Abstract
The dysregulation of lipid metabolism has emerged as a central component of many neurodegenerative diseases. Variants of the lipid transport protein, Apolipoprotein E (APOE), modulate risk and resilience for several neurodegenerative diseases including late-onset Alzheimer’s disease. APOE variants directly alter the lipid metabolism of cells and tissues and have been broadly associated with several other cellular and systemic phenotypes. Targeting APOE-associated metabolic pathways may offer opportunities to alter disease-related phenotypes and consequently, attenuate risk for and impart resilience against multiple neurodegenerative diseases. Here we review the molecular, cellular, and tissue-level alterations to lipid metabolism that arise from different APOE isoforms. These changes to lipid metabolism could help us understand disease mechanisms and tune neurodegenerative disease risk and resilience.
Keywords: Apolipoprotein E, lipid metabolism, neurodegenerative disease, Alzheimer’s disease
Lipid metabolism is a central player in neurodegenerative diseases
Lipids serve vital functions as membrane components, scaffolding molecules, and signaling molecules in every cell. Since the brain is the second-most lipid-rich organ [1,2], it is unsurprising that altered lipid homeostasis plays an important role in neurodegenerative diseases. In fact, the first described case of Alzheimer’s disease (AD) included the observation of aberrant lipid accumulation within glia [3]. Lipid dysregulation is also implicated in other late-onset neurodegenerative diseases like Parkinson’s disease (PD) and Lewy Body Dementia (LBD) [4-6]. Mutations in lipid-processing pathways also cause severe early-onset neurodegenerative diseases such as Tay-Sachs disease, Batten’s disease, Sandhoff disease, and Neimann-Pick disease [7]. Changes in lipid homeostasis have been linked to neuroinflammation, a central element of the pathology of many neurodegenerative diseases [8,9]. Several genetic risk factors for common neurodegenerative diseases like AD, PD, and LBD include lipid metabolism genes like APOE, CLU, INPP5D, ABCA7, TREM2, PLCG2, and GBA [10-12]. In this review, we detail not only how APOE variants perturb cellular and systemic lipid metabolism, but also how these perturbations may underlie and modulate risk for many neurological diseases.
APOE is a ubiquitous apolipoprotein
The human gene APOE encodes Apolipoprotein E (APOE), a ubiquitous lipid transport protein that binds a variety of lipid species, including cholesterol, phospholipids, and triglycerides in lipoprotein particles [13]. APOE transports lipids into cells via different cell surface receptors. Its mechanism of action with the low-density lipoprotein receptor (LDLR) is well described [14] while novel APOE receptors are emerging [15,16]. APOE is present in both the periphery and the central nervous system (CNS). In the brain, it is the most abundant lipoprotein as quantified by mass spectrometry [17].
In the periphery, APOE is primarily produced by hepatocytes and circulates in plasma [18]. In the CNS, APOE is mostly expressed in glia, which are key cellular mediators of metabolic homeostasis. Astrocytes, which modulate neuronal communication and metabolic health, produce the most APOE in the CNS, followed by microglia, the primary resident immune cells in the brain [19]. Other cell types in both the periphery (like macrophages and adipocytes) and CNS (like oligodendrocytes and pericytes) have been shown to produce APOE as well [20,21]. Although neurons do not produce an appreciable amount of APOE under basal conditions, studies have noted that neuronal stress and injury induce APOE expression [22-24].
APOE alleles modify disease risk
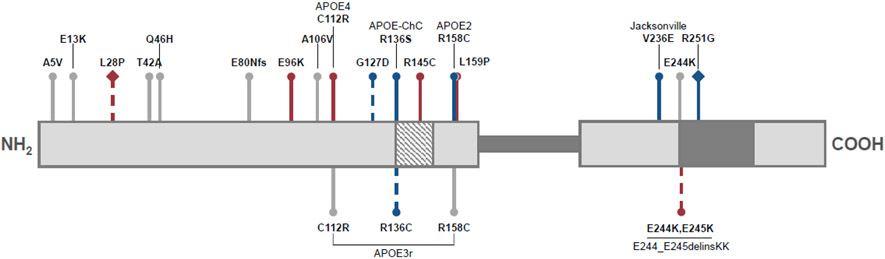
In humans, APOE exists in three major alleles – APOE2, APOE3, and APOE4 – which differ from each other at two amino acid positions (Figure 1, Box 1). APOE3 (rs7412 C / rs429358 T; C112 / R158) is the most common allele (allelic frequency of 78% in Caucasian populations) and is a neutral allele with respect to neurodegenerative disease risk [25]. APOE4 (rs7412 C /rs429358 C; R112 / R158) has an allelic frequency of 14% in Caucasian populations and is the strongest genetic risk factor for late-onset Alzheimer’s disease (LOAD). LOAD risk increases with APOE4 gene dose [26,27]. In contrast, APOE2 (rs7412 T / rs429358 T; C112 / C158) is present at 8% in Caucasian populations. APOE2 carriers display decreased risk for LOAD. The APOE alleles have strong associations with the key pathological hallmarks of LOAD, amyloid-beta and phosphorylated tau [28]. Although the association between APOE alleles and LOAD risk and protection exists across different ancestral backgrounds, the strength of the association varies (Box 2) [29,30]. Deep sequencing efforts and small familial studies have uncovered rare coding variants of APOE [31,32]. Some of these, like the Christchurch (rs121918393 ; R135S), Jacksonville (rs199768005; V236E), and R251G (rs26760666) variants [33-37], also have strong associations with resilience to LOAD while many others are of unknown clinical significance with respect to neurological diseases.
Figure 1. Numerous nonsynonymous APOE mutations modify neurological disease risk.
The mature APOE protein consists of two primarily alpha-helical domains (light grey sections), connected by an intrinsically disordered hinge region (dark grey bar; residues 200-215). The N-terminal domain contains the low-density lipoprotein receptor binding site (diagonal stripes; residues 136-150) while the C-terminal domain contains sites for lipid binding (dark grey; residues 244-272). In relation to APOE3 (C112; R158), risk mutations are depicted in red, protective mutations in blue, and mutations of unknown neurological effect are in grey. Dashed lines indicate predicted or mild directional effects. Diamond-headed pins reflect variants on an APOE4 (C112R) background. This diagram was adapted from data in the Alzforum APOE mutations database. Abbreviations: fs represents a frameshift mutation, ChC stands for Christchurch, delins is a deletion-insertion mutation.
BOX 1: Evolution of the APOE gene.
Although many vertebrate species have an APOE gene, current sequencing data suggests that, while it is polymorphic in humans, it is monomorphic in closely-related nonhuman primates [161]. In fact, APOE4 is the ancestral human allele with closely related variants present in non-human primates. Given that many neurodegenerative diseases occur late in life (post-reproductively), human APOE4 is unlikely to be under negative reproductive selection pressure. APOE3 and subsequently APOE2 evolved less than 200,000 years ago [162]. It is postulated that APOE2 and APOE3 were selected for since they confer advantages to changing lifestyles and diets – especially those including higher dietary lipids due to meat consumption [163]. The varied ability of different APOE isoforms to bind and internalize lipids could provide a molecular correlate for this hypothesis.
Despite its effects on disease risk, APOE4 is still maintained as the second most common allele in humans. Multiple studies have suggested that APOE4 may confer selective immune advantages early in life [164-167]. Given the intimate relationship between lipid metabolism and immune function, perhaps this connection occurs through APOE alleles impact on lipids [77,168,169]. Understanding the evolution of allelic diversity will better illuminate APOE’s effect on lipid metabolism and disease risk, at different life stages and ancestral backgrounds.
BOX 2: Effects of ancestry and sex on disease-associations of APOE alleles.
Most research on APOE alleles and LOAD genetics has been performed on Northern-European populations. Smaller studies in populations with diverse ancestral backgrounds have revealed that APOE4 allele frequency differs among different populations. Thus, while APOE4 is found in 14% of Caucasian American individuals, it is found in up to 40% of African Americans, 37% in Oceania, and 26% in Australia. In southern Asia and Europe the APOE4 allelic frequency is less than 10% whereas in Northern Europe it rises up to 25% [29,170-173]. The epidemiological effects of APOE alleles also vary by population. For example, in Korea, Japan, and Japanese-American populations, APOE4 confers a greater risk for LOAD than in Caucasian population [30]. In contrast, for Native Americans, Hispanic Americans, African Americans, and those with African ancestry, APOE4 is associated with lower LOAD risk than in Caucasian-American populations [30,174-177]. A recent study in a Chinese population demonstrated that APOE3 is more protective than APOE2 [178]. Some of these ancestry-specific effects have been attributed to the APOE haplotype [174,176]. One recent study has discovered a novel locus (19q13.31) that may contribute to the attenuation of APOE4-mediated AD risk in African Americans [179]. The diversity in epidemiological data demonstrates that APOE4-mediated risk can be mitigated, and discovery of the underlying mechanisms can present ancestry-specific strategies for therapeutic intervention.
Sex differences can also impact the association of APOE alleles and LOAD risk. Females are nearly twice as likely to be diagnosed with AD than males [180]. Although overall there are no clear clinical differences in AD incidence between male and female APOE4 carriers, a recent study demonstrated that the susceptibility of female APOE4 carriers may occur earlier than in males [181]. Further studies of APOE4 patients suggest that these sex differences may be related to different metabolite profiles [114].
Given the global rise of neurodegenerative disease and the variation in population demographics of APOE alleles, it is vital that future research addresses not only the genetic implications of ancestral background and sex but also the intersection of these variables on disease.
Although increased LOAD risk is the most notable consequence of APOE4, this allele also confers risk to many other neurological conditions, including LBD [11], poor recovery from traumatic brain injury [38,39], cerebral amyloid angiopathy [40-43], chronic traumatic encephalopathy [44], and chemotherapy-induced cognitive impairment [45-47]. APOE4, when compared to APOE3, is also associated with increased risk for non-neurological conditions like hypercholesterolemia, hypertriglyceridemia, cardiovascular disease, as well as severe and long COVID-19 [48-53]. Independent of its association with LOAD protection, APOE2, is also associated with longevity [54]. However, APOE2, when compared to APOE3, has been found to increase risk for hypercholesteremia, cardiovascular diseases, macular degeneration, and certain cancers [55-58]. In fact, a recent study of samples from the UK Biobank discovered that APOE alleles were associated with risk for a multitude of conditions including obesity, liver disease, chronic airway obstruction, and type-2 diabetes [29,59,60].
The extensive association between APOE alleles and disparate diseases suggests that APOE regulates general cellular processes. Recent studies have connected APOE alleles to differential effects on many central biological pathways like lipid homeostasis, glucose utilization [61], inflammation [62-64], cellular trafficking [65-67], and mitochondrial function [68]. These disruptions occur in many cell types and reveal the profound and pleiotropic effect of APOE variants on diverse aspects of cell biology. Given that lipid disruptions are present in multiple neurodegenerative diseases, that APOE alleles have distinct phenotypic consequences on multiple cell types, and that APOE binds and distributes lipids within and between cells, we hypothesize that APOE impacts cellular phenotypes and disease risk via its effects on lipid metabolism.
Perturbed lipid metabolism is one potential mechanism for how APOE influences neurodegenerative disease risk. Other hypotheses, including those that implicate APOE isoforms in altering protein aggregation and deposition, have had more years of exploration and evidence to support them. The hypothesis that effects on lipid metabolism may unify the pleotropic phenotypes of various APOE isoforms is emergent and requires more research to establish its primary mechanisms and causative effects on disease risk. Nevertheless, it is an attractive hypothesis given the broad influence of lipid metabolism on multiple areas of cell health; modulating lipid metabolism may be used to attenuate APOE-mediated risk for multiple diseases.
Researchers have studied the effects of APOE isoforms on amyloid-beta in the context of AD for many years [69-72]. Recent genetic and functional studies have allowed the field to recognize the impact of APOE isoforms on disease risk beyond amyloid-beta and AD. Mass spectrometry advances have enabled the quantitative profiling of many lipid species in various disease models. The confluence of the identification of genetic risk factors, the underexplored potential of targeting lipid state as a therapeutic option for neurodegenerative diseases, and new tools to study metabolites have all resulted in rising interest in understanding the biology of lipids in neurodegenerative disease. APOE, with its strong genetic and functional associations with many disease states, is at the heart of this interest. Although the research in this area is in its early stages, we discuss below what is known about how APOE isoforms impact lipid metabolism and neurodegenerative disease-associated disruptions in cellular systems, model organisms, and patient tissue.
Cellular effects of APOE isoforms on lipid metabolism
Impacts on Intracellular Metabolism
Multiple studies connect APOE4 and the accumulation of intracellular cholesterol (Table 1). Cholesterol accumulates in human induced pluripotent stem cell (iPSC)-derived APOE4 homozygous astrocytes and microglia [63,64,66], as well as in the endoplasmic reticulum of oligodendrocytes [20]. Both increased de novo synthesis [73] as well as reduced efflux [63,66] have been implicated in APOE4-associated cholesterol accumulation. A recent study on the epidemiologically-protective APOE3-Jacksonville variant suggested that its protection arises, in part, from its ability to promote cholesterol efflux [36]. Many studies have correlated changes in cholesterol biosynthesis, efflux, and retention to LOAD-associated phenotypes. Cholesterol accumulation in APOE4 astrocytes has been associated with decreased lysosomal function [66], increased pro-inflammatory cytokine production [66], increased matrisome signaling [73], impaired uptake of amyloid beta [64], and dysregulated cytoskeletal dynamics [66]. These correlations suggest that aberrant cholesterol levels may be central to neurodegenerative disease risk. Further work will be necessary to define a causal and mechanistic link between APOE4, glial cholesterol accumulation, and LOAD-associated pathologies.
Table 1.
APOE allele-associated metabolic phenotypes in different cell types and their relationships to neurodegeneration
| Cell Type | APOE isoforms examined |
Model System | Metabolic Phenotype | Relationship to neurodegeneration | References |
|---|---|---|---|---|---|
| Astrocytes | ApoE4, ApoE3 | human iPSC-derived astrocytes | ApoE4 leads to decreased cholesterol efflux and increased intracellular cholesterol accumulation compared to ApoE3 | Correlates with impaired lysosomal function, increased pro-inflammatory cytokine production, impaired amyloid-beta clearance, actin cytoskeletal defects | [64,66,73] |
| ApoE4 leads to increased triglyceride accumulation and lipid droplet formation compared to ApoE3 | Increased sensitivity to fatty acid lipotoxicity | [76] | |||
| ApoE4 leads to decreased oxygen consumption and increased glycolysis compared to ApoE3 | Correlates with pro-inflammatory astrocyte state | [196] | |||
| primary astrocyte culture from humanized APOE mouse | ApoE4 leads to more lipid droplet accumulation upon lipid challenge compared to ApoE3 | Decreased ability to oxidize excess fatty acids, increased sensitivity to lipotoxicity | [75] | ||
| ApoE3, ApoE3-V236E | AAV-ApoE treated amyloid mouse model, primary mouse astrocytes | ApoE3-V236E results in greater cholesterol efflux and better lipidation than ApoE3 | Correlates with reduced amyloid plaque load | [36] | |
| Microglia | ApoE4, ApoE3 | human iPSC-derived microglia | ApoE4 leads to triglyceride accumulation, lipid droplet formation, and cholesterol accumulation compared to ApoE3 | Results in microglial activation, neuroinflammation, decreased neuronal activity | [63,73,76] |
| primary microglia from humanized APOE mouse | ApoE4 leads to decreased oxidative phosphorylation, increased glycolysis compared to ApoE3 | Associated with increased microglial reactivity and similar transcriptome to disease-associated microglia | [62] | ||
| Oligodendrocytes | ApoE4, ApoE3 | human iPSC-derived oligodendrocytes | ApoE4 leads to cholesterol, triglyceride, diglyceride accumulation compared to ApoE3 | Leads to impaired axon myelination | [20] |
| human post-mortem tissue | ApoE4 leads to cholesterol accumulation, lipid droplet accumulation compared to ApoE3 | Associated with impaired myelination | [20] |
In addition to cholesterol, APOE4 homozygosity results in accumulation of triglycerides within astrocytes and microglia (Table 1). Many of these studies report the storage of triglycerides within lipid droplets. Lipid droplets are organelles that bud from the ER membrane into the cytosol, and are composed of a protein-functionalized phospholipid monolayer that encapsulates a neutral lipid core [74]. In astrocytes from humanized APOE4 mice as well as in iPSC-derived astrocytes and microglia, APOE4 induces the formation of triglyceride-rich lipid droplets [63,75,76]. Although it remains unclear why APOE4 glia accumulate lipid droplets, one study finds that APOE4 astrocytes exhibit a decreased rate of fatty acid beta-oxidation [75], suggesting a role for decreased lipid catabolism. Lipid droplets are commonly thought to buffer cells against cytotoxic lipids (reviewed in [74]). The presence of abundant lipid droplets in APOE4 glia under basal conditions may suggest that this buffering mechanism is impaired or overwhelmed, thus rendering APOE4 glia more sensitive to lipotoxic insults [76]. In both microglia and astrocytes, APOE4-induced triglyceride accumulation is associated with a proinflammatory state [62,63,73]. Lipid-associated proinflammatory states in microglia have also been implicated in neurodegenerative disease and aging models [77]. Current research links APOE4-associated triglyceride accumulation to disrupted cellular functions of stress tolerance and neuroinflammation. Further work will help understand how these disrupted cellular functions contribute to increased neurodegenerative disease risk.
APOE4 exacerbates AD-associated phenotypes (amyloid-beta and tau accumulation, vascular defects, neuroinflammation, behavioral phenotypes) in other non-glial human iPSC-derived cell types [20,73,78], and mouse models [79-82]. These include pericytes (part of the blood brain barrier; [21,83,84]), neurons [78,82], as well as neurovascular immune cells [80]. Although these diverse cell types are involved in neurodegenerative disease risk and progression, future work is needed to establish how changes to intracellular lipid metabolism impact their cellular function.
Effects on neuron-glia interactions
APOE isoforms can impact neuronal health through modulating the exchange of lipids between glia and neurons (Figure 2). Here we will examine impacts of APOE isoform on interactions between neuron and various glial cells—astrocytes, microglia, and oligodendrocytes.
Figure 2. APOE isoforms impact communication between glia and neurons.
(A) Astrocytes provide metabolic support to neurons by supplying neurons with lipids such as cholesterol and metabolizing lipid species secreted by neurons. These activities are impaired by APOE4. (B) APOE4, but not APOE3, impairs calcium signaling in both microglia and neurons. APOE4 microglia also accumulate triglycerides, exhibit decreased purinergic signaling, and secrete proinflammatory cytokines. (C) Oligodendrocytes myelinate neurons. APOE4 oligodendrocytes display lower viability and intracellular cholesterol accumulation which leads to decreased myelination capacity. This figure was created using Biorender.com.
Lipid metabolism mediates neuron-astrocyte interactions
APOE genotype has a strong effect on neuron-astrocyte interactions. Work in Drosophila and mouse primary culture models established the role of APOE in transporting toxic peroxidated lipids and excess triglyceride-rich lipid droplets from neurons to astrocytes for degradation [85-87]. Human APOE4 impairs the transport of toxic lipids as well as astrocytic lipolysis [85,87,88]. Deficient clearance of lipids is correlated with increased oxidative stress, mitochondrial dysfunction, bioenergetic defects, and hampered synaptic transmission in neurons [88]. Neurotoxicity can also be induced by reactive astrocytes, an important component of the neuroinflammatory response in many neurodegenerative diseases [89,90]. APOE and clusterin-coated saturated fatty acid-rich particles are the mediators of reactive astrocyte-induced neurotoxicity [91]. How different APOE variants impact this function is undetermined.
Neurons also require a large amount of cholesterol and depend on astrocytes and APOE for adequate supply [92,93]. As the primary lipoprotein in the CNS, APOE delivers cholesterol to neurons. Independently, alteration of neuronal cholesterol levels has been implicated in LOAD-associated tau and amyloid-beta pathology [94]. The role of APOE in distribution of cholesterol may offer a direct link between APOE4 and LOAD pathology.
Lipid metabolism mediates neuron-microglia interactions
The interactions between microglia and neurons are also modulated by APOE4-associated lipid dysregulation. TAG-rich lipid droplet accumulation was found to induce a proinflammatory state in microglia in mouse models [77]. APOE4 homozygous microglia that accumulate triglycerides and cholesterol display a proinflammatory phenotype, exhibit reduced homeostatic purinergic signaling, and impact neuronal activity [63]. Direct modulation of the microglial lipid droplet levels using acyl-coA synthase inhibitors impacts both microglial neuroinflammation and neuronal activity [63]. These findings mechanistically link perturbed microglial lipid metabolism and neuronal function. Further work in degenerative disease models will be needed to confirm whether these cellular-level modulation of lipids can impact disease progression.
Lipid metabolism mediates neuron-oligodendrocyte interactions
APOE4-influences myelination in cellular, mouse, and human tissue. Mixed and matched co-cultures of iPSC-derived neurons and oligodendrocytes of APOE3 and APOE4 homozygous genotypes identified that aberrant cholesterol accumulation in APOE4 oligodendrocytes resulted poor myelination of cocultured neurons [20]. This same study showed deficient myelination in APOE4 humanized mice compared to their APOE3 counterparts. Another study in humanized APOE mice suggested that APOE4 impacts the viability of the oligodendrocytes themselves [95]. Both outcomes were loss-of function effects and result in poor myelination of axons, which has been associated with poor neuronal transmission and connectivity. A third study in a APOE4 homozygous tauopathy mouse model identified a gain of toxic function effect of neuronal APOE4 in causing myelination defects as well as decreasing the number of oligodendrocyte and oligodendrocyte precursor cells [82]. Interestingly, these effects were specific to a tauopathy mouse model suggesting that the interaction between APOE4 genotype and tau pathology may have specific effects on myelination.
The studies described above have started to unravel the complex relationship between APOE isoforms, their effects on glial lipid metabolism and the consequent cell non-autonomous effects. Further mechanistic studies are needed to truly decipher how modulation of APOE-associated lipid defects impacts disease risk and progression.
APOE isoforms impact lipoproteins and lipid transport
In the CNS, APOE resides on high density lipoprotein (HDL) particles whereas in the periphery it binds both HDL and very low density lipoproteins (VLDLs) [96,97]. The nature of the HDL particles has been reported to impact neurodegenerative disease phenotypes. One study reported that high density lipoproteins (HDLs) increase vascular amyloid deposition in in vitro models and HDLs containing APOE exacerbate that effect [83]. Another study of human plasma lipoproteins reported lower APOE content within HDL particles in patients with at least one APOE4 allele [98]. Moreover, the same study observed that patients with lower levels of APOE in their plasma or within HDL particles performed worse on cognitive function tests. These and other studies suggest that lipoprotein particle composition may impact neurodegenerative disease phenotypes and further study is necessary to understand the mechanisms that underlie these correlations.
APOE is lipidated in the secretory pathway of the cell by a lipid efflux protein, ABCA1 [99,100]. The lipidation status of APOE isoforms is different, with APOE4 reported to be most poorly lipidated [101,102]. Consequently, APOE isoforms have been shown to alter lipoprotein size and type—APOE4 is associated with smaller HDL particles than APOE3, which, in turn, binds smaller particles than APOE2 [103-105].The lipid species bound in lipoproteins are dictated by the lipid content of the cell type of origin and the APOE isoform present [98]. APOE isoforms are also found to differentially regulate the levels of proteins involved in lipidation, revealing a cyclical relationship between lipidation and APOE genotype [19]. Deficient APOE4 lipidation may compromise the ability to export excess lipids from cells [106-108] and alter the amount and nature of lipids transported to neurons and the vasculature, thereby modulating essential neuronal functions like synaptic connectivity and vascular integrity [109-111]. Additionally, APOE4 protein levels are observed to be lower compared to APOE3 levels within and secreted from multiple cell types and in plasma [64,112]. This may further exacerbate impaired lipid efflux.
A few studies have characterized the plasma, CSF, and brain lipidome of APOE4 carriers compared to non-carriers. One such study of 58 individuals identified 6 plasma metabolites (including lysophospholipids and cardiolipin) that were downregulated in APOE4 carriers [113]. Another study of the plasma metabolome of APOE4 carriers identified a preference for aerobic glycolysis [61]. A larger study of over 1500 individuals also found a few metabolites, including many phosphatidylcholines, significantly associated with both APOE genotype and sex [114]. The variability between these studies is likely attributable to differences in the subjects profiled as well as in the metabolomic coverage achieved. Changes in peripheral metabolites could serve as risk or resilience biomarkers, however, more work must be done to understand how blood metabolites reflect CNS APOE-associated processes (Box 3).
BOX 3: APOE-dependent, peripheral effects on the CNS.
It has been long thought that the CNS and peripheral pools of APOE do not mix [182,183]. However, recent studies have revealed that the two pools influence each other. This may occur through direct leakage via a more permeable blood brain barrier [84] or through indirect effects to systemic metabolism and signaling. In humanized APOE knock-in mouse models and those crossed to tau models, knocking out peripheral APOE impacts brain pathology while restoring peripheral APOE in a knockout organism can rescue cognitive defects associated with APOE loss of function [184,185]. In another amyloid mouse model, removal of peripheral APOE had less of an effect on brain pathology [186]. Together, these mixed results suggest systemic crosstalk, perhaps through metabolite exchange, where peripheral APOE impacts brain pathology. However, how interactions between peripheral APOE and brain APOE affect pathology may be specific to which model is used.
Dietary changes have also been shown to impact the CNS in an APOE isoform-dependent manner. For example, in APOE3 but not APOE4 humanized mouse models, high fat diets have been shown to increase both gliosis and neuronal immediate-early gene expression [187]. In younger adults, obesity predisposes APOE4 individuals to greater cognitive decline when they get older [188]. On the contrary, in older adults, obesity in APOE4 carriers result in slower cognitive decline [189]. These and other studies reveal the complex interplay between diet and CNS impacts of APOE4 alleles.
Additional studies in mice have demonstrated that APOE genotype can influence the gut microbiome [190,191] and vice versa – that alterations in the gut microbiome can also influence the role of APOE4 in tau pathology [192,193]. The mechanisms of this association are sex-dependent and may operate through systemic metabolic alterations [194]. Similar observations have been seen in humans [195]. However, the specific effects on gut microbiota and their consequences on neurodegenerative disease pathology is an area ripe for future exploration.
APOE mediated lipid metabolism perturbations in the brain
While cell type-specific and pairwise interactions can shed light on mechanistic relationships between glia and neurons, tissue-level studies enable the investigation of the regional and systemic effects of APOE.
Studies profiling lipids in an APOE knockout mouse model revealed APOE as a key regulator of multiple lipid metabolic pathways, including accumulation of cholesterol esters in glia [115,116]. Transcriptomic and lipidomic profiling in humanized APOE targeted replacement mice revealed both cell type-specific, brain regional, and plasma differences in lipid metabolism [117,118]. These studies identified APOE4-associated decrease in free fatty acid levels, increases in many TCA cycle metabolites as well as plasma changes in both phosphatidylcholines and unsaturated fatty acids. Another study applied lipidomics to an amyloid mouse model harboring various humanized APOE alleles and identified key brain region-specific lipid changes that differentiate areas vulnerable to early AD pathology (like the entorhinal cortex) and those that are more resistant to amyloid pathology (like the primary visual cortex) [119]. In the entorhinal cortex, the authors discovered an APOE4-associated decrease in diacylglycerol levels concomitant with increases in sphingolipids and bis(monoacylglycerol)phosphates. Studies like these nominate lipids as potential markers of vulnerability or resilience of different cell types and brain regions. Much work remains to understand the neurological consequences of lipid profile changes and the mechanisms by which they contribute to disease risk, resilience, and selective vulnerability of brain regions.
CSF profiling in APOE-genotyped individuals revealed some genotype-specific metabolites and the influence of peripheral dietary lipids [120]. One metabolomic characterization of post-mortem brain tissue from both healthy individuals and AD patients reported that APOE4 resulted in detectable sterol and sphingolipid disruption specifically in AD patients [121]. Other metabolomic characterizations of post-mortem human brain tissue have failed to identify metabolites significantly associated with the APOE4 risk genotype [20,122], though they do hint at trends in increased cholesterol esters, unsaturated lipids, and a few sphingomyelin species. This finding suggests that the variability of post-mortem brain tissue samples requires much higher sample sizes, profiling of comparable brain regions, and control of acquisition conditions to generate reproducible genotype-specific lipid signatures.
Advances in mass spectrometry imaging (MALDI-MSI) techniques have enabled the spatial tracing of metabolites. Recent studies have employed a combination of omics techniques and MALDI-MSI imaging to reveal that APOE4 microglia in humanized mouse models show a significant metabolic shift contributing to disease pathology [62]. Studies like these pave the way for relating metabolic changes on a cell type and regional level to disease progression and pathology, and will help bridge the gap to better understand whole organism effects of APOE alleles (Box 3)
Targeting APOE-associated disruptions to lipid metabolism
Given the pronounced effects of APOE alleles on disease risk and lipid metabolism, modulation of lipid metabolism is an attractive prospect to attenuate risk and impart resilience to disease. Many of these genotype-targeted approaches are still in early, preclinical stages and require significantly more basic research to truly understand their underlying mechanisms and broader effects.
Depletion of APOE as a therapeutic approach has emerged from work in mouse models. In transgenic mice expressing human P301S tau and human APOE4, astrocyte-specific genetic depletion of APOE4 reduced phosphorylated tau deposition and tau-mediated neurodegeneration, reduced glial activation signatures, and rescued cognitive defects [123]. Another study using a similar model found that selective depletion of APOE4 from neurons likewise decreased phosphorylated tau deposition and neurodegeneration, improved oligodendrocyte progenitor cell-mediated myelin degeneration, and was associated with a shift of astrocytic and microglial populations from disease-associated to disease-protective profiles [82]. Taken together, these studies suggest that there are toxic gain-of-function mechanisms of APOE4 in multiple cell types that may have cell autonomous and non-autonomous effects. However, there are many other studies that point to the loss-of-function effects of APOE4, especially in lipid binding (reviewed above). It will be important to understand the relative balance between loss-of-function and gain-of-toxic-function in the context of neurodegeneration in human systems before APOE4 depletion can be a viable preventative strategy.
A clinical trial has recently begun to test whether adeno-associated virus (AAV)-mediated expression of APOE2 in the CNS can alleviate AD-associated metrics in APOE4 homozygous individuals with mild and moderate dementia (https://clinicaltrials.gov/ct2/show/NCT03634007). The initial phase of the trial is focused on evaluating adverse events and toxicity. The trial is based on preclinical evidence in mouse models that showed reduction in brain amyloid levels upon APOE2 AAV injection, especially at lower levels of preexisting pathology [124,125]. If successful, this trial would provide precedent that risk-to-resilience conversion can be accomplished through exogenous genotype supplementation.
The best-explored avenue to modulate APOE-associated lipid metabolism is by activating retinoid X receptor (RXR)/liver X receptor (LXR) signaling. The RXR/LXR signaling axis controls the biosynthesis of lipids through the expression of many genes, including APOE and ABCA1 [126,127]. Activation of the RXR/LXR pathway can promote APOE4 lipidation, partly compensating for its inherently deficient lipidation [128]. RXR/LXR agonists like bexarotene (an FDA approved drug for certain cancers) have shown promise in reversing APOE4 lipidation defects and consequent cognitive defects in mice [129,130]. However, bexarotene has shown disparate results in different mouse models [131-133] suggesting that its mechanism of action is complex and may result in pleiotropic effects [134]. Two clinical trials explored the effect of bexarotene on both healthy individuals and AD patients [135,136]. These small trials showed no clear cognitive benefit, mixed results regarding changes to pathology, and increased the risk of adverse events. Other RXR/LXR agonists have also proceeded to phase 1 clinical trials but many have not progressed further due to adverse effects. These include increased triglyceride levels, decreases in neutrophils in humans, and hepatic steatosis in mouse models [137,138].
Peroxisome proliferator activated receptors (PPARs) are another class of nuclear receptors that form heterodimers with RXRs to drive expression of several lipid-associated genes, including ABCA1 and APOE. Activation of PPAR-gamma signaling with the agonist rosiglitazone has been associated with an improvement in learning and memory deficits and cerebrovascular function in several transgenic mouse models of AD [139-141]. However, multiple clinical trials to investigate safety and efficacy of rosiglitazone and pioglitazone therapy showed no clear benefit to either APOE4 carriers or APOE4 non-carriers while inducing the detrimental side effect of oedema [142-144].
A more nuanced approach to targeting APOE lipidation is via the activation of ABCA1, the transport protein responsible for lipidating APOE. For example, CS-6253 is an ABCA1 agonist that mimics the C-terminal domain of APOE itself. Such ABCA1 agonists have shown reduced AD pathology and cognitive deficits in an APOE4 mouse model [145]. However, ABCA1 agonists also alter the lipoprotein distribution in the plasma. Studies on CS-6253 in rodents and primates have shown effects in altering peripheral lipoprotein distribution and non-significant trends in CSF amyloid-beta 42 reduction, but do not impact CSF lipoproteins [146]. These approaches are still in preclinical stages. They do offer more precise alternatives to RXR/LXR and PPAR-gamma modulation, both which increase efflux through ABCA1, but result in unwanted hepatic lipogenesis.
Given the excess cholesterol accumulation in multiple APOE4-associated contexts, modulation of cholesterol has also been explored as an APOE4-targeted therapeutic. In a recent study, hydroxypropyl beta cyclodextrin, a cholesterol-depleting drug, was fed to APOE4 mice resulting in fewer myelination defects and more favorable performance on behavioral tests [20]. Many trials have been performed to evaluate whether statins (which inhibit cholesterol biosynthesis) ameliorate APOE4-associated phenotypes. APOE genotype-stratified trials revealed that APOE4 carriers show some reduced disease progression and cognitive decline when on statins compared to other APOE genotypes [147], further highlighting the importance of the APOE genotype-lipid metabolism axis in neurological disease.
Other potential therapeutic candidates have emerged from cellular studies. Choline supplementation was shown to reduce lipid burden in APOE4 astrocytes [76]. Choline is a readily available nutritional supplement with few side effects even when taken at high doses (https://ods.od.nih.gov/factsheets/Choline-Consumer/). It also displays general neuroprotective effects in multiple mouse model studies [148-151] and has shown benefit in humans as well [152]. The benign nature of choline supplementation presents an attractive option as an APOE-specific preventative intervention. Although choline has been tested as a general AD therapeutic with mixed results [153-155], many of these studies were not conducted on APOE genotype-stratified patients. Those trials that did stratify on APOE genotype did note greater efficacy in APOE4 carriers [156]. Further work on genotype-specific mechanisms of neuroprotection needs to occur to establish choline as a promising modulator of APOE4-mediated risk.
Given that APOE4 is a disease risk factor, not a causative gene, a genotype-specific preventative treatment has to be highly tolerable and have a biomarker [157,158] that can indicate prevention of disease onset or progression.
Concluding Remarks & Future Directions
Over 100 years ago Alois Alzheimer initially observed glial lipid accumulation in a post-mortem AD brain [3]. Today, we recognize the central role lipid metabolism plays in APOE-mediated risk and resilience for neurodegenerative diseases. Studying the role of APOE alleles in neurodegenerative diseases independent of disease-specific pathology can enable the discovery of common nodes and targetable pathways for many conditions. Cell biology studies in cultured glia have revealed cell type-specific mechanisms and consequences of APOE4-associated lipid disruptions. Co-culture studies have revealed the role of APOE isoforms in transferring lipids between astrocytes and neurons and shaping microglial states. However, much work remains to bridge the gap between these mechanistic studies and understanding the consequences of modulating lipid state in complex tissues like the brain. Although existing work in brain tissue has begun to characterize lipidomic changes in response to APOE genotype, much work is still needed to connect modulation of specific lipid metabolic pathways to pathology or cognitive outcomes. Finally, being able to relate findings in model systems to human tissue and physiology is crucial to substantiate disease relevance.
In addition to the continuation of studies like those reviewed above, we propose select promising lines of investigation and outstanding questions for the field (see Outstanding Questions).
Outstanding Questions.
How do APOE alleles with different sequences give rise to changes in lipid metabolism in different cell types and in organisms as whole?
Does ancestral background and sex impact the effects of APOE alleles on lipid metabolism?
What are the functional consequences of APOE allele-induced changes to lipid metabolism?
What are genetic and chemical modifiers of lipid metabolism that may present viable preventative strategies?
In past years, various research groups have identified novel protective alleles of APOE. Identifying the effects that protective alleles have on lipid metabolism may hold the key to modulating cellular lipid state to promote disease resilience. We look forward to seeing the expansion of understanding in this area.
APOE has been studied in the context of perturbations to peripheral lipid metabolism by the cardiovascular disease research field for years. Fostering communication between the cardiovascular disease and the neurodegenerative disease fields may provide insights into how APOE isoforms regulate disease risk in a fundamental way. Cross-disciplinary meetings and grant mechanisms could foster such communication and further fundamental research into the functional consequences of APOE-mediated lipid perturbations. This approach could have particular significance to the area of vascular dementia.
Technical advances in studying metabolites have enabled researchers to identify how the various APOE alleles modify risk for neurodegenerative disease through actions on cellular and organism-wide metabolism. Metabolomics approaches, including spatial metabolomics, are only improving in their sensitivity and specificity of detection [159]. Further, high-powered lipidomic characterization of large post-mortem tissue cohorts will help distinguish genotype-specific lipid signatures from the natural sample-to-sample variability. As these approaches are widely adopted, standardization of pipelines and preparation methods is important to allow data to be comparable across technical platforms. These platforms can accelerate the identification of new drug targets, engagement of targets by drug treatments, and mechanisms of actions of novel modulators [160].
Ultimately, changes in lipid metabolism are central to the modulation of disease risk by APOE alleles. Many studies are just uncovering the key determinants of these metabolic changes. Further work is needed to substantiate causal links between APOE-associated lipid changes and disease risk or resilience via chemical or genetic modulation of relevant lipid species. A comprehensive understanding of the cellular regulators of lipid metabolic disruptions will be central to targeting these nodes for therapeutic or preventative benefit.
Highlights:
Human APOE alleles modify lipid metabolism and impact risk for multiple neurodegenerative diseases.
APOE4 is associated with triglyceride and cholesterol accumulation in glia, modulating their immune reactivity.
APOE4 is poorly lipidated compared to APOE3 and prevents efficient transport of lipids between glia and neurons. This impacts neuronal homeostasis and survival.
APOE4 alters brain lipid metabolism in mouse models and human tissue.
Targeting APOE-associated changes to lipid metabolism could form the basis of genotype-specific preventative strategies for a broad range of neurodegenerative diseases.
Acknowledgements
We thank Dr. Madhav Thambisetty, Dr. Richard Proia, and Dr. Mark Cookson for their input on this review. ZMM was supported by a NINDS Competitive Fellowship Award. RAS was supported by an NINDS Training Fellowship. All authors were supported by the Intramural Research Programs of the National Institute of Diabetes and Digestive and Kidney Diseases and the National Institute of Neurological Disease and Stroke as well as by the Chan-Zuckerberg Initiative.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Dawson G. (2015) Measuring brain lipids. Biochim. Biophys. Acta - Mol. Cell Biol. Lipids 1851, 1026–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamilton JA et al. (2007) Brain uptake and utilization of fatty acids, lipids and lipoproteins: Application to neurological disorders. J. Mol. Neurosci 33, 2–11 [DOI] [PubMed] [Google Scholar]
- 3.Alzheimer A. (1907) Über eine eigenartige Erkrankung der Hirnrinde (In German, original case report for Alzheimer’s Disease). Allg Z Psych Psych-gerich Med [Google Scholar]
- 4.Fanning S. et al. (2020) Parkinson’s disease: proteinopathy or lipidopathy? npj Park. Dis 6, 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alza NP et al. (2019) Lipids at the crossroad of α-synuclein function and dysfunction: Biological and pathological implications. Front. Cell. Neurosci 13, 1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marin R. et al. (2017) Anomalies occurring in lipid profiles and protein distribution in frontal cortex lipid rafts in dementia with Lewy bodies disclose neurochemical traits partially shared by Alzheimer’s and Parkinson’s diseases. Neurobiol. Aging 49, 52–59 [DOI] [PubMed] [Google Scholar]
- 7.Schulze H and Sandhoff K (2011) Lysosomal lipid storage diseases. Cold Spring Harb. Perspect. Biol 3, 1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loving BA and Bruce KD (2020) Lipid and Lipoprotein Metabolism in Microglia. Front. Physiol 11, 1–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JA et al. (2021) Lipid metabolism in astrocytic structure and function. Semin. Cell Dev. Biol 112, 123–136 [DOI] [PubMed] [Google Scholar]
- 10.Bellenguez C. et al. (2022) New insights into the genetic etiology of Alzheimer’s disease and related dementias. Nat. Genet 54, 412–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chia R. et al. (2021) Genome sequencing analysis identifies new loci associated with Lewy body dementia and provides insights into its genetic architecture. Nat. Genet 53, 294–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nalls MA et al. (2019) Identification of novel risk loci, causal insights, and heritable risk for Parkinson’s disease: a meta-analysis of genome-wide association studies. Lancet Neurol. 18, 1091–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahley RW et al. (2009) Apolipoprotein E: Structure determines function, from atherosclerosis to Alzheimer’s disease to AIDS. J. Lipid Res 50, 183–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lane-Donovan C and Herz J (2017) ApoE, ApoE Receptors, and the Synapse in Alzheimer’s Disease. Trends Endocrinol. Metab 28, 273–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yeh FL et al. (2016) TREM2 Binds to Apolipoproteins, Including APOE and CLU/APOJ, and Thereby Facilitates Uptake of Amyloid-Beta by Microglia. Neuron 91, 328–340 [DOI] [PubMed] [Google Scholar]
- 16.Zhou J. et al. (2023) LilrB3 is a putative cell surface receptor of APOE4. Cell Res. DOI: 10.1038/s41422-022-00759-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roher AE et al. (2009) Proteomics-derived cerebrospinal fluid markers of autopsy-confirmed Alzheimer’s disease. Biomarkers Biochem. Indie. Expo, response, susceptibility to Chem 14, 493–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahley RW (2016) Central nervous system lipoproteins: ApoE and regulation of cholesterol metabolism. Arterioscler. Thromb. Vase. Biol 36, 1305–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lanfranco MF et al. (2021) Expression and secretion of apoE isoforms in astrocytes and microglia during inflammation. Glia 69, 1478–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blanchard JW et al. (2022) APOE4 impairs myelination via cholesterol dysregulation in oligodendrocytes. Nature 611, 769–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blanchard JW et al. (2020) Reconstruction of the human blood–brain barrier in vitro reveals a pathogenic mechanism of APOE4 in pericytes. Nat. Med 26, 952–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu Q. et al. (2006) Profile and regulation of apolipoprotein E (ApoE) expression in the CNS in mice with targeting of green fluorescent protein gene to the ApoE locus. J. Neurosci 26, 4985–4994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Metzger RE et al. (1996) Neurons of the Human Frontal Cortex Display Apolipoprotein E Immunoreactivity: Implications for Alzheimer’s Disease. J. Neuropathol. Exp. Neurol 55, 372–380 [DOI] [PubMed] [Google Scholar]
- 24.Boschert U. et al. (1999) Apolipoprotein E expression by neurons surviving excitotoxic stress. Neurobiol. Dis 6, 508–514 [DOI] [PubMed] [Google Scholar]
- 25.Liu C. et al. (2013) Apolipoprotein E and Alzheimer disease: risk, mechanisms, and therapy. Nat. Rev. Neurosci 9, 106–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corder EH et al. (1993) Gene Dose of Apolipoprotein E Type 4 Allele and the Risk of Alzheimer’s Disease in Late Onset Families. Science (80-. ). 261, 921–923 [DOI] [PubMed] [Google Scholar]
- 27.Strittmatter WJ et al. (1993) Apolipoprotein E: High-avidity binding to β-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc. Natl. Acad. Sci. U. S. A 90, 1977–1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deming Y. et al. (2017) Genome-wide association study identifies four novel loci associated with Alzheimer’s endophenotypes and disease modifiers. Acta Neuropathol. 133, 839–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Belloy ME et al. (2019) A Quarter Century of APOE and Alzheimer’s Disease: Progress to Date and the Path Forward. Neuron 101, 820–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farrer LA et al. (1997) Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA 278, 1349–1356 [PubMed] [Google Scholar]
- 31.Sudmant PH et al. (2015) An integrated map of structural variation in 2,504 human genomes. Nature 526, 75–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karczewski KJ et al. (2020) The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 581, 434–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wardell MR et al. (1987) Apolipoprotein E2-Christchurch (136 Arg----Ser). New variant of human apolipoprotein E in a patient with type III hyperlipoproteinemia. J. Clin. Invest 80, 483–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arboleda-Velasquez JF et al. (2019) Resistance to autosomal dominant Alzheimer’s disease in an APOE3 Christchurch homozygote: a case report. Nat. Med 25, 1680–1683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van den Maagdenberg AMJM et al. (1993) Characterization of five new mutants in the carboxyl-terminal domain of human apolipoprotein E: No cosegregation with severe hyperlipidemia. Am. J. Hum. Genet 52, 937–946 [PMC free article] [PubMed] [Google Scholar]
- 36.Liu C-C et al. (2021) APOE3-Jacksonville (V236E) variant reduces self-aggregation and risk of dementia. Sci. Transl. Med 13, eabc9375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Le Guen Y. et al. (2022) Association of Rare APOE Missense Variants V236E and R251G With Risk of Alzheimer Disease. JAMA Neurol. 79, 652–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jordan BD et al. (1997) Apolipoprotein E epsilon4 associated with chronic traumatic brain injury in boxing. JAMA 278, 136–140 [PubMed] [Google Scholar]
- 39.Teasdale GM et al. (2005) The association between APOE epsilon4, age and outcome after head injury: a prospective cohort study. Brain 128, 2556–2561 [DOI] [PubMed] [Google Scholar]
- 40.Shinohara M. et al. (2016) Impact of sex and APOE4 on cerebral amyloid angiopathy in Alzheimer’s disease. Acta Neuropathol. 132, 225–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Greenberg SM et al. (1995) Apolipoprotein E epsilon 4 and cerebral hemorrhage associated with amyloid angiopathy. Ann. Neurol 38, 254–259 [DOI] [PubMed] [Google Scholar]
- 42.Ringman JM et al. (2014) Clinical predictors of severe cerebral amyloid angiopathy and influence of APOE genotype in persons with pathologically verified Alzheimer disease. JAMA Neurol. 71, 878–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Premkumar DR et al. (1996) Apolipoprotein E-epsilon4 alleles in cerebral amyloid angiopathy and cerebrovascular pathology associated with Alzheimer’s disease. Am. J. Pathol 148, 2083–2095 [PMC free article] [PubMed] [Google Scholar]
- 44.Atherton K. et al. (2022) Association of APOE Genotypes and Chronic Traumatic Encephalopathy. JAMA Neurol. 79, 787–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Amidi A. et al. (2017) Changes in cognitive functions and cerebral grey matter and their associations with inflammatory markers, endocrine markers, and APOE genotypes in testicular cancer patients undergoing treatment. Brain Imaging Behav. 11, 769–783 [DOI] [PubMed] [Google Scholar]
- 46.Mandelblatt JS et al. (2018) Cancer-Related Cognitive Outcomes Among Older Breast Cancer Survivors in the Thinking and Living With Cancer Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol 36, JCO1800140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fernandez HR et al. (2020) Cancer Chemotherapy Related Cognitive Impairment and the Impact of the Alzheimer’s Disease Risk Factor APOE. Cancers (Basel). 12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carvalho-Wells AL et al. (2012) APOE genotype influences triglyceride and C-reactive protein responses to altered dietary fat intake in UK adults. Am. J. Clin. Nutr 96, 1447–1453 [DOI] [PubMed] [Google Scholar]
- 49.Dallongeville J. et al. (1992) Modulation of plasma triglyceride levels by apoE phenotype: a meta-analysis. J. Lipid Res 33, 447–454 [PubMed] [Google Scholar]
- 50.Bennet AM et al. (2007) Association of apolipoprotein E genotypes with lipid levels and coronary risk. JAMA 298, 1300–1311 [DOI] [PubMed] [Google Scholar]
- 51.Kurki SN et al. (2021) APOE ε4 associates with increased risk of severe COVID-19, cerebral microhaemorrhages and post-COVID mental fatigue: a Finnish biobank, autopsy and clinical study. Acta Neuropathol. Commun 9, 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Safdari Lord J. et al. (2022) The association of APOE genotype with COVID-19 disease severity. Sci. Rep 12, 13483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ostendorf BN et al. (2022) Common human genetic variants of APOE impact murine COVID-19 mortality. Nature 611, 346–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shinohara M. et al. (2020) APOE2 is associated with longevity independent of Alzheimer’s disease. Elife 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brewer HBJ et al. (1983) NIH conference. Type III hyperlipoproteinemia: diagnosis, molecular defects, pathology, and treatment. Ann. Intern. Med 98, 623–640 [DOI] [PubMed] [Google Scholar]
- 56.Mahley RW (2016) Apolipoprotein E: from cardiovascular disease to neurodegenerative disorders. J. Mol. Med. (Berl) 94, 739–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Klaver CC et al. (1998) Genetic association of apolipoprotein E with age-related macular degeneration. Am. J. Hum. Genet 63, 200–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ostendorf BN et al. (2020) Common germline variants of the human APOE gene modulate melanoma progression and survival. Nat. Med 26, 1048–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lumsden AL et al. (2020) Apolipoprotein E (APOE) genotype-associated disease risks: a phenome-wide, registry-based, case-control study utilising the UK Biobank. EBioMedicine 59, 102954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu HM et al. (2021) Heterogeneous effects of genetic risk for Alzheimer’s disease on the phenome. Transl. Psychiatry 11, 406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Farmer BC et al. (2021) APOE4 lowers energy expenditure in females and impairs glucose oxidation by increasing flux through aerobic glycolysis. Mol. Neurodegener 16, 1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee S. et al. (2023) APOE modulates microglial immunometabolism in response to age, amyloid pathology, and inflammatory challenge. Cell Rep. 42, 112196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Victor MB et al. (2022) Lipid accumulation induced by APOE4 impairs microglial surveillance of neuronal-network activity. Cell Stem Cell 29, 1197–1212.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lin YT et al. (2018) APOE4 Causes Widespread Molecular and Cellular Alterations Associated with Alzheimer’s Disease Phenotypes in Human iPSC-Derived Brain Cell Types. Neuron 98, 1141–1154.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Narayan P. et al. (2020) PICALM Rescues Endocytic Defects Caused by the Alzheimer’s Disease Risk Factor APOE4. Cell Rep. 33, 108224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.de Leeuw SM et al. (2022) APOE2, E3, and E4 differentially modulate cellular homeostasis, cholesterol metabolism, and inflammatory response in isogenic iPSC-derived astrocytes. Stem cell reports 17, 110–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nuriel T. et al. (2017) The Endosomal-Lysosomal Pathway Is Dysregulated by APOE4 Expression in Vivo. Front. Neurosci 11, 702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen H-K et al. (2011) Apolipoprotein E4 domain interaction mediates detrimental effects on mitochondria and is a potential therapeutic target for Alzheimer disease. J. Biol. Chem 286, 5215–5221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schmechel DE et al. (1993) Increased amyloid beta-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Proc. Natl. Acad. Sci. U. S. A 90, 9649–9653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Namba Y. et al. (1991) Apolipoprotein E immunoreactivity in cerebral amyloid deposits and neurofibrillary tangles in Alzheimer’s disease and kuru plaque amyloid in Creutzfeldt-Jakob disease. Brain Res. 541, 163–166 [DOI] [PubMed] [Google Scholar]
- 71.Polvikoski T et al. (1995) Apolipoprotein E, dementia, and cortical deposition of beta-amyloid protein. N. Engl. J. Med 333, 1242–1247 [DOI] [PubMed] [Google Scholar]
- 72.Holtzman DM et al. (2000) Apolipoprotein E facilitates neuritic and cerebrovascular plaque formation in an Alzheimer’s disease model. Ann. Neurol 47, 739–747 [PubMed] [Google Scholar]
- 73.Tcw J. et al. (2022) Cholesterol and matrisome pathways dysregulated in astrocytes and microglia. Cell 185, 2213–2233.e25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Olzmann JA and Carvalho P (2019) Dynamics and functions of lipid droplets. Nat. Rev. Mol. Cell Biol 20, 137–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Farmer BC et al. (2019) Apolipoprotein E4 Alters Astrocyte Fatty Acid Metabolism and Lipid Droplet Formation. DOI: 10.3390/cells8020182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sienski G. et al. (2021) APOE4 disrupts intracellular lipid homeostasis in human iPSC-derived glia. Sci. Transl. Med 13, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Marschallinger J. et al. (2020) Lipid-droplet-accumulating microglia represent a dysfunctional and proinflammatory state in the aging brain. Nat. Neurosci 23, 194–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang C. et al. (2018) Gain of toxic apolipoprotein E4 effects in human iPSC-derived neurons is ameliorated by a small-molecule structure corrector. Nat. Med 24, 647–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jackson RJ et al. (2022) APOE4 derived from astrocytes leads to blood-brain barrier impairment. Brain 145, 3582–3593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Koizumi K. et al. (2018) Apoε4 disrupts neurovascular regulation and undermines white matter integrity and cognitive function. Nat. Commun 9, 3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Montagne A. et al. (2021) APOE4 accelerates advanced-stage vascular and neurodegenerative disorder in old Alzheimer’s mice via cyclophilin A independently of amyloid-β. Nat. Aging 1, 506–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Koutsodendris N. et al. (2023) Neuronal APOE4 removal protects against tau-mediated gliosis, neurodegeneration and myelin deficits. Nat. Aging DOI: 10.1038/s43587-023-00368-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Robert J. et al. (2020) Cerebrovascular amyloid Angiopathy in bioengineered vessels is reduced by high-density lipoprotein particles enriched in Apolipoprotein E. Mol. Neurodegener 15, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Montagne A et al. (2020) APOE4 leads to blood–brain barrier dysfunction predicting cognitive decline. Nature 581, 71–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu L. et al. (2017) The Glia-Neuron Lactate Shuttle and Elevated ROS Promote Lipid Synthesis in Neurons and Lipid Droplet Accumulation in Glia via APOE/D. Cell Metab. 26, 719–737.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ioannou MS et al. (2019) Neuron-Astrocyte Metabolic Coupling Protects against Activity-Induced Fatty Acid Toxicity. Cell 177, 1522–1535.e14 [DOI] [PubMed] [Google Scholar]
- 87.Moulton MJ et al. (2021) Neuronal ROS-induced glial lipid droplet formation is altered by loss of Alzheimer’s disease-associated genes. Proc. Natl. Acad. Sci. U. S. A 118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Qi G. et al. (2021) ApoE4 Impairs Neuron-Astrocyte Coupling of Fatty Acid Metabolism. Cell Rep. 34, 108572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Guttenplan KA et al. (2020) Knockout of reactive astrocyte activating factors slows disease progression in an ALS mouse model. Nat. Commun 11, 3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liddelow SA et al. (2017) Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Guttenplan KA et al. (2021) Neurotoxic reactive astrocytes induce cell death via saturated lipids. Nature 599, 102–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nieweg K. et al. (2009) Marked differences in cholesterol synthesis between neurons and glial cells from postnatal rats. J. Neurochem 109, 125–134 [DOI] [PubMed] [Google Scholar]
- 93.Pfrieger FW and Lingerer N (2011) Cholesterol metabolism in neurons and astrocytes. Prog. Lipid Res 50, 357–371 [DOI] [PubMed] [Google Scholar]
- 94.van der Kant R. et al. (2019) Cholesterol Metabolism Is a Druggable Axis that Independently Regulates Tau and Amyloid-β in iPSC-Derived Alzheimer’s Disease Neurons. Cell Stem Cell 24, 363–375.e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cheng GW-Y et al. (2022) Apolipoprotein E ε4 Mediates Myelin Breakdown by Targeting Oligodendrocytes in Sporadic Alzheimer Disease. J. Neuropathol. Exp. Neurol 81, 717–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.van’t Hooft F and Havel RJ (1981) Metabolism of chromatographically separated rat serum lipoproteins specifically labeled with 125I-apolipoprotein E. J. Biol. Chem 256, 3963–3968 [PubMed] [Google Scholar]
- 97.Pitas RE et al. (1987) Lipoproteins and their receptors in the central nervous system. Characterization of the lipoproteins in cerebrospinal fluid and identification of apolipoprotein B,E(LDL) receptors in the brain. J. Biol. Chem 262, 14352–14360 [PubMed] [Google Scholar]
- 98.Koch M. et al. (2020) Association of Apolipoprotein e in Lipoprotein Subspecies with Risk of Dementia. JAMA Netw. Open 3, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Flowers SA and Rebeck GW (2020) APOE in the normal brain. Neurobiol. Dis 136, 104724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Courtney R and Landreth GE (2016) LXR Regulation of Brain Cholesterol: From Development to Disease. Trends Endocrinol. Metab 27, 404–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Heinsinger NM et al. (2016) Apolipoprotein E Genotype Affects Size of ApoE Complexes in Cerebrospinal Fluid. J. Neuropathol. Exp. Neurol 75, 918–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hu J. et al. (2015) Opposing effects of viral mediated brain expression of apolipoprotein E2 (apoE2) and apoE4 on apoE lipidation and Aβ metabolism in apoE4-targeted replacement mice. Mol. Neurodegener 10, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sakamoto T et al. (2008) Contributions of the Carboxyl-Terminal Helical Segment to the Self-Association and Lipoprotein Preferences of Human Apolipoprotein E3 and E4 Isoforms. Biochemistry 47, 2968–2977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nguyen D. et al. (2010) Molecular Basis for the Differences in Lipid and Lipoprotein Binding Properties of Human Apolipoproteins E3 and E4. Biochemistry 49, 10881–10889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Weisgraber KH (1990) Apolipoprotein E distribution among human plasma lipoproteins: Role of the cysteine-arginine interchange at residue 112. J. Lipid Res 31, 1503–1511 [PubMed] [Google Scholar]
- 106.Michikawa M. et al. (2000) Apolipoprotein E Exhibits Isoform-Specific Promotion of Lipid Efflux from Astrocytes and Neurons in Culture. J. Neurochem 74, 1008–1016 [DOI] [PubMed] [Google Scholar]
- 107.Minagawa H. et al. (2009) Mechanism underlying apolipoprotein E (ApoE) isoform-dependent lipid efflux from neural cells in culture. J. Neurosci. Res 87, 2498–2508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lindner K. et al. (2022) Isoform- and cell-state-specific lipidation of ApoE in astrocytes. Cell Rep. 38, 110435. [DOI] [PubMed] [Google Scholar]
- 109.Linetti A. et al. (2010) Cholesterol reduction impairs exocytosis of synaptic vesicles. J. Cell Sci 123, 595–605 [DOI] [PubMed] [Google Scholar]
- 110.Liu Q. et al. (2010) Neuronal LRP1 knockout in adult mice leads to impaired brain lipid metabolism and progressive, age-dependent synapse loss and neurodegeneration. J. Neurosci. Off. J. Soc. Neurosci 30, 17068–17078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liu Q. et al. (2007) Amyloid precursor protein regulates brain apolipoprotein E and cholesterol metabolism through lipoprotein receptor LRP1. Neuron 56, 66–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Riddell DR et al. (2008) Impact of apolipoprotein E (ApoE) polymorphism on brain ApoE levels. J. Neurosci. Off. J. Soc. Neurosci 28, 11445–11453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.peña-bautista carmen et al. (2020) Metabolomics study to identify plasma biomarkers in alzheimer disease: ApoE genotype effect. J. Pharm. Biomed. Anal 180, 113088. [DOI] [PubMed] [Google Scholar]
- 114.Arnold M. et al. (2020) Sex and APOE ε4 genotype modify the Alzheimer’s disease serum metabolome. Nat. Commun 11, 1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fitzner D. et al. (2020) Cell-Type- and Brain-Region-Resolved Mouse Brain Lipidome. Cell Rep. 32, 108132. [DOI] [PubMed] [Google Scholar]
- 116.Nugent AA et al. (2020) TREM2 Regulates Microglial Cholesterol Metabolism upon Chronic Phagocytic Challenge. Neuron 105, 837–854.e9 [DOI] [PubMed] [Google Scholar]
- 117.Area-Gomez E. et al. (2020) APOE4 is Associated with Differential Regional Vulnerability to Bioenergetic Deficits in Aged APOE Mice. Sci. Rep 10, 4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhao N. et al. (2020) Alzheimer’s Risk Factors Age, APOE Genotype, and Sex Drive Distinct Molecular Pathways. Neuron 106, 727–742.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Miranda AM et al. (2022) Effects of APOE4 allelic dosage on lipidomic signatures in the entorhinal cortex of aged mice. Transl. Psychiatry 12, 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hanson AJ et al. (2019) Cerebrospinal fluid lipidomics: effects of an intravenous triglyceride infusion and apoE status. Metabolomics 16, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bandaru VVR et al. (2009) ApoE4 disrupts sterol and sphingolipid metabolism in Alzheimer’s but not normal brain. Neurobiol. Aging 30, 591–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Novotny BC et al. (2022) Metabolomic and lipidomic signatures in autosomal dominant and late-onset Alzheimer’s disease brains. Alzheimers. Dement DOI: 10.1002/alz.12800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang C. et al. (2021) Selective removal of astrocytic APOE4 strongly protects against tau-mediated neurodegeneration and decreases synaptic phagocytosis by microglia. Neuron 109, 1657–1674.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rosenberg JB et al. (2018) AAVrh.10-Mediated APOE2 Central Nervous System Gene Therapy for APOE4-Associated Alzheimer’s Disease. Hum. Gene Ther. Clin. Dev 29, 24–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhao L. et al. (2016) Intracerebral adeno-associated virus gene delivery of apolipoprotein E2 markedly reduces brain amyloid pathology in Alzheimer’s disease mouse models. Neurobiol. Aging 44, 159–172 [DOI] [PubMed] [Google Scholar]
- 126.Zelcer N and Tontonoz P (2006) Liver X receptors as integrators of metabolic and inflammatory signaling. J. Clin. Invest 116, 607–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dawson MI and Xia Z (2012) The retinoid X receptors and their ligands. Biochim. Biophys. Acta 1821, 21–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Suon S. et al. (2010) Systemic treatment with liver X receptor agonists raises apolipoprotein E, cholesterol, and amyloid-β peptides in the cerebral spinal fluid of rats. Mol. Neurodegener 5, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Carter AY et al. (2017) Liver X receptor agonist treatment significantly affects phenotype and transcriptome of APOE3 and APOE4 Abca1 haplo-deficient mice. PLoS One 12, e0172161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Boehm-Cagan A and Michaelson DM (2014) Reversal of apoE4-Driven Brain Pathology and Behavioral Deficits by Bexarotene. J. Neurosci 34, 7293 LP – 7301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Balducci C. et al. (2015) The Continuing Failure of Bexarotene in Alzheimer’s Disease Mice. J. Alzheimer’s Dis 46, 471–482 [DOI] [PubMed] [Google Scholar]
- 132.O’Hare E. et al. (2016) Lack of support for bexarotene as a treatment for Alzheimer’s disease. Neuropharmacology 100, 124–130 [DOI] [PubMed] [Google Scholar]
- 133.LaClair KD et al. (2013) Treatment with bexarotene, a compound that increases apolipoprotein-E, provides no cognitive benefit in mutant APP/PS1 mice. Mol. Neurodegener 8, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mounier A. et al. (2015) Bexarotene-Activated Retinoid X Receptors Regulate Neuronal Differentiation and Dendritic Complexity. J. Neurosci. Off. J. Soc. Neurosci 35, 11862–11876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ghosal K. et al. (2016) A randomized controlled study to evaluate the effect of bexarotene on amyloid-β and apolipoprotein E metabolism in healthy subjects. Alzheimer’s Dement. Transl. Res. Clin. Interv 2, 110–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cummings JL et al. (2016) Double-blind, placebo-controlled, proof-of-concepttrial of bexarotene Xin moderate Alzheimer’s disease. Alzheimers. Res. Ther 8, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Groot PHE et al. (2005) Synthetic LXR agonists increase LDL in CETP species. J. Lipid Res 46, 2182–2191 [DOI] [PubMed] [Google Scholar]
- 138.Kirchgessner TG et al. (2016) Beneficial and Adverse Effects of an LXR Agonist on Human Lipid and Lipoprotein Metabolism and Circulating Neutrophils. Cell Metab. 24, 223–233 [DOI] [PubMed] [Google Scholar]
- 139.Toledo EM and Inestrosa NC (2010) Activation of Wnt signaling by lithium and rosiglitazone reduced spatial memory impairment and neurodegeneration in brains of an APPswe/PSEN1DeltaE9 mouse model of Alzheimer’s disease. Mol. Psychiatry 15, 228,272–285 [DOI] [PubMed] [Google Scholar]
- 140.Pedersen WA et al. (2006) Rosiglitazone attenuates learning and memory deficits in Tg2576 Alzheimer mice. Exp. Neurol 199, 265–273 [DOI] [PubMed] [Google Scholar]
- 141.Nicolakakis N. et al. (2008) Complete rescue of cerebrovascular function in aged Alzheimer’s disease transgenic mice by antioxidants and pioglitazone, a peroxisome proliferator-activated receptor gamma agonist. J. Neurosci. Off. J. Soc. Neurosci 28, 9287–9296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Risner ME et al. (2006) Efficacy of rosiglitazone in a genetically defined population with mild-to-moderate Alzheimer’s disease. Pharmacogenomics J. 6, 246–254 [DOI] [PubMed] [Google Scholar]
- 143.Geldmacher DS et al. (2011) A randomized pilot clinical trial of the safety of pioglitazone in treatment of patients with Alzheimer disease. Arch. Neurol 68, 45–50 [DOI] [PubMed] [Google Scholar]
- 144.Gold M. et al. (2010) Rosiglitazone monotherapy in mild-to-moderate Alzheimer’s disease: results from a randomized, double-blind, placebo-controlled phase III study. Dement. Geriatr. Cogn. Disord 30, 131–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Boehm-Cagan A. et al. (2016) ABCA1 Agonist Reverses the ApoE4-Driven Cognitive and Brain Pathologies. J. Alzheimers. Dis 54, 1219–1233 [DOI] [PubMed] [Google Scholar]
- 146.Noveir SD et al. (2022) Effect of the ABCA1 agonist CS-6253 on amyloid-β and lipoprotein metabolism in cynomolgus monkeys. Alzheimers. Res. Ther 14, 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Geifman N. et al. (2017) Evidence for benefit of statins to modify cognitive decline and risk in Alzheimer’s disease. Alzheimers. Res. Ther 9, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Blusztajn JK et al. (2017) Neuroprotective Actions of Dietary Choline Nutrients, 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Velazquez R. et al. (2019) Lifelong choline supplementation ameliorates Alzheimer’s disease pathology and associated cognitive deficits by attenuating microglia activation. Aging Cell 18, e13037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Velazquez R et al. (2020) Maternal choline supplementation ameliorates Alzheimer’s disease pathology by reducing brain homocysteine levels across multiple generations. Mol. Psychiatry 25, 2620–2629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Conant R and Schauss AG (2004) Therapeutic applications of citicoline for stroke and cognitive dysfunction in the elderly: a review of the literature. Altern. Med. Rev 9, 17–31 [PubMed] [Google Scholar]
- 152.Yuan J. et al. (2022) Is dietary choline intake related to dementia and Alzheimer’s disease risks? Results from the Framingham Heart Study. Am. J. Clin. Nutr 116, 1201–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Cacabelos R et al. (1996) Therapeutic Effects of CDP-Choline in Alzheimer’s Disease-Cognition, Brain Mapping, Cerebrovascular Hemodynamics, and Immune Factorsa. Ann. N. Y. Acad. Sci 777, 399–403 [DOI] [PubMed] [Google Scholar]
- 154.Amenta F. et al. (2001) Treatment of cognitive dysfunction associated with Alzheimer’s disease with cholinergic precursors. Ineffective treatments or inappropriate approaches? Mech. Ageing Dev 122, 2025–2040 [DOI] [PubMed] [Google Scholar]
- 155.Maurice D. (1987) A Review of Recent Clinical Trials in the Treatment of Alzheimer’s Dementia. Psychiatr. Ann 17, 178–191 [Google Scholar]
- 156.Alvarez XA et al. (1999) Double-blind placebo-controlled study with citicoline in APOE genotyped Alzheimer’s disease patients. Effects on cognitive performance, brain bioelectrical activity and cerebral perfusion. Methods Find. Exp. Clin. Pharmacol 21, 633–644 [PubMed] [Google Scholar]
- 157.Dai J. et al. (2018) Effects of APOE Genotype on Brain Proteomic Network and Cell Type Changes in Alzheimer’s Disease. Front. Mol. Neurosci 11, 454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ferguson AC et al. (2020) Alzheimer’s Disease Susceptibility Gene Apolipoprotein E (APOE) and Blood Biomarkers in UK Biobank (N = 395,769). J. Alzheimers. Dis 76, 1541–1551 [DOI] [PubMed] [Google Scholar]
- 159.Alexandrov T. (2020) Spatial Metabolomics and Imaging Mass Spectrometry in the Age of Artificial Intelligence. Annu. Rev. Biomed. Data Sci 3, 61–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Alarcon-Barrera JC et al. (2022) Recent advances in metabolomics analysis for early drug development. Drug Discov. Today 27, 1763–1773 [DOI] [PubMed] [Google Scholar]
- 161.McIntosh AM et al. (2012) The apolipoprotein E (APOE) gene appears functionally monomorphic in chimpanzees (Pan troglodytes). PLoS One 7, e47760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fullerton SM et al. (2000) Apolipoprotein E variation at the sequence haplotype level: implications for the origin and maintenance of a major human polymorphism. Am. J. Hum Genet 67, 881–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Finch CE and Stanford CB (2004) Meat-adaptive genes and the evolution of slower aging in humans. Q. Rev. Biol 79, 3–50 [DOI] [PubMed] [Google Scholar]
- 164.Trumble BC et al. (2017) Apolipoprotein E4 is associated with improved cognitive function in Amazonian forager-horticulturalists with a high parasite burden. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol 31, 1508–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Oriá RB et al. (2007) Role of apolipoprotein E4 in protecting children against early childhood diarrhea outcomes and implications for later development. Med. Hypotheses 68, 1099–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gharbi-Meliani A. et al. (2021) The association of APOE ε4 with cognitive function over the adult life course and incidence of dementia: 20 years follow-up of the Whitehall II study. Alzheimers. Res. Ther 13, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Garcia AR et al. (2021) APOE4 is associated with elevated blood lipids and lower levels of innate immune biomarkers in a tropical Amerindian subsistence population. Elife 10, e68231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hubler MJ and Kennedy AJ (2016) Role of lipids in the metabolism and activation of immune cells. J. Nutr. Biochem 34, 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bernardi S. et al. (2018) The Complex Interplay between Lipids, Immune System and Interleukins in Cardio-Metabolic Diseases. Int. J. Mol. Sci 19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Corbo RM and Scacchi R (1999) Apolipoprotein E (APOE) allele distribution in the world. Is APOE*4 a “thrifty” allele? Ann. Hum. Genet 63, 301–310 [DOI] [PubMed] [Google Scholar]
- 171.Egert S. et al. (2012) ApoE genotype: from geographic distribution to function and responsiveness to dietary factors. Proc. Nutr. Soc 71, 410–424 [DOI] [PubMed] [Google Scholar]
- 172.Eisenberg DTA et al. (2010) Worldwide allele frequencies of the human apolipoprotein E gene: climate, local adaptations, and evolutionary history. Am. J. Phys. Anthropol 143, 100–111 [DOI] [PubMed] [Google Scholar]
- 173.Logue MW et al. (2011) A Comprehensive Genetic Association Study of Alzheimer Disease in African Americans. Arch. Neurol 68, 1569–1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Blue EE et al. (2019) Local ancestry at APOE modifies Alzheimer’s disease risk in Caribbean Hispanics. Alzheimer’s Dement. 15, 1524–1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Naslavsky MS et al. (2022) Global and local ancestry modulate APOE association with Alzheimer’s neuropathology and cognitive outcomes in an admixed sample. Mol. Psychiatry 27, 4800–4808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Rajabli F. et al. (2018) Ancestral origin of ApoE ε4 Alzheimer disease risk in Puerto Rican and African American populations. PLoS Genet. 14, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Suchy-Dicey A. et al. (2022) APOE genotype, hippocampus, and cognitive markers of Alzheimer’s disease in American Indians: Data from the Strong Heart Study. Alzheimers. Dement 18, 2518–2526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Chen Q. et al. (2022) Protective effect of apolipoprotein E epsilon 3 on sporadic Alzheimer’s disease in the Chinese population: a meta-analysis. Sci. Rep 12, 13620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Rajabli F. et al. (2022) A locus at 19q13.31 significantly reduces the ApoE ε4 risk for Alzheimer’s Disease in African Ancestry. PLoS Genet. 18, e1009977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.AlzheimersAssociation Women and Alzheimer’s[Online]. Available: https://www.alz.org/alzheimers-dementia/what-is-alzheimers/women-and-alzheimer-s
- 181.Neu SC et al. (2017) Apolipoprotein E Genotype and Sex Risk Factors for Alzheimer Disease: A Meta-analysis. JAMA Neurol. 74, 1178–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sullivan PM et al. (1997) Targeted replacement of the mouse apolipoprotein E gene with the common human APOE3 allele enhances diet-induced hypercholesterolemia and atherosclerosis. J. Biol. Chem 272, 17972–17980 [DOI] [PubMed] [Google Scholar]
- 183.Linton MF et al. (1991) Phenotypes of apolipoprotein B and apolipoprotein E after liver transplantation. J. Clin. Invest 88, 270–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Liu C-C et al. (2022) Peripheral apoE4 enhances Alzheimer’s pathology and impairs cognition by compromising cerebrovascular function. Nat. Neurosci 25, 1020–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lane-Donovan C. et al. (2016) Genetic Restoration of Plasma ApoE Improves Cognition and Partially Restores Synaptic Defects in ApoE-Deficient Mice. J. Neurosci. Off. J. Soc. Neurosci 36, 10141–10150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Huynh T-PV et al. (2019) Lack of hepatic apoE does not influence early Aβ deposition: observations from a new APOE knock-in model. Mol. Neurodegener 14, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Jones NS et al. (2021) High-fat diet increases gliosis and immediate early gene expression in APOE3 mice, but not APOE4 mice. J. Neuroinflammation 18, 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ghebranious N. et al. (2011) A pilot study of gene/gene and gene/environment interactions in Alzheimer disease. Clin. Med. Res 9, 17–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Rajan KB et al. (2014) Gene-environment interaction of body mass index and apolipoprotein E ε4 allele on cognitive decline. Alzheimer Dis. Assoc. Disord 28, 134–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zajac DJ et al. (2022) APOE genetics influence murine gut microbiome. Sci. Rep 12, 1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Tran TTT et al. (2019) APOE genotype influences the gut microbiome structure and function in humans and mice: relevance for Alzheimer’s disease pathophysiology. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol 33, 8221–8231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Seo D-O et al. (2023) ApoE isoform- and microbiota-dependent progression of neurodegeneration in a mouse model of tauopathy. Science 379, eadd1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Maldonado Weng J. et al. (2019) Synergistic effects of APOE and sex on the gut microbiome of young EFAD transgenic mice. Mol. Neurodegener 14, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Russo R. et al. (2018) Gut-brain Axis: Role of Lipids in the Regulation of Inflammation, Pain and CNS Diseases. Curr. Med. Chem 25, 3930–3952 [DOI] [PubMed] [Google Scholar]
- 195.Hou M. et al. (2021) APOE-ε4 Carrier Status and Gut Microbiota Dysbiosis in Patients With Alzheimer Disease. Front. Neurosci 15, 619051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Lee S. et al. (2023) APOE4 drives transcriptional heterogeneity and maladaptive immunometabolic responses of astrocytes. bioRxiv DOI: 10.1101/2023.02.06.527204 [DOI] [Google Scholar]