Highlights
-
•
Exercise training attenuates angiotensin II-induced cardiac fibrosis.
-
•
POU2F1 is the key factor involved in the inhibitory effect of exercise training on cardiac fibrosis.
-
•
POU2F1 promotes cardiac fibroblast transdifferentiation and cardiac fibrosis.
-
•
Exercise training attenuates cardiac fibrosis by reducing POU2F1.
-
•
Exercise training reduces the POU2F1 level by activating AMPK to downregulate C/EBPβ, which is the transcriptional factor of POU2F1.
Keywords: AMPK, C/EBPβ, Cardiac fibrosis, Exercise, POU2F1
Abstract
Background
Exercise training protects against heart failure. However, the mechanism underlying the protective effect of exercise training on angiotensin II (Ang II)-induced cardiac fibrosis remains unclear.
Methods
An exercise model involving C57BL/6N mice and 6 weeks of treadmill training was used. Ang II (1.44 mg/kg/day) was administered to induce cardiac fibrosis. RNA sequencing and bioinformatic analysis were used to identify the key factors mediating the effects of exercise training on cardiac fibrosis. Primary adult mouse cardiac fibroblasts (CFs) were used in vitro. Adeno-associated virus serotype 9 was used to overexpress POU domain, class 2, transcription factor 1 (POU2F1) in vivo.
Results
Exercise training attenuated Ang II-induced cardiac fibrosis and reversed 39 gene expression changes. The transcription factor regulating the largest number of these genes was POU2F1. Compared to controls, POU2F1 was shown to be significantly upregulated by Ang II, which is itself reduced by exercise training. In vivo, POU2F1 overexpression nullified the benefits of exercise training on cardiac fibrosis. In CFs, POU2F1 promoted cardiac fibrosis. CCAAT enhancer-binding protein β (C/EBPβ) was predicted to be the transcription factor of POU2F1 and verified using a dual-luciferase reporter assay. In vivo, exercise training activated AMP-activated protein kinase (AMPK) and alleviated the increase in C/EBPβ induced by Ang II. In CFs, AMPK agonist inhibited the increase in C/EBPβ and POU2F1 induced by Ang II, whereas AMPK inhibitor reversed this effect.
Conclusion
Exercise training attenuates Ang II-induced cardiac fibrosis by reducing POU2F1. Exercise training inhibits POU2F1 by activating AMPK, which is followed by the downregulation of C/EBPβ, the transcription factor of POU2F1.
Graphical Abstract
1. Introduction
Exercise training imparts significant cardiovascular health benefits by improving aerobic fitness and quality of life and by decreasing disability, hospitalization, and mortality attributable to cardiovascular diseases.1 Increasing evidence has demonstrated that exercise training has cardiovascular benefits, attracts more attention from clinicians, and is recommended as a non-drug therapy.2 The American Heart Association includes physical activity as one of its “Life's Simple 7” metrics,3 and recommends that adults perform at least 150−300 min/week of moderate-intensity physical activity or 75−150 min/week of vigorous-intensity physical activity.4 However, the mechanism underlying the effects of exercise training on cardiovascular disease is not fully understood. Uncovering it could aid in the exploration of key targets and interventions to improve cardiovascular health. It could also lead to new treatment strategies for patients who are unable to perform regular exercise training.
Heart failure, as a clinical syndrome, is the end-stage manifestation of many cardiovascular diseases and is associated with remarkable mortality, morbidity, and healthcare expenditures.5 Cardiac fibrosis is the main pathological change associated with heart failure. It is mainly characterized by the unbalanced production and degradation of extracellular matrix (ECM) proteins,6 and it causes cardiac dysfunction, arrhythmogenesis, and adverse outcomes.7,8 Transdifferentiation of cardiac fibroblasts (CFs) to myofibroblasts is a critical pathological change associated with cardiac fibrosis. This change is characterized by the expression of alpha-smooth muscle actin (α-SMA) and an increase in the synthesis of ECM proteins such as fibronectin and Collagen I.9 Attenuating cardiac fibrosis is an important strategy for maintaining cardiac function and improving the prognosis of patients with heart failure. Clinical evidence supports the beneficial effects of exercise training, including improved ventricular compliance and alleviation of cardiac fibrosis.10
The mechanisms underlying the beneficial effects of exercise training have not yet been elucidated. To solve this problem, we established the mouse model of cardiac fibrosis induced by angiotensin II (Ang II) and intervened with long-term exercise training. Following exercise training, the differentially expressed gene (DEG) profiles were obtained by RNA sequencing the myocardium of the model mice. The prediction was that POU domain, class 2, transcription factor 1 (POU2F1) would be the transcription factor regulating the largest number of DEGs. It has been reported that POU2F1 participates in regulating genes associated with cell growth,11 cellular stress response,12 immune regulation,13 and metabolic regulation.14,15 Additionally, POU2F1 is upregulated in various tumor tissues and participates in the process of epithelial-mesenchymal transition.16 However, whether POU2F1 is involved in Ang II-induced cardiac fibrosis and how it is regulated by exercise training are unknown.
This study aimed to investigate whether POU2F1 mediates the beneficial effects of exercise training on attenuating Ang II-induced cardiac fibrosis, and to explore possible underlying molecular mechanisms.
2. Methods
2.1. Materials
Ang II was purchased from Sigma-Aldrich (A9525; St. Louis, MO, USA), as well as metformin (D-150959; Sigma-Aldrich). Compound C was purchased from Calbiochem (171260; EMD Biosciences Inc., San Diego, CA, USA).
2.2. Animals
The animal experiments were approved by the Committee on Animal Experimentation of the Peking University Health Science Center (approval ID: LA2020161). All procedures conformed to the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publication No. 85-23, revised 1996) and the Guidelines for Animal Experiments of the Peking University Health Science Center. Male C57BL/6N mice were purchased from Beijing Vital River Laboratory Animal Technology Corporation (Beijing, China). Six-week-old mice were used for the adeno-associated virus (AAV) injection experiment. Eight-week-old male C57BL/6 mice were used to dissociate primary adult mouse CFs. Ten-week-old mice were used for cardiac fibrosis models. Mice were fed a standard diet and exposed to a 12-h light–dark cycle in a controlled environment (20 ± 2 °C; 12-h light–dark cycles).
2.3. Mice exercise model and Ang II-induced cardiac fibrosis model
A total of 24 mice were randomly assigned to 4 experimental groups (6 mice per group): saline plus sedentary group (Saline + Sed); saline plus running group (Saline + Run); Ang II plus sedentary group (Ang II + Sed); and Ang II plus running group (Ang II + Run). To establish the cardiac fibrosis model, mice were infused for 6 weeks with either saline (IN9001; Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) or Ang II (Sigma-Aldrich) at a dose of 1.44 mg/kg/day by subcutaneous implantation of an osmotic pump (Model 1004 plus Model 1002; Alzet, Durect, Cupertino, CA, USA) in the dorsal region.17 The surgical procedures were performed under anesthesia with 1%−2% isoflurane (CN2L9100; Baxter Healthcare Corporation, Deerfield, IL, USA). For the exercise model, the exercise training protocol was formulated as described previously.18 Briefly, the maximal oxygen uptake (VO2max) of the mice was evaluated using a respiratory metabolic analysis system (Panlab, Barcelona, Spain) during a progressive exercise test on a treadmill with increments of 5 cm/s every 2 min until exhausted. The infusion of Ang II (Sigma-Aldrich) had no effect on the VO2max of mice (Supplementary Fig. 1). The experiment started with 5 days of adaptive exercise training on a motorized rodent treadmill (Model ZH-PT; Anhui Zhenghua Technology, Hefei, China) at 9−15 cm/s for 30 min/day. One day after the osmotic pump was implanted, trained animals were subjected to 6 weeks of treadmill exercise at a speed of 15 cm/s (at an intensity of 65%–75% VO2max) for 90 min/day, 6 days/week (Fig. 1A).
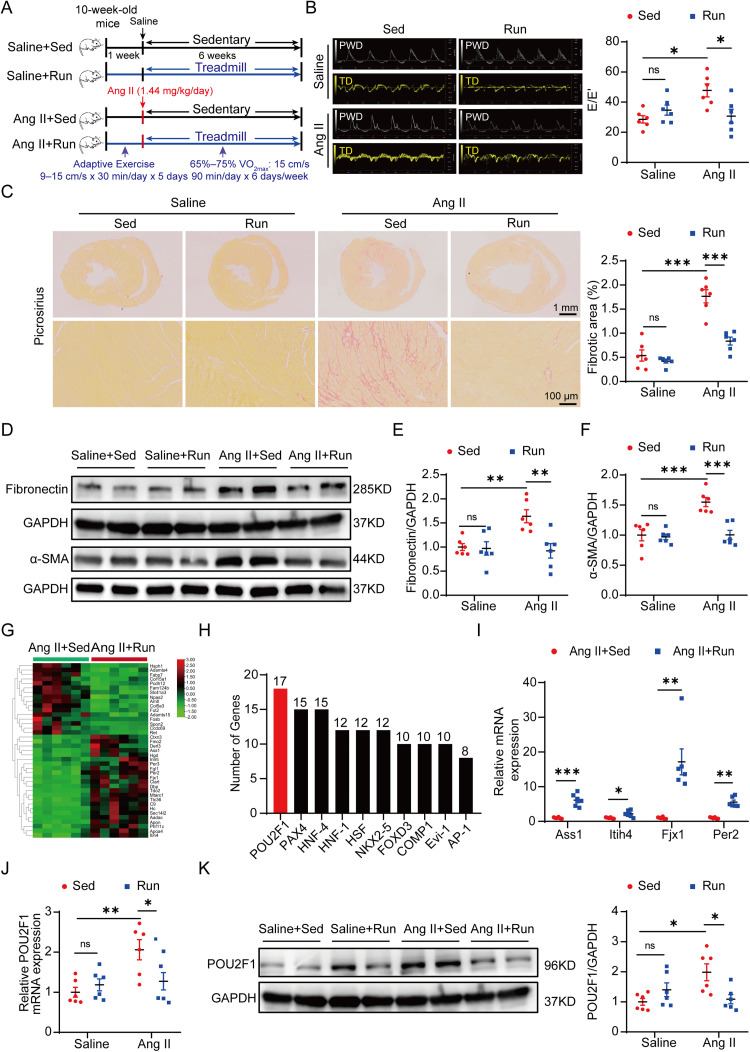
Fig. 1.
POU2F1 is involved in exercise training and the attenuation of Ang II-induced cardiac fibrosis. (A) a pattern diagram of the experimental protocol; (B) representative pulsed wave Doppler images across the mitral flow and tissue Doppler images of the mitral valve ring and measurements of E/E’; (C) picrosirius red staining of heart tissues and quantification of the fibrotic area (scale bars: top = 1 mm, bottom = 100 μm); (D) protein levels of fibronectin and α-SMA in heart tissues were detected using the Western blot; Quantitative analysis of the relative protein expressions of (E) fibronectin and (F) α-SMA in heart tissues; (G) heat map of DEGs; (H) the top 10 transcription factors predicted to bind to the greatest number of DEGs according to TRANSFAC database. The number indicates the amount of DEGs with predicted binding sites for the indicative transcription factor; (I) four genes regulated by POU2F1 and associated with fibrosis were validated by qPCR. (J) qPCR analysis of the POU2F1 mRNA levels in heart tissues; (K) the protein level of POU2F1 in heart tissues was detected using the Western blot. Data are presented as mean ± SEM. n = 6. A two-way analysis of variance followed by Tukey's post hoc test was used in (B), (C), (E), (F), (J), and (K). The t test or Mann–Whitney U test was used in (I). * p < 0.05; ** p < 0.01; *** p < 0.001. α-SMA = α-smooth muscle actin; Ang II = angiotensin II; AP-1 = activator protein 1; Ass1 = argininosuccinate synthase 1; COMP1 = protein kinase domain-containing protein; DEG = differentially expressed gene; Evi-1 = ecotropic viral integration site 1; Fjx1 = four-jointed box kinase 1; FOXD3 = forkhead box protein d3; GAPDH = glyceraldehyde-3-phosphate dehydrogenase; HNF-1 = hepatocyte nuclear factor 1; HNF-4 = hepatocyte nuclear factor 4; HSF = heat shock factor; Itih4 = inter-alpha-trypsin inhibitor heavy chain 4; NKX2-5 = NK2 transcription factor related, locus 5; ns = not significant; PAX4 = paired box homologous gene 4; Per2 = recombinant period circadian protein 2; POU2F1 = POU domain, class 2, transcription factor 1; PWD = pulsed-wave doppler; qPCR = quantitative polymerase chain reaction; Run = running; Sed = sedentary; SEM = standard error of mean; TD = tissue doppler.
2.4. AAV injection
To determine whether POU2F1 is involved in the effect exercise on cardiac fibrosis, cardiotropic AAV serotype 9 (AAV9) encoding either a recombinant green fluorescent protein (AAV9-GFP) or a mouse POU2F1 (AAV9-POU2F1) were packaged and synthesized (HanBio, Shanghai, China). Mice were injected with 1.4 × 1011 vector genomes per mL of AAV9-POU2F1 or AAV9-GFP via the tail vein at 4 weeks before Ang II infusion. Then the mice were randomly assigned to 4 experimental groups (total of 24 mice, 6 mice per group) and treated with Ang II before being subjected either to exercise training or to a sedentary lifestyle: Ang II plus AAV9-GFP injection plus sedentary group (Ang II + AAV9-GFP + Sed); Ang II plus AAV9-GFP injection plus running group (Ang II + AAV9-GFP + Run); Ang II plus AAV9-POU2F1 injection plus sedentary group (Ang II + AAV9-POU2F1 + Sed); and Ang II plus AAV9-POU2F1 injection plus running group (Ang II + AAV9-POU2F1 + Run).
2.5. Echocardiography
To evaluate cardiac function, echocardiography was performed before the mice were euthanized. Mice were anesthetized with 1%−2% isoflurane (Baxter Healthcare Corporation) via inhalation using a laboratory animal anesthesia machine (VetEquip, Vivermore, CA, USA). Echocardiographic images were acquired using a Vevo 2100 system (VisualSonics, Toronto, Ontario, Canada). In brief, the mice were anesthetized with isoflurane until the heart rate was stabilized at 350−400 beats per minute. An apical four-chamber view was acquired, and peak early diastolic transmitral flow velocity (E wave) and peak early diastolic mitral annulus velocity (E’ wave) were measured across the mitral valve. Then, the E/E’ was calculated to evaluate cardiac diastolic function.
2.6. Picrosirius red staining
The myocardial interstitial collagen was quantified using the picrosirius red staining technique. Fresh heart tissues were fixed with 4% paraformaldehyde (G1101; Servicebio, Wuhan, China) overnight and embedded in paraffin (YA0026; Beijing Solarbio Science & Technology Co., Ltd.). Subsequently, the paraffin was cut into 7–8 mm thick sections and stained with picrosirius red to evaluate the distributions and areas of collagen fibers in the heart tissue. Images were acquired using Nano Zoomer-SQ (Hamamatsu Photonics, Hamamatsu, Japan), and cardiac fibrosis quantification was performed using Image-Pro Plus 6.0 software (Media Cybernetics, Rockville, MD, USA).
2.7. RNA sequencing and bioinformatics
Total RNA was isolated from the heart tissues of the Ang II + Sed and Ang II + Run groups before RNA sequencing was performed by the Beijing Genomics Institute (Beijing, China) using the BGISEQ-500 to sequence the library products according to standard protocols; they also performed the standard bioinformatics analysis. The RNA sequencing datasets produced during this study are available in the National Center for Biotechnology Information Gene Expression Omnibus database under accession number PRJNA835821. DEGs were screened according to the following criteria: fold change >2 and adjusted Q < 0.05. The TRANSFAC database (http://gene-regulation.com/) was used to predict the potential transcription factor.
2.8. Isolation and culture of primary adult mouse CFs
Eight-week-old male C57BL/6 mice were used to dissociate primary adult mouse CFs. First, adult mouse hearts were harvested and cut into small pieces (1–2 mm3); then, they were digested repeatedly with 300 U/mL collagenase type II (17101015; Invitrogen, Carlsbad, CA, USA) at 37 °C. After centrifugation at 1000 r/min for 5 min, the supernatant was removed and the obtained cells were transferred to 10-cm dishes, where they were cultured in the Dulbecco's modified Eagle's medium (C11995500BT; Invitrogen) containing 10% fetal bovine serum (WS500T; Shanghai Viansaga biotech Ltd., Shanghai, China) at 37 °C in a humidified atmosphere containing 5% CO2. After 24 h, cells were washed with phosphate-buffered saline (P1020; Beijing Solarbio Science & Technology Co., Ltd.) to remove cell debris and non-adherent cells. Cells from the first passage were cultured in Dulbecco's modified Eagle's medium (Invitrogen) containing 10% fetal bovine serum (Shanghai Viansaga biotech Ltd.) for subsequent experiments.
2.9. Construction of the adenoviral vectors used in cell culture
Replication-deficient adenoviral vectors encoding a recombinant GFP, mouse POU2F1 (Ad-POU2F1), or mouse CCAAT enhancer-binding protein β (Ad-C/EBPβ) were packaged and synthesized (HanBio). A replication-deficient adenovirus vector carrying a short hairpin RNA against mouse POU2F1 (Ad-sh-POU2F1) was used to knock down POU2F1, whereas a replication-deficient adenovirus vector containing a scrambled sequence was used as a negative control (Ad-NC). The scrambled sequence was 5ʹ-TTCTCCGAACGTGTCACGTAA-3ʹ, and the shRNA sequence for POU2F1 was 5ʹ-GTACAGTCTAAATCCAGTGAA-3ʹ. CFs were infected with the adenovirus for 24−48 h, cultured in serum-free medium for 3−4 h, and treated with saline or Ang II (10−6 mol/L) for 48 h before being subjected to assays.
2.10. Immunofluorescence staining
The cells were fixed with 4% paraformaldehyde (Servicebio) for 10 min, permeabilized with 0.2% Triton X-100 (DH351-2; Beijing Dingguochangsheng Biotechnology, Co., Ltd., Beijing, China) for 20 min, and blocked with 1% bovine serum albumin (FA016; Beijing Dingguochangsheng Biotechnology, Co., Ltd.) for 30 min. Then, the fixed cells were incubated with diluted primary antibodies overnight at 4 °C. The primary antibodies used in this study are listed in Supplementary Table 1. Then the cells were incubated with the appropriate secondary antibody (1:1000 dilution; A21202; Invitrogen) for 1 h at 37 °C and protected from light. Hoechst 33342 (H1399; Invitrogen) was used to stain the cell nuclei for 10 min, and fluorescence intensity was detected and analyzed using the Cellomics ArrayScanVTI HCS Reader (ThermoFisher Scientific, Rockford, IL, USA). Target protein expression levels are expressed as the mean average fluorescence intensity.
2.11. Enzyme-linked immunosorbent assay (ELISA)
Levels of collagen I in the heart tissues and cell culture supernatants were measured using a commercial ELISA kit (SEA571Mu; Wuhan Cloud-Clone Corp., Wuhan, China). This procedure was performed according to the manufacturer's instructions. All values were within the linear range of the standard curve and calibrated using the total protein concentration.
2.12. Western blotting
Heart tissues and cells were extracted with RIPA buffer (R0010; Beijing Solarbio Science & Technology Co., Ltd.; 980 μL RIPA buffer, 10 μL phenylmethanesulfonyl fluoride, and 10 μL phosphatase inhibitors in a final volume of 1 mL). The lysates were centrifuged, and the resulting supernatant was collected to represent the total protein. Protein levels were quantified using a bicinchoninic acid protein kit (P1511-2; Applygen Technologies, Beijing, China). Cell lysates and heart tissues were subjected to 10% SDS gel electrophoresis (WB1102; Biotides, Beijing, China) and transferred to nitrocellulose membranes (P-N66485; Pall Corp., Port Washington, NY, USA). Non-fat milk (5%) (BE6250; EASYBIO, Beijing, China) or bovine serum albumin (Beijing Dingguochangsheng Biotechnology, Co., Ltd.) was used to block the nitrocellulose membranes for 1 h at room temperature. After blocking, membranes were incubated with primary antibodies at 4 °C overnight. Then, the nitrocellulose membranes (Pall Corp.) were incubated with horseradish peroxidase-conjugated secondary antibodies (Zhong Shan Golden Bridge Biological Technology, Beijing, China) for 1 h at room temperature. The immunoblotted proteins were visualized using an enhanced chemiluminescence Western blotting luminal reagent (WBKLS0500; Millipore, Billerica, MA, USA), and the images were acquired using GeneSys software (Syngene, Frederick, MD, USA). Protein expression was quantified by calculating the grayscale value of each band using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
2.13. Quantitative polymerase chain reaction (qPCR)
Total RNAs were extracted from heart tissues and cells using TRIZOL reagent (15596018; Invitrogen). The synthesis of cDNA was done using a reverse transcription reaction system (R323-01; Vazyme Biotech, Nanjing, China). qPCR was performed on the Quant Studio™ 3 System (ThermoFisher Scientific) using SYBR Green Mix (Q712-03; Vazyme Biotech). All procedures were performed in accordance with manufacturers’ instructions. The primer sequences used in this study are listed in Supplementary Table 2. The housekeeping gene glyceraldehyde-3-phosphate dehydrogenase was used to normalize the expression of the target genes.
2.14. Dual-luciferase reporter gene assay
Luciferase reporter plasmids were purchased from HanBio. The experiment used human embryonic kidney 293A (HEK 293A) cells. A total of 105 HEK 293A cells were inoculated into 24-well culture plates containing 10% fetal bovine serum (Shanghai Viansaga biotech Ltd.) at 37 °C and 5% CO2. When the cell density reached approximately 60%−70%, HEK 293A cells were transfected with C/EBPβ overexpression plasmid, control plasmid, wild-type plasmid (containing the POU2F1 promoter region with an intact −1457 to −1448 base pair (bp) fragment), or mutant luciferase reporter plasmid (in which the −1457 to −1448 bp fragment was deleted) using a hiPerFect transfection reagent (301705; QIAGEN, Dusseldorf, Germany). Renilla luciferase plasmid was used as a reference. After 24 h of transfection, cells were harvested to perform the assay using the dual-luciferase reporter assay kit (E1910; Promega, Madison, WI, USA). Results were presented as the luminescence ratio of firefly (Luc)/Renilla luciferase.
2.15. Chromatin immunoprecipitation assay
Chromatin immunoprecipitation assay (ChIP) kit (ab500; Abcam, Cambridge, UK) and high-Sensitivity ChIP Kit (ab185913; Abcam) were used to perform ChIP assays for cell samples and cardiac tissue, respectively. These assays followed the manufacturer's protocols.
About 50 mg of each cardiac tissue sample was taken and then cut into small pieces (1–2 mm3), while 3 × 106 CF cells were collected for each treatment. Next, proteins and their bound DNA were crosslinked using 1% formaldehyde (200-001-8; Xilong Chemical Co., Ltd, Guangzhou, China). The lysates were then collected and sonicated to shear DNA into fragments of 500–600 bp in length. The C/EBPβ antibody (ab32358; Abcam) and a non-immune IgG antibody (from the High-Sensitivity ChIP Kit; Abcam) were used for immunoprecipitation. The forward primer for the POU2F1 promoter region probe is 5ʹ-ATTTGACTGAATGTGGCATCCTTTTCC-3ʹ; the reverse primer is 5ʹ-GGATAGGGCCTCTCACATATTTCCC-3ʹ.
2.16. Statistical analysis
Statistical analyses were performed using Graph Prism 9.0 (GraphPad Software, La Jolla, CA, USA). All data are presented as mean ± standard error of mean. During a comparison of 2 groups, the Student unpaired t tests were used for normally distributed data; otherwise, the Mann–Whitney U test was selected to analyze differences among nonparametric data. For comparisons between multiple groups, an analysis of variance was performed, followed by Tukey's post hoc test. Statistical significance was set at p < 0.05.
3. Results
3.1. POU2F1 plays a role in the protective effect of exercise training on Ang II-induced cardiac fibrosis
To investigate the key mechanism underlying exercise training's ability to alleviate Ang II-induced cardiac fibrosis, 10-week-old male mice were continuously infused with a regimen of Ang II (1.44 mg/kg/day) accompanied by a 6-week moderate exercise training (intensity of 65%–75% VO2max) intervention. Our study showed that exercise training significantly attenuated Ang II-induced cardiac fibrosis. First, echocardiography indicated that Ang II-induced cardiac diastolic dysfunction, as reflected by increased E/E’, was markedly improved by exercise training (Fig. 1B). Moreover, exercise training significantly alleviated the Ang II-induced increase in the fibrotic area of heart tissues and expressions of collagen I, fibronectin, and α-SMA (Fig. 1C–1F and Supplementary Fig. 2). Additionally, Ang II-induced cardiac hypertrophy was markedly attenuated by exercise training (Supplementary Fig. 3A–3H). However, neither Ang II nor exercise training had a significant effect on the cardiac systolic function of mice as reflected by left ventricular ejection fraction and fractional shortening (Supplementary Fig. 3I and 3J).
RNA sequencing analysis demonstrated that exercise training reversed 39 DEGs in the myocardium of Ang II-treated mice, including 23 upregulated genes and 16 downregulated genes (Fig. 1G and Supplementary Table 3). Subsequently, the TRANSFAC database was used to predict the potential transcription factors of these DEGs. The top 10 transcription factors predicted to bind to the greatest number of DEGs are listed in Fig. 1H. Predicted to regulate the largest number of DEGs (17 DEGs). Among the 17 DEGs regulated by POU2F1, the 4 associated with fibrosis were validated using qPCR. Expression changes were consistent with the results of RNA sequencing (Fig. 1I). The expression of POU2F1 was also measured in the myocardium of Ang II-treated mice. Western blot analysis and qPCR showed that protein and mRNA levels in POU2F1 were both upregulated by Ang II, whereas exercise training downregulated their expression (Fig. 1J and 1K). Collectively, these results suggest that POU2F1 is involved in the attenuation by exercise training of Ang II-induced cardiac fibrosis.
Western blot analysis showed that POU2F1 was expressed in neonatal mouse cardiomyocytes, adult mouse cardiomyocytes, and CFs (Supplementary Fig. 4A and 4B). There was no significant change in the expression level of POU2F1 under Ang II (10−6 mol/L) treatment in neonatal mouse cardiomyocytes or adult mouse cardiomyocytes (Supplementary Fig. 4C–4F). Therefore, our study focused on the role of POU2F1 in CFs.
3.2. POU2F1 promotes CF transdifferentiation and ECM protein synthesis in vitro
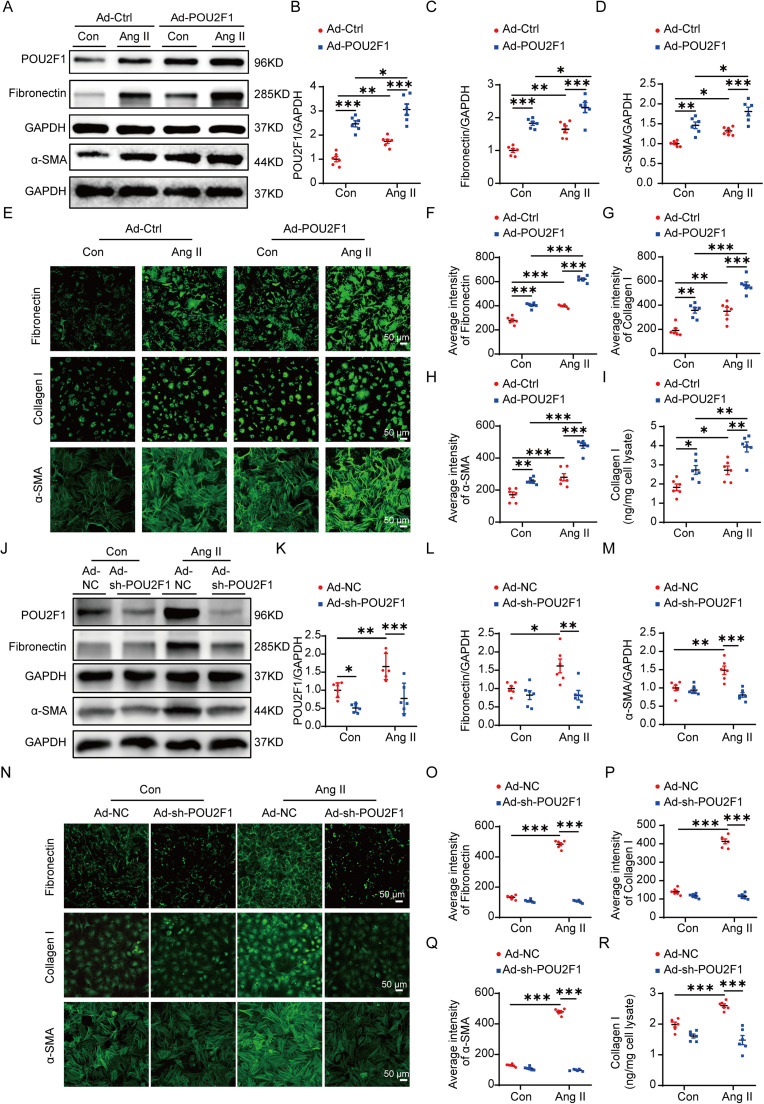
Pretreatment of CFs with Ad-POU2F1 significantly increased the protein expression of POU2F1 (Fig. 2A and 2B). Western blot analysis demonstrated that, compared to control cells, overexpression of POU2F1 increased the protein level of fibronectin and α-SMA in CFs with or without Ang II treatment (Fig. 2C and 2D). Additionally, immunofluorescence imaging showed that the average intensities of fibronectin, Collagen I, and α-SMA increased significantly in CFs overexpressing POU2F1 in both control and Ang II treatment conditions (Fig. 2E–2H). Enzyme-linked immunosorbent assay results showed that overexpression of POU2F1 promoted the secretion of collagen I in CFs with or without Ang II treatment (Fig. 2I). Pretreatment of CFs with Ad-sh-POU2F1 for 48 h significantly decreased the protein expression of POU2F1 (Fig. 2J and 2K). Knockdown of POU2F1 significantly reduced expressions of α-SMA, collagen I, and fibronectin in CFs treated by Ang II (Fig. 2L–2R). These results suggest that POU2F1 mediates the CF transdifferentiation and ECM protein synthesis induced by Ang II.
Fig. 2.
POU2F1 promoted CF differentiation and enhanced extracellular matrix protein synthesis. (A−I) CFs were infected with Ad-Ctrl or Ad-POU2F1 then treated with saline or Ang II for 48 h. (A) the expressions of POU2F1, fibronectin, and α-SMA were determined using the Western blot; (B−D) quantitative analysis of the relative protein expressions of (B) POU2F1, (C) fibronectin, and (D) α-SMA; (E) representative images and quantifications of the immunofluorescence analysis of (F) fibronectin, (G) Collagen I, and (H) α-SMA in CFs; (I) the secretion of Collagen I in the cell culture supernatant of CFs was determined by ELISA; (J−R) CFs were infected with Ad-NC or Ad-sh-POU2F1 to knock down POU2F1 then treated with saline or Ang II for 48 h; (J) Western blot analysis of POU2F1, fibronectin, and α-SMA in CFs. Quantitative analysis of relative protein expression of (K) POU2F1, (L) fibronectin, and (M) α-SMA in CFs; (N) Representative images and quantifications of the immunofluorescence analysis of (O) fibronectin, (P) Collagen I, and (Q) α-SMA in CFs; (R) the secretion of Collagen I in the cell culture supernatant of CFs was determined by an ELISA. Data are presented as mean ± SEM. n = 6. A two-way analysis of variance followed by Tukey's post hoc test was used. * p < 0.05; ** p < 0.01; *** p < 0.001. α-SMA = α-smooth muscle actin; Ad-Ctrl = control adenovirus; Ad-NC = negative control adenovirus; Ad-POU2F1 = POU2F1-overexpressed adenovirus; Ad-sh-POU2F1 = sh-POU2F1 adenovirus; Ang II = angiotensin II; CF = cardiac fibroblast; ELISA = enzyme-linked immunosorbent assay; GAPDH = glyceraldehyde-3-phosphate dehydrogenase; POU2F1 = POU domain, class 2, transcription factor 1; SEM = standard error of mean.
3.3. Protective effects of exercise training on Ang II-induced cardiac fibrosis are nullified by POU2F1 overexpression
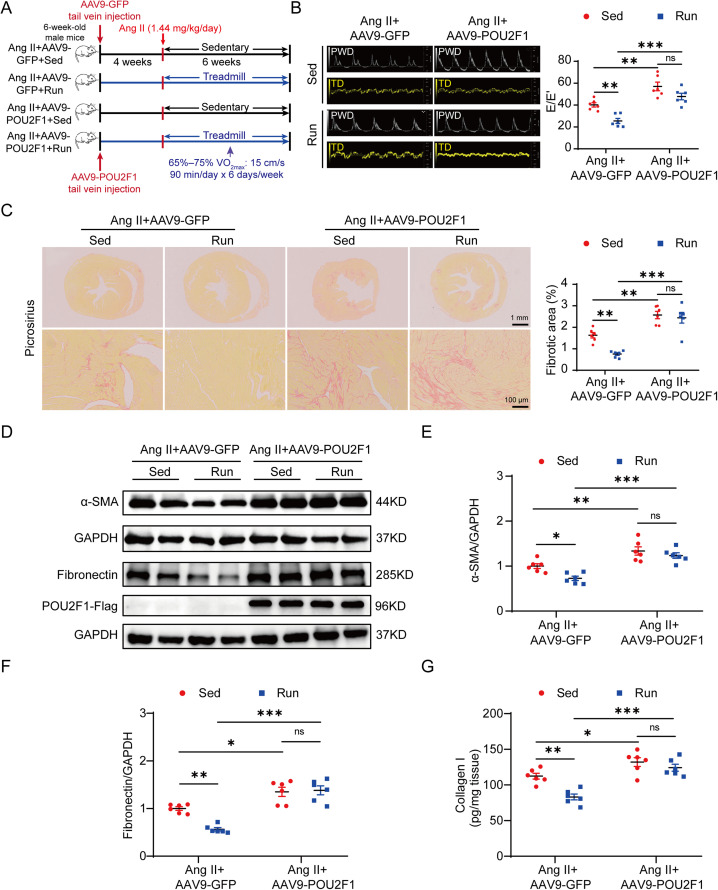
To clarify whether a decrease in POU2F1 contributes to the protective effect of exercise training on cardiac fibrosis induced by Ang II, an AAV9 vector carrying either the POU2F1 or GFP gene was injected into 2 groups (sedentary and exercise training) of 6-week-old male mice via the tail vein in vivo. After 4 weeks, when the POU2F1 gene was stably expressed in mouse hearts, the mice were treated with Ang II for 6 consecutive weeks (Fig. 3A). It was then confirmed that POU2F1 was overexpressed in AAV9-POU2F1 mice (Supplementary Fig. 5). Compared to the Ang II + AAV9-GFP + Sed group, cardiac diastolic dysfunction (reflected by E/E’) was markedly aggravated in Ang II + AAV9-POU2F1 + Sed mice. In fact, the beneficial effects of exercise training on cardiac diastolic dysfunction appear to have been nullified by AAV9-mediated POU2F1 overexpression (Fig. 3B). The fibrotic area of the heart tissues and the expressions of fibronectin, α-SMA, and Collagen I were also markedly higher in the Ang II + AAV9-POU2F1 + Sed group than those in the Ang II + AAV9-GFP + Sed group. Again, the alleviation of Ang II-induced cardiac fibrosis by exercise training appears to have been nullified by the overexpression of POU2F1, as there was no significant difference in the fibrotic area of the heart tissues or the expressions of fibronectin, α-SMA, and Collagen I between the Ang II + AAV9-POU2F1 + Sed and Ang II + AAV9-POU2F1 + Run groups (Fig. 3C–3G). Overall, these findings demonstrate that exercise training likely reduces Ang II-induced cardiac fibrosis by inhibiting POU2F1 expression. However, AAV9-POU2F1 had no significant effect on Ang II-induced cardiac hypertrophy or cardiac systolic function (Supplementary Fig. 6).
Fig. 3.
The protective effects of exercise training in Ang II-induced cardiac fibrosis are abolished by POU2F1 overexpression. (A) a pattern diagram of the experimental protocol; (B) representative pulsed wave Doppler images across the mitral flow and tissue Doppler images of the mitral valve ring and measurements of E/E’; (C) picrosirius red staining and quantification of the fibrotic area (scale bars: top = 1 mm, bottom = 100 μm); (D) the protein levels of α-SMA and fibronectin in heart tissues were detected using the Western blot; Quantitative analysis of relative protein expressions of (E) α-SMA and (F) fibronectin in heart tissues; (G) the protein levels of Collagen I in heart tissues were determined using an ELISA. Data are presented as mean ± SEM. n = 6. A two-way analysis of variance followed by Tukey's post hoc test was used. * p < 0.05; ** p < 0.01; *** p < 0.001. α-SMA = α-smooth muscle actin; AAV9 = adeno-associated virus serotype-9; Ang II = angiotensin II; ELISA = enzyme-linked immunosorbent assay; GAPDH = glyceraldehyde-3-phosphate dehydrogenase; GFP = green fluorescent protein; ns = not significant; POU2F1 = POU domain, class 2, transcription factor 1; PWD = pulsed-wave doppler; Run = running; Sed = sedentary; SEM = standard error of mean; TD = tissue doppler; VO2max = maximal oxygen uptake.
3.4. Exercise training reduces POU2F1 by activating AMPK and downregulating C/EBPβ
Next, we explored potential mechanisms through which exercise training regulates POU2F1. Through a TRANSFAC database analysis, we predicted C/EBPβ to be the upstream transcription factor of POU2F1 and located a putative binding site for C/EBPβ at the −1457 to −1448 bp upstream sequence of the mouse POU2F1 gene promoter (Fig. 4A). The results of luciferase reporter assays showed that in HEK 293A cells transfected with the wild-type POU2F1 promoter sequence plasmid, overexpression of C/EBPβ significantly increased luciferase activity. In contrast, overexpression of C/EBPβ did not affect the luciferase activity in cells transfected with the mutant POU2F1 promoter sequence plasmid. These results suggest that C/EBPβ is the transcriptional factor for POU2F1 (Fig. 4B). ChIP results showed that the enrichment of the POU2F1 promoter region to C/EBPβ was significantly increased following C/EBPβ overexpression in CFs (Fig. 4C). Meanwhile, ChIP assays showed that the binding of C/EBPβ to the POU2F1 promoter region was increased under Ang II treatment, whereas exercise could attenuate this regulation in vivo (Fig. 4D).
Fig. 4.
Exercise training reduced POU2F1 by activating AMPK and downregulating C/EBPβ. (A) schematic of the binding site between C/EBPβ and the mouse POU2F1 promoter region and the structures of the reporter plasmids carrying the full POU2F1 promoter region (POU2F1 wild type) or the −1457 to −1448 bp site deletion fragment (POU2F1 binding site deletion); (B) HEK 293A cells were transfected with the wild-type or mutant plasmid and then treated with control plasmid or C/EBPβ overexpression plasmid, respectively. A dual-luciferase reporter assay was performed (n = 3); (C) ChIP analysis using anti-C/EBPβ antibody or IgG, soluble chromatin (500−600 bp in length) from CFs overexpressing C/EBPβ, and primers targeting the region spanning the C/EBPβ binding sites in the POU2F1 promoter; (D) ChIP analysis using anti-C/EBPβ antibody or IgG, soluble chromatin (500−600 bp in length) from mouse heart tissues, and primers targeting the region spanning the C/EBPβ binding sites in the POU2F1 promoter; (E) the expression of C/EBPβ was determined by qPCR; (F) the expression of POU2F1 was determined by qPCR; (G) representative images and quantitative analysis of the Western blot for (H) C/EBPβ and POU2F1, as well as (I) fibronectin and α-SMA; (J) the secretion of collagen I in the cell culture supernatant of CFs was determined by ELISA; (K) representative images and quantitative analysis of the Western blot for (L) p-AMPK to total AMPK and (M) C/EBPβ to GAPDH in the Ang II-induced cardiac fibrosis model; (N−Q) CFs were pretreated with compound C (1 μmol/L) for 0.5 h and treated with metformin (1 mmol/L) for 0.5 h. Then, Ang II (10−6 mol/L) was added to CFs for 48 h; (N) representative images and quantitative analysis of the relative protein expressions of (O) p-AMPK to total AMPK, (P) C/EBPβ to GAPDH, and (Q) POU2F1 to GAPDH in harvested CFs. (R) ChIP analysis using anti-C/EBPβ antibody or IgG, soluble chromatin (500−600 bp in length) from CFs treated with AngII and/or metformin, and primers targeting the region spanning the C/EBPβ binding sites in the POU2F1 promoter. Data are presented as mean ± SEM. n = 6 in (C−R). The t test was used in (C), (E), (F), and (H−J). A two-way analysis of variance followed by Tukey's post hoc test was used in (B), (L), and (M). A one-way analysis of variance was used in (O−R). * p < 0.05; ** p < 0.01; *** p < 0.001. Ad-Ctrl = control adenovirus; AMPK = AMP-activated protein kinase; Ang II = angiotensin II; bp = base pair; C/EBPβ = CCAAT enhancer-binding protein β; CFs = cardiac fibroblasts; ChIP = chromatin immunoprecipitation; ELISA = enzyme-linked immunosorbent assay; GAPDH = glyceraldehyde-3-phosphate dehydrogenase; IgG = immunoglobulin G; ns = not significant; p-AMPK = phospho-AMP-activated protein kinase Thr 172; POU2F1 = POU domain, class 2, transcription factor 1; qPCR = quantitative polymerase chain reaction; Run = running; Sed = sedentary; SEM = standard error of mean.
Additionally, pretreatment of CFs with Ad-C/EBPβ for 24 h or 48 h significantly increased the mRNA and protein levels of C/EBPβ and POU2F1 (Fig. 4E–4H). Overexpression of C/EBPβ significantly increased the expressions of fibronectin and α-SMA and promoted the secretion of collagen I (Fig. 4G, 4I, and 4J).
Furthermore, we found that exercise training activated AMPK and downregulated C/EBPβ expression induced by Ang II in vivo (Fig. 4K–4M). In vitro, AMPK was activated to mimic the effects of exercise training on CFs. Western blot analysis demonstrated that the AMPK agonist metformin inhibited the increase of C/EBPβ and POU2F1 induced by Ang II in CFs, whereas the AMPK inhibitor compound C reversed this effect (Fig. 4N–4Q). In vitro, ChIP assays demonstrated that AMPK agonist metformin prevented the expected increase in the binding of C/EBPβ to the POU2F1 promoter region upon Ang II exposure (Fig. 4R). These data strongly suggest that exercise training reduces the expression of POU2F1 by activating AMPK and downregulating C/EBPβ.
4. Discussion
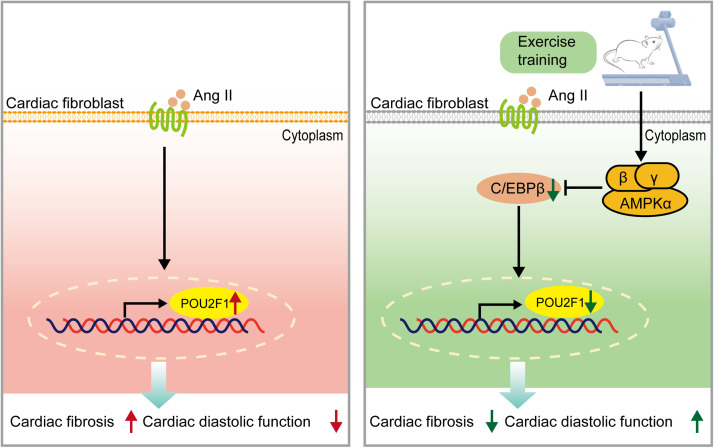
Our study showed that exercise training attenuated Ang II-induced cardiac fibrosis in mice by reducing the expression of POU2F1, which could promote CF transdifferentiation and cardiac fibrosis. Additionally, exercise training reduced the level of POU2F1 via activation of AMPK, which downregulated C/EBPβ, thought to be the transcription factor for POU2F1 (Fig. 5).
Fig. 5.
A working model of the role of POU2F1 in the protective effect of exercise training (attenuation of Ang II-induced cardiac fibrosis). AMPK = AMP-activated protein kinase; Ang II = angiotensin II; POU2F1 = POU domain, class 2, transcription factor 1; C/EBPβ = CCAAT enhancer-binding protein β.
Generally, moderate-intensity exercise training is defined as exercise during which 50%−70% VO2max is reached.19 Long-term moderate-intensity exercise training is the most common exercise mode used to explore the cellular and molecular mechanisms of exercise-induced cardiac protection,20 which is consistent with the exercise protocol of this study. Exercise training has been reported previously to have a role in cardiac protection in various animal models. In mice with myocardial infarction, for example, exercise training was found to alleviate cardiac fibrosis and improve cardiac function by increasing fibroblast growth factor 21 and inhibiting the transforming growth factor β1 (TGFβ1)-Smad2/3 signaling pathway.21 Our previous study found that exercise training could inhibit acute β-adrenergic overactivation-induced cardiac fibrosis by reducing the expression of inflammatory cytokines.18 Moderate-intensity exercise is generally safe and usually recommended for healthy adults of all ages. European Guidelines recommend that adults perform a minimum of 150 min of moderate-intensity exercise training over 5 days each week; additional benefits can be derived by doubling the amount (300 min) of moderate-intensity exercise per week.22 Although risk assessments should be performed in order to prescribe proper exercise programs or to provide advice for individuals with heart failure, moderate continuous exercise is the most commonly recommended exercise mode in uncomplicated cases.23
An important neurohormone in heart failure, Ang II causes cardiac fibrosis and dysfunction. In the present study, we found that exercise training can attenuate Ang II-induced cardiac fibrosis and improve cardiac diastolic dysfunction. We also found that POU2F1 is a potential mediator of the beneficial effects of exercise training on Ang II-induced cardiac fibrosis.
It has been reported previously that POU2F1 has an important role in tumors and inflammation. Previous studies demonstrate that POU2F1 promotes growth and metastasis of various tumors such as hepatocellular carcinoma and gastric cancer.24,25 In addition, POU2F1 could promote sepsis-induced inflammatory response by inhibiting macrophage apoptosis.26 However, the effect of POU2F1 on cardiac fibrosis has not been studied or identified. A widely expressed protein in the POU domain transcription factor family, POU2F1 is characterized by the presence of a substantial affinity for an 8-bp DNA site (octamer motif) and, therefore, is also known as octamer binding protein 1.27 It was first recognized for its role in target genes related to immune regulation (e.g., cytokines, including interleukin-2 (IL-2), IL-4, IL-8),28 but we know that some pro-inflammatory mediators are also regulated by POU2F1.29 Since myocardial inflammation and the recruitment of various immune cells contribute to the pathogenesis of cardiac fibrosis,30 it can be reasonably hypothesized that reducing POU2F1 through exercise may lead to a reduction in cardiac inflammation and, subsequently, an improvement in cardiac fibrosis. We previously showed that pathological stiffness can increase the expression of POU2F1, which inhibits the transcription of fibrosis repressor factors.31 In the present study, we found that POU2F1 mediated CF transdifferentiation and ECM protein synthesis induced by Ang II in vitro. Moreover, we saw that POU2F1 promoted cardiac fibrosis in vivo. It is also worth noting that overexpression of POU2F1 nullified the benefits exercise training might have otherwise had for cardiac fibrosis. Together, these results suggest that POU2F1 can be upregulated by both mechanical and hormonal factors, and that it is the key factor involved in the inhibitory effect exercise training has on cardiac fibrosis.
It has been reported that POU2F1 is itself regulated by multiple factors. High-throughput sequencing revealed that POU2F1 is a target gene of microRNA-205-5p (miR-205-5p) and miR-146a-5p.32,33 Another study revealed that the expressions of miR-205-5p and miR-146a-5p were increased by physical training.34 Wang et al.35 found that exercise training could decrease the level of long noncoding RNA taurine upregulated gene 1. Another study reported that taurine upregulated gene 1 upregulated the expression of POU2F1 by inhibiting the expression of miR-9-5p.36 These studies indirectly indicate that exercise might reduce the level of POU2F1 by targeting several noncoding RNAs. Our study provides direct evidence that exercise training can decrease the Ang II-induced expression of POU2F1 by inhibiting the transcription factor C/EBPβ.
Indeed, C/EBPβ plays a critical role in autoimmune myocarditis-induced CF differentiation37 and bleomycin-induced pulmonary fibrosis.38 Our study revealed that C/EBPβ can upregulate POU2F1 to mediate Ang II-induced cardiac fibrosis. This is a novel mechanism of C/EBPβ. Additionally, we found that exercise training inhibits the upregulated expression of C/EBPβ in a model of cardiac fibrosis induced by Ang II. Our results are consistent with those reported by Bostrom et al.,39 who, in a previous murine model of physiological hypertrophy, attributed the cardiomyocyte hypertrophy and proliferation induced by endurance exercise to a reduction in C/EBPβ. They found that C/EBPβ heterozygous mice displayed substantial resistance to pathological cardiac hypertrophy induced by pressure overload. Therefore, C/EBPβ appears to be another key factor mediating the inhibitory effect of exercise training on cardiac remodeling. Indeed, our results identified that the inhibition of C/EBPβ-POU2F1 axis contributes to the protective role exercise training plays in cardiac fibrosis. Finally, we demonstrated that exercise training reduced POU2F1 via AMPK activation and, subsequently, C/EBPβ downregulation.
AMPK, an endogenous protective factor for the heart, can be activated by several stimulations, such as exercise, hypoxia, and metformin.40 Many studies have attributed the benefits of exercise training to the activation of AMPK.41,42 In the present study, we found that exercise training could activate AMPK in vivo; we also found that the activation of AMPK could reverse the increase of C/EBPβ and POU2F1 induced by Ang II in vitro. We demonstrated in a previous study that exercise training alleviated cardiac fibrosis by reducing reactive oxygen species production in an AMPK-dependent manner.43 Our previous study also showed that AMPK can negatively regulate C/EBPβ, which is the transcription factor of TGFβ1, a major pro-fibrotic factor.44 In pancreatic β cells, AMPK activation led to the dephosphorylation of C/EBPβ T188, followed by the destabilization and reduction of C/EBPβ.45 We speculated that AMPK might modulate the phosphorylation status of C/EBPβ, ultimately decreasing its expression and activity in the present study. These aforementioned studies support our current results because they indicate that exercise training activates AMPK, which leads to a reduction in C/EBPβ expression and decreased cardiac fibrosis. According to the outcome of our present study, C/EBPβ not only suppresses the expression of TGFβ1, but it also reduces the level of POU2F1 to inhibit cardiac fibrosis.
In this study, we reported the opposing effects of exercise and Ang II on expressions of C/EBPβ–POU2F1 and cardiac fibrosis. Although exercise was shown to attenuate Ang II-induced cardiac hypertrophy, AAV9-POU2F1 had no significant effect on Ang II-induced cardiac hypertrophy. Moreover, there was no significant change in the expression level of POU2F1 under Ang II treatment in cardiomyocytes in vitro. That is, in Ang-II induced hypertrophic cardiomyocytes, Ang II had no effect on POU2F1 expression. Ang II increased the expression of POU2F1 only in CFs, promoting CF transdifferentiation and ECM protein synthesis and resulting in cardiac fibrosis. Therefore, we speculated that cardiac hypertrophic remodeling itself was not the main impetus for POU2F1 expression changes. Meanwhile, POU2F1 showed no impact on cardiac hypertrophy but was sufficient to induce cardiac fibrosis. Taken together, the change in C/EBPβ–POU2F1 does not appear to be an indirect consequence of changes in cardiac hypertrophic remodeling; it appears to be a cause but not a consequence of cardiac fibrosis.
A major limitation of this study is that only male mice were used in our model. However, a previous study found that exercise attenuated chronic Ang II-induced cardiac fibrosis in both sham-operated and ovariectomized female rats,46 indicating that exercise could alleviate Ang II-induced cardiac fibrosis in both males and females. Nevertheless, it remains unknown whether reducing POU2F1 would also be the key mechanism for alleviating Ang II-induced cardiac fibrosis through exercise training in female animals. This would need to be clarified by further study.
5. Conclusion
We found that exercise training inhibits Ang II-induced cardiac fibrosis by negatively regulating POU2F1. Exercise training reduces the expression of POU2F1 by activating AMPK and decreasing C/EBPβ, a transcription factor for POU2F1. Our findings indicate that POU2F1 is a novel mechanism through which exercise training attenuates cardiac fibrosis and suggest that it should be a therapeutic target for future treatment of cardiac fibrosis.
Acknowledgments
Acknowledgments
This work was supported by the National Natural Science Foundation of China (82030072 to HX, 81871850 to HY, 81972149 to WG, and 81830009 to YZ), Beijing Natural Science Foundation (No. 7212125 to HY), National Key R&D Program of China (Grant No. 2020YFA0803800 to HY), the Key Clinical Projects of Peking University Third Hospital (BYSYZD2019022 to HX), and Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Sciences (No. 2021-I2M-5-003 to HX and YZ).
Authors’ contributions
NF and HY contributed significantly to accomplishing the project through analysis and manuscript preparation; HX and WG contributed to designing the experiment, performing the analysis with constructive discussions, and revising the manuscript; YW and YZ participated in the design of the study. All authors have read and approved the final manuscript, and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
Supplementary materials associated with this article can be found in the online version at doi:10.1016/j.jshs.2022.10.004.
Contributor Information
Han Xiao, Email: xiaohan@bjmu.edu.cn.
Wei Gao, Email: weigao@bjmu.edu.cn.
Supplementary materials
References
- 1.Milutinović K, Stojiljković S, Ćuk J, et al. Athlete's heart. Fizicka kultura. 2018;72:139–147. [Google Scholar]
- 2.Villella M, Villella A. Exercise and cardiovascular diseases. Kidney Blood Press Res. 2014;39:147–153. doi: 10.1159/000355790. [DOI] [PubMed] [Google Scholar]
- 3.Lloyd-Jones DM, Hong Y, Labarthe D, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: The American Heart Association's strategic impact goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 4.Piercy KL, Troiano RP, Ballard RM, et al. The physical activity guidelines for Americans. JAMA. 2018;320:2020–2028. doi: 10.1001/jama.2018.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orso F, Fabbri G, Maggioni AP. Epidemiology of heart failure. Handb Exp Pharmacol. 2017;243:15–33. doi: 10.1007/164_2016_74. [DOI] [PubMed] [Google Scholar]
- 6.Kong P, Christia P, Frangogiannis NG. The pathogenesis of cardiac fibrosis. Cell Mol Life Sci. 2014;71:549–574. doi: 10.1007/s00018-013-1349-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frangogiannis NG. Cardiac fibrosis. Cardiovasc Res. 2021;117:1450–1488. doi: 10.1093/cvr/cvaa324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rubart M, Tao W, Lu XL, et al. Electrical coupling between ventricular myocytes and myofibroblasts in the infarcted mouse heart. Cardiovasc Res. 2018;114:389–400. doi: 10.1093/cvr/cvx163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Habiel DM, Hogaboam CM. Heterogeneity of fibroblasts and myofibroblasts in pulmonary fibrosis. Curr Pathobiol Rep. 2017;5:101–110. doi: 10.1007/s40139-017-0134-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grochulska A, Glowinski S, Bryndal A. Cardiac rehabilitation and physical performance in patients after myocardial infarction: Preliminary research. J Clin Med. 2021;10:2253. doi: 10.3390/jcm10112253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perri A, Catalano S, Bonofiglio D, et al. T3 enhances thyroid cancer cell proliferation through TRβ1/Oct-1-mediated cyclin D1 activation. Mol Cell Endocrinol. 2014;382:205–217. doi: 10.1016/j.mce.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Forbes SA, Beare D, Gunasekaran P, et al. Cosmic: Exploring the world's knowledge of somatic mutations in human cancer. Nucleic Acids Res. 2015;43:D805–D811. doi: 10.1093/nar/gku1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shakya A, Goren A, Shalek A, et al. Oct1 and OCA-B are selectively required for CD4 memory T cell function. J Exp Med. 2015;212:2115–2131. doi: 10.1084/jem.20150363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang HB, Fan J, Lin R, et al. Metabolic rewiring by oncogenic BRAF V600E links ketogenesis pathway to BRAF-MEK1 signaling. Mol Cell. 2015;59:345–358. doi: 10.1016/j.molcel.2015.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhar A, Haubrock M, Mukhopadhyay A, Maulik U, Bandyopadhyay S, Wingender E. Coexpression and coregulation analysis of time-series gene expression data in estrogen-induced breast cancer cell. Algorithms Mol Biol. 2013;8:9. doi: 10.1186/1748-7188-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhong Y, Huang H, Chen M, et al. POU2F1 over-expression correlates with poor prognoses and promotes cell growth and epithelial-to-mesenchymal transition in hepatocellular carcinoma. Oncotarget. 2017;8:44082–44095. doi: 10.18632/oncotarget.17296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daugherty A, Manning MW, Cassis LA. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J Clin Invest. 2000;105:1605–1612. doi: 10.1172/JCI7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alemasi A, Cao N, An X, et al. Exercise attenuates acute β-adrenergic overactivation-induced cardiac fibrosis by modulating cytokines. J Cardiovasc Transl Res. 2019;12:528–538. doi: 10.1007/s12265-019-09894-1. [DOI] [PubMed] [Google Scholar]
- 19.Kemi OJ, Loennechen JP, Wisloff U, Ellingsen O. Intensity-controlled treadmill running in mice: Cardiac and skeletal muscle hypertrophy. J Appl Physiol (1985) 2002;93:1301–1309. doi: 10.1152/japplphysiol.00231.2002. [DOI] [PubMed] [Google Scholar]
- 20.Rolim N, Skardal K, Hoydal M, et al. Aerobic interval training reduces inducible ventricular arrhythmias in diabetic mice after myocardial infarction. Basic Res Cardiol. 2015;110:44. doi: 10.1007/s00395-015-0502-9. [DOI] [PubMed] [Google Scholar]
- 21.Ma Y, Kuang Y, Bo W, et al. Exercise training alleviates cardiac fibrosis through increasing fibroblast growth factor 21 and regulating TGF-β1-Smad2/3-MMP2/9 signaling in mice with myocardial infarction. Int J Mol Sci. 2021;22:12341–12357. doi: 10.3390/ijms222212341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piepoli MF, Hoes AW, Agewall S, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR) Eur Heart J. 2016;37:2315–2381. doi: 10.1093/eurheartj/ehw106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gayda M, Ribeiro PA, Juneau M, Nigam A. Comparison of different forms of exercise training in patients with cardiac disease: Where does high-intensity interval training fit? Can J Cardiol. 2016;32:485–494. doi: 10.1016/j.cjca.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 24.Zhu HY, Cao GY, Wang SP, et al. POU2F1 promotes growth and metastasis of hepatocellular carcinoma through the fat1 signaling pathway. Am J Cancer Res. 2017;7:1665–1679. [PMC free article] [PubMed] [Google Scholar]
- 25.Wang J, Hou F, Tang L, et al. The interaction between long non-coding RNA LINC01564 and POU2F1 promotes the proliferation and metastasis of gastric cancer. J Transl Med. 2022;20:220. doi: 10.1186/s12967-022-03391-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang Y, Xue J, Qin L, Zhang J, Liu J, Yu J. LncRNA NEAT1 promotes inflammatory response in sepsis via the miR-31-5p/POU2F1 axis. Inflammation. 2021;44:1518–1528. doi: 10.1007/s10753-021-01436-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vazquez-Arreguin K, Tantin D. The Oct1 transcription factor and epithelial malignancies: Old protein learns new tricks. Biochim Biophys Acta. 2016;1859:792–804. doi: 10.1016/j.bbagrm.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pfeuffer I, Klein-Hessling S, Heinfling A, et al. Octamer factors exert a dual effect on the IL-2 and IL-4 promoters. J Immunol. 1994;153:5572–5585. [PubMed] [Google Scholar]
- 29.Ballard DW, Bothwell A. Mutational analysis of the immunoglobulin heavy chain promoter region. Proc Natl Acad Sci U S A. 1986;83:9626–9630. doi: 10.1073/pnas.83.24.9626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frangogiannis NG. Cardiac fibrosis: Cell biological mechanisms, molecular pathways and therapeutic opportunities. Mol Aspects Med. 2019;65:70–99. doi: 10.1016/j.mam.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 31.Li M, Wu J, Hu G, et al. Pathological matrix stiffness promotes cardiac fibroblast differentiation through the POU2F1 signaling pathway. Sci China Life Sci. 2021;64:242–254. doi: 10.1007/s11427-019-1747-y. [DOI] [PubMed] [Google Scholar]
- 32.Guo J, Li X, Miao L, et al. High-throughput sequencing reveals the differential microRNA expression profiles of human gastric cancer SGC7901 cell xenograft nude mouse models treated with traditional Chinese medicine Si Jun Zi Tang Decoction. Evid Based Complement Alternat Med. 2021;2021:6119212–6119222. doi: 10.1155/2021/6119212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gan K, Wu W, Li J, et al. Positive feedback loop of lncRNA FAM201A/miR‑146a‑5p/POU2F1 regulates IL-1β-induced chondrocyte injury in vitro. Mol Med Rep. 2022;25:12536–12546. doi: 10.3892/mmr.2021.12536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olson J, Sheean P, Matthews L, et al. Circulating miRNAs as early indicators of diet and physical activity response in women with metastatic breast cancer. Future Sci OA. 2021;7:FSO694. doi: 10.2144/fsoa-2020-0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang J, Niu Y, Tao H, Xue M, Wan C. Knockdown of lncRNA TUG1 inhibits hippocampal neuronal apoptosis and participates in aerobic exercise-alleviated vascular cognitive impairment. Biol Res. 2020;53:53. doi: 10.1186/s40659-020-00320-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xie CH, Cao YM, Huang Y, et al. Long non-coding RNA TUG1 contributes to tumorigenesis of human osteosarcoma by sponging miR-9-5p and regulating POU2F1 expression. Tumour Biol. 2016;37:15031–15041. doi: 10.1007/s13277-016-5391-5. [DOI] [PubMed] [Google Scholar]
- 37.Li X, Sun M, Men S, et al. The inflammatory transcription factor C/EBPβ plays a critical role in cardiac fibroblast differentiation and a rat model of cardiac fibrosis induced by autoimmune myocarditis. Int Heart J. 2018;59:1389–1397. doi: 10.1536/ihj.17-446. [DOI] [PubMed] [Google Scholar]
- 38.Hu B, Wu Z, Nakashima T, Phan SH. Mesenchymal-specific deletion of C/EBPβ suppresses pulmonary fibrosis. Am J Pathol. 2012;180:2257–2267. doi: 10.1016/j.ajpath.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bostrom P, Mann N, Wu J, et al. C/EBPβ controls exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell. 2010;143:1072–1083. doi: 10.1016/j.cell.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu S, Zou MH. AMPK, mitochondrial function, and cardiovascular disease. Int J Mol Sci. 2020;21:4987. doi: 10.3390/ijms21144987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kumar S, Behera S, Basu A, Dey S, Ghosh-Roy A. Swimming exercise promotes post-injury axon regeneration and functional restoration through AMPK. eNeuro. 2021;8:414–420. doi: 10.1523/ENEURO.0414-20.2021. ENEURO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ma Z, Qi J, Gao L, Zhang J. Role of exercise on alleviating pressure overload-induced left ventricular dysfunction and remodeling via AMPK-dependent autophagy activation. Int Heart J. 2020;61:1022–1033. doi: 10.1536/ihj.19-443. [DOI] [PubMed] [Google Scholar]
- 43.Ma X, Fu Y, Xiao H, et al. Cardiac fibrosis alleviated by exercise training is AMPK-dependent. PLoS One. 2015;10 doi: 10.1371/journal.pone.0129971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiao H, Piao CS, Chen RF, Zhang YY. AMP-activated kinase activation inhibits transforming growth factor-β1 production in cardiac fibroblasts via targeting C/EBPβ. Sheng Li Xue Bao. 2017;69:123–128. [in Chinese] [PubMed] [Google Scholar]
- 45.Matsuda T, Takahashi H, Mieda Y, et al. Regulation of pancreatic β cell mass by cross-interaction between CCAAT enhancer binding protein β induced by endoplasmic reticulum stress and AMP-activated protein kinase activity. PLoS One. 2015;10 doi: 10.1371/journal.pone.0130757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jitmana R, Raksapharm S, Kijtawornrat A, Saengsirisuwan V, Bupha-Intr T. Role of cardiac mast cells in exercise training-mediated cardiac remodeling in angiotensin II-infused ovariectomized rats. Life Sci. 2019;219:209–218. doi: 10.1016/j.lfs.2019.01.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.