Abstract
Objective and design
Preclinical studies suggest learned immune system responses to alcohol cues and consumption may contribute to alcohol's pharmacodynamic properties and/or Alcohol Use Disorder (AUD) pathogenesis. Mechanistically, these immune alterations may be associated with increased craving and alcohol consumption, both acutely and over time. We sought to characterize this relationship in a randomized, counter-balanced, crossover neuroimaging experiment which took place between June 2020–November 2021.
Methods
Thirty-three binge drinkers (BD) and 31 non-binge, social drinkers (SD), matched for demographic and psychological variables, were exposed to alcohol cues and water cues in two separate 7 T functional magnetic resonance imaging (fMRI) scans. Each scan was followed by the Alcohol Taste Test (ATT) of implicit motivation for acute alcohol. Craving measures and blood cytokine levels were collected repeatedly during and after scanning to examine the effects of alcohol cues and alcohol consumption on craving levels, Tumor necrosis factor alpha (TNF-α), and Interleukin 6 (IL-6) levels. A post-experiment one-month prospective measurement of participants’ “real world” drinking behavior was performed to approximate chronic effects.
Results
BD demonstrated significantly higher peak craving and IL-6 levels than SD in response to alcohol cues and relative to water cues. Ventromedial Prefrontal Cortex (VmPFC) signal change in the alcohol-water contrast positively related to alcohol cue condition craving and IL-6 levels, relative to water cue condition craving and IL-6 levels, in BD only. Additionally, peak craving and IL-6 levels were each independently related to ATT alcohol consumption and the number of drinks consumed in the next month for BD, again after controlling for craving and IL-6 repones to water cues. However, TNF-α release in the alcohol cue condition was not related to craving, neural activation, IL-6 levels, immediate and future alcohol consumption in either group after controlling for water cue condition responses.
Conclusions
In sum, BD show greater craving and IL-6 release in the alcohol cue condition than SD, both of which were associated with prefrontal cue reactivity, immediate alcohol consumption, and future alcohol consumption over the subsequent 30 days. Alcohol associated immune changes and craving effects on drinking behavior may be independent of one another or may be indicative of a common pathway by which immune changes in BD could influence motivation to consume alcohol.
Trial registration
Clinical Trials NCT04412824.
Keywords: Alcohol, Cues, fMRI, IL-6, TNF-α
Highlights
-
•
We used 7 T fMRI, repeated blood sampling, and ecological momentary assessment.
-
•
Craving and IL-6 were associated with prefrontal cue reactivity and future alcohol consumption.
-
•
Neural and immune changes in binge drinkers may influence motivation to consume alcohol.
-
•
TNF- α was not related to binge drinking behavior or alcohol cue reactivity.
1. Introduction
The immune consequences of severe Alcohol Use Disorders (AUDs) are well-known (Coleman and Crews, 2018), and preclinical findings have indicated that disrupted immune system responses to alcohol cues and alcohol consumption may contribute to the increased alcohol consumption seen in models of binge drinking (Vore et al., 2017). For example, although acute alcohol is generally immunosuppressive, as evidenced by its suppression of tumor necrosis factor alpha TNF-α (Nelson et al., 1989), adolescent and adult rats with adolescent binge drinking exposure display interleukin 6 (IL-6) release in the amygdala and paraventricular nucleus in response to alcohol intake (Doremus-Fitzwater et al., 2015).
When alcohol is consumed acutely, it passes from the stomach and intestines into the blood, entering the liver through the portal vein. In the liver, alcohol dehydrogenase (ADH), the key enzyme in alcohol metabolism, mediates the conversion of alcohol to acetaldehyde (Garcin et al., 1985). Acetaldehyde is a toxic intermediate which has a greater toxicity than ethanol and leads to liver injury. An alternate pathway independent of ADH, the microsomal ethanol oxidizing system (MEOS), is also responsible for alcohol metabolism. MEOS is a cytochrome P450 enzyme system, mainly expressed in the liver, although the MEOS is also located in other organs, including the brain (Lieber, 1994; Neve and Ingelman-Sundberg, 2000).
The MEOS is overexpressed in response to chronic and binge alcohol consumption (Lieber and DeCARLI, 1972). Importantly, increased expression and stimulation of MEOSs results in production of excess reactive oxygen species (ROS), lipid peroxidation, protein and DNA oxidation, and a proinflammatory state. Indeed, oxidant stress has been implicated as a pathogenic factor for the onset of alcohol liver disease (Kawaratani et al., 2013). Moreover, repeated intoxication sensitizes the immune system which can result in low-grade systemic inflammation (Carbia et al., 2021), including in the prefrontal cortex (Kraynak et al., 2018; Crews et al., 2011).
Two robust markers of low-grade systemic inflammation across different physical and psychiatric conditions are TNF-α and IL-6 (Jones et al., 2001; Zelová and Hošek, 2013; Maachi et al., 2004). TNF-α is mainly produced by Kupffer cells in the liver and is a critical pro-inflammatory cytokine which is a mediator in various physiological processes, such as inflammation, cell proliferation, and apoptosis. While acute consumption is associated with immediate reductions in TNF- α levels in both healthy controls and those with AUDs, preclinical models of heavy drinking and individuals with AUD show elevated basal levels of TNF- α (Laso et al., 2007; Khoruts et al., 1991; Portelli et al., 2019). This suggests that chronic alcohol may increase TNF- α production, which can lead to glutamatergic excitotoxicity and demyelination in neurons, thereby contributing to alcohol induced brain damage (Crews et al., 2011; Probert et al., 1997; Marshall et al., 2016). This upregulation of TNF- α may represent an allostatic response to the effects of alcohol consumption (Koob and Le Moal, 2001).
In contrast to TNF- α, both acute and chronic alcohol consumption increases IL-6 release in those with AUDs (Lee et al., 2021). IL-6 is an important influencer of neuroendocrine activity that easily passes the blood-brain barrier (Banks et al., 1994) to interact with central neurotransmitters and induce chronic inflammation (Erta et al., 2012; Reissner and Kalivas, 2010). Specifically, microglia in the brain express IL-6 more than other CNS cell types, and secrete IL-6 during peripheral immune activation, i.e., in response to alcohol consumption (Marshall et al., 2016; Zou and Crews, 2010). Human post mortem brain studies have suggested microglia activation may contribute to alcohol pathogenesis (He and Crews, 2008) and may influence alcohol's psychodynamic properties via effects on synaptic transmission (Grifasi et al., 2019). Moreover, IL-6 pathways are upregulated in alcohol preferring rodents (Blednov et al., 2012; Kimpel et al., 2007), while ablation of the IL-6 gene in mice decreases ethanol consumption (Blednov et al., 2005). Finally, circulating IL-6 levels at rest are higher in heavy drinkers than social drinkers (Zago et al., 2016) and Karoly et al. (2018), found that IL-6 levels correlated with self-reported alcohol use over several weeks. Thus, the upregulation of IL-6 may also represent an allostatic response to the effects of binge alcohol consumption (Koob and Le Moal, 2005).
Binge drinking represents an intermediate psychological and allostatic phenotype between social drinking and AUD and is a risk factor for developing an AUD. Physiologically, individuals who engage in binge drinking, demonstrate the dysregulated hypothalamic-pituitary-adrenal (HPA) axis responses to both alcohol consumption and alcohol cues seen in AUDs (for review, see Blaine and Sinha, 2017). Specifically, individuals who engage in binge drinking show elevated baseline cortisol levels and baseline craving, and a blunted cortisol response to both alcohol cues and acute alcohol consumption, which is accompanied by further increases in craving (Blaine et al., 2019). In AUDs, these altered HPA axis responses are associated with blunted ventromedial prefrontal cortex (VmPFC) activation to alcohol cues, which is predictive of time to relapse in treatment seeking individuals (Blaine et al., 2017, 2020).
While previous research has established differential responses in IL6 and TNF- α to alcohol cues and consumption in preclinical models and individuals with AUDs, these responses have not been observed in at-risk binge drinkers who are otherwise healthy. Further, the degree to which the relationship between immune responses and neural responses to alcohol cues, craving, or acute alcohol consumption differs between binge drinkers and healthy controls is unknown. Understanding this relationship may help elucidate mechanisms by which immune and neural changes associated with binge drinking leads to enhanced risk for AUD.
Therefore, the purpose of this prospective investigation was to investigate the immune and neural responses to alcohol cues, their relationship to craving, and their association with immediate and future alcohol consumption in social and binge drinkers, in the context of a within-subjects crossover study (NCT04412824). To this end we recruited beer drinking, non-smoking men and women ages 21–45 (N = 64, 34 males, 30 female), who were either social (SD) or binge drinking individuals (BD) without AUDs, for two multimodal neuroimaging sessions to compare the immediate effects of alcohol cues and consumption on immune and neural responses and their relationship with “real world” drinking behavior over a one month follow up. Water cues were used as an active control and session order was counterbalanced and randomized among participants.
We hypothesized that BD and SD would not have different baseline circulating levels of TNF-α or IL-6, as all participants were healthy young adults without AUD diagnoses. We hypothesized that BD would show greater cue induced craving, greater cue induced VmPFC activation, and greater immediate alcohol consumption, accompanied by lower TNF-α and greater IL-6 release after alcohol cue exposure and consumption when compared to SD, and relative to alcohol consumption after water cue exposure. We further hypothesized that in BD, IL-6, VmPFC and craving responses to alcohol cues and immediate alcohol consumption would be positively associated with drinking behavior in the real world, while TNF-α would be negatively related to drinking behavior in the real world.
2. Materials and methods
2.1. Participants
Participants were recruited from the greater Auburn-Opelika, Alabama area via advertisements on social media platforms. Screening of 1340 individuals occurred via Qualtrics survey to determine potential eligibility. At intake, beer drinking, non-smoking men and women ages 21–45 were categorized as non-binging Social Drinkers (SD; <7 standard drinks/week for women or 14 standard drinks/week for males, with no occasions of binge drinking) or as Binge Drinkers (BD; binges of ≥4 drinks in a 2 h time span and ≥8 standard drinks/week for women or ≥5 drinks for men in a 2-h time span and ≥15 standard drinks/week for men). There was a requirement of at least 3 binges per month in the last 3 months for BD, as indicated by the Timeline FollowBack (Sobell et al., 1992) and Cahalan Quantity and Frequency Variability Index (Cahalan et al., 1969) interviews. Current DSM-5 psychiatric disorders, as assessed by the Structured Clinical Interview for DSM-5 (SCID-5) (First et al., 2015), and any prescription medications were exclusionary (except hormonal birth control). To control for the possible influence of high estrogen and/or progesterone levels due to menstrual phase, female participants not using hormonal birth control (n = 10) were scanned on days 1–10 (follicular phase) of their menstrual cycles. Eighty-eight right-handed individuals with no magnetic resonance imaging (MRI) contraindications (metallic foreign objects in the body, etc.) enrolled in the study and completed intake and baseline assessments, 64 of whom (34 male, 30 female; 33 BD, 31 SD) completed both scans and at least 85% of follow-up surveys (See Supplemental Fig. 1 for CONSORT Diagram). This final sample size of N = 64 was determined a priori based on previous studies published by the first author correlating blood biomarkers with blood oxygen level dependent (BOLD) response to cues (Blaine et al., 2020). Groups were matched for sex, race, years of education, family history of AUD, years of regular drinking, and number of drinking days in the past month. Groups were not different on current subjective stress levels, depression and anxiety, impulsivity and number of childhood and lifetime traumatic events (Table 1). All group comparisons involved χ2 tests of frequency or independent t-tests based on mean, standard deviation, and number of participants per group. At baseline, the BD group was slightly younger and showed significantly higher number of drinking days in the past month, higher total amount of alcohol consumed in the past month, and higher usual and maximum number of drinks per drinking episode (Table 1).
Table 1.
Participant demographic, drinking, and psychological characteristics.
| DEMOGRAPHIC VARIABLES | SOCIAL DRINKERS (N = 31) | BINGE DRINKERS (N = 33) | Cohen's d/Chi square |
|---|---|---|---|
| SEX | |||
| FEMALE | 14 (45%) | 16 (48%) | |
| MALE | 17 (55%) | 17 (52%) | |
| RACE and ETHNICITY | |||
| BLACK/AFRICAN AMERICAN | 5 (16%) | 1 (3%) | |
| CAUCASIAN | 23 (74%) | 32 (97%) | |
| ASIAN AMERICAN | 3 (10%) | 0 (0%) | |
| HISPANIC | 6 (19%) | 6 (18%) | |
| YEARS OF EDUCATION | 17 (2.5) | 16 (1.6) | |
| AGE* | 28(7) | 24 (4.5) | 0.68 |
| DRINKING VARIABLES | |||
| NUMBER OF AUD FIRST DEGREE RELATIVES | 0.26 (0.6) | 0.24 (0.6) | |
| YEARS OF REGULAR DRINKING | 7.5 (6.9) | 5.2 (5) | |
| DRINKING DAYS IN PAST MONTH | 9.9 (7.4) | 14.6 (6.4) | |
| TOTAL AMOUNT CONSUMED IN PAST MONTH* | 23.5 (19.2) | 78.3 (63.7) | 1.16 |
| CAHALAN QFVI USUAL NUMBER OF DRINKS* | 2.5 (0.93) | 5.1 (2.8) | 1.25 |
| CAHALAN QFVI MAX NUMBER OF DRINKS* | 5 (1.8) | 9.2 (2.8) | 1.78 |
| LIFETIME MILD ALCOHOL USE DISORDER* | 7 (22.6%) | 18 (54.5%) | 6.86 |
| ALCOHOL USE DISORDERS IDENTIFICATION TEST (AUDIT)* | 4.6 (1.8) | 11(4) | 2.06 |
| PSYCHOLOGICAL VARIABLES | |||
| PERCEIVED STRESS SCALE (PSS) | 31.6 (4.4) | 31.4 (4.3) | |
| BECK DEPRESSION INVENTORY (BDI) | 5.6 (4.9) | 5.8 (5.8) | |
| BARRET IMPULSIVINESS SCALE (BIS) | 68.9 (8.4) | 71 (5.7) | |
| CHILDHOOD TRAUMA QUESTIONNAIRE (CTQ) | 63.4 (4.6) | 65.2 (4.6) | |
| STATE-TRAIT ANXIETY INDEX (STAI)- TRAIT | 30.6 (3.8) | 29.7 (2.8) | |
| STATE-TRAIT ANXIETY INDEX (STAI)- STATE | 60.4 (11) | 62 (5.4) | |
Note * denotes significantly different at p < 0.05. All group comparisons involved χ2 tests of frequency or independent t-tests based on mean, standard deviation, and number of participants per group.
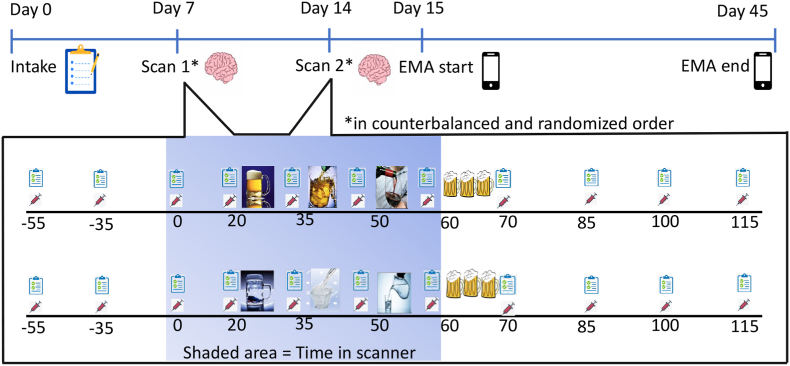
2.2. Within person and between participants experimental design (Fig. 1)
Fig. 1.
Between Subject and Repeated Measures Study Design. Two groups of participants, SD and BD, categorized on the basis of NIAAA criteria (NIAAA, 2012), were randomly assigned to view alcohol pictures during one fMRI scan and water pictures during another scan on a different day. An Alcohol Taste Test followed each scan (Blaine et al., 2019). Before, during, and after the scans and ATT, blood samples were taken from participants, in addition to measures of craving and alcohol effects at the specific timepoints shown. After completion of the two scans, participants answered questions on craving and drinking behavior via a smartphone app for 30 days.
In counter-balanced and randomized order, participants underwent the alcohol (ALC) and water (H2O) cue fMRI sessions. Randomization of condition order was performed by the first author using a random number sequence generator which is freely available on the internet. Prior to each experimental scan session, participants were required to be drug free, as tested by a urine drug screen for cannabis, benzodiazepines, opiates, and stimulants, and have a breath alcohol concentration of 0.000 g/L, as assessed via a Draeger Alcotest 6820 Breathalyzer test (Lubeck, Germany). At the start of the session, a registered nurse or nurse practitioner inserted an in-dwelling intravenous catheter into the participant's non-dominant arm to allow for repeated blood measurements. A baseline blood sample was drawn 55 min later to reduce the effects of needle insertion on baseline blood cortisol levels. Baseline alcohol craving was measured at this same time point using the Alcohol Urge Questionnaire (Bohn et al., 1995) and then again at each timepoint throughout the experiment. Participants were then placed in the 7 T S Magnetom MRI and underwent three, 10-min Blood Oxygen Level Dependent (BOLD) functional runs, with exposure to visual cues, accompanied by regular measurement of craving and blood draws for TNF-α and IL-6. To increase attention to the cues during each BOLD run, participants were instructed to click on a button with their dominant hand whenever they saw red wine (alcohol cue) or water bottles (water cue). Data on click number or accuracy were not collected. The number of alcohol and water cues was matched (see Supplemental Materials for Cue Image selection and valence/arousal evaluation). This crossover design allowed us to isolate the effect of alcohol cues and thus the ALC-H2O contrast was utilized to assess the alcohol cue effect in all analyses.
After each MRI scan, participants underwent a post-scan alcohol taste test (ATT) to isolate the effect alcohol versus water cues (ALC-H2O) on implicit motivation to consume alcohol. The 10-min ATT involved presenting the participant with 3 mugs of alcoholic beers (total of 1440 ml, equivalent to 4 cans of beer) and instructing them to taste the beers to assess if they are the “same or different” kind of beer. Participants were instructed to “drink as much as they need to” to make that determination and that they would be paid $10 if they were correct. Participants received beer with a 4.2% alcohol concentration and the 3 mugs of beers presented were always the same as each other. Therefore, a participant could take a small sip from each beer glass and be able to make their determination. Notably, participants often chose to consume more than a sip of each beer, and the amount consumed served as a behavioral index of alcohol motivation (Blaine et al., 2019; Jones et al., 2016). After the ATT, the Biphasic Alcohol Effects Questionnaire, Drug Effects Questionnaire and Alcohol Urge Questionnaire (Bohn et al., 1995; Martin et al., 1993; Morean et al., 2013) were administered, in addition to breath alcohol level measurements and blood draws for TNF-α and IL-6 levels, every 15 min for 45 min after alcohol consumption.
2.3. Neuroimaging procedures and analysis
Scanning occurred in a 7 T Siemens MAGNETOM MRI system equipped with a standard 32 channel head coil, using the T1 magnetization-prepared rapid gradient-echo (MPRAGE) sequence for structural scanning. High resolution structural images were acquired with the following parameters: TR = 2200ms, TE = 2.89ms, TI = 1050ms, bandwith = 240 Hz/pixel, flip angle = 7°, field of view = 190 × 190mm, matrix = 256 × 256, slice thickness = .7 mm, gap = .35 mm, 256 sagittal slices, 0.7 mm3 isotropic voxels.
Each scan consisted of 3 functional blocks with two types of visual stimuli (i.e., neutral pictures and pictures of water cues or pictures of alcohol cues). Each run lasted 10:24 and included a 90 s fixation cross, 3:18 of neutral stimuli presentation (33 images shown for 5 s each, in randomized order per block, with a 1 s fixation displayed between images), and 6:36 of alcohol or water cues (66 images shown for 5 s each in randomized order per block, with a 1 s fixation displayed between images). All visual stimuli were used only once. An echo planar (T2*) sequence was used to collect functional images. Two-hundred twenty-eight volumes (TR = 3000 ms, TE = 2.8 ms, bandwidth = 1124 Hz/pixel, flip angle = 70°, field of view = 200 × 200mm, matrix = 234 × 234, slice thickness = 1.5 mm, gap = .9 mm, 37 axial slices parallel to the anterior commissure-posterior commissure line, voxel size = 0.9 × 0.9 × 1.5 mm) were collected for each functional block (total time = 10 min, 24 s).
Neuroimaging data was preprocessed using FMRIPREP version 20.2.0 (Esteban et al., 2019) [RRID:SCR_016216], a Nipype (Gorgolewski et al., 2011) [RRID:SCR_002502] based tool. Each T1w (T1-weighted) volume was corrected for INU (intensity non-uniformity) using N4BiasFieldCorrection v2.1.0 (Tustison et al., 2010) and skull-stripped using antsBrainExtraction.sh v2.1.0 (using the OASIS template). Spatial normalization to the ICBM 152 Nonlinear Asymmetrical template version 2009c (Dale et al., 1999) [RRID:SCR_008796] was performed through nonlinear registration with the antsRegistration tool of ANTs v2.1.0 (Avants et al., 2008) [RRID:SCR_004757], using brain-extracted versions of both T1w volume and template. Brain tissue segmentation of cerebrospinal fluid (CSF), white-matter (WM) and gray-matter (GM) was performed on the brain-extracted T1w using fast (Zhang et al., 2001) (FSL v5.0.9, RRID:SCR_002823).
Functional data was slice time corrected using 3dTshift from AFNI v16.2.07 [11, RRID:SCR_005927] and motion corrected using mcflirt (FSL v5.0.9) (Jenkinson, 2003). This was followed by co-registration to the corresponding T1w using boundary-based registration (Greve and Fischl, 2009) with six degrees of freedom, using flirt (FSL). Motion correcting transformations, BOLD-to-T1w transformation and T1w-to-template (MNI) warp were concatenated and applied in a single step using antsApplyTransforms (ANTs v2.1.0) using Lanczos interpolation.
General linear modeling (GLM) was used for first-level analyses (e.g., individual-level) on each voxel in the entire brain volume using FSL FEAT (FMRI Expert Analysis Tool) Version 6.00, part of FSL (55) (FMRIB's Software Library, www.fmrib.ox.ac.uk/fsl). Preprocessed functional images were temporally filtered using a high pass filter of 120 s and images were spatially smoothed using a 6-mm Gaussian kernel. Individual runs of the task were analyzed using FMRIB's Improved Linear Model (FILM), with explanatory variables that included neutral and alcohol (water); contrast maps representing water – neutral and alcohol – neutral were derived from the beta maps generated in this analysis. Six movement regressors and their derivatives were included in first level models.
For higher-level (e.g., group level) data analysis, linear effects modeling using FSL FEAT was implemented with a 2 (average of runs for each session: Alcohol, Water) x 2 (group: BD, SD) design while covarying for age and sex. Session and run were treated as within-person fixed-effect factors, group as a between-person factor, and participant as a random factor. Results were cluster corrected at p < 0.05, with an initial whole brain analysis threshold of p < 0.001 for the ALC-H2O contrast. All associations presented are with each participants’ ALC-H2O average percent signal change for each significant cluster.
2.4. IL-6 and TNF-α measurement
Plasma levels of TNFα and IL-6 were determined by enzyme-linked immunosorbent assay (ELISA) (Quantikine, R&D Systems, Minneapolis, MN, USA). Briefly, blood samples were collected in 10.0-ml EDTA separator tubes (BD Vacutainer; Franklin NJ USA) from an indwelling venous catheter in the forearm, and timepoint collections were made in series according to the study design timeline presented in Fig. 1. Blood samples were centrifuged at 3000 rpm for 12 min at 4 °C and plasma aliquots were stored at −80 °C for biochemical analysis at the completion of the study. Plasma samples for each participant and timepoint were analyzed in duplicate in the same run. The intra- and inter-assay coefficients of variation (CVs), calculated from the original data, were 3.3% and 4.2% for IL-6 and 4.5% and 6.3% for TNFα, respectively. A 10% cutoff was used for all CVs.
2.5. Ecological momentary assessment
The MetricWire app (metricwire.com; Waterloo, Ontario, Canada), which can be used on Android or Apple devices, was used to collect a waking survey, 2 random prompt surveys, and an end of day survey from participants for a period of 30 days. Participants reported on the number of drinks since last survey during the waking survey, random prompts, and the end of day surveys. Waking survey number of drinks reported were assigned to the previous day and added to the values from the random prompts and end of day to survey to get a total number of drinks per day. Average response rate for the entire sample was 85% completion of surveys. In total, 6792 surveys were completed, of which 8% (538) represented unique drinking occasions. All participants reported at least one drinking occasion in the month following the scans. The average number of drinking days in the 30 day follow up was 8.4±4.1 and was not different between groups, t (62) = 1.041, p = 0.3. In contrast, the average number of drinks per drinking day was 5.1±1.2 for BD and 1.42±1.25 for SD, which was significantly different between groups, t (62) = 2.1, p = 0.037, Cohen's d = 0.59.
2.6. Statistical analysis
Group differences by condition and timepoint for outcome variables (craving, IL-6) were examined in R 3.0.3 using repeated measures ANOVA. A p-value of 0.05 or less was considered significant. Associations between outcome variables and future drinking, and among outcome variables, were examined using linear regression with group dummy coded as a continuous variable. If group was a significant predictor of the relationship between IL-6, craving, VmPFC activation, and future drinking, the data was split by groups and linear regression was performed to determine the relationship between experimental outcomes and future drinking within each group.
3. Results
3.1. Participants
A total of 64 (33 BD and 31 SD) participants completed 2 MRI scanning sessions with viable blood samples and completed at least 85% of surveys during the 30 days follow up phase. Seventeen participants were excluded from analysis due to excessive head movement in the scanner (greater than 1.5 mm in any direction). 11 BD and 6 SD were excluded from neuroimaging analyses due to excessive motion. A chi square test revealed that this was not a significant difference in the number of participants excluded per group. Therefore, N = 47 participants were used for fMRI analyses.
3.2. Baseline craving, IL-6, and TNF-α levels
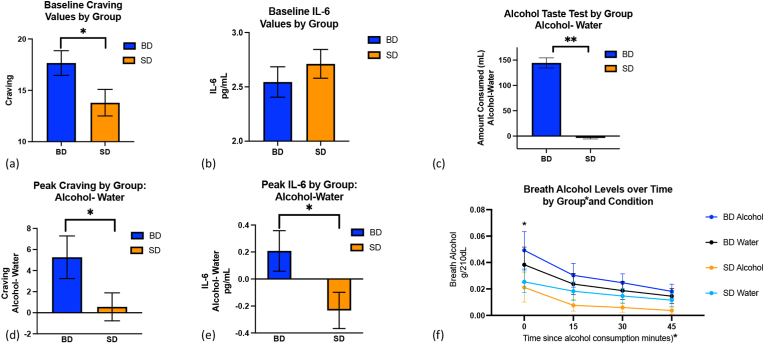
Initial craving for alcohol was not different between conditions (i.e., water cue vs. alcohol cue), F (1,63) = 0.64, p = 0.73 nor was there an interaction between group (BD vs. SD) and condition, F (1,63) = 0.42, p = 0.52. However, a main effect of group was observed for baseline craving, F (1,63) = 6.53, p = 0.01, η2 = 0.07 (Fig. 2a), such that BD had higher baseline craving than SD. This was confirmed by the relationship between baseline craving to number of drinks in the past three months, which was positive across all participants, F (1,63) = 11.05, p = 0.001, R2 = 0.151. However, this positive relationship was driven by the significant association in BD (F (1,32) = 4.87, p = 0.035, R2 = 0.136, whereas in SD, the relationship was not significant, F (1,30) = 3.68, p = 0.065.
Fig. 2.
Craving, IL-6, and Breath Alcohol Responses to Alcohol Cues and Consumption. (a) BD had greater initial craving for alcohol when compared to SD, F (1,63) = 6.53, p = 0.01, η2 = 0.07. (b) No difference in IL-6 levels between groups was observed at baseline, F (1,62) = 0.01, p = 0.92. (c). BD drank significantly greater alcohol in the alcohol cue condition relative to the water cue condition, while the amount of alcohol consumed by SD did not differ by condition, F (1,60) = 6.9, p = 0.01, η2 = 0.015. (d) BD showed greater craving in response to alcohol cues relative to water cues and SD, F (1,63) = 5.6, p = 0.02, η2 = 0.05, and (e) greater IL-6 release after alcohol consumption relative to water cues and SD, F (1,55) = 4.3, p = 0.039, η2 = 0.009. (f) BD showed higher breath alcohol concentrations than SD immediately after alcohol consumption, F (3,189) = 4.6, p = 0.004, η2 = 0.004. Note. Amount of alcohol consumed, IL-6, and craving are shown in response to alcohol cues after controlling for the response to water cues. *Indicates significant at p<0.05.
IL-6 baseline levels were not different between groups, F (1,62) = 0.01, p = 0.92 (Fig. 2b), or between conditions, F (1,62) = 0.09, p = 0.77, and no interaction was observed between group and condition, F (1,62) = 0.76, p = 0.39. This lack of relationship was confirmed by the finding that baseline IL-6 values were not related to number of drinks in the past three months across all participants, F (1,59) = 0.23, p = 0.64, or in each group separately, (BD: F (1,31) = 0.4, p = 0.55; SD: F (1,27) = 0.04, p = 0.85).
Finally, Baseline levels of TNF-α were not different between groups, F (1,61) = 0.006, p = 0.94, or conditions, F (1,61) = 0.011, p = 0.91, and no interaction was observed between group and condition, F (1,61) = 0.1,p = 0.92. Baseline TNF-α levels were not related to the number of drinks consumed in the past three months across all participants, F (1,61) = 1.7, p = 0.19, or in each group separately, (BD: F (1,32) = 0.16, p = 0.68; SD: F (1,29) = 0.32, p = 0.73).
3.3. Craving, IL-6, and TNF-α responses to alcohol cues and consumption
There were both group and condition effects on craving. The condition effect demonstrated the successful experimental manipulation, F (1,63) = 9.8, p = 0.003, η2 = 0.01, such that the alcohol cue condition resulted in higher levels of craving than the water cue condition (alcohol cue: 21.1 ± 1.1, water cue:18.9± 0.92). The group effect was such that BD showed higher peak craving than SD, F (1,63) = 5.6, p = 0.02, η2 = 0.05 (BD: 5.26±2, SD 0.56± 1.33; Fig. 2d).
A group by condition interaction was seen for IL-6 levels, F (1,55) = 4.3, p = 0.039, η2 = 0.009, such that the BD had greater IL-6 release in response to alcohol consumption after alcohol cues, whereas SD showed greater IL-6 release to alcohol consumption after water cues (BD:0.21± 0.85, SD: 0.23±0.75; Fig. 2e). On the other hand, TNF-α were not different by group, F (1,61) = 0.2, = p = 0.66, or condition, F (1,61) = 0.57, p = 0.46.
3.4. Alcohol taste test
A group by condition interaction was seen in the 2 × 2 repeated measures ANOVA for alcohol consumption in the ATT, (F (1,60) = 6.9, p = 0.01, η2=0.015; Fig. 2c), such that BD drank significantly greater alcohol in the alcohol cue condition relative to the water cue condition, while the amount of alcohol consumed by SD did not differ by condition. There were also significant effects of group, such that BD consumed more alcohol than SD (1,60) = 10.7, p = 0.002, η2 = 0.13), and condition, such that more alcohol was consumed in the alcohol cue condition relative to the water cue condition (F (1,60 = 8.5, p = 0.005, η2 = 0.02). Correspondingly, the repeated measures ANOVA revealed a timepoint by group effect for breath alcohol levels, whereby BD showed higher breath alcohol concentrations than SD immediately after alcohol consumption, (F (3,189) = 4.6, p = 0.004, η2 = 0.004; Fig. 2f). There was also a significant main effect of timepoint, such that breath alcohol levels dropped over time, F (3,189) = 29.46, p < 0.001, η2 = 0.03, and a significant main effect of group, such that BD had higher overall breath alcohol levels than SD, F (1,63) = 5.5, p = 0.02, η2 = 0.026.
3.5. Associations between immediate and future alcohol consumption, craving, IL-6, and TNF-α
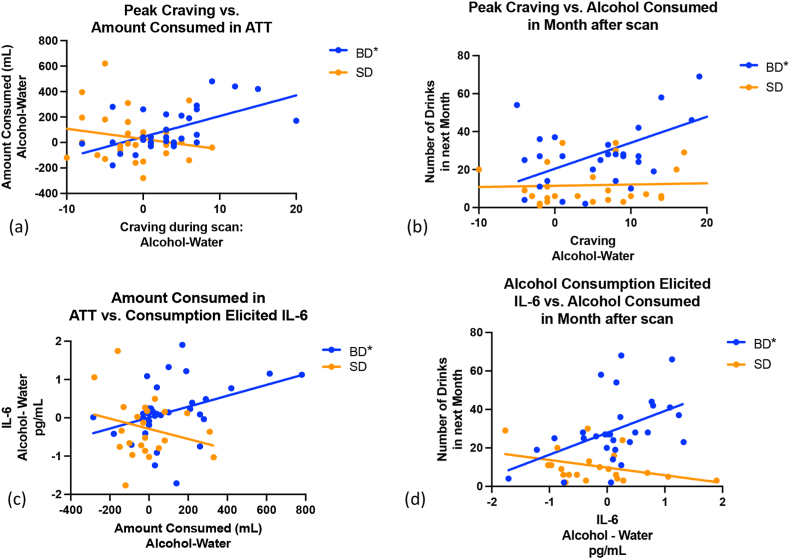
The amount of alcohol consumed in the ATT after alcohol cue exposure, relative to water cue exposure, was significantly related to the number of drinks consumed in the month following experimental appointments across all participants F (1,58) = 5.16, p = 0.03, R2 = 0.105 and for the BD group (1,27), = 6.43,p = 0.018 R2 = 0.21. The relationship was not significant when considering the SD group alone, F (1,29) = 1.05, p = 0.32.
The relationship between peak craving and the amount of alcohol consumed in the ATT differed between BD and SD, F (1,58) = 8.4, p = 0.005. Specifically, alcohol cue induced craving was associated with the amount of alcohol consumed in the ATT in BD, F (1,27) = 4.5, p = 0.04, R2 = 0.32, but not in SD, F (1,29) = 0.34, p = 0.57 (Fig. 3a). Similarly, peak craving was differently related to alcohol consumption in the real world between the two groups, F (1,56) = 5.98, p = 0.02. Alcohol consumption post alcohol cue related to the number of drinks consumed in the next month for BD, F (1,27) = 8.34, p = 0.007, R2 = 0.22 (Fig. 3b), but not SD F (1,29) = 0.02, p = 0.9.
Fig. 3.
Associations between Craving and IL-6 Release with Immediate and Future Drinking in BD only. (a) Peak craving was positively related to the amount of alcohol consumed during the Alcohol Taste Test in BD only, F (1,29) = 4.5, p = 0.04, R2 = 0.32. (b) Peak craving was also related to the amount of alcohol consumed in the following month for BD only, F (1,29) = 16.8, p = 0.0003, R2 = 0.37. (c) IL-6 release in response to alcohol consumption was positively related to the amount of alcohol consumed during the Alcohol Taste Test for BD only, F (1,27) = 5.6, p = 0.02, R2 = 0.15. (d) IL-6 release in response to alcohol consumption, F (1,27) = 7.7, p = 0.01, R2 = 0.22, was also associated with number of alcohol beverages consumed in the next month for BD only. Note. IL-6 and craving are shown in response to alcohol cues after controlling for the response to water cues. *Indicates significant at p<0.05.
The pattern of results was similar for IL-6. Groups differed in the relationship between alcohol consumption during the ATT and IL-6 release, F (1,55) = 4.9, p = 0.03. BD showed increased IL-6 release with greater alcohol consumption in the ATT, F (1,27) = 5.6, p = 0.02, R2 = 0.15 (Fig. 3c). SD showed no relationship between IL-6 release and immediate alcohol consumption in the ATT, F (1,29) = 2.51. P = 0.126. Groups also differed in the relationship between IL-6 release in response to alcohol consumption and future drinking in the real world, F (1,55) = 9.94,p = 0.003, and only BD alcohol consumption in the month following completion of the second scan was related to IL-6 release post alcohol consumption, F (1,27) = 7.7, p = 0.001, R2 = 0.22 (Fig. 3d). SD showed no relationship between IL-6 release and real-world alcohol consumption, F (1,27) = 0.2, p = 0.66. Interestingly, the relationship between peak craving and peak IL-6 response was significantly different by group, F (Coleman and Crews, 2018; Woolrich et al., 2004) = 10.98, p = 0.002, R2 = 0.23. Within each group, however, the relationship between IL-6 levels and craving were not significant, BD F (Coleman and Crews, 2018; He and Crews, 2008) = 0.27, p = 0.613; SD F (Coleman and Crews, 2018; Blednov et al., 2012) = 2.26, p = 0.146.
Finally, groups did not differ in the relationship between TNF-α levels and immediate alcohol consumption, F (Coleman and Crews, 2018; Goldstein and Volkow, 2002) = 2.7, p = 0.07, nor were there significant relationships between TNF-α levels and alcohol consumption in the ATT within each group, BD: F (1,32) = 0.3, p = 0.6; SD F (1,29) = 0.85, p = 0.37. The relationship between TNF-α levels and alcohol consumption in the following month was also not different by groups, F (1,62) = 0.27, p = 0.6, or significant in either group when examined separately, BD F (1,31) = 6.87, p = 0.06; SD: F (1,29) = 0.08, p = 0.78. Moreover, TNFα responses were not related IL-6 responses across all participants, F (1,55) = 0.33, p = 0.57, or within groups, BD F (1,27) = 0.93, p = 0.34; SD F (1,29) = 0.68, p = 0.42.
3.6. Neural alcohol cue reactivity
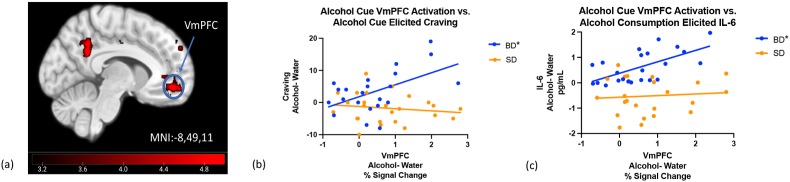
Contrary to our hypotheses, there were no group differences in blood oxygen level dependent (BOLD) response to alcohol cues after controlling for the effects of water cues. Both participant groups exhibited increased activation in the left Ventral Anterior Cingulate (Brodmann Area [BA] 24; Local maximum Montreal Neurological Institute [MNI] coordinates (x, y, z) −3, −16, 39), right Dorsomedial Prefrontal Cortex (BA 9; 0, 41, 30), right Lateral Orbitofrontal Cortex (Brodmann area 47; 32,39,-9), left Ventromedial Prefrontal Cortex (BA 10; −29,57,15; Fig. 4a), left Angular Gyrus (BA 39; −52,-72,25), left Frontal Eyefields (BA 8; −17,35,39), left Ventral Posterior Cingulate Cortex (BA23; −9,-50,30), and left Dorsal Anterior Cingulate Cortex (BA 32; −1,49,-11; Table 2).
Fig. 4.
VmPFC Cue Reactivity positively associated with Cue elicited craving, and IL-6 Release post alcohol consumption in BD only. (a) No group differences in whole brain and cluster corrected ALC-H20 contrast when the SD alcohol-water activation was subtracted from the BD alcohol-water. (b) However, greater VmPFC activation in response to alcohol cues, after controlling for water cue activation, was correlated with cue elicited craving in BD, F (1, 21) = 6.10, p = 0.02, R2 = 0.2 and (c) IL-6 release in response to alcohol consumption in BD, F (1, 21) = 7.60, p = 0.01, R2 = 0.23. Note: Final imaging sample N = 47, with 11 BD and 6 SD excluded for excessive motion.
Table 2.
Significant Clusters in Alcohol- Water Contrast in All Participants, whole brain p < 0.001, cluster p < 0.05.
| Region | BA | Max Z | Voxels | x | y | z |
|---|---|---|---|---|---|---|
| Left Ventral Anterior Cingulate | 24 | 4.19 | 190 | −3.3 | −16.2 | 39.1 |
| Right Dorsomedial Prefrontal Cortex | 9 | 5.58 | 237 | 0.1 | 41.1 | 29.5 |
| Right Lateral Orbitofrontal Cortex | 47 | 4.92 | 347 | 31.7 | 38.5 | −8.9 |
| Left Ventromedial Prefrontal Cortex | 10 | 4.76 | 532 | −29.0 | 56.5 | 15.1 |
| Left Angular Gyrus | 39 | 5.86 | 742 | −52.0 | −71.8 | 24.7 |
| Left Frontal Eyefields | 8 | 5.16 | 1615 | −17.0 | 34.5 | 39.1 |
| Left Ventral Posterior Cingulate Cortex | 23 | 6.02 | 2944 | −9.3 | −50.4 | 29.5 |
| Left Dorsal Anterior Cingulate Cortex | 32 | 5.64 | 3635 | −0.4 | 48.8 | −11.3 |
Note: BA= Brodmann Area. Max Z = Z statistic for peak voxel in cluster. x,y,z = MNI Coordinates.
3.7. Associations between neural cue reactivity and craving, immediate and future alcohol consumption, IL-6, and TNF-α
Despite a lack of overall group differences in neural cue reactivity, relationships between VmPFC activation and craving differed between the two groups, F (1,46) = 4.4, p = 0.04, such that BD showed a significant positive relationship F (1, 21) = 7.8, p = 0.01, R2 = 0.25 (Fig. 4b), and SD showed no relationship, F (1,25) = 0.02,p = 0.91. Similarly, the correlation between VmPFC response and IL-6 release post alcohol consumption differed between the two groups, F (1,46) = 4.33, p = 0.04. In BD, VmPFC cue reactivity was positively associated with IL-6 release post alcohol consumption, F (1, 21) = 14.6, p = 0.0008, R2 = 0.38 (Fig. 4c), whereas the relationship in SD was not significant, F (1,25) = 0.15, p = 0.71. Contrary to our hypotheses, VmPFC cue reactivity was not associated with alcohol consumption during the ATT or in the next 30 days across all participants (F (1,46) <0.001, p = 0.9822), nor within each group (BD: (F (1,21) = 1.76,p = 0.2); SD: (F (1,25) = 3.34,p = 0.09). Finally, the TNF-α cue response was also not related to VmPFC activation in response to alcohol cues, after controlling for both responses to water cues, in either group, BD: F (1,23) = 1.19, p = 0.29, SD: F (1,24) = 0.05, p = 0.82.
4. Discussion
The results of the this randomized, counter-balanced, clinical investigation suggest acute alcohol induction of IL-6 may be a specific immune process modulator that contributes to increased alcohol motivation and consumption in young adult, at-risk BD. Specifically, the results of the present study suggest that acute IL-6 release, craving, and neural alcohol cue reactivity are positively associated with immediate and future alcohol consumption in BD, but not SD, after controlling for baseline differences within participants and across groups, and for non-specific effects of water cues.
Evidence for conditioned IL-6 responses to acute intoxication can be found in the recent preclinical studies of Gano et al. (2017), in which unconditioned ethanol induced IL-6 release in the amygdala and hippocampus was paired with a neutral flavor stimulus. After only 4 pairings, conditioned IL-6 release was seen in response to the neutral flavor. Furthermore, while IL-6 release in humans has been measured in other studies 3 or more hours post consumption, a recent study suggests IL-6 steadily and linearly increases during the initial 4 h post consumption and can increase up to 40% 12 h post consumption, even after low doses of alcohol like those consumed in our study (van de Loo et al., 2020).
However, the exact mechanistic relationship between IL-6 release to acute alcohol and neuroinflammation, however, remains unclear. Studies of artificially induced peripheral inflammation have indicated that systemic inflammation alters extracellular norepinephrine and pro-inflammatory mRNA levels in the amygdala, thereby increasing hypothalamic release of corticotropin-releasing hormone (CRH) and contributing to associated excitotoxic cascades (Bienkowski and Rinaman, 2011; Engler et al., 2011; Serrats and Sawchenko, 2006). In preclinical studies, alcohol also directly elicits glial production of immune factors (Gruol et al., 2021). Thus, the link between IL-6 release and AUDs may be that repeated alcohol intoxication triggers neural inflammation (Kraynak et al., 2018) by disrupting pre-synaptic neurotransmitter release (Gruol et al., 2021).
Importantly, prefrontal cortex neuroinflammation has been linked to deficits in reward responding in substance use disorders (Goldstein and Volkow, 2002, 2011; Seney et al., 2021) and may underlie the altered relationship between VmPFC cue activation, craving, and the IL-6 release seen in BD. VmPFC cue activation has been linked to craving and drinking behaviors previously (Blaine et al., 2017) and influences behavioral and emotional coping responses to alcohol and stress cues via direct synaptic connections to the extended amygdala (Etkin et al., 2011). If these synaptic relays are damaged via glucocorticoid-induced excitotoxicity, in combination with associated neuroinflammatory processes, there may be loss of gray matter volume and functional integrity in the VmPFC (Fein et al., 2002).
Unlike the VmPFC, we did not find a relationship between craving and IL-6 release to cues or after alcohol consumption in BD or SD. Tonic IL-6 suppression has also been linked to craving in those with moderate AUDs (Milivojevic et al., 2017), but our results suggest there is an acute increase in IL-6 post alcohol consumption in BD which is associated with future drinking. Deletion of the IL-6 gene in mice specifically reduces ethanol consumption, unlike deletion of other cytokine receptor genes (Blednov et al., 2012), and high tonic levels of IL-6 are associated with craving during withdrawal in those with severe AUDs (Heberlein et al., 2014). A recent meta-analysis suggested that IL-6 and other cytokine level abnormalities, in addition to their relationship to craving, may vary by stage of disease progression (Adams et al., 2020). Thus, the directionality of the relationship between IL-6 levels and symptoms of AUDs remains unclear.
Finally, in contrast to IL-6, TNF-α cue and consumption responses were not different between groups. These results are similar to previous reports that suggest TNF-α levels are unaltered after moderate doses of alcohol acutely in healthy subjects (Monnig et al., 2020). Recently, Hillmer et al. (2020), reported decreases in TNF-α in response to alcohol consumption however, the time course of alcohol consumption in their investigation was significantly longer (i.e., 6 h) than the present study, suggesting a delayed TNF-α response to alcohol. Elevated baseline TNF-α levels are often seen in those with severe AUDs, coupled with a reliable decrease in TNF-α in response to alcohol consumption (Heberlein et al., 2014), so TNF-α may be related to AUD progression at later stages or may only result from greater chronic consumption. TNF-α is associated with acute alcohol liver injury (Kitazawa et al., 2003; Hansen et al., 1994), and increased TNF-α production precedes liver injury in chronic AUDs, (Kawaratani et al., 2013; Iimuro et al., 1997), but further studies are required to elucidate the relationship between neural inflammation and both acute and chronic alcohol consumption.
It is important to note several limitations of the current study. First, the study design did not fully allow for a complete disentanglement of alcohol expectancy effects due to cues from the effects of consumption of alcohol on IL-6 levels, as active alcohol was used in both conditions. To fully address any expectancy and consumption confounds, future studies should include additional conditions where placebo alcohol is administered in combination with water cues and separately with alcohol cues (i.e., 4 conditions total: alcohol cues plus placebo, alcohol cues plus alcohol, water cues plus placebo, and water cues plus alcohol). Nevertheless, our results support numerous previous findings in the addiction literature suggesting the neural response to the rewarding properties of drugs shift from the drug itself to cues (for review, see Volkow et al., 2010). Second, the current study was not powered to explore sex-related differences in neural and immune response to alcohol cues and future investigations are warranted to examine these comparisons. Additionally, although we carefully obtained drinking histories using multiple measures prior to SD and BD group inclusion, no biochemical verification of current alcohol use was performed to confirm binge/social drinking status. Our results indicated that IL-6 release to alcohol consumption was not related to recent (past month) drinking history, but was associated with future (next month) drinking behavior. This might be because 4 daily surveys provided a more accurate assessment than participant recall, but this result will need to be replicated. Also, the SD group was slightly older than the BD group. Therefore, it is possible that age differences may have some effect on alcohol cue salience. However, no differences in neural cue reactivity were observed between groups. These limitations notwithstanding, our results suggest IL6, but not TNF-α, may be related to the increased motivation to consume alcohol seen in BD and therefore may also contribute to the risk of development of AUDs.
Funding
National Institutes of Health grant R00-AA025401 (SKB).
Study approval
The protocol was approved by the Auburn University Human Subjects Research Institutional Review Board. All participants provided informed, written consent before participation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbih.2023.100645.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
Data availability
Data will be made available on request.
References
- Adams C., Conigrave J.H., Lewohl J., Haber P., Morley K.C. Alcohol use disorder and circulating cytokines: a systematic review and meta-analysis. Brain Behav. Immun. 2020;89:501–512. doi: 10.1016/j.bbi.2020.08.002. [DOI] [PubMed] [Google Scholar]
- Avants B.B., Epstein C.L., Grossman M., Gee J.C. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med. Image Anal. 2008;12:26–41. doi: 10.1016/j.media.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks W.A., Kastin A.J., Gutierrez E.G. Penetration of interleukin-6 across the murine blood-brain barrier. Neurosci. Lett. 1994;179:53–56. doi: 10.1016/0304-3940(94)90933-4. [DOI] [PubMed] [Google Scholar]
- Bienkowski M.S., Rinaman L. Immune challenge activates neural inputs to the ventrolateral bed nucleus of the stria terminalis. Physiol. Behav. 2011;104:257–265. doi: 10.1016/j.physbeh.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaine S.K., Sinha R. Alcohol, stress, and glucocorticoids: from risk to dependence and relapse in alcohol use disorders. Neuropharmacology. 2017;122:136–147. doi: 10.1016/j.neuropharm.2017.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaine S.K., Seo D., Sinha R. Peripheral and prefrontal stress system markers and risk of relapse in alcoholism. Addiction Biol. 2017;22(2):468–478. doi: 10.1111/adb.12320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaine S.K., Seo D., Sinha R. Peripheral and prefrontal stress system markers and risk of relapse in alcoholism. Addiction Biol. 2017;22:468–478. doi: 10.1111/adb.12320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaine S.K., Nautiyal N., Hart R., Guarnaccia J., Sinha R. Craving, cortisol and behavioral alcohol motivation responses to stress and alcohol cue contexts and discrete cues in binge and non‐binge drinkers. Addiction Biol. 2019;24:1096–1108. doi: 10.1111/adb.12665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaine S.K., et al. Association of prefrontal-striatal functional pathology with alcohol abstinence days at treatment initiation and heavy drinking after treatment initiation. Am. J. Psychiatr. 2020;177:1048–1059. doi: 10.1176/appi.ajp.2020.19070703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov Y.A., et al. Perturbation of chemokine networks by gene deletion alters the reinforcing actions of ethanol. Behav. Brain Res. 2005;165:110–125. doi: 10.1016/j.bbr.2005.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov Y.A., et al. Neuroimmune regulation of alcohol consumption: behavioral validation of genes obtained from genomic studies. Addiction Biol. 2012;17:108–120. doi: 10.1111/j.1369-1600.2010.00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn M.J., Krahn D.D., Staehler B.A. Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcohol Clin. Exp. Res. 1995;19:600–606. doi: 10.1111/j.1530-0277.1995.tb01554.x. [DOI] [PubMed] [Google Scholar]
- Cahalan D., Cisin I.H., Crossley H.M. American drinking practices: a national study of drinking behaviors and attitudes. Monogr. Rutgers Cent. Alcohol Stud. 1969;6 [Google Scholar]
- Carbia C., et al. A biological framework for emotional dysregulation in alcohol misuse: from gut to brain. Mol. Psychiatr. 2021;26:1098–1118. doi: 10.1038/s41380-020-00970-6. [DOI] [PubMed] [Google Scholar]
- Coleman L.G., Crews F.T. The Neuropharmacology of Alcohol. Springer; 2018. Innate immune signaling and alcohol use disorders; pp. 369–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews F., Zou J., Qin L. Induction of innate immune genes in brain create the neurobiology of addiction. Brain Behav. Immun. 2011;25:S4–S12. doi: 10.1016/j.bbi.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale A.M., Fischl B., Sereno M.I. Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater T.L., Gano A., Paniccia J.E., Deak T. Male adolescent rats display blunted cytokine responses in the CNS after acute ethanol or lipopolysaccharide exposure. Physiol. Behav. 2015;148:131–144. doi: 10.1016/j.physbeh.2015.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler H., et al. Acute amygdaloid response to systemic inflammation. Brain Behav. Immun. 2011;25:1384–1392. doi: 10.1016/j.bbi.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Erta M., Quintana A., Hidalgo J. Interleukin-6, a major cytokine in the central nervous system. Int. J. Biol. Sci. 2012;8:1254. doi: 10.7150/ijbs.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban O., et al. fMRIPrep: a robust preprocessing pipeline for functional MRI. Nat. Methods. 2019;16:111–116. doi: 10.1038/s41592-018-0235-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A., Egner T., Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cognit. Sci. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G., et al. Cortical gray matter loss in treatment‐naive alcohol dependent individuals. Alcohol Clin. Exp. Res. 2002;26:558–564. [PMC free article] [PubMed] [Google Scholar]
- First M.B., William J.B.W., Karg R.S., Spitzer R.L. American Psychiatric Association; Arlington, VA: 2015. Structured Clinical Interview for DSM-V- Research Version (SCID 5 for DSM-5, Research Version; SCID-5-RV. Version 1.0.0. [Google Scholar]
- Gano A., Pautassi R.M., Doremus-Fitzwater T.L., Deak T. Conditioned effects of ethanol on the immune system. Exp. Biol. Med. 2017;242:718–730. doi: 10.1177/1535370217694097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcin F., et al. Aldehyde dehydrogenase in drosophila: developmental and functional aspects. Alcohol. 1985;2:85–89. doi: 10.1016/0741-8329(85)90021-7. [DOI] [PubMed] [Google Scholar]
- Goldstein R.Z., Volkow N.D. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am. J. Psychiatr. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein R.Z., Volkow N.D. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat. Rev. Neurosci. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgolewski K., et al. Nipype: a flexible, lightweight and extensible neuroimaging data processing framework in python. Front. Neuroinf. 2011;13 doi: 10.3389/fninf.2011.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve D.N., Fischl B. Accurate and robust brain image alignment using boundary-based registration. Neuroimage. 2009;48:63–72. doi: 10.1016/j.neuroimage.2009.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grifasi I.R., et al. Characterization of the hippocampal neuroimmune response to binge-like ethanol consumption in the drinking in the dark model. Neuroimmunomodulation. 2019;26:19–32. doi: 10.1159/000495210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruol D.L., Hernandez R.V., Roberts A. Alcohol enhances responses to high frequency stimulation in hippocampus from transgenic mice with increased astrocyte expression of IL-6. Cell. Mol. Neurobiol. 2021;41:1299–1310. doi: 10.1007/s10571-020-00902-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J., Cherwitz D.L., Allen J.I. The role of tumor necrosis factor-alpha in acute endotoxin-induced hepatotoxicity in ethanol-fed rats. Hepatology. 1994;20:461–474. [PubMed] [Google Scholar]
- He J., Crews F.T. Increased MCP-1 and microglia in various regions of the human alcoholic brain. Exp. Neurol. 2008;210:349–358. doi: 10.1016/j.expneurol.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heberlein A., et al. TNF-α and IL-6 serum levels: neurobiological markers of alcohol consumption in alcohol-dependent patients? Alcohol. 2014;48:671–676. doi: 10.1016/j.alcohol.2014.08.003. [DOI] [PubMed] [Google Scholar]
- Hillmer A.T., Nadim H., Devine L., Jatlow P., O'Malley S.S. Acute alcohol consumption alters the peripheral cytokines IL-8 and TNF-α. Alcohol. 2020;85:95–99. doi: 10.1016/j.alcohol.2019.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iimuro Y., Gallucci R.M., Luster M.I., Kono H., Thurman R.G. Antibodies to tumor necrosis factor alfa attenuate hepatic necrosis and inflammation caused by chronic exposure to ethanol in the rat. Hepatology. 1997;26:1530–1537. doi: 10.1002/hep.510260621. [DOI] [PubMed] [Google Scholar]
- Jenkinson M. Fast, automated, N‐dimensional phase‐unwrapping algorithm. Magn. Reson. Med.: An Official Journal of the International Society for Magnetic Resonance in Medicine. 2003;49:193–197. doi: 10.1002/mrm.10354. [DOI] [PubMed] [Google Scholar]
- Jones S.A., Horiuchi S., Topley N., Yamamoto N., Fuller G.M. The soluble interleukin 6 receptor: mechanisms of production and implications in disease. Faseb. J. 2001;15:43–58. doi: 10.1096/fj.99-1003rev. [DOI] [PubMed] [Google Scholar]
- Jones A., et al. The ad-libitum alcohol ‘taste test’: secondary analyses of potential confounds and construct validity. Psychopharmacology. 2016;233:917–924. doi: 10.1007/s00213-015-4171-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karoly H.C., Bidwell L.C., Mueller R.L., Hutchison K.E. Investigating the relationships between alcohol consumption, cannabis use, and circulating cytokines: a preliminary analysis. Alcohol Clin. Exp. Res. 2018;42:531–539. doi: 10.1111/acer.13592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaratani H., et al. The effect of inflammatory cytokines in alcoholic liver disease. Mediat. Inflamm. 2013;2013 doi: 10.1155/2013/495156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoruts A., Stahnke L., McClain C.J., Logan G., Allen J.I. Circulating tumor necrosis factor, interleukin‐1 and interleukin‐6 concentrations in chronic alcoholic patients. Hepatology. 1991;13:267–276. [PubMed] [Google Scholar]
- Kimpel M.W., et al. Functional gene expression differences between inbred alcohol-preferring and–non-preferring rats in five brain regions. Alcohol. 2007;41:95–132. doi: 10.1016/j.alcohol.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitazawa T., et al. The production of tumor necrosis factor-alpha by macrophages in rats with acute alcohol loading. Alcohol Clin. Exp. Res. 2003;27:72S–75S. doi: 10.1097/01.ALC.0000078611.55696.F0. [DOI] [PubMed] [Google Scholar]
- Koob G.F., Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Koob G.F., Le Moal M. Plasticity of reward neurocircuitry and the'dark side'of drug addiction. Nat. Neurosci. 2005;8:1442–1444. doi: 10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- Kraynak T.E., Marsland A.L., Wager T.D., Gianaros P.J. Functional neuroanatomy of peripheral inflammatory physiology: a meta-analysis of human neuroimaging studies. Neurosci. Biobehav. Rev. 2018;94:76–92. doi: 10.1016/j.neubiorev.2018.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laso F.J., Vaquero J.M., Almeida J., Marcos M., Orfao A. Chronic alcohol consumption is associated with changes in the distribution, immunophenotype, and the inflammatory cytokine secretion profile of circulating dendritic cells. Alcohol Clin. Exp. Res. 2007;31:846–854. doi: 10.1111/j.1530-0277.2007.00377.x. [DOI] [PubMed] [Google Scholar]
- Lee M.R., Abshire K.M., Farokhnia M., Akhlaghi F., Leggio L. Effect of oral alcohol administration on plasma cytokine concentrations in heavy drinking individuals. Drug Alcohol Depend. 2021;225 doi: 10.1016/j.drugalcdep.2021.108771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber C.S. Hepatic and metabolic effects of ethanol: pathogenesis and prevention. Ann. Med. 1994;26:325–330. doi: 10.3109/07853899409148346. [DOI] [PubMed] [Google Scholar]
- Lieber C.S., DeCARLI L.M. The role of the hepatic microsomal ethanol oxidizing system (MEOS) for ethanol metabolism in vivo. J. Pharmacol. Exp. Therapeut. 1972;181:279–287. [PubMed] [Google Scholar]
- Maachi M., et al. Systemic low-grade inflammation is related to both circulating and adipose tissue TNFα, leptin and IL-6 levels in obese women. Int. J. Obes. 2004;28:993–997. doi: 10.1038/sj.ijo.0802718. [DOI] [PubMed] [Google Scholar]
- Marshall S.A., Geil C.R., Nixon K. Prior binge ethanol exposure potentiates the microglial response in a model of alcohol-induced neurodegeneration. Brain Sci. 2016;6:16. doi: 10.3390/brainsci6020016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C.S., Earleywine M., Musty R.E., Perrine M.W., Swift R.M. Development and validation of the biphasic alcohol effects scale. Alcohol Clin. Exp. Res. 1993;17:140–146. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Milivojevic V., et al. Peripheral immune system adaptations and motivation for alcohol in non‐dependent problem drinkers. Alcohol Clin. Exp. Res. 2017;41:585–595. doi: 10.1111/acer.13317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnig M.A., et al. Immune response to an acute moderate dose of alcohol in healthy young adults. Alcohol Alcohol. 2020;55:616–623. doi: 10.1093/alcalc/agaa079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morean M.E., et al. The drug effects questionnaire: psychometric support across three drug types. Psychopharmacology. 2013;227:177–192. doi: 10.1007/s00213-012-2954-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson S., Bagby G.J., Bainton B.G., Summer W.R. The effects of acute and chronic alcoholism on tumor necrosis factor and the inflammatory response. JID (J. Infect. Dis.) 1989;160:422–429. doi: 10.1093/infdis/160.3.422. [DOI] [PubMed] [Google Scholar]
- Neve E.P., Ingelman-Sundberg M. Molecular basis for the transport of cytochrome P450 2E1 to the plasma membrane. J. Biol. Chem. 2000;275:17130–17135. doi: 10.1074/jbc.M000957200. [DOI] [PubMed] [Google Scholar]
- NIAAA . US Department of Health and Human Services; 2012. Rethinking Drinking: Alcohol and Your Health. [Google Scholar]
- Portelli J., et al. Peripheral proinflammatory markers are upregulated in abstinent alcohol-dependent patients but are not affected by cognitive bias modification: preliminary findings. Drug Alcohol Depend. 2019;204 doi: 10.1016/j.drugalcdep.2019.107553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probert L., et al. TNF-α transgenic and knockout models of CNS inflammation and degeneration. J. Neuroimmunol. 1997;72:137–141. doi: 10.1016/s0165-5728(96)00184-1. [DOI] [PubMed] [Google Scholar]
- Reissner K.J., Kalivas P.W. Using glutamate homeostasis as a target for treating addictive disorders. Behav. Pharmacol. 2010;21:514. doi: 10.1097/FBP.0b013e32833d41b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seney M.L., et al. Transcriptional alterations in dorsolateral prefrontal cortex and nucleus accumbens implicate neuroinflammation and synaptic remodeling in opioid use disorder. Biol. Psychiatr. 2021;90:550–562. doi: 10.1016/j.biopsych.2021.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrats J., Sawchenko P.E. CNS activational responses to staphylococcal enterotoxin B: T‐lymphocyte‐dependent immune challenge effects on stress‐related circuitry. J. Comp. Neurol. 2006;495:236–254. doi: 10.1002/cne.20872. [DOI] [PubMed] [Google Scholar]
- Sobell L.C., Sobell M.B. In: Measuring Alcohol Consumption: Psychosocial and Biological Methods. Allen L., Litten R., editors. Humana Press; Totowa, NJ: 1992. Timeline Follow-back: a technique for assessing self-reported ethanol consumption; pp. 41–72. [Google Scholar]
- Tustison N.J., et al. N4ITK: improved N3 bias correction. IEEE Trans. Med. Imag. 2010;29:1310–1320. doi: 10.1109/TMI.2010.2046908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Loo A.J., et al. The inflammatory response to alcohol consumption and its role in the pathology of alcohol hangover. J. Clin. Med. 2020;9:2081. doi: 10.3390/jcm9072081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N.D., et al. Addiction: decreased reward sensitivity and increased expectation sensitivity conspire to overwhelm the brain's control circuit. Bioessays. 2010;32:748–755. doi: 10.1002/bies.201000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vore A.S., Doremus-Fitzwater T., Gano A., Deak T. Adolescent ethanol exposure leads to stimulus-specific changes in cytokine reactivity and hypothalamic-pituitary-adrenal axis sensitivity in adulthood. Front. Behav. Neurosci. 2017;11:78. doi: 10.3389/fnbeh.2017.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich M.W., Behrens T.E., Beckmann C.F., Jenkinson M., Smith S.M. Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimage. 2004;21:1732–1747. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Zago A., et al. Alcohol use disorder and inflammatory cytokines in a population sample of young adults. Journal of Alcoholism & Drug Dependence. 2016:1–5. [Google Scholar]
- Zelová H., Hošek J. TNF-α signalling and inflammation: interactions between old acquaintances. Inflamm. Res. 2013;62:641–651. doi: 10.1007/s00011-013-0633-0. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Brady M., Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans. Med. Imag. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- Zou J., Crews F. Induction of innate immune gene expression cascades in brain slice cultures by ethanol: key role of NF‐κB and proinflammatory cytokines. Alcohol Clin. Exp. Res. 2010;34:777–789. doi: 10.1111/j.1530-0277.2010.01150.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.