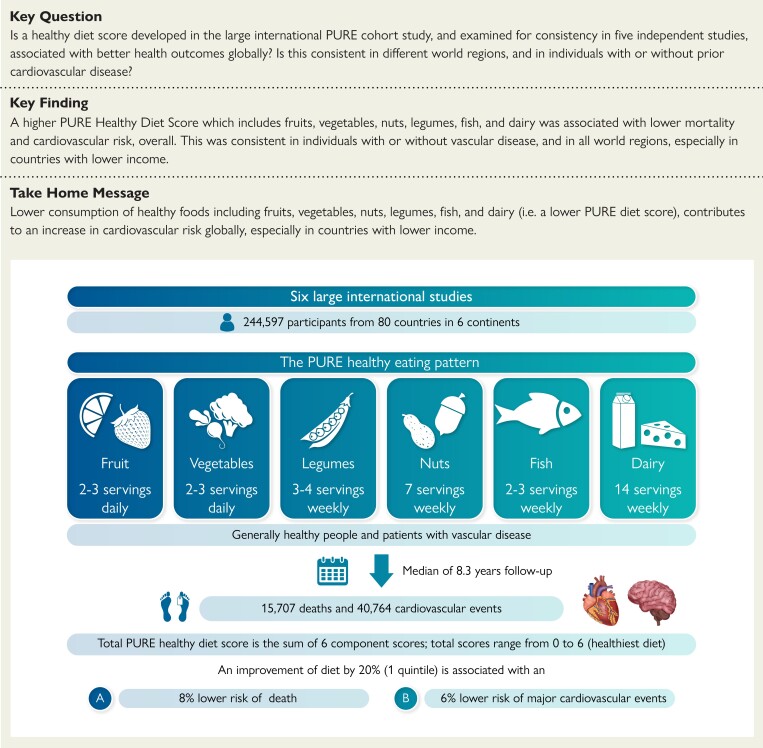
Abstract
Aims
To develop a healthy diet score that is associated with health outcomes and is globally applicable using data from the Prospective Urban Rural Epidemiology (PURE) study and replicate it in five independent studies on a total of 245 000 people from 80 countries.
Methods and results
A healthy diet score was developed in 147 642 people from the general population, from 21 countries in the PURE study, and the consistency of the associations of the score with events was examined in five large independent studies from 70 countries. The healthy diet score was developed based on six foods each of which has been associated with a significantly lower risk of mortality [i.e. fruit, vegetables, nuts, legumes, fish, and dairy (mainly whole-fat); range of scores, 0–6]. The main outcome measures were all-cause mortality and major cardiovascular events [cardiovascular disease (CVD)]. During a median follow-up of 9.3 years in PURE, compared with a diet score of ≤1 points, a diet score of ≥5 points was associated with a lower risk of mortality [hazard ratio (HR) 0.70; 95% confidence interval (CI) 0.63–0.77)], CVD (HR 0.82; 0.75–0.91), myocardial infarction (HR 0.86; 0.75–0.99), and stroke (HR 0.81; 0.71–0.93). In three independent studies in vascular patients, similar results were found, with a higher diet score being associated with lower mortality (HR 0.73; 0.66–0.81), CVD (HR 0.79; 0.72–0.87), myocardial infarction (HR 0.85; 0.71–0.99), and a non-statistically significant lower risk of stroke (HR 0.87; 0.73–1.03). Additionally, in two case-control studies, a higher diet score was associated with lower first myocardial infarction [odds ratio (OR) 0.72; 0.65–0.80] and stroke (OR 0.57; 0.50–0.65). A higher diet score was associated with a significantly lower risk of death or CVD in regions with lower than with higher gross national incomes (P for heterogeneity <0.0001). The PURE score showed slightly stronger associations with death or CVD than several other common diet scores (P < 0.001 for each comparison).
Conclusion
A diet comprised of higher amounts of fruit, vegetables, nuts, legumes, fish, and whole-fat dairy is associated with lower CVD and mortality in all world regions, especially in countries with lower income where consumption of these foods is low.
Keywords: Diet quality, Dietary patterns, Cardiovascular events, Mortality, Diverse populations, Global
Structured Graphical Abstract
Structured Graphical Abstract.
In a combined analysis of data from six international studies involving 245 000 people from 80 countries, a diet comprised of higher amounts of fruit, vegetables, nuts, legumes, fish, and dairy foods is associated with lower risk of cardiovascular disease and mortality in all world regions, especially in lower income countries. PURE, Prospective Urban Rural Epidemiology.
See the editorial comment for this article ‘Identifying nutritional priorities for global health: time for a more PURE focus on protective foods', by D. Mozaffarian, https://doi.org/10.1093/eurheartj/ehad325.
Introduction
Unhealthy diets have been ranked as a major factor for death and cardiovascular disease (CVD) globally.1,2 Contemporary nutrition recommendations are based on the associations of individual foods, nutrients, and dietary patterns with CVD in many older and contemporary prospective cohort studies conducted mostly in North America, Europe, and East Asia; numerous short-term randomized intervention trials of physiologic risk factors; and a few randomized clinical trials of dietary patterns on clinical outcomes but conducted mainly in Western countries.3–8
Previous diet pattern scores [Dietary Approaches to Stop Hypertension (DASH), Mediterranean, Healthy Eating Index (HEI), and, more recently, the EAT-Lancet Planetary diet] have been described and their relationship to CVD and mortality has been tested mainly in Western countries.6,8–16 These diet scores combine consumption of foods that are believed to be protective with foods (or nutrients) considered to be harmful, and so no diet score is focused exclusively on protective foods despite a recent increased focus on higher intakes of protective foods for disease prevention.3 Additionally, these previous diet scores all have in common an emphasis on increased fruit, vegetables, legumes, nuts, and fish, with some differences in their focus on different types of fats and consumption of dairy or red meat.
Recent data from cohort studies have challenged conventional recommendations about which dietary components are protective or harmful. Dietary exposures (such as whole-fat dairy) that were previously thought to increase CVD have recently been shown to be either neutral or protective in large cohort studies.17–25 This new information has not been incorporated into nutrition guidelines. Additionally, it is not known whether conclusions drawn from diet score studies conducted predominantly in USA, Europe, and East Asia are applicable to other world regions (e.g. Africa, South America, Middle East or South Asia), including parts of the globe where dietary patterns differ markedly (Africa and South Asia).26
Our aims were to (i) develop a healthy diet score from the large Prospective Urban Rural Epidemiology (PURE) cohort study involving 147 642 people from 21 countries in 5 continents; (ii) examine the consistency of the associations of the PURE healthy diet score with events in three independent prospective studies (n = 43 834 in 50 countries) and 2 case–control studies of myocardial infarction (MI) (n = 26 191 in 52 countries) or stroke (n = 26 930 in 33 countries); (iii) assess whether the PURE healthy diet score is applicable to people from high, middle and low income countries, from various regions of the world, and to those with and without prior CVD; and (iv) compare the performance of the PURE healthy diet score with that of other commonly used diet scores (Mediterranean diet, HEI-2010 and 2015, DASH and Planetary Diet scores).3–6,8–10,12,13
Methods
Study design and participants
Details of the studies’ designs and population characteristics have been published before and are described in the Supplementary data online, Appendix. In brief, the PURE Study1,27 is an ongoing large-scale epidemiological cohort study that has enrolled 166 762 individuals, 35–70 years of age, from the general population in 21 low-, middle-, and high-income countries on 5 continents (see Supplementary data online, Appendices S1 and S2). Participants were enrolled into the study between 1 January 2003, and 31 July 2018. For the current analysis, we developed the healthy diet score in 147 642 participants with complete information on their diet (see Supplementary data online, Figure S1). We included all outcome events known until 31 July 2019 (see Supplementary data online, Appendix S3).
We examined the consistency of the associations of the diet score with events in 43 834 vascular patients in 3 prospective studies from 50 countries, and in 2 case–control studies of MI (n = 26 191 in 52 countries) or stroke (n = 26 930 in 33 countries). Ongoing Telmisartan Alone and in combination with Ramipril Global End point Trial (ONTARGET) was a randomized controlled trial of anti-hypertension medication (ramipril, telmisartan, and their combination) in 25 620 patients, aged 55 years or older, with vascular disease or diabetes enrolled between October 2002 and April 2004.28 Telmisartan Randomized AssesmeNt Study in ACE iNtolerant subject with cardiovascular Disease (TRANSCEND) was a randomized controlled trial of telmisartan vs. placebo in 5926 participants enrolled between October 2002 and April 2004.29 For this analysis, we included 31 429 participants from ONTARGET and TRANSCEND with dietary assessments in 40 countries on 6 continents. Of these, 20 195 were from 20 high-income countries, and 11 234 from 18 middle-income countries (see Supplementary data online, Appendix S1).
Outcome Reduction With Initial Glargine Intervention (ORIGIN) was a randomized controlled trial of insulin glargine or standard care and n-3 fatty acids or placebo (2-by-2 factorial design) in 12 405 people (mean age, 63.5 years) with cardiovascular risk factors plus impaired fasting glucose or diabetes enrolled between 5 February 2004 and 27 December 2005.30,31 Of these, 4763 were from 20 high-income countries on 6 continents, 7255 from 19 middle-income countries, and 387 from 1 low-income country (see Supplementary data online, Appendix S1).
The INTERHEART study was a standardized case–control study involving 11 931 cases of first acute MI and 14 260 controls from 52 countries on 6 continents enrolled between February 1999 and March 2003.32,33 Of these, 6333 were from 21 high-income countries, 15 911 from 20 middle-income countries, and 3947 from 11 low-income countries.
The INTERSTROKE study was a standardized case-control study involving 13 444 cases of acute first stroke and 13 486 controls from 33 countries on 6 continents enrolled between 11 January 2007 and 8 August 2015.34,35 Of these, 4849 were from 11 high-income countries, 14 598 from 15 middle-income countries, and 7483 from 5 low-income countries. These studies collectively included people from all inhabited continents of the world (see Supplementary data online, Appendix S1).
Collectively, our analysis includes a broad population drawn from 80 countries involving all inhabited continents with good representation from high-income, middle-income, and low-income countries. This includes 21% of participants from high-income, 60% from middle-income, and 19% from low-income countries, which is similar to the global population distribution (16% high-income, 65% middle-income, and 19% low-income countries in 2008, the median time point of participant recruitment).
All studies were co-ordinated by the Population Health Research Institute, Hamilton Health Sciences and McMaster University, Hamilton, ON, Canada.
Procedures
The information about study variables was collected with similar approaches and data collection forms in each of the studies. Information about demographic factors, lifestyle, health history, and medication use was recorded. Physical assessments included standardized measurements of weight, height, waist and hip circumferences, and blood pressure.17,18
In PURE, participants’ habitual food intake was recorded using country-specific validated food frequency questionnaires (FFQs) at baseline. The number of food items in the FFQs varied from 95 to 250 items (see Supplementary data online, Appendix S4).17,18,28,29,31,32,34 In ONTARGET, TRANSCEND, ORIGIN, INTERHEART, and INTERSTROKE, dietary information at baseline was obtained using a 19-item qualitative FFQ with information on individual foods or food groups and alcohol intake.28–35 In ORIGIN, we also collected repeat measures of diet after 2 years, which we used to adjust for regression dilution biases (see ‘Statistical analyses’ section).
PURE healthy diet score
In the PURE cohort, we derived a healthy diet score based on six food categories each of which have been associated with a lower risk of mortality (summarized in Supplementary data online, Appendix S5). These food categories consisted of fruit, vegetables, legumes, nuts, fish, and dairy.
While diet is complex, a simple dietary score is most practical in communicating what is a healthy dietary pattern. We used an unweighted score similar to previous dietary indices (e.g. Mediterranean, DASH, HEI, Recommended Foods Score, etc).11–14 Further, our scoring scheme was similar to numerous other cohort studies of diet scores and health outcomes (e.g. Mediterranean diet).12,14 A value of 0 or 1 was assigned to each of the six components of the score with the use of the median in the study cohort as the cut-off. A score of 1 (healthy) was assigned when an individual’s intake of the food component was above the median in the cohort. A score of 0 (unhealthy) was assigned when intake was at or below the median. The total PURE healthy diet score was the unweighted sum of the six component scores. The healthy diet scores range from 0 to 6 points, with higher scores indicating a healthier diet. Additionally, we conducted separate analyses where we used quintile cut-offs of each food (instead of the median), which results in a score with a wider range (i.e. from 0 to 30). With this use of this scoring method, the results were similar and so the results using the simpler median cut-off is presented. (A weighted score provides nearly identical results, and so the unweighted score is used for simplicity.)
To translate the PURE diet scores into a healthy eating pattern for the public (i.e. intake amounts of each of the six foods that are needed to achieve the ‘healthiest’ diet), we used the mean intake of each food category among people in the upper quintile of the PURE diet score as the intake target. These intake amounts represent the average intake of each of the six foods in people in the top 20% of the PURE diet score, which represents an eating pattern associated with the lowest risk of outcome events.
The Mediterranean, HEI-2010 and 2015, DASH, and Planetary Health scores have been described previously3–6,9,10,12 and are summarized in Supplementary data online, Appendix S6.
Outcomes
Myocardial infarctions, strokes, heart failure, and cardiovascular and other deaths were recorded during structured follow-up using standard forms within each prospective study, and these events were centrally adjudicated. In the two case–control studies, specific criteria for cases and for controls were used.32–35
In the prospective studies, the outcomes included in the analyses were major cardiovascular events (fatal CVD, non-fatal MI, stroke, and heart failure) and total mortality. The median duration of follow-up was 9.3 years [interquartile range (IQR) 7.5–10.8] in PURE, 4.5 years (IQR 4.4–5.0) in ONTARGET and TRANSCEND, and 6.2 years (IQR 5.8–6.7) in ORIGIN.
Statistical analyses
Continuous variables were expressed as mean (±SD) and categorical variables as percentages. In PURE, we computed the mean PURE healthy diet score overall and by geographical region. ANOVA was conducted, with tests for linear trend, to compare the mean values of the PURE healthy diet score by country gross national income. Participants were categorized into fifths (quintiles) of the healthy diet score. To characterize dietary profiles from ‘least healthy’ to ‘most healthy’ diet, we calculated the mean intake of foods (g per day) and nutrients (% of energy) by quintile group of the dietary score. The food and nutrient intakes of individuals in the lowest and highest quintile groups (i.e. lowest and highest fifths) of the healthy diet score characterizes the dietary profile of the least and most healthy diet, respectively.
In PURE, we used restricted cubic-spline plots with four knots to explore the shape of the association between the diet scores and the risk of mortality and major CVD.36 Cox frailty models with random effects (to account for clustering within study centres) were used to assess the association between diet scores and the outcomes. We identified a priori confounding variables for adjustment in the multivariable models. In a minimally adjusted model, we adjusted for age, sex, and study centre (as a random effect). The primary model adjusted for age, sex, study centre (as a random effect), energy intake, waist-to-hip ratio, education, wealth index, current smoking status, urban or rural location, physical activity, baseline diabetes, and use of statin or blood pressure lowering medications. We verified the assumption of proportionality of hazards using standard log (-log survival) vs. log time plots. To test for linear trends, the median value was assigned to each level category of the diet score (i.e. 0–6) and included the variable as a quantitative risk factor. A one-point increase (i.e. one-point change) in the diet score was calculated from the median value of each level category (0–6).
In sensitivity analyses, we tested the impact of removing potential mediators (body mass index, waist to hip ratio, diabetes, and hypertension) on the estimates in the primary models. Additionally, we assessed whether diet scores had variable impact by geographical region using tests of interaction.
Independent replication of the results was tested in three prospective studies of patients with vascular disease (ONTARGET, TRANSCEND, and ORIGIN), and in two case–control studies of first MI (INTERHEART) and first stroke (INTERSTROKE).
In ONTARGET and TRANSCEND, since the entry criteria and study conduct were similar between the two trials, other than ACE inhibitor intolerance in the TRANSCEND trial, we pooled the data from both studies. For these studies and ORIGIN, as in the PURE analyses, we used Cox frailty models with similar adjustment models, but additionally adjusted for treatment allocation.
In INTERHEART and INTERSTROKE (case–control studies), we used hierarchical logistic regression with random intercepts to account for centre clustering and adjusted for the matching criteria (age and sex), education, physical activity, current smoker, diabetes, waist-to-hip ratio, hypertension, and statin use. We tested the collinearity of variables in each study previously.17,18,28–35,37–39
For the combined analyses of the six studies, we used a two-stage individual participant data meta-analysis.40 First, we assessed the associations between the healthy diet score and events in each cohort separately (as described above). Second, the study-specific hazard ratios (HRs) and 95% confidence intervals (CIs) were pooled in a random-effects meta-analysis. We used the DerSimonian-Laird approach with the Hartung-Knapp-Sidik-Jonkman variance correction method.41 Tests of heterogeneity were conducted using the I2 statistic.
For the analyses of Mediterranean, HEI-2010 and 2015, DASH, and Planetary Health Diet scores, we used the same restricted cubic-spline and Cox frailty modelling approaches described above for the PURE healthy diet score to model associations with clinical outcomes.
To determine which diet scores are most strongly associated with major CVD and death, we conducted a receiver-operating characteristic analysis to assess the predictive ability of the different diet scores vs. the PURE score.42 The predictive performance of each Cox model was measured by the area under the receiver-operating characteristic curve (AUC). The significance level of differences in AUC values were calculated using the DeLong test.43 In initial analyses, we included data only from PURE (n = 147 642), in which dietary information was collected using full-length, country-specific FFQs and therefore is better suited for capturing most of the dietary components of each diet score (see Supplementary data online, Appendix S6). In secondary analyses, we compared the various diet scores with the PURE Healthy Diet Score based on data from all four prospective cohorts (i.e. including the three cohorts that used the shorter qualitative FFQ) (n = 191 476 overall).
Statistical analyses were conducted using SAS Statistical Package (version 9.4; SAS Institute, Inc., Cary, NC).
Results
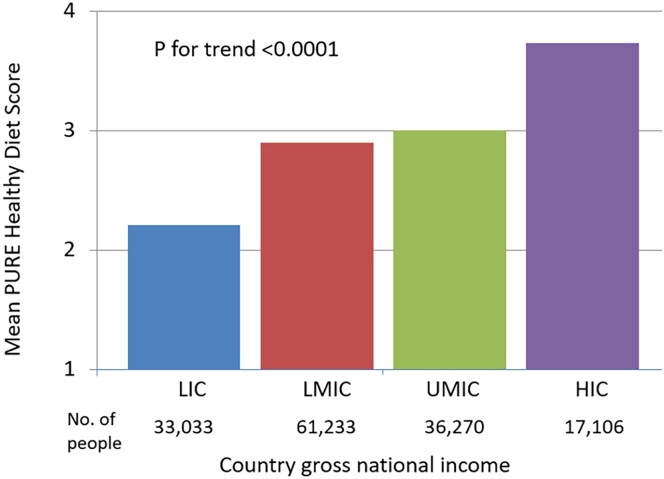
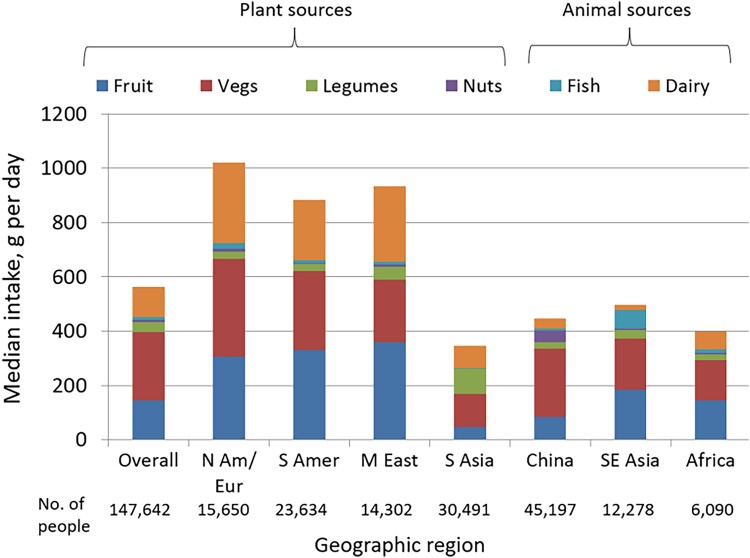
The characteristics of the participants from PURE are shown in Table 1. The mean PURE Healthy Diet Score was 2.95 (SD 1.50). A higher healthy diet score was associated with higher per capita gross national income (P-trend <0.0001) (Figure 1). The highest median diet scores and intake of food components in the diet score were found in North America and Europe, Middle East, and South America, while South Asia, Africa, Southeast Asia, and China had lower scores and intake of its component foods (Figure 2).
Table 1.
Baseline characteristics of participants by category of PURE healthy diet score in a general population (PURE; n = 147 642) a
| Overall | PURE Healthy Diet Score, quintile category | |||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | ||
| Numbers | 147 642 | 25 996 | 34 952 | 33 442 | 28 133 | 25 119 |
| Median diet score (range) | 3 (0–6) | 1 (0–1) | 2 | 3 | 4 | 5 (5–6) |
| Age, years | 50.6 ± 9.8 | 49.8 ± 10.0 | 50.3 ± 10.0 | 50.7 ± 9.8 | 51.0 ± 9.7 | 51.3 ± 9.6 |
| % women | 85 852 (58.2) | 15 444 (59.4) | 20 723 (59.3) | 19 355 (57.9) | 15 951 (56.7) | 14 379 (57.2) |
| % urban | 78 455 (53.1) | 8877 (34.2) | 15 642 (44.8) | 18 424 (55.1) | 17 915 (63.7) | 17 597 (70.1) |
| Country income region | ||||||
| Low-income country | 33 033 | 9498 (28.8) | 12 273 (37.2) | 6758 (20.5) | 2836 (8.6) | 1668 (5.1) |
| Middle-income country | 97 503 | 15 742 (16.1) | 20 998 (21.5) | 23 574 (24.2) | 20 811 (21.3) | 16 378 (16.8) |
| High-income country | 17 106 | 756 (4.4) | 1681 (9.8) | 3110 (18.2) | 4486 (26.2) | 7073 (41.4) |
| Geographic regions | ||||||
| North America/Europe | 15 650 | 839 (5.4) | 1757 (11.2) | 2883 (18.4) | 3916 (25.0) | 6255 (40.0) |
| South America | 23 634 | 4636 (19.6) | 4914 (20.8) | 5000 (21.2) | 5018 (21.2) | 4066 (17.2) |
| Middle East | 14 302 | 830 (5.8) | 1852 (13.0) | 3357 (23.5) | 3769 (26.4) | 4494 (31.4) |
| South Asia | 30 491 | 9280 (30.4) | 11 952 (39.2) | 6280 (20.6) | 2303 (7.6) | 676 (2.2) |
| China | 45 197 | 6211 (13.7) | 10 322 (22.8) | 11 753 (26.0) | 9602 (21.2) | 7309 (16.2) |
| Southeast Asia | 12 278 | 2058 (16.8) | 3103 (25.3) | 3148 (25.6) | 2757 (22.5) | 1212 (9.9) |
| Africa | 6090 | 2142 (35.2) | 1052 (17.3) | 1021 (16.8) | 768 (12.6) | 1107 (18.2) |
| Education | ||||||
| None or primary | 62 915 (42.7) | 16 670 (64.5) | 16 756 (48.1) | 13 294 (39.9) | 9453 (33.7) | 6742 (26.9) |
| Secondary/high/higher secondary | 55 767 (37.9) | 7394 (28.6) | 13 599 (39.0) | 13 944 (41.8) | 11 681 (41.6) | 9149 (36.5) |
| Trade or College/University | 28 586 (19.4) | 1799 (7.0) | 4499 (12.9) | 6121 (18.4) | 6957 (24.8) | 9210 (36.7) |
| Wealth index, median (IQR)b | 0.11 (−0.73 to 0.76) | −0.67 (−1.51 to 0.22) | −0.22 (−1.02 to 0.45) | 0.10 (−0.57 to 0.72) | 0.39 (−0.22 to 1.01) | 0.73 (0.14–1.31) |
| Body mass index, kg/m2 | 25.8 ± 5.3 | 24.8 ± 5.7 | 25.3 ± 5.3 | 26.0 ± 5.0 | 26.4 ± 5.0 | 26.8 ± 5.0 |
| Waist to hip ratio | 0.874 ± 0.085 | 0.866 ± 0.089 | 0.873 ± 0.085 | 0.878 ± 0.083 | 0.878 ± 0.083 | 0.876 ± 0.085 |
| Systolic blood pressure, mmHg | 131.3 ± 22.4 | 130.4 ± 23.4 | 131.5 ± 22.5 | 132.2 ± 22.4 | 131.7 ± 22.5 | 130.6 ± 21.1 |
| Diastolic blood pressure, mmHg | 81.9 ± 15.4 | 81.8 ± 14.4 | 81.9 ± 13.6 | 82.2 ± 15.6 | 82.1 ± 16.3 | 81.7 ± 17.2 |
| Current smoker | 30 220 (20.6) | 6486 (25.2) | 7627 (22.0) | 6730 (20.3) | 5384 (19.3) | 3993 (16.0) |
| Alcohol | ||||||
| Never | 102 384 (69.7) | 19 213 (74.6) | 26 132 (75.2) | 23 359 (70.2) | 18 667 (66.6) | 15 013 (60.0) |
| Former | 6548 (4.5) | 1073 (4.2) | 1299 (3.7) | 1629 (4.9) | 1387 (5.0) | 1160 (4.6) |
| Current | 37 911 (25.8) | 5471 (21.2) | 7312 (21.1) | 8289 (24.9) | 7977 (28.5) | 8862 (35.4) |
| Physical activity | ||||||
| Low | 24 942 (18.1) | 5020 (21.5) | 6302 (19.8) | 5987 (18.9) | 4588 (17.0) | 3045 (12.8) |
| Moderate | 51 911 (37.7) | 7788 (33.3) | 11 816 (37.2) | 12 413 (39.2) | 10 628 (39.4) | 9266 (38.8) |
| High | 60 895 (44.2) | 10 595 (45.3) | 13 678 (43.0) | 13 304 (42.0) | 11 762 (43.6) | 11 556 (48.4) |
| Diabetes, self-reported or on glucose lowering medication or fasting glucose ≥ 7 mmol/L | 16 193 (11.1) | 2151 (8.3) | 4080 (11.7) | 3954 (11.8) | 3282 (11.7) | 2726 (10.9) |
| Hypertension, self-reported or on BP lowering medication or BP ≥ 140/90 mm Hg | 56 808 (40.8) | 9390 (38.7) | 13 249 (40.4) | 13 534 (42.7) | 11 061 (41.9) | 9574 (39.8) |
| Cardiovascular disease | 7594 (5.2) | 1242 (4.8%) | 1565 (4.5) | 1758 (5.3) | 1614 (5.7) | 1415 (5.6) |
| Cancer | 2312 (1.6) | 285 (1.1) | 378 (1.1) | 490 (1.5) | 500 (1.8) | 659 (2.6) |
| Blood pressure lowering medications | 21 201 (14.4) | 2745 (10.6%) | 4313 (12.3) | 5001 (15.0) | 4748 (16.9) | 4394 (17.5) |
| Statin medications | 4881 (3.3) | 378 (1.5%) | 730 (2.1) | 1103 (3.3) | 1232 (4.4) | 1438 (5.7) |
| Energy intake, kcals per day | 2147 ± 818 | 1676 ± 705 | 1996 ± 741 | 2163 ± 771 | 2332 ± 769 | 2617 ± 822 |
Values are mean ± SD or n (%).
Wealth index is based on information collected on household possessions such as electricity, car, computer, television, phone, etc.25,26.
Physical activity categories are low, < 600; moderate, 600–3000; and high, > 3000 metabolic equivalent of task per minute per week.
Figure 1.
Prospective Urban Rural Epidemiology healthy diet score by country gross national income (n = 147 642).
Figure 2.
Median intake of the food categories of the Prospective Urban Rural Epidemiology healthy diet score, overall and by geographic region (n = 147 642).
Nutrition profiles of low (least healthy) and high (most healthy) diet scores
Based on the mean intake of foods by level of diet score, a ‘most healthy’ diet (i.e. diet score in the highest fifth; ≥ 5 points) contains 563.1 g/day (5 servings) of fruit and vegetables, 48.0 g/day (0.5 servings) of legumes, 28.2 g/day (1.2 servings) of nuts, 26.1 g/day (0.3 servings) of fish, 185.5 g/day (2.0 servings) of dairy [of which 130.5 g/day (1.4 servings) is whole-fat dairy], 54.5 g/day (0.5 servings) of red meat, and 22.1 g/day (0.3 servings) of poultry. This corresponds to a diet of variety consisting of each nutrient in moderate amounts [i.e. 56% of energy from carbohydrates, 27% from fats (including 8.9% from saturated and 15.0% from unsaturated fats), 17.2% from protein].
By contrast, a ‘least healthy’ diet (i.e. diet score in the lowest fifth; ≤ 1 points) is comprised of markedly lower amounts of each food group (Table 2). This corresponds to a diet high in carbohydrates (66% of energy), and with lower fat (20% of energy; including 6.3% from saturated and 10.7% from unsaturated fats), lower protein (13.5% of energy), and lower red meat (24.1 g/day) and poultry (10.3 g/day).
Table 2.
Nutritional profiles by PURE healthy diet score category in a general population (from ‘least healthy’ to ‘most healthy’) (PURE; n = 147 642)a,b
| PURE Healthy Diet Score, quintile category | |||||
|---|---|---|---|---|---|
| ≤1 (least healthy) | 2 | 3 | 4 | ≥5 (most healthy) | |
| Fruit, g per day | 45.6 | 82.4 | 133.3 | 185.5 | 256.8 |
| Vegetables, g per day | 85.0 | 126.1 | 180.6 | 232.4 | 306.3 |
| Legumes, g per day | 21.0 | 31.9 | 32.9 | 36.5 | 48.0 |
| Nuts, g per day | 3.9 | 7.0 | 13.9 | 20.2 | 28.2 |
| Fish, g per day | 6.1 | 16.4 | 18.0 | 20.8 | 26.1 |
| Dairy, g per day | 31.2 | 75.5 | 94.2 | 132.0 | 185.5 |
| Red meat, unprocessed, g per day | 24.1 | 27.2 | 35.0 | 43.3 | 54.5 |
| White meat, g per day | 10.3 | 11.1 | 13.8 | 18.0 | 22.1 |
| Eggs, g per day | 9.6 | 12.7 | 16.0 | 17.5 | 18.0 |
| Whole wheat foods, g per day | 42.7 | 40.8 | 35.0 | 32.3 | 40.9 |
| Refined wheat foods, g per day | 101.9 | 105.8 | 116.1 | 124.9 | 119.4 |
| Rice, g per dayc | 178.4 | 166.6 | 139.1 | 107.8 | 80.8 |
| Potatoes and tubers, g per day | 22.5 | 20.9 | 25.0 | 30.0 | 34.6 |
| Sweets, g per dayd | 70.4 | 66.3 | 78.9 | 104.2 | 128.6 |
| Cholesterol, mg per day | 187.9 | 248.0 | 293.7 | 344.3 | 394.6 |
| Alcohol, g per day | 76.0 | 37.8 | 42.9 | 49.9 | 65.2 |
| Carbohydrates, %E | 65.8 | 62.8 | 61.0 | 58.7 | 56.4 |
| Fats, %E | 20.1 | 22.8 | 23.7 | 25.2 | 27.1 |
| Saturated, %E | 6.3 | 7.8 | 7.9 | 8.2 | 8.9 |
| Monounsaturated, %E | 6.3 | 7.0 | 7.8 | 8.5 | 9.5 |
| Polyunsaturated, %E | 4.4 | 4.9 | 5.0 | 5.1 | 5.5 |
| Polyunsaturated-to-saturated fat ratio | 0.89 | 0.76 | 0.74 | 0.71 | 0.68 |
| Other, %Ee | 3.1 | 3.0 | 3.1 | 3.4 | 3.1 |
| Protein, %E | 13.5 | 14.3 | 15.3 | 16.4 | 17.2 |
Table shows mean values for each food and nutrient.
Energy intakes (mean ± SD) by quintile category are: Q1: 1676 ± 705; Q2: 1996 ± 741; Q3: 2163 ± 771; Q4: 2332 ± 769; Q5: 2617 ± 822.
Rice is mainly white rice, as brown rice intake was captured only in Argentina and Brazil, where its intake was low (2 g/day in Argentina and 16 g/day in Brazil).
Sweets include cakes, cookies, biscuits, gelatins, pastries, pies, puddings, and candies.
‘Other’ is comprised of glycerol and other aldehydes.
%E, % of total energy intake.
The other diet scores similarly include higher amounts of fruits, vegetables and nuts, but differ in the consumption of other foods. Higher Mediterranean and HEI scores correspond with lower intakes of red meat, DASH relates to lower red meat, poultry, and dairy, while the Planetary diet is the most restrictive and characterized by lower red meat, poultry, dairy, fish, and legumes. The DASH and Planetary diets are markedly higher in carbohydrates and lower in fat (mainly from saturated fat) (see Supplementary data online, Appendices S6 and S7).
Healthy diet score vs. outcome events in PURE
During a median of 9.3 years (IQR 7.5–10.8) of follow-up in PURE, 8201 major CVD events and 10 076 total deaths were documented (Table 3). At least one follow-up visit was completed for 98% of participants.
Table 3.
Associations of the PURE healthy diet score with outcomes in a general population (PURE; n = 147 642)a
| All participants (n = 147 642) | Hazard ratio (95% CI) | P for trend | Hazard ratio (95% CI) per 20 percentile increment | ||||
|---|---|---|---|---|---|---|---|
| Q1 (n = 25 996) | Q2 (n = 34 952) | Q3 (n = 33 442) | Q4 (n = 28 133) | Q5 (n = 25 119) | |||
| Median score (range) | 1 (0–1) | 2 | 3 | 4 | 5 (5–6) | ||
| Total mortality | |||||||
| No. of deaths (%) (n = 10 076) | 2936 (11.29) | 2879 (8.24) | 1932 (5.78) | 1372 (4.88) | 957 (3.81) | ||
| Incidence rate (events per 1000 person-years) | 12.14 | 8.86 | 6.22 | 5.25 | 4.10 | ||
| Age and sex adjusted | 1.00 | 0.82 (0.78–0.86) | 0.67 (0.63–0.72) | 0.62 (0.58–0.67) | 0.54 (0.50–0.59) | <0.0001 | 0.85(0.84–0.87) |
| Multivariable | 1.00 | 0.89 (0.84–0.94) | 0.79 (0.73–0.83) | 0.75 (0.69–0.81) | 0.70 (0.63–0.77) | <0.0001 | 0.91(0.89–0.93) |
| No history of CVD | 1.00 | 0.89 (0.84–0.95) | 0.78 (0.73–0.84) | 0.76 (0.70–0.83) | 0.71 (0.64–0.79) | <0.0001 | 0.92(0.89–0.94) |
| History of CVD | 1.00 | 0.83 (0.69–1.00) | 0.73 (0.60–0.89) | 0.63 (0.50–0.79) | 0.55 (0.42–0.72) | <0.0001 | 0.86(0.81–0.92) |
| Major CVD | |||||||
| No. of major CVD (%) (n = 8201) | 1659 (6.38) | 21 516.15) | 1778 (5.32) | 1451 (5.16) | 1162 (4.63) | ||
| Incidence rate (events per 1000 person-years) | 6.86 | 6.61 | 5.72 | 5.55 | 4.98 | ||
| Age and sex adjusted | 1.00 | 0.94 (0.88–1.00) | 0.81 (0.76–0.87) | 0.79 (0.73–0.85) | 0.73 (0.67–0.80) | <0.0001 | 0.92(0.91–0.94) |
| Multivariable | 1.00 | 0.96 (0.90–1.03) | 0.84 (0.78–0.91) | 0.82 (0.76–0.90) | 0.82 (0.75–0.91) | <0.0001 | 0.94(0.92–0.97) |
| No history of CVD | 1.00 | 0.96 (0.89–1.03) | 0.84 (0.78–0.92) | 0.85 (0.78–0.94) | 0.84 (0.76–0.94) | 0.0001 | 0.95(0.93–0.98) |
| History of CVD | 1.00 | 0.96 (0.80–1.15) | 0.81 (0.67–0.98) | 0.71 (0.57–0.87) | 0.70 (0.56–0.89) | 0.0002 | 0.90(0.85–0.95) |
| MI | |||||||
| No. of MI (%) (n = 3806) | 809 (3.11) | 1080 (3.09) | 810 (2.42) | 598 (2.13) | 509 (2.03) | ||
| Incidence rate (events per 1000 person-years) | 3.34 | 3.32 | 2.60 | 2.29 | 2.18 | ||
| Age and sex adjusted | 1.00 | 0.98 (0.89–1.07) | 0.84 (0.76–0.93) | 0.76 (0.68–0.85) | 0.75 (0.66–0.86) | <0.0001 | 0.92 (0.89–0.95) |
| Multivariable | 1.00 | 1.01 (0.92–1.12) | 0.89 (0.79–0.99) | 0.82 (0.72–0.93) | 0.86 (0.75–0.99) | 0.0014 | 0.95 (0.92–0.98) |
| No history of CVD | 1.00 | 1.03 (0.92–1.14) | 0.91 (0.80–1.03) | 0.87 (0.76–1.01) | 0.92 (0.79–1.09) | 0.0581 | 0.97 (0.93–1.00) |
| History of CVD | 1.00 | 0.93 (0.73–1.18) | 0.74 (0.57–0.97) | 0.57 (0.42–0.77) | 0.58 (0.42–0.81) | <0.0001 | 0.85 (0.79–0.92) |
| Stroke | |||||||
| No. of stroke (%) (n = 3925) | 756 (2.91) | 953 (2.73) | 866 (2.59) | 769 (2.73) | 581 (2.31) | ||
| Incidence rate (events per 1000 person-years) | 3.13 | 2.94 | 2.78 | 2.94 | 2.48 | ||
| Age and sex adjusted | 1.00 | 0.89 (0.81–0.98) | 0.80 (0.72–0.88) | 0.83 (0.75–0.93) | 0.74 (0.65–0.83) | <0.0001 | 0.94 (0.91–0.96) |
| Multivariable | 1.00 | 0.91 (0.82–1.01) | 0.82 (0.73–0.91) | 0.86 (0.76–0.97) | 0.81 (0.71–0.93) | 0.0034 | 0.95 (0.92–0.98) |
| No history of CVD | 1.00 | 0.89 (0.79–0.99) | 0.81 (0.71–0.91) | 0.85 (0.75–0.97) | 0.80 (0.69–0.93) | 0.0059 | 0.95 (0.92–0.99) |
| History of CVD | 1.00 | 1.04 (0.79–1.37) | 0.91 (0.68–1.22) | 0.94 (0.69–1.29) | 0.88 (0.62–1.25) | 0.3649 | 0.96 (0.89–1.04) |
| Composite of death or major CVD | |||||||
| No. of composite events (%) (n = 15 019) | 3770 (14.50%) | 4046 (11.58%) | 3023 (9.04%) | 2374 (8.44%) | 1806 (7.19%) | ||
| Incidence rate (events per 1000 person-years) | 15.59 | 12.45 | 9.72 | 9.08 | 7.73 | ||
| Age and sex adjusted | 1.00 | 0.86 (0.82–0.90) | 0.73 (0.69–0.76) | 0.70 (0.66–0.74) | 0.64 (0.60–0.68) | <0.0001 | 0.89 (0.88–0.90) |
| Multivariable | 1.00 | 0.91 (0.86–0.95) | 0.79 (0.75–0.84) | 0.79 (0.74–0.85) | 0.78 (0.72–0.84) | <0.0001 | 0.93 (0.92–0.95) |
| No history of CVD | 1.00 | 0.91 (0.86–0.96) | 0.79 (0.75–0.84) | 0.81 (0.75–0.86) | 0.79 (0.73–0.86) | <0.0001 | 0.94 (0.92–0.96) |
| History of CVD | 1.00 | 0.90 (0.77–1.04) | 0.78 (0.67–0.92) | 0.71 (0.60–0.85) | 0.67 (0.55–0.82) | <0.0001 | 0.90 (0.86–0.94) |
Age and sex adjusted: adjusted for age, sex, and centre (latter as random effect).
Multivariable models are adjusted for age, sex, study centre (random effect), waist-to-hip ratio, education level (primary or less; secondary; trade, college, or university), wealth index, urban or rural location, physical activity (low, < 600; moderate, 600–3000; high, > 3000 metabolic equivalent of task per minute per week), smoking status (never, former, or current), history of diabetes, use of statin or anti-hypertension medications, and total energy intake.
Higher PURE healthy diet score was associated with lower risk of mortality, major CVD, MI, stroke, CVD mortality, non-CVD mortality, and the composite of death or major CVD, in the age, sex, and study centre adjusted models (all P for trend < 0.001) (Table 3).
These associations were attenuated after adjusting for additional lifestyle factors and co-morbidities but remained statistically significant for each outcome. Compared with a healthy diet score in the lowest fifth (≤1 points; reference category), a healthy diet score in the highest fifth (≥5 points) was associated with a lower risk of total mortality (HR = 0.70; 95% CI: 0.63–0.77; P-trend <0.0001), major CVD (HR = 0.82; 0.75–0.91; P-trend < 0.0001), MI (HR = 0.86; 0.75–0.99), stroke (HR = 0.81; 0.71–0.93), CVD mortality (HR = 0.72; 0.60–0.85), non-CVD mortality (HR = 0.68; 0.60–0.78), and the composite of death or CVD (HR = 0.78; 0.72–0.84; P-trend <0.0001) (Table 3).
A quintile increase in the healthy diet score (an important but achievable change in score for an entire population) was associated with a lower risk of total mortality (HR = 0.91; 0.89–0.93), major CVD (HR = 0.94; 0.92–0.97), MI (HR = 0.95; 0.92–0.98), stroke (HR = 0.95; 0.92–0.98), CVD mortality (HR = 0.91; 0.88–0.95), non-CVD mortality (HR = 0.91; 0.88–0.93), and composite of death or CVD (HR = 0.93; 0.92–0.95) (Table 3 and Figure 3). Removal of any one component of the diet score generally results in slightly weaker associations between the diet score and the composite of CVD or death, so that each food makes a similar modest contribution to the score (see Supplementary data online, Appendix S8).
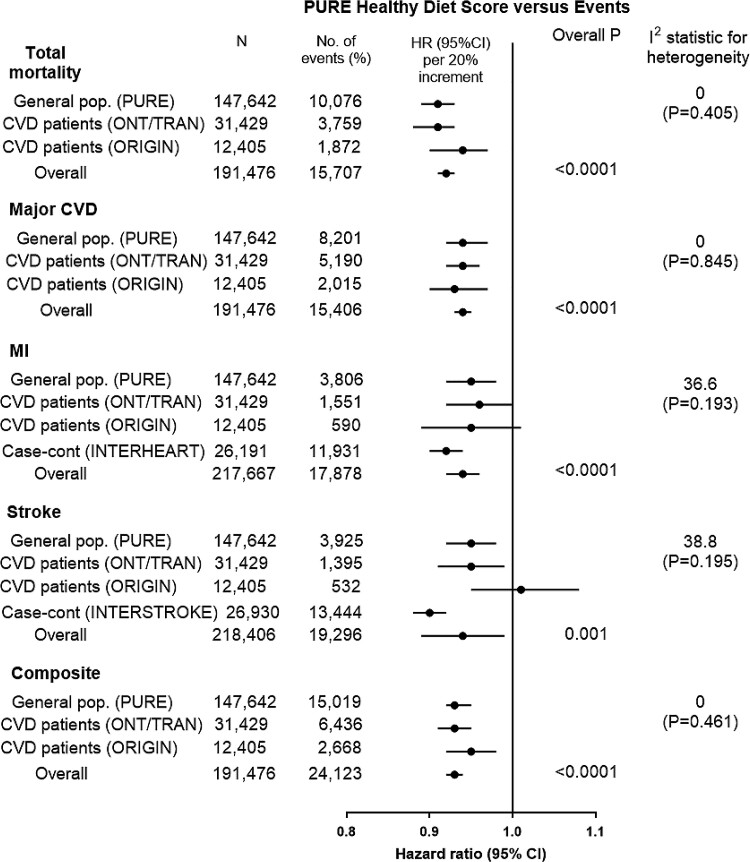
Figure 3.
Association of Prospective Urban Rural Epidemiology healthy diet score vs. events across studies (n = 244 597). Hazard ratios (95%) are per 20 percentile increment in the diet score. Hazard ratios (95% CI) are multivariable adjusted.
The associations of the PURE healthy diet score with lower risk of events were found both in people with and without prior vascular disease (Table 3).
In sensitivity analyses, after excluding waist-to-hip ratio, body mass index, history of diabetes, or history of hypertension from the models (i.e. considering these variables as possible mediators), the results were similar. Further, when participants who had an event in the first 2 years of follow-up were excluded, the associations remained similar (see Supplementary data online, Appendix S9). Lastly, when unprocessed red meat or whole grains were included from the PURE healthy diet score, the results were again similar (see Supplementary data online, Appendix S10), indicating that a modest amount of meat or whole grains can be part of a healthy diet.
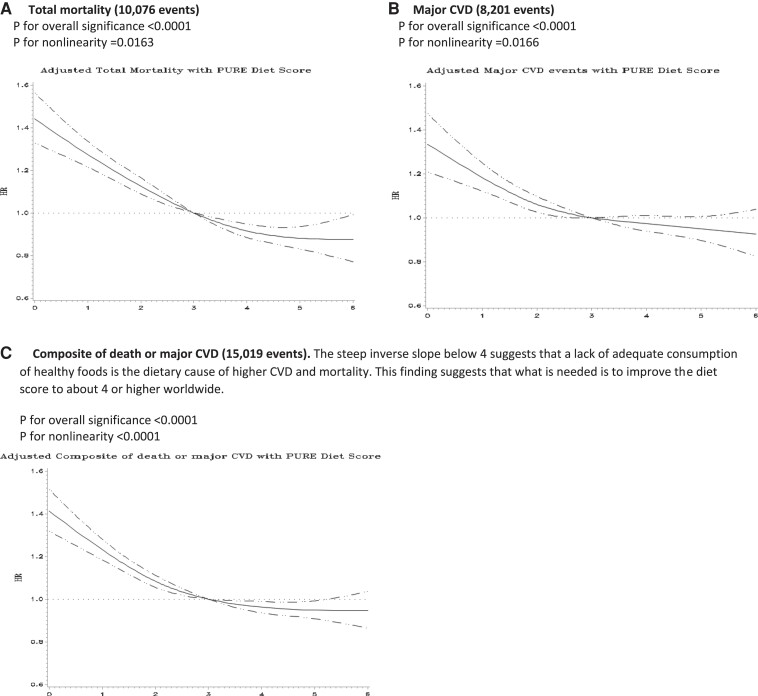
Restricted cubic splines showed significant non-linearity for associations between the healthy diet score and risk of total mortality (P = 0.0163), major CVD events (P = 0.0166), and the composite of death or major CVD (P < 0.0001) (Figure 4). The association with composite events was twice as steep amongst participants with diet scores below than above the median (per quintile increment in the diet score HR = 0.89; 0.87–0.92 vs. HR = 0.96; 0.93–0.99).
Figure 4.
Cubic splines for the association of the Prospective Urban Rural Epidemiology healthy diet score with A) total mortality, B) major CVD, and C) composite of death or major CVD in a general population (Prospective Urban Rural Epidemiology, n = 147 642).
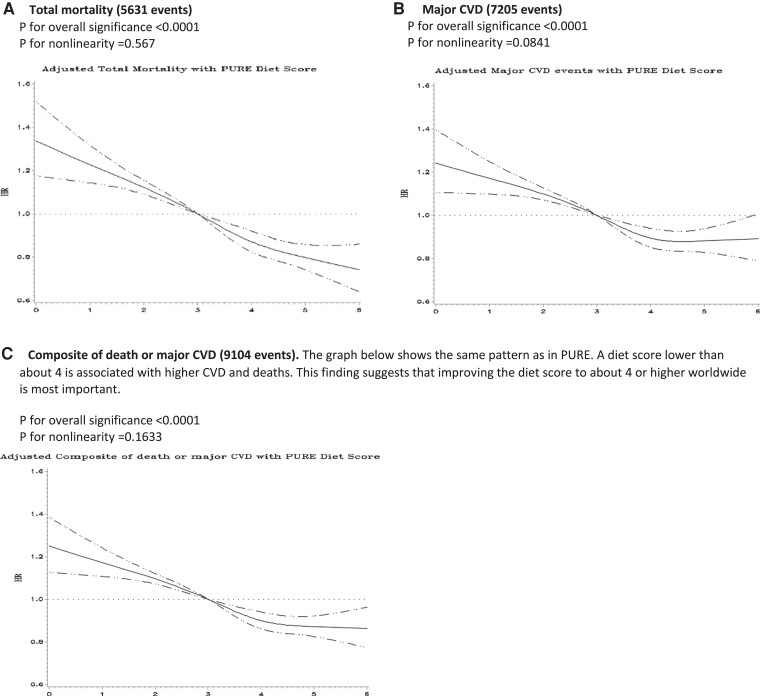
Independent replication of results of the PURE healthy diet score
The PURE Healthy Diet Score showed consistent associations with mortality, major CVD, MI, and stroke in ONTARGET/TRANSCEND, ORIGIN, INTERHEART, and INTERSTROKE, with risk estimates that were similar to those observed in PURE (Figures 3 and 5; Supplementary data online, Appendices S11–S13).
Figure 5.
Cubic splines for the association of the Prospective Urban Rural Epidemiology healthy diet score with A) total mortality, B) major CVD, and C) composite of death or major CVD in those with previous vascular disease drawn from ONTARGET, TRANSCEND, and ORIGIN (n = 43 834).
Combined analysis of all prospective studies and by prior CVD status and study design
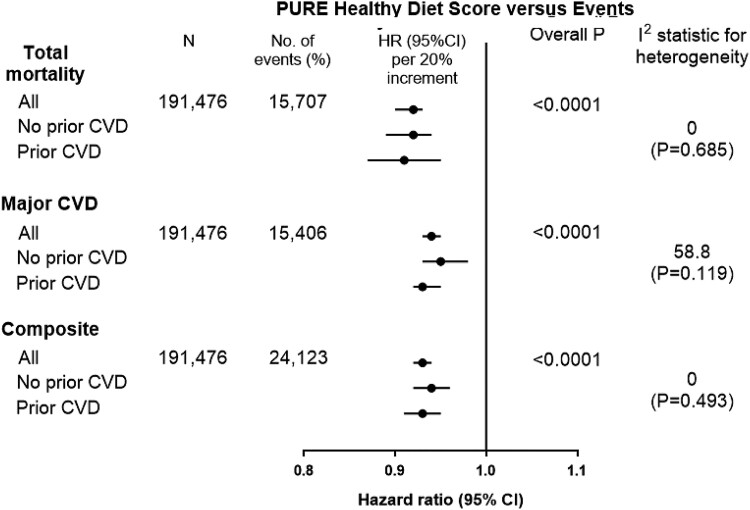
The associations of the PURE healthy diet score with primary events were similar across the four prospective studies (median follow-up of 8.3 years; for major CVD, overall P < 0.0001, I2 = 0, P = 0.845; for death, overall P < 0.0001, I2 = 0, P = 0.405, and for composite of death or CVD, overall P < 0.0001, I2 = 0, P = 0.461) (Figure 3).
A higher healthy diet score was associated with a lower risk of total mortality (comparing ≥5 points vs. ≤1 points, HR = 0.72; 0.67–0.77), major CVD (HR = 0.81; 0.76–0.86), MI (HR = 0.85; 0.77–0.95), stroke (HR = 0.83; 0.75–0.92), CVD mortality (HR = 0.71; 0.61–0.99), non-CVD mortality (HR = 0.78; 0.61–0.99), and the composite outcome (HR = 0.78; 0.74–0.83) (Figure 3). A quintile increase in the healthy diet score was associated with a lower risk of total mortality (HR = 0.92; 0.90–0.93), major CVD (HR = 0.94; 95% CI: 0.93–0.95), MI (HR = 0.94; 0.92–0.96), stroke (HR = 0.94; 0.89–0.99), and composite of death or CVD (HR = 0.93; 0.92–0.94) (Figure 3).
In these studies, the associations were consistent in people with and without prior CVD (for major CVD, I2 = 46.8, P = 0.119; for death, I2 = 0, P = 0.685, and for composite of death or CVD, I2 = 0, P = 0.493) (Figure 6).
Figure 6.
Association of Prospective Urban Rural Epidemiology healthy diet score vs. Events in those with and without prior cardiovascular disease in the four independent prospective studies (n = 191 476). Hazard ratios (95%) are per 20 percentile increment in the diet score. Hazard ratios (95% CI) are multivariable adjusted.
Cubic splines show significant non-linearity for the associations between the healthy diet score and the composite outcome (P < 0.0001 for non-linearity; P < 0.0001 for overall significance of the curve), with significantly steeper slopes amongst people with scores below vs. above the median (HR = 0.88; 0.86–0.90 vs. HR = 0.96; 0.94–0.98, respectively, per quintile increment in the PURE healthy diet score) (P for heterogeneity <0.001) (see Supplementary data online, Appendix S14). The curve flattens out at about a diet score of 3 indicating that most of the observed differences in outcomes were associated with modestly higher consumption of healthy foods, compared to little or none, and that the observed potential health differences among those who already consumed a moderate amount of these foods (e.g. people with a diet score of 4 or higher) is less.
Other dietary scores vs. outcome events
In analyses comparing different diet scores, the Mediterranean, HEI-2010, HEI-2015, and DASH scores showed beneficial associations with all outcome events, while the Planetary Health score showed neutral associations with events (see Supplementary data online, Appendix S15). The PURE healthy diet score was most similar to the HEI-2010 and HEI-2015 diet scores, with only slightly larger HRs found for the PURE score (Table 4). In tests comparing the differences in AUC values between diet scores,43 the PURE score showed significantly stronger associations with composite events and mortality risk compared to the HEI-2010 and HEI-2015, significantly stronger associations with composite events, mortality risk, and major CVD risk compared to the Mediterranean and DASH diet scores and substantially stronger associations with all three types of events than the Planetary diet score (Table 4). In secondary analyses, when included all four prospective cohorts in the analysis (n = 191 476), the results were similar (see Supplementary data online, Appendix S16).
Table 4.
Associations of various diet scores with specific health outcome compared to the PURE healthy diet score based in PURE study participants (n = 147 642). The HR is per 20 percentile increment in the diet scorea
| PURE score | Mediterranean score | HEI 2010 score | HEI 2015 score | DASH score | Planetary health score | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AUC (95% CI); HR (95% CI) per 20 percentile increment of diet score | AUC (95% CI); HR (95% CI) per 20 percentile increment of diet score | ROC contrast estimation of this score vs. PURE diet score | AUC (95% CI); HR (95% CI) per 20 percentile increment of diet score | ROC contrast estimation of this score vs. PURE diet score | AUC (95% CI); HR (95% CI) per 20 percentile increment of diet score | ROC contrast estimation of this score vs. PURE diet score | AUC (95% CI); HR (95% CI) per 20 percentile increment of diet score | ROC contrast estimation of this score vs. PURE diet score | AUC (95% CI); HR (95% CI) per 20 percentile increment of diet score | ROC contrast estimation of this score vs. PURE diet score | |
| Total mortality | AUC = 0.61 (0.60–0.62); HR 0.91 (0.89–0.93) | AUC = 0.55 (0.54–0.56); HR 0.96 (0.94–0.98) | P < 0.001 | AUC = 0.55 (0.54–0.56); HR 0.93 (0.91–0.95) | P < 0.001 | AUC = 0.54 (0.53–0.55); HR 0.94 (0.92–0.96) | P < 0.001 | AUC = 0.53 (0.52–0.54); HR 0.96 (0.94–0.98) | P < 0.001 | AUC = 0.52 (0.51–0.53); HR 1.00 (0.98–1.03) | P < 0.001 |
| Major CVD | AUC = 0.54 (0.53–0.55); HR 0.94 (0.92–0.97) | AUC = 0.51 (0.50–0.52); HR 0.99 (0.97–1.01) | P < 0.001 | AUC = 0.52 (0.51–0.53); HR 0.95 (0.92–0.97) | P = 0.002 | AUC = 0.54 (0.53–0.55); HR 0.95 (0.93–0.97) | P = 0.185 | AUC = 0.52 (0.51–0.53); HR 0.99 (0.97–1.01) | P < 0.001 | AUC = 0.52 (0.51–0.53); HR 1.01 (0.99–1.03) | P = 0.002 |
| Composite of death or CVD | AUC = 0.58 (0.57–0.58); HR 0.93 (0.92–0.95) | AUC = 0.53 (0.52–0.54); HR 0.97 (0.96–0.99) | P < 0.001 | AUC = 0.54 (0.53–0.55); HR 0.94 (0.93–0.96) | P < 0.001 | AUC = 0.54 (0.53–0.55); HR 0.95 (0.93–0.96) | P < 0.001 | AUC = 0.53 (0.52–0.53); HR 0.97 (0.96–0.99) | P < 0.001 | AUC = 0.52 (0.51–0.53); HR 1.00 (0.98–1.02) | P < 0.001 |
Multivariable models are adjusted for age, sex, study centre (random effect), waist-to-hip ratio, education level (primary or less; secondary; trade, college, or university), wealth index, urban or rural location, physical activity (low, < 600; moderate, 600–3000; high, > 3000 metabolic equivalent of task per minute per week), smoking status (never, former, or current), history of diabetes, use of statin or anti-hypertension medications, and total energy intake.
AUC, area under ROC curve; ROC, receiver operating characteristic; HEI, Health eating Index; HR, hazard ratio; DASH, Dietary Approaches to Stop Hypertension.
Subgroup analyses by income and geographic regions
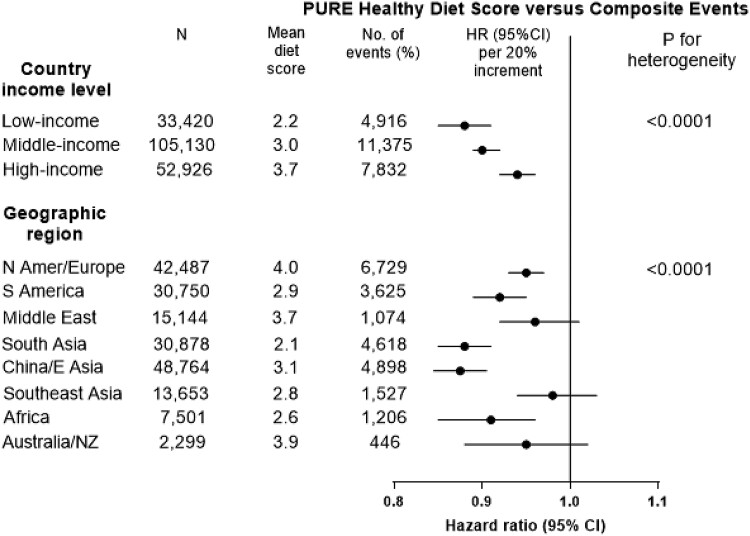
The PURE healthy diet score was associated with a lower risk of composite events across geographic regions and in countries grouped by income levels, but the associations were stronger in South Asia, China, and Africa which are also countries with low diet scores (mean score of 2.1 in South Asia, 2.6 in Africa and 3.1 in China) compared to most other regions (which have a mean diet score of 3.5) (P for heterogeneity <0.0001) and in countries with lower gross national incomes than in countries with higher national incomes (P for heterogeneity <0.0001) (Figure 7).
Figure 7.
Association of diet scores with composite of death or major cardiovascular disease, by country income region and geographic region in the four prospective studies (n = 191 476). Hazard ratios (95% CI) are multivariable adjusted. The top panel (by country income level) shows that an inadequate intake of key foods is of greatest importance in the countries with lower gross national incomes.
The other diet scores showed directionally consistent associations by geographic and income regions, except for the Planetary diet score which was associated with a higher risk of composite events in South Asia and China (P for heterogeneity = 0.040).
Discussion
In this combined analysis of data from four large, international prospective cohort studies from 80 countries and two case–control studies from 62 countries including a total of 244 597 individuals among whom nearly 50 000 events were recorded, from all inhabited continents, we showed that a 20% (1 quintile) higher PURE healthy diet score was associated with a 6% lower risk of major CVD, and 8% lower risk of mortality (Structured Graphical Abstract). The score includes foods that are part of other diet scores (i.e. fruit, vegetables, legumes, nuts, and fish), but also an element that previous scores do not include (i.e. whole-fat dairy) (see Box for the PURE Healthy Diet eating pattern). Our analyses adjusted for a large number of potential confounders including socioeconomic status (at the individual level using education and an index of wealth) and at the country level by grouping countries into categories of income and demonstrating consistent findings across country income groups. The PURE score appears to be slightly more predictive of composite events than the Mediterranean, HEI-2010, HEI-2015, and DASH diet scores and substantially more predictive than the Planetary diet score. The planetary diet score is the most restrictive and substantially limits animal foods, whereas the PURE diet score permits a moderate amount of animal foods (e.g. 1 cup milk or yogurt; 3 ounce cooked red or white meat daily). Our findings suggest that globally the key to a healthy diet is probably one that includes diverse natural foods in moderation, rather than restricting intake to a small number of food categories. Additionally, the PURE diet score was associated with lower events globally and in all continents of the world. By contrast, very little data exist on how the other diet scores perform in low and middle income countries and in populations other than those from Western countries, apart from Japan. The PURE diet score was predictive of outcomes in those with and without vascular disease or diabetes and in all world regions. However, the associations were significantly stronger in South Asia, China, and Africa, regions where the PURE diet score is low. These findings suggest that an inadequate level of consumption of key healthy foods is a larger problem than over-consumption of some nutrients or foods (such as saturated fats or whole-fat dairy and meats—all of which are consumed in lower amounts with a lower diet score) for mortality and CVD risk around the world. On this basis, given the low intake of fats and especially saturated fat (i.e. whole-fat dairy) among people with the lowest diet score (i.e. mean saturated fat intake in the lowest quintile group of the diet score was only 6.3% of energy), current targeted dietary guidance limiting the consumption of saturated fat and dairy in many populations of the world may not be warranted. Furthermore, if these associations are causal, it suggests that increasing consumption of most natural foods including whole-fat dairy, together with fruits, vegetables, nuts, legumes, and fish, in countries with lower gross national incomes (where intakes are low partly due to cultural or economic factors) would most likely produce important reductions in CVD and death. This conclusion contrasts with the usual recommendations from the Western guidelines which have largely focused on avoiding over-nutrition or excess of foods including whole-dairy rather than addressing the low intake of these foods.22
Box.
The PURE Healthy Diet score translated into a healthy eating pattern
| Eat More | Amount a,b | What counts as a serving? |
|---|---|---|
| Fruits and vegetables | 4 to 5 servings daily | 1 medium apple, banana, pear; 1 cup leafy vegs; 1/2 cup other vegs |
| Legumes | 3 to 4 servings weekly | 1/2 cup beans or lentils |
| Nuts | 7 servings weekly | 1 oz., tree nuts or peanuts |
| Fish | 2 to 3 servings weekly | 3 oz. cooked (pack of cards size) |
| Dairy | 14 servings weekly | 1 cup milk or yogurt; 1 ½ oz cheese |
| Whole grainsc | Moderate amounts (e.g. 1 serving daily) can be part of a healthy diet | 1 slice (40 g) bread; ½ medium (40 g) flat bread; ½ cup (75–120 g) cooked rice, barley, buckwheat, semolina, polenta, bulgur or quinoa |
| Unprocessed meatsc | Moderate amounts (e.g. 1 serving daily) can be part of a healthy diet | 3 oz. cooked red meat or poultry |
Amounts shown are based on intakes among people in the upper quintile category of the PURE Healthy Diet score (i.e. a diet score of 5 or higher).
Median daily intake values of food components in the overall PURE cohort are: Fruit, 145 g; vegetables, 250 g; legumes, 38 g; nuts, 9 g; fish, 12 g; dairy, 113 g; whole grains, 35 g; and unprocessed red meat or poultry, 58 g.
When red meat or whole grains are included in the diet score in a sensitivity analysis, the findings were similar (neither stronger nor weaker) (Appendix 9), indicating that a moderate amount of whole grains or unprocessed meats can be part of a healthy diet. To this end, a healthy diet can be achieved in a number of ways which does not necessarily require either including or excluding any specific food category.
Comparison with other studies
Several studies have reported that higher diet quality, as assessed by the HEI-2010, HEI-2015 and AHEI-2010 scores, a Mediterranean Diet score, and the DASH score, when comparing the extremes of quintiles or quartiles, was associated with a 10%–20% lower risk of death from any cause and a 20%–30% lower risk of CVD death.9–16 However, these findings are mainly based on observational data from the USA, Europe, and East Asia, but none from Africa, South America, Middle East, or South Asia. These patterns all have in common an emphasis on increased fruit, vegetables, legumes, nuts, whole grains, and fish, with some differences in their focus on different types of fats and the consumption of dairy or red meat. Our findings show that a similar dietary pattern but which also includes dairy (consumed mostly as whole fat dairy globally; i.e. 130.5 g/day out of 185.5 g/day of dairy consumed as whole-fat among people in the highest diet score category; ≥ 5 points) may have favourable associations with health outcomes in a global population. The PURE score showed slightly stronger associations with events than most other diet scores but markedly stronger associations with events than the Planetary score. The Mediterranean and HEI diet scores were the least restrictive, as they included higher amounts of most foods that are included in the PURE score (including total dairy), whereas the DASH diet score includes specifically low-fat dairy consumption, which is probably less preferable to the inclusion of ‘total dairy’ on a global basis. Each of these scores also included whole grains which in several cohort studies showed favourable associations with CVD events, but whole grains did not contribute to the PURE score’s usefulness in predicting risk of CVD or death (see Supplementary data online, Appendices S6, S10 and S9and S17). Therefore including a moderate amount of whole grains is optional for a healthy diet. We did not include the AHEI-201011 in our analyses as it includes alcohol as a component in the score and reliable responses were not expected as alcohol consumption is prohibited in several countries. Additionally, recent studies including PURE44 have suggested limiting or eliminating alcohol consumption. However, since the AHEI-2010 (which does not include dairy) is similar to the HEI-2010 and HEI-2015 scores (which include dairy—a food group associated with lower risk of death and CVD in PURE,17), it would not be expected that the different versions of AHEI or HEI would materially differ in predicting CVD or death. The Planetary diet was the most restrictive, additionally recommending less legumes. The DASH and Planetary diets were also higher in carbohydrates and lower in fat (mainly from saturated fat). By contrast, a high PURE score is compatible with an overall less restricted diet [56% of energy from carbohydrates, 27% from fats (9% saturated and 15% unsaturated) and 17% protein] that includes a variety of natural foods in moderate amounts. A low PURE score includes very high amounts of carbohydrates (∼two-thirds of total energy), predominantly from refined carbohydrates in the countries with lower income. Since not one diet suits all individuals, and since there are cultural preferences for different types of foods, dietary guidelines need to reflect these global variations. Further, given that different components of the healthy diet have variable availability and costs in different countries (e.g. extra-virgin olive oil and wine are consumed in very low amounts in many countries distant from the Mediterranean region), a diet pattern that includes a variety of healthy food choices may be more practical to meeting the needs of diverse populations globally than highly restrictive diets derived largely from Western and East Asian populations. This also might help to address the continuing large problem of under-nutrition in many countries or the poorer segments of high-income countries.
Implications for nutrition policy
In recent years, major new dietary recommendations have been revised to drop upper limits on total fat or dietary cholesterol, and a greater focus has been placed on protective foods (and the accompanying food matrix found in whole foods) and healthy diet patterns.3,45 Despite these changes, public purchasing choices, industry formulations, and policy actions have not yet been updated with this newer evidence. For example, the public and industry remain heavily focused on low-fat foods and have avoided nuts as they are considered to be ‘energy dense’. Similarly, policy actions (e.g. front-of-package nutrition labels in the UK seek, Chile’s black box warning labels, and recently proposed warning labels in Canada) remain mainly focused on reducing certain nutrients, such as fat, saturated fat, added sugar, and salt.46–48 These recommendations are similar to the World Health Organization’s recent proposed major focus on low-fat, low-saturated fat diets.49 By contrast, there are almost no national or international strategies and policies to increase a number of protective foods (e.g. nuts, fish or dairy). Therefore, while the findings from PURE are largely consistent with the nutrition science and modern dietary recommendations to focus on protective foods, the public’s understanding of healthy eating and relevant global policies have not yet caught up to this science.
We found that the associations of diet scores with events are markedly steeper at lower levels of the diet score (i.e. below the global median), largely represented by world regions with lower gross national incomes (e.g. mean diet score of 2.1 in South Asia, 2.6 in Africa, and 3.1 in China). The substantially lower intake of key foods among those with the lowest diet scores (i.e. people with scores in the lowest 20%) is only partly compensated with higher intake of other foods in the diet (i.e. higher rice and grains) and accompanied by markedly lower overall energy intake (1700 kcal/day in the lowest quintile compared to 2600 kcal/day in the fifth quintile). Although FFQs are not precise tools to estimate energy intake, this large difference in energy consumption and foods between those at the lowest and highest diet score category may suggest that a significant proportion of deaths and vascular events in adults around the world may be due to under-nutrition (i.e. a low intake of protective foods including dairy and low energy intake) rather than over-nutrition, which would be contrary to some current beliefs. This would also mean that recommendations to increase, decrease, or not change intake of any given food or nutrient (e.g. dairy or fats) in the population must consider the current level of intake of various foods in a country and whether consumption of specific foods is low, high or optimal. This emphasizes the need for context specific policies and priorities for different populations globally. Changes in food policies to improve the availability and affordability of healthy natural foods are needed, particularly in countries with lower income.50
Animal foods such as dairy products and meats are a major source of saturated fats, which have been presumed to adversely affect blood lipids and increase CVD and mortality.49,51–54 However, recent data suggest that the effects on lipids and BP are much more modest than previously thought. While higher intake of saturated fats is associated with slightly higher LDL cholesterol, it does not increase the atherogenic particles such as small dense LDL or Apo B.55,56 Further, recent reviews of observational studies and our findings in PURE showed that dairy foods, especially whole-fat dairy, may be protective against risk of hypertension and metabolic syndrome.19,57,58 These foods also contain potentially beneficial compounds including quality protein, milk fat globule phospholipids (mainly in whole-fat dairy), unsaturated and branched-chain fats, and numerous vitamins and minerals.50 Our findings show that intakes of dairy (up to 185 g/day; or ∼two servings/day, mainly from whole-fat dairy17) can be included with other beneficial foods as part of a healthy diet. It is noteworthy that when we included red meat in the diet score in a sensitivity analysis, the findings were similar (neither stronger nor weaker) (see Supplementary data online, Appendix S10), in keeping with our finding of a neutral association between red meat and CVD59 and providing evidence that unprocessed red meats are not a priority target for health to either avoid (as strongly emphasized by the EAT-Lancet report) nor to include (as strongly emphasized by ‘paleo’ and ‘keto’ diets) in a healthy diet. These findings are also in keeping with previous data from observational studies19,57–60 which allowed up to one daily serving of red meat in the Mediterranean diet in Prevención con Dieta Mediterránea and was associated with lower CVD and mortality.8 [Similarly, as with red meat, when whole grains are included in the diet score in a sensitivity analysis, the findings were similar, indicating that a moderate amount of whole grains can be part of a healthy diet (see Supplementary data online, Appendix S10).] To this end, a diet score of 4 (i.e. the level at which most major gains in health are observed and beyond which there is modest additional health gain) can be achieved in a number of ways which does not necessarily require either including or excluding animal foods from the diet. For instance, vegetarians can reach a diet score of 4 by consuming plenty of fruits, vegetables, legumes, nuts, whole grains, and dairy foods. Conversely, non-vegetarians can achieve the same score by consuming plenty of fruits, vegetables, and legumes together with any one of dairy or fish, or even moderate amounts of red meat or poultry. In populations globally and especially in disadvantaged populations, moderate amounts of whole-fat dairy are not harmful and can be beneficial. The ideal diet for each population is likely one of variety and moderation, which are characteristics of the PURE diet.
Strengths and limitations of this study
Our study is large and includes 244 597 people from 80 countries at different economic levels and from all inhabited continents of the world, and so our results are globally applicable and can be adapted by different regions based on available foods, taste and cultural preferences. The diet score derived from the PURE study was replicated in five independent studies with different designs and populations and was observed within different groups of countries by income category. Our analysis is based on 15 707 deaths and 17 878 MIs and 19 294 strokes. Therefore our results are robust.
Our study has some potential limitations. First, diet (as in most large epidemiologic studies) was self-reported and variations in reporting might lead to random errors that could dilute real associations between diet scores and clinical outcomes. Therefore, the beneficial effects of a healthier diet may be larger than what we have estimated. In some of the studies (e.g. ORIGIN), we have repeat measures of diet and adjusting for regression dilution biases slightly strengthens the associations (see Supplementary data online, Appendix S18). Second, we did not examine the role of individual types of fruits and vegetables as components in the diet score, since the power to detect associations of the different types of fruits and vegetables vs. CVD or mortality is low (i.e. given that the number of events per type of fruit and vegetable was relatively low). Recent evidence suggests that bioactive compounds and, in particular, polyphenols which are found in certain fruit or vegetables (e.g. berries, spinach, and beans) may be especially protective against CVD.61 Future work is needed to sort out what proportion of fruit and vegetable-related health benefits are driven by delivering dietary polyphenols or other nutrients. Third, in observational studies, the possibility of residual confounding from unmeasured or imprecise measurement of covariates cannot be completely ruled out—especially given that the differences in risk of clinical events is modest (∼10%–20% relative differences). Ideally, large randomized trials are essential to definitively clarify the clinical impact on events of a policy of proposing a dietary pattern in populations. While such trials are difficult and expensive to conduct, they are justifiable given the important public health impact of clarifying the health effects of diet (an essential exposure globally). Fourth, the use of the median intake of each food component as a cut-off in the scoring scheme for each diet may not reflect the full range of consumption or provide a meaningful indicator of consumption associated with the disease. However, the use of quintiles instead of medians within each study or within each region yielded the same results indicating the robustness of our findings. Fifth, the level of intake to meet the cut-off threshold for each food group in the diet score may differ between countries. However, in sensitivity analyses where we used region-specific median cut-offs to classify participants on each component of the diet score, the results were similar to using the overall cohort median of each food component. Further, with unprocessed red meat and whole grains included or excluded from the diet score in these sensitivity analyses, the results were again similar (see Supplementary data online, Appendix S10). Sixth, misclassification of exposures cannot be ruled out as we did not have repeat measures of diet in all studies and a full length FFQ was used only in PURE. However, the ORIGIN study in which we conducted repeat diet assessments at 2 years showed similar results based on the first vs. second diet assessments. This indicates that misclassification of dietary intake during follow-up was not sufficiently of major concern to undermine our findings (see Supplementary data online, Appendix S19). Lastly, one unique aspect of the study is the focus on only protective foods, i.e. a dietary pattern score that highlights what is missing from the food supply, especially in poorer world regions, but this does not negate the importance of limiting the consumption of harmful foods such as highly processed foods.62 While the PURE diet score had significantly stronger associations with events than other diet scores, the HRs were only slightly larger for PURE than for most other diet scores. However, the Planetary score was the least predictive of events. Our analyses provide empirical evidence that all diet scores (other than the Planetary diet score) are of value to predict death or CVD globally and in all regions of the world.
Conclusions
Consumption of a diet comprised of higher amounts of fruits, vegetables, nuts, legumes, and a moderate amount of fish and whole-fat dairy is associated with a lower risk of CVD and mortality in all world regions, but especially in countries with lower income where consumption of these natural foods is low. Similar associations were found with the inclusion of meat or whole grain consumption in the diet score (in the ranges common in the six studies that we included). Our findings indicate that the risks of deaths and vascular events in adults globally are higher with inadequate intake of protective foods.
Authors’ contributions
A.M. designed the present analyses, performed its statistical analysis, and wrote the first draft of the manuscript. M.D. reviewed and provided critical comments on drafts. S.Y. designed all the studies included in the present analyses, conceived and initiated the overall PURE study, supervised its conduct and data analysis, and provided critical comments on all drafts of the manuscript. S.R. co-ordinated the worldwide PURE study and reviewed and commented on drafts. K.T. was the co-principal investigator of the PURE study and reviewed and commented on drafts. H.G. and S.Y. were joint PIs of the ORIGIN study; S.Y. and K.T. lead the ONTARGET/TRANSCEND studies; S.Y. was the PI of the INTERHEART study and M.O.D. and S.Y. were the PIs of the INTERSTROKE study. All other authors co-ordinated the study and collected data for the PURE study in their respective countries and provided comments on drafts of the manuscript.
Supplementary data
Supplementary data is available at European Heart Journal online.
Pre-registered clinical trial number
None supplied.
Ethical approval
This study was approved by the research ethics committees at all participating centres and at Hamilton Health Sciences, Hamilton, Ontario, Canada.
Supplementary Material
Contributor Information
Andrew Mente, Population Health Research Institute, Hamilton Health Sciences and McMaster University, 2nd Floor, Room C2-105, 237 Barton St East, Hamilton, Ontario L8L 2X2, Canada; Department of Health Research Methods, Evidence, and Impact, McMaster University, 1280 Main St W, Hamilton, Ontario L8S 4L8, Canada.
Mahshid Dehghan, Population Health Research Institute, Hamilton Health Sciences and McMaster University, 2nd Floor, Room C2-105, 237 Barton St East, Hamilton, Ontario L8L 2X2, Canada.
Sumathy Rangarajan, Population Health Research Institute, Hamilton Health Sciences and McMaster University, 2nd Floor, Room C2-105, 237 Barton St East, Hamilton, Ontario L8L 2X2, Canada.
Martin O’Donnell, Department of Medicine, McMaster University, Hamilton, Ontario, Canada; HRB-Clinical Research Facility, University of Galway, Galway, Connacht, Ireland.
Weihong Hu, Population Health Research Institute, Hamilton Health Sciences and McMaster University, 2nd Floor, Room C2-105, 237 Barton St East, Hamilton, Ontario L8L 2X2, Canada.
Gilles Dagenais, Department of Medicine, Université Laval Institut universitaire de cardiologie et de pneumologie de Québec, Quebec City G1V 4G5, Canada.
Andreas Wielgosz, Department of Medicine, University of Ottawa, Ottawa, Ontario, Canada.
Scott A. Lear, Faculty of Health Sciences, and Department of Biomedical Physiology & Kinesiology, Simon Fraser University Vancouver, Burnaby, British Columbia, Canada.
Li Wei, State Key Laboratory of Cardiovascular Disease, Fuwai Hospital, National Center for Cardiovascular Disease, Peking Union Medical College & Chinese Academy of Medical Sciences, Beijing, Xicheng District, China.
Rafael Diaz, Estudios Clinicos Latinoamerica ECLA, Universidad Nacional de Rosario, Rosario, Santa Fe, Argentina.
Alvaro Avezum, International Research Center, Hospital Alemão Oswaldo Cruz & UNISA, Sao Paulo, São Paulo estado, SP Brazil.
Patricio Lopez-Jaramillo, Masira Research Institute, Medical School, Universidad de Santander (UDES), Bucaramanga, Santander, Colombia.
Fernando Lanas, Francisco Salazar, Universidad de La Frontera, Temuco, Araucanía, Chile.
Sumathi Swaminathan, Division of Nutrition, St John's Research Institute, Koramangala, Bangalore, Karnataka, India.
Manmeet Kaur, Postgraduate Institute of Medical Education and Research, School of Public Health, Chandigarh, Punjab and Haryana, India.
K Vijayakumar, Health Action by People, Amrita Institute of Medical Sciences, Trivandrum, Kerala, India.
Viswanathan Mohan, Director and Chief of Diabetes Research, Madras Diabetes Research Foundation and Dr. Mohan’s Diabetes Specialities Centre, Chennai, Tamil Nadu, India.
Rajeev Gupta, Eternal Heart Care Centre and Research Institute, Rajasthan University of Health Sciences, Jaipur, Rajasthan, India.
Andrzej Szuba, Department of Internal Medicine, Wroclaw Medical University, 4th Military Hospital, Wroclaw, Lower Silesian Voivodeship, Poland.
Romaina Iqbal, Department of Community Health Sciences and Medicine, Aga Khan University, Karachi, Sindh, Pakistan.
Rita Yusuf, Department of Life Sciences, Independent University, Bangladesh, Bashundhara, Dhaka, Dhaka District, Bangladesh.
Noushin Mohammadifard, Isfahan Cardiovascular Research Center, Cardiovascular Research Institute, Isfahan University of Medical Sciences, Isfahan, Isfahan Province, Iran.
Rasha Khatib, Departments of Neurology, Northwestern University Feinberg School of Medicine, Chicago, IL, USA.
Nafiza Mat Nasir, Department of Primary Care Medicine, Faculty of Medicine, Universiti Teknologi MARA (UiTM), Shah Alam, Selangor, Malaysia.
Kubilay Karsidag, Department of Internal Medicine, Division of Endocrinology, Medical Faculty of Istanbul University, Istanbul, Istanbul Province, Turkey.
Annika Rosengren, Department of Molecular and Clinical Medicine, Sahlgrenska Academy, University of Gothenburg and Sahlgrenska University Hospital/Östra Hospital, Gothenburg, Västergötland, Sweden.
Afzalhussein Yusufali, Hatta Hospital, Dubai Health Authority, Dubai Medical University, Dubai, United Arab Emirates.
Edelweiss Wentzel-Viljoen, Faculty of Health Sciences, Centre of Excellence for Nutrition, Potchefstroom, North West Province, South Africa.
Jephat Chifamba, College of Health Sciences, Physiology Department, University of Zimbabwe, Harare, Harare Metropolitan Province, Zimbabwe.
Antonio Dans, Department of Medicine, University of the Philippines, Ermita, Manila, Metro Manila, Philippines.
Khalid F Alhabib, Department of Cardiac Sciences, King Fahad Cardiac Center, College of Medicine, King Saud University, Riyadh, Riyadh Province, Saudi Arabia.
Karen Yeates, Department of Medicine, Queen's University, 94 Stuart Street, Etherington Hall, Kingston, Ontario, Canada.
Koon Teo, Population Health Research Institute, Hamilton Health Sciences and McMaster University, 2nd Floor, Room C2-105, 237 Barton St East, Hamilton, Ontario L8L 2X2, Canada; Department of Health Research Methods, Evidence, and Impact, McMaster University, 1280 Main St W, Hamilton, Ontario L8S 4L8, Canada; Department of Medicine, McMaster University, Hamilton, Ontario, Canada.
Hertzel C Gerstein, Population Health Research Institute, Hamilton Health Sciences and McMaster University, 2nd Floor, Room C2-105, 237 Barton St East, Hamilton, Ontario L8L 2X2, Canada; Department of Health Research Methods, Evidence, and Impact, McMaster University, 1280 Main St W, Hamilton, Ontario L8S 4L8, Canada; Department of Medicine, McMaster University, Hamilton, Ontario, Canada.
Salim Yusuf, Population Health Research Institute, Hamilton Health Sciences and McMaster University, 2nd Floor, Room C2-105, 237 Barton St East, Hamilton, Ontario L8L 2X2, Canada; Department of Health Research Methods, Evidence, and Impact, McMaster University, 1280 Main St W, Hamilton, Ontario L8S 4L8, Canada; Department of Medicine, McMaster University, Hamilton, Ontario, Canada.
Data availability
No additional data are available. The PURE study is a large international prospective cohort study that is still ongoing. Data are not publicly available at this time.
Funding
S.Y. is supported by the Marion W Burke endowed chair of the Heart and Stroke Foundation of Ontario.
The PURE study is an investigator-initiated study that is funded by the Population Health Research Institute, Hamilton Health Sciences Research Institute (HHSRI), the Canadian Institutes of Health Research, Heart and Stroke Foundation of Ontario, Support from Canadian Institutes of Health Research’s Strategy for Patient Oriented Research, through the Ontario SPOR Support Unit, as well as the Ontario Ministry of Health and Long-Term Care and through unrestricted grants from several pharmaceutical companies [with major contributions from AstraZeneca (Canada), Sanofi-Aventis (France and Canada), Boehringer Ingelheim (Germany and Canada), Servier, and GlaxoSmithKline], and additional contributions from Novartis and King Pharma and from various national or local organizations in participating countries.
These include: Argentina: Fundacion ECLA (Estudios Clínicos Latino America); Bangladesh: Independent University, Bangladesh and Mitra and Associates; Brazil: Hospital Alemão Oswaldo Cruz, São Paulo, Brazil; Canada: This study was supported by an unrestricted grant from Dairy Farmers of Canada and the National Dairy Council (U.S.), Public Health Agency of Canada and Champlain Cardiovascular Disease Prevention Network; Chile: Universidad de La Frontera [EXD05-0003]; China: National Center for Cardiovascular Diseases and ThinkTank Research Center for Health Development; Colombia: Colciencias (grant 6566-04-18062 and grant 6517-777-58228); India: Indian Council of Medical Research; Malaysia: Ministry of Science, Technology and Innovation of Malaysia (grant number: 100-IRDC/BIOTEK 16/6/21 [13/2007], and 07-05-IFN-BPH 010), Ministry of Higher Education of Malaysia (grant number: 600-RMI/LRGS/5/3 [2/2011]), Universiti Teknologi MARA, Biostatistics & Data Repository Sector, National Institute of Health, Setia Alam—for the data linkage service, and National Registration Department (JPN) for their willingness to share their mortality records for research purposes, Universiti Kebangsaan Malaysia (UKM-Hejim-Komuniti-15-2010); occupied Palestinian territory: the United Nations Relief and Works Agency for Palestine Refugees in the Near East, occupied Palestinian territory; International Development Research Centre, Canada; Philippines: Philippine Council for Health Research and Development; Poland: Polish Ministry of Science and Higher Education (grant number: 290/W-PURE/2008/0), Wroclaw Medical University; Saudi Arabia: Saudi Heart Association, Dr. Mohammad Alfagih Hospital, The Deanship of Scientific Research at King Saud University (Research group number: RG-1436-013), Riyadh; Saleh Hamza Serafi Chair for Research of Coronary Heart Disease, Umm AlQura University, Makkah, Saudi Arabia; South Africa: The North-West University, SA and Netherlands Programme for Alternative Development, National Research Foundation, Medical Research Council of South Africa, The South Africa Sugar Association, Faculty of Community and Health Sciences; Sweden: Grants from the Swedish state under an agreement between the Swedish government and the county councils concerning economic support of research and education of doctors (ALFGBG-966211); the Swedish Heart and Lung Foundation (2021-0345); the Swedish Research Council (2018-02527); AFA insurance (16-0334); and the Swedish Council for Health, Working Life and Welfare (2013-0325); Turkey: Metabolic Syndrome Society, AstraZeneca, Sanofi Aventis; United Arab Emirates: Sheikh Hamdan Bin Rashid Al Maktoum Award For Medical Sciences and Dubai Health Authority, Dubai.
Patient and public involvement
No patients were involved in setting the research question or outcome measures, in the design and implementation of the study, or in dissemination plans of this research.
Dissemination declaration
The findings of this study will be disseminated to relevant audiences through McMaster University communications and Twitter. Each of the co-authors will disseminate the findings in their countries through local presentations. The authors who are invited speakers will present the findings at national and international conferences.
Transparency
The lead author (A.M.) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted and that any discrepancies from the study as planned have been explained.
References
- 1. Yusuf S, Rangarajan S, Teo K, Islam S, Li W, Liu L, et al. Cardiovascular risk and events in 17 low-, middle-, and high-income countries. N Engl J Med 2014;371:818–827. 10.1056/NEJMoa1311890 [DOI] [PubMed] [Google Scholar]
- 2. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation 2015;131:e29–e322. 10.1161/CIR.0000000000000152 [DOI] [PubMed] [Google Scholar]
- 3. Dietary Guidelines Advisory Committee . Scientific Report of the 2015 Dietary Guidelines Advisory Committee. 2015. http://www.health.gov/dietaryguidelines/2015-scientific-report/. Accessed 12 Nov 2019.
- 4. GBD 2017 Diet Collaborators . Health effects of dietary risks in 195 countries, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet 2019;393:1958–1972. 10.1016/S0140-6736(19)30041-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Micha R, Shulkin ML, Peñalvo JL, Khatibzadeh S, Singh GM, Rao M, et al. Etiologic effects and optimal intakes of foods and nutrients for risk of cardiovascular diseases and diabetes: systematic reviews and meta-analyses from the nutrition and chronic diseases expert group (NutriCoDE). PLoS One 2017;12:e0175149. 10.1371/journal.pone.0175149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Willett W, Rockström J, Loken B, Springmann M, Lang T, Vermeulen S, et al. Food in the anthropocene: the EAT–lancet commission on healthy diets from sustainable food systems. Lancet 2019;393:447–492. 10.1016/S0140-6736(18)31788-4 [DOI] [PubMed] [Google Scholar]
- 7. Howard BV, Van Horn L, Hsia J, Manson JE, Stefanick ML, Wassertheil-Smoller S, et al. Low-fat dietary pattern and risk of cardiovascular disease: the women’s health initiative randomized controlled dietary modification trial. JAMA 2006;295:655–666. 10.1001/jama.295.6.655 [DOI] [PubMed] [Google Scholar]
- 8. Estruch R, Ros E, Salas-Salvadó J, Covas MI, Corella D, Arós F, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 2013;368:1279–1290. 10.1056/NEJMoa1200303 [DOI] [PubMed] [Google Scholar]
- 9. Guenther PM, Casavale KO, Reedy J, Kirkpatrick SI, Hiza HA, Kuczynski KJ, et al. Update of the healthy eating Index: hEI-2010. J Acad Nutr Diet 2013;113:569–580. 10.1016/j.jand.2012.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Krebs-Smith SM, Pannucci TE, Subar AF, Kirkpatrick SI, Lerman JL, Tooze JA, et al. Update of the healthy eating Index: hEI-2015. J Acad Nutr Diet 2018;118:1591–1602. 10.1016/j.jand.2018.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr 2012;142:1009–1018. 10.3945/jn.111.157222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med 2003;348:2599–2608. 10.1056/NEJMoa025039 [DOI] [PubMed] [Google Scholar]
- 13. Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med 2008;168:713–720. 10.1001/archinte.168.7.713 [DOI] [PubMed] [Google Scholar]
- 14. Sotos-Prieto M, Bhupathiraju SN, Mattei J, Fung TT, Li Y, Pan A, et al. Association of changes in diet quality with total and cause-specific mortality. N Engl J Med 2017;377:143–153. 10.1056/NEJMoa1613502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Akbaraly TN, Ferrie JE, Berr C, Brunner EJ, Head J, Marmot MG, et al. Alternative healthy eating index and mortality over 18 y of follow-up: results from the Whitehall II cohort. Am J Clin Nutr 2011;94:247–253. 10.3945/ajcn.111.013128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fung TT, Rexrode KM, Mantzoros CS, Manson JE, Willett WC, Hu FB. Mediterranean diet and incidence of and mortality from coronary heart disease and stroke in women. Circulation 2009;119:1093–1100. 10.1161/CIRCULATIONAHA.108.816736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dehghan M, Mente A, Rangarajan S, Sheridan P, Mohan V, Iqbal R, et al. Association of dairy intake with cardiovascular disease and mortality in 21 countries from five continents (PURE): a prospective cohort study. Lancet 2018;392:2288–2297. 10.1016/S0140-6736(18)31812-9 [DOI] [PubMed] [Google Scholar]
- 18. Dehghan M, Mente A, Zhang X, Swaminathan S, Li W, Mohan V, et al. Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): a prospective cohort study. Lancet 2017;390:2050–2062. 10.1016/S0140-6736(17)32252-3 [DOI] [PubMed] [Google Scholar]
- 19. Mente A, Dehghan M, Rangarajan S, McQueen M, Dagenais G, Wielgosz A, et al. Association of dietary nutrients with blood lipids and blood pressure in 18 countries: a cross-sectional analysis from the PURE study. Lancet Diabetes Endocrinol 2017. ;5:774–787. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- 20. Siri-Tarino PW, Sun Q, Hu FB, Krauss RM. Meta-analysis of prospective cohort studies evaluating the association of saturated fat with cardiovascular disease. Am J Clin Nutr 2010;91:535–546. 10.3945/ajcn.2009.27725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Micha R, Wallace SK, Mozaffarian D. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: a systematic review and meta-analysis. Circulation 2010;121:2271–2283. 10.1161/CIRCULATIONAHA.109.924977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. de Goede J, Soedamah-Muthu SS, Pan A, Gijsbers L, Geleijnse JM. Dairy consumption and risk of stroke: a systematic review and updated dose-response meta-analysis of prospective cohort studies. J Am Heart Assoc 2016;5:e002787. 10.1161/JAHA.115.002787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. de Oliveira Otto MC, Lemaitre RN, Song X, King IB, Siscovick DS, Mozaffarian D. Serial measures of circulating biomarkers of dairy fat and total and cause-specific mortality in older adults: the cardiovascular health study. Am J Clin Nutr 2018;108:476–484. 10.1093/ajcn/nqy117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tong TY, Appleby PN, Key TJ, Dahm CC, Overvad K, Olsen A, et al. The associations of major foods and fibre with risks of ischaemic and haemorrhagic stroke: a prospective study of 418 329 participants in the EPIC cohort across nine European countries. Eur Heart J 2020;41:2632–2640. 10.1093/eurheartj/ehaa007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tong TYN, Appleby PN, Bradbury KE, Perez-Cornago A, Travis RC, Clarke R, et al. Risks of ischaemic heart disease and stroke in meat eaters, fish eaters, and vegetarians over 18 years of follow-up: results from the prospective EPIC-Oxford study. BMJ 2019;366:l4897. 10.1136/bmj.l4897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Anand SS, Hawkes C, de Souza RJ, Mente A, Dehghan M, Nugent R, et al. Food consumption and its impact on cardiovascular disease: importance of solutions focused on the globalized food system: a report from the workshop convened by the world heart federation. J Am Coll Cardiol 2015;66:1590–1614. 10.1016/j.jacc.2015.07.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yusuf S, Islam S, Chow CK, Rangarajan S, Dagenais G, Diaz R, et al. Use of secondary prevention drugs for cardiovascular disease in the community in high-income, middle-income, and low-income countries (the PURE study): a prospective epidemiological survey. Lancet 2011;378:1231–1243. 10.1016/S0140-6736(11)61215-4 [DOI] [PubMed] [Google Scholar]
- 28. Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, Schumacher H, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008;358:1547–1559. 10.1056/NEJMoa0801317 [DOI] [PubMed] [Google Scholar]
- 29. Yusuf S, Teo K, Anderson C, Pogue J, Dyal L, Copland I, et al. Effects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high risk patients intolerant to angiotensin-converting enzyme inhibitors. Lancet 2008;372:1174–1183. 10.1016/S0140-6736(08)61242-8 [DOI] [PubMed] [Google Scholar]
- 30. Gerstein H, Yusuf S, Riddle MC, Ryden L, Bosch J; Origin Trial Investigators . Rationale, design, and baseline characteristics for a large international trial of cardiovascular disease prevention in people with dysglycemia: the ORIGIN trial (outcome reduction with an initial glargine intervention). Am Heart J 2008;155:26–32. 10.1016/j.ahj.2007.09.009 [DOI] [PubMed] [Google Scholar]
- 31. ORIGIN Trial Investigators; Gerstein HC, Bosch J, Dagenais GR, Díaz R, Jung H, Maggioni AP, et al. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med 2012;367:319–328. 10.1056/NEJMoa1203858 [DOI] [PubMed] [Google Scholar]
- 32. Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 2004;364:937–952. 10.1016/S0140-6736(04)17018-9 [DOI] [PubMed] [Google Scholar]
- 33. Rosengren A, Hawken S, Ounpuu S, Sliwa K, Zubaid M, Almahmeed WA, et al. Association of psychosocial risk factors with risk of acute myocardial infarction in 11119 cases and 13648 controls from 52 countries (the INTERHEART study): case-control study. Lancet 2004;364:953–962. 10.1016/S0140-6736(04)17019-0 [DOI] [PubMed] [Google Scholar]
- 34. O’Donnell MJ, Xavier D, Liu L, Zhang H, Chin SL, Rao-Melacini P, et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet 2010;376:112–123. 10.1016/S0140-6736(10)60834-3 [DOI] [PubMed] [Google Scholar]
- 35. O’Donnell MJ, Chin SL, Rangarajan S, Xavier D, Liu L, Zhang H, et al. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study. Lancet 2016;388:761–775. 10.1016/S0140-6736(16)30506-2 [DOI] [PubMed] [Google Scholar]
- 36. Harrell FE. Regression Modeling Strategies with Applications to Linear Models, Logistic Regression and Survival Analysis. New York: Springer-Verlag; 2001. [Google Scholar]
- 37. Dehghan M, Mente A, Teo KK, Gao P, Sleight P, Dagenais G, et al. Relationship between healthy diet and risk of cardiovascular disease among patients on drug therapies for secondary prevention: a prospective cohort study of 31 546 high-risk individuals from 40 countries. Circulation 2012;126:2705–2712. 10.1161/CIRCULATIONAHA.112.103234 [DOI] [PubMed] [Google Scholar]
- 38. Iqbal R, Anand S, Ounpuu S, Islam S, Zhang X, Rangarajan S, et al. Dietary patterns and the risk of acute myocardial infarction in 52 countries: results of the INTERHEART study. Circulation 2008;118:1929–1937. 10.1161/CIRCULATIONAHA.107.738716 [DOI] [PubMed] [Google Scholar]
- 39. Jenkins DJA, Dehghan M, Mente A, Bangdiwala SI, Rangarajan S, Srichaikul K, et al. Glycemic index, glycemic load, and cardiovascular disease and mortality. N Engl J Med 2021;384:1312–1322. 10.1056/NEJMoa2007123 [DOI] [PubMed] [Google Scholar]
- 40. Burke DL, Ensor J, Riley RD. Meta-analysis using individual participant data: one-stage and two-stage approaches, and why they may differ. Stat Med 2017;36:855–875. 10.1002/sim.7141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. IntHout J, Ioannidis JP, Borm GF. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-laird method. BMC Med Res Methodol 2014;14:25. 10.1186/1471-2288-14-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hanley JA, McNeil BJ. A method of comparing the areas under receive operating characteristic curves derived from the same cases. Radiology 1983;148:839–843. 10.1148/radiology.148.3.6878708 [DOI] [PubMed] [Google Scholar]
- 43. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837–845. 10.2307/2531595 [DOI] [PubMed] [Google Scholar]
- 44. Smyth A, Teo KK, Rangarajan S, O'Donnell M, Zhang X, Rana P, et al. Alcohol consumption and cardiovascular disease, cancer, injury, admission to hospital, and mortality: a prospective cohort study. Lancet 2015;386:1945–1954. 10.1016/S0140-6736(15)00235-4 [DOI] [PubMed] [Google Scholar]
- 45. Brazil. Ministry of Health of Brazil. Secretariat of Health Care. Primary Health Care Department. Dietary Guidelines for the Brazilian Population. Brasília: Ministry of Health of Brazil, 2015. Internet. https://bvsms.saude.gov.br/bvs/publicacoes/dietary_guidelines_brazilian_population.pdf (9 February 2022, date last accessed).
- 46. Reyes M, Garmendia ML, Olivares S, Aqueveque C, Zacarías I, Corvalán C. Development of the Chilean front-of-package food warning label. BMC Public Health 2019;19:906. 10.1186/s12889-019-7118-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Department of Health. Food Standards Agency. Guide to Creating a Front of Pack (FoP) Nutrition Label for Pre-packed Products Sold Through Retail Outlets. London: UK-FSA, 2016. Updated Nov 2016. Internet. https://www.food.gov.uk/sites/default/files/media/document/fop-guidance_0.pdf(12 November 2019, date last accessed).
- 48. Consultation on front-of-package nutrition labelling . Health Canada. Internet: https://www.canada.ca/en/health-canada/programs/front-of-package-nutrition-labelling.html(12 November 2019, date last accessed).
- 49. World Health Organization . Healthy diet: Key facts. 23 October 2018. Internet: https://www.who.int/news-room/fact-sheets/detail/healthy-diet(1 August 2019, date last accessed).
- 50. Mozaffarian D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: a comprehensive review. Circulation 2016;133:187–225. 10.1161/CIRCULATIONAHA.115.018585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dietary Guidelines for Americans, 2015–2020 . United States Department of Agriculture.
- 52. Internet: https://health.gov/dietaryguidelines/2015/guidelines/(1 August 2019, date last accessed).
- 53. Eckel RH, Jakicic JM, Ard JD, de Jesus JM, Houston Miller N, Hubbard VS, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation 2014;129:S76–S99. 10.1161/01.cir.0000437740.48606.d1 [DOI] [PubMed] [Google Scholar]
- 54. Keys A, Anderson JT, Grande F. Serum cholesterol response to changes in the diet: IV. Particular saturated fatty acids in the diet. Metabolism 1965;14:776–787. 10.1016/0026-0495(65)90004-1 [DOI] [PubMed] [Google Scholar]
- 55. Chiu S, Bergeron N, Williams PT, Bray GA, Sutherland B, Krauss RM. Comparison of the DASH (dietary approaches to stop hypertension) diet and a higher-fat DASH diet on blood pressure and lipids and lipoproteins: a randomized controlled trial. Am J Clin Nutr 2016;103:341–347. 10.3945/ajcn.115.123281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gijsbers L, Ding EL, Malik VS, de Goede J, Geleijnse JM, Soedamah-Muthu SS. Consumption of dairy foods and diabetes incidence: a dose-response meta-analysis of observational studies. Am J Clin Nutr 2016;103:1111–1124. 10.3945/ajcn.115.123216 [DOI] [PubMed] [Google Scholar]
- 57. Alexander DD, Bylsma LC, Vargas AJ, Cohen SS, Doucette A, Mohamed M, et al. Dairy consumption and CVD: a systematic review and meta-analysis. Br J Nutr 2016;115:737–750. 10.1017/S0007114515005000 [DOI] [PubMed] [Google Scholar]
- 58. Bhavadharini B, Dehghan M, Mente A, Rangarajan S, Sheridan P, Mohan V, et al. Association of dairy consumption with metabolic syndrome, hypertension and diabetes in 147 812 individuals from 21 countries. BMJ Open Diabetes Res Care 2020;8:e000826. 10.1136/bmjdrc-2019-000826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Iqbal R, Dehghan M, Mente A, Rangarajan S, Wielgosz A, Avezum A, et al. Associations of unprocessed and processed meat intake with mortality and cardiovascular disease in 21 countries [prospective urban rural epidemiology (PURE) study]: a prospective cohort study. Am J Clin Nutr 2021;114:1049–1058. 10.1093/ajcn/nqaa448 [DOI] [PubMed] [Google Scholar]
- 60. Lee JE, McLerran DF, Rolland B, Chen Y, Grant EJ, Vedanthan R, et al. Meat intake and cause-specific mortality: a pooled analysis of Asian prospective cohort studies. Am J Clin Nutr 2013;98:1032–1041. 10.3945/ajcn.113.062638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sesso HD, Manson JE, Aragaki AK, Rist PM, Johnson LG, Friedenberg G, et al. Effect of cocoa flavanol supplementation for the prevention of cardiovascular disease events: the COCOA supplement and multivitamin outcomes study (COSMOS) randomized clinical trial. Am J Clin Nutr 2022;115:1490–1500. 10.1093/ajcn/nqac055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bonaccio M, Di Castelnuovo A, Ruggiero E, Costanzo S, Grosso G, De Curtis A, et al. Joint association of food nutritional profile by nutri-score front-of-pack label and ultra-processed food intake with mortality: Moli-Sani prospective cohort study. BMJ 2022;378:e070688. 10.1136/bmj-2022-070688 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No additional data are available. The PURE study is a large international prospective cohort study that is still ongoing. Data are not publicly available at this time.