Abstract
Maintenance peritoneal dialysis (PD) is commonly associated with cardiovascular diseases (CVDs), whose risk is assessed via LDL-C. Nonetheless, oxidized LDL (oxLDL), as being a key component of atherosclerotic lesions, could be also associated with atherosclerosis and related CVDs. However, its predictive value for CVDs risk assessment is subject of research studies due to the lack of specific methods to measure oxLDL status from its individual lipid/protein components. In the present study, six novel oxLDL markers, representative of certain oxidative modifications on the LDL protein and lipid components, are measured in atherosclerosis-prone PD patients (39) versus those in chronic kidney disease patients (61) under hemodialysis (HD) and healthy controls (40). LDL from serum of PD, HD and control subjects were isolated and fractionated into cholesteryl esters, triglycerides, free cholesterol, phospholipids and apolipoprotein B100 (apoB100). Subsequently the oxLDL markers cholesteryl ester hydroperoxides (-OOH), triglyceride-OOH, free cholesterol-OOH, phospholipid-OOH, apoB100 malondialdehyde and apoB100 dityrosines were measured. LDL carotenoid levels and LDL particle serum concentration were also measured. The levels of all oxLDL lipid-OOH markers were significantly elevated in PD patients versus control, while the levels of cholesteryl ester-/triglyceride-/free cholesterol-OOH were significantly elevated in PD versus HD patients, regardless of patients’ underlying medical conditions, sex, age, PD type, clinical biochemical markers and medication. It should be noted that all fractionated lipid-OOH levels were inversely correlated with LDL-P concentration, while LDL-P concentration was not correlated with LDL-C in PD patients. Moreover, LDL carotenoids were significantly lower in PD patients versus control. The increased levels of oxLDL status specific markers in both PD and HD patients (compared to control), support a potential prognostic value of oxLDL regarding CVD risk assessment in both patient groups. Lastly, the study introduces the oxLDL peroxidation markers free cholesterol-OOH and cholesteryl ester-OOH as complementary to LDL-P number, and as possible alternatives to LDL-C.
Keywords: Atherosclerosis, Peritoneal dialysis, Oxidized LDL, Oxidative stress, Clinical markers
Graphical abstract
Highlights
-
•
Association of oxidized LDL (oxLDL) with peritoneal dialysis (PD) patients.
-
•
Six novel oxLDL markers based on oxLDL lipid/protein components.
-
•
OxLDL markers are higher in PD versus hemodialysis patients.
-
•
OxLDL markers do not correlate with LDL-C and LDL-C/HDL-C in PD patients.
-
•
Most oxLDL marker levels correlate inversely with LDL particle number.
1. Introduction
Chronic kidney disease (CKD) is an adverse medical condition affecting 11–13% of the world's population [1]. Gradual deterioration of kidney function in CKD patients leads to end stage kidney disease (ESKD) which is mainly treated with hemodialysis (HD) or peritoneal dialysis (PD). Although HD is the most commonly followed procedure, PD is followed by 12–15% of ESKD patients [1]. A high prevalent adverse medical comorbidity in ESKD patients, including PD patients, is atherosclerosis [2]. Oxidized LDL (oxLDL) could be associated with atherosclerosis and related cardiovascular diseases (CVDs) because they are key components of atherosclerotic lesions. OxLDL are known to accumulate in the arterial subendothelial space, where their impeding phagocytosis by macrophages transform them into apoptotic foam cells, leading to atherosclerotic lesions formation [3]. These are responsible for the development and progression of CVD, which is the main cause of death in ESKD patients [4,5] and affect over half of the dialysis population [6]. The main CVD risk factors present in this population consist of traditional risk factors such as hypertension, diabetes, dyslipidemia, obesity, smoking, sedentary lifestyle [1,6,7], and non-traditional uremia-related risk factors such as inflammation, endothelial dysfunction, malnutrition, hypoalbuminemia, anemia, abnormal calcium metabolism, uremic toxins and oxidative stress (OS) [1,6].
From all the aforementioned risk factors, OS seems to play a major role regarding CVD development in PD patients [[8], [9], [10]], since it leads to the formation of, the atherosclerotic lesion-initiating, oxLDL particles [11]. It has been shown that CKD patients, and especially ESKD patients, present high levels of OS [1,12], attributed to factors such as: use of bioincompatible PD solutions that induce an oxidative response of peritoneal cells [1,8,9], high-temperature sterilization of glucose-containing PD solutions [leading to the formation of pro-oxidants advanced glycation end-products (AGEs) and glucose degradation products (GDPs)] [1,8,9], chronic inflammation [8], uremia [1,13], reduced pro-oxidants clearance from blood due to kidney dysfunction [1] and reduced levels of blood antioxidants [1]. Therefore, oxLDL status evaluation can be a, complementary to the already established ones, clinical marker that could contribute to a better CVD development risk assessment in patients on PD, especially when considering that several studies pinpoint the inadequacy of the currently used LDL-C clinical marker [14] and of the LDL particle (LDL-P) number (plasma concentration) marker that is limited to research studies [15]. It is worth mentioning that several studies show that the LDL-P number marker proves a better atherosclerosis risk indicator when two more parameters are taken into account. These are mostly the size and especially the oxidative modifications of LDL-P, known to play crucial role in atherosclerotic lesion development [15].
Despite OS's crucial role in CVD development in CKD patients, there is a limited number of studies on the correlation between OS and CKD [12], and even fewer regarding oxLDL and CVD development in CKD, the majority of which have been performed on ESKD patients on HD. The number of studies regarding the evaluation of oxLDL levels in patients on PD is even more limited [1]. Some of these studies measure oxLDL levels directly in the blood of PD patients [3,10,13,[16], [17], [18], [19], [20], [21]], while the others measure the generated in the blood autoantibodies against oxLDL (anti-oxLDL Ab) [4,[20], [21], [22], [23], [24], [25], [26], [27]]. However, all these studies are not in unison in their oxLDL marker related conclusions, with the main problem being the fact that they report contradictory results due to the use of non-specific and non-standardized methodologies for evaluating oxLDL status [28]. Furthermore, anti-oxLDL Ab levels are questionable as atherosclerotic marker, since some studies consider them as atheroprotective while others as atherogenic molecules [11].
In this study, we evaluated oxLDL in PD patients (versus a control group) by the quantification of specific oxidative modifications on LDL lipid and protein components and by the quantification of LDL carotenoid content, using novel, simple and clinically applicable methods previously developed [28]. These methods include the isolation of LDL particles (with >90% recovery and at 90% purity [28]) by a heparin/MgCl2-based precipitation method in order to avoid the time-consuming and cumbersome ultracentrifugation alternative. Subsequently, LDL particles are for the first-time fractionated into their main components [apolipoprotein B100 (apoB100), cholesteryl esters, triglycerides, free cholesterol, phospholipids, carotenoids and tocopherols] using an organic solvent extraction methodology. This allows the characterization and quantification of the specific oxidative modifications present on the LDL components and thus allowing a more specific characterization of the, so far undefined, oxLDL status. In the present study, the oxidative markers lipid hydroperoxides (-OOH), on the four LDL lipid fractions, as well as malondialdehyde (MDA) and dityrosines (DiTyr) on apoB100 were quantified using simple photometric and fluorometric methods, respectively, while LDL carotenoids were also quantified by a photometric method instead of the cumbersome HPLC alternative. Furthermore, the levels of the tested oxLDL markers in PD patients were also compared with those of CKD on HD patients, which have been previously studied by our group [28]. Our goal was to investigate possible correlation between oxLDL levels and PD and to evaluate possible use of oxLDL as a more reliable clinical marker for CVD prevalence in patients on PD.
2. Materials and methods
2.1. Subjects
In the present study, 39 PD patients (22–92 years old) and 40 healthy, sex and age matched, control adults (23–67 years old) were recruited from the Department of Nephrology and Kidney Transplantation of the University Hospital of Patras, Greece. Additionally, data from 61 HD patients participating in a recent study of our group [28] were also used. Demographic and clinical data for all PD, HD and control group subjects are presented in Table 1. Participation in the study was voluntary and all the subjects gave a written consent, after thorough briefing on the purposes of this particular study. The protocol of the study was approved by the Scientific Committee of the University Hospital of Patras, Greece (No.353/02/09/2015). The employed experimental procedures are in accordance with the ethical standards of the Helsinki Declaration of 1975, as revised in 2013.
Table 1.
PD, HD and control subjects’ age, sex and medical status. As medical status is defined the type of peritoneal dialysis followed, the underlying medical conditions and the medication received.
| PD patients | HD patients | Control | ||
|---|---|---|---|---|
| Age (years) Mean (±SD) | 22 to 92 59.4 (±17) | 20 to 94 61.9 (±15.2) | 23 to 67 45.2 (±14) | |
| N | ||||
| Sex | ||||
| Male | 25 | 41 | 25 | |
| Female | 14 | 20 | 15 | |
| Medical status | ||||
| Peritoneal dialysis type | CAPD | 25 | ||
| NIPD | 10 | |||
| IPD | 2 | |||
| CCPD | 2 | |||
| Medical conditions | ||||
| Hypertension | 38 | 26 | – | |
| Coronary heart disease (CHD) | 6 | 11 | – | |
| Peripheral artery disease (PAD) | 11 | 24 | – | |
| Cardiovascular disease (CVD) | 16 | 34 | – | |
| Diabetes | 10 | 19 | – | |
| Medication | ||||
| Statin treatment | 27 | 22 | – | |
| Alfacalcidol | 5 | 8 | – | |
| Paricalcitol | 13 | 11 | – | |
| ACE inhibitors | 6 | 3 | – | |
| Angiotensin receptor blockers (ARBs) | 20 | 11 | – | |
| Calcium channel blockers (CaChBI) | 22 | 15 | – | |
| Beta blockers | 36 | 35 | – | |
| Vitamin B supplement | 1 | 19 | – | |
Table Notes:
CAPD: continuous ambulatory peritoneal dialysis.
NIPD: nocturnal intermittent peritoneal dialysis.
IPD: intermittent peritoneal dialysis.
CCPD: continuous cycling peritoneal dialysis.
2.2. Methods
The methods employed in the present study, briefly outlined below, involve the application of innovative protocols for the isolation of LDL particles and their further fractionation into their main components, as well as innovative assays for the quantification of specific LDL oxidative modifications.
Blood from PD patients and control subjects was separated into serum, was supplemented with 1/1 mM BHA/BHT (in order to avoid LDL oxidation during isolation and handling) and can be used immediately for LDL isolation, or stored frozen at −80 °C. LDL were selectively isolated from 2 ml blood serum by an innovative precipitation methodology, which combines a heparin-citrate-based LDL precipitation at pH 5.12 and a second MgCl2-based LDL precipitation, as described in detail in a previous publication of our group [28]. Then, the isolated LDL were fractionated into their protein and total lipid plus antioxidant components [28]: The protein fraction consists of a single protein (apoB100), while the total LDL lipid fraction consists of cholesteryl esters, triglycerides, free cholesterol and phospholipids and the LDL antioxidant molecules carotenoids and a-tocopherol. Subsequently, an innovative method was applied to sub-fractionate the resulting total lipids into cholesteryl esters, triglycerides, free cholesterol and phospholipids as well as carotenoids and a-tocopherol [28].
Then, LDL apoB100 and lipid sub-fractions were assayed for the following six specific oxidative modifications/markers [assays details in Ref. [28]]: (i) the hydroperoxides (-OOH) of cholesteryl esters (cholesteryl ester-OOH), triglycerides (triglyceride-OOH), free cholesterol (free cholesterol-OOH) and phospholipids (phospholipid-OOH), (ii) the product of peroxidized lipids, MDA, bound to apoB100 (apoB100-MDA) and (iii) DiTyr formed on apoB100 (apoB100-DiTyr). Finally, the levels of LDL carotenoids were measured [28] and LDL-P number was calculated for each PD patient using a novel approach based on the weight of the isolated apoB100 (combined with apoB100 molecular weight), as described elsewhere [28].
2.3. Statistical analysis
Data are presented as mean (M) ± standard deviation (SD). All statistical analysis was performed with the use of IBM SPSS Statistics 26. A normality test (Kolmogorov-Smirnov when sample size is ≥ 50 or Shapiro-Wilk when sample size is ≤ 50) was performed on all numerical data. For the comparison of the not normally distributed LDL oxidation markers between control and PD patients, as well as between PD and HD, the Mann-Whitney U test was performed, after having checked and confirmed their homogeneity of variance by the Levene's test. Furthermore, in order to search for possible association between the medical status of PD patients and LDL oxidation markers, the Mann-Whitney U test was also performed (since data are also not normally distributed), and the homogeneity of variance was checked and confirmed by the Levene's test. Regarding the correlation between the tested LDL oxidation markers and PD patients' LDL-carotenoid levels, age and recorded clinical biochemical markers (LDL-C, HDL-C, total cholesterol, triglycerides, CRP, vitamin D, iPTH, Ca, P, CaxP), the Linear Regression Analysis was performed. Also, for checking possible correlation between the four peritoneal dialysis types (in which PD patients are subjected) and the tested LDL oxidation markers the Kruskal-Wallis test was performed, since the data are not normally distributed. The significance level for all tests was set at p ≤ 0.05.
Finally, a precision statistical analysis with time (between-run and between-day repeatability) as well as within a sole analytical run was performed for the LOOH, apoB100-MDA and apoB100-DiTyr assays. For the three aforementioned assays, we measured three successive dilutions for each sample and calculated their mean value.
3. Results
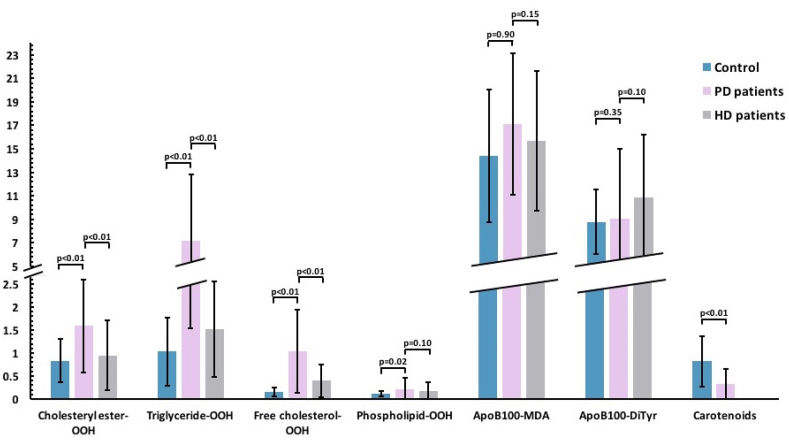
The following six oxLDL markers (four on LDL lipid sub-classes and two on LDL apoB100) were measured in LDL from the blood serum of PD patients compared to the control group: cholesteryl ester-OOH, triglyceride-OOH, free cholesterol-OOH, phospholipid-OOH, apoB100-MDA, apoB100-DiTyr (Fig. 1).
Fig. 1.
OxLDL markers in PD and HD patients versus control group. Cholesteryl ester-OOH levels are significantly higher in PD versus HD patients and control group (1.59 ± 1.01 vs 0.95 ± 0.76 and 0.83 ± 0.47), as are also free cholesterol-OOH levels (0.74 ± 0.90 vs 0.40 ± 0.35 and 0.16 ± 0.10), and triglyceride-OOH levels (7.17 ± 5.64 vs 1.52 ± 1.04 and 1.03 ± 0.74), respectively. Phospholipid-OOH levels are significantly higher in PD patients versus control (0.22 ± 0.24 vs 0.12 ± 0.06) and higher but not significantly in PD versus HD patients (0.18 ± 0.19). All these markers are expressed as nmoles cum-OOH equivalent/mg apoB100. ApoB100-MDA levels (expressed as pmoles MDA/mg apoB100) are higher, but not significantly, in PD versus HD patients and control (0.22 ± 0.24 vs 0.18 ± 0.19 and 0.12 ± 0.06). ApoB100-DiTyr levels (expressed as pmoles DiTyr/mg apoB100) are higher, but not significantly, in PD patients versus control (9.06 ± 5.97 vs 8.79 ± 2.78) and lower, but not significantly in PD versus HD patients (10.84 ± 5.40). LDL carotenoid levels (expressed as nmoles carotenoids/mg apoB100) are significantly higher in PD patients versus control (0.82 ± 0.55 vs 0.32 ± 0.33). OxLDL markers' average level comparisons are shown in a numerical Y-axis (with units ± standard deviation presented as error bars).
Regarding the comparison of PD patients with the control group, we found that all four LDL lipid sub-classes markers (cholesteryl ester-OOH, triglyceride-OOH, free cholesterol-OOH, phospholipid-OOH) were significantly higher (2-fold, 7-fold, 4.5-fold and 2-fold respectively), while the two oxLDL apoB100 markers (apoB100-MDA, apoB100-DiTyr) were slightly, but not significantly, higher in PD patients compared to the control group (Table 2). In more detail, the Mann-Whitney U test showed that cholesteryl ester-OOH (U = 138.00, z = −4.04, p < 0.01), triglyceride-OOH (U = 18.00, z = −5.96, p < 0.01), free cholesterol-OOH (U = 12.50, z = −6.05, p < 0.01) and phospholipid-OOH (U = 247.50, z = −2.29, p = 0.02) levels were significantly elevated in PD patients compared to the control group, while apoB100-MDA (U = 382.00, z = −0.13, p = 0.90) and apoB100-DiTyr (U = 332.00, z = −0.93, p = 0.35) levels did not differ significantly between the two groups.
Table 2.
OxLDL markers and LDL carotenoid levels in PD patients compared to the control group.
| OxLDL marker | Subject | Value | p-value | Fold change (PD vs control) |
|---|---|---|---|---|
| Cholesteryl ester-OOH | Control | 0.83 (±0.47) | <0.01 | 1.92 |
| PD | 1.59 (±1.01) | |||
| Triglyceride-OOH | Control | 1.03 (±0.74) | <0.01 | 6.96 |
| PD | 7.17 (±5.64) | |||
| Free cholesterol-OOH | Control | 0.16 (±0.10) | <0.01 | 4.63 |
| PD | 0.74 (±0.90) | |||
| Phospholipid-OOH | Control | 0.12 (±0.06) | 0.02 | 1.83 |
| PD | 0.22 (±0.24) | |||
| apoB100-MDA | Control | 14.40 (±5.68) | 0.90 | 1.19 |
| PD | 17.11 (±6.01) | |||
| apoB100-DiTyr | Control | 8.79 (±2.78) | 0.35 | 1.03 |
| PD | 9.06 (±5.97) | |||
| Carotenoids | Control | 0.82 (±0.55) | <0.01 | 0.39 |
| PD | 0.32 (±0.33) |
Table notes:
Values are presented as mean (M) ± standard deviation (SD).
Cholesteryl ester-OOH, triglyceride-OOH, free cholesterol-OOH, phospholipid-OOH markers are expressed as nmoles cum-OOH equivalent/mg apoB100. ApoB100-MDA marker is expressed as pmoles MDA/mg apoB100 and apoB100-DiTyr marker is expressed as pmoles DiTyr/mg apoB100.
Carotenoids are expressed as nmoles carotenoids/mg apoB100.
Subsequently, we investigated whether the significantly increased levels of the four LDL lipid sub-classes oxidation markers in PD group correlated with demographics and medical status. Specifically, there was no correlation between sex and LDL lipid sub-classes oxidation markers (Supplement, Table S1). Regarding PD patients' underlying medical conditions, we found no statistically significant correlation between CHD, PAD, CVD and diabetes and the levels of the four LDL lipid sub-classes oxidation markers (Supplement, Table S1). Regarding PD patients' medication, we investigated whether alfacalcidol, paricalcitol, ACE inhibitors, ARBs, CaChBI and statin correlated with the elevated levels of the four LDL lipid sub-classes oxidation markers. No such correlation was found as well (Supplement, Table S2). Lastly, no correlation was found between LDL lipid sub-classes oxidation markers and PD patients’ PD type (Supplement, Table S3).
Α possible correlation between the increased oxidation levels of the four LDL lipid sub-classes markers and age, as well as the clinically measured biochemical markers of each PD patient was also investigated, using Linear Regression Analysis. The analysis showed that LDL cholesteryl ester-OOH, triglyceride-OOH, free cholesterol-OOH and phospholipid-OOH levels did not correlate in any way with PD patients’ age and the following clinical biochemical markers: LDL-C, HDL-C, total cholesterol, triglycerides, CRP, vitamin D, iPTH and CaxP (Supplement, Table S4).
Furthermore, the levels of LDL carotenoids between PD patients and the control group were compared. Our findings suggest that the control group presented significantly elevated (by 2.6-fold) LDL carotenoid levels compared to PD patients (Fig. 1, Table 2). Specifically, the Mann-Whitney U test shows that LDL carotenoid levels were significantly elevated in the control group compared to PD patients (U = 158.00, z = −3.72, p < 0.01). A possible correlation between the levels of the six measured oxLDL markers and the levels of LDL-carotenoids was also investigated using Linear Regression Analysis. The results of this analysis showed that none of the six tested oxLDL markers correlated with PD patients’ LDL carotenoid levels (Table 3) whereas in the control group only phospholipid-OOH levels showed an inverse correlation with LDL-carotenoid levels (Table 4).
Table 3.
Correlation of LDL carotenoid levels with oxLDL markers in PD patients using the Linear Regression Analysis.
| Unstandardized coefficient |
Standardized coefficient |
||||
|---|---|---|---|---|---|
| OxLDL marker | B | Std. Error | Beta | t-value | p-value |
| Chosteryl ester-OOH | −0.82 | 0.49 | −0.27 | −1.68 | 0.10 |
| Triglyceride-OOH | 3.18 | 2.78 | 0.19 | 1.14 | 0.26 |
| Free cholesterol-OOH | 0.47 | 0.44 | 0.17 | 1.06 | 0.30 |
| Phospilipid-OOH | 0.12 | 0.12 | 0.17 | 1.03 | 0.31 |
| ApoB100-MDA | 11.58 | 7.42 | 0.25 | 1.56 | 0.13 |
| ApoB100-DiTyr | 9.36 | 8.87 | 0.30 | 1.21 | 0.10 |
| N | 39 | ||||
Table 4.
Correlation of LDL carotenoid levels with oxLDL markers in control group using the Linear Regression Analysis.
| Unstandardized coefficient |
Standardized coefficient |
||||
|---|---|---|---|---|---|
| OxLDL marker | Β | Std. Error | Beta | t-value | p-value |
| Chosteryl ester-OOH | −0.04 | 0.28 | −0.04 | −0.16 | 0.88 |
| Triglyceride-OOH | −0.03 | 0.17 | −0.04 | −0.18 | 0.86 |
| Free cholesterol-OOH | −1.19 | 1.21 | −0.23 | −0.99 | 0.34 |
| Phospilipid-OOH | −4.14 | 1.96 | −0.45 | −2.11 | 0.049 |
| ApoB100-MDA | −0.01 | 0.01 | −0.09 | −0.37 | 0.72 |
| ApoB100-DiTyr | −0.03 | 0.05 | −0.17 | −0.74 | 0.47 |
| N | 40 | ||||
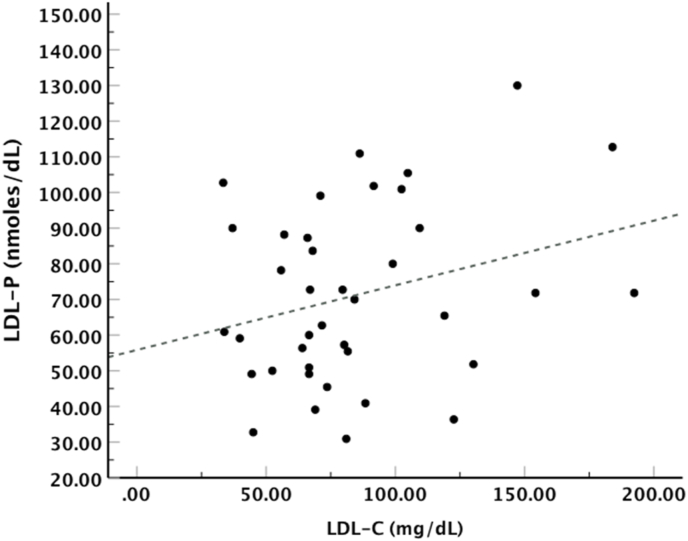
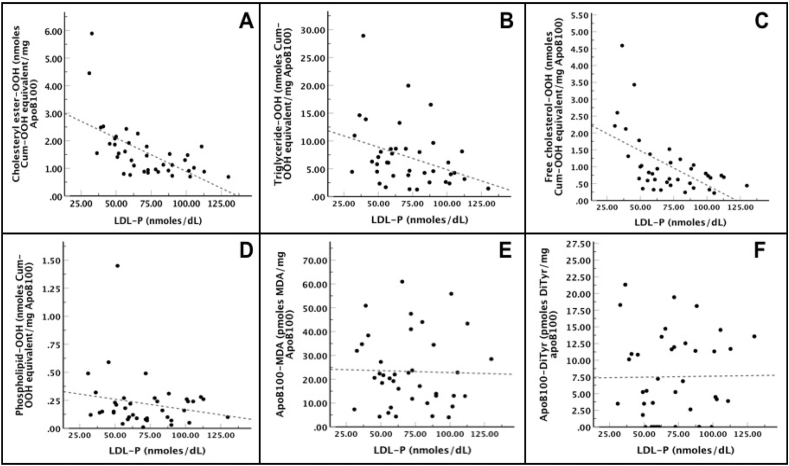
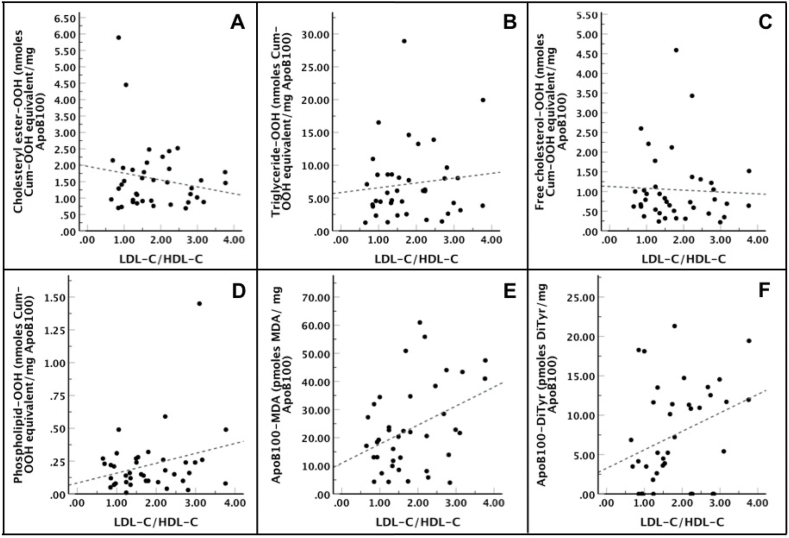
Additionally, LDL-P serum concentration was calculated at 64 ± 22.3 nmoles/dL for PD patients, while LDL-P serum concentration for HD patients was calculated at 133 ± 50 nmoles/dL and for control subjects at 134.3 ± 57.8 nmoles/dL. Subsequently, the correlation of LDL-C with LDL-P number was also investigated in PD group using Linear Regression Analysis, and our results showed that there was a proportional but not statistically significant [R2 = 0.05, F(1,37) = 3.11, p = 0.09] correlation between those two parameters (Fig. 2). Regarding LDL-P number correlation with the tested oxLDL markers, we found by Linear Regression Analysis a statistically significant inverse correlation between this number and cholesteryl ester-OOH, triglyceride-OOH and free cholesterol-OOH, while LDL-P number correlated inversely but with no statistical significance with phospholipid-OOH, and did not correlate with either apoB100-MDA or apoB100-DiTyr (Fig. 3). Of note, the relation between the tested oxLDL markers with the LDL-C/HDL-C ratio was also investigated. Using Linear Regression Analysis in PD patients, we found that only the two apoB100 oxidation markers, apoB100-MDA and apoB100-DiTyr, correlated proportionally with the LDL-C/HDL-C ratio (Fig. 4).
Fig. 2.
LDL-C versus LDL-P number in PD patients. Linear Regression Analysis showed that LDL-C levels do not significantly correlate with LDL-P concentration (β = 0.18, t = 1.76, p = 0.09).
Fig. 3.
LDL-P number versus oxLDL markers in PD patients. The Linear Regression Analysis showed that: (i) cholesteryl ester-OOH [R2 = 0.34, F(1,37) = 20.16, β = −0.02, t = −4.49, p = 0.001] (panel A), triglyceride-OOH [R2 = 0.10, F(1,37) = 5.33, β = −0.08, t = −2.31, p = 0.03] (panel B) and free cholesterol-OOH [R2 = 0.30, F(1,37) = 16.99, β = −0.02, t = −4.12, p = 0.001] (panel C) correlated inversely, in a statistically significant way, with LDL-P number. (ii) Phospholipid-OOH correlated inversely but not statistically significant with LDL-P [R2 = 0.01, F(1,37) = 1.41, β = −0.002, t = −1.19, p = 0.24] (panel D). (iii) apoB100-MDA [R2 = 0.00, F(1,37) = 0.03, β = −0.02, t = −0.16, p = 0.88] and apoB100-DiTyr [R2 = 0.00, F(1,37) = 0.01, β = 0.003, t = 0.07, p = 0.94] did not correlate with LDL-P (panel E and F, respectively).
Fig. 4.
LDL-C/HDL-C ratio versus oxLDL markers in PD patients. The Linear Regression Analysis showed that: (i) cholesteryl ester-OOH [R2 = 0.01, F(1,37) = 1.20, β = −0.21, t = −1.10, p = 0.28] (panel A), triglyceride-OOH [R2 = 0.00, F(1,37) = 0.47, β = 0.73, t = 0.68, p = 0.50] (panel B), free cholesterol-OOH [R2 = 0.00, F(1,37) = 0.07, β = −0.05, t = −0.27, p = 0.79] (panel C) and phospholipid-OOH [R2 = 0.05, F(1,37) = 2.93, β = 0.08, t = 1.71, p = 0.10] (panel D) did not statistically correlate with LDL-C/HDL-C ratio. (ii) ApoB100-MDA [R2 = 0.12, F(1,37) = 6.31, β = 6.77, t = 2.51, p = 0.02] and apoB100-DiTyr [R2 = 0.08, F(1,37) = 4.18, β = 2.35, t = 2.04, p = 0.048] correlated proportionally and statistically with LDL-C/HDL-C ratio (panel E and F, respectively).
Finally, in a previous study from our group the aforementioned six selected oxLDL markers were also measured in LDL from the blood serum of 61 HD patients [28]. In the present study we compared the levels of these oxLDL markers between the previously studied HD group and the PD group (Table 5). The levels of cholesteryl ester-OOH (U = 551.50, z = −4.51), triglyceride-OOH (U = 167.50, z = −7.22) and free cholesterol-OOH (U = 439.50, z = −5.30) were significantly higher (1.7-fold, 4.7-fold and 2.6-fold respectively) in PD group compared to the HD group (Table 5). On the other hand, the levels of phospholipid-OOH (U = 956.00, z = −1.65) and apoB100-MDA (U = 872.00, z = −2.24) presented a slight but not statistically important increase in PD group, while apoB100 DiTyr levels (U = 810.50, z = −2.68) presented a slight but not statistically important decrease in PD group compared to the HD group (Table 5).
Table 5.
OxLDL markers comparison between PD and HD patients.
| OxLDL marker | Subject | Value | p-value | Fold change (PD vs HD) |
|---|---|---|---|---|
| Cholesteryl ester-OOH | HD | 0.95 (±0.76) | <0.01 | 1.67 |
| PD | 1.59 (±1.01) | |||
| Triglyceride-OOH | HD | 1.52 (±1.04) | <0.01 | 4.72 |
| PD | 7.17 (±5.64) | |||
| Free cholesterol-OOH | HD | 0.40 (±0.35) | <0.01 | 2.60 |
| PD | 1.04 (±0.90) | |||
| Phospholipid-OOH | HD | 0.18 (±0.19) | 0.10 | 1.20 |
| PD | 0.22 (±0.24) | |||
| ApoB100-MDA | HD | 15.68 (±5.96) | 0.15 | 1.09 |
| PD | 17.11 (±6.01) | |||
| ApoB100-DiTyr | HD | 10.84 (±5.40) | 0.10 | 0.84 |
| PD | 9.06 (±5.97) |
Table notes.
Values are presented as mean (M) ± standard deviation (SD).
Cholesteryl ester-OOH, triglyceride-OOH, free cholesterol-OOH, phospholipid-OOH markers are expressed as nmoles cum-OOH equivalent/mg apoB100. ApoB100-MDA marker is expressed as pmoles MDA/mg apoB100 and apoB100-DiTyr marker is expressed as pmoles DiTyr/mg apoB100.
4. Discussion
The present study measures, for the first time, specific OS-induced oxidative modifications on the main LDL components (cholesteryl esters, triglycerides, free cholesterol, phospholipids, apoB100) and LDL carotenoid levels isolated from the blood serum of PD patients (versus healthy subjects and HD patients).
Of the six oxLDL markers tested, the four lipid peroxidation markers (cholesteryl ester-OOH, triglyceride-OOH, free cholesterol-OOH, phospholipid-OOH) emerge as potential clinical markers for atherosclerosis assessment in PD patients, since they were significantly elevated (2-fold, 7-fold, 4.5-fold and 2-fold respectively) in this patient group compared to the control group (Table 2). The elevated PD patients’ LDL lipid oxidation levels are justified by the known high OS present in this group of patients [8], which further intensifies by the reduced activity of various antioxidant enzymes [3,4] and the reduced levels of certain serum antioxidants [8]. It is also worth mentioning that three (cholesteryl ester-OOH, triglyceride-OOH, free cholesterol-OOH) of our novel lipid oxLDL markers correlated inversely with LDL-P concentration (Fig. 3). This result argues in favor of previous findings that LDL-P concentration could better contribute to the assessment of atherosclerosis risk development if combined with other LDL parameters important to the atherosclerotic lesion formation mechanism such as LDL oxidative state [15]. This is the first study to measure specific oxidative modifications on the LDL components of atherosclerosis-prone PD patients using simple, novel and with clinical applicability methodologies, that for their implementation require a minimum of 1 ml (2 ml optimal) of human blood serum due to the sensitivity of the oxidation determination methodologies applied. The existing studies use inadequate methodologies for assessing oxLDL status [28] and this is reflected on their discrepant results regarding oxLDL and anti-oxLDL Ab predictive value; specifically, some conclude that oxLDL [3,10,17,19] or/and anti-oxLDL Ab [4,22] levels are elevated in PD patients compared to the control group, others find no difference between PD patients and control group oxLDL [16,18,20] or/and anti-oxLDL Ab [20,24,25] levels, while one study concludes that oxLDL and anti-oxLDL Ab levels are higher in the control group compared to PD patients group [21].
The aforementioned elevated lipid oxLDL markers do not correlate with any of PD patients' underlying medical conditions (Supplement, Table S1), suggesting that the effect of PD on LDL oxidation is more pronounced itself than any underlying medical condition that seem to play secondary role. In fact, our findings are supported by the fact that CKD itself is considered an independent risk factor for CVD development [2]. Furthermore, the elevated lipid oxLDL markers do not correlate with patients’ sex (Supplement, Table S1), age (Supplement, Table S4), medication received - including statins - (Supplement, Table S2) and PD type (Supplement, Table S3). Particularly, the lack of correlation between oxLDL markers and statin intake is supported by the known absence of beneficial effects regarding CVD development in dialysis patients by statin treatment [7,[29], [30], [31]].
Notably, no correlation existed between the elevated lipid oxLDL markers and several clinical biomarkers (Supplement, Table S4), including LDL-C and HDL-C levels. The lack of LDL-C correlation with a key component of atherosclerotic lesion formation is in accordance with previous studies [3,14,28], argues in favor of the increasingly prevailing position that LDL-C may not be the best indicator for CVD risk assessment [5,14,[32], [33], [34], [35], [36]] and points out to the need for new complementary markers such as oxLDL. Adding to that, several studies indicate that LDL-C lowering agents do not have a beneficial effect on CVD incidence in dialysis patients [5,14]. Another finding of our study is that LDL-C did not correlate with the, related to oxLDL status, LDL-P concentration of PD patients (Fig. 2). This result is in accordance with the finding that the cholesterol content of LDL-P is not representative to their number [15]. Regarding HDL-C, in the present study a lack of correlation between oxLDL markers and its levels was observed, in contrast to a previous study of our group where HDL-C correlated inversely with oxLDL markers in HD patients [28]. This can be attributed to the fact that PD patients present increased loss of HDL particles in the peritoneal effluent (while LDL particles are not affected) and also to the fact that PD patients present more lipid abnormalities, such as low HDL-C levels, compared to HD patients [7,11]. The last thing this study checked regarding LDL-C and HDL-C levels was the LDL-C/HDL-C ratio which correlated proportionally with the non-significantly increased apoB100 oxidation markers (apoB100-MDA and apoB100-DiTyr). However, this ratio was not correlated with the lipid oxLDL markers that were significantly elevated in PD patients (Fig. 4), as it would be expected since it has been suggested as a probable novel marker for CVD risk assessment [37].
When it comes to LDL antioxidant defense, we showed that LDL carotenoid levels were significantly elevated (2.6-fold) in the control group compared to PD group (Table 2), while their levels did not correlate with any of the tested oxLDL markers in PD group (Table 3) and correlated inversely only with phospholipid-OOH marker in the control group (Table 4). This inverse correlation in the control group may be justified by the fact that carotenoids reside mostly on LDL particle phospholipid shell [38], therefore protecting it from oxidation. This correlation was expectedly absent in the PD group probably because of decrease of their antioxidant defense due to carotenoid depletion. It should be also noted that a-tocopherol is reported to constitute the main antioxidant defense of LDL particles [25] and is associated with low CVD risk development [20], while ubiquinol-10 is also reported as the “first-line” of LDL antioxidant defense [39], therefore it would important to measure and compare their levels with our novel oxLDL markers in future studies.
In this study, we also compared the levels of the six tested novel oxLDL markers between PD and HD patients and found that three of the lipid oxidation markers (cholesteryl ester-OOH, triglyceride-OOH and free cholesterol-OOH) were significantly elevated (1.7-fold, 4.7-fold and 2.6-fold, respectively) in PD patients compared to HD patient group (Table 5), thus pointing out that LDL are more prone to oxidation in PD patients compared to HD patients, which advances to the possible assumption that PD patients present higher risk in developing atherosclerotic lesions that HD patients. Our results are in accordance with some studies comparing OS levels between PD and HD patients [[40], [41], [42]], and also with a study showing that oxLDL particles from PD patients were more atherogenic than oxLDL particles from HD patients [20]. Nevertheless, there are several other studies reporting that OS levels do not differ between PD and HD and others that find more elevated OS in HD compared to PD patients [8,13]. Therefore, it is clear that the existing data regarding the comparison of OS levels between PD and HD are conflicting, probably due to the small number of relevant studies [43], and also due to the fact that each study evaluates OS levels by measuring different OS markers and/or with different methodologies, making practically impossible the comparison between them. Comparison between each different OS marker rather than between the general OS state appears as a more accurate approach when comparing PD and HD oxidative status.
To conclude, the present study is the first to evaluate the levels of six novel oxLDL markers in PD patients, compared to a control group and a HD group, by measuring specific oxidative modifications of the LDL individual lipid and protein components that collectively define oxLDL status. Our results show increased levels of four oxLDL specific markers (cholesteryl ester-OOH, triglyceride-OOH, free cholesterol-OOH, phospholipid-OOH) in PD patients (versus control), with three of them (cholesteryl ester-OOH, triglyceride-OOH, free cholesterol-OOH) being also higher in PD patients than their counterparts in HD patients. Since these markers appear elevated in both PD and HD patients they support the viewing of oxLDL as a promising clinical marker for CVD risk assessment in both groups of patients, but also for comparing this risk in PD vs HD. Moreover, the study introduces free cholesterol-OOH and cholesteryl ester-OOH as possible alternative markers to LDL-C and complementary to LDL-P number, given that both –OOH markers can be readily measured in clinical practice by simple and reproducible assays already developed by our laboratory [28]. Our data need further verification by extending our study to a higher number (>100) of participants, which will also allow setting more conclusively the normal levels of oxLDL markers in the healthy population. These normal levels can then be used as reference control levels for assessing the risk of atherosclerotic lesion development in PD patients and in general in every individual. Setting reliable normal levels for the studied markers, may also establish higher statistical significance for the slightly increased levels of the apoB100-MDA and apoB100-DiTyr markers in PD patients. Moreover, to strengthen further the role of oxLDL in any CVDs, as well as for assessing the risk of atherosclerotic lesion formation, the development of clinically applicable methodologies for measuring additional markers, such as 7-keto-cholesterol (free/esterified), 7-beta-hydroxyl-cholesterol (free/esterified), triglyceride/phospholipid carbonyl groups (-C O), apoB100-C O, apoB100 disulfide bridges (S–S, resulting from oxidation of the –SH group in adjacent Cyt's) and the LDL antioxidant a-tocopherol, will help fully define oxLDL status. Additionally, the bis-allylic hydrogen content of LDL lipid sub-fractions, as being indicative of the extent of lipid peroxidation [44], will be explored for its possible correlation with the lipid oxidation markers of the present study and as an additional oxLDL marker in future studies.
Funding
This work was supported by Greece and the European Union (European Social Fund-ESF) through the Operational Programme « Human Resources Development, Education and Lifelong Learning» in the context of the Act “Enhancing Human Resources Research Potential by undertaking a Doctoral Research” Sub-action 2: IKY Scholarship Programme for PhD candidates in the Greek Universities [grant numbers: 2022-050-0502-52508, 2022-050-0502-52518]; and the “Andreas Mentzelopoulos Foundation” by University of Patras, Greece.
CRediT authorship contribution statement
Polyxeni Papadea: Methodology, Investigation, Formal analysis, Visualization, Formal analysis, Data curation, Funding acquisition, Writing – original draft, Writing – review & editing. Electra Kalaitzopoulou: Investigation, Formal analysis. Marianna Skipitari: Investigation, Formal analysis. Athina Varemmenou: Investigation, Formal analysis. Marios Papasotiriou: Methodology, Writing – review & editing. Evangelos Papachristou: Methodology, Writing – review & editing. Dimitrios Goumenos: Methodology, Writing – review & editing. Tilman Grune: Resources, Writing – review & editing. Christos D. Georgiou: Conceptualization, Methodology, Validation, Formal analysis, Visualization, Supervision, Project administration, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare to be in a relationship with State Scholarship Foundation (IKY) (Personal Scholarship awarded by IKY Foundation’s program “Scholarship Program for PhD candidates in the Greek Universities”)
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2023.102762.
Abbreviations
- AGEs
advanced glycation end-products
- Anti-oxLDL Ab
autoantibodies against oxLDL
- ApoB100
apolipoprotein B100
- ApoB100-DiTyr
dityrosines formed on apoB100
- ApoB100-MDA
malondialdehyde bound to apoB100
- ARBs
angiotensin receptor blockers
- CaChBI
calcium channel blockers
- CAPD
continuous ambulatory peritoneal dialysis
- CCPD
continuous cycling peritoneal dialysis
- CHD
coronary heart disease
- CKD
chronic kidney disease
- CVD
cardiovascular disease
- ESKD
end-stage chronic kidney disease
- GDPs
glucose degradation products
- HD
hemodialysis
- IPD
intermittent peritoneal dialysis
- LDL-P
LDL particle
- M
mean
- MDA
malondialdehyde
- NIPD
nocturnal intermittent peritoneal dialysis
- -OOH
hydroperoxides
- OS
oxidative stress
- OxLDL
oxidized LDL
- PAD
peripheral artery disease
- PD
peritoneal dialysis
- SD
standard deviation
Appendix A. Supplementary data
The following is the Supplementary data to this article.
Data availability
Data will be made available on request.
References
- 1.Liakopoulos V., Varouktsi G., Tsinari A., Veljkovic A., Lazarevic G., Perisic Z., Hadzi-Djokic J., Kocic G., Roumeliotis S. Implications of oxidative stress in end-stage kidney disease patients: a review of causative mechanisms, current concepts. Acta Medica Medianae. 2022;61:53–59. [Google Scholar]
- 2.Mekki K., Taleb W., Bouzidi N., Kaddous A., Bouchenak M. Effect of hemodialysis and peritoneal dialysis on redox status in chronic renal failure patients: a comparative study. Lipids Health Dis. 2010;9:93. doi: 10.1186/1476-511X-9-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Samouilidou C.E., Karpouza P.A., Kostopoulos V., Bakirtzi T., Pantelias K., Petras D., Tzanatou-Exarchou H., Grapsa J.E. Lipid abnormalities and oxidized LDL in chronic kidney disease patients on hemodialysis and peritoneal dialysis. Ren. Fail. 2012;34(2):160–164. doi: 10.3109/0886022X.2011.641515. [DOI] [PubMed] [Google Scholar]
- 4.Krishnaswamy P.R., Rao A., Murali W., Sudarshan Ballal H. Paraoxonase activity and antibodies to oxidized-LDL in chronic renal failure patients on renal replacement therapy, ndian. J. Clin. Biochem. 2006;21:173–176. doi: 10.1007/BF02912937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lluesa H.J., López-Romero C.L., Monzó B.J.J., Marugan R.M., Boyano V.I., Rodriguez-Espinosa D., Gomez-Bori A., Orient S.A., Such D.R., Perez S.P., Jaras H.J. Lipidic profiles of patients starting peritoneal dialysis suggest an increased cardiovascular risk beyond classical dyslipidemia biomarkers. Sci. Rep. 2022;12 doi: 10.1038/s41598-022-20757-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rysz J., Franczyk B., Ławiński J., Gluba-Brzózka A. Oxidative stress in ESRD patients on dialysis and the risk of cardiovascular diseases. Antioxidants. 2020;9(11):1079. doi: 10.3390/antiox9111079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prichard S.S. Management of hyperlipidemia in patients on peritoneal dialysis: current approaches. Kidney Int. Suppl. 2006;70:S115–S117. doi: 10.1038/sj.ki.5001926. [DOI] [PubMed] [Google Scholar]
- 8.Liakopoulos V., Roumeliotis S., Gorny X., Dournousi E., Mertens R.P. Oxidative stress in hemodialysis patients: a review of the literature. Oxid. Med. Cell. Longev. 2017;2017 doi: 10.1155/2017/3081856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roumeliotis S., Eleftheriadis T., Liakopoulos V. Is oxidative stress an issue in peritoneal dialysis? Semin. Dial. 2019;32:463–466. doi: 10.1111/sdi.12818. [DOI] [PubMed] [Google Scholar]
- 10.Sozer V., Korkmaz Guntas G., Konukoglu D., Dervisoglu E., Gelisgen R., Tabak O., Kalender B., Uzun H. Effects of peritoneal-and hemodialysis on levels of plasma protein and lipid oxidation markers in diabetic patients. Minerva Med. 2013;104:75–84. [PubMed] [Google Scholar]
- 11.Omran J., Al-Dadah A., Dellsperger K.C. Dyslipidemia in patients with chronic and end-stage kidney disease. Cardiorenal Med. 2013;3:165–177. doi: 10.1159/000351985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehmetoglu I., Yerlikaya F.H., Kurban S., Erdem S.S., Tonbul Z. Oxidative stress markers in hemodialysis and peritoneal dialysis patients, including coenzyme Q10 and ischemia-modified albumin. Int. J. Artif. Organs. 2012;35:226–232. doi: 10.5301/ijao.5000078. [DOI] [PubMed] [Google Scholar]
- 13.Yonova D., Trendafilov I., Papazov V., Stanchen I., Zidarov R., Antonov S. Comparative study of oxidative stress in peritoneal dialysis and hemodialysis patients. Hippokratia. 2004;8:170–172. [Google Scholar]
- 14.Lin T., Xia X., Yu J., Qiu Y., Yi C., Lin J., Mao H., Yang X., Huang F. The predictive study of the relation between elevated low-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio and mortality in peritoneal dialysis. Lipids Health Dis. 2020;19:51. doi: 10.1186/s12944-020-01240-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qiao Y.N., Zou Y.L., Guo S.D. Low-density lipoprotein particles in atherosclerosis. Front. Physiol. 2022;13 doi: 10.3389/fphys.2022.931931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castoldi G., Antolini L., Bombardi C., Perego L., Mariani P., Viganò M.R., Torti G., Casati M., Corti A., Zerbini G., Valsecchi M.G., Stella A. Oxidative stress biomarkers and chromogranin A in uremic patients: effects of dialytic treatment. Clin. Biochem. 2010;43:1387–1392. doi: 10.1016/j.clinbiochem.2010.08.028. [DOI] [PubMed] [Google Scholar]
- 17.Göçmen A.Y., Sahin E., Koçak H., Tuncer M., Gümüşlü S. Levels of asymmetric dimethylarginine, nitric oxide and lipid peroxidation markers in patients with end-stage renal disease having peritoneal dialysis treatment. Clin. Biochem. 2008;41:836–840. doi: 10.1016/j.clinbiochem.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 18.Johnson-Davis K., Fernelius C., Eliason B.N., Wilson A., Beddhu S., Roberts L.W. Blood enzymes and oxidative stress in chronic kidney disease: a cross sectional study. Ann. Clin. Lab. Sci. 2011;41(4):331–339. [PubMed] [Google Scholar]
- 19.Meier P., Spertini F., Blanc E., Burnier M. Oxidized low-density lipoproteins activate CD4+ T cell apoptosis in patients with end-stage renal disease through Fas engagement. J. Am. Soc. Nephrol. 2007;18:331–342. doi: 10.1681/ASN.2006050514. [DOI] [PubMed] [Google Scholar]
- 20.O'Byrne D., Devaraj S., Islam K.N., Collazo R., McDonald L., Grundy S., Jialal I. Low-density lipoprotein (LDL)-induced monocyte-endothelial cell adhesion, soluble cell adhesion molecules, and autoantibodies to oxidized-LDL in chronic renal failure patients on dialysis therapy. Metabolism. 2001;50:207–215. doi: 10.1053/meta.2001.19486. [DOI] [PubMed] [Google Scholar]
- 21.Pawlak K., Mysliwiec M., Pawlak D. Oxidized LDL to autoantibodies against oxLDL ratio - the new biomarker associated with carotid atherosclerosis and cardiovascular complications in dialyzed patients. Atherosclerosis. 2012;224:252–257. doi: 10.1016/j.atherosclerosis.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 22.Maggi E., Bellazzi R., Falaschi F., Frattoni A., Perani G., Finardi G., Gazo A., Nai M., Romanini D., Bellomo G. Enhanced LDL oxidation in uremic patients: an additional mechanism for accelerated atherosclerosis? Kidney Int. 1994;45:876–883. doi: 10.1038/ki.1994.115. [DOI] [PubMed] [Google Scholar]
- 23.Maggi E., Bellazzi R., Gazo A., Seccia M., Bellomo G. Autoantibodies against oxidatively-modified LDL in uremic patients undergoing dialysis. Kidney Int. 1994;46:869–876. doi: 10.1038/ki.1994.344. [DOI] [PubMed] [Google Scholar]
- 24.Pawlak K., Pawlak D., Mysliwiec M. Cu/Zn superoxide dismutase plasma levels as a new useful clinical biomarker of oxidative stress in patients with end-stage renal disease. Clin. Biochem. 2005;38:700–705. doi: 10.1016/j.clinbiochem.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 25.Roob J.M., Rabold T., Hayn M., Khoschsorur G., Resch U., Holzer H., Winklhofer-Roob B.M. Ex vivo low-density lipoprotein oxidizability and in vivo lipid peroxidation in patients on CAPD. Kidney Int. Suppl. 2001;78:S128–S136. doi: 10.1046/j.1523-1755.2001.59780128.x. [DOI] [PubMed] [Google Scholar]
- 26.Teng Y.-Y., Sari S.M., Wang C.-H., Kuo C., Hsu B.-G. MO740: decrease serum IgG anti-oxLDL antibodies levels are associated with peripheral arterial disease in patients with peritoneal dialysis. Nephrol. Dial. Transplant. 2022;37 gfac079.01. [Google Scholar]
- 27.Wang C.-H., Kuo C.-H., Hsu B.-G. Decrease serum anti-oxLDL antibody is an independent marker of peripheral arterial disease in peritoneal dialysis patients. Atherosclerosis. 2022;355:e285. [Google Scholar]
- 28.Papadea P., Skipitari M., Kalaitzopoulou E., Varemmenou A., Spiliopoulou M., Papasotiriou M., Papachristou E., Goumenos D., Onoufriou A., Rosmaraki E., Margiolaki I., Georgiou C.D. Methods on LDL particle isolation, characterization, and component fractionation for the development of novel specific oxidized LDL status markers for atherosclerotic disease risk assessment. Front. Med. - Transl. 2023;9 doi: 10.3389/fmed.2022.1078492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fellström C.B., Jardine G.A., Schmieder E.R., Holdaas H., Bannister K., Beutler J., Chae D., Chevaile A., Cobbe M.S., Crönhagen-Riska C., De Lima J.J., Lins R., Mayer G., McMahon W.A., Parving H., Remuzzi G., Samuelsson O., Sonkodi S., Süleymanlar G., Tsakiris D., Tesar V., Todorov V., Wiecek A., Wüthrich P.R., Gottlow M., Johnsson E., Zannad F. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N. Engl. J. Med. 2009;360:1395–1407. doi: 10.1056/NEJMoa0810177. [DOI] [PubMed] [Google Scholar]
- 30.Group S.C. Study of Heart and Renal Protection (SHARP): randomized trial to assess the effects of lowering low-density lipoprotein cholesterol among 9,438 patients with chronic kidney disease. Am. Heart J. 2010;160(5):785–794. doi: 10.1016/j.ahj.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 31.Wanner C., Krane V., März W., Olschewski M., Asmus H.G., Krämer W., Kühn K.W., Kütemeyer H., Mann J.F., Ruf G., Ritz E. vol. 27. Kidney Blood Press; 2004. pp. 259–266. (Deutsche Diabetes-Dialyse-Studie (4D) Study Group. Randomized Controlled Trial on the Efficacy and Safety of Atorvastatin in Patients with Type 2 Diabetes on Hemodialysis (4D Study): Demographic and Baseline Characteristics). Res. [DOI] [PubMed] [Google Scholar]
- 32.Blankstein R., Budoff J.M., Shaw J.L., Goff C.D., Polak F.J., Lima J., Blumenthal S.R., Nasir K. Predictors of coronary heart disease events among asymptomatic persons with low low-density lipoprotein cholesterol. MESA (Multi-Ethnic Study of Atherosclerosis) J. Am. Coll. Cardiol. 2011;58(4):364–374. doi: 10.1016/j.jacc.2011.01.055. [DOI] [PubMed] [Google Scholar]
- 33.Leslie M. Is it time to retire cholesterol tests? - measuring a blood protein, apoB, might save more lives. Science. 2017 doi: 10.1126/science.358.6368.1237. https://www.sciencemag.org/news/2017/12/it-time-retire-cholesterol-tests?r3f986=https://www.google.com/ [DOI] [PubMed] [Google Scholar]
- 34.Navarese P.E., Robinson G.J., Kowalewski M., Kolodziejczak M., Andreotti F., Bliden K., Tantry U., Kubica J., Raggi P., Gurbel A.P. Association between baseline LDL-C level and total and cardiovascular mortality after LDL-C lowering. A systematic review and meta-analysis. JAMA. 2018;319(15):1566–1579. doi: 10.1001/jama.2018.2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ravnskov U., de Lorgeril M., Diamond M.D., Hama R., Hamazaki T., Hammarskjold B., Hynes N., Kendrick M., Langsjoen H.P., Mascitelli L., McCully S.M., Okuyama H., Rosch J.P., Schersten T., Sultan S., Sundberg R. LDL-C does not cause cardiovascular disease: a comprehensive review of current literature. Expet Rev. Clin. Pharmacol. 2018;11(10):959–970. doi: 10.1080/17512433.2018.1519391. [DOI] [PubMed] [Google Scholar]
- 36.Wang B., Chen S., Liu J., Liang Y., Meng L., Yan X., Huang H., Chen G., Huang Z., Xu D., Li M., Liang J., Liu S., Chen J., Liu Y., Tan N. Association between baseline LDL-C and prognosis among patients with coronary artery disease and advanced kidney disease. BMC Nephrol. 2021;22:168. doi: 10.1186/s12882-021-02375-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhong Z., Hou J., Zhang Q., Zhong W., Li B., Li C., Liu Z., Yang M., Zhao P. Assessment of the LDL-C/HDL-C ratio as a predictor of one year clinical outcomes in patients with acute coronary syndromes after percutaneous coronary intervention and drug-eluting stent implantation. Lipids Health Dis. 2019;18(1):40. doi: 10.1186/s12944-019-0979-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin S., Quaroni L., White W.S., Cotton T., Chumanov G. Localization of carotenoids in plasma low-density lipoproteins studied by surface-enhanced resonance Raman spectroscopy. Biopolymers. 2000;57(4):249–256. doi: 10.1002/1097-0282(2000)57:4<249::AID-BIP6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 39.Stocker R., Bowry V.W., Frei B. Ubiquinol-10 protects human low density lipoprotein more efficiently against lipid peroxidation than does alpha-tocopherol. Proc. Natl. Acad. Sci. USA. 1991;88:1646–1650. doi: 10.1073/pnas.88.5.1646. Proc. Nati. Acad. Sci. 88: 1646-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Al-Hweish A., Sultan S.S., Mogazi K., Elsammak M.Y. Plasma myeloperoxidase, NT-proBNP, and troponin-I in patients on CAPD compared with those on regular hemodialysis. Hemodial. Int. 2010;14:308–315. doi: 10.1111/j.1542-4758.2010.00455.x. [DOI] [PubMed] [Google Scholar]
- 41.McGrath L.T., Douglas A.F., McClean E., Brown J.H., Doherty C.C., Johnston G.D., Archbold G.P. Oxidative stress and erythrocyte membrane fluidity in patients undergoing regular dialysis. Clin. Chim. Acta. 1995;235:179–188. doi: 10.1016/0009-8981(95)06027-x. [DOI] [PubMed] [Google Scholar]
- 42.Taylor J.E., Scott N., Bridges A., Henderson I.S., Stewart W.K., Belch J.J. Lipid peroxidation and antioxidants in continuous ambulatory dialysis patients. Perit. Dial. Int. 1992;12:252–256. [PubMed] [Google Scholar]
- 43.Capusa C., Stoian I., Rus E., Lixandru D., Barbulescu C., Mircescu G. Does dialysis modality influence the oxidative stress of uremic patients? Kidney Blood Press. Res. 2012;35:220–225. doi: 10.1159/000331560. [DOI] [PubMed] [Google Scholar]
- 44.Wagner B.A., Buettner G.R., Burns C.P. Free radical-mediated lipid peroxidation in cells: oxidizability is a function of cell lipid bis-allylic hydrogen content. Biochemistry. 1994;33(15):4449–4453. doi: 10.1021/bi00181a003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.