Abstract
Background:
Cell therapy has been proposed as part of the therapeutic arsenal to assist bone formation and remodeling in the early stages of osteonecrosis of the femoral head. The purpose of this study is to determine the effects of intraosseous inoculation of mesenchymal stem cells on bone formation and remodeling in an established experimental model of osteonecrosis of the femoral head in immature pigs.
Methods:
Thirty-one 4-week-old immature Yorkshire pigs were used. Experimental osteonecrosis of the femoral head was created in the right hip of all included animals (n = 31). The month after surgery, hip and pelvis radiographs were taken to confirm osteonecrosis of the femoral head. Four animals were excluded following surgery. Two groups were established: (A) mesenchymal stem cell-treated group (n = 13) and (B) saline-treated group (n = 14). One month after surgery the mesenchymal stem cell-group received an intraosseous injection of 10 × 106 mesenchymal stem cell (5 cc) and the saline-treated group of 5 cc of physiological saline solution. Osteonecrosis of the femoral head progression was assessed by monthly X-rays (1-, 2-, 3- and 4-months post-surgery). The animals were sacrificed 1 or 3 months following the intraosseous injection. Repair tissue and osteonecrosis of the femoral head were histologically evaluated immediately after sacrifice.
Results:
At time of sacrifice, radiographic images showed evident osteonecrosis of the femoral head with associated severe femoral head deformity in 11 of the 14 animals (78%) in the saline group and in only 2 of the 13 animals (15%) in the mesenchymal stem cell group. Histologically, the mesenchymal stem cell group showed less osteonecrosis of the femoral head and less flattening. In the saline group, there was pronounced femoral head flattening and the damaged epiphyseal trabecular bone was largely replaced with fibrovascular tissue.
Conclusion:
Intraosseous mesenchymal stem cells inoculation improved bone healing and remodeling in our immature pig osteonecrosis of the femoral head model. This work supports further investigation to determine whether mesenchymal stem cells enhance the healing process in immature osteonecrosis of the femoral head.
Keywords: Femoral head, osteonecrosis, pig model, hip, development, mesenchymal stem cells, Legg–Calvé–Perthes disease
Introduction
Intracapsular surgical ligation at the base of the femoral neck is an excellent model for inducing ischaemia and osteonecrosis of the femoral head (ONFH) of the piglet and creating Legg–Calve–Perthes disease (LCPD) like changes. After induction of ischaemic necrosis, osteoclastic resorption of necrotic tissue has been recognized to initiate collapse and deformation of the femoral head (FH). Abnormal proximal femoral growth during the healing process can lead to severe FH deformity and early hip osteoarthritis. The etiology of this disease is still controversial.1–5 Many previous clinical and experimental studies have suggested that ischaemic injury to the immature capital femoral epiphysis plays a major role in LCPD pathogenesis. Osteonecrotic lesions are characterized by increased apoptosis in the trabecular bone of the FH. 6 Moreover, it has been observed that the activity and number of mesenchymal stem cells (MSCs) in adult avascular necrosis are below normal7–9 and osteoblast proliferative capacity is significantly reduced. 10
Studies in human FH7–9,11 and experimental LCPD models 12 report that MSCs are carried by capillaries into necrotic bone areas where they differentiate into osteoblasts and form new bone by apposition on the surface of the existing necrotic trabecular bone. Later, the centre of the necrotic area is reabsorbed by osteoclasts and replaced with newly formed bone. This remodeling process explains the changes observed in immature necrotic FH, including trabecular widening, increased bone mass, and repair tissue density. Nevertheless, newly formed bone is fragile, which means that weight bearing may flatten and/or deform the FH possibly leading to articular cartilage damage.13–15 Thus, unlike adult ONFH, LCPD occurs in immature bone that has a characteristic vascularization, a greater capacity for plastic deformity, a reversible evolution due to a phenomenon of tissue regeneration by penetration of MSCs and a regenerative process following ischaemic necrosis carried out by apposition of new layers of bone from the necrotic bone itself.
MSCs have been shown to improve clinical outcomes, relieve pain, decrease disease progression, lower the hip arthroplasty conversion rate and improve recovery in adult patients with a clinical diagnosis of osteonecrosis.16–19 Radiographically, compared to controls, MSCs inoculation reduced lesion size.6,9 However, the effect of MSCs inoculation in an immature hip with idiopathic ONFH has not been studied yet. Recent studies on the outcomes of intraosseous application of autologous MSC in paediatric or young adult patients with avascular necrosis secondary to corticosteroids or chemotherapy report the need for further prospective studies. 20 The purpose of this study is to (a) induce ONFH in immature pigs using an established ischaemic model and (b) determine whether an intraosseous MSCs injection improves bone remodeling in the context of established ONFH model at 3 months post-inoculation.
Materials and methods
The prospective study design was approved by the Institutional Animal Care and Use Committees (CEA-UCM 122/2012) and the Research Committee of our Institution and the Regional Government (S-BIO-0204-2006, MesenCAM; P2010/BMD-240, CellCAM). All applicable international, national, and/or institutional guidelines for animal care and use were followed. Thirty-one 4-week-old and 5–6 kg weight immature Yorkshire pigs were used. Three animals were excluded from the study due to premature death from causes unrelated to the study and another was sacrificed before the end of the experiment due to a femoral neck fracture. Finally, a total of 27 animals were included. The study was conducted at the Faculty of Veterinary Medicine of the Complutense University of Madrid, between 2015 and 2017.
Experiment design
ONFH was surgically induced as previously described 15 in the right hip of each animal. Animals were then randomly allocated to one of two groups, using computer-generated random numbers: (a) MSC-treated group (n = 13) and (b) saline-treated group (n = 14). One month after surgery the MSC group received an intraosseous injection of 10 × 106 MSC (5 cc) and the SS group of 5 cc of physiological saline solution. Before the injection, all animals underwent an anteroposterior (AP) pelvis radiograph (35 × 45cm CR 30-X, AGFA Healthcare, Barcelona) to confirm ONFH. Injection into the central area of the FH was performed under radiological control with an 8G-biopsy trocar to confirm the adequate application (Figure 1). Afterward, they were sacrificed sequentially: Half of each group at 4 weeks post-injection and the other half at 12 weeks, and the proximal portion of both femurs were harvested.
Figure 1.
Intraosseous injection of the femoral head (a) under radiographic control (b). Through a percutaneous hip approach, with a 1 cm incision next to the greater trochanter, the femoral head is accessed with the trocar for injection.
Induction of ischaemic insult to the capital femoral epiphysis
Anaesthesia induction was performed using diazepam (0.5 mg/kg), ketamine (2 mg/kg), and propofol (0.5 mg/kg). Prophylactic antibiotic was administered. The animal was positioned in lateral decubitus with the operative side up, which was prepped and draped in the usual sterile fashion. A lateral approach was performed, the gluteus and hip external rotator muscles were incised, and a lateral capsulotomy was performed without damaging or excising the labrum. Using a curved clamp, a titanium metal cerclage was placed around the femoral neck without dislocating the FH or transecting the Ligamentum teres. The cerclage was tightened with a cable tensioner (DePuy Synthes®, Johnson & Johnson) set at 20 kg to disrupt the blood supply. Resorbable sutures (Vicryl Plus® 0-0 and Monocryl, Johnson & Johnson Medical, Belgium) were used for closure. The surgical site was protected with aluminium wound spray (Aluspray®). Skin dressings and post-surgical splinting or casting were not used. Immediately after closure, a total of 60 ml of bone marrow was aspirated from the ipsilateral posterosuperior iliac crest of the animals of the MSC group, using an 8G-biopsy trocar. The aspirated marrow was transferred to a sterile container and taken to the laboratory. Following surgical ischaemia induction, they were allowed to bear weight as tolerated.
MSCs preparation
Bone marrow aspirate was centrifuged to isolate mononuclear cells using density gradients (Ficoll-Hypaque Plus®, Amersham Bioscience, Piscataway, NJ, USA). The samples were centrifuged at 2.700 r/min for 30 min. Once the mononuclear cells had been obtained, they were seeded at a concentration of 1.6 × 105 cells/cm2, in DMEM at 10% FBS with an antibiotic. After incubation for 48 h the non-adhering cells were discarded before adding fresh medium. When the cells reached 80%–90% confluence, they were trypsinized and cultured to obtain 4 × 103 cells/cm2. Remaining cells were frozen under viable conditions for use throughout the project. MSCs were transduced with a lentiviral vector coding for green fluorescent protein gene (GFP; culture concentration 34 lentivirus/MSC). 105 cells/well were plated onto a P6-well plate and, the following day, GFP-LV supernatant was added to the cells at an MOI of 10 for 16 h of incubation.
Imaging studies
At 1-month post-surgery, ONFH was confirmed using plain pelvis AP and axial frog views of the affected hip (right side). Radiographs were repeated monthly until sacrifice. All images were taken by the same experienced veterinary radiologist to ensure image reliability. In both groups, the images were used to take the following measurements for bone remodeling and ONFH assessment: (a) FH width: Growth plate length at the FH; (b) central column height: Distance from the centre of the base of the physis to the top of the FH above it; (c) lateral column height: Distance from a point 1/3 lateral to the exterior edge of the physis to the top of the FH above it; (d) epiphyseal quotient (EQ), was used to quantify the degree of FH collapse in both groups compared with the contralateral normal FH (auto-control). EQ was calculated as described by Kong et al. 21 as the height of the osseous capital femoral epiphysis divided by the maximum transverse diameter. A good result is between 0.75 and 1, fair is between 0.5 and 0.75, and poor is less than 0.5.
For greater reliability, all radiographs were evaluated by 2 independent and blinded paediatric orthopedic surgeons, not involved in the surgical procedures. Each investigator performed the radiologic measurements three times, with a minimum 1-week interval between them, and an average of these values was used.
Histology
The proximal portions of both femurs were analyzed macroscopically before sagittal sectioning. Specimens were fixed in formaldehyde (10%) and Bouin's solution for 24 h, and decalcificed (10%EDTA-75%PVP). They were then embedded in paraffin and divided into 4-micron sections. Finally, the sections were stained with Massońs trichrome and haematoxylin–eosin. Immunohistochemical studies were performed on formalin-fixed paraffin-embedded tissue using antigen retrieval with the PT Link System (Dako). Antibody anti-GFP (Invitrogen) was applied at a dilution of 1–200. Staining was performed on a Dako Autostainer (Dako) and was visualized with a DAB chromogen. Figure 2 shows the successful expression of GFP protein by the sample of MSCs that were implanted in the animals. Positive and negative controls were appropriately used. The diagnosis of osteonecrosis was established based on the presence of empty lacunae or pyknotic osteocyte nuclei in the trabecular bone accompanied by necrosis of the surrounding bone marrow.
Figure 2.
Transduction efficiency of MSCs infected with the lenti-GFP vector. Flow cytometry analysis of MSCs infected with the lenti-GFP vector. In green, MSCs expressing the GFP protein in the sample that was implanted in the animals. MSC: mesenchymal stem cell; GFP: green fluorescent protein gene.
Statistical study
Measurements of the two groups were assessed using STATA (version 12.0, StataCorp LP, CollegeStation, TX). Radiographs were obtained and measured before and at 1-,2-,3- and 4- months after surgery. Statistical analysis evaluated the differences found in radiographic measurements between the different groups and with the contralateral healthy FH (auto-control). Values were presented as continuous variables using the mean and standard deviation. For comparative analysis, non-parametric tests were used. The Wilcoxon signed-rank test was used to compare the different measurements in each group and the Mann-Whitney U test was used for independent samples, with statistical significance set at p < 0.05. Fisher's test was used for categorical values.
Results
Radiographic measurements
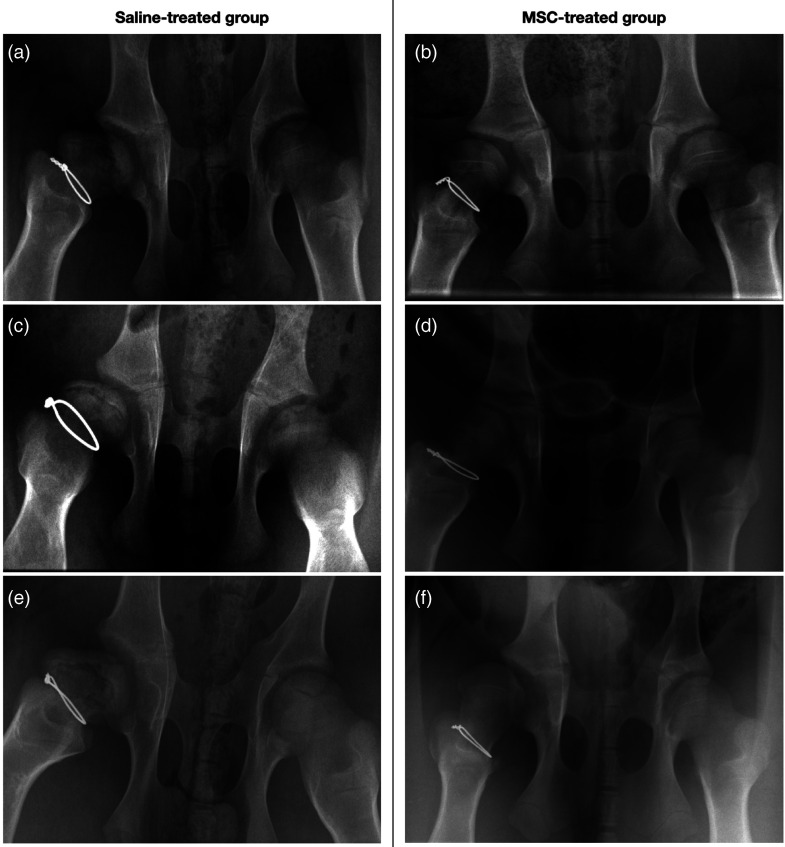
The proximal femur of the piglet had a well-developed secondary ossification centre at 4 weeks of age when surgery was done. Pelvic radiographs of both groups showed, from the second week after surgery onwards, an increase in the FH density, which is consistent with a pattern of necrosis. After 4 weeks, we noted a reduction in the height of the epiphysis, in both the central and the lateral column, an increase in the FH width and in the medial articular space, and osteoporosis in the epiphysis (Figure 3).
Figure 3.
(a) Pelvis X-rays of various piglets of the control saline group that shows severe FH deformity with flattening and collapse. (b) Pelvis X-rays of various specimen of the MSCs group showing the more spherical shape of the FH. FH: femoral head; MSC: mesenchymal stem cell.
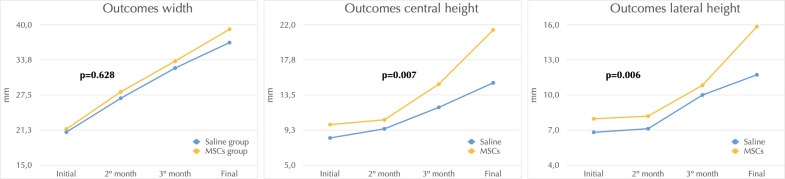
The radiological measurements collected one month after surgery and three months after infiltration showed increases in both groups (Table 1, Figure 4). The EQ in the MSC group was 0.84 (0.03) for the non-operated and 0.71 (0.06) for the operated side, whereas in the saline group it was 0.80 (0.04) for the non-operated and 0.24 (0.06) for the operated side (p = 0.021), reflecting a severe FH deformity. Considering only operated hips a statistically significant difference was found (p = 0.036).
Table 1.
Results of radiological measurements in each group.
| MSC group | SS group | P value | |||
|---|---|---|---|---|---|
| Initial | Final | Initial | Final | ||
| Width (mm) | 22.11 (1.5) | 39.22 (6.9) | 21.71 (3.4) | 36.84 (3.0) | 0.628 |
| Central column height (mm) | 10.76 (1,0) | 21.38 (4,7) | 8.67 (1.0) | 14.97 (1.9) | 0.007 |
| Lateral column height (mm) | 8.52 (1,0) | 15.85 (2.6) | 7.12 (0.9) | 11.73 (1.8) | 0.006 |
| Epiphyseal quotient | 0.71 (0.06) | 0.24 (0.06) | 0.036 | ||
All values are displayed as mean and standard deviation. MSC: mesenchymal stem cell treated group; SS: saline-treated group.
Figure 4.
Graphs showing the results of FH width, central column height, and lateral column height between the saline group (blue) and the MSC group (yellow). FH: femoral head; MSC: mesenchymal stem cell.
Macroscopic findings
Gross examination at sacrifice revealed cervical and cephalic deformation in all operated femurs, with significant changes compared to non-operated side. The deformed head was flatter and wider than normal, with the medial portion better preserved than the lateral. The articular cartilage was delustrated and showed increased thickness in the saline group, being less evident in the MSC group (Figure 5).
Figure 5.
Photograph illustrating the macroscopic appearance of both proximal femurs (right: operated; left: auto-control) at 12 weeks after infiltration. (a) The saline-group specimen showed a shorter neck and a markedly deformed FH, with flattening of the lateral 2/3 of the FH compared to the medial 1/3, and a relative increase of coxa vara. (b) In the MSC-group specimen, the FH preserves greater sphericity. The cerclage placed in the lower part of the intracapsular neck, adjacent to the capsular attachment. FH: femoral head; MSC: mesenchymal stem cell.
The saline and MSC groups showed marked macroscopic deformities at 4 months after surgery, but the MSC group was less affected than the SS group, maintaining the sphericity of the FH. A 100% of the FH of the group SS showed coxa magna versus 33% of the group MSC (p = 0.02) and FH flattening was observed in all the saline group and in half of the MSC group.
Microscopic findings
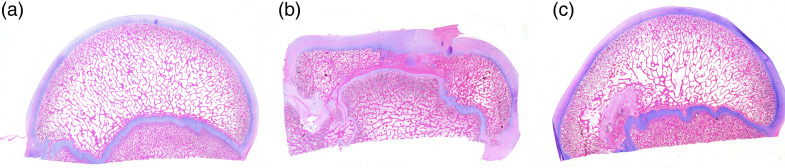
All specimens showed growth arrest of the bony epiphysis, necrotic changes in the marrow space, empty lacunae in the trabecular bone and fibrovascular repair tissue (Figure 6). Immunohistochemistry showed an absence of GFP-positive cells in all specimens. In the contralateral FHs (auto-control), the articular cartilage showed a well-preserved architecture (Figure 7A).
Saline group (Figure 7B): The FHs showed thickening of the articular cartilage. The formation of accessory ossification centres within the epiphysis was also observed. These bore a resemblance to healthy cartilage, being made up of cartilage arranged into columns with signs of enchondral ossification. In other cases, these foci consisted only of a nidus of cartilage surrounded by trabecular bone. Animals sacrificed at three months had similar changes to those observed in animals sacrificed after one month.
MSC group: FHs were found to have a slight flattening and small foci of fibrovascular penetration, with joint cartilage thinning, intra-epiphyseal accessory ossification foci, thickened vessels, and a wavy outline (Figure 7C) The changes in the animals sacrificed at three months were similar to those observed at one month. In one case, we identified the formation of a new line of cartilage located inside the epiphysis, close to the growth cartilage and running parallel to it. The bone remaining between this new line and the joint cartilage had smaller trabecula containing osteoblasts, osteoclasts and a fibromixoid stroma.
Figure 6.
(a) Photomicrograph of normal FH of immature piglet. (b) Photomicrograph of the saline-treated group FH of immature piglet at 4 months after induction of ischaemia. (c) Photomicrograph of the MSC-treated group FH of immature piglet at 4 months after induction of ischaemia. All images are macro-microphotographs (1×) with HE staining. FH: femoral head; HE: haematoxylin-eosin; MSC: mesenchymal stem cell.
Figure 7.
(a) Non-operated hips: The contralateral non-operated FG showed articular cartilage with a well-preserved architecture (i). The physeal cartilage had a straight outline and adequate columnar layout (ii). The epiphyseal bone showed adipose and haematopoietic intertrabecular tissue as usual (iii and iv). (b) Saline-treated group: Penetration of vascularized fibrous tissue from the epiphysis is shown at the tidemark of the articular cartilage (i). Replacement of most of the epiphyseal trabeculae by fibrous tissue, with collapse of the epiphysis and narrowing of the distance between the articular cartilage and the physeal cartilage (ii). Hyperplastic stromal vessels surrounded by abundant fibrous tissue (iii). Formation of accessory ossification centres in the thickness of the epiphysis, in relation to the articular cartilage; unstructured physeal cartilage interrupted by vascularized fibrous tissue (iv). (c) MSC-treated group: Articular cartilage slightly thinned, with small foci of fibrovascular tissue penetration and epiphyseal bone trabeculae thinner than in healthy heads (i). Physeal cartilage with wavy contour (ii). Thick vessels in the intertrabecular adipose stroma of the epiphysis (iii). Accessory focus of newly formed bone in the thickness of the epiphyseal trabeculae, slightly thickened (iv). All images are HE, 4×. MSC: mesenchymal stem cell; HE: haematoxylin-eosin; MSC: mesenchymal stem cell.
Discussion
Many research groups, including ours, are currently exploring the treatment of ONFH with MSCs, all employing different procedures, at different time points and using different doses.22–26 The decreased numbers and impaired activity of MSCs in the necrotic area27,28 justify the use of cell therapy in this setting. MSCs have the potential to differentiate into bone, cartilage, or fat tissue, provide osteogenic precursors to the necrotic area, and have also demonstrated a healing capability to enhance angiogenesis and prevent fibrosis. 5 The plasticity and remodeling potential of the immature hip provides the FH the opportunity to restore the spherical congruency by remaining within the acetabular cavity, unlike the ONFH in the adult. An in vivo model of ONFH in an immature hip to study the effects of MSCs therapy could greatly help to optimize the development of clinical therapies for this condition.
We present the results of treatment with MSCs in an experimental model of avascular necrosis of the FH in immature pigs. The titanium cerclage ensured that the blood supply was cut off when tension was applied since this material is more resistant than a normal suture. Moreover, we could radiographically ascertain correct cerclage positioning without producing further artefacts. We did not section the Ligamentum teres, so that we could better simulate the conditions in LCPD, avoiding coxofemoral dislocation and instability. Hip dislocation would worsen the injuries, lead to immobilization of the animal, and make infection more likely. 15
Intraosseous therapy with MSCs was a safe procedure without acute or prolonged adverse effects, which corroborates previous clinical experience [ 29 ]. We used imaging techniques to assess the effect of therapy on the evolution of ONFH. We observed no changes in the first two months after surgery. The changes began around the third month with an increase in epiphyseal bone density and a reduction in the size of the immature FH without flattening. Kim et al. 12 observed no statistically significant changes between weeks 4 and 8, and a slight flattening at 4 weeks after surgery, which became severe after 8 weeks. In the saline group, we observed greater deformation of the FH with more pronounced flattening, particularly in the central and lateral areas, corresponding with the load-bearing zones. We observed a widening of the metaphysis leading to FH extrusion in some cases. In the most severe cases, we found subluxation of the FH due to the poor fit between the deformed FH and the acetabulum. 30
Serial radiographs showed significant differences between groups at the final follow-up. The saline group showed significant deformity due to decreased central and lateral column height, with all animals presenting flattening and coxa magna. Meanwhile, in the MSC group only half of the FH showed flattening and 33% coxa magna. Comparing only the operated hips, the EQ was significantly higher for the animals receiving cell therapy. All these quantitative indexes showed that this therapy resulted in a better recovery in terms of sphericity of the necrotic FH.
Invasion of the medullary vascular spaces of the necrotic area of the FH is carried out by proliferative MSCs and through the capillaries of the adjacent metaphysis; these MSCs can differentiate towards the osteoblastic line and form new bone by apposition thus replacing the necrotic bone. Only when the appositional bone completely surrounds the FH can resorption of the central bone take place, allowing the central area of necrotic bone to be replaced by new bone and the osteolytic areas to disappear. Furthermore, when epiphyseal density increases, vascular supply is reestablished. 28 The phenomenon of bone repair moves from the periphery towards the centre of the lesion, and osteolytic areas disappear. At the end of the study, the MSC group showed slightly thin articular cartilage, with small foci of fibrovascular tissue penetration. In the epiphysis, the bone trabeculae were thinner than in the unoperated heads (auto-control).
In our study, as other authors, 12 we found an increase in bone resorption accompanied by a limited capacity for new bone formation. This imbalance could be related to the loss of the mechanical properties of the FH and the loss of its structural integrity. Our findings suggest that the FH tends to become deformed after the establishment of ischaemia because bone formation is insufficient after resorption. The lower degree of deformity found in the MSCs group could be related to the ‘premature’ differentiation of the MSCs into osteoblastic cells that are capable of restoring the structural integrity of the FH. Moreover, the new bone perforation and MSCs infiltration, seem to have triggered the vascular repair mechanism, since we found vascularized fibrous tissue from the metaphysis into the epiphysis. Histologically, we observed that the epiphysis collapsed, with cartilage thickening and fibrovascular tissue penetrating both the growth cartilage and the accessory ossification foci.9,12,13,30 We marked the cells with GFP in order to facilitate the MSC identification after administration. However, we did not find any GFP-positive cells in the studied samples, strongly suggesting that the infused MSCs did not engraft long-term. An alternative explanation is that the immune system of the animals recognized the GFP protein as ‘ not self’, destroying the GFP-infused MSCs. Other authors 10 have previously reported that the numbers of MSCs decreased with time after transplantation to undetectable levels, suggesting that MSCs appear to mediate their effects by a ‘hit and run’ mechanism.
Our results are promising but should be considered with caution. The small sample presents the potential risk of type II errors. In addition, no sample size calculation was performed beforehand. Also, a qualitative assessment of remodeling potential was not possible. Moreover, the immunohistochemical study has not been able to detect the presence of MSCs at the infiltration sites. And finally, we did not attempt to evaluate the impact of our experimental therapy on the animals’ quality of life.
Conclusion
The present experimental model of avascular necrosis in immature pigs by disrupting the blood supply to the FH resembles the deformity of the FH in children with LCPD. We were able to follow the evolution of therapy by imaging and studying the FHs pathology. Intraosseous MSCs injection improved bone healing and remodeling in this preclinical model of ONFH in immature specimens. This work supports further investigation to determine how MSCs may enhance the healing process in immature ONFH and should help in optimizing this regenerative cell therapy.
Supplemental Material
Supplemental material, sj-pdf-1-sci-10.1177_00368504231179790 for Intraosseous injection of mesenchymal stem cells for the treatment of osteonecrosis of the immature femoral head and prevention of head deformity: A study in a pig model by Sergio Martínez-Álvarez and María Galán-Olleros, Daniel Azorín-Cuadrillero, Ángel Palazón-Quevedo, África González-Murillo, Gustavo J Melen-Frajlich, Manuel Ramírez-Orellana, Tomás Epeldegui-Torre, Francisco Forriol in Science Progress
Acknowledgments
Gabriel Manso-Díaz, Isabel Santiago, Isabel García-Real, José Luis Martínez Rubio, Stefano Gambera, Javier García-Castro, Consuelo Serres-Dalmau, Antonio Rodríguez-Bertos, Nieves Lorenzo Lorenzo, José Luis Sánchez Castilla, Caroline Fox and Harry Kim.
Author biographies
Sergio Martínez-Álvarez, MD, is a pediatric orthopaedic surgeon.
María Galán-Olleros, MD, is a pediatric orthopaedic surgeon.
Daniel Azorín-Cuadrillero, MD, is a specialist in pathology.
Ángel Palazón-Quevedo, MD, is a pediatric orthopaedic surgeon.
África González-Murillo, PhD, is a biologist specialized in cellular and molecular biology and cancer research.
Gustavo J Melen-Frajlich, PhD, is a biologist specialized in cell therapy.
Manuel Ramírez-Orellana, MD., PhD., is a specialist Pediatric Oncohematology and cell therapy.
Tomás Epeldegui-Torre, MD., PhD., is a pediatric orthopaedic surgeon.
Francisco Forriol, MD., PhD., is Chairman for Orthopaedics.
Footnotes
Contributorship: Sergio Martínez-Álvarez1, María Galán-Olleros1,, Daniel Azorín-Cuadrillero2, Ángel Palazón-Quevedo1, África González-Murillo3, Gustavo J Melen-Frajlich3, Manuel Ramírez-Orellana3, Tomás Epeldegui-Torre1 and Francisco Forriol4 SM-A, TE-T and FF contributed to the research design. SM-A performed all the surgical procedures. MG-O and AP-Q contributed to the radiological evaluation and measurements. DA-C contributed to the histopathology examination. MR-O, AG-M and GJM-F worked on culture and preparation of MSCs. SM-A, MG-O and AP-Q contributed to the analysis and interpretation of the data and drafting of the paper. All authors contributed to reading and approving the final version of the submitted manuscript. All authors agreed to be responsible for all aspects of the work, ensuring that issues related to the accuracy or integrity of any part of the work were properly investigated and resolved.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: The study design was approved by the Institutional Animal Care and Use Committees (CEA-UCM 122/2012). All applicable international, national, and/or institutional guidelines for animal care and use were followed. All procedures performed in the studies involving animals were in accordance with the ethical standards of the institution where the studies were conducted. The project was also approved by the Research Committee of our Institution and Madrid Regional Government (S-BIO-0204-2006, MesenCAM; P2010/BMD-240, CellCAM) to Javier García-Castro and Manuel Ramirez-Orellana.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project was supported by the Fondo para la Investigación Científica y Tecnológica (FIS PI10/02805) of the Spanish Government for Health Research.
ORCID iD: María Galán-Olleros https://orcid.org/0000-0001-9074-9215
Supplemental material: Supplemental material for this article is available online.
References
- 1.Glueck CJ, Freiberg RA, Boppana Set al. et al. Thrombophilia, hypofibrinolysis, the eNOS T-786C polymorphism, and multifocal osteonecrosis. J Bone Joint Surg Am 2008; 90: 2220–2229. [DOI] [PubMed] [Google Scholar]
- 2.Vosmaer A, Pereira RR, Koenderman JS, et al. Coagulation abnormalities in Legg–Calvé–Perthes disease. J Bone Joint Surg Am 2010; 92: 121–128. [DOI] [PubMed] [Google Scholar]
- 3.Simkin PA, Downey DJ. Hypothesis: retrograde embolization of marrow fat may cause osteonecrosis. J Rheumatol 1987; 14: 870–872. [PubMed] [Google Scholar]
- 4.Glimcher MJ, Kenzora JE. Nicolas andry award. The biology of osteonecrosis of the human femoral head and its clinical implications: 1. Tissue biology. Clin Orthop Relat Res 1979; 138: 284–309. [PubMed] [Google Scholar]
- 5.Hernigou P, Trousselier M, Roubineau F, et al. Stem Cell Therapy for the Treatment of Hip Osteonecrosis: a 30-Year Review of Progress. Clin Orthop Surg 2016; 8: –8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gangji V, Hauzeur JP, Schoutens A, et al. Abnormalities in the replicative capacity of osteoblastic cells in the proximal femur of patients with osteonecrosis of the femoral head. J Rheumatol 2003; 30: 348–351. [PubMed] [Google Scholar]
- 7.Lee HS, Huang GT, Chiang H, et al. Multipotential mesenchymal stem cells from femoral bone marrow near the site of osteonecrosis. Stem Cells 2003; 21: 190–199. [DOI] [PubMed] [Google Scholar]
- 8.Kenzora JE, Steele RE, Yosipovitch ZHet al. et al. Experimental osteonecrosis of the femoral head in adult rabbits. Clin Orthop Relat Res 1978; 130: 8–46. [PubMed] [Google Scholar]
- 9.Kim HK, Stephenson N, Garces A, et al. Effects of disruption of epiphyseal vasculature on the proximal femoral growth plate. J Bone Joint Surg Am 2009; 91: 1149–1158. [DOI] [PubMed] [Google Scholar]
- 10.Mastro-Martínez I, Pérez-Suárez E, Melen G, et al. Effects of local administration of allogenic adipose tissue-derived mesenchymal stem cells on functional recovery in experimental traumatic brain injury. Brain Inj 2015; 29: 1497–1510. [DOI] [PubMed] [Google Scholar]
- 11.Boss JH, Misselevich I. Osteonecrosis of the femoral head of laboratory animals: the lessons learned from a comparative study of osteonecrosis in man and experimental animals. Vet Pathol 2003; 40: 345–354. [DOI] [PubMed] [Google Scholar]
- 12.Kim HK, Su PH. Development of flattening and apparent fragmentation following ischemic necrosis of the capital femoral epiphysis in a piglet model. J Bone Joint Surg Am 2002; 84: 1329–1334. [DOI] [PubMed] [Google Scholar]
- 13.Rowe SM, Lee JJ, Chung JY, et al. Deformity of the femoral head following vascular infarct in piglets. Acta Orthop 2006; 77: 33–38. [DOI] [PubMed] [Google Scholar]
- 14.Rowe SM, Chung JY, Moon ES, et al. The effects of subluxation of the femoral head with avascular necrosis in growing rabbits. J Pediatr Orthop 2004; 24: 645–650. [DOI] [PubMed] [Google Scholar]
- 15.Martínez-Álvarez S, Epeldegui-Torre T, Manso-Díaz G, et al. Inducción experimental de la enfermedad de perthes en corderos [experimental induction of Perthes disease in lambs]. Rev Esp Cir Ortop Traumatol 2014; 58: 68–77. [DOI] [PubMed] [Google Scholar]
- 16.Wang Z, Sun QM, Zhang FQ, et al. Core decompression combined with autologous bone marrow stem cells versus core decompression alone for patients with osteonecrosis of the femoral head: a meta-analysis. Int J Surg 2019; 69: 23–31. [DOI] [PubMed] [Google Scholar]
- 17.Kang JS, Suh YJ, Moon KH, et al. Clinical efficiency of bone marrow mesenchymal stem cell implantation for osteonecrosis of the femoral head: a matched pair control study with simple core decompression. Stem Cell Res Ther 2018; 9: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hernigou P, Dubory A, Homma Y, et al. Cell therapy versus simultaneous contralateral decompression in symptomatic corticosteroid osteonecrosis: a thirty year follow-up prospective randomized study of one hundred and twenty five adult patients. Int Orthop 2018; 42: 1639–1649. [DOI] [PubMed] [Google Scholar]
- 19.Houdek MT, Wyles CC, Smith JH, et al. Hip decompression combined with bone marrow concentrate and platelet-rich plasma for corticosteroid-induced osteonecrosis of the femoral head: mid-term update from a prospective study. Bone Jt Open 2021; 2: 926–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Döring M, Kluba T, Cabanillas Stanchi KM, et al. Longtime outcome after intraosseous application of autologous mesenchymal stromal cells in pediatric patients and young adults with avascular necrosis after steroid or chemotherapy. Stem Cells Dev 2020; 29: 811–822. [DOI] [PubMed] [Google Scholar]
- 21.Kong SY, Kim HW, Park HW, et al. Effects of multiple drilling on the ischemic capital femoral epiphysis of immature piglets. Yonsei Med J 2011; 52: 809–817. Erratum in: Yonsei Med J. 2012; 53(1): 240. Gong, Sun Young [corrected to Kong, Sun Young]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feitosa ML, Fadel L, Beltrão-Braga PC, et al. Successful transplant of mesenchymal stem cells in induced osteonecrosis of the ovine femoral head: preliminary results. Acta Cir Bras 2010; 25: 416–422. [DOI] [PubMed] [Google Scholar]
- 23.Aimaiti A, Saiwulaiti Y, Saiyiti M, et al. Therapeutic effect of osteogenically induced adipose derived stem cells on vascular deprivation-induced osteonecrosis of the femoral head in rabbits. Chin J Traumatol 2011; 14: 215–220. [PubMed] [Google Scholar]
- 24.Vélez R, Hernández-Fernández A, Caminal M, et al. Treatment of femoral head osteonecrosis with advanced cell therapy in sheep. Arch Orthop Trauma Surg 2012; 132: 1611–1618. [DOI] [PubMed] [Google Scholar]
- 25.Poignard A, Lebouvier A, Cavet M, et al. New preclinical porcine model of femoral head osteonecrosis to test mesenchymal stromal cell efficiency in regenerative medicine. Int Orthop 2014; 38: 1837–1844. [DOI] [PubMed] [Google Scholar]
- 26.Song H, Tao L, Wang F, et al. Effect of bone mesenchymal stem cells transplantation on the micro-environment of early osteonecrosis of the femoral head. Int J Clin Exp Pathol 2015; 8: 14528–14534. [PMC free article] [PubMed] [Google Scholar]
- 27.Hernigou P, Beaujean F, Lambotte JC. Decrease in the mesenchymal stem-cell pool in the proximal femur in corticosteroid-induced osteonecrosis. J Bone Joint Surg Br 1999; 81: 349–355. [DOI] [PubMed] [Google Scholar]
- 28.Suh KT, Ahn JM, Lee JS, et al. MRI Of the proximal femur predicts marrow cellularity and the number of mesenchymal stem cells. J Magn Reson Imaging 2012; 35: 218–222. [DOI] [PubMed] [Google Scholar]
- 29.de Rojas T, Martínez-Álvarez S, Lerma-Lara S, et al. Outcome of childhood leukaemia survivors and necrosis of the femoral head treated with autologous mesenchymal stem cells. Clin Transl Oncol 2018; 20: 584–590. [DOI] [PubMed] [Google Scholar]
- 30.Shapiro F, Connolly S, Zurakowski D, et al. Femoral head deformation and repair following induction of ischemic necrosis: a histologic and magnetic resonance imaging study in the piglet. J Bone Joint Surg Am 2009; 91: 2903–2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-sci-10.1177_00368504231179790 for Intraosseous injection of mesenchymal stem cells for the treatment of osteonecrosis of the immature femoral head and prevention of head deformity: A study in a pig model by Sergio Martínez-Álvarez and María Galán-Olleros, Daniel Azorín-Cuadrillero, Ángel Palazón-Quevedo, África González-Murillo, Gustavo J Melen-Frajlich, Manuel Ramírez-Orellana, Tomás Epeldegui-Torre, Francisco Forriol in Science Progress