Abstract
This report describes a procedure for characterizing membrane protein topology which combines the analysis of reporter protein hybrids and trypsin-sensitive 31-amino-acid insertions generated by using transposons ISphoA/in and ISlacZ/in. Studies of the F factor TraD protein imply that the protein takes on a structure with two membrane-spanning sequences and amino and carboxyl termini facing the cytoplasm. It was possible to assign the subcellular location of one region for which the behavior of fused reporter proteins was ambiguous, based on the trypsin cleavage behavior of a 31-residue insertion.
A variety of biochemical, genetic, and immunological methods have been devised to assay integral membrane protein topology (9, 17, 19, 38). One method consists of the construction of gene fusions encoding hybrid proteins in which C-terminal sequences of the membrane protein being analyzed are replaced by a reporter enzyme whose activity reflects its subcellular location, such as alkaline phosphatase, β-lactamase, or β-galactosidase. The absence of the normal membrane protein C-terminal sequences can complicate such studies if the C-terminal residues contribute to a protein’s topology (2, 35). An alternative topology assay in which membrane protein sequences are not lost utilizes membrane proteins carrying inserted sequences that can be modified in a subcellular location-dependent fashion (reviewed in reference 19). Insertions are often tolerated without disrupting a membrane protein’s activity, indicating that the altered proteins retain the normal topology (20, 25). An efficient transposon-based method for producing insertions sensitive to tobacco etch virus protease has been used to analyze outer membrane protein topology (8, 10).
We recently described transposons that generate alkaline phosphatase or β-galactosidase gene fusions which can be converted into in-frame 31-codon insertions (25). We report here that the inserted 31-amino-acid sequence is highly sensitive to cleavage by trypsin and that the cleavage behavior of insertion derivatives of two well-characterized inner membrane proteins accurately reflects their topological structures. The combined analysis of gene fusions and 31-codon insertions was also used to characterize the topology of the F factor TraD protein, a protein required for the DNA transfer step of conjugation (13, 16, 29).
MATERIALS AND METHODS
Growth media, strains, and plasmids.
Growth media were as described previously (25). The Escherichia coli strains and plasmids used in this study are listed in Table 1. The traD gene was subcloned from pMP1 (30) into pBR322 on a HpaI/BamHI fragment and then further subcloned on a smaller NgoMI-HincII fragment into pBAD18 (15) between the XmaI and HincII sites of the polylinker to make pNLK2. This placed traD under control of the araBAD promoter. The single BamHI site in pNLK2 was eliminated by site-directed mutagenesis (GGATCC→CGATCC) to form pNLK5 (18).
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Description |
|---|---|
| E. coli strains | |
| BT8 | F− Δ(lac)U169 araD139 rpsL thiA relA malT(Con) zhe::Tn10 |
| CC118 | araD139 Δ(ara leu)7697 ΔlacX74 phoA20 galE galK thi rpsE rpoB argE(Am) recA1 |
| CC762 | Δ(lac)U169 rpsL thi Δ(ara leu)7697 pcnB zad::Tn10 phoR zaj::Tn10kan |
| CC874 | F′ lacIq Δ(lacZ lacY)::cat/araD139 Δ(ara leu)7697 ΔlacX74 phoAΔ20 galE galK thi rpsE rpoB argE(Am) recA1 |
| EM1014 | JCFL14 traD(Am)/ΔlacX74 galK his trp lys rpsL λr |
| Plasmids | |
| pCM389 | pBR322-based plasmid carrying wild-type lacY (25) |
| pCM701 | pCM389 carrying a 31-codon insertion in lacY corresponding to residue 38 (25) |
| PCM702 | pCM389 carrying a 31-codon insertion in lacY corresponding to residue 71 (25) |
| pJFG5 | pBR322-derived plasmid carrying tsr (14) |
| pNLK5 | pBAD18-derived plasmid carrying wild-type traD expressed under control of the araBAD promoter |
| pNLK6 | Deletion derivative (SphI-SphI) of pNLK5 encoding approximately the N-terminal 10% of TraD |
Generation of gene fusions and 31-codon insertions.
TnlacZ, TnphoA/in, a sacB mutant derivative of TnphoA/in and TnlacZ/in were used to generate gene fusions using phage lambda derivatives as delivery vectors (24, 25). Several traD-phoA fusions were also produced from TnlacZ or ISlacZ/in insertions by fusion switching (24). Gene fusions to tsr were generated by transposition into plasmid pJFG5 (14), and most fusions to traD were generated by transposition into pNLK5. Several fusions corresponding to the N-terminal region of TraD were generated after transposition into plasmid pNLK6, an SphI-SphI deletion derivative of pNLK5 which carries only the 5′ 206 bp of traD. Thirty-one-codon insertion derivatives of these fusions were generated after the missing SphI-SphI fragment carrying the 3′ end of traD had been replaced in each plasmid. ISphoA/in and ISlacZ/in insertions were converted into 31-codon in-frame insertions by cleavage with BamHI and DNA ligase treatment (25).
Assay of trypsin sensitivity in spheroplasts.
Trypsin treatment was carried out by using one of two procedures. In procedure 1, cells grown overnight in Luria-Bertani medium (LB) supplemented with ampicillin (100 μg/ml) or M63 supplemented with thiamine (1 μg/ml), all amino acids (20 μg/ml), and ampicillin (50 μg/ml) were diluted to an optical density at 600 nm (OD600) of 0.1 in fresh medium and grown at 37°C to an OD600 of approximately 0.9. Isopropyl-β-d-thiogalactopyranoside (2 mM, final concentration) (for lacY) or arabinose (0.2% final) (for traD) was added as inducer, and cells were incubated at 37°C for an additional 45 to 60 min. Approximately equal numbers of cells were pelleted by centrifugation at 4°C in a Sorvall SH-MT rotor (10 min at 5,000 × g), washed in sucrose buffer (18% sucrose, 100 mM Tris HCl [pH 8.0]), and centrifuged as before. Pellets were resuspended in 500 μl of sucrose buffer, to which was added lysozyme (207 μg/ml, final concentration) and EDTA (17 mM, final concentration), and then incubated 30 min on ice. The resulting spheroplast preparations were divided into two 290-μl aliquots; one aliquot was incubated with trypsin (30 μg/ml, final concentration), and the second was incubated with inactivated trypsin (30 μg/ml, final concentration). (Inactivation was achieved by mixing 32 μl trypsin [300 μg/ml] with 32 μl of soybean trypsin inhibitor [STI; 1 mg/ml] and 20 μl of phenylmethylsulfonyl fluoride [PMSF; 20 mg/ml].) After 30 min of incubation at room temperature, the reactions with active trypsin were terminated by the addition of STI (90 μg/ml, final concentration) and PMSF (1.1 mg/ml, final concentration). The trypsin-treated spheroplasts were then centrifuged for 3 min at 16,000 × g at 4°C. The resulting pellets were washed in 250 μl of sucrose buffer containing STI (90 μg/ml, final concentration) and PMSF (360 μg/ml), centrifuged as before, and resuspended in 600 μl of freeze-thaw buffer (10 mM Tris HCl [pH 8.0], 16 μg of STI per ml, 320 μg of PMSF per ml). Spheroplasts were lysed with three freeze-thaw cycles in dry ice-ethanol baths. To each sample was added MgCl2 (32 mM, final concentration) and bovine pancreatic DNase (10 μg/ml, final concentration), followed immediately by centrifugation (25 min at 16,000 × g at 4°C) to produce a membrane pellet. Supernatant (cytoplasmic) proteins were recovered after precipitation with an equal volume of cold 10% trichloroacetic acid. The proteins in both the membrane and cytoplasmic fractions were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and either stained with Coomassie blue or subjected to Western blotting using antibodies directed against the target protein or the 31-residue inserted sequence (25). Trypsin cleavage of the OmpA protein periplasmic domain was monitored to determine the efficiency of spheroplast formation and proteolysis. Trypsin cleavage of an abundant 45-kDa cytoplasmic protein, tentatively identified as elongation factor Tu, was monitored to control for cell lysis before or during proteolysis.
Procedure 2 for assay of trypsin sensitivity used cells that had been grown in M63 minimal medium supplemented with 0.2% glycerol, thiamine (1 μg/ml), and all amino acids except methionine and cysteine (20 μg/ml). Cultures grown overnight were diluted 1:100 into fresh medium and grown with aeration to a final OD600 of approximately 0.35. Cells were harvested from chilled cultures by centrifugation, then resuspended in an equal volume of 40% sucrose–35 mM Tris HCl (pH 8.0)–2.5 mM EDTA containing egg white lysozyme (5 μg/ml), and incubated at 0°C for 15 min. Two 1.25-ml portions of spheroplasted cells were prepared. The first served as the untreated control, and the second was incubated with freshly prepared trypsin (25 μg/ml) for 20 to 30 min at 0°C. After the incubation, PMSF (200 μg/ml, final concentration) was added to all samples. Spheroplasts were then harvested by centrifugation, resuspended in SDS-PAGE sample buffer, heated to 65°C for 20 min, and subjected to Western blot analysis. The efficiency of spheroplast formation and proteolysis and the integrity of spheroplasts during the manipulations were monitored as described above for procedure 1.
Assay of TraD-PhoA synthesis rates.
Pulse-labeling experiments were performed to determine the rate of production of each of the TraD-PhoA hybrid proteins (24). The strains used in these assays (CC762 carrying traD-phoA plasmids) expressed wild-type alkaline phosphatase constitutively, which was used as an internal control for the recovery of hybrid protein in the anti-alkaline phosphatase immunoprecipitation step.
Quantitative mating assays.
The ability of the plasmids carrying traD insertions to complement the JCFL14 traD F plasmid for transfer from EM1014 was measured in conjugation assays with strain BT8 as the recipient. Donor and recipient cultures were grown overnight in LB at 37°C. Before mixing with the recipient culture, the donor cultures were incubated with 0.2% l-arabinose for 1 h to induce expression of the plasmid-borne traD allele. Aliquots (0.1 ml) of recipient and arabinose-induced donor cultures were diluted with 0.8 ml of LB and rolled slowly at 37°C for 1 h. At the beginning of the matings, the donor cultures were diluted and plated onto LB agar containing 5-bromo-4-chloro-3-indolyl-β,d-galactoside (XG) and ampicillin for enumeration. At the end of the matings, samples of the mating mixtures were diluted and plated onto lactose-minimal agar with tetracycline to determine the number of Flac transconjugants.
RESULTS
Transposons ISphoA/in and ISlacZ/in allow the generation of alkaline phosphatase or β-galactosidase gene fusions which may be converted into in-frame 31-codon insertions (25). Since active alkaline phosphatase fusions generally correspond to exported sequences and active β-galactosidase fusions correspond to cytoplasmic regions (38), the combined use of ISphoA/in and ISlacZ/in make it possible to generate 31-amino-acid insertions at sites distributed throughout an inner membrane protein. We have observed that the 31-amino-acid sequence is quite sensitive to trypsin cleavage (see below). We thus expected that examining the pattern of trypsin sensitivity in spheroplasts of a set of such insertions would provide an assay of membrane topology which was independent of the analysis of reporter protein hybrids.
Studies of topologically characterized proteins.
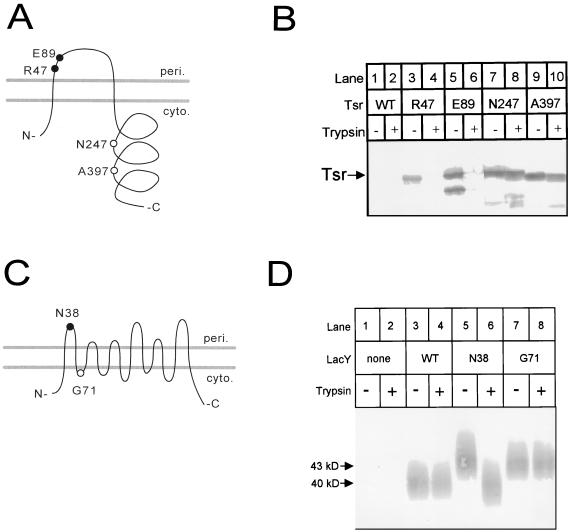
To examine whether the trypsin sensitivity of 31-amino-acid insertions accurately reflected membrane topology, we examined derivatives of two E. coli proteins with relatively well established topologies, the Tsr serine chemoreceptor and lac permease (3, 11, 14, 23, 26). Four ISphoA/in insertions situated in regions corresponding to the periplasmic and C-terminal cytoplasmic domains of Tsr were generated and converted into 31-codon insertions (Materials and Methods). Strains producing the tsr-phoA hybrid proteins expressed alkaline phosphatase activities in accord with the topology (see the legend to Fig. 1). Cells producing the chemoreceptor 31-amino-acid insertion derivatives were converted into spheroplasts, exposed to trypsin, and analyzed by Western blotting with an antiserum directed against the 31-amino-acid insert sequence (Fig. 1A and B). Protein from cells producing Tsr without an insertion did not react with the antiserum, as expected (Fig. 1B, lanes 1 and 2). In contrast, the R47 and E89 insertion mutants produced proteins migrating at approximately 70 kDa which were sensitive to trypsin (lanes 3 to 6). The N247 and A397 insertion mutant proteins were not significantly degraded by exposure to trypsin (lanes 7 to 10), although OmpA in the same cells was cleaved (not shown), showing that trypsin was active at periplasmic sites. These results show that the trypsin cleavage behavior of the Tsr insertions is consistent with that expected from the membrane topology of Tsr.
FIG. 1.
Trypsin cleavage of insertion derivatives of lac permease and Tsr. (A) Positions in the Tsr topology of the 31-residue insertions examined. Closed circles denote trypsin sensitivity in spheroplasts, and open circles denote trypsin resistance. The alkaline phosphatase activities of cells expressing the tsr-ISphoA/in insertions used to generate the 31-residue insertions indicated were measured for plasmids in strain CC118. The alkaline phosphatase activities (expressed in activity units ± standard deviation) were as follows: no plasmid, 1.7 ± 0.1; R47, 2162 ± 49; E89, 2589 ± 22; N247, 14 ± 1; and A397, 8 ± 0.4. peri., periplasmic; cyto., cytoplasmic. (B) Western blot of Tsr insertion derivatives following trypsin treatment of spheroplasts. A polyclonal antiserum directed against the inserted sequence was used to develop the blot (25). The trypsin treatment led to the production of polypeptides approximately the sizes expected for N-terminal fragments resulting from cleavage within the two periplasmic inserts (∼6.5 kDa for R47 and ∼15 kDa for E89) (not shown). Trypsin treatment procedure 2 with cells grown at 30°C was used. (C) Positions in the lac permease topology of the N38 and G71 31-residue insertions. The closed circle indicates trypsin sensitivity in spheroplasts; the open circle indicates resistance. (D) Western blot of lac permease insertion derivatives following trypsin treatment of spheroplasts. A polyclonal antiserum directed against the C terminus of lactose permease was used to develop the blot. lac permease runs as a broad band in SDS-PAGE. Strain CC874 carrying different plasmids was analyzed by trypsin treatment procedure 1 with cells grown at 37°C. WT, wild type.
As a second test, we examined the trypsin sensitivity of two insertions in the lac permease which had been generated earlier, N38 and G71 (Fig. 1C and D) (25). These insertion mutants are highly active for lactose transport and therefore probably assume the same membrane topology as the wild-type protein. Following spheroplast formation and trypsin treatment, proteins were analyzed by Western blotting using an anti-lac permease antiserum (Fig. 1D). The strain deleted of the lac permease gene did not show any bands (Fig. 1D, lanes 1 and 2), whereas a strain expressing wild-type lac permease produced a heterodisperse band migrating at 40 kDa which was unaffected by exposure to trypsin (lanes 3 and 4), as observed earlier (20). The strain expressing the N38 periplasmic insertion derivative produced a band migrating somewhat more slowly than the wild type (at ∼45 kDa) (lane 5), consistent with its carrying a 31-amino-acid insertion. Exposure to trypsin converted this protein to a species migrating with an apparent mass slightly less than 40 kDa (lane 6), as expected for cleavage within the inserted sequence (predicted to remove an approximately 6.5-kDa polypeptide). In contrast, the G71 cytoplasmic domain insertion was unaffected by exposure to trypsin (lanes 7 and 8).
The results of the lac permease trypsin accessibility assays, summarized in Fig. 1C, are compatible with membrane topology studies carried out using alkaline phosphatase gene fusions (3). Previous studies demonstrated that insertions engineered using site-directed mutagenesis into the fourth and sixth periplasmic domains of the protein were sensitive to trypsin added to spheroplasts (20, 20a). The cleavage properties of introduced factor Xa sites were also compatible with the topology (32). These results indicate that proteolysis at introduced cleavage sites, including 31-amino-acid insertions, provides a reliable assay of the transmembrane arrangement of a topologically complex inner membrane protein.
TraD topology.
We next sought to analyze the topology of F factor TraD protein, a protein required for DNA transfer in conjugation. The traD gene encodes a protein predicted from hydrophobicity analysis to have either two or three membrane-spanning sequences, with strongly predicted spanning sequences at residues 28 to 47 and 105 to 130 and a more weakly predicted sequence at 392 to 412 (14). We first constructed a plasmid (pNLK5) with traD under control of the araBAD promoter (Materials and Methods). The plasmid complemented the conjugation defect of a traD amber mutant (see below), indicating that TraD was expressed in active form. Plasmids carrying traD-phoA and traD-lacZ fusions were generated by transposition of ISphoA/in, TnlacZ, or ISlacZ/in into pNLK5 or a deletion derivative of pNLK5 (Materials and Methods). Additional traD-phoA fusions were generated by fusion switching from traD-lacZ fusions (23) (Materials and Methods).
The alkaline phosphatase activities of strains producing the different hybrid proteins indicate that residues K6, I14, G282, E620, and N702 are cytoplasmic (<25 activity units) and that residues I59, T65, and E67 are periplasmic (>150 U) (Table 2 and Fig. 2A). Curiously, the Q94 hybrid exhibited an intermediate level of activity (46 U) (Table 2). The different hybrids were synthesized at similar rates except for K6, which was synthesized at a somewhat higher level (Table 2). These findings thus suggest a topology for TraD in which there are two membrane-spanning segments, with cytoplasmic N and C termini (Fig. 2A).
TABLE 2.
Alkaline phosphatase activities of TraD-PhoA hybrid proteins
| Hybrid proteina | Alkaline phosphatase activityb (SD) | Hybrid protein synthesis ratec (SD) |
|---|---|---|
| pNLK5 | 2.5 (0.62) | NA |
| K6 | 21 (9.9) | 5.0 (2.2) |
| I14 | 11 (4.1) | 2.1 (0.4) |
| I59 | 750 (190) | 0.76 (0.1) |
| T65 | 250 (270) | ND |
| E67 | 160 (5) | 1.0 (0.1) |
| Q94 | 46 (5.0) | 0.73 (0.2) |
| G282 | 5.1 (1.3) | 0.77 |
| E620 | 6.0 (0.65) | 0.65 |
| N702 | 4.9 (2.3) | ND |
Encoded by pNLK5 derivatives.
Expressed in arbitrary units (24). Values are averages of 3 to 14 assays each.
Expressed relative to alkaline phosphatase encoded by the chromosome and expressed constitutively. TraD expression values are the average of one to two assays each. NA, not applicable; ND, not determined.
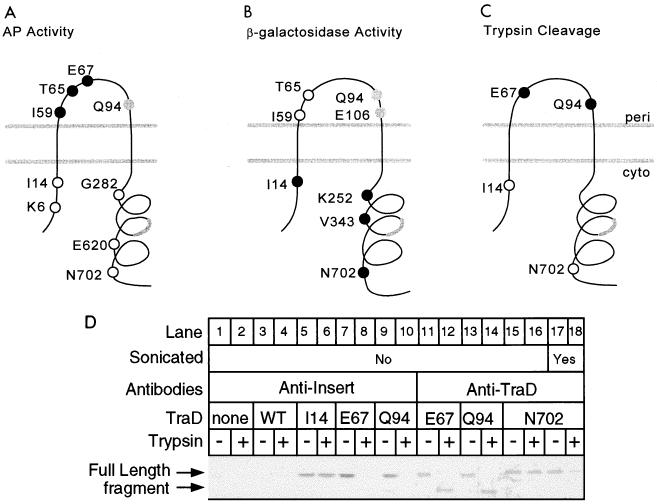
FIG. 2.
Analysis of TraD topology. (A) Locations in the favored two-span model and alkaline phosphatase activities of nine TraD-alkaline phosphatase hybrids. Closed circles denote high activity (greater than 150 Miller units), open circles denote low activity (less than 25 Miller units), and the gray circle denotes an intermediate value (46 Miller units) (Table 2). The thick gray line denotes the location of a potential third membrane-spanning sequence. (B) Locations and β-galactosidase activities of eight TraD–β-galactosidase hybrids. Closed circles denote high activity (I14, 183 U, K252, 133 U; V343, 145 U; N702, 127 U), open circles denote low activity (I59, 2 U; T65, 6 U), and the gray circles denote intermediate activity (Q94, 48 U; E106, 70 U). (C) Positions in the topology and cleavage by trypsin in spheroplasts of four 31-amino-acid insertions in TraD. Closed circles represent insertions that were trypsin sensitive; open circles represent trypsin-resistant insertions. (D) Western blot of TraD insertion derivatives following trypsin treatment of spheroplasts. A polyclonal antiserum directed against either the inserted sequence or wild-type TraD was used as indicated previously (25). A strain deleted for traD was used as a negative control (none). A strain expressing wild-type TraD (WT) served as a negative control in the antiinsert blot. An aliquot of the spheroplast preparation expressing the N702 insertion derivative was sonicated before the addition of trypsin (lanes 17 and 18). Trypsin treatment procedure 1 was used with cells grown at 37°C.
The activities of the TraD–β-galactosidase chimeras were also consistent with a topology for TraD in which there are two membrane-spanning sequences (Fig. 2B). Cells producing hybrid proteins with junctions at I14, K252, V343, and N702 showed high activity (>125 Miller units), suggesting that these sites are situated in the cytoplasm. Hybrid proteins with junctions at I59 and T65 exhibited low activities (<20 Miller units). Interesting, cells producing hybrid proteins with fusion junctions at Q94 and E106 exhibited intermediate β-galactosidase activities (48 and 70 U, respectively). Thus, for both alkaline phosphatase and β-galactosidase fusions, hybrids with junctions at the end of the putative periplasmic domain showed intermediate activities, leading to some uncertainty as to the correct subcellular assignment of this region.
To provide additional testing of the topology model shown in Fig. 2A and B, we examined the trypsin sensitivity of several 31-amino-acid insertion derivatives (Fig. 2C and D). Cells expressing TraD with insertions at I14, E67, Q94, and N702 were exposed to trypsin after spheroplast formation, followed by Western blot analysis (Fig. 2D) (Materials and Methods). No signal from the antiinsert antiserum was observed for the traD(Am) strain with the vector plasmid (pBAD18) (Fig. 2D, lanes 1 and 2) or plasmid expressing TraD lacking an insertion (pNLK5) (lanes 3 and 4). However, cells expressing the I14 insertion mutant produced a protein migrating at approximately 82 kDa which was unaffected by trypsin (lanes 5 and 6). In contrast, the E67 and Q94 mutant proteins were efficiently cleaved by trypsin (lanes 7 to 14). Furthermore, products of the size expected for C-terminal fragments resulting from cleavage in the 31-residue insertions (∼74 kDa) were observed when the TraD antiserum was used for the blot (lanes 12 and 14). Finally, the 31-residue insertion at N702 was resistant to trypsin cleavage (lanes 15 and 16), although it was degraded if the spheroplast preparation was disrupted by sonication prior to trypsin exposure (lanes 17 and 18). We assume that the nearly complete degradation of TraD following sonication reflects cleavage at trypsin-sensitive cytoplasmic sites in addition to the 31-residue insertion. Taken as a whole, these results are in excellent agreement with the analysis of TraD-PhoA and TraD-LacZ hybrid proteins. The cleavage of the insertion at Q94 supports assignment of the region which includes Q94 (and in which fused reporter proteins gave intermediate activities) to the periplasm. The TraD periplasmic domain is relatively rich in positively charged residues (6 of 60 residues) (14), and it may be that C-terminal sequences absent in the Q94 and E106 hybrid proteins contribute to the export of the domain in the wild-type protein.
We examined the abilities of the different traD insertions to complement a traD(Am) strain for conjugal transfer (Table 3). The traD(Am) mutation does not cause complete loss of TraD function (36) but was used because it is relatively nonpolar on the expression downstream tra operon genes. Insertions at four positions in TraD were active, identifying permissive sites and indicating that at least a fraction of the corresponding proteins insert into the membrane in the correct (functional) topology.
TABLE 3.
Complementation efficiencies of TraD 31-codon insertion alleles
| traD allele | Transfer efficiencya (transconjugants/donor) |
|---|---|
| Vector (pBAD18) | 7.4 × 10−5 |
| K6 | 9.8 × 10−4 |
| I14 | 1.1 × 10−3 |
| I59 | 1.0 × 10−3 |
| T65 | 0.23 |
| E67 | 0.25 |
| Q94 | 0.74 |
| G282 | 2.6 × 10−4 |
| E620b | 1.5 × 10−3 |
| N702 | 1.5 |
Relative to EM1014 carrying the wild-type traD parent plasmid pNLK5, assigned a value of 1. This strain gave ∼3 transconjugants/donor under the conditions used.
The 31-codon insertion generates a frameshift mutation (rendering the C-terminal traD sequence out of frame) because 8 rather than 9 bp were duplicated during insertion of ISlacZ/in (not shown).
DISCUSSION
This report describes a method for combining the analysis of reporter protein hybrids and trypsin-sensitive insertions to characterize cytoplasmic membrane protein topology in E. coli. The use of the two independent measures of the topology of a membrane protein provides a more rigorous analysis than use of either method alone and makes it possible objectively to assign sites whose subcellular location based on either method alone is uncertain.
Both reporter protein hybrids and engineered protease sensitive sites have been used previously to analyze membrane topology in prokaryotes and eukaryotes (17, 19, 38). The most frequently used reporter enzymes for membrane topology studies in bacteria are alkaline phosphatase, β-galactosidase, and β-lactamase, and the corresponding gene fusions are simply generated by insertion of appropriate transposon derivatives or in vitro mutagenesis (38). In a topology study analyzing a set of such fusions, sites may sometimes be difficult to assign because the corresponding hybrid proteins express enzymatic activities intermediate between that of most periplasmic and cytoplasmic hybrids. It has generally been possible to account for such exceptional behavior by the absence of C-terminal sequences in the hybrid proteins which contribute to the topology (2, 4, 19, 22, 31).
The proteolytic sensitivity of engineered insertions has been used to assay the topologies of a variety of membrane proteins (reviewed in reference 19). The analysis of such insertions provides the advantage that sequences C terminal to the inserted sequence are present in the derivatives analyzed. The main disadvantage has been that relatively laborious procedures have been required to construct such derivatives and analyze their behavior. In this regard, the 31-codon insertion derivatives examined here are relatively simple to construct, and they are situated at exactly the same sites as the alkaline phosphatase or β-galactosidase gene fusions used to generate them (25). Transposons for creating gene fusions which may be converted into insertions encoding tobacco etch virus protease cleavage sites were described earlier and have been used to analyze the topologies of the outer membrane proteins TolC and LamB (8, 10). A high proportion of 31-amino-acid insertion derivatives of several cytoplasmic membrane proteins exhibit significant activity (typically 30 to 50%) (references 25 and 35a and this report). In general, the retention of activity indicates that such a protein has inserted into the membrane at least in part in the wild-type topology.
To test the use of 31-amino-acid insertions for analyzing membrane protein topology, we examined derivatives of two topologically well-characterized proteins, lac permease and the serine chemoreceptor. We observed that the trypsin sensitivity of the insertion derivatives in spheroplasts was in accord with topology models established earlier using a variety of methods. Antisera recognizing the 31-amino-acid inserted sequence could be used to follow trypsin cleavage (Fig. 1 and 2), an advantage for the analysis of proteins for which antibodies are unavailable. The most obvious limitation of the method is that it may be difficult or impossible to use to analyze membrane proteins that are sensitive to trypsin at their periplasmic faces. However, periplasmic sensitivity to trypsin under the relatively mild conditions used here to cleave the 31-amino-acid insertion does not appear to be particularly common in inner membrane proteins (20, 27, 34, 37).
We next used a combination of 31-codon insertions and gene fusions to characterize the topology of the F factor TraD protein. TraD is thought to play a central role in DNA binding and transfer steps of the conjugation process. Our results indicated that TraD takes on a membrane topology with two membrane-spanning segments with cytoplasmic amino and carboxyl termini. The properties of the different alkaline phosphatase and β-galactosidase hybrid proteins and 31-amino-acid insertions were in excellent general agreement. However, analysis of one of the insertion derivatives made it possible to assign to the periplasm a region at which fused reporter enzymes showed intermediate enzymatic activities.
The TraD topology implied by our results differs from that proposed earlier for a TraD homologue, the VirD4 protein encoded by the Agrobacterium tumefaciens Ti plasmid, which assumed that the VirD4 C-terminal region protrudes into the periplasm (28). The VirD4 model was based largely on arguments made to rationalize the complex fractionation behavior of VirD4 and VirD4-PhoA hybrid proteins. Although interpreted otherwise, the enzymatic activities of the VirD4-PhoA hybrid proteins described in the study imply that the VirD4 C-terminal region is actually cytoplasmic. A second study of VirD4-PhoA hybrid proteins also implied that the C-terminal region of TraD is cytoplasmic (5). We thus suspect that the fractionation results of the first study were misleading and that the VirD4 topology is analogous to that proposed here for TraD.
TraD is a DNA binding protein which is thought to function directly in the DNA transfer step in conjugation through an interaction with a relaxosome complex constructed at the F factor transfer origin oriT (6, 7, 12, 13, 16, 29, 30). Homologues of TraD are widespread in conjugal systems and may also be involved in protein export (1, 21, 40). TraD has potential nucleotide binding site sequence motifs at residues 192 to 199 and 421 to 426 (13), and alterations in these conserved sites have been shown to inactivate a TraD homologue (1). Our model for the TraD topology places both nucleotide binding motifs in the large C-terminal cytoplasmic domain. A region at the very C terminus of TraD which helps determine the specificity of plasmid transfer and may be involved in the interaction with the oriT complex is also predicted to be cytoplasmic (33). As these examples help illustrate, the TraD topology provides a simple structural context for interpreting the properties of mutations altering the behavior of this fascinating protein.
ACKNOWLEDGMENTS
This work was supported by grants MCB-506989 and MCB-9818189 from the National Science Foundation and by Public Health Service grant GM-46493.
We are grateful to Lynn Dansey and Ned Minkley for providing TraD antisera and to Michael Ehrmann for communicating unpublished results.
REFERENCES
- 1.Balzer D, Pansegrau W, Lanka E. Essential motifs of relaxase (TraI) and TraG proteins involved in conjugative transfer of plasmid RP4. J Bacteriol. 1994;176:4285–4295. doi: 10.1128/jb.176.14.4285-4295.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyd D, Manoil C, Beckwith J. Determinants of membrane protein topology. Proc Natl Acad Sci USA. 1987;84:8525–8529. doi: 10.1073/pnas.84.23.8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calamia J, Manoil C. lac permease of Escherichia coli: topology and sequence elements promoting membrane insertion. Proc Natl Acad Sci USA. 1990;87:4937–4941. doi: 10.1073/pnas.87.13.4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calamia J, Manoil C. Membrane protein spanning segments as export signals. J Mol Biol. 1992;224:539–543. doi: 10.1016/0022-2836(92)90542-r. [DOI] [PubMed] [Google Scholar]
- 5.Das A, Xie Y-H. Construction of transposon Tn3phoA: its application in defining the membrane topology of the Agrobacterium tumefaciens DNA transfer proteins. Mol Microbiol. 1998;27:405–414. doi: 10.1046/j.1365-2958.1998.00688.x. [DOI] [PubMed] [Google Scholar]
- 6.Dash P, Traxler B, Panicker M, Hackney D, Minkley E G. Biochemical characterization of Escherichia coli DNA helicase I. Mol Microbiol. 1992;6:1163–1172. doi: 10.1111/j.1365-2958.1992.tb01555.x. [DOI] [PubMed] [Google Scholar]
- 7.Disque-Kochem C, Dreiseikelmann B. The cytoplasmic DNA-binding protein TraM binds to the inner membrane protein TraD in vitro. J Bacteriol. 1997;179:6133–6137. doi: 10.1128/jb.179.19.6133-6137.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ehrmann, M. Personal communication.
- 9.Ehrmann M, Boyd D, Beckwith J. Genetic analysis of membrane protein topology by a sandwich gene fusion approach. Proc Natl Acad Sci USA. 1990;87:7574–7578. doi: 10.1073/pnas.87.19.7574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ehrmann M, Bplek P, Mondigler M, Boyd D, Lange R. TnTIN and TnTAP: mini-transposons for site-specific proteolysis in vivo. Proc Natl Acad Sci USA. 1997;94:13111–13115. doi: 10.1073/pnas.94.24.13111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Falke J, Dernburg A, Sternberg D, Zalkin N, Milligan D, Koshland D E. Structure of a bacterial sensory receptor. J Biol Chem. 1988;263:14850–14858. [PubMed] [Google Scholar]
- 12.Firth N, Ippen-Ihler K, Skurray R A. Structure and function of the F factor and mechanism of conjugation. In: Neidhardt F, Curtiss III R, Ingraham J, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: molecular and cellular biology. 2nd ed. Washington, D.C.: American Society for Microbiology; 1996. pp. 2377–2401. [Google Scholar]
- 13.Frost L, Ippen-Ihler K, Skurray R. Analysis of the sequence and gene products of the transfer region of the F sex factor. Microbiol Rev. 1994;58:162–210. doi: 10.1128/mr.58.2.162-210.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gebert J, Overhoff B, Manson M, Boos W. The Tsr chemosensory transducer of Escherichia coli assembles into the cytoplasmic membrane via a SecA-dependent process. J Biol Chem. 1988;263:16652–16660. [PubMed] [Google Scholar]
- 15.Guzman L-M, Belin D, Carson M, Beckwith J. Tight regulation, modulation and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol. 1995;177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ippen-Ihler K, Minkley E G. The conjugation system of F, the fertility factor of Escherichia coli. Annu Rev Genet. 1986;20:593–624. doi: 10.1146/annurev.ge.20.120186.003113. [DOI] [PubMed] [Google Scholar]
- 17.Jennings M. Topography of membrane proteins. Annu Rev Biochem. 1989;58:999–1027. doi: 10.1146/annurev.bi.58.070189.005031. [DOI] [PubMed] [Google Scholar]
- 18.Kunkel T A, Roberts J D, Zakour R A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- 19.Lee M H, Manoil C. Molecular genetic analysis of membrane protein topology. In: Kaback H R, Konigs W N, Lolkema J S, editors. Transport processes in eukaryotic and prokaryotic organisms. Amsterdam, The Netherlands: Elsevier; 1996. pp. 189–201. [Google Scholar]
- 20.Lee M H, Manoil C. Engineering protease sensitive sites in a membrane transport protein. Protein Eng. 1997;10:715–723. doi: 10.1093/protein/10.6.715. [DOI] [PubMed] [Google Scholar]
- 20a.Lee, M. H., and C. Manoil. Unpublished data.
- 21.Lessl M, Pansegrau W, Lanka E. Relationship of DNA-transfer systems: essential transfer factors of plasmids RP4, Ti and F share common sequences. Nucleic Acids Res. 1992;22:6099–6100. doi: 10.1093/nar/20.22.6099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis M, Chang J, Simoni R. A topological analysis of subunit alpha from Escherichia coli F1F0 ATPase predicts eight transmembrane segments. J Biol Chem. 1990;265:10541–10550. [PubMed] [Google Scholar]
- 23.Manoil C. Analysis of protein localization by use of gene fusions with complementary properties. J Bacteriol. 1990;172:1035–1042. doi: 10.1128/jb.172.2.1035-1042.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manoil C. Analysis of membrane protein topology using alkaline phosphatase and β-galactosidase gene fusions. Methods Cell Biol. 1991;34:61–75. doi: 10.1016/s0091-679x(08)61676-3. [DOI] [PubMed] [Google Scholar]
- 25.Manoil C, Bailey J. A simple screen for permissive sites in proteins: analysis of E. coli lac permease. J Mol Biol. 1997;267:250–263. doi: 10.1006/jmbi.1996.0881. [DOI] [PubMed] [Google Scholar]
- 26.Manoil C, Beckwith J. A genetic approach to analyzing membrane protein topology. Science. 1986;233:1403–1408. doi: 10.1126/science.3529391. [DOI] [PubMed] [Google Scholar]
- 27.Nilsson I, von Heijne G. Fine-tuning the topology of a polytopic membrane protein: role of positively and negatively charged amino acids. Cell. 1990;62:1135–1141. doi: 10.1016/0092-8674(90)90390-z. [DOI] [PubMed] [Google Scholar]
- 28.Okamoto S, Toyoda-Yamamoto A, Ito K, Takebe I, Machida Y. Localization and orientation of the VirD4 protein of Agrobacterium tumefaciens in the cell membrane. Mol Gen Genet. 1991;228:24–32. doi: 10.1007/BF00282443. [DOI] [PubMed] [Google Scholar]
- 29.Panicker M, Minkley E G. DNA transfer occurs during a cell surface contact stage of F sex factor-mediated bacterial conjugation. J Bacteriol. 1985;162:584–590. doi: 10.1128/jb.162.2.584-590.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Panicker M, Minkley E G. Purification and properties of the F sex factor TraD protein, an inner membrane conjugal transfer protein. J Biol Chem. 1992;267:12761–12766. [PubMed] [Google Scholar]
- 31.Prinz W, Beckwith J. Gene fusion analysis of membrane protein topology: a direct comparison of alkaline phosphatase and β-lactamase fusions. J Bacteriol. 1994;176:6410–6413. doi: 10.1128/jb.176.20.6410-6413.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sahin-Toth M, Dunten R, Kaback H R. Design of a membrane protein for site-specific proteolysis: properties of engineered factor Xa protease sites in the lactose permease of Escherichia coli. Biochemistry. 1995;34:1107–1112. doi: 10.1021/bi00004a001. [DOI] [PubMed] [Google Scholar]
- 33.Sasstre J I, Cabexon E, de la Cruz F. The carboxyl terminus of protein TraD adds specificity and efficiency to F-plasmid conjugative transfer. J Bacteriol. 1998;180:6039–6042. doi: 10.1128/jb.180.22.6039-6042.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seligman L. Sequence determinants of a simple membrane protein topology. Ph.D. thesis. Seattle, Wash: Department of Genetics, University of Washington; 1994. [Google Scholar]
- 35.Seligman L, Manoil C. An amphipathic sequence determinant of membrane protein topology. J Biol Chem. 1994;269:19888–19896. [PubMed] [Google Scholar]
- 35a.Traxler, B. Unpublished data.
- 36.Traxler B, Minkley E. Revised genetic map of the distal end of the F transfer operon: implications for DNA helicase I, nicking at oriT, and conjugal DNA transport. J Bacteriol. 1987;169:3251–3259. doi: 10.1128/jb.169.7.3251-3259.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Traxler B, Beckwith J. Assembly of a hetero-oligomeric membrane protein complex. Proc Natl Acad Sci USA. 1992;89:10852–10856. doi: 10.1073/pnas.89.22.10852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Traxler B, Boyd D, Beckwith J. The topological analysis of integral cytoplasmic membrane proteins. J Membr Biol. 1993;132:1–11. doi: 10.1007/BF00233047. [DOI] [PubMed] [Google Scholar]
- 39.von Heijne G. The distribution of positively charged residues in bacterial inner membrane proteins correlates with the trans-membrane topology. EMBO J. 1986;5:3021–3027. doi: 10.1002/j.1460-2075.1986.tb04601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Winans S C, Burns D, Christie P J. Adaptation of a conjugal transfer system for the export of pathogenic macromolecules. Trends Microbiol. 1996;4:64–68. doi: 10.1016/0966-842X(96)81513-7. [DOI] [PMC free article] [PubMed] [Google Scholar]