Abstract
Background
Diabetes mellitus (DM) is a worldwide public health problem. The burden of diabetes has been continuously increasing from day to day, especially in developing countries like Ethiopia. Globally, half of all cases of diabetes mellitus are undiagnosed. Diabetes mellitus can be easily handled if it is detected early. There is limited evidence on the magnitude of undiagnosed diabetics and prediabetes at the community level in Ethiopia, particularly in the study area.
Objective
To assess the magnitude of undiagnosed diabetes mellitus, prediabetes, and associated factors among adults living in Debre Tabor town.
Methods
A community-based cross-sectional study was conducted in Debre Tabor town from October to December 2021. A total of 407 participants were selected using a multistage sampling technique. A pretested structural questionnaire was used to collect demographic, behavioral, and clinical data. Anthropometric measurements were taken with standardized and calibrated equipment. A fasting venous blood sample was collected for blood glucose level determination. Logistic regression was used to identify risk factors. A P-value ≤0.05 was considered statistically significant.
Result
The magnitude of undiagnosed diabetes mellitus and prediabetes was found to be 4.5% (95% CI: 2.9–7.4) and 14.5% (95% CI: 11.1–18.1), respectively. Older age (AOR: 6.50, 95% CI: 1.82–23.21), abnormal body mass index (AOR: 6.84, 95% CI: 1.91–24.54), systolic hypertension (AOR: 8.74, 95% CI: 2.53–30.19), and family history of diabetes mellitus (FHDM) (AOR: 12.45, 95% CI: 3.63–42.65) were significantly associated with undiagnosed diabetes mellitus. Using saturated oil (AOR: 1.97, 95% CI: 1.09–3.55), having a high waist circumference (AOR: 2.16, 95% CI: 1.20–3.87), and being hypertensive (AOR: 2.26, 95% CI: 1.04–4.96) were all significantly associated with Prediabetes.
Conclusion
Adults in Debre Tabor town have a high prevalence of undiagnosed diabetes and prediabetes. A variety of modifiable risk factors were also identified. As a result, focusing the prevention strategy on such modifiable risk factors may help to minimize the prevalence of undiagnosed diabetes mellitus and prediabetes as well as future disease complications.
Keywords: Undiagnosed diabetes mellitus, Prediabetes, Magnitude, Associated factors, Northcentral Ethiopia
1. Introduction
Diabetes mellitus (DM) is a heterogeneous metabolic disorder characterized by the presence of hyperglycemia due to impairment of insulin secretion, defective insulin action, or both, and disturbances of carbohydrate, fat, and protein metabolism [1,2]. Chronic hyperglycemia is associated with long-term microvascular (retinopathy, nephropathy, and neuropathy) and macrovascular (ischemic heart disease, stroke, and peripheral vascular disease) complications [3]. In the absence of an early diagnosis, the complications of diabetes can be severe [4]. Comorbidities of DM lead to a substantial decrease in the patient's quality of life as well as socioeconomic consequences [5]. Global diabetes-related health expenditures were estimated at 966 billion USD in 2021 and are projected to reach 1054 billion USD by 2045 [6].
The prevalence of diabetes is increasing worldwide. The International Diabetes Federation (IDF) estimates that 536.6 million people were living with diabetes in 2021, and this number is projected to increase by 46%, reaching 783.2 million by 2045 [6,7]. Diabetes was responsible for around 6.7 million adult deaths in 2021. In addition, 541 million people were estimated to have impaired glucose tolerance or prediabetes worldwide [6]. Low- and middle-income countries (LMICs) account for the vast majority of people with prediabetes (72.2%), and the European region had the lowest prevalence (5.1%) [8]. People with prediabetes are more likely to acquire diabetes in the future. Prediabetics are also more likely to develop diabetic complications and cardiovascular disease [1].
As IDF estimates and other studies have shown, approximately 50% of all individuals with diabetes are thought to be undiagnosed [[9], [10], [11]]. Undiagnosed diabetes is nearly twice as common in Africa (66.7%) as it is in developed countries (37.5%) [12]. This is because of a lack of testing facilities and equipment, a shortage of educated medical workers, limited access to health facilities, and a lack of knowledge about diabetes in Africa [13]. Furthermore, in the African region, 77.0% of all diabetes-related fatalities occur before the age of 60 [14].
Diabetic prevalence is currently increasing more rapidly in poor nations than in industrialized countries, where resources for diabetic management are low [11]. According to a 2022 IDF report, more than 80% of diabetics live in low- and middle-income countries [15]. The growth of DM in poor nations is being attributed to sedentary lives, expanding urbanized cultures, and associated changes in dietary and physical exercise habits [16].
Even though the prevalence of diabetes in Sub-Saharan Africa (3%) was lower than the global prevalence (8.5%) in 2016, there has been a rising trend in recent decades due to the consequences of rapid urbanization, globalization, and lifestyle changes [17]. Furthermore, it is concerning that more than two-thirds of diabetes mellitus cases in sub-Saharan Africa remain undiagnosed [18,19]. Ethiopia, a sub-Saharan African country, has a high prevalence of diabetes, and diabetes morbidity affected 3.8% of the population in 2016, according to World Health Organization (WHO) diabetes country profiles. Furthermore, diabetes accounts for 1% of all deaths [20], and undiagnosed diabetes is very common in the country [[20], [21], [22], [23]].
Diabetes currently has no known single cause. However, some factors, such as advanced age, obesity, dyslipidemia, sedentary lifestyle, and genetic factors, are likely to play an essential role in developing DM in most populations [24]. The American Diabetes Association recommends that people with a body mass index (BMI) greater than 25 kg/m2, regardless of age, be screened for diabetes. In addition, those who have high blood pressure, live a sedentary lifestyle, have a family history of diabetes mellitus (FHDM), are over 45 years old, and have been diagnosed with prediabetes should be screened for diabetes once a year [25].
Only a few community-based research studies [21,22,26] have published data on undiagnosed diabetes and prediabetes prevalence in Ethiopia. However, neither in the study area nor in Northcentral Ethiopia as a whole has a community-based investigation of diabetes and its associated variables been conducted. As a result, this study was conducted in Debre Tabor Town, Northcentral Ethiopia, to investigate the magnitude of undiagnosed diabetes, prediabetes, and their related risk factors. Thus, this paper provides evidence about the magnitude of undiagnosed DM, prediabetes, and their risk factors in northcentral Ethiopia as a whole for policymakers to plan prevention, early diagnosis, intervention methods, and community-based screening programs.
2. Materials and methods
2.1. Study area
The research was carried out in Debre Tabor. It is located 667 km from Addis Ababa, Ethiopia's capital city, in the South Gondar Zone of Amhara Regional State, north-central Ethiopia, about 109 km from Bahir Dar, and 50 km east of Lake Tana. The surface area of Debre Tabor city is about 31.87 km2. The town has a latitude and longitude of 110 51′ N and 380 1′ E, with an elevation of 2706 m (8878feet) above sea level. There is only one sports ground in the city. There are 36,285 houses in the town, which is divided into six kebeles (small administrative divisions). According to the population and household survey, the town had a total population of 87,627 people, with 47,319 women and 40,308 men. In town, there is one hospital, three health centers, and four private clinics (Fig. 1).
Fig. 1.
Map of the study area (Debre Tabor town).
2.2. Study design, period, and populations
A community-based cross-sectional study design was conducted from October 1 to December 30, 2021, to assess undiagnosed diabetes mellitus, prediabetes, and their associated factors among adults in Debre Tabor town, north-central Ethiopia. Adults aged 18 years and older who lived at least half a year in the study area, volunteered to give informed written consent, and fulfilled the eligibility criteria were included in the study.
3. Eligibility criteria
Individuals who were severely ill due to other medical conditions, pregnant women, individuals who ate food, individuals currently taking any drugs that may affect glucose metabolism (i.e., steroids, B-blockers, and thiazide diuretics), individuals with diagnosed diabetes mellitus, those with a mental disorder, and those unable to hear and/or speak were all excluded from the study.
4. Sample size and sampling technique
The sample size was generated using a single population proportion calculation with the following assumptions: 6.8% prevalence of diabetes mellitus from a previous study in Dessie town, northeast Ethiopia [24], 0.03 desired precision, 95% level of confidence, and a design effect of 1.5. As a result, the ultimate sample size was 407. The study participants were chosen using a multistage cluster sampling method. The six kebeles were considered as clusters, and the lottery method was used to choose 50% of the clusters (three clusters). The number of households in each selected cluster was 5020, 6500, and 6010. The sample was then distributed proportionally to the total number of households in each selected kebele. The systematic sampling technique was used to choose households in each kebele, utilizing the list of households as a sampling frame. Finally, if there was more than one eligible person in the household, a research participant was chosen by a lottery system.
5. Data collection
After informed consent had been obtained, the data were collected. The participants were free to withdraw from the study. The questionnaire followed the World Health Organization's step-by-step approach to non-communicable disease surveillance. To collect data on socio-demographic, behavioral, and clinical characteristics, face-to-face interviews with a pretested and semi-structured questionnaire were employed. After the interview in the evening, the participants were instructed to fast for the next 10–16 h. A standard vacutainer tube was used to collect 3–5 mL of venous blood the next morning. The blood sample was clotted for 15–20 min at room temperature before being centrifuged for 10 min at 3000 rpm. The fasting serum glucose level was then measured in the Debre Tabor Hospital Clinical Chemistry Laboratory using the Siemens Dimension EXL 200 Integrated Chemistry Analyzer (Siemens Healthcare Diagnostics Ltd. USA) using the hexokinase method. Prediabetes refers to a level of blood glucose between 100 and 125 mg/dl with no diabetic medication. When the fasting blood glucose (FBG) level is 126 mg/dl or higher or if a history of diabetic treatment is present, diabetes is diagnosed. Undiagnosed diabetes mellitus is defined as an elevated glucose level that meets the diabetes mellitus criteria but has not been diagnosed [27].
5.1. Variable measurements
Anthropometric measures were taken using standardized methodologies and calibrated equipment. In light indoor clothing and bare feet, each eligible participant was weighed to the nearest 0.1 kg. The study participants' height and weight were used to calculate their body mass index (BMI) (kg/m2). A handheld stadiometer was used to determine the height. BMI <18.5; 18.5–24.9; 25–29.9; and ≥30 were used to define underweight, normal weight, overweight, and obesity, respectively [28,29]. The waist circumference (WC) was measured at the midpoint between the lower margin of the least palpable rib and the top of the iliac crest. According to the WHO report, male and female waist circumferences of >102 cm and >88 cm, respectively, were considered abnormal [30].
Each participant's systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured twice with a mercury sphygmomanometer, first in a sitting position and once after a 15-min rest. The second BP reading was taken 5 min after the first reading. The average value was then calculated and used to determine the BP result. Hypertension was defined as a systolic blood pressure of ≥140 mmHg or a diastolic blood pressure of ≥90 mmHg or current usage of blood pressure-lowering medication [31].
The WHO global recommendation on physical activity was used to quantify physical activity. As a result, a cut-off of 600 metabolic equivalents (MET)-minutes per week was chosen to distinguish physically active people (achieving 600 or more MET minutes per week) from sedentary or physically inactive people (achieving fewer than 600 MET minutes per week) [32,33]. When a participant said he or she smoked tobacco products every day during the data collection period, that was considered a current tobacco smoker. Participants who had consumed alcohol within the previous 30 days of the data collection period were also deemed current drinkers [32].
6. Data quality assurance
Before real data collection, the questionnaire was pre-tested on 5% of the sample size in Woreta town, which is located outside of the study area, for correctness and consistency. Data collectors received a one-day training on the study's purpose and relevance, confidentiality, study participants' rights, consenting, interview techniques, laboratory test procedures, and quality control. Six trained BSc nurses gathered socio-demographic, behavioral, clinical, and anthropometric data under the supervision of investigators. The laboratory test was carried out in the lab by four senior medical laboratory technologists. Furthermore, the investigators extensively monitored and evaluated the data-gathering process regularly to guarantee the completeness and consistency of the data obtained, and they provided daily comments to the data collectors. The collected data was thoroughly examined for completeness, accuracy, and clarity.
After the quality control sample was run, the laboratory test was analyzed to ensure that the method was safe. The normal and pathological quality control samples for the Siemens Dimension EXL 200 Integrated Chemistry Analyzer were performed daily before the research samples were run. Each quality control reagent was handled according to the manufacturer's instructions. The instrument was calibrated when the quality control results failed and according to the manufacturer's instructions. Using laboratory manuals and standard operating procedures from the Debre Tabor Comprehensive specialized hospital laboratory, the pre-analytical, analytical, and post-analytical stages of quality assurance were meticulously followed.
6.1. Data analysis and interpretation
The data was cleaned, corrected, and double-checked for accuracy. Then, the data were entered into Epi Info version 7 and transferred to the statistical software SPSS version 20 for analysis. Categorical variables were presented using frequency and percentages. For continuous variables, the mean and standard deviation were employed. The associations between independent and outcome variables were determined using bivariate and multivariate logistic regression. To control the effect of confounding variables, variables with a p-value of ≤0.2 in the bivariable analysis were kept in the multivariable model. The model's fitness was determined using the Hosmer–Lemeshow goodness-of-fit statistic. The strength of the associations between factors was assessed using crude and adjusted odds ratios, as well as their respective 95% confidence intervals (CI). Statistical significance was defined as a p-value of less than 0.05.
6.2. Ethical consideration
Debre Tabor University's Research and Ethical Review Committee granted ethical approval; the letter's reference number was CHS/1827/2021. Additionally, the Debre Tabor town administration, Debre Tabor town Kebele administration, Debre Tabor town health office, and Debre Tabor Comprehensive Specialized Hospital senior management have all written letters of support. The study was conducted as per the Declaration of Helsinki. The data was collected after getting written informed consent from both the literate and legal guardians of all illiterate participants. The participants in the study received no financial compensation or benefit whatsoever. The study participants were identified using codes to protect data confidentiality, and no unauthorized individuals had access to the information obtained. Those participants who had elevated blood glucose levels and raised blood pressure were liked to Debre Tabor Hospital for further diagnosis and management of their condition.
7. Results
7.1. The sociodemographics and habits of the study participants
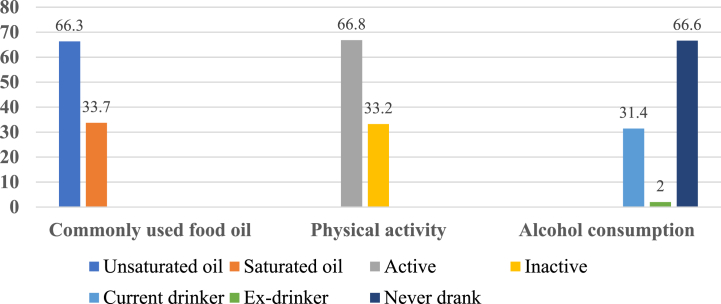
The study had a total of 407 participants, with a 100% response rate. The average age of the participants was 35 ± 12.7 years old. About 46.2% of all participants in the study were over the age of 35. The majority of those who took part, 93.4%, were Orthodox Christians, and 52.1% of those who took part were female. In addition, 74% of the participants were married, and 9.6% had no formal education (Table 1). Moreover, 33.7% of the participants used saturated oil, 33.2% were physically inactive, and 33.4% drank alcohol (Fig. 2).
Table 1.
Socio-demographic characteristics of the study participants in debre tabor town, northcentral Ethiopia, 2021 (N = 407).
| Variables | Category | Frequency | Percentage |
|---|---|---|---|
| Age | ≤35 years | 219 | 53.8 |
| >35 years | 188 | 46.2 | |
| Sex | Male | 195 | 47.9 |
| Female | 212 | 52.1 | |
| Religion | Orthodox | 380 | 93.4 |
| Muslim | 20 | 4.9 | |
| Protestant | 7 | 1.7 | |
| Marital status | Married | 301 | 74.0 |
| Never married | 64 | 15.7 | |
| Widowed | 31 | 7.6 | |
| Divorced/separated | 11 | 2.7 | |
| Educational status | No formal education | 36 | 8.8 |
| Primary school | 50 | 12.3 | |
| High school | 101 | 24.8 | |
| College/University | 220 | 54.1 | |
| Occupation | Government employee | 163 | 40.0 |
| Private sector employee | 76 | 18.7 | |
| Housewife | 85 | 20.9 | |
| Merchant | 51 | 12.5 | |
| Others | 32 | 7.9 | |
| Monthly income | <1000 ETB | 4 | 1.0 |
| 100-1800 ET B | 83 | 20.4 | |
| 1801-2400 ET B | 60 | 14.7 | |
| >2400 ETB | 260 | 63.9 |
Abbreviation: ETB, Ethiopian Birr.
Fig. 2.
Habits of the study participants in Debre Tabor town, Northcentral Ethiopia,2021(N = 407).
7.2. Anthropometrics and clinical characteristics
About 20.6% of the total participants had a BMI of 25 kg/m2 or higher, 42.3% had an abnormal waist circumference, 12% had systolic hypertension, 11.5% had a family history of DM, and 10.3% had a previous diagnosis of hypertension (Table 3).
Table 3.
The Distribution of DM and Prediabetes by anthropometric measurement and Clinical Characteristics of Study Participants in Debre Tabor Town, Northcentral Ethiopia, 2021 (N = 407).
| Characteristics | Category | Total N (%) | Undiagnosed DM (n = 407) |
Prediabetes (n = 387) |
||
|---|---|---|---|---|---|---|
| Yes |
No |
Yes |
No |
|||
| n (%) | n (%) | n (%) | n (%) | |||
| BMI (kg/m2) | <25 | 323 (79.4) | 12 (2.9) | 311 (76.4) | 42 (10.9) | 269 (69.5) |
| ≥25 | 84 (20.6) | 8 (2) | 76 (18.7 | 14 (3.6) | 62 (16.0) | |
| SBP | Non hypertensive | 358 (88.0) | 8 (2) | 350 (86) | 54 (14.0) | 296 (76.5) |
| Hypertensive | 49 (12.0) | 12 (2.9) | 37 (9.1) | 2 (0.5) | 35 (9.0) | |
| DBP | Non hypertensive | 375 (92.1) | 18 (4.4) | 357 (87.7) | 53 (13.7) | 303 (78.3) |
| Hypertensive | 32 (7.9) | 2 (0.5) | 30 (7.4) | 3 (0.8) | 28 (7.2) | |
| WC | Normal | 235 (57.7) | 9 (2.2) | 226 (55.5) | 24 (6.2) | 202 (52.2) |
| Abnormal | 172 (42.3) | 11 (2.7) | 161 (39.6) | 32 (8.3) | 129 (33.3) | |
| FHDM | Yes | 47 (11.5) | 12 (2.9) | 35 (8.6) | 7 (1.8) | 28 (7.2) |
| No | 360 (88.5) | 8 (2) | 352 (86.5) | 49 (12.7) | 303 (78.3) | |
| Diagnosed Hypertension | Yes | 42 (10.3) | 2 (0.5) | 40 (9.8) | 11 (2.9) | 29 (7.5) |
| No | 365 (89.7) | 18 (4.4) | 347 (85.3) | 45 (11.6) | 302 (78.0) | |
Abbreviations: FHDM, Family History of Diabetes Mellitus; SBP, Systolic Blood Pressure; DBP, Diastolic Blood Pressure; WC, Waist Circumference; DM, Diabetes Mellitus.
7.3. Undiagnosed diabetes mellitus and prediabetes
The magnitude of undiagnosed DM was 4.9% (95% CI: 2.9–7.4). In addition, the magnitude of prediabetes was 14.5% (95% CI: 11.1–18.1). The magnitude of undiagnosed DM was high among males (3.4%) and those over the age of 35 (3.7%). However, the magnitude of prediabetes was higher in those who were married (10.9%) and in those participants who had an abnormal waist circumference (8.3%) (Table 2).
Table 2.
The distribution of DM and prediabetes by socio-demographic and behavioral characteristics of the study participants in debre tabor town, northcentral Ethiopia, 2021 (N = 407).
| Characteristics | Category | Total N (%) | Undiagnosed DM(n = 407) |
Prediabetes (n = 387) |
||
|---|---|---|---|---|---|---|
| Yes |
No |
Yes |
No |
|||
| n (%) | n (%) | n (%) | n (%) | |||
| Age | ≤35 years | 219 (53.8) | 5 (1.2) | 214 (52.6) | 32 (8.3) | 182 (47.0) |
| >35 years | 188 (46.2) | 15 (3.7) | 173 (42.5) | 24 (6.2) | 149 (38.5) | |
| Sex | Male | 195 (47.9) | 14 (3.4) | 181 (44.5) | 26 (6.7) | 155 (40.0) |
| Female | 212 (52.1) | 6 (1.5) | 206 (50.6) | 30 (7.8) | 176 (45.5) | |
| Religions | Orthodox | 380 (93.4) | 19 (4.7) | 361 (88.7) | 52 (13.5) | 309 (79.8) |
| Muslim | 20 (4.9) | 1 (0.3) | 19 (4.7) | 4 (1.0) | 15 (3.9) | |
| Protestant | 7 (1.7) | 0 (0) | 7 (1.7) | 0 (0) | 7 (1.8) | |
| Marital status | Married | 301 (74.0) | 18 (4.4) | 283 (69.5) | 42 (10.9) | 241 (62.3) |
| Never married | 64 (15.7) | 1 (0.3) | 63 (15.5) | 7 (1.8) | 56 (14.5) | |
| Widowed | 31 (7.6) | 1 (0.3) | 30 (7.4) | 5 (1.3) | 25 (6.5) | |
| Divorced/Separated | 11 (2.7) | 0 (0) | 11 (2.7) | 2 (0.5) | 9 (2.3) | |
| Educational status | No formal education | 36 (8.8) | 1 (0.3) | 35 (8.6) | 9 (2.3) | 26 (6.7) |
| Primary school | 50 (12.3) | 3 (0.7) | 47 (11.6) | 9 (2.3) | 38 (9.8) | |
| High school | 101 (24.8) | 2 (0.5) | 99 (24.3) | 13 (3.4) | 86 (22.2) | |
| College/University | 220 (54.1) | 14 (3.4) | 206 (50.6) | 25 (6.5) | 181 (46.8) | |
| Occupation | Government employee | 163 (40.0) | 10 (2.5) | 153 (37.6 | 16 (4.1) | 137 (35.4) |
| Private sector employee | 76 (18.7) | 3 (0.7) | 73 (17.9) | 9 (2.3) | 64 (16.5) | |
| Housewife | 85 (20.9) | 0 (0) | 85 (20.9) | 15 (3.9) | 70 (18.1) | |
| Merchant | 51 (12.5) | 5 (1.2) | 46 (11.3) | 6 (1.6) | 40 (10.3) | |
| Others | 32 (7.9) | 2 (0.5) | 30 (7.4) | 10 (2.6) | 20 (5.2) | |
| Income | <1000 ETB | 4 (1.0) | 1 (0.3) | 3 (0.7) | 1 (0.3) | 2 (0.5) |
| 100-1800 ET B | 83 (20.4) | 4 (0.9) | 79 (19.4) | 11 (2.8) | 68 (17.6) | |
| 1801-2400 ET B | 60 (14.7) | 5 (1.2) | 55 (13.5) | 9 (2.3) | 46 (11.9) | |
| >2400 ETB | 260 (63.9) | 10 (2.5) | 250 (61.4) | 35 (9.0) | 215 (55.6) | |
| Commonly used food oil | Unsaturated oil | 270 (66.3) | 11 (2.7) | 259 (63.6) | 30 (7.8) | 229 (59.2) |
| Saturated oil | 137 (33.7) | 9 (2.2) | 128 (31.5) | 26 (6.7) | 102 (26.3) | |
| Physical activity | Active | 272 (66.8) | 18 (4.4) | 254 (62.4 | 34 (8.8) | 220 (56.8) |
| Inactive | 135 (33.2) | 2 (0.5) | 133 (32.7) | 22 (5.7) | 111 (28.7) | |
| Alcohol consumption | Current drinker | 128 (31.4) | 6 (1.5) | 122 (30) | 17 (4.4) | 105 (27.1) |
| Ex-drinker | 8 (2.0) | 0 (0) | 8 (2) | 1 (0.3) | 7 (1.8) | |
| Never drank | 271 (66.6) | 14 (3.4) | 257 (63.1 | 38 (9.8) | 219 (56.6) | |
7.4. Factors associated with undiagnosed diabetes mellitus
Older age, being male, having a BMI ≥25 kg/m2, having increased systolic blood pressure (SBP), being physically inactive, and having a family history of DM (FHDM) were all found to be significantly associated with undiagnosed DM in a bivariate logistic regression analysis. However, in the multivariable logistic regression analysis, older age, BMI≥25 kg/m2, increased systolic blood pressure, and FHDM were independently associated with undiagnosed DM. As a result, those participants who were aged >35 years were 6.5 (AOR = 6.50, 95% CI: 1.82 to 23.21) times more likely to have undiagnosed DM than participants aged ≤35 years. Similarly, participants with systolic hypertension were 8.7 (AOR = 8.74, 95% CI: 2.53 to 30.19) times more likely to have undiagnosed DM compared to non-hypertensive participants. Furthermore, participants with a BMI of ≥25 kg/m2 were 6.8 (AOR = 6.84, 95% CI: 1.91–2.54) times more likely to have undiagnosed DM than those with a BMI of less than 24.9 kg/m2. Moreover, those participants who had a family history of DM were 12.4 (AOR = 12.45, 95% CI: 3.63 to 42.65) times more likely to have undiagnosed diabetes than the participants who hadn't had a family history of DM (Table 4).
Table 4.
Bivariate and multivariable analysis of factors associated with undiagnosed DM among the population at Debre Tabor Town, Northcentral Ethiopia, 2021 (n = 407).
| Variables | DM n (%) |
COR (95% CI) | AOR (95% CI) | P-Value | ||
|---|---|---|---|---|---|---|
| Yes | No | |||||
| Age | ≤35 years | 5 (1.2) | 214 (52.6) | 1 | 1 | |
| >35 years | 15 (3.7) | 173 (42.5) | 3.71 (1.32–10.41) | 6.50 (1.82–23.21) * | 0.004 | |
| Sex | Male | 14 (3.4) | 181 (44.5) | 2.66 (1.02–7.05) | 1.97 (0.54–6.63) | 0.275 |
| Female | 6 (1.5) | 206 (50.6) | 1 | 1 | ||
| BMI (kg/m2) | <25 | 12 (2.9) | 311 (76.4 | 1 | 1 | |
| ≥25 | 8 (2) | 76 (18.7 | 2.73 (1.08–6.91) | 6.84 (1.91–24.54) * | 0.003 | |
| SBP | <140 mmHg | 8 (2) | 350 (86) | 1 | 1 | |
| ≥140 mmHg | 12 (2.9) | 37 (9.1) | 14.19 (5.45–36.93) | 8.74 (2.53–30.19) * | 0.001 | |
| Physical activity | Active | 18 (4.4) | 254 (62.4 | 4.71 (1.08–20.62) | 4.10 (0.84–19.95) | 0.080 |
| Inactive | 2 (0.5) | 133 (32.7) | 1 | 1 | ||
| FHDM | Yes | 12 (2.9) | 35 (8.6) | 15.09 (5.78–39.39) | 12.45 (3.63–42.65) * | <0.001 |
| No | 8 (2) | 352 (86.5) | 1 | |||
Note: *p < 0.05, statistically significant association.
Abbreviations: AOR, Adjusted Odds Ratio; CI, Confidence Interval; COR, Crude Odds Ratio; BMI, Body Mass Index, FHDM, Family History of Diabetes Mellitus; SBP, Systolic Blood Pressure; DM, Diabetes Mellitus.
7.5. Factors associated with prediabetes
In the bivariate logistic regression analysis, educational status, using saturated oil, abnormal WC, and previously diagnosed hypertension were found to be significantly associated with prediabetes. In multivariate analysis, however, using saturated oil, abnormal WC, and previously diagnosed hypertension were all independently associated with prediabetes. Those participants who used saturated oil were 1.97 (AOR = 1.97, 95% CI: 1.09 to 3.55) times more likely to have prediabetes than unsaturated oil consumers. In addition, participants who had an abnormal waist circumference were 2.16 (AOR = 2.16, 95% CI: 1.20 to 3.87) times more likely to have prediabetes than the participants who had normal WC. Moreover, study participants with previously diagnosed hypertension had 2.26 (AOR = 2.26, 95% CI: 1.04 to 4.96) times higher odds of prediabetes than undiagnosed participants (Table 5).
Table 5.
Bivariate and multivariable analysis of factors associated with prediabetes among the population at Debre Tabor Town, Northcentral Ethiopia, 2021 (n = 387).
| Variables | Prediabetes n (%) |
COR (95% CI) | AOR (95% CI) | P-Value | ||
|---|---|---|---|---|---|---|
| Yes | No | |||||
| Educational status | No formal education | 9 (2.3) | 26 (6.7) | 2.51 (1.05–5.96) | 2.28 (0.93–5.60) | 0.071 |
| Primary school | 9 (2.3) | 38 (9.8) | 1.72 (0.74–3.97) | 1.78 (0.75–4.22) | 0.190 | |
| High school | 13 (3.4) | 86 (22.2) | 1.09 (0.53–2.24 | 1.06 (0.51–2.23) | 0.869 | |
| College/University | 25 (6.5) | 181 (46.8) | 1 | |||
| Commonly used food oil | Unsaturated oil | 30 (7.8) | 229 (59.2) | 1 | ||
| Saturated oil | 26 (6.7) | 102 (26.3) | 1.95 (1.10–3.46) | 1.97 (1.09–3.55) * | 0.025 | |
| WC | Normal | 24 (6.2) | 202 (52.2) | 1 | 1 | |
| High | 32 (8.3) | 129 (33.3) | 2.09 (1.18–3.71) | 2.16 (1.20–3.87) * | 0.010 | |
| Diagnosed Hypertension | Yes | 11 (2.9) | 29 (7.5) | 2.55 (1.19–5.45) | 2.26 (1.04–4.96) * | 0.041 |
| No | 45 (11.6) | 302 (78.0) | 1 | 1 | ||
Note: *p < 0.05, statistically significant association.
Abbreviations: AOR, Adjusted Odds Ratio; CI, Confidence Interval; COR, Crude Odds Ratio; WC, Waist Circumference.
Others: Includes students, and retired.
8. Discussion
The present magnitude of undiagnosed diabetes was 4.5% (95% CI: 2.9–7.4). This finding was in line with those of studies conducted in Mizan-Aman,southwest Ethiopia (5.8%) [34]; Dessie, northeast Ethiopia (4.9%) [24]; Gilgel Gibe, southwest Ethiopia (3.8%) [35]; Gondar, northwest Ethiopia (3.5%) [36]; Dire Dawa, eastern Ethiopia (6.2%) [20]; Dabat, northwest Ethiopia (5.1%) [37]; Jimma, southwest Ethiopia (5.7%) [38]; Bishoftu, Ethiopia (5%) [39]; South Africa (3.3%) [40]; Henan, China (4.2%) [41]; Quebec, Canada (6.2%) [42]; Tehran, Iran (5.1%) [43]; Qatar (5.9%) [44]; Punjab, India (6.8%) [45]; Pakistan (7.1%) [46]; and Bangladesh (5.4%) [47].
However, this result was higher than those found in studies conducted in Hosanna, Southern Ethiopia (2.1%) [18], Sidama, Southern Ethiopia (1.0%) [48], Koladiba, Northwest Ethiopia (2.3%) [26], and Benin (0.7%) [49]. This disparity could be due to a variety of factors, including differences in the study population, sample size, and socio-demographic features of study participants. In contrast to research undertaken in Benin, Sidama, and Koladiba (Ethiopia), the current study focused solely on city dwellers. People's lifestyles are influenced by urbanization, and it is one of the most common risk factors for diabetes mellitus progression [[49], [50], [51]]. Furthermore, the Sidama, Ethiopia, study includes people aged 15 and above, whereas our study includes people aged 18 and up.
In the current study, however, the magnitude of undiagnosed diabetes was lower than in Bahir Dar, northwest Ethiopia (10.2%) [22], East Gojjam, Ethiopia (11.5%) [21], Texas, USA (11%) [52], and Augsburg, Germany (8.2%) [53]. This disparity could be attributed to differences in lifestyle, genetics, sample size, socioeconomics, and sociodemographics. The study population also varied in age; we included adults 18 and up, whereas the German study covered an older population of 55–74 years, and the East Gojjam, Ethiopia, study included adults 25 and up. The onset of DM is linked to advanced age [20,36,37,[45], [46], [47], [48]]. However, the higher prevalence of undiagnosed DM in the USA and Germany studies could be attributable to the use of oral glucose tolerance tests (OGTT) and HbA1C to diagnose DM, whereas only fasting blood glucose was used to diagnose DM in our study, thereby underestimating the magnitude of undiagnosed DM.
In the present study, the magnitude of prediabetes was 14.5% (95% CI: 11.1–18.1). This finding was supported by research conducted in Mizan-Aman, southern Ethiopia (15.9%) [34], Dessie, northeast Ethiopia (15.7%) [24], Koladiba, northwest Ethiopia (12%) [26], and Bahir Dar, northwest Ethiopia (12.9%) [22], Jimma, southwest Ethiopia (12.9%) [38], Augsburg, Germany (16.4%) [53], Qatar (12.5%) [44], and Pakistan (14.4%) [46]. However, this result is lower than that of research conducted in Ningbo, China (28.5%) [54], Texas, USA (32%) [52], and Bangladesh (22.4%) [47]. This variation could be due to a variety of factors, including differences in lifestyle, genetics, sample size, socioeconomic, and sociodemographic factors. Moreover, this discrepancy could be due to differences in the ages of study participants; in the current study, we only include people aged 18 and up, whereas in the China Study, the participants' ages ranged from 20 to 80 years, and in the Bangladeshi study, the participants' ages ranged from 35 to 80 years. Another explanation for variance could be related to the use of different diagnostic methodologies; both the Ningbo (China) and Texas (USA) investigations employed oral glucose tolerance tests and HbA1C to diagnose DM and prediabetes, but we only used fasting blood glucose in our study, which could be understating the magnitude of prediabetes.
The magnitude of prediabetes in the present study was higher than the studies conducted in Dabat, northwestern Ethiopia (9.31%) [37]; Sidama, southern Ethiopia (2.6%) [48]; Gilgel Gibe, southwest Ethiopia (9.7%) [35]; Iganga, Uganda (3.9%) [55]; Jeddah, Saudi Arabia (10.2%) [56]; Henan, China (7.22%) [41]; Tehran, Iran (8.7%) [43]; South Africa (4.8%) [40]; and Punjab, India (6.3%) [45]. This disagreement might be due to variations in lifestyle, genetics, sample size, socioeconomic, and sociodemographic factors. In addition, the difference might be because of the study setting and age variation of the study participants; the present study was done only on urban residents, while the studies done in Sidama, southern Ethiopia; Gilgel Gibe, southwest Ethiopia; Henan, China; South Africa; and Punjab, India, include both urban and rural residents. Moreover, in the current study, the age of the study participants was 18 years and above, while in the Sidama and South Africa studies, the age of the study participants was 15 years and above, and in the Uganda study, the age of the study participants spanned from 13 to 60 years.
The current research found an association between older age and undiagnosed diabetes. Similar findings were obtained in other studies [20,37,43,[45], [46], [47], [48], [49],52,21,36]. Because of the commonly seen decline in physical activity, aging is known to be associated with increased adiposity and decreased muscle mass. Insulin sensitivity is thought to be reduced as a result of these alterations, predisposing people to metabolic syndrome or diabetes [56]. Furthermore, the aging process is linked to a decline in B-cell proliferative potential and an increase in apoptotic sensitivity [57].
In the present study, undiagnosed DM was associated with a higher BMI (BMI 25 kg/m2), which was supported by other studies [18,20,22,24,34,39,41,43,47,49,53,56]. Obesity can cause a rise in the production of adipokines and cytokines, which contribute to insulin resistance, as well as a decrease in the levels of adiponectin, which acts as an insulin sensitizer [58]. Obesity is also associated with fat deposition, especially in the liver, which leads to insulin resistance [59].
In the current study, systolic hypertension was revealed to be highly associated with undiagnosed diabetes. This result was consistent with earlier studies [24,40,43,48,52,53]. Higher blood pressure has been linked to microvascular and endothelial dysfunction, which can lead to insulin resistance [60].
Undiagnosed diabetes was associated with a family history of diabetes, according to our findings. This result was similar to that of previous investigations [21,22,24,36,[39], [40], [41],[43], [44], [45], [46],56]. If one parent has diabetes, the lifetime chance of any offspring having diabetes is roughly 40%, and if both parents have diabetes, the lifetime risk is nearly 70% [61]. The exact mechanism by which genetic predisposition causes DM is unknown, but lifestyle and living situations within families may play a role [62].
The magnitude of prediabetes was significantly associated with abdominal obesity, and this finding was in line with the report from other studies [35,41,44,56]. Abdomen obesity is caused by the accumulation of adipocytes in the abdominal area, which causes the release of highly active hormones known as adipokines, which have been linked to insulin resistance and impaired glucose homeostasis [35,41].
In our study, hypertension was also found to be a risk factor for prediabetes. Study participants with previously diagnosed hypertension had 2.26 (AOR = 2.26; 95% CI: 1.04 to 4.96) times higher odds of prediabetes than undiagnosed participants. This finding was similar to those of studies conducted in Uganda [55] and Ningbo, China [54]. Angiotensin II activity is elevated in the circulatory system of hypertension patients, which could be one probable reason. Angiotensin II influences the function of the pancreatic islets by activating the renin-angiotensin-aldosterone system, resulting in islet fibrosis and reduced insulin production, ultimately leading to insulin resistance [63,64].
Another modifiable risk factor for prediabetes identified in this investigation was the use of saturated oil. Saturated oil users were 1.97 times more likely than unsaturated oil users to have prediabetes (AOR = 1.97, % CI: 1.09 to 3.55). Increased circulating and/or hepatic saturated fatty acids have been linked to the development and progression of nonalcoholic fatty liver disease (NAFLD) as well as liver damage via apoptosis activation [65]. It is strongly linked to visceral obesity and insulin resistance, with postprandial hyperinsulinemia and abnormal glucose tolerance being common in NAFLD patients. Furthermore, in the general adult population, NAFLD is a substantial and independent risk factor for pre-DM [66].
8.1. Limitation of the study
Firstly, this study was a cross-sectional study and therefore was not able to establish a causal relationship between undiagnosed DM or prediabetes and the risk factors identified. Secondly, because of a lack of resources, we didn't use an oral glucose tolerance test or HbA1c, but we only used fasting blood glucose for diagnosis, so the magnitude of Undiagnosed DM and Prediabetes may be underestimated. Thirdly, the study did not address lipid profile measurement because of a lack of resources. Fourthly, the study did not address c-peptide measurement because of a lack of resources, so we couldn't differentiate between type 1 DM and type 2 DM.
9. Conclusion
Adults in Debre Tabor town have a high magnitude of undiagnosed diabetes and prediabetes. Undiagnosed DM was associated with being older, having a higher BMI, having systolic hypertension, and having FHDM. Abdominal obesity, hypertension, and the use of saturated oil were all associated with prediabetes. As a result, focusing the prevention strategy on such modifiable risk factors may help to minimize the magnitude of undiagnosed diabetes mellitus and prediabetes, as well as future disease complications.
Author contribution statement
Shewaneh Damtie: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools, or data; Wrote the paper.
Lemma Workineh; Tegenaw Tiruneh; Tahir Eyayu: Analyzed and interpreted the data; Wrote the paper.
Ayenew Berhan; Biruk Legese; Teklehaimanot Kiros: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools, or data; Wrote the paper.
Birhanu Getie: Contributed reagents, materials, analysis tools, or data; Wrote the paper.
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
Acknowledgments
We would like to express our deepest gratitude to a staff member of the Medical Laboratory Science Department of Debre Tabor Comprehensive Specialized Hospital as well as all study participants for their cooperation.
References
- 1.Punthakee Z., Goldenberg R., Katz P. Definition, classification and diagnosis of diabetes, prediabetes and metabolic syndrome. Can. J. Diabetes. 2018;42(Suppl 1):S10–S15. doi: 10.1016/j.jcjd.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization . 2019. Classification of Diabetes Mellitus. [Google Scholar]
- 3.Chawla A., Chawla R., Jaggi S. Microvascular and macrovascular complications in diabetes mellitus: distinct or continuum? Indian J Endocrinol Metab. 2016;20(4):546–551. doi: 10.4103/2230-8210.183480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization . World Health Organization; 2014. Global Status Report on Noncommunicable Diseases 2014. [Google Scholar]
- 5.Boyle J.P., Honeycutt A.A., Narayan K.M., Hoerger T.J., Geiss L.S., Chen H., et al. Projection of diabetes burden through 2050: impact of changing demography and disease prevalence in the U.S. Diabetes Care. 2001;24(11):1936–1940. doi: 10.2337/diacare.24.11.1936. [DOI] [PubMed] [Google Scholar]
- 6.Sun H., Saeedi P., Karuranga S., Pinkepank M., Ogurtsova K., Duncan B.B., et al. IDF Diabetes Atlas: global, regional, and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022;183 doi: 10.1016/j.diabres.2021.109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogurtsova K., Guariguata L., Barengo N.C., Ruiz P.L., Sacre J.W., Karuranga S., et al. IDF diabetes Atlas: global estimates of undiagnosed diabetes in adults for 2021. Diabetes Res. Clin. Pract. 2022;183 doi: 10.1016/j.diabres.2021.109118. [DOI] [PubMed] [Google Scholar]
- 8.Echouffo-Tcheugui J.B., Selvin E. Prediabetes and what it means: the epidemiological evidence. Annu. Rev. Publ. Health. 2021;42:59–77. doi: 10.1146/annurev-publhealth-090419-102644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saeedi P., Petersohn I., Salpea P., Malanda B., Karuranga S., Unwin N., et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the international diabetes federation diabetes atlas. Diabetes Res. Clin. Pract. 2019;157 doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 10.Beagley J., Guariguata L., Weil C., Motala A.A. Global estimates of undiagnosed diabetes in adults. Diabetes Res. Clin. Pract. 2014;103(2):150–160. doi: 10.1016/j.diabres.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Cho N.H., Shaw J.E., Karuranga S., Huang Y., da Rocha Fernandes J.D., Ohlrogge A.W., et al. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018;138:271–281. doi: 10.1016/j.diabres.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 12.Ogurtsova K., da Rocha Fernandes J.D., Huang Y., Linnenkamp U., Guariguata L., Cho N.H., et al. IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017;128:40–50. doi: 10.1016/j.diabres.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 13.Sun H., Saeedi P., Karuranga S., Pinkepank M., Ogurtsova K., Duncan B.B., et al. IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022;183 doi: 10.1016/j.diabres.2021.109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wou C., Unwin N., Huang Y., Roglic G. Implications of the growing burden of diabetes for premature cardiovascular disease mortality and the attainment of the Sustainable Development Goal target 3.4. Cardiovasc. Diagn. Ther. 2019;9(2):140–149. doi: 10.21037/cdt.2018.09.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Federation I.D. tenth ed. International Diabetes Federation; 2022. IDF Diabetes Atlas. [Google Scholar]
- 16.Animaw W., Seyoum Y. Increasing prevalence of diabetes mellitus in a developing country and its related factors. PLoS One. 2017;12(11) doi: 10.1371/journal.pone.0187670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bickler S.W., Wang A., Amin S., Halbach J., Lizardo R., Cauvi D.M., et al. Urbanization in sub-saharan Africa: declining rates of chronic and recurrent infection and their possible role in the origins of non-communicable diseases. World J. Surg. 2018;42(6):1617–1628. doi: 10.1007/s00268-017-4389-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dereje N., Earsido A., Temam L., Abebe A. Prevalence and associated factors of diabetes mellitus in Hosanna town, southern Ethiopia. Ann Glob Health. 2020;86(1):18. doi: 10.5334/aogh.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lozano R., Fullman N., Abate D., Abay S.M., Abbafati C., Abbasi N., et al. Measuring progress from 1990 to 2017 and projecting attainment to 2030 of the health-related Sustainable Development Goals for 195 countries and territories: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):2091–2138. doi: 10.1016/S0140-6736(18)32281-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ayele B.H., Roba H.S., Beyene A.S., Mengesha M.M. Prevalent, uncontrolled, and undiagnosed diabetes mellitus among urban adults in Dire Dawa, Eastern Ethiopia: a population-based cross-sectional study. SAGE Open Med. 2020;8 doi: 10.1177/2050312120975235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wondemagegn A.T., Bizuayehu H.M., Abie D.D., Ayalneh G.M., Tiruye T.Y., Tessema M.T. Undiagnosed diabetes mellitus and related factors in East Gojjam (NW Ethiopia) in 2016: a community-based study. J Public Health Res. 2017;6(1):834. doi: 10.4081/jphr.2017.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bantie G.M., Wondaye A.A., Arike E.B., Melaku M.T., Ejigu S.T., Lule A., et al. Prevalence of undiagnosed diabetes mellitus and associated factors among adult residents of Bahir Dar city, northwest Ethiopia: a community-based cross-sectional study. BMJ Open. 2019;9(10) doi: 10.1136/bmjopen-2019-030158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Asmelash D., Asmelash Y. The burden of undiagnosed diabetes mellitus in adult african population: a systematic review and meta-analysis. J. Diabetes Res. 2019 doi: 10.1155/2019/4134937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Endris T., Worede A., Asmelash D. Prevalence of diabetes mellitus, prediabetes and its associated factors in Dessie town, northeast Ethiopia: a community-based study. Diabetes Metab Syndr Obes. 2019;12:2799–2809. doi: 10.2147/DMSO.S225854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(Suppl 1):S81–S90. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- 26.Worede A., Alemu S., Gelaw Y.A., Abebe M. The prevalence of impaired fasting glucose and undiagnosed diabetes mellitus and associated risk factors among adults living in a rural Koladiba town, northwest Ethiopia. BMC Res. Notes. 2017;10(1):251. doi: 10.1186/s13104-017-2571-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013;36(Suppl 1):S67–S74. doi: 10.2337/dc13-S067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Health Organization . World Health Organization; 2009. Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks. [Google Scholar]
- 29.Stevens G., Mascarenhas M., Mathers C. Global health risks: progress and challenges. Bull. World Health Organ. 2009;87(9):646. doi: 10.2471/blt.09.070565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: executive summary. Expert Panel on the Identification, Evaluation, and Treatment of Overweight in Adults. Am. J. Clin. Nutr. 1998;68(4):899–917. doi: 10.1093/ajcn/68.4.899. [DOI] [PubMed] [Google Scholar]
- 31.National Cholesterol Education Program (NCEP) Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). Third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation. 2002;106(25):3143–3421. [PubMed] [Google Scholar]
- 32.World Health Organization . World Health Organization; Geneva: 2015. The WHO Stepwise Approach to Non-communicable Disease Risk Factor Surveillance (STEPS) [Google Scholar]
- 33.WHO . World Health Organization; Geneva: 2010. GPAQ: Global Physical Activity Questionnaire (Version 2.0) [Google Scholar]
- 34.Aynalem S.B., Zeleke A.J. Prevalence of diabetes mellitus and its risk factors among individuals aged 15 Years and above in mizan-aman town, southwest Ethiopia, 2016: a cross-sectional study. Internet J. Endocrinol. 2018 doi: 10.1155/2018/9317987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seifu W., Woldemichael K., Tsehaineh B. Prevalence and risk factors for diabetes mellitus and impaired fasting glucose among adults aged 15–64 years in Gilgel Gibe Field Research Center, Southwest Ethiopia, 2013: through a WHO stepwise approach. MOJ Public Health. 2015;2(4) [Google Scholar]
- 36.Abebe S.M., Berhane Y., Worku A., Assefa A. Diabetes mellitus in North West Ethiopia: a community-based study. BMC Publ. Health. 2014;14:97. doi: 10.1186/1471-2458-14-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wolde H.F., Derso T., Biks G.A., Yitayal M., Ayele T.A., Gelaye K.A., et al. High hidden burden of diabetes mellitus among adults aged 18 Years and above in urban northwest Ethiopia. J. Diabetes Res. 2020;2020 doi: 10.1155/2020/9240398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zenebe T., Merga H., Habte E. A community-based cross-sectional study of the magnitude of dysglycemia and associated factors in Southwest Ethiopia. Int. J. Diabetes Dev. Ctries. 2019;39(4):749–755. [Google Scholar]
- 39.Yoseph C.M., Mistire W.G., Samuel K.B., Ahmed R.G., Demo Y.T. Prevalence of undiagnosed diabetes mellitus and its risk factors in selected institutions at Bishofu Town, East Shoa, Ethiopia. J. Diabetes Metabol. 2013;S12:8. doi: 10.4172/2155-6156.S12-008. [DOI] [Google Scholar]
- 40.Motala A.A., Esterhuizen T., Gouws E., Pirie F.J., Omar M.A. Diabetes and other disorders of glycemia in a rural South African community: prevalence and associated risk factors. Diabetes Care. 2008;31(9):1783–1788. doi: 10.2337/dc08-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abdulai T., Li Y., Zhang H., Tu R., Liu X., Zhang L., et al. Prevalence of impaired fasting glucose, type 2 diabetes and associated risk factors in undiagnosed Chinese rural population: the Henan Rural Cohort Study. BMJ Open. 2019;9(8) doi: 10.1136/BMJopen-2019-029628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leong A., Dasgupta K., Chiasson J.L., Rahme E. Estimating the population prevalence of diagnosed and undiagnosed diabetes. Diabetes Care. 2013;36(10):3002–3008. doi: 10.2337/dc12-2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hadaegh F., Bozorgmanesh M.R., Ghasemi A., Harati H., Saadat N., Azizi F. High prevalence of undiagnosed diabetes and abnormal glucose tolerance in the Iranian urban population: Tehran Lipid and Glucose Study. BMC Publ. Health. 2008;8:176. doi: 10.1186/1471-2458-8-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bener A., Zirie M., Janahi I.M., Al-Hamaq A.O., Musallam M., Wareham N.J. Prevalence of diagnosed and undiagnosed diabetes mellitus and its risk factors in a population-based study of Qatar. Diabetes Res. Clin. Pract. 2009;84(1):99–106. doi: 10.1016/j.diabres.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 45.Tripathy J.P., Thakur J.S., Jeet G., Chawla S., Jain S., Pal A., et al. Prevalence and risk factors of diabetes in a large community-based study in North India: results from a STEPS survey in Punjab, India. Diabetol. Metab. Syndrome. 2017;9:8. doi: 10.1186/s13098-017-0207-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Basit A., Fawwad A., Qureshi H., Shera A.S., Members N.D.S.P. Prevalence of diabetes, pre-diabetes and associated risk factors: second National Diabetes Survey of Pakistan (NDSP), 2016-2017. BMJ Open. 2018;8(8) doi: 10.1136/BMJopen-2017-020961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Akter S., Rahman M.M., Abe S.K., Sultana P. Prevalence of diabetes and prediabetes and their risk factors among Bangladeshi adults: a nationwide survey. Bull. World Health Organ. 2014;92:204. doi: 10.2471/BLT.13.128371. 13A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zekewos A., Loha E., Egeno T., Wubshet K., Merga Z. Prevalence of diabetes mellitus and associated factors in southern Ethiopia: a community based study. Ethiop J Health Sci. 2018;28(4):451–460. doi: 10.4314/ejhs.v28i4.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kpozehouen A., Djrolo F., Sossa C.J., Gbary A.R., Chouehanou Y., Fambo D., et al. Prevalence and associated factors of diabetes mellitus in Benin. OJEpi. 2015;5(3):163. [Google Scholar]
- 50.Hall V., Thomsen R.W., Henriksen O., Lohse N. Diabetes in Sub Saharan Africa 1999-2011: epidemiology and public health implications. A systematic review. BMC Publ. Health. 2011;11:564. doi: 10.1186/1471-2458-11-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sicree R., Shaw J. Zimmet: the Global Burden: diabetes and impaired glucose tolerance. Diabetes Atlas, IDF. 2009;4 [Google Scholar]
- 52.Fisher-Hoch S.P., Vatcheva K.P., Rahbar M.H., McCormick J.B. Undiagnosed diabetes and pre-diabetes in health disparities. PLoS One. 2015;10(7) doi: 10.1371/journal.pone.0133135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rathmann W., Haastert B., Icks A., Löwel H., Meisinger C., Holle R., et al. High prevalence of undiagnosed diabetes mellitus in Southern Germany: target populations for efficient screening. The KORA survey 2000. Diabetologia. 2003;46(2):182–189. doi: 10.1007/s00125-002-1025-0. [DOI] [PubMed] [Google Scholar]
- 54.Zhao M., Lin H., Yuan Y., Wang F., Xi Y., Wen L.M., et al. Prevalence of pre-diabetes and its associated risk factors in rural areas of Ningbo, China. Int. J. Environ. Res. Publ. Health. 2016;13(8):808. doi: 10.3390/ijerph13080808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aramo C., Oyom A.P., Okello E., Acam V., Okiria J.C., Mwambi B., et al. Assessing the prevalence and risk factors of pre-diabetes among the community of Iganga Municipality, Uganda: a cross-sectional study. BMC Res. Notes. 2019;12(1):553. doi: 10.1186/s13104-019-4589-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bahijri S.M., Jambi H.A., Al Raddadi R.M., Ferns G., Tuomilehto J. The prevalence of diabetes and prediabetes in the adult population of Jeddah, Saudi arabia--A community-based survey. PLoS One. 2016;11(4) doi: 10.1371/journal.pone.0152559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maedler K., Schumann D.M., Schulthess F., Oberholzer J., Bosco D., Berney T., et al. Aging correlates with decreased beta-cell proliferative capacity and enhanced sensitivity to apoptosis: a potential role for Fas and pancreatic duodenal homeobox-1. Diabetes. 2006;55(9):2455–2462. doi: 10.2337/db05-1586. [DOI] [PubMed] [Google Scholar]
- 58.Deng Y., Scherer P.E. Adipokines as novel biomarkers and regulators of metabolic syndrome. Ann. N. Y. Acad. Sci. 2010;1212:E1–E19. doi: 10.1111/j.1749-6632.2010.05875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Larson-Meyer D.E., Newcomer B.R., Ravussin E., Volaufova J., Bennett B., Chalew S., et al. Intrahepatic and intramyocellular lipids are determinants of insulin resistance in prepubertal children. Diabetologia. 2011;54(4):869–875. doi: 10.1007/s00125-010-2022-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim M.J., Lim N.K., Choi S.J., Park H.Y. Hypertension is an independent risk factor for type 2 diabetes: the Korean genome and epidemiology study. Hypertens. Res. 2015;38(11):783–789. doi: 10.1038/hr.2015.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.InterAct Consortium. Scott R.A., Langenberg C., Sharp S.J., Franks P.W., Rolandsson O., et al. The link between family history and risk of type 2 diabetes is not explained by anthropometric, lifestyle, or genetic risk factors: the EPIC-InterAct study. Diabetologia. 2013;56(1):60–69. doi: 10.1007/s00125-012-2715-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ferrannini E., Gastaldelli A., Iozzo P. Pathophysiology of prediabetes. Med. Clin. 2011;95(2):327–339. doi: 10.1016/j.mcna.2010.11.005. vii-viii. [DOI] [PubMed] [Google Scholar]
- 63.Ip S.P., Chan Y.W., Che C.T., Leung P.S. Effect of chronic hypoxia on glutathione status and membrane integrity in the pancreas. Pancreatology. 2002;2(1):34–39. doi: 10.1159/000049446. [DOI] [PubMed] [Google Scholar]
- 64.Wang X.P., Zhang R., Wu K., Wu L., Dong Y. Angiotensin II mediates acinar cell apoptosis during the development of rat pancreatic fibrosis by AT1R. Pancreas. 2004;29(4):264–270. doi: 10.1097/00006676-200411000-00004. [DOI] [PubMed] [Google Scholar]
- 65.Gentile C.L., Pagliassotti M.J. The role of fatty acids in the development and progression of nonalcoholic fatty liver disease. J. Nutr. Biochem. 2008;19(9):567–576. doi: 10.1016/j.jnutbio.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zelber-Sagi S., Lotan R., Shibolet O., Webb M., Buch A., Nitzan-Kaluski D., et al. Non-alcoholic fatty liver disease independently predicts prediabetes during a 7-year prospective follow-up. Liver Int. 2013;33(9):1406–1412. doi: 10.1111/liv.12200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.