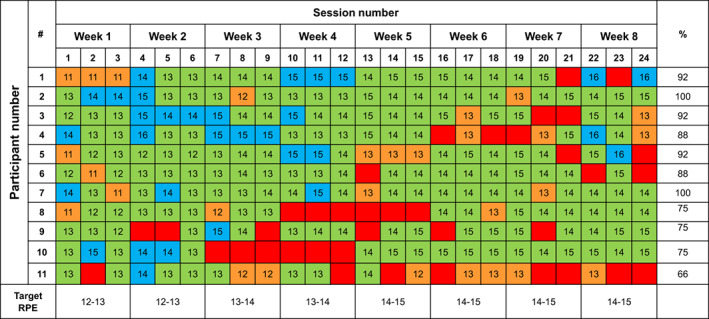
Intervention attendance and session intensity (RPE) per participant. Green squares represent that patient completed the exercise session at the prescribed rating of perceived effort; blue squares represent that patient completed the exercise sessions above the prescribed rating of perceived effort; orange squares represent that patient completed the exercise sessions below the prescribed rating of perceived effort; red squares represent missed or interrupted sessions.
No severe or life‐threatening adverse events were attributed to the exercise intervention. Adverse events are presented in Table 2. The most common checkpoint inhibitor treatment‐related side effects/adverse events were fatigue (20.0%) and diarrhoea (15.0%). Two participants (one completed the intervention, and one withdrew from the intervention) experienced a transient ischaemic attack. There was one minor exercise‐related exacerbation during the intervention sessions, which was the reopening of a surgical site (calf) 1 week after removal of suspected melanoma. In addition, one participant (participant #10 in Figures 2, 3, 4) discontinued combination PD‐1/CTLA‐4 inhibitor treatment due to an elevated liver function test result after 2 weeks into the exercise intervention. The treating oncologist suggested a 2‐week hiatus before recommencing the exercise intervention. Steroids were prescribed to manage the elevated liver enzymes and checkpoint inhibitor treatment was not recommenced before the completion of the intervention.
TABLE 2.
Frequency of participant‐reported adverse events/side effects.
| Adverse event | n (%) |
|---|---|
| Alopecia | 1 (2.5%) |
| Diarrhoea | 6 (15.0%) |
| Elevated liver enzymes | 2 (5.0%) |
| Fatigue (moderate/severe) | 8 (20.0%) |
| Gastrointestinal pain | 1 (2.5%) |
| Grand mal seizure | 1 (2.5%) |
| Hepatitis | 1 (2.5%) |
| Hypothyroidism | 1 (2.5%) |
| Migraine | 1 (2.5%) |
| Nausea | 2 (5.0%) |
| Night sweats | 1 (2.5%) |
| Peripheral neuropathy | 1 (2.5%) |
| Psoriasis | 1 (2.5%) |
| Psychological distress | 3 (7.5%) |
| Rash | 4 (10.0%) |
| Seroma | 1 (2.5%) |
| Surgical wound reopening a | 1 (2.5%) |
| Tachycardia | 1 (2.5%) |
| Transient ischaemic attack | 2 (5.0%) |
| Vertigo | 1 (2.5%) |
A direct result of the exercise intervention.
FIGURE 4.
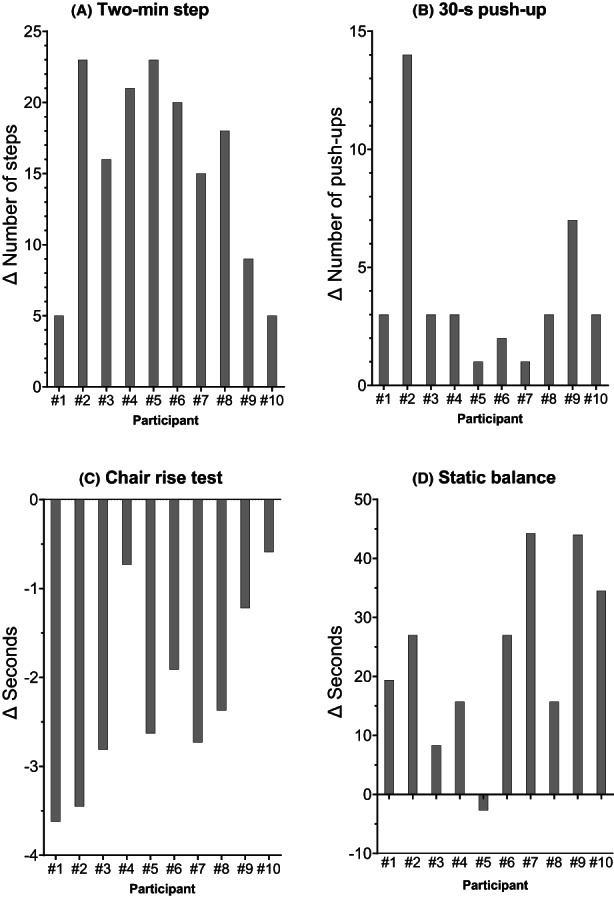
Waterfall plots of individual participants showing change in (A) 2‐min step, (B) 30‐s push‐up, (C) chair rise test, and (D) static balance over an 8‐week telehealth exercise programme. Individual patient numbers are identified in association with the bars.
3.4. Exploratory endpoints
We observed statistically significant improvements in multiple exploratory endpoints: cardiovascular capacity, upper body strength/endurance, functional performance and static balance. An increase of 15.5 steps (17.6%) was observed in the 2‐min step test (cardiovascular capacity), 4.0 repetitions (39.6%) during the 30‐s push‐up (upper body strength/endurance), 7.6 s (3.8%) in the static balance test (static balance), and a reduction of 2.9 s (23.2%) was observed in the chair rise test (functional performance) (Table 3). Individual changes in these outcomes are presented in Figure 4. There were no significant changes in global health status or any of the functional or symptom subscales following the intervention (Table 4). In addition, we did not observe improvements in perceived balance or change in body mass. The median fatigue level before each exercise intervention session was 1.9 pts (IQR: 0.9–3.8 pts). Following the intervention, a significant median increase in physical activity levels from 516 to 1374 metabolic equivalent min (MET.min) per week was observed.
TABLE 3.
Physiological outcomes and change over 8 weeks.
| Variables | Baseline | Post‐intervention | Mean difference | ||
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean | 95% CI | p | |
| Two‐minute step, steps | 88.3 ± 27.9 | 103.8 ± 29.5 | 15.5 | 10.5–20.5 | <0.001 |
| 30‐s push‐up, reps | 10.1 ± 4.8 | 14.1 ± 3.6 | 4.0 | 1.2–6.8 | 0.010 |
| Chair rise test, sec | 12.5 ± 3.3 | 9.6 ± 2.8 | −2.9 | −4.7 to −1.1 | 0.006 |
| Static balance a , sec | 190.2 (129.3–201.3) | 197.8 (160.6–219.2) | – | – | 0.007 |
Abbreviations: 95% CI, 95% confidence interval; SD, standard deviation.
Median (interquartile range).
TABLE 4.
Patient‐reported outcomes and change over 8 weeks.
| Variables | Baseline | Post‐intervention | |
|---|---|---|---|
| Median (IQR) | Median (IQR) | p | |
| EORTC QLQ‐C30 | |||
| Global health status | 66.7 (47.9–83.3) | 75.0 (58.3–83.3) | 0.888 |
| Physical functioning | 93.3 (65.0–100.0) | 96.7 (70.0–100.0) | 0.315 |
| Role functioning | 75.0 (29.2–100.0) | 91.7 (62.5–100.0) | 0.306 |
| Emotional functioning | 75.0 (58.3–91.7) | 83.3 (58.3–93.8) | 1.000 |
| Cognitive functioning | 83.3 (62.5–100.0) | 91.7 (62.5–100.0) | 0.279 |
| Social functioning | 66.7 (29.2–100.0) | 83.3 (16.7–100.0) | 0.496 |
| Fatigue | 44.4 (22.2–66.7) | 33.3 (19.4–47.2) | 0.440 |
| Nausea/vomiting | 0.0 (0.0–16.7) | 0.0 (0.0–16.67) | 0.705 |
| Pain | 25.0 (12.5–25.0) | 16.7 (0.0–37.5) | 0.786 |
| Dyspnoea | 0.0 (0.0–33.3) | 16.7 (0.0–41.7) | 0.084 |
| Insomnia | 33.3 (25.0–66.7) | 33.3 (0.0–66.7) | 1.000 |
| Appetite loss | 0.0 (0.0–33.3) | 0.0 (0.0–8.33) | 0.157 |
| Constipation | 33.3 (0.0–33.3) | 0.0 (0.0–33.3) | 0.046 |
| Diarrhoea | 0.0 (0.0–33.3) | 0.0 (0.0–8.3) | 0.705 |
| Financial difficulties | 0.0 (0.0–100.0) | 33.3 (0.0–100.0) | 0.157 |
| ABC Scale, pts | 140.5 (115.0–150.0) | 147.0 (122.3–149.0) | 0.192 |
| IPAQ‐SF | |||
| Physical activity levels a | 516 (363–1292) | 1374 (435–1763) | 0.047 |
| Sedentary behaviour | 3225 (2363–4500) | 2550 (1650–3450) | 0.107 |
Abbreviations: ABC Scale, Activities‐Specific Balance Confidence Scale; EORTC QLQ‐C30, The European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30; IPAQ‐SF, International Physical Activity Questionnaire–Short Form; IQR, interquartile range.
MET‐min/week.
4. DISCUSSION
The present study examined the feasibility, safety and preliminary efficacy of an 8‐week telehealth supervised exercise programme in patients with melanoma receiving checkpoint inhibitor therapy. The telehealth exercise intervention was feasible, tolerable and safe for patients with melanoma receiving checkpoint inhibitors. In addition, physical function measures were significantly improved while QoL was preserved despite patients undergoing checkpoint inhibitor therapy, well‐known to cause reductions in QoL.
This is the first study to report on supervised telehealth exercise in advanced melanoma patients or those undertaking checkpoint inhibitor therapy. Except for the recruitment rate which was just below the target of 50%, all the feasibility outcomes indicate that telehealth exercise is a feasible and safe intervention for this patient group. The telehealth exercise intervention exceeded the pre‐determined criteria for study outcomes (>3) with a completion rate of 91%, programme attendance of 88%, median exercise compliance of 82.1% (resistance exercise) and 84.9% (aerobic exercise), and tolerance at 88%, without causing any severe or life‐threatening adverse events. While two participants self‐reported experiencing a transient ischaemic attack, neither occurred during exercise and neither participant's GP attributed the event to the intervention.
It has been consistently reported that patients with advanced or metastatic cancer are under‐represented in survivorship research and services, given the complexity of their supportive care needs. 35 In this small cohort study, we found that a short‐term telehealth exercise programme may result in meaningful physical function and balance improvements while preserving QoL in advanced melanoma patients. Our results, if confirmed by further research, could provide clinicians with information on additional tools to support these patients achieve better physical and health‐related outcomes during checkpoint inhibitor therapy. This could potentially result in a better prognosis by more effectively managing cardiovascular, metabolic and musculoskeletal health. 36 , 37
Moreover, delivering exercise through telehealth is also a novel aspect of the study. A number of health‐related services have had to be changed during the COVID‐19 pandemic, 38 including access to supervised exercise. Most cancer patients have limited access to health services due to distance, transport, inconvenience, and financial capacity, resulting in an unacceptable disparity and suboptimal QoL for those patients who cannot access the best practice in melanoma care. Although our results need to be confirmed with a larger randomised controlled trial, the telehealth supervised exercise programme implemented in the present study is an important strategy to remove the disadvantage of patients unable to access clinic‐based facilities due to financial or geographic constraints. 38 Additionally, previous telehealth exercise interventions in a variety of cancer populations have reported symptom relief without causing severe adverse events. 14 , 39 , 40 Interestingly, even with an expected demanding cancer treatment regime due to a higher number of consultations, we observed that exercise intervention completion rates, programme attendance and exercise compliance were moderate to high, resulting in a relatively high tolerance of this group of patients. This may be partially explained by the supervised telehealth component of the intervention, enabling participants to complete sessions in their chosen location without the need to travel or attend a physical venue. Therefore, our data support future studies examining how telehealth exercise can improve treatment‐related outcomes in patients with advanced melanoma and, ultimately, be part of future exercise recommendations for cancer patients.
Our findings on preliminary efficacy may be important given the association between cardiorespiratory fitness and muscle strength with independent living and survival in older patients and those living with cancer. 41 , 42 , 43 , 44 Additionally, increased aerobic capacity, lower body muscle power, upper body strength and balance are associated with decreased fall risk among older adults. 45 , 46 These observed gains may represent the translation of exercise effects to potential health benefits in this group of patients, however, we must consider the small sample size. Also, our finding that body weight was maintained during the intervention is important as reductions in body weight during checkpoint inhibitor therapy are related to poorer survival. 47 Exercise programmes involving resistance training (i.e. anabolic exercise) might prove effective in this context, through maintenance of body weight while increasing lean body mass (i.e. muscle mass). 48 , 49 , 50 Therefore, using resistance‐based exercise programmes through telehealth may be a potential strategy to reduce the risk of sarcopenia and cachexia 51 , 52 for advanced melanoma patients.
Since advanced melanoma patients receiving checkpoint inhibitor therapy can have lower QoL than matched controls, 53 managing patient‐reported symptoms and outcomes is an important aspect of holistic health care. Among the potential mediators, intense treatment 54 and an advanced tumour 55 are associated with reductions in QoL. Exercise has been shown to significantly improve QoL when treating various cancer types. 56 Our exploratory analysis on QoL aligns with that of the Lacey et al. 57 study, which reported patients with melanoma receiving checkpoint inhibitor therapy preserved QoL over an 8‐week supportive care intervention that included exercise, dietary advice, psycho‐oncology services and complementary therapies. Similar to the study of Lacey et al., 57 the small sample size of the present study may limit the ability to detect meaningful change in this outcome. Nevertheless, maintaining QoL would still benefit advanced cancer patients. Interestingly, it has been suggested that advanced cancer patients may prioritise QoL over the length of life when receiving cancer treatment, potentially even refusing treatment to maintain QoL. 58 Maintaining QoL with exercise is feasible and a low‐cost intervention, which may improve treatment adoption and reduce treatment modification/cessation.
The strength of the present study is that it comprised a structured multimodal design (i.e. aerobic, resistance and balance activities) for patients with advanced melanoma receiving checkpoint inhibitor therapy. In addition, the utilisation of telehealth to provide supervised exercise is particularly relevant for individuals with advanced cancer (potentially immuno‐compromised), enabling access to exercise services for patients in metropolitan, rural and remote settings. The information provided regarding exercise metrics such as attendance, exercise compliance and tolerance enables the replication of this study on a larger scale. However, some limitations are worthy of comment. Changes to hospital recruitment procedures (following COVID‐19) and disease progression among many patients with advanced melanoma made recruitment difficult; consequently, our sample size was small, and a control group was not utilised. Moreover, due to the COVID‐19 pandemic and patients advanced melanoma status, assessments were undertaken via telehealth. As a result, the learning effect within the physiological testing outcomes could not be measured without a non‐exercise control group, potentially contributing to the changes in physical function observed. Given the number of comparisons/analyses undertaken, we cannot discount that at least one of the significant findings may be due to chance. Future studies should utilise a randomised controlled design to further investigate the effects on QoL and other patient‐reported symptoms during a longer exercise intervention. Additionally, we recommend that future studies examine the effect of exercise on muscle mass/body composition during checkpoint inhibitor treatment using techniques such as dual X‐ray absorptiometry. Finally, monitoring treatment compliance/tolerance during an exercise intervention will provide important clinical insights.
In conclusion, an 8‐week online telehealth exercise intervention is feasible and well tolerated by patients with melanoma receiving checkpoint inhibitor therapy with no major adverse events. Further, the intervention appeared to improve physical function in this group of patients while preserving QoL. These are important findings to inform the design of future randomised trials, which should include larger patient numbers, a usual care control group, a longer exercise intervention, and objective measures of body composition.
AUTHOR CONTRIBUTIONS
Brendan J Crosby: Conceptualization (lead); data curation (lead); formal analysis (lead); investigation (lead); methodology (lead); project administration (lead); software (lead); validation (lead); writing – original draft (lead); writing – review and editing (lead). Robert U. Newton: Conceptualization (supporting); investigation (supporting); methodology (supporting); supervision (equal); validation (supporting); visualization (supporting); writing – review and editing (supporting). Daniel A Galvao: Conceptualization (supporting); investigation (supporting); methodology (supporting); supervision (equal); validation (supporting); visualization (supporting); writing – review and editing (supporting). Dennis R Taaffe: Conceptualization (supporting); formal analysis (supporting); investigation (supporting); methodology (supporting); supervision (equal); validation (supporting); visualization (supporting); writing – review and editing (supporting). Pedro Lopez: Conceptualization (supporting); data curation (supporting); formal analysis (lead); investigation (supporting); methodology (supporting); software (supporting); visualization (supporting); writing – review and editing (supporting). Tarek Meniawy: Conceptualization (supporting); methodology (supporting); supervision (supporting); validation (supporting); visualization (supporting); writing – review and editing (supporting). Muhammad A Khattak: Conceptualization (supporting); methodology (supporting); supervision (supporting); validation (supporting); visualization (supporting); writing – review and editing (supporting). Wei‐Sen Lam: Conceptualization (supporting); methodology (supporting); supervision (supporting); validation (supporting); visualization (supporting); writing – review and editing (supporting). Elin S. Gray: Conceptualization (supporting); investigation (supporting); methodology (supporting); supervision (equal); validation (supporting); visualization (supporting); writing – review and editing (supporting). Favil Singh: Conceptualization (supporting); data curation (supporting); formal analysis (supporting); investigation (supporting); methodology (supporting); project administration (supporting); supervision (lead); validation (supporting); writing – review and editing (supporting).
CONFLICT OF INTEREST STATEMENT
No financial support was received to conduct the present study, or for the preparation or publication of this manuscript. Sponsors were not involved in the study design, analysis or interpretation of data, manuscript writing and decision to submit the manuscript for publication.
ETHICS STATEMENT
The research project was approved by the Edith Cowan University Human Research Ethics Committee (2019‐00795‐CROSBY) and the Sir Charles Gairdner and Osborne Park Health Care Group Human Research Ethics Committee (RGS0000004232).
Supporting information
Table S1.
Table S2.
Table S3.
ACKNOWLEDGEMENTS
Brendan J. Crosby is supported by the Cancer Council WA through the Paul Katris Honours and Masters Scholarship. Pedro Lopez is supported by the National Health and Medical Research Council (NHMRC) Centre of Research Excellence (CRE) in Prostate Cancer Survivorship Scholarship. Daniel A. Galvão and Robert U. Newton are funded by a NHMRC CRE in Prostate Cancer Survivorship. Elin S. Grey is supported by a fellowship from Cancer Council WA. The authors would like to thank Anna Reid for her support with patient recruitment. Open access publishing facilitated by Edith Cowan University, as part of the Wiley ‐ Edith Cowan University agreement via the Council of Australian University Librarians.
Crosby BJ, Newton RU, Galvão DA, et al. Feasibility of supervised telehealth exercise for patients with advanced melanoma receiving checkpoint inhibitor therapy. Cancer Med. 2023;12:14694‐14706. doi: 10.1002/cam4.6091
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Michielin O, Atkins MB, Koon HB, Dummer R, Ascierto PA. Evolving impact of long‐term survival results on metastatic melanoma treatment. J Immunother Cancer. 2020;8(2):e000948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dickson PV, Gershenwald JE. Staging and prognosis of cutaneous melanoma. Surg Oncol Clin N Am. 2011;20(1):1‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kottschade LA. Incidence and management of immune‐related adverse events in patients undergoing treatment with immune checkpoint inhibitors. Curr Oncol Rep. 2018;20(3):24. [DOI] [PubMed] [Google Scholar]
- 4. Hayes SC, Newton RU, Spence RR, Galvao DA. The Exercise and Sports Science Australia position statement: exercise medicine in cancer management. J Sci Med Sport. 2019;22(11):1175‐1199. [DOI] [PubMed] [Google Scholar]
- 5. Campbell KL, Winters‐Stone KM, Wiskemann J, et al. Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med Sci Sports Exerc. 2019;51(11):2375‐2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rock CL, Thomson C, Gansler T, et al. American Cancer Society guideline for diet and physical activity for cancer prevention. CA Cancer J Clin. 2020;70(4):245‐271. [DOI] [PubMed] [Google Scholar]
- 7. Schmitz KH, Campbell AM, Stuiver MM, et al. Exercise is medicine in oncology: engaging clinicians to help patients move through cancer. CA Cancer J Clin. 2019;69(6):468‐484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Crosby BJ, Lopez P, Galvao DA, et al. Associations of physical activity and exercise with health‐related outcomes in patients with melanoma during and after treatment: a systematic review. Integr Cancer Ther. 2021;20:15347354211040757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lunski MJ, Burton J, Tawagi K, et al. Multivariate mortality analyses in COVID‐19: comparing patients with cancer and patients without cancer in Louisiana. Cancer. 2021;127(2):266‐274. [DOI] [PubMed] [Google Scholar]
- 10. Imam Z, Odish F, Gill I, et al. Older age and comorbidity are independent mortality predictors in a large cohort of 1305 COVID‐19 patients in Michigan United States. J Intern Med. 2020;288(4):469‐476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Department of Health [Internet].2022. Available from:https://www.health.gov.au/topics/health‐technologies‐and‐digital‐health/about/telehealth#resources. [Google Scholar]
- 12. Newton RU, Hart NH, Clay T. Keeping patients with cancer exercising in the age of COVID‐19. J Oncol Pract. 2020;16(10):656‐664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Galvao DA, Taaffe DR, Hayne D, et al. Weight loss for overweight and obese patients with prostate cancer: a study protocol of a randomised trial comparing clinic‐based versus telehealth delivered EXercise and nutrition intervention (the TelEX trial). BMJ Open. 2022;12(6):e058899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Morrison KS, Paterson C, Toohey K. The feasibility of exercise interventions delivered via telehealth for people affected by cancer: a rapid review of the literature. Semin Oncol Nurs. 2020;36(6):151092. [DOI] [PubMed] [Google Scholar]
- 15. Winters‐Stone KM, Boisvert C, Li F, et al. Delivering exercise medicine to cancer survivors: has COVID‐19 shifted the landscape for how and who can be reached with supervised group exercise? Support Care Cancer. 2022;30(3):1903‐1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. American College of Sports Medicine . ACSM's guidelines for exercise testing and prescription. Lippincott Williams & Wilkins. 2013;455. [DOI] [PubMed] [Google Scholar]
- 17. Borg G. Ratings of perceived exertion and heart rates during short‐term cycle exercise and their use in a new cycling strength test. Int J Sports Med. 1982;3(3):153‐158. [DOI] [PubMed] [Google Scholar]
- 18. Dittus KL, Gramling RE, Ades PA. Exercise interventions for individuals with advanced cancer: a systematic review. Prev Med. 2017;104:124‐132. [DOI] [PubMed] [Google Scholar]
- 19. Heywood R, McCarthy AL, Skinner TL. Safety and feasibility of exercise interventions in patients with advanced cancer: a systematic review. Support Care Cancer. 2017;25(10):3031‐3050. [DOI] [PubMed] [Google Scholar]
- 20. Fairman CM, Nilsen TS, Newton RU, et al. Reporting of resistance training dose, adherence, and tolerance in exercise oncology. Med Sci Sports Exerc. 2020;52(2):315‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nilsen TS, Scott JM, Michalski M, et al. Novel methods for reporting of exercise dose and adherence: an exploratory analysis. Med Sci Sports Exerc. 2018;50(6):1134‐1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guidarelli C, Lipps C, Stoyles S, Dieckmann NF, Winters‐Stone KM. Remote administration of physical performance tests among persons with and without a cancer history: establishing reliability and agreement with in‐person assessment. J Geriatr Oncol. 2022;13(5):691‐697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bohannon RW, Crouch RH. Two‐minute step test of exercise capacity: systematic review of procedures, performance, and clinimetric properties. J Geriatr Phys Ther. 2019;42(2):105‐112. [DOI] [PubMed] [Google Scholar]
- 24. Uher I, Liba J. Correlation between functional fitness of older people and environmental and accommodation conditions. J Phys Educ Sport. 2017;17(4):2365‐2371. [Google Scholar]
- 25. Coombes JS, Skinner T. ESSA's Student Manual for Health, Exercise and Sport Assessment‐eBook. Elsevier Health Sciences; 2014:319‐350. [Google Scholar]
- 26. Galvao DA, Taaffe DR. Resistance exercise dosage in older adults: single‐versus multiset effects on physical performance and body composition. J Am Geriatr Soc. 2005;53(12):2090‐2097. [DOI] [PubMed] [Google Scholar]
- 27. Kellner P, Neubauer J, Polách M. Objectivity of push‐up tests and technique assessment. J Phys Educ Sport. 2021;21(4):1629‐1634. [Google Scholar]
- 28. Powell LE, Myers AM. The Activities‐Specific Balance Confidence (ABC) Scale. J Gerontol A Biol Sci Med Sci. 1995;50A(1):M28‐M34. [DOI] [PubMed] [Google Scholar]
- 29. Sprangers MA, Cull A, Bjordal K, Groenvold M, Aaronson NK. The European Organization for Research and Treatment of Cancer. Approach to quality of life assessment: guidelines for developing questionnaire modules. EORTC study group on quality of life. Qual Life Res. 1993;2(4):287‐295. [DOI] [PubMed] [Google Scholar]
- 30. Mendoza TR, Wang XS, Cleeland CS, et al. The rapid assessment of fatigue severity in cancer patients: use of the brief fatigue inventory. Cancer. 1999;85(5):1186‐1196. [DOI] [PubMed] [Google Scholar]
- 31. Lee PH, Macfarlane DJ, Lam TH, Stewart SM. Validity of the International Physical Activity Questionnaire Short Form (IPAQ‐SF): a systematic review. Int J Behav Nutr Phys Act. 2011;8:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42(7):1409‐1426. [DOI] [PubMed] [Google Scholar]
- 33. Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ‐C30: a quality‐of‐life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365‐376. [DOI] [PubMed] [Google Scholar]
- 34. Committee IR. Guidelines for data processing and analysis of the International Physical Activity Questionnaire (IPAQ)‐short and long forms. http://www.ipaq.ki.se/scoring.pdf 2005.
- 35. Lustberg MB, Carlson M, Nekhlyudov L. Introduction to special section: living with incurable cancer: addressing gaps in cancer survivorship. J Cancer Surviv. 2021;15(3):367‐369. [DOI] [PubMed] [Google Scholar]
- 36. Newman AB, Simonsick EM, Naydeck BL, et al. Association of long‐distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006;295(17):2018‐2026. [DOI] [PubMed] [Google Scholar]
- 37. Cesari M, Kritchevsky SB, Newman AB, et al. Added value of physical performance measures in predicting adverse health‐related events: results from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2009;57(2):251‐259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Collins IM, Burbury K, Underhill CR. Teletrials: implementation of a new paradigm for clinical trials. Med J Aust. 2020;213(6):263‐5. e1. [DOI] [PubMed] [Google Scholar]
- 39. Galiano‐Castillo N, Cantarero‐Villanueva I, Fernández‐Lao C, et al. Telehealth system: a randomized controlled trial evaluating the impact of an internet‐based exercise intervention on quality of life, pain, muscle strength, and fatigue in breast cancer survivors. Cancer. 2016;122(20):3166‐3174. [DOI] [PubMed] [Google Scholar]
- 40. Toohey K, Paterson C, Moore M, Hunter M. Towards best practice in the delivery of prescribed exercise via telehealth for individuals diagnosed with cancer: a randomised controlled trial protocol. Contemp Clin Trials. 2022;119:106833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Spirduso WW, Cronin DL. Exercise dose‐response effects on quality of life and independent living in older adults. Med Sci Sports Exerc. 2001;33(6 Suppl):S598‐S608. discussion S9‐10. [DOI] [PubMed] [Google Scholar]
- 42. Jensen MT, Holtermann A, Bay H, Gyntelberg F. Cardiorespiratory fitness and death from cancer: a 42‐year follow‐up from the Copenhagen Male Study. Br J Sports Med. 2017;51(18):1364‐1369. [DOI] [PubMed] [Google Scholar]
- 43. Kim Y, White T, Wijndaele K, et al. The combination of cardiorespiratory fitness and muscle strength, and mortality risk. Eur J Epidemiol. 2018;33(10):953‐964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Versteeg KS, Blauwhoff‐Buskermolen S, Buffart LM, et al. Higher muscle strength is associated with prolonged survival in older patients with advanced cancer. Oncologist. 2018;23(5):580‐585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Toraman A, Yildirim NU. The falling risk and physical fitness in older people. Arch Gerontol Geriatr. 2010;51(2):222‐226. [DOI] [PubMed] [Google Scholar]
- 46. Ejupi A, Brodie M, Gschwind YJ, Lord SR, Zagler WL, Delbaere K. Kinect‐based five‐times‐sit‐to‐stand test for clinical and in‐home assessment of fall risk in older people. Gerontology. 2016;62(1):118‐124. [DOI] [PubMed] [Google Scholar]
- 47. McQuade JL, Daniel CR, Hess KR, et al. Association of body‐mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: a retrospective, multicohort analysis. Lancet Oncol. 2018;19(3):310‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lopez P, Taaffe DR, Galvao DA, et al. Resistance training effectiveness on body composition and body weight outcomes in individuals with overweight and obesity across the lifespan: a systematic review and meta‐analysis. Obes Rev. 2022;23(5):e13428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Keilani M, Hasenoehrl T, Baumann L, et al. Effects of resistance exercise in prostate cancer patients: a meta‐analysis. Support Care Cancer. 2017;25(9):2953‐2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Padilha CS, Marinello PC, Galvao DA, et al. Evaluation of resistance training to improve muscular strength and body composition in cancer patients undergoing neoadjuvant and adjuvant therapy: a meta‐analysis. J Cancer Surviv. 2017;11(3):339‐349. [DOI] [PubMed] [Google Scholar]
- 51. Adams SC, Segal RJ, McKenzie DC, et al. Impact of resistance and aerobic exercise on sarcopenia and dynapenia in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. Breast Cancer Res Treat. 2016;158(3):497‐507. [DOI] [PubMed] [Google Scholar]
- 52. Cao A, Ferrucci LM, Caan BJ, Irwin ML. Effect of exercise on sarcopenia among cancer survivors: a systematic review. Cancer. 2022;14(3):786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Coens C, Suciu S, Chiarion‐Sileni V, et al. Health‐related quality of life with adjuvant ipilimumab versus placebo after complete resection of high‐risk stage III melanoma (EORTC 18071): secondary outcomes of a multinational, randomised, double‐blind, phase 3 trial. Lancet Oncol. 2017;18(3):393‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Joseph RW, Liu FX, Shillington AC, et al. Health‐related quality of life (QoL) in patients with advanced melanoma receiving immunotherapies in real‐world clinical practice settings. Qual Life Res. 2020;29(10):2651‐2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lisy K, Lai‐Kwon J, Ward A, et al. Patient‐reported outcomes in melanoma survivors at 1, 3 and 5 years post‐diagnosis: a population‐based cross‐sectional study. Qual of Life Res. 2020;29(8):2021‐2027. [DOI] [PubMed] [Google Scholar]
- 56. Buffart LM, Kalter J, Sweegers MG, et al. Effects and moderators of exercise on quality of life and physical function in patients with cancer: an individual patient data meta‐analysis of 34 RCTs. Cancer Treat Rev. 2017;52:91‐104. [DOI] [PubMed] [Google Scholar]
- 57. Lacey J, Lomax AJ, McNeil C, et al. A supportive care intervention for people with metastatic melanoma being treated with immunotherapy: a pilot study assessing feasibility, perceived benefit, and acceptability. Support Care Cancer. 2019;27(4):1497‐1507. [DOI] [PubMed] [Google Scholar]
- 58. Shrestha A, Martin C, Burton M, Walters S, Collins K, Wyld L. Quality of life versus length of life considerations in cancer patients: a systematic literature review. Psychooncology. 2019;28(7):1367‐1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Table S2.
Table S3.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


