ABSTRACT
Essential food workers experience elevated risks of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection due to prolonged occupational exposures in food production and processing areas, shared transportation (car or bus), and employer-provided shared housing. Our goal was to quantify the daily cumulative risk of SARS-CoV-2 infection for healthy susceptible produce workers and to evaluate the relative reduction in risk attributable to food industry interventions and vaccination. We simulated daily SARS-CoV-2 exposures of indoor and outdoor produce workers through six linked quantitative microbial risk assessment (QMRA) model scenarios. For each scenario, the infectious viral dose emitted by a symptomatic worker was calculated across aerosol, droplet, and fomite-mediated transmission pathways. Standard industry interventions (2-m physical distancing, handwashing, surface disinfection, universal masking, ventilation) were simulated to assess relative risk reductions from baseline risk (no interventions, 1-m distance). Implementation of industry interventions reduced an indoor worker’s relative infection risk by 98.0% (0.020; 95% uncertainty interval [UI], 0.005 to 0.104) from baseline risk (1.00; 95% UI, 0.995 to 1.00) and an outdoor worker’s relative infection risk by 94.5% (0.027; 95% UI, 0.013 to 0.055) from baseline risk (0.487; 95% UI, 0.257 to 0.825). Integrating these interventions with two-dose mRNA vaccinations (86 to 99% efficacy), representing a worker’s protective immunity to infection, reduced the relative infection risk from baseline for indoor workers by 99.9% (0.001; 95% UI, 0.0002 to 0.005) and outdoor workers by 99.6% (0.002; 95% UI, 0.0003 to 0.005). Consistent implementation of combined industry interventions, paired with vaccination, effectively mitigates the elevated risks from occupationally acquired SARS-CoV-2 infection faced by produce workers.
IMPORTANCE This is the first study to estimate the daily risk of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection across a variety of indoor and outdoor environmental settings relevant to food workers (e.g., shared transportation [car or bus], enclosed produce processing facility and accompanying breakroom, outdoor produce harvesting field, shared housing facility) through a linked quantitative microbial risk assessment framework. Our model has demonstrated that the elevated daily SARS-CoV-2 infection risk experienced by indoor and outdoor produce workers can be reduced below 1% when vaccinations (optimal vaccine efficacy, 86 to 99%) are implemented with recommended infection control strategies (e.g., handwashing, surface disinfection, universal masking, physical distancing, and increased ventilation). Our novel findings provide scenario-specific infection risk estimates that can be utilized by food industry managers to target high-risk scenarios with effective infection mitigation strategies, which was informed through more realistic and context-driven modeling estimates of the infection risk faced by essential food workers daily. Bundled interventions, particularly if they include vaccination, yield significant reductions (>99%) in daily SARS-CoV-2 infection risk for essential food workers in enclosed and open-air environments.
KEYWORDS: COVID-19, quantitative microbial risk assessment (QMRA), vaccinations, nonpharmaceutical interventions (NPIs), produce industry worker
INTRODUCTION
Essential food worker populations have been disproportionately affected by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) illness and death (1–5). For instance, Canadian migrant farmworkers experienced a 20-fold higher incidence of SARS-CoV-2 infections relative to the general population (July 2020) (6). Similarly, food and agricultural workers in California, USA, experienced a 39% increase in excess mortality compared to a 12% increase for workers in nonessential sectors (March to November 2020) (7). Workplace practices such as close proximity and long shifts may be contributing factors to this increased infection risk (1, 8). Additional environmental exposures may occur among food workers during shared transportation (carpooling, work bus) (9–11) and in employer-provided housing (e.g., crowding, poor ventilation) (12–18). For example, a 2022 study found that of nearly 2.5 million confirmed SARS-CoV-2 infections in the Czech Republic, 27% of the infections originated either from work-related causes or contacts, while 73% originated from out-of-work contacts, with food workers noted as one of the highest incidence populations (19). Protecting essential food workers from SARS-CoV-2 is necessary to reduce the burden of disease among this often understudied and marginalized population and to ensure stability in the global food supply chain (20–22).
Global agricultural organizations (Food and Agriculture Organization of the United Nations, U.S. Department of Agriculture) and food trade associations (Global Cold Chain Alliance, American Frozen Food Institute) have recommended integrating SARS-CoV-2 infection control strategies (e.g., distancing, masking, symptom screening, vaccination, ventilation) to reduce transmission among food workers (23–27). However, despite the demonstrated effectiveness of these interventions among the general population during short-duration activities, their impact on protecting essential food workers during extended-duration (e.g., a whole day) activities has been poorly characterized (28–37). For example, outdoor seasonal and migratory workers involved in produce production, harvest, and processing have extended SARS-CoV-2 exposure during their daily activities (e.g., field harvesting, shared transportation, crowded employer-provided housing) (38–40). To our knowledge, there are limited studies quantifying the impact of SARS-CoV-2 infection control strategies to reduce transmission among food workers. One study, within a pork processing plant, found that SARS-CoV-2 PCR screening averted 7 to 40% of clinical SARS-CoV-2 cases among food workers (41). A second study, for indoor food workers, found that multiple SARS-CoV-2 infection control strategies (masking, distancing, vaccination) reduced worker infection risk below 1.0% within an indoor food processing and packaging environment (42). A third study, for indoor cold-chain food workers, found that standard SARS-CoV-2 infection control strategies (masking, distancing, vaccination) protected food workers from fomite-mediated transmission during indoor cold-chain packaging and transport activities (43). These studies focused on an indoor environment and only the processing or packaging activity but did not consider the outdoor environment or other daily food worker activities (e.g., field harvesting, shared transportation, crowded employer-provided housing) that may heighten SARS-CoV-2 infection risk among produce workers, including seasonal and migratory workers.
To address this need, the purpose of this study was to evaluate the impact of combined food industry interventions and worker vaccinations on reducing the daily cumulative risk of SARS-CoV-2 infection from occupational exposures to SARS-CoV-2 in the agricultural environment. Using a novel modular stochastic quantitative microbial risk assessment (QMRA) approach, we characterized the cumulative risk of SARS-CoV-2 infection across sequential environmental scenarios experienced daily by both indoor and outdoor food workers. This novel QMRA approach was then used to quantify the effect of individual and combined risk mitigation interventions at reducing SARS-CoV-2 infection risk across all indoor and outdoor scenarios. The findings of this work can be used by the produce industry to protect the health and well-being of this essential workforce from infection comprehensively across daily, rather than solely one-time, exposure to SARS-CoV-2 and future novel respiratory pathogens.
RESULTS
Scenario-specific and cumulative daily SARS-CoV-2 infection risks for an indoor produce worker with and without infection control measures.
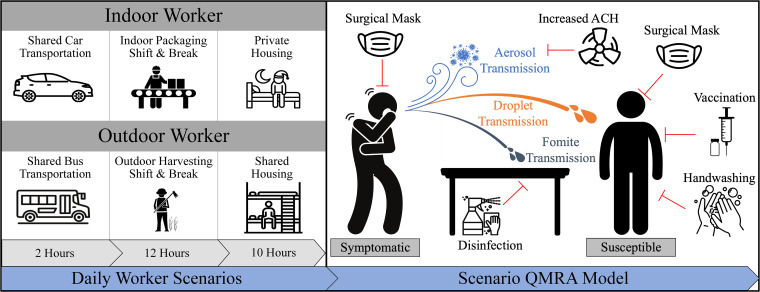
We investigated the individual and cumulative SARS-COV-2 infection risk to a susceptible indoor produce worker (Fig. 1) across four daily scenarios: shared car transportation (2 h), an indoor facility work shift (11 h), break in the indoor facility breakroom (1 h), and private housing (10 h). With no infection control strategies applied, the lowest-risk scenario was the indoor breakroom (Table 1), whereas the highest-risk scenario was at 1-m distancing during the enclosed work shift. In the 11-h work shift scenario, the relative risk decreased by 47% when the distance was increased between workers from 1 m (1.00) to 2 m (0.530). However, due to the other daily exposures, the 24-h daily cumulative risk remained above 0.86 for both 1- and 2-m work shift distancing. After implementing individual infection control strategies across each modeled scenario, for the 24-h daily cumulative risk at 1 m, universal double masking resulted in the greatest relative risk reduction of 62%, while surgical masking reduced the risk by 22%, and cloth masking by 10%. For the 24 h daily cumulative risk at 1 m, increasing ventilation rates across each scenario resulted in a 8% relative risk reduction, while hourly handwashing and surface disinfection twice during the work shift provided no relative risk reduction (0%). The 24-h daily cumulative risk at 2 m was lower for each intervention assessed compared to the 24-h daily cumulative risk at 1 m.
FIG 1.
Environmental scenarios affecting daily cumulative risks of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) exposure for an indoor and outdoor produce worker. (Left) Daily worker scenarios. Simulations were conducted in which workers were engaged in three overarching environmental scenarios: 2 h of shared transportation to and from work (aerosol exposure only), a 12-h working shift (aerosol, droplet, fomite exposure), and 10 h of private (no exposure) or shared housing (aerosol, droplet, fomite exposure). (Top left) The indoor worker was assumed to travel in the back of an enclosed, midsized car for 2 h total with an infectious symptomatic passenger seated in front of them, allowing for exposure to virus-containing aerosols. During the indoor work shift, the uninfected susceptible and infectious symptomatic worker were assumed to spend 11 h physically distanced (2 m) while working on a conveyor line, with 1 h spent in an indoor breakroom. The indoor worker was assumed to not participate in shared housing. (Bottom left) The outdoor worker was assumed to travel in an enclosed school bus for 2 h total with an infectious symptomatic passenger seated >3 m away from the uninfected susceptible worker, allowing for exposure to virus-containing aerosols. Given the nature of the outdoor work shift, the uninfected susceptible and infectious symptomatic workers were assumed to spend 11 h in close-contact range (1 m) while working in the open-air harvesting field, with 1 h spent on the school bus functioning as a breakroom. The outdoor uninfected susceptible worker was assumed to participate in employer-provided shared housing for 10 h with an infectious symptomatic co-worker. Of the 10 h, it was assumed that the workers would spend 2 h physically distanced (2 m) in the space, while the remaining 8 h would be spent sleeping in the same room (aerosol exposure only). (Right) Scenario quantitative microbial risk assessment (QMRA) model. Each scenario simulates the risk of SARS-CoV-2 infection for an uninfected and susceptible worker after exposure to an infected and symptomatic co-worker across viral transmission (aerosol-, droplet-, and/or fomite-mediated) pathways. Each scenario also quantifies the impact of infection control strategies (red lines), adjusted for the context of each scenario. For example, universal masking (defined as both workers wearing the same type of mask) was applied to both the infected and susceptible worker, increased air exchange and surface disinfection was applied to the modeled environmental scenario, and both the hand hygiene and vaccination interventions were applied to the susceptible worker alone (see Materials and Methods). ACH, air exchange rate.
TABLE 1.
Cumulative risk and risk reduction of SARS-CoV-2 infection for indoor workersa
| Infection control strategy | Shared car transportation | Work shift distancing |
Break in breakroom | Private housing | Daily cumulative riskb |
||
|---|---|---|---|---|---|---|---|
| 1 m | 2 m | 1-m distancing | 2-m distancing | ||||
| Nonec | 0.698 (0.356 to 0.955) | 1.00 (0.983 to 1.00) | 0.530 (0.246 to 0.850) | 0.024 (0.009 to 0.058) | None | 1.00 (0.995 to 1.00) | 0.865 (0.571 to 0.992) |
| Handwashing and surface disinfectiond | No | 1.00 (0.00%) | 0.426 (19.7%) | No | None | 1.00 (0.00%) | 0.833 (3.78%) |
| Cloth maskd | 0.163 (76.7%) | 0.866 (13.4%) | 0.148 (72.0%) | No | None | 0.896 (10.4%) | 0.303 (65.0%) |
| Surgical maskd | 0.108 (84.5%) | 0.749 (25.1%) | 0.076 (85.7%) | No | None | 0.785 (21.5%) | 0.186 (78.6%) |
| Double maskd | 0.025 (96.4%) | 0.332 (66.8%) | 0.025 (95.3%) | No | None | 0.378 (62.2%) | 0.058 (93.3%) |
| Increased air exchanged | 0.126 (81.9%) | 0.904 (9.64%) | 0.081 (84.7%) | 0.004 (81.7%) | None | 0.923 (7.74%) | 0.217 (75.0%) |
The table shows the cumulative risk and risk reduction (%) of SARS-CoV-2 infection for a susceptible indoor produce worker exposed to an infected co-worker. “No” indicates that the infection control strategy was not applied (see Methods). “None” means that there was no risk of infection in the private housing scenario. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Assuming there is contact between an infected and susceptible individual in every environmental scenario except for private housing.
The values represent median (95% uncertainty interval) SARS-CoV-2 infection risk.
The values represent median (% reduction) SARS-CoV-2 infection risk compared to no infection control strategy.
Scenario-specific and cumulative daily SARS-CoV-2 infection risks for an outdoor produce worker with and without infection control measures.
Next, we investigated the individual and cumulative infection risk to a susceptible outdoor food worker (Fig. 1) across four daily scenarios: shared bus transportation (2 h), an outdoor field work shift (11 h), a rest break in the bus (1 h), and shared housing (10 h). Without infection control measures, the lowest-risk scenario was at the 2-m distance during the outdoor field work shift (Table 2), whereas the highest infection risk was the outdoor field shift at 1-m distancing. In the 11-h outdoor work shift, the relative risk decreased by 97% when the distance was increased between workers from 1 m (0.322) to 2 m (0.009). However, due to the other daily exposures, the 24-h daily cumulative risk remained above 0.21 for both 1- and 2-m distancing. After implementing individual infection control strategies across each modeled scenario, for the 24-h daily cumulative risk at 1 m, universal double masking resulted in the greatest relative risk reduction of 92%, while surgical masking reduced the risk by 84%, and cloth masking reduced the risk by 77%. For the 24-h daily cumulative risk at 1 m, increasing ventilation rates in the shared bus transportation and shared housing scenarios resulted in a 15% relative risk reduction, while hourly handwashing and surface disinfection twice during the 11-h outdoor work shift provided a nominal relative risk reduction of <1%. The 24-h daily cumulative risk at 2 m was lower for each intervention assessed compared to the 24-h daily cumulative risk at 1 m.
TABLE 2.
Cumulative risk and risk reduction of SARS-CoV-2 infection for outdoor workersa
| Infection control strategy | Shared bus transportation | Work shift distancing |
Rest break in the bus | Shared housing | Daily cumulative riskb |
||
|---|---|---|---|---|---|---|---|
| 1 m | 2 m | 1-m distancing | 2-m distancing | ||||
| Nonec | 0.010 (0.003 to 0.032) | 0.322 (0.097 to 0.779) | 0.009 (0.004 to 0.021) | 0.012 (0.005 to 0.029) | 0.189 (0.074 to 0.415) | 0.487 (0.257 to 0.825) | 0.216 (0.096 to 0.445) |
| Handwashing and surface disinfectiond | No | 0.320 (0.56%) | 0.008 (13.1%) | No | No | 0.486 (0.25%) | 0.215 (0.49%) |
| Cloth maskd | 0.001 (85.4%) | 0.056 (82.7%) | 0.002 (81.0%) | No | 0.034 (82.1%) | 0.112 (77.1%) | 0.051 (76.5%) |
| Surgical maskd | 9.5 × 10−4 (90.7%) | 0.035 (89.1%) | 0.001 (86.8%) | No | 0.024 (87.3%) | 0.080 (83.7%) | 0.040 (81.6%) |
| Double maskd | 2.2 × 10−4 (97.8%) | 0.009 (97.1%) | 3.7 × 10−4 (95.9%) | No | 0.009 (95.3%) | 0.038 (92.2%) | 0.024 (89.0%) |
| Increased air exchanged | 0.006 (45.3%) | NA | NA | No | 0.101 (46.4%) | 0.413 (15.1%) | 0.128 (40.8%) |
The table shows the cumulative risk and risk reduction (%) of SARS-CoV-2 infection for a susceptible outdoor produce worker exposed to an infected co-worker. “No” indicates that the infection control strategy was not applied (see Methods). “NA” means that it is not applicable to apply increased air exchange in an outdoor environment. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Assuming there is contact between an infected and susceptible individual in every worker scenario.
The values represent median (95% uncertainty interval) SARS-CoV-2 infection risk.
The values represent median (% reduction) SARS-CoV-2 infection risk compared to no infection control strategy.
Impact of combined infection control strategies and vaccination on the daily cumulative risk of SARS-CoV-2 infection for essential produce workers.
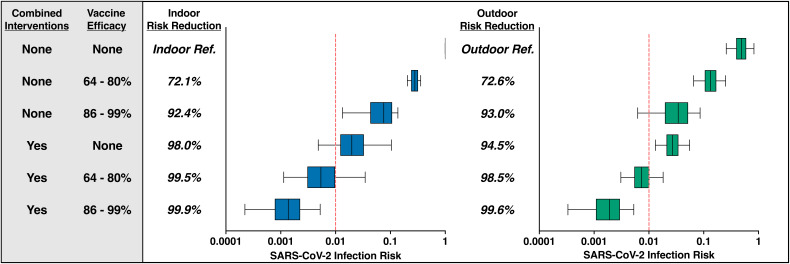
We then investigated the impact of combined infection control measures with and without vaccination on the daily cumulative SARS-CoV-2 risk for the indoor produce worker (Fig. 2, left). Without vaccination, combining hand hygiene, surface disinfection, universal surgical mask usage, physical distancing to 2 m, and increased ventilation strategies across the indoor work scenarios reduced the relative daily infection risk by 98.0% for the indoor worker (0.020; 95% uncertainty interval [UI], 0.005 to 0.104) compared to the baseline risk at 1 m (1.00; 95% UI, 0.995 to 1.00). These combined infection control measures were found to be more effective than vaccination alone (72.1 to 92.4% suboptimal and optimal vaccination relative risk reduction, respectively). Combining infection control strategies with vaccination further reduced the relative daily infection risks between 99.5 and 99.9% for the indoor worker (suboptimal vaccine, 0.005 [95% UI, 0.001 to 0.035]; optimal vaccine, 0.001 [95% UI, 0.0002 to 0.005]). We also investigated the impact of these combined infection control measures with and without vaccination on the daily cumulative infection risk for the outdoor produce worker (Fig. 2, right). Combining hand hygiene, surface disinfection, universal surgical mask usage, physical distancing to 2 m, and increased ventilation strategies across the applicable outdoor worker scenarios reduced the relative daily infection risk by 94.5% (0.027; 95% UI, 0.013 to 0.055) compared to the baseline risk at 1 m (0.487; 95% UI, 0.257 to 0.825). Combining infection control strategies with vaccination further reduced the relative daily infection risks between 98.5 and 99.6% for the outdoor worker (suboptimal vaccine, 0.007 [95% UI, 0.003 to 0.018]; optimal vaccine, 0.002 [95% UI, 0.0003 to 0.005]). For both the indoor and outdoor worker, the combined infection control measures and vaccinations led to an absolute daily risk of ≤0.002.
FIG 2.
Essential produce workers experience the greatest reduction in SARS-CoV-2 infection risk when vaccinations are applied in combination with recommended infection control strategies. The combined interventions column represents simultaneous surgical mask usage, hourly handwashing, surface disinfection (twice per work shift), increased physical distancing to 2 m, and an increased air exchange rate per scenario. Within the vaccine efficacy column, the 64 to 80% vaccine efficacy range represents the suboptimal vaccine, while the 86 to 99% vaccine efficacy represents the optimal vaccine, both of which represent a worker’s level of immunity to any infection, asymptomatic or symptomatic (see Materials and Methods for details). Relative risk reductions were calculated by comparing the risk estimate per intervention scenario against the baseline (no intervention, 1-m work shift distance) risk estimate. The top row represents the baseline (reference) daily cumulative infection risk for an indoor (left) and outdoor (right) produce worker across the 2 h of shared transportation, 12-h work shift at 1 m, and 10 h of shared housing scenario (outdoor worker only) with no infection control strategies applied. The median risk of infection is denoted by the line within each box plot, with error bars representing the 95% uncertainty interval. Finally, a vertical line has been added to denote a 24-h infection risk of 1.0%.
Sensitivity analyses.
Spearman rank correlation coefficients were calculated for each scenario to identify the parameters that were the most influential to the final SARS-CoV-2 exposure dose estimate. Across all scenarios, the parameters identified as most influential at increasing virus exposure were the viral shedding rate per hour (ρavg = 0.94), the infected worker’s salivary virus concentration (ρavg = 0.85), and the coughing frequency (ρavg = 0.34). The parameters identified as most influential in decreasing virus exposure across all scenarios were the infected worker’s double masking (ρavg = −0.74), the susceptible worker’s surgical masking (ρavg = −0.69), and the susceptible worker’s cloth masking (ρavg = −0.55). The car transportation and outdoor work shift scenarios were found to be affected the most by parameter variability, with overall variability ratios calculated to be 4.24 (aerosol transmission) and 4.48 (droplet transmission), respectively. Finally, the overall uncertainty ratio, representing the combined effect of parameter variability and uncertainty, was largest for the viral dose on a susceptible worker’s hand (6.13) and the Brownian diffusivity (4.29) calculated for aerosol particle exposure. Please see supplemental material for additional details on sensitivity analyses (Fig. S2A and B and S3A and B) or uncertainty (Table S3).
DISCUSSION
The goal of this study was to estimate the impact of recommended infection control interventions on the daily cumulative SARS-CoV-2 infection risk to produce workers (i.e., working in an indoor facility or open outdoor farm) when exposed to various environmental scenarios (i.e., 1- or 2-m distancing, shared transportation, with or without shared housing). Among the environmental scenarios assessed for an indoor worker, we found the highest risk to be associated with the 1-m distancing indoor work shift, followed by shared car transportation. For an outdoor worker, the highest risk was associated with the 1-m distancing outdoor work shift, followed by shared housing. When evaluating the scenario-specific impact of each infection control intervention, we found that double masking provided the greatest protection for the indoor worker, while increased physical distancing between workers provided the greatest protection for outdoor workers. However, the greatest reduction (>99%) in daily SARS-CoV-2 infection risk (≤0.002) was observed when the combined infection control interventions were paired with optimal SARS-CoV-2 vaccinations for both indoor and outdoor workers. Despite elevated SARS-CoV-2 exposures throughout daily produce worker activities, these results suggest that current risk mitigation strategies, when implemented together, can effectively protect essential food workers from infection.
Our findings for the indoor packaging facility and outdoor harvesting field are consistent with data from well documented outbreaks across multiple farms and food processing facilities globally (1, 2, 22, 44, 45), which have highlighted the increased duration of contact and close proximity of workers on assembly lines as factors contributing to an elevated infection risk. In our study, one contributing factor for this elevated risk is likely the extended exposure duration (11-h work shift and 1-h break) to a coughing infected worker compared to other scenarios for which exposure durations are in the 1- to 2-h range. We also explored the impact of physical distancing during the work shifts and found that in the indoor facility and outdoor field, increasing the distance between workers from 1 to 2 m provided a 47.0% (indoor) and 97.2% (outdoor) relative reduction in infection risk. Consistent with modeling work by Wei and Li (46), there is an attenuation in distance traveled by respiratory particles with increasing size: small-diameter (<50 μm) respiratory aerosols can travel beyond 4 m after coughing, whereas 99% of large-diameter droplets (>100 μm) fall to adjacent surfaces within 2 m of their point of origin. As demonstrated here and in our previous modeling work, increasing the distance between workers allows for a shift in the predominant transmission route from close-contact droplets and aerosols to exclusively aerosols (<50 μm; responsible for 1.3% of expelled viral dose), further exemplifying the importance of physical distancing to reduce infection risk (42).
The elevated infection risk found in the shared car transportation scenario is consistent with the work of Ng et al. (47), who found that sharing a vehicle with an individual infected with SARS-CoV-2 was associated with a 3.05-fold increase in the odds of infection. This finding is likely due to the relatively low volume of air space (2.6 m3) within the car, combined with the elevated persistence of infectious aerosols over multiple hours (48). For example, after increasing the volume inside the modeled car by 3-fold to 7.8 m3 and keeping all other parameters constant, we observed a 55.4% reduction in the 2-h infection risk (0.311; 95% UI, 0.130 to 0.616) attributed to volume alone. Given our assumption of a well mixed environment in which the persistent aerosol particles are homogeneously distributed, one would expect aerosol exposures and subsequent infection risks to decrease as the air volume within the scenario increases. Similarly, conditions such as poor ventilation and small, enclosed employer-provided housing have been reported in various outbreaks among outdoor produce workers (14–18). For example, in July 2020, a California farmworker housing facility with five workers per room documented a 204-person SARS-CoV-2 outbreak (49). As others have reported (50–53), the smaller the size of the enclosed space and the more individuals in that space, the greater the risk of SARS-CoV-2 transmission. Taken together, these scenarios highlight the interconnected relationship between duration of exposure, distancing between individuals, and the size/volume of the enclosed space on SARS-CoV-2 infection risk.
Increased ventilation, relative to other infection control strategies, resulted in the greatest relative reduction in daily infection risk for an indoor worker (75.0% reduction at 2 m), while double masking provided the largest protective effect for both indoor (93.3% reduction at 2 m) and outdoor workers (92.2% reduction at 1 m). Our findings are consistent with the increasing evidence that higher ventilation rates, when applied appropriately and without recirculation, can reduce SARS-CoV-2 exposure and subsequent infection risk (54–57). Mechanistically, the overall airborne concentration of virus-containing particles can be reduced through natural or mechanical increases in ventilation rates, thereby reducing the viral dose exposure to a susceptible worker (36). An important limitation to this intervention has been noted by Lee and Ahn (55), who found that even at high ventilation rates of 20 h−1, this intervention could not overcome the high infection risk associated with an infected and susceptible worker sustaining contact in small, enclosed office spaces (20 m3). This suggests that larger facilities, like the indoor packing facility modeled here (460 m3), would likely see a greater reduction in SARS-CoV-2 infection risk upon increasing their ventilation rate, compared to a smaller facility or office spaces.
Consistent with numerous laboratory (58–60) and empirical (28, 61) studies testing risk reductions associated with wearing face masks, our findings demonstrate that masks are an effective tool for reducing SARS-CoV-2 infection risk. Of the masks modeled, double masking (surgical mask followed by cloth mask) provided the greatest relative reduction in daily risk, followed by surgical and cloth masks. Given the increased transmissibility of newer SARS-CoV-2 variants (Delta: B.1.617.2; and Omicron: B.1.1.159 [62, 63]), the CDC has recently revised masking recommendations and has transitioned from promoting double masking to now promoting N95 and KN95 respirators, while surgical masks continue to be recommended for industry by Occupational Safety and Health Administration (OSHA) (64–66). After incorporating the universal usage of optimally fit N95 respirators (58) throughout our model (data not shown), the 24-h daily cumulative risk at 1 m for an indoor (0.033; 95% UI, 0.013 to 0.098) and outdoor (0.016; 95% UI, 0.007 to 0.033) produce worker was reduced by 96.7 and 96.8%, respectively. Given the documented facemask accessibility issues faced by produce workers at the onset of the pandemic (67, 68), the implementation of N95 respirators, though of superior risk reduction, is likely not feasible in this occupational setting. However, our findings support the promotion of double masking in these occupational scenarios to further minimize infection risk beyond the use of cloth or surgical masks alone.
Our work provides novel insight into the effectiveness of combining vaccination with food industry-recommended infection control strategies to mitigate daily SARS-CoV-2 infection risk among essential produce workers. As demonstrated with the agent-based modeling studies of Farthing and Lanzas (69) and Kerr et al. (70), while individual interventions (e.g., physical distancing, masking, vaccinations, symptomatic testing, etc.) provided modest reductions in the expected number of SARS-CoV-2 infections, implementing multiple interventions simultaneously provided synergistic reductions in expected infections. It is important to note that this multipronged approach assumes absolute compliance with the interventions described; however, the layering of risk reduction practices is not new to the produce industry (71). As demonstrated by Mogren et al. (72), the “hurdle approach” of enforcing multiple risk reduction strategies with variable degrees of compliance can provide greater reductions in risk than a single intervention with poor compliance.
The strengths of our model include a detailed exposure assessment designed to simulate the environmental scenarios experienced daily by indoor and outdoor produce workers through a linked-modeling approach, vetting by academic and food industry partners to inform and endorse the design of our modeled environmental scenarios, and a versatile modeling framework that can be leveraged to evaluate novel SARS-CoV-2 strains or future respiratory pathogens and additional settings outside the food production and processing industry. To our knowledge, this is the first QMRA for SARS-CoV-2 that has established a linked-modeling approach to quantify the risk of infection both within and across a variety of environmental scenarios to estimate the scenario-specific and daily cumulative risks of infection. In doing so, we have also been able to elucidate the variable impact of infection control interventions across these individual, realistic, and context-driven environmental scenarios, which can be utilized by food industry managers to apply targeted, personalized, and data-informed interventions that would best protect their workforce from infection in their specific occupational context. Furthermore, the framework of our model not only allows for the estimation of infection risk across a variety of additional indoor (e.g., schools, hospitals, restaurants) and outdoor (e.g., open-air markets, recreational venues) environmental scenarios but also establishes a foundational modeling structure that could be adjusted for novel SARS-CoV-2 strains (e.g., increased salivary titers, lower infectious dose) or even other future respiratory pathogen outbreaks. While these multiscenario features are not commonly applied to QMRA models and have been the focus of expansive agent-based modeling work (70), this study highlights the capabilities of QMRA modeling to extend beyond one-time scenario interactions between an infectious and susceptible individual to assess interactions across multiple scenarios.
As for potential limitations, the first would be our assumption of a uniform distribution of viral concentration across all respiratory particle size classes. While this assumption has been implemented by the aerosol modeling work of Zhang et al. (73), it is probably not entirely representative of what is happening in real-world situations. This could have led to an artificially increased risk associated with close proximity (≤1 m) exposure to droplets, given their large diameters (100 to 750 μm) and, by assumption, a higher concentration of infectious SARS-CoV-2 virus. A second limitation is that our model uses the dose-response relationship of SARS-CoV-1 to assess infection risk. Given that the SARS-CoV-2 dose-response parameter(s) have yet to be defined in the literature, this approach has become common practice across multiple SARS-CoV-2 QMRA studies (73–76). We took a multipronged approach to address this limitation, first by generating an approximate SARS-CoV-2 dose-response parameter that translated to an 50% infective dose (ID50) of 102 viral particles, which is within the 10 to 1,000 viral particle range for similar respiratory pathogens (77, 78). Next, we calibrated the viral shedding parameter by using clinical data collected from patients during the first week of symptom onset and a 1:100 conversion factor from PCR-based genome equivalent copies to PFU (79–82), resulting in viral titers of 6.1 to 7.4 log10 PFU. Finally, we calculated the average indoor risk of infection after a 1-h exposure at 1 m to be 0.678 (95% UI, 0.270, 0.984), which is within the attack rate range of well documented SARS-CoV-2 outbreaks in meat processing (attack rate [AR], 30.2%) (8), households (AR, 52.7%) (83), recreational spaces (AR, 53.3 to 86.8%) (84), and food preparation facilities (AR, 60.0%) (85). Taken together, our approach ensures that we are providing risk estimates in realistic, context-specific scenarios that are within the range of documented SARS-CoV-2 outbreaks, while not underestimating the potential infection risk. Another limitation is that we assumed discordant vaccination status (e.g., only the susceptible worker had received a vaccination) among the infected and susceptible produce workers modeled. While vaccine hesitancy has been well documented among the food worker population (86, 87), these findings suggest that daily infection risk can be reduced by promoting a single-dose (73%) or two-dose vaccine (93%). This is of particular importance for the outdoor agricultural worker community, as the transient nature of this worker population highlights the need for public health efforts to continue promoting and advocating for vaccinations in these communities that can reduce infection risk, regardless of occupational setting. Future work is needed to understand the SARS-CoV-2 vaccination coverage levels within this essential worker community, the findings of which could then be incorporated into the present model to increase the generalizability of our results. Lastly, quantitative microbial risk assessment models are not able to easily incorporate the diverse characteristics of groups of susceptible individuals that could lead to a heightened baseline risk of infection (i.e., age or comorbidities such as hypertension, obesity, or diabetes mellitus [88]) or convey the severity and outcome of the infection (i.e., mild asymptomatic or symptomatic disease, moderate disease requiring hospitalization, or severe disease resulting in death). Future work could address these limitations by integrating QMRA models with compartment-based modeling approaches (e.g., susceptible-exposed-infected-recovered [SEIR]). This integration could evaluate the SARS-CoV-2 interventions, infection disease course, and outcomes at the individual and population levels for susceptible produce workers.
Our model has demonstrated that the elevated daily SARS-CoV-2 infection risk experienced by indoor and outdoor produce workers can be reduced to ≤2 × 10−3 when vaccinations (optimal vaccine efficacy, 86 to 99%) are combined with recommended infection control strategies. Given their consistency in reducing infection risk for both indoor and outdoor produce workers (92.4 to 93.0% reduction), vaccinations should continue to be prioritized as an effective means by which to maintain a healthy workforce and reliable global food supply. However, our findings show that vaccinations alone are not sufficient to bring the cumulative daily infection risk below a 0.01 threshold for either indoor or outdoor worker populations. This underscores the need for vaccinations to be combined with additional infection control interventions (e.g., handwashing, surface disinfection, universal masking, physical distancing, and increased ventilation) implemented by the food industry to further reduce the potential for SARS-CoV-2 transmission. These findings provide additional evidence supporting international (European Union OSHA, WHO), domestic (CDC, U.S. OSHA), and industry-specific (FDA, American Frozen Food Institute [AFFI]) recommendations for preventing SARS-CoV-2 transmission during food production and processing shifts and across various day-to-day exposure scenarios (e.g., shared transportation, employer-provided housing, etc.) (21, 27, 66, 89–96). Taken together, these results highlight the need for produce industry management to continue promoting vaccine uptake in their workforce and support the use of industry-recommended infection control strategies when managing SARS-CoV-2 transmission among essential workers.
MATERIALS AND METHODS
Model overview.
The model outcomes include (i) the scenario-specific and the daily cumulative SARS-CoV-2 infection risk for a susceptible produce worker quantified across three viral transmission pathways (aerosol-, droplet-, and fomite-mediated) and (ii) the individual and combined impact of recommended infection control strategies on reducing the daily cumulative risk of SARS-CoV-2 infection for a susceptible produce worker. Leveraging calculations described in our previous SARS-CoV-2 QMRA model (42), we quantified the risk of infection across three transmission pathways (aerosols, droplets, and fomites) for the environmental scenarios pertinent to both indoor and outdoor food workers (e.g., shared transportation, work shift, work break, private/shared housing). Each scenario assumed a single infected symptomatic worker either coughed or breathed virus-laden respiratory aerosols (defined here as particles with a diameter <50 μm) and droplets (particle diameter 50 to 750 μm) that could infect a susceptible worker. We did not model an infected asymptomatic worker because of their low risk to a susceptible worker, due to low emission of viral particles from breathing (42). Briefly, the aerosol transmission pathway assumed a well mixed environment in which all virally contaminated aerosols would be homogenously distributed throughout the volume of the modeled scenario and would accumulate in the environment over time (73, 97, 98). The aerosols could be removed from the scenario by the scenario-specific air exchange rate or through the relative humidity- and temperature-specific viral decay rate. The droplet-mediated transmission pathway assumed that the virally contaminated droplets would be emitted from the infected worker’s cough and reach distances of 1 to 3 m based on their size and would not accumulate in the air over time (99–101). Finally, the fomite-mediated transmission pathway assumed that contamination would occur through the fallout of both aerosols and droplets onto the fomite surface based on the particle’s settling velocity and gravitational trajectory (102). These particles could be removed from the fomite through either the surface-specific viral decay rate or by surface disinfection. For each modeled scenario, the susceptible worker’s viral dose was combined across the aerosol-, droplet-, and fomite-mediated transmission pathways to obtain a cumulative, scenario-specific viral dose. These environmental scenarios were linked for either an indoor or an outdoor worker, and indoor and outdoor workers had a different set of linked environmental scenarios as described below. All models were constructed in R (version 4.0.3; R Development Core Team; Vienna, Austria) using 10,000 Monte Carlo iterations of literature-derived parameters and their probability distributions, to provide daily cumulative infection risks for each type of worker. Details regarding the QMRA modeling parameters are summarized in Table 3 (79, 80, 103–111). Additional model parameters used to inform the SARS-CoV-2 viral transmission pathways are summarized in Table S1 and grouped into three categories: (i) viral shedding through coughing respiratory events, (ii) fomite-mediated transmission and dose-response parameters, and (iii) risk mitigation interventions (infection control interventions and vaccinations).
TABLE 3.
QMRA parameters for SARS-CoV-2 viral transmissiona
| Parameter | Units | Description | Input valuesb | Distribution | References |
|---|---|---|---|---|---|
| Log10(Cvirus) | PFU·mL−1 | Concn of virus in saliva | 6.80 (6.10, 7.40) | Triangular | 79, 103 |
| FC | Cough·h−1 | Coughing rate per hour | 24.7 (10.0, 39.3) | Uniform | 104 |
| Handsa | m2 | Surface area of two palms | 1.8 × 10−2 (1.5 × 10−2, 2.2 × 10−2) | Triangular | 105, 106 |
| λhand | min−1 | Viral decay on hand surface | 1.20 (0.92, 1.47) | Uniform | 107 |
| Freq.hs | Contact·min−1 | Contact frequency between hands and fomite | 8.4 (5.4, 11.4) | Triangular | 108 |
| Freq.hf | Contact·min−1 | Contact frequency between hands and face | 0.261 (0.072, 0.450) | Triangular | 109 |
| F 23 | Proportion | Proportion of virus transferred from hand to face | 0.200 (0.063) | Normal | 110 |
| L dep | Proportion | Deposition fraction of virus into the lungs | 1.00 | Point | Assumed |
| I R | m3·h−1 | Inhalation rate per hour | 2.40 (1.62, 3.18) | Uniform | 111 |
| k risk c | PFU−1 | Dose-response parameter to determine infection risk at a given viral dose | 6.80 × 10−3 | Point | 80 |
QMRA, quantitative microbial risk assessment; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
The values presented as mode (minimum, maximum) for triangular distributions, mean (minimum, maximum) for uniform distributions, and mean (SD) for normal distributions.
This upper estimate, within the published krisk range (80), was used to calibrate our model to documented SARS-CoV-2 outbreaks, as described in the materials and methods.
Sequential environmental scenarios for indoor and outdoor produce workers.
The daily cumulative infection risk was estimated for a susceptible produce worker exposed to a symptomatic SARS-CoV-2-infected co-worker throughout all representative daily environmental scenarios. Susceptibility was defined herein as an individual with no pre-existing health conditions, prior vaccinations, or infections altering their level of susceptibility. Additionally, the infection risk estimates reported for the susceptible worker represents any form of infection (e.g., asymptomatic, mild symptoms, severe symptoms) or timing of illness. These daily environmental scenarios were identified through discussions with industry trade associations, including the AFFI and the Produce Marketing Association (PMA); research and extension experience provided by representatives of the Arizona Cooperative Extension Organization, the Western Regional Center to Enhance Food Safety, and the Emory Farmworker project; and our past research on farms and packing facilities along the United States and Mexico border (112–114). Each scenario (Fig. 1) was modeled as an independent QMRA and expanded from our previous modeling work of SARS-CoV-2 transmission in an indoor food manufacturing facility (42). The SARS-CoV-2 exposure dose was combined across each environmental scenario experienced by the susceptible produce worker to estimate the daily infection risk. Scenario-specific parameters, such as the volume of space modeled, temperature and relative humidity, baseline air exchange rate (ACH), and fomite-specific viral decay, can be found in Table 4.
TABLE 4.
Environmental scenario-specific QMRA model parametersa
| Parameter | Shared transportation |
Occupational location |
Residential |
|||
|---|---|---|---|---|---|---|
| Car | Bus | Indoor facility | Indoor breakroom | Outdoor field | Shared housing | |
| Total exposure time (h) | 2.00 | 3.00 | 11.0 | 1.00 | 11.0 | 10.0 |
| Room vol (m3) | 2.60 | 81.4 | 460 | 139 | 17.0 | 34.0 |
| Temp | 75.0°F (23.9°C) | 75.0°F (23.9°C) | 65.0°F (18.3°C) | 65.0°F (18.3°C) | 85.0°F (29.4°C) | 75.0°F (23.9°C) |
| Relative humidity | Low (≤30%) | Low (≤30%) | Low (≤30%) | Low (≤30%) | High (≥50%) | High (≥50%) |
| Kinematic viscosity (m2/s) | 1.54 × 10−5 | 1.54 × 10−5 | 1.49 × 10−5 | 1.49 × 10−5 | 1.59 × 10−5 | 1.54 × 10−5 |
| Dynamic viscosity (N⋅s/m2) | 1.83 × 10−5 | 1.83 × 10−5 | 1.80 × 10−5 | 1.80 × 10−5 | 1.86 × 10−5 | 1.83 × 10−5 |
| Air viral decay (h−1)b (129) | 0.478 (0.150, 0.806) | 0.478 (0.150, 0.806) | 0.114 (0.048, 0.180) | 0.114 (0.048, 0.180) | 11.8 (11.1, 12.5) | 2.79 (2.13, 3.45) |
| Baseline ACH (exchange/h)c | 2.55 (0.98 to 4.10) (122) | 11.1 (8.96 to 13.4) (123) | 0.10d | 0.10d | 991 (584 to 1,298) (130) | 0.35d (131) |
| Increased ACH (exchange/h)c | 29.1 (6.50 to 51.7) (122) | 21.6 (10.5 to 37.3) (123) | 6.0d | 6.0d | NA | 3.90 (2.08 to 5.72) (131) |
| Fomite material | Polyester | Plastic | Stainless steel | Stainless steel | Stainless steel | Glass |
| Fomite viral decay (h−1) (132, 133)e | 0.222 (0.177, 0.267) | 0.148 (0.134, 0.162) | 0.293 (0.164, 0.422) | 0.293 (0.164, 0.422) | 0.293 (0.164, 0.422) | 0.179 (0.147, 0.211) |
| Fomite transfer efficacy (134)c | 0.003 (0.001 to 0.007) | 0.217 (0.001 to 0.511) | 0.076 (0.017 to 0.139) | 0.076 (0.017 to 0.139) | 0.374 (0.056 to 0.688) | 0.673 (0.176 to 0.839) |
The references are used to inform parameter estimates. ACH, air exchange rate; QMRA, quantitative microbial risk assessment.
The uniform distribution values are presented as mean (minimum, maximum).
The values represent mean (95% uncertainty interval).
Point distribution with no standard deviation.
The triangular distribution values are presented as mean (minimum, maximum).
SARS-CoV-2 dose characterization.
The daily cumulative risk of SARS-CoV-2 infection to a susceptible produce worker was calculated by summing the viral dose across each environmental scenario designated to either an indoor or outdoor produce worker. The scenario-specific risk of SARS-CoV-2 infection was calculated by summing the viral dose across each transmission pathway (aerosol, droplet, fomite) for each environmental scenario (shared transportation, work shift, work break, shared housing). Both the scenario-specific and daily cumulative viral doses from aerosol-, droplet-, and fomite-mediated transmission were then converted into risk estimates, as described below.
A susceptible worker may be exposed to aerosol (Daero) and droplet (Ddrop) viral doses. Aerosol (Daero) and droplet (Ddrop) doses were both calculated from the concentration of virus in the scenario (Ct) at time t for the specified transmission pathway (aerosol [aero], droplet [drop]), the deposition fraction of particles into the lung mucosa (Ldep), the inhalation rate (IR), and the duration of exposure (Et). The sum of aerosol (Daero) and droplet (Ddrop) is defined as Dair.
The fomite-mediated (Dhand) viral dose was calculated by using the frequency of hand-to-face contacts (Hface), the ratio of finger (Fsa)-to-hand (Hsa) surface area, the concentration of virus on the hand at time t (Chand) for both aerosol and droplet exposure pathways, the fraction of pathogens transferred from the hand to the facial mucosal membrane (F23), and the exposure duration in hours (t) as follows:
The scenario-specific viral dose was calculated by combining the viral dose for the aerosol and droplet pathways (Dair) with the fomite-mediated pathway (Dhand) for a specified environmental scenario. For instance, the scenario-specific viral dose for the 11-h indoor processing facility shift (Dprocessing) was calculated by:
Finally, the daily cumulative viral dose for an indoor (DIndoor) or an outdoor (DOutdoor) produce worker was calculated by combining the scenario-specific viral dosages relevant to each worker as follows:
SARS-CoV-2 transmission model calibration and risk characterization.
To calculate the probability of SARS-CoV-2 infection for a susceptible worker at a given viral dose, we calibrated our SARS-CoV-2 transmission model as follows. First, we modeled the SARS-CoV-2 viral shedding parameter after peak viral titers in saliva by converting PCR-based genome equivalent copies reported within the first week of symptom onset (4.3 to 7.4 log10 viral copies per mL) to PFU using a 1:100 conversion (6.1 to 7.4 log10 PFU) (79–82). Second, we calibrated the SARS-CoV-2 dose-response parameter to a median infectious dose (ID50) of 102 viral particles (within the documented ID50 range of 10 to 1,000 viral particles for similar respiratory pathogens) by leveraging the upper 99.5% bound estimate from 10,000 iterations of the SARS-CoV-1 dose-response parameter (6.80 × 10−3; Table 3), as previously described by Sobolik et al. (42, 77, 78, 80). To validate these calibrated risk estimates, we calculated the average indoor risk of infection after a 1-h exposure at 1 m to be 0.678 (95% UI, 0.270, 0.984), which fell within the AR range of well documented SARS-CoV-2 outbreaks in meat processing facilities (AR, 30.2%) (8), households (AR, 52.7%) (83), recreational spaces (AR, 53.3 to 86.8%) (84), and food preparation facilities (AR, 60.0%) (85). It should be noted that in a recent human challenge study, which used a single dose of wild-type SARS-CoV-2, the ID50 was estimated to be 10 viral particles (115). Although we cannot establish a dose-response relationship based on a single inoculation dose, this ID50 estimate (which approximates to 7 PFU) (116) does align with the range of inoculation values (5 to 12,000 PFU) used to establish the dose-response relationship for SARS-CoV-1. Thus, given that our calibrated model aligns with the documented outbreaks described above, we will continue to utilize the ID50 of 102 viral particles in our calculations. With our validated model, we then calculated the scenario-specific infection risk (e.g., Rcar transportation) and the daily cumulative infection risk (e.g., RIndoor) as follows:
The results are presented as the median risk values with the 95% UI, which is comprised of the 2.5 and 97.5 percentiles. Additionally, the daily cumulative risk estimates are stratified by the distance between the infected and susceptible worker during their 11-h work shift. For example, the daily cumulative risk reported for an indoor worker at 1 m utilizes the dose calculated for the 11-h indoor work shift in which the infected and susceptible workers are separated by a 1-m distance, whereas the daily cumulative risk at 2 m utilizes the dose from the 11-h work shift with the workers separated by a 2-m distance. Given that increased distancing was not applied to other individual scenarios (e.g., transportation, housing, etc.), these did not affect the daily cumulative risk estimates stratified by distance.
Selection of infection control strategies for SARS-CoV-2.
Recommended infection control strategies, such as physical distancing from 1 m (3 feet) to 2 m (6 feet), surface disinfection and handwashing during work shifts, masking for both infected and susceptible worker (i.e., universal masking), increased air exchange rates, and vaccination were all identified from a systematic review, conducted by one of the co-authors, of 1,847 United Nations and English-speaking government websites to distill global guidelines or recommendations to prevent food workers from acquiring COVID-19 (117). These strategies were then vetted with our trade association partners (AFFI, PMA) and extension co-authors (J.K. and C.M.R.) to ensure their alignment and efficacy with the interventions assessed in our models. The impact of these interventions (individually or combined) was evaluated based on the percentage of reduction in infection risk relative to the baseline scenario with no interventions.
Increasing the distance (physical distancing) between workers from 1 to 2 m, based on U.S. Centers for Disease Control and Prevention (CDC) and U.S. OSHA guidance (118), was analyzed in the indoor packing facility and outdoor harvesting field scenarios. It should be noted that in regard to physical distancing recommendations, the World Health Organization (WHO) maintains a 1-m distancing recommendation (119), highlighting the inherent variability in global public health guidance throughout the pandemic. Within these work shift scenarios, the impact of hourly handwashing (2-log10 viral removal) (120) and surface disinfection twice per shift (3-log10 viral removal) (121) was also assessed. The efficacy of various facemask materials (cloth, surgical) and double masking (surgical mask followed by cloth mask) was assessed across all scenarios except for the indoor and outdoor 1-h breaks to allow for eating and in the 8 h in the shared residential scenario to allow for sleeping. The range in efficacy for each facemask material can be found in Table 5 and Table S1.
TABLE 5.
QMRA parameters for risk mitigation intervention efficaciesa
| Parameter | Units | Description | Input valuesb | Distribution | References |
|---|---|---|---|---|---|
| HW eff | Log reduction | Hand washing efficacy | 0.552 (0.0, 2.0) | Triangular | 135 |
| SC eff | Log reduction | Surface disinfection efficacy | 0.95 (0.90, 0.999) | Uniform | 121, 135 |
| C mask(R) | % Reduction | Recipient cloth mask efficacy | 52.9 (17.0, 88.7) | Uniform | 58, 59, 136 |
| S mask(R) | % Reduction | Recipient surgical mask efficacy | 68.0 (37.0, 99.8) | Uniform | 58, 59, 136 |
| D mask(R) | % Reduction | Recipient double mask efficacy | 68.5 (40.0, 96.8) | Uniform | 58, 59, 136 |
| V Opt | % Reduction | Optimal SARS-CoV-2 vaccine efficacy | 92.3 (86.0, 99.0) | Uniform | 32, 137 |
| V Sub | % Reduction | Suboptimal SARS-CoV-2 vaccine efficacy | 72.0 (64.0, 80.0) | Uniform | 33, 138, 139 |
QMRA, quantitative microbial risk assessment.
The values are presented as mean (minimum, maximum) for uniform and triangular distributions.
Scenario-specific increases in air exchange rate (ACH) based on particulate exposure studies were assessed across each environmental scenario except for the 11-h outdoor harvesting field shift and 1-h bus break scenario, given the inability to adjust outdoor ventilation rates. For the shared car transportation scenario, the baseline ACH of 2.55 h−1 (95% UI, 0.98 to 4.10) was increased to 29.1 h−1 (95% UI, 7.67 to 50.6), which was representative of a 2005 Ford Taurus midsize car traveling from 0 to 50 mph with all windows open to simulate a method for increasing the ventilation rate inside the vehicle (122). For both the indoor packaging facility and associated breakroom, the ACH was increased from 0.1 h−1, representing a facility with negligible ventilation, to 6.0 h−1, representing the facilities surveyed through our conversations with food industry managers across the United States (data not shown). For the shared bus transportation scenario, the baseline ACH of 11.1 h−1 (95% UI, 8.96 to 13.4) was increased to 21.6 h−1 (95% UI, 10.5 to 37.3), which was representative of a school bus driving under realistic conditions with all windows open to simulate a method for increasing the ventilation rate inside the vehicle (123). For the shared residential scenario, the baseline ACH of 0.35 h−1 (point estimate) was increased to 3.9 h−1 (95% UI, 2.08 to 5.72), which is representative of opening a window to increase natural ventilation in an apartment-style scenario (124). Additional information on the risk mitigation intervention parameters can be found in Table 5.
Finally, we evaluated the impact of two vaccination scenarios (suboptimal and optimal vaccine efficacies) on reducing the daily cumulative risk of SARS-CoV-2 infection for a susceptible worker. For the suboptimal vaccine (VSub), the mean vaccine efficacy was 72.0% (95% UI, 64.4 to 79.6%) and represented either receiving one dose of the Johnson & Johnson vaccine, one of the two-dose vaccine series, a reduced vaccine efficacy to novel variants, or incomplete immunity due to a prior SARS-CoV-2 infection (31, 32, 125). For the optimal vaccine (VOpt), the mean vaccine efficacy was 92.3% (95% UI, 86.3 to 98.7%) and represented two doses (≥14 days after final vaccination) of Pfizer-BioNTech or Moderna-NIAD mRNA series vaccines received by the susceptible worker (33, 34). We assumed that only the susceptible worker would be vaccinated across all scenarios and that the vaccine efficacy parameters in Table 5 would represent the susceptible worker’s level of immunity to infection. For example, assuming that the indoor susceptible worker was vaccinated with the optimal efficacy vaccine (VOpt), the daily cumulative risk of infection for the optimal vaccine efficacy scenario is represented as:
Although the impact of booster vaccinations or waning immunity to infection was not specifically assessed in the modeled scenarios, these factors are captured by proxy due to the vaccine efficacy ranges against infection assessed in our calculations. For example, receiving three doses of the Pfizer-BioNTech BNT162b2 mRNA vaccine was recently shown to provide a vaccine efficacy against infection of 89.1% (95% confidence interval [CI], 87.5 to 90.5%) (126), which falls within the range of the optimal vaccine efficacy scenario assessed in our models. Furthermore, a recent meta-analysis found that 4 months after receiving the two-dose mRNA vaccine regimen, vaccine efficacy against infection decreased to 62% (95% CI, 53.0 to 69.0%) (127), which falls within the range of the suboptimal vaccine efficacy scenario assessed in our models.
Sensitivity analysis.
Sensitivity analyses were conducted for each modeled scenario to determine the most influential parameters in estimating the SARS-CoV-2 viral exposure dose. Parameters identified as being most influential in the final cumulative dose estimate were reported as Spearman rank correlational coefficients using the “tornado” function in the mc2d R package (128). To investigate the propagation of variability and uncertainty throughout the models, the “mcratio” function was used to calculate the variability and overall uncertainty ratio for each modeled parameter. Model stability was achieved after 10,000 Monte Carlo iterations, which was applied across all modeled scenarios (Fig. S1).
Data availability.
The code developed and utilized throughout this analysis is available through GitHub at the following DOI: https://doi.org/10.5281/zenodo.8003697.
ACKNOWLEDGMENTS
We thank Sanjay Gummalla (American Frozen Food Institute), Lory Reveil (American Frozen Food Institute), and Max Teplitski (Produce Marketing Association) for their valuable time and input as food and produce production and processing experts and for conducting surveys of facilities.
This work was partially supported by T32 grant 2T32ES012870-16 from the National Institutes of Health (J.S.S.), grants 2019-67017-29642 (J.S.L.) and 2020-67034-31728 (J.S.S.) from the National Institute of Food and Agriculture at the U.S. Department of Agriculture, grants R01 GM124280 and R01 GM124280-03S1 (B.A.L.) from the National Institute of General Medical Sciences, grant T32AI138952 (E.T.S.) from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health, and funds from Emory University and the Infectious Disease Across Scales Training Program (E.T.S.). The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health, or the U.S. Department of Agriculture.
Footnotes
Supplemental material is available online only.
Contributor Information
D. Kane Cooper, Email: derrick.kane.cooper@emory.edu.
Nicole R. Buan, University of Nebraska-Lincoln
REFERENCES
- 1.Dyal JW, Grant MP, Broadwater K, Bjork A, Waltenburg MA, Gibbins JD, Hale C, Silver M, Fischer M, Steinberg J, Basler CA, Jacobs JR, Kennedy ED, Tomasi S, Trout D, Hornsby-Myers J, Oussayef NL, Delaney LJ, Patel K, Shetty V, Kline KE, Schroeder B, Herlihy RK, House J, Jervis R, Clayton JL, Ortbahn D, Austin C, Berl E, Moore Z, Buss BF, Stover D, Westergaard R, Pray I, DeBolt M, Person A, Gabel J, Kittle TS, Hendren P, Rhea C, Holsinger C, Dunn J, Turabelidze G, Ahmed FS, deFijter S, Pedati CS, Rattay K, Smith EE, Luna-Pinto C, Cooley LA, et al. 2020. COVID-19 among workers in meat and poultry processing facilities―19 states, April 2020. Morb Mortal Wkly Rep 69:557–561. doi: 10.15585/mmwr.mm6918e3. [DOI] [PubMed] [Google Scholar]
- 2.Waltenburg MA, Rose CE, Victoroff T, Butterfield M, Dillaha JA, Heinzerling A, Chuey M, Fierro M, Jervis RH, Fedak KM, Leapley A, Gabel JA, Feldpausch A, Dunne EM, Austin C, Pedati CS, Ahmed FS, Tubach S, Rhea C, Tonzel J, Krueger A, Crum DA, Vostok J, Moore MJ, Kempher H, Scheftel J, Turabelidze G, Stover D, Donahue M, Thomas D, Edge K, Gutierrez B, Berl E, McLafferty M, Kline KE, Martz N, Rajotte JC, Julian E, Diedhiou A, Radcliffe R, Clayton JL, Ortbahn D, Cummins J, Barbeau B, Carpenter S, Pringle JC, Murphy J, Darby B, Graff NR, Dostal TKH, et al. 2021. Coronavirus disease among workers in food processing, food manufacturing, and agriculture workplaces. Emerg Infect Dis 27:243–249. doi: 10.3201/eid2701.203821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kavanagh KT, Pare J, Pontus C. 2020. COVID-19: through the eyes through the front line, an international perspective. Antimicrob Resist Infect Control 9:179. doi: 10.1186/s13756-020-00850-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Conference of State Legislature. 2020. COVID-19: essential workers in the States. National Conference of State Legislature. https://www.ncsl.org/research/labor-and-employment/covid-19-essential-workers-in-the-states.aspx. Accessed 15 March, 2021.
- 5.U.S. Department of Homeland Security. 2020. Advisory memorandum on identification of essential critical infrastructure workers during COVID-19 response. https://www.cisa.gov/publication/guidance-essential-critical-infrastructure-workforce. Accessed 22 April, 2021.
- 6.Vosko LF, Spring C. 2022. COVID-19 outbreaks in Canada and the crisis of migrant farmworkers’ social reproduction: transnational labour and the need for greater accountability among receiving states. J Int Migr Integr 23:1765–1791. doi: 10.1007/s12134-021-00905-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y-H, Glymour M, Riley A, Balmes J, Duchowny K, Harrison R, Matthay E, Bibbins-Domingo K. 2021. Excess mortality associated with the COVID-19 pandemic among Californians 18–65 years of age, by occupational sector and occupation: March through November 2020. PLoS One 16:e0252454. doi: 10.1371/journal.pone.0252454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steinberg J, Kennedy ED, Basler C, Grant MP, Jacobs JR, Ortbahn D, Osburn J, Saydah S, Tomasi S, Clayton JL. 2020. COVID-19 outbreak among employees at a meat processing facility—South Dakota, March–April 2020. Morb Mortal Wkly Rep 69:1015–1019. doi: 10.15585/mmwr.mm6931a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reid A, Schenker MB. 2016. Hired farmworkers in the US: demographics, work organisation, and services. Am J Ind Med 59:644–655. doi: 10.1002/ajim.22613. [DOI] [PubMed] [Google Scholar]
- 10.Lan FY, Wei CF, Hsu YT, Christiani DC, Kales SN. 2020. Work-related COVID-19 transmission in six Asian countries/areas: a follow-up study. PLoS One 15:e0233588. doi: 10.1371/journal.pone.0233588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pongpirul WA, Pongpirul K, Ratnarathon AC, Prasithsirikul W. 2020. Journey of a Thai taxi driver and novel coronavirus. N Engl J Med 382:1067–1068. doi: 10.1056/NEJMc2001621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gmehlin C, Munoz-Price LS. 2022. COVID-19 in long term care facilities: a review of epidemiology, clinical presentations, and containment interventions. Infect Control Hosp Epidemiol 43:504–509. doi: 10.1017/ice.2020.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roxby AC, Greninger AL, Hatfield KM, Lynch JB, Dellit TH, James A, Taylor J, Page LC, Kimball A, Arons M, Munanga A, Stone N, Jernigan JA, Reddy SC, Lewis J, Cohen SA, Jerome KR, Duchin JS, Neme S. 2020. Outbreak investigation of COVID-19 among residents and staff of an independent and assisted living community for older adults in Seattle, Washington. JAMA Intern Med 180:1101–1105. doi: 10.1001/jamainternmed.2020.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gentry AL, Grzywacz JG, Quandt SA, Davis SW, Arcury TA. 2007. Housing quality among North Carolina farmworker families. J Agric Saf Health 13:323–337. doi: 10.13031/2013.23355. [DOI] [PubMed] [Google Scholar]
- 15.Arcury TA, Weir M, Chen H, Summers P, Pelletier LE, Galván L, Bischoff WE, Mirabelli MC, Quandt SA. 2012. Migrant farmworker housing regulation violations in North Carolina. Am J Ind Med 55:191–204. doi: 10.1002/ajim.22011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quandt SA, Brooke C, Fagan K, Howe A, Thornburg TK, McCurdy SA. 2015. Farmworker housing in the United States and its impact on health. New Solut 25:263–286. doi: 10.1177/1048291115601053. [DOI] [PubMed] [Google Scholar]
- 17.Accorsi EK, Samples J, McCauley LA, Shadbeh N. 2020. Sleeping within six feet: challenging Oregon’s labor housing COVID-19 guidelines. J Agromed 25:413–416. doi: 10.1080/1059924X.2020.1815622. [DOI] [PubMed] [Google Scholar]
- 18.Quandt SA, Wiggins MF, Chen H, Bischoff WE, Arcury TA. 2013. Heat index in migrant farmworker housing: implications for rest and recovery from work-related heat stress. Am J Public Health 103:e24–e26. doi: 10.2105/AJPH.2012.301135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tuček M, Vaněček V. 2021. COVID-19 in the Czech Republic 2020: probable transmission of the coronavirus SARS-CoV-2. Cent Eur J Public Health 29:159–161. doi: 10.21101/cejph.a6963. [DOI] [PubMed] [Google Scholar]
- 20.Fan M, Pena AA. 2021. How vulnerable are U.S. crop workers?: Evidence from representative worker data and implications for COVID-19. J Agromed 26:256–265. doi: 10.1080/1059924X.2021.1890293. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization, Food and Agriculture Organization of the United Nations. 2020. COVID-19 and food safety: guidance for food businesses. https://apps.who.int/iris/handle/10665/331705. Accessed 22 April, 2021.
- 22.Lusk JL, Chandra R. 2021. Farmer and farm worker illnesses and deaths from COVID-19 and impacts on agricultural output. PLoS One 16:e0250621. doi: 10.1371/journal.pone.0250621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Food and Agriculture Organization of the United Nations. 2020. Migrant workers and the COVID-19 pandemic. FAO, Rome, Italy. doi: 10.4060/ca8559en. Accessed 15 August, 2021. [DOI] [Google Scholar]
- 24.Food and Agriculture Organization of the United Nations. 2021. COVID-19: guidance for preventing transmission of COVID-19 within food businesses. FAO, Rome, Italy. doi: 10.4060/cb6030en. Accessed 15 August, 2021. [DOI] [Google Scholar]
- 25.U.S. Department of Agriculture. 2021. USDA COVID-19 workplace safety plan. https://www.usda.gov/sites/default/files/documents/usda-covid-19-workplace-safety-plan.pdf. Accessed 30 May, 2021.
- 26.Global Cold Chain Alliance. 2021. GCCA fact sheet: OSHA ETS on COVID-19 vaccination and testing. https://www.gcca.org/sites/default/files/OSHAETSFactSheet.pdf. Accessed 12 November, 2021.
- 27.American Frozen Food Institute. 2020. Proper usage of face masks/coverings to protect against COVID-19. https://www.idfa.org/wordpress/wp-content/uploads/2020/04/ProperUsageofFaceMasks_6Apr2020_Version2_SIGNED.pdf. Accessed 23 May, 2021.
- 28.Chu DK, Akl EA, Duda S, Solo K, Yaacoub S, Schünemann HJ, COVID-19 Systematic Urgent Review Group Effort (SURGE) study authors . 2020. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet 395:1973–1987. doi: 10.1016/S0140-6736(20)31142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herstein JJ, Degarege A, Stover D, Austin C, Schwedhelm MM, Lawler JV, Lowe JJ, Ramos AK, Donahue M. 2021. Characteristics of SARS-CoV-2 transmission among meat processing workers in Nebraska, USA, and effectiveness of risk mitigation measures. Emerg Infect Dis 27:1032–1038. doi: 10.3201/eid2704.204800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prunas O, Warren JL, Crawford FW, Gazit S, Patalon T, Weinberger DM, Pitzer VE. 2022. Vaccination with BNT162b2 reduces transmission of SARS-CoV-2 to household contacts in Israel. Science 375:1151–1154. doi: 10.1126/science.abl4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lopez Bernal J, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S, Stowe J, Tessier E, Groves N, Dabrera G, Myers R, Campbell CNJ, Amirthalingam G, Edmunds M, Zambon M, Brown KE, Hopkins S, Chand M, Ramsay M. 2021. Effectiveness of COVID-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med 385:585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Corchado-Garcia J, Zemmour D, Hughes T, Bandi H, Cristea-Platon T, Lenehan P, Pawlowski C, Bade S, O’Horo JC, Gosres GJ, Williams AW, Badley AD, Halamka J, Virk A, Swift MD, Wagner T, Soundararajan V. 2021. Analysis of the effectiveness of the Ad26.COV2.S adenoviral vector vaccine for preventing COVID-19. JAMA Netw Open 4:e2132540. doi: 10.1001/jamanetworkopen.2021.32540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW, Hammitt LL, Türeci Ö, Nell H, Schaefer A, Ünal S, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Jansen KU, Gruber WC, C4591001 Clinical Trial Group . 2020. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med 383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, McGettigan J, Khetan S, Segall N, Solis J, Brosz A, Fierro C, Schwartz H, Neuzil K, Corey L, Gilbert P, Janes H, Follmann D, Marovich M, Mascola J, Polakowski L, Ledgerwood J, Graham BS, Bennett H, Pajon R, Knightly C, Leav B, Deng W, Zhou H, Han S, Ivarsson M, Miller J, Zaks T, COVE Study Group . 2021. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parhizkar H, Dietz L, Olsen-Martinez A, Olsen-Martinez A, Horve PF, Barnatan L, Northcutt D, Van Den Wymelenberg KG. 2022. Quantifying environmental mitigation of aerosol viral load in a controlled chamber with participants diagnosed with COVID-19. Clin Infect Dis 75:e174–e184. doi: 10.1093/cid/ciac006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morawska L, Tang JW, Bahnfleth W, Bluyssen PM, Boerstra A, Buonanno G, Cao J, Dancer S, Floto A, Franchimon F, Haworth C, Hogeling J, Isaxon C, Jimenez JL, Kurnitski J, Li Y, Loomans M, Guy M, Marr LC, Mazzarella L, Melikov AK, Miller S, Milton DK, Nazaroff W, Nielsen PV, Noakes C, Peccia J, Querol X, Sekhar C, Seppänen O, Tanabe S-I, Tellier R, Tham KW, Wargocki P, Wierzbicka A, Yao M. 2020. How can airborne transmission of COVID-19 indoors be minimised? Environ Int 142:105832. doi: 10.1016/j.envint.2020.105832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ingram C, Downey V, Chen Y, Archibald M, Kallas K-A, Kumar J, Naughton P, Uteh CO, Rojas-Chaves A, Shrestha S, Syed S, Büttner FC, Buggy C, Perrotta C. 2021. COVID-19 prevention and control measures in workplace settings: a rapid review and meta-analysis. Int J Environ Res Public Health 18:7847. doi: 10.3390/ijerph18157847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson C, Dukes K, Sinnwell E, Culp K, Zinnel D, Corwin C. 2022. Innovative cohort process to minimize COVID-19 infection for migrant farmworkers during travel to Iowa. Workplace Health Saf 70:17–23. doi: 10.1177/21650799211045308. [DOI] [PubMed] [Google Scholar]
- 39.Chicas R, Xiuhtecutli N, Houser M, Glastra S, Elon L, Sands JM, McCauley L, Hertzberg V. 2022. COVID-19 and agricultural workers: a descriptive study. J Immigr Minor Health 24:58–64. doi: 10.1007/s10903-021-01290-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fielding-Miller RK, Sundaram ME, Brouwer K. 2020. Social determinants of COVID-19 mortality at the county level. PLoS One 15:e0240151. doi: 10.1371/journal.pone.0240151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.VanderWaal K, Black L, Hodge J, Bedada A, Dee S. 2021. Modeling transmission dynamics and effectiveness of worker screening programs for SARS-CoV-2 in pork processing plants. PLoS One 16:e0249143. doi: 10.1371/journal.pone.0249143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sobolik JS, Sajewski ET, Jaykus L-A, Cooper DK, Lopman BA, Kraay ANM, Ryan PB, Leon JS. 2022. Controlling risk of SARS-CoV-2 infection in essential workers of enclosed food manufacturing facilities. Food Control 133:108632. doi: 10.1016/j.foodcont.2021.108632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sobolik JS, Sajewski ET, Jaykus L-A, Cooper DK, Lopman BA, Kraay ANM, Ryan PB, Guest JL, Webb-Girard A, Leon JS. 2022. Decontamination of SARS-CoV-2 from cold-chain food packaging provides no marginal benefit in risk reduction to food workers. Food Control 136:108845. doi: 10.1016/j.foodcont.2022.108845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bade B, Ramirez S, Saxton D. 2021. Always essential, perpetually disposable: California farmworkers and the COVID-19 pandemic. https://cirsinc.org/wp-content/uploads/2021/08/COFS-_Phase-Two-Preliminary-Report.pdf. Accessed 18 June, 2021.
- 45.Günther T, Czech-Sioli M, Indenbirken D, Robitaille A, Tenhaken P, Exner M, Ottinger M, Fischer N, Grundhoff A, Brinkmann MM. 2020. SARS-CoV-2 outbreak investigation in a German meat processing plant. EMBO Mol Med 12:e13296. doi: 10.15252/emmm.202013296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wei J, Li Y. 2015. Enhanced spread of expiratory droplets by turbulence in a cough jet. Build Environ 93:86–96. doi: 10.1016/j.buildenv.2015.06.018. [DOI] [Google Scholar]
- 47.Ng OT, Marimuthu K, Koh V, Pang J, Linn KZ, Sun J, De Wang L, Chia WN, Tiu C, Chan M, Ling LM, Vasoo S, Abdad MY, Chia PY, Lee TH, Lin RJ, Sadarangani SP, Chen MI-C, Said Z, Kurupatham L, Pung R, Wang L-F, Cook AR, Leo Y-S, Lee VJ. 2021. SARS-CoV-2 seroprevalence and transmission risk factors among high-risk close contacts: a retrospective cohort study. Lancet Infect Dis 21:333–343. doi: 10.1016/S1473-3099(20)30833-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, Tamin A, Harcourt JL, Thornburg NJ, Gerber SI, Lloyd-Smith JO, de Wit E, Munster VJ. 2020. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med 382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rode E. 2020. Farmworker housing outbreak: 154 workers released from isolation Tuesday. Ventura County Star. https://www.vcstar.com/story/news/local/2020/07/07/oxnard-farmworker-housing-covid-outbreak-coronavirus-recovery-rate/5387928002/. Accessed 7 July, 2021.
- 50.Qian H, Miao T, Liu L, Zheng X, Luo D, Li Y. 2021. Indoor transmission of SARS-CoV-2. Indoor Air 31:639–645. doi: 10.1111/ina.12766. [DOI] [PubMed] [Google Scholar]
- 51.Bazant MZ, Bush JWM. 2021. A guideline to limit indoor airborne transmission of COVID-19. Proc Natl Acad Sci USA 118:e2018995118. doi: 10.1073/pnas.2018995118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vlacha V, Feketea G, Petropoulou A, Trancá SD. 2021. The significance of duration of exposure and circulation of fresh air in SARS-CoV-2 transmission among healthcare workers. Front Med 8:664297. doi: 10.3389/fmed.2021.664297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Flocks J. 2020. The potential impact of COVID-19 on H-2A agricultural workers. J Agromed 25:367–369. doi: 10.1080/1059924X.2020.1814922. [DOI] [PubMed] [Google Scholar]
- 54.Correia G, Rodrigues L, Gameiro da Silva M, Gonçalves T. 2020. Airborne route and bad use of ventilation systems as non-negligible factors in SARS-CoV-2 transmission. Med Hypotheses 141:109781. doi: 10.1016/j.mehy.2020.109781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee H, Ahn KH. 2021. Estimate of the critical exposure time based on 70 confirmed COVID-19 cases. J Korean Phys Soc 79:492–498. doi: 10.1007/s40042-021-00225-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kennedy M, Lee SJ, Epstein M. 2021. Modeling aerosol transmission of SARS-CoV-2 in multi-room facility. J Loss Prev Process Ind 69:104336. doi: 10.1016/j.jlp.2020.104336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Curtius J, Granzin M, Schrod J. 2021. Testing mobile air purifiers in a school classroom: reducing the airborne transmission risk for SARS-CoV-2. Aerosol Sci Technol 55:586–599. doi: 10.1080/02786826.2021.1877257. [DOI] [Google Scholar]
- 58.Lindsley WG, Blachere FM, Law BF, Beezhold DH, Noti JD. 2021. Efficacy of face masks, neck gaiters and face shields for reducing the expulsion of simulated cough-generated aerosols. Aerosol Sci Technol 55:449–457. doi: 10.1080/02786826.2020.1862409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fischer EP, Fischer MC, Grass D, Henrion I, Warren WS, Westman E. 2020. Low-cost measurement of face mask efficacy for filtering expelled droplets during speech. Sci Adv 6:2–7. doi: 10.1126/sciadv.abd3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ueki H, Furusawa Y, Iwatsuki-Horimoto K, Imai M, Kabata H, Nishimura H, Kawaoka Y. 2020. Effectiveness of face masks in preventing airborne transmission of SARS-CoV-2. mSphere 5:e00637-20. doi: 10.1128/mSphere.00637-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Doung-Ngern P, Suphanchaimat R, Panjangampatthana A, Janekrongtham C, Ruampoom D, Daochaeng N, Eungkanit N, Pisitpayat N, Srisong N, Yasopa O, Plernprom P, Promduangsi P, Kumphon P, Suangtho P, Watakulsin P, Chaiya S, Kripattanapong S, Chantian T, Bloss E, Namwat C, Limmathurotsakul D. 2020. Case-control study of use of personal protective measures and risk for SARS-CoV 2 infection, Thailand. Emerg Infect Dis 26:2607–2616. doi: 10.3201/eid2611.203003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moghaddar M, Radman R, Macreadie I. 2021. Severity, pathogenicity and transmissibility of delta and lambda variants of SARS-CoV-2, toxicity of spike protein and possibilities for future prevention of COVID-19. Microorganisms 9:2167. doi: 10.3390/microorganisms9102167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Centers for Disease Control and Prevention COVID-19 Response Team. 2021. SARS-CoV-2 B.1.1.529 (Omicron) variant — United States, December 1–8, 2021. Morb Mortal Wkly Rep 70:1731–1734. doi: 10.15585/mmwr.mm7050e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Centers for Disease Control and Prevention. 2021. Improve how your mask protects you. https://www.cdc.gov/coronavirus/2019-ncov/your-health/effective-masks.html. Accessed 15 December, 2021.
- 65.Centers for Disease Control and Prevention. 2022. COVID-19 types of masks and respirators key messages: choosing a mask or respirator for different situations. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/types-of-masks.html#print. Accessed 16 March, 2022.
- 66.Occupational Safety and Health Administration. 2021. Protecting workers: guidance on mitigating and preventing the spread of COVID-19 in the workplace. https://www.osha.gov/coronavirus/safework. Accessed 16 March, 2022.
- 67.COVID-19 Farmworker Study. 2020. Oregon fires exacerbate COVID-19 impact on farmworkers’ health, housing, and livelihoods. https://cirsinc.org/wp-content/uploads/2021/08/Preliminary-Data-Brief_OR_COFS_-9.22.20.pdf. Accessed 18 June, 2021.
- 68.Howard J, Huang A, Li Z, Tufekci Z, Zdimal V, van der Westhuizen H-M, von Delft A, Price A, Fridman L, Tang L-H, Tang V, Watson GL, Bax CE, Shaikh R, Questier F, Hernandez D, Chu LF, Ramirez CM, Rimoin AW. 2021. An evidence review of face masks against COVID-19. Proc Natl Acad Sci USA 118:e2014564118. doi: 10.1073/pnas.2014564118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Farthing TS, Lanzas C. 2021. Assessing the efficacy of interventions to control indoor SARS-CoV-2 transmission: an agent-based modeling approach. Epidemics 37:100524. doi: 10.1016/j.epidem.2021.100524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kerr CC, Stuart RM, Mistry D, Abeysuriya RG, Rosenfeld K, Hart GR, Núñez RC, Cohen JA, Selvaraj P, Hagedorn B, George L, Jastrzębski M, Izzo AS, Fowler G, Palmer A, Delport D, Scott N, Kelly SL, Bennette CS, Wagner BG, Chang ST, Oron AP, Wenger EA, Panovska-Griffiths J, Famulare M, Klein DJ. 2021. Covasim: an agent-based model of COVID-19 dynamics and interventions. PLoS Comput Biol 17:e1009149. doi: 10.1371/journal.pcbi.1009149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liggans G, Dutilly D, Carrington-Liggans K, Cartagena M, Idjagboro C, Williams L, Lewis G, Russell M, Sudler R. 2020. Interventions and compliance: how the response to COVID-19 reflects decades of retail food protection efforts. J Agric Food Syst Community Dev 10:227–231. doi: 10.5304/jafscd.2020.101.007. [DOI] [Google Scholar]
- 72.Mogren L, Windstam S, Boqvist S, Vågsholm I, Söderqvist K, Rosberg AK, Lindén J, Mulaosmanovic E, Karlsson M, Uhlig E, Håkansson Å, Alsanius B. 2018. The hurdle approach—a holistic concept for controlling food safety risks associated with pathogenic bacterial contamination of leafy green vegetables. A review. Front Microbiol 9:1965. doi: 10.3389/fmicb.2018.01965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang X, Ji Z, Yue Y, Liu H, Wang J. 2021. Infection risk assessment of COVID-19 through aerosol transmission: a case study of South China seafood market. Environ Sci Technol 55:4123–4133. doi: 10.1021/acs.est.0c02895. [DOI] [PubMed] [Google Scholar]
- 74.Shi K-W, Huang Y-H, Quon H, Ou-Yang Z-L, Wang C, Jiang SC. 2021. Quantifying the risk of indoor drainage system in multi-unit apartment building as a transmission route of SARS-CoV-2. Sci Total Environ 762:143056. doi: 10.1016/j.scitotenv.2020.143056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wilson AM, Weir MH, Bloomfield SF, Scott EA, Reynolds KA. 2021. Modeling COVID-19 infection risks for a single hand-to-fomite scenario and potential risk reductions offered by surface disinfection. Am J Infect Control 49:846–848. doi: 10.1016/j.ajic.2020.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dada AC, Gyawali P. 2021. Quantitative microbial risk assessment (QMRA) of occupational exposure to SARS-CoV-2 in wastewater treatment plants. Sci Total Environ 763:142989. doi: 10.1016/j.scitotenv.2020.142989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yezli S, Otter JA. 2011. Minimum infective dose of the major human respiratory and enteric viruses transmitted through food and the environment. Food Environ Virol 3:1–30. doi: 10.1007/s12560-011-9056-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schröder I. 2020. COVID-19: a risk assessment perspective. ACS Chem Health Saf 27:160–169. doi: 10.1021/acs.chas.0c00035. [DOI] [PubMed] [Google Scholar]
- 79.To KK-W, Tsang OT-Y, Leung W-S, Tam AR, Wu T-C, Lung DC, Yip CC-Y, Cai J-P, Chan JM-C, Chik TS-H, Lau DP-L, Choi CY-C, Chen L-L, Chan W-M, Chan K-H, Ip JD, Ng AC-K, Poon RW-S, Luo C-T, Cheng VC-C, Chan JF-W, Hung IF-N, Chen Z, Chen H, Yuen K-Y. 2020. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis 20:565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pitol AK, Julian TR. 2021. Community transmission of SARS-CoV-2 by surfaces: risks and risk reduction strategies. Supporting information. Environ Sci Technol Lett 8:263–269. https://pubs.acs.org/doi/suppl/10.1021/acs.estlett.0c00966/suppl_file/ez0c00966_si_001.pdf. doi: 10.1021/acs.estlett.0c00966. [DOI] [PubMed] [Google Scholar]
- 81.Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, Niemeyer D, Jones TC, Vollmar P, Rothe C, Hoelscher M, Bleicker T, Brünink S, Schneider J, Ehmann R, Zwirglmaier K, Drosten C, Wendtner C. 2020. Virological assessment of hospitalized patients with COVID-2019. Nature 581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 82.He X, Lau EHY, Wu P, Deng X, Wang J, Hao X, Lau YC, Wong JY, Guan Y, Tan X, Mo X, Chen Y, Liao B, Chen W, Hu F, Zhang Q, Zhong M, Wu Y, Zhao L, Zhang F, Cowling BJ, Li F, Leung GM. 2020. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med 26:672–675. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- 83.Baker JM, Nakayama JY, O’Hegarty M, McGowan A, Teran RA, Bart SM, Mosack K, Roberts N, Campos B, Paegle A, McGee J, Herrera R, English K, Barrios C, Davis A, Roloff C, Sosa LE, Brockmeyer J, Page L, Bauer A, Weiner JJ, Khubbar M, Bhattacharyya S, Kirking HL, Tate JE. 2022. SARS-CoV-2 B.1.1.529 (Omicron) variant transmission within households — four U.S. jurisdictions, November 2021–February 2022. Morb Mortal Wkly Rep 71:341–346. doi: 10.15585/mmwr.mm7109e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hamner L, Dubbel P, Capron I, Ross A, Jordan A, Lee J, Lynn J, Ball A, Narwal S, Russell S, Patrick D, Leibrand H. 2020. High SARS-CoV-2 attack rate following exposure at a choir practice. Morb Mortal Wkly Rep 69:606–610. https://www.cdc.gov/mmwr/volumes/69/wr/mm6919e6.htm. doi: 10.15585/mmwr.mm6919e6. [DOI] [PubMed] [Google Scholar]
- 85.Hou L, Zhou H, Meng N, Yu X, Wang X, Wang T, Zhang J, Wang Y, Li S, Guo S, Yu J, Chen M, Shi W, Xiao N, Yang C, Liu J. 2021. A COVID-19 outbreak emerging in a food processing company - Harbin City, Heilongjiang Province, China, January–February 2021. China CDC Wkly 3:681–687. https://pubmed.ncbi.nlm.nih.gov/34594967/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.King WC, Rubinstein M, Reinhart A, Mejia R. 2021. Time trends, factors associated with, and reasons for COVID-19 vaccine hesitancy: a massive online survey of US adults from January–May 2021. PLoS One 16:e0260731. doi: 10.1371/journal.pone.0260731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gehlbach D, Vázquez E, Ortiz G, Li E, Sánchez CB, Rodríquez S, Pozar M, Cheney AM. 2021. COVID-19 testing and vaccine hesitancy in Latinx farm-working communities in the Eastern Coachella Valley. Res Sq rs.3.rs-587686. https://www.researchsquare.com/article/rs-587686/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ng WH, Tipih T, Makoah NA, Vermeulen J-G, Goedhals D, Sempa JB, Burt FJ, Taylor A, Mahalingam S. 2021. Comorbidities in SARS-CoV-2 patients: a systematic review and meta-analysis. mBio 12:e03647-20. doi: 10.1128/mBio.03647-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.U.S. Food & Drug Administration, Occupational Safety and Health Adminstration. 2020. Employee health and food safety checklist for human and animal food operations during the COVID-19 pandemic. U.S. Food & Drug Administration, Washington, DC. [Google Scholar]
- 90.European Union Occupational Safety and Health Administration. 2020. COVID-19: Back to the workplace – adapting workplaces and protecting workers. https://osha.europa.eu/en/publications/covid-19-back-workplace-adapting-workplaces-and-protecting-workers/view. Accessed 15 March, 2021.
- 91.World Health Organization. 2020. Transmission of SARS-CoV-2: implications for infection prevention precautions. https://www.who.int/news-room/commentaries/detail/transmission-of-sars-cov-2-implications-for-infection-prevention-precautions. Accessed 21 March, 2021.
- 92.World Health Organization. 2021. Coronavirus disease (COVID-19) advice for the public: when and how to use masks. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public/when-and-how-to-use-masks. Accessed 21 March, 2021.
- 93.Centers for Disease Control and Prevention. 2020. Agriculture workers and employers: interim guidance from CDC and the U.S. Department of Labor. https://www.cdc.gov/coronavirus/2019-ncov/community/guidance-agricultural-workers.html. Accessed 18 March, 2021.
- 94.Centers for Disease Control and Prevention. 2021. Guidance for organizing large events and gatherings risk factors to consider. https://www.cdc.gov/coronavirus/2019-ncov/community/large-events/considerations-for-events-gatherings.html#print. Accessed 18 March, 2021.
- 95.Centers for Disease Control and Prevention. 2020. COVID-19 guidance for shared or congregate housing review, update, and implement emergency operations plans (EOPs). Centers for Disease Control and Prevention, Atlanta, GA. https://stacks.cdc.gov/view/cdc/92388. Accessed 15 March, 2021. [Google Scholar]
- 96.Oregon Occupational Safety and Health Adminstration. 2020. Temporary administrative rule addressing the COVID-19 public health emergency in labor housing and agricultural employment. https://osha.oregon.gov/OSHARules/adopted/2020/ao2-2020-text-emergency-rules-ag-covid.pdf. Accessed 2 April, 2021.
- 97.Azimi P, Keshavarz Z, Cedeno Laurent JG, Stephens B, Allen JG. 2021. Mechanistic transmission modeling of COVID-19 on the Diamond Princess cruise ship demonstrates the importance of aerosol transmission. Proc Natl Acad Sci USA 118:e2015482118. doi: 10.1073/pnas.2015482118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nicas M, Nazaroff WW, Hubbard A. 2005. Toward understanding the risk of secondary airborne infection: emission of respirable pathogens. J Occup Environ Hyg 2:143–154. doi: 10.1080/15459620590918466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Han ZY, Weng WG, Huang QY. 2013. Characterizations of particle size distribution of the droplets exhaled by sneeze. J R Soc Interface 10:20130560. doi: 10.1098/rsif.2013.0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang B, Wu H, Wan XF. 2020. Transport and fate of human expiratory droplets—A modeling approach. Phys Fluids 32:083307. doi: 10.1063/5.0021280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jayaweera M, Perera H, Gunawardana B, Manatunge J. 2020. Transmission of COVID-19 virus by droplets and aerosols: a critical review on the unresolved dichotomy. Environ Res 188:109819. doi: 10.1016/j.envres.2020.109819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bourouiba L, Dehandschoewercker E, Bush JWM. 2014. Violent expiratory events: on coughing and sneezing. J Fluid Mech 745:537–563. doi: 10.1017/jfm.2014.88. [DOI] [Google Scholar]
- 103.Zheng S, Fan J, Yu F, Feng B, Lou B, Zou Q, Xie G, Lin S, Wang R, Yang X, Chen W, Wang Q, Zhang D, Liu Y, Gong R, Ma Z, Lu S, Xiao Y, Gu Y, Zhang J, Yao H, Xu K, Lu X, Wei G, Zhou J, Fang Q, Cai H, Qiu Y, Sheng J, Chen Y, Liang T. 2020. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang Province, China, January–March 2020: retrospective cohort study. BMJ 369:m1443. doi: 10.1136/bmj.m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Leung NHL, Chu DKW, Shiu EYC, Chan K-H, McDevitt JJ, Hau BJP, Yen H-L, Li Y, Ip DKM, Peiris JSM, Seto W-H, Leung GM, Milton DK, Cowling BJ. 2020. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med 26:676–680. doi: 10.1038/s41591-020-0843-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bouwknegt M, Verhaelen K, Rzeżutka A, Kozyra I, Maunula L, von Bonsdorff C-H, Vantarakis A, Kokkinos P, Petrovic T, Lazic S, Pavlik I, Vasickova P, Willems KA, Havelaar AH, Rutjes SA, de Roda Husman AM. 2015. Quantitative farm-to-fork risk assessment model for norovirus and hepatitis A virus in European leafy green vegetable and berry fruit supply chains. Int J Food Microbiol 198:50–58. doi: 10.1016/j.ijfoodmicro.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 106.U.S. Environmental Protection Agency. 2011. Dermal exposure studies. In U.S. EPA exposure factors handbook. U.S. Environmental Protection Agency, Washington, DC. [Google Scholar]
- 107.Nicas M, Jones RM. 2009. Relative contributions of four exposure pathways to influenza infection risk. Risk Anal 29:1292–1303. doi: 10.1111/j.1539-6924.2009.01253.x. [DOI] [PubMed] [Google Scholar]
- 108.Zhang N, Chen X, Jia W, Jin T, Xiao S, Chen W, Hang J, Ou C, Lei H, Qian H, Su B, Li J, Liu D, Zhang W, Xue P, Liu J, Weschler LB, Xie J, Li Y, Kang M. 2021. Evidence for lack of transmission by close contact and surface touch in a restaurant outbreak of COVID-19. J Infect 83:207–216. doi: 10.1016/j.jinf.2021.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nicas M, Best D. 2008. A study quantifying the hand-to-face contact rate and its potential application to predicting respiratory tract infection. J Occup Environ Hyg 5:347–352. doi: 10.1080/15459620802003896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pitol AK, Bischel HN, Boehm AB, Kohn T, Julian TR. 2018. Transfer of enteric viruses adenovirus and coxsackievirus and bacteriophage MS2 from liquid to human skin. Appl Environ Microbiol 84:e01809-18. doi: 10.1128/AEM.01809-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.U.S. Environmental Protection Agency. 2011. EPA/600/R-090/052F. In Exposure factors handbook. U.S. Environmental Protection Agency, Washington, DC. [Google Scholar]
- 112.Ailes EC, Leon JS, Jaykus L-A, Johnston LM, Clayton HA, Blanding S, Kleinbaum DG, Backer LC, Moe CL. 2008. Microbial concentrations on fresh produce are affected by postharvest processing, importation, and season. J Food Prot 71:2389–2397. doi: 10.4315/0362-028x-71.12.2389. [DOI] [PubMed] [Google Scholar]
- 113.Johnston LM, Jaykus LA, Moll D, Anciso J, Mora B, Moe CL. 2006. A field study of the microbiological quality of fresh produce of domestic and Mexican origin. Int J Food Microbiol 112:83–95. doi: 10.1016/j.ijfoodmicro.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 114.Newman KL, Bartz FE, Johnston L, Moe CL, Jaykus LA, Leon JS. 2017. Microbial load of fresh produce and paired equipment surfaces in packing facilities near the U.S. and Mexico border. J Food Prot 80:582–589. doi: 10.4315/0362-028X.JFP-16-365. [DOI] [PubMed] [Google Scholar]
- 115.Killingley B, Mann AJ, Kalinova M, Boyers A, Goonawardane N, Zhou J, Lindsell K, Hare SS, Brown J, Frise R, Smith E, Hopkins C, Noulin N, Löndt B, Wilkinson T, Harden S, McShane H, Baillet M, Gilbert A, Jacobs M, Charman C, Mande P, Nguyen-Van-Tam JS, Semple MG, Read RC, Ferguson NM, Openshaw PJ, Rapeport G, Barclay WS, Catchpole AP, Chiu C. 2022. Safety, tolerability and viral kinetics during SARS-CoV-2 human challenge in young adults. Nat Med 28:1031–1041. doi: 10.1038/s41591-022-01780-9. [DOI] [PubMed] [Google Scholar]
- 116.Karimzadeh S, Bhopal R, Huy NT. 2021. Review of infective dose, routes of transmission, and outcome of COVID-19 caused by the SARS-COV-2: comparison with other respiratory viruses. Epidemiol Infect 149:e96. doi: 10.1017/S0950268821000790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kawamoto K, Leon JS. Summary of global guidance, guidelines or recommendations to prevent food workers from acquiring COVID-19. 2021. https://etd.library.emory.edu/concern/etds/3f462661j?locale=en. Accessed 5 August, 2021.
- 118.Centers for Disease Control and Prevention, Occupational Safety and Health Administration. 2020. Manufacturing workers and employers—interim guidance from CDC and the Occupational Safety and Health Administration. https://stacks.cdc.gov/view/cdc/85488. Accessed 18 March, 2021.
- 119.World Health Organization. 2020. Coronavirus disease (COVID-19) advice for the public. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public. Accessed 7 April, 2023.
- 120.Grove SF, Suriyanarayanan A, Puli B, Zhao H, Li M, Li D, Schaffner DW, Lee A. 2015. Norovirus cross-contamination during preparation of fresh produce. Int J Food Microbiol 198:43–49. doi: 10.1016/j.ijfoodmicro.2014.12.023. [DOI] [PubMed] [Google Scholar]
- 121.U.S. Environmental Protection Agency. 2020. List N: disinfectants for coronavirus (COVID-19). Pesticide registration. https://www.epa.gov/coronavirus/about-list-n-disinfectants-coronavirus-covid-19-0. Accessed 15 March, 2021.
- 122.Ott W, Klepeis N, Switzer P. 2008. Air change rates of motor vehicles and in-vehicle pollutant concentrations from secondhand smoke. J Expo Sci Environ Epidemiol 18:312–325. doi: 10.1038/sj.jes.7500601. [DOI] [PubMed] [Google Scholar]
- 123.Chaudhry SK, Elumalai SP. 2020. The influence of school bus ventilation scenarios over in-cabin PM number concentration and air exchange rates. Atmos Pollut Res 11:1396–1407. doi: 10.1016/j.apr.2020.05.021. [DOI] [Google Scholar]
- 124.Zhu Y, Hinds WC, Krudysz M, Kuhn T, Froines J, Sioutas C. 2005. Penetration of freeway ultrafine particles into indoor environments. J Aerosol Sci 36:303–322. doi: 10.1016/j.jaerosci.2004.09.007. [DOI] [Google Scholar]
- 125.Nasreen S, Chung H, He S, Brown KA, Gubbay JB, Buchan SA, Fell DB, Austin PC, Schwartz KL, Sundaram ME, Calzavara A, Chen B, Tadrous M, Wilson K, Wilson SE, Kwong JC, Canadian Immunization Research Network Provincial Collaborative Network Investigators . 2021. Effectiveness of mRNA and ChAdOx1 COVID-19 vaccines against symptomatic SARS-CoV-2 infection and severe outcomes with variants of concern in Ontario. medRxiv. doi: 10.1101/2021.06.28.21259420. [DOI] [PubMed] [Google Scholar]
- 126.Saciuk Y, Kertes J, Shamir Stein N, Ekka Zohar A. 2022. Effectiveness of a third dose of BNT162b2 mRNA vaccine. J Infect Dis 225:30–33. doi: 10.1093/infdis/jiab556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wu N, Joyal-Desmarais K, Ribeiro PAB, Vieira AM, Stojanovic J, Sanuade C, Yip D, Bacon SL. 2023. Long-term effectiveness of COVID-19 vaccines against infections, hospitalisations, and mortality in adults: findings from a rapid living systematic evidence synthesis and meta-analysis up to December, 2022. Lancet Respir Med 11:439–452. doi: 10.1016/S2213-2600(23)00015-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pouillot R, Delignette-Muller ML. 2010. Evaluating variability and uncertainty separately in microbial quantitative risk assessment using two R packages. Int J Food Microbiol 142:330–340. doi: 10.1016/j.ijfoodmicro.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 129.Dabisch P, Schuit M, Herzog A, Beck K, Wood S, Krause M, Miller D, Weaver W, Freeburger D, Hooper I, Green B, Williams G, Holland B, Bohannon J, Wahl V, Yolitz J, Hevey M, Ratnesar-Shumate S. 2021. The influence of temperature, humidity, and simulated sunlight on the infectivity of SARS-CoV-2 in aerosols. Aerosol Sci Technol 55:142–153. doi: 10.1080/02786826.2020.1829536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Blanco MN, Fenske RA, Kasner EJ, Yost MG, Seto E, Austin E. 2017. Real-time monitoring of spray drift from three different orchard sprayers. Physiol Behav 176:139–148. doi: 10.1016/j.physbeh.2017.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.ANSI/ASHRAE. 2019. Ventilation and acceptable indoor air quality in residential buildings. https://ashrae.iwrapper.com/ASHRAE_PREVIEW_ONLY_STANDARDS/STD_62.2_2019. Accessed 20 March, 2021.
- 132.Biryukov J, Boydston JA, Dunning RA, Yeager JJ, Wood S, Reese AL, Ferris A, Miller D, Weaver W, Zeitouni NE, Phillips A, Freeburger D, Hooper I, Ratnesar-Shumate S, Yolitz J, Krause M, Williams G, Dawson DG, Herzog A, Dabisch P, Wahl V, Hevey MC, Altamura LA. 2020. Increasing temperature and relative humidity accelerates inactivation of SARS-COV-2 on surfaces. mSphere 5:e00441-20. doi: 10.1128/mSphere.00441-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kwon T, Gaudreault NN, Richt JA. 2020. Environmental stability of SARS-CoV-2 on different types of surfaces under indoor and seasonal climate conditions. bioRxiv. doi: 10.1101/2020.08.30.274241. [DOI] [PMC free article] [PubMed]
- 134.Lopez GU, Gerba CP, Tamimi AH, Kitajima M, Maxwell SL, Rose JB. 2013. Transfer efficiency of bacteria and viruses from porous and nonporous fomites to fingers under different relative humidity conditions. Appl Environ Microbiol 79:5728–5734. doi: 10.1128/AEM.01030-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mokhtari A, Jaykus LA. 2009. Quantitative exposure model for the transmission of norovirus in retail food preparation. Int J Food Microbiol 133:38–47. doi: 10.1016/j.ijfoodmicro.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 136.Konda A, Prakash A, Moss GA, Schmoldt M, Grant GD, Guha S. 2020. Aerosol filtration efficiency of common fabrics used in respiratory cloth masks. ACS Nano 14:6339–6347. doi: 10.1021/acsnano.0c03252. [DOI] [PubMed] [Google Scholar]
- 137.Swift MD, Breeher LE, Tande AJ, Tommaso CP, Hainy CM, Chu H, Murad MH, Berbari EF, Virk A. 2021. Effectiveness of messenger RNA coronavirus disease 2019 (COVID-19) vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in a cohort of healthcare personnel. Clin Infect Dis 73:e1376–e1379. doi: 10.1093/cid/ciab361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.U.S. Food & Drug Administration. 2021. Janssen COVID-19 vaccine. Vaccines and related biological products advisory committee February 26, 2021 meeting briefing document. https://www.fda.gov/media/146217/download. Accessed 25 March, 2021.
- 139.U.S. Food & Drug Administration. 2021. Janssen COVID-19 vaccine. Vaccines and related biological products advisory committee February 26, 2021 meeting briefing addendum document. https://www.fda.gov/media/146218/download. Accessed 25 March, 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download aem.00128-23-s0001.docx, DOCX file, 1.1 MB (1.1MB, docx)
Data Availability Statement
The code developed and utilized throughout this analysis is available through GitHub at the following DOI: https://doi.org/10.5281/zenodo.8003697.