Abstract
Introduction
Amyotrophic Lateral Sclerosis (ALS) is a devastating neurodegenerative disorder that progressively leads to motor neuron degeneration at the neuromuscular junctions, resulting in paralysis in the patients. The clinical diagnosis of ALS is time taking and further delays the therapeutics that can be helpful if the disease is diagnosed at an early stage. Changes in plasma composition can be reflected upon CSF composition and hence, can be used to study the diagnosis and prognosis markers for the disease.
Aim
To develop a simple model system using motor neuron like cell line after plasma induction.
Method
Neuroblastoma × Spinal Cord hybridoma cell line (NSC34) was cultured under appropriate conditions. 10% ALS patients’ plasma was added to the media, and cells were conditioned for 12 h. Cell survival analysis and differential gene expression of a panel of molecules (published previously, VEGF, VEGFR2, ANG, OPTN, TDP43, and MCP-1) were done.
Results
ALS patients’ plasma impacted the life of the cells and reduced survival to nearly 50% after induction. VEGF was found to be significantly down-regulated in the cells, which can be explained as a reason for reduced cell survival.
Conclusion
ALS plasma altered the expression of an essential neuroprotective and growth factor VEGF in NSC34 cells leading to reduced viability.
Keywords: ALS, NSC34, VEGF, ALS plasma, ALS CSF
1. Introduction
Amyotrophic Lateral Sclerosis (ALS) is a degenerative disorder that includes neuromuscular interactions. The neurons degenerate, and the muscles get atrophied, causing paralysis. The disease grows rapidly in some cases and relatively very slowly in others. ALS is sporadic in approximately 90% of the cases and familial in rest of the 10%, where C9orf72 and SOD1 are the most studied genes for the familial origin of the disease [1]. Mutations in various other genes have been studied for sporadic ALS. However, no single molecule has been assigned to the pathology of the disease to date, making the prognosis more difficult.
Proteomic studies on the biofluids from ALS patients have shown altered levels of various physiologically important proteins [[2], [3], [4]]. Our previous studies have focused on a specific panel of the molecules studied in relation to ALS [[5], [6], [7]]. The levels of these molecules were analysed in the CSF and plasma of ALS patients. The specificities of the molecules in the panel have been explained well in our previous studies. The panel contains Vascular Endothelial growth factor (VEGF) and its receptor VEGFR2, Angiogenin (ANG), Optineruin (OPTN), Transactive Response DNA Binding Protein 43 (TDP43), and Chemokine Ligand 2 (CCL2).
The first three proteins in the panel are involved in vascularisation and also have been found to play neuroprotective roles in CNS [8,9]. VEGF is a growth factor and acts as a trophic factor for the survival and proliferation of the surrounding cells [9]. OPTN and TDP43 proteins are involved in protein inclusions formed inside the dying neurons. TDP43 is an important transcription factor, and its mislocalisation affects cell survival [10]. OPTN is another molecule that is involved in regulating autophagy [11] and is found to be involved in the process of neuroinflammation along with CCL2, an important marker for neuroinflammation.
Neuroblastoma × spinal cord hybrid (NSC34) cell line is a model for studying cellular-level ALS pathology as the cell line has characteristic features of motor neurons. Hence, ALS pathology can be created in the cell line, and different prognosis and therapeutic approaches can be studied with its help (details in method section). Although some previous studies have shown the toxicity of ALS patients’ CSF in the in-vitro [12] and in-vivo [13] system, no previous studies have tested the effect of plasma from patients in-vitro. However, some early studies have studied the serum cytotoxicity in-vitro and in-vivo [14,15]. The CSF of the ALS patients reduced the cell viability of the NSC34 cells and also caused ALS-like symptoms in mice. Some studies have shown that ALS CSF-induced neurodegeneration in the NSC34 cells can be reversed after the administration of neuroprotective molecules such as VEGF, BDNF [[16], [17], [18], [19]].
Altered biochemical changes in the systemic circulation are representative of the altered composition of CSF and can be used for biomarker discovery for neurodegenerative diseases. Studies combining CSF and plasma analysis can be more helpful in studying brain pathology [20,21]. Plasma can be collected non-invasively and may be used to create the ALS pathology, as shown by the current study. The plasma of ALS subjects has been shown to have varying protein configurations than normal healthy individuals [7]. To see how the composition of the plasma affects the cells, the cell viability was analysed using MTT assay. Further, the gene expression of the proteins proposed in the panel was analysed in the cells. However, blood-brain barrier has an important role to play in this interaction of plasma with neurons; hence in-vivo studies for plasma are warranted along with CSF.
2. Methods
2.1. Cell line
Neuroblastoma × Spinal Cord Hybridoma cell line is commonly abbreviated and mentioned as NSC34 cells. The cell line is a hybrid of spinal cord cells taken from 12 to 14 days old embryonic mice and the mice neuroblastoma N18TG2 cells. The cell line developed by Cashman et al. in 1992 is an appropriate model for the studies concerning motor neurons as the cells mimic the properties of motor neurons [22]. The cell line is a mixture of large motor neurons and small neurons. The cell line was provided to us by Dr. Vegasna Radha and Dr. Archana from CCMB, Hyderabad. The cells were cultured using the DMEM Glutamax (Dulbecco's Minimum Essential Medium with Glutamax, 10569-010, Gibco, Grand Island, US) supplemented with 10% Fetal Bovine Serum (FBS, 10270106, Gibco, Thermo, Brazil) and 1% Penicillin-Streptomycin (Pen-Strep, (10,000 U/mL),15140122, Gibco, Thermo, Grand Island, US) at 37°c with 5% CO2 in the incubator.
2.2. Subjects
The plasma of nine ALS patients was used for inducing the cultured cells in various combinations of plasma samples. The study was approved by the Institutional Ethics Committee (Ethical reference no. NK/5365/PhD/382). Mean ALS FRS R score and mean disease duration (in months) of ALS patients were 31.9 ± 10.46 and 21.87 ± 13.74, respectively. The disease progression rate (ΔFS) for the group of ALS patients was 0.736 [23]. Healthy individuals’ plasma was used as a control for the induction experiment of cells (eight). Also the non-induced or unstimulated cells were used as an additional experimental control. The blood samples were collected from the participants after they consented to participate. The plasma was isolated from the whole blood by centrifugation at 1500 RPM for 30 min.
2.3. Induction of cells
The cultured cells were induced with media containing the plasma of patients and controls for 12 h. 10% plasma media was used for the induction of cells. The complete media (DMEM with 10% FBS) was further supplemented with 10% plasma from the subjects. The plasma samples were pooled for three subjects in each category for all the experimental setups in different combinations. Before induction, the cells were subjected to serum starvation for 12 h by culturing in DMEM only (without FBS supplementation). Serum starvation was done to bring all the cells on the same cell cycle stage, i.e. G0, to avoid any effect of different cell cycle stages on MTT results [24]. After serum starvation, the cells were cultured for 12 h with plasma media. Further assessments were done after this induction.
2.4. MTT assay
To analyse the effect of plasma from human subjects on the cell line or to test the toxicity of the plasma of ALS patients, the cell viability was analysed using MTT assay. MTT assay helps to assess the percentage cell survival . MTT is a colorimetric assay, and results are based on the color intensity of the end products. Cells were seeded in a 96-well plate at a density 10,000 cells/well. The cells were allowed to be confluent. Thereafter, the cells were serum starved for 12 h and then treated with plasma for 12 h before adding MTT substrate. MTT substrate i.e. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (Thiazolyl Blue Tetrazolium Bromide, M2128-500 MG, Sigma-Aldrich, Merck KGaA, Darmstadt, Germany), 0.5 mg/ml working solution with 10% DMEM-FBS media, when added to the culture media, it got reduced by live cells and got converted from yellow solution to formazan crystals, which were then dissolved with the help of DMSO and color intensity (Optical density, OD) was measured at 595 nm with a microplate reader (Bio-Rad Laboratories, California, USA). Succinate dehydrogenase in the mitochondria of live cells causes the reduction of MTT reagent. Untreated cells were used as the experimental control. The percentage cell viability for MTT assay can be calculated using the below formula:
2.5. mRNA expression
After analyzing the cell viability, mRNA expression was analysed for genes referred to in the introduction section. RT-qPCR was done for mRNA expression. The cells were seeded in T-25 flasks till they became confluent. The cells were treated in the same manner as for MTT assay. After the treatment was complete, the images of cells were studied for morphological alterations, after which the media was discarded. Untreated cells were used as experimental controls. Cells were washed with PBS and trypsinised using Trypsin-EDTA (0.25%, 1×, 25200056, Gibco, Thermo, Canada). Immediately RNA isolation was done using the standard kit protocol for RNeasy Mini Kit (Qiagen, Hilden, Germany). cDNA synthesis was done thereafter immediately using the Verso cDNA synthesis kit (Thermo Fischer Scientific, Waltham, Massachusetts, USA).
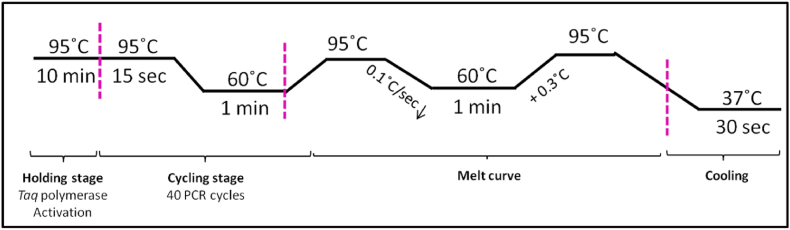
Primer sequence and details have been reproduced in Table 1. 10 μl of the reaction mixture was prepared by adding cDNA samples, Power up SYBR Green Master Mix reagent (Applied Biosystems, Foster City, California, USA), primers (forward-reverse, Eurofins Genomics India Pvt. Ltd.) and RNAase free water. The PCR was done using the Step one Real-Time PCR System (Applied Biosystems, Foster City, California, USA). 50 ng of cDNA concentration was used for the reaction. The PCR was done in triplicates. β-actin was used for the normalization of the gene expression, and relative fold change was calculated in the gene expression. The PCR program setup is shown in Fig. 1; the default program of the ABI step one software for calculating ΔΔCt with SYBR green reagent has been used for the gene expression.
Table 1.
Primer sequences of the genes quantified using RTq-PCR. The genes tested are Vascular Endothelial Growth Factor (VEGF), VEGF receptor 2 (VEGFR2), Angiogenin (ANG), Optineurin (OPTN), Transactive Response DNA Binding Protein 43 (TDP43) and Chemokine Ligand 2 (CCL2).
| Gene | Primer Sequence | |
|---|---|---|
| VEGF | F R |
5′-CGATTGAGACCCTGGTGGA-3′ 5′-GTCTTTCTTTGGTCTGCATTCAC-3′ |
| VEGFR2 | F R |
5′-TCATAATAGAAGGTGCCCAGGA-3′ 5′-CGTAGGACAATGACAAGAAGGA-3′ |
| ANG | F R |
5′-AACCTCACCCTGCAAAGATG-3′ 5′-GTGGACAGGCAAACCATTCT-3′ |
| OPTN | F R |
5′-TGTTTCAAAGAGGAGCCGAG-3′ 5′-ATCACATGGATCTGAAGCGT-3′ |
| TDP43 | F R |
5′-CAACTCTAAGCAAAGCCCAG-3′ 5′-ATCTACCACTTCTCCATACTGAC-3′ |
| MCP1 | F R |
5′-GCCAACTCTCACTGAAGCC-3′ 5′-CGTTAACTGCATCTGGCTGAG-3′ |
| β-actin | F R |
5′-AGCCATGTACGTAGCCATCC-3′ 5′-CTCTCAGCTGTGGTGGTGAA-3′ |
F-Forward, R-Reverse.
Fig. 1.
PCR program used for the quantification using ΔΔCt method with SYBR Green dye. First is the holding stage for the activation of reagents, then the cycling stage with 40 PCR cycles, each having denaturation, extension, and annealing. Melt Curve was also tested to analyse the integrity of the amplifications.
2.6. Statistical analysis
The data was tested for distribution using the one-sample K–S test. It was found to be parametric, and hence, ANOVA was used to compare the cell viability followed by post-hoc analysis (Tukey HSD test), and Independent t-test was applied to compare the fold change for genes in the ALS plasma-treated cells and control plasma-treated cells using the ΔΔCt values. The data from minimum three experiments was considered for the analysis. Each experiment was done in triplicates.
3. Results
3.1. ALS patient plasma is toxic for the NSC34 cells
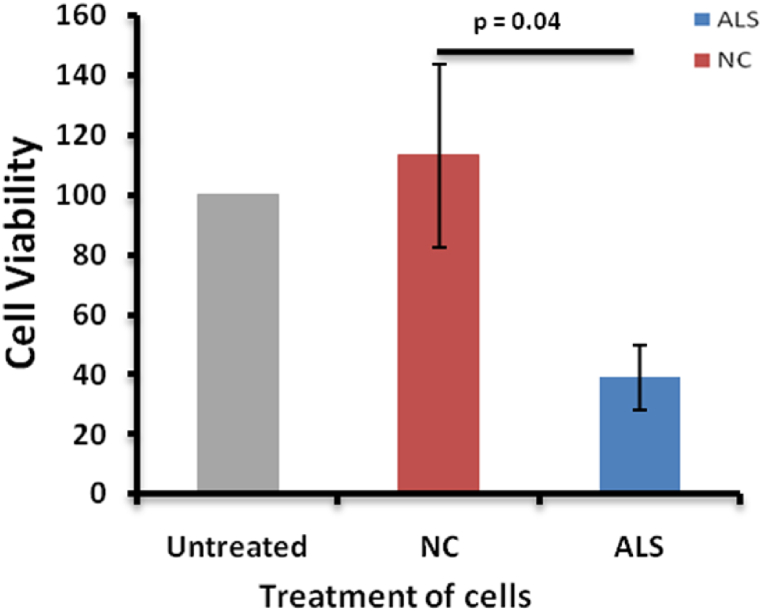
Decreased cell viability was seen in the cells treated with ALS patients' plasma in comparison to healthy individuals’ plasma and untreated cells (Fig. 2). Plasma has been found to be toxic to motor neuron-like cells. Considering this loss in the cell viability of the cells treated with ALS plasma (p = 0.04), gene expression was analysed in the cells.
Fig. 2.
The cultured NSC 34 cells were serum starved for 12 h and then kept under treatment for 12 h. MTT assay was done to estimate cell viability. There was a reduction in the percentage of live cells in the ALS plasma-treated cells in comparison to untreated and normal control treated plasma cells. n = 5 (five experiments were done in triplicates). Untreated cells have been used as an experimental negative control. Abbreviations; NC, Normal control (NSC34 cells treated with healthy individuals plasma), ALS, Amyotrophic Lateral Sclerosis (Cells treated with ALS patients plasma).
3.2. ALS plasma treatment altered the morphology of the NSC34 cells
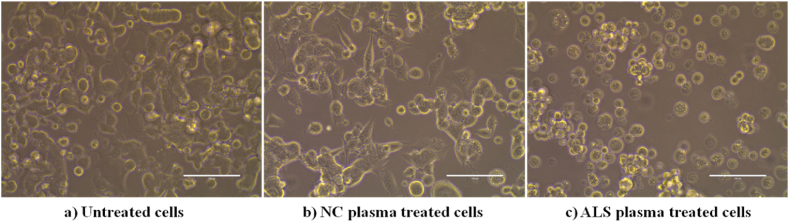
The characteristic morphology of the NSC34 cells is neuron-like with dendrites, and axons. This cell line is an adherent cell line and the cells are found adhered to the culture dishes and attain their characteristic neuron-like shape. However, the ALS plasma treatment resulted in an alteration of the morphology of the cells. Instead of having the characteristic shape, the cells lost their adherent property and started floating as circular cells suspended in the media. Also, the cell density was reduced in the culture dish treated with ALS plasma (Fig. 3).
Fig. 3.
Altered morphology of the cells treated with ALS patients' plasma. (a) The characteristic morphology of the untreated NSC34 cells. The cells are rounded but they are adherent cells and are in the diving stage. (b) The cells after the control plasma treatment have acquired the characteristic neuronal shape. (c) The cells are more rounded in structure and are floating as a suspension in the media after treatment with the ALS patients' plasma. Abbreviations, NC, Normal control, ALS, Amyotrophic Lateral Sclerosis.
3.3. Treatment of cells with ALS plasma significantly reduced the VEGF mRNA expression in the cells
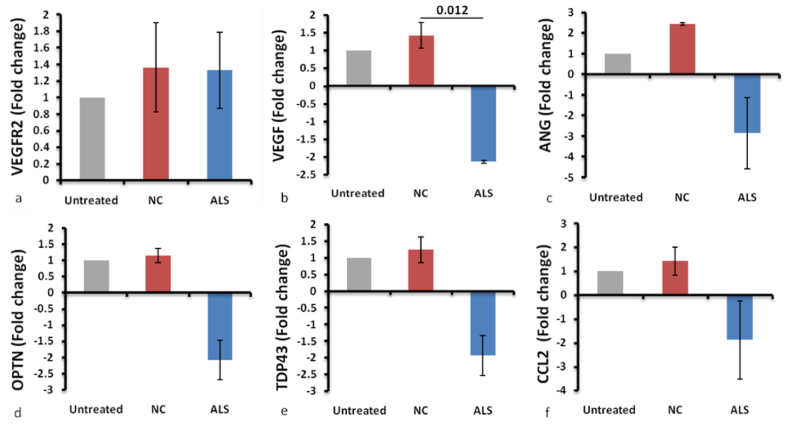
The relative fold change in the mRNA expression was calculated using the SYBR method. Fold change was analysed for the following genes: VEGF, VEGFR2, ANG, OPTN, TDP43, and CCL2 (Fig. 4). The fold change for all the genes in the untreated cells was considered as one, and the fold change for the cells treated with plasma was normalized to that. VEGF was significantly downregulated in the ALS plasma-treated cells in comparison to cells treated with control plasma (Fig. 4b). A decrease in the VEGF expression was noted, mimicking the reduced VEGF levels in the ALS plasma as shown in our previous study [5]. The difference in fold change for the other genes was not significant (Fig. 4a, c-f).
Fig. 4.
Fold change in the mRNA expression of VEGFR2 (a), VEGF (b), ANG (c), OPTN (d), TDP43 (e), and CCL2 (f). (a) VEGFR2 has not shown much change in the expression. (b) VEGF has been significantly downregulated in ALS patients' plasma-treated cells. p-0.012. (c–f) Other genes (ANG, OPTN, TDP43 and CCL2) have shown downregulation though not significant. n = 3 (Three experiments done in triplicates). Abbreviations, VEGF, Vascular Endothelial Growth Factor, VEGFR2, VEGF receptor 2, ANG, Angiogenin, OPTN, Optineurin, TDP43 Transactive Response DNA Binding Protein 43, CCL2, Chemokine Ligand 2.
4. Discussion
ALS was first defined by Charcot as a Neuromuscular disorder in which neuronal degeneration leads to muscle wasting and ultimately paralysis. Within three to five years of the onset of the disease, the patients got choked to death because of respiratory failure. Various proteins till now have been explored for the biomarker potential for studying prognosis and therapeutic studies. CSF from ALS patients has shown to be toxic in the in-vitro and in-vivo systems. Also, the toxicity caused by CSF has shown to be rescued after adding the neurotrophic factors, VEGF [12] and Brain Derived Neurotrophic Factor (BDNF) [18] to these systems.
However, in our case, no CSF toxicity was observed even when tested at higher dosages (Supplementary Fig. 1). This observation might be explained by the lower progression rate of disease in our ALS patients in comparison to previous studies where CSF toxicity was shown [17,23]. The disease pattern in India has been described as a slow progressing disease in comparison to western counterparts in various studies [25,26]. Galán et al. observed that CSF toxicity was patient-specific and not all patients’ CSF was toxic. They had also shown that there is no correlation between CSF toxicity and the survival of ALS patients [27]. Since the changes in the CSF were presumably reflected in the plasma [20,21], the potential of ALS plasma could also be explored for creating such a model system. Some earlier studies have shown the serum of ALS patients to be toxic for in vitro systems [28,29].
Our previous studies have analysed a panel of proteins in the ALS plasma (completely) and CSF (partially) of the same cohort. In the study, a similar decreasing trend for VEGF in both fluids was reported [5,7]. A predictive logistic regression model developed using the demographics and the protein levels in both fluids of the cohort showed the combined role of all three markers, demographics, protein levels in the plasma and CSF, in the prediction of the disease [6]. Hence, the analysis of plasma from ALS patients in the in-vitro system is warranted. The current study has shown that the ALS plasma is toxic for the NSC34 cells and it decreases the cell viability to almost half of the untreated cells. Along with the decrease in cell viability, the cells also lose their characteristic shape as well as the adherent property, as shown by the cells in suspension (Fig. 2).
Since the panel of the molecules we have studied till now play an important role in the pathology besides providing neuroprotection to the cells, the same panel has been analysed in the NSC34 cells in the present study. VEGF is found to be significantly downregulated in cells treated with ALS plasma in comparison to cells treated with control plasma. However, protein level estimation in the NSC34 cells or in the supernatant, after stimulation with plasma could not be done in the present study. Absence of protein level correlation with mRNA expression is a limitation of the current study.
The downregulation of VEGF is in concert with the decrease in VEGF levels in the plasma of ALS patients of our cohort as reported in our previous study [5]. Different studies have shown that VEGF administration could reverse the degeneration in the NSC34 cells although VEGF mRNA expression was not observed in the NSC34 in these studies [17,19]. The downregulation of VEGF in our study is consistent with this role of VEGF. However, there are contrasting reports regarding the VEGF levels in the biofluids of ALS patients. Gao et al. reported elevated VEGF levels in the ALS CSF and serum. They could observe an inverse correlation between the VEGF levels and disease progression rate. VEGF is a neuroprotective molecule and the downregulation of which can be attributed to the reduced viability of the NSC34 cells in our study.
Another reason for studying the plasma in-vitro system for ALS is to explore the possibility of ALS progression to be a dying back phenomenon [[30], [31], [32]]. Since dying back theory of ALS progression suggests that the degeneration starts at the muscular or the neuromuscular junction level. Fischer et al. in a case report of a sporadic ALS patients’ autopsy, has shown that there were indications of denervation and re-innervation of muscles, but the motor neurons did not indicate any abnormality [33]. It may be speculated that there is some altered composition of plasma or reduction in certain growth factors in the plasma that might trigger the degeneration in ALS patients. Also, there might be a possibility that degenerating muscles release some degenerative factors in the plasma and at the neuromuscular junction, which leads to the degeneration of motor neurons [34]. The degeneration first starts at the muscular level and then moves toward neurons. This warrants the need to target plasma composition as a biomarker for ALS and also this warrants more studies targeting the therapeutic approaches for muscles. This can further be supported by the fact that only motor neurons get affected in ALS and not the other neurons.
5. Conclusion
ALS plasma altered the expression of an essential neuroprotective and growth factor VEGF in NSC34 cells leading to reduced viability.
Author contribution statement
Radhika Khosla: Performed the experiments; Analysed and interpreted the data; Wrote the paper.
Hemant Bhagat: Parth Lal: Contributed reagents, materials, analysis tools or data.
Akshay Anand: Conceived and designed the experiments; Analysed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Data availability statement
Data will be made available on request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was funded by Indian Council of Medical Research, New Delhi, India (No.5/4–5/122Neuro/2013-NCD-I). We thank Dr. Archana B Siva, Head BDG & Coordinator SDP, Centre for Cellular & Molecular Biology (CCMB), Hyderabad and Dr. Vegesna Radha, Emeritus Scientist, Centre for Cellular & Molecular Biology (CCMB), Hyderabad for providing the cell line NSC34.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e18287.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Renton A.E., Chiò A., Traynor B.J. State of play in amyotrophic lateral sclerosis genetics. Nat. Neurosci. 2014;17(1):17–23. doi: 10.1038/nn.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hedl T.J., San Gil R., Cheng F., Rayner S.L., Davidson J.M., De Luca A., et al. Proteomics approaches for biomarker and drug target discovery in ALS and FTD. Front. Neurosci. 2019;13:548. doi: 10.3389/fnins.2019.00548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thompson A.G., Gray E., Mäger I., Thézénas M.-L., Charles P.D., Talbot K., et al. CSF extracellular vesicle proteomics demonstrates altered protein homeostasis in amyotrophic lateral sclerosis. Clin. Proteonomics. 2020;17(1):1–12. doi: 10.1186/s12014-020-09294-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leoni E., Bremang M., Mitra V., Zubiri I., Jung S., Lu C.-H., et al. Combined tissue-fluid proteomics to unravel phenotypic variability in amyotrophic lateral sclerosis. Sci. Rep. 2019;9(1):1–16. doi: 10.1038/s41598-019-40632-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Modgil S., Khosla R., Tiwari A., Sharma K., Anand A. Association of plasma biomarkers for angiogenesis and proteinopathy in Indian amyotrophic lateral sclerosis patients. J. Neurosci. Rural Pract. 2020;11(4):573–580. doi: 10.1055/s-0040-1714314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khosla R., Rain M., Sharma S., Anand A. Amyotrophic Lateral Sclerosis (ALS) prediction model derived from plasma and CSF biomarkers. PLoS One. 2021;16(2) doi: 10.1371/journal.pone.0247025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khosla R., Rain M., Chawathey S., Modgil S., Tyagi R., Thakur K., et al. Identifying putative cerebrospinal fluid biomarkers of amyotrophic lateral sclerosis in a north Indian population. Muscle Nerve. 2020;62(4):528–533. doi: 10.1002/mus.27026. [DOI] [PubMed] [Google Scholar]
- 8.Wu D., Yu W., Kishikawa H., Folkerth R.D., Iafrate A.J., Shen Y., et al. Angiogenin loss‐of‐function mutations in amyotrophic lateral sclerosis. Ann. Neurol.: Official Journal of the American Neurological Association and the Child Neurology Society. 2007;62(6):609–617. doi: 10.1002/ana.21221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pronto-Laborinho A.C., Pinto S., de Carvalho M. Roles of vascular endothelial growth factor in amyotrophic lateral sclerosis. BioMed Res. Int. 2014;2014 doi: 10.1155/2014/947513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Winton M.J., Igaz L.M., Wong M.M., Kwong L.K., Trojanowski J.Q., Lee V.M.-Y. Disturbance of nuclear and cytoplasmic TAR DNA-binding protein (TDP-43) induces disease-like redistribution. sequestration, and aggregate formation. 2008;283(19):13302–13309. doi: 10.1074/jbc.M800342200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moharir S.C., Bansal M., Ramachandran G., Ramaswamy R., Rawat S., Raychaudhuri S., et al. Identification of a splice variant of optineurin which is defective in autophagy and phosphorylation. 2018;1865(11):1526–1538. doi: 10.1016/j.bbamcr.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 12.Vijayalakshmi K., Alladi P.A., Sathyaprabha T., Subramaniam J.R., Nalini A., Raju TJBr. Cerebrospinal fluid from sporadic amyotrophic lateral sclerosis patients induces degeneration of a cultured motor. neuron cell line. 2009;1263:122–133. doi: 10.1016/j.brainres.2009.01.041. [DOI] [PubMed] [Google Scholar]
- 13.Mishra P.S., Boutej H., Soucy G., Bareil C., Kumar S., Picher-Martel V., et al. Transmission of ALS pathogenesis by the cerebrospinal fluid. Acta neuropathologica communications. 2020;8(1):65. doi: 10.1186/s40478-020-00943-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolfgram F., Myers L. Amyotrophic lateral sclerosis: effect of serum on anterior horn cells in tissue culture. Science. 1973;179(4073):579–580. doi: 10.1126/science.179.4073.579. [DOI] [PubMed] [Google Scholar]
- 15.Obál I., Nógrádi B., Meszlényi V., Patai R., Ricken G., Kovacs G.G., et al. Experimental motor neuron disease induced in mice with long-term repeated intraperitoneal injections of serum from ALS patients. Int. J. Mol. Sci. 2019;20(10):2573. doi: 10.3390/ijms20102573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kulshreshtha D., Vijayalakshmi K., Alladi P.A., Sathyaprabha T., Nalini A., Raju T.J.N.D. Vascular endothelial growth factor attenuates neurodegenerative changes in the NSC-34 motor neuron cell line induced by cerebrospinal fluid of sporadic amyotrophic lateral sclerosis patients. 2011;8(5):322–330. doi: 10.1159/000323718. [DOI] [PubMed] [Google Scholar]
- 17.Vijayalakshmi K., Ostwal P., Sumitha R., Shruthi S., Varghese A.M., Mishra P., et al. Role of VEGF and VEGFR2 receptor in reversal of ALS-CSF induced degeneration of NSC-34 motor neuron cell line. 2015;51(3):995–1007. doi: 10.1007/s12035-014-8757-y. [DOI] [PubMed] [Google Scholar]
- 18.Shruthi S., Sumitha R., Varghese A.M., Ashok S., Sagar B.C., Sathyaprabha T., et al. Brain-derived neurotrophic factor facilitates functional recovery from ALS-cerebral spinal fluid-induced neurodegenerative changes in the NSC-34 motor neuron cell line. 2017;17(1):44–58. doi: 10.1159/000447559. [DOI] [PubMed] [Google Scholar]
- 19.Shantanu S., Vijayalakshmi K., Shruthi S., Sagar B.C., Sathyaprabha T., Nalini A., et al. VEGF alleviates ALS-CSF induced cytoplasmic accumulations of TDP-43 and FUS/TLS in NSC-34 cells. J. Chem. Neuroanat. 2017;81:48–52. doi: 10.1016/j.jchemneu.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 20.Aluise C.D., Sowell R.A., DajbeBA-MboD Butterfield. Peptides and proteins in plasma and cerebrospinal fluid as biomarkers for the prediction, diagnosis, and monitoring of therapeutic. efficacy of Alzheimer's disease. 2008;1782(10):549–558. doi: 10.1016/j.bbadis.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jankovska E., Svitek M., Holada K., Petrak J. Affinity depletion versus relative protein enrichment: a side-by-side comparison of two major strategies for increasing human cerebrospinal fluid proteome coverage. Clinical proteomics. 2019;16:1–10. doi: 10.1186/s12014-019-9229-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cashman N.R., Durham H.D., Blusztajn J.K., Oda K., Tabira T., Shaw I.T., et al. Neuroblastoma× spinal cord (NSC) hybrid cell lines resemble developing motor neurons. 1992;194(3):209–221. doi: 10.1002/aja.1001940306. [DOI] [PubMed] [Google Scholar]
- 23.Labra J., Menon P., Byth K., Morrison S., Vucic S. Rate of disease progression: a prognostic biomarker in ALS. J. Neurol. Neurosurg. Psychiatr. 2016;87(6):628–632. doi: 10.1136/jnnp-2015-310998. [DOI] [PubMed] [Google Scholar]
- 24.Baghdadchi N. The effects of serum starvation on cell cycle synchronization. OSR Journal of Student Research. 2013;1(1):4. [Google Scholar]
- 25.Sondhi S., Sharma S., Kaushal S., Mehta A., Banayal V. The profile of amyotrophic lateral sclerosis in natives of Western Himalayas: hospital-based cohort study. J. Neurosci. Rural Pract. 2018;9(3):305–311. doi: 10.4103/jnrp.jnrp_8_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nalini A., Thennarasu K., Gourie-Devi M., Shenoy S., Kulshreshtha D. Clinical characteristics and survival pattern of 1153 patients with amyotrophic lateral sclerosis: experience over 30 years from India. Journal of the neurological sciences. 2008;272(1–2):60–70. doi: 10.1016/j.jns.2008.04.034. [DOI] [PubMed] [Google Scholar]
- 27.Galán L., Matías-Guiu J., Matias-Guiu J., Yanez M., Pytel V., Guerrero-Sola A., et al. Cerebrospinal fluid cytotoxicity does not affect survival in amyotrophic lateral sclerosis. Acta Neurol. Scand. 2017;136(3):212–216. doi: 10.1111/ane.12717. [DOI] [PubMed] [Google Scholar]
- 28.Roisen F.J., Bartfeld H., Donnenfeld H., Baxter J. Neuron specific in vitro cytotoxicity of sera from patients with amyotrophic lateral sclerosis. Muscle Nerve: Official Journal of the American Association of Electrodiagnostic Medicine. 1982;5(1):48–53. doi: 10.1002/mus.880050109. [DOI] [PubMed] [Google Scholar]
- 29.Yi F., Lautrette C., Vermot-Desroches C., Bordessoule D., Couratier P., Wijdenes J., et al. In vitro induction of neuronal apoptosis by anti-Fas antibody-containing sera from amyotrophic lateral sclerosis patients. J. Neuroimmunol. 2000;109(2):211–220. doi: 10.1016/s0165-5728(00)00288-5. [DOI] [PubMed] [Google Scholar]
- 30.Chou S.M., Norris F.H.J.M. Medicine NojotAAoE. Issues & opinions: amyotrophic lateral sclerosis. Lower motor neuron disease spreading to upper motor neurons. 1993;16(8):864–869. doi: 10.1002/mus.880160810. [DOI] [PubMed] [Google Scholar]
- 31.Dadon-Nachum M., Melamed E. Offen DjjoMN. The “dying-back” phenomenon of motor neurons in ALS. 2011;43(3):470–477. doi: 10.1007/s12031-010-9467-1. [DOI] [PubMed] [Google Scholar]
- 32.Piotrkiewicz M., Hausmanowa-Petrusewicz I. Frontiers Media SA; 2013. Amyotrophic Lateral Sclerosis: a Dying Motor Unit? p. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fischer L.R., Culver D.G., Tennant P., Davis A.A., Wang M., Castellano-Sanchez A., et al. Amyotrophic lateral sclerosis is a distal axonopathy. evidence in mice and man. 2004;185(2):232–240. doi: 10.1016/j.expneurol.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 34.Tsitkanou S., Lindsay A., Della Gatta P. The role of skeletal muscle in amyotrophic lateral sclerosis. a ‘dying‐back’or ‘dying‐forward’phenomenon? 2019;597(23):5527–5528. doi: 10.1113/JP278835. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.