Abstract
Avian campylobacteriosis is a vandal infection that poses human health hazards. Campylobacter is usually colonized in the avian gut revealing mild signs in the infected birds, but retail chicken carcasses have high contamination levels of Campylobacter spp. Consequently, the contaminated avian products constitute the main source of human infection with campylobacteriosis and result in severe clinical symptoms such as diarrhea, abdominal pain, spasm, and deaths in sensitive cases. Thus, the current review aims to shed light on the prevalence of Campylobacter in broiler chickens, Campylobacter colonization, bird immunity against Campylobacter, sources of poultry infection, antibiotic resistance, poultry meat contamination, human health hazard, and the use of standard antimicrobial technology during the chicken processing of possible control strategies to overcome such problems.
Key words: broiler processing, Campylobacter, control strategies, foodborne infection, natural compounds
INTRODUCTION
Campylobacter is a gram-negative, facultative intracellular bacteria which belongs to epsilon proteobacteria which is a class of proteobacteria (Mota-Gutierrez et al., 2022). At the same time, it has been described as an animal pathogen since the early 1900s due to its particular growing conditions (Skirrow, 2006). Its importance as a causative agent of bacterial gut illness was not established until the 1970s with a selective medium that could isolate and sustain the growth of Campylobacter in the laboratory (Allos, 2001).
Campylobacter was previously taxonomically grouped as Vibrio spp., and its infections in humans and animals were described as “related Vibrio infections” (Johnson et al., 2017; Wales et al., 2019). In 1963, the genus Campylobacter had been proposed by Sebald and Veron (1963). Subsequently, Vibrio-like organisms, including Campylobacter coli and Campylobacter jejuni, were regrouped as Campylobacter spp., in 1973 (Véron and Chatelain, 1973).
Campylobacter is a strain of bacteria that thrives only under very specific conditions, and it requires extraordinary conditions to grow (Hazeleger et al., 1998). Campylobacter is a thermophilic bacterium, growing optimally at temperature of 42°C, while minimal growth appears at 31°C to 32°C (Hazeleger et al., 1998). Furthermore, it is a microaerobic bacteria that requires approximately 5% O2, 10% CO2, and 85% N2 to grow (Bolton et al., 1997). Another distinctive characteristic that separates Campylobacter from other enteric bacteria is its inability to metabolize glucose (Hofreuter, 2014).
In addition, Campylobacter utilizes tricarboxylic acid cycle intermediates and amino acids as carbon resources (Guccione et al., 2008; Hofreuter, 2014). Despite the metabolic limitations, Campylobacter can colonize wild and domestic animal hosts (Sheppard and Maiden, 2015). The avian species is the preferred host species of Campylobacter, as its optimal growth is supported by the avian species' natural intestinal temperature (Wagenaar et al., 2015). Campylobacter is also sensitive to several environmental stressors, including exposure to atmospheric oxygen levels (Smith et al., 2016; Hsieh et al., 2018), dehydration (Line, 2006), as well as heat (Klančnik et al., 2014) and cold shocks (Josefsen et al., 2015).
However, using different survival mechanisms, Campylobacter can survive extended periods in unfavorable environmental conditions (Hughes et al., 2009). Campylobacter has been noticed to be a viable but nonculturable (VBNC) conditions upon subject to unfavorable circumstances (Hazeleger et al.,1994). Morphological changes characterize the VBNC state, reduced metabolic rates, increased chemical and physical stressors tolerance, and decreased culture ability in growth media (Lv et al., 2020).
It is also debatable whether VBNC Campylobacter cells can be resuscitated to colonize the host and initiate infection in the host (Ballou et al., 2016). Additionally, it was noticed that Campylobacter could perform biofilm when subjected to an aerobic environment (Bezek et al., 2016). Ica et al. (2012) revealed that Campylobacter cells might enter a VBNC state once the biofilm is formed to protect the cells from dangerous conditions. However, Teh et al. (2014) argued that there is not enough evidence to conclude that biofilm formation in Campylobacter is a part of the survival mechanism; instead, the observed formation of biofilm may be a result of simple attachment to abiotic surfaces or food matrices (Teh et al., 2014). Nonetheless, the capability of Campylobacter to live outside the host and adapt to different environmental circumstances demonstrates how Campylobacter can survive via the food production chain (Firlieyanti et al., 2016; EFSA and ECDPC, 2019; Elgamoudi et al., 2020; Habib et al., 2023).
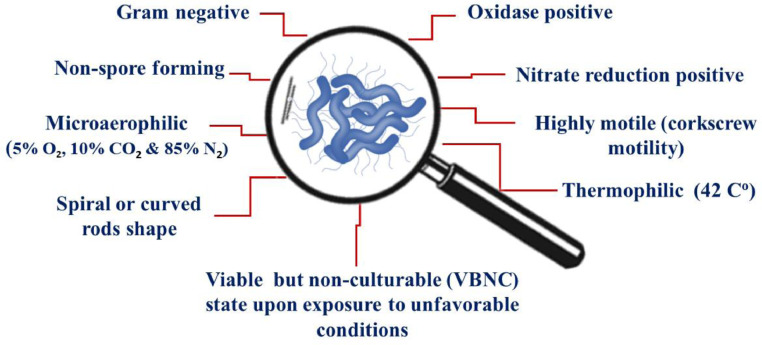
The morphological, biochemical, and physiological characteristics of Campylobacter spp. are presented in Figure 1. Recently molecular tools were known as the best methods used for microbial identification (Hong et al., 2023). The world is directed to the use of natural materials which have been proved to have antimicrobial effects against different species of Campylobacter (Balta et al., 2021). The antimicrobial activity of some natural compounds against different species of Campylobacter biofilm, their minimal inhibitory concentration (MIC), and their mode of action are summarized in Table 1.
Figure 1.
The morphological, biochemical, and physiological characteristics of Campylobacter species.
Table 1.
The antimicrobial activity of some natural compounds against different species of Campylobacter biofilm, their minimal inhibitory concentration (MIC), and their mode of action.
| Natural compounds | MIC | Campylobacter strain | Mechanism | References |
|---|---|---|---|---|
| Essential oils and polyphenolic extracts | ||||
| Cinnamaldehyde | 1.76 µg mL−1 | Campylobacter coli | A substance deposited on the bilayer surface that causes membrane instability | Wagle et al., 2019 |
| Eugenol | 2.69 µg mL−1 | Campylobacter jejuni NCTC 81–176 | Degradation of the extracellular matrix | Klančnik et al., 2021 |
| Carvacrol | 31.25 µg mL−1 | Campylobacter jejuni NCTC 11168 | Disintegration of hydrophobicity by binding membrane hydrophobic groups | Šimunović et al., 2020 |
| (−)-α-Pinene | 125 µg mL−1 | Campylobacter jejuni RC039 | A substance deposited on the bilayer surface that causes membrane instability | Salehi et al., 2019 |
| Resveratrol | 200 µg mL−1 | Campylobacter jejuni S-8 | Inhibition of AI-2 molecule activity | Roila et al., 2019 |
| Diallyl sulfide | 40 µg mL−1 | Campylobacter jejuni F38011 | Disintegration of hydrophobicity by binding membrane hydrophobic groups | Duarte et al., 2015 |
| Clove oil | 400 µg mL−1 | Campylobacter jejuni 180ip | A substance deposited on the bilayer surface that causes membrane instability | Wagle et al., 2021 |
| Lavender essential oil | 1000 µg mL−1 | Campylobacter jejuni 238ip | Inhibition of AI-2 molecule activity | Gahamanyi et al., 2020 |
| Juniper essential oil | 1000 µg mL−1 | Campylobacter coli | A substance deposited on the bilayer surface that causes membrane instability | Gahamanyi et al., 2020 |
| Grapefruit seed extract | 60 µg mL−1 | Campylobacter jejuni NCTC 81–176 | A substance deposited on the bilayer surface that causes membrane instability | Silveira et al., 2019 |
| Citrus lemon peel extract | 225 µg mL−1 | Campylobacter jejuni NCTC 11168 | Degradation of the extracellular matrix | Castillo et al., 2014 |
| Green tea extract | 50 µg mL−1 | Campylobacter jejuni RC039 | Disintegration of hydrophobicity by binding membrane hydrophobic groups | Wagle et al., 2021 |
| Antimicrobial peptides and amino acids | ||||
| Puroindoline A (PinA) | 512 µg mL−1 |
Campylobacter jejuni 81–176 Campylobacter jejuni RC039 Campylobacter jejuni S-8 |
Reacting with specific membrane components, such as anionic phospholipids and lipopolysaccharides, which break down the membrane and kills bacteria. The reaction may depend on the hydrophobicity of peptides by binding hydrophobic membrane groups. Additionally, peptides are deposited on the bilayer surface due to ionic/electrostatic interactions, resulting in membrane instability and disintegration. When peptides contain both hydrophobic and hydrophilic residues, a property known as amphipathicity, both of the preceding mechanisms may be at work. In addition, peptide length is crucial; short peptides have an excellent amphipathic structure and potent antimicrobial activity. | McDougald et al., 2012 |
| White kidney bean protein hydrolysate | 90 µg mL−1 | Campylobacter jejuni NCTC 11168 | Reacting with specific membrane components, such as anionic phospholipids and lipopolysaccharides, which break down the membrane and kills bacteria. The reaction may depend on the hydrophobicity of peptides by binding hydrophobic membrane groups. Additionally, peptides are deposited on the bilayer surface due to ionic/electrostatic interactions, resulting in membrane instability and disintegration. When peptides contain both hydrophobic and hydrophilic residues, a property known as amphipathicity, both of the preceding mechanisms may be at work. In addition, peptide length is crucial; short peptides have an excellent amphipathic structure and potent antimicrobial activity. | Saad et al., 2021 |
| Red kidney bean protein hydrolysate | 75 µg mL−1 | Campylobacter jejuni NCTC 11168 | Reacting with specific membrane components, such as anionic phospholipids and lipopolysaccharides, which break down the membrane and kills bacteria. The reaction may depend on the hydrophobicity of peptides by binding hydrophobic membrane groups. Additionally, peptides are deposited on the bilayer surface due to ionic/electrostatic interactions, resulting in membrane instability and disintegration. When peptides contain both hydrophobic and hydrophilic residues, a property known as amphipathicity, both of the preceding mechanisms may be at work. In addition, peptide length is crucial; short peptides have an excellent amphipathic structure and potent antimicrobial activity. | Saad et al., 2021 |
| Black kidney bean protein hydrolysate | 45 µg mL−1 | Campylobacter jejuni NCTC 11168 | Reacting with specific membrane components, such as anionic phospholipids and lipopolysaccharides, which break down the membrane and kills bacteria. The reaction may depend on the hydrophobicity of peptides by binding hydrophobic membrane groups. Additionally, peptides are deposited on the bilayer surface due to ionic/electrostatic interactions, resulting in membrane instability and disintegration. When peptides contain both hydrophobic and hydrophilic residues, a property known as amphipathicity, both of the preceding mechanisms may be at work. In addition, peptide length is crucial; short peptides have an excellent amphipathic structure and potent antimicrobial activity. | Saad et al., 2021 |
| D-Tryptophan | 1000 µg mL−1 |
Campylobacter jejuni 180ip Campylobacter jejuni 238ip |
Reacting with specific membrane components, such as anionic phospholipids and lipopolysaccharides, which break down the membrane and kills bacteria. The reaction may depend on the hydrophobicity of peptides by binding hydrophobic membrane groups. Additionally, peptides are deposited on the bilayer surface due to ionic/electrostatic interactions, resulting in membrane instability and disintegration. When peptides contain both hydrophobic and hydrophilic residues, a property known as amphipathicity, both of the preceding mechanisms may be at work. In addition, peptide length is crucial; short peptides have an excellent amphipathic structure and potent antimicrobial activity. | Elgamoudi et al., 2020; Saad et al., 2021 |
| D-Serine | 1000 µg mL−1 |
Campylobacter jejuni RC039 Campylobacter jejuni S-8 |
Reacting with specific membrane components, such as anionic phospholipids and lipopolysaccharides, which break down the membrane and kills bacteria. The reaction may depend on the hydrophobicity of peptides by binding hydrophobic membrane groups. Additionally, peptides are deposited on the bilayer surface due to ionic/electrostatic interactions, resulting in membrane instability and disintegration. When peptides contain both hydrophobic and hydrophilic residues, a property known as amphipathicity, both of the preceding mechanisms may be at work. In addition, peptide length is crucial; short peptides have an excellent amphipathic structure and potent antimicrobial activity. | Elgamoudi et al., 2020; Saad et al., 2021 |
| D-Alanine | 1000 µg mL−1 |
Campylobacter jejuni RC039 Campylobacter jejuni S-8 |
Reacting with specific membrane components, such as anionic phospholipids and lipopolysaccharides, which break down the membrane and kills bacteria. The reaction may depend on the hydrophobicity of peptides by binding hydrophobic membrane groups. Additionally, peptides are deposited on the bilayer surface due to ionic/electrostatic interactions, resulting in membrane instability and disintegration. When peptides contain both hydrophobic and hydrophilic residues, a property known as amphipathicity, both of the preceding mechanisms may be at work. In addition, peptide length is crucial; short peptides have an excellent amphipathic structure and potent antimicrobial activity. | Elgamoudi et al., 2020; Saad et al., 2021 |
| D-Methionine | 1000 µg mL−1 |
Campylobacter jejuni NCTC 11168 Campylobacter jejuni 180ip Campylobacter jejuni 238ip |
Reacting with specific membrane components, such as anionic phospholipids and lipopolysaccharides, which break down the membrane and kills bacteria. The reaction may depend on the hydrophobicity of peptides by binding hydrophobic membrane groups. Additionally, peptides are deposited on the bilayer surface due to ionic/electrostatic interactions, resulting in membrane instability and disintegration. When peptides contain both hydrophobic and hydrophilic residues, a property known as amphipathicity, both of the preceding mechanisms may be at work. In addition, peptide length is crucial; short peptides have an excellent amphipathic structure and potent antimicrobial activity. | Elgamoudi et al., 2020; Saad et al., 2021 |
| Probiotics derivatives | ||||
| Bacteriocin Reuterin |
32 µg mL−1 580 µg mL−1 |
Campylobacter jejuni Campylobacter coli |
DNA synthesis interfering with the membrane integrity of bacterial cells | Asare et al., 2020 |
|
Campylobacter jejuni Campylobacter jejuni |
DNA synthesis interfering with the membrane integrity of bacterial cells | Asare et al., 2020 | ||
| Glycolipid biosurfactant | ||||
|
Sophorolipid |
32 µg mL−1 |
Campylobacter jejuni subsp. jejuni 33560 |
Lysis of the cell membrane | Silveira et al., 2019 |
EPIDEMIOLOGY OF CAMPYLOBACTER IN BROILER PRODUCTION
Avian Campylobacteriosis
Campylobacter can multiply in the gut epithelium of almost warm-blooded host species (Biswas et al., 2019; Barker et al., 2020). Poultry is the predominant host for Campylobacter spp., possibly due to their elevated body temperature (Kers et al., 2018; Tram et al., 2020; Beterams et al., 2023). Even while all commercial bird species may host Campylobacter spp., the risk is greater in chicken due to the massive volumes consumed (Ijaz et al., 2018; Dubovitskaya et al., 2023). Campylobacter is a commensal in broiler chickens that forms a benign condition with multiplication up to 1010 colony-forming units (CFU) g droppings−1 (Dhillon et al., 2006; Battersby et al., 2016). Campylobacter was collected from practically every area of the broiler's gut; however, it is instantly present in the caeca and cloaca, which do not attach to the villus epithelium but rather to the mucus coating the intestinal villi (Achen et al., 1998).
In contrast to the illness in humans, Campylobacter do not cause any lesion in chickens and cohabits with the lower gut in a commensal state (Ingresa-Capaccioni et al., 2016; Myintzaw et al., 2023). Histopathological investigations showed no pathological lesions or marked alteration in crypt features (Shaughnessy et al., 2011; Golz et al., 2020). Furthermore, multiplication persists, implying that immunity is inefficient in eradicating infection, at least in these conditions. At the same time, older birds, such as layers, may have had less colonization over time (Johannessen et al., 2020). In summary, this situation has enormous advantages for Campylobacter and no adverse consequences for the host (Beterams et al., 2023).
Campylobacter Colonization and Immunity
Ingestion of 35 CFU of Campylobacter was sufficient to induce multiplication in chicken tissues (Umar et al., 2016; Urdaneta et al., 2023). After ingestion, Campylobacter were directed to cecal and cloacal crypts, revealing an established Campylobacter multiplication for 24 h postintroduction (Coward et al., 2008). Campylobacter is rarely noticed in chicken below 2 to 3 wk old (van Gerwe et al., 2009). This young age-linked resistance (commonly called lag phase) expanded against various Campylobacter spp., which is not fully known (Sahin et al., 2002). Campylobacter specified maternal antibodies are predominant in chicken and might be included in this resistance (Šimunović et al., 2020). The high concentration of antibodies detected within the first 2 wk declines to negligible levels within 3 to 4 wk (Sahin et al., 2002; Šimunović et al., 2020).
In addition, the phase of intestinal growth was hypothesized to be included in this age-related resistance, as bird gut tissues undergo physiological modifications within the brooding time (van Der Wielen et al., 2000; Natsos et al., 2019). Alterations in the gut microbiota and competitive cecal microbiome (Mead, 2002) are also related to the lag stage and raising techniques, such as ration and medication changes that occur during the rearing period (Mead, 2002).
Chickens are easily colonized with Campylobacter due to their rapid reproduction (Bailey et al., 2019). Most (>95%) of the chickens of that flock are multiplied for days (Stern et al., 2001) and stay so until slaughter (Coward et al., 2008; Bailey et al., 2019). However, after 8 wk, colonization could lower the bacterial count and number of colonized chickens, accompanied by adaptive immunity and modification in the nutritious tract microbiome (Sahin et al., 2003a; Vandeplas et al., 2010).
However, no lesion was noticed with chicken colonization, and gut immunity to the disease was detected with elevated cytokine expression (Borrmann et al., 2007; Larson et al., 2008; Li et al., 2008) and toll-like receptor (TLR) activation (de Zoete et al., 2010). Campylobacter can stimulate both systemic and mucosal immunity in birds, as various research reported the presence of immune-accompanied gene and protein expression post-Campylobacter multiplication of birds (de Zoete et al., 2007).
However, it is still unknown how Campylobacter reacts with the bird's immune organs to excite immunity (Lin, 2009). Analysis of collected chicken samples showed increased cytokine expression (Smith et al., 2008) and circulating monocytes/macrophages (Meade et al., 2009; Pumtang-On et al., 2021). Various avian cells provoke or upregulate cytokines throughout in vitro challenges (Li et al., 2008). However, the chicken host's Campylobacter-specific antibody response was slow and moderate since the infection did not cause obvious intestinal inflammation or cell death (Lin, 2009; Gorain et al., 2020). In some investigations, Campylobacter was also recovered from the thymus, bursa of Fabricius, reproductive tract, spleen, hepatic tissue, and blood in chicken, supposing that Campylobacter may attack gut tissue and induce systemic reaction (Meade et al., 2009; Nothaft et al., 2021).
Recent research established that C. jejuni could attach to and enter the villi of birds in vitro and in vivo. However, the C. jejuni strains that attack chicken epithelium tissues could not colonize intracellularly but rapidly escape from the cells (Byrne et al., 2007). Thus, Van Deun et al. (2008) supposed a new multiplication procedure of C. jejuni by evading short-term clearance via fast epithelial invasion and then evasion, with rapid multiplication in the mucus. Other studies have demonstrated that chicken immunity may be ineffectively stimulated upon Campylobacter multiplication, and expression of different antimicrobial peptide genes may be lowered (Hermans et al., 2012; Gorain et al., 2020). All these remarks may suggest that C. jejuni is correctly acclimatized to the avian host, and bacteria can be considered a commensal gut microbiota. This fact may contribute to the continuous multiplication of Campylobacter in the bird's alimentary tract (Gorain et al., 2020). Campylobacter colonization and bird immune response against the infection with campylobacteriosis are expressed in Figure 2.
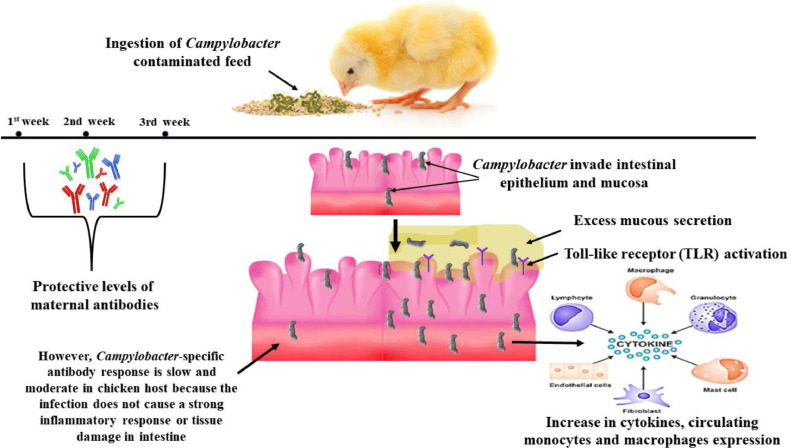
Figure 2.
Campylobacter colonization and bird immune response against the infection with campylobacteriosis. At the first 3 wk of age, the bird has a protective level of maternal antibodies against Campylobacter. After this age, the bird ingests contaminated food with Campylobacter. The bacteria then invade intestinal epithelium and mucosa stimulating excess mucous secretion and activation of toll-like receptors. The bird immunity stimulates cytokines, circulating monocytes, and macrophage expression.
Broiler Production and Campylobacter Jejuni
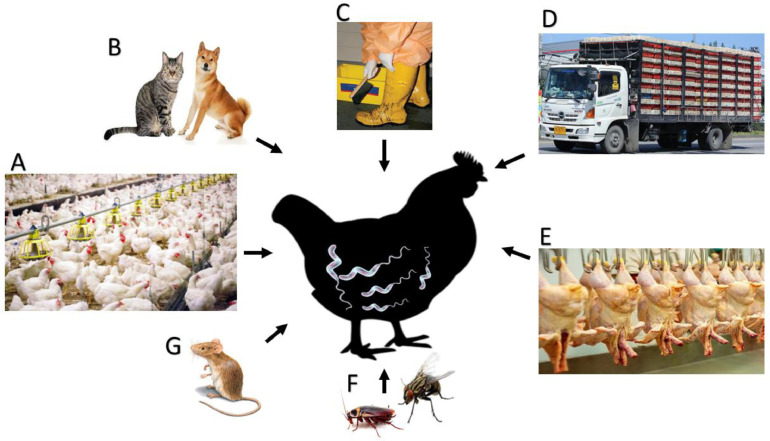
The different mechanisms of Campylobacter transmission in chickens are summarized in Figure 3.
Figure 3.
The mechanisms of Campylobacter transmission in chickens. (A) Contaminated feed, water, and utensils at the farm level. (B) Carnivorous, especially dogs and cats. (C) Human movement between different farms with contaminated utensils. (D) During birds’ transportation from previously contaminated cages or contaminated environments. (E) At slaughterhouses level during bird evisceration and processing with previously contaminated machines. (F) Insects and (G) rodents (insects and rodents can transmit infection during all stages of bird rearing, transportation, slaughtering, processing, and handling).
Infection With Campylobacter During the Rearing Period (at the Farm Level)
Independent farmers grow broiler chickens until the birds reach the market weight, which takes about 6 to 7 wk. During the grow-out and processing stages, Campylobacter can enter the production chain and contaminate either flock on the farm or carcasses at the processing area. C. jejuni is commensal in broilers due to the absence of inflammatory reactions induced by C. jejuni colonization and the failure of chickens to clear C. jejuni out from the intestines (National Chicken Council, 2019). Because of this, there are no clinical indications linked with the multiplication of C. jejuni in broilers (Salem et al., 2019; Abd El-Hack et al., 2021a).
In broilers, C. jejuni multiplication takes place in the lower gastrointestinal tissue. It is thought to persist in the intestine by undergoing fast replication in the mucus after invasion and evasion from the cecal crypt, thereby avoiding expulsion from the intestine (Van Deun et al., 2008; Wysok et al., 2020). Broilers can harbor high concentrations up to 109 CFU of C. jejuni g cecal matter−1 (Beery et al.,1988). Once C. jejuni enters a farm, the whole flock is expected to become colonized by C. jejuni in a few days (Newell and Fearnley, 2003; Gorain et al., 2020). However, several studies pointed out that not all chickens become colonized with Campylobacter within a positive flock, and colonization levels may vary broadly within flocks (Hansson et al., 2004, 2007, 2010). The multiplication of C. jejuni in broilers is not observed until 2 to 3 wk of broiler age (Shange et al., 2019). Because the timing of colonization coincides with maternal immunity, maternal antibodies may protect broilers from C. jejuni multiplication at a young age (Sahin et al., 2001; Singh et al., 2019).
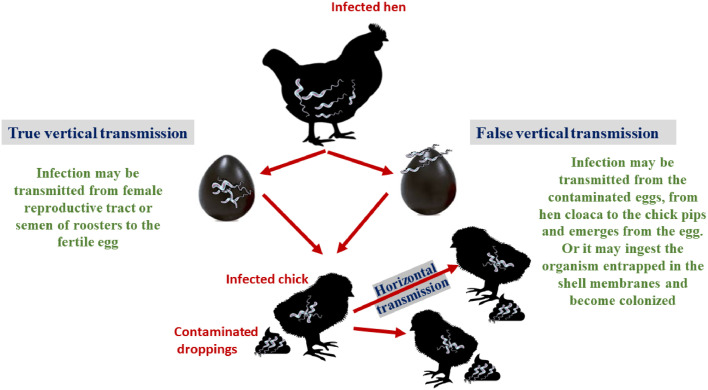
Furthermore, as broilers age, there is a decrease in Campylobacter shedding (Dipineto et al., 2011). Dipineto et al. (2011) revealed that Campylobacter multiplication lowered over time in laying hens, resulting in some birds being free of Campylobacter (Dipineto et al., 2011; Taha-Abdelaziz et al., 2018). This finding provides support for the hypothesis that age-related improvements in immunity and gut bacterial stability contribute to the more obvious Campylobacter colonization in older birds (Taha-Abdelaziz et al., 2018). Figure 4 provides a concise summary of the various modes of transmission of the Campylobacter infection.
Figure 4.
Transmission of Campylobacter disease hypothesis. True vertical transmission via female reproductive tract or contaminated roosters’ semen. False vertical transmission via contaminated eggs and the bacteria penetrates the eggshell. Horizontal transmission from infected bird to susceptible bird.
The potential of the vertical route of transmission of Campylobacter in broilers was studied extensively (Callicott et al., 2006; Vandeputte et al., 2019). Shanker et al. (1986) discovered that unless C. jejuni was directly injected into albumin, natural colonization did not occur, even when C. jejuni-challenged eggshells were used. In addition, a number of investigations have failed to differentiate the identical genotypes of C. jejuni in parent farms and their chicken offspring (Petersen et al., 2001; Vandeputte et al., 2020). The presence of anti-Campylobacter antibodies in the mother reduces both the vertical transmission and proliferation of the bacteria in newly hatched chickens (Sahin et al., 2001).
Campylobacter is most commonly spread from one flock of broilers to another through horizontal transmission (Gregory et al., 1997). There has been a significant amount of epidemiological research done to shed light on the identification of origins of Campylobacter in bird farms, and various possible sources have been found. These sources reported are insects (Dale et al., 2015), rodents (Hiett et al., 2002a,b,c; Gorain et al., 2020), wild birds (Hald et al., 2016), other livestock and avian farms (Zweifel et al., 2008), the environment around and within the poultry farm (e.g., soil, puddle, and drinking water) (Messens et al., 2009), and residual Campylobacter population from previous flocks (Hald et al., 2016; Taha-Abdelaziz et al., 2018).
Despite numerous attempts, researchers have been unable to differentiate Campylobacter from other possible sources until after the flock had been infested (Vandeputte et al., 2019). This failure could be because 1) many research had inadequate study design and sampling schemes, 2) previous studies failed to identify unknown sources of Campylobacter around and within the poultry farm, 3) Current laboratory techniques are incapable of reviving and cultivating Campylobacter in the VBNC state, or 4) the current genotyping techniques are not sensitive enough to differentiate Campylobacter strains due to high frequency of DNA recombination observed in Campylobacter genome (Agunos et al., 2014; Vandeputte et al., 2019).
Depending on the investigations that were conducted to determine the origins of the outbreak, multiple on-farm danger agents together with Campylobacter colonization in broiler farms have been found (Natsos et al., 2019). The majority of these risk factors are associated with poor biosecurity and hygiene standards in the chicken house (Chowdhury et al., 2012; Natsos et al., 2019). The failure to sanitize drinking water, the absence of anteroom and boot dips, and the absence of adequate pest treatments all contribute to the easy access that natural Campylobacter reservoirs have to the farm (Bailey et al., 2019).
Some other on-farm risk factors include the time of year (summer vs. other season) (Ellis-Iversen et al., 2012; Bailey et al., 2019), the practice of reusing litter (Sandberg et al., 2015), the length of the downtime period (the time between flocks when the broiler houses are cleaned and disinfected) (Sommer et al., 2016), the routine of thinning practice (Näther et al., 2009; Pumtang-On et al., 2021; Taha-Abdelaziz et al., 2023), general conditions of broiler farms (Chowdhury et al., 2012), and broilers age (Chowdhury et al., 2012).
Campylobacter Incidence in Broiler Chickens
The incidence in commercial broiler farms differs mostly according to the age of the animals (Koutsoumanis et al., 2020; Elhelw et al., 2022). Under the commercial system, Campylobacter is hardly detected in broilers 1 to 3 wk of age, but newly born chicken can be in vitro challenged with C. jejuni (Sahin et al., 2001). Campylobacter infection is commonly noticed post the third week of age for most commercial farms. While some chicken become infected, C. jejuni spreads throughout the rest of the farm and stays there until slaughter, polluting the meat in the processing facilities (Shreeve et al., 2000; Nothaft et al., 2021).
C. jejuni is most commonly seen in commercial birds, and as many as 100% of broilers at slaughter may be infected (Deng et al., 2020). Results from a European union survey in member states to evaluate the incidence of Campylobacter in broilers revealed a mean prevalence of 71.2% (95% confidence intervals: 68.5; 73.7), with results varying from 2% (Estonia) to 100% (Luxembourg). The median member states prevalence of Campylobacter-colonized broiler batches was 57.1% (EFSA and ECDC, 2021a,b).
In addition, results from the European summary report on trends and sources of zoonoses, zoonotic agents, and foodborne outbreaks in 2014 reported an overall prevalence of Campylobacter in fresh broiler flesh collected at slaughter, processing, and retail of 38.4% of the 6,703 examined units (EFSA and ECDC, 2015). Different recording member states had very different rates of Campylobacter-positive chicken meat samples. Campylobacter was found in 35.5% of single samples at retail, and 6 of the 11 member states that took samples at retail found that less than 50% of them were positive. At the slaughterhouse level, Campylobacter was found in 44.4% of the single samples that were checked (EFSA and ECDC, 2015).
Birds' Campylobacter shedding rates fluctuate throughout the year, with summer seeing the highest rates (Wedderkopp et al., 2000). However, C. jejuni is prevalent in broilers, and some flocks remain Campylobacter-negative during their raising period (Stern et al., 2001; Gorain et al., 2020). Campylobacter is prevalent in organic and free-range chicken flocks (Heuer et al., 2001; Clavijo and Florez, 2018), suggesting that all manufacture techniques are susceptible to Campylobacter infection.
Broiler Farms: Potential Sources of Contamination and Risk Factors
Campylobacter potential origins and transmission routes in bird farms have been widely investigated. However, no apparent factor(s) has been discovered to explain the organism's prevalence in commercial avian farms (Higham et al., 2018; Chintoan-Uta et al., 2020). According to numerous studies, the most likely cause of C. jejuni infection in chickens is horizontal dissemination from the surrounding environment (O'Mahony et al., 2011). Possible sources include old litter (Thakur et al., 2013), untreated drinking water (Zimmer et al., 2003), other farm animals or domestic pets (Ellis-Iversen et al., 2009, 2012), insects (Hazeleger et al., 2008), rodents (McDowell et al., 2008), instruments, and transport vehicles and farm laborers (Ridley et al., 2008; Sibanda et al., 2018).
Due to extreme sensitivity to oxygen and moisture, Campylobacter cannot thrive in the natural environment (Singh et al., 2019). Campylobacter is seldom found in fresh litter or feed testing before birds become ill (Thakur et al., 2013). Campylobacter is a bacterium that may be spread from contaminated used litter to agricultural settings (Montrose et al., 1985). Due to frequent cleaning, disinfection, and the replacement of litter between flocks, litter is seldom a cause of contamination in EU broiler production (Evans, 1992). Furthermore, in the USA, national epidemiologic studies found no significant variations in the prevalence and onset of Campylobacter among flocks on different grow-out farms employing different litter utilization practices (Stern et al., 2001). Cattle, swine, and other poultry have all been found to serve as important reservoirs for Campylobacter (Lyngstad et al., 2008; Vandeputte et al., 2019).
Molecular epidemiological research on farm animals has shown that strains spreading to bird flocks are sometimes discovered alongside other livestock, such as cows and swine (Korsak et al., 2015; Di Giannatale et al., 2019; Ito and Kishimoto, 2023). In longitudinal investigations, the route of transfer from the animals to the broilers may be seen before avian flock multiplication (Katsma et al., 2007). Removing other livestock from a chicken farm will only lower infection by 41 to 44% (Katsma et al., 2007). However, this appears to be a rather infrequent event (Johnsen et al., 2006; Vandeputte et al., 2020), and most of the strains discovered near cattle are not detected later in broilers. In addition, domestic animals such as dogs and cats were seen to harbor on a regular basis (Vandeputte et al., 2020). They shed C. coli and C. jejuni and have been identified as a risk agent of Campylobacter disease in broiler farms (Torralbo et al., 2014; Hwang and Singer, 2020).
Insects, like beetles, flies, may act as mechanical vectors for Campylobacter transmission from livestock to chickens (Hald et al., 2008). Shane et al. (1985) was the first to demonstrate Campylobacter transmission by flies across chicken flocks in a controlled laboratory setting. Hald et al. (2004) showed that C. jejuni was mainly transported by house flies from animals to the broiler farms via ventilation inlets. Furthermore, flies may have a linking effect in Campylobacter epidemiology, since several studies have proven the influence of hygiene barriers and fly screens in reducing the prevalence of Campylobacter spp., between farms of broiler chickens (Bahrndorff et al., 2013; Gölz et al., 2018). However, the role of flies as a key cause of Campylobacter infection in broilers was controversial (Gölz et al., 2018).
Although some Campylobacter serotypes and genotypes were found from insects and broilers on farms, the path of spread was unclear (Bailey et al., 2019). Prior to the positive confirmation of Campylobacter in the broilers, it was unusual for insects to test positive in a chicken farm, suggesting that insects are not the predominant source of Campylobacter in a broiler farm (Nesbit et al., 2001). The larvae of darkling beetles have also been linked to the transmission of C. jejuni between breeding populations (Refrégier-Petton et al., 2001).
There is also evidence that rats and mice can spread Campylobacter (Arsenault et al., 2007; McDowell et al., 2008; Newell et al., 2011). In addition, there have been reports of rodents in certain contemporary poultry farms (Evans and Sayers, 2000). This threat may be minimal because Campylobacter carriage is seldom identified in trapped rats (Vandeputte et al., 2020). The risk of Campylobacter being introduced to poultry farms through the hands of a farm worker is high (Ridley et al., 2008; Hakeem et al., 2021). Humans providing poultry through a nonsterile environment increase the risk of Campylobacter multiplication, as shown by Johnsen et al. (2006).
Campylobacter may spread easily from the outside environment into the avian farm, and human traffic is a major vector for this pathogen (Cardinale et al., 2004; Hofshagen and Kruse, 2005). Another study by Hansson et al. (2010) confirmed the importance of agricultural workers following thorough hygiene protocols and using appropriate hygiene. There is a clear correlation between the number of agricultural employees and the number of tourists and the prevalence of Campylobacter infections (Huneau-Salaun et al., 2007).
Campylobacter was isolated from the hands, boots, and clothing of agricultural workers, catchers, and managers, as well as truck drivers (Ramabu et al., 2004). Human visitors to the avian home may bring Campylobacter in from the outside ecosystem, since molecular epidemiology shows that these strains are commonly followed by flock expansion (Ridley et al., 2008, 2011). Workers in the agricultural sector may not be the only ones concerned about the spread of Campylobacter in the birdhouse. The most recent European baseline assessment of Campylobacter in bird farms confirms the widespread belief that thinning or partial depopulation is a significant risk factor for infectious disease (EFSA, 2010; Kittler et al., 2021a). Many catching crews work for poultry companies. Like workers, they move from one farm to another with their trucks, tools, boots, and clothes, often without caring about their own hygiene (Harris et al., 2021; Malavi et al., 2021).
Both the sources of drinking water and the treatment procedure can have an effect on the proliferation of Campylobacter (Zimmer et al., 2003). Ogden et al. (2007) used multilocus sequence typing (MLST) to discover C. jejuni sequence types in water tanks and broiler houses, providing more evidence that water is likely a source of infection in broilers. In addition to finding C. coli in groundwater, Pérez-Boto et al. (2010) concluded that drinking water was a potential source of C. coli on bird farms. Even though Campylobacter has been found in water systems, whether or not this indicates recent fecal contamination from cattle or wild birds is still up for debate (Zimmer et al., 2003).
Drinking water in avian dwellings is often positive after chickens have been grown, raising questions about the potential significance of this source in introducing Campylobacter into avian environments (Nothaft et al., 2021). Consequently, the contaminated water does not support the growth of Campylobacter; rather, it only serves as a passive channel for the bacteria to spread (Sahin et al., 2002). Water treatments with disinfectants may prevent Campylobacter from accessing a farm rather than spreading within a livestock (Ellis-Iversen et al., 2009).
Campylobacter may also be introduced to poultry farms by a vertical pathway, from the hen to the egg to the fowl (Takeshita et al., 2021). The possibility that Campylobacter may be transmitted from birds' eggs to the next generation was, nevertheless, examined. Researchers have noted the potential for Campylobacter to be transmitted from birds to humans through contaminated egg products (Takeshita et al., 2021). Campylobacter was successfully found in 10% of 275 semen samples from commercial broiler breeder roosters, with quantities as high as 1,000 CFU mL−1, as reported by Cox et al. (2002b). Furthermore, Campylobacter has been detected in the oviducts and other reproductive organs of breeder chickens (Hiett et al., 2002a; Cox et al., 2005; Natsos et al., 2019). Campylobacter may survive in the moist membranes of eggshells. The chicken may eat the bacterium as soon as it pips and emerges from the egg, becoming infected and spreading it to other chickens in the flock through its cecal droppings (Tang et al., 2020). It is hypothesized that Campylobacter can be transmitted from one generation of broilers to the next via the egg, which becomes contaminated with droppings as it travels through the cloaca (Allen and Griffiths, 2001; Cox et al., 2012; Marotta et al., 2015; Tang et al., 2020).
Cox et al. (2002a) detected ribotypes and flaA short-variable-region alleles in a commercial broiler breeder flock and its progeny broiler flock, providing molecular evidence of the occurrence of vertical transmission. Further evidence that Campylobacter might be present in chicken before to transit to the farm comes from the finding of amplifiable Campylobacter DNA in samples taken from hatchery fluff, a gut of developing embryos, and newly hatched chicken (Thibodeau et al., 2015).
On the other hand, Dion et al. (2020) concluded that while vertical transmission between breeders and broilers is necessary, it is infrequent and poses little threat to commercial farms. Newell and Fearnley (2003) observed that the vertical spread of C. jejuni is unusual because of a constant lag stage in recognizing C. jejuni multiplication in birds. However, Campylobacter may be present in the hatching chicken in very low numbers, and factors such as maternal antibodies keep the population from expanding too rapidly (Sahin et al., 2003a). This is supported by the fact that, when using standard culture methods, many laboratories have been unable to identify Campylobacter in day-old chicks (Herman et al., 2003; Mazengia et al., 2015).
The ability to reliably isolate Campylobacter from a variety of biological and dietary samples is presently unavailable because to the lack of a suitable culture technique. Most culture methods were developed to isolate Campylobacter from highly contaminated feces (Taha-Abdelaziz et al., 2018). However, these techniques may not identify Campylobacter in foods or biological materials if the quantity of cells is minimal, the cells are sublethally injured or stressed, or if they are living but cannot be cultured (Taha-Abdelaziz et al., 2018).
It has been hypothesized by Agunos et al. (2014) that a major roadblock in studying Campylobacter in broilers is the difficulty to cultivate the bacteria from younger birds (>2 wk old). There is still a significant prejudice against the assumption that viable eggs might generate Campylobacter infection in breeder and broiler farms due to the inability to grow Campylobacter. The evidence that there are other modes of egg transmission than the traditional transovarial route has been largely ignored by previous investigations (Cox et al., 2012; Gorain et al., 2020).
Campylobacter Contamination of Broiler Flocks During Transport and Slaughter
Transport from farm to processing plant, live haul, bleeding, scalding, plucking (defeathering), evisceration, and chilling are the traditional primary processing stages for broiler chickens (Barbut, 2016). There is a high probability of intra- and intercongregational Campylobacter infection during the transportation of a flock from the farm to the processing plant (Slader et al., 2002; Hansson et al., 2005). According to epidemiological study, insufficient cleaning and disinfection of transportation modules and trucks can lead to Campylobacter-negative birds becoming contaminated with persistent Campylobacter observed on this equipment during transit, since the surface of broilers has the potential to get contaminated with Campylobacter (Shang et al., 2018; Karki et al., 2019; Rasschaert et al., 2020).
Thinning is a prevalent practice in European countries when a subset of a flock is slaughtered early or late to satisfy varied market weight standards. Without adequate biosecurity measures being taken during the capture and transportation of broiler chickens, there is a considerable increase in the danger of introducing Campylobacter to the remaining broilers (Newell et al., 2011). Campylobacter concentrations on carcasses are known to fluctuate widely throughout the various stages of processing. While it may be challenging to eliminate all Campylobacter from carcasses during processing, it is possible to significantly reduce the amount of Campylobacter present (Newell et al., 2011). However, there is also a chance that harmful carcasses will be cross-contaminated. Scalding, plucking, and eviscerating are practices that are known to increase the spread of foodborne pathogens (Berrang et al., 2011; Nothaft et al., 2021). Scalding entails immersing carcasses in hot water to open feather follicles and allow feathers to be easily removed during the harvesting process. The defeathering phase occurs prior to the evisceration stage and entails the removal of feathers and the top layer of the integument (USDA-FSIS, 2008).
Feathers with a high concentration of feces can contaminate slaughterhouse scalding tanks and spread disease to meat (Singh et al., 2019). When picking fingertips rub against carcasses, feces can be released, resulting in cross-contamination between carcasses (Berrang et al., 2001; Vandeputte et al., 2019). Evisceration is a technique that eliminates internal viscera and any processing errors from carcasses before chilling. Because high levels of Campylobacter are commonly found in broiler chicken intestines, rupturing the intestine can release Campylobacter, contaminating carcasses (Izat et al., 1988). Carcasses are tested for contamination and reprocessed if any is found. It has been shown, however, that reprocessing does not always reduce bacterial levels in carcasses (Fletcher et al., 1997). It is critical to avoid both indirect contact between contaminated and uncontaminated carcasses and the release of Campylobacter from contaminated carcasses. Reducing Campylobacter levels on carcasses and minimizing cross-contamination throughout each processing step requires the use of appropriate antimicrobial and sanitary techniques. Frequently rinsing the processing equipment is recommended by USDA-FSIS (2015) to prevent the accumulation of contaminants and bacteria.
Chloride and peroxides are two examples of antimicrobial agents that could be added to chilling tanks and washing water to prevent the growth of bacteria (USDA-FSIS, 2015; Bailey et al., 2019). It is also recommended to use countercurrent water flow so that carcasses are gradually exposed to cleaner water (Buhr et al., 2014). Another recommended technique is maintaining a healthy pH in water tanks to prevent the establishment of Campylobacter and other foodborne bacteria (USDA-FSIS, 2015). Lastly, it has been established that less contamination of one carcass by another can be achieved through better machine and equipment maintenance (USDA-FSIS, 2015).
Potential Risks During and Just Prior to Transport and Slaughter
When they are approximately 6 wk old, broilers are placed in crates, transported, and then processed at the butchery. Movement, crowding, temperature changes, and lack of water and food all contribute to the high levels of stress that animals experience during travel (Mainali et al., 2009; Dogan et al., 2019). Stress during transport from the farm to the processing facility disrupts gastrointestinal function, lowers an animal's resistance, and promotes the spread of gastrointestinal bacteria (Klančnik et al., 2013). Transportation stress has been linked to a 1,000-fold increase in bacterial populations on carcasses (Altekruse et al., 1999). Pollution levels could rise and Campylobacter could be introduced to wild farms if crates are not properly cleaned (Stern et al., 2001; Ridley et al., 2011). Even after cleaning and disinfecting, the contents of transport boxes are still microbiologically contaminated, even though the boxes have a clean outward appearance (Gorain et al., 2020). Some studies found that, even after cleaning and disinfecting, 60 and 71% of the transport crates examined were positive for Campylobacter (Rasschaert et al., 2007, 2020).
Furthermore, 80% of chicken farmers do not sterilize containers, and only 18.3% clean trucks and trailers properly, according to a survey conducted by Auburn University, USA among more than 10,000 poultry enterprises of varying sizes (O'Mahony et al., 2011; Nothaft et al., 2021). Frequent detection of organic residue on truck crates after washing indicates that the sanitizing process is ineffective and the germs are able to survive (O'Mahony et al., 2011). Consequently, the transmission in these contaminated crates may cause external contamination in Campylobacter-negative birds (Hansson et al., 2005; Rasschaert et al., 2020). Genotypes recovered from sanitized containers were also discovered on the carcasses of slaughtered chickens after shipment and slaughter (Vandeputte et al., 2019). According to Schroeder et al. (2014), the concentration of these pathogens on the surface of birds correlates with the concentrations found on thoroughly processed carcasses. Therefore, it is crucial to reduce the farm prevalence of these pathogens and transport stress throughout the process in order to lower the risk of contaminated meat products entering the food chain (Vandeputte et al., 2019).
Risk Factors During Slaughter, Dressing, and Processing
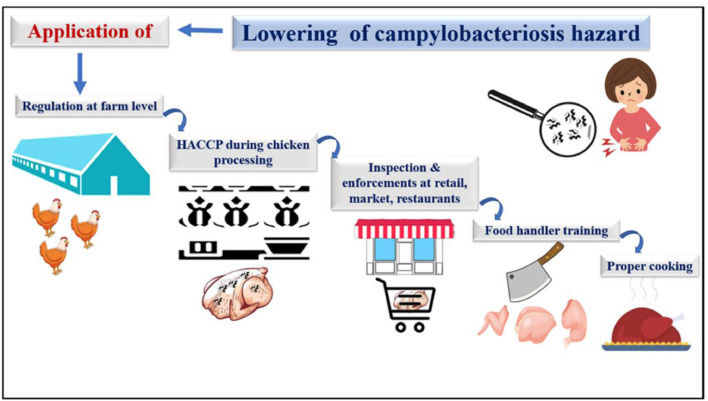
Using data from 2008, the European Food Safety Authorities were able to estimate the prevalence of Campylobacter and Salmonella-contaminated broiler carcasses across the community and in each EU member state, and they released those findings in 2010 (EFSA and ECDC, 2021b). The investigation centered on birds entering the food chain at the level of broiler batches in slaughterhouses (Thames and Sukumaran, 2020; Lindqvist et al., 2022). Campylobacter contamination of broiler carcasses was studied on a national scale by analyzing the contamination rate, sample size, and concentration of slaughterhouses. Carcass samples from 9,324 broiler batches were analyzed for Campylobacter in the European Union (Bertram et al., 2019). An increase in Campylobacter in the intestine's points to the possibility of contamination of bird carcasses during slaughter, most likely due to the spillage of droppings during defeathering and evisceration and the spread of disease in the slaughterhouse environment (Hakeem et al., 2020).
However, the European Union has yet to adopt the use of Hazard Analysis and Critical Control Points Programs (HACCP) to reduce Campylobacter contamination of meat carcasses (EFSA and ECDC, 2015; Giangaspero, 2018). Campylobacter is transported to the corpses during the slaughtering process, and the load may decrease during scalding, cooling, and freezing while increasing during defeathering and disemboweling (Gonzalez-Fandos et al., 2020). There have been multiple studies looking at how scalding affects Campylobacter contamination of carcasses. However, there have been reports of contrasting findings (Gonzalez-Fandos et al., 2020).
However, scalding has been identified by some research as a possible source of Campylobacter cross-contamination. The pathogen was found in scald tank water before the first birds arrived, indicating that contamination persists even after cleanliness measures have been taken (Schroeder et al., 2014; Perez-Arnedo and Gonzalez-Fandos, 2019). Scald tank water had an average of 2.90 CFU mL−1 of C. jejuni (Osiriphun et al., 2012). Thus, even after the tank was thoroughly cleaned, some Campylobacter remained. Previous research has documented the bacterium's ability to survive in this setting (Rahimi et al., 2010). The attachment of the Campylobacter to the broiler skin may be aided by the feather follicles in the integument, which are thought to shield the Campylobacter and prevent the loss of stratum corneum at high scalding temperatures (Chantarapanont et al., 2004; Vandeputte et al., 2020).
Campylobacter spp. are particularly well-adapted to life on the skin of broilers, where they can thrive in organic biofilms. Furthermore, the temperature in subcutis is frequently 3°C to 4°C lower than the scalding temperature (Yang et al., 2001). Similarly, Ellerbroek et al. (2010) found that scalding had no effect on the eradication of Campylobacter, even though the postscalding isolation rate was 91.1%. However, it has been reported that the total number of bacteria on skin carcasses decreases after scalding (Guerin et al., 2010; Lawes et al., 2012). Berrang et al. (2007) found a mean reduction of 0.43 log CFU after chilling, while Guerin et al. (2010) found the largest reduction of 2.9 CFU postscalding and 1.7 CFU after chilling. Campylobacter contamination has also been prevalent during the defeathering process (Duffy et al., 2014; Vandeputte et al., 2020).
Previous research has indicated that the disembowelment process may greatly enhance the cross-contamination of Campylobacter due to the shattering of internal organs and stomach contents (Seliwiorstow et al., 2015; Da Rosa et al., 2021). Droppings are expelled from the cloaca by the selector's fingers pressing on the abdomen during defeathering, resulting in high broiler carcass and slaughter equipment contamination (Mendes et al., 2020). Furthermore, the surface of the finger becomes rough with use, allowing bacteria to colonize the crevices on the surface of the rubber finger and multiply overnight if not adequately disinfected. This results in cross-contamination between flocks as bacteria on the rubber fingers are transferred to the corpses during the subsequent defeathering (Golden and Mishra, 2020). There has been no recent agreement on the trend in Campylobacter counts following evisceration, and Rosenquist et al. (2006) observed an increase in Campylobacter populations following evisceration. However, other authors reported a decrease (Reich et al., 2008; Taha-Abdelaziz et al., 2018) or no difference in Campylobacter numbers postevisceration (Berrang and Dickens, 2000; Zhang et al., 2020).
Processing facilities have various options for chilling corpses to lower their temperature (Berrang et al., 2008; Zeiger et al., 2017). Meat that has been immersed in cold water has a significantly lower Campylobacter counts than air-chilled meat (Berrang et al., 2008; Lee et al., 2017). Sanitizers like chlorine are used in some immersion chill tanks to help reduce contaminants like blood and tissue fragments (Guerin et al., 2010; Urdaneta et al., 2023). Chlorine use in the chill tank has been shown to significantly reduce the amount of Campylobacter but not completely remove bacteria (Berrang et al., 2007). Samples of water from the chill tank were found to contain Campylobacter, which suggests that this is the most likely source of cross-contamination (Lindblad et al., 2006; Vandeputte et al., 2019).
Pollution of Campylobacter can occur in uncontaminated carcasses brought to the chill tank, although extensively contaminated carcasses will have less bacteria when they are removed from the tank. However, the elimination of Campylobacter from the surface of the carcass through immersion freezing does not prevent cross-contamination (Bashor et al., 2004; Bailey et al., 2019). Chilling air has been shown to have either no microbiological effects (Vandeputte et al., 2019) or just minor effects (Bailey et al., 2019) on Campylobacter levels.
One final important consideration for this pathogen is the possibility of cross-contamination during manufacture. Close contact between carcasses and equipment results in the accumulation of tissue fragments harboring Campylobacter, which in turn contaminate succeeding carcasses (Oh et al., 2018; Nothaft et al., 2021). Furthermore, there is a risk of cross-contamination between bird carcasses when their outer surfaces come into contact with one another, with personnel hands, and with trimming mesh gloves and knives (Furukawa et al., 2017; Zhong et al., 2020). Slaughterhouse workers become vectors for the development of these diseases as soon as they come into contact with livestock (Myintzaw et al., 2020). Ellerbroek et al. (2010) discovered that processing instruments and laborers are a source of cross-contamination, and that Campylobacter can be found on staff's hands, slaughtering implements, and transport crates. Many researchers have also observed cross-contamination between batches from different flocks, as well as contamination of noninfected batches from previously slaughtered batches (Ellerbroek et al., 2010). Specifically, the first carcasses of subsequent negative batches are contaminated by Campylobacter-positive batches, as reported by Hue et al. (2010). Aerosols and droplets created by excessive washing during the hanging, defeathering, and evisceration stages of slaughter may also contribute to the spread of disease (Peyrat et al., 2008).
Campylobacter Jejuni as a Human Foodborne Disease
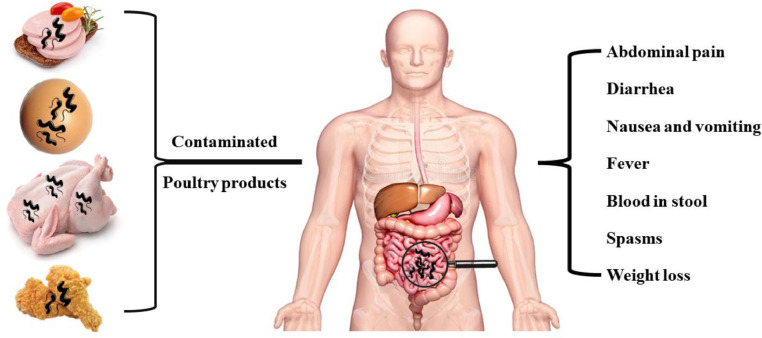
In affluent countries, Campylobacter is a common cause of acute gastroenteritis (Lynch et al., 2022). Figure 5, Figure 6 provide a summary of the avian reservoirs of human Campylobacter infections and the associated human symptoms. Campylobacteriosis is mostly caused by C. jejuni and is frequently encountered in solitary instances. However, campylobacteriosis outbreaks have previously been observed (Lahti et al., 2017; Šimunovi´c et al., 2022). Most people infected with campylobacteriosis either show no symptoms or experience only moderate ones, including diarrhea (which may or may not be bloody), nausea, fever, and abdominal pain (Allos, 2001). These moderate clinical manifestations can last for up to a week without demanding special antibiotic treatments or alternatives such as probiotics (ISO, 2017a; Neijat et al., 2019).
Figure 5.
Avian sources of Campylobacter infection in humans. This can be through contaminated eggs, chicken meat, and chicken meat products. The related symptoms in humans including abdominal pain, diarrhea, nausea, vomiting, fever, blood in stool, spams, and weight loss.
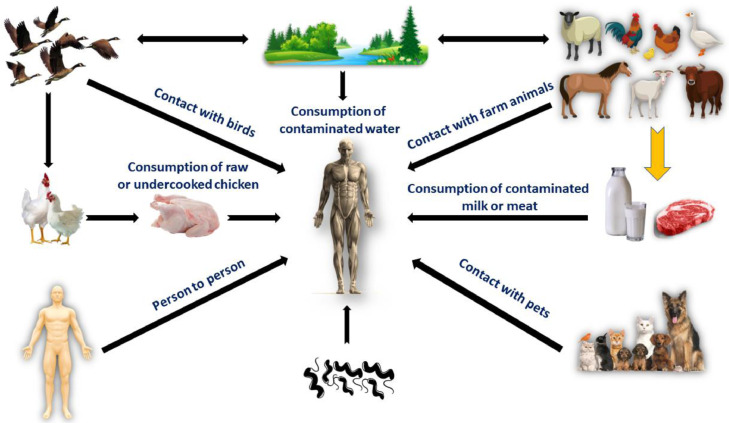
Figure 6.
Campylobacter infection sources in humans. Consumption of contaminated water; contact with infected farm animals, pets, or wild birds; consumption of contaminated milk, eggs, meat; consumption of undercooked or raw chicken or chicken products; and contact of diseased human with susceptible person.
Pancreatitis, peritoneal inflammation, bacteremia, reactive arthritis, and Guillain-Barré syndrome are only some of the more serious complications that could arise, with potentially devastating results (Ahmed et al., 2018). The precise mechanism by which an infection with Campylobacter brings about these long-term effects is unclear. The estimated incidence of campylobacteriosis in the USA is 14 cases per 100,000 people (CDC, 2019). In 2015, 13 deaths were attributed to Campylobacter infections, with 6,289 confirmed cases recorded by the Food Net surveillance system in the USA (CDC, 2017). The true incidence of campylobacteriosis is estimated to be 9 million cases annually in Europe due to the disease's asymptomatic character (Facciolà et al., 2017; ISO, 2017b).
Campylobacteriosis cases are most common in July and August, which coincides with the peak season for isolating Campylobacter from chickens and other fowl in industrialized countries (Sibanda et al., 2018). In contrast, campylobacteriosis is common and mild in third-world nations, where the vast majority of infections are asymptomatic (Platts-Mills and Kosek, 2014). Campylobacteriosis is estimated to be more prevalent in developing countries (Kaakoush et al., 2015). In a self-inflicted experiment, Robinson (1981) demonstrated that as little as 500 C. jejuni cells were sufficient to cause diarrhea and stomach pain, suggesting that the infective dose of C. jejuni is low. Similarly, investigations in healthy adults demonstrated that exposure to 800 to 105 CFU of C. jejuni could result in clinical symptoms (Tribble et al., 2010).
In addition, several experimental tests demonstrated that greater doses resulted in a higher frequency of attacks (Tribble et al., 2010; Weerasooriya et al., 2022). C. jejuni has an estimated incubation time of 2 to 4 d, while infections caused by more potent strains tend to have shorter incubation periods (Tribble et al., 2010). Campylobacteriosis is usually self-limiting, therefore patients recover without special therapy in most cases (Silva et al., 2011). Electrolyte and fluid replacement is suggested for mild cases (Vandeputte et al., 2019). Antibiotics may be advised for severe instances, particularly in vulnerable groups such as the young, the old, and those with impaired immune systems (Pacanowski et al., 2008; Pumtang-On et al., 2021; Abd El-Hack et al., 2022a; Taha-Abdelaziz et al., 2023).
Macrolides are a commonly used antibiotic for treating campylobacteriosis, especially in children when fluoroquinolones cannot be used (Pacanowski et al., 2008; Gorain et al., 2020). Many investigators of disease etiology have concluded that the most significant risk factor for campylobacteriosis is the ingestion or handling of raw or undercooked avian products (Rodrigues et al., 2001; Domingues et al., 2012). Avian spp. is the most important source of Campylobacter in humans due to the high levels of colonization they can maintain and the rising global consumption of avian items (Ramakrishnan et al., 2019; Kittler et al., 2021b).
Campylobacteriosis is caused by several factors, including consuming raw dairy products (Domingues et al., 2012), coming into contact with animals or pets, swimming in polluted water (Ravel et al., 2016; Campagnolo et al., 2018), and consuming untreated water (Domingues et al., 2012; Ravel et al., 2016). Travel to third-world countries may be responsible for a significant number of cases of USA campylobacteriosis, according to research by Ricotta et al. (2014). In the yr 2005 to 2011, international travel was linked to approximately 18% of campylobacteriosis cases in the USA. Antibiotic-resistant Campylobacter was more likely to be the source of these infections than common Campylobacter (Ricotta et al., 2014).
Reducing Campylobacter contamination in avian goods is seen as an effective technique for reducing the public health burden of campylobacteriosis in people, as chicken is the primary source of sporadic campylobacteriosis (Hankel et al., 2022). The success of countries like Iceland and New Zealand in drastically reducing campylobacteriosis rates shows that this disease is complex and requires a wide range of approaches (Sears et al., 2011; Tustin et al., 2011). There was a nationwide outbreak of campylobacteriosis in Iceland in 1998, just 1 yr after the disease was made reportable (Tustin et al., 2011; Hankel et al., 2022).
Within 4 yr, the campylobacteriosis rate increased from 12.2 to 116 per 100,000 people, and the increase was blamed on the accessibility of fresh poultry products (Stern et al., 2003). Control measures in the food production chain include a number of activities implemented at different stages (Sakaridis et al., 2018; Béjaoui et al., 2022). To reduce the amount of viable Campylobacter on items sold to the public, pre- and postharvest surveillance and monitoring systems were put in place, and goods from farms that tested positive for Campylobacter were mandated to be frozen before being sold (García-Sánchez et al., 2017, 2018; Gorain et al., 2020). Producer education initiatives improved the biosecurity measures taken on farms and the general cleanliness of farms and processing plants (Tustin et al., 2011; Eriksson et al., 2023).
Campylobacter awareness and safe food handling and preparation practices have been the subject of consumer education efforts (Stern et al., 2003; Tustin et al., 2011). Campylobacteriosis cases dropped by 72% in Iceland after these measures were taken (Wagenaar et al., 2013). New Zealand had a 54% decrease in the average prevalence of campylobacteriosis after implementing similar measures that targeted both pre- and postharvest sectors as well as retail and consumers (Sears et al., 2011). Even though the decline in these two countries is remarkable, it is unclear whether or not similar results can be achieved elsewhere (Wagenaar et al., 2013; Myintzaw et al., 2020).
Campylobacter infections are more likely to occur when raw poultry is handled improperly in a home kitchen (Kingsbury et al., 2023). According to Bull et al. (2006), C. jejuni may be present in 98% of commercially available chicken flesh in the USA and Europe. Table 2 presents information about the prevalence of Campylobacter spp. in raw chicken in various countries.
Table 2.
Campylobacter species prevalence in retail raw poultry meat in various countries.
Antimicrobial Resistance in Campylobacter Spp
Antibiotics are a type of antibacterial that either prevent the proliferation of bacteria or slow its progression (Lynch et al., 2020; Abd El-Hack et al., 2022b). Since the discovery of penicillin by Alexander Fleming in 1928 (Tan and Tatsumura, 2015; Kovács et al., 2019), antibiotics have greatly improved the prevention and treatment of infectious diseases in both human and veterinary medicine due to their ability to kill off harmful bacteria (Qin et al., 2023). Antibiotics were first used in animal production in the 1940s when they were introduced as growth boosters (Casagrande Proietti et al., 2018; Dai et al., 2020).
In response to the widespread use of antibiotics in hospitals and farms, Campylobacter has evolved multiple methods of resistance. Since then, the abuse of antibiotics in agriculture has led to an increase of antimicrobial-resistant foodborne bacteria, such as Campylobacter, which poses a severe threat to the efficacy of antibiotic therapies and raises serious public health issues (Van Boeckel et al., 2015; Khan et al., 2018; García-Sánchez et al., 2019). Antibiotics that are medically important to people were banned as growth promoters in food animal production in the USA in 2017. This was done in response to growing public health concerns about the spread of antibiotic-resistant foodborne bacteria to humans along the food chain (WHO, 2015, 2020; USDA, 2017).
In both underdeveloped and developed nations, Campylobacter isolates resistant to many antibiotics have been found (Bolinger and Kathariou, 2017; Myintzaw et al., 2023). Naturally occurring Campylobacter strains have developed resistance to many medications, including vancomycin, trimethoprim, rifampin, and bacitracin (Myintzaw et al., 2023). However, the precise resistance mechanisms remain unclear (Sproston et al., 2018; Santos-Ferreira et al., 2022). In addition, Campylobacter is resistant to fluoroquinolone, macrolides, and tetracycline (Tang et al., 2017; Vizzini et al., 2021). Less than 10% of Campylobacter isolates in the USA and other industrialized nations are resistant to macrolides (Engberg et al., 2001; Cha et al., 2016). However, this trend was more pronounced in developing countries (Okeke et al., 2005; Buiatte et al., 2023). Most macrolide-resistant C. coli strains have been found in pigs because tylosin is commonly used as a growth stimulant in the pig industry (Natsos et al., 2019). Campylobacter isolated from other animals, appears to have poor macrolide resistance (Tedersoo et al., 2022).
Expected low levels of macrolide resistance in Campylobacter suggest that longer courses of treatment are necessary (Caldwell et al., 2008; García-Sánchez et al., 2020). Another factor is that the resistance phenotype is less permanent because to the large fitness cost associated with macrolide resistance, so Campylobacter might quickly lose the phenotypic in the absence of selection pressure (Luangtongkum et al., 2009; Vandeputte et al., 2020). Unlike with macrolides, there has been a consistent worldwide increase in fluoroquinolone resistance among Campylobacter isolates (Sproston et al., 2018). In the 1980s, fluoroquinolone was initially introduced to the chicken industry as a means of preventing and treating respiratory illnesses (Kovács et al., 2016).
Resistance to fluoroquinolones in Campylobacter can be challenging to treat because of the antibiotic's broad range activity and widespread usage in the treatment of infectious diseases, including campylobacteriosis (Andersson and MacGowan, 2003). Since then, however, there has been an alarming increase in cases of human and avian fluoroquinolone-resistant Campylobacter (Engberg et al., 2001). Nachamkin et al. (2002) conducted a study and found that the percentage of C. jejuni isolates showing resistance to fluoroquinolones has increased dramatically from 5% in 1992 to 40.5% in 2002. Between 2008 and 2014, studies in China found that among Campylobacter isolates from chicken, nearly 100% were resistant to fluoroquinolones (Wang et al., 2016).
Multiple studies have linked campylobacteriosis to fluoroquinolones-resistant Campylobacter found in contaminated poultry products as the likely source of the bacteria (Nothaft et al., 2021). Patients identified with fluoroquinolone-resistant campylobacteriosis were less likely to have taken fluoroquinolones prior to diagnosis than those diagnosed with fluoroquinolone-susceptible campylobacteriosis, according to Kassenborg et al. (2004). In addition, those who developed fluoroquinolone-resistant campylobacteriosis were ten times more likely to eat poultry items in the week prior to getting sick than the healthy controls were. Nothaft et al. (2021) hypothesized that the infection originated from ingesting fluoroquinolone-resistant Campylobacter from contaminated bird items.
Results from in vitro and in vivo studies by Luo et al. (2003) and Han et al. (2012) suggested that resistance to fluoroquinolones improves the fitness of Campylobacter. In 2005, the USA Food and Drug Administration (FDA) banned the use of fluoroquinolones in the USA chicken sector due to this problem (Han et al., 2012). However, in spite of these efforts, no less fluoroquinolone-resistant Campylobacter has been found in either poultry or humans in the USA. In the absence of such selective forces, it was hypothesized that fluoroquinolone-resistant Campylobacter would persist in broilers (Han et al., 2012).
Several in vivo and in vitro experiments demonstrated that less than 24 h is required to detect fluoroquinolones-resistant C. jejuni following drug uptake, demonstrating the rapidity with which Campylobacter acquires resistance to these antibiotics (Gencay et al., 2018). Point mutation accumulation in the gyrA and parC genes is a necessary and sufficient condition for the development of fluoroquinolones resistance in other enteric bacteria, such as Salmonella and E. coli (Morgan-Linnell et al., 2009).
Campylobacter, on the other hand, only needs a single point mutation in gyrA to become resistant to fluoroquinolones. The most commonly documented point mutation in Campylobacter is a change from threonine to isoleucine at position 86 in the gyrA (Yan et al., 2006). This gyrA mutation alters DNA architecture during transcription, replication, and recombination by decreasing negative supercoiling (Han et al., 2012).
DNA supercoiling variations affect a wide range of promoter functions and transcriptome expressions, both in vitro and in vivo (Nothaft et al., 2021). Variable gene expressions were also observed in C. jejuni, according to another in vitro investigation that evaluated the transcriptome expressions of fluoroquinolone-resistant and -susceptible Campylobacter (Han, 2009). Results showed that negative supercoiling and regulatory mechanisms in C. jejuni were attenuated in the absence of fluoroquinolones, suggesting that resistance to these antibiotics improves iron uptake (Han, 2009). Although fluoroquinolone-resistant Campylobacter has emerged and persisted in USA broiler production, our understanding of the ecological drivers of fluoroquinolones resistance dynamics within the Campylobacter population and the hazard agents associated with this phenomenon is limited (Han, 2009).
Using commercial antimicrobials to reduce Campylobacter contamination in poultry processing
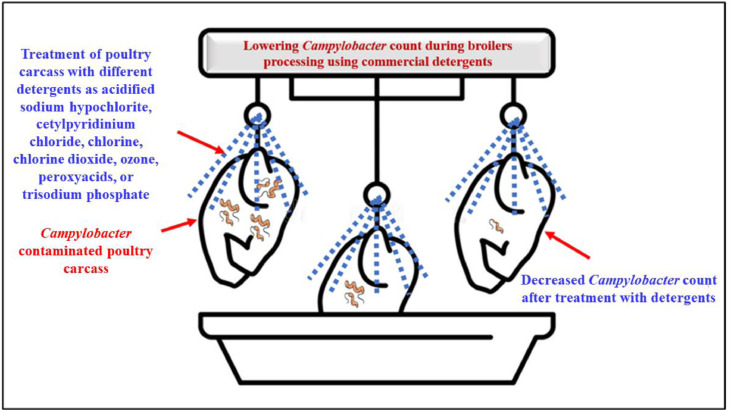
Reductions in Campylobacter spp. have been attributed to the use of commercially available antimicrobial technologies throughout the processing of broiler chickens (McKenna et al., 2020; Gichure et al., 2022). Kemp et al. (2001) reduced microbial levels on contaminated chicken carcasses utilizing an online acidified sodium chlorite spray system and an inside-outside broiler chickens carcass washer. Several agents, such as acidified sodium hypochlorite, cetylpyridinium chloride, ozone, chlorine dioxide, chlorine, peroxy acids, or trisodium phosphate, have been used to decrease the number of Campylobacter spp., in carcasses (Oyarzabal, 2005). Figure 7 shows the results of utilizing commercial antimicrobials to reduce Campylobacter contamination in broilers throughout processing.
Figure 7.
Using commercial antimicrobials such acidified sodium chloride, chlorine, chlorine dioxide, ozone, peroxy-acids, or trisodium phosphate to reduce Campylobacter contamination during broiler production.
Campylobacter load in raw chicken carcasses was observed to be reduced by Arritt et al. (2002). Commercial use of an effective antibacterial chemical spray consisting of 10% trisodium phosphate, 0.1% acidified sodium hypochlorite, 0.1 and 0.5% cetylpyridinium chloride, or 1% tween 80 could reduce the amount of rinse water necessary for cleaning carcasses (Arritt et al., 2002). Adding chlorine to cold water during chicken processing has been investigated and shown to be effective by Yang et al. (2001). They demonstrated that it did not drastically lower the number of S. typhimurium and Campylobacter on the chicken integument (Yang et al., 2001).
Chilling water with chlorine dioxide has been shown to reduce microbiological contamination in commercial broiler processing plants by Doyle and Waldroup (1996). The number of Campylobacter was reduced by 1.5 logs in 1 d and 1.2 logs in 6 d storage on trisodium phosphate-treated carcasses, according to Slavik et al. (1994). Iwata et al. (2023) reported that tryptanthrin reduced the number of C. jejuni bacteria in the intestines of chickens.
Control of Campylobacter Infection in Poultry
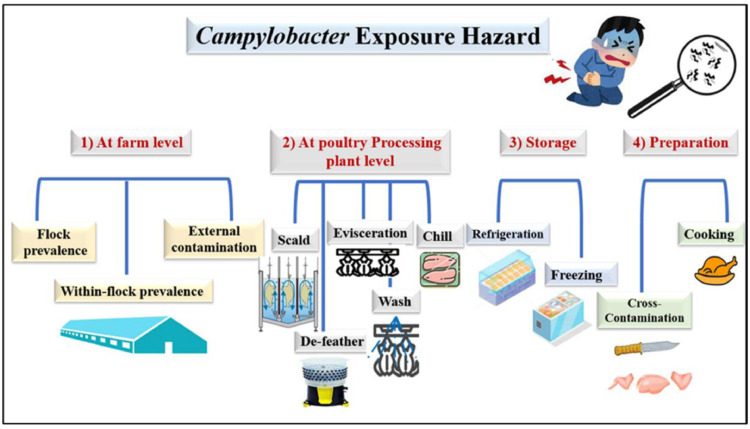
Figure 8 shows that the risk of Campylobacter in broiler chickens exists from the time they are raised on farms through the time they are processed in poultry plants, stored, and finally cooked. Figure 9 provides a concise overview of the methods used to prevent the spread of Campylobacter illness in broiler chickens.
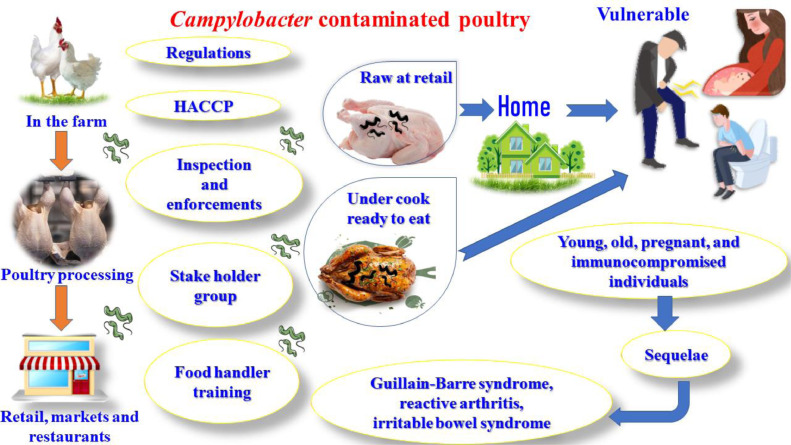
Figure 8.
Campylobacter exposure risk at various stages of broiler chicken rearing, such as the farm (flock prevalence, within flock prevalence, external contamination), the processing plant (scald, evisceration, chill), storage (refrigeration, freezing), and preparation (cross contamination, cooking).
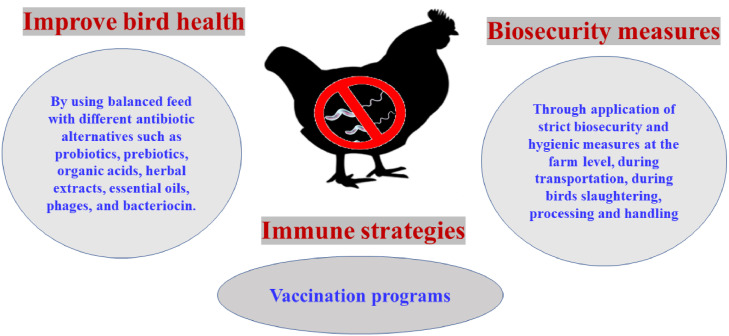
Figure 9.
Control of Campylobacter infection in broiler chickens includes increasing immunity in birds through vaccination, improving bird health through balanced feed with additives (antimicrobial or natural antibiotic alternatives), and implementing biosecurity measures at various levels of rearing, transportation, processing, and handling.
The most effective on-farm intervention to prevent the introduction of Campylobacter into broilers is an increase in biosecurity and general hygiene standards (D'angelantonio et al., 2021; Dogan et al., 2022). To prevent the spread of Campylobacter in broilers, even the most stringent on-farm biosecurity measures have proven ineffective (Sibanda et al., 2018; Poudel et al., 2022).
However, these precautions only appear to work in the Scandinavian countries, where the prevalence of Campylobacter in broilers is already low, and severe weather patterns may prevent the survival of certain insect and rodent populations that may be harboring Campylobacter (Lianou et al., 2017; Zhang et al., 2018). Feed and water acidification are two more examples of on-farm interventions that have proven ineffective (Hermans et al., 2011; El Haddad et al., 2019). To a lesser extent, it appears that acidifying litter treatments, which are meant to make the litter environment less hospitable to Campylobacter and other foodborne pathogens such as Salmonella, have only a modest effect on Campylobacter levels in broilers (Chinivasagam et al., 2020; Hwang and Singer, 2020). Probiotics have been shown to drastically lower Campylobacter count, according to studies by Arsi et al. (2015) and Taha-Abdelaziz et al. (2019). Bacillus subtilis PS-216 showed substantial anti-Campylobacter activities, as determined by Šimunovi´c et al. (2022), although the medium growth temperature and presence of oxygen are both crucial for B. subtilis.
The effectiveness of several dietary supplements against Campylobacter in broilers was also assessed by Guyard-Nicodème et al. (2016) throughout the whole of the rearing process. Khan et al. (2020) showed that prebiotics significantly decreased Campylobacter colonization in the guts of layering organisms and Erega et al. (2021) demonstrated that probiotics could be used to control Campylobacter infection. A similar antibacterial effect was obtained for carrots essential oil against Campylobacter infection by Luc et al. (2020). Glycyrrhiza glabra (Liquorice) extract was shown to decrease C. jejuni colonization in the intestines of broiler chickens in a separate investigation conducted by Ibrahim et al. (2020). According to Peh et al. (2020), organic acids have strong antibacterial action and may be able to lower the number of Campylobacter spp. When tested against C. jejuni in sterile distilled water, sodium caprate, thymol, carvacrol, and potassium sorbate were all found to diminish C. jejuni count when used at various concentrations and exposure times by Greene et al. (2022). Bacteriophages have been studied for their potential to reduce the prevalence of Campylobacter in broiler chickens by Richards et al. (2019), Chinivasagam et al. (2020), Ushanov et al. (2020), and Nafarrate et al. (2021).
There are currently no on-farm therapies proven to be effective and cost-efficient in reducing Campylobacter occurrence and levels in broilers (Thomas et al., 2020; Beterams et al., 2023). Bacteriocins, immunization, and probiotics were three more innovative methods used to reduce Campylobacter colonization on farms and in slaughterhouses (Nishiyama et al., 2014; Meunier et al., 2017; Helmy et al., 2022). The most encouraging outcomes have been shown with the immunization of broiler chicks against Campylobacter (Helmy et al., 2022). Using a Campylobacter vaccine created from multiplication proteins exposed on the surface of C. jejuni, Neal-McKinney et al. (2014) demonstrated a 2 log reduction in the degree of C. jejuni colonization in 20 d old chickens following intramuscular injection (Neal-McKinney et al., 2014). Two recent studies have employed an effective vaccine against Campylobacter infection in broilers (Meunier et al., 2017; Nothaft et al., 2017).
To transport Campylobacter immunogenic surface proteins, Wyszyńska et al. (2004) used attenuated Salmonella as a vector. Broiler chicks as young as 4 wk old were vaccinated orally, and the results demonstrated a significant decrease in the prevalence and levels of C. jejuni multiplication compared to control groups (Wyszyńska et al., 2004; Poudel et al., 2023). However, these novel interventions are still in the early stages of development, so more study is needed to optimize the best delivery system and test efficacy in real-world settings (Djennad et al., 2017; Mota-Gutierrez et al., 2022). Producers will have to rely on biosecurity improvements to poultry farms until these interventions are ready for widespread application (Mota-Gutierrez et al., 2022).
Table 3 briefly explains the advantages and disadvantages of various methods to reduce Campylobacter dissemination. Figure 10, Figure 11 demonstrated that implementing the regulations of HACCP, inspecting chicken meat, enforcing these regulations, forming stakeholder groups, and providing food handlers with training can lower the risk of campylobacteriosis.
Table 3.
The advantages and disadvantages of various Campylobacter species dissemination control strategies.
| Feed and water treatments | Study location | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Organic and fatty acids, essential oils, plant extracts | Farm |
|
|
Dedieu et al., 2020 |
| Probiotics | Farm |
|
Variability in their effectiveness across studies. More on-farm research is required to improve this method | Mañes-Lázaro et al., 2017 |
| Bacteriocin | Farm |
|
|
Dai et al., 2020 |
| Bacteriophage | Farm |
|
|
Richards et al., 2019; Chinivasagam et al., 2020 |
| Vaccine | Farm |
|
|
Ramakrishnan et al., 2019 |
| Slaw and fast freezing | Slaughterhouse |
|
|
Haughton et al., 2012; Harrison et al., 2013 |
| Steam-ultrasound | Slaughterhouse | Reducing Campylobacter in the carcasses >2.51 log | Changes in chicken skins but it is acceptable | Musavian et al., 2014 |
Figure 10.
Avian campylobacteriosis poses a risk to humans at various stages of rearing and processing, and it can be controlled by implementing regulations, a hazard analysis and critical control points programs (HACCP), initiating and enforcing it, forming a stakeholder group, and providing food handler training.
Figure 11.
Lowering the risk of avian campylobacteriosis at various stages of rearing and processing by implementing farm-level regulations, hazard analysis and critical control points (HACCP) systems during chicken processing, retail, market, and restaurant inspections and enforcements, stakeholder groups, food handler training, and proper cooking.
The Importance of Natural Antibiotics in the Prevention and Treatment of Campylobacteriosis
The growing concern surrounding antibiotic resistance in Campylobacter has led to an increase in the number of research efforts being put toward the creation of new and alternative control methods for campylobacteriosis (Dai et al., 2020). Numerous studies have been carried out up to this point to reduce the amount of Campylobacter colonization in animals that are used in the production of food, with the end goal of improving public health by eliminating potential sources of infection (Dai et al., 2020). Both prebiotics and probiotics have been utilized extensively in an effort to enhance bird performance and reduce the proliferation of pathogens (Khan et al., 2020; Abd El-Hack et al., 2021b; Erega et al., 2021).
In a mouse model, Morrow et al. (2005) showed that human milk oligosaccharides (HMOs) inhibit the proliferation of C. jejuni. When mannan-oligosaccharide was introduced as a feed supplement at a concentration of 0.2%, Baurhoo et al. (2009) observed that the level of Campylobacter detected in chicken cecal contents and litter samples dropped by a significant amount. Wyszyńska and Godlewska (2021) demonstrated the role of lactic acid bacteria in minimizing the incidence of Campylobacter infection in hens. Butyrate was the most effective short chain fatty acids against C. jejuni in culture media, according to experimental research conducted by Van Deun et al. (2008). However, adding butyrate to feed for broiler chicken had no effect on C. jejuni colonization. In postharvest chicken, Wagle et al. (2021) discovered that the phytochemicals turmeric, curcumin, allyl sulfide, garlic oil, and ginger oil could reduce the number of C. jejuni and pinpoint the underlying mechanisms of action.
Despite the promising results seen with some of these antibiotic natural alternatives, reliable alternatives with known doses, well-known mechanisms of action, and known side effects have not yet been created; therefore, more research is needed to clear out these points.
CONCLUSIONS
The primary cause of human campylobacteriosis worldwide is chicken products. Along with the rise in Campylobacter-related illnesses, the prevalence of antibiotic-resistant Campylobacter spp. has also risen. Therefore, it is crucial to develop innovative natural antimicrobial treatments together with appropriate biosecurity and hygiene practices at the farm level to prevent Campylobacter colonization in commercial avian farms, which is the main cause of human sickness. Some feed additives and immunizations are necessary in order to affect the virulence of Campylobacter and the survival variables that are associated with microbial pathogenesis. Campylobacteriosis risks will be reduced by the implementation of HACCP regulations, chicken meat inspection, enforcement, stakeholder group, and food handler training. It is possible that the combination of these preventative measures will lead to a successful intervention that will stop the spread of Campylobacter infections into the food chain and reduce the associated risks to human health. However, additional research is required before these treatments may be considered completely effective.
ACKNOWLEDGMENTS
This publication was made possible by grant number NC.X-267-5-12-170-1 from the National Institute of Food and Agriculture (NIFA) and the Department of Family and Consumer Sciences and the Agriculture Research Station at North Carolina Agriculture and Technical State University (Greensboro, NC, USA 27411). This work was also supported, in part, by 1890 Capacity Building Program grant no. (2020-38821-31113/project accession no. 021765). Also, the authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through Small Group Research Project under grant number 44/487, and the authors acknowledge the Research Center for Advance Materials (RCAMS) at King Khalid University, Saudi Arabia for their valuable technical support. This review was also funded by the Abu Dhabi Award for Research Excellence—Department of Education and Knowledge (Grant #: 21S105) to K. A. El-Tarabily.
Author contributions: All authors were equal contributors in writing this review article. All of them read and approved the final manuscript.
DISCLOSURES
All authors declare no conflicts of interest.
REFERENCES
- Abd El-Hack M.E., El-Saadony M.T., Shehata A.M., Arif M., Paswan V.K., Batiha G.E.-S., Khafaga A.F., Elbestawy A.R. Approaches to prevent and control Campylobacter spp. colonization in broiler chickens: a review. Environ. Sci. Pollut. Res. Int. 2021;28:4989–5004. doi: 10.1007/s11356-020-11747-3. [DOI] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Shafi M.E., Alshahrani O.A., Saghir S.A., Al-Wajeeh A.S., Abdel-Moneim A.M.E. Prebiotics can restrict Salmonella populations in poultry: a review. Anim. Biotechnol. 2021;7:1668–1677. doi: 10.1080/10495398.2021.1883637. [DOI] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Salem H.M., El-Tahan A.M., Soliman M.M., Youssef G.B.A., Youssef G.B., Taha A.E., Soliman S.M., Ahmed A.E., El-kott A.F., AlSyaad K.M., Swelum A.A. Alternatives to antibiotics for organic poultry production: types, modes of action and impacts on bird's health and production. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Elbestawy A.R., El-Shall N.A., Saad A.M., Salem H.M., El-Tahan A.M., Khafaga A.F., Taha A.E., AbuQamar S.F., El-Tarabily K.A. Necrotic enteritis in broiler chickens: disease characteristics and prevention using organic antibiotic alternatives—a comprehensive review. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achen M., Morishita T.Y., Ley E.C. Shedding and colonization of Campylobacter jejuni in broilers from day-of-hatch to slaughter age. Avian Dis. 1998;42:732–737. [PubMed] [Google Scholar]
- Agunos A., Waddell L., Léger D., Taboada E. A systematic review characterizing on-farm sources of Campylobacter spp. for broiler chickens. PLoS One. 2014;9 doi: 10.1371/journal.pone.0104905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed J., Arfat Y.A., Bher A., Mulla M., Jacob H., Auras R. Active chicken meat packaging based on polylactide films and bimetallic Ag-Cu nanoparticles and essential oil. J. Food Sci. 2018;83:1299–1310. doi: 10.1111/1750-3841.14121. [DOI] [PubMed] [Google Scholar]
- Allen K.J., Griffiths M.W. Use of luminescent Campylobacter jejuni ATCC 33291 to assess eggshell colonization and penetration in fresh and retail eggs. J. Food Prot. 2001;64:2058–2062. doi: 10.4315/0362-028x-64.12.2058. [DOI] [PubMed] [Google Scholar]
- Allos B.M. Campylobacter jejuni infections: update on emerging issues and trends. Clin. Infect. Dis. 2001;32:1201–1206. doi: 10.1086/319760. [DOI] [PubMed] [Google Scholar]
- Altekruse S.F., Stern N.J., Fields P.I., Swerdlow D.L. Campylobacter jejuni an emerging foodborne pathogen. Emerg. Infect. Dis. 1999;5:28–35. doi: 10.3201/eid0501.990104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson M.I., MacGowan A.P. Development of the quinolones. J. Antimicrob. Chemother. 2003;51(Suppl. 1):1–11. doi: 10.1093/jac/dkg212. [DOI] [PubMed] [Google Scholar]
- Arritt F.M., Eifert J.D., Pierson M.D., Sumner S.S. Efficacy of antimicrobials against Campylobacter jejuni on chicken breast skin. J. Appl. Poult. Res. 2002;11:358–366. [Google Scholar]
- Arsenault J., Letellier A., Quessy S., Normand V., Boulianne M. Prevalence and risk factors for Salmonella spp. and Campylobacter spp. caecal colonization in broiler chicken and turkey flocks slaughtered in Quebec, Canada. Prev. Vet. Med. 2007;81:250–264. doi: 10.1016/j.prevetmed.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Arsi K., Donoghue A.M., Woo-Ming A., Blore P.J., Donoghue D.J. Intracloacal inoculation, an effective screening method for determining the efficacy of probiotic bacterial isolates against Campylobacter colonization in broiler chickens. J. Food Prot. 2015;78:209–2013. doi: 10.4315/0362-028X.JFP-14-326. [DOI] [PubMed] [Google Scholar]
- Asare P.T., Zurfluh K., Greppi A., Lynch D., Schwab C., Stephan R., Lacroix C. Reuterin demonstrates potent antimicrobial activity against a broad panel of human and poultry meat Campylobacter spp. isolates. Microorganisms. 2020;8:78. doi: 10.3390/microorganisms8010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahrndorff S., Rangstrup-Christensen L., Nordentoft S., Hald B. Foodborne disease prevention and broiler chickens with reduced Campylobacter infection. Emerg. Infect. Dis. 2013;19:425–430. doi: 10.3201/eid1903.111593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey M.A., Taylor R.M., Brar J.S., Corkran S.C., Velasquez C., Rama E.N., Oliver H.F., Singh M. Prevalence and antimicrobial resistance of Campylobacter from antibiotic-free broilers during organic and conventional processing. Poult. Sci. 2019;98:1447–1454. doi: 10.3382/ps/pey486. [DOI] [PubMed] [Google Scholar]
- Ballou A.L., Ali R.A., Mendoza M.A., Ellis J.C., Hassan H.M., Croom W.J., Koci M.D. Development of the chick microbiome: how early exposure influences future microbial diversity. Front. Vet. Sci. 2016;3:2. doi: 10.3389/fvets.2016.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balta I., Linton M., Pinkerton L., Kelly C., Stef L., Pet I., Stef D., Cristete A., Gundogdu O., Corcionivoschi N. The effect of natural antimicrobials against Campylobacter spp. and its similarities to Salmonella spp., Listeria spp., Escherichia coli, Vibrio spp., Clostridium spp. and Staphylococcus spp. Food Control. 2021;121 [Google Scholar]
- Barbut S. CRC Press; Boca Raton, FL: 2016. Pages 541 in Poultry Products Processing: An Industry Guide. [Google Scholar]
- Barker C.R., Painset A., Swift C., Jenkins C., Godbole G., Maiden M.C.J., Dallman T.J. Microevolution of Campylobacter jejuni during long-term infection in an immunocompromised host. Sci. Rep. 2020;10:10109. doi: 10.1038/s41598-020-66771-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashor M.P., Curtis P.A., Keener K.M., Sheldon B.W., Kathariou S., Osborne J.A. Effects of carcass washers on Campylobacter contamination in large broiler processing plants. Poult. Sci. 2004;83:1232–1239. doi: 10.1093/ps/83.7.1232. [DOI] [PubMed] [Google Scholar]
- Battersby T., Whyte P., Bolton D.J. The pattern of Campylobacter contamination on broiler farms; external and internal sources. J. Appl. Microbiol. 2016;120:1108–1118. doi: 10.1111/jam.13066. [DOI] [PubMed] [Google Scholar]
- Baurhoo B., Ferket P.R., Zhao X. Effects of diets containing different concentrations of mannanoligosaccharide or antibiotics on growth performance, intestinal development, cecal and litter microbial populations, and carcass parameters of broilers. Poult. Sci. 2009;88:2262–2272. doi: 10.3382/ps.2008-00562. [DOI] [PubMed] [Google Scholar]
- Beery J.T., Hugdahl M.B., Doyle M.P. Colonization of gastrointestinal tracts of chicks by Campylobacter jejuni. Appl. Environ. Microbiol. 1988;54:2365–2370. doi: 10.1128/aem.54.10.2365-2370.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béjaoui A., Gharbi M., Bitri S., Nasraoui D., Ben Aziza W., Ghedira K., Rfaik M., Marzougui L., Ghram A., Maaroufi A. Virulence profiling, multidrug resistance and molecular mechanisms of Campylobacter strains from chicken carcasses in Tunisia. Antibiotics. 2022;11:830. doi: 10.3390/antibiotics11070830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrang M.E., Bailey J.S., Altekruse S.F., Patel B., Shaw Jr W.K., Meinersmann R.J., Fedorka-Cray P.J. Prevalence and numbers of Campylobacter on broiler carcasses collected at rehang and post chill in 20 U.S. processing plants. J. Food Prot. 2007;70:1556–1560. doi: 10.4315/0362-028x-70.7.1556. [DOI] [PubMed] [Google Scholar]
- Berrang M.E., Buhr R.J., Cason J.A., Dickens J.A. Broiler carcass contamination with Campylobacter from feces during defeathering. J. Food Prot. 2001;64:2063–2066. doi: 10.4315/0362-028x-64.12.2063. [DOI] [PubMed] [Google Scholar]
- Berrang M.E., Dickens J.A. Presence and level of Campylobacter spp. on broiler carcasses throughout the processing plant. J. Appl. Poult. Res. 2000;9:43–47. [Google Scholar]
- Berrang M.E., Meinersmann R.J., Smith D.P., Zhuang H. The effect of chilling in cold air or ice water on the microbiological quality of broiler carcasses and the population of Campylobacter. Poult. Sci. 2008;87:992–998. doi: 10.3382/ps.2007-00406. [DOI] [PubMed] [Google Scholar]
- Berrang M.E., Smith D.P., Meinersmann R.J. Variations on standard broiler processing in an effort to reduce Campylobacter numbers on post pick carcasses. J. Appl. Poult. Res. 2011;20:197–202. [Google Scholar]
- Bertram R., Kehrenberg C., Seinige D., Krischek C. Peracetic acid reduces Campylobacter spp. numbers and total viable counts on broiler breast muscle and drumstick skins during modified atmosphere package storage. Poult. Sci. 2019;98:5064–5073. doi: 10.3382/ps/pez266. [DOI] [PubMed] [Google Scholar]
- Beterams A., Tolksdorf T., Martin A., Stingl K., Bandick N., Reich F. Change of Campylobacter, Escherichia coli and Salmonella counts in packaged broiler breast meat stored under modified atmosphere and vacuum conditions at 4 and 10°C based on cultural and molecular biological quantification. Food Control. 2023;145 [Google Scholar]
- Bezek K., Kurinčič M., Knauder E., Klančnik A., Raspor P., Bucar F., Možina S.S. Attenuation of adhesion, biofilm formation and quorum sensing of Campylobacter jejuni by Euodia ruticarpa. Phyther. Res. 2016;30:1527–1532. doi: 10.1002/ptr.5658. [DOI] [PubMed] [Google Scholar]
- Biswas C., Leboveic A., Burke K., Biswas D. In: Pages 123–138 in Food Safety in Poultry Meat Production, Food Microbiology and Food Safety. Venkitanarayanan K., Thakur S., Ricke S.C., editors. Springer; Cham; Switzerland: 2019. Post-harvest approaches to improve poultry meat safety. [Google Scholar]
- Bolinger H., Kathariou S. The current state of macrolide resistance in Campylobacter spp.: trends and impacts of resistance mechanisms. Appl. Environ. Microbiol. 2017;83:e00416–e00417. doi: 10.1128/AEM.00416-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton F.J., Wareing D.R., Sails A.D. Comparison of a novel microaerobic system with three other gas-generating systems for the recovery of Campylobacter species from human faecal samples. Eur. J. Clin. Microbiol. Infect. Dis. 1997;16:839–842. doi: 10.1007/BF01700415. [DOI] [PubMed] [Google Scholar]
- Borrmann E., Berndt A., Hanel I., Kohler H. Campylobacter-induced interleukin-8 responses in human intestinal epithelial cells and primary intestinal chick cells. Vet. Microbiol. 2007;124:115–124. doi: 10.1016/j.vetmic.2007.04.041. [DOI] [PubMed] [Google Scholar]
- Buhr R.J., Walker J.M., Bourassa D.V., Caudill A.B., Kiepper B.H., Zhuang H. Impact of broiler processing scalding and chilling profiles on carcass and breast meat yield. Poult. Sci. 2014;93:1534–1541. doi: 10.3382/ps.2013-03535. [DOI] [PubMed] [Google Scholar]
- Buiatte A.B.G., de Melo R.T., Peres P.A.B.M., Bastos C.M., Grazziotin A.L., Rodriguez P.M.A., Barreto F., Rossi D.A. Virulence, antimicrobial resistance, and dissemination of Campylobacter coli isolated from chicken carcasses in Brazil. Food Control. 2023;147 [Google Scholar]
- Bull S.A., Allen V.M., Domingue G., Jørgensen F., Frost J.A., Ure R., Whyte R., Tinker D., Corry J.E., Gillard-King J., Humphrey T.J. Sources of Campylobacter spp. colonizing housed broiler flocks during rearing. Appl. Environ. Microbiol. 2006;72:645–652. doi: 10.1128/AEM.72.1.645-652.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne C.M., Clyne M., Bourke B. Campylobacter jejuni adhere to and invade chicken intestinal epithelial cells in vitro. Microbiology. 2007;153:561–569. doi: 10.1099/mic.0.2006/000711-0. [DOI] [PubMed] [Google Scholar]
- Caldwell D.B., Wang Y., Lin J. Development, stability, and molecular mechanisms of macrolide resistance in Campylobacter jejuni. Antimicrob. Agents Chemother. 2008;52:3947–3954. doi: 10.1128/AAC.00450-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callicott K.A., Friethriksdottir V., Reiersen J., Lowman R., Bisaillon J.R., Gunnarsson E., Berndtson E., Hiett K.L., Needleman D.S., Stern N.J. Lack of evidence for vertical transmission of Campylobacter spp. in chickens. Appl. Environ. Microbiol. 2006;72:5794–5798. doi: 10.1128/AEM.02991-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campagnolo E.R., Philipp L.M., Long J.M., Hanshaw N.L. Pet-associated campylobacteriosis: a persisting public health concern. Zoonoses Public Health. 2018;65:304–311. doi: 10.1111/zph.12389. [DOI] [PubMed] [Google Scholar]
- Cardinale E., Tall F., Gueye E.F., Cisse M., Salvat G. Risk factors for Campylobacter spp. infection in Senegalese broiler-chicken flocks. Prev. Vet. Med. 2004;64:15–25. doi: 10.1016/j.prevetmed.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Casagrande Proietti P., Pergola S., Bellucci S., Menchetti L., Miraglia D., Franciosini M.P. Occurrence and antimicrobial susceptibility of Campylobacter spp. on fresh and refrigerated chicken meat products in Central Italy. Poult. Sci. 2018;97:2895–2901. doi: 10.3382/ps/pey147. [DOI] [PubMed] [Google Scholar]
- Castillo S., Heredia N., Arechiga-Carvajal E., García S. Citrus extracts as inhibitors of quorum sensing, biofilm formation and motility of Campylobacter jejuni. Food Biotechnol. 2014;28:106–122. [Google Scholar]
- CDC . Department of Health and Human Services, CDC; Atlanta, GA: 2017. Foodborne Diseases Active Surveillance Network (FoodNet): FoodNet 2015 Surveillance Report (Final Data) [Google Scholar]
- CDC. 2019. Questions and Answers: Campylobacter (Campylobacteriosis). Accessed Jan. 2023. https://www.cdc.gov/campylobacter/faq.html.
- Cha W., Mosci R., Wengert S.L., Singh P., Newton D.W., Salimnia H., Lephart P., Khalife W., Mansfield L.S., Rudrik J.T., Manning S.D. Antimicrobial susceptibility profiles of human Campylobacter jejuni isolates and association with phylogenetic lineages. Front. Microbiol. 2016;7:589. doi: 10.3389/fmicb.2016.00589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantarapanont W., Berrang M.E., Frank J.F. Direct microscopic observation of viability of Campylobacter jejuni on chicken skin treated with selected chemical sanitizing agent. J. Food Prot. 2004;67:1146–1152. doi: 10.4315/0362-028x-67.6.1146. [DOI] [PubMed] [Google Scholar]
- Chinivasagam H.N., Estella W., Maddock L., Mayer D.G., Weyand C., Connerton P.L., Connerton I.F. Bacteriophages to control Campylobacter in commercially farmed broiler chickens, in Australia. Front. Microbiol. 2020;11:632. doi: 10.3389/fmicb.2020.00632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chintoan-Uta C., Wisedchanwet T., Glendinning L., Bremner A., Psifidi A., Vervelde L., Watson K., Watson M., Stevens M.P. Role of cecal microbiota in the differential resistance of inbred chicken lines to colonization by Campylobacter jejuni. Appl. Environ. Microbiol. 2020;86:e02607–e02619. doi: 10.1128/AEM.02607-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury S., Sandberg M., Themudo G.E., Ersboll A.K. Risk factors for Campylobacter infection in Danish broiler chickens. Poult. Sci. 2012;91:2701–2709. doi: 10.3382/ps.2012-02412. [DOI] [PubMed] [Google Scholar]
- Clavijo V., Florez M.J.V. The gastrointestinal microbiome and its association with the control of pathogens in broiler chicken production: a review. Poult. Sci. 2018;97:1006–1021. doi: 10.3382/ps/pex359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook A., Odumeru J., Lee S., Pollari F. Campylobacter, Salmonella, Listeria monocytogenes, verotoxigenic Escherichia coli, and Escherichia coli prevalence, enumeration, and subtypes on retail chicken breasts with and without skin. J. Food Prot. 2012;75:34–40. doi: 10.4315/0362-028X.JFP-11-206. [DOI] [PubMed] [Google Scholar]
- Coward C., van Diemen P.M., Conlan A.J.K., Gog J.R., Stevens M.P., Jones M.A., Maskell D.J. Competing isogenic Campylobacter strains exhibit variable population structures in vivo. Appl. Environ. Microbiol. 2008;74:3857–3867. doi: 10.1128/AEM.02835-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox N.A., Bailey J.S., Richardson L.J., Buhr R.J., Cosby D.E., Wilson J.L., Hiett K.L., Siragusa G.R., Bourassa D.V. Presence of naturally occurring Campylobacter and Salmonella in the mature and immature ovarian follicles of late-life broiler breeder hens. Avian Dis. 2005;49:285–287. doi: 10.1637/7324-011005. [DOI] [PubMed] [Google Scholar]
- Cox N.A., Richardson L.J., Maurer J.J., Berrang M.E., Fedorka-Cray P.J., Buhr R.J., Byrd J.A., Lee M.D., Hofacre C.L., O'Kane P.M., Lammerding A.M., Clark A.G., Thayer S.G., Doyle M.P. Evidence for horizontal and vertical transmission in Campylobacter passage from hen to her progeny. J. Food Prot. 2012;75:1896–1902. doi: 10.4315/0362-028.JFP-11-322. [DOI] [PubMed] [Google Scholar]
- Cox N.A., Stern N.J., Hiett K.L., Berrang M.E. Identification of a new source of Campylobacter contamination in poultry: transmission from breeder hens to broiler chickens. Avian Dis. 2002;46:535–541. doi: 10.1637/0005-2086(2002)046[0535:IOANSO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Cox N.A., Stern N.J., Wilson J.L., Musgrove M.T., Buhr R.J., Hiett K.L. Isolation of Campylobacter spp. from semen samples of commercial broiler breeder roosters. Avian Dis. 2002;46:717–720. doi: 10.1637/0005-2086(2002)046[0717:IOCSFS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Dai L., Sahin O., Grover M., Zhang Q. New and alternative strategies for the prevention, control, and treatment of antibiotic-resistant Campylobacter. Transl. Res. 2020;223:76–88. doi: 10.1016/j.trsl.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale E.L., Nolan S.P., Berghaus R.D., Hofacre C.L. On farm prevention of Campylobacter and Salmonella: lessons learned from basic biosecurity interventions. J. Appl. Poult. Res. 2015;24:222–232. [Google Scholar]
- D'angelantonio D., Scattolini S., Boni A., Neri D., Di Serafino G., Connerton P., Connerton I., Pomilio F., Di Giannatale E., Migliorati G., Aprea G. Bacteriophage therapy to reduce colonization of Campylobacter jejuni in broiler chickens before slaughter. Viruses. 2021;13:1428. doi: 10.3390/v13081428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Rosa P.P., Ávila B.P., Angelo I.D.V., Chesini R.G., Fernandes T.A., da Silva Camacho J., Bugoni M., Roll V.F.B., Gularte M.A. Impact of different chicken meat production systems on consumers’ purchase perception. Br. Poult. Sci. 2021;62:387–395. doi: 10.1080/00071668.2020.1857335. [DOI] [PubMed] [Google Scholar]
- Dedieu L., Brunel J.M., Lorenzi V., Muselli A., Berti L., Bolla J.M. Antibacterial mode of action of the Daucus carota essential oil active compounds against Campylobacter jejuni and efflux-mediated drug resistance in Gram-negative bacteria. Molecules. 2020;25:5448. doi: 10.3390/molecules25225448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W., Dittoe D.K., Pavilidis H.O., Chaney W.E., Yang Y., Ricke S.C. Current perspectives and potential of probiotics to limit foodborne Campylobacter in poultry. Front. Microbiol. 2020;11 doi: 10.3389/fmicb.2020.583429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Zoete M.R., Keestra A.M., Roszczenko P., van Putten J.P. Activation of human and chicken toll-like receptors by Campylobacter spp. Infect. Immun. 2010;78:1229–1238. doi: 10.1128/IAI.00897-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Zoete M.R., van Putten J.P.M., Wagenaar J.A. Vaccination of chickens against Campylobacter. Vaccine. 2007;25:5548–5557. doi: 10.1016/j.vaccine.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Dhillon A.S., Shivaprasad H.L., Schaberg D., Wier F., Weber S., Bandli D. Campylobacter jejuni infection in broiler chickens. Avian Dis. 2006;50:55–58. doi: 10.1637/7411-071405R.1. [DOI] [PubMed] [Google Scholar]
- Di Giannatale E., Calistri P., Di Donato G., Decastelli L., Goffredo E., Adriano D., Mancini M.E., Galleggiante A., Neri D., Antoci S., Marfoglia C., Marotta F., Nuvoloni R., Migliorati G. Thermotolerant Campylobacter spp. in chicken and bovine meat in Italy: prevalence, level of contamination and molecular characterization of isolates. PLoS One. 2019;14 doi: 10.1371/journal.pone.0225957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dion M.B., Oechslin F., Moineau S. Phage diversity, genomics and phylogeny. Nat. Rev. Microbiol. 2020;18:125–138. doi: 10.1038/s41579-019-0311-5. [DOI] [PubMed] [Google Scholar]
- Dipineto L., Gargiulo A., Russo T.P., De Luca Bossa L.M., Borrelli L., Menna L.F., Fioretti A. Campylobacter jejuni, Campylobacter coli, and cytolethal distending toxin genes in laying hens. Avian Dis. 2011;55:103–105. doi: 10.1637/9525-091510-ResNote.1. [DOI] [PubMed] [Google Scholar]
- Djennad A., Nichols G., Loiacono G., Fleming L., Kessel A., Kovats S., Lake I., Sarran C., Elson R., Lane C., Hoeser C., Bailey T. The seasonality and effects of temperature and rainfall on Campylobacter infections. Int. J. Popul. Data Sci. 2017;1:034. [Google Scholar]
- Dogan O.B., Aditya A., Ortuzar J., Clarke J., Wang B. A systematic review and meta-analysis of the efficacy of processing stages and interventions for controlling Campylobacter contamination during broiler chicken processing. Compr. Rev. Food Sci. Food Saf. 2022;21:227–271. doi: 10.1111/1541-4337.12860. [DOI] [PubMed] [Google Scholar]
- Dogan O.B., Clark J., Mattos F., Wang B. A quantitative microbial risk assessment model of Campylobacter in broiler chickens: evaluating processing interventions. Food Control. 2019;100:97–110. [Google Scholar]
- Domingues A.R., Pires S.M., Halasa T., Hald T. Source attribution of human campylobacteriosis using a meta-analysis of case-control studies of sporadic infections. Epidemiol. Infect. 2012;140:970–981. doi: 10.1017/S0950268811002676. [DOI] [PubMed] [Google Scholar]
- Doyle M.L., Waldroup A.L. Microbiological evaluation of chlorine dioxide in commercial poultry plant. Pages 97 in Proceedings of the 88th Annual Meeting of the Poultry Science Association; Louisville, KY; 1996. [Google Scholar]
- Duarte A., Alves A.C., Ferreira S., Silva F., Domingues F.C. Resveratrol inclusion complexes: antibacterial and anti-biofilm activity against Campylobacter spp. and Arcobacter butzleri. Food Res. Int. 2015;77:244–250. [Google Scholar]
- Dubovitskaya O., Seinige D., Valero A., Reich F., Kehrenberg C. Quantitative assessment of Campylobacter spp. levels with real-time PCR methods at different stages of the broiler food chain. Food Microbiol. 2023;110 doi: 10.1016/j.fm.2022.104152. [DOI] [PubMed] [Google Scholar]
- Duffy L.L., Blackall P.J., Cobbold R.N., Fegan N. Quantitative effects of in-line operations on Campylobacter and Escherichia coli through two Australian broiler processing plants. Int. J. Food Microbiol. 2014;188:128–134. doi: 10.1016/j.ijfoodmicro.2014.07.024. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) Analysis of the baseline survey on the prevalence of Campylobacter in broiler batches and of Campylobacter and Salmonella on broiler carcasses, in the EU, 2008-Part B: analysis of factors associated with Campylobacter colonisation of broiler batches and with Campylobacter contamination of broiler carcasses; and investigation of the culture method diagnostic characteristics used to analyze broiler carcass samples. EFSA J. 2010;8:1522. [Google Scholar]
- EFSA (European Food Safety Authority) and ECDC (European Centre for Disease Prevention and Control) The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2014. EFSA J. 2015;13:4329. [Google Scholar]
- EFSA (European Food Safety Authority) and ECDC (European Centre for Disease Prevention and Control) The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2015. EFSA J. 2016;14:4634. doi: 10.2903/j.efsa.2017.5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) and ECDPC (European Centre of Disease Prevention and Control) The European Union one health 2018 zoonoses report. EFSA J. 2019;17:5926. doi: 10.2903/j.efsa.2019.5926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) and ECDC (European Centre for Disease Prevention and Control) The European Union one health 2019 zoonoses report. EFSA J. 2021;19:e06406. doi: 10.2903/j.efsa.2021.6406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) and ECDPC (European Centre for Disease Prevention and Control) The European Union one health 2020 zoonoses report. EFSA J. 2021;19:6971. doi: 10.2903/j.efsa.2021.6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgamoudi B.A., Taha T., Korolik V. Inhibition of Campylobacter jejuni biofilm formation by D-amino acids. Antibiotics. 2020;9:836. doi: 10.3390/antibiotics9110836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Haddad L., Harb C.P., Gebara M.A., Stibich M.A., Chemaly R.F. A systematic and critical review of bacteriophage therapy against multidrug-resistant ESKAPE organisms in humans. Clin. Infect. Dis. 2019;69:167–178. doi: 10.1093/cid/ciy947. [DOI] [PubMed] [Google Scholar]
- Elhelw R., Elhariri M., Salem H.M., Khalefa H.S., Hamza D.A., Ahmed Z.S. Molecular screening of gastric Helicobacter pullorum recovered from different avian species in Egypt. Pol. J. Vet. Sci. 2022;25:369–374. doi: 10.24425/pjvs.2022.142019. [DOI] [PubMed] [Google Scholar]
- Ellerbroek L.I., Lienau J.A., Klein G. Campylobacter spp. in broiler flocks at farm level and the potential for cross-contamination during slaughter. Zoonoses Public Health. 2010;57:e81–e88. doi: 10.1111/j.1863-2378.2009.01267.x. [DOI] [PubMed] [Google Scholar]
- Ellis-Iversen J., Jorgensen F., Bull S., Powell L., Cook A.J., Humphrey T.J. Risk factors for Campylobacter colonization during rearing of broiler flocks in Great Britain. Prev. Vet. Med. 2009;89:178–184. doi: 10.1016/j.prevetmed.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Ellis-Iversen J., Ridley A., Morris V., Sowa A., Harris J., Atterbury R., Sparks N., Allen V. Persistent environmental reservoirs on farms as risk factors for Campylobacter in commercial poultry. Epidemiol. Infect. 2012;140:916–924. doi: 10.1017/S095026881100118X. [DOI] [PubMed] [Google Scholar]
- Engberg J., Aarestrup F.M., Taylor D.E., Gerner-Smidt P., Nachamkin I. Quinolone and macrolide resistance in Campylobacter jejuni and C. coli: resistance mechanisms and trends in human isolates. Emerg. Infect. Dis. 2001;7:24–34. doi: 10.3201/eid0701.010104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erega A., Stefanic P., Dogsa I., Danevˇciˇc T., Simunovic K., Klanˇcnik A., Možina S.S., Mulec I.M. Bacillaene mediates the inhibitory effect of Bacillus subtilis on Campylobacter jejuni biofilms. Appl. Environ. Microbiol. 2021;87 doi: 10.1128/AEM.02955-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson D., Råhlén E., Bergenkvist E., Skarin M., Fernström L.L., Ryden J., Hansson I. Survival of Campylobacter jejuni in frozen chicken meat and risks associated with handling contaminated chicken in the kitchen. Food Control. 2023;145 [Google Scholar]
- Evans S.J. Introduction and spread of thermophilic campylobacters in broiler flocks. Vet. Rec. 1992;131:574–576. [PubMed] [Google Scholar]
- Evans S.J., Sayers A.R. A longitudinal study of Campylobacter infection of broiler flocks in Great Britain. Prev. Vet. Med. 2000;46:209–223. doi: 10.1016/s0167-5877(00)00143-4. [DOI] [PubMed] [Google Scholar]
- Facciolà A., Riso R., Avventuroso E., Visalli G., Delia S.A., Laganà P. Campylobacter: from microbiology to prevention. J. Prev. Med. Hyg. 2017;58:E79–E92. [PMC free article] [PubMed] [Google Scholar]
- Firlieyanti A.S., Connerton P.L., Connerton I.F. Campylobacters and their bacteriophages from chicken liver: the prospect for phage biocontrol. Int. J. Food Microbiol. 2016;237:121–127. doi: 10.1016/j.ijfoodmicro.2016.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher D.L., Craig E.W., Arnold J.W. An evaluation of on-line “reprocessing” on visual contamination and microbiological quality of broilers. J. Appl. Poult. Res. 1997;6:436–442. [Google Scholar]
- Furukawa I., Ishihara T., Teranishi H., Saito S., Yatsuyanagi J., Wada E., Kumagai Y., Takahashi S., Konno T., Kashio H., Kobayashi A., Kato N., Hayashi K., Fukushima K., Ishikawa K., Horikawa K., Oishi A., Izumiya H., Ohnishi T., Konishi Y., Kuroki T. Prevalence and characteristics of Salmonella and Campylobacter in retail poultry meat in Japan. Jpn. J. Infect. Dis. 2017;70:239–247. doi: 10.7883/yoken.JJID.2016.164. [DOI] [PubMed] [Google Scholar]
- Gahamanyi N., Song D.-G., Cha K.H., Yoon K.-Y., Mboera L.E.G., Matee M.I., Mutangana D., Amachawadi R.G., Komba E.V.G., Pan C.-H. Susceptibility of Campylobacter strains to selected natural products and frontline antibiotics. Antibiotics. 2020;9:790. doi: 10.3390/antibiotics9110790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Sánchez L., Melero B., Diez A.M., Jaime I., Canepa A., Rovira J. Genotyping, virulence genes and antimicrobial resistance of Campylobacter spp. isolated during two seasonal periods in Spanish poultry farms. Prev. Vet. Med. 2020;176 doi: 10.1016/j.prevetmed.2020.104935. [DOI] [PubMed] [Google Scholar]
- García-Sánchez L., Melero B., Diez A.M., Jaime I., Rovira J. Characterization of Campylobacter species in Spanish retail from different fresh chicken products and their antimicrobial resistance. J. Food Microbiol. 2018;76:457–465. doi: 10.1016/j.fm.2018.07.004. [DOI] [PubMed] [Google Scholar]
- García-Sánchez L., Melero B., Jaime I., Hänninen M.L., Rossi M., Rovira J. Campylobacter jejuni survival in a poultry processing plant environment. Food Microbiol. 2017;65:185–192. doi: 10.1016/j.fm.2017.02.009. [DOI] [PubMed] [Google Scholar]
- García-Sánchez L., Melero B., Jaime I., Rossi M., Ortega I., Rovira J. Biofilm formation, virulence and antimicrobial resistance of different Campylobacter jejuni isolates from a poultry slaughterhouse. Food Microbiol. 2019;83:193–199. doi: 10.1016/j.fm.2019.05.016. [DOI] [PubMed] [Google Scholar]
- Gencay Y.E., Sørensen M.C.H., Wenzel C.Q., Szymanski C.M., Brøndsted L. Phase variable expression of a single phage receptor in Campylobacter jejuni NCTC12662 influences sensitivity toward several diverse CPS-dependent phages. Front. Microbiol. 2018;9:82. doi: 10.3389/fmicb.2018.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giangaspero M. A cosmopolitan one health issue: campylobacteriosis. Clin. Microbiol. 2018;7:1. [Google Scholar]
- Gichure J.N., Njage P.M.K., Wambui J.M., Dykes G.A., Buys E.M., Coorey R. Systematic-review and meta-analysis on effect of decontamination interventions on prevalence and concentration of Campylobacter spp. during primary processing of broiler chickens. Food Microbiol. 2022;102 doi: 10.1016/j.fm.2021.103923. [DOI] [PubMed] [Google Scholar]
- Golden C.E., Mishra A. Prevalence of Salmonella and Campylobacter spp. in alternative and conventionally produced chicken in the United States: a systematic review and meta-analysis. J. Food Prot. 2020;83:1181–1197. doi: 10.4315/JFP-19-538. [DOI] [PubMed] [Google Scholar]
- Golz J.C., Epping L., Knüver M.T., Borowiak M., Hartkopf F., Deneke C., Malorny B., Semmler T., Stingl K. Whole genome sequencing reveals extended natural transformation in Campylobacter impacting diagnostics and the pathogens adaptive potential. Sci. Rep. 2020;10:3686. doi: 10.1038/s41598-020-60320-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gölz G., Kittler S., Malakauskas M., Alter T. Survival of Campylobacter in the food chain and the environment. Curr. Clin. Microbiol. Rep. 2018;5:126–134. [Google Scholar]
- Gonzalez-Fandos E., Maya N., Martínez-Laorden A., Perez-Arnedo I. Efficacy of lactic acid and modified atmosphere packaging against Campylobacter jejuni on chicken during refrigerated storage. Foods. 2020;9:109. doi: 10.3390/foods9010109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorain C., Singh A., Bhattacharyya S., Kundu A., Lahiri A., Gupta S., Mallick A.I. Mucosal delivery of live Lactococcus lactis expressing functionally active JlpA antigen induces potent local immune response and prevent enteric colonization of Campylobacter jejuni in chickens. Vaccine. 2020;38:1630–1642. doi: 10.1016/j.vaccine.2019.12.064. [DOI] [PubMed] [Google Scholar]
- Greene G., Koolman L., Whyte P., Lynch H., Coffey A., Lucey B., Egan J., O'Connor L., Bolton D. The efficacy of organic acid, medium chain fatty acid and essential oil-based broiler treatments; in vitro anti-Campylobacter jejuni activity and the effect of these chemical-based treatments on broiler performance. J. Appl. Microbiol. 2022;132:687–695. doi: 10.1111/jam.15204. [DOI] [PubMed] [Google Scholar]
- Gregory E., Barnhart H., Dreesen D.W., Stern N.J., Corn J.L. Epidemiological study of Campylobacter spp. in broilers: source, time of colonization, and prevalence. Avian Dis. 1997;41:890–898. [PubMed] [Google Scholar]
- Guccione E., Leon-Kempis M.D.R., Pearson B.M., Hitchin E., Mulholland F., Van Diemen P.M., Stevens M.P., Kelly D.J. Amino acid-dependent growth of Campylobacter jejuni: key roles for aspartase (AspA) under microaerobic and oxygen-limited conditions and identification of AspB (Cj0762), essential for growth on glutamate. Mol. Microbiol. 2008;69:77–93. doi: 10.1111/j.1365-2958.2008.06263.x. [DOI] [PubMed] [Google Scholar]
- Guerin M.T., Sir C., Sargeant J.M., Waddell L., O'Connor A.M., Wills R.W., Bailey R.H., Byrd J.A. The change in prevalence of Campylobacter on chicken carcasses during processing: a systematic review. Poult. Sci. 2010;89:1070–1084. doi: 10.3382/ps.2009-00213. [DOI] [PubMed] [Google Scholar]
- Guyard-Nicodème M., Keita A., Quesne S., Amelot M., Poezevara T., Le Berre B., Sánchez J., Vesseur P., Martín Á., Medel P., Chemaly M. Efficacy of feed additives against Campylobacter in live broilers during the entire rearing period. Poult. Sci. 2016;95:298–305. doi: 10.3382/ps/pev303. [DOI] [PubMed] [Google Scholar]
- Habib I., Ibrahim Mohamed M.Y., Ghazawi A., Lakshmi G.B., Khan M., Li D., Sahibzada S. Genomic characterization of molecular markers associated with antimicrobial resistance and virulence of the prevalent Campylobacter coli isolated from retail chicken meat in the United Arab Emirates. Curr. Res. Food Sci. 2023;6 doi: 10.1016/j.crfs.2023.100434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakeem M.J., Feng J., Nilghaz A., Ma L., Seah H.C., Konkel M.E., Lu X. Active packaging of immobilized zinc oxide nanoparticles controls Campylobacter jejuni in raw chicken meat. Appl. Environ. Microbiol. 2020;86 doi: 10.1128/AEM.01195-20. e01195-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakeem M.J., Lu X. Survival and control of Campylobacter in poultry production environment. Front. Cell. Infect. Microbiol. 2021;10 doi: 10.3389/fcimb.2020.615049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hald B., Skov M.N., Nielsen E.M., Rahbek C., Madsen J.J., Wainø M., Chriél M., Nordentoft S., Baggesen D.L., Madsen M. Campylobacter jejuni and Campylobacter coli in wild birds on Danish livestock farms. Acta Vet. Scand. 2016;58:11. doi: 10.1186/s13028-016-0192-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hald B., Skovgård H., Bang D.D., Pedersen K., Dybdahl J., Jespersen J.B., Madsen M. Flies and Campylobacter infection of broiler flocks. Emerg. Infect. Dis. 2004;10:1490–1492. doi: 10.3201/eid1008.040129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hald B., Skovgård H., Pedersen K., Bunkenborg H. Influxed insects as vectors for Campylobacter jejuni and Campylobacter coli in Danish broiler houses. Poult. Sci. 2008;87:1428–1434. doi: 10.3382/ps.2007-00301. [DOI] [PubMed] [Google Scholar]
- Han J. Campylobacter jejuni. PhD Dissertation. Iowa State University; Ames, USA: 2009. Molecular mechanisms involved in the emergence and fitness of fluoroquinolone-resistant. [Google Scholar]
- Han J., Wang Y., Sahin O., Shen Z., Guo B., Shen J., Zhang Q. A fluoroquinolone resistance associated mutation in gyrA affects DNA supercoiling in Campylobacter jejuni. Front. Cell Infect. Microbiol. 2012;2:21. doi: 10.3389/fcimb.2012.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankel J., Gibson T., Skov J., Andersen K.B., Dargatz M., Kappel A., Thiemann F., Curtis B., Chuppava B., Visscher C. Monitoring of Campylobacter jejuni in a chicken infection model by measuring specific volatile organic compounds and by qPCR. Sci. Rep. 2022;12:1175. doi: 10.1038/s41598-022-15863-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson I., Ederoth M., Andersson L., Vagsholm I., Engvall E.O. Transmission of Campylobacter spp. to chickens during transport to slaughter. J. Appl. Microbiol. 2005;99:1149–1157. doi: 10.1111/j.1365-2672.2005.02689.x. [DOI] [PubMed] [Google Scholar]
- Hansson I., Engvall E.O., Lindblad J., Gunnarsson A., Vagsholm I. Surveillance programme for Campylobacter species in Swedish broilers, July 2001 to June 2002. Vet. Rec. 2004;155:193–196. doi: 10.1136/vr.155.7.193. [DOI] [PubMed] [Google Scholar]
- Hansson I., Forshell L.P., Gustafsson P., Boqvist S., Lindblad J., Engvall E.O., Andersson Y., Vågsholm I. Summary of the Swedish Campylobacter program in broilers, 2001 through 2005. J. Food Prot. 2007;70:2008–2014. doi: 10.4315/0362-028x-70.9.2008. [DOI] [PubMed] [Google Scholar]
- Hansson I., Pudas N., Harbom B., Engvall E.O. Within-flock variations of Campylobacter loads in caeca and on carcasses from broilers. Int. J. Food Microbiol. 2010;141:51–55. doi: 10.1016/j.ijfoodmicro.2010.04.019. [DOI] [PubMed] [Google Scholar]
- Harris K., Taylor S., DiPietro R.B. Antecedents and outcomes of restaurant employees’ food safety intervention behaviors. Int. J. Hosp. Manag. 2021;94 [Google Scholar]
- Harrison D., Corry J.E.L., Tchórzewska M.A., Morris V.K., Hutchison M.L. Freezing as an intervention to reduce the numbers of Campylobacter isolated from chicken livers. Lett. Appl. Microbiol. 2013;57:206–213. doi: 10.1111/lam.12098. [DOI] [PubMed] [Google Scholar]
- Haughton P.N., Lyng J., Cronin D., Fanning S., Whyte P. Effect of crust freezing applied alone and in combination with ultraviolet light on the survival of Campylobacter on raw chicken. Food Microbiol. 2012;32:147–151. doi: 10.1016/j.fm.2012.05.004. [DOI] [PubMed] [Google Scholar]
- Hazeleger W., Arkesteijn C., Toorop-Bouma A., Beumer R. Detection of the coccoid form of Campylobacter jejuni in chicken products with the use of the polymerase chain reaction. Int. J. Food Microbiol. 1994;24:273–281. doi: 10.1016/0168-1605(94)90125-2. [DOI] [PubMed] [Google Scholar]
- Hazeleger W.C., Bolder N.M., Beumer R.R., Jacobs-Reitsma W.F. Darkling beetles (Alphitobius diaperinus) and their larvae as potential vectors for the transfer of Campylobacter jejuni and Salmonella enterica serovar paratyphi B variant Java between successive broiler flocks. Appl. Environ. Microbiol. 2008;74:6887–6891. doi: 10.1128/AEM.00451-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazeleger W.C., Wouters J.A., Rombouts F.M., Abee T. Physiological activity of Campylobacter jejuni far below the minimal growth temperature. Appl. Environ. Microbiol. 1998;64:3917–3922. doi: 10.1128/aem.64.10.3917-3922.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmy Y.A., Closs Jr G., Jung K., Kathayat D., Vlasova A., Rajashekara G. Effect of probiotic E. coli Nissle 1917 supplementation on the growth performance, immune responses, intestinal morphology, and gut microbes of Campylobacter jejuni infected chickens. Infect. Immun. 2022;90 doi: 10.1128/iai.00337-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman L., Heyndrickx M., Grijspeerdt K., Vandekerchove D., Rollier I., De Zutter L. Routes for Campylobacter contamination of poultry meat: epidemiological study from hatchery to slaughterhouse. Epidemiol. Infect. 2003;131:1169–1180. doi: 10.1017/s0950268803001183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans D., Pasmans F., Messens W., Martel A., Van Immerseel F., Rasschaert G., Heyndrickx M., Van Deun K., Haesebrouck F. Poultry as a host for the zoonotic pathogen Campylobacter jejuni. Vector Borne Zoonotic Dis. 2012;12:89–98. doi: 10.1089/vbz.2011.0676. [DOI] [PubMed] [Google Scholar]
- Hermans D., Van Deun K., Messens W., Martel A., Van Immerseel F., Haesebrouck F., Rasschaert G., Heyndrickx M., Pasmans F. Campylobacter control in poultry by current intervention measures ineffective: urgent need for intensified fundamental research. Vet. Microbiol. 2011;152:219–228. doi: 10.1016/j.vetmic.2011.03.010. [DOI] [PubMed] [Google Scholar]
- Heuer O.E., Pedersen K., Andersen J.S., Madsen M. Prevalence and antimicrobial susceptibility of thermophilic Campylobacter in organic and conventional broiler flocks. Lett. Appl. Microbiol. 2001;33:269–274. doi: 10.1046/j.1472-765x.2001.00994.x. [DOI] [PubMed] [Google Scholar]
- Hiett K.L., Cox N.A., Buhr R.J., Stern N.J. Genotype analyses of Campylobacter isolated from distinct segments of the reproductive tracts of broiler breeder hens. Curr. Microbiol. 2002;45:400–404. doi: 10.1007/s00284-002-3771-0. [DOI] [PubMed] [Google Scholar]
- Hiett K.L., Cox N.A., Stern N.J. Direct polymerase chain reaction detection of Campylobacter spp. in poultry hatchery samples. Avian Dis. 2002;46:219–223. doi: 10.1637/0005-2086(2002)046[0219:DPCRDO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Hiett K.L., Stern N.J., Fedorka-Cray P., Cox N.A., Musgrove M.T., Ladely S. Molecular subtype analyses of Campylobacter spp. from Arkansas and California poultry operations. Appl. Environ. Microbiol. 2002;68:6220–6236. doi: 10.1128/AEM.68.12.6220-6236.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higham L.E., Scott C., Akehurst K., Dring D., Parnham A., Waterman M., Bright A. Effects of financial incentives and cessation of thinning on prevalence of Campylobacter: a longitudinal monitoring study on commercial broiler farms in the UK. Vet. Rec. 2018;183 doi: 10.1136/vr.104823. 595-595. [DOI] [PubMed] [Google Scholar]
- Hofreuter D. Defining the metabolic requirements for the growth and colonization capacity of Campylobacter jejuni. Front. Cell Infect. Microbiol. 2014;4 doi: 10.3389/fcimb.2014.00137. 137-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofshagen M., Kruse H. Reduction in flock prevalence of Campylobacter spp. in broilers in Norway after implementation of an action plan. J. Food Prot. 2005;68:2220–2223. doi: 10.4315/0362-028x-68.10.2220. [DOI] [PubMed] [Google Scholar]
- Hong S.H., Seo K.H., Yoon S.H., Kim S.K., Chon J. Gold nanoparticle and polymerase chain reaction (PCR)-based colorimetric assay for the identification of Campylobacter spp. in chicken carcass. Food Sci. Anim. Resour. 2023;43:73–84. doi: 10.5851/kosfa.2022.e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh Y.H., Simpson S., Kerdahi K., Sulaiman I.M. A comparative evaluation study of growth conditions for culturing the isolates of Campylobacter spp. Curr. Microbiol. 2018;75:71–78. doi: 10.1007/s00284-017-1351-6. [DOI] [PubMed] [Google Scholar]
- Hue O., Bouquin S.Le, Laisney M.J., Allain V., Lalande F., Petetin I., Rouxel S., Quesne S., Gloaguen P.Y., Picherot M., Santolini J., Salvat G., Bougeard S., Chemaly M. Prevalence of and risk factors for Campylobacter spp. contamination of broiler chicken carcasses at the slaughterhouse. Food Microbiol. 2010;27:992–999. doi: 10.1016/j.fm.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Hughes R.A., Hallett K., Cogan T., Enser M., Humphrey T. The response of Campylobacter jejuni to low temperature differs from that of Escherichia coli. Appl. Environ. Microbiol. 2009;75:6292–6298. doi: 10.1128/AEM.00993-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huneau-Salaun A., Denis M., Balaine L., Salvat G. Risk factors for Campylobacter spp. colonization in French free-range broiler-chicken flocks at the end of the indoor rearing period. Prev. Vet. Med. 2007;80:34–48. doi: 10.1016/j.prevetmed.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Hwang H., Singer R.S. Survey of the U.S. broiler industry regarding pre- and postharvest interventions targeted to mitigate Campylobacter contamination on broiler chicken products. J. Food Prot. 2020;83:1137–1148. doi: 10.4315/JFP-19-527. [DOI] [PubMed] [Google Scholar]
- Ibrahim D., Sewid A.H., Arisha A.H., Abd El-Fattah A.H., Abdelaziz A.M., Al-Jabr O.A., Kishawy A.T.Y. Influence of Glycyrrhiza glabra extract on growth, gene expression of gut integrity, and Campylobacter jejuni colonization in broiler chickens. Front. Vet. Sci. 2020;7 doi: 10.3389/fvets.2020.612063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ica T., Caner V., Istanbullu O., Nguyen H.D., Ahmed B., Call D.R., Beyenal H. Characterization of mono-and mixed-culture Campylobacter jejuni biofilms. Appl. Environ. Microbiol. 2012;78:1033–1038. doi: 10.1128/AEM.07364-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ijaz U.Z., Sivaloganathan L., McKenna A., Richmond A., Kelly C., Linton M., Stratakos A.C., Lavery U., Elmi A., Wren B.W., Dorrell N., Corcionivoschi N., Gundogdu O. Comprehensive longitudinal microbiome analysis of the chicken cecum reveals a shift from competitive to environmental drivers and a window of opportunity for Campylobacter. Front. Microbiol. 2018;9:2452. doi: 10.3389/fmicb.2018.02452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingresa-Capaccioni S., Jim´enez-Trigos E., Marco-Jim´enez F., Catal´a P., Vega S., Marin C. Campylobacter epidemiology from breeders to their progeny in Eastern Spain. Poult. Sci. 2016;95:676–683. doi: 10.3382/ps/pev338. [DOI] [PubMed] [Google Scholar]
- ISO . International Organization for Standardization; Geneva, Switzerland: 2017. Microbiology of the Food Chain-Horizontal Method for the Detection and Enumeration of Listeria Monocytogenes and of Listeria Spp. – Part 2: Enumeration Method. ISO Norm 11290-2:2017. [Google Scholar]
- ISO . International Organization for Standardization; Geneva, Switzerland: 2017. Microbiology of the Food Chain-Horizontal Method for the Detection and Enumeration of Listeria Monocytogenes and of Listeria Spp. – Part 1: Detection Method. ISO Norm 11290-1:2017. [Google Scholar]
- Ito S., Kishimoto M. Development of a sampling and real-time PCR method for the quantitative detection of Campylobacter spp. in retail chicken meat without DNA extraction. J. Food Prot. 2023;86 doi: 10.1016/j.jfp.2022.100028. [DOI] [PubMed] [Google Scholar]
- Iwata T., Watanabe-Yanai A., Tamamura-Andoh Y., Arai N., Akiba M., Kusumoto M. Tryptanthrin reduces Campylobacter jejuni colonization in the chicken gut by a bactericidal mechanism. Appl. Environ. Microbiol. 2023;89:1701–1722. doi: 10.1128/aem.01701-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izat A.L., Gardner F.A., Denton J.H., Golan F.A. Incidence and level of Campylobacter jejuni in broiler processing. Poult. Sci. 1988;67:1568–1572. doi: 10.3382/ps.0671568. [DOI] [PubMed] [Google Scholar]
- Johannessen G.S., Garofolo G., Di Serafino G., Koláčková I., Karpíšková R., Wieczorek K., Osek J., Christensen J., Torp M., Hoorfar J. Campylobacter in chicken – critical parameters for international, multicentre evaluation of air sampling and detection methods. Food Microbiol. 2020;90 doi: 10.1016/j.fm.2020.103455. [DOI] [PubMed] [Google Scholar]
- Johnsen G., Kruse H., Hofshagen M. Genetic diversity and description of transmission routes for Campylobacter on broiler farms by amplified-fragment length polymorphism. J. Appl. Microbiol. 2006;101:1130–1139. doi: 10.1111/j.1365-2672.2006.02995.x. [DOI] [PubMed] [Google Scholar]
- Johnson T.J., Shank J.M., Johnson J.G. Current and potential treatments for reducing Campylobacter colonization in animal hosts and disease in humans. Front. Microbiol. 2017;8:487. doi: 10.3389/fmicb.2017.00487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josefsen M.H., Bhunia A.K., Engvall E.O., Fachmann M.S.R., Hoorfar J. Monitoring Campylobacter in the poultry production chain-from culture to genes and beyond. J. Microbiol. Methods. 2015;112:118–125. doi: 10.1016/j.mimet.2015.03.007. [DOI] [PubMed] [Google Scholar]
- Kaakoush N.O., Castaño-Rodríguez N., Mitchell H.M., Man S.M. Global epidemiology of Campylobacter infection. Clin. Microbiol. Rev. 2015;28:687–720. doi: 10.1128/CMR.00006-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki A.B., Wells H., Fakhr M.K. Retail liver juices enhance the survivability of Campylobacter jejuni and Campylobacter coli at low temperatures. Sci. Rep. 2019;9:2733. doi: 10.1038/s41598-018-35820-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassenborg H.D., Smith K.E., Vugia D.J., Rabatsky-Ehr T., Bates M.R., Carter M.A., Dumas N.B., Cassidy M.P., Marano N., Tauxe R.V., Angulo F.J., Emerging Infections Program FoodNet Working Group Fluoroquinolone-resistant Campylobacter infections: eating poultry outside of the home and foreign travel are risk factors. Clin. Infect. Dis. 2004;38(Suppl. 3):S279–S284. doi: 10.1086/381597. [DOI] [PubMed] [Google Scholar]
- Katsma W.E., De Koeijer A.A., Jacobs-Reitsma W.F., Mangen M.J., Wagenaar J.A. Assessing interventions to reduce the risk of Campylobacter prevalence in broilers. Risk Anal. 2007;27:863–876. doi: 10.1111/j.1539-6924.2007.00928.x. [DOI] [PubMed] [Google Scholar]
- Kemp G.K., Aldrich M.L., Guerra M.L., Schneider K.R. Continuous online processing of fecal-and ingesta-contaminated poultry carcasses using an acidified sodium chlorite antimicrobial intervention. J. Food Prot. 2001;64:807–812. doi: 10.4315/0362-028x-64.6.807. [DOI] [PubMed] [Google Scholar]
- Kers J.G., Velkers F.C., Fischer E.A.J., Hermes G.D.A., Stegeman J.A. Host and environmental factors affecting the intestinal microbiota in chickens. Front. Microbiol. 2018;9:235. doi: 10.3389/fmicb.2018.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S., Moore R.J., Stanley D., Chousalkar K.K. The gut microbiota of laying hens and its manipulation with prebiotics and probiotics to enhance gut health and food safety. Appl. Environ. Microbiol. 2020;86 doi: 10.1128/AEM.00600-20. e00600-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan J.A., Rathore R.S., Abulreesh H.H., Qais F.A., Ahmad I. Prevalence and antibiotic resistance profiles of Campylobacter jejuni isolated from poultry meat and related samples at retail shops in Northern India. Foodborne Pathog. Dis. 2018;15:218–225. doi: 10.1089/fpd.2017.2344. [DOI] [PubMed] [Google Scholar]
- Kingsbury J.M., Horn B., Armstrong B., Midwinter A., Biggs P., Callander M., K.Mulqueen M.Brooks, van der Logt P., Biggs R. The impact of primary and secondary processing steps on Campylobacter concentrations on chicken carcasses and portions. Food Microbiol. 2023;110 doi: 10.1016/j.fm.2022.104168. [DOI] [PubMed] [Google Scholar]
- Kittler S., Shakeri G., Peh E., Plötz M. A one health perspective on a multi-hurdle approach to combat Campylobacter spp. in broiler meat. Curr. Clin. Micro. Rep. 2021;8:49–61. [Google Scholar]
- Kittler S., Steffan S., Peh E., Plötz M. Phage biocontrol of Campylobacter: a one health approach. Curr. Top. Microbiol. Immunol. 2021;431:127–168. doi: 10.1007/978-3-030-65481-8_6. [DOI] [PubMed] [Google Scholar]
- Klančnik A., Šimunović K., Sterniša M., Ramić D., Možina S.Smole, Bucar F. Anti-adhesion activity of phytochemicals to prevent Campylobacter jejuni biofilm formation on abiotic surfaces. Phytochem. Rev. 2021;20:55–84. [Google Scholar]
- Klančnik A., Vučković D., Jamnik P., Abram M., Možina S.S. Stress response and virulence of heat-stressed Campylobacter jejuni. Microb. Environ. 2014;29:338–345. doi: 10.1264/jsme2.ME14020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klančnik A., Vučković D., Plankl M., Abram M., Smole Možina S. In vivo modulation of Campylobacter jejuni virulence in response to environmental stress. Foodborne Pathog. Dis. 2013;10:566–572. doi: 10.1089/fpd.2012.1298. [DOI] [PubMed] [Google Scholar]
- Korsak D., Maćkiw E., Rożynek E., Żyłowska M. Prevalence of Campylobacter spp. in retail chicken, Turkey, pork, and beef meat in Poland between 2009 and 2013. J. Food Prot. 2015;78:1024–1028. doi: 10.4315/0362-028X.JFP-14-353. [DOI] [PubMed] [Google Scholar]
- Koutsoumanis K., Allende A., Alvarez-Ordóñez A., Bolton D., Bover-Cid S., Davies R., Cesare A.D., Herman L., Hilbert F., Lindqvist R., Nauta M., Peixe L., Ru G., Simmons M., Skandamis P., Suffredini E., Alter T., Crotta M., Ellis-Iversen J., Hempen M., Messens W., Chemaly M. Update and review of control options for Campylobacter in broilers at primary production. EFSA J. 2020;18:e06090. doi: 10.2903/j.efsa.2020.6090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovács J.K., Felső P., Horváth G., Schmidt J., Dorn Á., Ábrahám H., Cox A., Márk L., Emődy L., Kovács T., Schneider G. Stress response and virulence potential modulating effect of peppermint essential oil in Campylobacter jejuni. Biomed. Res. Int. 2019;2019 doi: 10.1155/2019/2971741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovács J.K., Felső P., Makszin L., Pápai Z., Horváth G., Ábrahám H., Palkovics T., Böszörményi A., Emődy L., Schneider G. Antimicrobial and virulence-modulating effects of clove essential oil on the foodborne pathogen Campylobacter jejuni. Appl. Environ. Microbiol. 2016;82:6158–6166. doi: 10.1128/AEM.01221-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahti E., Rehn M., Ockborn G., Hansson I., Ågren J., Engvall E.O., Jernberg C. Outbreak of campylobacteriosis following a dairy farm visit: confirmation by genotyping. Foodborne Pathog. Dis. 2017;14:326–332. doi: 10.1089/fpd.2016.2257. [DOI] [PubMed] [Google Scholar]
- Larson C.L., Shah D.H., Dhillon A.S., Call D.R., Ahn S., Haldorson G.J., Davitt C., Konkel M.E. Campylobacter jejuni invade chicken LMH cells inefficiently and stimulate differential expression of the chicken CXCLi1 and CXCLi2 cytokines. Microbiology. 2008;154:3835–3847. doi: 10.1099/mic.0.2008/021279-0. [DOI] [PubMed] [Google Scholar]
- Lawes J.R., Vidal A., Clifton-Hadley F.A., Sayers R., Rodgers J., Snow L., Evans S.J., Powell L.F. Investigation of prevalence and risk factors for Campylobacter in broiler flocks at slaughter: results from a UK survey. Epidemiol. Infect. 2012;140:1725–1737. doi: 10.1017/S0950268812000982. [DOI] [PubMed] [Google Scholar]
- Lee S.K., Park H.J., Lee J.H., Lim J.S., Seo K.H., Heo E.J., Kim Y.J., Wee S.H., Moon J.S. Distribution and molecular characterization of Campylobacter species at different processing stages in two poultry processing plants. Foodborne Pathog. Dis. 2017;14:141–147. doi: 10.1089/fpd.2016.2218. [DOI] [PubMed] [Google Scholar]
- Li Y.P., Ingmer H., Madsen M., Bang D.D. Cytokine responses in primary chicken embryo intestinal cells infected with Campylobacter jejuni strains of human and chicken origin and the expression of bacterial virulence associated genes. BMC Microbiol. 2008;8:107. doi: 10.1186/1471-2180-8-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lianou A., Panagou E.Z., Nychas G.J.E. In: Pages 521–552 in Lawrie’s Meat Science. 8th ed. Toldra´ F., editor. Woodhead Publishing; Sawston, United Kingdom: 2017. Meat safety—I foodborne pathogens and other biological issues. [Google Scholar]
- Lin J. Novel approaches for Campylobacter control in poultry. Foodborne Pathog. Dis. 2009;6:755–765. doi: 10.1089/fpd.2008.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindblad M., Hansson I., Vågsholm I., Lindqvist R. Postchill Campylobacter prevalence on broiler carcasses in relation to slaughter group colonization level and chilling system. J. Food Prot. 2006;69:495–499. doi: 10.4315/0362-028x-69.3.495. [DOI] [PubMed] [Google Scholar]
- Lindqvist R., Cha W., Dryselius R., Lahti E. The temporal pattern and relationship of Campylobacter prevalence in broiler slaughter batches and human campylobacteriosis cases in Sweden 2009–2019. Int. J. Food Microbiol. 2022;378 doi: 10.1016/j.ijfoodmicro.2022.109823. [DOI] [PubMed] [Google Scholar]
- Line J.E. Influence of relative humidity on transmission of Campylobacter jejuni in broiler chickens. Poult. Sci. 2006;85:1145–1150. doi: 10.1093/ps/85.7.1145. [DOI] [PubMed] [Google Scholar]
- Luangtongkum T., Jeon B., Han J., Plummer P., Logue C.M., Zhang Q. Antibiotic resistance in Campylobacter: emergence, transmission and persistence. Future Microbiol. 2009;4:189–200. doi: 10.2217/17460913.4.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luc D., Michel B.J., Vanina L., Alain M., Liliane B., Jean M.B. Antibacterial mode of action of the Daucus carota essential oil active compounds against Campylobacter jejuni and efflux mediated drug resistance in gram-negative bacteria. Molecules. 2020;25:5448. doi: 10.3390/molecules25225448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo N., Sahin O., Lin J., Michel L.O., Zhang Q. In vivo selection of Campylobacter isolates with high levels of fluoroquinolone resistance associated with gyrA mutations and the function of the CmeABC efflux pump. Antimicrob. Agents Chemother. 2003;47:390–394. doi: 10.1128/AAC.47.1.390-394.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv R., Wang K., Feng J., Heeney D.D., Liu D., Lu X. Detection and quantification of viable but non-culturable Campylobacter jejuni. Front. Microbiol. 2020;10:2920. doi: 10.3389/fmicb.2019.02920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch H., Franklin-Hayes P., Koolman L., Egan J., Gutierrez M., Byrne W., Golden O., Bolton D., Reid P., Coffey A., Lucey B., O'Connor L., Unger K., Whyte P. Prevalence and levels of Campylobacter in broiler chicken batches and carcasses in Ireland in 2017–2018. Int. J. Food Microbiol. 2022;372 doi: 10.1016/j.ijfoodmicro.2022.109693. [DOI] [PubMed] [Google Scholar]
- Lynch C.T., Lynch H., Egan J., Whyte P., Bolton D., Coffey A., Lucey B. Antimicrobial resistance of Campylobacter isolates recovered from broilers in the Republic of Ireland in 2017 and 2018: an update. Br. Poult. Sci. 2020;61:550–556. doi: 10.1080/00071668.2020.1758300. [DOI] [PubMed] [Google Scholar]
- Lyngstad T.M., Jonsson M.E., Hofshagen M., Heier B.T. Risk factors associated with the presence of Campylobacter species in Norwegian broiler flocks. Poult. Sci. 2008;87:1987–1994. doi: 10.3382/ps.2008-00132. [DOI] [PubMed] [Google Scholar]
- Ma L., Wang Y., Shen J., Zhang Q., Wu C. Tracking Campylobacter contamination along a broiler chicken production chain from the farm level to retail in China. Int. J. Food Microbiol. 2014;181:77–84. doi: 10.1016/j.ijfoodmicro.2014.04.023. [DOI] [PubMed] [Google Scholar]
- Mäesaar M., Praakle K., Meremäe K., Kramarenko T., Sõgel J., Viltrop A., Muutra K., Kovalenko K., Matt D., Hörman A., Hänninen M.L., Roasto M. Prevalence and counts of Campylobacter spp. in poultry meat at retail level in Estonia. Food Cont. 2014;44:72–77. [Google Scholar]
- Mainali C., Gensler G., McFall M., King R., Irwin R., Senthilselvan A. Evaluation of associations between feed withdrawal and other management factors with Salmonella contamination of broiler chickens at slaughter in Alberta. J. Food Prot. 2009;72:2202–2207. doi: 10.4315/0362-028x-72.10.2202. [DOI] [PubMed] [Google Scholar]
- Malavi D.N., Abong G.O., Muzhingi T. Effect of food safety training on behavior change of food handlers: a case of orange-fleshed sweet potato purée processing in Kenya. Food Control. 2021;119 doi: 10.1016/j.foodcont.2020.107500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mañes-Lázaro R., Van Diemen P.M., Pin C., Mayer M.J., Stevens M.P., Narbad A. Administration of Lactobacillus johnsonii FI9785 to chickens affects colonization by Campylobacter jejuni and the intestinal microbiota. Br. Poult. Sci. 2017;58:373–381. doi: 10.1080/00071668.2017.1307322. [DOI] [PubMed] [Google Scholar]
- Marotta F., Garofolo G., Di Donato G., Aprea G., Platone I., Cianciavicchia S., Alessiani A., Di Giannatale E. Population diversity of Campylobacter jejuni in poultry and its dynamic of contamination in chicken meat. BioMed. Res. Int. 2015 doi: 10.1155/2015/859845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazengia E., Fisk C., Liao G., Huang H., Meschke J. Direct observational study of the risk of cross-contamination during raw poultry handling: practices in private homes. Food Prot. Trends. 2015;35:8–23. [Google Scholar]
- McDougald D., Rice S.A., Barraud N., Steinberg P.D., Kjelleberg S. Should we stay or should we go: mechanisms and ecological consequences for biofilm dispersal. Nat. Rev. Microbiol. 2012;10:39–50. doi: 10.1038/nrmicro2695. [DOI] [PubMed] [Google Scholar]
- McDowell S.W., Menzies F.D., McBride S.H., Oza A.N., McKenna J.P., Gordon A.W., Neill S.D. Campylobacter spp. in conventional broiler flocks in Northern Ireland: epidemiology and risk factors. Prev. Vet. Med. 2008;84:261–276. doi: 10.1016/j.prevetmed.2007.12.010. [DOI] [PubMed] [Google Scholar]
- McKenna A., Ijaz U.Z., Kelly C., Linton M., Sloan W.T., Green B.D., Lavery U., Dorrell N., Wren B.W., Richmond A., Corcionivoschi N., Gundogdu O. Impact of industrial production system parameters on chicken microbiomes: mechanisms to improve performance and reduce Campylobacter. Microbiome. 2020;8:128. doi: 10.1186/s40168-020-00908-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead G.C. Factors affecting intestinal colonization of poultry by Campylobacter and role of microflora in control. World's Poult. Sci. J. 2002;58:169–178. [Google Scholar]
- Meade K.G., Narciandi F., Cahalane S., Reiman C., Allan B., O'Farrelly C. Comparative in vivo infection models yield insights on early host immune response to Campylobacter in chickens. Immunogenetics. 2009;61:101–110. doi: 10.1007/s00251-008-0346-7. [DOI] [PubMed] [Google Scholar]
- Mendes Â.J., Santos-Ferreira N.L., Costa F.M., Lopes E.P., Freitas-Silva J., Inácio Â.S., Moreira F., Martins da Costa P. External contamination of broilers by Campylobacter spp. increases from the farm to the slaughterhouse. Br. Poult. Sci. 2020;61:550–556. doi: 10.1080/00071668.2020.1736264. [DOI] [PubMed] [Google Scholar]
- Messens W., Herman L., De Zutter L., Heyndrickx M. Multiple typing for the epidemiological study of contamination of broilers with thermotolerant Campylobacter. Vet. Microbiol. 2009;138:120–131. doi: 10.1016/j.vetmic.2009.02.012. [DOI] [PubMed] [Google Scholar]
- Meunier M., Guyard-Nicodème M., Vigouroux E., Poezevara T., Beven V., Quesne S., Bigault L., Amelot M., Dory D., Chemaly M. Promising new vaccine candidates against Campylobacter in broilers. PLoS One. 2017;12 doi: 10.1371/journal.pone.0188472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezher Z., Saccares S., Marcianò R., De Santis P., Rodas E.M., De Angelis V., Condoleo R. Occurrence of Campylobacter spp. in poultry meat at retail and processing plants’ levels in Central Italy. Ital. J. Food Saf. 2016;5:5495. doi: 10.4081/ijfs.2016.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montrose M.S., Shane S.M., Harrington K.S. Role of litter in the transmission of Campylobacter jejuni. Avian Dis. 1985;29:392–399. [PubMed] [Google Scholar]
- Morgan-Linnell S.K., Becnel Boyd L., Steffen D., Zechiedrich L. Mechanisms accounting for fluoroquinolone resistance in Escherichia coli clinical isolates. Antimicrob. Agents Chemother. 2009;53:235–241. doi: 10.1128/AAC.00665-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow A.L., Ruiz-Palacios G.M., Jiang X., Newburg D.S. Human-milk glycans that inhibit pathogen binding protect breast-feeding infants against infectious diarrhea. J. Nutr. 2005;135:1304–1307. doi: 10.1093/jn/135.5.1304. [DOI] [PubMed] [Google Scholar]
- Mota-Gutierrez J., Lis L., Lasagabaster A., Nafarrate I., Ferrocino I., Cocolin L., Rantsiou K. Campylobacter spp. prevalence and mitigation strategies in the broiler production chain. Food Microbiol. 2022;104 doi: 10.1016/j.fm.2022.103998. [DOI] [PubMed] [Google Scholar]
- Musavian H.S., Krebs N.H., Nonboe U., Corry J.E., Purnell G. Combined steam and ultrasound treatment of broilers at slaughter: a promising intervention to significantly reduce numbers of naturally occurring campylobacters on carcasses. Int. J. Food Microbiol. 2014;176:23–28. doi: 10.1016/j.ijfoodmicro.2014.02.001. [DOI] [PubMed] [Google Scholar]
- Myintzaw P., Jaiswal A.K., Jaiswal S. A review on campylobacteriosis associated with poultry meat consumption. Food Rev. Int. 2023;39:2107–2121. [Google Scholar]
- Myintzaw P., Moran F., Jaiswal A.K. Campylobacteriosis, consumer's risk perception, and knowledge associated with domestic poultry handling in Ireland. J. Food Saf. 2020;40:e12799. [Google Scholar]
- Nachamkin I., Ung H., Li M. Increasing fluoroquinolone resistance in Campylobacter jejuni, Pennsylvania, USA,1982-2001. Emerg. Infect. Dis. 2002;8:1501–1503. doi: 10.3201/eid0812.020115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nafarrate I., Mateo E., Miranda-Cadena K., Lasagabaster A. Isolation, host specificity and genetic characterization of Campylobacter specific bacteriophages from poultry and swine sources. Food Microbiol. 2021;97 doi: 10.1016/j.fm.2021.103742. [DOI] [PubMed] [Google Scholar]
- Näther G., Alter T., Martin A., Ellerbroek L. Analysis of risk factors for Campylobacter species infection in broiler flocks. Poult. Sci. 2009;88:1299–1305. doi: 10.3382/ps.2008-00389. [DOI] [PubMed] [Google Scholar]
- National Chicken Council . The National Chicken Council; Washington, DC: 2019. Vertical Integration.https://www.nationalchickencouncil.org/industry-issues/vertical-integration Accessed Mar. 2019. [Google Scholar]
- Natsos G., Mouttotou N.K., Ahmad S., Kamran Z., Ioannidis A., Koutoulis K.C. The genus Campylobacter: detection and isolation methods, species identification and typing techniques. J. Hell. Vet. Med. Soc. 2019;70:1327–1338. [Google Scholar]
- Neal-McKinney J.M., Samuelson D.R., Eucker T.P., Nissen M.S., Crespo R., Konkel M.E. Reducing Campylobacter jejuni colonization of poultry via vaccination. PLoS One. 2014;9 doi: 10.1371/journal.pone.0114254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neijat M., Shirley R.B., Welsher A., Barton J., Thiery P., Kiarie E. Growth performance, apparent retention of components, and excreta dry matter content in Shaver White Pullets (5 to 16 week of age) in response to dietary supplementation of graded levels of a single strain Bacillus subtilis probiotic. Poult. Sci. 2019;98:3777–3786. doi: 10.3382/ps/pez080. [DOI] [PubMed] [Google Scholar]
- Nesbit E.G., Gibbs P., Dreesen D.W., Lee M.D. Epidemiologic features of Campylobacter jejuni isolated from poultry broiler houses and surrounding environments as determined by use of molecular strain typing. Am. J. Vet. Res. 2001;62:190–194. doi: 10.2460/ajvr.2001.62.190. [DOI] [PubMed] [Google Scholar]
- Newell D.G., Elvers K.T., Dopfer D., Hansson I., Jones P., James S., Gittins J., Stern N.J., Davies R., Connerton I., Pearson D., Salvat G., Allen V.M. Biosecurity-based interventions and strategies to reduce Campylobacter spp. on poultry farms. Appl. Environ. Microbiol. 2011;77:8605–8614. doi: 10.1128/AEM.01090-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell D.G., Fearnley C. Sources of Campylobacter colonization in broiler chickens. Appl. Environ. Microbiol. 2003;69:4343–4351. doi: 10.1128/AEM.69.8.4343-4351.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama K., Seto Y., Yoshioka K., Kakuda T., Takai S., Yamamoto Y., Mukai T. Lactobacillus gasseri SBT2055 reduces infection by and colonization of Campylobacter jejuni. PLoS One. 2014;9 doi: 10.1371/journal.pone.0108827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nothaft H., Perez-Muñoz M.E., Gouveia G.J., Duar R.M., Wanford J.J., Lango-Scholey L., Panagos C.G., Srithayakumar V., Plastow G.S., Coros C., Bayliss C.D., Edison A.S., Walter J., Szymanski C.M. Coadministration of the Campylobacter jejuni N-glycan-based vaccine with probiotics improves vaccine performance in broiler chickens. Appl. Environ. Microbiol. 2017;83 doi: 10.1128/AEM.01523-17. e01523-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nothaft H., Perez-Munoz M.E., Yang T.F., Murugan A.V.M., Miller M., Kolarich D., Plastow G.S., Walter J., Szymanski C.M. Improving chicken responses to glycoconjugate vaccination against Campylobacter jejuni. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.734526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden I.D., MacRae M., Johnston M., Strachan N.J., Cody A.J., Dingle K.E., Newell D.G. Use of multilocus sequence typing to investigate the association between the presence of Campylobacter spp. in broiler drinking water and Campylobacter colonization in broilers. Appl. Environ. Microbiol. 2007;73:5125–5129. doi: 10.1128/AEM.00884-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh E., Chui L., Bae J., Li V., Ma A., Mutschall S.K., Taboada E.N., McMullen L.M., Jeon B. Frequent implication of multistress-tolerant Campylobacter jejuni in human infections. Emerg. Infect. Dis. 2018;6:1037–1044. doi: 10.3201/eid2406.171587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okeke I.N., Laxminarayan R., Bhutta Z.A., Duse A.G., Jenkins P., O'Brien T.F., Pablos-Mendez A., Klugman K.P. Antimicrobial resistance in developing countries. Part I: recent trends and current status. Lancet Infect. Dis. 2005;5:481–493. doi: 10.1016/S1473-3099(05)70189-4. [DOI] [PubMed] [Google Scholar]
- O'Mahony E., Buckley J.F., Bolton D., Whyte P., Fanning S. Molecular epidemiology of Campylobacter isolates from poultry production units in southern Ireland. PLoS One. 2011;6:e28490. doi: 10.1371/journal.pone.0028490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osiriphun S., Tuitemwong P., Koetsinchai W., Tuitemwong K., Erickson L.E. Model of inactivation of Campylobacter jejuni in poultry scalding. J. Food Eng. 2012;110:38–43. [Google Scholar]
- Oyarzabal O.A. Reduction of Campylobacter spp. by commercial antimicrobials applied during the processing of broiler chickens: a review from the United States perspective. J. Food Prot. 2005;68:1752–1760. doi: 10.4315/0362-028x-68.8.1752. [DOI] [PubMed] [Google Scholar]
- Pacanowski J., Lalande V., Lacombe K., Boudraa C., Lesprit P., Legrand P., Trystram D., Kassis N., Arlet G., Mainardi J.L., Doucet-Populaire F., Girard P.M., Meynard J.L., CAMPYL Study Group Campylobacter bacteremia: clinical features and factors associated with fatal outcome. Clin. Infect. Dis. 2008;47:790–796. doi: 10.1086/591530. [DOI] [PubMed] [Google Scholar]
- Peh E., Kittler S., Reich F., Kehrenberg C. Antimicrobial activity of organic acids against Campylobacter spp. and development of combinations—a synergistic effect? PLoS One. 2020;15 doi: 10.1371/journal.pone.0239312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Arnedo I., Gonzalez-Fandos E. Prevalence of Campylobacter spp. in poultry in three Spanish farms, a slaughterhouse and a further processing plant. Foods. 2019;8:111. doi: 10.3390/foods8030111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Boto D., García-Peña F.J., Abad-Moreno J.C., Hurtado-Pizarro M.D., Pérez-Cobo I., Echeita M.A. Drinking water as the source of Campylobacter coli infection in grandparent heavy breeders. Avian Pathol. 2010;39:483–487. doi: 10.1080/03079457.2010.518138. [DOI] [PubMed] [Google Scholar]
- Petersen L., Nielsen E.M., On S.L. Serotype and genotype diversity and hatchery transmission of Campylobacter jejuni in commercial poultry flocks. Vet. Microbiol. 2001;82:141–154. doi: 10.1016/s0378-1135(01)00382-0. [DOI] [PubMed] [Google Scholar]
- Peyrat M.B., Soumet C., Maris P., Sanders P. Recovery of Campylobacter jejuni from surfaces of poultry slaughterhouses after cleaning and disinfection procedures: analysis of a potential source of carcass contamination. Int. J. Food Microbiol. 2008;124:188–194. doi: 10.1016/j.ijfoodmicro.2008.03.030. [DOI] [PubMed] [Google Scholar]
- Platts-Mills J.A., Kosek M. Update on the burden of Campylobacter in developing countries. Curr. Opin. Infect. Dis. 2014;27:444–450. doi: 10.1097/QCO.0000000000000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poudel S., Jia L., Arick M.A., II, Hsu C.Y., Thrash A., Sukumaran A.T., Adhikari P., Kiess A.S., Zhang L. In silico prediction and expression analysis of vaccine candidate genes of Campylobacter jejuni. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2023.102592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poudel S., Li T., Chen S., Zhang X., Cheng W.H., Sukumaran A.T., Kiess A.S., Zhang L. Prevalence, antimicrobial resistance, and molecular characterization of Campylobacter isolated from broilers and broiler meat raised without antibiotics. Microbiol. Spectr. 2022;10 doi: 10.1128/spectrum.00251-22. e00251-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pumtang-On P., Mahony T.J., Hill R.A., Vanniasinkam T. A systematic review of Campylobacter jejuni vaccine candidates for chickens. Microorganisms. 2021;9:397. doi: 10.3390/microorganisms9020397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin X., Wang X., Shen Z. The rise of antibiotic resistance in Campylobacter. Curr. Opin. Gastroenterol. 2023;39:9–15. doi: 10.1097/MOG.0000000000000901. [DOI] [PubMed] [Google Scholar]
- Rahimi E., Momtaz H., Ameri M., Ghasemian-Safaei H., Ali-Kasemi M. Prevalence and antimicrobial resistance of Campylobacter species isolated from chicken carcasses during processing in Iran. Poult. Sci. 2010;89:1015–1020. doi: 10.3382/ps.2009-00090. [DOI] [PubMed] [Google Scholar]
- Ramabu S.S., Boxall N.S., Madie P., Fenwick S.G. Some potential sources for transmission of Campylobacter jejuni to broiler chickens. Lett. Appl. Microbiol. 2004;39:252–256. doi: 10.1111/j.1472-765X.2004.01573.x. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan A., Schumack N.M., Gariepy C.L., Eggleston H., Nunez G., Espinoza N., Nieto M., Castillo R., Rojas J., Mccoy A.J., Beck Z., Matyas G.R., Alving C.R., Guerry P., Poly F., Laird R.M. Enhanced immunogenicity and protective efficacy of a Campylobacter jejuni conjugate vaccine co-administered with liposomes containing monophosphoryl lipid A and QS-21. mSphere. 2019;4:e00101–e00119. doi: 10.1128/mSphere.00101-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasschaert G., De Zutter L., Herman L., Heyndrickx M. Campylobacter contamination of broilers: the role of transport and slaughterhouse. Int. J. Food Microbiol. 2020;322 doi: 10.1016/j.ijfoodmicro.2020.108564. [DOI] [PubMed] [Google Scholar]
- Rasschaert G., Houf K., De Zutter L. External contamination of Campylobacter-free flocks after transport in cleaned and disinfected containers. J. Food Prot. 2007;70:40–46. doi: 10.4315/0362-028x-70.1.40. [DOI] [PubMed] [Google Scholar]
- Ravel A., Pintar K., Nesbitt A., Pollari F. Nonfood-related risk factors of campylobacteriosis in Canada: a matched case-control study. BMC Public Health. 2016;16 doi: 10.1186/s12889-016-3679-4. 1016-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refrégier-Petton J., Rose N., Denis M., Salvat G. Risk factors for Campylobacter spp. contamination in French broiler-chicken flocks at the end of the rearing period. Prev. Vet. Med. 2001;50:89–100. doi: 10.1016/s0167-5877(01)00220-3. [DOI] [PubMed] [Google Scholar]
- Reich F., Atanassova V., Haunhorst E., Klein G. The effects of Campylobacter numbers in caeca on the contamination of broiler carcasses with Campylobacter. Int. J. Food Microbiol. 2008;127:116–120. doi: 10.1016/j.ijfoodmicro.2008.06.018. [DOI] [PubMed] [Google Scholar]
- Richards P.J., Connerton P.L., Connerton I.F. Phage biocontrol of Campylobacter jejuni in chickens does not produce collateral effects on the gut microbiota. Front. Microbiol. 2019;10:476. doi: 10.3389/fmicb.2019.00476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricotta E.E., Palmer A., Wymore K., Clogher P., Oosmanally N., Robinson T., Lathrop S., Karr J., Hatch J., Dunn J., Ryan P., Blythe D. Epidemiology and antimicrobial resistance of international travel-associated Campylobacter infections in the United States, 2005–2011. Am. J. Public Health. 2014;104:e108–e114. doi: 10.2105/AJPH.2013.301867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley A., Morris V., Gittins J., Cawthraw S., Edge S., Allen V. Potential sources of Campylobacter infection on chicken farms: contamination and control of broiler harvesting equipment, vehicles and personnel. J. Appl. Microbiol. 2011;111:233–244. doi: 10.1111/j.1365-2672.2011.05038.x. [DOI] [PubMed] [Google Scholar]
- Ridley A.M., Toszeghy M.J., Cawthraw S.A., Wassenaar T.M., Newell D.G. Genetic instability is associated with changes in the colonization potential of Campylobacter jejuni in the avian intestine. J. Appl. Microbiol. 2008;105:95–104. doi: 10.1111/j.1365-2672.2008.03759.x. [DOI] [PubMed] [Google Scholar]
- Robinson D.A. Infective dose of Campylobacter jejuni in milk. Br. Med. J. (Clin. Res. Ed.) 1981;282:1584. doi: 10.1136/bmj.282.6276.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues L.C., Cowden J.M., Wheeler J.G., Sethi D., Wall P.G., Cumberland P., Tompkins D.S., Hudson M.J., Roberts J.A., Roderick P.J. The study of infectious intestinal disease in England: risk factors for cases of infectious intestinal disease with Campylobacter jejuni infection. Epidemiol. Infect. 2001;127:185–193. doi: 10.1017/s0950268801006057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roila R., Ranucci D., Valiani A., Galarini R., Servili M., Branciari R. Antimicrobial and anti-biofilm activity of olive oil by-products against Campylobacter spp. isolated from chicken meat. Acta Sci. Pol. Technol. Ailment. 2019;18:43–52. doi: 10.17306/J.AFS.0629. [DOI] [PubMed] [Google Scholar]
- Rosenquist H., Sommer H.M., Nielsen N.L., Christensen B.B. The effect of slaughter operations on the contamination of chicken carcasses with thermotolerant Campylobacter. Int. J. Food Microbiol. 2006;108:226–232. doi: 10.1016/j.ijfoodmicro.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Saad A.M., Sitohy M.Z., Ahmed A.I., Rabie N.A., Amin S.A., Aboelenin S.M., Soliman M.M., El-Saadony M.T. Biochemical and functional characterization of kidney bean protein alcalase-hydrolysates and their preservative action on stored chicken meat. Molecules. 2021;26:4690. doi: 10.3390/molecules26154690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin O., Luo N., Huang S., Zhang Q. Effect of Campylobacter-specific maternal antibodies on Campylobacter jejuni colonization in young chickens. Appl. Environ. Microbiol. 2003;69:5372–5379. doi: 10.1128/AEM.69.9.5372-5379.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin O., Morishita T.Y., Zhang Q. Campylobacter colonization in poultry: sources of infection and modes of transmission. Anim. Health Res. Rev. 2002;3:95–105. doi: 10.1079/ahrr200244. [DOI] [PubMed] [Google Scholar]
- Sahin O., Zhang Q., Meitzler J.C., Harr B.S., Morishita T.Y., Mohan R. Prevalence, antigenic specificity, and bactericidal activity of poultry anti-Campylobacter maternal antibodies. Appl. Environ. Microbiol. 2001;67:3951–3957. doi: 10.1128/AEM.67.9.3951-3957.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaridis I., Ellis R.J., Cawthraw S.A., van Vliet A.H., Stekel D.J., Penell J., Chambers M., La Ragione R.M., Cook A.J. Investigating the association between the caecal microbiomes of broilers and Campylobacter burden. Front. Microbiol. 2018;9:927. doi: 10.3389/fmicb.2018.00927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi B., Upadhyay S., Orhan I.E., Jugran A.K., Jayaweera S.L.D., Dias D.A., Sharopov F., Taheri Y., Martins N., Baghalpour N. Therapeutic potential of α-and β-pinene: a miracle gift of nature. Biomolecules. 2019;9:738. doi: 10.3390/biom9110738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem L., Nashwa M.A.K., Mona S.A., Barakat A.M.A. Antimicrobial resistance of Campylobacter jejuni isolated from chicken, some animal products and human in Kalyoubia, Egypt, with special reference to its viability. Benhav. Vet. Med. J. 2019;36:156–163. [Google Scholar]
- Sandberg M., Sorensen L.L., Steenberg B., Chowdhury S., Ersboll A.K., Alban L. Risk factors for Campylobacter colonization in Danish broiler flocks, 2010 to 2011. Poult. Sci. 2015;94:447–453. doi: 10.3382/ps/peu065. [DOI] [PubMed] [Google Scholar]
- Santos-Ferreira N., Ferreira V., Teixeira P. Occurrence and multidrug resistance of Campylobacter in chicken meat from different production systems. Foods. 2022;11:1827. doi: 10.3390/foods11131827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder M.W., Eifert J.D., Ponder M.A., Schmale III D.G. Association of Campylobacter spp. levels between chicken grow-out environmental samples and processed carcasses. Poult. Sci. 2014;93:734–741. doi: 10.3382/ps.2013-03646. [DOI] [PubMed] [Google Scholar]
- Sears A., Baker M.G., Wilson N., Marshall J., Muellner P., Campbell D.M., Lake R.J., French N.P. Marked campylobacteriosis decline after interventions aimed at poultry. N. Z. Emerg. Infect. Dis. 2011;17:1007–1015. doi: 10.3201/eid1706.101272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebald M., Veron M. Base DNA content and classification of Vibrios. Ann. Inst. Pasteur (Paris) 1963;105:897–910. [PubMed] [Google Scholar]
- Seliwiorstow T., Baré J., Van Damme I., Uyttendaele M., De Zutter L. Campylobacter carcass contamination throughout the slaughter process of Campylobacter-positive broiler batches. Int. J. Food Microbiol. 2015;194:25–31. doi: 10.1016/j.ijfoodmicro.2014.11.004. [DOI] [PubMed] [Google Scholar]
- Shane S.M., Montrose M.S., Harrington K.S. Transmission of Campylobacter jejuni by the housefly (Musca domestica) Avian Dis. 1985;29:384–391. [PubMed] [Google Scholar]
- Shang Y., Kumar S., Oakley B., Kim W.K. Chicken gut microbiota: importance and detection technology. Front. Vet. Sci. 2018;5:254. doi: 10.3389/fvets.2018.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shange N., Gouws P., Hoffman L.C. Campylobacter and Arcobacter species in food-producing animals: prevalence at primary production and during slaughter. World J. Microbiol. Biotechnol. 2019;35:146. doi: 10.1007/s11274-019-2722-x. [DOI] [PubMed] [Google Scholar]
- Shanker S., Lee A., Sorrell T.C. Campylobacter jejuni in broilers: the role of vertical transmission. J. Hyg. 1986;96:153–159. doi: 10.1017/s002217240006592x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaughnessy R.G., Meade K.G., McGivney B.A., Allan B., O'Farrelly C. Global gene expression analysis of chicken caecal response to Campylobacter jejuni. Vet. Immunol. Immunopathol. 2011;142:64–71. doi: 10.1016/j.vetimm.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Sheppard S.K., Maiden M.C.J. The evolution of Campylobacter jejuni and Campylobacter coli. Cold Spring Harb. Perspect. Biol. 2015;7 doi: 10.1101/cshperspect.a018119. a018119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shreeve J.E., Toszeghy M., Pattison M., Newell D.G. Sequential spread of Campylobacter infection in a multipen broiler house. Avian Dis. 2000;44:983–988. [PubMed] [Google Scholar]
- Sibanda N., McKenna A., Richmond A., Ricke S.C., Callaway T., Stratakos A.C., Gundogdu O., Corcionivoschi N. A review of the effect of management practices on Campylobacter prevalence in poultry farms. Front. Microbiol. 2018;9:2002. doi: 10.3389/fmicb.2018.02002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva J., Leite D., Fernandes M., Mena C., Gibbs P.A., Teixeira P. Campylobacter spp. as a foodborne pathogen: a review. Front. Microbiol. 2011;2:200. doi: 10.3389/fmicb.2011.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveira V.A.I., Nishio E.K., Freitas C.A.U.Q., Amador I.R., Kobayashi R.K.T., Caretta T., Macedo F., Celligoi M.A.P.C. Production and antimicrobial activity of sophorolipid against Clostridium perfringens and Campylobacter jejuni and their additive interaction with lactic acid. Biocatal. Agric. Biotechnol. 2019;21 [Google Scholar]
- Šimunović K., Ramić D., Xu C., Smole Možina S. Modulation of Campylobacter jejuni motility, adhesion to polystyrene surfaces, and invasion of INT407 cells by quorum-sensing inhibition. Microorganisms. 2020;8:104. doi: 10.3390/microorganisms8010104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šimunovi´c K., Stefanic P., Klanˇcnik A., Erega A., Mulec I.M., Možina S.S. Bacillus subtilis PS-216 antagonistic activities against Campylobacter jejuni NCTC 11168 are modulated by temperature, oxygen, and growth medium. Microorganisms. 2022;10:289. doi: 10.3390/microorganisms10020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A., Nisaa K., Bhattacharyya S., Mallick A.I. Immunogenicity and protective efficacy of mucosal delivery of recombinant hcp of Campylobacter jejuni Type VI secretion system (T6SS) in chickens. Mol. Immunol. 2019;111:182–197. doi: 10.1016/j.molimm.2019.04.016. [DOI] [PubMed] [Google Scholar]
- Skirrow M.B. John McFadyean and the centenary of the first isolation of Campylobacter species. Clin. Infect. Dis. 2006;43:1213–1217. doi: 10.1086/508201. [DOI] [PubMed] [Google Scholar]
- Slader J., Domingue G., Jorgensen F., McAlpine K., Owen R.J., Bolton F.J., Humphrey T.J. Impact of transport crate reuse and of catching and processing on Campylobacter and Salmonella contamination of broiler chickens. Appl. Environ. Microbiol. 2002;68:713–719. doi: 10.1128/AEM.68.2.713-719.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavik M.F., Kim J.W., Pharr M.D., Raben D.P., Tsai S., Lobsinger C.M. Effect of trisodium phosphate on Campylobacter attached to post-chill chicken carcasses. J. Food Prot. 1994;57:324–326. doi: 10.4315/0362-028X-57.4.324. [DOI] [PubMed] [Google Scholar]
- Smith C.K., Abuoun M., Cawthraw S.A., Humphrey T.J., Rothwell L., Kaiser P., Barrow P.A., Jones M.A. Campylobacter colonization of the chicken induces a proinflammatory response in mucosal tissues. FEMS Immunol. Med. Microbiol. 2008;54:114–121. doi: 10.1111/j.1574-695X.2008.00458.x. [DOI] [PubMed] [Google Scholar]
- Smith S., Meade J., Gibbons J., McGill K., Bolton D., Whyte P. The impact of environmental conditions on Campylobacter jejuni survival in broiler faeces and litter. Infect. Ecol. Epidemiol. 2016;6 doi: 10.3402/iee.v6.31685. 31685-31685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer H.M., Høg B.B., Larsen L.S., Sørensen A.I.V., Williams N., Merga J.Y., Cerdà-Cuéllar M., Urdaneta S., Dolzd R., Wieczorek K., Osek J., David B., Hofshagen M., Jonsson M., Wagenaarg J.A., Bolder N., Rosenquist H. Analysis of farm specific risk factors for Campylobacter colonization of broilers in six European countries. Microb. Risk Anal. 2016;2-3:16–26. [Google Scholar]
- Sproston E.L., Wimalarathna H.M.L., Sheppard S.K. Trends in fluoroquinolone resistance in Campylobacter. Microb. Genom. 2018;4 doi: 10.1099/mgen.0.000198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern N.J., Fedorka-Cray P., Bailey J.S., Cox N.A., Craven S.E., Hiett K.L., Musgrove M.T., Ladely S., Cosby D., Mead G.C. Distribution of Campylobacter spp. in selected U.S. poultry production and processing operations. J. Food Prot. 2001;64:1705–1710. doi: 10.4315/0362-028x-64.11.1705. [DOI] [PubMed] [Google Scholar]
- Stern N.J., Hiett K.L., Alfredsson G.A., Kristinsson K.G., Reiersen J., Hardardottir H., Briem H., Gunnarsson E., Georgsson F., Lowman R., Berndtson E., Lammerding A.M., Paoli G.M., Musgrove M.T. Campylobacter spp. in Icelandic poultry operations and human disease. Epidemiol. Infect. 2003;130:23–32. doi: 10.1017/s0950268802007914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taha-Abdelaziz K., Astill J., Kulkarni R.R., Read L.R., Najarian A., Farber J.M., Sharif S. In vitro assessment of immunomodulatory and anti-Campylobacter activities of probiotic lactobacilli. Sci. Rep. 2019;9:17903. doi: 10.1038/s41598-019-54494-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taha-Abdelaziz K., Hodgins D.C., Alkie T.N., Quinteiro W., Yitbarek A., Astill J., Sharif S. Oral administration of PLGA-encapsulated CpG ODN and Campylobacter jejuni lysate reduces cecal colonization by Campylobacter jejuni in chickens. Vaccine. 2018;36:388–394. doi: 10.1016/j.vaccine.2017.11.073. [DOI] [PubMed] [Google Scholar]
- Taha-Abdelaziz K., Singh M., Sharif S., Sharma S., Kulkarni R.R., Alizadeh M., Yitbarek A., Helmy Y.A. Intervention strategies to control Campylobacter at different stages of the food chain. Microorganisms. 2023;11:113. doi: 10.3390/microorganisms11010113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita N., Watanabe T., Ishida-Kuroki K., Sekizaki T. Transition of microbiota in chicken cecal droppings from commercial broiler farms. BMC Vet. Res. 2021;17:10. doi: 10.1186/s12917-020-02688-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan S.Y., Tatsumura Y. Alexander Fleming (1881-1955): discoverer of penicillin. Singapore Med. J. 2015;56:366–367. doi: 10.11622/smedj.2015105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y., Fang L., Xu C., Zhang Q. Antibiotic resistance trends and mechanisms in the foodborne pathogen, Campylobacter. Anim. Health Res. Rev. 2017;18:87–98. doi: 10.1017/S1466252317000135. [DOI] [PubMed] [Google Scholar]
- Tang Y., Jiang Q., Tang H., Wang Z., Yin Y., Ren F., Kong L., Jiao X., Huang J. Characterization and prevalence of Campylobacter spp. from broiler chicken rearing period to the slaughtering process in eastern China. Front. Vet. Sci. 2020;7:227. doi: 10.3389/fvets.2020.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedersoo T., Roasto M., Mäesaar M., Kisand V., Ivanova M., Meremäe K. The prevalence, counts, and MLST genotypes of Campylobacter in poultry meat and genomic comparison with clinical isolates. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teh A.H.T., Lee S.M., Dykes G.A. Does Campylobacter jejuni form biofilms in food-related environments? Appl. Environ. Microbiol. 2014;80:5154–5160. doi: 10.1128/AEM.01493-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur S., Brake J., Keelara S., Zou M., Susick E. Farm and environmental distribution of Campylobacter and Salmonella in broiler flocks. Res. Vet. Sci. 2013;94:33–42. doi: 10.1016/j.rvsc.2012.07.014. [DOI] [PubMed] [Google Scholar]
- Thames H.T., Sukumaran A.T. A review of Salmonella and Campylobacter in broiler meat: emerging challenges and food safety measures. Foods. 2020;11:776. doi: 10.3390/foods9060776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thépault A., Guyard-Nicodème M., Rose V., Quesne S., Queguiner M., Houard E., Mégraud F., Rivoal K., Chemaly M. A representative overview of the genetic diversity and lipooligosaccharide sialylation in Campylobacter jejuni along the broiler production chain in France and its comparison with human isolates. Int. J. Food Microbiol. 2018;274:20–30. doi: 10.1016/j.ijfoodmicro.2018.03.010. [DOI] [PubMed] [Google Scholar]
- Thibodeau A., Fravalo P., Yergeau E., Arsenault J., Lahaye L., Letellier A. Chicken caecal microbiome modifications induced by Campylobacter jejuni colonization and by a non-antibiotic feed additive. PLoS One. 2015;10:e0131978. doi: 10.1371/journal.pone.0131978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C., Martin A., Sachsenroder J., Bandick N. Effects of modified atmosphere packaging on an extended-spectrum beta-lactamase-producing Escherichia coli, the microflora, and shelf life of chicken meat. Poult. Sci. 2020;99:7004–7014. doi: 10.1016/j.psj.2020.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torralbo A., Borge C., Allepuz A., García-Bocanegra I., Sheppard S.K., Perea A., Carbonero A. Prevalence and risk factors of Campylobacter infection in broiler flocks from southern Spain. Prev. Vet. Med. 2014;114:106–113. doi: 10.1016/j.prevetmed.2014.01.019. [DOI] [PubMed] [Google Scholar]
- Tram G., Day C.J., Korolik V. Bridging the gap: a role for Campylobacter jejuni biofilms. Microorganisms. 2020;8:452. doi: 10.3390/microorganisms8030452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribble D.R., Baqar S., Scott D.A., Oplinger M.L., Trespalacios F., Rollins D., Walker R.I., Clements J.D., Walz S., Gibbs P., Burg 3rd E.F., Moran A.P., Applebee L., Bourgeois A.L. Assessment of the duration of protection in Campylobacter jejuni experimental infection in humans. Infect. Immun. 2010;78:1750–1759. doi: 10.1128/IAI.01021-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tustin J., Laberge K., Michel P., Reiersen J., Dađadóttir S., Briem H., Harđardóttir H., Kristinsson K., Gunnarsson E., Friđriksdóttir V., Georgsson F. A national epidemic of campylobacteriosis in Iceland, lessons learned. Zoonoses Public Health. 2011;58:440–447. doi: 10.1111/j.1863-2378.2010.01387.x. [DOI] [PubMed] [Google Scholar]
- Umar S., Maiyah A.T., Mushtaq A. Campylobacter infections in poultry: update on challenges and potential immune interventions. Worlds Poult. Sci. J. 2016;72:381–390. [Google Scholar]
- Urdaneta S., Lorca-Oró C., Dolz R., López-Soria S., Cerdà-Cuéllar M. In a warm climate, ventilation, indoor temperature and outdoor relative humidity have significant effects on Campylobacter spp. colonization in chicken broiler farms which can occur in only 2 days. Food Microbiol. 2023;109 doi: 10.1016/j.fm.2022.104118. [DOI] [PubMed] [Google Scholar]
- USDA . USDA; Silver Spring, MD: 2017. Fact Sheet: Veterinary Feed Directive Final Rule and Next Steps. [Google Scholar]
- USDA-FSIS . U.S. Department of Agriculture (USDA)-Food Safety and Inspection Service (FSIS); Washington, DC: 2008. Compliance Guideline for Controlling Salmonella and Campylobacter in Poultry – Second Edition. [Google Scholar]
- USDA-FSIS . U.S. Department of Agriculture (USDA)-Food Safety and Inspection Service (FSIS); Washington, DC: 2015. DRAFT FSIS Compliance Guideline for Controlling Salmonella and Campylobacter in Raw Poultry. [Google Scholar]
- Ushanov L., Lasareishvili B., Janashia I., Zautner A.E. Application of Campylobacter jejuni phages: challenges and perspectives. Animals. 2020;11:279. doi: 10.3390/ani10020279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Boeckel T.P., Brower C., Gilbert M., Grenfell B.T., Levin S.A., Robinson T.P., Teillant A., Laxminarayan R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. 2015;112:5649–5654. doi: 10.1073/pnas.1503141112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandeplas S., Dubois-Dauphin R., Palm R., Beckers Y., Thonart P., Théwis A. Prevalence and sources of Campylobacter spp. contamination in free-range broiler production in the southern part of Belgium. Biotechnol. Agron. Soc. Environ. 2010;14:279–288. [Google Scholar]
- Vandeputte J., Martel A., Antonissen G., Verlinden M., De Zutter L., Heyndrickx M., Haesebrouck F., Pasmans F., Garmyn A. Research Note: Lyophilization of hyperimmune egg yolk: effect on antibody titer and protection of broilers against Campylobacter colonization. Poult. Sci. 2020;99:2157–2161. doi: 10.1016/j.psj.2019.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandeputte J., Martel A., Van Rysselberghe N., Antonissen G., Verlinden M., De Zutter L., Heyndrickx M., Haesebrouck F., Pasmans F., Garmyn A. In ovo vaccination of broilers against Campylobacter jejuni using a bacterin and subunit vaccine. Poult. Sci. 2019;98:5999–6004. doi: 10.3382/ps/pez402. [DOI] [PubMed] [Google Scholar]
- van Der Wielen P.W., Biesterveld S., Notermans S., Hofstra H., Urlings B.A., van Knapen F. Role of volatile fatty acids in development of the cecal microflora in broiler chickens during growth. Appl. Environ. Microbiol. 2000;66:2536–2540. doi: 10.1128/aem.66.6.2536-2540.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Deun K., Pasmans F., Ducatelle R., Flahou B., Vissenberg K., Martel A., Van den Broeck W., Van Immerseel F., Haesebrouck F. Colonization strategy of Campylobacter jejuni results in persistent infection of the chicken gut. Vet. Microbiol. 2008;130:285–297. doi: 10.1016/j.vetmic.2007.11.027. [DOI] [PubMed] [Google Scholar]
- Van Gerwe T., Miflin J.K., Templeton J.M., Bouma A., Wagenaar J.A., Jacobs-Reitsma W.F., Stegeman A., Klinkenberg D. Quantifying transmission of Campylobacter jejuni in commercial broiler flocks. Appl. Environ. Microbiol. 2009;75:625–628. doi: 10.1128/AEM.01912-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Véron M., Chatelain R. Taxonomic study of the genus Campylobacter sebald and Véron and designation of the neotype strain for the type species, Campylobacter fetus (Smith and Taylor) Sebald and Véron. Int. J. Syst. Evol. Micr. 1973;23:122–134. [Google Scholar]
- Vizzini P., Manzano M., Farre C., Meylheuc T., Chaix C., Ramarao N., Vidic J. Highly sensitive detection of Campylobacter spp. in chicken meat using a silica nanoparticle enhanced dot blot DNA biosensor. Biosens. Bioelectron. 2021;171 doi: 10.1016/j.bios.2020.112689. [DOI] [PubMed] [Google Scholar]
- Wagenaar J.A., French N.P., Havelaar A.H. Preventing Campylobacter at the source: why is it so difficult? Clin. Infect. Dis. 2013;57:1600–1606. doi: 10.1093/cid/cit555. [DOI] [PubMed] [Google Scholar]
- Wagenaar J.A., Newell D.G., Kalupahana R.S., Mughini-Gras L. In: Pages 159–177 in Zoonoses – Infections Affecting Humans and Animals: Focus on Public Health Aspects. Sing A., editor. Springer; Dordrecht, Netherlands: 2015. Campylobacter: animal reservoirs, human infections, and options for control. [Google Scholar]
- Wagle B.R., Donoghue A.M., Jesudhasan P.R. Select phytochemicals reduce Campylobacter jejuni in postharvest poultry and modulate the virulence attributes of C. jejuni. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.725087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagle B.R., Upadhyay A., Upadhyaya I., Shrestha S., Arsi K., Liyanage R., Venkitanarayanan K., Donoghue D.J., Donoghue A.M. Trans-cinnamaldehyde, eugenol and carvacrol reduce Campylobacter jejuni biofilms and modulate expression of select genes and proteins. Front. Microbiol. 2019;10:1837. doi: 10.3389/fmicb.2019.01837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wales A.D., Vidal A.B., Davies R.H., Rodgers J.D. Field interventions against colonization of broilers by Campylobacter. Compr. Rev. Food Sci. Food Saf. 2019;18:167–188. doi: 10.1111/1541-4337.12397. [DOI] [PubMed] [Google Scholar]
- Wang Y., Dong Y., Deng F., Liu D., Yao H., Zhang Q., Shen J., Liu Z., Gao Y., Wu C., Shen Z. Species shift and multidrug resistance of Campylobacter from chicken and swine, China, 2008–14. J. Antimicrob. Chemother. 2016;71:666–669. doi: 10.1093/jac/dkv382. [DOI] [PubMed] [Google Scholar]
- Wedderkopp A., Rattenborg E., Madsen M. National surveillance of Campylobacter in broilers at slaughter in Denmark in 1998. Avian Dis. 2000;44:993–999. [PubMed] [Google Scholar]
- Weerasooriya G., Khan S., Chousalkar K.K., McWhorter A.R. Invasive potential of sub-lethally injured Campylobacter jejuni and Salmonella typhimurium during storage in chicken meat juice. Food Control. 2022;135 [Google Scholar]
- Wei B., Cha S.Y., Yoon R.H., Kang M., Roh J.H., Seo H.S., Lee J.A., Jang H.K. Prevalence and antimicrobial resistance of Campylobacter spp. isolated from retail chicken and duck meat in South Korea. Food Control. 2016;62:63–68. [Google Scholar]
- World Health Organization (WHO) WHO; 2015. Pages 255 in Who Estimates of the Global Burden of Foodborne Diseases: Foodborne Disease Burden Epidemiology Reference Group 2007-2015.https://apps.who.int/iris/handle/10665/199350 Accessed Jan. 2023. [Google Scholar]
- World Health Organization (WHO). 2020. Campylobacter. Accessed May 2021. https://www.who.int/news-room/fact-sheets/detail/campylobacter.
- Wysok B., Wojtacka J., Kivistö R. Pathogenicity of Campylobacter strains of poultry and human origin from Poland. Int. J. Food Microbiol. 2020;334 doi: 10.1016/j.ijfoodmicro.2020.108830. [DOI] [PubMed] [Google Scholar]
- Wyszyńska A.K., Godlewska R. Lactic acid bacteria – a promising tool for controlling chicken Campylobacter infection. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.703441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyszyńska A., Raczko A., Lis M., Jagusztyn-Krynicka E.K. Oral immunization of chickens with avirulent Salmonella vaccine strain carrying C. jejuni 72Dz/92 cjaA gene elicits specific humoral immune response associated with protection against challenge with wild-type Campylobacter. Vaccine. 2004;22:1379–1389. doi: 10.1016/j.vaccine.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Yan M., Sahin O., Lin J., Zhang Q. Role of the CmeABC efflux pump in the emergence of fluoroquinolone-resistant Campylobacter under selection pressure. J. Antimicrob. Chemother. 2006;58:1154–1159. doi: 10.1093/jac/dkl412. [DOI] [PubMed] [Google Scholar]
- Yang H., Li Y., Johnson M.G. Survival and death of Salmonella typhimurium and Campylobacter jejuni in processing water and on chicken skin during poultry scalding and chilling. J. Food Prot. 2001;64:770–776. doi: 10.4315/0362-028x-64.6.770. [DOI] [PubMed] [Google Scholar]
- Zeiger K., Popp J., Becker A., Hankel J., Visscher C., Klein G., Meemken D. Lauric acid as feed additive – an approach to reducing Campylobacter spp. in broiler meat. PLoS One. 2017;12 doi: 10.1371/journal.pone.0175693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Peng Z., Li P., Mao Y., Shen R., Tao R., Diao X., Liu L., Zhao Y., Luo X. Complex internal microstructure of feather follicles on chicken skin pro-motes the bacterial cross-contamination of carcasses during the slaughtering process. Front. Microbiol. 2020;11 doi: 10.3389/fmicb.2020.571913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Tang M., Zhou Q., Zhang J., Yang X., Gao Y. Prevalence and characteristics of Campylobacter throughout the slaughter process of different broiler batches. Front. Microbiol. 2018;9:2092. doi: 10.3389/fmicb.2018.02092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong X., Wu Q., Zhang J., Ma Z., Wang J., Nie X., Ding Y., Xue L., Chen M., Wu S., Wei X., Zhang Y. Campylobacter jejuni biofilm formation under aerobic conditions and inhibition by ZnO nanoparticles. Front. Microbiol. 2020;11:207. doi: 10.3389/fmicb.2020.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer M., Barnhart H., Idris U., Lee M.D. Detection of Campylobacter jejuni strains in the water lines of a commercial broiler house and their relationship to the strains that colonized the chickens. Avian Dis. 2003;47:101–107. doi: 10.1637/0005-2086(2003)047[0101:DOCJSI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Zweifel C., Scheu K.D., Keel M., Renggli F., Stephan R. Occurrence and genotypes of Campylobacter in broiler flocks, other farm animals, and the environment during several rearing periods on selected poultry farms. Int. J. Food Microbiol. 2008;125:182–187. doi: 10.1016/j.ijfoodmicro.2008.03.038. [DOI] [PubMed] [Google Scholar]