Abstract
Background
Historically, quality-of-care monitoring was performed separately for transcatheter and surgical aortic valve replacement (TAVR, SAVR). Using consensus indicators, we provide a global report on the quality of care for treatment of aortic stenosis across the highest-volume treatments: transfemoral (TF) TAVR, isolated SAVR, and SAVR combined with coronary artery bypass graft.
Methods
Retrospective observational cohort study of consecutive patients in a regional system of care. Primary endpoint was 30-day and 1-year mortality (2015-2019). Secondary endpoints included rate of new pacemaker, rate of readmission, and length of stay (2012-2019). Following multivariable logistic regressions, we developed mortality case-mix adjustment models to report risk estimates.
Results
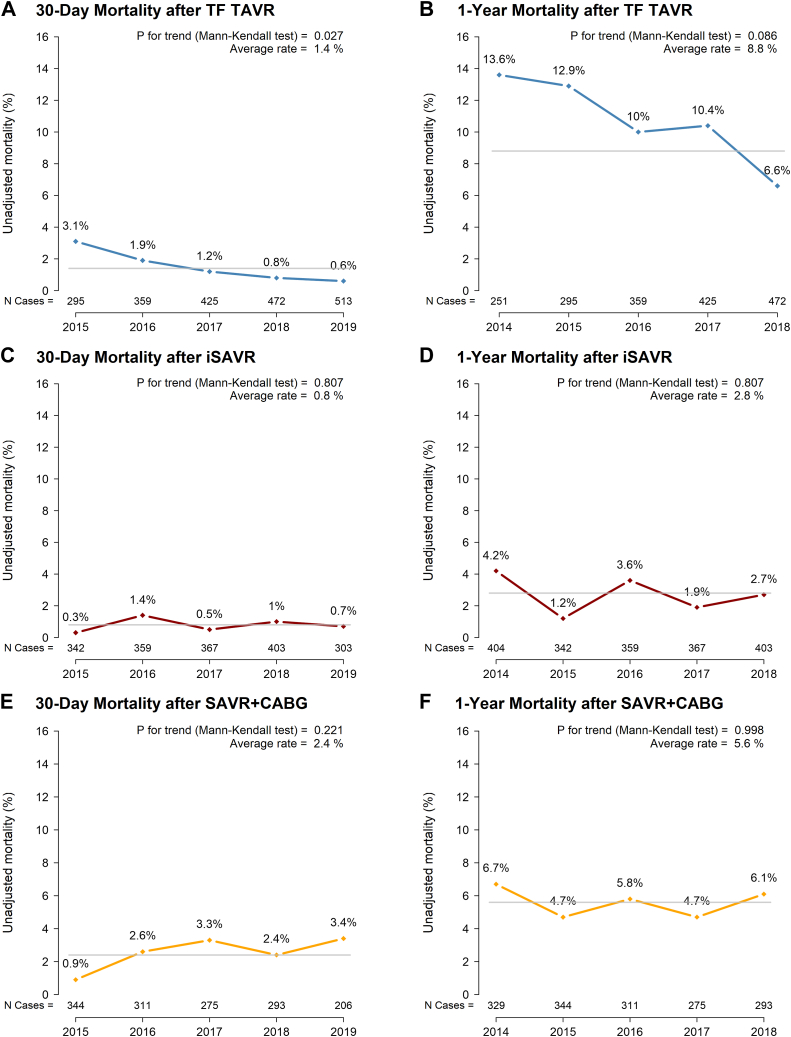
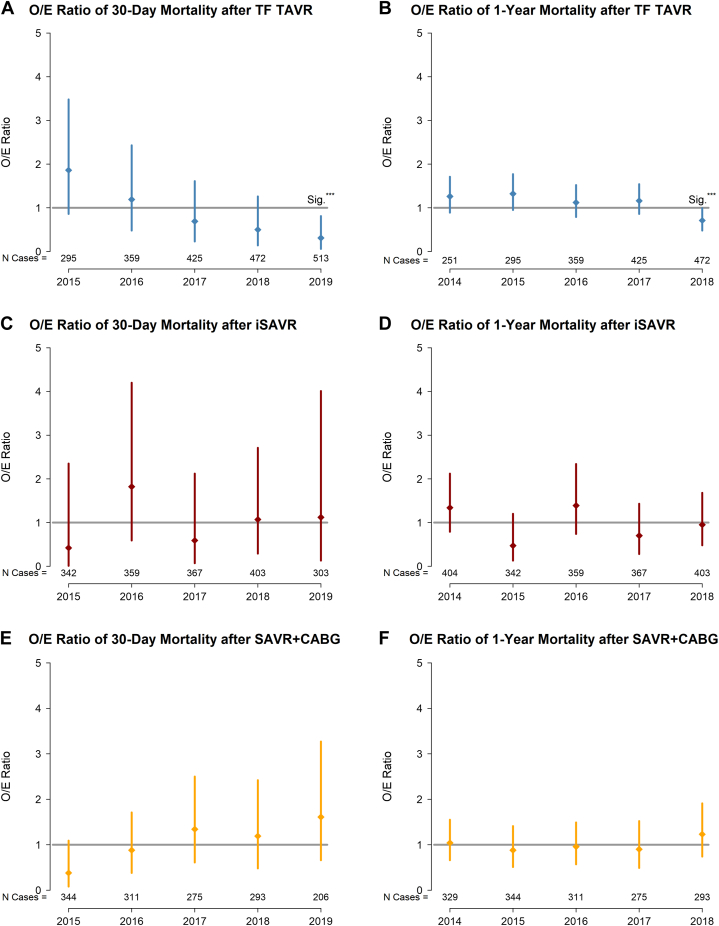
The proportion of patients receiving TAVR grew from 32% to 53% (2015-2019). Those receiving TF TAVR were significantly older, with higher rates of comorbidities. Observed 30-day and 1-year all-cause mortality after TF TAVR decreased from 3.1% to 0.6% (P = 0.03), and 13.6% to 6.6% (P = 0.09), respectively; surgical mortality rates for isolated SAVR and SAVR combined with coronary artery bypass graft were low and did not change significantly over time, ranging from 0.3% to 1.4% and from 0.9% to 3.4%, respectively at 30 days, and from 0.9% to 3.4% and from 4.7% to 6.7 at 1 year. In the TF TAVR cohort, the observed vs expected ratio for 30-day and 1-year mortality decreased significantly from 1.9 (95% confidence interval [CI] 0.9, 3.5) to 0.3 (95% CI 0.1, 0.8), and from 1.3 (95% CI 0.9, 1.7) to 0.7 (95% CI 0.5, 0.99), respectively; no change occurred in risk-adjusted surgical mortality.
Conclusions
Consensus quality indicators provide unique insights on the quality of care for patients receiving treatment for aortic stenosis.
Résumé
Contexte
Par le passé, la surveillance de la qualité des soins était réalisée séparément pour l’implantation valvulaire aortique par cathéter (IVAC) et la chirurgie de remplacement valvulaire aortique (CRVA). À l’aide d’indicateurs consensuels, nous dressons un rapport général de la qualité des soins dans les traitements les plus courants de la sténose aortique : IVAC fémorale, CRVA seule et CRVA combinée à un pontage coronarien.
Méthodologie
Une étude de cohorte observationnelle et rétrospective a été menée pour évaluer les patients consécutifs ayant fréquenté un système de santé régional. Le critère d’évaluation principal était le taux de mortalité à 30 jours et à 1 an (2015 à 2019). Les critères d’évaluation secondaires comprenaient le taux de nouveaux sti-mulateurs cardiaques, le taux de réadmission et la durée du séjour (2012 à 2019). Après des régressions logistiques multivariées, nous avons élaboré des modèles d’ajustement selon les groupes de cas pour le taux de mortalité afin d’estimer les risques.
Résultats
La proportion de patients qui ont subi une IVAC est passée de 32 % à 53 % (2015 à 2019). Les patients qui ont subi une IVAC transfémorale étaient significativement plus vieux que ceux des autres groupes et présentaient un plus haut taux d’affections concomitantes. Les taux de mortalité de toute cause observés à 30 jours et à 1 an après une IVAC transfémorale ont respectivement diminué de 3,1 % à 0,6 % (P = 0,03) et de 13,6 % à 6,6 % (P = 0,09). Les taux de mortalité pour une CRVA seule et une CRVA combinée à un pontage coronarien étaient faibles et n’ont pas changé de manière significative au fil du temps : les taux de mortalité à 30 jours sont passés de 0,3 % à 1,4 % et de 0,9 % à 3,4 %, respectivement, et les taux de mortalité à 1 an, de 0,9 % à 3,4 % et de 4,7 % à 6,7 %, respectivement. Dans la cohorte ayant subi une IVAC transfémorale, le rapport du taux de mortalité observé par rapport au taux de mortalité attendu à 30 jours et à 1 an a diminué de manière significative, soit de 1,9 (intervalle de confiance [IC] à 95 % : 0,9 à 3,5) à 0,3 (IC à 95 % : 0,1 à 0,8), et de 1,3 (IC à 95 % : 0,9 à 1,7) à 0,7 (IC à 95 % : 0,5 à 0,99), respectivement. Aucune variation n’a été notée quant au taux de mortalité ajusté selon les risques pour une intervention chirurgicale.
Conclusions
Les indicateurs consensuels de la qualité fournissent des informations uniques sur la qualité des soins chez les patients traités pour une sténose aortique.
Clinical trials have reported excellent outcomes after surgical aortic valve replacement (SAVR) and transcatheter aortic valve replacement (TAVR) across the range of surgical risk profiles.1,2 Historically, health policy organizations and other authorities mandated the reporting of quality outcomes of those with severe symptomatic aortic stenosis (AS), stratified by treatment modality. Drivers of these isolated processes include health policy infrastructure (eg, established evaluation frameworks and quality indicators, separate registries, and funding models), the challenges of accounting for temporal changes in differences in the SAVR and TAVR patient cohorts, and the delay between rapidly evolving evidence and clinical care and health policy.
The British Columbia (BC, Canada) TAVR program was implemented in 2011 to support a centrally coordinated, funded, and evaluated health service led by a provincial agency.3 The mandate of this regional system of care was to leverage local expertise and the coordinating resources of the provincial agency to expand access to TAVR across the western Canada province’s 5 cardiac hospitals in a province of 5 million people, while optimizing quality of care and timely access to health services.4 Concurrently, the BC SAVR program has benefited from the sustained engagement of all provincial programs and surgeons; in collaboration with the same provincial agency, it developed a surgical registry that houses over 25 years of robust data to regularly report quality by procedure, hospital, and surgeon, and informs local and provincial quality improvement initiatives.5,6 Previously, monitoring of quality of care was performed separately for TAVR and SAVR, coordinated by Cardiac Services BC, a program of the Provincial Health Services Authority responsible for planning, coordinating, monitoring, funding, and evaluating cardiac disease treatment in BC.
In response to evolving evidence and changes in practice, clinical and policy stakeholders developed the BC aortic stenosis (BC-AS) quality report and suggested the following common set of aortic valve replacement (AVR) quality indicators to produce a single quality report of the treatment of AS: 30-day and 1-year all-cause mortality, 30-day new permanent pacemaker rates, postprocedure length of stay, and 30-day all-cause and 1-year cardiac-specific hospital readmission. The primary objectives of this work were to help shift the culture of health services to a more disease-centred approach, and to strengthen the quality of care across treatment modalities for people with AS, while recognizing the nuances of treatment decisions, patient characteristics, and procedural challenges. We report on the temporal changes in standardized quality indicators for SAVR and TAVR in this regional system of care.
Methods
AVR quality indicators and evaluation cohorts
The selection of common quality indicators for TAVR and SAVR was informed by historical local SAVR and TAVR evaluation frameworks and further refined with clinicians’ input, the Canadian Cardiovascular Society Quality Project,7 the US Society of Thoracic Surgeons (STS)/American College of Cardiology (ACC) Transcatheter Valve Therapy (STS/ACC TVT) Registry,8 and the Donabedian model for measuring quality of care.9 The goal was to identify a limited list of indicators that could be measured reliably, inform quality improvement across systems of care and within hospitals, and establish a preliminary consensus quality framework with clinical stakeholders. Separate quality reports were generated for TAVR, with differentiation of transfemoral (TF) TAVR and alternative surgical access (non-TF) TAVR, and SAVR, with distinction between isolated SAVR (iSAVR) and SAVR combined with coronary artery bypass graft (SAVR+CABG), exclusive of any concomitant procedures. For the purposes of this study, we report on outcomes after TF TAVR, iSAVR, and SAVR+CABG to capture the largest-volume cohorts. The BC-AS quality framework and patient cohorts are illustrated in Supplemental Figure S1.
The consensus decision was made to avoid premature comparisons between the transcatheter and surgical approaches, given the heterogeneity of patient groups, significant changes that occurred in TAVR devices and procedural approaches during the study period, and the current absence of a common contemporary administrative risk-adjustment model for SAVR and TAVR. We aimed to identify opportunities for quality improvement within each program and initiate the process of standardizing data collection and variable definitions in the surgical and transcatheter administrative registries. The objective also was to maximize the use of established SAVR risk models developed over time, while acknowledging that the application of a surgical risk model to contemporary TAVR was inadequate; emerging TAVR-specific risk-adjustment models remain of limited use in the rapidly evolving context of indications. In addition, consideration of differences in data definitions of selected variables limited robust comparisons. Thus, quality was reported along identically defined indicators, but in separate reports, without inferential analyses of statistical differences between treatment modalities.
Study design and population
We conducted a retrospective observational cohort study of consecutive patients who presented with AS/aortic insufficiency who underwent an index TAVR or SAVR as follows: (i) between 2015 and 2019, to report 30-day and 1-year mortality up to 2019, to capture contemporary outcomes; and (ii) between 2012 and 2019, to report on temporal changes in new permanent pacemaker, length of stay, and hospital readmission. The index hospitalization for AVR indicated the first time point within the study period. Inclusion criteria were being a patient older than 18 years at the time of AVR and having a completed AVR device implantation. We excluded the following patients: (i) non-BC residents; (ii) those who had a prior AVR within a year of the index procedure in the analysis of mortality, to wash out the effect of the previous procedures and reduce bias10; and (iii) those who could not be identified in the Canadian Institute for Health Information Discharge Abstract Database for data linkage.
Endpoints and data sources
The combined primary endpoint of the study was 30-day and 1-year all-cause mortality for TF TAVR, iSAVR, and SAVR+CABG, to report on contemporary indications for allocation of funding, devices, and practice. Secondary endpoints included the following: annual 30-day new permanent pacemaker rates (in patients without a preexisting device implant); postprocedure length of stay; and 30-day all-cause and 1-year cardiac-specific readmission (defined as myocardial infarction, unstable angina, congestive heart failure). Mandatory reporting of all procedures, patient characteristics, procedural details, and in-hospital outcomes to provincial registries was enhanced with linkages to administrative databases, including BC Vital Statistics to report mortality, and the Canadian Institute for Health Information Discharge Abstract Database for length of stay and readmission data (Supplemental Table S1).
Statistical methods
Multivariable logistic regression analyses were performed to model the log-odds of 30-day and 1-year all-cause mortality for each group. Candidate variables, such as patient demographics, pre-procedural clinical factors, and comorbidities, were incorporated in the variable-selection process. Backwards elimination and stepwise selection of significant predictors were performed. Covariates with P values < 0.1 in the univariate analysis were retained in the final multivariable model. Age and sex were forced into the model, to control for unmeasured confounding effects. In addition, hospital effects were adjusted for the primary outcome.
Separate mortality case-mix adjustment models were developed based on the model cohorts that leveraged the available data (iSAVR and SAVR+CABG: 2000-2019; TF TAVR: 2012-2019); the expected mortality rates were estimated using the risk-adjusted model for the evaluation cohort (SAVR and TAVR: 2015-2019). We calculated the observed vs expected (O/E) mortality ratio and computed the confidence interval of the O/E ratio based on the normal approximation to the binomial distribution to compare risk-adjusted performance across all hospitals.11
Categorical variables expressed as frequencies and percentages were compared using the χ2 test. Continuous variables are presented as mean (standard deviation) and were compared using the Student t test. A linear regression model was used to compare continuous variables over time; the Cochran-Armitage and the Mann-Kendall tests were used for trend. All statistical analyses were conducted using SAS, version 9.4 (SAS Institute, Cary, NC) and R, version 4.0.2 (R Development Core Team).
The study was reviewed by the Providence Health Care/University of British Columbia Research Ethics office and was assessed as meeting the criteria for quality improvement.
Results
Procedural volumes
The mean annual procedural volumes were 389 (range: 214, 622) and 673 (range: 549, 735) for all TAVR (TF and non-TF), and SAVR with or without coronary artery bypass graft (CABG [SAVR+/-CABG]), respectively. A total of 8495 consecutive patients had AVR in BC between 2012 and 2019; of these, 3115 (36.7%) had TAVR (TF: 86.6%; non-TF: 13.4%), and 5380 (63.3%) had SAVR (iSAVR: 54.3%; SAVR+CABG: 45.7%). Over the 5-year study period, the proportion of those with all TAVR grew from 32% to 53% of those with all AVR (Supplemental Fig. S2).
Study cohorts
Supplemental Figure S3 illustrates the study cohorts used to develop the evaluation models of risk-adjusted mortality (2015-2019) and describe temporal trends (2012-2019).
Patient baseline characteristics
The evaluation model cohorts included 2070, 1774, and 1429 participants who had TF TAVR, iSAVR, and SAVR+CABG, respectively. On average, TF TAVR patients were older (81.4 ± 7.6 years vs 68.1 ± 10.3 years for iSAVR, and 72.5 ± 7.7 years for SAVR+CABG) and included a higher proportion of women (43.3% vs 36.4% for iSAVR; 18.7% for SAVR+CABG). The proportion of patients with New York Heart Association (NYHA) class III or IV symptoms was higher in the TAVR group (62.5%) than in the SAVR groups (iSAVR: 39.9%; SAVR+CABG: 38.3%). TAVR patients had higher rates of the following: prior coronary revascularization (percutaneous coronary intervention—25.5% vs 5.2% for iSAVR and 16.4% for SAVR+CABG; CABG—15.9% vs 2.5% for iSAVR and 2.7% for SAVR+CABG); prior pacemaker rates (11.1% vs 3%); and prior SAVR (ie, “valve-in-valve”: 9.3% vs 5.7% for iSAVR and 2.2% for SAVR+CABG; Table 1). The comorbidity profile of the surgical cohorts remained relatively unchanged, whereas significantly more TAVR patients presented as urgent inpatients or following a failed surgical bioprosthesis, with decreasing burden of atrial fibrillation, home oxygen, and concomitant mitral valve disease (P < 0.05). Over time, the mean age of patients decreased significantly across all treatment options (Supplemental Table S2).
Table 1.
Patient characteristics of the evaluation cohorts for TF TAVR, iSAVR, and SAVR+CABG (2015-2019)
| Characteristic | TF TAVR (N = 2070) | iSAVR (N = 1774) | SAVR+CABG (N = 1429) |
|---|---|---|---|
| Age, y | 81.4 ± 7.6 | 68.1 ± 10.3 | 72.5 ± 7.7 |
| Age ≥ 80 y | 1397 (67.5) | 878 (49.5) | 963 (67.4) |
| Female sex | 897 (43.3) | 643 (36.2) | 267 (18.7) |
| Body mass index, kg/ m2 | 28.1 ± 6.3 | 27.6 ± 5.7 | 28.2 ± 5.2 |
| Body surface area, m2 | 1.9 ± 0.3 | 2.0 ± 0.3 | 2.0 ± 0.2 |
| Atrial fibrillation/flutter | 679 (33.7) | 181 (10.2) | 157 (11) |
| LVEF < 35% | 195 (9.4) | 96 (5.4) | 101 (7.1) |
| NYHA functional class III or IV | 1180 (62.5) | 707 (39.9) | 548 (38.3) |
| Congestive heart failure | N/A | 589 (33.2) | 493 (34.5) |
| eGFR, mL/min | 58.6 ± 19.8 | 75.7 ± 47.5 | 70.6 ± 21.6 |
| Dialysis | 42 (2.1) | 22 (1.2) | 25 (1.7) |
| COPD | N/A | 340 (19.2) | 294 (20.6) |
| Diabetes | 580 (28.6) | 413 (23.3) | 560 (39.2) |
| Liver disease | N/A | 128 (7.2) | 90 (6.3) |
| Pulmonary hypertension | N/A | 1177 (66.3) | 1175 (82.2) |
| PCI | 517 (25.5) | 92 (5.2) | 234 (16.4) |
| CABG | 322 (15.9) | 45 (2.5) | 39 (2.7) |
| Prior AVR | 193 (9.3) | 101 (5.7) | 32 (2.2) |
| PVD | N/A | 113 (6.4) | 210 (14.7) |
| Pacemaker | 147 (11.1) | 53 (3) | 43 (3) |
| Preoperative ventilation | N/A | 9 (0.5) | 7 (0.5) |
| AV gradient | 42.3 ± 15.8 | 49.1 ± 25.9 | 41.2 ± 17 |
| AV area | 0.8 ± 1.4 | 0.8 ± 1.4 | 1 ± 3.3 |
| Moderate or severe anemia∗ | 106 (7.5) | 129 (7.3) | 129 (9) |
| Malignant disease < 5 y | N/A | 172 (9.7) | 165 (11.5) |
| History of substance use disorder | N/A | 43 (2.4) | 18 (1.3) |
| Depression | N/A | 103 (5.8) | 39 (2.7) |
| KCCQ-OS | 47.5 ± 24.6 | N/A | N/A |
| Elective outpatient | 1730 (84) | N/A | N/A |
| Priority II or III∗ | N/A | 1636 (92.3) | 1273 (89.1) |
Values are n (%) or mean (± standard deviation), unless otherwise indicated.
AV, atrioventricular; AVR, AV replacement; CABG, coronary artery bypass graft; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; iSAVR isolated SAVR; KCCQ-OS, Kansas City Cardiomyopathy Questionnaire overall score; LVEF, left ventricular ejection fraction; N/A, not available (variable not routinely collected for procedure); NYHA, New York Heart Association; PCI, percutaneous coronary intervention; PVD, peripheral vascular disease; SAVR, surgical aortic valve replacement; TAVR, transcatheter aortic valve replacement; TF, transfemoral.
Derived variables: SAVR priority level II: elective outpatient wait time < 6 weeks; SAVR priority level III: elective outpatient wait time < 12 weeks; moderate or severe anemia: hemoglobin < 110 g/L.
Mortality
TF TAVR
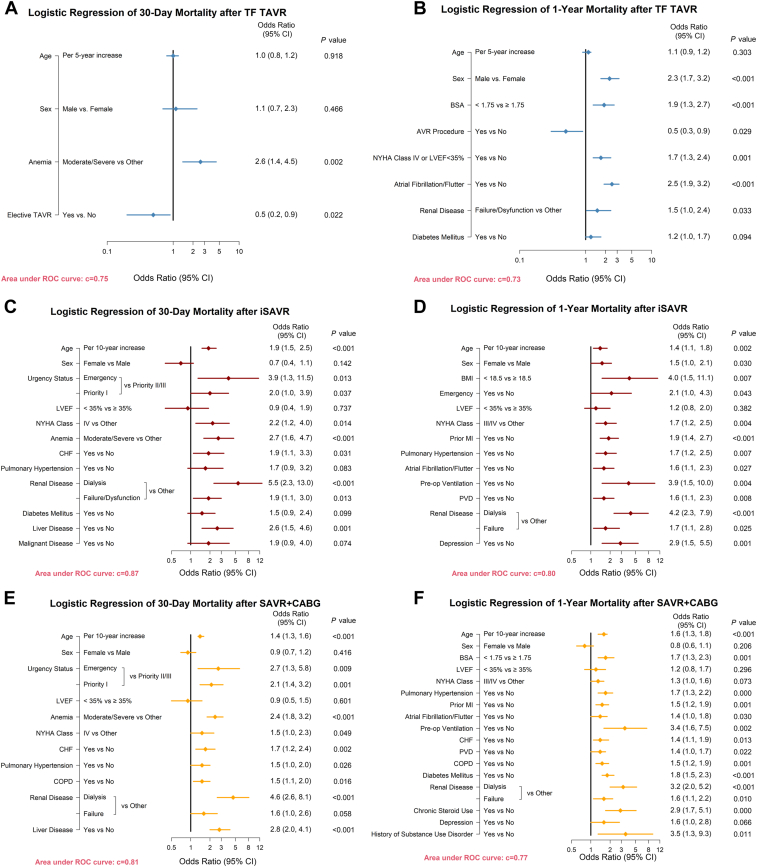
Between 2015 and 2019, observed 30-day and 1-year all-cause mortality after TF TAVR decreased, from 3.1% to 0.6% (P for trend = 0.03), and 13.6% to 6.6% (P for trend = 0.09), respectively (Fig. 1, A and B). In the 30-day mortality logistic regression model, the presence of moderate/severe anemia (hemoglobin < 110 g/L; odds ratio [OR], 2.6; 95% confidence interval [CI] 1.4, 4.5; P = 0.002) and procedure urgency (elective vs urgent, OR, 0.5; 95% CI 0.2, 0.9; P = 0.02) were associated with mortality. In the 1-year model, male sex, lower body surface area, NYHA class IV functional status or left ventricular ejection fraction < 35%, atrial fibrillation, renal disease (estimated glomerular filtration rate < 30 mL/min), and diabetes were additional significant predictors of mortality, whereas TAVR in a failed bioprosthesis was associated with decreased mortality risk (OR, 0.5; 95% CI 0.3, 0.9; Fig. 2, A and B). After controlling for significant predictors, the O/E ratio for TF TAVR decreased from 1.9 (95% CI 0.9, 3.5) in 2015 to 0.3 (95% CI 0.1, 0.8), indicating a statistically significantly lower than average 30-day mortality after risk adjustment in 2019 (c-index = 0.75). A similar pattern in the by-year analysis demonstrated significantly lower 1-year mortality in 2018 (2014: O/E ratio, 1.3; 95% CI 0.9, 1.7; 2018: O/E ratio, 0.7; 95% CI 0.5, 0.99; c-index = 0.73; Fig. 3, A and B).
Figure 1.
Temporal change in unadjusted 30-day and 1-year mortality after transcatheter aortic valve replacement (TAVR), isolated surgical aortic valve replacement (iSAVR) and SAVR+ coronary artery bypass graft (CABG) (2015-2019). TF, transfemoral.
Figure 2.
Logistic regression models for 30-day and 1-year mortality. AVR, aortic valve replacement; BMI, body mass index; BSA, body surface area; CABG, coronary artery bypass graft; CHF, congestive heart failure; CI, confidence interval; COPD, chronic obstructive pulmonary disease; iSAVR, isolated SAVR; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NYHA, New York Heart Association; PVD, peripheral vascular disease; ROC, receiver operating characteristic; SAVR, surgical AVR; TAVR, transcatheter AVR; TF, transfemoral.
Figure 3.
Temporal change in risk-adjusted 30-day (2015-2019) and 1-year (2014-2018) mortality by treatment modality, reported as observed/expected (O/E) ratio. The O/E ratio estimates the risk of mortality by comparing the observed (observed events/eligible population) against the expected (expected events based on risk model/eligible population). An O/E ratio > 1 indicates worse performance than the reference population with an equivalent case mix (model); an O/E ratio < 1 indicates better-than-expected performance. When interpreting results, note that the difference may be spurious if the 95% confidence interval (CI) overlaps with the reference population. ∗∗∗Significant difference (Sig.). CABG, coronary artery bypass graft; iSAVR, isolated SAVR; SAVR, surgical aortic valve replacement; TAVR, transcatheter aortic valve replacement; TF, transfemoral.
iSAVR and SAVR+CABG
Rates of observed 30-day and 1-year mortality remained low and did not change significantly over time in the surgical cohorts, ranging from 0.3% to 1.4%, and 1.2% to 4.2%, respectively, after iSAVR, whereas mortality after SAVR+CABG ranged from 0.9% to 3.4% at 30 days, and 4.7% to 6.7% at 1 year (Fig. 1, C-F). Common risk factors across the 2 surgical cohorts in the 30-day logistic regression models included the following: age; procedure urgency; left ventricular ejection fraction < 35%; moderate/severe anemia; NYHA class IV functional status; heart failure; renal disease; and liver disease (defined as indicated in Supplemental Table S1; c-index = 0.87 for iSAVR; c-index = 0.80 for SAVR+CABG). In the 1-year model, pulmonary hypertension, preoperative ventilation, and depression were additional variables that significantly increased patients’ risk of mortality (c-index = 0.81 for iSAVR; c-index = 0.77 for SAVR+CABG; Fig. 2, C-F). In the risk-adjusted analyses, the O/E ratio did not change significantly between 2015 and 2019, indicating that the 30-day and 1-year mortality rates were comparable over time (Fig. 3, C-F).
30-day new permanent pacemaker
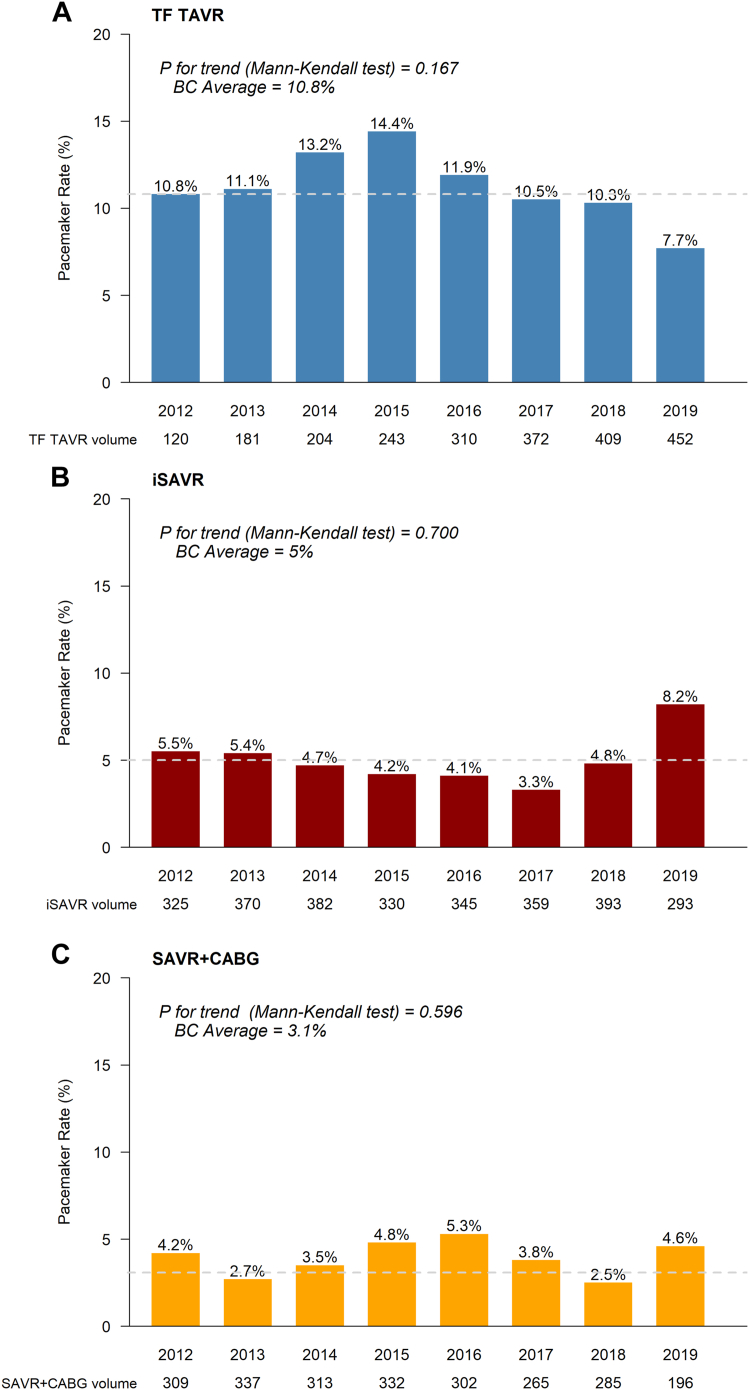
Provincial rates of new permanent pacemaker (in the absence of a preexisting device) after TF TAVR declined from 14.4% to 7.7%. The mean provincial rate was 10.8% between 2012 and 2019; nonsignificant increases were noted in 2014 (13.2%) and 2015 (14.4%). A similar pattern was seen recently for surgical patients; overall, the mean provincial rate was 4.8% after iSAVR, and 4.2% after SAVR+CABG, with a spike noted in 2019 (iSAVR: 8.2%; Fig. 4).
Figure 4.
Temporal change in 30-day new permanent pacemaker (2012-2019). BC, British Columbia; CABG, coronary artery bypass graft; iSAVR, isolated SAVR; SAVR, surgical aortic valve replacement; TAVR, transcatheter aortic valve replacement; TF, transfemoral.
Hospital length of stay
The median length of stay after TAVR decreased from 3 days (interquartile range [IQR]: 3, 6) in 2012 to 1 day (IQR: 1, 3) in 2012-2016; the average rate of next-day discharge increased significantly, from 11.2% in 2012 to 69.4% in 2019 (P for trend: 0.03; Supplemental Fig. S4). The 6-day median (IQR: 5, 7-9) length of stay after iSAVR remained unchanged, whereas a decrease occurred after 2012, from a median of 8 (IQR: 6, 14) to 7 (IQR: 6, 11) for SAVR+CABG (Supplemental Table S3).
Hospital readmission
Between 2012 and 2018, the average rates of all-cause 30-day and cardiac-specific 1-year readmission after TF TAVR were 14.7% and 15.1%, respectively. Temporal trends demonstrated a sustained decline after 2015. For patients who had iSAVR, 30-day all-cause and 1-year cardiac readmission rates remained stable around the provincial averages (30-day: 5.4%; 1-year: 11%); however, we observed fluctuations of readmission rates after SAVR+CABG, with 2018 rates exceeding the average 6.9% 30-day and 13.1% 1-year rates (Fig. 5).
Figure 5.
Temporal change in readmission (2012-2018). CABG, coronary artery bypass graft; iSAVR, isolated SAVR; SAVR, surgical aortic valve replacement; TAVR, transcatheter aortic valve replacement.
Discussion
In this analysis of temporal changes in standardized quality indicators in a regional system of care treating patients with AS, we found that the same indicators, as measured in separate reports, demonstrated a high quality of care in an integrated regional system of care. The findings highlight the following important recent trends in the treatment of AS in a real-world setting: (i) TF TAVR patients were older, included more women, had a higher burden of comorbidities, and were more likely to have had a prior AVR than surgical patients; (ii) over time, a significant decrease occurred in the mean age in all AVR patients; (iii) between 2015 and 2019, a narrowing of differences occurred in observed mortality rates across treatment modalities, with significant decreases over time for TF TAVR and low stable rates for SAVR; (iv) in 2019, observed 30-day mortality after TAVR was 0.6%, and patients had a significantly lower-than-expected mortality rate after risk adjustment; and (v) a temporal trend occurred of decreasing rates of mortality, pacemaker and hospital readmission, and median length of stay after TF TAVR, whereas SAVR patients had similar outcomes over time.
Registry-based evaluation of treatment of aortic stenosis
In addition to providing important regional information on clinical outcomes following treatment of AS, our study demonstrates the feasibility and value of a disease-focused evaluation framework with standardized quality indicators. To date, outcome assessment across treatment modalities has been driven primarily by clinical trials; most regional and national registries have focused on the distinct evaluation of TAVR (eg, the STS/ACC TVT Registry of TAVR in the US;8 the French Transcatheter Aortic Valve Implantation [FRANCE TAVI] registry and the French Aortic National CoreValve and Edwards 2 [FRANCE 2] registry in France;12 and the German Aortic Valve Registry [GARY] in Germany13) or SAVR (eg, STS National Database in the US,14 the National Adult Cardiac Surgery Audit in the United Kingdom15). This historical approach fails to capture common variables with standardized definitions across treatment modalities required to build robust predictive models in the rapidly evolving clinical context of the treatment of valvular heart disease. The BC-AS quality report presented in this study illustrates a more disease- and patient-centred approach, and a feasible strategy to address these challenges and ensure the monitoring of patient selection and outcomes across the lifetime management of AS. These efforts reflect evidence that outcomes registries can be powerful drivers of quality and can foster multiple uses and exciting opportunities—ranging from informing individual care to promoting research and knowledge mobilization.16
Healthcare quality measurement is fundamental to systems performance, and helps overcome the ongoing challenges of lack of alignment on patients’ needs, fragmentation of care, and inequitable access to the range of treatment options.17 The registry-based monitoring of quality of care provides information to clinicians, hospitals, health funders, and consumers that can be utilized to ensure that patients receive the highest-quality cardiac care and to improve quality, in addition to informing the understanding of disease progression and timing of intervention, determining safety or harm, and assessing clinical cost effectiveness.18 The availability of timely and targeted quality reports presents opportunities to track important alerts for continuous quality improvement. This tracking is particularly pertinent to advance “real-world” evidence about the experiences of women with valvular heart disease, beyond what is known from clinical trials.19 For example, action items resulting from the BC-AS report presented in this study resulted in various initiatives, including the distribution of site-specific quality reports with comparators to provincial standards, implementation of standardization of care, the examination of barriers to early discharge, and opportunities to further shift from a procedure-based to a more patient/disease-centred approach in patients’ journey of care.20
In our study, we found that the ratio of women increased in the TAVR cohort. This finding is in keeping with previous research that has hypothesized that women may develop heart valve disease later than men for physiological reasons related to sex,21 present with a lower comorbid burden, and achieve better outcomes, independently of baseline characteristics and procedural approaches.22 Further efforts are required to parse the intersectional effects of sex and gender on detection, diagnosis, referral patterns, and timely access to treatment, and a disease-centred evaluation of longitudinal outcomes for men and women with AS.
An increasing need is for policy makers and clinicians to ensure the standardized risk-adjusted comparison of TAVR and SAVR to inform health services planning and funding models, monitor quality of care, scrutinize long-term outcomes and device performance, and inform local cost-effectiveness evaluation.23,24 To achieve this shift, a pressing need exists to standardize the selection of registry-based variables, definitions, and data-quality monitoring to support the development of disease-focused data repositories and effective risk models to guide clinical care. Integrated health information systems are essential to support the planning, implementation, monitoring, and evaluation of integrated health services focused on the multimodality and lifetime management of valvular heart disease.25
Selection of quality indicators
In the BC-AS framework, the selection of mortality was augmented with additional process and structure indicators that recognize contemporary issues and the essential contributions of the multidisciplinary team. The inclusion of temporal changes in new pacemakers was informed by previous provincial registry-driven quality improvement that resulted in the overall decrease of new conduction delays after TAVR, and narrowing of the new pacemaker utilization rates between hospitals due to changes in devices and implantation techniques.26 Similarly, the scrutiny of in-hospital length of stay provides a surrogate marker of the effectiveness of processes of care, the potential in-hospital complications related to the treatment of AS, frailty, and other unique health vulnerabilities of older patients with valvular heart disease, and health-resource utilization during patients’ admission to improve program efficiencies.27,28
Nevertheless, the value of additional quality indicators should be assessed to reflect evidence supporting the use of the Donabedian model in guiding the improvement of health services.9 Stroke metrics are important patient-centred outcomes for which there remain significant challenges to accurately and effectively measure events to inform appropriate interpretation.29 Although initially considered in the BC-AS quality report, issues of data definition and quality precluded the inclusion of stroke as one of the indicators, and prompted stakeholder to consider initiatives for future evaluation. In real-world data, longitudinal evaluation of device performance was similarly highlighted by the working group, and will require the adoption of standardized echocardiographic surveillance to be included in future quality reporting.
Important structure and process indicators pertinent to the management of AS may include the evaluation of a patient’s journey of care from the onset of symptoms to follow-up,20 monitoring of wait times from referral to assessment and procedure,30 and availability of program infrastructure that promotes a disease-focused and patient-centred approach to the assessment pathway and treatment.31 The monitoring of changes in patient-reported outcomes (PROs) adopted in the early era of TAVR clinical trials,32,33 and established in clinical care,8,34 has yielded important evidence about patients’ perspectives on changes in self-reported health status. In our study, the availability of the Kansas City Cardiomyopathy Questionnaire overall score for TAVR patients was not matched in the surgical group, and this prevented the adoption of PROs in the BC-AS report. PROs provide insights into patients’ experiences of symptoms, their quality of life and overall functioning, and their values, preferences, and goals for healthcare.35 In addition to augmenting the monitoring of outcomes, PROs support patient-provider engagement and facilitate a shared decision-making approach, to help achieve a high-quality treatment decision. Ongoing efforts are required to facilitate meaningful patient engagement in the reporting of quality of care, to ensure that indicators reflect what matters most to patients with valvular heart disease, and shift to a more patient-centred approach. As indications for TAVR and SAVR continue to evolve, the adoption of a pertinent structure, processes, and patient-centred outcomes will strengthen a comprehensive approach to the monitoring of quality of care in the treatment of valvular heart disease.
Shifting the treatment of AS
Our findings are consistent with previous research that has reported the increasing use of TAVR, and the acceleration of improvement in TAVR outcomes in recent years. Other regions have reported a similar pattern of temporal increase in the proportion of TAVR compared to SAVR, and the increased magnitude of mortality benefit and accelerated rate of improvement in TAVR.8,36 Across studies, TAVR patients remain older and have higher rates of comorbidities than surgical patients.37,38 Our study augments existing evidence that mortality after TAVR is not driven by patients’ comorbid burden, therebyreinforcing the safety and effectiveness of the procedure, and the importance of mitigating the risks of hospitalization, to facilitate an accelerated return to baseline function and discharge home.
In this context, the approval of TAVR for patients with a higher risk of predicted surgical mortality, and the improved results with TAVR, which now equal those with iSAVR, attest to the joint success of the regional program in standardizing a multidisciplinary approach to treatment decision, and allocating resources and processes to improve the overall population’s outcomes in the short term. Longitudinal outcome studies in randomised trials and large registries are important to monitor long-term outcomes in evolving patient cohorts. Important changes over time that were not fully accounted for in this study include the burden of coronary artery disease in surgical cohorts, regional practices and patterns of initial referrals for treatment (eg, cardiac surgery vs TAVR programs), evolving indications and funding for TAVR, and improvements in TAVR technology.
The impact of quality improvement
The pace of improved outcomes after TAVR has been driven by significant advances in technology, imaging, procedural approaches, and rapid reconditioning of clinical pathways.39 The excellent longstanding outcomes achieved by surgical programs have not resulted in similar scrutiny of all aspects of the patient’s journey of care, which may present opportunities to further augment advances in surgical protocols and the implementation of enhanced recovery protocols through quality improvement.40
Implications for health policy
Lastly, our findings may have implications for future health policy decisions. Indeed, healthcare funding across regions remains driven by procedure-based reimbursement that may incentivize separate silos of program planning and patient care, rather than requiring a collective approach responsive to patient need and contemporary evidence. The funding model reflected in this study likely mirrors the structures and processes of multiple jurisdictions in public health systems. Conventionally, funders allocate separate surgical and transcatheter heart valve procedure volumes in their service-level agreements, with varying funding rates based on different case mixes. This approach results in largely “disconnected” internal hospital budgets allocated to procedure rooms (eg, surgical vs interventional cardiology programs), separate funding of staffing and equipment and other cost drivers, and internal political pressures to compete for resources.41 These patterned choices are commonly informed by historical experiences and constrain opportunities to shift the culture of care to a more disease-centred approach that supports lifelong management of heart valve disease.
Inequities in access to timely treatment driven by hospital TAVR/SAVR ratios and wait times have been reported.30,42 The current focus on value-based healthcare may present a road map that can help shift health policy and support cardiac programs to examine the lifetime management of valvular heart disease.43 In addition, endorsement of shared decision-making is increasing, and patient interest in participating in their treatment decisions is rising.44 These changes in health policy have important implications for examining current referral pathways, access to integrated heart teams, and a more patient-focused approach that considers tailored risk considerations, the preferences, values, and priorities of patients, and lifetime management.45 In addition, intersecting factors, including geography/residence, race, ethnicity, indigeneity, rurality, and socioeconomic status are known to impact equity, access to care, referral trajectories, and treatment options.46 To adapt to this evolving context, health policy must ensure the following (i) that patients are informed and empowered to participate in their care decisions; (ii) that access to TAVR and SAVR is equitable and timely; (iii) that TAVR and SAVR care is evidence-based and efficient; and (iv) that outcomes are excellent across treatment options.47
Limitations
These findings should be examined in the context of a certain number of limitations. The study was a retrospective observational study of site-reported administrative data augmented by data linkages, using different time windows for 30-day and 1-year assessment because of the delay in data linkage. Study time points reflect a program in evolution. We highlighted important issues related to the availability of common covariates across the separate TAVR and SAVR registries that could not be addressed in this study; we may not have captured the full complement of determinants of outcomes, including frailty. In addition, issues related to sex-stratified differences in the cohorts, evolving technology, indications and case selection, physician- and/or hospital-level factors, complex in-hospital complications, and operator experience may not be accounted for fully. We excluded non-TF TAVR due to the consistently low volumes of this approach and the evolving change from a primarily transapical approach to the default use of less-invasive approaches (subclavian, transcarotid), and we recognize that this approach warrants future examination.
Conclusion
Our study offers novel evidence to support a shift to a disease-centred evaluation framework, and proposes a set of consensus quality indicators that are reliably measurable and relevant to clinicians, policymakers, and patients. As indications continue to evolve, we highlight opportunities for quality improvement across treatment options, and comprehensive health service planning to ensure access and quality of care in the treatment of valvular heart disease. Future research is required to inform the development of real-world risk-predictor models to support the best procedural treatment of AS across diverse populations, monitor long-term outcomes, and promote the role of a comprehensive multidisciplinary team to ensure appropriate treatment recommendations.
Acknowledgements
The authors gratefully acknowledge the significant contributions of Cardiac Services BC, a program of the British Columbia (BC) Provincial Health Services Agency, their leadership of the described project, stewardship of data, data analytics, and strong support for quality improvement. The authors further acknowledge the contributions of the 5 BC cardiac programs that provide access to aortic valve replacement in BC: Kelowna General Hospital (Kelowna, BC), Royal Columbian Hospital (New Westminster, BC), Royal Jubilee Hospital (Victoria, BC), St. Paul’s Hospital (Vancouver, BC), and Vancouver General Hospital (Vancouver, BC).
Ethics Statement
The study was reviewed by the Providence Health Care/University of British Columbia Research Ethics office and was assessed as meeting the criteria for quality improvement.
Funding Sources
D.M. is supported by the Swiss National Science Foundation (grant P2LAP3_199561) and the SICPA foundation. The other authors have no funding sources to declare.
Disclosures
S.B.L. reports being a consultant for Edwards and Medtronic. D.M. is a cardiac fellow who was funded by the Swiss Government Foundation is located in Berne CH (https://www.snf.ch/en/GrjwOKMdGiigVhgY/page/theSNSF/profile). J.S. reports being a consultant to Edwards, Medtronic, and Boston Scientific; and receiving research funding from Edwards and Medtronic. D.A.W. reports receiving research grants from Abbott and Edwards. J.G.W. reports being a consultant and/or receiving research support from Edwards, Abbott, Boston Scientific, and Vivitro Medical. The other authors have no conflicts of interest to disclose.
Footnotes
See page 519 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2023.03.015.
Supplementary Material
References
- 1.Kundi H., Strom J.B., Valsdottir L.R., et al. Trends in isolated surgical aortic valve replacement according to hospital-based transcatheter aortic valve replacement volumes. JACC Cardiovasc Interv. 2018;11:2148–2156. doi: 10.1016/j.jcin.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Kolkailah A.A., Doukky R., Pelletier M.P., et al. Transcatheter aortic valve implantation versus surgical aortic valve replacement for severe aortic stenosis in people with low surgical risk. Cochrane Database Syst Rev. 2019;12:CD013319. doi: 10.1002/14651858.CD013319.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stub D., Lauck S., Lee M., et al. Regional systems of care to optimize outcomes in patients undergoing transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2015;8:1944–1951. doi: 10.1016/j.jcin.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 4.Mack M. Balancing optimal outcomes with access to care: It can be done. JACC CardiovascInterv. 2015;8:1952–1953. doi: 10.1016/j.jcin.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 5.Lamarche Y., Elmi-Sarabi M., Ding L., et al. A score to estimate 30-day mortality after intensive care admission after cardiac surgery. J Thorac Cardiovasc Surg. 2017;153:1118–1125.e4. doi: 10.1016/j.jtcvs.2016.11.039. [DOI] [PubMed] [Google Scholar]
- 6.Pu A., Ding L., Shin J., et al. Long-term outcomes of multiple arterial coronary artery bypass grafting: a population-based study of patients in British Columbia, Canada. JAMA Cardiol. 2017;2:1187–1196. doi: 10.1001/jamacardio.2017.3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asgar A.W., Lauck S., Ko D., et al. Quality of care for transcatheter aortic valve implantation: development of Canadian Cardiovascular Society quality indicators. Can J Cardiol. 2016;32:1038.e1–1038.e4. doi: 10.1016/j.cjca.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 8.Carroll J.D., Mack M.J., Vemulapalli S., et al. STS-ACC TVT Registry of Transcatheter Aortic Valve Replacement. J Am Coll Cardiol. 2020;76:2492–2516. doi: 10.1016/j.jacc.2020.09.595. [DOI] [PubMed] [Google Scholar]
- 9.Donabedian A. The seven pillars of quality. Archiv Pathol Lab Med. 1990;114:1115–1118. [PubMed] [Google Scholar]
- 10.Harvey R.D., Mileham K.F., Bhatnagar V., et al. Modernizing clinical trial eligibility criteria: recommendations of the ASCO-Friends of Cancer Research Washout Period and Concomitant Medication Work Group. Clin Cancer Res. 2021;27:2400–2407. doi: 10.1158/1078-0432.CCR-20-3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hosmer D., Lemeshow S. Confidence interval estimation of interaction. Epidemiology. 1992;3:452–456. doi: 10.1097/00001648-199209000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Didier R., Le Breton H., Eltchaninoff H., et al. Evolution of TAVI patients and techniques over the past decade: the French TAVI registries. Arch Cardiovasc Dis. 2022;115:206. doi: 10.1016/j.acvd.2022.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Hamm C.W., Beyersdorf F. GARY—the largest registry of aortic stenosis treatment worldwide. Eur Heart J. 2020;41:733–735. doi: 10.1093/eurheartj/ehaa048. [DOI] [PubMed] [Google Scholar]
- 14.Bowdish M.E., D’Agostino R.S., Thourani V.H., et al. STS Adult Cardiac Surgery Database: 2021 update on outcomes, quality, and research. Ann Thorac Surg. 2021;111:1770–1780. doi: 10.1016/j.athoracsur.2021.03.043. [DOI] [PubMed] [Google Scholar]
- 15.Grant S.W., Kendall S., Goodwin A.T., et al. Trends and outcomes for cardiac surgery in the United Kingdom from 2002 to 2016. JTCVS Open. 2021;7:259–269. doi: 10.1016/j.xjon.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ludvigsson J.F. Quality registries: exciting opportunities. J Intern Med. 2016;279:130–131. doi: 10.1111/joim.12463. [DOI] [PubMed] [Google Scholar]
- 17.Burstin H., Leatherman S., Goldmann D. The evolution of healthcare quality measurement in the United States. J Intern Med. 2016;279:154–159. doi: 10.1111/joim.12471. [DOI] [PubMed] [Google Scholar]
- 18.Mulder D.S., Spicer J. Registry-based medical research: data dredging or value building to quality of care? Ann Thorac Surg. 2019;108:274–282. doi: 10.1016/j.athoracsur.2018.12.060. [DOI] [PubMed] [Google Scholar]
- 19.Pilote L., Humphries K.H. Incorporating sex and gender in cardiovascular research: The time has come. Can J Cardiol. 2014;30:699–702. doi: 10.1016/j.cjca.2013.09.021. [DOI] [PubMed] [Google Scholar]
- 20.Pibarot P., Lauck S., Morris T., et al. Patient care journey for patients with heart valve disease. Can J Cardiol. 2022;38:1296–1299. doi: 10.1016/j.cjca.2022.02.025. [DOI] [PubMed] [Google Scholar]
- 21.Humphries K.H., Toggweiler S., Rodés-Cabau J., et al. Sex differences in mortality after transcatheter aortic valve replacement for severe aortic stenosis. J Am Coll Cardiol. 2012;60:882–886. doi: 10.1016/j.jacc.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 22.Conrotto F., D’Ascenzo F., Presbitero P., et al. Effect of gender after transcatheter aortic valve implantation: a meta-analysis. Ann Thorac Surg. 2015;99:809–816. doi: 10.1016/j.athoracsur.2014.09.089. [DOI] [PubMed] [Google Scholar]
- 23.Kaul S. Raising the evidentiary bar for guideline recommendations for TAVR: JACC review topic of the week. J Am Coll Cardiol. 2020;76:985–991. doi: 10.1016/j.jacc.2020.05.085. [DOI] [PubMed] [Google Scholar]
- 24.Baron S.J., Wang K., House J.A., et al. Cost-effectiveness of transcatheter versus surgical aortic valve replacement in patients with severe aortic stenosis at intermediate risk. Circulation. 2019;139:877–888. doi: 10.1161/CIRCULATIONAHA.118.035236. [DOI] [PubMed] [Google Scholar]
- 25.Reynolds H.W., Sutherland E.G. A systematic approach to the planning, implementation, monitoring, and evaluation of integrated health services. BMC Health Serv Res. 2013;13:168. doi: 10.1186/1472-6963-13-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sathananthan J., Ding L., Yu M., et al. Implications of transcatheter heart valve selection on early and late pacemaker rate and on length of stay. Can J Cardiol. 2018;34:1165–1173. doi: 10.1016/j.cjca.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 27.Afilalo J., Lauck S., Kim D.H., et al. Frailty in older adults undergoing aortic valve replacement: the FRAILTY-AVR study. J Am Coll Cardiol. 2017;70:689–700. doi: 10.1016/j.jacc.2017.06.024. [DOI] [PubMed] [Google Scholar]
- 28.Lauck S.B., Wood D.A., Baumbusch J., et al. Vancouver transcatheter aortic valve replacement clinical pathway: minimalist approach, standardized care, and discharge criteria to reduce length of stay. Circ Cardiovasc Qual Outcomes. 2016;9:312–321. doi: 10.1161/CIRCOUTCOMES.115.002541. [DOI] [PubMed] [Google Scholar]
- 29.Parker C., Schwamm L.H., Fonarow G.C., Smith E.E., Reeves M.J. Stroke quality metrics systematic reviews of the relationships to patient-centered outcomes and impact of public reporting. Stroke. 2012;43:155–162. doi: 10.1161/STROKEAHA.111.635011. [DOI] [PubMed] [Google Scholar]
- 30.Wijeysundera H.C., Henning K.A., Qiu F., et al. Inequity in access to transcatheter aortic valve replacement: a pan-Canadian evaluation of wait-times. Can J Cardiol. 2020;36:844–851. doi: 10.1016/j.cjca.2019.10.018. [DOI] [PubMed] [Google Scholar]
- 31.Lauck S.B., Achtem L., Boone R.H., et al. Implementation of processes of care to support transcatheter aortic valve replacement programs. Eur J Cardiovasc Nurs. 2013;12:33–38. doi: 10.1016/j.ejcnurse.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 32.Reynolds M.R., Magnuson E.A., Wang K., et al. Health-related quality of life after transcatheter or surgical aortic valve replacement in high-risk patients with severe aortic stenosis. J Am Coll Cardiol. 2012;60:548–558. doi: 10.1016/j.jacc.2012.03.075. [DOI] [PubMed] [Google Scholar]
- 33.Baron S.J., Arnold S.V., Wang K., et al. Health status benefits of transcatheter vs surgical aortic valve replacement in patients with severe aortic stenosis at intermediate surgical risk: results from the PARTNER 2 randomized clinical trial. JAMA Cardiol. 2017;2:837–845. doi: 10.1001/jamacardio.2017.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lauck S.B., Arnold S.V., Borregaard B., et al. Very early changes in quality of life after transcatheter aortic valve replacement: results from the 3M TAVR trial. Cardiovasc Revascular Med. 2020;21:1573–1578. doi: 10.1016/j.carrev.2020.05.044. [DOI] [PubMed] [Google Scholar]
- 35.Lavallee D.C., Chenok K.E., Love R.M., et al. Incorporating patient-reported outcomes into health care to engage patients and enhance care. Health Affairs. 2016;35:575–582. doi: 10.1377/hlthaff.2015.1362. [DOI] [PubMed] [Google Scholar]
- 36.Lauck S.B., Baron S.J., Irish W., et al. Temporal changes in mortality after transcatheter and surgical aortic valve replacement: retrospective analysis of US Medicare patients (2012-2019) J Am Heart Assoc. 2021;10 doi: 10.1161/JAHA.120.021748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mori M., Gupta A., Wang Y., et al. Trends in transcatheter and surgical aortic valve replacement among older adults in the United States. J Am Coll Cardiol. 2021;78:2161–2172. doi: 10.1016/j.jacc.2021.09.855. [DOI] [PubMed] [Google Scholar]
- 38.Durko A., Osnabrugge R., van Mieghem N., et al. Annual number of candidates for transcatheter aortic valve implantation per country: current estimates and future projections. Eur Heart J. 2018;39:2635–2642. doi: 10.1093/eurheartj/ehy107. [DOI] [PubMed] [Google Scholar]
- 39.Wood D.A., Lauck S.B., Cairns J.A., et al. The Vancouver 3M (Multidisciplinary, Multimodality, But Minimalist) Clinical Pathway Facilitates Safe Next-Day Discharge Home at Low-, Medium-, and High-Volume Transfemoral Transcatheter Aortic Valve Replacement Centers: the 3M TAVR Study. JACC Cardiovasc Interv. 2019;12:459–469. doi: 10.1016/j.jcin.2018.12.020. [DOI] [PubMed] [Google Scholar]
- 40.D’Agostino R.S., Jacobs J.P., Badhwar V., et al. The Society of Thoracic Surgeons Adult Cardiac Surgery Database: 2019 update on outcomes and quality. Ann Thorac Surg. 2019;107:24–32. doi: 10.1016/j.athoracsur.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 41.Smith N., Mitton C., Hall W., et al. High performance in healthcare priority setting and resource allocation: a literature- and case study-based framework in the Canadian context. Social Sci Med. 2016;162:185–192. doi: 10.1016/j.socscimed.2016.06.027. [DOI] [PubMed] [Google Scholar]
- 42.Albassam O., Henning K., Qiu F., et al. Increasing wait-time mortality for severe aortic stenosis: a population-level study of the transition in practice from surgical aortic valve replacement to transcatheter aortic valve replacement. Circ Cardiovasc Interv. 2020;13 doi: 10.1161/CIRCINTERVENTIONS.120.009297. [DOI] [PubMed] [Google Scholar]
- 43.Mjåset C., Ikram U., Nagra N., Feeley T.W. Value-based health care in four different health care systems. NEJM Catalyst Innov Care Deliv. 2020;1 [Google Scholar]
- 44.Lauck S.B., Lewis K.B. Shared decision-making in cardiac care: Can we close the gap between good intentions and improved outcomes? Heart. 2022;109:4–5. doi: 10.1136/heartjnl-2022-321482. [DOI] [PubMed] [Google Scholar]
- 45.Lauck S.B., Lewis K.B., Borregaard B., de Sousa I. What is the right decision for me?” Integrating patient perspectives through shared decision-making for valvular heart disease therapy. Can J Cardiol. 2021;37:1054–1063. doi: 10.1016/j.cjca.2021.02.022. [DOI] [PubMed] [Google Scholar]
- 46.Anand S.S., Abonyi S., Arbour L., et al. Explaining the variability in cardiovascular risk factors among First Nations communities in Canada: a population-based study. Lancet Planetary Health. 2019;3:e511–e520. doi: 10.1016/S2542-5196(19)30237-2. [DOI] [PubMed] [Google Scholar]
- 47.Tsevat J., Moriates C. Value-based health care meets cost-effectiveness analysis. Ann Intern Med. 2018;169:329–332. doi: 10.7326/M18-0342. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.