Abstract
Background
Schizophrenia and other psychoses are thought to be associated with a substantial increase in aggressive behaviour, violence and violent offending. However, acts of aggression or violence committed by people with severe mental illness are rare and circumscribed to a small minority of individuals. We know little about the frequency and variability of violent episodes for people with schizophrenia who present chronic or recurrent aggressive episodes, and of available interventions to reduce such problems. A psychological intervention, cognitive behavioural therapy (CBT), aims to challenge dysfunctional thoughts and has been used since the mid‐1970s to improve mental health and emotional disorders. CBT includes different interventional procedures, such as cognitive therapy, elements of behavioural therapy, problem‐solving interventions, and coping skills training, among others. Although CBT presents much diversity, interventions are characteristically problem‐focused, goal‐directed, future‐oriented, time‐limited (about 12 to 20 sessions over four to six months), and empirically based. CBT has shown clinically beneficial effects in persistent positive and negative symptoms of schizophrenia and its use as an add‐on therapy to medication in the treatment of schizophrenia is supported by treatment guidelines. However, several Cochrane Reviews recently concluded that, due to the low quality of evidence available, no firm conclusions can currently be made regarding the effectiveness of adding CBT to standard care for people with schizophrenia, or about CBT compared to other psychosocial treatments for people with schizophrenia. Whereas CBT is not an emergency or crisis intervention that acts immediately on the known or unknown triggers underlying aggressive behaviour, might be a timely treatment used to manage persistent aggression or repeated aggressive episodes in people with schizophrenia.
Objectives
To assess the efficacy and safety of cognitive behavioural therapy(CBT) plus standard care versus standard care alone for people with schizophrenia and persistent aggression.
Search methods
On 18 January 2023, we searched the Cochrane Schizophrenia Group's Study‐Based Register of Trials which is based on CENTRAL, CINAHL, ClinicalTrials.Gov, Embase, ISRCTN, MEDLINE, PsycINFO, PubMed, and WHO ICTRP. We also inspected references of all identified studies for more studies.
Selection criteria
All randomised controlled trials comparing CBT plus standard care with standard care alone for people with schizophrenia and persistent aggression.
Data collection and analysis
We independently inspected citations, selected studies, extracted data and appraised study quality. For binary outcomes, we calculated risk ratios (RR) and their 95% confidence intervals (CIs). For continuous outcomes we calculated mean differences (MD) and their 95%CIs for outcomes reported with the same measurement scale. Post hoc, for counts over person‐time outcomes, we calculated incidence rate ratios (IRRs) and their 95%CIs. If feasible, we combined study outcomes with the random‐effects model. We assessed the risk of bias for included studies and created a summary of findings table using the GRADE approach.
Main results
We included two studies with 184 participants with psychotic disorder (mainly schizophrenia) and violence. The studies were run in forensic units and prison. Both studies were at high risk of bias on blinding (performance and detection bias).
CBT plus standard care as compared with standard care may result in little to no difference in the frequency of physical violence at end of trial (IRR 0.52; 95% CI 0.23 to 1.18) and follow‐up (IRR 0.86; 95% CI 0.44 to 1.68). The confidence interval did not exclude the null effect, and the certainty of the evidence is very low due to lack of blinding and to the small sample size.
One study reported no deaths in both arms and zero serious and other adverse events. The other study did not report any figure for deaths or adverse events.
CBT plus standard care as compared with standard care may result in little to no difference in leaving the study early for any reason (RR 1.04; 95% CI 0.53 to 2.00). Confidence interval did not exclude the null effect and the certainty of the evidence is low due to lack of blinding and the small sample size.
Authors' conclusions
Whereas the evidence from only two studies with 184 participants suggests the use of CBT plus standard care may reduce some aggressive behaviours in patients with schizophrenia, the grading of the certainty of the evidence is very low. It implies that there is not yet reliable evidence to guide clinical decisions and therefore more evidence is needed to get a more precise estimate of the effect of the intervention. Currently, we have very little confidence in the effect estimate, and the true effect could be substantially different from its estimate.
Keywords: Humans, Aggression, Anxiety, Cognitive Behavioral Therapy, Cognitive Behavioral Therapy/methods, Psychotic Disorders, Schizophrenia, Schizophrenia/complications, Schizophrenia/therapy
Plain language summary
Cognitive behavioural therapy plus standard care versus standard care for persistent aggressive behaviour or agitation in people with schizophrenia
Is cognitive behavioural therapy better than conventional treatment for treating aggression or agitation in people with schizophrenia?
Key messages
• We did not find enough good‐quality evidence about the benefits of cognitive behavioural therapy on aggression in people with schizophrenia. We found only two studies with not enough participants enroled to give reliable results.
• Larger, well‐designed studies are needed to give better estimates of the benefits and potential harms of cognitive behavioural interventions.
How important is aggression in people with schizophrenia?
Schizophrenia is a mental disorder characterised by disruptions in thought processes, perceptions, emotional responsiveness, and social interactions. It is typically persistent and can be severe and disabling. Whereas the risk of aggression (self‐aggression and aggression to others) in persons with schizophrenia is rare and circumscribed to a small minority of individuals, aggression if present adds to the burden of illness by increasing the risk of injuries and death. Cognitive behavioural therapy aims to challenge dysfunctional thoughts and is used to improve mental health and emotional disorders; it has shown beneficial effects in persistent symptoms of schizophrenia and its use as an add‐on therapy to medication in the treatment of schizophrenia is supported by treatment guidelines. However, no firm conclusions can currently be made regarding the effectiveness of adding cognitive behaviour therapy to standard care for people with schizophrenia and aggressive behaviours. Whereas cognitive behaviour therapy is not an emergency or crisis intervention that acts immediately on the known or unknown triggers underlying aggressive behaviour, it might be a timely treatment used to manage persistent aggression or repeated aggressive episodes in people with schizophrenia.
How is aggression in people with schizophrenia treated?
Treatments for the condition include:
• medicine‐based treatments;
• non‐medicine‐based treatments;
• physical treatments (restraint and seclusion).
What did we want to find out?
We wanted to find out if cognitive behavioural therapy was better than standard care to reduce:
• aggressive behaviours;
• agitation;
• self‐harm;
• dropouts from treatment.
We wanted to find out if cognitive behavioural therapy was better than standard care to improve:
• overall mental state;
• well‐being.
We also wanted to find out if cognitive behavioural therapy was associated with any unwanted effects.
What did we do? We searched for studies that investigated cognitive behavioural therapy compared with standard care for treating aggression in people with schizophrenia.
We compared and summarised the results of the eligible studies and rated our confidence in the evidence based on factors such as study methods and sizes.
What did we find?
We found two studies that involved 184 people with schizophrenia and aggression and lasted between three and six months. One study was conducted in the UK and the other in the USA. The main results of the review are:
• cognitive behavioural therapy may result in little to no difference in the frequency of acts of physical violence;
• cognitive behavioural therapy may reduce slightly the frequency of acts of verbal aggression;
• cognitive behavioural therapy does not change the mean score on self‐reported aggression scales;
• cognitive behavioural therapy may result in little to no difference in leaving the study for any reason.
What are the limitations of the evidence?
We have little confidence in the evidence because:
• people in the studies were aware of which treatment they were getting.;
• not all studies provided data about everything that we were interested in;
• studies were few and very small and the null effect could not be excluded for most of the outcomes.
There is uncertainty about the results of the outcomes.
How up to date is this evidence?
The evidence is up‐to‐date to 18 January 2023.
Summary of findings
Summary of findings 1. CBT plus standard care compared to standard care for persistent aggressive behaviour or agitation in people with schizophrenia.
| CBT plus standard care compared to standard care for persistent aggressive behaviour or agitation in people with schizophrenia | |||
| Patient or population: persistent aggressive behaviour or agitation in people with schizophrenia Setting: any clinical setting Intervention: CBT plus standard care Comparison: standard care | |||
| Outcomes | Impact | № of participants (studies) | Certainty of the evidence (GRADE) |
| Aggression ‐ frequency of physical violence. Assessed with: frequency of aggressive episodes over person‐time. Follow up: median 6 months. | Rate ratio 0.52 (0.23 to 1.18) amongst 84 participants included in one RCT. Data was reported as incidence rate ratios with 95% CI. Absolute effect estimation was not allowed due that frequencies of aggressions were not reported. CBT may result in little to no difference in the frequency of physical violence, but the evidence is very uncertain. Participants allocated to CBT had 0.52 times the rate of events compared to participants allocated to standard care (0.23 times fewer to 1.18 times more). |
84 (1 RCT) | ⨁◯◯◯ VERY LOW 1 2 |
| Agitation ‐ frequency of agitation | No study reported on this important outcome. | 0 (0 RCTs) |
Not estimable |
| Clinically important adverse effect or event. Assessed with: number of adverse effects recorded. Follow up: 6 months. |
Absolute and relative effect sizes are not estimable. No serious adverse effects or events were recorded in both arms. | 100 (1 RCT) |
Not estimable |
| Self harm ‐ frequency of self harm | No study reported on this important outcome. | 0 (0 RCTs) |
Not estimable |
| Mental state ‐ clinically important change in mental state | No study reported on this important outcome. | 0 (0 RCTs) |
Not estimable |
| Leaving the study early for any reason. Follow‐up: range 3 to 6 months. |
Risk ratio 1.04 (0.53 to 2.00) amongst 184 participants included in two RCTs. CBT may result in little to no difference in leaving the study early for any reason. |
184 (2 RCTs) |
⨁⨁◯◯ LOW 3 4 |
| Quality of life ‐ clinically important change in overall quality of life | No study reported on this important outcome. | 0 (0 RCTs) |
Not estimable |
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||
1 We downgraded two levels due to very serious risk of bias for lack of blinding of participants and personnel (open trial), and for lack of blinding of outcome assessment (clinical notes from clinicians involved in the treatment or self‐reported scales from unblinded participants).
2 We downgraded one level due to information based on only one study with a total of 84 participants.
3 We downgraded one level due to serious risk of bias for lack of blinding of participants and personnel (open trial).
4 We downgraded one level due to information based on two studies with a total of 184 participants.
Background
Description of the condition
Schizophrenia is a mental disorder characterised by disruptions in thought processes, perceptions, emotional responsiveness, and social interactions. Although the course of schizophrenia varies amongst individuals, it is typically persistent and can be both severe and disabling (The National Institute of Mental Health). Schizophrenia presents a worldwide prevalence in the range of 1.4 to 4.6 per 1000 population, and a yearly incidence rate in the range of 0.16 to 0.42 per 1000 population (Jablensky 2000; Moreno‐Küstner 2018; Saha 2005). It is a leading cause of disability with an important global burden (Chong 2016), estimated at 13.6 million absolute disability‐adjusted life years (DALYs) (Whiteford 2015). This represents 0.5% of all disease DALYs and 5.3% of mental, neurological, and substance use DALYs (Whiteford 2015). Clinically, schizophrenia presents a cluster of symptoms from different domains. Positive symptoms include delusions (fixed beliefs not amenable to change because of conflicting evidence) and hallucinations (perception‐like experiences without an external stimulus). Other domains include negative symptoms (emotional flatness, lack of motivation), cognitive alterations (attention and information processing deficits), and mood disorders. Whereas positive symptoms reflect an excess or distortion of normal functions, negative symptoms reflect a diminution or loss of normal function (flattening of affect and poverty of speech) (APA 2013; Fuller 2003).
Aggression is defined as "a disposition, a willingness to inflict harm, regardless of whether this is behaviourally expressed and physical harm is sustained" (Serper 2011). Schizophrenia and other psychoses are thought to be associated with a substantial increase in aggressive behaviour, violence and violent offending (Fazel 2009). However, acts of aggression or violence committed by people with severe mental illness are rare and circumscribed to a small minority of individuals (Walsh 2002). Of the several risk factors that have been linked with aggression in schizophrenia, failure to adequately treat episodes and relapses is a major predictor for aggression (Serper 2011). However, we know little about the frequency and variability of violent episodes for people with schizophrenia who present chronic or recurrent aggressive episodes, and of available interventions to reduce such problems.
The risk of violence (self‐aggression and aggression to others) in persons with schizophrenia adds to the burden of the illness by increasing the risk of injuries and death (Olfson 2015), and is higher in populations with comorbid substance use disorders (Fazel 2009). Physical aggression implies motor behaviours that take physical form in motor action to physically harm others and requires deliberate intention. Verbal aggression implies the presence of verbal abuse or threats (Serper 2011), agitation (restlessness with excessive motor activity, irritability, and greater responsiveness to internal and external stimuli) which, if severe, could also lead to aggressive and violent behaviours (Garriga 2016).
Description of the intervention
Cognitive behavioural therapy (CBT) aims to challenge dysfunctional thoughts and has been used since the mid‐1970s as a psychological intervention to improve mental health and emotional disorders (Beck 1976). CBT assumes that cognitive and emotional processes mediate the acquisition and maintenance of behaviours and that cognitions that do not correspond to specific environmental conditions are maladaptive (Beck 1976). CBT allows the participant to work jointly with a therapist to address current maladaptive cognitions and understand the causes behind those cognitions, related maladaptive behaviours, and identify new strategies to reduce distress and cope with difficult situations (Ali 2015). CBT includes different interventional procedures, such as cognitive therapy, elements of behavioural therapy, problem‐solving interventions, and coping skills training, amongst others. Although CBT presents much diversity, interventions are characteristically problem‐focused, goal‐directed, future‐oriented, time‐limited (about 12 to 20 sessions over 4 to 6 months), and empirically‐based (Grant 2005). CBT has shown clinically relevant efficacy for the treatment of a variety of mental health‐related problems, including a beneficial effect of CBT in persistent positive and negative symptoms of schizophrenia, and functioning (Bighelli 2018; Bighelli 2022; Hofmann 2012; Jauhar 2014; Rathod 2010). The use of CBT as an add‐on therapy to medication in the treatment of schizophrenia is supported by treatment guidelines (NICE 2014). However, several Cochrane Reviews recently concluded that, due to the low quality of evidence available, no firm conclusions can currently be made regarding the effectiveness of adding CBT to standard care for people with schizophrenia (Jones 2018a), or about CBT compared to other psychosocial treatments for people with schizophrenia (Jones 2018b).
How the intervention might work
CBT for schizophrenia is similar to using the technique for other types of mental health disorders. It involves a collaborative therapeutic relationship, which leads to the development of a shared understanding of the problem, and allows participants to set goals and develop techniques or strategies to adequately manage the problematic experiences or behaviours. CBT acts by modifying erroneous cognitions that lead to problematic and maladaptive behaviours in specific environmental conditions. It works in a therapeutic milieu, where people with schizophrenia and their therapists are active participants who identify and manage ways to solve problematic behaviours (Ali 2015). CBT, as applied to people with schizophrenia and aggression, aims to remediate distressing emotional experiences or dysfunctional behaviour, by changing the way in which the individual interprets and evaluates the experience or cognates on its consequence and meaning. CBT encourages the person to identify and challenge biased interpretations of experiences that may be maintaining symptoms (Jones 2018a). CBT is not an emergency or crisis intervention that acts immediately on the known or unknown triggers underlying aggressive behaviour but can be a timely treatment used to manage persistent aggression or repeated aggressive episodes, and perhaps prevent future aggressive behaviour in people with schizophrenia.
Why it is important to do this review
There are several Cochrane Reviews that focus on the use of emergency pharmacological treatments to control psychosis‐induced agitation or aggression, or both, in people with schizophrenia (e.g. Ahmed 2010; Khushu 2016; Ostinelli 2017; Zaman 2017). A recent non‐Cochrane Review that focuses on short‐acting intramuscular treatments is Paris 2021. However, these reviews do not focus on interventions for persistent aggression, or repeated aggressive episodes. There are other reviews on the issue of the efficacy of non‐pharmacological interventions for violence or aggression in severe mental illness (Du 2017; Gaynes 2016; Hockenhull 2012; Muralidharan 2006; Rampling 2016; Sailas 2000). However, these reviews do not focus on CBT as an intervention for people with schizophrenia. A narrative review by Rampling 2016 suggested that non‐pharmacological structured interventions, such as CBT, could decrease the severity and frequency of aggression (fewer incidents of physical or verbal aggression in the intervention group), but evidence of quality is currently lacking. Reviews of CBT interventions that go beyond symptom reduction are needed (Nowak 2016). We think that updated evidence focussing specifically on the efficacy of CBT plus standard care for the long‐term management of aggression in people diagnosed with schizophrenia is of interest, and would fill a knowledge gap. This review will also add to the findings a suite of Cochrane Reviews that have assessed the overall effectiveness of CBT for people with schizophrenia (Jones 2018a; Jones 2018b; Naeem 2015).
Objectives
To assess the efficacy and safety of Cognitive behavioural therapy (CBT) plus standard care versus standard care alone for people with schizophrenia and persistent aggression.
Methods
Criteria for considering studies for this review
Types of studies
We considered all relevant randomised controlled trials (RCTs). We excluded quasi‐randomised studies, such as those that allocated intervention by alternate days of the week. Where people were given additional treatments as well as CBT plus standard care, we only included data if the adjunct treatment was evenly distributed between groups, and it was only the CBT that was randomised.
Types of participants
Adults (18+ years), regardless of gender, with schizophrenia or related disorders, including schizophreniform disorder, schizoaffective disorder and delusional disorder, by any means of diagnosis, and presenting with chronic or persistent agitation or aggression in either hospital settings (emergency services and wards), ambulatory, or community settings.
Types of interventions
1. Cognitive behavioural therapy
We defined CBT as a psychological therapy for cognitive restructure of thoughts, emotions, and behaviours directed ‐ in this review ‐ to manage actual situations and actions of aggressive and agitated behaviours (cognitive therapy, problem‐solving interventions, and coping skills training, amongst others). CBT normally includes elements of cognitive restructuring or cognitive therapy (CT) and elements of behavioural therapy (BT) that are delivered together. However, CT and BT elements could also be approached separately in the therapeutic process. Therefore, we considered any intervention that included CT or BT elements alone, as well as CBT interventions that included both components, as an eligible intervention under the term 'CBT'. In this review, we considered CBT to be a component of comprehensive treatment intervention, that is in addition to standard care and involving short‐term administration (20 sessions or fewer) as it is usual for CBT‐focussed interventions (Grant 2005). We reported the main characteristics of the interventions according to the template for intervention description and replication (TIDieR) (Hoffmann 2014) (see Table 2; Table 3).
1. TIDieR ‐ Cullen 2012.
| Study | Cullen, 2012 | |
| TIDieR item | Experimental intervention | Control intervention |
| BRIEF NAME | R&R | Standard care (TAU) |
| WHY | R&R is a highly structured, manualized program targeting social problem‐solving skills and thinking styles. | Not described |
| WHAT materials | Not described | Not described |
| PROCEDURES | The R&R program includes 8 core modules:
|
Not described |
| WHO provided | Delivered by experienced staff who had received training during intensive 5‐day workshops provided by the program authors | Not described |
| HOW delivered | Group format (5 to 8 patients per group) | Not described |
| WHERE occurred | At medium‐secure forensic hospitals | |
| WHEN and HOW MUCH | A minimum of 36 two‐hour sessions. Sessions were held 2 or 3 times weekly. | Not described |
| TAILORING | Not tailored | Not described |
| MODIFICATIONS | Not declared | Not described |
| HOW WELL planned | Program developers emphasise the need to ensure treatment integrity; when possible (i.e. when all participants in the group signed a release of confidential information form), sessions were recorded using audiovisual equipment, and randomly selected sessions were assessed by one of the authors using an objective rating scale developed by the Cognitive Centre Foundation (www.cognitivecentre.com). Formal feedback based on these ratings was provided in supervision sessions, and strategies to improve delivery were also discussed. On the basis of the total number of R&R sessions attended, participants were defined as completers (those who attended 30 or more sessions) and noncompleters (those who attended fewer than 30 sessions). | Not described |
| HOW WELL actual | Reviews completed throughout the trial indicated that the underlined procedure ensured that a high standard of program delivery was maintained. The percentage of completers (48%, 21 of 44) was slightly less than the percentage of noncompleters (52%, 23 of 44). | Not described |
R&R: reasoning and rehabilitation; TAU: treatment as usual.
2. TIDieR ‐ NCT03713398.
| Study | NCT03713398 | |
| TIDieR item | Experimental intervention | Control intervention |
| BRIEF NAME | Thinking for a Change delivered to serious mental illness (T4C‐SMI) plus standard prison mental health services | Standard prison mental health services |
| WHY | T4C‐SMI is an intervention that address criminogenic risk factors trying to improve impulsivity, criminal attitudes, interpersonal problem‐solving, levels of aggression and behavioural infractions. | Not described |
| WHAT materials | Not described | Not described |
| PROCEDURES | T4C‐SMI includes 3 modules:
|
Not described |
| WHO provided | Not described | Not described |
| HOW delivered | Group format | |
| WHERE occurred | Prison | |
| WHEN and HOW MUCH | T4C‐SMI entails a 25‐session, manualized intervention that is delivered in a closed‐group format at least twice a week over a 3‐month period. | Not described |
| TAILORING | Not tailored | |
| MODIFICATIONS | Not declared | Not described |
| HOW WELL planned | Not described | Not described |
| HOW WELL actual | Not described | Not described |
2. Standard care
For this review, we considered standard care to be the normal level of care a participant not in a trial would receive for their condition. For people with schizophrenia, this normally includes a biological, psychological and social approach to care, including antipsychotic medication, and utilisation of services such as hospital stay, day hospital attendance and community psychiatric nursing involvement.
Types of outcome measures
We aimed to divide all outcomes according to the following time points: up to one month (short term), up to three months (medium‐term), up to six months (long term), and over six months (very long term). If feasible, we also aimed to single out outcomes reported at 12 months or more to inform whether effects are sustainable over long time periods.
We endeavoured to report binary outcomes recording clear and clinically meaningful degrees of change (e.g. global impression of much improved, or more than 50% improvement on a rating scale ‐ as defined within the trials) before any others. Thereafter, we listed other binary outcomes, and then those that were continuous.
For binary outcomes such as 'clinically important change', 'any change', and 'relapse', we used the definition used by each of the trials.
We only included data from validated scales (see Data extraction and management).
Primary outcomes
1. Specific behaviours
Outcomes under this heading rely on behavioural observation, clinical notes or validated scales.
1.1 Aggression (physical or verbal)
1.1.1 Another episode of aggression
1.2 Agitation
1.2.1 Another episode of agitation
2. Adverse effect/event(s)
2.1 Clinically important adverse effect or event
Secondary outcomes
1. Specific behaviours
Outcomes under this heading rely on behavioural observations, clinical notes or validated scales.
1.1 Aggression
1.1.1 Frequency of aggressive episodes 1.1.2 Clinically important change in aggression 1.1.3 Any change in aggression 1.1.4 Average endpoint or change score on aggression scale (e.g., Aggression Questionnaire ‐ Short Form)
1.2 Agitation
1.2.1 Frequency of agitation 1.2.2 Clinically important change in agitation 1.2.3 Any change in agitation 1.2.4 Average endpoint or change score on agitation scale (e.g., Agitated Behavior Scale)
1.3 Self‐harm, including suicide
1.3.1 Another episode of self harm 1.3.2 Frequency of self harm
1.4 Injury to others
1.4.1 Frequency of injury to others 1.4.2 Another episode of injury to others
2. Tranquillisation
Outcomes under this heading rely on clinical notes.
2.1 Needing additional administration of intervention medication
3. Global State
Outcomes under this heading rely on validated scales of ill‐health or clinical notes.
3.1 Clinically important change in global state 3.2 Any change in global state 3.3 Average endpoint or change score on global state scale (e.g., Clinical Global Impression Scale) 3.4 Use of additional medication ‐ not intervention medication 3.5 Use of restraints or seclusion 3.6 Relapse ‐ as defined by each study 3.7 Recurrence of violent incidents 3.8 Needing extra visits from the doctor 3.9 Refusing medication
4. Mental state
Outcomes under this heading rely on validated scales of mental health.
4.1 Clinically important change in general mental state 4.2 Any change in general mental state 4.3 Average endpoint or change score on general mental state scale (e.g., Brief Psychiatric Rating Scale) 4.4 Clinically important change in specific symptoms (positive/negative/cognitive) 4.5 Any change in specific symptoms (positive/negative/cognitive) 4.6 Average endpoint or change score on specific mental state scale (e.g., Positive and Negative Syndrome Scale)
5. Service use
Outcomes under this heading rely on registered information or validated scales.
5.1 Hospital admission 5.2 Clinically important engagement with services 5.3 Average endpoint or change score on engagement scale (e.g., Client Engagement and Service Use Scale)
6. Adverse effects
Outcomes under this heading rely on registered adverse effects or validated scales.
6.1 Death ‐ not suicide 6.2 Any general adverse effects 6.3 Any specific adverse effects 6.4 Average endpoint or change score on general adverse effect scale (any reported scale for non‐drug general adverse effects) 6.5 Clinically important change in specific adverse effects 6.6 Any change in specific adverse effects 6.7 Average endpoint or change score on specific adverse effects scale (any reported scale for non‐drug specific adverse effects)
7. Leaving the study early
Outcomes under this heading rely on the studies flow chart.
7.1 For any reason 7.2 For specific reason
8. Satisfaction with treatment (recipient/informal caregiver/professional provider of care)
Outcomes under this heading rely on participants self‐report or validated scales.
8.1 Satisfied with treatment 8.2 Average endpoint or change score on satisfaction scale (e.g., Short Assessment of Patient Satisfaction)
9. Acceptance of treatment
Outcomes under this heading rely on participants decisions (registered) or validated scales.
9.1 Accepting treatment 9.2 Average endpoint or change score on acceptance scale (e.g., Treatment Acceptability/Adherence Scale)
10. Quality of life
Outcomes under this heading rely on validated quality of life scales.
10.1 Clinically important change in overall quality of life 10.2 Any change in overall quality of life 10.3 Average endpoint or change score on quality of life scale (e.g., Schizophrenia Quality of Life Scale) 10.4 Clinically important change in specific aspects of quality of life 10.5 Any change in specific aspects of quality of life 10.6 Average endpoint or change score on specific aspects of quality of life scale (e.g., Satisfaction with Life Domains Scale)
11. Economic outcomes
Outcomes under this heading rely on observed/estimated costs.
11.1 Direct costs 11.2 Indirect costs
Search methods for identification of studies
Electronic searches
Cochrane Schizophrenia Group's Study‐Based Register of Trials
On 3 February 2020, 10 February 2021, and 06 March 2022, the Information Specialist searched the register using the following search strategy:
(*Cogniti* in Intervention) AND ((*Aggression* OR *Agitation*) in Health Care Condition) of STUDY.
In such study‐based register, searching the major concept retrieves all the synonyms and relevant studies because all the studies have already been organised based on their interventions and linked to the relevant topics (Shokraneh 2017; Shokraneh 2021; Roberts 2021). This allows rapid and accurate searches that reduce waste in the next steps of systematic reviewing (Shokraneh 2019). Following the methods from Cochrane (Lefebvre 2019), this register is compiled by systematic searches of major resources (CENTRAL, CINAHL, ClinicalTrials.Gov, Embase, ISRCTN, MEDLINE, PsycINFO, PubMed, WHO ICTRP) and their monthly updates, ProQuest Dissertations and Theses A&I and its quarterly update, hand‐searches, grey literature, and conference proceedings (Shokraneh 2020; see Group's website). There is no language, date, document type, or publication status limitations for inclusion of records into the register.
An additional search containing specific terms pertaining to agitation and aggression was performed on 18 January 2023. The databases searched, the search strategy for each one of the databases, and the references obtained are reported in Appendix 1 (Appendix 1). This last search included all the references located previously.
Searching other resources
1. Reference searching
We inspected references of all included studies for further relevant studies.
2. Personal contact
We tried to contact the corresponding author of each included study for additional information regarding incomplete or unpublished data. If done, we noted the outcome of this contact in the 'Included studies' or 'Studies awaiting classification' tables.
Data collection and analysis
Selection of studies
Review authors (MCMC, EGF) independently inspected citations from the searches and identified relevant abstracts; JB independently re‐inspected these abstracts to ensure the reliability of selection. Where disputes arose, we acquired the full report for more detailed scrutiny. EGF obtained and inspected full reports of the abstracts or reports meeting the review criteria. JB re‐inspected these full reports in order to ensure the reliability of the selection.
Data extraction and management
1. Extraction
Review authors (EGF, BS) extracted study characteristics and data from all included studies. In addition, to ensure reliability, JB independently extracted data from all included studies. We discussed any disagreement and documented our decisions. If necessary, we attempted to contact authors through an open‐ended request in order to obtain missing information, or for clarification.
2. Management
2.1 Forms
We extracted data onto standard, pre‐designed, simple forms supplied by Cochrane Schizophrenia.
2.2 Scale‐derived data
We included continuous data from rating scales only if:
the psychometric properties of the measuring instrument were described in a peer‐reviewed journal (Marshall 2000);
the measuring instrument was not written or modified by one of the trialists for that particular trial; and
the instrument was a global assessment of an area of functioning and not subscores which were not, in themselves, validated or shown to be reliable.
Ideally, the measuring instrument should either be a self‐report or completed by an independent rater or relative (not the therapist). We realised that this was not often reported clearly; and note if this was the case or not in Description of studies.
2.3 Endpoint versus change data
There are advantages of both endpoint and change data: change data can remove a component of between‐person variability from the analysis; however, calculation of change needs two assessments (baseline and endpoint) that can be difficult to obtain in unstable and difficult‐to‐measure conditions such as schizophrenia. We decided to use endpoint data primarily, and only use change data if the former were not available. We only combined endpoint and change data in the analysis if necessary, as we preferred to use mean differences (MDs) rather than standardised mean differences (SMDs) throughout (Deeks 2011).
2.4 Skewed data
Continuous data on clinical and social outcomes are often not normally distributed. To avoid the pitfall of applying parametric tests to non‐parametric data, we applied the following standards to relevant continuous data before inclusion.
We took the following approach for endpoint data from studies including fewer than 200 participants.
When a scale started from the finite number zero, we planned to subtract the lowest possible value from the mean, and divide this by the standard deviation (SD). If this value was lower than one, it strongly suggested that the data were skewed, and we would have excluded these data. If this ratio was higher than one but less than two, there was the suggestion that the data were skewed: we would have entered these data and tested whether their inclusion or exclusion would change the results substantially. If such data changed results we would have entered as 'other data'. Finally, if the ratio was larger than two we would have included these data because it was less likely that they were skewed (Altman 1996).
If a scale started from a positive value (such as the Positive and Negative Syndrome Scale (PANSS), which can have values from 30 to 210 (Kay 1986)), we planned to modify the calculation described above to take the scale starting point into account. In these cases, skewed data were present if 2 SD > (S − S min), where S was the mean score and 'S min' was the minimum score.
We planned to enter all relevant data from studies of more than 200 participants in the analysis irrespective of the above rules because skewed data pose less of a problem in large studies. We also entered all relevant change data, as when continuous data are presented on a scale that includes a possibility of negative values (such as change data), it is difficult to tell whether data were skewed.
2.5 Common measurement
To facilitate comparison between trials, we aimed to convert variables that could be reported in different metrics, such as days in the hospital (mean days per year, per week or per month) to a common metric (e.g. mean days per month), where relevant.
2.6 Conversion of continuous to binary
Where possible, we made efforts to convert outcome measures to dichotomous data. This was planned to be done by identifying cut‐off points on rating scales and dividing participants accordingly into 'clinically improved' or 'not clinically improved' categories. It is generally assumed that if there is a 50% reduction in a scale‐derived score such as the Brief Psychiatric Rating Scale (BPRS) (Overall 1962), or the PANSS (Kay 1986), this could be considered to be a clinically significant response (Leucht 2005a; Leucht 2005b). If data based on these thresholds were not available, we planned to use the primary cut‐off presented by the original authors.
2.7 Direction of graphs
Where possible, we entered data in such a way that the area to the left of the line of no effect indicates a favourable outcome for CBT plus standard care. Where keeping to this make it impossible to avoid outcome titles with clumsy double‐negatives (e.g. 'not un‐improved'), we reported data where the left of the line indicated an unfavourable outcome and noted this in the relevant graphs.
Assessment of risk of bias in included studies
Review authors (JB, EGF) worked independently to assess the risk of bias by using criteria described in the Cochrane Handbook for Systematic Reviews of Interventions to assess trial quality (Higgins 2011a). This set of criteria is based on evidence of associations between potential overestimation of effect and the level of risk of bias of the article that may be due to aspects of sequence generation, allocation concealment, blinding, incomplete outcome data and selective reporting, or the way in which these 'domains' are reported.
If the raters disagreed, we made the final rating by consensus. Where the trials provided inadequate details of randomisation and other characteristics, we attempted to contact the authors of the studies in order to obtain further information. We reported non‐concurrence in quality assessment, but if disputes arose regarding the category to which a trial was to be allocated, we resolved this by discussion.
We noted the level of risk of bias in both the text of the review, in figures, and the Table 1.
Measures of treatment effect
1. Binary data
For binary outcomes, we calculated a standard estimation of the risk ratio (RR) and its 95% confidence interval (CI), as it has been shown that RR is more intuitive than odds ratios (ORs) (Boissel 1999); and that clinicians tend to interpret OR as RR (Deeks 2000). Although the number needed to treat for an additional beneficial outcome (NNTB) and the number needed to treat for an additional harmful outcome (NNTH), with their CIs, are intuitively attractive to clinicians, they are problematic to calculate and interpret in meta‐analyses (Hutton 2009). For binary data presented in the Table 1we, where possible, calculated illustrative comparative risks.
2. Continuous data
For continuous outcomes, we estimated the mean difference (MD) between groups. We preferred not to calculate effect size measures (SMD). However, if scales of very considerable similarity were used, we would have presumed that there was a small difference in measurement, calculated the effect size, then transformed the effect back into the units of one or more of the specific instruments.
3. Other metrics
Other measure of treatment effect ‐ not contemplated in the published protocol ‐ was the rate of aggressive behaviours over time. In this case, we calculated the Incidence Rate Ratio (IRR) and its CIs from the available data (aggregated frequency counts of events and total person‐time follow‐up) (Deeks 2011). This was considered in a generic inverse variance meta‐analysis.
Unit of analysis issues
1. Cluster trials
Studies increasingly employ 'cluster randomisation' (such as randomisation by clinician or practice), but analysis and pooling of clustered data poses problems. Authors often fail to account for intraclass correlation in clustered studies, leading to a unit of analysis error whereby P values are spuriously low, CIs unduly narrow and statistical significance overestimated (Divine 1992). This causes type I errors (Bland 1997; Gulliford 1999).
Where primary studies incorporated clustering into the analysis, we planned to present these data as if from a non‐cluster‐randomised study but adjusting for the clustering effect.
Where the primary studies did not account for clustering, we planned to present data in a table, with a (*) symbol to indicate the presence of a probable unit of analysis error. We planned to to contact the first authors of studies to obtain intraclass correlation coefficients for their clustered data and to adjust for this using accepted methods (Gulliford 1999).
We have sought statistical advice and have been advised that the binary data from cluster trials presented in a report should be divided by a 'design effect'. This is calculated using the mean number of participants per cluster (m) and the intraclass correlation coefficient (ICC): thus design effect = 1 + (m − 1) * ICC (Donner 2002). If the ICC was not reported, we assumed it to be 0.1 (Ukoumunne 1999).
If trial authors analysed cluster studies appropriately and taken into account the ICCs and relevant data documented in the report, we planned to synthesis these with other studies using the generic inverse variance technique.
2. Cross‐over trials
A major concern regarding cross‐over trials is the carry‐over effect. This occurs if an effect (e.g. pharmacological, physiological or psychological) of the treatment in the first phase is carried over to the second phase. As a consequence, participants can differ significantly from their initial state at entry into the second phase, despite a wash‐out phase. For the same reason, cross‐over trials are not appropriate if the condition of interest is unstable (Elbourne 2002). As both carry‐over and unstable conditions are very likely in severe mental illness, we planned to only use data from the first phase of cross‐over studies.
3. Studies with multiple treatment groups
Where a study involves more than two treatment arms, if relevant, we planned to present the additional treatment arms in comparisons. If data were binary, we would have simply added these and combined them within the two‐by‐two table. If data were continuous, we planned to combine data following the formula in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). Where additional treatment arms were not relevant, we planned to not reproduce these data.
Dealing with missing data
1. Overall loss of credibility
At some degree of loss of follow‐up, data must lose credibility (Xia 2009). We choose that, should more than 50% of data for any particular outcome be unaccounted for, we will not reproduce these data or use them within analyses. If, however, more than 50% of those in one arm of a study were lost, but the total loss was less than 50%, we planned to address this within the summary of findings table by down‐rating certainty. Finally, we also planned to downrate certainty within the 'Summary of findings' table should the loss be 25% to 50% in total.
2. Binary
In the case where attrition for a binary outcome was between 0% and 50%, and where these data were not clearly described, we planned to present data on a 'once‐randomised‐always‐analyse' basis (an intention‐to‐treat analysis (ITT)). We assumed that those leaving the study early all had the same rates of the negative outcome as those who completed. We planned to use the rate of those who stayed in the study ‐ in that particular arm of the trial ‐ and also applied this to those who did not. We planned to undertake a sensitivity analysis testing how prone the primary outcomes were to change when data only from people who complete the study to that point were compared to theITT analysis using the above assumptions.
3. Continuous
3.1 Attrition
We used data where attrition for a continuous outcome was between 0% and 50%, and the study only reported data from people who complete the study to that point.
3.2 Standard deviations
If standard deviations (SDs) were not reported, we planned to obtain the missing values from the authors. If these were not available, where there were missing measures of variance for continuous data, but an exact standard error (SE) and CIs available for group mean, and either P value or t value available for differences in mean, we would have calculated SDs according to the rules described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). When only the SE was reported, SDs would have been calculated by the formula SD = SE * √(n). The Cochrane Handbook for Systematic Reviews of Interventions presents detailed formulae for estimating SDs from P, t or F values, CIs, ranges or other statistics (Higgins 2011b). If these formulae did not apply, we planned to calculate the SDs according to a validated imputation method, based on the SDs of the other included studies (Furukawa 2006). Although some of these imputation strategies can introduce error, the alternative would be to exclude a given study’s outcome and thus to lose information. Nevertheless, we planned to examine the validity of the imputations in a sensitivity analysis that excluded imputed values.
3.3 Assumptions about participants who left the trials early or were lost to follow‐up
Various methods are available to account for participants who leave the trials early or are lost to follow‐up. Some trials just present the results of study completers; others use the method of last observation carried forward (LOCF); while more recently, methods such as multiple imputation or mixed‐effects models for repeated measurements (MMRM) have become more of a standard. While the latter methods seem to be somewhat better than LOCF (Leon 2006), we feel that the high percentage of participants leaving the studies early, and differences between groups in their reasons for doing so, are often the core problem in randomised schizophrenia trials. We therefore did not exclude studies based on the statistical approach they used. However, by preference we planned to use the more sophisticated approaches, i.e. we preferred to use MMRM or multiple‐imputation to LOCF, and we only presented completer analyses if ITT data were not available. Moreover, we addressed this issue in the item 'Incomplete outcome data' of the risk of bias tool.
Assessment of heterogeneity
1. Clinical heterogeneity
We considered all included studies initially, without seeing comparison data, to judge clinical heterogeneity. We simply inspected all studies for participants who were clearly outliers or situations that we had not predicted would arise and, where found, discussed such situations or participant groups.
2. Methodological heterogeneity
We considered all included studies initially, without seeing comparison data, to judge methodological heterogeneity. We simply inspected all studies for clearly outlying methods which we had not predicted would arise and discussed any such methodological outliers.
3. Statistical heterogeneity
3.1 Visual inspection
We inspected graphs visually to investigate the possibility of statistical heterogeneity.
3.2 Employing the I² statistic
We investigated heterogeneity between studies by considering the I² statistic alongside the Chi² P value. The I² statistic provides an estimate of the percentage of inconsistency thought to be due to chance (Higgins 2003). The importance of the observed value of I² depends on the magnitude and direction of effects as well as the strength of evidence for heterogeneity (e.g. P value from Chi² test, or a confidence interval for I²). We interpreted an I² estimate of 50% or more, accompanied by a statistically significant Chi² statistic, as evidence of substantial heterogeneity (Chapter 9. Cochrane Handbook for Systematic Reviews of Interventions) (Deeks 2011). When we identified substantial levels of heterogeneity in the primary outcome, we planned to explore the reasons for heterogeneity (Subgroup analysis and investigation of heterogeneity).
Assessment of reporting biases
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results (Egger 1997). These are described in section 10.1 of the Cochrane Handbook for Systemic reviews of Interventions (Sterne 2011).
1. Protocol versus full study
We tried to locate protocols of included randomised trials. If the protocol was available, we compared outcomes in the protocol and in the published report. If the protocol was not available, we compared the outcomes listed in the methods' section of the trial report with actually reported results.
2. Funnel plot
We are aware that funnel plots may be useful in investigating reporting biases but are of limited power to detect small‐study effects. We did not use funnel plots for outcomes where there were 10 or fewer studies, or where all studies were of similar size. In other cases, where funnel plots were possible, we planned to seek statistical advice in their interpretation.
Data synthesis
We understand that there is no closed argument for preference for use of fixed‐effect or random‐effects models. The random‐effects method incorporates an assumption that the different studies are estimating different, yet related, intervention effects. This often seems to be true to us, and the random‐effects model takes into account differences between studies, even if there is no statistically significant heterogeneity. There is, however, a disadvantage to the random‐effects model: it puts added weight onto small studies, which are often the most biased ones. Depending on the direction of effect, these studies can either inflate or deflate the effect size. When feasible (presence of at least two studies to allow for the estimation of between‐studies variability of effects) we used a random‐effects model for all analyses.
Subgroup analysis and investigation of heterogeneity
1. Subgroup analyses
1.1 Primary outcomes
If the necessary data were available, we planned to carry out subgroup analyses separately for three prespecified study categories:
the type of CBT intervention (e.g. categorisation of CBTs in terms of types, length and follow‐up of interventions);
the type of randomisation performed within studies (randomisation with parallel groups, cluster randomisation, or period randomisation for cross‐over studies);
the risk of bias (we assessed outcome results for studies at low risk of bias versus studies at unknown or high risk of bias for each primary outcome).
2. Investigation of heterogeneity
We planned to report if inconsistency was high. Firstly, we investigated whether we entered the data correctly. Secondly, if data were correct, we inspected the graph visually and removed outlying studies successively to see if homogeneity was restored. For this review we decided that should this occur with data contributing no more than 10% of the total weighting, we would leave these data in the analyses. If not, we did not pool these data and discussed any issues. We know of no supporting research for this 10% cut‐off, but are investigating the use of prediction intervals as an alternative to this unsatisfactory state.
When unanticipated clinical or methodological heterogeneity was obvious, we planned to simply state hypotheses regarding these for future reviews or versions of this review. We did not anticipate undertaking analyses relating to these.
Sensitivity analysis
We planned to carry out sensitivity analyses, for primary outcomes only, to explore the influence of the factors listed below. If there were substantial differences in the direction or precision of effect estimates in any of the sensitivity analyses listed below, we planned to remove data from the lower‐quality trials from analyses, present these data separately and discuss issues. Where there were no substantial differences in the direction or precision of effect estimates, we planned to keep data from the lower‐quality trials in the relevant analyses.
1. Assumptions for lost data
We planned to analyse the effects of including data where we made assumptions regarding lost data (see Dealing with missing data).
2. Risk of bias
We planned to analyse the effects of including data from trials that were at high risk of bias across one or more of the domains (see Assessment of risk of bias in included studies).
3. Imputed values
We planned to analyse the effects of including data from trials where we used imputed values for the intraclass correlation (ICC) to calculate the design effect in cluster‐randomised trials (see Unit of analysis issues).
4. Fixed‐ and random‐effects
We intended to synthesise data using random‐effects models; however, we planned to also synthesise data using fixed‐effect models, to evaluate whether this altered the size or direction of effect estimates.
Summary of findings and assessment of the certainty of the evidence
We used the GRADE approach to interpret findings (Schünemann 2011), and used GRADEpro GDT to export data from our review to create a summary of findings table. These tables provide outcome‐specific information concerning the overall certainty of evidence from each included study in the comparison, the magnitude of the effect of the interventions examined, and the sum of available data on all outcomes we rated as important to patient care and decision‐making. We aimed to select the following main outcomes for inclusion in the summary of findings table.
Aggression: frequency of aggressive episode
Agitation: frequency of agitation
Adverse effect or event: clinically important adverse effect or event
Self‐harm: frequency of self‐harm
Mental state: clinically important change in mental state
Leaving the study early for any reason
Quality of life: clinically important change in overall quality of life
If data were not available for these prespecified outcomes, but were available for ones that were similar, we planned to present the closest outcome to the prespecified one in the table but took this into account when grading the finding.
Our final summary of findings table includes the closest outcome reported to aggression. We did not find available information on the other main outcomes. Differences between protocol and review are reported in the Differences between protocol and review.
Results
Description of studies
For detailed descriptions of the studies, see Characteristics of included studies, and Characteristics of excluded studies.
Results of the search
The search, current to 18 January 2023, identified 22 records corresponding to 17 different studies. We excluded 15 studies with 18 references with reasons. We included two studies (four references) in the review (Figure 1).
1.
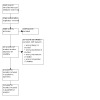
Included studies
1. Design and duration
The two studies included in this review (Cullen 2012; NCT03713398) used a randomised parallel‐group design. Cullen 2012 was conducted in the UK, it assessed outcomes at end of the trial at six months and at 12‐month follow‐up. NCT03713398 was conducted in the USA, it assessed outcomes at the end of the trial at three months and at six‐month follow‐up.
2. Participants
Cullen 2012 included men with schizophrenia (80% of the total sample), schizoaffective disorder, and other psychotic disorder, and used operational criteria for the diagnosis (Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM‐IV), International Classification of Disorders, tenth edition (ICD‐10). Mean age was 35.4 years (SD 10), and participants were people with a history of violent behaviour leading to confinement in medium‐secure forensic units. NCT03713398 included mainly men (72% of participants) 18 years or older, with diagnosis of schizophrenia, schizoaffective disorder, psychotic disorder, bipolar disorder with psychotic features or major depressive disorder with psychotic features as inclusion criteria. Participants were people in prison with moderate to high risk levels of criminogenic risk factors including aggression.
3. Size
Cullen 2012 included a total of 84 participants (44 allocated to the experimental reasoning and rehabilitation programme, 40 allocated to treatment as usual). NCT03713398 included a total of 100 participants (50 allocated to the experimental Thinking for a Change, 50 allocated to standard prison mental health services). Overall, 184 participants are included in this review.
4. Setting
Cullen 2012 conducted the study in medium‐secure forensic units of psychiatric hospitals. NCT03713398 conducted the study in prison.
5. Interventions
5.1 Cognitive behavioural therapy (CBT) plus standard care
Cullen 2012 applied a CBT program named "Reasoning and Rehabilitation". The program is a highly structured, manualised program including eight core modules targeting social problem‐solving skills and thinking styles. It was delivered by experienced staff who were trained in the program (Table 2). NCT03713398 applied a CBT program named "Thinking for a Change" that is a structured manualised program including modules on social skills training, cognitive restructuring, and problem‐solving methods (Table 3).
5.2 Standard care
Participants in the Cullen 2012 study received standard care defined as treatment as usual. All participants in the control group were free to receive any interventions considered to be part of their usual treatment. They were forbidden to attend the "Reasoning and Rehabilitation" sessions. Participants in the NCT03713398 study received standard care defined as standard prison mental health services.
TIDieR of interventions
Table 2 and Table 3 present the template for intervention description.
6. Outcomes
6.1 General
We found no data for the majority of the outcomes prespecified in the protocol of the review. Cullen 2012 reported the frequency over time of aggressive behaviours (physical violence and verbal aggression), which was a prespecified secondary outcome in the protocol of this review. No other reported efficacy outcome from Cullen 2012 fitted our list of primary or secondary outcomes. NCT03713398 reported the change in levels of the 12‐item Aggression Questionnaire ‐ Short Form that includes information on physical and verbal aggression, anger and hostility. No other reported efficacy outcome from NCT03713398 fitted our list of primary or secondary outcomes. We decided to include those secondary outcomes in this review and justify our decision in the Differences between protocol and review.
6.2 Outcome scales providing useable data
Cullen 2012 provided no summary data from outcome scales but estimates of incidence rate ratios (IRR) and their 95% CIs for events of physically violent behaviours and verbal aggressions. The study reports other scale measures not prespecified as outcomes to evaluate in this review (change in attitude to offending, response to frustration and aggressive response to everyday stress, attribute blame for criminal acts, problem‐solving style, change in risk of violence to others, change in emotional responses, change in irritability and anger to everyday stresses, change in probabilistic reasoning). NCT03713398 provided mean changes (and SDs) from baseline for the 12‐item Aggression Questionnaire ‐ Short Form. No other reported outcome measure was prespecified as outcomes to evaluate in this review (number of participants with post test behavioural infractions, median number of days in administrative segregation, change in overall interpersonal problem‐solving score, change in overall criminal attitudes score, change in overall impulsivity score).
6.3 Missing outcomes
There were not any other scale sincluding outcomes prespecified in our protocol.
Excluded studies
Excluded studies
We excluded 15 studies, including 18 references, with reasons. In most studies the cognitive intervention was not compared to standard care (ACTRN12613001126707; Ahmed 2015; Ahmed 2018; Ahmed 2019; Fleming 1982; Haddock 2009; Inchausti 2018; ISRCTN43585723; Khan 2022; Lindenmayer 2019; Moulden 2020; O'Reilly 2019; Putkonen 2013; Swanson 2006). ACTRN12613001126707 and Hodel 2003 are non‐randomised, non‐comparative studies. Fleming 1982 included children as participants who were not eligible for this review. ISRCTN43585723 included participants with a first episode of schizophrenia, but did not include the presence of aggressive behaviour. Moulden 2020 is a retrospective and descriptive study of a cohort of offender patients. O'Reilly 2019 excluded people judged too dangerous to participate because of positive symptoms combined with aggressive or self‐harming behaviour. In Putkonen 2013 , the intervention is non‐cognitive and directed to personnel. Swanson 2006 is a prevalence study of violent behaviour in people with schizophrenia.
Ongoing studies
There are no ongoing studies.
Awaiting assessment
There are no studies awaiting assessment.
Risk of bias in included studies
See also Figure 2 and Figure 3.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We considered Cullen 2012 at low risk on random sequence generation and allocation concealment. Participants in the study were randomly allocated to interventions with block randomisation stratified by centre, and only after randomisation was the research team informed of the allocation status for each participant. We considered NCT03713398 at low risk on random sequence generation and allocation concealment. The prison where the study was conducted did not allow electronic equipment to be brought into facilities, therefore the study team used shuffled envelops to randomise participants on site using computer‐generated random numbers and block randomisation procedures.
Blinding
We considered both studies at high risk. It was not possible to blind the delivered interventions to participants and personnel. And, even with outcome assessors blinded to treatment allocation, the main outcomes of interest were extracted from case notes of therapists not blinded to interventions (Cullen 2012) or from self‐reported scales from participants not blinded to interventions (NCT03713398).
Incomplete outcome data
We considered Cullen 2012 study at low risk, with very few dropouts with reasons. We considered NCT03713398 study at high risk since more than 50% of participants did not complete the study (52% in the experimental group, 54% in the control group).
Selective reporting
We considered Cullen 2012 at unclear risk since its protocol was retrospectively registered. We considered NCT03713398 at low risk since all prespecified outcomes were afterward reported.
Other potential sources of bias
We did not find other potential sources of bias.
Effects of interventions
See: Table 1
Reported Comparison: CBT plus standard care versus standard care
Only the outcomes with data available are reported below, any other outcome recorded in methods but not shown is not reported or available. See Table 1 for main outcomes.
Primary outcomes
1. Clinically important adverse effect or event
Cullen 2012 failed to report adverse effects in the published studies. NCT03713398 reported all‐cause mortality with zero events in both arms. See Analysis 1.1.
1.1. Analysis.

Comparison 1: CBT plus standard care versus standard care, Outcome 1: Clinically important adverse effect or event
Secondary outcomes
1. Frequency of aggressive episodes
1.1 Frequency of physical violence (3‐6 months)
Outcome assessed at the end of treatment. Results with very low certainty of evidence (1 study, 84 participants) suggest that CBT may result in little to no difference in the frequency of physical violence when compared with the control condition, the 95%confidence interval ( CI) is compatible with null effect (Incidence Rate Ratio (IRR)0.52, 95% CI 0.23 to 1.18). Participants allocated to CBT had 0.52 times fewer the rate of events compared to participants allocated to standard care (0.23 times fewer to 1.18 times more). See Analysis 1.2.
1.2. Analysis.

Comparison 1: CBT plus standard care versus standard care, Outcome 2: Frequency of physical violence (3‐6 months)
1.2 Frequency of physical violence (12 months)
Outcome assessed at follo‐ up. Results with very lo w certainty of evidence (1 study, 84 participants) suggest that CBT may result in little to no difference in the frequency of physical violence when compared with the control condition, the 95% CI is compatible with null effect (IRR 0.86, 95% CI 0.44 to 1.68). Participants allocated to CBT had 0.86 times fewer the rate of events compared to participants allocated to standard care (0.44 times fewer to 1.68 times more). See Analysis 1.3.
1.3. Analysis.

Comparison 1: CBT plus standard care versus standard care, Outcome 3: Frequency of physical violence (12 months)
1.3 Frequency of verbal aggression (3‐6 months)
Outcome assessed at the end of treatment. Results with very low certainty of evidence (1 study, 84 participants) suggest that CBT may reduce the frequency of verbal aggression when compared with the control condition (IRR 0.49, 95% CI 0.28 to 0.86). Participants allocated to CBT had 0.49 times fewer the rate of events compared to participants allocated to standard care (0.28 times fewer to 0.86 times fewer). See Analysis 1.4.
1.4. Analysis.

Comparison 1: CBT plus standard care versus standard care, Outcome 4: Frequency of verbal aggression (3‐6 months)
1.4 Frequency of verbal aggression (12 months)
Outcome assessed at follow up. Results with very low certainty of evidence (1 study, 84 participants) suggest that CBT may reduce the frequency of verbal aggression when compared with the control condition (IRR 0.56, 95% CI 0.34 to 0.92). Participants allocated to CBT had 0.56 fewer times the rate of events compared to participants allocated to standard care (0.34 times fewer to 0.92 times fewer). See Analysis 1.5.
1.5. Analysis.

Comparison 1: CBT plus standard care versus standard care, Outcome 5: Frequency of verbal aggression (12 months)
2. Average endpoint or change score on aggression scale
2.1 Change score on aggression scale (3 months)
Outcome assessed at the end of treatment. Results with very low certainty of evidence (1 study, 45 participants) suggest no difference between CBT and control condition on the total score on aggression scale (mean difference (MD) 2.13 lower over a 60 point range, 95% CI ‐8.59 to 4.33). See Analysis 1.6.
1.6. Analysis.

Comparison 1: CBT plus standard care versus standard care, Outcome 6: Change score on aggression scale (3 months)
2.2 Change score on aggression scale (6 months)
Outcome assessed at follow up. Results with very low certainty of evidence (1 study, 45 participants) suggest no difference between CBT and control condition on the total score on aggression scale (MD 3.95 lower over a 60 point range, 95% CI ‐11.1 to 3.2). See Analysis 1.7.
1.7. Analysis.

Comparison 1: CBT plus standard care versus standard care, Outcome 7: Change score on aggression scale (6 months)
3. Adverse effects
3.1 Death ‐ not suicide
NCT03713398 reported no deaths in both arms. See Analysis 1.8.
1.8. Analysis.

Comparison 1: CBT plus standard care versus standard care, Outcome 8: Death ‐ not suicide
3.2 Any general adverse effects
NCT03713398 reported no other adverse effects in both arms. See Analysis 1.9.
1.9. Analysis.

Comparison 1: CBT plus standard care versus standard care, Outcome 9: Any general adverse effects
4. Leaving the study early
4.1 For any reason
Results with low certainty of evidence (2 studies, 184 participants) suggest that CBT may result in little to no difference in leaving the study early for any reason when compared with the control condition (risk ratio (RR) 1.04, 95% CI 0.53 to 2.00). See Analysis 1.10.
1.10. Analysis.

Comparison 1: CBT plus standard care versus standard care, Outcome 10: Leaving the study early for any reason
Discussion
Summary of main results
This review presents the comparison of cognitive behavioural therapy(CBT) versus standard care on aggression exerted either as physical violence or as verbal aggression in people with schizophrenia. We have found very scarce evidence regarding efficacy with only two trials (Cullen 2012, NCT03713398) including 184 participants in total and conducted with people restrained in a forensic unit (Cullen 2012) or in prison (NCT03713398). Whereas the effect estimates favour CBT intervention to reduce verbal aggression and shows a favourable trend for CBT to reduce physical violence, the certainty of the evidence is very low due to performance and detection bias because of the lack of blinding, and imprecision. In summary, very limited current evidence supports CBT interventions to reduce physical and verbal aggressive behaviours in people with schizophrenia.
We have not found evidence regarding potential harms of the CBT intervention. NCT03713398 did not report any death or the presence of any general or serious adverse effects.
Overall completeness and applicability of evidence
A possible limitation of the review is that we did not do an extensive search of reference lists from the included studies or previous reviews. However, as this review is part of a current ongoing project that focuses on non‐pharmacological interventions in severe mental illness (see protocols in Moreno‐Calvete 2020; Moreno‐Calvete 2021, also described in the Open Science Framework https://osf.io/d56a2 and https://osf.io/myzd9), we consider it unlikely that we could have lost any relevant eligible trials.
The main limitation of this review concerns the small evidence with only two studies included (Cullen 2012, NCT03713398) with a relatively small size (84 and 100 participants, respectively) and outcomes assessed with different types of effects (incidence rate ratio and mean difference). These limitations preclude the generalisation of results and the replication of findings across trials. The results of this review are imprecise and limit the applicability of evidence to answer whether CBT might work for persistent aggressive behaviour or agitation in people with schizophrenia.
Quality of the evidence
We had planned to assess the efficacy and safety of CBT as compared with standard care for persistent aggressive behaviour in people with schizophrenia on a large set of primary and secondary outcomes related to aggression, agitation, self‐harm, injury to others, tranquillisation, global state, mental state, service use, adverse effects, leaving the study early, satisfaction with treatment, acceptance of treatment, quality of life, and economic outcomes. However, we identified only two eligible trials with outcomes restricted to a very few of our potential outcomes. The impossibility to blind the interventions to participants and personnel, as well as to outcome assessors, and the imprecision in the effect estimates resulting from the small sample sizes accrued in the included trials, explain the very low certainty of evidence attained in this review.
Potential biases in the review process
We have followed the methods expected in a Cochrane Review of interventions. After receiving the literature searches run by the Information Specialist of the Cochrane Schizophrenia Group, we did all further review processes prone to potential biases in duplicate: screening and selection of eligible studies, data extraction, assessment of the risk of bias, and grading the evidence. All discrepancies were resolved by discussion. We followed the methods stated in the published protocol with deviations from it noted in the Differences between protocol and review section. Therefore, we do not think there could be potential biases in the review process that invalidate the review findings or, at least, none we are aware of.
Agreements and disagreements with other studies or reviews
There are not many focussed reviews to which to compare our results. Most reviews on the efficacy of non‐pharmacological interventions for violence or aggression in severe mental illness do not focus on CBT as an intervention for people with schizophrenia (Du 2017; Gaynes 2016; Hockenhull 2012; Muralidharan 2006; Sailas 2000). Two narrative reviews by Rampling 2016 and Darmedru 2017 suggested that non‐pharmacological structured interventions, such as CBT, cognitive remediation and social cognitive training could decrease the severity and frequency of aggression (fewer incidents of physical or verbal aggression in the intervention group), but evidence of magnitude and direction of effect as well as the certainty of the evidence is currently lacking. We know of an umbrella review on interventions in general and forensic psychiatry on violence prevention that included five trials in a qualitative synthesis (Wolf 2017). However, we cannot compare our results with this overview since it is not focussed on severe mental illness and the intervention studies included non‐randomised as well as randomised controlled trials. A recent review (Slamaning 2021) includes three studies under the heading of Cognitive‐Behavioral Treatment Program, but two of them are not randomised and then do not fit our inclusion criteria and the third is the Cullen 2012 study already included in this review. A meta‐analysis focussing on the effectiveness of psychological and psychosocial interventions for forensic mental health inpatients (McIntosh 2021) quotes several studies which focus on aggression, however, the only study fitting our inclusion criteria is again Cullen 2012. We do not know of other focussed systematic reviews.
Authors' conclusions
Implications for practice.
1. For people with schizophrenia
People with schizophrenia or related disorders should be aware that the evidence we have found is of very low certainty and currently very limited evidence supports cognitive behavioural therapy (CBT) interventions to reduce physical or verbal aggressive behaviours.
2. For clinicians
Given the results of this systematic review and the very low certainty of the evidence, clinicians should understand that the current evidence is very limited to support CBT interventions to reduce physical and verbal aggressive behaviours in people with schizophrenia. We encourage clinicians working with people with schizophrenia and a history of aggressive behaviours to participate in randomised trials conducted in appropriate settings.
3. For policymakers
Given the very low certainty of evidence, the implementation of CBT interventions is not yet fully supported by the current evidence.
Implications for research.
1. General
Whereas the evidence might suggest the use of CBT may reduce aggressive behaviours in patients with schizophrenia, the grading of the certainty of the evidence is very low. It implies that there is not yet reliable evidence to guide clinical decisions and therefore more evidence is needed to get a more precise estimate of the effect of the intervention. Currently, we have very little confidence in the effect estimate, and the true effect could be substantially different from the estimate of effect.
2. Specific
More randomised controlled trials are required to reliably ascertain the effect of CBT on aggressive behaviours in people with schizophrenia. Future trials must be adequately powered to assess a variety of outcomes considered to be relevant to evaluate the use of CBT to reduce aggressive behaviours. These outcomes and the underlying measurement dimensions should be agreed by appropriate stakeholders (at least, people with schizophrenia, clinicians, and policymakers). Also, minimal important differences for measurement scales should be derived to better assess changes in aggressive behaviour. One suggested design for a study is outlined in Table 4.
3. Suggested design for future trial.
| Methods | Participants | Interventions | Outcomes |
| Allocation: randomised, clearly described | Diagnosis: people with schizophrenia or related disorders and history of aggressive behaviours | 1. CBT plus standard care1 | Aggression:
Agitation:
|
| Blinding: outcome assessors (due to nature of intervention) | N ~ 3002 | 2. Standard care3 | Self‐harm:
Adverse effects/events:
|
| Duration: 3 to 6 months (endpoint), 6 to 12 months (follow‐up) | Global state:
Leaving the study early:
Others:
|
CBT: cognitive behavioural therapy.
1Psychological therapy for cognitive restructure of thoughts, emotions and behaviours that usually includes cognitive therapy and elements of behavioural therapy delivered individually or concurrently
32 Powered to be able to identify a difference of ~20% between groups for primary outcome with adequate degree of certainty.
3Normal level of care participants would receive for their condition
What's new
| Date | Event | Description |
|---|---|---|
| 7 August 2023 | Amended | Typographical errors fixed in Abstract and Plain Language Summary |
History
Protocol first published: Issue 1, 2020 Review first published: Issue 7, 2023
| Date | Event | Description |
|---|---|---|
| 3 February 2020 | Amended | Search was done and 7 Studies (including 15 References) were sent to the authors. |
Acknowledgements
The Cochrane Schizophrenia Editorial Base is situated across the University of Melbourne, Australia, Technical University of Munich, Germany and the University of Nottingham, UK and produces and maintains standard text for use in the Methods section of their reviews. We have used this text as the basis of what appears here and adapted it as required.
The following people conducted the editorial process for this article:
Sign‐off Editor (final editorial decision): Irene Bighelli, Technical University of Munich
Managing Editor (selected peer reviewers, collated peer‐reviewer comments, provided editorial guidance to authors, edited the article): Hui Wu, Technical University of Munich
Contact Editor (provided editorial guidance to authors): Spyridon Siafis, Technical University of Munich
Copy Editor (copy‐editing and production): [NAME, AFFILIATION]
Information Specialist (search strategy and search results): Farhad Shokraneh, Systematic Review Consultants
The previous Cochrane Schizophrenia Group editorial base also supported this work: Co‐ordinating Editor, Clive Adams (before 2020), Managing Editor, Claire Irving (before 2020), Assistant Managing Editor, Ghazaleh Aali, University College London (before April 2021)
Peer‐reviewers* (provided comments and recommended an editorial decision): Alessandra Santoro (clinical/content review). The other content reviewer wished to remain anonymous.
*AS is a member of Cochrane Schizophrenia, and provided peer‐review comments on this article, but was not otherwise involved in the editorial process or decision‐making for this article.
Appendices
Appendix 1. Search strategy
| Database | Date range | Search date | # hits | # deduplicated |
| Cochrane Library CENTRAL | Complete database | 18012023 | 565 | 472 |
| Cochrane Library REVIEWS | Complete database | 18012023 | 55 | 51 |
| Embase Classic+Embase 1947 to 2023 January 13 | Complete database | 18012023 | 655 | 602 |
| Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process, In‐Data‐Review & Other Non‐Indexed Citations and Daily 1946 to January 13, 2023 | Complete database | 18012023 | 119 | 117 |
| APA PsycInfo 1806 to January Week 2 2023 | Complete database | 18012023 | 38 | 23 |
| 1432 | 1265 |
Cochrane Library (CENTRAL & REVIEWS)
#1 MeSH descriptor: [Clinical Trials as Topic] explode all trees
#2 MeSH descriptor: [Randomized Controlled Trial] explode all trees
#3 MeSH descriptor: [Double‐Blind Method] explode all trees
#4 MeSH descriptor: [Single‐Blind Method] explode all trees
#5 MeSH descriptor: [Cross‐Over Studies] explode all trees
#6 (randomized controlled trial):pt (Word variations have been searched)
#7 (controlled clinical trial):pt (Word variations have been searched)
#8 (clinical trial):pt (Word variations have been searched)
#9 ((clinic$ NEAR/2 trial)):ti,ab,kw (Word variations have been searched)
#10 ((random$ NEAR/5 control$ NEAR/5 trial$)):ti,ab,kw (Word variations have been searched)
#11 ((crossover or cross‐over)):ti,ab,kw (Word variations have been searched)
#12 (((singl$ or double$ or trebl$ or tripl$) NEAR (blind$ or mask$))):ti,ab,kw (Word variations have been searched)
#13 (randomi$):ti,ab,kw (Word variations have been searched)
#14 (random$ NEAR/5 (assign$ or allocat$ or assort$ or reciev$))
#15 {or #1‐#14}
#16 MeSH descriptor: [Schizophrenia] explode all trees
#17 MeSH descriptor: [Paranoid Disorders] explode all trees
#18 (schizo$):ti,ab,kw (Word variations have been searched)
#19 (hebephreni$):ti,ab,kw (Word variations have been searched)
#20 (oligophreni$):ti,ab,kw (Word variations have been searched)
#21 (psychotic$):ti,ab,kw (Word variations have been searched)
#22 (psychosis):ti,ab,kw (Word variations have been searched)
#23 (psychoses):ti,ab,kw (Word variations have been searched)
#24 (((chronic$ or sever$) NEAR/2 mental$ NEAR/2 (ill$ or disorder$))):ti,ab,kw (Word variations have been searched)
#25 MeSH descriptor: [Dyskinesia, Drug‐Induced] explode all trees
#26 MeSH descriptor: [Psychomotor Agitation] explode all trees
#27 MeSH descriptor: [Neuroleptic Malignant Syndrome] explode all trees
#28 MeSH descriptor: [Diagnosis, Dual (Psychiatry)] explode all trees
#29 ((tardiv$ NEAR dyskine$)):ti,ab,kw (Word variations have been searched)
#30 (akathisi$):ti,ab,kw (Word variations have been searched)
#31 (acathisi$):ti,ab,kw (Word variations have been searched)
#32 ((neuroleptic$ and (malignant NEAR/2 syndrome))):ti,ab,kw (Word variations have been searched)
#33 ((neuroleptic$ and (movement and disorder$))):ti,ab,kw (Word variations have been searched)
#34 (parkinsoni$):ti,ab,kw (Word variations have been searched)
#35 (neuroleptic‐induc$):ti,ab,kw (Word variations have been searched)
#36 {or #29‐#35}
#37 ((parkinson's NEAR/1 disease)):ti,ab,kw (Word variations have been searched)
#38 #36 NOT #37
#39 {or #16‐#28}
#40 #38 OR #39
#41 #15 AND #40
#42 MeSH descriptor: [Behavior Therapy] explode all trees
#43 ((behavior therapy) or (cognitive therapy) or (cognitive behavior therapy) or (cognitive behaviour therapy) or (behaviour therapy)):ti,ab,kw
#44 #42 OR #43
#45 MeSH descriptor: [Aggression] explode all trees
#46 MeSH descriptor: [Psychomotor Agitation] explode all trees
#47 MeSH descriptor: [Self‐Injurious Behavior] explode all trees
#48 ((aggress* OR agitat* OR harm* OR injur*)):ti,ab,kw
#49 {or #45‐#48}
#50 #41 AND #44 AND #49 in Trials
Embase
1. (schizo$ or psychotic$ or psychosis or psychoses).mp.
2. ((chronic$ or severe$ or persistent$) adj (mental$ or psychological$) adj (disorder$ or ill$)).mp.
3. exp schizophrenia/
4. exp psychosis/
5. mental patient/
6. (tardiv$ adj dyskine$).mp.
7. neuroleptic agent/
8. (neuroleptic$ and (malignant adj2 syndrome)).mp.
9. tardive dyskinesia/
10. akathisia/
11. exp neuroleptic malignant syndrome/
12. (neuroleptic$ and movement and disorder$).mp.
13. parkinsoni$.mp.
14. parkinson's.mp.
15. or/1‐14
16. 15 not parkinson's.ti.
17. (clin$ adj2 trial).mp.
18. ((singl$ or doubl$ or trebl$ or tripl$) adj (blind$ or mask$)).mp.
19. (random$ adj5 (assign$ or allocat$)).mp.
20. randomi$.mp.
21. crossover.mp.
22. exp randomized‐controlled‐trial/
23. exp double‐blind‐procedure/
24. exp crossover‐procedure/
25. exp single‐blind‐procedure/
26. exp randomization/
27. or/17‐26
28. and/16,27
29. exp Behavior Therapy/
30. (behavior therapy or cognitive therapy or cognitive behavior therapy or cognitive behaviour therapy or behaviour therapy).mp.
31. or/29‐30
32. exp Aggression/
33. exp Agitation/
34. exp automutilation/
35. (aggress* or agitat* or harm* or injur*).mp.
36. or/32‐35
37. 28 and 31 and 36
MEDLINE
1. randomized controlled trial.pt.
2. controlled clinical trial.pt.
3. randomized.ab.
4. placebo.ab.
5. clinical trials as topic.sh.
6. randomly.ab.
7. trial.ab.
8. or/1‐7
9. exp animals/ not humans.sh.
10. 8 not 9
11. exp SCHIZOPHRENIA/
12. exp Paranoid Disorders/
13. schizo$.mp.
14. hebephreni$.mp.
15. oligophreni$.mp.
16. psychotic$.mp.
17. psychosis.mp.
18. psychoses.mp.
19. ((chronic$ or sever$) adj2 mental$ adj2 (ill$ or disorder$)).mp.
20. exp dyskinesia, drug‐induced/
21. exp psychomotor agitation/
22. exp neuroleptic malignant syndrome/
23. exp "diagnosis, dual (psychiatry)"/
24. (tardiv$ adj dyskine$).mp.
25. akathisi$.mp.
26. acathisi$.mp.
27. (neuroleptic$ and (malignant adj2 syndrome)).mp.
28. (neuroleptic$ and (movement and disorder$)).mp.
29. parkinsoni$.mp.
30. neuroleptic‐induc$.mp.
31. or/24‐30
32. 31 not (parkinson's adj1 disease).ti.
33. or/11‐23
34. or/32‐33
35. and/10,34
36. exp Behavior Therapy/
37. (behavior therapy or cognitive therapy or cognitive behavior therapy or cognitive behaviour therapy or behaviour therapy).mp.
38. or/36‐37
39. exp Aggression/
40. exp Psychomotor Agitation/
41. exp Self‐Injurious Behavior/
42. (aggress* or agitat* or harm* or injur*).mp.
43. or/39‐42
44. 35 and 38 and 43
PsycINFO
1. randomi$.mp.
2. ((singl$ or doubl$ or trebl$ or tripl$) adj (blind$ or mask$)).mp.
3. placebo$.mp.
4. exp placebo/
5. crossover.mp.
6. exp treatment effectiveness evaluation/
7. exp mental health program evaluation/
8. (random$ adj (assign$ or allocate$)).mp.
9. or/1‐8
10. schizo$.mp.
11. hebephreni$.mp.
12. oligophreni$.mp.
13. psychotic$.mp.
14. psychosis.mp.
15. psychoses.mp.
16. ((chronic$ or sever$) adj2 mental$ adj2 (ill$ or disorder$)).mp.
17. exp psychosis/
18. exp schizophrenia/
19. exp schizoaffective disorder/
20. (tardiv$ adj dyskine$).mp.
21. akathisi$.mp.
22. acathisi$.mp.
23. (neuroleptic$ and (malignant adj2 syndrome)).mp.
24. (neuroleptic$ and (movement and disorder$)).mp.
25. exp neuroleptic malignant syndrome/
26. exp dyskinesia/
27. exp tardive dyskinesia/
28. exp akathisia/
29. neuroleptic‐induc$.mp.
30. parkinsoni$.mp.
31. parkinsonism.sh.
32. (parkinson's adj1 disease).ti.
33. or/10‐31
34. 33 not 32
35. 9 and 34
36. exp Behavior Therapy/
37. (behavior therapy or cognitive therapy or cognitive behavior therapy or cognitive behaviour therapy or behaviour therapy).mp.
38. or/36‐37
39. exp Aggressiveness/
40. exp Agitation/
41. exp Self‐Destructive Behavior/ or exp Self‐Injurious Behavior/
42. or/39‐41
43. and/35,38,42
Data and analyses
Comparison 1. CBT plus standard care versus standard care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Clinically important adverse effect or event | 1 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.2 Frequency of physical violence (3‐6 months) | 1 | Rate Ratio (IV, Random, 95% CI) | Totals not selected | |
| 1.3 Frequency of physical violence (12 months) | 1 | Rate Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 1.4 Frequency of verbal aggression (3‐6 months) | 1 | Rate Ratio (IV, Random, 95% CI) | Totals not selected | |
| 1.5 Frequency of verbal aggression (12 months) | 1 | Rate Ratio (IV, Random, 95% CI) | Totals not selected | |
| 1.6 Change score on aggression scale (3 months) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.7 Change score on aggression scale (6 months) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.8 Death ‐ not suicide | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.9 Any general adverse effects | 1 | 0 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.10 Leaving the study early for any reason | 2 | 184 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.53, 2.00] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cullen 2012.
| Study characteristics | ||
| Methods | Allocation: randomised Blindness: unblinded; researchers were not blind to group status. Duration: 3 to 4 months, further 12‐month follow‐up post treatmentdesign: superiority, parallel group, randomised trial, multisite Country: UK |
|
| Participants | Diagnosis: DSM‐IV or ICD‐10 criteria for schizophrenia (n = 69), schizoaffective disorder (n = 0) or other psychotic disorder ( = 5) N =84 Age: mean age 35.4 years (SD = 10) Sex: all participants were male. History: history of violent behaviour leading to the current admission Ethnicity: 50% African or African‐Caribbean, 32% white, 18% other Setting: medium‐secure forensic units in psychiatric hospitals Exclusion: having participated in R&R or a similar programme previously, actively psychotic, presence of significant cognitive impairments, and not having sufficient proficiency in English |
|
| Interventions |
|
|
| Outcomes | Short term (end of treatment:3 to 4 months):
Medium term (12‐month post‐treatment):
|
|
| Notes | Trial registration: ISRCTN 46561083 Funding: NHS National Research and Development programme on Forensic Mental Health, UK Declaration of interest: none Contact of authors: not pursued; the study was reported extensively in 3 independent publications. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Within each site, participants were randomly allocated (1:1) to interventions with block randomisation stratified by centre using equal block sizes. |
| Allocation concealment (selection bias) | Low risk | Randomisation was done for a yet recruited cohort which controlled for allocation concealment. Only after randomisation, the research team of each unit were informed of the allocation status for each participant. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The interventions delivered in this study do not permit to blind them to participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Researchers were not blind to allocation status, as this was often revealed in the clinical notes or by the patients themselves. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall, the attrition rate was low for the outcomes of interest; 42 of 44 participants in the R&R group provided information at end of treatment (4.5% attrition), whereas all 40 participants randomised to TAU provided information (0% attrition). At 12‐month post‐treatment, 42 of 44 participants in the R&R group provided information (4.5% attrition) as well as 38 of 40 participants in the TAU group (5% attrition). |
| Selective reporting (reporting bias) | Unclear risk | This study has a retrospective trial registration, and this makes unclear if all prespecified outcomes have been reported. |
| Other bias | Low risk | No other source of bias was identified. |
NCT03713398.
| Study characteristics | ||
| Methods | Open‐label, parallel, randomised clinical trial | |
| Participants | Male and female prison inmates, 18 years and older, with moderate to high risk levels of criminogenic risk factors and a diagnosis of schizophrenia, schizoaffective disorder, psychotic disorder, bipolar disorder with psychotic features or major depressive disorder with psychotic features | |
| Interventions | 1. T4C 2. Standard prison mental health services Intervention will be delivered over a 3‐month period. |
|
| Outcomes | Primary outcomes:
Secondary outcomes:
|
|
| Notes | ClinicalTrials.gov Identifier: NCT03713398 Funding: R34MH111855 (U.S. NIH Grant/Contract) Declaration of interest: none Contact of authors: PI was contacted on issues regarding administration of the experimental intervention and risk of bias. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The author gave us the following explanation on randomisation: "In this study randomization will take place on‐site at the prison, which does not allow electronic equipment to be brought into facilities. Therefore, the study team will use shuffled envelopes to randomize participants on‐site at the prison. The study team will use a table of computer‐generated random numbers and block randomization procedures to ensure an equal distribution of subjects to each arm of the study". |
| Allocation concealment (selection bias) | Low risk | Use of shuffled envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial; masking was not possible. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessments are based on unblinded participant reported outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High level of attrition in both treatment arms. More than 50% of participants who started the trial did not complete it. |
| Selective reporting (reporting bias) | Low risk | All registered outcomes are reported. |
| Other bias | Low risk | None found |
DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders, fourth edition; ICD: International Classification of Diseases; R&R: reasoning and rehabilitation; T4C: Thinking for a Change; TAU: treatment as usual.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ACTRN12613001126707 | Ineligible design: nonrandomised noncomparative study |
| Ahmed 2015 | Ineligible comparator: the intervention is not compared to standard care (computer games control activities) |
| Ahmed 2018 | Ineligible comparator: the intervention is not compared to standard care (cognitive remediation training). |
| Ahmed 2019 | Ineligible comparator: the intervention is not compared to standard care (computerised cognitive remediation). |
| Fleming 1982 | Ineligible population: aggressive children in residential treatment |
| Haddock 2009 | Ineligible comparator: the intervention is not compared to standard care (social activity therapy). |
| Hodel 2003 | Ineligible design: nonrandomised noncomparative study |
| Inchausti 2018 | Ineligible comparator: the intervention is not compared to standard care (conventional social skills training). |
| ISRCTN43585723 | Ineligible population: the study includes participants with a first episode of schizophrenia but does not include the presence of agitation or aggressive behavior. |
| Khan 2022 | Ineligible comparator: the intervention is not compared to standard care (cognitive remediation plus a computer‐based control). |
| Lindenmayer 2019 | Ineligible comparator: the intervention is not compared to standard care (cognitive remediation training). |
| Moulden 2020 | Ineligible design: retrospective and descriptive study |
| O'Reilly 2019 | Ineligible population: people judged too dangerous to participate because of positive symptoms combined with aggressive or self‐harming behaviour in the last month were excluded. |
| Putkonen 2013 | Ineligible intervention: the intervention is directed to personnel to prevent seclusion and restraint. |
| Swanson 2006 | Ineligible design: prevalence study of violent behaviour in people with schizophrenia. |
Differences between protocol and review
We did not consider the Incidence Rate Ratio (IRR) as an effect measure in the protocol of this review. The results reported in the Cullen 2012 study did not allow us to estimate the prespecified dichotomous or numeric outcomes. However, the report of overall frequencies of aggression and estimates of population‐time did allow us to estimate IRRs (ratio of event counts to total person‐time of follow‐up) to compare intervention arms.
The protocol included seven outcomes to report in the summary of findings table. However, information on most of them was not available. The included studies reported primarily physical violence and verbal aggression at the end of the intervention and at follow‐up, and we used this information as the closest to the prespecified outcome: aggression ‐ frequency of aggressive episode. Explicitly, we included (i) the frequency of aggressive episodes over six months as the outcome to report in the table of summary of findings as well as the mean change score at three months from a self‐reported aggression scale. The frequency of aggressive episodes over 12 months of follow‐up, the frequency of episodes of verbal aggression (at six and 12 months), and the mean change score at six months in the self‐reported aggression scale were reported in full in the results section.
Contributions of authors
Javier Ballesteros: wrote the protocol; screened the literature for eligibility; did data extraction and management; assessed risk of bias; did the statistical analyses and drafted the text of the review.
María Concepción Moreno‐Calvete: reviewed and drafted parts of the protocol; screened the literature for eligibility and management; assessed risk of bias and drafted the text of the review.
Eduardo González‐Fraile: reviewed and drafted parts of the protocol; screened the literature for eligibility; did data extraction and management; assessed risk of bias and drafted the text of the review.
Borja Santos: reviewed and drafted parts of the protocol; did data extraction and management and participated in the statistical analyses.
Sources of support
Internal sources
-
University of the Basque Country, UPV/EHU (Leioa), Spain
Grants UFI 11/35, GIU 14/27, PPGA18/03
-
Department of Mental Health, Basque Health Service (Bilbao), Spain
Institutional
-
International University of La Rioja (Logroño), Spain
Institutional
-
National Institute for Health and Care Research (NIHR), UK
provided funding for Cochrane Schizophrenia Group
External sources
-
Department of Health of the Government of the Basque Country, Spain
Grant number 2017111101
Declarations of interest
Javier Ballesteros: none known
María Concepción Moreno‐Calvete: none known
Eduardo González‐Fraile: none known
Borja Santos: none known
Edited (no change to conclusions)
References
References to studies included in this review
Cullen 2012 {published data only}
- Cullen AE, Clarke AY, Kuipers E, Hodgins S, Dean K, Fahy T. A multi-site randomized controlled trial of a cognitive skills programme for male mentally disordered offenders: ocial-cognitive outcomes. Psychological Medicine 2012;42(3):557-69. [DOI] [PubMed] [Google Scholar]
- Cullen AE, Clarke AY, Kuipers E, Hodgins S, Dean K, Fahy T. A multisite randomized trial of a cognitive skills program for male mentally disordered offenders: violence and antisocial behavior outcomes. Journal of Consulting and Clinical Psychology 2012;80(6):1114-20. [DOI] [PubMed] [Google Scholar]
- Cullen AE, Soria C, Clarke AY, Dean K, Fahy T. Factors predicting dropout from the reasoning and rehabilitation program with mentally disordered offenders. Criminal Justice and Behavior 2011;38(3):217-30. [Google Scholar]
NCT03713398 {unpublished data only}
- NCT03713398. Mental health services for prisoners with SMI. https://ClinicalTrials.gov/show/NCT03713398 (first received 19 October 2018).
References to studies excluded from this review
ACTRN12613001126707 {unpublished data only}https://www.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12613001126707
- ACTRN12613001126707. The effects of a psychological intervention for delusions on reducing aggression in schizophrenia patients in a forensic psychiatric setting. ACTRN12613001126707 (first received 18 January 2023).
Ahmed 2015 {published data only}
- Ahmed AO, Hunter KM, Goodrum NM, Batten N-J, Birgenheir D, Hardison E, et al. A randomised study of cognitive remediation for forensic and mental health patients with schizophrenia. Journal of Psychiatric Research 2015;68:8-18. [DOI] [PubMed] [Google Scholar]
Ahmed 2018 {published data only}
- Ahmed A, Hoptman M, Lindenmayer J-P. Can cognitive training decrease reactive aggression in schizophrenia? In: Schizophrenia Bulletin. Vol. 44 (Suppl 1). 2018:S175. [DOI: 10.1093/schbul/sby016.429] [DOI]
Ahmed 2019 {published data only}
- Ahmed A, Kirstie-Kulsa M, Hefner A, Jones O, Ljuri I, Budgazad M, et al. Cognitive training for impulsive aggression in schizophrenia. In: Schizophrenia Bulletin. Vol. Suppl 2. 2019:S271-2. [DOI: 10.1093/schbul/sbz018.455] [DOI]
Fleming 1982 {published data only}
- Fleming CC. Evaluation of an anger management program with aggressive children in residential treatment [Dissertation]. 1982.
Haddock 2009 {published data only}
- Haddock G, Barrowclough C, Shaw JJ, Dunn G, Novaco RW, Tarrier N. Cognitive-behavioural therapy v. social activity therapy for people with psychosis and a history of violence: randomised controlled trial. British Journal of Psychiatry 2009;194(2):152-7. [DOI] [PubMed] [Google Scholar]
- Haddock G. Cognitive behaviour therapy for schizophrenia and violence (the PICASSO project). National Research Register 2004;3.
Hodel 2003 {published data only}
- Hodel B, West A. A cognitive training for mentally ill offenders with treatment-resistant schizophrenia. The Journal of Forensic Psychiatry & Psychology 2003;14(3):554-68. [DOI: 10.1080/1478994031000152745] [DOI] [Google Scholar]
Inchausti 2018 {published data only}
- Inchausti F, García-Poveda NV, Ballesteros-Prados A, Ortuño-Sierra J, Sánchez-Reales S, Prado-Abril J, et al. The effects of metacognition-oriented social skills training on psychosocial outcome in schizophrenia-spectrum disorders: a randomized controlled trial. Schizophrenia Bulletin 2018;44(6):1235-44. [DOI: 10.1093/schbul/sbx168] [DOI] [PMC free article] [PubMed] [Google Scholar]
ISRCTN43585723 {published data only}
- ISRCTN43585723. Promoting personal recovery from psychosis: a randomised controlled trial in first episode schizophrenia. www.isrctn.com/ISRCTN43585723 (first received 23 January 2004).
- Jackson C. Promoting personal recovery from psychosis: a randomised control trial in first episode schizophrenia. National Research Register 2003.
Khan 2022 {published data only}
- Khan A, Lindenmayer JP, Insel B, Seddo M, Demirli E, DeFazio K, et al. Computerized cognitive and social cognition training in schizophrenia for impulsive aggression. Schizophrenia Research 2022;s256:117-25. [DOI: 10.1016/j.schres.2022.11.004] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lindenmayer 2019 {published data only}
- Lindenmayer J-P, Khan A, Ljuri I, Jones O, Yoon J, Hefner A, et al. Cognitive training for social cognition in impulsive aggression in schizophrenia. In: Schizophrenia Bulletin. Vol. Suppl 2. 2019:S217-8. [DOI: 10.1093/schbul/sbz019.317] [DOI]
Moulden 2020 {published data only}
- Moulden HM, Mamak M, Chaimowitz G. A preliminary evaluation of the effectiveness of dialectical behaviour therapy in a forensic psychiatric setting. Criminal Behaviour and Mental Health 2020;30(2-3):141-50. [DOI] [PubMed] [Google Scholar]
O'Reilly 2019 {published data only}
- NCT02360813. Cognitive remediation therapy within a secure forensic setting. https://clinicaltrials.gov/ct2/show/NCT02360813.
- O'Reilly K, Donohoe G, O'Sullivan D, Coyle C, Corvin A, O'Flynn P, et al. A randomized controlled trial of cognitive remediation for a national cohort of forensic patients with schizophrenia or schizoaffective disorder. BMC Psychiatry 2019;19(1):27. [DOI: 10.1186/s1288-019-2018-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly K, Donohoe G, O'Sullivan D, Coyle C, Mullaney R, O'Connell P, et al. Study protocol: a randomised controlled trial of cognitive remediation for a national cohort of forensic mental health patients with schizophrenia or schizoaffective disorder. BMC Psychiatry 2016;16(5):5. [DOI: 10.1186/s12888-016-0707-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
Putkonen 2013 {published data only}
- Putkonen A, Kuivalainen S, Louheranta O, Repo-Tiihonen E, Ryynänen O-P, Kautiainen H, et al. Cluster-randomized controlled trial of reducing seclusion and restraint in secured care of men with schizophrenia. Psychiatric Services 2013;64(9):850-5. [DOI: 10.1176/appi.ps.201200393] [DOI] [PubMed] [Google Scholar]
Swanson 2006 {published data only}
- Swanson JW, Swartz MS, Van Dorn RA, Elbogen EB, Wagner HR, Rosenheck RA, et al. A national study of violent behavior in persons with schizophrenia. Archives of General Psychiatry 2006;63:490-9. [DOI] [PubMed] [Google Scholar]
Additional references
Ahmed 2010
- Ahmed U, Jones H, Adams CE. Chorpromazine for psychosis induced aggression or agitation. Cochrane Database of Systematic Reviews 2010, Issue 4. Art. No: CD007445. [DOI: 10.1002/14651858.CD007445.pub2] [DOI] [PubMed] [Google Scholar]
Ali 2015
- Ali A, Hall I, Blickwedel J, Hassiotis A. Behavioural and cognitive-behavioural interventions for outwardly-directed aggressive behaviour in people with intellectual disabilities. Cochrane Database of Systematic Reviews 2015, Issue 4. Art. No: CD003406. [DOI: 10.1002/14651858.CD003406.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Altman 1996
- Altman DG, Bland JM. Detecting skewness from summary information. BMJ 1996;313(7066):1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
APA 2013
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fifth edition. Arlington, VA: American Psychiatric Association, 2013. [Google Scholar]
Beck 1976
- Beck AT. Cognitive therapy and the emotional disorders. London: Penguin, 1976. [Google Scholar]
Bighelli 2018
- Bighelli I, Salanti G, Huhn M, Schneider-Thoma J, Krause M, Reitmeir C, Wallis S, Schwermann F, Pitschel-Walz G, Barbui C, Furukawa TA, Leucht S. Psychological interventions to reduce positive symptoms in schizophrenia: systematic review and network meta-analysis. World Psychiatry 2018;17:316-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bighelli 2022
- Bighelli I, Wallis S, Reitmeir C, Schwermann F, Salahuddin NH, Leucht S. Effects of psychological treatments on functioning in people with schizophrenia: a systematic review and meta-analysis of randomized controlled trials. European Archives of Psychiatry and Clinical Neuroscience 2022;273(4):779-810. [DOI: 10.1007/s00406-022-01526-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bland 1997
- Bland JM. Statistics notes. Trials randomised in clusters. BMJ 1997;315(7108):600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boissel 1999
- Boissel JP, Cucherat M, Li W, Chatellier G, Gueyffier F, Buyse M, et al. The problem of therapeutic efficacy indices. 3. Comparison of the indices and their use [Aperçu sur la problématique des indices d'efficacité thérapeutique, 3: comparaison des indices et utilisation. Groupe d'Etude des Indices D'efficacite]. Therapie 1999;54(4):405-11. [PMID: ] [PubMed] [Google Scholar]
Chong 2016
- Chong HY, Teoh SL, Wu DB, Kotirum S, Chiou CF, Chaiyakunapruk N. Global economic burden of schizophrenia: a systematic review. Neuropsychiatric Disease and Treatment 2016;12:357-73. [DOI: 10.2147/NDT.996649] [DOI] [PMC free article] [PubMed] [Google Scholar]
Darmedru 2017
- Darmedru C, Demily C, Franck N. Cognitive remediation and social cognitive training for violence in schizophrenia: a systematic review. Psychiatry Research 2017;251:266-74. [DOI: 10.1016/j.psychres.2016.12.062] [DOI] [PubMed] [Google Scholar]
Deeks 2000
- Deeks J. Issues in the selection for meta-analyses of binary data. In: 8th International Cochrane Colloquium; 2000 Oct 25-28; Cape Town. Cape Town (South Africa): The Cochrane Collaboration, 2000.
Deeks 2011
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Divine 1992
- Divine GW, Brown JT, Frazier LM. The unit of analysis error in studies about physicians' patient care behavior. Journal of General Internal Medicine 1992;7(6):623-9. [DOI] [PubMed] [Google Scholar]
Donner 2002
- Donner A, Klar N. Issues in the meta-analysis of cluster randomized trials. Statistics in Medicine 2002;21(19):2971-80. [DOI] [PubMed] [Google Scholar]
Du 2017
- Du M, Wang X, Yin S, Shu W, Hao R, Zhao S, et al. De-escalation techniques for psychosis-induced aggression or agitation. Cochrane Database of Systematic Reviews 2017, Issue 4. Art. No: CD009922. [DOI: 10.1002/14651858.CD009922.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7109):629-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne D, Altman DG, Higgins JP, Curtina F, Worthingtond HV, Vaile A. Meta-analyses involving cross-over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140-9. [DOI] [PubMed] [Google Scholar]
Fazel 2009
- Fazel S, Gulati G, Linsell L, Geddes JR, Grann M. Schizophrenia and violence: systematic review and meta-analysis. PLoS Medicine 2009;6(8):e1000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fuller 2003
- Fuller RL, Schultz SK, Andreasen NC. The symptoms of schizophrenia. In: Hirsch SR, Weinberger D, editors(s). Schizophrenia. Second edition. Oxford, UK: Blackwell Science Ltd, 2003:25-33. [Google Scholar]
Furukawa 2006
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta-analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59(1):7-10. [DOI] [PubMed] [Google Scholar]
Garriga 2016
- Garriga M, Pacchiarotti I, Kasper S, Zeller SL, Allen MH, Vázquez G, et al. Assessment and management of agitation in psychiatry: expert consensus. The World Journal of Biological Psychiatry 2016;17(2):86-128. [DOI: 10.3109/15622975.2015.1132007] [DOI] [PubMed] [Google Scholar]
Gaynes 2016
- Gaynes BN, Brown C, Lux LJ, Brownley K, Van Dorn R, Edlund M, et al. Strategies to de-escalate aggressive behavior in psychiatric patients. Comparative Effectiveness Review 2016;180:16-EHC032-EF. [www.effectivehealthcare.ahrq.gov/reports/final.cfm] [PubMed] [Google Scholar]
Grant 2005
- Grant P, Young PR, DeRubeis RJ. Cognitive and behavioral therapies. In: Gabbard GO, Beck JS, Holmes J, editors(s). Oxford Textbook of Psychotherapy. Oxford (UK): Oxford University Press, 2005:15-25. [Google Scholar]
Gulliford 1999
- Gulliford MC. Components of variance and intraclass correlations for the design of community-based surveys and intervention studies: data from the Health Survey for England 1994. American Journal of Epidemiology 1999;149(9):876-83. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Higgins 2011b
- Higgins JP, Green S, editor(s). Chapter 7: Selecting studies and collecting data. In: Higgins JP, Green S, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Hockenhull 2012
- Hockenhull JC, Whittington R, Leitner M, Barr W, McGuire J, Cherry MG, et al. A systematic review of prevention and intervention strategies for populations at high risk of engaging in violent behaviour: update 2002-8. Health Technology Assessment 2012;16(3):1-152. [DOI: 10.3310/hta16030] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hoffmann 2014
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. British Medical Journal 2014;348:g1687. [DOI: 10.1136/bmj.g1687] [DOI] [PubMed] [Google Scholar]
Hofmann 2012
- Hofmann SG, Asnaani A, Vonk IJ, Sawyer AT, Fang A. The efficacy of cognitive behavioral therapy: a review of meta-analyses. Cognitive Therapy Research 2012;36:427-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hutton 2009
- Hutton JL. Number needed to treat and number needed to harm are not the best way to report and assess the results of randomised clinical trials. British Journal of Haematology 2009;146(1):27-30. [PMID: ] [DOI] [PubMed] [Google Scholar]
Jablensky 2000
- Jablensky A. Epidemiology of schizophrenia: the global burden of disease and disability. European Archives of Psychiatry and Clinical Neurosciences 2000;250:274-85. [DOI] [PubMed] [Google Scholar]
Jauhar 2014
- Jauhar S, McKenna J, Radua J, Fung E, Salvador R, Laws KR. Cognitive-behavioural therapy for the symptoms of schizophrenia: systematic review and meta-analysis with examination of potential bias. British Journal of Psychiatry 2014;204:20-9. [DOI: 10.1192/bjp.bp.112.116285] [DOI] [PubMed] [Google Scholar]
Jones 2018a
- Jones C, Hacker D, Xia J, Meaden A, Irving CB, Zhao S, et al. Cognitive behavioural therapy plus standard care versus standard care for people with schizophrenia. Cochrane Database of Systematic Reviews 2018, Issue 12. Art. No: CD007964. [DOI: 10.1002/14651858.CD007964.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 2018b
- Jones C, Hacker D, Xia J, Meaden A, Irving CB, Zhao S, et al. Cognitive behavioural therapy plus standard care versus standard care plus other psychosocial treatments for people with schizophrenia. Cochrane Database of Systematic Reviews 2018, Issue 11. Art. No: CD008712. [DOI: 10.1002/14651858.CD008712.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kay 1986
- Kay SR, Opler LA, Fiszbein A. Positive and Negative Syndrome Scale (PANSS) Manual. North Tonawanda, NY: Multi-Health Systems, 1986. [Google Scholar]
Khushu 2016
- Khushu A, Powney MJ. Haloperidol for long-term aggression in psychosis. Cochrane Database of Systematic Reviews 2016, Issue 11. Art. No: CD009830. [DOI: 10.1002/14651858.CD009830.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2019
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M, et al. Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors(s). Cochrane Handbook for Systematic Reviews of Interventions. 2 edition. John Wiley and Sons, 2019:67-107. [DOI: 10.1002/9781119536604.ch4] [DOI] [Google Scholar]
Leon 2006
- Leon AC, Mallinckrodt CH, Chuang-Stein C, Archibald DG, Archer GE, Chartier K. Attrition in randomized controlled clinical trials: methodological issues in psychopharmacology. Biological Psychiatry 2006;59(11):1001-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Leucht 2005a
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel RR. What does the PANSS mean? Schizophrenia Research 2005;79(2-3):231-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Leucht 2005b
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel R. Clinical implications of brief psychiatric rating scale scores. British Journal of Psychiatry 2005;187:366-71. [PMID: ] [DOI] [PubMed] [Google Scholar]
Marshall 2000
- Marshall M, Lockwood A, Bradley C, Adams C, Joy C, Fenton M. Unpublished rating scales: a major source of bias in randomised controlled trials of treatments for schizophrenia. British Journal of Psychiatry 2000;176:249-52. [DOI] [PubMed] [Google Scholar]
McIntosh 2021
- McIntosh LG, Janes S, O'Rourke S, Thomson LDG. Effectiveness of psychological and psychosocial interventions for forensic mental health inpatients a meta-analysis. Aggression and Violent Behavior 2021;58:101551. [DOI: 10.1016/j.avb.2021.101551] [DOI] [Google Scholar]
Moreno‐Calvete 2020
- Moreno-Calvete MC, Ruiz-Ibañez I, Uriarte-Uriarte JJ. Scoping review protocol of non-pharmacological interventions for interpersonal and self-directed violence in adults with severe mental illness. BMJ Open 2020;10:e37006. [DOI: 10.1136/bmjopen-2020-037006] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moreno‐Calvete 2021
- Moreno-Calvete MC, Ballesteros-Rodríguez FJ. Non-pharmacological strategies for self-directed and interpersonal violence in people with severe mental illness: a rapid overview of systematic reviews (protocol). BMJ Open 2021;11:e043576. [DOI: 10.1136/bmjopen-2020-043576] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moreno‐Küstner 2018
- Moreno-Küstner B, Martín C, Pastor L. Prevalence of psychotic disorders and its association with methodological issues. A systematic review and meta-analyses. PLoS One 2018;13(4):e0195687. [DOI] [PMC free article] [PubMed] [Google Scholar]
Muralidharan 2006
- Muralidharan S, Fenton M. Containment strategies for people with serious mental illness. Cochrane Database of Systematic Reviews 2006, Issue 3. Art. No: CD002084. [DOI: 10.1002/14651858.CD002084.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Naeem 2015
- Naeem F, Farooq S, Kingdon D. Cognitive behavioural therapy (brief versus standard duration) for schizophrenia. Cochrane Database of Systematic Reviews 2015, Issue 10. Art. No: CD010646. [DOI: 10.1002/14651858.CD010646.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2014
- NICE. Psychosis and schizophrenia in adults: prevention and management. NICE clinical guideline 2014. [www.nice.org.uk/guidance/cg178] [PubMed]
Nowak 2016
- Nowak I, Sabariego C, Świtaj P, Anczewska M. Disability and recovery in schizophrenia: a systematic review of cognitive behavioral therapy interventions. BMC Psychiatry 2016;16:228. [DOI: 10.1186/s12888-016-0912-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
Olfson 2015
- Olfson M, Gerhard T, Huang C, Crystal S, Stroup TS. Premature mortality among adults with schizophrenia in the United States. JAMA Psychiatry 2015;72:1172-81. [DOI] [PubMed] [Google Scholar]
Ostinelli 2017
- Ostinelli EG, Brooke-Powney MJ, Li X, Adams CE. Haloperidol for psychosis-induced aggression or agitation (rapid tranquillisation). Cochrane Database of Systematic Reviews 2017, Issue 7. Art. No: CD009377. [DOI: 10.1002/14651858.CD009377.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Overall 1962
- Overall JE, Gorham DR. The brief psychiatric rating scale. Psychological Reports 1962;10:799-812. [Google Scholar]
Paris 2021
- Paris G, Bighelli I, Deste G, Siafis S, Schneider-Thoma J, Zhu Y, Davis JM, Vita A, Leucht S. Short-acting intramuscular second-generation antipsychotic drugs for acutely agitated patients with schizophrenia spectrum disorders. A systematic review and network meta-analysis. Schizophrenia Research 2021;229:3-11. [DOI: 10.1016/j.schres.2021.01.021] [DOI] [PubMed] [Google Scholar]
Rampling 2016
- Rampling J, Furtado V, Winsper C, Marwaha S, Lucca G, Livanou M, et al. Non-pharmacological interventions for reducing aggression and violence in serious mental illness: a systematic review and narrative synthesis. European Psychiatry 2016;34:17-28. [DOI] [PubMed] [Google Scholar]
Rathod 2010
- Rathod S, Phiri P, Kingdon D. Cognitive behavioral therapy for schizophrenia. Psychiatric Clinics of North America 2010;33(3):527-36. [DOI] [PubMed] [Google Scholar]
Roberts 2021
- Roberts MT, Shokraneh F, Sun Y, Groom M, Adams CE. Classification of psychotherapy interventions for people with schizophrenia: development of the Nottingham Classification of Psychotherapies. Evidence-Based Mental Health 2021;24:62-9. [DOI: 10.1136/ebmental-2020-300151] [DOI] [PMC free article] [PubMed] [Google Scholar]
Saha 2005
- Saha S, Chant D, Welham J, McGrath J. A systematic review of the prevalence of schizophrenia. PLoS Medicine 2005;2(5):e141. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sailas 2000
- Sailas E, Fenton M. Seclusion and restraint for people with serious mental illnesses. Cochrane Database of Systematic Reviews 2000, Issue 2. Art. No: CD001163. [DOI: 10.1002/14651858.CD001163] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In Higgins JP, Green S, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Serper 2011
- Serper MR. Aggression in schizophrenia. Schizophrenia Bulletin 2011;37(5):897-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shokraneh 2017
- Shokraneh F, Adams CE. Study-based registers of randomized controlled trials: starting a systematic review with data extraction or meta-analysis. BioImpacts 2017;7(4):209-17. [DOI: 10.15171/bi.2017.25] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shokraneh 2019
- Shokraneh F, Adams CE. Study-based registers reduce waste in systematic reviewing: discussion and case report. Systematic Reviews 2019;8:129. [DOI: 10.1186/s13643-019-1035-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shokraneh 2020
- Shokraneh F, Adams CE. Cochrane Schizophrenia Group’s Study-Based Register of Randomized Controlled Trials: Development and Content Analysis. Schizophrenia Bulletin Open 2020;1:sgaa061. [DOI: 10.1093/schizbullopen/sgaa061] [DOI] [Google Scholar]
Shokraneh 2021
- Shokraneh F, Adams CE. Classification of all pharmacological interventions tested in trials relevant to people with schizophrenia: study-based analysis. Health Information and Libraries Journal 2021;In Press:In Press. [DOI: 10.1111/hir.12366] [DOI] [PubMed] [Google Scholar]
Slamaning 2021
- Slamaning R, Reisegger A, Winkler H, Girolamo G, Carrà G, Crocamo C, et al. A systematic review of non-pharmacological strategies to reduce the risk of violence in patients with schizophrenia spectrum disorders in forensic settings. Frontiers in Psychiatry 2021;12:618860. [DOI: 10.3389/fpsyt.2021.618860] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D, editor(s). Chapter 10: Addressing reporting biases. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Intervention. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Ukoumunne 1999
- Ukoumunne OC, Gulliford MC, Chinn S, Sterne JA, Burney PG. Methods for evaluating area-wide and organisation-based intervention in health and health care: a systematic review. Health Technology Assessment 1999;3(5):iii-92. [PubMed] [Google Scholar]
Walsh 2002
- Walsh E, Fahy T. Violence in society. Contribution of mental illness is low. BMJ 2002;325:507-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Whiteford 2015
- Whiteford HA, Ferrari AJ, Degenhardt L, Feigin V, Vos T. The global burden of mental, neurological and substance use disorders: an analysis from the Global Burden of Disease Study 2010. PLOS One 2015;10(2):e0116820. [DOI: 10.1371/journal.pone.0116820] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wolf 2017
- Wolf A, Whiting D, Fazel S. Violence prevention in psychiatry: un umbrella review of interventions in general and forensic psychiatry. Journal of Forensic Psychiatry and Psychology 2017;28(5):659-73. [DOI: 10.1080/14789949.2017.1284886] [DOI] [PMC free article] [PubMed] [Google Scholar]
Xia 2009
- Xia J, Adams CE, Bhagat N, Bhagat V, Bhoopathi P, El-Sayeh H, et al. Loss to outcomes stakeholder survey: the LOSS study. Psychiatric Bulletin 2009;33(7):254-7. [Google Scholar]
Zaman 2017
- Zaman H, Sampson SJ, Beck AL, Sharma T, Clay FJ, Spyridi S, et al. Benzodiazepines for psychosis-induced aggression or agitation. Cochrane Database of Systematic Reviews 2017, Issue 12. Art. No: CD003079. [DOI: 10.1002/14651858.CD003079.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Ballesteros 2020
- Ballesteros J, Moreno-Calvete MC, Gonzalez-Fraile E, Santos B. Cognitive behavioural therapy plus standard care versus standard care for persistent aggressive behaviour or agitation in people with schizophrenia (Protocol). Cochrane Database of Systematic Reviews 2020;1:CD013511. [DOI: 10.1002/14651858.CD013511] [DOI] [PMC free article] [PubMed] [Google Scholar]


