Abstract
We report 2 rare cases of male breast cancer with bloody nipple discharge. Patient 1, a 32-year-old male, presented with a bloody nipple discharge from the left breast. Diagnostic workup revealed papillary ductal carcinoma in situ. Patient 1 underwent bilateral mastectomy with left axillary sentinel lymph node biopsy and has been doing well ever since. Patient 2, a 70-year-old male with concomitant metastatic prostate cancer, presented with a palpable right breast mass and with initially serous, then bloody nipple discharge. Diagnostic workup revealed invasive ductal carcinoma with ductal carcinoma in situ of the right breast. Patient 2 received aromatase inhibitor therapy prior to right total mastectomy with SLN biopsy followed by adjuvant tamoxifen therapy. Patient 2 recovered without complication for 2 years until metastatic disease recurrence was detected. This case report's purpose is to increase awareness and enhance understanding of the presentation, diagnosis, treatment, and outcomes of rare malignant pathologies.
Keywords: Male breast cancer, Nipple discharge, DCIS, Invasive ductal carcinoma, Papillary ductal carcinoma
Introduction
Male breast cancer (MBC) is one of the rarest forms of breast cancer, only accounting for <1% of all breast cancer diagnoses [1]. In contrast to female breast cancer, MBC is more often diagnosed at advanced stages. The rarity and lack of awareness of MBC contribute to delay in detection and subsequent advanced stage at diagnosis [1].
MBC most often presents with a painless palpable mass, and less commonly presents with nipple discharge [1]. Nipple discharge is a presenting symptom in only 6% of cases of MBC [1]. Nipple discharge can be milky, clear, or bloody, with the latter 2 being strongly associated with underlying malignancy in males [2].
Much of the management of MBC is based on what is known about female breast cancer [3]. Here we present 2 cases of MBC with nipple discharge at diagnosis, with 1 patient exhibiting papillary ductal carcinoma in situ (DCIS) and the other exhibiting invasive ductal carcinoma (IDC) with DCIS.
Case presentation
Case 1
A 32-year-old man, with no personal history of cancer and no family history of breast cancer, was seen for bloody nipple discharge and a palpable abnormality in the left breast. The discharge had been occurring for the previous year, though the patient had neglected it until being seen at the clinic.
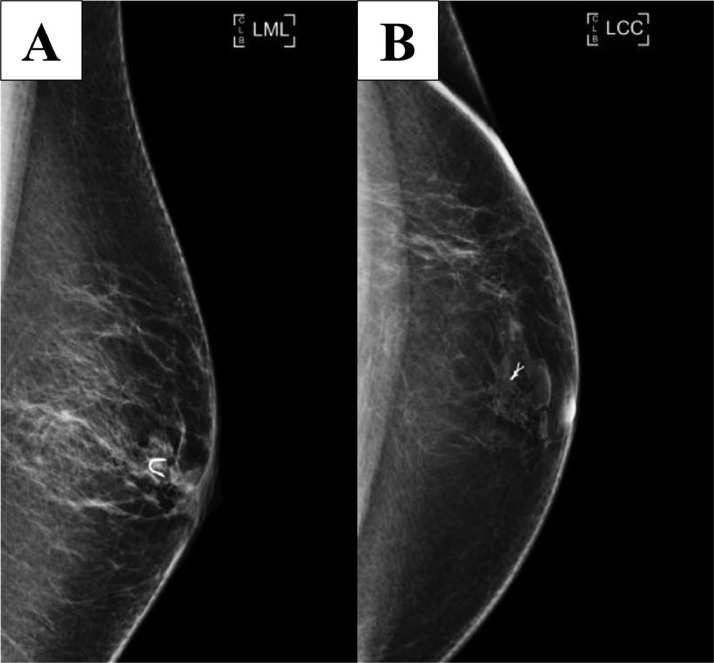
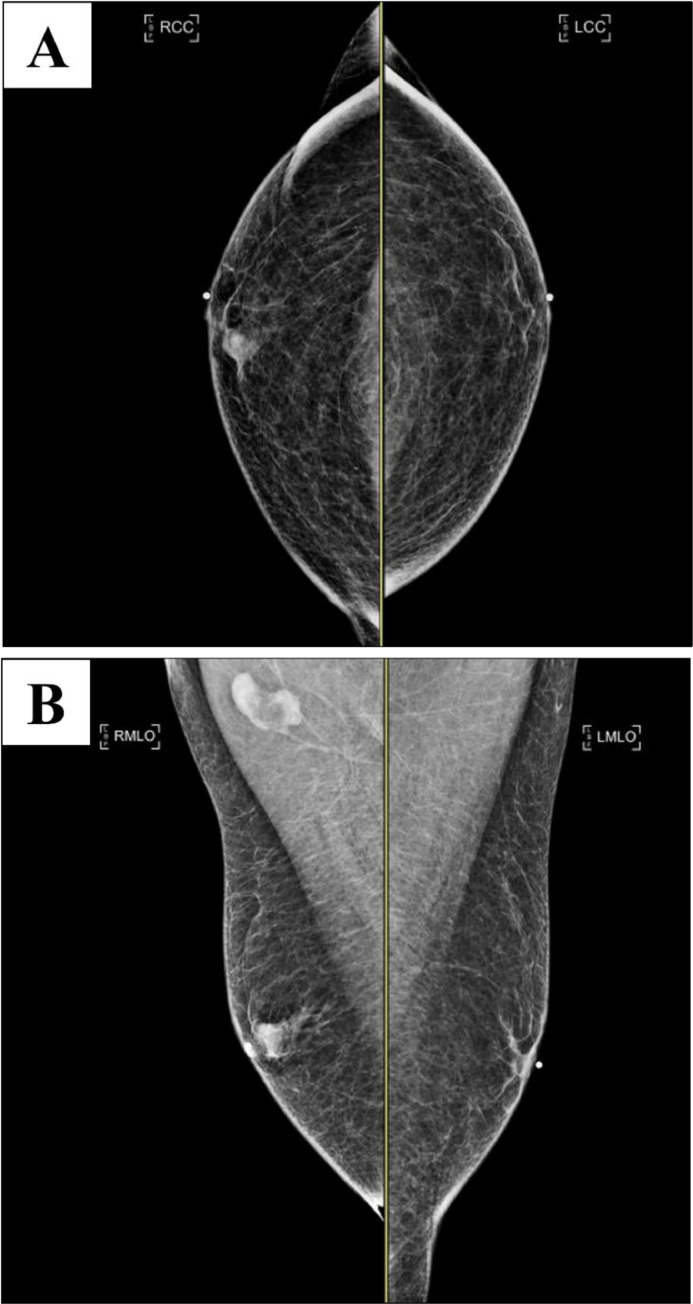
Bilateral diagnostic mammograms (craniocaudal and mediolateral oblique; see Fig. 1) were obtained and revealed 1 round mass with mostly circumscribed margins and another round mass with obscured margins and associated coarse calcification in the left anterior breast subareolar region 3:00 position in the area of palpable concern.
Fig. 1.
Bilateral craniocaudal (A) and mediolateral oblique (B) mammograms show 1 round mass with mostly circumscribed margins and another adjacent mass with obscured margin and with associated coarse calcification in the left breast subareolar region. BB markers are placed on the nipples and a triangular marker is placed on the area of palpable concern.
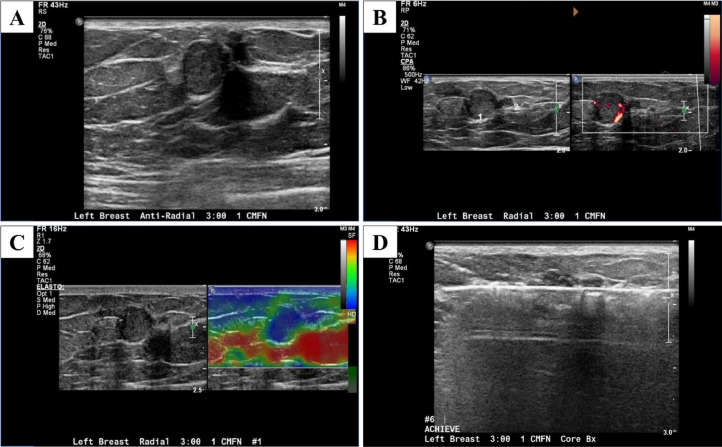
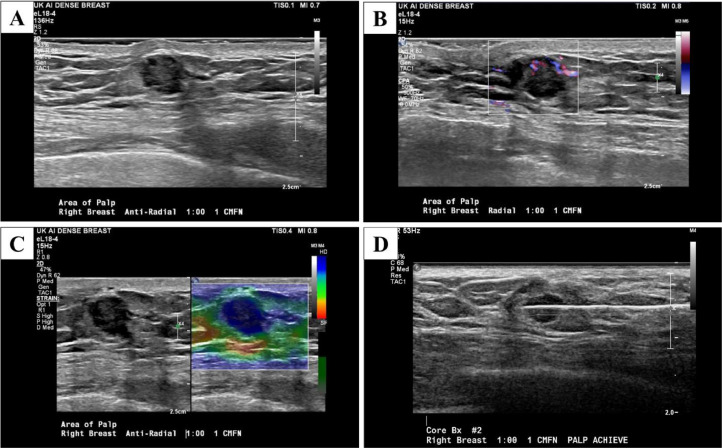
Focused Ultrasound was done to further evaluate these abnormal mammogram findings and the periareolar region of the left breast for the nipple discharge using both a radial approach and an antiradial approach (Fig. 2). This revealed 2 round, circumscribed, isoechoic masses with internal vascularity measuring 10 and 4 mm at 3-o'clock, located 1 cm from the nipple in the area of palpable concern. Elastography was used and showed the masses were hard (Fig. 2).
Fig. 2.
Focused grayscale ultrasound in the left breast in the area of palpable concern showed 2 adjacent 10 mm and 4 mm round, circumscribed, isoechoic masses in the left breast 3: 00 position 1 cm from the nipple (A), which have increased blood flow on the Doppler ultrasound (B) and are hard on the elastography (C). Ultrasound-guided core biopsy with a 12-gauge needle was done in the left breast at 3-o'clock and 1 cm from the nipple (D).
Ultrasound-guided core biopsy was performed (Fig. 2), and a biopsy clip was placed (Fig. 3).
Fig. 3.
Left mediolateral (A) and left craniocaudal (B) postbiopsy mammograms show the clip in the appropriate position.
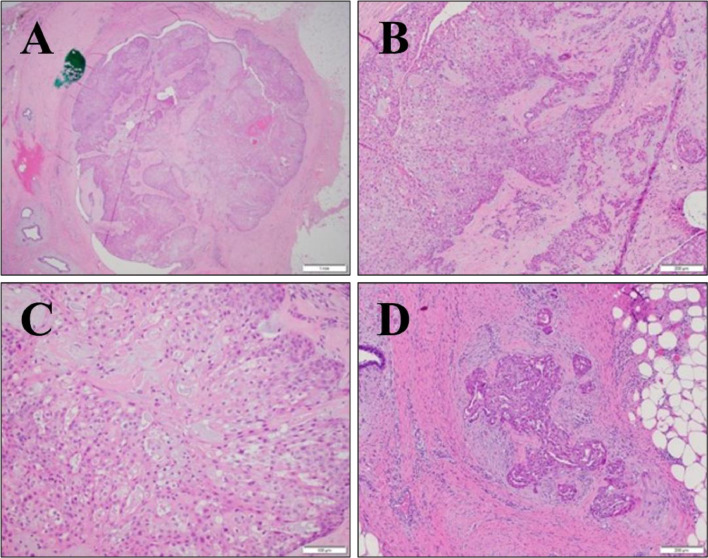
Histological pathology report was released and revealed fragments of sclerosing papillomatous neoplasm with apocrine features (Fig. 4), and regressing changes (Fig. 5), which is consistent with papillary DCIS without invasive components.
Fig 4.
Low-power image of partially sclerosed papilloma involved by intermediate-grade DCIS (A). Borders are well-circumscribed, and there is no invasive disease in this focus. Intermediate-power view of DCIS with squamous metaplasia (B). Acellular, eosinophilic areas represent sclerosis. Nuclear detail of intermediate-grade DCIS (C). A small focus of invasive carcinoma located 2 mm from targeted sclerosed papilloma with DCIS (D). Desmoplastic stroma surrounds invasive tumors.
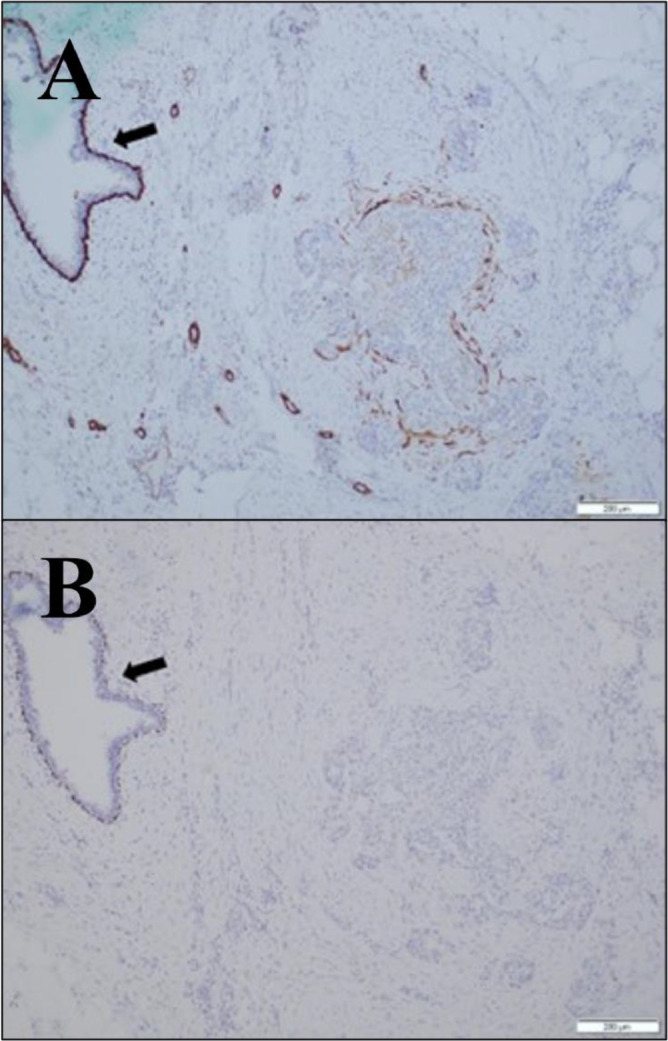
Fig. 5.
Invasive nature of focus is confirmed with myosin heavy chain stain, which fails to highlight a convincing layer of myoepithelial cells in this focus, thereby suggesting that the patient does not have papilloma with DCIS (A). Adjacent benign ductal structures show complete circumferential staining of the myoepithelial layer (A; arrow). Invasive nature of focus is confirmed with p63 immunostain, which fails to highlight a convincing layer of myoepithelial cells in this focus (B). Adjacent benign ductal structures show complete circumferential staining of the myoepithelial layer (B; arrow).
Pathological analysis initially suggested papilloma with DCIS, due to the benign nature of the ducts within the breasts, and an apparent lack of invasive nature (Fig. 4). Closer inspection, however, revealed a lack of a layer of myoepithelial cells, suggesting that papilloma with DCIS was not observed in this patient (Fig. 5) and that papillary DCIS was the correct diagnosis.
The patient underwent a bilateral mastectomy and left axillary sentinel lymph node (SLN) biopsy. The surgical pathological analysis showed papillary DCIS and negative axillary lymph node. Patient recovered well after surgery without hormonal or chemotherapy and has been doing well on the yearly follow-up.
The patient was seen by a genetic counselor to evaluate the probability of him having inherited DCIS. The determination was that the disease was unlikely to have been inherited, due to the patient lacking a history of any cancer, not just breast cancer.
Case 2
A 70-year-old man, with a past medical history of metastatic prostate cancer status, postradical retropubic prostatectomy with bilateral pelvic lymph node dissection, salvage radiation therapy, with ongoing androgen deprivation therapy, presents to surgical oncology with 1-month history of palpable right breast mass and initially serious, now bloody nipple discharge. He has a family history of breast cancer in his maternal aunt and 2 maternal first cousins.
Bilateral diagnostic mammograms were obtained and showed a right breast mass at 1-o'clock located 1 cm from the nipple with indistinct margins (Fig. 6). The mass correlated to the palpable mass and bloody nipple discharge in the right breast.
Fig. 6.
Bilateral craniocaudal (A) and mediolateral oblique (B) mammograms display a right breast mass with indistinct margins at 1-o'clock located 1 cm from the nipple. BB markers are placed on the nipples and a triangular marker is placed at the area of palpable concern. There is a mild enlargement of right axillary lymph nodes.
There is a mild enlargement of the right axillary lymph nodes (Fig. 6). A focused ultrasound was obtained for further evaluation of right breast mass. A round, isoechoic, mass with indistinct margins, and internal vascularity measuring 12 mm in the right breast at 1-o'clock located 1 cm from the nipple was observed (Fig. 7). Elastography was used and showed the mass was hard (Fig. 7).
Fig. 7.
Focused ultrasound in the right breast in the area of palpable concern showed a single round 12×8×9 mm hypoechoic mass in the 1: 00 position 1 cm from the nipple (A), which has increased blood flow on the Doppler ultrasound (B) and is hard on the elastography (C) Ultrasound-guided core biopsy with a 14-gauge needle was done in the left breast at 1-o'clock and 1 cm from the nipple (D).
A focused ultrasound was performed in the right axilla in transverse and longitudinal planes. There were a few abnormal lymph nodes in the right breast. Ultrasound-guided core biopsy was performed (Fig. 7), and a biopsy clip was placed in the right breast. A fine needle aspiration was performed in the lymph node of the right axilla.
The biopsy of the right breast revealed ER+, PR+, and HER2- IDC with apocrine features. In-situ carcinoma with papillary and solid types were present. Axillary fine needle aspiration was unremarkable.
A right total mastectomy with SLN biopsy was the initial treatment decision, but cardiac complications requiring stent placement delayed this procedure. The patient was put on aromatase inhibitor therapy for 6 months before undergoing the mastectomy with SLN biopsy. The SLN biopsy was negative for any tumor. The patient was started on adjuvant tamoxifen following the procedure. He recovered without complication from breast cancer for 2 years follow-up.
The patient was concurrently experiencing complications from metastatic prostate cancer. A CT scan revealed right pleural effusion and extensive pleural-based metastatic disease with biopsy consistent with breast primary. Tamoxifen was discontinued and anastrozole/ibrance was started. Pleural disease progressed, and the patient was switched to pembrolizumab/paclitaxel, then docetaxel. Improvement in pleural disease was achieved on pembrolizumab/docetaxel. An episode of malignant right pleural disease recurrence was treated with fulvestrant. Patient has since been without further progression of metastatic breast cancer.
The patient underwent genetic counseling due to a history of both male breast cancer and metastatic prostate cancer. Genetic testing was negative for known deleterious mutations.
Discussion
We have reported 2 rare cases of breast cancer in a male with uncommon presentations of nipple discharge and palpable masses. These symptoms are often overlooked by patients despite the fact they are usually quite suspicious. One case was noninvasive pure papillary DCIS while the other case contained both IDC and DCIS. Few cases of MBC are reported in the literature, and even fewer are reported with nipple discharge as the presenting symptom.
Nipple discharge alone is an insufficient indicator of MBC. MBC most commonly presents as a painless lump in the breast [4,5]. Further diagnostic work, including imaging, biopsy, and pathology, is needed if the only notable presentation is nipple discharge [6]. According to the American College of Radiology (ACR) Appropriateness Criteria for the symptomatic male breast, it is recommended to perform mammography and ultrasound for initial imaging in males with physical exam findings suspicious of breast cancer [7]. These exam findings include nipple discharge and palpable masses, as seen in our 2 cases. MBC often presents on mammography as a radiodense irregular retroareaolar mass with ill-defined margins [8]. The tissue pathology report is used to confirm the diagnosis of MBC.
MBC has distinctly different pathology characteristics than FBC. Anatomical differences between males and females result in differences in pathology prevalence in MBC and FBC [9]. Normal male breast tissue contains ducts without terminal lobules, resulting in IDC making up about 85% of MBC diagnoses, while invasive lobular carcinoma (ILC) is only 1%-2% [9]. Normal female breasts, containing lobules, result in FBC diagnoses being 80% IDC and 15% ILC [9,10]. The variation in ER subtypes expressed by MBC and FBC can also be affected by anatomical differences [9]. ER-beta receptors are often found in the kidney, brain, bone, and prostate tissue, while ER-alpha receptors are often found in endometrium and ovarian tissue [9]. MBC most commonly expresses ER-beta while FBC most often expresses the ER-alpha receptor subtype [9].
Since literature on MBC is far less abundant than on FBC, the treatment of MBC has historically been guided by FBC treatment. Recommended FBC treatment usually involves lumpectomy followed by radiation therapy or mastectomy, [11], [12], [13]. Mastectomy is a recommended treatment for men for its consistently good prognosis [14]. However, more recent studies suggest that breast-conserving surgery (BCS) may be another viable treatment option [15,16]. In a systematic review of 9 retrospective cohort studies ranging from 7 to 6039 patients, it was concluded that BCS is as effective as mastectomy in overall survival time [15]. In a retrospective study of 8445 males with early-stage invasive breast cancer, BCS with radiation therapy showed an increased overall survival time compared to mastectomy +/- radiation [16]. Hormonal therapy can be effective as another adjuvant therapy when the neoplasm tests positive for hormone receptors [17].
However, a unique approach may need to be considered for MBC due to predominant ER-beta expression [9]. It is unclear how effective chemotherapy can be as a treatment for MBC, though it appears to be less effective than FBC [18].
MBC patients also see a genetic counselor and undergo genetic testing to evaluate the probability of having inherited cancer-related gene mutations, as the result can play a key role in the determination of the treatment and the prognosis of the patient's cancer and risk of contracting future malignancies [17,19]. The results of genetic testing may also warrant genetic screening of family members [19].
MBC has about the same prognosis at each cancer stage as FBC when adjusted for staging and other relevant factors [2]. This is an interesting finding, given that MBC tends to have a worse prognosis than FBC does when no adjustments are made [4]. This worse prognosis can be explained by a later diagnosis of MBC. One key reason for this is low awareness amongst men that they can contract breast cancer [4]. Another reason may be embarrassment over having breast cancer, as male patients can be reluctant to discuss symptoms of the breast with their doctors [2]. In Patient 1, these observations on outcome were also observed. The patient experienced nipple discharge for 1 year prior to feeling the lump in the breast. However, the patient chose to ignore it, suggesting a lack of awareness of, and a potential sense of embarrassment over, contracting breast cancer.
Greater efforts must be taken to ensure that more men are aware that they can contract breast cancer and that bloody nipple discharge is one of the concerning symptoms of breast cancer. The potential stigma around MBC should be addressed, and the awareness of suspicious symptoms of breast cancer should be raised so that patients can receive early diagnoses and increased likelihood of survival.
Patient consent
The University Of Kentucky College Of Medicine and the Markey Cancer Center both express that consent was acquired from the 2 patients.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
References
- 1.Yalaza M, İnan A, Bozer M. Male breast cancer. J Breast Health. 2016;12(1):1–8. doi: 10.5152/tjbh.2015.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramakrishna KN, Durland J, Ramos C, Dhamoon AS. Unilateral nipple discharge in a man without a palpable mass diagnosed as breast cancer. BMJ Case Rep. 2020;13(11) doi: 10.1136/bcr-2020-236223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cardoso F, Bartlett JMS, Slaets L, van Deurzen CHM, van Leeuwen-Stok E, et al. Characterization of male breast cancer: results of the EORTC 10085/TBCRC/BIG/NABCG International Male Breast Cancer Program. Ann Oncol. 2018;29(2):405–417. doi: 10.1093/annonc/mdx651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brents M, Hancock J. Ductal carcinoma in situ of the male breast. Breast Care. 2016;11(4):288–290. doi: 10.1159/000447768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farooq A, Horgan K. Male breast cancer presenting as nipple discharge. Case Rep Surg. 2011;2011 doi: 10.1155/2011/804843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morrogh M, King TA. The significance of nipple discharge of the male breast. Breast J. 2009;15:632–638. doi: 10.1111/j.1524-4741.2009.00818.x. [DOI] [PubMed] [Google Scholar]
- 7.Niell BL, Lourenco AP, Moy L, Baron P, Didwania AD, et al. ACR appropriateness criteria evaluation of the symptomatic male breast. J Am Coll Radiol. 2018;15(11S):S313–S320. doi: 10.1016/j.jacr.2018.09.017. [DOI] [PubMed] [Google Scholar]
- 8.Yen PPW, Sinha N, Barnes PJ, Butt R, Iles S. Benign and malignant male breast diseases: radiologic and pathologic correlation. Can Assoc Radiol J. 2015;266(3):198–207. doi: 10.1016/j.carj.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Zheng G, Leone JP. Male breast cancer: an updated review of epidemiology, clinicopathology, and treatment. J Oncol. 2022;2022 doi: 10.1155/2022/1734049. –11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kao Y, Wu YJ, Hsu CC, Lin HJ, Wang JJ, Tian YF, et al. Short- and long-term recurrence of early-stage invasive ductal carcinoma in middle-aged and old women with different treatments. Sci Rep. 2022;12(1):4422. doi: 10.1038/s41598-022-08328-4. -10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Groen EJ, Elshof LE, Visser LL, Rutgers EJT, Winter-Warnars HAO, et al. Finding the balance between over- and under-treatment of ductal carcinoma in situ (DCIS) Breast. 2017;31:274–283. doi: 10.1016/j.breast.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Wapnir IL, Dignam JJ, Fisher B, Mamounas EP, Anderson SJ, Julian TB, et al. Long-term outcomes of invasive ipsilateral breast tumor recurrences after lumpectomy in NSABP B-17 and B-24 randomized clinical trials for DCIS. J Natl Cancer Inst. 2011;103(6):478–488. doi: 10.1093/jnci/djr027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barrio AV, Van Zee KJ. Controversies in the treatment of ductal carcinoma in situ. Annu Rev Med. 2017;68:197–211. doi: 10.1146/annurev-med-050715-104920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakhri S, Jaidane O, Bouhani M, Adouni O, Kammoun S, Chargui R, et al. Pure ductal carcinoma in situ in the male breast: a rare entity. Eur J Breast Health. 2019;16(1):77–80. doi: 10.5152/ejbh.2019.4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sauder CAM, Bateni SB, Davidson AJ, Nishijima DK. Breast conserving surgery compared to mastectomy in male breast cancer: a systematic review. Clin Breast Cancer. 2020;20(3):e309–e314. doi: 10.1016/j.clbc.2019.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bateni SB, Davidson AJ, Arora A, Daly ME, Stewart S L, Bold RJ, et al. Is breast-conserving therapy appropriate for male breast cancer patients? A National Cancer Database Analysis. Ann Surg Oncol. 2019;26(7):2144–2153. doi: 10.1245/s10434-019-07159-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doke K, Butler S, Mitchell MP. Current therapeutic approaches to DCIS. J Mammary Gland Biol Neoplasia. 2018;23(4):279–291. doi: 10.1007/s10911-018-9415-1. [DOI] [PubMed] [Google Scholar]
- 18.Leone JP, Hassett MJ, Leone J, Tolaney SM, Vallejo C T, Leone BA, et al. Efficacy of neoadjuvant chemotherapy in male breast cancer compared with female breast cancer. Cancer. 2022;128(21):3796–3803. doi: 10.1002/cncr.34448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Desmond A, Kurian A W, Gabree M, Mills M A, Anderson MJ, Kobayashi Y, et al. Clinical actionability of multigene panel testing for hereditary breast and ovarian cancer risk assessment. JAMA Oncol. 2015;1(7):943–951. doi: 10.1001/jamaoncol.2015.2690. [DOI] [PubMed] [Google Scholar]