Abstract
Body weight is under physiological regulation. When body fat mass decreases, a series of responses are triggered to promote weight regain by increasing food intake and decreasing energy expenditure. Analogous, in response to experimental overfeeding, excessive weight gain is counteracted by a reduction in food intake and possibly by an increase in energy expenditure. While low blood leptin and other hormones defend against weight loss, the signals that oppose overfeeding-induced fat mass expansion are still unknown. In this article, we discuss insights gained from overfeeding interventions in humans and intragastric overfeeding studies in rodents. We summarize the knowledge on the relative contributions of energy intake, energy expenditure and energy excretion to the physiological defence against overfeeding-induced weight gain. Furthermore, we explore literature supporting the existence of unidentified endocrine and non-endocrine pathways that defend against weight gain. Finally, we discuss the physiological drivers of constitutional thinness and suggest that overfeeding of individuals with constitutional thinness represents a gateway to understand the physiology of weight gain resistance in humans. Experimental overfeeding, combined with modern multi-omics techniques, has the potential to unveil the long-sought signalling pathways that protect against weight gain. Discovering these mechanisms could give rise to new treatments for obesity.
This article is part of a discussion meeting issue ‘Causes of obesity: theories, conjectures and evidence (Part I)’.
Keywords: overfeeding, obesity, body weight, energy homeostasis, constitutional thinness, endocrinology
1. Introduction
Disturbances in mammalian energy balance, for example through voluntary or involuntary reduction in energy intake, elicit a cascade of counterregulatory responses serving to prevent an organismal energy crisis [1]. These feedback mechanisms affect both energy intake and energy expenditure and the strength by which they correct energy balance is not tightly determined by adiposity status. In other words, no matter the level of body fatness, most humans are subjected to powerful increases in hunger and food-motivation in response to even a short-term negative energy balance [1]. In the face of a fattening modern society, this ubiquitous homeostatic defence against loss of body weight represents one of the biggest challenges for the management of obesity [1].
The feedback mechanisms that restore body weight are not only triggered by a negative energy balance. Conscious attempts to gain weight are also countered by corrective homeostatic mechanisms [2–4]. This is illustrated by numerous overfeeding studies in which weight gain (induced by a controlled positive energy balance) is recovered once overfeeding is replaced by ad libitum eating [2]. This protection against overfeeding-induced weight gain is well documented in both animals [5] and humans [2] and seems to involve several different physiological responses, as discussed below. Whereas the mediators that protect against weight loss have been under intense scrutiny since the 1990s, there has been much less research addressing the molecular mechanisms that drive the physiological defence against forced weight gain [5–7]. In this article, we provide an overview of the current state of this research area and explore future directions and translational implications for overfeeding research.
2. Experimental overfeeding in animals and humans
Human experimental overfeeding is an intervention by which a positive energy balance is established in a controlled setting. In the literature, overfeeding interventions are sometimes referred to as ‘voluntary overfeeding’ or ‘conscious overfeeding’. Typically, the interventions range in duration from single energy-rich meals to months of caloric surplus in which energy intake is often 30–50% above the energy needs for weight stability [2]. These types of controlled studies differ from the spontaneous increase in food intake and the resulting weight gain that can be seen for example in response to changes in life and/or environmental circumstances. The latter, we assume, more closely resembles the emergence of common obesity. Experimental overfeeding on the other hand enables clamping of energy balance at a positive level that would be undesirable in free-living conditions and accordingly the study of homeostatic mechanisms that defend against an experimentally induced weight gain.
In contrast to the voluntary nature of human overfeeding studies (i.e. informed consent to participate), experimental overfeeding in animals is involuntary and executed via controlled intragastric nutrient infusion or forced gavage of food [5]. A common level of overfeeding in mice is 150% [5]. This corresponds to infusion of 1.5 times the energy needed to maintain a stable weight and leads to a weight gain of approximately 30% within two weeks [5]. Intragastric overfeeding in rodents is a powerful model to clamp food intake and assess how energetic surplus, macronutrient composition and specific dietary components influence energy homeostasis and cardiometabolic health [5]. It is important to note that the term ‘overfeeding’ is loosely used in the animal literature and that countless studies use ‘overfeeding’ to refer to voluntary overeating of a palatable high-fat diet [5]. Diet-induced obesity in rodents emerges in ‘agreement’ with mechanisms that regulate energy homeostasis and the polygenic susceptibility of a given model. Thus, the excess fat accumulation does not violate the homeostatic defence system in the diet-induced obesity model. In contrast, a key feature of experimental (intragastric) overfeeding is the infusion of energy beyond what the animal is willing to consume, even on the most palatable cafeteria diet. Analogous to experimental overfeeding in humans, this set-up enables the study of homeostatic mechanisms engaged to actively counter weight gain. Intragastric overfeeding in animals, however, is not without limitations. Intragastric infusion of food bypasses the oral cavity and will therefore not elicit the same gustatory and cephalic effects seen in response to oral food intake. Moreover, intragastric overfeeding requires an invasive surgical procedure and the frequent clogging of the infusion tube by the liquid diet renders it challenging to run long-term overfeeding trials in rodents. In addition to overfeeding of mice, rats and humans, overfeeding studies have also been conducted in guinea pigs, swine, dogs, monkeys and different bird species [5].
3. Experimental overfeeding reveals large weight variability in humans
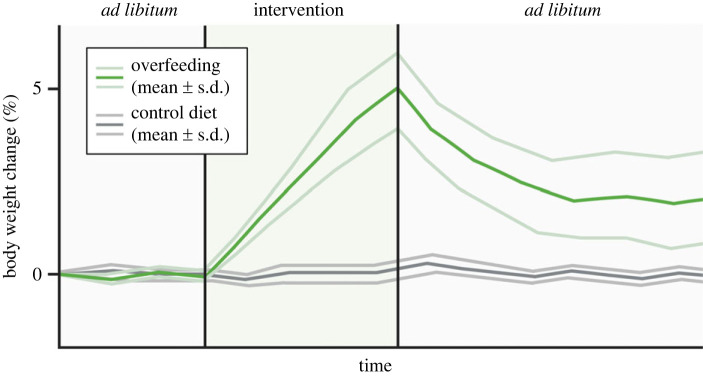
Analogues to the outcomes from lifestyle- and drug-induced weight loss trials, there is large inter-individual variation in the magnitude of overfeeding-induced weight gain [2,4] (figure 1). Likewise, there is some variability in the weight recovery response that is frequently observed once the overfeeding has been terminated [2] (figure 1). These findings emphasize heterogeneity in the compensatory responses to overfeeding. Of note, when examining the mechanisms that underpin protection from weight gain, it might be important to distinguish between mechanisms that defend against weight gain during the active overfeeding period and mechanisms that drive weight loss in the recovery period after overfeeding.
Figure 1.
Physiological response to experimental overfeeding in humans. Changes in body weight during and after overfeeding; the light coloured lines reflect the inter-individual variation in weight gain and weight recovery in response to overfeeding.
(a) . Body weight gain: inter-individual variation during overfeeding
Weight gain in response to experimental overfeeding in humans typically ranges from 1.4 to 8.1 kg [2] and in some extreme cases up to 15 kg [8], still with large inter-individual differences within each study. This variation between individuals is emphasized by a seminal 100-day overfeeding study in 12 pairs of monozygotic twins [9]. This study found a weight gain range of 4.3 to 13.3 kg and that the majority of this variation was attributed to genetic factors [9]. On a group basis, there is a close relationship between the amount of overfed calories and the weight gain observed (see [2] for an excellent systematic summary). However, one neglected caveat that could potentially underlie some of the large variation is the lack of sufficient attention and/or ability to closely determine the exact energy requirements for body weight stability. Over time, an error of e.g. 10% in estimating caloric requirements for keeping body weight stable can amount to a relatively large variation in the level of overfeeding between individuals in a long-term overfeeding study. The weight that is gained during overfeeding reflects an increase in body fat mass of 60–70% and a 30–40% increase in lean mass [2]. In rodents, the weight gain in response to intragastric overfeeding is oftentimes more pronounced. As such, a few weeks of 150% overfeeding in rats or mice usually increases body weight by 20–40% and the weight gain variability is much less than in the human studies [5]. The homogeneous overfeeding-induced weight gain in rodents is likely the result of e.g. low genetic variability (inbreeding), single-housing of animals in a highly controlled environment, and the experimental conditions in which the intragastric infusion of liquid food through a tube not only enables high-energetic overfeeding accuracy, but likely also minimizes variation in spontaneous physical activity between animals.
(b) . Body weight recovery: inter-individual variation after overfeeding
There is scarce literature on the weight recovery phase from experimental overfeeding in humans. Most studies have focused on the weight gain that occurs during the overfeeding period [2]. This might reflect that it is challenging to quantify energy intake once subjects are released to ad libitum eating in the recovery phase. Nonetheless, the available data in human studies point to a relatively rapid initial weight loss [2,10,11]. For some individuals, the entire weight gain is lost within weeks of stopping the overfeeding [10], yet for others, the initial rapid weight loss seems to be followed by a more slow and gradual return towards the pre-overfeeding body weight [2,10,11]. While some people seem to fully recover their body weight within a few months, others fail to do so [10,12–14]. Whether they eventually lose all overfeeding-induced weight is unclear but there are studies in animals demonstrating remnant adiposity compared to preintervention levels, which implies an incomplete recovery from the energy homeostatic insult [5]. This contrasts with ‘unsuccessful’ weight loss interventions that are typically countered by homeostatic forces ensuring complete recovery of body fat levels and it highlights that energy balance regulation, in many but not all individuals, is biased toward weight gain [15].
4. Physiological defence mechanisms against weight gain
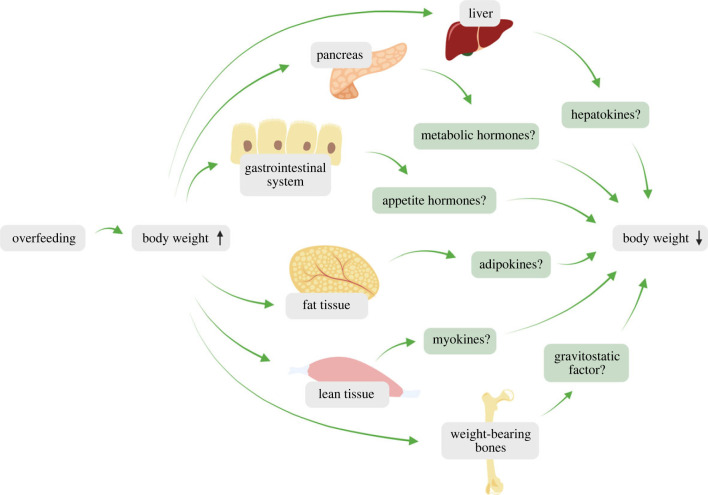
The physiological effectors and the molecular mediators responsible for weight loss following a period of overfeeding are incompletely understood (figure 2). As we shall explore in the following, it is challenging in humans to quantify relative and time-resolved changes in appetite, energy expenditure and energy excretion in response to overfeeding. Rodent intragastric overfeeding studies, on the other hand, provide very accurate information on changes in body weight and food intake during and after overfeeding.
Figure 2.
Suggested physiological mechanisms governing weight loss following overfeeding-induced weight gain in humans. Defence against overfeeding might involve reduced food intake, increased non-shivering thermogenesis, increased non-exercise activity thermogenesis and increased energy excretion.
(a) . Energy intake: does overfeeding lower appetite?
It is evident that a potent and prolonged reduction in voluntary food intake plays a key role for weight loss following intragastric overfeeding in rodents [5]. Two weeks of 150% overfeeding in rats or mice almost eliminates food intake during overfeeding and for 3–5 days post-overfeeding, upon which food intake then slowly increases back to its normal level over a prolonged period until body weight is finally recovered [5]. More extreme studies such as a 200% overfeeding for 3 months in rats elicited a powerful suppression of food intake for more than two weeks post-overfeeding [16]. Larger animals also display pronounced suppression of food intake following overfeeding. In dogs, one week of 133% overfeeding completely suppressed feeding for a couple of days following overfeeding and, two weeks after overfeeding, the dogs still showed a 13–26% reduction in food intake [17]. In another overfeeding study, Rhesus monkeys basically consumed no food for several weeks after overfeeding had ceased, indicating a very powerful response in this primate species [18]. However, whereas pronounced and prolonged post-overfeeding hypophagia is evident in animal studies, the human data are less clear (figure 2). Most studies in which post-overfeeding food intake has been quantified have been of short duration and the data suggest an incomplete compensation for the excess energy [2,10]. One study reported that food intake was significantly suppressed following 21 days of overfeeding in young men, however changes of dietary conditions during the recovery phase might have confounded these data [19]. Another study in which the dietary conditions following overfeeding were controlled for, examined the effects of 13 days of overfeeding in men and women. They found that 35% overfeeding for 13 days on average elicited a 2.3 kg weight gain [20]. Perhaps surprisingly, the energy intake was not significantly suppressed following overfeeding but merely returned to baseline levels. This finding might be in line with a study demonstrating that individuals that are resistant to obesity display a stronger hypophagic compensation than individuals that are prone to obesity [21]. Based on the data on energy intake and changes in body weight post-overfeeding, the authors estimated that the weight loss could be attributed to a 14% increase in energy expenditure [20].
(b) . Energy expenditure: is weight gain counteracted by adaptive thermogenesis?
In humans, effects of overfeeding on energy expenditure have frequently been estimated from the assessment of resting metabolic rate, typically measured at distinct time-points using indirect calorimetry following a brief period of rest [2]. In general, there is no consensus regarding the effects of experimental overfeeding on resting metabolic rate when the duration of the overfeeding intervention is less than one month. By contrast, studies ranging from 30 to 100 days of overfeeding have consistently observed increases in metabolic rate, typically ranging from 5% to 12% [2]. However, once corrected for body weight or fat-free mass, the increase in metabolic rate disappears, implying that the thermogenic effect exclusively reflects the increase in body weight induced by overfeeding and not a compensatory increase in energy expenditure [2]. This also aligns with the lack of consensus from the short-term studies in which the subtle increase in weight has a smaller confounding effect on metabolic rate. Similar to the marked variation in overfeeding-induced weight gain between people, there is large inter-individual differences in the energetic response to overfeeding [2]. Data from more comprehensive studies that have employed 24 h measures of calorimetry or used doubly labelled water to assess energy expenditure in response to experimental overfeeding also exist [2,22]. In agreement with the studies measuring resting metabolic rate, the evidence for overfeeding-induced increases in energy expenditure is strongest with long-term overfeeding studies. However, whether an adaptive increase in energy expenditure that is greater than what is gauged from a larger body mass (so-called ‘Luxuskonsumption’) contributes to offset overfeeding-induced weight gain remains uncertain [2].
Previous work on this topic has also attempted to quantify components of energy expenditure like diet-induced thermogenesis (DIT) and non-exercise activity thermogenesis (NEAT) [2]. While DIT refers to an increase in energy expenditure above basal metabolic rate after a test meal, NEAT refers to the energy expenditure that is derived from both unconscious movements, such as fidgeting and other restless behaviours and ‘spontaneous’ physical activity outside of formal exercise programmes, such as walking and standing [23,24]. Studies of DIT in response to ingestion of food with different macronutrient composition have revealed that metabolization of ingested protein is more energetically costly than metabolizing carbohydrate or fat [2]. At this point, it is unclear if overfeeding induces an adaptive increase in DIT and if this might oppose experimental weight gain. However, it is interesting to note that studies suggest that protein-enriched overfeeding diets lead to a lower weight gain than that induced by overfeeding diets that are rich in other macronutrients [25]. Overall, it is estimated that DIT constitutes around 10% of energy intake for a typical Western diet [24]. In response to overfeeding, the 10% from DIT will obviously give rise to a higher absolute DIT, but not in a compensatory fashion to oppose the overfeeding-induced weight gain [26]. Whereas DIT thermogenesis seems dispensable for the defence against weight gain, there is some data to support that NEAT is engaged to oppose weight gain in response to overfeeding [27]. Estimates of NEAT stem from subtracting basal metabolic rate (BMR) and DIT from total energy expenditure in response to overfeeding and the initial studies proposed that NEAT promotes resistance to weight gain [27]. Subsequent studies have challenged the role of NEAT and found less pronounced effects in response to overfeeding [2,28] (figure 2). These observations might align with more recent evidence implying that total energy expenditure is constrained [29,30] and that an increase in energy expenditure from NEAT might thus be countered by a suppression of BMR.
Parallel to the human literature, animal studies are also relatively inconclusive when it comes to overfeeding-induced effects on energy expenditure. Some rodent studies fail to identify increased total energy expenditure measured by indirect calorimetry whereas other studies report an increase [5]. However, we have yet to see chronic intragastric overfeeding studies integrated with high-resolution indirect calorimetry systems. Such a study in combination with measures of excreted energy (as described below) will enable an accurate assessment of the homeostatic compensatory changes to food intake, energy expenditure and energy excretion during and after overfeeding in rodents.
(c) . Energy excretion: an overlooked factor defending against weight gain?
Changes in body weight and energy balance are often exclusively attributed to alterations in energy intake and energy expenditure. However, energy (in the form of macronutrients) can also be lost through e.g. faeces and urine and this calorie excretion is an understudied aspect of energy balance regulation [31]. Bomb calorimetry analyses of faeces from healthy humans show that around 5–8% of the energy consumed is lost in faeces on average [12,32]. However, there are large inter-individual differences in energy excretion [33] with some people losing up to 11% while others only lose around 1–2% of the ingested energy [32,34]. This large variation between people was also found in a study from 2020 that exposed adults with obesity to 3 days of 150% overfeeding [35]. Quite remarkably, one individual in this study excreted approximately 500 kcal day−1 whereas another individual excreted only approximately 80 kcal day−1 [31,35]. Accordingly, these subjects were not submitted to the same amount of metabolizable energy from overfeeding and this might partly explain the heterogeneous weight gain observed in humans in response to experimental overfeeding [31]. Another human study has further indicated a role of energy excretion in weight regulation by observing that people with a Ruminococcaceae enterotype have a lower body weight and display a higher stool energy density than people with a Bacteroides enterotype [36]. In contrast with these observations, a six-week overfeeding study from 1980 did not find remarkable inter-individual differences in faecal energy excretion [12]. Moreover, other studies in humans have not found differences in energy excretion between subjects with leanness and obesity [37], nor observed an increase in faecal energy and fat excretion in response to overfeeding [27,38,39]. Thus, it is currently unclear if increased energy excretion contributes to the defence against weight gain (figure 2). The absence of overfeeding-induced lipid excretion in faeces might suggest that faecal excretion of carbohydrates, peptides and amino acids could be more important than the excretion of dietary lipids. Here, it is important to highlight that it is difficult to discriminate between non-absorbed energy from ingested food and energy derived from excreted intestinal cells and bacteria in the faeces [40]. In addition to energy loss via the gut, excretion of energy through other means such as urine, gasses and skin might also make smaller contributions to overall energy balance. Intriguingly, a study in mice has shown that overexpression of thymic stromal lymphopoietin reverses high-fat diet-induced obesity via increased lipid secretion in the sebaceous glands of the skin [41].
5. The dual intervention point model and hormonal defence against weight gain
The notion that homeostatic forces ensure energy balance by matching energy intake with energy expenditure gave rise to the widespread set-point theory of weight regulation [42]. The discovery of leptin enhanced the popularity of this theory which states that the body has a fixed weight that it strives to maintain [43,44]. While the set-point theory provides a useful and simple framework for public communication of body weight biology and explaining why weight loss is difficult to sustain, the theory fails to account for the fact that many individuals experience non-corrected weight fluctuations in response to socio-environmental changes [45,46]. This highlights that human body weight might not be regulated around a specific set-point, but rather within a weight range, as proposed by the dual intervention point model [47–49]. The dual intervention point model implies the existence of upper and lower boundaries or intervention points that when encountered in response to perturbations in energy balance will elicit a corrective homeostatic response to prevent the body weight from breaching these boundaries. In between the upper and the lower boundary, there is a ‘zone of indifference’ in which the biological mechanisms that regulate body weight seem less stringent and where socio-environmental components act as the predominant factors modulating weight [50]. The dual intervention point model is consistent with leptin biology as it stipulates that leptin is a key hormonal guard of the lower intervention point [50]. Thus, in response to a prolonged negative energy balance, a decline in fat mass leads to a decrease in circulating leptin levels, which is perceived by the central leptin-melanocortin system as a threat to survival. Accordingly, the brain initiates a strong homeostatic response to counter this potentially dangerous situation by increasing hunger, stimulating food-motivated behaviours, and by decreasing energy expenditure in an adaptive manner [51–53].
(a) . Evidence for a hormonal defence against weight gain
While the ‘starvation response’ that counteracts a breach of the lower weight boundary is well described, it is unclear what mechanisms that keep body weight from crossing the upper weight boundary and ensure weight recovery into the ‘zone of indifference’ [50,54]. Experiments with rodents that are surgically connected in a manner that establishes a shared blood circulatory system, so-called parabiosis studies, support the existence of a hormonal system that protects against overfeeding-induced weight gain [55,56]. Several parabiosis studies have shown that overfeeding of one parabiotic partner induces hypophagia and weight loss in the non-overfed partner [57,58]. Additional support of the existence of circulating signals of the overfed state comes from a line of other publications [3,4,7,59–62] and studies in the 1990s that have demonstrated weight-lowering and appetite-suppressing effects when administering adipose-conditioned medium and fat extracts from overfed animals to naïve animals [63–65]. These findings are in line with the lipostatic theory [66] and suggest that adipose tissue responds to overfeeding by secreting a hormone that counteracts weight gain (figure 3). Yet lean body mass, including organs and tissues such as skeletal muscle, liver, pancreas and weight-bearing bones have also been proposed to release signalling molecules that affect appetite and defend against weight gain (figure 2) [4,5,67–69]. Research on the endocrine factors that defend against weight gain has excluded a series of well-known metabolic hormones as sole mediators of the upper intervention point. This includes leptin, insulin, cholecystokinin, somatostatin, amylin, pancreatic polypeptide, ghrelin and glucagon [4,5,63,64,70,71]. For example, studies in rats have found that while the circulating level of leptin increases dramatically with overfeeding, it rapidly returns to the ‘baseline’ level seen in non-overfed control animals [71]. This rapid decrease in blood leptin is uncoupled from the prolonged hypophagia seen in rats following overfeeding [70,71]. Another research group has pharmacologically clamped blood leptin in leptin-deficient ob/ob mice and found that these mice retained an ability to lower body weight following intragastric overfeeding [72]. Several other hormones are acknowledged to be involved in energy balance regulation. These include fibroblast growth factor 21 (FGF21), growth differentiation factor 15 (GDF15), liver-expressed antimicrobial peptide 2 (LEAP2), adiponectin, glucagon-like peptide-1 (GLP-1) and gastric inhibitory polypeptide (GIP)—to name a few [73]. Most of these protein and peptide hormones remain to be studied in the context of overfeeding and only a few studies have to our knowledge explored GDF15, adiponectin and FGF21 in relation to this. These studies show that overfeeding does not elevate plasma GDF15 [74,75], and it is currently unclear if GDF15 helps counteract a positive energy balance [76]. By contrast, overfeeding increases plasma adiponectin and FGF21 [77–80] and because FGF21 has been linked to weight gain resistance in humans [81], this hormone might be a physiological signal of the overfed state. Further, it is important to stress that the work revolving around the endocrine defence against weight gain almost exclusively has been done in rodents. Given the apparent differences in post-overfeeding hypophagia between rodents and humans, the rodent model might have some translational drawbacks. Moreover, the rodent literature is quite underwhelming with less than 10 published original articles on intragastric overfeeding in mice of which only two studies have combined the use of transgenic animals and experimental overfeeding to probe pathway causality.
Figure 3.
Potential circulating regulators of overfeeding. Cartoon depicting possible tissues and secreted factors that might be involved in the protection against overfeeding-induced weight gain.
6. Non-endocrine defence systems against weight gain
The parabiosis studies clearly suggest the existence of hormones that protect against overfeeding-induced obesity. But hormonal mediators might not act alone. In other words, is it possible that non-endocrine signals contribute to the homeostatic defence against experimental weight gain? There is abundant neuronal crosstalk between peripheral organs and the brain. The sympathetic nervous system (SNS) affects metabolic homeostasis by influencing the activity and function of several tissues and organs including skeletal muscle, liver and adipose tissue [82,83]. There is some evidence that overfeeding has a modest effect on the activity of the SNS, but this is not corroborated by studies measuring catecholamine excretion in urine [2]. The effect of overfeeding on thermogenic adipose tissue has been explored for decades—since brown fat was first suggested to mediate resistance to obesity in rats [84]. Today, several studies have shown that cold activates human brown adipose tissue and that low brown fat activity is linked to adiposity [85–88]. Yet, there are profound inter-species differences that challenge the translatability of the anti-obesity effects of brown fat from rodents to humans [89]. Thus, it remains uncertain if activation of non-shivering thermogenesis is an adaptive response that attenuates overfeeding-induced weight gain in humans. While one short-term overfeeding study has suggested that overfeeding-induced thermogenesis in humans might be mediated by non-shivering thermogenesis in brown fat [90], other human studies have questioned the popular notion that overfeeding leads to an adaptive activation of brown fat thermogenesis [91–94] (figure 2). In addition to SNS control of metabolic organs, there is also sensory innervation of peripheral organs that could directly convey energetic status to the brain without involving any blood-borne hormones. For example, adipose tissue afferents could serve to inform the brain on body fatness or catabolic/anabolic status of fat depots, by for example sensing local free fatty acids or glycerol [95]. Finally, a non-endocrine defence system against weight gain might involve a gut-vagal-brain axis. In this context, studies have found that macronutrients directly engage vagal signalling and spinal signalling to influence activity of the hunger-promoting Agouti-related protein-expressing neurons in the hypothalamus [96,97].
7. Overfeeding of people with constitutional thinness: a gateway to uncover the causes of weight gain resistance in humans
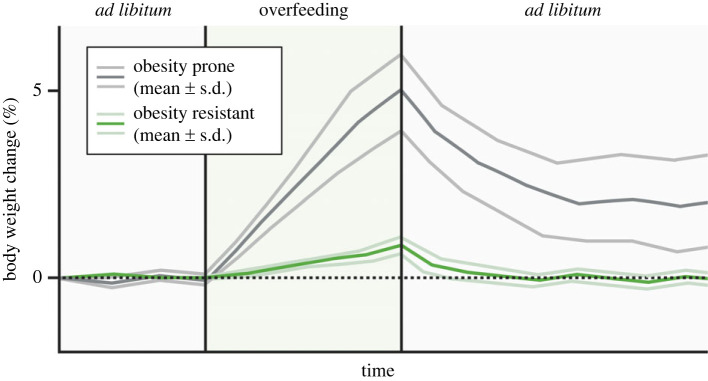
Despite the highly conducive environment for obesity that characterizes modern society, a substantial portion of the population still maintains a remarkably low body weight throughout their lives [98]. Many of these individuals, we believe, are born with an overlooked and understudied type of persistent underweight termed ‘constitutional thinness’ [99]. People with constitutional thinness report being resistant to overfeeding-induced weight gain (figure 4) and have always been in the lower percentiles for BMI [99–101]. Thus, like individuals in the upper end of the BMI spectrum that struggle with maintaining a weight loss, people with constitutional thinness find it very hard, if not impossible, to maintain a forced weight gain. Unlike pathological forms of underweight, this type of thinness is not driven by diseases or eating disorders and it comes with normal plasma levels of leptin [102] and a body fat percentage that is in the normal range (albeit in the lower end) [103]. The causes of constitutional thinness are currently unknown. Revealing why individuals with constitutional thinness are protected against weight gain might lead to novel and more efficacious ways to prevent and treat obesity. Constitutional thinness is a heritable condition that runs in families [98,100,104,105]. Although several genetic variants in e.g. MC4R, GIPR and GPR75 have been associated with protection from obesity [106–108] and a few studies have started to explore the genetics of constitutional thinness [105,109,110], the exact genetic variants that underlie constitutional thinness are still largely unknown. Constitutional thinness does not seem to be driven by high levels of physical exercise [38,111,112] and specific dietary preferences are also unlikely explanations, given that people with constitutional thinness display the same nutrient and energy intake as controls with normal weight [38,100,111]. Some studies suggest that increased energy expenditure might explain why people with constitutional thinness seemingly eat just as many calories per day while at the same time weighing markedly less than individuals with normal weight [100]. This notion is supported by studies showing that constitutional thinness is associated with higher glucose uptake in brown fat [113], increased norepinephrine levels and mitochondrial respiration in white fat [38,109], and slightly elevated blood levels of thyroid hormones [112]. Other potential explanations for the obesity resistance in constitutional thinness include higher levels of fidgeting [114], increased energy excretion [31], reduced responsiveness to food cues [115,116] and/or better sensing of a positive energy balance, i.e. enhanced sensitivity to overeating-induced satiety signals [4,21,117].
Figure 4.
Experimental overfeeding in obesity prone versus resistant subjects. Individuals that are resistant to weight gain such as individuals with constitutional thinness might have an exaggerated homeostatic defence against overfeeding-induced weight gain.
In 1929, the Danish physiologist and Nobel laureate, August Krogh, stated that ‘for a large number of problems there will be some animal of choice, or a few such animals, on which it can most conveniently be studied’ [118]. An analogous clinical approach with enhanced translational potential is to study extreme human phenotypes [119]. Thus, in this context, experimental overfeeding specifically in individuals with constitutional thinness represents a valuable gateway to explore the physiological phenomenon of weight gain resistance in humans. The rationale behind this is that people with constitutional thinness likely display much more modest fluctuations in body weight and fat mass compared to people that are prone to weight gain. Thus, in accordance with the dual intervention point model, less overfeeding-induced weight gain is required to breach their upper weight boundary. Moreover, the physiological response to overfeeding might be especially powerful in people with constitutional thinness (figure 4). In comparison to individuals with normal weight, it should therefore be easier to provoke and unveil the unknown mechanisms that protect against weight gain in people with this specific type of thinness. In other words, individuals with constitutional thinness might be ideal ‘models’ for studying the biological defence against weight gain. We believe that the anti-obesity mechanisms uncovered by this experimental approach would also operate in people with normal weight (and possibly also overweight), perhaps just at a lower strength. To our knowledge, only a few overfeeding studies have been conducted in individuals with constitutional thinness [38,120–123]. Although one of these overfeeding studies have provided evidence of weight gain resistance [122], another study did not find clear differences in relative weight gain between people with constitutional thinness and controls with normal weight [38]. This weak inter-group weight gain variability reported in the latter of these two overfeeding studies [38] might be explained by the lack of a highly controlled inpatient setting [124]. Interestingly, while increased energy expenditure and NEAT (spontaneous movements) have been observed in people with constitutional thinness when they are studied in an ‘energetically unperturbed’ state, as highlighted above, there is conflicting evidence as to whether these processes are activated in response to overfeeding [38,120–122,125]. In summary, the limited number of overfeeding experiments in individuals with constitutional thinness highlights that there is a need for better controlled overfeeding studies and perhaps also acute studies with more profound overeating in single meals and in a single day. Such future studies might help uncover why people with constitutional thinness are protected from excessive weight gain.
8. Conclusion and future directions
Experimental overfeeding studies have greatly enhanced our understanding of the complex physiological mechanisms that regulate energy balance and prevent weight gain. Yet, with an escalating obesity pandemic, the overfeeding literature outlines a noticeable conundrum: how do we reconcile the notion of a powerful biological protection against weight gain with the fact that we are hastily approaching 1 billion individuals with obesity globally? The dual intervention point model suggests that individuals that are genetically predisposed to obesity have inherited an upper intervention point that is located at a much higher level than others. Thus, in these individuals, adiposity can supposedly evolve without violating the upper intervention point and, compared to people with leanness, it requires a very high level of body fat mass before weight gain defence mechanisms are activated. But what are the enigmatic mechanisms that defend against such excess adiposity? As outlined in this article, there is evidence to suggest that unidentified endocrine signals are important for the protection against weight gain. These hormonal signals might act in concert with bidirectional nervous communication between the brain and the periphery. Irrespective of the uncertainties about the homeostatic drivers of overfeeding, the existence of overfeeding-induced mechanisms that potently reduce body weight renders it intriguing to speculate whether this biology can be exploited pharmacologically for the management of obesity. Toward this objective it is key that we do not lose sight of potential translational pitfalls. It is evident that pronounced and prolonged hyperphagia is a key feature of the weight recovery following overfeeding in rodents. However, the human data on overfeeding-induced suppression of appetite are currently less clear. Thus, for ambitious overfeeding multi-omics endeavours that aim at identifying new druggable pathways for prevention of weight gain and for inducing weight loss, it is a prerequisite to continuously ensure that key results are not lost in translation. This can be achieved through a combination of overfeeding studies across the weight spectrum in rodents and humans.
Acknowledgements
Figures were created using http://BioRender.com.
Data accessibility
This article has no additional data.
Authors' contributions
J.L.: conceptualization, writing—original draft and writing—review and editing; C.C.: conceptualization, funding acquisition, writing—original draft and writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
C.C. is a co-founder of Ousia Pharma ApS, a biotech company developing therapeutics for treatment of metabolic disease. J.L. has no competing interests.
Funding
The Novo Nordisk Foundation Center for Basic Metabolic Research is an independent Research Center, based at the University of Copenhagen, Denmark, and partially funded by an unconditional donation from the Novo Nordisk Foundation (www.cbmr.ku.dk) (grant no. NNF18CC0034900).
References
- 1.Schwartz MW, et al. 2017. Obesity pathogenesis: an endocrine society scientific statement. Endocr Rev. 38, 267-296. ( 10.1210/er.2017-00111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bray GA, Bouchard C. 2020. The biology of human overfeeding: a systematic review. Obes. Rev. 21, e13040. ( 10.1111/obr.13040) [DOI] [PubMed] [Google Scholar]
- 3.Ravussin Y, Leibel RL, Ferrante AW Jr. 2014. A missing link in body weight homeostasis: the catabolic signal of the overfed state. Cell Metab. 20, 565-572. ( 10.1016/j.cmet.2014.09.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lund J, Lund C, Morville T, Clemmensen C. 2020. The unidentified hormonal defense against weight gain. PLoS Biol. 18, e3000629. ( 10.1371/journal.pbio.3000629) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ranea-Robles P, Lund J, Clemmensen C. 2022. The physiology of experimental overfeeding in animals. Mol. Metab. 64, 101573. ( 10.1016/j.molmet.2022.101573) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woods SC, Seeley RJ, Porte D Jr, Schwartz MW. 1998. Signals that regulate food intake and energy homeostasis. Science 280, 1378-1383. ( 10.1126/science.280.5368.1378) [DOI] [PubMed] [Google Scholar]
- 7.Harris RB. 2013. Is leptin the parabiotic ‘satiety’ factor? Past and present interpretations. Appetite 61, 111-118. ( 10.1016/j.appet.2012.08.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pasquet P, et al. 1992. Massive overfeeding and energy balance in men: the Guru Walla model. Am. J. Clin. Nutr. 56, 483-490. ( 10.1093/ajcn/56.3.483) [DOI] [PubMed] [Google Scholar]
- 9.Bouchard C, et al. 1990. The response to long-term overfeeding in identical twins. N. Engl. J. Med. 322, 1477-1482. ( 10.1056/NEJM199005243222101) [DOI] [PubMed] [Google Scholar]
- 10.Bray GA. 2020. The pain of weight gain: self-experimentation with overfeeding. Am. J. Clin. Nutr. 111, 17-20. ( 10.1093/ajcn/nqz264) [DOI] [PubMed] [Google Scholar]
- 11.Pasquet P, Apfelbaum M. 1994. Recovery of initial body weight and composition after long-term massive overfeeding in men. Am. J. Clin. Nutr. 60, 861-863. ( 10.1093/ajcn/60.6.861) [DOI] [PubMed] [Google Scholar]
- 12.Norgan NG, Durnin JV. 1980. The effect of 6 weeks of overfeeding on the body weight, body composition, and energy metabolism of young men. Am. J. Clin. Nutr. 33, 978-988. ( 10.1093/ajcn/33.5.978) [DOI] [PubMed] [Google Scholar]
- 13.Roberts SB, et al. 1990. Energy expenditure and subsequent nutrient intakes in overfed young men. Am. J. Physiol. 259, R461-R469. ( 10.1152/ajpregu.1990.259.3.R461) [DOI] [PubMed] [Google Scholar]
- 14.Bouchard C, et al. 1996. Overfeeding in identical twins: 5-year postoverfeeding results. Metabolism 45, 1042-1050. ( 10.1016/s0026-0495(96)90277-2) [DOI] [PubMed] [Google Scholar]
- 15.Schwartz MW, et al. 2003. Is the energy homeostasis system inherently biased toward weight gain? Diabetes 52, 232-238. ( 10.2337/diabetes.52.2.232) [DOI] [PubMed] [Google Scholar]
- 16.Cohn C, Joseph D. 1962. Influence of body weight and body fat on appetite of ‘normal’ lean and obese rats. Yale J. Biol. Med. 34, 598-607. [PMC free article] [PubMed] [Google Scholar]
- 17.Janowitz HD, Hollander F. 1955. The time factor in the adjustment of food intake to varied caloric requirement in the dog: a study of the precision of appetite regulation. Ann. N. Y. Acad. Sci. 63, 56-67. ( 10.1111/j.1749-6632.1955.tb36545.x) [DOI] [PubMed] [Google Scholar]
- 18.Jen KL, Hansen BC. 1984. Feeding behavior during experimentally induced obesity in monkeys. Physiol. Behav. 33, 863-869. ( 10.1016/0031-9384(84)90220-8) [DOI] [PubMed] [Google Scholar]
- 19.Roberts SB, et al. 1996. Effects of age on energy expenditure and substrate oxidation during experimental overfeeding in healthy men. J. Gerontol. A 51, B148-B157. ( 10.1093/gerona/51a.2.b148) [DOI] [PubMed] [Google Scholar]
- 20.Levitsky DA, Obarzanek E, Mrdjenovic G, Strupp BJ. 2005. Imprecise control of energy intake: absence of a reduction in food intake following overfeeding in young adults. Physiol. Behav. 84, 669-675. ( 10.1016/j.physbeh.2005.01.004) [DOI] [PubMed] [Google Scholar]
- 21.Halliday TM, et al. 2020. Appetite-related responses to overfeeding and longitudinal weight change in obesity-prone and obesity-resistant adults. Obesity 28, 259-267. ( 10.1002/oby.22687) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joosen AM, Bakker AH, Westerterp KR. 2005. Metabolic efficiency and energy expenditure during short-term overfeeding. Physiol. Behav. 85, 593-597. ( 10.1016/j.physbeh.2005.06.006) [DOI] [PubMed] [Google Scholar]
- 23.Levine JA, et al. 2005. Interindividual variation in posture allocation: possible role in human obesity. Science 307, 584-586. ( 10.1126/science.1106561) [DOI] [PubMed] [Google Scholar]
- 24.Johannsen DL, Ravussin E. 2008. Spontaneous physical activity: relationship between fidgeting and body weight control. Curr. Opin. Endocrinol. Diabetes Obes. 15, 409-415. ( 10.1097/MED.0b013e32830b10bb) [DOI] [PubMed] [Google Scholar]
- 25.Leaf A, Antonio J. 2017. The effects of overfeeding on body composition: the role of macronutrient composition - a narrative review. Int. J. Exerc. Sci. 10, 1275-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Westerterp KR. 2013. Metabolic adaptations to over- and underfeeding—still a matter of debate? Eur. J. Clin. Nutr. 67, 443-445. ( 10.1038/ejcn.2012.187) [DOI] [PubMed] [Google Scholar]
- 27.Levine JA, Eberhardt NL, Jensen MD. 1999. Role of nonexercise activity thermogenesis in resistance to fat gain in humans. Science 283, 212-214. ( 10.1126/science.283.5399.212) [DOI] [PubMed] [Google Scholar]
- 28.Apolzan JW, et al. 2014. Effects of weight gain induced by controlled overfeeding on physical activity. Am. J. Physiol. Endocrinol. Metab. 307, E1030-E1037. ( 10.1152/ajpendo.00386.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Careau V, et al. 2021. Energy compensation and adiposity in humans. Curr. Biol. 31, 4659-4666. ( 10.1016/j.cub.2021.08.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pontzer H, et al. 2016. Constrained total energy expenditure and metabolic adaptation to physical activity in adult humans. Curr. Biol. 26, 410-417. ( 10.1016/j.cub.2015.12.046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lund J, Gerhart-Hines Z, Clemmensen C. 2020. Role of energy excretion in human body weight regulation. Trends Endocrinol. Metab. 31, 705-708. ( 10.1016/j.tem.2020.06.002) [DOI] [PubMed] [Google Scholar]
- 32.Jumpertz R, et al. 2011. Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. Am. J. Clin. Nutr. 94, 58-65. ( 10.3945/ajcn.110.010132) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wierdsma NJ, et al. 2014. Bomb calorimetry, the gold standard for assessment of intestinal absorption capacity: normative values in healthy ambulant adults. J. Hum. Nutr. Diet 27(Suppl. 2), 57-64. ( 10.1111/jhn.12113) [DOI] [PubMed] [Google Scholar]
- 34.Heymsfield SB, et al. 1981. Energy malabsorption: measurement and nutritional consequences. Am. J. Clin. Nutr. 34, 1954-1960. ( 10.1093/ajcn/34.9.1954) [DOI] [PubMed] [Google Scholar]
- 35.Basolo A, et al. 2020. Effects of underfeeding and oral vancomycin on gut microbiome and nutrient absorption in humans. Nat. Med. 26, 589-598. ( 10.1038/s41591-020-0801-z) [DOI] [PubMed] [Google Scholar]
- 36.Boekhorst J, et al. 2022. Stool energy density is positively correlated to intestinal transit time and related to microbial enterotypes. Microbiome 10, 223. ( 10.1186/s40168-022-01418-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Webb P, Annis JF. 1983. Adaptation to overeating in lean and overweight men and women. Hum. Nutr. Clin. Nutr. 37, 117-131. [PubMed] [Google Scholar]
- 38.Ling Y, et al. 2019. Persistent low body weight in humans is associated with higher mitochondrial activity in white adipose tissue. Am. J. Clin. Nutr. 110, 605-616. ( 10.1093/ajcn/nqz144) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Es AJ, et al. 1984. Human energy metabolism below, near and above energy equilibrium. Br. J. Nutr. 52, 429-442. ( 10.1079/bjn19840111) [DOI] [PubMed] [Google Scholar]
- 40.Murphy JL, Wootton SA, Bond SA, Jackson AA. 1991. Energy content of stools in normal healthy controls and patients with cystic fibrosis. Arch. Dis. Child. 66, 495-500. ( 10.1136/adc.66.4.495) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choa R, et al. 2021. Thymic stromal lymphopoietin induces adipose loss through sebum hypersecretion. Science 373, abd2893. ( 10.1126/science.abd2893) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keesey RE, Hirvonen MD. 1997. Body weight set-points: determination and adjustment. J. Nutr. 127, 1875S-1883S. ( 10.1093/jn/127.9.1875S) [DOI] [PubMed] [Google Scholar]
- 43.Friedman J. 2016. The long road to leptin. J. Clin. Invest. 126, 4727-4734. ( 10.1172/JCI91578) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Y, et al. 1994. Positional cloning of the mouse obese gene and its human homologue. Nature 372, 425-432. ( 10.1038/372425a0) [DOI] [PubMed] [Google Scholar]
- 45.Speakman JR. 2013. Evolutionary perspectives on the obesity epidemic: adaptive, maladaptive, and neutral viewpoints. Annu. Rev. Nutr. 33, 289-317. ( 10.1146/annurev-nutr-071811-150711) [DOI] [PubMed] [Google Scholar]
- 46.Speakman JR. 2018. Why lipostatic set point systems are unlikely to evolve. Mol. Metab. 7, 147-154. ( 10.1016/j.molmet.2017.10.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Levitsky DA. 2002. Putting behavior back into feeding behavior: a tribute to George Collier. Appetite 38, 143-148. ( 10.1006/appe.2001.0465) [DOI] [PubMed] [Google Scholar]
- 48.Herman CP, Polivy J. 1984. A boundary model for the regulation of eating. Res. Publ. Assoc. Res. Nerv. Ment. Dis. 62, 141-156. [PubMed] [Google Scholar]
- 49.Speakman JR. 2007. A nonadaptive scenario explaining the genetic predisposition to obesity: the ‘predation release’ hypothesis. Cell Metab. 6, 5-12. ( 10.1016/j.cmet.2007.06.004) [DOI] [PubMed] [Google Scholar]
- 50.Speakman JR, Elmquist JK. 2022. Obesity: an evolutionary context. Life Metab. 1, 10-24. ( 10.1093/lifemeta/loac002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leibel RL, Rosenbaum M, Hirsch J. 1995. Changes in energy expenditure resulting from altered body weight. N. Engl. J. Med. 332, 621-628. ( 10.1056/NEJM199503093321001) [DOI] [PubMed] [Google Scholar]
- 52.Rosenbaum M, Kissileff HR, Mayer LE, Hirsch J, Leibel RL. 2010. Energy intake in weight-reduced humans. Brain Res. 1350, 95-102. ( 10.1016/j.brainres.2010.05.062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Polidori D, Sanghvi A, Seeley RJ, Hall KD. 2016. How strongly does appetite counter weight loss? Quantification of the feedback control of human energy intake. Obesity 24, 2289-2295. ( 10.1002/oby.21653) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Flier JS, Maratos-Flier E. 2017. Leptin's physiologic role: does the emperor of energy balance have no clothes? Cell Metab. 26, 24-26. ( 10.1016/j.cmet.2017.05.013) [DOI] [PubMed] [Google Scholar]
- 55.Hervey GR. 1959. The effects of lesions in the hypothalamus in parabiotic rats. J. Physiol. 145, 336-352. ( 10.1113/jphysiol.1959.sp006145) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harris RB. 2013. Contribution made by parabiosis to the understanding of energy balance regulation. Biochim. Biophys. Acta 1832, 1449-1455. ( 10.1016/j.bbadis.2013.02.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nishizawa Y, Bray GA. 1980. Evidence for a circulating ergostatic factor: studies on parabiotic rats. Am. J. Physiol. 239, R344-R351. ( 10.1152/ajpregu.1980.239.3.R344) [DOI] [PubMed] [Google Scholar]
- 58.Harris RB, Martin RJ. 1984. Specific depletion of body fat in parabiotic partners of tube-fed obese rats. Am. J. Physiol. 247, R380-R386. ( 10.1152/ajpregu.1984.247.2.R380) [DOI] [PubMed] [Google Scholar]
- 59.Hervey GR. 2013. Control of appetite. Personal and departmental recollections. Appetite 61, 100-110. ( 10.1016/j.appet.2012.10.008) [DOI] [PubMed] [Google Scholar]
- 60.Harris RB, Bruch RC, Martin RJ. 1989. In vitro evidence for an inhibitor of lipogenesis in serum from overfed obese rats. Am. J. Physiol. 257, R326-R336. ( 10.1152/ajpregu.1989.257.2.R326) [DOI] [PubMed] [Google Scholar]
- 61.Fruhbeck G, Gomez-Ambrosi J. 2001. Rationale for the existence of additional adipostatic hormones. FASEB J. 15, 1996-2006. ( 10.1096/fj.00-0829hyp) [DOI] [PubMed] [Google Scholar]
- 62.Smith GP. 2013. Hervey, Harris, and the parabiotic search for lipostatic signals. Appetite 61, 97-99. ( 10.1016/j.appet.2012.08.024) [DOI] [PubMed] [Google Scholar]
- 63.Weigle DS, et al. 1998. Leptin does not fully account for the satiety activity of adipose tissue-conditioned medium. Am. J. Physiol. 275, R976-R985. ( 10.1152/ajpregu.1998.275.4.R976) [DOI] [PubMed] [Google Scholar]
- 64.Hulsey MG, Martin RJ. 1992. An anorectic agent from adipose tissue of overfed rats: effects on feeding behavior. Physiol. Behav. 52, 1141-1149. ( 10.1016/0031-9384(92)90473-f) [DOI] [PubMed] [Google Scholar]
- 65.Goodner GC, Goodner CJ. 1996. Demonstration that acid-ethanol extracts of rat adipose tissue contain an inhibitor of food intake in the mouse. J. Lab. Clin. Med. 128, 246-250. ( 10.1016/s0022-2143(96)90025-6) [DOI] [PubMed] [Google Scholar]
- 66.Kennedy GC. 1953. The role of depot fat in the hypothalamic control of food intake in the rat. Proc. R. Soc. Lond. B 140, 578-596. ( 10.1098/rspb.1953.0009) [DOI] [PubMed] [Google Scholar]
- 67.Jansson JO, et al. 2018. Body weight homeostat that regulates fat mass independently of leptin in rats and mice. Proc. Natl Acad. Sci. USA 115, 427-432. ( 10.1073/pnas.1715687114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Grannell A, De Vito G, Murphy JC, le Roux CW. 2019. The influence of skeletal muscle on appetite regulation. Expert Rev. Endocrinol. Metab. 14, 267-282. ( 10.1080/17446651.2019.1618185) [DOI] [PubMed] [Google Scholar]
- 69.Dulloo AG. 2021. Physiology of weight regain: lessons from the classic Minnesota Starvation Experiment on human body composition regulation. Obes. Rev. 22(Suppl. 2), e13189. ( 10.1111/obr.13189) [DOI] [PubMed] [Google Scholar]
- 70.Gloy VL, Lutz TA, Langhans W, Geary N, Hillebrand JJ. 2010. Basal plasma levels of insulin, leptin, ghrelin, and amylin do not signal adiposity in rats recovering from forced overweight. Endocrinology 151, 4280-4288. ( 10.1210/en.2010-0439) [DOI] [PubMed] [Google Scholar]
- 71.White CL, Purpera MN, Ballard K, Morrison CD. 2010. Decreased food intake following overfeeding involves leptin-dependent and leptin-independent mechanisms. Physiol. Behav. 100, 408-416. ( 10.1016/j.physbeh.2010.04.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ravussin Y, et al. 2018. Evidence for a non-leptin system that defends against weight gain in overfeeding. Cell Metab. 28, 289-299. ( 10.1016/j.cmet.2018.05.029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Muller TD, Bluher M, Tschop MH, DiMarchi RD. 2022. Anti-obesity drug discovery: advances and challenges. Nat. Rev. Drug Discov. 21, 201-223. ( 10.1038/s41573-021-00337-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Patel S, et al. 2019. GDF15 provides an endocrine signal of nutritional stress in mice and humans. Cell Metab. 29, 707-718. ( 10.1016/j.cmet.2018.12.016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Klein AB, et al. 2021. Pharmacological but not physiological GDF15 suppresses feeding and the motivation to exercise. Nat. Commun. 12, 1041. ( 10.1038/s41467-021-21309-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Klein AB, Kleinert M, Richter EA, Clemmensen C. 2022. GDF15 in appetite and exercise: essential player or coincidental bystander? Endocrinology 163, bqab242. ( 10.1210/endocr/bqab242) [DOI] [PubMed] [Google Scholar]
- 77.Heilbronn LK, Campbell LV, Xu A, Samocha-Bonet D. 2013. Metabolically protective cytokines adiponectin and fibroblast growth factor-21 are increased by acute overfeeding in healthy humans. PLoS ONE 8, e78864. ( 10.1371/journal.pone.0078864). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Willis SA, et al. 2020. Acute hyperenergetic, high-fat feeding increases circulating FGF21, LECT2, and fetuin-A in healthy men. J. Nutr. 150, 1076-1085. ( 10.1093/jn/nxz333) [DOI] [PubMed] [Google Scholar]
- 79.Lundsgaard AM, et al. 2017. Circulating FGF21 in humans is potently induced by short term overfeeding of carbohydrates. Mol. Metab. 6, 22-29. ( 10.1016/j.molmet.2016.11.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hollstein T, et al. 2019. Metabolic response to fasting predicts weight gain during low-protein overfeeding in lean men: further evidence for spendthrift and thrifty metabolic phenotypes. Am. J. Clin. Nutr. 110, 593-604. ( 10.1093/ajcn/nqz062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vinales KL, et al. 2019. FGF21 is a hormonal mediator of the human ‘Thrifty’ metabolic phenotype. Diabetes 68, 318-323. ( 10.2337/db18-0696) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Clemmensen C, et al. 2017. Gut-brain cross-talk in metabolic control. Cell 168, 758-774. ( 10.1016/j.cell.2017.01.025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Martinez-Sanchez N, et al. 2022. The sympathetic nervous system in the 21st century: neuroimmune interactions in metabolic homeostasis and obesity. Neuron 110, 3597-3626. ( 10.1016/j.neuron.2022.10.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rothwell NJ, Stock MJ. 1979. A role for brown adipose tissue in diet-induced thermogenesis. Nature 281, 31-35. ( 10.1038/281031a0) [DOI] [PubMed] [Google Scholar]
- 85.van Marken Lichtenbelt WD, et al. 2009. Cold-activated brown adipose tissue in healthy men. N. Engl. J. Med. 360, 1500-1508. ( 10.1056/NEJMoa0808718) [DOI] [PubMed] [Google Scholar]
- 86.Cypess AM, et al. 2009. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 360, 1509-1517. ( 10.1056/NEJMoa0810780) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Virtanen KA, et al. 2009. Functional brown adipose tissue in healthy adults. N. Engl. J. Med. 360, 1518-1525. ( 10.1056/NEJMoa0808949) [DOI] [PubMed] [Google Scholar]
- 88.Saito M, et al. 2009. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 58, 1526-1531. ( 10.2337/db09-0530) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Carpentier AC, Blondin DP, Haman F, Richard D. 2022. Brown adipose tissue—a translational perspective. Endocr. Rev. 44, 143-192. ( 10.1210/endrev/bnac015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wijers SL, Saris WH, van Marken Lichtenbelt WD. 2007. Individual thermogenic responses to mild cold and overfeeding are closely related. J. Clin. Endocrinol. Metab. 92, 4299-4305. ( 10.1210/jc.2007-1065) [DOI] [PubMed] [Google Scholar]
- 91.Vosselman MJ, et al. 2013. Brown adipose tissue activity after a high-calorie meal in humans. Am. J. Clin. Nutr. 98, 57-64. ( 10.3945/ajcn.113.059022) [DOI] [PubMed] [Google Scholar]
- 92.Schlogl M, et al. 2013. Overfeeding over 24 h does not activate brown adipose tissue in humans. J. Clin. Endocrinol. Metab. 98, E1956-E1960. ( 10.1210/jc.2013-2387) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Peterson CM, et al. 2016. The thermogenic responses to overfeeding and cold are differentially regulated. Obesity 24, 96-101. ( 10.1002/oby.21233) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Peterson CM, Orooji M, Johnson DN, Naraghi-Pour M, Ravussin E. 2017. Brown adipose tissue does not seem to mediate metabolic adaptation to overfeeding in men. Obesity 25, 502-505. ( 10.1002/oby.21721) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bartness TJ, Song CK. 2007. Brain-adipose tissue neural crosstalk. Physiol. Behav. 91, 343-351. ( 10.1016/j.physbeh.2007.04.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bai L, et al. 2019. Genetic identification of vagal sensory neurons that control feeding. Cell 179, 1129-1143. ( 10.1016/j.cell.2019.10.031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Goldstein N, et al. 2021. Hypothalamic detection of macronutrients via multiple gut-brain pathways. Cell Metab. 33, 676-687. ( 10.1016/j.cmet.2020.12.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bulik CM, Allison DB. 2001. The genetic epidemiology of thinness. Obes. Rev. 2, 107-115. ( 10.1046/j.1467-789x.2001.00030.x) [DOI] [PubMed] [Google Scholar]
- 99.Bailly M, et al. 2020. Definition and diagnosis of constitutional thinness: a systematic review. Br. J. Nutr. 124, 531-547. ( 10.1017/S0007114520001440) [DOI] [PubMed] [Google Scholar]
- 100.Bossu C, et al. 2007. Energy expenditure adjusted for body composition differentiates constitutional thinness from both normal subjects and anorexia nervosa. Am. J. Physiol. Endocrinol. Metab. 292, E132-E137. ( 10.1152/ajpendo.00241.2006) [DOI] [PubMed] [Google Scholar]
- 101.Estour B, et al. 2017. Differentiating constitutional thinness from anorexia nervosa in DSM 5 era. Psychoneuroendocrinology 84, 94-100. ( 10.1016/j.psyneuen.2017.06.015) [DOI] [PubMed] [Google Scholar]
- 102.Germain N, et al. 2007. Constitutional thinness and lean anorexia nervosa display opposite concentrations of peptide YY, glucagon-like peptide 1, ghrelin, and leptin. Am. J. Clin. Nutr. 85, 967-971. ( 10.1093/ajcn/85.4.967) [DOI] [PubMed] [Google Scholar]
- 103.Bailly M, et al. 2021. Is constitutional thinness really different from anorexia nervosa? A systematic review and meta-analysis. Rev. Endocr. Metab. Disord. 22, 913-971. ( 10.1007/s11154-021-09650-4) [DOI] [PubMed] [Google Scholar]
- 104.Whitaker KL, Jarvis MJ, Boniface D, Wardle J. 2011. The intergenerational transmission of thinness. Arch. Pediatr. Adolesc. Med. 165, 900-905. ( 10.1001/archpediatrics.2011.147) [DOI] [PubMed] [Google Scholar]
- 105.Riveros-McKay F, et al. 2019. Genetic architecture of human thinness compared to severe obesity. PLoS Genet. 15, e1007603. ( 10.1371/journal.pgen.1007603) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Turcot V, et al. 2018. Protein-altering variants associated with body mass index implicate pathways that control energy intake and expenditure in obesity. Nat. Genet. 50, 26-41. ( 10.1038/s41588-017-0011-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lotta LA, et al. 2019. Human gain-of-function MC4R variants show signaling bias and protect against obesity. Cell 177, 597-607. ( 10.1016/j.cell.2019.03.044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Akbari P, et al. 2021. Sequencing of 640 000 exomes identifies GPR75 variants associated with protection from obesity. Science 373. ( 10.1126/science.abf8683). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Orthofer M, et al. 2020. Identification of ALK in thinness. Cell 181, 1246-1262. ( 10.1016/j.cell.2020.04.034) [DOI] [PubMed] [Google Scholar]
- 110.Hübel CEA et al. 2021. Constitutional thinness and anorexia nervosa differ on a genomic level. medRxiv. ( 10.1101/2021.03.08.21253137) [DOI]
- 111.Galusca B, et al. 2018. Reduced fibre size, capillary supply and mitochondrial activity in constitutional thinness' skeletal muscle. Acta Physiol. 224, e13097. ( 10.1111/apha.13097) [DOI] [PubMed] [Google Scholar]
- 112.Hu S, et al. 2022. Higher than predicted resting energy expenditure and lower physical activity in healthy underweight Chinese adults. Cell Metab. 34, 1413-1415. ( 10.1016/j.cmet.2022.05.012) [DOI] [PubMed] [Google Scholar]
- 113.Pasanisi F, et al. 2013. Evidence of brown fat activity in constitutional leanness. J. Clin. Endocrinol. Metab. 98, 1214-1218. ( 10.1210/jc.2012-2981) [DOI] [PubMed] [Google Scholar]
- 114.Marra M, et al. 2007. BMR variability in women of different weight. Clin. Nutr. 26, 567-572. ( 10.1016/j.clnu.2007.03.006) [DOI] [PubMed] [Google Scholar]
- 115.Cornier MA, Von Kaenel SS, Bessesen DH, Tregellas JR.. 2007. Effects of overfeeding on the neuronal response to visual food cues. Am. J. Clin. Nutr. 86, 965-971. ( 10.1093/ajcn/86.4.965) [DOI] [PubMed] [Google Scholar]
- 116.Cornier MA, et al. 2009. The effects of overfeeding on the neuronal response to visual food cues in thin and reduced-obese individuals. PLoS ONE 4, e6310. ( 10.1371/journal.pone.0006310) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cornier MA, Grunwald GK, Johnson SL, Bessesen DH. 2004. Effects of short-term overfeeding on hunger, satiety, and energy intake in thin and reduced-obese individuals. Appetite 43, 253-259. ( 10.1016/j.appet.2004.06.003) [DOI] [PubMed] [Google Scholar]
- 118.Krebs HA. 1975. The August Krogh Principle: ‘For many problems there is an animal on which it can be most conveniently studied’. J. Exp. Zool. 194, 221-226. ( 10.1002/jez.1401940115) [DOI] [PubMed] [Google Scholar]
- 119.O'Rahilly S. 2021. ‘Treasure Your Exceptions’—studying human extreme phenotypes to illuminate metabolic health and disease: the 2019 Banting Medal for Scientific Achievement lecture. Diabetes 70, 29-38. ( 10.2337/dbi19-0037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Schmidt SL, Harmon KA, Sharp TA, Kealey EH, Bessesen DH. 2012. The effects of overfeeding on spontaneous physical activity in obesity prone and obesity resistant humans. Obesity 20, 2186-2193. ( 10.1038/oby.2012.103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schmidt SL, Kealey EH, Horton TJ, VonKaenel S, Bessesen DH. 2013. The effects of short-term overfeeding on energy expenditure and nutrient oxidation in obesity-prone and obesity-resistant individuals. Int. J. Obes. 37, 1192-1197. ( 10.1038/ijo.2012.202) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Germain N, et al. 2014. Specific appetite, energetic and metabolomics responses to fat overfeeding in resistant-to-bodyweight-gain constitutional thinness. Nutr. Diabet. 4, e126. ( 10.1038/nutd.2014.17) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ling Y, et al. 2020. Resistance to lean mass gain in constitutional thinness in free-living conditions is not overpassed by overfeeding. J. Cachexia Sarcopenia Muscle 11, 1187-1199. ( 10.1002/jcsm.12572) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hall KD. 2020. Challenges of human nutrition research. Science 367, 1298-1300. ( 10.1126/science.aba3807) [DOI] [PubMed] [Google Scholar]
- 125.Creasy SA, Rynders CA, Bergouignan A, Kealey EH, Bessesen DH. 2018. Free-living responses in energy balance to short-term overfeeding in adults differing in propensity for obesity. Obesity 26, 696-702. ( 10.1002/oby.22121) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.