Abstract
Background
Garlic (Allium sativum), the underground bulb of the Allium genus, has been consumed on Earth for thousands of years. Many clinical trials of garlic supplementation on components of metabolic syndrome (MetS) have emerged in recent years, but there is no consensus on the effect. This meta-analysis aimed at systematically evaluating the effect of garlic supplementation on components of MetS.
Methods
In this meta-analysis, we searched Pubmed, Embase, Cochrane, Medline, Web of Science databases, and clinical trials online sites from inception to November 1, 2022, with language restrictions to English. We engaged participants > 18 years and eligible for the clinical diagnosis of MetS or those with metabolic disorders and garlic was the only intervention. Outcomes included waist circumference, and body mass index, triglycerides, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, blood pressure, and fasting blood glucose. Meta-regression and subgroup analyses were conducted based on six covariates (total sample size, the mean age, the mean dose, the duration of intervention, the oral form of garlic, and the dietary intervention).
Results
Results from 19 RCTs were included engaging 999 participants. Compared to placebo, garlic significantly reduced TG [SMD (95%CI) = -0.66 (-1.23, -0.09)], TC [SMD (95%CI) = -0.43 (-0.86, -0.01)], LDL [SMD (95%CI) = -0.44(-0.88, -0.01)], DBP [SMD (95%CI) = -1.33 (-2.14, -0.53)], BMI [SMD (95%CI) = -1.10(-1.90, -0.20)], and WC [SMD (95%CI) = -0.78(-1.09, -0.47)]. Meta-regression showed age and sample size are potential effect modifiers.
Conclusion
According to the results of meta-analysis, the modulatory effect of garlic on some MetS components is evident. More high-quality, large-scale RCTs are needed to confirm iat based on the high heterogeneity and potential publication bias of the current data.
Trial registration
https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=373228, ID: CRD42022373228.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12906-023-04038-0.
Keywords: Garlic, Metabolic syndrome, Systematic review, AMETA-analysis, Randomized controlled trials
Introduction
Metabolic syndrome(MetS) is a condition of metabolic disorder in which central obesity, dyslipidemia, insulin resistance, and hypertension exist as a cluster [1]. As a breeding ground for various serious diseases (cardiovascular disease, type 2 diabetes, polycystic ovary syndrome) [2–4], the four major components of the MetS were previously named by scientists as the "deadly quartet" [5]. Pathophysiological mechanisms such as inflammation, endoplasmic reticulum stress, activation of the renin angiotensin aldosterone system, and dysbiosis of the intestinal flora are intertwined in the formation of MetS [6–9]. Regions around the world are witnessing the epidemic of MetS, with an adult prevalence of approximately 35% in the United States and 14% in China [10], and the number continues to grow at an alarming rate. Currently, no comprehensive treatment and management plan is available for MetS, and modification of dietary habits is a simple, feasible, and efficient intervention.
Garlic (Allium sativum), the underground bulb of the Allium genus, originated in Central Asia and the Mediterranean region thousands of years ago and is nowadays loved by people worldwide [11]. Many researchers hypothesize and verify that the high concentration of sulfur-containing bioactive compounds(alliin, allicin, S-allyl cysteine, and diallyl trisulfide) in its bulb can alleviate oxidative stress, apoptosis, inflammation, and vascular remodeling to ameliorate MetS [11, 12]. And the growing clinical evidence also suggests that the effect of garlic and its extracts on MetS [13]. A. A. Sangouni, et al. conducted an RCT finding that garlic lowered metabolic components, insulin resistance, fatty liver index and appetite in patients with MetS [14]. Meanwhile, a systematic review negated the lipid-lowering effect of garlic [15]. Therefore, no consistent conclusion has been reached and it is necessary to employ thorough meta-analysis on relevant RCTs to quantitatively evaluate the effect of garlic on MetS components.
Materials and methods
This meta-analysis and systematic evaluation were performed in strict compliance with the Cochrane Handbook [16] and registered in PROSPERO (ID: CRD42022373228). The results were presented according to the 27 entries listed in the PRISMA checklist. (Supplementary Table S1) Literature retrieval, inclusion, data extraction, and quality evaluation were performed by two researchers simultaneously and independently. Any discrepancy was resolved through discussion or consulting a third researcher to reach a consensus.
Information sources and retrieval strategy
Two members independently searched Pubmed, Embase, COCHRANE, Medline, and Web of science, with the time frame limited to the date of establishment to November 1, 2022, and the publication language limited to English. The search strategy used a combination of subject words plus free words, including garlic, allicin, metabolic syndrome, hypertension, hyperlipidemia, insulin resistance, waist circumference, and body mass index. (Supplementary Data 1) Additional searches were conducted at the National Institutes of Health (http://clinicaltrials.gov/) and the WHO International Clinical Trials Registry Platform (www.who.int/clinical-trials-registry-platform) to find ongoing clinical studies. References in relevant published systematic reviews or reviews were also scanned to reduce omissions.
Eligibility criteria
The inclusion criteria for this study were strictly based on the PICOS principles and contained the following entries. 1) Participants: participants > 18 years and eligible for the clinical diagnosis of MetS or those with a state of disordered glucose, lipid, and blood pressure, the diagnostic criteria for MetS can be derived from any of the international authoritative organizations; 2) Intervention: garlic and its derivatives or extracts (row garlic, allicin, produced garlic powder, aged garlic) as the only intervention; 3) Control: placebo with no evidence to affect outcomes; 4) Outcomes: include one or more of the following indices: waist circumference (WC), and body mass index (BMI), triglycerides (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-c), high-density lipoprotein cholesterol(HDL-c), blood pressure (SBP, DBP), and fasting blood glucose (FBG); 5) Study: parallel or crossover RCTs.
Exclusion criteria were: 1) The control group received non-pharmacological therapies such as exercise; 2) Participants with heart disease, chronic kidney disease, gastrointestinal disorders, participants who are pregnant or breastfeeding, or participants who smoke or abuse alcohol; 3) Taking other medications that impact weight, blood pressure, lipids, and blood glucose; 4) Outcomes are not available; 5) Reviews, commentaries, conference abstracts, and case reports.
Data extraction and quality evaluation
We entered the following information in the standardized data extraction form: 1) Basic information: first author's name, nationality, institution, and year of publication; 2) Baseline information: sample size, male/female ratio, mean age, health status, and baseline disease; 3) Trial information: the oral form of garlic, placebo composition, dose, duration of intervention and dietary intervention; 4) Outcomes: WC, BMI, TG, TC, LDL-c, HDL-c, FBG, SBP, DBP. 5) Trial process: randomization method, implementation of allocation concealment, blinded format.
Included studies were independently evaluated according to the criteria of the Cochrane Handbook (version 5.1.0). The evaluation entries included: the generation of random sequences, allocation concealment, blinding, selection bias, incomplete outcome information, selective reporting of outcomes, and other sources of bias. The risk of bias for each entry was evaluated as "low risk," "high risk," and "unclear risk".
Data process and analysis
Data analysis was conducted according to the statistical guidelines referenced in the current version of the Cochrane Handbook. To avoid errors caused by unit inconsistency, we used Cohen's standardized mean difference (SMD) to evaluate the effect value [16]. The cut-off values of 0.2, 0.5, and 0.8 were used to divide SMD, corresponding to low, medium, and high effects. For data measured multiple times, the most recent data after the trial completion was selected. For studies with multiple intervention dose groups, the highest-dose group was selected. For presenting data results as means and standard deviations, they were transformed into means and standard deviations of pre- and post-intervention differences. For quartile data, they were transformed into mean and standard deviation format using the method developed by Hozo SP [17]. For crossover RCTs, the first-stage data were extracted to avoid the cumulative effects on the later results.
Data analysis was performed by Review Manager 5.4, Stata17 (StataCorp LP, College Station, US), and R 4.2.1. Data in this study were all continuous variables and effect sizes were presented as SMD and 95% CI. Low, medium, and high levels of heterogeneity were decided by the I2 statistic of 25%, 50%, and 75%. If I2 > 50%, significant heterogeneity was indicated, and the effect sizes were combined using a random-effects model. Subgroups were divided based on total sample size, the mean age of the intervention group, the mean daily dose of garlic, the duration of intervention, and the oral form of garlic. Meta-regression and subgroup analysis were performed to detect and elucidate the sources of high heterogeneity. Sensitivity analysis was performed to screen the RCT’s impact on the robustness of the results. Contour-enhanced funnel plot and Egger’s test were used to detect publication bias. In the Contour-enhanced funnel plot, the dark to light gray represents 90%, 95%, and 99% CIs, and the white area in the middle is the invalid interval. If most of the scatter falls in the white interval, we consider that no publication bias of negative results exists [18].
Quality of evidence and GRADE approach
The evidence for each index of the meta-analysis was assessed based on the five entries (risk of bias, inconsistency, indirectness, imprecision, publication bias) listed in the GRADE (Grading of Recommendations Assessment, Development and Evaluation) handbook (https://gdt.gradepro.org/app/handbook/handbook.html) and classified into four levels: high quality, moderate quality, low quality, and very low quality [19]. This process was performed on the GRADEpro GDT online website (https://gdt.gradepro.org).
Results
Research screening and description
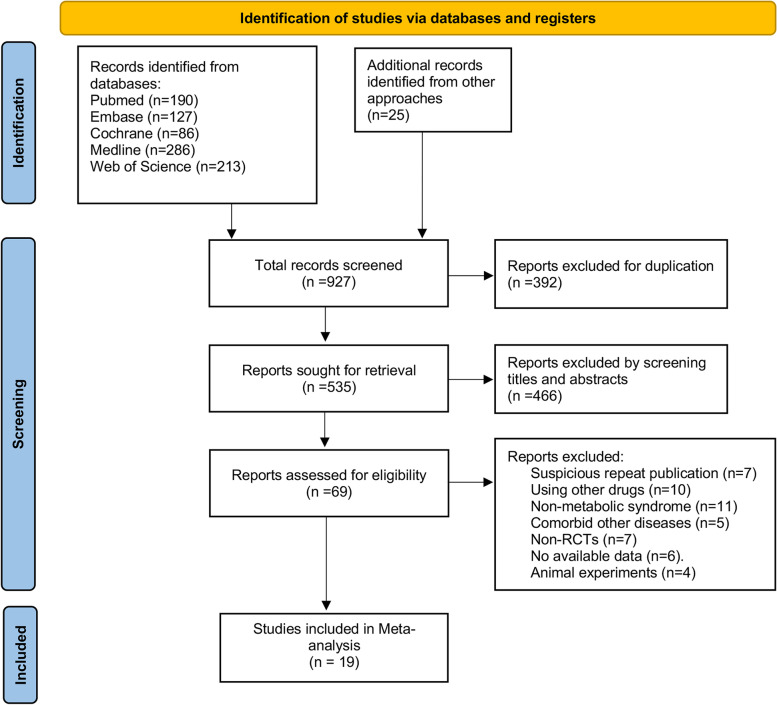
A total of 927 papers were retrieved, and 392 duplications were removed. 466 irrelevant papers were excluded after reading the titles and abstracts. We read the full text of the remaining 69 articles, and 50 papers were excluded for various reasons. The remaining 19 papers were included in the meta-analysis (Fig. 1).
Fig. 1.
Flow chart of literature screening
Basic characterization and quality evaluation
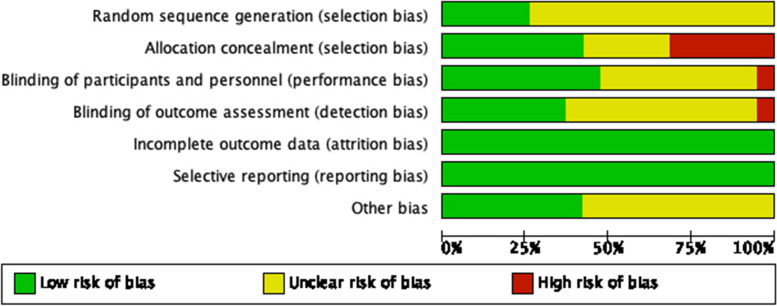
A total of 19 RCTs were included [14, 20–37], including 17 in a parallel design [14, 20–34, 36] and 2 in a crossover design [35, 37]. The total number of participants was 999, including 497 in the treatment group versus 502 in the control group. The mean age fluctuated from 39 to 63. Eight studies were conducted in Asia [14, 21, 25, 28, 30, 31, 33, 34], five in North America [20, 24, 26, 27, 36], and three each in Oceania [29, 32, 35] and Europe [22, 23, 37]. Baseline diseases included hyperlipidemia, hypertension, MetS, and nonalcoholic fatty liver disease. One treatment group used raw garlic orally [21], three used aged garlic extract [28, 32, 37], one used processed garlic [25], and the remainder used garlic powder tablets. The intervention doses of garlic powder ranged from 188 to 2400 mg. The duration of each group ranged from 6 to 24 weeks (Table 1). The results of the quality evaluation of each RCT are shown below (Fig. 2).
Table 1.
Basic information of the included randomized controlled trials
| Author year | Country | Trial design | Sample | Mean age | Baseline disease | Intervention | Dosage | Duration | Outcomes | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T | C | T | C | T | C | mg/d | /w | |||||
| Adler, A. J.,1997 [20] | Canada | parallel | 11 | 11 | 45.9 | 45.4 | hyperlipidemia | GT | placebo | 900 | 12 | TG,TC,LDL,HDL |
| Aslani, N.,2016 [21] | Iran | parallel | 27 | 28 | 45.3 | 39.3 | hyperlipidemia | RG | none | 8 | BMI,TG,TC,LDL,HDL | |
| Auer 1990 [22] | Germany | parallel | 24 | 23 | NR | NR | hypertension | GT | placebo | 600 | 12 | SBP,DBP |
| Byrne, DJ.,1999 [23] | UK | parallel | 20 | 11 | NR | NR | hyperlipidemia | GT | placebo | 900 | 24 | TG,TC,LDL,HDL |
| Gardner, CD.,2001 [24] | USA | parallel | 16 | 18 | 50.2 | 51.6 | hyperlipidemia | GT | placebo | 999 | 12 | TG,TC,LDL,HDL |
| Higashikawa, F., 2012 [25] | Japan | parallel | 28 | 26 | 52 | 51.4 | hyperlipidemia | PG | placebo | 900 | 12 | BMI,TG,TC,LDL,HDL,FBG |
| Isaacsohn, JL.,1998 [26] | USA | parallel | 24 | 18 | 58 | 57.4 | hyperlipidemia | GT | placebo | 900 | 12 | BMI,TG,TC,LDL,HDL,SBP,DBP |
| Jain, AK.,1993 [27] | USA | parallel | 20 | 22 | 48 | 55 | hyperlipidemia | GT | placebo | 900 | 12 | TG,TC,LDL,HDL,FBG,SBP,DBP |
| Jung, ES.,2014 [28] | Korea | parallel | 28 | 27 | 50.13 | 50.83 | hyperlipidemia | AGE | placebo | 12 | TG,TC,LDL,HDL | |
| Kannar, D.,2001 [29] | Australia | parallel | 19 | 22 | 52.6 | 57.4 | hyperlipidemia | GT | placebo | 800 | 12 | TG,TC,LDL,HDL |
| Nakasone,Yasushi, 2013 [30] | Japan | parallel | 19 | 21 | 58 | 59 | hypertension | GT | placebo | 188 | 12 | SBP,DBP |
| Peleg, A.,2003 [31] | Israel | parallel | 13 | 20 | 52.4 | 54.7 | hyperlipidemia | GT | placebo | 16 | TG,TC,LDL,HDL | |
| Riad, Karin,2018 [32] | Australia | parallel | 23 | 26 | 62.8 | 61.9 | hypertension | AGE | placebo | 2400 | 12 | WC,BMI,TG,LDL,HDL,FBG,SBP,DBP |
| Sangouni, Abbas A,2020 [33] | Iran | parallel | 45 | 43 | 45.2 | 44.2 | NAFLD | GT | placebo | 1600 | 12 | WC,BMI,TG,TC,LDL,HDL |
| Sangouni, Abbas A,2021 [14] | Iran | parallel | 42 | 42 | 46.9 | 44.6 | MetS | GT | placebo | 1600 | 12 | TG,TC,LDL,HDL |
| Sharifi, F.,2010 [34] | Iran | parallel | 20 | 20 | 47.9 | 50.5 | MetS | GT | placebo | 1800 | 6 | WC,BMI,TG,TC,HDL,FBG,SBP,DBP |
| Simons, LA.,1995 [35] | Australia | crossover | 12 | 17 | 53.6 | 53.6 | hyperlipidemia | GT | placebo | 900 | 12 | TG,TC,LDL,HDL,SBP,DBP |
| Superko, HR. 2000 [36] | USA | parallel | 25 | 25 | 53 | 53 | hyperlipidemia | GT | placebo | 900 | 12 | SBP,DBP |
| Valls, RM.,2022 [37] | Spain | crossover | 32 | 34 | 53.7 | 52.7 | hyperlipidemia | AGE | placebo | 250 | 6 | SBP,DBP |
T Treatment group, C Control group, NR: not reported, NAFLD Non-alcoholic fatty liver disease, MetS Metabolic syndrome, GT Garlic tablet, RG Row garlic, PG Processed garlic, AGE Aged garlic extract, WC Waist circumference, BMI Body mass index, TG Triglycerides, TC Total cholesterol, LDL-c Low-density lipoprotein cholesterol, HDL-c High-density lipoprotein cholesterol, FBG Fasting blood glucose, SBP Systolic blood pressure, DBP Diastolic blood pressure
Fig. 2.
Risk of bias of assessment
Effect of garlic on the components of MetS
Effect of garlic on anthropometric indices
The results showed that BMI [SMD (95%CI) = -1.10(-1.90, -0.20), p < 0.05, I2 = 92.1%, 6 trials, 324 participants], and WC [SMD (95%CI) = -0.78(-1.09, -0.47), p < 0.05, I2 = 0.00, 3trials, 173 participants] were significantly lower in the treatment group (Table 2 and 3).
Table 2.
Meta-analysis of the effect of garlic on WC
Table 3.
Meta-analysis of the effect of garlic on BMI
| Study | SMD (95% CI) | Weight % |
|---|---|---|
| Higashikawa, Fumiko., 2012 [25] | -0.35 (-0.89, 0.19) | 20.96 |
| Isaacsohn, JL., 1998 [26] | -0.08 (-0.69, 0.53) | 20.5 |
| Riad, Karin., 2018 [32] | -0.73 (-1.17, -0.29) | 21.48 |
| Sangouni, Abbas Ali., 2021 [14] | -0.35 (-0.78, 0.07) | 21.58 |
| Sharifi, F., 2010 [34] | -5.00 (-6.28, -3.72) | 15.47 |
| Aslani, N., 2016 [21] | (excluded) | 0 |
| Overall (I-squared = 92.1%, p=0.000) | 1.10 (-1.99, -0.20) | 100 |
Effect of garlic on blood lipids
In random effects model, we found that garlic supplementation was associated with significant reductions in TG [SMD (95%CI) = -0.66 [-1.23, -0.09], p < 0.05,15trials, 701 participants], TC [SMD (95%CI) = -0.43 (-0.86, -0.01), p < 0.05,14trials, 617 participants], and LDL [SMD (95%CI) = -0.44(-0.88, -0.01), p < 0.05, 14trials, 577 participants]. We also observed a slight rise of HDL in the treatment group [SMD (95%CI) = 0.1(-0.28, 0.48), p = 0.59, 15 trials, 701 participants] with no significance (Tables 4, 5, 6 and 7).
Table 4.
Meta-analysis of the effect of garlic on TG
| Study | SMD (95% CI) | Weight % |
|---|---|---|
| Adler, A. J., 1997 [20] | -3.15 (-4.44, -1.87) | 5.11 |
| Aslani, N., 2016 [21] | -0.54 (-1.07, 0.00) | 7.03 |
| Byrne, DJ., 1999 [23] | -0.09 (-0.82, 0.65) | 6.57 |
| Gardner, CD., 2001 [24] | -0.13 (-0.70, 0.44) | 6.96 |
| Higashikawa, Fumiko., 2012 [25] | -0.33 (-0.87, 0.21) | 7.03 |
| Isaacsohn, JL., 1998 [26] | -0.30 (-0.91, 0.32) | 6.86 |
| Jain, AK., 1993 [27] | 0.10 (-0.50, 0.71) | 6.88 |
| Jung, ES.,2014 [28] | -0.40 (-0.93, 0.14) | 7.04 |
| Kannar, D., 2001 [29] | 0.54 (-0.09, 1.17) | 6.84 |
| Peleg, A., 2003 [31] | 0.90 (-0.14, 1.94) | 5.77 |
| Riad, Karin., 2018 [32] | -0.93 (-1.38, -0.48) | 7.2 |
| Sangouni, Abbas Ali., 2021 [14] | -3.19 (-3.83, -2.56) | 6.82 |
| Sangouni, Abbas Ali., 2020 [33] | -0.34 (-0.90, 0.22) | 6.98 |
| Sharifi, F., 2010 [34] | -2.35 (-3.17, -1.54) | 6.37 |
| Simons, LA., 1995 [35] | 0.25 (-0.99, 0.49) | 6.56 |
| Overall (I-squared = 89.5%, p=0.000) | -0.66 (-1.17, -0.16) | 100 |
Table 5.
Meta-analysis of the effect of garlic on TC
| Study | SMD (95% CI) | Weight % |
|---|---|---|
| Adler, A. J., 1997 [20] | -20.48 (-26.89, -14.08) | 0.42 |
| Aslani, N., 2016 [21] | -1.09 (-1.66, -0.52) | 8.61 |
| Byrne, DJ., 1999 [23] | -0.48 (-1.23, 0.26) | 7.75 |
| Gardner, CD., 2001 [24] | -0.25 (-0.83, 0.32) | 8.59 |
| Higashikawa, Fumiko., 2012 [25] | -0.83 (-1.38, -0.27) | 8.66 |
| Isaacsohn, JL., 1998 [26] | 0.19 (-0.43, 0.80) | 8.4 |
| Jain, AK., 1993 [27] | -0.37 (-0.99, 0.24) | 8.41 |
| Jung, ES., 2014 [28] | -0.23 (-0.76, 0.30) | 8.78 |
| Kannar, D., 2001 [29] | -0.66 (-1.29, -0.03) | 8.31 |
| Peleg, A., 2003 [31] | 0.20 (-0.79, 1.19) | 6.55 |
| Sangouni, Abbas Ali., 2021 [14] | -0.93 (-1.37, -0.49) | 9.17 |
| Sangouni, Abbas Ali., 2020 [33] | -0.16 (-0.72, 0.39) | 8.67 |
| Simons, LA., 1995 [35] | 0.71 (-0.05, 1.47) | 7.67 |
| Sharifi, F., 2010 [34] | (excluded) | 0 |
| Overall (I-squared = 81.9%, p=0.000) | -0.43 (-0.86, -0.01) | 100 |
Table 6.
Meta-analysis of the effect of garlic on LDL
| Study | SMD (95% CI) | Weight % |
|---|---|---|
| Adler, A. J., 1997 [20] | -9.33 (-12.34, -6.32) | 1.74 |
| Aslani, N., 2016 [21] | -0.99 (-1.55, -0.3) | 8.52 |
| Byrne, DJ., 1999 [23] | -0.31 (-1.05, 0.43) | 7.72 |
| Gardner, CD., 2001 [24] | -0.18 (-0.76, 0.39) | 8.7 |
| Higashikawa, Fumiko., 2012 [25] | -1.11 (-11.68, -0.53) | 8.46 |
| Isaacsohn, JL., 1998 [26] | 0.22 (-0.39, 0.84) | 8.3 |
| Jain, AK., 1993 [27] | -0.39 (-1.01, 0.22) | 8.3 |
| Jung, ES., 2014 [28] | -0.05 (-0.58, 0.48) | 8.66 |
| Kannar, D., 2001 [29] | -0.87 (-1.51, -0.23) | 8.16 |
| Peleg, A., 2003 [31] | 0.17 (-0.82, 1.16) | 6.57 |
| Sangouni, Abbas Ali., 2021 [14] | -0.75 (-1.18, -0.32) | 9.04 |
| Sangouni, Abbas Ali., 2020 [33] | 0.07 (-0.48, 0.63) | 8.55 |
| Simons, LA., 1995 [35] | 0.99 (0.20, 1.77) | 7.51 |
| Overall (I-squared = 83.0%, p=0.000) | -0.44 (-0.88, -0.01) | 100 |
Table 7.
Meta-analysis of the effect of garlic on HDL
| Study | SMD (95% CI) | Weight % |
|---|---|---|
| Adler, A. J., 1997 [20] | -1.90 (-2.92, -0.88) | 5.18 |
| Aslani, N., 2016 [21] | 0.51 (-0.03, 1.05) | 7.1 |
| Byrne, DJ., 1999 [23] | -0.37 (-1.12, 0.37) | 6.29 |
| Gardner, CD., 2001 [24] | 0.05 (-0.52, 0.62) | 6.97 |
| Higashikawa, Fumiko., 2012 [25] | 0.23 (-0.31, 0.76) | 7.11 |
| Isaacsohn, JL., 1998 [26] | 0.41 (-0.21, 1.03) | 6.79 |
| Jain, AK., 1993 [27] | -0.14 (-0.75, 0.47) | 6.84 |
| Jung, ES., 2014 [28] | 0.41 (-0.12, 0.95) | 7.11 |
| Kannar, D., 2001 [29] | -0.88 (-1.53, -0.24) | 6.69 |
| Peleg, A., 2003 [31] | -0.06 (-1.04, 0.93) | 5.31 |
| Riad, Karin., 2018 [32] | 1.34 (0.86, 1.81) | 7.33 |
| Sangouni, Abbas Ali., 2021 [14] | 1.04 (0.60, 1.49) | 7.43 |
| Sangouni, Abbas Ali., 2020 [33] | -0.04 (-0.59, 0.52) | 7.04 |
| Sharifi, F., 2010 [34] | -0.74 (-1.38, -0.10) | 6.7 |
| Simons, LA., 1995 [35] | 1.00 (0.21, 1.79) | 6.12 |
| Overall (I-squared = 82.8%, p=0.000) | 0.10 (-0.28, 0.48) | 100 |
Effect of garlic on blood pressure
In nine RCTs (423 participants), we found garlic supplementation was associated with a dramatic decline of DBP [SMD (95%CI) = -1.33 (-2.14, -0.53), p < 0.05, I2 = 92.1%], but with a slight decline of SBP [SMD (95%CI) = -0.56(-1.58, 0.47), p = 0.29, I2 = 95.1%] (Tables 8 and 9).
Table 8.
Meta-analysis of the effect of garlic on SBP
| Study | SMD (95% CI) | Weight % |
|---|---|---|
| Auer, W., 1990 [22] | -0.69 (-1.28, -0.10) | 12.02 |
| Isaacsohn, JL., 1998 [26] | 0.22 (-0.39, 0.84) | 11.99 |
| Jain, AK., 1993 [27] | 0.15 (-0.46, 0.76) | 12 |
| Nakasone, Yasushi, 2013 [30] | -0.69 (-1.41, 0.03) | 11.8 |
| Riad, Karin., 2018 [32] | -1.23 (-1.70, -0.77) | 12.2 |
| Sharifi, F., 2010 [34] | 5.81 (4.37, 7.26) | 10.04 |
| Simons, LA., 1995 [35] | -0.55 (-1.31, 0.20) | 11.73 |
| Superko, HR., 2000 [36] | -0.10 (-0.58, 0.38) | 12.18 |
| Valls, RM., 2022 [37] | -13.11 (-16.12, -10.10) | 6.04 |
| Overall (I-squared = 95.1%, p=0.000) | -0.56 (-1.58, 0.47) | 100 |
Table 9.
Meta-analysis of the effect of garlic on DBP
| Study | SMD (95% CI) | Weight % |
|---|---|---|
| Auer, W., 1990 [22] | -0.89 (-1.49, -0.29) | 12.28 |
| Isaacsohn, JL., 1998 [26] | -0.42 (-1.04, 0.20) | 12.23 |
| Jain, AK., 1993 [27] | 0.00 (-0.61,0.61) | 12.27 |
| Nakasone, Yasushi, 2013 [30] | -0.96 (-1.70, -0.22) | 11.87 |
| Riad, Karin., 2018 [32] | -0.98 (-1.43, -0.52) | 12.65 |
| Sharifi, F., 2010 [34] | -2.77 (-3.65, -1.89) | 11.39 |
| Simons, LA., 1995 [35] | 0.00 (-0.74, 0.74) | 11.86 |
| Superko, HR., 2000 [36] | -0.69 (-1.19, -0.19) | 12.55 |
| Valls, RM.,2022 [37] | -18.40 (-22.58, -14.22) | 2.9 |
| Overall (I-squared = 92.1%, p=0.000) | -1.33 (-2.14, -0.53) | 100 |
Effect of garlic on FBG
The effect of garlic on FBG was reported in five studies involving 214 individuals. According to the forest plot, garlic supplementation reduces FBG mildly [SMD (95%CI) = -0.26(-0.95, 0.44), P = 0.469, I2 = 85.3%] with no statistical significance (Table 10).
Table 10.
Meta-analysis of the effect of garlic on FBG
| Study | SMD (95% CI) | Weight % |
|---|---|---|
| Higashikawa, Fumiko., 2012 [25] | 0.00 (-0.53, 0.53) | 20.9 |
| Jain, AK., 1993 [27] | -0.10 (-0.71, 0.51) | 20.18 |
| Riad, Karin., 2018 [32] | -0.39 (-0.83, 0.04) | 21.83 |
| Sharifi, F., 2010 [34] | -1.76 (-2.49, -1.02) | 18.82 |
| Simons, LA., 1995 [35] | 0.99 (0.20, 1.77) | 18.28 |
| Overall (I-squared = 85.3%, p=0.000) | -0.26 (-0.95, 0.44) | 100 |
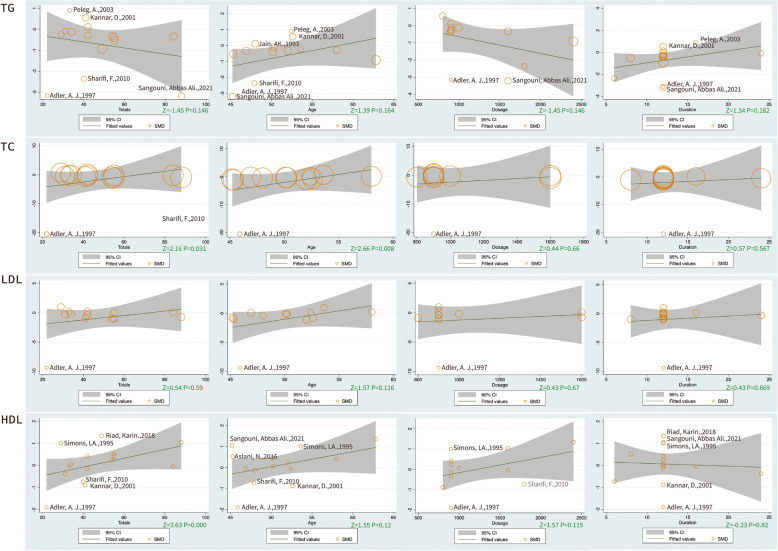
Meta-regression and subgroup analysis
Based on the high heterogeneity of the serological and anthropometric indices, we grouped covariates (potential effect modifiers) such as total sample size, the mean age, mean daily dose, duration of intervention, the oral form of garlic, and the dietary intervention that might affect the results. Indices that included more than 10 RCTs were analyzed by meta-regression based on these covariates. The results show that TC may be positively modulated by age (Z = 2.16, P < 0.05). Both TC (Z = 2.66, P < 0.05) and HDL (Z = 3.63, P < 0.05) showed an ascending trend as the sample size increased. However, there was no significant difference in the benefits of different garlic intervention doses for each index (Fig. 3).
Fig. 3.
The results of meta-regression
To further explore the effect of potential covariates in participants with MetS, we did subgroup analysis for TG, TC, LDL, HDL, SBP, and DBP. Dividing the sample size by 50, we found thatin the larger sample trial (≥ 50), TG, TC, LDL, SBP, and DBP reduced significantly. In participants younger than 50 years old, we found TG, TC, LDL reduced significantly. To our surprise, during shorter interventions (< 10 weeks), TG, TC, LDL, SBP, and DBP showed significant decreases. Only the elevated HDL seemed associated with longer interventions. We found that row garlic and aged garlic may be more effective than produced garlic for their significant effect on the reduction of TC, LDL, SBP, DBP and the elevation of HDL. Strikingly, we found that under the advised diet, garlic played a weak role in regulating blood sugar and blood lipids (Table 11).
Table 11.
Subgroup analysis of the included randomized controlled trials
| TG | TC | LDL | |||||||
| Number | Combined Effect Value | Heterogeneity | Number | Combined Effect Value | Heterogeneity | Number | Combined Effect Value | Heterogeneity | |
| SMD (95%CI) mmol/L | I2 | SMD (95%CI) mmol/L | I2 | SMD (95%CI) mmol/L | I2 | ||||
| overall | 15 | -0.66(-1.17, -0.16)* | 89.50% | 14 | -0.43(-0.86, -0.01)* | 81.90% | 13 | -0.44(-0.88, -0.01)* | 83.00% |
| Totals | |||||||||
| ≥ 50 | 5 | -0.83(-1.08, -0.58)* | 85.90% | 5 | -0.65(-1.02, -0.01)* | 58.90% | 5 | -0.56(-1.02, -0.11)* | 73.40% |
| < 50 | 10 | -0.41(-0.62, -0.2)* | 93.70% | 9 | -0.35(-1.07, 0.38) | 85.70% | 8 | -0.46(-1.19, 0.27) | 86.30% |
| Age | |||||||||
| ≥ 50 | 8 | -0.29(-0.5, -0.08)* | 64.80% | 7 | -0.18(-0.55, 0.2) | 58.70% | 7 | -0.15(-0.65, 0.35) | 76.20% |
| < 50 | 7 | -1.02(-1.28, -0.77)* | 93.60% | 7 | -0.93(-1.76, -0.09)* | 88.80% | 6 | -0.93(-1.71, -0.15)* | 87.90% |
| Dosage | |||||||||
| ≥ 1000 | 4 | -1.41(-1.7,-1.13)* | 94.40% | 3 | -0.53(-1.32, 0.19) | 78.10% | 2 | -0.36(-1.16, 0.45) | 80.90% |
| < 1000 | 8 | -0.17(-0.4, 0.06) | 74.10% | 8 | -0.47(-1.17, 0.22) | 86.60% | 8 | -0.63(-1.36, 0.1) | 88.00% |
| Duration | |||||||||
| ≥ 10w | 13 | -0.51(-0.69, -0.34)* | 89.50% | 13 | -0.37(-0.82, 0.07) | 81.90% | 12 | -0.40(-0.86, 0.07) | |
| < 10w | 2 | -1.09(-1.54, -0.64)* | 92.50% | 2 | -1.09(-1.66, -0.52)* | 1 | -0.99(-1.55, -0.43)* | 83.30% | |
| Dietary intervention | |||||||||
| none | -1.82(-3.66, 0.03) | 91.90% | 3 | -10.22(-29.81, 9.37) | 97.30% | 2 | -4.70(-13.54, 4.14) | 96.90% | |
| regular | -0.40(-0.70, -0.11) | 44.30% | 5 | -0.55(-0.90, -0.21) | 44.70% | 5 | -0.54(-0.97, -0.12) | 64.40% | |
| advised | -0.46(-1.61, 0.69) | 94.30% | 6 | -0.16(-0.67, 0.35) | 74.40% | 6 | -0.07(-0.62, 0.48) | 77.70% | |
| Oral form | |||||||||
| row | 1 | -0.54(-1.07, 0.00) | 1 | '-1.09(-1.66, -0.53) | 1 | '-0.99(-1.55, -0.43) | |||
| aged | 2 | -0.68(-1.20, -0.17) | 54.30% | 1 | '-0.23(-0.76, 0.30) | 1 | '-0.05(-0.58, 0.48) | ||
| produced | 12 | -0.69(-1.36, -0.01) | 91.60% | 13 | '-0.40(-0.90, 0.10) | 83.40% | 11 | '-0.45(-0.97, 0.07) | 84.60% |
| HDL | SBP | DBP | |||||||
| Number | Combined Effect Value | Heterogeneity | Number | Combined Effect Value | Heterogeneity | Number | Combined Effect Value | Heterogeneity | |
| SMD (95%CI) mmol/L | I2 | SMD (95%CI) mmol/L | I2 | SMD (95%CI) mmol/L | I2 | ||||
| overall | 15 | 0.1(-0.28, 0.48) | 82.80% | 9 | -0.56(-1.58, 0.47) | 95.1 | 9 | -1.33(-2.14, -0.53)* | 92.10% |
| Totals | |||||||||
| ≥ 50 | 5 | 0.45(0.08, 0.82)* | 61.50% | 2 | -6.52(-19.26, 6.23) | 95.10% | 2 | -9.42(-26.77, 7.94) | 98.50% |
| < 50 | 10 | -0.09(-0.66, 0.47) | 86.10% | 7 | 0.27(-0.71, 1.25) | 93.50% | 7 | -0.83(-1.39, -0.26)* | 81.70% |
| Age | |||||||||
| ≥ 50 | 8 | 0.33(-0.16, 0.81) | 80.10% | 6 | -1.51(-2.65, -0.37)* | 94.10% | 6 | -1.52(-2.62, -0.43)* | 93.30% |
| < 50 | 7 | -0.16(-0.79, 0.46) | 86.00% | 3 | 1.64(-0.92, 4.20) | 97.00% | 3 | -1.19(-2.61, 0.23) | 92.30% |
| Dosage | |||||||||
| ≥ 1000 | 4 | 0.42(-0.47, 1.31) | 91.40% | 2 | 2.25(-4.65, 9.16) | 98.80% | 2 | -1.23(-2.21, -0.26)* | 92.30% |
| < 1000 | 8 | -0.15(-0.64, 0.34) | 76.60% | 7 | -1.08(-2.03, -0.12)* | 92.30% | 7 | -1.83(-3.59, -0.07)* | 92.10% |
| Duration | |||||||||
| ≥ 10w | 13 | 0.14(-0.28, 0.55) | 83.10% | 7 | -0.42(-0.85, 0.01) | 73.10% | 7 | -0.59(-0.90, -0.29)* | 46.30% |
| < 10w | 2 | -0.10(-1.32, 1.12) | 88.30% | 2 | -3.60(-22.14, 14.94) | 99.20% | 2 | -10.45(-25.76,4.86) | 98.10% |
| Dietary intervention | |||||||||
| none | 3 | -0.92(-1.69, -0.16) | 65.00% | 2 | 2.53(-3.85, 8.90) | 98.50% | -1.80(-3.65, 0.04) | 91.70% | |
| regular | 6 | 0.42(-0.02, 0.86) | 74.60% | 3 | -3.82(-6.62, -1.03) | 97.40% | -4.33(-7.18, -1.48) | 97.30% | |
| advised | 6 | 0.26(-0.36, 0.88) | 82.50% | 4 | -0.23(-0.62, 0.16) | 34.90% | -0.54(-0.90, -0.19) | 20.80% | |
| Oral form | |||||||||
| row | 1 | 0.51(-0.03, 1.05) | - | - | - | - | - | - | - |
| aged | 2 | 0.88(-0.03, 1.79) | 84.60% | 2 | -7.07(-18.71, 4.56) | 98.30% | 2 | -9.56(-26.63, 7.52) | 98.50% |
| produced | 12 | -0.07(-0.49, 0.35) | 80.30% | 7 | 0.41(-0.48, 1.29) | 91.90% | 7 | '-0.78(-1.35, -0.22) | 81% |
*p < 0.05, statistically significant, CI Confidence interval, TG Triglycerides, TC Total cholesterol, LDL-c Low-density lipoprotein cholesterol, HDL-c High-density lipoprotein cholesterol, SBP Systolic blood pressure, DBP Diastolic blood pressure
Sensitivity analysis and publication bias
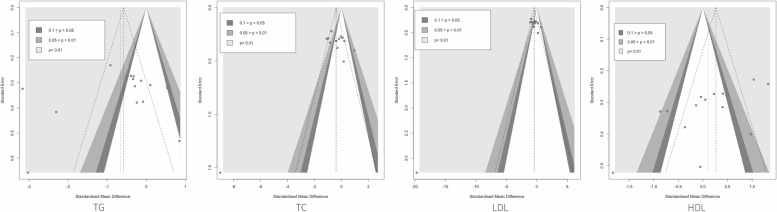
To assess the robustness and reliability of the meta-analysis results, we performed a sensitivity analysis by removing the RCTs one by one. The results showed that removing any studies of TC, LDL, HDL, and DBP did not affect the results. While the robustness of TG may be affected by the data of Sangouni Abbas Ali and the robustness of SBP may be affected by the data of Riad Karin. While removing trials that may affect the results, we found the direction of combined statistics didn’t change (Supplementary Figure S1). In addition, we performed the trim and filling method for TG and SBP and found no censoring and addition.
We used contour-enhanced funnel plots and Egger’s tests to detect the presence of publication bias. Most of the indices are symmetrically distributed, but we find asymmetry in the funnel plot of TG. However, most of its scatter fell in the right white invalid interval. We used Egger’s tests to avoid the possibility of a statistical Type I error with low test efficacy and find no publication bias of TG (p > 0.05) and potential publication bias of HDL(p < 0.05) (Fig. 4).
Fig. 4.
The results of publication bias
Quality of evidence
Although the RCTs we included were all high-grade evidence, their quality declined with reporting bias and publication bias. The GRADE approach showed moderate-quality evidence for major indices, but low-quality evidence for the HDL due to publication bias (Table 12).
Table 12.
Quality of evidence based on GRADE approach
| Certainty assessment | № of patients | Effect | Certainty | Importance | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| № of | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Treatment | Control | Absolute | ||
| study | (95% CI) | ||||||||||
| TG | Randomized trials | Serious | Not serious | Not serious | Not serious | None | 362 | 339 | SMD 0.66 SD lower | ⨁⨁⨁◯ | important |
| 15 | (1.17 lower to 0.16 lower) | Moderate | |||||||||
| TC | Randomized trials | Serious | Not serious | Not serious | Not serious | None | 320 | 297 | SMD 0.43 SD lower | ⨁⨁⨁◯ | important |
| 14 | (0.86 lower to 0.01 lower) | Moderate | |||||||||
| LDL | Randomized trials | Serious | Not serious | Not serious | Not serious | None | 300 | 277 | SMD 0.44 SD lower | ⨁⨁⨁◯ | important |
| 13 | (0.88 lower to 0.01 lower) | Moderate | |||||||||
| HDL | Randomized trials | Serious | Not serious | Not serious | Not serious | Publication bias strongly suspected | 362 | 339 | SMD 0.1 SD higher | ⨁⨁◯◯ | important |
| 15 | (0.28 lower to 0.48 higher) | Low | |||||||||
| SBP | Randomized trials | Serious | Not serious | Not serious | Not serious | None | 206 | 217 | SMD 0.56 SD lower | ⨁⨁⨁◯ | important |
| 9 | (1.58 lower to 0.47 higher) | Moderate | |||||||||
| DBP | Randomized trials | Serious | Not serious | Not serious | Not serious | None | 206 | 217 | SMD 1.33 SD lower | ⨁⨁⨁◯ | important |
| 9 | (2.14 lower to 0.53 lower) | Moderate | |||||||||
CI Confidence interval, SMD Standard mean difference
Disscusion
Summary of evidence
MetS was first proposed in 1988, and its content has gradually been clarified over decades of development [5]. Currently, the WHO Diabetes Group, the US National Cholesterol Education Program: Adult Treatment Panel III, and the International Diabetes Federation Consensus Group formed a consensus that obesity and metabolic disorders of glucose, lipid, and blood pressure together contribute to MetS [38–40]. Nowadays, there is no comprehensive management for MetS, and improving lifestyle and dietary habits may be the best way to prevent and manage it [41, 42]. Lack of intervention will lay down the risk of CVD and diabetes [2]. In recent years, research has been emerging on food supplementation and plant-food-derived bioactive to intervene in MetS and its components [43]. Among them, garlic is a popular daily spicy condiment, and researchers are curious and enthusiastic about its potential to improve the human metabolic profile.
For an in-depth, extensive, and comprehensive review of the effect of garlic on the MetS components, we retrieved 19 RCTs (including 999 participants) from 10 countries by strictly limiting inclusion and exclusion criteria. We consumed garlic and its extract(row garlic, produced garlic powder, aged garlic) and placebo in the treatment and control groups and found garlic supplementation can modulate anthropometric indices (BMI, WC) and lipids (TG, TC, LDL), and lower DBP in MetS. Meta-regression showed that most indices wouldn’t be regulated by mean daily dose, duration of intervention, the oral form of garlic, and the dietary intervention. During subgroup analysis, we found lipid indices fall more in the older age group and prolonged intervention does not produce better modulatory effects of metabolic indices.
Overview of underlying mechanisms
The pharmacological activity of garlic depends on the abundance of non-volatile sulfur-containing compounds such as alliin and S-allyl cysteine in the cytoplasm of its bulb, which accounts for about 3.5% of the weight of raw garlic and 80% of the total sulfur-containing compounds [44]. After the destruction process (squeezing and cutting), organosulfur compounds(allicin and S-allylcysteine) are catalyzed by alliinase to produce allicin and jointly play a role in regulating blood lipids, blood pressure, and blood sugar [45]. However, studies have shown that allicin is produced after 6 s of squeezing and cutting in raw garlic, and after digestion in the intestine with oral administration of processed garlic, and the process may be affected by the gastrointestinal environment (stomach acid, protease). Therefore, processed garlic may be less effective than raw garlic [46]. In the vitro model of metabolic syndrome, organosulfur compounds extracted from garlic can increase the abundance of acidophilus in the intestinal flora to improve insulin sensitivity and increase the production of taurine to upregulate PPARγ and CPT1A in the liver to promote β-oxidation of fatty acids [47]. Studies have also demonstrated that organosulfur compounds may synergistically exert various inhibitory effects in the process of cholesterol synthesis in the liver, such as inhibiting key enzymes in cholesterol synthesis (HMG-CoA reductase, cholesterol 7α-hydroxylase, fatty acid synthase) [15, 48, 49]. Meanwhile, allicin can also regulate lipid metabolic signals and modulate metabolic organs. In animal models with high-fat diet, allicin can up-regulate the expression of lipolytic genes (lipoprotein lipase and adipose triglyceride lipase), down-regulate the expression of fat degradation genes (fatty acid synthase, PPARγ), and promotes browning of white adipose tissue [50, 51]. For hypertension, garlic and its derivatives can alleviate oxidative stress by inhibiting the activity of ROS and NADPH oxidase [52]. Sulfur-containing compounds can produce H2S after erythrocyte action, and allicin can mediate the upregulation of angiotensin II receptors and eNOS, both of which have been shown to dilate blood vessels and lower blood pressure [53, 54]. Moreover, allicin can modulate insulin concentrations in STZ-induced diabetic rats with a dose-dependent response [55]. And garlic extract can bind to the druggable region of DPP-4 to inhibit its activity, exerting a hypoglycemic effect similar to that of DPP-4 inhibitors (selegiline) [56].
Comparison with previous studies
There are no meta-analyses exploring the efficacy of garlic in treating MetS, but studies of garlic for a single MetS component (lipids, blood pressure, blood glucose) have been widely conducted. The benefits of garlic in lowering total cholesterol, blood pressure, and blood glucose are clear, but different findings existed for other lipid parameters [57–59]. The earliest meta-analysis showed that garlic reduced TC by 9% [60]. In subsequent large-scale meta-analyses, garlic showed significant reductions in TC and TG, but slight reductions in HDL and LDL [61, 62]. These inconsistent results may be related to insufficient participant samples and inconsistent baseline disease. In a recent meta-analysis investigating the association between garlic intake and cardiovascular disease, garlic could exert better lipid-regulating effects in CVD participants at low intervention times, which is consistent with the conclusions we obtained [63]. Nowadays, there is no comprehensive management for MetS, and improving lifestyle and dietary habits may be the best way to prevent and manage it. All evidence proves that daily garlic supplementation can be a potential modulator of metabolic disorders.
Limitations
Undeniably, the present meta-analysis has potential limitations. The first is the high heterogeneity, we conducted subgroup analysis and meta-regression, but the source of heterogeneity still cannot be pinpointed completely. Based on our current evidence, sample size and age may be the potential components influencing heterogeneity. Secondly, the sensitivity analysis identified RCTs that could affect the robustness of the results, but the removal of the RCT did not change the direction of the effect and the trim and filling method suggested reliable results. Third, Egger’s tests showed the presence of publication bias for HDL. Fourth, according to the available information, we cannot conduct a further meta-analysis on the oral form of garlic. And a lack of consistency in garlic's origin and extraction process may also influence the results. Finally, based on the low to moderate level of evidence from the GRADE approach, we cannot draw a robust quantitative conclusion yet.
Implications for future research
Based on the limitations encountered in this study, we make the following recommendations for subsequent research in this area. First, we suggest that RCTs should be reported strictly according to the standardization in the CONSORT statement [64] so that they can be evaluated and interpreted in subsequent studies. Second, more high-quality and large-scale meta-analyses are needed to improve the credibility of the outcomes. The possibility of risks in the Cochrane Handbook (version 5.1.0) should be reduced during the study design process. Studies focusing on optimal dose and intervention duration should also be conducted to construct a standard paradigm for the administration of garlic supplementation. Finally, based on the current lack of management and treatment for MetS, researchers could explore other medicinal and edible products that may alleviate MetS.
Conclusion
In conclusion, quantitative analysis of 19 RCTs (999 participants) revealed that garlic supplementation partially modulated serum lipid profile (TG, TGL, HDL), blood pressure (SBP), and anthropometric parameters (WC, BMI) of MetS. It demonstrated that garlic is a potentially beneficial medicinal food product for MetS. However, based on the current evidence, we cannot draw a robust conclusion on the beneficial extent of garlic supplementation on MetS, and further large-scale RCTs are still needed to support this conclusion.
Supplementary Information
Additional file 1: Supplementary Table S1. PRISMA checklist of the Systematic Review and Meta-Analysis of garlic supplementation with MetS. Supplementary Figure S1. The results of sensitivity analysis. Supplementary Data.
Acknowledgements
Not applicable.
Authors’ contributions
Zhenyue Fu analyzed the data and wrote the full text; Jiayu Lv and Xiya Gao did the literature search and data extraction, Haoran Zheng; Shuqing Shi, and Xia Xu drew the pictures and tables, Bingxuan Zhang, Huaqin Wu, and Qingqiao Song did the second-time revision and polishing of the article.
Funding
This review is supported by Scientific and technological innovation project of China Academy of Chinese Medical Sciences(CI2021A01603, CI2021A03608).
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its additional information files.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhenyue Fu, Jiayu Lv and Xiya Gao are the co-first authors.
References
- 1.Cornier MA, Dabelea D, Hernandez TL, Lindstrom RC, Steig AJ, Stob NR, Van Pelt RE, Wang H, Eckel RH. The metabolic syndrome. Endocr Rev. 2008;29(7):777–822. doi: 10.1210/er.2008-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, Rinfret S, Schiffrin EL, Eisenberg MJ. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56(14):1113–1132. doi: 10.1016/j.jacc.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 3.Moran LJ, Misso ML, Wild RA, Norman RJ. Impaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2010;16(4):347–363. doi: 10.1093/humupd/dmq001. [DOI] [PubMed] [Google Scholar]
- 4.Lim SS, Kakoly NS, Tan JWJ, Fitzgerald G, Bahri Khomami M, Joham AE, Cooray SD, Misso ML, Norman RJ, Harrison CL, et al. Metabolic syndrome in polycystic ovary syndrome: a systematic review, meta-analysis and meta-regression. Obes Rev. 2019;20(2):339–352. doi: 10.1111/obr.12762. [DOI] [PubMed] [Google Scholar]
- 5.Kaplan NM. The deadly quartet. Upper-body obesity, glucose intolerance, hypertriglyceridemia, and hypertension. Arch Intern Med. 1989;149(7):1514–1520. doi: 10.1001/archinte.1989.00390070054005. [DOI] [PubMed] [Google Scholar]
- 6.Saltiel AR, Olefsky JM. Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest. 2017;127(1):1–4. doi: 10.1172/JCI92035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shan B, Wang X, Wu Y, Xu C, Xia Z, Dai J, Shao M, Zhao F, He S, Yang L, et al. The metabolic ER stress sensor IRE1α suppresses alternative activation of macrophages and impairs energy expenditure in obesity. Nat Immunol. 2017;18(5):519–529. doi: 10.1038/ni.3709. [DOI] [PubMed] [Google Scholar]
- 8.Thethi T, Kamiyama M, Kobori H. The link between the renin-angiotensin-aldosterone system and renal injury in obesity and the metabolic syndrome. Curr Hypertens Rep. 2012;14(2):160–169. doi: 10.1007/s11906-012-0245-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dabke K, Hendrick G, Devkota S. The gut microbiome and metabolic syndrome. J Clin Invest. 2019;129(10):4050–4057. doi: 10.1172/JCI129194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCullough AJ. Epidemiology of the metabolic syndrome in the USA. J Dig Dis. 2011;12(5):333–340. doi: 10.1111/j.1751-2980.2010.00469.x. [DOI] [PubMed] [Google Scholar]
- 11.Jeremic JN, Jakovljevic VL, Zivkovic VI, Srejovic IM, Bradic JV, Milosavljevic IM, Mitrovic SL, Jovicic NU, Bolevich SB, Svistunov AA, et al. garlic derived diallyl trisulfide in experimental metabolic syndrome: metabolic effects and cardioprotective role. Int J Mol Sci. 2020;21(23):9100. doi: 10.3390/ijms21239100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vazquez-Prieto MA, González RE, Renna NF, Galmarini CR, Miatello RM. Aqueous garlic extracts prevent oxidative stress and vascular remodeling in an experimental model of metabolic syndrome. J Agric Food Chem. 2010;58(11):6630–6635. doi: 10.1021/jf1006819. [DOI] [PubMed] [Google Scholar]
- 13.Shang A, Cao SY, Xu XY, Gan RY, Tang GY, Corke H, Mavumengwana V, Li HB. Bioactive Compounds and Biological Functions of Garlic (Allium sativum L.) Foods. 2019;8(7):246. doi: 10.3390/foods8070246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sangouni AA, Alizadeh M, Jamalzehi A, Parastouei K. Effects of garlic powder supplementation on metabolic syndrome components, insulin resistance, fatty liver index, and appetite in subjects with metabolic syndrome: a randomized clinical trial. Phytother Res. 2021;35(8):4433–4441. doi: 10.1002/ptr.7146. [DOI] [PubMed] [Google Scholar]
- 15.Zeng T, Zhang CL, Zhao XL, Xie KQ. The roles of garlic on the lipid parameters: a systematic review of the literature. Crit Rev Food Sci Nutr. 2013;53(3):215–230. doi: 10.1080/10408398.2010.523148. [DOI] [PubMed] [Google Scholar]
- 16.Cohen J. A power primer. Psychol Bull. 1992;112(1):155–159. doi: 10.1037/0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 17.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol. 2008;61(10):991–996. doi: 10.1016/j.jclinepi.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 19.Brozek JL, Akl EA, Alonso-Coello P, Lang D, Jaeschke R, Williams JW, Phillips B, Lelgemann M, Lethaby A, Bousquet J, et al. Grading quality of evidence and strength of recommendations in clinical practice guidelines. Part 1 of 3. An overview of the GRADE approach and grading quality of evidence about interventions. Allergy. 2009;64(5):669–677. doi: 10.1111/j.1398-9995.2009.01973.x. [DOI] [PubMed] [Google Scholar]
- 20.Adler AJ, Holub BJ. Effect of garlic and fish-oil supplementation on serum lipid and lipoprotein concentrations in hypercholesterolemic men. Am J Clin Nutr. 1997;65(2):445–450. doi: 10.1093/ajcn/65.2.445. [DOI] [PubMed] [Google Scholar]
- 21.Aslani N, Entezari MH, Askari G, Maghsoudi Z, Maracy MR. Effect of garlic and lemon juice mixture on lipid profile and some cardiovascular risk factors in people 30-60 years old with moderate hyperlipidaemia: a randomized clinical trial. Int J Prev Med. 2016;7:95. doi: 10.4103/2008-7802.187248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Auer W, Eiber A, Hertkorn E, Hoehfeld E, Koehrle U, Lorenz A, Mader F, Merx W, Otto G, Schmid-Otto B, et al. Hypertension and hyperlipidaemia: garlic helps in mild cases. Br J Clin Pract Suppl. 1990;69:3–6. [PubMed] [Google Scholar]
- 23.Byrne DJ, Neil HA, Vallance DT, Winder AF. A pilot study of garlic consumption shows no significant effect on markers of oxidation or sub-fraction composition of low-density lipoprotein including lipoprotein(a) after allowance for non-compliance and the placebo effect. Clin Chim Acta. 1999;285(1–2):21–33. doi: 10.1016/S0009-8981(99)00063-7. [DOI] [PubMed] [Google Scholar]
- 24.Gardner CD, Lawson LD, Block E, Chatterjee LM, Kiazand A, Balise RR, Kraemer HC. Effect of raw garlic vs commercial garlic supplements on plasma lipid concentrations in adults with moderate hypercholesterolemia: a randomized clinical trial. Arch Intern Med. 2007;167(4):346–353. doi: 10.1001/archinte.167.4.346. [DOI] [PubMed] [Google Scholar]
- 25.Higashikawa F, Noda M, Awaya T, Ushijima M, Sugiyama M. Reduction of serum lipids by the intake of the extract of garlic fermented with Monascus pilosus: a randomized, double-blind, placebo-controlled clinical trial. Clin Nutr (Edinburgh, Scotland) 2012;31(2):261–266. doi: 10.1016/j.clnu.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 26.Isaacsohn JL, Moser M, Stein EA, Dudley K, Davey JA, Liskov E, Black HR. Garlic powder and plasma lipids and lipoproteins: a multicenter, randomized, placebo-controlled trial. Arch Intern Med. 1998;158(11):1189–1194. doi: 10.1001/archinte.158.11.1189. [DOI] [PubMed] [Google Scholar]
- 27.Jain AK, Vargas R, Gotzkowsky S, McMahon FG. Can garlic reduce levels of serum lipids? A controlled clinical study. Am J Med. 1993;94(6):632–635. doi: 10.1016/0002-9343(93)90216-C. [DOI] [PubMed] [Google Scholar]
- 28.Jung ES, Park SH, Choi EK, Ryu BH, Park BH, Kim DS, Kim YG, Chae SW. Reduction of blood lipid parameters by a 12-wk supplementation of aged black garlic: a randomized controlled trial. Nutrition. 2014;30(9):1034–1039. doi: 10.1016/j.nut.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 29.Kannar D, Wattanapenpaiboon N, Savige GS, Wahlqvist ML. Hypocholesterolemic effect of an enteric-coated garlic supplement. J Am Coll Nutr. 2001;20(3):225–231. doi: 10.1080/07315724.2001.10719036. [DOI] [PubMed] [Google Scholar]
- 30.Nakasone Y, Nakamura Y, Yamamoto T, Yamaguchi H. Effect of a traditional Japanese garlic preparation on blood pressure in prehypertensive and mildly hypertensive adults. Exp Ther Med. 2013;5(2):399–405. doi: 10.3892/etm.2012.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peleg A, Hershcovici T, Lipa R, Anbar R, Redler M, Beigel Y. Effect of garlic on lipid profile and psychopathologic parameters in people with mild to moderate hypercholesterolemia. Isr Med Assoc J. 2003;5(9):637–640. [PubMed] [Google Scholar]
- 32.Riad K, Travica N, Sali A. The effect of kyolic aged garlic extract on gut microbiota, inflammation, and cardiovascular markers in hypertensives: the GarGIC trial. Front Nutr. 2018;5:122. doi: 10.3389/fnut.2018.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sangouni AA. Mohammad Hosseini Azar MR, Alizadeh M: Effect of garlic powder supplementation on hepatic steatosis, liver enzymes and lipid profile in patients with non-alcoholic fatty liver disease: a double-blind randomised controlled clinical trial. Br J Nutr. 2020;124(4):450–456. doi: 10.1017/S0007114520001403. [DOI] [PubMed] [Google Scholar]
- 34.Sharifi F, Sheikhi A, Behdad M, Mousavinasab N. Effect of garlic on serum adiponectin and interleukin levels in women with metabolic syndrome. Int J Endocrinol Metabolism. 2010;8(2):68–73. [Google Scholar]
- 35.Simons LA, Balasubramaniam S, Von Koningsmark M, Parfitt A, Simons J, Peters W. On the effect of garlic on plasma lipids and lipoproteins in mild hypercholesterolaemia. Atherosclerosis. 1995;113(2):219–225. doi: 10.1016/0021-9150(94)05449-S. [DOI] [PubMed] [Google Scholar]
- 36.Superko HR, Krauss RM. Garlic powder, effect on plasma lipids, postprandial lipemia, low-density lipoprotein particle size, high-density lipoprotein subclass distribution and lipoprotein(a) J Am Coll Cardiol. 2000;35(2):321–326. doi: 10.1016/S0735-1097(99)90541-7. [DOI] [PubMed] [Google Scholar]
- 37.Valls RM, Companys J, Calderón-Pérez L, Salamanca P, Pla-Pagà L, Sandoval-Ramírez BA, Bueno A, Puzo J, Crescenti A, Del Bas JM, et al. Effects of an optimized aged garlic extract on cardiovascular disease risk factors in moderate hypercholesterolemic subjects: a randomized, crossover, double-blind, sustainedand controlled study. Nutrients. 2022;14(3):405. doi: 10.3390/nu14030405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15(7):539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 39.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143-421. [PubMed]
- 40.Zimmet P, Magliano D, Matsuzawa Y, Alberti G, Shaw J. The metabolic syndrome: a global public health problem and a new definition. J Atheroscler Thromb. 2005;12(6):295–300. doi: 10.5551/jat.12.295. [DOI] [PubMed] [Google Scholar]
- 41.Samson SL, Garber AJ. Metabolic syndrome. Endocrinol Metab Clin North Am. 2014;43(1):1–23. doi: 10.1016/j.ecl.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 42.Xu H, Li X, Adams H, Kubena K, Guo S. Etiology of metabolic syndrome and dietary intervention. Int J Mol Sci. 2018;20(1):128. doi: 10.3390/ijms20010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Butnariu M, Fratantonio D, Herrera-Bravo J, Sukreet S, Martorell M, Ekaterina Robertovna G, Les F, López V, Kumar M, Pentea M, et al. Plant-food-derived bioactives in managing hypertension: From current findings to upcoming effective pharmacotherapies. Curr Top Med Chem. 2023;23(8):589–617. doi: 10.2174/1568026623666230106144509. [DOI] [PubMed] [Google Scholar]
- 44.Sánchez-Gloria JL, Arellano-Buendía AS, Juárez-Rojas JG, García-Arroyo FE, Argüello-García R, Sánchez-Muñoz F, Sánchez-Lozada LG, Osorio-Alonso H. Cellular mechanisms underlying the cardioprotective role of allicin on cardiovascular diseases. Int J Mol Sci. 2022;23(16):9082. doi: 10.3390/ijms23169082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Melguizo-Rodríguez L, García-Recio E, Ruiz C, De Luna-Bertos E, Illescas-Montes R, Costela-Ruiz VJ. Biological properties and therapeutic applications of garlic and its components. Food Funct. 2022;13(5):2415–2426. doi: 10.1039/D1FO03180E. [DOI] [PubMed] [Google Scholar]
- 46.Lawson LD, Gardner CD. Composition, stability, and bioavailability of garlic products used in a clinical trial. J Agric Food Chem. 2005;53(16):6254–6261. doi: 10.1021/jf050536+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen K, Nakasone Y, Yi S, Ibrahim HR, Sakao K, Hossain MA, Hou DX. Natural garlic organosulfur compounds prevent metabolic disorder of lipid and glucose by increasing gut commensal bacteroides acidifaciens. J Agric Food Chem. 2022;70(19):5829–5837. doi: 10.1021/acs.jafc.2c00555. [DOI] [PubMed] [Google Scholar]
- 48.Gebhardt R, Beck H. Differential inhibitory effects of garlic-derived organosulfur compounds on cholesterol biosynthesis in primary rat hepatocyte cultures. Lipids. 1996;31(12):1269–1276. doi: 10.1007/BF02587912. [DOI] [PubMed] [Google Scholar]
- 49.Yeh YY, Liu L. Cholesterol-lowering effect of garlic extracts and organosulfur compounds: human and animal studies. J Nutr. 2001;131(3s):989s–993s. doi: 10.1093/jn/131.3.989S. [DOI] [PubMed] [Google Scholar]
- 50.Shi X, Zhou X, Chu X, Wang J, Xie B, Ge J, Guo Y, Li X, Yang G. Allicin improves metabolism in high-fat diet-induced obese mice by modulating the gut microbiota. Nutrients. 2019;11(12):2909. doi: 10.3390/nu11122909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee CG, Rhee DK, Kim BO, Um SH, Pyo S. Allicin induces beige-like adipocytes via KLF15 signal cascade. J Nutr Biochem. 2019;64:13–24. doi: 10.1016/j.jnutbio.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 52.Liu C, Cao F, Tang QZ, Yan L, Dong YG, Zhu LH, Wang L, Bian ZY, Li H. Allicin protects against cardiac hypertrophy and fibrosis via attenuating reactive oxygen species-dependent signaling pathways. J Nutr Biochem. 2010;21(12):1238–1250. doi: 10.1016/j.jnutbio.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 53.García Trejo E, Arellano Buendía AS, Sánchez Reyes O, García Arroyo FE, ArguelloGarcía R, Loredo Mendoza ML, Tapia E, Sánchez Lozada LG, Osorio Alonso H. The beneficial effects of allicin in chronic kidney disease are comparable to losartan. Int J Mol Sci. 2017;18(9):1980. doi: 10.3390/ijms18091980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Benavides GA, Squadrito GL, Mills RW, Patel HD, Isbell TS, Patel RP, Darley-Usmar VM, Doeller JE, Kraus DW. Hydrogen sulfide mediates the vasoactivity of garlic. Proc Natl Acad Sci U S A. 2007;104(46):17977–17982. doi: 10.1073/pnas.0705710104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thomson M, Al-Qattan KK, Js D, Ali M. Anti-diabetic and anti-oxidant potential of aged garlic extract (AGE) in streptozotocin-induced diabetic rats. BMC Complement Altern Med. 2016;16:17. doi: 10.1186/s12906-016-0992-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kalhotra P, Chittepu V, Osorio-Revilla G, Gallardo-Velazquez T. Phytochemicals in garlic extract inhibit therapeutic enzyme dpp-4 and induce skeletal muscle cell proliferation: a possible mechanism of action to benefit the treatment of diabetes mellitus. Biomolecules. 2020;10(2):305. doi: 10.3390/biom10020305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Silagy CA, Neil HA. A meta-analysis of the effect of garlic on blood pressure. J Hypertens. 1994;12(4):463–468. doi: 10.1097/00004872-199404000-00017. [DOI] [PubMed] [Google Scholar]
- 58.Ried K. Garlic lowers blood pressure in hypertensive individuals, regulates serum cholesterol, and stimulates immunity: an updated meta-analysis and review. J Nutr. 2016;146(2):389s–396s. doi: 10.3945/jn.114.202192. [DOI] [PubMed] [Google Scholar]
- 59.Shabani E, Sayemiri K, Mohammadpour M. The effect of garlic on lipid profile and glucose parameters in diabetic patients: a systematic review and meta-analysis. Prim Care Diabetes. 2019;13(1):28–42. doi: 10.1016/j.pcd.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 60.Warshafsky S, Kamer RS, Sivak SL. Effect of garlic on total serum cholesterol. A meta-analysis. Ann Intern Med. 1993;119(7 Pt 1):599–605. doi: 10.7326/0003-4819-119-7_Part_1-199310010-00009. [DOI] [PubMed] [Google Scholar]
- 61.Reinhart KM, Talati R, White CM, Coleman CI. The impact of garlic on lipid parameters: a systematic review and meta-analysis. Nutr Res Rev. 2009;22(1):39–48. doi: 10.1017/S0954422409350003. [DOI] [PubMed] [Google Scholar]
- 62.Zeng T, Guo FF, Zhang CL, Song FY, Zhao XL, Xie KQ. A meta-analysis of randomized, double-blind, placebo-controlled trials for the effects of garlic on serum lipid profiles. J Sci Food Agric. 2012;92(9):1892–1902. doi: 10.1002/jsfa.5557. [DOI] [PubMed] [Google Scholar]
- 63.Li S, Guo W, Lau W, Zhang H, Zhan Z, Wang X, Wang H. The association of garlic intake and cardiovascular risk factors: A systematic review and meta-analysis. Crit Rev Food Sci Nutr. 2022;14:1–19. doi: 10.1080/10408398.2022.2053657. [DOI] [PubMed] [Google Scholar]
- 64.Antes G. The new CONSORT statement. Bmj. 2010;340:c1432. doi: 10.1136/bmj.c1432. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary Table S1. PRISMA checklist of the Systematic Review and Meta-Analysis of garlic supplementation with MetS. Supplementary Figure S1. The results of sensitivity analysis. Supplementary Data.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its additional information files.