Abstract
Ophiothrix angulata (Say, 1825) is one of the most common and well-known ophiuroids in the Western Atlantic, with a wide geographic and bathymetric range. The taxonomy of this species has been controversial for a century because of its high morphological variability. Here we integrate information from DNA sequence data, color patterns, and geometric morphometrics to assess species delimitation and geographic differentiation in O. angulata. We found three deeply divergent mtDNA-COI clades (K2P 17.0–27.9%). ITS2 nuclear gene and geometric morphometrics of dorsal and ventral arm plates differentiate one of these lineages, as do integrative species delineation analyses, making this a confirmed candidate species.
Keywords: Nuclear DNA, Mitochondrial DNA, Morphology, Geometrics morphometrics, Integrative taxonomy, Caribbean, Gulf of Mexico, Coral reefs
Introduction
Members of the Class Ophiuroidea, brittle stars, basket stars, and snake stars, are the most diverse of the five echinoderm classes, and are often abundant and, at times, dominant members of marine benthic communities (Kissling & Taylor, 1977; Lewis & Bray, 1983; Hendler & Littman, 1986; Aronson, 1988; Stöhr, O’Hara & Thuy, 2012; Boissin et al., 2016). While the taxonomy of ophiuroids was thought to be well known, sequence data has demonstrated that numerous well-known brittle stars are complexes of cryptic species (e.g., the cosmopolitan Amphipholis squamata (Boissin, Féral & Chenuil, 2008) and the Northeastern Atlantic Ophiothrix fragilis (Pérez-Portela, Almada & Turon, 2013; Taboada & Pérez-Portela, 2016)).
The tropical Western Atlantic (WA) is home to the second major coral reef realm, has very high diversity and endemicity, and hosts a remarkably abundant, rich, and ecologically diverse brittle star fauna (Kissling & Taylor, 1977; Stöhr, O’Hara & Thuy, 2012; Sobha, Vibija & Fahima, 2023). The Ophiotrichidae (Ljungman, 1867) is the third-largest family of brittle stars and is well-represented in the WA. Ophiuroids have undergone extensive systematic revision following recent phylogenomic studies, and ophiotrichids were found to be “genetically and morphologically coherent” (O’Hara et al., 2018). Although ophiuroids have become well-defined at the family level, substantial work remains on many genera and species. Ophiothrix is the largest brittle star genus with 96 accepted species (Dos Santos Alitto et al., 2019; Santana et al., 2020; Stöhr, O’Hara & Thuy, 2023), but neither the genus nor its subgenera are monophyletic (O’Hara et al., 2017; O’Hara et al., 2018). The high diversity and morphological variability of ophiotrichids have made their species-level taxonomy challenging (Clark, 1946; Clark, 1967; Tommasi, 1970; Hoggett, 1991; Hendler, 2005).
Traditional species delineation of ophiuroids has focused on macro-morphological characters that can show substantial variability and overlap among species, causing taxonomic uncertainty (Arlyza et al., 2013). An integrative approach that combines information from live appearance, microstructure, life history, ecology, ethology, physiology, distribution, and especially DNA sequence data provides information to resolve species in challenging groups like Ophiothrix (Bickford et al., 2007; Boissin, Féral & Chenuil, 2008; Padial et al., 2010; Pérez-Portela, Almada & Turon, 2013; O’Hara et al., 2014a; Richards, De Biasse & Shivji, 2015; Taboada & Pérez-Portela, 2016; Dos Santos Alitto et al., 2019; Newton et al., 2020). Over the past decade, the use of microstructural characters has emerged as a valuable tool in systematic studies of brittle stars, revealing their phylogenetic value (O’Hara et al., 2014b; Thuy & Stöhr, 2016). Among these characters, arm plates have proven to be particularly important in establishing a congruence with molecular data, enabling the inference of phylogenetic relationships even at the genus level (Thuy & Stöhr, 2016). Therefore, these characters offer a valuable approach to analyze species complexes and contribute to species delimitation.
Ophiothrix angulata (Say, 1825) was one of the first brittle stars and the first ophiotrichid described for the Americas. It is nearly ubiquitous along the Atlantic coasts of North and South America in warm temperate to tropical waters, from North Carolina, USA to at least Venezuela, and throughout the Caribbean islands, Bahamas, and Bermuda (Devaney, 1974; Herrera-Moreno & Betancourt Fernández, 2004; Alvarado, Solís-Marín & Ahearn, 2008; Borrero-Pérez et al., 2008; Del Valle García et al., 2008; Laguarda-Figueras et al., 2009; Alvarado & Solís-Marín, 2013; Noriega & Fuentes-Carrero, 2014; Sandino et al., 2017; GBIF, 2022). Records of O. angulata further south are now attributed to other species (Santana et al., 2017; Santana et al., 2020). The species also has a broad bathymetric distribution from intertidal to bathyal depths (∼1,000 m; ICML-UNAM 3.34.40: 770 m; MCZ OPH-30910: 1,499 m). This species is capable of inhabiting corals, sponges, live under rocks, and among turf algae, which gives it a great capacity to adapt to different micro-habitats.
Ophiothrix angulata is highly variable and has a broad latitudinal and bathymetric distribution that has attracted taxonomic attention (Tommasi, 1970; Clark, 1933; Hendler et al., 1995; Hendler et al., 1999; Hendler, 2005; Santana et al., 2017). Variation is especially notable in color (Lyman, 1865; Verrill, 1899; Clark, 1901), and Clark (1918) named five varieties based on this. The species has also been noted to vary in disc shape, and arrangement of spinelets around the disc, but Hendler et al. (1995), concluded that this variation does not seem to sort into species-level units.
The goal of this study was to assess whether the great morphological diversity of O. angulata is the result of high intra-specific variation or differentiation among multiple cryptic or pseudo-cryptic species. We tested species boundaries using an integrative taxonomic approach, by combining mtDNA COI and nrDNA ITS2 sequence data, color patterns, and geometric morphometrics of dorsal and ventral arm plates. We combined results from genetic and morphological assessments for species delimitation using an integrated Bayesian phylogenetic and phylogeographic approach (iBPP) (Solís-Lemus, Knowles & Ané, 2015). We also analyzed the population diversity and demographic history of the clades discovered.
Materials & Methods
Sampling sites and collections
We used 146 Ophiothrix angulata specimens from 24 localities across the West Atlantic (Fig. 1; Table S1). Thirty-five samples were collected specifically for this project; others were obtained from collections at the Invertebrate Zoology Collection, Florida Museum of Natural History, University of Florida (UF); University of West Florida (UWF); Natural History Museum of Los Angeles County (LACM); Colección Nacional de Equinodermos “Dra. María Elena Caso Muñoz”, Instituto de Ciencias del Mar y Limnología, UNAM, México (ICML-UNAM); Colección Regional de Equinodermos de la Península de Yucatán, UMDI-Sisal, UNAM, México (COREPY-UNAM), and Museo de Zoología, Escuela de Biología, Universidad de Costa Rica (MZ-UCR). Field sample collection was approved by Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación (SAGARPA: Permission number: PPF/DGOPA-082/19). All 146 individuals were sequenced for COI, 14 for ITS2, 46 were selected for geometrics morphometric analyses while living color pattern was examined for 46 specimens from the UF and COREPY photographic collections. Twelve additional sequences available from GenBank were also used (Table S1). Nine terminals from five outgroup species were also sequenced: Ophiothrix cimar Hendler, 2005; Ophiothrix lineata Lyman, 1860; Ophiothrix stri Hendler, 2005; Ophiothrix suensonii Lütken, 1856 and Ophiactis savignyi (Müller & Troschel, 1842).
Figure 1. Sampling locations (see Table S1 for details), with the proportion of specimens per clade are given as pie charts.
Clade 1 (green), Clade 2 (blue), and Clade 3 (pink). OS: off Steinhatchee, North of St. Petersburg and Cedar Key. NWSP, Northwest of St. Petersburg; NSP and SSP, North and South of St. Petersburg; NFK, North of Florida Keys; FK, Florida Keys; PB, Palm Beach; JA, near Jacksonville; LU, near Louisiana; TV, Tuxpan Veracruz; SV, Sistema Arrecifal Veracruzano and Monte Pio; CAC, Cayo Arcas area; CAN, Cayo Arenas; ALA, Alacranes Reef; MR, Mayan Riviera; MH, Mahahual; BE, Belize; NI, Nicaragua; CR, Costa Rica; PA, Panama; StM, St. Martin; CU, Curaçao; GU, Guadeloupe; FG, French Guiana. ©OpenStreetMap 2022.
DNA extraction and sequence alignment
DNA was extracted from ethanol-fixed arm tissue using the Chelex protocol (Walsh, Metzger & Higuchi, 1991) or the Omega Bio-Tek E.Z.N.A. Mollusc DNA kit according to the manufacturer’s instructions. The echinoderm barcoding primers COIceF and COIceR (Hoareau & Boissin, 2010) were used to amplify a 655-base pair (bp) region of COI as described by Michonneau & Paulay (2014). Electropherograms were checked, assembled into contigs, and manually edited using Sequencher 4.6 (Gene Code Corps, Ann Arbor, MI, USA). Consensus sequences were aligned using Muscle (Edgar, 2004), and alignment was verified by eye using PhyDE v.10.0 (Müller et al., 2010). The sequence alignment was converted to protein using Genius v8.1.7 (Kearse et al., 2012) to ensure a proper reading frame and to verify the absence of stop codons. A 527 pb section of the nuclear DNA internal transcribed spacer-2 (ITS2) was amplified using the primers OphITS2F and OphITS2R as described by Naughton et al. (2014). Sequences were deposited in GenBank (COI accession numbers: MT338285–MT338398, ON245084–ON245096; ITS2 accession numbers: OQ225473–OQ225482).
Phylogenetic analyses
Phylogenetic analyses using Bayesian Inference (BI) and Maximum Likelihood (ML) methods were performed separately for COI and ITS2 sequence datasets on the CIPRES Science Gateway portal (Miller, Pfeiffer & Schwartz, 2010). jModelTest2 (Darriba et al., 2012) was used on the CIPRES portal to select the best model of molecular evolution based on Akaike information criteria tests (AIC). ML analyses were done using RAxML-HPC2 on XSEDE (Stamatakis, 2006; Stamatakis, Hoover & Rougemont, 2008) with the GTR+GAMMA model of sequence evolution; nodal support values were assessed with 1,000 rapid bootstraps (Felsenstein, 1985). BI analyses were performed using MrBayes v.3.2.7a on XSEDE (Ronquist et al., 2012), using the GTR+I+G model. The MCMC search was based on two independent runs of four chains each and 6,000,000 generations (sampled every 1,000 generations) until the final average and standard deviation were close to 0.01. Twenty-five percent of the initial trees were discarded as Burn-in. Results were summarized in Tracer v.1.7.1 (Rambaut et al., 2018) based on the Effective Sample Size-ESS for each parameter. The gene phylogenies were represented using FigTree v.1.4.4 (Rambaut, 2018) and annotated using Adobe Illustrator CC v.2017-22.0.1.
Geometric morphometric analyses
We analyzed dorsal arm plates (DAP) and ventral arm plates (VAP) from 46 specimens. Images of DAP and VAP were obtained through scanning electron microscopy (SEM) from intermediate-sized specimens with disc diameters (DD) of 2.5 to 5.5 mm to assess the size-independent variability. The integument was removed from the 4th–8th arm segments (counted from the first arm segment that contained a regular vertebra and lateral plates Stöhr, 2005), due to the adult proximal arm plates showing the highest degree of morphological differentiation, reflecting differences between species (Thuy & Stöhr, 2011). The integument removal was performed by submergence in 0.3% sodium hypochlorite solution for 2–8 h, washed with distilled water and 98% ethanol, air-dried, and mounted on aluminum stubs using carbon tape. The samples were then gold-coated and scanned using a Hitachi-SU1510 SEM at the LANABIO facility at the Instituto de Biología, UNAM.
In order to organize the data for analyses, the file format of the plate images was imported into TPS file format using tpsUtil v.1.58. Landmarks and curves were digitized using tpsDig2 v.2.17 (Rohlf, 2015). Along the external margin of each DAP, five homologous type II landmarks and sixty-eight evenly-spaced semi-landmarks were digitized (Fig. 2A). The same procedure was followed for VAP but using four landmarks type II and 66 semi-landmarks evenly-spaced (Fig. 2B). Geometric morphometric (GM) analyses followed the outlined by Masonick & Weirauch (2019), using the R package geomorph v.3.2.1 R package (Adams & Otárola-Castillo, 2013). The GM analyses compared four clades and subclades defined by COI sequence data set (Table S2): Clade 1A, Clade 1B, Clade 2A, and Clade 3. Generalized Procrustes analysis was used to extract the shape data for comparison, removal, translation, scaling, and rotation of all selected landmarks. A proxy of size in GM is centroid size which is the square root of the sum of squared distances of an object’s landmarks from their centroid or center of gravity.
Figure 2. DAP and VAP landmarks.
Landmarks type II (red) and semi-landmarks (yellow) used for geometric morphometrics to investigate variation in arm plates in Ophiothrix angulata. Numbers indicate landmark position. (A) Dorsal arm plate (DAP). (B) Ventral arm plate (VAP). Pe, Proximal edge; De, Distal edge. Photos credit: Y. Quetzalli Hernández-Díaz.
The semi-landmarks digitized for each arm plate were optimized to reduce bending energy using the function ProcD = False in geomorph, thus providing the best fit during optimization. Shape variation was analyzed through principal component analysis (PCA) using the covariance matrix of the individual, and a graphical scatterplot was performed using the two principal components, which accumulated the maximum variance of the data (PC1 vs. PC2; S3 Appendix). Thin-plate spline deformation grids were calculated from the mean shape variance along each PC axis with the shapes v.1.2.5 R package (Dryden, 2018), representing the overall shape modification. To enhance the visualization of data dispersion, two scatterplots in 3D were constructed based on the first three PCs using the R package car v.3.0-6 (Fox, Weisberg & Price, 2018; Masonick & Weirauch, 2019). A Procrustes analysis of variance (ANOVA) was performed on all analyses using the “procD.lm” function in geomorph. The resulting pairwise Procrustes distances were compared to assess the significance of differences in mean arm plate shape among the groups. The statistical significance of the observed variation was assessed through a permutation test of the randomized model residuals with 999 iterations at an α-value of 0.05.
Measurement error and allometry-test
To evaluate the impact of measurement error, the selected landmarks were digitized twice on each image (dorsal and ventral arm plates), on different days, by the same observer (Viscosi & Cardini, 2011). The error was calculated as percent measurement error (%ME) by comparing the variation among measurements based on a formula developed by (Bailey & Byrnes, 1990; Yezerinac, Lougheed & Handford, 1992). A Procrustes ANOVA on the residuals of Procrustes distances was used to compare within and among individual shape variance components. The allometry was evaluated by regression of the Procrustes shape coordinates on centroid size using a log10 scale. Interaction between centroid size and “clade” (dorsal or ventral arm plates grouped by clade) factor was estimated with Procrustes ANOVA in geomorph v.3.2.1 (Klingenberg, 2016).
Dorsal arm color pattern analysis
Color patterns were examined from live-taken images associated with 46 sequenced specimens from the UF and COREPY invertebrate image collections (Table S1). Twenty-five color characters were selected from dorsal arm views (Figs. S1, S2, and S3). All characters were treated as discrete and unordered (Appendix S1). Disc color characters were not selected because they showed a great amount of individual variation. Ophiactis savignyi was used as outgroup species (Fig. S4).
The character matrix was edited in Mesquite v.3.51 (Maddison & Maddison, 2018), with inapplicable data scored with “-” (Appendix S2). Parsimony analysis was conducted using TNT v.1.5 (Goloboff & Catalano, 2016). Optimal trees were searched using random addition sequences of Wagner trees, followed by the TBR algorithm, using 500 replicates, and saving 10 trees per replicate. The resulting trees were used as starting points for a round of TBR branch swapping. Bootstrap support values for the strict consensus tree were determined through 1,000 iterations, with default settings. Visualization, interpretation, and annotation of the cladogram were performed with FigTree, TNT, and Illustrator, respectively.
Molecular species delimitation
Two sequence-based species delimitation methods were employed using mtDNA data only. Multi-rate Poisson tree processes (mPTP) is a non-coalescent, sequence-based, maximum likelihood method that does not require pre-determined taxonomic designations and uses statistical cutoffs to delineate taxa on a phylogenetic input tree (Zhang et al., 2013; Kapli et al., 2017). It identifies variation in the pace of branching events, modeling speciation based on the number of substitutions. The online version of mPTP (https://mptp.h-its.org/#/tree) was used to calculate species delimitation, using the Bayesian tree as input, mPTP model selected, with nine specimens as outgroup taxa. The resulting trees were visualized using FigTree v.1.4.4.
The Bayeasian program BPP v.4.1.4 (Yang, 2015) was used to infer phylogenetic and phylogeographic patterns under the multispecies coalescent model (MSC) and to calculate potential species’ posterior probabilities delimitation (Yang & Rannala, 2010; Yang, 2015). BPP appears to be relatively robust to the influence of unequal population sizes, rates of population growth, unbalanced sampling, and mutation rate heterogeneity (Luo et al., 2018), and has proven effective in analyses of taxon evolution and divergence (Zhang et al., 2013; Moritz et al., 2018). A 151-taxon dataset and the Bayesian tree were used for all BPP analyses, because sequences with missing data were eliminated. The estimation of appropriate starting species divergence times (τs) and population size parameters (θs) was initially performed through A00 BPP analysis (Masonick & Weirauch, 2019). This estimation was based on the expected number of mutations per kilobase, as suggested by Yang (2015). The parameters for the MSC model were estimated using BPP v4.1.4 (following the A00 analysis from Yang, 2015). The joint species tree estimation and species delimitation analyses (A11) were carried out with inverse-gamma parameters of θ (5, 0.05) and τ (5, 0.02). The A11 analysis was run for 100,000 generations, sampling every two steps after discarding the initial 10,000 generations as burn-in. The analysis results were confirmed by conducting three independent runs for each analysis. Only lineages with a posterior probability (pp) of ≥ 0.95 were considered well-supported (Masonick & Weirauch, 2019).
Integrative species delimitation
Morphological and mtDNA data were analyzed in a common coalescent Bayesian framework using the program iBPP v.2.1.3 (Solís-Lemus, Knowles & Ané, 2015). This method has been shown to improve species delimitation accuracy by incorporating molecular and quantitative phenotypic data in the assessment of a priori species assignments using a guide tree. iBPP analyses were performed using the Bayesian topology as a guide tree. The same values for demographic parameters θ and τ as in the BPP analysis were used. The total evidence analyses described below utilized two datasets, as iBPP is capable of incorporating morphological data represented by quantitative, continuous traits: (a) multistate character matrix with the 25 arm color-characters as trait data scored through the color pattern analysis, and (b) PC1 + PC2 values for DAP and VAP as trait data obtained through the GM analysis. For both analyses, 46 specimens were used that included sequences and GM data, and the second included the specimens with sequences and the photographic record in vivo. To determine if different data types result in congruent delimitations, the following comparisons were made among data types: sequence data only, coloration data only, GM data only, sequence and coloration data (iBPPSeq+COL), and sequence and GM data (iBPPSeq+GM) (Masonick & Weirauch, 2019). Posterior probabilities (pp) at each node were averaged after performing each analysis three times. After a burn-in phase of 10,000 iterations, every second tree was sampled for a total of 100,000 trees. Well-supported delimitations were only considered for nodes on the guide tree that were recovered with pp values of ≥ 0.95 (Masonick & Weirauch, 2019).
Population diversity
Standard measures of genetic diversity (number of haplotypes, haplotype diversity h, and nucleotide diversity π) were calculated using Arlequin v.3.5.2.2 (Excoffier & Lischer, 2010). Unique haplotypes were identified using DnaSP v6.12 (Rozas et al., 2017). Geographical relationships of mtDNA haplotypes were summarized using the TCS algorithm (Clement et al., 2002) in the software PopART v.1.7 (Leigh & Bryant, 2015). To perform the homologous character comparison, missing data were excluded by trimming sequences to 632 bp. For comparison of the extent of divergence with other ophiuroid species, evolutionary distance values were generated in MEGA 11 (Tamura, Stecher & Kumar, 2021) using the Kimura 2-parameter model (Kimura, 1980), support values based on 1,000 bootstraps, including both transitions and transversions, the rate variation among sites was modeled with a gamma distribution (shape parameter = 1), codon positions included were 1st + 2nd + 3rd, and missing data were treated as pairwise deletion (Table 1).
Table 1. Genetic distances (±standard error) between recovered clades of O. angulata based on a Kimura 2-Parameters model for COI.
Inter-specific distance values are presented below the diagonal. Numbers along the diagonal in bold and brackets represent intra-specific variation. Genetic distances were compared with the congeneric species Ophiothrix lineata and O. suensonii distributed in the Caribbean and Gulf of Mexico; Ophiothrix cimar distributed in the Caribbean and Ophiactis savignyi (outgroup).
| COI | Clade 1 | Clade 2 | Clade 3 | O. cimar | O. lineata | O. suensonii | O. savignyi |
|---|---|---|---|---|---|---|---|
| Clade 1 | [0.032 ± 0.004] | ||||||
| Clade 2 | 0.170 ± 0.019 | [0.007 ± 0.001] | |||||
| Clade 3 | 0.279 ± 0.028 | 0.264 ± 0.026 | [0.053 ± 0.006] | ||||
| O. cimar | 0.316 ± 0.032 | 0.310 ± 0.033 | 0.278 ± 0.028 | − | |||
| O. lineata | 0.276 ± 0.028 | 0.253 ± 0.027 | 0.254 ± 0.026 | 0.133 ± 0.016 | − | ||
| O. suensonii | 0.304 ± 0.030 | 0.278 ± 0.029 | 0.274 ± 0.028 | 0.243 ± 0.027 | 0.264 ± 0.028 | − | |
| O. savignyi | 0.323 ± 0.032 | 0.302 ± 0.031 | 0.319 ± 0.032 | 0.279 ± 0.030 | 0.263 ± 0.027 | 0.302 ± 0.032 | − |
Demographic history
To test for past population expansions, the neutrality Fu’s Fs test (Fu, 1997) was implemented in Arlequin v.3.5.2.2, and significance was assessed with 1,000 permutations. In addition, the frequency of the distribution of mismatches was obtained in Arlequin and plotted with the R package ggplot2 (Wickham, 2016) to determine whether the populations exhibit evidence of spatial/demographic expansions or a stationary population history (Tajima, 1989). The Raggedness index and the sum of squared deviations (SSD) obtained in Arlequin were used to analyze the goodness of fit for the population expansion model, according to Harpending (1994).
Results
Genetic differentiation and spatial distribution
The consensus COI phylogenetic trees showed three deeply divergent (K2P distances 17.0–27.9%; Table 1), highly supported (PP/bootstrap at 100/≥90) clades in Ophiothrix angulata with both methods (BI and ML) (Fig. 3). Two clades (Clade 2 and Clade 3) are widespread, whereas Clade 1 has a more restricted range (Fig. 1). The three clades have overlapping depth distributional ranges down to 45 m, with only Clade 3 extending deeper, with five sequenced specimens from 45–135 m (Fig. S5). COI haplotype networks recovered the same groups obtained in the phylogenetic tree. Haplogroup 1 (Clade 1) was separated from Haplogroup 2 (Clade 2) by 64 mutational steps (m-s), while Haplogroup (Clade 3) was separated by 110 m-s from Clade 1, and by 96 m-s from Clade 2. Clades 1 and 2 were not differentiated in ITS2, but Clade 3 was divergent (Fig. 4).
Figure 3. MtDNA bayesian consensus tree.
Bayesian consensus tree of COI sequences produced using the GTR+I+G model in MrBayes v.3.2.7a on XSEDE for Ophiothrix angulata and outgroups. Clade 1 (green), Clade 2 (blue), Clade 3 (pink), and Outgroup (gray). Bayesian posterior probabilities (above), followed by ML bootstrap support (below; 1,000 replicates), are indicated at nodes.
Figure 4. MtDNA vs nrDNA trees.
A comparison of mtDNA (COI) (left) and nrDNA (ITS2) (right) phylogenies from the BI analysis are displayed as cladograms for Ophiothrix angulata with Bayesian posterior probabilities displayed above and ML bootstrap (1,000 replicates) support displayed below the nodes.
Clade 1
Clade 1 (n = 76) was encountered only in the Gulf of Mexico and east Florida, including the Veracruz platform reefs in the Southern Gulf of Mexico and Northern Gulf of Mexico at 0–41 m depths (Fig. S5). Although it overlaps in distribution and depth with clades 2 and 3, the latter are widely distributed across the sampled areas (Fig. 1). Three sympatric subclades can be differentiated within Clade 1 (Figs. 3 and 5), that cooccur in the Northern Gulf of Mexico and the Florida Keys.
Figure 5. TCS haplotype network clades 1 and 2.
Clade 1A (n = 42), Clade 1B (n = 26), Clade 1C (n = 3), Clade 2A (n = 42), and Clade 2B (n = 2). TCS haplotype network of COI sequences for two Ophiothrix angulata clades (632 bp). The number of specimens is superimposed onto the more abundant haplotypes. Northern Gulf of Mexico (green). Florida Keys (orange). Eastern Florida (vermilion). Southern Gulf of Mexico (grey). Campeche Bank (yellow). Western Caribbean (blue). Southwestern Caribbean (sky blue). Eastern Caribbean (pink).
Clade 2
Clade 2 (n = 51) was collected in almost all areas sampled (Northern Gulf of Mexico, Florida Keys, East Florida, Southern Gulf of Mexico, Western Caribbean, Southwestern Caribbean, and the Eastern Caribbean; Fig. 1) at 0.5–42 m depths (Fig. S5). Two deeply divergent (5.1% K2P), allopatric subclades can be differentiated in Clade 2: a widely distributed subclade 2A (n = 49) that displays a star-like haplotype network (Fig. 5), and subclade 2B (n = 2) sampled only in Guadeloupe (Eastern Caribbean).
Clade 3
Clade 3 included 31 specimens from Northwest Florida, Florida Keys, Campeche Bank, Western Caribbean, and Eastern Caribbean (Fig. 1) at 1.5–135 m depths (Fig. S5). This clade shows high levels of differentiation, with almost all specimens having distinct COI sequences, separated by multiple substitutions up to ∼5.3% K2P (Fig. 6).
Figure 6. TCS haplotype network Clade 3 (n = 31).
TCS haplotype network of COI sequences for one Ophiothrix angulata clade (632 pb). The number of specimens is superimposed onto the more abundant haplotypes. Northern Gulf of Mexico (green). Florida Keys (orange). Campeche Bank (yellow). Western Caribbean (blue). Eastern Caribbean (pink).
Geometric morphometrics
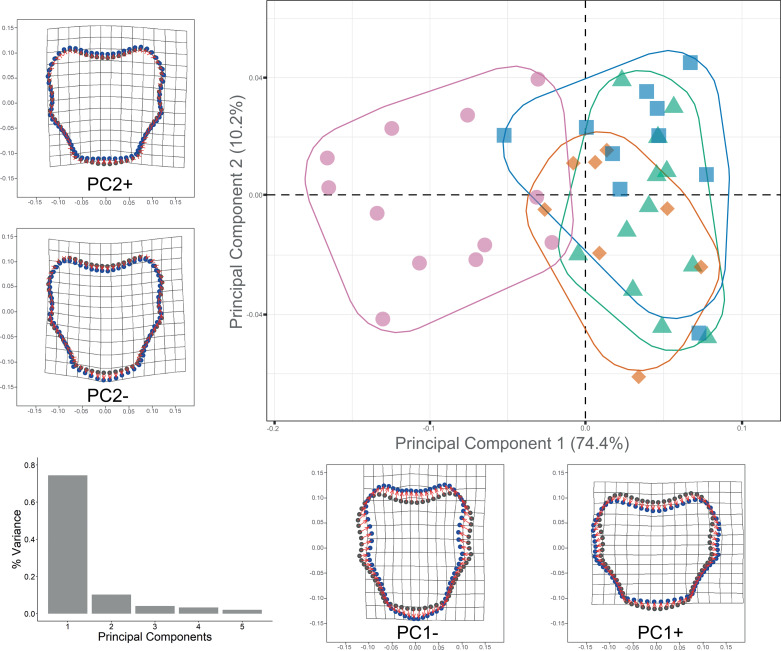
Procrustes ANOVA showed significant differentiation among clades for DAP and VAP (p-value < 0.05). Clades 1A, 1B, and 2A clustered together, while Clade 3 separated in the morpho-space and was significantly different based on pairwise comparisons among clades for both arm plates (Table S3). Corresponding thin-plate spline (TPS) deformation grids for PC1 in DAP analysis illustrate the extremes of variation with the extension/shortening of proximal and lateral edges, while PC2 shows the extension/shortening of the distal edge (Fig. 7). TPS deformation grids in VAP indicate that a considerable portion of the variance in PC1 is attributed to the extension/shortening of both, the proximal and distal edges, as well as and the lateral edges. In contrast, PC2 shows the extension/shortening of proximal and distal edges (Fig. 8). To further illustrate how clades groups in morpho-space, three-dimensional scatterplots of the three principal components (PCs) for both DAP and VAP analyses are provided (Figs. S6 and S7). Delimitations based on significantly different DAP and VAP shapes tested in GM analysis are shown in Figs. 7 and 8, respectively; the DAP and VAP analysis results show a correlation with molecular evidence supporting the three clades’ relationship. The Multivariate Regression showed that 16.7% of the variation for DAP and 9.0% for VAP was attributable to allometric variation in shape; this variation showed no significant interaction for any plate between Centroid size and “clade” factor in Procrustes ANOVA (p-value > 0.05). Measurement errors were low, 1.53% for DAP and 3.05% for VAP.
Figure 7. Dorsal arm plate geometric morphometrics.
Scatterplots showing shape variation along principal component axes. Clade 1A (green). Clade 1B (orange). Clade 2 (blue). Clade 3 (pink). Thin-plate spline deformation grids accompany each PC axis to show the specimens’ shape at their positive and negative ends; the arm plate consensus shape is gray. The bar graph depicts the percentage of variance explained by PC axes.
Figure 8. Ventral arm plate geometric morphometrics.
Scatterplots showing shape variation along principal component axes. Clade 1A (green). Clade 1B (orange). Clade 2 (blue). Clade 3 (pink). Thin-plate spline deformation grids accompany each PC axis to show the specimens’ shape at their positive and negative ends; the arm plate consensus shape is gray. The bar graph depicts the percentage of variance explained by PC axes.
Dorsal arm color pattern analysis
All but four (characters 16, 19, 22, and 25) of the 25 selected color-characters were parsimony informative, but none showed concordant differences with genetic clade assignment (Fig. S8). The consensus tree length was 170 steps, with CI = 0.201 and RI = 0.479. Specimens from all clades clustered together.
Molecular species delimitation
BPP analyses recovered Clades 1, 3, and subclades 2A, and 2B as distinct, with posterior probability values of > 99 for all clades. mPTP species delimitations recovered the same clades as BPP (Fig. 9).
Figure 9. Species delimitation for Ophiothrix angulata clades based on molecular, morphological, and integrative approaches.
Clade 1A (UF7632), Clade 1B (UF11608), Clade 2A (UF10248), and Clade 3 (UF10250). COI phylogeny on the left, representative specimen based on DAP and VAP consensus shape of major clades in the middle, species delineations on the right. Delineated species are represented by separate colors. iBPP results are based on (A) COI and arm color information (Seq+COL) and (B) COI and geometric morphometric data (Seq+GM). ND, no data. Photo credit: Invertebrate Zoology Collection, Florida Museum of Natural History, University of Florida.
Integrative species delimitation.
iBPP analyses recovered Clades 1A, 1B, 2, and 3 as distinct for both iBPPSeq+GM and iBPPSeq, with high support in all runs. iBPPGM separated Clade 3 with high support, but low support was found between clades 1A, 1B, and 2. iBPPSeq+COL and iBPPCOL did not show congruence in runs, so all clade specimens clustered together (Fig. S9).
Population diversity
Haplotype diversity (h) and nucleotide diversity (π) ranged from 0.743 to 1.000 and from 0.007 to 0.048, respectively, among the clades (Table 2). TCS network recovered the same three clades as the phylogenetic analysis of mtDNA. Nucleotide (π) and haplotype (h) diversity values in the TCS network indicated that Clade 3 was more genetically diverse, followed by Clade 1, while Clade 2 exhibited lower nucleotide and haplotype diversity (Table 2).
Table 2. Summary statistics and demographic analyses for the largest clades of O. angulata;
N = number individuals, Number of haplotypes, h = haplotype diversity, π = nucleotide diversity ± standard deviation, SSD = Sum of squared differences in mismatch analysis, Mismatch distribution raggedness index (r), results of Fu’s Fs. Clade 1C was not considered because its n = 3.
| Clades | N | Number of haplotypes | h | π | SSD | Raggedness r | Fu’s Fs | |||
|---|---|---|---|---|---|---|---|---|---|---|
| SSD | p-value | r | p-value | Fs | p-value | |||||
| Clade 1 | 76 | 74 | 0.999 ± 0.003 | 0.032 ± 0.001 | 0.023 | 0.129 | 0.005 | 0.320 | −72.71 | 0.000 |
| Clade 1A | 44 | 43 | 0.998 ± 0.005 | 0.010 ± 0.001 | 0.004 | 0.143 | 0.018 | 0.156 | −34.17 | 0.000 |
| Clade 1B | 29 | 29 | 1.000 ± 0.009 | 0.026 ± 0.002 | 0.013 | 0.187 | 0.007 | 0.889 | −18.64 | 0.000 |
| Clade 2 | 51 | 23 | 0.743 ± 0.068 | 0.007 ± 0.002 | 0.006 | 0.810 | 0.015 | 0.972 | −11.73 | 0.000 |
| Clade 3 | 31 | 30 | 0.998 ± 0.009 | 0.048 ± 0.003 | 0.004 | 0.914 | 0.009 | 0.574 | −08.73 | 0.007 |
Demographic history
Results of Fu’s Fs tests, Raggedness index, and SSD analysis are provided in Table 2. Fu’s neutrality test gave significant negative values for all clades, and subclades 1A and 1B, suggesting past demographic expansions. The Raggedness index and SSD were low and non-significant for all clades and subclades 1A and 1B, respectively, suggesting an unimodal distribution of mismatches as expected for a demographic expansion. The distribution of mismatches for Clades 1 and 3 was bimodal (Fig. S10), which may indicate demographic balance. However, when clades 1A and 1B were analyzed separately, mismatches showed a unimodal distribution for 1A while continued bimodal for 1B (Fig. S11). The mismatch distribution of Clade 2 was unimodal (Fig. S10).
Discussion
Species delimitation in Ophiothrix angulata
Analysis of COI sequence data revealed three deeply-divergent clades within Ophiothrix angulata in the tropical Western Atlantic, one of which (Clade 3) was also divergent in ITS2 and geometric morphometrics. Species delineation algorithms recovered the same three clades using genetic and combined genetic and morphometric data. The deep level of genetic differentiation with COI among these clades is also consistent with the three lineages representing separate species (Table 1).
The consistency between morphological and genetic data on differentiating Clade 3, together with its co-occurrence with clades 1 and 2, demonstrates the lack of gene flow between Clade 3 and the others, and clearly establishes that it is a separate biological species; thus, it can be considered a confirmed candidate species (CCS) (Padial et al., 2010). All three clades co-occur in the Florida Keys, where they were collected on the same day, site, depth, and habitat (UF10247-1A, UF10248-2A, and UF10250-3 in Table S1), which further suggests reproductive isolation for Clade 3.
The status of clades 1 and 2 remain open, as they were separated only by COI sequences, and thus could be species or deep conspecific lineages (DCL) (Padial et al., 2010). While the distribution of these two clades overlaps and they are sympatric at several localities surveyed, Clade 1 is mostly known from around central and North Florida, while Clade 2 was encountered in South Florida, Southern Gulf of Mexico, and the Caribbean. This distribution largely reflects the differentiation of Gatunian and Callosahatchian faunas that have been established since the Miocene (Vermeij, 2005) and suggest allopatric differentiation along this boundary. Additional studies are needed to assess their status.
Clustering sequences into three subclades in Clade 1 and two subclades in Clade 2 is more challenging to interpret. In Clade 2, the two subclades are allopatric, with one represented by two specimens from the Lesser Antilles, the other widespread, suggesting geographic differentiation.
Geometric morphometrics and Dorsal arm color pattern
The shape of both the dorsal and ventral arm plates proved to be a useful indicator for distinguishing Clade 3 from Clades 1-2. Geometric morphometrics has been employed in echinoderm studies to investigate different approaches to understanding the biology and classification of the different orders and families. For instance, Martínez-Melo, De Luna & Buitrón-Sánchez (2017) used GM methods to differentiate between genera in the Cassidulidae family based on the cryptic morphology of plate shapes in Echinoidea. De los Palos-Peña et al. (2021), combined scanning electron microscopy and ontogenetic studies of the odontophore in Luidia superba to understand patterns of size and shape variation. Similarly, Swisher (2021) studied ontogeny in fossil clypeasteroids and confirmed size and shape changes in the oral/aboral plates using GM methods. However, our study incorporates the concept of allometry, which considers the effect of size on shape variation due to ontogeny and other ecological factors influencing morphology (Benítez et al., 2013; Klingenberg, 2022).
Closely related species frequently differ in color pattern and color differences are often among the first visible morphological changes that appear among differentiating species (Benavides-Serrato & O’Hara, 2008; Hoareau et al., 2013). Ophiothrix angulata displays high polymorphism in color patterns (Figs. S1, S2, and S3), and color differences have been suggested to potentially reflect cryptic species differentiation in this species (Clark, 1918; Tommasi, 1970). The absence of correlation between color pattern and genetic clade in our study suggests that color variation is an intra-specific trait. This finding is consistent with previous studies on the Ophiothrix fragilis complex, a widely distributed species in the Northeastern Atlantic Ocean. Previous attempts to link genetic lineages (Baric & Sturmbauer, 1999; Muths et al., 2009; Taboada & Pérez-Portela, 2016, for lineages 1 and 2) with some of the color variants identified by Koehler (1921) were unsuccessful.
COI divergence in ophiuroids
COI K2P distances among the three clades (17.0–27.9%) were higher than the mean inter-specific divergence among most ophiuroid species. In a DNA barcoding study of 503 specimens of 191 ophiuroid species, Ward, Holmes & O’Hara (2008) found intra-specific variation to range 0–3% (mean = 0.62%), whereas inter-specific divergence within genera averaged 15%. High levels of genetic divergence encountered in other ophiuroid species have generally led to the recognition of multiple cryptic species. Examples include the Ophiothrix fragilis complex: K2P distance = 18.6% (Muths et al., 2009), 15–17% (Pérez-Portela, Almada & Turon, 2013), 19–22% (Taboada & Pérez-Portela, 2016); Ophioderma longicaudum complex: K2P distance = 2.2–10.2% (Boissin, Stöhr & Chenuil, 2011), 0.8–10.7% (Weber, Stöhr & Chenuil, 2019); Ophiomyxa vivipara, Ophiacantha vivipara, Ophiura ooplax, Ophiactis abyssicola and Ophiothrix aristulata complexes: K2P distance = 2.9–3.7%, 14.1–16.7%, 22%, 6.7%, and 22.9%, respectively (O’Hara et al., 2014a); and Ophiacantha wolfarntzi complex: K2P distance = 5.4–25.7% (Martín-Ledo, Sands & López-González, 2013). The differentiation among Clades 1, 2, and 3 in COI is thus in line with species-level differences in other ophiuroids.
Haplotype diversity and demographic history
Haplotype networks display contrasting topologies, with Clades 1 and 3 showing higher intra-specific diversity than Clade 2. Clade 1 shows a great diversity of haplotypes in the Northern Gulf of Mexico off Florida, where all three subclades were present. In contrast, specimens from Eastern Florida, the Florida Keys, and the Southern Gulf of Mexico each fell into single and different subclades. This pattern is suggestive of allopatric differentiation along the periphery of the Clade’s range, but few samples were available from these areas making interpretation challenging. Historical demography results show evidence of recent expansion (Fu’s, r, SSD, and mismatches) for subclade 1A.
Clade 2 showed a star-like network suggesting recent population expansion (Allcock & Strugnell, 2012). Most haplotypes were within 3 m-s of the common one, except for the two specimens from Guadeloupe that were 30 m-s distant. These results suggest that continental populations along North and Central America are isolated from the insular population sampled in the Lesser Antilles, and that the former, at least, underwent a recent expansion, potentially following the Last Glacial Maximum.
Clade 3 showed the highest haplotype diversity and did not display signs of recent expansion in all analyses but showed a high diversity of haplotypes. It is noteworthy that this is the only clade that was represented among deeper water samples, suggesting that it may have the most extensive depth range, and thus potentially greater physiological tolerance. The high gene diversity has resulted in a larger population size than the other clades, suggesting success in exploitation and colonization of habitats. Some haplotypes in this clade were shared between localities separated by more than 500 km.
Conclusions
This study provides a broad evaluation of the systematics of one of the most common ophiuroids in the Tropical Western Atlantic, Ophiothrix angulata. COI sequence data revealed three deeply divergent genetic lineages. The high color variability exhibited by this group did not correlate with lineages suggesting that it represents intra-specific polymorphism. Clade 3 was separated by mtDNA, nrDNA, molecular species delimitation, the shape of dorsal and ventral arm plates, and the integrative analysis with mtDNA and geometric morphometric data. Therefore, we consider it as a confirmed candidate species. Results demonstrate that a thorough arm morphology analysis can help differentiate clades within this species complex. Molecular analyses and in situ records show that all three clades co-occur in some areas. For Clades 1A and 2, Fu’s, r, SSD, and mismatches showed evidence of recent expansion. Additional geographic sampling combined with physiological, reproductive, and ecological data incorporating phylogeographic analysis may further resolve this species complex.
Supplemental Information
In vivo, dorsal arm color patterns of 25 characters were used in the parsimony analysis. Characters (Appendix S1): 1, 2, 3, 4, 5, 7, 12, 15, 17, 20, and 23. Outgroup characters are available in Fig. 6. (A) UF7631. (B) UF13163. (C) UF10250. (D) UF10825. Photo credit: Invertebrate Zoology Collection, Florida Museum of Natural History, University of Florida.
In vivo, dorsal arm color patterns of 25 characters were used in the parsimony analysis. Characters (Appendix S1): 10, 11, 14, and 18. Outgroup characters are available in Fig. 6. (A) COREPY25a. (B) UF10823. (C) UF9013. (D) COREPY207. Photo credit: Invertebrate Zoology Collection, Florida Museum of Natural History, University of Florida, and Y. Quetzalli Hernández-Díaz.
In vivo, dorsal arm color patterns of 25 characters were used in the parsimony analysis. Characters (Appendix S1): 6, 8, 9, 13, 21, and 24. Outgroup characters are available in Fig. 6. (A) UF11605. (B) UF11953. (C) UF10248. (D) UF13948. Photo credit: Invertebrate Zoology Collection, Florida Museum of Natural History, University of Florida.
In vivo, dorsal arm color patterns of 25 characters were used in the parsimony analysis. Ophiactis savignyi ICML-UNAM 18084 used as outgroup in the maximum parsimony cladogram. Characters (Appendix S1): 16, 19, 22, and 25. Photo credit: Y. Quetzalli Hernández-Díaz.
Sample collection depths (in meters). Clade 1 (green), Clade 2 (blue), and Clade 3 (pink).
Three-dimensional scatter plot of the first three PCs from dorsal arm plates.
Three-dimensional scatter plot of the first three PCs from ventral arm plates.
Maximum parsimony cladogram based on morphological coloration matrix, followed by bootstrap values from 1,000 replicates. Clade 1A (green), Clade 1B (orange), Clade 2A (blue), and Clade 3 (pink). IC = 0.201, IR = 0.479. Length 170 steps.
Individual units of the 5-unit box plots correspond to a unique combination or type of data analyzed. Blue indicates that the node was highly supported (>95 posterior probability). Orange indicates that the node had a posterior probability of 70 or below. (A) Seq + GM = mtDNA and geometric morphometric data. (B) Seq + COL = mtDNA and arm coloration data. (C) Seq only = mtDNA. (D) GM only = geometric morphometric data. (E) COL only = arm coloration data.
The distribution of mismatches depicting each clade’s demographic history using COI gene sequence. Red lines show the distribution expected under the sudden expansion model. (A) Clade 1. (B) Clade 2. (C) Clade 3.
Mismatch distributions depicting the demographic history for subclades (A) 1A and (B) 1B using COI gene sequence. Red lines show the distribution expected under the sudden-expansion model.
Molecular voucher information and associated GenBank accession numbers for all used specimens. *Depths obtained with relief information in google earth.
Genetic morphometric analysis voucher information.
P-values associated with pairwise Procrustes distances between the mean shapes of dorsal arm plate (up) and ventral arm plate (below) of Ophiothrix angulata from the subclades 1A, 1B, 2A, and Clade 3. Significance levels: *P < 0.05, **P < 0.005.
List of characters of dorsal arm coloration.
Character matrix of the 47 individuals and 25 characters used in the Parsimony analysis. Out = outgroup.
GenBank: MT338285– MT3388398. Data in FASTA format can be opened in the program MEGA.
GenBank: ON245084– ON245096. Data in FASTA format can be opened in the program MEGA.
GenBank: OQ225473– OQ225482. Data in FASTA format can be opened in the program MEGA.
Acknowledgments
We thank David Pawson, Christopher Pomory, Gordon Hendler, Juan José Alvarado, and Nuno Simões for their contribution with samples for sequencing. We thank Tania Pineda, Daniel Janies, and Antar Pérez for their help in sequencing extra material; Tania Pineda, Carolina Martín, Andrea Caballero, and Tayra Parada for images of collection labels; Berenit Mendoza (Laboratorio de Microscopía Electrónica, IB-UNAM) for their technical support during the acquisition of SEM images, Susana Guzmán (Laboratorio de Microscopía y Fotografía de la Biodiversidad II, IB-UNAM) and Miguel Pérez (Laboratorio de Cómputo de Alto Rendimiento, FC-UNAM) for their technical support; Antar Pérez (PopArt, BPP), Sandra Ospina (geomorph), Jesús Díaz (TNT), Maite Mascaró and Dorottya Angyal for their constructive data analysis advice. We thank to the museum curators and collection managers for facilitating access to their collections Amanda Bemis, John Slapcinsky, Adam Baldinger, Penny Benson, Chad Walter, Alicia Durán, Pedro Homa, Raúl Castillo, and Leonardo Chacón.
Funding Statement
This work was supported by CONACYT scholarship CVU 271645 through the Posgrado de Ciencias del Mar y Limnología, UNAM, and the Ernst Mayr Grant of the Museum of Comparative Zoology of Harvard University. Sequencing costs were supported by NSF DEB 0529724. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare that there are no competing interests.
Author Contributions
Yoalli Quetzalli Hernández-Díaz conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft.
Francisco Solis conceived and designed the experiments, authored or reviewed drafts of the article, and approved the final draft.
Rosa G. Beltrán-López analyzed the data, authored or reviewed drafts of the article, and approved the final draft.
Hugo A. Benítez analyzed the data, authored or reviewed drafts of the article, and approved the final draft.
Píndaro Díaz-Jaimes analyzed the data, authored or reviewed drafts of the article, and approved the final draft.
Gustav Paulay analyzed the data, authored or reviewed drafts of the article, and approved the final draft.
Field Study Permissions
The following information was supplied relating to field study approvals (i.e., approving body and any reference numbers):
Field sample collection where approved by Secretaria de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación (SAGARPA).
DNA Deposition
Data Deposition
The following information was supplied regarding data availability:
The raw data employed for Parsimony analysis, for depths and frequency and for geometric morphometrics analysis are in the Supplemental Files.
References
- Adams & Otárola-Castillo (2013).Adams DC, Otárola-Castillo E. Geomorph: an R package for the collection and analysis of geometric morphometric shape data. Methods in Ecology and Evolution. 2013;4:393–399. doi: 10.1111/2041-210X.12035. [DOI] [Google Scholar]
- Allcock & Strugnell (2012).Allcock AL, Strugnell JM. Southern Ocean diversity: new paradigms from molecular ecology. Trends in Ecology and Evolution. 2012;27:520–528. doi: 10.1016/j.tree.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Alvarado & Solís-Marín (2013).Alvarado JJ, Solís-Marín FA, editors. Echinoderms research and diversity in Latin America. Springer; Berlin: 2013. [Google Scholar]
- Alvarado, Solís-Marín & Ahearn (2008).Alvarado JJ, Solís-Marín FA, Ahearn C. Equinodermos (Echinodermata) del Caribe Centroamericano. Revista de Biología Tropical. 2008;56:37–55. [PubMed] [Google Scholar]
- Arlyza et al. (2013).Arlyza IS, Shen KN, Solihin DD, Soedharma D, Berrebi P, Borsa P. Species boundaries in the Himantura uarnak species complex (Myliobatiformes: Dasyatidae) Molecular Phylogenetics and Evolution. 2013;66:429–435. doi: 10.1016/j.ympev.2012.09.023. [DOI] [PubMed] [Google Scholar]
- Aronson (1988).Aronson RB. Palatability of five Caribbean ophiuroids. Bulletin of Marine Science. 1988;43:93–97. [Google Scholar]
- Bailey & Byrnes (1990).Bailey RC, Byrnes J. A new, old method for assessing measurement error in both univariate and multivariate morphometric studies. Systematic Zoology. 1990;39:124–130. doi: 10.2307/2992450. [DOI] [Google Scholar]
- Baric & Sturmbauer (1999).Baric S, Sturmbauer C. Ecological parallelism and cryptic species in the genus Ophiothrix derived from mitochondrial DNA sequences. Molecular Phylogenetics and Evolution. 1999;11:157–162. doi: 10.1006/mpev.1998.0551. [DOI] [PubMed] [Google Scholar]
- Benavides-Serrato & O’Hara (2008).Benavides-Serrato M, O’Hara TD. A new species in the Ophiocoma erinaceus complex from the South-west Pacific Ocean (Echinodermata: Ophiuroidea: Ophiocomidae) Memoirs of Museum Victoria. 2008;65:51–56. doi: 10.24199/j.mmv.2008.65.4. [DOI] [Google Scholar]
- Benítez et al. (2013).Benítez HA, Avaria-Llautureo J, Canales-Aguirre CB, Jerez V, Parra LE, Hernández CE. Evolution of sexual size dimorphism and its relationship with sex ratio in carabid beetles of Genus Ceroglossus Solier. Current Zoology. 2013;59:769–777. doi: 10.1093/czoolo/59.6.769. [DOI] [Google Scholar]
- Bickford et al. (2007).Bickford D, Lohman DJ, Sodhi NS, Ng PKL, Meier R, Winker K, Ingram KK, Das I. Cryptic species as a window on diversity and conservation. Trends in Ecology & Evolution. 2007;22:148–155. doi: 10.1016/j.tree.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Boissin, Féral & Chenuil (2008).Boissin E, Féral JP, Chenuil A. Defining reproductively isolated units in a cryptic and syntopic species complex using mitochondrial and nuclear markers: the brooding brittle star, Amphipholis squamata (Ophiuroidea) Molecular Ecology. 2008;17:1732–1744. doi: 10.1111/j.1365-294X.2007.03652.x. [DOI] [PubMed] [Google Scholar]
- Boissin et al. (2016).Boissin E, Hoareau TB, Paulay G, Bruggemann JH. Shallow-water reef ophiuroids (Echinodermata: Ophiuroidea) of Réunion (Mascarene Islands), with biogeographic considerations. Zootaxa. 2016;4098:273–297. doi: 10.11646/zootaxa.4098.2.4. [DOI] [PubMed] [Google Scholar]
- Boissin, Stöhr & Chenuil (2011).Boissin E, Stöhr S, Chenuil A. Did vicariance and adaptation drive cryptic speciation and evolution of brooding in Ophioderma longicauda (Echinodermata: Ophiuroidea), a common Atlanto-Mediterranean ophiuroid? Molecular Ecology. 2011;20:4737–4755. doi: 10.1111/j.1365-294X.2011.05309.x. [DOI] [PubMed] [Google Scholar]
- Borrero-Pérez et al. (2008).Borrero-Pérez GH, Benavides-Serrato M, Solano Ó, Navas SGR. Brittle-stars (Echinodermata: Ophiuroidea) from the continental shelf and upper slope of the Colombian Caribbean. Revista de Biologia Tropical. 2008;56:169–204. [Google Scholar]
- Clark (1967).Clark AM. Notes on the family ophiotrichidae (Ophiuroidea) Journal of Natural History Series. 1967;13(9):637–655. doi: 10.1080/00222936608651676. [DOI] [Google Scholar]
- Clark (1901).Clark HL. The Echinoderms of Porto Rico. US Fish Commission Bulletin for 1900. 1901;2:231–263. [Google Scholar]
- Clark (1918).Clark HL. Brittle-stars, new and old. Bulletin of the Museum of Comparative of Zoology At Harvard College. 1918;62:265–338. [Google Scholar]
- Clark (1933).Clark HL. Scientific survey of Porto Rico and the Virgin Islands. New York Academy of Sciences; New York: 1933. [Google Scholar]
- Clark (1946).Clark HL. The echinoderm fauna of Australia. Its composition and its origin. Carnegie Institution of Washington Publication; Washington, D.C.: 1946. Family Ophiotrichidae; pp. 213–228. [Google Scholar]
- Clement et al. (2002).Clement M, Snell Q, Walker P, Posada D, Crandall K. TCS: estimating gene genealogies. Parallel and distributed processing symposium, international proceedings, vol. 184.2002. [Google Scholar]
- Darriba et al. (2012).Darriba D, Taboada GL, Doallo R, Posada D. JModelTest 2: more models, new heuristics and parallel computing. Nature Methods. 2012;9:772. doi: 10.1038/nmeth.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De los Palos-Peña et al. (2021).De los Palos-Peña M, Solís-Marín FA, Laguarda-Figueras A, Durán-González A. Ontogenetic variation of the odontophore of Luidia superba (Asteroidea: Paxillosida) and its taxonomic implications. Revista De Biología Tropical. 2021;69:89–100. doi: 10.15517/rbt.v69iSuppl.1.46330. [DOI] [Google Scholar]
- Del Valle García et al. (2008).Del Valle García R, Abreu Pérez M, Rodríguez R, Solís-Marín FA, Laguarda-Figueras A, Durán-González A. Equinodermos (Echinodermata) del occidente del Archipiélago Sabana-Camagüey, Cuba. Revista De Biología Tropical. 2008;56:19–35. [Google Scholar]
- Devaney (1974).Devaney DM. Shallow-water echinoderms from British Honduras, with a description of a new species of Ophiocoma (Ophiuroidea) Bulletin of Marine Science. 1974;22:122–164. [Google Scholar]
- Dos Santos Alitto et al. (2019).Dos Santos Alitto RA, Zacagnini Amaral AC, Dias de Oliveira L, Serrano H, Seger KR, Borges Guilherme PD, Di Domenico M, Christensen AB, Bolsoni Lourenço L, Tavares M, Borges M. Atlantic west Ophiothrix spp. In the scope of integrative taxonomy: Confirming the existence of Ophiothrix trindadensis Tommasi, 1970. PLOS ONE. 2019;14:1–28. doi: 10.1371/journal.pone.0210331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryden (2018).Dryden IL. R Foundation for Statistical Computing; Vienna: 2018. [Google Scholar]
- Edgar (2004).Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Excoffier & Lischer (2010).Excoffier L, Lischer HEL. Arlequin suite version 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Molecular Ecology Resources. 2010;10:564–567. doi: 10.1111/j.1755-0998.2010.02847.x. [DOI] [PubMed] [Google Scholar]
- Felsenstein (1985).Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.2307/2408678. [DOI] [PubMed] [Google Scholar]
- Fox, Weisberg & Price (2018).Fox J, Weisberg S, Price B. Car: companion to applied regression. https://cran.r-project.org/web/packages/car/index.html 2018
- Fu (1997).Fu YX. Statistical tests of neutrality of mutations against population growth, hitchhiking and background selection. Genetics Society of America. 1997;147:915–925. doi: 10.1093/genetics/147.2.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBIF (2022).Gbif Ophiothrix angulata (Say, 1825) in GBIF Secretariat (2022) 2022 GBIF Backbone Taxonomy. Checklist dataset. https://doi.org/10.15468/39omei. (accessed via GBIF.org on May 26th, 2022)
- Goloboff & Catalano (2016).Goloboff PA, Catalano SA. TNT version 1.5, including a full implementation of phylogenetic morphometrics. Cladistics. 2016;32:221–238. doi: 10.1111/cla.12160. [DOI] [PubMed] [Google Scholar]
- Harpending (1994).Harpending HC. Signature of ancient population growth in a low-resolution mitochondrial DNA mismatch distribution. Human Biology. 1994;66:591–600. [PubMed] [Google Scholar]
- Hendler (2005).Hendler G. Two new brittle star species of the genus Ophiothrix (Echinodermata: Ophiuroidea: Ophiotrichidae) from coral reefs in the Southern Caribbean Sea, with notes on their biology. Caribbean Journal of Science. 2005;41:583–599. [Google Scholar]
- Hendler et al. (1999).Hendler G, Baldwin CC, Smith DG, Thacker CE. Planktonic dispersal of juvenile brittle stars (Echinodermata: Ophiuroidea) on a Caribbean reef. Bulletin of Marine Science. 1999;65:283–288. [Google Scholar]
- Hendler & Littman (1986).Hendler G, Littman BS. The ploys of sex: relationships among the mode of reproduction, body size and habitats of coral-reef brittlestars. Coral Reefs. 1986;5:31–42. doi: 10.1007/BF00302169. [DOI] [Google Scholar]
- Hendler et al. (1995).Hendler G, Miller JE, Pawson DL, Kier PM. Echinoderms of Florida and the Caribbean: Sea stars, sea urchins, and allies. Smithsonian Institution Press; Washington, D.C.: 1995. [Google Scholar]
- Herrera-Moreno & Betancourt Fernández (2004).Herrera-Moreno A, Betancourt Fernández L. Especies de equinodermos recientes (Echinodermata: Crinoidea, Asteroidea, Ophiuroidea, Echinoidea y Holothuroidea) conocidas para la Hispaniola. Revista Ciencia Y Sociedad. 2004;29:506–533. doi: 10.22206/cys.2004.v29i3.pp506-533. [DOI] [Google Scholar]
- Hoareau & Boissin (2010).Hoareau TB, Boissin E. Design of phylum-specific hybrid primers for DNA barcoding: addressing the need for efficient COI amplification in the Echinodermata. Molecular Ecology Resources. 2010;10:960–967. doi: 10.1111/j.1755-0998.2010.02848.x. [DOI] [PubMed] [Google Scholar]
- Hoareau et al. (2013).Hoareau TB, Boissin E, Paulay G, Bruggemann JH. The Southwestern Indian Ocean as a potential marine evolutionary hotspot: perspectives from comparative phylogeography of reef brittle-stars. Journal of Biogeography. 2013;40:2167–2179. doi: 10.1111/jbi.12155. [DOI] [Google Scholar]
- Hoggett (1991).Hoggett AK. The genus Macrophiothrix (Ophiuroidea: Ophiotrichidae) in Australian waters. Invertebrate Taxonomy. 1991;4:1077–1146. doi: 10.1071/IT9901077. [DOI] [Google Scholar]
- Kapli et al. (2017).Kapli P, Lutteropp S, Zhang J, Kobert K, Pavlidis P, Stamatakis A, Flouri T. Multi-rate Poisson tree processes for single-locus species delimitation under maximum likelihood and Markov chain Monte Carlo. Bioinformatics. 2017;33:1630–1638. doi: 10.1093/bioinformatics/btx025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearse et al. (2012).Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Meintjes P, Drummond A. Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28:1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura (1980).Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. Journal of Molecular Evolution. 1980;16:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- Kissling & Taylor (1977).Kissling DL, Taylor GT. Habitat factors for reef dwelling ophiuroids in the Florida Keys. Symposium RS of M and A ed. Third international coral reef symposium; Miami, Florida. 1977. pp. 225–231. [Google Scholar]
- Klingenberg (2016).Klingenberg CP. Size, shape, and form: concepts of allometry in geometric morphometrics. Development Genes and Evolution. 2016;226:113–137. doi: 10.1007/s00427-016-0539-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingenberg (2022).Klingenberg CP. Methods for studying allometry in geometric morphometrics: a comparison of performance. Evolutionary Ecology. 2022;36:439–470. doi: 10.1007/s10682-022-10170-z. [DOI] [Google Scholar]
- Koehler (1921).Koehler R. Faune De France. 1. Echinodermes. Paris: Office Central de Faunistique, Fédération Francaise des Sociétés de Sciences Naturelles; 1921. [Google Scholar]
- Laguarda-Figueras et al. (2009).Laguarda-Figueras A, Hernández-Herrejón LA, Solís-Marín FA, Durán-González A. Ofiuroideos del Caribe mexicano y Golfo de México. ICML-UNAM; Cancún: 2009. [Google Scholar]
- Leigh & Bryant (2015).Leigh JW, Bryant D. POPART: full-feature software for haplotype network construction. Methods in Ecology and Evolution. 2015;6:1110–1116. doi: 10.1111/2041-210X.12410. [DOI] [Google Scholar]
- Lewis & Bray (1983).Lewis J, Bray R. Community structure of ophiuroids (Echinodermata) from three different habitats on a coral reef in Barbados, West Indies. Marine Biology. 1983;73:171–176. doi: 10.1007/BF00406885. [DOI] [Google Scholar]
- Ljungman (1867).Ljungman A. Ophiuroidea viventia huc usque cognita enumerat. Öfversigt Af Kgl. Vetenskaps-Akademiens FörhandLingar. 1867;23:303–336. [Google Scholar]
- Luo et al. (2018).Luo A, Ling C, Ho SYW, Zhu CD. Comparison of methods for molecular species delimitation across a range of speciation scenarios. Systematic Biology. 2018;67:830–846. doi: 10.1093/sysbio/syy011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lütken (1856).Lütken CF. Bidrag til Kundskab om Slangestjernerne. II. Oversigt over de vestindiske Ophiurer. Videnskabelige Meddelelser Fra Dansk Naturhistorisk Förening I KjøBenhavn. 1856;7:1–19. [Google Scholar]
- Lyman (1860).Lyman T. Descriptions of new Ophiuridae, belonging to the Smithsonian Institution and to the Museum of Comparative Zoology at Cambridge. Proceedings of the Boston Society of Natural History. 1860;1859–1861:193–204. [Google Scholar]
- Lyman (1865).Lyman T. Ophiuridae and Astrophytidae. University Press; Cambridge, Welch: 1865. Ophiothrix angulata; pp. 162–164. Bigelow & Co. [Google Scholar]
- Maddison & Maddison (2018).Maddison WP, Maddison DR. Mesquite: a modular system for evolutionary analysis. Version 3.51http://www.mesquiteproject.org/ 2018
- Martín-Ledo, Sands & López-González (2013).Martín-Ledo R, Sands CJ, López-González PJ. A new brooding species of brittle star (Echinodermata: Ophiuroidea) from Antarctic waters. Polar Biology. 2013;36:115–126. doi: 10.1007/s00300-012-1242-z. [DOI] [Google Scholar]
- Martínez-Melo, De Luna & Buitrón-Sánchez (2017).Martínez-Melo A, De Luna E, Buitrón-Sánchez BE. Morfometría de los equinodeos de la Familia Cassidulidae (Echinoidea: Cassiduloida) Revista de Biología Tropical. 2017;65:S233–S243. doi: 10.15517/rbt.v65i1-1.31691. [DOI] [Google Scholar]
- Masonick & Weirauch (2019).Masonick P, Weirauch C. Integrative species delimitation in Nearctic ambush bugs (Heteroptera: Reduviidae: Phymatinae): insights from molecules, geometric morphometrics and ecological associations. Systematic Entomology. 2019;45:205–223. doi: 10.1111/syen.12388. [DOI] [Google Scholar]
- Michonneau & Paulay (2014).Michonneau F, Paulay G. Revision of the genus Phyrella (Holothuroidea: Dendrochirotida) with the description of a new species from Guam. Zootaxa. 2014;3760:101–140. doi: 10.11646/zootaxa.3760.2.1. [DOI] [PubMed] [Google Scholar]
- Miller, Pfeiffer & Schwartz (2010).Miller MA, Pfeiffer W, Schwartz T. Creating the CIPRES Science gateway for inference of large phylogenetic trees. Proceedings of the gateway computing environments workshop (GCE); New Orleans, LA. 2010. pp. 1–8. [DOI] [Google Scholar]
- Moritz et al. (2018).Moritz CC, Pratt RC, Bank S, Bourke G, Bragg JG, Doughty P, Keogh JS, Laver RJ, Potter S, Teasdale LC, Tedeschi LG, Oliver PM. Cryptic lineage diversity, body size divergence, and sympatry in a species complex of Australian lizards (Gehyra) Evolution. 2018;72:54–66. doi: 10.1111/evo.13380. [DOI] [PubMed] [Google Scholar]
- Müller & Troschel (1842).Müller J, Troschel FH. System der Asteriden.1. Asteriae. 2. Ophiuridae. Braunschweig; Vieweg: 1842. p. 115. [Google Scholar]
- Müller et al. (2010).Müller K, Müller J, Neinhuis C, Quandt D. PhyDE—Phylogenetic Data Editor, version 0.9971. 2010. http://www.phyde.de http://www.phyde.de
- Muths et al. (2009).Muths D, Jollivet D, Gentil F, Davoult D. Large-scale genetic patchiness among NE Atlantic populations of the brittle star Ophiothrix fragilis. Aquatic Biology. 2009;5:117–132. doi: 10.3354/ab00138. [DOI] [Google Scholar]
- Naughton et al. (2014).Naughton KM, O’Hara TD, Appleton B, Cisternas PA. Antitropical distributions and species delimitation in a group of ophiocomid brittle stars (Echinodermata: Ophiuroidea: Ophiocomidae) Molecular Phylogenetics and Evolution. 2014;78:232–244. doi: 10.1016/j.ympev.2014.05.020. [DOI] [PubMed] [Google Scholar]
- Newton et al. (2020).Newton LG, Starrett J, Hendrixson BE, Derkarabetian S, Bond JE. Integrative species delimitation reveals cryptic diversity in the southern Appalachian Antrodiaetus unicolor (Araneae: Antrodiaetidae) species complex. Molecular Ecology. 2020;2:2269–2287. doi: 10.1111/mec.15483. [DOI] [PubMed] [Google Scholar]
- Noriega & Fuentes-Carrero (2014).Noriega N, Fuentes-Carrero Y. Contribución al conocimiento de la diversidad de los equinodermos del Parque Nacional Archipiélago De Los Roques, Venezuela. Acta Biologica Venezuelica. 2014;34:285–292. [Google Scholar]
- O’Hara et al. (2014a).O’Hara TD, England PR, Gunasekera RM, Naughton KM. Limited phylogeographic structure for five bathyal ophiuroids at continental scales. Deep Sea Research Part I: Oceanographic Research Papers. 2014a;84:18–28. doi: 10.1016/j.dsr.2013.09.009. [DOI] [Google Scholar]
- O’Hara et al. (2014b).O’Hara TD, Hugall AF, Thuy B, Moussalli A. Phylogenomic resolution of the Class Ophiuroidea unlocks a global microfossil record. Current Biology. 2014b;24:1874–1879. doi: 10.1016/j.cub.2014.06.060. [DOI] [PubMed] [Google Scholar]
- O’Hara et al. (2017).O’Hara TD, Hugall AF, Thuy B, Stöhr S, Martynov AV. Restructuring higher taxonomy using broad-scale phylogenomics: the living Ophiuroidea. Molecular Phylogenetics and Evolution. 2017;107:415–430. doi: 10.1016/j.ympev.2016.12.006. [DOI] [PubMed] [Google Scholar]
- O’Hara et al. (2018).O’Hara TD, Stöhr S, Hugall AF, Thuy B, Martynov A. Morphological diagnoses of higher taxa in Ophiuroidea (Echinodermata) in support of a new classification. European Journal of Taxonomy. 2018;416:1–35. doi: 10.5852/ejt.2018.416. [DOI] [Google Scholar]
- Padial et al. (2010).Padial JM, Miralles A, De la Riva I, Vences M. The integrative future of taxonomy. Frontiers in Zoology. 2010;7:1–14. doi: 10.1186/1742-9994-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Portela, Almada & Turon (2013).Pérez-Portela R, Almada V, Turon X. Cryptic speciation and genetic structure of widely distributed brittle stars (Ophiuroidea) in Europe. Zoologica Scripta. 2013;42:151–169. doi: 10.1111/j.1463-6409.2012.00573.x. [DOI] [Google Scholar]
- Rambaut (2018).Rambaut A. Institute of Evolutionary Biology, University of Edinburgh; Edinburgh, U.K: 2018. FigTree version 1.4.4. [Google Scholar]
- Rambaut et al. (2018).Rambaut A, Drummond AJ, Xie D, Baele G, Suchard MA. Posterior summarization in Bayesian phylogenetics using tracer 1.7. Systematic Biology. 2018;67:901–904. doi: 10.1093/sysbio/syy032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards, De Biasse & Shivji (2015).Richards VP, De Biasse MB, Shivji MS. Genetic evidence supports larval retention in the Western Caribbean for an invertebrate with high dispersal capability (Ophiothrix suensonii: Echinodermata, Ophiuroidea) Coral Reefs. 2015;34:313–325. doi: 10.1007/s00338-014-1237-z. [DOI] [Google Scholar]
- Rohlf (2015).Rohlf FJ. tpsDIG2, digitize landmarks and outlines, version 2.17. Department of Ecology and Evolution, State University of New York at Stony Brook; New York, NY: 2015. [Google Scholar]
- Ronquist et al. (2012).Ronquist F, Teslenko M, Van Der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. MrBayes version 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozas et al. (2017).Rozas J, Ferrer-Mata A, Sánchez-Del Barrio JC, Guirao-Rico S, Librado P, Ramos-Onsins SE, Sánchez-Gracia A. DnaSP 6: DNA sequence polymorphism analysis of large datasets. Molecular Biology and Evolution. 2017;34:3299–3302. doi: 10.1093/molbev/msx248. [DOI] [PubMed] [Google Scholar]
- Sandino et al. (2017).Sandino OB, Solís-Marín FA, Caballero-Ochoa AA, Conejeros-Vargas CA, Márquez-Borrás F, Ayala-Aguilera AP, Laguarda-Figueras A. Equinodermos De Nicaragua: nuevos registros del Pacífico y Caribe Sur. Revista De Biología Tropical. 2017;65:S288–S298. doi: 10.15517/rbt.v65i1-1.31696. [DOI] [Google Scholar]
- Santana et al. (2017).Santana A, Manso CLC, Almeida ACS, Alves OFS. Redescription and designation of a neotype for Ophiothrix angulata (Say, 1825) (Echinodermata: Ophiuroidea: Ophiotrichidae) Zootaxa. 2017;4344:291–307. doi: 10.11646/zootaxa.4344.2.5. [DOI] [PubMed] [Google Scholar]
- Santana et al. (2020).Santana A, Manso CLC, Almeida ACS, Alves OFS. Taxonomic review of Ophiothrix Müller & Troschel, 1840 (Echinodermata: Ophiuroidea) from Brazil, with the description of four new species. Zootaxa. 2020;4808:51–78. doi: 10.11646/zootaxa.4808.1.3. [DOI] [PubMed] [Google Scholar]
- Say (1825).Say T. On the species of the Linnean genus Aterias, inhabiting the coast of the United States. Journal of the Academy of Natural Sciences of Philadelphia. 1825;5:141–154. [Google Scholar]
- Sobha, Vibija & Fahima (2023).Sobha TR, Vibija CP, Fahima P. Coral reef: a hot spot of marine biodiversity. In: Sukumaran ST, Keerthi TR, editors. Conservation and sustainable utilization of bioresources. Sustainable development and biodiversity. Springer; Singapore: 2023. [DOI] [Google Scholar]
- Solís-Lemus, Knowles & Ané (2015).Solís-Lemus C, Knowles LL, Ané C. Bayesian species delimitation combining multiple genes and traits in a unified framework. Evolution. 2015;69:492–507. doi: 10.1111/evo.12582. [DOI] [PubMed] [Google Scholar]
- Stamatakis (2006).Stamatakis A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22:2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- Stamatakis, Hoover & Rougemont (2008).Stamatakis A, Hoover P, Rougemont J. A rapid bootstrap algorithm for the RAxML web servers. Systematic Biology. 2008;57:758–771. doi: 10.1080/10635150802429642. [DOI] [PubMed] [Google Scholar]
- Stöhr (2005).Stöhr S. Who’s who among baby brittle stars (Echinodermata: Ophiuroidea): Postmetamorphic development of some North Atlantic forms. Zoological Journal of the Linnean Society. 2005;143:543–576. doi: 10.1111/j.1096-3642.2005.00155.x. [DOI] [Google Scholar]
- Stöhr, O’Hara & Thuy (2012).Stöhr S, O’Hara TD, Thuy B. Global diversity of brittle stars (Echinodermata: Ophiuroidea) PLOS ONE. 2012;7:e31940. doi: 10.1371/journal.pone.0031940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stöhr, O’Hara & Thuy (2023).Stöhr S, O’Hara TD, Thuy B. World Ophiuroidea Database. http://www.marinespecies.org/ophiuroidea. [16 April 2023];2023
- Swisher (2021).Swisher RE. Convergent discoidal sand dollars from isolated regions: a geometric morphometric analyses of Dendraster and Arachnoides. Terrestrial Atmospheric and Oceanic Sciences. 2021;32:1117–1130. [Google Scholar]
- Taboada & Pérez-Portela (2016).Taboada S, Pérez-Portela R. Contrasted phylogeographic patterns on mitochondrial DNA of shallow and deep brittle stars across the Atlantic-Mediterranean area. Scientific Reports. 2016;6:32425. doi: 10.1038/srep32425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajima (1989).Tajima F. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics Society of America. 1989;123:585–595. doi: 10.1093/genetics/123.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura, Stecher & Kumar (2021).Tamura K, Stecher G, Kumar S. MEGA11: molecular evolutionary genetics analysis version 11. Molecular Biology and Evolution. 2021;38:3022–3027. doi: 10.1093/molbev/msab120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuy & Stöhr (2011).Thuy B, Stöhr S. Lateral arm plate morphology in brittle stars (Echinodermata: Ophiuroidea): new perspectives for ophiuroid micropalaeontology and classification. Zootaxa. 2011;3013:1–47. doi: 10.11646/zootaxa.3013.1.1. [DOI] [Google Scholar]
- Thuy & Stöhr (2016).Thuy B, Stöhr S. A new morphological phylogeny of the Ophiuroidea (Echinodermata) accords with molecular evidence and renders microfossils accessible for cladistics. PLOS ONE. 2016;11:e0156140. doi: 10.1371/journal.pone.0156140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommasi (1970).Tommasi LR. Os ofiuróides recentes do Brasil e de regiões vizinhas. Contribuições Avulsas do Instituto Oceanográfico Universidade de São Paulo, série Oceanografia Biológica. 1970;20:1–146. [Google Scholar]
- Vermeij (2005).Vermeij GJ. One-way traffic in the western Atlantic: causes and consequences of Miocene to early Pleistocene molluscan invasions in Florida and the Caribbean. Paleobiology. 2005;31:624–642. doi: 10.1666/0094-8373(2005)031[0624:OTITWA]2.0.CO;2. [DOI] [Google Scholar]
- Verrill (1899).Verrill AE. Report on the Ophiuroidea collected by the Bahama Expedition in 1893. Bulletin from the Laboratories of Natural History of the State University of Iowa. 1899;5:1–86. [Google Scholar]
- Viscosi & Cardini (2011).Viscosi V, Cardini A. Leaf morphology, taxonomy and geometric morphometrics: a simplified protocol for beginners. PLOS ONE. 2011;6:e25630. doi: 10.1371/journal.pone.0025630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh, Metzger & Higuchi (1991).Walsh PS, Metzger DA, Higuchi R. Chelex 100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. Biotechniques. 1991;10:506–513. [PubMed] [Google Scholar]
- Ward, Holmes & O’Hara (2008).Ward RD, Holmes BH, O’Hara TD. DNA barcoding discriminates echinoderm species. Molecular Ecology Resources. 2008;8:1202–1211. doi: 10.1111/j.1755-0998.2008.02332.x. [DOI] [PubMed] [Google Scholar]
- Weber, Stöhr & Chenuil (2019).Weber AAT, Stöhr S, Chenuil A. Species delimitation in the presence of strong incomplete lineage sorting and hybridization: lessons from Ophioderma (Ophiuroidea: Echinodermata) Molecular Phylogenetics and Evolution. 2019;131:138–148. doi: 10.1016/j.ympev.2018.11.014. [DOI] [PubMed] [Google Scholar]
- Wickham (2016).Wickham H. Springer-Verlag; New York: 2016. [Google Scholar]
- Yang (2015).Yang Z. The BPP program for species tree estimation and species delimitation. Current Zoology. 2015;61:854–865. doi: 10.1093/czoolo/61.5.854. [DOI] [Google Scholar]
- Yang & Rannala (2010).Yang Z, Rannala B. Bayesian species delimitation using multilocus sequence data. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:9264–9269. doi: 10.1073/pnas.0913022107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yezerinac, Lougheed & Handford (1992).Yezerinac SM, Lougheed SC, Handford P. Measurement error and morphometric studies: statistical power and observer experience. Systematic Biology. 1992;41:471–482. doi: 10.1093/sysbio/41.4.471. [DOI] [Google Scholar]
- Zhang et al. (2013).Zhang J, Kapli P, Pavlidis P, Stamatakis A. A general species delimitation method with applications to phylogenetic placements. Bioinformatics. 2013;29:2869–2876. doi: 10.1093/bioinformatics/btt499. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
In vivo, dorsal arm color patterns of 25 characters were used in the parsimony analysis. Characters (Appendix S1): 1, 2, 3, 4, 5, 7, 12, 15, 17, 20, and 23. Outgroup characters are available in Fig. 6. (A) UF7631. (B) UF13163. (C) UF10250. (D) UF10825. Photo credit: Invertebrate Zoology Collection, Florida Museum of Natural History, University of Florida.
In vivo, dorsal arm color patterns of 25 characters were used in the parsimony analysis. Characters (Appendix S1): 10, 11, 14, and 18. Outgroup characters are available in Fig. 6. (A) COREPY25a. (B) UF10823. (C) UF9013. (D) COREPY207. Photo credit: Invertebrate Zoology Collection, Florida Museum of Natural History, University of Florida, and Y. Quetzalli Hernández-Díaz.
In vivo, dorsal arm color patterns of 25 characters were used in the parsimony analysis. Characters (Appendix S1): 6, 8, 9, 13, 21, and 24. Outgroup characters are available in Fig. 6. (A) UF11605. (B) UF11953. (C) UF10248. (D) UF13948. Photo credit: Invertebrate Zoology Collection, Florida Museum of Natural History, University of Florida.
In vivo, dorsal arm color patterns of 25 characters were used in the parsimony analysis. Ophiactis savignyi ICML-UNAM 18084 used as outgroup in the maximum parsimony cladogram. Characters (Appendix S1): 16, 19, 22, and 25. Photo credit: Y. Quetzalli Hernández-Díaz.
Sample collection depths (in meters). Clade 1 (green), Clade 2 (blue), and Clade 3 (pink).
Three-dimensional scatter plot of the first three PCs from dorsal arm plates.
Three-dimensional scatter plot of the first three PCs from ventral arm plates.
Maximum parsimony cladogram based on morphological coloration matrix, followed by bootstrap values from 1,000 replicates. Clade 1A (green), Clade 1B (orange), Clade 2A (blue), and Clade 3 (pink). IC = 0.201, IR = 0.479. Length 170 steps.
Individual units of the 5-unit box plots correspond to a unique combination or type of data analyzed. Blue indicates that the node was highly supported (>95 posterior probability). Orange indicates that the node had a posterior probability of 70 or below. (A) Seq + GM = mtDNA and geometric morphometric data. (B) Seq + COL = mtDNA and arm coloration data. (C) Seq only = mtDNA. (D) GM only = geometric morphometric data. (E) COL only = arm coloration data.
The distribution of mismatches depicting each clade’s demographic history using COI gene sequence. Red lines show the distribution expected under the sudden expansion model. (A) Clade 1. (B) Clade 2. (C) Clade 3.
Mismatch distributions depicting the demographic history for subclades (A) 1A and (B) 1B using COI gene sequence. Red lines show the distribution expected under the sudden-expansion model.
Molecular voucher information and associated GenBank accession numbers for all used specimens. *Depths obtained with relief information in google earth.
Genetic morphometric analysis voucher information.
P-values associated with pairwise Procrustes distances between the mean shapes of dorsal arm plate (up) and ventral arm plate (below) of Ophiothrix angulata from the subclades 1A, 1B, 2A, and Clade 3. Significance levels: *P < 0.05, **P < 0.005.
List of characters of dorsal arm coloration.
Character matrix of the 47 individuals and 25 characters used in the Parsimony analysis. Out = outgroup.
GenBank: MT338285– MT3388398. Data in FASTA format can be opened in the program MEGA.
GenBank: ON245084– ON245096. Data in FASTA format can be opened in the program MEGA.
GenBank: OQ225473– OQ225482. Data in FASTA format can be opened in the program MEGA.