The concomitant use of the monoclonal antibodies (mAbs) tixagevimab-cilgavimab has been originally authorized for pre-exposure prophylaxis of COVID-19.1 Although limited by a weak neutralizing activity,2 tixagevimab-cilgavimab has been the only efficient combination available in France during the BA.1 and BA.2 breakthrough. The aim of this single-center retrospective study is to describe the use of tixagevimab-cilgavimab as an early treatment for COVID-19 in kidney transplant recipients (KTRs).
The study cohort consisted of all adult KTRs diagnosed with COVID-19 from December 22, 2021, to April 27, 2022 (ie, during the BA.1 and BA.2 breakthrough). Tixagevimab-cilgavimab (600 mg) was given intravenously. Participants were retrospectively divided into 2 groups according to their risk to develop moderate-to-severe COVID-19. Patients with at least 1 comorbid condition (ie, age >60 y, diabetes, obesity, or a history of cardiovascular disease) who were unvaccinated or showed a weak vaccine-induced humoral response (<264 BAU/mL after the last dose vaccine and before mAb administration) were considered at high risk. The remaining participants were deemed to be at low risk.
A total of 197 KTRs with COVID-19 were identified. Of them, 63 were excluded for the following reasons: treatment with sotrovimab (n = 31), administration of mAbs on hospitalization or in a late phase after symptom onset (ie, >8 d, n = 17), and missing information (n = 15). No adverse effect was observed at follow-up in any patient.
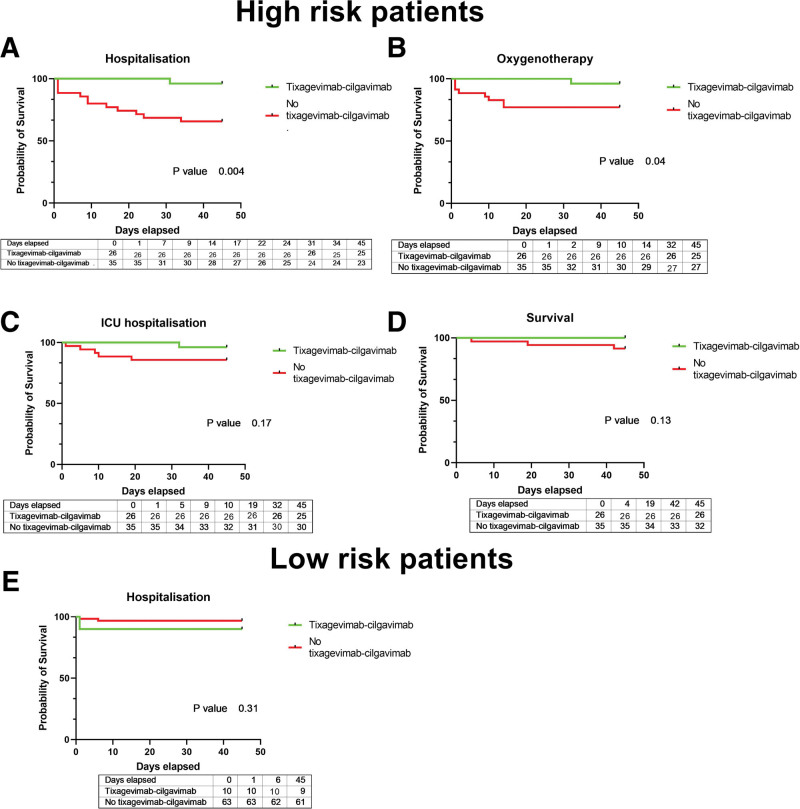
Among high-risk KTRs (n = 61), 26 received tixagevimab-cilgavimab within a median of 3 d (range: 2–4.75 d) from symptom onset. The remaining 35 patients were not treated with tixagevimab-cilgavimab for the following reasons: late notification of diagnosis (n = 19), clinical management in a different hospital (n = 4), COVID-19 infection before tixagevimab-cilgavimab availability (n = 7), patient refusal (n = 2), and unknown reasons (n = 3). Patients who received tixagevimab-cilgavimab benefited more frequently from early immunosuppressive therapy reduction (Table 1). COVID-19–related hospitalizations (3.8% versus 34%, respectively, P = 0.006; Figure 1A) and oxygen need (3.8% versus 23%, respectively, P = 0.04; Figure 1B) were significantly less frequent in KTRs treated with tixagevimab-cilgavimab. Similar, albeit not significant, trends were observed for intensive care unit admissions (3.8% versus 14.3%, respectively, P = 0.17; Figure 1C) and mortality (0 versus 3 patients, respectively, P = 0.13; Figure 1D). Of the low-risk KTRs (n = 73), 10 received tixagevimab-cilgavimab as an early treatment (Table 1). Three were hospitalized and 1 required oxygen treatment. No patient required intensive care unit admission or showed COVID-19–related mortality (Figure 1E).
TABLE 1.
General characteristics of kidney transplant recipients at high risk and low risk of moderate-to-severe COVID-19 according to early treatment with tixagevimab-cilgavimab
| High-risk group (n = 61) | Patient who did not receive curative tixagevimab-cilgavimab (n = 35) | Patient who received curative tixagevimab-cilgavimab (n = 26) | P value | Statistical test | Low-risk group (n = 73) | Patient who did not receive curative tixagevimab-cilgavimab (n = 63) | Patient who received curative tixagevimab-cilgavimab (n = 10) | P value | Statistical test | |
|---|---|---|---|---|---|---|---|---|---|---|
| Age, y | 61.0 [52.0–71.0] | 60 [52–71.5] | 65.5 [51.2–70.8] | 0.75 | Welch test | 49.0 [38–57] | 49 [38–57.5] | 49.5 [41.5–55.5] | 0.83 | Welch test |
| Men, n, %) | 42 (69%) | 25 (71%) | 17 (65%) | 0.61 | χ2 | 49 (67%) | 44 (70%) | 5 (50%) | 0.28 | Fisher exact test |
| BMI, kg/m2 | 28.4 [25.0–30.9] | 27.6 [24.9–30.6] | 28.5 [25.3–31.6] | 0.74 | Welch test | 25.1 [22.0–29.7] | 25.3 [22.1–30] | 24.9 [21.7–27.1] | 0.47 | Welch test |
| Serum creatinine, μmol/L | 133 [115–173] | 140 [116–186] | 125 [98–159] | 0.22 | Mann-Whitney U test | 115 [96–146] | 121 [96.0–146] | 98.5 [85.3–195] | 0.46 | Mann-Whitney U test |
| Cardiovascular disease, n, % | 21 (34%) | 8 (23%) | 13 (50%) | 0.03 | χ2 | 14 (19%) | 14 (22%) | 0 (0%) | 0.19 | Fisher exact test |
| Diabetes, n, % | 34 (56%) | 20 (57%) | 14 (54%) | 0.8 | χ2 | 18 (25%) | 18 (29%) | 0 (0%) | 0.06 | Fisher exact test |
| High blood pressure, n, % | 50 (82%) | 29 (83%) | 21 (81%) | 1 | Fisher exact test | 61 (84%) | 53 (84%) | 8 (80%) | 0.67 | Fisher exact test |
| First kidney transplantation, n, % | 54 (89%) | 33 (94%) | 21 (81%) | 0.13 | Fisher exact test | 62 (85%) | 54 (86%) | 8 (80%) | 0.64 | Fisher exact test |
| Time from kidney transplantation, y | 4.50 [1.75–8.73] | 2.9 [1.39–5.65] | 7.49 [266–12.5] | 0.02 | Mann-Whitney U test | 7.06 [4.14–11.8] | 7.06 [3.46–11.0] | 7.28 [5.40–17.6] | 0.5 | Mann-Whitney U test |
| Immunosuppressive drugs at diagnosis, n, % | ||||||||||
| Tacrolimus | 40 (66%) | 23 (66%) | 17 (65%) | 0.98 | χ2 | 48 (66%) | 41 (65%) | 7 (70%) | 1 | Fisher exact test |
| Cyclosporine | 14 (23%) | 7 (20%) | 7 (27%) | 0.52 | χ2 | 20 (27%) | 17 (27%) | 3 (30%) | 1 | Fisher exact test |
| MMF/MPA | 53 (87%) | 33 (87%) | 23 (85%) | 1 | Fisher exact test | 60 (82%) | 53 (84%) | 7 (70%) | 0.37 | Fisher exact test |
| mTOR inhibitors | 7 (11%) | 4 (11%) | 3 (12%) | 1 | Fisher exact test | 8 (11%) | 7 (11%) | 1 (10%) | 1 | Fisher exact test |
| Steroids | 45 (74%) | 26 (74%) | 19 (73%) | 0.92 | χ2 | 50 (68%) | 44 (70%) | 6 (60%) | 0.72 | Fisher exact test |
| Belatacept | 7 (11%) | 5 (14%) | 2 (7.7%) | 0.69 | Fisher exact test | 4 (5.5%) | 4 (6.3%) | 0 (0%) | 1 | Fisher exact test |
| Azathioprine | 0 | 0 | 0 | 5 (6.8%) | 4 (6.3%) | 1 (10%) | 0.53 | Fisher exact test | ||
| COVID-19 prevention, n, % | ||||||||||
| Vaccination | 57 (93%) | 32 (91%) | 25 (96%) | 0.64 | Fisher exact test | 69 (95%) | 60 (95%) | 9 (90%) | 0.45 | Fisher exact test |
| Pre-exposure tixagevimab-cilgavimab prophylaxis | 24 (39%) | 19 (54%) | 5 (19%) | <0.01 | χ2 | 7 (9.6%) | 7 (11%) | 0 (0%) | 0.58 | Fisher exact test |
| Pre-exposure casirivimab-imdevimab prophylaxis | 28 (46%) | 16 (46%) | 12 (46%) | 0.97 | χ2 | 8 (11%) | 6 (9.5%) | 2 (20%) | 0.3 | Fisher exact test |
| Antibody titer, BAU/mLa | 33 [1–117] | 8 [0–70] | 47 [16–133] | 0.12 | Mann-Whitney U test | 710 [240–1627] | 945 [285–1722] | 281 [47–407] | 0.02 | Mann-Whitney U test |
| History of COVID-19, n, % | 3 (4.9%) | 2 (5.7%) | 1 (3.8%) | 1 | Fisher exact test | 7 (9.6%) | 7 (11%) | 0 (0%) | 0.58 | Fisher exact test |
| COVID-19 presentation and clinical evolution, n, % | ||||||||||
| Symptomatic COVID-19 | 55 (92%) | 32 (94%) | 23 (88%) | 0.64 | Fisher exact test | 58 (88%) | 51 (88%) | 9 (90%) | 1 | Fisher exact test |
| COVID-19–related hospitalization | 13 (21%) | 12 (34%) | 1 (3.8%) | <0.01 | Log-rank | 3 (4.1%) | 2 (3.2%) | 1 (10%) | 0.31 | Log-rank |
| Oxygen need | 9 (5%) | 8 (23%) | 1 (3.8%) | 0.04 | Log-rank | 1 (1.4%) | 0 (0%) | 1 (10%) | 0.69 | Log-rank |
| ICU admission | 6 (9.8%) | 5 (14.3%) | 1 (3.8%) | 0.17 | Log-rank | 0 (0%) | 0 (0%) | 0 (0%) | 1 | Log-rank |
| Death | 3 (4.9%) | 3 (8.6%) | 0 (0%) | 0.13 | Log-rank | 0 (0%) | 0 (0%) | 0 (0%) | 1 | Log-rank |
| COVID-19 management, n, % | ||||||||||
| Dexamethasone | 7 (11%) | 6 (17%) | 1 (3.8%) | 0.22 | Fisher exact test | 1 (1.4%) | 1 (1.6%) | 0 (0%) | 1 | Fisher exact test |
| Immunosuppressive therapy reduction | 24 (41%) | 12 (36%) | 12 (46%) | 0.45 | χ2 | 7 (9.9%) | 4 (6.6%) | 3 (30%) | 0.06 | Fisher exact test |
| Early immunosuppressive therapy reductionb | 15 (24.6%) | 4 (1.1%) | 11 (42.3%) | 0.006 | χ2 | 5 (8.1%) | 2 (5.7%) | 3 (30%) | 0.016 | Fisher exact test |
Continuous variables are presented as medians (interquartile ranges), whereas categorical variables are presented as counts (percentages).
aOnly for patients not previously treated with casirivimab-imdevimab or tixagevimab-cilgavimab for pre-exposure prophylaxis.
bPatients who benefitted from a reduction of immunosuppression on hospitalization were excluded.
BAU, binding antibody unit; BMI, body mass index; ICU, intensive care unit; MMF, mycophenolate mofetil; MPA, mycophenolic acid; mTOR, mammalian target of rapamycin.
FIGURE 1.
Kaplan-Meier plots of hospitalization-free survival (A), oxygen need-free survival (B), ICU admission-free survival (C), and survival (D) in high-risk patients stratified according to the use (yes vs no) of tixagevimab-cilgavimab as an early treatment for COVID-19. Kaplan-Meier plots of hospitalization-free survival in low-risk patients stratified according to the use (yes vs no) of tixagevimab-cilgavimab as an early treatment for COVID-19 (E). ICU, intensive care unit.
Our data suggest that early administration of monoclonal anti–severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies, in combination with reduction of immunosuppression, may be clinically useful in high-risk KTRs with COVID-19. This is also supported by the observation that peak tixagevimab-cilgavimab concentrations are reached at 1 h after intravenous administration and are followed by rapid action.3 In contrast, our data provide an argument against the necessity to treat low-risk patients in the Omicron era. This is supported by the favorable clinical course observed in our study participants, and so even in the absence of treatment. As mAbs could drive the emergence of SARS-CoV-2 variants,4 we believe that their use should be limited to high-risk cases. However, our results should be interpreted in light of several limitations. This is a retrospective nonrandomized single-center study with a relatively limited sample size. Although the emergence of new variants (eg, BQ.1 and XBB), which are resistant to neutralization by most of the currently available mAbs (including tixagevimab-cilgavimab), puts this strategy into question,2 sotrovimab maintains a weak activity and can be used in high-risk patients as a curative strategy at the beginning of infection.2 This approach can also undergo future adaptations according to SARS-CoV-2 evolution and the in vitro efficacy of mAbs against novel variants. Fortunately, antiviral drugs continue to retain their activity against all variants and can provide an alternative strategy for the protection of KTRs. However, it should be kept in mind that the use of nirmatrelvir-ritonavir is challenging in patients being treated with calcineurin inhibitors and/or mammalian target of rapamycin inhibitors, and that remdesivir is contraindicated in patients with an estimated glomerular filtration rate <30 mL/min.5 A benefit-risk assessment tailored to the individual patient should be considered to guide the choice of the most appropriate option.
ACKNOWLEDGMENTS
The authors thank the clinical research team (Danielle Roy, Annie Menguy, and Fanny Husselstein) and the nursing staff (Sandra Ludwiller, Lucile Steinmetz, Christelle Appenzeller, and Fanny Petitjean) for their contribution to the study.
Footnotes
I.B. and S.C. were responsible for the study concept and design, data analysis, and manuscript writing; all authors were responsible for data collection and critical revision of the manuscript for important intellectual content.
S.C. has received consulting fees from Astra Zeneca. The other authors declare no conflicts of interest.
Data supporting the findings from this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Levin MJ, Ustianowski A, De Wit S, et al. LB5. PROVENT: phase 3 study of efficacy and safety of AZD7442 (tixagevimab/cilgavimab) for pre-exposure prophylaxis of COVID-19 in adults. Open Forum Infect Dis. 2021;8(Suppl_1):S810. [Google Scholar]
- 2.Planas D, Bruel T, Staropoli I, et al. Resistance of Omicron subvariants BA.2.75.2, BA.4.6, and BQ.1.1 to neutralizing antibodies. Nat Commun. 2023;14:824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bender Ignacio RA, Wohl DA, Arends R, et al. Comparative pharmacokinetics of tixagevimab/cilgavimab (AZD7442) administered intravenously versus intramuscularly in symptomatic SARS-CoV-2 infection. Clin Pharmacol Ther. 2022;112:1207–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caillard S, Laugel E, Benotmane I, et al. Molecular evolution of the SARS-CoV-2 omicron BA.2 variant in kidney transplant recipients with prolonged viral shedding. J Infect. 2023;86:513–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The BMJ. A living WHO guideline on drugs for covid-19. Available at https://www.bmj.com/content/370/bmj.m3379.long. Accessed February 15, 2023.