Abstract
Gene therapy holds great promise for curing cancer by editing the deleterious genes of tumor cells, but the lack of vector systems for efficient delivery of genetic material into specific tumor sites in vivo has limited its full therapeutic potential in cancer gene therapy. Over the past two decades, increasing studies have shown that lentiviral vectors (LVs) modified with different glycoproteins from a donating virus, a process referred to as pseudotyping, have altered tropism and display cell-type specificity in transduction, leading to selective tumor cell killing. This feature of LVs together with their ability to enable high efficient gene delivery in dividing and non-dividing mammalian cells in vivo make them to be attractive tools in future cancer gene therapy. This review is intended to summarize the status quo of some typical pseudotypings of LVs and their applications in basic anti-cancer studies across many malignancies. The opportunities of translating pseudotyped LVs into clinic use in cancer therapy have also been discussed.
Keywords: Cancer therapy, Clinical translation, Gene delivery, Lentiviral vector, Pseudotype
Background
Delivering curative genetic material into deleterious cells to combat disease, i.e., gene therapy, has shown remarkable therapeutic benefit and safety record in clinical trials, and is also rapidly emerging as a revolutionary modality for treating a wide range of genetic disorders and acquired human diseases.1,2 However, despite satisfactory results in preclinical studies and early-stage clinical testing, gene therapy fails to become a routine option in cancer treatment under most circumstances, principally due to the inability to selectively and efficiently deliver transgenes to specific cancerous cells in vitro and in vivo, one of the most formidable challenges for gene therapy application.3, 4, 5 A desired gene therapy essentially necessitates a delivery system capable of efficient gene transfer in a variety of cells or tissues, meanwhile, without causing any unwanted side effects. Currently, different delivery systems including viral and non-viral vectors and some physical approaches have been designed to offer a choice for gene therapy, and improving their performance remains a seminal concern for researchers.6 Viral vectors derived from naturally evolved viruses have great applicable potentials as they display high gene transfer capability in a variety of cells. Among them, lentiviral vectors (LVs) have emerged as powerful tools for stable gene delivery due to several advantages including the ability to transduce both dividing and non-dividing cells. Another prominent advantage is that their tropism can be modified through pseudotyping, a process by which a native surface protein is replaced with a viral envelope glycoprotein of interest, allowing selective transfer of transgene to specific population of cells, such as neural cells, lymphocytes, hepatic cells or tumor cells, based on the specific interactions between pseudotyping glycoproteins and corresponding cellular receptors for vector entry.7 This feature of LVs provides an extraordinary strength that could be harnessed to meet the urgent need for enabling specific delivery into tumor sites in vivo.
Over the last two decades, accumulating basic studies have shown the potentials of numerous pseudotypes of LVs in improving the efficacy of gene therapy against malignancies, particularly those LVs pseudotyped with glycoproteins from viruses including the vesicular stomatitis virus (VSV), lymphocytic choriomeningitis virus (LCMV), measles viral (MV), and hepatitis C virus (HCV). In addition, studies have also yielded a mass of advancements in the engineering approaches of these pseudotypes for successful targeted transduction of specific tumor cells. In this review, we attempt to summarize the altered tropism and optimized use of LVs in gene delivery by pseudotyping, and revisit the research status quo of applying LV pseudotypes in targeting specific cancer. We also discuss the promising prospects of their translation in future gene therapy for cancer treatment.
LVs are effective gene delivery vehicles
As many human diseases have been revealed to be determined genetically, a rational hypothesis was firstly proposed in 1972 for genetic manipulation in humans that replaces the defective DNA with exogenous “good” DNA for ameliorating inherited monogenic disorders caused by genetic defects.8 In the following years, growing interest has been evoked in gene therapy, which is believed to achieve persistent production of endogenous proteins in targeted cells, whereby yielding durable and curative clinical benefit. The upsurge in this research field can be exemplified most vividly by numerous endeavors and clinical trials devoted into developing gene therapies for hemophilia since the successful isolation of susceptible genes encoding the factor IX and VIII.9 However, the prospect of clinical application of gene therapy was once plagued by unresponsiveness and overt toxicities in early experimental trials launched in the 1990s.2 These setbacks in translational studies were concluded to be partially due to insufficient knowledge of viral vectors in that era.10 Since then, with the continuous improvements in developing new vectors and methods of gene delivery and safety modifications, thousands of clinical trials concerning gene therapy have been completed worldwide, leading to substantial progresses and particularly turning several gene therapies into approved drugs and novel treatment options that revolutionize multiple fields of modern medicine.3
Gene therapy is now being applied to treat diverse diseases by introducing foreign genomic materials into the targeted cell/tissue through an appropriate delivering system, mainly including the viral vectors, non-viral vectors, and physical approaches. Among them, viral vectors have long been explored as potential tools to transfer loaded genetic materials into desired host cells, because viruses have evolved naturally specialized mechanisms for integrating their genome and enabling gene expression after viral infection and multiplication.11 Dating back to 1990, viral vectors have already been employed in the first clinical trial of gene therapy to transfer the adenosine deaminase (ADA) gene into the T cells for treating the severe combined immunodeficiency. In this pioneering study, a persisted expression of integrated viral vector and ADA gene in T cells was detected.12 Owing to their natural features, one remarkable advantage of viral vectors is the high efficiency of gene delivery into a variety of cells. However, viral vectors have some limitations, such as toxicity, immunogenicity, mutagenicity and restricted carrying capacity. To circumvent these limitations, investigators also have been attempting to discover other alternative gene carriers. In recent decades, tremendous advancements have indeed been witnessed in the development of non-viral based systems and physical approaches for delivering exogenous gene, including cationic liposomes, cationic polymers, synthetic peptides and inorganic nanoparticles, or employing physical forces to facilitate gene transfer into cells.13, 14, 15 Nevertheless, another critical problem still existing is that compared with viral carriers, these emerging non-viral and physical methods demonstrate relatively lower efficiency, especially for in vivo gene delivery, which hinders their widespread use in future clinical treatment.16,17 A more thorough comparison between viral vectors and non-viral vectors could refer to some systematic reviews published recently elsewhere.18, 19, 20
To date, a majority of utilized viral vectors for gene therapy are based on three classes of viruses, including the adenovirus, adeno-associated virus and lentivirus. It has been iteratively proved that these viral vectors are able to confer a higher efficiency of gene transduction and permit longer duration of transgene expression than non-viral delivery systems, hence making them very attractive optional therapies for diseases.21 Among viral vectors, LVs have several advantages. Firstly, LVs can stably integrate into both dividing and non-dividing cells, thus ensuring sustained transgene expression within a wide spectrum of target cells and their progeny cells; secondly, LVs have a large packaging capacity up to 8 kb, which offers them a superiority with regard to expressing large genes for treating associated diseases; thirdly, the integration of prototypical LVs into the host cells exerts low oncogenic potential,22 hence increasing their biosafety; at last, LVs can be pseudotyped with heterologous envelope glycoproteins, through which the tropism of LVs can be deliberately modified for broadening the scope of their utility or achieving specific targeting of host cells.23,24
The recombinant LVs were first constructed in the 1990s from the genome of human immunodeficiency virus-1 (HIV-1).25, 26, 27 The resulting HIV-1-based LVs possess a large packaging capacity of 8–10 kb, and are able to integrate their viral particles into host genomes after viral infection. It is worth mentioning that among various retroviral vectors, these HIV-1-based LVs remain the most commonly used delivery vehicles for experimental studies and also valuable tools for gene therapy trials.28 Ever since the first clinical evaluation of LVs in a phase I open-label nonrandomized clinical trial for HIV in 2006,29 LVs have now been tested successfully in many clinical trials to treat a broad range of human diseases, for example, multiple types of malignancies (Table 1).23 And encouragingly, the number of ongoing clinical trials in regard to the therapeutic evaluation of LVs for cancer treatment is also dramatically increasing, and more related reports are retrievable at the clinicaltrials.gov database (https://clinicaltrials.gov/). The research status quo therefore indicates that harnessing LVs for gene delivery to humans holds great promise for the treatment of a wide variety of disorders, including human cancers.
Table 1.
Some representative completed and ongoing clinical trials exploiting LVs in cancer therapy.
| Conditions | Interventions | Locations | Phase | Identifier |
|---|---|---|---|---|
| Hematopoietic/Lymphoid cancer and 22 more malignancies | CART-19 | Abramson Cancer Center of The University of Pennsylvania, Philadelphia, Pennsylvania, United States | Phase 1 | NCT01029366 |
| B-cell non-Hodgkin lymphoma | CART-19 | Peking Union Medical College Hospital, Beijing, China | Phase 1 | NCT03483688 |
| Lymphoma | rHIV7-shI-TAR-CCR5RZ LV-transduced HPCs + carmustine + cyclophosphamide + etoposide | City of Hope Medical Center, Duarte, California, United States | Phase 1 | NCT00569985 |
| Advanced solid tumor | TCRT-ESO-A2 | Baylor University Medical Cancer Hospital, Dallas, Texas, United States | Phase 1 | NCT04878484 |
| Hematologic malignancy and 4 more malignancies | cCARTs cells | The General Hospital of Western Theater Command, Chengdu, China; Peking University Shenzhen Hospital, Shenzhen, China | Early Phase 1 | NCT03795779 |
| Metastatic breast cancer | huMNC2-CAR44 CARTs | City of Hope Medical Center, Duarte, California, United States | Phase 1 | NCT04020575 |
| Malignant neoplasm of nasopharynx and breast cancer recurrent | CAR-T cells recognizing EpCAM | West China Hospital, Sichuan University, Chengdu, Sichuan, China | Phase 1 | NCT02915445 |
| Hepatocellular carcinoma and metastatic liver cancer | ECT204 T cells | City of Hope, Duarte, California, United States Kansas University Cancer Center, Westwood, Kansas, United States |
Phase 1/2 | NCT04864054 |
| Glioblastoma multiforme | Interferon-α2-modified HSPCs + temferon | Ospedale San Raffaele, Milan, Italy Fondazione IRCCS Istituto Neurologico “Carlo Besta”, Milan, Italy Policlinico Universitario Fondazione Agostino Gemelli, Rome, Italy |
Phase 1/2 | NCT03866109 |
| Multiple myeloma | bb2121 | Mayo Clinic Arizona Scottsdale, Arizona, United States UCSF Medical Center, San Francisco, California, United States Moffitt Cancer Center, Tampa, Florida, United States and 24 more centers |
Phase 2 | NCT03601078 |
| Relapsed/Refractory B-cell Lymphoma, Childhood | CART-19 | Children's Hospital of Fudan University, Shanghai, China | Phase 1/2 | NCT03265106 |
| BPDCN | MB-102 + Fludarabine + Cyclophosphamide | City of Hope Medical Center, Duarte, California, United States Dana-Farber Cancer Institute, Boston, Massachusetts, United States Duke University, Durham, North Carolina, United States MD Anderson Cancer Center, Houston, Texas, United States |
Phase 1/2 | NCT04109482 |
| Relapsed or refractory B-ALL | CART-19 | Abramson Cancer Center of the University of Pennsylvania, Philadelphia, Pennsylvania, United States | Phase 2 | NCT02030847 |
| Recurrent ALL and CLL | Autologous anti-CD19CAR-4-1BB-CD3zeta-EGFRt-expressing T lymphocytes | Fred Hutch/University of Washington Cancer Consortium, Seattle, Washington, United States | Phase 1/2 | NCT01865617 |
| Multiple Myeloma | JNJ-68284528 | Mayo Clinic Cancer Center–Scottsdale, Phoenix, Arizona, United States and 20 more centers | Phase 1/2 | NCT03548207 |
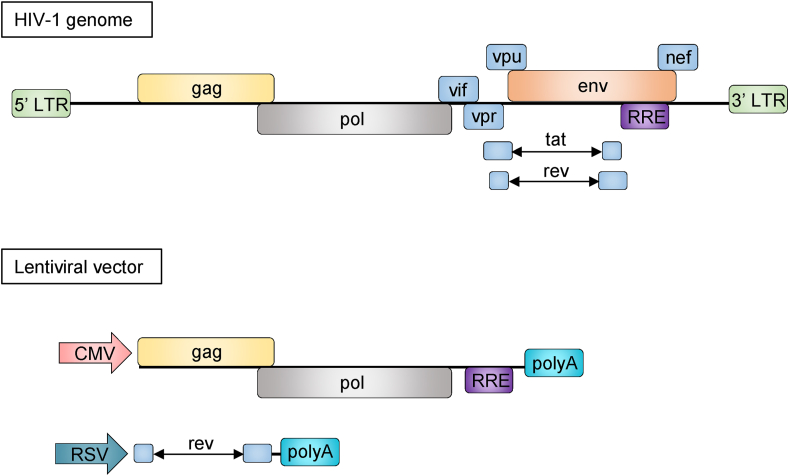
It is well agreed that an ideal gene therapy is extremely dependent on a safe, efficient and target-specific delivery system, therefore, viral vectors are mostly replication-deficient viruses and genetically modified to improve their safety, efficiency, and cellular uptake.30 For instance, LVs have been intensively modified for improving safety through multiple methods, including separating critical components of the HIV-1 genome into distinct plasmids, removing the accessory genes (vif, vpr, vpu and nef) and regulatory tat gene, and substituting the 5′UTR in the LV transfer vector with a tat-independent constitutive promoter (Fig. 1).31,32 These modifications together insure the generation of replication-deficient LVs. Although clinical trials using LVs with improved safety features support the feasibility and safety of LV-based gene therapies for human diseases,23,28 there are several major challenges to overcome for improving future clinical use. For instance, one risk is that the insertional mutagenesis may be lower when using LVs, but the theoretical possibility is not eliminated. The potential exacerbation of off-target effects due to LV-mediated persistent expression of the gene editing machinery poses another concern.23 Moreover, the long-term safety and transduction efficacy of LVs are still uncertain in patients who have already received LV-based gene therapies. Further, the limited host tropism of LVs has restricted the range of cellular targets and narrowed their applications in clinical practice.24 In fact, since the primary construction of LVs, the genome of LVs has also been repeatedly modified for improving their transduction efficiency and application range. In the next section, we will focus on illustrating how LVs are pseudotyped in varying forms to alter the host tropism and also discussing the relevant significance in optimizing their application in gene delivery.
Figure 1.
Modifications of HIV-1 genome for developing a vector plasmid with improved safety. Various critical accessory and regulatory components within the HIV-1 genome are removed or substituted in order to construct a safe viral vector. LTR, long-terminal repeats; gag, group-specific antigen; pol, DNA polymerase; vif, viral infectivity factor; vpr, viral protein R; vpu, viral protein u; nef, negative regulator factor; env, viral envelope; RRE, rev response element; tat, transactivator of transcription; rev, transactivating protein; CMV, cytomegalovirus; RSV, respiratory syncytial virus.
Pseudotyping LVs alters host tropism and optimizes their use in gene delivery
Basically, the host tropism of viral vectors is defined by the specific interaction between the viral envelope glycoproteins with corresponding receptors of the host cells. The HIV-1-based LVs are encapsidated by the HIV envelope glycoprotein consisting of an outer membrane protein gp120, which preferentially binds to the human CD4 receptors and co-receptors, resulting in a further conformation change in an inner membrane protein gp41 that eventually mediates viral entry into the target cells.33,34 Due to this reason, the tropism of primary LVs is confined to some CD4-expressing cells, such as T cells and monocytes.35 Correspondingly, however, this innate limitation obviously restricts the potential utilities of primary LVs in transducing genes into other numerous types of cells and therefore hinders their clinical application for many diseases. To solve this problem, attempts to construct the genome of LVs with the encapsidation of envelope protein of a second virus have resulted in numerous heterologous pseudotypes, which are transformed artificially to have the host tropism of the virus endowing the envelope protein.36 The glycoprotein of the vesicular stomatitis virus (VSV-G) has been the firstly used material to replace the envelope glycoprotein of viral-derived vectors.37 VSV is a single-stranded and negative-sense RNA virus pertaining to the Rhabdoviridae family, which exhibits remarkably robust and pantropic infectivity mediated by VSV-G, a trimeric protein that binds to the low-density lipoprotein receptor (LDLR) family members for allowing VSV entry.38 Because of the broad tropism conferred by a widespread expression of LDLR family members, VSV-G has now become the most widely used envelope glycoprotein to pseudotype LVs for altering the host tropism and expanding their range of application.39 Yet, VSV-G-pseudotyped LVs have several drawbacks, and many emerging pseudotypes of LVs engaging alternative envelopes have been developed and evaluated (Fig. 2). These pseudotypes display many differences in terms of vector titer, particle stability, cytotoxicity, host tropism, and transduction efficiency when compared with VSV-G pseudotypes.24,36 Among numerous types of pseudotypes, those that have been demonstrated to mediate successful targeted transduction of specific cell types particularly merit an updated review. The following contents are dedicated to summarize the recent advances in pseudotyped LVs whose glycoproteins are derived from viruses including the VSV, lymphocytic choriomeningitis virus (LCMV), measles viral (MV), and hepatitis C virus (HCV). The overview of these pseudotyped-LVs is listed in Table 2.
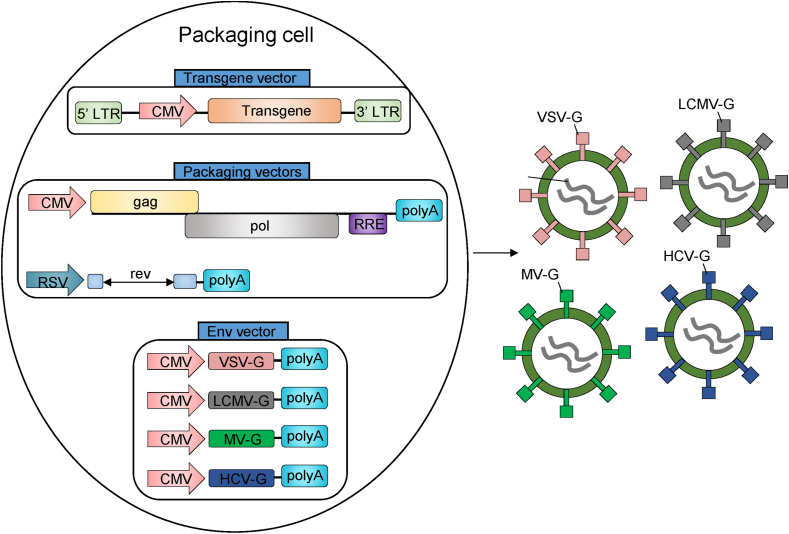
Figure 2.
The pseudotypings of LVs and their production. Packaging cells are transfected with a three-plasmid system consisting of a transgene vector, packaging vectors and an envelope vector, which encodes VSV-G, LCMV-G, MV-G or HCV-G, allowing production of different pseudotypes of LVs that have distinct tropisms. These assembled vector particles can be harvested from the supernatants and purified or concentrated further.
Table 2.
The overview of LVs pseudotyped with VSV-G, LCMV-G, MV-G and HCV-G.
| Virus Family | Envelope | Receptor | Tropism | Ref |
|---|---|---|---|---|
| Rhabdovirus | VSV-G | LDLR family members | Extremely broad | 38,40 |
| Arenavirus | LCMV-G | α-DG and additional unconfirmed receptors | Malignant glioma, astrocytes, neural progenitors and DCs | 41,42 |
| Paramyxovirus | MV-G | SLAM and CD46 | Lymphoid cells/tissues | 43,44 |
| Flavivirus | HCV-G | CD81 and coreceptors | Hepatocytes and hepatocarcinoma | 45,46 |
VSV-G-pseudotyped LVs
The primary design of VSV-G-pseudotyped HIV-1-based LVs was inspired by an early observation of a phenotypic mixing between HIV and VSV during coinfection in H9 cells that forms HIV hybrid virions bearing the VSV-G.47 In the following three studies, HIV-1 vector backbone was packaged into VSV-G to construct a pseudotype via transient cotransfection of plasmids expressing the VSV-G with an envelope-defective HIV-1 genome into packaging cells, such as 293T human kidney cells or African green monkey COS7 cells.26,48,49 The production of third-generation of VSV-G-pseudotyped LVs has been improved in terms of biosafety through using a conditional packaging system.50 At present, the transient transfection of adherent 293T cells with multiple plasmids remains the widely used approach to obtain VSV-G-pseudotyped LVs.51 A progress has recently been achieved in this field that the suspension producer cell lines from 293T cells can be rapidly generated via a stable transfection of a single large DNA construct encoding all components of LVs, and produce high titers with extended cell culture in stirred-tank bioreactors.52 This work offers an attractive alternative technique for massively manufacturing LVs. The development of novel methods for efficient production of LVs could also be motivated by new insights into the underlying mechanisms. For example, one study has shown that the glycosylation of VSV-G at N336 maximizes the expression of VSV-G in 293T cells, and thus promoting vector pseudotyping, and the level of VSV-G incorporated into LVs substantially boosts the transduction of primate cell lines, indicating that the incorporation of N336-glycosylated VSV-G into virions should be optimized for efficient production of VSV-G-pseudotyped LVs.53 Moreover, it has been found that the unphosphorylated ezrin proteins could inhibit VSV-G-mediated stabilization of HIV-1 Gag protein, and on the contrary, ezrin silencing yields higher amount of VSV-G-pseudotyped LVs.54 Considering that host cells can evolve natural defense mechanisms against viral infections and disturb production of HIV-1 vector,55 this study opens a new avenue toward high-amount production of LVs by inactivation of certain host restriction factors. Except high-titer production, VSV-G pseudotyping also has a purification advantage over other envelopes due to increased stability of vector particle, which allows concentration of LVs by ultracentrifugation.37
The complement resistance is another prerequisite for developing broadly-used LVs in human gene therapy. However, the VSV-G pseudotyped LVs produced in human cells were once found to be inactivated substantially by human serum complement.56 Fortunately, this caveat was subsequently resolved by multiple methods, such as using PEGylated VSV-G,57 or a fusion VSV-G containing the complement-regulatory protein CD55,58 or directly introducing mutations into surface exposed residues within VSV-G,59 or encapsulating within in situ synthesized polymer shell.60 These novel solutions are able to protect the viral particles from nonspecific complement-mediated neutralization. Although VSVG-pseudotyped LVs display variable biodistribution, and cause no significant toxicity when administered intravenously,61 they have been found contaminated heavily with tubulovesicular structures of cellular origin, which stimulate innate antiviral responses in host cells.62 One study has implied that optimizing the procedure of LVs’ production and purification to remove by-products during production may be an approach to diminish immune response after transduction of LVs.63
A recently published structural analysis has suggested that VSV-G specifically evolves to interact with the receptor cysteine-rich domains of the LDLR, which serves as a major receptor for VSV entry.40 Consistently, in zebra finches who lose the LDLR functional domain, the infectivity by VSVG-pseudotyped LVs is inefficient.64 Apart from LDLR, some regulators have also been shown to determine the infectivity of VSVG-pseudotyped LVs. The cholesterol represents one of them as its supplementation during viral production increases the infectivity of VSVG-pseudotyped LVs, although the detailed mechanisms require further clarification.65 Further, by prolonging the half-life of viral particles upon attachment to the cell surface, the infectivity of VSVG-pseudotyped LVs is also associated with cell–cell surface transmission prior to their cellular uptake.66 Moreover, the infectivity of VSVG-pseudotyped LVs was found to be facilitated by the host protein leucine-rich repeat-containing G protein-coupled receptor 4 (Lgr4) via its direct binding to VSVG.67 Furthermore, by a CRISPR screening assay, the interferon induced transmembrane (IFITM) proteins were validated as potent inhibitors of VSV-G-mediated entry of LVs.68 The identification of these novel host factors regulating VSV entry and infectivity lays a significant foundation for enhancing the transduction efficiency of VSVG-pseudotyped LVs in gene therapy.
VSVG-pseudotyped LVs were initially demonstrated to enable high-efficient gene delivery into some types of nondividing cells, including peripheral blood CD34+ cells, skin fibroblasts, macrophages, and neurons.26,48,49 A broader scope of cells/tissues that can be transferred effectively in vitro and/or in vivo have also been verified afterwards, such as airway epithelia,69, 70, 71, 72, 73 primary alveolar epithelia cells,74 blood hematopoietic cells,75,76 mouse brain tissues,77, 78, 79 spinal cord,80 liver and muscle,81 human islets,82 skin,83 intestinal mucosa,84 as well as mesenchymal stem cells.85 In these studies, VSV-G-pseudotyped LVs were evaluated alone or often used as standard references which display more or less efficiency in gene transduction compared to other LV pseudotypes. This extremely broad tropism of VSV-G pseudotypes renders them to be powerful tools in gene therapy.
LCMV-G-pseudotyped LVs
The LCMV is a noncytopathic RNA arenavirus that is capable of infecting a wide variety of mammalian cells from different tissues and species, and it has been extensively exploited as a model pathogen for investigating virus-induced immune responses.86 Miletic et al pseudotyped the early oncoretroviral vectors based on the murine leukemia virus (MLV) with the glycoproteins of replication-competent LCMV.87 In this attempt, the LCMV-G-pseudotyped vector remained highly stable when concentrated by ultracentrifugation and efficiently infected several different cell lines, suggesting it as a promising alternative to retroviral pseudotype. Later, this same research group established efficient recombinant packaging systems for generating LCMV-G pseudotype using LCMV-G-expressing plasmids combined with three sets of packaging constructs transiently transfected in 293T cells.88 Notably, the LCMV-G-pseudotyped LVs can be produced in high titers similar to that with VSV-G, and can also be concentrated efficiently by ultracentrifugation without loss of stability. They also efficiently transduced many cell lines from different species and tissues relevant to gene therapy. In addition, the low toxicity of LCMV-G allowed establishment of sustainable packaging cell line.88 The low toxicity of LCMV-G was also validated upon in vivo administration.89 Overall, these advantages represent LCMV-G-pseudotyped LVs as promising alternatives to VSV-G pseudotype in gene transfer applications.
Unlike VSV-G, LCMV-G is posttranslationally processed into GP-1 subunit, which acts to meditate binding to the alpha-dystroglycan (α-DG) as a cellular receptor for LCMV, and is also essential for the infectivity of pseudotyped viral vectors.41,90 An in-depth molecular analysis of the interaction of LCMV with α-DG has shown the involvement of globular protein domains and mucin-related structures of α-DG and a pivotal role of virus affinity for binding to α-DG.42 In accordance, altering α-DG affinity of LCMV-G-pseudotyped LVs has been revealed to confer unique cell and tissue tropism.91 Moreover, LCMV-G pseudotype could transduce 293T cells and murine dendritic cells (DCs) much more efficiently due to their high-affinity binding with α-DG.92 Nevertheless, although α-DG is widely expressed in most tissues,93 the tropism of LCMV-G-pseudotyped LVs is relatively narrow, displaying preference in infecting malignant glioma rather than normal neurons, compared with VSV-G pseudotype.94,95 In agreement, pseudotyping VSV with LCMV-G was found to enhance infectivity for glioma cells while sparing primary human and rat neurons in vitro and in vivo.96 This discrepancy suggests the existence of additional viral receptor(s) that may participate in cellular entry of LCMV. Indeed, one study has shown that the infectivity of LCMV-G-pseudotyped LVs can be enhanced by other four cell surface molecules, such as Axl and Tyro3,97 plausibly explaining the relatively narrowed tropism of LCMV-G-pseudotyped LVs.
The transduction pattern of LCMV-G-pseudotyped LVs in a given tissue has been determined empirically. For instance, compared to VSV-G-pseudotype, LCMV-G-pseudotyped LVs transduce similar populations, including striatum, thalamus, and white matter in the mouse brain.77 While, LCMV-G-pseudotyped LVs exhibit moderate and weak transduction in the striatum of adult rats.78 Additionally, it has been shown that in contrast to VSV-G pseudotype transducing midbrain neurons, LCMV-G-pseudotyped LVs induce transgene expression exclusively in astrocytes in the rat substantia nigra.98 Besides, by pseudotyping with LCMV-G, LVs can also be targeted to neural progenitors in the murine brain.99 In human islets, LCMV-pseudotyped LVs exert higher transduction efficiency and lower toxicity than those pseudotyped with VSV-G.82 These lines of evidence indicate that the transduction pattern of LCMV-G-pseudotyped LVs is quite distinct from that of VSV-G-pseudotype. This may presumably be dependent on expression of α-DG in target cells. However, given the complicacy of LCMV-G receptors, α-DG-independent mechanism may not be ruled out easily, which needs to be clarified by future studies. Furthermore, LVs pseudotyped with LCMV-G have been engineered to preferentially delivery antigen to the DCs for eliciting vaccine-directed immune responses in vivo,92 supporting their possible application for vaccine-directed therapy against malignancies.
MV-G-pseudotyped LVs
The measles virus (MV) is a kind of paramyxovirus causing measles disease in humans. It is well established that the infection of MV depends on two surface glycoproteins, the haemagglutinin (H) and fusion (F) protein, that mediate viral binding to the cellular receptor and subsequent entry into the host cells.100 The natural receptor of MV is the signaling lymphocyte activating molecule (SLAM),101 which is selectively expressed on the immature thymocytes, activated lymphocytes and monocytes, macrophages and mature DCs.102 This pattern of SLAM expression underlies the lymphoid tissue tropism of MV. On the other hand, due to the lack of expression of LDL receptor, VSV-G-pseudotyped LVs cannot transfer gene efficiently into quiescent T and B cells, and hematopoietic stem cells (HSCs),103 which limits their application in gene therapy targeting the immune system. Given the tropism feature of MV, two independent groups established efficient pseudotyping of LVs with both H and F glycoproteins of MV.104, 105, 106, 107 The resulting MV-G-pseudotyped LVs can not only be produced in substantial high-titer, but also retain the restricted tropism of MV for lymphocytes, particularly outperforming VSV-G pseudotype for transducing quiescent and stimulated T cells, and resting B lymphocytes and DCs.
Attaining high efficient production of viral vector is a major challenge in gene therapy. The vaccine strain of MV predominantly binds the ubiquitously expressed CD46 for cellular entry,108 and it has been found that utilizing CD46 null 293T cells produces 2-fold higher titer vectors and results in 2- to 3-fold higher efficacy in transducing HSCs.109 This observation provides an optimized condition to improve the production of MV-G-pseudotyped LVs. Another vital issue, however, is that the pre-existing MV-specific immunity aroused by vaccination or natural infection potentially obstructs the systemic administration of MV-G-pseudotyped LVs in humans. To address this, two studies have shown a promising approach which induces point mutations in the ectodomain of H glycoprotein for blunting the antibody binding sites, whereby assisting the MV-G-pseudotyped LVs escape MV antibody neutralization, and thus resulting in efficient in vivo transduction of the quiescent human T and B lymphocytes.110,111 According to these findings, using the modified MV-G other than the wild-type to pseudotype LVs is expected to provide more attractive tools for in vivo lymphocyte-based gene therapy and immunotherapy.
HCV-G-pseudotyped LVs
The HCV is a parenterally transmitted positive-stranded RNA virus that belongs to the flaviviridae family, and can induce acute and chronic liver diseases in humans, the only natural hosts for HCV.112 Two surface envelope glycoproteins, E1 and E2, form a functional noncovalent heterodimer to mediate HCV entry into cells.113 Several studies have demonstrated that HCV glycoproteins mediate viral entry and infection via a CD81-dependent manner.114, 115, 116, 117 Besides CD81, multiple lines of evidence have indicated that additional hepatocyte-specific coreceptors associated with CD81, including the C-type lectins L-SIGN and DC-SIGN, scavenger class B type I receptor (SRBI), tight junction protein claudin-1 (CLDN1), CLDN6, and CLDN9 also are essential for facilitating HCV entry.118,119,120, 121, 122, 123, 124, 125 Despite of these cell-surface components, other host factors, such as the epidermal growth factor receptor (EGFR), ephrin receptor A2 (EphA2),126 cell death-inducing DFFA-like effector b (CIDEB),127 fatty acid synthase (FASN),128 MAPK interacting serine/threonine kinase 1 (MKNK1),129 and the tripartite motif 26 (TRIM26),130 have also been shown to contribute to HCV entry. These findings indicate that some specific receptors, including CD81 and related coreceptors, contribute to a preferential tropism of E1 and E2 glycoproteins for hepatic cells.
Within some pseudotyped viral particles engineered with chimeric E1 and E2 glycoproteins bearing modifications in the transmembrane domains, the conformation and function of the E1/E2 complexes are disrupted.131,132 Therefore, to assemble HCV pseudo-particles, another tactic was employed, wherein the structurally unmodified and full-length functional HCV E1 and E2 glycoproteins were displayed onto the LVs through cotransfecting HCV E1 and E2 expression constructs with MLV-based packaging and transfer vectors.133 As a result, the generated HCV pseudo-particles were found highly infectious, with the primary hepatocytes and hepatocarcinoma being the major infection targets in vitro.133 There is also evidence manifesting that such particles can undergo proper receptor binding, and that E1/E2 complexes retain the mature conformation similar to that on the native HCV.134 Hence, this pseudotyping of LVs with HCV envelops argues that for fulfilling a correct assembling on LVs and the generation of high-titer chimerical infectious pseudo-particles retaining HCV hepatic tropism, no structurally modified E1 and E2 glycoproteins are required during pseudotyping. These HCV-G-pseudotyped LVs were also found to trigger high-titer anti-E1 and anti-E2 antibodies and broadly neutralizing antibodies in mice and macaques.135,136 As being devoid of any viral genome or enzymes, such as reverse transcriptase and integrase, HCV-G-pseudotyped LVs lack the viral replication machinery and thus are much safer than inactivated HCV to induce antibody responses by expressing substantial amounts of the two envelope proteins, which makes them attractive platforms for developing therapeutic HCV vaccines.
Applying pseudotyped LVs for cancer gene therapy
Gene therapy has been attracting enormous attention in the field of cancer therapeutics as an appealing treatment at the genetic level, due to its extraordinary properties in effectively modulating the expression of specific target genes, and simultaneously causing limited side effects, as demonstrated by experimental animals and clinical trials.137 Delivering therapeutic nucleic acids into cancer cells to silence oncogene expression or enhance tumor-suppressor expression is the central theme for cancer gene therapy. But, the shortage of efficient and safe delivery vectors remains a major challenge stymying cancer gene therapy. One the other hand, increasing studies have unraveled that some pseudotyped LVs, particularly those mentioned above, show a promising therapeutic potential in cancer gene therapy. However, as far as we can know, hitherto there is no thematic thesis reviewing this research topic. In the following, we aim to revisit the status quo of the preclinical studies of pseudotyped LVs in treating a variety of malignancies, and also discuss their opportunities in future clinical gene therapy for fighting cancer (Fig. 3).
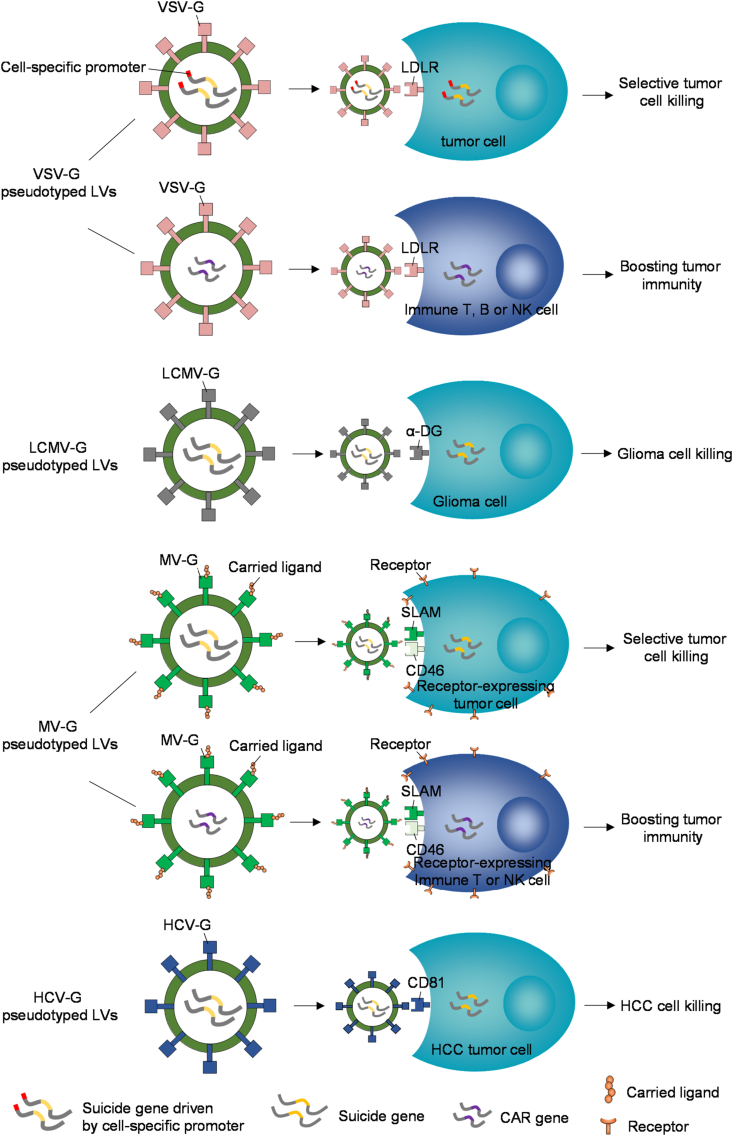
Figure 3.
Applying pseudotyped LVs for cancer gene therapy. Four types of pseudotypes depicted here have been demonstrated to delivery transgenes, including suicide genes, CAR genes or other therapeutic genes into targeted cells, based on the specific interactions between pseudotyping glycoproteins and corresponding cellular receptors for vector entry. The suicide gene transduction of tumor cells leads to direct cell killing. While the transduction of immune cells, such as T cells, B cells or NK cells, can boost cancer immunotherapy, thus evoking tumoricidal activities. CAR, chimeric antigen receptor.
VSV-G-pseudotyped LVs in cancer gene therapy
After the demonstration of high efficiency and safety of VSV-G-pseudotyped HIV-1-derived LVs in transducing the quiescent and dividing cells,138 these LVs were initially found to induce efficient transduction of the human hepatocellular carcinoma (HCC) cell lines,139 and HT1080 human fibrosarcoma xenografts,140 representing them as possible therapeutic tools for gene therapy of human HCC and fibrosarcoma. One recent study has also shown that the VSV-G-pseudotyped LVs can genetically modify the mesenchymal stromal cells to express microRNA-125b (miR-125b), which is loaded into the extracellular vesicles and specifically reduces HCC cell proliferation in vitro.141 Analogically, the small interfering RNA (siRNA) targeting the human telomerase reverse transcriptase (hTERT) was reported to be efficiently delivered by the VSV-G pseudotyped LVs and significantly inhibited the growth of xenografted human glioma tumors.142 Besides, an efficient and stable delivery of short hairpin RNA (shRNA) targeting the regulatory subunit R2Alpha of protein kinase A by VSV-G pseudotyped LVs reduces the viability of glioblastoma non-stem and stem-like cells.143 These proof-of-concept studies support an innovative strategy of using VSV-G-pseudotyped LVs for gene therapy of HCC and glioma via delivering miRNA, siRNA or shRNA. Despite of these, studies have also revealed the therapeutic potential of VSV-G pseudotyped LVs in many other malignancies. For example, VSV-G pseudotyped LVs can successfully transduce pancreatic cancer cell lines in vitro and pancreatic patient xenografts in nude mice,144 and display the highest transduction efficiency in lung cancer cells in a comparative analysis,145 suggesting that these vectors can mediate expression of a transgene for clinical gene therapy of pancreatic and lung cancers. Moreover, VSV-G pseudotyped LVs efficiently deliver CASP8 into breast cancer cells, leading to massive cell apoptosis and death and suppressed tumor growth of breast cancer model.146,147 These LVs also cause significant growth inhibition of human renal cell carcinoma (RCC) cells by transfecting tumor suppressor genes p53, p16, and PTEN simultaneously.148 Thus, the transfection of tumor suppressor genes mediated by VSV-G pseudotyped LVs exerts promising anti-tumor effects on human breast cancer and RCC, providing a reference for gene therapy of these cancers. One study has also depicted that the VSV-G-pseudotyped LVs expressing two antiangiogenic factors, angiostatin and endostatin, can efficiently and stably transduce the T24 human bladder cancer cells and result in significant inhibition of proliferation when cocultured with endothelial cells (ECs),149 hence showing the potentials of antiangiogenic gene therapy with VSV-G-pseudotyped LVs for bladder cancer treatment. Furthermore, the VSV-G-pseudotyped LVs also demonstrate therapeutic efficacy in some lymphoid malignancies, including multiple myeloma,150 and primary effusion lymphoma,151 underscoring an opportunity in immune-based gene therapy. There are some lines of evidence proving that the VSV-G-pseudotyped LVs would be useful in tuning up combinatorial cancer gene therapies. For instance, synchronous Bcl-2 downregulation and TRAIL upregulation mediated by VSV-G-pseudotyped LVs leads to enhanced eradication of gliomas compared to TRAIL monotherapy in athymic nude mice.152 Additionally, delivery of interferons and STAT3 siRNA using VSV-G-pseudotyped LVs efficiently suppresses the establishment of inoculated melanoma, and however, delivery of IFNs alone elicits no therapeutic effects on established melanoma.153
The broad tropism of VSV-G-pseudotyped LVs, however, poses a huge concern that the widespread transduction of many cell types is about to give rise to poor tumor distribution in vivo and undesirable effects. Some studies have suggested that this caveat could be overcome by alternative methods of vector construction for implementing targeted cellular expression. One example is that the VSV-G-pseudotyped LVs carrying the diphtheria toxin A gene (DTA) driven by the prostate-specific antigen (PSA) promoter were shown to specifically induce high-level expression in prostate cells and also efficiently infect the lymph node carcinoma of the prostate (LNCaP) tumors in mice without causing long-term pathogenic effects, and that the tissue-specific DTA expression can eradicate LNCaP prostate cancer cells.154 Further, taking a similar tactic, Palma et al generated VSV-G-pseudotyped LVs engineered for EC-specific expression by cloning transcription regulatory sequences from Tie2 gene preferentially expressed in ECs, and found that these vectors achieved remarkable specificity of expression in ECs in vitro and targeted expression to the ECs of tumor vessels in tumor-bearing mice.155 This vector construction method to some extent circumvents the nonspecificity issue of VSV-G-pseudotyped LVs and provides a strategy for selective delivery of gene therapy to tumor sites in vivo.
The ability of VSV-G-pseudotyped LVs to efficiently deliver genes into primary immune cells endows them a superiority in tumor immunotherapy. For example, one study has shown that owing to transducing the human peripheral blood lymphocytes (PBLs) in vitro with a high degree of efficiency, VSV-G-pseudotyped LVs carrying a chimeric T cell receptor could serve as useful tools to generate human anti-cancer chimeric T cells, which cause dramatic regression of malignant human colon carcinoma in nude mice.156 Similarly, VSV-G-pseudotyped LVs expressing two-gene T-cell receptors (TCRs) directed against the melanoma antigens gp100 and MART-1 were shown to afford robust anti-melanoma activities to engineered PBLs.157 TCR alpha and beta chains can also be inserted into VSV-G-pseudotyped LVs for obtaining robust and coordinated expression, and thus exerting significant therapeutic effects in vivo upon adoptive transfer in melanoma-bearing mice.158 The cord blood CD8+ T cells were also reported to be transduced by the VSV-G-pseudotyped LVs efficiently and exert a robust and specific anti-tumor capacity.159 Hence, VSV-G-pseudotyped LVs have great potential for constructing chimeric anti-tumor T cells by introducing transgenic expression, establishing an important basis for targeted adoptive cancer immunotherapy. Another notable virtue of LVs making them attractive tools for immunotherapy is that the pre-existing immunity to them is rare, which would enable their direct in vivo administration. Indeed, it has been demonstrated that a direct in vivo administration of VSV-G-pseudotyped LVs encoding the tumor-associated antigens (TAAs) triggers potent primary cytotoxic T-lymphocyte response and a memory CTL response, which retards the growth of grafted B16 melanoma in mice.160 This suggests that the VSV-G-pseudotyped LVs can induce therapeutic tumor immunity and the in vivo injection is an attractive approach for developing cancer vaccination.
LCMV-G-pseudotyped LVs in cancer gene therapy
As described above, LCMV-G-pseudotyped LVs show infection preference toward glioma over normal neurons, it therefore may not be surprising that so far most studies concentrated on evaluating the potential efficacy of LCMV-G-pseudotyped LVs in gene therapy against human glioma, the most frequent primary brain tumor with dismal prognosis. Two studies firstly discovered the selective and efficient transduction of malignant glioma by LVs pseudotyped with LCMV-G, and therefore proposed them to be attractive candidates for gene therapy of malignant glioma.94,95 It was further demonstrated that the LCMV-G pseudotypes could mediate a complete eradication of gliomas in a rat model using a suicide gene therapy.161 Moreover, these LVs were found to specifically and efficiently transduce cultured spheroids from human glioblastoma and invasive human glioblastoma xenografts in an animal model.162 Similarly, a suicide gene therapy mediated by LCMV-G-pseudotyped LVs results in a remission of invasive and cancer stem-like glioblastoma xenografts in a clinically relevant animal model.163 Altogether, these studies validate these vectors as attractive options for malignant glioma gene therapy.
Until now, the therapeutic evaluations of LCMV-G-pseudotyped LVs in other malignancies are scarce relative to glioma. However, as LCMV-G-pseudotyped LVs were demonstrated to efficiently transduce DCs and induce a strong antigen-specific T-cell immune response that can be further enhanced by boost injection in vivo,92 together with another evidence revealing a strong and long-lasting T cell response and a potent antitumor immunity induced by immunization with LV-transduced DCs,164 it is reasonable to assume that the LCMV-G-pseudotyped LVs could be useful vehicles for DC-based cancer immunotherapy. Since the actual clinical benefit of DC therapy for cancer patients is presently marginal, it is therefore of clinical importance to explore in the future whether these LVs can be used alone or in synergy with other modalities to assist to improve the therapeutic efficacy. In addition, it is also worth noting that several lines of current available evidence have shown the differential tissue transduction patterns of LVs pseudotyped with LCMV-G and other glycoproteins, while their transfection spectrum in diverse human tumor cells remains less investigated. More efforts are required to address this issue, which would help to broaden the utility of LCMV-G-pseudotyped LVs in cancer gene therapy.
MV-G-pseudotyped LVs in cancer gene therapy
The first hint to relate the MV-G-pseudotyped LVs to cancer gene therapy derives from an observation reporting that these pseudotypes could enable an efficient and stable transduction in the malignant plasma cells (PCs),165 some abnormal cells progressing into multiple myeloma due to their primary accumulation in the bone marrow.166 Two entry receptors CD46 and SLAM for MV-G binding are thought to contribute to this efficient transduction, as both of them are expressed by malignant PCs and also are indispensable for efficient transduction of MV-G-pseudotyped LVs in primary lymphocytes.43 This ability to modify PCs genetically unfolds a possibility to use these vectors for treating multiple myeloma by introducing a therapeutic gene. In addition, the high flexibility of the MV H glycoprotein allows accommodation of cell-targeting ligands to deliver transgene to specific populations of cancer cells. For example, one study designed MV-G-pseudotyped LVs with H protein bearing the interleukin-13 (IL-13), and found that these retargeted vectors specifically transduced in vitro cancer cells expressing high levels of IL-13 receptor α2 (IL-13Rα2) and IL-13Rα2-positive glioma xenografts in vivo.167 This targeting strategy of MV-G-pseudotyped LVs would enlighten their future use for transfecting other tumors expressing specific cell surface receptors. On the other hand, LVs have been used as successful vehicles in many clinical trials testing the effect of hematopoietic stem cell (HSC)-based gene therapy for many diseases.168 Strikingly, MV-G-pseudotyped LVs were shown to transduce HSC with an unprecedented efficiency.169 This discovery may facilitate future clinical applications requiring HSC gene modification, including the treatment of metastatic brain tumors, as a therapeutic potential for the clinical translation of HSC gene therapy for brain metastases originating from breast and lung cancer has recently been demonstrated.170
MV-G-pseudotyped LVs also hold promise to be engaged in tumor immunotherapy. For example, by engineering a CD4-specific designed ankyrin repeat protein (DARPin) on the modified H protein, the MV-G-pseudotyped LVs were converted to be highly specific and effective in transferring CAR genes into the human CD4+ T cells upon systemic administration, rendering them functionally active in lysing specific tumor cells.171,172 This exclusive gene transfer into T cell subsets upon systemic administration of MV-G-pseudotyped LVs offers a novel tactic to facilitate tumoricidal CAR T cell generation for immunotherapy. Further, the MV-G-pseudotyped LVs have also been found to mediate efficient CAR expression in NK cells, which exert pronounced anti-tumor activity against malignant triple-negative breast cancer cells,173 also implying that these LVs might be useful tools in NK-cell immunotherapy.
HCV-G-pseudotyped LVs in cancer gene therapy
The hepatocellular carcinoma (HCC) is the most common liver cancer, and chemotherapy remains the first-line treatment for patients who cannot receive surgical resection or liver transplantation. But, the therapeutic efficacy of chemotherapy for HCC is disappointing.174 This gloomy situation makes gene therapy an alternative option to treat HCC.175 Whereas, achieving HCC-specific gene delivery in vivo with minimal unwanted toxicity to healthy liver is very challenging. A breakthrough was achieved in this field by a recent research that developed the HCV-G-pseudotyped LVs for preferential recognition and gene delivery of HCC.176 In light of the hepatic tropism of HCV-G-pseudotyped LVs, this study constructed HCV-G-pseudotyped LVs as attractive alternatives to VSV-G-pseudotyping for transfecting the HCC. To this end, a three-plasmid system consisting of the HCV-E1E2 expression vector encoding the E1 and E2 glycoproteins from a 1b-type HCV and packaging plasmids encoding the gag and pol genes, together with the lentiCRISPR plasmids containing a specific sgRNA targeting the kinesin spindle protein (KSP) gene, whose inactivation causes cell cycle arrest and eventual tumor cell killing, was co-transfected into 293T cells to assemble the HCV-G-pseudotyped LVs carrying sgKSP.
In this systematic study, the authors firstly demonstrated the HCC-specific transduction of these pseudoparticles, which however had low or no infection rate in primary hepatocytes or human ovarian or cervical carcinoma cells. Mechanistically, data indicated that this transduction specificity could be attributed to varied expression of putative receptors for HCV entry, including CD81, CLDN1 and SRBI in these cells. Next, this study confirmed that the HCV-G-pseudotyped LVs retained a considerable stability in human serum and had little transduction efficiency in mature DCs and also elicited low innate immune response in human blood cells. Further, the authors proved that the intraperitoneally injected LVs could effectively deliver the transgenes specifically to orthotopic HCC tumors in the liver and potently inhibit tumor growth and progression, simultaneously causing negligible toxicity in major organs, including the liver.176 Hence, due to the superior features of HCV-G-pseudotyped LVs, such as the high stability in human serum, minimum immunologic barrier and HCC-specific gene delivery and antitumor activity, the systemic administration of these viral vectors represents a promising approach of gene therapy for eradicating HCC. Future studies should learn a valuable lesson from this study to pseudotype LVs for altering their tropism and thus yielding efficient in vivo delivery of tumoricidal genes to a specific type of tumor cell, ultimately achieving selective killing of cancer.
Conclusions and perspectives
Gene therapy holds high promise to treat cancer at the genetic level, since cancer is in essence an acquired genetic disorder. However, the success of using gene therapy for curing cancer lags far behind the achievements in the treatment of some monogenic disorders, for example the haemophilia B.177 This unmet expectation is largely due to the current failure to fulfill high efficient targeted gene transduction to cancer cells of interest without evoking undesired systemic toxicity, the ultimate goal of gene therapy in cancer treatment. As documented above, LVs are biological systems derived from natural retroviruses and show superiority in gene therapy over other delivery systems in several aspects, such as enabling transduction of human dividing and non-dividing cells with high efficiency in vitro and in vivo, and engendering low immunogenicity and genotoxic risk that permits their direct in vivo administration. Moreover, pseudotyped LVs with different glycoproteins can be endowed with the capacity to transduce specific cell types and tumor cells for implementing targeted cancer killing. For example, the LVs pseudotyped with the VSV-G can be constructed to be driven in a cell-specific manner and induce specific transgene expression in targeted tumor cells, or transfer CAR genes into immune cells for facilitating adoptive cancer immunotherapy. In addition, LCMV-G pseudotyping confers LVs with a preferred tropism toward malignant glioma, and the pseudotyping MV H glycoprotein can also be engineered to allow cell-specific targeting of LVs to some cancer cells or modify immune cells to acquire anti-tumor activities. Moreover, the hepatic tropism of HCV-G pseudotypes can retarget the transduction of suicide genes specifically into HCC tumors, resulting in inhibition of tumor growth with negligible toxicity in other healthy organs. Thus, by circumventing key issues concerning the efficiency, biosafety and specificity of vectors to certain extent, these evident advantages together would make pseudotyped LVs as appropriate and powerful gene delivery vectors for clinical applications in cancer gene therapy. Of note, except the above highlighted pseudotypes, some recent studies have also shown encouraging results of other pseudotypes of LVs in cancer treatment, such as the Sindbis virus,178,179 Zikavirus,180 and Tupaia paramyxovirus.181 Collectively, these established preclinical foundations suggest a prospective therapeutic impact of pseudotyped LVs in cancer treatment and also shed light on further studies for exploring their utility in cancer gene therapy (Fig. 3). We look forward to relevant clinical tests that evaluate the therapeutic efficacy and safety profile of these pseudotyped LVs incorporated into gene therapies for multiple human cancers.
A key barrier to efficacious cancer gene therapy is the low transduction efficiency achieved in local tumor sites.182 In vivo gene transfer via viral vectors can be administrated through different routes such as intratumoral injection and systemic delivery (e.g., intravenous and intraperitoneal injection), the most frequently used methods for administration.183 The intravenous route, however, is prone to induce side effects at off-target tissues and also needs to overcome additional biological barriers before reaching the tumor microenvironment.184 Although might confront with the challenge of inefficient diffusion,185,186 several studies have shown that in contrast to systemic delivery, direct intratumoral administration ensures optimal specificity and local vector concentration and overall transduction efficiency in the tumor.187, 188, 189, 190 Many clinical trials have also demonstrated the efficacy of intratumoral delivery of viral vectors for cancer gene therapy against primary tumors.191 It is also conceivable that for commonly used VSV-G-pseudotyped LVs which are not engineered to induce transgene expression in a tissue-specific manner, the delivery route has to be limited to intratumoral injection, as their broad tropism can transduce nonspecific cells and cause side-effects upon systemic administration. However, the direct intratumoral injection is not applicable in patients with widespread cancer cells or metastases,192 the primary causes responsible for about 90% of cancer deaths.193 In clinical applications, prescribing an optimal administration route is thus complicated and should be applied according to the condition of each patient in order to improve gene delivery efficiency. On the other hand, studies have revealed that upon systemic administration, the cell-type specificity of pseudotyped LVs such as LCMV-G and HCV-G allows highly selective and efficient delivery of transgenes to malignant glioma and hepatocarcinoma in animal models,161,162,176 implying that these LV pseudotypes may serve as superior tools over non-pseudotyped LVs when systemic administration is the only option for treatment. The selective transduction afforded by pseudotypings could be largely due to the specific interactions between glycoproteins and cellular receptors on tumor cells. Nevertheless, the explicit mechanisms by which pseudotypings enhance transduction efficiency remain to be clarified. Except the potential role of specific interactions between pseudotyped LVs and tumor cells which may reduce vector exhaustion in non-specific targets, one possibility is that the greater stability of pseudotyped LVs may allow longer exposure of target tumor cells to these vectors, which can result in more efficient transfer. Further, endosomal escape of viral vectors is also a possible strategy, since it has been described that the improved transduction efficiency by VSV-G pseudotyping results from increased escape from the endosomes.194 Further studies are warranted to test these hypothesizes. It is also of importance to investigate how various routes of administration might enable the use of pseudotyped LVs for cancer gene therapy.
Furthermore, despite the high translational promise of pseudotyped LVs, additional basic and clinical studies remain to be done before they reach the bedside. For example, developing preclinical safety models to test the oncogenic potential of LVs may further improve the designing of safer LVs to reduce the risk of insertional mutagenesis.195 Moreover, it needs to be ascertained that the non-integrating LVs and the short-term delivery could be helpful to circumvent the concern of potential exacerbation of off-target effects due to LV-mediated persistent expression of the gene editing machinery. A self-restricted CRISPR system was also developed recently to reduce LV-mediated durable expression.196 Alternatively, cell-specific or -inducible promoters turned on or off through administration of antibiotics, such as tetracycline,197 could also be useful tools to redirect transgene expression to a particular target, for example, a tumor cell, which may represent another strategy to reduce undesirable off-target effects of LVs. Importantly, clinical trials aimed to determine the long-term safety and transduction efficacy in patients who have received the LV-based gene therapy are also desperately required. Addressing these challenges concerning LVs would pave the way toward successful clinical applications of pseudotyped LVs in treating human cancers and other diseases.
Author contributions
LD conceived and wrote the manuscript. PL and HC reviewed and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Conflict of interests
Authors declare no conflict of interests.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81872071), the Fundamental Research Funds for the Central Universities (China) (No. SWU120054), the Natural Science Foundation of Chongqing (China) (No. cstc2019jcyj-zdxmX0033), and the Fundamental Research Funds for the Central Universities (China) (No. XYDS201912).
Acknowledgements
We thank all members of the Cui group for their helpful discussions. We apologize to those authors whose relevant work could not be included in this review article due to space limitations.
Footnotes
Peer review under responsibility of Chongqing Medical University.
Contributor Information
Ping Liang, Email: liangping868@sina.com.
Hongjuan Cui, Email: hcui@swu.edu.cn.
Abbreviations
- α-DG
alpha-dystroglycan
- B-ALL
B cell acute lymphoblastic leukemia
- bb2121
autologous T lymphocytes transduced with anti-BCMA CAR LVs to express a chimeric antigen receptor targeting the human B cell maturation antigen
- BPDCN
blastic plasmacytoid dendritic cell neoplasm
- CART-19
CD19 TCR-ζ/4-1BB LV-transduced autologous T cells
- CLL
chronic lymphocytic leukemia
- CLL1-CD33 cCARTs
cCAR T cells transduced with LVs to express two distinct units of anti-CLL1 and CD33 CARs
- DC
dendritic cells
- HCV-G
hepatitis C virus glycoprotein
- HPCs
hematopoietic progenitor cells
- HSPCs
hematopoietic stem and progenitor cells
- huMNC2-CAR44 CARTs
autologous T cells transduced with LVs coding for humanized MNC2-scFv
- JNJ-68284528
autologous T lymphocytes transduced with LCAR-B38M LV to express a chimeric antigen receptor targeting the human B cell maturation antigen
- LCMV-G
lymphocytic choriomeningitis virus glycoprotein
- LDLR
low-density lipoprotein receptor
- MB-102
adoptively transferred T cells transduced using LVs to express a CD123-specific, CD28-costimulatory CAR and a truncated human epidermal growth factor receptor
- MV-G
measle virus glycoprotein
- SLAM
signaling lymphocyte activating molecule
- TCRT-ESO-A2
autologous T cells transduced with LVs encoding an affinity enhanced TCR targeting tumor-associated antigen NY-ESO-1
- VSV-G
vesicular stomatitis virus glycoprotein
References
- 1.Naldini L. Gene therapy returns to centre stage. Nature. 2015;526(7573):351–360. doi: 10.1038/nature15818. [DOI] [PubMed] [Google Scholar]
- 2.Dunbar C.E., High K.A., Joung J.K., Kohn D.B., Ozawa K., Sadelain M. Gene therapy comes of age. Science. 2018;359(6372):eaan4672. doi: 10.1126/science.aan4672. [DOI] [PubMed] [Google Scholar]
- 3.Ginn S.L., Amaya A.K., Alexander I.E., Edelstein M., Abedi M.R. Gene therapy clinical trials worldwide to 2017: an update. J Gene Med. 2018;20(5):e3015. doi: 10.1002/jgm.3015. [DOI] [PubMed] [Google Scholar]
- 4.Goswami R., Subramanian G., Silayeva L., et al. Gene therapy leaves a vicious cycle. Front Oncol. 2019;9:297. doi: 10.3389/fonc.2019.00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poorebrahim M., Sadeghi S., Fakhr E., et al. Production of CAR T-cells by GMP-grade lentiviral vectors: latest advances and future prospects. Crit Rev Clin Lab Sci. 2019;56(6):393–419. doi: 10.1080/10408363.2019.1633512. [DOI] [PubMed] [Google Scholar]
- 6.Sung Y.K., Kim S.W. Recent advances in the development of gene delivery systems. Biomater Res. 2019;23:8. doi: 10.1186/s40824-019-0156-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duvergé A., Negroni M. Pseudotyping lentiviral vectors: when the clothes make the virus. Viruses. 2020;12(11):1311. doi: 10.3390/v12111311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedmann T., Roblin R. Gene therapy for human genetic disease? Science. 1972;175(4025):949–955. doi: 10.1126/science.175.4025.949. [DOI] [PubMed] [Google Scholar]
- 9.High K.A., Anguela X.M. Adeno-associated viral vectors for the treatment of hemophilia. Hum Mol Genet. 2016;25(R1):R36–R41. doi: 10.1093/hmg/ddv475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orkin S., Motulsky A. Report and recommendations of the panel to assess the NIH investment in research on gene therapy. Curr Sci. 1996;71(9):658–659. [Google Scholar]
- 11.Vogt V.M. In: Retroviruses. Coffin J.M., Hughes S.H., Varmus H.E., editors. Cold Spring Harbor; NY: 1997. Retroviral virions and genomes. [PubMed] [Google Scholar]
- 12.Blaese R.M., Culver K.W., Miller A.D., et al. T lymphocyte-directed gene therapy for ADA- SCID: initial trial results after 4 years. Science. 1995;270(5235):475–480. doi: 10.1126/science.270.5235.475. [DOI] [PubMed] [Google Scholar]
- 13.Kamimura K., Suda T., Zhang G., Liu D. Advances in gene delivery systems. Pharmaceut Med. 2011;25(5):293–306. doi: 10.2165/11594020-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nayerossadat N., Maedeh T., Ali P.A. Viral and nonviral delivery systems for gene delivery. Adv Biomed Res. 2012;1:27. doi: 10.4103/2277-9175.98152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Babaei M., Eshghi H., Abnous K., Rahimizadeh M., Ramezani M. Promising gene delivery system based on polyethylenimine-modified silica nanoparticles. Cancer Gene Ther. 2017;24(4):156–164. doi: 10.1038/cgt.2016.73. [DOI] [PubMed] [Google Scholar]
- 16.Ramamoorth M., Narvekar A. Non viral vectors in gene therapy- an overview. J Clin Diagn Res. 2015;9(1):GE01–GE06. doi: 10.7860/JCDR/2015/10443.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slivac I., Guay D., Mangion M., Champeil J., Gaillet B. Non-viral nucleic acid delivery methods. Expet Opin Biol Ther. 2017;17(1):105–118. doi: 10.1080/14712598.2017.1248941. [DOI] [PubMed] [Google Scholar]
- 18.Patil S., Gao Y.G., Lin X., et al. The development of functional non-viral vectors for gene delivery. Int J Mol Sci. 2019;20(21):5491. doi: 10.3390/ijms20215491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohammadinejad R., Dehshahri A., Sagar Madamsetty V., et al. In vivo gene delivery mediated by non-viral vectors for cancer therapy. J Contr Release. 2020;325:249–275. doi: 10.1016/j.jconrel.2020.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caffery B., Lee J.S., Alexander-Bryant A.A. Vectors for glioblastoma gene therapy: viral & non-viral delivery strategies. Nanomaterials. 2019;9(1):105. doi: 10.3390/nano9010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bulcha J.T., Wang Y., Ma H., Tai P.W.L., Gao G. Viral vector platforms within the gene therapy landscape. Signal Transduct Targeted Ther. 2021;6(1):53. doi: 10.1038/s41392-021-00487-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Montini E., Cesana D., Schmidt M., et al. Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration. Nat Biotechnol. 2006;24(6):687–696. doi: 10.1038/nbt1216. [DOI] [PubMed] [Google Scholar]
- 23.Milone M.C., O'Doherty U. Clinical use of lentiviral vectors. Leukemia. 2018;32(7):1529–1541. doi: 10.1038/s41375-018-0106-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joglekar A.V., Sandoval S. Pseudotyped lentiviral vectors: one vector, many guises. Hum Gene Ther Methods. 2017;28(6):291–301. doi: 10.1089/hgtb.2017.084. [DOI] [PubMed] [Google Scholar]
- 25.Naldini L., Blömer U., Gage F.H., Trono D., Verma I.M. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc Natl Acad Sci U S A. 1996;93(21):11382–11388. doi: 10.1073/pnas.93.21.11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naldini L., Blömer U., Gallay P., et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272(5259):263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 27.Blömer U., Naldini L., Kafri T., Trono D., Verma I.M., Gage F.H. Highly efficient and sustained gene transfer in adult neurons with a lentivirus vector. J Virol. 1997;71(9):6641–6649. doi: 10.1128/jvi.71.9.6641-6649.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marquez Loza L.I., Yuen E.C., McCray P.B., Jr. Lentiviral vectors for the treatment and prevention of cystic fibrosis lung disease. Genes. 2019;10(3):218. doi: 10.3390/genes10030218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levine B.L., Humeau L.M., Boyer J., et al. Gene transfer in humans using a conditionally replicating lentiviral vector. Proc Natl Acad Sci U S A. 2006;103(46):17372–17377. doi: 10.1073/pnas.0608138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yahya E.B., Alqadhi A.M. Recent trends in cancer therapy: a review on the current state of gene delivery. Life Sci. 2021;269:119087. doi: 10.1016/j.lfs.2021.119087. [DOI] [PubMed] [Google Scholar]
- 31.Kumar P., Woon-Khiong C. Optimization of lentiviral vectors generation for biomedical and clinical research purposes: contemporary trends in technology development and applications. Curr Gene Ther. 2011;11(2):144–153. doi: 10.2174/156652311794940782. [DOI] [PubMed] [Google Scholar]
- 32.Sakuma T., Barry M.A., Ikeda Y. Lentiviral vectors: basic to translational. Biochem J. 2012;443(3):603–618. doi: 10.1042/BJ20120146. [DOI] [PubMed] [Google Scholar]
- 33.Dalgleish A.G., Beverley P.C., Clapham P.R., Crawford D.H., Greaves M.F., Weiss R.A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984;312(5996):763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- 34.Moore J.P., Trkola A., Dragic T. Co-receptors for HIV-1 entry. Curr Opin Immunol. 1997;9(4):551–562. doi: 10.1016/s0952-7915(97)80110-0. [DOI] [PubMed] [Google Scholar]
- 35.Verhoeyen E., Cosset F.L. Engineering the surface glycoproteins of lentiviral vectors for targeted gene transfer. Cold Spring Harb Protoc. 2009;2009(8):pdb.top59. doi: 10.1101/pdb.top59. [DOI] [PubMed] [Google Scholar]
- 36.Cronin J., Zhang X.Y., Reiser J. Altering the tropism of lentiviral vectors through pseudotyping. Curr Gene Ther. 2005;5(4):387–398. doi: 10.2174/1566523054546224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burns J.C., Friedmann T., Driever W., Burrascano M., Yee J.K. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc Natl Acad Sci U S A. 1993;90(17):8033–8037. doi: 10.1073/pnas.90.17.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Finkelshtein D., Werman A., Novick D., Barak S., Rubinstein M. LDL receptor and its family members serve as the cellular receptors for vesicular stomatitis virus. Proc Natl Acad Sci U S A. 2013;110(18):7306–7311. doi: 10.1073/pnas.1214441110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dautzenberg I.J.C., Rabelink M.J.W.E., Hoeben R.C. The stability of envelope-pseudotyped lentiviral vectors. Gene Ther. 2021;28(1–2):89–104. doi: 10.1038/s41434-020-00193-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nikolic J., Belot L., Raux H., Legrand P., Gaudin Y., Albertini A.A. Structural basis for the recognition of LDL-receptor family members by VSV glycoprotein. Nat Commun. 2018;9(1):1029. doi: 10.1038/s41467-018-03432-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cao W., Henry M.D., Borrow P., et al. Identification of alpha-dystroglycan as a receptor for lymphocytic choriomeningitis virus and Lassa fever virus. Science. 1998;282(5396):2079–2081. doi: 10.1126/science.282.5396.2079. [DOI] [PubMed] [Google Scholar]
- 42.Kunz S., Sevilla N., McGavern D.B., Campbell K.P., Oldstone M.B. Molecular analysis of the interaction of LCMV with its cellular receptor [alpha]-dystroglycan. J Cell Biol. 2001;155(2):301–310. doi: 10.1083/jcb.200104103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frecha C., Lévy C., Costa C., et al. Measles virus glycoprotein-pseudotyped lentiviral vector-mediated gene transfer into quiescent lymphocytes requires binding to both SLAM and CD46 entry receptors. J Virol. 2011;85(12):5975–5985. doi: 10.1128/JVI.00324-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Navaratnarajah C.K., Generous A.R., Yousaf I., Cattaneo R. Receptor-mediated cell entry of paramyxoviruses: mechanisms, and consequences for tropism and pathogenesis. J Biol Chem. 2020;295(9):2771–2786. doi: 10.1074/jbc.REV119.009961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ding Q., von Schaewen M., Ploss A. The impact of hepatitis C virus entry on viral tropism. Cell Host Microbe. 2014;16(5):562–568. doi: 10.1016/j.chom.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Douam F., Lavillette D., Cosset F.L. The mechanism of HCV entry into host cells. Prog Mol Biol Transl Sci. 2015;129:63–107. doi: 10.1016/bs.pmbts.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 47.Zhu Z.H., Chen S.S., Huang A.S. Phenotypic mixing between human immunodeficiency virus and vesicular stomatitis virus or herpes simplex virus. J Acquir Immune Defic Syndr (1988) 1990;3(3):215–219. [PubMed] [Google Scholar]
- 48.Akkina R.K., Walton R.M., Chen M.L., Li Q.X., Planelles V., Chen I.S. High-efficiency gene transfer into CD34+ cells with a human immunodeficiency virus type 1-based retroviral vector pseudotyped with vesicular stomatitis virus envelope glycoprotein G. J Virol. 1996;70(4):2581–2585. doi: 10.1128/jvi.70.4.2581-2585.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reiser J., Harmison G., Kluepfel-Stahl S., Brady R.O., Karlsson S., Schubert M. Transduction of nondividing cells using pseudotyped defective high-titer HIV type 1 particles. Proc Natl Acad Sci U S A. 1996;93(26):15266–15271. doi: 10.1073/pnas.93.26.15266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dull T., Zufferey R., Kelly M., et al. A third-generation lentivirus vector with a conditional packaging system. J Virol. 1998;72(11):8463–8471. doi: 10.1128/jvi.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perletti G., Osti D., Marras E., Tettamanti G., de Eguileor M. Generation of VSV-G pseudotyped lentiviral particles in 293T cells. J Cell Mol Med. 2004;8(1):142–143. doi: 10.1111/j.1582-4934.2004.tb00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen Y.H., Pallant C., Sampson C.J., et al. Rapid lentiviral vector producer cell line generation using a single DNA construct. Mol Ther Methods Clin Dev. 2020;19:47–57. doi: 10.1016/j.omtm.2020.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Farley D.C., Iqball S., Smith J.C., Miskin J.E., Kingsman S.M., Mitrophanous K.A. Factors that influence VSV-G pseudotyping and transduction efficiency of lentiviral vectors-in vitro and in vivo implications. J Gene Med. 2007;9(5):345–356. doi: 10.1002/jgm.1022. [DOI] [PubMed] [Google Scholar]
- 54.Izumida M., Togawa K., Hayashi H., Matsuyama T., Kubo Y. Production of vesicular stomatitis virus glycoprotein-pseudotyped lentiviral vector is enhanced by Ezrin silencing. Front Bioeng Biotechnol. 2020;8:368. doi: 10.3389/fbioe.2020.00368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Simon V., Bloch N., Landau N.R. Intrinsic host restrictions to HIV-1 and mechanisms of viral escape. Nat Immunol. 2015;16(6):546–553. doi: 10.1038/ni.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.DePolo N.J., Reed J.D., Sheridan P.L., et al. VSV-G pseudotyped lentiviral vector particles produced in human cells are inactivated by human serum. Mol Ther. 2000;2(3):218–222. doi: 10.1006/mthe.2000.0116. [DOI] [PubMed] [Google Scholar]
- 57.Croyle M.A., Callahan S.M., Auricchio A., et al. PEGylation of a vesicular stomatitis virus G pseudotyped lentivirus vector prevents inactivation in serum. J Virol. 2004;78(2):912–921. doi: 10.1128/JVI.78.2.912-921.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schauber-Plewa C., Simmons A., Tuerk M.J., Pacheco C.D., Veres G. Complement regulatory proteins are incorporated into lentiviral vectors and protect particles against complement inactivation. Gene Ther. 2005;12(3):238–245. doi: 10.1038/sj.gt.3302399. [DOI] [PubMed] [Google Scholar]
- 59.Hwang B.Y., Schaffer D.V. Engineering a serum-resistant and thermostable vesicular stomatitis virus G glycoprotein for pseudotyping retroviral and lentiviral vectors. Gene Ther. 2013;20(8):807–815. doi: 10.1038/gt.2013.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liang M., Yan M., Lu Y., Chen I.S. Retargeting vesicular stomatitis virus glycoprotein pseudotyped lentiviral vectors with enhanced stability by in situ synthesized polymer shell. Hum Gene Ther Methods. 2013;24(1):11–18. doi: 10.1089/hgtb.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pan D., Gunther R., Duan W., et al. Biodistribution and toxicity studies of VSVG-pseudotyped lentiviral vector after intravenous administration in mice with the observation of in vivo transduction of bone marrow. Mol Ther. 2002;6(1):19–29. doi: 10.1006/mthe.2002.0630. [DOI] [PubMed] [Google Scholar]
- 62.Pichlmair A., Diebold S.S., Gschmeissner S., et al. Tubulovesicular structures within vesicular stomatitis virus G protein-pseudotyped lentiviral vector preparations carry DNA and stimulate antiviral responses via Toll-like receptor 9. J Virol. 2007;81(2):539–547. doi: 10.1128/JVI.01818-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baekelandt V., Eggermont K., Michiels M., Nuttin B., Debyser Z. Optimized lentiviral vector production and purification procedure prevents immune response after transduction of mouse brain. Gene Ther. 2003;10(23):1933–1940. doi: 10.1038/sj.gt.3302094. [DOI] [PubMed] [Google Scholar]
- 64.Velho T.A.F., Lovell P.V., Friedrich S.R., et al. Divergent low-density lipoprotein receptor (LDLR) linked to low VSV G-dependent viral infectivity and unique serum lipid profile in zebra finches. Proc Natl Acad Sci U S A. 2021;118(18) doi: 10.1073/pnas.2025167118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen Y., Ott C.J., Townsend K., Subbaiah P., Aiyar A., Miller W.M. Cholesterol supplementation during production increases the infectivity of retroviral and lentiviral vectors pseudotyped with the vesicular stomatitis virus glycoprotein (VSV-G) Biochem Eng J. 2009;44(2–3):199–207. doi: 10.1016/j.bej.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Skinner A.M., Chakkaramakkil Verghese S., Kurre P. Cell-cell transmission of VSV-G pseudotyped lentivector particles. PLoS One. 2013;8(9) doi: 10.1371/journal.pone.0074925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang N., Huang H., Tan B., et al. Leucine-rich repeat-containing G protein-coupled receptor 4 facilitates vesicular stomatitis virus infection by binding vesicular stomatitis virus glycoprotein. J Biol Chem. 2017;292(40):16527–16538. doi: 10.1074/jbc.M117.802090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Roesch F., OhAinle M., Emerman M. A CRISPR screen for factors regulating SAMHD1 degradation identifies IFITMs as potent inhibitors of lentiviral particle delivery. Retrovirology. 2018;15(1):26. doi: 10.1186/s12977-018-0409-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Johnson L.G., Olsen J.C., Naldini L., Boucher R.C. Pseudotyped human lentiviral vector-mediated gene transfer to airway epithelia in vivo. Gene Ther. 2000;7(7):568–574. doi: 10.1038/sj.gt.3301138. [DOI] [PubMed] [Google Scholar]
- 70.Kremer K.L., Dunning K.R., Parsons D.W., Anson D.S. Gene delivery to airway epithelial cells in vivo: a direct comparison of apical and basolateral transduction strategies using pseudotyped lentivirus vectors. J Gene Med. 2007;9(5):362–368. doi: 10.1002/jgm.1025. [DOI] [PubMed] [Google Scholar]
- 71.Copreni E., Castellani S., Palmieri L., Penzo M., Conese M. Involvement of glycosaminoglycans in vesicular stomatitis virus G glycoprotein pseudotyped lentiviral vector-mediated gene transfer into airway epithelial cells. J Gene Med. 2008;10(12):1294–1302. doi: 10.1002/jgm.1248. [DOI] [PubMed] [Google Scholar]
- 72.Copreni E., Palmieri L., Castellani S., Conese M. A VSV-G pseudotyped last generation lentiviral vector mediates high level and persistent gene transfer in models of airway epithelium in vitro and in vivo. Viruses. 2010;2(8):1577–1588. doi: 10.3390/v2081577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Carpentieri C., Farrow N., Cmielewski P., et al. The effects of conditioning and lentiviral vector pseudotype on short- and long-term airway reporter gene expression in mice. Hum Gene Ther. 2021;32(15–16):817–827. doi: 10.1089/hum.2021.031. [DOI] [PubMed] [Google Scholar]
- 74.Borok Z., Harboe-Schmidt J.E., Brody S.L., et al. Vesicular stomatitis virus G-pseudotyped lentivirus vectors mediate efficient apical transduction of polarized quiescent primary alveolar epithelial cells. J Virol. 2001;75(23):11747–11754. doi: 10.1128/JVI.75.23.11747-11754.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hanawa H., Kelly P.F., Nathwani A.C., et al. Comparison of various envelope proteins for their ability to pseudotype lentiviral vectors and transduce primitive hematopoietic cells from human blood. Mol Ther. 2002;5(3):242–251. doi: 10.1006/mthe.2002.0549. [DOI] [PubMed] [Google Scholar]
- 76.Kim Y.S., Wielgosz M.M., Hargrove P., et al. Transduction of human primitive repopulating hematopoietic cells with lentiviral vectors pseudotyped with various envelope proteins. Mol Ther. 2010;18(7):1310–1317. doi: 10.1038/mt.2010.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Watson D.J., Kobinger G.P., Passini M.A., Wilson J.M., Wolfe J.H. Targeted transduction patterns in the mouse brain by lentivirus vectors pseudotyped with VSV, Ebola, Mokola, LCMV, or MuLV envelope proteins. Mol Ther. 2002;5(5 Pt 1):528–537. doi: 10.1006/mthe.2002.0584. [DOI] [PubMed] [Google Scholar]
- 78.Wong L.F., Azzouz M., Walmsley L.E., et al. Transduction patterns of pseudotyped lentiviral vectors in the nervous system. Mol Ther. 2004;9(1):101–111. doi: 10.1016/j.ymthe.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 79.Watson D.J., Passini M.A., Wolfe J.H. Transduction of the choroid plexus and ependyma in neonatal mouse brain by vesicular stomatitis virus glycoprotein-pseudotyped lentivirus and adeno-associated virus type 5 vectors. Hum Gene Ther. 2005;16(1):49–56. doi: 10.1089/hum.2005.16.49. [DOI] [PubMed] [Google Scholar]
- 80.Schoderboeck L., Riad S., Bokor A.M., et al. Chimeric rabies SADB19-VSVg-pseudotyped lentiviral vectors mediate long-range retrograde transduction from the mouse spinal cord. Gene Ther. 2015;22(5):357–364. doi: 10.1038/gt.2015.3. [DOI] [PubMed] [Google Scholar]
- 81.MacKenzie T.C., Kobinger G.P., Kootstra N.A., et al. Efficient transduction of liver and muscle after in utero injection of lentiviral vectors with different pseudotypes. Mol Ther. 2002;6(3):349–358. doi: 10.1006/mthe.2002.0681. [DOI] [PubMed] [Google Scholar]
- 82.Kobinger G.P., Deng S., Louboutin J.P., et al. Transduction of human islets with pseudotyped lentiviral vectors. Hum Gene Ther. 2004;15(2):211–219. doi: 10.1089/104303404772680010. [DOI] [PubMed] [Google Scholar]
- 83.Hachiya A., Sriwiriyanont P., Patel A., et al. Gene transfer in human skin with different pseudotyped HIV-based vectors. Gene Ther. 2007;14(8):648–656. doi: 10.1038/sj.gt.3302915. [DOI] [PubMed] [Google Scholar]
- 84.Matsumoto H., Kimura T., Haga K., Kasahara N., Anton P., McGowan I. Effective in vivo and ex vivo gene transfer to intestinal mucosa by VSV-G-pseudotyped lentiviral vectors. BMC Gastroenterol. 2010;10:44. doi: 10.1186/1471-230X-10-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Petersen G.F., Hilbert B., Trope G., Kalle W., Strappe P. Efficient transduction of equine adipose-derived mesenchymal stem cells by VSV-G pseudotyped lentiviral vectors. Res Vet Sci. 2014;97(3):616–622. doi: 10.1016/j.rvsc.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 86.Zhou X., Ramachandran S., Mann M., Popkin D.L. Role of lymphocytic choriomeningitis virus (LCMV) in understanding viral immunology: past, present and future. Viruses. 2012;4(11):2650–2669. doi: 10.3390/v4112650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Miletic H., Bruns M., Tsiakas K., et al. Retroviral vectors pseudotyped with lymphocytic choriomeningitis virus. J Virol. 1999;73(7):6114–6116. doi: 10.1128/jvi.73.7.6114-6116.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Beyer W.R., Westphal M., Ostertag W., von Laer D. Oncoretrovirus and lentivirus vectors pseudotyped with lymphocytic choriomeningitis virus glycoprotein: generation, concentration, and broad host range. J Virol. 2002;76(3):1488–1495. doi: 10.1128/JVI.76.3.1488-1495.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Park F. Correction of bleeding diathesis without liver toxicity using are naviral-pseudotyped HIV-1-based vectors in hemophilia A mice. Hum Gene Ther. 2003;14(15):1489–1494. doi: 10.1089/104303403769211691. [DOI] [PubMed] [Google Scholar]
- 90.Beyer W.R., Pöpplau D., Garten W., von Laer D., Lenz O. Endoproteolytic processing of the lymphocytic choriomeningitis virus glycoprotein by the subtilase SKI-1/S1P. J Virol. 2003;77(5):2866–2872. doi: 10.1128/JVI.77.5.2866-2872.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dylla D.E., Xie L., Michele D.E., Kunz S., McCray P.B., Jr. Altering alpha-dystroglycan receptor affinity of LCMV pseudotyped lentivirus yields unique cell and tissue tropism. Genet Vaccine Ther. 2011;9:8. doi: 10.1186/1479-0556-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang C., Hu B., Xiao L., Liu Y., Wang P. Pseudotyping lentiviral vectors with lymphocytic choriomeningitis virus glycoproteins for transduction of dendritic cells and in vivo immunization. Hum Gene Ther Methods. 2014;25(6):328–338. doi: 10.1089/hgtb.2014.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Barresi R., Campbell K.P. Dystroglycan: from biosynthesis to pathogenesis of human disease. J Cell Sci. 2006;119(Pt 2):199–207. doi: 10.1242/jcs.02814. [DOI] [PubMed] [Google Scholar]
- 94.Miletic H., Fischer Y.H., Neumann H., et al. Selective transduction of malignant glioma by lentiviral vectors pseudotyped with lymphocytic choriomeningitis virus glycoproteins. Hum Gene Ther. 2004;15(11):1091–1100. doi: 10.1089/hum.2004.15.1091. [DOI] [PubMed] [Google Scholar]
- 95.Steffens S., Tebbets J., Kramm C.M., Lindemann D., Flake A., Sena-Esteves M. Transduction of human glial and neuronal tumor cells with different lentivirus vector pseudotypes. J Neuro Oncol. 2004;70(3):281–288. doi: 10.1007/s11060-004-6046-8. [DOI] [PubMed] [Google Scholar]
- 96.Muik A., Kneiske I., Werbizki M., et al. Pseudotyping vesicular stomatitis virus with lymphocytic choriomeningitis virus glycoproteins enhances infectivity for glioma cells and minimizes neurotropism. J Virol. 2011;85(11):5679–5684. doi: 10.1128/JVI.02511-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shimojima M., Kawaoka Y. Cell surface molecules involved in infection mediated by lymphocytic choriomeningitis virus glycoprotein. J Vet Med Sci. 2012;74(10):1363–1366. doi: 10.1292/jvms.12-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cannon J.R., Sew T., Montero L., Burton E.A., Greenamyre J.T. Pseudotype-dependent lentiviral transduction of astrocytes or neurons in the rat substantia nigra. Exp Neurol. 2011;228(1):41–52. doi: 10.1016/j.expneurol.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stein C.S., Martins I., Davidson B.L. The lymphocytic choriomeningitis virus envelope glycoprotein targets lentiviral gene transfer vector to neural progenitors in the murine brain. Mol Ther. 2005;11(3):382–389. doi: 10.1016/j.ymthe.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 100.Bester J.C. Measles and measles vaccination: a review. JAMA Pediatr. 2016;170(12):1209–1215. doi: 10.1001/jamapediatrics.2016.1787. [DOI] [PubMed] [Google Scholar]
- 101.Tatsuo H., Ono N., Tanaka K., Yanagi Y. SLAM (CDw150) is a cellular receptor for measles virus. Nature. 2000;406(6798):893–897. doi: 10.1038/35022579. [DOI] [PubMed] [Google Scholar]
- 102.Tatsuo H., Yanagi Y. The morbillivirus receptor SLAM (CD150) Microbiol Immunol. 2002;46(3):135–142. doi: 10.1111/j.1348-0421.2002.tb02678.x. [DOI] [PubMed] [Google Scholar]
- 103.Amirache F., Lévy C., Costa C., et al. Mystery solved: VSV-G-LVs do not allow efficient gene transfer into unstimulated T cells, B cells, and HSCs because they lack the LDL receptor. Blood. 2014;123(9):1422–1424. doi: 10.1182/blood-2013-11-540641. [DOI] [PubMed] [Google Scholar]
- 104.Funke S., Schneider I.C., Glaser S., et al. Pseudotyping lentiviral vectors with the wild-type measles virus glycoproteins improves titer and selectivity. Gene Ther. 2009;16(5):700–705. doi: 10.1038/gt.2009.11. [DOI] [PubMed] [Google Scholar]
- 105.Frecha C., Costa C., Nègre D., et al. Stable transduction of quiescent T cells without induction of cycle progression by a novel lentiviral vector pseudotyped with measles virus glycoproteins. Blood. 2008;112(13):4843–4852. doi: 10.1182/blood-2008-05-155945. [DOI] [PubMed] [Google Scholar]
- 106.Frecha C., Costa C., Lévy C., et al. Efficient and stable transduction of resting B lymphocytes and primary chronic lymphocyte leukemia cells using measles virus gp displaying lentiviral vectors. Blood. 2009;114(15):3173–3180. doi: 10.1182/blood-2009-05-220798. [DOI] [PubMed] [Google Scholar]
- 107.Humbert J.M., Frecha C., Amirache Bouafia F., et al. Measles virus glycoprotein-pseudotyped lentiviral vectors are highly superior to vesicular stomatitis virus G pseudotypes for genetic modification of monocyte-derived dendritic cells. J Virol. 2012;86(9):5192–5203. doi: 10.1128/JVI.06283-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dörig R.E., Marcil A., Chopra A., Richardson C.D. The human CD46 molecule is a receptor for measles virus (Edmonston strain) Cell. 1993;75(2):295–305. doi: 10.1016/0092-8674(93)80071-l. [DOI] [PubMed] [Google Scholar]
- 109.Ozog S., Chen C.X., Simpson E., et al. CD46 null packaging cell line improves measles lentiviral vector production and gene delivery to hematopoietic stem and progenitor cells. Mol Ther Methods Clin Dev. 2018;13:27–39. doi: 10.1016/j.omtm.2018.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kneissl S., Abel T., Rasbach A., Brynza J., Schneider-Schaulies J., Buchholz C.J. Measles virus glycoprotein-based lentiviral targeting vectors that avoid neutralizing antibodies. PLoS One. 2012;7(10):e46667. doi: 10.1371/journal.pone.0046667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lévy C., Amirache F., Costa C., et al. Lentiviral vectors displaying modified measles virus gp overcome pre-existing immunity in in vivo-like transduction of human T and B cells. Mol Ther. 2012;20(9):1699–1712. doi: 10.1038/mt.2012.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rehermann B., Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol. 2005;5(3):215–229. doi: 10.1038/nri1573. [DOI] [PubMed] [Google Scholar]
- 113.Douam F., Dao Thi V.L., Maurin G., et al. Critical interaction between E1 and E2 glycoproteins determines binding and fusion properties of hepatitis C virus during cell entry. Hepatology. 2014;59(3):776–788. doi: 10.1002/hep.26733. [DOI] [PubMed] [Google Scholar]
- 114.Zhang J., Randall G., Higginbottom A., Monk P., Rice C.M., McKeating J.A. CD81 is required for hepatitis C virus glycoprotein-mediated viral infection. J Virol. 2004;78(3):1448–1455. doi: 10.1128/JVI.78.3.1448-1455.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.McKeating J.A., Zhang L.Q., Logvinoff C., et al. Diverse hepatitis C virus glycoproteins mediate viral infection in a CD81-dependent manner. J Virol. 2004;78(16):8496–8505. doi: 10.1128/JVI.78.16.8496-8505.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Flint M., von Hahn T., Zhang J., et al. Diverse CD81 proteins support hepatitis C virus infection. J Virol. 2006;80(22):11331–11342. doi: 10.1128/JVI.00104-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lavillette D., Tarr A.W., Voisset C., et al. Characterization of host-range and cell entry properties of the major genotypes and subtypes of hepatitis C virus. Hepatology. 2005;41(2):265–274. doi: 10.1002/hep.20542. [DOI] [PubMed] [Google Scholar]
- 118.Pöhlmann S., Zhang J., Baribaud F., et al. Hepatitis C virus glycoproteins interact with DC-SIGN and DC-SIGNR. J Virol. 2003;77(7):4070–4080. doi: 10.1128/JVI.77.7.4070-4080.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lozach P.Y., Amara A., Bartosch B., et al. C-type lectins L-SIGN and DC-SIGN capture and transmit infectious hepatitis C virus pseudotype particles. J Biol Chem. 2004;279(31):32035–32045. doi: 10.1074/jbc.M402296200. [DOI] [PubMed] [Google Scholar]
- 120.Régeard M., Trotard M., Lepère C., Gripon P., Le Seyec J. Entry of pseudotyped hepatitis C virus into primary human hepatocytes depends on the scavenger class B type I receptor. J Viral Hepat. 2008;15(12):865–870. doi: 10.1111/j.1365-2893.2008.01048.x. [DOI] [PubMed] [Google Scholar]
- 121.Bartosch B., Vitelli A., Granier C., et al. Cell entry of hepatitis C virus requires a set of co-receptors that include the CD81 tetraspanin and the SR-B1 scavenger receptor. J Biol Chem. 2003;278(43):41624–41630. doi: 10.1074/jbc.M305289200. [DOI] [PubMed] [Google Scholar]
- 122.Harris H.J., Farquhar M.J., Mee C.J., et al. CD81 and claudin 1 coreceptor association: role in hepatitis C virus entry. J Virol. 2008;82(10):5007–5020. doi: 10.1128/JVI.02286-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zeisel M.B., Koutsoudakis G., Schnober E.K., et al. Scavenger receptor class B type I is a key host factor for hepatitis C virus infection required for an entry step closely linked to CD81. Hepatology. 2007;46(6):1722–1731. doi: 10.1002/hep.21994. [DOI] [PubMed] [Google Scholar]
- 124.Haid S., Windisch M.P., Bartenschlager R., Pietschmann T. Mouse-specific residues of claudin-1 limit hepatitis C virus genotype 2a infection in a human hepatocyte cell line. J Virol. 2010;84(2):964–975. doi: 10.1128/JVI.01504-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zheng A., Yuan F., Li Y., et al. Claudin-6 and claudin-9 function as additional coreceptors for hepatitis C virus. J Virol. 2007;81(22):12465–12471. doi: 10.1128/JVI.01457-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lupberger J., Zeisel M.B., Xiao F., et al. EGFR and EphA2 are host factors for hepatitis C virus entry and possible targets for antiviral therapy. Nat Med. 2011;17(5):589–595. doi: 10.1038/nm.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wu X., Lee E.M., Hammack C., et al. Cell death-inducing DFFA-like effector b is required for hepatitis C virus entry into hepatocytes. J Virol. 2014;88(15):8433–8444. doi: 10.1128/JVI.00081-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yang W., Hood B.L., Chadwick S.L., et al. Fatty acid synthase is up-regulated during hepatitis C virus infection and regulates hepatitis C virus entry and production. Hepatology. 2008;48(5):1396–1403. doi: 10.1002/hep.22508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kim S., Ishida H., Yamane D., et al. Contrasting roles of mitogen-activated protein kinases in cellular entry and replication of hepatitis C virus: MKNK1 facilitates cell entry. J Virol. 2013;87(8):4214–4224. doi: 10.1128/JVI.00954-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Liang Y., Zhang G., Li Q., et al. TRIM26 is a critical host factor for HCV replication and contributes to host tropism. Sci Adv. 2021;7(2):eabd9732. doi: 10.1126/sciadv.abd9732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Matsuura Y., Tani H., Suzuki K., et al. Characterization of pseudotype VSV possessing HCV envelope proteins. Virology. 2001;286(2):263–275. doi: 10.1006/viro.2001.0971. [DOI] [PubMed] [Google Scholar]
- 132.Lagging L.M., Meyer K., Owens R.J., Ray R. Functional role of hepatitis C virus chimeric glycoproteins in the infectivity of pseudotyped virus. J Virol. 1998;72(5):3539–3546. doi: 10.1128/jvi.72.5.3539-3546.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bartosch B., Dubuisson J., Cosset F.L. Infectious hepatitis C virus pseudo-particles containing functional E1-E2 envelope protein complexes. J Exp Med. 2003;197(5):633–642. doi: 10.1084/jem.20021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Op De Beeck A., Voisset C., Bartosch B., et al. Characterization of functional hepatitis C virus envelope glycoproteins. J Virol. 2004;78(6):2994–3002. doi: 10.1128/JVI.78.6.2994-3002.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Deng Y., Guan J., Wen B., et al. Induction of broadly neutralising HCV antibodies in mice by integration-deficient lentiviral vector-based pseudotyped particles. PLoS One. 2013;8(4):e62684. doi: 10.1371/journal.pone.0062684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Garrone P., Fluckiger A.C., Mangeot P.E., et al. A prime-boost strategy using virus-like particles pseudotyped for HCV proteins triggers broadly neutralizing antibodies in macaques. Sci Transl Med. 2011;3(94):94ra71. doi: 10.1126/scitranslmed.3002330. [DOI] [PubMed] [Google Scholar]
- 137.Yin H., Kanasty R.L., Eltoukhy A.A., Vegas A.J., Dorkin J.R., Anderson D.G. Non-viral vectors for gene-based therapy. Nat Rev Genet. 2014;15(8):541–555. doi: 10.1038/nrg3763. [DOI] [PubMed] [Google Scholar]
- 138.Chang L.J., Urlacher V., Iwakuma T., Cui Y., Zucali J. Efficacy and safety analyses of a recombinant human immunodeficiency virus type 1 derived vector system. Gene Ther. 1999;6(5):715–728. doi: 10.1038/sj.gt.3300895. [DOI] [PubMed] [Google Scholar]
- 139.Gerolami R., Uch R., Jordier F., et al. Gene transfer to hepatocellular carcinoma: transduction efficacy and transgene expression kinetics by using retroviral and lentiviral vectors. Cancer Gene Ther. 2000;7(9):1286–1292. doi: 10.1038/sj.cgt.7700225. [DOI] [PubMed] [Google Scholar]
- 140.Diaz R.M., Bateman A., Emiliusen L., et al. A lentiviral vector expressing a fusogenic glycoprotein for cancer gene therapy. Gene Ther. 2000;7(19):1656–1663. doi: 10.1038/sj.gt.3301277. [DOI] [PubMed] [Google Scholar]
- 141.Baldari S., Di Rocco G., Magenta A., Picozza M., Toietta G. Extracellular vesicles-encapsulated microRNA-125b produced in genetically modified mesenchymal stromal cells inhibits hepatocellular carcinoma cell proliferation. Cells. 2019;8(12):1560. doi: 10.3390/cells8121560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhao P., Wang C., Fu Z., et al. Lentiviral vector mediated siRNA knock-down of hTERT results in diminished capacity in invasiveness and in vivo growth of human glioma cells in a telomere length-independent manner. Int J Oncol. 2007;31(2):361–368. [PubMed] [Google Scholar]
- 143.Zorzan M., Del Vecchio C., Vogiatzis S., et al. Targeting the regulatory subunit R2Alpha of protein kinase A in human glioblastoma through shRNA-expressing lentiviral vectors. Viruses. 2021;13(7):1361. doi: 10.3390/v13071361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Saraga G., Mafficini A., Ghaneh P., Sorio C., Costello E. Both HIV- and EIAV-based lentiviral vectors mediate gene delivery to pancreatic cancer cells and human pancreatic primary patient xenografts. Cancer Gene Ther. 2007;14(9):781–790. doi: 10.1038/sj.cgt.7701066. [DOI] [PubMed] [Google Scholar]
- 145.Chen C., Akerstrom V., Baus J., Lan M.S., Breslin M.B. Comparative analysis of the transduction efficiency of five adeno associated virus serotypes and VSV-G pseudotype lentiviral vector in lung cancer cells. Virol J. 2013;10:86. doi: 10.1186/1743-422X-10-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ao Z., Chen W., Tan J., et al. Lentivirus-based virus-like particles mediate delivery of caspase 8 into breast cancer cells and inhibit tumor growth. Cancer Biother Radiopharm. 2019;34(1):33–41. doi: 10.1089/cbr.2018.2566. [DOI] [PubMed] [Google Scholar]
- 147.Wang M., Li X., Xie W., et al. Inhibitory effect of lentivirus-mediated Gag-Caspase-8 on the growth of HER-2-overexpressing primary human breast cancer cells. Cancer Biother Radiopharm. 2021 doi: 10.1089/cbr.2021.0124. [DOI] [PubMed] [Google Scholar]
- 148.Matsunaga W., Ichikawa M., Ishikawa T., Gotoh A. Lentiviral vector-mediated transfection of p53, p16 and PTEN genes against human renal cell carcinoma cell lines. Pers Med Universe. 2019;8:10–14. [Google Scholar]
- 149.Shichinohe T., Bochner B.H., Mizutani K., et al. Development of lentiviral vectors for antiangiogenic gene delivery. Cancer Gene Ther. 2001;8(11):879–889. doi: 10.1038/sj.cgt.7700388. [DOI] [PubMed] [Google Scholar]
- 150.Bovia F., Salmon P., Matthes T., et al. Efficient transduction of primary human B lymphocytes and nondividing myeloma B cells with HIV-1-derived lentiviral vectors. Blood. 2003;101(5):1727–1733. doi: 10.1182/blood-2001-12-0249. [DOI] [PubMed] [Google Scholar]
- 151.Godfrey A., Anderson J., Papanastasiou A., Takeuchi Y., Boshoff C. Inhibiting primary effusion lymphoma by lentiviral vectors encoding short hairpin RNA. Blood. 2005;105(6):2510–2518. doi: 10.1182/blood-2004-08-3052. [DOI] [PubMed] [Google Scholar]
- 152.Kock N., Kasmieh R., Weissleder R., Shah K. Tumor therapy mediated by lentiviral expression of shBcl-2 and S-TRAIL. Neoplasia. 2007;9(5):435–442. doi: 10.1593/neo.07223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wang X., Liu P., Liu H., et al. Delivery of interferons and siRNA targeting STAT3 using lentiviral vectors suppresses the growth of murine melanoma. Cancer Gene Ther. 2012;19(12):822–827. doi: 10.1038/cgt.2012.65. [DOI] [PubMed] [Google Scholar]
- 154.Yu D., Chen D., Chiu C., Razmazma B., Chow Y.H., Pang S. Prostate-specific targeting using PSA promoter-based lentiviral vectors. Cancer Gene Ther. 2001;8(9):628–635. doi: 10.1038/sj.cgt.7700344. [DOI] [PubMed] [Google Scholar]
- 155.De Palma M., Venneri M.A., Naldini L. In vivo targeting of tumor endothelial cells by systemic delivery of lentiviral vectors. Hum Gene Ther. 2003;14(12):1193–1206. doi: 10.1089/104303403322168028. [DOI] [PubMed] [Google Scholar]
- 156.Simmons A., Whitehead R.P., Kolokoltsov A.A., Davey R.A. Use of recombinant lentivirus pseudotyped with vesicular stomatitis virus glycoprotein G for efficient generation of human anti-cancer chimeric T cells by transduction of human peripheral blood lymphocytes in vitro. Virol J. 2006;3:8. doi: 10.1186/1743-422X-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yang S., Cohen C.J., Peng P.D., et al. Development of optimal bicistronic lentiviral vectors facilitates high-level TCR gene expression and robust tumor cell recognition. Gene Ther. 2008;15(21):1411–1423. doi: 10.1038/gt.2008.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bobisse S., Rondina M., Merlo A., et al. Reprogramming T lymphocytes for melanoma adoptive immunotherapy by T-cell receptor gene transfer with lentiviral vectors. Cancer Res. 2009;69(24):9385–9394. doi: 10.1158/0008-5472.CAN-09-0494. [DOI] [PubMed] [Google Scholar]
- 159.Lo Presti V., Cornel A.M., Plantinga M., et al. Efficient lentiviral transduction method to gene modify cord blood CD8(+) T cells for cancer therapy applications. Mol Ther Methods Clin Dev. 2021;21:357–368. doi: 10.1016/j.omtm.2021.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Dullaers M., Van Meirvenne S., Heirman C., et al. Induction of effective therapeutic antitumor immunity by direct in vivo administration of lentiviral vectors. Gene Ther. 2006;13(7):630–640. doi: 10.1038/sj.gt.3302697. [DOI] [PubMed] [Google Scholar]
- 161.Miletic H., Fischer Y.H., Giroglou T., et al. Normal brain cells contribute to the bystander effect in suicide gene therapy of malignant glioma. Clin Cancer Res. 2007;13(22 Pt 1):6761–6768. doi: 10.1158/1078-0432.CCR-07-1240. [DOI] [PubMed] [Google Scholar]
- 162.Miletic H., Huszthy P., Giroglou T., Bjerkvig R., von Laer D. 205. lentiviral pseudotyped vectors specifically and efficiently transduce invasive human glioblastoma xenografts. Mol Ther. 2008;16:S77–S78. [Google Scholar]
- 163.Huszthy P.C., Giroglou T., Tsinkalovsky O., et al. Remission of invasive, cancer stem-like glioblastoma xenografts using lentiviral vector-mediated suicide gene therapy. PLoS One. 2009;4(7):e6314. doi: 10.1371/journal.pone.0006314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.He Y., Zhang J., Mi Z., Robbins P., Falo L.D., Jr. Immunization with lentiviral vector-transduced dendritic cells induces strong and long-lasting T cell responses and therapeutic immunity. J Immunol. 2005;174(6):3808–3817. doi: 10.4049/jimmunol.174.6.3808. [DOI] [PubMed] [Google Scholar]
- 165.Schoenhals M., Frecha C., Bruyer A., et al. Efficient transduction of healthy and malignant plasma cells by lentiviral vectors pseudotyped with measles virus glycoproteins. Leukemia. 2012;26(7):1663–1670. doi: 10.1038/leu.2012.36. [DOI] [PubMed] [Google Scholar]
- 166.Laubach J., Richardson P., Anderson K. Multiple myeloma. Annu Rev Med. 2011;62:249–264. doi: 10.1146/annurev-med-070209-175325. [DOI] [PubMed] [Google Scholar]
- 167.Ou W., Marino M.P., Suzuki A., et al. Specific targeting of human interleukin (IL)-13 receptor alpha2-positive cells with lentiviral vectors displaying IL-13. Hum Gene Ther Methods. 2012;23(2):137–147. doi: 10.1089/hgtb.2012.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Morgan R.A., Gray D., Lomova A., Kohn D.B. Hematopoietic stem cell gene therapy: progress and lessons learned. Cell Stem Cell. 2017;21(5):574–590. doi: 10.1016/j.stem.2017.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lévy C., Amirache F., Girard-Gagnepain A., et al. Measles virus envelope pseudotyped lentiviral vectors transduce quiescent human HSCs at an efficiency without precedent. Blood Adv. 2017;1(23):2088–2104. doi: 10.1182/bloodadvances.2017007773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Andreou T., Rippaus N., Wronski K., et al. Hematopoietic stem cell gene therapy for brain metastases using myeloid cell-specific gene promoters. J Natl Cancer Inst. 2020;112(6):617–627. doi: 10.1093/jnci/djz181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhou Q., Uhlig K.M., Muth A., et al. Exclusive transduction of human CD4+ T cells upon systemic delivery of CD4-targeted lentiviral vectors. J Immunol. 2015;195(5):2493–2501. doi: 10.4049/jimmunol.1500956. [DOI] [PubMed] [Google Scholar]
- 172.Jamali A., Kapitza L., Schaser T., Johnston I.C.D., Buchholz C.J., Hartmann J. Highly efficient and selective CAR-gene transfer using CD4- and CD8-targeted lentiviral vectors. Mol Ther Methods Clin Dev. 2019;13:371–379. doi: 10.1016/j.omtm.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kravchenko Y.E., Frolova E.I., Chumakov S.P. Dual CAR-targeted natural killer cell lines demonstrate potent cytotoxic properties towards breast cancer cells. Int J Appl Exerc Physiol. 2020;9(12):13–19. [Google Scholar]
- 174.Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380(15):1450–1462. doi: 10.1056/NEJMra1713263. [DOI] [PubMed] [Google Scholar]
- 175.Reghupaty S.C., Sarkar D. Current status of gene therapy in hepatocellular carcinoma. Cancers. 2019;11(9):1265. doi: 10.3390/cancers11091265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lee S., Kim Y.Y., Ahn H.J. Systemic delivery of CRISPR/Cas9 to hepatic tumors for cancer treatment using altered tropism of lentiviral vector. Biomaterials. 2021;272:120793. doi: 10.1016/j.biomaterials.2021.120793. [DOI] [PubMed] [Google Scholar]
- 177.Brenner M.K., Gottschalk S., Leen A.M., Vera J.F. Is cancer gene therapy an empty suit? Lancet Oncol. 2013;14(11):e447–e456. doi: 10.1016/S1470-2045(13)70173-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ahani R., Roohvand F., Cohan R.A., et al. Sindbis virus-pseudotyped lentiviral vectors carrying VEGFR2-specific nanobody for potential transductional targeting of tumor vasculature. Mol Biotechnol. 2016;58(11):738–747. doi: 10.1007/s12033-016-9973-7. [DOI] [PubMed] [Google Scholar]
- 179.Bryson P.D., Han X., Truong N., Wang P. Breast cancer vaccines delivered by dendritic cell-targeted lentivectors induce potent antitumor immune responses and protect mice from mammary tumor growth. Vaccine. 2017;35(43):5842–5849. doi: 10.1016/j.vaccine.2017.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kretschmer M., Kadlubowska P., Hoffmann D., Schwalbe B., Auerswald H., Schreiber M. Zikavirus pr ME envelope pseudotyped human immunodeficiency virus type-1 as a novel tool for glioblastoma-directed virotherapy. Cancers. 2020;12(4):1000. doi: 10.3390/cancers12041000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Argaw T., Marino M.P., Timmons A., et al. In vivo targeting of lentiviral vectors pseudotyped with the Tupaia paramyxovirus H glycoprotein bearing a cell-specific ligand. Mol Ther Methods Clin Dev. 2021;21:670–680. doi: 10.1016/j.omtm.2021.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kotterman M.A., Chalberg T.W., Schaffer D.V. Viral vectors for gene therapy: translational and clinical outlook. Annu Rev Biomed Eng. 2015;17:63–89. doi: 10.1146/annurev-bioeng-071813-104938. [DOI] [PubMed] [Google Scholar]
- 183.Liang X., Liu L., Wei Y.Q., Gao G.P., Wei X.W. Clinical evaluations of toxicity and efficacy of nanoparticle-mediated gene therapy. Hum Gene Ther. 2018;29(11):1227–1234. doi: 10.1089/hum.2018.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.De Lombaerde E., De Wever O., De Geest B.G. Delivery routes matter: safety and efficacy of intratumoral immunotherapy. Biochim Biophys Acta Rev Cancer. 2021;1875(2):188526. doi: 10.1016/j.bbcan.2021.188526. [DOI] [PubMed] [Google Scholar]
- 185.Mok W., Stylianopoulos T., Boucher Y., Jain R.K. Mathematical modeling of herpes simplex virus distribution in solid tumors: implications for cancer gene therapy. Clin Cancer Res. 2009;15(7):2352–2360. doi: 10.1158/1078-0432.CCR-08-2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Smith E., Breznik J., Lichty B.D. Strategies to enhance viral penetration of solid tumors. Hum Gene Ther. 2011;22(9):1053–1060. doi: 10.1089/hum.2010.227. [DOI] [PubMed] [Google Scholar]
- 187.Zhong Y., Meng F., Deng C., Zhong Z. Ligand-directed active tumor-targeting polymeric nanoparticles for cancer chemotherapy. Biomacromolecules. 2014;15(6):1955–1969. doi: 10.1021/bm5003009. [DOI] [PubMed] [Google Scholar]
- 188.Kim Y.I., Ahn B.C., Ronald J.A., et al. Intratumoral versus intravenous gene therapy using a transcriptionally targeted viral vector in an orthotopic hepatocellular carcinoma rat model. J Vasc Intervent Radiol. 2012;23(5):704–711. doi: 10.1016/j.jvir.2012.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Baliaka A., Zarogoulidis P., Domvri K., et al. Intratumoral gene therapy versus intravenous gene therapy for distant metastasis control with 2-diethylaminoethyl-dextran methyl methacrylate copolymer non-viral vector-p53. Gene Ther. 2014;21(2):158–167. doi: 10.1038/gt.2013.68. [DOI] [PubMed] [Google Scholar]
- 190.Smith S.N., Schubert R., Simic B., et al. The SHREAD gene therapy platform for paracrine delivery improves tumor localization and intratumoral effects of a clinical antibody. Proc Natl Acad Sci U S A. 2021;118(21) doi: 10.1073/pnas.2017925118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kasala D., Yoon A.R., Hong J., Kim S.W., Yun C.O. Evolving lessons on nanomaterial-coated viral vectors for local and systemic gene therapy. Nanomedicine. 2016;11(13):1689–1713. doi: 10.2217/nnm-2016-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zhang W.W., Li L., Li D., et al. The first approved gene therapy product for cancer Ad-p53 (gendicine):12 years in the clinic. Hum Gene Ther. 2018;29(2):160–179. doi: 10.1089/hum.2017.218. [DOI] [PubMed] [Google Scholar]
- 193.Guan X. Cancer metastases: challenges and opportunities. Acta Pharm Sin B. 2015;5(5):402–418. doi: 10.1016/j.apsb.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kaikkonen M.U., Räty J.K., Airenne K.J., Wirth T., Heikura T., Ylä-Herttuala S. Truncated vesicular stomatitis virus G protein improves baculovirus transduction efficiency in vitro and in vivo. Gene Ther. 2006;13(4):304–312. doi: 10.1038/sj.gt.3302657. [DOI] [PubMed] [Google Scholar]
- 195.Zhou S., Fatima S., Ma Z., et al. Evaluating the safety of retroviral vectors based on insertional oncogene activation and blocked differentiation in cultured thymocytes. Mol Ther. 2016;24(6):1090–1099. doi: 10.1038/mt.2016.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Chen Y., Liu X., Zhang Y., et al. A self-restricted CRISPR system to reduce off-target effects. Mol Ther. 2016;24(9):1508–1510. doi: 10.1038/mt.2016.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Gossen M., Freundlieb S., Bender G., Müller G., Hillen W., Bujard H. Transcriptional activation by tetracyclines in mammalian cells. Science. 1995;268(5218):1766–1769. doi: 10.1126/science.7792603. [DOI] [PubMed] [Google Scholar]