Abstract
Aquatic eutrophication, often with anthropogenic causes, facilitates blooms of cyanobacteria including cyanotoxin producing species, which profoundly impact aquatic ecosystems and human health. An emerging concern is that aquatic eutrophication may interact with other environmental changes and thereby lead to unexpected cascading effects on terrestrial systems. Here, we synthesize recent evidence showing the possibility that accelerating eutrophication will spill over from aquatic ecosystems to the atmosphere via “air eutrophication”, a novel concept that refers to a process promoting the growth of airborne algae, some of them with the capacity to produce toxic compounds for humans and other organisms. Being catalyzed by various anthropogenic forcings—including aquatic eutrophication, climate warming, air contamination, and artificial light at night—accelerated air eutrophication may be expected in the future, posing a potentially increasing risk of threat to public health and the environment. So far knowledge of this topic is sparse, and we therefore consider air eutrophication a potentially important research field and propose an agenda of cross-discipline research. As a contribution, we have calculated a tolerable daily intake of 17 ng m–3 day–1 for the nasal intake of microcystins by humans.
Keywords: air eutrophication, airborne algae, microcystins, public health
Short abstract
Minimal research has been done on airborne algal toxins. This study reports that “air eutrophication” may be accelerated under the catalyst of anthropogenic forcings, posing a threat to public health and the environment.
Aquatic Eutrophication, Cyanotoxins, and Their Health Impacts
The great acceleration of the Anthropocene leaves severe imprints on our biosphere.1,2 Aquatic eutrophication induced by excessive inputs of nutrients (e.g., nitrogen and phosphorus) is among the most conspicuous of the anthropogenic forcings.3,4 Thus, eutrophication has profoundly impacted aquatic ecosystems and societies and led to, i.a., water quality deterioration, biodiversity loss, and human health problems caused by toxins produced by harmful algal blooms. This has resulted in declines of various ecosystem services,5,6 which compromises the UN’s Sustainable Development Goals. Cyanobacteria can produce a variety of toxins called cyanotoxins, such as microcystin (MC), which are detrimental and even lethal to invertebrates, fish, birds, and mammals7,8 (see more in Chart 1). Microcystin-leucine-arginine (MC-LR) is one of the most toxic and common microcystin variants.9 For humans, oral and dermal contact with water containing high concentrations of cyanobacterial toxins can lead to hepatic dysfunction and other digestive maladies.10 In 1996, contamination of water supplies with cyanotoxins resulted in the death of more than 60 people in the hemodialysis unit in Caruaru, North-East Brazil.11 To protect public health, the World Health Organization has proposed a guideline value of MC-LR below 1 μg L–1 in drinking water.12
Chart 1. Types of Cyanotoxins and Toxicity of Microcystins.
Another additional concern is that eutrophication may interact with other anthropogenic forcings and thereby have led to unexpected cascading environmental changes such as a spillover effect of aquatic eutrophication to terrestrial systems catalyzed by climate change. Promoted by increased frequency and intensity of hot-dry climate extremes, massive growth of cyanobacteria in waters elevates cyanotoxins to extreme levels that may be lethal to terrestrial megafauna. Examples of this are the mass death of African elephants in Botswana in 2020, which was attributed to an amplified effect of eutrophication and harmful cyanobacterial blooms,13 and the death of bald eagles (Haliaeetus leucocephalus) in Lake DeGray, Arkansas, after feeding on fish and waterfowl containing cyanotoxins cascading from cyanobacteria through the food chain.14
Airborne Algae, Algal Toxins, and the Risk of Impacts on Public Health
Algae can be emitted from aquatic ecosystems into the atmosphere by wind-driven or ecosystem disturbances linked to animal movement and human activity15−17 (Figure 1). Once emitted, the algae will be further dragged into the atmosphere by airborne turbulent kinetic energy,18 even into the troposphere.19 Recent studies have shown that 10% of microbes, including algae (0.5–5 μm in size) emitted from the sea, remain in the air 4 days after emission and that they can travel up to 11 000 km.20 Airborne algae have also been detected in indoor environments.21 Inhalation of these small-sized airborne algae is potentially harmful to animals and humans as they are deposited in the respiratory tract.22 A high occurrence frequency of algae was observed in the upper respiratory tract in 92% of 77 study individuals who received a nasal swab and in the central airways in 79% of 29 individuals inspected with bronchoscopy, suggesting that airborne algae can be lodged in the nostrils and lungs after inhalation.23
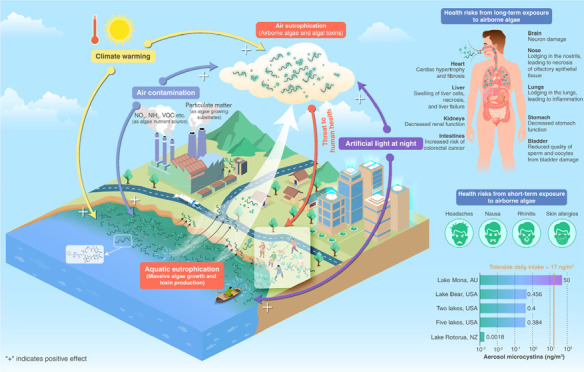
Figure 1.
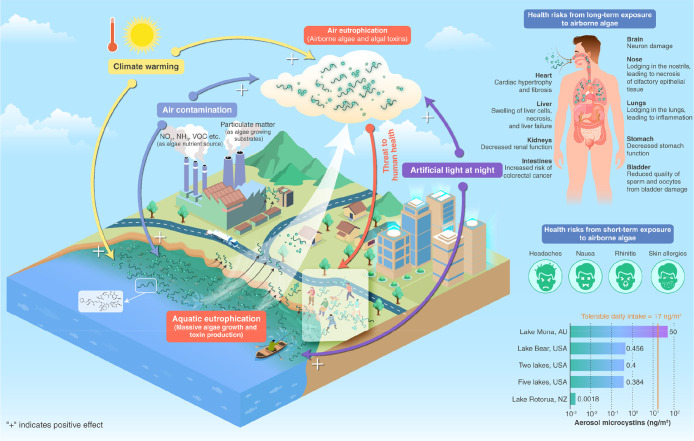
Development of “air eutrophication” and the potential impacts on human health and the maximum concentrations of microcystins measured in aerosols surrounding lakes. Aquatic eutrophication along with excessive nutrient loading promotes massive growth of algae, some of which are toxin-producing cyanobacteria. Aquatic algae can be emitted to the atmosphere where they form airborne assemblages. Air eutrophication develops along with the aquatic eutrophication and thereby provides algae sources, air contamination provides nutrients for airborne algae, and climate warming and artificial light at night stimulate the growth. Airborne algae and cyanotoxins can enter the body through human respiration, posing a potential threat to human health. Maximum concentrations of microcystins measured in aerosols surrounding lakes (see Chart 2 for the derivation of TDI—tolerable daily intake; see refs (25 and 73−77) for the source of measured aerosol microcystins).
Phytoplankton-generated aquatic toxins can be transferred to the air through aerosolization.17 In addition, algal toxins produced by certain airborne algae constitute an important threat to human health.24 In situ measurements in some lakes suggested a range of cyanotoxins between 0.00023 and 50 ng m–3 in aerosols (Figure 1, Table 1). When spraying lake water containing 230 μg L–1 MC into the air in the lab, MC in aerosol samples reached a level as high as 50 ng m–3.25 When female larval fruit flies (Drosophila melanogaster) were exposed to aerosolized cyanobacteria blooms, reduced lifespan and significant signs of cerebral degeneration were observed.26 In mice where MC-LR was administered by inhalation or intratracheally, necrosis of the respiratory epithelium, bleeding in the liver, and damage to kidneys and intestines were observed.27,28 For humans, inhalation of airborne algae and algal toxins may cause skin irritation, allergies, rhinitis, and respiratory problems29 (Figure 1).
Table 1. Collected Studies on Cyanotoxin Concentrations in Aerosolsa.
| cyanotoxin | aquatic microcystin (μg/L) | airborne microcystin (ng/m3) | quantification method | aerosol collection method | source of water studied | study location | reference |
|---|---|---|---|---|---|---|---|
| microcystin | 50 | 0.2 | ELISA | personal sampler | laboratory | laboratory | (74) |
| microcystin | 2.125 | 0.05 | ELISA | high-volume sampler | field | Lake Bear, Michigan | (74, 75) |
| 2.625 | 0.023 | ||||||
| 3 | 0.057 | ||||||
| microcystin | 82.275 | 0.0052 | ELISA | high-volume sampler | field | Bloom Lake 1, California | (76) |
| 82.275 | 0.4 | personal sampler | Bloom Lake 2, California | ||||
| 142.75 | 0.1 | ||||||
| 67.525 | 0.2 | ||||||
| microcystin | 230 | 50 | LC-MS/MS | lake spray aerosol generator | field (Lake Mona, AU) | laboratory | (25) |
| microcystin | 155.987 | 0.0003 | ELISA | high-volume sampler | field | Lake Rotorua, South Island, NZ | (77) |
| 1548.529 | 0.0018 | ||||||
| 447.839 | 0.0009 | ||||||
| nodularin | 4.718 | 0.0073 | Lake Forsyth, South Island, NZ | ||||
| 0.952 | 0.00023 | ||||||
| anatoxin-a | 21 | 0.16 | LC-MS/MS | air sampling device | field | Capaum Pond, Massachusetts | (78) |
Note: Data are average values.
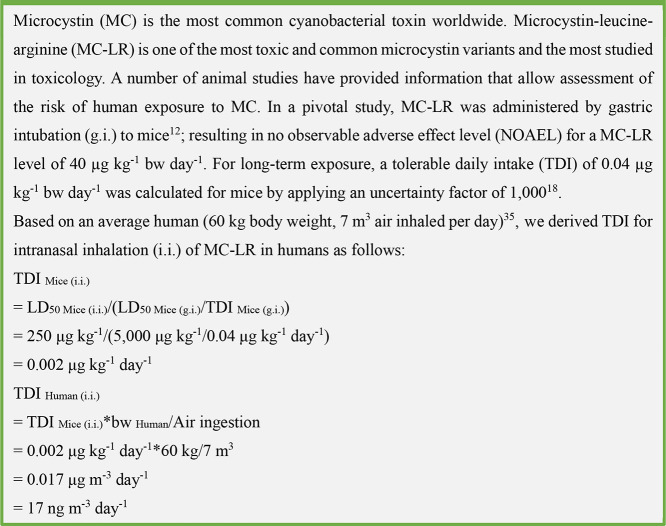
Despite their aquatic origin, most cyanotoxins tended to be more hazardous to terrestrial mammals (the median lethal dose of MC-LR by the intraperitoneal route was i.p. LD50 = 50 μg kg–1 for tested mice) than to aquatic biota (i.p. LD50 = 270–790 μg kg–1 for the tested fish Hypophthalmichthys molitrix and Oreochromnis niloticus).30 Furthermore, toxin inhalation might be more dangerous than oral intake as suggested by the observed median lethal dose of 250 μg kg–1 MC-LR through intratracheal administration to mice compared to 3000 μg kg–1 MC-LR through oral administration.25,31 Inhalation of MC-LR in mice was found to induce necrosis of the respiratory epithelium, which further promoted the absorption of the toxin into the bloodstream.27 Inhalation directly impacts the respiratory system, and inhaled toxins can directly enter the organs. In addition, studies on the immunological effects of vaccines by different routes of administration have revealed that inhaled aerosol vaccines provide better protection and stimulate stronger immune responses than nasal spray vaccines.32 The inhaled aerosol passes through the nasal passage and delivers the vaccine droplets deep into the airways, inducing a broad protective immune response.33 This also reinforces the view that nasal inhalation of aerosolized algae and algal toxins may have more potent toxic effects. To test this, we calculated a tolerable daily intake (TDI) of 17 ng m–3 day–1 for the nasal intake of MC-LR in humans, based on the TDI in mice18 and the average human body weight (60 kg body weight, 7 m3 of air inhaled per day)34 (see Chart 2 for the derivation of TDI).
Chart 2. TDI of MC-LR for Humans.
Multiple Drivers Potentially Accelerating Air Eutrophication
There is growing evidence that algal blooms in eutrophic aquatic ecosystems have increased in diversity, frequency, size, and geographical extent in recent decades,35 implying an enriched source of airborne algae.36,37 Climate warming, with the additional light hours and richer light intensities provided by artificial light at night (ALAN), may directly favor the formation, transportation, and development of airborne algae and increase the potential of toxin production and hence the risk of human exposure to toxins. In the future, humans and wildlife are expected to face an increasing risk of exposure to airborne algae and the cyanotoxins that they produce in cyanobacteria-impacted aquatic ecosystems.
It is widely recognized that eutrophication is accelerating in response to global warming, particularly in recent years.38,39 Widespread, prolonged, and unprecedented extreme heat has occurred in 2022 in the Northern Hemisphere, exceeding the expectations of many climate scientists.40 The preliminary results of a study of airborne algae conducted in the southern coastal zone of the Baltic Sea from January to December 2022 suggest that the total amount of airborne cyanobacteria was positively correlated with the concentration of MC-LR, and the increase of airborne cyanobacteria and airborne algae was mainly related to the rise in temperatures.41 The proportion of toxic species or strains and the release of toxins tend to increase in a hotter climate,42,43 potentially enhancing the risk of airborne algal toxin production. Climate warming may also favor the growth and competition of small-sized freshwater and marine algae species,44,45 which are more likely to be emitted into the atmosphere and carried to distant locations away from their sources than large-sized species.46
Light availability is another key factor controlling the growth of algae in aquatic ecosystems.47 Urbanization has led to rapid expansion of ALAN in space at a rate of 2–6% per year.48−50 With the advent of a wide range of lighting devices, both cool and warm white ALAN has become increasingly abundant.51,52 For freshwater cyanobacteria, warmer white ALAN means a higher photosystem II:photosystem I ratio and hence stronger photosynthesis activities.53 A positive correlation was observed between photosynthetically active radiation and toxin production in Microcystis aeruginosa.54 The proportion of cyanobacteria was found to increase by 17%, while the proportion of diatoms and chrysophytes decreased in spring when exposed to ALAN.55 A study conducted in the southern Baltic Sea region from 2018 to 2020 showed a positive effect of increased light intensity on the growth of the cyanobacterium Nostoc sp. and the diatoms Nitzschia sp., Amphora sp., and Halamphora sp.56 Therefore, the expansion of ALAN may have a positive effect on the development of “air eutrophication” through enhancing the growth of airborne algae: stimulating the growth of some algae and the production of algal toxins in the aquatic ecosystem, hence providing a richer source of algal and toxin emissions to the atmosphere.
Atmospheric deposition of pollutants has received attention as an important nutrient source for phytoplankton in aquatic ecosystems.57 The contribution of airborne particles is high and increasing in various nutrients such as nitrogen, phosphorus, and carbonaceous compounds.58−60 A global increase of 12.8 ± 1.3% of atmospheric ammonia (NH3) was recorded between 2008 and 2018.61 Significant increases in nitrogen dioxide (NO2) and reactive volatile organic compounds have also been widely reported, particularly in tropical cities.62 It has been shown that nitrogen-enriched acid rain and anthropogenic atmospheric nitrogen deposition can enhance marine primary production.63,64 For example, it was found that atmospheric dry deposition of nutrients along the coastal Bay of Bengal resulted in an increase in primary production from 3% to 19%.65 Aerosol deposits from wildfires, metals carried by volcanic lava, and deposition of nitrogen and phosphorus from burning fossil fuels have also been reported to stimulate the growth of aquatic phytoplankton.66,67 But what about the stimulation of the growth of airborne algae? A strong positive correlation was found between aerosol bacterial gene abundance and air pollutant concentrations and the air quality index in a study on the atmosphere of Hefei City, China.68 Whether a similar direct stimulating effect on the development of airborne algae and toxins occurs with increasing nutrient pollution in the air is an open question but important to elucidate given the potential health problems associated with some algae and their toxins in regions with high production of such algae in the water.
In contrast to the widely reported fundamental role of nutrients in the growth of aquatic algae, few studies are available on airborne algae, unfortunately.
Urgent Need for Research into Air Eutrophication and Development of Solutions
Currently, little research has been carried out on airborne algae, their prevalence, and impacts. However, we cannot wait for consensus based on ample evidence when it comes to potentially major threats to public health. Atmospheric bacteria and fungi are an important component of bioaerosols, and their community composition is influenced by a complex set of environmental factors.69 At the same time, they are more abundant than airborne algae and can pose a serious threat to human health and the environment as pathogens and allergens.70,71 As such, atmospheric bacteria and fungi will also play a non-negligible role in promoting air eutrophication. Therefore, we consider “air eutrophication” as a potentially important research field requiring a cross-discipline research effort, including medical, physical, hydrological, geological, and ecological expertise (Chart 3). This research agenda is timely for applying the precautionary principle as a critical risk management strategy for ensuring the safety of public health and the environment worldwide, particularly in the areas with extremely high risk of cyanotoxin exposure and climate changes.72
Chart 3. Research Agenda.
Acknowledgments
We thank Prof. Hans W. Paerl for his editorial contribution and valuable comments and Anne Mette Poulsen for English editions. This research was supported by the Yunnan Provincial Department of Science and Technology (202001BB050078; 202103AC100001). H.W. was supported by the Youth Innovation Association of the Chinese Academy of Sciences as an excellent member (Y201859). E.J. was supported by the TÜBITAK program BIDEB 2232 (project 118C250). Y.G. was supported by the Career Development Fellowship (number APP1163693) and Leader Fellowship (number APP2008813) of the Australian National Health and Medical Research Council. C.X. was supported by the National Natural Science Foundation of China (No. 32061143014) and the Fundamental Research Funds for the Central Universities of China.
Biographies

Prof. Dr. Haijun Wang has been working on aquatic eutrophication and cyanobacteria bloom for 22 years. Before moving to Yunnan University with a Grant of High-level Talented Scientist, he had been working for 20 years at the Institute of Hydrobiology, Chinese Academy of Sciences. He is now the deputy director of Institute for Ecological Research and Pollution Control of Plateau Lakes, School of Ecology and Environmental Sciences, Yunnan University. He was selected as an excellent member of the Youth Innovation Promotion Association of Chinese Academy of Sciences in 2018.

Prof. Dr. Ping Xie is a principal scientist at the Institute of Hydrobiology, Chinese Academy of Sciences, and is the director of Water Subcenter of Chinese Ecosystem Research Network (CERN) and vice director for the State Key Laboratory of Freshwater Ecology and Biotechnology, China, and the dean of Institute for Ecological Research and Pollution Control of Plateau Lakes, School of Ecology and Environmental Sciences, Yunnan University. He has very strong background in aquatic eutrophication, cyanobacteria blooms, and ecological impacts of cyanobacteria and cyanotoxins. Prof. Xie received his Ph.D. from University of Tsukuba, Japan, in 1989. He won an international award, Biwako Prize for Ecology, in 1999. He has published more than 250 SCI-indexed papers as the first or corresponding author. His articles have been cited over 10 000 times with an H index of 50. From 2014 to 2021, he has been on the list of highly cited Chinese scholars in Environmental Science or Biology released by Elsevier.
Author Contributions
Y.F.S., Y.G., and H.W. conceptualized and designed the study. Y.F.S. and H.W. visualized the tables and figures and wrote the original draft manuscript. Y.G., C.X., Y.L., X.Z., Q.L., E.J., H.W., and P.X. reviewed and edited the manuscript. All authors revised the manuscript, approved the final manuscript, and agreed to take responsibility for all aspects of the work.
The authors declare no competing financial interest.
Special Issue
Published as part of the Environmental Science & Technologyvirtual special issue “The Exposome and Human Health”.
References
- Costanza R.; de Groot R.; Sutton P.; van der Ploeg S.; Anderson S. J.; Kubiszewski I.; Farber S.; Turner R. K. Changes in the global value of ecosystem services. Glob. Environ. Change-Human Policy Dimens. 2014, 26, 152–158. 10.1016/j.gloenvcha.2014.04.002. [DOI] [Google Scholar]
- Steffen W.; Broadgate W.; Deutsch L.; Gaffney O.; Ludwig C. The trajectory of the Anthropocene: The Great Acceleration. Anthr. Rev. 2015, 2 (1), 81–98. 10.1177/2053019614564785. [DOI] [Google Scholar]
- Foley J. A.; DeFries R.; Asner G. P.; Barford C.; Bonan G.; Carpenter S. R.; Chapin F. S.; Coe M. T.; Daily G. C.; Gibbs H. K.; Helkowski J. H.; Holloway T.; Howard E. A.; Kucharik C. J.; Monfreda C.; Patz J. A.; Prentice I. C.; Ramankutty N.; Snyder P. K. Global consequences of land use. Science 2005, 309 (5734), 570–574. 10.1126/science.1111772. [DOI] [PubMed] [Google Scholar]
- Paerl H. W.; Gardner W. S.; McCarthy M. J.; Peierls B. L.; Wilhelm S. W. Algal blooms: Noteworthy nitrogen. Science 2014, 346 (6206), 175–175. 10.1126/science.346.6206.175-a. [DOI] [PubMed] [Google Scholar]
- Johnson C. N.; Balmford A.; Brook B. W.; Buettel J. C.; Galetti M.; Guangchun L.; Wilmshurst J. M. Biodiversity losses and conservation responses in the Anthropocene. Science 2017, 356 (6335), 270–275. 10.1126/science.aam9317. [DOI] [PubMed] [Google Scholar]
- Le Moal M.; Gascuel-Odoux C.; Menesguen A.; Souchon Y.; Etrillard C.; Levain A.; Moatar F.; Pannard A.; Souchu P.; Lefebvre A.; Pinay G. Eutrophication: A new wine in an old bottle?. Sci. Total Environ. 2019, 651, 1–11. 10.1016/j.scitotenv.2018.09.139. [DOI] [PubMed] [Google Scholar]
- Codd G. A.; Morrison L. F.; Metcalf J. S. Cyanobacterial toxins: Risk management for health protection. Toxicol. Appl. Pharmacol. 2005, 203 (3), 264–272. 10.1016/j.taap.2004.02.016. [DOI] [PubMed] [Google Scholar]
- Xie P.Microcystins in aquatic animals with potential risk to human health; Scientific Press: Beijing, 2006. [Google Scholar]
- Funari E.; Testai E. Human health risk assessment related to cyanotoxins exposure. Crit. Rev. Toxicol. 2008, 38 (2), 97–125. 10.1080/10408440701749454. [DOI] [PubMed] [Google Scholar]
- Stewart I.; Webb P. M.; Schluter P. J.; Shaw G. R. Recreational and occupational field exposure to freshwater cyanobacteria—A review of anecdotal and case reports, epidemiological studies and the challenges for epidemiologic assessment. Environ. Health 2006, 5 (6), 16563159. 10.1186/1476-069X-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouria S.; de Andrade A.; Barbosa J.; Cavalcanti R. L.; Barreto V. T. S.; Ward C. J.; Preiser W.; Poon G. K.; Neild G. H.; Codd G. A. Fatal microcystin intoxication in haemodialysis unit in Caruaru, Brazil. Lancet 1998, 352 (9121), 21–26. 10.1016/S0140-6736(97)12285-1. [DOI] [PubMed] [Google Scholar]
- Cyanobacterial toxins: Microcystins. Background document for development of WHO guidelines for drinking-water quality and guidelines for safe recreational water environments; World Health Organization: Geneva, 2020. https://apps.who.int/iris/bitstream/handle/10665/338066/WHO-HEP-ECH-WSH-2020.6-eng.pdf.
- Wang H. J.; Xu C.; Liu Y.; Jeppesen E.; Svenning J. C.; Wu J. G.; Zhang W. X.; Zhou T. J.; Wang P. Z.; Nangombe S.; Ma J. G.; Duan H. T.; Fang J. Y.; Xie P. From unusual suspect to serial killer: Cyanotoxins boosted by climate change may jeopardize megafauna. Innovation 2021, 2 (2), 100092. 10.1016/j.xinn.2021.100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breinlinger S.; Phillips T. J.; Haram B. N.; Mares J.; Martinez Yerena J. A.; Hrouzek P.; Sobotka R.; Henderson W. M.; Schmieder P.; Williams S. M.; Lauderdale J. D.; Wilde H. D.; Gerrin W.; Kust A.; Washington J. W.; Wagner C.; Geier B.; Liebeke M.; Enke H.; Niedermeyer T. H. J.; Wilde S. B. Hunting the eagle killer: A cyanobacterial neurotoxin causes vacuolar myelinopathy. Science 2021, 371 (6536), eaax9050 10.1126/science.aax9050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R. M. Jr; Larson D. A.; Bold H. C. Airborne algae: Their abundance and heterogeneity. Science 1964, 143 (3606), 583–585. 10.1126/science.143.3606.583. [DOI] [PubMed] [Google Scholar]
- Burrows S. M.; Hoose C.; Poschl U.; Lawrence M. G. Ice nuclei in marine air: Biogenic particles or dust?. Atmos. Chem. Phys. 2013, 13 (1), 245–267. 10.5194/acp-13-245-2013. [DOI] [Google Scholar]
- Plaas H. E.; Paerl H. W. Toxic cyanobacteria: A growing threat to water and air quality. Environ. Sci. Technol. 2021, 55 (1), 44–64. 10.1021/acs.est.0c06653. [DOI] [PubMed] [Google Scholar]
- Rosas I.; Royocotla G.; Mosino P. Meteorological effects on variation of airborne algae in Mexico. Int. J. Biometeorol. 1989, 33 (3), 173–179. 10.1007/BF01084602. [DOI] [Google Scholar]
- Sassen K.; Arnott W. P.; Starr O. C.; Mace G. G.; Wang Z.; Poellot M. R. Midlatitude cirrus clouds derived from Hurricane Nora: A case study with implications for ice crystal nucleation and shape. J. Atmos. Sci. 2003, 60 (7), 873–891. . [DOI] [Google Scholar]
- Mayol E.; Jimenez M. A.; Herndl G. J.; Duarte C. M.; Arrieta J. M. Resolving the abundance and air- sea fluxes of airborne microorganisms in the North Atlantic Ocean. Front. Microbiol. 2014, 5, 557. 10.3389/fmicb.2014.00557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu W. L.; Tneh S. Y.; Ambu S. A survey of airborne algae and cyanobacteria within the indoor environment of an office building in Kuala Lumpur, Malaysia. Grana 2013, 52 (3), 207–220. 10.1080/00173134.2013.789925. [DOI] [Google Scholar]
- Tesson S. V. M.; Skjoth C. A.; Santl-Temkiv T.; Londahl J. Airborne microalgae: Insights, opportunities, and challenges. Appl. Environ. Microbiol. 2016, 82 (7), 1978–1991. 10.1128/AEM.03333-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facciponte D. N.; Bough M. W.; Seidler D.; Carroll J. L.; Ashare A.; Andrew A. S.; Tsongalis G. J.; Vaickus L. J.; Henegan P. L.; Butt T. H.; Stommel E. W. Identifying aerosolized cyanobacteria in the human respiratory tract: A proposed mechanism for cyanotoxin-associated diseases. Sci. Total Environ. 2018, 645, 1003–1013. 10.1016/j.scitotenv.2018.07.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman K. Seasick lungs - How airborne algal toxins trigger asthma symptoms. Environ. Health Perspect. 2005, 113 (5), A324–A324. 10.1289/ehp.113-a324a. [DOI] [Google Scholar]
- Olson N. E.; Cooke M. E.; Shi J. H.; Birbeck J. A.; Westrick J. A.; Ault A. P. Harmful algal bloom toxins in aerosol generated from inland lake water. Environ. Sci. Technol. 2020, 54 (8), 4769–4780. 10.1021/acs.est.9b07727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J. M.; Liu J. Q.; Zhu Y.; Diaz-Perez Z.; Sheridan M.; Royer H.; Leibensperger R. III; Maizel D.; Brand L.; Popendorf K. J.; Gaston C. J.; Zhai R. G. Exposure to aerosolized algal toxins in south florida increases short- and long-term health risk in Drosophila model of aging. Toxins 2020, 12 (12), 787. 10.3390/toxins12120787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson J. M.; Hutt J. A.; Rein K.; Boggs S. E.; Barr E. B.; Fleming L. E. The toxicity of microcystin LR in mice following 7 days of inhalation exposure. Toxicon 2005, 45 (6), 691–698. 10.1016/j.toxicon.2005.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito E.; Kondo F.; Harada K. I. Intratracheal administration of microcystin-LR, and its distribution. Toxicon 2001, 39 (2–3), 265–271. 10.1016/S0041-0101(00)00124-0. [DOI] [PubMed] [Google Scholar]
- Genitsaris S.; Kormas K. A.; Moustaka-Gouni M. Airborne algae and cyanobacteria: Occurrence and related health effects. Front. Biosci. 2011, 3 (2), 772–787. [DOI] [PubMed] [Google Scholar]
- Shen Q.; Liu Y. D.; Li D. H.; Li S. X. The acute and subacute response of sliver carp and tilapia to microcystin. China Environ. Sci. 2019, 39 (06), 2633–2643. [Google Scholar]
- Fitzgeorge R. B.; Clark S. A.; Keevil C. W.. Routes of Intoxication. Indetection methods for cyanobacterial toxins; The Royal Society of Chemistry: Cambridge, 1994; pp 69–74. [Google Scholar]
- Xu F.; Wu S. P.; Yi L. N.; Peng S. D.; Wang F.; Si W. X.; Hou L. H.; Zhu T. Safety, mucosal and systemic immunopotency of an aerosolized adenovirus-vectored vaccine against SARS-CoV-2 in rhesus macaques. Emerg. Microbes Infect. 2022, 11 (1), 439–442. 10.1080/22221751.2022.2030199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyanathan V.; Afkhami S.; D’Agostino M. R.; Zganiacz A.; Feng X. Y.; Miller M. S.; Jeyanathan M.; Thompson M. R.; Xing Z. Differential biodistribution of adenoviral-vectored vaccine following intranasal and endotracheal deliveries leads to different immune outcomes. Front. Immunol. 2022, 13, 860399. 10.3389/fimmu.2022.860399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumpitsch C.; Koskinen K.; Schopf V.; Moissl-Eichinger C. The microbiome of the upper respiratory tract in health and disease. BMC Biol. 2019, 17 (1), 87. 10.1186/s12915-019-0703-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisman J.; Codd G. A.; Paerl H. W.; Ibelings B. W.; Verspagen J. M. H.; Visser P. M. Cyanobacterial blooms. Nat. Rev. Microbiol. 2018, 16 (8), 471–483. 10.1038/s41579-018-0040-1. [DOI] [PubMed] [Google Scholar]
- Sahu N.; Tangutur A. D. Airborne algae: Overview of the current status and its implications on the environment. Aerobiologia 2015, 31 (1), 89–97. 10.1007/s10453-014-9349-z. [DOI] [Google Scholar]
- May N. W.; Olson N. E.; Panas M.; Axson J. L.; Tirella P. S.; Kirpes R. M.; Craig R. L.; Gunsch M. J.; China S.; Laskin A.; Ault A. P.; Pratt K. A. Aerosol emissions from great lakes harmful algal blooms. Environ. Sci. Technol. 2018, 52 (2), 397–405. 10.1021/acs.est.7b03609. [DOI] [PubMed] [Google Scholar]
- Ho J. C.; Michalak A. M.; Pahlevan N. Widespread global increase in intense lake phytoplankton blooms since the 1980s. Nature 2019, 574 (7780), 667–670. 10.1038/s41586-019-1648-7. [DOI] [PubMed] [Google Scholar]
- Visser P. M.; Verspagen J. M. H.; Sandrini G.; Stal L. J.; Matthijs H. C. P.; Davis T. W.; Paerl H. W.; Huisman J. How rising CO2 and global warming may stimulate harmful cyanobacterial blooms. Harmful Algae 2016, 54, 145–159. 10.1016/j.hal.2015.12.006. [DOI] [PubMed] [Google Scholar]
- Be prepared for more heat. Lancet Planet. Health 2022, 6 (9), e706 10.1016/S2542-5196(22)00201-7. [DOI] [PubMed] [Google Scholar]
- Wisniewska K.; Sliwinska-Wilczewska S.; Savoie M.; Lewandowska A. U. Quantitative and qualitative variability of airborne cyanobacteria and microalgae and their toxins in the coastal zone of the Baltic Sea. Sci. Total Environ. 2022, 826, 154152. 10.1016/j.scitotenv.2022.154152. [DOI] [PubMed] [Google Scholar]
- Mantzouki E.; Lurling M.; Fastner J.; Domis L. d. S.; Wilk-Wozniak E.; Koreiviene J.; Seelen L.; Teurlincx S.; Verstijnen Y.; Krzton W.; Walusiak E.; Karosiene J.; Kasperoviciene J.; Savadova K.; Vitonyte I.; Cillero-Castro C.; Budzynska A.; Goldyn R.; Kozak A.; Rosinska J.; Szelag-Wasielewska E.; Domek P.; Jakubowska-Krepska N.; Kwasizur K.; Messyasz B.; Pelechata A.; Pelechaty M.; Kokocinski M.; Garcia-Murcia A.; Real M.; Romans E.; Noguero-Ribes J.; Duque D. P.; Fernandez-Moran E.; Karakaya N.; Haggqvist K.; Demir N.; Beklioglu M.; Filiz N.; Levi E. E.; Iskin U.; Bezirci G.; Tavsanoglu U. N.; Ozhan K.; Gkelis S.; Panou M.; Fakioglu O.; Avagianos C.; Kaloudis T.; Celik K.; Yilmaz M.; Marce R.; Catalan N.; Bravo A. G.; Buck M.; Colom-Montero W.; Mustonen K.; Pierson D.; Yang Y.; Raposeiro P. M.; Goncalves V.; Antoniou M. G.; Tsiarta N.; McCarthy V.; Perello V. C.; Feldmann T.; Laas A.; Panksep K.; Tuvikene L.; Gagala I.; Mankiewicz-Boczek J.; Yagci M. A.; Cinar S.; Capkin K.; Yagci A.; Cesur M.; Bilgin F.; Bulut C.; Uysal R.; Obertegger U.; Boscaini A.; Flaim G.; Salmaso N.; Cerasino L.; Richardson J.; Visser P. M.; Verspagen J. M. H.; Karan T.; Soylu E. N.; Maraslioglu F.; Napiorkowska-Krzebietke A.; Ochocka A.; Pasztaleniec A.; Antao-Geraldes A. M.; Vasconcelos V.; Morais J.; Vale M.; Koker L.; Akcaalan R.; Albay M.; Maronic D. S.; Stevic F.; Pfeiffer T. Z.; Fonvielle J.; Straile D.; Rothhaupt K. O.; Hansson L. A.; Urrutia-Cordero P.; Blaha L.; Geris R.; Frankova M.; Kocer M. A. T.; Alp M. T.; Remec-Rekar S.; Elersek T.; Triantis T.; Zervou S. K.; Hiskia A.; Haande S.; Skjelbred B.; Madrecka B.; Nemova H.; Drastichova I.; Chomova L.; Edwards C.; Sevindik T. O.; Tunca H.; Onem B.; Aleksovski B.; Krstic S.; Vucelic I. B.; Nawrocka L.; Salmi P.; Machado-Vieira D.; de Oliveira A. G.; Delgado-Martin J.; Garcia D.; Cereijo J. L.; Goma J.; Trapote M. C.; Vegas-Vilarrubia T.; Obrador B.; Grabowska M.; Karpowicz M.; Chmura D.; Ubeda B.; Galvez J. A.; Ozen A.; Christoffersen K. S.; Warming T. P.; Kobos J.; Mazur-Marzec H.; Perez-Martinez C.; Ramos-Rodriguez E.; Arvola L.; Alcaraz-Parraga P.; Toporowska M.; Pawlik-Skowronska B.; Niedzwiecki M.; Peczula W.; Leira M.; Hernandez A.; Moreno-Ostos E.; Blanco J. M.; Rodriguez V.; Montes-Perez J. J.; Palomino R. L.; Rodriguez-Perez E.; Carballeira R.; Camacho A.; Picazo A.; Rochera C.; Santamans A. C.; Ferriol C.; Romo S.; Soria J. M.; Dunalska J.; Sienska J.; Szymanski D.; Kruk M.; Kostrzewska-Szlakowska I.; Jasser I.; Zutinic P.; Udovic M. G.; Plenkovic-Moraj A.; Frak M.; Bankowska-Sobczak A.; Wasilewicz M.; Ozkan K.; Maliaka V.; Kangro K.; Grossart H. P.; Paerl H. W.; Carey C. C.; Ibelings B. W. Temperature effects explain continental scale distribution of cyanobacterial toxins. Toxins 2018, 10 (4), 156. 10.3390/toxins10040156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls J. T.; Wyatt K. H.; Doll J. C.; Rubenstein E. M.; Rober A. R. Hot and toxic: Temperature regulates microcystin release from cyanobacteria. Sci. Total Environ. 2018, 610, 786–795. 10.1016/j.scitotenv.2017.08.149. [DOI] [PubMed] [Google Scholar]
- Zohary T.; Flaim G.; Sommer U. Temperature and the size of freshwater phytoplankton. Hydrobiologia 2021, 848 (1), 143–155. 10.1007/s10750-020-04246-6. [DOI] [Google Scholar]
- Flombaum P.; Gallegos J. L.; Gordillo R. A.; Rincon J.; Zabala L. L.; Jiao N. Z.; Karl D. M.; Li W. K. W.; Lomas M. W.; Veneziano D.; Vera C. S.; Vrugt J. A.; Martiny A. C. Present and future global distributions of the marine cyanobacteria Prochlorococcus and Synechococcus. Proc. Natl. Acad. Sci. U. S. A. 2013, 110 (24), 9824–9829. 10.1073/pnas.1307701110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandowska A. U.; Sliwinska-Wilczewska S.; Wozniczka D. Identification of cyanobacteria and microalgae in aerosols of various sizes in the air over the Southern Baltic Sea. Mar. Pollut. Bull. 2017, 125 (1–2), 30–38. 10.1016/j.marpolbul.2017.07.064. [DOI] [PubMed] [Google Scholar]
- Edwards K. F.; Thomas M. K.; Klausmeier C. A.; Litchman E. Light and growth in marine phytoplankton: Allometric, taxonomic, and environmental variation. Limnol. Oceanogr. 2015, 60 (2), 540–552. 10.1002/lno.10033. [DOI] [Google Scholar]
- Holker F.; Moss T.; Griefahn B.; Kloas W.; Voigt C. C.; Henckel D.; Hanel A.; Kappeler P. M.; Volker S.; Schwope A.; Franke S.; Uhrlandt D.; Fischer J.; Klenke R.; Wolter C.; Tockner K. The dark side of light: A transdisciplinary research agenda for light pollution policy. Ecol. Soc. 2010, 15 (4), 13. 10.5751/ES-03685-150413. [DOI] [Google Scholar]
- Kyba C. C. M.; Kuester T.; de Miguel A. S.; Baugh K.; Jechow A.; Holker F.; Bennie J.; Elvidge C. D.; Gaston K. J.; Guanter L. Artificially lit surface of Earth at night increasing in radiance and extent. Sci. Adv. 2017, 3 (11), e1701528 10.1126/sciadv.1701528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segrestin J.; Mondy N.; Boisselet C.; Guigard L.; Lengagne T.; Poussineau S.; Secondi J.; Puijalon S. Effects of artificial light at night on the leaf functional traits of freshwater plants. Freshw. Biol. 2021, 66 (12), 2264–2271. 10.1111/fwb.13830. [DOI] [Google Scholar]
- Gaston K. J.; Davies T. W.; Bennie J.; Hopkins J. REVIEW: Reducing the ecological consequences of night-time light pollution: Options and developments. J. Appl. Ecol. 2012, 49 (6), 1256–1266. 10.1111/j.1365-2664.2012.02212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamantopoulou C.; Christoforou E.; Dominoni D. M.; Kaiserli E.; Czyzewski J.; Mirzai N.; Spatharis S. Wavelength-dependent effects of artificial light at night on phytoplankton growth and community structure. Proc. R. Soc. B-Biol. Sci. 2021, 288 (1953), 20210525. 10.1098/rspb.2021.0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin C.; Bruyant F.; Laprise M. H.; Cockshutt A. M.; Marie-Rose Vandenhecke J. M. R.; Huot Y. The impact of light pollution on diel changes in the photophysiology of Microcystis aeruginosa. J. Plankton Res. 2014, 36 (1), 286–291. 10.1093/plankt/fbt088. [DOI] [Google Scholar]
- Wiedner C.; Visser P. M.; Fastner J.; Metcalf J. S.; Codd G. A.; Mur L. R. Effects of light on the microcystin content of Microcystis strain PCC 7806. Appl. Environ. Microbiol. 2003, 69 (3), 1475–1481. 10.1128/AEM.69.3.1475-1481.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubisic M.; Singer G.; Bruno M. C.; van Grunsven R. H. A.; Manfrin A.; Monaghan M. T.; Holker F. A pigment composition analysis reveals community changes in pre-established stream periphyton under low-level artificial light at night. Limnologica 2018, 69, 55–58. 10.1016/j.limno.2017.10.004. [DOI] [Google Scholar]
- Wisniewska K.; Sliwinska-Wilczewska S.; Lewandowska A.; Konik M. The effect of abiotic factors on abundance and photosynthetic performance of airborne cyanobacteria and microalgae isolated from the southern Baltic Sea region. Cells 2021, 10 (1), 103. 10.3390/cells10010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav K.; Sarma V. V. S. S.; Rao D. B.; Kumar M. D. Influence of atmospheric dry deposition of inorganic nutrients on phytoplankton biomass in the coastal Bay of Bengal. Mar. Chem. 2016, 187, 25–34. 10.1016/j.marchem.2016.10.004. [DOI] [Google Scholar]
- Chen H. Y.; Huang L. M.; Ho T. Y.; Chiang K. P.; Chou W. C. A study of the nitrogen and phosphorus imbalance in East Asia based on the distribution patterns of and stoichiometric variation in global atmospheric nitrogen and phosphorus. Atmos. Environ. 2021, 266, 118691. 10.1016/j.atmosenv.2021.118691. [DOI] [Google Scholar]
- Decina S. M.; Templer P. H.; Hutyra L. R. Atmospheric inputs of nitrogen, carbon, and phosphorus across an urban area: Unaccounted fluxes and canopy influences. Earths Future 2018, 6 (2), 134–148. 10.1002/2017EF000653. [DOI] [Google Scholar]
- McDuffie E. E.; Martin R. V.; Spadaro J. V.; Burnett R.; Smith S. J.; O’Rourke P.; Hammer M. S.; van Donkelaar A.; Bindle L.; Shah V.; Jaegle L.; Luo G.; Yu F. Q.; Adeniran J. A.; Lin J. T.; Brauer M. Source sector and fuel contributions to ambient PM2.5 and attributable mortality across multiple spatial scales. Nat. Commun. 2021, 12 (1), 3594. 10.1038/s41467-021-23853-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme M.; Clarisse L.; Franco B.; Sutton M. A.; Erisman J. W.; Wichink Kruit R.; van Zanten M.; Whitburn S.; Hadji-Lazaro J.; Hurtmans D.; Clerbaux C.; Coheur P. F. Global, regional and national trends of atmospheric ammonia derived from a decadal (2008–2018) satellite record. Environ. Res. Lett. 2021, 16 (5), 055017. 10.1088/1748-9326/abd5e0. [DOI] [Google Scholar]
- Vohra K.; Marais E. A.; Bloss W. J.; Schwartz J.; Mickley L. J.; Van Damme M.; Clarisse L.; Coheur P. F. Rapid rise in premature mortality due to anthropogenic air pollution in fast-growing tropical cities from 2005 to 2018. Sci. Adv. 2022, 8 (14), eabm4435 10.1126/sciadv.abm4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paerl H. W.; Whitall D. R. Anthropogenically-derived atmospheric nitrogen deposition, marine eutrophication and harmful algal bloom expansion: Is there a link?. Ambio 1999, 28 (4), 307–311. [Google Scholar]
- Paerl H. W. Enhancement of marine primary production by nitrogen-enriched acid rain. Nature 1985, 315 (6022), 747–749. 10.1038/315747a0. [DOI] [Google Scholar]
- Kumari V. R.; Neeraja B.; Rao D. N.; Ghosh V. R. D.; Rajula G. R.; Sarma V. V. S. S. Impact of atmospheric dry deposition of nutrients on phytoplankton pigment composition and primary production in the coastal Bay of Bengal. Environ. Sci. Pollut. Res. 2022, 29 (54), 82218–82231. 10.1007/s11356-022-21477-3. [DOI] [PubMed] [Google Scholar]
- Tang W. Y.; Llort J.; Weis J.; Perron M. M. G.; Basart S.; Li Z. C.; Sathyendranath S.; Jackson T.; Rodriguez E. S.; Proemse B. C.; Bowie A. R.; Schallenberg C.; Strutton P. G.; Matear R.; Cassar N. Widespread phytoplankton blooms triggered by 2019–2020 Australian wildfires. Nature 2021, 597 (7876), 370–375. 10.1038/s41586-021-03805-8. [DOI] [PubMed] [Google Scholar]
- Wilson S. T.; Hawco N. J.; Armbrust E. V.; Barone B.; Bjorkman K. M.; Boysen A. K.; Burgos M.; Burrell T. J.; Casey J. R.; DeLong E. F.; Dugenne M.; Dutkiewicz S.; Dyhrman S. T.; Ferron S.; Follows M. J.; Foreman R. K.; Funkey C. P.; Harke M. J.; Henke B. A.; Hill C. N.; Hynes A. M.; Ingalls A. E.; Jahn O.; Kelly R. L.; Knapp A. N.; Letelier R. M.; Ribalet F.; Shimabukuro E. M.; Tabata R. K. S.; Turk-Kubo K. A.; White A. E.; Zehr J. P.; John S.; Karl D. M. Kilauea lava fuels phytoplankton bloom in the North Pacific Ocean. Science 2019, 365 (6457), 1040–1044. 10.1126/science.aax4767. [DOI] [PubMed] [Google Scholar]
- Jiang S. Y.; Sun B. W.; Zhu R. B.; Che C. S.; Ma D. W.; Wang R. F.; Dai H. T. Airborne microbial community structure and potential pathogen identification across the PM size fractions and seasons in the urban atmosphere. Sci. Total Environ. 2022, 831, 154665. 10.1016/j.scitotenv.2022.154665. [DOI] [PubMed] [Google Scholar]
- Bai W. Y.; Li Y. P.; Xie W. W.; Ma T. F.; Hou J. L.; Zeng X. L. Vertical variations in the concentration and community structure of airborne microbes in PM2.5. Sci. Total Environ. 2021, 760, 143396. 10.1016/j.scitotenv.2020.143396. [DOI] [PubMed] [Google Scholar]
- Wisniewska K.; Lewandowska A. U.; Sliwinska-Wilczewska S. The importance of cyanobacteria and microalgae present in aerosols to human health and the environment - Review study. Environ. Int. 2019, 131, 104964. 10.1016/j.envint.2019.104964. [DOI] [PubMed] [Google Scholar]
- Zhai Y. B.; Li X.; Wang T. F.; Wang B.; Li C. T.; Zeng G. M. A review on airborne microorganisms in particulate matters: Composition, characteristics and influence factors. Environ. Int. 2018, 113, 74–90. 10.1016/j.envint.2018.01.007. [DOI] [PubMed] [Google Scholar]
- Zhao X.; Liu Y.; Guo Y. M.; Xu C.; Chen L.; Codd G. A.; Chen J.; Wang Y.; Wang P. Z.; Yang L. W.; Zhou L.; Li Y.; Xiao S. M.; Wang H. J.; Paerl H. W.; Jeppesen E.; Xie P. Meta-analysis reveals cyanotoxins risk across African inland water. J. Hazard. Mater. 2023, 451, 131160. 10.1016/j.jhazmat.2023.131160. [DOI] [PubMed] [Google Scholar]
- Murby A. L.; Haney J. F. Field and laboratory methods to monitor lake aerosols for cyanobacteria and microcystins. Aerobiologia 2016, 32 (3), 395–403. 10.1007/s10453-015-9409-z. [DOI] [Google Scholar]
- Cheng Y. S.; Yue Z.; Irvin C. M.; Kirkpatrick B.; Backer L. C. Characterization of aerosols containing microcystin. Mar. Drugs 2007, 5 (4), 136–150. 10.3390/md504136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backer L. C.; Carmichael W.; Kirkpatrick B.; Williams C.; Irvin M.; Zhou Y.; Johnson T. B.; Nierenberg K.; Hill V. R.; Kieszak S. M.; Cheng Y. S. Recreational exposure to low concentrations of microcystins during an algal bloom in a small lake. Mar. Drugs 2008, 6 (2), 389–406. 10.3390/md6020389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backer L. C.; McNeel S. V.; Barber T.; Kirkpatrick B.; Williams C.; Irvin M.; Zhou Y.; Johnson T. B.; Nierenberg K.; Aubel M.; LePrell R.; Chapman A.; Foss A.; Corum S.; Hill V. R.; Kieszak S. M.; Cheng Y. S. Recreational exposure to microcystins during algal blooms in two California lakes. Toxicon 2010, 55 (5), 909–921. 10.1016/j.toxicon.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Wood S. A.; Dietrich D. R. Quantitative assessment of aerosolized cyanobacterial toxins at two New Zealand lakes. J. Environ. Monit. 2011, 13 (6), 1617–1624. 10.1039/c1em10102a. [DOI] [PubMed] [Google Scholar]
- Sutherland J. W.; Turcotte R. J.; Molden E.; Moriarty V.; Kelly M.; Aubel M.; Foss A. The detection of airborne anatoxin-a (ATX) on glass fiber filters during a harmful algal bloom. Lake Reserv. Manag. 2021, 37 (2), 113–119. 10.1080/10402381.2021.1881191. [DOI] [Google Scholar]