Abstract
Mood disorders are recurrent/chronic diseases with variable clinical remission rates. Available antidepressants are not effective in all patients and often show a relevant response latency, with a range of adverse events, including weight gain and sexual dysfunction. Novel rapid agents were developed with the aim of overcoming at least in part these issues. Novel drugs target glutamate, gamma-aminobutyric acid, orexin, and other receptors, providing a broader range of pharmacodynamic mechanisms, that is, expected to increase the possibility of personalizing treatments on the individual clinical profile. These new drugs were developed with the aim of combining a rapid action, a tolerable profile, and higher effectiveness on specific symptoms, which were relatively poorly targeted by standard antidepressants, such as anhedonia and response to reward, suicidal ideation/behaviours, insomnia, cognitive deficits, and irritability. This review discusses the clinical specificity profile of new antidepressants, namely 4-chlorokynurenine (AV-101), dextromethorphan-bupropion, pregn-4-en-20-yn-3-one (PH-10), pimavanserin, PRAX-114, psilocybin, esmethadone (REL-1017/dextromethadone), seltorexant (JNJ-42847922/MIN-202), and zuranolone (SAGE-217). The main aim is to provide an overview of the efficacy/tolerability of these compounds in patients with mood disorders having different symptom/comorbidity patterns, to help clinicians in the optimization of the risk/benefit ratio when prescribing these drugs.
Keywords: antidepressants, bipolar depression, clinical trials, gamma-aminobutyric acid, major depressive disorder, novel rapid acting antidepressant, pharmacodynamic mechanisms, postpartum depression, treatment-resistant depression
Introduction
Globally, mood disorders are a severe and chronic cause of disability, morbidity, and mortality (James et al., 2018). They impact social functioning, as well as work productivity, resulting in a significant socioeconomic burden (Egede et al., 2016). Moreover, these disorders have a strong impact on physical health and significantly increase the risk of developing other medical conditions, such as heart disease, diabetes, and obesity (Fanelli and Serretti, 2022; Wimberley et al., 2022). We cannot forget to mention one of the most urgent clinical concerns associated with depression, that is, suicide, as the pooled lifetime prevalence of suicide attempts is between 27 and 34% in people with depressive disorders (WHO depressive disorder, 2023). Antidepressant drugs, such as selective serotonin reuptake inhibitors, serotonin and norepinephrine reuptake inhibitors, and tricyclic antidepressants, are widely prescribed in the general population (Dörks et al., 2022) and used to treat depressive symptoms, as well as anxiety and obsessive symptoms, among others (Castle et al., 2021); however, they are associated with variable treatment outcomes that are difficult to predict (Fanelli et al., 2022). Consequently, finding an effective treatment for all patients with major depressive disorder (MDD), bipolar depression, postpartum depression (PPD), perimenopausal depression (PMD), and depression associated with general medical conditions remains a major unmet need.
Treatment-resistant depression (TRD) is usually defined as an ineffective response to at least two antidepressant drugs of adequate dose and duration (Gaynes et al., 2020), but also in patients who do achieve remission, residual symptoms are common (McClintock et al., 2011). TRD is particularly common in patients suffering from bipolar depression. The high burden of depressive phases in bipolar disorder is partly linked to the fact that there is only a limited number of approved treatment options for bipolar depression, and in most cases the evidence is controversial (McIntyre and Calabrese, 2019). Poor response to treatment (residual symptoms, slow improvement, or TRD) may be highly burdensome and associated with additional risks in some groups of patients, such as women in the peripartum. If not successfully treated, serious consequences can negatively affect the well being and health of both mother and child (Beck, 2006). More specifically, it increases the risk of preeclampsia, preterm delivery, maternal substance abuse, and suicidality (Vliegen et al., 2014), as well as causing significant impairment in both maternal functioning and mother-infant attachment (Gagliardi et al., 2012). This can have a negative impact on child cognitive, behavioural, and emotional development, with long-lasting repercussions (Frieder et al., 2019).
Poor treatment response is not the only issue of drugs currently approved for depressive disorders. Adverse events are indeed quite common, and not infrequently they compromise treatment adherence (Hu et al., 2004). Standard antidepressant treatments also show delayed action, as they require 4–6 weeks before reaching the complete therapeutic effects, and in the meantime, patients remain vulnerable to the risk of suicide (Machado-Vieira et al., 2009). The discussed points emphasize the urgent need for more broadly effective and rapid interventions in depression, especially with a rapid antisuicidal and antianhedonic effect. Recent preclinical and clinical studies are testing new glutamate drugs, for example, acting as n-methyl-d-aspartate receptor (NMDAR) antagonists (Salituro et al., 1994). Other new compounds target gamma-aminobutyric acid (GABA) neurotransmission, such as GABAA positive allosteric modulators (PAMs) (Althaus et al., 2020), and the orexin system, such as seltorexant (Recourt et al., 2019). Psychedelic drugs such as psilocybin have also attracted renewed interest in treating resistant cases (Griffiths et al., 2016), based on their effects on cognitive flexibility (Boulougouris et al., 2008) and associative learning (Harvey, 2003). Despite new drugs having general mechanisms alternative to the ‘classic’ monoaminergic hypothesis, pimavanserin is a new compound studied for depression that principally acts on the well studied serotonergic system.
The purpose of this review is to summarize and discuss the available literature on novel rapid acting antidepressants, in various stages of development, with respect to their antidepressant efficacy, safety, and tolerability, in patients with different mood disorders, also considering each drug-specific pharmacodynamic profile.
Search strategy and inclusion criteria
The search was conducted using the National Institutes of Health US National Library of Medicine Clinical Trials Database and PubMed, retrieving relevant data up to September 2022. The used keywords were: ‘av-101’, ‘dextromethorphan-bupropion’, ‘AXS-05’, ‘REL-1017’, ‘esmethadone’, ‘zuranolone’, ‘SAGE-17’, ‘PRAX-114’, ‘pimavanserin’, ‘psilocybin’, ‘seltorexant’, ‘MIN-202’, ‘PH-10’; each of these was combined with each of the following: ‘major depressive disorder’, ‘depression’, ‘antidepressant’, ‘depressive symptoms’, ‘bipolar depression’, ‘post-partum depression’. The drugs of interest were selected based on the availability of at least one clinical trial (any phase), completed or in progress, studying their possible efficacy in the treatment of depressive disorders. We did not include drugs already approved for the treatment of depressive disorders, except for dextromethorphan-bupropion (AXS-05) which was recently approved by the Food and Drug Administration (FDA) and can be still considered a novel rapid acting antidepressant. We did not consider drugs with insufficient evidence of efficacy on specific depressive symptoms, or without an innovative mechanism that would suggest a potential effectiveness.
The eligibility criteria were: published (including congress abstracts) or unpublished clinical trials (relevant information was also extracted from reviews, meta-analysis, and websites of biopharmaceutical companies); focused on the clinical effects on the mentioned new compounds in patients with depressive disorders as main diagnosis or comorbidity, or in healthy volunteers; written in English. This is a narrative review, therefore a quantitative synthesis and systematic evaluation of the quality of the included studies are not among the scope of this work.
Results
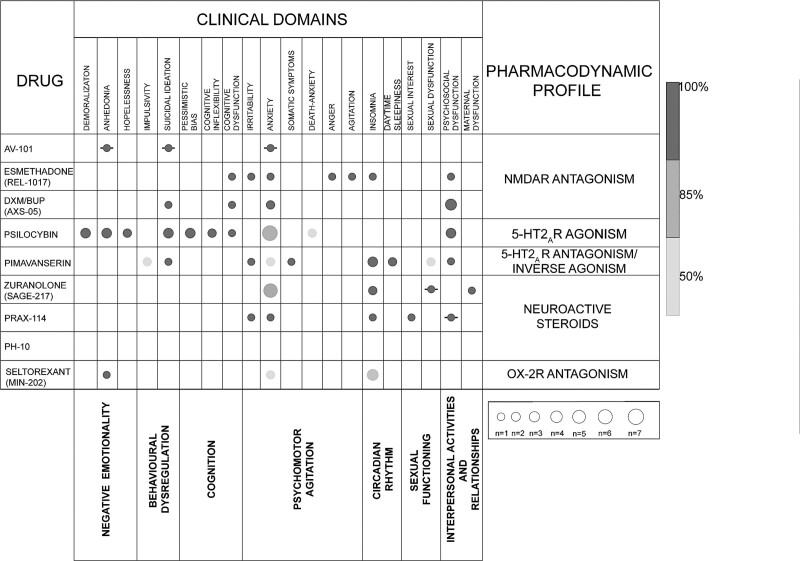
The main characteristics and studied indications of the nine compounds included in our review are presented in Table 1. They have been studied mostly for the treatment of MDD, as adjunctive treatment or monotherapy, often for TRD cases. Despite this leaving a gap regarding their potential benefits in other depressive disorders, the focus of this review is on the efficacy profile in terms of specific symptoms with cross-diagnostic relevance, rather than for specific categorical diagnoses. As one of the key parameters for the evaluation of new compounds is the time of antidepressant effect onset, we represented this in Fig. 1, together with the longest observed period in which the antidepressant effect was sustained. Completed and published clinical studies are summarized in Table 2. Completed clinical studies but not published in peer-reviewed journals are shown in Table 3 and incomplete studies until September 2022 are presented in Table 4.
Table 1.
Characteristics and studied indications of the included novel rapid acting antidepressant drugs according to the results of at least one study considered
| Drug | Mechanism of action/pharmacology | Route of administration | Recommended dose per day | Possible condition of effectiveness in human samples |
|---|---|---|---|---|
| 4-Chlorokynurenine (AV-101) | Competitive NMDAR antagonism | Orally | 1080 or 1440 mg | None |
| Esmethadone (REL-1017) | Noncompetitive NMDAR antagonism | Orally | 25 or 50 mg | Adjunctive treatment to standard antidepressants in MDD |
| Dextromethorphan-bupropion (AXS-05) | Noncompetitive NMDAR antagonism | Orally | 45 mg dextromethorphan/105 mg bupropion | Monotherapy in MDD and TRD |
| Psilocybin | 5-HT2AR agonism | Orally | 10 or 20 or 25 or 30 mg | Monotherapy in MDD and TRD monotherapy in cancer and AIDS-related depression and anxiety (PAP) |
| Pimavanserin | 5-HT2AR antagonism/inverse agonism | Orally | 34 mg | Adjunctive treatment to standard antidepressants in MDD monotherapy and adjunctive treatment to standard antidepressants in MDD associated with Parkinson’s disease |
| Zuranolone (SAGE-217) | GABAAR PAM | Orally | 30 or 50 mg | Monotherapy and adjunctive treatment to standard antidepressants in MDD monotherapy in PPD monotherapy in bipolar depression |
| Prax-114 | GABAAR PAM | Orally | 10 or 20 or 40 or 60 mg | Monotherapy in PMD |
| Ph-10 (pregn-4-en-20-yn-3-one) | Nasal chemosensory receptor modulator | Intranasally | 3.2 or 6.4 µg | Monotherapy in MDD |
| Seltorexant (JNJ-42847922/MIN-202) | Selective OX-2R antagonism | Orally | 10 or 20 or 40 mg | Adjunctive treatment to standard antidepressants in MDD |
GABAAR, gamma-aminobutyric acid type A receptor; MDD, major depressive disorder; NMDAR, n-methyl-d-aspartate receptor; OX-2R, orexin type 2 receptor; PAM, positive allosteric modulator; PAP, psilocybin assisted psychotherapy; PMD, perimenopausal depression; PPD, postpartum depression; R, receptor; TRD, treatment-resistant depression.
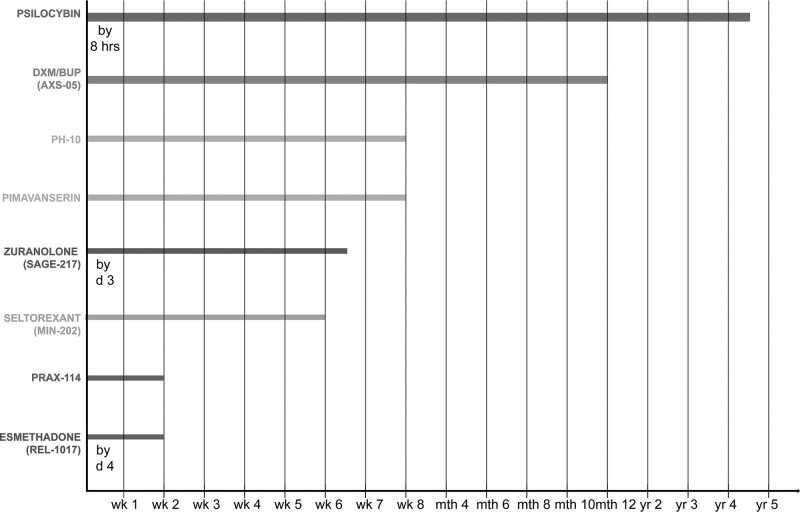
Fig. 1.
Earliest time of onset (indicated as the week of initial antidepressant effect) and longest duration of antidepressant efficacy (indicated as the longest period, that is, weeks, months, or years, of sustained antidepressant effect) reported for the included drugs in at least study. For psilocybin, zuranolone, and esmethadone, data are available on the onset of therapeutic effect before the first week of treatment. For AXS-05, PH-10, pimavanserin, seltorexant (MIN-202), and PRAX-114 there are no data about a possible early onset of the therapeutic effect (i.e. during the first hours or first days of treatment). BUP, bupropion; d, day; DXM, dextromethorphan; hr, hour; mth, month; wk, week; yr, year.
Table 2.
Completed clinical trials until September 2022 and published in peer-reviewed journals
| Mood disorder | Drug | Dose | Phase | Type of study | Durationa | Follow upb | NCT number | Trial design | Adjunctive treatment or monotherapy | Depressive symptoms severty scale at baseline | Population | Drug effectiveness on depressive symptoms | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Sex (F/M) | Mean age or range | ||||||||||||
| MDD | REL-1017 | 25–50 mg | II | Multicenter randomized double-blind placebo-controlled trial | 1 week | 15–21 days | NCT03051256 | 3 arms REL-1017 50 (n 21) REL-1017 25 (n 19) Placebo (n 22) |
Adjunctive treatment | MADRS 33.8 | 62 | 45.2%/54.8% | 49.2 | Reduction in depressive symptoms at week 1 MADRS: −17.4 for REL-1017 25 [P = 0.0122] [d = 0.8] vs −15.9 for REL-1017 50 [P = 0.0308] [d = 0.7] vs −8 for placebo (Primary outcome) |
| AXS-05 | 45/105 mg | II | Multicenter randomized double-blind parallel-group trial | 6 weeks | At week 7 | NCT03595579 | 2 arms AXS-05 (n 43) Bupropion (n 37) |
Monotherapy | MADRS 31.8 | 97 | 64%/38.1% | 37.5 | Reduction in depressive symptoms at week 6 MADRS: −17.3 for AXS-05 vs -12.1 for BUP [P = 0.013] [d = 0.5] (Primary outcome) |
|
| 45/105 mg | III | Multicenter randomized double-blind placebo-controlled trial | 6 weeks | At week 7 | NCT04019704 | 2 arms AXS-05 (n 156) Placebo (n 162) |
Monotherapy | MADRS 33 | 327 | 66%/34% | 42 | Reduction in depressive symptoms at week 6 MADRS: −16.6 for AXS-05 vs −11.9 for placebo [P = 0.002][d = 0.38] (Primary outcome) |
||
| Psilocybin | 25 mg 1 mg |
II | Randomized double-blind controlled trial | 6 weeks | 6 months | NCT03429075 | 2 arms Psilocybin + placebo (n 30) Psilocybin + escitalopram (n 29) |
Adjunctive treatment and monotherapy (PAP) | QIDS-SR-16 14.5 | 59 | 33.8%/66.1% | 41.2 | No difference in antidepressant effect between psilocybin and escitalopram at week 6 QIDS-SR-16: −8.0 for psilocybin vs −6.0 for escitalopram [P = 0.17] (Primary outcome) |
|
| 20 mg/70 kg 30 mg/70 kg |
II | Randomized waiting-list controlled trial | 8 weeks (immediate treatment) 13 weeks (delayed treatment) | 4 weeks | NCT03181529 | 2 arms Immediate treatment (n 13) Delayed treatment (n 11) |
Monotherapy (PAP) | GRID-HDRS 22.8 | 27 | 67%/33% | 39.8 | Reduction in depressive symptoms GRID-HDRS: 8.0 at week 5 [d = 2.5], 8.5 at week 8 [2.6] for immediate treatment group [P < 0,001] GRID- HDRS: 23.8 at week 5 [d = 2.5], 23.5 at week 8 [2.6] for delayed treatment group [P < 0.001] (Primary outcome) |
||
| 25 mg | I | Randomized double-blind placebo-controlled crossover trial | 2 weeks | 0 | NCT03912974 | 2 arms Escitalopram + psilocybin Placebo + psilocybin |
Monotherapy (PAP) | Healthy subjects | 27 | 47.9%/52.1% | 34 | Escitalopram pretreatment reduced psilocybin adverse effects [P = 0.004], fear [P = 0.004], anxiety [P < 0.05], adverse autonomic effects [P < 0.02] (Primary outcome) |
||
| Pimavanserin | 34 mg | II | Randomized double-blind placebo-controlled trial | 10 weeks | 4 weeks | NCT03018340 | 2 arms Pimavanserin (n 52) Placebo (n 155) |
Adjunctive treatment | MADRS > 20 | 207 | 72.9%/27.1% | ≥ 18 | Reduction in depressive symptoms at week 5 HDRS-17: −11.5 for pimavanserin vs −7.5 for placebo [P = 0.003] [d = 0.63] (Primary outcome) |
|
| Zuranolone | 30 mg | II | Randomized double-blind placebo-controlled trial | 2 weeks | 6 weeks | NCT03000530 | 2 arms Zuranolone (n 45) Placebo (n 44) |
Monotherapy | HDRS-17 25.2 | 89 | 62.7%/37.3% | 44.3 | Reduction in depressive symptoms at day 15 HDRS-17: −17.4 for zuranolone vs −10.3 for placebo [P = 0.0005] [d = 81] |
|
| Ph-10 | 3.2 µg 6.4 µg |
NA | Randomized double-blind placebo-controlled parallel-group trial | 8 weeks | 1 week | ISSN2456-9836 | 3 arms PH-10 6. PH-10 3.2 Placebo |
Monotherapy | HDRS-17 PH10 6.4 24.7 HDRS-17 PH10 3.2 22.4 |
30 | 60%/40% | PH10 6.4 46.6 PH10 3.2 33.2 |
Reduction in depressive symptoms at week 8 HDRS-17: −17.80 for PH-10 6.4 [P = 0.022] [d = 0.95] vs −16.3 for PH-10 3.2 [P = 0.101] [d = 0.74] vs −10.9 for placebo (Primary outcome) |
|
| Seltorexant | 20 mg | I | Multicenter randomized double-blind placebo-controlled parallel-group trial | 10 days or 28 days | 2 weeks | NCT02476058 | 3 arms Seltorexant (n 22) Placebo (n 12) Active placebo (n 13) |
Monotherapy and adjunctive treatment | IDS-C30 36.8 | 47 | 34%/66% | 18–64 |
Reduction in depressive symptoms at day 11 HDRS-17: −5.5 for seltorexant vs −3.6 for placebo vs −4.1 for active placebo [P < 0.05] (Primary outcome) |
|
| 10 mg 20 mg 40 mg |
I | Double-blind placebo-controlled four-way crossover trial | Single dose | 1 week | NCT02067299 | 4 arms Placebo-seltorexant 10-20-40 Seltorexant 10-40-placebo-20 Seltorexant 20-placebo-40-10 Seltorexant 40-20-10-placebo |
Adjunctive treatment | HDRS-17 9.35 | 20 | 60%/40% | 43 | Failed to improve depressive symptoms at week 1 QIDS-SR: −2.1 for Seltorexant 40 vs −0.7 for placebo | ||
| 10 mg 20 mg 40 mg |
II | Multicenter randomized double-blind placebo-controlled dose-finding trial | 6 weeks | 2 weeks | NCT03227224 | 4 arms: Seltorexant 10 (n 33) Seltorexant 20 (n 61) Seltorexant 40 (n 52) Placebo (n 137) |
Adjunctive treatment | MADRS ≥ 25 | 287 | 53.7%/46.3% | 49.1 | Reduction in depressive symptoms at week 6 MADRS: −3.1 for SLX 20 vs -1.5 for SLX 40 [P = 0.083] (Primary outcome) |
||
| TRD | AV-101 | 1080 mg 1440 mg |
II | Randomized double-blind placebo-controlled crossover Trial |
2 weeks | 0 | NCT02484456 | 2 arms AV-101-placebo Placebo-AV-101 |
Monotherapy | HDRS ≥ 18 | 19 | 47.4%/52.6% | 41.28 | Failed to improve depressive symptoms at day 15 in the HDRS [P = 0.16] [d = 0.22] (Primary outcome) |
| 720 mg 1440 mg |
I | Randomized double-blind placebo-controlled crossover trial | 3 weeks | 0 | NCT03583554 | 3 arms Placebo-AV-101 720- 1440 AV-101 720-1440-placebo AV-101 1440-placebo 720 |
Monotherapy | Healthy subjects | 10 | 8.3%/91.7% | 32.6 | 1440 mg showed consistent NMDAR blockade (Primary outcome) |
||
| Psilocybin | 10 mg 25 mg |
NA | Open-label trial | 2 psilocybin sessions 1 week apart | 3 months | ISRCTN14426797 | Single arm | Monotherapy (PAP) | QIDS 19.2 | 12 | 50%/50% | 44.7 | Reduction in depressive symptoms at 1 week and month 3 QIDS psilocybin 25: −11.8 at week 1 [P = 0.002] [g = 3.1] vs −9.2 at month 3 [P = 0.003] [g = 2] (Primary outcome) |
|
| PPD | Zuranolone | 30 mg | III | Randomized double-blind placebo-controlled trial | 2 weeks | 4 weeks | NCT02978326 | 2 arms Zuranolone (n 76) Placebo (n 74) |
Monotherapy | HDRS-17 ≥ 28.4 | 153 | 100%/0% | 28.3 | Reduction in depressive symptoms at day 15 HDRS-17: -17.8 for ZRN vs -13.6 for placebo [P = 0.003] [d = 0.53] (Primary outcome) |
| MDD and Parkinson’s disease | Pimavanserin | 34 mg | II | Open-label trial | 8 weeks | 2 weeks | NCT03482882 | Single arm | Monotherapy (n 21) adjunctive treatment (n 24) | HDRS 19.2 | 45 | 48.9%/51.1% | 69.3 | Reduction in depressive symptoms at week 8 HDRS-17: -11.2 for monotherapy vs -10.2 for adjunctive therapy [P < 0.0001] |
| Cancer-related depression and anxiety | Psilocybin | 0.2 mg/kg | I/II | Randomized double-blind placebo-controlled trial | 2 psilocybin sessions, several weeks apart |
6 months | NCT00302744 | 2 arms Psilocybin Active placebo |
Monotherapy (PAP) | BDI ≥ 15 | 12 | 91.7%/8.3% | 36–58 | Failed to improve depressive symptoms (BDI) and anxiety symptoms (STAI) at week 2 (Primary outcome) STAI reduced at month 1 [P = 0.001] and at month 3 [P = 0.03] BDI reduced at month 6 [P = 0.03] |
| 1 or 3 mg/70 kg 22 or 30 mg/70 kg |
II | Randomized double-blind crossover trial | 2 psilocybin sessions, 5 weeks apart |
6 months | NCT00465595 | 2 arms Psilocybin 1 or 3 first (n 25) Psilocybin 22 or 30 first (n 26) |
Monotherapy (PAP) | GRID-HDRS-17 22 | 56 | 49%/51% | 56.3 | Reduction in depressive symptoms at 6 months GRID-HDRS-17: clinical response rate of 78% for two-dose sequence group [d = 1.55] (Primary outcome) Reduction in anxiety symptoms at 6 months HAM-A: clinical response rate of 83% for two-dose sequence group [d = 1.55] (Primary outcome) |
||
| 0.3 mg/kg | I | Randomized double-blind placebo-controlled crossover Trial |
2 psilocybin sessions, 7 weeks apart |
6 months | NCT00957359 | 2 arms Psilocybin first (n 14) Active placebo first (n 15) |
Monotherapy (PAP) | BDI ≥ 15 | 31 | 62.1%/37.9% | 56.28 | Reduction in depressive symptoms at week 7 (prior the crossover) BDI: clinical response rate of 83% for psilocybin group first vs 14% for active placebo group first [P < 0.05][d = 0.82] (Primary outcome) Reduction in anxiety symptoms at week 7 (prior the crossover) HADS: clinical response rate of 58% for PSY group first vs 14% for active placebo group first [P ≤ 0.01][d = 1.07] (Primary outcome) |
||
| 0.3 mg/kg | NA | Randomized double-blind placebo-controlled crossover trial | 2 psilocybin sessions, 7 weeks apart |
4.5 years | NA | 2 arms Psilocybin first (n 6) Active placebo first (n 5) |
Monotherapy (PAP) | NA | 11 | 63.6%/36.4% | 60.3 | Reduction in suicidal ideation at 8 h [P < 0.001] and sustained for 6.5 months [P < 0.001] (Primary outcome) Reduction in LoM at 2 weeks [P = 0.005] and sustained for 6.5 months [P < 0.001], 3.2 years [P < 0.001], and 4.5 years [P < 0.001] |
||
| AIDS-related depression | Psilocybin | 0.3 mg/kg 0.36 mg/kg |
I | Open-label trial | 1 psilocybin session | 3 months | NCT02950467 | Single arm | Monotherapy (PAP) | DS-II ≥ 8/32 | 18 | 0%/100% | 59.2 | Reduction in demoralization at week 3 DS-II: −5.78 [hp2 = 0.47] (Primary outcome) |
For some studies, some data is not available (i.e. phase study, depressive symptoms severity scale at baseline, P value, effect size).
BDI, Beck Depression Inventory total score; DS-II, Demoralization Scale-II total score; GRID-HDRS, Grid-Hamilton Depression Rating Scale total score; HADS, Hospital Anxiety and Depression Scale total score; HAM-A, Hamilton Anxiety Rating Scale total score; HDRS, Hamilton Depression Rating Scale total score; IDS-C, Inventory of Depressive Symptomatology Clinician Rating total score; LoM, loss of meaning; MADRS, Montgomery-Asberg Depression Rating Scale total score; MDD, major depressive disorder; NA, not available; PAP, psilocybin assisted psychotherapy; PPD, postpartum depression; QIDS, Quick Inventory of Depressive Symptomatology total score; QIDS-SR, Quick Inventory of Depressive Symptomatology-Self Reported total score; STAI, State-Trait Anxiety Inventory total score; TRD, treatment-resistant depression.
Refers to the length over time of the pharmacological intervention.
Refers to the length of monitoring over time of participant’s health after the end of the pharmacological intervention.
Table 3.
Completed clinical trials but not published in peer-reviewed journals until September 2022
| Mood disorder | Drug | Dose | Phase | Type of study | Durationa | Follow upb | NCT number | Trial design | Adjunctive treatment or monotherapy | Depressive symptoms severity scale at baseline | Population | Drug effectiveness on depressive symptoms | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Sex (F/M) | Age range or mean age | ||||||||||||
| MDD | AV-101 | 1440 mg | II | Multicenter randomized double-blind placebo-controlled parallel-group Trial |
2 weeks | NA | NCT03078322 | 2 arms AV-101 Placebo |
Adjunctive treatment | HDRS-17 > 20 | 199 | NA | 18–65 | Failed to improve depressive symptoms at day 15 in the MADRS (Primary outcome) |
| AXS-05 | 45/105 mg | III | Multicenter open-label trial | 12 months | NA | NCT04039022 | Single arm | Monotherapy | MADRS 33 | 876 | 62%/38% | 42.4 | Reduction in depressive symptoms at week 6 MADRS: −21.1 (Primary outcome) |
|
| 45/105 mg | II | Multicenter open-label trial | 12 months | NA | Substudy of NCT04039022 (antidepressant unresponsive patients) |
Single arm | Monotherapy | MADRS 33.3 | 115 | NA | NA | Reduction in depressive symptoms at week 6 MADRS: −19.1 (Primary outcome) |
||
| 45/105 mg | III | Multicenter open-label trial | 12 months | NA | Substudy of NCT04039022 (patients with suicidal ideation) |
Single arm | Monotherapy | MADRS-suicidal ideation 3.4 MADRS 36.8 |
37 | NA | NA | Reduction in suicidal ideation at week 3 MADRS-SI: -2.8 [P = 0,001] (Primary outcome) |
||
| Pimavanserin | 34 mg | III | Multicenter randomized double-blind placebo-controlled parallel-group trials | 6 weeks | NA |
NCT03968159 and NCT03999918 |
2 arms Pimavanserin (n 148) Placebo (n 150) |
Adjunctive treatment | NA | 298 | 69.8%/30.2% | ≥ 18 | Failed to improve depressive symptoms at week 5 HDRS-17: −9.0 for pimavanserin vs −8.1 for placebo [P = 0.296] (Primary outcome) |
|
| Zuranolone | 50 mg | III | Multicenter randomized double-blind placebo-controlled trial | 2 weeks | Day 42 | NCT04442490 | 2 arms Zuranolone (n 27) Placebo (n 272) |
Monotherapy | HDRS-17 26.8 | 543 | 66%/34% | 39.7 | Reduction in depressive symptoms at day 15 HDRS-17: −14.1 for ZRN vs −12.3 for placebo [P = 0.0141] (Primary outcome) |
|
| 30 mg 50 mg |
III | Open-label trial | 2 weeks | 1 year | NCT03864614 | Single arm | Monotherapy (n 421) Adjunctive treatment (n 304) |
HDRS-17 25.3 | 725 | NA | 18–75 | Reduction in depressive symptoms at day 15 HDRS-17: −14.9 for zuranolone 30 vs −15.9 for zuranolone 50 |
||
| 50 mg | III | Randomized double-blind placebo-controlled trial | 2 weeks | 6 weeks | NCT04476030 | 2 arms Zuranolone (n 210) Placebo (n 215) |
Adjunctive treatment | HDRS-17 26.8 | 440 | NA | 18–64 | Reduction in depressive symptoms at day 3 HDRS-17: −8.9 for zuranolone vs −7.0 for placebo [P = 0.0004] (Primary outcome) |
||
| 20 mg 30 mg |
III | Multicenter randomized double-blind placebo-controlled trial | 2 weeks | Days 43–182 | NCT03672175 | 3 arms Zuranolone 20 (n 159) Zuranolone 30 (n 166) Placebo (n 157) |
Monotherapy | HDRS-17 ≥ 22 | 581 | 70.3%/29.7% | 18–65 | Failed to improve depressive symptoms at day 15 HDRS-17: –12.5 for zuranolone 30 vs -11.1 for placebo [P = 0.116] [d = 0.17] vs –11.5 for zuranolone 20 [P = 0.664] [d = 0.03] (Primary outcome) |
||
| Prax-114 | 40 mg | II/III | Randomized double-blind placebo-controlled trial | 4 weeks | 2 weeks | NCT04832425 | 2 arms PRAX-114 Placebo |
Monotherapy | HDRS-17 ≥ 23 | 216 | NA | 18–65 | Failed to improve depressive symptoms at day 15 in the HDRS-17 (Primary outcome) |
|
| Seltorexant | 20 mg 40 mg |
II | Multicenter randomized double-blind flexible-dose parallel-group trial | 6 months | 2 weeks | NCT03321526 | 2 arms Seltorexant (n 52) Quetiapine (extended-release) (n 52) |
Adjunctive treatment | NA | 104 | 66.3%/33.7% | 18–84 | Failed to improve depressive symptoms at week 12 in the MADRS | |
| TRD | AXS-05 | 45/105 mg | II | Multicenter randomized double-blind placebo-controlled trial | 52 weeks | 0 | NCT04608396 | 2 arms AXS-05 (n 22) Placebo (n 22) |
Monotherapy | NA | 44 | NA | ≥ 18 | Delayed the time to relapse of depressive symptoms up to 52 weeks [P = 0.002] (Primary outcome) |
| 45/105 mg | III | Randomized double-blind active-controlled two-period trial | 12 weeks | 0 | NCT02741791 | 2 arms AXS-05 (n 156) Bupropion (n 156) |
Monotherapy | NA | 312 | NA | 18–65 | Failed to improve depressive symptoms at week 6 in the MADRS [P = 0.117] [d = 0.21] (Primary outcome) |
||
| 45/105 mg | II | Multicenter open-label trial | 12 months | 0 | NCT04634669 | Single arm | Monotherapy | MADRS 32.2 | 150 | 60.7%/39.3% | 45.6 | Reduction in depressive symptoms at 12 months MADRS: −24.5 [P < 0.001] (Primary outcome) |
||
| Psilocybin | 1 mg 10 mg 25 mg |
II | Randomized double-blind controlled trial | Single treatment session | 12 weeks | NCT03775200 | 3 arms Psilocybin 1 Psilocybin 10 Psilocybin 25 |
Monotherapy (PAP) | NA | 233 | NA | ≥18 | Reduction in depressive symptoms at week 3 MADRS: −6.6 Psilocybin 25 vs psilocybin 1 [P < 0.001] (Primary outcome) |
|
| PPD | Zuranolone | 50 mg | III | Randomized double-blind placebo-controlled trial | 2 weeks | 4 weeks | NCT04442503 | 2 arms Zuranolone (n 97) Placebo (n 98) |
Monotherapy | HDRS-17 ≥ 26 | 195 | NA | 18–45 | Reduction in depressive symptoms at day 15 HDRS-17: −15.6 for zuranolone vs −11.6 for placebo [P = 0.0007] (Primary outcome) |
| Bipolar depression | Zuranolone | 30 mg | III | Open-label trial | 2 weeks | 4 weeks | NCT03692910 | 2 arms Zuranolone Placebo |
Monotherapy | HDRS 25.7 | 35 | 23%/12% | 47.6 | Reduction in depressive symptoms MADRS: −7.7 on day 3, -15.5 on day 15, -16.4 on day 42 |
| PMD | Prax-114 | 60 mg | II | Open-label trial | 2 weeks | 2 weeks | NA | Single arm | Monotherapy | HDRS-17 25.3 | 6 | 100%/0% | NA | Reduction in depressive symptoms at day 15 HDRS-17: −12 (Primary outcome) |
For some studies, some data is not available (i.e. follow-up, depressive symptoms severity scale at baseline, sex, age range or mean age, P value, effect size).
HDRS, Hamilton Depression Rating Scale total score; MADRS, Montgomery-Asberg Depression Rating Scale total score; MADRS-SI, Montgomery-Asberg Depression Rating Scale-Suicidal Ideation total score; MDD, major depressive disorder; NA, not available; PAP, psilocybin assisted psychotherapy; PMD, perimenopausal depression; PPD, postpartum depression; TRD, treatment-resistant depression.
Refers to the length over time of the pharmacological intervention.
Refers to the length of monitoring over time of participant’s health after the end of the pharmacological intervention.
Table 4.
Ongoing, terminated, or completed clinical trials without results posted until September 2022
| Mood disorder | Drug | Dose | Phase | Type of study | NCT number | Trial design | Adjunctive treatment or monotherapy | Depressive symptoms severity scale at baseline | Population | Drug effectiveness on depressive symptoms | Status | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Range age | |||||||||||
| MDD | REL-1017 | 25 mg | III | Multicenter randomized double-blind placebo-controlled trial | NCT04688164 | 2 arms REL-1017 Placebo |
Adjunctive treatment | NA | 400 | 18–65 | Reduction in depressive symptoms at day 28 in the MADRS (Primary outcome) |
No results posted |
| 25 mg | III | Multicenter randomized double-blind placebo-controlled trial | NCT04855747 | 2 arms REL-1017 Placebo |
Adjunctive treatment | NA | 400 | 18–65 | Reduction in depressive symptoms at day 28 in the MADRS (Primary outcome) |
Recruiting | ||
| 25 mg | III | Multicenter randomized double-blind placebo-controlled trial | NCT05081167 | 2 arms REL-1017 Placebo |
Monotherapy | NA | 400 | 18–65 | Reduction in depressive symptoms at day 28 in the MADRS (Primary outcome) |
No results posted | ||
| 25 mg | III | Open-label trial | NCT04855760 | Single arm | Adjunctive treatment | NA | 600 | 18–65 | Incidence of TEAEs (Primary outcome) | Recruiting | ||
| Psilocybin | 25 mg | II | Randomized double-blind support-of-concept trial | NCT03866174 | 2 arms Psilocybin Active placebo |
Monotherapy (PAP) | NA | 100 | 21–65 | Reduction in depressive symptoms at day 43 in the MADRS (Primary outcome) |
Not recruiting | |
| 0.215 mg/kg | II | Randomized double-blind placebo-controlled trial | NCT03715127 | 2 arms Psilocybin Placebo |
Monotherapy (PAP) | MADRS ≥ 10/≤40 | 55 | 18–60 | Reduction in depressive symptoms at day 32 in the MADRS and BDI (Primary outcome) |
No results posted | ||
| 0.1 mg/kg 0.3 mg/kg |
I | Randomized double-blind placebo-controlled crossover trial | NCT03554174 | 4 arms Placebo-psilocybin 0.1 Placebo-psilocybin 0.3 Psilocybin 0.1-placebo Psilocybin 0.3-placebo |
Monotherapy (PAP) | NA | 18 | 18–65 | Reduction in depressive symptoms at weeks 1 and 2 after each experimental session in the GRID-HDRS | Not recruiting | ||
| 25 mg | II | Randomized double-blind placebo-controlled trial | NCT03380442 | 3 arms Psilocybin Ketamine No treatment group |
Monotherapy (PAP) | HDRS ≥ 17 | 60 | 18–64 | Reduction in depressive symptoms at months 3 and 6 in the QIDS (Primary outcome) |
Unknown | ||
| 25 mg | II | Randomized double-blind placebo-controlled trial | NCT04620759 | 2 arms Psilocybin Placebo |
Monotherapy (PAP) | GRID-HDRS ≥ 18 | 90 | 21–65 | Reduction in depressive symptoms at month 1 in the GRID-HDRS (Primary outcome) |
Recruiting | ||
| 25 mg | II | Randomized double-blind placebo-controlled trial | NCT04630964 | 2 arms Psilocybin Active placebo |
Monotherapy (PAP) | MADRS > 22/≤30 | 35 | 20–65 | Reduction in depressive symptoms at day 8 in the MADRS (Primary outcome) |
Not recruiting | ||
| Pimavanserin | 34 mg | III | Open-label trial | NCT04000009 | Single arm | Monotherapy | NA | 235 | >18 | Number of participants with TEAEs (Primary outcome) |
Terminated for business reasons and not due to safety concerns | |
| Zuranolone | 30 mg | III | Randomized double-blind placebo-controlled trial | NCT03771664 | 2 arms Zuranolone Placebo |
Monotherapy | HDRS ≥ 20 | 87 | 18–64 | Improvement of sleep efficiency assessed by polysomnography on day 14 (Primary outcome) |
Terminated (internal company decisions) | |
| 30 mg | III | Randomized double-blind placebo-controlled trial | NCT04007367 | 2 arms Zuranolone Placebo |
Monotherapy | HDRS ≥ 20 | 52 | 18–65 | Time to relapse of depressive symptoms (Primary outcome) |
Terminated (internal company decisions) | ||
| PRAX-114 | 10 mg 20 mg 40 mg 60 mg |
II | Randomized double-blind placebo-controlled dose-ranging trial | NCT04969510 | 5 arms PRAX-114 10 PRAX-114 20 PRAX-114 40 PRAX-114 60 Placebo |
Monotherapy adjunctive treatment | HDRS-17 ≥ 23 | 110 | 18–65 | Reduction in depressive symptoms at day 15 in the HDRS-17 (Primary outcome) |
No results posted | |
| Seltorexant | NA | I | Randomized double-blind placebo-controlled trial | NCT04951609 | 2 arms Seltorexant Placebo |
Adjunctive treatment | NA | 52 | 12–17 | Reduction in depressive symptoms at week 6 in the MADRS | Recruiting | |
| NA | I | Multicenter randomized double-blind Placebo and positive controlled four-way crossover Trial |
NCT04451187 | 4 arms Seltorexant dose 1 Seltorexant dose 2 Placebo Zoplicone |
Adjunctive treatment | MADRS ≥ 18 | 63 | 21–80 | Driving performance as assessed in an on-road driving test (Primary outcome) |
Not recruiting | ||
| NA | III | Multicenter randomized double-blind placebo-controlled parallel-group trial | NCT04532749 | 2 arms Seltorexant Placebo |
Adjunctive treatment | HDRS-17 ≥ 20 | 212 | 18–74 | Reduction in depressive symptoms at day 43 in the MADRS (Primary outcome) |
Stopped as a result of the interim analysis -no results posted | ||
| NA | III | Multicenter randomized double-blind placebo-controlled parallel-group trial | NCT04533529 | 2 arms Seltorexant Placebo |
Adjunctive treatment | HDRS-17 ≥ 20 | 550 | 18–74 | Reduction in depressive symptoms at week 6 in the MADRS (Primary outcome) |
Recruiting | ||
| NA | III | Randomized double-blind parallel-group trial | NCT04513912 | 2 arms Seltorexant quetiapine (extended-release) |
Adjunctive treatment | HDRS-17 ≥ 20 | 720 | 18–74 | Treatment response at week 26 in the MADRS (Primary outcome) |
Recruiting | ||
| TRD | AXS-05 | 45/105 mg | II | Randomized double-blind active placebo-controlled Trial |
NCT04971291 | 2 arms AXS-05 Bupropion |
Monotherapy | NA | 312 | 18–65 | Reduction in depressive symptoms at week 6 in the MADRS (Primary outcome) |
Enrolling by invitation |
| Psilocybin | 5 mg 25 mg |
II | Randomized double-blind active placebo-controlled parallel-group trial | NCT04670081 | 3 arms Psilocybin 5 Psilocybin 25 Placebo |
Monotherapy (PAP) | NA | 144 | 25–65 | Treatment response at week 6 in the HDRS (Primary outcome) |
Recruiting | |
For some studies, some data is not available (i.e. drug’s dose and depressive symptoms severity scale at baseline).
BDI, Beck Depression Inventory total score; GRID-HDRS, Grid-Hamilton Depression Rating Scale total score; HDRS, Hamilton Depression Rating Scale total score; MADRS, Montgomery-Asberg Depression Rating Scale total score; MDD, major depressive disorder; NA, not available; NMDAR, n-methyl-d-aspartate receptor; PAP, psilocybin assisted psychotherapy; QIDS, Quick Inventory of Depressive Symptomatology total score; TEAE, treatment-emergent adverse event; TRD, treatment-resistant depression.
Compounds acting on the glutamatergic system
4-Chlorokynurenine
Av-101 (4-chlorokyneurine, 4-Cl-KYN) is a prodrug, rapidly converted to 7-chloro-kynurenic acid (7-Cl-KYNA) by kynurenine aminotransferase (Salituro et al., 1994). 7-Cl-KYNA exerts a neuroprotective action by a full antagonism activity on the glycine B co-agonist site of the NMDAR (Zanos et al., 2015). The effects of this drug are also linked to the metabolite 4-chloro-3-hydroxyanthranilic acid (4-Cl-3-HAA), which inhibits the quinolinic acid, a neurotoxic NMDAR agonist (Walsh et al., 1994). Considering the evidence implicating the kynurenine pathway in the aetiology of mood disorders (Brown et al., 2021), and the rapid, dose-dependent, and sustained antidepressant effect following a single administration in an animal study (Zanos et al., 2015), AV-101 was tested in humans; however, it failed to demonstrate benefits on depressive symptoms at week 2, according to two randomized clinical trials (RCTs) in patients with MDD (ClinicalTrials.gov Identifier NCT03078322 and NCT02484456). The drug failed both as adjunctive treatment to standard antidepressants (1440 mg/day for 2 weeks) (VistaGen Therapeutics, 2019) and in TRD as monotherapy (1080 mg/day for the first week, then 1440 mg/day for the second week) (Park et al., 2020). These negative results raised the question of whether AV-101 could penetrate the blood-brain barrier. To address this question, dose-related effects of AV-101 (720 and 1440 mg) on NMDARs engagement were examined in a small sample of healthy individuals (ClinicalTrials.gov Identifier NCT03583554) (Murphy et al., 2021). The dose of 1440 mg, but not 720 mg, showed consistent NMDAR blockade measured by an increase in electroencephalogram γ-band activity. Therefore, the dose of 1440 mg shows antagonist activity on the NMDARs, but no antidepressant effect was demonstrated in the available RCTs, which were both short-term studies.
Esmethadone
Rel-1017 is a dextro-isomer of racemic methadone (esmethadone or dextromethadone), with a low affinity and noncompetitive NMDARs antagonism profile (Bernstein et al., 2019); it also shows a micromolar affinity for the serotonin transporter (SERT) and norepinephrine transporter (NET) (Nemeroff, 2022). Compared to its stereoisomer (levomethadone), it shows a lower affinity for opioid receptors and therefore a lack of opioid-like effects such as analgesic, tolerance and dependence properties, or sedation/respiratory depression (Gorman et al., 1997). After demonstrating antidepressant activity in animal models, with modulation of synaptic connections and plasticity in the medial prefrontal cortex (Fogaça et al., 2019), REL-1017 was tested in phase I clinical trials. In two multiple doses ascending studies, single doses of up to 150 mg and repeated dosing of up to 75 mg daily for 10 days exhibited linear pharmacokinetics and a good safety profile, characterized by dose-related nausea and somnolence at higher doses (Bernstein et al., 2019). Rel-1017 received the FDA fast-track designation as an adjunctive treatment for MDD. There are two ongoing RCTs assessing the efficacy of this drug in MDD as an adjunctive treatment to the ongoing antidepressant in the short term (28 days) (ClinicalTrials.gov Identifier NCT04688164 and NCT04855747), and one well powered open-label study in the long term (1 year) (ClinicalTrials.gov Identifier NCT04855760). Other RCTs will consider REL-1017 as monotherapy (ClinicalTrials.gov Identifier NCT05081167) (Hecking et al., 2021). While these studies are still ongoing and do not have results, a 1-week small pilot study reported promising findings (ClinicalTrials.gov Identifier NCT03051256). In this study, REL-1017 showed a rapid antidepressant effect (by day 4), sustained up to 7 days after the last dose, in patients with MDD maintained on their previous antidepressant regimen (to which they have had an inadequate response). Both 25 and 50 mg showed mood improvement associated with an enhancement in cognitive and social functioning, anxiety, agitation, anger, irritability, and sleep quality (Fava et al., 2022).
Dextromethorphan-bupropion
AXS-05 is an oral combination of dextromethorphan and bupropion (Stahl, 2019). Dextromethorphan integrates the uncompetitive antagonism towards NMDARs with the sigma-1 receptor (S1R) agonism, the nicotinic acetylcholine receptors antagonism, and with activities on the SERT and NET (Taylor et al., 2016). Bupropion primarily acts selectively blocking neuronal reuptake of noradrenaline and dopamine (Stahl, 2019). Additionally, bupropion prevents dextromethorphan from being rapidly metabolized by cytochrome P450 2D6 (CYP2D6), leading to an increase in its bioavailability (Hecking et al., 2021). This innovative combination suggests a powerful pharmacological synergy and wide clinical use of AXS-05 across various psychiatric conditions (Stahl, 2019). The effectiveness of AXS-05 was proved with positive results in well powered studies of moderate or severe MDD, also in comorbidity with anxiety disorders. When compared with bupropion (Tabuteau et al., 2022) or placebo (Iosifescu et al., 2022), it had a more rapid onset of action (by week 1) and durable effect in the short-medium term (by week 6) in two phases II large RCTs (ClinicalTrials.gov Identifier NCT03595579 and NCT04019704), leading to FDA approval for MDD in August 2022. One open-label study (ClinicalTrials.gov Identifier NCT04039022) confirmed these promising findings in the long term (up to 12 months), showing an improvement in work/study, social and family functioning as well (Mardi, 2022), also in a sample unresponsive to other antidepressants (Axsome Therapeutics, 2020a). A very small study in a group of patients with suicidal ideation found a rapid remission of suicidal ideation by week 1, as well as an improvement in overall functioning by week 6 (Axsome Therapeutics, 2020b). AXS-05 demonstrated encouraging results also in TRD by week 1, with effects on depression, anxiety, and psychosocial functioning lasting up to 12 months (ClinicalTrials.gov Identifier NCT04634669) (Axsome Therapeutics, 2022). The drug consistently reduced the risk of depressive symptoms relapse (ClinicalTrials.gov Identifier NCT04608396) (Axsome Therapeutics, 2021). The primary antidepressant action of this drug was associated with an enhancement in cognitive function (Axsome Therapeutics, 2020c), a durable improvement in psychosocial functioning (Axsome Therapeutics, 2022), as well as a rapid and progressive relief of anxiety symptoms associated with TRD (Axsome Therapeutics, 2020c, 2022); however, one RCT in a well powered sample did not confirm AXS-05 benefits in TRD in a 12-week study (ClinicalTrials.gov Identifier NCT02741791) (Axsome Therapeutics, 2020c). An additional RCT is currently ongoing and aims to evaluate AXS-05 for MDD not responding to standard antidepressants and MDD with suicidal ideation (ClinicalTrials.gov Identifier NCT04971291) (Clinicaltrials.gov, 2021c). To summarize, AXS-05 has good evidence of efficacy on overall depressive and anxiety symptoms but targets well also suicidal ideation and cognitive dysfunction, two symptoms that often do not respond well to standard antidepressants, with resulting benefits on functioning in relevant areas of life.
Compounds acting on the serotonergic system
Psilocybin
Psilocybin (4-phosphoryloxy-N,N-dimethyltryptamine) is a tryptamine alkaloid hallucinogen naturally present in the psilocybe genus of mushrooms (Passie et al., 2002). Its antidepressant effect is putatively mediated by the serotonergic system, specifically by serotonin 2A receptors (5HT2ARs) agonism that leads to a major glutamate release by pyramidal neurons that express these receptors in the PFC. This activation results in a modulation of brain circuit connections (Carhart-Harris et al., 2014); however, the mechanisms behind the persistence over time of these neuroplasticity phenomena remain unknown. Imbalances of serotonin in the brain underpin and modulate depression, as well as emotional and physical symptoms of anxiety, cognition, learning, memory, reward processing, and sleep (Pourhamzeh et al., 2022).
Psilocybin has been studied for the treatment of MDD, but also for depression associated with general medical comorbidities. In addition to its potential therapeutic role in mood disorders, psilocybin showed preliminary benefits in obsessive-compulsive disorder (Moreno et al., 2006) and substance dependence (Veen et al., 2017).
All studies evaluated the effects of psilocybin administered in supportive and controlled environments, in combination with behavioural psychological support provided by trained therapists (psilocybin-assisted psychotherapy, PAP).
Major depressive disorder
PAP may represent a successful option for reducing depressive symptoms, with or without anxiety, with rapid effects lasting for about 6 months. This efficacy was confirmed in a recent meta-analysis of four small trials (Goldberg et al., 2020). Psilocybin was recognized as a breakthrough therapy for TRD by the FDA in 2018 and for MDD in 2019.
Positive effects of psilocybin in TRD, especially for the dose of 25 mg/day, are supported by a pilot study (ISRCTN14426797) (Carhart-Harris et al., 2016) and two well powered RCTs (ClinicalTrials.gov Identifier: NCT03775200 and NCT04670081, which is still ongoing) (Clinicaltrials.gov, 2021b; Compass Pathways, 2021). The results showed a rapid reduction of depressive and anxiety symptoms (by week 1 and week 3) and sustained improvement until months 3 and 6 in some participants. Importantly, anhedonia improved together with depressive symptoms (Carhart-Harris et al., 2016). Similarly, pessimism bias (unrealistic pessimism when predicting the occurrence of future life events) is a manifestation deeply connected with severe depression and decreased after administration of psilocybin, as reported in a post-hoc study with a small sample size of patients with TRD (Lyons and Carhart-Harris, 2018). Consistent with these cognitive changes, in a recent study psilocybin showed benefits on cognitive empathy in TRD patients with a subsequent improvement in emotional face recognition; this effect correlated also with reduced anhedonia (Stroud et al., 2018). These findings suggest that psilocybin may enhance cognitive flexibility (the capacity to adapt thoughts and behaviours to multiple environmental demands), as confirmed in another study, with an improvement found to persist for at least 4 weeks posttreatment (ClinicalTrials.gov Identifier NCT03181529) (Doss et al., 2021). Therefore, psilocybin seems to act positively on symptoms poorly targeted by standard antidepressants, but another important question is if it is overall superior to standard antidepressants in terms of depressive symptoms improvement. According to two RCTs, psilocybin is not more beneficial than escitalopram on overall depressive symptoms over a short period of time, but a different profile of action on depressive symptoms was confirmed. In a 6-week study (ClinicalTrials.gov Identifier NCT03429075) (Carhart-Harris et al., 2021), two administrations of psilocybin improved the perceived ability to cry and to feel compassion and intensified emotion and pleasure compared to escitalopram, as well as reduced suicidal ideation more than then escitalopram. In a crossover study (ClinicalTrials.gov Identifier NCT03912974) (Becker et al., 2022), a single dose of psilocybin was administered after a 2-week pretreatment with escitalopram or placebo. Escitalopram reduced psilocybin-induced adverse events [increased blood pressure and heart rate (HR), pupil dilatation, and other acute autonomic events], and negative psychic effects such as anxiety (‘anxious ego-dissolution’); however, escitalopram did not show additional efficacy on positive mood effects of psilocybin versus placebo. Finally, various phase I (ClinicalTrials.gov Identifier: NCT03554174) and phase II RCTs (ClinicalTrials.gov Identifier: NCT03866174, NCT03715127, NCT04620759, NCT04630964, NCT03380442) are currently ongoing. These trials aim to confirm the efficacy of psilocybin on depressive symptoms and some of them will also investigate neuroplasticity changes and brain mechanisms underpinning these changes (Clinicaltrials.gov, 2018, 2019a, 2019b, 2021d, 2021e, 2021f).
Major depressive disorder associated with general medical comorbidities or life-threatening illnesses
There is strong evidence supporting the efficacy of psilocybin on depressive and anxiety symptoms associated with life-threatening illnesses, such as neoplastic diseases or AIDS.
Clinical trials focused on psilocybin use in patients with advanced-stage cancers, located in the breast, colon, ovary, peritoneum, or salivary gland. Lymphoma, leukemia, and myeloma were also included (Doblin et al., 2019). Robust efficacy was confirmed in two systematic reviews as well (Reiche et al., 2018; Ross, 2018). The antidepressant and anxiolytic effects of psilocybin were immediate and persisted in a large percentage of patients for at least 6 months after a single active treatment [ClinicalTrials.gov Identifier: NCT00465595 (Griffiths et al., 2016) and ClinicalTrials.gov Identifier: NCT00957359 (Ross et al., 2016)] or even up to 4.5 years following a single dose, as demonstrated by a long-term follow-up study (Agin-Liebes et al., 2020). Such enduring improvements in anxiety and depression led to a consequent reduction in demoralization, hopelessness, and death anxiety (Ross et al., 2016; Agin-Liebes et al., 2020) and an improvement in optimism, death acceptance, and quality of life (Griffiths et al., 2016); however, these positive findings followed the administration of a high dose of psilocybin (22 or 30 mg/70 kg) and did not occur with a low dose (1 or 3 mg/70 kg) (Griffiths et al., 2016), in line with the results of a pilot study (ClinicalTrials.gov Identifier: NCT00302744). Only trait anxiety decreased at months 1 and 3, whereas a significant decrease in measures of depression was demonstrated only at month 6 (Grob et al., 2011). Of note, there are reports of a major reduction in negative emotionality and increased creativity when assuming psilocybin micro-dosing rather than a single active treatment (Anderson et al., 2019). For micro-dosing, the FDA refers to a dose of the drug that is 1% of the pharmacologically active dose, up to a maximum of 100 µg (EMA 2003); however, no advantage of micro-dosing over placebo was found in patients with depressive/anxious disorders not related to neoplastic diseases (Marschall et al., 2022), while one RCT reported benefits in patients with life-threatening cancer. In this study, a single dose of psilocybin was associated with acute and sustained reductions in suicidal ideation as early as 8 h and sustained for 6.5 months posttreatment. Loss of meaning (LoM), a component of demoralization, had similar reduction as early as 2 weeks after treatment and was sustained at 4.5 years of follow-up. A positive relationship was found between reductions in LoM and suicidal ideation, and between suicidal ideation and hopelessness and demoralization (Ross et al., 2021). These encouraging results prompted another recent pilot study (ClinicalTrials.gov Identifier: NCT02950467), that showed robust reductions in self-reported demoralization, grief, and psychological trauma in AIDS survivors. Over 7 weeks, patients were treated with both individual and group psilocybin sessions (8 and 12–15 h respectively). The self-reported demoralization, the primary clinical outcome, linearly decreased from baseline to the last follow-up assessment point at month 3 (Anderson et al., 2020).
Pimavanserin
Pimavanserin is an atypical antipsychotic that does not directly target dopamine receptors (Rissardo et al., 2022). Indeed, it predominantly acts on the serotonergic neurotransmission, through an inverse agonism and antagonism on the 5-HT2ARs, and the 5-HT2CRs at higher doses (Vanover et al., 2006). It is approved by the FDA to treat hallucinations and delusions associated with psychosis in Parkinson’s disease with a recommended dose range between 34 and 40 mg once daily.
Major depressive disorder
In light of the serotonergic profile, pimavanserin effectiveness was also tested in mood disorders, particularly in MDD. Short- to medium-term benefits (weeks 1–5) on depressive symptoms were demonstrated by the first stage of one RCT performed in a well powered sample (Clinical trial identification number NCT03018340); however, the second stage did not confirm these positive results, probably due to the smaller sample size (Fava et al., 2019). In this study, pimavanserin was administered as adjunctive treatment to standard antidepressants (SSRI or SNRI) and produced benefits on depressive symptoms that did not respond to previous antidepressant treatments. Moreover, significant improvement was observed for several secondary endpoints including impulsivity, irritability, anxiety, insomnia and daytime sleepiness, sexual and psychosocial functioning, and quality of life. In this regard, results from a secondary analysis have shown a reduction of anxiety and somatic symptoms in a sub-group of patients with severe and anxious MDD at baseline (Papakostas et al., 2020). Of note, in another secondary analysis, pimavanserin reduced both night sleep impairments and excessive day sleepiness and improved global functioning. Therefore, the efficacy of pimavanserin on psychosocial functioning may at least partly be mediated by the reduction of excessive daytime sleepiness (Jha et al., 2020). Interestingly, according to another post-hoc analysis, sexual function improved after treatment with pimavanserin. Women reported increased sexual desire, sexual arousal, orgasmic propensity, and general sexual satisfaction; similar results were not observed in male participants, probably due to the small number of men in the sample (Freeman et al., 2020). Noteworthy, the results of a secondary analysis demonstrated that pimavanserin determined a greater reduction in suicidal ideation by week 3 compared to placebo, despite the exclusion of patients with a history of previous suicide attempts and the small number of participants who reported suicidal ideation during the study (Shelton et al., 2020). Despite these promising results, three subsequent trials (Clinical trial identification number NCT03968159, NCT03999918, and NCT04000009) did not find any antidepressant effect, in the short or in the long term, and the sponsor decided not to pursue the development of pimavanserin for MDD; however, among secondary outcomes, an improvement in subjective quality of sleep was reported (Acadia, 2020).
Major depressive disorder associated with general medical comorbidities
Notwithstanding pimavanserin was withdrawn from development for MDD, there is currently one trial supporting potential benefits in reducing depressive occurrences in Parkinson’s disease, in the short-medium term (week 8), both in monotherapy and as adjunctive therapy to standard antidepressants (SSRI or SNRI) (ClinicalTrials.gov Identifier NCT03482882). This study also reported amelioration in secondary clinical outcomes such as global disease severity, sleep quality, overall quality of life, and motor function, though the sample size was small, and the psychometric instrument used in the study (Hamilton Depression Rating Scale or HDRS) was not developed for use in Parkinson’s disease (DeKarske et al., 2020).
Compounds acting as neuroactive steroids
Zuranolone
Zuranolone, an allopregnanolone analogous, is a neurosteroid that displays most of its therapeutic effects acting as a PAM of the synaptic and extra-synaptic GABAARs. It increases tonic and phasic GABAA currents (Botella et al., 2017). The facilitation of GABA circuits in the central nervous system (CNS) is a common mechanism of action shared also by endogenous neurosteroids (Abramian et al., 2014). Zuranolone is under investigation as a monotherapy for MDD (including PPD) and bipolar depression, using a treatment paradigm of 2 weeks. Its long half-life allows once-daily oral dosing, facilitating its administration and enhancing the therapeutic adherence (Althaus et al., 2020).
Major depressive disorder
Zuranolone shows antidepressant effects, especially in patients with depression and anxiety features, as demonstrated by three RCTs in well powered samples (ClinicalTrials.gov Identifier NCT03000530, NCT04442490, NCT04476030). Both 30 and 50 mg showed a rapid onset of action at day 3 and major benefits at day 15 in monotherapy (Gunduz-Bruce et al., 2019; Sage Therapeutics and Biogen, 2021b). When it was co-initiated with an SSRI or SNRI, benefits were reported during a 2-week treatment period, but not after, suggesting a nonsustained efficacy over time (Sage Therapeutics and Biogen, 2022). Although in one study the stabilization of symptoms persisted until day 42–45 (Sage Therapeutics and Biogen, 2021b), in another RCT in a larger sample (ClinicalTrials.gov Identifier NCT03672175), zuranolone 30 mg demonstrated benefits only in the very short term, from day 3 to day 12, and the dose of 20 mg was totally ineffective. Post-hoc analysis indicated that approximately 9% of the patients treated with 30 mg were nonadherent to the treatment. When the data were reanalyzed after excluding these patients, the dose of 30 mg improved depressive symptoms at day 15 (Sage Therapeutics, 2019). Zuranolone demonstrated a dose-dependently effect also in an open-label study, where the dose of 50 mg exhibited a stronger and more prolonged effect than the 30 mg dose in reducing depressive core symptoms, regardless of the co-therapy with an SSRI or SNRI (ClinicalTrials.gov Identifier NCT03864614). The majority of patients did not require more than two 2-week treatment cycles to reach remission (Sage Therapeutics and Biogen, 2021a). Zuranolone 30 mg gave initial evidence of effectiveness in cases with residual insomnia, with improvement in objective measures of quality of sleep, for example, total sleep time, latency to persistent sleep, the median number of awakenings, and mean duration of awakenings (ClinicalTrials.gov Identifier NCT03771664 and NCT04007367); however, both studies were terminated to advance the program with the 50 mg dose (Sage Therapeutics, 2022).
Postpartum depression
Zuranolone, both 30 and 50 mg doses, showed encouraging results as a treatment for PPD in two RCTs with well powered samples of outpatient women with sever mood impairment (ClinicalTrials.gov Identifier NCT02978326 and NCT04442503). The first trial included a sample of women who were up to the sixth month postpartum and had depression with onset between the last trimester and 4 weeks after delivery. Rapid (as soon as day 3) and persistent antidepressant effects (day 45) were demonstrated after 2 weeks of treatment with zuranolone 30 mg, as well as rapid improvement in anxiety, global functioning, and self-reported maternal function, despite a high placebo response (Deligiannidis et al., 2021). In the second trial, zuranolone 50 mg confirmed benefits on depression and anxiety, assessed at the same time points (Clinicaltrials.gov, 2020d); however, the possible persistence of the effect beyond 45 days and the long-term safety and efficacy are currently unknown.
Bipolar depression
Zuranolone 30 mg may provide rapid (by day 3) and prolonged (as far as day 42) relief of depressive symptoms in patients with bipolar depression type I or II, with a current major depressive episode, as suggested by an exploratory open-label study (ClinicalTrials.gov Identifier NCT03692910). In this study, the 2 weeks treatment paradigm was used, and two patients reported mild hypomania in the follow-up period, but no cases of mania, increased suicidal ideation, or suicidal behaviour were observed (Gunduz-Bruce et al., 2020). There is still no evidence of potential benefits for anxiety or insomnia associated with bipolar depression.
Prax-114
Prax-114 is a novel extra-synaptic GABAARs PAM, currently in phase II of development for the treatment of MDD (Hecking et al., 2021). In-vitro models it was demonstrated that PRAX-114 potentiates GABA-induced currents at both extra-synaptic and synaptic GABAARs, but with 10.5-fold greater potentiation of the former compared to the latter Rs. This pharmacodynamic property was suggested to potentially reduce the risk of sedative side effects, providing a wider therapeutic window versus other GABAARs PAMs, while keeping the antidepressant and anxiolytic benefits (Hughes et al., 2021).
Despite this promising pharmacodynamic mechanism, to our best knowledge PRAX-114 did not show any antidepressant effect in MDD when tested as monotherapy in a well powered RCT (ClinicalTrials.gov Identifier NCT04832425) (Praxis, 2022). Another well powered RCT (ClinicalTrials.gov Identifier NCT04969510) will evaluate the efficacy of PRAX-114 10–20–40–60 mg compared to placebo as adjunctive therapy to the current antidepressant treatment but no results are available (Clinicaltrials.gov, 2021a). Efficacy of PRAX-114, apart from mood disorders, is investigated also in posttraumatic stress disorder and essential tremor.
Menopausal and mood symptoms
There is preliminary evidence of efficacy of PRAX-114 in menopausal and mood symptoms. An open-label study used a single daily 60 mg dose in a small sample of outpatient women with menopausal distress and PMD. Prax-114 gradually improved mood symptoms and decreased moderate-to-severe vasomotor symptoms (e.g. hot flashes), in a 2-week treatment paradigm. Participants reported enhancement in energy and volition, sexual interest, sleep, anxiety, and irritability; however, symptoms worsened when treatment was discontinued, suggesting the need for a longer therapy (Praxis, 2021); however, these results were not published in a peer-reviewed journal, and apparently, the study was not preregistered.
Ph-10
Ph-10 (pregn-4-en-20-yn-3-one) is a neuroactive steroid that belongs to the group of ‘pherines’. Its intranasal administration engages local nasal chemosensory receptors that connect the olfactory system with limbic circuits through oligosynaptic neural connections (Monti et al., 2019). Extensive evidence demonstrated the fundamental role of the human olfactory system in social interactions, vegetative functions, emotions, and mood (Pause et al., 2001; Krusemark et al., 2013). Ph-10 demonstrated a good safety and tolerability profile versus placebo in a dose escalation study (from 0.8 to 6.4 µg), in a small sample of healthy volunteers (Monti et al., 2019). To the best of our knowledge, only one RCT testing this drug in MDD was published. The study included patients with moderate MDD and showed that self-administrated intranasal PH-10 at high dose (6.4 µg) had antidepressant effects by week 1, which were sustained in the short-to-medium-term (up to the study endpoint at week 8). A small dose-dependent effect favoured the high dose over the low dose (3.2 µg). Amelioration of depressive symptoms was not strongly supported by a significant improvement in quality and life satisfaction. These results cannot be generalized to patients with suicidal ideation, TRD, and bipolar depression, as these groups were excluded from the trial (Monti et al., 2019). Possible benefits of anhedonia, anxious or irritability features, sleep disturbances, or other specific depressive domains were not investigated.
Compound acting on the orexinergic system
Seltorexant (JNJ-42847922/MIN-202)
Seltorexant is a highly selective antagonist of the human orexin-2 receptor (OX-2R). There are two different orexin types: orexin A (OX-A) and orexin B (OX-B). Both are excitatory neuropeptide hormones, also known as hypocretins, that derive from a common pre-pro-orexin precursor produced in the posterior lateral hypothalamus. Their target is represented by orexin-1 receptor (OX-1R) and OX-2R, which are differently expressed through the CNS. OX-A displays a similar affinity for both receptors, and OX-B shows a greater affinity for OX-2R (Nollet and Leman, 2013).
The orexin system is increasingly being recognized as a key factor involved in hyperarousal and wake/sleeping transition and disturbances, with important implications in stressful or threatening situations. Orexins are involved in multiple functions often compromised during depressive episodes, such as the regulation of circadian rhythms, cognitive (including attention, learning, and memory) and sexual functioning, positive emotions and social interactions, hedonic capabilities and reward process, visceral functions, sensory systems, and pain (Nollet and Leman, 2013), as well as in anxiety and panic states (Johnson et al., 2012). Seltorexant is in phase III of development for MDD and has shown efficacy in normalizing sleep (Bonaventure et al., 2015). In an exploratory clinical study (Clinical trial identification number NCT02067299), the drug demonstrated a dose-dependent normalization of sleep after a single dose of 10–20 or 40 mg in MDD patients treated with SSRI or SNRI with residual insomnia (Brooks et al., 2019); the dose-dependent effect is in line with previous studies of OX2R antagonists in heathy subjects (Ark et al., 2018). The aforementioned study was not designed to test the possible antidepressant effect of seltorexant; however, it was reported that the 40 mg dose as monotherapy improved mood compared with other dosages. In a relatively small sample of patients with mild to moderate depression (Clinical trial identification number NCT02476058), seltorexant showed antidepressant effects as early as day 11 of treatment with 20 mg, with an improvement in self-reported sleep quality. The antidepressant efficacy was sustained with continued treatment for up to 28 days (Recourt et al., 2019). Higher benefits on core symptoms of depression and anxiety were demonstrated by seltorexant as adjunctive therapy to ongoing antidepressants in patients with MDD (Clinical trial identification number and NCT03227224). During these 6-week-period studies, the 20 mg dose showed higher antidepressant efficacy than the 10 and 40 mg doses, and it was even more effective in patients having sleep disturbances. The 40 mg dose improved sleep impairments, but it showed lower efficacy on depressive symptoms (Savitz et al., 2021). This result is in contrast with the findings previously mentioned, therefore it remains unclear which dose is more effective in case of residual insomnia in MDD.
Despite the positive results presented, one long follow-up RCT in a well powered sample did not find any benefit of seltorexant on depression and anxiety, with or without insomnia, when 20 and 40 mg were tested as adjunctive treatment to the ongoing antidepressant versus quetiapine extended-release 150–300 mg (ClinicalTrials.gov Identifier NCT03321526) (Clinicaltrials.gov, 2017).
Other phase II and phase III studies in adolescent, adult, and elderly patients with residual insomnia in MDD are ongoing (Clinical trial identification number NCT04532749, NCT04533529 NCT04451187, NCT04513912, NCT04951609) (Clinicaltrials.gov, 2020a, 2020b, 2020c, 2020e, 2021d). These studies will test seltorexant as adjunctive therapy to the ongoing antidepressant. To our current knowledge, there is no evidence of efficacy for seltorexant in TRD.
Adverse events, safety, and tolerability
Considering the impact of the safety profile on treatment adherence, in this paragraph we provide an overview of the most frequent adverse events associated with the drugs of interest, to guide the personalization of treatment choice also based on the tolerability profile, and to properly inform patients.
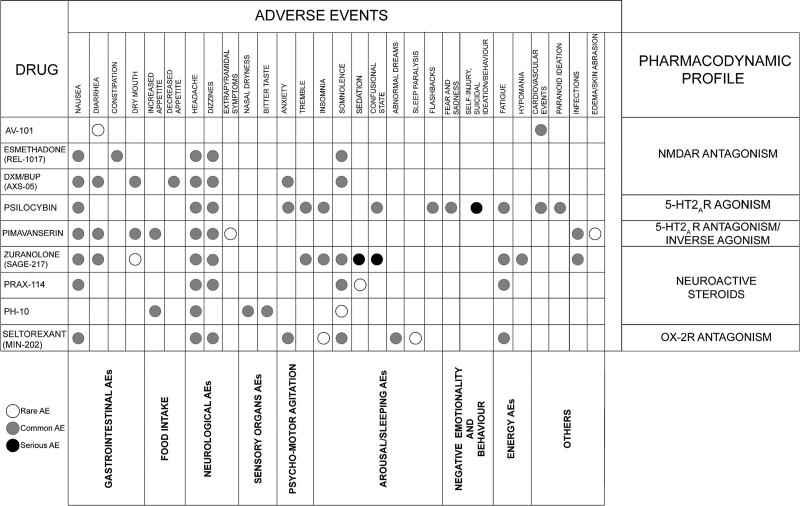
Overall, the discussed medications were safe and well tolerated, with a few serious adverse events. Details on the adverse events profile of each drug of interest are reported in Fig. 2; however, there is still limited evidence, particularly in the long term, and longitudinal studies are needed.
Fig. 2.
Description of adverse events of novel antidepressant drugs. Common adverse events were defined as those having a frequency ≥5%, while rare adverse events as those with a frequency <5%. Serious adverse events are defined as any adverse event causing clinically significant disability and incapacity or immediately life-threatening. BUP, bupropion; DXM, dextromethorphan; NMDAR, n-methyl-d-aspartate receptor; OX-2R, orexin type 2 receptor; R, receptor.
The neurological safety profile appears to be the most affected by new rapid agents. Headache was one of the most frequently reported adverse events for all compounds (apart from AV-101), with a frequency exceeding 10%; the risk seems to be more elevated in subjects exposed to psilocybin (67%) (Carhart-Harris et al., 2021) and seltorexant (40%) (Brooks et al., 2019). Dizziness was reported by 25% of patients treated with seltorexant (Brooks et al., 2019), despite being a common adverse event of other medications, with a frequency higher than 5%. Extrapyramidal symptoms (EPS), that is, akathisia, bradykinesia, and rigidity, were observed for pimavanserin, and ranged from 1.3% to 3.4%, without compromising motor function even in patients with Parkinson’s disease (DeKarske et al., 2020). Tremor is another adverse event that might represent an EPS or be associated with psychomotor agitation. In patients receiving psilocybin, it was mainly coupled with fear, sadness, and a physical experience of feeling the body shake (Doss et al., 2021). In patients treated with zuranolone, it was often associated with insomnia in 7% of patients and, similarly to the other adverse events of zuranolone, occurred predominantly during the 2-week treatment paradigm and then was no longer reported (Sage Therapeutics and Biogen, 2021a).
Pimavanserin and AV-101 appeared to be the safest with respect to psychomotor agitation, arousal and sleeping disturbances. On the other hand, 17–23.2% of patients with MDD and life-threatening illnesses treated with psilocybin, especially at higher doses, developed a mild activation syndrome at the beginning of treatment, mainly characterized by anxiety (Griffiths et al., 2016; Ross et al., 2016); however, in one trial anxiety affected 100% of patients (Carhart-Harris et al., 2016). Anxiety was generally acute and transient, but it also appeared several days after treatment (Anderson et al., 2020). It was mainly attributable to the psychological discomfort during psilocybin sessions, and it improved with reassurance from the clinical team.
Rates of insomnia were found to be over 10% for psilocybin and were sometimes accompanied by posttraumatic stress flashbacks (Anderson et al., 2020). In patients treated with seltorexant, few cases of unexpected insomnia (2.1%), sleep paralysis (1.4%), abnormal dreams (13%), and nightmares (1.4%) led to treatment discontinuation (Recourt et al., 2019; Savitz et al., 2021). In another trial of seltorexant, up to 13% of patients experienced abnormal dreams (Clinicaltrials.gov, 2017). No hypnagogic nor hypnopompic hallucinations were observed and there was no clear evidence of dose-related effect on these adverse events. Seltorexant safety profile seems similar to the first-in-class orexin receptor antagonist, namely suvorexant, already approved by FDA for the treatment of insomnia (Kuriyama and Tabata, 2017); however, unlike seltorexant, suvorexant has a longer half-life (about 12 h), with more severe adverse events, higher rates of sleeping disturbances (Sutton, 2015), as well as a functioning impairment, caused by symptoms similar to cataplexy that may occur the day after treatment (Recourt et al., 2019).
Although somnolence was common for different compounds, there are substantial differences. For seltorexant, somnolence appeared to be dose-related within the dose range of 10–40 mg/day, and was reported by 25% of patients (Brooks et al., 2019); however, somnolence was most frequent in patients treated with the GABAARs modulators PRAX-114 and zuranolone, with maximum rates of 37.5% and 26.5%, respectively (Clinicaltrials.gov, 2020d; Praxis, 2021). Despite resolving within a few hours, sedation may represent a serious adverse event for both compounds, especially when associated with confusion (Deligiannidis et al., 2021; Praxis, 2021). Transient confusion induced by psilocybin, albeit frequent (75%), can probably be considered a psychological effect that subsided after a few hours (60–180 min) (Carhart-Harris et al., 2016). These sleeping and arousal adverse events must be differentiated from fatigue, intended as the lack of energy and motivation to perform daily activities. Although it often associates with the daytime somnolence and sedation induced by GABAergic and orexinergic compounds, fatigue achieves higher rates during psilocybin trials (7–10%), suggesting more complicated mechanisms that extend beyond the alteration of the hypnic pattern (Carhart-Harris et al., 2021; Compass Pathways, 2021).
Regarding cardiovascular adverse events, psilocybin, and AV-101 caused minimal reductions in SBP/DBP and basal HR that can be related to a mild sympathomimetic effect (Grob et al., 2011; Griffiths et al., 2016; Ross et al., 2016; Doss et al., 2021). For AV-101 these events were not generalizable (Murphy et al., 2021), whereas patients receiving psilocybin reported cardiovascular adverse events in 76% of cases according to one RCT (Ross et al., 2016). No effects on QT interval prolongation emerged for any of the considered drugs.
Gastrointestinal adverse events were common for all the discussed agents. Psilocybin was associated with the highest rates of nausea (33%) (Carhart-Harris et al., 2016) and REL-1017 with the highest rates of constipation (14%), showing a dose-response relationship probably linked to the weak stimulation of opioid receptors (Fava et al., 2022). Dry mouth was a frequent adverse event experienced by 10.3% of patients treated with AXS-05 (Tabuteau et al., 2022) and by 9.6% of subjects with pimavanserin (Fava et al., 2019). Conversely, PH-10 and AV-101 were less likely to cause gastrointestinal adverse events: PH-10 showed a low trend to increase appetite that did not result in weight gain (Monti et al., 2019) and only one participant experienced transient diarrhoea with AV-101 1440 mg (Murphy et al., 2021). In general, more than 5% of patients treated with the other compounds experienced gastrointestinal adverse events, without resulting in hospitalization or treatment discontinuation. No gastrointestinal bleeding was reported.
Despite the limited data concerning PH-10, local nose irritation due to the intranasal administration, and bitter taste were reported during the treatment period (Monti et al., 2019). No other adverse events related to sensory organs were reported for the other compounds.
Considering all the discussed medications, there was no evidence of abuse or dependency potential, increased suicidal ideation, and/or suicidal behaviour compared with baseline. Only in one trial of psilocybin, 12 patients reported suicidal ideation or suicidal behaviour and intentional self-injury, which are however quite common in patients with TRD (Compass Pathways, 2021). During treatment with zuranolone, one case of suicide attempt and one of bile duct stones were reported; however, these patients had a previous history of suicide attempt and of bile duct repair, respectively (Sage Therapeutics, 2019).
Participants exposed to the discussed NMDAR antagonists experienced no worsening in depression, dissociative or psychotomimetic effects. In reference to this, it has been hypothesized that the unusually rapid rate of NMDAR unblocking may explain the low association of these drugs with psychotomimetic adverse events (Parsons et al., 1995). Only after psilocybin administration, a few cases of paranoid ideation and transient thought disorders were described, but they were generally mild and transient (Ross et al., 2016; Anderson et al., 2020); only in one trial 75% of participants underwent through a transient thought disorder (Carhart-Harris et al., 2016). No hallucinogen-persisting perception disorder or visual perceptual changes were observed, probably because of the safe environment wherein psilocybin sessions were tested. Accurately informing patients about possible adverse events contributed to avoiding psychotic reactions (Studerus et al., 2012).
Another noteworthy safety consideration is the low tendency of the drugs of interest to produce clinically significant changes in vital signs or consciousness, sexual dysfunction, cognitive impairment, euphoria, or manic episodes (only two cases of hypomania occurred during the follow-up period in the trial testing zuranolone in bipolar depression) (Gunduz-Bruce et al., 2020), withdrawal symptoms and weight gain or metabolic changes. Despite the time of observation being fairly limited to make firm conclusions, novel antidepressant drugs may represent metabolically and sexually friendly antidepressants. The relevance of the latter issue, among the general safety profile, acquires even more importance considering the limitations in the use of conventional antidepressants because of weight gain (Serretti and Mandelli, 2010), sexual impairments (Serretti and Chiesa, 2009) and (hypo)manic switches when used in bipolar depression (Bauer, 2022).
Discussion
Rapid acting antidepressants represent promising alternatives to standard medications for mood disorders, with various levels of evidence and in different phases of development, with overall encouraging findings about their efficacy and tolerability. This review provides an overview of the clinical trials that investigated new rapid acting antidepressants, with the aim to provide clinically useful information to tailor the prescription of these new compounds, based on the individual clinical profile. The specific clinical features targeted by each novel antidepressant drug were identified according to the current evidence and are reported in Fig. 3. There is not sufficient evidence to demonstrate if AV-101 and PH-10 positively target any specific depressive symptoms. Among new drugs acting on the glutamatergic system, we discussed AV-101, AXS-05, and REL-1017. Despite these three compounds primarily targeting the glutamatergic signal, AV-101 and AXS-05 appear to exhibit a major involvement in inflammatory pathways underpinning mood disorders, while the antidepressant action of REL-1017 seems to be primarily related to neuroplasticity phenomena, since the persistence of the therapeutic effect after treatment discontinuation.
Fig. 3.
Clinical features targeted by novel antidepressant drugs based on the availability of studies investigating a specific symptom domain even as a secondary outcome. An empty box indicates no studies exploring a specific symptom, while a larger diameter of dots indicates a progressively higher number of studies. Darker shades of grey indicate a progressively higher percentage of the number of studies reporting a positive result referred to the total number of studies investigating the specific symptom. Barred dot indicates studies with negative results in the explored symptom. BUP, bupropion; DXM, dextromethorphan; NMDAR, n-methyl-d-aspartate receptor; OX-2R, orexin type 2 receptor; R, receptor.
Av-101 principally acts through the kynurenine pathway, which plays an important role in various biological functions behind depression. Additionally, it also modulates brain-derived neurotrophic factor (BDNF) levels (Réus et al., 2015). Response to this drug appears to be compromised by comorbid anxiety disorders (Park et al., 2020) unless used as adjunctive treatment to standard antidepressants or with an appropriate dose increment. Higher doses (1440 mg/day) are indeed potentially more effective than lower doses (720 mg/day) in a preclinical study (Murphy et al., 2021). To further increase bioavailability, despite not overcoming the limitations reported in animal studies, the combination of AV-101 with probenecid (a uricosuric agent) has recently been investigated in rodents, with safe results (Murphy et al., 2021). Despite the drug was not associated with an improvement in suicidal ideation and/or suicidal behaviour, it was hypothesized to be a promising option for suicidality treatment in emergency settings, because of its ketamine-like glutamatergic mechanism of action, and the lack of dissociative adverse events (Zanos et al., 2015). Additionally, its antisuicidality effect may be not limited to the acute phase. Research in cases of suicide attempts shows that pro-inflammatory cytokines, frequently increased in these patients, lead to a persistent increase in quinolinic acid, which binds to NMDAR, and to a decrease in kynurenic acid (Bay-Richter et al., 2015). Consequently, AV-101 may rebalance quinolinic and kynurenic acids equilibrium, displaying a protective action on suicide risk also in the medium-long term.
Rel-1017, another NMDAR antagonist, demonstrated rapid efficacy in MDD, with the persistence of antidepressant activity up to 1 week after treatment discontinuation (Fava et al., 2022). Additionally, changes in anhedonia, motivation, and reward have accompanied antidepressant-like activity in rodent models, after a single dose (Fogaça et al., 2019). Rel-1017 may represent therefore a valid therapeutic option not only in MDD but also in TRD. Importantly, the potential of abuse should be better explored since the low affinity to the opioid receptors does not automatically exclude this issue (Nemeroff, 2022).
AXS-05, presumably due to the multimodal pharmacological activity, was beneficial in rapidly reducing depressive symptoms, even when associated with anxiety features and when unresponsive to other antidepressants, both in the short (by week 1) and long term (up to 12 months). Noteworthy AXS-05, albeit in a limited way, reduced cognitive dysfunctions and produced a tangible improvement in work/family functioning, ameliorating life quality (Axsome Therapeutics, 2020c, 2022). Among the pharmacodynamic mechanisms beyond AXS-05 efficacy in MDD, there is also a potential action towards α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (Nguyen and Matsumoto, 2015), shared also by ketamine (Maeng et al., 2008) and esketamine, approved by the FDA in 2019 for adults with TRD (Stachowicz and Sowa-Kućma, 2022). These two compounds demonstrated clear evidence of efficacy in reducing the severity of suicidal ideation with active intent (Siegel et al., 2021), as well as AXS-05 was reported to contrast suicidal ideation since the first week of therapy (Axsome Therapeutics, 2020b). Dextromethorphan itself has also evidence of preliminary efficacy in bipolar depression, both as monotherapy (Majeed et al., 2021) and as add-on therapy with memantine (Lee et al., 2020). Consequently, although AXS-05 clinical profile appears to be useful only in MDD and has not yet been investigated in bipolar depression, it owns the potential of being pursued for the treatment of bipolar depression, especially considering the efficacy in the management of cognitive dysfunction and suicidal ideation; however, it remains to be clarified whether AXS-05 efficacy may be extended to other domains typical of depressive experiences, such as anhedonia, demotivation, and alterations in reward processing. According to several studies reporting a correlation between the reduction of anhedonia and suicidal ideation (Ballard et al., 2017), we can hypothesize that AXS-05 may improve anhedonia.
Other drugs discussed in this review, namely psilocybin and pimavanserin, act mostly by modulating the serotoninergic system. Psilocybin in conjunction with psychotherapy demonstrated efficacy within 8 h of taking a single dose in the alleviation of demoralization and hopelessness (Ross et al., 2021). Initial self-perceived effects occur in approximately 30–60 min upon oral administration (Griffiths et al., 2006), and according to most trials, positive effects on anxiety and depressive experiences persist for up to 6 months after a few psilocybin sessions. Such long-lasting positive changes may be related to psilocybin-induced synaptic remodelling. Consistent with psilocybin-induced neuroplasticity, in some trials patients found relief in reconnecting to their emotionality (Anderson et al., 2019; Carhart-Harris et al., 2021; Ross et al., 2021) and improved cognitive flexibility (Griffiths et al., 2016; Doss et al., 2021) that may facilitate, potentially after a single dose, the recognition of feelings (Stroud et al., 2018), thus improving cognitive empathy. Additionally, one RCT in healthy individuals outlined the enhancement in emotional empathy (Pokorny et al., 2017). As multiple deficits in empathic abilities are extremely common in mood disorders and lead to social impairments (Cusi et al., 2011), these pro-empathic properties may be particularly relevant to enhance social skills in patients with MDD, TRD, and depression associated with neoplastic illnesses. In the latter group of patients, anxious and depressive symptoms are often accompanied by cognitive inflexibility with hopelessness, negative cognitive bias, and suicidal ideation. In these cases, as well as in TRD, the rapid and sustained reduction in suicidal ideation of psilocybin is particularly relevant (Carhart-Harris et al., 2021; Doss et al., 2021; Ross et al., 2021). The long-term antisuicidality potential may be linked to the increase of synaptic plasticity and BDNF levels, through modulation of glutamatergic prefrontal-limbic neural circuits. Another pharmacodynamic mechanism potentially behind this antisuicidal effect is the increase of dopamine levels in mesoaccumbens circuits, which may ameliorate reward process impairments (Sakashita et al., 2015). Of note, subjective and mystical experiences are considered to reduce suicidal ideation in the short term (Ross et al., 2021) and to be potentially implied in the risk of abuse (Griffiths et al., 2018); however, the potential of abuse and risk of dependence is fairly reduced by the lack of psilocybin-induced withdrawal and rebound symptoms.
In contrast to psilocybin, pimavanserin exerts an antagonism action mostly towards 5HT2ARs. It showed major efficacy in treating depressive episodes associated with Parkinson’s disease and inducing mood improvement associated with amelioration in sleeping and in overall quality of life (DeKarske et al., 2020). Despite the efficacy of pimavanserin in treating psychic symptoms associated with Parkinson’s disease, its benefits in MDD remain uncertain, due to the negative results of phase III trials (Acadia, 2020). It is however worth mentioning its positive effect on sexual dysfunction. Although it shares monoaminergic activity with other antidepressants, in contrast to them pimavanserin does not exhibit adrenergic, histaminergic, muscarinic, or dopaminergic activity (Vanover et al., 2006), to which sexual dysfunctions are attributed (Serretti and Chiesa, 2009), and the specific 5-HT2 antagonism may even improve sexual functioning. Another point to consider is that pimavanserin reduced suicidal ideation more than placebo (Shelton et al., 2020). This antisuicidality potential may be correlated to its efficacy in targeting irritation and insomnia, which in turn are associated with agitation and with depression with mixed features and an increased risk of suicide (McIntyre et al., 2016). To summarize, despite the development of pimavanserin for MDD was discontinued, future studies may clarify if this compound could be a useful option as adjunctive treatment to another antidepressant for MDD with compromised sexual functioning or sexual side effects, anxiety, irritability, agitation, insomnia, or excessive day sleepiness.
Finally, we discussed new compounds acting as neuroactive steroids, which were developed based on the evidence that dysregulation in neurosteroidogenis and GABAergic transmission are involved in anxiety and depressive disorders, including PPD (Luscher et al., 2011).
Zuranolone is a synthetic neurosteroid and targets GABAARs, similarly to other neurosteroids and benzodiazepines (Goetz et al., 2007); however, zuranolone shows a major affinity to extra-synaptic GABAARs, responsible for tonic inhibition, rather than synaptic GABAARs, mediators of phasic inhibition. Consequently, despite sharing with benzodiazepines phasic channel inhibition (clinically resulting in an anxiolytic and sedative action), it differs since it also exerts a tonic inhibition (responsible for the antidepressant action) (Reddy, 2018). This pharmacodynamic profile may hypothetically provide also anxiolytic, hypnotic, and sedative properties. In line with this, the major benefits of zuranolone 50 mg in a 2-week treatment regimen were reported in patients with anxious depression and those with residual insomnia. Efficacy in alleviating sleeping impairments was reported also in a crossover study of healthy adults with sleep disturbances (Bullock et al., 2022); however, improvements in anxiety and insomnia were not confirmed by all trials (Gunduz-Bruce et al., 2019). Despite in a few cases benefits persisted until days 42–45, generally the rapid improvement in MDD symptoms slightly decreased after the end of the 2-week treatment paradigm, thus requiring additional courses of treatment. Zuranolone was also tested in the treatment of PPD, with rapid reduction of depressive symptoms and anxiety, a common feature in PPD (Radoš, 2018), with sustained effects until day 45. The improvement of global and maternal functioning makes zuranolone a valid option for PPD, especially considering that none of the standard antidepressants have a specific indication for this condition. The oral route of administration facilitates the intake compared to its intravenous analogous brexanolone, already approved by FDA in 2019 for PPD. Positive results of zuranolone in bipolar depression open new horizons about the involvement of neurosteroids in this condition. The current evidence suggests that neurosteroids may act as endogenous mood stabilizers and low levels of neuroactive steroids characterize depressive and mixed episodes in bipolar depression. Consistently, preclinical evidence showed that lithium and olanzapine/fluoxetine modify the levels of neurosteroids, and these are all approved therapeutic compounds in bipolar depression (Carta et al., 2012). In summary, zuranolone was effective in reducing depressive symptoms within 15 days of treatment and may represent a viable therapeutic option with a rapid onset of action, especially in agitated depression, depression with anxious features, and sleep impairment. Benefits of zuranolone in TRD and suicidal ideation are still unknown. Of note, co-therapy with benzodiazepines should be avoided, for a consistent risk of increasing sedative effects.
Ph-10 is another neuroactive steroid, that demonstrated a rapid antidepressant potential in unipolar depression; however, only one trial is available, and it is characterized by several limitations that compromised the generalizability of results (Monti et al., 2019). Unlike zuranolone, PH-10 is not a direct GABARs modulator and consequently has a less sedative profile. Early findings demonstrated that ph-94b, another neuroactive compound, had therapeutic potential in patients with social anxiety disorder, because of a rapid decrease in sympathetic tone (Liebowitz et al., 2016). According to the common neurosteroid structure between PH-10 and ph-94b, and to the administration in µg dosages of both agents, similar efficacy of PH-10 in reducing social anxiety could be hypothesized, but it should be tested in future trials. Notably, PH-10 could represent a rapid onset and easy-to-administer (intranasal) option in this group of patients.
Prax-114 is another neurosteroid modulating the GABAergic system, but it currently shows poor evidence of efficacy in treating mood disorders, as it failed in a phase II/III RCT of MDD (Praxis, 2022). This failure underlines the complexity of developing compounds with effective clinical benefits. Despite the failure to demonstrate efficacy in MDD, the drug was tested for treating mood and perimenopausal symptoms in a small preliminary study, suggesting possible improvement in mood, energy, irritability, anxiety, insomnia, and sexual interest, with an overall safety profile (Praxis, 2021). Probably increasing the dosage up to 60 mg, compared with the 40 mg dose used in the MDD trial, contributed to reducing the high rates of insomnia that affect affective changes during menopausal transition, with consequent benefits on mood (Caruso et al., 2019).
Finally, we discussed seltorexant, a novel antidepressant acting on the orexinergic system. This drug demonstrated efficacy as an adjunctive treatment to the ongoing antidepressant in MDD, especially when associated with insomnia. In these cases, apart from showing a major effect on mood, it immediately ameliorated both self-reported and objective parameters of sleep experience within a few hours after administration (Brooks et al., 2019). Improvements in other depressive and anxiety symptoms were not as rapid, but generally started within the first week of treatment, or as early as day 11 according to another study, with the greatest amelioration at weeks 3 and 6 (Savitz et al., 2021). Whether seltorexant displays a dose-dependent effect across doses of 10–20–40 mg remains unclear. In this regard, a curvilinear dose-response relationship was hypothesized as previously noted also for esketamine and nortryptiline, with the maximum antidepressant response at 20 mg and a reduction of the therapeutic effects of seltorexant at the doses of 10 and 40 mg (Savitz et al., 2021). Consistently with the role of orexin pathways in the aetiology of mood, anxiety, and panic symptoms, we suggest that seltorexant might be particularly useful for improving depressive and anxiety symptoms associated with panic distress, as well as for inducing an immediate hypnotic effect. The ideal time of administration should consequently be 1 h before bedtime. These positive effects on sleep may also have benefits in preventing depressive relapses/recurrences, as there is a bidirectional connection between depression and insomnia (Oh et al., 2019). The principal mechanism of alleviating sleeping disturbances consists in reducing sleep latency and increasing sleep duration with very low evidence of altering sleep architecture, thus differing from the currently available hypnotic drugs that significantly alter the rapid eye movement phase (Brooks et al., 2019). Additionally, the involvement of orexin neurons in the modulation of irritability/aggressive behaviours supports the idea of possible effectiveness in these clinical features (Flanigan et al., 2020).
To summarize the clinical specificity profiles targeted by the discussed medications, we considered seven clinical domains: negative emotionality, behavioural dysregulation (impulsive or excessive behaviours that may even put life at risk), cognition, psychomotor agitation, circadian rhythm, sexual dysfunction, and interpersonal activities and relationships (Fig. 3). All compounds, except AV-101 and PH-10, may constitute an appropriate option to reduce psychomotor agitation, and REL-1017 shows the widest profile in terms of the number of targeted symptoms. Psilocybin and zuranolone show the strongest potential to target anxiety. No medication with effectiveness in reducing psychomotor retardation was identified, despite this being one of the main features of severe depressive episodes (Buyukdura et al., 2011). Several drugs (pimavanserin, zuranolone, PRAX-114, and seltorexant) showed efficacy for the regularization of the circadian rhythm, with pimavanserin not only favouring sleeping at night but also reducing excessive daytime sleepiness. Pimavanserin also showed effects on behavioural dysregulation, sexual and global functioning. Psilocybin was found to be the only drug with a strong specificity in reducing negative emotionality and in counteracting pessimism and cognitive inflexibility of depression. It also was effective in reducing behavioural dysregulation. AXS-05 displays a wide profile of efficacy among the domains of behavioural dysregulation, cognition, psychomotor agitation, and interpersonal functioning.
Limitations and future directions
The results of discussed studies should be interpreted in the context of several limitations. Specifically, the lack of complete data, significant placebo response rates, short-term treatments (Fava et al., 2019), and limited follow-up periods (Clinicaltrials.gov, 2020d; Park et al., 2020; Deligiannidis et al., 2021; Doss et al., 2021) reduced the possibility of finding differences in the experimental arm. Trials with longer follow-ups might be necessary to elucidate long-term antidepressant effects, as well as safety and tolerability in the long term. It should also be noted that some trials were pilot studies (Anderson et al., 2020), with small sample sizes (Ross et al., 2016; Fava et al., 2019; Gunduz-Bruce et al., 2019; Monti et al., 2019; Anderson et al., 2020; DeKarske et al., 2020; Park et al., 2020; Doss et al., 2021; Savitz et al., 2021), and lacked adequate double blinding (Ross et al., 2016) or an independent control group (Anderson et al., 2020; DeKarske et al., 2020; Ross et al., 2021; Sage Therapeutics and Biogen, 2021a; Axsome Therapeutics, 2022). Additionally, the drugs of interest were often tested only as adjunctive therapy to the ongoing antidepressants (Clinicaltrials.gov, 2017; Brooks et al., 2019; Fava et al., 2019, 2022; VistaGen Therapeutics, 2019; Acadia, 2020; Savitz et al., 2021; Sage Therapeutics and Biogen, 2022) and further trials should demonstrate whether the antidepressant effect is maintained in monotherapy. Most studies excluded patients having depression with mixed features, psychotic and substance use disorders, previous suicide attempts, or current suicidal ideation and/or significant medical comorbidities, thus limiting generalizability (Doss et al., 2021; Tabuteau et al., 2022). Several trials included only cases with moderate depression, thus limiting extrapolations to patients with severe depressive symptoms or TRD (Brooks et al., 2019; Fava et al., 2019; Gunduz-Bruce et al., 2019; Monti et al., 2019; Recourt et al., 2019; Sage Therapeutics, 2019; VistaGen Therapeutics, 2019; Acadia, 2020; Carhart-Harris et al., 2021; Doss et al., 2021; Sage Therapeutics and Biogen, 2021b; 2021a; Savitz et al., 2021; Sage Therapeutics and Biogen, 2022; Becker et al., 2022; Iosifescu et al., 2022; Praxis, 2022; Tabuteau et al., 2022). Overall, patients were not fully representative of the real-world population, and this limitation should be considered for future studies.
Regarding psilocybin, it has been tested as part of structured psychotherapy. A therapeutic approach including psychotherapy requires a significant effort in terms of time and costs, with consequent possible limitations in the applicability to public health services (Doss et al., 2021). Furthermore, it remains unclear whether psilocybin could have an antidepressant effect alone since there were no experimental conditions omitting supportive psychotherapy. The effects of micro-dosing should also be clarified, in relation to positive emotionality/beliefs and in reducing anxiety and depression compared to a single macro-dose.
Concerning some psilocybin trials, strategies to improve the recruitment of more diverse study populations are needed (Carhart-Harris et al., 2021; Doss et al., 2021; Ross et al., 2021). The available evidence supports the efficacy of psilocybin in patients with cancer, but these results may not be generalizable to other end-of-life medical diseases (e.g. neurodegenerative diseases) or in severe pain conditions. Additionally, psilocybin, like other psychedelics, is known to promote suggestibility, which might have enhanced positive outcomes (Carhart-Harris et al., 2015). Future studies should address the role of expectancy and suggestibility by measuring and controlling for these variables. Nonetheless, the approval of psilocybin remains extremely challenging due to the lack of appropriate ethical guidelines that can prevent its misuse and the consequent restrictions imposed by the Drug Enforcement Administration, despite no potential to cause addiction was found in the discussed trials. In this regard, there are no conflicts of interest for the authors of this manuscript.
Regarding seltorexant, although it showed benefits on residual insomnia in MDD, some trials were not primarily powered to demonstrate an antidepressant effect or efficacy on sleep disturbances (Brooks et al., 2019; Recourt et al., 2019).
The discussed drugs were all developed for nonparenteral administration. Considering the more robust and rapid effect of standard antidepressants when administered intravenously versus orally (Buoli et al., 2019), future development of parental formulations (other than oral) should be considered. Although most trials assessed objective symptoms, the evaluation of subjective well being, functional endpoints, and quality of life would be important in future studies. Additionally, most clinical trials used the HDRS (Hamilton, 1960), Mongtomery-Asberg Rating Scale (Montgomery and Åsberg, 1979), or the Quick Inventory of Depressive Symptomatology–Self Report (Rush et al., 2003); however, these instruments were designed to evaluate symptoms over a period of weeks; the use/development of different psychometric instruments to assess changes in symptoms severity within hours to days should be considered.
Conclusion
Novel rapid acting antidepressants represent a promising option, particularly for patients unresponsive to standard medications or those who discontinue treatment due to side effects.
The lack of psychotomimetic, dissociative, metabolic, and rebound side effects greatly increases the safety and tolerability profile of new antidepressants, favouring a good therapeutic adherence; however, some side effects need longer follow-ups to be reliably assessed, such as weight gain and withdrawal symptoms.
The benefits of each compound on specific symptom domains/profiles were discussed, as this should be a key objective of clinical trials, to facilitate the implementation of precision psychiatry.
Acknowledgements
This work was supported by #NEXTGENERATIONEU (NGEU) and funded by the Italian Ministry of University and Research, National Recovery and Resilience Plan, project MNESYS (PE0000006) – a multiscale integrated approach to the study of the nervous system in health and disease (DN. 1553 11.10.2022).
Conflicts of interest
Prof A.S. is or has been a consultant to or has received honoraria or grants unrelated to the present work from Abbott, Abbvie, Angelini, Astra Zeneca, Clinical Data, Boheringer, Bristol Myers Squibb, Eli Lilly, GlaxoSmithKline, Innovapharma, Italfarmaco, Janssen, Lundbeck, Naurex, Pfizer, Polifarma, Sanofi, Servier, and Taliaz. Dr C.F. was a speaker for Janssen. For the remaining authors, there are no conflicts of interest.
References
- Abramian AM, Comenencia-Ortiz E, Modgil A, Vien TN, Nakamura Y, Moore YE, et al. (2014). Neurosteroids promote phosphorylation and membrane insertion of extrasynaptic GABA A receptors. Proc Natl Acad Sci USA 111:7132–7137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acadia . (2020). ACADIA Pharmaceuticals Announces Top-line Results from the Phase 3 CLARITY Study Evaluating Pimavanserin for the Adjunctive Treatment of Major Depressive Disorder. Acadia Pharmaceuticals. https://acadia.com/media/news-releases/acadia-pharmaceuticals-announces-top-line-results-from-the-phase-3-clarity-study-evaluating-pimavanserin-for-the-adjunctive-treatment-of-major-depressive-disorder/. [Accessed 12 September 2022] [Google Scholar]
- Agin-Liebes GI, Malone T, Yalch MM, Mennenga SE, Ponté KL, Guss J, et al. (2020). Long-term follow-up of psilocybin-assisted psychotherapy for psychiatric and existential distress in patients with life-threatening cancer. J Psychopharmacol 34:155–166. [DOI] [PubMed] [Google Scholar]
- Althaus AL, Ackley MA, Belfort GM, Gee SM, Dai J, Nguyen DP, et al. (2020). Preclinical characterization of zuranolone (SAGE-217), a selective neuroactive steroid GABAA receptor positive allosteric modulator. Neuropharmacology 181:108333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson T, Petranker R, Rosenbaum D, Weissman CR, Dinh-Williams L-A, Hui K, et al. (2019). Microdosing psychedelics: personality, mental health, and creativity differences in microdosers. Psychopharmacology (Berl) 236:731–740. [DOI] [PubMed] [Google Scholar]
- Anderson BT, Danforth A, Daroff PR, Stauffer C, Ekman E, Agin-Liebes G, et al. (2020). Psilocybin-assisted group therapy for demoralized older long-term AIDS survivor men: an open-label safety and feasibility pilot study. EClinicalMedicine 27:100538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ark PD van der, Golor G, Nueten L van, Nandy P, Boer P de. (2018). Multiple daytime administration of the selective orexin-2 receptor antagonist JNJ-42847922 induces somnolence in healthy subjects without residual central effects. J Psychopharmacol 32:1330–1340. [DOI] [PubMed] [Google Scholar]
- Axsome Therapeutics 2020a). Axsome therapeutics announces positive efficacy and safety results from phase 3 COMET long-term trial and COMET-AU trial of AXS-05 in major depressive disorder. GlobeNewswire News Room; https://www.globenewswire.com/en/news-release/2020/12/01/2137234/33090/en/Axsome-Therapeutics-Announces-Positive-Efficacy-and-Safety-Results-from-Phase-3-COMET-Long-Term-Trial-and-COMET-AU-Trial-of-AXS-05-in-Major-Depressive-Disorder.html. [Accessed 14 September 2022] [Google Scholar]
- Axsome Therapeutics 2020b). Axsome Therapeutics announces positive results from the COMET-SI trial of AXS-05 in patients with major depressive disorder who have suicidal ideation. https://www.globenewswire.com/news-release/2020/12/8/2141143/0/en/Axsome-Therapeutics-Announces-Positive-Results-from-the-COMET-SI-Trial-of-AXS-05-in-Patients-with-Major-Depressive-Disorder-Who-Have-Suicidal-Ideation.html. [Accessed 14 September 2022] [Google Scholar]
- Axsome Therapeutics 2020c). Axsome therapeutics announces topline results of the STRIDE-1 phase 3 trial in treatment resistant depression and expert call to discuss clinical implications. GlobeNewswire News Room; https://www.globenewswire.com/en/news-release/2020/03/30/2008163/33090/en/Axsome-Therapeutics-Announces-Topline-Results-of-the-STRIDE-1-Phase-3-Trial-in-Treatment-Resistant-Depression-and-Expert-Call-to-Discuss-Clinical-Implications.html. [Accessed 14 September 2022] [Google Scholar]
- Axsome Therapeutics . (2021). Axsome therapeutics announces AXS-05 achieves primary and key secondary endpoints in the MERIT phase 2 trial in treatment resistant depression. GlobeNewswire News Room; https://www.globenewswire.com/news-release/2021/08/09/2276951/33090/en/Axsome-Therapeutics-Announces-AXS-05-Achieves-Primary-and-Key-Secondary-Endpoints-in-the-MERIT-Phase-2-Trial-in-Treatment-Resistant-Depression.html. [Accessed 14 September 2022] [Google Scholar]
- Axsome Therapeutics . (2022). Axsome therapeutics announces late-breaking presentations of positive results of the EVOLVE trial of AXS-05 in major depressive disorder after prior treatment failures at the American Society of Clinical Psychopharmacology (ASCP) 2022 Annual Meeting. GlobeNewswire News Room; https://www.globenewswire.com/en/news-release/2022/06/01/2454061/33090/en/Axsome-Therapeutics-Announces-Late-Breaking-Presentations-of-Positive-Results-of-the-EVOLVE-Trial-of-AXS-05-in-Major-Depressive-Disorder-After-Prior-Treatment-Failures-at-the-Ameri.html. [Accessed 14 September 2022] [Google Scholar]
- Ballard ED, Wills K, Lally N, Richards EM, Luckenbaugh DA, Walls T, et al. (2017). Anhedonia as a clinical correlate of suicidal thoughts in clinical ketamine trials. J Affect Disord 218:195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer MS. (2022). Bipolar disorder. Ann Intern Med 175:ITC97–ITC112. [DOI] [PubMed] [Google Scholar]
- Bay-Richter C, Linderholm KR, Lim CK, Samuelsson M, Träskman-Bendz L, Guillemin GJ, et al. (2015). A role for inflammatory metabolites as modulators of the glutamate N-methyl-d-aspartate receptor in depression and suicidality. Brain Behav Immun 43:110–117. [DOI] [PubMed] [Google Scholar]
- Beck CT. (2006). Postpartum depression. Am J Nurs 106:40–50. [DOI] [PubMed] [Google Scholar]
- Becker AM, Holze F, Grandinetti T, Klaiber A, Toedtli VE, Kolaczynska KE, et al. (2022). Acute effects of psilocybin after escitalopram or placebo pretreatment in a randomized, double-blind, placebo-controlled, crossover study in healthy subjects. Clin Pharmacol Ther 111:886–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein G, Davis K, Mills C, Wang L, McDonnell M, Oldenhof J, et al. (2019). Characterization of the safety and pharmacokinetic profile of d-methadone, a novel N-Methyl-d-aspartate receptor antagonist in healthy, opioid-naive subjects. J Clin Psychopharmacol 39:226–237. [DOI] [PubMed] [Google Scholar]
- Bonaventure P, Shelton J, Yun S, Nepomuceno D, Sutton S, Aluisio L, et al. (2015). Characterization of JNJ-42847922, a selective orexin-2 receptor antagonist, as a clinical candidate for the treatment of insomnia. J Pharmacol Exp Ther 354:471–482. [DOI] [PubMed] [Google Scholar]
- Botella GM, Salituro FG, Harrison BL, Beresis RT, Bai Z, Blanco M-J, et al. (2017). Neuroactive steroids. 2. 3α-Hydroxy-3β-methyl-21-(4-cyano-1 H -pyrazol-1′-yl)-19-nor-5β-pregnan-20-one (SAGE-217): a clinical next generation neuroactive steroid positive allosteric modulator of the (γ-Aminobutyric Acid) A receptor. J Med Chem 60:7810–7819. [DOI] [PubMed] [Google Scholar]
- Boulougouris V, Glennon JC, Robbins TW. (2008). Dissociable effects of selective 5-HT2A and 5-HT2C receptor antagonists on serial spatial reversal learning in rats. Neuropsychopharmacology 33:2007–2019. [DOI] [PubMed] [Google Scholar]
- Brooks S, Jacobs GE, Boer P. de, Kent JM, Nueten LV, Amerongen G van, et al. (2019). The selective orexin-2 receptor antagonist seltorexant improves sleep: An exploratory double-blind, placebo controlled, crossover study in antidepressant-treated major depressive disorder patients with persistent insomnia. J Psychopharmacol 33:202–209. [DOI] [PubMed] [Google Scholar]
- Brown SJ, Huang X-F, Newell KA. (2021). The kynurenine pathway in major depression: what we know and where to next. Neurosci Biobehav Rev 127:917–927. [DOI] [PubMed] [Google Scholar]
- Bullock A, Gunduz-Bruce H, Zammit GK, Qin M, Li H, Sankoh AJ, et al. (2022). A phase 1 double-blind, placebo-controlled study of zuranolone (SAGE-217) in a phase advance model of insomnia in healthy adults. Hum Psychopharmacol - Clin Exp 37:e2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buoli M, Rovera C, Pozzoli SM, Fiorentini A, Cremaschi L, Caldiroli A, et al. (2019). Is trazodone more effective than clomipramine in major depressed outpatients? A single-blind study with intravenous and oral administration. CNS Spectr 24:258–264. [DOI] [PubMed] [Google Scholar]
- Buyukdura JS, McClintock SM, Croarkin PE. (2011). Psychomotor retardation in depression: biological underpinnings, measurement, and treatment. Prog Neuropsychopharmacol Biol Psychiatry 35:395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Leech R, Hellyer PJ, Shanahan M, Feilding A, Tagliazucchi E, et al. (2014). The entropic brain: a theory of conscious states informed by neuroimaging research with psychedelic drugs. Front Hum Neurosci 8:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Kaelen M, Whalley MG, Bolstridge M, Feilding A, Nutt DJ. (2015). LSD enhances suggestibility in healthy volunteers. Psychopharmacology (Berl) 232:785–794. [DOI] [PubMed] [Google Scholar]
- Carhart-Harris RL, Bolstridge M, Rucker J, Day CMJ, Erritzoe D, Kaelen M, et al. (2016). Psilocybin with psychological support for treatment-resistant depression: an open-label feasibility study. Lancet Psychiatry 3:619–627. [DOI] [PubMed] [Google Scholar]
- Carhart-Harris R, Giribaldi B, Watts R, Baker-Jones M, Murphy-Beiner A, Murphy R, et al. (2021). Trial of psilocybin versus escitalopram for depression. N Engl J Med 384:1402–1411. [DOI] [PubMed] [Google Scholar]
- Carta MG, Bhat KM, Preti A. (2012). GABAergic neuroactive steroids: a new frontier in bipolar disorders? Behav Brain Funct 8:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso D, Masci I, Cipollone G, Palagini L. (2019). Insomnia and depressive symptoms during the menopausal transition: theoretical and therapeutic implications of a self-reinforcing feedback loop. Maturitas 123:78–81. [DOI] [PubMed] [Google Scholar]
- Castle D, Beilharz F, Phillips KA, Brakoulias V, Drummond LM, Hollander E, et al. (2021). Body dysmorphic disorder: a treatment synthesis and consensus on behalf of the International College of Obsessive-Compulsive Spectrum Disorders and the Obsessive Compulsive and Related Disorders Network of the European College of Neuropsychopharmacology. Int Clin Psychopharmacol 36:61–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- clinicaltrials.gov . (2017). A 6-month, multicenter, double-blind, randomized, flexible-dose, parallel-group study to compare the efficacy, safety, and tolerability of JNJ-42847922 versus quetiapine extended-release as adjunctive therapy to antidepressants in adult subjects with major depressive disorder who have responded inadequately to antidepressant therapy. clinicaltrials.gov. [Accessed 16 September 2022] [Google Scholar]
- clinicaltrials.gov . (2018). Psilocybin and depression – assessing the long-term effects of a single administration of psilocybin on the psychiatric symptoms and brain activity of patients with severe depression. clinicaltrials.gov. [Accessed 16 September 2022] [Google Scholar]
- clinicaltrials.gov 2019a). A randomized, double-blind, support-of-concept phase 2 study of single-dose psilocybin for major depressive disorder (MDD). clinicaltrials.gov. [Accessed 16 September 2022] [Google Scholar]
- clinicaltrials.gov 2019b). Phase II, randomized, double blind, placebo controlled, parallel group, single center study of psilocybin efficacy in major depression. clinicaltrials.gov. [Accessed 16 September 2022] [Google Scholar]
- clinicaltrials.gov 2020a). A multicenter, double-blind, randomized, parallel-group, placebo-controlled, study to evaluate the efficacy and safety of seltorexant 20 mg as adjunctive therapy to antidepressants in adult and elderly patients with major depressive disorder with insomnia symptoms who have responded inadequately to antidepressant therapy. clinicaltrials.gov. [Accessed 16 September 2022] [Google Scholar]
- clinicaltrials.gov 2020b). A multicenter, double-blind, randomized, parallel-group, placebo-controlled, study to evaluate the efficacy and safety of seltorexant 20 mg as adjunctive therapy to antidepressants in adult and elderly patients with major depressive disorder with insomnia symptoms who have responded inadequately to antidepressant therapy and an open-labeled long-term safety extension treatment with seltorexant. clinicaltrials.gov. [Accessed 16 September 2022] [Google Scholar]
- clinicaltrials.gov 2020c). A multicentric, randomized, double-blind, placebo- and positive-controlled 4-way crossover study to evaluate the effects of single and repeated administration of oral seltorexant as an add-on medication to an antidepressant on on-road driving performance in participants with major depressive disorder. clinicaltrials.gov. [Accessed 16 September 2022] [Google Scholar]
- clinicaltrials.gov 2020d). A randomized, double-blind, placebo-controlled study evaluating the efficacy and safety of SAGE-217 in the treatment of adults with severe postpartum depression. clinicaltrials.gov. [Accessed 16 September 2022] [Google Scholar]
- clinicaltrials.gov 2020e). Double-blind, randomized, parallel-group study with quetiapine extended release as comparator to evaluate the efficacy and safety of seltorexant 20 mg as adjunctive therapy to antidepressants in adult and elderly patients with major depressive disorder with insomnia symptoms who have responded inadequately to antidepressant therapy. clinicaltrials.gov. [Accessed 16 September 2022] [Google Scholar]
- clinicaltrials.gov 2021a). A phase 2 double-blind, placebo-controlled, dose-ranging clinical trial to evaluate the efficacy and safety of PRAX-114 in adjunctive and monotherapy treatment of participants with major depressive disorder and inadequate response to antidepressant treatment. clinicaltrials.gov. [Accessed 16 September 2022] [Google Scholar]
- clinicaltrials.gov 2021b). A phase II randomized, double-blind, active placebo-controlled parallel group trial to examine the efficacy and safety of psilocybin in treatment-resistant major depression. clinicaltrials.gov. [Accessed 16 September 2022] [Google Scholar]
- clinicaltrials.gov 2021c). A randomized, active-controlled, evaluation of AXS-05 for the treatment of treatment resistant depression in treatment-adherent patients. clinicaltrials.gov. [Accessed 16 September 2022] [Google Scholar]
- clinicaltrials.gov 2021d). A short-term exploratory study to evaluate safety, tolerability and pharmacokinetics of seltorexant as adjunctive therapy to antidepressants in adolescents with major depressive disorder who have an inadequate response to SSRI monotherapy and psychotherapy. clinicaltrials.gov. [Accessed 16 September 2022] [Google Scholar]
- clinicaltrials.gov 2021e). Psilocybin treatment of major depressive disorder with co-occurring alcohol use disorder. clinicaltrials.gov. [Accessed 16 September 2022] [Google Scholar]
- clinicaltrials.gov 2021f). The effect of psilocybin on MDD symptom severity and synaptic density - a single dose randomized, double blind, placebo- controlled phase 2 positron emission tomography study. clinicaltrials.gov. [Accessed 16 September 2022] [Google Scholar]
- COMPASS Pathways , (2021). COMPASS pathways announces positive topline results from groundbreaking phase 2b trial of investigational COMP360 psilocybin therapy for treatment-resistant depression.. https://compasspathways.com/ https://compasspathways.com/positive-topline-results/.[Accessed 10 September 2022] [Google Scholar]
- Cusi AM, MacQueen GM, Spreng RN, McKinnon MC. (2011). Altered empathic responding in major depressive disorder: relation to symptom severity, illness burden, and psychosocial outcome. Psychiatry Res 188:231–236. [DOI] [PubMed] [Google Scholar]
- DeKarske D, Alva G, Aldred JL, Coate B, Cantillon M, Jacobi L, et al. (2020). An open-label, 8-week study of safety and efficacy of pimavanserin treatment in adults with Parkinson’s disease and depression. J Parkinsons Dis 10:1751–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deligiannidis KM, Meltzer-Brody S, Gunduz-Bruce H, Doherty J, Jonas J, Li S, et al. (2021). Effect of zuranolone vs placebo in postpartum depression. JAMA Psychiatry 78:951–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doblin RE, Christiansen M, Jerome L, Burge B. (2019). The past and future of psychedelic science: an introduction to this issue. J Psychoactive Drugs 51:93–97. [DOI] [PubMed] [Google Scholar]
- Dörks M, Hoffmann F, Jobski K. (2022). Antidepressant drug use and regional prescribing patterns in Germany: results from a large population-based study. Int Clin Psychopharmacol 37:185–192. [DOI] [PubMed] [Google Scholar]
- Doss MK, Považan M, Rosenberg MD, Sepeda ND, Davis AK, Finan PH, et al. (2021). Psilocybin therapy increases cognitive and neural flexibility in patients with major depressive disorder. Transl Psychiatry 11:574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egede LE, Bishu KG, Walker RJ, Dismuke CE. (2016). Impact of diagnosed depression on healthcare costs in adults with and without diabetes: United States, 2004-2011. J Affect Disord 195:119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Medicines Agency (EMA) (2003). Evaluation of Medicines for human use, positio paper on non-clinical safety studies to support clinical trials with a single microdose. https://iaa-ams.co.jp/wpcontent/uploads/2020/10/MD1.pdf. [Accessed 29 September 2022].
- Fanelli G, Serretti A. (2022). Depression, antidepressants, and insulin resistance: which link? Eur Neuropsychopharmacol 60:4–6. [DOI] [PubMed] [Google Scholar]
- Fanelli G, Domschke K, Minelli A, Gennarelli M, Martini P, Bortolomasi M, et al. (2022). A meta-analysis of polygenic risk scores for mood disorders, neuroticism, and schizophrenia in antidepressant response. Eur Neuropsychopharmacol 55:86–95. [DOI] [PubMed] [Google Scholar]
- Fava M, Dirks B, Freeman MP, Papakostas GI, Shelton RC, Thase ME, et al. (2019). A phase 2, randomized, double-blind, placebo-controlled study of adjunctive pimavanserin in patients with major depressive disorder and an inadequate response to therapy (CLARITY). J Clin Psychiatry 80:19m12928. [DOI] [PubMed] [Google Scholar]
- Fava M, Stahl S, Pani L, Martin SD, Pappagallo M, Guidetti C, et al. (2022). REL-1017 (Esmethadone) as adjunctive treatment in patients with major depressive disorder: a phase 2a randomized double-blind trial. Am J Psychiatry 179:122–131. [DOI] [PubMed] [Google Scholar]
- Flanigan ME, Aleyasin H, Li L, Burnett CJ, Chan KL, LeClair KB, et al. (2020). Orexin signaling in GABAergic lateral habenula neurons modulates aggressive behavior in male mice. Nat Neurosci 23:638–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogaça MV, Fukumoto K, Franklin T, Liu R-J, Duman CH, Vitolo OV, et al. (2019). N-Methyl-D-aspartate receptor antagonist d-methadone produces rapid, mTORC1-dependent antidepressant effects. Neuropsychopharmacology 44:2230–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman MP, Fava M, Dirks B, Jha MK, Papakostas GI, Shelton RC, et al. (2020). Improvement of sexual functioning during treatment of MDD with adjunctive pimavanserin: a secondary analysis. Depress Anxiety 37:485–495. [DOI] [PubMed] [Google Scholar]
- Frieder A, Fersh M, Hainline R, Deligiannidis KM. (2019). Pharmacotherapy of postpartum depression: current approaches and novel drug development. CNS Drugs 33:265–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliardi L, Petrozzi A, Rusconi F. (2012). Symptoms of maternal depression immediately after delivery predict unsuccessful breast feeding: Figure 1. Arch Dis Child 97:355–357. [DOI] [PubMed] [Google Scholar]
- Gaynes BN, Lux L, Gartlehner G, Asher G, Forman-Hoffman V, Green J, et al. (2020). Defining treatment-resistant depression. Depress Anxiety 37:134–145. [DOI] [PubMed] [Google Scholar]
- Goetz T., Arslan A., Wisden W., Wulff P. (2007). GABAA receptors: structure and function in the basal ganglia. In: Progress in brain research. Elsevier. pp. 21–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg SB, Pace BT, Nicholas CR, Raison CL, Hutson PR. (2020). The experimental effects of psilocybin on symptoms of anxiety and depression: a meta-analysis. Psychiatry Res 284:112749. [DOI] [PubMed] [Google Scholar]
- Gorman AL, Elliott KJ, Inturrisi CE. (1997). The d- and l- isomers of methadone bind to the non-competitive site on the N-methyl-d-aspartate (NMDA) receptor in rat forebrain and spinal cord. Neurosci Lett 223:5–8. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Richards WA, McCann U, Jesse R. (2006). Psilocybin can occasion mystical-type experiences having substantial and sustained personal meaning and spiritual significance. Psychopharmacology (Berl) 187:268–83; discussion 284. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Johnson MW, Carducci MA, Umbricht A, Richards WA, Richards BD, et al. (2016). Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: a randomized double-blind trial. J Psychopharmacol 30:1181–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Johnson MW, Richards WA, Richards BD, Jesse R, MacLean KA, et al. (2018). Psilocybin-occasioned mystical-type experience in combination with meditation and other spiritual practices produces enduring positive changes in psychological functioning and in trait measures of prosocial attitudes and behaviors. J Psychopharmacol 32:49–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grob CS, Danforth AL, Chopra GS, Hagerty M, McKay CR, Halberstadt AL, et al. (2011). Pilot study of psilocybin treatment for anxiety in patients with advanced-stage cancer. Arch Gen Psychiatry 68:71–78. [DOI] [PubMed] [Google Scholar]
- Gunduz-Bruce H, Silber C, Kaul I, Rothschild AJ, Riesenberg R, Sankoh AJ, et al. (2019). Trial of SAGE-217 in patients with major depressive disorder. N Engl J Med 381:903–911. [DOI] [PubMed] [Google Scholar]
- Gunduz-Bruce H, Lasser R, Nandy I, Sankoh A, Jonas J, Doherty J, Kanes S. (2020). Open-label, phase 2 trial of the oral neuroactive steroid GABAA receptor positive allosteric modulator zuranolone in bipolar disorder I and II. HMP Global Learning Network. https://www.hmpgloballearningnetwork.com/site/pcn/posters/open-label-phase-2-trial-oral-neuroactive-steroid-gabaa-receptor-positive-allosteric. [Accessed 1 September 2022] [Google Scholar]
- Hamilton M. (1960). A rating scale for depression. J Neurol Neurosurg Psychiatry 23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey JA. (2003). Role of the serotonin 5-HT 2A receptor in learning. Learn Mem 10:355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecking J, Davoudian PA, Wilkinson ST. (2021). Emerging therapeutics based on the amino acid neurotransmitter system: an update on the pharmaceutical pipeline for mood disorders. Chronic Stress 5:247054702110204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu XH, Bull SA, Hunkeler EM, Ming E, Lee JY, Fireman B, et al. (2004). Incidence and duration of side effects and those rated as bothersome with selective serotonin reuptake inhibitor treatment for depression. J Clin Psychiatry 65:959–965. [DOI] [PubMed] [Google Scholar]
- Hughes Z, Scott L, Kahlig K, Wittmann M. (2021). PRAX-114 is a novel extrasynaptic GABA-A receptor preferring positive allosteric modulator with a wide separation between a translational biomarker signature associated with antidepressant-like activity, and sedative effects. Biol Psychiatry 89:S204. [Google Scholar]
- Iosifescu DV, Jones A, O’Gorman C, Streicher C, Feliz S, Fava M, et al. (2022). Efficacy and safety of AXS-05 (dextromethorphan-bupropion) in patients with major depressive disorder. J Clin Psychiatry 83:21m14345. [DOI] [PubMed] [Google Scholar]
- James SL, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. (2018). Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392:1789–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha MK, Fava M, Freeman MP, Thase ME, Papakostas GI, Shelton RC, et al. (2020). Effect of adjunctive pimavanserin on sleep/wakefulness in patients with major depressive disorder. J Clin Psychiatry 82:20m13425. [DOI] [PubMed] [Google Scholar]
- Johnson PL, Molosh A, Fitz SD, Truitt WA, Shekhar A. (2012). Orexin, stress, and anxiety/panic states. Prog Brain Res 198:133–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krusemark EA, Novak LR, Gitelman DR, Li W. (2013). When the sense of smell meets emotion: anxiety-state-dependent olfactory processing and neural circuitry adaptation. J Neurosci 33:15324–15332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama A, Tabata H. (2017). Suvorexant for the treatment of primary insomnia: a systematic review and meta-analysis. Sleep Med Rev 35:1–7. [DOI] [PubMed] [Google Scholar]
- Lee S-Y, Wang T-Y, Chen S-L, Chang Y-H, Chen P-S, Huang S-Y, et al. (2020). Combination of dextromethorphan and memantine in treating bipolar spectrum disorder: a 12-week double-blind randomized clinical trial. Int J Bipolar Disord 8:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebowitz MR, Hanover R, Draine A, Lemming R, Careri J, Monti L. (2016). Effect of as-needed use of intranasal PH94B on social and performance anxiety in individuals with social anxiety disorder. Depress Anxiety 33:1081–1089. [DOI] [PubMed] [Google Scholar]
- Luscher B, Shen Q, Sahir N. (2011). The GABAergic deficit hypothesis of major depressive disorder. Mol Psychiatry 16:383–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons T, Carhart-Harris RL. (2018). More realistic forecasting of future life events after psilocybin for treatment-resistant depression. Front Psychol 9:1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado-Vieira R, Salvadore G, DiazGranados N, Zarate CA. (2009). Ketamine and the next generation of antidepressants with a rapid onset of action. Pharmacol Ther 123:143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeng S, Zarate CA, Du J, Schloesser RJ, McCammon J, Chen G, et al. (2008). Cellular mechanisms underlying the antidepressant effects of ketamine: role of α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry 63:349–352. [DOI] [PubMed] [Google Scholar]
- Majeed A, Xiong J, Teopiz KM, Ng J, Ho R, Rosenblat JD, et al. (2021). Efficacy of dextromethorphan for the treatment of depression: a systematic review of preclinical and clinical trials. Expert Opin Emerg Drugs 26:63–74. [DOI] [PubMed] [Google Scholar]
- Mardi J. (2022). BioPharma Media, 2022. BioPharma Media. https://biopharma.media/auvelity-new-antidepressant-for-most-severe-depression-4068/. [Accessed 8 September 2022] [Google Scholar]
- Marschall J, Fejer G, Lempe P, Prochazkova L, Kuchar M, Hajkova K, et al. (2022). Psilocybin microdosing does not affect emotion-related symptoms and processing: a preregistered field and lab-based study. J Psychopharmacol 36:97–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintock SM, Husain MM, Wisniewski SR, Nierenberg AA, Stewart JW, Trivedi MH, et al. (2011). Residual symptoms in depressed outpatients who respond by 50% but do not remit to antidepressant medication. J Clin Psychopharmacol 31:180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre RS, Calabrese JR. (2019). Bipolar depression: the clinical characteristics and unmet needs of a complex disorder. Curr Med Res Opin 35:1993–2005. [DOI] [PubMed] [Google Scholar]
- McIntyre RS, Lee Y, Mansur RB. (2016). A pragmatic approach to the diagnosis and treatment of mixed features in adults with mood disorders. CNS Spectr 21:25–33. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Åsberg M. (1979). A new depression scale designed to be sensitive to change. Br J Psychiatry 134:382–389. [DOI] [PubMed] [Google Scholar]
- Monti L, Nicolini H, Liebowitz MR, Hanover R. (2019). A placebo controlled trial of PH10: test of a new rapidly acting intranasally administered antidepressant. Br J Pharm Res 04:2157–2168. [Google Scholar]
- Moreno FA, Wiegand CB, Taitano EK, Delgado PL. (2006). Safety, tolerability, and efficacy of psilocybin in 9 patients with obsessive-compulsive disorder. J Clin Psychiatry 67:1735–1740. [DOI] [PubMed] [Google Scholar]
- Murphy N, Ramakrishnan N, Vo-Le B, Vo-Le B, Smith MA, Iqbal T, et al. (2021). A randomized cross-over trial to define neurophysiological correlates of AV-101 N-methyl-d-aspartate receptor blockade in healthy veterans. Neuropsychopharmacology 46:820–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeroff CB. (2022). Back to the future: esmethadone, the (maybe) nonopiate opiate, and depression. Am J Psychiatry 179:83–84. [DOI] [PubMed] [Google Scholar]
- Nguyen L, Matsumoto RR. (2015). Involvement of AMPA receptors in the antidepressant-like effects of dextromethorphan in mice. Behav Brain Res 295:26–34. [DOI] [PubMed] [Google Scholar]
- Nollet M, Leman S. (2013). Role of orexin in the pathophysiology of depression: potential for pharmacological intervention. CNS Drugs 27:411–422. [DOI] [PubMed] [Google Scholar]
- Oh C-M, Kim HY, Na HK, Cho KH, Chu MK. (2019). The effect of anxiety and depression on sleep quality of individuals with high risk for insomnia: a population-based study. Front Neurol 10:849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papakostas GI, Fava M, Freeman MP, Shelton RC, Thase ME, Jha MK, et al. (2020). Effect of pimavanserin on anxious depression in patients with major depression and an inadequate response to previous therapy: secondary analysis of the clarity study. Int Clin Psychopharmacol 35:313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park LT, Kadriu B, Gould TD, Zanos P, Greenstein D, Evans JW, et al. (2020). A randomized trial of the N-Methyl-d-aspartate receptor glycine site antagonist prodrug 4-chlorokynurenine in treatment-resistant depression. Int J Neuropsychopharmacol 23:417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons CG, Quack G, Bresink I, Baran L, Przegalinski E, Kostowski W, et al. (1995). Comparison of the potency, kinetics and voltage-dependency of a series of uncompetitive NMDA receptor antagonists in vitro with anticonvulsive and motor impairment activity in vivo. Neuropharmacology 34:1239–1258. [DOI] [PubMed] [Google Scholar]
- Passie T, Seifert J, Schneider U, Emrich HM. (2002). The pharmacology of psilocybin. Addict Biol 7:357–364. [DOI] [PubMed] [Google Scholar]
- Pause BM, Miranda A, Göder R, Aldenhoff JB, Ferstl R. (2001). Reduced olfactory performance in patients with major depression. J Psychiatr Res 35:271–277. [DOI] [PubMed] [Google Scholar]
- Pokorny T, Preller KH, Kometer M, Dziobek I, Vollenweider FX. (2017). Effect of psilocybin on empathy and moral decision-making. Int J Neuropsychopharmacol 20:747–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourhamzeh M, Moravej FG, Arabi M, Shahriari E, Mehrabi S, Ward R, et al. (2022). The roles of serotonin in neuropsychiatric disorders. Cell Mol Neurobiol 42:1671–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praxis . (2021). Praxis precision medicines reports PRAX-114 perimenopausal depression (PMD) phase 2a proof-of-concept trial results and announces plans to advance to phase 2b study in women with menopausal and mood symptoms. Praxis Precision Medicines, Inc. https://investors.praxismedicines.com/news-releases/news-release-details/praxis-precision-medicines-reports-PRAX-114-perimenopausal/. [Accessed 17 September 2022] [Google Scholar]
- Praxis . (2022). Praxis precision medicines reports negative results from PRAX-114 phase 2/3 monotherapy aria study in patients with major depressive disorder. Praxis Precision Medicines, Inc. https://investors.praxismedicines.com/news-releases/news-release-details/praxis-precision-medicines-reports-negative-results-PRAX-114/. [Accessed 17 September 2022] [Google Scholar]
- Radoš SN. (2018). Anxiety during pregnancy and postpartum: course, predictors and comorbidity with postpartum depression. Acta Clinica Croatica 57:39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recourt K, Boer P. de, Zuiker R, Luthringer R, Kent J, Ark P. van der, et al. (2019). The selective orexin-2 antagonist seltorexant (JNJ-42847922/MIN-202) shows antidepressant and sleep-promoting effects in patients with major depressive disorder. Transl Psychiatry 9:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy D.S. (2018). GABA-A receptors mediate tonic inhibition and neurosteroid sensitivity in the brain. In: Vitamins and hormones. Elsevier, pp. 177–191. [DOI] [PubMed] [Google Scholar]
- Reiche S, Hermle L, Gutwinski S, Jungaberle H, Gasser P, Majić T. (2018). Serotonergic hallucinogens in the treatment of anxiety and depression in patients suffering from a life-threatening disease: a systematic review. Prog Neuropsychopharmacol Biol Psychiatry 81:1–10. [DOI] [PubMed] [Google Scholar]
- Réus GZ, Jansen K, Titus S, Carvalho AF, Gabbay V, Quevedo J. (2015). Kynurenine pathway dysfunction in the pathophysiology and treatment of depression: evidences from animal and human studies. J Psychiatr Res 68:316–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissardo JP, Durante I, Sharon I, Caprara ALF. (2022). Pimavanserin and Parkinson’s disease psychosis: a narrative review. Brain Sci 12:1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross S. (2018). Therapeutic use of classic psychedelics to treat cancer-related psychiatric distress. Int Rev Psychiatry 30:317–330. [DOI] [PubMed] [Google Scholar]
- Ross S, Bossis A, Guss J, Agin-Liebes G, Malone T, Cohen B, et al. (2016). Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: a randomized controlled trial. J Psychopharmacol 30:1165–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross S, Agin-Liebes G, Lo S, Zeifman RJ, Ghazal L, Benville J, et al. (2021). Acute and sustained reductions in loss of meaning and suicidal ideation following psilocybin-assisted psychotherapy for psychiatric and existential distress in life-threatening cancer. ACS Pharmacol Transl Sci 4:553–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, et al. (2003). The 16-item quick inventory of depressive symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry 54:573–583. [DOI] [PubMed] [Google Scholar]
- Sage Therapeutics . (2019). Sage therapeutics reports topline results from pivotal phase 3 MOUNTAIN study of SAGE-217 in major depressive disorder. Sage Therapeutics, Inc. https://investor.sagerx.com/news-releases/news-release-details/sage-therapeutics-reports-topline-results-pivotal-phase-3. [Accessed 23 September 2022] [Google Scholar]
- Sage Therapeutics . (2022). Sage therapeutics announces second quarter 2022 financial results and highlights pipeline and business progress. Sage Therapeutics, Inc. https://investor.sagerx.com/news-releases/news-release-details/sage-therapeutics-announces-second-quarter-2022-financial. [Accessed 23 September 2022] [Google Scholar]
- Sage Therapeutics, Biogen 2021a). Sage therapeutics and biogen announce positive, one-year zuranolone 50 mg data in the ongoing open-label SHORELINE study in patients with MDD. https://www.businesswire.com/news/home/20211201005365/en/Sage-Therapeutics-and-Biogen-Announce-Positive-One-Year-Zuranolone-50-mg-Data-in-the-Ongoing-Open-Label-SHORELINE-Study-in-Patients-with-MDD. [Accessed 23 September 2022]
- Sage Therapeutics, Biogen 2021b). Sage therapeutics and biogen announce positive pivotal phase 3 results for zuranolone, an investigational two-week, once-daily therapeutic being evaluated for major depressive disorder. Biogen. https://investors.biogen.com/news-releases/news-release-details/sage-therapeutics-and-biogen-announce-positive-pivotal-phase-3. [Accessed 23 September 2022] [Google Scholar]
- Sage Therapeutics, Biogen . (2022). Sage therapeutics and biogen announce the phase 3 CORAL study met its primary and key secondary endpoints – comparing zuranolone 50 mg co-initiated with standard of care antidepressant vs. standard of care co-initiated with placebo in people with MDD. Biogen. https://investors.biogen.com/news-releases/news-release-details/sage-therapeutics-and-biogen-announce-phase-3-coral-study-met. [Accessed 23 September 2022] [Google Scholar]
- Sakashita Y, Abe K, Katagiri N, Kambe T, Saitoh T, Utsunomiya I, et al. (2015). Effect of psilocin on extracellular dopamine and serotonin levels in the mesoaccumbens and mesocortical pathway in awake rats. Biol Pharm Bull 38:134–138. [DOI] [PubMed] [Google Scholar]
- Salituro FG, Tomlinson RC, Baron BM, Palfreyman MG, McDonald IA, Schmidt W, et al. (1994). Enzyme-activated antagonists of the strychnine-insensitive glycine/NMDA receptor. J Med Chem 37:334–336. [DOI] [PubMed] [Google Scholar]
- Savitz A, Wajs E, Zhang Y, Xu H, Etropolski M, Thase ME, et al. (2021). Efficacy and safety of seltorexant as adjunctive therapy in major depressive disorder: a phase 2b, randomized, placebo-controlled, adaptive dose-finding study. Int J Neuropsychopharmacol 24:965–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serretti A, Chiesa A. (2009). Treatment-emergent sexual dysfunction related to antidepressants. J Clin Psychopharmacol 29:259–266. [DOI] [PubMed] [Google Scholar]
- Serretti A, Mandelli L. (2010). Antidepressants and body weight. J Clin Psychiatry 71:1259–1272. [DOI] [PubMed] [Google Scholar]
- Shelton RC, Fava M, Freeman MP, Thase ME, Papakostas GI, Jha MK, et al. (2020). Effect of adjunctive pimavanserin on suicidal ideation in patients with major depression: analysis of the CLARITY study. J Affect Disord 277:478–485. [DOI] [PubMed] [Google Scholar]
- Siegel AN, Vincenzo JDD, Brietzke E, Gill H, Rodrigues NB, Lui LMW, et al. (2021). Antisuicidal and antidepressant effects of ketamine and esketamine in patients with baseline suicidality: a systematic review. J Psychiatr Res 137:426–436. [DOI] [PubMed] [Google Scholar]
- Stachowicz K, Sowa-Kućma M. (2022). The treatment of depression – searching for new ideas. Front Pharmacol 13:988648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl SM. (2019). Dextromethorphan/bupropion: a novel oral NMDA (N-methyl-d-aspartate) receptor antagonist with multimodal activity. CNS Spectr 24:461–466. [DOI] [PubMed] [Google Scholar]
- Stroud JB, Freeman TP, Leech R, Hindocha C, Lawn W, Nutt DJ, et al. (2018). Psilocybin with psychological support improves emotional face recognition in treatment-resistant depression. Psychopharmacology (Berl) 235:459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studerus E, Gamma A, Kometer M, Vollenweider FX. (2012). Prediction of psilocybin response in healthy volunteers. PLoS One 7:e30800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton E. (2015). Profile of suvorexant in the management of insomnia. Drug Des Devel Ther 9:6035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabuteau H, Jones A, Anderson A, Jacobson M, Iosifescu DV. (2022). Effect of AXS-05 (dextromethorphan-bupropion) in major depressive disorder: a randomized double-blind controlled trial. Am J Psychiatry 179:490–499. [DOI] [PubMed] [Google Scholar]
- Taylor CP, Traynelis SF, Siffert J, Pope LE, Matsumoto RR. (2016). Pharmacology of dextromethorphan: relevance to dextromethorphan/quinidine (Nuedexta®) clinical use. Pharmacol Ther 164:170–182. [DOI] [PubMed] [Google Scholar]
- Vanover KE, Weiner DM, Makhay M, Veinbergs I, Gardell LR, Lameh J, et al. (2006). Pharmacological and behavioral profile of N -(4-Fluorophenylmethyl)-N-(1-methylpiperidin-4-yl)-N′-(4-(2-methylpropyloxy)phenylmethyl) carbamide (2 R,3 R)-dihydroxybutanedioate (2:1) (ACP-103), a novel 5-hydroxytryptamine 2A receptor inverse agonist. J Pharmacol Exp Ther 317:910–918. [DOI] [PubMed] [Google Scholar]
- Veen B.T.H. de, Schellekens AFA, Verheij MMM, Homberg JR. (2017). Psilocybin for treating substance use disorders? Expert Rev Neurother 17:203–212. [DOI] [PubMed] [Google Scholar]
- VistaGen Therapeutics . (2019). VistaGen reports topline phase 2 results for AV-101 as an adjunctive treatment of major depressive disorder. https://www.prnewswire.com/news-releases/vistagen-reports-topline-phase-2-results-for-av-101-as-an-adjunctive-treatment-of-major-depressive-disorder-300958317.html. [Accessed 25 September 2022]
- Vliegen N, Casalin S, Luyten P. (2014). The course of postpartum depression. Harv Rev Psychiatry 22:1–22. [DOI] [PubMed] [Google Scholar]
- Walsh JL, Hui-Qiu W, Ungerstedt U, Schwarcz R. (1994). 4-chloro-3-hydroxyanthranilate inhibits quinolinate production in the rat hippocampus in vivo. Brain Res Bull 33:513–516. [DOI] [PubMed] [Google Scholar]
- WHO depressive disorder . (2023). Fact sheets/detail/depressive disorder (depression). https://www.who.int/news-room/fact-sheets/detail/depression. [Accessed 2 September 2022]
- Wimberley T, Horsdal HT, Brikell I, Laursen TM, Astrup A, Fanelli G, et al. (2022). Temporally ordered associations between type 2 diabetes and brain disorders – a Danish register-based cohort study. BMC Psychiatry 22:573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Piantadosi SC, Wu H-Q, Pribut HJ, Dell MJ, Can A, et al. (2015). The prodrug 4-chlorokynurenine causes ketamine-like antidepressant effects, but not side effects, by NMDA/Glycine B-site inhibition. J Pharmacol Exp Ther 355:76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]