Abstract
Objectives
High altitude exposure decreases the incidence of obesity and metabolic syndrome, but increases the expression of the thermogenic adipokines (leptin, fat cell fatty acid-binding protein (A-FABP) and visfatin). This study investigated the correlation of these adipokines with obesity and metabolic syndrome (MetS) in populations residing in a plateau-specific environment.
Design
Case–control study.
Setting
We cross-sectionally analysed data from the China Multi-Ethnic Cohort.
Participants
A total of 475 obese (OB, body mass index (BMI)≥28.0 kg/m2) plateau Han people and 475 age, sex and region-matched non-obese (NO, 18.5≤BMI<24.0 kg/m2) subjects were recruited. MetS was defined according to the National Cholesterol Education Program Adult Treatment Panel III guidelines.
Primary and secondary outcome measures
Data with normal distributions were expressed as the mean (Stanard Deviation, SD), and data with skewed distributions were expressed as the median (Interquartile Range, IQR). The participants were grouped and the rank-sum test, χ2 test or t-tests was used for comparing groups. Spearman correlation coefficients were estimated to assess the relationships among leptin, A-FABP, visfatin and the components of MetS in each group.
Results
A-FABP was an independent predictor of OB (OR, 1.207; 95% CI, 1.170 to 1.245; p<0.05), ABSI (OR, 1.035; 95%CI, 1.019 to 1.052; p<0.05) and MetS (OR, 1.035; 95% CI, 1.013 to 1.057; p<0.05). Leptin was an independent predictor of MetS in the NO group. Visfatin was an independent predictor of increased ABSI, but not for OB or MetS.
Conclusion
An abnormally elevated plasma A-FABP level, but not leptin or visfatin is a potential risk factor for MetS in high-altitude populations.
Keywords: nutrition, nutritional support, epidemiology
Strengths and limitations of this study.
This study is based on a China Multi-Ethnic Cohort study in the southwest, and focuses on a case–control epidemiological study in the plateau region, making up for the lack of research on obesity and metabolic syndrome in the plateau region.
This study was cross-sectional and cannot be translated into definitive causal inferences.
Our study population included only subjects living at high altitudes, and comparisons with subjects at lower altitudes are lacking. Therefore, it is unclear whether the correlations are changed in a high-altitude environment, and, if so, the degree of change remains unclear.
The non-obese subjects in this study were matched with the OB subjects, which may not fully reflect the prevalence and risk factors of MetS in the NO group.
Introduction
Obesity (OB) is traditionally defined as a body mass index (BMI)≥28.0 kg/m2 in China1 and, metabolic syndrome (MetS) is a complex pathological group of metabolic disorders relating to protein, fat and carbohydrates. OB and MetS are interconnected, heterogeneous diseases that represent the leading causes of non-communicable deaths worldwide.2 3It is estimated that more than 50% of the global deaths are related to OB and MetS, with an increasing contribution from low-income and middle-income countries.4 The prevalence and incidence of OB and MetS are unevenly distributed between and within countries because of variations in environment, ethnicity and lifestyle.5
China has been confronting a growing burden of OB and MetS, with strong regional variation.6 Most primary data on the prevalence and risk factors for OB and MetS come from Central and Eastern China,7 with geography, ethnicity and lifestyle factors that vary significantly compared with those of Western China. Geographical environments and climates in particular, are increasingly seen as important risk factors for OB and MetS worldwide.8 The Han is the ethnic majority group in China who mainly live in the central and eastern regions, with a low altitude and warm climate. Some have migrated to the western regions, which have higher altitudes and colder climates. High-altitude/cold populations have different outcomes for OB and MetS compared with low-altitude/warm populations.9 However, little is known about the geographical variation in the prevalence, incidence and/or risk factors for OB and MetS of Han people in China. This study was launched based on the China Multi-Ethnic Cohort (CMEC) to address the urgent need for data on the risk factors for OB and MetS across various geographical environments and climates.10
Obesity is an important risk factor for MetS. In addition, there are significant differences in the risk of MetS among individuals with similar BMI. While some obese individuals may have normal metabolism, some non-obese (NO) individuals may have metabolism disorders. Since BMI alone cannot consistently predict the risk of MetS, researchers focus on the changes of endocrine function of adipose tissue to understand the pathogenesis of MetS in OB and NO individuals, and search for better adipose factors to predict the risk of MetS.11 12 In the components of MetS, WC is used as one of the judgment criteria. Some scholars13 have pointed out that both WC and BMI have certain deficiencies in judging the adverse outcomes of metabolic syndrome, and abdominal obesity has a better correlation with the adverse outcomes of MetS. Therefore, a body shape index (ABSI) was proposed to reflect the degree of abdominal obesity and the adverse outcomes of MetS. Therefore, we will also discuss the relationship between adipokines and ABSI from multiple perspectives, with the aim of finding stable adipokines for predicting MetS.14
Adipokines are secreted by adipose tissue and are involved in regulating the energy metabolism. Thermogenic adipokines are known as adipokines which regulate energy consumption through promoting non-shivering heat production.15 Leptin, an adipokine secreted by white adipose tissue, regulates diet, glucose and lipid metabolism through the central nervous system.16 Fat cell fatty acid-binding protein (A-FABP) is mainly expressed by adipocytes and macrophages and is involved in lipid regulation and metabolism.17 Visfatin is mainly secreted by visceral adipose tissue, can promote the transformation of preadipocytes to adipocytes and has an insulin-like function.18 Leptin, A-FABP and visfatin are thermogenic adipokines, and are suspected modifiable risk factors in OB and MetS.19 20 The plateau natural environment, characterised by hypoxia and cold, is an important activation method for adipose tissue thermogenesis. Many studies have confirmed that adipose tissue thermogenesis reduces OB and MetS, while simultaneously increasing the expression of leptin, A-FABP and visfatin.21 22 However, whether the associations among plasma leptin, A-FABP and visfatin levels and OB/MetS are affected by adipose tissue thermogenesis in the high-altitude region remains unclear. Therefore we studied this correlation in the plateau Han population and provided a theoretical basis for the prevention and control of OB and MetS in the plateau population.
Methods
Participants
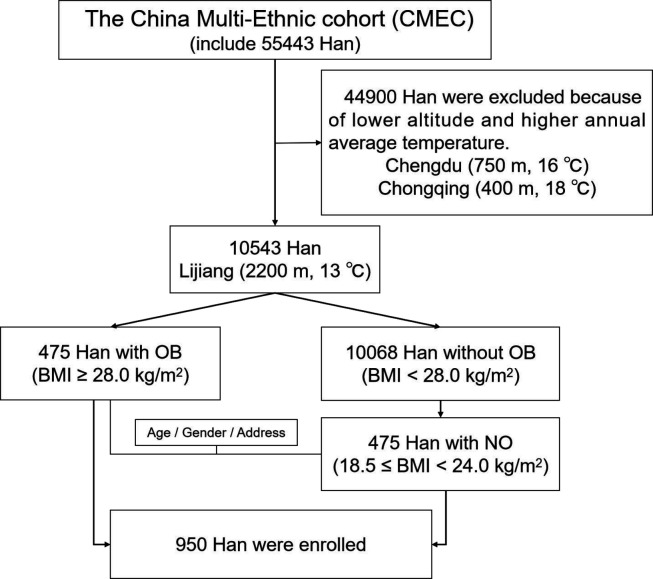
As figure 1 shows, the study subjects were from the China Multi-Ethnic Cohort (CMEC), the inclusion and the exclusion criteria of which have been described previously.10 Overall, 55 443 Han ethnic people were enrolled in the cohort. The study received ethical approval from the Sichuan University Medical Ethical Review Board (K2016038) and written informed consent was provided by all participants. A total of 10 543 Han ethnic people living in the plateau area (average altitude 2200 m, annual average temperature 13°C) were enrolled in the study. Of these, 475 Han ethnic people with obesity were assigned to the OB group. A further 475 Han ethnic people with standard BMI matched by age, sex and region were recruited and assigned to the NO group. The exclusion criteria as follows: pregnancy, lactation, having any recent surgery such as bariatric, or taking any medications and supplements effective on weight and variables studied such as slimming drugs, hypoglycaemic drugs, lipid-lowering drugs within 12 hours.
Figure 1.
Inclusion process of the enrolled subjects. BMI, body mass index; OB, obese; No, non-obese
Data collection
The major content of our questionnaire included clinical characteristics, medical history, habitual diet and socio-demographic details. The questionnaire information was collected using a tablet computer by a face-to-face interview implemented by well-trained interviewers. The data quality was assessed by listening to the audio records.
Physical activity was assessed using the MESA Typical Week Physical Activity Survey (MESA-TWPAS).23 According to the mode and duration of physical activity in the questionnaire, calculated the individual metabolic equivalent (METs) and the daily physical activity level (Kcal/day): METs×0.0175×time (min/day)×weight (kg).
A standardised physical examination, including anthropometry and blood pressure (BP) measurement, was conducted for all participants. After the participant had rested in a seated position for 10 min, a physician measured the participant’s BP using a standardised protocol with an OMRON (model: U30) BP monitor and an appropriately sized cuff on the participant’s right arm. Two other BP readings were taken at 1 min intervals, and the average of three readings was reported as the examination BP. The height, weight and waist and hip circumference of the participants were also measured using standardised protocols with an ultrasonic body fat analyzer (model: EF08) and a soft measuring tape. Attendees underwent laboratory assessments of other risk factors in the fasting state. Phlebotomy was performed after the participants had rested in the seated position for 10 min.
Participants provided venous blood samples after overnight fasting (at least 8 hours). All blood samples were collected and stored at -80°C without freeze-thaw cycles until required for use in the assay. Plasma high-density lipoprotein cholesterol (HDL-C), triglycerides (TG) and fasting plasma glucose (FPG) was measured using standardised assays by a biochemical autoanalyzer (cobas 8000 Biochemical Autoanalyzer, Roche, Germany). According to the manufacturer instructions, plasma levels of leptin were measured using a sandwich ELISA (R&D Systems, model: DLP00; USA); plasma levels of A-FABP were measured using a sandwich ELISA (R&D Systems, model: DLP00; P215509; USA); plasma levels of visfatin were measured using a competitive ELISA (RayBiotech Systems, model: EIA-VIS; 120718 702; USA).
Outcome definition
BMI was defined as: weight/height2; obesity (OB) was defined as BMI≥28.0 kg/m2; and non-obese (NO) was defined as 18.5≤BMI<24.0 kg/m2.24
Using the National Cholesterol Education Program Adult Treatment Panel III guidelines,25 MetS was defined as the presence of ≥3 of the following: (1) increased waist circumference (WC): WC≥90 cm for men and ≥80 cm for women; (2) elevated BP: systolic blood pressure ≥130 mmHg or diastolic blood pressure ≥85 mmHg or treatment for hypertension; (3) elevated TG: TG≥1.7 mmol/L or treatment for hypertriglyceridaemia; (4) low HDL-C: HDL-C<1.03 mmol/L in men and <1.29 mmol/L in women or using lipid-lowering treatment; (5) elevated FPG: FPG≥5.6 mmol/L or treatment with oral hypoglycaemic agents or insulin.
A body shape index (ABSI) was defined as: WC/(BMI2/3 × height1/2).26 27 Increased ABSI: ABSI ≥ 0.080 for both sexes.28
Participants were grouped according to BMI and MetS or one component of MetS.
Statistical analysis
Analyses were performed using SPSS 20.0 statistical software (SPSS Inc., Chicago, USA). Data with normal distributions were expressed as the mean (Standard Deviatioon, SD), and data with skewed distributions were expressed as the median (Interquartile Range, IQR). The participants were grouped and the rank-sum test, χ2 test or t-tests was used for comparing groups. Spearman correlation coefficients were estimated to assess the relationships among leptin, A-FABP, visfatin and the components of MetS in each group. Conditional multiple logistic regression analysis (for OB) and stepwise multiple logistic regression analysis (for MetS, the components of MetS and increased ABSI) were used to examine the association (OR) of A-FABP, visfatin with OB, MetS, the components of MetS and increased ABSI. The multivariable model 1 was adjusted for age, sex, higher education, smoking, alcohol consumption and physical activity levels. Model 2 was adjusted for model 1 plus BMI. For all analyses, p-values<0.05 were considered statistically significant.
Patient and public involvement
Participants in this study gave informed consent for participation. However, the participant and public were not involved in the design or conduct of the study.
Results
Baseline characteristics
In this study, 950 Han residents were surveyed, including 320 men (33.7%) and 630 women (66.3%). The median (IQR) age of the participants was 51 (46 to 61) years. The baseline plasma leptin, A-FABP and visfatin levels and basic clinical characteristics of the participants are shown according to the case–control group in table 1. The plasma HDL-C levels were markedly lower in the OB group than in the NO group. The WC, HC, WHR, BP, TC, TG, LDL-C, FPG, ABSI and glycated haemoglobin levels were markedly higher in the OB group. Compared with the NO group, the OB participants generally had higher rates of MetS and a proportion of metabolic derangements. Among participants in the NO group, 12.0% had MetS, 11.2% showed increased WC, 34.7% showed elevated BP, 33.9% showed elevated TG, 18.3% showed low HDL-C, 11.4% showed elevated FPG and 24.4% showed increased ABSI. In the OB group, the rate of MetS was 69.3%, 91.8% showed increased WC, 65.9% showed elevated BP, 72.0% showed elevated TG, 50.5% performed low HDL-C, 25.1% showed elevated FPG and 30.3% showed increased ABSI. The plasma leptin, A-FABP and visfatin levels were higher in the OB group than in the NO group. The median (IQR) BMI of residents living in 2140 m (n=884) was 26.0 (21.4, 29.2) kg/m², and that of residents living in 2250 m (n=66) was 25.8 (21.7, 29.4) kg/m², there is no significant difference between the two (Z=−1.244, p=0.213). The median (interquartile range) ABSI of residents living in 2140 m was 0.077 (0.074, 0.081), was markedly higher than living in 2250 m (Z=−2.140, p=0.032).
Table 1.
Baseline characteristics of the participants
| Clinical features | NO (n=475) | OB (n=475) | P value |
| High education lever, n (%) | 155 (32.6) | 163 (34.3) | 0.254 |
| Smoking, n (%) | 114 (24.0) | 93 (19.6) | 0.192 |
| Alcohol use, n (%) | 72 (15.2) | 56 (11.8) | 0.422 |
| Physical activity level, Kcal/day | 784.7 (142.9, 1382.4) | 1369.5 (669.5, 2127.7) | <0.001 |
| WC, cm | 74.0 (70.0, 79.0) | 93.0 (89.0, 98.0) | <0.001 |
| HC, cm | 87.5 (84.5, 90.0) | 100.0 (97.0, 103.0) | <0.001 |
| WHR | 0.9 (0.8, 0.9) | 0.9 (0.9, 1.0) | <0.001 |
| Weight, kg | 53.0 (49.5, 58.3) | 73.7 (68.6, 78.8) | <0.001 |
| BMI, kg/m² | 21.4 (20.2, 22.8) | 29.3 (28.5, 30.4) | <0.001 |
| ABSI |
0.08 (0.07, 0.08) | 0.08 (0.07, 0.08) | <0.001 |
| SBP, mm Hg | 117.3 (107.0, 130.3) | 129.3 (117.7, 142.3) | <0.001 |
| DBP, mm Hg | 76.7 (69.7, 84.7) | 83.3 (76.3, 91.7) | <0.001 |
| Plasma TC, mmol/L | 4.9 (4.3, 5.6) | 5.1 (4.5, 5.8) | <0.001 |
| Plasma TG, mmol/L | 1.4 (1.0, 1.9) | 2.3 (1.6, 3.3) | <0.001 |
| Plasma HDL-C, mmol/L | 1.5 (0.4) | 1.2 (0.3) | <0.001 |
| Plasma LDL-C, mmol/L | 2.9 (0.8) | 3.1 (0.9) | 0.004 |
| FPG, mmol/L | 4.9 (4.6, 5.2) | 5.1 (4.7, 5.6) | <0.001 |
| Glycated haemoglobin, g/L | 5.6 (5.4, 6.0) | 5.9 (5.5, 6.2) | <0.001 |
| Increased WC, n (%) | 53 (11.2) | 436 (91.8) | <0.001 |
| Elevated BP, n (%) | 165 (34.7) | 313 (65.9) | <0.001 |
| Elevated TG, n (%) | 161 (33.9) | 342 (72.0) | <0.001 |
| Low HDL-C, n (%) | 87 (18.3) | 240 (50.5) | <0.001 |
| Elevated FPG, n (%) | 54 (11.4) | 119 (25.1) | <0.001 |
| MetS, n (%) | 57 (12.0) | 329 (69.3) | <0.001 |
| Leptin, ng/mL | 3.6 (1.8, 6.3) | 12.0 (7.2, 17.3) | <0.001 |
| A-FABP, ng/mL | 11.1 (8.3, 14.8) | 18.1 (14.0, 23.4) | <0.001 |
| Visfatin, ng/mL | 16.2 (13.1, 19.2) | 16.7 (13.7, 20.4) | 0.025 |
Total n=950. OB: Obesity (BMI≥28.0 kg/m2), NO: Non-obese (18.5≤BMI<24.0 kg/m2), high education lever: Junior high school or above; Alcohol use: Alcohol use, monthly and above, WC: Waist circumference, HC: Hip circumference, WHR: Waist to hip ratio, BMI: Body mass index, ABSI: A body shape index, SBP: Systolic blood pressure, DBP: Diastolic blood pressure, TC: Total cholesterol, TG: Triglycerides, HDL-C: High-density lipoprotein, LDL-C: Low-density lipoprotein, FPG: Fasting plasma glucose, Increased WC: WC ≥ 0.900 m for men and ≥ 0.800 m for women, Elevated BP: SBP ≥ 130 mmHg or DBP ≥ 85 mmHg or treatment for hypertension, Elevated TG: TG ≥ 1.7 mmol/L or treatment for hypertriglyceridemia, Low HDL-C: HDL-C < 1.03 mmol/L in men and < 1.29 mmol/L in women or using lipid-lowering treatment, Elevated FPG: FPG ≥ 5.6 mmol/L or treatment with oral hypoglycemic agents or insulin, Increased ABSI: ABSI ≥ 0.080 for both sexes, MetS: Metabolic syndrome, A-FABP: Fat cell fatty acid-binding protein. Data with normal distributions are expressed as the mean (standard deviation); data with skewed distributions are expressed as the median (interquartile range); the enumeration data is expressed as n (%). Measurement data were tested with t-test or rank sum test; enumeration data were tested with chi-square test. p < 0.05, the difference was statistically significant.
ABSI, a body shape index; A-FABP, fat cell fatty acid-binding protein; BMI, body mass index; DBP, diastolic blood pressure; FPG, fasting plasma glucose; HC, hip circumference; HDL-C, high-density lipoprotein; LDL-C, low-density lipoprotein; MetS, metabolic syndrome; SBP, systolic blood pressure; TC, total cholesterol; TG, triglycerides; WC, waist circumference; WHR, waist to hip ratio.
Group differences in plasma thermogenic adipokines levels and their correlation with MetS
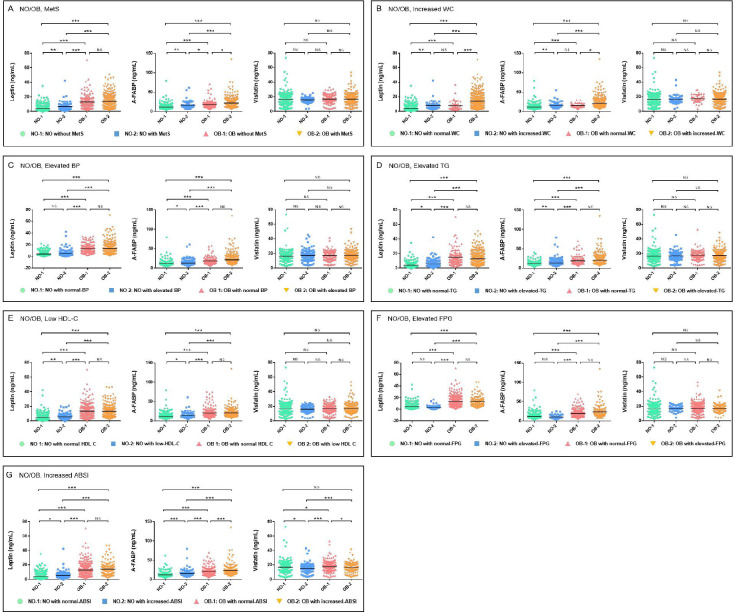
The mean values of leptin, A-FABP and visfatin according to with/without MetS, its components and ABSI among the OB and NO groups are represented in figure 2. The plasma leptin and A-FABP levels were significantly higher in OB participants with MetS compared among the four groups, although there were no significant differences in plasma visfatin levels.
Figure 2.
Plasma thermogenic adipokines levels of the four groups. Data are presented as a scatter chart. Plasma thermogenic adipokines levels were detected by ELISA. Differences between groups were compared using the rank-sum test, and stratified by obesity, MetS and its components. p<0.05, the difference was statistically significant; *p<0.05; **p<0.01; ***p<0.001; NS, p≥0.05, the difference was not statistically significant. (A) Serum thermogenic adipokines levels in the four groups divided by BMI and MetS. NO-1 (n=418): Non-obese subjects without MetS; NO-2 (n=57): Non-obese subjects with MetS; OB-1 (n=146): Obese subjects without MetS; OB-2 (n=329): Obese subjects with MetS. (B) Serum thermogenic adipokines levels in the four groups divided by BMI and increased WC. NO-1 (n=422): Non-obese subjects with normal-WC; NO-2 (n=53): Non-obese subjects with increased-WC; OB-1 (n=39): Obese subjects with normal-WC; OB-2 (n=436): Obese subjects with increased-WC. (C)Serum thermogenic adipokines levels in the four groups divided by BMI and elevated BP. NO-1 (n = 310): Non-obese subjects with normal-BP; NO-2 (n = 165): Non-obese subjects with elevated-BP; OB-1 (n = 162): Obese subjects with normal-BP; OB-2 (n = 313): Obese subjects with elevated-BP. (D)Serum thermogenic adipokines levels in the four groups divided by BMI and elevated TG. NO-1 (n = 314): Non-obese subjects with normal-TG; NO-2 (n = 161): Non-obese subjects with elevated-TG; OB-1 (n = 133): Obese subjects with normal-TG; OB-2 (n = 342): Obese subjects with elevated-TG. (E)Serum thermogenic adipokines levels in the four groups divided by BMI and low HDL-C. NO-1 (n = 388): Non-obese subjects with normal-HDL-C; NO-2 (n = 87): Non-obese subjects with low-HDL-C; OB-1 (n = 235): Obese subjects with normal-HDL-C; OB-2 (n = 240): Obese subjects with low-HDL. (F) Serum thermogenic adipokines levels in the four groups divided by BMI and elevated FPG. NO-1 (n=421): Non-obese subjects with normal-FPG; NO-2 (n=54): Non-obese subjects with elevated-FPG; OB-1 (n=356): Obese subjects and normal-FPG; OB-2 (n=119): Obese subjects with elevated-FPG. (G)Serum thermogenic adipokines levels in the four groups divided by BMI and increased ABSI. NO-1 (n = 359): Non-obese subjects with normal-ABSI; NO-2 (n = 116): Non-obese subjects with increased-ABSI; OB-1 (n = 331): Obese subjects with normal-ABSI; OB-2 (n = 144): Obese subjects with increased-ABSI.BMI, body mass index; BP, blood pressure; FPG, fasting plasma glucose; HDL-C, high-density lipoprotein cholesterol; MetS, metabolic syndrome; NO, non-obese; OB, obese; TG, triglycerides; WC, waist circumference; ABSI, a body shape index.
Correlation analysis of plasma leptin, A-FABP and visfatin levels with BMI, MetS and its components, and ABSI under all, the NO and the OB groups, is demonstrated in table 2. BMI, MetS were positively correlated with plasma leptin and A-FABP levels in all and the NO group. Meanwhile, BMI and MetS were positively correlated with plasma A-FABP levels in OB patients. However, MetS was not correlated with plasma leptin level in the OB patients. For both groups, the NO and the OB patients, MetS were not associated with plasma visfatin level.ABSI was positively correlated with plasma A-FABP levels, and negatively correlated with plasma visfatin levels in both the NO and the OB group. ABSI was positively correlated with plasma leptin levels in all and the NO group, but not in the OB patients.
Table 2.
Correlation of plasma thermogenic adipokines levels with MetS
| BMI (r) | MetS (r) | Increased WC (r) | Elevated BP (r) | Elevated TG (r) | Low HDL-C (r) | Elevated FPG (r) | Increaded ABSI (r) | |
| Leptin | ||||||||
| ALL (n=950) P value |
0.677 <0.001 |
0.450 <0.001 |
0.648 <0.001 |
0.200 <0.001 |
0.277 <0.001 |
0.292 <0.001 |
0.065 0.044 |
0.156 <0.001 |
| NO (n=475) P value |
0.382 <0.001 |
0.193 <0.001 |
0.318 <0.001 |
0.014 0.754 |
0.168 <0.001 |
0.193 <0.001 |
−0.144 0.002 |
0.150 0.001 |
| OB (n=475) P value |
0.315 <0.001 |
0.061 0.182 |
0.262 <0.001 |
−0.013 0.781 |
−0.097 0.034 |
0.014 0.765 |
−0.117 0.714 |
0.058 0.208 |
| A-FABP | ||||||||
| ALL (n=950) P value |
0.564 <0.001 |
0.427 <0.001 |
0.526 <0.001 |
0.258 <0.001 |
0.306 <0.001 |
0.258 <0.001 |
0.130 <0.001 |
0.251 <0.001 |
| NO (n=475) P value |
0.273 <0.001 |
0.177 <0.001 |
0.195 <0.001 |
0.162 <0.001 |
0.170 <0.001 |
0.155 0.001 |
−0.034 0.457 |
0.249 <0.001 |
| OB (n=475) P value |
0.220 <0.001 |
0.176 <0.001 |
0.166 <0.001 |
0.086 0.062 |
0.094 0.040 |
0.053 0.250 |
0.117 0.011 |
0.205 <0.001 |
| Visfatin | ||||||||
| ALL (n=950) P value |
0.062 0.055 |
0.031 0.337 |
0.042 0.199 |
0.061 0.061 |
0.029 0.379 |
−0.023 0.487 |
0.064 0.049 |
−0.099 0.002 |
| NO (n=475) P value |
0.090 0.049 |
−0.013 0.785 |
−0.020 0.671 |
0.041 0.371 |
0.051 0.263 |
−0.025 0.583 |
0.077 0.092 |
−0.119 0.010 |
| OB (n=475) P value |
−0.092 0.045 |
−0.015 0.751 |
−0.039 0.391 |
0.033 0.476 |
−0.047 0.304 |
−0.067 0.147 |
0.034 0.463 |
−0.103 0.025 |
ALL, n = 950; NO: Non-obese subjects, n = 475; OB: Obesity, n = 475. Components of MetS: increased WC, elevated BP, elevated TG, low HDL-C, elevated FPG and increased ABSI. Spearman correlation analysis was used to analyze the associations of plasma thermogenic adipokines levels with MetS. r: Correlationcoefficient; r > 0, a positive correlation between the two parameters; r < 0, the negative correlation between the two parameters. P < 0.05, the difference was statistically significant.
ABSI, a body shape index; BMI, body mass index; BP, blood pressure; FPG, fasting plasma glucose; HDL-C, high-density lipoprotein cholesterol; MetS, metabolic syndrome; NO, non-obese subjects; OB, obesity; TG, triglycerides; WC, waist circumference.
Analysis of influencing factors of plasma thermogenic adipokines levels
Regarding the stratified analysis of the data, the results shown in table 3 and online supplemental tables 1.1–1.3, and show that male, younger age, smoking and alcohol consumption correlated with lower plasma leptin and A-FABP levels, while male, living at a lower altitude, smoking and alcohol consumption correlated with lower plasma visfatin levels in. Therefore, we combined these variables in the logistic regression model.
Table 3.
Analysis of influencing factors of plasma thermogenic adipokines levels
| ALL (n=950) | Leptin (ng/mL) | A-FABP (ng/mL) | Visfatin (ng/mL) | |||
| C (ng/mL) | Z/H | C (ng/mL) | Z/H | C (ng/mL) | Z/H | |
| Sex | ||||||
| Male | 3.2 (1.2, 6.0) | −15.532 | 12.1 (8.2, 17.0) | −7.216 | 17.3 (14.3, 20.3) | −2.791 |
| Female | 9.4 (5.1, 15.9)* | 15.4 (11.4, 20.5)* | 16.2 (13.1, 19.3)* | |||
| Altitude of residence | ||||||
| 2140 m | 6.7 (3.2, 12.8) | −1.291 | 14.3 (10.3, 19.8) | −0.548 | 16.3 (13.2, 19.5) | −4.954 |
| 2250 m | 6.0 (2.3, 12.2) | 14.1 (10.3, 19.7) | 18.7 (16.5, 21.2)† | |||
| Age | ||||||
| 30–44 years | 7.4 (3.4, 13.3) | 2.158 | 13.1 (8.8, 16.2) | 23.618 | 16.5 (12.9, 19.5) | 0.639 |
| 45–64 years | 6.6 (3.1, 12.3) | 14.3 (10.3, 19.9)‡ | 16.5 (13.5, 19.7) | |||
| 65–79 years | 8.1 (3.1, 16.7) | 17.2 (12.1, 27.5)‡ | 16.5 (13.4, 19.9) | |||
| Smoking | ||||||
| Yes | 2.8 (1.0, 5.2) | −12.993 | 12.1 (7.9, 16.3) | −6.787 | 17.7 (14.5, 20.3) | −3.090 |
| No | 8.4 (4.4, 14.7)§ | 15.0 (11.1, 20.5)§ | 16.3 (13.2, 19.4)§ | |||
| Alcohol use | ||||||
| Yes | 3.5 (1.5, 6.2) | −7.416 | 12.3 (8.5, 18.6) | −4.635 | 17.2 (14.0, 20.5) | −2.082 |
| No | 7.4 (3.6, 13.7)¶ | 14.5 (10.6, 20.1)¶ | 16.4 (13.4, 19.6)¶ | |||
Using the rank-sum test to analyse the effect of various factors on plasma visfatin levels.
*Compared with the same group of men, p<0.05.
†Compared with the same group of 2140 m altitude, p<0.05.
‡Compared with the same group of 30–44 years of age, p<0.05.
§Compared with the same group of smokers, p<0.05.
¶Compared with the same group of alcohol users, p<0.05.
bmjopen-2022-066789supp001.pdf (96.1KB, pdf)
Discriminant analysis of plasma thermogenic adipokines levels and MetS
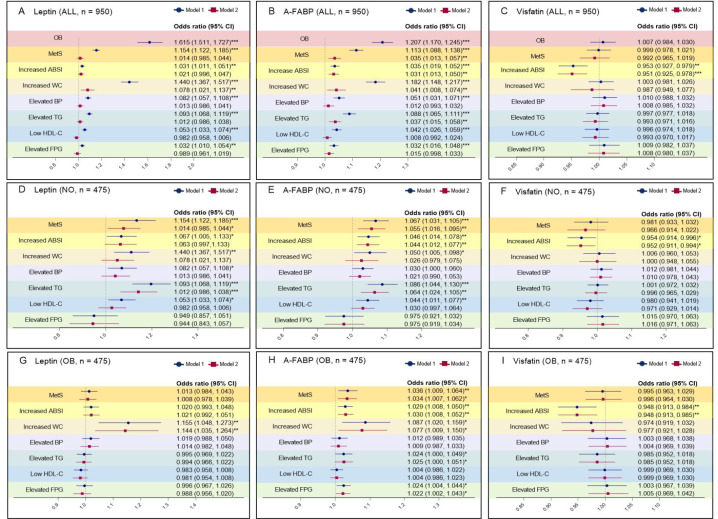
In the model 1 adjusted for age, sex, high education lever, smoking, alcohol use and physical activity level; in the model 2 adjusted plus BMI, discriminant analysis of plasma leptin, A-FABP and visfatin with obesity, MetS, increased ABSI for all, the NO and the OB patients are shown in figure 3. Plasma leptin and A-FABP levels were demonstrated to be the independent predictive factors for OB. Plasma leptin and A-FABP levels were demonstrated to be the independent predictive factors for increased WC, but plasma visfatin levels were not. Plasma leptin levels was an independent predictive factor for increased ABSI only in the model 1, but plasma leptin and visfatin levels were demonstrated to be the independent predictive factors for increased ABSI. In the model 1, plasma A-FABP levels was an independent predictive factor for MetS (odds ratio, 1.154; 95% CI, 1.122–1.185; P < 0.05); but not in the model 2 (odds ratio, 1.014; 95% CI, 0.985–1.044; P > 0.05). Plasma A-FABP levels were shown to be the independent predictive factors for Mets in all plateau patients (odds ratio, 1.035; 95% CI, 1.013–1.057; P < 0.05). Plasma visfatin levels were at least a weak indicator to distinguish obesity or MetS (odds ratio, 0.999; 95% CI, 0.978–1.021; P > 0.05). And there were no sexual dimorphism on the discriminant analysis of plasma thermogenic adipokines levels with obesity and MetS shown in table 4.
Figure 3.
Discriminant analysis of plasma thermogenic adipokines levels with obesity, MetS and its components. ALL, n = 950, NO: Non-obese, n = 475, OB: Obesity, n = 475. In the multivariate logistic regression analysis of obesity, MetS, its components and ABSI, model 1 was adjusted for age, sex, higher education, smoking, alcohol consumption and physical activity levels, indicated by the blue ovals; model 2 was adjusted for model 1 plus BMI, indicated by the red squares; odds ratio and 95% CI are shown in the forest illustration; odds ratio value > 1, risk factor; odds ratio value < 1, protective factor; 95% CI does not contain 1, *P < 0.05; **P < 0.01; ***P < 0.001, the difference was statistically significant. A/B/C. Discriminant analysis of plasma leptin, A-FABP and visfatin with obesity, ABSI, MetS, and its components for all; D/E/F. Discriminant analysis of plasma leptin, A-FABP and visfatin with ABSI, MetS, and its components for the NO; G/H/I. Discriminant analysis of plasma leptin, A-FABP and visfatin with ABSI, MetS, and its components for the OB patients.
Table 4.
Sexual dimorphism on discriminant analysis of plasma thermogenic adipokines levels with obesity and MetS
| Leptin | A-FABP | Visfatin | ||||
| OB | MetS | OB | MetS | OB | MetS | |
| OR (95% CI) P value |
OR (95% CI) P value |
OR (95% CI) P value |
OR (95% CI) P value |
OR (95% CI) P value |
OR (95% CI) P value |
|
| Male (n=320) |
3.712 (2.716 to 5.074) <0.001 |
1.096 (0.976 to 1.231) 0.122 |
1.195 (1.136 to 1.257) <0.001 |
1.038 (1.001 to 1.078) 0.045 |
1.025 (0.982 to 1.070) 0.252 |
0.994 (0.941 to 1.050) 0.835 |
| Female (n=630) |
1.472 (1.380 to 1.570) <0.001 |
1.015 (0.984 to 1.046) 0.347 |
1.223 (1.174 to 1.273) <0.001 |
1.031 (1.005 to 1.058) 0.021 |
0.995 (0.969 to 1.022) 0.732 |
0.993 (0.962 to 1.025) 0.669 |
In the multivariate logistic regression analysis of MetS, adjusted for age, high education lever, smoking, alcohol use, physical activity level and BMI; in the multivariate logistic regression analysis of OB, adjusted for age, high education level, smoking, alcohol use and physical activity level; OR value>1, risk factor; OR value<1, protective factor; 95% CI does not contain 1, p<0.05, the difference was statistically significant.
BMI, body mass index; F-AFBP, fat cell fatty acid-binding protein; MetS, metabolic syndrome; OB, obesity.
Discussion
Our results suggested that abnormally elevated plasma A-FABP levels were positively associated with OB and MetS in plateau subjects and this is consistent with the results of some previous studies.29 30 A-FABP is mainly expressed in adipocytes and macrophages and plays important roles in the development of insulin resistance and atherosclerosis.31 In mature adipocytes, A-FABP is a predominant cytosolic protein, accounting for 6% of total cellular proteins. This protein may be an important regulator of systemic insulin sensitivity and lipid and glucose metabolism.32 In one study, serum A-FABP concentrations in 100 underweight and 129 overweight or OB individuals were measured, and it was found that the serum A-FABP concentrations in OB/overweight individuals were significantly lower in men than in women, but not in underweight individuals. There was a strong positive correlation between serum A-FABP concentration and BMI in both men and women.17 Other research work using tissue-specific fatty acid binding protein 4-deficient mice has demonstrated a significant role of A-FABP in the cellular metabolism of polar and neutral lipids, and that its deficiency leads to a metabolic shift towards TGs, lipoproteins and lipolysis products.33 Thus, a high-altitude environment alone could not affect the relationship between abnormally elevated A-FABP and OB, MetS.
Our results suggested that leptin had the strongest association with OB among the three thermogenic adipokines. Leptin is directly secreted by white adipose tissue, and the expansion of adipose tissue directly increases leptin level, hence it can most directly respond to OB and the MetS in plateau subjects, consistent with the results of Obradovic et al.16 29 30 However, our results suggested that the relationship between leptin and MetS was different in the NO and OB groups. Leptin was independently associated with MetS only in the NO group, but this association disappeared in the OB group. Elevated leptin is often accompanied by OB, leading to disorders of glucose and lipid metabolism, which is closely related to MetS.34 The disappearance of this association in OB patients, may be related to high and ineffective leptin, known as leptin resistance.35 When leptin is abnormally elevated and leptin receptors cannot be increased adaptively, leptin receptor-related pathways fail and cannot effectively regulate the central nervous system through the blood-brain barrier.36 Furthermore, leptin was independently associated with MetS only in model 1, but this association disappeared in model 2 which was adjusted additionally for BMI. The volume of adipose tissue directly determines the concentration of leptin.37 It has been suggested that leptin may be a consequence of OB, rather than a cause of MetS.38 An increase in adiposity may lead to increased leptin levels in the absence of a parallel increase of MetS. Similar to A-FABP, the relationship between leptin and OB and MetS was unaffected by the high-altitude natural environment. Unlike A-FABP, the results largely lost significance after adjustment for BMI suggesting that the relation between leptin and MetS is dependent OB.
However, our study found that visfatin was not associated with OB and MetS, either in the population, OB or NO group. Recent research on the association of visfatin with OB has been controversial as several studies found no significant differences in plasma visfatin levels among people with different BMI groups.39 40 Berndt et al studies have shown that the plasma visfatin level in OB patients was lower than that of the control group, and the expression of visfatin in the adipose tissue of the OB group was also decreased.41 42 Still, Chen et al found that plasma visfatin levels were positively correlated with body fat content and WHR and in vitro experiments also confirmed the close relationship between visfatin and adipocyte differentiation and lipid accumulation.41 43 It was considered the differences in these conclusions may be due to differences in sex,39 living habits (smoking, napping)44 45 and natural environment (cold), among others, while it is also worth considering the impact of the high-altitude environment. Visfatin is mainly derived from visceral adipose tissue, so previous studies may have concentrated on factors such as the volume and distribution of visceral non-thermogenic adipose tissue, while ignoring the effect of thermogenic adipose tissue. When studying the effect of visfatin on adipose tissue thermogenesis, visfatin was also found to be expressed in thermogenic adipose tissue and visfatin mRNA was higher than that in the liver and visceral adipose tissue. Visfatin is related to the differentiation and function of thermogenic adipose tissue. With the mutual promotion, the level of visfatin increases in differentiated thermogenic adipocytes and the increase in visfatin further enhances thermogenesis.19 It was found that the physiological processes and energy metabolism change with altitude.46 Our results also found that the altitude of Shunzhou town (2250 m) is about 100 m higher than that of Yongbei town (2140 m). Unlike leptin and A-FABP, visfatin showed obvious altitude-dependent differences. We speculate that visfatin is more susceptible to the high-altitude natural environment and the activation degree of thermogenic adipose tissue influence, sequentially in turn interferes with this prediction performance for OB and MetS. In conclusion, plasma visfatin level is a poor stability predictor of OB and combined risk.
ABSI was an indicator reflecting abdominal obesity. The study compared the advantages of BMI, WC, and ABSI in predicting poor prognosis of MetS. Therefore, in this study, we used it to demonstrate the predictive efficacy of three thermogenic related adipokines again, and the results still showed that A-FABP is the most stable predictor among the three. A-FABP was a risk factor for abdominal obesity, the research results of Nagayama D et al. also show that A-FABP is a risk factor for abdominal obesity.47 48At the same time, we also found that visfatin serves as a protective factorfor abdominal obesity. In a study targeting abdominal obesity, Straburzy ń Ska Lupa A et al. found that patients with abdominal obesity had significantly lower levels of visceral adiponectin compared to those without abdominal obesity. The positive correlation between visfatin and resistin may indicate that visfatin plays a role in inflammation, similar to our research findings.49 Comparing the relationship between three types of adipokines and WC, BMI, and ABSI, it was found that there is a significant correlation between A-FABP, leptin and WC, BMI. Zaghlool SB et al found a correlation between leptin and BMI, with weak correlation between WC and A-FABP. Similar to our research findings, both have demonstrated an association between two factors and BMI and WC.50 While the visfatin has a significant correlation with ABSI. Zaidi H et al. found that an increase in visfatin can lead to the development of abdominal obesity, which is similar to our research findings.51
There are several limitations in this study. First, this study was cross-sectional and cannot be translated into definitive causal inferences. Thus, large-scale and prospective clinical trial should be performed to investigate the subject further. Second, our study population included only Han subjects living at high altitudes, and comparisons with Han subjects at lower altitudes are lacking. Therefore, it is unclear whether the correlation between thermogenic adipokines and OB/MetS are changed in a high-altitude environment, and, if so, the degree of change remains unclear. It will be necessary to study the Han population in low-altitude areas to clarify the correlation between adipokines and disease distribution from the geographical differences. It is also necessary to study non-Han populations. Third, previous studies were not conducted in a plateau-specific environment, and it is necessary to simulate the plateau environment to further study and clarify its unique mechanisms. Fourth, the NO subjects in this study were matched with the OB subjects, which may not fully reflect the prevalence and risk factors of MetS in the NO group. Cluster sampling in all NO populations will be performed in the future to strengthen our findings.
Conclusions
Supplementary Material
Acknowledgments
We thank all the participants in this study.
Footnotes
ZZ, JH, DZ and YW contributed equally.
Contributors: JY and QM conceived and designed the study. ZZ, JH, DZ and YW conducted research. ZC and YX analysed data. RH and YQ performed statistical analysis. ZZ, JH, DZ and YW wrote paper draft, and revised the manuscript. JY responsible for the overall content as the guarantor. All authors read and approved the final manuscript.
Funding: This work was supported by grants from the National Natural Science Foundation of China (82260641, 81860597); National Key R&D Program of China (2017YFC0907302).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The study received Eethical approval from the Sichuan University Medical Ethical Review Board (K2016038) and written informed consent was provided by all participants
References
- 1.Pydi SP, Jain S, Barella LF, et al. Β-arrestin-1 suppresses myogenic reprogramming of brown fat to maintain euglycemia. Sci Adv 2020;6:eaba1733. 10.1126/sciadv.aba1733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gowshall M, Taylor-Robinson SD. The increasing prevalence of non-communicable diseases in low-middle income countries: the view from Malawi. Int J Gen Med 2018;11:255–64. 10.2147/IJGM.S157987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bennett JE, Stevens GA, Mathers CD, et al. NCD countdown 2030: worldwide trends in non-communicable disease mortality and progress towards sustainable development goal target 3.4. Lancet 2018;392:1072–88. 10.1016/S0140-6736(18)31992-5 [DOI] [PubMed] [Google Scholar]
- 4.Wang Y fa, Lim H, Wu Y. Growing global burden of chronic noncommunicable diseases and an alarming situation in China. Beijing Da Xue Xue Bao Yi Xue Ban 2012;44:688–93. [PubMed] [Google Scholar]
- 5.Rembert N, He K, Judd SE, et al. The geographic distribution of trace elements in the environment: the REGARDS study. Environ Monit Assess 2017;189:84. 10.1007/s10661-016-5733-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou M, Wang H, Zeng X, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019;394:1145–58. 10.1016/S0140-6736(19)30427-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu X, Mao Z, Li Y, et al. Cohort profile: the Henan rural cohort: a prospective study of chronic non-communicable diseases. Int J Epidemiol 2019;48:1756–1756j. 10.1093/ije/dyz039 [DOI] [PubMed] [Google Scholar]
- 8.Perini W, Snijder MB, Peters RJG, et al. Ethnic disparities in estimated cardiovascular disease risk in Amsterdam, the Netherlands: the HELIUS study. Neth Heart J 2018;26:252–62. 10.1007/s12471-018-1107-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.West JB. High altitude medicine and biology in Peru. High Alt Med Biol 2010;11:173. 10.1089/ham.2010.11301 [DOI] [PubMed] [Google Scholar]
- 10.Zhao X, Hong F, Yin J, et al. Cohort profile: the China multi-ethnic cohort (CMEC) study. Int J Epidemiol 2021;50:721–721l. 10.1093/ije/dyaa185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin BY, Genden K, Shen W, et al. The prevalence of obesity and metabolic syndrome in Tibetan immigrants living in high altitude areas in Ladakh, India. Obes Res Clin Pract 2018;12:365–71. 10.1016/j.orcp.2017.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X, Song J, Gao Y, et al. Association between weight gain from young to middle adulthood and metabolic syndrome across different BMI categories at young adulthood. Front Endocrinol (Lausanne) 2021;12:812104. 10.3389/fendo.2021.812104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gurka MJ, Filipp SL, Musani SK, et al. Use of BMI as the marker of Adiposity in a metabolic syndrome severity score: derivation and validation in predicting long-term disease outcomes. Metabolism 2018;83:68–74. 10.1016/j.metabol.2018.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagayama D, Fujishiro K, Tsuda S, et al. Enhanced prediction of renal function decline by replacing waist circumference with "A body shape index (ABSI)” in diagnosing metabolic syndrome: a retrospective cohort study in Japan. Int J Obes (Lond) 2022;46:564–73. 10.1038/s41366-021-01026-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chouchani ET, Kajimura S. Metabolic adaptation and maladaptation in adipose tissue. Nat Metab 2019;1:189–200. 10.1038/s42255-018-0021-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Obradovic M, Sudar-Milovanovic E, Soskic S, et al. Leptin and obesity: role and clinical implication. Front Endocrinol (Lausanne) 2021;12:585887. 10.3389/fendo.2021.585887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu A, Wang Y, Xu JY, et al. Adipocyte fatty acid-binding protein is a plasma biomarker closely associated with obesity and metabolic syndrome. Clin Chem 2006;52:405–13. 10.1373/clinchem.2005.062463 [DOI] [PubMed] [Google Scholar]
- 18.Fukuhara A, Matsuda M, Nishizawa M, et al. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science 2005;307:426–30. 10.1126/science.1097243 [DOI] [PubMed] [Google Scholar]
- 19.Dimitriadis GK, Adya R, Tan BK, et al. Effects of visfatin on brown adipose tissue energy regulation using T37I cells. Cytokine 2019;113:248–55. 10.1016/j.cyto.2018.07.013 [DOI] [PubMed] [Google Scholar]
- 20.Shu L, Hoo RLC, Wu X, et al. A-FABP mediates adaptive thermogenesis by promoting intracellular activation of thyroid hormones in brown adipocytes. Nat Commun 2017;8:14147. 10.1038/ncomms14147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim MH, Seong JB, Huh J-W, et al. Peroxiredoxin 5 ameliorates obesity-induced non-alcoholic fatty liver disease through the regulation of oxidative stress and AMP-activated protein kinase signaling. Redox Biol 2020;28:101315. 10.1016/j.redox.2019.101315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin GG, Landrock D, McIntosh AL, et al. High glucose and liver fatty acid binding protein gene ablation differentially impact whole body and liver phenotype in high-fat pair-Fed mice. Lipids 2020;55:309–27. 10.1002/lipd.12238 [DOI] [PubMed] [Google Scholar]
- 23.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc 2000;32:S498–504. 10.1097/00005768-200009001-00009 [DOI] [PubMed] [Google Scholar]
- 24.Apovian CM. Obesity: definition, comorbidities, causes, and burden. Am J Manag Care 2016;22:s176–85. [PubMed] [Google Scholar]
- 25.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005;112:2735–52. 10.1161/CIRCULATIONAHA.105.169404 [DOI] [PubMed] [Google Scholar]
- 26.Krakauer JC. Expansion of waist circumference in medical literature: potential clinical application of a body shape index. J Obes Weight Loss Ther 2014;04:02. 10.4172/2165-7904.1000216 [DOI] [Google Scholar]
- 27.Krakauer NY, Krakauer JC. An anthropometric risk index based on combining height, weight, waist, and hip measurements. J Obes 2016;2016:8094275. 10.1155/2016/8094275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagayama D, Watanabe Y, Yamaguchi T, et al. Issue of waist circumference for the diagnosis of metabolic syndrome regarding arterial stiffness: possible utility of a body shape index in middle-aged nonobese Japanese urban residents receiving health screening. Obes Facts 2022;15:160–9. 10.1159/000520418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niu G, Li J, Wang H, et al. Associations of A-FABP with anthropometric and metabolic indices and inflammatory cytokines in obese patients with newly diagnosed type 2 diabetes. Biomed Res Int 2016;2016:9382092. 10.1155/2016/9382092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.El Husseny MWA, Mamdouh M, Shaban S, et al. Adipokines: potential therapeutic targets for vascular dysfunction in type II diabetes mellitus and obesity. J Diabetes Res 2017;2017:8095926. 10.1155/2017/8095926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Furuhashi M. Fatty acid-binding protein 4 in cardiovascular and metabolic diseases. J Atheroscler Thromb 2019;26:216–32. 10.5551/jat.48710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Makowski L, Hotamisligil GS. Fatty acid binding proteins--the evolutionary crossroads of inflammatory and metabolic responses. J Nutr 2004;134:2464S–2468S. 10.1093/jn/134.9.2464S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li H, Herrmann T, Seeßle J, et al. Role of fatty acid transport protein 4 in metabolic tissues: insights into obesity and fatty liver disease. Biosci Rep 2022;42:BSR20211854. 10.1042/BSR20211854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim J-E, Kim J-S, Jo M-J, et al. The roles and associated mechanisms of adipokines in development of metabolic syndrome. Molecules 2022;27:334. 10.3390/molecules27020334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grasso P. Harnessing the power of Leptin: the biochemical link connecting obesity, diabetes, and cognitive decline. Front Aging Neurosci 2022;14:861350. 10.3389/fnagi.2022.861350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jais A, Brüning JC. Arcuate nucleus-dependent regulation of metabolism-pathways to obesity and diabetes mellitus. Endocr Rev 2022;43:314–28. 10.1210/endrev/bnab025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao L-J, Jiang H, Papasian CJ, et al. Correlation of obesity and osteoporosis: effect of fat mass on the determination of osteoporosis. J Bone Miner Res 2008;23:17–29. 10.1359/jbmr.070813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weiss R, Bremer AA, Lustig RH. What is metabolic syndrome, and why are children getting it Ann N Y Acad Sci 2013;1281:123–40. 10.1111/nyas.12030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saboori S, Hosseinzadeh-Attar MJ, Yousefi Rad E, et al. The comparison of serum vaspin and visfatin concentrations in obese and normal weight women. Diabetes Metab Syndr 2015;9:320–3. 10.1016/j.dsx.2013.10.009 [DOI] [PubMed] [Google Scholar]
- 40.Hosseinzadeh-Attar MJ, Golpaie A, Foroughi M, et al. The relationship between Visfatin and serum concentrations of C-reactive protein, interleukin 6 in patients with metabolic syndrome. J Endocrinol Invest 2016;39:917–22. 10.1007/s40618-016-0457-1 [DOI] [PubMed] [Google Scholar]
- 41.Berndt J, Klöting N, Kralisch S, et al. Plasma Visfatin concentrations and fat depot-specific mRNA expression in humans. Diabetes 2005;54:2911–6. 10.2337/diabetes.54.10.2911 [DOI] [PubMed] [Google Scholar]
- 42.Pisani DF, Dumortier O, Beranger GE, et al. Visfatin expression analysis in association with recruitment and activation of human and rodent brown and brite adipocytes. Adipocyte 2016;5:186–95. 10.1080/21623945.2015.1122854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen M-P, Chung F-M, Chang D-M, et al. Elevated plasma level of Visfatin/pre-B cell colony-enhancing factor in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab 2006;91:295–9. 10.1210/jc.2005-1475 [DOI] [PubMed] [Google Scholar]
- 44.Bai X-J, Fan L-H, He Y, et al. Nicotine may affect the secretion of adipokines Leptin, Resistin, and visfatin through activation of KATP channel. Nutrition 2016;32:645–8. 10.1016/j.nut.2015.12.001 [DOI] [PubMed] [Google Scholar]
- 45.Kazem YMI, Shebini SME, Moaty MIA, et al. Sleep deficiency is a modifiable risk factor for obesity and cognitive impairment and associated with elevated visfatin. Open Access Maced J Med Sci 2015;3:315–21. 10.3889/oamjms.2015.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bai J, Li L, Li Y, et al. Genetic and immune changes in Tibetan high-altitude populations contribute to biological adaptation to hypoxia. Environ Health Prev Med 2022;27:39. 10.1265/ehpm.22-00040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nagayama D, Sugiura T, Choi S-Y, et al. Various obesity indices and arterial function evaluated with CAVI - is waist circumference adequate to define metabolic syndrome? Vasc Health Risk Manag 2022;18:721–33. 10.2147/VHRM.S378288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nagayama D, Watanabe Y, Yamaguchi T, et al. New index of abdominal obesity, a body shape index, is BMI-independently associated with systemic arterial stiffness in real-world Japanese population. Int J Clin Pharmacol Ther 2020;58:709–17. 10.5414/CP203778 [DOI] [PubMed] [Google Scholar]
- 49.Straburzyńska-Lupa A, Nowak A, Pilaczyńska-Szcześniak Ł, et al. Visfatin, Resistin, hsCRP and insulin resistance in relation to abdominal obesity in women with rheumatoid arthritis. Clin Exp Rheumatol 2010;28:19–24. [PubMed] [Google Scholar]
- 50.Zaghlool SB, Sharma S, Molnar M, et al. Revealing the role of the human blood plasma Proteome in obesity using genetic drivers. Nat Commun 2021;12:1279. 10.1038/s41467-021-21542-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zaidi H, Aksnes T, Åkra S, et al. Abdominal adipose tissue associates with adiponectin and TNFα in middle-aged healthy men. Front Endocrinol (Lausanne) 2022;13:874977. 10.3389/fendo.2022.874977 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-066789supp001.pdf (96.1KB, pdf)
Data Availability Statement
Data are available upon reasonable request. The data that support the findings of this study are available from the corresponding author upon reasonable request.