Abstract
Introduction
Cell processing operators (CPOs) use a variety of disinfectants that vaporize in the workspace environment. These disinfectants can induce allergic reactions in CPOs, due to their long working hours at cell processing facilities (CPFs). Ionic substances such as CH3COO− generated from peracetic acid, nitrogen oxides (NOx) and sulfur oxides (SOx) from outdoor environment are also known to pollute air. Therefore, our objective was to assess the air quality in CPFs and detect volatile organic compounds (VOCs) from disinfectants and building materials, and airborne ionic substances from outdoor air.
Methods
Sampling was conducted at three CPFs: two located in medical institutions and one located at a different institution. Air samples were collected using a flow pump. Ion chromatographic analysis of the anionic and cationic compounds was performed. For VOC analysis, a thermal desorption analyzer coupled with capillary gas chromatograph and flame ionization detector was used.
Results
Analysis of the ionic substances showed that Cl−, NOx, and SOx, which were detected in large amounts in the outdoor air, were relatively less in the CPFs. Ethanol was detected as the main component in the VOC analysis. Toluene was detected at all sampling points. As compared to the other environments, air in the incubator contained larger amounts of VOCs, that included siloxane, tetradecane, and aromatics.
Conclusions
No VOCs or ionic substances of immediate concern to the health of the CPOs were detected during the non-operating period. However, new clinical trials of cell products are currently underway in Japan, and a variety of new cell products are expected to be approved. With an increase in cell processing, health risks to CPOs that have not been considered previously, may become apparent. We should continue to prepare for the future expansion of the industry using a scientific approach to collect various pieces of information and make it publicly available to build a database.
Keywords: Disinfectant odor, Cell processing operators, Volatile organic compounds (VOCs), Ionic substances, Exposure assessment, Air quality
Highlights
-
•
Air quality in cell-processing facilities was assessed for the first time.
-
•
Volatile organic compounds from disinfectants are toxic to humans.
-
•
The main VOC was ethanol, some other VOCs exceeded MHLW's advisable value.
-
•
No toxic ionic substances were detected in the processing area.
-
•
Various VOCs, such as siloxanes and aromatics, were detected in the incubator.
Abbreviations
- BSCs
biosafety cabinets
- CPOs
cell processing operators
- CPFs
cell processing facilities
- FID
Flame ionization detector
- GC
gas chromatograph
- IC
Ion chromatograph
- Inc
incubator
- NOx
nitrogen oxides
- MHLW
Ministry of health labor and welfare
- MS
mass Mass spectrometer
- SOx
sulfur oxides
- TD
thermal desorption analyzer
- UA
uncontrolled areas
- VOCs
volatile organic compounds
1. Introduction
For the processing of cell products, that cannot be sterilized, it is important to guarantee sterility during the process. To achieve this, cell products must be protected from several risks, including bacteria present in cell-processing facilities [[1], [2], [3], [4]] and from bacteria and fungi in raw materials [5,6]. In addition, residues such as culture fluid droplets may be present in biosafety cabinets (BSCs), and it is essential to prevent cross-contamination [7,8]. Because of this unique environment, cell processing operators (CPOs), who use a variety of disinfectants such as alcohol, hypochlorous acid, hydrogen peroxide, and peracetic acid, may be exposed to these agents. Thus, there are concerns that excessive use of disinfectants to prevent cell contamination can lead to health hazards. Moreover, residues of hydrogen peroxide used as disinfectants can affect the culture cells [9]. Therefore, investigating the air quality is necessary.
Several CPOs working in these environments are affected by the odors of disinfectants [10]. In terms of health, it has been reported that healthcare workers in hospitals, that have similar environments due to frequent use of disinfectants, show more indoor air-related problems than those working in office buildings [11,12]. Disinfectants used in cell processing facilities can induce allergic reactions such as asthma and contact dermatitis upon exposure [[13], [14], [15], [16], [17]]. Alcohol-based hand disinfectants are commonly used in manufacturing to reduce the contamination of cell products. During hand sanitization, users are exposed to a rapid increase in ethanol concentration over a short period of time [18,19]. In cell-processing facilities (CPFs), as in other buildings, the indoor air quality can be affected by the emission of volatile organic compounds (VOCs) from building materials [20,21]. Furthermore, exposure to VOCs such as aromatics represented by BTX (benzene, toluene, and xylene), aliphatic compounds, and aldehydes used in these building materials is of particular concern because of their potentially harmful effects on human health [[22], [23], [24]]. Ionic substances, such as CH3COO− generated from peracetic acid used in decontaminants, and nitrogen oxides (NOx) and sulfur oxides (SOx) from outdoor environment, are also known to affect the air quality [25,26]. For example, NO3− and SO42− are the main contributors to urban air pollution [27], and urban enter CPFs during outdoor air intake. However, data on the air contamination in CPFs are unknown.
Although the environment and hands must be disinfected regularly to process the cell products, the specific nature of the activities, such as disinfection and decontamination, can cause air contamination. In addition, CPOs often work in cleanroom environments for long periods, increasing their exposure. Because there are several types of equipment installed inside CPFs than in a typical office or hospital setup, these equipment may also act as sources of air contamination. Furthermore, the CPF circulates most of its internal air to maintain clean air, which can make the environment susceptible to residual contaminants from outside, building materials or equipment. However, there is a lack of knowledge regarding the nature and concentration of these induced air pollutants. Therefore, this study mainly aims to assess the quality of airborne ionic substances and VOC contamination in CPFs to prevent exposure to CPOs.
2. Materials and methods
2.1. Overview of monitored facilities
In the current study, sampling was conducted at Facility A, located within a hospital, Facility B, located in a healthcare organization, both of which were established in March 2015. Sampling was also conducted Facility C, which was established in March 2017. Each sampling point was divided into controlled and uncontrolled areas (UA). The controlled area was divided into four categories (Grade D, Grade C, Grade B, and Grade A) in accordance with the definition of cleanliness in "Consideration of Aseptic Manipulation in Cell Culture Processing Facilities" based on the "Safety Law" published by the Japanese Society for Regenerative Medicine. Grade A was the area where aseptic cell processing was performed. A summary map of each CPF is shown in Fig. 1A and B, and C.
Fig. 1.
Summary map of sampling locations. (A, B and C) Schematic diagram of facilities A, B, and C. Each color represents cleanliness grade definition and environment. Classification for comparison analysis is divided into outside-CPF group (n = 5), inside-CPF group (n = 14), and equipment-related group (n = 7). Inc: Incubator, BSCs: Biosafety cabinets. (D) The photo shows air sampling. Air flow rate for VOC 0.5 L/min for 20 min and for ions 1.0 L/min for 12 h.
The data for each facility are graphically shown as the mean of the values for each group as follows: AB1 and C11 were defined as Outdoor, located outside CPF; A1 was defined as a clean, non-classified, and uncontrolled area located within CPF; B1, C1 were defined as UA; AB2 was defined as Laboratory located outside CPF; A2, B2 were defined as Grade D; A3, A4, B3, C2, and C3 were defined as Grade C; and A5, B4, C4, C5, C6, and C7 were defined as Grade B. The values of the incubator (Inc.) were taken from A6, B5, C8, and C9, and the values of the BSCs were taken from A7, B6, and C11 (Fig. 1).
For the comparative analysis, the subjects were classified into three groups: outside CPF, inside CPF, and equipment-related. The outside-CPF group, is an uncontrolled area that includes outdoors and laboratory, and consists of five locations. The inside-CPF group consisted of 14 locations including Grades D, C, and B as environments where CPOs could work for long durations, and the equipment-related group consisted of 7 locations including BSCs and incubators installed in the CPF. The manufacturers, installation dates, time to measure and model numbers of the incubators and safety cabinets classified in the equipment-related group are listed in Table S1.
2.2. Sampling
Air samples were actively collected using a flow pump (MP-Σ100NHII and MP-Σ300NII, Sibata Scientific Technology Ltd, Tokyo, Japan). For VOCs, air samples were collected using a glass thermal desorption tube packed with a Tenax GR (Camsco, TX, USA) with a pumping flow rate of 0.5 L/min for 20 min. Before sampling, sample tube conditioners (STC-4000, GL Sciences Inc., Tokyo, Japan) were preconditioned by heating at 300 °C for 1 h with helium gas at a flow rate of 30 mL/min. For ions, air samples were collected using a hand-made polypropylene impinger containing deionized water (18.2 MΩ cm) supplied by Milli-Q IQ7010 (Merck Millipore Corporation, MA, USA) with a pumping flow rate of 1.0 L/min for 12 h (Fig. 1D).
Sampling was conducted during non-operating period termed as “at rest”. “At-rest” cleanroom is defined in ISO 14644 as a cleanroom that is complete, functional and ready for operation, with the equipment inside, but without the personnel. Hand disinfection and floor wiping with ethanol spray were performed when entering and exiting the sampling equipment installation. Sampling at Facilities A and B was conducted on the same days (October 20–21, 2022), and sampling at Facility C was conducted on May 9, 2017. Analyses were conducted within one day of sampling.
2.3. Ion analysis
Standard solutions of anionic compounds such as fluorides, acetates, formates, chlorides, nitrites, bromides, nitrates, phosphates, and sulfates, and cationic compounds such as lithium, sodium, ammonium, potassium, calcium, and magnesium were obtained from Kanto Chemical Co., Inc. (Tokyo, Japan). Ion chromatograph (IC) analysis of anionic and cationic compounds was performed using an IC coupled with an automatic eluent generator, concentrator column, guard column, analytical column, suppressor, and conductivity detector (Thermo Fisher Scientific Inc., MA, USA). The analytical conditions for the anions and cations are presented in Table 1.
Table 1.
Analysis conditions of anions and cations.
|
IC |
Anions |
Cations |
|---|---|---|
| Dionex ICS-5000 | Dionex Integrion RFIC | |
| Injection volume | 0.050 mL | 0.36 mL |
| Eluent | Potassium hydroxide | Methanesulfonic acid |
| Flow rate of eluent | 0.012 mL/min | 0.36 mL/min |
| Concentration profile of eluent | 6 mM for 10 min, | 30 mM |
| 4 mM/min up to 30 mM, | ||
| 1.4 mM/min up to 50 mM, | ||
| 3.3 mM/min up to 60 mM, | ||
| 60 mM for 7 min | ||
| Concentrator column | Dionex IonSwift MAC-200 (0.75 mm × 80 mm) | Dionex IonPac TCC-LP1 (4 mm × 35 mm) |
| Guard column | Dionex IonPac AG15 (0.4 mm × 50 mm) | Dionex IonPac CG16 (3 mm × 50 mm) |
| Analytical column | Dionex IonPac AS15 (0.4 mm × 250 mm) | Dionex IonPac CS16 (3 mm × 250 mm) |
| Column temperature | 30 °C | 40 °C |
| Suppressor | ACES | CERS_2 mm |
| Electrical current | 13 mA | 32 mA |
| Detector temperature | 35 °C | 35 °C |
| Carbonate removal device | CRD-200 |
2.4. VOC analysis
Standard solutions of VOCs such as toluene, ethylbenzene, o-xylene, m-xylene, p-xylene, styrene, p-dichlorobenzene, and tetradecane were obtained from Fujifilm Wako Pure Chemical Corporation (Osaka, Japan). TD/GC/FID analysis was performed using a thermal desorption (TD) analyzer (TD-20, Shimadzu Corporation, Kyoto, Japan) coupled with a capillary gas chromatograph (GC) and a flame ionization detector (FID; GC-2010 Plus, Shimadzu Corporation, Kyoto, Japan). The concentration of total VOCs (TVOC) was estimated, as a toluene-equivalent value (μg・Tol/m3) using an external standard calibration method, based on the areas of the detected peaks with their retention times longer than that of n-hexane and earlier than that of n-hexadecane, as defined in ISO 16000–6 (2011). The concentration of all of VOCs (TVOC-all) was estimated likewise based on all the detected peaks. The concentration of VOCs in the standard solution was estimated using an external standard calibration method based on the areas of the detected peaks. The xylene concentration was estimated as the total amount of its isomers (o-, m-, p-xylene).
TD/GC/MS analysis was performed using a TD analyzer (TD-30R, Shimadzu Corporation, Kyoto, Japan) coupled with a capillary GC and a quadrupole mass spectrometer (MS) as a detector (GCMS-QP2020, Shimadzu Corporation, Kyoto, Japan). Mass scanning in electron impact mode was conducted in the range of 30–450 m/z at a rate of 909 scans/s. The mass spectra were compared with the National Institute of Standards and Technology database for compound identification. The compounds with a similarity index of 90% or more were used for further analyses. The concentration of VOCs, such as ethanol, eucalyptol, nonanal, and siloxane was estimated as a toluene equivalent value (μg・Tol/m3) by the external standard calibration method based on the areas of the detected peaks. The analysis conditions for the VOCs are presented in Table 2.
Table 2.
Experimental parameters for TD/GC/FID and TD/GC/MS analysis.
| TD/GC/FID | TD/GC/MS | |
|---|---|---|
| TD | ||
| Desorption temperature | 280 °C | 250 °C |
| Temperature of cold trap | −14 °C | −20 °C |
| Injection temperature | 280 °C | 250 °C |
| Control mode | Pressure | Pressure |
| Carrier gas | Helium | Helium |
| Pressure | 100 kPa | 100 kPa |
| Split ratio | 1/20 | 1/20 |
| Temperature profile of column oven | 40 °C for 5 min, | 40 °C for 5 min, |
| 10 °C/min up to 300 °C, | 10 °C/min up to 300 °C, | |
| 300 °C for 15 min | 300 °C for 15 min | |
| GC | ||
| Column | DB-1 (Agilent J&W Corp.) | DB-1MS (Agilent J&W Corp.) |
| Film thickness | 0.25 μm | 0.25 μm |
| Length | 30 m | 60 m |
| Inner diameter | 0.32 mm | 0.32 mm |
| Detector | FID | MS |
| Detector temperature | 320 °C | |
| Makeup gas | Nitrogen | |
| Flow rate of makeup gas | 30 mL/min | |
| Flow rate of hydrogen gas | 40 mL/min | |
| Flow rate of air | 400 mL/min | |
| Temperature of ion source | 200 °C | |
| Detector gain | −0.10 kV | |
2.5. Statistical analysis
Statistical analyses were performed using GraphPad Prism version 9.5.1 (GraphPad Software, La Jolla, CA, USA). The data were presented as the mean ± standard deviation (SD). For multiple comparisons, the non-parametric ANOVA (Kruskal-Wallis test) was followed by two-stage linear step-up procedure of Benjamini, Krieger, and Yekutieli. Statistical significance was defined as P < 0.05.
3. Results
3.1. Ionic substances in CPFs
In the current study, F−, CH3COO−, HCOO−, Cl−, NOx + SOx, and NH4+ as representative ionic substances, are shown (Fig. 2). No significant differences were observed in F−, CH3COO−, HCOO−, NOx + SOx, and NH4+.Significantly high Cl− was detected in outside-CPF group with a concentration of 698 ± 1315 ng/m3, and significantly high values were observed in the outdoor air of Facility C (Fig. 2 and Fig. S1).
Fig. 2.
Ionic substance in CPFs. Data are presented as mean ± SD for sampling data from multiple locations. Each group is composed of outside-CPF group (n = 5), inside-CPF group (n = 14), and equipment-related group (n = 7).
The other ionic substances Br+, Na+, K+, and Mg2+ were below the detection limit. The concentrations of PO43−, Ca2+ were quite low at 400 ng/m3 and showed no characteristic trends (Figs. S1 and S2); NO2, NO3, and SO4 followed the same trend and were detected more in the outdoor air (Figs. S1 and S2).
3.2. Total VOC in CPFs
The concentrations of TVOC-all were analyzed and calculated for each sampling location. The TVOC-all compounds were categorized into seven groups: Alcohols, Aldehydes, Aliphatics, Aromatics, Siloxanes, Terpenes, and Unidentified. The visualization of a proportion of the TVOC-all compounds at each sampling point was presented for each facility (refer to Fig. 3A and B, and C). The equipment-related group exhibited the highest concentrations of TVOC-all and TVOC values across all facilities, with particularly notable levels observed in the incubator (Fig. 3D and S3). The advisable value of 400 μg・Tol/m3 set by the Japanese Ministry of Health, Labor and Welfare (MHLW), was exceeded in incubator, grade B and BSC at Facility A; in incubator and grade C at Facility B; and in incubator at Facility C (Fig. S3). The majority of these VOCs were alcohols, particularly in facilities A and B, which are located within healthcare facilities (Fig. 3).
Fig. 3.
Total VOCs and the classification ratio of VOCs detected at each sampling point. (A, B and C) Data representation for each facility. TVOC-all was classified into seven groups: Alcohols, Aldehydes, Aliphatics, Aromatics, Siloxanes, Terpenes, and Unidentified, and a part of whole at each sampling point was visualized. (D) TVOC-all, which is composed of total VOC and alcohol concentrations, is calculated using toluene conversion value. The values for outside-CPF group (n = 5), inside-CPF group (n = 14), and equipment-related group (n = 7) are presented as mean ± SD. For multiple comparisons, the two-stage linear step-up procedure of Benjamini, Krieger and Yekutieli was applied after performing a non-parametric ANOVA (Kruskal-Wallis test). Statistical significance∗ was set at P < 0.05. (E) TVOC are calculated using toluene conversion value. The data for outside-CPF group (n = 5), inside-CPF group (n = 14), and equipment-related group (n = 7) are presented as mean ± SD. For multiple comparisons, the two-stage linear step-up procedure of Benjamini, Krieger and Yekutieli was applied after performing a non-parametric ANOVA (Kruskal-Wallis test). Statistical significance∗ was set at P < 0.05.
3.3. Most detected VOCs in CPFs
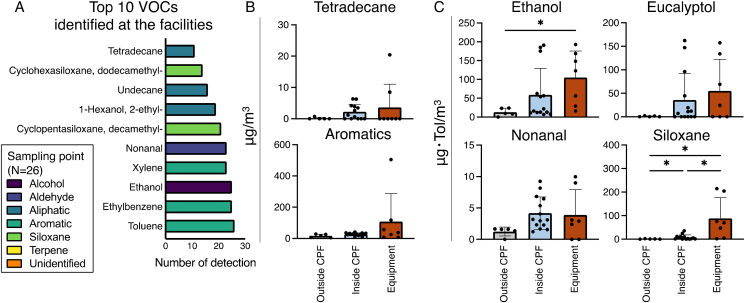
Toluene was detected at all 26 sampling points, and was particularly high in one particular incubator (C8) at Facility C (Fig. 4A and S4). Large quantities of other aromatics such as xylenes, ethylbenzene, and styrene were also detected in this incubator. Aromatics, the most detected chemicals, tended to be more abundant in the incubators (Fig. 4A and B, and S4). However, aromatics did not differ significantly in group comparisons (Fig. 4B). The VOCs in the aromatics were individually compared. Styrene in C8 incubator was the only VOC detected in this study that exceeded the guideline value of 220 μg/m3 set by Japanese MHLW (Fig. S4 and Table 3). Toluene, xylene, and ethylbenzene did not exceed the guidelines; however, their values were high in the C8 incubator (Fig. S4 and Table 3). Tetradecane, which belongs to the aliphatic group, also exhibited high concentration in the same incubator; however, no significant differences were observed in group comparisons (Fig. 4B and S5). Ethanol was the most frequently used alcohol in CPF and, was significantly higher in the equipment-related group as compared to the outside-CPF group (Fig. 4C). Eucalyptol, a terpene, which is used as an aromatic in hand sanitizers, was detected only in facilities A and B and correlated with ethanol (Fig. 4B, S5 and S6). Nonanal, a causative agent of body smell and classified as an aldehyde, was detected in Grade B and BSC samples; however, it did not vary significantly (Fig. 4C, and S5). Siloxanes, which are raw materials of silicone, were also detected and were significantly higher in concentration in the equipment-related group (Fig. 4B). Siloxanes tended to be particularly abundant in the incubators (Fig. S5).
Fig. 4.
Frequently detected VOCs in CPFs. (A) Top 10 VOCs identified at 26 sampling points. The color of the bar is classified according to the type of the chemical. (B) VOCs detected the most in each area. The color of the bar indicates the detected area. These data are shown by toluene conversion value. The data for outside-CPF group (n = 5), inside-CPF group (n = 14), and equipment-related group (n = 7) are presented as mean ± SD. For multiple comparisons, the two-stage linear step-up procedure of Benjamini, Krieger and Yekutieli were applied after performing a non-parametric ANOVA (Kruskal-Wallis test). Statistical significance∗ was set at P < 0.05. (C) Amount of frequently identified VOCs detected in each area. Aromatics show the sum of toluene, ethylbenzene, and xylene. These data are shown by quantitative values. Each group consisted of outside-CPF (n = 5), inside-CPF (n = 14), and equipment-related (n = 7), they are presented as mean ± SD. For multiple comparisons, the two-stage linear step-up procedure of Benjamini, Krieger and Yekutieli were applied after performing a non-parametric ANOVA (Kruskal-Wallis test). Statistical significance∗ was set at P < 0.05.
Table 3.
Guideline value of VOCs for indoor air concentration by Japanese MHLW.
| VOCs | Guideline value for indoor air concentration (at 25 °C) |
|---|---|
| Formaldehyde | 100 μg/m3 (0.08 ppm) |
| Acetaldehyde | 48 μg/m3 (0.03 ppm) |
| Toluene | 260 μg/m3 (0.07 ppm) |
| Xylene | 200 μg/m3 (0.05 ppm) |
| Ethylbenzene | 3800 μg/m3 (0.88 ppm) |
| Styrene | 220 μg/m3 (0.05 ppm) |
| p-Dichlorobenzene | 240 μg/m3 (0.04 ppm) |
| Tetradecane | 330 μg/m3 (0.04 ppm) |
| Chlorpyrifos | 1 μg/m3 (0.07 ppb) For children: 0.1 μg/m3 (0.007 ppb) |
| Fenobucarb | 33 μg/m3 (3.8 ppb) |
| Diazinon | 0.29 μg/m3 (0.02 ppb) |
| Di-n-butyl phthalate | 17 μg/m3 (1.5 ppb) |
| Di-(2-ethylhexyl) phthalate | 100 μg/m3 (6.3 ppb) |
4. Discussion
In this study, air contamination by VOCs and ionic substances in CPFs during the non-operating period was analyzed. The results of the analysis showed different trends in Grade B areas, where CPOs stayed for a long time — in incubators where cells were cultured, and in BSCs where cells were opened. Ionic substances are affected by the decontaminant and outdoor air; VOC values exceeding the MHLW guideline values were observed in some areas, where ethanol was the main component. Aromatic substances were detected more frequently in the incubators. The MHLW guideline values do not pertain to immediate toxicity effects, but rather serve as management standards that consider potential toxicity risks during extended periods of exposure. Therefore, in terms of long-time exposure to CPOs, some operational regimes may require review.
A characteristic of CPF is that it facilitates the amount of recirculating ventilation to about 90–95% by reducing the amount of outside air introduced. However, this causes VOCs to remain in the environment once they are generated. To target CPFs prone to such VOC residue, sampling was conducted at three locations at rest period in the present study. Facilities A and B were located within the same healthcare organization, whereas Facility C was located at a different institution. Therefore, management policies for each CPFs differed. For example, Facilities A and B had a policy of cleaning with ethanol wipes in the room after processing, leading to a higher concentration of ethanol. In addition to these differences in policy, Facility C was located near the sea and had different outdoor air conditions. The builder, buildings and equipment also differed, and differences in the detection trends were observed for substances derived from building or equipment materials. As there are several CPFs [10], analyzing more of them will help identify the overall trends.
The effects of ionic substances are often reported in semiconductor cleanrooms because their presence has a negative impact on semiconductor manufacturing [25,26]. However, the presence of ionic substances in CPFs has not been evaluated. In this study, we analyzed the environment inside CPFs as a potential source of air contamination because of multiple equipment installed and frequent disinfection, which is different from that of an office or hospital. Ionic substances are highly water-soluble and readily dissolve in the culture medium. Therefore, the presence of large amounts of ionic substances may affect cell culture. Ionic substances are present in the outdoor air, and many are trapped by the intake filters [28]. NO3− and SO42−, which are the main air pollutants, were detected in the outdoor air. Cl− was detected at high levels in the outdoor air of Facility C, which is located near the sea, although these ionic substances were not introduced into the CPFs. These results indicate that there is no contamination of ionic substances from inside CPF or from the installed equipment. In addition, this indicates that exposure to CPOs to these ionic substances detected during the non-operating period is unlikely to cause any immediate health problems.
TVOC management varies widely across countries and there are no uniform international standards [[29], [30], [31]]. Although the advisable value of the MHLW in Japan is set in terms of lifetime exposure to houses, it can be adapted to CPFs too, where CPOs spend a long time. In this study, all four incubators analyzed exceeded the advisable value of 400 μg・Tol/m3. As CPOs do not operate or work in incubators, their immediate health risk is low, but manager of CPFs need to be alerted. Because CPFs showed high values of TVOCs and styrene in the incubator (C8) immediately after introduction, it might be necessary to confirm that there were no problems in the operation by pre-culturing cells at the first time of use. Because cell culture problems may arise when the production site is changed, a detailed analysis will be necessary in the future.
The types of VOCs detected differed according to the sampling point. In particular, large amounts of alcohol were detected in Facilities A and B, which may have originated from the standard operating procedures of the facilities. The use of alcohol and other disinfectants in CPFs is unavoidable, partly because of the nature of the cell products. Although the use of alcohol has some positive hygienic aspects, such as its effectiveness in eliminating bacteria, high exposure to alcohols has been reported to pose a risk of increasing the incidence of allergies in offspring [14]. Furthermore, respiratory problems have been reported in workers in environments where disinfectants, such as hydrogen peroxide and peracetic acid, are frequently used [32,33]. In animal studies, it is also known that individuals with respiratory impairment have a lower ozone toxicity threshold [34]. Therefore, it is very important to check and inform CPOs in advance whether they have any respiratory conditions or are allergic to alcohol or other disinfectants. As alcohols or other disinfectants such as ethanol are not always the best disinfectants for cell product manufacturing sites that use high-protein serum or human tissue [35], it may be necessary to consider alternative methods as well. A survey estimated that 50% of CPOs working in the country had two to three years of work experience, suggesting a high attrition rate [10]. This high personnel mobility could be due to these working conditions; hence, further causal investigations and measures to protect CPOs are necessary.
The top ten most commonly detected VOCs in each facility were derived from building or equipment materials, such as toluene, ethylbenzene, and xylene, chemicals, such as ethanol, a disinfectant, nonanal, which is produced by humans, and siloxane, raw materials for silicone. Disinfectants were detected more frequently in Grade B areas, the incubator, and the BSC, suggesting that ethanol used for room cleaning leaked into the incubator and the BSC. Several VOCs were also detected at high levels in the incubator at C8. The elevated values observed in the incubator can potentially be attributed to the presence of silicone in the lid, which is intended to prevent the leakage of CO2 and humidity. There is a possibility that the raw materials used in the incubator, such as toluene, commonly found in paints, and tetradecane, occasionally used as an adhesive, contain aromatic compounds. However, the exact cause of the increased values could not be determined. VOC levels of incubator were immediately non-toxic to CPOs. Although there are concerns about their effects on cells, toluene, siloxane, and tetradecane are insoluble in water and do not dissolve in culture medium. With the exception of the incubator immediately after installation, the data obtained in this study showed that, in terms of toxicity to CPOs, CPFs can be expected to operate safely by preventing the abuse of disinfectants.
This study had three limitations. First, the number of sampling points was just 26. In addition, the analysis was not conducted under uniform conditions because the incubators and BSCs were installed at different times and were of different models. This study is the first to investigate VOCs and ionic substances in CPFs, and it was concluded that its adverse effects were minor.
Second, these are short-term data from a non-operating period, and not from operating period measured over a long period. CPOs use large amounts of ethanol spray during cell processing. Therefore, further operational measurements should be conducted in the future. In addition, based on the results of this study, it may be necessary to wear a badge specialized for ethanol detection, like "luminescence badge” used for radiation exposure measurement. Because cell culture is expected to be continued to be performed manually, we must consider developing an environment that safeguards the health of CPOs.
Third, air quality measurements were taken from the perspective of worker protection, and their effects on cell culture were beyond the scope of this study. We hope that the data from this study will provide evidence that VOCs and ionic substances may have adverse effects on cell cultures.
5. Conclusions
No VOCs or ionic substances of immediate concern to the health of CPOs were detected in this study. However, new clinical trials of cell products are currently underway in Japan [36,37], and a variety of new cell products are expected to be approved. With an increase in cell processing, problems related to CPOs that have not been previously considered, may become apparent. Moreover, in design stage itself, it may be necessary to consider facility planning based on the user characteristics of cell processing and the use of many disinfectants. We should continue to prepare for the future expansion of the industry using a scientific approach to collect various pieces of information and make it publicly available by building a database.
Funding
This research was funded by a joint research grant from the SHIMIZU CORPORATION. One of the authors (MM) of this paper is employed by the Japan Agency for Medical Research and Development (AMED) under grant number JP22bk0304003, which was provided to IS.
Authors' contributions
MM, KA, TK, KY, YO, and KT: Data acquisition, analysis, and interpretation. KY and YO: Acquisition of IC and VOC data. MM: Drafting the manuscript. MM, KY, NK and IS: Manuscript revision for important intellectual content. All the authors have read and approved the final manuscript.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Ichiro Sekiya reports financial support was provided by Shimizu Corporation.
Acknowledgements
We thank Hisako Katano and Sayaka Komura for managing laboratory work. We also thank Ayako Tsuji and Jun Kusano for management of CPFs.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.reth.2023.07.002.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
References
- 1.Raval J.S., Koch E., Donnenberg A.D. Real-time monitoring of non-viable airborne particles correlates with airborne colonies and represents an acceptable surrogate for daily assessment of cell-processing cleanroom performance. Cytotherapy. 2012;14(9):1144–1150. doi: 10.3109/14653249.2012.698728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Negre H., Pinte L., Manduke R., Cunningham A., Anderson H., Richard S., et al. Personnel environmental monitoring during manufacture of manipulated cell therapy products. Cytotherapy. 2018;20(5) [Google Scholar]
- 3.Mizuno M., Endo K., Katano H., Tsuji A., Kojima N., Watanabe K., et al. The environmental risk assessment of cell-processing facilities for cell therapy in a Japanese academic institution. PLoS One. 2020;15(8) doi: 10.1371/journal.pone.0236600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin P.G., Gonzalez M.B., Martinez A.R., Lara V.G., Naveros B.C. Isolation and characterization of the environmental bacterial and fungi contamination in a pharmaceutical unit of mesenchymal stem cell for clinical use. Biologicals. 2012;40(5):330–337. doi: 10.1016/j.biologicals.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Mizutani M., Samejima H., Terunuma H., Kino-oka M. Experience of contamination during autologous cell manufacturing in cell processing facility under the Japanese Medical Practitioners Act and the Medical Care Act. Regen Ther. 2016;5:25–30. doi: 10.1016/j.reth.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takagi R., Kobayashi S., Yamato M., Owaki T., Kasai Y., Hosoi T., et al. How to prevent contamination with Candida albicans during the fabrication of transplantable oral mucosal epithelial cell sheets. Regen Ther. 2015;1:1–4. doi: 10.1016/j.reth.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogawa Y., Mizutani M., Okamoto R., Kitajima H., Ezoe S., Kino-oka M. Understanding the formation and behaviors of droplets toward consideration of changeover during cell manufacturing. Regen Ther. 2019;12:36–42. doi: 10.1016/j.reth.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mizuno M., Yori K., Takeuchi T., Yamaguchi T., Watanabe K., Tomaru Y., et al. Cross-contamination risk and decontamination during changeover after cell-product processing. Regen Ther. 2023;22:30–38. doi: 10.1016/j.reth.2022.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chihara R., Kitajima H., Ogawa Y., Nakamura H., Tsutsui S., Mizutani M., et al. Effects of residual H2O2 on the growth of MSCs after decontamination. Regen Ther. 2018;9:111–115. doi: 10.1016/j.reth.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mizuno M., Sugahara Y., Iwayama D., Miyashita N., Katano H., Sekiya I. Stress and motivation of cell processing operators: a pilot study of an online questionnaire survey. Regen Ther. 2022;21:547–552. doi: 10.1016/j.reth.2022.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Veysi R., Heibati B., Jahangiri M., Kumar P., Latif M.T., Karimi A. Indoor air quality-induced respiratory symptoms of a hospital staff in Iran. Environ Monit Assess. 2019;191(2):50. doi: 10.1007/s10661-018-7182-5. [DOI] [PubMed] [Google Scholar]
- 12.Hellgren U.-M., Reijula K. Indoor air problems in hospitals: a challenge for occupational health. AAOHN J. 2011;59(3):111–117. doi: 10.3928/08910162-20110223-01. [DOI] [PubMed] [Google Scholar]
- 13.Goh C.F., Ming L.C., Wong L.C. Dermatologic reactions to disinfectant use during the COVID-19 pandemic. Clin Dermatol. 2021;39(2):314–322. doi: 10.1016/j.clindermatol.2020.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kojima R., Shinohara R., Kushima M., Horiuchi S., Otawa S., Yokomichi H., et al. Japan Environment and Children's Study Group. Children's Study, Prenatal occupational disinfectant exposure and childhood allergies: the Japan Environment and Children's study. Occup Environ Med. 2022;79(8):521–526. doi: 10.1136/oemed-2021-108034. [DOI] [PubMed] [Google Scholar]
- 15.Lau A., Tarlo S.M. Update on the management of occupational asthma and work-exacerbated asthma. Allergy Asthma Immunol Res. 2019;11(2):188–200. doi: 10.4168/aair.2019.11.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dumas O., Boggs K.M., Quinot C., Varraso R., Zock J.P., Henneberger P.K., et al. Occupational exposure to disinfectants and asthma incidence in U.S. nurses: a prospective cohort study. Am J Ind Med. 2020;63(1):44–50. doi: 10.1002/ajim.23067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dumas O., Wiley A.S., Quinot C., Varraso R., Zock J.P., Henneberger P.K., et al. Occupational exposure to disinfectants and asthma control in US nurses. Eur Respir J. 2017;50(4) doi: 10.1183/13993003.00237-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bessonneau V., Thomas O. Assessment of exposure to alcohol vapor from alcohol-based hand rubs. Int J Environ Res Publ Health. 2012;9(3):868–879. doi: 10.3390/ijerph9030868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang J., Ding X., Isaacson K.P., Tasoglou A., Huber H., Shah A.D., et al. Ethanol-based disinfectant sprays drive rapid changes in the chemical composition of indoor air in residential buildings. J Hazard Mater Lett. 2021;2 doi: 10.1016/j.hazl.2021.100042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Redlich C.A., Sparer J., Cullen M.R. Sick-building syndrome. Lancet. 1997;349(9057):1013–1016. doi: 10.1016/S0140-6736(96)07220-0. [DOI] [PubMed] [Google Scholar]
- 21.Levin J.-O., Bessonneau V., Mosqueron L., Berrubé A., Mukensturm G., Buffet-Bataillon S., et al. Voc contamination in hospital, from stationary sampling of a large panel of compounds, in view of healthcare workers and patients exposure assessment. PLoS One. 2013;8(2) doi: 10.1371/journal.pone.0055535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niaz K., Bahadar H., Maqbool F., Abdollahi M. A review of environmental and occupational exposure to xylene and its health concerns. EXCLI J. 2015;14:1167–1186. doi: 10.17179/excli2015-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henderson L., Brusick D., Ratpan F., Veenstra G. A review of the genotoxicity of ethylbenzene. Mutat Res. 2007;635(2–3):81–89. doi: 10.1016/j.mrrev.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Davidson C.J., Hannigan J.H., Bowen S.E. Effects of inhaled combined Benzene, Toluene, Ethylbenzene, and Xylenes (BTEX): toward an environmental exposure model. Environ Toxicol Pharmacol. 2021;81 doi: 10.1016/j.etap.2020.103518. [DOI] [PubMed] [Google Scholar]
- 25.Den W., Bai H., Kang Y. Organic airborne molecular contamination in semiconductor fabrication clean rooms. J Electrochem Soc. 2006;153(2) [Google Scholar]
- 26.Chen R.Q., Shiue A., Liu J.J., Zhi Y., Zhang D.C., Xia F., et al. Integrated on-site collection and off-site analysis of airborne molecular contamination in cleanrooms for integrated circuit manufacturing processes. Build Environ. 2022;214 [Google Scholar]
- 27.Bernstein J.A., Alexis N., Bacchus H., Bernstein I.L., Fritz P., Horner E., et al. The health effects of non-industrial indoor air pollution. J Allergy Clin Immunol. 2008;121(3):585–591. doi: 10.1016/j.jaci.2007.10.045. [DOI] [PubMed] [Google Scholar]
- 28.Fujimoto T., Takeda K., Nonaka T. 2008. Airborne molecular contamination, developments in surface contamination and cleaning; pp. 329–474. [Google Scholar]
- 29.Umweltbundesamt [Evaluation of indoor air contaminants by means of reference and guideline values] Bundesgesundheitsblatt - Gesundheitsforsch - Gesundheitsschutz. 2007;50(7):990–1005. doi: 10.1007/s00103-007-0290-y. [DOI] [PubMed] [Google Scholar]
- 30.Persily A. Challenges in developing ventilation and indoor air quality standards: the story of ASHRAE standard 62. Build Environ. 2015;91:61–69. doi: 10.1016/j.buildenv.2015.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolkoff P., Nielsen G.D. Organic compounds in indoor air—their relevance for perceived indoor air quality? Atmos Environ. 2001;35(26):4407–4417. [Google Scholar]
- 32.Hawley B., Casey M., Virji M.A., Cummings K.J., Johnson A., Cox-Ganser J. Respiratory symptoms in hospital cleaning staff exposed to a product containing hydrogen peroxide, peracetic acid, and acetic acid. Ann Work Expo Health. 2017;62(1):28–40. doi: 10.1093/annweh/wxx087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dumas O., Varraso R., Boggs K.M., Quinot C., Zock J.P., Henneberger P.K., et al. Association of occupational exposure to disinfectants with incidence of chronic obstructive pulmonary disease among US female nurses. JAMA Netw Open. 2019;2(10) doi: 10.1001/jamanetworkopen.2019.13563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tomita K., Okawara T., Ohira C., Morimoto A., Aihara R., Kurihara T., et al. An acceptable concentration (0.1 ppm) of ozone exposure exacerbates lung injury in a mouse model. Am J Respir Cell Mol Biol. 2021;65(6):674–676. doi: 10.1165/rcmb.2021-0302LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mizuno M., Matsuda J., Watanabe K., Shimizu N., Sekiya I. Effect of disinfectants and manual wiping for processing the cell product changeover in a biosafety cabinet. Regen Ther. 2023;22:169–175. doi: 10.1016/j.reth.2023.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mizuno M., Endo K., Katano H., Amano N., Nomura M., Hasegawa Y., et al. Transplantation of human autologous synovial mesenchymal stem cells with trisomy 7 into the knee joint and 5 years of follow-up. Stem Cells Transl Med. 2021;10(11):1530–1543. doi: 10.1002/sctm.20-0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sekiya I., Koga H., Otabe K., Nakagawa Y., Katano H., Ozeki N., et al. Additional use of synovial mesenchymal stem cell transplantation following surgical repair of a complex degenerative tear of the medial meniscus of the knee: a case report. Cell Transplant. 2019;28(11):1445–1454. doi: 10.1177/0963689719863793. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.