Abstract
N-Acetylneuraminic acid (Neu5Ac), the most common type of Sia, generally acts as the terminal sugar in cell surface glycans, glycoconjugates, oligosaccharides, lipo-oligosaccharides, and polysaccharides, thus exerting numerous physiological functions. The extensive applications of Neu5Ac in the food, cosmetic, and pharmaceutical industries make large-scale production of this chemical desirable. Biosynthesis which is associated with important application potential and environmental friendliness has become an indispensable approach for large-scale synthesis of Neu5Ac. In this review, the physiological roles of Neu5Ac was first summarized in detail. Second, the safety evaluation, regulatory status, and applications of Neu5Ac were discussed. Third, enzyme-catalyzed preparation, whole-cell biocatalysis, and microbial de novo synthesis of Neu5Ac were comprehensively reviewed. In addition, we discussed the main challenges of Neu5Ac de novo biosynthesis, such as screening and engineering of key enzymes, identifying exporters of intermediates and Neu5Ac, and balancing cell growth and biosynthesis. The corresponding strategies and systematic strategies were proposed to overcome these challenges and facilitate Neu5Ac industrial-scale production.
Keywords: N-Acetylneuraminic acid, Sia, Physiological effects, Biosynthesis, Metabolic engineering strategies
1. Introduction
Sialic acids (Sia) are a class of α-keto acid sugars with a nine-carbon backbone [1]. Different sialyl linkages and Sia forms result in the formation of complex types of natural modifications. They widely occur in vertebrates and higher invertebrates as terminal components of glycans on the cell-surface glycoconjugates [2]. To date, over 90 naturally occurring forms of Sia have been identified [3,4]. The high structural complexity and diverse properties of Sia contribute to a variety of biological functions, including antiviral activity [3], immunomodulatory activity [5], beneficial effects on brain and cognitive function [6], and skin whitening [7]. N-acetylneuraminic acid (Neu5Ac), systematically named 5-acetamido-3,5-dideoxy-d-glycero-d-galacto-non-2-ulopyranosonic acid, is the dominant type of Sia [8,9]. Neu5Ac occurs in a variety of organisms, such as viruses, bacteria, fungi, protozoa, and higher animals, and is also present in human milk [10]. In human milk, Neu5Ac is predominantly bound to proteins, lipids, and oligosaccharides, forming sialylated structures. Neu5Ac is the key monosaccharide block of sialylated human milk oligosaccharides (HMOs). For example, 3′-sialyllactose (3′-SL) and 6′-sialyllactose (6′-SL), two simplest sialylated HMOs, incorporate Neu5Ac residue with galactose molecule of lactose via α2,6- and α2,3-linkages, respectively [11]. Approximately 14%–33% of human milk oligosaccharides are cross-linked with Neu5Ac [12]. Neu5Ac is also present in free (unbound) form in the human body, including human milk [13].
Neu5Ac is involved in many physiological and pathological events, such as cellular recognition, cell adhesion, fertilization, viral invasion, and tumorigenesis [14]. It has attracted increasing attention from researchers owing to its multifunctional biological properties and great contribution to the field of therapeutics. Owing to its important roles, Neu5Ac possesses vast potential for applications in the food, cosmetic, and pharmaceutical industries. Clinical studies have demonstrated that Neu5Ac is safe and well tolerated as an ingredient in food supplements with no significant side effects [15,16]. Neu5Ac is also a key precursor for producing some anti-influenza viral drugs, such as zanamivir and oseltamivir [17]. Neu5Ac is also often used in targeted cancer therapy.
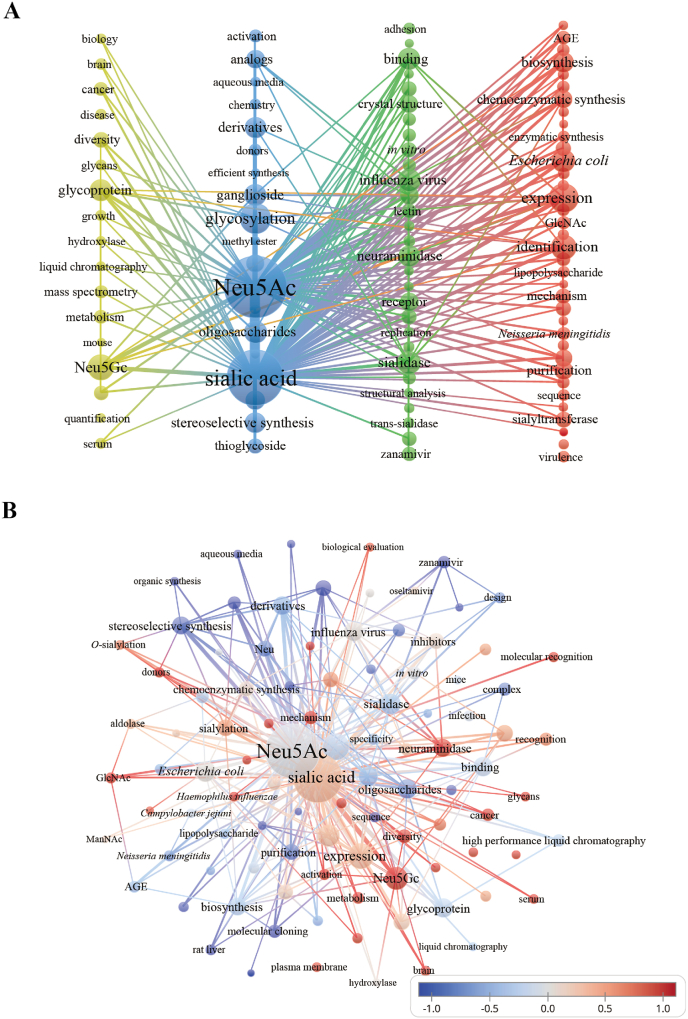
In light of the vital role of Neu5Ac, we reviewed the relevant literature published between 2000 and 2023 by searching the core database of Web of Science using the keyword “N-acetylneuraminic acid” to explore the present and evolving trends in the field of Neu5Ac research. Altogether, 2062 literature items were retrieved. After data cleaning, VOSviewer was used to visualize and analyze trends (Fig. 1). Based on keyword co-occurrence analysis (Fig. 1A), it was revealed that the study of Neu5Ac revolves around four key themes: 1) the physiological roles of Neu5Ac in brain development and cancer treatment, 2) Neu5Ac in the Sia family, 3) antiviral activity of Neu5Ac, and 4) biosynthesis of Neu5Ac. An overlay visualization map uncovered three new streams of research in recent years: the antiviral mechanism of Neu5Ac, the molecular recognition process in cancer, and biosynthesis learning from native microorganisms (Fig. 1B).
Fig. 1.
(A) Keyword co-occurrence network visualization map for research on Neu5Ac. The sizes of the letters and circles represent the level of term frequency. The linkages between the nodes convey the co-occurrence relationship between them. Link strength denotes the frequency with which they co-occur. Nodes with the same color belong to one cluster (B) Overlay visualization map of keywords according to year. The color bar indicates the timeline of keywords, whereby the colors range from blue (published at the earliest) to red (published at the latest).
Based on the above discussion, the physiological roles of Neu5Ac were summarized, the safety evaluation, regulatory status, and applications of Neu5Ac were discussed, and the biosynthesis of Neu5Ac was reviewed in detail. In addition, we offered a comprehensive insight into the main challenges in microbial de novo biosynthesis of Neu5Ac and the efficient strategies used to overcome the limitations of metabolic engineering production.
2. Physiological roles of Neu5Ac
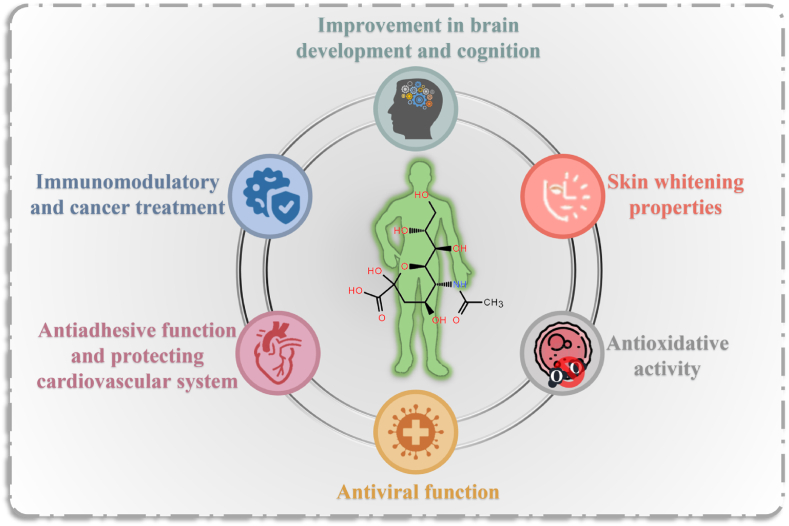
The exposed positions of Neu5Ac on macromolecules and cell membranes suggest its considerable potential for impacts on biological events. Numerous studies have confirmed the physiological functions of Neu5Ac (Fig. 2) [[18], [19], [20]].
Fig. 2.
Physiological roles of Neu5Ac.
2.1. Improvement of brain development and cognition
As early as 1941, Ernst Klenk discovered Sia in brain glycolipids, which he named “neuraminic acid” as it was found in neural tissue [21]. As the most abundant Sia in humans, Neu5Ac is particularly enriched in human brain and displays at the terminal position of brain gangliosides and polysialic acid, thus regulating molecular interactions and brain plasticity [22,23]. Exogenous Neu5Ac treatment has been proved to improve memory [24], elevate cognition and behavior performance [25], and increase brain ganglioside content. Yew et al. reported that Neu5Ac exerts neurotrophic effects by promoting the differentiation, migration, and proliferation of neuroectodermal cells in the neural stem cell model embryonic mice [26].
The impact of Neu5Ac supplementation on improving learning and memory has been widely investigated in rodents [25,27] and piglets [22]. Exogenous Neu5Ac administration has been reported to enhance frontal-cortex Neu5Ac content and to promote cognition and behavior performance, as evaluated by the Y-maze test [24]. Bian et al. administered Neu5Ac to pregnant mothers and rat pups to evaluate its effects on behavior. The mothers were supplemented with different doses of Neu5Ac from 15 d after the beginning of pregnancy until the end of lactation [28]. Based on the Morris water maze and shuttle box tests, the maternal Neu5Ac supplementation resulted in offspring with better cognitive performance, suggesting that maternal Neu5Ac administration improved learning and memory functions in their offspring [29]. However, there was no significant difference between the experimental group and the control group's rat pups, so it was concluded that Neu5Ac supplementation does not improve the cognitive ability of the rat pups. Therefore, Neu5Ac seems to play a cognitive improvement function in the early stages of life, a key window for brain development [30].
In addition, a series of studies carried out in piglets have revealed a close relationship between Neu5Ac and early brain development in newborn mammals [31,32]. An obvious dose-response relationship in the hippocampus was shown between the amount of dietary Neu5Ac supplementation and the mRNA levels of ST8SiaIV and GNE, which are two key genes linked to learning behavior [33,34].
2.2. Immunomodulatory activity and cancer treatment
The types of Sia present on molecules are involved in differentiating between self and foreign antigens in immune systems of many organisms [35]. An essential self-identifier in glycan coding sequences is Neu5Ac. A specific self-associated molecular pattern (SAMP) is generally generated in sialoglycans on the glycocalyx of healthy cells and recognized by Sia–binding immunoglobulin-like lectins (Siglecs), which are widely distributed throughout various immune-competent cells of primates and rodents and significantly contribute to immunology, inflammation, hemostasis, and cancer development [36]. The human Siglecs family comprise 15 receptors and they specifically recognize Neu5Ac moiety with respective glycosidic linkages, which consist of α2,3-, α2,6-, and α2,8-linkages, and thus activate specific immunological responses [37]. For example, as a regulator of the complement alternative pathway in innate immunity, serum complement factor H recognizes α2,3-Neu5Ac-SAMP and triggers the protection of “self” cells [38,39]. Remarkably, human-specific pathogens, commensals, and tumor tissues have the unique ability to display Neu5Ac on their surfaces. For example, Neisseria gonorrhoeae, the organism that causes the gonococcal infection, can transfer Neu5Ac moiety of host cell's CMP-Neu5Ac to their cell-surface lipooligosaccharide to evade human immune surveillance. However, through interacting with the lipooligosaccharide of the pathogenic bacteria, a non-protective Neu5Ac analog can be utilized to design complement-based antibacterial immunotherapeutic [40,41].
Additionally, Neu5Ac modifiers, such as N-glycolylneuraminic acid (Neu5Gc), ketodeoxynononic acid (Kdn), and O-acetylated Sia, are frequently expressed by tumor cells at levels that differ from those in the corresponding healthy cells. A novel strategy to suppress tumor growth involves the inhibition of sialyltransferases by injection of Ac53FaxNeu5Ac, a fluorinated Sia mimetic that can increase the number of CD8+ T-cell and natural killer cells while decreasing the number of regulatory T-cell [42].
2.3. Antiadhesive function and protection of the cardiovascular system
The adhesion of pathogenic bacteria, such as N. meningitidis, C. jejuni, and H. influenzae, to host tissues has long been known to be a prerequisite for initiating the infection [43]. As was previously indicated, some bacterial pathogens use Neu5Ac released by host sialidases or other bacteria, transferring free Neu5Ac to their own cell surface, thereby facilitating their attachment to the host cells [44]. As a result, Neu5Ac has the antiadhesive ability because the competitive adherence of Neu5Ac to Sia receptors on bacterial surface results in bacteria having fewer Sia receptors available and less ability to connect to specific Sia receptors on host cells. Salcedo et al. reported that Neu5Ac exhibited the most extraordinary interference with the adhesion of several newborn pathogens to Caco-2 adenocarcinoma cells compared with GD3, GM1, and GM3, three main gangliosides with well-known positive effects in human milk. Neu5Ac at the concentrations of both 32.0 and 261.7 mg/L, which correspond to the quantities seen in the bioaccessible portion of commercial infant formulae and human milk, respectively, showed anti-pathogenic effect [45].
In addition to competitive adherence to pathogen receptors, Neu5Ac helps cell membranes produce a negative charge on the surface, which results in the development of an electrostatic shield protective of host cells. This electrostatic repulsion is pivotal for avoiding unfavorable cell contact in blood circulation and anti-adhesive repulsion [46]. A striking example is that the interplay between low-density lipoprotein and the blood vessel wall is regulated by increased levels of Neu5Ac in the vessel wall and erythrocytes through increasing negative charges on these surfaces, thus preventing high-fat diet-induced hypercholesterolemia and hypercoagulation [47]. Furthermore, hydrophilic and negatively charged Sia, including Neu5Ac, promote red blood cell stabilization and prevent aggregation of blood components [10]. Wang et al. reported that Neu5Ac exhibited an apparent vasodilation, which was dose-dependent, in the mesenteric artery of Sprague-Dawley rats, demonstrating its anti-hypertensive capabilities [48]. Further clinical research is required to pave the way for Neu5Ac to serve as a useful supplement in food and drug to prevent and treat hypertension.
2.4. Antiviral function
The two primary routes by which the influenza virus attaches to the host cell surface are: avian viral hemagglutinins (HA) predominantly bind α2,3-Neu5Ac while influenza A viral HAbind to α2,6-Neu5Ac as the primary infectious step [49]. According to the research by Guo et al., Neu5Ac derived from edible bird's nest (EBN) following neuraminidase treatment showed a potent inhibitory effect against the infection of human, avian, or porcine influenza viruses to host organisms by inhibiting hemagglutination activity [50]. Another study characterized the mechanisms of this therapeutic effect, namely the inhibition of intracellular autophagy during the life cycle of influenza viruses, which can expeditiously lead to a reduction in their amplification [51]. Based on these findings, tremendous efforts have been made to use multivalent Sia materials as antiviral agents [52]. Neu5Ac has been conjugated to various scaffolds such as polymersomes, liposomes, gold nanoparticles, and nanogels to amplify the effect of interfering with the viral infection process [53].
2.5. Antioxidative activity
In 2004, Iijima et al. revealed the importance of Neu5Ac as a potent active oxygen scavenger. They claimed that monomeric Neu5Ac is oxidized in vitro at physiological conditions by an equimolar amount of cytotoxic hydrogen peroxide, generating a safe decarboxylated product. For this reaction, its characteristic free-ketocarboxylic acid group is essential [54]. Subsequently, it was shown that Neu5Ac could also attenuate the cytotoxicity of biologically relevant lipid hydroperoxides, which can result in undesirable biological disorders and chronic diseases [55]. Through neutralizing oxidative damage caused by reactive oxygen species and raising the transcriptional level of hepatic antioxidant genes, Neu5Ac dramatically reduced oxidative stress and mitigated lipopolysaccharide (LPS)-triggered kidney failure [56,57]. The antioxidative capacity of Neu5Ac suggests its potentially significant role in illnesses like inflammation and aging [58].
2.6. Skin whitening properties
It has been recommended that Neu5Ac is a powerful substance for skin whitening. Its skin-lightning property is on par with those of well-known skin-whitening substances, including kojic acid, arbutin, gallic acid, and hydroquinone [59]. In a skin-whitening test, Neu5Ac obtained by complete digestion of EBN exhibited significant inhibition to tyrosinase activity. In cultured B16 as well as A375 melanoma cells, where melanin-rich melanosomes are formed and released, a significant reduction in melanin concentration was observed after incubation with Neu5Ac [7]. Additionally, in a 3D model of human skin, Neu5Ac was found to have a lightning impact on skin color. This finding suggests that Neu5Ac can permeate the keratinocyte layer and act on melanocytes [59].
3. Applications of Neu5Ac
3.1. Safety of Neu5Ac
The safety evaluations of Neu5Ac have been performed in Sprague-Dawley rats [60] and N-acetylneuraminic acid synthase deficiency (NANSd) patients [16]. No evidence of toxicity or compound-related side effect was observed. When supplied with a daily dose of 1895 mg/kg bw, Neu5Ac is safe for female propagation and young development [60]. The single Generally Recognized as Safe (GRAS) notice for Neu5Ac (GRN 602), which is produced by an aldolase enzyme preparation and used as a component in term infant formulae at a maximum usage level of 50 mg per liter, was approved by American Food and Drug Administration (FDA) and given to Glycom A/S in 2016 [61]. Later in 2017, European Food Safety Authority (EFSA) certified synthetic Neu5Ac as a novel food (NF) with regulation No. 258/97. Following this regulation, the European Commission approved Neu5Ac for use as a food additive in the market at the prescribed amounts [3]. In addition, Neu5Ac has also been approved by China as a new food ingredient.
3.2. Applications in the food industry and cosmetics
Rapid research progress has provided mounting evidence of the role of Neu5Ac in human nutrition, opening up enormous prospects for the food industry to develop effective dietary supplements and nutraceuticals. However, Röhrig et al. reported that the concentrations of Neu5Ac in commercial infant formulae were still much lower than those in human milk [62]. Adding Neu5Ac to infant formulae would help them more closely resemble human milk. To date, main baby food manufacturers have designed new types of infant formulae using 3′-SL and 6′-SL as additives [11].
Furthermore, Neu5Ac dominates the quality differentiation of certain foods containing Sia. In the market, EBN, known as a traditional tonic ingredient with a high concentration of Neu5Ac, is graded by its free Neu5Ac content. EBN with abundant Neu5Ac tends to be more expensive [59]. In addition, because of the different lightning mechanisms and chemical structures of Neu5Ac compared to other skin-lightning agents, Neu5Ac, whose International Nomenclature Cosmetic Ingredient (INCI) name is ACETYLNEURAMINIC ACID, has been used as a safe and active ingredient in skin care cosmeceutical products owing to its skin-whitening effect and antioxidant activity [63].
3.3. Applications in the pharmaceutical industry
Relying on the multifunctional characteristics of Neu5Ac, innovative diagnostic and therapeutic materials have been created for autoimmune diseases, neurological disorders, hypertension, cancer, gastric diseases, and contagious diseases. Indeed, Neu5Ac has been used to synthesize several antiviral agents, such as zanamivir and oseltamivir, which have been recognized as potent medications to treat and prevent infections [64]. With the emergence of various resistant viruses, inhibiting the adhesion of the viral HA glycoprotein to host cells is an alternative strategy. Monomeric Neu5Ac and Neu5Ac-functionalized polymersomes have been regarded as promising inhibitors, which has fueled the demand for Neu5Ac-based pharmaceutical agents [52,53].
Moreover, Neu5Ac extended-release formulation therapies helped GNE myopathy patients ameliorate the decline in upper extremity muscle strength [65]. GNE myopathy, arising from variants in gene GNE, is a kind of neuromuscular disease. Ultragenyx Pharmaceutical carried out numerous practical clinical researches works to evaluate the safety and efficacy of this formulation in GNE myopathy patients and established its pharmacokinetics [66]. In a Phase II clinical trial involving 47 subjects with GNE myopathy, extended-release tablets of Neu5Ac at a dose of 6 g/day maintained upper extremity strength and this effect retained for 24 weeks [67]. Unfortunately, a follow-up Phase III study in 89 patients failed to confirm this effect [68]. The disparities in clinical outcomes may be explained by variations in the size and heterogeneity of these two trials. In the future, therapeutics focusing on the pathogenesis or the biosynthetic pathway of Neu5Ac should be developed. Further studies are warranted to explore the different formulations of extended-release Neu5Ac.
3.4. Biosynthesis of Neu5Ac
The extensive applications of Neu5Ac in nutraceuticals and pharmaceuticals makes large-scale production of this chemical desirable. Neu5Ac is tradicionally produced via extraction from natural sources or by chemical synthesis. Neu5Ac can be isolated from EBN, mucins, milk, eggs, red meat, and some fish species using certain methods [69]. However, extraction approach to Neu5Ac production includes multiple steps, such as ethanol extraction, acidic hydrolysis, chromatographic analysis, purification, and recrystallization, which makes NeuAc production both complicated and costly [70]. Neu5Ac can also be synthesized by chemical methods that require complex protection, deprotection, reduction, and oxidation steps. But the chemical methods are limited by their low production efficiency, unsatisfactory stereoselectivity, and high environmental pollution [71]. In recent decades, biosynthesis, which is associated with good application potential and environmentally friendly characteristics, has become an indispensable approach for large-scale production of Neu5Ac.
3.5. Enzymatic production
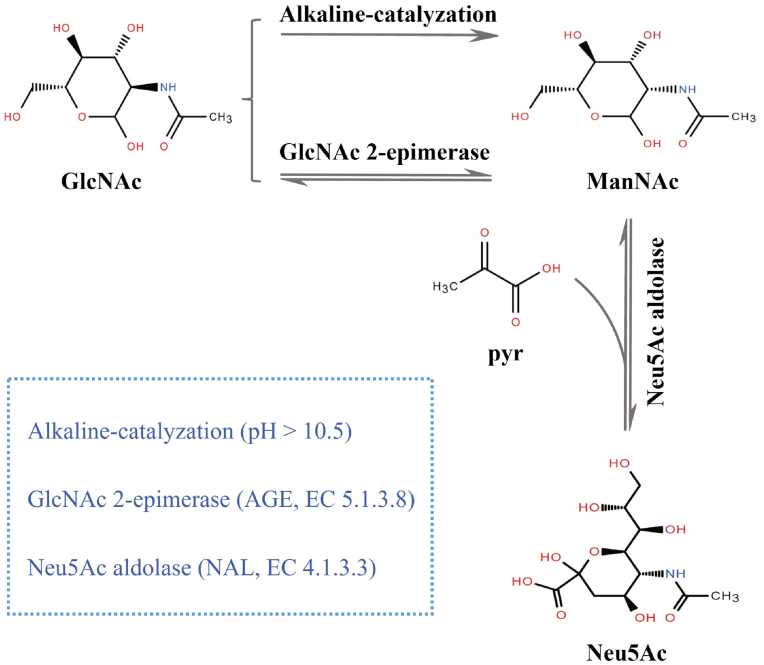
Neu5Ac can be enzymatically prepared through two approaches: 1) the chemoenzymatic approach and 2) the two-step enzymatic approach (Fig. 3). During the chemoenzymatic process, substrate N-acetylglucosamine (GlcNAc) firstly epimerizes to N-acetylmannosamine (ManNAc) by alkaline-catalyzation, and then ManNAc condenses with co-substrate pyruvate to generate Neu5Ac by Neu5Ac aldolase (NAL, EC 4.1.3.3). However, the chemical epimerization of GlcNAc to ManNAc requires large quantities of bases and organic solvents, which bring pollution to the environment [72]. With increasing GlcNAc 2-epimerases (AGE, EC 5.1.3.8) were identified and heterologous expression, AGE-catalyzed biotransformation gradually superseded the alkaline epimerization of GlcNAc to ManNAc. In the two-step enzymatic approach, Neu5Ac can be synthesized by two-enzyme cascade in one pot, in which AGE catalyzes the epimerization of GlcNAc and NAL catalyzes the condensation with pyruvate, therefore forming Neu5Ac [73]. Nucleotides, such as ATP, dATP, ddATP, ADP, and GTP, can not only act as AGE activators, but also inhibit AGE degradation by thermolysin [74]. The crystal structures and catalytic mechanism of NAL and AGE, two important enzymes involved in the two-step enzymatic approach to Neu5Ac synthesi, were comprehensively reviewed by Hussain et al., in 2022 [75].
Fig. 3.
Two approaches for enzymatic production of Neu5Ac.
Kragl et al. first attempted the coupling reaction of NAL and AGE to achieve Neu5Ac continuous production. By using 34 U/mL AGE and 1.19 U/mL NAL in an enzyme membrane reactor (EMR), Neu5Ac was obtained in yields of 67% from 200 mM ManNAc and 100 mM pyruvate with 5 mM ATP at pH 7.5 and 25 °C [76]. Since then, many developments have been made to enhance the productivity and efficiency of this process. AGEs from various sources have been widely identified and further analyzed, including AGE from porcine (pAGE), Synechocystis sp. (SnAGE), Anabaena sp. (bAGE), Bacteroides ovatus ATCC (BoAGE2), Nostoc sp. KVJ10 (nAGE10), Anabaena variabilis (AvaAGE), Pedobacter heparinus (PhGn2E), and Bacteroides thetaiotaomicron (BT0453). Similarly, different sources of NALs have been characterized, such as E. coli (EcNAL), Lactobacillus plantarum (LpNAL), Staphylococcus carnosus TM300 (ScNAL), C. glutamicum (CgNAL), Peptoclostridium difficile (PdNAL), Mycoplasma sp. (MyNAL), Aliivibrio salmonicida (AsNAL), Staphylococcus aureus (SaNAL), and Haemophilus influenza (HiNAL). Different AGEs and NALs possess different kinetic values, enzymatic properties, and specific activities [75].
Lee et al. devised a shifting reaction temperature method for a coupling reaction for efficient Neu5Ac production. When the reaction reached equilibrium at 30 °C in approximately 10 h, the temperature was set at 20 °C to change the reaction equilibrium, thus achieving a high conversion yield [77]. Zimmermann et al. devised a computer-based kinetic model to predict the reaction course and, therefore, optimize the reaction conditions for different purposes. In a fed-batch reactor, the productivity of Neu5Ac increased from 1.8 × 10−3 to 3.3 × 10−3 mol L−1 h−1 after modeling optimization [78]. In addition, immobilization has been incorporated into large-scale Neu5Ac production to reuse expensive enzymes and decrease enzyme autodigestion. Bloemendal et al. designed a continuous-flow bioreactor by immobilizing NAL on immobead 150P. The conversion ratio of ManNAc to Neu5Ac reached 82% from 500 mM ManNAc and 100 mM pyruvate at a flow rate of 0.05 mL/min [79]. To improve the soluble expression of AGE and NAL, fusion tags, molecular chaperones, and suitable host strains need to be carefully selected [80,81]. Protein engineering strategies, including rational design and directed evolution, have also been used to improve the catalytic properties, such as reducing the cleavage activity of NAL [82].
Enzyme synthesis provides many advantages, such as high conversion rates, low by-product formation, and mild reaction conditions. Nevertheless, enzyme purification and isolation of Neu5Ac from the equilibrium system are complex and time-consuming steps. Moreover, the requirement of ATP for AGE activity increases production costs.
3.6. Whole-cell catalysis
Whole‐cell biocatalysis using recombinant enzyme-expressing strains can simplify the production process and post-treatments and circumvent protein purification and the addition of ATP, which is more conducive for Neu5Ac production. In whole-cell catalysis, microbial cells are capable of in situ production of the key enzymes and cofactors for Neu5Ac synthesis in the culture medium. In 2002, a whole-cell catalytic process for Neu5Ac synthesis was constructed by coupling two engineered E. coli strains separately expressing AGE and Neu5Ac synthetase (NeuB, EC 4.1.3.19). Cell permeability was improved by the treatment of Nymeen S-215. After a 22-h reaction, 12.3 g/L Neu5Ac was generated with the conversion of GlcNAc to Neu5Ac at 5% [83]. To enhance the conversion rate of GlcNAc to Neu5Ac, Lee et al. expressed Anabaena sp. CH1 AGE (bAGE), a high-activity AGE with high ATP affinity, and Neu5Ac lyase (nanA) in E. coli BL21 (DE3) cells. The Neu5Ac yield was improved to 122.3 g/L and the conversion rate of GlcNAc to Neu5Ac was enhanced to 33.3% [84].
Different recombinant strains, carbon sources, substrates, and production strategies have been explored to improve Neu5Ac production and reduce costs (Table 1) [85]. Zhu et al. constructed an exogenous pyruvate-independent Neu5Ac biocatalytic process using glycerol as the carbon source to decrease downstream processes and environmental costs [86]. In addition, many studies have highlighted the importance of engineering the hosts for Neu5Ac synthesis. Deleting nag and poxB from the E. coli BL21 (DE3) chromosome, which are responsible for reducing GlcNAc and sodium pyruvate degradation, respectively, showed a synergistic effect, with the yield of Neu5Ac reaching 63.8% and a GlcNAc conversion rate of 66.8% [87]. Recently, emerging metabolic engineering approaches combined with protein engineering strategies have attracted much attention. Chen et al. identified an AGE mutant and a more efficient NAL using directed mutagenesis and molecular dynamics simulations, respectively. By further eliminating the transporter genes nagE and nanT and optimizing the growth conditions, 108.8 g/L Neu5Ac was produced with a GlcNAc conversion rate of 58.6% [88].
Table 1.
Whole‐cell biocatalysis for Neu5Ac production.
| Recombinant strains | Carbon sources | Substrates | Main strategies | GlcNAc conversion rate | Product concentration | Reference |
|---|---|---|---|---|---|---|
| two E. coli NM522 cells and a Corynebacterium ammoniagenes strain | glucose | 800 mM GlcNAc | Two E. coli NM522 expressed slr1975 from Synechocystis sp. PCC6803 and neuB from E. coli K1, respectively, coupling with Corynebacterium ammoniagenes to supply PEP. | 5% | 12.3 g/L | [83] |
| Two E. coli BL21 (DE3) cells | glucose | 1200 mM GlcNAc, 1200 mM pyruvate | Two E. coli BL21 (DE3) cells expressed age from Anabaena sp. CH1 and nanA from E. coli NovaBlue, respectively. | 33.3% | 122.3 g/L | [84] |
| E. coli BL21 (DE3) | glucose | 600 mM GlcNAc, 800 mM pyruvate | E. coli BL21 expressed slr1975 from Synechocystis sp. PCC6803 and nanA from E. coli K12. An AGE mutant and a more efficient NAL were identified by directed mutagenesis and molecular dynamics simulation, respectively. The transporter genes nanT and nagE were eliminated. | 58.6% | 108.8 g/L | [88] |
| S. marcescens | GlcNAc | 12.5 mM GlcNAc, 10 mM PEP | S. marcescens expressed slr1975 from Synechocystis sp. PCC6803 and neuB from Campylobacter jejuni NCTC11168. Pathways fluxes were balanced through promoter swapping. | 12.4% | 0.48 g/L | [85] |
| E. coli BL21 (DE3) | glucose | 800 mM GlcNAc, 1200 mM sodium pyruvate | A novel AGE BT0453 with high protein solubility cloned from Bacteroides thetaiotaomicron and E. coli NanA were expressed. | 5.36% | 13.27 g/L | [81] |
| E. coli MG1655 | glycerol | 45.2 mM GlcNAc | Production with different carbon sources was compared. A two-stage pH shift strategy was carried out. The GlcNAc concentration was optimized. | 43.35% | 18.17 g/L | [86] |
| E. coli BL21 (DE3) | glucose | 800 mM GlcNAc, 500 mM sodium pyruvate | Gene nag and poxB were deleted from the E. coli BL21 (DE3) chromosome. Precursor feeding were conducted. | 66.8% | 164.88 g/L | [87] |
3.7. De novo biosynthesis
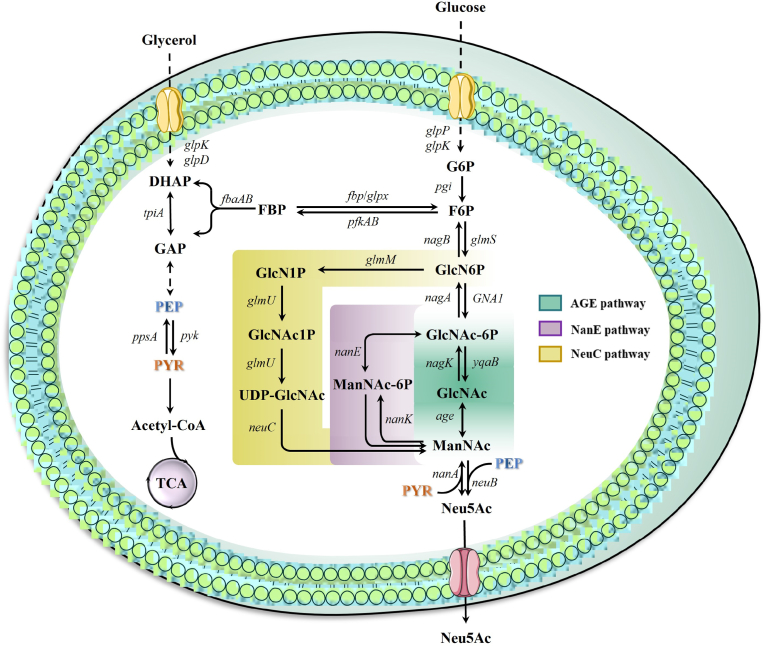
Unlike whole‐cell biocatalysis, production of Neu5Ac via de novo biosynthesis can utilize inexpensive carbon sources as substrates to meet the demand for large-scale production without the addition of any direct precursors. The carbon sources used for Neu5Ac synthesis include glucose, glycerol, malate, and fructose [89]. Recently, the metabolic engineering approach based on cell factories has been extensively studied as a preferred choice for industrial-scale production of Neu5Ac. Neu5Ac can be synthesized in cell factories by three routes: AGE, GlcNAc-6-phosphate 2-epimerase (NanE), and UDP-GlcNAc 2-epimerase (NeuC) pathways (Fig. 4) [90]. Glucosamine-6-phosphate (GlcN6P) is the common metabolic intermediate of three Neu5Ac synthetic pathways. In NeuC pathway, GlcN6P is converted to UDP-GlcNAc by phosphoglucosamine mutase GlmM and GlcNAc-1-phosphate uridyltransferase/glucosamine-1-phosphate acetyltransferase (GlmU), and then further converted to the direct precursor ManNAc by NeuC. GlcNAc6P is another common metabolic intermediate of AGE and NanE pathways. However, it is reported that GlcNAc6P can be converted to GlcNAc and then easily accumulated as byproduct [91]. Engineering individual or multiplexed pathways in various host strains have been used to biosynthesize Neu5Ac (Table 2).
Fig. 4.
Metabolic pathway for Neu5Ac biosynthesis in E. coli. Dashed arrows indicate multiple reactions. The green-colored box, purple-colored box, and yellow-colored box represent the AGE pathway, the NanE pathway and the NeuC pathway, respectively. Main metabolite abbreviations as follows: DHAP, dihydroxyacetone phosphate; GAP, glyceraldehyde 3-phosphate; PEP, phosphoenolpyruvic acid; PYR, pyruvate; G6P, glucose-6-phosphate; GlcN6P, phosphate glucosamine-6-phosphate; GlcNAc-6P, N-acetylglucosamine-6-phosphate; GlcNAc, N-acetylglucosamine; ManNAc, N-acetylmannosamine; ManNAc6P, N-acetylmannosamine 6-phosphate; UDP-GlcNAc, uridine 5′-diphospho-N-acetylglucosamine; Neu5Ac, N-acetylneuraminic acid. Key gene abbreviations: pyk, encoding pyruvate kinase; ppsA, encoding phosphoenolpyruvate synthetase; glmS, encoding glucosamine-6-phosphate synthase; nagB, encoding glucosamine-6-phosphate deaminase; glmM, encoding phosphoglucosamine mutase; glmU, encoding N-acetylglucosamine-1-phosphate uridyltransferase/glucosamine-1-phosphate acetyltransferase; neuC, encoding UDP-GlcNAc 2-epimerase; GNA1, encoding glucosamine-6-phosphate N-acetyltransferase; nagA, encoding N-acetylglucosamine-6-phosphate deacetylase; yqaB, encoding phosphatase; nagK, encoding N-acetylglucosamine kinase; age, encoding N-acetyl-d-glucosamine 2-epimerase; nanK, encoding N-acetylmannosamine kinase; nanE, encoding N-acetylmannosamine-6-phosphate epimerase; neuB, encoding Neu5Ac synthase; nanA, encoding Neu5Ac aldolase.
Table 2.
Summary on Neu5Ac de novo biosynthesis.
| Host | Pathways | Strategies | Carbon sources | GlcNAc titer | ManNAc titer | Neu5Ac titer | Reference |
|---|---|---|---|---|---|---|---|
| E. coli MG1655 | NeuC pathway |
|
glucose | N.M | N.M | 1.7 g/L (shake flask) | [9] |
| E. coli DH5α | AGE pathway |
|
glucose | 13.51 g/L | 3.49 g/L | 7.85 g/L (5 L bioreactor) | [93] |
| E. coli DH5α | AGE pathway |
|
glucose | ∼10 g/L | ∼2 g/L | 8.31 g/L (two-stage fermentation) | [94] |
| E. coli DH5α | AGE pathway |
|
glucose | ∼8 g/L | ∼1 g/L | 14.32 g/L (shake flask) | [95] |
| E. coli DH5α | AGE pathway |
|
glucose | ∼13 g/L | ∼3 g/L | 16.7 g/L (shake flask) | [96] |
| E. coli MG1655 | NeuC pathway |
|
glucose | N.M | N.M | 1.4 g/L (shake flask) | [97] |
| E.coli BL21 (DE3) | NeuC pathway |
|
glycerol | N.M | N.M | 23.46 g/L (3 L bioreactor) | [98] |
| B. subtilis | AGE pathway |
|
glucose | N.M | N.M | 2.18 g/L (shake flask) | [89] |
| B. subtilis | AGE pathway | 1) fine-tuning expression of GNA1, pfkA, and pyk via N-terminal coding sequences | glucose | N.M | N.M | 2.75 g/L (shake flask) | [99] |
| B. subtilis | AGE pathway | 1) dynamic regulation through genetic code expansion-based cell growth and biosynthesis balance engineering strategy | glucose | N.M | N.M | 4.72 g/L (3 L bioreactor) | [100] |
| B. subtilis | AGE pathway | 1) enriching the producing cell subpopulation through an inducible population quality control system | glucose | N.M | N.M | 4.23 g/L (3 L fermenter) | [101] |
| B. subtilis | AGE, NanE, and NeuC pathways |
|
glucose | 39.7 g/L | 14.4 g/L | 30.10 g/L (5 L bioreactor) | [90] |
| B. subtilis | NeuC pathway |
|
glucose and glycerol as dual-carbon sources | 0 g/L (shake flask) | 2.2 g/L (shake flask) |
|
[91] |
N.M: Not mentioned.
E. coli has been the most often used host for Neu5Ac synthesis because of its high expression, rapid growth, and thorough characterization [92]. E. coli is one of several bacterial species that can use Neu5Ac as a carbon source in cellular metabolism. By disrupting the native pathway of Neu5Ac catabolism and introducing an exogenous NeuC pathway, a metabolically engineered E. coli was obtained for the first time to produce Neu5Ac [9]. As mentioned above, GlcN6P is the common metabolic intermediate of three Neu5Ac synthetic pathways and it is synthesized by glucosamine synthase GlmS. The overexpression of glmS from E. coli obviously improved the Neu5Ac titer to 1.7 g/L in shake-flask feeding cultivation [9]. However, the glmS is under the tightly feedback inhibition of GlcN6P. In order to relief this feedback control, Kang et al. introduced a four-site variant of glmS (E14K, D386V, S449P and E524G). They designed AGE pathway in E. coli DH5α by introducing slr1975 from Synechocystis sp. PCC6803 and GNA1 from Saccharomyces cerevisiae EBY100 and the Neu5Ac titer reached 7.85 g/L in a 5-L bioreactor [93]. From then on, their group developed many strategies to improve Neu5Ac production based on the AGE pathway, such as NeuB evolution using an evolved Neu5Ac biosensor [94], evolution of Neu5Ac riboswitch [95], optimization of amino sugar metabolism and improvement of PEP supply [96]. Peters et al. also developed Neu5Ac biosensors and applied them in Neu5Ac production. They used different promoter engineering strategies to create novel Neu5Ac biosensors based on the transcription factor NanR and tested the applicability in high-throughput screening. Through NeuC pathway, the engineered E. coli MG1655 obtained 1.4 g/L Neu5Ac in shake-flask cultivation [97]. In addition, to improve the genetic stability of Neu5Ac production, efforts have been made to construct plasmid-free engineered strains to avoid the use of antibiotic markers and the loss of plasmids. Liu et al. constructed a plasmid-free engineered E. coli BL21 (DE3) strain using clustered regularly interspaced palindromic repeat (CRISPR)/CRISPR-associated protein 9 (Cas9)-mediated genome editing and produced 23.46 g/L Neu5Ac in a 3-L bioreactor without antibiotic addition [98]. This was the highest Neu5Ac titer ever reported by fed-batch cultivation of metabolically engineered E. coli.
As a GRAS strain, B. subtilis has been used as a potential host strain for food-grade Neu5Ac production. Through introducing a special glucose and malate coutilization pathway in B. subtilis, the supply of GlcNAc and PEP reached balance and the Neu5Ac titer improved to 1.65 g/L. Balancing cell growth and Neu5Ac biosynthesis further increased the Neu5Ac titer to 2.18 g/L in shake-flask cultivation [89]. Using N-terminal coding sequences for fine-tuning expression of GNA1, pfkA, and pyk enhanced the Neu5Ac titer to 2.75 g/L [99]. Furthermore, various strategies have been used for efficient production of Neu5Ac, such as elimination of byproducts accumulation [91], dynamic regulation of cell growth and population quality [100,101], and combination of three Neu5Ac synthesis pathways [90]. The highest Neu5Ac titer produced by engineered B. subtilis was 30.10 g/L using glucose as the sole carbon source in a 5-L fermenter [90].
3.8. Challenges and strategies of Neu5Ac de novo biosynthesis
3.8.1. Screening or engineering of key enzymes
During de novo synthesis of Neu5Ac, some enzymes, such as GlmS, AGE and NeuB, are critical for the improvement of the Neu5Ac titer. To date, many efforts have been focused on optimizing the substrate affinity and catalytic activity of key enzymes, including 1) mining for new enzymes from different sources, 2) analyzing structural features and crucial amino acid residues, 3) applying saturation mutagenesis at the key sites, 4) constructing kinetic models, 5) constructing a substrate channel by self-assembly of enzymes, and 6) optimizing the expression level of key enzymes through promoter engineering [75,88,90]. For example, an A506G-G420A variant of NeuB and a T1108G mutant of AGE led to a 1.30-fold and a 1.45-fold improvements in Neu5Ac titers, respectively [94,95].
3.8.2. Transport of intermediates and products
Many studies have reported a considerable amount of GlcNAc and ManNAc detected in the fermentation products (Table 2), indicating that they were exported to the medium by unknown transporters, which severely restricted the efficient biosynthesis of Neu5Ac and increased downstream purification costs [95]. In E. coli, the PTS transporters NagE and ManXYZ are responsible for GlcNAc uptake, whereas ManXYZ and NanT transporters are responsible for ManNAc and Neu5Ac import, respectively [102]. However, exporters of intermediates and Neu5Ac have not yet been completely elucidated. In the future, transporters and transport mechanisms should be continually studied in engineered strains or understood based on native microorganisms that can naturally use GlcNAc, ManNAc, and Neu5Ac as carbon and nitrogen sources.
3.8.3. Balancing cell growth and biosynthesis
De novo biosynthesis of Neu5Ac competes for cell wall synthesis pathways and precursors of glycolysis, and balancing cell growth and biosynthesis is significant for the efficiency of Neu5Ac production. To date, many studies have focused on static, dynamic, and systematic dynamic regulation of the Neu5Ac pathway (Table 2). Modular pathway enhancement strategies, metabolite or signal molecule-responsive biosensors, CRISPR interference (CRISPRi), and genetic code expansion-based cell growth and biosynthesis balance engineering (GCE-CGBBE) strategies have become powerful synthetic biology tools for balancing the precursor supply for cell growth and biosynthesis by activating or repressing the expression of key genes [100].
3.8.4. Systematic strategies
Although the above strategies could contribute to solving bottlenecks in the synthetic pathway, systematic strategies are still indispensable for the upscaling of Neu5Ac. The complexity of metabolism and its regulation require a global and systematic perspective of cell factories to help achieve a better understanding of microbial bioprocesses. Systems metabolic engineering, which combines traditional metabolic engineering with systems biology, synthetic biology, and evolutionary engineering, enables rapid development of cell factories [103]. A combination of machine learning, omics data, and CRISPR tools makes metabolic regulation feasible. For example, with the development of omics technologies, machine learning-based metabolic modes obtained from multi-omics data analysis can comprehensively identify crucial genes and explore potential metabolic routes, which could be used to identify exporters of intermediates and Neu5Ac [104].
4. Conclusion and outlook
Neu5Ac, the most dominant form of Sia, generally occupies the terminal position of cell-surface glycans, oligosaccharides, and glycoconjugates, thereby participating in many physiological and pathological processes. Owing to the various physiological roles and extensive applications of Neu5Ac, as reviewed in this article, there has been increasing demand for large-scale production of this chemical. Over the past few decades, biosynthesis of Neu5Ac has made considerable progress. However, Neu5Ac production remains inefficient and faces several challenges. Further work is needed to optimize the substrate affinity and catalytic activity of key enzymes such as AGE and NeuB. The transporter and transport mechanisms of GlcNAc, ManNAc, and Neu5Ac require further elucidation. Machine learning-based metabolic modes, CRISPRi, and transposon systems could be useful tools for screening potential genes in transport systems. Balancing cell growth and biosynthesis is another challenge that needs to be overcome. Further research is required to elucidate the metabolism and regulation of the engineered strains. Systems metabolic engineering will draw increasing attention to the construction of microbial cell factories for industrial-scale synthesis of Neu5Ac.
CRediT authorship contribution statement
Mingli Zhao: Writing - original draft, Writing - review & editing. Yingying Zhu: Conceptualization, Methodology. Hao Wang: Writing - review & editing. Wenli Zhang: Supervision, Resources. Wanmeng Mu: Supervision, Resources, Project administration, Funding acquisition, Writing - review & editing.
Data availability
No data was used for the research described in the article.
Declaration of competing interest
The authors declare no competing financial interest.
Acknowledgments
This work was supported by the National Key R&D Program of China (No. 2022YFC2104900) and the National Natural Science Foundation of China (No. 31922073).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.Li Y., Chen X. Sialic acid metabolism and sialyltransferases: natural functions and applications. Appl Microbiol Biotechnol. 2012;94:887–905. doi: 10.1007/s00253-012-4040-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Varki N.M., Strobert E., Dick E.J., Benirschke K., Varki A. Biomedical differences between human and nonhuman hominids: potential roles for uniquely human aspects of sialic acid biology. Annu Rev Pathol. 2011;6:365–393. doi: 10.1146/annurev-pathol-011110-130315. [DOI] [PubMed] [Google Scholar]
- 3.Guin S., Velasco-Torrijos T., Dempsey E. Explorations in a galaxy of sialic acids: a review of sensing horizons, motivated by emerging biomedical and nutritional relevance. Sens Diagn. 2022;1:10–70. doi: 10.1039/D1SD00023C. [DOI] [Google Scholar]
- 4.Schauer R., Kamerling J.P. In: Baker D.C., editor. vol. 75. Academic Press; 2018. Exploration of the sialic acid world; pp. 1–213. (Advances in carbohydrate chemistry and biochemistry). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ling A.J.W., Chang L.S., Babji A.S., Latip J., Koketsu M., Lim S.J. Review of sialic acid's biochemistry, sources, extraction and functions with special reference to edible bird's nest. Food Chem. 2022;367 doi: 10.1016/j.foodchem.2021.130755. [DOI] [PubMed] [Google Scholar]
- 6.Abdul Khalid S., Rashed A., Aziz S., Ahmad H. Effects of sialic acid from edible bird nest on cell viability associated with brain cognitive performance in mice. World J Tradit Chin Med. 2019;5:214–219. doi: 10.4103/wjtcm.wjtcm_22_19. [DOI] [Google Scholar]
- 7.Wong Z.C.F., Chan G.K.L., Wu K.Q.Y., Poon K.K.M., Chen Y., Dong T.T.X., et al. Complete digestion of edible bird's nest releases free N-acetylneuraminic acid and small peptides: an efficient method to improve functional properties. Food Funct. 2018;9:5139–5149. doi: 10.1039/C8FO00991K. [DOI] [PubMed] [Google Scholar]
- 8.McNaught A.D. Nomenclature of carbohydrates. Carbohydr Res. 1997;297:1–92. doi: 10.1016/S0008-6215(97)83449-0. [DOI] [PubMed] [Google Scholar]
- 9.Lundgren B.R., Boddy C.N. Sialic acid and N-acyl sialic acid analog production by fermentation of metabolically and genetically engineered Escherichia coli. Org Biomol Chem. 2007;5:1903–1909. doi: 10.1039/b703519e. [DOI] [PubMed] [Google Scholar]
- 10.Ghosh S. Sialic acid and biology of life: an introduction. Sialic Acids Sialoglycoconjug Biol Life, Health Dis. 2020:1–61. doi: 10.1016/B978-0-12-816126-5.00001-9. [DOI] [Google Scholar]
- 11.Zhu Y., Zhang J., Zhang W., Mu W. Recent progress on health effects and biosynthesis of two key sialylated human milk oligosaccharides, 3′-sialyllactose and 6′-sialyllactose. Biotechnol Adv. 2023;62 doi: 10.1016/j.biotechadv.2022.108058. [DOI] [PubMed] [Google Scholar]
- 12.Soyyilmaz B., Miks M.H., Rohrig C.H., Matwiejuk M., Meszaros-Matwiejuk A., Vigsnaes L.K. The mean of milk: a review of human milk oligosaccharide concentrations throughout lactation. Nutrients. 2021;13:2737. doi: 10.3390/nu13082737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galeotti F., Coppa G.V., Zampini L., Maccari F., Galeazzi T., Padella L., et al. On-line high-performance liquid chromatography–fluorescence detection–electrospray ionization–mass spectrometry profiling of human milk oligosaccharides derivatized with 2-aminoacridone. Anal Biochem. 2012;430:97–104. doi: 10.1016/j.ab.2012.07.027. [DOI] [PubMed] [Google Scholar]
- 14.Chen X., Varki A. Advances in the biology and chemistry of sialic acids. ACS Chem Biol. 2010;5:163–176. doi: 10.1021/cb900266r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turck D., Bresson J., Burlingame B., Dean T., Fairweather-Tait S., Heinonen M., et al. Safety of synthetic N‐acetyl‐D‐neuraminic acid as a novel food pursuant to Regulation (EC) No 258/97. EFS2. 2017;15:4918. doi: 10.2903/j.efsa.2017.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tran C., Turolla L., Ballhausen D., Buros S.C., Teav T., Gallart-Ayala H., et al. The fate of orally administered sialic acid: first insights from patients with N-acetylneuraminic acid synthase deficiency and control subjects. Mol Genet Metab Rep. 2021;28 doi: 10.1016/j.ymgmr.2021.100777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y., Tao F., Du M., Ma C., Qiu Jianhua, Gu Lichuan, et al. An efficient method for N-acetyl-D-neuraminic acid production using coupled bacterial cells with a safe temperature-induced system. Appl Microbiol Biotechnol. 2010;86:481–489. doi: 10.1007/s00253-009-2302-3. [DOI] [PubMed] [Google Scholar]
- 18.Zhang C., Chen J., Liu Y., Xu D. Sialic acid metabolism as a potential therapeutic target of atherosclerosis. Lipids Health Dis. 2019;18:173. doi: 10.1186/s12944-019-1113-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun X., Zhang S., Ren J., Udenigwe C.C. Sialic acid-based strategies for the prevention and treatment of Helicobacter pylori infection: emerging trends in food industry. Crit Rev Food Sci Nutr. 2022;62:1713–1724. doi: 10.1080/10408398.2020.1846157. [DOI] [PubMed] [Google Scholar]
- 20.Sokolovskaya O.M., Tan M.-W., Wolan D.W. Sialic acid diversity in the human gut: molecular impacts and tools for future discovery. Curr Opin Struct Biol. 2022;75 doi: 10.1016/j.sbi.2022.102397. [DOI] [PubMed] [Google Scholar]
- 21.Siddiqui S.S., Matar R., Merheb M., Hodeify R., Vazhappilly C.G., Marton J., et al. Siglecs in brain function and neurological disorders. Cells. 2019;8:1125. doi: 10.3390/cells8101125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang B., Yu B., Karim M., Hu H., Sun Y., McGreevy P., et al. Dietary sialic acid supplementation improves learning and memory in piglets. Am J Clin Nutr. 2007;85:561–569. doi: 10.1093/ajcn/85.2.561. [DOI] [PubMed] [Google Scholar]
- 23.Schnaar R.L., Gerardy-Schahn R., Hildebrandt H. Sialic acids in the brain: gangliosides and polysialic acid in nervous system development, stability, disease, and regeneration. Physiol Rev. 2014;94:461–518. doi: 10.1152/physrev.00033.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oliveros E., Vázquez E., Barranco A., Ramírez M., Gruart A., Delgado-García J.M., et al. Sialic acid and sialylated oligosaccharide supplementation during lactation improves learning and memory in rats. Nutrients. 2018;10:1519. doi: 10.3390/nu10101519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morgan B.L.G., Winick M. Effects of administration of N-acetylneuraminic acid (NANA) on brain NANA content and behavior. J Nutr. 1980;110:416–424. doi: 10.1093/jn/110.3.416. [DOI] [PubMed] [Google Scholar]
- 26.Yew M.Y., Koh R.Y., Chye S.M., Zainal Abidin S.A., Othman I., Ng K.Y. Neurotrophic properties and the de novo peptide sequencing of edible bird's nest extracts. Food Biosci. 2019;32 doi: 10.1016/j.fbio.2019.100466. [DOI] [Google Scholar]
- 27.Popov N., Toffano G., Riechert U., Matthies H. Effects of intraventricularly applied gangliosides and N-acetylneuraminic acid on acquisition and retention performance of a brightness discrimination task in rats. Pharmacol Biochem Behav. 1989;34:209–212. doi: 10.1016/0091-3057(89)90301-8. [DOI] [PubMed] [Google Scholar]
- 28.Bian D., Wang X., Huang J., Chen X., Li H. Maternal Neu5Ac supplementation during pregnancy improves offspring learning and memory ability in rats. Front Nutr. 2021;8 doi: 10.3389/fnut.2021.641027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xie Y., Zeng H., Huang Z., Xu H., Fan Q., Zhang Y., et al. Effect of maternal administration of edible bird's nest on the learning and memory abilities of suckling offspring in mice. Neural Plast. 2018;2018 doi: 10.1155/2018/7697261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu F., Simpson A.B., D'Costa E., Bunn F.S., van Leeuwen S.S. Sialic acid, the secret gift for the brain. Crit Rev Food Sci Nutr. 2022:1–20. doi: 10.1080/10408398.2022.2072270. [DOI] [PubMed] [Google Scholar]
- 31.Wang B., Downing J.A., Petocz P., Brand-Miller J., Bryden W.L. Metabolic fate of intravenously administered N-acetylneuraminic acid-6-14C in newborn piglets. Asia Pac J Clin Nutr. 2007;16:110–115. [PubMed] [Google Scholar]
- 32.Wang B., Hu H., Yu B. Molecular characterization of pig ST8Sia IV—a critical gene for the formation of neural cell adhesion molecule and its response to sialic acid supplement in piglets. Nutr Neurosci. 2006;9:147–154. doi: 10.1080/10284150600903594. [DOI] [PubMed] [Google Scholar]
- 33.Gal B., Ruano M.J., Puente R., García-Pardo L.A., Rueda R., Gil A., et al. Developmental changes in UDP-N-acetylglucosamine 2-epimerase activity of rat and Guinea-pig liver. Comp Biochem Physiol B Biochem Mol Biol. 1997;118:13–15. doi: 10.1016/s0305-0491(97)00016-3. [DOI] [PubMed] [Google Scholar]
- 34.Mühlenhoff M., Manegold A., Windfuhr M., Gotza B., Gerardy-Schahn R. The impact of N-glycosylation on the functions of polysialyltransferases. J Biol Chem. 2001;276:34066–34073. doi: 10.1074/jbc.M101022200. [DOI] [PubMed] [Google Scholar]
- 35.Tao F., Zhang Y., Ma C., Xu P. Biotechnological production and applications of N-acetyl-D-neuraminic acid: current state and perspectives. Appl Microbiol Biotechnol. 2010;87:1281–1289. doi: 10.1007/s00253-010-2700-6. [DOI] [PubMed] [Google Scholar]
- 36.Varki A. PAMPs, DAMPs and SAMPs: host glycans are self-associated molecular patterns, but subject to microbial molecular mimicry. FASEB J. 2021;35 doi: 10.1096/fasebj.2021.35.S1.00015. [DOI] [Google Scholar]
- 37.Pillai S., Netravali I.A., Cariappa A., Mattoo H. Siglecs and immune regulation. Annu Rev Immunol. 2012;30:357–392. doi: 10.1146/annurev-immunol-020711-075018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmidt C.Q., Hipgrave Ederveen A.L., Harder M.J., Wuhrer M., Stehle T., Blaum B.S. Biophysical analysis of sialic acid recognition by the complement regulator Factor H. Glycobiology. 2018;28:765–773. doi: 10.1093/glycob/cwy061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murugesan G., Weigle B., Crocker P.R. Siglec and anti-Siglec therapies. Curr Opin Chem Biol. 2021;62:34–42. doi: 10.1016/j.cbpa.2021.01.001. [DOI] [PubMed] [Google Scholar]
- 40.Ram S., Shaughnessy J., DeOliveira R.B., Lewis L.A., Gulati S., Rice P.A. Utilizing complement evasion strategies to design complement-based antibacterial immunotherapeutics: lessons from the pathogenic Neisseriae. Immunobiology. 2016;221:1110–1123. doi: 10.1016/j.imbio.2016.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shaughnessy J., Chabeda A., Tran Y., Zheng B., Nowak N., Steffens C., et al. An optimized Factor H-Fc fusion protein against multidrug-resistant Neisseria gonorrhoeae. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.975676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Büll C., Boltje T.J., Balneger N., Weischer S.M., Wassink M., van Gemst J.J., et al. Sialic acid blockade suppresses tumor growth by enhancing T-cell–mediated tumor immunity. Cancer Res. 2018;78:3574–3588. doi: 10.1158/0008-5472.CAN-17-3376. [DOI] [PubMed] [Google Scholar]
- 43.Sharon N. Carbohydrates as future anti-adhesion drugs for infectious diseases. Biochim Biophys Acta, Gen Subj. 2006;1760:527–537. doi: 10.1016/j.bbagen.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 44.Severi E., Hood D.W., Thomas G.H.Y. Sialic acid utilization by bacterial pathogens. Microbiology. 2007;153:2817–2822. doi: 10.1099/mic.0.2007/009480-0. 2007. [DOI] [PubMed] [Google Scholar]
- 45.Salcedo J., Barbera R., Matencio E., Alegría A., Lagarda M.J. Gangliosides and sialic acid effects upon newborn pathogenic bacteria adhesion: an in vitro study. Food Chem. 2013;136:726–734. doi: 10.1016/j.foodchem.2012.08.078. [DOI] [PubMed] [Google Scholar]
- 46.Cioffi D.L., Pandey S., Alvarez D.F., Cioffi E.A. Terminal sialic acids are an important determinant of pulmonary endothelial barrier integrity. Am J Physiol Cell Physiol. 2012;302:L1067–L1077. doi: 10.1152/ajplung.00190.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Yida, Mustapha U.I., Ismail M., Wong W., Abdullah M.A., Ideris A., et al. N-acetylneuraminic acid attenuates hypercoagulation on high fat diet-induced hyperlipidemic rats. Food Nutr Res. 2015;59 doi: 10.3402/fnr.v59.29046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang C., Cheng L., Shen B., Yuan Z., Feng Y., Lu S. Antihypertensive and antioxidant properties of sialic acid, the major component of edible bird's nests. Curr Top Nutraceutical Res. 2018;17:376–379. doi: 10.37290/ctnr2641-452X.17:376-379. [DOI] [Google Scholar]
- 49.Chen C.-H., Lin Y.-P., Lin J.-L., Li S.-T., Ren C.-T., Wu C.-Y., et al. Rapid identification of terminal sialic acid linkage isomers by Pseudo-MS3 mass spectrometry. Isr J Chem. 2015;55:412–422. doi: 10.1002/ijch.201400141. [DOI] [Google Scholar]
- 50.Guo C.-T., Takahashi T., Bukawa W., Takahashi N., Yagi H., Kato K., et al. Edible bird's nest extract inhibits influenza virus infection. Antivir Res. 2006;70:140–146. doi: 10.1016/j.antiviral.2006.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haghani A., Mehrbod P., Safi N., Kadir F.A.A., Omar A.R., Ideris A. Edible bird's nest modulate intracellular molecular pathways of influenza A virus infected cells. BMC Compl Alternative Med. 2017;17:22. doi: 10.1186/s12906-016-1498-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cao Y., Song W., Chen X. Multivalent sialic acid materials for biomedical applications. Biomater Sci. 2023;11:2620–2638. doi: 10.1039/D2BM01595A. [DOI] [PubMed] [Google Scholar]
- 53.Nazemi A., Haeryfar S.M.M., Gillies E.R. Multifunctional dendritic sialopolymersomes as potential antiviral agents: their lectin binding and drug release properties. Langmuir. 2013;29:6420–6428. doi: 10.1021/la400890f. [DOI] [PubMed] [Google Scholar]
- 54.Iijima R., Takahashi H., Namme R., Ikegami S., Yamazaki M. Novel biological function of sialic acid (N-acetylneuraminic acid) as a hydrogen peroxide scavenger. FEBS Lett. 2004;561:163–166. doi: 10.1016/S0014-5793(04)00164-4. [DOI] [PubMed] [Google Scholar]
- 55.Iijima R., Ichikawa T., Yamazaki M. Sialic acid attenuates the cytotoxicity of the lipid hydroperoxides HpODE and HpETE. Carbohydr Res. 2009;344:933–935. doi: 10.1016/j.carres.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 56.Yang C.-C., Yao C.-A., Yang J.-C., Chien C.-T. Sialic acid rescues repurified lipopolysaccharide-induced acute renal failure via inhibiting TLR4/PKC/gp91-mediated endoplasmic reticulum stress, apoptosis, autophagy, and pyroptosis signaling. Toxicol Sci. 2014;141:155–165. doi: 10.1093/toxsci/kfu121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yida Z., Imam M.U., Ismail M., Ismail N., Ideris A., Abdullah M.A. High fat diet-induced inflammation and oxidative stress are attenuated by N-acetylneuraminic acid in rats. J Biomed Sci. 2015;22:96. doi: 10.1186/s12929-015-0211-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yadav J., Verma A.K., Garg R.K., Ahmad K., Shiuli, Mahdi A.A., et al. Sialic acid associated with oxidative stress and total antioxidant capacity (TAC) expression level as a predictive indicator in moderate to severe Alzheimer's disease. Exp Gerontol. 2020;141 doi: 10.1016/j.exger.2020.111092. [DOI] [PubMed] [Google Scholar]
- 59.Chan G.K.L., Wong Z.C.F., Lam K.Y.C., Cheng L.K.W., Zhang L.M., Lin H., et al. Edible bird's nest, an asian health food supplement, possesses skin lightning activities: identification of N-acetylneuraminic acid as active ingredient. JCDSA. 2015;5:262–274. doi: 10.4236/jcdsa.2015.54032. [DOI] [Google Scholar]
- 60.Choi S.S.H., Baldwin N., Wagner V.O., Roy S., Rose J., Thorsrud B.A., et al. Safety evaluation of the human-identical milk monosaccharide sialic acid (N-acetyl-D-neuraminic acid) in Sprague-Dawley rats. Regul Toxicol Pharmacol. 2014;70:482–491. doi: 10.1016/j.yrtph.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 61.FDA GRAS exemption claim for N-Acetyl-D-Neuraminic acid (NANA) 2015. https://www.fda.gov/media/95353/download
- 62.Röhrig C.H., Choi S.S.H., Baldwin N. The nutritional role of free sialic acid, a human milk monosaccharide, and its application as a functional food ingredient. Crit Rev Food Sci Nutr. 2017;57:1017–1038. doi: 10.1080/10408398.2015.1040113. [DOI] [PubMed] [Google Scholar]
- 63.Fan Q., Lian J., Liu X., Zou F., Wang X., Chen M. A study on the skin whitening activity of digesta from edible bird's nest: a mucin glycoprotein. Gels. 2021;8:24. doi: 10.3390/gels8010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Świerczyńska M., Mirowska-Guzel D.M., Pindelska E. Antiviral drugs in influenza. Int J Environ Res Publ Health. 2022;19:3018. doi: 10.3390/ijerph19053018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yoshioka W., Nishino I., Noguchi S. Recent advances in establishing a cure for GNE myopathy. Curr Opin Neurol. 2022;35:629–636. doi: 10.1097/WCO.0000000000001090. [DOI] [PubMed] [Google Scholar]
- 66.Carrillo N., Malicdan M.C., Huizing M. GNE myopathy: etiology, diagnosis, and therapeutic challenges. Neurotherapeutics. 2018;15:900–914. doi: 10.1007/s13311-018-0671-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Argov Z., Caraco Y., Lau H., Pestronk A., Shieh P.B., Skrinar A., et al. Aceneuramic acid extended release administration maintains upper limb muscle strength in a 48-week study of subjects with GNE myopathy: results from a phase 2, randomized, controlled study. J Neuromuscul Dis. 2016;3:49–66. doi: 10.3233/JND-159900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lochmüller H., Behin A., Caraco Y., Lau H., Mirabella M., Tournev I., et al. A phase 3 randomized study evaluating sialic acid extended-release for GNE myopathy. Neurology. 2019;92:e2109–e2117. doi: 10.1212/WNL.0000000000006932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang H., Lu L., Chen X. An overview and future prospects of sialic acids. Biotechnol Adv. 2021;46 doi: 10.1016/j.biotechadv.2020.107678. [DOI] [PubMed] [Google Scholar]
- 70.Martinsson A., Raal A., Svennerholm L. Isolation of N-acetylsialic acid from normal liver. Biochim Biophys Acta. 1957;23:652. doi: 10.1016/0006-3002(57)90393-1. [DOI] [PubMed] [Google Scholar]
- 71.Zhao L., Tian R., Shen Q., Liu Y., Liu L., Li J., et al. Pathway engineering of Bacillus subtilis for enhanced N‐acetylneuraminic acid production via whole‐cell biocatalysis. Biotechnol J. 2019;14 doi: 10.1002/biot.201800682. [DOI] [PubMed] [Google Scholar]
- 72.Blayer S., Woodley J.M., Dawson M.J., Lilly M.D. Alkaline biocatalysis for the direct synthesis of N-acetyl-D-neuraminic acid (Neu5Ac) from N-acetyl-D-glucosamine (GlcNAc) Biotechnol Bioeng. 1999;66:131–136. doi: 10.1002/(SICI)1097-0290(1999)66:2<131::AID-BIT6>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 73.Datta A. Regulatory role of adenosine triphosphate on hog kidney N-acetyl-D-glucosamine 2-epimerase. Biochemistry. 1970;9:3363–3370. doi: 10.1021/bi00819a011. [DOI] [PubMed] [Google Scholar]
- 74.Lee Y.-C., Wu H.-M., Chang Y.-N., Wang W.-C., Hsu W.-H. The central cavity from the (α/α)6 barrel structure of Anabaena sp. CH1 N-acetyl-D-glucosamine 2-epimerase contains two key histidine residues for reversible conversion. J Mol Biol. 2007;367:895–908. doi: 10.1016/j.jmb.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 75.Hussain M.I., Zhang X., Lv X., Basharat S., Shahbaz U., Li J., et al. Enzymatic production of N-acetylneuraminic acid: advances and perspectives. Syst Microbiol Biomanuf. 2022;2:130–146. doi: 10.1007/s43393-021-00050-y. [DOI] [Google Scholar]
- 76.Kragl U., Gygax D., Ghisalba O., Wandrey C. Enzymatic two-step synthesis of N-acetyl-neuraminic acid in the enzyme membrane reactor. Angew Chem, Int Ed. 1991;30:827–828. doi: 10.1002/anie.199108271. [DOI] [Google Scholar]
- 77.Lee J.-O., Yi J.-K., Lee S.-G., Takahashi S., Kim B.-G. Production of N-acetylneuraminic acid from N-acetylglucosamine and pyruvate using recombinant human renin binding protein and sialic acid aldolase in one pot. Enzym Microb Technol. 2004;35:121–125. doi: 10.1016/j.enzmictec.2003.10.020. [DOI] [Google Scholar]
- 78.Zimmermann V., Hennemann H.-G., Daußmann T., Kragl U. Modelling the reaction course of N-acetylneuraminic acid synthesis from N-acetyl-D-glucosamine—new strategies for the optimisation of neuraminic acid synthesis. Appl Microbiol Biotechnol. 2007;76:597–605. doi: 10.1007/s00253-007-1033-6. [DOI] [PubMed] [Google Scholar]
- 79.Bloemendal V.R.L.J., Moons S.J., Jja Heming, Chayoua M., Niesink O., van Hest J.C.M., et al. Chemoenzymatic synthesis of sialic acid derivatives using immobilized N-acetylneuraminate lyase in a continuous flow reactor. Adv Synth Catal. 2019;361:2443–2447. doi: 10.1002/adsc.201900146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Klermund L., Riederer A., Groher A., Castiglione K. High-level soluble expression of a bacterial N-acyl-D-glucosamine 2-epimerase in recombinant Escherichia coli. Protein Expr Purif. 2015;111:36–41. doi: 10.1016/j.pep.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 81.Gao X., Zhang F., Wu M., Wu Z., Shang G. Production of N-acetyl-D-neuraminic acid by whole cells expressing Bacteroides thetaiotaomicron N-acetyl-D-glucosamine 2-epimerase and Escherichia coli N-acetyl-D-neuraminic acid aldolase. J Agric Food Chem. 2019;67:6285–6291. doi: 10.1021/acs.jafc.9b01839. [DOI] [PubMed] [Google Scholar]
- 82.Wang S., Li Y., Han Z., Chen X., Chen Q., Wang Y., et al. Molecular characterization of a novel N-acetylneuraminate lyase from a deep-sea symbiotic mycoplasma. Mar Drugs. 2018;16:80. doi: 10.3390/md16030080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tabata K., Koizumi S., Endo T., Ozaki A. Production of N-acetyl-D-neuraminic acid by coupling bacteria expressing N-acetyl-D-glucosamine 2-epimerase and N-acetyl-D-neuraminic acid synthetase. Enzym Microb Technol. 2002;30:327–333. doi: 10.1016/S0141-0229(01)00515-4. [DOI] [Google Scholar]
- 84.Lee Y.-C., Chien H.-C.R., Hsu W.-H. Production of N-acetyl-D-neuraminic acid by recombinant whole cells expressing Anabaena sp. CH1 N-acetyl-D-glucosamine 2-epimerase and Escherichia coli N-acetyl-D-neuraminic acid lyase. J Biotechnol. 2007;129:453–460. doi: 10.1016/j.jbiotec.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 85.Yan Q., Fong S.S. Design and modularized optimization of one‐step production of N‐ acetylneuraminic acid from chitin in Serratia marcescens. Biotechnol Bioeng. 2018;115:2255–2267. doi: 10.1002/bit.26782. [DOI] [PubMed] [Google Scholar]
- 86.Zhu D., Wu J., Zhan X., Zhu L., Jiang Y. Enhanced N-acetyl-D-neuraminic production from glycerol and N-acetyl-D-glucosamine by metabolically engineered Escherichia coli with a two-stage pH-shift control strategy. J Ind Microbiol Biotechnol. 2019;46:125–132. doi: 10.1007/s10295-018-02132-8. [DOI] [PubMed] [Google Scholar]
- 87.Zhang Q., Zhang J., Shao Y., Shang G. Escherichia coli BL21(DE3) optimized deletion mutant as the host for whole cell biotransformation of N-acetyl-D-neuraminic acid. Res Square. 2022 doi: 10.21203/rs.3.rs-2298932/v1. [DOI] [PubMed] [Google Scholar]
- 88.Chen X., Zhou J., Zhang L., Pu Z., Liu L., Shen W., et al. Development of an Escherichia coli-based biocatalytic system for the efficient synthesis of N-acetyl-D-neuraminic acid. Metab Eng. 2018;47:374–382. doi: 10.1016/j.ymben.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 89.Zhang X., Liu Y., Liu L., Wang M., Li J., Du G., et al. Modular pathway engineering of key carbon‐precursor supply‐pathways for improved N‐acetylneuraminic acid production in Bacillus subtilis. Biotechnol Bioeng. 2018;115:2217–2231. doi: 10.1002/bit.26743. [DOI] [PubMed] [Google Scholar]
- 90.Zhang X., Wang C., Lv X., Liu L., Li J., Du G., et al. Engineering of synthetic multiplexed pathways for high-level N-acetylneuraminic acid bioproduction. J Agric Food Chem. 2021;69:14868–14877. doi: 10.1021/acs.jafc.1c06017. [DOI] [PubMed] [Google Scholar]
- 91.Guo H., Tian R., Wang C., Zhao R., Lv X., Liu L., et al. Improved N-acetylneuraminic acid bioproduction by optimizing pathway for reducing intermediate accumulation. Food Bioeng. 2022;1:205–211. doi: 10.1002/fbe2.12030. [DOI] [Google Scholar]
- 92.Lu M., Mosleh I., Abbaspourrad A. Engineered microbial routes for human milk oligosaccharides synthesis. ACS Synth Biol. 2021;10:923–938. doi: 10.1021/acssynbio.1c00063. [DOI] [PubMed] [Google Scholar]
- 93.Kang J., Gu P., Wang Y., Li Y., Yang F., Wang Q., et al. Engineering of an N-acetylneuraminic acid synthetic pathway in Escherichia coli. Metab Eng. 2012;14:623–629. doi: 10.1016/j.ymben.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 94.Yang P., Wang J., Pang Q., Zhang F., Wang J., Wang Q., et al. Pathway optimization and key enzyme evolution of N-acetylneuraminate biosynthesis using an in vivo aptazyme-based biosensor. Metab Eng. 2017;43:21–28. doi: 10.1016/j.ymben.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 95.Pang Q., Han H., Liu X., Wang Z., Liang Q., Hou J., et al. In vivo evolutionary engineering of riboswitch with high-threshold for N-acetylneuraminic acid production. Metab Eng. 2020;59:36–43. doi: 10.1016/j.ymben.2020.01.002. [DOI] [PubMed] [Google Scholar]
- 96.Pang Q., Han H., Xu Y., Liu X., Qi Q., Wang Q. Exploring amino sugar and phosphoenolpyruvate metabolism to improve Escherichia coli N-acetylneuraminic acid production. J Agric Food Chem. 2020;68:11758–11764. doi: 10.1021/acs.jafc.0c04725. [DOI] [PubMed] [Google Scholar]
- 97.Peters G., Paepe B.D., Wannemaeker L.D., Duchi D., Maertens J., Lammertyn J., et al. Development of N-acetylneuraminic acid responsive biosensors based on the transcriptional regulator NanR. Biotechnol Bioeng. 2018;115:1855–1865. doi: 10.1002/bit.26586. [DOI] [PubMed] [Google Scholar]
- 98.Liu C., Lv X., Li J., Liu L., Du G., Liu Y. Metabolic engineering of Escherichia coli for increased bioproduction of N-acetylneuraminic acid. J Agric Food Chem. 2022;70:15859–15868. doi: 10.1021/acs.jafc.2c05994. [DOI] [PubMed] [Google Scholar]
- 99.Tian R., Liu Y., Chen J., Li J., Liu L., Du G., et al. 2019. Synthetic N-terminal coding sequences for fine-tuning gene expression and metabolic engineering in Bacillus subtilis; pp. 131–141. 55. [DOI] [PubMed] [Google Scholar]
- 100.Tian R., Liu Y., Cao Y., Zhang Z., Li J., Liu L., et al. Titrating bacterial growth and chemical biosynthesis for efficient N-acetylglucosamine and N-acetylneuraminic acid bioproduction. Nat Commun. 2020;11:5078. doi: 10.1038/s41467-020-18960-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cao Y., Tian R., Lv X., Li J., Liu L., Du G., et al. Inducible population quality control of engineered Bacillus Subtilis for improved N-acetylneuraminic acid biosynthesis. ACS Synth Biol. 2021;10:2197–2209. doi: 10.1021/acssynbio.1c00086. [DOI] [PubMed] [Google Scholar]
- 102.Plumbridge J., Vimr E. Convergent pathways for utilization of the amino sugars N-acetylglucosamine, N-acetylmannosamine, and N-acetylneuraminic acid by Escherichia coli. J Bacteriol. 1999;181:47–54. doi: 10.1128/JB.181.1.47-54.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ko Y.-S., Kim J.W., Lee J.A., Han T., Kim G.B., Park J.E., et al. Tools and strategies of systems metabolic engineering for the development of microbial cell factories for chemical production. Chem Soc Rev. 2020;49:4615–4636. doi: 10.1039/D0CS00155D. [DOI] [PubMed] [Google Scholar]
- 104.Lv X., Hueso-Gil A., Bi X., Wu Y., Liu Y., Liu L., et al. New synthetic biology tools for metabolic control. Curr Opin Biotechnol. 2022;76 doi: 10.1016/j.copbio.2022.102724. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.