Abstract
Background
Globally, metabolic syndrome (MS) and Helicobacter pylori (HP) infection, which have gained an epidemic status, are major challenges to human health, society, and medical professionals. Recent studies have demonstrated that MS is closely related to HP infection. Additionally, HP is an important risk factor for gastric cancer. However, systematic reviews on HP are lacking. This review aimed to summarize and analyze the potential correlation of HP infection with MS and its components, as well as the underlying mechanism, to provide reference and strategies for clinical prevention and treatment.
Methodology
Previous studies examining the correlation between HP and MS since 1990 were retrieved from the PubMed, Web of Science, and Embase databases. The potential correlation between HP infection and MS and its components was comprehensively analyzed. The keywords “Helicobacter pylori,” “HP,” “metabolic syndrome,” “hypertension,” “obesity,” “diabetes,” or “dyslipidemia” were used in all fields. No language restrictions were imposed.
Results
MS was strongly correlated to HP infection. The inflammatory response and inflammatory factors produced during HP infection are important etiological factors for insulin resistance and MS. The co-occurrence of long-term chronic inflammation and immune dysfunction with MS may be the predisposing factor for HP infection. MS components, such as diabetes, hypertension, dyslipidemia, and obesity were also correlated with HP infection in one or both directions.
Conclusions
HP infection and MS may promote the pathogenesis of each other. The contribution of HP infection and MS to gastric cancer cannot be ruled out based on co-occurrence. The MS components diabetes and obesity may be bidirectionally correlated with HP infection.
Keywords: Metabolic syndrome, Helicobacter pylori, Insulin resistance, Inflammatory factors
Introduction
Metabolic syndrome (MS) is a common clinical condition without a global unified definition. However, various associations and guidelines have reached a consensus on its components, namely metabolic aberrations characterized by hyperglycemia, hypertension, dyslipidemia, and obesity. MS affects approximately 25% of the global population (Saklayen, 2018). The clinical outcomes of MS and its components are a major challenge to the global healthcare system, society, and public health. The global number of cases of Helicobacter pylori (HP) infection, which is one of the most common bacterial infections, is as high as approximately 4.4 billion. The incidence of HP infection in developing countries is higher than that in developed countries (Burucoa & Axon, 2017; Hooi et al., 2017). Previous studies have demonstrated that HP infection is an important risk factor for chronic gastritis, gastric ulcer, gastric cancer, and other gastric diseases and that it is closely related to MS (Upala et al., 2016). Based on the disease burden and multiple related complications, MS and HP infection are major health concerns for the global population. The potential correlation between MS and HP infection will have important implications for the prognosis of patients with MS, HP infection, or both MS and HP infection.
Currently, limited studies have examined the correlation of MS and its components with HP infection. Additionally, a comprehensive analysis of the complex causal relationship between MS and HP infection has not been previously performed. This review describes the correlation of MS and its components with HP infection, especially the potential causal relationships and their possible mechanisms. Additionally, potential therapeutic targets have been suggested to enable clinicians and medical researchers to address the co-occurrence of these pathological conditions and consequently prevent the occurrence and development of diseases and related complications and improve the prognosis of MS and HP infection.
Survey methodology
In this study, literature examining the correlation between HP and MS since 1990 was retrieved from the PubMed, Web of Science, and Embase databases. Additionally, the potential correlation between HP infection and MS and its components, as well as the underlying mechanisms, were comprehensively analyzed. The free keywords “Helicobacter pylori,” “HP,” “metabolic syndrome,” “hypertension,” “obesity,” “diabetes,” or “dyslipidemia” were used in all fields. No language restrictions were imposed.
HP and MS
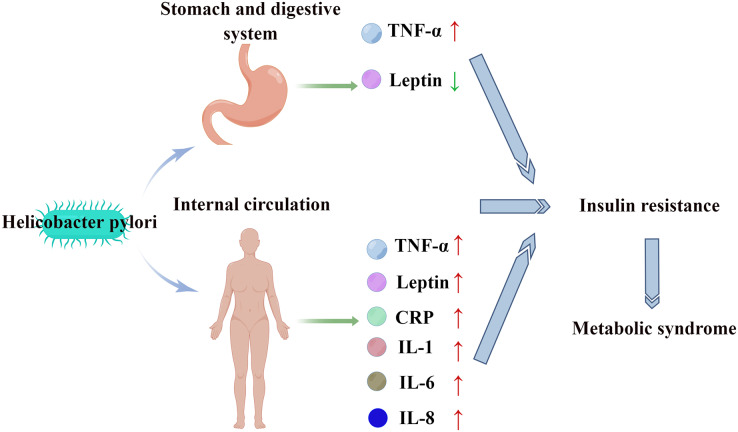
Studies in different countries and regions have demonstrated a positive correlation between HP infection and MS (Azami et al., 2021; Chen et al., 2019b; Gunji et al., 2008; Lim et al., 2019). The prevalence of MS in serum HP-positive patients (27.2%) was significantly higher than that in serum HP-negative patients (21.0%) (P < 0.001) (Lim et al., 2019). HP infection promotes chronic inflammation and immune responses in the stomach and digestive tract in which several inflammatory cytokines and adipokines, such as tumor necrosis factor-α (TNF-α) and leptin are involved (Crabtree, 1996; Ernst et al., 1994; Upala et al., 2016). Compared with those in patients without HP infection, the TNF-α levels are upregulated and the leptin levels are downregulated in patients with HP infection (Kusters, van Vliet & Kuipers, 2006; Roper et al., 2008). The upregulation of TNF-α and the downregulation of leptin can lead to insulin resistance (Matthaei et al., 2000; Shoelson, Herrero & Naaz, 2007). Additionally, HP infection can also induce systemic inflammatory responses, resulting in the upregulation of interleukin (IL)-1, IL-6, IL-8, C-reactive protein (CRP), TNF-α, leptin, and other inflammatory factors and cytokines and the development of insulin resistance (Franceschi et al., 2014; Mansori et al., 2020; Yu et al., 2019). Insulin resistance, which is one of the core pathophysiological mechanisms underlying MS pathogenesis (Cho et al., 2022) (Fig. 1), promotes the release of free fatty acids in adipose tissue, the upregulation of very-low-density lipoprotein, and the downregulation of high-density lipoprotein (HDL). The upregulation of free fatty acids, inflammatory cytokines, adipokines, and mitochondrial dysfunction results in impaired insulin signal transduction, decreased glucose uptake by skeletal muscles, increased hepatic gluconeogenesis, and β cell dysfunction, leading to the development of hyperglycemia. Additionally, insulin resistance promotes the development of hypertension by disrupting nitric oxide-induced vasodilation (Gallagher, Leroith & Karnieli, 2010). Therefore, HP infection may promote the development of MS by inducing insulin resistance.
Figure 1. HP stimulates the digestive tract and systemic circulation to release inflammatory factors and cytokines, leading to insulin resistance and MS.
The effects of MS and insulin resistance on HP infection have not been previously reported. However, we hypothesized that the correlation of MS and insulin resistance with HP infection is bidirectional. In patients with MS exhibiting long-term insulin resistance, the ability of MS to promote HP infection cannot be ruled out. Long-term chronic inflammation and immune dysfunction in patients with MS are potential susceptibility factors for HP infection. Further studies are needed to clarify these correlations.
Recent studies have demonstrated that MS is an important risk factor for tumors, especially for the occurrence and development of gastric cancer and other digestive system tumors (Belladelli, Montorsi & Martini, 2022). HP infection and MS may exert similar effects on gastric cancer. Thus, the synergistic effects of HP infection and MS on gastric cancer cannot be ruled out. Patients with both MS and HP infection should be carefully monitored, especially based on stable metabolic parameters and other controllable factors to reduce the risk of gastric cancer and HP infection.
The effect of HP eradication on MS is currently controversial. Mokhtare et al. (2017) demonstrated that the eradication of HP significantly alleviates the dysregulated fasting blood glucose and HbA1c levels, dyslipidemia, enhanced abdominal circumference, and other important components of MS. Chopeĭ et al. (2014) demonstrated that the eradication of HP can significantly alleviate dysregulated glucolipid metabolism, liver function, and CRP level in patients with MS. Liou et al. (2019) demonstrated that although the eradication of HP did not significantly affect the prevalence of MS, it exerted significant beneficial effects on metabolic parameters, including the alleviation of insulin resistance, the downregulation of triglyceride (TG) and low-density lipoprotein cholesterol (LDL) levels, and the upregulation of high-density lipoprotein cholesterol (HDL) levels. Therefore, several studies have demonstrated that HP eradication alleviates metabolic parameters. However, the effect of HP eradication on the prevalence of MS must be evaluated as it depends on various confounding factors, such as the current diagnostic criteria for MS, the degree of improvement of MS components, and the follow-up time after successful HP eradication. MS standards must be unified, and long-term, prospective, randomized, controlled studies must be designed to further clarify the effect of HP eradication on MS.
Some studies have also reported negative results. A cross-sectional survey revealed no correlation between serum HP antibody positivity and the occurrence of metabolic diseases. This negative result may be related to factors, such as research methodology, system error, and sample size. Thus, further studies are needed to clarify the correlation (Wawro et al., 2019).
HP and diabetes
The main manifestation of hyperglycemia, a component of MS, is diabetes mellitus (DM). Several studies have reported a positive correlation between HP infection and diabetes. HP infection, which affects the incidence of type 1 diabetes, type 2 diabetes, and gestational diabetes, increases the risk of diabetes by approximately 27% (Mansori et al., 2020; Wan et al., 2020; Xia et al., 2020). Meanwhile, the rate of HP infection in patients with diabetes was significantly higher than that in patients without diabetes (Chen et al., 2019a). Additionally, the HbA1c level in patients with HP infection was significantly higher than that in patients without HP infection (Chen et al., 2019a). HP infection was positively correlated with HbA1c upregulation in patients with diabetes (Chen et al., 2019a). Therefore, insulin resistance resulting from HP-induced inflammatory factors is a key etiological factor for diabetes. Additionally, the HP infection-induced dysregulation of cellular and humoral immunity is an etiological factor for diabetes (Borody et al., 2002).
Kim et al. (2022) indicated that HP eradication downregulated the HbA1c levels in patients with diabetes or pre-diabetes. The HbA1c level in the HP-eradicated group was significantly lower than that in the non-eradicated group in the first and fifth years of follow-up. Additionally, HP eradication alleviated hypertension and decreased body mass index (BMI). This may be related to the mitigation of systemic inflammation and insulin resistance. Cornejo-Pareja et al. (2019) demonstrated that HP eradication promotes the secretion of glucagon-like peptide-1 (GLP-1) by modulating the distribution of human intestinal flora. This is one of the potential reasons for the improvement of glucose metabolism after HP eradication. Finally, HP infection is reported to be related to proteinuria in patients with diabetes. HP infection eradication may be an important strategy to protect renal function in patients with diabetes (Shi et al., 2018) although the specific underlying mechanisms are unclear.
Song et al. (2021) demonstrated that the probability of HP eradication failure in patients with DM was two times higher than that in patients without DM. Additionally, the BMI was directly proportional to the HP eradication failure rate. Nam et al. (2019) suggested that poor glycemic control hinders the eradication of HP. Diabetes is an important risk factor for local or systemic infection, and hyperglycemia promotes microbial reproduction. The progression of diabetes is associated with decreased gastrointestinal peristalsis, impaired gastric acid secretion, delayed gastric emptying, and gastroparesis, which increase the risk of microbial colonization and infection in the digestive system (Jeon et al., 2012). Therefore, diabetes can increase the risk of HP infection. The adverse interaction between DM and HP infection cannot be ruled out. DM affects the incidence of HP infection and the efficacy of HP eradication treatment, while HP affects the incidence and metabolic parameters of DM.
Diabetes also increases the risk of gastric cancer development and gastric cancer-related mortality (Tseng, 2021). This suggests that patients with both diabetes and HP infection must be screened for the occurrence and development of gastric cancer.
HP and hypertension
The pathogenesis of hypertension, a component of MS, mainly involves vascular endothelial dysfunction, sympathetic nerve impairment, and the aberrant activation of the renin-angiotensin-aldosterone system (Gupta et al., 2022). Hypertension is also closely related to HP infection (Dore et al., 2022; Fang, Xie & Fan, 2022; Kountouras et al., 2022). Yue et al. (2022) reported that HP infection increased the risk of hypertension by 32%. Wan et al. (2018) demonstrated that HP infection was positively correlated with the risk of hypertension. Additionally, the average arterial pressure of patients with HP infection increased by 0.723 mmHg when compared with that of patients without HP infection. Migneco et al. (2003) revealed that the blood pressure of patients with hypertension after HP infection eradication was significantly lower than that at the baseline. Additionally, the 24-h average systolic blood pressure decreased from 135.5 ± 9.7 to 123.6 ± 11.8 mmHg, while the 24-h average diastolic blood pressure decreased from 85.6 ± 9.3 to 69.3 ± 7.8 mmHg after HP eradication. However, in patients with hypertension in whom HP was not successfully eradicated, the blood pressure at follow-up was not significantly different from that at the baseline (Migneco et al., 2003).
These findings indicated that HP infection is a potential cause of hypertension. Although no specific mechanism for this phenomenon has been reported, some theoretical evidence supports this hypothesis. HP infection-induced inflammatory response and inflammatory factors can damage the vascular endothelium. Dysfunctional vascular endothelium is an etiological factor for hypertension. Additionally, HP infection can affect the immune system and induce autoimmune reactions, which may also cause hypertension (Huang et al., 2021). The colonization of HP has been detected in the arterial wall (Kowalski, 2001). The direct injury to the vessel wall caused by HP infection may impair vascular elasticity and hemodynamics and consequently cause hypertension. HP infection is closely related to dyslipidemia. Long-term dyslipidemia-induced atherosclerosis is also an important factor affecting the occurrence and development of hypertension.
The role of hypertension in HP infection has not been examined. We hypothesize that hypertension and HP infection lack the bidirectional correlation as observed in diabetes. One exception may be hypertension-induced injury of the digestive system, especially the stomach, which increases the susceptibility to HP infection. However, this phenomenon has rarely been observed.
HP and dyslipidemia
Dyslipidemia, a component of MS, is an important risk factor for cardiovascular diseases. Long-term dyslipidemia can significantly increase the risk of acute cardiovascular and cerebrovascular events. Tali et al. (2022) reported that the incidence rates of hypercholesterolemia, hypertriglyceridemia, high LDL, and low HDL in patients with HP infection (73.21%, 68.75%, 77.04%, and 69.72%, respectively) were significantly higher than those in patients without HP infection (26.79%, 31.25%, 22.96%, and 30.28%, respectively). The TG and LDL levels in patients with HP infection were significantly higher than those in patients without HP infection. Similarly, the TC and HDL levels were downregulated in patients with HP infection (Tali et al., 2022). Nigatie et al. (2022) demonstrated that the prevalence of dyslipidemia in patients with HP infection was as high as 71.8%. The median TG, TC, and LDL levels in patients with HP infection (93, 143, and 108 mg/dL, respectively) were significantly higher than those in patients without HP infection (83, 125, and 95 mg/dL, respectively) (Nigatie et al., 2022). Previous studies have demonstrated that HP infection was positively correlated with TC, TG, and LDL levels and negatively correlated with HDL levels (Shimamoto et al., 2020).
Park et al. (2021) reported that the incidence of dyslipidemia in HP-eradicated cases (98.1 per 1,000 person-years) was significantly lower than that in patients with HP infection (130.5 per 1,000 person-years). Additionally, HP eradication decreased the risk of dyslipidemia. Other studies have demonstrated that HP eradication increased HDL levels and decreased TG and LDL levels (Iwai et al., 2019; Mokhtare et al., 2017). Dyslipidemia, especially high LDL and low HDL levels, is closely related to the occurrence and development of atherosclerosis and coronary heart disease. Thus, HP eradication can exert beneficial effects in patients with dyslipidemia.
Pih et al. (2021) demonstrated that LDL upregulation and HDL downregulation were associated with the risk of gastric cancer. These individuals with aberrant blood lipids were not tested for HP infection. If these patients had HP infection, it may be due to the impact of cancer rather than aberrant blood lipids. However, the influence of lipid components on gastric cancer cannot be ruled out. HDL is reported to exhibit anti-inflammatory and antioxidant activities in tumors (Soran, Schofield & Durrington, 2015).
These findings suggest that HP infection may affect the lipid profile through some mechanisms. Although the exact mechanism is not clear, it can be speculated. HP promotes the production of some inflammatory factors (such as TNF-α and IL-8), which can lead to the dysregulation of lipoproteins and lipids and the mobilization of lipids from tissues to blood (Gallin, Kaye & O’Leary, 1969; Park et al., 2021). Additionally, HP infection promotes the release of ghrelin and leptin, affecting the absorption of nutrients and consequently modulating the lipid profile (Shimamoto et al., 2020). Similar to hypertension, the effect of dyslipidemia on HP infection has not been examined. We believe that the possibility of dyslipidemia affecting HP infection is low.
HP and obesity
Obesity is closely related to other components of MS. The etiology and pathogenesis of obesity intersect with those of diabetes, hypertension, and dyslipidemia. Compared with that of the other three components, the risk of obesity is the highest among the general population. Baradaran et al. (2021) reported that the prevalence of obesity is positively correlated with HP infection. The risk of HP infection in patients with obesity is 46% higher than that in patients without obesity. Additionally, patients with HP infection are more prone to develop obesity than non-infected patients (Baradaran et al., 2021). One study demonstrated that HP infection is positively correlated with a high level of BMI and that HP infection is negatively correlated with BMI decline in patients with obesity (Zhang et al., 2020). Obesity is also associated with gastric cancer. The incidence of gastric cancer in patients with persistent obesity increased by 20% (Lim et al., 2022). This phenomenon may be related to the high HP infection rate and insulin resistance. However, the effects of obesity-related inherent factors, such as aberrant adipocyte cytokines, ectopic fat deposition, changes in gut microbiota, chronic inflammation, and oxidative stress on tumors cannot be ruled out (Avgerinos et al., 2019).
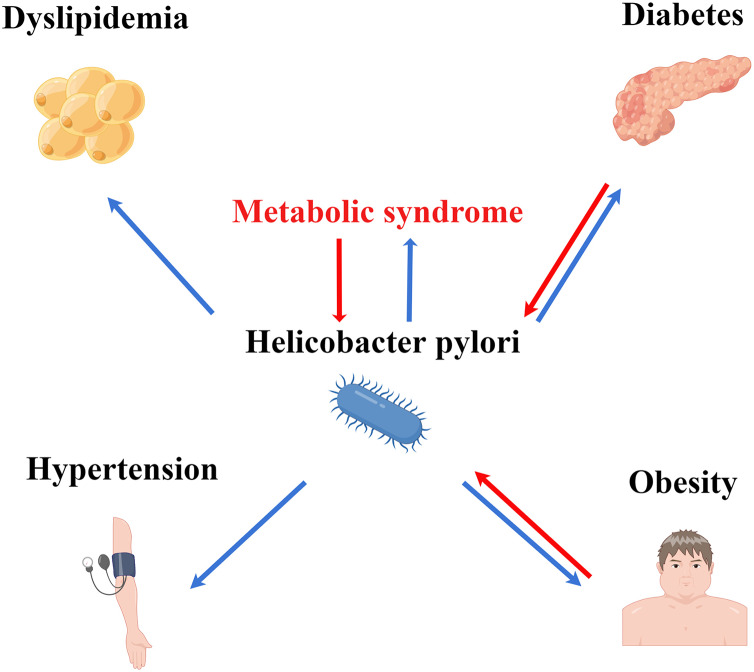
These findings indicate the correlation between obesity and HP infection. HP infection promotes the production of inflammatory factors, leading to a chronic systemic inflammatory state and insulin resistance, which can promote the occurrence and development of obesity. The aberrant secretion of serum leptin after HP infection delays the feeling of satiety, increasing energy intake and causing obesity (Baradaran et al., 2021). Additionally, patients with obesity exhibit imbalanced autoimmunity, impaired ability of monocytes to transform into macrophages, and suppressed cytotoxicity of natural killer cells. These factors may promote the occurrence of HP infection (Marti, Marcos & Martinez, 2001; Moulin et al., 2009). Similar to diabetes, the association between obesity and HP infection may be bidirectional (Fig. 2).
Figure 2. Association of HP infection with MS and its components. HP infection may interact with MS, diabetes, and obesity.
Conclusions
HP infection is closely related to MS. Additionally, HP infection may be correlated with the components of MS, such as diabetes and obesity. HP eradication enables the suppression of the occurrence and development of MS and its components, as well as the alleviation of the dysregulated metabolic parameters in vivo. Patients with MS and its components must be screened for HP infection to improve their metabolic status.
Acknowledgments
Figure support was provided by Figdraw.
Funding Statement
This work was supported by the Joint Medical Research Project of Chongqing Municipal Science and the Technology Bureau and Health Commission (2023QNXM044), the Scientific research project of Chongqing Municipal Sports Bureau (C202108,C202202,C202206), and the Chongqing Key Laboratory of Emergency Medicine (2022KFKT03). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Ying Deng, Email: 1137432251@qq.com.
Ruoqing Li, Email: lrqlyx@foxmail.com.
Additional Information and Declarations
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Qinli Xie conceived and designed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft.
Yangjun He conceived and designed the experiments, performed the experiments, analyzed the data, authored or reviewed drafts of the article, and approved the final draft.
Danni Zhou performed the experiments, analyzed the data, prepared figures and/or tables, and approved the final draft.
Yi Jiang conceived and designed the experiments, authored or reviewed drafts of the article, and approved the final draft.
Ying Deng performed the experiments, prepared figures and/or tables, and approved the final draft.
Ruoqing Li conceived and designed the experiments, authored or reviewed drafts of the article, and approved the final draft.
Data Availability
The following information was supplied regarding data availability:
This is a literature review.
References
- Avgerinos et al. (2019).Avgerinos KI, Spyrou N, Mantzoros CS, Dalamaga M. Obesity and cancer risk: emerging biological mechanisms and perspectives. Metabolism-Clinical and Experimental. 2019;92(4):121–135. doi: 10.1016/j.metabol.2018.11.001. [DOI] [PubMed] [Google Scholar]
- Azami et al. (2021).Azami M, Baradaran HR, Dehghanbanadaki H, Kohnepoushi P, Saed L, Moradkhani A, Moradpour F, Moradi Y. Association of Helicobacter pylori infection with the risk of metabolic syndrome and insulin resistance: an updated systematic review and meta-analysis. Diabetology & Metabolic Syndrome. 2021;13(1):145. doi: 10.1186/s13098-021-00765-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baradaran et al. (2021).Baradaran A, Dehghanbanadaki H, Naderpour S, Pirkashani LM, Rajabi A, Rashti R, Riahifar S, Moradi Y. The association between Helicobacter pylori and obesity: a systematic review and meta-analysis of case-control studies. Clinical Diabetes and Endocrinology. 2021;7(1):15. doi: 10.1186/s40842-021-00131-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belladelli, Montorsi & Martini (2022).Belladelli F, Montorsi F, Martini A. Metabolic syndrome, obesity and cancer risk. Current Opinion in Urology. 2022;32(6):594–597. doi: 10.1097/MOU.0000000000001041. [DOI] [PubMed] [Google Scholar]
- Borody et al. (2002).Borody T, Ren Z, Pang G, Clancy R. Impaired host immunity contributes to Helicobacter pylori eradication failure. American Journal of Gastroenterology. 2002;97(12):3032–3037. doi: 10.1111/j.1572-0241.2002.07121.x. [DOI] [PubMed] [Google Scholar]
- Burucoa & Axon (2017).Burucoa C, Axon A. Epidemiology of Helicobacter pylori infection. Helicobacter. 2017;22(Suppl 1):e12403. doi: 10.1111/hel.12403. [DOI] [PubMed] [Google Scholar]
- Chen et al. (2019a).Chen J, Xing Y, Zhao L, Ma H. The association between Helicobacter pylori infection and glycated hemoglobin A in Diabetes: a meta-analysis. Journal of Diabetes Research. 2019a;2019:3705264. doi: 10.1155/2019/3705264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al. (2019b).Chen YY, Fang WH, Wang CC, Kao TW, Chang YW, Wu CJ, Zhou YC, Sun YS, Chen WL. Helicobacter pylori infection increases risk of incident metabolic syndrome and diabetes: a cohort study. PLOS ONE. 2019b;14(2):e0208913. doi: 10.1371/journal.pone.0208913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho et al. (2022).Cho YH, Lee Y, Choi JI, Lee SR, Lee SY. Biomarkers in metabolic syndrome. Advances in Clinical Chemistry. 2022;111(5–6):101–156. doi: 10.1016/bs.acc.2022.07.003. [DOI] [PubMed] [Google Scholar]
- Chopeĭ et al. (2014).Chopeĭ IV, Mykhalko la O, Chubirko KI, Hechko MM, Varvarynets’ AV, Khanenko SS, Kanchiĭ VM. Influence of Helicobacter pylori eradication on the metabolic syndrome components. Wiadomości Lekarskie. 2014;67(2 Pt 2):226–229. [PubMed] [Google Scholar]
- Cornejo-Pareja et al. (2019).Cornejo-Pareja I, Martin-Nunez GM, Roca-Rodriguez MM, Cardona F, Coin-Araguez L, Sanchez-Alcoholado L, Gutierrez-Repiso C, Munoz-Garach A, Fernandez-Garcia JC, Moreno-Indias I, Tinahones FJ. H. pylori eradication treatment alters gut microbiota and GLP-1 secretion in humans. Journal of Clinical Medicine. 2019;8(4):451. doi: 10.3390/jcm8040451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree (1996).Crabtree JE. Gastric mucosal inflammatory responses to Helicobacter pylori. Alimentary Pharmacology & Therapeutics. 1996;10(Sup1):29–37. doi: 10.1046/j.1365-2036.1996.22164003.x. [DOI] [PubMed] [Google Scholar]
- Dore et al. (2022).Dore MP, Saba PS, Tomassini G, Niolu C, Monaco M, Pes GM. Increased risk to develop hypertension and carotid plaques in patients with long-lasting Helicobacter pylori gastritis. Journal of Clinical Medicine. 2022;11(9):2282. doi: 10.3390/jcm11092282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst et al. (1994).Ernst PB, Jin Y, Reyes VE, Crowe SE. The role of the local immune response in the pathogenesis of peptic ulcer formation. Scandinavian Journal of Gastroenterology. 1994;205:22–28. doi: 10.3109/00365529409091405. [DOI] [PubMed] [Google Scholar]
- Fang, Xie & Fan (2022).Fang Y, Xie H, Fan C. Association of hypertension with Helicobacter pylori: a systematic review and meta‐analysis. PLOS ONE. 2022;17(5):e0268686. doi: 10.1371/journal.pone.0268686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi et al. (2014).Franceschi F, Annalisa T, Teresa DR, Giovanna D, Ianiro G, Franco S, Viviana G, Valentina T, Riccardo LL, Antonio G. Role of Helicobacter pylori infection on nutrition and metabolism. World Journal of Gastroenterology. 2014;20(36):12809–12817. doi: 10.3748/wjg.v20.i36.12809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher, Leroith & Karnieli (2010).Gallagher EJ, Leroith D, Karnieli E. Insulin resistance in obesity as the underlying cause for the metabolic syndrome. Mount Sinai Journal of Medicine: A Journal of Translational and Personalized Medicine. 2010;77(5):511–523. doi: 10.1002/msj.20212. [DOI] [PubMed] [Google Scholar]
- Gallin, Kaye & O’Leary (1969).Gallin JI, Kaye D, O’Leary WM. Serum lipids in infection. New England Journal of Medicine. 1969;281(20):1081–1086. doi: 10.1056/NEJM196911132812001. [DOI] [PubMed] [Google Scholar]
- Gunji et al. (2008).Gunji T, Matsuhashi N, Sato H, Fujibayashi K, Okumura M, Sasabe N, Urabe A. Helicobacter pylori infection is significantly associated with metabolic syndrome in the Japanese population. American Journal of Gastroenterology. 2008;103(12):3005–3010. doi: 10.1111/j.1572-0241.2008.02151.x. [DOI] [PubMed] [Google Scholar]
- Gupta et al. (2022).Gupta R, Alcantara R, Popli T, Tariq U, Sood A, Mahajan S, Ayele H, Rajeswaran Y, Vyas AV. Firibastat: a novel brain aminopeptidase inhibitor—a new era of antihypertensive therapy. Current Problems in Cardiology. 2022;47(9):100859. doi: 10.1016/j.cpcardiol.2021.100859. [DOI] [PubMed] [Google Scholar]
- Hooi et al. (2017).Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, Tanyingoh D, Malfertheiner P, Graham DY, Wong VWS, Wu JCY, Chan FKL, Sung JJY, Kaplan GG, Ng SC. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology. 2017;153(2):420–429. doi: 10.1053/j.gastro.2017.04.022. [DOI] [PubMed] [Google Scholar]
- Huang et al. (2021).Huang M, Zhu L, Jin Y, Fang Z, Chen Y, Yao Y. Association between Helicobacter pylori infection and systemic arterial hypertension: a meta-analysis. Arquivos Brasileiros de Cardiologia. 2021;117(2):626–636. doi: 10.36660/abc.20200186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai et al. (2019).Iwai N, Okuda T, Oka K, Hara T, Inada Y, Tsuji T, Komaki T, Inoue K, Dohi O, Konishi H, Naito Y, Itoh Y, Kagawa K. Helicobacter pylori eradication increases the serum high density lipoprotein cholesterol level in the infected patients with chronic gastritis: a single-center observational study. PLOS ONE. 2019;14(8):e0221349. doi: 10.1371/journal.pone.0221349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon et al. (2012).Jeon CY, Haan MN, Cheng C, Clayton ER, Mayeda ER, Miller JW, Aiello AE. Helicobacter pylori infection is associated with an increased rate of diabetes. Diabetes Care. 2012;35(3):520–525. doi: 10.2337/dc11-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim et al. (2022).Kim WS, Choi Y, Kim N, Lim SH, Noh G, Kim KW, Park J, Jo H, Yoon H, Shin CM, Park YS, Lee DH. Long-term effect of the eradication of Helicobacter pylori on the hemoglobin A1c in type 2 diabetes or prediabetes patients. Korean Journal of Internal Medicine. 2022;37(3):579–590. doi: 10.3904/kjim.2021.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kountouras et al. (2022).Kountouras J, Papaefthymiou A, Polyzos SA, Kazakos E, Vardaka E, Touloumtzi M, Tzitiridou-Chatzopoulou M, Liatsos C, Sgantzou IK, Knuchel J, Doulberis M. Impact of active Helicobacter pylori infection-related metabolic syndrome on systemic arterial hypertension. Arquivos Brasileiros de Cardiologia. 2022;119(3):502–504. doi: 10.36660/abc.20210931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalski (2001).Kowalski M. Helicobacter pylori (H. pylori) infection in coronary artery disease: influence of H. pylori eradication on coronary artery lumen after percutaneous transluminal coronary angioplasty. The detection of H. pylori specific DNA in human coronary atherosclerotic plaque. Journal of Physiology and Pharmacology. 2001;52:3–31. [PubMed] [Google Scholar]
- Kusters, van Vliet & Kuipers (2006).Kusters JG, van Vliet AH, Kuipers EJ. Pathogenesis of Helicobacter pylori infection. Clinical Microbiology Reviews. 2006;19(3):449–490. doi: 10.1128/CMR.00054-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim et al. (2019).Lim SH, Kim N, Kwon JW, Kim SE, Baik GH, Lee JY, Park KS, Shin JE, Song HJ, Myung DS, Choi SC, Kim HJ, Lim JH, Yim JY, Kim JS. Positive association between Helicobacter pylori infection and metabolic syndrome in a Korean population: a multicenter nationwide study. Digestive Diseases and Sciences. 2019;64(8):2219–2230. doi: 10.1007/s10620-019-05544-3. [DOI] [PubMed] [Google Scholar]
- Lim et al. (2022).Lim JH, Shin CM, Han KD, Lee SW, Jin EH, Choi YJ, Yoon H, Park YS, Kim N, Lee DH. Association between the persistence of obesity and the risk of gastric cancer: a nationwide population-based study. Cancer Research and Treatment. 2022;54(1):199–207. doi: 10.4143/crt.2021.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou et al. (2019).Liou JM, Chen CC, Chang CM, Fang YJ, Bair MJ, Chen PY, Chang CY, Hsu YC, Chen MJ, Chen CC, Lee JY, Yang TH, Luo JC, Chen CY, Hsu WF, Chen YN, Wu JY, Lin JT, Lu TP, Chuang EY, El-Omar EM, Wu MS, Taiwan Gastrointestinal D, Helicobacter C. Long-term changes of gut microbiota, antibiotic resistance, and metabolic parameters after Helicobacter pylori eradication: a multicentre, open-label, randomised trial. The Lancet Infectious Diseases. 2019;19(10):1109–1120. doi: 10.1016/S1473-3099(19)30272-5. [DOI] [PubMed] [Google Scholar]
- Mansori et al. (2020).Mansori K, Moradi Y, Naderpour S, Rashti R, Moghaddam AB, Saed L, Mohammadi H. Helicobacter pylori infection as a risk factor for diabetes: a meta-analysis of case-control studies. BMC Gastroenterology. 2020;20(1):77. doi: 10.1186/s12876-020-01223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti, Marcos & Martinez (2001).Marti A, Marcos A, Martinez JA. Obesity and immune function relationships. Obesity Reviews. 2001;2(2):131–140. doi: 10.1046/j.1467-789x.2001.00025.x. [DOI] [PubMed] [Google Scholar]
- Matthaei et al. (2000).Matthaei S, Stumvoll M, Kellerer M, Haring HU. Pathophysiology and pharmacological treatment of insulin resistance. Endocrine Reviews. 2000;21(6):585–618. doi: 10.1210/edrv.21.6.0413. [DOI] [PubMed] [Google Scholar]
- Migneco et al. (2003).Migneco A, Ojetti V, Specchia L, Franceschi F, Candelli M, Mettimano M, Montebelli R, Savi L, Gasbarrini G. Eradication of Helicobacter pylori infection improves blood pressure values in patients affected by hypertension. Helicobacter. 2003;8(6):585–589. doi: 10.1111/j.1523-5378.2003.00180.x. [DOI] [PubMed] [Google Scholar]
- Mokhtare et al. (2017).Mokhtare M, Mirfakhraee H, Arshad M, Samadani Fard SH, Bahardoust M, Movahed A, Masoodi M. The effects of Helicobacter pylori eradication on modification of metabolic syndrome parameters in patients with functional dyspepsia. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2017;11(4):S1031–S1035. doi: 10.1016/j.dsx.2017.07.035. [DOI] [PubMed] [Google Scholar]
- Moulin et al. (2009).Moulin CM, Marguti I, Peron JP, Rizzo LV, Halpern A. Impact of adiposity on immunological parameters. Arquivos Brasileiros de Endocrinologia & Metabologia. 2009;53(2):183–189. doi: 10.1590/S0004-27302009000200010. [DOI] [PubMed] [Google Scholar]
- Nam et al. (2019).Nam SJ, Park SC, Lee SH, Choi DW, Lee SJ, Bang CS, Baik GH, Park JK. Helicobacter pylori eradication in patients with type 2 diabetes mellitus: multicenter prospective observational study. SAGE Open Medicine. 2019;7:2050312119832093. doi: 10.1177/2050312119832093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigatie et al. (2022).Nigatie M, Melak T, Asmelash D, Worede A. Dyslipidemia and its associated factors among Helicobacter pylori-infected patients attending at University of Gondar Comprehensive Specialized Hospital, Gondar, North-West Ethiopia: a comparative cross-sectional study. Journal of Multidisciplinary Healthcare. 2022;15:1481–1491. doi: 10.2147/JMDH.S368832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park et al. (2021).Park Y, Kim TJ, Lee H, Yoo H, Sohn I, Min YW, Min BH, Lee JH, Rhee PL, Kim JJ. Eradication of Helicobacter pylori infection decreases risk for dyslipidemia: a cohort study. Helicobacter. 2021;26(2):e12783. doi: 10.1111/hel.12783. [DOI] [PubMed] [Google Scholar]
- Pih et al. (2021).Pih GY, Gong EJ, Choi JY, Kim MJ, Ahn JY, Choe J, Bae SE, Chang HS, Na HK, Lee JH, Jung KW, Kim DH, Choi KD, Song HJ, Lee GH, Jung HY. Associations of serum lipid level with gastric cancer risk, pathology, and prognosis. Cancer Research and Treatment. 2021;53(2):445–456. doi: 10.4143/crt.2020.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper et al. (2008).Roper J, Francois F, Shue PL, Mourad MS, Pei Z, Olivares de Perez AZ, Perez-Perez GI, Tseng CH, Blaser MJ. Leptin and ghrelin in relation to Helicobacter pylori status in adult males. The Journal of Clinical Endocrinology & Metabolism. 2008;93(6):2350–2357. doi: 10.1210/jc.2007-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saklayen (2018).Saklayen MG. The global epidemic of the metabolic syndrome. Current Hypertension Reports. 2018;20(2):12. doi: 10.1007/s11906-018-0812-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi et al. (2018).Shi Y, Duan JY, Liu DW, Qiao YJ, Han QX, Pan SK, Tang L, Cai GY, Chen XM, Liu ZS, Zhu HY. Helicobacter pylori infection is associated with occurrence of proteinuria in type 2 diabetes patients: a systemic review and meta-analysis. Chinese Medical Journal. 2018;131(22):2734–2740. doi: 10.4103/0366-6999.245269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamoto et al. (2020).Shimamoto T, Yamamichi N, Gondo K, Takahashi Y, Takeuchi C, Wada R, Mitsushima T, Koike K. The association of Helicobacter pylori infection with serum lipid profiles: an evaluation based on a combination of meta-analysis and a propensity score-based observational approach. PLOS ONE. 2020;15(6):e0234433. doi: 10.1371/journal.pone.0234433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoelson, Herrero & Naaz (2007).Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007;132(6):2169–2180. doi: 10.1053/j.gastro.2007.03.059. [DOI] [PubMed] [Google Scholar]
- Song et al. (2021).Song X, Cai C, Jin Q, Chen X, Yu C. The efficacy of Helicobacter pylori eradication in diabetics and its effect on glycemic control: a systematic review and meta-analysis. Helicobacter. 2021;26(2):e12781. doi: 10.1111/hel.12781. [DOI] [PubMed] [Google Scholar]
- Soran, Schofield & Durrington (2015).Soran H, Schofield JD, Durrington PN. Antioxidant properties of HDL. Frontiers in Pharmacology. 2015;6(6 Pt B):222. doi: 10.3389/fphar.2015.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tali et al. (2022).Tali LDN, Faujo GFN, Konang JLN, Dzoyem JP, Kouitcheu LBM. Relationship between active Helicobacter pylori infection and risk factors of cardiovascular diseases, a cross-sectional hospital-based study in a Sub-Saharan setting. BMC Infectious Diseases. 2022;22(1):731. doi: 10.1186/s12879-022-07718-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng (2021).Tseng CH. The relationship between diabetes mellitus and gastric cancer and the potential benefits of metformin: an extensive review of the literature. Biomolecules. 2021;11(7):1022. doi: 10.3390/biom11071022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upala et al. (2016).Upala S, Jaruvongvanich V, Riangwiwat T, Jaruvongvanich S, Sanguankeo A. Association between Helicobacter pylori infection and metabolic syndrome: a systematic review and meta-analysis. Journal of Digestive Diseases. 2016;17(7):433–440. doi: 10.1111/1751-2980.12367. [DOI] [PubMed] [Google Scholar]
- Wan et al. (2018).Wan Z, Hu L, Hu M, Lei X, Huang Y, Lv Y. Helicobacter pylori infection and prevalence of high blood pressure among Chinese adults. Journal of Human Hypertension. 2018;32(2):158–164. doi: 10.1038/s41371-017-0028-8. [DOI] [PubMed] [Google Scholar]
- Wan et al. (2020).Wan Z, Song L, Hu L, Hu M, Lei X, Huang Y, Lv Y. Helicobacter pylori infection is associated with diabetes among Chinese adults. Journal of Diabetes Investigation. 2020;11(1):199–205. doi: 10.1111/jdi.13102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wawro et al. (2019).Wawro N, Amann U, Butt J, Meisinger C, Akmatov MK, Pessler F, Peters A, Rathmann W, Kaab S, Waterboer T, Linseisen J. Helicobacter pylori seropositivity: prevalence, associations, and the impact on incident metabolic diseases/risk factors in the population-based KORA study. Frontiers in Public Health. 2019;7:96. doi: 10.3389/fpubh.2019.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia et al. (2020).Xia B, Wang W, Lu Y, Chen C. Helicobacter pylori infection increases the risk of metabolic syndrome in pregnancy: a cohort study. Annals of Translational Medicine. 2020;8(14):875. doi: 10.21037/atm-20-4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu et al. (2019).Yu Y, Cai J, Song Z, Wang J, Wu L. Association of Helicobacter pylori infection with metabolic syndrome in aged Chinese females. Experimental and Therapeutic Medicine. 2019;17:4403–4408. doi: 10.3892/etm.2019.7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue et al. (2022).Yue L, Zhang R, Chen S, Duan G. Relationship between Helicobacter pylori and incident hypertension as well as blood pressure: a systematic review and meta-analysis. Digestive Diseases. 2022;41(1):124–137. doi: 10.1159/000524078. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2020).Zhang J, Chen Y, Chen W, Xu H, Wang H, Chen L, Ye Y, Wang Z, Ye J. Persistent infection of Helicobacter pylori affects weight loss in obese population compared with persistent negative: a case-control study based on healthy Chinese. Helicobacter. 2020;25(4):e12697. doi: 10.1111/hel.12697. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The following information was supplied regarding data availability:
This is a literature review.