ABSTRACT
Mounting evidence suggests that gut microbial composition and its metabolites, including short-chain fatty acids (SCFAs), have beneficial effects in regulating host immunogenicity to vaccines. However, it remains unknown whether and how SCFAs improve the immunogenicity of the rabies vaccine. In this study, we investigated the effect of SCFAs on the immune response to rabies vaccine in vancomycin (Vanco)-treated mice and found that oral gavage with butyrate-producing bacteria (C. butyricum) and butyrate supplementation elevated RABV-specific IgM, IgG, and virus-neutralizing antibodies (VNAs) in Vanco-treated mice. Supplementation with butyrate expanded antigen-specific CD4+ T cells and IFN-γ-secreting cells, augmented germinal center (GC) B cell recruitment, promoted plasma cells (PCs) and RABV-specific antibody-secreting cells (ASCs) generation in Vanco-treated mice. Mechanistically, butyrate enhanced mitochondrial function and activated the Akt-mTOR pathway in primary B cells isolated from Vanco-treated mice, ultimately promoting B lymphocyte-induced maturation protein-1 (Blimp-1) expression and CD138+ PCs generation. These results highlight the important role of butyrate in alleviating Vanco-caused humoral immunity attenuation in rabies-vaccinated mice and maintaining host immune homeostasis.
IMPORTANCE The gut microbiome plays many crucial roles in the maintenance of immune homeostasis. Alteration of the gut microbiome and metabolites has been shown to impact vaccine efficacy. SCFAs can act as an energy source for B-cells, thereby promoting both mucosal and systemic immunity in the host by inhibiting HDACs and activation of GPR receptors. This study investigates the impact of orally administered butyrate, an SCFA, on the immunogenicity of rabies vaccines in Vanco-treated mice. The results showed that butyrate ameliorated humoral immunity by facilitating the generation of plasma cells via the Akt-mTOR in Vanco-treated mice. These findings unveil the impact of SCFAs on the immune response of the rabies vaccine and confirm the crucial role of butyrate in regulating immunogenicity to rabies vaccines in antibiotic-treated mice. This study provides a fresh insight into the relationship of microbial metabolites and rabies vaccination.
KEYWORDS: rabies vaccine, gut microbiome, SCFA, vancomycin, B cells, Akt-mTOR pathway
INTRODUCTION
Rabies is a deadly zoonotic disease, and once symptoms of the disease begin to manifest, the mortality rate is almost 100% (1, 2). It causes around 59,000 deaths in the world every year, mostly in developing countries (3, 4). Dogs are the main source of human rabies, accounting for over 99% of cases. Massive vaccination in dogs is the most effective way to eliminate human rabies. The World Health Organization (WHO) suggests that rabies transmission can be effectively controlled when at least 70% of dogs are vaccinated, aiming to eliminate canine-mediated human rabies by 2030 (5–7).
Our previous studies uncovered that depletion of the gut microbiome by broad-spectrum antibiotics or Vanco solely attenuated the humoral immune response to rabies vaccines. Furthermore, the immunogenicity of rabies vaccines differed widely in the natural population group of mice with higher levels of Clostridiales and Lachnospiraceae positively correlating with higher antibody titers (8). Likewise, gut microbial dysbiosis or differences in microbial compositions can also affect the immune response to other vaccines via gut microbiome metabolites, such as influenza (9–11) and COVID-19 (12, 13). These findings confirm the crucial role of the gut microbiome in regulating host vaccine immunity. However, it remains unclear whether gut microbiome-derived metabolites can improve the immune efficacy of rabies vaccines.
As one of the most extensively studied metabolites originating from the gut microbiome, short-chain fatty acids (SCFAs), such as acetate (C2), propionate (C3), and butyrate (C4) (14), reach concentrations exceeding 0.1 mol/kg in the human colon (15) and have multiple beneficial effects on the immune response of the host (16), including powerful anti-inflammatory (17), anti-allergic (18), anti-tumor effects (19), as well as the maintenance of energy metabolism homeostasis (20). The mechanism by which SCFAs exert their biological functions is through inhibiting histone deacetylases (HDACs) and activating downstream signaling pathways as ligands for G protein-coupled receptors (GPRs, e.g., GPR41, GPR43, GPR109A, and Olfr78) (16). Notably, the conclusion regarding the impact of SCFAs on humoral immunity is conflicting, as some researchers suggest that SCFAs promote optimal antibody responses and immune reactions following Citrobacter infection by enhancing B cell metabolism, plasma cell differentiation, and antibody production (20). However, Sanchez et al. indicated that a low dose of SCFAs slightly assists the host immune response, while excessive SCFAs inhibit rather than promote the host immune response to ovalbumin (OVA) and autoimmunity in mouse lupus models by upregulating miRNAs targeting Acida and Prdm1 mRNA-3′ UTRs through HDAC inhibition (21). Therefore, it is worthwhile to investigate whether SCFAs contribute to the immunogenicity of vaccines, especially oral and parenteral vaccines, further confirming their immunomodulatory role.
Considering the impairment effect of Vanco on the humoral immune response to rabies vaccines and the important role of SCFAs in host immune response, the aim of this study was to investigate the beneficial effect of SCFA supplementation on the humoral immune response to rabies vaccine in Vanco-treated mice. Our results showed that Vanco significantly altered the SCFAs levels in cecal contents, serum, and inguinal lymph nodes based on targeted metabolomics, with butyrate exhibiting the highest fold change. Oral gavage of Vanco-treated mice with butyrate-producing bacteria (C. butyricum) and butyrate improved the production of RABV-specific IgM, IgG, and virus-neutralizing antibodies (VNAs), highlighting the important role of butyrate in maintaining host immune homeostasis.
RESULTS
Vanco treatment diminishes SCFA-producing bacteria and the abundance of SCFAs.
Vanco is an antibiotic that primarily targets Gram-positive bacteria, including Clostridia (8). Numerous studies have shown that the main metabolites produced by Clostridium are SCFAs, which have powerful immunomodulatory effects (22–24). Of note, The major metabolite of B. coccoides is acetate (corresponding to acetic acid) (22–25), and the major metabolite of C. butyricum is butyrate (corresponding to butyric acid) (26). Therefore, we first verified the abundance of representative SCFA-producing bacteria through qPCR to ensure the depletion of SCFA-producing bacteria by Vanco treatment. Compared with the untreated group, the abundance of B. coccoides (Fig. 1A) and C. butyricum (Fig. 1B) in the Vanco group was significantly reduced. Additionally, we found that the cecum tissue of the Vanco group was larger (Fig. 1C) and the contents (Fig. 1D) were more than that of the untreated group.
FIG 1.
Vanco treatment alters the abundance of SCFA-producing bacteria and SCFA levels. Mice were orally administered with 0.5g/L vancomycin for 4 weeks, while the untreated group served as control (Untreated, n = 3, Vanco, n = 3). The abundances of SCFA-producing bacteria and SCFA levels were analyzed by qPCR and GC-MS, respectively. (A) Changes in B. coccoides/total bacteria abundance in the two groups. (B) Changes in C. butyricum/total bacteria abundance in the two groups. (C) Representative images of the cecum in the two groups. (D) Weight of cecal content. Differences in SCFA levels (E) and relative abundances (H) in cecal contents between the two groups. Differences in SCFA levels (F) and relative abundances (I) in serum between the two groups. Differences in SCFA levels (G) and relative abundances (J) in inguinal lymph nodes between the two groups. The error bars indicate the standard deviations (SD) and “ns” represents no significant difference. The statistical significance was evaluated by unpaired two-tailed t test.
Vanco treatment has been shown to decrease the SCFAs content in cecal contents, thus increasing IL-17-producing γδ T cells (23) and altering the SCFAs content in stools, cecum, tumors, and tumor-draining lymph nodes (TDLNs), thereby changing the anti-tumor effect of radiation therapy (RT) (27). Based on these findings, we aimed to investigate whether Vanco treatment could also alter the SCFAs content in key indicators of serum and inguinal lymph nodes during mouse rabies vaccine immunization using gas chromatography-mass spectrometry (GC-MS). The results showed that SCFAs could be detected in the cecal contents (Fig. 1E), serum (Fig. 1F), and inguinal lymph nodes (Fig. 1G), with higher levels in the cecal contents than in other tissues. Acetic acid had a higher level than propionic acid and butyric acid, but the fold change of butyric acid (untreated/Vanco) was higher than that of acetic acid and propionic acid (Fig. 1H to J). Specifically, the average fold change of butyric acid in the cecal contents was 82.11 (Fig. 1H), in the serum was 1.25 (Fig. 1I), and in the inguinal lymph nodes was 1.40 (Fig. 1J). In summary, Vanco treatment significantly reduces the abundance of SCFA-producing bacteria and the content of SCFAs, with butyric acid exhibiting the highest fold change.
Butyrate-producing bacteria and butyrate supplementation enhance the RABV-specific antibody production in Vanco-treated mice.
Given the fact that Vanco treatment depletes SCFA-producing bacteria and the SCFAs contents mentioned above, we further explored whether SCFA-producing bacteria and SCFAs could mitigate Vanco-caused humoral immunity attenuation in rabies-vaccinated mice. Mice ceased Vanco treatment after 4 weeks of administration and oral gavage with B. coccoides, C. butyricum, fecal microbiota transplantation (FMT), or phosphate-buffered saline (PBS) for 4 consecutive days before being intramuscularly (i.m.) immunized with 107 fluorescent focus units (FFU) iLBNSE. Blood was collected at corresponding time points, and serum was isolated to detect RABV-specific antibodies and VNAs production (Fig. 2A). The results showed that compared with the Vanco+PBS group, the Vanco+FMT group and Vanco+C. butyricum (CBM588 strain) group, but not Vanco+B. coccoides group, had higher RABV-specific IgM (Fig. 2B), IgG (Fig. 2C), and VNA levels (Fig. 2D). These results suggest that butyrate-producing bacteria has a beneficial effect on Vanco-caused humoral immunity attenuation in rabies-vaccinated mice.
FIG 2.
Effects of butyrate-producing bacteria and butyrate on the production of RABV-specific antibodies. (A to D) Mice were discontinued from Vanco treatment after 4 weeks of administration, then orally gavage with B. coccoides, C. butyricum, FMT, or PBS as the control group for four consecutive days before being immunized with 107 FFU inactivated RABV strain (iLBNSE). Blood was collected at the corresponding time points, and serum was isolated to detect RABV-specific antibodies and VNAs production. (A) Schematic representation of gavage and immunization of four groups. Mouse serum was collected at different time points after immunization and RABV-specific IgM (B), IgG (C), and VNA (D) levels were detected by ELISA or FAVN (Vanco+PBS, n = 12, Vanco+FMT, n = 12, Vanco+B. coccoides, n = 12 and Vanco+C. butyricum, n = 12). (E to H) Mice in untreated (n = 12), Vanco (n = 12) and Vanco+Butyrate groups (n = 12) were i.m. immunized with 107 FFU iLBNSE and blood was collected at the corresponding time points for subsequent analysis. (E) Schematic representation of oral SCFA administration and immunization of three groups. Mouse serum was collected at different time points after immunization and RABV-specific IgM (F), IgG (G), and VNA (H) levels were detected by ELISA or FAVN. The error bars indicate the standard errors of the mean (SEM) and “ns” represents no significant difference. The statistical significance of Anti-RABV-IgM, Anti-RABV-IgG and VNA titer was evaluated by one-way analysis of variance (ANOVA) followed by Tukey’s multiple-comparison test.
To further investigate the main metabolite butyrate of C. butyricum on the immunogenicity of rabies vaccines, mice treated with Vanco for 4 weeks were simultaneously given butyrate for 1 week and immunized with rabies vaccine. This group was continuously treated with Vanco and butyrate until 28 days post-RABV immunization, and the Vanco-treated and untreated group were served as controls (Fig. 2E). The results showed that the Vanco+Butyrate group had higher RABV-specific IgM (Fig. 2F), IgG (Fig. 2G), and VNA levels (Fig. 2H) than Vanco group, suggesting that butyrate has a beneficial effect on Vanco-caused humoral immunity attenuation in rabies-vaccinated mice.
Butyrate supplementation modulates the Th1/Th2 balance in Vanco-treated mice.
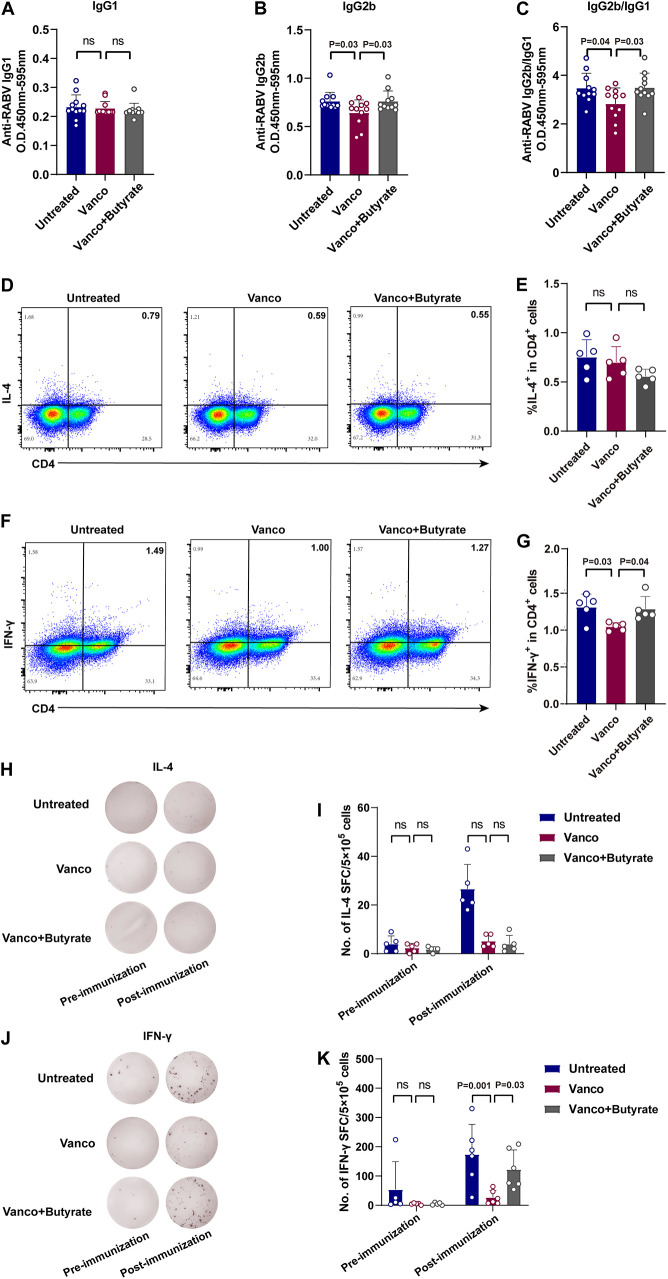
The class switching of immunoglobulins (Ig) is the foundation for the host to produce different types of antibodies (28). IL-4, secreted by T helper 2 (Th2) cells, promotes the production of IgG1 while IFN-γ, secreted by T helper 1 (Th1) cells, promotes the production of IgG2a/b. Both play important regulatory roles in Ig class switching (29, 30). Based on this, the RABV-specific IgG isotypes among three groups were evaluated by ELISA and the results showed that the Vanco+Butyrate group exhibited more RABV-specific IgG2b (Fig. 3B) and IgG2b/IgG1 (Fig. 3C) ratio than the Vanco group, while there was no significant difference in RABV-specific IgG1 production (Fig. 3A) among the three groups.
FIG 3.
Butyrate regulates the Th1/Th2 balance in Vanco-treated mice post-RABV immunization. (A to C) Mice were orally administered with 0.5g/L vancomycin for 4 weeks, followed by oral administration of SCFA metabolites before intramuscular injection of 107 FFU iLBNSE. Mouse serum was collected at different time points and RABV-specific IgG1 (A), IgG2b (B), and IgG2b/IgG1 (C) levels were detected by ELISA (Untreated, n = 12, Vanco, n = 12 and Vanco+Butyrate, n = 12). The statistical significance was evaluated by one-way ANOVA. The error bars of Anti-RABV IgG isotypes indicate the SEM. (D to K) Spleens single cells from three groups of mice (Untreated, n = 5, Vanco, n = 5 and Vanco+Butyrate, n = 5) were collected 7 dpi. The cells were stimulated with inactivated and purified RABV virions for 36 h, and antigen-specific T cell production and IL-4 or IFN-γ-secreting cells were assessed by intracellular cytokine staining (ICCS) and ELISpot assay. (D) Representative flow cytometry plot of IL-4+ CD4+ T cells. (E) Percentages of IL-4+ CD4+ T cells. (F) Representative flow cytometry plot of IFN-γ+ CD4+ T cells. (G) Percentages of IFN-γ+ CD4+ T cells. (H) Representative image of IL-4-secreting cells. (I) The number of IL-4-secreting cells. (J) Representative image of IFN-γ-secreting cells. (K) The number of IFN-γ-secreting cells. The error bars of antigen-specific T cells indicate the SD and “ns” represents no significant difference. The statistical significance was evaluated by one-way ANOVA followed by Tukey’s multiple-comparison test.
To further investigate the relationship between IgG isotypes and Th1/Th2 responses, splenic lymphocytes were harvested from untreated, Vanco, and Vanco+Butyrate groups of mice at 7 days postimmunization (dpi). IL-4- and IFN-γ-secreting cells were assessed by intracellular cytokine staining (ICCS) and ELISPOT after being stimulated with inactivated and purified RABV virions, respectively. The representative gating strategy of IL-4+ CD4+ T cells and IFN-γ+ CD4+ T cells were shown in Fig. 3D and F, and more IFN-γ+ CD4+ T cells (Fig. 3E) were observed in the Vanco+Butyrate group compared to the Vanco group, with no significant differences noted in IL-4+ CD4+ T cells (Fig. 3G) among the groups. Besides, the Vanco+Butyrate group produced more IFN-γ-secreting cells (Fig. 3J and K), but not IL-4-secreting cells (Fig. 3H and I) than the Vanco group of mice. These results collectively indicate that butyrate facilitates the generation of a Th1-biased immune response in Vanco-treated mice post-RABV immunization.
Butyrate supplementation is dispensable for DC maturation and cDC2 generation in Vanco-treated mice.
Effective activation of cDCs is critical for initiating vaccine immunity, among which cDC2 serves as an inducer of humoral immunity by directly presenting antigens to CD4+ T cells. The cDC2s and the major DC subset induce early follicular helper T (Tfh) cells differentiation in the T-B border by the expression of ICOSL and IL-6 (28, 31). Accordingly, we further examined whether butyrate can promote DC maturation and cDC2 generation. The draining lymph nodes (dLNs) were collected from untreated, Vanco, and Vanco+Butyrate groups of mice at 7 dpi and cDC, cDC1, and cDC2 were analyzed by flow cytometry with the gating strategies shown in Fig. 4A. As the result, there was no significant difference in cDC (MHC II+ in CD11c+) (Fig. 4B), cDC1 (CD8α+ CD11b- in cDCs) (Fig. 4C), or cDC2 (CD8α- CD11b+ in cDCs) (Fig. 4D) among three groups, indicating that butyrate does not impact the immunogenicity of rabies vaccines in Vanco-treated mice by enhancing DC maturation and cDC2 generation.
FIG 4.
Butyrate does not affect DC activation and cDC2 generation post-RABV immunization. Mice from three groups (Untreated, n = 6, Vanco, n = 5 or 6 and Vanco+Butyrate, n = 6) were intramuscularly injected with 107 FFU iLBNSE, and dLNs were collected on day 6 postimmunization to observe DC activation and cDC2 generation by flow cytometry. (A) Gating strategy for DC activation (MHC II+ in CD11c+), cDC1 (CD8α+ CD11b- in cDCs), and cDC2 (CD8α- CD11b+ in cDCs). (B) Percentages of DC activation. (C) Percentages of cDC1. (D) Percentages of cDC2. The error bars indicate the SD and “ns” represents no significant difference. The statistical significance was evaluated by one-way ANOVA followed by Tukey’s multiple-comparison test.
Butyrate supplementation contributes to humoral immune response in Vanco-treated mice.
The humoral immune response, mediated by CD4+ T cells, is pivotal in eradicating RABV (32, 33). Notably, Tfh cells are specialized providers of help to B cells by providing several signals, including CD40L, C-X-C Motif Chemokine Receptor 5 (CXCR5), and IL-21, which is ultimately critical for germinal center (GC) responses and the production of high-affinity antibodies in response to vaccine immunization (34–37). Thus, the Tfh cells, GC B cells, and plasma cells (PCs) generation among the three groups was investigated. The draining lymph nodes (dLNs) or bone marrows (BMs) were collected from untreated, Vanco, and Vanco+Butyrate groups of mice, and Tfh cells, GC B cells, and PCs were analyzed by flow cytometry at 7 and 14 dpi with the gating strategies shown in (Fig. 5A to C). As the result, significantly more GC B cells (B220+ CD95+ GL7+) (Fig. 5E) and PCs (CD138+ B220low Cells) (Fig. 5F) were observed in the Vanco+Butyrate group than in the Vanco group; however, no differences were noted among the three groups regarding Tfh cells (CD4+ PD1+ CXCR5+) (Fig. 5D). Furthermore, the generation of RABV-specific antibody-secreting cells (ASCs) in dLNs and BMs were evaluated by an enzyme-linked immunosorbent spot (ELISpot) assay at 14 dpi and representative imaging of RABV-specific ASCs were shown in Fig. 5G and I. As expected, compared with the Vanco group, the Vanco+Butyrate group possessed significantly more RABV-specific ASCs both in dLNs (Fig. 5H) and BMs (Fig. 5J). Together, these results demonstrate that butyrate can better facilitate the generation of GC B cells, PCs, and RABV-specific ASCs in Vanco-treated mice post-RABV immunization.
FIG 5.
The effect of butyrate on Tfh cells, GC B cells, PCs, and RABV-specific ASCs generation after RABV immunization. Mice from the untreated (n = 5 or 6), Vanco (n = 6), and Vanco+Butyrate group (n = 5 or 6) were i.m. immunized with 107 FFU iLBNSE, and Tfh cells, GC B cells, PCs, and RABV-specific ASCs in dLNs or BMs were detected by flow cytometry at 7 and 14 dpi. (A to C) Gating strategy for Tfh cells (CD4+ PD1+ CXCR5+) (A), GC B cells (B220+ CD95+ GL7+) (B), and PCs (CD138+ B220low Cells) (C). (D) Percentages of Tfh cells. (E) Percentages of GC B cells. (F) Percentages of PCs. (G) Representative images of RABV-specific ASCs in dLNs. (H) Numbers of RABV-specific ASCs in dLNs. (I) Representative images of RABV-specific ASCs in BMs. (J) Numbers of RABV-specific ASCs in BMs. The error bars indicate the SD and “ns” represents no significant difference. The statistical significance of Tfh cells, GC B cells, PCs, and RABV-specific ASCs was evaluated by one-way ANOVA followed by Tukey’s multiple-comparison test.
Butyrate promotes the generation of PCs via Akt-mTOR pathway.
Given that butyrate (NaBu) supplementation improved the humoral responses to rabies vaccines in Vanco-treated mice through promote GC B cells, PCs, and RABV-specific ASCs generation, we further investigated the role of butyrate in regulating PCs produced in vitro. Primary B cells isolated from Vanco-treated mice were treated with different doses of butyrate, and cell viability was evaluated by CCK-8 assay. The results showed that 62.5 μM and 125 μM butyrate did not impact the cell viability compared with the untreated group (Fig. 6A). Flow cytometry was used to detect PCs generation, and the results displayed that the 125 μM and 250 μM butyrate induced a higher proportion of CD138+ PCs (Fig. 6B and C), whereas 250 μM butyrate affected cell viability. B lymphocyte-induced maturation protein-1 (Blimp-1) is a critical factor that controls PC differentiation (38, 39); thus, we examined the mRNA (corresponding to Prdm1) and protein expression of Blimp-1, respectively. The results showed that the butyrate group exhibited higher expression of Prdm1 (Fig. 6D) and mean fluorescence intensity (MEI) of Blimp-1 (Fig. 6E and F) than that in the untreated group, indicating that butyrate contributes to PCs generation by upregulating Blimp-1.
FIG 6.
Butyrate improves mitochondrial function and activates the Akt-mTOR pathway, promoting the generation of PCs in primary B lymphocytes isolated from Vanco-treated mice. Primary B lymphocytes from mice treated with vancomycin for 4 weeks were isolated by negative selection beads and stimulated with 5 μg/mL LPS, 5 μg/mL anti-CD40, and 10 μg/mL anti-IgM. The cell viability, CD138+ PCs, mitochondrial function, and Akt-mTOR pathway were detected at indicated time points, respectively. (A) Cell viability was measured using a CCK-8 assay after 5 days of culture (n = 3). (B) Gating strategy for CD138+ PCs. (C) Percentages of CD138+ PCs (n = 3). (D) Relative expression levels of Prdm1 by qPCR (n = 3). (E) Representative images of Blimp-1 mean fluorescence intensity (MFI). (F) MFI of Blimp-1 (n = 4). (G) Mitochondrial membrane potential changes were analyzed by TMRE staining (n = 4). (H) Mitochondrial complex I activity was assessed by the multifunctional microplate reader (n = 4). (I) ATP production was analyzed by a luminometer (n = 3). (J) Expression of Akt and mTOR was detected by Western blotting. (K) Cell viability of Akt inhibitor VIII was detected using a CCK-8 assay (n = 3). (L) Cell viability of the mTOR inhibitor rapamycin was detected using a CCK-8 assay (n = 3). (M) Relative expression levels of Prdm1 by qPCR when treated with inhibitors (n = 3). (N) Gating strategy for CD138+ PCs when treated with inhibitors. (O) Percentages of CD138+ PCs when treated with inhibitors (n = 3). The error bars indicate the SD and “ns” represents no significant difference. The statistical significance was evaluated by unpaired two-tailed t test.
Given that germinal center selection and efficient affinity maturation require dynamic regulation of mTORC1 kinase, which contributes to the reciprocal balance between Tfh and Th1 cell fates and their respective metabolic activities following acute viral infection (40–42), we speculated that butyrate might be able to accelerate PCs generation by enhancing mitochondrial function and Akt-mTOR pathway in primary B cells isolated from Vanco-treated mice. As the result, butyrate-treated group exhibited higher mitochondrial membrane potential (Fig. 6G), mitochondrial complex I activity (Fig. 6H), and ATP contents (Fig. 6I) compared to the untreated group. In addition, the activity of Akt and mTOR was increased after treatment of B cells with butyrate (Fig. 6J). Accordingly, inhibition of AKT/mTOR signaling by AKT-inhibitor III (Fig. 6K) or rapamycin (Fig. 6L) substantially suppressed Prdm1 mRNA levels (Fig. 6M) and CD138+ PCs generation (Fig. 6N and O) in butyrate-treated B cells. Taken together, butyrate enhances mitochondrial function and promotes PC generation via Akt-mTOR pathway in Vanco-treated mice.
DISCUSSION
SCFAs are one of the main metabolic products of Clostridium, serving as the primary substrate for acetyl-CoA synthesis and participating in cellular energy metabolism, gene expression, and protein synthesis (15). SCFAs are water-soluble and diffusible gut-derived metabolites reaching their peak concentration in the cecum and gradually decreasing from the proximal to the distal colon (43). In this study, SCFA-producing bacteria and SCFA levels in cecum contents, serum, and inguinal lymph nodes had statistically significant reductions after Vanco treatment. Similarly, Vanco treatment also downregulated the SCFAs in TDLNs and spleens thus, influencing many aspects of host physiology (22, 23). Moreover, supplementation with butyrate-producing bacteria or butyrate was able to increase RABV-specific antibody and VNA production in Vanco-treated mice by promoting the generation of GC B cells, PCs, and RABV-specific ASCs.
SCFAs have a potent positive impact on B cells by promoting their activation and differentiation into plasma cells (20). Consistent with our results, supplementation of SCFAs in mice treated with broad-spectrum antibiotics (Abx) has been shown to promote the production of trivalent influenza vaccine (TIV)-specific IgG (10), indicating that the effect of SCFAs on vaccine efficacy is not RABV specific. However, their study also indicated that supplementation of SCFAs with a normal diet was insufficient to heighten the immunogenicity to TIV, suggesting the function of SCFAs in promoting humoral immunity occurs when the host is deficient. In this regard, we also found that SCFAs mix (acetate, 70 mM; propionate, 30 mM; butyrate, 20 mM) or butyrate alone (20 mM or 100 mM) did not enhance the immunogenicity of the rabies vaccine. Supplementation of SCFAs (mainly acetate) in Abx-treated mice could also promote the production of intestinal IgA+ B cells by activating the GPR43 and inducing DC expression of Aldh1a2 but did not affect splenic IgA+ B cells, indicating the important role of acetate in the maintenance of gut homeostasis (44). Similarly, our results showed that acetate-producing bacteria and acetate supplementation could not improve the immunogenicity of rabies vaccines in Vanco-treated mice when administering rabies vaccines via intramuscular injection. Supplementation of SCFAs (acetate, 67.5 mM; propionate, 25.9 mM; butyrate, 40 mM) in Vanco-treated mice augmented the generation of IgE in mouse serum and εGT in Peyer’s patches (PPs) but did not affect IgA and IgG2a in serum or εGT in the spleens (44). In our model, the serum total IgG was also unaffected after antibiotic treatment.
Conventional dendritic cells (cDCs) are one of the most crucial cells for initiating vaccine-induced immune responses and can be distinguished based on their phenotypic markers and immunological functions (45). Our previous studies have shown that recombinant RABV expressing DC-activating factors elevates humoral immune responses in mice, including GM-CSF, macrophage-derived chemokine (MDC), macrophage inflammatory protein 1α (MIP-1α), and Fms-like Tyrosine Kinase 3 Ligand (Flt3L) (46–49). RABV vaccines combined with adjuvants can also boost humoral immune responses by promoting DC activation and downstream germinal center reactions, including colloidal manganese salt (MnJ), aluminum, unlipidated outer membrane protein 19 (U-OMP19), and flagellin protein (50–52). cDC1 has a preference for antigen cross-presentation to CD8+ T cells via MHC-I molecules while cDC2 has a bias for presentation to CD4+ T cells via MHC-II molecules (28, 53). During the early stages of an immune response, peripheral cDC2 cells carry the largest amount of antigen peptide-MHC (pMHC) complex and prime naive CD4+ T cells, which ultimately lead to the development of humoral immunity (28, 54). However, our results showed no significant differences in cDCs, and cDC2, indicating butyrate did not contribute to DC activation. Previous studies have demonstrated that SCFAs inhibit the maturation and generation of DCs, including bone marrow stem cell-derived DCs and human monocyte-derived DCs, by inhibiting HDAC activity (18, 55) and downregulating transcription factors PU.1 and RelB that were associated with DC maturation (56). Nonetheless, acetate has been reported to induce RA in DCs and indirectly regulate B-cell IgA production (44). Additionally, SCFAs upregulated BAFF and ALDH1a2 in DCs to promote B-cell IgA and IgG production, as well as upregulated antibody responses elicited by cholera toxin (CT) and rescued antibody responses in Abx-treated mice (57). SCFAs (10 mM acetate or 1 mM butyrate) with a low concentration stimulated the production of pro-inflammatory cytokines crucial for inducing rapid Th1 responses against infections, including IL-6, tumor necrosis factor-α (TNF-α), and granulocyte-macrophage colony-stimulating factor (GM-CSF), as well as chemokines such as CXCL1 and CXCL10 (58).
Although SCFAs seem to be potent regulators of the immunosuppressive activity of CD4 T cells by inducing epigenetic reprogramming (59, 60), some recent reports suggest that SCFAs can also intensify the activity of CD4+ effector T cells (61, 62), indicating that the impact of microbial metabolites on the immune system may be more complex than previously believed. In our study, although butyrate supplementation did not upregulate the number of Tfh cells in Vanco-treated mice, an increase of antigen-specific IFN-γ+ CD4 T cells and IFN-γ-secreting cells were found in the Vanco+Butyrate group after stimulation with RABV virions. Additionally, it promoted class switching of Ig toward RABV-specific IgG2b isotype, and thus, contributed to Th1-mediated immune responses. Although SCFAs have potent anti-inflammatory and Treg-inducing functions, they have been shown to not affect the basal immune levels of mice (63, 64).
The PI3K pathway in the light zone (LZ) of the germinal center (GC) negatively regulates the expression of forkhead box protein (FOXO) transcription factors to control the differentiation of B cells in the GC, which can augment vaccine immune responses through promoting downstream Akt-mTOR pathway and mitochondrial energy metabolism (41, 65, 66). The Akt-mTOR pathway is an important pathway that regulates various metabolic pathways, including glucose metabolism, lipid metabolism, and protein metabolism, thereby regulating host energy metabolism (67–69) and antiviral humoral immunity (70, 71). SCFAs are substrates synthesized by acetyl-CoA and that participate in host energy metabolism through the tricarboxylic acid cycle while regulating lymphocyte metabolism-epigenetics by activating the Akt-mTOR pathway (60). Foh et al. found that adding butyrate to primary B lymphocytes could promote the generation of IL-10+ IgM+ PCs by altering mitochondrial function and HDAC3 (72). Following this idea, we further isolated primary B cells from Vanco-treated mice and found that butyrate could elevate Blimp-1 expression by promoting mitochondrial function and Akt-mTOR pathway, thereby promoting CD138+ PCs generation (Fig. 7). SCFAs fuel host antibody production and intensify the production of host intestinal IgA and systemic IgG by promoting mitochondrial quality and fatty acid oxidation (FAO) (20). However, Sanchez et al. emphasized that at high doses, SCFAs inhibited antibody production by regulating AID and Blimp-1 expression (21). These conflicting results suggest that the dosage of SCFAs has a significant impact on host antibody production. Our results also further confirmed the SCFAs in improving the immunogenicity of rabies vaccines in Vanco-treated mice.
FIG 7.
Proposed mechanism of butyrate in enhancing the humoral response to rabies vaccination in vancomycin-treated mice. Butyrate promotes mitochondrial function and activates the Akt-mTOR pathway in primary B lymphocytes, increasing the production of PCs, ultimately improving humoral immunity to rabies vaccine in Vanco-treated mice.
In addition to SCFAs, other metabolites may also promote vaccine immunogenicity. Based on our previous results of 16S rRNA gene sequencing, the abundance of Lactobacillus intestinalis was completely depleted after Vanco treatment (8). Lactobacillus intestinalis primarily uses dietary vitamin A (VA) as a substrate to produce retinoic acid (RA), which promotes host gene expression, such as RA-induced gene Stra6, and maintains host gene expression and metabolic homeostasis (73). Wu et al. found that SCFA (mainly acetate) induced DC expression of Aldh1a2, which converts VA into RA, promoting intestinal IgA responses and mucosal adjuvant activity against CT in a GPR43-dependent manner (44). Based on these research findings, we hypothesize that the downregulation of the rabies vaccine immunogenicity induced by Vanco treatment may be related to the sharp decrease in Lactobacillus intestinalis, resulting in reduced RA and ALDH production, and subsequently lowered expression of key genes associated with antibody production. Further experiments are needed to confirm the hypothesis. Furthermore, dietary fiber (DF) is the main source of SCFAs, and studies have suggested that DF may play a role in the immune response to TIV vaccines (10) and COVID-19 mRNA vaccines (74). Whether dietary fiber can magnify the immunogenicity of rabies vaccines is also worth further study.
In summary, this present study demonstrates the impact of the SCFAs on the immunogenicity of rabies vaccines and maintenance of immune homeostasis in Vanco-treated mice by promoting GC B cells, PCs, and RABV-specific ASCs generation, thus enhancing VNA production. These findings reinforce the link between vaccine immunogenicity and the gut microbiome and provide new insights for the microbial metabolites in maintaining host immune homeostasis.
MATERIALS AND METHODS
Viruses, cells, and antibodies.
The RABV vaccine strain LBNSE derived from the parental strain SAD-L16, with two mutations on amino acid positions 194 (aspartic acid to serine, TCC) and 333 (arginine to glutamic acid, GAA) in the gene encoding the G protein, resulting in a weakened pathogenicity (47, 75, 76). The LBNSE and lab-attenuated RABV strain CVS-11 were propagated in BSR cells. The inactivated LBNSE (iLBNSE) vaccine was prepared by adding 0.2% polyformaldehyde and incubating at 37°C for 24 h. BSR cells (kindly provided by Bernhard Dietzschold at Thomas Jefferson University, Philadelphia, PA, USA) were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS).
FITC anti-rabies monoclonal globulin was purchased from FUJIREBIO (Melvin, PA). The antibodies directly labeled fluorescein for flow cytometric analyses were as follows. FITC anti-mouse CD4 (catalog no. 100406), PE anti-mouse CD279 (PD-1) (catalog no. 109104), APC anti-mouse CD185 (CXCR5) (catalog no. 145506), PerCP/Cyanine5.5 anti-mouse IFN-γ Antibody (catalog no. 505822), Brilliant Violet 421 anti-mouse IL-4 Antibody (catalog no. 504119), APC anti-mouse CD3 Antibody (catalog no. 100236), FITC anti-mouse CD11c Antibody (catalog no. 117306), PerCP/Cyanine5.5 anti-mouse I-A/I-E Antibody (catalog no. 107626), APC/Cyanine7 anti-mouse/human CD11b Antibody (catalog no. 101226), PE anti-mouse CD8a Antibody (catalog no. 100707), FITC anti-mouse/human CD45R/B220 (catalog no. 103206), Alexa Fluor 647 anti-mouse/human GL7 (clone GL7) (catalog no. 144606), PE anti-mouse Blimp-1 Antibody (catalog no. 145506), and APC anti-mouse CD185 (CXCR5) Antibody (catalog no. 145506) were purchased from Biolegend (CA, USA). PE anti-mouse CD95 (APO-1/Fas) (catalog no. 152608) was purchased from eBioscience (San Diego, CA). Antibodies used in Western blot included Phospho-Akt (Ser473) Antibody (catalog no. 9271), Akt Antibody (catalog no. 9272), mTOR (7C10) Rabbit MAb (catalog no. 2983), Phospho-mTOR (Ser2448) Rabbit MAb (catalog no. 5536) were purchased from CST (Danvers, MA), and β-actin (6D1) (catalog no. M177-3) was purchased from MBL (Beijing, China). ELISA antibodies included Horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (catalog no. BA1050), IgG1 (catalog no. BF03002), IgG2b (catalog no. BF03004), and IgM (catalog no. BF03006) were purchased from Boster or Biodragon (Wuhan, China).
Animals, bacteria, and animal models.
Specific pathogen-free (SPF)-grade female ICR mice and C57BL/6 mice were obtained and housed in the animal facility of Huazhong Agricultural University. The mice were maintained under SPF conditions with a 12-h light/dark cycle and provided with sufficient water and food.
FMT was performed according to previous studies (25). In brief, 0.7 g of fresh fecal samples were collected from untreated mice and ground in a sterile EP tube containing steel beads for 1 min. After filtering through a 70-μm mesh, the fecal suspension was washed twice with 10 to 15 mL of sterile PBS. After final centrifugation at 100 × g for 5 min, the fecal pellets were thoroughly dissolved in sterile PBS (0.1 mg/mL) under anaerobic conditions and administered via oral gavage of 150 μL per mouse. The Blautia coccoides (ATCC 29236) (B. coccoides) strain was kindly provided by Professor Zhu Shu at Zhejiang University and was administered via oral gavage after culturing on BHI (catalog no. HBKP8297-5; HopeBio, China) solid medium in anaerobic conditions at 37°C for 24 h and administered via oral gavage of 2 × 108 CFU per mouse. The Clostridium butyricum (CBM588) strain was obtained from Miyarisan Pharmaceutical Co., Ltd. and administered via oral gavage of 500 mg/kg/day (3.4 × 108 CFU/g/mouse/day) following the method described by Ariyoshi et al. (77).
The experimental design included FMT/gavage experiments and SCFA supplementation experiments. In the FMT/gavage experiment, mice were randomly assigned to four groups: (i) Vanco+PBS group (n = 12): 3-week-old mice were treated with Vanco (0.5 g/L) (catalog no. S17059; Yuanye, China) for 4 weeks and then gavage with phosphate-buffered saline (PBS) daily for 4 consecutive days; (ii) Vanco+FMT group (n = 12): 3-week-old mice were treated with Vanco (0.5 g/L) for 4 weeks and then gavage with FMT daily for 4 consecutive days; (iii) Vanco+ B. coccoides group (n = 12): 3-week-old mice were treated with Vanco (0.5 g/L) for 4 weeks and then gavage with B. coccoides daily for 4 consecutive days; (iv) Vanco+ CBM588 group (n = 12): 3-week-old mice were treated with Vanco (0.5 g/L) for 4 weeks and then gavage with CBM588 daily for 4 consecutive days. All four groups of mice were subsequently i.m. immunized with 107 FFU iLBNSE (100 μL/mouse).
In the SCFA supplementation experiment, mice were randomly assigned to three groups: (i) untreated group (n = 12): 3-week-old mice were administered with sterile water as a control; (ii) Vanco group (n = 12): 3-week-old mice were treated with Vanco (0.5 g/L) until the end of the experiment; (iii) Vanco+Butyrate group (n = 12): 3-week-old mice were treated with Vanco (0.5 g/L) for 4 weeks, and then 40 mM sodium butyrate (NaBu) (catalog no. 303410; Sigma, USA) was added together to their drinking water until the end of the experiment. All three groups of mice were subsequently immunized with 107 FFU iLBNSE (100 μL/mouse).
Quantitative real-time PCR.
Quantification of B. coccoides (78) and C. butyricum (79) in stools were performed by quantitative real-time (qPCR). In short, genomic DNA from fecal samples was extracted using the TIANamp Stool DNA Kit (catalog no. DP328; TIANGEN, China). Quantification of Prdm1 (21) was performed by qPCR as well. Briefly, the RNA of primary B cells was extracted with TRIzol reagent (Thermo Fisher Scientific, USA) and reverse transcribed with HiScript II Q RT supermix (catalog no. R222-01; Vazyme, China) to obtain cDNA. The amplification reaction conditions were as follows: 95°C for 8 min, followed by 40 cycles at 95°C for 15 s, and annealing at 60°C for 45 s. The reaction mixture consisted of 2 μL DNA (<100 ng), 1 μL forward primer (10 μM), 1 μL reverse primer (10 μM), and 10 μL AceQ Universal SYBR qPCR Master Mix (2×) (catalog no. Q511-02; Vazyme, China), supplemented with ddH2O to a final volume of 20 μL. Primers for detecting B. coccoides, C. butyricum, and the Prdm1 gene were shown in Table 1.
Table 1.
Primers used for qPCR
| Primer | Sequence (5'-3') |
|---|---|
| 27F | AGAGTTGATCCTGGCTCAG |
| 338R | CTGCTGCCTCCCGTAGGAGT |
| B. coccoides-F | AAATGACGGTACCTGACTAA |
| B. coccoides-R | CTTTGAGTTTCATTCTTGCGAA |
| C. butyricum-F | GTGCCGCCGCTAACGCATTAAGTAT |
| C. butyricum-R | ACCATGCACCACCTGTCTTCCTGCC |
| Prdm1-F | GCTGCTGGGCTGCCTTTGGA |
| Prdm1-R | GGAGAGGAGGCCGTTCCCCA |
| β-actin-F | CACTGCCGCATCCTCTTCCTCCC |
| β-actin-R | CAATAGTGATGACCTGGCCGT |
Short-chain fatty acid measurement.
The concentrations of SCFAs in cecum contents, serum, and inguinal lymph nodes were measured using targeted metabolomics (80). Samples (100 mg or 100 μL) were mixed with 50 μL of 15% phosphoric acid and 10 μL of an internal standard solution (hexanoic acid) at a concentration of 75 μg/mL, then vortexed for 30 s together with 140 μL of ether. The mixture was centrifuged at 4°C, 12,000 rpm for 10 min, and the supernatant was collected and analyzed by GC-MS. Pure standards of acetic acid, propionic acid, and butyric acid were prepared in appropriate amounts and mixed to form a standard concentration gradient. The GC-MS chromatographic conditions were as follows: Agilent HP-INNOWAX capillary column (30 m × 0.25 mm ID × 0.25 μm), split injection mode with a 1 μL sample volume, and a split ratio of 10:1, an injection port temperature of 250°C, ion source temperature of 230°C, transfer line temperature of 250°C, and quadrupole temperature of 150°C. The temperature program started at 90°C, raised at a rate of 10°C/min to 120°C, followed by an increase of 5°C/min to 150°C, and finally an increase of 25°C/min to 250°C for 2 min. Helium gas was used as the carrier gas with a flow rate of 1.0 mL/min. MS conditions included electron impact ionization (EI) source, full scan and SIM scanning modes, and an electron energy of 70 eV.
VNA measurement.
Rabies virus-neutralizing antibodies (VNAs) were determined using a fluorescent antibody virus neutralization (FAVN) test described previously (8, 81). Briefly, mouse serum was separated and heat-inactivated at 56°C for 30 min. DMEM (100 μL) was added to a 96-well plate, and test or standard serum (50 μL) was added to the first column and serially diluted 3-fold. Each sample was added to four adjacent wells. A rabies challenge virus standard (CVS-11) containing 100 FFU was added to each well, and the plates were incubated at 37°C for 1 h. Following incubation, 2 × 104 BSR cells were added to each well, and solutions were incubated at 37°C for 72 h. Samples were then fixed with 80% ice-cold acetone for 30 min and stained with FITC anti-rabies monoclonal globulin. Fluorescence was observed under an Olympus IX51 fluorescence microscope (Olympus, Tokyo, Japan). Fluorescence values were compared to those of a reference serum (obtained from the National Institute for Biological Standards and Control, Hertfordshire, UK), and results were normalized and quantified in international units per mL (IU/mL).
ELISA.
RABV-specific IgM, IgG, and IgG subtypes were determined using ELISAs according to previously described methods (8). Briefly, ELISA plates were coated with 500 ng/well of inactivated and purified RABV virion proteins (25 mg/mL) in coating buffer (5 mM Na2CO3, pH 9.6) overnight at 4°C. The plate was washed three times with PBS-Tween (0.5% Tween 80, wt/vol) (PBST) and then blocked in 5% low-fat milk-PBS for 2 h at 37°C. Next, diluted serum (1:30 for IgM, 1:20,000 for IgG, 1:500 for IgG1, and 1:2,000 for IgG2b) was added to the plates and incubated for 2 h at 37°C. After incubation, plates were washed three times in PBST, and then HRP-conjugated goat anti-mouse IgM, IgG, IgG1, or IgG2b (100 μL per well, 1:1,500 dilution) was added to each well for 1 h at 37°C. The color was developed using a substrate solution TMB (100 μL per well) (catalog no. P0209; Beyotime, China). and reactions were stopped with 2M sulfuric acid. Optical densities (OD) were recorded at 450 nm using a SpectraMax 190 spectrophotometer (Molecular Devices, CA, USA).
ELISpot assay.
Commercial ELISpot kits from DAKEWE (Shenzhen, China) were used to measure IFN-γ (catalog no. CT317) and IL-4 (catalog no. CT319) secreting cells in mice. Splenocytes were isolated 7 dpi and seeded into a 96-well plate. The cells were stimulated with inactivated and purified RABV virions and incubated at 37°C with 5% CO2 for 24 h. The plates were washed and processed according to the manufacturer's instructions before being scanned and the spots quantitated. RABV-specific ASCs were determined through B cells ELISpot using Multiscreen-HA ELISpot plates (catalog no. MAHAS4510; Millipore, USA) coated with purified RABV virions (82). The blocked ELISpot plates were incubated with cell suspensions from draining lymph nodes and biotin-conjugated mouse IgG antibody, streptavidin-alkaline phosphatase (catalog no. 3310-10-1000; Mabtech, Sweden), and BCIP/NBT-plus (catalog no. 3650-10; Mabtech, Sweden).
Flow cytometry.
Flow cytometry was used to analyze the DC activation, immune cells, Blimp-1 expression, and CD4 T cell response proportions. To detect DC activation and immune cells, the surface staining protocol was followed. In short, cells were resuspended in PBS containing 0.2% (wt/vol) bovine serum albumin (BSA), filtered through a 40-mm nylon filter, centrifuged, and washed with PBS containing 0.2% (wt/vol) BSA. After two washes with PBS, the single-cell suspensions in PBS (containing 0.2% BSA [wt/vol]) were enumerated. A total of 1 × 106 cells were stained by flow cytometric antibody using the following staining protocol: cDC (MHC II+ in CD11c+), cDC1 (CD8α+ CD11b- in cDCs), cDC2 (CD8α- CD11b+ in cDCs), Tfh cells (CD4+ PD1+ CXCR5+), GC B cells (B220+ CD95+ GL7+), and PCs (CD138+ B220low). The cells were then incubated at 4°C for 30 min and washed twice with PBS containing 0.2% (wt/vol) BSA before being analyzed by BECKMAN FACS cytoflex instrument. For analysis of Blimp-1 expression and CD4 T cell responses, the surface and intracellular staining protocol was adopted. Following staining cells with a viability dye, cells were primarily surface stained; thereafter, fixation and permeabilization were executed using BD Fixation/Permeabilization solution (catalog no. 554714; BD Biosciences) or True-Nuclear Transcription Factor Buffer (catalog no. 424401; BioLegend, USA) by the manufacturer's recommendation. Lastly, cells were intracellularly stained and analyzed by BECKMAN FACS cytoflex instrument.
Isolation and culture of primary B cells.
C57BL/6 mice were euthanized, and their spleens were gently ground to obtain a single-cell suspension. Pan-B cells were negatively purified using a MojoSort mouse pan-B cell isolation kit (catalog no. 480052, BioLegend, USA). To sum up, a biotin antibody cocktail containing CD11c, CD49b, CD3e, CD4, CD8, Ter119, and Gr1 antibodies were added into cells and incubated on ice for 15 min, and then streptavidin nanobeads were added and incubated on ice for another 15 min. Magnetic separation was then employed to remove labeled non-B cells resulting in >90% purity. Finally, the pan-B cells were cultured at a density of 1 × 106 cells/mL in RPMI 1640 medium supplemented with 10% heat-inactivated FBS, 100 IU/mL penicillin/streptomycin, 50 mM β-Me, and 10 mM HEPES. For basic PC-inducing conditions in cell culture experiments, B cells were stimulated with 5 μg/mL LPS (catalog no. tlrl-3pelps, InvivoGen), 5 μg/mL anti-CD40 (catalog no. 157503, BioLegend, USA), and 10 μg/mL anti-IgM (catalog no. 157102, BioLegend, USA) for a period of 5 days.
Cell viability assay.
The effects of NaBu (catalog no. B5887, Sigma, USA), Akt inhibitor VIII (catalog no. SF2784, Beyotime, China), and Rapamycin (catalog no. S1842, Beyotime, China) on primary B cells viability were assessed using a cell proliferation/toxicity assay kit from Abbkine (catalog no. KTA1020). Concisely stated, 100 μL of cell suspension containing 5,000 cells was seeded into a 96-well plate and cultured overnight. Subsequently, 5 μL of the vehicle with various concentrations was added to each well and incubated at 37°C with 5% CO2 for an additional 48 h. Following this, 10 μL of CCK-8 solution was added to the 96-well plate, which was then incubated for another 4 h at 37°C with 5% CO2. The OD at 450 nm was measured using a SpectraMax 190 spectrophotometer (Molecular Devices, CA, USA), and the results were calculated accordingly.
Mitochondrial function analysis.
The mitochondrial membrane potential assay kit (catalog no. C2001S, Beyotime, China), mitochondrial complex I activity assay kit (catalog no. KTB1850, Abbkine, China) and ATP assay kit (catalog no. S0026B, Beyotime, China) were employed to evaluate mitochondrial function. For mitochondrial membrane potential assay, 0.2 × 106 cells were collected and washed with 200 μL of FACS-buffer (5 min, 400 × g, 4°C). An appropriate amount of TMRE mitochondrial stains were added to the cells, which were then incubated at 37°C for 15 to 45 min. After three washes using FACS buffer, the staining results were analyzed via flow cytometry. For mitochondrial complex I activity assay, 5 × 106 cells were collected and fresh mitochondria were isolated by ice bath homogenization followed with centrifugation at 4°C. The activity of mitochondrial complex I was assessed by multimode microplate reader (TECAN, USA). For ATP content assay, 2 × 106 cells were collected and lysed by 100 μL of ATP lysis solution, followed by centrifugation at 4°C and 12,000 rpm for 5 min. The supernatant was collected and then added to the ATP detection working solution, and the RLU was measured using a luminometer.
Western blot.
The protein levels of Akt and mTOR in primary mouse B cells were detected by western blot. Briefly, the cultured cells were lysed using RIPA lysis buffer (catalog no. P0013B; Beyotime, China) and the protein concentrations were determined using the BCA protein assay kit (catalog no. P0010S; Beyotime, China) according to the manufacturer's instructions. For Western blot, the SDS-PAGE gels were transferred onto a polyvinylidene fluoride (PVDF) membrane (catalog no. 1620177; Bio-Rad, USA). The membrane was blocked with 5% skim milk for 2 h at room temperature followed by three washes with TBST and incubated with primary antibody overnight at 4°C. After washing three times with TBST, the corresponding HRP-conjugated secondary antibody was added and incubated at room temperature for 45 min. Visualization of the blot was done using ECL reagent (catalog no. P0018AM; Beyotime, China) and detection was performed using the Amersham Imager 600 enhanced chemiluminescence (ECL) analysis system (GE, MA, USA).
Statistical analysis.
GraphPad Prism 8.0 (GraphPad Software, Inc., San Diego, CA) was used to determine the differences in concentration of SCFAs and partial immune cell numbers using an unpaired two-tailed t test. The statistical significance of DCs, Anti-RABV-IgM, VNAs, Anti-RABV-IgG and isotypes, Tfh cells, GC B cells and PCs was evaluated by one-way ANOVA followed by Tukey’s multiple-comparison test.
Ethics statement.
All animal experiments in this study were conducted in strict accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals of the Ministry of Science and Technology of the People's Republic of China. All animal experiments were approved by the Scientific Ethics Committee of Huazhong Agricultural University (Permit Number: HZAUMO-2019-079).
ACKNOWLEDGMENTS
We thank Shuaipeng He and Ruyi Ye in Experimental Animal Center, Huazhong Agricultural University for their careful management of the animals. This study was supported by the National Key Research and Development Program of China (No. 2022YFD1800100) and the Guangdong Major Project of Basic and Applied Basic Research (2020B0301030007 to Z.F.F.).
Contributor Information
Ling Zhao, Email: zling604@outlook.com, lingzhao@mail.hzau.edu.cn.
Rebecca Ellis Dutch, University of Kentucky College of Medicine.
REFERENCES
- 1.Blanchard T. 2004. Rabies and other lyssavirus diseases. Lancet 363:1906–1907. doi: 10.1016/S0140-6736(04)16367-8. [DOI] [PubMed] [Google Scholar]
- 2.Sellal F, Stoll-Keller F. 2005. Rabies: ancient yet contemporary cause of encephalitis. Lancet 365:921–923. doi: 10.1016/S0140-6736(05)71060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hampson K, Coudeville L, Lembo T, Sambo M, Kieffer A, Attlan M, Barrat J, Blanton JD, Briggs DJ, Cleaveland S, Costa P, Freuling CM, Hiby E, Knopf L, Leanes F, Meslin FX, Metlin A, Miranda ME, Muller T, Nel LH, Recuenco S, Rupprecht CE, Schumacher C, Taylor L, Vigilato MA, Zinsstag J, Dushoff J. Global Alliance for Rabies Control Partners for Rabies Protection. 2015. Estimating the global burden of endemic canine rabies. PLoS Negl Trop Dis 9:e0003709. doi: 10.1371/journal.pntd.0003709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fooks AR, Banyard AC, Horton DL, Johnson N, McElhinney LM, Jackson AC. 2014. Current status of rabies and prospects for elimination. Lancet 384:1389–1399. doi: 10.1016/S0140-6736(13)62707-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonilla-Aldana DK, Ruiz-Saenz J, Martinez-Gutierrez M, Villamil-Gomez W, Mantilla-Meluk H, Arrieta G, Leon-Figueroa DA, Benites-Zapata V, Barboza JJ, Munoz-Del-Carpio-Toia A, Franco OH, Cabrera M, Sah R, Al-Tawfiq JA, Memish ZA, Amer FA, Suarez JA, Henao-Martinez AF, Franco-Paredes C, Zumla A, Rodriguez-Morales AJ. 2023. Zero by 2030 and OneHealth: the multidisciplinary challenges of rabies control and elimination. Travel Med Infect Dis 51:102509. doi: 10.1016/j.tmaid.2022.102509. [DOI] [PubMed] [Google Scholar]
- 6.Thumbi SM, Blumberg L, Le Roux K, Salahuddin N, Abela B. 2022. A call to accelerate an end to human rabies deaths. Lancet 400:2261–2264. doi: 10.1016/S0140-6736(22)02487-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zinsstag J, Lechenne M, Laager M, Mindekem R, Naissengar S, Oussiguere A, Bidjeh K, Rives G, Tessier J, Madjaninan S, Ouagal M, Moto DD, Alfaroukh IO, Muthiani Y, Traore A, Hattendorf J, Lepelletier A, Kergoat L, Bourhy H, Dacheux L, Stadler T, Chitnis N. 2017. Vaccination of dogs in an African city interrupts rabies transmission and reduces human exposure. Sci Transl Med 9. doi: 10.1126/scitranslmed.aaf6984. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Wu Q, Zhou M, Luo Z, Lv L, Pei J, Wang C, Chai B, Sui B, Huang F, Fu ZF, Zhao L. 2020. Composition of the murine gut microbiome impacts humoral immunity induced by rabies vaccines. Clin Transl Med 10:e161. doi: 10.1002/ctm2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hagan T, Cortese M, Rouphael N, Boudreau C, Linde C, Maddur MS, Das J, Wang H, Guthmiller J, Zheng NY, Huang M, Uphadhyay AA, Gardinassi L, Petitdemange C, McCullough MP, Johnson SJ, Gill K, Cervasi B, Zou J, Bretin A, Hahn M, Gewirtz AT, Bosinger SE, Wilson PC, Li S, Alter G, Khurana S, Golding H, Pulendran B. 2019. Antibiotics-driven gut microbiome perturbation alters immunity to vaccines in humans. Cell 178:1313–1328. doi: 10.1016/j.cell.2019.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cait A, Mooney A, Poyntz H, Shortt N, Jones A, Gestin A, Gell K, Grooby A, O'Sullivan D, Tang JS, Young W, Thayabaran D, Sparks J, Ostapowicz T, Tay A, Poppitt SD, Elliott S, Wakefield G, Parry-Strong A, Ralston J, Beasley R, Weatherall M, Braithwaite I, Forbes-Blom E, Gasser O. 2021. Potential association between dietary fibre and humoral response to the seasonal influenza vaccine. Front Immunol 12:765528. doi: 10.3389/fimmu.2021.765528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oh JZ, Ravindran R, Chassaing B, Carvalho FA, Maddur MS, Bower M, Hakimpour P, Gill KP, Nakaya HI, Yarovinsky F, Sartor RB, Gewirtz AT, Pulendran B. 2014. TLR5-mediated sensing of gut microbiota is necessary for antibody responses to seasonal influenza vaccination. Immunity 41:478–492. doi: 10.1016/j.immuni.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ng SC, Peng Y, Zhang L, Mok CK, Zhao S, Li A, Ching JY, Liu Y, Yan S, Chan DLS, Zhu J, Chen C, Fung AC, Wong KK, Hui DS, Chan FK, Tun HM. 2022. Gut microbiota composition is associated with SARS-CoV-2 vaccine immunogenicity and adverse events. Gut 71:1106–1116. doi: 10.1136/gutjnl-2021-326563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang B, Tang L, He W, Jiang X, Hu C, Li Y, Zhang Y, Pang K, Lei Y, Li S, Liu S, Wang S, Yang M, Li Z, Zhao F, Yang S. 2022. Correlation of gut microbiota and metabolic functions with the antibody response to the BBIBP-CorV vaccine. Cell Rep Med 3:100752. doi: 10.1016/j.xcrm.2022.100752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim CH. 2023. Complex regulatory effects of gut microbial short-chain fatty acids on immune tolerance and autoimmunity. Cell Mol Immunol doi: 10.1038/s41423-023-00987-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Macfarlane S, Macfarlane GT. 2003. Regulation of short-chain fatty acid production. Proc Nutr Soc 62:67–72. doi: 10.1079/PNS2002207. [DOI] [PubMed] [Google Scholar]
- 16.Xie L, Alam MJ, Marques FZ, Mackay CR. 2023. A major mechanism for immunomodulation: dietary fibres and acid metabolites. Semin Immunol 66:101737. doi: 10.1016/j.smim.2023.101737. [DOI] [PubMed] [Google Scholar]
- 17.Yang W, Cong Y. 2021. Gut microbiota-derived metabolites in the regulation of host immune responses and immune-related inflammatory diseases. Cell Mol Immunol 18:866–877. doi: 10.1038/s41423-021-00661-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan J, McKenzie C, Vuillermin PJ, Goverse G, Vinuesa CG, Mebius RE, Macia L, Mackay CR. 2016. Dietary fiber and bacterial SCFA enhance oral tolerance and protect against food allergy through diverse cellular pathways. Cell Rep 15:2809–2824. doi: 10.1016/j.celrep.2016.05.047. [DOI] [PubMed] [Google Scholar]
- 19.He Y, Fu L, Li Y, Wang W, Gong M, Zhang J, Dong X, Huang J, Wang Q, Mackay CR, Fu YX, Chen Y, Guo X. 2021. Gut microbial metabolites facilitate anticancer therapy efficacy by modulating cytotoxic CD8(+) T cell immunity. Cell Metab 33:988–1000. doi: 10.1016/j.cmet.2021.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Kim M, Qie Y, Park J, Kim CH. 2016. Gut microbial metabolites fuel host antibody responses. Cell Host Microbe 20:202–214. doi: 10.1016/j.chom.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanchez HN, Moroney JB, Gan H, Shen T, Im JL, Li T, Taylor JR, Zan H, Casali P. 2020. B cell-intrinsic epigenetic modulation of antibody responses by dietary fiber-derived short-chain fatty acids. Nat Commun 11:60. doi: 10.1038/s41467-019-13603-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cait A, Hughes MR, Antignano F, Cait J, Dimitriu PA, Maas KR, Reynolds LA, Hacker L, Mohr J, Finlay BB, Zaph C, McNagny KM, Mohn WW. 2018. Microbiome-driven allergic lung inflammation is ameliorated by short-chain fatty acids. Mucosal Immunol 11:785–795. doi: 10.1038/mi.2017.75. [DOI] [PubMed] [Google Scholar]
- 23.Dupraz L, Magniez A, Rolhion N, Richard ML, Da Costa G, Touch S, Mayeur C, Planchais J, Agus A, Danne C, Michaudel C, Spatz M, Trottein F, Langella P, Sokol H, Michel ML. 2021. Gut microbiota-derived short-chain fatty acids regulate IL-17 production by mouse and human intestinal gammadelta T cells. Cell Rep 36:109332. doi: 10.1016/j.celrep.2021.109332. [DOI] [PubMed] [Google Scholar]
- 24.Cui C, Hong H, Shi Y, Zhou Y, Qiao CM, Zhao WJ, Zhao LP, Wu J, Quan W, Niu GY, Wu YB, Li CS, Cheng L, Hong Y, Shen YQ. 2022. Vancomycin pretreatment on MPTP-induced Parkinson's disease mice exerts neuroprotection by suppressing inflammation both in brain and gut. J Neuroimmune Pharmacol doi: 10.1007/s11481-021-10047-y. [DOI] [PubMed] [Google Scholar]
- 25.Yang XL, Wang G, Xie JY, Li H, Chen SX, Liu W, Zhu SJ. 2021. The intestinal microbiome primes host innate immunity against enteric virus systemic infection through type i interferon. mBio 12. doi: 10.1128/mBio.00366-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang JM, Sun YS, Zhao LQ, Chen TT, Fan MN, Jiao HC, Zhao JP, Wang XJ, Li FC, Li HF, Lin H. 2019. SCFAs-induced GLP-1 secretion links the regulation of gut microbiome on hepatic lipogenesis in chickens. Front Microbiol 10:2176. doi: 10.3389/fmicb.2019.02176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uribe-Herranz M, Rafail S, Beghi S, Gil-de-Gomez L, Verginadis I, Bittinger K, Pustylnikov S, Pierini S, Perales-Linares R, Blair IA, Mesaros CA, Snyder NW, Bushman F, Koumenis C, Facciabene A. 2020. Gut microbiota modulate dendritic cell antigen presentation and radiotherapy-induced antitumor immune response. J Clin Invest 130:466–479. doi: 10.1172/JCI124332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yin X, Chen S, Eisenbarth SC. 2021. Dendritic cell regulation of T helper cells. Annu Rev Immunol 39:759–790. doi: 10.1146/annurev-immunol-101819-025146. [DOI] [PubMed] [Google Scholar]
- 29.Erasmus JH, Khandhar AP, O'Connor MA, Walls AC, Hemann EA, Murapa P, Archer J, Leventhal S, Fuller JT, Lewis TB, Draves KE, Randall S, Guerriero KA, Duthie MS, Carter D, Reed SG, Hawman DW, Feldmann H, Gale M, Jr., Veesler D, Berglund P, Fuller DH. 2020. An Alphavirus-derived replicon RNA vaccine induces SARS-CoV-2 neutralizing antibody and T cell responses in mice and nonhuman primates. Sci Transl Med 12. doi: 10.1126/scitranslmed.abc9396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Higgins BW, Shuparski AG, Miller KB, Robinson AM, McHeyzer-Williams LJ, McHeyzer-Williams MG. 2022. Isotype-specific plasma cells express divergent transcriptional programs. Proc Natl Acad Sci USA 119:e2121260119. doi: 10.1073/pnas.2121260119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krishnaswamy JK, Gowthaman U, Zhang B, Mattsson J, Szeponik L, Liu D, Wu R, White T, Calabro S, Xu L, Collet MA, Yurieva M, Alsen S, Fogelstrand P, Walter A, Heath WR, Mueller SN, Yrlid U, Williams A, Eisenbarth SC. 2017. Migratory CD11b(+) conventional dendritic cells induce T follicular helper cell-dependent antibody responses. Sci Immunol 2. doi: 10.1126/sciimmunol.aam9169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perry LL, Lodmell DL. 1991. Role of CD4+ and CD8+ T cells in murine resistance to street rabies virus. J Virol 65:3429–3434. doi: 10.1128/JVI.65.7.3429-3434.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tantawichien T, Jaijaroensup W, Khawplod P, Sitprija V. 2001. Failure of multiple-site intradermal postexposure rabies vaccination in patients with human immunodeficiency virus with low CD4+ T lymphocyte counts. Clin Infect Dis 33:E122–4. doi: 10.1086/324087. [DOI] [PubMed] [Google Scholar]
- 34.Lynn DJ, Benson SC, Lynn MA, Pulendran B. 2022. Modulation of immune responses to vaccination by the microbiota: implications and potential mechanisms. Nat Rev Immunol 22:33–46. doi: 10.1038/s41577-021-00554-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Victora GD, Nussenzweig MC. 2022. Germinal centers. Annu Rev Immunol 40:413–442. doi: 10.1146/annurev-immunol-120419-022408. [DOI] [PubMed] [Google Scholar]
- 36.Vinuesa CG, Linterman MA, Yu D, MacLennan IC. 2016. Follicular helper T cells. Annu Rev Immunol 34:335–368. doi: 10.1146/annurev-immunol-041015-055605. [DOI] [PubMed] [Google Scholar]
- 37.Pollard AJ, Bijker EM. 2021. A guide to vaccinology: from basic principles to new developments. Nat Rev Immunol 21:83–100. doi: 10.1038/s41577-020-00479-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu J, Yang K, Cai S, Zhang X, Hu L, Lin F, Wu SQ, Xiao C, Liu WH, Han J. 2022. A p38alpha-BLIMP1 signalling pathway is essential for plasma cell differentiation. Nat Commun 13:7321. doi: 10.1038/s41467-022-34969-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tellier J, Shi W, Minnich M, Liao Y, Crawford S, Smyth GK, Kallies A, Busslinger M, Nutt SL. 2016. Blimp-1 controls plasma cell function through the regulation of immunoglobulin secretion and the unfolded protein response. Nat Immunol 17:323–330. doi: 10.1038/ni.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ray JP, Staron MM, Shyer JA, Ho PC, Marshall HD, Gray SM, Laidlaw BJ, Araki K, Ahmed R, Kaech SM, Craft J. 2015. The interleukin-2-mTORc1 kinase axis defines the signaling, differentiation, and metabolism of T helper 1 and follicular B helper T cells. Immunity 43:690–702. doi: 10.1016/j.immuni.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ersching J, Efeyan A, Mesin L, Jacobsen JT, Pasqual G, Grabiner BC, Dominguez-Sola D, Sabatini DM, Victora GD. 2017. Germinal center selection and affinity maturation require dynamic regulation of mTORC1 kinase. Immunity 46:1045–1058. doi: 10.1016/j.immuni.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keating R, Hertz T, Wehenkel M, Harris TL, Edwards BA, McClaren JL, Brown SA, Surman S, Wilson ZS, Bradley P, Hurwitz J, Chi H, Doherty PC, Thomas PG, McGargill MA. 2013. The kinase mTOR modulates the antibody response to provide cross-protective immunity to lethal infection with influenza virus. Nat Immunol 14:1266–1276. doi: 10.1038/ni.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haenen D, Zhang J, Souza da Silva C, Bosch G, van der Meer IM, van Arkel J, van den Borne JJ, Perez Gutierrez O, Smidt H, Kemp B, Muller M, Hooiveld GJ. 2013. A diet high in resistant starch modulates microbiota composition, SCFA concentrations, and gene expression in pig intestine. J Nutr 143:274–283. doi: 10.3945/jn.112.169672. [DOI] [PubMed] [Google Scholar]
- 44.Wu W, Sun M, Chen F, Cao AT, Liu H, Zhao Y, Huang X, Xiao Y, Yao S, Zhao Q, Liu Z, Cong Y. 2017. Microbiota metabolite short-chain fatty acid acetate promotes intestinal IgA response to microbiota which is mediated by GPR43. Mucosal Immunol 10:946–956. doi: 10.1038/mi.2016.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rodrigues PF, Kouklas A, Cvijetic G, Bouladoux N, Mitrovic M, Desai JV, Lima-Junior DS, Lionakis MS, Belkaid Y, Ivanek R, Tussiwand R. 2023. pDC-like cells are pre-DC2 and require KLF4 to control homeostatic CD4 T cells. Sci Immunol 8:eadd4132. doi: 10.1126/sciimmunol.add4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou M, Wang L, Zhou S, Wang Z, Ruan J, Tang L, Jia Z, Cui M, Zhao L, Fu ZF. 2015. Recombinant rabies virus expressing dog GM-CSF is an efficacious oral rabies vaccine for dogs. Oncotarget 6:38504–38516. doi: 10.18632/oncotarget.5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wen Y, Wang H, Wu H, Yang F, Tripp RA, Hogan RJ, Fu ZF. 2011. Rabies virus expressing dendritic cell-activating molecules enhances the innate and adaptive immune response to vaccination. J Virol 85:1634–1644. doi: 10.1128/JVI.01552-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao L, Toriumi H, Wang H, Kuang Y, Guo X, Morimoto K, Fu ZF. 2010. Expression of MIP-1alpha (CCL3) by a recombinant rabies virus enhances its immunogenicity by inducing innate immunity and recruiting dendritic cells and B cells. J Virol 84:9642–9648. doi: 10.1128/JVI.00326-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Y, Yang J, Li M, Cui M, Fu ZF, Zhao L, Zhou M. 2019. A recombinant rabies virus expressing Fms-like tyrosine kinase 3 ligand (Flt3L) induces enhanced immunogenicity in mice. Virol Sin 34:662–672. doi: 10.1007/s12250-019-00144-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou M, Zhang G, Ren G, Gnanadurai CW, Li Z, Chai Q, Yang Y, Leyson CM, Wu W, Cui M, Fu ZF. 2013. Recombinant rabies viruses expressing GM-CSF or flagellin are effective vaccines for both intramuscular and oral immunizations. PLoS One 8:e63384. doi: 10.1371/journal.pone.0063384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao J, Zhang Y, Chen Y, Zhang J, Pei J, Cui M, Fu ZF, Zhao L, Zhou M. 2021. A novel oral rabies vaccine enhances the immunogenicity through increasing dendritic cells activation and germinal center formation by expressing U-OMP19 in a mouse model. Emerg Microbes Infect 10:913–928. doi: 10.1080/22221751.2021.1923341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Z, Yuan Y, Chen C, Zhang C, Huang F, Zhou M, Chen H, Fu ZF, Zhao L. 2021. Colloidal manganese salt improves the efficacy of rabies vaccines in mice, cats, and dogs. J Virol 95:e0141421. doi: 10.1128/JVI.01414-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eisenbarth SC. 2019. Dendritic cell subsets in T cell programming: location dictates function. Nat Rev Immunol 19:89–103. doi: 10.1038/s41577-018-0088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cabeza-Cabrerizo M, Cardoso A, Minutti CM, Pereira da Costa M, Reis e Sousa C. 2021. Dendritic Cells Revisited. Annu Rev Immunol 39:131–166. doi: 10.1146/annurev-immunol-061020-053707. [DOI] [PubMed] [Google Scholar]
- 55.Radojevic D, Tomic S, Mihajlovic D, Tolinacki M, Pavlovic B, Vucevic D, Bojic S, Golic N, Colic M, Dokic J. 2021. Fecal microbiota composition associates with the capacity of human peripheral blood monocytes to differentiate into immunogenic dendritic cells in vitro. Gut Microbes 13:1–20. doi: 10.1080/19490976.2021.1921927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Singh N, Thangaraju M, Prasad PD, Martin PM, Lambert NA, Boettger T, Offermanns S, Ganapathy V. 2010. Blockade of dendritic cell development by bacterial fermentation products butyrate and propionate through a transporter (Slc5a8)-dependent inhibition of histone deacetylases. J Biol Chem 285:27601–27608. doi: 10.1074/jbc.M110.102947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang W, Xiao Y, Huang X, Chen F, Sun M, Bilotta AJ, Xu L, Lu Y, Yao S, Zhao Q, Liu Z, Cong Y. 2019. Microbiota metabolite short-chain fatty acids facilitate mucosal adjuvant activity of cholera toxin through GPR43. J Immunol 203:282–292. doi: 10.4049/jimmunol.1801068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim MH, Kang SG, Park JH, Yanagisawa M, Kim CH. 2013. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology 145:396–406. doi: 10.1053/j.gastro.2013.04.056. [DOI] [PubMed] [Google Scholar]
- 59.Coutzac C, Jouniaux JM, Paci A, Schmidt J, Mallardo D, Seck A, Asvatourian V, Cassard L, Saulnier P, Lacroix L, Woerther PL, Vozy A, Naigeon M, Nebot-Bral L, Desbois M, Simeone E, Mateus C, Boselli L, Grivel J, Soularue E, Lepage P, Carbonnel F, Ascierto PA, Robert C, Chaput N. 2020. Systemic short chain fatty acids limit antitumor effect of CTLA-4 blockade in hosts with cancer. Nat Commun 11:2168. doi: 10.1038/s41467-020-16079-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Luu M, Pautz S, Kohl V, Singh R, Romero R, Lucas S, Hofmann J, Raifer H, Vachharajani N, Carrascosa LC, Lamp B, Nist A, Stiewe T, Shaul Y, Adhikary T, Zaiss MM, Lauth M, Steinhoff U, Visekruna A. 2019. The short-chain fatty acid pentanoate suppresses autoimmunity by modulating the metabolic-epigenetic crosstalk in lymphocytes. Nat Commun 10:760. doi: 10.1038/s41467-019-08711-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kespohl M, Vachharajani N, Luu M, Harb H, Pautz S, Wolff S, Sillner N, Walker A, Schmitt-Kopplin P, Boettger T, Renz H, Offermanns S, Steinhoff U, Visekruna A. 2017. The microbial metabolite butyrate induces expression of Th1-associated factors in CD4(+) T cells. Front Immunol 8:1036. doi: 10.3389/fimmu.2017.01036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Park J, Kim M, Kang SG, Jannasch AH, Cooper B, Patterson J, Kim CH. 2015. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol 8:80–93. doi: 10.1038/mi.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kaiko GE, Horvat JC, Beagley KW, Hansbro PM. 2008. Immunological decision-making: how does the immune system decide to mount a helper T-cell response? Immunology 123:326–338. doi: 10.1111/j.1365-2567.2007.02719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wiechers C, Zou M, Galvez E, Beckstette M, Ebel M, Strowig T, Huehn J, Pezoldt J. 2021. The microbiota is dispensable for the early stages of peripheral regulatory T cell induction within mesenteric lymph nodes. Cell Mol Immunol 18:1211–1221. doi: 10.1038/s41423-021-00647-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen D, Wang Y, Manakkat Vijay GK, Fu S, Nash CW, Xu D, He D, Salomonis N, Singh H, Xu H. 2021. Coupled analysis of transcriptome and BCR mutations reveals role of OXPHOS in affinity maturation. Nat Immunol 22:904–913. doi: 10.1038/s41590-021-00936-y. [DOI] [PubMed] [Google Scholar]
- 66.Sander S, Chu VT, Yasuda T, Franklin A, Graf R, Calado DP, Li S, Imami K, Selbach M, Di Virgilio M, Bullinger L, Rajewsky K. 2015. PI3 kinase and FOXO1 transcription factor activity differentially control B cells in the germinal center light and dark zones. Immunity 43:1075–1086. doi: 10.1016/j.immuni.2015.10.021. [DOI] [PubMed] [Google Scholar]
- 67.Kumagai S, Togashi Y, Sakai C, Kawazoe A, Kawazu M, Ueno T, Sato E, Kuwata T, Kinoshita T, Yamamoto M, Nomura S, Tsukamoto T, Mano H, Shitara K, Nishikawa H. 2020. An oncogenic alteration creates a microenvironment that promotes tumor progression by conferring a metabolic advantage to regulatory T cells. Immunity 53:187–203. doi: 10.1016/j.immuni.2020.06.016. [DOI] [PubMed] [Google Scholar]
- 68.Chou WC, Guo Z, Guo H, Chen L, Zhang G, Liang K, Xie L, Tan X, Gibson SA, Rampanelli E, Wang Y, Montgomery SA, Brickey WJ, Deng M, Freeman L, Zhang S, Su MA, Chen X, Wan YY, Ting JP. 2021. AIM2 in regulatory T cells restrains autoimmune diseases. Nature 591:300–305. doi: 10.1038/s41586-021-03231-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sun Z, Yao Y, You M, Liu J, Guo W, Qi Z, Wang Z, Wang F, Yuan W, Yu S. 2021. The kinase PDK1 is critical for promoting T follicular helper cell differentiation. Elife 10. doi: 10.7554/eLife.61406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zeng H, Cohen S, Guy C, Shrestha S, Neale G, Brown SA, Cloer C, Kishton RJ, Gao X, Youngblood B, Do M, Li MO, Locasale JW, Rathmell JC, Chi H. 2016. mTORC1 and mTORC2 kinase signaling and glucose metabolism drive follicular helper T cell differentiation. Immunity 45:540–554. doi: 10.1016/j.immuni.2016.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ye L, Lee J, Xu L, Mohammed AU, Li W, Hale JS, Tan WG, Wu T, Davis CW, Ahmed R, Araki K. 2017. mTOR promotes antiviral humoral immunity by differentially regulating CD4 helper T cell and B cell responses. J Virol 91. doi: 10.1128/JVI.01653-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Foh B, Buhre JS, Lunding HB, Moreno-Fernandez ME, Konig P, Sina C, Divanovic S, Ehlers M. 2022. Microbial metabolite butyrate promotes induction of IL-10+IgM+ plasma cells. PLoS One 17:e0266071. doi: 10.1371/journal.pone.0266071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bonakdar M, Czuba LC, Han G, Zhong G, Luong H, Isoherranen N, Vaishnava S. 2022. Gut commensals expand vitamin A metabolic capacity of the mammalian host. Cell Host Microbe 30:1084–1092. doi: 10.1016/j.chom.2022.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lunken GR, Golding L, Schick A, Majdoubi A, Lavoie PM, Vallance BA. 2022. Gut microbiome and dietary fibre intake strongly associate with IgG function and maturation following SARS-CoV-2 mRNA vaccination. Gut:gutjnl-2022-328556. doi: 10.1136/gutjnl-2022-328556. [DOI] [PubMed] [Google Scholar]
- 75.Blanton JD, Self J, Niezgoda M, Faber ML, Dietzschold B, Rupprecht C. 2007. Oral vaccination of raccoons (Procyon lotor) with genetically modified rabies virus vaccines. Vaccine 25:7296–7300. doi: 10.1016/j.vaccine.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Conzelmann KK, Cox JH, Schneider LG, Thiel HJ. 1990. Molecular cloning and complete nucleotide sequence of the attenuated rabies virus SAD B19. Virology 175:485–499. doi: 10.1016/0042-6822(90)90433-r. [DOI] [PubMed] [Google Scholar]
- 77.Ariyoshi T, Hagihara M, Eguchi S, Fukuda A, Iwasaki K, Oka K, Takahashi M, Yamagishi Y, Mikamo H. 2020. Clostridium butyricum MIYAIRI 588-induced protectin D1 has an anti-inflammatory effect on antibiotic-induced intestinal disorder. Front Microbiol 11:587725. doi: 10.3389/fmicb.2020.587725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tye H, Yu CH, Simms LA, de Zoete MR, Kim ML, Zakrzewski M, Penington JS, Harapas CR, Souza-Fonseca-Guimaraes F, Wockner LF, Preaudet A, Mielke LA, Wilcox SA, Ogura Y, Corr SC, Kanojia K, Kouremenos KA, De Souza DP, McConville MJ, Flavell RA, Gerlic M, Kile BT, Papenfuss AT, Putoczki TL, Radford-Smith GL, Masters SL. 2018. NLRP1 restricts butyrate producing commensals to exacerbate inflammatory bowel disease. Nat Commun 9:3728. doi: 10.1038/s41467-018-06125-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bartosch S, Fite A, Macfarlane GT, McMurdo ME. 2004. Characterization of bacterial communities in feces from healthy elderly volunteers and hospitalized elderly patients by using real-time PCR and effects of antibiotic treatment on the fecal microbiota. Appl Environ Microbiol 70:3575–3581. doi: 10.1128/AEM.70.6.3575-3581.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tomioka S, Seki N, Sugiura Y, Akiyama M, Uchiyama J, Yamaguchi G, Yakabe K, Ejima R, Hattori K, Kimizuka T, Fujimura Y, Sato H, Gondo M, Ozaki S, Honme Y, Suematsu M, Kimura I, Inohara N, Nunez G, Hase K, Kim YG. 2022. Cooperative action of gut-microbiota-accessible carbohydrates improves host metabolic function. Cell Rep 40:111087. doi: 10.1016/j.celrep.2022.111087. [DOI] [PubMed] [Google Scholar]
- 81.Tian D, Luo Z, Zhou M, Li M, Yu L, Wang C, Yuan J, Li F, Tian B, Sui B, Chen H, Fu ZF, Zhao L. 2016. Critical role of K1685 and K1829 in the large protein of rabies virus in viral pathogenicity and immune evasion. J Virol 90:232–244. doi: 10.1128/JVI.02050-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Luo Z, Li Y, Zhou M, Lv L, Wu Q, Chen C, Zhang Y, Sui B, Tu C, Cui M, Chen H, Fu ZF, Zhao L. 2019. Toll-like receptor 7 enhances rabies virus-induced humoral immunity by facilitating the formation of germinal centers. Front Immunol 10:429. doi: 10.3389/fimmu.2019.00429. [DOI] [PMC free article] [PubMed] [Google Scholar]