Abstract
MicroRNAs (miRNAs) are short non-coding RNA molecules that regulate gene expression by targeting specific messenger RNAs (mRNAs) in distinct cell types. This review provides a com-prehensive overview of the current understanding regarding the involvement of miR-483-5p and miR-483-3p in various physiological and pathological processes. Downregulation of miR-483-5p has been linked to numerous diseases, including type 2 diabetes, fatty liver disease, diabetic nephropathy, and neurological injury. Accumulating evidence indicates that miR-483-5p plays a crucial protective role in preserving cell function and viability by targeting specific transcripts. Notably, elevated levels of miR-483-5p in the bloodstream strongly correlate with metabolic risk factors and serve as promising diagnostic markers. Consequently, miR-483-5p represents an appealing biomarker for predicting the risk of developing diabetes and cardiovascular diseases and holds potential as a therapeutic target for intervention strategies. Conversely, miR-483-3p exhibits significant upregulation in diabetes and cardiovascular diseases and has been shown to induce cellular apoptosis and lipotoxicity across various cell types. However, some discrepancies regarding its precise function have been reported, underscoring the need for further investigation in this area.
Keywords: miRNAs, miR-483-5p, miR-483-3p, diabetes, biomarker
1. Introduction
Type 2 diabetes (T2D) is a chronic metabolic disorder characterized by elevated blood glucose levels due to deficiencies in insulin secretion by pancreatic β-cells and/or impaired insulin sensitivity in peripheral tissues such as the liver, adipose tissue, and muscle [1,2]. Individuals with T2D face an augmented risk of developing severe secondary complications, including fatty liver disease, endothelial dysfunction, cardiovascular disease, and renal failure [3].
MicroRNAs (miRNAs) are a class of endogenous non-coding RNA approximately 17–23 nucleotides in length that bind to the 3′-untranslated region (3′UTR) of target mRNAs, leading to their degradation and/or translational repression [4]. Dysregulation of miRNA expression has been linked to a variety of diseases, including diabetes, making them potential biomarkers and therapeutic targets [5,6]. MiRNAs exhibit dynamic regulation of gene expression, which facilitates buffering of gene expression levels to achieve a stable state. However, miRNA-mediated inhibition of target transcripts is not universal among cell types, as it can be influenced by alternative splicing/polyadenylation events and the levels of cell type-specific factors that can alter the secondary structure of target transcripts [7].
MicroRNAs have the ability to circulate in a stable manner in the blood, either in association with protein complexes or encapsulated within exosome-like vesicles. They play a crucial role in facilitating communication between different cell types [8]. The accessibility of circulating miRNAs makes them promising biomarkers, as they can be detected in various bodily fluids, including blood plasma, serum, urine, cerebrospinal fluid, and saliva. To ensure their protection and transportation, miRNAs are enveloped by lipids, exosomes, or proteins such as Argonaute2 (AGO2) [9]. These circulating miRNAs may act as mediators of endocrine signaling between organs or serve as indicators of tissue-specific miRNA release through paracrine or autocrine signaling within an organ.
The dysregulation of miRNA expression is not the sole determinant of human disease development, yet it does contribute to the regulation of disease progression and prognosis. Consequently, rectifying the imbalance of miRNA expression levels holds significant therapeutic potential. Disease-modifying therapies that target miRNAs can be devised through two primary strategies: increasing protective mRNA levels by inhibiting miRNAs or decreasing detrimental mRNA through miRNA mimics [10]. Recent advancements in oligonucleotide delivery technology have demonstrated the ability to selectively target specific tissues, cells, and subcellular compartments, including the traversal of the blood–brain barrier for brain-specific delivery [10]. These breakthroughs in RNA therapeutics have instilled optimism that these approaches may eventually translate into viable treatment options in the future.
This review provides a comprehensive summary of the existing understanding regarding the tissue-specific expression patterns of miR-483 in various organs and underscores its diverse functions in numerous diseases including obesity, diabetes, fatty liver disease, heart failure, diabetic nephropathy, and brain injury.
2. MiR-483 Biogenesis
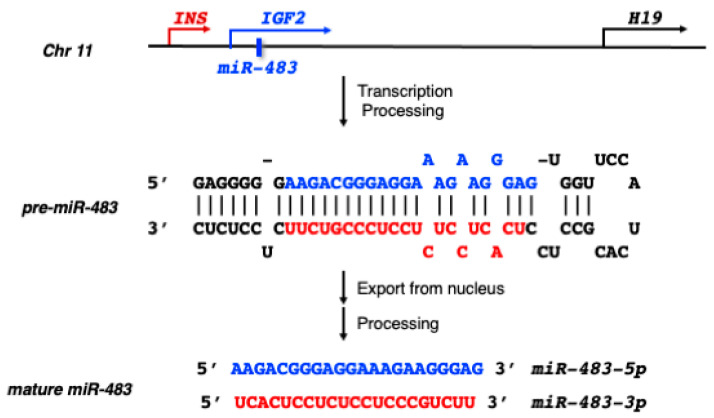
MiR-483 is located within the second intron of the human insulin-like growth factor 2 (IGF2) gene on chromosome 11 (Figure 1). It encodes for two mature microRNAs: miR-483-5p and miR-483-3p. The biogenesis of miR-483 follows the standard miRNA biogenesis process, involving transcription of the primary miRNA transcript (pri-miR-483), which is then cleaved by the nuclear microprocessor complex to produce a precursor miRNA (pre-miR-483). After export from the nucleus, the stem-loop pre-miR-483 is further processed by Dicer to produce double-stranded duplexes, which include miR-483-5p derived from the 5′-arm (previously called miR-483), and miR-483-3p derived from the 3′-arm (previously called miR-483*). Traditionally, it was believed that only one of the miRNA strands is stably produced and functional, while the other strand is degraded. However, more recent studies have shown that both mature miRNA strands can be expressed and have functional roles for most miRNAs. MiR-483-5p and miR-483-3p are both produced and functional in specific tissues and cells. They exhibit partial reverse complementarity and have distinct mRNA-targeting sequences, which makes them biologically different and capable of targeting different mRNA transcripts (Figure 1). The sequences of miR-483-5p and miR-483-3p are highly conserved among mammalian species, including humans, mice, and rats.
Figure 1.
The genomic location of miR-483 and Ins-Igf2-H19 gene clusters on human chromosome 11. The sequences of the stem-loop precursor miRNA (pre-miR-483) and two mature miRNAs, miR-483-5p (highlighted in blue) and miR-483-3p (highlighted in red) are from the miRBase database (https://www.mirbase.org/, accessed on 15 May 2023).
The genomic localization of miR-483 is intriguing, as it resides within the INS-IGF2 locus, which plays a critical role in the insulin pathway [11]. The host gene for miR-483, IGF2, is located within the IGF2/H19 genetic locus [12]. Notably, IGF2 is exclusively expressed from the paternal allele, while the H19 gene demonstrates maternal expression. IGF2 has established involvement in lipid metabolism and obesity [13].
Both miR-483-5p and miR-483-3p have demonstrated functional activity, although their differences in terms of transcription regulation and stability remain poorly understood. Studies have suggested that certain intragenic miRNAs are co-expressed and function in conjunction with their host genes, while others do not exhibit such coordination. The transcription of miR-483-5p may be coregulated with its host gene, IGF2, or regulated independently of IGF2 transcription [14]. Considering that IGF2 transcript levels are typically very low in adults, it is expected that IGF2 and miR-483 are independently regulated in adult tissues. Aberrant expressions of miR-483 regulated by DNA methylation have been observed in the liver and skeletal muscle [15]. In addition, miR-483-5p is reported to be upregulated by high glucose, insulin, and various stress conditions [16,17,18], suggesting the expression of miR-483-5p is regulated in response to changes in cellular environment in order to support adaptation to an altered microenvironment. The transcription of miR-483-3p can also be upregulated independently of IGF2 transcription via β-catenin/Wnt signaling [19], insulin-responsive/sensitive elements, or inflammatory transcription factors [20]. These data suggest that the expression patterns of miR-483-5p and miR-483-3p may vary across different cell types and can even differ within the same cells under various pathological stress conditions.
3. Role of MiR-483-5p in Diabetes
3.1. MiR-483-5p Protects Pancreatic β-Cells Function and Identity
Pancreatic β-cells are specialized cells in the pancreas that synthesize and secrete insulin, a hormone that regulates blood glucose level. In patients with T2D, β-cells gradually lose their function and secrete insufficient insulin, which leads to elevated blood glucose levels [21]. A common view is that the continuous loss of β-cells is a major feature in the development of type 2 diabetes. However, this principle has been challenged by studies suggesting that β-cells do not die in patients with diabetes but undergo alteration in β-cell identity (or β-cell dedifferentiation) [22,23]. The dedifferentiation of β-cells into glucagon-producing α-cells or the earlier-stage progenitor-like cells has been considered a feature during the development of T2D.
MiR-483-5p has been shown to exhibit highly differential expression between pancreatic β-cells and α-cells, with higher expression in β-cells than in α-cells [16]. This differential expression is critical for maintaining β-cell function since miR-483-5p targets the suppressor of cytokine signalling3 (SOCS3), leading to increased insulin transcription in β-cells and decreased glucagon transcription in α-cells (Table 1, Figure 2). Mice with β-cell-specific deletion of miR-483-5p display hyperglycemia and glucose intolerance, along with reduced insulin release when fed a high-fat diet [24].
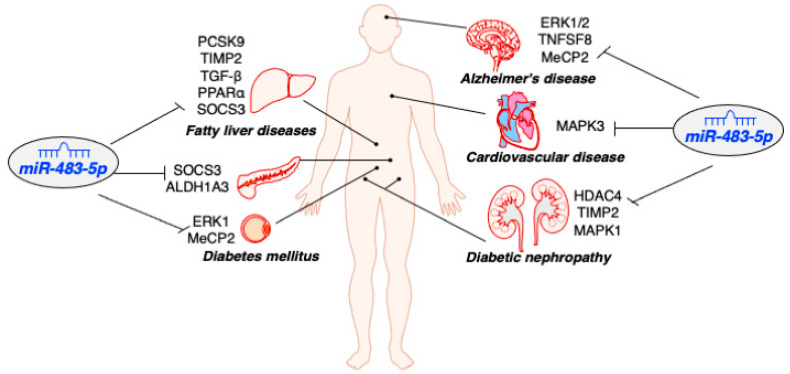
Figure 2.
Impacts of miR-483-5p in human diseases. The cartoon highlights the involvement of miR-483-5p in numerous human diseases through its regulation of specific targets in various cell types, including liver, pancreas, adipose, kidney, heart, and brain.
Deletion of miR-483 results in the loss of β-cell identity, as evidenced by elevated expression of aldehyde dehydrogenase family 1, subfamily A3 (ALDH1A3) [24]. ALDH1A3 has been validated as a novel marker of β-cell dedifferentiation, and elevated ALDH1A3 expression occurs in the islets of diabetic human subjects and various diabetic mouse models [25]. Further studies have validated ALDH1A3 as a direct target of miR-483-5p, and overexpression of miR-483-5p represses ALDH1A3 expression [24] (Table 1, Figure 2). However, the identity of ALDH1A3-positive β-cells and how miR-483-5p participates in ALDH1A3-mediated β-cell dedifferentiation remains to be investigated.
Hyperlipidemia, characterized by reduced circulating high-density lipoprotein (HDL) cholesterol and elevated circulating low-density lipoprotein (LDL) cholesterol, is a major risk factor for developing diabetes [26]. Genetic ablation of miR-483 induces unfavorable blood lipid profiles, including increased LDL and decreased HDL [24]. High serum levels of cholesterol are associated with increased islet cholesterol content and decreased insulin secretion [26]. Islets exposed to LDL show an increase in oxidative stress, leading to impaired mitochondrial function and insulin synthesis. These findings highlight the critical role of miR-483-5p in protecting β-cell function, and miR-483-5p inactivation induces dyslipidemia and initiates β-cell dedifferentiation.
Exosomes from human islets are enriched with miR-483-5p more than 10-fold compared to cellular content [27]. Furthermore, the level of miR-483-5p in serum is strongly associated with metabolic risk factors for diabetes, such as body mass index (BMI), waist circumference, insulin resistance, triglycerides, and cholesterol levels [18]. The circulating miR-483 levels are also coordinated with the change in glycated hemoglobin A1C (HbA1c) in T2D patients with short-term intensive insulin therapy [28]. These findings suggest that miR-483-5p holds immense potential as a novel diagnostic and prognostic tool in T2D treatment strategies.
3.2. MiR-483-5p Mitigates Hyperlipidemia-Associated Fatty Liver Disease
The liver plays a central role in lipid metabolism by secreting LDL via the LDL receptor (LDLR) on the liver surface [29]. Excessive hepatic lipid accumulation can trigger cellular stress responses, inflammation, cell death, and fibrosis [30]. Thus, hyperlipidemia in individuals with insulin resistance and T2D is a significant factor in the development of hepatic insulin resistance and the progression of non-alcoholic fatty liver disease (NAFLD) or alcoholic fatty liver disease (AFLD) [31].
Research has shown that hyperlipidemic human subjects with elevated LDL levels exhibit lower levels of circulatory miR-483-5p [32]. In hypercholesterolemic mouse models and HepG2 cells, overexpression of miR-483-5p significantly inhibits the expression of PCSK9, which subsequently increases hepatocyte expression of LDLR and enhances LDL uptake [33] (Figure 2). PCSK9 encodes the proprotein convertase subtilisin/kexin type 9 protein, which binds LDL and induces LDLR degradation. PCSK9 loss-of-function mutations are associated with hypocholesterolemia, while gain-of-function mutations are linked to familial hypercholesterolemia [27,34]. PCSK9 has been identified as a promising therapeutic target for patients who do not adequately respond to statins, a commonly used cholesterol-lowering medication [35]. Further studies have confirmed that PCSK9 is a direct target of miR-483-5p, and overexpression of miR-483-5p has also been shown to reduce plasma cholesterol and LDL levels in hypercholesterolemic mice (Table 1). These findings suggest that miR-483-5p can ameliorate hypercholesterolemia and may be a potential therapeutic target for this condition.
A previous study has found that overexpression of miR-483-5p inhibits the expression of SOCS3 in mouse Hepa1-6 cells [14] (Table 1). SOCS3 is a negative regulator of the JAK/STAT pathway and is implicated in hypertriglyceridemia associated with insulin resistance and leptin resistance [36,37]. New evidence shows that miR-483 is downregulated in rats with liver fibrosis [38] (Table 1, Figure 2). Overexpression of miR-483 inhibits mouse liver fibrosis by targeting tissue inhibitor of metalloproteinase 2 (TIMP2) and platelet-derived growth factor-β (PDGF-β). A recent report indicates that miR-483-5p expression is lower in NAFLD and AFLD mouse models and human hepatocellular carcinoma (HCC) tissue samples [39]. The downregulation of miR-483-5p increases not only TIMP2 expression but also peroxisome proliferator-activated receptor alpha (PPARα) and transforming growth factor beta (TGF-β) expression (Table 1, Figure 2). In the liver, PPARα regulates lipid metabolism and controls liver homeostasis, and its dysregulation may lead to hepatic steatosis and fibrosis [40]. These findings suggest that miR-483-5p can alleviate lipid deposition in the liver and mitigate hyperlipidemia-associated NAFLD. Therefore, miR-483 may be a potential therapeutic target for treating patients with fatty liver disease.
Table 1.
Impacts of miR-483-5p in human diseases.
| Type of Disease | Expression Pattern | Tissue | Cell Lines | Targets | Function | References |
|---|---|---|---|---|---|---|
| Type 2 Diabetes | down | pancreatic islets | MIN6 |
SOCS3 ALDH1A3 |
Induce insulin secretion, inhibit glucagon secretion, and maintain β-cell identity | [16,24] |
| Obesity/ Diabetes |
down | adipose | 3T3-L1 |
ERK1 MeCP2 |
Promote adipogenesis | [41,42,43] |
| Fatty liver disease (NAFLD/ AFLD) |
down | liver | HepG2 |
PCSK9 TIMP2 TGF-β PPARα SOCS3 |
Reduce lipid deposition and inhibit liver fibrosis | [32,33,38,39] |
| Diabetic nephropathy | down | Kidney tubule | HK2 TCMK-1 |
HDAC4 TIMP2 MAPK1 |
Prevent renal tubular damage and renal fibrosis | [44,45] |
| Alzheimer, Brain injury after cardiac arrest |
down | neuron | Neonatal Fibroblasts, PC12 |
ERK1/2 TNFSF8 MeCP2 |
Promote mitochondrial biogenesis, inhibit ROS generation, protect neurological function, and regulate fetal brain development |
[46] |
| Cardiovascular disease | up | serum, carotid bulb |
AC16 | MAPK3 | Induced cell apoptosis and oxidative stress | [18,47,48,49] |
3.3. MiR-483-5p Promotes Adipogenesis in the Adipose
Adipose tissue plays essential roles in maintaining lipid and glucose homeostasis. However, in obesity, adipose tissue becomes dysfunctional, promoting a pro-inflammatory, hyperlipidemic, and insulin-resistant environment that contributes to T2D and cardiovascular disease [50]. Adipogenesis is a complex series of steps involving the orchestrated activation of multiple transcription factors, which drive the typical physiological and morphological changes [51]. Among these factors, the expression of peroxisome proliferator-activated receptor gamma (PPARγ) plays a crucial role in facilitating the differentiation and maintenance of mature adipocytes. PPARγ induces the expression of genes associated with insulin sensitivity, lipogenesis, and lipolysis, thereby influencing the metabolic functions of adipocytes [52].
Recent studies have shown that miR-483-5p plays a significant role in regulating adipogenesis. The expression of miR-483-5p is significantly downregulated in subcutaneous adipose tissue obtained from human subjects with obesity compared to those without obesity [41] (Table 1). MiR-483-5p positively regulates the expression of PPARγ and facilitates adipogenesis in mouse pre-adipocyte 3T3-L1 cells and human adipose-derived MSCs by targeting ERK1 [42,53]. It also targets methyl CpG-binding protein 2 (MeCP2), an epigenetic negative regulator of adipose browning, and enhances adipogenesis in hBMSCs [43,54] (Table 1, Figure 2). Inhibition of miR-483-5p fails to stimulate the accumulation of marrow adipocytes and PPARγ expression.
Moreover, miR-483-5p can influence osteogenesis, a bone disease characterized by low bone mass and poor bone quality. It is highly enriched in extracellular vesicles derived from aged bone matrix, and its inhibition delays the development of osteoarthritis [55,56]. Additionally, miR-483-5p can be released from differentiating adipocytes and found in the plasma of subjects with osteoporosis, indicating its potential as a non-invasive biomarker for this condition.
4. Role of MiR-483-5p in Other Human Diseases
Hyperglycemia associated with diabetes can damage various organs, including the cardiovascular system and kidneys, over time. Diabetic complications include nephropathy, neuropathy, cardiomyopathy, and retinopathy [57]. They share similar pathological mechanisms, such as inflammation, mitochondrial dysfunction, and oxidative stress. Recent studies have demonstrated that miR-483-5p participates in the pathogenesis of diabetic complications.
4.1. MiR-483-5p Downregulation in Patients with Diabetic Nephropathy
Diabetic nephropathy (DN) is a common microvascular complication of diabetes and is characterized by progressive kidney disease. The disease initially manifests as glomerular hyperfiltration, followed by persistent albuminuria and microalbuminuria, ultimately leading to significant proteinuria, impaired renal function, and a gradual reduction in the glomerular filtration rate [58].
Recent studies have demonstrated that miR-483-5p is downregulated in kidney tissues of both type 1 and type 2 diabetic mouse models, as well as in proximal renal tubular cells of patients with DN [44] (Table 1, Figure 2). The level of miR-483-5p is positively correlated with estimated glomerular filtration rate (eGFR), an indicator of renal function. MiR-483-5p reduction is associated with lower eGFR and prevalence of proteinuria. Overexpression of miR-483-5p has been shown to have a protective effect on high glucose-induced damage in renal tubular cells by targeting HDAC4 [44]. Furthermore, miR-483-5p targets TIMP2 and MAPK1 in renal tubular epithelial cells [45], both of which are involved in the pathogenesis of renal disease (Table 1, Figure 2). Notably, the expression of TIMP2 is significantly higher in renal biopsies with renal disease [59]. Injection of AAV-miR-483-5p has been shown to alleviate interstitial fibrosis in diabetic mice by inhibiting the expression of TIMP2, MAPK1, and other fibrosis-related genes such as Col1a1, Col4a1, and fibronectin [59].
Interestingly, miR-483-5p is present in urine and is upregulated in the extracted urine exosomes of DN patients [45]. MiR-483-5p can be secreted from cellular tubular epithelial cells and transported into urine through exosomes. The process is mediated by hnRNPA1, a key player in miRNA sorting and transporting into extracellular vesicles [60]. Differences in miR-483-5p expression in tubular epithelial cells between intracellular and extracellular environments may provide new insights for the diagnosis and treatment of DN. The accumulation of miR-483-5p in urinary exosomes may serve as a new biomarker for effectively distinguishing DN patients from healthy individuals.
4.2. MiR-483-5p Protects Neurological Function against Oxidative Stress
According to recent studies, miR-483-5p plays a crucial role in protecting against neurological injury induced by cardiac arrest. Specifically, miR-483-5p is significantly downregulated in hippocampal samples from patients with neurological injury after cardiac arrest compared to the normal group [61] (Table 1, Figure 2). Brain injury following cardiac arrest is mainly caused by the generation of mitochondrial reactive oxygen species (ROS), which can be inhibited by promoting mitochondrial biogenesis and reducing ROS generation. MiR-483-5p targets TNFSF8 to achieve this effect and protect against neurological impairment. Overexpression of miR-483-5p reduces protein expression of Bax and cleaved caspase 3, thereby inhibiting cytochrome c release and ROS generation from mitochondria, which alleviates cell injury by ischemia–reperfusion [61]. The inhibition of miR-483-5p in rats results in more severe hippocampal damage than the control group after cardiac arrest. These findings demonstrate that miR-483-5p targets TNFSF8 to protect against neurological impairment by promoting mitochondrial biogenesis and inhibiting ROS generation.
Previous research also suggests that miR-483-5p elevation represents a compensatory neuroprotective mechanism during the early stage of Alzheimer’s disease [62]. During the early stage of Alzheimer’s disease, miR-483-5p upregulation inhibits TAU phosphorylation and prevents neurofibrillary pathology by targeting ERK1/2 expression (Table 1, Figure 2). In contrast, the depletion of miR-483-5p results in a concomitant increase in pathological TAU phosphorylation and neurofibrillary tangle formation in the brain. MiR-483-5p is co-upregulated with its host gene IGF2 in the cerebrospinal fluid of patients with Alzheimer’s disease. IGF2 upregulation can promote neurogenesis and synapse formation against oxidative stress, suggesting a potential neuroprotective role for miR-483-5p in Alzheimer’s disease.
Furthermore, miR-483-5p plays a crucial role in the regulation of MeCP2 levels for brain development during fetal stages [46] (Table 1). MeCP2 protein levels must be tightly regulated to ensure normal neurological function, and miR-483-5p precisely controls MeCP2 levels during fetal development. MeCP2 levels are repressed during fetal development and elevated during postnatal development in human brains. MiR-483-5p is enriched in human fetal brains compared to postnatal brains, indicating an inverse correlation between miR-483-5p and MeCP2 levels in developing human brains. In addition, overexpression of miR-483-5p in hippocampal neurons rescues the abnormal dendritic spine phenotype caused by elevated MeCP2. MiR-483-5p modulates MeCP2-interacting corepressor complexes, including HDAC4 and TBL1X, shedding light on its role in regulating MeCP2 levels and interacting proteins during human fetal development.
4.3. Elevation of Circulating MiR-483-5p as a Biomarker for Cardiovascular Disease
Cardiovascular disease (CVD) is the primary cause of mortality and morbidity, and atherosclerosis serves as the prevailing underlying factor in CVD-related manifestations, including heart attacks and strokes. Individuals with diabetes have historically exhibited a higher prevalence of CVD compared to those without diabetes, and the risk of CVD often coincides with comorbidities such as obesity, dyslipidemia, and hypertension [63].
Studies have shown that elevated serum levels of miR-483-5p are significantly associated with insulin resistance and hypertension, which are risk factors for the development of T2D, cardiovascular disease, and other associated complications [18,64] (Table 1). The serum concentration of miR-483-5p could reflect the degree of leakage from the source tissue due to varying degrees of permeability/leakage. In fact, miRNAs can be secreted as exosomes to transfer their effects to adjacent cells [9]. The increase in miR-483-5p in serum could be a consequence of tissue damage or inflammation during the development of diabetes and cardiovascular disease.
Indeed, elevated circulating levels of miR-483-5p are observed in patients with acute myocardial infarction (AMI) compared to those without AMI [47] (Table 1, Figure 2). AMI, commonly known as a heart attack, is a serious condition that occurs when a blood clot obstructs blood flow to the heart, leading to cardiac cell death and impaired heart function. Timely diagnosis is crucial for effective management and treatment. Research has shown that circulating miR-483-5p levels positively correlate with elevated cardiac troponin I, a widely used biomarker for AMI diagnosis [48]. In fact, miR-483-5p reaches peak expression much earlier than troponin I, suggesting miR-483-5p would provide more sensitive and valuable diagnostic information than traditional markers during the early phases of AMI.
Despite the observed rise in circulating levels of miR-483-5p, studies also showed a predominant increase in miR-483-5p expression in the atrial myocardium of patients diagnosed with atrial fibrillation (AF) compared to individuals maintaining sinus rhythm [49] (Figure 2). AF is a common type of cardiac arrhythmia characterized by irregular electrical activities in the atria. It often coexists with AMI. In vitro experiments demonstrated that hypoxia induces miR-483-5p expression in cardiomyocytes and miR-483-5p upregulation increases hypoxia-induced cell apoptosis and oxidative stress by targeting MAPK3 [48] (Table 1). Furthermore, miR-483-5p has been found to be significantly linked to intima media thickness of the carotid bulb and the number of plaques, both of which are indicators of atherosclerosis [65,66]. The available data collectively indicate that miR-483-5p holds promise as a promising biomarker for assessing the risk of CVD. However, the causal relationship between the increased levels of miR-483-5p and the cardiac rhythm disorder remains uncertain. Further investigations are necessary to elucidate the underlying mechanisms and fully understand the role of miR-483-5p in the pathogenesis of diabetes and CVD.
5. Implication of MiR-483-3p in Human Disease
MiR-483-3p has been extensively studied in the context of cancer [67]. More recent data have shown that mir-483-3p upregulation has been reported in adipose, cardiomyocytes, and endothelial cells in patients with diabetes or cardiovascular diseases. Its role can differ depending on the disease context or cell type, where it can act as a factor inducing apoptosis or supporting cell survival against inflammation.
In contrast to miR-483-5p, miR-483-3p has been demonstrated to inhibit adipocyte differentiation and promote insulin resistance [68] (Table 2). Studies conducted on adipose tissue from both adult humans with low-birth-weight and prediabetic adult rats exposed to suboptimal nutrition early in life have revealed a notable increase in miR-483-3p expression, suggesting a conserved programming of this miRNA between species [69] (Table 2). Overexpression of miR-483-3p hampers adipocyte differentiation by suppressing growth/differentiation factor-3 (GDF3), a member of the TGFβ superfamily. This leads to a reduction in the number of lipid droplet-containing cells, resulting in limited lipid storage capacity within the adipose tissue. Consequently, this dysregulation promotes lipotoxicity and insulin resistance, and it heightens vulnerability to metabolic diseases [69].
Table 2.
Implications of miR-483-3p in human diseases.
| Type of Disease | Expression Pattern | Tissue | Cell Lines | Targets | Function | Refs |
|---|---|---|---|---|---|---|
| prediabetes/ type 2 diabetes |
up | adipose | 3T3-L1 | GDF3 | Induce lipotoxicity and insulin resistance | [69] |
| diabetic vascular disease | up | vascular endothelium, endothelial progenitor cells, cardiomyocytes |
HAEC, H9C2 |
VEZF1
SRF1 IGF1 |
Induce apoptosis in cardiomyocytes and endothelial cells |
[20,70,71,72] |
| cardiovascular disease, hypertension, aortic valve calcification |
down | serum, heart/aortic valve endothelial cells, cardiomyocytes |
TGF-β
CTGF ACE1 ET1 UBE2C CDK9 |
Inhibit apoptosis, Protects endothelial function against inflammation | [73,74,75] |
Endothelial cell injury or dysfunction is a pivotal initial event in the pathogenesis of vascular complications associated with diabetes. The expression of miR-483-3p is elevated in the aortic endothelial wall of individuals with diabetes compared to control subjects without diabetes, and it is also higher in diabetic mice compared to nondiabetic mice [20] (Table 2). Overexpression of miR-483-3p induces increased apoptosis in both endothelial cells and macrophages, impairing reendothelialization processes. This effect is mediated through the targeting of vascular endothelial zinc finger 1 (VEZF1), an endothelial transcription factor that is diminished in the aorta of diabetic mice [20]. In a murine carotid-injury model, systemic inhibition of miR-483-3p leads to increased VEZF1 expression and rescues the impaired reendothelialization associated with diabetes, highlighting that heightened miR-483-3p levels limit endothelial repair capacity in patients with T2D. Furthermore, in endothelial progenitor cells (EPCs), miR-483-3p targets serum response factor (SRF) resulting in reduced cell migration and tube formation while promoting cell apoptosis [70] (Table 2).
The upregulation of miR-483-3p serum levels has also been observed in newly diagnosed diabetic cats, streptozotocin-induced diabetic mice, or high glucose-cultured cardiomyocytes [71,72] (Table 2). Upregulated miR-483-3p in transgenic mice with diabetes exacerbates cardiomyocyte apoptosis by transcriptionally repressing insulin growth factor 1 (IGF1) [72]. Taken together, the high-glucose-induced upregulation of miR-483-3p induces cell apoptosis and promotes cardiomyocytes and endothelial cell injury, which contributes to the high cardiovascular risk in patients with type 2 diabetes.
However, new studies suggest that miR-483-3p may play a protective effect against endothelial dysfunction in hypertension [73]. Hypertension is a significant risk factor for the development of coronary heart disease and stroke. Endothelial dysfunction and arterial remodeling contribute to increased vascular wall thickness and arterial stiffness. Analyses of patient cohorts have revealed a correlation between serum levels of miR-483-3p and the progression of hypertension [73] (Table 2). The overexpression of miR-483-3p in endothelial cells inhibits Ang II-induced endothelial dysfunction by targeting several molecules, such as transforming growth factor-beta (TGF-β), connective tissue growth factor (CTGF), angiotensin-converting enzyme 1 (ACE1), and endothelin-1 (ET-1) [73] (Table 2). Telmisartan, a drug used to treat hypertension, can also significantly induce the aortic and serum levels of miR-483-3p in hypertension patients and spontaneous hypertension rats.
MiR-483-3p also exerts a protective role against endothelial inflammation and prevents aortic valve (AV) calcification, a major cause of cardiac-related deaths in aging individuals [74]. The expression of miR-483-3p is reduced in AV endothelial cells during the process of porcine AV calcification, and miR-483-3p mimic protects against endothelial inflammation and inhibits AV calcification [74] (Table 2). In contrast, miR-483-3p reduction induces endothelial inflammation and subsequent AV calcification by increasing the expression of its target, ubiquitin E2 ligase-C (UBE2C). Moreover, a very recent report showed a similar downregulation of miR-483-3p in the cardiac tissue of mice with acute myocardial infarction (AMI) [75] (Table 2). Therapeutic hypothermia, a clinical therapy to reduce body temperature and suppress tissue injury from AMI, induces miR-483-3p expression, and elevated miR-483-3p inhibits hypoxia-induced cardiomyocyte apoptosis by directly binding and decreasing cyclin-dependent kinase 9 (CDK9). Together, these data suggest that miR-483-3p exerts a protective effect, and miR-483-3p mimic may serve as a potential therapeutic for cardiovascular disease. The discrepancies regarding the precise function of miR-483-3p in cardiomyocytes and endothelial cells require further investigation.
6. Conclusions and Perspectives
In summary, miR-483-5p and miR-483-3p exhibit distinct and tissue-specific functions in the pathogenesis of human diseases. The available evidence highlights the protective role of miR-483-5p against lipotoxicity and oxidative stress through its regulation of specific targets in various cell types. The reduced expression of miR-483-5p is associated with conditions such as type 2 diabetes, fatty liver, diabetic nephropathy, and neurological injuries. Conversely, elevated levels of miR-483-5p in serum are strongly correlated with obesity and hypertension, underscoring its potential as a valuable biomarker for the prediction and diagnosis of diabetes and CVS.
Conversely, miR-483-3p displays notable upregulation in the context of diabetes and CVS, and its involvement has been demonstrated in inducing cell apoptosis and lipotoxicity across diverse cell types. However, recent investigations have suggested a potential protective role of miR-483-3p against endothelial inflammation, highlighting the intricate nature of its function and emphasizing the need for additional research to fully comprehend its mechanisms.
Considering the expression and functional roles of both miR-483-5p and miR-483-3p in specific reported tissues and cells, further studies are needed to investigate their co-expression patterns and regulatory mechanisms. While they hold promise as biomarkers and potential therapeutic targets, it is crucial to elucidate the underlying mechanisms that contribute to their altered expression and circulation. Further research is necessary to advance our understanding in this regard.
Author Contributions
X.T. wrote the first draft; K.M., A.M., N.M. and E.S. edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The work was supported by National Institutes of Health Grants DK103197 and DK134992 (to X.T.) and King-Chavez-Parks Future Faculty Fellowship (to K.M.).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Handy R.M., Holloway G.P. Insights into the development of insulin resistance: Unraveling the interaction of physical inactivity, lipid metabolism and mitochondrial biology. Front. Physiol. 2023;14:647. doi: 10.3389/fphys.2023.1151389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dinić S., Jovanović J.A., Uskoković A., Mihailović M., Grdović N., Tolić A., Rajić J., Đorđević M., Vidaković M. Oxidative stress-mediated beta cell death and dysfunction as a target for diabetes management. Front. Endocrinol. 2022;13:1006376. doi: 10.3389/fendo.2022.1006376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sinclair A.J., Abdelhafiz A.H. Metabolic Impact of Frailty Changes Diabetes Trajectory. Metabolites. 2023;13:295. doi: 10.3390/metabo13020295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoshida T., Asano Y., Ui-Tei K. Modulation of MicroRNA Processing by Dicer via Its Associated dsRNA Binding Proteins. Non Coding RNA. 2021;7:57. doi: 10.3390/ncrna7030057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.LaPierre M.P., Stoffel M. MicroRNAs as stress regulators in pancreatic beta cells and diabetes. Mol. Metab. 2017;6:1010–1023. doi: 10.1016/j.molmet.2017.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang W. MicroRNAs: Biomarkers, Diagnostics, and Therapeutics. Methods Mol. Biol. 2017;1617:57–67. doi: 10.1007/978-1-4939-7046-9_4. [DOI] [PubMed] [Google Scholar]
- 7.O’Brien J., Hayder H., Zayed Y., Peng C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018;9:402. doi: 10.3389/fendo.2018.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guay C., Regazzi R. Circulating microRNAs as novel biomarkers for diabetes mellitus. Nat. Rev. Endocrinol. 2013;9:513–521. doi: 10.1038/nrendo.2013.86. [DOI] [PubMed] [Google Scholar]
- 9.Guay C., Menoud V., Rome S., Regazzi R. Horizontal transfer of exosomal microRNAs transduce apoptotic signals between pancreatic beta-cells. Cell Commun. Signal. 2015;13:17. doi: 10.1186/s12964-015-0097-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diener C., Keller A., Meese E. Emerging concepts of miRNA therapeutics: From cells to clinic. Trends Genet. 2022;38:613–626. doi: 10.1016/j.tig.2022.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Polychronakos C., Kukuvitis A., Giannoukakis N., Colle E. Parental imprinting effect at the INS-IGF2 diabetes susceptibility locus. Diabetologia. 1995;38:715–719. doi: 10.1007/BF00401845. [DOI] [PubMed] [Google Scholar]
- 12.Tabano S., Colapietro P., Cetin I., Grati F.R., Zanutto S., Mandò C., Antonazzo P., Pileri P., Rossella F., Larizza L. Epigenetic modulation of the IGF2/H19 imprinted domain in human embryonic and extra-embryonic compartments and its possible role in fetal growth restriction. Epigenetics. 2010;5:313–324. doi: 10.4161/epi.5.4.11637. [DOI] [PubMed] [Google Scholar]
- 13.Livingstone C., Borai A. Insulin-like growth factor-II: Its role in metabolic and endocrine dis-ease. Clin. Endocrinol. 2014;80:773–781. doi: 10.1111/cen.12446. [DOI] [PubMed] [Google Scholar]
- 14.Ma N., Wang X., Qiao Y., Li F., Hui Y., Zou C., Jin J., Lv G., Peng Y., Wang L., et al. Coexpression of an intronic microRNA and its host gene reveals a potential role for miR-483-5p as an IGF2 partner. Mol. Cell. Endocrinol. 2011;333:96–101. doi: 10.1016/j.mce.2010.11.027. [DOI] [PubMed] [Google Scholar]
- 15.Le F., Wang L.Y., Wang N., Li L., Li L.J., Zheng Y.M., Lou H.Y., Liu X.Z., Xu X.R., Sheng J.Z. In vitro fertilization alters growth and expression of Igf2/H19 and their epigenetic mechanisms in the liver and skeletal muscle of newborn and elder mice. Biol. Reprod. 2013;88:75. doi: 10.1095/biolreprod.112.106070. [DOI] [PubMed] [Google Scholar]
- 16.Mohan R., Mao Y., Zhang S., Zhang Y.W., Xu C.R., Gradwohl G., Tang X. Differentially Expressed MicroRNA-483 Confers Distinct Functions in Pancreatic beta- and alpha-Cells. J. Biol. Chem. 2015;290:19955–19966. doi: 10.1074/jbc.M115.650705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Emmerling V.V., Fischer S., Stiefel F., Holzmann K., Handrick R., Hesse F., Hörer M., Kochanek S., Otte K. Temperature-sensitive miR-483 is a conserved regulator of recombinant protein and viral vector production in mammalian cells. Biotechnol. Bioeng. 2015;113:830–841. doi: 10.1002/bit.25853. [DOI] [PubMed] [Google Scholar]
- 18.Gallo W., Esguerra JL S., Eliasson L., Melander O. miR-483-5p associates with obesity and insulin resistance and independently asso-ciates with new onset diabetes mellitus and cardiovascular disease. PLoS ONE. 2018;13:e0206974. doi: 10.1371/journal.pone.0206974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veronese A., Visone R., Consiglio J., Acunzo M., Lupini L., Kim T., Ferracin M., Lovat F., Miotto E., Balatti V., et al. Mutated beta-catenin evades a microRNA-dependent regulatory loop. Proc. Natl. Acad. Sci. USA. 2011;108:4840–4845. doi: 10.1073/pnas.1101734108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuschnerus K., Straessler E.T., Müller M.F., Lüscher T.F., Landmesser U., Kränkel N. Increased Expression of miR-483-3p Impairs the Vascular Response to Injury in Type 2 Diabetes. Diabetes. 2018;68:349–360. doi: 10.2337/db18-0084. [DOI] [PubMed] [Google Scholar]
- 21.Alejandro E.U., Gregg B., Blandino-Rosano M., Cras-Méneur C., Bernal-Mizrachi E. Natural history of beta-cell adaptation and failure in type 2 diabetes. Mol. Aspects Med. 2015;42:19–41. doi: 10.1016/j.mam.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Landsman L., Parent A., Hebrok M. Elevated Hedgehog/Gli signaling causes beta-cell dedif-ferentiation in mice. Proc. Natl. Acad. Sci. USA. 2011;108:17010–17015. doi: 10.1073/pnas.1105404108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Talchai C., Xuan S., Lin H.V., Sussel L., Accili D. Pancreatic beta cell dedifferentiation as a mechanism of diabetic beta cell fail-ure. Cell. 2012;150:1223–1234. doi: 10.1016/j.cell.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Z., Mohan R., Chen X., Matson K., Waugh J., Mao Y., Li W., Tang X., Satin L.S. microRNA-483 Protects Pancreatic beta-Cells by Targeting ALDH1A3. Endocrinology. 2021;162:bqab031. doi: 10.1210/endocr/bqab031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim-Muller J.Y., Fan J., Kim YJ R., Lee S.A., Ishida E., Blaner W.S., Accili D. Aldehyde dehydrogenase 1a3 defines a subset of failing pancreatic beta cells in diabetic mice. Nat. Commun. 2016;7:12631. doi: 10.1038/ncomms12631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bardini G., Rotella C.M., Giannini S. Dyslipidemia and Diabetes: Reciprocal Impact of Impaired Lipid Metabolism and Beta-Cell Dysfunction on Micro- and Macrovascular Complications. Rev. Diabet. Stud. 2012;9:82–93. doi: 10.1900/RDS.2012.9.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abifadel M., Varret M., Rabès J.-P., Allard D., Ouguerram K., Devillers M., Cruaud C., Benjannet S., Wickham L., Erlich D., et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat. Genet. 2003;34:154–156. doi: 10.1038/ng1161. [DOI] [PubMed] [Google Scholar]
- 28.Lopez Y.O.N., Retnakaran R., Zinman B., Pratley R.E., Seyhan A.A. Predicting and understanding the response to short-term intensive insulin therapy in people with early type 2 diabetes. Mol. Metab. 2018;20:63–78. doi: 10.1016/j.molmet.2018.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bourbon M., Alves A.C., Sijbrands E.J. Low-density lipoprotein receptor mutational analysis in diagnosis of familial hypercholesterolemia. Curr. Opin. Infect. Dis. 2017;28:120–129. doi: 10.1097/MOL.0000000000000404. [DOI] [PubMed] [Google Scholar]
- 30.Heeren J., Scheja L. Metabolic-associated fatty liver disease and lipoprotein metabolism. Mol. Metab. 2021;50:101238. doi: 10.1016/j.molmet.2021.101238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greenberg A.S., Coleman R.A., Kraemer F.B., McManaman J.L., Obin M.S., Puri V., Yan Q.-W., Miyoshi H., Mashek D.G. The role of lipid droplets in metabolic disease in rodents and humans. J. Clin. Investig. 2011;121:2102–2110. doi: 10.1172/JCI46069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiang Y., Mao L., Zuo M.L., Song G.L., Tan L.M., Yang Z.B. The Role of MicroRNAs in Hyperlipidemia: From Pathogenesis to Therapeutical Ap-plication. Mediators Inflamm. 2022;2022:3101900. doi: 10.1155/2022/3101900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dong J., He M., Li J., Pessentheiner A., Wang C., Zhang J., Sun Y., Wang W.-T., Zhang Y., Liu J., et al. microRNA-483 ameliorates hypercholesterolemia by inhibiting PCSK9 production. J. Clin. Investig. 2020;5:e143812. doi: 10.1172/jci.insight.143812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kwon H.J., Lagace T.A., McNutt M.C., Horton J.D., Deisenhofer J. Molecular basis for LDL receptor recognition by PCSK9. Proc. Natl. Acad. Sci. USA. 2008;105:1820–1825. doi: 10.1073/pnas.0712064105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang P.-Y. PCSK9 as a therapeutic target for cardiovascular disease. Exp. Ther. Med. 2017;13:810–814. doi: 10.3892/etm.2017.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Senn J.J., Klover P.J., Nowak I.A., Zimmers T.A., Koniaris L.G., Furlanetto R.W., Mooney R.A. Suppressor of cytokine signaling-3 (SOCS-3), a potential mediator of interleu-kin-6-dependent insulin resistance in hepatocytes. J. Biol. Chem. 2003;278:13740–13746. doi: 10.1074/jbc.M210689200. [DOI] [PubMed] [Google Scholar]
- 37.Bjørbæk C., Elmquist J.K., Frantz J.D., Shoelson S.E., Flier J.S. Identification of SOCS-3 as a potential mediator of central leptin resistance. Mol. Cell. 1998;1:619–625. doi: 10.1016/S1097-2765(00)80062-3. [DOI] [PubMed] [Google Scholar]
- 38.Li F., Ma N., Zhao R., Wu G., Zhang Y., Qiao Y., Han D., Xu Y., Xiang Y., Yan B. Overexpression of miR-483-5p/3p cooperate to inhibit mouse liver fibrosis by sup-pressing the TGF-beta stimulated HSCs in transgenic mice. J. Cell Mol. Med. 2014;18:966–974. doi: 10.1111/jcmm.12293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Niture S., Gadi S., Qi Q., Gyamfi M.A., Varghese R.S., Rios-Colon L., Chimeh U., Vandana, Ressom H.W., Kumar D. MicroRNA-483-5p Inhibits Hepatocellular Carcinoma Cell Proliferation, Cell Steatosis, and Fibrosis by Targeting PPARα and TIMP2. Cancers. 2023;15:1715. doi: 10.3390/cancers15061715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Y., Nakajima T., Gonzalez F.J., Tanaka N. PPARs as Metabolic Regulators in the Liver: Lessons from Liver-Specific PPAR-Null Mice. Int. J. Mol. Sci. 2020;21:2061. doi: 10.3390/ijms21062061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lacedonia D., Tartaglia N., Scioscia G., Soccio P., Pavone G., Moriondo G., Gallo C., Barbaro M.P.F., Ambrosi A. Different Expression of Micro-RNA in the Subcutaneous and Visceral Adipose Tissue of Obese Subjects. Rejuvenation Res. 2022;25:89–94. doi: 10.1089/rej.2022.0004. [DOI] [PubMed] [Google Scholar]
- 42.Chen K., He H., Xie Y., Zhao L., Zhao S., Wan X., Yang W. miR-125a-3p and miR-483-5p promote adipogenesis via suppressing the RhoA/ROCK1/ERK1/2 pathway in multiple symmetric lipomatosis. Sci. Rep. 2015;5:11909. doi: 10.1038/srep11909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giuliani A., Sabbatinelli J., Amatori S., Graciotti L., Silvestrini A., Matacchione G., Ramini D., Mensà E., Prattichizzo F., Babini L., et al. MiR-422a promotes adipogenesis via MeCP2 downregulation in human bone marrow mesenchymal stem cells. Cell. Mol. Life Sci. 2023;80:75. doi: 10.1007/s00018-023-04719-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu L., Chen H., Yun J., Song L., Ma X., Luo S., Song Y. miRNA-483-5p Targets HDCA4 to Regulate Renal Tubular Damage in Diabetic Nephropathy. Horm. Metab. Res. 2021;53:562–569. doi: 10.1055/a-1480-7519. [DOI] [PubMed] [Google Scholar]
- 45.Liu D., Liu F., Li Z., Pan S., Xie J., Zhao Z., Liu Z., Zhang J., Liu Z. HNRNPA1-mediated exosomal sorting of miR-483-5p out of renal tubular epithelial cells promotes the progression of diabetic nephropathy-induced renal interstitial fibrosis. Cell Death Dis. 2021;12:255. doi: 10.1038/s41419-021-03460-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Han K., Gennarino V.A., Lee Y., Pang K., Hashimoto-Torii K., Choufani S., Raju C.S., Oldham M.C., Weksberg R., Rakic P., et al. Human-specific regulation of MeCP2 levels in fetal brains by microRNA miR-483-5p. Genes Dev. 2013;27:485–490. doi: 10.1101/gad.207456.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li L., Li S., Wu M., Chi C., Hu D., Cui Y., Song J., Lee C., Chen H. Early diagnostic value of circulating microRNAs in patients with suspected acute myo-cardial infarction. J. Cell. Physiol. 2019;234:13649–13658. doi: 10.1002/jcp.28045. [DOI] [PubMed] [Google Scholar]
- 48.Hao Y., Yuan H., Yu H. Retracted article: Downregulation of miR-483-5p decreases hypoxia-induced injury in human cardiomyocytes by targeting MAPK3. Cell. Mol. Biol. Lett. 2020;25:20. doi: 10.1186/s11658-020-00213-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 49.Harling L., Lambert J., Ashrafian H., Darzi A., Gooderham N.J., Athanasiou T. Elevated serum microRNA 483-5p levels may predict patients at risk of post-operative atrial fibrillation. Eur. J. Cardio Thoracic Surg. 2017;51:73–78. doi: 10.1093/ejcts/ezw245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Artasensi A., Mazzolari A., Pedretti A., Vistoli G., Fumagalli L. Obesity and Type 2 Diabetes: Adiposopathy as a Triggering Factor and Thera-peutic Options. Molecules. 2023;28:3094. doi: 10.3390/molecules28073094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ambele M.A., Dhanraj P., Giles R., Pepper M.S. Adipogenesis: A Complex Interplay of Multiple Molecular Determinants and Pathways. Int. J. Mol. Sci. 2020;21:4283. doi: 10.3390/ijms21124283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gross B., Pawlak M., Lefebvre P., Staels B. PPARs in obesity-induced T2DM, dyslipidaemia and NAFLD. Nat. Rev. Endocrinol. 2016;13:36–49. doi: 10.1038/nrendo.2016.135. [DOI] [PubMed] [Google Scholar]
- 53.Zhang J., Huang Y., Shao H., Bi Q., Chen J., Ye Z. Grape seed procyanidin B2 inhibits adipogenesis of 3T3-L1 cells by targeting perox-isome proliferator-activated receptor gamma with miR-483-5p involved mechanism. Biomed. Pharmacother. 2017;86:292–296. doi: 10.1016/j.biopha.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 54.Liu C., Wang J., Wei Y., Zhang W., Geng M., Yuan Y., Chen Y., Sun Y., Chen H., Zhang Y., et al. Fat-Specific Knockout of Mecp2 Upregulates Slpi to Reduce Obesity by Enhancing Browning. Diabetes. 2019;69:35–47. doi: 10.2337/db19-0502. [DOI] [PubMed] [Google Scholar]
- 55.Wang Z.-X., Luo Z.-W., Li F.-X., Cao J., Rao S.-S., Liu Y.-W., Wang Y.-Y., Zhu G.-Q., Gong J.-S., Zou J.-T., et al. Aged bone matrix-derived extracellular vesicles as a messenger for calcification paradox. Nat. Commun. 2022;13:1453. doi: 10.1038/s41467-022-29191-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang H., Zhang H., Sun Q., Wang Y., Yang J., Yang J., Zhang T., Luo S., Wang L., Jiang Y. Intra-articular Delivery of Antago-miR-483-5p Inhibits Osteoarthritis by Modulating Matrilin 3 and Tissue Inhibitor of Metalloproteinase 2. Mol. Ther. 2017;25:715–727. doi: 10.1016/j.ymthe.2016.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 57.Usui I. Common metabolic features of hypertension and type 2 diabetes. Hypertens. Res. 2023;46:1227–1233. doi: 10.1038/s41440-023-01233-x. [DOI] [PubMed] [Google Scholar]
- 58.Samsu N. Diabetic Nephropathy: Challenges in Pathogenesis, Diagnosis, and Treatment. BioMed Res. Int. 2021;2021:1497449. doi: 10.1155/2021/1497449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schanz M., Kimmel M., Alscher M.D., Amann K., Daniel C. TIMP-2 and IGFBP7 in human kidney biopsies in renal disease. Clin. Kidney J. 2023;16:sfad010. doi: 10.1093/ckj/sfad010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sonoda H., Lee B.R., Park K.-H., Nihalani D., Yoon J.-H., Ikeda M., Kwon S.-H. miRNA profiling of urinary exosomes to assess the progression of acute kidney injury. Sci. Rep. 2019;9:4692. doi: 10.1038/s41598-019-40747-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang Q., Zhan H., Liu C., Zhang C., Wei H., Li B., Zhou D., Lu Y., Huang S., Cheng J. Neuroprotective Effect of miR-483-5p Against Cardiac Arrest-Induced Mitochon-drial Dysfunction Mediated Through the TNFSF8/AMPK/JNK Signaling Pathway. Cell. Mol. Neurobiol. 2022;43:2179–2202. doi: 10.1007/s10571-022-01296-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nagaraj S., Want A., Laskowska-Kaszub K., Fesiuk A., Vaz S., Logarinho E., Wojda U. Candidate Alzheimer’s Disease Biomarker miR-483-5p Lowers TAU Phosphoryla-tion by Direct ERK1/2 Repression. Int. J. Mol. Sci. 2021;22:3653. doi: 10.3390/ijms22073653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sattar N., McMurray J., Boren J., Rawshani A., Omerovic E., Berg N., Holminen J., Skoglund K., Eliasson B., Gerstein H.C., et al. Twenty Years of Cardiovascular Complications and Risk Factors in Patients with Type 2 Diabetes: A Nationwide Swedish Cohort Study. Circulation. 2023;147:1872–1886. doi: 10.1161/CIRCULATIONAHA.122.063374. [DOI] [PubMed] [Google Scholar]
- 64.Li R., Jiang L., Wang X. Aberrant expression of miR-483-5p in patients with asymptomatic carotid artery stenosis and its predictive value for cerebrovascular event occurrence. Exp. Ther. Med. 2021;22:1101. doi: 10.3892/etm.2021.10536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li S., Lee C., Song J., Lu C., Liu J., Cui Y., Liang H., Cao C., Zhang F., Chen H. Circulating microRNAs as potential biomarkers for coronary plaque rupture. Oncotarget. 2017;8:48145–48156. doi: 10.18632/oncotarget.18308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gallo W., Ottosson F., Kennbäck C., Jujic A., Esguerra J.L.S., Eliasson L., Melander O. Replication study reveals miR-483-5p as an important target in prevention of car-diometabolic disease. BMC Cardiovasc. Disord. 2021;21:162. doi: 10.1186/s12872-021-01964-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pepe F., Visone R., Veronese A. The Glucose-Regulated MiR-483-3p Influences Key Signaling Pathways in Cancer. Cancers. 2018;10:181. doi: 10.3390/cancers10060181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bork-Jensen J., Thuesen A.C.B., Bang-Bertelsen C.H., Grunnet L.G., Pociot F., Beck-Nielsen H., Ozanne S., Poulsen P., Vaag A. Genetic versus Non-Genetic Regulation of miR-103, miR-143 and miR-483-3p Expression in Adipose Tissue and Their Metabolic Implications-A Twin Study. Genes. 2014;5:508–517. doi: 10.3390/genes5030508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ferland-McCollough D., Twinn D., Cannell I., David H., Warner M., Vaag A., Bork-Jensen J., Brøns C., Gant T., Willis A., et al. Programming of adipose tissue miR-483-3p and GDF-3 expression by maternal diet in type 2 diabetes. Cell Death Differ. 2017;19:1003–1012. doi: 10.1038/cdd.2011.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kong L., Hu N., Du X., Wang W., Chen H., Li W., Wei S., Zhuang H., Li X., Li C. Upregulation of miR-483-3p contributes to endothelial progenitor cells dysfunction in deep vein thrombosis patients via SRF. J. Transl. Med. 2016;14:23. doi: 10.1186/s12967-016-0775-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fleischhacker S.N., Bauersachs S., Wehner A., Hartmann K., Weber K. Differential expression of circulating microRNAs in diabetic and healthy lean cats. Veter J. 2013;197:688–693. doi: 10.1016/j.tvjl.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 72.Qiao Y., Zhao Y., Liu Y., Ma N., Wang C., Zou J., Liu Z., Zhou Z., Han D., He J. miR-483-3p regulates hyperglycaemia-induced cardiomyocyte apoptosis in trans-genic mice. Biochem. Biophys. Res. Commun. 2016;477:541–547. doi: 10.1016/j.bbrc.2016.06.051. [DOI] [PubMed] [Google Scholar]
- 73.Shang F., Guo X., Chen Y., Wang C., Gao J., Wen E., Lai B., Bai L. Endothelial MicroRNA-483-3p Is Hypertension-Protective. Oxidative Med. Cell. Longev. 2022;2022:3698219. doi: 10.1155/2022/3698219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fernandez Esmerats J., Villa-Roel N., Kumar S., Gu L., Salim M.T., Ohh M., Taylor W.R., Nerem R.M., Yoganathan A.P., Jo H. Disturbed Flow Increases UBE2C (Ubiquitin E2 Ligase C) via Loss of miR-483-3p, Inducing Aortic Valve Calcification by the pVHL (von Hippel-Lindau Protein) and HIF-1alpha (Hypoxia-Inducible Factor-1alpha) Pathway in Endothelial Cells. Arterioscler. Thromb. Vasc. Biol. 2019;39:467–481. doi: 10.1161/ATVBAHA.118.312233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xue Q., Zhang Q., Guo Z., Wu L., Chen Y., Chen Z., Yang K., Cao J. Therapeutic Hypothermia Inhibits Hypoxia-Induced Cardiomyocyte Apoptosis Via the MiR-483-3p/Cdk9 Axis. J. Am. Heart Assoc. 2023;12:e026160. doi: 10.1161/JAHA.122.026160. [DOI] [PMC free article] [PubMed] [Google Scholar]