Abstract
Key Points
A collaborative nephrologist–pharmacist telehealth clinic significantly improved difficult-to-control hypertension in patients with CKD.
Reduction in systolic BP was achieved without significant and widespread worsening of renal function or change in electrolytes.
Background
Hypertension (HTN) is the most common chronic health condition worldwide and affects patients with CKD at increasing rates as kidney function falls. Uncontrolled BP can have a significant effect on cardiovascular disease, kidney disease progression, and mortality. We implemented an interdisciplinary team to assess the impact a fully virtual management system, on top of usual nephrology care, could have on HTN control among Veterans Administration patients with difficult to manage HTN.
Methods
Patients with difficult-to-control HTN were referred to a collaborative nephrology telemedicine clinic for care by a nephrologist and a clinical pharmacist. BP was managed through telephone visits conducted by the pharmacist every four to 12 weeks. Patients were sent a home BP monitor, provided education about its use, and were instructed to monitor home BP regularly. Those with at least three phone visits who had objective home BP measurements at each visit were included in the pragmatic analysis. Change in systolic BP from baseline was the primary outcome variable.
Results
Of the 55 patients meeting inclusion criteria, a mean reduction of 16±14 mm Hg in systolic BP and 6±7 mm Hg in diastolic BP was shown. In 12±7 months, 44% of patients achieved goal BP (<130/80) and 31% were discharged back to primary care management in an average of 8±5 months with apparent sustained effect.
Conclusions
An interdisciplinary team of a pharmacist and nephrologist using a virtual care model is an effective method for managing difficult-to-control HTN in this pragmatic assessment.
Keywords: hypertension, CKD, interdisciplinary, telehealth
Introduction
Hypertension (HTN) is the most common chronic disease worldwide and leads to significant morbidity and mortality.1 Despite the grave effect of uncontrolled HTN, achieving ideally controlled BP occurs in just 32%–50% of patients.2 Finding mechanisms to improve control is imperative to improving patient outcomes.3
CKD has significant prevalence in the world's population at approximately 13%–15%.4,5 HTN occurs at increasing rates as the degree of CKD worsens. More than 80% of those with CKD stage 3 have HTN, and this increases to >90% in those with CKD stage 4 or 5.6–8 Intensive BP control can have a positive effect on patients with CKD, reducing both cardiovascular events and all-cause mortality.9
Numerous methods to improve control of BP have been implemented with wide-ranging effects.10–15 A physician–pharmacist collaboration is one effective method to improve BP control over usual care methods. Using this model, one study reduced systolic BP approximately 13 mm Hg compared with a 5 mm Hg reduction with usual management. In addition, the physician–pharmacist intervention group showed an apparent sustained greater effect in BP lowering for at least a 9-month follow-up period.16 The increased acceptance of telecommunication methods to improve BP control has opened opportunities of alternative management methods. Expert opinion suggests that remote BP monitoring with transmission of data, a mechanism to track adherence, and using an interdisciplinary team is an ideal approach when implementing telemedicine to HTN management.17
In this article, we provide a proof-of-concept analysis and significant treatment effect after implementing this HTN management structure at one Veterans Administration (VA) hospital. This work was deemed not human subjects research by the University of Wisconsin Health Sciences Institutional Review Board.
Materials and Methods
In 2018, we established a collaborative nephrology telemedicine HTN clinic between a nephrologist and a clinical pharmacist at the William S. Middleton VA Hospital, Madison, WI. Patients with non–dialysis-dependent CKD and difficult-to-control HTN were referred to the clinic for HTN management between May 2018 and May 2020. Patients with at least three phone visits who had objective home BP measurements (method below) at each visit were included in the analysis (Figure 1). Objective home BPs were defined as numerical BP values recorded by the patient's monitor, written down in a BP log, or numerical BPs uploaded into the medical record from the VA home telehealth system.
Figure 1.
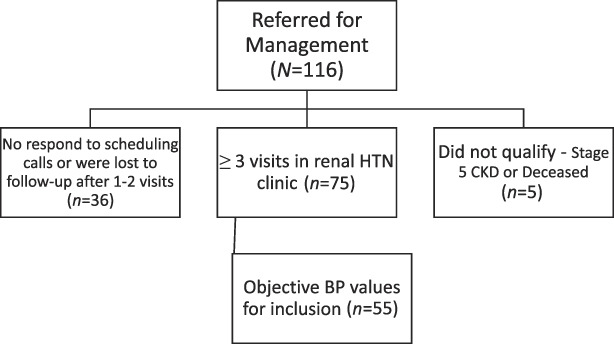
N=116 patients were referred for HTN management over a 2-year period.
Patients were referred to the renal HTN clinic for BP management by either VA nephrologists or primary care teams. The status of a patient's CKD was determined during office visits outside the HTN clinic. If they did not already have one, patients were provided with an upper arm BP monitor from the VA (UA-651-AC; A&D Medical, San Jose, CA) or received one as part of the VA home telehealth program (Commander Flex CD320, Medtronic Care Management Services, LLC; MCMS Omnivisor Pro System, Minneapolis, MN). Standardized review of correct BP measurement technique education16 was provided by the pharmacist and trainees who completed the televisits and was reviewed with all patients whether they previously had or were newly provided a BP monitor. Patients were advised to check their BP once daily and record those values. This clinic was designated to have 4 hours of clinic time per week. Patients were scheduled for 15–30-minute phone appointments with the pharmacist or supervised pharmacy trainee. BP values were communicated in one of three ways: (1) verbally, over the phone; (2) mailed in BP paper logs; or (3) uploaded over phone or digitally using the home telehealth service and then uploaded directly into the electronic medical record before each appointment. To have a visit included for analysis, a minimum of five BP readings since the last visit were required. Patient visits took place every 4–8 weeks depending on the severity of BP elevation. Patient data were not included if only subjective reports of BP were provided. Only patients with either systolic BP (SBP) ≥130 mm Hg or diastolic BP (DBP) ≥80 mm Hg at baseline were included in this analysis. The usual care provided by nephrologists remained unchanged for patients referred for HTN management. HTN visits with the pharmacist were added between regular nephrology visits, and more touchpoints accompanied the usual care performed by the nephrologist. Given that those with CKD 5 were sent outside the VA after dialysis was initiated (usual protocol), we excluded these patients from our analysis.
On the basis of reported BPs, the pharmacist adjusted BP medications to achieve BP goals <130/80 mm Hg for patients with CKD according to the 2017 American College of Cardiology/American Heart Association (ACC/AHA) HTN guidelines.18 In the VA system, pharmacists can prescribe medications and order laboratory tests under an approved scope of practice with a supervising physician. For the first 6 months, all notes and medication changes were approved by the clinic nephrologist. After that period, the pharmacist enacted medication changes largely independently, within the approved scope of practice and with nephrologist consultation when needed. The pharmacist's scope of practice was limited to HTN and factors related to BP medications (e.g., hypokalemia/hyperkalemia, potassium binders, and potassium supplements).
Mean BP values were calculated from home readings at each visit. Data are expressed as mean±SD, median (interquartile range), or percentages. The primary outcome (change in SBP from initial to last visit) was calculated by subtracting the initial visit from the last visit SBP and was analyzed using the Mann–Whitney test (Prism Graphpad v 9.0, San Diego, CA). The percent of patients who achieved BP <130/80 mm Hg and experienced 20, 15 and 10 mm Hg reductions in SBP over that time interval was also calculated. In addition, the percent of patients with SBP ≥150 mm Hg, 140–149 mm Hg, 130–139 mm Hg, and <130 mm Hg was calculated for the initial and last clinic visit. The mean number of antihypertensive medications at baseline and at the final patient visit was tabulated as well as the mean number of medication changes made during clinical management. Patients were discharged from clinic if they had two visits with average BP <130/80 separated by at least eight weeks. Follow-up data on discharged patients were collected using the last office BP measurement and compared with the discharge office BP to estimate consistency in control after clinic discharge.
Results
Over a two-year period, 116 patients were referred to the HTN clinic for management (Figure 1). Of these, 36 patients did not respond to initial scheduling attempts or did not respond to follow-up scheduling after their first or second visit. An additional five patients were referred to clinic but either had stage 5 CKD (not yet on dialysis) (n=4) at the time of referral or passed away (n=1) before being seen in clinic. There were 75 patients who completed at least one visit, and 55 met inclusion for analysis (Table 1). The most likely reason for not meeting inclusion criteria was lack of objective home BP values. In alignment with the population at our single-center VA hospital, 98% of patients were older White men. The mean age of patients was 78±7 years, and the mean body mass index was 31±6 kg/m2. Most (78%) patients had proteinuria (urinary protein:creatinine ratio ≥200 mg/g) with a mean urinary protein to creatinine ratio of 1231±1666 mg/g. On average, patients were taking three or more antihypertensive medications at the initial clinic visit, had CKD stage 3b, and had a baseline SBP of 150 mm Hg (Table 1).
Table 1.
Patient characteristics and demographics at initial visit, last visit, and after clinic discharge
| Variable | Initial Visit (n=55) | Last Visit (n=55) | Postclinic Discharge (n=13) |
|---|---|---|---|
| eGFR, (mL/min per 1.73 m2) | 38±14 | 37±16 | 40±18 |
| Serum potassium (mmol/L) | 4.4±0.5 | 4.3±0.4 | 4.2±0.4 |
| No. of antihypertensive medications | 4 (3,5) | 4 (3,5) | 3 (3,5) |
| Patients taking ≥3 antihypertensive medications (%) | 78 | 89 | 85 |
| SBP (mm Hg) | 150±12 | 134±14 | 133±10a |
| DBP (mm Hg) | 73±10 | 66±9 | 70±5a |
All data are presented as mean±SD, median (IQR), or N (%). IQR, interquartile range; SBP, systolic BP; DBP, diastolic BP.
Office BP values as patient did not have home BP values after discharge from clinic.
Of the 55 patients included in the analysis, 46% had an initial average home SBP ≥150 mm Hg, 33% had SBP between 140 and 149 mm Hg, and 21% had SBP between 130 and 139 mm Hg. The mean follow-up period from baseline to the last visit was 12±7 months, and both home SBP (P < 0.0001) and DBP (P < 0.001) were significantly reduced over the management period compared with baseline (Figure 2). Table 2 presents the percentage of patients taking individual classes of antihypertensive medications at the initial and final visits. As expected, the use of each class of medications increased from the initial visit. At the conclusion of the period for analysis, the percent of patients with SBP ≥150 mm Hg and 140–149 mm Hg was significantly lower, whereas the percent of patients with SBP 130–139 mm Hg and <130 mm Hg increased (Figure 3). Fewer patients had SBP ≥150 (P < 0.0002) and between 140 and 149 mm Hg (P = 0.02) at the last visit compared with the initial visit. Two thirds of patients experienced at least a 10 mm Hg reduction in SBP between initial and the last clinic visit (Figure 4). The mean changes in SBP and DBP between initial and final visit were −16±14 and −6±7 mm Hg, respectively. The mean change in SBP per clinic telehealth visit was −2.4±2.2 mm Hg per visit, while the mean change in SBP per medication change was ‐4.4±6.9 mm Hg. At the time of analysis, 24 (44%) patients had average home BP <130/80 mm Hg. Seventeen of those were discharged from clinic. The other seven patients were awaiting their second visit to meet discharge criteria (Table 3).
Figure 2.
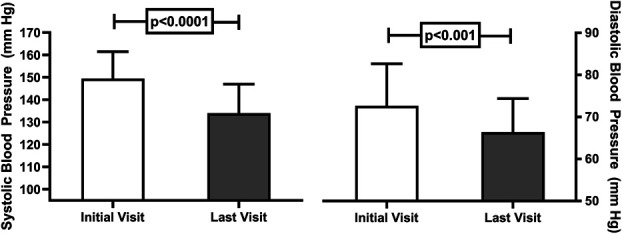
Systolic (left panel) and diastolic (right panel) BP at the initial telehealth visit (open bars) and last visit (gray bars). SBP (P < 0.0001) and DBP (P < 0.001) were significantly reduced at the last visit compared with initial visit.
Table 2.
Number and percentage of patients taking classes of antihypertensive medications across visits
| Medication Class | Initial Visit n (%) |
Last Visit n (%) |
Increase in Medication Use % |
|---|---|---|---|
| Calcium channel blocker | 38 (69) | 43 (78) | 9 |
| Beta-blocker | 39 (71) | 42 (76) | 5 |
| ACE-I or ARB | 36 (65) | 40 (73) | 8 |
| Loop diuretic | 19 (35) | 21 (38) | 3 |
| Hydralazine | 17 (31) | 22 (40) | 9 |
| Thiazide diuretic | 14 (25) | 16 (29) | 4 |
| α-1 antagonist | 8 (15) | 13 (24) | 9 |
| Aldosterone antagonist | 9 (16) | 12 (22) | 6 |
| Nitrate | 8 (15) | 8 (15) | 0 |
| α-2 agonist | (7) 13 | 11 (21) | 8 |
ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Figure 3.
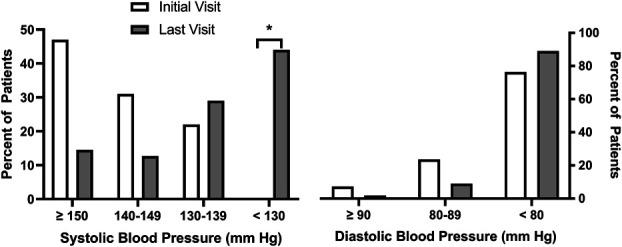
Percent of patients with SBP ≥150 mm Hg, between 140 and 149 mm Hg, 130–139 mm Hg, and <130 mm Hg at initial visit (open bars) and last visit (gray bars) (left panel). In the right panel is percent of patients with DBP ≥90 mm Hg, between 80 and 89 mm Hg, and <80 mm Hg at initial visit (open bars) and final visit (gray bars). *Zero patients had SBP <130 mm Hg at initial visit, and 44% of patients had SBP <130 mm Hg at last visit.
Figure 4.
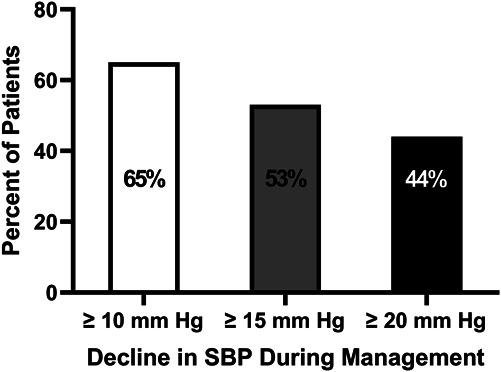
Percent of patients who experienced at least 10 mm Hg (open bar), 15 mm Hg (gray bar), and 20 mm Hg (dark bar) reduction in SBP from initial to last visit.
Table 3.
Summary of BP changes and clinic statistics
| Achieved BP Goal <130/80 mm Hg (%) | 44 |
| No. of medication changes made | 5 (2–10) |
| Follow-up period (mo) | 12±7 |
| Follow-up visits (No.) | 7 (4–9) |
| Discharged back to referring team (%) | 31 |
| Time to clinic discharge (mo) | 8±5 |
| Change in SBP in 12 mo (mm Hg) | −16±4 |
| Change in SBP per visit (mm Hg) | −2.4±2.2 |
| Change in SBP per medication change (mm Hg) | −4.4±6.9 |
All data are presented as mean±SD, median (IQR), or percent. IQR, interquartile range; SBP, systolic BP.
Despite significant medication changes including an increase in renin–angiotensin–aldosterone blockade, serum potassium was not changed from baseline to the last follow-up visit (4.4 to 4.3 mEq/L) (P = 0.33). Serum creatinine showed significant increase from baseline to the last follow-up visit (1.91 to 2.05 mg/dl) (P = 0.01), but eGFR did not significantly change between baseline and the final follow-up visit (38 to 37 ml/min per 1.73 m2) (P = 0.54). No patients progressed to doubling of serum creatinine or needing dialysis over the period of this study.
Of the 17 patients discharged from clinic, follow-up office BP data were available for 13 patients. The mean office systolic BP at clinic discharge (134±13 mm Hg) was not different compared with the average office systolic BP 12±7 months after clinic discharge (133±10 mm Hg) (P = 0.94 Mann–Whitney). DBP was similar at clinic discharge (70±8 mm Hg) compared with the average office DBP after clinic discharge (70±5 mm Hg) (P = 0.88). Data were unavailable for seven patients who did not have any VA office visits conducted after clinic discharge likely because of the lack of in-person office visits due to coronavirus disease 2019 (COVID-19) constraints.
Discussion
The collaborative pharmacist–physician telehealth HTN clinic implemented at a single VA hospital for patients with difficult-to-control HTN was shown to have a positive effect on BP control. Reduction in SBP over an average of 12 months of follow-up was ∼16 mm Hg, and 44% of patients reached ACC/AHA recommended BP goals (<130/80 mm Hg) at 2 years after implementation. Furthermore, analysis of persistence of BP control in a subset showed that reductions in BP were maintained for 12±7 months after clinic discharge (P = 0.94) by review of office readings. The reduction in BP observed is particularly encouraging considering that the referred patients with CKD had difficult-to-control HTN (uncontrolled despite ≥3 medications).
Most patients were able to successfully monitor and report home BP for analysis, but in those who could not, the availability of VA issued telemonitoring equipment was an option to broaden compliance. In addition, BP lowering was achieved and maintained after discharge from the clinic over 12±7 months, suggesting that HTN is a medical condition that is particularly amenable to telehealth management. Interestingly, despite a median of 5 medication changes made during management, at the last visit, patients were taking same median number of total BP medications and BP control was improved. Thus, it seems that patients were changed to more appropriate BP medications for clinical circumstances that effectively lowered BP. Of note, during the COVID-19 pandemic, telehealth endeavors have grown and measuring their effectiveness for managing chronic diseases is more important than ever to determine which conditions are best suited for this type of clinical intervention.
Although speculative, we attribute the reduction in BP to collaborative medication intensifications and more frequent visits enabling frequent monitoring and medication titrations. Telehealth visits minimized provider scheduling and geographic and economic factors that ordinarily present obstacles to frequent visits. The collaborative nature of the team was essential to successfully lowering BP. The nephrologist's role was to combine clinical aspects that affect BP and how those related to mechanisms of action of the medications in collaboration with the pharmacist. The nephrologist also ensured that appropriate patients who were suitable for management in the clinic were referred to the clinic for care and was available for collaborative conversations for medication selection, titration, and HTN workup.
HTN is a medical condition where team-based care leverages skills of different practitioners to assist patients in achieving goals of therapy. Several previous studies have published mixed results regarding the effects of pharmacist involvement in HTN care.16,19–23 Of these, two trials exclusively studied patients with CKD using office BP measurements. In one study, pharmacists did not control the adjustment of antihypertensive medications, and the pharmacist intervention did not lower SBP compared with the control group.22 In the second, Chang et al. studied the effect of medication therapy management interventions by pharmacists in patients with CKD stage 3a. Pharmacists did adjust antihypertensive medications, but BP control rates were similar between intervention and control patients.23 Our study is unique in that we exclusively used telehealth (pharmacist–patient) communications coupled with patient-measured home BP monitoring. This method more closely aligns with the ideal ambulatory BP monitoring and empowers patients to be part of the care team.24,25 Thus, this study provides data on the effectiveness of a collaborative nephrology telehealth-based HTN service partnering with patients to measure BP at home. Given the increased use and importance of telehealth modalities, our findings are timely and relevant, suggesting collaborative BP management using telehealth can positively affect BP control in patients with CKD with difficult-to-control HTN.
The described telehealth clinical service can be adapted to a health system that allows pharmacists to collaboratively manage HTN in patients within an approved scope of practice or under a collaborative practice agreement. Modifications to this approach would be appropriate to individualize the fit and functionality for different health systems and institutions. Referral to our clinical service came primarily from the VA-based nephrology group. A small number of patients were referred by primary care for difficult to manage HTN. Although the pharmacist made most of the medication adjustments, collaboration between the nephrologist and pharmacist through weekly in-clinic discussions was essential in effective management. Team-based care and coordination between pharmacist and nephrologist ensured that appropriate care for HTN, CKD, and other medical conditions was delivered. The described clinic model worked effectively with the inclusion of pharmacist trainees (students and residents). A single supervisor staffed all visits which included about half of the visits as a supervisor to pharmacist trainees. Thus, there was consistency in decision-making and intervention.
Strengths of our study include a selection bias toward a clinic population with difficult-to-control HTN as 77% of patients were taking three or more medications at the time of referral. Furthermore, one pharmacist was the consistent care provider and made decisions on BP medications, limiting variability between practice patterns. In addition, scheduling permitted the pharmacist to address any HTN-related patient care issues between nephrology visits, thus decreasing both the burden of in-basket messages and follow-up visits with the nephrologist. There was a relatively small financial commitment for the half day per week of work provided from the pharmacist. Finally, patients were supplied consistent BP monitors free of charge from the VA.
There are limitations to our study. First, this analysis is a pragmatic assessment of the effectiveness of our clinic as implemented in real-world conditions; therefore, our sample size was small as clinic time was limited due to this being a newly initiated clinic. We did not have a control group for comparison because those in the nephrology clinic alone did not have sufficient objective BP measurements recorded. Analysis of the BP control of patients before clinic referral was not a suitable comparison as there was a change in recommended BP treatment goals (from <140/90 to <130/80 mm Hg) by the ACC/AHA guidelines a few months before clinic implementation.18 Second, to assess BP control, patients were required to be adherent with home self-monitoring of BP and report objective measurements at each visit. Thus, the resulting data come from patients who are adherent to clinic recommendations and monitoring requirements. This creates a selection bias for adherent patients despite our ability to provide a wide range of equipment free of charge. Caution should be used when extrapolating the BP changes to all patients with CKD and HTN, but it does support the effect of patient engagement in BP control. Third, our follow-up duration did not provide sufficient time to measure the effect of BP management on renal function, cardiovascular, or mortality outcomes. Fourth, the study population consisted largely of older, White male veterans, which limits generalizability to broader populations outside the VA health care system. Fifth, education about BP measurement technique was standardized for all patients. Most patients already had a BP monitor when referred for care, but standardized education was reviewed for each patient. Patients did not visually demonstrate BP measurement technique, but correct technique was repeatedly reviewed, and patients were treated on the basis of home measurements. Finally, persistence of BP control is based on office BP levels instead of home readings, which is a different method than what was used for titration and monitoring; hence, comparison should be made with caution.
Almost all patients with CKD will develop HTN, increasing the risk for cardiovascular events and progression of CKD.26,27 A subset of patients with CKD has HTN that is difficult to control, necessitating creative approaches for HTN management. Our pragmatic analysis of a collaborative nephrology telemedicine HTN clinic with a nephrologist, a clinical pharmacist, and trainees demonstrates significant reduction of SBP by approximately 16 mm Hg over a 1-year period with at least 44% of patients reaching recommended BP goals (<130/80 mm Hg). This model can be implemented and adapted for any health system to provide improved HTN care and decrease the care burden placed on nephrologists.
Footnotes
See related editorial, “Telehealth and Management Support for Hypertension in CKD: Time to Raise the Bar,” on pages 722–724.
Disclosures
J.M. Dopp has a speaker's contract with Idorsia Pharmaceuticals and completed an advisory board with Vifor Pharmaceuticals. L. Maursetter reports the following: Honoraria: ASN for BRCU. The remaining author has nothing to disclose.
Funding
None.
Author Contributions
Conceptualization: John M. Dopp, Laura Maursetter.
Data curation: Austin Lange, Laura Maursetter.
Formal analysis: John M. Dopp, Austin Lange, Laura Maursetter.
Investigation: John M. Dopp, Laura Maursetter.
Methodology: John M. Dopp, Austin Lange, Laura Maursetter.
Project administration: John M. Dopp, Laura Maursetter.
Resources: John M. Dopp, Laura Maursetter.
Supervision: John M. Dopp, Laura Maursetter.
Writing - original draft: John M. Dopp, Austin Lange, Laura Maursetter.
Writing – review & editing: John M. Dopp, Austin Lange, Laura Maursetter.
References
- 1.Lamirault G, Artifoni M, Daniel M, Barber-Chamoux N; Nantes University Hospital Working Group on Hypertension. Resistant hypertension: novel insights. Curr Hypertens Rev. 2020;16(1):61–72. doi: 10.2174/1573402115666191011111402 [DOI] [PubMed] [Google Scholar]
- 2.Fryar C, Ostchega Y, Hales C, Zhang G, Kruszon-Moran D. Hypertension prevalence and control among adults: United States, 2015-2016. NCHS Data Brief. 2017:(289):1–8. [PubMed] [Google Scholar]
- 3.Cooper-DeHoff RM Gong Y Handberg EM, et al. Tight blood pressure control and cardiovascular outcomes among hypertensive patients with diabetes and coronary artery disease. JAMA. 2010;304(1):61–68. doi: 10.1001/jama.2010.884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horowitz B, Miskulin D, Zager P. Epidemiology of hypertension in CKD. Adv Chronic Kidney Dis. 2015;22(2):88–95. doi: 10.1053/j.ackd.2014.09.004 [DOI] [PubMed] [Google Scholar]
- 5.Braden GL Chapman A Ellison DH, et al. Advancing nephrology: division leaders advise ASN. Clin J Am Soc Nephrol. 2021;16(2):319–327. doi: 10.2215/CJN.01550220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muntner P Anderson A Charleston J, et al. Hypertension awareness, treatment, and control in adults with CKD: results from the chronic renal insufficiency cohort (CRIC) study. Am J Kidney Dis. 2010;55(3):441–451. doi: 10.1053/j.ajkd.2009.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rao MV, Qiu Y, Wang C, Bakris G. Hypertension and CKD: kidney early evaluation program (KEEP) and national health and nutrition examination survey (NHANES), 1999-2004. Am J Kidney Dis. 2008;51(4):S30–S37. doi: 10.1053/j.ajkd.2007.12.012 [DOI] [PubMed] [Google Scholar]
- 8.Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function--measured and estimated glomerular filtration rate. N Engl J Med. 2006;354(23):2473–2483. doi: 10.1056/nejmra054415 [DOI] [PubMed] [Google Scholar]
- 9.Cheung AK Rahman M Reboussin DM, et al. Effects of intensive BP control in CKD. J Am Soc Nephrol. 2017;28(9):2812–2823. doi: 10.1681/ASN.2017020148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Unda Villafuerte F Llobera Cànaves J Lorente Montalvo P, et al. Effectiveness of a multifactorial intervention, consisting of self-management of antihypertensive medication, self-measurement of blood pressure, hypocaloric and low sodium diet, and physical exercise, in patients with uncontrolled hypertension taking 2 or more antihypertensive drugs: the MEDICHY study. Medicine (Baltimore). 2020;99(17):e19769. doi: 10.1097/md.0000000000019769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carlson DJ, Dieberg G, Hess NC, Millar PJ, Smart NA. Isometric exercise training for blood pressure management: a systematic review and meta-analysis. Mayo Clinic Proc. 2014;89(3):327–334. doi: 10.1016/j.mayocp.2013.10.030 [DOI] [PubMed] [Google Scholar]
- 12.Carey RM, Muntner P, Bosworth HB, Whelton PK. Prevention and control of hypertension: JACC health promotion series. J Am Coll Cardiol. 2018;72(11):1278–1293. doi: 10.1016/j.jacc.2018.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chmielewski J, Carmody JB. Dietary sodium, dietary potassium, and systolic blood pressure in US adolescents. J Clin Hypertens (Greenwich). 2017;19(9):904–909. doi: 10.1111/jch.13014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burnier M, Egan BM. Adherence in hypertension. Circ Res. 2019;124(7):1124–1140. doi: 10.1161/circresaha.118.313220 [DOI] [PubMed] [Google Scholar]
- 15.Cramer H, Sellin C, Schumann D, Dobos G. Yoga in arterial hypertension. Dtsch Arztebl Int. 2018;115(50):833–839. doi: 10.3238/arztebl.2018.0833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderegg MD Gums TH Uribe L, et al. Pharmacist intervention for blood pressure control in patients with diabetes and/or chronic kidney disease. Pharmacotherapy. 2018;38(3):309–318. doi: 10.1002/phar.2083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Omboni S McManus RJ Bosworth HB, et al. Evidence and recommendations on the use of telemedicine for the management of arterial hypertension: an international expert position paper. Hypertension. 2020;76(5):1368–1383. doi: 10.1161/hypertensionaha.120.15873 [DOI] [PubMed] [Google Scholar]
- 18.Whelton PK Carey RM Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Hypertension. 2018;71(6):e13–e115. doi: 10.1161/HYP.0000000000000065 [DOI] [PubMed] [Google Scholar]
- 19.Magid DJ, Olson KL, Billups SJ, Wagner NM, Lyons EE, Kroner BA. A pharmacist-led, American Heart Association Heart360 Web-enabled home blood pressure monitoring program. Circ Cardiovasc Qual Outcomes. 2013;6(2):157–163. doi: 10.1161/circoutcomes.112.968172 [DOI] [PubMed] [Google Scholar]
- 20.Margolis KL Asche SE Bergdall AR, et al. Effect of home blood pressure telemonitoring and pharmacist management on blood pressure control: a cluster randomized clinical trial. JAMA. 2013;310(1):46–56. doi: 10.1001/jama.2013.6549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carter BL Coffey CS Ardery G, et al. Cluster-randomized trial of a physician/pharmacist collaborative model to improve blood pressure control. Circ Cardiovasc Qual Outcomes. 2015;8(3):235–243. doi: 10.1161/circoutcomes.114.001283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cooney D Moon H Liu Y, et al. A pharmacist based intervention to improve the care of patients with CKD: a pragmatic, randomized, controlled trial. BMC Nephrol. 2015;16(1):56. doi: 10.1186/s12882-015-0052-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang AR Evans M Yule C, et al. Using pharmacists to improve risk stratification and management of stage 3A chronic kidney disease: a feasibility study. BMC Nephrol. 2016;17(1):168. doi: 10.1186/s12882-016-0383-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Brien E, Parati G, Stergiou G. Ambulatory blood pressure measurement: what is the international consensus? Hypertension. 2013;62(6):988–994. doi: 10.1161/hypertensionaha.113.02148 [DOI] [PubMed] [Google Scholar]
- 25.Zhao J Hu Y Zhang X, et al. Efficacy of empowerment strategies for patients with hypertension: a systematic review and meta-analysis. Patient Educ Couns. 2020;103(5):898–907. doi: 10.1016/j.pec.2019.11.025 [DOI] [PubMed] [Google Scholar]
- 26.Gansevoort RT Correa-Rotter R Hemmelgarn BR, et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet. 2013;382(9889):339–352. doi: 10.1016/s0140-6736(13)60595-4 [DOI] [PubMed] [Google Scholar]
- 27.Pugh D, Gallacher PJ, Dhaun N. Management of hypertension in chronic kidney disease. Drugs. 2019;79(4):365–379. doi: 10.1007/s40265-019-1064-1 [DOI] [PMC free article] [PubMed] [Google Scholar]


