Abstract
The renin-angiotensin system (RAS) is a hormonal cascade that contributes to several disorders: systemic hypertension, heart failure, kidney disease, and neurodegenerative disease. Activation of the RAS can promote inflammation and fibrosis. Drugs that target the RAS can be classified into 3 categories, AT1 angiotensin receptor blockers (ARBs), angiotensin-converting enzyme (ACE) inhibitors, and renin inhibitors. The therapeutic efficacy of current RAS-inhibiting drugs is limited by poor penetration across the blood-brain barrier, low bioavailability, and to some extent, short half-lives. Nanoparticle-mediated drug delivery systems (DDSs) are possible emerging alternatives to overcome such limitations. Nanoparticles are ideally 1–100 nm in size and are considered efficient DDSs mainly due to their unique characteristics, including water dispersity, prolonged half-life in blood circulation, smaller size, and biocompatibility. Nano-scale DDSs can reduce the drug dosage frequency and acute toxicity of drugs while enhancing therapeutic success. Different types of nanoparticles, such as chitosan, polymeric, and nanofibers, have been examined in RAS-related studies, especially in hypertension, cardiovascular disease, and COVID-19. In this review article, we summarize the physical and chemical characteristics of each nanoparticle to elaborate on their potential use in RAS-related nano-drug delivery research and clinical application.
Keywords: Renin-angiotensin system, Nanoparticles, Drug delivery, Hypertension, Cardiovascular disease, COVID-19
Graphical abstract
1. Introduction
The Renin-Angiotensin System (RAS) is an enzymatic cascade first identified as a hormonal system of the kidney that regulates blood pressure [1]. It is now known to be a major endocrine system that regulates blood pressure, water, and electrolyte balance, mediating many other physiological and pathophysiological actions [2]. The classical RAS is activated by the release of renin from the juxtaglomerular cells of the kidney into circulation. Renin cleaves blood-borne angiotensinogen protein into angiotensin I (Ang I). Subsequently, Ang I is converted to Ang II by angiotensin-converting enzyme (ACE), which is found most abundantly in the lung tissues and vascular endothelium [3,4] (Fig. 1). The octapeptide Ang II is the most active hormone of the RAS, exerting its actions primarily on the AT1 Ang II type receptor (AT1R). The classical axis of the RAS, the ACE/Ang II/AT1R axis, activates homeostatic mechanisms to conserve and replenish fluid and sodium as well as restore normal blood pressure. However, it also activates several inflammatory pathways, the overactivation of which can contribute to hypernatremia, volume overload, and hypertension, with damage to the heart and kidneys. The counter-regulatory arm of the RAS, the ACE2/Ang (1–7)/Mas receptor axis, has anti-inflammatory and depressor actions [5,6].
Fig. 1.
The schematic diagram of the classical RAS (ACE/AngII/ATIR axis and the counterregulatory ACE2/Ang1-7/Mas axis arm). The figure represents only the AT1 receptor-rich organs.
Notably, angiotensinogen, a nonfunctional glycosylated serpin globulin protein composed of 452 amino acids, is secreted constitutively into the bloodstream, mainly from the liver and adipose tissues [7]. The angiotensinogen gene is also expressed in all the tissues where a local RAS has been reported. Brain angiotensinogen is synthesized and secreted extracellularly by astrocytes [8]. Angiotensinogen synthesis is stimulated by glucocorticoids, thyroid hormones, estrogen, and Ang II [9]. The codependency of angiotensinogen and Ang II levels in the bloodstream arise when Ang II binds to and activates hepatic AT1 receptors. The AT1 receptor plays a pivotal role in the RAS, regulating the cardiovascular and most other RAS functions upon binding Ang II [10]. AT2R is a less characterized receptor of the RAS. It is also activated by Ang II, Ang III, Ang (1–7) at higher concentrations, and potentially by the variant Ang peptides Alatensin (Ala [11] Ang II) and almandine (Ala [11] Ang (1–7)) [12]. AT2R is considered the functional antagonist of the predominantly expressed AT1R [13]. Recent studies indicate that selective activation of the AT2R mediates anti-inflammatory and pro-natriuretic effects that are cardioprotective, renoprotective, and anti-hypertensive, improving insulin sensitivity and reversing adiposity [[14], [15], [16]]. The RAS can also operate locally in organs such as the brain, kidney, heart, liver, and gastrointestinal tract [17].
The RAS plays its most vital role in regulating blood pressure and cardiometabolic function, but it also contributes to obesity, aging, cancer, cognitive impairment, and neurodegeneration [[18], [19], [20]]. The RAS causes cardiac diseases such as heart failure, coronary artery disease with myocardial infarction, and cardiomyopathy, largely arising from its hypertensive effects [21]. The RAS also generates reactive oxygen species leading to oxidative stress, which, in the central nervous system (CNS), contributes to neurodegenerative diseases. Angiotensin-converting enzyme-2 (ACE2) is a portal to the entrance of the SARS-CoV-2 virus into cells, as well as being the portal for SARS-CoV-1 [[22], [23], [24], [25]].
Drugs that target the RAS are classified into i) Renin inhibitors, e.g., aliskiren, ii) ACE inhibitors, e.g., captopril, iii) AT1 receptor (AT1R) antagonists, also known as AT1 angiotensin receptor blockers (ARBs), e.g., losartan. In addition, stimulation of aldosterone release is an integral component of the RAS such that it is sometimes referred to as the renin-angiotensin-aldosterone system (RAAS). Hence, one could add a 4th group as RAAS blockers: iv) Aldosterone (mineralocorticoid) receptor antagonists, e.g., spironolactone. There is also a dual inhibitor drug that blocks both the AT1R and neprilysin, the enzyme that metabolizes atrial natriuretic peptide and Ang II (valsartan and sacubitril combination, Entresto®) [26]. The therapeutic success of drugs that target the RAS is limited by the relative lack of ability to cross the blood-brain barrier (BBB), poor bioavailability, and relatively short half-life of some of these drugs [27,28]. Nanoscale-drug delivery systems (DDSs) appear to provide solutions for limitations of currently available RAS inhibitor therapies: facilitating BBB passage, augmenting bioavailability, and extending the duration of action (Table 1). Having a high surface area to volume ratio with better penetration and accumulation capacity in target tissues, nanoscale-DDSs can reduce drug dosing frequency and adverse side effects caused by peak drug levels [11]. Advanced multifunctional nanoparticles (NP) offer great potential to markedly improve the delivery of loaded drug molecules to target cells/tissues. Nanoscale-DDSs and gene delivery systems consistently provide higher efficacy in many therapeutic applications, including RAS-related diseases [29,30]. The most common NPs used in RAS-related research have been developed using synthetic polymers, natural polymers (chitosan), and nanofiber NPs [11,31,32]. Thus, this review systematically discusses the nanotechnological therapeutic approaches for RAS-related diseases.
Table 1.
Comparison between different RAS drug categories in clinical use, their actions, limitations, and possible nanoparticle approaches for their distribution limitations.
| RAS Drug Category | Drugs | Actions | Limitations | Nanoparticle approach | Ref. |
|---|---|---|---|---|---|
| Direct Renin Inhibitors | Aliskiren | Inhibit the initial rate-limiting Step of the RAS that converts Angiotensinogen to Angiotensin I by Renin enzyme. | Poor bioavailability Weak antihypertensive effect Short half-life |
Possibly will enhance the bioavailability and half-life to improve the antihypertensive effect | [[33], [34], [35]] |
| ACE Inhibitors (ACEI) | Captopril Enalapril Fosinopril Ramipril others |
Inhibit the catalysis of Angiotensin I to Angiotensin II Lowers high blood pressure to provide the cardiovascular protection |
Treatment-related adverse side effects such as dry cough and angioedema | Deliver lower dose of the drugs to the targeted location by using nanoparticles would help to reduce the side effects. | [33,36] |
| AT1 angiotensin receptor blockers (ARBs) | Losartan Candesartan Valsartan Eprosartan Telmisartan Olmesartan Irbesartan Azilsartan |
ARBs block the AT1 receptors reducing the effect of Angiotensin II on Blood pressure (BP) | ARBs cause fewer side effects than ACEI. Poor BBB penetrability (Except telmisartan and candesartan) |
Possibly enhance the BBB penetration by loading the ARBs on NPs | [28,37] |
| Mineralocorticoid receptor antagonists (MRAs) | Spiranolactone Finerenone Eplerenone Mexrenone Canrenone |
Decrease the sodium reabsorption to enhance the water excretion by the kidney. Eventually lowers the BP and fluid around the heart to provide cardiovascular protection. | Adverse side effects such as increased urination. | Possibly deliver the drug in a lower dosage to minimize the side effects while acquiring high treatment efficacy. | [38,39] |
2. Nanoparticles
Nanoparticles (NPs) are ideally 1–100 nm in size, with an outer layer of organic and inorganic coatings that determine their physical and chemical properties. Numerous studies currently in progress leverage the potential benefit of NPs for improved drug delivery, although only a few have been adapted for clinical use. The NPs are preferred for drug delivery due to their unique characteristics, including biocompatibility, water dispersibility, biodegradability, and smaller size [40]. NPs display drastically different characteristics than their bulk materials due to size reduction down to the atomic level [41]. Bulk materials display relatively constant properties, whereas NPs display variable characteristics due to surface atom enhancement arising from the size reduction [42]. Even though NP approaches are widespread for the experimental delivery of anticancer drugs for oncology studies [40,43], they have had limited use in the experimental administration of drugs for the treatment of RAS-related diseases. Hypertension is the RAS disease that is the greatest focus of NP research [32,44]. Thus, this section discusses several different types of NPs that have been used to administer RAS-inhibiting drugs.
2.1. Polymeric nanoparticles
Polymeric NPs are classified into two major categories; natural and synthetic. For example, natural NPs can be made from carbohydrate polymers, such as chitosan and cellulose-based polymers, or from proteinaceous materials, such as albumin and gelatin, whereas various types of synthetic polymers or copolymers are used to form synthetic NP matrices [45]. Natural polymeric NPs are mostly biodegradable and biocompatible. However, batch-to-batch inconsistency has limited their widespread usage. Synthetic polymeric NPs may be preferred drug delivery due to their superior tunability, which can generate more consistent chemical compositions [46].
2.1.1. Natural polymeric nanoparticles
2.1.1.1. Polysaccharide-based NPs (chitosan)
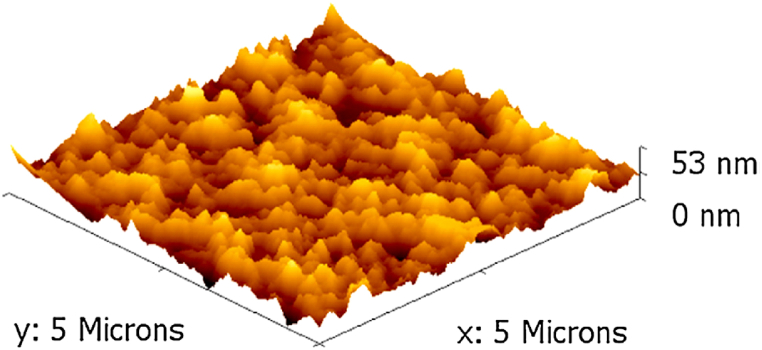
Polysaccharides are versatile nanomaterials with the potential for diverse biomedical applications [47]. Among these, chitosan has been one of the most extensively studied natural polymers for drug delivery for the last three decades. Chitosan is a natural polymer derived from the partial deacetylation of chitin. It is a cationic polysaccharide biopolymer composed of glucosamine and N-acetylglucosamine copolymers [48]. The common precursors for chitosan NPs are crustacean shells such as lobster, shrimp, or crab [49]. Lysozyme enzymes hydrolyze chitosan into nontoxic, non-immunogenic, non-carcinogenic amine sugar products readily absorbed and metabolized by humans [50]. More focused reviews on chitosan-based biomaterials and chitosan NPs are found elsewhere [51,52]. Interestingly, the chitosan NP particle size can be less than 70 nm (Fig. 2), displaying a high surface-to-volume ratio. Chitosan has been widely used for the development of advanced nano-DDSs [[53], [54], [55], [56]]. Drug encapsulation efficacy increases with the decrease in molecular weight of the chitosan [57]. One drawback of such chitosan NPs is that the rate of degradation increases with decreases in molecular weight [58]. Chitosan NPs have been used to treat RAS-related hypertension and COVID-19. Chitosan NPs have been used as carriers for ACE2 as well as RAS blockers e.g., for the delivery of valsartan, captopril, and non-RAS antihypertensives, e.g. amlodipine [11,59].
Fig. 2.
3-dimensional AFM image of the chitosan NPs. The figure was reprinted from Ref. [11] with the permission of the publisher.
2.1.1.2. Protein-based nanoparticles
Protein-based or proteinaceous materials are considered to be attractive candidate materials for nanoscale drug and gene delivery systems [60,61]. Some of the proteins used to make these NPs, such as albumin and gelatin, are listed as ‘generally recognized as safe (GRAS) materials, facilitating their applicability as DDSs. Proteins have diverse functional groups for further chemical modifications with amphiphilic properties for self-assembly [62]. Of note, albumin-based NPs have continued to draw increasing attention since the FDA approval of Abraxane®, the first-ever albumin-bound drug (paclitaxel) as an NP DDS to enter the market. The involvement of such protein-based NPs in RAS-inhibiting drugs has yet to be developed; hence this can be a novel nanomaterial that can be used to develop novel RAS-inhibiting DDSs.
2.1.2. Synthetic polymeric nanoparticles
Synthetic polymeric NPs are colloidal particles composed of synthetic polymers or copolymers that are either biodegradable such as poly (lactide-co-glycolide) (PLGA), poly (amino acid)-based polymers, poly (e-caprolactone), polyanhydrides, polyorthoesters, and polyphosphazene, or non-biodegradable, such as vinyl or acrylic polymers/copolymers and polyethylene glycols (PEG) with appropriate molecular weights, from which the drugs can diffuse out [[63], [64], [65], [66], [67], [68], [69], [70]]. The characteristic properties of synthetic polymeric NPs, such as lipophilicity, biocompatibility, and surface charge, can be easily tuned during synthesis [46]. Synthetic polymeric NPs are potential candidates for diagnostics and drug delivery due to advances in controlled drug release, theranostic potential, protection of drug molecule cargos, specific targeting moieties, and facilitation of a high therapeutic index [71,72]. The endocytic process drives the cellular uptake of polymeric NPs [73]. As with classical mechanisms, the controlled release kinetics from synthetic polymeric NPs is accomplished by a structural modification to promote drug diffusion out of the polymer matrix [74]. For hydrophobic polymers such as polyanhydrides and polyorthoesters, drug release is mainly modulated by the rate of surface erosion of the matrix, whereas for PLGA-based polymers, drug release is affected by bulk erosion and formation of water channels within the matrix [75,76]. However, contemporary researchers focus on exogenous and endogenous stimuli that trigger drug release mechanisms that are selective for microenvironments of specific diseases [77]. The major drawback of synthetic polymeric NPs is their larger size, normally >100 nm. By controlling co-monomers or block compositions (relative lengths of the hydrophilic and hydrophobic segment within a polymer chain), synthetic polymeric NPs can be made nontoxic by surface modifications such as coating their surfaces with polyethylene glycol (PEG)-phospholipid copolymers. Polylactide-co-glycolide (PLGA) polymers have also been used as carriers for selected antihypertensive small peptides and model drugs that treat RAS-related diseases, especially hypertension and COVID-19 3,4.
2.2. Metallic nanoparticles
Metallic NPs are synthesized from any metal or metal oxide, and the most commonly used metals are silver, gold, iron, and platinum [78]. Metallic NPs display remarkable physicochemical characteristics that differ from their bulk materials. The most advantageous metallic NP properties are wavelength-dependent photoluminescence, localized surface plasmon resonance, high water dispersity, and smaller size [79]. Metallic NPs are widely used for electronic chip manufacturing. Furthermore, they can function as enzymatic biosensors for biomedical applications [80]. The major limitation of metallic NPs is toxicity at high concentrations. There is a tendency for metallic NPs to alter their structure, becoming chemically modified toxins that can accumulate in tissues [81]. This limits the use of metallic NPs in biomedical nano-DDS in-vivo.
2.2.1. Gold nanoparticles (AuNPs)
Colloidal AuNP use dates back to Faraday’s studies in the 18th century [82]. AuNPs are the most widely used NP for biomedical applications. AuNPs are the nano-type that enables many unprecedented applications. Bulk gold is an inert yellow solid, whereas AuNPs form a wine-red color colloidal solution with antioxidant properties [83]. The size of AuNPs ranges from 1 to 100 nm. They can be found in many shapes, such as spherical, octahedral, decahedral, tetrahedral, nano-triangles, and nano-prisms [84]. AuNPs are commonly used in biotechnology, biomedicine, and radiation medicine due to their ability to conjugate probes and small molecules with low toxicity relative to other metal-based NPs. Cells take up colloidal AuNPs via specific receptor-ligand interactions or non-receptor-specific diffusion transporters [82]. AuNPs are stored inside cells in perinuclear vesicular compartments upon cellular uptake. However, a disadvantage is that AuNP exposure is cytotoxic for extended periods [85]. AuNPs have been used in RAS research as highly sensitive and selective plasmonic bio-sensors for ACE detection at concentrations as low as 0.4 mU/mL [86].
2.2.2. Silver nanoparticles (AgNPs)
Silver is a noble metal that has undisputed advantages, such as electrical conductivity and resistance to oxidation, featuring plasmonic and antibacterial properties [87]. Ag has been used as an antimicrobial agent for centuries; the Phoenicians used Ag vessels to store water and wine during their long voyages [88]. Also, ancient Egyptians used Ag compounds to prevent wound infections [89]. AgNPs are colloidal particles that feature interesting properties such as plasma waves, enabling surface plasmon resonance, interaction with an electromagnetic field, high diffusivity of the surface atoms, and melting at extremely low temperatures [90,91]. AgNPs generally range from 1 to 100 nm in diameter. AgNPs have broad applications, such as their antimicrobial properties, which make them popular in clothing manufacturing, food preservation, water purification, and biomedical fields. Smaller-sized AgNPs enable BBB penetration [92]. Thus, for brain delivery of RAS-blocking drugs, AgNPs not only facilitate their entry into the brain but also have potential antioxidant properties that reduce brain oxidative stress [30].
2.3. Nanofibers (NFs)
Nanofibers are made from carbon and/or semiconductor materials and most polymers [93]. NFs are applied in many fields, including material science, tissue engineering, chemical industry, and energy storage, due to their remarkable properties, such as high surface area, high mechanical stability, and good electrical conductivity. Thermal phase separation, electrospinning, vapor deposition, and self-assembly are some of the chemical, mechanical and synthetic methodologies for the formation of NFs [94]. Electrospinning is the most popular methodology; it exhibited promising results in tissue engineering. Electrospinning controls the thickness and composition of the NFs along with porosity [95]. In the electrospinning process, NFs with a diameter of 50–1000 nm can be produced by applying an electrical potential to the polymer solution [96]. When the electrical potential is applied, mutual charge repulsion of the polymer solution occurs, leading to a force opposite to the surface tension. Upon increasing the electrical potential, the opposite force overcomes the surface tension at a critical point, producing a jet-like ejection of NFs. The ejected charged jet-like particles form randomly oriented nanofibers that can be collected on a rotating or stationary grounded metallic collector [97]. Electrospinning can also use natural polymers such as collagen and chitosan for NF production. Carbon NFs can be prepared from the chemical vapor deposition method [98]. NFs have the potential for controlled nano-drug delivery to improve the therapeutic efficacy of the drugs. As noted below, NFs carrying RAS-interacting drugs have been developed, displaying promising outcomes [31].
3. Nanoparticle approaches for the treatment of RAS-related diseases
3.1. Hypertension
Hypertension is a global health crisis that leads to complications such as vascular damage, heart failure, stroke, and kidney failure, causing premature death and disability. Hypertension causes 7.6 million deaths per year worldwide [99,100]. The recommended systolic and diastolic blood pressures (BP) for healthy adults are 120/80 mmHg [101,102]. When the systolic and diastolic BPs are ≥140/90 mmHg, it is considered to be hypertension [100]. If hypertension is left untreated for a long period of time, it can lead to the impairments mentioned above. Currently, numerous antihypertensive therapeutics are in clinical practice. They can be classified into four main classes; 1) RAS inhibitors (Renin inhibitors, ARBs and ACEIs), 2) β-blockers (BBs), 3) calcium channel blockers (CCBs), and 4) diuretics [103,104]. ACEI, and renin inhibitors block steps in the metabolic cascade of the RAS, while BBs inhibit stimulation of renin release from the kidney, and ARBs block the AT1R that mediates the pressor actions of Ang II. CCBs reduce the influx of Ca2+ into cardiac myocytes, pacemaker cells, and vascular smooth muscle cells to reduce cardiac output and vascular resistance, while diuretics regulate the fluids by promoting the excretion of water and electrolytes [105,106]. Many antihypertensive drugs, such as valsartan, have relatively low oral bioavailability, while others have short half-lives [107,108]. Advanced drug delivery systems, such as NP-mediated delivery systems, can overcome these limitations, thereby increasing therapeutic efficacy.
In 2015, Antal et al., introduced aliskiren-loaded magnetic polymeric NPs for hypertension treatment [32]. Aliskiren is a direct renin inhibitor that binds to the active site of the renin, thereby decreasing the production of Ang I. However, a limiting factor of aliskiren in terms of therapeutic action is its low bioavailability [109]. They formulated aliskiren-loaded magnetic poly (D, L) lactide (PLA) NPs to study their effect on systolic blood pressure of male spontaneously hypertensive rats (SHR) [32]. The magnetic Fe3O4 NPs were synthesized by the coprecipitation of the Fe2+ and Fe3+ in an alkaline medium. They were coated with sodium oleate to create a stable water-based magnetic fluid. The magnetic NPs and aliskiren were entrapped into the PLA polymeric NPs. Scanning electron microscopy (SEM) demonstrated a spherical shape of the NPs, whereas dynamic light scattering (DLS), differential centrifugal sedimentation (DCS), and NP tracking analysis (NTA) determined that the particle diameters ranged between 58 and 227 nm. Loading of aliskiren was 6.3% weight/weight (w/w) drug content with 75% drug entrapment. In vivo systolic blood pressure (SBP) was measured in a 12-week-old male SHR. The free aliskiren (25 mg/kg per day) treated SHR group displayed a reduced SBP from ∼195 mmHg to 179 ± 1.8 mmHg compared to the non-drug treated control group. The aliskiren-loaded PLA-NPs (25 mg/kg/day) reduced SBP even lower (154 ± 3.9 mmHg) (Fig. 3). Thus, the authors claim nano-encapsulation may protect aliskiren from degradation while enhancing its bioavailability. However, a limitation of the study is the lack of cytotoxicity analysis of the PLA-NPs.
Fig. 3.
Systolic blood pressure measurements of the SHR for the non-treated control group, free aliskiren, free PLA-NP, and aliskiren-PLA-NP treated group [32]. Reprinted from Ref. [32] with the permission of the publisher.
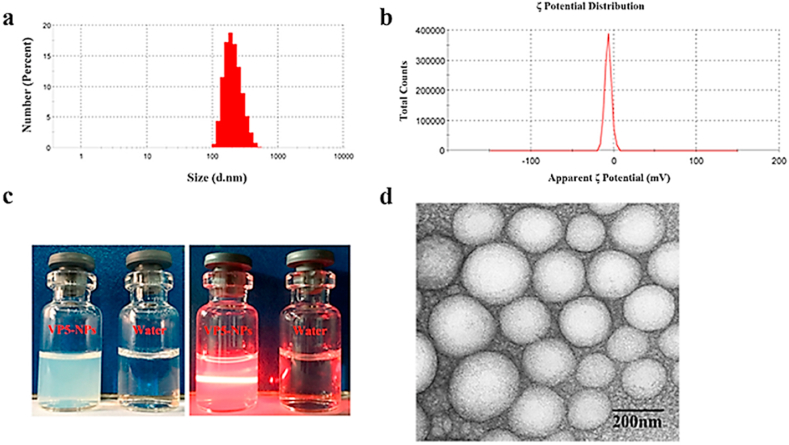
In 2016, Yang and co-workers [110] synthesized poly-(lactic-co-glycolic) acid NPs (PLGA) for the delivery of an antihypertensive small peptide VP5, a 5 amino acid chain (VLPVP), that has similarities to small proline-containing tripeptides reported to lower blood pressure in humans through ACE inhibition [111]. Yang and co-workers prepared the VP5 loaded PLGA NPs by the double emulsion solvent evaporation method [110]. The inner aqueous phase was prepared by dissolving the VP5 in deionized water, and the organic phase containing PLGA was emulsified with the inner aqueous phase to form the primary emulsion. Subsequently, the primary emulsion was added to a polyvinyl alcohol solution to prepare the double emulsion. The formed NPs were purified by ultracentrifugation. The obtained VP5-NPs were non-charged with a 223.7 ± 2.3 nm particle size with a narrow distribution of 0.12 ± 0.001 (mean ± SD) (Fig. 4a). The VP5 entrapment efficacy (EE%) and the drug loading capacity (DL%) were 87.37 ± 0.92% and 2.1 ± 0.17, respectively. TEM images confirmed the spherical shape of the particles (Fig. 4d). In vivo analyses were conducted with SHRs. Various dosages of free VP5 (0.4, 0.8, 1.6 mg/kg) and VP5-NP (0.8 mg/kg) were administered orally. Surprisingly, the 0.8 mg/kg dose of VP5-NPs reduced SBP significantly more than all doses of free VP5 over all time periods ranging from 2 to 72 h. The likely reasons behind this are 1) controlled release of VP5 by the NP system, and 2) NP-encapsulated VP5 was protected from enzymatic degradation, leading to enhanced bioavailability of the VP5. Thus, the authors claim their VP5-NP system will be a potential therapy for the administration of peptide drugs for hypertension. However, the authors have not reported the extent of ACE inhibition or the cytotoxicity profiles of the free NPs in vitro or in vivo.
Fig. 4.
The characteristics of the VP5-NPs a) Particle size, b) Potential distribution, c) The appearance under room light and the Tyndall effect, and d) the TEM image [110]. The figure was reprinted from Yu et al., 2016 [110] with the permission of the publisher.
In 2016, Niaz et al., introduced chitosan NPs to deliver the ACE inhibitor captopril, the calcium channel blocker amlodipine, or the angiotensin receptor blocker (ARB) valsartan as treatment for hypertension [11]. The drug-loaded chitosan NPs were formulated by the sonication-assisted ionic gelation method. Chitosan solution was prepared by dissolving 0.3% (w/v) chitosan in 1% (v/v) acetic acid solution. Separately, 1% tripolyphosphate (TPP) solutions with each drug solution were prepared. A 50 mg quantity of the drug was dissolved in 2 mL of TPP solution, which was then added to 25 mL of chitosan solution. The mixture was stirred at room temperature for 30 min followed by ultrasonication (25 kHz) for 30 min. The drug-loaded chitosan-NPs were obtained using centrifugation. The EE % of captopril, amlodipine and valsartan in chitosan NPs were 92 ± 1.6, 87 ± 0.5, and 91 ± 0.9%, respectively. The high EE reportedly depended on the hydrophobicity of the cross-linking of the –COOH groups of the drugs with the -NH2 groups of the chitosan NP. SEM images confirmed the drug-free chitosan NPs were smooth in shape and spherical. The captopril-loaded NPs are larger than the drug-free chitosan NPs, remaining in the spherical shape and nanometer scale with a homogeneous distribution. The amlodipine-loaded chitosan NPs was described as also being regular smooth particles. The valsartan-loaded NPs were irregular in shape, rough, and aggregated. The temperature sensitivity of the valsartan leads to a susceptibility to temperature-induced dryness and structural alterations of the NPs, ultimately leading to their degradation. In vitro drug release profiles of the captopril-loaded chitosan-NPs promisingly demonstrated higher retention of the drugs with slow release from the chitosan NP system. The drug release kinetics were higher at 37 °C compared to 4 °C. However, the authors reported only the captopril drug release profiles. Drug release kinetics data for amlodipine and valsartan were not presented. However, chitosan NP-captopril systems could enhance captopril’s bioavailability, absorption, and retention time, leading to reduced drug dosage and frequency of dosing with fewer side effects. In vivo assessments with toxicity screens will determine if these chitosan NP-captopril systems can live up to their potential for superior delivery of this antihypertensive drug.
3.2. Cardiovascular diseases
Cardiovascular diseases (CVDs) are still the foremost contributor to global mortality and morbidity. The world health organization (WHO) reported that CVDs are expected to be responsible for 22.2 million deaths annually by 2030 [112]. Heart attacks and strokes represent 7.3 and 6.2 million deaths annually [[113], [114], [115]]. CVDs lead to many complex disorders that affect normal heart function and blood flow, with the most serious clinical manifestation of CVD being a myocardial infarction. General risk factors involved in CVDs are hypertension, rheumatic heart disease, ischemic heart disease (angina), cerebrovascular disease (stroke), and inflammatory myocarditis [115]. The peptidergic enzymatic cascade of the RAS and its AT1 receptor homeostatically controls cardiovascular physiology but also plays a key role in cardiovascular pathogenesis and heart failure. Overactivation of the RAS causes adverse outcomes of fibrosis, intense vasoconstriction, and cardiac remodeling, necessitating effective pharmacological antagonism of the RAS. One of the cornerstones of clinical therapy for heart failure is ARBs [116]. Even though the current ARBs display a strong binding affinity for AT1 receptors, their limited tissue distribution precludes access to all AT1Rs mediating various disease states. Besides the ARBs, the bioactive peptide fragment of Ang II, Ang 1–7, acting as a counter-regulatory RAS ligand, contributes to cardioprotection [117]. However, the extremely short half-life, unfavorable pharmacokinetics, and non-target-specific delivery limit the efficacy of free bioactive peptides. Thus, the use of NP technology to improve peptidic RAS drug bioavailability and efficacy promises to provide better control of the pathophysiology of the RAS.
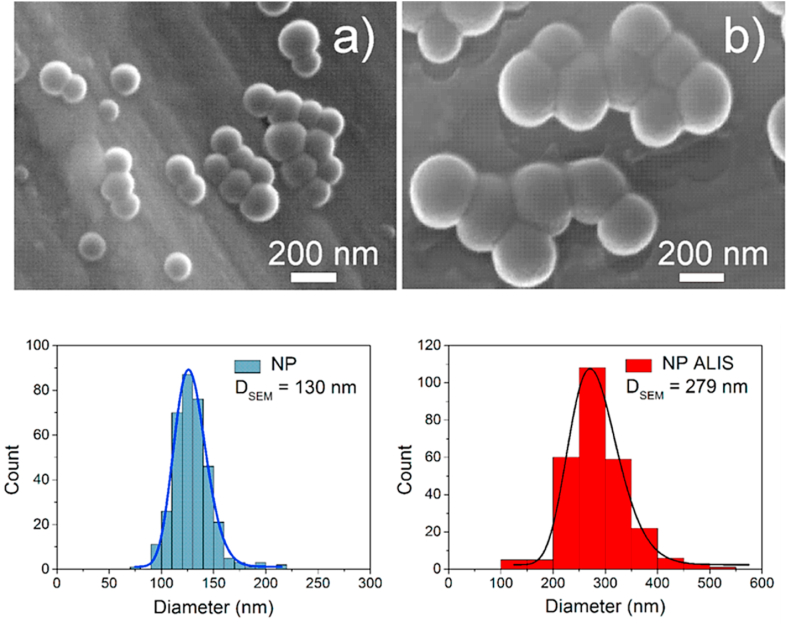
In 2019, Pechanova et al. loaded the renin inhibitor aliskiren onto polylactic acid (PLA) polymeric NPs to study its effects on blood pressure, nitric oxide synthase activity in the heart, vasoactivity of the mesenteric artery and collagen accumulation in the heart of the SHR [118]. PLA NPs were prepared by the nanoprecipitation method. The SEM images determined the average particle size of the free PLA NPs, and the aliskiren-loaded PLA NP to be 130 and 279 nm, respectively (Fig. 5 a-b). The free PLA NP zeta potential was −17 mV, while the drug-loaded PLA NP was −24 mV, suggesting that a portion of the drug is on the surface of the NP. The DLS hydrodynamic size confirmed that the aliskiren-loaded PLA NP was stable over 16 weeks, while temperature stability measurements verified the absence of thermal aggregation of the aliskiren-loaded PLA NP over an extended time. The external sink method drug release profiles demonstrated 85% and 95% cumulative aliskiren release by 24 h at pH 7.4 and 2.0, respectively. However, the drug release profile determined that the drug release took place in two phases; the first phase, representing ∼95% of the aliskiren released from the PLA NP occurred within 75 min reflecting aliskiren trapped on the surface of the NP, whereas the second phase represented the release of the drugs trapped in the PLA NP matrix. Free aliskiren, administered by oral gavage (25 mg/kg/day) for 3 weeks, lowered BP by 10%, whereas the same dose of aliskiren in the PLA-NPs lowered BP by 25%. Vasoactivity studies of mesenteric arteries displayed that the magnitude of the phenylephrine-induced (10−6 mol/L) precontraction force in arteries from PLA-NP-Aliskiren treated SHR was significantly smaller (3.44 ± 0.1 mN/mm2) than in the non-drug treated control group (4.93 ± 0.12 mN/mm2) and the free aliskiren treated group (4.86 ± 0.09 mN/mm2). The authors suggested that the PLA-NP-loaded aliskiren significantly increased its potency, which could decrease the likelihood of adverse side effects. The study provides an example of the superiority of NP-mediated RAS-inhibiting drug delivery. However, a limitation of this study is the lack of detail on the synthesis, purification steps, and toxicity of this NP preparation.
Fig. 5.
The SEM images with the corresponding histograms below the image of the (a) free PLA NP and (b) Aliskiren loaded PLA-NP. The figure was reprinted from Ref. [118] with permission of the publisher.
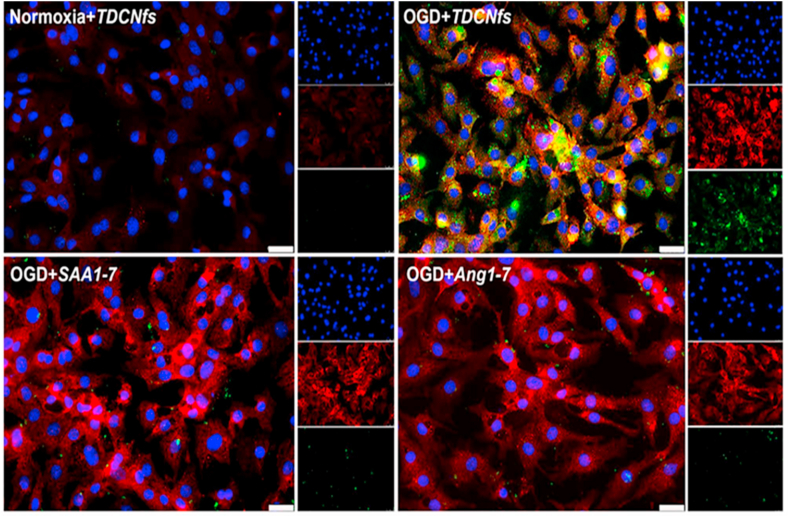
In 2021, Wen et al. introduced a supramolecular nanofiber to co-deliver the therapeutic heptapeptide angiotensin (1–7; DRVYIHP) and the ARB telmisartan to counter-regulate the RAS through combined therapy [31]. The supramolecular nanofiber was constructed with a customized fluorophore (NBD)-conjugated peptide NBD-DFDFDYDEDEG-DRVYIHP (SAA1-7). Supramolecular nano-fiber-telmisartan doped co-assembled nanofibers (TDCNfs) were prepared through a series of solvent exchange steps followed by heating incubation, gelation, centrifugation, and lyophilization. TDCNfs were characterized by circular dichroism, Fourier transforms infrared (FTIR), and fluorescence spectroscopy. The TEM images indicated a uniform nanofiber diameter of 7.6 ± 1.8 nm. The stability measurement indicated that the colloidal solution of the TDCNfs in PBS (pH 7.4) was stable for one month without precipitation or agglomeration. Cell viability measurements were carried out with primary neonatal rat cardiomyocytes (NRCMs) and neonatal rat cardio fibroblasts (NRCfs). Surprisingly, the cell viability was increased with increasing TDCNf concentration (0.01–10 μM), indicating that the TDCNfs had a pro-survival effect. However, cell viability decreased at 100 μM, suggesting that the pro-survival effect of TDCNfs occurs at a limited concentration range, with higher concentrations being cytotoxic. Using surface plasmon resonance imaging, the reported KD values for the binding affinity of the TDCNfs, SAA1-7, and Ang-1-7 to recombinant human MasR (rhMasR) were 16.5, 12.6, and 10.3 nM, respectively. The corresponding binding Response Units (RU) to Ang 1–7, SAA1-7, and TDCNfs were 65.1, 62.3, and 58.2, respectively, indicating similar affinities at the rhMasR. The co-localization of the TDCNfs in the cells was analyzed by confocal microscopy. The primary neonatal rat cardiomyocytes (NRCMs) expressed high levels of AT1R under oxygen/glucose-deprived (OGD) conditions displayed in red fluorescence (Fig. 6). TDCNfs displayed a high binding affinity for the AT1Rs, with co-localization observed in the plasma membrane region under OGD conditions of the NRCMs, whereas the Ang1-7 and SAA1-7 had scant co-localization with the AT1Rs. Thus, overall, TDCNfs have a strong affinity towards AT1R compared to the reference peptides, most likely facilitated by telmisartan binding to AT1R (Fig. 6). The study validated that TDCNfs bind AT1R making them a promising approach for future RAS-related research.
Fig. 6.
Confocal imaging of AT1R (red) and TDCNfs (green) inOGD rat cardiac myocytes. NBDAng1-7, NBDSAA1-7 showed no binding to the OGD myocytes, while TDCNfs colocalized with the OGD myocytes (yellow). The three single channel images are shown as smaller images to the right of the colocalization images. Scale bar 25 μM. The figure was reprinted from Ref. [31] upon the permission of the publisher.
4. Rationale for brain delivery of RAS blockers
The brain RAS includes the peptides angiotensin (Ang) I, Ang II, Ang III, Ang IV, Ang I-7, and Ang 3–7. The ligands interact with RAS receptors AT1, AT2, AT4, and Mas, localized within the brain. The AT1 and AT2 receptor types are well characterized, while the AT4 and Mas receptors are less well-defined. The ability of ARBs to protect cognitive function by inhibiting the neurotoxic effects of AT1R stimulation is of considerable interest as a prophylactic treatment for dementia [119]. Conversely, the AT4R has been suggested to have a neuroprotective role in the brain [120]. AT4R is found largely in the neocortex, hippocampus, amygdala, and nucleus basalis of Meynert [121,122]. The conversion of Ang II to Ang III and IV reportedly facilitates dopamine release in the striatum and ACh release in the hippocampus [123,124]. Defects in both dopaminergic and cholinergic signaling are associated with neurodegenerative diseases, leading to suggestions that drugs mimicking Ang IV may have a neuroprotective role in mitigating these diseases. Even though the synthetically made Ang IV peptide analogs displayed high cognitive-enhancing effects in several animal models of dementia, their use in clinical therapeutic practice has been limited due to their rapid degradation with short half-lives and poor BBB penetration due to their hydrophilicity. Thus, nano-drug delivery techniques that can transport RAS-related drugs have the potential to treat brain diseases.
4.1. Alzheimer’s disease
Alzheimer’s disease (AD) is an irreversible neurodegenerative disease leading to intellectual deterioration, with loss of memory and language ability. The primary pathological hallmarks of AD are amyloid-beta (Aβ) deposition, intraneuronal accumulation of neurofibrillary tangles (NFT) formed by hyperphosphorylated tau protein aggregates, degeneration of cholinergic neurons in the basal forebrain, along with the neurotoxic accumulation of reactive oxygen species (ROS) [[125], [126], [127], [128]]. However, the ACE gene has recently been identified as a risk factor for AD [129]. The ACE gain of function coding variant ACE1 R1279Q has recently been associated with the occurrence of AD, leading to experimental studies of mice with the ACE1 R1279Q knocking displaying hippocampal neurodegeneration and inflammation, with female mice having greater hippocampal neurodegeneration than males [130]. Recent studies in mice [131,132] suggest that one of the mechanisms for the beneficial effects of ARB losartan could be through the activation of the AT4 receptor. In past years, RAS inhibition therapy has been identified as a means to delay the progression of cognitive decline in AD, extending beyond the antihypertensive effects of RAS inhibitors [[133], [134], [135]]. Thus, the RAS may play a key role in AD pathogenesis.
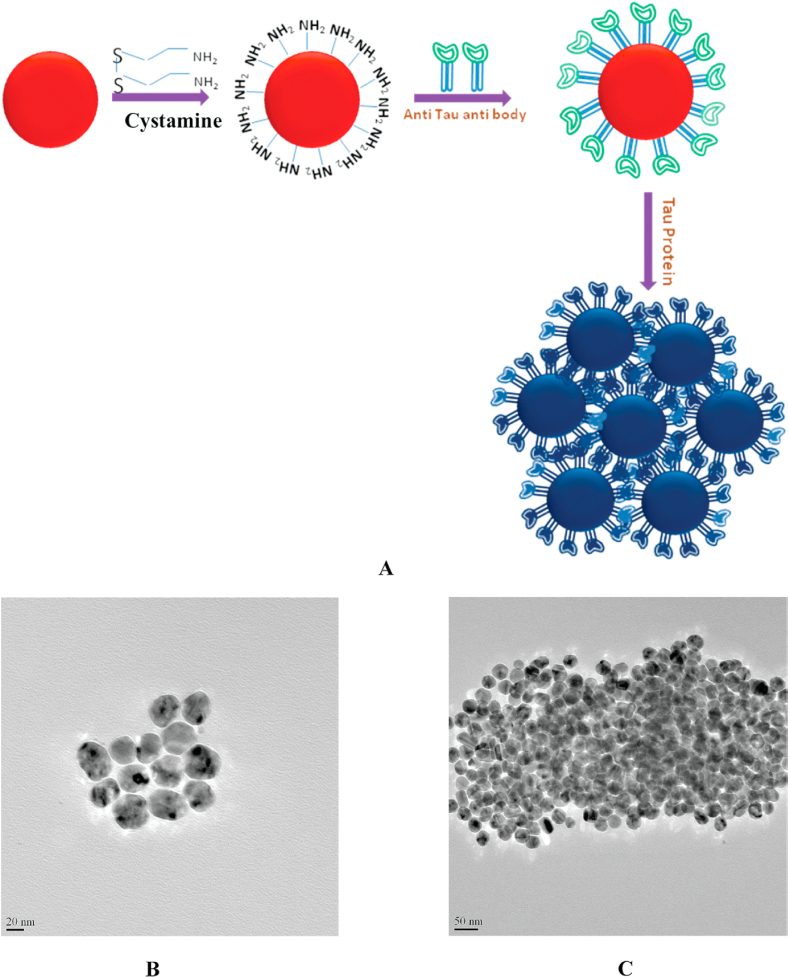
Nanoparticle approaches are of considerable interest for AD treatment, though they have not yet been applied to RAS-related AD research studies [128]. The most well-studied NPs in AD research are metallic and polymeric. They have been used for diagnostic purposes: for instance, in 2009, Neely et al. developed an AuNP-based NP two-photon Rayleigh scattering (TPRS) assay to analyze the tau protein concentration in the AD brain by introducing the AuNP as a colorimetric sensor [136]. Anti-tau antibody (tau-mab) conjugated AuNPs were used to detect the tau concentration through an antibody-antigen interaction. Antibody-conjugated AuNPs were aggregated in the tau NFT-rich regions and bound to multiple tau-mab antigen binding sites. The reddish color tau-mab conjugated AuNPs turned blue upon binding the antibody with the NFT antigen (Fig. 7). The results indicated that the antibody-conjugated AuNP was able to detect as little as 1 pg/mL of NFT aggregate in the AD brain. In another study in 2012, Brambilla et al. introduced a treatment using long-circulating PEG corona of poly (alkyl cyanoacrylate) (PACA) and poly (lactic acid) NPs (without any antibody) to decrease Aβ aggregates [137]. The authors claimed that the PLA and PACA NPs in the PEG corona enhanced the interaction with monomeric Aβ1-42. The particle size of the NPs was 110 nm, and the BBB penetration was facilitated by the adsorption of Apo-E protein from rat serum onto the NP surface. The Apo-E adsorption onto NPs also increases the affinity of the NPs for Aβ aggregates, which facilitates a complement activation elimination of the Aβ1-42 aggregates. However, as mentioned above, even though NPs have been used to eliminate Aβ and tau, NPs containing RAS inhibitors and related drugs for preventing AD pathology represent a promising but untested approach for AD mitigation.
Fig. 7.
A) First two steps represents the shematic diagram of the synthesis of the monoclonal anti-tau aggregate antibody conjugated gold nanoparticles wherease the last step represents the color change of the anti-tau aggregate antibody conjugated gold nanoparticles when they bind to the tau protein aggregate. B) TEM image of the anti-tau antibody conjugated gold nanoparticles C) TEM image of the anti-tau antibody conjugated gold nanoparticle after the binding with tau protein. Figure was reprinted from Ref. [136] under the persmison of the publisher.
4.2. Parkinson’s disease
Parkinson's disease (PD), the second-most prevalent neurodegenerative disease, is a chronic, progressive neurological disease that causes tremors, stiffness, akinesia and slow or hesitant speech. In 1992, Allen et al. first proposed the potential association between brain RAS activity and PD [138]. They analyzed the postmortem PD brains, describing a reduction of Ang II receptor binding in the substantia nigra and striatum in the brain. PD occurs due to the loss of dopaminergic (DA) neurons in the substantia nigra pars compacta. The striatum is the primary projection of the substantia nigra dopaminergic neurons, the loss of which leads to inadequate stimulation of stratal dopaminergic D1 and D2 receptors [139]. Regulatory interaction between the brain RAS and dopaminergic neurons in the striatum has been established in recent studies. PD patients treated with the ACE inhibitor perindopril have improved motor responses to the DA precursor, 3,4-dihydroxy-L-Phenylalanine (L-DOPA) [140]. Moreover, ACE also metabolizes bradykinin which can promote inflammation, an important contributing factor in PD. Additionally, high Ang II levels exacerbate dopaminergic cell death by overstimulating the AT1R, possibly leading to synergistic PD pathogenesis and progression [141,142]. Even though the NP-encapsulated RAS inhibitors have not been studied in PD, it has been shown that the liposomal antioxidant NPs positively affect PD [143].
In 2010, Zeevalk et al. reported that a reduced glutathione liposome conjugate (liposomal-GSH) replenished intracellular GSH after its depletion, potentially providing neuroprotection in PD [144]. The depletion of GSH and the optimum time for repletion were determined using cultured neurons from rat mesencephalon. To simulate PD, cells were exposed to the environmental toxins paraquat and maneb to deplete their dopamine. Reduced levels of GSH in the substantia nirgra are reported to be a contributing factor to the loss of midbrain dopamine neurons in PD [145]. The liposomal-GSH treatment sustained dose-dependent dopaminergic cell viability with an EC50 = 10.5 ± 1.08 μM.
4.3. COVID-19
The novel SARS-CoV-2 coronavirus enters the body by binding to plasma membrane-bound angiotensin-converting enzyme 2 (ACE2) of the renin-angiotensin system. This zinc metalloprotease (carboxypeptidase) provides the receptor binding pocket for SARS-COV-1 and 2 in its extracellular domain. Upon binding of SARS-CoV-2 to ACE-2, a fusion of the spike protein of the virus with the cell membrane occurs in concert with the host cell serine protease TMPRSS2 [146]. It has recently been shown that the SARS-CoV-2 variant spike proteins might have additional receptors, especially for the intra and extrapulmonary immune and non-immune cells that lack the ACE2 receptors, such as C-lectin (CLR), toll-like (TLR), neuropilin-1 (NRP1), and the non-immune receptor glucose-regulated protein 78 (GRP78) [147]. Age-associated conditions such as hypertension, diabetes, and non-alcoholic fatty liver, lung, and kidney diseases are comorbidities that are associated with increased COVID-19 severity. These comorbidities may predispose COVID-19 patients to “inflammaging” enhancing age-related systemic inflammation while weakening the adaptive immune response to COVID-19 infection [[148], [149], [150]]. Thus, enhancing the RAS pathway leading to increased AT1R activation can exacerbate COVID-19 infection. Initially, during the COVID-19 pandemic, it was debated whether ACE inhibitors or ARBs could be used as SARS-COV-2 medications [[151], [152], [153], [154]]. It was suspected that the ACEIs/ARBs might increase ACE2 expression, which could enhance SARS-COV-2 infectivity. However, the adverse effects of increased Ang II activation of the AT1R were considered to lead to worse outcomes than what might transpire from increased ACE2 expression [155]. Moreover, an imbalance of ACE-1 and ACE-2 and dysregulation in the RAS pathway were found in COVID-19 pathobiology studies [156]. The antiviral drugs initially used for primary therapy of COVID-19 fever, and pneumonia included oseltamivir phosphate (OP), a drug whose delivery can be enhanced using NP technology. The rate of oseltamivir binding to plasma protein is very low, with no drug interaction due to metabolism by the cytochrome P450 system or glucuronidase [157]. Thus, a recent study has shown the benefits of delivering oseltamivir through NPs mediation for COVID-19 treatments.
In 2021, Ucar et al. conducted a study on an oseltamivir phosphate (OP)-loaded biocompatible, a biodegradable polymeric nano-drug delivery system that has been developed [157]. OP was loaded onto PLGA NPs which are conjugated to spike-binding peptide SBP1 of SARS-COV-2. SBP1 is a first-class specific peptide linker for SARS-COV-2-RBD. The OP-loaded-PLGA NPs were synthesized by the solvent evaporation method, and the particle size of the bare OP-PLGA and targeted peptide SBP1 conjugated OP-PLGA were 162 ± 11.0 and 226.9 ± 21.4 nm, respectively. The drug entrapment efficacy was 61.4%, and the OP-PLGA and OP-PLGA-SBPI zeta potential was −23.9 ± 1.21 and −4.59 ± 0.728 nm, respectively. The high negativity of the OP-PLGA NPs indicated the high stability of the NPs, and the reduction of the negativity after binding the SBP1 peptide demonstrates successful conjugation of the amino group of the peptide onto the PLGA surface –COOH group. The FEG-SEM and FTIR conducted the morphological characterization of the NPs. In vitro stability of the OP-PLGA NPs was analyzed by storing the NPs in a sealed container at 5 ± 3 °C. The particle size and the polydisperse index (PDI) were measured over a month, and the results indicated there was no considerable change in the parameters, confirming the stability of the NPs over a month. In vitro OP release from the NP system was examined at pH 7.4 at 37 °C. The results indicated that there was no initial burst release occurred, and fast release was displayed during the first 30 days and reached the plateau at about 72 days. The OP-released rates of the OP-PLGA and OP-PLGA-SBPI NPs were 53.7% and 50.4%, respectively. The authors further reported that the SBP1 peptide conjugation on NPs is reported for the first time, which would be beneficial for administering newer antiviral drugs. However, the study would be more informative if the authors considered conducting Covid-19 infected in vitro cell lines of in vivo animal studies to compare the OP-PLGA and OP-PLGA-SBPI drug delivery models. In view of the current use of two antiviral agents (nirmatrelvir and ritonavir) in Paxlovid as well as the antiviral Molnupiravir to treat COVID-19 is of interest to determine if their distribution efficacy and duration of action could be enhanced using a similar PLGA-SBPI NP technology.
5. Conclusion and viewpoints
The RAS is an enzymatic cascade and signaling system that was first identified in 1898. While the RAS functions to maintain fluid and electrolyte homeostasis, it is also directly or indirectly involved in a number of diseases, including hypertension, cardiovascular disease, COVID-19, and AD. During the past century, pathobiological researchers have discovered several root causes for each disease, facilitating disease mitigation through diagnosis and pharmacotherapy. The primary limitations of the current RAS therapeutics are poor BBB penetration and, for some agents, low bioavailability. NP approaches for RAS drug delivery have been studied during the last two decades to overcome those limitations. NPs being developed for RAS-inhibiting drugs are synthetic polymeric NPs such as PLGA, PACA, and PEG. Natural polymeric NP chitosan has also been studied to deliver RAS inhibitors. All the NP studies discussed in this manuscript demonstrate a higher therapeutic efficacy of nano-drug delivery systems compared to free-drug delivery. However, NP research on RAS-inhibitor drugs is still preliminary, requiring many improvements before NPs can be used in pre-clinical and clinical studies. The current drawback of the NP studies discussed in this manuscript is the lack of information about the purification steps in the NP synthesis process and toxicity studies to prove that the NPs are nontoxic. Moreover, most RAS-related nano-drug delivery studies were limited to wet-lab scale experiments. It is necessary to conduct studies in vitro in cell lines or in vivo animal studies to test the therapeutic efficacy of nano-drug delivery systems. As discussed, the recent study by Wen et al., 2021 documented in vitro cell line efficacy of the Ang 1–7 nanofiber moiety as a potential therapy for cardiovascular disease, having a particle size of 7.6 ± 1.8 nm [31]. This study stands out over all other RAS-related NP studies due to the smaller particle size, high AT1R binding affinity of the telmisartan-containing NPs, and efficient cellular uptake. Thus, we also suggest NPs such as carbon dots (particle size <10 nm) and PEG-capped extruded smaller-size niosomes (particle size <20 nm) for the development of NP RAS inhibitor therapies. Smaller particle-size NPs protect the cell membrane and BBB from severe damage [128]. In addition, we introduce a novel research pathway for the application of NPs for the delivery of RAS-inhibiting drugs to the brain to treat AD and other neurodegenerative diseases. The ability of ARBs to enhance cognitive function shows great promise for treating and preventing AD.
Author contribution
Sajini Hettiarachchi structured the manuscript, gathered data, and wrote the manuscript. Robert Speth advised Sajini Hettiarachchi to find appropriate citations and provide the necessary biological explanations and knowledge. Also, Robert Speth thoroughly reviewed and edited the manuscript. Young Kwon wrote a section on protein-based nanoparticles and some parts on synthetic polymeric nanoparticles. Also, he reviewed and edited the manuscript. Yadollah Omidi reviewed and edited the manuscript, especially the abstract, introduction, and nanoparticle sections.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Robert C. Speth reports financial support was provided by National Institutes of Health. The authors declare no conflict of interest.
Acknowledgment
We thank the National Institute of Health for providing the grant funding (HL-441015) support to SDH and RCS. Also, we thank Dr. Kathryn Sandberg (Professor and vice chair of the research department of Medicine, Georgetown University, Washington DC) for her guidance. We thank the BioRender team for providing us with the software facility to draw the graphical abstract.
Contributor Information
Sajini D. Hettiarachchi, Email: shettiar@nova.edu.
Robert C. Speth, Email: rs1251@nova.edu.
References
- 1.Tigerstedt R., Bergmann P.G. Niere und kreislauf. Scand. Arch. Physiol. 1898;8:223–271. [Google Scholar]
- 2.Speth R.C. Reference Module in Biomedical Sciences. Elsevier; 2021. Renin-angiotensin-aldosterone system. [Google Scholar]
- 3.Yang T., Xu C. Physiology and pathophysiology of the intrarenal renin-angiotensin system: an update. J. Am. Soc. Nephrol. 2017;28:1040–1049. doi: 10.1681/ASN.2016070734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paz Ocaranza M., Riquelme J.A., García L., Jalil J.E., Chiong M., Santos R.A., Lavandero S. Counter-regulatory renin–angiotensin system in cardiovascular disease. Nat. Rev. Cardiol. 2020;17:116–129. doi: 10.1038/s41569-019-0244-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodrigues Prestes T.R., Rocha N.P., Miranda A.S., Teixeira A.L., Simoes-e-Silva A.C. The anti-inflammatory potential of ACE2/angiotensin-(1-7)/Mas receptor axis: evidence from basic and clinical research. Curr. Drug Targets. 2017;18:1301–1313. doi: 10.2174/1389450117666160727142401. [DOI] [PubMed] [Google Scholar]
- 6.Rocha N.P., Cleary C., Colpo G.D., Furr Stimming E., Teixeira A.L. Peripheral levels of renin-angiotensin system components are associated with cognitive performance in huntington’s disease. Front. Neurosci. 2020;1358 doi: 10.3389/fnins.2020.594945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yiannikouris F., Karounos M., Charnigo R., English V.L., Rateri D.L., Daugherty A., Cassis L.A. Adipocyte-specific deficiency of angiotensinogen decreases plasma angiotensinogen concentration and systolic blood pressure in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012;302:R244–R251. doi: 10.1152/ajpregu.00323.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stornetta R.L., Hawelu-Johnson C.L., Guyenet P.G., Lynch K.R. Astrocytes synthesize angiotensinogen in brain. Science. 1988;242:1444–1446. doi: 10.1126/science.3201232. [DOI] [PubMed] [Google Scholar]
- 9.Speth R.C. Reference Module in Biomedical Sciences. Elsevier; 2021. Renin-angiotensin-aldosterone system. [Google Scholar]
- 10.Miller A.J., Arnold A.C. The renin–angiotensin system in cardiovascular autonomic control: recent developments and clinical implications. Clin. Auton. Res. 2019;29:231–243. doi: 10.1007/s10286-018-0572-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niaz T., Shabbir S., Manzoor S., Rehman A., Rahman A., Nasir H., Imran M. Antihypertensive nano-ceuticales based on chitosan biopolymer: physico-chemical evaluation and release kinetics. Carbohydr. Polym. 2016;142:268–274. doi: 10.1016/j.carbpol.2016.01.047. [DOI] [PubMed] [Google Scholar]
- 12.Carey R.M. Blood pressure and the renal actions of AT 2 receptors. Curr. Hypertens. Rep. 2017;19:1–3. doi: 10.1007/s11906-017-0720-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.AbdAlla S., Lother H., Abdel-tawab A.M., Quitterer U. The angiotensin II AT2 receptor is an AT1receptor antagonist. J. Biol. Chem. 2001;276:39721–39726. doi: 10.1074/jbc.M105253200. [DOI] [PubMed] [Google Scholar]
- 14.Hakam A.C., Hussain T. Renal angiotensin II type-2 receptors are upregulated and mediate the candesartan-induced natriuresis/diuresis in obese Zucker rats. Hypertension. 2005;45:270–275. doi: 10.1161/01.HYP.0000151622.47814.6f. [DOI] [PubMed] [Google Scholar]
- 15.Dhande I., Ma W., Hussain T. Angiotensin AT2 receptor stimulation is anti-inflammatory in lipopolysaccharide-activated THP-1 macrophages via increased interleukin-10 production. Hypertens. Res. 2015;38:21–29. doi: 10.1038/hr.2014.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nag S., Patel S., Mani S., Hussain T. Role of angiotensin type 2 receptor in improving lipid metabolism and preventing adiposity. Mol. Cell. Biochem. 2019;461:195–204. doi: 10.1007/s11010-019-03602-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paul M., Poyan Mehr A., Kreutz R. Physiology of local renin-angiotensin systems. Physiol. Rev. 2006;86:747–803. doi: 10.1152/physrev.00036.2005. [DOI] [PubMed] [Google Scholar]
- 18.George A.J., Thomas W.G., Hannan R.D. The renin–angiotensin system and cancer: old dog, new tricks. Nat. Rev. Cancer. 2010;10:745–759. doi: 10.1038/nrc2945. [DOI] [PubMed] [Google Scholar]
- 19.Baltatu O.C., Campos L.A., Bader M. Local renin–angiotensin system and the brain—a continuous quest for knowledge. Peptides. 2011;32:1083–1086. doi: 10.1016/j.peptides.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 20.Forrester S.J., Booz G.W., Sigmund C.D., Coffman T.M., Kawai T., Rizzo V., Scalia R., Eguchi S. Angiotensin II signal transduction: an update on mechanisms of physiology and pathophysiology. Physiol. Rev. 2018;98:1627–1738. doi: 10.1152/physrev.00038.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raizada V., Skipper B., Luo W., Griffith J. Intracardiac and intrarenal renin-angiotensin systems: mechanisms of cardiovascular and renal effects. J. Invest. Med. 2007;55:341–359. doi: 10.2310/6650.2007.00020. [DOI] [PubMed] [Google Scholar]
- 22.Shang J., Ye G., Shi K., Wan Y., Luo C., Aihara H., Geng Q., Auerbach A., Li F. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581:221–224. doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang H., Penninger J.M., Li Y., Zhong N., Slutsky A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46:586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu R., Zhao J., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N. Escaping to nature during a pandemic: a natural experiment in asian cities during the COVID-19 pandemic with big social media data. SocArXiv. 2019;3 2020. [Google Scholar]
- 25.Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balakumar P., Jagadeesh G. Pathophysiology and Pharmacotherapy of Cardiovascular Disease. Springer; 2015. Drugs targeting RAAS in the treatment of hypertension and other cardiovascular diseases; pp. 751–806. [Google Scholar]
- 27.Ramya K., Suresh R., Kumar H.Y., Kumar B.P., Murthy N.S. Decades-old renin inhibitors are still struggling to find a niche in antihypertensive therapy. A fleeting look at the old and the promising new molecules. Bioorg. Med. Chem. 2020;28 doi: 10.1016/j.bmc.2020.115466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ho J.K., Moriarty F., Manly J.J., Larson E.B., Evans D.A., Rajan K.B., Hudak E.M., Hassan L., Liu E., Sato N. Blood-brain barrier crossing renin-angiotensin drugs and cognition in the elderly: a meta-analysis. Hypertension. 2021;78:629–643. doi: 10.1161/HYPERTENSIONAHA.121.17049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sepúlveda-Rivas S., Leal M.S., Pedrozo Z., Kogan M.J., Ocaranza M.P., Morales J.O. Nanoparticle-mediated angiotensin-(1-9) drug delivery for the treatment of cardiac hypertrophy. Pharmaceutics. 2021;13:822. doi: 10.3390/pharmaceutics13060822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krawczyńska A., Dziendzikowska K., Gromadzka-Ostrowska J., Lankoff A., Herman A.P., Oczkowski M., Królikowski T., Wilczak J., Wojewódzka M., Kruszewski M. Silver and titanium dioxide nanoparticles alter oxidative/inflammatory response and renin–angiotensin system in brain. Food Chem. Toxicol. 2015;85:96–105. doi: 10.1016/j.fct.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 31.Wen Z., Zhan J., Li H., Xu G., Ma S., Zhang J., Li Z., Ou C., Yang Z., Cai Y. Dual-ligand supramolecular nanofibers inspired by the renin-angiotensin system for the targeting and synergistic therapy of myocardial infarction. Theranostics. 2021;11:3725. doi: 10.7150/thno.53644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Antal I., Kubovcikova M., Zavisova V., Koneracka M., Pechanova O., Barta A., Cebova M., Antal V., Diko P., Zduriencikova M. Magnetic poly (d, l-lactide) nanoparticles loaded with aliskiren: a promising tool for hypertension treatment. J. Magn. Magn Mater. 2015;380:280–284. [Google Scholar]
- 33.Gullapalli N., Bloch M.J., Basile J. Renin-angiotensin-aldosterone system blockade in high-risk hypertensive patients: current approaches and future trends. Therapeut. Adv. Cardiovascul. Disease. 2010;4:359–373. doi: 10.1177/1753944710384430. [DOI] [PubMed] [Google Scholar]
- 34.Sanoski C.A. Aliskiren: an oral direct renin inhibitor for the treatment of hypertension. Pharmacotherapy. 2009;29:193–212. doi: 10.1592/phco.29.2.193. [DOI] [PubMed] [Google Scholar]
- 35.Azizi M., Webb R., Nussberger J., Hollenberg N.K. Renin inhibition with aliskiren: where are we now, and where are we going? J. Hypertens. 2006;24:243–256. doi: 10.1097/01.hjh.0000202812.72341.99. [DOI] [PubMed] [Google Scholar]
- 36.Dicpinigaitis P.V. Angiotensin-converting enzyme inhibitor-induced cough: ACCP evidence-based clinical practice guidelines. Chest. 2006;129:169S–173S. doi: 10.1378/chest.129.1_suppl.169S. [DOI] [PubMed] [Google Scholar]
- 37.Siragy H.M. A current evaluation of the safety of angiotensin receptor blockers and direct renin inhibitors. Vasc. Health Risk Manag. 2011:297–313. doi: 10.2147/VHRM.S15541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guichard J.L., Clark D., III, Calhoun D.A., Ahmed M.I. Aldosterone receptor antagonists: current perspectives and therapies. Vasc. Health Risk Manag. 2013:321–331. doi: 10.2147/VHRM.S33759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maron B.A., Leopold J.A. Aldosterone receptor antagonists: effective but often forgotten. Circulation. 2010;121:934–939. doi: 10.1161/CIRCULATIONAHA.109.895235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liyanage P.Y., Hettiarachchi S.D., Zhou Y., Ouhtit A., Seven E.S., Oztan C.Y., Celik E., Leblanc R.M. Nanoparticle-mediated targeted drug delivery for breast cancer treatment. Biochim. Biophys. Acta Rev. Canc. 2019;1871:419–433. doi: 10.1016/j.bbcan.2019.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abdel-Aziz H.M., Hasaneen M.N., Omer A.M. Nano chitosan-NPK fertilizer enhances the growth and productivity of wheat plants grown in sandy soil. Spanish J. Agric. Res. 2016;14:e0902. [Google Scholar]
- 42.Gupta A.K., Naregalkar R.R., Vaidya V.D., Gupta M. 2007. Recent Advances on Surface Engineering of Magnetic Iron Oxide Nanoparticles and Their Biomedical Applications. [DOI] [PubMed] [Google Scholar]
- 43.Hettiarachchi S.D., Graham R.M., Mintz K.J., Zhou Y., Vanni S., Peng Z., Leblanc R.M. Triple conjugated carbon dots as a nano-drug delivery model for glioblastoma brain tumors. Nanoscale. 2019;11:6192–6205. doi: 10.1039/c8nr08970a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu P., Yuan L., Wang Y., Du Q., Sheng J. Effect of GPE-AGT nanoparticle shRNA transfection system mediated RNAi on early atherosclerotic lesion. Int. J. Clin. Exp. Pathol. 2012;5:698. [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang L., Gu F., Chan J., Wang A., Langer R., Farokhzad O. Nanoparticles in medicine: therapeutic applications and developments. Clin. Pharmacol. Ther. 2008;83:761–769. doi: 10.1038/sj.clpt.6100400. [DOI] [PubMed] [Google Scholar]
- 46.Crucho C.I., Barros M.T. Polymeric nanoparticles: a study on the preparation variables and characterization methods. Mater. Sci. Eng. C. 2017;80:771–784. doi: 10.1016/j.msec.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 47.Plucinski A., Lyu Z., Schmidt B.V. Polysaccharide nanoparticles: from fabrication to applications. J. Mater. Chem. B. 2021;9:7030–7062. doi: 10.1039/d1tb00628b. [DOI] [PubMed] [Google Scholar]
- 48.Divya K., Jisha M. Chitosan nanoparticles preparation and applications. Environ. Chem. Lett. 2018;16:101–112. [Google Scholar]
- 49.Hajji S., Younes I., Ghorbel-Bellaaj O., Hajji R., Rinaudo M., Nasri M., Jellouli K. Structural differences between chitin and chitosan extracted from three different marine sources. Int. J. Biol. Macromol. 2014;65:298–306. doi: 10.1016/j.ijbiomac.2014.01.045. [DOI] [PubMed] [Google Scholar]
- 50.Zainol I., Ghani S., Mastor A., Derman M., Yahya M. Enzymatic degradation study of porous chitosan membrane. Mater. Res. Innovat. 2009;13:316–319. [Google Scholar]
- 51.Choi C., Nam J.-P., Nah J.-W. Application of chitosan and chitosan derivatives as biomaterials. J. Ind. Eng. Chem. 2016;33:1–10. [Google Scholar]
- 52.Matalqah S.M., Aiedeh K., Mhaidat N.M., Alzoubi K.H., Bustanji Y., Hamad I. Chitosan nanoparticles as a novel drug delivery system: a review article. Curr. Drug Targets. 2020;21:1613–1624. doi: 10.2174/1389450121666200711172536. [DOI] [PubMed] [Google Scholar]
- 53.Fathi M., Zangabad P.S., Majidi S., Barar J., Erfan-Niya H., Omidi Y. Stimuli-responsive chitosan-based nanocarriers for cancer therapy. Bioimpacts: BI. 2017;7:269. doi: 10.15171/bi.2017.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fathi M., Majidi S., Zangabad P.S., Barar J., Erfan‐Niya H., Omidi Y. Chitosan‐based multifunctional nanomedicines and theranostics for targeted therapy of cancer. Med. Res. Rev. 2018;38:2110–2136. doi: 10.1002/med.21506. [DOI] [PubMed] [Google Scholar]
- 55.Fathi M., Barar J., Erfan-Niya H., Omidi Y. Methotrexate-conjugated chitosan-grafted pH-and thermo-responsive magnetic nanoparticles for targeted therapy of ovarian cancer. Int. J. Biol. Macromol. 2020;154:1175–1184. doi: 10.1016/j.ijbiomac.2019.10.272. [DOI] [PubMed] [Google Scholar]
- 56.Pakzad Y., Fathi M., Omidi Y., Mozafari M., Zamanian A. Synthesis and characterization of timolol maleate-loaded quaternized chitosan-based thermosensitive hydrogel: a transparent topical ocular delivery system for the treatment of glaucoma. Int. J. Biol. Macromol. 2020;159:117–128. doi: 10.1016/j.ijbiomac.2020.04.274. [DOI] [PubMed] [Google Scholar]
- 57.Yang H.-C., Hon M.-H. The effect of the molecular weight of chitosan nanoparticles and its application on drug delivery. Microchem. J. 2009;92:87–91. [Google Scholar]
- 58.Taşkın P., Canısağ H., Şen M. The effect of degree of deacetylation on the radiation induced degradation of chitosan. Radiat. Phys. Chem. 2014;94:236–239. [Google Scholar]
- 59.Orkhan F., Melike U., Cihan G., Faruk D.O., Samet B., Ilknur U., Alemdar J. RBD and ACE2 embedded chitosan nanoparticles as a prevention approach for SARS-COV 2. Biomed. J. Sci. Techn. Res. 2021;37:29193–29197. [Google Scholar]
- 60.DeFrates K., Markiewicz T., Gallo P., Rack A., Weyhmiller A., Jarmusik B., Hu X. Protein polymer-based nanoparticles: fabrication and medical applications. Int. J. Mol. Sci. 2018;19:1717. doi: 10.3390/ijms19061717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tarhini M., Greige-Gerges H., Elaissari A. Protein-based nanoparticles: from preparation to encapsulation of active molecules. Int. J. Pharm. 2017;522:172–197. doi: 10.1016/j.ijpharm.2017.01.067. [DOI] [PubMed] [Google Scholar]
- 62.Martínez-López A.L., Pangua C., Reboredo C., Campión R., Morales-Gracia J., Irache J.M. Protein-based nanoparticles for drug delivery purposes. Int. J. Pharm. 2020;581 doi: 10.1016/j.ijpharm.2020.119289. [DOI] [PubMed] [Google Scholar]
- 63.Su Y., Zhang B., Sun R., Liu W., Zhu Q., Zhang X., Wang R., Chen C. PLGA-based biodegradable microspheres in drug delivery: recent advances in research and application. Drug Deliv. 2021;28:1397–1418. doi: 10.1080/10717544.2021.1938756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boddu S.H., Bhagav P., Karla P.K., Jacob S., Adatiya M.D., Dhameliya T.M., Ranch K.M., Tiwari A.K. Polyamide/poly (amino acid) polymers for drug delivery. J. Funct. Biomater. 2021;12:58. doi: 10.3390/jfb12040058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang J., Zhou Q., Shen K., Song N., Ni L. Controlling nanodomain morphology of epoxy thermosets templated by poly (caprolactone)-block-poly (dimethylsiloxane)-block-poly (caprolactone) ABA triblock copolymer. RSC Adv. 2018;8:3705–3715. doi: 10.1039/c7ra12826f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brenza T.M., Schlichtmann B.W., Bhargavan B., Vela Ramirez J.E., Nelson R.D., Panthani M.G., McMillan J.M., Kalyanaraman B., Gendelman H.E., Anantharam V. Biodegradable polyanhydride‐based nanomedicines for blood to brain drug delivery. J. Biomed. Mater. Res. 2018;106:2881–2890. doi: 10.1002/jbm.a.36477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li L., Xu Y., Milligan I., Fu L., Franckowiak E. Du W. Synthesis of highly pH-responsive glucose poly (orthoester) Angew. Chem. Int. Ed. 2013;52:13699–13702. doi: 10.1002/anie.201306391. [DOI] [PubMed] [Google Scholar]
- 68.Ogueri K.S., Ogueri K.S., Allcock H.R., Laurencin C.T. Polyphosphazene polymers: the next generation of biomaterials for regenerative engineering and therapeutic drug delivery. J. Vacuum Sci. Tech. B, Nanotech. Microelect.: Materials, Processing, Measurem. Phenomena. 2020;38 doi: 10.1116/6.0000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ray D., Mohapatra D.K., Mohapatra R.K., Mohanta G.P., Sahoo P.K. Synthesis and colon-specific drug delivery of a poly (acrylic acid-co-acrylamide)/MBA nanosized hydrogel. J. Biomater. Sci. Polym. Ed. 2008;19:1487–1502. doi: 10.1163/156856208786140382. [DOI] [PubMed] [Google Scholar]
- 70.Suk J., Xu Q., Kim N., Hanes J., Ensign L., Sciences H., Sciences M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016;99:28–51. doi: 10.1016/j.addr.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kamaly N., Xiao Z., Valencia P.M., Radovic-Moreno A.F., Farokhzad O.C. Targeted polymeric therapeutic nanoparticles: design, development and clinical translation. Chem. Soc. Rev. 2012;41:2971–3010. doi: 10.1039/c2cs15344k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Krasia-Christoforou T., Georgiou T.K. Polymeric theranostics: using polymer-based systems for simultaneous imaging and therapy. J. Mater. Chem. B. 2013;1:3002–3025. doi: 10.1039/c3tb20191k. [DOI] [PubMed] [Google Scholar]
- 73.Iversen T.-G., Skotland T., Sandvig K. Endocytosis and intracellular transport of nanoparticles: present knowledge and need for future studies. Nano Today. 2011;6:176–185. [Google Scholar]
- 74.Kamaly N., Yameen B., Wu J., Farokhzad O.C. Degradable controlled-release polymers and polymeric nanoparticles: mechanisms of controlling drug release. Chem. Rev. 2016;116:2602–2663. doi: 10.1021/acs.chemrev.5b00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Geraili A., Mequanint K. Systematic studies on surface erosion of photocrosslinked polyanhydride tablets and data correlation with release kinetic models. Polymers. 2020;12:1105. doi: 10.3390/polym12051105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Versypt A.N.F., Pack D.W., Braatz R.D. Mathematical modeling of drug delivery from autocatalytically degradable PLGA microspheres—a review. J. Contr. Release. 2013;165:29–37. doi: 10.1016/j.jconrel.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Crucho C.I. Stimuli‐responsive polymeric nanoparticles for nanomedicine. ChemMedChem. 2015;10:24–38. doi: 10.1002/cmdc.201402290. [DOI] [PubMed] [Google Scholar]
- 78.Dahman Y. 2017. Nanotechnology and Functional Materials for Engineers. (Elsevier) [Google Scholar]
- 79.Grumezescu A.M. 2016. Fabrication and Self-Assembly of Nanobiomaterials: Applications of Nanobiomaterials. (William Andrew) [Google Scholar]
- 80.Mordorski B., Friedman A. Functionalized Nanomaterials for the Management of Microbial Infection. Elsevier; 2017. Metal nanoparticles for microbial infection; pp. 77–109. [Google Scholar]
- 81.Auffan M., Rose J., Wiesner M.R., Bottero J.-Y. Chemical stability of metallic nanoparticles: a parameter controlling their potential cellular toxicity in vitro. Environ. Pollut. 2009;157:1127–1133. doi: 10.1016/j.envpol.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 82.Sperling R.A., Gil P.R., Zhang F., Zanella M., Parak W.J. Biological applications of gold nanoparticles. Chem. Soc. Rev. 2008;37:1896–1908. doi: 10.1039/b712170a. [DOI] [PubMed] [Google Scholar]
- 83.Ghosh P., Han G., De M., Kim C.K., Rotello V.M. Gold nanoparticles in delivery applications. Adv. Drug Deliv. Rev. 2008;60:1307–1315. doi: 10.1016/j.addr.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 84.Kumar A., Mazinder Boruah B., Liang X.-J. Gold nanoparticles: promising nanomaterials for the diagnosis of cancer and HIV/AIDS. J. Nanomater. 2011;2011:1–17. [Google Scholar]
- 85.Albrecht M.A., Evans C.W., Raston C.L. Green chemistry and the health implications of nanoparticles. Green Chem. 2006;8:417–432. [Google Scholar]
- 86.Su S., Yu T., Hu J., Xianyu Y. A bio-inspired plasmonic nanosensor for angiotensin-converting enzyme through peptide-mediated assembly of gold nanoparticles. Biosens. Bioelectron. 2022;195 doi: 10.1016/j.bios.2021.113621. [DOI] [PubMed] [Google Scholar]
- 87.Rajan K., Roppolo I., Chiappone A., Bocchini S., Perrone D., Chiolerio A. Silver nanoparticle ink technology: state of the art. Nanotechnol. Sci. Appl. 2016;9:1. doi: 10.2147/NSA.S68080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Russell A., Hugo W. 7 antimicrobial activity and action of silver. Prog. Med. Chem. 1994;31:351–370. doi: 10.1016/s0079-6468(08)70024-9. [DOI] [PubMed] [Google Scholar]
- 89.Murphy M., Ting K., Zhang X., Soo C., Zheng Z. Current development of silver nanoparticle preparation, investigation, and application in the field of medicine. J. Nanomater. 2015;2015:1–12. [Google Scholar]
- 90.Jain P.K., Huang X., El-Sayed I.H., El-Sayed M.A. Review of some interesting surface plasmon resonance-enhanced properties of noble metal nanoparticles and their applications to biosystems. Plasmonics. 2007;2:107–118. [Google Scholar]
- 91.Qi W., Wang M. Size and shape dependent melting temperature of metallic nanoparticles. Mater. Chem. Phys. 2004;88:280–284. [Google Scholar]
- 92.Dziendzikowska K., Gromadzka‐Ostrowska J., Lankoff A., Oczkowski M., Krawczyńska A., Chwastowska J., Sadowska‐Bratek M., Chajduk E., Wojewodzka M., Dušinská M. Time‐dependent biodistribution and excretion of silver nanoparticles in male Wistar rats. J. Appl. Toxicol. 2012;32:920–928. doi: 10.1002/jat.2758. [DOI] [PubMed] [Google Scholar]
- 93.Tan E., Lim C. Mechanical characterization of nanofibers–a review. Compos. Sci. Technol. 2006;66:1102–1111. [Google Scholar]
- 94.Vasita R., Katti D.S. Nanofibers and their applications in tissue engineering. Int. J. Nanomed. 2006;1:15. doi: 10.2147/nano.2006.1.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dzenis Y. Spinning continuous fibers for nanotechnology. Science. 2004;304:1917–1919. doi: 10.1126/science.1099074. [DOI] [PubMed] [Google Scholar]
- 96.Fridrikh S.V., Jian H.Y., Brenner M.P., Rutledge G.C. Controlling the fiber diameter during electrospinning. Phys. Rev. Lett. 2003;90 doi: 10.1103/PhysRevLett.90.144502. [DOI] [PubMed] [Google Scholar]
- 97.Kameoka J., Craighead H.G. Fabrication of oriented polymeric nanofibers on planar surfaces by electrospinning. Appl. Phys. Lett. 2003;83:371–373. [Google Scholar]
- 98.Elias K.L., Price R.L., Webster T.J. Enhanced functions of osteoblasts on nanometer diameter carbon fibers. Biomaterials. 2002;23:3279–3287. doi: 10.1016/s0142-9612(02)00087-x. [DOI] [PubMed] [Google Scholar]
- 99.Arima H., Barzi F., Chalmers J. Mortality patterns in hypertension. J. Hypertens. 2011;29:S3–S7. doi: 10.1097/01.hjh.0000410246.59221.b1. [DOI] [PubMed] [Google Scholar]
- 100.Al-Makki A., DiPette D., Whelton P.K., Murad M.H., Mustafa R.A., Acharya S., Beheiry H.M., Champagne B., Connell K., Cooney M.T. Hypertension pharmacological treatment in adults: a World Health Organization guideline executive summary. Hypertension. 2022;79:293–301. doi: 10.1161/HYPERTENSIONAHA.121.18192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Staessen J.A., Wang J., Bianchi G., Birkenhäger W.H. Essential hypertension. Lancet. 2003;361:1629–1641. doi: 10.1016/S0140-6736(03)13302-8. [DOI] [PubMed] [Google Scholar]
- 102.Widimský J. The SPRINT research. A randomized trial of intensive versus standard blood-pressure control. Vnitr. Lek. 2016;62:44–47. [PubMed] [Google Scholar]
- 103.Elliott W.J., Ram C.V.S. Calcium channel blockers. J. Clin. Hypertens. 2011;13:687–689. doi: 10.1111/j.1751-7176.2011.00513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Roush G.C., Sica D.A. Diuretics for hypertension: a review and update. Am. J. Hypertens. 2016;29:1130–1137. doi: 10.1093/ajh/hpw030. [DOI] [PubMed] [Google Scholar]
- 105.Martin N., Manoharan K., Davies C., Lumbers R.T. Beta‐blockers and inhibitors of the renin‐angiotensin aldosterone system for chronic heart failure with preserved ejection fraction. Cochrane Database Syst. Rev. 2021;6:1–175. doi: 10.1002/14651858.CD012721.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Arumugham V.B., Shahin M.H. StatPearls [Internet] StatPearls Publishing; 2021. Therapeutic uses of diuretic agents. [PubMed] [Google Scholar]
- 107.Puchalska P., Marina Alegre M.L., Garcia Lopez M.C. Isolation and characterization of peptides with antihypertensive activity in foodstuffs. Crit. Rev. Food Sci. Nutr. 2015;55:521–551. doi: 10.1080/10408398.2012.664829. [DOI] [PubMed] [Google Scholar]
- 108.Michel M.C., Foster C., Brunner H.R., Liu L. A systematic comparison of the properties of clinically used angiotensin II type 1 receptor antagonists. Pharmacol. Rev. 2013;65:809–848. doi: 10.1124/pr.112.007278. [DOI] [PubMed] [Google Scholar]
- 109.Waldmeier F., Glaenzel U., Wirz B., Oberer L., Schmid D., Seiberling M., Valencia J., Riviere G.-J., End P., Vaidyanathan S. Absorption, distribution, metabolism, and elimination of the direct renin inhibitor aliskiren in healthy volunteers. Drug Metab. Dispos. 2007;35:1418–1428. doi: 10.1124/dmd.106.013797. [DOI] [PubMed] [Google Scholar]
- 110.Yu T., Zhao S., Li Z., Wang Y., Xu B., Fang D., Wang F., Zhang Z., He L., Song X. Enhanced and extended anti-hypertensive effect of VP5 nanoparticles. Int. J. Mol. Sci. 2016;17:1977. doi: 10.3390/ijms17121977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cicero A.F., Aubin F., Azais-Braesco V., Borghi C. Do the lactotripeptides isoleucine–proline–proline and valine–proline–proline reduce systolic blood pressure in European subjects? A meta-analysis of randomized controlled trials. Am. J. Hypertens. 2013;26:442–449. doi: 10.1093/ajh/hps044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Şahin B., İlgün G. Risk factors of deaths related to cardiovascular diseases in World Health Organization (WHO) member countries. Health Soc. Care Community. 2022;30:73–80. doi: 10.1111/hsc.13156. [DOI] [PubMed] [Google Scholar]
- 113.Reed G.W., Rossi J.E., Cannon C.P. Acute myocardial infarction. Lancet. 2017;389:197–210. doi: 10.1016/S0140-6736(16)30677-8. [DOI] [PubMed] [Google Scholar]
- 114.Virani S.S., Alonso A., Benjamin E.J., Bittencourt M.S., Callaway C.W., Carson A.P., Chamberlain A.M., Chang A.R., Cheng S., Delling F.N. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 115.Roth G.A., Mensah G.A., Johnson C.O., Addolorato G., Ammirati E., Baddour L.M., Barengo N.C., Beaton A.Z., Benjamin E.J., Benziger C.P. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J. Am. Coll. Cardiol. 2020;76:2982–3021. doi: 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Song Y., Zhao Z., Zhang J., Zhao F., Jin P. Effects of sacubitril/valsartan on life quality in chronic heart failure: a systematic review and meta-analysis of randomized controlled trials. Fronti. Cardiovascul. Med. 2022;9 doi: 10.3389/fcvm.2022.922721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Santos R.A.S., Sampaio W.O., Alzamora A.C., Motta-Santos D., Alenina N., Bader M., Campagnole-Santos M.J. The ACE2/angiotensin-(1–7)/MAS axis of the renin-angiotensin system: focus on angiotensin-(1–7) Physiol. Rev. 2017;98:505–553. doi: 10.1152/physrev.00023.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pechanova O., Barta A., Koneracka M., Zavisova V., Kubovcikova M., Klimentova J., Tӧrӧk J., Zemancikova A., Cebova M. Protective effects of nanoparticle-loaded aliskiren on cardiovascular system in spontaneously hypertensive rats. Molecules. 2019;24:2710. doi: 10.3390/molecules24152710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Deng Z., Jiang J., Wang J., Pan D., Zhu Y., Li H., Zhang X., Liu X., Xu Y., Li Y. Angiotensin receptor blockers are associated with a lower risk of progression from mild cognitive impairment to dementia. Hypertension. 2022;79:2159–2169. doi: 10.1161/HYPERTENSIONAHA.122.19378. [DOI] [PubMed] [Google Scholar]
- 120.Wright J.W., Harding J.W. Contributions by the brain renin-angiotensin system to memory, cognition, and alzheimer's disease. J. Alzheim. Dis. : JAD. 2019;67:469–480. doi: 10.3233/jad-181035. [DOI] [PubMed] [Google Scholar]
- 121.Chai S.Y., Bastias M.A., Clune E.F., Matsacos D.J., Mustafa T., Lee J.H., McDowall S.G., Mendelsohn F.A., Albiston A.L., Paxinos G. Distribution of angiotensin IV binding sites (AT4 receptor) in the human forebrain, midbrain and pons as visualised by in vitro receptor autoradiography. J. Chem. Neuroanat. 2000;20:339–348. doi: 10.1016/s0891-0618(00)00112-5. [DOI] [PubMed] [Google Scholar]
- 122.Miller-Wing A.V., Hanesworth J.M., Sardinia M.F., Hall K.L., Wright J.W., Speth R.C., Grove K.L., Harding J.W. Central angiotensin IV binding sites: distribution and specificity in Guinea pig brain. J. Pharmacol. Exp. Therapeut. 1993;266:1718–1726. [PubMed] [Google Scholar]
- 123.Stragier B., Sarre S., Vanderheyden P., Vauquelin G., Fournié‐Zaluski M.C., Ebinger G., Michotte Y. Metabolism of angiotensin II is required for its in vivo effect on dopamine release in the striatum of the rat. J. Neurochem. 2004;90:1251–1257. doi: 10.1111/j.1471-4159.2004.02600.x. [DOI] [PubMed] [Google Scholar]
- 124.Lee J., Mustafa T., McDowall S.G., Mendelsohn F.A., Brennan M., Lew R.A., Albiston A.L., Chai S.Y. Structure-activity study of LVV-hemorphin-7: angiotensin AT4 receptor ligand and inhibitor of insulin-regulated aminopeptidase. J. Pharmacol. Exp. Therapeut. 2003;305:205–211. doi: 10.1124/jpet.102.045492. [DOI] [PubMed] [Google Scholar]
- 125.Herholz K. Acetylcholine esterase activity in mild cognitive impairment and Alzheimer’s disease. Eur. J. Nucl. Med. Mol. Imag. 2008;35:25–29. doi: 10.1007/s00259-007-0699-4. [DOI] [PubMed] [Google Scholar]
- 126.Buerger K., Ewers M., Pirttilä T., Zinkowski R., Alafuzoff I., Teipel S.J., DeBernardis J., Kerkman D., McCulloch C., Soininen H. CSF phosphorylated tau protein correlates with neocortical neurofibrillary pathology in Alzheimer's disease. Brain. 2006;129:3035–3041. doi: 10.1093/brain/awl269. [DOI] [PubMed] [Google Scholar]
- 127.Mohsenzadegan M., Mirshafiey A. The immunopathogenic role of reactive oxygen species in Alzheimer disease. Iran. J. Allergy, Asthma Immunol. 2012:203–216. [PubMed] [Google Scholar]
- 128.Hettiarachchi S.D., Zhou Y., Seven E., Lakshmana M.K., Kaushik A.K., Chand H.S., Leblanc R.M. Nanoparticle-mediated approaches for Alzheimer’s disease pathogenesis, diagnosis, and therapeutics. J. Contr. Release. 2019;314:125–140. doi: 10.1016/j.jconrel.2019.10.034. [DOI] [PubMed] [Google Scholar]
- 129.Kunkle B.W., Grenier-Boley B., Sims R., Bis J.C., Damotte V., Naj A.C., Boland A., Vronskaya M., Van Der Lee S.J., Amlie-Wolf A. Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Aβ, tau, immunity and lipid processing. Nat. Genet. 2019;51:414–430. doi: 10.1038/s41588-019-0358-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cuddy L.K., Prokopenko D., Cunningham E.P., Brimberry R., Song P., Kirchner R., Chapman B.A., Hofmann O., Hide W., Procissi D. Aβ-accelerated neurodegeneration caused by Alzheimer’s-associated ACE variant R1279Q is rescued by angiotensin system inhibition in mice. Sci. Transl. Med. 2020;12:eaaz2541. doi: 10.1126/scitranslmed.aaz2541. [DOI] [PubMed] [Google Scholar]
- 131.Royea J., Hamel E. Brain angiotensin II and angiotensin IV receptors as potential Alzheimer’s disease therapeutic targets. Geroscience. 2020;42:1237–1256. doi: 10.1007/s11357-020-00231-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Royea J., Zhang L., Tong X.-K., Hamel E. Angiotensin IV receptors mediate the cognitive and cerebrovascular benefits of losartan in a mouse model of Alzheimer's disease. J. Neurosci. 2017;37:5562–5573. doi: 10.1523/JNEUROSCI.0329-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Qiu C., Winblad B., Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol. 2005;4:487–499. doi: 10.1016/S1474-4422(05)70141-1. [DOI] [PubMed] [Google Scholar]
- 134.Petek B., Villa‐Lopez M., Loera‐Valencia R., Gerenu G., Winblad B., Kramberger M., Ismail M.A.M., Eriksdotter M., Garcia‐Ptacek S. Connecting the brain cholesterol and renin–angiotensin systems: potential role of statins and RAS‐modifying medications in dementia. J. Intern. Med. 2018;284:620–642. doi: 10.1111/joim.12838. [DOI] [PubMed] [Google Scholar]
- 135.Kehoe P.G. The coming of age of the angiotensin hypothesis in Alzheimer’s disease: progress toward disease prevention and treatment? J. Alzheim. Dis. 2018;62:1443–1466. doi: 10.3233/JAD-171119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Neely A., Perry C., Varisli B., Singh A.K., Arbneshi T., Senapati D., Kalluri J.R., Ray P.C. Ultrasensitive and highly selective detection of Alzheimer’s disease biomarker using two-photon Rayleigh scattering properties of gold nanoparticle. ACS Nano. 2009;3:2834–2840. doi: 10.1021/nn900813b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Brambilla D., Verpillot R., Le Droumaguet B., Nicolas J., Taverna M., Kóňa J., Lettiero B., Hashemi S.H., De Kimpe L., Canovi M. PEGylated nanoparticles bind to and alter amyloid-beta peptide conformation: toward engineering of functional nanomedicines for Alzheimer’s disease. ACS Nano. 2012;6:5897–5908. doi: 10.1021/nn300489k. [DOI] [PubMed] [Google Scholar]
- 138.Allen A.M., MacGregor D.P., Chai S.Y., Donnan G.A., Kaczmarczyk S., Richardson K., Kalnins R., Ireton J., Mendelsohn F.A. Angiotensin II receptor binding associated with nigrostriatal dopaminergic neurons in human basal ganglia. Ann. Neurol.: Official J. Am. Neurol. Associat. Child Neurol. Society. 1992;32:339–344. doi: 10.1002/ana.410320306. [DOI] [PubMed] [Google Scholar]
- 139.Labandeira‐Garcia J.L., Rodriguez‐Pallares J., Dominguez‐Meijide A., Valenzuela R., Villar‐Cheda B., Rodríguez‐Perez A.I. Dopamine‐angiotensin interactions in the basal ganglia and their relevance for Parkinson's disease. Mov. Disord. 2013;28:1337–1342. doi: 10.1002/mds.25614. [DOI] [PubMed] [Google Scholar]
- 140.Wright J.W., Kawas L.H., Harding J.W. A role for the brain RAS in Alzheimer’s and Parkinson’s diseases. Front. Endocrinol. 2013;4:158. doi: 10.3389/fendo.2013.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Labandeira-García J.L., Garrido-Gil P., Rodriguez-Pallares J., Valenzuela R., Borrajo A., Rodríguez-Perez A.I. Brain renin-angiotensin system and dopaminergic cell vulnerability. Front. Neuroanat. 2014;8:67. doi: 10.3389/fnana.2014.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kobiec T., Otero-Losada M., Chevalier G., Udovin L., Bordet S., Menéndez-Maissonave C., Capani F., Pérez-Lloret S. The renin–angiotensin system modulates dopaminergic neurotransmission: a new player on the scene. Front. Synaptic Neurosci. 2021;13 doi: 10.3389/fnsyn.2021.638519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Suntres Z.E. Liposomal antioxidants for protection against oxidant-induced damage. J. Toxicol. 2011;2011:1–16. doi: 10.1155/2011/152474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zeevalk G.D., Bernard L.P., Guilford F. Liposomal-glutathione provides maintenance of intracellular glutathione and neuroprotection in mesencephalic neuronal cells. Neurochem. Res. 2010;35:1575–1587. doi: 10.1007/s11064-010-0217-0. [DOI] [PubMed] [Google Scholar]
- 145.Jenner P., Dexter D., Sian J., Schapira A., Marsden C. Oxidative stress as a cause of nigral cell death in Parkinson's disease and incidental Lewy body disease. Ann. Neurol.: Offi. J. Am. Neurol. Associat. Child Neurol. Society. 1992;32:S82–S87. doi: 10.1002/ana.410320714. [DOI] [PubMed] [Google Scholar]
- 146.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.-H., Nitsche A. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. e278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gadanec L.K., McSweeney K.R., Qaradakhi T., Ali B., Zulli A., Apostolopoulos V. Can SARS-CoV-2 virus use multiple receptors to enter host cells? Int. J. Mol. Sci. 2021;22:992. doi: 10.3390/ijms22030992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ferrucci L., Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018;15:505–522. doi: 10.1038/s41569-018-0064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Fulop T., Larbi A., Dupuis G., Le Page A., Frost E.H., Cohen A.A., Witkowski J.M., Franceschi C. Immunosenescence and inflamm-aging as two sides of the same coin: friends or foes? Front. Immunol. 2018;8:1960. doi: 10.3389/fimmu.2017.01960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sriram K., Loomba R., Insel P.A. Targeting the renin− angiotensin signaling pathway in COVID-19: unanswered questions, opportunities, and challenges. Proc. Natl. Acad. Sci. USA. 2020;117:29274–29282. doi: 10.1073/pnas.2009875117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Speth R.C. Response to recent commentaries regarding the involvement of angiotensin-converting enzyme 2 (ACE2) and renin-angiotensin system blockers in SARS-CoV-2 infections. Drug Dev. Res. 2020;81:643–646. doi: 10.1002/ddr.21672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Speth R.C. Keep taking your ACE inhibitors and ARBs during the COVID 19 pandemic. J. Trav. Med. 2020;27 doi: 10.1093/jtm/taaa045. taaa045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Speth R.C. Angiotensin II administration to COVID-19 patients is not advisable. Crit. Care. 2020;24:296. doi: 10.1186/s13054-020-03032-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Vaduganathan M., Vardeny O., Michel T., McMurray J.J.V., Pfeffer M.A., Solomon S.D. Renin–angiotensin–aldosterone system inhibitors in patients with covid-19. N. Engl. J. Med. 2020:1–7. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sriram K., Insel P.A. Risks of ACE inhibitor and ARB usage in COVID‐19: evaluating the evidence. Clin. Pharmacol. Ther. 2020;108:236–241. doi: 10.1002/cpt.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sriram K., Insel P.A. A hypothesis for pathobiology and treatment of COVID‐19: the centrality of ACE1/ACE2 imbalance. Br. J. Pharmacol. 2020;177:4825–4844. doi: 10.1111/bph.15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ucar B., Acar T., Arayici P.P., Derman S. A nanotechnological approach in the current therapy of COVID-19: model drug oseltamivir-phosphate loaded PLGA nanoparticles targeted with spike protein binder peptide of SARS-CoV-2. Nanotechnology. 2021;32 doi: 10.1088/1361-6528/ac1c22. [DOI] [PubMed] [Google Scholar]