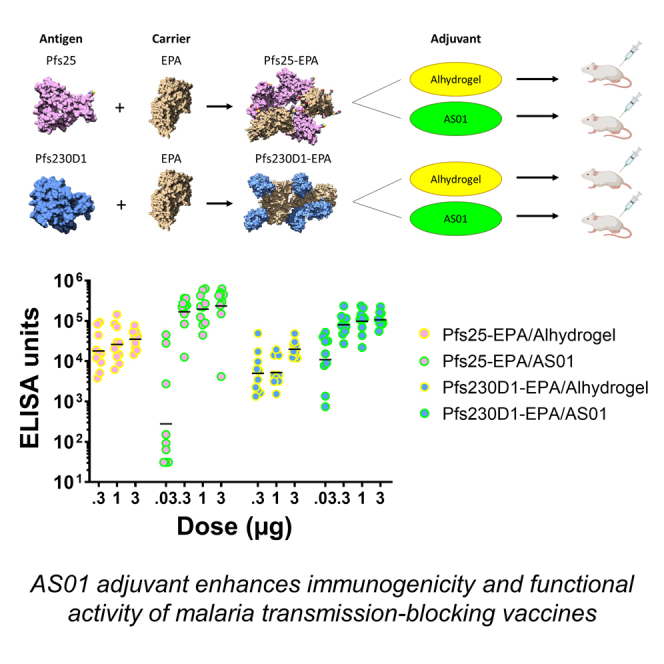
Summary
Malaria transmission-blocking vaccine candidates Pfs25-EPA and Pfs230D1-EPA target sexual stage development of Plasmodium falciparum parasites in the mosquito host, thereby reducing mosquito infectivity. When formulated on Alhydrogel, Pfs25-EPA has demonstrated safety and immunogenicity in a phase 1 field trial, while Pfs230D1-EPA has shown superior activity to Pfs25-EPA in a phase 1 US trial and has entered phase 2 field trials. Development continues to enhance immunogenicity of these candidates toward producing a vaccine to reduce malaria transmission (VRMT) with both pre-erythrocytic (i.e., anti-infection) and transmission-blocking components. GSK Adjuvant Systems have demonstrated successful potency in pre-erythrocytic vaccine trials and might offer a common platform for VRMT development. Here, we describe preclinical evaluations of Pfs25-EPA and Pfs230D1-EPA nanoparticles with GSK platforms. Formulations were stable after a series of assessments and induced superior antibody titers and functional activity in CD-1 mice, compared to Alhydrogel formulations of the same antigens.
Subject area(s): Parasitology, Molecular biology
Graphical abstract
Highlights
-
•
Pfs25-EPA or Pfs230D1-EPA induces higher titers in AS01 vs. alum adjuvants in mice
-
•
Pfs230D1-EPA induces higher complement-dependent activity in AS01 formulations
-
•
Pfs25-EPA induces plasma cells and durable antibody titers vs. Pfs25 monomer
-
•
Co-administration of Pfs25-EPA+Pfs230D1-EPA in AS01 did not reduce antibody titers
Parasitology; Molecular biology
Introduction
Malaria is a devastating global health problem; an estimated 241 million cases and 627,000 deaths occurred in 2020, with the majority of cases and deaths caused by Plasmodium falciparum.1 Global malaria cases have not seen any significant reduction since 2015, and converging threats including (but not limited to) the COVID-19 pandemic and emerging antimalarial drug resistance have contributed to recent increases in cases and deaths worldwide.1 In 2021, the World Health Organization (WHO) recommended broader deployment across Africa of RTS,S/AS01E, the world’s first malaria vaccine, but emphasized that new tools and approaches are still needed to pursue elimination.1
Vaccine development has targeted various stages of the parasite life cycle (e.g., pre-erythrocytic, blood stage, and sexual stage). RTS,S targets the pre-erythrocytic stage, to prevent or stop parasite infection before progression from the human liver to the bloodstream. Transmission-blocking vaccines (TBVs) focus on sexual stages of the parasite, designed to impede development of parasites inside the mosquito. By reducing mosquito infectivity, TBVs aim to halt propagation of the parasite to other human hosts, perhaps eliminating it from an affected population.2 Two leading TBV candidates are Pfs25 and Pfs230D1, currently being developed and evaluated in clinical trials.
Pfs25 is a P. falciparum surface protein expressed during zygote and ookinete stages in infected mosquitoes.3 Previous gene knockout experiments suggested its importance for parasite survival inside the mosquito midgut.2,4 Furthermore, a double-knockout study on P. berghei showed loss of Pfs25 results in reduced ookinete invasion into midgut epithelial cells.5,6 Lastly, antibodies raised against recombinant Pfs25 have been shown to halt parasite development within mosquitoes.7
Pfs230 is a large protein (>300 kDa) expressed early in gametocytogenesis, remaining associated with the surface of the emerged gamete after being ingested in a blood meal by a mosquito.8,9 The current vaccine candidate encompasses domain 1 of Pfs230 cloned and expressed in P. pastoris (referred to as Pfs230D1).10 Previous experiments demonstrated male gametes with a disrupted Pfs230 gene are unable to interact with erythrocytes and form exflagellation centers.11 Antibodies raised against Pfs230 bind the surface of gametes, reducing P. falciparum infectivity to mosquitoes, and can lyse gametes in the presence of complement.12 Anti-Pfs230 antibodies have also been observed in naturally exposed individuals.2,13,14 These data collectively supported Pfs25 and Pfs230 as important promising TBV candidates to advance into clinical trials.
TBV clinical development has focused on improving immunogenicity of Pfs25 and Pfs230D1 through conjugation to various carrier proteins and formulation with different adjuvants. Recent studies have demonstrated immunogenicity of these antigens can be significantly enhanced when conjugated to carrier proteins such as OMPC (the outer-membrane protein complex of Neisseria meningitidis) or rEPA (recombinant nontoxic Pseudomonas aeruginosa ExoProtein A) to form nanoparticles.15,16,17,18 Pfs25 formulations with adjuvants such as Montanide ISA 51 or Alhydrogel have been evaluated in phase 1 clinical trials.3,19 Pfs25 monomer formulated in ISA 51 adjuvant was found to be too reactogenic for further development.3 Pfs25-EPA formulated on Alhydrogel was well tolerated and immunogenic in both US naive volunteers19 and Mali exposed populations,20 but questions remained as to whether it was sufficiently potent and durable to reduce malaria transmission. Pfs230D1-EPA was recently shown to induce superior activity compared to Pfs25-EPA when formulated on Alhydrogel in US naive populations10 and has now advanced to phase 1 (clinicaltrials.gov ID NCT02334462) and phase 2 community field trials (clinicaltrials.gov ID NCT03917654) in Mali.
Preclinical evidence suggested TBV co-administrations might enhance vaccine activity,21 and we previously proposed that Pfs25 should be assessed in co-administration with other antigens to improve human vaccine activity.20 We evaluated Pfs25-EPA co-administered with Pfs230D1-EPA (both formulated in Alhydrogel) in US naive populations and found that co-administration conferred no advantage over single antigens.10 We investigate here whether this pattern remains consistent with other adjuvants.
The Adjuvant System AS01 from GSK emerged as a promising option due to its ability to safely enhance both humoral and cellular responses. The pediatric dose, called AS01E, is currently used for formulation of RTS,S,22,23,24,25,26 while the adult dose, called AS01B, is used in the licensed shingles vaccine SHINGRIX. AS01 is a liposomal preparation that incorporates the Toll-like receptor 4 (TLR4) ligand 3-O-desacyl-4′-monophosphoryl lipid A (MPL) and the saponin fraction Quillaja saponaria 21 (QS-21).23 Here, we report preclinical studies using nanoparticles Pfs25-EPA and Pfs230D1-EPA17,27 formulated with AS0123 as single antigens or as a co-administered antigen combination. We tested quality and stability of the formulations and evaluated immunogenicity and transmission-blocking activity (TBA) compared to other formulations including Alhydrogel and (for Pfs25-EPA) the alternative GSK adjuvant, AS04, that contains an aluminum salt and MPL.28 This comparison allowed us to evaluate the single TLR4 immunostimulant on a known stable delivery platform for Pfs25-EPA (aluminum salt) versus AS01 containing both MPL and the saponin QS-21 immunostimulants in a liposomal platform.
Results
Formulation studies
A series of formulation studies including assessments of aspecific adsorption, nanoparticle integrity, aggregation, stability, and liposome integrity (described in the supplementary text) confirmed that each antigen formulation was stable at varying temperatures. Detailed outcomes of each formulation study are provided in the supplementary text and presented in Figures S1–S7.
Study 1: Evaluation of Pfs25 monomer and nanoparticles in various adjuvants
Pfs25 vaccine formulations in AS01, AS04, Alhydrogel, as well as unconjugated Pfs25 monomer in AS01, were evaluated for immunogenicity and functional activity in CD-1 mice.
Immunogenicity in CD-1 mice
Antibody responses against Pfs25-EPA formulated in different adjuvants and administered at different dosages were measured by ELISA. High-dose groups were followed for 223 days (for Pfs25M-EPA/Alhydrogel and Pfs25/AS01) or 230 days (for Pfs25-EPA/AS01 and Pfs25-EPA/AS04) to determine duration of responses. On day 42 and for all doses tested, Pfs25-EPA formulated in AS01 induced significantly higher antibody levels than unconjugated Pfs25 formulated in AS01 (p < 0.0001 for each dose); both GSK vaccine formulations (AS01 and AS04) induced significantly higher antibody levels than Pfs25-EPA formulated in Alhydrogel (p < 0.05 for low and medium doses; p < 0.001 for high dose) (Figure 1A). In analyses of the decay rate between day 42 and day 223/230, titers induced by Pfs25-EPA formulated in AS01 were more durable than those induced by unconjugated Pfs25 formulated in AS01 (p < 0.05); no significant differences were observed among other formulations (Figure 1B).
Figure 1.
Immunogenicity of Pfs25 with or without conjugation to EPA, formulated with AS01, AS04, or Alhydrogel adjuvants, at varying doses in mice
(A) Antibody levels (ELISA units) are presented as box (median with lower and upper quartiles) and whisker (minimum to maximum) plots with individual data points overlaid, grouped by dose, and colored according to formulation and adjuvant. Significant differences were assessed between vaccine groups within each dosing group by Kruskal-Wallis with Dunn’s test for multiple comparisons. Significant Dunn’s adjusted p values are presented as asterisks: ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001.
(B) In animals that received 3 μg vaccine doses, antibody responses were measured to assess durability over 223 or 230 days post-vaccination, presented as medians with error bars indicating interquartile range. Differences between antibody decay rates resulting from each vaccine were assessed by comparing slopes of linear regression lines; significant difference is indicated by single asterisk (∗p < 0.05) between the Pfs25-EPA/AS01 and Pfs25/AS01 group.
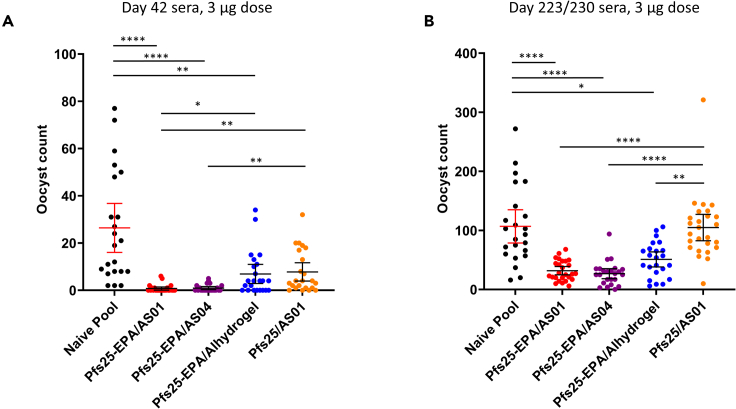
Serum functional activity post-vaccination and end of study
To assess TBA induced by vaccines, standard membrane feeding assays (SMFAs) were performed on pooled, heat-inactivated serum samples collected at day 42 (2 weeks post-dose 2) and at end of study (day 223 or day 230; ∼28 weeks post-dose 2), from CD-1 mice that received the 3 μg dose of Pfs25 vaccine formulations. At both time points, antisera against all conjugated Pfs25-EPA nanoparticles significantly reduced oocyst counts when compared to naive controls, unlike antisera against the Pfs25 monomer (Figure 2). At day 42, Pfs25-EPA/AS01 showed significant oocyst reduction compared to Pfs25-EPA/Alhydrogel, but the difference between these groups was not statistically significant at end of study (Figure 2A). At end of study, all conjugated Pfs25-EPA nanoparticles induced significantly greater serum TBA compared to both naive control and the Pfs25 monomer in AS01 (Figure 2B).
Figure 2.
Pfs25 conjugated nanoparticles in different adjuvants, but not unconjugated monomer in AS01, induce durable functional activity
Standard membrane feeding assays (SMFA) were performed to evaluate Pfs25 vaccine-induced transmission-reducing activity (oocyst reduction in mosquito midguts). SMFA were performed on (A) Day 42 and (B) at end of Study 1 (Day 223 or 230) in mice that received 3 µg vaccine doses. Data are presented as mean with error bars indicating 95%CI. Differences in SMFA oocyst counts between adjuvants were analyzed by Kruskal-Wallis with Dunn’s test for multiple comparisons. Significant Dunn’s adjusted p-values are presented as asterisks: ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p ≤ 0.0001.
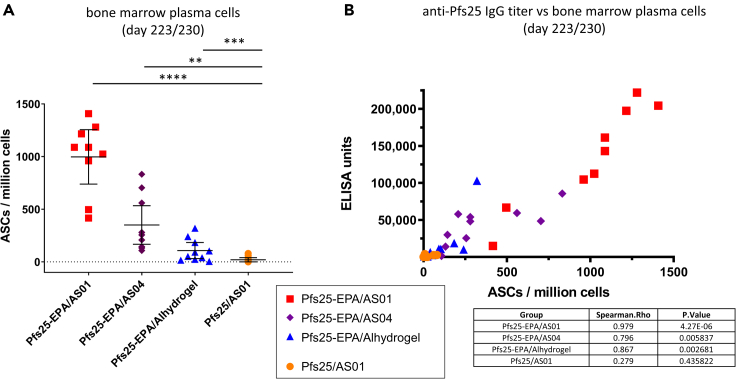
ELISpot analysis of bone marrow plasma cells (BMPCs)
BMPCs were recovered from 3 μg-dose groups on day 42 and at the end of study 1 (day 223 or day 230). Similar to the results for day 42 (data not shown), the mean number of antibody-secreting cells (ASCs)/million bone marrow cells at the end of the study was greatest (997) in the Pfs25-EPA/AS01-formulated group, and all EPA-conjugated antigens (regardless of adjuvant) induced significantly higher ASCs than the Pfs25 monomer in AS01 (Figure 3). AS04 produced a substantially lower mean number of ASCs (350), followed by Alhydrogel (∼30), but these differences were not statistically significant (Figure 3A). These BMPC levels correlated with ELISA units for all Pfs25 immunogens except the Pfs25 monomer (Figure 3B).
Figure 3.
Pfs25-specific bone marrow plasma cell numbers were highest with Pfs25-EPA nanoparticle formulated in AS01
(A) Bone marrow plasma cells induced by Pfs25 vaccine formulations were quantified by ELISpot; data are presented as mean with error bars indicating 95% CI, and differences between vaccine groups were assessed by Kruskal-Wallis with Dunn’s test for multiple comparisons. Significant Dunn’s adjusted p values are presented as asterisks: ∗∗p < 0.01; ∗∗∗p = 0.001; ∗∗∗∗p < 0.0001.
(B) Anti-Pfs25 IgG titers by bone marrow plasma cell count are presented. Spearman’s correlation values are presented in the embedded table.
Study 2: Evaluation of Pfs25 and Pfs230D1 conjugated nanoparticles, alone or as a co-administered combination, in AS01 versus Alhydrogel
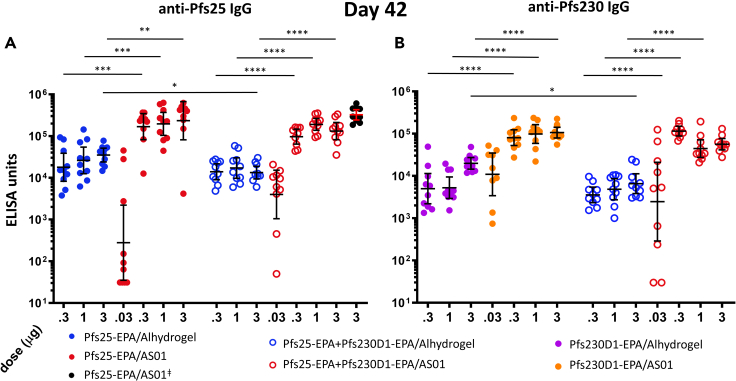
Antibody responses induced by Pfs25-EPA, by Pfs230D1-EPA, or by the combination (co-administered) were compared when formulated in either Alhydrogel or AS01 and administered to CD-1 mice. At each dose tested, AS01 formulations induced significantly higher anti-Pfs25 and anti-Pfs230D1 immunoglobulin G (IgG) levels (Figure 4) than the corresponding Alhydrogel formulations measured 2 weeks post-dose 2 (study day 42). In general, co-administration of Pfs25-EPA and Pfs230D1-EPA did not significantly alter the IgG levels measured by ELISA against either antigen, when compared to immunization with Pfs25 or Pfs230D1 antigen alone with AS01 (Figure 4). However, at the highest antigen dose (3 μg) with Alhydrogel, both anti-Pfs25 and anti-Pfs230D1 IgG levels were significantly lower in animals that received co-administered Pfs25+Pfs230D1 versus Pfs25 or Pfs230D1 alone (p < 0.05).
Figure 4.
AS01 induces higher IgG levels than Alhydrogel against Pfs25-EPA and Pfs230D1-EPA, when tested as single or co-administered antigens
(A) Anti-Pfs25 IgG and (B) anti-Pfs230 IgG antibody levels are presented as geometric means with error bars indicating 95%CI, with individual data points overlayed, grouped by dose and colored by adjuvant (blue/purple = Alhydrogel, red/orange = AS01 (containing a dose of 2.5 µg MPL/2.5 µg QS-21). Solid dots indicate mouse groups receiving Pfs25 alone or Pfs230 alone; empty dots indicate mouse groups receiving Pfs25 combined with Pfs230. ǂBlack dots indicate Pfs25-EPA alone formulated with the higher 5 µg MPL/5 µg QS-21 dose of AS01. Differences between adjuvant formulations for each dose received were analyzed by Kruskal-Wallis with Dunn’s test for multiple comparisons. Significant Dunn’s adjusted p values are presented as asterisks: ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p ≤ 0.0001.
See also Figures S8–S10.
Study 3: Evaluation of Pfs230D1 monomer versus conjugated nanoparticle in saline and Alhydrogel
We assessed the effect of protein-protein conjugation on the immunogenicity of Pfs230D1 antigen in saline and Alhydrogel formulations. We observed that Pfs230D1-EPA induces higher antibody levels than unconjugated Pfs230D1 monomer in either formulation, and these differences were significant at the 2.5 μg Pfs230D1 antigen dose (Figure S8).10
Study 4: Evaluation of Pfs230D1 conjugated nanoparticle in AS01 versus Alhydrogel
To confirm the effects of adjuvant on Pfs230D1-EPA immunogenicity, antibody responses were compared when Pfs230D1-EPA was formulated in either Alhydrogel or AS01 and administered to CD-1 mice. ELISA analyses confirmed superior immunogenicity of Pfs230D1-EPA formulated in AS01 compared to Alhydrogel (Figure S9), with total IgG levels similar to those measured in Study 2.
Study 5: Evaluation of two independent Pfs230D1-EPA conjugate lots in AS01
We assessed total IgG and IgG subclasses induced by two separate preparations of Pfs230D1-EPA (Research and Reference Lots) formulated with AS01 at 0.03, 0.3, 1, and 3 μg doses. Total IgG levels in these mice (Figure S10) were similar between the two vaccine lots and comparable to those measured earlier for our Pfs230D1-EPA/AS01 formulations at similar doses (Figures 4 and S9). These studies confirmed durability of anti-Pfs230D1 antibodies by ELISA through at least 18 weeks post-vaccination (Figure S10).
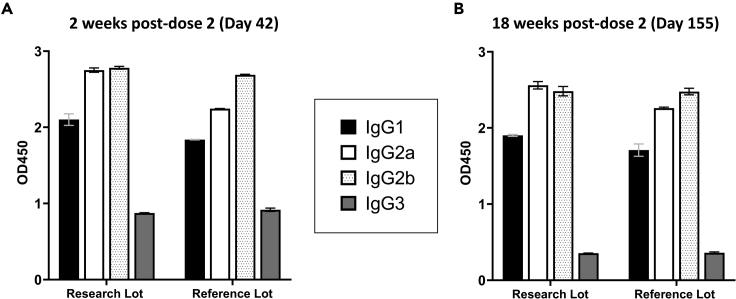
In our prior studies, we showed that mice immunized with Pfs230D1-EPA/Alhydrogel developed a dominant IgG1 isotype response,16,29 which is non-complement fixing in mice. Conversely, the profile induced by Pfs230D1-EPA/AS01 in this study indicated a mixed subclass response with preferential IgG2a/2b antibodies (Figure 5), similar to our prior studies of Pfs230D1-EPA formulated with the AS01-biosimilar adjuvant, Glucopyranosyl Lipid A-liposome Quillaja saponaria 21 (GLA-LSQ).29 In the current study, IgG1, IgG2a, IgG2b, and IgG3 levels were comparable at both 2 and 18 weeks post-dose 2 and between Research and Reference Lots.
Figure 5.
IgG subclasses show a mixed profile after immunization with Pfs230D1-EPA/AS01 (0.3 μg) in CD-1 mice
Sera from CD-1 mice immunized with Research and Reference Lots of Pfs230D1-EPA/AS01 were collected on (A) Day 42 (2 weeks post-dose 2) and on (B) Day 155 (18 weeks post-dose 2), and pooled to determine IgG subclasses. Research Lot refers to the vaccine formulation used in these preclinical studies, while the Reference Lot more closely resembles the cGMP-manufactured material for human use. Data are presented as means with error bars indicating standard error of the mean of two technical replicates.
Pooled sera from CD-1 mice immunized with either of two lots of Pfs230D1 conjugated nanoparticles in AS01 in study 5 were also studied in SMFA in the presence of complement. The results confirmed serum functional activity at 2 weeks post-vaccination, and activity persisted at least 18 weeks post-vaccination with antigen doses ranging from 0.3 to 3 μg Pfs230D1 (Figure S11).
Functional activity in the absence or presence of complement
Pfs230D1 anti-serum or antibody TBA has been shown to be complement dependent, including for Pfs230D1-EPA vaccine.10,30 SMFAs were performed without or with human complement. Each sample was a pool of sera from the group of 10 mice on day 42 (2 weeks post-dose 2) from study 2.
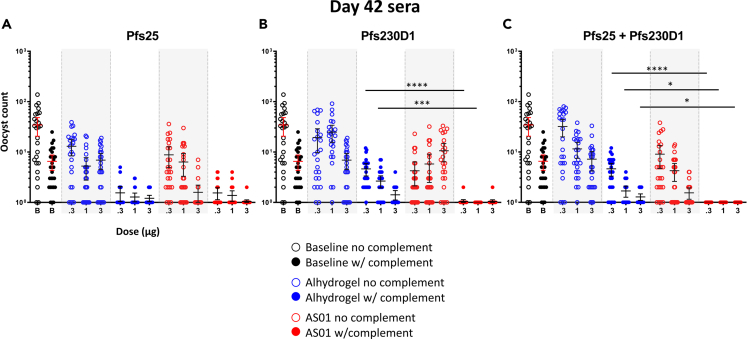
In the absence of complement, groups receiving Pfs25, Pfs230D1, or co-administered Pfs25+Pfs230D1 in either adjuvant did not significantly differ in their serum functional activity to reduce oocyst levels on mosquito midguts (Figure 6). However, in the presence of complement, AS01 formulations conferred significantly greater serum oocyst reduction activity than Alhydrogel formulations of Pfs230D1 alone (Figure 6B) and co-administered Pfs25+Pfs230D1 (Figure 6C), but not Pfs25 alone (Figure 6A). Pfs230D1-EPA/AS01 enhanced serum functional activity (compared to Pfs230D1-EPA/Alhydrogel) after vaccination with 0.3 and 1 μg doses, with significantly lower oocyst counts in the SMFA test (p < 0.0001, p < 0.001, respectively) (Figure 6B). Co-administration of Pfs25-EPA + Pfs230D1-EPA in AS01 induced 100% blocking activity at doses of 0.3, 1, and 3 μg, when assayed in the presence of intact human complement (Figure 6C). These data are presented in terms of functional activity (transmission-reducing activity [TRA] and TBA) in Figure S12.
Figure 6.
Functional activities of Pfs25-EPA and Pfs230D1-EPA are dependent on the presence of complement
Standard membrane feeding assays (SMFA) were performed to evaluate (A) Pfs25 and (B) Pfs230D1, and (C) Pfs25+Pfs230D1 vaccine-induced transmission-reducing activity (oocyst reduction in mosquito midguts). Sera from all animals within vaccine dosing groups (see Figure 4) were pooled for feeding assays. All sample pools were run in the same assay; baseline points are identical in all panels. Assays were run in either the presence or absence of complement. Data are presented as oocyst counts on a log10 scale; an offset of +1 was applied to all values to account for raw values of 0. Lines represent means with error bars indicating 95% CI. Differences in SMFA oocyst counts between adjuvants were analyzed by Kruskal-Wallis with Dunn’s test for multiple comparisons. Significant Dunn’s adjusted p-values are presented as asterisks: ∗p < 0.05; ∗∗∗p < 0.001; ∗∗∗∗p ≤ 0.0001.
See also Figures S11 and S12.
Discussion
Pfs25 and Pfs230 are two leading malaria TBV candidate antigens, targeting sexual stages of the parasite within the mosquito host. When conjugated to carrier protein EPA and formulated in Alhydrogel, Pfs25 has been shown to be well tolerated and immunogenic in phase 1 trials in both US naive volunteers19 and a malaria-exposed population in Mali20; Pfs230D1 has been shown to induce superior activity to Pfs25 in US naive volunteers10 and has progressed to phase 2 field trials in Mali (clinicaltrials.gov ID NCT02334462; NCT03917654). TBV development is now focused on enhancing immunogenicity, potency, and durability of response by testing antigen formulations with different carrier proteins and adjuvants. GSK Adjuvant Systems are promising platforms that have successfully enhanced immunogenicity of pre-erythrocytic malaria vaccines (i.e., WHO-recommended RTS,S) and might provide a common platform to combine pre-erythrocytic and transmission-blocking antigens toward a vaccine to reduce malaria transmission (VRMT). Here, we conducted preclinical studies comparatively assessing Pfs25 and Pfs230D1, formulated alone or in combination with Alhydrogel, AS01, or AS04 (for Pfs25 only).
We first assessed the stability of TBV antigens formulated in GSK adjuvants. Formulations of Pfs25-EPA and Pfs230D1-EPA in liposomal adjuvant AS01 proved to be stable at varying temperatures. Based on the observation that protein content decreased over time during our aspecific adsorption assessment, we limited storage of diluted protein to less than 24 h at +4°C for each antigen and in practice used preparations within 6 h. In addition, as differential scanning calorimetry results showed both antigens underwent transitions before 40°C, we limited working temperatures to approximately 20°C–25°C (ambient temperature) for up to 30 min to allow for injections at ambient. In practice all formulations were stored at 2°C–8°C for a period of less than 6 h from time of formulation to injection. Analyses were performed on samples taken on each day of injection (particle sizing and SDS-PAGE analysis of formulated antigens) to confirm formulation stability at the post-injection time point as compared with both reference standard and pre-immunization samples. All formulations remained stable from the time of formulation through immunization for studies 1, 2, 4, and 5 (see supplementary text).
We then assessed immunogenicity of Pfs25 with or without conjugation to EPA and formulated with Alhydrogel, AS01, or AS04 adjuvants at varying doses in CD-1 mice (study 1; Figure 1, Figure 2, Figure 3). Two weeks post-dose 2 and at each dose tested, the nanoparticle Pfs25-EPA adjuvanted with AS01 produced significantly higher titers to Pfs25 antigen than the Pfs25 monomer with AS01 or Pfs25-EPA on Alhydrogel. Antibody responses to Pfs25-EPA were higher at all time points in the AS01 group than in the AS04 group. In addition, antibody responses to the Pfs25-EPA nanoparticle in AS01 decayed at a significantly slower rate than the Pfs25 monomer in AS01. An ELISpot analysis of Pfs25-specific BMPCs showed the highest number of BMPCs were induced by AS01, followed by AS04 and Alhydrogel. The Pfs25-EPA nanoparticle induced higher levels of BMPCs (which correlated with ELISA units) than the Pfs25 monomer in AS01 (which did not correlate with ELISA units).
We subsequently compared immunogenicity conferred by Pfs25-EPA and Pfs230D1-EPA, formulated on Alhydrogel or AS01 and delivered either alone or co-administered (study 2; Figures 4 and 6). AS01 formulations induced higher anti-Pfs25 and anti-Pfs230D1 IgG titers versus Alhydrogel for all doses of antigen. Varying the dose of AS01 did not significantly affect titers for Pfs25. High-dose Pfs25 and Pfs230D1 single antigens gave higher titers than the co-administered Pfs25+Pfs230D1 formulations on Alhydrogel, but not in AS01. In SMFA testing, co-administered antigens had no evidence of increased activity over single antigens, as has also been reported in nonhuman primate studies and human trials,10,31 albeit here the Pfs230D1 single antigen induced nearly complete TBA versus the co-administered Pfs25+Pfs230D1 combination that induced complete TBA. This might be explained in part by the high activity of the antigens alone, particularly when formulated in AS01, which is difficult to improve on when measured by SMFA, and also by negative immunologic interactions between the combined antigens, as seen with the Alhydrogel formulations (Figure S12). In an independent study (study 4; Figure S9), we confirmed that AS01 formulations of Pfs230D1-EPA induced higher IgG titers versus Alhydrogel formulations for all doses of antigen.
Finally, we examined Pfs230D1-EPA/AS01 a third time (study 5; Figures 5, S10, and S11) to compare total IgG and IgG subclass responses induced by two independently prepared Pfs230D1-EPA vaccine lots. Total IgG responses measured in our standardized ELISA were consistent between the two vaccine lots, as well as between the three studies that assessed Pfs230D1-EPA/AS01. In assessing IgG subclasses, Pfs230D1-EPA/AS01 generated a balanced response, with relatively high IgG1, IgG2a, and IgG2b, as well as relatively low levels of IgG3 that decayed disproportionately to the other subclasses between 2 and 18 weeks post-vaccination. The Research Lot used in these preclinical studies was comparable to the Reference Lot, which resembles Good Manufacturing Practices (GMP)-manufactured material for human use, according to their isotype profile in AS01 adjuvant.
Human complement has enhanced serum functional activity induced by Pfs230D1-EPA in human trials.10 In the studies here, the superiority of AS01 adjuvant over Alhydrogel for inducing functional Pfs230D1 anti-sera activity was observed in the presence but not the absence of complement (study 2; Figure 6). Functional activity of Pfs230D1-EPA/AS01 antisera was confirmed in a second study, where it persisted for at least 18 weeks post-vaccination with Pfs230D1 doses of 0.3 μg and higher (study 5; Figure S10).
This study had limitations. Preclinical studies in animals are not fully predictive of human responses to vaccines and adjuvants, which can only be conclusively determined in clinical trials. Nevertheless, the dosing for AS01 in mice had been recommended for mouse studies by the manufacturer. Functional serum activity was measured in the SMFA, the gold standard assay for this purpose; however, the reproducibility of results is greatest with high levels of functional activity and is less reliable below 50%.
Overall, these results show that conjugation to carrier protein EPA confers greater immunogenicity and durability than unconjugated monomers of Pfs25 and Pfs230D1, and further, formulation of Pfs25 and Pfs230D1 conjugates in AS01 induces greater immunogenicity and functional activity compared to Alhydrogel at the adjuvant doses tested. TBV development should continue to examine alternate adjuvants and carriers to produce a more highly efficacious vaccine toward malaria elimination. AS01 is a promising platform for multicomponent malaria vaccines to pursue this goal.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| 4B7 monoclonal antibody | LMIV, NIAID, NIH | N/A |
| Coat Anti-mouse IgG1 | Southern Biotech. | Cat No 1073-04 |
| Coat Anti-mouse IgG2a | Southern Biotech. | Cat No 1083-04 |
| Coat Anti-mouse IgG2b | Southern Biotech. | Cat No 1093-04 |
| Coat Anti-mouse IgG2c | Southern Biotech. | Cat No 1077-04 |
| Coat Anti-mouse IgG3 | Southern Biotech. | Cat No 1130-04 |
| Biological samples | ||
| AB + naive human serum | Interstate Blood Bank, Inc., Memphis, TN, USA | 1/22/2016 |
| Diagnostic Red Blood Cells Group (O+) Anticoagulant: CPD | GRIFOLS BioSupplies, (formerly Interstate Blood Bank, Inc., 5065 Covington Way, Memphis TN, USA; | No cat number. |
| Chemicals, peptides, and recombinant proteins | ||
| Pfs25 | LMIV, NIAID, NIH | Ref #17 |
| Pfs25-EPA | LMIV, NIAID, NIH | Ref #17 |
| Pfs230D1-EPA | LMIV, NIAID, NIH | Ref #27 |
| 4X NuPAGE™ LDS Sample Buffer | ThermoFisher Scientific, Invitrogen | Cat: NP0007 |
| Critical commercial assays | ||
| Micro BCA Protein Assay Kit | ThermoFisher Scientific, Pierce | Cat: 23235 |
| ELISpot Flex: Mouse IgG (ALP) (ELISpot reagents and kit) | Mabtech, Inc | 3825-2A |
| Experimental models: Organisms/strains | ||
| CD-1 mice | Charles River | https://www.criver.com/products-services/find-model/cd-1r-igs-mouse?region=3611 |
| Anopheles stephensi (Nijmegen) | from Joep Meiwissen, The Catholic University of the Netherlands, Neimegen Netherlands and maintained since 1985 at NIH | |
| Plasmodium falciparum, strain NF54(SH) | Culture obtained in 2006 from Sanaria | |
| Software and algorithms | ||
| Prism | GraphPad Software Inc., La Jolla, CA | https://www.graphpad.com/ |
| R version 3.6.3. | R Core Team and the R Foundation for Statistical Computing | version 3.6.3 |
| SoftMax Pro7software SMp7 | Molecular Devices | SMp7 |
| Other | ||
| AS01 | Glaxo Smith Kline | Supplied by GSK and from SHINGRIX kit A4X57 |
| Alhydrogel® 2% | CRODA, Denmark | 4883, 5069, |
| AS04 | Glaxo Smith Kline | Supplied by GSK |
| Immuno 4 HBX plates | ThermoFisher Scientific | Lot 3855 |
| Corning polystyrene flat bottom reading plate | Corning | Product Number: 3679 |
| Zetasizer Nano-ZS, ZEN 3600 particle size analyzer | Malvern Panalytical, Inc. | Zetasizer Nano-ZS, ZEN 3600 |
| Immunospot analyzer | CTL, Shaker Heights, OH | |
| Spectramax M3 microplate reader | Molecular Devices Co | Spectramax M3 |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Patrick E. Duffy (patrick.duffy@nih.gov).
Materials availability
This study did not generate new unique reagents.
Experimental model details
All animal studies used healthy, female CD-1 mice aged 5 to 6 weeks old, and were performed in an AAALAC-accredited facility in accordance with an animal study protocol guided and approved by the Institutional Animal Care and Use Committee (IACUC) at the National Institutes of Health. Animals (obtained from Charles River Laboratories, Wilmington, MA or from Taconic Laboratories, Hudson, NY) were housed in SPF-filtered micro-isolators; nestlets were used for enrichment with food and water administered ad libitum.
Method details
Recombinant proteins and conjugated vaccine candidates
PpPfs25M is a Pichia-expressed recombinant Pfs25 with a molecular mass of 18,713 Da. EcEPA is an E. coli–expressed recombinant protein with molecular mass of 66,975 Da. The Pfs25M-EPA conjugate was produced by reaction between thiolated PpPfs25M and maleimide-activated EcEPA, followed by purification using size-exclusion chromatography.
PpPfs230D1M is a Pichia-expressed recombinant subsegment (S542–G736) of Pfs230 with a molecular mass of 21,854 Da. EcEPA is an E. coli–expressed recombinant protein with molecular mass of 66,975 Da. The Pfs230D1M-EPA conjugate was produced by reaction between thiolated PpPfs230D1M and maleimide-activated EcEPA, followed by purification using size-exclusion chromatography.
Research lot refers to the vaccine formulation used in these preclinical studies, while the Reference Lot used in the isotyping studies more closely resembles the cGMP-manufactured material for human use.
Formulation studies
Compatibility studies between Pfs25-EPA and Pfs230D1-EPA nanoparticles and AS01 were performed to determine formulation stability. Assessments included aspecific adsorption, protein content, and intrinsic fluorescence. Nanoparticle, liposome, and final formulation integrity were performed using SDS-PAGE and a high throughput panel including differential scanning calorimetry (DSC). Nephelometry, turbidimetry and dynamic light scattering (DLS) were performed on formulations prepared in duplicate. Detailed methods for each formulation study are provided in the supplementary text.
Immunization and sampling of CD-1 mice
Except where stated, Alhydrogel was delivered to CD-1 mice at a final dose of 80 μg; formulations were analyzed for antigen binding to Alhydrogel, and all products were 100% bound. Animals were bled via mandibular vein during the study, with a final bleed via cardiac puncture at end of study. No adverse effects were observed during study timepoints. Samples from these studies were tested for antibody responses by ELISA, cellular responses by ELISpot, and functional activity by Standard Membrane Feeding Assay (SMFA), to compare and evaluate various TBV antigens formulated with clinically tested adjuvant systems from GSK.
Study 1: Evaluation of Pfs25 monomer and nanoparticles in various adjuvants
In Study 1, groups of 10 or 15 CD-1 mice were immunized by intramuscular (IM) injection on Days 0 and 28 with 50 μL of Pfs25 or Pfs25-EPA adjuvanted formulations. All 10 animals in each low (0.3 μg) and middle (1 μg) dose group and 5 of the 15 animals in the high dose (3 μg) groups were terminated on Day 42. The remaining 10 animals in the high dose groups were sampled every 28 days through Day 223 (for Pfs25M-EPA/Alhydrogel and Pfs25/AS01) and Day 230 (for Pfs25-EPA/AS01 and Pfs25-EPA/AS04) to track the duration of the Pfs25 antibody response. In this study, AS01 was delivered at a final dose per animal per immunization day of 5 μg MPL and 5 μg QS-21 in the liposomal platform, AS04 was delivered at a final dose of 5 μg MPL/50 μg aluminum hydroxide.
Study 2: Evaluation of Pfs25 and Pfs230D1 conjugated nanoparticles, alone or as a co-administered combination, in AS01 versus Alhydrogel
In Study 2, groups of 10 mice were immunized IM on Days 0 and 28 with 50 μL of Pfs25-EPA, Pfs230D1-EPA, or with both formulations co-administered in separate legs. Antigen doses ranging from 0.03 to 3 μg of each target antigen were compared. In this study, an AS01 dose containing 2.5 μg MPL/2.5 μg QS-21 was used for individual formulations. Co-administered groups received a 5 μg MPL/5 μg QS-21 AS01 adjuvant dose, divided between the two syringes. A single group of Pfs25-EPA with 5 μg MPL/5 μg QS-21 AS01 was included to allow comparisons to Study 1.
Study 3: Evaluation of Pfs230D1 monomer versus conjugated nanoparticle in saline and Alhydrogel
In Study 3, groups of 10 mice were immunized IM with Pfs230D1 or Pfs230D1-EPA in a volume of 50 μL on days 0 and day 28 regimen. Pfs230D1 antigen was administered at doses of 0.5 μg (Pfs230D1 monomer and Pfs230D1-EPA) or 2.5 μg (Pfs230D1 monomer). Alhydrogel was delivered at a final dose of 45 μg.
Study 4: Evaluation of Pfs230D1 conjugated nanoparticle in AS01 versus Alhydrogel
In Study 4, groups of 10 or 15 mice were immunized IM on Days 0 and 28 with 50 μL of Pfs230D1-EPA. Two (Pfs230D1) antigen doses, 0.03 and 3 μg were compared. AS01 dose containing 2.5 μg MPL/2.5 μg QS-21 was used for individual formulations.
Study 5: Evaluation of two independent Pfs230D1-EPA conjugate lots in AS01
In Study 5, groups of 10 mice were immunized IM on Days 0 and 28 with 50 μL of Pfs230D1-EPA, using either of two independent lots of Pfs230D1-EPA (Research Lot and Reference Lot). Four antigen doses ranging from 0.03 to 3 μg of each target antigen were compared. An AS01 dose containing 2.5 μg MPL/2.5 μg QS-21 was used for individual formulations.
Samples from these studies were variously examined in the following assays:
-
•
Enzyme-linked Immunosorbent Assay (ELISA).
Antibody responses were measured by ELISA performed on Day 42 sera (2 weeks post-dose 2). Immulon 4 HBX flat bottom microtiter plates (Dynex Technologies) ELISA plates were coated with 1 μg/mL of antigen in a volume of 100 μL per well in carbonate coating buffer (pH 9.6) overnight at 4°C. After blocking in 5% skim milk in TBS blocking buffer in a volume of 320 μL per well for 2 h, samples were serially diluted in TBS/5% milk and plated in triplicate in a volume of 100 μL per well and incubated at room temperature for 2 h. Plates were washed 4 times and alkaline phosphatase–labeled goat anti-mouse IgG (H + L), goat anti-human IgG (H + L), or goat anti-monkey phosphatase labeled secondary antibody (Seracare Life Sciences, catalog 5220-0303) was added in a volume of 100 μL per well and incubated at room temperature for 2 h. After washing 4 times, dissolved phosphatase substrate tablets (MilliporeSigma) were added in a volume of 100 μL per well and plates were incubated for 20 min before the optical density (OD) was measured with a Spectramax 340PC (Molecular Devices). Each ELISA plate contained an internal serum standard from which a 4-parameter curve was calculated with Softmax software. According to laboratory standard operating procedures, any samples for which ELISA results from triplicate wells exceeded a prespecified coefficient of variation were repeated. ELISA units were assigned to test samples based on the sera dilution that gave an OD of 1.0, adjusted to the internal standard.
This assay uses standardized controls to account for interassay variability and provides reliable and consistent results for both preclinical and clinically obtained sera samples.
-
•
Standard Membrane Feeding Assays (SMFA).
To determine functional activity of Pfs25-EPA and Pfs230D1-EPA, a pool of 20 μL from the Day 42 sera from each of the high dose (3 μg) groups (Study 1) or from each group (Study 2 and Study 5) was diluted with 40 μL of a naive AB + human serum pool (purchased from Interstate Blood Bank, Inc., Memphis, TN, USA) with and without complement (Study 2) or with complement only (Study 5) and tested for the ability to block oocyst development in the mosquito midgut. Serum was mixed with 100 μL of red blood cells infected with P. falciparum parasite culture and fed to mosquitoes that were dissected 8 days later. Further details of SMFA procedures have been previously described.32
-
•
ELISpot analysis of bone marrow plasma cells.
Bone marrow plasma cells were recovered from the 3 μg dose groups on Days 42 and at the end of Study 1 (Day 223 and Day 230). The mouse IgG ELISpot was performed using reagents from Mabtech, Inc (Cincinnati, OH). Complete details of the assay are provided in the supplementary text.
-
•
Antibody Isotyping.
Sera from CD-1 mice in Study 5 immunized with a Research and Reference Lot of Pfs230D1-EPA antigen formulated with AS01 (2.5 μg MPL/2.5 μg QS-21) were collected on Day 42 (2 weeks post-dose 2) and on Day 155 (18 weeks post-dose 2), and pooled to determine IgG subclasses. Research lot refers to the vaccine formulation used in these preclinical studies, while the Reference Lot more closely resembles the cGMP-manufactured material for human use.
Quantification and statistical analysis
Statistical analysis was performed using GraphPad PRISM (San Diego, CA) software, version 8.0.1, and R version 3.6.3.33 Differences in ELISA titers between groups were analyzed by Kruskal-Wallis with Dunn’s test for multiple comparisons on Day 42 for both Study 1 and Study 2. p values < 0.05 were considered significant. Differences in SMFA oocyst counts between adjuvants were analyzed by Kruskal-Wallis with Dunn’s test for multiple comparisons. Differences between ELISpot ASC counts between vaccine formulations were analyzed by Kruskal-Wallis with Dunn’s test for multiple comparisons.
Differences in repeated measures of antibody levels over time were compared between groups by GEE modeling with exchangeable correlation. Differences in the durability of response were analyzed by comparisons of linear regression best fit slopes, using an established method for comparing two independent samples.34 For Study 4, differences in ELISA titer between groups were analyzed by Mann-Whitney for Day 42 and 154 results. p values <0.05 were considered significant.
Sample sizes are indicated by individual data points or indicated in the figure legend.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health. We are grateful to GSK for providing the Adjuvant Systems AS01 and AS04, as well as for preliminary stability testing, support, and guidance for its formulation and use for the preclinical studies.
Author contributions
Conceptualization: KMR, YW, and DLN.
Methodology: KMR and YW.
Formal Analysis: RDM.
Investigation: EKB, LEL, OM, CA, IZ, and KMR.
Writing – Original Draft: KMR.
Writing – Review and Editing: JPG, KMR, and PED.
Visualization: JPG and KMR.
Supervision: PED.
Declaration of interests
The authors declare no competing interests.
Inclusion and diversity
We support inclusive, diverse, and equitable conduct of research. One or more of the authors of this paper self-identifies as an underrepresented ethnic minority in their field of research or within their geographical location. One or more of the authors of this paper self-identifies as living with a disability.
Published: June 22, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.107192.
Supplemental information
Data and code availability
-
•
All data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.WHO . World Health Organization; 2021. World Malaria Report 2021.https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2021 [Google Scholar]
- 2.Chaturvedi N., Bharti P.K., Tiwari A., Singh N. Strategies & recent development of transmission-blocking vaccines against Plasmodium falciparum. Indian J. Med. Res. 2016;143:696–711. doi: 10.4103/0971-5916.191927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu Y., Ellis R.D., Shaffer D., Fontes E., Malkin E.M., Mahanty S., Fay M.P., Narum D., Rausch K., Miles A.P., et al. Phase 1 trial of malaria transmission blocking vaccine candidates Pfs25 and Pvs25 formulated with montanide ISA 51. PLoS One. 2008;3 doi: 10.1371/journal.pone.0002636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sidén-Kiamos I., Vlachou D., Margos G., Beetsma A., Waters A.P., Sinden R.E., Louis C. Distinct roles for pbs21 and pbs25 in the in vitro ookinete to oocyst transformation of Plasmodium berghei. J. Cell Sci. 2000;113:3419–3426. doi: 10.1242/jcs.113.19.3419. [DOI] [PubMed] [Google Scholar]
- 5.Baton L.A., Ranford-Cartwright L.C. Do malaria ookinete surface proteins P25 and P28 mediate parasite entry into mosquito midgut epithelial cells? Malar. J. 2005;4:15. doi: 10.1186/1475-2875-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tomas A.M., Margos G., Dimopoulos G., van Lin L.H., de Koning-Ward T.F., Sinha R., Lupetti P., Beetsma A.L., Rodriguez M.C., Karras M., et al. P25 and P28 proteins of the malaria ookinete surface have multiple and partially redundant functions. EMBO J. 2001;20:3975–3983. doi: 10.1093/emboj/20.15.3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stowers A.W., Keister D.B., Muratova O., Kaslow D.C. A region of Plasmodium falciparum antigen Pfs25 that is the target of highly potent transmission-blocking antibodies. Infect. Immun. 2000;68:5530–5538. doi: 10.1128/iai.68.10.5530-5538.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williamson K.C. Pfs230: from malaria transmission-blocking vaccine candidate toward function. Parasite Immunol. 2003;25:351–359. doi: 10.1046/j.1365-3024.2003.00643.x. [DOI] [PubMed] [Google Scholar]
- 9.Williamson K.C., Keister D.B., Muratova O., Kaslow D.C. Recombinant Pfs230, a Plasmodium falciparum gametocyte protein, induces antisera that reduce the infectivity of Plasmodium falciparum to mosquitoes. Mol. Biochem. Parasitol. 1995;75:33–42. doi: 10.1016/0166-6851(95)02507-3. [DOI] [PubMed] [Google Scholar]
- 10.Healy S.A., Anderson C., Swihart B.J., Mwakingwe A., Gabriel E.E., Decederfelt H., Hobbs C.V., Rausch K.M., Zhu D., Muratova O., et al. Pfs230 yields higher malaria transmission-blocking vaccine activity than Pfs25 in humans but not mice. J. Clin. Invest. 2021;131 doi: 10.1172/JCI146221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eksi S., Czesny B., van Gemert G.J., Sauerwein R.W., Eling W., Williamson K.C. Malaria transmission-blocking antigen, Pfs230, mediates human red blood cell binding to exflagellating male parasites and oocyst production. Mol. Microbiol. 2006;61:991–998. doi: 10.1111/j.1365-2958.2006.05284.x. [DOI] [PubMed] [Google Scholar]
- 12.Read D., Lensen A.H., Begarnie S., Haley S., Raza A., Carter R. Transmission-blocking antibodies against multiple, non-variant target epitopes of the Plasmodium falciparum gamete surface antigen Pfs230 are all complement-fixing. Parasite Immunol. 1994;16:511–519. doi: 10.1111/j.1365-3024.1994.tb00305.x. [DOI] [PubMed] [Google Scholar]
- 13.Healer J., McGuinness D., Carter R., Riley E. Transmission-blocking immunity to Plasmodium falciparum in malaria-immune individuals is associated with antibodies to the gamete surface protein Pfs230. Parasitology. 1999;119:425–433. doi: 10.1017/s0031182099005041. [DOI] [PubMed] [Google Scholar]
- 14.Ouédraogo A.L., Roeffen W., Luty A.J.F., de Vlas S.J., Nebie I., Ilboudo-Sanogo E., Cuzin-Ouattara N., Teleen K., Tiono A.B., Sirima S.B., et al. Naturally acquired immune responses to Plasmodium falciparum sexual stage antigens Pfs48/45 and Pfs230 in an area of seasonal transmission. Infect. Immun. 2011;79:4957–4964. doi: 10.1128/IAI.05288-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qian F., Wu Y., Muratova O., Zhou H., Dobrescu G., Duggan P., Lynn L., Song G., Zhang Y., Reiter K., et al. Conjugating recombinant proteins to Pseudomonas aeruginosa ExoProtein A: a strategy for enhancing immunogenicity of malaria vaccine candidates. Vaccine. 2007;25:3923–3933. doi: 10.1016/j.vaccine.2007.02.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scaria P.V., Rowe C.G., Chen B.B., Muratova O.V., Fischer E.R., Barnafo E.K., Anderson C.F., Zaidi I.U., Lambert L.E., Lucas B.J., et al. Outer membrane protein complex as a carrier for malaria transmission blocking antigen Pfs230. NPJ Vaccines. 2019;4:24. doi: 10.1038/s41541-019-0121-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shimp R.L., Jr., Rowe C., Reiter K., Chen B., Nguyen V., Aebig J., Rausch K.M., Kumar K., Wu Y., Jin A.J., et al. Development of a Pfs25-EPA malaria transmission blocking vaccine as a chemically conjugated nanoparticle. Vaccine. 2013;31:2954–2962. doi: 10.1016/j.vaccine.2013.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu Y., Przysiecki C., Flanagan E., Bello-Irizarry S.N., Ionescu R., Muratova O., Dobrescu G., Lambert L., Keister D., Rippeon Y., et al. Sustained high-titer antibody responses induced by conjugating a malarial vaccine candidate to outer-membrane protein complex. Proc. Natl. Acad. Sci. USA. 2006;103:18243–18248. doi: 10.1073/pnas.0608545103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Talaat K.R., Ellis R.D., Hurd J., Hentrich A., Gabriel E., Hynes N.A., Rausch K.M., Zhu D., Muratova O., Herrera R., et al. Safety and Immunogenicity of Pfs25-EPA/Alhydrogel(R), a Transmission Blocking Vaccine against Plasmodium falciparum: An Open Label Study in Malaria Naive Adults. PLoS One. 2016;11 doi: 10.1371/journal.pone.0163144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sagara I., Healy S.A., Assadou M.H., Gabriel E.E., Kone M., Sissoko K., Tembine I., Guindo M.A., Doucoure M., Niaré K., et al. Safety and immunogenicity of Pfs25H-EPA/Alhydrogel, a transmission-blocking vaccine against Plasmodium falciparum: a randomised, double-blind, comparator-controlled, dose-escalation study in healthy Malian adults. Lancet Infect. Dis. 2018;18:969–982. doi: 10.1016/S1473-3099(18)30344-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duffy P.E., Kaslow D.C. A novel malaria protein, Pfs28, and Pfs25 are genetically linked and synergistic as falciparum malaria transmission-blocking vaccines. Infect. Immun. 1997;65:1109–1113. doi: 10.1128/IAI.65.3.1109-1113.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chandramohan D., Zongo I., Sagara I., Cairns M., Yerbanga R.S., Diarra M., Nikièma F., Tapily A., Sompougdou F., Issiaka D., et al. Seasonal Malaria Vaccination with or without Seasonal Malaria Chemoprevention. N. Engl. J. Med. 2021;385:1005–1017. doi: 10.1056/NEJMoa2026330. [DOI] [PubMed] [Google Scholar]
- 23.Didierlaurent A.M., Laupèze B., Di Pasquale A., Hergli N., Collignon C., Garçon N. Adjuvant system AS01: helping to overcome the challenges of modern vaccines. Expert Rev. Vaccines. 2017;16:55–63. doi: 10.1080/14760584.2016.1213632. [DOI] [PubMed] [Google Scholar]
- 24.RTSS Clinical Trials Partnership Efficacy and safety of the RTS,S/AS01 malaria vaccine during 18 months after vaccination: a phase 3 randomized, controlled trial in children and young infants at 11 African sites. PLoS Med. 2014;11 doi: 10.1371/journal.pmed.1001685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.RTSS Clinical Trials Partnership Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet. 2015;386:31–45. doi: 10.1016/S0140-6736(15)60721-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.RTSS Clinical Trials Partnership. Agnandji S.T., Lell B., Fernandes J.F., Abossolo B.P., Methogo B.G.N.O., Kabwende A.L., Adegnika A.A., Mordmüller B., Issifou S., et al. A phase 3 trial of RTS,S/AS01 malaria vaccine in African infants. N. Engl. J. Med. 2012;367:2284–2295. doi: 10.1056/NEJMoa1208394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MacDonald N.J., Nguyen V., Shimp R., Reiter K., Herrera R., Burkhardt M., Muratova O., Kumar K., Aebig J., Rausch K., et al. Structural and Immunological Characterization of Recombinant 6-Cysteine Domains of the Plasmodium falciparum Sexual Stage Protein Pfs230. J. Biol. Chem. 2016;291:19913–19922. doi: 10.1074/jbc.M116.732305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Didierlaurent A.M., Morel S., Lockman L., Giannini S.L., Bisteau M., Carlsen H., Kielland A., Vosters O., Vanderheyde N., Schiavetti F., et al. AS04, an aluminum salt- and TLR4 agonist-based adjuvant system, induces a transient localized innate immune response leading to enhanced adaptive immunity. J. Immunol. 2009;183:6186–6197. doi: 10.4049/jimmunol.0901474. [DOI] [PubMed] [Google Scholar]
- 29.Scaria P.V., Chen B.B., Rowe C.G., Alani N., Muratova O.V., Barnafo E.K., Lambert L.E., Zaidi I.U., Lees A., Rausch K.M., et al. Comparison of carrier proteins to conjugate malaria transmission blocking vaccine antigens, Pfs25 and Pfs230. Vaccine. 2020;38:5480–5489. doi: 10.1016/j.vaccine.2020.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coelho C.H., Tang W.K., Burkhardt M., Galson J.D., Muratova O., Salinas N.D., Alves E Silva T.L., Reiter K., MacDonald N.J., Nguyen V., et al. A human monoclonal antibody blocks malaria transmission and defines a highly conserved neutralizing epitope on gametes. Nat. Commun. 2021;12:1750. doi: 10.1038/s41467-021-21955-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sagara I., Healy S.A., Assadou M.H., Gabriel E.E., Kone M., Sissoko K., Tembine I., Guindo M.A., Doucoure M., Niaré K., et al. A randomized controlled phase 1 trial of malaria transmission-blocking vaccines Pfs230D1-EPA and Pfs25-EPA in Alhydrogel® in healthy Malian adults. Lancet Infect. Dis. 2018;18:969–982. In press. [Google Scholar]
- 32.Kapulu M.C., Da D.F., Miura K., Li Y., Blagborough A.M., Churcher T.S., Nikolaeva D., Williams A.R., Goodman A.L., Sangare I., et al. Comparative assessment of transmission-blocking vaccine candidates against Plasmodium falciparum. Sci. Rep. 2015;5 doi: 10.1038/srep11193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Team R.C. R Foundation for Statistical Computing; 2020. A Language and Environment for Statistical Computing. [Google Scholar]
- 34.Zaiontz C. Comparing the slopes for two independent samples. 2018. https://www.real-statistics.com/regression/hypothesis-testing-significance-regression-line-slope/comparing-slopes-two-independent-samples/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
All data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.