Abstract
In men > ~35 years, aging is associated with perturbations in the hypothalamus-pituitary–testicular axis and declining serum testosterone concentrations. The major changes are decreased gonadotropin-releasing hormone (GnRH) outflow and decreased Leydig cell responsivity to stimulation by luteinizing hormone (LH). These physiologic changes increase the prevalence of biochemical secondary hypogonadism—a low serum testosterone concentration without an elevated serum LH concentration. Obesity, medications such as opioids or corticosteroids, and systemic disease further reduce GnRH and LH secretion and might result in biochemical or clinical secondary hypogonadism. Biochemical secondary hypogonadism related to aging often remits with weight reduction and avoidance or treatment of other factors that suppress GnRH and LH secretion. Starting at age ~65–70, progressive Leydig cell dysfunction increases the prevalence of biochemical primary hypogonadism—a low serum testosterone concentration with an elevated serum LH concentration. Unlike biochemical secondary hypogonadism in older men, biochemical primary hypogonadism is generally irreversible. The evaluation of low serum testosterone concentrations in older men requires a careful assessment for symptoms, signs and causes of male hypogonadism. In older men with a body mass index (BMI) ≥ 30, biochemical secondary hypogonadism and without an identifiable cause of hypothalamus or pituitary pathology, weight reduction and improvement of overall health might reverse biochemical hypogonadism. For older men with biochemical primary hypogonadism, testosterone replacement therapy might be beneficial. Because aging is associated with decreased metabolism of testosterone and increased tissue-specific androgen sensitivity, lower dosages of testosterone replacement therapy are often effective and safer in older men.
Keywords: Aging, Hypogonadism, Testosterone, Dihydrotestosterone, Estradiol, Late-onset hypogonadism
1. Introduction
Serum total and free testosterone concentrations decline in aging men, and this decline typically begins by their 4th decade. This decline tends to be gradual and progressive and is due to perturbations primarily at the levels of the hypothalamus and testes. The focus of this review is to describe the physiological mechanisms of aging-related decline of serum testosterone concentrations, the health and lifestyle factors that contribute to this aging-related decline, and the aging-related changes in testosterone metabolism and androgen sensitivity that potentially attenuate the effects of this decline. We also discuss whether aging-related decline in serum testosterone is a cause or a marker of poor health and whether aging-related male hypogonadism exists. Finally, we integrate the evidence for the pathophysiology of these aging-related changes of testosterone concentrations, testosterone metabolism and androgen sensitivity into a rational clinical approach for the assessment and management of an older man with a low serum testosterone concentration.
2. Physiological changes of the hypothalamus-pituitary–testicular axis that affect serum testosterone concentrations associated with aging in men: an overview
The entire male hypothalamus-pituitary–testicular axis is directly or indirectly affected by aging with a net effect of decreased testosterone and sperm production in most older men. With aging, there is impaired hypothalamic secretion of gonadotropin-releasing hormone (GnRH) that results in decreased pituitary gonadotropin secretion of luteinizing hormone (LH), and Leydig cells become less responsive to LH stimulation compared to young men (Fig. 1). The net effect is a lower mean circulating serum total testosterone concentration of aging men compared to younger men and an attenuation of the normal rise of serum LH concentrations that would be typical of normal physiology and classic primary hypogonadism. Although serum LH concentrations tend to rise, they remain in the normal range for the majority of aging men even in the 10–20% with low serum testosterone concentrations [1–4]. In men > 70 years, the proportion of men with high serum LH concentrations increases significantly but remains the minority [2].
Fig. 1.
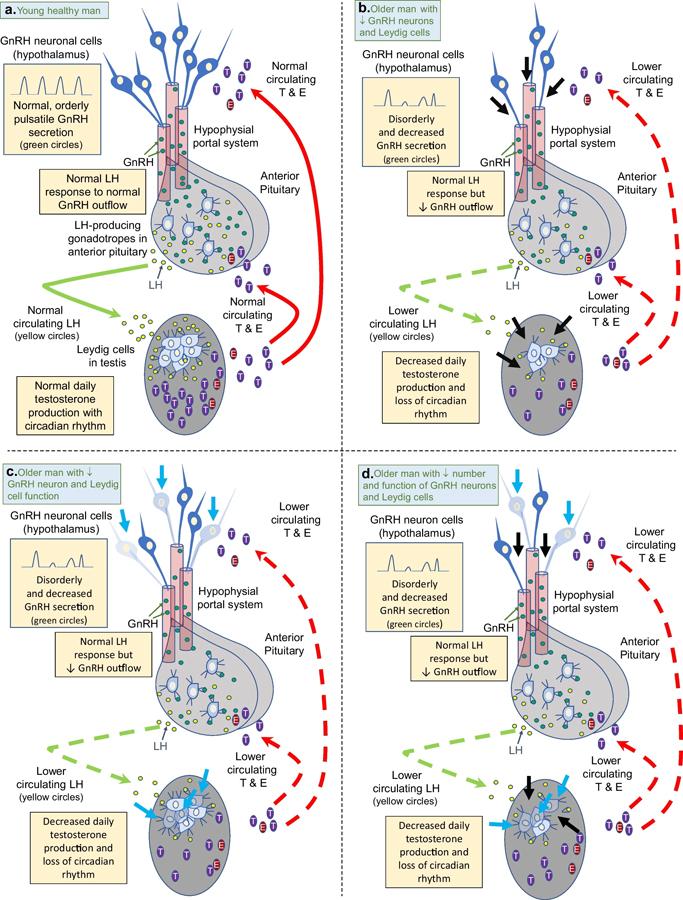
The hypothalamic-pituitary–testicular axis in normal healthy younger men (<50 years) vs. aging men (≥50 years). Figure 1a shows the normal axis with orderly pulsatile gonadotropin-releasing hormone (GnRH) secretion from hypothalamic GnRH neurons flowing into the hypophysial portal system to the pituitary to stimulate gonadotropes to produce luteinizing hormone (LH) that then flows into general circulation and to the testes to stimulate Leydig cells to produce normal amounts of testosterone and estradiol daily with an early morning peak. Circulating testosterone (T) and estradiol (E) regulate GnRH and LH release by negative feedback. In aging men, GnRH is secreted in a disorderly pattern with diminished average amplitude resulting in decreased stimulation of LH secretion from the pituitary. Although gonadotrope response to GnRH is normal, LH secretion is decreased relative to serum T concentrations. There is also a primary testicular defect in Leydig cell production of T in aging men. The decrease in LH secretion and Leydig responsivity to LH results in decrease average serum T concentrations as men age. The mechanism of decreased GnRH and Leydig cells might be due to an aging-related decreased number of GnRH neurons and Leydig cells (Fig. 1b) decreased function of GnRH neurons and Leydig cells (Fig. 1c) or a combination of decreased number and function of GnRH and Leydig cells (Fig. 1d). Thick black arrows point to where GnRH neurons and/or Leydig cells have atrophied with aging. Thick blue arrows point to GnRH neurons and/or Leydig cells have significantly decreased function associated with aging. Dashed green and red arrows indicate lower circulating LH (and less positive feedback) and lower circulating T and E (and less negative feedback), respectively.
In general, free testosterone concentrations tend to decrease more than total testosterone concentrations because of the association between aging and higher sex hormone binding globulin (SHBG) concentrations. However, aging is also associated with increasing body mass index (BMI) and an increased prevalence of obesity, a condition that decreases serum SHBG. The negative effect of obesity is significantly greater than the positive effect of aging on serum SHBG concentrations [5]. Compared to older men with a BMI ≥ 30, lean, older men have a disproportionately greater decrease in serum free testosterone vs. serum total testosterone concentrations. In addition, testosterone metabolism decreases and organ-specific androgen sensitivity might increase with aging in men; these two effects attenuate the effects of declining serum total testosterone production and amplify the effects of androgen replacement or supplementation therapy.
2.1. Aging-related perturbations of hypothalamic secretion of gonadotropin-releasing hormone concentrations associated with aging in men
Hypothalamic secretion of gonadotropin-releasing hormone (GnRH) is attenuated in healthy, aging men [6–9]. A mathematical model estimated a decline of 33–50% from age 20 to age 80 years in men [8, 9]. The conclusion that GnRH output decreases in aging men is based on inferential data because it is not feasible to measure GnRH concentrations in the human hypophyseal portal system. LH pulse patterns and amplitude reflect hypothalamic GnRH neuron function. Alterations in LH pulse patterns and smaller LH pulse amplitudes imply that aging is associated with decreased GnRH secretion in men [9, 10]. A 2020 study demonstrated that LH pulse outflow (measured by the change in pulsatile LH secretion and mass) was negatively associated with age, confirming previous studies that used different experimental models) [6–8, 10]. Studies of male rodents are confirmatory, demonstrating decreased density of GnRH neuronal synapses, decreased in vitro hypothalamic GnRH release and decreased in vivo LH pulse amplitude [9, 11].
In healthy older men, there is loss of the normal circadian rhythm of serum testosterone in men beginning in their mid-60 s and early 70 s [12–14]. The circadian rhythm of testosterone secretion is regulated by the hypothalamus, and this aging-related perturbation of the circadian rhythm is likely due to the aging-related decrease in GnRH outflow and changes in kisspeptin-neurokinin B neuronal system that regulates human GnRH secretion pulsatility. It is not known if this aging-related loss of circadian rhythm is an early manifestation of the effects aging on the hypothalamus or whether obesity, co-morbidities or poor overall health accelerate the loss of circadian rhythm. It is likely, though, there is interplay between aging and health factors that additively or synergistically disturb normal hypothalamic regulation of the daily pattern of gonadotropin and testosterone secretion.
It is not known why hypothalamic secretion of GnRH decreases as men age, but hypotheses include decreased numbers of GnRH neurons or perturbations of kisspeptin and neurokinin B neuronal cell function. In a male rat model, aging is associated with a decrease in the number of hypothalamic GnRH-expressing neurons and hypothalamic GnRH expression [11]. The finding that aging is associated with fewer hypothalamic GnRH-expressing neurons has not been confirmed in humans. There are very small numbers of hypothalamic GnRH neurons in young men making quantitative studies of human aging-related changes very difficult [15, 16].
Kisspeptin is a major regulator (stimulator) of GnRH secretion, and the evidence indicates that circulating concentrations of kisspeptin decrease as men age [16–19]. However, hypothalamic kisspeptin, not circulating kisspeptin, is the most important regulator of GnRH neuronal cell secretion of GnRH. In animal models, hypothalamic kisspeptin-producing neurons tend to decrease with aging, but these neurons increase in humans with a greater increase seen in postmenopausal women than older men [19, 20]. In humans, hypothalamic kisspeptin secretion is modulated by neurokinin B (NKB) produced by NKB neurons that may also co-secrete kisspeptin. The precise role of NKB in the regulation of kisspeptin production and secretion in men is unknown, but it is an important regulator (stimulator) of kisspeptin [17]. In younger men (< 50 years old), about 35% of NKB cells co-secrete kisspeptin, but in men over age 50, the percentage of NKB cells that co-secrete kisspeptin nearly doubles to almost 70% [18]. The physiologic effects of these histologic changes in aging men are still unknown, but they suggest a role of hypothalamic NKB cells in the decreased GnRH outflow of aging men.
Estrogen receptor α and androgen receptors are found on kisspeptin neurons, but GnRH neurons do not have estrogen receptor α and androgen receptors [19]. Although estrogen receptor α has not been found on human GnRH neurons, estrogen receptor β has been identified on these cells, but its physiologic role has not been established [15]. Therefore, it is likely that sex steroid hormone regulation of GnRH secretion is largely mediated indirectly by regulation of kisspeptin secretion, but there might also be direct negative feedback by estrogen [15, 19]. As serum testosterone and dihydrotestosterone concentrations decline in aging men, serum estradiol concentrations remain relatively unchanged until the middle of the 7th decade when they begin to increase (see Sect. 4). Therefore, increased circulating estradiol concentrations might play a role in the decreased GnRH outflow in older men by binding the estrogen receptor α on kisspeptin neurons and the estrogen receptor β on GnRH neurons.
2.2. Aging-related perturbations of pituitary secretion of luteinizing hormone concentrations associated with aging in men
The pituitary gonadotropes respond normally to stimulus by exogenous GnRH in aging men compared to younger men. Pulsatile administration of GnRH for two weeks resulted incomparable pulsatile secretion of immunoreactive and bioactive LH in two different studies [21, 22]. However, there have been inconsistent results from studies of GnRH infusions and LH responsivity of older vs. young men [8, 9]. Notably, the studies with disparate results did not account for the baseline differences of endogenous serum testosterone concentrations available for negative feedback on pituitary gonadotrope secretion of LH between older and younger men. In a 2010 study, investigators addressed this confounding variable by “clamping” serum testosterone concentrations in 8 healthy younger men (19–39 years) and 8 healthy older men (64–70 years) [8]. In this study, ketoconazole was administered to suppress endogenous testosterone production, and transdermal testosterone was administered to maintain serum testosterone in the mid-normal range. The investigators then compared the effects of escalating dosages (from physiologic to a dosage that was 75–300 times greater than physiologic) of GnRH vs. placebo on serum LH responsivity. There were no differences in mean, peak, incremental or pulsatile LH responses between the younger and older groups of men across the broad range of GnRH doses. This study demonstrated that GnRH-dose-dependent gonadotrope secretion of LH was preserved in older men compared to younger men when testosterone concentrations were clamped (and maintained in the mid-normal range). This testosterone “clamp and give-back study” resulted in similar serum concentrations of testosterone and only small differences (~20%) in serum estradiol between the two groups. Thus, this study using a testosterone clamp eliminated or at least minimized the variation in negative feedback by differential serum concentrations of endogenous testosterone and its metabolites in older men compared to young men. This key study permits the definitive conclusion that pituitary gonadotrope responsivity to GnRH (vis à vis LH secretion) remains normal in older, healthy men compared to young, healthy men.
In summary, although pituitary gonadotrope response to exogenous GnRH remains preserved in aging men, LH secretion is subnormal compared to young men because of diminished and less orderly endogenous GnRH outflow due to decreased number and/or function of GnRH neurons in older men (Fig. 1b–d) [6, 23]. Thus, decreased hypothalamic GnRH outflow is a major factor in the relatively lower serum LH concentrations for a given serum testosterone concentration that is seen in many older men.
2.3. Aging-related perturbations of Leydig cell secretion of testosterone concentrations associated with aging in men
Numerous studies have demonstrated that aging men have decreased Leydig secretion of testosterone in response to endogenous or exogenous LH (or LH activity in the form of human chorionic gonadotropin) compared to younger men [23–27]. In addition, the administration of oral clomiphene, an estrogen receptor α blocker when administered at high dosages, 50 mg twice daily for one week raised serum immunoreactive and bioactive LH concentrations similarly in healthy young men and men ≥ 65 years old. However, clomiphene raised serum total testosterone and free testosterone by 100% and 304% in healthy young men, but only by 32% and 8% in the older group [28].
It is unclear whether this aging-related diminished testicular response to LH stimulation is due to Leydig cell dysfunction, decreased numbers of Leydig cells or both (Fig. 1b–d). Some small human studies of post-mortem testicular samples or samples of infertile men with complete spermatogenesis have shown an association between aging and loss of Leydig cells, but others have not [29–33]. A 2022 post-mortem study of the whole testicular transcriptome and histology of 4 young (17–22 years old) and 8 older men (62–76 years) demonstrated an association between increasing age with loss of Leydig cells and evidence of dysregulation of testosterone production [34]. In addition, in the older group only, increasing BMI was associated with these changes. The findings from these human histology studies are limited by small numbers, inherent selection bias in participants and the lack of uniform examination processes.
2.4. Aging-related changes in testosterone metabolism and androgen sensitivity associated with aging in men
There is a decline in testosterone production that is partially mitigated by a decline in testosterone clearance as men age from 20 and 30 years to 50 years and older [35–38]. This difference in testosterone clearance seems to include decreased clearance during exogenous testosterone administration. There is no difference in testosterone production or clearance between white and Asian men, and it is likely there are no clinically important differences between any races or ethnicity [35, 39, 40]. In a study of different dosage regimens of weekly intramuscular testosterone enanthate, apparent testosterone clearance was calculated based on the amount of testosterone delivered weekly, trough testosterone concentrations and correction by absorption kinetics. The mean clearance was ~25% lower in the older group (ages 60–75 years) than the younger group (ages 19–35 years) [41].
There is indirect evidence of increased, organ-specific, androgen sensitivity in aging men. Investigators systematically compared the differential effects of 5 different dosages of intramuscular testosterone enanthate (ranging from 25 to 600 mg) weekly plus a GnRH agonist to suppress endogenous testosterone production to a group of older men (ages 60–75 years) and a group of younger men (ages 19–35 years) [42]. The older group had significantly greater percentage increases in serum hematocrit and hemoglobin concentrations than the younger group during testosterone therapy. There was no aging-related difference in muscle mass or strength with both groups having similar dose-dependent increases of fat free mass and leg press strength. However, at the two sub-physiologic replacement dosages, 25 and 50 mg weekly, the older group gained less fat mass (i.e., more androgen effect) than the younger group [43, 44]. Finally, there was a dose-dependent effect of testosterone therapy on libido and erectile function in the older group with no significant difference seen between dosages in the younger group [45].
2.5. Summary of aging-related changes of hypothalamus-pituitary gonadal axis and clinical implications
In healthy aging men, there is decreased and less orderly hypothalamic GnRH outflow that results in decreased LH secretion from pituitary gonadotropes. There is also decreased Leydig cell secretion of testosterone in response to LH stimulation. These effects of aging might be attenuated or absent in very healthy older men. Furthermore, these aging-related physiologic changes in the hypothalamus-pituitary–testicular axis likely vary between individuals, and deficits might be variably expressed within individuals. Overall, there is declining function in the hypothalamic GnRH neurons and testicular Leydig cells in aging men. This decline of hypothalamic-pituitary–testicular axis function is partially mitigated by decreased metabolic clearance of testosterone and possibly by increased organ-specific androgen sensitivity. Although there are limited data, there do not appear to be clinically important differences in these aging-related changes of the hypothalamus-pituitary–gonadal axis in men of different races [35, 39, 40, 46, 47].
3. Epidemiology of serum testosterone concentrations associated with aging in men
Several studies have demonstrated a gradual longitudinal decline in serum total and free testosterone concentration in men that begins (at least by) age 35 and is followed by a sharper decline starting at age ~65 [3, 48–54]. The decline has been observed in most studies using immunoassays or mass spectrometry assays, but the measured decline is greater in the studies that have used the more accurate mass spectrometry assays [47]. Cross-sectional studies are generally consistent with the findings of longitudinal studies although the observed annual decline of total testosterone concentrations is smaller than observed in longitudinal studies [47, 54]. In a cross-sectional study of more than 10,000 Australian men (ages 35–100) and more than 5,000 men ≥ 70 years old, there was a gradual decline (~0.5% per year) in the median serum total testosterone (measured by mass spectrometry) beginning at age 35 with a sharper decline between ages 60–70 and an even more marked decline after age 80 [47]. A 5-year mass spectrometry longitudinal study of men ≥ 70 years old (baseline mean age = 76 years) confirmed the finding of a sharp decline in serum total testosterone beginning at 80 years [53]. This study demonstrated a 2.6% and 2.8% average annual decline of total testosterone and calculated free testosterone, respectively men ≥ 80 years. A longitudinal mass spectrometry study with 9 years by follow-up demonstrated a 2.0% and 2.9% average annual decline of serum total and calculated free testosterone concentrations in men with a median baseline age of 75 years [54].
Two recent longitudinal studies are worth highlighting: one because it is the largest longitudinal study, and the other because the long follow-up interval and use of mass spectrometry to measure testosterone concentrations [3, 55]. The largest published study did not show an association between aging and a decrease in serum total testosterone [55]. In this 2022 study of more than 6000 British men (40–69 years old), there was a negligible change in mean serum total testosterone concentration (0.06 ± 0.03 nmol/L) over the 4.3-year follow-up interval. The men were relatively healthy, and the study was limited by using an immunoassay for measurement of total testosterone and a relatively short follow-up. In a 2021 longitudinal study of Belgian men with a follow-up of 12 years demonstrated a 1.2% per year decline in mean serum total testosterone concentrations measured by mass spectrometry and a 1.6% per year decline in mean calculated free testosterone concentrations [3]. This study had a smaller number of men (~700) and were younger (24–46 years old) than 2022 British study. Cumulatively, the epidemiological studies suggest that serum total testosterone begins to decline ~0.5%−0.1.2% (in mass spectrometry studies) per year in the 4th decade with a greater rate decline that begins in the mid-seventh decade and accelerates to a decline of ~2–3% by the eighth decade [3, 47, 51–54, 56].
Epidemiological studies of older men have consistently demonstrated an increase in serum SHBG and decrease in free testosterone concentration, too [3, 51–53, 55–58]. Serum SHBG avidly binds circulating testosterone, and there is generally an inverse relationship between serum free testosterone and serum SHBG concentrations in older men [59]. Because serum SHBG concentrations increase with age, serum free testosterone concentrations decrease more than total testosterone concentrations in aging men [47, 51, 52, 55–57]. Even in the large 2022 British longitudinal study that demonstrated minimal change in serum total testosterone in men aged 40–69 over a 4.3-year interval, there was a significant increase in mean serum SHBG concentrations and a decrease in calculated free testosterone concentrations [55].
3.1. Epidemiological studies of the relationship between aging, obesity, co-morbidities, and lifestyle
Several epidemiological studies suggest that much, if not all, of the association between declining testosterone and aging in men is due to obesity, co-morbidities, and lifestyle factors. Very healthy men might not have alterations in the circadian rhythm of testosterone secretion or decreases in serum total testosterone concentrations [12, 55, 57, 60, 61]. However, serum free testosterone concentrations decrease and serum SHBG concentrations increase even in groups of very healthy aging men [3, 55, 57, 60, 61]. Aging-related decline of testosterone secretion might be prevented, delayed or reversed with measures that maintain good health or effectively treat obesity and co-morbidities.
3.1.1. Obesity, serum testosterone and SHBG concentrations and aging-related changes in aging men
There is a complex interaction between aging, increasing BMI, and serum total testosterone, free testosterone and SHBG concentrations. In men, aging is associated with increasing BMI and visceral adiposity, physical factors that are associated with lower serum SHBG concentrations and lower total testosterone concentrations. In men with normal hypothalamic-pituitary–testicular axes, obesity results in low serum total testosterone and SHBG concentrations, but normal free (unbound) serum testosterone concentrations. In older men (particularly those with severe class 3 obesity, BMI ≥ 40) with impaired hypothalamic-pituitary–testicular axes, obesity is associated low serum total testosterone and free testosterone. Obesity likely directly reduces GnRH outflow, and these effects are mediated by leptin receptors in the hypothalamic ventral pre-mamillary neurons and hypothalamic kisspeptin neurons (Fig. 2) and potentially via direct effects on testicular leptin receptors to decrease testosterone biosynthesis [62, 63]. Finally, obesity is associated with decreased SHBG concentrations. Although aging is associated with increased serum SHBG concentrations, the effects of BMI to suppress SHBG prevail as the BMI increases. Older men with BMIs > 30 have lower serum SHBG concentrations than older, lean men [5, 60, 61]. The complex interaction between aging, obesity and the male hypothalamic-pituitary axis can lead to a discordance in the decrease of total and free testosterone that depends on the amount of underlying dysfunction of the axis and the BMI.
Fig. 2.
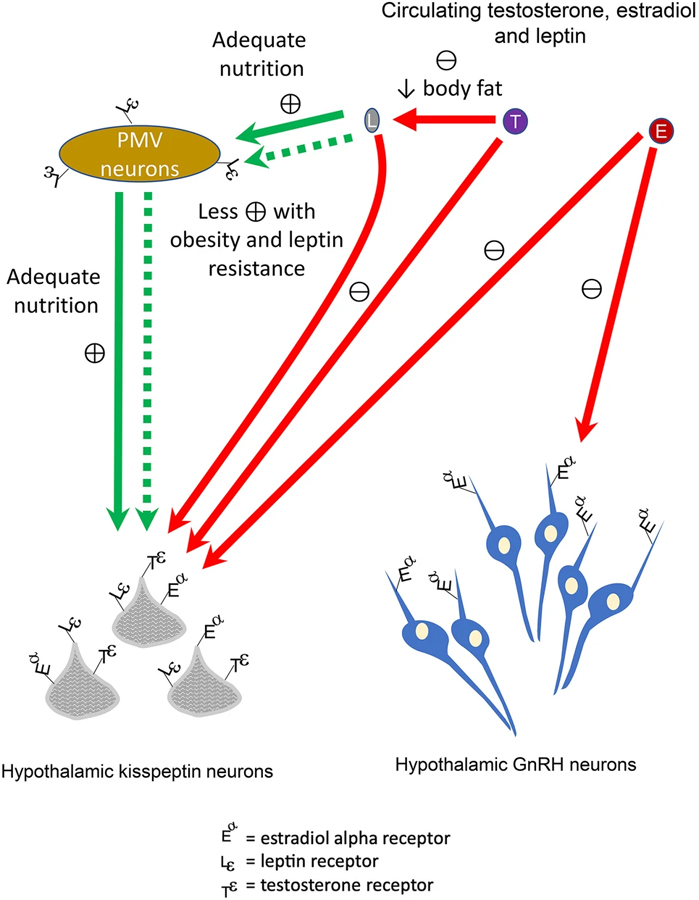
Regulation of gonadotropin-releasing hormone (GnRH) secretion by kisspeptin, leptin, testosterone, and estradiol. Kisspeptin is the most important stimulator of hypothalamic GnRH secretion. Normal adult serum leptin concentrations induce stimulation of hypothalamic kisspeptin neurons by pre-mamillary ventral neurons (PMV) in men. Leptin is secreted by fat cells, and normal serum adult leptin concentrations depend on adequate nutrition. Thus, anorexia and cachexia are associated with diminished GnRH outflow. Obesity is associated with high serum leptin concentrations is also associated with diminished stimulation of GnRH; this effect might be due to obesity-related leptin resistance or indirect and direct feedback via PMV and hypothalamic kisspeptin neuron cells. Testosterone exerts negative feedback on androgen receptors expressed by hypothalamic kisspeptin cells whereas estradiol may suppress GnRH secretion indirectly via hypothalamic kisspeptin neuron cell estradiol alpha receptors and directly via hypothalamic GnRH receptors
A higher BMI might account for much of the aging-related decline in serum total testosterone concentration [51, 52, 55, 61, 64–67]. A 2017 cross-sectional study of nearly 3000 Chinese Han men suggests that leaner aging men are more likely maintain serum total testosterone concentrations [60]. In this study, median serum total testosterone concentrations (measured by mass spectrometry) were slightly higher in older men than younger men whereas median serum SHBG concentrations in men over age 60 were 2 to 3 times the concentrations of men ages 20–45. Calculated free testosterone concentrations were not reported, but they presumably were lower in the older group than the younger group (based on small increases in median serum total testosterone concentrations and the very large increases in median serum SHBG concentrations). Only 5% of this cohort of Chinese Han men had a BMI ≥ 30, and the mean BMI was 24. Although BMI was negatively correlated with serum total testosterone concentrations, this relatively lean population did not demonstrate an association between age and declining total testosterone concentrations. The mean BMI and prevalence of obesity (BMI ≥ 30) in this study was lower than other epidemiological studies of older men whose mean BMIs were≥ 26 and/or the prevalence of obesity was ≥20% [47, 61, 64–68]. The cross-sectional design of this study of Chinese men significantly limits any conclusion about longitudinal changes of serum testosterone associated with aging.
3.1.2. Overall health, co-morbidities, lifestyle factor and aging-related changes in serum testosterone
Epidemiological studies have demonstrated an association between lower serum total testosterone concentrations in aging men and self-reported poor health and co-morbidities including metabolic syndrome, diabetes mellitus, hypertension, and polypharmacy (a marker of multiple co-morbidities) [52, 61, 65, 67, 68]. In addition, lifestyle factors such as heavy alcohol consumption, smoking cessation, lack of employment, being single or widowed, and lower level of education have been associated with lower serum total testosterone concentrations in aging men [3, 49, 51, 52, 57, 62, 67].
3.2. Summary of obesity, co-morbidities, lifestyle factors and aging-related changes in testosterone
Although it has been tempting to invoke a causal relationship between declining serum testosterone concentrations in the decline of androgen-responsive endpoints such as strength, bone density and sexual function, epidemiology studies suggest that low serum testosterone concentrations are more likely to be a marker of poor health, obesity and co-morbidities than caused by aging per se. Although obesity and co-morbidities might contribute significantly to the aging-related decline of serum testosterone, there is an independent effect of aging on the hypothalamus-pituitary–testicular axis. The physiology studies demonstrating decreased hypothalamic GnRH outflow and Leydig cell secretion of testosterone were in healthy, non-obese young and older men [6–9, 21, 22, 25, 26, 69]. Because aging is associated with decreased overall function of the hypothalamus-pituitary–testicular axis, men become more vulnerable to factors that further suppress testosterone production and serum testosterone concentrations.
4. Epidemiology of serum dihydrotestosterone and estradiol concentrations associated with aging in men
There are fewer studies of the epidemiology of changes in the concentrations of the major metabolites of testosterone, dihydrotestosterone, and estradiol. Because these hormones circulate at much lower concentrations than testosterone, we will focus on the studies that have used mass spectrometry assays that can accurately measure serum dihydrotestosterone and estradiol concentrations at the concentrations typical of healthy men [70]. A cross-sectional study of over 10,000 Australian men demonstrated that serum dihydrotestosterone concentrations decline at about the same rate as testosterone concentrations decline (0.5% per year) and a small decline of serum estradiol concentrations (0.2% per year) beginning at age ~35 [47]. A smaller cross-sectional study demonstrated no decrease of serum dihydrotestosterone or estradiol concentrations in men, ages 40–97 (mean age 60), who self-reported very good or excellent healthy compared to healthy younger men, ages 40–50 [57]. In a cross-sectional study of nearly 1500 United States men (mean age 61 years and mean BMI 29), serum total estradiol and estrone concentrations were 3% higher per decade-age increase, but calculated serum free estradiol declined by 4% per decade-age increase [71]. A cross-sectional study of Australian men ≥ 70 years (mean age 77) demonstrated an inverse relationship between serum dihydrotestosterone and estradiol concentrations and age [72]. A 5-year longitudinal study of serum dihydrotestosterone and estradiol measurements in Australian men ≥ 70 years demonstrated a decrease of serum dihydrotestosterone of > 2% per year and an increase of serum estradiol concentrations of ~3% per year [68].
Based on a small number of studies using accurate assay methods, there seems to be a decline of serum dihydrotestosterone concentrations that is similar to the decline in serum total testosterone (0.5–1.2% per year in mass spectrometry studies) in men starting at ~35 years and that decreases more rapidly in the middle of the 7th decade of life. Serum estradiol concentrations, on the other hand, tend to remain relatively stable and begin to increase in the middle of the 7th decade of life.
5. The epidemiology of biochemical primary and secondary hypogonadism in aging men
As with younger men, accurate assessment of serum gonadotropins is essential to assess for the cause of low serum testosterone concentrations in older men. We will use the terms “biochemical primary hypogonadism” and “biochemical secondary hypogonadism” to describe older men without symptoms and signs of androgen deficiency and no classic cause of hypothalamus-pituitary–testicular pathology, but who have low serum testosterone concentrations with elevated serum gonadotropin concentrations or low serum testosterone concentrations without elevated serum gonadotropin concentrations, respectively. There is uncertainty about whether older men with biochemical hypogonadism benefit from exogenous testosterone therapy.
We use the term “biochemical hypogonadism” because the epidemiological studies of the association between aging and longitudinal changes of serum testosterone generally have not reported data on symptoms, signs or outcomes of androgen deficiency and have been based on a single baseline serum testosterone concentration. The identifiable classic causes of hypothalamic-pituitary–testicular pathology is an exclusion criterion for these epidemiology studies. The failure to confirm low serum testosterone concentrations and the lack of data from randomized, placebo-controlled trials of the effects of testosterone on men with low testosterone concentrations and without an identifiable pathology of hypothalamus-pituitary–testicular preclude making a diagnosis of hypogonadism [73]. Thus, we will use the more conservative term of “biochemical hypogonadism”.
Three landmark epidemiology studies, the Massachusetts Male Aging Study (MMAS), the Boston Area Community Health study (BACH), and the European Male Ageing Study (EMAS) characterized the prevalence and/or incidence of hypogonadism based on symptoms suggestive of hypogonadism; the MMAS and BACH study also included signs or outcomes of hypogonadism such as osteoporosis [1, 4, 50, 51, 65, 67, 74–78]. The MMAS was an important early epidemiology study examining the prevalence of low serum testosterone concentrations and 8 symptoms suggestive of androgen deficiency; hypogonadism was defined by the presence of 3 of the 8 symptoms and a low serum testosterone concentration [74]. Over 1000 men, baseline ages 40–70, were followed for ~9 years; a follow-up study extended the follow-up to ~15 years [74, 75]. Limitations of the MMAS include the lack of specificity of 6 of the 8 symptoms, the use of an immunoassay to measure serum total testosterone and to calculate serum free testosterone concentrations, and the lack of data for serum gonadotropin concentrations [70, 73, 76]. The BACH study was a cross-sectional study of nearly 1500 men, ages 30–79, and this study defined hypogonadism based on 1 of 3 more specific symptoms or signs (low libido, erectile dysfunction, or osteoporosis) or ≥ 2 less specific symptoms of hypogonadism (sleep disturbance, depressed mood, lethargy or low physical performance plus a serum total testosterone) [50]. The BACH study was limited by the inclusion of non-specific symptoms to define hypogonadism, the use of an immunoassay to measure testosterone, the lack of measurement of serum gonadotropins and lack of data by age subgroup.
Several of the longitudinal EMAS results are discussed in the remainder of Sect. 5 below. However, the symptoms and signs of hypogonadism and low serum testosterone are reported and analyzed as aggregate data and are not reported and analyzed at the individual patient level (i.e., prospectively divided into groups of individuals with low serum testosterone concentrations and with or without symptoms and signs of hypogonadism) in these follow-up studies of EMAS. For MMAS, BACH and EMAS, the findings are limited by lack of confirmation of low serum testosterone concentrations at each time point of the follow-up [73].
5.1. Biochemical primary hypogonadism associated with aging in men
The life-time prevalence of primary hypogonadism has been estimated to be less than 1% in the overall adult male population, and the life-time prevalence of Klinefelter syndrome, the most common cause of primary hypogonadism is about 0.2% [79]. However, in men ≥ 70 years old, the prevalence of biochemical primary hypogonadism (defined by a low serum testosterone and elevated LH concentration) might rise to 2–7% [1, 2].
In a 4.3-year longitudinal study of 1991 European men, ages 40–79, who did not have pituitary, testicular or adrenal disease identified at baseline or during follow-up, the baseline prevalence of biochemical primary hypogonadism was 1.35% at baseline and 2% at the end of follow-up [4]. Men with biochemical secondary hypogonadism (defined by low serum total testosterone and normal serum gonadotropins) were excluded from the analysis; these men constituted about 10% of the original cohort. Men were classified into eugonadism or biochemical primary hypogonadism based on a single sample obtained upon entry into the study and a second blood sample at the end of the follow-up. The men who had baseline and persistent biochemical primary hypogonadism at follow-up were typically > 70 years old at baseline (mean age 75 years) and had very low baseline serum total testosterone concentrations (mean serum total testosterone < 220 ng/dL or 7.3 nmol/L), significantly elevated serum LH concentrations (mean serum LH= 20 IU/L, about twice the upper limit of normal) and symptoms suggestive of hypogonadism (e.g., decreased sexual thoughts, decreased erectile dysfunction). These men with persistent primary hypogonadism had even lower serum testosterone concentrations at the end of the study. In about 20% men with baseline biochemical primary hypogonadism, serum total testosterone concentrations were normal at the end of the study. These men who “recovered” from primary hypogonadism were younger (mean age 62 years) and had baseline low-to-low normal serum total testosterone concentrations (mean serum total testosterone ~260 ng/dl or 9.3 nmol/L), near-normal baseline serum free testosterone concentrations and slightly elevated serum LH concentrations; it cannot be determined whether normalization was due to the natural variation of serum testosterone concentrations or a change in gonadal function. The men with persistent primary hypogonadism had worse health, more co-morbidities and higher mortality.
Two Australian studies confirmed that serum LH rises after age 70 and that primary biochemical hypogonadism increases after age 70 [2, 53]. In a longitudinal study that included 958 men followed for 5 years, mean serum LH concentrations steadily rose from age 70 to age 89, and mean serum LH was > 12 IU/L at ages 80–85 [53]. In a second Australian Health in Men study of over 1000 men with a median baseline age of ~75 years, mean serum testosterone concentrations fell 2% per year and mean LH concentrations increased 7.5% year after 8.6 years of follow-up [2]. Primary biochemical hypogonadism (defined as serum total testosterone < 184 ng/dL [< 6.4 nmol/L] and LH > 16 nmol/L) rose from 0.6% to 7% of the cohort at the end of follow-up. At the end of follow-up, ~12% of this cohort had low serum testosterone and non-elevated serum LH concentrations (≤ 16 IU/L), and ~10% had normal serum testosterone and elevated serum LH concentrations (> 16 IU/L).
Collectively, these studies suggest that primary biochemical and clinical hypogonadism becomes more common in men > 65–70 years old and that biochemical findings of primary hypogonadism persist in the majority of men > 65–70 years. The mechanism of this decline has not been elucidated, but it is likely that progressive aging-related declines in Leydig cell reserve are contributory. Men who have biochemical primary hypogonadism with low serum testosterone concentrations and symptoms of hypogonadism are likely to experience progressively declining serum testosterone concentrations. The benefits and risks of testosterone therapy have not been studied in older men with primary hypogonadism, but it is reasonable to evaluate and treat these patients similarly to younger men with primary hypogonadism.
5.1.1. Compensated biochemical primary hypogonadism associated with aging in men
Two studies have demonstrated that there is high percentage of older men with persistently high serum LH concentrations, but normal serum testosterone concentrations. This biochemical pattern is sometimes referred to as “compensated primary hypogonadism” and “subclinical primary hypogonadism”. In the European Male Aging Study, ~9.5% of the men (baseline mean age = 67) had compensated biochemical primary hypogonadism at baseline [77]. After the 4.3-year follow-up, ~66% of the men with compensated biochemical primary hypogonadism had persistent compensated primary hypogonadism, ~25% recovered to eugonadism (with normal serum LH), and ~8% developed primary biochemical hypogonadism with low serum total testosterone and high LH concentrations. The incidence of new compensated biochemical primary hypogonadism was 1.3% per year during the follow-up. Compared to those men who were eugonadal (normal serum testosterone and LH concentrations) at baseline and follow-up, those men with persistent compensated biochemical hypogonadism had baseline worse overall health with more co-morbidities, and worse sexual function, cognitive processing speed, and physical function; these differences remained at the end of follow-up. The strongest predictors of compensated primary hypogonadism were age > 70 years, diabetes mellitus, chronic pain, and low physical activity. Recovery to eugonadism was predicted by age < 60 years, better health, non-smoking status, and higher sexual function, cognitive processing, and physical function at baseline. In The Health in Men Study, 1.8% of older men (mean age = 75 years) had low serum testosterone and elevated serum LH concentrations at baseline, and this pattern rose to 10.4% of the cohort at the end of the ~9-year follow-up [2]. These studies confirm a progressive decline of Leydig cell function that is associated with worse sexual dysfunction, physical function, and overall health in the oldest old men.
Because the benefits of testosterone are not proven and the risks are unknown for men with compensated primary hypogonadism, the clinician should focus the management of compensated primary hypogonadism on limiting or avoiding causes of decreased hypothalamus-pituitary–testicular function such as glucocorticoids, opioids, drugs that induce hyperprolactinemia or anti-androgens such as spironolactone. Because many older men with compensated hypogonadism maintain normal serum testosterone concentrations for many years, testosterone therapy should be withheld until serum testosterone concentrations have declined below normal, or if the clinician has high degree of suspicion for hypogonadism.
5.2. Secondary hypogonadism associated with aging in men
In aging men with low serum testosterone concentrations and no classic cause of hypothalamus-pituitary–testicular disorder, the most common biochemical profile is secondary hypogonadism (with inappropriately normal serum gonadotropin concentrations) [1, 3]. The principal factor that seems to affect the risk of secondary hypogonadism in aging men is obesity (whereas increasing age > 70 years is the principal risk factor for primary hypogonadism as discussed in Sects. 5.1 and 5.1.1. above). In the baseline cross-sectional analysis of the European Male Ageing Study, biochemical secondary hypogonadism was 4–5 times more common in than primary hypogonadism in men 40–79 years old [1]. Greater BMI was the primary risk factor for secondary hypogonadism with an odds ratio that increased from > 3 in men with BMI 25.0–29.9 to > 7.0 in men with a BMI > 30. In the 4.3-year longitudinal follow-up of the European Male Ageing Study cohort, recovery from biochemical secondary hypogonadism occurred in 42.9% of the men. Predictors of recovery to eugonadism included a baseline BMI < 30, normal baseline waist circumference (< 94 cm), baseline age < 60 years, weight loss during follow-up and higher education.
Because recovery from biochemical secondary hypogonadism is common in aging men without a classic cause of hypogonadism, clinicians should focus on lifestyle measures for weight loss and improved overall health and treatment of systemic diseases [78, 80]. Testosterone therapy should not be routinely prescribed to aging men with biochemical secondary hypogonadism and no identifiable cause hypogonadism.
6. Does late-onset male hypogonadism exist?
Late-onset hypogonadism has been defined as a clinical and biochemical syndrome associated with advancing age and symptoms of male hypogonadism and low serum testosterone concentrations [81]. It is implicit, but not explicit, in the term and definition that aging per se is a cause of hypogonadism. It is not a useful term or concept for several reasons. Aging has not been proven to be a permanent cause of male hypogonadism. The evidence suggests that many older men with biochemical hypogonadism might (over time) have normalization of serum testosterone concentrations; men < 60 years with biochemical secondary hypogonadism seem particularly likely to revert a eugonadal state with weight loss or improvement in overall health. Secondly, the term “late-onset hypogonadism” facilitates potential errors in clinical care because the term suggests that all older men with low serum testosterone concentrations might benefit from testosterone therapy.
The term “functional hypogonadism” has also been proposed [82]. This term has been defined in 2020 European Andrology Society guidelines:
“Functional hypogonadism (also referred to late-onset, age-related, or adult-onset hypogonadism) is defined as the coexistence of androgen deficiency-like features and low serum T concentrations occurring in the absence of both intrinsic structural hypothalamic-pituitary-testis (HPT) axis pathology and of specific pathologic conditions suppressing the HPT axis (such as microprolactinoma, endogenous Cushing syndrome) in middle-aged or older men… Functional hypogonadism may be potentially reversible if the underlying causes are identified and adequately treated or removed, whereas organic hypogonadism is generally an irreversible condition secondary to genetic faults or pathological perturbations of the HPT axis.” [78]
Functional hypogonadism and organic hypogonadism as defined are not useful terms. So-called “functional causes” of hypogonadism might not be effectively reversible. For example, sufficient weight loss might not be achievable in men with obesity-related hypogonadism, and discontinuation of high dosages of opioids might not be possible in patients with chronic pain and opioid-induced hypogonadism. Conversely, “organic causes” of hypogonadism such as Cushing syndrome or hyperprolactinemia due to a macroprolactinoma are often reversible. Even hypogonadism due to a pituitary macroadenoma is sometimes reversible.
A more useful typology is to characterize hypogonadism as primary or secondary and irreversible or potentially reversible. For example, Klinefelter syndrome is due to a genetic abnormality with supernumerary X sex chromosome(s); this syndrome typically results in small testes and irreversible primary hypogonadism. A man who has low serum testosterone and LH concentrations 3 years after high-dosage external beam radiation 3 years after treatment of a brain tumor has irreversible secondary hypogonadism due to radiation injury of the pituitary. A 60-year-old man with a BMI > 30, a low serum testosterone and normal serum LH and no identifiable cause of hypothalamic-pituitary–testicular pathology has potentially reversible secondary hypogonadism due to aging, obesity, and comorbidities.
7. Summary of clinical implications of aging-related changes of the hypothalamus-pituitary–testicular axis
The clinical implications of the aging-related changes are summarized in Table 1. The clinical implications include the following: 1) decreased hypothalamic outflow of GnRH leads to greater vulnerability to development of biochemical secondary hypogonadism due to medications, obesity, and systemic disease; 2) progressive Leydig cell dysfunction results in a higher incidence and prevalence of persistent primary biochemical hypogonadism in men over age 65; 3) and because there is decreased metabolism of testosterone and increased tissue-specific androgen sensitivity associated with aging, older men with hypogonadism often require lower testosterone replacement therapy dosages.
Table 1.
Clinical implications of physiologic changes of hypothalamus-pituitary–testicular axis in aging men
| Physiologic change | Clinical implication |
|---|---|
| ↓ GnRH outflow | Aging men have increased vulnerability to secondary hypogonadism due to mild to moderate insults and injuries to the hypothalamus and pituitary including head trauma; head and neck irradiation; drugs and medications such as opioids, corticosteroids, and anti-dopaminergic agents; obesity; and systemic disease |
| ↓ Leydig cell daily production of testosterone | Aging men have increased vulnerability to primary hypogonadism due to testicular Leydig cell insult or suppression including drugs that decrease testosterone synthesis, pelvic radiation, and chemotherapy. A minority of men > 65 years old with baseline low Leydig cell reserve might experience primary hypogonadism due to aging alone |
| ↑ Tissue-specific androgen sensitivity | Optimal, safe and effective testosterone replacement therapy dosages might be 50–75% that of younger men |
| ↓ Testosterone metabolism | For many older, hypogonadal men, optimal, safe and effective testosterone replacement therapy dosages might be 50–75% that of younger men |
The largest evidence gaps about the clinical implications of aging-related hypogonadism are the following: 1) the lack of prospective longitudinal epidemiological studies of healthy, racially and geographically diverse men with baseline data on symptoms, signs and outcomes of hypogonadism (e.g., bone density) and serum testosterone (measured on at least two occasions by a validated, harmonized assay) [73], and serum gonadotropins with long-term follow-up of these metrics; and 2) placebo-controlled studies of the effects of testosterone therapy in healthy, racially and geographically diverse men with repeatedly and consistently low serum testosterone concentrations (measured in a validated, harmonized assay), no identifiable cause of hypogonadism and a broad range of ages and BMIs.
8. Conclusions and clinical guidance
Aging is associated with decreased function of the hypothalamus-pituitary–testicular axis that leads to increased incidence and prevalence of biochemical secondary hypogonadism in men 35–65 years old and increased incidence and prevalence of biochemical primary hypogonadism in men >65 years old. Biochemical secondary hypogonadism occurs predominantly in men with higher BMIs and is likely to remit with weight loss or interventions that improve overall health. Clinicians should be cautious about initiation of testosterone therapy for older men with biochemical secondary hypogonadism and should focus on interventions to improve overall health including increased exercise and lifestyle changes (Fig. 3a). Biochemical primary hypogonadism is more likely to persist in men > 65 years old. Because Klinefelter syndrome is the most common cause of primary hypogonadism and it is commonly underdiagnosed, serum karyotyping should be considered in all older men with biochemical primary hypogonadism [79]. Testosterone therapy should be initiated in older men with primary hypogonadism due to Klinefelter syndrome. There are no clinical trials of testosterone therapy in older men with biochemical primary hypogonadism. Based on the benefit-to-detriment ratio observed in younger with primary hypogonadism and the likelihood that biochemical primary hypogonadism is a permanent condition in men > 65 years, testosterone therapy is reasonable to consider in older men with persistently low serum testosterone concentrations and high serum gonadotropin concentrations (Fig. 3b).
Fig. 3.
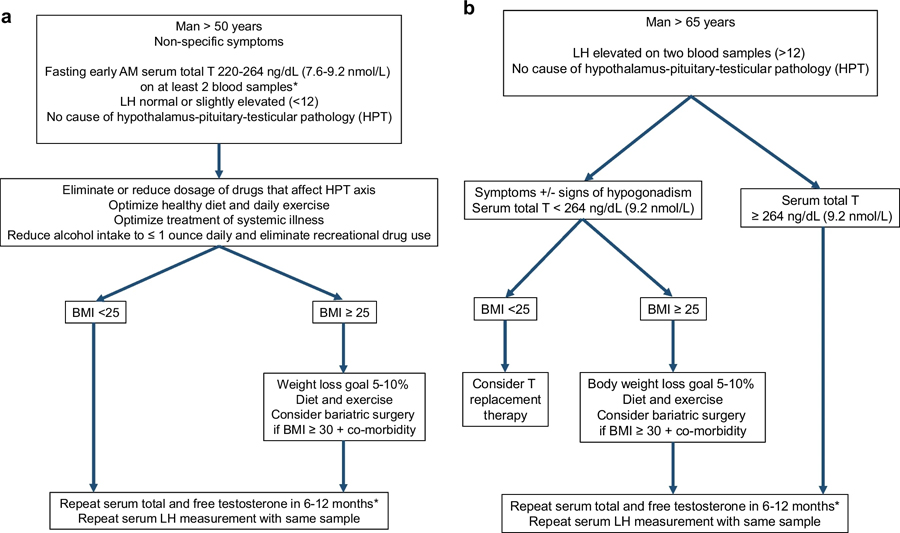
Management algorithm for biochemical secondary hypogonadism (Fig. 3a), primary hypogonadism (left side of Fig. 3b) and compensated primary hypogonadism (right side of Fig. 3b) in aging men. Biochemical secondary hypogonadism is common in aging men, particularly men > 50 years and BMI ≥ 30. Biochemical secondary hypogonadism associated with obesity is often reversible. Biochemical primary hypogonadism is more common in men > 65 years old, and it is much less commonly reversible than biochemical secondary hypogonadism. Biochemical compensated primary hypogonadism commonly persists with stable, normal serum testosterone concentrations; these men can be followed with annual assessment of serum testosterone concentrations. *An accurate testosterone assay is defined by one that has been validated and harmonized by an accuracy-based, quality control program such as the one established by the United States Center of Disease of Control [73]. The lower limit of a normal serum total testosterone concentration should be 264 ng/dL (9.16 nmol/L) [73].
Compensated biochemical primary hypogonadism may persist indefinitely in older men.
Because the benefits of testosterone are not proven and the risks are unknown for older men with compensated primary hypogonadism, the clinician should manage compensated primary hypogonadism similarly to biochemical secondary hypogonadism in older men by promoting healthy lifestyle choices and limiting or avoiding causes of decreased hypothalamus-pituitary–testicular function such as glucocorticoids, opioids, drugs that induce hyperprolactinemia or anti-androgens such as spironolactone. Because many older men with compensated hypogonadism maintain normal serum testosterone concentrations for many years, testosterone therapy generally should be withheld until serum testosterone concentrations have declined below normal (unless there is a high degree of suspicion for hypogonadism).
Understanding the physiological changes of aging in the male hypothalamus-pituitary–testicular axis is essential for the management of low serum testosterone concentrations in aging men. There is a spectrum in the pathophysiologic effects of aging on a given individual man’s gonadal axis that depends on the man’s baseline hypothalamic-pituitary–testicular reserve function, differential rates of physiologic aging and co-morbidities including obesity and systemic disease. On one end of the spectrum, some very healthy aging men with baseline normal hypothalamus-pituitary–testicular axis function might experience little or no change in serum testosterone concentrations into their 8th, 9th, or 10th decade. In contrast to these rare men, many more aging men with baseline normal hypothalamus-pituitary–testicular axis function might experience declining serum testosterone concentrations as their BMI rises and systemic diseases (and their therapies that might affect GnRH outflow) accrue. These men may eventually develop biochemical secondary hypogonadism that might remit with treatment of obesity or systemic disease and avoidance of drugs that affect the hypothalamus-pituitary axis. On the other hand, aging may unmask a baseline compromised hypothalamus or pituitary dysfunction (e.g., a patient with radiotherapy of head neck cancer who suffers some hypothalamic-pituitary injury due to radiation scatter to the sellar region) might develop irreversible secondary hypogonadism with aging. Finally, a significant minority of aging men >65 years seem to develop irreversible primary hypogonadism due to progressive aging-related decline in overall Leydig cell function; again, a decreased baseline reserve of Leydig cell function might contribute to or accelerate the onset of aging-related primary hypogonadism. Based on the physiology of aging, it is best for the clinician to consider advancing age and obesity as potential contributors as opposed to sole causes of the increasing incidence of low serum testosterone concentrations in aging men. Because obesity is potentially treatable, lifestyle measures represent a sensible preventive and therapeutic intervention for most aging men that present with biochemical secondary hypogonadism and no identifiable pathology of the hypothalamus-pituitary–testicular axis.
Knowledge of the physiological changes of the hypothalamus-pituitary–testicular axis is important for the evaluation and management of aging men with low serum testosterone concentrations. It is essential to base the diagnosis of clinical hypogonadism on symptoms, signs, and biochemical evidence of androgen deficiency and to restrict testosterone therapy to men who are mostly likely to benefit. At any age, the men most likely to benefit from testosterone therapy are those who have symptoms, signs, or outcomes of androgen deficiency and who have consistently low to very low serum testosterone concentrations.
Funding
Dr. Anawalt receives funding from the NIH-NICHD (HHSN275000251) and as the site PI on NIH-RO1HL1343653 (Kanias, PI).
Footnotes
Declarations
Conflicts of interest Drs. Anawalt and Matsumoto report no commercial or financial conflicts of interest.
References
- 1.Tajar A, Forti G, O’Neill TW, Lee DM, Silman AJ, Finn JD, et al. Characteristics of secondary, primary, and compensated hypogonadism in aging men: evidence from the European Male Ageing Study. J Clin Endocrinol Metab 2010;95:1810–8. [DOI] [PubMed] [Google Scholar]
- 2.Yeap BB, Manning L, Chubb SAP, Handelsman DJ, Almeida OP, Hankey GJ, et al. Progressive impairment of testicular endocrine function in ageing men: Testosterone and dihydrotestosterone decrease, and luteinizing hormone increases, in men transitioning from the 8th to 9th decades of life. Clin Endocrinol (Oxf) 2018;88:88–95. [DOI] [PubMed] [Google Scholar]
- 3.Banica T, Verroken C, Reyns T, Mahmoud A, T’Sjoen G, Fiers T, Kaufman JM, Lapauw B. Early decline of androgen levels in healthy adult men: An effect of aging per se? A prospective cohort study. J Clin Endocrinol Metab 2021;106:1074–83. [DOI] [PubMed] [Google Scholar]
- 4.Ahern T, Swiecicka A, Eendebak RJ, Carter EL, Finn JD, Pye SR, et al. Natural history, risk factors and clinical features of primary hypogonadism in ageing men: Longitudinal data from the European Male Ageing Study. Clin Endocrinol (Oxf) 2016;85:891–901. [DOI] [PubMed] [Google Scholar]
- 5.Cooper LA, Page ST, Amory JK, Anawalt BD, Matsumoto AM. The association of obesity with sex hormone-binding globulin is stronger than the association with ageing–implications for the interpretation of total testosterone measurements. Clin Endocrinol (Oxf) 2015;83:828–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roelfsema F, Liu PY, Takahashi PY, Yang RJ, Veldhuis JD. Dynamic interactions between LH and testosterone in healthy community-dwelling men: Impact of age and body composition. J Clin Endocrinol Metab 2020;105:e628–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keenan DM, Takahashi PY, Liu PY, Roebuck PD, Nehra AX, Iranmanesh A, et al. An ensemble model of the male gonadal axis: illustrative application in aging men. Endocrinology 2006;147:2817–28. [DOI] [PubMed] [Google Scholar]
- 8.Iranmanesh A, Mulligan T, Veldhuis JD. Age in men does not determine gonadotropin-releasing hormone’s dose-dependent stimulation of luteinizing hormone secretion under an exogenous testosterone clamp. J Clin Endocrinol Metab 2010;95:2877–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Veldhuis JD, Keenan DM, Liu PY, Iranmanesh A, Takahashi PY, Nehra AX. The aging male hypothalamic-pituitary-gonadal axis: pulsatility and feedback. Mol Cell Endocrinol 2009;299:14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keenan DM, Veldhuis JD. Age-dependent regression analysis of male gonadal axis. Am J Physiol Regul Integr Comp Physiol 2009;297:R1215–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gruenewald DA, Naai MA, Marck BT, Matsumoto AM. Age-related decrease in hypothalamic gonadotropin-releasing hormone (GnRH) gene expression, but not pituitary responsiveness to GnRH, in the male Brown Norway rat. J Androl 2000;21:72–84. [PubMed] [Google Scholar]
- 12.Diver MJ, Imtiaz KE, Ahmad AM, Vora JP, Fraser WD. Diurnal rhythms of serum total, free and bioavailable testosterone and of SHBG in middle-aged men compared with those in young men. Clin Endocrinol (Oxf) 2003;58:710–7. [DOI] [PubMed] [Google Scholar]
- 13.Bremner WJ, Vitiello MV, Prinz PN. Loss of circadian rhythmicity in blood testosterone levels with aging in normal men. J Clin Endocrinol Metab 1983;56:1278–81. [DOI] [PubMed] [Google Scholar]
- 14.Plymate SR, Tenover JS, Bremner WJ. Circadian variation in testosterone, sex hormone-binding globulin, and calculated non-sex hormone-binding globulin bound testosterone in healthy young and elderly men. J Androl 1989;10:366–71. [DOI] [PubMed] [Google Scholar]
- 15.Hrabovszky E, Kalló I, Szlávik N, Keller E, Merchenthaler I, Liposits Z. Gonadotropin-releasing hormone neurons express estrogen receptor-beta. J Clin Endocrinol Metab 2007;92:2827–30. [DOI] [PubMed] [Google Scholar]
- 16.Campbell RE, Coolen LM, Hoffman GE, Hrabovszky E. Highlights of neuroanatomical discoveries of the mammalian gonadotropin-releasing hormone system. J Neuroendocrinol 2022;34: e13115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderson RA, Millar RP. The roles of kisspeptin and neurokinin B in GnRH pulse generation in humans, and their potential clinical application. J Neuroendocrinol 2022;34: e13081. [DOI] [PubMed] [Google Scholar]
- 18.Hrabovszky E, Takács S, Göcz B, Skrapits K. New Perspectives for Anatomical and Molecular Studies of Kisspeptin Neurons in the Aging Human Brain. Neuroendocrinology 2019;109:230–41. [DOI] [PubMed] [Google Scholar]
- 19.Lee EB, Dilower I, Marsh CA, Wolfe MW, Masumi S, Upadhyaya S, et al. Sexual dimorphism in kisspeptin signaling. Cells 2022;11:1146–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hrabovszky E, Molnár CS, Sipos MT, Vida B, Ciofi P, Borsay BA, et al. Sexual dimorphism of kisspeptin and neurokinin B immunoreactive neurons in the infundibular nucleus of aged men and women. Front Endocrinol (Lausanne) 2011;2(80):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mulligan T, Iranmanesh A, Kerzner R, Demers LW, Veldhuis JD. Two-week pulsatile gonadotropin releasing hormone infusion unmasks dual (hypothalamic and Leydig cell) defects in the healthy aging male gonadotropic axis. Eur J Endocrinol 1999;141:257–66. [DOI] [PubMed] [Google Scholar]
- 22.Veldhuis JD, Iranmanesh A, Mulligan T. Age and testosterone feedback jointly control the dose-dependent actions of gonadotropin-releasing hormone in healthy men. J Clin Endocrinol Metab 2005;90:302–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rubens R, Dhont M, Vermeulen A. Further studies on Leydig cell function in old age. J Clin Endocrinol Metab 1974;39:40–5. [DOI] [PubMed] [Google Scholar]
- 24.Harman SM, Tsitouras PD. Reproductive hormones in aging men. I. Measurement of sex steroids, basal luteinizing hormone, and Leydig cell response to human chorionic gonadotropin. J Clin Endocrinol Metab 1980;51:35–40. [DOI] [PubMed] [Google Scholar]
- 25.Veldhuis JD, Liu PY, Keenan DM, Takahashi PY. Older men exhibit reduced efficacy of and heightened potency downregulation by intravenous pulses of recombinant human LH: a study in 92 healthy men. Am J Physiol Endocrinol Metab 2012;302:E117–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takahashi PY, Votruba P, Abu-Rub M, Mielke K, Veldhuis JD. Age attenuates testosterone secretion driven by amplitude-varying pulses of recombinant human luteinizing hormone during acute gonadotrope inhibition in healthy men. J Clin Endocrinol Metab 2007;92:3626–32. [DOI] [PubMed] [Google Scholar]
- 27.Mulligan T, Iranmanesh A, Veldhuis JD. Pulsatile iv infusion of recombinant human LH in leuprolide-suppressed men unmasks impoverished Leydig-cell secretory responsiveness to mid-physiological LH drive in the aging male. J Clin Endocrinol Metab 2001;86:5547–53. [DOI] [PubMed] [Google Scholar]
- 28.Tenover JS, Matsumoto AM, Plymate SR, Bremner WJ. The effects of aging in normal men on bioavailable testosterone and luteinizing hormone secretion: response to clomiphene citrate. J Clin Endocrinol Metab 1987;65:1118–26. [DOI] [PubMed] [Google Scholar]
- 29.Mularoni V, Esposito V, Di Persio S, Vicini E, Spadetta G, Berloco P, et al. Age-related changes in human Leydig cell status. Hum Reprod 2020;35:2663–76. [DOI] [PubMed] [Google Scholar]
- 30.Kaler LW, Neaves WB. Attrition of the human Leydig cell population with advancing age. Anat Rec 1978;192:513–8. [DOI] [PubMed] [Google Scholar]
- 31.Neaves WB, Johnson L, Porter JC, Parker CR Jr, Petty CS. Leydig cell numbers, daily sperm production, and serum gonadotropin levels in aging men. J Clin Endocrinol Metab 1984;59:756–63. [DOI] [PubMed] [Google Scholar]
- 32.Paniagua R, Martín A, Nistal M, Amat P. Testicular involution in elderly men: comparison of histologic quantitative studies with hormone patterns. Fertil Steril 1987;47:671–9. [DOI] [PubMed] [Google Scholar]
- 33.Petersen PM, Seierøe K, Pakkenberg B. The total number of Leydig and Sertoli cells in the testes of men across various age groups - a stereological study. J Anat 2015;226:175–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nie X, Munyoki SK, Sukhwani M, Schmid N, Missel A, Emery BR, et al. Single-cell analysis of human testis aging and correlation with elevated body mass index. Dev Cell 2022;57:1160–1176.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang C, Catlin DH, Starcevic B, Leung A, DiStefano E, Lucas G, et al. Testosterone metabolic clearance and production rates determined by stable isotope dilution/tandem mass spectrometry in normal men: influence of ethnicity and age. J Clin Endocrinol Metab 2004;89:2936–41. [DOI] [PubMed] [Google Scholar]
- 36.Vermeulen A, Rubens R, Verdonck L. Testosterone secretion and metabolism in male senescence. J Clin Endocrinol Metab 1972;34:730–5. [DOI] [PubMed] [Google Scholar]
- 37.Baker HW, Burger HG, de Kretser DM, Hudson B, O’Connor S, Wang C, et al. Changes in the pituitary- testicular system with age. Clin Endocrinol (Oxf) 1976;5:349–72. [DOI] [PubMed] [Google Scholar]
- 38.Ishimaru T, Pages L, Horton R. Altered metabolism of androgens in elderly men with benign prostatic hyperplasia. J Clin Endocrinol Metab 1977;45:695–701. [DOI] [PubMed] [Google Scholar]
- 39.Santner S, Albertson B, Zhang GY, Zhang GH, Santulli M, Wang C, et al. Comparative rates of androgen production and metabolism in Caucasian and Chinese subjects. J Clin Endocrinol Metab 1998;83:2104–9. [DOI] [PubMed] [Google Scholar]
- 40.Heald AH, Patel J, Anderson SG, Vyas A, Rudenski A, Hughes E, et al. Migration is associated with lower total, but not free testosterone levels in South Asian men. Clin Endocrinol (Oxf) 2007;67:651–5. [DOI] [PubMed] [Google Scholar]
- 41.Coviello AD, Lakshman K, Mazer NA, Bhasin S. Differences in the apparent metabolic clearance rate of testosterone in young and older men with gonadotropin suppression receiving graded doses of testosterone. J Clin Endocrinol Metab 2006;91:4669–75. [DOI] [PubMed] [Google Scholar]
- 42.Coviello AD, Kaplan B, Lakshman KM, Chen T, Singh AB, Bhasin S. Effects of graded doses of testosterone on erythropoiesis in healthy young and older men. J Clin Endocrinol Metab 2008;93:914–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bhasin S, Woodhouse L, Casaburi R, Singh AB, Mac RP, Lee M, et al. Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle. J Clin Endocrinol Metab 2005;90:678–88. [DOI] [PubMed] [Google Scholar]
- 44.Bhasin S, Woodhouse L, Casaburi R, Singh AB, Bhasin D, Berman N, et al. Testosterone dose-response relationships in healthy young men. Am J Physiol Endocrinol Metab 2001;281:E1172–81. [DOI] [PubMed] [Google Scholar]
- 45.Gray PB, Singh AB, Woodhouse LJ, Storer TW, Casaburi R, Dzekov J, et al. Dose-dependent effects of testosterone on sexual function, mood, and visuospatial cognition in older men. J Clin Endocrinol Metab 2005;90:3838–46. [DOI] [PubMed] [Google Scholar]
- 46.Richard A, Rohrmann S, Zhang L, Eichholzer M, Basaria S, Selvin E, et al. Racial variation in sex steroid hormone concentration in black and white men: a meta- analysis. Andrology 2014;2:428–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Handelsman DJ, Yeap B, Flicker L, Martin S, Wittert GA, Ly LP. Age-specific population centiles for androgen status in men. Eur J Endocrinol 2015;173:809–17. [DOI] [PubMed] [Google Scholar]
- 48.Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR, Baltimore Longitudinal Study of Aging. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore longitudinal study of aging. J Clin Endocrinol Metab 2001;86:724–31. [DOI] [PubMed] [Google Scholar]
- 49.Feldman HA, Longcope C, Derby CA, Johannes CB, Araujo AB, Coviello AD, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts Male Aging study. J Clin Endocrinol Metab 2002;87:589–98. [DOI] [PubMed] [Google Scholar]
- 50.Araujo AB, Esche GR, Kupelian V, O’Donnell AB, Travison TG, Williams RE, et al. Prevalence of symptomatic androgen deficiency in men. J Clin Endocrinol Metab 2007;92:4241–7. [DOI] [PubMed] [Google Scholar]
- 51.Camacho EM, Huhtaniemi IT, O’Neill TW, Finn JD, Pye SR, Lee DM, et al. Age-associated changes in hypothalamic-pituitary-testicular function in middle-aged and older men are modified by weight change and lifestyle factors: longitudinal results from the European Male Ageing Study. Eur J Endocrinol 2013;168:445–55. [DOI] [PubMed] [Google Scholar]
- 52.Shi Z, Araujo AB, Martin S, O’Loughlin P, Wittert GA. Longitudinal changes in testosterone over five years in community-dwelling men. J Clin Endocrinol Metab 2013;98:3289–97. [DOI] [PubMed] [Google Scholar]
- 53.Hsu B, Cumming RG, Hirani V, Blyth FM, Naganathan V, Le Couteur DG, et al. Temporal trend in androgen status and androgen-sensitive outcomes in older men. J Clin Endocrinol Metab 2016;101:1836–46. [DOI] [PubMed] [Google Scholar]
- 54.Kaufman JM, Lapauw B, Mahmoud A, T’Sjoen G, Huhtaniemi IT. Aging and the male reproductive system. Endocr Rev 2019;40:906–72. [DOI] [PubMed] [Google Scholar]
- 55.Marriott RJ, Murray K, Hankey GJ, Manning L, Dwivedi G, Wu FCW, et al. Longitudinal changes in serum testosterone and sex hormone-binding globulin in men aged 40–69 years from the UK Biobank. Clin Endocrinol (Oxf) 2022;96:589–98. [DOI] [PubMed] [Google Scholar]
- 56.Yeap BB, Manning L, Chubb SAP, Handelsman DJ, Almeida OP, Hankey GJ, et al. Progressive impairment of testicular endocrine function in ageing men: testosterone and dihydrotestosterone decrease, and luteinizing hormone increases, in men transitioning from the 8th to 9th decades of life. Clin Endocrinol (Oxf) 2018;88:88–95. [DOI] [PubMed] [Google Scholar]
- 57.Sartorius G, Spasevska S, Idan A, Turner L, Forbes E, Zamojska A, et al. Serum testosterone, dihydrotestosterone and estradiol concentrations in older men self- reporting very good health: the Healthy Man Study. Clin Endocrinol 2012;77:755–63. [DOI] [PubMed] [Google Scholar]
- 58.Fabbri E, An Y, Gonzalez-Freire M, Zoli M, Maggio M, Studenski SA, et al. Bioavailable testosterone linearly declines over a wide age spectrum in men and women from the Baltimore Longitudinal Study of Aging. J Gerontol A Biol Sci Med Sci 2016;71:1202–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goldman AL, Bhasin S, Wu FCW, Krishna M, Matsumoto AM, Jasuja R. A reappraisal of testosterone’s binding in circulation: physiological and clinical implications. Endocr Rev 2017;38:302–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xia F, Wang N, Han B, Li Q, Chen Y, Zhu C, et al. Hypothalamic-pituitary gonadal axis in aging men and women: Increasing total testosterone in aging men. Neuroendocrinology 2017;104:291–301. [DOI] [PubMed] [Google Scholar]
- 61.Yeap BB, Marriott RJ, Antonio L, Bhasin S, Dobs AS, Dwivedi G, et al. Sociodemographic, lifestyle and medical influences on serum testosterone and sex hormone-binding globulin in men from UK Biobank. Clin Endocrinol (Oxf) 2021;94:290–302. [DOI] [PubMed] [Google Scholar]
- 62.Childs GV, Odle AK, MacNicol MC, MacNicol AM. The importance of leptin to reproduction. Endocrinology 2021;162(2):bqaa204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Van de Velde F, Deventer K, Van Gansbeke W, Van Eenoo P, Van Renterghem P, Fiers T, et al. Metabolism of testosterone during weight loss in men with obesity. J Steroid Biochem Mol Biol 2021;209: 105851. [DOI] [PubMed] [Google Scholar]
- 64.Mohr BA, Bhasin S, Link CL, O’Donnell AB, McKinlay JB. The effect of changes in adiposity on testosterone levels in older men: longitudinal results from the Massachusetts Male Aging Study. Eur J Endocrinol 2006;155:443–52. [DOI] [PubMed] [Google Scholar]
- 65.Travison TG, Araujo AB, Kupelian V, O’Donnell AB, McKinlay JB. The relative contributions of aging, health, and lifestyle factors to serum testosterone decline in men. J Clin Endocrinol Metab 2007;92:549–55. [DOI] [PubMed] [Google Scholar]
- 66.Derby CA, Zilber S, Brambilla D, Morales KH, McKinlay JB. Body mass index, waist circumference and waist to hip ratio and change in sex steroid hormones: the Massachusetts Male Ageing Study. Clin Endocrinol (Oxf) 2006;65:125–33. [DOI] [PubMed] [Google Scholar]
- 67.Tajar A, Huhtaniemi IT, O’Neill TW, Lee DM, Silman AJ, Finn JD, et al. Characteristics of androgen deficiency in late-onset hypogonadism: results from the European Male Aging Study (EMAS). J Clin Endocrinol Metab 2012;97:1508–16. [DOI] [PubMed] [Google Scholar]
- 68.Hsu B, Cumming RG, Blyth FM, Naganathan V, Le Couteur DG, Seibel MJ, et al. Longitudinal and cross-sectional relationships of circulating reproductive hormone levels to self-rated health and health-related quality of life in community-dwelling older men. J Clin Endocrinol Metab 2014;99:1638–47. [DOI] [PubMed] [Google Scholar]
- 69.Liu PY, Pincus SM, Takahashi PY, Roebuck PD, Iranmanesh A, Keenan DM, et al. Aging attenuates both the regularity and joint synchrony of LH and testosterone secretion in normal men: analyses via a model of graded GnRH receptor blockade. Am J Physiol Endocrinol Metab 2006;290:E34–41. [DOI] [PubMed] [Google Scholar]
- 70.Handelsman DJ, Wartofsky L. Requirement for mass spectrometry sex steroid assays in the Journal of Clinical Endocrinology & Metabolism. J Clin Endocrinol Metab 2013;98:3971–3. [DOI] [PubMed] [Google Scholar]
- 71.Jasuja GK, Travison TG, Davda M, Murabito JM, Basaria S, Zhang A, et al. Age trends in estradiol and estrone levels measured using liquid chromatography tandem mass spectrometry in community-dwelling men of the Framingham Heart Study. J Gerontol A Biol Sci Med Sci 2013;68:733–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yeap BB, Alfonso H, Chubb SA, Handelsman DJ, Hankey GJ, Norman PE, Flicker L. Reference ranges and determinants of testosterone, dihydrotestosterone, and estradiol levels measured using liquid chromatography-tandem mass spectrometry in a population-based cohort of older men. J Clin Endocrinol Metab 2012;97:4030–9. [DOI] [PubMed] [Google Scholar]
- 73.Bhasin S, Brito JP, Cunningham GR, Hayes FJ, Hodis HN, Matsumoto AM, et al. Testosterone therapy in men with hypogonadism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2018;103:1715–44. [DOI] [PubMed] [Google Scholar]
- 74.Araujo AB, O’Donnell AB, Brambilla DJ, Simpson WB, Longcope C, Matsumoto AM, et al. Prevalence and incidence of androgen deficiency in middle-aged and older men: estimates from the Massachusetts Male Aging Study. J Clin Endocrinol Metab 2004;89:5920–6. [DOI] [PubMed] [Google Scholar]
- 75.Travison TG, Shackelton R, Araujo AB, Hall SA, Williams RE, Clark RV, et al. The natural history of symptomatic androgen deficiency in men: onset, progression, and spontaneous remission. J Am Geriatr Soc 2008;5:831–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu FC, Tajar A, Beynon JM, Pye SR, Silman AJ, Finn JD, EMAS Group, et al. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med 2010;363:123–35. [DOI] [PubMed] [Google Scholar]
- 77.Eendebak RJAH, Ahern T, Swiecicka A, Pye SR, O’Neill TW, Bartfai G. Elevated luteinizing hormone despite normal testosterone levels in older men-natural history, risk factors and clinical features. Clin Endocrinol (Oxf) 2018;88:479–90. [DOI] [PubMed] [Google Scholar]
- 78.Rastrelli G, Carter EL, Ahern T, Finn JD, Antonio L, O’Neill TW, et al. Development of and recovery from secondary hypogonadism in aging men: Prospective results from the EMAS. J Clin Endocrinol Metab 2015;100:3172–82. [DOI] [PubMed] [Google Scholar]
- 79.Thirumalai A, Anawalt BD. Epidemiology of male hypogonadism. Endocrinol Metab Clin North Am 2022;51:1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yeap BB, Almeida OP, Hyde Z, et al. Healthier lifestyle predicts higher circulating testosterone in older men: the Health In Men Study. Clin Endocrinol (Oxf) 2009;70:455–63. [DOI] [PubMed] [Google Scholar]
- 81.Nieschlag E Late-onset hypogonadism: a concept comes of age. Andrology 2020;8:1506–11. [DOI] [PubMed] [Google Scholar]
- 82.Corona G, Goulis DG, Huhtaniemi I, Zitzmann M, Toppari J, Forti G, et al. European Academy of Andrology (EAA) guidelines on investigation, treatment and monitoring of functional hypogonadism in males: Endorsing organization: European Society of Endocrinology. Andrology 2020;8:970–87. [DOI] [PubMed] [Google Scholar]


