INTRODUCTION
Pain is both common and challenging to manage in patients with cancer. Disease progression, oncologic treatment (e.g., chemotherapy, surgery), and co-morbid conditions may contribute to inter-individual variation in pain, as well as to fluctuating needs for clinical management [13, 35]. The majority of patients with cancer require emergency department (ED) visits and hospitalization during the course of their illness [24, 35]. Over two thirds of cancer patients visit the ED within 6 months of diagnosis [39], and up to 60% are hospitalized [13, 35]. Studies suggest that up to 40% of ED visits during cancer are for pain [13, 35].
Opioid analgesia is a pharmacologic mainstay of cancer pain management, with patients self-titrating opioid consumption on an as needed basis, with variable success [31]. Unfortunately, increased restrictions on opioid prescribing, although not necessarily intended for patients with cancer, may dissuade clinicians from prescribing opioids, while stigma may simultaneously discourage patients from using opioids [4]. Opioid prescriptions for cancer pain have substantially declined [2, 22] and one study demonstrated a 50% rise in cancer pain-related ED visits over the past decade [22]. Previous studies in patients with chronic non-cancer pain suggest that higher psychological distress and pain catastrophizing are associated with worse pain outcomes, including during hospitalization [21, 38]. Yet, to our knowledge, no study has examined whether these and other salient factors are associated with worse pain outcomes among hospitalized cancer patients.
Demographic, clinical, and psychological factors may modulate pain and explain inter-individual variability in patients’ pain experiences. An increased understanding of the impact of patient-level risk factors on pain may help identify hospitalized patients at highest risk, and inform personalized, early intervention. As many of these risk factors may be interrelated, a simultaneous and comprehensive assessment of a wide range of potential pain-modulatory factors is important when evaluating their influence. In this prospective study, we aimed to evaluate demographic, clinical, and psychological characteristics as potential predictors of worse daily pain and greater daily opioid administration in a sample of cancer patients who presented to the ED with pain and were subsequently hospitalized. We hypothesized that greater psychological symptoms would be associated with greater pain and increased opioid administration during admission.
METHODS
Patients were recruited for this prospective observational cohort study upon presentation to the ED at Brigham and Women’s Hospital (BWH), an academic, urban hospital in Boston, MA that serves as the primary ED for the Dana-Farber Cancer Institute. This ED evaluates approximately 60,000 patients annually, a third of whom are oncology patients. Study procedures were approved by the Massachusetts General Brigham Institutional Review Board.
Participants and Study Procedures
Patients presenting to the ED with pain were screened for eligibility. Inclusion criteria were: age≥18, cancer requiring treatment within the last two years, pain of ≥4/10, English proficiency, and medical and psychiatric stability confirmed by the treating clinician. A research assistant approached the patient to explain the study. After providing consent, patients completed a 10-15 minute questionnaire using REDCap (Research Electronic Data Capture), a secure, web-based software platform [26]. A study assistant collected basic demographic and clinical information from the chart at baseline, and completed a pain-focused structured abstraction of the medical record after hospital discharge.
Information about the 178 patients enrolled in the larger parent ED study have been described previously [5]. The current analysis was restricted to the 111 patients (62%) who were admitted to the hospital after this ED visit.
Primary Outcomes
Primary outcomes were daily average pain severity and opioid administration during hospitalization. Pain scores were recorded by clinicians in the electronic medical record (EMR) routinely with vital signs and during intermittent clinical assessments, (e.g., before and after analgesic administration). Therefore, the total number of pain scores recorded per patient per day was variable, typically at least 5 scores per day, but ranging up to 17 per day. Opioid analgesics administered during the hospital stay were recorded with a timestamp in the EMR. All pain scores within each calendar day (24-hour time period from midnight-midnight) were averaged for a total daily pain score. All opioids administered during each calendar day were converted to morphine milligram equivalents (MMEs), allowing calculation of a total daily opioid administration, then divided by the number of hours inpatient on that calendar day (i.e., typically 24hrs, but fewer on days of admission or discharge), and expressed as MME/hr.
Independent Variables
Patients completed questionnaires to self-report their race, education, income, and health literacy [17], as well as cancer treatment (e.g., chemotherapy, surgery, immunotherapy, radiation) received in the last two years, cancer-related surgical procedures in the last 3 months, and chronic pain predating their cancer. Patients also self-reported current analgesic use, including opioids. NIH-PROMIS short-forms were used to assess symptoms of depression, anxiety, and sleep disturbance in the past 7 days. Scores were converted to t-scores to compare to standard populations using PROMIS conversion tables [15], The Pain Catastrophizing Scale (PCS) assessed rumination, helplessness, and magnification of pain in the past 7 days [42], using a 5-point Likert scale, with higher scores representing worse symptoms (range 0-52). The 4-item Perceived Stress Scale (PSS-4) [18] measured perceived stress within the last week using a 5-point Likert scale (range 0-20). Age, gender, insurance type, cancer treatment in the last 6 weeks, and metastatic disease status were abstracted from the EMR.
Statistical Analysis
To estimate associations between independent and dependent variables (i.e., daily pain severity, daily opioid administration) collected longitudinally (daily) across the hospital stay, we performed univariable generalized estimating equation (GEE) analyses with an autoregressive correlation structure, which allows estimation of an IV with the DV across multiple days and can accommodate missing data or unequal numbers of observations (e.g., variations in total number of inpatient days). Association effect sizes are reported as GEE model beta coefficients (B) and confidence intervals (CI). Each individual univariable GEE evaluated the relationship between a single baseline independent variable (age, gender, race, education, income, smoking status, insurance type, health literacy, metastatic disease, history of recent surgery, previous history of chronic pain, current outpatient opioid use, depression, anxiety, pain catastrophizing, sleep disturbance, and stress) assessed at time of ED admission, and the outcome of interest across all days of admission (daily average pain or daily opioids administered). Independent variables that were associated with both the outcomes at the p≤0.1, or with one outcome at the p≤0.05 level, were included in subsequent multivariable GEE analyses. These multivariable GEE analyses allowed for an evaluation of the independent contribution of baseline variables to explaining variance in each outcome, while controlling for the impact of other variables included in the multivariable model. As an additional exploratory analysis, we compared characteristics of patients who were or were not discharged with a new opioid prescription using Mann-Whitney U, Wilcoxon rank sum, and Pearson Chi-square tests. All analyses were performed using Statistical Package for the Social Sciences version-27, IBM corporation, Armonk, NY.
RESULTS
Of the 330 patients screened upon arrival to the ED, 250 were eligible, 178 consented, and 175 completed their baseline survey (76%) [5]. Of those consenting in the ED, 111(62%) were subsequently hospitalized and had inpatient pain scores and opioid utilization data, and were included in the current analysis (Table 1). Mean hospital length of stay was 5.6 days (median=3, SD=6.6, range:1-37 days), with the majority (90%) of patients staying 15 days or less. Approximately half of these patients were female (54%), 85% identified as White, and most (73%) reported pain as the primary reason for presenting to the ED. Patients had various cancer types, including colorectal (21%), ovarian (13%), lung (12%), pancreatic/liver (10%), and breast (8%) as the most common types. Metastatic disease was common (80%) and most patients (70%) had undergone some treatment for cancer in the last 6 weeks, and about a third (32%) reported having cancer-related surgery in the previous 3 months. About half (46%) reported taking prescription opioids for pain before their admission (referred to as “outpatient opioid use”), 26% reported using outpatient non-opioid over-the-counter pain medications, and 9.7% reported using cannabis (Table 1). Reported symptoms of depression, anxiety, and sleep disturbance were similar to other clinical samples (t-scores in 54th-56th percentile), slightly above average compared to population means, [14] and patients reported relatively high levels of pain catastrophizing (18.5±12.5), similar to chronic pain samples [6].
Table 1.
Patient Characteristics (n=111)
| n (%) | |
|---|---|
| Gender (female) | 61 (54%) |
| Age, m (SD) | 57.9 (13.9) |
| Race | |
| White | 94 (85%) |
| Black/African American | 10 (9%) |
| Asian | 3 (3%) |
| Don’t know | 1 (0.9%) |
| Ethnicity | |
| Non-Hispanic/Latino | 101 (91%) |
| Hispanic/Latino | 6 (5%) |
| Highest education level | |
| Some high school | 3 (3%) |
| High school diploma | 22 (20%) |
| Some college | 33 (29%) |
| College degree | 23 (21%) |
| Higher level degree | 30 (27%) |
| Marital Status | |
| Married | 68 (61%) |
| Single | 21 (19%) |
| Divorced | 15 (14%) |
| Widowed | 7 (6%) |
| Average Income per person in household | |
| 0-15K | 35 (31%) |
| 15-25K | 22 (20%) |
| 25-40K | 27 (24%) |
| 40-60K | 5 (5%) |
| 60-75K | 0 (0%) |
| 75-100K | 3 (3%) |
| >100K | 4 (4%) |
| Insurance Type | |
| Private | 55 (49%) |
| Medicare | 37 (31%) |
| Medicaid | 16 (17%) |
| Other | 3 (2%) |
| Cancer type | |
| Colorectal | 23 (21%) |
| Other cancer type | 16 (15%) |
| Ovarian | 15 (13%) |
| Lung | 14 (12%) |
| Pancreatic/ Liver | 11 (10%) |
| Breast | 9 (8%) |
| Prostate | 6 (5%) |
| Uterine/urinary | 4 (4%) |
| Head and Neck | 3 (3%) |
| Leukemia/Leukemia | 7 (6%) |
| Esophageal/gastric | 2 (2%) |
| Cancer stage at diagnosis | |
| I | 13 (12%) |
| II | 3 (3%) |
| III | 14 (12%) |
| IV | 72 (64%) |
| N/A | 11 (10%) |
| Metastatic disease | 89 (80%) |
| Years since diagnosis | |
| ≤1 | 54 (48.6%) |
| 1-3 | 25 (23%) |
| >3 | 29 (27%) |
| Any treatment history | |
| Chemotherapy | 93 (83%) |
| Immunotherapy | 30 (27%) |
| Radiation | 46 (41%) |
| Surgery | 71 (64%) |
| Treatment last 6 weeks | 78 (70%) |
| Surgery last 3 months | 35 (32%) |
| Outpatient pain medication use (self-report) | |
| Opioids | 75 (43%) |
| Short-acting opioids | 41 (36%) |
| Long-acting opioids | 24 (21%) |
| OTC | 45 (26%) |
| Acetaminophen | 35 (20%) |
| Ibuprofen | 19 (11%) |
| Other RX pain meds | 16 (9%) |
| Cannabis | 15 (9%) |
| Prescription(s) given at discharge | |
| Opioids (for those without previous use) | |
| Single opioid on discharge | 19 (17%) |
| Non-opioid pain medication | 41 (36%) |
| Acetaminophen | 31 (28%) |
| Gabapentin | 8 (7%) |
| Ibuprofen | 1 (1%) |
| Pain on admission to ED | |
| Chronic pain prior to cancer diagnosis | 31 (27%) |
| Pain severity | 5.4 ± 1.9 |
| Psychological symptoms on admission to ED | |
| Depression PROMIS (T-score) | 54.1 ± 9.7 |
| Anxiety PROMIS (T-score) | 54.9 ± 10.9 |
| Sleep PROMIS (T-score) | 57.0 ± 8.4 |
| Pain Catastrophizing | 18.7 ± 12.5 |
| Perceived Stress Scale | 6.9 ± 3.2 |
The median number of EMR pain scores on hospital day 1 was 7 (n=109, M=7.27, SD=3.66, range: 1-17) and this number remained stable (6-8 scores/day) on subsequent hospital days (Table 2). For each patient, all pain scores for a 24-hour period were averaged (daily pain severity score). While there was a large amount of inter-individual variability, on average pain scores fell in the moderate severity range (3.4-5.0/10, Figure 1A). Most patients (87%) received opioid analgesics during their hospitalization (Figure 1B). Median daily dose of opioids administered was 20 MMEs (M=75.4, SD=159.9, range:0-1136), with the majority of patients receiving less than 60 MMEs per day. Average daily opioids administered across the group varied over time: 28.6 MMEs (SD=55.7) on hospital day 1, with a trend towards higher average MME/day across time (116 MMEs (SD= 300.6) on the 12th day of admission), possibly reflecting a shift in the distribution of patients as length of stay increased (Figure 1B). Only 3 patients received a pain intervention (e.g., nerve block) while hospitalized. The majority of patients were discharged home (87.6%), with the remaining discharged to hospice (6.1%) or a skilled nursing facility (4.4%), and 2 individuals died during hospitalization.
Table 2.
Number of pain scores collected per day during hospitalization
| Day inpatient | N | Mean | Std. Deviation | Median | Minimum | Maximum |
|---|---|---|---|---|---|---|
| 0 | 102 | 3.6 | 2.6 | 3.0 | 1.0 | 9.0 |
| 1 | 109 | 7.3 | 3.7 | 7.0 | 1.0 | 17.0 |
| 2 | 92 | 7.5 | 3.8 | 7.0 | 1.0 | 15.0 |
| 3 | 76 | 7.8 | 3.8 | 7.0 | 1.0 | 13.0 |
| 4 | 62 | 6.2 | 2.2 | 7.0 | 1.0 | 8.0 |
| 5 | 47 | 6.6 | 1.8 | 8.0 | 2.0 | 8.0 |
| 6 | 40 | 6.2 | 2.2 | 7.0 | 1.0 | 8.0 |
| 7 | 33 | 6.2 | 2.1 | 8.0 | 3.0 | 8.0 |
| 8 | 28 | 6.3 | 1.7 | 7.0 | 2.0 | 8.0 |
| 9 | 26 | 5.9 | 2.2 | 6.5 | 1.0 | 8.0 |
| 10 | 21 | 5.8 | 2.3 | 6.0 | 2.0 | 8.0 |
| 11 | 17 | 6.0 | 2.5 | 8.0 | 1.0 | 8.0 |
| 12 | 14 | 6.3 | 1.7 | 6.5 | 4.0 | 8.0 |
| 13 | 13 | 6.2 | 1.9 | 7.0 | 3.0 | 8.0 |
| 14 | 11 | 6.3 | 1.9 | 6.0 | 3.0 | 8.0 |
| 15 | 10 | 6.8 | 1.8 | 8.0 | 4.0 | 8.0 |
Figure 1.
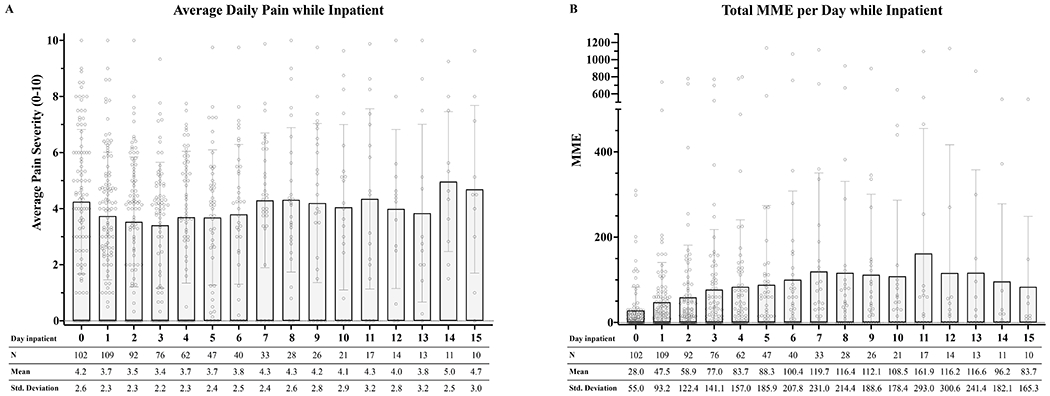
A. Daily average pain severity during hospitalization.
B. Daily average opioids administered (MMEs) during hospitalization.
Univariable GEE Analyses
Patient characteristics that were significantly associated with both daily pain severity and daily opioid administration during hospitalization on univariable GEE analysis included younger age, lower education, time since last recent cancer surgery, metastasis, outpatient opioid use, and greater depression, anxiety, and pain catastrophizing (p≤.05). Additionally, lower income, lower health literacy, greater sleep disturbance and perceived stress, and chronic pain predating a patient’s cancer diagnosis were significantly associated with greater daily pain during hospitalization, but not greater opioid administration (p≤.05, Table 3).
Table 3.
Univariable GEE analyses identifying risk factors of average daily pain severity and opioids administered (MMEs) in the hospital
| Average pain per day while inpatient | Total MME per day while inpatient | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| B (LCI, UCI) | Std. Error | Wald Chi-square | p | B (LCI, UCI) | Std. Error | Wald Chi-square | p | |
| Demographics | ||||||||
| Age | −0.0 (−0.1, 0.0) | 0.0 | 4.5 | 0.034 | −0.6 (−1.2, 0.0) | 0.3 | 4.0 | 0.046 |
| Male gender | −0.2 (−1.0, 0.6) | 0.4 | 0.2 | 0.651 | −8.7 (−27.6, 10.2) | 9.6 | 0.8 | 0.367 |
| Caucasian | −1.0 (−2.4, 0.5) | 0.8 | 1.7 | 0.197 | −30.9 (−87.6, 25.8) | 28.9 | 1.1 | 0.286 |
| College graduate | −0.9 (−1.7, −0.1) | 0.4 | 4.7 | 0.030 | −26.2 (−44.7, −7.7) | 9.4 | 7.7 | 0.006 |
| Currently smoke | 0.6 (−0.5, 1.8) | 0.6 | 1.2 | 0.275 | 0.1 (−23.1, 23.4) | 11.9 | 0.0 | 0.991 |
| Average income | −0.3 (−0.6, −0.1) | 0.1 | 5.6 | 0.018 | 0.9 (−6.7, 8.5) | 3.9 | 0.1 | 0.813 |
| Health literacy | 0.6 (0.1, 1.2) | 0.3 | 4.6 | 0.032 | 14.2 (−2.4, 30.9) | 8.5 | 2.8 | 0.094 |
| Insurance type | ||||||||
| Medicare | −0.2 (−1.2, 0.8) | 0.5 | 0.1 | 0.707 | −1.9 (−27.9, 24.2) | 13.3 | 0.0 | 0.888 |
| Medicaid | 0.2 (−0.7, 1.1) | 0.4 | 0.1 | 0.700 | −1.0 (−20.3, 18.2) | 9.8 | 0.0 | 0.916 |
| Other | −0.3 (−2.5, 1.8) | 1.1 | 0.1 | 0.766 | 20.9 (−23.9, 65.8) | 22.9 | 0.8 | 0.360 |
| Cancer and Surgical History | ||||||||
| Cancer treatment within the last 6 weeks | −0.8 (−1.8, 0.2) | 0.5 | 2.3 | 0.129 | −26.9 (−56.4, 2.6) | 15.1 | 3.2 | 0.074 |
| Time since most recent cancer surgery | −0.3 (−0.4, −0.1) | 0.1 | 8.6 | 0.003 | −3.4 (−6.8, 0.0) | 1.7 | 3.9 | 0.048 |
| Metastatic cancer | 1.3 (0.5, 2.2) | 0.4 | 9.6 | 0.002 | 26.9 (14.1, 39.6) | 6.5 | 17.0 | <.001 |
| Psychological | ||||||||
| Depression | 0.2 (0.1, 0.3) | 0.0 | 18.7 | <.001 | 2.3 (0.1, 4.5) | 1.1 | 4.3 | 0.038 |
| Anxiety | 0.2 (0.1, 0.3) | 0.1 | 10.6 | 0.001 | 2.8 (0.3, 5.3) | 1.3 | 4.7 | 0.030 |
| Sleep disturbance | 0.1 (0.0, 0.2) | 0.1 | 6.5 | 0.011 | 1.3 (−0.9, 3.6) | 1.2 | 1.3 | 0.246 |
| Pain catastrophizing | 0.1 (0.0, 0.1) | 0.0 | 19.6 | <.001 | 1.5 (0.1, 2.9) | 0.7 | 4.3 | 0.039 |
| Perceived stress | 0.2 (0.1, 0.3) | 0.1 | 9.5 | 0.002 | 2.0 (−0.3, 4.3) | 1.2 | 2.9 | 0.087 |
| Pain | ||||||||
| Previous history of chronic pain before cancer | 1.2 (0.4, 2.0) | 0.4 | 9.4 | 0.002 | 9.7 (−9.2, 28.6) | 9.6 | 1.0 | 0.314 |
| Outpatient opioid use | 2.2 (1.4, 2.9) | 0.4 | 34.1 | <.001 | 43.3 (23.8, 62.7) | 9.9 | 19.0 | <.001 |
| Length of stay | 0.1 (−0.0, 0.1) | 0.0 | 3.0 | 0.08 | 2.1 (−0.4, 4.7) | 1.3 | 2.6 | 0.11 |
Note: Reference group = female, private insurance, stage I cancer, non-metastatic cancer, and no history of previous chronic pain before cancer. Measures: Higher scores indicated higher values of that symptom PROMIS depression, anxiety, and sleep disturbance; Pain Catastrophizing Scale, Perceived Stress Scale, higher scores for health literacy indicate lower health literacy.
Abbreviations: LCI, lower confidence interval (95%); UCI, upper confidence interval (95%); MME, morphine milligram equivalents.
Multivariable GEE Analyses
Risk Factors for Higher Daily Pain Severity during Hospitalization
Patient characteristics that emerged as independently associated with greater daily pain severity on multivariable analysis included: recent surgery (B=−0.2 p=0.016), higher pain catastrophizing (B=0.1, p=0.001), outpatient opioid use (B=1.4, p≤0.001), and chronic pain predating a patient’s cancer diagnosis (B=0.8, p=0.022). Other demographic, clinical, and psychological factors were not independently related to daily pain severity in the multivariable model (Table 4, Figure 2).
Table 4.
Multivariable GEE analyses identifying risk factors of average daily pain severity and opioids administered (MMEs) during hospitalization
| Average daily pain | Average daily opioids administered | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| B (LCI, UCI) | Std. Error | Wald Chi-square | p | B (LCI, UCI) | Std. Error | Wald Chi-square | p | |
| Demographics | ||||||||
| Age | 0.0 (0.0, 0.0) | 0.0 | 2.3 | 0.129 | −0.4 (−1.0, 0.1) | 0.3 | 2.2 | 0.138 |
| College graduate | 0.0 (−0.7, 0.7) | 0.4 | 0.0 | 0.947 | −6.2 (−20.6, 8.1) | 7.3 | 0.7 | 0.396 |
| Average income | −0.1 (−0.2, 0.1) | 0.1 | 0.6 | 0.430 | -- | -- | -- | -- |
| Health literacy | 0.4 (0.0, 0.8) | 0.2 | 3.5 | 0.060 | 10.8 (−3.1, 24.8) | 7.1 | 2.3 | 0.128 |
| Cancer and surgical history | ||||||||
| Time since most recent cancer surgery | −0.2 (−0.3, 0.0) | 0.1 | 5.8 | 0.016 | −1.4 (−4.5, 1.7) | 1.6 | 0.8 | 0.377 |
| Metastatic cancer | 0.4 (−0.5, 1.4) | 0.5 | 0.7 | 0.387 | 16.2 (0.9, 31.5) | 7.8 | 4.3 | 0.038 |
| Psychological | ||||||||
| Depression | 0.0 (−0.2, 0.1) | 0.1 | 0.2 | 0.617 | −4.9 (−9.2, −0.5) | 2.2 | 4.8 | 0.028 |
| Anxiety | 0.0 (−0.1, 0.2) | 0.1 | 0.1 | 0.769 | 3.7 (0.4, 7.0) | 1.7 | 4.7 | 0.030 |
| Sleep disturbance | 0.0 (−0.1, 0.1) | 0.0 | 0.2 | 0.673 | -- | -- | -- | -- |
| Pain catastrophizing | 0.1 (0.0, 0.1) | 0.0 | 10.1 | 0.001 | 1.6 (0.0, 3.2) | 0.8 | 3.9 | 0.049 |
| Perceived stress | 0.0 (−0.1, 0.1) | 0.1 | 0.0 | 0.911 | −1.5 (−4.3, 1.3) | 1.4 | 1.2 | 0.280 |
| Pain | ||||||||
| Previous history of chronic pain before cancer | 0.8 (0.1, 1.5) | 0.4 | 5.2 | 0.022 | -- | -- | -- | -- |
| Outpatient opioid use | 1.4 (0.6, 2.1) | 0.4 | 13.3 | <.001 | 32.8 (17.2, 48.3) | 7.9 | 17.0 | <.001 |
Note: Reference group = non-metastatic cancer, and no history of previous chronic pain before cancer. Measures: Higher scores indicated higher values of that symptom PROMIS depression, anxiety, and sleep disturbance; Pain Catastrophizing Scale, Perceived Stress Scale, higher scores for health literacy indicate worse literacy.
Abbreviations: LCI, lower confidence interval (95%); UCI, upper confidence interval (95%); MME, morphine milligram equivalents.
Figure 2.
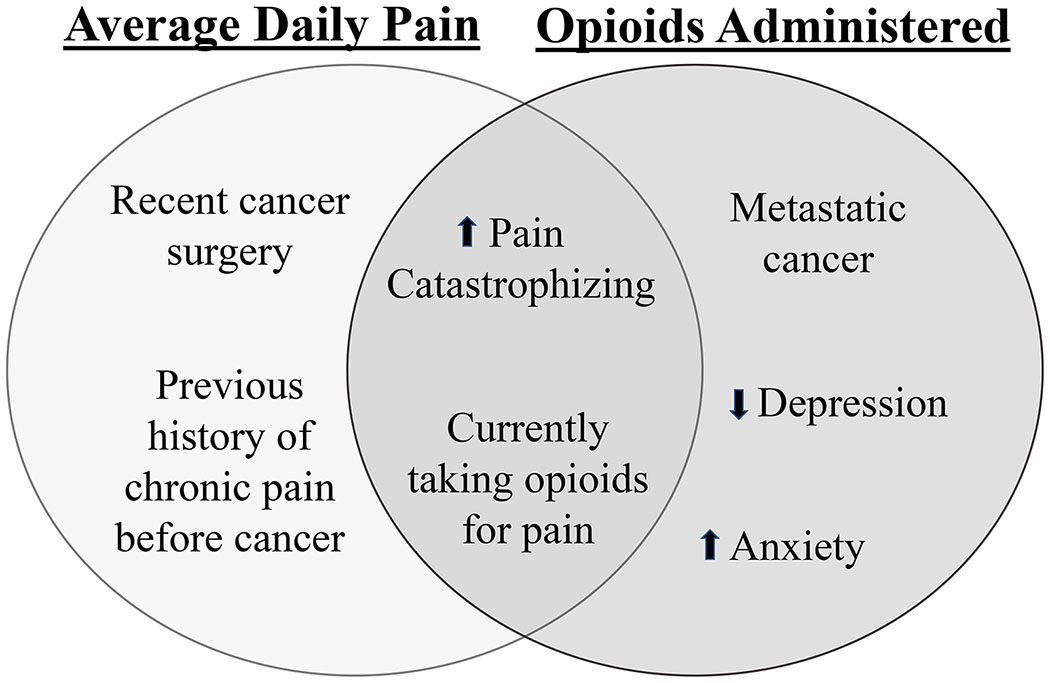
Risk factors of higher average daily pain severity and daily opioids administered during hospital admission.
Risk Factors of Higher Daily Opioid Administration during Hospital
Patient characteristics that emerged as independently associated with higher daily opioid administration on multivariable analysis included: metastatic disease (B=16.2, p=0.038), higher pain catastrophizing (B=1.6, p=0.049), higher anxiety symptoms (B=3.7, p=0.030), lower depressive symptoms (B=−4.9, p=0.028), and outpatient opioid use (B=32.8, p≤0.001). Other demographic, clinical, and psychological factors were not independently related to daily opioid administration in the multivariable model (Table 4, Figure 2).
New Analgesic Prescriptions at Hospital Discharge
A subset of patients who were not taking opioids when presenting to the hospital, subsequently received a new analgesic prescription at discharge. Of the 113 hospitalized patients, 17% (n=19) were discharged with a new opioid prescription (reported no previous outpatient opioid use before admission). These patients were more likely to have more advanced disease (15/19 with stage≥3 cancer; χ2=12.6, p=0.006). No other demographic, clinical, or psychological characteristics distinguished these patients from the larger group. A small proportion of the whole cohort were discharged with new anxiolytics (16%, n=18), antidepressants (8%, n=9), Gabapentin (7%, n=8), acetaminophen (28%, n=32), and Non-Steroidal Anti-Inflammatory Drugs (<1%, n=1).
DISCUSSION
In this prospective cohort study of hospitalized cancer patients, we observed that greater psychological symptoms were independently associated with worse pain and greater opioid administration. Specifically, controlling for demographic and clinical characteristics, higher pain catastrophizing was independently associated with both greater daily pain severity and daily opioid administration, while higher anxiety was associated with greater opioid administration. These findings, that both catastrophizing and anxiety are associated with worse pain outcomes among hospitalized cancer patients, may suggest that treatments which also address psychological symptoms could be helpful to incorporate into pain management plans.
Pain catastrophizing, a psychological process that includes rumination on and magnification of pain, as well as helpless thoughts about pain [32] was independently associated with both worse daily pain and greater daily opioid administration during hospitalization. For many, experiencing the diagnosis and treatment of cancer is life-altering and triggers significant distress, which can worsen pain [12]. It is understandable that patients with a cancer diagnosis experience significant worry when pain worsens, [28] as such worsening could potentially denote disease progression, resulting in fear, anxiety, and a desire for further evaluation. Our results highlight that catastrophic cognitions about pain have a significant, consistent, and independent association with worse pain outcomes, and may represent a potential target for interventions that address such cognitions.
It is plausible that patients who experience higher anxiety and higher catastrophic thoughts about pain may verbalize more about their pain experience, and thus, potentially receive more opioids during hospitalization. Interestingly, in contrast, greater depressive symptoms were associated with lower daily opioid administration in this sample. Possible explanations for this somewhat counterintuitive finding may be that depressive symptoms may result in patients being less vocal about their pain, an increased awareness of clinicians regarding the relationship between depression and opioid misuse which encourages less treatment of depressed affect with opioids, or a generally lesser or more complex association of depression with acute pain and opioid use. Several other studies have also identified that while anxiety or catastrophizing were associated with greater opioid use after surgery or during patients’ hospitalizations, depression in fact was not [6, 20, 21, 38, 40, 45]. While opioids should continue to be readily available to treat cancer-related pain, oncology care teams need to screen and treat psychological symptoms that co-occur with cancer pain because these symptoms are associated with worse pain outcomes and research has shown that psychological symptoms are risk factors for opioid misuse [12, 41]. Future studies which explicitly investigate interactions between depression and other psychological symptoms in the treatment of cancer-related pain are merited.
Not surprisingly, patients who reported taking opioids before hospitalization (43% of this sample) had greater daily opioid consumption and reported greater daily pain, highlighting the challenge of effective pain management in the presence of opioid tolerance. Currently, a consensus on “appropriate” opioid use for successful pain management remains a challenge in the context of cancer. Many patients struggle with outpatient self-management, with a resultant occasional over- or under- dosing of opioid analgesics, or use of opioids to manage psychological symptoms [31, 36]. Underutilization or insufficient dosing of analgesics may be related to fears of addiction/dependence and stigma related to the opioid epidemic, significant side effects (e.g., constipation), and/or lack of education on complex dosing instructions [4, 11]. Interestingly, only a quarter of patients in this cohort reported taking over-the-counter pain medications, and very few reported the use of cannabis. While opioids are the pharmacological mainstay for the management of moderate-to-severe cancer pain, evidence suggests that the use of opioids as a single modality may not be sufficient, and that use of multimodal treatments that target various aspects of the experience of pain may improve cancer pain management [25, 43].
Having metastatic disease was associated with greater opioid administration, but not worse pain severity. Interestingly, the sole distinguishing factor of patients receiving new opioid prescriptions on discharge was metastatic disease, perhaps indicating practitioners’ willingness to prescribe opioids to patients at a more advanced stage, and a willingness to employ opioids for the indication of acute worsening pain from metastatic disease.
Another notable independent predictor of worse pain was more recent cancer-related surgery. During oncologic treatment, surgery, radiation, and chemotherapy treatment are aimed to eliminate or slow cancer progression, thus theoretically alleviating the source of cancer pain. At times, these treatments may exacerbate pain and result in acute pain worsening that is unresponsive to treatment [31]. While we did not observe a significant relationship between recent cancer treatment (within the last 6 weeks) and pain during hospitalization, more recent surgery remained a consistent predictor in the multivariable model. Persistent Post-Surgical Pain (PPSP) is a recognized cause of new chronic pain, with incidence of PPSP ranging up to 50%, [30] and may put patients at greater risk of chronic opioid use [10]. Managing acute postsurgical pain in patients with chronic pain (whether from cancer or another source) is challenging, as patients with a history of chronic pain may have a greater degree of central sensitization [44]. The fact that proximity to a more recent cancer surgery was associated with greater pain underscores this challenge, and emphasizes the importance of preventive perioperative analgesic management and postoperative pain management for patients with cancer. Multidisciplinary transitional pain services, including a diverse team of clinicians, who perform comprehensive pain evaluations and offer multi-modal pain management approaches are becoming more common to help manage acute exacerbations of pain in ED or perioperative settings [23].
Psychological factors, which have been consistently associated with greater pain, may serve as rational targets for intervention [25, 41, 43]. From a clinical perspective, evaluating and openly discussing patients’ concerns about the underlying cause of their pain may decrease pain-related catastrophic thoughts. Pain education and brief meditation/relaxation exercises in the hospital may reduce pain-related anxiety [16]. The efficacy of a multimodal approach to pain treatment, including both pharmacologic and non-pharmacologic treatments for patients, is clear [8, 37]. Patient-centered, accessible psychological interventions have previously been shown to improve cancer pain [1, 19, 25, 43]. Development of personalized, culturally relevant, and accessible pain interventions [34], for example employing telehealth for rural populations, may allow a greater reach to patients who have traditionally had less access to palliative and specialized pain care [7, 33]. Increasing the education of clinicians about a variety of adjunctive therapies may improve accessibility of these resources as well [9].
Limitations
Despite the strengths of this prospective, observational design, several limitations warrant consideration. First, pain scores in the medical record may not reflect the entire pain experience, with some pain scores recorded both directly before and after receiving opioids, or at intervals around shift change. However, the estimation of daily pain was relatively frequent (typically 7 times/day) and employed averaging across an entire inpatient day. The common practice of recording both before and after opioid administration, together with the relatively random assignment of nursing staff to patients, may in fact potentially guard against spurious extremes, allowing this pragmatic methodology to perhaps give a more balanced view of pain. The numerical pain scores that are typically used in clinical practice also do not capture patients’ pain-related distress or pain interference. Although somewhat cumbersome to employ on a daily basis in the context of a busy inpatient hospitalization, more comprehensive evaluation of pain-related distress and interference might be feasible intermittently and should be included in future prospective studies. Second, while relatively balanced by gender, our sample had a higher percentage of patients who were white and well-educated. Future studies need to recruit a more diverse sample to allow for greater generalizability [29]. Third, while patients self-reported outpatient opioid use, the exact dosage was not ascertained, which did not allow for an estimation of the degree of opioid tolerance. Lastly, patients in this study presented to the ED with significant pain, which may limit the generalizability of our findings to the outpatient or other contexts. Interestingly, rates of opioid use [3, 6] and previous chronic pain [27, 40] in our sample are similar to those previously reported in postoperative and outpatient oncology pain samples.
Conclusion
The prospective evaluation of a wide range of characteristics of individual cancer patients revealed significant independent associations of both psychological factors (catastrophizing and anxiety) and medical factors (recent surgery, previous opioid use and chronic pain) with greater daily pain severity and opioid administration during hospitalization. As pain continues to be a difficult-to-treat symptom in patients with cancer, our findings may help inform more comprehensive and personalized pain treatment.
ACKNOWLEDGEMENTS
The authors would like the thank the participants for engaging in this work, and with the research team at BWH emergency medicine department to collect the data for this work.
Funding:
NIH/NIGMS: R35 GM128691 (KLS), The National Palliative Care Research Center Kornfeld Scholars Award – grant number not applicable (DRA), NIH/NCI: K08 CA266937 (DRA), NIH/NIDA: K23 DA044874 (PRC), DP2DA056107 (PRC), Defense Advanced Research Projects Agency – grant number not applicable (PRC).
Footnotes
Conflict of Interest Statement: The authors have no conflicts of interest to declare.
REFERENCES
- [1].Aapro M, Bossi P, Dasari A, Fallowfield L, Gascón P, Geller M, Jordan K, Kim J, Martin K, Porzig S. Digital health for optimal supportive care in oncology: benefits, limits, and future perspectives. 28 2020;Supportive Care in Cancer (10):4589–4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Agarwal A, Roberts A, Dusetzina S, Royce T. Changes in opioid prescribing patterns among generalists and oncologists for Medicare part D beneficiaries from 2013 to 2017. JAMA Oncology 2020;6(8):1271–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Apolone G, Corli O, Caraceni A, Negri E, Deandrea S, Montanari M, Greco MT. Pattern and quality of care of cancer pain management. Results from the Cancer Pain Outcome Research Study Group. British Journal of Cancer 2009;100(10):1566–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Azizoddin D, Knoerl R, Adam R, Kessler D, Tulsky J, Edwards R, Enzinger A. Cancer pain self-management in the context of a national opioid epidemic: Experiences of patients with advanced cancer using opioids. Cancer 2021;127(17):3254–3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Azizoddin DR, Beck M, Flowers KM, Wilson JM, Chai P, Johnsky L, Cremone G, Edwards R, Hasdianda A, Boyer E, Schreiber KL. Psychological Evaluation of Patients With Cancer Presenting to the Emergency Department With Pain: Independent Predictors of Worse Pain Severity, Interference, and Higher Hourly Opioid Administration. JCO Oncology Practice 2022;18(10):e1648–e1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Azizoddin DR, Schreiber K, Beck MR, Enzinger AC, Hruschak V, Darnall BD, Edwards RR, Allsop MJ, Tulsky JA, Boyer E, Mackey S. Chronic pain severity, impact, and opioid use among patients with cancer: An analysis of biopsychosocial factors using the CHOIR learning health care system. Cancer-Am Cancer Soc 2021;127(17):3254–3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bakitas M, Watts K, Malone E, Dionne-Odom J, McCammon S, Taylor R, Tucker R, Elk R. Forging a new frontier: providing palliative care to people with cancer in rural and remote areas. Journal of Clinical Oncology 2020;38(9):963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bennett M, Paice J, Wallace M. Pain and opioids in cancer care: benefits, risks, and alternatives American Society of Clinical Oncology Educational Book 2017;37:705–713. [DOI] [PubMed] [Google Scholar]
- [9].Breuer B, Fleishman S, Cruciani R, Portenoy R. Medical oncologists’ attitudes and practice in cancer pain management: a national survey. J Clin Oncol 2011;29(36):4769–4775. [DOI] [PubMed] [Google Scholar]
- [10].Brummett C, Waljee J, Goesling J, Moser S, Lin P, Englesbe M, Bohnert A, Kheterpal S, Nallamothu B. New Persistent Opioid Use After Minor and Major Surgical Procedures in US Adults. JAMA Surgery 2017;152(6):e170504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bulls H, Chu E, Goodin B, Liebschutz J, Wozniak A, Schenker Y, Merlin J. Framework for opioid stigma in cancer pain. Pain 2021;163(2):e182–e189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Carlson LE, Waller A, Mitchell AJ. Screening for distress and unmet needs in patients with cancer: review and recommendations. J Clin Oncol 2012;30(11):1160–1177. [DOI] [PubMed] [Google Scholar]
- [13].Caterino JM, Adler D, Durham DD, Yeung SJ, Hudson MF, Bastani A, Bernstein SL, Baugh CW, Coyne CJ, Grudzen CR, Henning DJ, Klotz A, Madsen TE, Pallin DJ, Reyes-Gibby CC, Rico JF, Ryan RJ, Shapiro NI, Swor R, Venkat A, Wilson J, Thomas CR Jr., Bischof JJ, Lyman GH. Analysis of Diagnoses, Symptoms, Medications, and Admissions Among Patients With Cancer Presenting to Emergency Departments. Jama Netw Open 2019;2(3):e190979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Cella D, Choi S, Condon D, Schalet B, Hays R, Rothrock N, Yount S, Cook K, Gershon R, Amtmann D. PROMIS® adult health profiles: efficient short-form measures of seven health domains. Value in health 2019;22(5):537–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Cella D, Yount S, Rothrock N, Gershon R, Cook K, Reeve B, Ader D, Fries JF, Bruce B, Rose M, Group PC. The Patient-Reported Outcomes Measurement Information System (PROMIS): progress of an NIH Roadmap cooperative group during its first two years. Med Care 2007;45(5 Suppl 1):S3–S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chai P, Schwartz E, Hasdianda M, Azizoddin D, Kikut A, Jambaulikar G, Edwards R, Boyer E, Schreiber K. A brief music app to address pain in the emergency department: Prospective study. Journal of Medical Internet Research 2020;22(5):e18537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chew L, Bradley K, Boyko E. Brief questions to identify patients with inadequate health literacy. Family Medicine 2004;36(8):588–594. [PubMed] [Google Scholar]
- [18].Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav 1983;24(4):385–396. [PubMed] [Google Scholar]
- [19].Coyne C, Reyes-Gibby C, Durham D, Abar B, Adler D, Bastani A, Bernstein S, Baugh C, Bischof J, Grudzen C, Henning D, Hudson M, Klotz A, Lyman G, Madsen T, Pallin D, Rico J, Ryan R, Shapiro N, Swor R, Thomas CJ, Venkat A, Wilson J, Yeung S, Caterino J. Cancer pain management in the emergency department: a multicenter prospective observational trial of the Comprehensive Oncologic Emergencies Research Network (CONCERN). Support Care Cancer 2021;29(8):4543–4553. [DOI] [PubMed] [Google Scholar]
- [20].Darnall BD, Ziadni MS, Flood PKP, Heathcote L, Mackey IG, Taub CJ, Wheeler A. “My Surgical Success”: Effect of a Digital Behavioral Pain Medicine Intervention on Time to Opioid Cessation After Breast Cancer Surgery—A Pilot Randomized Controlled Clinical Trial. Pain Medicine 2019;0(0):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Dunn L, Durieux M, Fernández L, Tsang S, Smith-Straesser E, Jhaveri H, Spanos S, Thames M, Spencer C, Lloyd A. Influence of catastrophizing, anxiety, and depression on in-hospital opioid consumption, pain, and quality of recovery after adult spine surgery. Journal of Neurosurgery: Spine 2017;28(1):119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Enzinger A, Ghosh K, Keating N, Cutler D, Landrum M, Wright A. US Trends in Opioid Access Among Patients With Poor Prognosis Cancer Near the End-of-Life. Journal of Clinical Oncology 2021;39(26). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Featherall J, Anderson J, Anderson L, Bayless K, Anderson Z, Brooke B, Gililland J, Buys M. A Multidisciplinary Transitional Pain Management Program Is Associated With Reduced Opioid Dependence After Primary Total Joint Arthroplasty. The Journal of Arthroplasty 2022;37(6):1048–1053. [DOI] [PubMed] [Google Scholar]
- [24].Gallaway MS, Idaikkadar N, Tai E, Momin B, Rohan EA, Townsend J, Puckett M, Stewart SL. Emergency department visits among people with cancer: Frequency, symptoms, and characteristics. Journal of the American College of Emergency Physicians Open 2021;2(3):e12438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gorin S, Krebs P, Badr H, Janke EA, Jim HS, Spring B, Mohr DC, Berendsen MA, Jacobsen PB. Meta-analysis of psychosocial interventions to reduce pain in patients with cancer. J Clin Oncol 2012;30(5):539–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, McLeod L, Delacqua G, Delacqua F, Kirby J, Duda SN, Consortium RE. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hasuo H, Sakai K. Clinical Characteristics of Noncancer-Related Upper Back Pain on Initiation of Palliative Care in Patients with Incurable Cancer. Palliat Med Rep 2021;2(1):335–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hay J, Buckley T, Ostroff J. The role of cancer worry in cancer screening: a theoretical and empirical review of the literature. Psycho-Oncology: Journal of the Psychological, Social and Behavioral Dimensions of Cancer 2005;14(7):517–534. [DOI] [PubMed] [Google Scholar]
- [29].Janevic M, Mathur V, Booker S, Morais C, Meints S, Yeager K, Meghani S. Making Pain Research More Inclusive: Why and How. The Journal of Pain 2021;23(5):707–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kehlet H, Jensen T, Woolf C. Persistent postsurgical pain: risk factors and prevention. The Lancet 2006;367(9522):1618–1625. [DOI] [PubMed] [Google Scholar]
- [31].Kwon JH. Overcoming barriers in cancer pain management. J Clin Oncol 2014;32(16):1727–1733. [DOI] [PubMed] [Google Scholar]
- [32].Lazaridou A, Koulouris A, Devine J, Haack M, Jamison R, Edwards R, Schreiber K. Impact of daily yoga-based exercise on pain, catastrophizing, and sleep amongst individuals with fibromyalgia. Journal of Pain Research 2019;12:2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Levit L, Byatt L, Lyss A, Paskett E, Levit K, Kirkwood K, Schenkel C, Schilsky R. Closing the rural cancer care gap: three institutional approaches. JCO oncology practice 2020;16(7):422–430. [DOI] [PubMed] [Google Scholar]
- [34].Ludwick A, Corey K, Meghani S. Racial and socioeconomic factors associated with the use of complementary and alternative modalities for pain in cancer outpatients: an integrative review. Pain Management Nursing 2020;21(2):142–150. [DOI] [PubMed] [Google Scholar]
- [35].Mayer DK, Travers D, Wyss A, Leak A, Waller A. Why do patients with cancer visit emergency departments? Results of a 2008 population study in North Carolina. J Clin Oncol 2011;29(19):2683–2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Miaskowski C, Dodd M, West C, Paul S, Tripathy D, Koo P, Schumacher K. Lack of adherence with the analgesic regimen: a significant barrier to effective cancer pain management. Journal of clinical oncology 2001;19(23):4275–4279. [DOI] [PubMed] [Google Scholar]
- [37].Mishra S, Bhatnagar S, Goyal G, Rana S, Upadhya S. A comparative efficacy of amitriptyline, gabapentin, and pregabalin in neuropathic cancer pain: a prospective randomized double-blind placebo-controlled study. American Journal of Hospice and Palliative Medicine® 2012;29(3):177–182. [DOI] [PubMed] [Google Scholar]
- [38].Pagé M, Karanicolas P, Cleary S, Wei A, McHardy P, Ladak S, Ayach N, Sawyer J, McCluskey S, Srinivas C. In-hospital opioid consumption, but not pain intensity scores, predicts 6-month levels of pain catastrophizing following hepatic resection: A trajectory analysis. European Journal of Pain 2019;23(3):503–514. [DOI] [PubMed] [Google Scholar]
- [39].Panattoni L, Fedorenko C, Greenwood-Hickman MA, Kreizenbeck K, Walker JR, Martins R, Eaton KD, Rieke JW, Conklin T, Smith B, Lyman G, Ramsey SD. Characterizing Potentially Preventable Cancer- and Chronic Disease–Related Emergency Department Use in the Year After Treatment Initiation: A Regional Study. J Oncol Pract 2018;14(3):e176–e185. [DOI] [PubMed] [Google Scholar]
- [40].Schreiber KL, Zinboonyahgoon N, Xu XL, Spivey T, King T, Dominici L, Partridge A, Golshan M, Strichartz G, Edwards RR. Preoperative Psychosocial and Psychophysical Phenotypes as Predictors of Acute Pain Outcomes After Breast Surgery. J Pain 2019;20(5):540–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Sharifzadeh Y, Kao M, Sturgeon J, Rico T, Mackey S, Darnall B. Pain Catastrophizing Moderates Relationships between Pain Intensity and Opioid Prescription: Nonlinear Sex Differences Revealed Using a Learning Health System. Anesthesiology 2017;127(1):136–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Sullivan MJL, Bishop SR, & Pivik J. The Pain Catastrophizing Scale: Development and validation. Psychological Assessment, 1995;7(4), 524–532. [Google Scholar]
- [43].Syrjala KL, Jensen MP, Mendoza ME, Yi JC, Fisher HM, Keefe FJ. Psychological and behavioral approaches to cancer pain management. J Clin Oncol 2014;32(16):1703–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Woolf C Central sensitization: implications for the diagnosis and treatment of pain. PAIN 2011;152(3):S2–S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Yennurajalingam S, Arthur J, Reddy S, Edwards T, Lu Z, Moraes ARd, Wilson SM, Erdogan E, Joy MP, Ethridge SD, Kuriakose L, Malik JS, Najera JM, Rashid S, Qian Y, Kubiak MJ, Nguyen K, Wu J, Hui D, Bruera E. Frequency of and factors associated with nonmedical opioid use behavior among patients with cancer receiving opioids for cancer pain. JAMA Oncology 2021. 7(3):401–411. [DOI] [PMC free article] [PubMed] [Google Scholar]


