Abstract
Background:
Consumption of an organic diet reduces exposure to a range of agricultural pesticides. Only three studies have examined the effect of an organic diet intervention on exposure to the herbicide glyphosate, the most heavily used agricultural chemical in the world. Despite its widespread use, the primary sources of glyphosate exposure in humans are poorly understood.
Objective:
Our objective was to examine the effect of an organic diet intervention on urinary glyphosate concentrations among pregnant individuals.
Methods:
We conducted a 2-wk randomized crossover trial in which 39 pregnant participants living near () and far () from agricultural fields received a 1-wk supply of conventional groceries and 1 wk of organic groceries, randomized to order. We collected daily first morning void urine samples and analyzed composite samples from each week for glyphosate. We examined differences in urinary glyphosate concentrations between the conventional week and the organic week among all participants and stratified by residential proximity to an agricultural field.
Results:
Median specific gravity–adjusted glyphosate concentrations were and during the conventional and organic weeks, respectively. We observed modest decreases in urinary glyphosate concentrations from the conventional to organic week among far-field participants, but no difference among near-field participants. In secondary analyses excluding participants who did not meet a priori criteria of compliance with the intervention, we observed significant decreases in urinary glyphosate concentrations, particularly among far-field participants (, depending on exclusion criteria).
Discussion:
This trial is the first to examine the effect of an organic diet intervention on glyphosate among people living near and far from agricultural fields. Our results suggest that diet is an important contributor to glyphosate exposure in people living from agricultural fields; for people living near crops, agriculture may be a dominant exposure source during the pesticide spray season. https://doi.org/10.1289/EHP12155
Introduction
Glyphosate, a broad-spectrum herbicide that is the active ingredient in Roundup, is the most heavily used agricultural chemical in history.1,2 Globally, the use of glyphosate has increased since the late 1990s1 with its expanded use as a preharvest desiccant and in “Roundup Ready” genetically engineered glyphosate-tolerant crops.3 Despite its frequent detection in food samples4 and environmental samples from both agricultural and nonagricultural communities,5,6 relatively little data on human exposure to glyphosate exist.7,8 In response to increasing evidence that prenatal glyphosate exposure may be associated with adverse birth outcomes such as shortened gestational age,9–11 recent editorials and consensus statements have called for more biomonitoring and epidemiological research on glyphosate, particularly during vulnerable periods such as pregnancy and early childhood.12,13 Although additional evidence from larger studies is needed, if the previously observed associations of prenatal glyphosate exposure and adverse birth outcomes9–11 are true, it is imperative to identify effective exposure-reduction interventions during this critical window of exposure, particularly given the widespread use of glyphosate and potential population impacts.
Evidence of adverse health effects associated with glyphosate dosing during the prenatal period has also been reported in toxicological studies. For example, studies have shown a far greater gut microbiome dysbiosis in the offspring of laboratory animals following prenatal exposure to glyphosate and glyphosate-based herbicides (GBHs), as opposed to exposure starting in early adulthood.14,15 Other studies have demonstrated teratogenic and carcinogenic effects of in utero glyphosate exposure in animals through mechanisms such as the disruption of retinoic acid signaling, estrogen biosynthesis, and enzymatic pathways,16 as well as inducing DNA damage and oxidative stress17,18 and DNA methylation, which could result in epigenetic modifications.19
Previous observational and intervention exposure assessment studies, which have largely focused on nonagricultural populations, have shown that an organic diet is associated with lower exposures to a range of insecticides, herbicides, and fungicides among children and adults.20–32 These findings are consistent with studies showing lower pesticide residues on organic food,33,34 because synthetic pesticides such as glyphosate are prohibited in organic farming.35 Only three organic-diet intervention studies have examined exposure to glyphosate21,29,36; one of these studies enrolled only two participants.29 In addition, most organic diet interventions have focused on insecticides, which are typically applied to different crops using different application methods than herbicides. For example, although fruit and vegetable consumption has consistently been associated with urinary insecticide concentrations,20,23,24,37 glyphosate has been detected more commonly in grain and legume products, including processed foods.1,38,39 Furthermore, only one study has examined the effect of an organic diet intervention on urinary pesticide concentrations among participants living in both urban and rural/agricultural areas.20 Although diet is one of the primary sources of exposure to most pesticides in nonagricultural populations,31,40–42 nonoccupationally exposed individuals living in agricultural regions are exposed to pesticides via multiple sources and pathways in addition to diet, including the direct contamination of soil43 or drinking water,44 and from inhalation and dermal absorption following pesticide spray drift.45,46 The primary sources of pesticide exposure and effective exposure-reduction strategies among people living near pesticide-treated agricultural fields are poorly understood.
The purpose of this trial was to assess the effect of an organic diet intervention on urinary glyphosate concentrations among pregnant people in Idaho, including individuals living within of and farther than from an agricultural field. We hypothesized that urinary glyphosate concentrations would be lower during the organic diet period in comparison with the conventional diet period. We also hypothesized that residential proximity to agricultural fields would modify the effect of the intervention, resulting in a larger change in glyphosate concentrations between the diet periods among participants living farther from agricultural fields in comparison with those living closer.
Methods
Study Participants
We recruited 40 pregnant people in their first trimester from Women, Infants, and Children (WIC) clinics from Southwest District Health (serving the towns of Nampa and Caldwell), South Central District Health (serving Twin Falls, Shoshone, Gooding, Jerome, and Heyburn), and Central District Health (serving Boise, Meridian, and Garden City) in Idaho for a longitudinal study to examine urinary glyphosate concentrations during pregnancy. WIC is a federal program administered through local agencies that provides supplemental nutritious food, nutrition counseling, and health screenings and referrals to low-income nutritionally at-risk pregnant, breastfeeding, and postpartum women, as well as infants and children up to age 5 y.47 Our research team prepared videos in English and Spanish that WIC staff shared with clients who might be interested in participating in the study. If WIC staff spoke with a pregnant person who was interested in learning more about the study, the staff member asked the individual if they could share their contact information with our research team. Our research team contacted interested individuals directly to describe the study and assess their interest and eligibility. The principal investigator of the study, C.L.C., and her research team, including a lab manager, two graduate students, and two undergraduate students (including a native Spanish speaker) were responsible for enrolling all participants.
We enrolled participants between 23 February and 3 June 2021 and followed them until they gave birth between 5 August and 28 December 2021. Participants were eligible if they were in their first trimester of pregnancy; over the age of 18 y; spoke English or Spanish; had not been told by a medical professional that they had a high-risk pregnancy; did not work with pesticides or live with anyone who worked with pesticides; had access to a smartphone, tablet, or computer that could connect to the internet; and consumed a mostly conventional (nonorganic) diet.
As part of this longitudinal study, we conducted a 2-wk nested randomized crossover trial of an organic diet. This paper focuses specifically on this 2-wk dietary intervention trial in which we collected daily first morning void (FMV) urine samples during the intervention from 16 June to 30 June 2021 (during the pesticide spray season). All participants received 1 wk of conventional groceries and 1 wk of organic groceries, randomized to order. The outcome of interest for this trial was changes in urinary glyphosate concentrations between the conventional week and the organic week.
We calculated a sample size of 40 participants to provide a power of 0.80 at a 0.05 significance level in a two-sided test. This calculation was based on reported geometric mean glyphosate concentrations in urine samples from mothers in 48 farm and nonfarm households from a 2007 study in Iowa.48 We assumed a standard deviation (SD) of , based on the ratio of mean to variance in organophosphate (OP) pesticide concentrations from a longitudinal study that analyzed multiple samples from children.49 We used the SD from a study of OPs rather than glyphosate because no comparable glyphosate data exist, and the half-lives are similar ( h).50,51
The Boise State University institutional review board reviewed and approved all study procedures. This study was registered, and the protocol can be accessed, on ClinicalTrials.gov (NCT: 04155463). The Consolidated Standards of Reporting Trials (CONSORT) checklist is available in the supplementary material.
Survey Data and Food Logs
We administered a brief questionnaire at enrollment to assess sociodemographic factors and the use of pesticides at the participant’s home in the past year (by the participant or by others). We also asked participants to notify us if they moved or resided at any other homes during the study period. Prior to each of the 2 wk of the dietary intervention, participants received a Food Log in which they were asked to a) rate on a five-point Likert scale how much of the food that they ate each day was from the groceries provided by the study (i.e., everything, most, about half, a little bit, none), and b) write down any foods or drinks they consumed each day that were not from the groceries provided by the study (See Supplementary Material for example of Food Log from Week 1).
Assessment of Proximity to Agricultural Fields
We geocoded each participant’s address where the participant reported living during the 2-wk dietary intervention and verified the existence and location of all fields within a radius of their home, as previously described.52 Briefly, in August 2021, we used Google Earth to identify all potential agricultural fields (areas of green or brown that did not contain homes or other structures) within a radius of each participant’s home and then visually inspected each of the fields to determine whether it was currently being used for crop production. We plotted the geocoded address of each residence and the locations of all agricultural fields in current cultivation in ArcGIS and calculated the distance from each residence to the nearest agricultural field. We henceforth refer to participants living within of an agricultural field as “near-field” and those living farther than from an agricultural field as “far-field” participants, based on categorizations used in previous exposure assessment and epidemiology studies.53–56 Although Idaho does not have publicly available pesticide use data and we are not able to confirm that glyphosate was sprayed in fields surrounding near-field participants’ homes during the dietary intervention period, we intentionally conducted the sampling during the agricultural spray season and confirmed that each near-field participant had at least one “Round-Up Ready” crop that is typically sprayed with glyphosate (e.g., corn, alfalfa)57 within a radius of their home. One participant lived at two different houses during the intervention, but both residences were within of an agricultural field, and she was thus classified as near-field for the trial period.
Randomization
We randomly assigned participants to receive 1 wk of either organic or conventional groceries (16 June–22 June), followed by one washout day (based on glyphosate’s estimated half-life of 5.5 to 9 h),58 then 1 wk of groceries of the opposite food type (24 June–30 June).
C.H. was responsible for randomizing participants to receive either organic or conventional groceries during the first week. All study staff and participants were informed of group assignment, because participants needed to be informed of which type of groceries to order and study staff needed to ensure grocery orders aligned with the dietary allocation each week. We conducted stratified randomization59 by residential location using a random number generator.
IDs for participants from each recruitment area were entered into separate columns in a spreadsheet. Using a random number generator from 1 to 1,000, we entered a random number in separate columns for each of 18 pairs, grouped by location. We sorted each of these columns in ascending order and assigned the first nine participants to receive organic food the first week, and the second nine participants to receive conventional food the first week. Using the random number generator from 1 to 1,000, we then entered a random number for the remaining four participants, sorted the numbers in descending order, and assigned the first two participants to receive organic food the first week and the last two to receive conventional food the first week.
Dietary Intervention
Figure 1 illustrates the study design and sample collection by day. Participants received their grocery orders on Days 1 and 9. Daily FMV urine samples were collected on Days 3–9 for Week 1 and Days 11–17 for Week 2; Days 2 and 10 were washout days.
Figure 1.
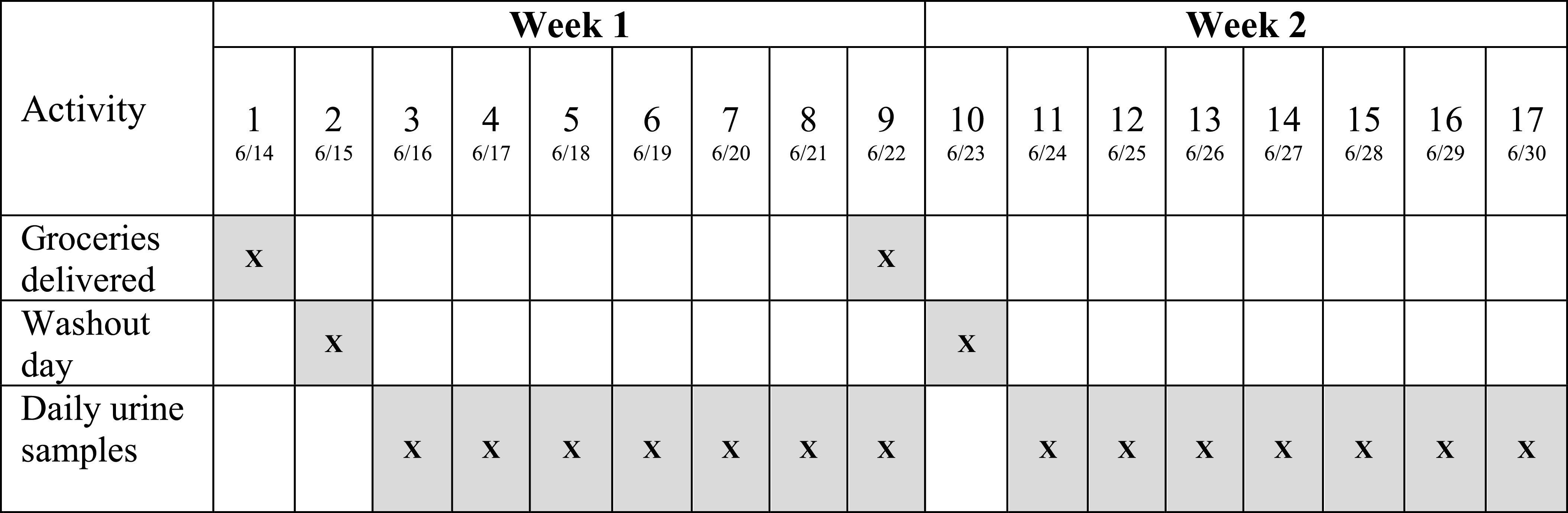
Dietary intervention study design and sample collection by day among 39 pregnant participants in a randomized crossover conventional vs. organic dietary intervention trial.
Study staff created a unique online account from a local grocery store chain for each participant. Prior to the start of each week, we provided participants a flyer with the account information and instructions on how to log into the account and shop for all the food they anticipated eating that week, up to USD . Participants were asked to order all organic or all conventional items in accordance with whether they had been randomized to receive organic or conventional food that week. Study staff logged into each participant’s account, confirmed that all the food items corresponded with their dietary randomization that week, and ordered the groceries to be delivered to the participant’s home, when possible. For participants living in areas in which delivery was not available (generally rural areas), study staff picked up the food orders at the grocery store and delivered them to the participants’ homes.
Urine Collection
We collected daily FMV urine samples from each participant during the dietary intervention, from 16 June 16 to 30 June 2021 (except Day 10, which was a washout day when no urine was collected). In accordance with COVID-19 protocols, we implemented procedures to retrieve urine samples in a contactless manner. Participants were provided a urine cup with a unique barcode and label with their Participant ID number and the day of the study period. We sent participants a video link with instructions regarding how to collect their urine samples, including a reminder to write the date and time of collection on the label on the urine cup. We provided participants with a cooler and ice packs and asked them to place their urine sample inside a plastic sealable bag in the cooler each morning after collection. Each day, study staff drove to each participant’s home to collect the sample, placed it in a transport cooler with ice packs to be transported to the laboratory, and left in the participant’s cooler a new barcoded urine cup in a sealable plastic bag for the following day’s sample collection. Each night prior to sample collection, study staff sent participants a text reminder to collect the following morning’s first urine sample and to place it in their cooler with ice packs and to complete their Food Logs.
After returning to the lab, we determined the specific gravity of each sample at 5°C and aliquoted samples into cryovials. We aliquoted of each sample into two separate cryovials and stored these samples at . We also created composite samples for each week for every participant. For the composite samples, we aliquoted of each participant’s FMV daily sample into two separate cryovials and stored the samples at . After collecting the following day’s sample, we removed the composite cryovials from the freezer, aliquoted of the sample on top of the previous day’s frozen sample, and returned the cryovials to the freezer. We repeated this process for all participants’ seven daily urine samples to form a composite sample intended to represent each participant’s mean exposure during each week of the dietary intervention, without requiring a freeze–thaw cycle.
Quantification of Urinary Glyphosate
We shipped a vial of each participant’s FMV composite urine for each week of the dietary intervention overnight on dry ice to the U.S. Centers for Disease Control and Prevention (U.S. CDC) for analysis. Details of the glyphosate quantification method have been described previously.60 Briefly, urinary glyphosate was determined by ion chromatography-isotope dilution–tandem mass spectrometry using a Dionex chromatography system (using polyether ether ketone materials to prevent carryover and interaction with metal surfaces) and an AB Sciex 5500 triple quadrupole mass spectrometer. The limit of detection (LOD) was . The accuracy of the method was established by spiking two different urine samples at zero, low, mid, and high glyphosate concentrations. The mean relative recovery was 99% (range 97%–103%). Accuracy of the method has been confirmed with repeated successful participation of the U.S. CDC laboratory in two international external quality assessment programs since 2019. Along with the study samples and analytical standards, each analytical batch included high- and low-concentration quality control materials (QCs) and reagent blanks to assure the accuracy and reproducibility of the data. The concentrations of the QCs were evaluated using standard statistical probability rules.61 If the QC samples failed the statistical evaluation, all the samples in the batch were reextracted. The U.S. CDC laboratory used the same approach to analyze samples from the National Health and Nutrition Examination Survey (NHANES).62,63 The analysis of de-identified specimens at the U.S. CDC laboratory was determined not to constitute human subjects research.
Evaluating the Effect of Composite Sampling
We conducted a small assessment to evaluate whether the composite samples provided an acceptable alternative to analyzing and averaging individual FMV daily samples. We analyzed all 14 individual daily FMV spot urine samples for one randomly selected participant and confirmed that the mean glyphosate concentration from the two sets of seven individual daily samples was similar to the value from the corresponding weekly composite urine samples (nonspecific gravity–adjusted concentrations: Week 1 Composite , Average of seven individual daily samples ; Week 2 Composite , Average of seven individual daily ). Based on these results, we are confident that the composite samples were appropriate representations of the average of the glyphosate concentrations in the FMV daily urine samples.
Evaluation of Compliance with the Intervention
We developed a protocol to assess compliance with the dietary intervention (see Supplementary Material) and developed a priori exclusion criteria for secondary analyses. In the main secondary analyses, we excluded the following: a) participants missing urine samples from either week and b) participants who did not turn in at least one of the two weekly food logs.
In subsequent secondary analyses, we excluded participants with low compliance based on self-report on the Likert scale regarding how much of the food they ate from each day was from the groceries provided by the study [total Likert scores of on the Food Log, corresponding to an average of self-reported category of “About half of what I ate was from the study” across that week (see Food Log Protocol in the Supplementary Material for detailed calculation of score)]; participants who listed large amounts of food as consumed from outside of the study [total score , corresponding to more than one-third of the snacks and meals consumed during the organic week being from nonorganic food outside of the grocery order (see “Food Log Protocol” in Supplementary Material for detailed calculation of score)]; or participants who did not write down anything for food consumed from outside the study on the Food Log. We prespecified evaluation of these exclusions separately and in various combinations. The full set of subsequent secondary analyses is shown in Table S1.
Data Analysis
We calculated the specific gravity of each composite FMV urine sample as the mean of the specific gravity of the seven individual FMV samples of which the composite was composed. We imputed values below the LOD as 64 and adjusted urinary concentrations for specific gravity using the following equation: ,65 where is the adjusted result (), is the original concentration (), 1.017 is the mean specific gravity measured within the study population during the dietary intervention, and is the mean specific gravity of the individual composite sample. All urinary glyphosate concentrations henceforth refer to specific gravity–adjusted concentrations.
We calculated geometric means (GM) and the percent difference in glyphosate concentrations between the conventional and organic week for each participant as: , where is the urinary concentration during the organic week, and is the urinary concentration during the conventional week. Data were not normally distributed (p-value from Shapiro-Wilk normality test ). We conducted two-tailed Wilcoxon signed rank tests for paired data to assess differences in urinary glyphosate concentrations between the conventional and organic weeks among all participants and among participants stratified by proximity to agriculture. Finally, we ran two-tailed Wilcoxon rank sum tests for independent data to evaluate differences in urinary glyphosate concentrations between the far- and near-field participants separately during the organic and conventional diet weeks. Our primary “Intention to Treat” analysis included the entire study population (). In secondary analyses, we excluded participants based on a priori exclusion criteria as described above. We also evaluated the effect of diet order in the crossover trial and found that urinary concentrations did not differ between participants receiving organic groceries in the first week vs. the second week or between those receiving conventional groceries in the first week vs. the second week, both among all participants and among near- and far-field participants. We used Stata (version 14.2; StataCorp.) for all analyses.
Results
Of the 58 individuals referred by WIC clinics, 53 (91%) were eligible; 40 (75%) of eligible individuals agreed to participate and were enrolled in the study (Figure 2).
Figure 2.
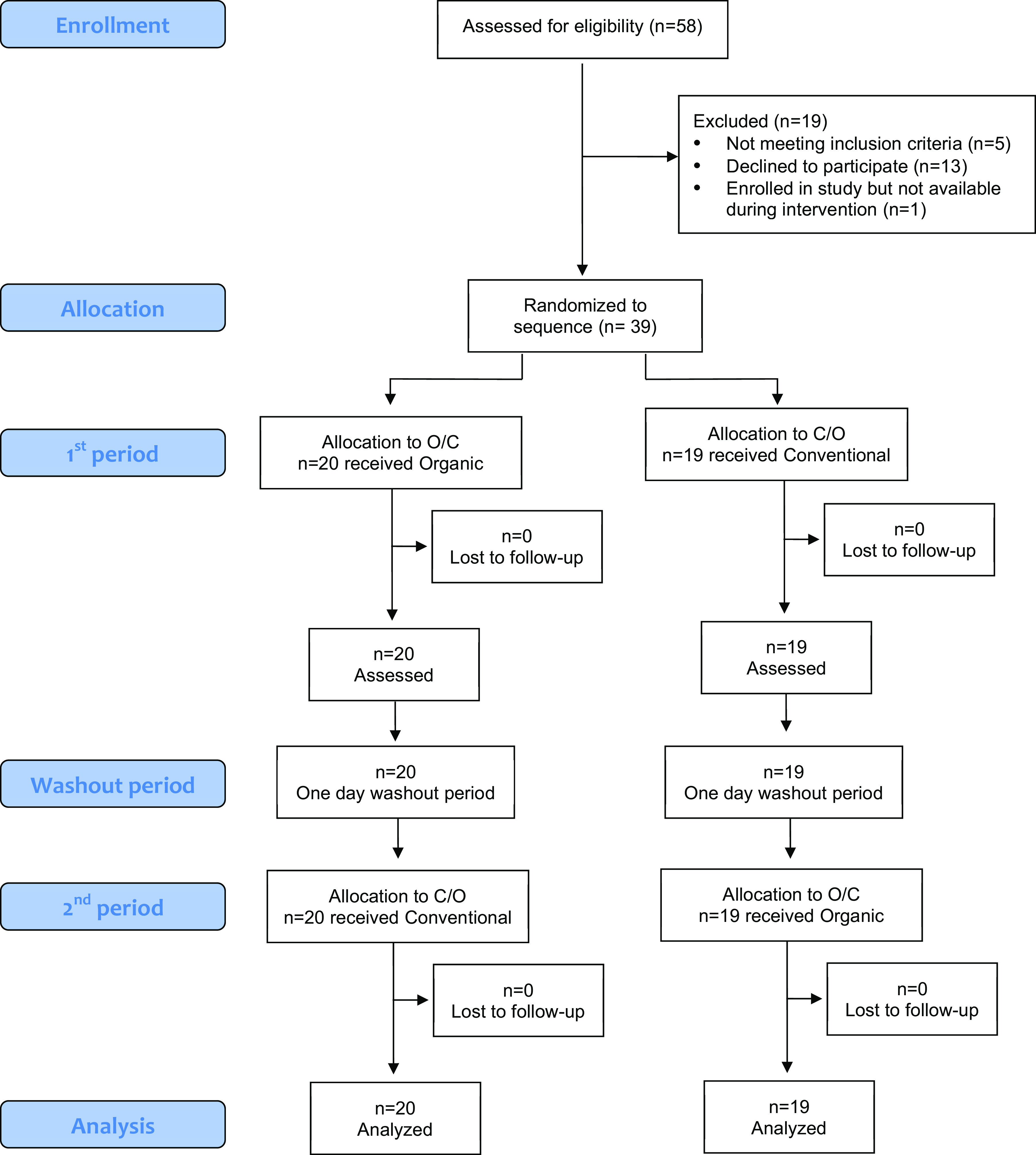
CONSORT enrollment, random assignment, and retention of study participants among 39 pregnant participants in a randomized crossover conventional vs. organic dietary intervention trial. Note: CONSORT, Consolidated Standards of Reporting Trials; C/O, conventional/organic; O/C, organic/conventional.
Of the 40 participants enrolled in the longitudinal study, 39 took part in the dietary intervention (one participant was out of town during the intervention period; this participant had been randomized to receive conventional groceries during the first week prior to notifying study staff she would not be available during the dietary intervention). During the intervention, 35 participants were in their second trimester of pregnancy, and 4 participants were in their third trimester. From these 39 participants, we collected a total of 531 urine samples (97% of the maximum possible 546), an average of 13.6 of 14 possible samples from each participant, including an average of 6.8 during both the conventional and organic weeks. Thirty-three participants (85%) provided all 14 urine samples; 1 participant (2.5%) provided 13 samples; 2 (5.1%) provided 12 samples; 2 (5.1%) provided 11 samples; and 1 (2.6%) provided 10 samples.
Table 1 shows the demographic characteristics of the 39 individuals who participated in the dietary intervention, stratified by whether they were randomized to receive organic or conventional groceries first. Participants mostly identified as White (; 51.3%) or Hispanic/Latina (; 48.7%) and reported having less than a college education (; 94.9%) and a household income of in the previous year (; 69.2%). No participants reported using herbicides themselves in their home within the last year, but eight participants reported that someone else had sprayed herbicides at their residence in the last year, including professional pesticide control companies. Five of these participants did not know what type of herbicide had been sprayed, whereas one reported that glyphosate was used, one reported that the product Cheetah—which contains glufosinate ammonium—was used, and one reported that two different Spectracide products containing a combination of nonglyphosate herbicides were used.
Table 1.
Demographic characteristics of 39 pregnant people participating in a randomized crossover diet intervention study of urinary glyphosate concentrations following consumption of a conventional vs. organic diet in Idaho, 2021 [n (%)].
| Characteristic | All () | O/C ()a | C/O ()b |
|---|---|---|---|
| Age (y) | |||
| 18–22 | 11 (28.2) | 6 (30.0) | 5 (26.3) |
| 23–27 | 10 (25.6) | 4 (20.0) | 6 (31.6) |
| 28–32 | 13 (33.3) | 6 (30.0) | 7 (36.8) |
| 33–37 | 5 (12.8) | 4 (20.0) | 1 (5.3) |
| Race/ethnicityc | |||
| Caucasian or White | 20 (51.3) | 9 (42.9) | 11 (55.0) |
| Hispanic or Latina | 19 (48.7) | 10 (47.6) | 9 (45.0) |
| Asian | 1 (2.6) | 1 (4.8) | 0 (0.0) |
| American Indian or Alaskan Native | 1 (2.6) | 1 (4.8) | 0 (0.0) |
| African American or Black | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Highest level of education | |||
| Less than high school | 5 (12.8) | 2 (10.0) | 3 (15.8) |
| Graduated high school/earned GED | 14 (35.9) | 9 (45.0) | 5 (26.3) |
| Some college | 18 (46.2) | 8 (40.0) | 10 (52.6) |
| Bachelor’s degree | 1 (2.6) | 1 (5.0) | 0 (0.0) |
| Graduate school/advanced degree | 1 (2.6) | 0 (0.0) | 1 (5.3) |
| Household income in previous year (USD) | |||
| 9 (23.1) | 5 (25.0) | 4 (21.1) | |
| 10 (25.6) | 3 (15.0) | 7 (36.8) | |
| 8 (20.5) | 3 (15.0) | 5 (26.3) | |
| 5 (12.8) | 4 (20.0) | 1 (5.3) | |
| 5 (12.8) | 3 (15.0) | 2 (10.5) | |
| Missing or prefer not to answer | 2 (5.1) | 2 (10.0) | 0 (0.0) |
| Number living in household | |||
| 1–3 | 15 (38.5) | 9 (45.0) | 6 (31.6) |
| 4–6 | 22 (56.4) | 10 (50.0) | 12 (63.2) |
| 7–9 | 2 (5.1) | 1 (5.0) | 1 (5.3) |
| Personally used herbicides at residence in last year | |||
| Yes | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| No | 39 (100.0) | 20 (100.0) | 19 (100.0) |
| Someone else used herbicides at residence in last year | |||
| Yes | 8 (20.5) | 6 (30.0) | 2 (10.5) |
| No | 28 (71.8) | 13 (65.0) | 15 (79.0) |
| Don’t know | 3 (7.7) | 1 (5.0) | 2 (10.5) |
| Type of herbicides used at residence in last year | |||
| Do not know or unknown pesticides sprayed by pesticide control company | 5 (62.5) | 4 (66.7) | 1 (50.0) |
| Roundup (glyphosate) | 1 (11.1) | 0 (0.0) | 1 (50.0) |
| Cheetah (glufosinate ammonium) | 1 (11.1) | 1 (16.7) | 0 (0.0) |
| Spectracide (combination of nonglyphosate herbicides) | 1 (11.1) | 1 (16.7) | 0 (0.0) |
Note: All characteristics were self-reported by participants at enrollment. C/O, conventional/organic; GED, general equivalency diploma; O/C, organic/conventional; USD, U.S. dollars.
n (%) among participants who received organic groceries the first week.
n (%) among participants who received conventional groceries the first week.
Participant could select more than one option.
We analyzed participants’ grocery orders and food logs and found participants ordered relatively similar levels of grain and legume products, which have been found to have high levels of glyphosate residues,1,38,39 during the conventional and organic weeks. Specifically, 54% of participants ordered a greater proportion of legumes and grain products during the conventional week, and the remaining 46% ordered a greater or similar proportion of grains and legumes during the organic week.
Table 2 shows the detection frequency and the geometric mean (GM) and interquartile range (IQR) urinary glyphosate concentrations during the organic and conventional weeks among all participants (), far-field participants (), and near-field participants (). Glyphosate was detected in 74.4% of the 78 total weekly FMV composite urine samples. Among all participants, the detection frequency was 76.9% during the conventional phase and 71.8% during the organic phase. For near-field participants, the detection frequency was 75% during both weeks; among participants living far from fields, glyphosate was detected in 78.9% of the conventional week samples and 68.4% of the samples collected during the organic week. Urinary concentrations were higher among near-field participants in comparison with far-field participants, though the difference was not statistically significant (organic week: 0.20 vs. , ; conventional week: 0.21 vs. , ).
Table 2.
Detection frequency and specific gravity–adjusted urinary glyphosate concentrations (micrograms per liter) from pregnant participants () consuming a conventional and organic diet ( samples).
| Conventional () | Organic () | |||
|---|---|---|---|---|
| Detection frequency (%) | GM (IQR)a | Detection frequency (%) | GM (IQR)a | |
| All participants | 76.9 | 0.19 (0.11, 0.30) | 71.8 | 0.17 (0.10, 0.26) |
| Far-field | 78.9 | 0.18 (0.11, 0.31) | 68.4 | 0.14 (0.10, 0.23) |
| Near-field | 75.0 | 0.21 (0.12, 0.29) | 75.0 | 0.20 (0.12, 0.27) |
Note: Far-field is defined as living from an agricultural field; near-field is defined as living from an agricultural field. FMV, first morning void; GM, geometric mean; IQR, interquartile range; LOD, limit of detection.
Urine samples were pooled FMV; values included and imputed as .
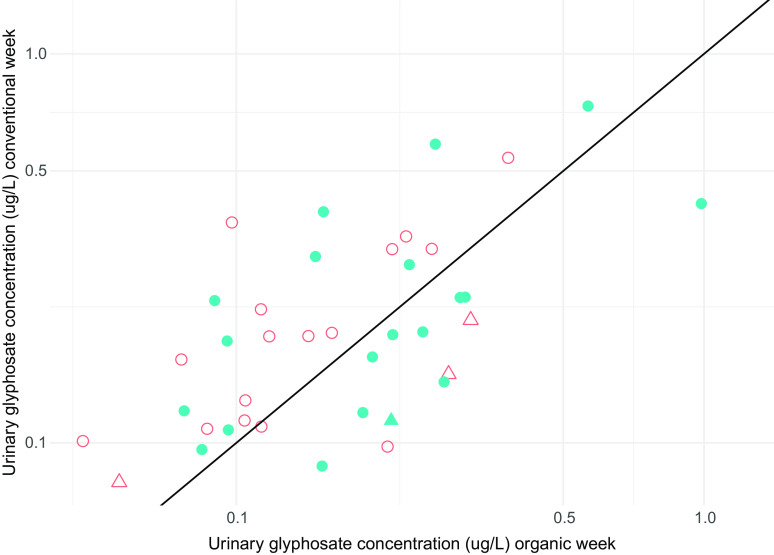
Figure 3 shows a scatter plot of the urinary glyphosate concentrations during the conventional and organic week on a log-log scale, with red dots representing far-field participants and blue dots representing near-field participants. Most participants, and particularly those living far from an agricultural field, fell above the diagonal identity line, indicating higher urinary glyphosate concentrations during the conventional week in comparison with the organic week. Participants who were excluded in secondary analyses due to missing urine samples for either week or who did not turn in a food log are represented with triangle symbols; four of these five participants were below the diagonal identity line.
Figure 3.
Specific gravity–adjusted urinary glyphosate concentrations () during conventional and organic week on log-log scale among 39 pregnant participants in a randomized crossover conventional vs. organic dietary intervention trial. Blue filled dots represent participants living near () from an agricultural field, and red hollow dots represent participants living far () from an agricultural field. Triangles represent participants excluded from secondary analyses due to missing samples or who did not turn in Food Log. Dots below the diagonal identity line indicate participants who had higher glyphosate concentrations during the organic week; dots above the diagonal identity line indicate participants who had higher glyphosate concentrations during the conventional week. Data can be found in Table S3.
Table 3 shows the results of the Wilcoxon signed rank test and the median and IQR percent change in urinary glyphosate concentrations between the conventional and organic diet weeks among all participants and separately among far- and near-field participants. This table shows both the results of the Intention to Treat analyses in which we included all 39 participants, as well as the main secondary analyses. Among all participants (i.e., Intention to Treat), consumption of an organic diet decreased urinary glyphosate concentrations by . For near-field participants, no reduction in glyphosate concentrations was evident with an organic diet, whereas consumption of an organic diet reduced urinary glyphosate concentrations by nearly 25% for far-field participants [median (IQR) percent [95% confidence interval (CI) , ]; Wilcoxon signed rank ] in the Intention to Treat analysis.
Table 3.
Change in urinary glyphosate concentrations from conventional diet to organic diet in 39 pregnant participants, stratified by far-field vs. near-field residential location.
| Median (IQR) percent change from conventional to organic diet |
Wilcoxon signed rank test p-Valuea | |||||
|---|---|---|---|---|---|---|
| All participants | Far-field | Near-field | All participants | Far-field | Near-field | |
| All participantsb | (, 29.8) | (, ) | 1.4 (, 43.0) | 0.17 | 0.06 | 0.83 |
| Exclude participants missing samples from either weekc | (, 29.8) | (, ) | 1.4 (, 43.0) | 0.18 | 0.09 | 0.82 |
| Exclude participants who did not turn in Food Log for either weekc | (, 15.7) | (, ) | (, 30.2) | 0.04 | 0.66 | |
| Exclude participants missing samples or who did not turn in Food Log for either weekd | (, 17.5) | (, ) | (, 30.2) | 0.04 | 0.66 | |
Note: Far-field is defined as living from an agricultural field; near-field defined as living from an agricultural field. IQR, interquartile range.
p-Value comparing glyphosate concentrations from conventional week to organic week.
participants; 18 far-field and 20 near-field participants.
participants; 17 far-field and 19 near-field participants.
participants; 16 far-field and 19 near-field participants.
We observed even greater decreases in urinary glyphosate concentrations, particularly among far-field participants (median percent decrease ; Wilcoxon signed rank ; Table 3), in secondary analyses in which we excluded participants who did not meet a priori criteria of compliance with the intervention. However, the organic diet intervention still had almost no effect on urinary glyphosate levels among near-field participants, even in secondary analyses ( in main secondary analyses) when we restricted to participants who adhered to the intervention.
Figure 4 shows the percent change in specific gravity–adjusted glyphosate concentrations from the conventional to organic week for each participant. Figure 4A shows the percentage change from the Intention to Treat analysis (i.e., no exclusion criteria), and Figure 4B shows the percentage change when excluding five participants who missed urine samples or who did not turn in a food log. Blue dots above the horizontal line at 0% (i.e., no change) indicate participants whose glyphosate concentration was higher during the organic week, and red dots below the horizontal line indicate participants whose concentration was lower during the organic week. As seen in Figure 4A, a greater number of near-field participants had higher glyphosate concentrations during the organic week () in comparison with far-field participants (). Among all groups, we did observe some participants in which the magnitude of increased concentrations during the organic week was quite large () ( for all participants; three near-field and five far-field). Findings were similar in Figure 4B after implementing a priori exclusion criteria; however, even fewer far-field participants () had higher urinary glyphosate concentrations during the organic week in comparison with the conventional week.
Figure 4.
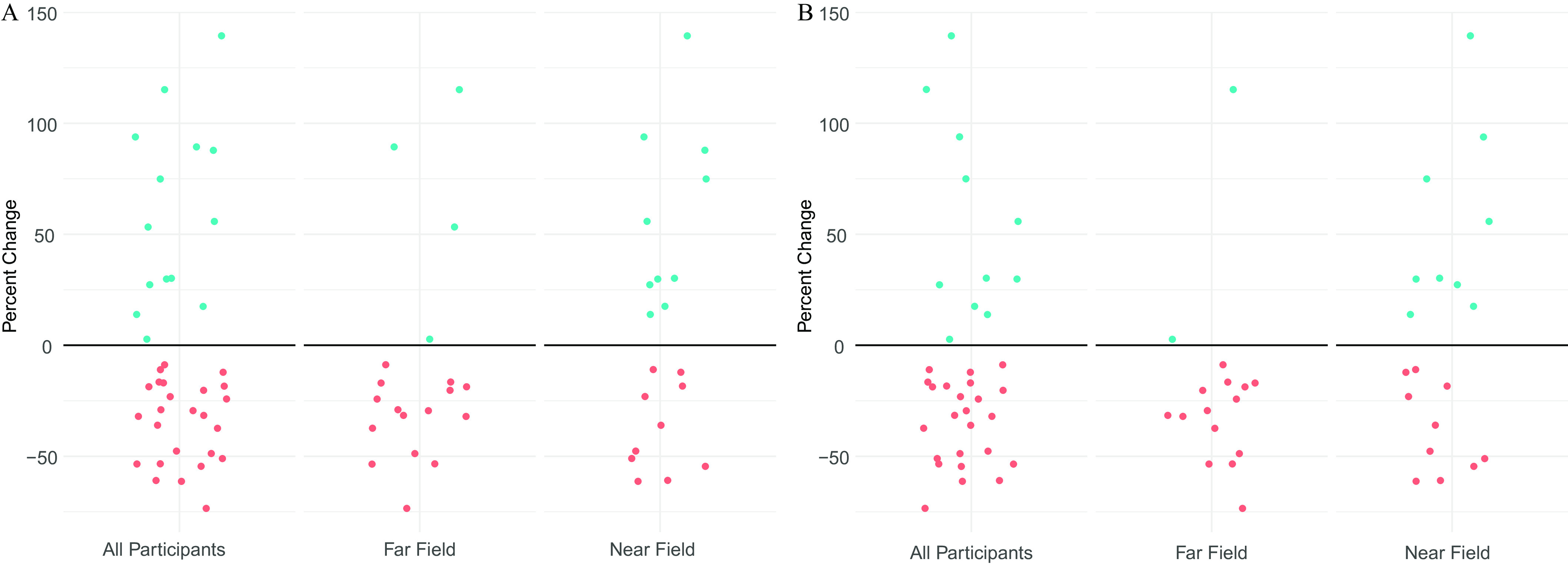
(A) Percent change in urinary specific gravity–adjusted glyphosate concentrations from conventional week to organic week among all participants (), far-field participants (), and near-field participants () in randomized crossover conventional vs. organic dietary intervention trial. Blue dots above the horizontal line indicate individuals in whom concentrations increased during organic week and red dots below the horizontal line indicate individuals in whom concentrations decreased during the organic week. Data can be found in Table S3. (B) Percent change in urinary specific gravity–adjusted glyphosate concentrations from conventional week to organic week among participants in a randomized crossover conventional vs. organic dietary intervention trial after excluding those missing samples or who did not turn in Food Log among all participants (), far-field participants (), and near-field participants (). Blue dots above the horizontal line indicate individuals in whom concentrations increased during organic week, and red dots below the horizontal line indicate individuals in whom concentrations decreased during the organic week. Data can be found in Table S3.
We observed even greater reductions in urinary glyphosate concentrations from the conventional to organic week among all participants and far-field participants in subsequent secondary analyses in which we excluded combinations of participants or other groups of participants who did not meet a priori criteria of compliance with the intervention based on their self-reported Likert Scale or the amount of food they reported consuming from outside of the study on the food log (Table S1).
Discussion
In this randomized crossover trial of organic food, we observed a decrease in urinary glyphosate concentrations among pregnant participants, driven by a reduction among individuals who lived farther than from an agricultural field. It is notable that the organic diet intervention had almost no effect on urinary glyphosate concentrations among participants living within of an agricultural field, suggesting that other sources of exposure contribute more than dietary intake in this population.
The current study builds on previous dietary interventions and addresses novel scientific questions by examining the influence of an organic diet intervention a) with the herbicide glyphosate, which is used on different crops and applied via different applications methods than those of insecticides that have been the focus of most previous studies; b) among agricultural and nonagricultural populations exposed to pesticides via different sources and pathways; and c) during pregnancy, a critical window of vulnerability for exposure to pesticides and other environmental chemicals. The widespread use of glyphosate has resulted in ubiquitous human exposure,66 and growing evidence suggests that higher prenatal glyphosate concentrations, even in nonoccupationally exposed populations, may be associated with an increased risk of adverse birth outcomes.9–11 It is thus imperative to investigate and identify effective exposure-reduction strategies during pregnancy among populations with different sources of pesticide exposure.
Previous intervention and observational studies have provided consistent evidence that an organic diet is associated with decreased exposure to a range of herbicides, insecticides, and fungicides among both children20–27 and adults.21,28–32 However, only three studies focused on glyphosate. In one study involving seven adults and nine children from four families, investigators reported that urinary concentrations of glyphosate and its metabolite aminomethylphosphonic acid (AMPA) decreased by 71% and 77%, respectively, over a 6-day organic diet intervention.21 In another investigation involving two Swiss adults, glyphosate concentrations decreased during the organic diet period in one participant; however, concentrations of both glyphosate and AMPA were nondetectable for most samples.29 A recent parallel-group dietary intervention trial with 27 adults that aimed to examine the effects of diet (Western vs. Mediterranean) and food type (organic vs. conventional) reported that urinary glyphosate and AMPA concentrations both decreased significantly among the organic diet intervention group, with glyphosate being detected in 38% of the conventional group samples but 0% of the organic samples.36 There are a variety of potential explanations for why we observed a smaller percentage decrease in urinary glyphosate levels in our study in comparison with these previous studies, including differences in baseline levels of glyphosate during the conventional period, differences in the delivery of and adherence to the intervention, and the likely impact that compositing our samples had on reducing single-day outlier concentrations in comparison with studies that analyzed individual spot urine samples.
Only one study has directly compared the effects of an organic diet intervention among participants living in agricultural and nonagricultural areas. In a 2015 examination of 20 children living in Oakland, California, and 20 children living in the agricultural region of Salinas, California, investigators reported that urinary concentrations of biomarkers of exposure to OP insecticides and the herbicide 2,4-dichlorophenoxyacetic acid decreased during a 7-day organic diet intervention, and then increased again following the reintroduction of conventional food.20 The investigators did not observe a significant interaction between diet and location (Oakland vs. Salinas) for most pesticides, but they did report a nonsignificant decrease in urinary concentrations of 3-phenoxybenzoic acid (3-PBA)—a nonspecific metabolite of several pyrethroid insecticides—among children living in Oakland, whereas 3-PBA concentrations actually increased slightly among Salinas children during the organic diet period.20 Collectively, these results suggest that an organic diet may not decrease exposure to all pesticides among people living in agricultural regions.
We observed slightly higher glyphosate concentrations among near-field participants in comparison with far-field participants; glyphosate concentrations were nearly identical among near-field participants during the conventional and organic diet weeks. It is unlikely that noncompliance with the dietary intervention or other confounding factors contributed significantly to the lack of a decrease in urinary glyphosate concentrations among near-field participants during the organic diet week. Near- and far-field participants were recruited from the same WIC clinics and had similar sociodemographic characteristics. We also attempted to account for dietary compliance with a series of secondary analyses. Although the strength of the association increased among far-field participants with additional exclusion criteria, the relationship among near-field participants remained unchanged.
Diet is consistently a primary predictor of total pesticide exposure in nonagricultural populations,31,40–42 but the contribution of diet vs. para-occupational exposures (e.g., pesticide drift, take-home exposures) for people living near agricultural fields is poorly understood. Meta-analyses and systematic reviews have suggested that para-occupational and agricultural drift pathways, and, to a lesser extent, residential pesticide use, contribute to pesticide exposure among individuals living in agricultural regions.45,46,67 However, data have been insufficient to characterize the role of dietary exposures.46 Although previous studies have examined determinants of exposure to pesticides such as OPs, pyrethroids, and some herbicides and fungicides among individuals living in agricultural areas, few have examined glyphosate, which has different physicochemical properties, application methods, and use patterns than those of other pesticides. Glyphosate binds to soil particles and accumulates in top-soil layers,68 and it is possible that people living in agricultural areas may be exposed to glyphosate through windblown dust that accumulates in the home, where it may not degrade as quickly as outdoors.69 We hypothesized that reductions in urinary glyphosate concentrations would be greater among participants living far from agricultural fields, but we also hypothesized that the intervention would reduce exposure among participants living near agricultural fields. However, our data indicate that nondietary sources dominate glyphosate exposure among people living near agricultural fields during the pesticide-spray season. Investigating the contribution of specific sources and pathways of glyphosate exposure in agricultural communities is essential to understand the most effective exposure-reduction measures.
Previous organic diet intervention trials have taken various approaches to implementing and monitoring compliance with the intervention, from providing participants with organic produce ordered through a website built by the study staff28 to providing prepared organic meals from a licensed chef or caterer.21,22 In this trial, we delivered the intervention to maximize adherence while maintaining feasibility because participants lived in an approximately 4-h driving radius, with many living in rural areas far from other participants. A secondary goal of ours was to provide participants with a “real-world” method to order food to assess the scalability of such an intervention.
We instructed participants to order the food that they would normally order, with the goal that this approach would lead to similar diets between the two study weeks. We found that participants ordered similar proportions of grains and legumes during the conventional and organic weeks of the study. However, we are not able to quantitatively assess the type and amount of each food item consumed because we did not collect a full 24-h dietary recall. We expect that any changes in dietary habits between the conventional and organic week would have negligible, if any, impact on our overall findings.
We observed stronger decreases in urinary glyphosate levels among all participants and far-field participants after excluding those who did not meet various a priori criteria of compliance with the intervention. Although evaluating compliance with the dietary intervention is difficult without a detailed 24-h dietary recall, our findings highlight that an organic diet may reduce urinary glyphosate levels among those living far from agriculture, even in populations who do not adhere to a full organic diet, which likely more closely mirrors dietary habits in the general population.
Our intervention delivery method had many advantages. Participants had full autonomy over the items they ordered, and participants ordered from a familiar grocery store chain with a well-established food delivery system. However, we discovered some logistical challenges that would limit the utility of such a method in a larger intervention study. The website did not have a Spanish-language translation, and although study staff were able to assist Spanish-speaking participants, some may still have had some challenges in finding the items they would normally order. In addition, not all study participants lived within the delivery range, and we had to pick up grocery orders and deliver them directly to study participants who lived in rural areas. Although study staff reviewed all the orders to ensure compliance during the week of the intervention, some participants occasionally received a few conventional items in the week they were supposed to receive organic food because of errors by the grocery shopping system; in these situations, study staff purchased organic versions of the incorrect items and delivered them directly to participants and asked them to wait to eat the conventional items until their organic week was completed. We found solutions for each of these issues, but they were labor intensive. In future studies, we would recommend working directly with grocery stores in advance, rather than relying on the general public–facing systems, and recommend preferentially working with stores whose websites could easily be converted into the primary languages spoken by all participants.
By employing an intervention that more closely mirrored a real-world diet, our findings almost certainly underrepresent the effect of a strict organic diet. This underrepresentation may contribute to the smaller changes we observed in our study in comparison with previous dietary interventions. These findings have broad implicants for the general population and suggest that diets that incorporate some organic food can still reduce urinary glyphosate concentrations.
In this study, we observed that both near- and far-field participants had lower urinary glyphosate concentrations than participants in previous studies of pregnant individuals, as well as the general U.S. population from NHANES in 2013–2014 and 2015–201662,70 (see Table S1). We found slightly lower urinary concentrations among our participants in comparison with two studies of pregnant participants in the United States9 and Puerto Rico11 and significantly lower concentrations in our study in comparison with an investigation of 71 pregnant people in Indiana.10 The reasons for these differences are unknown. They may relate, at least in part, to the extent of glyphosate use across the United States, with higher use in the Midwest in comparison with other areas71; to differences in study design and quantification methods used; and to dietary habits of participants and year(s) of sample collection. Further, NHANES had a much larger sample size ( samples); is nationally representative of the overall U.S. population; and includes children, who tend to have higher concentrations of glyphosate and other environmental chemicals in comparison with adults.72,73 It is notable that glyphosate urinary concentrations display high day-to-day intraindividual variation because of the likely episodic nature of exposures and its short half-life.58 Therefore, by compositing urine samples we did not observe extreme outlier concentrations that are likely captured in studies that collect single spot samples, such as NHANES.
This study has some limitations. First, the trial was small but was comparable with other trials of organic diet, which ranged from 2 to 40 participants, with most enrolling between 16 and 23 participants.20–25,28–30 In addition, the data presented here are based on the collection of 539 urine samples; previous organic diet intervention studies have ranged from 1629 to 85474 samples; the majority collected fewer than 200 samples. Due to budget constraints, we did not analyze individual urine samples, preventing us from examining changes in concentrations throughout the intervention. Nevertheless, analyzing a composite of each participant’s weekly urine allowed us to maximize sample size while minimizing laboratory costs. Moreover, pooling each week’s urine samples allowed us to minimize the influence of intraindividual variability75 that is common in studies of environmental chemicals with relatively short biological half-lives76 such as glyphosate. If compositing the urine samples had any effect on our findings, it likely decreased the influence of extreme outlier variables. In addition, we measured concentrations in 14 daily urine samples and found excellent agreement between the mean concentrations in the daily and weekly pooled samples.
Another limitation is that we did not measure AMPA, the primary metabolite of glyphosate, formed by microbial degradation in the soil.77,78 However, AMPA is a nonspecific metabolite of glyphosate, and recent studies suggest that humans may be primarily exposed to AMPA directly through food and water and only to a lesser extent from metabolism of glyphosate.66 Thus, assessment of changes in AMPA concentrations from a conventional to an organic diet may not directly reflect changes in glyphosate exposure. Finally, we did not measure exposure to other coformulants used in GBHs. Our findings suggest that nondietary, para-occupational exposures contributed heavily to glyphosate concentrations among those living in agricultural regions, suggesting that those living near agricultural fields may also be exposed to high levels of coformulants, which may be highly toxic.79–82 There has been an increasing emphasis on investigating the toxicity of adjuvants and inert ingredients in pesticides,83–85 and future studies should examine exposure to coformulants present in GBHs.
Finally, this study was conducted with a relatively small sample of participants who had been recruited from WIC clinics and whose dietary habits may not be generalizable to the broader population. For example, some research suggests that lower-income households tend to purchase fewer fruits and vegetables and larger quantities of processed foods,86 which likely have higher glyphosate levels. However, other research has reported high intake of ultraprocessed food across income levels in the United States.87 It is difficult to ascertain how similar dietary habits were among participants in our study to those of different socioeconomic groups in the United States; however, it is likely that populations who consume greater proportions of processed foods and grain products would likely have greater decreases in urinary glyphosate levels following an organic intervention.
Our study also has notable strengths. It is the first to analyze the impact of an organic diet intervention on glyphosate exposure during pregnancy, a critical window of susceptibility for exposure to glyphosate and other environmental chemicals. By conducting this study in a heavily agricultural state like Idaho, we were able to recruit approximately half the cohort who lived near agricultural fields () and the other half from urban/suburban locations. In addition, we conducted this study in June, during the peak of the agricultural pesticide spray season, allowing us to investigate nondietary sources of pesticide exposure (e.g., pesticide drift) while minimizing the influence of dietary exposures during the organic week. Finally, we were able to collect 97% of total daily samples among 39 individuals living in an over 200-mile radius.
In summary, we observed that an organic diet intervention led to decreased urinary glyphosate concentrations among pregnant individuals who lived far from agricultural fields in Idaho, particularly among those who complied with the intervention. These findings are in line with previous studies, which have primarily focused on urban populations without exposure from agricultural sources. Combined with previous observations, our results suggest that an organic diet is effective at reducing exposure to pesticides for the majority of the U.S. population. In contrast, the intervention had no effect on glyphosate exposure for near-field participants. Prenatal glyphosate exposure has recently been associated with adverse birth outcomes such as shortened gestational age,9–11 and it is necessary to understand sources of exposure in diverse populations to develop effective exposure-reduction recommendations.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the study participants and the Women Infants and Children (WIC) clinics who helped us recruit participants. The authors also acknowledge P. Morales-Agudelo and M. Vidal for technical assistance in measuring glyphosate. The authors thank A. Hernandez, B. Ellison, A. O’Brien, and A. Balentine for all of their efforts in conducting the fieldwork, including collection of daily urine samples.
C.H. conducted investigation, project administration, formal analysis, data collection, writing for original draft. M.S. worked on conceptualization and manuscript writing, review, and editing. L.S. worked on conceptualization and manuscript writing, review, and editing. B.P.L. worked on conceptualization and manuscript writing, review, and editing. M.A. worked on conceptualization and manuscript writing, review, and editing. M.O. worked on conceptualization; manuscript writing, review, and editing; and laboratory analysis. A.M.C. worked on conceptualization; manuscript writing, review, and editing; and laboratory analysis. C.L.C. conducted project administration, conceptualization, data collection, supervision, funding acquisition, and manuscript review and editing.
To protect the confidentiality of our study participants, data from this study are not available.
This study was funded by NIEHS grant K01ES028745.
The findings and conclusions of this report are those of the authors and do not necessarily represent the official position of the U.S. CDC. The use of trade names is for identification only and does not imply endorsement by the U.S. CDC, the Public Health Service, or the U.S. Department of Health and Human Services.
References
- 1.Benbrook CM. 2016. Trends in glyphosate herbicide use in the United States and globally. Environ Sci Eur 28(1):3, PMID: , 10.1186/s12302-016-0070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duke SO, Powles SB. 2008. Glyphosate: a once-in-a-century herbicide. Pest Manag Sci 64(4):319–325, PMID: , 10.1002/ps.1518. [DOI] [PubMed] [Google Scholar]
- 3.Vandenberg LN, Blumberg B, Antoniou MN, Benbrook CM, Carroll L, Colborn T, et al. 2017. Is it time to reassess current safety standards for glyphosate-based herbicides? J Epidemiol Community Health 71(6):613–618, PMID: , 10.1136/jech-2016-208463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.U.S. Food and Drug Administration. 2019. Pesticide Residue Monitoring Program Fiscal Year 2019 Pesticide Report. https://www.fda.gov/media/153142/download [accessed 10 August 2022].
- 5.Curwin BD, Hein MJ, Sanderson WT, Nishioka MG, Reynolds SJ, Ward EM, et al. 2005. Pesticide contamination inside farm and nonfarm homes. J Occup Environ Hyg 2(7):357–367, PMID: , 10.1080/15459620591001606. [DOI] [PubMed] [Google Scholar]
- 6.Tzanetou E, Karasali H. 2020. Glyphosate residues in soil and air: an integrated review. In: Pests, Weeds and Diseases in Agricultural Crop and Animal Husbandry Production. Kontogiannatos D, Kourti A, Mendes KF, eds. IntechOpen. 10.5772/intechopen.93066. [DOI] [Google Scholar]
- 7.Gillezeau C, Lieberman-Cribbin W, Taioli E. 2020. Update on human exposure to glyphosate, with a complete review of exposure in children. Environ Health 19(1):115, PMID: , 10.1186/s12940-020-00673-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gillezeau C, van Gerwen M, Shaffer RM, Rana I, Zhang L, Sheppard L, et al. 2019. The evidence of human exposure to glyphosate: a review. Environ Health 18(1):2, PMID: , 10.1186/s12940-018-0435-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lesseur C, Pathak KV, Pirrotte P, Martinez MN, Ferguson KK, Barrett ES, et al. 2022. Urinary glyphosate concentration in pregnant women in relation to length of gestation. Environ Res 203:111811, PMID: , 10.1016/j.envres.2021.111811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parvez S, Gerona RR, Proctor C, Friesen M, Ashby JL, Reiter JL, et al. 2018. Glyphosate exposure in pregnancy and shortened gestational length: a prospective Indiana birth cohort study. Environ Health 17(1):23, PMID: , 10.1186/s12940-018-0367-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silver MK, Fernandez J, Tang J, McDade A, Sabino J, Rosario Z, et al. 2021. Prenatal exposure to glyphosate and its environmental degradate, aminomethylphosphonic acid (AMPA), and preterm birth: a nested case–control study in the PROTECT cohort (Puerto Rico). Environ Health Perspect 129(5):57011, PMID: , 10.1289/EHP7295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kogevinas M. 2021. Glyphosate exposure during pregnancy and preterm birth (more research is needed). Environ Health Perspect 129(5):51301, PMID: , 10.1289/EHP9428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Myers JP, Antoniou MN, Blumberg B, Carroll L, Colborn T, Everett LG, et al. 2016. Concerns over use of glyphosate-based herbicides and risks associated with exposures: a consensus statement. Environ Health 15(1):19, PMID: , 10.1186/s12940-016-0117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mesnage R, Panzacchi S, Bourne E, Mein CA, Perry MJ, Hu J, et al. 2022. Glyphosate and its formulations Roundup Bioflow and RangerPro alter bacterial and fungal community composition in the rat caecum microbiome. Front Microbiol 13:888853, PMID: , 10.3389/fmicb.2022.888853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mesnage R, Teixeira M, Mandrioli D, Falcioni L, Ducarmon QR, Zwittink RD, et al. 2021. Use of shotgun metagenomics and metabolomics to evaluate the impact of glyphosate or roundup Mon 52276 on the gut microbiota and serum metabolome of Sprague-Dawley rats. Environ Health Perspect 129(1):17005, PMID: , 10.1289/EHP6990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paganelli A, Gnazzo V, Acosta H, López SL, Carrasco AE. 2010. Glyphosate-based herbicides produce teratogenic effects on vertebrates by impairing retinoic acid signaling. Chem Res Toxicol 23(10):1586–1595, PMID: , 10.1021/tx1001749. [DOI] [PubMed] [Google Scholar]
- 17.Benachour N, Sipahutar H, Moslemi S, Gasnier C, Travert C, Séralini GE, et al. 2007. Time- and dose-dependent effects of roundup on human embryonic and placental cells. Arch Environ Contam Toxicol 53(1):126–133, PMID: , 10.1007/s00244-006-0154-8. [DOI] [PubMed] [Google Scholar]
- 18.Richard S, Moslemi S, Sipahutar H, Benachour N, Seralini G-E. 2005. Differential effects of glyphosate and roundup on human placental cells and aromatase. Environ Health Perspect 113(6):716–720, PMID: , 10.1289/ehp.7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rossetti MF, Canesini G, Lorenz V, Milesi MM, Varayoud J, Ramos JG, et al. 2021. Epigenetic changes associated with exposure to glyphosate-based herbicides in mammals. Front Endocrinol (Lausanne) 12:671991, PMID: , 10.3389/fendo.2021.671991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bradman A, Quirós-Alcalá L, Castorina R, Aguilar Schall R, Camacho J, Holland NT, et al. 2015. Effect of organic diet intervention on pesticide exposures in young children living in low-income urban and agricultural communities. Environ Health Perspect 123(10):1086–1093, PMID: , 10.1289/ehp.1408660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fagan J, Bohlen L, Patton S, Klein K. 2020. Organic diet intervention significantly reduces urinary glyphosate levels in U.S. children and adults. Environ Res 189:109898, PMID: , 10.1016/j.envres.2020.109898. [DOI] [PubMed] [Google Scholar]
- 22.Hyland C, Bradman A, Gerona R, Patton S, Zakharevich I, Gunier RB, et al. 2019. Organic diet intervention significantly reduces urinary pesticide levels in U.S. children and adults. Environ Res 171:568–575, PMID: , 10.1016/j.envres.2019.01.024. [DOI] [PubMed] [Google Scholar]
- 23.Lu C, Barr Dana B, Pearson Melanie A, Waller Lance A. 2008. Dietary intake and its contribution to longitudinal organophosphorus pesticide exposure in urban/suburban children. Environ Health Perspect 116(4):537–542, PMID: , 10.1289/ehp.10912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu C, Toepel K, Irish R, Fenske RA, Barr DB, Bravo R, et al. 2006. Organic diets significantly lower children’s dietary exposure to organophosphorus pesticides. Environ Health Perspect 114(2):260–263, PMID: , 10.1289/ehp.8418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu C, Barr DB, Pearson MA, Walker LA, Bravo R. 2009. The attribution of urban and suburban children’s exposure to synthetic pyrethroid insecticides: a longitudinal assessment. J Expo Sci Environ Epidemiol 19(1):69–78, PMID: , 10.1038/jes.2008.49. [DOI] [PubMed] [Google Scholar]
- 26.Curl CL, Fenske RA, Elgethun K. 2003. Organophosphorus pesticide exposure of urban and suburban preschool children with organic and conventional diets. Environ Health Perspect 111(3):377–382, PMID: , 10.1289/ehp.5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holme F, Thompson B, Holte S, Vigoren EM, Espinoza N, Ulrich A, et al. 2016. The role of diet in children’s exposure to organophosphate pesticides. Environ Res 147:133–140, PMID: , 10.1016/j.envres.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Curl CL, Porter J, Penwell I, Phinney R, Ospina M, Calafat AM, et al. 2019. Effect of a 24-week randomized trial of an organic produce intervention on pyrethroid and organophosphate pesticide exposure among pregnant women. Environ Int 132:104957, PMID: , 10.1016/j.envint.2019.104957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Göen T, Schmidt L, Lichtensteiger W, Schlumpf M. 2017. Efficiency control of dietary pesticide intake reduction by human biomonitoring. Int J Hyg Environ Health 220(2 pt A):254–260, PMID: , 10.1016/j.ijheh.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 30.Oates L, Cohen M, Braun L, Schembri A, Taskova R. 2014. Reduction in urinary organophosphate pesticide metabolites in adults after a week-long organic diet. Environ Res 132:105–111, PMID: , 10.1016/j.envres.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 31.Curl CL, Beresford SAA, Fenske RA, Fitzpatrick AL, Lu C, Nettleton JA, et al. 2015. Estimating pesticide exposure from dietary intake and organic food choices: the Multi-Ethnic Study of Atherosclerosis (MESA). Environ Health Perspect 123(5):475–483, PMID: , 10.1289/ehp.1408197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berman T, Göen T, Novack L, Beacher L, Grinshpan L, Segev D, et al. 2016. Urinary concentrations of organophosphate and carbamate pesticides in residents of a vegetarian community. Environ Int 96:34–40, PMID: , 10.1016/j.envint.2016.08.027. [DOI] [PubMed] [Google Scholar]
- 33.Brantsæter AL, Ydersbond TA, Hoppin JA, Haugen M, Meltzer HM. 2017. Organic food in the diet: exposure and health implications. Annu Rev Public Health 38:295–313, PMID: , 10.1146/annurev-publhealth-031816-044437. [DOI] [PubMed] [Google Scholar]
- 34.Barański M, Srednicka-Tober D, Volakakis N, Seal C, Sanderson R, Stewart GB, et al. 2014. Higher antioxidant and lower cadmium concentrations and lower incidence of pesticide residues in organically grown crops: a systematic literature review and meta-analyses. Br J Nutr 112(5):794–811, PMID: , 10.1017/S0007114514001366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.U.S. Department of Agriculture. 2022. National List of Allowed and Prohibited Substances. https://www.ecfr.gov/current/title-7/subtitle-B/chapter-I/subchapter-M/part-205/subpart-G [accessed 18 January 2023].
- 36.Rempelos L, Wang J, Barański M, Watson A, Volakakis N, Hoppe H-W, et al. 2022. Diet and food type affect urinary pesticide residue excretion profiles in healthy individuals: results of a randomized controlled dietary intervention trial. Am J Clin Nutr 115(2):364–377, PMID: , 10.1093/ajcn/nqab308. [DOI] [PubMed] [Google Scholar]
- 37.Bradman A, Castorina R, Barr DB, Chevrier J, Harnly ME, Eisen EA, et al. 2011. Determinants of organophosphorus pesticide urinary metabolite levels in young children living in an agricultural community. Int J Environ Res Public Health 8(4):1061–1083, PMID: , 10.3390/ijerph8041061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kolakowski BM, Miller L, Murray A, Leclair A, Bietlot H, van de Riet JM, et al. 2020. Analysis of glyphosate residues in foods from the Canadian retail markets between 2015 and 2017. J Agric Food Chem 68(18):5201–5211, PMID: , 10.1021/acs.jafc.9b07819. [DOI] [PubMed] [Google Scholar]
- 39.Louie F, Jacobs NFB, Yang LGL, Park C, Monnot AD, Bandara SB, et al. 2021. A comparative evaluation of dietary exposure to glyphosate resulting from recommended U.S. diets. Food Chem Toxicol 158:112670, PMID: , 10.1016/j.fct.2021.112670. [DOI] [PubMed] [Google Scholar]
- 40.Riederer AM, Bartell SM, Barr DB, Ryan PB. 2008. Diet and nondiet predictors of urinary 3-phenoxybenzoic acid in NHANES 1999–2002. Environ Health Perspect 116(8):1015–1022, PMID: , 10.1289/ehp.11082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Papadopoulou E, Haug LS, Sakhi AK, Andrusaityte S, Basagaña X, Brantsaeter AL, et al. 2019. Diet as a source of exposure to environmental contaminants for pregnant women and children from six European countries. Environ Health Perspect 127(10):107005, PMID: , 10.1289/EHP5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu H, Campana AM, Wang Y, Kannan K, Liu M, Zhu H, et al. 2021. Organophosphate pesticide exposure: demographic and dietary predictors in an urban pregnancy cohort. Environ Pollut 283:116920, PMID: , 10.1016/j.envpol.2021.116920. [DOI] [PubMed] [Google Scholar]
- 43.Simcox NJ, Fenske RA, Wolz SA, Lee IC, Kalman DA. 1995. Pesticides in household dust and soil: exposure pathways for children of agricultural families. Environ Health Perspect 103(12):1126–1134, PMID: , 10.1289/ehp.951031126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Syafrudin M, Kristanti RA, Yuniarto A, Hadibarata T, Rhee J, Al-Onazi WA, et al. 2021. Pesticides in drinking water–a review. Int J Environ Res Public Health 18(2):468, PMID: , 10.3390/ijerph18020468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deziel NC, Freeman LEB, Graubard BI, Jones RR, Hoppin JA, Thomas K, et al. 2017. Relative contributions of agricultural drift, para-occupational, and residential use exposure pathways to house dust pesticide concentrations: meta-regression of published data. Environ Health Perspect 125(3):296–305, PMID: , 10.1289/EHP426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deziel NC, Friesen MC, Hoppin JA, Hines CJ, Thomas K, Freeman LE, et al. 2015. A review of nonoccupational pathways for pesticide exposure in women living in agricultural areas. Environ Health Perspect 123(6):515–524, PMID: , 10.1289/ehp.1408273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.U.S. Food and Nutrition Service. About WIC – WIC at a Glance. https://www.fns.usda.gov/wic/about-wic-glance [accessed 11 July 2022].
- 48.Curwin BD, Hein MJ, Sanderson WT, Striley C, Heederik D, Kromhout H, et al. 2007. Urinary pesticide concentrations among children, mothers and fathers living in farm and non-farm households in Iowa. Ann Occup Hyg 51(1):53–65, PMID: , 10.1093/annhyg/mel062. [DOI] [PubMed] [Google Scholar]
- 49.Griffith W, Curl CL, Fenske RA, Lu CA, Vigoren EM, Faustman EM, et al. 2011. Organophosphate pesticide metabolite levels in pre-school children in an agricultural community: within- and between-child variability in a longitudinal study. Environ Res 111(6):751–756, PMID: , 10.1016/j.envres.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anadón A, Martínez-Larrañaga MR, Martínez MA, Castellano VJ, Martínez M, Martin MT, et al. 2009. Toxicokinetics of glyphosate and its metabolite aminomethyl phosphonic acid in rats. Toxicol Lett 190(1):91–95, PMID: , 10.1016/j.toxlet.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 51.Spaan S, Pronk A, Koch HM, Jusko TA, Jaddoe VWV, Shaw PA, et al. 2015. Reliability of concentrations of organophosphate pesticide metabolites in serial urine specimens from pregnancy in the Generation R Study. J Expo Sci Environ Epidemiol 25(3):286–294, PMID: , 10.1038/jes.2014.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hyland C, McConnell K, DeYoung E, Curl CL. 2022. Evaluating the accuracy of satellite-based methods to estimate residential proximity to agricultural crops. J Expo Sci Environ Epidemiol, PMID: , 10.1038/s41370-022-00467-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Simões M, Huss A, Brouwer M, Krop E, Janssen N, Vermeulen R, et al. 2022. Residential proximity to crops and agricultural pesticide use and cause-specific mortality: a prospective census-based cohort study in The Netherlands. Sci Total Environ 817:152932, PMID: , 10.1016/j.scitotenv.2022.152932. [DOI] [PubMed] [Google Scholar]
- 54.Teysseire R, Manangama G, Baldi I, Carles C, Brochard P, Bedos C, et al. 2020. Assessment of residential exposures to agricultural pesticides: a scoping review. PLoS One 15(4):e0232258, PMID: , 10.1371/journal.pone.0232258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ward MH, Lubin J, Giglierano J, Colt JS, Wolter C, Bekiroglu N, et al. 2006. Proximity to crops and residential exposure to agricultural herbicides in Iowa. Environ Health Perspect 114(6):893–897, PMID: , 10.1289/ehp.8770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rappazzo KM, Warren JL, Davalos AD, Meyer RE, Sanders AP, Brownstein NC, et al. 2019. Maternal residential exposure to specific agricultural pesticide active ingredients and birth defects in a 2003–2005 North Carolina birth cohort. Birth Defects Res 111(6):312–323, PMID: , 10.1002/bdr2.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cuhra M. 2015. Review of GMO safety assessment studies: glyphosate residues in roundup ready crops is an ignored issue. Environ Sci Eur 27(1):20, 10.1186/s12302-015-0052-7. [DOI] [Google Scholar]
- 58.Connolly A, Jones K, Basinas I, Galea KS, Kenny L, McGowan P, et al. 2019. Exploring the half-life of glyphosate in human urine samples. Int J Hyg Environ Health 222(2):205–210, PMID: , 10.1016/j.ijheh.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 59.Suresh K. 2011. An overview of randomization techniques: an unbiased assessment of outcome in clinical research. J Hum Reprod Sci 4(1):8–11, PMID: , 10.4103/0974-1208.82352. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 60.Schütze A, Morales-Agudelo P, Vidal M, Calafat AM, Ospina M. 2021. Quantification of glyphosate and other organophosphorus compounds in human urine via ion chromatography isotope dilution tandem mass spectrometry. Chemosphere 274:129427, PMID: , 10.1016/j.chemosphere.2020.129427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Caudill SP, Schleicher RL, Pirkle JL. 2008. Multi-rule quality control for the age-related eye disease study. Stat Med 27(20):4094–4106, PMID: , 10.1002/sim.3222. [DOI] [PubMed] [Google Scholar]
- 62.U.S. Centers for Disease Control and Prevention. 2022. NHANES 2013–2014 Laboratory Data–Continuous NHANES. Atlanta, GA: National Center for Health Statistics. https://wwwn.cdc.gov/Nchs/Nhanes/2013-2014/SSGLYP_H.htm [accessed 25 January 2023]. [Google Scholar]
- 63.U.S. Centers for Disease Control and Prevention. 2022. NHANES 2015–2016 Laboratory Data–Continuous NHANES. Atalanta, GA: National Center for Health Stastistics. https://wwwn.cdc.gov/Nchs/Nhanes/2015-2016/SSGLYP_I.htm [accessed 25 January 2023]. [Google Scholar]
- 64.Hornung RW, Reed LD. 1990. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg 5(1):46–51, 10.1080/1047322X.1990.10389587. [DOI] [Google Scholar]
- 65.Duty SM, Ackerman RM, Calafat AM, Hauser R. 2005. Personal care product use predicts urinary concentrations of some phthalate monoesters. Environ Health Perspect 113(11):1530–1535, PMID: , 10.1289/ehp.8083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Connolly A, Coggins MA, Koch HM. 2020. Human biomonitoring of glyphosate exposures: state-of-the-art and future research challenges. Toxics 8(3):60, PMID: , 10.3390/toxics8030060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Teysseire R, Manangama G, Baldi I, Carles C, Brochard P, Bedos C, et al. 2021. Determinants of non-dietary exposure to agricultural pesticides in populations living close to fields: a systematic review. Sci Total Environ 761:143294, PMID: , 10.1016/j.scitotenv.2020.143294. [DOI] [PubMed] [Google Scholar]
- 68.Kanissery R, Gairhe B, Kadyampakeni D, Batuman O, Alferez F. 2019. Glyphosate: its environmental persistence and impact on crop health and nutrition. Plants (Basel) 8(11):499, PMID: , 10.3390/plants8110499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kuiper G, Young BN, WeMott S, Erlandson G, Martinez N, Mendoza J, et al. 2022. Factors associated with levels of organophosphate pesticides in household dust in agricultural communities. Int J Environ Res Public Health 19(2):862, PMID: , 10.3390/ijerph19020862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ospina M, Schütze A, Morales-Agudelo P, Vidal M, Wong L-Y, Calafat AM, et al. 2022. Exposure to glyphosate in the United States: data from the 2013–2014 National Health and Nutrition Examination Survey. Environ Int 170:107620, PMID: , 10.1016/j.envint.2022.107620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.U.S. Geological Survey. Estimated Agricultural Pesticide Use: Pesticide Use Maps – Estimated Agricultural Use for Glyphosate, 2019 (Preliminary). https://water.usgs.gov/nawqa/pnsp/usage/maps/show_map.php?year=2019&map=GLYPHOSATE&hilo=H%22 [accessed11 May 2023].
- 72.Bellinger DC. 2018. An overview of environmental chemical exposures and neurodevelopmental impairments in children. Pediatr Med 1:9–9, 10.21037/pm.2018.11.03. [DOI] [Google Scholar]
- 73.Etzel RA. 2020. The special vulnerability of children. Int J Hyg Environ Health 227:113516, PMID: , 10.1016/j.ijheh.2020.113516. [DOI] [PubMed] [Google Scholar]
- 74.Makris KC, Konstantinou C, Andrianou XD, Charisiadis P, Kyriacou A, Gribble MO, et al. 2019. A cluster-randomized crossover trial of organic diet impact on biomarkers of exposure to pesticides and biomarkers of oxidative stress/inflammation in primary school children. PLoS One 14(9):e0219420, PMID: , 10.1371/journal.pone.0219420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vernet C, Philippat C, Agier L, Calafat AM, Ye X, Lyon-Caen S, et al. 2019. An empirical validation of the within-subject biospecimens pooling approach to minimize exposure misclassification in biomarker-based studies. Epidemiology 30(5):756–767, PMID: , 10.1097/EDE.0000000000001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bradman A, Kogut K, Eisen EA, Jewell NP, Quirós-Alcalá L, Castorina R, et al. 2013. Variability of organophosphorous pesticide metabolite levels in spot and 24-hr urine samples collected from young children during 1 week. Environ Health Perspect 121(1):118–124, PMID: , 10.1289/ehp.1104808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Grandcoin A, Piel S, Baurès E. 2017. AminoMethylPhosphonic acid (AMPA) in natural waters: its sources, behavior and environmental fate. Water Res 117:187–197, PMID: , 10.1016/j.watres.2017.03.055. [DOI] [PubMed] [Google Scholar]
- 78.Kanissery RG, Welsh A, Sims GK. 2015. Effect of soil aeration and phosphate addition on the microbial bioavailability of carbon-14-glyphosate. J Environ Qual 44(1):137–144, PMID: , 10.2134/jeq2014.08.0331. [DOI] [PubMed] [Google Scholar]
- 79.Mesnage R, Benbrook C, Antoniou MN. 2019. Insight into the confusion over surfactant co-formulants in glyphosate-based herbicides. Food Chem Toxicol 128:137–145, PMID: , 10.1016/j.fct.2019.03.053. [DOI] [PubMed] [Google Scholar]
- 80.Mesnage R, Ferguson S, Brandsma I, Moelijker N, Zhang G, Mazzacuva F, et al. 2022. The surfactant co-formulant POEA in the glyphosate-based herbicide RangerPro but not glyphosate alone causes necrosis in Caco-2 and HepG2 human cell lines and ER stress in the ToxTracker assay. Food Chem Toxicol 168:113380, PMID: , 10.1016/j.fct.2022.113380. [DOI] [PubMed] [Google Scholar]
- 81.Defarge N, Takács E, Lozano VL, Mesnage R, Spiroux de Vendômois J, Séralini GE, et al. 2016. Co-formulants in glyphosate-based herbicides disrupt aromatase activity in human cells below toxic levels. Int J Environ Res Public Health 13(3):264, PMID: , 10.3390/ijerph13030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Defarge N, Spiroux de Vendômois J, Séralini GE. 2018. Toxicity of formulants and heavy metals in glyphosate-based herbicides and other pesticides. Toxicol Rep 5:156–163, PMID: , 10.1016/j.toxrep.2017.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mesnage R, Antoniou MN. 2017. Ignoring adjuvant toxicity falsifies the safety profile of commercial pesticides. Front Public Health 5:361, PMID: , 10.3389/fpubh.2017.00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Freeman LEB. 2022. Invited perspective: pesticide adjuvants and inert ingredients – a missing piece of the puzzle. Environ Health Perspect 130(8):81301, PMID: , 10.1289/EHP11512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cox C, Zeiss M. 2022. Health, pesticide adjuvants, and inert ingredients: California case study illustrates need for data access. Environ Health Perspect 130(8):85001, PMID: , 10.1289/EHP10634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.French SA, Tangney CC, Crane MM, Wang Y, Appelhans BM. 2019. Nutrition quality of food purchases varies by household income: the SHoPPER study. BMC Public Health 19(1):231, PMID: , 10.1186/s12889-019-6546-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Juul F, Parekh N, Martinez-Steele E, Monteiro CA, Chang V. 2021. Current intake of ultra-processed foods in the U.S. adult population according to education-level and income. Curr Dev Nutr 5(suppl 2):418, 10.1093/cdn/nzab038_030. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.