Summary
Background
Cognitive deficits are among the main disabling symptoms in COVID-19 patients and post-COVID syndrome (PCS). Within brain regions, the hippocampus, a key region for cognition, has shown vulnerability to SARS-CoV-2 infection. Therefore, in vivo detailed evaluation of hippocampal changes in PCS patients, validated on post-mortem samples of COVID-19 patients at the acute phase, would shed light into the relationship between COVID-19 and cognition.
Methods
Hippocampal subfields volume, microstructure, and perfusion were evaluated in 84 PCS patients and compared to 33 controls. Associations with blood biomarkers, including glial fibrillary acidic protein (GFAP), myelin oligodendrocyte glycoprotein (MOG), eotaxin-1 (CCL11) and neurofilament light chain (NfL) were evaluated. Besides, biomarker immunodetection in seven hippocampal necropsies of patients at the acute phase were contrasted against eight controls.
Findings
In vivo analyses revealed that hippocampal grey matter atrophy is accompanied by altered microstructural integrity, hypoperfusion, and functional connectivity changes in PCS patients. Hippocampal structural and functional alterations were related to cognitive dysfunction, particularly attention and memory. GFAP, MOG, CCL11 and NfL biomarkers revealed alterations in PCS, and showed associations with hippocampal volume changes, in selective hippocampal subfields. Moreover, post mortem histology showed the presence of increased GFAP and CCL11 and reduced MOG concentrations in the hippocampus in post-mortem samples at the acute phase.
Interpretation
The current results evidenced that PCS patients with cognitive sequalae present brain alterations related to cognitive dysfunction, accompanied by a cascade of pathological alterations in blood biomarkers, indicating axonal damage, astrocyte alterations, neuronal injury, and myelin changes that are already present from the acute phase.
Funding
Nominative Grant FIBHCSC 2020 COVID-19. Department of Health, Community of Madrid. Instituto de Salud Carlos III through the project INT20/00079, co-funded by European Regional Development Fund “A way to make Europe” (JAMG). Instituto de Salud Carlos III (ISCIII) through Sara Borrell postdoctoral fellowship Grant No. CD22/00043) and co-funded by the European Union (MDC). Instituto de Salud Carlos III through a predoctoral contract (FI20/000145) (co-funded by European Regional Development Fund “A way to make Europe”) (MVS). Fundación para el Conocimiento Madri+d through the project G63-HEALTHSTARPLUS-HSP4 (JAMG, SOM).
Keywords: Post-COVID syndrome, Cognition, Hippocampus, Neuroimaging, Blood biomarkers, Histopathology
Research in context.
Evidence before this study
We searched PubMed for articles with the following keywords: “post-COVID” AND “neuroimaging” AND “cognition” from 2020 to April 2023. Search resulted in 20 articles, from which only three shared the same objective, but did not focus on the hippocampus and did not perform a multidisciplinary analysis. Few studies have been conducted using neuroimaging, revealing that cognitive dysfunction in COVID-19 patients is linked to brain alterations, including the hippocampus, from the acute to post-acute phases, suggesting that hippocampus might be a target of COVID-19. Previous research includes studies performed in shorter follow-up periods and lack of interdisciplinary and multimodal analysis, without blood biomarkers analysis or comparisons with histopathological samples of post-mortem patients at the acute phase.
Added value of this study
We conducted an interdisciplinary and multimodal long-term follow-up analysis of patients with post-COVID syndrome and compared with post-mortem patients at the acute phase. We found that patients with post-COVID syndrome at one year from the infection present hippocampal volume loss which was related to other grey and white matter brain alterations, linked to cognitive impairment. These alterations were supported by neuroimaging and biomarker analysis, revealing axonal damage, astrocyte alterations, neuronal injury, and myelin changes. All these changes were also found in necropsies from acute COVID-19 patients after death.
Implications of all the available evidence
Presence of several pathophysiological mechanisms are involved in the hippocampal damage form the acute phase and some of them still activated in post-COVID syndrome patients after one year. This damage is linked to neuropsychological deficits. Results suggest presence of neuroinflammatory process or neurodegeneration. Understanding the pathophysiological mechanisms of post-COVID syndrome is essential for the correct diagnosis and treatment of patients. Longitudinal follow-up assessments are needed to establish prognostic profiles of post-COVID syndrome patients.
Introduction
SARS-CoV-2 virus infection has been associated with a wide range of neurological manifestations present from the acute phase and may persist over time leading to residual effects. Patients with a history of SARS-CoV-2 infection with persistent symptoms over 12 weeks are diagnosed with post-COVID syndrome (PCS).1 PCS patients present a wide range of symptoms among which cognitive dysfunction is one of the most frequent symptoms,2 having a negative impact in patient’s quality of life.3 Cognitive domains showing greater impairment in PCS include attention, processing speed, executive functions, and memory.4, 5, 6
Cognitive deficits in PCS have been related to structural and functional brain alterations,7, 8, 9 distributed in cortical and subcortical areas. Particularly, the hippocampus showed changes at 4-month and 6-month follow-up,7,10 but also since the acute and post-acute phases.11, 12, 13, 14 These findings suggest that hippocampus might be a target of COVID-19. However, although some functional neuroimaging investigations have suggested the involvement of this region in more extended follow-up periods,8 structural neuroimaging with the needed resolution to evaluate the hippocampus and its subfields is still lacking.
Several pathophysiological mechanisms that may underlie brain alterations following SARS-CoV-2 infection have been proposed, including neuroinflammation.15 A recent study evidenced impaired microglial reactivity in both mice and humans. In mice, alterations in the neurogenesis of the hippocampus and reduced oligodendrocytes and axon myelin were observed and accompanied by increased levels of CSF cytokines, especially eotaxin-1 (CCL11), which showed associations with cognitive symptoms in COVID patients.16 Indeed, a previous PCS study reported significant associations between hippocampal volume atrophy at three months after infection, and systemic inflammatory marker alterations in the acute stage in COVID-19 patients.11 In this line, astrocyte and neuronal injury have also been found related to SARS-CoV-2 infection, reflected in elevated neurofilament light chain (NfL), and glial fibrillary acidic protein (GFAP),17,18 and myelin oligodendrocyte glycoprotein (MOG) alterations19; respective biomarkers of axonal damage,20 astrocyte activation/injury21 or myelin changes.22 Additionally, the hippocampus is a grey matter region especially susceptible to hypoxia, and hypoperfusion, showing greater alterations compared to other brain regions, especially in the cornus amonis and dentate gyrus subfields.23,24 Neuropathological studies in postmortem examinations are very helpful in the understanding and providing insight into the brain pathology. In this case, neuropathological studies also suggested a relationship between COVID-19 symptoms and a systemic inflammation.25
Among brain regions, the hippocampus is a core region for cognition, widely related to memory but also to attention, processing speed, and executive functions.26 Therefore, because cognitive dysfunction is one of the main disabling symptoms in PCS patients and given the susceptibility of the hippocampus to SARS-CoV-2 infection, we considered important to investigate more in depth hippocampal alterations in PCS in vivo, and in post-mortem samples from the acute phase. Performing a comprehensive study of hippocampal abnormalities in vivo and post mortem may shed light about the pathophysiological mechanisms underlying PCS. In this line, analyses of hippocampal subfields, which have its own specialization, may help elucidate different patterns of atrophy related to infection and different relationships with cognitive or clinical symptoms.26,27 Accordingly, we consider that distinct subfields may be more vulnerable to different pathophysiological mechanisms involved in SARS-CoV-2 infection.23,24
Overall, the present study aimed to investigate hippocampal subfield abnormalities in PCS patients within about 1-year follow-up, and in post-mortem samples at the acute phase to test whether hippocampal alterations were present since the first symptoms of the infection. The study aims were: 1) to evaluate the hippocampal subfields volume changes in PCS compared to healthy controls (HC) and its association with cognition; 2) to assess hippocampal perfusion and microstructural characteristics in PCS and HC; 3) to determine hippocampal volume associations with further structural and functional brain alterations in PCS; 4) to investigate hippocampal volume associations with blood biomarkers in PCS; 5) finally, to analyze the hippocampal neuropathology of post-mortem samples after COVID-19 acute infection.
Methods
Participants
One-hundred and twenty-two participants were recruited, including 86 PCS with subjective cognitive complaints after SARS-CoV-2 and 36 HC, after exclusions, final sample size was 84 PCS and 33 HC. Recruitment flowchart was included in Supplementary Fig. S1. Patients were consecutively recruited through the department of Neurology at Hospital Clínico San Carlos between November 2020 and December 2021, with mean evolution since first symptoms of 11.08 ± 4.47 months. Serological analysis was conducted in HC to exclude both anamnestic and serological cases of previous SARS-CoV-2 infection. Additionally, another HC group (n = 37) was recruited to compare blood biomarker data. Inclusion and exclusion criteria can be found in Supplementary Materials. The main clinical and demographic characteristics of PCS patients are shown in Table 1. Regarding post-mortem analysis, seven autopsies of patients diagnosed with COVID-19 (dead in acute phase), and eight donors considered as controls were analysed.
Table 1.
Sociodemographics of PCS and HC.
| PCS (n = 84) | HC (n = 33) | U/Fisher | p | |
|---|---|---|---|---|
| Age | 50.89 (11.25) | 49.18 (16.14) | 1332.50 | 0.746 |
| Sex (women: n, %) | 58 (69.04%) | 20 (60.60%) | – | 0.392 |
| Education (years) | 14.20 (3.83) | 15.39 (3.74) | 1137.50 | 0.107 |
| Premorbid risk factors | ||||
| Hypertension (n, %) | 20 (23.80%) | 4 (12.12%) | – | 0.210 |
| Diabetes (n, %) | 9 (10.71%) | 1 (3.03%) | – | 0.280 |
| Dyslipidemia (n, %) | 22 (26.19%) | 4 (12.12%) | – | 0.140 |
| Neurological symptoms in the acute stage | ||||
| Headache (n, %) | 67 (79.76%) | – | – | – |
| Hyposmia + ageusia (n, %) | 46 (54.76%) | – | – | – |
Values are expressed in mean and standard deviation (SD) otherwise noted.
Neuropsychological and clinical assessment
PCS patients underwent a comprehensive neuropsychological evaluation. A trained neuropsychologist administered the cognitive protocol including attention, working memory, processing speed, executive functions, memory, language, and visuo perceptive and visuospatial abilities. Clinical assessment included fatigue, depression, olfaction, and sleep disorders. Specific tests can be found in Supplementary Materials.
Neuroimaging acquisition
Patients were scanned using a 3.0T Magnet (GE Signa Architect) and a 48-channel head coil. T1-weighted images, a high resolution in-plane T2-weighted images perpendicular to the hippocampal axis in order to adequate the output, diffusion-weighted images, arterial spin labeling (ASL) and resting-state fMRI, were acquired in a single session. Acquisition parameters are shown in Supplementary Materials.
Hippocampal segmentation
T1-weighted images were preprocessed and analysed with freesurfer software version v7.2.0. The processing of T1 high-resolution images for the cortical surface reconstruction followed the Freesurfer analysis pipeline.28,29 Segmentation of hippocampal subfields was performed with T1 (isotropic 1 mm3 voxel) and additional T2 (high resolution slices in plane transverse to the hippocampus axis) following the latest automated algorithm from freesurfer, which addresses the shortcomings from the previous method.30 The hippocampal subfield atlas was derived from a novel atlas algorithm based on high resolution (0.13 mm) and ex vivo MRI data from autopsy brains using a 7-T scanner. In the current study, 19 hippocampal subfields, divided in head, body and tail, were included for analysis. Subdivisions were as follows 1) HEAD: parasubiculum, presubiculum, subiculum, CA1, CA3, CA4, the molecular and granule cell layers of the dentate gyrus (GC-ML-DG), molecular layer HP and HATA subfields; 2) BODY: precubiculum, subiculum, CA1, CA3, CA4, GC-ML-DG, molecular layer HP and fimbria subfields; 3) TAIL: hippocampal tail. In addition, hippocampal fissure volume was also calculated (Supplementary Fig. S2). Further, whole hippocampal body and head and whole hippocampus volume were extracted. All volumes were extracted for left and right hemispheres separately, and the mean bilateral volume was calculated. Each of the hippocampal subfield volumes group statistical comparison of PCS patients and controls was performed in SPSS. Analyses were performed with bilateral hippocampal subfields volume values, and left and right subfields volume are shown in Supplementary Table S1.
Quality check
We performed a two-step quality control for the hippocampal subfield segmentation process, similar to previous studies.27 First, outliers were considered when volume was ±5 standard deviations of the mean value for each subfield. In a second step, each patient’s hippocampal segmentation was visually inspected by two researchers independently, and overlaid on each patient’s T1 image in order to exclude for errors in the registration or assignment of the subfields. We excluded one patient due to outlier detection, and one HC was excluded due to segmentation errors.
ASL analysis
The ASL acquisition and 3D T1 series from each patient were processed with the ASAP (Automatic Software for ASL Processing) 2.0 toolbox. After an initial step of skull-stripping of the high resolution T1 weighted scan with tissue segmentation for generation of GM and WM probability maps, computation of cerebral blood flow (CBF) map was performed.31 In order to estimate the CBF for the gray and white matter independently, the rigid registration between the low spatial resolution ASL series and the high resolution T1-weighted scan was followed by partial volume correction for different tissues, performed using a previously validated algorithm,32 with a regression kernel of 5 × 5 × 1 voxels. The partial volume correction corrected maps were then normalized to MNI space. Then, perfusion values from subject-specific bilateral hippocampus mask were extracted with the REX toolbox and exported into SPSS for analysis.
Neurite orientation dispersion and density imaging (NODDI) characteristics
Diffusion data were preprocessed and analysed following FDT processing pipeline in FMRIB Software Library (FSL) (v.6.0.5).33 First, each subject’s images were concatenated and radiologically oriented, topup was applied to estimate and correct susceptibility-induced distortions (fieldmap estimation),34 followed by BET brain extraction35 and eddy command to correct for distortion36 with a fieldmap estimated by topup. The current study examined hippocampal grey matter integrity using the NODDI Matlab toolbox.37 NODDI is an advanced non-gaussian diffusion model that quantifies the microstructural characteristics of dendrites and axons, using a three-compartment diffusion model (intracellular, extracellular and free water compartments). FICVF and ODI maps were obtained from the subject-specific bilateral hippocampus mask and included in SPSS for analysis.
White matter volume
Whole-brain white matter volume was calculated with the DARTEL tool (Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra) in SPM12.38 After orientation and segmentation, the mean template was created, then performed spatial normalization into the Montreal Neurological Institute (MNI) template space. Then, images were modulated, and smoothing with isotropic Gaussian kernel of 8 mm full-width at half maximum (FWHM) was applied. Total Intracranial Volume (TIV) was calculated. Bilateral hippocampal volume values were included in the statistical model for correlation analysis with whole-brain white matter volume, with age and TIV as covariates.
Functional connectivity
Functional connectivity (FC) analysis was performed using CONN Functional Connectivity Toolbox 18.b.39 After removing the first 5 scans, each subject’ 200 functional images were realigned and unwrapped, non-linear coregistered with structural data, slice timing corrected (interleaved bottom-up), and spatially normalized into the standard MNI space (Montreal Neurological Institute), then, outliers were detected (ART-based scrubbing) and finally, images were smoothed using a Gaussian kernel of 8 mm FWMH. As recommended, band-pass filtering was performed with a frequency window of 0.008–0.09 Hz.40 Subject’ specific left and right hippocampal volume masks were included in CONN toolbox for analysis. SEED-to-voxel analysis approach was performed, with subject-specific hippocampal volume mask as SEED and cortical and subcortical areas from the Harvard-Oxford atlas as targets.
Determination of blood biomarkers measurement
Blood samples were acquired from PCS patients at time of enrollment in the study. Briefly, for the GFAP, MOG and CCL11 biomarkers we used patient serum and for NfL we used plasma. Biomarker concentrations were measured using a high-sensitivity enzyme-linked immunosorbent assay (ELISA) kit for human samples. Each plate contained a standard curve. Samples were suitably diluted to ensure they fell within the range of the standard curve. FLUOstar Omega plate reader (BMG Labtech) was used to determine the concentrations of the curves, HC and patients, using the MARS—Data analysis program, for their analysis comparing the optical density (OD) of the samples with the standard curve. Among PCS sample, GFAP, MOG and CCL11 was analysed from 57 PCS patients and NfL was analysed from 56 PCS patients (see Supplementary Table S2). Blood biomarker levels from PCS patients were compared with a larger sample of HC (n = 37), equivalent in age (p = 0.168) and sex (p = 0.822). Outliers were detected and excluded, one HC for GFAP and MOG, and three patients and two HC for NfL analysis. Detailed information can be found in Supplementary Materials.
Brain tissue autopsy
Seven autopsies of patients diagnosed with COVID-19 (dead in acute phase), and eight donors considered as controls were analysed. Control patients had no history of neurological disease or with a cause of death unrelated to any neurological condition, including 4 women with an age range of 30–72 years and 4 men from 50 to 65 years of age. COVID-19 patients included 3 women with an age range of 22–33 years and 4 men from 62 to 73 years of age. All patients died due to complications of COVID-19 infection. Clinical details of COVID-19 patients are specified in Supplementary Table S3. Presence of GFAP, MOG and CCL11 in the hippocampus was investigated. Autopsy procedure, preparation and storing of biological material are described in Supplementary Materials.
Statistics
Statistical analyses were conducted using IBM SPSS Statistics for Windows, version 26.0 (IBM Corp., Armonk, NY: IBM Corp.). Normality of data was tested with the Kolmogorov–Smirnov test. Sociodemographic, clinical, and cognitive characteristics of the sample were compared using Mann–Whitney U or Chi-squared tests for quantitative or categorical data, respectively. Regarding hippocampal subfields volume differences between PCS and HC, multivariate analysis of covariance (MANCOVA) was performed and results are reported at p < 0.05 FDR-corrected. FC analyses were reported at p < 0.05 FDR-corrected. Hippocampal volume correlations with white matter volume were reported at p < 0.05 FWE-corrected and K > 250 voxels. Neuroimaging analyses included age as covariate and Total Intracranial Volume (TIV) was also included as nuisance covariate in brain structural analysis. Regarding blood biomarkers, measurements were calculated in pg/ml and significant differences were set at p < 0.05, and differences between groups were performed with age as covariate. Correlations between neuroimaging measurements and cognition were performed (p < 0.05). More restricted significance at p < 0.01 and Bonferroni correction (p < 0.0017) was also labelled for correlation analyses in the Figures. Partial correlation analyses were performed with age (and TIV when required) as covariates (two-sided). R scores were considered small, moderate, and large when scores were 0.10, 0.30 and 0.50, respectively.41 Finally, GFAP, MOG and CCL11 presence in post-mortem samples was evaluated with OD values, and significant differences were computed with U-Mann–Whitney due to the small sample size. Effect sizes were reported with partial Eta-Squared (ηp2) for MANCOVA analyses, interpreted as small, medium, or large when scores were 0.01, 0.06 and 0.14, respectively, or r = z/√N for U-Mann–Whitney analyses, interpreted as 0.10, 0.30 and 0.50, respectively.
Ethics
The present study was approved by the ethics committee from Hospital Clínico San Carlos (reference: code 20/633-E, 21/062-E, and 20/651-E-COVID, Ref Biobank 22001) and participants provided written informed consent prior to research participation.
Role of funders
The funders had no role in study design, data collection, data analysis, data interpretation or writing of the report. All authors accept responsibility for the decision to submit for publication.
Results
Sociodemographic and neuropsychological profile of PCS
PCS patients were recruited at mean of 11.08 ± 4.47 months after first symptoms, and had a mean of 50.89 ± 11.25 years old and 69% (58/84) were women. PCS patients and HC were equivalent in age, sex, and education and no significant differences were found in premorbid risk factors (Table 1). During the acute phase, 33.33% (28/84) of PCS were hospitalized and 10.71% (9/84) received assisted ventilation. The hospitalized patients were older compared to non-hospitalized patients but similar in clinical symptoms (Supplementary Table S4).
Neuropsychological profile of PCS is shown in Supplementary Table S5. PCS patients presented with cognitive impairment, mostly in attention (42.9%; 36/84), memory (40.5%; 34/84), executive functions (38.1%; 32/84), but also in visuospatial ability (31%; 26/84), processing speed (28.6%; 24/84), and language (19%; 16/84).
Clinical profile of PCS patients included presence of fatigue in 80.95% (68/84) of patients (mean (m) = 53.27 ± 14.97), depression in 25% (21/84) (m = 14.39 ± 9.04), sleep quality dysfunction in 82.14% (69/84) (m = 9.61 ± 4.70), and olfactory problems in 69.04% (58/84) (m = 9.18 ± 2.34).
Hippocampal subfields volume and cognition
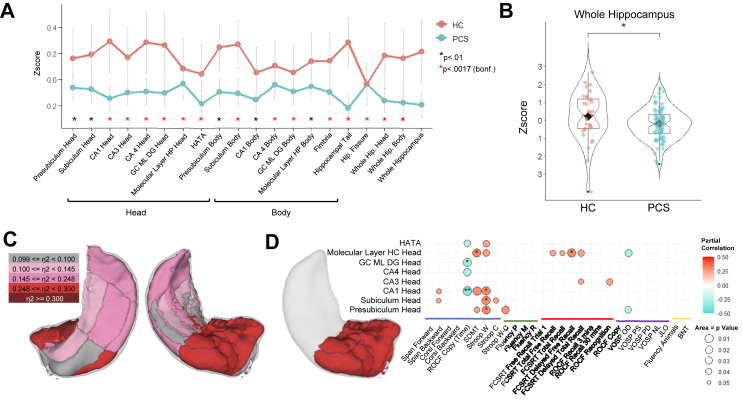
PCS patients presented with lower volume in almost all subfields of the hippocampus as compared to HC, except for CA3 body and parasubiculum subfields. Fig. 1A visualizes the hippocampal subfields that showed significant volume differences between PCS and HC, and whole hippocampal volume differences between PCS and HC are shown in Fig. 1B. All hippocampal subfields volume (bilateral, left and right) are shown in Supplementary Table S1. Effect size of hippocampal subfields volume differences was also calculated, and shown in Fig. 1C. Hippocampal subfields of the head of the hippocampus, including CA1, CA3, CA4, and dentate gyrus showed the largest effect sizes (CA1: ηp2 = 0.248; CA3: ηp2 = 0.253; CA4: ηp2 = 0.292; dentate Gyrus: ηp2 = 0.302), in addition to fimbria (ηp2 = 0.383) from the hippocampal body.
Fig. 1.
Hippocampal subfield volume differences in PCS and HC. (A) Mean (z-scores) and standard error of hippocampal subfield volume differences in PCS and HC. (B) Mean (z-score) of the bilateral whole hippocampus volume in PCS and HC; (C) Visual representation of the effect size of the hippocampal volume differences between PCS and HC, measured with η2; (D) Correlations between head of the hippocampus and cognition (p < 0.05); ∗p < 0.01 and ∗∗p < 0.0017 (Bonferroni corrected). Blue = Attention, processing speed and working memory; Green = Executive Functions; Red = Learning and Memory; Purple = Visuo perceptive, visuospatial and visuoconstructive ability; Yellow = Language; ROCF = Rey–Osterrieth complex figure; SDMT = Symbol digit modalities test; Stroop W = Stroop word subtest; Stroop C = Stroop color subtest; Stroop W-C = Stroop word-color interference subtest; FCSRT = Free and cued selective reminding test; VOSP = Visual object and space perception battery; JLO = Judgment line orientation; BNT = Boston naming test.
Regarding possible effects of disease severity, hippocampal volume differences were more accentuated in hospitalized patients compared to non-hospitalized patients in most of the hippocampal subfields. However, hospitalized patients also showed increased volume in specific subfields, such as presubiculum and subiculum of the head and body of the hippocampus. Supplementary Fig. S3 shows significant volume differences between hospitalized and non-hospitalized patients (p < 0.05).
Focusing on hippocampal volume associations with cognition, head hippocampal subfield volumes revealed stronger and positive associations with cognition, except for ROCF copy (time) that correlated negatively, showing the more time to complete the task, the more reduced volume. Fig. 1D presents the correlations between the head of the hippocampus and cognition. Supplementary Fig. S4 shows a correlation heatmap between whole hippocampal subfields and cognition in PCS patients.
Hippocampal NODDI characteristics and perfusion
Regarding neurite orientation dispersion and density imaging (NODDI) characteristics of the hippocampus, PCS showed higher intracellular volume fraction (FICVF) (PCS = 0.529 ± 0.027; HC = 0.512 ± 0.027; F = 21.544; p < 0.001: ηp2 = 0.152), and higher orientation dispersion index (ODI) values (PCS = 0.356 ± 0.017; HC = 0.351 ± 0.017; F = 21.620; p < 0.001; ηp2 = 0.279) compared to HC—reflecting changes of grey matter organization. Supplementary Fig. S4 shows associations between FICVF and ODI with cognition. Specifically, FICVF inversely correlated with attention, working memory, and memory. Similarly, ODI values showed negative associations with attention, working memory, processing speed and memory.
Regarding hippocampal perfusion, PCS patients showed lower perfusion in the hippocampus compared to HC (PCS = 28.94 ± 6.94; HC = 30.79 ± 7.73; F = 9.023; p < 0.001; ηp2 = 0.137). Reduced perfusion was related to memory performance, specifically to Rey Figure Recognition (r = 0.267; p = 0.016) and FCSRT Total Recall (r = 0.218; p = 0.050).
Hospitalized patients compared to non-hospitalized patients showed lower hippocampal perfusion values (F = 5.413; p = 0.006; ηp2 = 0.118), and increased FICVF (F = 7.085; p = 0.001; ηp2 = 0.152) and ODI values (F = 12.176; p < 0.001; ηp2 = 0.236).
Hippocampal volume and white matter volume
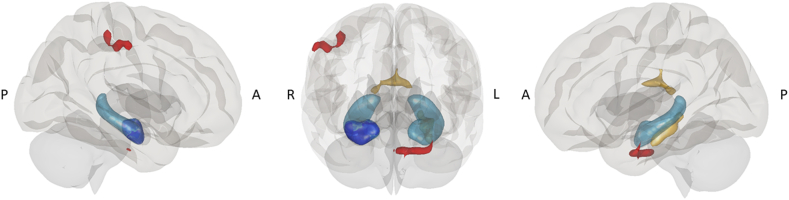
Bilateral hippocampal volume was significantly and positively related to white matter volume adjacent to the left parahippocampal and fusiform gyrus area (peak coordinate: x = −26; y = −30; z = 15; p < 0.05 FWE-corrected), and thalamus (peak coordinate: x = −4; y = −16; z = 18; p < 0.05 FWE-corrected) (Fig. 2). This association was not significant for HC.
Fig. 2.
Hippocampal volume and neuroimaging associations. Schematic representation of hippocampal volume associations with white matter volume and FC from hippocampal head. Whole bilateral hippocampal volume (represented in light blue) was associated with white matter volume in left parahippocampal and fusiform gyrus and thalamus (yellow). FC from the head of the hippocampus (dark blue) showed reduced connectivity with parahippocampal and parietal areas (red) in PCS compared to HC. A = Anterior; P = Posterior; R = Right; L = Left.
In addition, in PCS patients, white matter volume in the left parahippocampal and fusiform area showed significant relationships with attention, processing speed, memory, and working memory, specifically, with SDMT (r = 0.298; p = 0.007), Span Backwards (r = 0.233; p = 0.037), ROCF (time) (r = 0.236; p = 0.035), FCSRT Free Recall Trial 1 (r = 0.229; p = 0.041), FCSRT total free recall (r = 0.294; p = 0.008), FCSRT Total Recall (r = 0.261; p = 0.019), FCSRT delayed free recall (r = 0.330; p = 0.003), FCSRT Delayed Total Recall (r = 0.236; p = 0.035), Fluency (M) (r = 0.229; p = 0.041), and VOSP OD (r = 0.244; p = 0.029), showing higher white matter volume, better cognitive performance.
Functional connectivity of the hippocampus
Due to the stronger associations between hippocampal head volume and cognition, we aimed to investigate FC alterations from the hippocampal head. Results revealed reduced connectivity in PCS patients compared to HC between the right head of the hippocampus and the left anterior parahippocampal division (peak coordinate: x = −18; y = −4; z = −34; 310 voxels; p < 0.05 FDR-corrected), and the parietal area, including supramarginal and postcentral areas, overlapping the dorsal attention network (peak coordinate: x = 52; y = −24; z = 46; 254 voxels; p < 0.05 FDR-corrected) (Fig. 2).
Blood-biomarkers associations with hippocampal characteristics in PCS
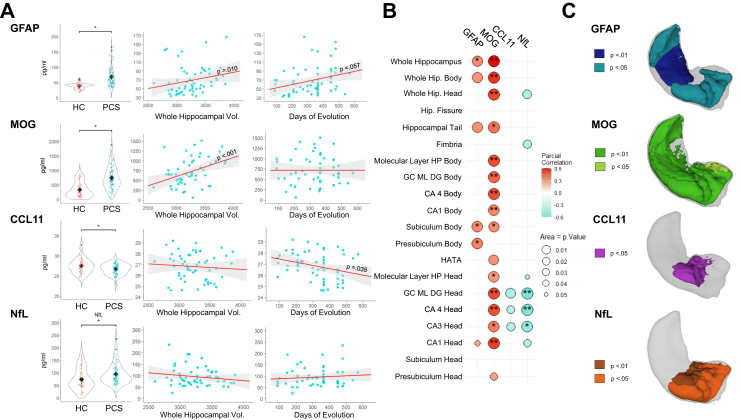
PCS patients compared to HC showed increased values of GFAP (PCS = 68.97 ± 31.54; HC = 39.44 ± 8.28; F = 15.324; p < 0.001; ηp2 = 0.254), MOG (PCS = 757.36 ± 392.49; HC = 338.35 ± 259.73; F = 15.868; p < 0.001; ηp2 = 0.261) and NfL (PCS = 95.66 ± 41.75; HC = 73.90 ± 43.73; F = 7.684; p < 0.001; ηp2 = 0.168), as well as reduced values of CCL11 (PCS = 26.74 ± 1.34; HC = 27.50 ± 1.82; F = 4.529; p = 0.013; ηp2 = 0.091). Distribution of scores is shown in Fig. 3A. Also, hospitalized patients showed increased GFAP and NfL values compared to non-hospitalized patients (GFAP: F = 7.086; p = 0.002; ηp2 = 0.208; NfL: F = 4.090; p = 0.023; ηp2 = 0.141).
Fig. 3.
Blood-biomarkers associations with hippocampal volume. (A) Blood biomarker differences between groups, correlation with whole hippocampal volume and days of evolution in PCS. Black rhomb indicates mean value; (B) Significant correlations between blood biomarkers and hippocampal subfields volume in PCS [∗p < 0.01; ∗∗p < 0.0017 (Bonferroni corrected)]; (C) Visual representation of blood biomarkers associations with hippocampal subfield areas in PCS.
Regarding GFAP, PCS patients showed significant, positive, and moderate correlation with whole hippocampal volume (r = 0.345; p = 0.010) and trends to significance with days of evolution (r = 0.256; p = 0.057). Similarly, MOG showed significant, positive, and large correlation with whole hippocampal volume (r = 0.597; p < 0.001). On the contrary, CCL11 showed a negative and significant correlation with days of evolution (r = −0.282; p = 0.035) (Fig. 3A). Associations between blood biomarkers and hippocampal volume subfields were also analysed in PCS patients and shown in Fig. 3B. GFAP was significantly associated with presubiculum and subiculum body subfields. MOG revealed significant associations with almost all hippocampal volume subfields, CCL11 showed negative and significant associations with dentate gyrus, CA3 head and CA4 head volumes of the hippocampus and NfL showed negative and significant associations with hippocampal head subfields. A visual representation of hippocampal subfields significantly associated with blood biomarkers is presented in Fig. 3C.
Associations between blood biomarkers and NODDI characteristics revealed negative relationships between MOG and FICVF (r = −0.336; p = 0.012), suggesting that higher MOG is related with lower FICVF. Additionally, MOG was positively associated with white matter volume in the thalamus (r = 0.337; p = 0.013) and with white matter volume in the parahippocampal area (r = 0.270; p = 0.049).
Moreover, correlations between blood biomarkers and cognition were performed. Results revealed significant and positive correlations between CCL11 and MOG with cognition, and significant and negative correlations between GFAP and NfL with cognition (Supplementary Fig. S5).
Histopathology of the hippocampus in the acute phase
To examine neuropathological features, histological examination of brain specimens derived from seven deceased COVID-19 patients (aged from 20 to 73 years old, median age 49.4) and eight control brains from normal donors (aged from 30 to 72 years old, median age 57 was performed (Supplementary Materials). In general, perivascular infiltrates and an increase in microglial cells were observed in COVID-19 patients. An interesting histological finding is the apparent neuronal loss in the granular neurons of the dentate gyrus, observing the neuropil loosed and oedematous.
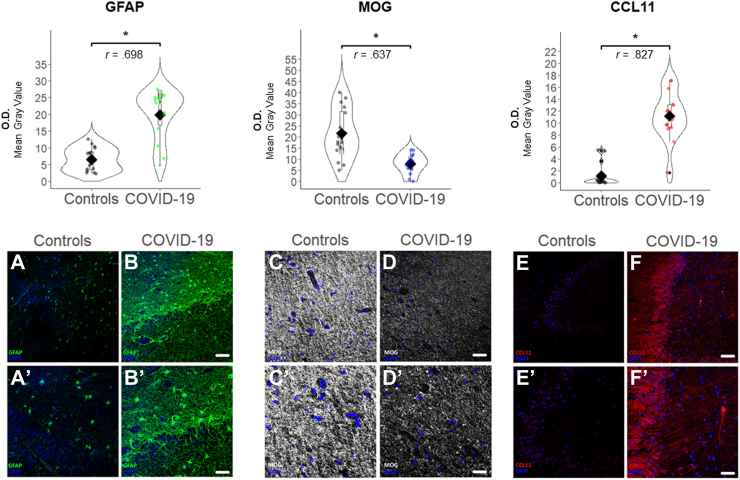
Regarding the GFAP expression in the hippocampus, we found in patients with SARS-CoV-2 infection a marked increase in the number of astrocytic cells, which also showed an increase in the expression of their cytoskeleton visualized with GFAP immunostaining, specifically in the CA4-polymorphic layer-granular cell layer, with histological data compatible with reactive gliosis (Fig. 4). Significant differences were found, showing COVID-19 patients elevated GFAP expression (OD mean = 19.86 ± 7.48) compared to controls (mean = 6.57 ± 3.43) (p < 0.001).
Fig. 4.
GFAP, MOG, and CCL11 expression in the hippocampus tissue samples. Top: Mean values of GFAP, MOG and CCL11 expression in necropsies of COVID-19 patients and controls, measured with O.D. (Optical Density, mean gray value). Mann–Whitney-U test effect size is shown with “r” values. Black rhomb indicates mean value. Bottom: Images of hippocampus tissue sample of GFAP (green), MOG (white), and CCL11 (red) in patients and controls. Example of control tissue sample is shown in A, C, and E. Example of COVID-19 tissue sample is shown in B, D, and F. A′, B′, C′, D′, E′ and F′ represent image detail. Scale bar = 150 μm: A, B, C, D, E, and F; Scale bar = 75 μm: A′, B′, C′, D′, E′, and F′ (∗p < 0.001).
COVID-19 patients showed low expression of immunomarking for MOG (mainly in subiculum, CA1, alveus, fimbria) (OD mean = 7.99 ± 4.43) compared to controls (OD mean = 21.59 ± 11.01) (p < 0.001) (Fig. 4).
At the same time, COVID-19 patients presented with higher expression of CCL11 in neurons and dendrites, especially in the pyramidal ones of CA1, CA4 and, to a lesser extent, in the granule neurons of the hippocampus (Fig. 4). Significant differences were found, showing COVID-19 patients increased CCL11 expression (mean = 11.18 ± 4.10 OD) compared to controls (mean = 1.17 ± 1.85 OD) (p < 0.001).
Discussion
The present study aimed to in vivo investigate hippocampal abnormalities in post-COVID syndrome patients and post mortem samples of patients at acute COVID-19 infection. Findings revealed lower hippocampal subfields grey matter volume accompanied by altered microstructural integrity, hypoperfusion, and functional connectivity changes in PCS compared to controls. Hippocampal subfields volume were correlated with the levels of GFAP, MOG, NfL and CCL11, which showed significant changes against controls. Moreover, histological samples confirmed the presence of increased GFAP and CCL11 and reduced MOG in the hippocampus at the acute phase.
PCS revealed lower volume in most of the subfields of the hippocampus, including head, body and tail compared to HC. Previous studies reported hippocampal volume alterations in COVID-19 patients since the acute and post-acute phases11,14 and long COVID,7,10 which reinforces the hypothesis that the hippocampus may be particularly vulnerable during SARS-CoV-2 infection.
Additionally, these hippocampal abnormalities were related to cognitive dysfunction in PCS. Hippocampal head volume showed greater volume loss and stronger associations with cognitive decline. This goes in line with previous studies that found associations between head of the hippocampus and cognitive deficits in other disorders.42,43 Hippocampal volume reductions were related with attention, processing speed, and working memory deficits, as well as with memory to a lesser extent. Although traditionally hippocampus was described as a structure that contributes to memory ability, more recent studies also report a broader role of the hippocampus in cognition, including attention,44,45 information processing,46 and executive functions.46,47
The lower hippocampal volume in PCS was accompanied by GM microstructural alterations, specifically with increased FICVF and ODI values. ODI is a measure of dispersion of dendrites and axons. Usually FICVF is interpreted as “neurite density” and appears to be reduced in neurodegenerative diseases.48,49 However, in the present study, PCS patients showed higher FICVF compared to controls, which was more accentuated in hospitalized patients, and inversely correlated with cognition, suggesting that increased FICVF in PCS is related to greater cognitive decline. In the same line, ODI values were elevated in PCS patients compared to controls, showing hospitalized patients greater ODI values, which were related to poorer cognitive performance. A previous study in ischemic stroke also found increased FICVF and ODI values during the acute and post-acute phases, and authors suggested the FICVF not be interpreted as neurite density, but rather as an alteration in the intracellular space volume and, thus, associated with grey matter microstructural alterations.50 Similarly, another hypoxic study also found increased FICVF and ODI values.51 The increased intracellular space volume might represent water accumulation inside the cell, consequence of the membrane permeability changes reflected in the altered perfusion, and linked to the increased axonal and dendrite dispersion which might not allow good perfusion and possibly accumulate more water.
In this regard, bilateral hippocampus also revealed hypoperfusion in patients compared to HC, which was related to memory deficits in PCS. Previous studies also reported hypoperfusion in PCS patients in different brain regions,52, 53, 54 including frontal areas and the hippocampus.52,54 In addition, these alterations are likely linked to the severity of symptoms in the acute phase, because hospitalized patients showed greater reduction in perfusion compared to non-hospitalized patients. A previous study suggested that SARS-CoV-2 infection may be linked to a vasoconstricting effect which triggers a reduced blood flow.55 In addition, a potential role of the glymphatic system and the interplay with perivascular spaces and choroid plexus in the homeostasis of fluid circulation and metabolic waste could be hypothesized to explain these findings.
These results suggest that hippocampal volume loss was accompanied by microstructural alterations, showing changes in the intracellular volume, together with hypoperfusion in PCS. Previous studies indicated that cerebral blood flow was associated with white matter microstructural integrity.56 Remarkably, hospitalized patients presented with greater hippocampal alterations compared to non-hospitalized patients, showing lower hippocampal volume in most subfields, accompanied by greater hypoperfusion and grey matter microstructural alterations. The more accentuated alterations in hospitalized patients suggest a relationship between the severity of symptoms in the acute phase and increased brain alterations at follow-up. Previous studies also revealed greater deterioration in hospitalized patients after COVID-19 infection.7,9
The hippocampal volume alterations in PCS were also linked to alterations in other brain areas. On one hand, hippocampal volume reduction was associated with lower white matter volume in adjacent areas to the parahippocampal area and thalamus. The lower white matter volume in the parahippocampus was also related to cognitive deficits, mostly with attention, processing speed and memory. On the other hand, the head of the hippocampus showed reduced connectivity with the right parietal area including postcentral, and supramarginal areas and with the left parahippocampal area in PCS compared to controls. The intraparietal sulcus is located in the parietal area, next to the postcentral and supramarginal areas, and is part of the dorsal attention network.57 Interestingly, the volume of the head of the hippocampus showed greater associations with attention deficits in PCS. Additionally, the parahippocampal gyrus has been found to be a mediational structure in the FC between hippocampus and the default-mode network,58 and previous studies demonstrated relationships between the default-mode network and cognition, including attention and memory.59
Moreover, blood-biomarkers were also acquired at follow-up to investigate possible pathophysiological mechanisms that underlie these brain alterations in PCS. Previous studies suggested microglia reactivity in COVID-19 patients, which is related with astrocyte reactivity, myelin alterations, and impairment of hippocampal neurogenesis.15,16 In the present study, we investigated GFAP, MOG, CCL11 and NfL biomarkers.
On the one hand, GFAP, as an indicator of astrogliosis,60 was increased in PCS patients compared to controls, showing hospitalized patients higher values compared to non-hospitalized patients, similar to previous studies that found increased GFAP values in more severe patients.18,61 The increment in GFAP values in PCS was associated with enlarged whole hippocampal volume, and showed trends to significance with days of evolution, suggesting that GFAP values may increase over time in PCS. Noteworthy, GFAP values showed stronger associations with two specific subfields of the hippocampus, the presubiculum and subiculum. These subfields revealed greater volume in hospitalized patients compared to non-hospitalized patients. Unfortunately, no previous study evaluated GFAP values at longitudinal follow-up (>11 months). Previous studies in COVID-19 patients found increased GFAP values compared to HC in the acute phase,18,61 and its alterations were associated with disease severity.61 Longitudinal studies performed assessments at shorter follow-up periods, and found reduced GFAP values in recovered COVID-19 patients compared to the acute phase,62,63 but also elevated values at long-term.17 However, patients from the present study present neurological complications, including cognitive impairment after 11 months from the infection. A recent PET study with translocator protein total distribution volume (TSPO VT) radiotracer, a marker of gliosis, revealed increased TSPO VT in PCS patients compared to controls, in several areas including the hippocampus.64
On the other hand, MOG biomarker has been linked to myelination,22 and PCS patients showed increased MOG values compared to controls and large associations with hippocampal volume, showing that the higher the MOG values, the greater the hippocampal volume, and this relationship was significant for all the hippocampal subfields. Moreover, higher MOG values were also related to higher white matter volume and lower FICVF. In this case, FICVF change might be understood as deterioration of the intracellular space volume, which may suggest neuroinflammation at this level.
Moreover, CCL11 has been described as an inhibitor of hippocampal neurogenesis.65 In the present study, PCS patients at 11 months follow-up revealed reduced CCL11 compared to controls and inverse associations with days of evolution, suggesting a reduction in CCL11 over time. Interestingly, CCL11 values showed inverse and significant associations with subfields of the head of the hippocampus, with lower CCL11 values indicating increased volume in the dentate gyrus, CA3 and CA4. The dentate gyrus subfield has been described as a neurogenesis area of the hippocampus,66 and this region is directly connected to CA4 and CA3, creating new axonal and synaptic connections between areas. A previous study also found elevated CCL11 values in post-COVID patients with brain fog.16
Furthermore, NfL, a measure of neuronal injury,67 revealed increased values in PCS compared to controls, and these values were more accentuated in hospitalized patients. The combination of increased GFAP and NfL values in blood has been previously found in long COVID patients with neurological symptoms.17 Previous studies assessing shorter follow-up periods also found increased NfL values in severe patients compared to mild or controls.61,63 In fact, elevated NfL values in serum have also been found in hospitalized patients since the acute infection.68 In addition, the increased NfL values in the present study were inversely associated with hippocampal head volume, as previous findings in other disorders.69
GFAP, MOG and CCL11 concentrations in hippocampal necropsies from COVID-19 patients who died at the acute phase were also analysed. Results showed increased GFAP concentration in COVID-19 patients compared to control subjects which goes in line with the elevated values of GFAP in blood at the acute phase found in the literature.18,61 Moreover, reduced MOG was found in post-mortem samples compared to controls, which suggests myelin damage at the acute phase, also found in mice COVID-19 model.16 In addition, post-mortem samples revealed increased concentration of CCL11 compared to controls. Interestingly, biomarker presence in post-mortem samples was analysed in the subgranular zone of the hippocampus, which is consistent with previous studies suggesting its role as an inhibitor of hippocampal neurogenesis.65
Overall, these results suggest that after 11 months from the acute infection, PCS patients with cognitive deficits present brain alterations evidenced by multimodal imaging and several blood biomarkers. These brain alterations are related to neuropsychological deficits. The specific alterations suggest grey and white matter changes, including axonal damage, astrocyte alterations, neuronal injury, and myelin changes. All these changes appear to be present also in necropsies from acute COVID-19 patients after death, therefore, these alterations are present in the acute phase but also at longitudinal follow-up after 11 months. These findings suggest that several pathophysiological processes are involved in hippocampal damage in PCS, and this damage is linked to cognitive deficits. These processes may be summarised in the following three main hypotheses. Firstly, these patients may have experienced an acute damage, such as hypoxia or acute neuroinflammation. This may explain some neuroimaging findings, such as the involvement of CA1, CA3, CA4 and subiculum subfields and NODDI characteristics, previously described in stroke.24,70, 71, 72 However, an isolated acute event would not explain all the findings of our study, such as the elevation of NfL after several months, the neuroimaging correlates of blood biomarkers, or the lack of correlation between NfL and MOG with time. A second hypothesis is the presence of some activated mechanisms over time, especially neuroinflammatory. The presence of biomarker changes several months after the infection and the long-lasting nature of symptoms support this hypothesis. Persistent activation of the immunological system could induce blood-brain barrier disruption, neuroinflammation and brain damage and may justify the elevated values of NfL in the long-term. Parallelly, the positive association between GFAP with hippocampal volume, and negative association with CCL11, added to the reduced CCL11 over time, may also show a possible compensatory mechanism driven by astrocyte activation, and reduction of neurogenesis inhibition in the hippocampus. MOG function is still not well understood. In this case, the association between increased MOG and increased volume in the hippocampus could be a consequence of neuroinflammation, but also to a remyelination process,22,73 reinforced by the positive associations between MOG and white matter volumes in the parahippocampal and thalamus areas and the negative associations with FICVF. However, a third hypothesis should also bear in mind: the possibility of unchaining neurodegenerative mechanisms. White matter microstructure findings seem opposite to the pattern seen in neurodegenerative disorders, but GFAP and NfL are sensitive and early biomarkers of these diseases.74 However, these hypotheses are not mutually exclusive because several mechanisms may be activated. Interestingly, patients from this study were relatively young, and recent studies found higher frequency of cognitive impairment in younger patients with PCS.75,76 The fact that these patients are relatively young could suggest that is less probable the hypothesis of unmasked neurodegeneration as the main cause of cognitive deficits in post-COVID syndrome, and points towards an actual association between PCS and cognitive dysfunction by other mechanisms such as neuroinflammation. The interpretation of the pathophysiological mechanisms should be taken with caution and longitudinal follow-up assessments are needed to better understand the underlying processes and confirm these hypotheses.
Some limitations should be considered. First, the present study is a cross-sectional design, and a longitudinal follow-up of these patients could help in the better understanding of the pathophysiological mechanisms that underlie these brain alterations, and whether these changes are dynamic. Regarding biomarkers, they were evaluated in blood, and not in cerebrospinal fluid. However, correlations between blood biomarkers with neuroimaging results reinforce their usefulness. Also, blood-biomarkers were obtained at PCS but not at the acute phase, which could have helped in the better interpretation of the results. Nevertheless, histological samples of post-mortem patients at the acute phase were assessed. In addition, due to pandemic restrictions, HC group for neuroimaging analyses was relatively small and blood biomarkers were assessed in a secondary HC group. Finally, body mass index may influence GFAP values and it was not taken into account77; therefore, this should be considered in future studies.
In conclusion, patients with post-COVID syndrome at 11 months from the infection present hippocampal volume loss which was related to other grey and white matter brain alterations, linked to cognitive impairment. These alterations were supported by neuroimaging and biomarker analysis, revealing axonal damage, astrocyte alterations, neuronal injury, and myelin changes. All these changes were also found in necropsies from acute COVID-19 patients after death. Our findings suggest the presence of several pathophysiological mechanisms, some of them still activated in the long-term.
Contributors
The study design and administration was performed by MDC, JMG, UGP, JAMG. Recruitment was performed by RSS, JJGR, CDA, MJGM, MVS, UGP, JAMG. Data Curation was carried out by MYF, RSS, JJGR, LGM, CDA, MJGM, MVS, FCC, DOH, NGR, SOM, MSBM, MJ, SF, CP, BSC, UGP, JAMG. The analyses were performed by MDC, MYF, LGM, JJGR, FCC, UGP, JAMG. Investigation and interpretation of data was performed by all authors. Visualization of results was performed by MDC, LGM, UGP, JAMG. Whole process was supervised by MDC, UGP, JAMG. MDC, JAMG, UGP wrote the first draft. All authors reviewed and edited the manuscript. All authors have confirmed that they had full access to all the data in the study and accept responsibility to submit for publication.
Data sharing statement
The anonymized data will be provided in compliance with Health Research Institute “San Carlos” (IdISCC) from Hospital Clinico San Carlos, available from the corresponding author upon reasonable request. Requests for the data should be submitted to JAMG.
Declaration of interests
The authors declare that they have no competing interests.
Acknowledgments
Nominative Grant FIBHCSC 2020 COVID-19. Department of Health, Community of Madrid (JMG, JAMG).
Instituto de Salud Carlos III through the project INT20/00079, co-funded by European Regional Development Fund “A way to make Europe” (JAMG).
Instituto de Salud Carlos III (ISCIII) through Sara Borrell postdoctoral fellowship Grant No. CD22/00043) and co-funded by the European Union (MDC).
Instituto de Salud Carlos III through a predoctoral contract (FI20/000145) (co-funded by European Regional Development Fund “A way to make Europe”) (MVS).
Fundación para el Conocimiento Madri+d through the project G63-HEALTHSTARPLUS-HSP4 (JAMG, SOM).
Authors want to thank all the participants and donors involved in the study.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ebiom.2023.104711.
Contributor Information
Maria Díez-Cirarda, Email: mdcirarda@salud.madrid.org.
Jordi A. Matias-Guiu, Email: jordi.matias-guiu@salud.madrid.org.
Appendix A. Supplementary data
References
- 1.Soriano J.B., Murthy S., Marshall J.C., Relan P., Diaz J.V. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis. 2022;22(4):e102–e107. doi: 10.1016/S1473-3099(21)00703-9. https://www.sciencedirect.com/science/article/pii/S1473309921007039 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Premraj L., Kannapadi N.V., Briggs J., et al. Mid and long-term neurological and neuropsychiatric manifestations of post-COVID-19 syndrome: a meta-analysis. J Neurol Sci. 2022;434 doi: 10.1016/j.jns.2022.120162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delgado-Alonso C., Cuevas C., Oliver-Mas S., et al. Fatigue and cognitive dysfunction are associated with occupational status in post-COVID syndrome. Int J Environ Res Public Health. 2022;19 doi: 10.3390/ijerph192013368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.García-Sánchez C., Calabria M., Grunden N., et al. Neuropsychological deficits in patients with cognitive complaints after COVID-19. Brain Behav. 2022;12(3) doi: 10.1002/brb3.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ariza M., Cano N., Segura B., et al. Neuropsychological impairment in post-COVID condition individuals with and without cognitive complaints. Front Aging Neurosci. 2022;14 doi: 10.3389/fnagi.2022.1029842. https://www.frontiersin.org/articles/10.3389/fnagi.2022.1029842 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delgado-Alonso C., Valles-Salgado M., Delgado-Álvarez A., et al. Cognitive dysfunction associated with COVID-19: a comprehensive neuropsychological study. J Psychiatr Res. 2022;150:40–46. doi: 10.1016/j.jpsychires.2022.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Douaud G., Lee S., Alfaro-Almagro F., et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature. 2022;604(7907):697–707. doi: 10.1038/s41586-022-04569-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goehringer F., Bruyere A., Doyen M., et al. Brain 18F-FDG PET imaging in outpatients with post-COVID-19 conditions: findings and associations with clinical characteristics. Eur J Nucl Med Mol Imaging. 2022;50(4):1084–1089. doi: 10.1007/s00259-022-06013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Díez-Cirarda M., Yus M., Gómez-Ruiz N., et al. Multimodal neuroimaging in post-COVID syndrome and correlation with cognition. Brain. 2022;146(5):2142–2152. doi: 10.1093/brain/awac384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tu Y., Zhang Y., Li Y., et al. Post-traumatic stress symptoms in COVID-19 survivors: a self-report and brain imaging follow-up study. Mol Psychiatry. 2021;26(12):7475–7480. doi: 10.1038/s41380-021-01223-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qin Y., Wu J., Chen T., et al. Long-term microstructure and cerebral blood flow changes in patients recovered from COVID-19 without neurological manifestations. J Clin Invest. 2021;131(8) doi: 10.1172/JCI147329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hafiz R., Gandhi T.K., Mishra S., et al. Higher limbic and basal ganglia volumes in surviving COVID-negative patients and the relations to fatigue. Neuroimage Rep. 2022;2 doi: 10.1016/j.ynirp.2022.100095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siciliano M., de Micco R., Trojano L., et al. Cognitive impairment is associated with Hoehn and Yahr stages in early, de novo Parkinson disease patients. Parkinsonism Relat Disord. 2017;41:86–91. doi: 10.1016/j.parkreldis.2017.05.020. [DOI] [PubMed] [Google Scholar]
- 14.Lu Y., Li X., Geng D., et al. Cerebral micro-structural changes in COVID-19 patients – an MRI-based 3-month follow-up study. EClinicalMedicine. 2020;25 doi: 10.1016/j.eclinm.2020.100484. https://www.sciencedirect.com/science/article/pii/S2589537020302285 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monje M., Iwasaki A. The neurobiology of long COVID. Neuron. 2022;110(21):3484–3496. doi: 10.1016/j.neuron.2022.10.006. https://www.sciencedirect.com/science/article/pii/S0896627322009102 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernández-Castañeda A., Lu P., Geraghty A.C., et al. Mild respiratory COVID can cause multi-lineage neural cell and myelin dysregulation. Cell. 2022;185(14):2452–2468.e16. doi: 10.1016/j.cell.2022.06.008. https://www.sciencedirect.com/science/article/pii/S0092867422007139 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lai Y.J., Liu S.H., Manachevakul S., Lee T.A., Kuo C.T., Bello D. Biomarkers in long COVID-19: a systematic review. Front Med. 2023;10 doi: 10.3389/fmed.2023.1085988. https://www.frontiersin.org/articles/10.3389/fmed.2023.1085988 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanberg N., Ashton N.J., Andersson L.M., et al. Neurochemical evidence of astrocytic and neuronal injury commonly found in COVID-19. Neurology. 2020;95(12):e1754–e1759. doi: 10.1212/WNL.0000000000010111. http://n.neurology.org/content/95/12/e1754.abstract Available from: [DOI] [PubMed] [Google Scholar]
- 19.Ide T., Kawanami T., Eriguchi M., Hara H. SARS-CoV-2-related myelin oligodendrocyte glycoprotein antibody-associated disease: a case report and literature review. Intern Med. 2022;61(8):1253–1258. doi: 10.2169/internalmedicine.8709-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaetani L., Blennow K., Calabresi P., di Filippo M., Parnetti L., Zetterberg H. Neurofilament light chain as a biomarker in neurological disorders. J Neurol Neurosurg Psychiatry. 2019;90(8):870–881. doi: 10.1136/jnnp-2018-320106. http://jnnp.bmj.com/content/90/8/870.abstract Available from: [DOI] [PubMed] [Google Scholar]
- 21.Hol E.M., Pekny M. Glial fibrillary acidic protein (GFAP) and the astrocyte intermediate filament system in diseases of the central nervous system. Curr Opin Cell Biol. 2015;32:121–130. doi: 10.1016/j.ceb.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 22.Solly S.K., Thomas J.L., Monge M., et al. Myelin/oligodendrocyte glycoprotein (MOG) expression is associated with myelin deposition. Glia. 1996;18(1):39–48. doi: 10.1002/(SICI)1098-1136(199609)18:1%3C39::AID-GLIA4%3E3.0.CO. [DOI] [PubMed] [Google Scholar]
- 23.Fotuhi M., Do D., Jack C. Modifiable factors that alter the size of the hippocampus with ageing. Nat Rev Neurol. 2012;8(4):189–202. doi: 10.1038/nrneurol.2012.27. [DOI] [PubMed] [Google Scholar]
- 24.Lana D., Ugolini F., Giovannini M.G. An overview on the differential interplay among neurons-astrocytes-microglia in CA1 and CA3 hippocampus in hypoxia/ischemia. Front Cell Neurosci. 2020;14 doi: 10.3389/fncel.2020.585833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maiese A., Manetti A.C., Bosetti C., et al. SARS-CoV-2 and the brain: a review of the current knowledge on neuropathology in COVID-19. Brain Pathol. 2021;31(6) doi: 10.1111/bpa.13013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Evans T.E., Adams H.H.H., Licher S., et al. Subregional volumes of the hippocampus in relation to cognitive function and risk of dementia. Neuroimage. 2018;178:129–135. doi: 10.1016/j.neuroimage.2018.05.041. https://www.sciencedirect.com/science/article/pii/S1053811918304488 Available from: [DOI] [PubMed] [Google Scholar]
- 27.Cao B., Passos I.C., Mwangi B., et al. Hippocampal subfield volumes in mood disorders. Mol Psychiatry. 2017;22(9):1352–1358. doi: 10.1038/mp.2016.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dale A.M., Fischl B., Sereno M.I. Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 29.Fischl B., Sereno M.I., Dale A.M. Cortical surface-based analysis: II: inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9(2):195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- 30.Iglesias J.E., Augustinack J.C., Nguyen K., et al. A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: application to adaptive segmentation of in vivo MRI. Neuroimage. 2015;115:117–137. doi: 10.1016/j.neuroimage.2015.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alsop D.C., Detre J.A., Golay X., et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med. 2015;73(1):102–116. doi: 10.1002/mrm.25197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Asllani I., Borogovac A., Brown T.R. Regression algorithm correcting for partial volume effects in arterial spin labeling MRI. Magn Reson Med. 2008;60(6):1362–1371. doi: 10.1002/mrm.21670. [DOI] [PubMed] [Google Scholar]
- 33.Smith S.M., Jenkinson M., Johansen-Berg H., et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 34.Andersson J.L.R., Skare S., Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage. 2003;20(2):870–888. doi: 10.1016/S1053-8119(03)00336-7. https://www.sciencedirect.com/science/article/pii/S1053811903003367 Available from: [DOI] [PubMed] [Google Scholar]
- 35.Smith S.M. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andersson J.L.R., Sotiropoulos S.N. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage. 2016;125:1063–1078. doi: 10.1016/j.neuroimage.2015.10.019. https://www.sciencedirect.com/science/article/pii/S1053811915009209 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang H., Schneider T., Wheeler-Kingshott C.A., Alexander D.C. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage. 2012;61(4):1000–1016. doi: 10.1016/j.neuroimage.2012.03.072. https://www.sciencedirect.com/science/article/pii/S1053811912003539 Available from: [DOI] [PubMed] [Google Scholar]
- 38.Ashburner J., Barnes G., Chen C.C., et al. Wellcome Trust Centre for Neuroimaging; London, UK: 2014. SPM12 manual. 2464, 4. [Google Scholar]
- 39.Whitfield-Gabrieli S., Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2(3):125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- 40.Weissenbacher A., Kasess C., Gerstl F., Lanzenberger R., Moser E., Windischberger C. Correlations and anticorrelations in resting-state functional connectivity MRI: a quantitative comparison of preprocessing strategies. Neuroimage. 2009;47(4):1408–1416. doi: 10.1016/j.neuroimage.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 41.Hojat M., Xu G. A visitor’s guide to effect sizes–statistical significance versus practical (clinical) importance of research findings. Adv Health Sci Educ. 2004;9(3):241–249. doi: 10.1023/B:AHSE.0000038173.00909.f6. [DOI] [PubMed] [Google Scholar]
- 42.Ibarretxe-Bilbao N., Ramirez-Ruiz B., Tolosa E., et al. Hippocampal head atrophy predominance in Parkinson’s disease with hallucinations and with dementia. J Neurol. 2008;255(9):1324–1331. doi: 10.1007/s00415-008-0885-8. [DOI] [PubMed] [Google Scholar]
- 43.Apostolova L.G., Dutton R.A., Dinov I.D., et al. Conversion of mild cognitive impairment to Alzheimer disease predicted by hippocampal atrophy maps. Arch Neurol. 2006;63(5):693–699. doi: 10.1001/archneur.63.5.693. [DOI] [PubMed] [Google Scholar]
- 44.Perosa V., Priester A., Ziegler G., et al. Hippocampal vascular reserve associated with cognitive performance and hippocampal volume. Brain. 2020;143(2):622–634. doi: 10.1093/brain/awz383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Serkova V.V., Nikol’skaya K.A., Eremina L.V. The hippocampus as an organizer of operative attention. Neurosci Behav Physiol. 2016;46(9):997–1004. doi: 10.1007/s11055-016-0344-4. [DOI] [Google Scholar]
- 46.Papp K.V., Kaplan R.F., Springate B., et al. Processing speed in normal aging: effects of white matter hyperintensities and hippocampal volume loss. Aging Neuropsychol Cognit. 2014;21(2):197–213. doi: 10.1080/13825585.2013.795513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Frodl T., Schaub A., Banac S., et al. Reduced hippocampal volume correlates with executive dysfunctioning in major depression. J Psychiatry Neurosci. 2006;31(5):316–323. [PMC free article] [PubMed] [Google Scholar]
- 48.Sone D., Shigemoto Y., Ogawa M., et al. Association between neurite metrics and tau/inflammatory pathology in Alzheimer’s disease. Alzheimers Dement. 2020;12(1) doi: 10.1002/dad2.12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kamagata K., Hatano T., Okuzumi A., et al. Neurite orientation dispersion and density imaging in the substantia nigra in idiopathic Parkinson disease. Eur Radiol. 2016;26(8):2567–2577. doi: 10.1007/s00330-015-4066-8. [DOI] [PubMed] [Google Scholar]
- 50.Wang Z., Zhang S., Liu C., et al. A study of neurite orientation dispersion and density imaging in ischemic stroke. Magn Reson Imaging. 2019;57:28–33. doi: 10.1016/j.mri.2018.10.018. [DOI] [PubMed] [Google Scholar]
- 51.Ohki A., Saito S., Hata J., Okano H.J., Higuchi T., Fukuchi K. Neurite orientation dispersion and density imaging for evaluating the severity of neonatal hypoxic-ischemic encephalopathy in rats. Magn Reson Imaging. 2019;62:214–219. doi: 10.1016/j.mri.2019.07.013. [DOI] [PubMed] [Google Scholar]
- 52.Ardellier F.D., Baloglu S., Sokolska M., et al. Cerebral perfusion using ASL in patients with COVID-19 and neurological manifestations: a retrospective multicenter observational study. J Neuroradiol. 2023 doi: 10.1016/j.neurad.2023.01.005. https://www.sciencedirect.com/science/article/pii/S0150986123000068 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yus M., Matias-Guiu J.A., Gil-Martínez L., et al. Persistent olfactory dysfunction after COVID-19 is associated with reduced perfusion in the frontal lobe. Acta Neurol Scand. 2022;146(2):194–198. doi: 10.1111/ane.13627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tian T., Wu J., Chen T., et al. Long-term follow-up of dynamic brain changes in patients recovered from COVID-19 without neurological manifestations. JCI Insight. 2022;7(4) doi: 10.1172/jci.insight.155827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hirunpattarasilp C., James G., Kwanthongdee J., et al. SARS-CoV-2 triggers pericyte-mediated cerebral capillary constriction. Brain. 2023;146(2):727–738. doi: 10.1093/brain/awac272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen J.J., Rosas H.D., Salat D.H. The relationship between cortical blood flow and sub-cortical white-matter health across the adult age span. PLoS One. 2013;8(2) doi: 10.1371/journal.pone.0056733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Allan P.G., Briggs R.G., Conner A.K., et al. Parcellation-based tractographic modeling of the dorsal attention network. Brain Behav. 2019;9(10) doi: 10.1002/brb3.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ward A.M., Schultz A.P., Huijbers W., Van Dijk K.R.A., Hedden T., Sperling R.A. The parahippocampal gyrus links the default-mode cortical network with the medial temporal lobe memory system. Hum Brain Mapp. 2014;35(3):1061–1073. doi: 10.1002/hbm.22234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smallwood J., Bernhardt B.C., Leech R., Bzdok D., Jefferies E., Margulies D.S. The default mode network in cognition: a topographical perspective. Nat Rev Neurosci. 2021;22(8):503–513. doi: 10.1038/s41583-021-00474-4. [DOI] [PubMed] [Google Scholar]
- 60.Sofroniew M.V. Astrogliosis. Cold Spring Harb Perspect Biol. 2015;7(2) doi: 10.1101/cshperspect.a020420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bonetto V., Pasetto L., Lisi I., et al. Markers of blood-brain barrier disruption increase early and persistently in COVID-19 patients with neurological manifestations. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.1070379. https://www.frontiersin.org/articles/10.3389/fimmu.2022.1070379 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lennol M.P., Ashton N.J., Moreno-Pérez O., et al. Transient changes in the plasma of astrocytic and neuronal injury biomarkers in COVID-19 patients without neurological syndromes. Int J Mol Sci. 2023;24:2715. doi: 10.3390/ijms24032715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kanberg N., Simrén J., Edén A., et al. Neurochemical signs of astrocytic and neuronal injury in acute COVID-19 normalizes during long-term follow-up. EBioMedicine. 2021;70 doi: 10.1016/j.ebiom.2021.103512. https://www.sciencedirect.com/science/article/pii/S2352396421003054 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Braga J., Lepra M., Kish S.J., et al. Neuroinflammation after COVID-19 with persistent depressive and cognitive symptoms. JAMA Psychiatry. 2023 doi: 10.1001/jamapsychiatry.2023.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Villeda S.A., Luo J., Mosher K.I., et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477(7362):90–94. doi: 10.1038/nature10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Toda T., Parylak S.L., Linker S.B., Gage F.H. The role of adult hippocampal neurogenesis in brain health and disease. Mol Psychiatry. 2019;24(1):67–87. doi: 10.1038/s41380-018-0036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Abu-Rumeileh S., Abdelhak A., Foschi M., et al. The multifaceted role of neurofilament light chain protein in non-primary neurological diseases. Brain. 2023;146(2):421–437. doi: 10.1093/brain/awac328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Prudencio M., Erben Y., Marquez C.P., et al. Serum neurofilament light protein correlates with unfavorable clinical outcomes in hospitalized patients with COVID-19. Sci Transl Med. 2021;13(602) doi: 10.1126/scitranslmed.abi7643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sánchez-Benavides G., Suárez-Calvet M., Milà-Alomà M., et al. Amyloid-β positive individuals with subjective cognitive decline present increased CSF neurofilament light levels that relate to lower hippocampal volume. Neurobiol Aging. 2021;104:24–31. doi: 10.1016/j.neurobiolaging.2021.02.026. https://www.sciencedirect.com/science/article/pii/S019745802100083X Available from: [DOI] [PubMed] [Google Scholar]
- 70.Wu W., Brickman A.M., Luchsinger J., et al. The brain in the age of old: the hippocampal formation is targeted differentially by diseases of late life. Ann Neurol. 2008;64(6):698–706. doi: 10.1002/ana.21557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gemmell E., Tam E., Allan L., et al. Neuron volumes in hippocampal subfields in delayed poststroke and aging-related dementias. J Neuropathol Exp Neurol. 2014;73(4):305–311. doi: 10.1097/NEN.0000000000000054. [DOI] [PubMed] [Google Scholar]
- 72.Wang Z., Zhang S., Liu C., et al. A study of neurite orientation dispersion and density imaging in ischemic stroke. Magn Reson Imaging. 2019;57:28–33. doi: 10.1016/j.mri.2018.10.018. https://www.sciencedirect.com/science/article/pii/S0730725X18301863 Available from: [DOI] [PubMed] [Google Scholar]
- 73.Lindner M., Heine S., Haastert K., et al. Sequential myelin protein expression during remyelination reveals fast and efficient repair after central nervous system demyelination. Neuropathol Appl Neurobiol. 2008;34(1):105–114. doi: 10.1111/j.1365-2990.2007.00879.x. [DOI] [PubMed] [Google Scholar]
- 74.Beyer L., Stocker H., Rujescu D., et al. Amyloid-beta misfolding and GFAP predict risk of clinical Alzheimer’s disease diagnosis within 17 years. Alzheimers Dement. 2022 doi: 10.1002/alz.12745. [DOI] [PubMed] [Google Scholar]
- 75.Prabhakaran D., Day G.S., Munipalli B., et al. Neurophenotypes of COVID-19: risk factors and recovery outcomes. Brain Behav Immun Health. 2023;30 doi: 10.1016/j.bbih.2023.100648. https://www.sciencedirect.com/science/article/pii/S2666354623000625 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Matias-Guiu J.A., Herrera E., González-Nosti M., et al. Development of criteria for cognitive dysfunction in post-COVID syndrome: the IC-CoDi-COVID approach. Psychiatry Res. 2023;319 doi: 10.1016/j.psychres.2022.115006. https://www.sciencedirect.com/science/article/pii/S0165178122005972 Available from: [DOI] [PubMed] [Google Scholar]
- 77.Yalachkov Y., Schäfer J.H., Jakob J., et al. Effect of estimated blood volume and body mass index on GFAP and NfL levels in the serum and CSF of patients with multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. 2023;10(1) doi: 10.1212/NXI.0000000000200045. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.