Abstract
Background
Emollients are universally recommended for atopic dermatitis/eczema (‘eczema’), to improve the skin barrier and reduce symptoms. However, our knowledge of the frequency and nature of adverse effects associated with their use is limited.
Objectives
We sought to determine how well adverse events are reported in randomized controlled trials (RCTs) of emollients for eczema.
Methods
MEDLINE was searched from inception (1946) to May 2022. Inclusion criteria were RCTs of moisturizers or emollients used as a leave-on treatment (as the intervention or control) in adults or children with eczema. Exclusion criteria were non-RCTs; patients with other diagnoses included; use of emollient as bath additives, soap substitutes or as preventative; and not published in English. References of eligible papers were reviewed for any additional, relevant research. Data were extracted into an Excel spreadsheet and analysed descriptively. An assessment of study quality was carried out using the Joanna Briggs Institute tool for RCTs.
Results
From 369 potential papers, 35 papers (reporting on 34 studies) were included. Most research was conducted in research centres or hospitals (unclear in 34%). In total, 89% reported collecting data on adverse events related to emollient treatment use but the methods used were poorly reported (40% unclear). Four papers used patient questionnaires/diaries. However, it was unclear how and what was collected as only two studies showed the questionnaires used.
Conclusions
Reporting of adverse events related to emollient use in trials of patients with eczema is poor and inconsistent. Agreement should be reached on how and what adverse events should be collected, to standardize reporting across studies.
Emollients are universally accepted as a treatment for atopic dermatitis; however, our knowledge of the frequency and nature of adverse effects associated with their use is limited. We sought to determine how well adverse events are reported in randomized controlled trials of emollients for eczema. We concluded that they are poorly reported and agreement should be reached on how and what adverse events should be collected to standardize reporting across studies.
What is already known about this topic?
Emollients use in people with eczema is determined by effectiveness and acceptability.
Adverse events associated with emollient use are usually mild but estimates of their frequency vary widely.
What does this study add?
Adverse event reporting in trials of emollients for eczema is poor and data are collected inconsistently across randomized controlled trials.
Standardization of the methods of collecting and reporting emollient adverse effects would help with comparisons across different studies and products.
Atopic dermatitis/eczema (‘eczema’) is a chronic, inflammatory skin condition, affecting children and adults. Its global prevalence is steadily increasing.1 It is characterized by dry and itchy skin, prone to lichenification and skin infections.2 Emollients are recommended in the treatment of eczema; to soothe pruritis, to improve the skin’s barrier function and to help reduce the recurrence of disease flares.1–4 However, underuse is common and may be related to the acceptability of different products, including any adverse effects.5 A 2017 Cochrane review of randomized controlled trials (RCTs) comparing emollients in eczema reported that adverse events were only reported in half of studies (41/77), and these were uninformative.1 A more recent systematic review (2019) that included RCTs, cohort studies, case–control studies and case reports, also concluded that adverse events were poorly reported, with estimates of how common adverse events were widely varying (2–59% of patients).6
Extending the above work, the aim of this systematic review was to look at how RCTs captured data on adverse events associated with emollient use and what symptoms they discussed and reported on when they did.
Materials and methods
The review was prospectively registered on Research Registry (unique number: reviewregistry1441) and conducted/reported in line with PRISMA guidance (Appendix S1; see the Supporting Information).7
Literature search
The MEDLINE database (Ovid) was searched from 1946 to May 2022. The search strategy used is given in Appendix S2; see the Supporting Information.
The citations were exported into Microsoft Excel, and their eligibility assessed. Firstly, they were screened using the title and abstract. Next, the remaining papers were read in full to create a list of eligible papers. Lastly, these were then reviewed for any additional papers, which were then added to create the final list to be used for data extraction and analysis. Papers were independently screened by two authors (E.R.E. and S.C. or M.A.) and any questions were discussed with a fourth author (M.J.R.) until a decision was made.
Quality assessment
An overall quality assessment of the papers included was carried out using the Joanna Briggs Institute checklist for RCTs.8 M.A. led this assessment and ambiguities were resolved with E.R.E. and M.J.R.
Inclusion and exclusion criteria
Inclusion criteria were: RCTs of moisturizers or emollients used as a leave-on treatment (as the intervention or control) in adults or children with atopic eczema or atopic dermatitis. Papers were excluded if: they did not study leave-on emollients or moisturizers (e.g. bath additives or soap substitutes); were not RCTs; included patients with other diagnoses; the use of emollients as preventative treatments; were not published in English; and were not in humans.
Emollients or moisturizers used in the treatment of eczema do not have a strict definition, but for the purposes of this review were defined as products applied directly to the skin to help retain moisture. They did not contain recognized anti-inflammatory agents such as topical corticosteroids or calcineurin inhibitors.
The adverse events focused on in this report are those related to the treatment.
Data extraction
Microsoft Excel was used to extract and analyse the data. One author, E.R.E, piloted the data extraction tables, before they were finalized. E.R.E. first completed the data extraction then, a second author, M.A., independently checked them. Discrepancies were discussed with a third author, M.J.R. The main outcomes assessed were: how many studies reported on adverse events, if and how those studies collected these data and what the adverse events as a result of emollients were. Data extraction focused on treatment-related adverse events, specifically localized skin reactions. Data about the study characteristics were also collected including: patient demographics, setting, type of emollient and if they reported broad or detailed data on adverse events.
We collected data on any of the treatment-related adverse events because of either the emollient used as the control or intervention on the assumption (where not stated) that the same methodology was used in all trial arms.
Results
Literature search
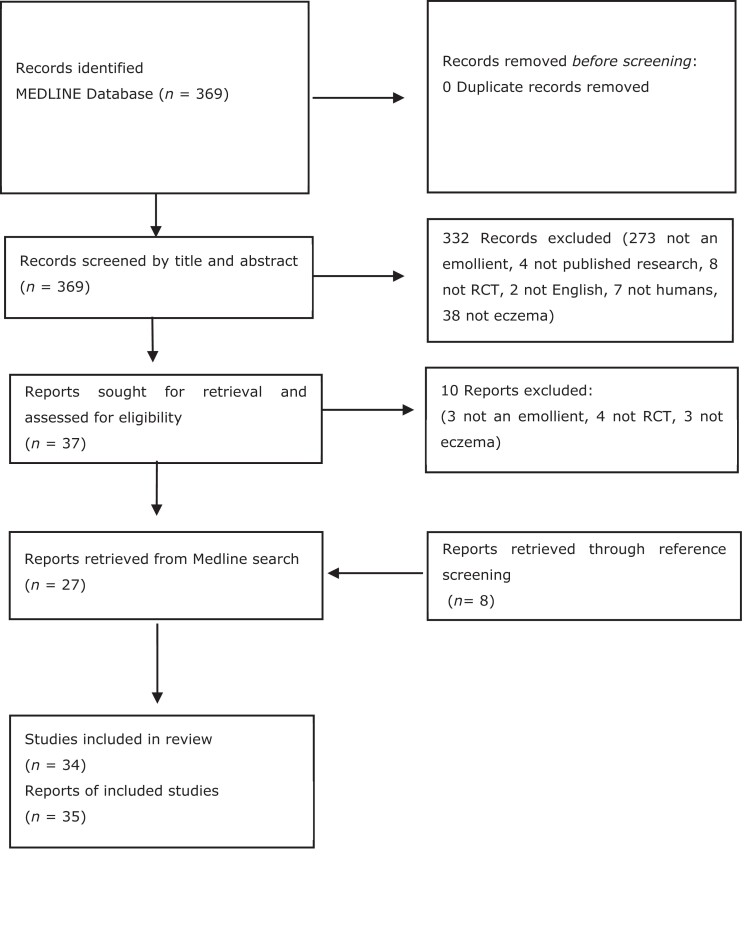
From 369 results, 332 papers were excluded. In total, 37 papers were retrieved in full, and a further 10 papers excluded. Eight additional articles were included after screening the references of eligible papers, creating 35 as the final list of eligible reports, or 34 studies (see Figure 1).
Figure 1.
PRISMA flowchart – eligibility. RCT, randomized controlled trial.
Study characteristics
The 35 papers were published from 1977 to 2021 (Table S1; see Supporting Information). The majority, 43% (15/35) were focused on paediatric patients9–23 compared with 26% (9/35) on adults24–32 and 31% (11/35) that included both.33–43 Four (11%) were conducted in multiple countries12,13,24,41 and 14% (5/35) did not clearly state where the research took place.15,17,27,30,38 Of those conducted in one country, 20% (7/35) were in the USA11,18,22,23,34–36,43 and 17% (6/35) in Germany.10,16,26,29,32,33
The research settings were primarily in research centres (37%) 13/3511–13,18,19,26,29,30,32,35,36,39,41 or hospitals (26%) 9/359,10,16,24,25,28,31,40,42 and one took place across both (3%).23 There was no research in a community setting, although 12 papers (34%) were unclear.14,15,17,20–22,27,33,34,37,38,43
Five studies were within-patient studies, where both the emollient and control were used on the same patient.15,27,29,35,43
Assessment of overall study quality
In 57% (20/35) of the papers studied, allocation was either not concealed or it was unclear.9,14,16,18,20–22,24,26–28,30–34,37,39,41,43 In 26% (9/35) of the total papers included, patients were not blind to allocation9,10,12,13,15,16,29,33,35 and for 9% (3/35) it was unclear if any participants were blind to allocation or not.19,21,27 In two papers – one using a patient diary10 and another a questionnaire33 – the participants were not blind to allocation. In 14% (5/35) the assessor was not blind to allocation9,12,13,16,28 and in 9% (3/35) it was unclear.19,21,27 In three papers with investigator assessed data collection, the investigators were not blinded.12,16,28
In all but one of the papers31 it was unclear whether outcomes were measured in a reliable way as the number and training of the assessors was not mentioned (Table S2; see Supporting Information).
Emollients studied
The 35 papers included 46 different emollients (Appendix S3; see Supporting Information). AtopiclairTM was the most commonly used emollient (three papers).13,18,21 The emollients used included a range of formulations, the most common was cream (41%; 19/46)10–14,16,18,21,22,24,25,27,31,32,34,35,38,39,42,43 followed by lotion (13%; 6/46).9,15,23,26,30,43 The ingredients in the emollients varied from botanicals, ceramides and ‘active’ ingredients (Appendix S3).
Adverse events data capture and methodology
The majority of reports, 89% (31/35), did capture data on adverse events related to the treatment, although only 58% (18/31) provided a specific report into the nature of the adverse events experienced. The other papers provided a broad summary of adverse events explaining either there were no adverse events experienced or highlighting that the product was deemed safe.
The methods used to capture the data on adverse events were poorly explained. Most reports (14/35; 40%) were unclear.9,13,15,17,21,22,25,29,30,32,34,38,42,43 Where stated, 26% (9/35) were investigator assessed,12,16,23,24,28,35,36,40,41 6% (2/35) were patient reported14,18 and 1 study39 used both the investigator and the patient to capture adverse event data (Table 1).
Table 1.
Methodology and reporting on adverse events (AEs) (n = 35)
| Methodology | Studies using this methodology, n | Reported on the nature of AEs experienced, n (%) |
|---|---|---|
| Investigator assessed | 9 | 6 (67) |
| Investigator assessed and patient reported | 1 | 1 (100) |
| Patient case report forms | 1 | 1 (100) |
| Patient diaries | 1 | 0 (0) |
| Patient reported | 2 | 2 (100) |
| Questionnaire | 3 | 1 (33) |
| Unclear | 14 | 7 (50) |
| No data collection | 4 | 0 (0) |
A range of other methods were mentioned such as patient diaries,10 case report forms,11 and questionnaires.19,20,33 Two studies shared their questionnaire on adverse events.19,20 One focused on the frequency of adverse events,20 the other had a graph, which showed the question used for data capture (‘How bothered were you by the study regime’s side effects?’) and the results.19 Angelova-Fischer et al. did not share their questionnaire, but present the results of the frequency and nature of the adverse events for the three products used in the trial.33
Nature of adverse events
In 31% (11/35) of the studies, one or more patients stopped using the product because of treatment-related adverse events.13,14,16,18,21,23,24,28,30,32,41 In each of these studies a more detailed report of adverse events experienced was reported. In five of the studies, it was unclear if patients stopped using the product or not.17,20,26,29,31
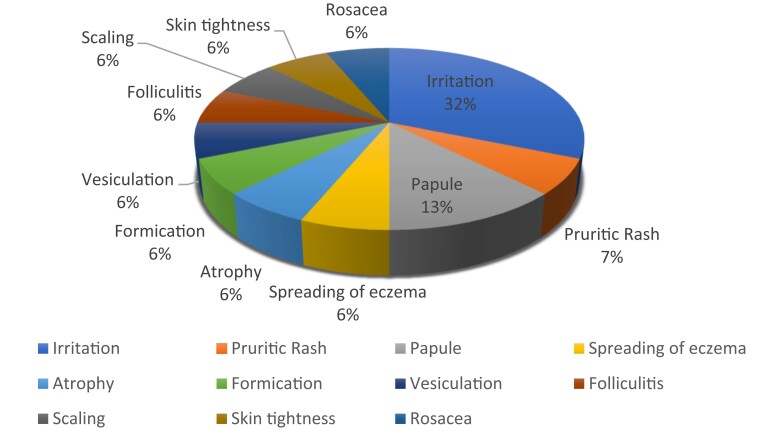
Where studies did provide information on the type of side-effects experienced from the emollient the most common were ‘pruritis’,11,12,14,16,24,25,30,32,33,41,42 ‘burning on application’12,14,18,21,23,24,32,33,41 and ‘erythema’ (Table 2).12,14,16,23,24,28,30,32,33 Eleven studies mentioned ‘other’ symptoms, of these ‘irritation’ was the most common, followed by ‘papule’ (Figure 2).9,11,14,16,23,24,28,32,33,38,41
Table 2.
Symptoms reported as adverse events (n = 35)
| Symptom | Papers reporting, n (%) |
|---|---|
| Itching/pruritis | 11 (31) |
| Erythema | 9 (26) |
| Burning or warm on application | 9 (26) |
| Stinging on/after application | 6 (17) |
| Worsening of eczema/flare ups | 6 (17) |
| Allergic reactions (e.g. contact dermatitis/hypersensitivity) | 2 (6) |
| Skin infections | 2 (6) |
| Dryness | 2 (6) |
| New rash | 2 (6) |
| Pain | 1 (3) |
| Swelling | 1 (3) |
| Tingling | 0 (0) |
| Peeling of skin | 0 (0) |
Figure 2.
Breakdown of ‘other’ adverse symptoms.
Discussion
We identified 35 papers reporting on 34 trials of 46 emollients. They primarily included children and took place in research centres and secondary care sites. Four papers did not discuss adverse events at all,26,27,31,37 and of the remaining 31 papers, 58% provided detail on the adverse events experienced. The methodology to collect data on adverse events was unclear in 40% of the papers and only two papers shared their data-collection tools. Where studies provided detailed information about the type of adverse events experienced with emollients, pruritis, erythema and burning on application were the most common, whereas ‘irritation’ was the most commonly mentioned ‘other’ symptom.
This study has some limitations in addition to its strengths. To the best of our knowledge, this review is the first to focus on how and what adverse events are reported in trials of emollients used in the treatment of eczema. Because of the lack of consensus about how or what symptoms should be sought and reported, and variation across countries and cultures in how terms are understood or used, there is likely to be inconsistency in the use of terminology. For instance, one study may have referred to ‘stinging’ and another study may have referred to ‘burning’ in reference to the same side-effect. The generally poor reporting of adverse events was also reflected in poor reporting generally, as noted by the overall quality assessment.
This review collected data on the adverse events that were reported. If adverse events were experienced but there was no method to collect this information then it would have been missed. This review was also limited by only including papers published in English and only searching one database (MEDLINE). This review only focused on RCTs, which means that other study types with relevant information will have been missed.
The van Zuuren et al. Cochrane review of trials comparing moisturizers in the treatment of eczema, concluded that adverse event reporting for emollients needed to be more ‘complete’.1 This more in-depth assessment highlights a lack of consistency in the methodology used to collect data on adverse events.
Previous research has also highlighted that adverse events were generally mild in nature and uncommon with emollients and moisturizers.1,6 Yet 31% of the papers in this review had a participant stop using a product because of treatment-related side-effects. Adverse events maybe mild but they are not insignificant. It is therefore important that adverse events are accurately recorded and reported to improve clinical practice. Since running the searches, the Best Emollients for Eczema (BEE) trial has been published,44 which reported that 37% of children experienced one or more adverse event, although (with the exception of stinging) this did not differ between lotions, creams, gels or ointments.
A study looking at unwanted side-effects of emollients also found stinging or burning on application to be one of the more commonly experienced adverse events; however, this symptom could be considered a normal response to an emollient and associated with the severity of the eczema rather than an adverse event.45
Conclusions
This review highlights a lack of consistency in the methods used to assess and report on adverse events. One way to reduce the variability in methodology would be to agree on a list of events to be routinely reported and create standardized tools to aid their collection. The BEE trial summarized the findings from patient-completed questionnaires by symptom and emollient type, clearly demonstrating the frequency and nature of the adverse event.44 This was similar to Angelova-Fischer et al. but included a more extensive list of symptoms such as worsening of eczema, peeling of skin and swelling.33,44
Further research into the nature of adverse events from use of emollients would be welcome to help shed light on what could be considered a ‘normal’ but unpleasant effect of an emollient on the skin and what is an adverse event. This will improve the ability of clinicians to educate patients as to what to expect from their emollient and hopefully improve adherence to treatment.
Adverse events in RCTs about emollients used in the treatment of eczema are poorly reported. The methodology used to collect data on adverse events varies and often was unclear. Improving the quality of data collection and reporting using standardized tools could help improve our understanding of the relative merits of different emollients.
Supplementary Material
Contributor Information
Elizabeth R Emmett, Gloucestershire NHS Trust, Cheltenham, UK.
Megan Allen, Bristol Medical School, University of Bristol, Bristol, UK.
Sarah Crownshaw, Great Western Hospitals NHS Trust, Swindon, UK.
Matthew J Ridd, Bristol Medical School, University of Bristol, Bristol, UK.
Funding sources
M.A. was supported by the Academy of Medical Sciences and the Wellcome Trust (INSPIRE).
Data availability
Data are available on request from the corresponding author.
Ethics statement
Ethical approval: not applicable. Informed consent: not applicable.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher’s website.
References
- 1. van Zuuren EJ, Fedorowicz Z, Christensen R et al. Emollients and moisturisers for eczema. Cochrane Database Syst Rev 2017; 2:CD012119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. NICE . BNF. Eczema. Treatment summaries. Available at: https://bnf.nice.org.uk/treatment-summaries/eczema/ (last accessed 27 April 2023).
- 3. van Halewijn KF, Lahnstein T, Bohnen AM et al. Recommendations for emollients, bathing and topical corticosteroids for the treatment of atopic dermatitis: a systematic review of guidelines. Eur J Dermatol 2022; 32:113–36. [DOI] [PubMed] [Google Scholar]
- 4. LePoidevin LM, Lee DE, Shi VY. A comparison of international management guidelines for atopic dermatitis. Pediatr Dermatol 2019; 36:36–65. [DOI] [PubMed] [Google Scholar]
- 5. Capozza K, Schwartz A. Does it work and is it safe? Parents’ perspectives on adherence to medication for atopic dermatitis. Pediatr Dermatol 2020; 37:58–61. [DOI] [PubMed] [Google Scholar]
- 6. Bhanot A, Huntley A, Ridd MJ. Adverse events from emollient use in eczema: a restricted review of published data. Dermatol Ther (Heidelb ) 2019; 9:193–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Page MJ, McKenzie JE, Bossuyt PM et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Joanna Briggs Institute . Critical appraisal tools. Available at: https://jbi.global/critical-appraisal-tools (last accessed 27 April 2023).
- 9. Liu L, Ong G. A randomized, open-label study to evaluate an intermittent dosing regimen of fluticasone propionate 0.05% cream in combination with regular emollient skin care in reducing the risk of relapse in pediatric patients with stabilized atopic dermatitis. J Dermatol Treat 2018; 29:501–9. [DOI] [PubMed] [Google Scholar]
- 10. Stettler H, Kurka P, Kandzora J et al. A new topical panthenol-containing emollient for maintenance treatment of childhood atopic dermatitis: results from a multicenter prospective study. J Dermatolog Treat 2017; 28:774–9. [DOI] [PubMed] [Google Scholar]
- 11. Lisante TA, Nuñez C, Zhang P et al. Efficacy and safety of an over-the-counter 1% colloidal oatmeal cream in the management of mild to moderate atopic dermatitis in children: a double-blind, randomized, active-controlled study. J Dermatolog Treat 2017; 28:659–67. [DOI] [PubMed] [Google Scholar]
- 12. Boralevi F, Saint Aroman M, Delarue A et al. Long-term emollient therapy improves xerosis in children with atopic dermatitis. J Eur Acad Dermatol Venereol 2014; 28:1456–62. [DOI] [PubMed] [Google Scholar]
- 13. Tiplica GS, Kaszuba A, Malinauskienė L et al. Prevention of flares in children with atopic dermatitis with regular use of an emollient containing glycerol and paraffin: a randomized controlled study. Pediatr Dermatol 2017; 34:282–9. [DOI] [PubMed] [Google Scholar]
- 14. Gayraud F, Sayag M, Jourdan E. Efficacy and tolerance assessment of a new type of dermocosmetic in infants and children with moderate atopic dermatitis. J Cosmet Dermatol 2015; 14:107–12. [DOI] [PubMed] [Google Scholar]
- 15. Udompataikul M, Srisatwaja W. Comparative trial of moisturizer containing licochalcone A vs. hydrocortisone lotion in the treatment of childhood atopic dermatitis: a pilot study. J Eur Acad Dermatol Venereol 2010; 25:660–5. [DOI] [PubMed] [Google Scholar]
- 16. Korting HC, Schöllmann C, Cholcha W et al. Efficacy and tolerability of pale sulfonated shale oil cream 4% in the treatment of mild to moderate atopic eczema in children: a multicentre, randomized vehicle-controlled trial. J Eur Acad Dermatol Venereol 2010; 24:1176–82. [DOI] [PubMed] [Google Scholar]
- 17. Haider SA. Treatment of atopic eczema in children: clinical trial of 10% sodium cromoglycate ointment. BMJ 1977; 1:1570–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Boguniewicz M, Zeichner JA, Eichenfield LF et al. MAS063DP is effective monotherapy for mild to moderate atopic dermatitis in infants and children: a multicenter, randomized, vehicle-controlled study. J Pediatr 2008; 152:854–9. [DOI] [PubMed] [Google Scholar]
- 19. Ma L, Li P, Tang J et al. Prolonging time to flare in pediatric atopic dermatitis: a randomized, investigator-blinded, controlled, multicenter clinical study of a ceramide-containing moisturizer. Adv Ther 2017; 34:2601–11. [DOI] [PubMed] [Google Scholar]
- 20. Giordano-Labadie F, Cambazard F, Guillet G et al. Evaluation of a new moisturizer (Exomega milk®) in children with atopic dermatitis. J Dermatol Treat 2009; 17:78–81. [DOI] [PubMed] [Google Scholar]
- 21. Patrizi A, Capitanio B, Neri I et al. A double-blind, randomized, vehicle-controlled clinical study to evaluate the efficacy and safety of MAS063DP (ATOPICLAIRTM) in the management of atopic dermatitis in paediatric patients. Pediatr Allergy Immunol 2008; 19:619–25. [DOI] [PubMed] [Google Scholar]
- 22. Draelos ZD, Traub M, Fabno ND et al. Efficacy of topical botanical treatment of children with mild to moderate atopic dermatitis. J Drugs Dermatol 2019; 18:1038–45. [PubMed] [Google Scholar]
- 23. Stainer R, Matthews S, Arshad SH et al. Efficacy and acceptability of a new topical skin lotion of sodium cromoglicate (Altoderm) in atopic dermatitis in children aged 2–12 years: a double-blind, randomized, placebo-controlled trial. Br J Dermatol 2005; 152:334–41. [DOI] [PubMed] [Google Scholar]
- 24. Åkerström U, Reitamo S, Langeland T et al. Clinical report: comparison of moisturizing creams for the prevention of atopic dermatitis relapse: a randomized double-blind controlled multicentre clinical trial. Acta Derm Venereol 2015; 95:587–92. [DOI] [PubMed] [Google Scholar]
- 25. Spada F, Harrison IP, Barnes TM et al. A daily regimen of a ceramide-dominant moisturizing cream and cleanser restores the skin permeability barrier in adults with moderate eczema: a randomized trial. Dermatol Ther 2021; 34:e14970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Angelova-Fischer I, Rippke F, Richter D et al. Stand-alone emollient treatment reduces flares after discontinuation of topical steroid treatment in atopic dermatitis: a double-blind, randomized, vehicle-controlled, left-right comparison study. Acta Derm Venereol 2018; 98:517–23. [DOI] [PubMed] [Google Scholar]
- 27. Nistico SP, del Duca E, Tamburi F et al. Superiority of a vitamin B12-barrier cream compared with standard glycerol-petrolatum-based emollient cream in the treatment of atopic dermatitis: a randomized, left-to-right comparative trial. Dermatol Ther 2017; 30:e12523. [DOI] [PubMed] [Google Scholar]
- 28. Tamura M, Kawasaki H, MAsunaga T et al. Equivalence-evaluation-moisturizers-atopic-dermatitis. J Cosmet Sci 2015; 66:295–303. [PubMed] [Google Scholar]
- 29. Simpson E, Böhling A, Bielfeldt S et al. Improvement of skin barrier function in atopic dermatitis patients with a new moisturizer containing a ceramide precursor. J Dermatol Treat 2012; 24:122–5. [DOI] [PubMed] [Google Scholar]
- 30. Bissonnette R, Maari C, Provost N et al. A double-blind study of tolerance and efficacy of a new urea-containing moisturizer in patients with atopic dermatitis. J Cosmet Dermatol 2010; 9:16–21. [DOI] [PubMed] [Google Scholar]
- 31. Wirén K, Nohlgård C, Nyberg F et al. Treatment with a barrier-strengthening moisturizing cream delays relapse of atopic dermatitis: a prospective and randomized controlled clinical trial. J Eur Acad Dermatol Venereol 2009; 23:1267–72. [DOI] [PubMed] [Google Scholar]
- 32. Stücker M, Pieck C, Stoerb C et al. Topical vitamin B12—a new therapeutic approach in atopic dermatitis – evaluation of efficacy and tolerability in a randomized placebo-controlled multicentre clinical trial. Br J Dermatol 2004; 150:977–83. [DOI] [PubMed] [Google Scholar]
- 33. Angelova-Fischer I, Neufang G, Jung K et al. A randomized, investigator-blinded efficacy assessment study of stand-alone emollient use in mild to moderately severe atopic dermatitis flares. J Eur Acad Dermatol Venereol 2014; 28:9–15. [DOI] [PubMed] [Google Scholar]
- 34. Draelos ZD, Traub M, Fabno ND et al. Validation of botanical treatment efficiency for adults and children suffering from mild to moderate atopic dermatitis. J Drugs Dermatol 2019; 18:557–61. [PubMed] [Google Scholar]
- 35. Emer JJ, Frankel A, Sohn A et al. A bilateral comparison study of pimecrolimus cream 1% and a topical medical device cream in the treatment of patients with atopic dermatitis. J Drugs Dermatol 2011; 10:735–43. [PubMed] [Google Scholar]
- 36. Frankel A, Sohn A, Patel R et al. Bilateral comparison study of pimecrolimus cream 1% and a ceramide-hyaluronic acid emollient foam in the treatment of patients with atopic dermatitis. J Drugs Dermatol 2011; 10:666. [PubMed] [Google Scholar]
- 37. Khiljee S, Ur Rehman N, Khiljee T et al. Formulation and clinical evaluation of topical dosage forms of Indian Penny Wort, walnut and turmeric in eczema. Pak J Pharm Sci 2015; 28:2001–7. [PubMed] [Google Scholar]
- 38. Hyo Kwon S, Jin Lim C, Jung J et al. The effect of autophagy-enhancing peptide in moisturizer on atopic dermatitis: a randomized controlled trial. J Dermatol Treat 2019; 30:558–64. [DOI] [PubMed] [Google Scholar]
- 39. Lee J, Jung E, Koh J et al. Effect of rosmarinic acid on atopic dermatitis. J Dermatol 2008; 35:768–71. [DOI] [PubMed] [Google Scholar]
- 40. Lin YK, Chang SH, Yang CY et al. Efficacy and safety of indigo naturalis ointment in treating atopic dermatitis: a randomized clinical trial. J Ethnopharmacol 2020; 250:112477. [DOI] [PubMed] [Google Scholar]
- 41. Peserico A, Städtler G, Sebastian M et al. Reduction of relapses of atopic dermatitis with methylprednisolone aceponate cream twice weekly in addition to maintenance treatment with emollient: a multicentre, randomized, double-blind, controlled study. Br J Dermatol 2008; 158:801–7. [DOI] [PubMed] [Google Scholar]
- 42. Tan WP, Suresh S, Tey HL et al. A randomized double-blind controlled trial to compare a triclosan-containing emollient with vehicle for the treatment of atopic dermatitis. Clin Exp Dermatol 2010; 35:e109–12. [DOI] [PubMed] [Google Scholar]
- 43. Zirwas M, Barkovic S. Anti-pruritic efficacy of itch relief lotion and cream in patients with atopic history – comparison with hydrocortisone cream. J Drugs Dermatol 2017; 16:243–7. [PubMed] [Google Scholar]
- 44. Ridd MJ, Wells S, Webb D et al. Effectiveness and safety of lotion, cream, gel, and ointment emollients for childhood eczema: a pragmatic, randomised, phase 4, superiority trial. Lancet Child Adolesc Health 2022; 6:522–32. [DOI] [PubMed] [Google Scholar]
- 45. Oakley R, Lawton S. Views on unwanted effects of leave-on emollients and experiences surrounding their incidence. Dermatol Nurs 2016; 15:38–43. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on request from the corresponding author.