Abstract
Immunometabolism considers the relationship between metabolism and immunity. Typically, researchers focus on either the metabolic pathways within immune cells that affect their function or the impact of immune cells on systemic metabolism. A more holistic approach that considers both these viewpoints is needed. On September 5–8, 2022, experts in the field of immunometabolism met for the Keystone symposium “Immunometabolism at the Crossroads of Obesity and Cancer” to present recent research across the field of immunometabolism, with the setting of obesity and cancer as an ideal example of the complex interplay between metabolism, immunity, and cancer. Speakers highlighted new insights on the metabolic links between tumor cells and immune cells, with a focus on leveraging unique metabolic vulnerabilities of different cell types in the TME as therapeutic targets and demonstrated the effects of diet, the microbiome, and obesity on immune system function and cancer pathogenesis and therapy. Finally, speakers presented new technologies to interrogate the immune system and uncover novel metabolic pathways important for immunity.
Keywords: cancer, immunity, immunometabolism, immunotherapy, metabolism, obesity
Graphical Abstract

Immunometabolism considers the relationship between metabolism and immunity. Typically, researchers focus on either the metabolic pathways within immune cells that affect their function or the impact of immune cells on systemic metabolism. On September 5–8, 2022, experts in the field of immunometabolism met for the Keystone symposium “Immunometabolism at the Crossroads of Obesity and Cancer” to present recent research across the field of immunometabolism, with the setting of obesity and cancer as an ideal example of the complex interplay between metabolism, immunity, and cancer.
Introduction
Immunometabolism has generally been viewed through two lenses: the intrinsic cellular metabolic pathways that drive the immune response and the impact of immune cells on systemic metabolism. Merging these two viewpoints is critical to gaining a holistic view of the interplay between metabolism and immunity. On September 5–8, 2022, experts in the field of immunometabolism met for the Keystone symposium “Immunometabolism at the Crossroads of Obesity and Cancer” to do just that, using obesity and obesity-driven cancer as examples to highlight the links between immune cells, signaling, metabolism, and cancer.
The Centers for Disease Control considers thirteen cancers as linked to being overweight or obese.1 While some of the increased risk of cancer in obesity may be a direct result of increased nutrients—tumor cells require more nutrients to grow and proliferate—there are also more subtle impacts. Metabolic transformation is a hallmark of cancer cells as cells adapt to grow in a cell-autonomous manner and survive the often-harsh conditions of the tumor microenvironment (TME). At the same time, the unique fuels present affect other cells in the TME, including infiltrating T cells. In particular, the accumulation of inhibitory metabolites often leads to an immunosuppressive environment, enabling tumor cells to evade the cytotoxic T cell response.2 As obesity also represents an altered inflammatory state, the interplay between tumor and immune cells in the setting of obesity is similarly altered. One key sign of this is the so-called obesity-immunotherapy paradox, in which obesity is associated with an increased response to immunotherapy.3,4
During the symposium, speakers discussed new insights on the metabolic links between tumor cells and immune cells. In particular, several talks demonstrated how unique metabolic vulnerabilities of different cell types in the TME may pave the way for new therapies, either in targeting tumor cells directly or in activating dysfunctional immune cells to reinvigorate anti-tumor immunity. Another key theme was understanding the effects of diet, the microbiome, and obesity in immune system function and cancer pathogenesis and therapy. Finally, speakers presented new technologies to interrogate the immune system and uncover novel metabolic pathways important for immunity.
Metabolic Regulation of the Anti-Cancer Immune Response
Metabolic regulation of T cell activity in the tumor microenvironment and inflammation
Jeffrey C. Rathmell from Vanderbilt University presented unpublished work on how metabolic programs change during T cell differentiation. T cell activation is accompanied by a metabolic shift from a catabolic or resting state to a proliferative, anabolic state that promotes nutrient uptake and cell metabolism. Rathmell discussed several efforts in his lab to understand the connections between metabolism and immunity. One approach is to look for genes involved in both inborn errors of metabolism and inborn errors of immunity. While previous research shows limited overlap between these two,5,6 Rathmell believes there is an opportunity to use inborn errors as a guide to identify novel ways in which the immune system integrates with metabolism. He also described efforts to understand the connection between obesity, cancer, and the immune system. Rathmell showed data on the effects of obesity on the immune infiltrate in mouse models of cancer and how these differences impact immune cell metabolism and potentially sensitivity to immunotherapy. Finally, Rathmell discussed the impact of heat in the inflammatory microenvironment, which is often overlooked. Febrile temperatures have previously been shown to impact differentiation and pathogenicity of some T cell subsets.7 Rathmell’s group is investigating the impact of fever temperatures on effector T cell phenotypes and metabolic signaling.
Impact of phosphoinositide acyl chain saturation on T cell activation
Erika Pearce from Johns Hopkins University presented work on the role of lipid metabolism in T cell function. The membrane phospholipids phosphatidylinositide phosphate (PIP) are part of the intracellular signaling pathways involved in T cell activation.8 Much of the research on understanding their role has focused on the impact of the phosphate head group while less is known about the role of the composition of the lipid acyl chain. Pearce presented unpublished data demonstrating that the saturation state of the phosphoinositide acyl chain can define distinct pools of PIP2 that are involved in initial and sustained CD8+ T cell signaling.
Metabolic pressures and immunosuppression
Ping-Chih Ho from the University of Lausanne discussed how the immune system impacts the metabolic preference of cancer cells and enables them to evade T cell immunity. During tumor progression, the TME shifts from an immunosupportive state, in which T cells can recognize neoantigens presented by cancer cells and destroy them, to an immunosuppressive state, in which T cells are exhausted and/or dysfunctional and the tumor is essentially invisible to the immune system. This shift is partially due to upregulated of immunosuppressive molecules by the tumor; however, Ho argued that metabolic processes within the TME can also promote immune evasion. Cancer cells with altered metabolism have been shown to drive metabolic stress within the TME and suppress anti-tumor immunity—as tumor cells use up available nutrients and resources, T cells within the immune infiltrate cannot maintain their metabolic fitness and perform their anti-tumor activities.9 Ho presented unpublished data on the pressures that drive metabolic changes early in tumor development and ultimately contribute to immune evasion.
Identifying immune cell-specific metabolic vulnerabilities in the TME
Marcia Haigis from Harvard Medical School presented work on the role of mitochondrial metabolism in T cells in cancer. Haigis’s group has shown that T cell activation induces mitochondrial biogenesis and one-carbon metabolism to support the energy needs of increased proliferation and anabolic metabolism.10,11 Since T cells use many of the same fuels and signaling networks as cancer cells, it is difficult to specifically target metabolism in cancer cells without negatively impacting anti-tumor immunity. Haigis’s group is working to identify different metabolic vulnerabilities in cytotoxic T cells and tumor cells in the TME. They developed a co-culture system of T cells and tumor cells to identify metabolic dependencies within the two cell types.12 More recently, they devised a method to rapidly separate these co-cultured cells to study signaling pathways and metabolism. Haigis showed that tumor cell-derived lactate induced cytotoxic T cells to switch their pyruvate utilization via downregulation of pyruvate dehydrogenase (PDH) and upregulation of pyruvate carboxylase (PC). Inhibiting PDH further upregulated PC activity and enhanced T cell–mediated anti-tumor toxicity.13 Haigis’s group is continuing to understand how this change in metabolism in CD8+ T cells impacts their cytotoxic activity to identify mechanisms that can be targeted to restore T cell–mediated cytotoxicity in the TME.
Metabolic regulation of γδ T cell anti-tumor activity
Murad Mamedov from Alex Marson’s lab at the University of California, San Francisco presented unpublished results from a CRISPR screen to understand metabolic regulation of cancer cell interactions with γδ T cells. γδ T cells represent a small proportion of circulating T cells but have several advantages over the more common ɑβ T cells when it comes to immunotherapy. First, because γδ T cells are not MHC restricted, they have the potential to be used as off-the-shelf T cell therapies. In contrast, current T-cell therapies are produced from a patient’s own T cells, sometimes leading to long manufacturing times. γδ T cells also recognize several ligands important in tumorigenic pathways and have broad anti-tumor activity.14 Mamedov focused on the Vγ9Vδ2 subset of γδ T cells, which require assembly of the butyrophilin (BTN) complex for activation. Expression of BTN proteins is induced by the mevalonate pathway, which is often upregulated in cancer.15 Using a CRISPR screen, Mamedov identified metabolic pathways that regulate Vγ9Vδ2 T cell–mediated cytotoxicity via BTN expression.
Importance of T cells in targeting mIDH1 in cholangiocarcinoma
Meng-Ju Wu from Nabeel Bardeesy’s lab at Massachusetts General Hospital presented work in understanding how mutant IDH1 (mIDH1) promotes immunoevasion and tumor maintenance in cholangiocarcinoma. Normally, IDH1 converts isocitrate to ɑ-ketoglutarate. However, in glioblastoma and intrahepatic cholangiocarcinoma (ICC), mutation of IDH1 alters its activity, leading to accumulation of R-enantiomer of 2-hydroxyglutarate (R-2HG). R-2HG acts as a competitive inhibitor of many enzymes that use ɑ-ketoglutarate as a substrate, leading to epigenetic genes that impact cell differentiation. A mIDH1 inhibitor, ivosidenib, was approved for mIDH1 advanced or metastatic cholangiocarcinoma in 2021. While this agent improves clinical outcomes, patients ultimately relapse.16 Wu developed the first genetically engineered mouse model for mIDH1 ICC to understand the impacts of mIDH1 inhibition and identify strategies to improve the efficacy of mIDH1 inhibitors. He showed that the predominant IDH1 mutant allele in cholangiocarcinoma produces higher levels of R-2HG than the allele commonly found in glioblastoma. The efficacy of ivosidenib in this model was dependent on an intact immune system. Single-cell RNA sequencing (scRNAseq) showed that mIDH1 inhibition increased CD8+ T cell effector function and CD8+ T cell infiltration as well as the tumor-intrinsic IFNγ response. Notably, longer-term, ivosidenib increased immune checkpoint activation and regulatory T (Treg) cell recruitment, which potentially dampened its therapeutic effect. Combining ivosidenib with anti-CTLA-4 antibody (immune checkpoint blockade) led to long-term tumor shrinkage that persisted even after treatment ended (Figure 1). Wu hopes that this work will inform strategies to improve the clinical efficacy of ivosidenib on patients with mutant IDH1 cancers.17,18
Figure 1.
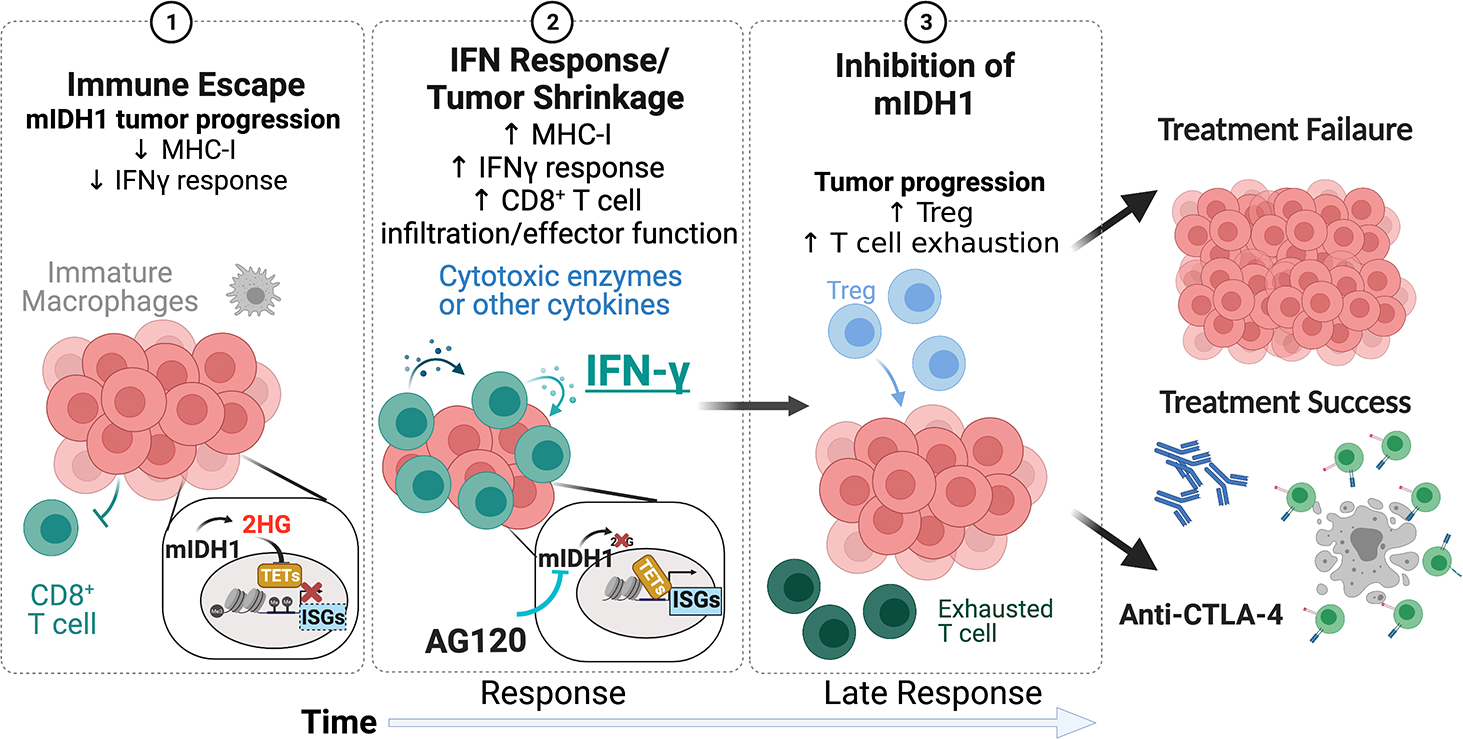
Model of AG120 (ivosidenib).
Metabolic Reprogramming for Improved Immunotherapy
A metabolic code for acetyl-CoA in T cell activation
Susan M. Kaech from The Salk Institute discussed work on understanding the metabolic mechanisms involved in CD8+ T cell exhaustion. CD8+ T cell differentiation is determined by how the cells are activated. Acute stimulation causes naïve cells to differentiate into effector cells and eventually memory T cells. Chronic stimulation, however, promotes differentiation into exhausted T cells, which have lost much of their cytotoxic potential. While this can be a protective mechanism to protect against pathologic inflammation, in settings like the TME or chronic infection, it renders the immune system incapable of controlling pathologic cells. Because different T cell subsets have different epigenetic states19, Kaech focused on the production of acetyl-CoA in T cells, which can be produced by various acetyl-CoA synthetases and is essential for histone acetylation. She presented unpublished data indicating that different acetyl-CoA synthetases may have distinct roles during T cell differentiation, thus impacting epigenetic modifications and, subsequently, gene expression. Ultimately, Kaech hopes that this work will inform strategies to rejuvenate exhausted T cells via metabolic and/or epigenetic reprogramming.
Impact of lipids on immune cell function
Lydia Lynch from Harvard Medical School presented work on linking the metabolic state in obesity to immune function and cancer. A high-fat diet changes the nutrients available to tumor cells and subsequently alters their metabolism.20 This leads to insulin resistance and increased levels of circulating insulin and glucose, which can promote pathways in tumor cells associated with cell migration and survival.21 In mice, both calorie restriction and a ketogenic diet have been shown to reduce insulin and glucose levels, but only calorie restriction impacts tumor growth.22 Lynch showed that lipids, which are abundant in a ketogenic diet, are a common feature of the pro-tumor response in obesity. They accumulate in the tumor interstitial fluid, serving as an important energy source for tumor cells23–25 and are also taken up by immune cells. This causes changes in lipid metabolism that are associated with dysfunction in several immune cells, including NK cells, CD8+ T cells, dendritic cells, and macrophages, limiting their anti-tumor response.26,27 Lynch presented unpublished data looking at the impact of different types of high-fat diets on immune function and tumor growth.
A link between fructose, the gut, and liver disease
Mark Febbraio from the Monash Institute of Pharmaceutical Sciences presented work on understanding the role of the gut in nonalcoholic steatohepatitis (NASH), a disease characterized by accumulation of fat in the liver. While there is no approved treatment for NASH, several agents are under investigation. Febbraio noted that many therapies have failed in clinical trials because they target lipid metabolism in the liver, resulting in high levels of circulating lipids and cholesterol. Febbraio’s group is taking an alternate view of NASH by focusing on excess sugar, specifically fructose. They have shown that fructose is toxic to both the gut and the liver. Working in a mouse model in which a high-fat diet is known to induce NASH and HCC28–30, Febbraio’s group showed that excess fructose can induce similar effects in the liver while also disrupting gut barrier integrity and the microbiome. Targeting the microbiome in this model reduced the incidence of fructose-mediated NASH, suggesting a link between the gut and the liver.31,32 Febbraio’s group is investigating ways to protect the gut against fructose-mediated gut dysbiosis. Previous work has shown that IL-6 signaling can restore epithelial barrier function via YAP activity.33 In Febbraio’s mouse model, upregulating IL-6 signaling in intestinal epithelial cells restored the epithelial barrier and decreased fructose-derived hepatic steatosis and HCC.32 His group is now working to target IL-6 activity pharmacologically. They have designed a IL-6-like cytokine, IC7Fc, that promotes IL-6 signaling without its inflammatory effects.34 He presented unpublished preclinical data on the impact of IC7Fc on the gut and the development of fructose-derived NASH and HCC. Febbraio is also involved in a multi-center, multi-omics project to identify new biomarkers and drug targets in NASH and HCC.
The impact of obesity in inflammatory diseases
Sagar Bapat from the University of California, San Francisco presented work on the impact of obesity on the immunological response to inflammatory disease, specifically atopic dermatitis (AD). Individuals with obesity and AD have more severe disease and are more likely to be intractable to treatment than nonobese individuals. Bapat showed that in a mouse model of AD, obese mice similarly had more severe disease, characterized by an expanded dermis and epidermis as well as increased infiltration of leukocytes. Further characterization of the immune response showed that lean mice primarily mount a Th2-mediated response while obese mice mount a Th17-predominant response. This difference had important implications for treatment response. Conventional biologic therapies that target Th2 cytokines worsened disease in obese mice. Using an integrated multi-omics approach, Bapat found that dysregulation of PPARγ in T cells in obese mice likely causes an imbalance in Th2/Th17-mediated inflammation. Treating obese mice with the PPARγ agonist rosiglitazone restored the therapeutic efficacy of Th2-targeted therapies in obese animals.35 These studies reveal how obesity might alter immune responses in inflammatory disease and suggest a precision medicine approach to target these alterations in therapeutically meaningful ways.
The role of metabolism in apoptotic resistance in Th17 cells
Hanna Hong from Costas Lyssiotis’s lab at the University of Michigan presented unpublished work on the role of metabolism in apoptotic resistance in Th17 cells. Th17 cells mediate chronic inflammation via production of pro-inflammatory cytokines. They also may play a key role in immune homeostasis at barrier sites, such as the intestinal epithelium.36 Previous work in Lyssiotis’s lab showed that Th17 cells primarily use oxidative phosphorylation (OXPHOS) as an energy source in vitro while they use glycolysis in vivo.a Metabolically distinct Th17 cells are also found in vivo. In the intestine, homeostatic cells are primarily oxidative while inflammatory cells use OXPHOS and glycolysis.37 Hong presented unpublished data comparing these two metabolic populations of Th17 cells, particularly their sensitivity to apoptosis.
Targeting metabolically distinct macrophage subsets to improve response to immunotherapy
Weiping Zou from the University of Michigan presented work leveraging metabolic patterns in tumor-infiltrating immune cells to improve responses to immunotherapy. Nearly 20 years ago, Zou’s group demonstrate that PD-L1 expression in the TME led to an immunosuppressive milieu and that blockade of the PD-L1/PD-1 pathway could normalize T cell function and restore antitumor immunity.38 In the TME, myeloid cells are the major PD-L1-expressing immune cells and immune targets of anti-PD(L)1 agents.38–40 Zou showed that tumor cells themselves are at least partially responsible for the development of myeloid-derived suppressor cells (MDSC). Aerobic glycolysis in tumor cells promotes G-CSF and GM-CSF expression and secretion into the TME, which control myeloid cell development.41 Zou also presented unpublished data focused on tumor-associated macrophages (TAM). He showed that different TAM subsets within the TME display different metabolic profiles. This heterogeneity within TAMs may provide a way to specifically target immunosuppressive populations and shift them toward a more immunoreactive phenotype.
Microbial Metabolites, Nutrients, and Immunity
Impact of diet on immune responses to commensal bacteria
Yasmine Belkaid from the NIAID presented work on the link between microbiota, nutrition, and immunity. The immune system is commonly regarded through the lens of infection and its interactions with pathogens; however, as Belkaid pointed out, most interactions between immune cells and non-host cells are with the commensal microbiota. Belkaid’s group has shown that, in mice, T cells that recognize skin microbiota accumulate in the tissue and protect it against infection. These microbiota-specific T cells are highly plastic, expressing genes that allow them to participate in the barrier function while also maintaining the ability to shift to a repair program in the context of injury.42–46 Additional work on the mechanism of this T cell response revealed that Staphylococcus epidermidis promotes the transcription of endogenous retroviruses (ERV) in keratinocytes. Reverse transcription of ERVs to DNA triggers an immune response via the cGAS/STING pathway. In lean mice, this T cell response is homeostatic; however, in mice fed a high-fat diet, the T cell response to S. epidermidis shifts to an inflammatory response.47 Belkaid presented unpublished data exploring whether diet can impact ERV expression in humans and how this may affect inflammation and immunity. She also presented work on understanding how the nervous system may be impacted by homeostatic immunity to the microbiota as well.
Diet-mediated intestinal remodeling via immune signaling
Zuri Sullivan from Catherine Dulac’s lab at Harvard University presented work performed while she was in a PhD student in Ruslan Medzhitov’s lab at Yale on understanding how the intestine adapts to and is regulated by diet. Sullivan showed that the nutrient-handling machinery in the intestine can be rapidly induced on demand based on nutrient availability. In mice fed a high-carbohydrate diet, intestinal epithelial cells upregulate genes involved in carbohydrate handling while in those fed a high-protein diet, intestinal epithelial cells upregulate genes involved in protein handling. Sullivan showed that this transcriptional response is due to remodeling of the intestinal epithelium. Since lymphocytes have been shown to regulate tissue adaptation to intestinal pathogens, Sullivan explored the possibility that T cells could regulate diet-induced epithelial remodeling as well. She found that γδ T cells were responsible for regulating the carbohydrate-handling transcriptional program in intestinal epithelial cells. She put forth a model in which IL-22 negatively regulates carbohydrate-handling genes. Under conditions of high carbohydrates, γδ T cells limit IL-22 expression, thus promoting transcription of carbohydrate-handling genes.48
The role of gasdermin C in the intestine
Andrea Keller from Maria Mihavlova’s lab at The Ohio State University presented unpublished data on the effect of nutrient status and the immune environment on intestinal gasdermin C. Gasdermin proteins induce pyroptosis in response to infection, creating pores in the cell membrane and releasing pro-inflammatory cytokines.49–53 Much of the research on gasdermin proteins has focused on gasdermin D. Keller’s work focuses on what cues in the intestinal environment trigger gasdermin C expression and whether gasdermin C activates pyroptotic pathways in a similar mechanism as gasdermin D.
Understanding metabolism in tissue-resident macrophages
Stefanie Wculek from David Sancho’s group at CNIC Spain presented work on the metabolic requirements of tissue-resident macrophages in homeostasis. Activated macrophages can adopt either a pro-inflammatory M1 phenotype, which is metabolically characterized by increased glycolysis and a blocked TCA cycle, or an anti-inflammatory M2 phenotype, which is characterized by an increase in OXPHOS and an intact TCA cycle.54 However, less is known about the metabolic requirements of tissue-resident, homeostatic macrophages. Wculek showed that tissue-resident macrophages have diverse metabolic phenotypes, dependent on their tissue of origin. She also showed that it may be possible to selectively target metabolic vulnerabilities in pro-inflammatory macrophages found in adipose tissue in the setting of obesity.
A potential link between sleep and immunity
Douglas Green from St. Jude Children’s Research Hospital presented unpublished work on the relationship between sleep and immunity. Green’s group is working to understand why infection induces sleep and whether sleep during illness is beneficial to the immune response and recovery.
Interactions between diet and systemic and tissue immunometabolism
OGT as a nutrient sensor in the liver
Catherine Postic from INSERM Institut Cochin presented work on the role of O-linked N-acetylglucosaminyltransferase (OGT) on nutrient sensing in the liver. Postic’s group is particularly interested in the pathophysiology of non-alcoholic fatty liver disease (NAFLD) in which hyperglycemia triggers de novo fatty acid synthesis in the liver, ultimately leading to inflammation, steatosis, fibrosis, and cirrhosis. Her lab has shown that carbohydrate response element binding protein (ChREBP) is a key mediator of steatosis via lipogenesis but that it can also protect against steatosis by buffering lipotoxic fatty acids.55–58 To better understand the role of ChREBP in the liver, Postic’s group has looked at how post-translational modifications impact its function. ChREBP can be modified by OGT via O-GlyNAcylation. The OGT signaling pathway has emerged as a major regulator of energy homeostasis under both physiological and pathological conditions.59 In the liver, O-GlyNAcylation by OGT increases ChREBP levels in the liver and upregulates the expression of ChREBP target genes.60 Postic showed unpublished data on the impact of OGT expression in nutrient sensing and fatty liver disease.
Novel single-cell technologies to understand immunity
Ido Amit from the Weizmann Institute presented work on using single-cell approaches to understand immunity and its role in disease. His group incorporates data from single-cell transcriptomics, proteomics, and cell signaling data with patient-specific data to build predictive models of disease and identify novel immune targets and pathways. For example, in multiple myeloma cells, single-cell approaches were used to identify immune-related signatures of treatment resistance, which revealed new targets that may help sensitize patients to treatment.61 In another study, single-cell analyses of skin and blood samples helped to identify new disease-related cell subsets in patients with scleroderma.62 Amit described two novel single-cell technologies developed in his lab—physically interacting cells sequencing (PIC-seq)63 and intracellular staining and sequencing (INs-seq).64 PIC-seq combines cell sorting of physically interacting cells with scRNAseq to provide single-cell resolution of immune interactions in situ. Using this technology, Amit’s group identified a CD4+ helper T cell population (Tht) that interacts with dendritic cells in large immune aggregates within the TME and shares many of the features of dysfunctional CD8+ T cells. Amit showed that PD-l blockade can promote Tht cell-mediated tumor killing, highlighting the importance of T cell–dendritic cell interactions in the response to immunotherapy.65 The second technology, INs-seq, couples scRNAseq and intracellular protein activity. Amit’s group has used this to understand immunometabolism in tumors by characterizing MDSCs, which are often dysfunctional in the TME. MDSCs are difficult to characterize via cell surface markers but have clear metabolic dysfunction. Using INs-seq, Amit’s group has identified two new subsets of tumor-infiltrating MDSCs that express both TREM2 and arginase-1. These myeloid regulatory cells localized to necrotic/hypoxic areas of the tumor. Depletion of these cells by targeting TREM2 promoted immune activation and tumor ablation. Amit proposed that the TREM2+ myeloid regulatory cells are important for tumor-immune escape.64
Impact of lipid metabolism on metastasis
Salvador Aznar Benitah from ICREA presented work on the role of lipid metabolism in metastasis. Benitah’s group has shown that a population of cells that express the fatty acid transporter CD36+ is responsible for initiating metastasis in several tumor types. These cells show a high preference for fatty acids as a fuel—several components involved in fatty acid uptake, synthesis, storage, and oxidation are upregulated.25,66 As a result of these findings, Benitah has co-founded a company, ONA therapeutics, to explore the potential of CD36+ cells as an anti-metastatic therapeutic target. Recently, in a collaborative work headed by Michaela Frye from the DKFZ in Heidelberg, the Frye and Benitah groups showed that high expression of the mitochondrial tRNA methyltransferase NSUN3 increases the efficiency of OXPHOS in CD36+ cells. Inhibiting NSUN3 shifted the metabolic preference from OXPHOS toward glycolysis resulting in the ablation of CD36+ metastatic potential.67
Benitah’s group has also investigated whether specific fatty acids are more pro-metastatic than others. Both ex vivo and in vivo administration of various fatty acids demonstrated that palmitic acid could enhance the metastatic potential of metastatic-initiating cells in oral tumors and melanoma. Other fatty acids, such as linoleic or oleic acid, had no impact on metastasis. The impact of palmitate on metastasis persisted even after it was removed from the culture media or when primary tumors were transferred into another animal, indicating a role for epigenetics. Benitah’s group found, indeed, palmitate induces several persistent epigenetic markers in CD36+ cells, mainly trimethylation of histone H3 at lysine 4, particularly at the promoters of genes associated with neurogenesis and gliogenesis. Such a transcriptional signature has been shown to stimulate intratumoral Schwann cells and innervation. Benitah showed that activation of Schwann cells by CD36+ cells facilitates metastasis via aberrant perineuronal nets.68
Metabolic flexibility during T cell activation
Russell Jones from the Van Andel Research Institute discussed research on using metabolomics to understand how different nutrients impact T cell function. Jones’s group uses stable isotope tracing and rapid cell sorting to study physiologic immune cell metabolism at various time points during infection.69,70 He showed that in mice, CD8+ T cells show evidence of glucose partitioning during infection. While glucose is heavily used as a fuel in the early steps of glycolysis, which serves to generate intermediates for nucleotide and serine production, it is not generally incorporated into the TCA cycle. This differs from what is seen during T cell activation in vitro. They also found that CD8+ T cells prefer different fuels over the course of infection. During the priming stages of T cell activation, cells prefer glucose to fuel glycolysis and glutamine to fuel the TCA cycle. However, during the peak of the T cell response, acetate becomes an important fuel for the TCA cycle.71 Jones’s group developed a physiologic cell culture medium to better understand the impact of nutrient availability on T cell metabolism. The medium includes physiologic carbon sources that are not typically found in cell culture media like acetate, citrate, lactate, and pyruvate. In the absence of these carbon sources, glucose was a major contributor to the TCA cycle. However, when these carbon sources were included, it was not, consistent with the previous in vivo results. The inclusion of physiologic carbon sources also augmented T cell cytokine production.72 Finally, Jones showed unpublished data on the use of ketone bodies as fuel by CD8+ T cells. He stressed that metabolic flexibility is an important feature of highly functional effector T cells as it allows them to adapt to their environment and use the substrates available.
Metabolic adaptations in tissue-resident T cells
Miguel Reina-Campos from Ananda Goldrath’s lab at the University of California, San Diego presented unpublished work on the metabolic adaptations of tissue-resident memory CD8+ T cells (TRM). Like Jones, Reina-Campos stressed that the metabolic flexibility of CD8+ T cells enables them to adapt to diverse tissue environments. TRM cells in particular are long-lived cells that mount a rapid, frontline defense against reinfection. Because of their ubiquitous presence in various organs long term, Trm cells have adapted different metabolic changes based on their tissue of residence73. Importantly, tumor-infiltrating lymphocytes with TRM features have superior antitumor properties. Reina-Campos’s work is focused on leveraging these metabolic adaptations to improve the function of CD8+ T cells for vaccination and immunotherapeutic strategies (Figure 2).
Figure 2.
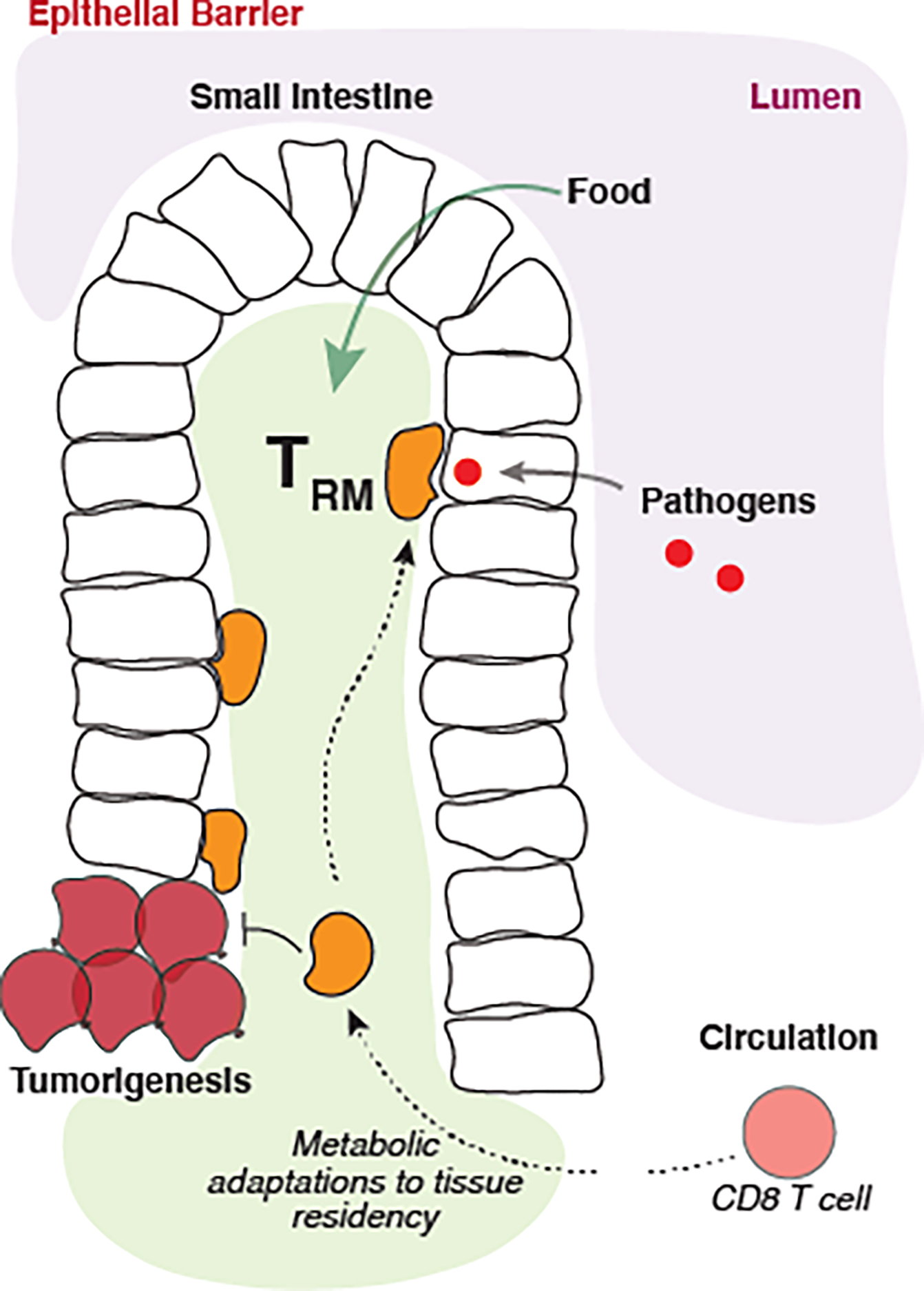
Metabolic adaptations of tissue-resident memory CD8+ T cells.
Regulatory T cells in visceral adipose tissue
Santiago Valle Torres from Axel Kallies’s lab at the Peter Doherty Institute presented work on regulatory T cell populations in visceral adipose tissue (VAT). The VAT is an endocrine organ that regulates functions like appetite, glucose metabolism, insulin secretion, and lipid metabolism.74 Kallies’s lab previously demonstrated sex-specific differences in VAT regulatory T cells in mice. In male mice, the VAT is more inflammatory than in female mice, which influences the phenotype of regulatory T cells. For example, VAT regulatory T cells do not express the canonical markers of VAT regulatory T cells.75 Valle Torres has further characterized the sex differences in VAT regulatory T cells, describing different cell subsets and elucidating their roles in metabolism and inflammation.
Frontiers in Immunometabolism
Mechanisms of fat-induced tumorigenesis in the intestine
Semir Beyaz from Cold Spring Harbor Laboratory presented work on how fatty acids influence cell fate. Beyaz’s group is broadly interested in how nutrients impact cell fate and function at the cell, tissue, and organism level. They have developed several conceptual and experimental tools to explore these connections that have narrowed down interactions between epithelial stem cells, immune cells, and microbes that are responsive to dietary fat. Work in a mouse model of obesity revealed both cell-intrinsic pathways and microbiome-related mechanisms that link a high-fat diet to intestinal cancer. A high-fat diet activated the lipid-sensing transcription factor PPAR in intestinal stem cells, thus increasing fatty acid oxidation and enhancing stemness and tumorigenicity of tumor-initiating cells. High-fat diet–related perturbations to the microbiome also induced changes in immune cells that increased cancer risk.23,76,77 Beyaz presented unpublished work focused on the impact of dietary fatty acids on stem cell plasticity in the intestinal epithelium and their potential impact on tissue repair and tumorigenesis.
Weight loss as an intervention for endometrial cancer
Donal Brennan from University College Dublin presented a clinical perspective on the potential of weight loss as an intervention in endometrial cancer. Endometrial cancer has a strong association with obesity, and some data indicate that weight loss may reduce this risk. For example, in a recent meta-analysis, several recent retrospective cohort studies consistently demonstrate a reduction in cancer incidence among those who undergo metabolic surgery.78,79 There are several potential ways by which weight loss may positively impact outcomes in endometrial cancer. First, epidemiologic data support a protective role for weight loss, suggesting a biological role. Also, many patients present in early stages and can be cured via surgery (usually a hysterectomy). Weight loss can reduce the risk of surgical complications and improve overall metabolic health. Finally, many younger patients with endometrial cancer want to avoid surgery and preserve fertility. Weight loss may offer an adjunctive approach to medical therapies. Brennan described a randomized clinical trial in women with obesity and endometrial cancer who were treated with either progestin, progestin and metformin, or progestin and weight loss. Interestingly, all three groups lost weight, and rates of pathological complete response across the study were encouraging. While the study was not designed to determine the efficacy of weight loss, it did demonstrate the safety and feasibility of delaying surgery to study weight loss interventions in this patient population.80 In a second study in which patients underwent metabolic surgery, weight loss was associated with tumor regression and a more immune active TME.26 As many patients are hesitant to undergo metabolic surgery, Brennan’s group is investigating the potential of weight management via GLP-1 which is known to induce weight loss in humans81, as an adjunct to other systemic treatments.
The impact of Kreb’s cycle intermediates on inflammation
Luke O’Neill from Trinity Biomedical Sciences Institute presented work on the role of fumarate and other Kreb’s cycle intermediates in autoimmunity and inflammation. O’Neill noted that there are common links between many autoimmune diseases and it may be possible to find a target or pathway that provides benefit across multiple conditions.82 One potential common target is the inflammasome. O’Neill’s group has shown that the small molecule inflammasome inhibitor, CRID3, impacts several preclinical models of inflammation.83 Another common feature of inflammation and autoimmunity is mitochondrial dysfunction. O’Neill’s group has been linking the function of Kreb’s cycle intermediates to cytokine production. They have found that, broadly, succinate is pro-inflammatory, itaconate is anti-inflammatory, and fumarate is immunomodulatory.84,85 He focused primarily on the impact of fumarate. Mutations in fumarate hydratase (FH), the enzyme that catalyzes the conversion of fumarate to malate, are associated with several types of cancer.86 FH deficiency causes an accumulation of fumarate, which can modify and inhibit histone demethylases, ultimately driving HIF-1ɑ expression.87 O’Neill presented data on the role of fumarate in regulating cytokine and interferon production in macrophages.
Antitumor-immunity in obesity-dependent and -independent cancers
Rachel Perry from Yale University presented work on understanding the link between obesity, cancer, and immunity. She noted that many highly immunogenic cancers, such as NSCLC and melanoma are typically not associated with obesity. Perry’s group and others have shown that overweight may be a positive prognostic factor in these immunologically hot cancers and correlates with response to immunotherapy, but not to other treatment modalities.88–90 Perry focused on two metabolic changes in obesity as potentially contributing to these effects–the increase in endogenous glucose production and increased fatty acid oxidation. In tumor cell lines, physiologic variations in glucose or insulin did not impact glucose metabolism in cancers not associated with obesity but did increase glucose metabolism in obesity-related cancers. Insulin also increased cell division in a dose-dependent manner in these obesity-dependent cancers. Perry showed that obesity-associated tumors respond to insulin by increasing mitochondrial glucose oxidation and increasing cell division; however, no impact was seen in obesity-independent cancers. Blocking glucose oxidation reduced cell division in obesity-dependent cell lines but not obesity-independent cells. Similar effects were seen in mice.91 Perry also showed that increased glucose oxidation is a hallmark of T cell activation. She purported that in obesity, increased glucose production contributes to increased glucose metabolism in immune cells and increased T cell activation. Perry also proposed that the higher fatty acid oxidation that occurs in obesity may be associated with reduced T cell exhaustion, and thus a more robust antitumor immune response. Finally, Perry is investigating the potential to treat obesity-driven cancers with the SGLT2 inhibitor dapagliflozin. Dapagliflozin blocks glucose uptake in the renal tubules, allowing it to be excreted in the urine. She showed that in a mouse model of triple-negative breast cancer (TNBC), dapagliflozin enhanced the efficacy of chemotherapy specifically in tumors driven by mutations upstream of insulin signaling.92 They are designing a clinical trial in obese women with TNBC to see if similar effects are seen in humans.
Frontiers in Immunometabolism: Treg cell metabolism
Dirk Brenner from the Luxembourg Institute of Health presented work on reactive oxygen species (ROS) in Treg metabolism. T cell activation promotes the production of ROS, which must be tightly regulated to enable expression of proteins involved in the metabolic reprogramming from OXPHOS to glycolysis. Brenner showed that glutathione (GSH) plays a key role in buffering ROS levels in effector T cells.93 More recent work has shown that the concentration of GSH in Treg cells is much higher than in effector T cells, suggesting that GSH may play an important role in Treg cells. In mice, knocking out glutamate cysteine ligase (Gclc), an enzyme involved in GSH production, in Treg cells abrogated GSH production and led to the accumulation of ROS. While this did not impact Treg cell differentiation, it did result in the accumulation of effector T cells and a decrease in the number of Tregs, which is a hallmark of autoimmunity. The mice eventually developed lethal auto-inflammatory disease. Brenner showed that Gclc is critical for the immunosuppressive activity of Treg cells. Gclc-deficient Treg cells showed downregulation of the transcription factor Foxp3 and upregulation of serine metabolism and mTOR activation. Brenner put forth a model in which accumulation of ROS in stressed Treg cells drives an increase in serine levels via both upregulation of a serine importer and increased serine synthesis. Serine accumulation in turn activates mTOR, which inhibits Foxp3 and negatively impacts Treg cell function. Restricting serine in vitro and in vivo rescued the effect of Gclc deficiency on Treg cell function and spontaneous autoimmunity in mice. This work reveals a role for GSH in modulating Treg cell function by restricting serine metabolism.94
Acknowledgments
Sagar P. Bapat was supported by; U.S. National Institutes of Health (NIH) grants F30 DK096828, T32 GM007198, R38 HL143581 and K38 HL154202.
Footnotes
Competing Interests
Jeffrey Rathmell is a founder, scientific advisory board member, and stockholder of Sitryx Therapeutics, a scientific advisory board member and stockholder of Caribou Biosciences, a member of the scientific advisory board of Nirogy Therapeutics, has consulted for Merck, Pfizer, and Mitobridge within the past three years, and has received research support from Incyte Corp., Calithera Biosciences, and Tempest Therapeutics.
Franchi L. et al., J Immunol 2017.
References
- 1.Obesity and Cancer | CDC. 2022.July 13, 2022 Accessed November 16, 2022. https://www.cdc.gov/cancer/obesity/index.htm. [Google Scholar]
- 2.Elia I & Haigis MC. 2021. Metabolites and the tumour microenvironment: from cellular mechanisms to systemic metabolism. Nat. Metab. 3: 21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Z, Aguilar EG, Luna JI, et al. 2019. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat. Med. 25: 141–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dudzinski SO, Bader JE, Beckermann KE, et al. 2021. Leptin Augments Antitumor Immunity in Obesity by Repolarizing Tumor-Associated Macrophages. J. Immunol. Baltim. Md 1950 207: 3122–3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tangye SG, Al-Herz W, Bousfiha A, et al. 2020. Human Inborn Errors of Immunity: 2019 Update on the Classification from the International Union of Immunological Societies Expert Committee. J. Clin. Immunol. 40: 24–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferreira CR, van Karnebeek CDM, Vockley J, et al. 2019. A proposed nosology of inborn errors of metabolism. Genet. Med. Off. J. Am. Coll. Med. Genet. 21: 102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang X, Ni L, Wan S, et al. 2020. Febrile Temperature Critically Controls the Differentiation and Pathogenicity of T Helper 17 Cells. Immunity 52: 328–341.e5. [DOI] [PubMed] [Google Scholar]
- 8.Shyer JA, Flavell RA & Bailis W. 2020. Metabolic signaling in T cells. Cell Res. 30: 649–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang C-H, Qiu J, O’Sullivan D, et al. 2015. Metabolic Competition in the Tumor Microenvironment Is a Driver of Cancer Progression. Cell 162: 1229–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ron-Harel N, Santos D, Ghergurovich JM, et al. 2016. Mitochondrial biogenesis and proteome remodeling promote one-carbon metabolism for T cell activation. Cell Metab. 24: 104–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ron-Harel N, Notarangelo G, Ghergurovich JM, et al. 2018. Defective respiration and one-carbon metabolism contribute to impaired naïve T cell activation in aged mice. Proc. Natl. Acad. Sci. U. S. A. 115: 13347–13352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drijvers JM, Gillis JE, Muijlwijk T, et al. 2021. Pharmacologic Screening Identifies Metabolic Vulnerabilities of CD8+ T Cells. Cancer Immunol. Res. 9: 184–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elia I, Rowe JH, Johnson S, et al. 2022. Tumor cells dictate anti-tumor immune responses by altering pyruvate utilization and succinate signaling in CD8+ T cells. Cell Metab. 34: 1137–1150.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saura-Esteller J, de Jong M, King LA, et al. 2022. Gamma Delta T-Cell Based Cancer Immunotherapy: Past-Present-Future. Front. Immunol. 13: 915837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rigau M, Ostrouska S, Fulford TS, et al. 2020. Butyrophilin 2A1 is essential for phosphoantigen reactivity by γδ T cells. Science 367: eaay5516. [DOI] [PubMed] [Google Scholar]
- 16.Tian W, Zhang W, Wang Y, et al. 2022. Recent advances of IDH1 mutant inhibitor in cancer therapy. Front. Pharmacol. 13: 982424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu M-J, Shi L, Merritt J, et al. 2022. Biology of IDH mutant cholangiocarcinoma. Hepatol. 75: 1322–1337. [DOI] [PubMed] [Google Scholar]
- 18.Wu M-J, Shi L, Dubrot J, et al. 2022. Mutant IDH Inhibits IFNγ-TET2 Signaling to Promote Immunoevasion and Tumor Maintenance in Cholangiocarcinoma. Cancer Discov. 12: 812–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Y, Zander R, Khatun A, et al. 2018. Transcriptional and Epigenetic Regulation of Effector and Memory CD8 T Cell Differentiation. Front. Immunol. 9: 2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lien EC & Vander Heiden MG. 2019. A framework for examining how diet impacts tumour metabolism. Nat. Rev. Cancer 19: 651–661. [DOI] [PubMed] [Google Scholar]
- 21.Hopkins BD, Goncalves MD & Cantley LC. 2016. Obesity and Cancer Mechanisms: Cancer Metabolism. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol 34: 4277–4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lien EC, Westermark AM, Zhang Y, et al. 2021. Low glycaemic diets alter lipid metabolism to influence tumour growth. Nature 599: 302–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beyaz S, Mana MD, Roper J, et al. 2016. High-fat diet enhances stemness and tumorigenicity of intestinal progenitors. Nature 531: 53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Z & Kang Y. 2017. Lipid Metabolism Fuels Cancer’s Spread. Cell Metab. 25: 228–230. [DOI] [PubMed] [Google Scholar]
- 25.Pascual G, Avgustinova A, Mejetta S, et al. 2017. Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature 541: 41–45. [DOI] [PubMed] [Google Scholar]
- 26.Dyck L, Prendeville H, Raverdeau M, et al. 2022. Suppressive effects of the obese tumor microenvironment on CD8 T cell infiltration and effector function. J. Exp. Med. 219: e20210042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michelet X, Dyck L, Hogan A, et al. 2018. Metabolic reprogramming of natural killer cells in obesity limits antitumor responses. Nat. Immunol. 19: 1330–1340. [DOI] [PubMed] [Google Scholar]
- 28.Nakagawa H, Umemura A, Taniguchi K, et al. 2014. ER stress cooperates with hypernutrition to trigger TNF-dependent spontaneous HCC development. Cancer Cell 26: 331–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shalapour S, Lin X-J, Bastian IN, et al. 2017. Inflammation-induced IgA+ cells dismantle anti-liver cancer immunity. Nature 551: 340–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Febbraio MA, Reibe S, Shalapour S, et al. 2019. Preclinical Models for Studying NASH-Driven HCC: How Useful Are They? Cell Metab. 29: 18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Febbraio MA & Karin M. 2021. “Sweet death”: Fructose as a metabolic toxin that targets the gut-liver axis. Cell Metab. 33: 2316–2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Todoric J, Di Caro G, Reibe S, et al. 2020. Fructose stimulated de novo lipogenesis is promoted by inflammation. Nat. Metab. 2: 1034–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taniguchi K, Wu L-W, Grivennikov SI, et al. 2015. A gp130-Src-YAP module links inflammation to epithelial regeneration. Nature 519: 57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Findeisen M, Allen TL, Henstridge DC, et al. 2019. Treatment of type 2 diabetes with the designer cytokine IC7Fc. Nature 574: 63–68. [DOI] [PubMed] [Google Scholar]
- 35.Bapat SP, Whitty C, Mowery CT, et al. 2022. Obesity alters pathology and treatment response in inflammatory disease. Nature 604: 337–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ho AW & Kupper TS. 2019. T cells and the skin: from protective immunity to inflammatory skin disorders. Nat. Rev. Immunol. 19: 490–502. [DOI] [PubMed] [Google Scholar]
- 37.Franchi L, Monteleone I, Hao L-Y, et al. 2017. Inhibiting Oxidative Phosphorylation In Vivo Restrains Th17 Effector Responses and Ameliorates Murine Colitis. J. Immunol. Baltim. Md 1950 198: 2735–2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Curiel TJ, Wei S, Dong H, et al. 2003. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat. Med. 9: 562–567. [DOI] [PubMed] [Google Scholar]
- 39.Lin H, Wei S, Hurt EM, et al. 2018. Host expression of PD-L1 determines efficacy of PD-L1 pathway blockade-mediated tumor regression. J. Clin. Invest. 128: 1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang H, Liang Y, Anders RA, et al. 2018. PD-L1 on host cells is essential for PD-L1 blockade-mediated tumor regression. J. Clin. Invest. 128: 580–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li W, Tanikawa T, Kryczek I, et al. 2018. Aerobic Glycolysis Controls Myeloid-Derived Suppressor Cells and Tumor Immunity via a Specific CEBPB Isoform in Triple-Negative Breast Cancer. Cell Metab. 28: 87–103.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Naik S, Bouladoux N, Wilhelm C, et al. 2012. Compartmentalized control of skin immunity by resident commensals. Science 337: 1115–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Naik S, Bouladoux N, Linehan JL, et al. 2015. Commensal-dendritic-cell interaction specifies a unique protective skin immune signature. Nature 520: 104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Linehan JL, Harrison OJ, Han S-J, et al. 2018. Non-classical Immunity Controls Microbiota Impact on Skin Immunity and Tissue Repair. Cell 172: 784–796.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harrison OJ, Linehan JL, Shih H-Y, et al. 2019. Commensal-specific T cell plasticity promotes rapid tissue adaptation to injury. Science 363: eaat6280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Constantinides MG, Link VM, Tamoutounour S, et al. 2019. MAIT cells are imprinted by the microbiota in early life and promote tissue repair. Science 366: eaax6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lima-Junior DS, Krishnamurthy SR, Bouladoux N, et al. 2021. Endogenous retroviruses promote homeostatic and inflammatory responses to the microbiota. Cell 184: 3794–3811.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sullivan ZA, Khoury-Hanold W, Lim J, et al. 2021. γδ T cells regulate the intestinal response to nutrient sensing. Science 371: eaba8310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kayagaki N, Stowe IB, Lee BL, et al. 2015. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526: 666–671. [DOI] [PubMed] [Google Scholar]
- 50.Shi J, Zhao Y, Wang K, et al. 2015. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526: 660–665. [DOI] [PubMed] [Google Scholar]
- 51.Liu X, Zhang Z, Ruan J, et al. 2016. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 535: 153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kuang S, Zheng J, Yang H, et al. 2017. Structure insight of GSDMD reveals the basis of GSDMD autoinhibition in cell pyroptosis. Proc. Natl. Acad. Sci. U. S. A. 114: 10642–10647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xia S, Zhang Z, Magupalli VG, et al. 2021. Gasdermin D pore structure reveals preferential release of mature interleukin-1. Nature 593: 607–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Russell DG, Huang L & VanderVen BC. 2019. Immunometabolism at the interface between macrophages and pathogens. Nat. Rev. Immunol. 19: 291–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Benhamed F, Denechaud P-D, Lemoine M, et al. 2012. The lipogenic transcription factor ChREBP dissociates hepatic steatosis from insulin resistance in mice and humans. J. Clin. Invest. 122: 2176–2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Iroz A, Montagner A, Benhamed F, et al. 2017. A Specific ChREBP and PPARα Cross-Talk Is Required for the Glucose-Mediated FGF21 Response. Cell Rep. 21: 403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bricambert J, Alves-Guerra M-C, Esteves P, et al. 2018. The histone demethylase Phf2 acts as a molecular checkpoint to prevent NAFLD progression during obesity. Nat. Commun. 9: 2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Parlati L, Regnier M, Guillou H, et al. 2021. New targets for NAFLD. JHEP Rep. Innov. Hepatol 3:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bond MR & Hanover JA. 2015. A little sugar goes a long way: the cell biology of O-GlcNAc. J. Cell Biol. 208: 869–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guinez C, Filhoulaud G, Rayah-Benhamed F, et al. 2011. O-GlcNAcylation increases ChREBP protein content and transcriptional activity in the liver. Diabetes 60: 1399–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cohen YC, Zada M, Wang S-Y, et al. 2021. Identification of resistance pathways and therapeutic targets in relapsed multiple myeloma patients through single-cell sequencing. Nat. Med. 27: 491–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gur C, Wang S-Y, Sheban F, et al. 2022. LGR5 expressing skin fibroblasts define a major cellular hub perturbed in scleroderma. Cell 185: 1373–1388.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Giladi A, Cohen M, Medaglia C, et al. 2020. Dissecting cellular crosstalk by sequencing physically interacting cells. Nat. Biotechnol. 38: 629–637. [DOI] [PubMed] [Google Scholar]
- 64.Katzenelenbogen Y, Sheban F, Yalin A, et al. 2020. Coupled scRNA-Seq and Intracellular Protein Activity Reveal an Immunosuppressive Role of TREM2 in Cancer. Cell 182: 872–885.e19. [DOI] [PubMed] [Google Scholar]
- 65.Cohen M, Giladi A, Barboy O, et al. 2022. The interaction of CD4+ helper T cells with dendritic cells shapes the tumor microenvironment and immune checkpoint blockade response. Nat. Cancer 3: 303–317. [DOI] [PubMed] [Google Scholar]
- 66.Martin-Perez M, Urdiroz-Urricelqui U, Bigas C, et al. 2022. The role of lipids in cancer progression and metastasis. Cell Metab. 34: 1675–1699. [DOI] [PubMed] [Google Scholar]
- 67.Delaunay S, Pascual G, Feng B, et al. 2022. Mitochondrial RNA modifications shape metabolic plasticity in metastasis. Nature 607: 593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pascual G, Domínguez D, Elosúa-Bayes M, et al. 2021. Dietary palmitic acid promotes a prometastatic memory via Schwann cells. Nature 599: 485–490. [DOI] [PubMed] [Google Scholar]
- 69.Kaymak I, Williams KS, Cantor JR, et al. 2021. Immunometabolic Interplay in the Tumor Microenvironment. Cancer Cell 39: 28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sheldon RD, Ma EH, DeCamp LM, et al. 2021. Interrogating in vivo T-cell metabolism in mice using stable isotope labeling metabolomics and rapid cell sorting. Nat. Protoc. 16: 4494–4521. [DOI] [PubMed] [Google Scholar]
- 71.Ma EH, Verway MJ, Johnson RM, et al. 2019. Metabolic Profiling Using Stable Isotope Tracing Reveals Distinct Patterns of Glucose Utilization by Physiologically Activated CD8+ T Cells. Immunity 51: 856–870.e5. [DOI] [PubMed] [Google Scholar]
- 72.Kaymak I, Luda KM, Duimstra LR, et al. 2022. Carbon source availability drives nutrient utilization in CD8+ T cells. Cell Metab. 34: 1298–1311.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Reina-Campos M, Scharping NE & Goldrath AW. 2021. CD8+ T cell metabolism in infection and cancer. Nat. Rev. Immunol. 21: 718–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Scheja L & Heeren J. 2019. The endocrine function of adipose tissues in health and cardiometabolic disease. Nat. Rev. Endocrinol. 15: 507–524. [DOI] [PubMed] [Google Scholar]
- 75.Vasanthakumar A, Chisanga D, Blume J, et al. 2020. Sex-specific adipose tissue imprinting of regulatory T cells. Nature 579: 581–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Beyaz S, Mana MD & Yilmaz ÖH. 2021. High-fat diet activates a PPAR-δ program to enhance intestinal stem cell function. Cell Stem Cell 28: 598–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Beyaz S, Chung C, Mou H, et al. 2021. Dietary suppression of MHC class II expression in intestinal epithelial cells enhances intestinal tumorigenesis. Cell Stem Cell 28: 1922–1935.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ross RC, Akinde YM, Schauer PR, et al. 2022. The role of bariatric and metabolic surgery in the development, diagnosis, and treatment of endometrial cancer. Front. Surg. 9: 943544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nagle CM, Marquart L, Bain CJ, et al. 2013. Impact of weight change and weight cycling on risk of different subtypes of endometrial cancer. Eur. J. Cancer Oxf. Engl. 1990 49: 2717–2726. [DOI] [PubMed] [Google Scholar]
- 80.Janda M, Robledo KP, Gebski V, et al. 2021. Complete pathological response following levonorgestrel intrauterine device in clinically stage 1 endometrial adenocarcinoma: Results of a randomized clinical trial. Gynecol. Oncol. 161: 143–151. [DOI] [PubMed] [Google Scholar]
- 81.Vosoughi K, Atieh J, Khanna L, et al. 2021. Association of Glucagon-like Peptide 1 Analogs and Agonists Administered for Obesity with Weight Loss and Adverse Events: A Systematic Review and Network Meta-analysis. EClinicalMedicine 42: 101213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Buckley CD, Chernajovsky L, Chernajovsky Y, et al. 2021. Immune-mediated inflammation across disease boundaries: breaking down research silos. Nat. Immunol. 22: 1344–1348. [DOI] [PubMed] [Google Scholar]
- 83.Coll RC, Robertson A, Butler M, et al. 2011. The cytokine release inhibitory drug CRID3 targets ASC oligomerisation in the NLRP3 and AIM2 inflammasomes. PloS One 6: e29539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ryan DG, Murphy MP, Frezza C, et al. 2019. Coupling Krebs cycle metabolites to signalling in immunity and cancer. Nat. Metab. 1: 16–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lin J, Ren J, Gao DS, et al. 2021. The Emerging Application of Itaconate: Promising Molecular Targets and Therapeutic Opportunities. Front. Chem. 9: 669308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ylisaukko-oja SK, Cybulski C, Lehtonen R, et al. 2006. Germline fumarate hydratase mutations in patients with ovarian mucinous cystadenoma. Eur. J. Hum. Genet. EJHG 14: 880–883. [DOI] [PubMed] [Google Scholar]
- 87.Xiao M, Yang H, Xu W, et al. 2012. Inhibition of α-KG-dependent histone and DNA demethylases by fumarate and succinate that are accumulated in mutations of FH and SDH tumor suppressors. Genes Dev. 26: 1326–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Leitner BP & Perry RJ. 2020. The Impact of Obesity on Tumor Glucose Uptake in Breast and Lung Cancer. JNCI Cancer Spectr. 4: pkaa007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kichenadasse G, Miners JO, Mangoni AA, et al. 2020. Association Between Body Mass Index and Overall Survival With Immune Checkpoint Inhibitor Therapy for Advanced Non-Small Cell Lung Cancer. JAMA Oncol. 6: 512–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McQuade JL, Daniel CR, Hess KR, et al. 2018. Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: a retrospective, multicohort analysis. Lancet Oncol. 19: 310–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rabin-Court A, Rodrigues MR, Zhang X-M, et al. 2019. Obesity-associated, but not obesity-independent, tumors respond to insulin by increasing mitochondrial glucose oxidation. PloS One 14: e0218126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Akingbesote ND, Norman A, Zhu W, et al. 2022. A precision medicine approach to metabolic therapy for breast cancer in mice. Commun. Biol. 5: 478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mak TW, Grusdat M, Duncan GS, et al. 2017. Glutathione Primes T Cell Metabolism for Inflammation. Immunity 46: 675–689. [DOI] [PubMed] [Google Scholar]
- 94.Kurniawan H, Franchina DG, Guerra L, et al. 2020. Glutathione Restricts Serine Metabolism to Preserve Regulatory T Cell Function. Cell Metab. 31: 920–936.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]


