Abstract
Chemical bath deposition (CBD) has been demonstrated as a remarkable technology to fabricate high‐quality SnO2 electron transport layer (ETL) for large‐area perovskite solar cells (PSCs). However, surface defects always exist on the SnO2 film coated by the CBD process, impairing the devices’ performance. Here, a facile periodic acid post‐treatment (PAPT) method is developed to modify the SnO2 layer. Periodic acid can react with hydroxyl groups on the surface of SnO2 films and oxidize Tin(II) oxide to Tin(IV) oxide. With the help of periodic acid, a better energy level alignment between the SnO2 and perovskite layers is achieved. In addition, the PAPT method inhibits interfacial nonradiative recombination and facilitates charge transportation. Such a multifunctional strategy enables to fabricate PSC with a champion power conversion efficiency (PCE) of 22.25%, which remains 93.32% of its initial efficiency after 3000 h without any encapsulation. Furthermore, 3 × 3 cm2 perovskite mini‐modules are presented, achieving a champion efficiency of 18.10%. All these results suggest that the PAPT method is promising for promoting the commercial application of large‐area PSCs.
Keywords: chemical bath deposition, large area, periodic acid, perovskite solar cells, SnO2
Chemical bath deposition (CBD) is extraordinarily suitable for depositing large‐area, dense and uniform SnO2 films, which is beneficial for the future commercialization of perovskite solar cells. A periodic acid post‐treatment is developed to modify the CBD‐SnO2 films. Periodic acid can not only react with the hydroxyl groups on the surface of SnO2 but also oxidize the Sn(II) to Sn(IV).
![]()
1. Introduction
Perovskite solar cells (PSCs) have made great progress over the past ten years,[ 1 , 2 , 3 ] while the highest certified power conversion efficiency (PCE) exceeded 25% recently.[ 4 ] A typical planar PSC device includes an electron transport layer (ETL), perovskite layer, hole transport layer (HTL), and electrodes. ETL plays a critical role in extracting and transporting the photogenerated electrons as well as blocking holes to reduce charge recombination.[ 5 ] Therefore, the ETL's intrinsic optical and electronic properties will influence the photovoltaic performance and stability of a PSC. TiO2 is one of the most popular ETLs in PSCs due to its favorable electronic and optical properties. For a long period, the compact TiO2 (c‐TiO2)/mesoporous TiO2 (mp‐TiO2) stack occupies an important position in high‐efficiency PSCs.[ 6 , 7 ] However, TiO2 still has a few disadvantages, such as relatively low bulk electron mobility (<1 cm2 V−1 s−1), high processing temperature, and high photocatalytic activity, which may decrease the long‐term stability of PSCs.[ 8 ] Recently, SnO2 has been considered as a promising ETL material owing to its outstanding bulk electron mobility (≈250 cm2 V−1 s−1), low‐temperature processability (<200 °C), and superb long‐term operational stability.[ 9 ] Spin coating is the most typical technique to deposit SnO2 thin films. In 2015, Fang and co‐workers used solution‐processed SnO2 as ETL material of PSCs for the first time, achieving a champion PCE of 17.21%. They synthesized SnO2 films by a facile sol–gel approach: spin coating SnCl2·2H2O precursor solutions and followed by thermal annealing at 180 °C.[ 10 ] Later, much work reported the deposition of synthesized SnO2 nanoparticles (NPs) or quantum dots (QDs) to form SnO2 films with better crystallization and less recombination centers.[ 11 ] You and co‐workers used commercial SnO2 colloidal precursor solutions to fabricate a dense and pinhole‐free SnO2 film. The PSCs based on this low‐temperature solution‐processed SnO2 film achieved a certified PCE of 19.9%.[ 12 ]
Chemical bath deposition (CBD) is another good technique for fabricating SnO2 thin films. Different from the spin‐coating method, the CBD approach is extraordinarily suitable for depositing large‐area, dense and uniform SnO2 films without any area limits.[ 13 ] It is very suitable for the future commercialization of perovskite solar cells. In 2016, Correa‐Baena and co‐workers firstly introduced the CBD technique for depositing the SnO2 layer in planar PSCs, which yielded a high open‐circuit voltage of 1.21 V at a bandgap of 1.62 eV, and achieved a stabilized PCE of 20.7%.[ 14 ] Recently, Seo and co‐workers fabricated highly efficient PSCs based on CBD‐coated SnO2, with a certified PCE of 25.2%, indicating that CBD‐SnO2 could potentially improve photovoltaic performances.[ 15 ] However, surface defects, such as oxygen vacancies and hydroxyl groups, always exist on the surface of CBD‐coated SnO2, which not only increase the non‐radiative recombination and impair the device's performances but also accelerate the degradation of the devices.[ 16 ] Sargent and co‐workers reported surface modification of CBD‐SnO2 by ammonium fluoride, leading to reduced defects sites and better energy level match. This treatment achieved higher open circuit voltages and a champion PCE of 23.2%.[ 17 ] Qi and co‐workers reported the incorporation of KMnO4 into the CBD process of SnO2 films. The strong oxidizing nature of KMnO4 promoted the conversion from Sn(II) to Sn(IV), leading to reduced trap defects and higher carrier mobility of SnO2. In addition, K+ ions and Mn2+ ions improved perovskite films’ crystallinity and phase stability.[ 16 ]
In this work, we developed a periodic acid post‐treatment (PAPT) method to modify the CBD‐SnO2 films. Periodic acid (H5IO6), with acidity and strong oxidizing properties, shows a bifunctional effect on SnO2 layers. It can not only react with the hydroxyl groups on the surface of SnO2 films but also oxidize the Tin(II) oxide (SnO) to Tin(IV) oxide (SnO2). With the help of periodic acid, a better energy level alignment between the SnO2 and perovskite layers and reduced defect sites in the SnO2 layer were achieved simultaneously. Furthermore, we investigated the effect of periodic acid on the photovoltaic performances and stability of the corresponding perovskite devices.
2. Results and Discussions
Figure 1 shows the schematic illustration of SnO2 films fabricated by the chemical bath deposition and PAPT method. Etched FTO glasses were immersed in dilute SnCl2 aqueous solution with urea, thioglycolic acid, and hydrochloric acid, and then heated at 70 °C for 4 h. For PAPT‐modified substrates, they were subsequently immersed in periodic acid solution with different concentrations for 5 min. After that, the substrates were thoroughly washed with deionized water to remove the residual reagents and annealed at 150 °C for 40 min.
Figure 1.
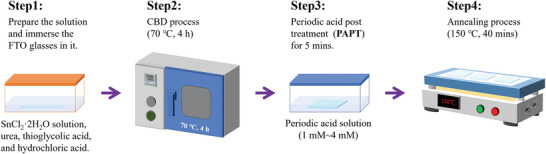
Schematic illustration of SnO2 films fabricated by the CBD process and PAPT method.
To study the treatment mechanism of periodic acid to SnO2 films, we conducted X‐ray photoelectron spectroscopy (XPS) measurements. For the Sn 3d XPS spectra (Figure 2a), the two peaks of Sn 3d5/2 and 3d3/2 in pristine SnO2 shifted from 487.2 and 495.6 eV to the higher binding energies of 487.6 and 496.1 eV, with the spin–orbit splitting energy of 8.4 eV.[ 18 ] The higher binding energy of Sn 3d indicates more Sn (II)’s conversion to Sn (IV),[ 16 , 19 ] potentially widening the band gap and decreasing the number of oxygen vacancies.[ 20 , 21 ] In terms of O 1s spectra (Figure 2b), two peaks of around 532.5 and 531.1 eV refer to the hydroxyl group (–OH) on the SnO2 surface and the saturated lattice oxygen in SnO2 film, respectively.[ 17 , 20 ] The calculated areas of the –OH in pristine SnO2 reduced from 82.9% to 35.3% in the PAPT‐modified SnO2, indicating more chemisorbed non‐lattice oxygen transferred to lattice oxygen and existed in the form of SnO2. It is known that surface –OH introduces a deep energy level into the bandgap that is near the valence band, bringing the generation of non‐radiative recombination and energy losses in the devices.[ 22 ] As shown in Figure 2c, two typical peaks of I 3d orbitals appeared in the PAPT‐modified SnO2 sample. In contrast, the pristine SnO2 sample showed no signal, indicating iodide remained on the PAPT‐modified SnO2 surface by chemical doping. The two peaks of I 3d5/2 and 3d3/2 located at 619.8 and 631.3 eV refer to iodine anion (I−), which were consistent with the referenced SnI2 signals in the previous reports.[ 23 , 24 ] According to the XPS results of Sn 3d and O 1s, we believe that the PAPT treatment urges the transformation of Sn(II) to Sn(IV) and the conversion of chemically absorbed hydroxyl to lattice oxygen, which lead to reduced trap states and potentially higher V OC and FF of the PSCs.
Figure 2.
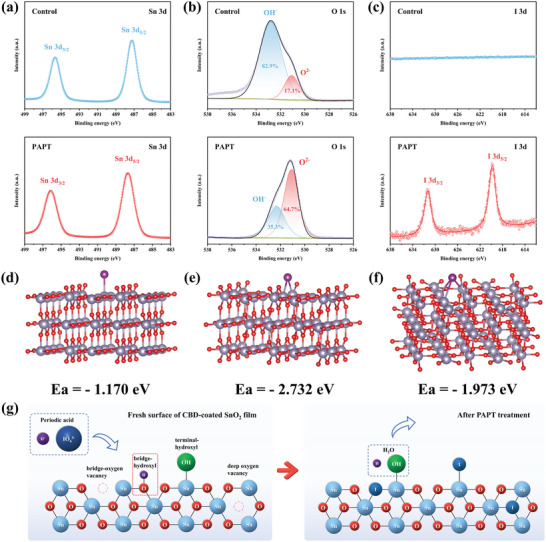
XPS spectra of a) Sn 3d, b) O 1s, and c) I 3d for the pristine SnO2 and PAPT‐modified SnO2 films. Hollow circle: raw data. Line: fitted data. All the SnO2 films were prepared via the CBD method. Absorption energy and corresponding structures. d) Iodine atom absorbed at the terminal Sn site. e) The iodine atom replaces bridge O site (iodine fills in the bridge oxygen vacancy). f) The iodine atom fills in the deep oxygen vacancy. g) Schematic image of the PAPT modification on the SnO2 surface.
As previously reported,[ 17 , 22 ] four kinds of oxygen defects exist on the surface of the CBD‐SnO2 films, including two kinds of hydroxyl defects and two kinds of oxygen vacancy defects (V O): 1) terminal hydroxyl (OHT), which is bonded to a single Sn atom on the surface (Figure S1a, Supporting Information); 2) bridge hydroxyl (OHB), including a bridge oxygen and an absorbed hydrogen atom (Figure S1b, Supporting Information); 3) bridge‐oxygen vacancy (V OB), i.e., the missing of the bridge oxygen atom (Figure S1c, Supporting Information); 4) deep oxygen vacancy (V OD), i.e., the missing of another oxygen atom located at a deeper layer than bridge oxygen (Figure S1d, Supporting Information). To figure out if the iodine atom will remain at the hydroxyl site or fill the V O, we conducted density functional theory (DFT) calculations. The stoichiometric (110) lattice plane was chosen as the studied surface due to that it is the most thermodynamically stable configuration.[ 12 , 25 ] According to the results of DFT calculations, the absorption energies (E a) of the iodine atom absorbed on terminal Sn (Figure 2d) and bridge O site (Figure 2e) are −1.170 and −2.732 eV. The negative value of E a indicates that the iodine atom is prone to be absorbed in the same site after the hydroxyl groups were eliminated by periodic acid. Also, the more negative E a implies iodine atoms are more apt to be absorbed in the bridge oxygen sites. As for V O, the E a of the iodine atom substituted the bridge V OB is exactly the same as previously calculated results, which is −2.732 eV. And the E a of the iodine atom substituted at V OD (−1.973 eV) showed a similar result (Figure 2f), indicating that iodine atoms can effectively passivate the V O defects on the SnO2 surface. Above all, the schematic image of the PAPT method is shown in Figure 2g.
From the above analysis, we found that periodic acid has the following three functions: 1) H+ can decrease the surface hydroxyl groups; 2) The strong oxidation of can oxidize Sn2+ into Sn4+; 3) The reduced I− can also reduce the surface –OH and fill in the oxygen vacancies. To figure out which one plays a more critical role, we treated SnO2 films with sulfuric acid (H2SO4) and hydroiodic acid (HI), respectively. It should be noted that the concentrations of H2SO4 and HI are 5 and 2 mm, respectively, which are consistent with the H+ and I− concentrations in the optimal concentration of periodic acid (2 mm, which will be discussed later).
XPS spectra of SnO2 films treated by H2SO4 and HI are shown in Figure S2 (Supporting Information). The double peaks of the Sn 3d of both samples are located at 487.2 and 495.6 eV (Figure S2a, Supporting Information), which is consistent with the position of the control sample (Figure 2a), indicating that a small amount of Sn2+ may exist in the SnO2 films. It is because dilute H2SO4 and HI cannot oxidize Sn2+ into Sn4+. As shown in Figure S2b (Supporting Information), the O 1s peak can be divided into two peaks corresponding to –OH and lattice oxygen, respectively. It can be seen that the –OH peak area of H2SO4‐modified SnO2 samples reduces from 82.9% to 40.1%, while that of HI‐modified samples only reduces it to 53.2%, indicating that H+ is more effective for eliminating the surface –OH than I−. Finally, we observed weak I 3d double peaks on the surface of the HI‐treated sample (Figure S2c, Supporting Information), the intensity of which is much lower than that of the H5IO6‐treated sample, suggesting that more iodine atoms in periodic acid stay on the SnO2 films. Above all, periodic acid has a stronger ability to eliminate surface oxygen defects than H2SO4 and HI. Furthermore, we carried out XPS depth analysis measurements to study the oxygen defects inside the SnO2 film. The etching depth is around 10 nm. It can be seen that the peak of O 1s is located around 531.1 eV (Figure S3, Supporting Information), corresponding to the position of lattice oxygen, while no –OH peaks are observed. In addition, I 3d double peaks are observed in PAPT‐modified SnO2 samples, indicating that only the periodic acid can fill the oxygen vacancies inside the SnO2 films.
Ultraviolet photoelectron spectroscopy (UPS) measurements were performed to investigate the influence of energy level by periodic acid. The valence band maximum (VBM) values of samples can be calculated by the formula of VBM = 21.22 eV − E cutoff + E onset.[ 26 ] As shown in Figure 3a, the corresponding VBM values of pristine SnO2 and PAPT‐modified SnO2 are 8.20 and 8.12 eV, respectively. In combination with the optical bandgap values of samples (Figure 3b), the conduction band minimum (CBM) values of pristine SnO2 and PAPT‐modified SnO2 are 4.30 and 4.19 eV, respectively. Similarly, the perovskite's CBM and optical bandgap can be calculated as 4.15 eV (Figure S4, Supporting Information) and 1.56 eV (Figure S5, Supporting Information), respectively. Therefore, the schematic energy level diagram of FTO, the pristine SnO2, PAPT‐modified SnO2, and perovskite is shown in Figure 3c. A better energy band alignment between the PAPT‐modified SnO2 and perovskite layers was achieved due to the upshift of the conduction band and Fermi level of SnO2. As a result, a reduced energy barrier and faster electron extraction at the SnO2 and perovskite interface could be realized.[ 27 ]
Figure 3.
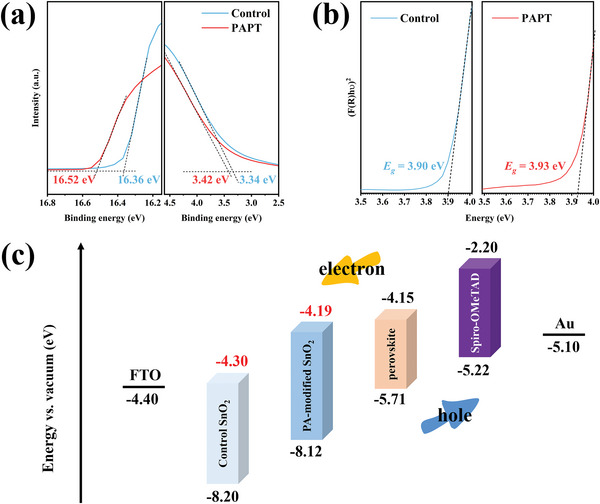
a) UPS spectra and b) UV–vis reflection spectra of the pristine SnO2 and PAPT‐modified SnO2 films. c) Schematic energy level diagram of FTO, the pristine SnO2, PAPT‐modified SnO2, and perovskite.
Grazing incidence X‐ray diffraction (GIXRD) was conducted to investigate the change in the crystal structure of the SnO2 films. As shown in Figure 4a, several peaks of the PAPT‐modified SnO2 film are slightly higher than that of the pristine sample, indicating a better crystallinity was obtained after PAPT treatment. We carried out electron conductivity and space‐charge‐limited current (SCLC) measurements of devices based on the FTO/SnO2/Au structure, as shown in Figure 4b and Figure S6 (Supporting Information). The PAPT‐modified SnO2 film exhibit higher conductivity and electron mobility than those of the pristine SnO2 film, suggesting the PAPT treatment could improve the electron transport of the SnO2 films. As shown in Figure 4c, Hall effect measurements demonstrate a similar result. The PAPT‐modified SnO2 film showed a higher hall mobility µ H (3.07 × 10−3 m2 V−1 s−1) and larger carrier concentration n (5.63 × 1027 m−3) than those of the pristine SnO2 film (µ H = 2.88 × 10−3 m2 V−1 s−1 and n = 5.36 × 1027 m−3).
Figure 4.
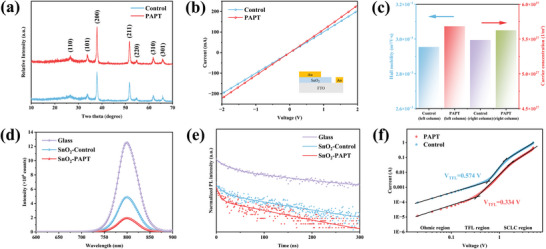
a) Grazing incidence X‐ray diffraction (GIXRD) spectra of the pristine SnO2 and PAPT‐modified SnO2 films. b) I–V curves of the devices based on the FTO/SnO2/Au structure for evaluating the conductivity of the pristine SnO2 and PAPT‐modified SnO2 films. c) Hall mobility and carrier concentration results of the pristine SnO2 and PAPT‐modified SnO2 films. d) Steady‐state PL spectra and e) Time‐resolved PL (TRPL) spectra of the perovskite films on glass, the pristine SnO2 and PAPT‐modified SnO2 films. f) Dark J–V characteristics of the electron‐only devices based on different SnO2 layer. (The device structure: FTO/SnO2/perovskite/PCBM/Ag).
According to the UV–visible light transmittance spectra of SnO2 films (Figure S7, Supporting Information), the PAPT treatment would not influence the high visible light transmission of SnO2 films. Atomic force microscopy (AFM) measurements were carried out to investigate the surface morphology of the chemical‐bath deposited SnO2 layers (Figure S8, Supporting Information). The PAPT‐modified SnO2 film showed a relatively lower roughness with a root mean square (RMS) of 9.91 nm compared to the pristine SnO2 film (RMS = 11.08 nm), indicating the PAPT treatment would not impair the crystal growth process of the upper‐level perovskite layers. Scanning electron microscope (SEM) of perovskite films (Figure S9, Supporting Information) deposited on the surface of the pristine and PAPT‐modified SnO2 films could also prove this point of view.
Steady‐state photoluminescence (PL) and time‐resolved PL (TRPL) measurements were carried out to study the charge transfer dynamics at the SnO2/perovskite interfaces. As shown in Figure 4d, the perovskite film on the PAPT‐modified SnO2 film exhibited only 23.1% PL intensity of that of the perovskite on the pristine SnO2 film. It indicates more efficient charge extraction of PAPT‐modified SnO2 film, owing to the better energy level match. For the TRPL spectra, both samples showed bi‐exponential decay curves (Figure 4e). The average carrier lifetime (τ avg) was fitted and listed in Table S1 (Supporting Information). The perovskite film deposited on the PAPT‐modified SnO2 film exhibited the τ avg of 50.55 ns, which is about 60% shorter than that of the perovskite film on the pristine SnO2 film, 82.70 ns. It also suggests faster electron collection and transportation.
We also estimated the trap density by measuring the dark current density–voltage (J–V) curves based on electron‐only devices (FTO/SnO2/perovskite/PCBM/Ag). The typical dark J–V curves can be divided into three regions: ohmic region, trap‐filling limited (TFL) region, and trap‐free space charge limited current region. The electron defect density (N t) can be estimated by the trap‐filling limit voltage (V TFL).[ 25 , 28 ] The electron‐only devices based on the PAPT‐modified SnO2 film obtained a much lower V TFL (0.334 V) and N t (3.51 × 1015 cm−3) compared to those based on the pristine SnO2 film (V TFL = 0.574 V and N t = 6.04 × 1015 cm−3), as shown in Figure 4f. The fitting parameters (R 2) were listed in Table S2 (Supporting Information). Obviously, the PAPT treatment helps reduce the electron defect density and facilitates the charge transport between the SnO2 and perovskite films.
To investigate the photovoltaic performances of PSCs with the PAPT‐modified SnO2 films, we fabricated devices with a structure of FTO/SnO2/perovskite/OAI/Spiro‐OMeTAD/Au, as shown in Figure 5a,b. After careful optimization of the periodic acid concentration (1–4 mm) (Table S3, Supporting Information), an optimal concentration of the PAPT treatment (2 mm) led to a champion PCE of 22.25% (Figure 5c), with a J SC, V OC, and FF of 25.02 mA cm−2, 1.09 V, and 81.55%, respectively. Meanwhile, PSCs based on the pristine SnO2 layer only achieved a champion PCE of 19.77% with a J SC, V OC, and FF of 25.09 mA cm−2, 1.06 V, and 74.28%, respectively. The external quantum efficiency (EQE) spectrum of PSCs based on the PAPT‐modified SnO2 film led to an integrated J SC of 22.45 mA cm−2, as shown in Figure 5d. The steady‐state PCE of the champion PSC is measured to be 22.10% under a constant bias voltage of 0.949 V (Figure 5e). Statistic distributions of the PCE, FF, and V OC of the PSCs based on different concentrations of periodic acid are shown in Figure 5f,g and Figure S10 (Supporting Information).
Figure 5.
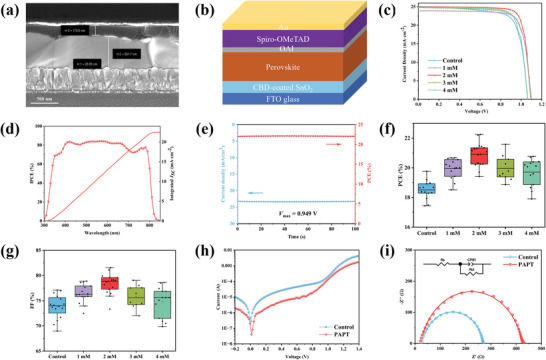
a) The cross‐section SEM image of the fabricated PSCs. b) The structure schematic image of a typical PSC device. (The device structure: FTO/PAPT‐modified SnO2/perovskite/OAI/Spiro‐OMeTAD/Au). c) J–V curves of the champion PSCs based on the different amounts of periodic acid treatments. d) EQE spectra for the PSCs based on the PAPT‐modified SnO2 film. e) Steady‐state photocurrent measurement of the champion PSC based on the PAPT‐modified SnO2 film. The applied bias voltage was 0.949 V, which was the voltage at the maximum power point. f) PCE and g) FF statistic distributions of the PSCs with different amounts of periodic acid treatments. h) Dark I–V curves of the PSCs based on the pristine SnO2 and PAPT‐modified SnO2 films. i) Nyquist plots of the PSCs based on the pristine SnO2 and PAPT‐modified SnO2 films, which was measured at a bias potential of 1.0 V in the frequency range of 1 MHz to 0.1 Hz in the dark.
The PCE enhancement is mainly attributed to the increment of FF and V OC, which benefited from the interface modification by the periodic acid treatment. In addition, we investigated the role of periodic acid on the transport properties in PSCs by dark I‐V characterization. As shown in Figure 5h, PAPT‐modified devices exhibited a significantly lower dark current, indicating the reduction of leakage current between the SnO2/perovskite interface, which could be attributed to the higher compactness and uniformity of the PAPT‐modified SnO2 films.[ 25 ] Moreover, we conducted electrochemical impedance spectroscopy (EIS) to investigate the charge transfer and recombination processes in the PSCs. Figure 5i shows the Nyquist plots of devices based on the pristine SnO2 and PAPT‐modified SnO2 films, measured at a bias potential of −1.0 V under dark conditions. It is worth mentioning that the semicircle at the high‐frequency region representing the charge transfer resistance (R ct) was too small to be identified.[ 29 ] Therefore, only one semicircle at the low‐frequency region was observed from the Nyquist plots, reflecting the interfacial recombination resistance (R rec) between the SnO2 layer and the perovskite layer. According to the fitting results (Table S4, Supporting Information) employing the equivalent circuit (inset of Figure 5h), the R rec value increased from 240.6 to 399.7 Ω after the PAPT modification. The higher R rec indicates a lower charge recombination rate,[ 29 ] thus contributing to the improved FF and V OC. In addition, the fitted series resistance (R S) values are 29.49 and 23.96 Ω for PSCs based on the pristine SnO2 and PAPT‐modified SnO2 films, respectively. The smaller R S is ascribed to increased conductivity of the SnO2 layer, consistent with the previous measurement. We also performed the electric capacity–voltage (C–V) measurement to analyze the built‐in potential (V bi) by the Mott–Schottky equation. As shown in Figure S11 (Supporting Information), the PAPT‐modified device displayed a higher V bi (0.94 V) than the control device (0.77 V), indicating more efficient charge separation and collection and, thus, higher V OC.[ 30 ] Drive‐level capacitance profiling (DLCP) measurement was conducted to obtain spatial distributions of trap densities in SnO2 films.[ 31 , 32 ] As shown in Figure S12 (Supporting Information), the trap density in PAPT‐SnO2 is relatively lower than that of the control‐SnO2 film, indicating PAPT modification is beneficial for reducing the defect density in the SnO2 layer. All these characterization results suggest the PAPT modification optimizes the interfacial charge transportation and inhibits the interfacial recombination between the ETL and perovskite layers, leading to better photovoltaic performances.
Finally, we fabricated the laser‐etched perovskite solar mini‐modules (PSMs) with an area of 3 × 3 cm2 using a slot‐die coating method, as shown in Figure 6a. The champion PSM based on the PAPT‐modified SnO2 film achieved an excellent PCE of 18.10% (J SC = 7.16 mA cm−2, V OC = 3.45 V, and FF = 73.42%) with an aperture area of 2.29 cm2, while the control one only achieved a PCE of 16.13% (J SC = 7.08 mA cm−2, V OC = 3.35 V, and FF = 68.02%). These results reveal that the CBD process with the periodic acid treatment is quite suitable for large‐area production of PSCs and PSMs. Next, we evaluated the stabilities of PSCs according to the International Summit on Organic PV Stability (ISOS) protocols.[ 33 ] As shown in Figure 6b, the unencapsulated device based on the PAPT‐modified SnO2 film remained at 93.32% of its initial PCE after 3000 h of exposure to a relative humidity of 20–25% at 20 °C in an ambient atmosphere (conform to ISOS‐D‐1 procedure). The control device only kept 29.79% of its original PCE under the same conditions. The excellent stability of PAPT‐modified devices could be ascribed to the more compact and smoother interface between the SnO2 and perovskite films, with fewer defects and channels for moisture.
Figure 6.
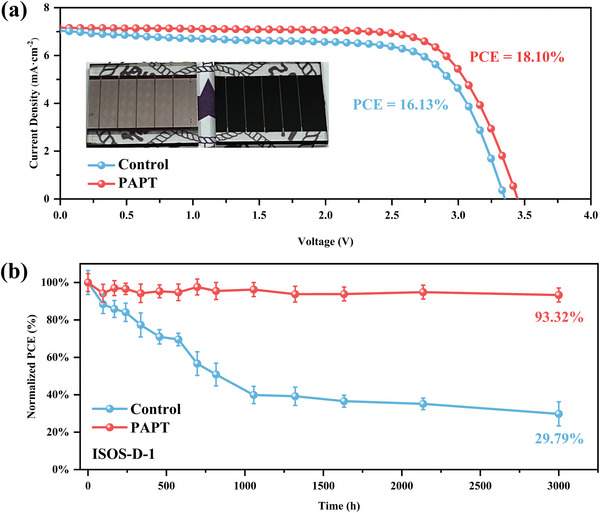
a) The photograph and the of the J–V curves of 3 × 3 cm2 modules based on control and PAPT‐modified SnO2 films. b) Stability tests of PSCs stored in 20 °C, 20–25% RH for 3000 h (ISOS‐D‐1 procedure).
3. Conclusions
In summary, we developed a powerful periodic acid post‐treatment (PAPT) method to modify the SnO2 films fabricated by the CBD process. According to XPS results, it is found that periodic acid promoted the transformation of Sn(II) to Sn(IV) and the conversion of chemically absorbed hydroxyl to lattice oxygen, which could lead to reduced trap states and potentially higher V OC of the PSCs. In addition, a better energy band alignment between the PAPT‐modified SnO2 and perovskite layers was achieved due to the upshift of the conduction band and Fermi level of SnO2. As a result, a reduced energy barrier and faster electron extraction at the SnO2 and perovskite interface could be realized. PSCs based on PAPT‐modified SnO2 layer achieved a champion PCE of 22.25%, with J SC, V OC, and FF of 25.02 mA cm−2, 1.09 V, 81.55%, respectively. Meanwhile, PSCs based on pristine SnO2 layer only achieved a champion PCE of 19.77% with J SC, V OC, and FF of 25.09 mA cm−2, 1.06 V, 74.28%, respectively. The PCE enhancement is mainly attributed to the increment of FF and V OC, benefited from the interface modification by the periodic acid treatment. In addition, the devices based on PAPT‐modified SnO2 exhibited excellent stability, which remain 93.32% of its initial efficiency stored at 20 °C, 20–25% RH after 3000 h without any encapsulation. Furthermore, we fabricated 3 × 3 cm2 perovskite mini‐modules based on PAPT‐modified SnO2 layers, achieving a high efficiency of 18.10% with the aperture area of 2.29 cm2, while the control one only achieved PCE of 16.13%. These results indicate that PAPT method efficiently boost the large‐scale production of SnO2 ETL and perovskite modules.
Conflict of Interest
The authors declare no conflict of interest.
Author Contributions
Z.W. and J.S. conceived the project, fabricated, and characterized the perovskite solar cells. N.C. provided support for the CBD process. X.W., Z.Z., X.L., H.Z., and J.Y. assisted to analyze and discuss the experiments results. J.L. contributed to the DFT calculations. S.C. and Z.W. contributed to CV and DLCP measurements. Z.W. wrote the manuscript. X.L. and H.L. supervised the work and revised the manuscript. All the authors discussed the results and commented on the manuscript.
Supporting information
Supporting Information
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (NSFC 52072207, 62175204), Science and Technology Project of Fujian Province (No. 2021H6018) and the Innovation Platform for the Academician of Hainan Province (HD‐YSZX‐202007 and HD‐YSZX‐202008). The authors also thank Yuqian Zhou and Jia Yang (Tsinghua University) for their contributions of DFT calculations.
Wu Z., Su J., Chai N., Cheng S., Wang X., Zhang Z., Liu X., Zhong H., Yang J., Wang Z., Liu J., Li X., Lin H., Periodic Acid Modification of Chemical‐Bath Deposited SnO2 Electron Transport Layers for Perovskite Solar Cells and Mini Modules. Adv. Sci. 2023, 10, 2300010. 10.1002/advs.202300010
Contributor Information
Xin Li, Email: lixin01@xmu.edu.cn.
Hong Lin, Email: hong-lin@tsinghua.edu.cn.
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.
References
- 1. Min H., Lee D., Kim J., Kim G., Lee K., Kim J., Paik M., Kim Y., Kim K., Kim M., Shin T., Seok S., Nature 2021, 598, 444. [DOI] [PubMed] [Google Scholar]
- 2. Liu X., Tai M., Gu J., Wu Z., Zhong H., Wang X., Wang Z., Lin H., J. Mater. Chem. C 2022, 10, 10964. [Google Scholar]
- 3. Yang L., Xiong Q., Li Y., Gao P., Xu B., Lin H., Li X., Miyasaka T., J. Mater. Chem. A 2021, 9, 1574. [Google Scholar]
- 4. Zhao Y., Ma F., Qu Z., Yu S., Shen T., Deng H.‐X., Chu X., Peng X., Yuan Y., Zhang X., You J., Science 2022, 377, 531. [DOI] [PubMed] [Google Scholar]
- 5. Zhou Y., Li X., Lin H., Small 2020, 16, 1902579. [DOI] [PubMed] [Google Scholar]
- 6. Jeong J., Kim M., Seo J., Lu H., Ahlawat P., Mishra A., Yang Y., Hope M. A., Eickemeyer F. T., Kim M., Yoon Y. J., Choi I. W., Darwich B. P., Choi S. J., Jo Y., Lee J. H., Walker B., Zakeeruddin S. M., Emsley L., Rothlisberger U., Hagfeldt A., Kim D. S., Grätzel M., Kim J. Y., Nature 2021, 592, 381. [DOI] [PubMed] [Google Scholar]
- 7. Jeong M., Choi I. W., Go E. M., Cho Y., Kim M., Lee B., Jeong S., Jo Y., Choi H. W., Lee J., Bae J.‐H., Kwak S. K., Kim D. S., Yang C., Science 2020, 369, 1615. [DOI] [PubMed] [Google Scholar]
- 8. Lin L., Jones T. W., Yang T. C.‐J., Duffy N. W., Li J., Zhao L., Chi B., Wang X., Wilson G. J., Adv. Funct. Mater. 2020, 31, 2008300. [Google Scholar]
- 9. Xiong L., Guo Y., Wen J., Liu H., Yang G., Qin P., Fang G., Adv. Funct. Mater. 2018, 28, 1802757. [Google Scholar]
- 10. Ke W., Fang G., Liu Q., Xiong L., Qin P., Tao H., Wang J., Lei H., Li B., Wan J., Yang G., Yan Y., J. Am. Chem. Soc. 2015, 137, 6730. [DOI] [PubMed] [Google Scholar]
- 11. Jiang Q., Zhang X., You J., Small 2018, 14, 1801154. [Google Scholar]
- 12. Jiang Q., Zhang L., Wang H., Yang X., Meng J., Liu H., Yin Z., Wu J., Zhang X., You J., Nat. Energy 2016, 2, 16177. [Google Scholar]
- 13. Wu Z., Li W., Ye Y., Li X., Lin H., Sustainable Energy Fuels 2021, 5, 1926. [Google Scholar]
- 14. Anaraki E. H., Kermanpur A., Steier L., Domanski K., Matsui T., Tress W., Saliba M., Abate A., Grätzel M., Hagfeldt A., Correa‐Baena J.‐P., Energy Environ. Sci. 2016, 9, 3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yoo J. J., Seo G., Chua M. R., Park T. G., Lu Y., Rotermund F., Kim Y.‐K., Moon C. S., Jeon N. J., Correa‐Baena J.‐P., Bulović V., Shin S. S., Bawendi M. G., Seo J., Nature 2021, 590, 587. [DOI] [PubMed] [Google Scholar]
- 16. Tong G., Ono L. K., Liu Y., Zhang H., Bu T., Qi Y., Nano‐Micro Lett. 2021, 13, 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jung E. H., Chen B., Bertens K., Vafaie M., Teale S., Proppe A., Hou Y., Zhu T., Zheng C., Sargent E. H., ACS Energy Lett. 5, 2796. [Google Scholar]
- 18. Dutta D., Bahadur D., J. Mater. Chem. 2012, 22, 24545. [Google Scholar]
- 19. Bang H. I., Seo H. B., Bae B. S., Yun E.‐J., Phys. Status Solidi RRL 2019, 216, 1800863. [Google Scholar]
- 20. Kim S., Yun Y. J., Kim T., Lee C., Ko Y., Jun Y., Chem. Mater. 2021, 33, 8194. [Google Scholar]
- 21. Li N., Du K., Liu G., Xie Y., Zhou G., Zhu J., Li F., Cheng H.‐M., J. Mater. Chem. A 2013, 1, 1536. [Google Scholar]
- 22. Jia J., Qian C., Dong Y., Li Y. F., Wang H., Ghoussoub M., Butler K. T., Walsh A., Ozin G. A., Chem. Soc. Rev. 2017, 46, 4631. [DOI] [PubMed] [Google Scholar]
- 23. Cao X., Li J., Dong H., Li P., Fan Q., Xu R., Li H., Zhou G., Wu Z., Adv. Funct. Mater. 2021, 31, 2104344. [Google Scholar]
- 24. Yuan Q.‐Q., Zheng F., Shi Z.‐Q., Li Q.‐Y., Lv Y.‐Y., Chen Y., Zhang P., Li S.‐C., Adv. Sci. 2021, 8, 2100009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Deng J., Zhang H., Wei K., Xiao Y., Zhang C., Yang L., Zhang X., Wu D., Yang Y., Zhang J., Adv. Funct. Mater. 2022, 32, 2209516. [Google Scholar]
- 26. Tong G., Son D.‐Y., Ono L. K., Liu Y., Hu Y., Zhang H., Jamshaid A., Qiu L., Liu Z., Qi Y., Adv. Energy Mater. 2021, 11, 2003712. [Google Scholar]
- 27. Zhou Y., Yang S., Yin X., Han J., Tai M., Zhao X., Chen H., Gu Y., Wang N., Lin H., J. Mater. Chem. A 2019, 7, 1878. [Google Scholar]
- 28. Wu Z., Liu X., Zhong H., Wu Z., Chen H., Su J., Xu Y., Wang X., Li X., Lin H., Small Methods 2022, 6, 2200669. [DOI] [PubMed] [Google Scholar]
- 29. Zhou Y., Zhong H., Han J., Tai M., Yin X., Zhang M., Wu Z., Lin H., J. Mater. Chem. A 2019, 7, 26334. [Google Scholar]
- 30. Yang L., Feng J., Liu Z., Duan Y., Zhan S., Yang S., He K., Li Y., Zhou Y., Yuan N., Ding J., Liu S. F., Adv. Mater. 2022, 34, 2201681. [DOI] [PubMed] [Google Scholar]
- 31. Ni Z., Bao C., Liu Y., Jiang Q., Wu W.‐Q., Chen S., Dai X., Chen B., Hartweg B., Yu Z., Holman Z., Huang J., Science 2020, 367, 1352. [DOI] [PubMed] [Google Scholar]
- 32. Xiong Q., Yang L., Zhou Q., Wu T., Mai C.‐L., Wang Z., Wu S., Li X., Gao P., ACS Appl. Mater. Interfaces 2020, 12, 46306. [DOI] [PubMed] [Google Scholar]
- 33. Khenkin M. V., Katz E. A., Abate A., Bardizza G., Berry J. J., Brabec C., Brunetti F., Bulović V., Burlingame Q., Di Carlo A., Cheacharoen R., Cheng Y.‐B., Colsmann A., Cros S., Domanski K., Dusza M., Fell C. J., Forrest S. R., Galagan Y., Di Girolamo D., Grätzel M., Hagfeldt A., Von Hauff E., Hoppe H., Kettle J., Köbler H., Leite M. S., Liu S., Loo Y.‐L., Luther J. M., et al., Nat. Energy 2020, 5, 35. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.


