Abstract
SETTING:
According to reports in South Africa, treatment failure rates for rifampicin-resistant TB (RR-TB) are significant and below the WHO target of ≥70%. HIV infection and the use of highly active antiretroviral therapy (HAART) influence how patients receiving anti-TB drugs respond to therapy. In the treatment of RR-TB, more recent medications, including bedaquiline, pretomanid and linezolid (BPaL), have shown promising results.
OBJECTIVE:
To assess treatment outcomes in RR-TB patients using BPaL and other second-line anti-TB drugs as recommended by the WHO in the South African population.
DESIGN:
The databases Medline, PubMed, Google Scholar and Embase were searched for studies between 2015 and 2022, which investigated BPaL outcomes in South Africa.
RESULTS:
Of the 27,259 participants, 21% were on bedaquiline, 1% were taking pretomanid and 9% were taking linezolid as part of their background regimen. About 68% of the patients were HIV-positive, with 59% of them taking HAART.
CONCLUSION:
Overall, 66% of patients taking BPaL drugs as part of their background regimen had favourable treatment outcomes. Additionally, patients with RR-TB who were HIV-positive and taking HAART while receiving BPaL drugs as part of a background regimen had improved treatment outcomes.
Keywords: tuberculosis, drug resistance, bedaquiline, pretomanid, linezolid, HAART, rifampicin-resistant
Abstract
CONTEXTE :
Selon des rapports en Afrique du Sud, les taux d’échec du traitement de la TB résistante à la rifampicine (RR-TB) sont considérables et inférieurs à l’objectif de ≥70% fixé par l’OMS. L’infection par le VIH et l’utilisation d’une thérapie antirétrovirale hautement active (HAART) influencent la manière dont les patients recevant des médicaments anti-TB répondent au traitement. Dans le traitement de la RR-TB, des médicaments plus récents, notamment la bédaquiline, le prétomanid et le linézolide (BPaL), ont donné des résultats prometteurs.
OBJECTIF :
Évaluer les résultats du traitement des patients atteints de RR-TB à l’aide de BPaL et d’autres médicaments anti-TB de deuxième intention, conformément aux recommandations de l’OMS, dans la population sud-africaine.
MÉTHODE :
Les bases de données Medline, PubMed, Google Scholar et Embase ont été consultées pour trouver des études réalisées entre 2015 et 2022 sur les résultats de la BPaL en Afrique du Sud.
RÉSULTATS :
Sur les 27 259 participants, 21% prenaient de la bédaquiline, 1% du prétomanid et 9% du linézolide dans le cadre de leur traitement de fond. Environ 68% des patients étaient séropositifs, et 59% d’entre eux suivaient un traitement HAART.
CONCLUSION :
Dans l’ensemble, 66% des patients prenant des médicaments BPaL dans le cadre de leur traitement de fond ont obtenu des résultats favorables. En outre, les patients atteints de RR-TB qui étaient séropositifs et prenaient le traitement HAART tout en recevant des médicaments BPaL dans le cadre d’un traitement de fond ont eu de meilleurs résultats thérapeutiques.
Since 2013, bedaquiline (BDQ) is recommended by the WHO for the treatment of selected patients with rifampicin-resistant TB (RR-TB).1 In June 2015, South Africa became one of the first countries to use newly available BDQ for RR-TB. Following this, the South African programmatic data were used to update the WHO recommendations for the treatment of drug-resistant TB (DR-TB) in 2018 and 2019.2 This led to the most recent WHO recommendation for an all-oral shorter regimen of BDQ thus replacing the injectables.2 At the end of 2018, South Africa modified its all-oral regimen, recommending 6 months of BDQ and 2 months of linezolid (LZD) for all TB patients initiating the shorter 9–12 months DR-TB regimen.3 The US Food and Drug Administration (FDA) approved a new, all-oral regimen to treat RR-TB in August 2019. The combination of BDQ, pretomanid (Pa) and LZD, collectively referred to as BPaL, was tested in the Nix-TB trial, which involved patients with extensively drug-resistant TB (XDR-TB), alongside patients who cannot tolerate or do not respond to multidrug-resistant TB (MDR-TB) therapy. The WHO definition for XDR-TB was changed in 2021.4 BDQ and LZD (BPaL) regimens for DR-TB have been studied in several trials, including the Zenix, BEAT-TB, and TB-Practecal trials, to better understand their efficacy in the treatment of TB.36,37 BDQ has a relatively long half-life (6 months), which may make it particularly vulnerable to resistance acquisition, especially in settings with high treatment loss to follow-up.5 The emergence of BDQ resistance is well-documented.6 Nonetheless, in August 2018, supported by a meta-analysis of collaborative group for the Meta-analysis of individual patient data in RR-TB treatment and observational studies,7,8 the WHO recommended including BDQ as a Group A drug in long course RR-TB regimens. In 2022, the WHO recommended the programmatic use of BPaL/M,38 necessitating the evaluation of BPaL effectiveness in the country. This study examined BPaL introduction to patient regimens and available treatment outcome data on BPaL initiation among patients being treated for RR-TB and patients with HIV-TB coinfection and the use of highly active antiretroviral therapy (HAART) during treatment in South Africa. These experiences could be useful to other country programmes and for the introduction of other novel regimens, including the shortened regimens recently recommended by the WHO.9,10
METHODS
Data sources and inclusion criteria
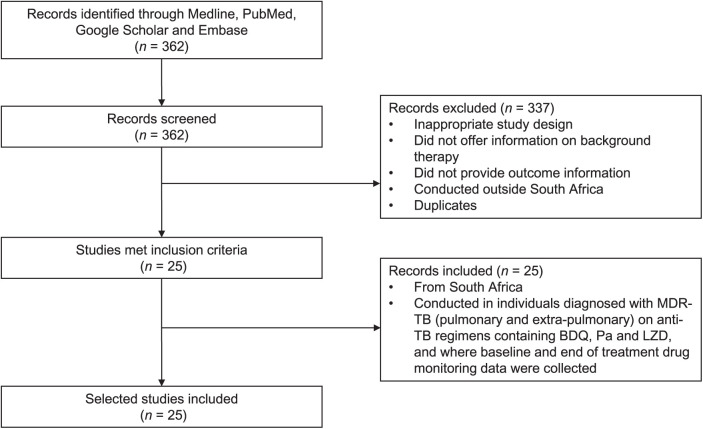
For the period between June 2015 and June 2022, the databases Medline, PubMed, Google Scholar and Embase were searched using the following terms: “multi-drug resistance” + “tuberculosis” + “resistance” + “drug” + “treatment” + “South Africa” + “prevalence” + “effectiveness” + “safety” + “bedaquiline” + “pretomanid” + “linezolid” + “BPaL”. Full published studies were reviewed and evaluated for eligibility using the following inclusion criteria: 1) studies carried out in South Africa; 2) studies that aimed to assess the safety of patients, as well as the effectiveness of treatment regimen based on BPaL and other second-line drugs for MDR-TB; 3) studies reported between June 2015 to June 2022; 4) studies that assessed “BPaL” as part of a background regimen’s effectiveness in either monotherapy or combination therapy with other anti-TB drugs; and 5) studies reported in English (Figure 1). The exclusion criteria were as follows: 1) research that was not carried out in South Africa, 2) animal studies 3) studies that did not offer information on the background regimen, 4) studies that did not provide outcome information, and 5) study duplicates.
Figure 1.
Flow diagram of literature search and review process for study selection. MDR-TB = multidrug-resistant TB; BDQ = bedaquiline; Pa = pretomanid; LZD = linezolid.
A total of 362 studies were retrieved from the search. However, 337 of these studies had to be eliminated due to inclusion requirements (Figure 1). The protocol for this study followed PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines, as previously reported.11 The Table gives the 25 studies that met the inclusion criteria. The following variables were extracted from the included studies to generate the current systematic review: author, period, population size, age, sex, HIV status, antiretroviral treatment, patient with MDR/XDR-TB, treatment regimen (BDQ, Pa, LZD) and treatment result of patients (favourable outcome vs. unfavourable outcome).
Statistical analysis
All data analysis and visualisation were performed using the R programming environment v3.5.0 (R Computing, Vienna, Austria).12 A meta-analysis was conducted using the included studies. For statistical analysis, the R packages metafor and meta were utilised. The log risk ratios and sample variances for DR-TB patients treated with BPaL optimised background regiment therapy and the use of HAART were calculated using the escalc function. To quantify heterogeneity among the included studies, random effect sizes were calculated. The odds ratios (ORs) and random effect sizes of study variables (BPaL, HAART and HIV) and patients treatment outcomes (favourable and unfavourable) were calculated using the escalc function in R to generate a forest plot of the included studies.
RESULTS
Baseline characteristics of included studies
The Table gives the baseline characteristics of the 25 studies included in the review. All 25 studies were carried out in South Africa (Table).
Table.
Characteristics of the 25 selected studies containing BPaL as alternative treatment regimen for RR-TB
| A) Study population and characteristics | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Reference | Study period | Population size n |
Age years |
Sex | HIV status (positive) n/N (%) |
On antiretroviral therapy (HAART) n/N (%) |
|||
| 13 | 27 | ≤18 | Male: 12 Female: 15 |
— | — | ||||
| 14 | 2008–2016 | 428 | ≥15 | Male: 263 Female: 165 |
94/428 (22) | 92/94 (97.9) | |||
| 15 | 2016–2018 | 195 | ≥18 | Male: 84 Female: 111 |
123/195 (63) | 113/195 (58) | |||
| 16 | 2015–2017 | 109 | ≥14 | Male: 57 Female: 52 |
56/109 (51) | — | |||
| 17 | 2008–2012 | 273 | — | Male: 154 Female: 119 |
119/273 (43) | 108/273 (39) | |||
| 18 | 2016 | 28 | ≥ 18 | Male: 17 Female: 11 |
11/28 (39) | — | |||
| 19 | 2015–2018 | 472 | ≥18 | Male: 289 Female: 183 |
471/472 (99) | 54/472 (11) | |||
| 20 | 2013–2017 | 108 | — | Female: 108 | 88/108 (81) | 74/108 (68) | |||
| 9 | 2013–2014 | 91 | ≥18 | Male: 55 Female:36 |
54/91 (59) | 54/91 (59) | |||
| 21 | 2017 | 1,387 | ≥18 | Male: 812 Female: 575 |
967/1,387 (70) | 918/1,387 (66) | |||
| 22 | 2016–2019 | 297 | ≥18 | Male: 151 Female: 147 |
137/297 (46) | 297/297 (100) | |||
| 23 | 2008–2017 | 272 | ≥18 | Male: 161 Female: 111 |
135/272 (50) | 125/272 (46) | |||
| 24 | 2014–2018 | 63 | ≥18 | Male: 39 Female: 24 |
37/63 (59) | — | |||
| 25 | 2014–2018 | 122 | ≥18 | Male: 74 Female: 48 |
64/122 (52) | — | |||
| 26 | 2014–2016 | 19,617 | ≥15 | Male: 10 Female: 959/8,658 |
13,893/19,617 (71) | 12,430/19,617 (63) | |||
| 27 | 2018–2019 | 117 | ≥14 | Male: 70 Female: 47 |
80/117 (68) | 80/117 (68) | |||
| 28 | 2015–2017 | 330 | ≥18 | Male: 190 Female: 140 |
204/330 (62) | 233/330 (70.6) | |||
| 29 | 2013–2018 | 537 | ≥18 | Male: 342 Female: 195 |
138/537 (25.7) | — | |||
| 30 | 2016 | 30 | ≥18 | Male: 10 Female: 20 |
14/30 (46.7) | — | |||
| 31 | 2021 | 5 | ≥18 | Female: 5 | 3/5 (60) | 1/5 (20) | |||
| 32 | 2015–2017 | 211 | ≥18 | Male: 122 Female: 89 |
108/211 (51) | ||||
| 33 | 2008–2019 | 2008 | ≥18 | Male: 1,055 Female: 953 |
1,445/2008 (72) | 1,351/2008 (67.3) | |||
| 34 | 2014–2015 | 151 | Male: 72 Female: 79 |
116/151 (76.8) | 116/151 (76.8) | ||||
| 35 | 2013–2015 | 200 patients | ≥18 | Female: 99 Male: 101 |
134/200 (67.0) | 134/200 (67.0) | |||
| 36 | 2017–2019 | 181 | ≥14 | Male: 112 Female: 69 |
36 /181 (20) |
— | |||
| Total | 27,259 | — | 15,199/12,061 | 18,527 | 16,180 | ||||
| B) TB type, treatment regimen and treatment outcome | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Reference | MDR-TB n/N (%) |
Pre-XDR-TB n/N (%) |
XDR-TB n/N (%) |
BDQ n/N (%) |
Pa n/N (%) |
LZD n/N (%) |
Optimised background regiment | Favourable treatment outcome | Unfavourable treatment outcome |
| 13 | — | 9/27 (33) | 18/27 (67) | 27/27 (100) | — | 26/27 (96) | Yes | 27/27 (100) | — |
| 14 | 233/428 (54) | — | 195/428 (45.6) | 428/428 (100) | — | 4/428 (1) | Yes | 276/428 (64) | 152/428 (35) |
| 15 | 29/195 (15) | 78/195 (40) | 80/195 (41) | 195/195 (100) | — | 179/195 (92) | Yes | 145/195 (74) | 50/195 (26) |
| 16 | 38/109 (35) | — | 71/109 (65) | 109/109 (100) | — | 88/109 (81) | Yes | 97/109 (89) | 11/109 (10) |
| 17 | — | — | 273/273 (100) | — | — | 179/273 (65) | Yes | 70/273 (26) | 203/273 (74) |
| 18 | 2/28 (7) | 10/28 (36) | 14/28 (50) | 28/28 (100) | — | 23/28 (82) | Yes | 22/28 (79) | 6/28 (21) |
| 19 | 472/472 (100) | — | — | 472/472 (100) | — | 423/472 (89.6) | Yes | 358/ 458 (78) | 100/458 (22) |
| 20 | 83/108 (77) | 25/108 (23) | 25/108 (23) | 58/108 (54) | — | — | Yes | 72/108 (67) | 36/108 (33) |
| 9 | — | 57/91 (63) | 34/91 (37) | 91/91 (100) | — | 64/91 (70) | Yes | 58/91 (64) | 33/91 (36) |
| 21 | 1387/1387 (100) | — | — | 688/1387 (50) | — | — | Yes | 928/1387 (67) | 459/1,387 (33) |
| 22 | 297/297 (100) | — | — | 92/297 (31) | — | — | Yes | 73/92 (79) | 19/92 (21) |
| 23 | — | — | 272/272 (100) | 68/272 (25) | — | 55/272 (20) | Yes | 45/68 (66) | 23/68 (34) |
| 24 | — | — | 63/63 (100) | 41/63 (65) | — | 22/63 (35) | Yes | 45/63 (71) |
18/63 (28) |
| 25 | 11/122 (9) | 25/122 (20) | 86/122 (70) | 122/122 (100) | — | 103/122 (84) | Yes | 79/122 (65) | 43/122 (35) |
| 26 | 18,542/1,9617 (94) | — | 1,075/19,617 (5) | 1016/19 617 (5) | — | — | Yes | 479/1016 (47) | 128/1,016 (12) |
| 27 | 117/117 (100) | — | — | 108/117 (92.3) | — | 107/117 (91) | Yes | 88/117 (75) |
29/117 (25) |
| 28 | 330/330 (100) | — | — | 162/330 (49) | — | — | — | 42/330 (13) | 288/330 (87) |
| 29 | 349/537 (65) | — | 188/537 (35) | 537/537 (100) | — | — | Yes | 325/537 (60) | 212/537 (39) |
| 30 | — | — | 30/30 (100) | 30/30 (100) | — | — | Yes | 30/30 (100) | — |
| 31 | 5/5 (100) | — | — | 5/5 (100) | — | 5/5 (100) | Yes | 3/5 (60) | 2/5 (40) |
| 32 | 109/211 (52) | — | 102/211 (48) | 211/211 (100) | 109/ (52) | 195/211 (92) | Yes | 164/211 (78) | 47/211 (22) |
| 33 | 2008/2008 (100) | — | — | 619/2008 (31) | — | 619/2008 (31) | Yes | 1772/2,008 (88) | 236/2,008 (12) |
| 34 | — | 45/151 (29.8) | 106/151 (70.2) | 151/151 (100) | — | 151/151 (100) | Yes | 96/151 (63) | 55/151 (36) |
| 35 | — | 113/200 (56.5) | 87/200 (43) | 200/200 (100) | — | 128/200 (64) | Yes | 146/200 (73) | 54/200 (27) |
| 36 | 21/181 (12) | 85/181 (47) | 75/181 (41) | 181/181 (100) | 181/181 (100) | 181/181 (100) | Yes | 170/181 (93) | 11/181 (6) |
| Total | 24,033 | 447 | 2,794 | 5,639 | 290 | 2,552 | — | 5,610 | 2,215 |
BPaL = BDQ+Pa+LZD; RR-TB= rifampicin-resistant TB; HAART = highly active antiretroviral therapy; MDR-TB = multidrug-resistant TB; XDR-TB = extensively drug-resistant TB; BDQ = bedaquiline; Pa = pretomanid; LZD = linezolid.
In total, 27,259 individuals participated in the included studies (including the control groups from the individual studies), which were conducted between 2015 and 2022; most of the participants (16/25, 64%) were ≥18 years old. A total of 21% (5,639/27,259) patients were on BDQ, 1% (290/27,259) were on Pa and 9% (2,552/27,259) treated with LZD. The BPaL drugs used in combination with other second-line anti-TB drugs (isoniazid, rifampicin [RIF], moxifloxacin, levofloxacin, para-aminosalicylic acid, terizidone, ethionamide and delamanid). The BPaL combination treatment regimens varied depending on the individual cases being treated.
Among the participants, 56% (15,199/27,259) were males while 44% (12,061/27,259) were females (Table). Approximately 68% of (18,527/27,259) patients were HIV-positive, while antiretroviral drugs were administered to 59% (16,180/27,259) of the participants. However, not all studies indicated whether antiretroviral medications were being taken by all patients in the study (Table). In total, 27,259 patients with TB were categorised as MDR, pre-XDR and XDR-TB; 88% (24,033/27,259) of patients had RR-TB, 2% (447/27,259) had pre-XDR-TB and 10% (2,794/27,259) had XDR-TB (Table). A total of 66% (5,610/8,481) patients had favourable treatment outcomes and 26% (2,215/8,481) patients had unfavourable treatment outcomes among the recipients of the BPaL optimal background regimen.
Definitions
The included studies categorised HIV and ART status as HIV-negative, HIV-positive on ART, HIV-positive no ART reported, or HIV status unknown. Characteristics of TB included whether diagnosed as MDR-, RR- or XDR-TB, as well as history of previous first-line or second-line TB treatment. Favourable outcomes included patients who achieved cure (defined as treatment completed without evidence of failure, with three or more consecutive negative sputum culture results after the intensive phase of treatment) or treatment completion. Unfavourable outcomes were assigned to patients who died, failed treatment, relapse or were lost to follow-up (LTFU).23
Meta-analysis
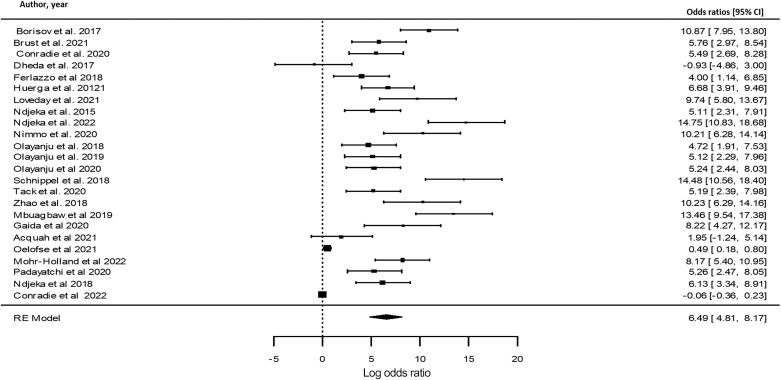
To assess the impact of BPaL drugs use as part of a background regimen and treatment outcomes, meta-analysis was used to evaluate the impact of BPaL on favourable treatment outcomes during therapy. The strength of the association between the occurrences were measured using ORs. Figure 2 illustrates the outcomes of the meta-analysis, which revealed a positive association in the use of BPaL and favourable treatment outcomes in the included studies.
Figure 2.
Forest plot indicating positive association between BPaL use and favourable treatment outcomes. BPaL use associated with favourable treatment outcomes. CI = confidence interval; RE = random effects; BPaL = bedaquiline, pretomanid and linezolid.
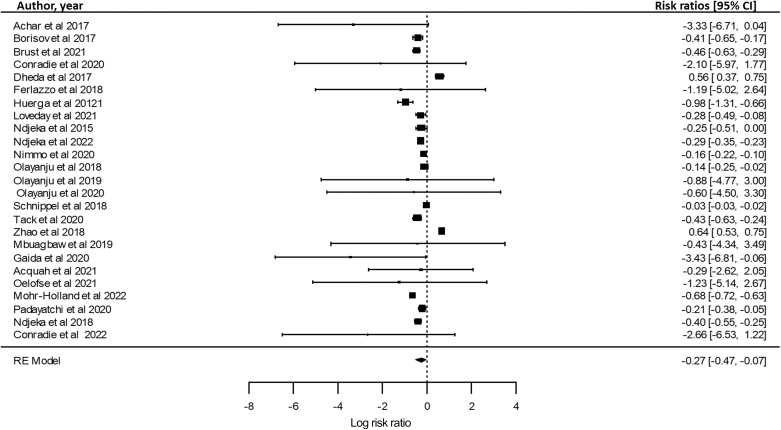
To evaluate how BPaL and HAART use affects the treatment outcome, a meta-analysis was carried out to offer transparent and objective findings of the included studies in this review (Figure 3). Relative risk ratios (RRs) were used to determine the strength of the association of BPaL and HAART use to the treatment outcomes of RR-TB patients. When assessed, RR-TB patients who received BPaL and HAART drugs showed favourable treatment outcome as seen in the forest plot (Figure 3). Overall, the data indicate that the use of BPaL with HAART drugs leads to increased therapeutic success in the treatment of RR-TB, thus suggesting that the BPaL regimen; combined with HAART drugs may increase treatment success in the treatment of RR-TB.
Figure 3.
Forest plot indicating favourable outcome with BPaL regimen + HAART use: relative risk of favourable outcome in patients taking BPaL regimen + ARVs. CI = confidence interval; RE = random effects; BPaL = bedaquiline, pretomanid and linezolid; HAART = highly active antiretroviral therapy; ARV = antiretroviral.
DISCUSSION
The WHO 2022 policy guidelines have proposed accelerating the use of BPaL/M in shorter and completely oral treatment regimens for multidrug-resistant/rifampicin-resistant TB (MDR- or RR-TB) as a replacement for the second-line injectable medication, or as a full novel shorter regimen under operational research settings.38 Therefore, this review assessed treatment outcomes in RR-TB patients using BPaL and other second-line anti-TB drugs as recommended by the WHO in the South African population (cohort studies, programmatic, expanded access).
The WHO proposed that by simplifying the treatment regimen, patients may be able to adhere to therapy much better and achieve more favourable treatment outcomes.2 For both individual patients with TB and national TB programmes, a shorter duration of treatment that is effective is beneficial.16 Visits to healthcare facilities place a financial and time burden on patients. Income loss often constitutes the largest financial risk for patients. For TB programmes, a shorter duration of treatment translates into fewer patients being in care at any one time, with the potential for reduced loss to follow-up.16
The review included 27,259 participants: 21% (5,639/27,259) patients were on BDQ, 1% (290/27,259) patients on Pa and approximately 9% (2,552/27,259) were on LZD respectively. In a setting with a high HIV burden, the South African BPaL-oral short regimen yielded promising results. Among the recipients of the BPaL optimal regimen, a total of 66% (5,610/8,481) had favourable treatment outcomes. The included studies suggested that the detection of RR-TB and use of BPaL drugs may lead to increased therapeutic success or favourable treatment outcomes. These findings are in line with other BPaL deployment experiences from high HIV and TB burden areas,32 which reported that the use of BPaL has resulted in decreases in DR-TB-related mortality and treatment failure.32 Approximately 68% (18,527/27,259) of participants were HIV-positive, while 59% (16,180/27,259) of participants were receiving antiretroviral drugs. Patients with TB were classified into three categories: 88% (24,033/27,259) had RR-TB, 2% (447/27,259) had pre-XDR-TB and 10% (2,794/27,259) had XDR-TB (Table). The current review showed that HAART usage in patients with HIV-TB coinfection had a positive impact on the treatment outcomes of patients taking BPaL regimens.
A meta-analysis was performed in the current review to provide transparent, objective and repeatable replicable summaries of the included study findings. Figure 2 shows that the majority of the included studies using BPaL therapy had favourable outcomes, while only one study showed unfavourable outcomes in the BPaL therapy in the meta-analysis (Figure 2). This finding is supported by the recent study report by Conradie et al. on BPaL in South Africa.16 The statistical findings revealed that BPaL may support increased therapeutic success in the treatment of RR-TB. A meta-analysis was used to calculate the ORs using a statistical analytic tool (R Studio) to measure the association between HIV and the use of HAART drugs in patients receiving BPaL therapy. The association between BPaL exposure and treatment outcome was assessed to understand the impact of HIV and the use of HAART treatment (Figure 3). The meta-analysis showed positive association between HIV, HAART and BPaL use which impacted the favourable treatment outcome in individuals taking BPaL therapy (Figure 3).
The review findings revealed that RR-TB patients taking BPaL drugs with an optimal background regimen stand a favourable treatment outcome. However, there are a number of limitations, including the limited number of published studies assessing treatment outcomes in RR-TB patients using BPaL and other second-line anti-TB drugs in the South African population. The lack of further BPaL patient data is another limitation of the review. Not all the included studies reported on the patient history up to treatment success or failure in either the MDR-, pre-XDR- or the XDR-TB groups, as well as the period of initiation of treatment to completion. As the review only focused on South Africa, which potentially limits the generalisability of the findings compared to other countries in the continent. South Africa was selected because it has a developed regulatory framework, good clinical trial capacity and historically poor outcomes among patients with RR-TB and a high background prevalence of HIV-positive cases in the population.16 Future research is needed to evaluate whether adding BPaL and the proper use of the second-line treatment regimen, as recommended by the WHO, improves patient outcomes in Africa. The WHO’s recent conditional recommendation to employ a 6-month treatment regimen comprising BDQ, Pa, LZD (600 mg) and moxifloxacin (BPaLM), instead of the 9-month or longer (18-month) regimens in RR-TB patients necessitate more research into the efficacy of these medications.38 While it is very critical to offer BPaL in DR-TB patients, WHO standards should be strictly followed by healthcare practitioners, and patient adherence monitored to prevent the development of BPaL-resistant Mycobacterium tuberculosis strains in South Africa.
CONCLUSION
In conclusion, these prospective long-term outcome data from a TB-endemic setting indicate that a BDQ, Pa and LZD-based treatment regimen can result in substantial and remarkable improvement in treatment outcomes among patients with RR-TB. These data inform clinical practice in endemic settings and make a strong case for the immediate roll-out of these drugs for the treatment of RR-TB in endemic settings. Given the importance of BPaL treatment regimen in current and future treatment, routine BPaL resistance monitoring is urgently needed and available programmes require strengthening to ensure full adherence and prevent widespread transmission of BPaL-resistant TB. The advent of new and repurposed bactericidal drugs such as LZD, Pa and BDQ can offer new hope for patients with DR-TB in South Africa and the rest of the world. Further investigations looking into the effectiveness of BPaL treatment in Africa are required.
Acknowledgements
The authors acknowledge the University of Venda, Stellenbosch University, Cape Town, South Africa, and the SAMRC for the works done in collaboration.
Funding Statement
Research reported in this article was supported by the South African Medical Research Council (SAMRC) through its Division of Research Capacity Development under the Research Capacity Development Initiative from funding received from the South African National Treasury. The content and findings reported/illustrated are the sole deduction, view and responsibility of the researcher and do not reflect the official position and sentiments of the SAMRC.
Footnotes
Conflicts of interest: none declared.
References
- 1.World Health Organization The use of bedaquiline in the treatment of multidrug-resistant tuberculosis: interim policy guidance. Geneva, Switzerland: WHO; 2013. [PubMed] [Google Scholar]
- 2.World Health Organization WHO consolidated guidelines on drug-resistant tuberculosis treatment. Geneva, Switzerland: WHO; 2019. [PubMed] [Google Scholar]
- 3.Department of Health, Republic of South Africa Interim clinical guidance for the implementation of injectable-free regimens for rifampicin-resistant tuberculosis in adults, adolescents, and children. Pretoria, South Africa: DoH; 2018. [Google Scholar]
- 4.Burki T. BPaL approved for multidrug-resistant tuberculosis. Lancet Infect Dis. 2019;19(10):1063–1064. doi: 10.1016/S1473-3099(19)30489-X. [DOI] [PubMed] [Google Scholar]
- 5.Dheda K, et al. Recent controversies about MDR and XDR-TB: Global implementation of the WHO shorter MDR-TB regimen and bedaquiline for all with MDR-TB? Respirology. 2018;23(1):36–45. doi: 10.1111/resp.13143. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen TVA, et al. Bedaquiline resistance: its emergence, mechanism, and prevention. Clin Infect Dis. 2018;66(10):1625–1630. doi: 10.1093/cid/cix992. [DOI] [PubMed] [Google Scholar]
- 7.Murray CJL, et al. Global, regional, and national incidence and mortality for HIV, tuberculosis, and malaria during 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9947):1005–1070. doi: 10.1016/S0140-6736(14)60844-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmad N, et al. Collaborative Group for the Meta-Analysis of Individual Patient Data in MDR-TB treatment-2017 Treatment correlates of successful outcomes in pulmonary multidrug-resistant tuberculosis: an individual patient data meta-analysis. Lancet. 2018;392(10150):821–834. doi: 10.1016/S0140-6736(18)31644-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ndjeka N, et al. Treatment of drug-resistant tuberculosis with bedaquiline in a high HIV prevalence setting: an interim cohort analysis. Int J Tuberc Lung Dis. 2015;19(8):979–985. doi: 10.5588/ijtld.14.0944. [DOI] [PubMed] [Google Scholar]
- 10.Guglielmetti L, et al. Compassionate use of bedaquiline for the treatment of multidrug-resistant and extensively drug-resistant tuberculosis: interim analysis of a French cohort. Clin Infect Dis. 2015;60(2):188–194. doi: 10.1093/cid/ciu786. [DOI] [PubMed] [Google Scholar]
- 11.Moher D, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 12.Schwarzer G. Meta-analysis in R. In: Systematic reviews in health research: meta-analysis in context. Hoboken, NJ, USA & Chichester, UK: John Wiley; 2022. pp. 510–534. [Google Scholar]
- 13.Achar J, et al. Off-label use of bedaquiline in children and adolescents with multidrug-resistant tuberculosis. Emerg Infect Dis. 2017;23(10):1711. doi: 10.3201/eid2310.170303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borisov SE, et al. Effectiveness and safety of bedaquiline-containing regimens in the treatment of MDR- and XDR-TB: a multicentre study. Eur Respir J. 2017;49(5):1700387. doi: 10.1183/13993003.00387-2017. [DOI] [PubMed] [Google Scholar]
- 15.Brust JCM, et al. Effectiveness and cardiac safety of bedaquiline-based therapy for drug-resistant tuberculosis: a prospective cohort study. Clin Infect Dis. 2021;73(11):2083–2092. doi: 10.1093/cid/ciab335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conradie F, et al. Treatment of highly drug-resistant pulmonary tuberculosis. N Engl J Med. 2020;382(10):893–902. doi: 10.1056/NEJMoa1901814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dheda K, et al. Outcomes, infectiousness, and transmission dynamics of patients with extensively drug-resistant tuberculosis and home-discharged patients with programmatically incurable tuberculosis: a prospective cohort study. Lancet Respir Med. 2017;5(4):269–281. doi: 10.1016/S2213-2600(16)30433-7. [DOI] [PubMed] [Google Scholar]
- 18.Ferlazzo G, et al. Early safety and efficacy of the combination of bedaquiline and delamanid for the treatment of patients with drug-resistant tuberculosis in Armenia, India, and South Africa: a retrospective cohort study. Lancet Infect Dis. 2018;18(5):536–544. doi: 10.1016/S1473-3099(18)30100-2. [DOI] [PubMed] [Google Scholar]
- 19.Huerga H, et al. Safety and effectiveness outcomes from a 14-country cohort of patients with multi-drug resistant tuberculosis treated concomitantly with bedaquiline, delamanid, and other second-line drugs. Clin Infect Dis. 2022;75(8):1307–1314. doi: 10.1093/cid/ciac176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loveday M, et al. Maternal and infant outcomes among pregnant women treated for multidrug/rifampicin-resistant tuberculosis in South Africa. Clin Infect Dis. 2021;72(7):1158–1168. doi: 10.1093/cid/ciaa189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ndjeka N, et al. Treatment outcomes 24 months after initiating short, all-oral bedaquiline-containing or injectable-containing rifampicin-resistant tuberculosis treatment regimens in South Africa: a retrospective cohort study. Lancet Infect Dis. 2022;22(7):1042–1051. doi: 10.1016/S1473-3099(21)00811-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nimmo C, et al. Dynamics of within-host Mycobacterium tuberculosis diversity and heteroresistance during treatment. EBioMedicine. 2020;55:102747. doi: 10.1016/j.ebiom.2020.102747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olayanju O, et al. Long-term bedaquiline-related treatment outcomes in patients with extensively drug-resistant tuberculosis from South Africa. Eur Respir J. 2018;51(5):1800544. doi: 10.1183/13993003.00544-2018. [DOI] [PubMed] [Google Scholar]
- 24.Olayanju O, et al. Linezolid interruption in patients with fluoroquinolone-resistant tuberculosis receiving a bedaquiline-based treatment regimen. Int J Infect Dis. 2019;85:74–79. doi: 10.1016/j.ijid.2019.04.028. [DOI] [PubMed] [Google Scholar]
- 25.Olayanju O, et al. A regimen containing bedaquiline and delamanid compared to bedaquiline in patients with drug-resistant tuberculosis. Eur Respir J. 2020;55(1):1901181. doi: 10.1183/13993003.01181-2019. [DOI] [PubMed] [Google Scholar]
- 26.Schnippel K, et al. Effect of bedaquiline on mortality in South African patients with drug-resistant tuberculosis: a retrospective cohort study. Lancet Respir Med. 2018;6(9):699–706. doi: 10.1016/S2213-2600(18)30235-2. [DOI] [PubMed] [Google Scholar]
- 27.Tack I, et al. Safety and effectiveness of an all-oral, bedaquiline-based, shorter treatment regimen for rifampicin-resistant tuberculosis in high human immunodeficiency virus (HIV) burden rural South Africa: a retrospective cohort analysis. Clin Infect Dis. 2021;73(9):e3563–e3571. doi: 10.1093/cid/ciaa1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao Y, et al. Improved treatment outcomes with bedaquiline when substituted for second-line injectable agents in multidrug-resistant tuberculosis: a retrospective cohort study. Clin Infect Dis. 2019;68(9):1522–1529. doi: 10.1093/cid/ciy727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mbuagbaw L, et al. Outcomes of bedaquiline treatment in patients with multidrug-resistant tuberculosis. Emerg Infect Dis. 2019;25(5):936. doi: 10.3201/eid2505.181823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gaida R, Truter I, Peters CA. Adverse effects of bedaquiline in patients with extensively drug-resistant tuberculosis. S Afr J Infect Dis. 2020;35(1):1–6. doi: 10.4102/sajid.v35i1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Acquah R, et al. Outcomes of children born to pregnant women with drug-resistant tuberculosis treated with novel drugs in Khayelitsha, South Africa: a report of five patients. Pediatr Infect Dis J. 2021;40(5):e191. doi: 10.1097/INF.0000000000003069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oelofse S, et al. Pretomanid with bedaquiline and linezolid for drug-resistant TB: a comparison of prospective cohorts. Int J Tuberc Lung Dis. 2021;25(6):453–460. doi: 10.5588/ijtld.21.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mohr-Holland E, et al. Early mortality during rifampicin-resistant TB treatment. Int J Tuberc Lung Dis. 2022;26(2):150–157. doi: 10.5588/ijtld.21.0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Padayatchi N, et al. Treatment outcomes in patients with drug-resistant TB-HIV co-infection treated with bedaquiline and linezolid. Int J Tuberc Lung Dis. 2020;24(10):1024–1031. doi: 10.5588/ijtld.20.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ndjeka N, et al. High treatment success rate for multidrug-resistant and extensively drug-resistant tuberculosis using a bedaquiline-containing treatment regimen. Eur Respir J. 2018;52(6):1801528. doi: 10.1183/13993003.01528-2018. [DOI] [PubMed] [Google Scholar]
- 36.Conradie F, et al. Bedaquiline–pretomanid–linezolid regimens for drug-resistant tuberculosis. N Engl J Med. 2022;387(9):810–823. doi: 10.1056/NEJMoa2119430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berry C, et al. TB-PRACTECAL: study protocol for a randomised, controlled, open-label, phase II–III trial to evaluate the safety and efficacy of regimens containing bedaquiline and pretomanid for the treatment of adult patients with pulmonary multidrug-resistant tuberculosis. Trials. 2022;23(1):484. doi: 10.1186/s13063-022-06331-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.World Health Organization WHO consolidated guidelines on tuberculosis. Module 4: treatment-drug-resistant tuberculosis treatment, 2022 update. Geneva, Switzerland: WHO; 2022. [PubMed] [Google Scholar]