Abstract
Background
Anastomotic leak (AL) is a common but severe complication after oesophagectomy. It is unknown how to determine the severity of AL objectively at diagnosis. Determining leak severity may guide treatment decisions and improve future research. This study aimed to identify leak-related prognostic factors for mortality, and to develop a Severity of oEsophageal Anastomotic Leak (SEAL) score.
Methods
This international, retrospective cohort study in 71 centres worldwide included patients with AL after oesophagectomy between 2011 and 2019. The primary endpoint was 90-day mortality. Leak-related prognostic factors were identified after adjusting for confounders and were included in multivariable logistic regression to develop the SEAL score. Four classes of leak severity (mild, moderate, severe, and critical) were defined based on the risk of 90-day mortality, and the score was validated internally.
Results
Some 1509 patients with AL were included and the 90-day mortality rate was 11.7 per cent. Twelve leak-related prognostic factors were included in the SEAL score. The score showed good calibration and discrimination (c-index 0.77, 95 per cent c.i. 0.73 to 0.81). Higher classes of leak severity graded by the SEAL score were associated with a significant increase in duration of ICU stay, healing time, Comprehensive Complication Index score, and Esophagectomy Complications Consensus Group classification.
Conclusion
The SEAL score grades leak severity into four classes by combining 12 leak-related predictors and can be used to the assess severity of AL after oesophagectomy.
The Severity of oEsophageal Anastomotic Leak (SEAL) score was developed using data from the TENTACLE—Esophagus study, an international, multicentre retrospective cohort study including 1509 patients with anastomotic leak after oesophagectomy. The SEAL score was developed to determine anastomotic leak severity at diagnosis, and combines 12 leak-related parameters at diagnosis. The score may be useful in clinical practice and could improve future research.
Introduction
Anastomotic leak (AL) is a major complication after oesophagectomy, and leak rates of between 10 and 20 per cent have been reported1–3. Annually, an estimated 20 000 patients worldwide may develop AL after oesophagectomy1,4–6. AL is associated with increased postoperative morbidity, high postoperative mortality rates, prolonged hospital admission, and increased hospital costs1,7–10. In addition, it has been shown to decrease long-term quality of life and oncological survival11–13.
The clinical presentation of patients with AL is diverse and its impact ranges widely. Patients may or may not present with signs of systemic infection, contaminated fluid collections, and/or conduit necrosis. Currently, it is not known how to determine the severity of AL at diagnosis, but a better understanding of its severity may guide treatment strategies. Furthermore, a tool to assess leak severity could improve the comparability of future research and may be used to correct for leak severity. Therefore, a tool to assess the severity of AL at diagnosis is needed.
AL is currently classified according to the definition of the Esophagectomy Complications Consensus Group (ECCG)14. Aimed at improving benchmarking and consistent reporting of research outcomes, this classification is based on the invasiveness of the leak treatment (conservative, radiological or endoscopic intervention, and surgical intervention). However, this system does not reflect the severity of AL in terms of outcome. In addition, as the score is determined in hindsight by the treatment performed, this classification cannot guide treatment decisions in clinical practice. The ultimate measure of leak severity is the risk of death. AL severity should be determined by leak-related parameters that are available at diagnosis to enable adequate decision-making based on AL severity. However, only small and heterogeneous cohort studies1,9,15–19 have investigated which leak-related factors are associated with leak severity, and no comprehensive severity score has been developed.
The aims of this study were to identify leak-related prognostic factors for 90-day mortality in patients with AL after oesophagectomy with gastric tube reconstruction for cancer, and to develop a Severity of oEsophageal Anastomotic Leak (SEAL) score to classify the severity of AL at diagnosis in these patients.
Methods
Study design
TENTACLE—Esophagus (TreatmENT of AnastomotiC Leakage after Esophagectomy) is an international multicentre retrospective cohort study. Centres performing oesophageal cancer surgery were invited to participate and the study was performed in collaboration with the Dutch Upper Gastrointestinal Cancer Audit, Oesophago-Gastric Anastomosis Audit, and European Minimally Invasive Oesophagectomy Think Tank initiative. Participation was allowed irrespective of geography and patient volume. Seventy-one centres from 20 different countries participated in this study (Table S1).
The study protocol was approved by the institutional review board of Radboud University Medical Centre, and by local ethics committees of participating centres if additional approval was needed. The need for individual informed consent was waived owing to the retrospective study design and anonymous data collection. This study was performed according to TRIPOD guidelines20. The TENTACLE—Esophagus study is registered in the Clinical Trials registry (NCT03829098) and the full study protocol is accessible at https://www.tentaclestudy.com.
Population
Adults who developed AL after oesophagectomy with gastric tube reconstruction for resectable cancer of the oesophagus or gastro-oesophageal junction (cT1–4a N0–3 M0) between January 2011 and June 2019 were included consecutively. AL was defined as a ‘full-thickness gastrointestinal defect involving oesophagus, anastomosis, staple line or conduit, irrespective of presentation or method of identification’14. Patients who were diagnosed with AL after death or who underwent an emergency oesophagectomy or oesophagectomy for benign disease were excluded.
Data collection, verification, and quality validation
Data were collected by local investigators at each participating centre and recorded in an online database (www.castoredc.com). Data were pseudoanonymized and traceable patient data were stored locally.
To ensure robustness of data and to minimize the risk of incomplete patient inclusion, data verification and data quality validation were undertaken (Appendix S1). For data verification, an algorithm was developed to screen all data fields for inconsistent entries, typographical errors, and missing values. Data quality validation was performed by independent local validators to assess case ascertainment and data accuracy. Case ascertainment was assessed by comparing a centre's number of included patients with the expected number of leaks based on the centre's annual resection rate and expected minimum leak rate of 5 per cent. Data accuracy was assessed by retrieving 15 key parameters from medical records and comparing these with study data.
Outcome measures
The primary outcome was 90-day mortality, defined as death from any cause within 90 days after oesophagectomy. Mortality was used as a proxy for leak severity, and 90-day mortality was chosen because it reflects most complication-related deaths without including deaths unrelated to AL (such as early recurrence)21,22. Secondary outcomes were used to assess the clinical relevance of the SEAL score and included in-hospital, 30-day, and 180-day mortality, duration of ICU and hospital stay, time to leak healing (confirmed by imaging or clinically if patients were put on (at least) a non-clear liquid diet), Comprehensive Complication Index (CCI) score, and ECCG classification. The CCI represents the severity of all complications, ranging from 0 (no complications) to 100 (death)23. The ECCG classification consists of three leak types: type I, AL requiring medical, dietary or no therapy; type II, AL requiring reintervention but not surgical reintervention; and type III, requiring surgical therapy14.
Prognostic factors and predictors
Potential leak-related prognostic factors for 90-day mortality and predictors for the SEAL score were selected based on the literature and expert opinion. Although preoperative parameters (such as age or co-morbidity) may affect outcomes, these factors do not reflect the severity of the leak itself, and so were not included as predictors in the SEAL score. Grading of AL severity may be considered analogously to grading of tumours; although parameters such as age or co-morbidity affect outcomes and are often taken into account when making treatment decisions, the tumour itself is graded by its size and extent of dissemination24. Similarly, in the SEAL score, the leak itself is graded by leak-related parameters at diagnosis and preoperative parameters were not included in this score (Table 1).
Table 1.
Potential leak-related prognostic factors and SEAL score predictors
| Leak-related parameters | |
|---|---|
| Nasogastric tube at diagnosis | |
| Anastomotic reinforcement | |
| C-reactive protein | |
| SEAL score predictors | |
| POD of diagnosis* | |
| Level of care at diagnosis* | |
| Diet at diagnosis* | |
| Leucocyte count* | |
| Resection type* | |
| qSOFA score* | |
| Haemodynamic failure* | |
| Respiratory failure* | |
| Renal failure* | |
| Intrathoracic fluid collections* | |
| Defect circumference* | |
| Overall condition conduit* |
Leak-related parameters identified as prognostic factors after adjusting for confounders were included as predictors of the Severity of oEsophageal Anastomotic leak (SEAL) score to determine leak severity. POD, postoperative day; qSOFA, quick Sequential Organ Failure Assessment.
Statistical analysis
Multiple imputation using chained equations was used to reduce bias in results owing to missing data. Additional information on (handling of) missing data is presented in Appendix S2 and Table S225.
Prognostic factors for 90-day mortality were identified by assessing the effect of individual parameters on 90-day mortality. Crude and adjusted ORs and 95 per cent confidence intervals were estimated using univariable and multivariable logistic regression analyses. Confounding variables used for adjustment of each potential prognostic factor were selected using a directed acyclic graph (DAG) (Appendix S3 and Fig. S1)26.
The SEAL score was developed using multivariable logistic regression to determine the risk of 90-day mortality (Appendix S4). Leak-related parameters identified as prognostic factors after adjustment for confounders were included as predictors for the SEAL score. Predictors for the SEAL score were not selected using backwards selection as described in the online protocol, but were selected using the DAG approach as inclusion of causal predictors may benefit model performance27,28. The model was validated internally using bootstrapping techniques (500 replicates). Model coefficients were adjusted for the shrinkage factor found during bootstrapping. After internal validation, the model performance was re-evaluated. Model performance was assessed in terms of discrimination expressed as concordance index (c-index) and calibration using a calibration plot29. A sensitivity analysis was undertaken, assessing model performance in subgroups based on geographical location.
The SEAL score was divided into four classes of AL severity: mild, moderate, severe, and critical. Class cut-off values were predefined by the study group based on clinical judgement of AL severity in relation to the risk of 90-day mortality: mild, less than 5 per cent; moderate, ≥5–<15 per cent; severe, ≥15–<25 per cent; and critical, at least 25 per cent predicted risk of 90-day mortality. Secondary outcomes were compared between different classes of leak severity using the χ2 test for ordinal outcomes and one-way ANOVA for continuous outcomes. P < 0.050 was considered statistically significant. All statistical analyses were performed in each imputed data set and pooled subsequently using R version 3.6.2 with packages rms and mice (R Foundation for Statistical Computing, Vienna, Austria)30.
Results
Preoperative characteristics
A total of 1514 patients with AL were included in the database. Five patients were excluded owing to locally advanced disease (cT4b) (3 patients), oesophagojejunal reconstruction (1), and post-mortem diagnosis of AL (1). Preoperative characteristics of 1509 patients included in the analysis are shown in Table 2.
Table 2.
Preoperative patient characteristics
| Overall (n = 1509) | |
|---|---|
| Age (years), median (i.q.r.) | 66 (59–71) |
| Sex | |
| F | 287 (19.0) |
| M | 1222 (81.0) |
| BMI (kg/m2), median (i.q.r.) | 26 (23–29) |
| Missing | 123 (8.2) |
| Comorbidity | |
| ASA 1 | 143 (9.5) |
| ASA 2 | 845 (56.0) |
| ASA 3 | 469 (31.1) |
| ASA 4 | 19 (1.3) |
| Missing | 33 (2.2) |
| Performance status | |
| ECOG 0 | 666 (44.1) |
| ECOG 1 | 425 (28.2) |
| ECOG 2 | 88 (5.8) |
| ECOG 3 | 8 (0.5) |
| ECOG 4 | 3 (0.2) |
| Missing | 319 (21.1) |
| Tumour type | |
| Adenocarcinoma | 1088 (72.1) |
| SCC | 377 (25.0) |
| Other | 38 (2.5) |
| Missing | 6 (0.4) |
| Tumour location | |
| Cervical | 19 (1.3) |
| Upper thoracic | 54 (3.6) |
| Mid thoracic | 229 (15.2) |
| Lower thoracic | 779 (51.6) |
| GOJ | 418 (27.7) |
| Missing | 10 (0.7) |
| Clinical T-stage | |
| cTis | 17 (1.1) |
| cT1 | 169 (11.2) |
| cT2 | 298 (19.7) |
| cT3 | 926 (61.4) |
| cT4a | 48 (3.2) |
| cTx | 51 (3.4) |
| Clinical N-stage | |
| cN0 | 595 (39.4) |
| cN1 | 522 (34.6) |
| cN2 | 215 (14.2) |
| cN3 | 51 (3.4) |
| cN+ | 87 (5.8) |
| cNx | 39 (2.6) |
| Neoadjuvant treatment | |
| None | 301 (19.9) |
| Chemoradiotherapy | 352 (22.3) |
| Chemotherapy | 842 (55.8) |
| Radiotherapy | 6 (0.4) |
| Missing | 8 (0.5) |
Values are n (%) unless otherwise indicated. BMI, body mass index; ASA, American Society of Anesthesiologists; ECOG, Eastern Cooperative Oncology Group; SCC, squamous cell carcinoma; GOJ, gastro-oesophageal junction.
Data quality validation
Of the 71 participating centres, 9 (13 per cent) included a smaller sample than expected. The patient screening procedures at these centres were reviewed and were found appropriate in 8 centres. One centre, which included 3 patients, rescreened their records and included 3 additional patients. Of 1514 records, 182 (12.0 per cent) were validated for data accuracy assessment and overall data accuracy was 96.5 per cent (Appendix S1).
Leak-related parameters at diagnosis of anastomotic leak
The median postoperative day (POD) of diagnosis (defined as confirmation of AL by clinical, imaging or other assessment) was day 8 (i.q.r. 5–11) days after oesophagectomy. Most patients (56.3 per cent) had undergone transthoracic oesophagectomy with intrathoracic anastomosis; 27.0 per cent had undergone transthoracic oesophagectomy with cervical anastomosis, and 16.0 per cent transhiatal oesophagectomy. At diagnosis of AL, 57.1 per cent of patients were on the surgical ward and 36.0 per cent in a high-care department. AL was most often diagnosed by CT, drain fluids, oesophagography, and endoscopy (49.2, 17.2, 13.6, and 12.1 per cent respectively). Some 54.7 per cent of patients had intrathoracic fluid collections (i.e. drained or undrained, mediastinal or pleural), 16.3 per cent had cervical fluid collections, and 20.6 per cent had no fluid collections. A defect circumference of 25 per cent or more was observed in 12.8 per cent of patients. Overall conduit ischaemia/necrosis was found in 9.8 per cent (Table 3).
Table 3.
Leak-related parameters at diagnosis of anastomotic leak
| Leak parameter | Value | Leak parameter | Value |
|---|---|---|---|
| POD of diagnosis, median (i.q.r.) | 8 (5–11) | Leucocyte count (× 109/l), median (i.q.r.) | 12.3 (9.4, 16.1) |
| Missing | 140 (9.3) | Missing | 87 (5.8) |
| Diagnostic modality | CRP (mg/l), median (i.q.r.) | 190 (114, 278) | |
| Endoscopy | 182 (12.1) | Missing | 175 (11.6) |
| Oesophagography | 205 (13.6) | Haemodynamic failure | |
| CT | 743 (49.2) | No | 1226 (81.2) |
| Fluids from drain/wound | 260 (17.2) | Yes | 137 (9.1) |
| Reoperation | 23 (1.5) | Missing | 146 (9.7) |
| Other | 54 (3.6) | Respiratory failure | |
| None | 14 (0.9) | No | 1135 (75.2) |
| Missing | 28 (1.9) | Yes | 255 (16.9) |
| Resection type | Missing | 119 (7.9) | |
| TTO-CA | 407 (27.0) | Renal failure | |
| TTO-IA | 849 (56.3) | No | 1329 (88.1) |
| THO-CA | 241 (16.0) | Yes | 55 (3.6) |
| Missing | 12 (0.8) | Missing | 125 (8.3) |
| Resection approach | qSOFA score | ||
| Open | 528 (35.0) | 0 | 711 (47.1) |
| Hybrid, thoracoscopic | 73 (4.8) | 1 | 234 (15.5) |
| Hybrid, laparoscopic | 167 (11.1) | 2 | 97 (6.4) |
| TMIO | 664 (44.0) | 3 | 51 (3.4) |
| RAMIO | 69 (4.6) | Missing | 416 (27.6) |
| Missing | 8 (0.5) | Intrathoracic fluid collections | |
| Omental wrap | None | 519 (34.4) | |
| No | 986 (65.3) | Drained | 139 (9.2) |
| Yes | 380 (25.2) | Undrained | 686 (45.5) |
| Missing | 143 (9.5) | Missing | 165 (10.9) |
| Level of care at diagnosis | Defect circumference (%) | ||
| Surgical ward | 862 (57.1) | <25 | 570 (37.8) |
| ICU, MC, HC, PACU | 543 (36.0) | ≥25 | 193 (12.8) |
| ED, other | 61 (4.0) | Not available | 745 (49.4) |
| Missing | 43 (2.8) | Missing | 1 (0.0) |
| Diet at diagnosis | Overall conduit condition | ||
| No restriction | 168 (11.1) | Well perfused | 1084 (71.8) |
| Liquids | 371 (24.6) | Ischaemic/necrotic | 148 (9.8) |
| Water | 158 (10.5) | Not available | 276 (18.4) |
| Nil by mouth | 612 (40.6) | Missing | 1 (0.0) |
| Missing | 200 (13.3) |
Values are n (%) unless otherwise indicated. POD, postoperative day; CT, computed tomography; TTO, transthoracic oesophagectomy; CA, cervical anastomosis, IA, intrathoracic anastomosis; THO, transhiatal oesophagectomy; TMIO, total minimally invasive oesophagectomy; RAMIO, robot-assisted minimally invasive oesophagectomy; ICU, intensive care unit; MC, medium care; HC, high care; PACU, postanaesthesia care unit; ED, emergency department; CRP, C-reactive protein; qSOFA, quick Sequential Organ Failure Assessment.
Outcomes
The 90-day mortality rate was 11.7 per cent (176 deaths), and 30-day, 180-day and in-hospital mortality rates were 5.2, 15.9, and 10.7 per cent respectively. The median duration of hospital stay was 30 (i.q.r. 20–49) days and of ICU stay was 6 (2–15) days. The anastomotic defect healed in 1270 patients (84.2 per cent) and the median time to healing was 26 (i.q.r. 13–46) days. The median CCI score was 44 (i.q.r. 31–64). Of all patients, 27.3 per cent had an ECCG type I leak, 36.8 per cent a type II leak, and 36.0 per cent a type III leak.
Prognostic factors for mortality
Crude and adjusted ORs for possible prognostic factors for 90-day mortality are presented in Table S3. The following leak-related parameters at diagnosis of AL were identified as prognostic factors: early or late diagnosis of AL (before POD 5 and after POD 11), organ failure (respiratory failure, haemodynamic failure, renal failure or raised quick Sequential Organ Failure Assessment (qSOFA) score), admission to a high-care unit, leucopenia and leucocytosis, presence of intrathoracic fluid collections (drained and/or undrained), defect circumference at least 25 per cent, and overall conduit ischaemia/necrosis. AL after transhiatal oesophagectomy and a diet consisting of water only (versus nil by mouth, liquid or unrestricted diet) at diagnosis were associated with reduced 90-day mortality. Anastomotic reinforcement (omental wrap/pleural flap), nasogastric tube at diagnosis, and C-reactive protein (CRP) levels at diagnosis were not identified as leak-related prognostic factors.
SEAL score development and internal validation
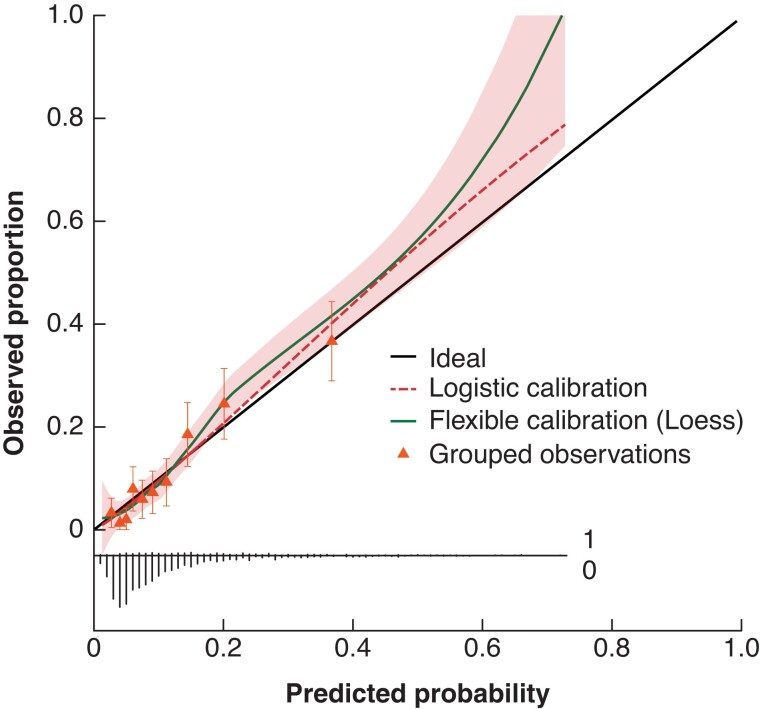
The SEAL score was developed by including all 12 identified prognostic leak-related parameters in a multivariable logistic regression model (Table 4). After internal validation, the c-index for the SEAL score was 0.77 (95 per cent c.i. 0.73 to 0.81) and the SEAL score showed good calibration (Fig. 1).
Table 4.
The SEAL score after internal validation
| Predictor | Odds ratio |
|---|---|
| POD of diagnosis* | 1.43 (0.89, 2.29) |
| Level of care at diagnosis | |
| Surgical ward | 1.00 (reference) |
| ICU, MC, HC, PACU | 1.83 (1.19, 2.82) |
| Other | 1.31 (0.52, 3.29) |
| Diet at diagnosis | |
| Unrestricted | 1.00 (reference) |
| Liquids | 0.87 (0.46, 1.65) |
| Water | 0.45 (0.17, 1.21) |
| Nil by mouth | 1.02 (0.54, 1.90) |
| qSOFA score (per point) | 1.21 (0.88, 1.66) |
| Respiratory failure | 1.53 (0.90, 2.60) |
| Haemodynamic failure | 1.14 (0.62, 2.13) |
| Renal failure | 2.48 (1.30, 4.73) |
| Leucocyte count* | 0.92 (0.70, 1.20) |
| Resection type | |
| TTO-CA | 1.00 (reference) |
| TTO-IA | 0.69 (0.46, 1.04) |
| THO-CA | 0.68 (0.37, 1.26) |
| Defect circumference ≥25% | 1.66 (1.08, 2.57) |
| Overall conduit ischaemia/necrosis | 1.20 (0.73, 1.98) |
| Intrathoracic collections | |
| None | 1.00 (reference) |
| Drained | 1.48 (0.78, 2.82) |
| Undrained | 1.52 (0.98, 2.37) |
Values in parentheses are 95 per cent confidence intervals. *Interquartile OR, i.q.r.: postoperative day (POD) of diagnosis 5–11 days, leucocyte count 9.3–16.1 × 109/l. SEAL, Severity of oEsophageal Anastomotic Leak; ICU, intensive care unit; MC, medium care; HC, high care; PACU, postanaesthesia care unit; qSOFA, quick Sequential Organ Failure Assessment; TTO, transthoracic oesophagectomy; CA, cervical anastomosis; IA, intrathoracic anastomosis; THO, transhiatal oesophagectomy.
Fig. 1.
Calibration plot of the SEAL score
The calibration slope was 1.12 (95 per cent c.i. 0.93 to 1.31) and resembles the strength of predictors. The calibration intercept was 0.00 (95 per cent c.i. –0.17 to 0.17), and resembles the ‘calibration in the large’ indicating whether the model systematically overpredicts or underpredicts. Discrimination: c-index 0.77 (95 per cent c.i. 0.73 to 0.81). The shaded area displays the 95 per cent confidence interval of the flexible calibration curve. The triangles plotted in the calibration curve represent observed proportion versus predicted probabilities for decile predictions, with error bars indicating 95 per cent confidence interval for a specific decile. The broom plot at the bottom shows the distribution of predicted probabilities for 90-day mortality in patients who did (1) or did not (0) die within 90 days. SEAL, Severity of oEsophageal Anastomotic Leak.
Outcomes for patients in different AL severity classes according to the SEAL score are summarized in Table 5, including an example of a patient in each class. The majority of patients (51.2 per cent) were classified as having a moderate AL; 25.4 per cent had mild, 13.4 per cent severe, and 10.1 per cent had critical leak. There was good alignment between the observed and predicted mortality rate within each leak severity class. Patients with greater leak severity had a statistically significantly longer duration of hospital (and ICU) stay, longer time to leak healing, higher mortality rate (in-hospital, 30-day, 90-day, and 180-day mortality), and higher CCI score. Furthermore, the percentage of ECCG type I leaks declined, whereas the percentage of ECCG type III leaks increased. Model performance was good among 1401 patients treated in European centres, but could not be assessed accurately in 108 patients treated in non-European centres owing to the limited number of patients (Fig. S2).
Table 5.
Pooled outcomes according to the SEAL score
| SEAL score (predicted risk of 90-day mortality) | ||||
|---|---|---|---|---|
| Mild (<5%) | Moderate (≥5–<15%) | Severe (≥15–<25%) | Critical (≥25%) | |
| Example | Patient diagnosed on surgical ward, on a water diet and with a small defect and viable conduit. No organ failure or intrathoracic fluid collections | Patient presenting with intrathoracic collections or a large defect, with leucocytosis and tachypnoea or hypotension | Patient diagnosed on POD 3 in ICU with single organ failure, together with intrathoracic fluid collections or a large anastomotic defect | Complex presentation, patient with multiple organ failure and combination of intrathoracic fluid collections, large defect and/or conduit necrosis |
| % of patients, range * | 25.4 (21–30) | 51.2 (47–56) | 13.4 (12–15) | 10.1 (9–11) |
| Predicted mortality risk (%), range*† | 3.6 (3–4) | 8.8 (8–9) | 19.2 (19–20) | 36.6 (36–38) |
| 90-day mortality (%), range *† | 3.0 (2–4) | 8.0 (7–9) | 23.5 (18–27) | 36.6 (33–40) |
| 30-day mortality (%), range*† | 0.5 (0–1) | 3.3 (3–4) | 8.3 (6–11) | 22.1 (20–24) |
| 180-day mortality (%), range*† | 5.8 (4–7) | 12.3 (11–13) | 28.5 (23–33) | 43.6 (39–47) |
| In-hospital mortality (%), range*† | 1.8 (1–3) | 7.2 (6–8) | 21.3 (16–25) | 37.6 (35–41) |
| Duration of hospital stay (mean, days), range*† | 27 (26–29) | 39 (38–41) | 52 (48–57) | 50 (48–53) |
| Duration of ICU stay (mean, days), range*† | 5 (4–5) | 11 (10–12) | 22 (19–24) | 28 (27–30) |
| Leak healing time (mean, days), range*† | 30 (28–32) | 35 (33–36) | 41 (36–48) | 43 (39–51) |
| CCI score (mean), range*† | 34 (33–35) | 46 (45–47) | 64 (62–66) | 77 (75–79) |
| ECCG classification (%), range*† | ||||
| Type I | 42.8 (40–48) | 28.1 (25–31) | 12.5 (9–16) | 3.5 (1–7) |
| Type II | 36.8 (34–40) | 38.7 (36–42) | 33.9 (28–38) | 30.4 (25–34) |
| Type III | 20.5 (17–23) | 33.2 (31–36) | 53.6 (50–60) | 66.1 (62–72) |
Range across imputations. SEAL, Severity of oEsophageal Anastomotic Leak; ICU, intensive care unit; POD, postoperative day; CCI, Comprehensive Complication index; ECCG, Esophagectomy Complications Consensus Group. †P < 0.010 (χ2 test for ordinal outcomes, 1-way ANOVA for continuous outcomes).
The internally validated SEAL score was incorporated into an online application, which enables determination of leak severity and is available at https://www.tentaclestudy.com/seal-score.
Discussion
The SEAL score classifies patients with AL into four classes of leak severity according to the predicted risk of 90-day mortality. The score combines 12 leak-related prognostic factors at diagnosis, and showed good performance after internal validation. A more severe leak according to the SEAL score was associated with a longer duration of hospital and ICU stay, longer healing time, higher CCI score, and higher ECCG grade, indicating that this score is relevant beyond 90-day mortality. This unique cohort enabled identification of prognostic factors, and development and internal validation of the SEAL score.
Some limitations should, however, be discussed. There is a risk of bias owing to patient selection, but the patients were consecutive and the data validated. Patients with subclinical AL may be under-represented as they may not have been identified during screening for eligible patients. Such patients may not require admission or treatment; this is not the targeted population for the SEAL score, and omission of these patients therefore has few consequences. A recent prospective international audit1, which included all patients undergoing oesophagectomy, found an AL rate of 14.2 per cent, with an AL mortality rate similar to that in the present study, indicating comparability of cohorts.
Appropriate measures were taken to ensure data quality; meticulous data verification was performed to ensure uniform data collection and data quality validation showed good data accuracy, similar to other surgical studies31–33. Multiple imputation was used to increase precision and avoid bias during the analysis25. Still, the high rate of unavailable data on defect circumference reflects current diagnostic strategies and limited reporting of endoscopic findings. The prognostic impact of defect circumference and conduit condition underscores the importance of endoscopy as a diagnostic assessment, and detailed reporting of imaging assessments may further improve the data quality of future studies. Even though the SEAL score was developed in a large international cohort and was validated internally, it is unknown whether the findings can be generalized29. Independent external validation of the SEAL score should be undertaken as model performance could not be assessed accurately in centres outside Europe. However, external validation requires an additional large and detailed data set, which is not currently available.
The study has identified an array of leak-related prognostic factors in patients with AL, highlighting the complex and multifactorial character of AL severity. The present findings have confirmed previous suggestions that defect circumference, intrathoracic fluid collections, and conduit condition have a high prognostic impact1,15,17–19. Patients with a diet consisting of water only at diagnosis were found to have better outcomes. As drinking water may help limit contamination, these patients could be less prone to developing sepsis. Interestingly, although CRP may have an important role in diagnosing AL34–36, the present findings indicated that CRP levels do not reflect AL severity at diagnosis. Contrary to popular belief, anastomotic reinforcement (omental wrap) was not found to reduce the severity of AL37–40. Transhiatal oesophagectomy was identified as a favourable prognostic factor, and the risk of developing intrathoracic manifestations may indeed be lower as no transthoracic resection is performed18,41,42. However, after transthoracic oesophagectomy no difference was found regarding outcomes of cervical and intrathoracic AL, supporting the recent ‘chute hypothesis’, proposing that thoracic dissection may increase the risk of intrathoracic manifestations in cervical AL19,42,43.
The SEAL score adopts a novel approach to determining AL severity by combining leak-related prognostic factors at diagnosis of AL. A higher SEAL score was associated with higher ECCG leak type, and the SEAL score expresses explicitly at diagnosis what the ECCG classification indicates implicitly once treatment has been completed14. The applicability of the ECCG classification is limited in clinical practice, whereas the SEAL score may be used to determine the severity of AL for individual patients. Moreover, use of the SEAL score may unify understanding of the impact of AL on patients, and surgeons, gastroenterologists and other physicians may monitor patients with severe or critical AL more closely. In the future, the SEAL score may guide treatment decisions, but its role is yet to be established. For example, patients with a higher SEAL score may benefit from more aggressive (surgical) treatment. For research purposes, the ECCG classification and the SEAL score may be complementary; the ECCG classification may be used to standardize reporting of AL treatments, and the SEAL score to report leak severity. Moreover, previous studies1,44 were unable to assess the efficacy of AL treatments owing to confounding bias, whereas the SEAL score can be used to adjust for leak severity and may consequently reduce bias.
Development of the SEAL score is the first step towards finding evidence for the optimal treatment of AL. Future analyses of TENTACLE—Esophagus will investigate the efficacy of leak treatment taking into account AL severity; assess the association between practice variation and outcomes; and aim to develop a prediction model incorporating the SEAL score and preoperative factors to accurately predict mortality risk based on patient characteristics and leak severity.
In conclusion, the SEAL score combines leak-related parameters at diagnosis and grades leak severity into four classes based on individual predictions of 90-day mortality; the score reflects morbidity in terms of duration of hospital (and ICU) stay, healing time, CCI score, and ECCG leak type. In clinical practice, the score is instrumental in determining the severity of AL at diagnosis and may guide clinical decision-making in the future. In research, the SEAL score may be used to report and adjust for the severity of AL after oesophagectomy.
Collaborators
TENTACLE—Esophagus Collaborative Group: E. Matthée, C. A. M. Slootmans, G. Ultee, J. Schouten (Radboud university medical centre, Nijmegen, The Netherlands); S. S. Gisbertz, W. J. Eshuis, M. C. Kalff, M. L. Feenstra (Amsterdam UMC, University of Amsterdam, Cancer Centre Amsterdam, Amsterdam, The Netherlands); D. L. van der Peet, W. T. Stam (AmsterdamUMC, location VUmc, Amsterdam, The Netherlands); B. van Etten, F. Poelmann, N. Vuurberg, J. W. van den Berg (University Medical Centre Groningen, University of Groningen, Groningen, The Netherlands); I. S. Martijnse, R. M. Matthijsen (Elisabeth-Tweesteden Ziekenhuis, Tilburg, The Netherlands); M. Luyer, W. Curvers, T. Nieuwenhuijzen (Catharina Ziekenhuis Eindhoven, Eindhoven, The Netherlands); A.k E. Taselaar (Erasmus medical centre, Rotterdam, The Netherlands); E. A. Kouwenhoven, M. Lubbers (Ziekenhuisgroep Twente, Almelo, The Netherlands); M. Sosef, F. Lecot, T. C. M. Geraedts (Zuyderland Medisch Centrum, Heerlen, The Netherlands); S. van Esser, J. W. T. Dekker (Reinier de Graaf Gasthuis, Delft, The Netherlands); F. van den Wildenberg (Canisius-Wilhelmina Ziekenhuis, Nijmegen, The Netherlands); W. Kelder, M. Lubbers, P. C. Baas, J. W. A. de Haas (Martini Ziekenhuis, Groningen, The Netherlands); H. H. Hartgrink, R. R. Bahadoer (Leiden University Medical Centre, Leiden, The Netherlands); J. W. van Sandick, K. J. Hartemink, X. Veenhof (Netherlands Cancer Institute - Antoni van Leeuwenhoek, Amsterdam, The Netherlands); H. Stockmann, B. Gorgec, P. Weeder (Spaarne Gasthuis, Haarlem, The Netherlands); M. J. Wiezer, C. M .S. Genders (St. Antonius Ziekenhuis, Nieuwegein, The Netherlands); E. Belt, B. Blomberg (Albert Schweitzer, Dordrecht, The Netherlands); P. van Duijvendijk, L. Claassen, D. Reetz (Gelre, Apeldoorn, The Netherlands); P. Steenvoorde, W. Mastboom, H. J. Klein Ganseij (Medisch Spectrum Twente, Enschede, The Netherlands); A. D. van Dalsen, A. Joldersma, M. Zwakman (Isala, Zwolle, The Netherlands); R. P. R. Groenendijk, M. Montazeri (IJsselland Ziekenhuis, Cappelle aan de Ijssel, The Netherlands); St. Mercer, B. Knight, G. van Boxel (Portsmouth Hospital University Trust, Portsmouth, United Kingdom); R. J. McGregor, R. J. E. Skipworth, C. Frattini, A. Bradley (University of Edinburgh, Royal Infirmary of Edinburgh, Edinburgh, United Kingdom); M. Nilsson, M. Hayami, B. Huang (Karolinska University Hospital, Stockholm, Sweden); J. Bundred, R. Evans (Queen Elizabeth Hospital, Birmingham, United Kingdom); P. P. Grimminger, P. C. van der Sluis, U. Eren (University Medical Centre Mainz, Mainz, Germany); J. Saunders, E. Theophilidou, Z. Khanzada (Nottingham University Hospitals NHS Trust, Nottingham, United Kingdom); J. A. Elliott, J. Ponten, S. King, J. V. Reynolds (Trinity St. James's Cancer Institute, Dublin, Ireland); B. Sgromo, K. Akbari, S. Shalaby (Churchill Hospital, Oxford University Hospitals, Oxford, United Kingdom); C. A Gutschow, H. Schmidt, D. Vetter (University Hospital Zurich, Zurich, Switzerland); K. Moorthy, M. A. H. Ibrahim, G. Christodoulidis (Imperial, London, United Kingdom); J. V. Räsänen, J. Kauppi, H. Söderström (Helsinki University Hospital, Helsinki University, Helsinki, Finland); D. K. Manatakis, D. P. Korkolis, D. Balalis, A. Rompu (Saint Savvas Cancer Hospital, Athens, Greece); B. Alkhaffaf, M. Alasmar, M. Arebi (Salford Royal NHS Foundation Trust, Division of Cancer Sciences, University of Manchester, Manchester, United Kingdom); G. Piessen, F. Nuytens, S. Degisors (University Lille, Claude Huriez University Hospital, CHU de Lille, Lille, France); A. Ahmed, A. Boddy, S. Gandhi, O. Fashina (University of Leicester, Leicester, United Kingdom); E. Van Daele, P. Pattyn (Ghent University Hospital, Ghent, Belgium); W. B. Robb, M. Arumugasamy, M. Al Azzawi, J. Whooley (Beaumont Hospital, Dublin, Ireland); E. Colak, E. Aybar, A. C. Sari, M. S. Uyanik, A. B. Ciftci (Samsun Training and Research Hospital, Samsun, Turkey); R. Sayyed, B. Ayub, G. Murtaza, A. Saeed, P. Ramesh (Patel Hospital, Karachi, Pakistan); A. Charalabopoulos, T. Liakakos, D. Schizas, E. Baili, A. Kapelouzou (Laiko General Hospital, Athens, Greece); M. Valmasoni, E. S. Pierobon, G. Capovilla, S. Merigliano (University Hospital of Padova, Padova, Italy); C. Silviu, B. Rodica, A. Florin, R. Cristian Gelu, H. Petre (Sf. Maria Hospital, Bucharest, Romania); R. Guevara Castro, A. F. Salcedo (Clinica Universitaria Colombia, Bogota, Colombia); I. Negoi, V. M. Negoita, C. Ciubotaru, B. Stoica, S. Hostiuc (Carol Davila University of Medicine and Pharmacy Bucharest, Emergency Hospital of Bucharest, Bucharest, Romania); N. Colucci, S. P. Mönig, C. H. Wassmer, J. Meyer (Geneva University Hospitals, Geneva, Switzerland); F. R. Takeda, R. A. Aissar Sallum, U. Ribeiro, I. Cecconello (University of Sao Paulo, Sao Paulo, Brazil); E. Toledo, M. S. Trugeda, M. J. Fernández, C. Gil, S. Castanedo (Valdecilla Hospital, Santander, Spain); A. Isik, E. Kurnaz (Erzincan Binali Yildirim University, Erzincan, Turkey); J. F. Videira, M. Peyroteo, R. Canotilho (Instituto Português de Oncologica, Porto, Portugal); J. Weindelmayer, S. Giacopuzzi, C. A. De Pasqual (Verona University Hospital, Verona, Italy); M. Bruna, F. Mingol, J. Vaque, C. Pérez (La Fe Hospital, Valencia, Spain); A. W. Phillips, J. Chmelo, J. Brown, L. E. Han (Royal Victoria Infirmary, Newcastle upon Tyne, United Kingdom); J. A. Gossage, A. R. Davies, C. R. Baker, M. Kelly, M. Saad (Guy's and St Thomas' Hospitals, London, United Kingdom); D. Bernardi, L. Bonavina, E. Asti, C. Riva, R. Scaramuzzo (University of Milan, Milan, Italy); M. Elhadi, H. Abdelkarem Ahmed, A. Elhadi, F. A. Elnagar, A. A. A. Msherghi (Tripoli University Hospital, Tripoli, Libya); V. Wills, C. Campbell, M. Perez Cerdeira, S. Whiting (John Hunter New England LHD, Newcastle, Australia); N. Merrett, A. Das, C. Apostolou, A. Lorenzo (South Western Sydney Local Health District, Sydney, Australia); F. Sousa, J. Adelino Barbosa, V. Devezas, E. Barbosa, C. Fernandes (Centro Hospitalar Universitário São João, Oporto, Portugal); G. Smith, E. Y. Li, N. Bhimani, P. Chan, K. Kotecha (Royal North Shore Hospital, Sydney, Australia); M. W. Hii, S. M. Ward, M. Johnson, M. Read, L. Chong (St Vincent’s Hospital, Melbourne, Australia); M. J. Hollands, M. Allaway, A. Richardson, E. Johnston, A. Z. L. Chen (Westmead Hospital Hospital, Sydney, Australia); H. Kanhere, S. Prasad, P. McQuillan, T. Surman (Royal Adelaide Hospital, Adelaide, Australia); M. I. Trochsler, W. A. Schofield, S. K. Ahmed, J. L. Reid, M. C. Harris (The Queen Elizabeth Hospital, Adelaide, Australia); S. Gananadha, J. Farrant, N. Rodrigues, J. Fergusson (Canberra Hospital, Canberra, Australia); A. Hindmarsh, Z. Afzal, P. Safranek, V. Sujendran, S. Rooney (Addenbrooke’s hospital, Cambridge, United Kingdom); C. Loureiro, S. Leturio Fernández, I. Díez del Val (University Hospital Basurto, Bilbao, Spain); S. Jaunoo, L. Kennedy, A. Hussain (Brighton & Sussex University Hospitals, Brighton, United Kingdom); D. Theodorou, T. Triantafyllou, C. Theodoropoulos, T. Palyvou (Hippocration hospital, Athens, Greece); M. Elhadi, F. Abdullah Ben Taher, M. Ekheel, A. A. A. Msherghi (Sabratha National Cancer Institute, Sabratha, Libya).
Supplementary Material
Acknowledgements
S.U. and M.V. contributed equally as first authors; F.v.W and C.R. contributed equally as senior authors. The authors thanks all those involved in the TENTACLE—Esophagus study for their contribution to this large international study. The TENTACLE—Esophagus study was registered in the Clinical Trials registry (NCT03829098) before the start of the study. Study data are not openly available. The authors are willing to share data upon reasonable request to the corresponding authors.
Contributor Information
Sander Ubels, Department of Surgery, Radboud Institute for Health Sciences, Radboud University Medical Centre, Nijmegen, the Netherlands.
Moniek Verstegen, Department of Surgery, Radboud Institute for Health Sciences, Radboud University Medical Centre, Nijmegen, the Netherlands.
Bastiaan Klarenbeek, Department of Surgery, Radboud Institute for Health Sciences, Radboud University Medical Centre, Nijmegen, the Netherlands.
Stefan Bouwense, Department of Surgery, Maastricht University Medical Centre+, Maastricht, the Netherlands.
Mark van Berge Henegouwen, Department of Surgery, Amsterdam UMC, Cancer Centre Amsterdam, University of Amsterdam, Amsterdam, the Netherlands.
Freek Daams, Department of Surgery, Amsterdam UMC, Cancer Centre Amsterdam, University of Amsterdam, Amsterdam, the Netherlands.
Marc J van Det, Department of Surgery, ZGT hospital group, Almelo, the Netherlands.
Ewen A Griffiths, Department of Upper Gastrointestinal Surgery, University Hospitals Birmingham NHS Foundation Trust, Queen Elizabeth Hospital Birmingham, Birmingham, UK; Institute of Cancer and Genomic Sciences, College of Medical and Dental Sciences, University of Birmingham, Birmingham, UK.
Jan W Haveman, Department of Surgery, University Medical Centre Groningen, University of Groningen, Groningen, the Netherlands.
Joos Heisterkamp, Department of Surgery, Elisabeth-TweeSteden Hospital, Tilburg, the Netherlands.
Renol Koshy, Department of Surgery, Newcastle upon Tyne Hospital NHS Trust, Newcastle upon Tyne, UK; Department of Surgery, University Hospitals of Coventry and Warwickshire NHS Trust, Coventry, UK.
Grard Nieuwenhuijzen, Department of Surgery, Catharina Hospital, Eindhoven, the Netherlands.
Fatih Polat, Department of Surgery, Canisius-Wilhelmina Hospital, Nijmegen, the Netherlands.
Peter D Siersema, Department of Gastroenterology and Hepatology, Radboud Institute for Health Sciences, Radboud University Medical Centre, Nijmegen, The Netherlands.
Pritam Singh, Department of Surgery, Nottingham University Hospitals NHS Trust, Nottingham, UK; Department of Surgery, Regional Oesophago-Gastric Unit, Royal Surrey County Hospital, Guildford, UK.
Bas Wijnhoven, Department of Surgery, Erasmus University Medical Centre, Rotterdam, the Netherlands.
Gerjon Hannink, Department of Operating Rooms, Radboud Institute for Health Sciences, Radboud University Medical Centre, Nijmegen, The Netherlands.
Frans van Workum, Department of Surgery, Radboud Institute for Health Sciences, Radboud University Medical Centre, Nijmegen, the Netherlands; Department of Surgery, Canisius-Wilhelmina Hospital, Nijmegen, the Netherlands.
Camiel Rosman, Department of Surgery, Radboud Institute for Health Sciences, Radboud University Medical Centre, Nijmegen, the Netherlands.
the TENTACLE—Esophagus Collaborative Group:
E Matthée, C A M Slootmans, G Ultee, J Schouten, S S Gisbertz, W J Eshuis, M C Kalff, M L Feenstra, D L van der Peet, W T Stam, B van Etten, F Poelmann, N Vuurberg, J W van den Berg, I S Martijnse, R M Matthijsen, M Luyer, W Curvers, T Nieuwenhuijzen, A K E Taselaar, E A Kouwenhoven, M Lubbers, M Sosef, F Lecot, T C M Geraedts, S van Esser, J W T Dekker, F van den Wildenberg, W Kelder, M Lubbers, P C Baas, J W A de Haas, H H Hartgrink, R R Bahadoer, J W van Sandick, K J Hartemink, X Veenhof, H Stockmann, B Gorgec, P Weeder, M J Wiezer, C M S Genders, E Belt, B Blomberg, P van Duijvendijk, L Claassen, D Reetz, P Steenvoorde, W Mastboom, H J Klein Ganseij, A D van Dalsen, A Joldersma, M Zwakman, R P R Groenendijk, M Montazeri, St Mercer, B Knight, G van Boxel, R J McGregor, R J E Skipworth, C Frattini, A Bradley, M Nilsson, M Hayami, B Huang, J Bundred, R Evans, P P Grimminger, P C van der Sluis, U Eren, J Saunders, E Theophilidou, Z Khanzada, J A Elliott, J Ponten, S King, J V Reynolds, B Sgromo, K Akbari, S Shalaby, C A Gutschow, H Schmidt, D Vetter, K Moorthy, M A H Ibrahim, G Christodoulidis, J V Räsänen, J Kauppi, H Söderström, D K Manatakis, D P Korkolis, D Balalis, A Rompu, B Alkhaffaf, M Alasmar, M Arebi, G Piessen, F Nuytens, S Degisors, A Ahmed, A Boddy, S Gandhi, O Fashina, E Van Daele, P Pattyn, W B Robb, M Arumugasamy, M Al Azzawi, J Whooley, E Colak, E Aybar, A C Sari, M S Uyanik, A B Ciftci, R Sayyed, B Ayub, G Murtaza, A Saeed, P Ramesh, A Charalabopoulos, T Liakakos, D Schizas, E Baili, A Kapelouzou, M Valmasoni, E S Pierobon, G Capovilla, S Merigliano, C Silviu, B Rodica, A Florin, R Cristian Gelu, H Petre, R Guevara Castro, A F Salcedo, I Negoi, V M Negoita, C Ciubotaru, B Stoica, S Hostiuc, N Colucci, S P Mönig, C H Wassmer, J Meyer, F R Takeda, R A Aissar Sallum, U Ribeiro, I Cecconello, E Toledo, M S Trugeda, M J Fernández, C Gil, S Castanedo, A Isik, E Kurnaz, J F Videira, M Peyroteo, R Canotilho, J Weindelmayer, S Giacopuzzi, C A De Pasqual, M Bruna, F Mingol, J Vaque, C Pérez, A W Phillips, J Chmelo, J Brown, L E Han, J A Gossage, A R Davies, C R Baker, M Kelly, M Saad, D Bernardi, L Bonavina, E Asti, C Riva, R Scaramuzzo, M Elhadi, H Abdelkarem Ahmed, A Elhadi, F A Elnagar, A A A Msherghi, V Wills, C Campbell, M Perez Cerdeira, S Whiting, N Merrett, A Das, C Apostolou, A Lorenzo, F Sousa, J Adelino Barbosa, V Devezas, E Barbosa, C Fernandes, G Smith, E Y Li, N Bhimani, P Chan, K Kotecha, M W Hii, S M Ward, M Johnson, M Read, L Chong, M J Hollands, M Allaway, A Richardson, E Johnston, A Z L Chen, H Kanhere, S Prasad, P McQuillan, T Surman, M I Trochsler, W A Schofield, S K Ahmed, J L Reid, M C Harris, S Gananadha, J Farrant, N Rodrigues, J Fergusson, A Hindmarsh, Z Afzal, P Safranek, V Sujendran, S Rooney, C Loureiro, S Leturio Fernández, I Díez del Val, S Jaunoo, L Kennedy, A Hussain, D Theodorou, T Triantafyllou, C Theodoropoulos, T Palyvou, M Elhadi, F Abdullah Ben Taher, M Ekheel, and A A A Msherghi
Funding
The TENTACLE—Esophagus study received funding from Medtronic. The study was performed independently, and Medtronic had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript.
Disclosure. All authors declare funding from Medtronic for the submitted work. P.S. reports grants from The Enose Company, grants and other from Motus GI, grants from Pentax, grants from Micro-Tech, and other from Boston Scientific, outside the submitted work; B.K. reports grants from Medtronic and ZonMw, outside the submitted work; M.v.B.H. reports other from Mylan, other from Alesi Surgical, other from Johnson and Johnson, other from BBraun, other from Medtronic, grants from Olympus, and grants from Stryker, outside the submitted work; all fees unrelated to submitted work, paid to institution. The authors declare no other conflict of interest.
Supplementary material
Supplementary material is available at BJS online.
References
- 1. Oesophago-Gastric Anastomosis Study Group; West Midlands Research Collaborative . Rates of anastomotic complications and their management following esophagectomy: results of the Oesophago-Gastric Anastomosis Audit (OGAA). Ann Surg 2022;275:382–390 [DOI] [PubMed] [Google Scholar]
- 2. van Workum F, Verstegen MHP, Klarenbeek BR, Bouwense SAW, van Berge Henegouwen MI, Daams F et al. Intrathoracic vs cervical anastomosis after totally or hybrid minimally invasive esophagectomy for esophageal cancer: a randomized clinical trial. JAMA Surg 2021;156:601–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kuppusamy MK, Low DE; International Esodata Study Group . Evaluation of international contemporary operative outcomes and management trends associated with esophagectomy: a 4-year study of > 6000 patients using ECCG definitions and the online Esodata database. Ann Surg 2020;275:515–525 [DOI] [PubMed] [Google Scholar]
- 4. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424 [DOI] [PubMed] [Google Scholar]
- 5. Dutch Upper Gastrointestinal Cancer Audit . Core Figures 2015–2019 (Basis Tabel 2015–2019). Leiden: Dutch Institute for Cancer Auditing, 2020
- 6. Netherlands Cancer Registry (NCR) . NCR Figures (NKR Cijfers). Netherlands Comprehensive Cancer Organisation (IKNL), 2019. https://iknl.nl/nkr-cijfers (accessed 10 December 2021)
- 7. Biere SSAY, van Berge Henegouwen MI, Maas KW, Bonavina L, Rosman C, Garcia JR et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012;379:1887–1892 [DOI] [PubMed] [Google Scholar]
- 8. Goense L, Meziani J, Ruurda JP, van Hillegersberg R. Impact of postoperative complications on outcomes after oesophagectomy for cancer. Br J Surg 2018;106:111–119 [DOI] [PubMed] [Google Scholar]
- 9. Turkyilmaz A, Eroglu A, Aydin Y, Tekinbas C, Muharrem Erol M, Karaoglanoglu N. The management of esophagogastric anastomotic leak after esophagectomy for esophageal carcinoma. Dis Esophagus 2009;22:119–126 [DOI] [PubMed] [Google Scholar]
- 10. Lubbers M, Workum F, Berkelmans G, Rosman C, Luyer M, Nieuwenhuijzen G et al. Variations in treatment of an anastomotic leakage after Ivor Lewis esophagectomy. J Clin Images Med Case Rep 2021;2:1417 [Google Scholar]
- 11. Fransen LFC, Berkelmans GHK, Asti E, van Berge Henegouwen MI, Berlth F, Bonavina L et al. The effect of postoperative complications after minimally invasive esophagectomy on long-term survival: an international multicenter cohort study. Ann Surg 2021;274:e1129–e1137 [DOI] [PubMed] [Google Scholar]
- 12. Derogar M, Orsini N, Sadr-Azodi O, Lagergren P. Influence of major postoperative complications on health-related quality of life among long-term survivors of esophageal cancer surgery. J Clin Oncol 2012;30:1615–1619 [DOI] [PubMed] [Google Scholar]
- 13. Scarpa M, Saadeh LM, Fasolo A, Alfieri R, Cagol M, Cavallin F et al. Health-related quality of life in patients with oesophageal cancer: analysis at different steps of the treatment pathway. J Gastrointest Surg 2013;17:421–433 [DOI] [PubMed] [Google Scholar]
- 14. Low DE, Alderson D, Cecconello I, Chang AC, Darling GE, D'Journo XB et al. International consensus on standardization of data collection for complications associated with esophagectomy: Esophagectomy Complications Consensus Group (ECCG). Ann Surg 2015;262:286–294 [DOI] [PubMed] [Google Scholar]
- 15. Manghelli JL, Ceppa DP, Greenberg JW, Blitzer D, Hicks A, Rieger KM et al. Management of anastomotic leaks following esophagectomy: when to intervene? J Thorac Dis 2019;11:131–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guo J, Chu X, Liu Y, Zhou N, Ma Y, Liang C. Choice of therapeutic strategies in intrathoracic anastomotic leak following esophagectomy. World J Surg Oncol 2014;12:402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alanezi K, Urschel JD. Mortality secondary to esophageal anastomotic leak. Ann Thorac Cardiovasc Surg 2004;10:71–75 [PubMed] [Google Scholar]
- 18. Korst RJ, Port JL, Lee PC, Altorki NK. Intrathoracic manifestations of cervical anastomotic leaks after transthoracic esophagectomy for carcinoma. Ann Thorac Surg 2005;80:1185–1190 [DOI] [PubMed] [Google Scholar]
- 19. van Rossum PSN, Haverkamp L, Carvello M, Ruurda JP, van Hillegersberg R. Management and outcome of cervical versus intrathoracic manifestation of cervical anastomotic leakage after transthoracic esophagectomy for cancer. Dis Esophagus 2017;30:1–8 [DOI] [PubMed] [Google Scholar]
- 20. Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMC Med 2015;13:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. In H, Palis BE, Merkow RP, Posner MC, Ferguson MK, Winchester DP et al. Doubling of 30-day mortality by 90 days after esophagectomy: a critical measure of outcomes for quality improvement. Ann Surg 2016;263:286–291 [DOI] [PubMed] [Google Scholar]
- 22. Talsma AK, Lingsma HF, Steyerberg EW, Wijnhoven BP, Van Lanschot JJ. The 30-day versus in-hospital and 90-day mortality after esophagectomy as indicators for quality of care. Ann Surg 2014;260:267–273 [DOI] [PubMed] [Google Scholar]
- 23. Slankamenac K, Graf R, Barkun J, Puhan MA, Clavien PA. The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg 2013;258:1–7 [DOI] [PubMed] [Google Scholar]
- 24. Rice TW, Patil DT, Blackstone EH. 8th edition AJCC/UICC staging of cancers of the esophagus and esophagogastric junction: application to clinical practice. Ann Cardiothorac Surg 2017;6:119–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Van Buuren S. Flexible Imputation of Missing Data. Boca Raton, FL: CRC Press, 2018 [Google Scholar]
- 26. Shrier I, Platt RW. Reducing bias through directed acyclic graphs. BMC Med Res Methodol 2008;8:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Thornley S. Causation and statistical prediction: perfect strangers or bedfellows? J Biometric Biostat 2012;3 [Google Scholar]
- 28. Heinze G, Dunkler D. Five myths about variable selection. Transpl Int 2017;30:6–10 [DOI] [PubMed] [Google Scholar]
- 29. Collins GS, de Groot JA, Dutton S, Omar O, Shanyinde M, Tajar A et al. External validation of multivariable prediction models: a systematic review of methodological conduct and reporting. BMC Med Res Methodol 2014;14:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wood AM, Royston P, White IR. The estimation and use of predictions for the assessment of model performance using large samples with multiply imputed data. Biometric J 2015;57:614–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. van der Werf LR, Busweiler LAD, van Sandick JW, van Berge Henegouwen MI, Wijnhoven BPL. Reporting national outcomes after esophagectomy and gastrectomy according to the Esophageal Complications Consensus Group (ECCG). Ann Surg 2020;271:1095–1101 [DOI] [PubMed] [Google Scholar]
- 32. STARSurg Collaborative . Impact of postoperative non-steroidal anti-inflammatory drugs on adverse events after gastrointestinal surgery. Br J Surg 2014;101:1413–1423 [DOI] [PubMed] [Google Scholar]
- 33. Bhangu A, Ademuyiwa AO, Aguilera ML, Alexander P, Al-Saqqa SW, Borda-Luque G et al. Surgical site infection after gastrointestinal surgery in high-income, middle-income, and low-income countries: a prospective, international, multicentre cohort study. Lancet Infect Dis 2018;18:516–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Barbaro A, Eldredge TA, Shenfine J. Diagnosing anastomotic leak post-esophagectomy: a systematic review. Dis Esophagus 2021;34:doaa076 [DOI] [PubMed] [Google Scholar]
- 35. Aiolfi A, Asti E, Rausa E, Bonavina G, Bonitta G, Bonavina L. Use of C-reactive protein for the early prediction of anastomotic leak after esophagectomy: systematic review and Bayesian meta-analysis. PLoS One 2018;13:e0209272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liesenfeld LF, Sauer P, Diener MK, Hinz U, Schmidt T, Muller-Stich BP et al. Prognostic value of inflammatory markers for detecting anastomotic leakage after esophageal resection. BMC Surg 2020;20:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sepesi B, Swisher SG, Walsh GL, Correa A, Mehran RJ, Rice D et al. Omental reinforcement of the thoracic esophagogastric anastomosis: an analysis of leak and reintervention rates in patients undergoing planned and salvage esophagectomy. J Thorac Cardiovasc Surg 2012;144:1146–1151 [DOI] [PubMed] [Google Scholar]
- 38. Zhou D, Liu QX, Deng XF, Zheng H, Lu X, Dai JG et al. Anastomotic reinforcement with omentoplasty reduces anastomotic leakage for minimally invasive esophagectomy with cervical anastomosis. Cancer Manag Res 2018;10:257–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zheng QF, Wang JJ, Ying MG, Liu SY. Omentoplasty in preventing anastomotic leakage of oesophagogastrostomy following radical oesophagectomy with three-field lymphadenectomy. Eur J Cardiothorac Surg 2013;43:274–278 [DOI] [PubMed] [Google Scholar]
- 40. Chen X, Liu S, Chen P, He H, Wang F. Application of pleural flaps in laparoscopic–thoracoscopic esophagectomy for esophageal cancer. J Thorac Dis 2020;12:973–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. van Heijl M, van Wijngaarden AKS, Lagarde SM, Busch ORC, van Lanschot JJB, van Berge Henegouwen MI. Intrathoracic manifestations of cervical anastomotic leaks after transhiatal and transthoracic oesophagectomy. Br J Surg 2010;97:726–731 [DOI] [PubMed] [Google Scholar]
- 42. Verstegen MHP, Slaman AE, Klarenbeek BR, van Berge Henegouwen MI, Gisbertz SS, Rosman C et al. Outcomes of patients with anastomotic leakage after transhiatal, Mckeown or Ivor Lewis esophagectomy: a nationwide cohort study. World J Surg 2021;45:3341–3349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Blewett CJ, Miller JD, Young JE, Bennett WF, Urschel JD. Anastomotic leaks after esophagectomy for esophageal cancer: a comparison of thoracic and cervical anastomoses. Ann Thorac Cardiovasc Surg 2001;7:75–78 [PubMed] [Google Scholar]
- 44. Verstegen MHP, Bouwense SAW, van Workum F, Ten Broek R, Siersema PD, Rovers M et al. Management of intrathoracic and cervical anastomotic leakage after esophagectomy for esophageal cancer: a systematic review. World J Emerg Surg 2019;14:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.