Abstract
The article offers a survey of currently notable artificial intelligence methods (released between 2019-2023), with a particular emphasis on the latest advancements in detecting rheumatoid arthritis (RA) at an early stage, providing early treatment, and managing the disease. We discussed challenges in these areas followed by specific artificial intelligence (AI) techniques and summarized advances, relevant strengths, and obstacles. Overall, the application of AI in the fields of RA has the potential to enable healthcare professionals to detect RA at an earlier stage, thereby facilitating timely intervention and better disease management. However, more research is required to confirm the precision and dependability of AI in RA, and several problems such as technological and ethical concerns related to these approaches must be resolved before their widespread adoption.
Keywords: rheumatoid arthritis, artificial intelligence, early diagnosis, early intervention, disease management
Introduction
Rheumatoid arthritis (RA) is a complex chronic autoimmune disease characterized by persistent inflammation with unclear etiology. It affects many joints, including the hands, feet, and wrists, and also causes pericarditis, pulmonary fibrosis, peripheral nephropathy, etc.[1,2] The disease is associated with considerable morbidity, disability, and mortality, posing a significant burden on the mental level, physical level and social well-being of the patients.[3] While there have been notable advancements in treatment, there is room for improvement in early diagnosis, timely treatment, and effective disease management. The slow progression and nonspecific initial symptoms of RA hinder accurate and prompt diagnosis. It is crucial to enhance the performance of diagnostic tools and the identify reliable biomarkers for early detection. Timely intervention is essential to delaying disease progression and minimizing irreversible joint damage. Challenges include drug selection difficulties, low treatment adherence, and patient variability in medication response. There is a need for personalized precision medicine systems and biomarkers to predict drug efficacy. Additionally, RA is a chronic condition that requires long-term management to control symptoms, prevent joint damage, and improve overall quality of life. However, poor treatment adherence, low follow-up rate, and inadequate chronic disease management contribute to a high disease recurrence rate. Therefore, extensive efforts in early diagnosis, timely intervention, prediction of disease progression, and disease management are important to improve the long-term outcomes of RA patients.
Artificial intelligence (AI) is defined as computers and other technology that mimic human intelligence-assisted mechanisms such as thinking, deep learning, reasoning, and sensory understanding.[4] Machine learning, a subgroup of AI, is a powerful analytic approach, as stated by Dr. Stoel in his article, that is developed to automatically learn from the data and make decisions.[5] In recent years, AI and its subclass machine learning (ML) have gained much attention as analytic methods and advanced techniques to improve efficacy and efficiency in healthcare. The medical AI/ML techniques are ranging from simple online appointment booking, health mentoring to complex drug development, disease diagnostics, digital consultation, and personalized treatment, etc.[3] Moreover, the AI/ML systems have shown promise in various data types, such as electronic health records (EHRs), randomized controlled trials (RCTs), medical images and so on.[4] For example, the medical AL/ML algorithms have been used to assist the selection of an appropriate antibiotic prescription based on the large number of patient’s demographic information and clinical history.[6] Additionally, decision-making based on ML improves the accuracy and efficiency of decision-making by physicians.[3] Medical AI techniques have been applied across various diseases including RA, including medical applications such as image analysis and diagnosis. These efforts illustrate that AI approaches have the potential to transform the treatment of rheumatic diseases. However, the use of AI in rheumatology is not as mature as in other medical areas. More studies are needed on the development and implementation of medical AI techniques to help diagnose and manage rheumatic diseases.[7]
We conducted an online search using PubMed in March 2023 using the following keywords “rheumatoid arthritis” and “artificial intelligence”. The study selection was limited to full-text studies published between 2019 to 2023 and the aim was related to the diagnosis, intervention, and management of RA. Our objective is to provide a survey of recent studies that have employed AI in RA, along with considering the potential role that AI might play in the future. Specifically, we will focus on key analyses, advanced methods, relevant strengths and limitations, and future directions in the following areas of RA: (1) diagnosis; (2) intervention; and (3) disease management.
AI in the Diagnosis of RA
As the condition of RA progress, structural damage becomes a primary factor lead to functional disability.[8] Late diagnosis is highly correlated with adverse long-term outcomes for RA patients. Therefore, the timely diagnosis of RA is crucial for the treatment of patients to better manage and improve the outcome of the patient.[2] However, there are many barriers that existed which lead to delayed diagnosis of RA. One of the main difficulties is that signs and symptoms of RA in an early stage can be nonspecific, such as joint pain and stiffness, which can also be caused by other conditions.[9] Furthermore, the progression of the disease can vary widely between individuals, making it difficult to identify and diagnose in its early stages.[9] Another challenge is that there is no definitive test for RA. Diagnosis typically involves a combination of clinical evaluation, imaging tests, and laboratory tests, such as rheumatoid factor (RF) and anti-cyclic citrullinated peptide (anti-CCP) antibodies.[2] However, these tests can have false results, which can lead to delayed or incorrect diagnoses. Therefore, there are significant challenges associated with early diagnosis of RA, emphasizing the critical importance of early detection for initiating timely treatment and preventing the progression to chronic RA.
Many machine learning works have been conducted in the diagnosis of RA to aid clinicians in making more accurate and efficient diagnoses (Table 1). First, AI algorithms, such as naive Bayes, convolutional neural network (CNN), logistic regression, and support vector machine (SVMs) or deep learning can analyze imaging data, such as X-rays, magnetic resonance imaging (MRI), and computer tomography (CT), to detect subtle changes in the joints that may indicate early RA.[10,11] MRI can provide accurate data on early signs of inflammatory arthritis, particularly in the identification of bone marrow edema and tenosynovitis.[12] X-rays is also most commonly used and easily accessible non-invasive method for monitoring disease progression, and can also detect bone structural changes in early stages.[13] Bai et al.[14] developed a method for detecting finger joint involvement in patients with RA using hand X-ray images, and they achieved an accuracy of 91.8% by training an artificial neural network (ANN) for this task. The automated analysis helps rheumatologists in the diagnosis process, replacing previous manual inspection of clinical images, which have greatly increased the efficacy and efficiency in the classification of RA.
Table 1.
Summary of the studies of artificial intelligence (AI) in early diagnosis of rheumatoid arthritis (RA), including authors, data types, methods, and main findings of the same category
| Main findings | Data types | Methods | Author (Month/Year) |
|---|---|---|---|
| Studies have demonstrated the effectiveness of CNN, Bayesian, or deep | Radiographs | CNN | Hirano et al.[17] (Nov. 2019) |
| learning in detecting and classifying RA from radiographs. ML models have been developed to quantify radiographic joint damage, discriminate between RA, osteoarthritis and normal hand radiographs, and | CNN, DL | Üreten et al.[18,19] (Apr. 2020, 2022) | |
| detect joint ankylosis and subluxation. In addition, deep learning-based | Bayesian | Sun et al. [20] (Aug. 2022) | |
| systems have been proposed for automatic assessment of bone destruction | |||
| and computer-aided diagnosis of RA using hand radiographs. These | DL | Izumi et al.[21] (Feb. 2023) | |
| results demonstrate the potential of ML to improve the diagnosis and assessment of RA through automated image analysis. | DNN | Miyama et al.[22] (Oct. 2022) | |
| DL | Wang et al.[23] (Jun. 2022) | ||
| These studies focus on the use of ML, specifically deep learning or CNN, in analyzing ultrasound images for RA. They include automatic localization of anatomical regions, estimation of metacarpal-head cartilage thickness, and classification of synovial proliferation in metacarpophalangeal joints using ultrasound images. ML shows promise as a valuable tool for analyzing ultrasound images in diagnosing and assessing rheumatoid arthritis. | Ultrasonography | CNN | Hemalatha et al.[24] (Apr. 2019) |
| CNN | Fiorentino et al.[25] (Feb. 2022) | ||
| DL | Wu et al.[26] (Feb. 2022) | ||
| Machine learning techniques applied to genetic data have identified immune-related biomarkers for rheumatoid arthritis, including diagnostic | Genetic data | SVM-RFE | Chen et al.[27] (Nov. 2021) |
| signatures, immune cell infiltration characteristics, and specific markers | SVM-RFE, random forest | Yu et al.[28] (Oct. 2021) | |
| such as PSMB9 and CXCL13. Robust machine learning models have also | |||
| been developed to predict RA using optimized polygenic risk scores. These findings advance our understanding of RA and demonstrate the | Linear regression, elastic Net, random forest | Rychkov et al.[16] (Jun. 2021) | |
| potential of machine learning in biomarker discovery. | Lasso, SVM-RFE | Li et al.[29] (Dec. 2022) | |
| Bayesian | Lim et al.[30] (Feb. 2023) | ||
| The studies demonstrate that ML methods, such as ABC analysis, random forest, logistic regression, and neural network, effectively differentiate | EHRs | Computed ABC analysis, random forest | Lötsch et al.[31] (Jan. 2020) |
| between RA patients and non-RA individuals. These methods rely on utilizing EHR data, which includes clinical laboratory test results, demographic data, and clinical reports, etc. These studies highlight | SVM | Maarseveen et al.[32] (Jun. 2021) | |
| the potential of employing machine learning techniques and EHR to enhance the early diagnosis of RA. | SVM, logistic regression, and AdaBoost | Olatunji, et al.[33] (Sep. 2022) | |
| ANN | Bai et al.[14] (Jun. 2022) | ||
| CSNN | Geng et al.[34] (Aug. 2022) |
CNN, convolutional neural network; SVM, support vector machine; DL, deep learning; DNN, deep neural networks; SVM-RFE, support vector machine-recursive feature elimination; AdaBoost, adaptive boosting; ANN, artificial neural network; CSNN, cost-sensitive neural network; EHRs, electronic health records; PSMB9, proteasome 20S subunit beta type-9; CXCL13, CXC chemokine ligand 13; EHR, electronic health record; ML, machine learning.
Furthermore, AI-based tools have been developed to analyze blood test results and clinical data from EHRs to assist in distinguishing RA from other inflammatory conditions. Many AI tools can help identify biomarkers that are indicative of RA and provide more accurate diagnoses. Mc Ardle et al.[15] developed a random forest model that evaluated serum protein biomarkers and demonstrated good performance in discriminating between patients with RA and those with psoriatic arthritis. The model achieved an area under the curve (AUC) of 0.79 in the initial phase and 0.85 in the subsequent validation phase. The researchers created a robust matching learning feature selection pipeline, which identified new biomarkers for RA in over 2000 blood samples from RA patients.[16]
Overall, by utilizing AI in the diagnostic process, clinicians may be able to identify RA earlier, which can assist specialized physicians in developing tailored treatment strategies to prevent disease progression. This approach enables timely administration of antirheumatic drugs to achieve remission in rheumatoid arthritis patients. However, further research is needed to validate the accuracy and reliability of AI in diagnosing RA and to evaluate its feasibility and cost-effectiveness in clinical settings.
AI in Early Intervention of RA
Early intervention in RA is important to slow down or even prevent the progression of joint damage and other complications associated with the disease, which can potentially bring benefits to both patients and society. Delayed treatment can result in irreversible joint damage, considerably impacting the patient’s ability to perform daily activities and causing long-term disability. Moreover, no treatment that has been demonstrated to be effective in preventing the development of RA, so a pressing requirement exists for the development of new biomarkers to initiate early intervention. Early treatment of non-steroidal anti-inflammatory drugs (NSAIDs) and disease-modifying anti-rheumatic drugs (DMARDs), which can slow or stop the progression of the disease and reduce joint damage. Frazzei et al.[35] conducted a comprehensive systematic literature review and identified 1821 articles. The findings indicated that no treatment could prevent the onset of RA, but administering rituximab and abatacept at an early stage resulted in a delay in the onset of fully developed RA. Another research conducted by van der Linden et al.[36] have demonstrated that starting treatment within the first 12 weeks of the onset of symptoms can lead to better outcomes, such as enhanced physical functioning and quality of life, as well as reduced rates of disability and joint damage. Therefore, treating RA early through appropriate interventions is crucial for improving long outcomes of the patient.
However, the early intervention of RA encounters various challenges, including the complexity of selecting appropriate medications, and the variability in medication response among individuals. In recent years, AI has shown the potential to improve early intervention of RA, as summarized in Table 2, which outlines various ML techniques applied in this context. As mentioned earlier, RA is an autoimmune disease that can affect the whole body, and usually affects joint first. However, the course of the disease can vary among individuals, and non-joint symptoms can be present at the early stages.[37] Many ML methods have been investigated to detect disease progression.[3] In a study conducted by Norgeot et al.,[38] a longitudinal deep learning model was used to predict disease activity in RA using EHR data. Disease activity was classified using the clinical disease activity index (CDAI). The study demonstrated a strong predictive performance, with an area under the curve (AUC) of 0.91 in the test cohort. The most influential predictors for disease activity were found to be CDAI, cortisone injections, and C-reactive protein (CRP) levels.
Table 2.
Summary of the studies of artificial intelligence (AI) in early intervention of rheumatoid arthritis (RA), including authors, data types, methods, and main findings of the same category
| Main findings | Data types | Methods | Author (Month/Year) |
|---|---|---|---|
| ML techniques have demonstrated their value in the field of early intervention in RA by analyzing EHRs, clinical trial data and claims data. These | EHRs, claims data, clinical cohort, RCTs | DL | Norgeot et al.[38] (Mar. 2019) |
| studies have used ML models to predict clinical | NLP | Spencer et al.[42] (Nov. 2021) | |
| outcomes, estimate disease activity scores, predict treatment response, identify predictors of severe COVID-19 outcomes, and cluster comorbidities in RA patients. These findings | |||
| highlight the potential of ML to improve early intervention strategies for RA by leveraging | XGBoost, SVM | Morid et al.[43] (Jul. 2021) | |
| multiple healthcare data sources. | Random forests | Johansson et al.[44] (May. 2021) | |
| Logistic regression | Burns et al.[45] (Nov. 2022) | ||
| Hierarchical clustering, factor analysis, k-means clustering, and network analysis | Crowson et al.[46] (Feb. 2023) | ||
| Factor analysis | England et al.[47] (Feb. 2023) | ||
| Linear regression, lasso and ridge, SVM, random forest, and XGBoost | Koo et al.[48] (Jul. 2021) | ||
| Logistic regression, k-nearest neighbors, naïve Bayes classifier and random forests | Vodencarevic et al.[49] (Feb. 2021) | ||
| ML methods applied to genetic data in RA have demonstrated the potential to predict drug response, uncover molecular mechanisms of | Genetic data | GPR | Guan et al.[50] (Dec. 2019) |
| therapy, identify genetic markers associated | Text mining | Wang et al.[51] (Jul. 2021) | |
| with clinical treatment response outcomes, to specific and treatments. accurately predict | Random forest, SVM | Kim et al.[52] (Oct. 2021) | |
| Random forest | Tao et al.[53] (Oct. 2021) | ||
| Multivariate logistic regression, elastic net, random forest, and SVM | Kim et al.[54] (Oct. 2022) | ||
| Random forest | Lim et al.[55] (Oct. 2022) | ||
| Random forest | Lim et al.[56] (Jan. 2022) | ||
| Random forest | Myasoedova et al.[40] (Jun. 2022) |
DL, deep learning; NLP, natural language processing; SVM, support vector machine; GPR, Gaussian process regression; EHRs, electronic health records; RCTs, randomized controlled trials; XGBoost, eXtreme gradient boosting; COVID-19, coronavirus disease 2019; ML, machine learning.
Additionally, ML have been utilized to predict treatment responses to DMARDs, which classified into conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), biologic disease-modifying antirheumatic drugs (bDMARDs) and targeted synthetic DMARDs (tsDMARDs).[39] The research conducted by Myasoedova et al.[40] is among the initial attempts to use machine learning techniques that combine clinical and genomic information to make personalized predictions about the response to methotrexate in individuals diagnosed with early RA. Along with baseline Disease Activity Score in 28 joints (DAS28) scores, intergenic single nucleotide polymorphisms (SNPs) such as rs12446816, rs13385025, rs113798271, and ATIC (rs2372536) demonstrated high importance (above 60.0) in predicting methotrexate response in RA patients. Yoosuf et al.[41] used multi-omics analyses and ML models including linear model and kernel-based models to identify new biomarkers for the prediction response to anti-TNF treatment, which found pathways influenced by treatment. This information can provide valuable insights to physicians during evaluation and improve the decision-making process.
AI in Disease Management of RA
Aside from early diagnosis and early intervention, proper management of RA is crucial to avoid long-term adverse outcomes, such as functional disability and increased mortality risk.[57] In 2010, the European League Against Rheumatism (EULAR) published updated guidelines for the management of RA with DMARDs. These guidelines cover various aspects of RA management, including the use of conventional DMARDs, biologic DMARDs, and targeted synthetic DMARDs, as well as the timing and sequencing of treatments, monitoring of disease activity, and management of comorbidity.[39] Effective and personalized management of RA is essential to enhance patients’ outcomes and quality of life.
However, poor treatment adherence, low compliance with follow-up appointments, and a lack of chronic disease management systems are common challenges in the effective management of chronic conditions such as RA.[58] This can lead to sub-optimal outcomes, such as increased disease activity, joint damage, and functional disability, as well as reduced quality of life. Various factors that pose challenges to the effective management of RA, such as forgetfulness and misconception about the disease treatment at a patient level, and lack of patient education and support at a healthcare system level. The absence of a chronic disease management system poses a significant challenge in the effective management of RA. First, education is a critical component of early intervention, as it can help patients better understand their condition and the importance of adhering to treatment. In addition, regular monitoring and follow-up are important in early intervention, as they can help to ensure that patients are responding to treatment and that any changes in their condition are identified and addressed promptly.
Overcoming these barriers is crucial for effective RA management. In recent years, AI has shown a potential to improve the disease management of RA through the following avenues (Table 3). One application of AI in RA disease management is monitoring the disease, including scheduling follow-ups, notification for medications, etc. In the past decades, the field of mobile health (mHealth) has made significant advancements, resulting in a multitude of a smartphone (apps) designed for individuals with RA.[59] Gossec et al.[60] utilized data from wearable activity trackers that used machine-generated models for predicting flare sensitivity, and a connected monitoring interface on a smartphone developed by Pers et al.[61] significantly lowered the number of physical visits. ML techniques have the ability to transform RA-related research and enhance disease management. However, these models are not currently prepared to fully contribute to daily practice, many issues like technique and ethical concerns with these methods need to be addressed before their implementation.[62]
Table 3.
Summary of the studies of artificial intelligence (AI) in disease management of rheumatoid arthritis (RA), including authors, data types, methods, and main findings
| Main findings | Data types | Methods | Author (Month/Year) |
|---|---|---|---|
| ML is being used in the field of RA to support dis- | Observational cohort | Bayes, random forests | Gossec et al.[60] (Oct. 2019) |
| ease management. Applications include detecting flares based on physical activity data, predicting flares using ultrasound and blood test data, | Ultrasound images, blood test | Logistic regression, random forest, and XGBoost | Matsuo et al.[63] (May. 2022) |
| extracting results from clinical notes using natural | EHRs | NLP | Humbert-Droz et al.[64] (Mar. 2023) |
| language processing, and developing AI-based | |||
| flare prediction systems. These approaches have the potential to improve disease monitoring in RA. | Clinical cohort | AI-powered RA clinical decision support tool | Labinsky et al.[65] (Jan. 2023) |
NLP, natural language processing; EHRs, electronic health records; XGBoost, eXtreme gradient boosting; ML, machine learning.
Discussion
The objective of this study is to provide a concise overview of the role of AI in RA, specifically in the areas of early diagnosis, early intervention and disease management. Based on our overview, AI is a highly efficient and effective method for analyzing complex data in RA. We summarized various literature on key analyses, advanced methods, relevant strengths in these fields. As shown in Figure 1, ML has been widely used in the field of RA to improve diagnosis and treatment by using diverse types of data. EHRs, genetic data, and imaging data has been used to analyze and classify patients with RA, enabling more accurate and efficient diagnosis. ML techniques have also been used to predict treatment response using genetic data, enabling personalized and targeted interventions. By harnessing the power of ML, healthcare professionals can gain value insights from large datasets, identify patterns and make informed decisions about patients care. The integration of ML in RA has the potential to significantly improve patient outcomes and the overall management of the disease.
Figure 1.
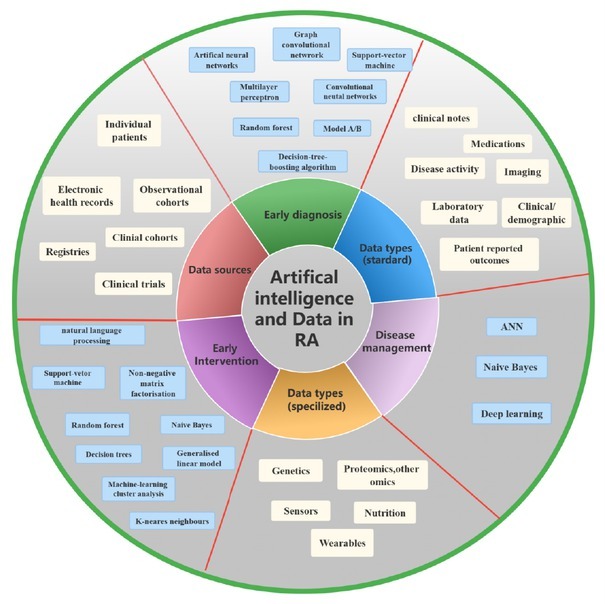
Artificial intelligence (AI) techniques, data types and data sources in early intervention, diagnosis and disease management in rheumatoid arthritis (RA); ANN, artificial neural network.
Despite the potential benefits of applying ML to RA, there are several barriers for wide-spread implementation. A major concern is the accuracy of the health data for ML analysis. Ensuring data quality is crucial for accurate results,[62] but the available data from different sources maybe insufficient or inconsistent. In addition, standard class labels used for classification can be inconsistent between experts and can change over time as the understanding of RA evolves.[66] Another significant obstacle is data security and privacy, particularly for sensitive information contained in EHRs. Decentralized data storage approaches, such as federated learning, have been proposed to minimize the impact of data breaches or hacking incident.[67] However, there remains a risk of privacy attacks on the AI system. AI approaches have been explored in RA research, but few methods were practical in real medical settings. Achieving accurate diagnosis, early intervention and effective disease management in RA presents ongoing challenges.
Future studies of AI in RA should address above challenges. The establishment of a large-scale RA patient cohort with long-term follow-up may allow the development and validation of predictive models for the risk of occurrence and prognosis of major commodities, such as interstitial lung disease, cardiovascular disease, osteoporosis, and fragility fractures. The results of these model can inform the development of early warning strategies, and appropriate interventions, supporting the development of a comprehensive chronic disease prevention and management system for RA. In addition, due to the explosive growth of clinical data and the rapid development of AI, the development of an intelligent fusion analysis platform and the decision support system holds great potential for enhancing the precision and effectiveness of clinical diagnosis and treatment. However, further research is needed to fully develop and evaluate such a system.
Conclusion
Overall, current AI has the potential to detect RA earlier, facilitate early intervention, and better disease management, but further research is needed to validate its accuracy and address ethical concerns.
Funding Statement
This work was supported by the National Key Research and Development Program of China (No. 2022YFC2504605), the National Natural Science Foundation of China (No. 82172069), the Key Research Project of Zhejiang Lab Grants (No. 2022ND0AC01).
Footnotes
Author Contributions
Li J: Conceptualization. Wang J and Tian Y: Original draft. Zhou T, Tong D, Ma J, Li J: Reviewing and editing. Wang J and Tian Y: Visualization. All authors have read and approve the final manuscript.
Informed Consent
Not applicable.
Ethical Statement
Not applicable.
Conflict of Interest
The authors declare no conflict of interest.
Data Sharing
No additional data are available.
References
- [1].Klareskog L, Catrina AI, Paget S. Rheumatoid arthritis. Lancet. 2009;373:659. doi: 10.1016/S0140-6736(09)60008-8. - [DOI] [PubMed] [Google Scholar]
- [2].Avramidis GP, Avramidou MP, Papakostas GA. Rheumatoid arthritis diagnosis: Deep learning vs. humane. Appl Sci. 2022;12:10. [Google Scholar]
- [3].Hügle M, Omoumi P, van Laar JM. Applied machine learning and artificial intelligence in rheumatology. Rheumatol Adv Pract. 2020;4:rkaa005. doi: 10.1093/rap/rkaa005. et al . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Rajpurkar P, Chen E, Banerjee O. AI in health and medicine. Nat Med. 2022;28:31. doi: 10.1038/s41591-021-01614-0. et al . - [DOI] [PubMed] [Google Scholar]
- [5].Stoel B. Use of artificial intelligence in imaging in rheumatology - current status and future perspectives. RMD Open. 2020;6:e001063. doi: 10.1136/rmdopen-2019-001063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Didelot X, Pouwels KB. Machine-learning-assisted selection of antibiotic prescription. Nat Med. 2019;25:1033. doi: 10.1038/s41591-019-0517-0. - [DOI] [PubMed] [Google Scholar]
- [7].Medrano I, Choy E, Gossec L. Artificial Intelligence in Rheumatic Diseases: Can It Solve the Treatment Management Puzzle? EMJ Rheumatol. 2021;8:28. et al . - [Google Scholar]
- [8].Stack RJ, Nightingale P, Jinks C. Delays between the onset of symptoms and first rheumatology consultation in patients with rheumatoid arthritis in the UK: an observational study. BMJ Open. 2019;9:e024361. doi: 10.1136/bmjopen-2018-024361. et al . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9]. https://www.ncbi.nlm.nih.gov/books/NBK384455/ 2023. Accessed at Mar 6.
- [10].Ahalya RK, Snekhalatha U, Dhanraj V. Automated segmentation and classification of hand thermal images in rheumatoid arthritis using machine learning algorithms: A comparison with quantum machine learning technique. J Therm Biol. 2023;111:103404. doi: 10.1016/j.jtherbio.2022.103404. [DOI] [PubMed] [Google Scholar]
- [11].Imtiaz M, Shah SAA. Rahman Z. A review of arthritis diagnosis techniques in artificial intelligence era: Current trends and research challenges. Neurosci Inform. 2022;2:100079. ur. [Google Scholar]
- [12].Nieuwenhuis WP, Krabben A, Stomp W. Evaluation of magnetic resonance imaging-detected tenosynovitis in the hand and wrist in early arthritis. Arthritis Rheumatol. 2015;67:869. doi: 10.1002/art.39000. et al . - [DOI] [PubMed] [Google Scholar]
- [13].Maziarz K, Krason A, Wojna Z. Deep learning for rheumatoid arthritis: Joint detection and damage scoring in x-rays. arXiv. 2021 https://arxiv.org/abs/2104.13915 arXiv:2104.13915. Accessed at Mar 6, 2023. [Google Scholar]
- [14].Bai L Zhang Y Wang P et alImproved diagnosis of rheumatoid arthritis using an artificial neural network Sci Rep 2022129810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mc Ardle A, Kwasnik A, Szentpetery A. Identification and Evaluation of Serum Protein Biomarkers That Differentiate Psoriatic Arthritis From Rheumatoid Arthritis. Arthritis Rheumatol. 2022;74:81. doi: 10.1002/art.41899. et al . - [DOI] [PubMed] [Google Scholar]
- [16].Rychkov D, Neely J, Oskotsky T. Cross-Tissue Transcriptomic Analysis Leveraging Machine Learning Approaches Identifies New Biomarkers for Rheumatoid Arthritis. Front Immunol. 2021;12:638066. doi: 10.3389/fimmu.2021.638066. et al . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hirano T, Nishide M, Nonaka N. Development and validation of a deep-learning model for scoring of radiographic finger joint destruction in rheumatoid arthritis. Rheumatol Adv Pract. 2019;3:rkz047. doi: 10.1093/rap/rkz047. et al . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Üreten K, Erbay H, Maraş HH. Detection of rheumatoid arthritis from hand radiographs using a convolutional neural network. Clin Rheumatol. 2020;39:969. doi: 10.1007/s10067-019-04487-4. - [DOI] [PubMed] [Google Scholar]
- [19].Üreten K, Maraş HH. Automated Classification of Rheumatoid Arthritis, Osteoarthritis, and Normal Hand Radiographs with Deep Learning Methods. J Digit Imaging. 2022;35:193. doi: 10.1007/s10278-021-00564-w. - [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sun D, Nguyen TM, Allaway RJ. A Crowdsourcing Approach to Develop Machine Learning Models to Quantify Radiographic Joint Damage in Rheumatoid Arthritis. JAMA Netw Open. 2022;5:e2227423. doi: 10.1001/jamanetworkopen.2022.27423. et al . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Izumi K, Suzuki K, Hashimoto M. Detecting hand joint ankylosis and subluxation in radiographic images using deep learning: A step in the development of an automatic radiographic scoring system for joint destruction. PLoS One. 2023;18:e0281088. doi: 10.1371/journal.pone.0281088. et al . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Miyama K, Bise R, Ikemura S. Deep learning-based automatic-bone-destruction-evaluation system using contextual information from other joints. Arthritis Res Ther. 2022;24:227. doi: 10.1186/s13075-022-02914-7. et al . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wang HJ, Su CP, Lai CC. Deep Learning-Based Computer-Aided Diagnosis of Rheumatoid Arthritis with Hand X-ray Images Conforming to Modified Total Sharp/van der Heijde Score. Biomedicines. 2022;10:1355. doi: 10.3390/biomedicines10061355. et al . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hemalatha RJ, Vijaybaskar V, Thamizhvani TR. Automatic localization of anatomical regions in medical ultrasound images of rheumatoid arthritis using deep learning. Proc Inst Mech Eng H. 2019;233(6):657. doi: 10.1177/0954411919845747. - [DOI] [PubMed] [Google Scholar]
- [25].Fiorentino MC, Cipolletta E, Filippucci E. A deep-learning framework for metacarpal-head cartilage-thickness estimation in ultrasound rheumatological images. Comput Biol Med. 2022;141:105117. doi: 10.1016/j.compbiomed.2021.105117. et al . [DOI] [PubMed] [Google Scholar]
- [26].Wu M, Wu H, Wu L. A deep learning classification of metacarpophalangeal joints synovial proliferation in rheumatoid arthritis by ultrasound images. J Clin Ultrasound. 2022;50:296–301. doi: 10.1002/jcu.23143. et al . [DOI] [PubMed] [Google Scholar]
- [27].Chen Y, Liao R, Yao Y. Machine learning to identify immune-related biomarkers of rheumatoid arthritis based on WGCNA network. Clin Rheumatol. 2022;41:1057. doi: 10.1007/s10067-021-05960-9. et al . - [DOI] [PubMed] [Google Scholar]
- [28].Yu R, Zhang J, Zhuo Y. Identification of Diagnostic Signatures and Immune Cell Infiltration Characteristics in Rheumatoid Arthritis by Integrating Bioinformatic Analysis and Machine-Learning Strategies. Front Immunol. 2021;12:724934. doi: 10.3389/fimmu.2021.724934. et al . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Li Z, Chen Y, Zulipikaer M. Identification of PSMB9 and CXCL13 as Immune-related Diagnostic Markers for Rheumatoid Arthritis by Machine Learning. Curr Pharm Des. 2022;28:2842. doi: 10.2174/1381612828666220831085608. et al . - [DOI] [PubMed] [Google Scholar]
- [30].Lim AJW, Tyniana CT, Lim LJ. Robust SNP-based prediction of rheumatoid arthritis through machine-learning-optimized polygenic risk score. J Transl Med. 2023;21:92. doi: 10.1186/s12967-023-03939-5. et al . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lötsch J, Alfredsson L, Lampa J. Machine-learning-based knowledge discovery in rheumatoid arthritis-related registry data to identify predictors of persistent pain. Pain. 2020;161:114. doi: 10.1097/j.pain.0000000000001693. - [DOI] [PubMed] [Google Scholar]
- [32].Maarseveen TD, Maurits MP, Niemantsverdriet E. Handwork vs machine: a comparison of rheumatoid arthritis patient populations as identified from EHR free-text by diagnosis extraction through machine-learning or traditional criteria-based chart review. Arthritis Res Ther. 2021;23:174. doi: 10.1186/s13075-021-02553-4. et al . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Olatunji SO, Alansari A, Alkhorasani H. A Novel Ensemble-Based Technique for the Preemptive Diagnosis of Rheumatoid Arthritis Disease in the Eastern Province of Saudi Arabia Using Clinical Data. Comput Math Methods Med. 2022;2022:2339546. doi: 10.1155/2022/2339546. et al . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Geng L, Qu W, Wang S. Prediction of diagnosis results of rheumatoid arthritis patients based on autoantibodies and costsensitive neural network. Clin Rheumatol. 2022;41:2329. doi: 10.1007/s10067-022-06109-y. et al . - [DOI] [PubMed] [Google Scholar]
- [35].Frazzei G, Musters A, de Vries N. Prevention of rheumatoid arthritis: A systematic literature review of preventive strategies in at-risk individuals. Autoimmun Rev. 2023;22:103217. doi: 10.1016/j.autrev.2022.103217. et al . [DOI] [PubMed] [Google Scholar]
- [36].van der Linden MP, le Cessie S, Raza K. Long-term impact of delay in assessment of patients with early arthritis. Arthritis Rheum. 2010;62:3537. doi: 10.1002/art.27692. et al . - [DOI] [PubMed] [Google Scholar]
- [37].Heidari B. Rheumatoid Arthritis: Early diagnosis and treatment outcomes. Caspian J Intern Med. 2011;2:161. - [PMC free article] [PubMed] [Google Scholar]
- [38].Norgeot B, Glicksberg BS, Trupin L. Assessment of a Deep Learning Model Based on Electronic Health Record Data to Forecast Clinical Outcomes in Patients With Rheumatoid Arthritis. JAMA Netw Open. 2019;2:e190606. doi: 10.1001/jamanetworkopen.2019.0606. et al . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Smolen JS, Landewé RBM, Bijlsma JWJ. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79:685. doi: 10.1136/annrheumdis-2019-216655. et al . - [DOI] [PubMed] [Google Scholar]
- [40].Myasoedova E, Athreya AP, Crowson CS. Toward Individualized Prediction of Response to Methotrexate in Early Rheumatoid Arthritis: A Pharmacogenomics-Driven Machine Learning Approach. Arthritis Care Res. 2022;74:879. doi: 10.1002/acr.24834. et al . - [DOI] [PubMed] [Google Scholar]
- [41].Yoosuf N, Maciejewski M, Ziemek D. Early prediction of clinical response to anti-TNF treatment using multi-omics and machine learning in rheumatoid arthritis. Rheumatology (Oxford) 2022;61:1680. doi: 10.1093/rheumatology/keab521. et al . - [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Spencer AK, Bandaria J, Leavy MB. Validation of a machine learning approach to estimate Clinical Disease Activity Index Scores for rheumatoid arthritis. RMD Open. 2021;7:e001781. doi: 10.1136/rmdopen-2021-001781. et al . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Morid MA, Lau M, Del Fiol G. Predictive analytics for step-up therapy: Supervised or semi-supervised learning? J Biomed Inform. 2021;119:103842. doi: 10.1016/j.jbi.2021.103842. [DOI] [PubMed] [Google Scholar]
- [44].Johansson FD, Collins JE, Yau V. Predicting Response to Tocilizumab Monotherapy in Rheumatoid Arthritis: A Real-world Data Analysis Using Machine Learning. J Rheumatol. 2021;48:1364. doi: 10.3899/jrheum.201626. et al . - [DOI] [PubMed] [Google Scholar]
- [45].Burns SM, Woodworth TS, Icten Z. A Machine Learning Approach to Identify Predictors of Severe COVID-19 Outcome in Patients With Rheumatoid Arthritis. Pain Physician. 2022;25:593. et al . - [PubMed] [Google Scholar]
- [46].Crowson CS, Gunderson TM, Davis JM. Using Unsupervised Machine Learning Methods to Cluster Comorbidities in a Population-Based Cohort of Patients With Rheumatoid Arthritis. Arthritis Care Res (Hoboken) 2023;75:210. doi: 10.1002/acr.24973. 3rd. et al . - [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].England BR, Yang Y, Roul P. Identification of Multimorbidity Patterns in Rheumatoid Arthritis Through Machine Learning. Arthritis Care Res (Hoboken) 2023;75:220. doi: 10.1002/acr.24956. et al . - [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Koo BS, Eun S, Shin K. Machine learning model for identifying important clinical features for predicting remission in patients with rheumatoid arthritis treated with biologics. Arthritis Res Ther. 2021;23:178. doi: 10.1186/s13075-021-02567-y. et al . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Vodencarevic A, Tascilar K, Hartmann F. Advanced machine learning for predicting individual risk of flares in rheumatoid arthritis patients tapering biologic drugs. Arthritis Res Ther. 2021;23:67. doi: 10.1186/s13075-021-02439-5. et al . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Guan Y, Zhang H, Quang D. Machine Learning to Predict Anti-Tumor Necrosis Factor Drug Responses of Rheumatoid Arthritis Patients by Integrating Clinical and Genetic Markers. Arthritis Rheumatol. 2019;71:1987. doi: 10.1002/art.41056. et al . - [DOI] [PubMed] [Google Scholar]
- [51].Wang Q, Fan Z, Li J. Systematic analysis of the molecular mechanisms of methotrexate therapy for rheumatoid arthritis using text mining. Clin Exp Rheumatol. 2021;39:829. et al . - [PubMed] [Google Scholar]
- [52].Kim W, Kim TH, Oh SJ. Association of TLR 9 gene polymorphisms with remission in patients with rheumatoid arthritis receiving TNF-α inhibitors and development of machine learning models. Sci Rep. 2021;11:20169. doi: 10.1038/s41598-021-99625-x. et al . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Tao W, Concepcion AN, Vianen M. Multiomics and Machine Learning Accurately Predict Clinical Response to Adalimumab and Etanercept Therapy in Patients With Rheumatoid Arthritis. Arthritis Rheumatol. 2021;73:212. doi: 10.1002/art.41516. et al . - [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Kim W, Jin Oh S, Thi Trinh N. Effects of RETN polymorphisms on treatment response in rheumatoid arthritis patients receiving TNF-α inhibitors and utilization of machine-learning algorithms. Int Immunopharmacol. 2022;111:109094. doi: 10.1016/j.intimp.2022.109094. et al . [DOI] [PubMed] [Google Scholar]
- [55].Lim LJ, Lim AJW, Ooi BNS. Machine learning using genetic and clinical data identifies a signature that robustly predicts methotrexate response in rheumatoid arthritis. Rheumatology (Oxford) 2022;61:4175. doi: 10.1093/rheumatology/keac032. et al . - [DOI] [PubMed] [Google Scholar]
- [56].Lim AJW, Lim LJ, Ooi BNS. Functional coding haplotypes and machine-learning feature elimination identifies predictors of Methotrexate Response in Rheumatoid Arthritis patients. EBio-Medicine. 2022;75:103800. doi: 10.1016/j.ebiom.2021.103800. et al . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Aletaha D, Smolen JS. Diagnosis and Management of Rheumatoid Arthritis: A Review. JAMA. 2018;320:1360. doi: 10.1001/jama.2018.13103. - [DOI] [PubMed] [Google Scholar]
- [58].Fernandez-Lazaro CI, García-González JM, Adams DP. Adherence to treatment and related factors among patients with chronic conditions in primary care: a cross-sectional study. BMC Fam Pract. 2019;20:132. doi: 10.1186/s12875-019-1019-3. et al . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Druce KL, Dixon WG, McBeth J. Maximizing Engagement in Mobile Health Studies: Lessons Learned and Future Directions. Rheum Dis Clin North Am. 2019;45:159. doi: 10.1016/j.rdc.2019.01.004. - [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Gossec L, Guyard F, Leroy D. Detection of Flares by Decrease in Physical Activity, Collected Using Wearable Activity Trackers in Rheumatoid Arthritis or Axial Spondyloarthritis: An Application of Machine Learning Analyses in Rheumatology. Arthritis Care Res (Hoboken) 2019;71:1336. doi: 10.1002/acr.23768. et al . - [DOI] [PubMed] [Google Scholar]
- [61].Pers YM, Valsecchi V, Mura T. A randomized prospective open-label controlled trial comparing the performance of a connected monitoring interface versus physical routine monitoring in patients with rheumatoid arthritis. Rheumatology (Oxford) 2021;60:1659. doi: 10.1093/rheumatology/keaa462. et al . - [DOI] [PubMed] [Google Scholar]
- [62].Kedra J, Davergne T, Braithwaite B. Machine learning approaches to improve disease management of patients with rheumatoid arthritis: review and future directions. Expert Rev Clin Immunol. 2021;17:1311. doi: 10.1080/1744666X.2022.2017773. et al . - [DOI] [PubMed] [Google Scholar]
- [63].Matsuo H, Kamada M, Imamura A. Machine learning-based prediction of relapse in rheumatoid arthritis patients using data on ultrasound examination and blood test. Sci Rep. 2022;12:7224. doi: 10.1038/s41598-022-11361-y. et al . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Humbert-Droz M, Izadi Z, Schmajuk G. Development of a Natural Language Processing System for Extracting Rheumatoid Arthritis Outcomes From Clinical Notes Using the National Rheumatology Informatics System for Effectiveness Registry. Arthritis Care Res (Hoboken) 2023;75:608. doi: 10.1002/acr.24869. et al . - [DOI] [PubMed] [Google Scholar]
- [65].Labinsky H, Ukalovic D, Hartmann F. An AI-Powered Clinical Decision Support System to Predict Flares in Rheumatoid Arthritis: A Pilot Study. Diagnostics (Basel) 2023;13:148. doi: 10.3390/diagnostics13010148. et al . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Kingsmore KM, Puglisi CE, Grammer AC. An introduction to machine learning and analysis of its use in rheumatic diseases. Nat Rev Rheumatol. 2021;17:710. doi: 10.1038/s41584-021-00708-w. et al . - [DOI] [PubMed] [Google Scholar]
- [67].Nguyen TV, Dakka MA, Diakiw SM. A novel decentralized federated learning approach to train on globally distributed, poor quality, and protected private medical data. Sci Rep. 2022;12:8888. doi: 10.1038/s41598-022-12833-x. et al . [DOI] [PMC free article] [PubMed] [Google Scholar]


