Abstract
The CRISPR-Cas system is commonly known for its ability to cleave DNA in a programmable manner, which has democratized gene editing and facilitated recent breakthroughs in gene therapy. However, newer iterations of the technology using nuclease-disabled Cas enzymes have spurred a variety of different types of genetic engineering platforms such as transcriptional modulation using the CRISPR activation (CRISPRa) and CRISPR interference (CRISPRi) systems. This review introduces the creation of these programmable transcriptional modulators, various methods of delivery utilized for these systems, and recent technological developments. CRISPRa and CRISPRi have also been implemented in genetic screens for interrogating gene function and discovering genes involved in various biological pathways. We describe recent compelling examples of how these tools have become powerful means to unravel genetic networks and uncovering important information about devastating diseases. Finally, we provide an overview of preclinical studies in which transcriptional modulation has been used therapeutically, and we discuss potential future directions of these novel modalities.
Keywords: CRISPR, CRISPRa, CRISPRi, transcriptional modulation, technology, preclinical, epigenetic, screen
Graphical abstract
Bak and colleagues describe recent developments in two technologies for CRISPR-Cas-based transcriptional modulation termed CRISPR activation (CRISPRa) and CRISPR interference (CRISPRi).
Introduction
2022 celebrated the first decade since the advent of the CRISPR-Cas gene editing platform, and during this period the system has been expanded, modified, and improved as a diverse toolbox for genetic engineering and gene therapy. CRISPR-Cas was originally developed for gene editing but has been repurposed for other types of genetic manipulations, including transcriptional regulation of specific genes. Historically, manipulation of gene activity has been performed using different approaches such as gene targeting/gene knockout (KO), gene supplementation using cDNA delivery, RNA interference (RNAi), antisense oligonucleotides, and zinc finger proteins (ZFPs) or transcription activator-like effector (TALE) proteins fused to transcriptional modulators.1 However, all systems have major drawbacks when it comes to production time, cost, or specificity. CRISPR-Cas-based transcriptional engineering has proven highly specific, enables both up- and downregulation of genes, and is easy to produce and utilize. With the maturation of these tools and delivery platforms, transcriptional modulation has found wide biomedical use in studies of gene function, large genetic screens, and in preclinical studies for genetic disorders and regenerative stem cell-based medicine.
Transient transcriptional modulation using the CRISPR-Cas system was first shown by Qi et al., who showed that the binding of an endonucleolytically deactivated Cas9 (dCas9) to the downstream region of a transcriptional start site (TSS) inhibited transcription by causing a steric hindrance for the RNA polymerase, thus leading to temporary inhibition of gene expression.2 This method was termed CRISPR interference (CRISPRi). dCas9 was then linked to negative transcriptional regulation domains, thereby improving the inhibitory effect of CRISPRi, and later it was linked to positive transcriptional regulation domains to generate a system for transcriptional upregulation termed CRISPR activation (CRISPRa).3,4 In the following years, epigenetic regulation domains have been fused to the dCas9 protein, for example for targeted methylation and demethylation of DNA, creating the opportunity for persistent, epigenetic, transcriptional regulation of target genes.5,6,7,8,9 It can be difficult to distinguish these systems from each other because of their similarities and fast developing nomenclature. In fact, the term CRISPRon has been used for both transient transcriptional activation and epigenetic long-term transcriptional activation, while the term CRISPRoff, CRISPRi, and CRISPR inhibition have all been used for epigenetic long-term transcriptional inhibition.5,10 For this review, the focus will be on transient transcriptional up- and downregulation that returns to baseline upon loss of the dCas-coupled transcriptional effectors, and we term these two systems CRISPR activation and interference.
The conventional CRISPR-Cas gene editing platform
The first CRISPR-Cas gene editing studies made use of the Cas9 endonuclease from Streptococcus pyogenes to generate site specific, genomic double-strand breaks (DSBs) guided by two small RNAs: the CRISPR RNA (crRNA) and the trans-activating crRNA (tracrRNA). These two RNAs carry mutually complementary regions and hybridize to form a guide RNA (gRNA) that is recognized and complexed with Cas9 to form a ribonucleoprotein (RNP). Twenty nucleotides (nt) in the 5′ end of the crRNA constitute the so-called spacer sequence of the gRNA and direct the Cas9 RNP complex to genomic target sites with complementarity to the spacer, also known as the protospacer. The protospacer must be located next to a protospacer adjacent motif (PAM), which for S. pyogenes is 5′-NGG-3′, which is specifically recognized and bound by the Cas9 enzyme.11 CRISPR systems from other microbial strains have different PAM sequence requirements, they may not have a tracrRNA, and the spacer sequence may differ in length from the 20 nt of the S. pyogenes Cas9 system and be located in the 3′ end of the crRNA.12 To simplify the S. pyogenes Cas9 system, the crRNA and tracrRNA have been fused to a single guide RNA (sgRNA).13
Conventional genome editing relies on cellular repair mechanisms to restore the DNA sequence following a DNA DSB. Non-homologous end-joining is the default repair pathway during which the two DNA ends are ligated back together. However, before ligation occurs, end-processing enzymes may cause small insertion/deletions (INDELs) to be created, which can disrupt the reading frame and lead to gene KO.13 For precise gene editing, the homology-directed repair (HDR) pathway may be utilized in which a homologous repair template is supplied to the cells, which acts as a DNA donor from which new DNA sequences can be copy-pasted into the DNA DSB during repair in a seamless manner.14
Biochemical understanding of Cas9 endonucleolytic activity revealed that Cas9 consists of two nuclease domains, RuvC1 and HNH, which each facilitates cleavage of one DNA strand. Inactivating mutations in these domains, D10A (RuvC1) and H840A (HNH),13 generated endonuclease deactivated or “dead” Cas9 (dCas9), and other dCas variants from other CRISPR systems have been generated with a similar approach.15,16,17 These mutations neither affect the gRNA interaction with the target DNA nor the DNA binding capability of Cas9.2 These dCas systems, or partially inactivated nicking Cas enzymes,18 have since enabled a diverse genetic engineering toolbox containing base and prime editors,19,20 and both transient and long-term transcriptional regulators.1,2,3,4,5
Cas variants and considerations for CRISPR-based gene regulation
As the CRISPR field is rapidly expanding, the consensus about nomenclature is still lagging, although steps have been made to streamline the categorization and naming of Cas genes and proteins (e.g., the renaming of Cpf1 to Cas12a). For this review, the nomenclature of Cas proteins and effectors will be streamlined for ease of reading. For example, the nuclease dead S. pyogenes Cas9 linked C-terminally to VP64 will be termed dSpCas9-VP64, whereas it will be termed VP64-dSpCas9 when fused to the N terminus. Cas effector enzymes are often characterized through a system describing their class, type, and subtype, all depending on the protein function—a classification that has been extensively reviewed.21,22,23 As for canonical CRISPR-Cas-based gene editing, various Cas proteins have been utilized for CRISPRa/i, with the most popular variants originating from S. pyogenes and S. aureus, from here on referred to as SpCas9 and SaCas9, respectively. Other variants, such as AsCas12a and LbCas12a (previously known as AsCpf1 and LbCpf1), originating from Acidaminococcus sp. and Lachnospiraceae bacterium, respectively, have also been adapted for transcriptional regulation to expand the utility of CRISPR-Cas system.24 Unlike Cas9 variants, Cas12a variants have only one endonuclease domain, the RuvC, thus lacking the HNH domain found in Cas9. As for dCas9, dCas12a variants are created by mutating the RuvC endonuclease domain to inactivate the nuclease.25 A list of different Cas proteins and effector domains used in CRISPRa and CRISPRi studies are shown in Table 1.
Table 1.
Overview of CRISPRa and CRISPRi platforms
| Cas variant | PAM | Effector/recruiters | Total size (bp) | Reference |
|---|---|---|---|---|
| dSpCas9 | 5′-NGG-3′ | – | ∼4,101 | Qi et al.; Jinek et al.2,13 |
| KRAB | ∼4,341 | Jensen et al.; Tian et al.; le Sage et al.; Dräger et al.; Jung et al.; Schmidt et al.; Cui et al.; Drobna-Śledzińska et al.; Chung et al.; Heman-Ackah et al.; Moreno et al.; Yoshida et al.; Escobar et al.; Leng et al.26,27,28,29,30,31,32,33,34,35,36,37,38,39 | ||
| KRAB-MeCP2 | ∼5,313 | Yeo et al.; Alerasool et al.; de Solis et al.; Ding et al.40,41,42,43 | ||
| ZIM3 | ∼4,599 | Alerasool et al.41 | ||
| VP64a | ∼4,380 | Ding et al.; Matharu et al.; Black et al.; Sokka et al.; Zhou et al.; Colasante et al.; Zhu et al.; Thompson et al.; Jaudon et al.43,44,45,46,47,48,49,50,51 | ||
| VPR | ∼5,799 | Jensen et al.; Cui et al.; Heman-Ackah et al.; Escobar et al.; Strezoska et al.; Urrutia-Cabrera et al.; Nguyen et al.; Unlu et al.; Susco et al.; Schoger et al.; Di Maria et al.26,32,35,38,52,53,54,55,56,57,58 | ||
| SunTag (GCN4-VP64) | ∼4,998 + ∼1,749b | Jung et al.; Tanenbaum et al.; Liu et al.; Boettcher et al.; Liu et al.; Danziger et al.; Siepe et al.30,59,60,61,62,63,64 | ||
| SunTag (GCN4-p65-HSF1) | ∼5,157 + ∼1,929b | Zhou et al.65 | ||
| VP64 (MS2-p65-HSF1) (SAM) | ∼4,380 + ∼1,416c | le Sage et al.; Schmidt et al.; Li et al.; Hsu et al.; Thege et al.; Yang et al.; Deng et al.; Wang et al.28,31,66,67,68,69,70,71 | ||
| VPR (MS2-p65-HSF1) (SAM) | ∼5,799 + ∼1,416c | Giehrl-Schwab et al.72 | ||
| VPH | ∼5,745 | Tian et al.27 | ||
| VPH-dSpCas9-SS18 | ∼6,471 | Tian et al.; Beyersdorf et al.27,73 | ||
| dxCas9 (dSpCas9 derivative) | 5′-NG-3′, 5′-GAA-3′, 5′-GAT-3′ |
VPR | ∼5,799 | Hu et al.74 |
| dSaCas9 | 5′-NNGRRT-3′ | – | ∼3,159 | |
| KRAB | ∼3,399 | Escobar et al.; Black et al.; Gemberling et al.38,45,75 | ||
| VP64 | ∼3,438 | Matharu et al.44 | ||
| VPR | ∼4,854 | Jensen et al.; Cui et al.; Escobar et al.; Nguyen et al.; Di Maria et al.26,32,38,54,58 | ||
| VPR (Mini) | ∼4,104 | Vora et al.76 | ||
| dAsCas12a | 5′-TTTV-3′ | – | ∼3,921 | |
| KRAB | ∼4,314 | Escobar et al.38 | ||
| VPR | ∼5,658 | Escobar et al.38 | ||
| Sp65p3-HSF1 (Activ) | ∼4,659 | Campa et al.77 | ||
| dLbCas12a | 5′-TTTV-3′ | – | ∼3,684 | |
| KRAB | ∼4,077 | Escobar et al.38 | ||
| VPR | ∼5,421 | Escobar et al.38 | ||
| dimpLbCas12a (dLbCas12a-RVRR) | 5′-TNTN-3′ 5′-TACV-3′ 5′-TTCV-3′ 5′-CTCV-3′ 5′-CCCV-3′ |
SunTag (GCN4-VP64) | ∼4,644 + ∼2,034b | Tóth et al.78 |
| enAsCas12a | 5′-TTYN-3′ 5′-VTTV-3′ 5′-TRTV-3′ |
VPR | 5,658 | Kleinstiver et al.25 |
Many variations of VP16 repeats exist and have been used for CRISPRa, such as VP48, VP160, and VP192, but the most utilized is the VP64 variant.
The first number indicates the size of the dCas-Suntag construct with 10xGCN4 binding sites, and the second denotes the size of the scFv-containing construct.
The first number is the size of the dSpCas9-effector construct, the second is the size of the MS2-p65-HSF1 construct.
Different factors should be taken into consideration when choosing a Cas variant for CRISPR gene regulation. For in vivo use, the large size of SpCas9 at ∼4.1 kb has made people gravitate to the smaller Cas9 variant SaCas9 at ∼3.2 kb, as it can be packed with a sgRNA cassette into most viral vectors commonly used for in vivo gene editing.79 The Cas12a variants are approximately the same size as the SpCas9 variant at ∼3.9 kb, depending on the organism from which Cas12a originates.79 For viral vector delivery, these sizes are of particular importance as addition of the transcriptional effector domains add to the size of the constructs resulting in very large, complex constructs that may be too large to pack into viral vectors together with the sgRNA cassettes. Particularly the popular AAV vectors are challenged by their packaging constraint of around 4.7 kb. Size considerations and choice of effector domain could also be important parameters for the ability to produce recombinant protein for direct RNP delivery, which will be discussed later.16,26
Another important consideration for choosing a Cas variant lies in the PAM sequence utilized, which is unique to individual CRISPR systems and essential for the ability of Cas to bind the target DNA. PAM site availability can be a limiting factor and can make it difficult to target an exact position or desired region. This can often be mitigated by choosing another Cas protein with a different PAM sequence.80 Some Cas proteins have short, common PAM sequences, such as SpCas9 with the PAM 5′-NGG-3′.11 Other variants have longer, more specific PAM sequences, such as SaCas9 (5′-NNGRRT-3′).16,81 Both AsCas12a and LbCas12a recognize the PAM 5′-TTTV-3′.24,82 A short, unspecific PAM can allow for many possible target sites, but can also increase the likelihood of off-target sites in the genome, which are PAM-containing sites with imperfect complementarity to the gRNA. More stringent PAMs limit the possible gRNA target positions, but also make off-target binding less likely. Multiple Cas variants have been engineered to alter or simplify the canonical PAM sequences for various Cas variants allowing for greater utility of the CRISPR system.25,74,78,83 It has also been found that some Cas variants tolerate some non-canonical PAMs for recognition of the target sequence.84,85,86
Another recently reported Cas variant for CRISPR-based gene regulation is the dCasMINI variant which was shown to be active when used for CRISPRa and base editing.87 The dCasMINI system is an engineered variant of Cas12f with a size of ∼1.6 kb. Xu et al. performed four rounds of mutations to increase the performance of the protein, resulting in the Cas variant they termed dCasMINI-V4, later referred to only as dCasMINI.87 This CRISPR system is normally not active in mammals, but truncations and modifications to the gRNA scaffold enabled targeted transcriptional upregulation in human cell lines when fused to a transcriptional activation domain. While an effective addition to the CRISPRa toolbox, the nuclease-active variant of CasMINI was not nearly as effective at cleaving DNA as its Cas12a counterpart. dCasMINI uses a 5′-TTTR-3′ PAM, not unlike the PAM utilized by the Cas12a variants (5′-TTTV-3′), although a bit stricter, limiting the sgRNA design options.
While both CRISPRa and CRISPRi are relatively fast acting once in the cell, the kinetics of the transcriptional effect relies on both the method of delivery, which will be touched on later, but also on whether activation or inhibition is performed.26 The effect of CRISPRa can usually be observed much quicker than that of CRISPRi, as transcription and translation occur within hours. On the other hand, gene repression cannot be detected until degradation of the existing mRNA and protein has taken place.
gRNAs—The navigational system for CRISPR-Cas gene manipulation
The gRNA is what allows for the broad utility of the CRISPR-Cas gene modification system, as the gRNA is the programmable part of the Cas:gRNA RNP complex. Along with the first report of the CRISPR-Cas9 system for programmable gene editing came also the fusion of the tracrRNA and crRNA into a sgRNA. This created a two-component system that could be easily reprogrammed by changing the short spacer region toward the desired target site. However, some CRISPR systems only require a crRNA, such as Cas12a. gRNAs are made up of a constant scaffold sequence specific for the Cas protein variant and a programmable spacer region. The gRNA scaffolds include several stem-loop structures within the sequence depending on the Cas variant, which allow for binding of Cas and formation of the RNP complex. The spacer length needed for effective CRISPR-Cas gene modification also differs between Cas variants. The canonical Cas9 sgRNA spacers for SpCas9 and SaCas9 are 20–21 nt long, whereas the Cas12a crRNA spacers are longer (23–25 nt).24 Interestingly, for SpCas9 it has been found that sgRNAs with truncated 5′ ends, i.e., spacers of 14–15 nt, still allow for binding of the Cas protein and localization of the RNP complex to the target site but are not able to facilitate DNA cleavage.88,89 Thus, the use of these truncated sgRNAs allows for transcriptional modulation using an endonuclease-active SpCas9 fused to a transcriptional regulation domain. This can be utilized to perform simultaneous CRISPRa or interference and gene editing, when using truncated sgRNAs for the CRISPRa/i target gene and full-length sgRNA for targeted DNA cleavage.88,89
An important consideration when choosing sgRNAs is the possibility for off-target activity. Proper selection of the spacer sequence can minimize or in some cases completely eradicate detectable off-target activity, but this can be difficult depending on the Cas variant used or the desired target site. The sgRNA design for the CRISPRa and CRISPRi systems is somewhat more flexible than for canonical CRISPR-Cas gene editing as there is no strict consideration as to the positioning of the cut site as when using a functional Cas nuclease for precise HDR-based gene editing. One of the main considerations when designing CRISPRa and CRISPRi sgRNAs is the positioning of the sgRNA relative to the TSS of the target gene, as the effector can only regulate transcription within a specific window at the TSS. High-throughput screens have identified high-activity windows for both CRISPRa and CRISPRi in the regions [TSS –300, TSS +0] or [TSS –400, TSS –50] for CRISPRa and [TSS –50, TSS +300] for CRISPRi.90,91 The CRISPick online sgRNA design tool from the Broad Institute that predicts specific and efficient CRISPRa and CRISPRi sgRNA designs for a given input gene for multiple different popular Cas variants uses the [TSS –300, TSS +0] and [TSS –50, TSS +300] for CRISPRa and CRISPRi, respectively. The relatively broad targeting regions allow for the design of multiple functional sgRNAs for each target gene and pooling of sgRNAs, which have previously proven to lead to more efficient transcriptional modulation than a single sgRNA. The requirement for proximity to a TSS drastically limits the number of possible off-target sites. However, CRISPRa has also been found active when targeting distant enhancers of the target gene, although the specific design requirements for this have not been investigated.44 As enhancers are less characterized and defined than TSSs, in silico prediction of off-target sites in enhancer elements may be inadequate, and experimental investigation of potential off-target gene regulation of specific sgRNAs would be needed. In general, experimental assessment of off-target effects is performed by RNA sequencing to identify deregulated genes beyond the target gene,87 but scrutiny of these data in terms of identification of putative sgRNA binding sites and further validation might be necessary to rule out indirect effects caused by the intended target gene regulation.
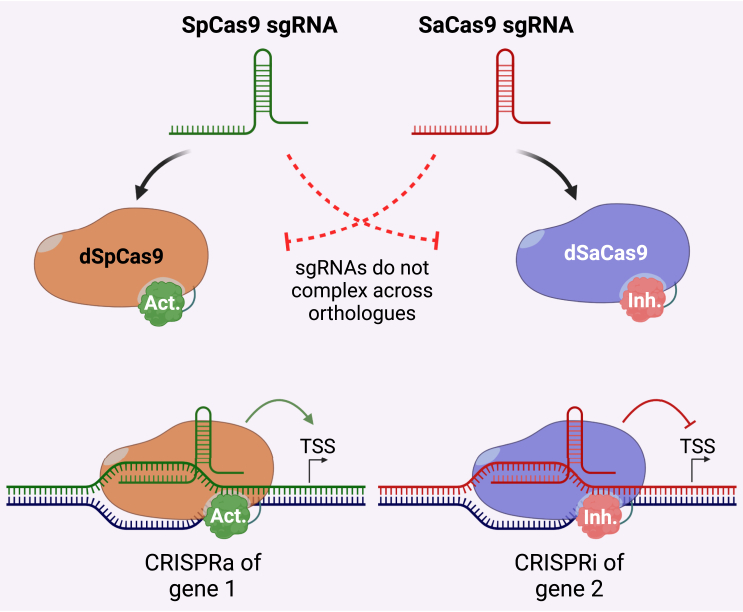
Since different Cas variants require different gRNA scaffolds, CRISPR-Cas systems can be combined to perform multi-modal genetic engineering, for example, orthogonal gene regulation by simultaneous up- and downregulation of different target genes by dedicating dSpCas9 for CRISPRi and dSaCas9 for CRISPRa (Figure 1),26 or simultaneous induction of DSBs using one Cas variant, while regulating transcription with another.92 The CRISPR-Cas gene modification systems have also been used for multiplexing, both for canonical gene editing at multiple genetic loci,93 but also for CRISPRa and CRISPRi, allowing for transcriptional modulation of multiple genes simultaneously by delivery of a single Cas variant with a pool of sgRNAs with different spacers.10 This mix-and-match approach to the CRISPR-Cas systems showcases the many possibilities and broad utility of the toolbox as it becomes more popular for both in vitro and in vivo applications.
Figure 1.
Orthogonal gene regulation by CRISPRa and CRISPRi
Simultaneous CRISPRa and CRISPRi is possible using Cas orthologous, e.g., SpCas9 and SaCas9, since the sgRNAs do not complex with orthologous Cas enzymes. In this example, dSpCas9 is fused to a transcriptional activation domain (Act.) and dSaCas9 is fused to a transcriptional inhibitor (Inh.). Combined delivery of all components in the same cells can lead to upregulation of target gene 1 and downregulation of gene 2.
For RNA and RNP delivery, sgRNAs can be produced either through in vitro transcription (IVT) or as synesthetic, chemically modified sgRNAs.94 While the IVT gRNAs are cheaper to produce than commercially available synthetic sgRNAs, they are also less stable within the cell, as the unprotected RNAs are more prone to degradation, whereas synthetic sgRNAs can be chemically modified to increase stability and activity in the cell. When delivering preassembled RNP complexes, the sgRNAs are somewhat protected from degradation when embedded in Cas9, enabling the use of unmodified IVT sgRNAs for this use, but for sgRNA and Cas9 mRNA delivery, the use of chemically modified synthetic sgRNAs is essential as unprotected sgRNAs are rapidly degraded.94 These synthetic sgRNAs can be acquired from several commercial sources and are available within 1–2 weeks after ordering. Most commonly they are produced with chemical modifications that reduce innate immune sensing and protect the ends of the sgRNAs, e.g., using 2′-O-methyl bases and phosphorothioate linkages at the three terminal bases in each end. In contrast, IVT sgRNAs have been shown to be highly immunostimulatory through the RIG-I pathway and induce interferon responses that compromise cell viabilities.95,96,97
CRISPRi—Domains and structure
As mentioned previously, the CRISPRi system was originally described using only dCas9 to sterically block the RNA polymerase to interfere with transcription of a target gene (Figure 2).2,13 Later, a transcriptional repressor domain was linked to dCas9, improving the inhibitory effect of dCas9.3 The domain originally used was a KRAB domain, which originates from the human ZFP Kox1.98,99 Several KRAB domains have been derived from different ZFPs, but most CRISPRi studies use the KOX1 variant, which has also been used for gene repression using TALEs.100,101,102 These KRAB domains bind the corepressor protein KAP1, which in turn recruits the histone methyltransferase SETDB1 that promotes tri-methylation of histone H3 lysine 9 (H3K9), leading to powerful transcriptional repression.103 Despite being regarded as an epigenetic modification, these deposited histone marks are not stable and gene expression returns to baseline once the repression complex is gone.91 Yeo et al. tested different combinations of more than 20 different effector domains and identified the KOX1 KRAB domain linked to methyl CpG binding protein 2 (MeCP2), a protein involved in gene repression in nerve cells, to promote the most powerful transcriptional interference.40 Subsequently, Alerasool et al. screened 57 different KRAB domains and identified the ZIM3 KRAB domain as the most efficient, even superior to the KOX1 KRAB-MeCP2 bipartite domain.41
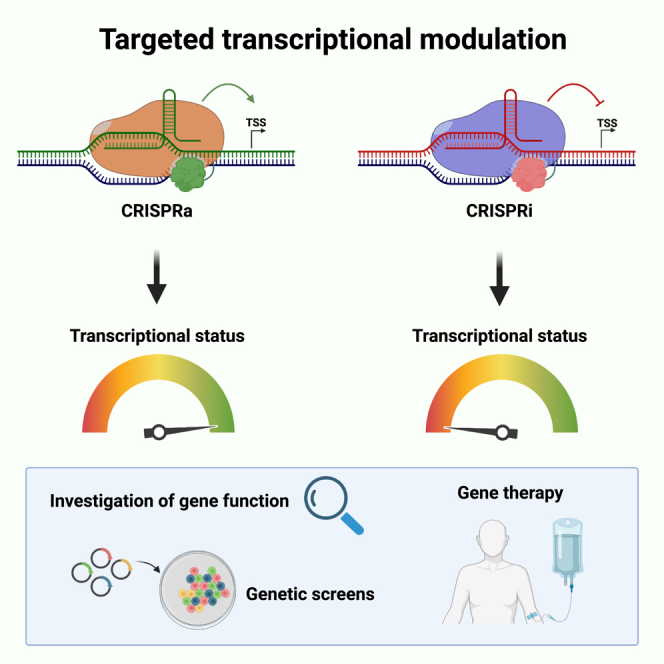
Figure 2.
Overview of different CRISPRa and CRISPRi platforms
Transcriptional modulation is mediated by localization of transcriptional activators or inhibitors to the region around the transcriptional start site (TSS). For CRISPRa, multiple activators can be recruited using either the sgRNA scaffold (SAM) or an array of epitopes fused to dCas (SunTag). For CRISPRi, steric hindrance can interfere with transcription using a dCas protein alone, but transcriptional inhibition is improved using a fused transcriptional repression domain.
CRISPRa—Domains and structure
The current CRISPRa systems can be divided into two categories: one which utilizes only the dCas protein fused to a transcriptional activation domain (cis), and one which employs recruitment of transcriptional activators to adaptors located either on the dCas protein or the sgRNA scaffold (trans) (Figure 2).
The first CRISPRa system to be developed was the addition of VP48 or VP64 to dSpCas9.3,4,10,104 Shortly thereafter, the name CRISPRa was coined. VP64 is an activation domain made up of four VP16 domains that facilitate the induction of transcription in herpes simplex virus.105 While dSpCas9-VP64 promoted transcriptional upregulation of the target gene, the induced upregulation was moderate and not as effective as targeted gene activation using VP64-based TALE activators.4 Other activation domains have been combined and tested in different configurations, with the tripartite activator, VP64-p65-Rta (VPR) promoting the most powerful upregulation in the first category where the transcriptional activator is fused directly to dCas9.106 p65 is part of the transcription factor NF-κB and is the domain responsible for transcriptional activation, whereas replication and transcription activator (Rta) is an activator of viral genes in Epstein-Barr virus.107
While these CRISPRa systems are two-component systems made up of only the sgRNA and dCas protein fused to a transcriptional regulator, the second category consists of other powerful and popular CRISPRa systems that involve a third component. The SunTag system was originally developed both for CRISPR-mediated transcriptional upregulation and chromosome labeling for fluorescent imaging purposes.59 It consists of dCas9 fused to a SunTag, a polypeptide chain containing an array of epitopes able to recruit an antibody-derived single-chain variable fragment (scFv) termed GCN4, which is linked to VP64. This scFv(GCN4)-VP64 effector is delivered along the dCas9-SunTag and the sgRNA. This allows the recruitment of multiple VP64 copies to a single dCas9 protein, leading to a more potent upregulation of the target gene than can be achieved using dCas9-VP64 alone. Originally designed to have either 10× or 24× antibody binding sites, both constructs showed similar activation of the tested target genes, leading most studies to utilize the 10× variant to limit the size of the construct.
Like SunTag, the synergistic activation mediator (SAM) system is also a three-component CRISPRa system, utilizing the basic dSpCas9-VP64 fusion protein. For the SAM system, the hairpin structure of the sgRNA has been modified to contain two aptamers that each carry two binding sites for the MS2 protein.108 The third component of the SAM system is an MS2-p65-HSF1 fusion protein that can be recruited to the MS2 aptamers, thus theoretically delivering four copies of the p65 and human heat-shock factor 1 (HSF1) bipartite transcriptional activation domain to each dCas9-VP64 complex, allowing potent transcriptional activation. HSF1 is a highly conserved protein that works as a regulator of the transcriptional response to proteolytic stress,109 and contains a transcriptional activation domain. As for the SunTag system, the efficiency of the SAM system lies in the ability to recruit multiple copies of the transcriptional activators than can be fused directly to the dCas protein.
Variations of the VPR effector have recently been developed, one being the VPR mini effector featuring truncated versions of the p65 and Rta domains.76 Another newly reported effector, termed VPH, combines four repeats of VP48, p65, and HSF1.27 The VPH system later was fused N-terminally to dSpCas9 along with a C-terminal fusion of a domain called SS18.73 The SS18 is a component of the SWI/SNF chromatin remodeling complex which can expose binding sites for transcription factors. The SS18 is sufficient for recruitment of the full SWI/SNF complex, allowing for improved target gene activation by the VPH tripartite domain.73
Delivery methods for CRISPR-Cas-based gene regulators
RNP delivery
Many CRISPR-Cas gene editing strategies rely on delivery of the Cas effector and sgRNA precomplexed as an RNP complex prior to delivery by transfection (Figure 3). The use of RNPs for CRISPR-Cas gene editing is highly effective and widely used because the short duration of RNP exposure minimizes the risk of off-target edits in the cells.110 A similar decrease in off-target activity for RNP-delivered CRISPRa and CRISPRi effectors would be expected, although this has not been experimentally validated. In contrast to gene editing, RNPs are rarely used for CRISPR-Cas-based transcriptional regulation, likely because recombinant multi-domain Cas9 fusion proteins are difficult to produce. However, the relatively small dSpCas9-VP64 protein (VP64 is 54 amino acids) is commercially available for the CRISPRa system, and we also previously reported the production and use of this.26 Our comparison between recombinant dSpCas9-VP64 RNP complex and dSpCas9-VPR mRNA showed barely any difference in the proportion of cells with target gene upregulation analyzed by flow cytometry. However, RNP delivery led to a significantly lower mean fluorescent intensity (MFI) of the induced gene upon RNP delivery, using the same sgRNAs for both RNP and mRNA delivery.26 This signifies that >90% of cells are transfected with both delivery modalities, but the lower MFI observed with RNP delivery might be explained by the use of VP64 vs. VPR used in the mRNA, or that mRNA delivery gives rise to more effector molecules per cell. More comparisons would need to be performed to accurately determine the relative efficiency of these two delivery modalities. Both the RNP and mRNA delivery showed a return to baseline expression levels by around day 7 post-electroporation. So far, no reports have been made of the production of recombinant dSpCas9-VPR protein, nor any other CRISPRa/i effector proteins.
Figure 3.
Delivery modalities for CRISPR-Cas-based transcriptional modulators
Retro/lentiviral, AAV, and plasmid vectors encode both dCas-effector and sgRNA, and whereas lentiviral vectors integrate these expression cassettes into the genome, AAV and plasmid vectors are transcribed episomally. RNA delivery relies on in-vitro-transcribed RNA encoding dCas-effector and synthetic sgRNA delivery. Some dCas-effector proteins can be generated as recombinant protein and precomplexed into an RNP complex using synthetic sgRNAs prior to delivery.
Plasmid
Plasmid delivery is by far the most common in vitro delivery mode of the CRISPRa and CRISPRi components (Figure 3). Plasmid constructs of most CRISPRa and CRISPRi effectors can easily be acquired from the nonprofit plasmid repository Addgene as well as plasmids for expressing gRNAs. gRNA-encoding plasmids carrying a spacer sequence for a target of interest can be cloned within a few days with simple and affordable general cloning protocols. While not as effective as RNP or mRNA delivery on a per-cell basis,26,94 plasmid delivery is easily approachable and allows for delivery of effectors that cannot be produced as recombinant protein.
An important consideration when choosing plasmid delivery is the efficiency of the transfection. While it can be effective for some immortalized cell lines, it can be difficult or almost impossible to apply in primary cells due to low delivery efficiency or toxicity due to immunological innate DNA sensing pathways.111 Therefore, plasmid delivery is difficult to apply in a therapeutic setting.94 Selecting the transfection method most suitable for the target cells can improve efficiency and decrease cellular toxicity.112 Another concern with plasmid delivery is the chance, albeit low, of random plasmid insertion into chromosomal DNA of the target cells which would lead to permanent activity of the CRISPRa/i effectors in a small fraction of the cells.113 Other delivery options such as mRNA delivery seem more suitable for therapeutic approaches for in vivo or ex vivo gene regulation.
mRNA
Another way to deliver the components for CRISPR-Cas transcriptional regulators is the transfection of IVT mRNA (Figure 3). The mRNA constructs can be transfected along with synthetic sgRNAs that are chemically modified at both ends to reduce innate immune sensing and increase stability as discussed previously.
The use of IVT mRNA allows for effective delivery of constructs that cannot be produced as recombinant protein. The IVT mRNA can be produced using different 5′ caps for reduced immunogenicity and increased translation of the mRNA, with CleanCap and ARCA caps being regularly used for IVT of mRNA constructs. Along with 5′ capping, modified nucleotides are commonly used to improve the stability and translation of the mRNA while reducing anti-RNA immune responses, with notable modifications being pseudouridine and N1-methyl-pseudouridine,26 the latter which has recently been used in the Pfizer-BioNTech and Moderna Therapeutics mRNA Coronavirus vaccines.114
While mRNA delivery of the CRISPRa and CRISPRi effectors with pooled sgRNAs lead to more efficient transcriptional regulation than plasmid delivery, it is not as widely used as plasmid. The process of producing IVT mRNA is not nearly as widespread as regular plasmid cloning, and many combinations of Cas protein and transcriptional regulation domains reported for plasmid delivery have not been reported for IVT mRNA. Although both dSpCas9 and dSaCas9 constructs for CRISPRa and CRISPRi are extensively used for plasmid or viral vector delivery, only dSpCas9 have been reported for mRNA delivery of both the CRISPRa and CRISPRi systems. dSaCas9-KRAB, while active for plasmid and viral vector delivery, has not been described for mRNA delivery, nor has the use of dCas12a mRNA.26,73,115
CRISPRa and CRISPRi RNA systems are easily transfected into the cells, especially with the use of electroporation or lipofection reagents optimized for RNA delivery.26,73,116 Many cell types also show improved viability post-transfection with mRNA in contrast to plasmid, as mRNA is less toxic. The electroporation of mRNA components can efficiently be achieved in both immortalized cell lines used for in vitro work, but also in harder to transfect, more therapeutically relevant primary cells such as T cells, hematopoietic stem cells, human neuronal stem cells, and natural killer cells.26
mRNA systems also lead to a faster activation or inhibition of gene expression. mRNA delivery of dSpCas9-VPR with synthetic sgRNAs led to detectable CRISPRa 3 h post-electroporation and keeping the full activation going for more than 48 h post-transfection.26 While studies are lacking on the kinetics of CRISPRa/i by plasmid DNA delivery, experience from gene editing systems shows that it takes longer to reach the full effect than delivery of RNP or mRNA due to the need for the Cas effector to be transcribed and translated before the system is active. The dynamics of CRISPRi is somewhat different from CRISPRa. In studies delivering mRNA of dSpCas9-KRAB by electroporation with synthetic sgRNAs, maximum target gene repression was observed 2–5 days post-electroporation depending on cell type. This is most likely due to the requirement of endogenous mRNA and protein of the target gene to be turned over before a repression is detectable.26
Viral vector delivery
Viral vectors have become very popular for delivery of the CRISPR-Cas systems, especially for in vivo purposes or for CRISPR-based screens. Viral vectors with large capacity such as adenovirus (AdV), retrovirus, or lentivirus (LV) vectors can contain all components of the CRISPR system (Figure 3). However, adeno-associated virus (AAV) vectors, which have gained much attraction for in vivo therapies due to their high delivery efficiencies, suffer from their relatively small packaging capacity at around 4.7 kb and remain a major bottleneck for CRISPR-Cas systems. This challenge becomes particularly important for CRISPRa and CRISPRi systems since the addition of large transcriptional regulators adds considerable size to the already large Cas enzymes. Hence, important considerations of effector type as well as Cas variant and promoter choice must be taken; and, even with careful design considerations, two AAV vectors must be used to accommodate all components. Due to its smaller size, the SaCas9 variant has become increasingly popular for use in AAV.117 While dSpCas9 constructs have been packaged into AAVs, they are large and usually require multiple AAV constructs using intein peptides to split dSpCas9-effector genes,118 or using a single AAV to deliver nuclease-active SpCas9 along with a SAM sgRNA with a truncated spacer sequence, allowing for Cas9 binding to the target, but no cleavage.119
The duration of the imposed transcriptional modulation is also important to consider. While the CRISPRa and CRISPRi systems are often described as transient, the effects of the systems can become permanent depending on the delivery method. AAV vector delivery leads to the presence of stable episomal vector DNA that provides persistent expression in postmitotic tissues. The use of integrating LV vectors also leads to sustained expression of the CRISPRa or CRISPRi system. If a transient effect is desired, one needs to consider other vector systems like mRNA delivery or integration-deficient LV (IDLV) vectors.
LV vectors are preferred when performing pooled CRISPRa or CRISPRi screens because they allow for integration of sgRNA libraries into the target cells that commonly are pre-engineered with stable expression of the CRISPRa or CRISPRi effectors. Here, the integrated sgRNA sequence serves as the barcode for the target gene that has been regulated, and phenotype-genotype relationships can be unraveled by NGS-based determination of sgRNA representation following an applied selective pressure such as drug treatment, as described in the next section.
Loss- and gain-of-function screens
In recent years, one of the most prevalent uses of CRISPRa and CRISPRi has been for the purpose of performing loss- and gain-of-function genetic screens. Loss- and gain-of-function screens allow for the identification of genes that are either sufficient or necessary, respectively, to confer a specific phenotype, which might be a response to different treatments or conditioning of cells. As mentioned above, LV vectors are commonly used for pooled genetic screens as they facilitate chromosomal integration of the gRNA library (Figure 4). The use of combined CRISPRa and CRISPRi screens have become more common because having both overexpression and knockdown data for the same selection condition is a powerful means to elucidate gene functionality and unravel genetic pathways. These combined screens usually utilize cells with stable expression of either a CRISPRa or CRISPRi construct, which are then transduced with LV sgRNA libraries, and selected and sequenced in parallel with a control population, allowing for determination of both the effect of the upregulation of target genes from CRISPRa and the knockdown from CRISPRi. While CRISPRi screens have natural analogous counterparts in the widely utilized CRISPR KO and RNAi screens, which function in much the same way as CRISPRi, some important differences between the three systems are relevant to consider when choosing which kind of screen to perform. RNAi acts on the RNA level to degrade the target mRNA or inhibit translation of the mRNA into protein, leading to a partial knockdown of gene expression. CRISPR KO screens affect the DNA by creating permanent INDELs, which might cause a complete KO of the target gene if open reading frame (ORF)-disrupting INDELs are installed at all alleles. This is desirable for some studies as the results are permanent and have a higher specificity than the RNAi system, although full gene KO can also be problematic for essential genes for which a full KO is lethal. RNAi has also been shown to be less specific than CRISPR-based screening methods, which can produce misleading data.120 CRISPRi screens can be designated as a happy medium. As with CRISPR KO, CRISPRi affects the DNA but makes no permanent changes to the genome; however, it inhibits transcription, causing a knockdown of the target gene with a higher specificity than the RNAi system while still making it possible to make readouts on essential genes due to incomplete downregulation. It is important to consider that the use of integrating LVs for these loss- and gain-of-function CRISPRa/i screens will result in stable expression of the Cas effector and sgRNA, thus losing the transient quality of the CRISPRa/i systems. This could be achieved by combining LV vector sgRNA library delivery with, for example, transient dCas9-effector delivery as has been done in KO screens using nuclease-active Cas9 protein.121
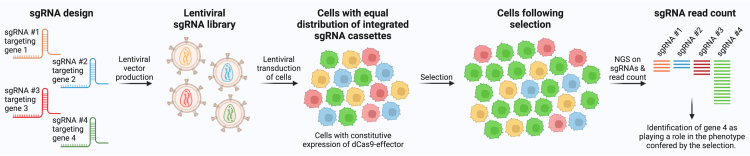
Figure 4.
CRISPRa and CRISPRi pooled screens
Pooled CRISPRa or CRISPRi screens begin with designing the sgRNA library, which can be genome-wide or target a subset of genes, for example transcription factors or a custom gene panel. This simple example contains four different sgRNAs each targeting a different gene. Lentiviral plasmids are cloned containing the sgRNA expression cassette and then lentiviral vectors are produced and used to transduce cells that usually already constitutively express the dCas9-effector that facilitates gene modulation. Transduction is performed at a low multiplicity of infection ensuring that only a single sgRNA expression cassette is integrated in each cell. The vector also contains a selection marker enabling depletion of untransduced cells. Throughout the process, it is important to confirm abundant and similar representation of sgRNAs. Next, a selection is applied to the cells either by means of increased or decreased survival/proliferation or FACS-based selection based on a specific phenotype or function. This usually follows a period of exposure to a stimulus such as drug treatment. Following selection, sgRNA cassettes are sequenced and quantified by NGS, and selection for or against a specific sgRNA signifies an involvement of the sgRNA’s target gene in the process that was investigated, for example drug resistance.
Generally, the scalability of pooled CRISPR screens to encompass the full genome makes them very cost efficient on a per-gene basis. However, they also require significant expertise, particularly in the bioinformatic analysis. An alternative to pooled screens is arrayed or parallel screens. Arrayed CRISPR screens are performed by targeting one single gene per sample and then performing arrayed experiments in 96- or 384-well plates. This screening approach is more expensive and usually encompasses much fewer genes than a pooled screen. However, it is more specific and accurate than a pooled screen since there is no risk that cells carrying different genetic perturbations affect one another in the same sample, which can confound the results. Arrayed screens can therefore also be used at a smaller scale to verify the results of a pooled screen when the list of genes of interest has been reduced. Strezoska et al. performed a small arrayed CRISPRa screen based on 153 target genes to identify regulators of interleukin-6 (IL-6) production.52
The screening system most related to the CRISPRa gain-of-function screens is based on ORF overexpression by cDNA delivery. Much like CRISPR KO and CRISPRi screens, many ORF and CRISPRa screens are performed using LV libraries to deliver the cDNAs or CRISPR components to the cells. Delivery of the ORF library depends heavily on the genes, as very large genes or genes with complex sequences can be difficult or impossible to clone. Another consideration when choosing ORF screens is that many genes have multiple transcript variants arising from different promoters and alternative splicing. Differential and simultaneous actions of several splice variants cannot be recapitulated using simple cDNA ORF screens where usually only one transcript variant is expressed per cell. Furthermore, non-coding regulatory elements in the UTRs may be difficult to include due to size constraints. Many of these issues are addressed with CRISPRa screens. In general, CRISPRa libraries have reduced complexity as only short 20 nt spacer sequences constitute the library. Furthermore, as CRISPRa acts on the endogenous gene, all transcript variants arising from alternative splicing will be maintained. These considerations have made CRISPRa a popular platform to perform gain-of-function screens, leading to an abundance of reported CRISPRa screens for identifying genes related to everything from drug resistance to cell-fate differentiation and reprogramming.45,60,66
CRISPRa and CRISPRi screens have been used in several studies to uncover genes related to different cellular functions and pathways (Table 2). Exemplary studies include identification of genes involved in resistance and sensitivity to the BRAF inhibitor vemurafenib,28 identification of genes related to neurodegeneration by probing microglia survival, activation, and phagocytosis,29 and genes associated with resistance to different HDAC inhibitors.30 These screens have even been applied to primary human cells where Schmidt et al. performed CRISPRa and CRISPRi screens in primary human T cells to identify genes involved in regulation of IL-2 and interferon-γ secretion. Here, results from genome-wide LV vector-based screens were confirmed using smaller arrayed screens.31 Finally, orthogonal CRISPR screens utilizing dSpCas9 for CRISPRa and nuclease-active SaCas9 for KO in the same cell demonstrated the ability to investigate genetic networks and interdependence of genes, e.g., if an activated gene can compensate for the loss of another.61 The knowledge extracted from these powerful screens have led to a better understanding of genotype-phenotype relationships and identified novel genetic targets that may be druggable and could enable new therapies or improve existing drugs.
Table 2.
An overview of CRISPRa and CRISPRi genetic screens
| Reference | CRISPRa/i construct | Target cells | Purpose |
|---|---|---|---|
| Le Sage et al.28 | dSpCas-KRAB, dSpCas9-VP64 (SAM) | A375 melanoma cells | uncovering gene networks driving drug resistance |
| Boettcher et al.61 | dSpCas9-10xGCN4 (SunTag) + scFv-GCN4-sfGFP-VP64 | K562 cells | analyzing the quantitative effects of activation and KO to reconstruct directional gene dependency networks for K562 cells |
| Liu et al.60 | dSpCas9-10xGCN4 (SunTag) + scFv-GCN4-sfGFP-VP64 | mouse embryonic stem cells | identification of factors that drive neuronal fate and reprogramming |
| Yang et al.69 | dSpCas9-VP64 (SAM) | primed human epiblast stem cells | identification of novel factors related to cellular reprogramming |
| Strezoska et al.52 | dSpCas9-VPR | U2OS cells | identification of regulators of IL-6 cytokine secretion |
| Black et al.45 | VP64-dSpCas9-VP64, dSaCas9-KRAB | human iPSCs | identification of master regulators and cofactors related to human neuronal cell fate specification |
| Tian et al.27 | dSpCas9-VPH, dSpCas9-KRAB | human iPSCs | lysosomal failure linked to ferroptosis in human neurons |
| Li et al.66 | dSpCas9-VP64 (SAM) | human HCC cell lines | identification of regulators of sorafenib resistance |
| Zhu et al.49 | dSpCas9-VP64 | HEK293T, Huh7 | identification of candidate receptors for SARS-CoV-2 entry |
| Dräger et al.29 | dSpCas9-KRAB, | human iPSCs | uncovering regulators of microglial states, enabling their functional characterization and therapeutic targeting |
| Thompson et al.50 | dSpCas9-VP64 | keratinocytes | identification of genes and pathways that increase type VII collagen (C7) expression. |
| Danziger et al.63 | dSpCas9-10xGCN4 (SunTag) + scFv-GCN4-sfGFP-VP64 | A549 | identification of interferon-stimulated genes, SARS-CoV-2 restriction factor identification |
| Unlu et al.55 | dSpCas9-VPR | Jurkat, HepG2, HEK293T, MDA-ME-231, PaTu-8988t | identification of SLCO2B1 as a heme transporter that enhances cellular iron availability |
| Siepe et al.64 | dSpCas9-10xGCN4 (SunTag) + scFv-GCN4-sfGFP-VP64 | K562 | identification of orphan ligand-receptor relationships |
| Leng et al.39 | dSpCas9-KRAB | HiPSC-derived astrocytes “iAstrocytes” | identifying cellular pathways controlling inflammatory astrocyte reactivity induced by IL-1α+TNF0C1q |
| Schmidt et al.31 | dSpCas9-VP64 (SAM), dSpCas9-KRAB | primary human T cells | identifying regulators of cytokine production |
| Jung et al.30 | dSpCas9-10xGCN4 (SunTag) + scFv-GCN4-sfGFP-VP64, dSpCas9-KRAB | 32Dcl2 | identifying genetic networks in HDAC-inhibitor-resistant cells |
| Zhang et al.122 | dSpCas9-VP64 (SAM) | HEPG2 | in vivo study to screen for drivers of hepatocellular carcinoma growth and metastasis |
CRISPRa in a pre-clinical setting
One possible use of the CRISPRa system is for controlling cell fate and directing cell differentiation (Table 3). A well-known example is the conversion of somatic cells into induced pluripotent stem cells (iPSCs) and differentiation of iPSCs into specific adult cell types.46,60,62 The dSpCas9-VPR-mediated upregulation of the neurogenic differentiation factor 1 (NEUROD1) gene in iPSCs was shown to enable differentiation into adult neurons.53 In addition, directed differentiation of mouse astrocytes or embryonic fibroblasts into motor neurons was shown by CRISPRa (dCas9-VP64) of the genes Ngn2 and Isl1.47 The latter study also showed motor neuron generation after AAV delivery of CRISPRa reagents in vivo, indicating that these findings could lead to a possible CRISPRa-based gene therapy to regenerate neuronal cells after brain or spinal damage. Other reports of using CRISPRa for cell fate differentiation and tissue regeneration include improved bone, heart, and brain tissue regeneration.48,54,67 The CRISPRa system has also been utilized to rescue the obesity phenotype of Sim1 and Mc4r haploinsufficient mice. Matharu et al.44 found that dCas9-VP64-mediated CRISPRa of the single active allele could increase gene expression levels and rescue the haploinsufficiency phenotype. Interestingly, they found that targeting the Sim1 promoter caused a systematic upregulation of Sim1 in tissues where it was expressed, whereas targeting the hypothalamus-specific enhancer caused a tissue-specific upregulation of Sim1 expression. This finding adds an additional layer to the control of CRISPRa.
Table 3.
Pre-clinical therapeutic CRISPRa and CRISPRi studies
| Reference | CRISPRa/i construct | Cells/model | Purpose |
|---|---|---|---|
| Heman-Ackah et al.35 | dSpCas9-VPR, dSpCas9-KRAB | human iPSC-derived neurons | CRISPRa/i for precise modulations of endogenous gene expression in fate-committed iPSC-derived neurons |
| Liu et al.62 | dSpCas9-10xGCN4 (SunTag) + scFv-GCN4-sfGFP-VP64 | mouse embryonic fibroblasts (MEFs), tail tip fibroblasts (TTFs) | reprogramming of fibroblasts toward pluripotency |
| Yoshida et al.123 | dSpCas9-KRAB | lung SCC cells, pulmonary adenocarcinoma cells, esophageal SCC cells, foreskin fibroblasts |
CRISPRi targeting of ΔNp63 for treatment of squamous cell carcinoma |
| Moreno et al.36 | KRAB-dSpCas9 | C57BL/6J mice | targeted repression of Nrl to mediate in situ reprogramming of rod cells into cone-like cells |
| Matharu et al.44 | dSpCas9-VP64, dSaCas9-VP64 | neuro-2a, Sim+/− mice | promoter or enhancer activation to rescue haploinsufficiency |
| Chung et al.34 | dSpCas9-KRAB | white adipocytes | inhibition of Fabp4 to ameliorate obesity, inflammation, hepatic steatosis, and insulin resistance |
| Wang et al.71 | dSpCas9-VP64, MCP-p65-HSF1 (SAM) | C57BL/6J mice | multiplexed activation of endogenous genes to elicit potent antitumor immunity |
| Hsu et al.67 | dSpCas9-VP64, MCP-p65-HSF1 (SAM) | rat bone marrow-derived stem cells | enhancement of BMSC osteogenesis and promotion of calvarial bone regeneration |
| Di Maria et al.58 | dSaCas9-VPR, dSpCas9-VPR | C57BL/6 mice | overexpression of CB1 receptors in neurons |
| Colasante et al.48 | dSpCas9-VP160 | in vivo mouse study | in vivo CRISPRa to decrease seizures and rescues cognitive deficits in a rodent model of epilepsy |
| Nguyen et al.54 | dSpCas9-VPR, dSaCas9-VPR, dSt1Cas9-VPR, dNmCas9-VPR | adipose-derived stem cells | activation of long non-coding RNA DANCR to promote bone regeneration |
| Zhou et al.47 | dSpCas9-VP64 | mice astrocytes (in vivo) | in vivo reprogramming of astrocytes to motor neurons |
| Gemberling et al.75 | dSpCas9-p300, dSaCas9-KRAB | in vivo mouse model | creation of transgenic mouse models for CRISPRa and CRISPRi |
| Cui et al.32 | dSpCas9-VPR, dSaCas9-VPR, dSpCas9-KRAB | MCF7, MDA-MB-231, HCC202, HEK293T | dual CRISPR interference and activation for targeted reactivation of X-linked endogenous FOXP3 in human breast cancer cells |
| Sokka et al.46 | dSpCas9-VP192 | human fibroblasts | reprogramming of fibroblasts into human pluripotent stem cells |
| Urrutia-Cabrera et al.53 | dSpCas9-VPR | PBMC-iPSCs | improved induced pluripotent stem cell differentiation into neurons |
| Drobna-Śledzińska et al.33 | dSpCas9-KRAB | Jurkat, ALL-SIL | inhibition of miRNAs and miRNA clusters |
| Thege et al.68 | dSpCas9-VP64, MCP-p65-HSF1 (SAM) | in vivo mouse model | activation enables rapid evaluation and functional validation of putative oncogenes in vivo |
| Giehrl-Schwab et al.72 | dSpCas9-VPR, MCP-p65-HSF1 (SAM) | mouse primary cortical astrocytes | rescue of Parkinson’s disease motor symptoms by astrocyte reprogramming |
| Deng et al.70 | dSpCas9-VP64, MCP-p65-HSF1 (SAM) | dSpCas9-VP64 (SAM) transgenic mice | mouse model for aggressive lymphoma and interrogation of venetoclax resistance |
| Schoger et al.57 | dSpCas9-VPR | transgene mouse model | enhancing cardiomyocyte transcription in vivo |
| Ding et al.43 | dSpCas9-VP64, dSpCas9-KRAB-MeCP2 | KAI3, NUNK1, SNT16 cells | insight into the regulation of Epstein-Barr virus latency gene expression |
| Jaudon et al.51 | dSpCas9-VP64 | Itgb3 KO mice/HET mice | activation of autism gene Itgb3 restores cortical network excitability via mGluR5 signaling |
| Susco et al.56 | dSpCas9-VPR | hPSCs | uncovering specific areas of molecular convergence between Down syndrome and Fragile X syndrome |
| Zhou et al.65 | dSpCas9-10xGCN4 (SunTag) + scFv-GCN4-sfGFP-p65-HSF1 | mouse astrocytes and neurons in vivo | activation of multiple genes and long noncoding RNAs in mice to convert astrocytes into neurons for studying complex gene networks in the brain |
| Wangensteen et al.124 | dSpCas9-10xGCN4 (SunTag) + scFv-GCN4-sfGFP-VP64 | mouse hepatocytes in vivo | activation of genes that influence liver repopulation and carcinogenesis |
Beyersdorf et al. used in vivo delivery of RNA-based CRISPRa components (VPH-dCas9-SS18) using lipid nanoparticles (LNPs) to target the liver of mice.73 They initially showed potent gene activation of B4galnt2 with more than 90% of hepatocytes showing gene activation, and they also observed gene activation in muscle tissues and draining lymph nodes. Gene activation was evident 24 h after delivery and declined to base line at 9–12 days post-injection, whereafter redosing demonstrated efficient gene reactivation. As an interesting safety switch, the authors showed rapid termination of CRISPRa activity 24 h following delivery of mRNA encoding the “anti-CRISPR” protein AcrIIA4, which blocks the sgRNA binding site in the Cas9 protein. Finally, the authors showed potent upregulation of erythropoietin (Epo), which regulates erythropoiesis and would be therapeutically relevant in several clinical scenarios where a burst in red blood cell production is warranted. While peak blood levels of Epo were 10% of that obtained in previous studies using direct Epo mRNA delivery, the duration of Epo activation was longer with CRISPRa and showed return to baseline at around 7 days post-delivery, while direct mRNA delivery peaked at 6 h post-delivery followed by a rapid decline. This demonstrates that CRISPRa has a broader kinetic profile than direct mRNA delivery and that therapeutic protein levels can be obtained with fewer doses and without sharp peaks in protein amounts.
Development and breeding of animal models such as mice for use in preclinical research can be a long and costly process,68,125 but with the use of CRISPRa and CRISPRi that process can be much less taxing when producing mice for gain- or loss-of-function in vivo studies. As an example, orthotopic xenografted human breast cancer tumors were studied in NSG female mice with dual CRISPRa (dCas9-VPR) and CRISPRi (dCas9-KRAB) in the transplanted cells to study X-linked tumor suppressor genes. Here, the rationale was that reactivation of the tumor suppressor gene FOXP3 from the inactivated X chromosome could inhibit tumor growth, which was shown with simultaneous upregulation of FOXP3 and silencing of XIST using CRISPRa and CRISPRi, respectively.32 The dual transcriptional regulation led to reactivation of the inactivated X chromosome, a finding that could be beneficial for further study of disorders and diseases associated with X-linked inactivation, and it might be therapeutically relevant for treatment of female breast cancers.
Finally, different transgenic mouse models have been reported and some are also commercially available such as a Rosa26 SunTag knockin carrying a Cre-inducible dCas9-SunTag with 24xGCN4 (Gt(ROSA)26Sortm1(CAG-dCas9-SunTag)Khk) and another with Cre-inducible dCas9-VPR (Gt(ROSA)26Sorem1(CAG-LSL-dCas9-VPR-IRES-EGFP-WPRE-pA)). The former was used to study the impact of Myc, Slc7a11, and Tp53 activation on liver repopulation following liver injury and the possible initiation of carcinogenesis. Liver-specific induction of the SunTag system was facilitated by injection of AAV8 vectors encoding Cre expressed from a hepatocyte-specific (TBG) promoter. Delivery of sgRNA and the scFv(GCN4)-VP64 was facilitated by hydrodynamic plasmid injection, which consisted of a transposon system allowing genomic integration of the cassettes and the possibility to screen several target genes by sequencing the sgRNA.124 A similar transgenic mouse carrying both the dCas9-SunTag system (10xGCN4) and the scFv(GCN4)-p65-HSF1 activation domain in one Cre-inducible cassette was used in conjunction with AAV delivery of sgRNAs and Cre to study the effect of activating eight genes and two long-noncoding RNA (lncRNA) simultaneously in the nervous system.65
CRISPRi in a pre-clinical setting
As mentioned, the use of CRISPRi improves specificity over RNAi. This was also evident in a study inhibiting miRNA transcription. CRISPRi using dCas9-KRAB led to more consistent silencing when silencing clusters of miRNAs compared with short hairpin RNAs and short interfering RNAs.33 The use of clustered silencing of multiple miRNAs could be envisaged for treating certain cancers for which miRNAs are upregulated and make treatment less effective.
With its higher specificity, CRISPRi could become more popular with time and be tested in many of the same therapeutic settings that RNAi have found relevance and even shown to be effective (Table 3). As an example, Gemberling et al. created a transgenic Cre-inducible dSpCas9-KRAB mouse to test the effect of targeted CRISPR-mediated repression of Pcsk9 in vivo.75 The loss of Pcsk9 protein is known to cause a decrease in low-density lipoprotein (LDL) cholesterol serum levels. They found that CRISPRi of Pcsk9 reduced the level of Pcsk9 and in turn decreased serum levels of LDL cholesterol. Similarly, dSpCas9 was used by Chung et al., however, without using any repressor domain, to improve obesity, inflammation, hepatic steatosis, and insulin resistance in mice by inhibition of Fabp4.34 The Fabp4 repression was made specific to adult adipocytes by delivering dSpCas9- and sgRNA-encoding plasmids using an oligopeptide complex termed ATS-9R, which contains a targeting peptide with specificity toward prohibitin present on the surface of adipocytes. Heman-Ackah et al. similarly utilized dSpCas9 without a repressor domain to perform multiplexed inhibition of SNCA, MAPT, HTT, and APP, four genes associated with neurodegenerative diseases.35 While this study was performed in a cancer cell line, further development in in vivo neuronal delivery techniques could bring CRISPRi forward as a possible novel treatment of these devastating diseases. CRISPRi has also found use in preclinical studies of retinal disorders. Moreno et al. used KRAB-dSpCas9 in an in vivo mouse model for retinitis pigmentosa (RP), which is genetic disorder that causes progressive degeneration of rod photoreceptors and consequent secondary death of cone photoreceptors.36 They delivered the CRISPRi components by subretinal injection employing dual AAV vectors with a split-intein approach to inhibit the expression of the Nrl gene. Nrl inhibition can reprogram rods into cone-like cells that are resistant to the RP mutations. The authors found that inhibition of Nrl reversed photoreceptor degeneration and led to improved visual function. Finally, CRISPRi has also been used in preclinical cancer studies. Yoshida et al. used dSpCas9-KRAB to repress expression of ΔNp63, which is a potent oncogenic variant of the tumor suppressor TAp63.37 The targeted repression of ΔNp63 using an AdV vector for delivery of sgRNAs and dSpCas9-KRAB decreased cell proliferation and colony formation of different squamous cell carcinomas both in vitro and in a xenograft model of human lung squamous cell carcinoma. However, for the in vivo experiment, cells were transduced with the AdV vector prior to inoculation of the mice with the cancer cells. Potent in vivo delivery strategies for targeting cancer cells are warranted, but, nevertheless, this study showcases an approach that might generalize to other known oncogenes and could lead to personalized therapies for cancer.
Perspectives and discussion
As highlighted in the articles described above, there are many possible applications of CRISPR-mediated transcriptional regulation. In the years to come, many more will likely follow with the development of smaller and more efficient Cas variants and effectors that would lead to an improvement of delivery methods and development of genetic transcriptional modulation both in vitro and in vivo. Other technologies such as epigenome editors and RNA-targeting Cas13 systems are also undergoing refinements, such as development of compact Cas enzymes and light-inducible activation and may compete with or complement existing CRISPRa and CRISPRi methods.126,127,128
One of the main uncertainties with CRISPR-based gene editing and one of the most discussed is the issue of off-target activity of the Cas RNP complex. CRISPRa and CRISPRi systems have largely stayed clear from these discussions for a few reasons. One, when performed with transiently active vectors, i.e., without genomic integration or stable episomal presence of Cas and gRNA cassettes, the transcriptional modulation is not permanent, and the effect will last only as long as the Cas enzyme and gRNAs are expressed, whether it be intentional or off-target transcriptional modulation. Two, off-target binding of the Cas RNP complex would have to occur at unique positions in relation to the promoter and TSS of a given gene to mediate off-target gene activation or silencing, which reduces the likelihood of off-target effects. While these are important points that mitigate the risks associated with off-target activity, it is also important to point out the prevalent use of CRISPRa/i systems for cell differentiation. Hence, even transient off-target modulation of endogenous genes related to cell-fate and differentiation could have more lasting or permanent impacts on cell states. While CRISPRa and CRISPRi studies rarely report any investigation into off-target activity, some studies have assessed the specificity of the systems.44,129,130 In general, these studies find both systems to be highly specific. However, a single study showed off-target activation of IL-6 with the use of dCas9-VPR when targeting the lncRNA RP11-326A19.4.131 The authors could not bioinformatically predict the off-target activation of IL-6, but the finding encourages future studies to use both bioinformatic analysis of sgRNA design and unbiased methods such as RNA sequencing to detect any off-target activity.
As mentioned previously, the sgRNA design for CRISPRa and CRISPRi is somewhat different from that of canonical CRISPR-Cas gene editing. The desired up- or downregulation can only be achieved when utilizing sgRNAs that target the area around the transcription start site or, for CRISPRa, at distant enhancer regions. While the optimal targeting windows for CRISPRa and CRISPRi used in the CRISPick online design tool were devised using the dSpCas9-SunTag VP16 and dSpCas9-KRAB systems,91 respectively, these ranges might not accurately reflect optimal windows for other dCas-effector variants. Other ranges have been reported,90,132 and some studies have also obtained efficient gene modulation using sgRNA targeting outside these ranges.26,38 Future screening efforts may improve sgRNA design tools and delineate the biological causes for why some genes are difficult or impossible to modulate using these tools.26
One potential challenge to overcome for clinical implementation of CRISPRa and CRISPRi for in vivo therapy is the immunogenicity of the Cas enzyme that may hinder repeated dosing of dCas9-effectors or stable expression from persistent vector types. Studies have found most people to have preexisting adaptive immunity to SpCas9 and SaCas9 since they are derived from bacteria from common infections.133,134 However, a large proportion of Cas9-reactive T cells have been shown to be regulatory T cells that may promote tolerance to Cas9. Many transcriptional effector domains are also derived from microorganisms and there may already be prevalent immunity toward these proteins or repeated dosing might cause this. Future studies should characterize this phenomenon further, and even though ongoing in vivo clinical trials using nuclease-active Cas9 and base editors are based on transient delivery, these studies might provide clues to the significance of immunogenicity. If necessary, strategies to circumvent this could be implemented, for example by elimination of immunodominant epitopes in dCas-effector proteins.135
For decades, in vivo genetic therapies have been hampered by the lack of efficient and tissue-specific delivery tools. However, recent efforts have seen a rise in the reports of efficacious gene therapies that rely on LNP- or AAV-based gene delivery, and some of these have even obtained marketing authorization. Gene modulation technologies can certainly piggyback on these technological advances, but also come with specific challenges such as the need for large genetic cargo capacity—challenges that are shared with newly developed gene editing tools such as prime editors and CRISPR-associated transposases. A growing interest from the biotech industry in treatment modalities relying on transcriptional or epigenetic modulation will likely spur means to overcome current challenges, and several companies have recently joined the scene with large investments from venture capitalists.136 The major attractive feature of CRISPRa and CRISPRi is the ability to modulate gene expression without causing permanent changes to the DNA. This is particularly compelling when multiplexing and orthogonalizing the technology, which is likely to be a major focus for the next wave of studies. Next directions of the technology will probably also be directed at showcasing and utilizing the better control of gene expression with fine-tunable modulators, which might be advantageous compared with other overexpression methods that may cause unphysiological changes to gene expression.
Conclusions
There is still much to be learned about basic features and advanced application of the CRISPRa and CRISPRi systems, especially for in vivo and ex vivo therapeutic use. While these technologies are still some years from being used therapeutically, they show vast applications for example in functional gene studies, understanding and manipulating cell-fate trajectories in regenerative medicine, and elucidating drug-gene interactions by high-throughput screening methods. This developing toolbox will improve and speed up the way new drug targets are discovered and facilitate development of new therapies—both genetic and conventional. There is no denying the potential beneficial uses of these systems as means to fine-tune gene expression in a DNA break-free manner, and further engineering of gene modulation systems combined with improved delivery methods can hopefully lead to more widespread use of these tools in a therapeutic setting.
Acknowledgments
Funding in the Bak Lab is supported by a grant from the Danish health authorities (SST) (4-1612-391/1), the EU Commission in the form of an ERC Starting Grant (project 101041231, Horizon Europe Pillar I) and a grant from the Horizon Research and Innovation Actions (project 101057438, Horizon Europe Pillar II), the Lundbeck Foundation (R238-2016-3349 and R303-2018-3571), the Independent Research Fund Denmark (0134-00113B, 0242-00009B, and 9144-00001B), the Novo Nordisk Foundation (NNF19OC0058238 and NNF21OC0071259), Innovation Fund Denmark (8056-00010B), the Carlsberg Foundation (CF20-0424 and CF17-0129), Agnes og Poul Friis Fond, Slagtermester Max Wørzner og Hustru Inger Wørzners Mindelegat, the A.P. Møller Foundation, the Riisfort Foundation, and a Genome Engineer Innovation Grant from Synthego. All figures were created with Biorender.com.
Author contributions
L.B. took lead in writing the manuscript with assistance from T.I.J. and R.O.B. T.I.J. and R.O.B. drafted the figures with input from L.B. All authors reviewed, edited, and approved the final manuscript.
Declaration of interests
R.O.B. holds equity in Graphite Bio and UNIKUM Tx. T.I.J. and R.O.B. are employees of UNIKUM Tx. None of the companies were involved in this study.
References
- 1.Laufer B.I., Singh S.M. Strategies for precision modulation of gene expression by epigenome editing: an overview. Epigenetics Chromatin. 2015;8:34. doi: 10.1186/s13072-015-0023-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qi L.S., Larson M.H., Gilbert L.A., Doudna J.A., Weissman J.S., Arkin A.P., Lim W.A. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell. 2013;152:1173–1183. doi: 10.1016/j.cell.2013.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gilbert L.A., Larson M.H., Morsut L., Liu Z., Brar G.A., Torres S.E., Stern-Ginossar N., Brandman O., Whitehead E.H., Doudna J.A., et al. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell. 2013;154:442–451. doi: 10.1016/j.cell.2013.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maeder M.L., Linder S.J., Cascio V.M., Fu Y., Ho Q.H., Joung J.K. CRISPR RNA-guided activation of endogenous human genes. Nat. Methods. 2013;10:977–979. doi: 10.1038/nmeth.2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nuñez J.K., Chen J., Pommier G.C., Cogan J.Z., Replogle J.M., Adriaens C., Ramadoss G.N., Shi Q., Hung K.L., Samelson A.J., et al. Genome-wide programmable transcriptional memory by CRISPR-based epigenome editing. Cell. 2021;184:2503–2519.e17. doi: 10.1016/j.cell.2021.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giménez C.A., Ielpi M., Mutto A., Grosembacher L., Argibay P., Pereyra-Bonnet F. CRISPR-on system for the activation of the endogenous human INS gene. Gene Ther. 2016;23:543–547. doi: 10.1038/gt.2016.28. [DOI] [PubMed] [Google Scholar]
- 7.Hilton I.B., D'Ippolito A.M., Vockley C.M., Thakore P.I., Crawford G.E., Reddy T.E., Gersbach C.A. Epigenome editing by a CRISPR-Cas9-based acetyltransferase activates genes from promoters and enhancers. Nat. Biotechnol. 2015;33:510–517. doi: 10.1038/nbt.3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu X., Tao Y., Gao X., Zhang L., Li X., Zou W., Ruan K., Wang F., Xu G.L., Hu R. A CRISPR-based approach for targeted DNA demethylation. Cell Discov. 2016;2:16009. doi: 10.1038/celldisc.2016.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu X.S., Wu H., Ji X., Stelzer Y., Wu X., Czauderna S., Shu J., Dadon D., Young R.A., Jaenisch R. Editing DNA methylation in the mammalian genome. Cell. 2016;167:233–247.e17. doi: 10.1016/j.cell.2016.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng A.W., Wang H., Yang H., Shi L., Katz Y., Theunissen T.W., Rangarajan S., Shivalila C.S., Dadon D.B., Jaenisch R. Multiplexed activation of endogenous genes by CRISPR-on, an RNA-guided transcriptional activator system. Cell Res. 2013;23:1163–1171. doi: 10.1038/cr.2013.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mojica F.J.M., Díez-Villaseñor C., García-Martínez J., Almendros C. Short motif sequences determine the targets of the prokaryotic CRISPR defence system. Microbiology. 2009;155:733–740. doi: 10.1099/mic.0.023960-0. [DOI] [PubMed] [Google Scholar]
- 12.Kim H.K., Song M., Lee J., Menon A.V., Jung S., Kang Y.-M., Choi J.W., Woo E., Koh H.C., Nam J.-W., Kim H. In vivo high-throughput profiling of CRISPR–Cpf1 activity. Nat. Methods. 2017;14:153–159. doi: 10.1038/nmeth.4104. [DOI] [PubMed] [Google Scholar]
- 13.Jinek M., Chylinski K., Fonfara I., Hauer M., Doudna J.A., Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337:816–821. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hou Z., Zhang Y., Propson N.E., Howden S.E., Chu L.F., Sontheimer E.J., Thomson J.A. Efficient genome engineering in human pluripotent stem cells using Cas9 from Neisseria meningitidis. Proc. Natl. Acad. Sci. USA. 2013;110:15644–15649. doi: 10.1073/pnas.1313587110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mali P., Aach J., Stranges P.B., Esvelt K.M., Moosburner M., Kosuri S., Yang L., Church G.M. CAS9 transcriptional activators for target specificity screening and paired nickases for cooperative genome engineering. Nat. Biotechnol. 2013;31:833–838. doi: 10.1038/nbt.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ran F.A., Cong L., Yan W.X., Scott D.A., Gootenberg J.S., Kriz A.J., Zetsche B., Shalem O., Wu X., Makarova K.S., et al. In vivo genome editing using Staphylococcus aureus Cas9. Nature. 2015;520:186–191. doi: 10.1038/nature14299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang X., Wang J., Cheng Q., Zheng X., Zhao G., Wang J. Multiplex gene regulation by CRISPR-ddCpf1. Cell Discov. 2017;3:17018. doi: 10.1038/celldisc.2017.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cong L., Ran F.A., Cox D., Lin S., Barretto R., Habib N., Hsu P.D., Wu X., Jiang W., Marraffini L.A., Zhang F. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Komor A.C., Kim Y.B., Packer M.S., Zuris J.A., Liu D.R. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature. 2016;533:420–424. doi: 10.1038/nature17946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anzalone A.V., Randolph P.B., Davis J.R., Sousa A.A., Koblan L.W., Levy J.M., Chen P.J., Wilson C., Newby G.A., Raguram A., Liu D.R. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature. 2019;576:149–157. doi: 10.1038/s41586-019-1711-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koonin E.V., Makarova K.S., Zhang F. Diversity, classification and evolution of CRISPR-Cas systems. Curr. Opin. Microbiol. 2017;37:67–78. doi: 10.1016/j.mib.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Makarova K.S., Wolf Y.I., Koonin E.V. Classification and nomenclature of CRISPR-Cas systems: where from here? CRISPR J. 2018;1:325–336. doi: 10.1089/crispr.2018.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nidhi S., Anand U., Oleksak P., Tripathi P., Lal J.A., Thomas G., Kuca K., Tripathi V. Novel CRISPR–Cas systems: an updated review of the current achievements, applications, and future research perspectives. Int. J. Mol. Sci. 2021;22:3327. doi: 10.3390/ijms22073327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zetsche B., Gootenberg J.S., Abudayyeh O.O., Slaymaker I.M., Makarova K.S., Essletzbichler P., Volz S.E., Joung J., van der Oost J., Regev A., et al. Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system. Cell. 2015;163:759–771. doi: 10.1016/j.cell.2015.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kleinstiver B.P., Sousa A.A., Walton R.T., Tak Y.E., Hsu J.Y., Clement K., Welch M.M., Horng J.E., Malagon-Lopez J., Scarfò I., et al. Engineered CRISPR–Cas12a variants with increased activities and improved targeting ranges for gene, epigenetic and base editing. Nat. Biotechnol. 2019;37:276–282. doi: 10.1038/s41587-018-0011-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jensen T.I., Mikkelsen N.S., Gao Z., Foßelteder J., Pabst G., Axelgaard E., Laustsen A., König S., Reinisch A., Bak R.O. Targeted regulation of transcription in primary cells using CRISPRa and CRISPRi. Genome Res. 2021;31:2120–2130. doi: 10.1101/gr.275607.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tian R., Abarientos A., Hong J., Hashemi S.H., Yan R., Dräger N., Leng K., Nalls M.A., Singleton A.B., Xu K., et al. Genome-wide CRISPRi/a screens in human neurons link lysosomal failure to ferroptosis. Nat. Neurosci. 2021;24:1020–1034. doi: 10.1038/s41593-021-00862-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.le Sage C., Lawo S., Panicker P., Scales T.M.E., Rahman S.A., Little A.S., McCarthy N.J., Moore J.D., Cross B.C.S. Dual direction CRISPR transcriptional regulation screening uncovers gene networks driving drug resistance. Sci. Rep. 2017;7:17693. doi: 10.1038/s41598-017-18172-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dräger N.M., Sattler S.M., Huang C.T.L., Teter O.M., Leng K., Hashemi S.H., Hong J., Aviles G., Clelland C.D., Zhan L., et al. A CRISPRi/a platform in human iPSC-derived microglia uncovers regulators of disease states. Nat. Neurosci. 2022;25:1149–1162. doi: 10.1038/s41593-022-01131-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jung P., Schmalbrock L., Wirth M. CRISPR activation/interference screen to identify genetic networks in HDAC-inhibitor-resistant cells. Methods Mol. Biol. 2023;2589:429–454. doi: 10.1007/978-1-0716-2788-4_28. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt R., Steinhart Z., Layeghi M., Freimer J.W., Bueno R., Nguyen V.Q., Blaeschke F., Ye C.J., Marson A. CRISPR activation and interference screens decode stimulation responses in primary human T cells. Science. 2022;375:eabj4008. doi: 10.1126/science.abj4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cui X., Zhang C., Xu Z., Wang S., Li X., Stringer-Reasor E., Bae S., Zeng L., Zhao D., Liu R., et al. Dual CRISPR interference and activation for targeted reactivation of X-linked endogenous FOXP3 in human breast cancer cells. Mol. Cancer. 2022;21:38. doi: 10.1186/s12943-021-01472-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drobna-Śledzińska M., Maćkowska-Maślak N., Jaksik R., Dąbek P., Witt M., Dawidowska M. CRISPRi for specific inhibition of miRNA clusters and miRNAs with high sequence homology. Sci. Rep. 2022;12:6297. doi: 10.1038/s41598-022-10336-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chung J.Y., Ain Q.U., Song Y., Yong S.B., Kim Y.H. Targeted delivery of CRISPR interference system against Fabp4 to white adipocytes ameliorates obesity, inflammation, hepatic steatosis, and insulin resistance. Genome Res. 2019;29:1442–1452. doi: 10.1101/gr.246900.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heman-Ackah S.M., Bassett A.R., Wood M.J.A. Precision modulation of neurodegenerative disease-related gene expression in human iPSC-derived neurons. Sci. Rep. 2016;6:28420. doi: 10.1038/srep28420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moreno A.M., Fu X., Zhu J., Katrekar D., Shih Y.R.V., Marlett J., Cabotaje J., Tat J., Naughton J., Lisowski L., et al. In situ gene therapy via AAV-CRISPR-cas9-mediated targeted gene regulation. Mol. Ther. 2018;26:1818–1827. doi: 10.1016/j.ymthe.2018.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoshida M., Yokota E., Sakuma T., Yamatsuji T., Takigawa N., Ushijima T., Yamamoto T., Fukazawa T., Naomoto Y. Development of an integrated CRISPRi targeting DeltaNp63 for treatment of squamous cell carcinoma. Oncotarget. 2018;9:29220–29232. doi: 10.18632/oncotarget.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Escobar M., Li J., Patel A., Liu S., Xu Q., Hilton I.B. Quantification of genome editing and transcriptional control capabilities reveals hierarchies among diverse CRISPR/Cas systems in human cells. ACS Synth. Biol. 2022;11:3239–3250. doi: 10.1021/acssynbio.2c00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leng K., Rose I.V.L., Kim H., Xia W., Romero-Fernandez W., Rooney B., Koontz M., Li E., Ao Y., Wang S., et al. CRISPRi screens in human iPSC-derived astrocytes elucidate regulators of distinct inflammatory reactive states. Nat. Neurosci. 2022;25:1528–1542. doi: 10.1038/s41593-022-01180-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yeo N.C., Chavez A., Lance-Byrne A., Chan Y., Menn D., Milanova D., Kuo C.C., Guo X., Sharma S., Tung A., et al. An enhanced CRISPR repressor for targeted mammalian gene regulation. Nat. Methods. 2018;15:611–616. doi: 10.1038/s41592-018-0048-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alerasool N., Segal D., Lee H., Taipale M. An efficient KRAB domain for CRISPRi applications in human cells. Nat. Methods. 2020;17:1093–1096. doi: 10.1038/s41592-020-0966-x. [DOI] [PubMed] [Google Scholar]
- 42.de Solis C.A., Ho A., Holehonnur R., Ploski J.E. The development of a viral mediated CRISPR/Cas9 system with doxycycline dependent gRNA expression for inducible in vitro and in vivo genome editing. Front. Mol. Neurosci. 2016;9:70. doi: 10.3389/fnmol.2016.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ding W., Wang C., Narita Y., Wang H., Leong M.M.L., Huang A., Liao Y., Liu X., Okuno Y., Kimura H., et al. The epstein-barr virus enhancer interaction landscapes in virus-associated cancer cell lines. J. Virol. 2022;96:e0073922. doi: 10.1128/jvi.00739-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matharu N., Rattanasopha S., Tamura S., Maliskova L., Wang Y., Bernard A., Hardin A., Eckalbar W.L., Vaisse C., Ahituv N. CRISPR-mediated activation of a promoter or enhancer rescues obesity caused by haploinsufficiency. Science. 2019;363:eaau0629. doi: 10.1126/science.aau0629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Black J.B., McCutcheon S.R., Dube S., Barrera A., Klann T.S., Rice G.A., Adkar S.S., Soderling S.H., Reddy T.E., Gersbach C.A. Master regulators and cofactors of human neuronal cell fate specification identified by CRISPR gene activation screens. Cell Rep. 2020;33:108460. doi: 10.1016/j.celrep.2020.108460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sokka J., Yoshihara M., Kvist J., Laiho L., Warren A., Stadelmann C., Jouhilahti E.M., Kilpinen H., Balboa D., Katayama S., et al. CRISPR activation enables high-fidelity reprogramming into human pluripotent stem cells. Stem Cell Rep. 2022;17:413–426. doi: 10.1016/j.stemcr.2021.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou M., Tao X., Sui M., Cui M., Liu D., Wang B., Wang T., Zheng Y., Luo J., Mu Y., et al. Reprogramming astrocytes to motor neurons by activation of endogenous Ngn2 and Isl1. Stem Cell Rep. 2021;16:1777–1791. doi: 10.1016/j.stemcr.2021.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Colasante G., Qiu Y., Massimino L., Di Berardino C., Cornford J.H., Snowball A., Weston M., Jones S.P., Giannelli S., Lieb A., et al. In vivo CRISPRa decreases seizures and rescues cognitive deficits in a rodent model of epilepsy. Brain. 2020;143:891–905. doi: 10.1093/brain/awaa045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu S., Liu Y., Zhou Z., Zhang Z., Xiao X., Liu Z., Chen A., Dong X., Tian F., Chen S., et al. Genome-wide CRISPR activation screen identifies candidate receptors for SARS-CoV-2 entry. Sci. China. Life Sci. 2022;65:701–717. doi: 10.1007/s11427-021-1990-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thompson E.L., Pickett-Leonard M., Riddle M.J., Chen W., Albert F.W., Tolar J. Genes and compounds that increase type VII collagen expression as potential treatments for dystrophic epidermolysis bullosa. Exp. Dermatol. 2022;31:1065–1075. doi: 10.1111/exd.14555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jaudon F., Thalhammer A., Zentilin L., Cingolani L.A. CRISPR-mediated activation of autism gene Itgb3 restores cortical network excitability via mGluR5 signaling. Mol. Ther. Nucleic Acids. 2022;29:462–480. doi: 10.1016/j.omtn.2022.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Strezoska Ž., Dickerson S.M., Maksimova E., Chou E., Gross M.M., Hemphill K., Hardcastle T., Perkett M., Stombaugh J., Miller G.W., et al. CRISPR-mediated transcriptional activation with synthetic guide RNA. J. Biotechnol. 2020;319:25–35. doi: 10.1016/j.jbiotec.2020.05.005. [DOI] [PubMed] [Google Scholar]
- 53.Urrutia-Cabrera D., Hsiang-Chi Liou R., Lin J., Shi Y., Liu K., Hung S.S.C., Hewitt A.W., Wang P.Y., Ching-Bong Wong R. Combinatorial approach of binary colloidal crystals and CRISPR activation to improve induced pluripotent stem cell differentiation into neurons. ACS Appl. Mater. Inter. 2022;14:8669–8679. doi: 10.1021/acsami.1c17975. [DOI] [PubMed] [Google Scholar]
- 54.Nguyen N.T.K., Chang Y.H., Truong V.A., Hsu M.N., Pham N.N., Chang C.W., Wu Y.H., Chang Y.H., Li H., Hu Y.C. CRISPR activation of long non-coding RNA DANCR promotes bone regeneration. Biomaterials. 2021;275:120965. doi: 10.1016/j.biomaterials.2021.120965. [DOI] [PubMed] [Google Scholar]
- 55.Unlu G., Prizer B., Erdal R., Yeh H.W., Bayraktar E.C., Birsoy K. Metabolic-scale gene activation screens identify SLCO2B1 as a heme transporter that enhances cellular iron availability. Mol. Cell. 2022;82:2832–2843.e7. doi: 10.1016/j.molcel.2022.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Susco S.G., Ghosh S., Mazzucato P., Angelini G., Beccard A., Barrera V., Berryer M.H., Messana A., Lam D., Hazelbaker D.Z., Barrett L.E. Molecular convergence between Down syndrome and fragile X syndrome identified using human pluripotent stem cell models. Cell Rep. 2022;40:111312. doi: 10.1016/j.celrep.2022.111312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schoger E., Zelarayán L.C. Enhancing cardiomyocyte transcription using in vivo CRISPR/Cas9 systems. Methods Mol. Biol. 2022;2573:53–61. doi: 10.1007/978-1-0716-2707-5_5. [DOI] [PubMed] [Google Scholar]
- 58.Di Maria V., Moindrot M., Ryde M., Bono A., Quintino L., Ledri M. Development and validation of CRISPR activator systems for overexpression of CB1 receptors in neurons. Front. Mol. Neurosci. 2020;13:168. doi: 10.3389/fnmol.2020.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tanenbaum M.E., Gilbert L.A., Qi L.S., Weissman J.S., Vale R.D. A protein-tagging system for signal amplification in gene expression and fluorescence imaging. Cell. 2014;159:635–646. doi: 10.1016/j.cell.2014.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu Y., Yu C., Daley T.P., Wang F., Cao W.S., Bhate S., Lin X., Still C., 2nd, Liu H., Zhao D., et al. CRISPR activation screens systematically identify factors that drive neuronal fate and reprogramming. Cell Stem Cell. 2018;23:758–771.e8. doi: 10.1016/j.stem.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boettcher M., Tian R., Blau J.A., Markegard E., Wagner R.T., Wu D., Mo X., Biton A., Zaitlen N., Fu H., et al. Dual gene activation and knockout screen reveals directional dependencies in genetic networks. Nat. Biotechnol. 2018;36:170–178. doi: 10.1038/nbt.4062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu P., Chen M., Liu Y., Qi L.S., Ding S. CRISPR-based chromatin remodeling of the endogenous Oct4 or Sox2 locus enables reprogramming to pluripotency. Cell Stem Cell. 2018;22:252–261.e4. doi: 10.1016/j.stem.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 63.Danziger O., Patel R.S., DeGrace E.J., Rosen M.R., Rosenberg B.R. Inducible CRISPR activation screen for interferon-stimulated genes identifies OAS1 as a SARS-CoV-2 restriction factor. Plos Pathog. 2022;18:e1010464. doi: 10.1371/journal.ppat.1010464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Siepe D.H., Henneberg L.T., Wilson S.C., Hess G.T., Bassik M.C., Zinn K., Garcia K.C. Identification of orphan ligand-receptor relationships using a cell-based CRISPRa enrichment screening platform. Elife. 2022;11:e81398. doi: 10.7554/eLife.81398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou H., Liu J., Zhou C., Gao N., Rao Z., Li H., Hu X., Li C., Yao X., Shen X., et al. In vivo simultaneous transcriptional activation of multiple genes in the brain using CRISPR-dCas9-activator transgenic mice. Nat. Neurosci. 2018;21:440–446. doi: 10.1038/s41593-017-0060-6. [DOI] [PubMed] [Google Scholar]
- 66.Li L., Yu S., Hu Q., Hai Y., Li Y. Genome-scale CRISPRa screening identifies MTX1 as a contributor for sorafenib resistance in hepatocellular carcinoma by augmenting autophagy. Int. J. Biol. Sci. 2021;17:3133–3144. doi: 10.7150/ijbs.62393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hsu M.N., Huang K.L., Yu F.J., Lai P.L., Truong A.V., Lin M.W., Nguyen N.T.K., Shen C.C., Hwang S.M., Chang Y.H., Hu Y.C. Coactivation of endogenous Wnt10b and Foxc2 by CRISPR activation enhances BMSC osteogenesis and promotes calvarial bone regeneration. Mol. Ther. 2020;28:441–451. doi: 10.1016/j.ymthe.2019.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thege F.I., Rupani D.N., Barathi B.B., Manning S.L., Maitra A., Rhim A.D., Wörmann S.M. A programmable in vivo CRISPR activation model elucidates the oncogenic and immunosuppressive functions of MYC in lung adenocarcinoma. Cancer Res. 2022;82:2761–2776. doi: 10.1158/0008-5472.Can-21-4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang J., Rajan S.S., Friedrich M.J., Lan G., Zou X., Ponstingl H., Garyfallos D.A., Liu P., Bradley A., Metzakopian E. Genome-scale CRISPRa screen identifies novel factors for cellular reprogramming. Stem Cell Rep. 2019;12:757–771. doi: 10.1016/j.stemcr.2019.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Deng Y., Diepstraten S.T., Potts M.A., Giner G., Trezise S., Ng A.P., Healey G., Kane S.R., Cooray A., Behrens K., et al. Generation of a CRISPR activation mouse that enables modelling of aggressive lymphoma and interrogation of venetoclax resistance. Nat. Commun. 2022;13:4739. doi: 10.1038/s41467-022-32485-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang G., Chow R.D., Bai Z., Zhu L., Errami Y., Dai X., Dong M.B., Ye L., Zhang X., Renauer P.A., et al. Multiplexed activation of endogenous genes by CRISPRa elicits potent antitumor immunity. Nat. Immunol. 2019;20:1494–1505. doi: 10.1038/s41590-019-0500-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Giehrl-Schwab J., Giesert F., Rauser B., Lao C.L., Hembach S., Lefort S., Ibarra I.L., Koupourtidou C., Luecken M.D., Truong D.J.J., et al. Parkinson's disease motor symptoms rescue by CRISPRa-reprogramming astrocytes into GABAergic neurons. EMBO Mol. Med. 2022;14:e14797. doi: 10.15252/emmm.202114797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beyersdorf J.P., Bawage S., Iglesias N., Peck H.E., Hobbs R.A., Wroe J.A., Zurla C., Gersbach C.A., Santangelo P.J. Robust, durable gene activation in vivo via mRNA-encoded activators. ACS Nano. 2022;16:5660–5671. doi: 10.1021/acsnano.1c10631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hu J.H., Miller S.M., Geurts M.H., Tang W., Chen L., Sun N., Zeina C.M., Gao X., Rees H.A., Lin Z., Liu D.R. Evolved Cas9 variants with broad PAM compatibility and high DNA specificity. Nature. 2018;556:57–63. doi: 10.1038/nature26155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gemberling M.P., Siklenka K., Rodriguez E., Tonn-Eisinger K.R., Barrera A., Liu F., Kantor A., Li L., Cigliola V., Hazlett M.F., et al. Transgenic mice for in vivo epigenome editing with CRISPR-based systems. Nat. Methods. 2021;18:965–974. doi: 10.1038/s41592-021-01207-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vora S., Cheng J., Xiao R., VanDusen N.J., Quintino L., Pu W.T., Vandenberghe L.H., Chavez A., Church G. Rational design of a compact CRISPR-Cas9 activator for AAV-mediated delivery. bioRxiv. 2018 doi: 10.1101/298620. Preprint at. [DOI] [Google Scholar]
- 77.Campa C.C., Weisbach N.R., Santinha A.J., Incarnato D., Platt R.J. Multiplexed genome engineering by Cas12a and CRISPR arrays encoded on single transcripts. Nat. Methods. 2019;16:887–893. doi: 10.1038/s41592-019-0508-6. [DOI] [PubMed] [Google Scholar]
- 78.Tóth E., Varga É., Kulcsár P.I., Kocsis-Jutka V., Krausz S.L., Nyeste A., Welker Z., Huszár K., Ligeti Z., Tálas A., Welker E. Improved LbCas12a variants with altered PAM specificities further broaden the genome targeting range of Cas12a nucleases. Nucleic Acids Res. 2020;48:3722–3733. doi: 10.1093/nar/gkaa110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pyzocha N.K., Chen S. Diverse class 2 CRISPR-Cas effector proteins for genome engineering applications. ACS Chem. Biol. 2018;13:347–356. doi: 10.1021/acschembio.7b00800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Karvelis T., Gasiunas G., Siksnys V. Methods for decoding Cas9 protospacer adjacent motif (PAM) sequences: a brief overview. Methods. 2017;121-122:3–8. doi: 10.1016/j.ymeth.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 81.Kleinstiver B.P., Prew M.S., Tsai S.Q., Nguyen N.T., Topkar V.V., Zheng Z., Joung J.K. Broadening the targeting range of Staphylococcus aureus CRISPR-Cas9 by modifying PAM recognition. Nat. Biotechnol. 2015;33:1293–1298. doi: 10.1038/nbt.3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen P., Zhou J., Wan Y., Liu H., Li Y., Liu Z., Wang H., Lei J., Zhao K., Zhang Y., et al. A Cas12a ortholog with stringent PAM recognition followed by low off-target editing rates for genome editing. Genome Biol. 2020;21:78. doi: 10.1186/s13059-020-01989-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kleinstiver B.P., Prew M.S., Tsai S.Q., Topkar V.V., Nguyen N.T., Zheng Z., Gonzales A.P.W., Li Z., Peterson R.T., Yeh J.R.J., et al. Engineered CRISPR-Cas9 nucleases with altered PAM specificities. Nature. 2015;523:481–485. doi: 10.1038/nature14592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hsu P.D., Scott D.A., Weinstein J.A., Ran F.A., Konermann S., Agarwala V., Li Y., Fine E.J., Wu X., Shalem O., et al. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat. Biotechnol. 2013;31:827–832. doi: 10.1038/nbt.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang Y., Ge X., Yang F., Zhang L., Zheng J., Tan X., Jin Z.B., Qu J., Gu F. Comparison of non-canonical PAMs for CRISPR/Cas9-mediated DNA cleavage in human cells. Sci. Rep. 2014;4:5405. doi: 10.1038/srep05405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tóth E., Czene B.C., Kulcsár P.I., Krausz S.L., Tálas A., Nyeste A., Varga É., Huszár K., Weinhardt N., Ligeti Z., et al. Mb- and FnCpf1 nucleases are active in mammalian cells: activities and PAM preferences of four wild-type Cpf1 nucleases and of their altered PAM specificity variants. Nucleic Acids Res. 2018;46:10272–10285. doi: 10.1093/nar/gky815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xu X., Chemparathy A., Zeng L., Kempton H.R., Shang S., Nakamura M., Qi L.S. Engineered miniature CRISPR-Cas system for mammalian genome regulation and editing. Mol. Cell. 2021;81:4333–4345.e4. doi: 10.1016/j.molcel.2021.08.008. [DOI] [PubMed] [Google Scholar]
- 88.Dahlman J.E., Abudayyeh O.O., Joung J., Gootenberg J.S., Zhang F., Konermann S. Orthogonal gene knockout and activation with a catalytically active Cas9 nuclease. Nat. Biotechnol. 2015;33:1159–1161. doi: 10.1038/nbt.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kiani S., Chavez A., Tuttle M., Hall R.N., Chari R., Ter-Ovanesyan D., Qian J., Pruitt B.W., Beal J., Vora S., et al. Cas9 gRNA engineering for genome editing, activation and repression. Nat. Methods. 2015;12:1051–1054. doi: 10.1038/nmeth.3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sanson K.R., Hanna R.E., Hegde M., Donovan K.F., Strand C., Sullender M.E., Vaimberg E.W., Goodale A., Root D.E., Piccioni F., Doench J.G. Optimized libraries for CRISPR-Cas9 genetic screens with multiple modalities. Nat. Commun. 2018;9:5416. doi: 10.1038/s41467-018-07901-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gilbert L.A., Horlbeck M.A., Adamson B., Villalta J.E., Chen Y., Whitehead E.H., Guimaraes C., Panning B., Ploegh H.L., Bassik M.C., et al. Genome-scale CRISPR-mediated control of gene repression and activation. Cell. 2014;159:647–661. doi: 10.1016/j.cell.2014.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Najm F.J., Strand C., Donovan K.F., Hegde M., Sanson K.R., Vaimberg E.W., Sullender M.E., Hartenian E., Kalani Z., Fusi N., et al. Orthologous CRISPR-Cas9 enzymes for combinatorial genetic screens. Nat. Biotechnol. 2018;36:179–189. doi: 10.1038/nbt.4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bak R.O., Dever D.P., Reinisch A., Cruz Hernandez D., Majeti R., Porteus M.H. Multiplexed genetic engineering of human hematopoietic stem and progenitor cells using CRISPR/Cas9 and AAV6. Elife. 2017;6:e27873. doi: 10.7554/eLife.27873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hendel A., Bak R.O., Clark J.T., Kennedy A.B., Ryan D.E., Roy S., Steinfeld I., Lunstad B.D., Kaiser R.J., Wilkens A.B., et al. Chemically modified guide RNAs enhance CRISPR-Cas genome editing in human primary cells. Nat. Biotechnol. 2015;33:985–989. doi: 10.1038/nbt.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kim S., Koo T., Jee H.G., Cho H.Y., Lee G., Lim D.G., Shin H.S., Kim J.S. CRISPR RNAs trigger innate immune responses in human cells. Genome Res. 2018;28:367–373. doi: 10.1101/gr.231936.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mu W., Tang N., Cheng C., Sun W., Wei X., Wang H. In vitro transcribed sgRNA causes cell death by inducing interferon release. Protein Cell. 2019;10:461–465. doi: 10.1007/s13238-018-0605-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wienert B., Shin J., Zelin E., Pestal K., Corn J.E. In vitro-transcribed guide RNAs trigger an innate immune response via the RIG-I pathway. PLoS Biol. 2018;16:e2005840. doi: 10.1371/journal.pbio.2005840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Moosmann P., Georgiev O., Thiesen H.J., Hagmann M., Schaffner W. Silencing of RNA polymerases II and III-dependent transcription by the KRAB protein domain of KOX1, a Krüppel-type zinc finger factor. Biol. Chem. 1997;378:669–677. doi: 10.1515/bchm.1997.378.7.669. [DOI] [PubMed] [Google Scholar]
- 99.Lorenz P., Koczan D., Thiesen H.J. Transcriptional repression mediated by the KRAB domain of the human C2H2 zinc finger protein Kox1/ZNF10 does not require histone deacetylation. Biol. Chem. 2001;382:637–644. doi: 10.1515/bc.2001.075. [DOI] [PubMed] [Google Scholar]
- 100.Zhang Z., Wu E., Qian Z., Wu W.S. A multicolor panel of TALE-KRAB based transcriptional repressor vectors enabling knockdown of multiple gene targets. Sci. Rep. 2014;4:7338. doi: 10.1038/srep07338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fink K.D., Deng P., Gutierrez J., Anderson J.S., Torrest A., Komarla A., Kalomoiris S., Cary W., Anderson J.D., Gruenloh W., et al. Allele-specific reduction of the mutant Huntingtin allele using transcription activator-like effectors in human Huntington's disease fibroblasts. Cell Transpl. 2016;25:677–686. doi: 10.3727/096368916x690863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Leben K., Strmšek Ž., Lebar T., Verbič A., Dragovan M., Omersa N., Anderluh G., Jerala R. Binding of the transcription activator-like effector augments transcriptional regulation by another transcription factor. Nucleic Acids Res. 2022;50:6562–6574. doi: 10.1093/nar/gkac454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Iyengar S., Farnham P.J. KAP1 protein: an enigmatic master regulator of the genome. J. Biol. Chem. 2011;286:26267–26276. doi: 10.1074/jbc.R111.252569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Perez-Pinera P., Kocak D.D., Vockley C.M., Adler A.F., Kabadi A.M., Polstein L.R., Thakore P.I., Glass K.A., Ousterout D.G., Leong K.W., et al. RNA-guided gene activation by CRISPR-Cas9-based transcription factors. Nat. Methods. 2013;10:973–976. doi: 10.1038/nmeth.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Beerli R.R., Segal D.J., Dreier B., Barbas C.F., 3rd Toward controlling gene expression at will: specific regulation of the erbB-2/HER-2 promoter by using polydactyl zinc finger proteins constructed from modular building blocks. Proc. Natl. Acad. Sci. USA. 1998;95:14628–14633. doi: 10.1073/pnas.95.25.14628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chavez A., Scheiman J., Vora S., Pruitt B.W., Tuttle M., P R Iyer E., Lin S., Kiani S., Guzman C.D., Wiegand D.J., et al. Highly efficient Cas9-mediated transcriptional programming. Nat. Methods. 2015;12:326–328. doi: 10.1038/nmeth.3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hong Y., Qi J., Gong D., Han C., Deng H. Replication and transcription activator (RTA) of Murine Gammaherpesvirus 68 binds to an RTA-responsive element and activates the expression of ORF18. J. Virol. 2011;85:11338–11350. doi: 10.1128/jvi.00561-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Konermann S., Brigham M.D., Trevino A.E., Joung J., Abudayyeh O.O., Barcena C., Hsu P.D., Habib N., Gootenberg J.S., Nishimasu H., et al. Genome-scale transcriptional activation by an engineered CRISPR-Cas9 complex. Nature. 2015;517:583–588. doi: 10.1038/nature14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Marinho H.S., Real C., Cyrne L., Soares H., Antunes F. Hydrogen peroxide sensing, signaling and regulation of transcription factors. Redox Biol. 2014;2:535–562. doi: 10.1016/j.redox.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vakulskas C.A., Dever D.P., Rettig G.R., Turk R., Jacobi A.M., Collingwood M.A., Bode N.M., McNeill M.S., Yan S., Camarena J., et al. A high-fidelity Cas9 mutant delivered as a ribonucleoprotein complex enables efficient gene editing in human hematopoietic stem and progenitor cells. Nat. Med. 2018;24:1216–1224. doi: 10.1038/s41591-018-0137-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dever D.P., Bak R.O., Reinisch A., Camarena J., Washington G., Nicolas C.E., Pavel-Dinu M., Saxena N., Wilkens A.B., Mantri S., et al. CRISPR/Cas9 β-globin gene targeting in human haematopoietic stem cells. Nature. 2016;539:384–389. doi: 10.1038/nature20134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhao Z., Anselmo A.C., Mitragotri S. Viral vector-based gene therapies in the clinic. Bioeng. Transl. Med. 2022;7:e10258. doi: 10.1002/btm2.10258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang Z., Troilo P.J., Wang X., Griffiths T.G., Pacchione S.J., Barnum A.B., Harper L.B., Pauley C.J., Niu Z., Denisova L., et al. Detection of integration of plasmid DNA into host genomic DNA following intramuscular injection and electroporation. Gene Ther. 2004;11:711–721. doi: 10.1038/sj.gt.3302213. [DOI] [PubMed] [Google Scholar]
- 114.Morais P., Adachi H., Yu Y.T. The critical contribution of pseudouridine to mRNA COVID-19 vaccines. Front. Cell Dev. Biol. 2021;9:789427. doi: 10.3389/fcell.2021.789427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fueller J., Herbst K., Meurer M., Gubicza K., Kurtulmus B., Knopf J.D., Kirrmaier D., Buchmuller B.C., Pereira G., Lemberg M.K., Knop M. CRISPR-Cas12a-assisted PCR tagging of mammalian genes. J. Cell Biol. 2020;219:e201910210. doi: 10.1083/jcb.201910210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Van Tendeloo V.F., Ponsaerts P., Lardon F., Nijs G., Lenjou M., Van Broeckhoven C., Van Bockstaele D.R., Berneman Z.N. Highly efficient gene delivery by mRNA electroporation in human hematopoietic cells: superiority to lipofection and passive pulsing of mRNA and to electroporation of plasmid cDNA for tumor antigen loading of dendritic cells. Blood. 2001;98:49–56. doi: 10.1182/blood.v98.1.49. [DOI] [PubMed] [Google Scholar]
- 117.Thakore P.I., Kwon J.B., Nelson C.E., Rouse D.C., Gemberling M.P., Oliver M.L., Gersbach C.A. RNA-guided transcriptional silencing in vivo with S. aureus CRISPR-Cas9 repressors. Nat. Commun. 2018;9:1674. doi: 10.1038/s41467-018-04048-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chew W.L., Tabebordbar M., Cheng J.K.W., Mali P., Wu E.Y., Ng A.H.M., Zhu K., Wagers A.J., Church G.M. A multifunctional AAV-CRISPR-Cas9 and its host response. Nat. Methods. 2016;13:868–874. doi: 10.1038/nmeth.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liao H.K., Hatanaka F., Araoka T., Reddy P., Wu M.Z., Sui Y., Yamauchi T., Sakurai M., O'Keefe D.D., Núñez-Delicado E., et al. In vivo target gene activation via CRISPR/Cas9-Mediated trans-epigenetic modulation. Cell. 2017;171:1495–1507.e15. doi: 10.1016/j.cell.2017.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Boettcher M., McManus M.T. Choosing the right tool for the job: RNAi, TALEN, or CRISPR. Mol. Cell. 2015;58:575–585. doi: 10.1016/j.molcel.2015.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shifrut E., Carnevale J., Tobin V., Roth T.L., Woo J.M., Bui C.T., Li P.J., Diolaiti M.E., Ashworth A., Marson A. Genome-wide CRISPR screens in primary human T cells reveal key regulators of immune function. Cell. 2018;175:1958–1971.e15. doi: 10.1016/j.cell.2018.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang B., Ren Z., Zheng H., Lin M., Chen G., Luo O.J., Zhu G. CRISPR activation screening in a mouse model for drivers of hepatocellular carcinoma growth and metastasis. iScience. 2023;26:106099. doi: 10.1016/j.isci.2023.106099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yoshida M., Yokota E., Sakuma T., Yamatsuji T., Takigawa N., Ushijima T., Yamamoto T., Fukazawa T., Naomoto Y. Development of an integrated CRISPRi targeting ΔNp63 for treatment of squamous cell carcinoma. Oncotarget. 2018;9:29220–29232. doi: 10.18632/oncotarget.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wangensteen K.J., Wang Y.J., Dou Z., Wang A.W., Mosleh-Shirazi E., Horlbeck M.A., Gilbert L.A., Weissman J.S., Berger S.L., Kaestner K.H. Combinatorial genetics in liver repopulation and carcinogenesis with a in vivo CRISPR activation platform. Hepatology. 2018;68:663–676. doi: 10.1002/hep.29626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sakashita A., Ariura M., Namekawa S.H. CRISPR-mediated activation of transposable elements in embryonic stem cells. Methods Mol. Biol. 2022;2509:171–194. doi: 10.1007/978-1-0716-2380-0_11. [DOI] [PubMed] [Google Scholar]
- 126.Abudayyeh O.O., Gootenberg J.S., Essletzbichler P., Han S., Joung J., Belanto J.J., Verdine V., Cox D.B.T., Kellner M.J., Regev A., et al. RNA targeting with CRISPR-Cas13. Nature. 2017;550:280–284. doi: 10.1038/nature24049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Konermann S., Lotfy P., Brideau N.J., Oki J., Shokhirev M.N., Hsu P.D. Transcriptome engineering with RNA-targeting type VI-D CRISPR effectors. Cell. 2018;173:665–676.e14. doi: 10.1016/j.cell.2018.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhao W., Wang Y., Liang F.S. Chemical and light inducible epigenome editing. Int. J. Mol. Sci. 2020;21:998. doi: 10.3390/ijms21030998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chavez A., Tuttle M., Pruitt B.W., Ewen-Campen B., Chari R., Ter-Ovanesyan D., Haque S.J., Cecchi R.J., Kowal E.J.K., Buchthal J., et al. Comparison of Cas9 activators in multiple species. Nat. Methods. 2016;13:563–567. doi: 10.1038/nmeth.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Savell K.E., Bach S.V., Zipperly M.E., Revanna J.S., Goska N.A., Tuscher J.J., Duke C.G., Sultan F.A., Burke J.N., Williams D., et al. A neuron-optimized CRISPR/dCas9 activation system for robust and specific gene regulation. eNeuro. 2019;6 doi: 10.1523/eneuro.0495-18.2019. ENEURO.0495-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Soubeyrand S., Lau P., Peters V., McPherson R. Off-target effects of CRISPRa on interleukin-6 expression. PLoS One. 2019;14:e0224113. doi: 10.1371/journal.pone.0224113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Horlbeck M.A., Gilbert L.A., Villalta J.E., Adamson B., Pak R.A., Chen Y., Fields A.P., Park C.Y., Corn J.E., Kampmann M., Weissman J.S. Compact and highly active next-generation libraries for CRISPR-mediated gene repression and activation. Elife. 2016;5:e19760. doi: 10.7554/eLife.19760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Charlesworth C.T., Deshpande P.S., Dever D.P., Camarena J., Lemgart V.T., Cromer M.K., Vakulskas C.A., Collingwood M.A., Zhang L., Bode N.M., et al. Identification of preexisting adaptive immunity to Cas9 proteins in humans. Nat. Med. 2019;25:249–254. doi: 10.1038/s41591-018-0326-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wagner D.L., Amini L., Wendering D.J., Burkhardt L.M., Akyüz L., Reinke P., Volk H.D., Schmueck-Henneresse M. High prevalence of Streptococcus pyogenes Cas9-reactive T cells within the adult human population. Nat. Med. 2019;25:242–248. doi: 10.1038/s41591-018-0204-6. [DOI] [PubMed] [Google Scholar]
- 135.Ferdosi S.R., Ewaisha R., Moghadam F., Krishna S., Park J.G., Ebrahimkhani M.R., Kiani S., Anderson K.S. Multifunctional CRISPR-Cas9 with engineered immunosilenced human T cell epitopes. Nat. Commun. 2019;10:1842. doi: 10.1038/s41467-019-09693-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fine-tuning epigenome editors. Nat. Biotechnol. 2022;40:281. doi: 10.1038/s41587-022-01270-w. [DOI] [PubMed] [Google Scholar]