Abstract
Purpose of Review:
Social and environmental factors have been related to both symptom expression of disordered eating in individuals as well as changes in the prevalence of eating disorders (EDs) in populations. Neural differences in processing social information may contribute to EDs. This review assesses the evidence for aberrant neural responses during social processing in EDs.
Recent Findings:
This review examines how constructs within the social processing domain have been evaluated by neuroimaging paradigms in EDs, including communication, affiliation, and understanding of both oneself and others.
Summary:
Differences related to social processing in EDs include altered processing for self-relevant stimuli, in the context of identity, valence, expectations and affiliative relationships. Future work is needed to integrate how differences in processing social stimuli relate to alterations in cognitive control and reward as well as specific disordered eating symptoms.
Keywords: fMRI, identity, social cognition, anorexia nervosa, bulimia nervosa, self-esteem
Introduction
Eating disorders (ED) are serious mental illnesses that include altered behaviors and cognitions surrounding eating. The key behaviors can include: restriction of food intake, compensatory behaviors to remove food from one’s body, and binge-eating episodes including eating excessively and with loss of control over eating. Cognitive issues defining of EDs include both preoccupations and ruminations about body-image, food, and eating, often in conjunction with shame and guilt. When EDs are treated, acute improvements in both cognitions and behaviors are observed [1]. However, EDs have a high relapse rate, with more than half of patients with anorexia nervosa (AN) and bulimia nervosa (BN) showing persistence of symptoms even after treatment [2, 3]. Nevertheless, EDs do resolve over time, with approximately thirty percent of individuals with AN and nearly seventy percent for BN no longer meeting disorder criteria nine years later [4]. A better understanding of the processes that enable sustained recovery might shorten the course of illness, inform prevention efforts, and improve targets in therapeutic treatments.
Eating disorders typically begin during adolescence [5], a developmental period characterized by socio-emotional development and reorientation towards peers. Self-esteem as well as socializing problems are related to ED symptoms [6]. Social stressors are often reported as coinciding with ED symptom onset. One of the more common stressors reported is bullying about shape/weight/appearance [7, 8]. Changes in social environment can change disordered eating behaviors. For example, the introduction of cable-television to Fiji resulted in an immediate increase in exposure to Western culture and values through television programs. Before this exposure, none of the adolescent women surveyed reported inducing vomiting to control weight, but three years later 11% of a similar cohort endorsed this behavior [9]. Interviews with Fijian adolescents suggested body image concerns and disordered eating were associated with experiencing increased social pressures from a changing society [10].
Difficulties in the social domain play key roles as maintenance factors in a number of models of ED development/maintenance e.g., Transdiagnostic Cognitive Behavioral Model [11, 12], Cognitive Interpersonal Maintenance Model [13, 14], and Interpersonal Psychotherapy - Eating Disorders [15]. In all, interpersonal difficulties, deficits in social cognition, negative evaluations by others, and avoidance of social interactions are highlighted as factors contributing to the development and maintenance of EDs.
In the last two decades, neuroimaging research has improved our understanding of the neural circuitry engaged when people process social information [16–18]. Adolescence and young adulthood are an especially critical period for neurodevelopment of a stable sense of identity and positive self-esteem [19, 20]. Understanding how the social problems observed in EDs are related to brain development may help clarify psychopathological mechanisms in EDs and inform prevention and treatment of EDs. This review considers the state of evidence for aberrant processing of social information in EDs, identifying promising approaches for future attention.
Conceptualization of Social Dimensions
Social processing is one of the six domains within the Research Domain organizational Criteria (RDoC) created to organize experimental research on the brain by the National Institute of Mental Health. Four constructs lie within the Social Processes Domain. The Social Communication construct (Communication) refers to the dynamic interplay of interactions between individuals that includes facial and non-facial communication. The Affiliation and Attachment construct (Affiliation) considers how positive social interactions lead to increased approach motivation and the development of deeper social bonds. The Perception and Understanding of Self construct (Self) includes both agency and self-knowledge as related to interpreting one’s own cognitive, emotional, and physical state and attributes. The Perception and Understanding of Others construct (Other) assesses how an individual evaluates the cognitive/emotional states, behaviors and traits in other people.
Social processing is dynamic, emerging as interactions between oneself, others, and environment lead to internalization of beliefs about oneself, other people and one’s social network (Figure 1). Individuals develop a sense of identity that is influenced both by their real-world interactions and abstracted knowledge. For example, when considering the word “family”, a person has stored information from both their own experiences of spending time with real-world family members and knowledge about what families are like from books, television, and discussions about families with peers. These two conceptualizations of ‘family’ are merged and set up beliefs and expectations about families and how people in given roles should behave towards each other. These beliefs about family may then change how a person interacts with people labeled as such, altering in-the-moment behavioral interactions. People often change their behaviors based on whether interactions are with people who share a common group identity with them [21].
Figure 1.
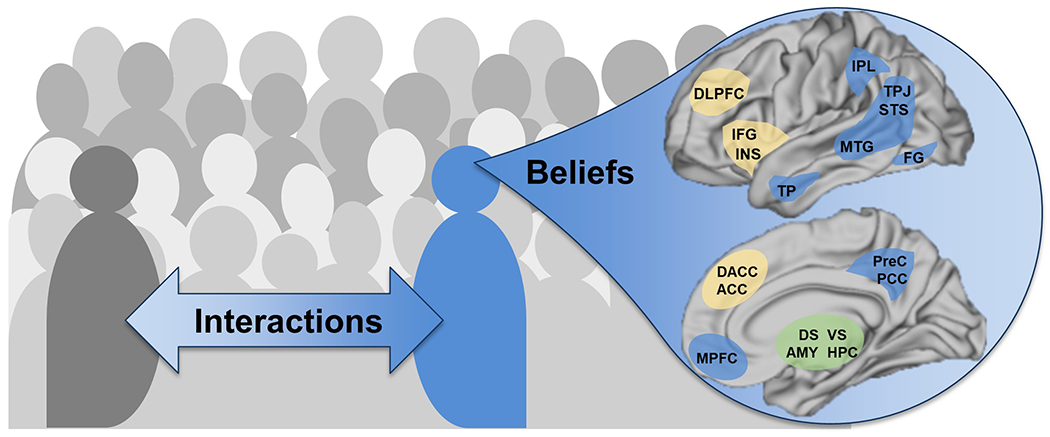
Key features in social processing paradigms as well as the neural regions involved. On left, an individual (blue) interacts with other people (gray) within a social environment. Each individual also internalizes beliefs about themselves, other people, and the world in a generalized sense over their lifetime. Many different brain regions and networks are involved in processing social information which requires both internalizing beliefs and reacting to discrete experiences, with roles for the default mode network (blue), salience network (yellow) and subcortical nuclei (green). Key regions include medial prefrontal cortex (MPFC), precuneus (PreC), posterior cingulate (PCC), intraparietal lobule (IPL), temporoparietal junction (TPJ), superior temporal sulcus (STS), middle temporal gyrus (MTG), temporal pole (TP), fusiform gyrus (FG), anterior cingulate (ACC), dorsal anterior cingulate (DACC), inferior frontal gyrus (IFG), insula (INS), dorsolateral prefrontal cortex (DLPFC), amygdala (AMY), dorsal striatum (DS), ventral striatum (VS), hippocampus (HPC).
Further increasing the complexity of this domain, social stimuli and their resulting responses depend upon information obtained and processed at many different levels within our brain. At the sensory level, the visual and auditory cortices prioritize signals related to communication, such as faces and language [22–24]. Thus, neural processing of social information integrates in-the-moment responses to sensory stimuli, in relation to both an individual’s current affective state, and their beliefs about self and world. The primary tool deployed is functional magnetic resonance imaging (fMRI), a technique that measures blood oxygenation level dependent (BOLD) signals in the brain, a proxy for changes in neural activity. Social processing fMRI paradigms connect changes in neural activations to events the participant is experiencing within that paradigm, allowing inferences about the function of neural regions to be connected to behaviors and cognitions within a well-controlled task paradigm.
Many different cortical and subcortical regions (Figure 1, right) have been identified in reviews of social processing paradigms in healthy people [16–19], including areas in the default mode network (blue), salience network (yellow), and subcortical nuclei (green). A separate type of fMRI study, resting state, defines neural networks by identifying neural regions that are activated and deactivated simultaneously [25]. The two neural networks most prominent in studies with social paradigms, are the default mode network, commonly engaged by self-directed introspection [26], and the salience network, associated with complex decision-making [27]. Subcortical regions associated with fear, reward, and memory can also be activated during social paradigms. An additional level of inference is added when connecting psychopathology to brain differences. Most commonly, a cohort with psychopathology is associated with reduced or heightened activation of regions in response to the same events within a paradigm, suggesting a different internal experience. Neural differences during these tasks may also be related to clinical, psychological, or behavioral assessments. This review considers the evidence for altered neural processing of social stimuli in EDs, focusing on cognitive neuroimaging paradigms.
Methods
We evaluated existing neuroimaging studies in EDs in relation to the organizing constructs of the RDoC Social Domain. Articles were initially identified with a PubMed search (July 2022) with the following terms: “neuroimaging” or “fMRI”; “social”; with the additional terms of “anorexia nervosa” (103 articles) or “bulimia nervosa” (22) or “binge-eating disorder” (85). Abstracts were screened to identify case-control fMRI studies with both an ED and healthy comparison group. The remaining articles (39 for AN; 9 for BN; 23 for BED) were then reviewed to determine if the paradigm related to the four RDoC constructs (Communication, Affiliation, Self, Other). Similar paradigms were grouped: Social/Non-Social Stimuli, Facial Emotion, Emotional Self-Regulation, Appraisals, Self/Other Stimuli, and Interactions.
Results
Interpreting Social & Non-Social Stimuli
The earliest studies in social processing in EDs compared social to non-social stimuli; this paradigm probes both the Communication and Other constructs. One paradigm, Social Attributions, involves watching short videos of moving geometric shapes that move either as if they are people interacting or as inanimate objects bumping into each other. Neurotypical people demonstrate pronounced differences in modulation of many regions including medial prefrontal cortex (MPFC), temporoparietal junction (TPJ), and temporal poles (TP) when comparing the two types of shape videos [28]. Variants of this type of task have been deployed in five studies in EDs (Table 1, Social and Non-Social Stimuli). In four studies participants made social decisions: answering a question after the social video (People: All Friends?) and after physical videos (Bumper Cars: Same Weight?). Adults recovering from anorexia nervosa had less activation in the right TPJ, precuneus, and inferior frontal gyri relative to comparison participants when attention was directed to the animate relative to inanimate [29]. A follow-up study showed diminished processing in the right TPJ for adults with BN [30]. The study in adolescents with AN showed reduced processing in the social condition in MPFC and less activation in this region at baseline was associated with worse outcomes a year later [31]. A more recent study [32] evaluated adolescents with AN and BN, observing reduced activation in the superior temporal sulcus (STS) in AN and hyperactivation in this area in BN. A separate study used similar social/non-social shape stimuli as the other four, but participants passively viewed the social and non-social movements, not responding or making any decisions about interactions [33], and reported no differences in brain activations in ED cohort relative to comparison women. These data suggest a hypothesis that neural differences in processing social relative to non-social stimuli is related to decision-making rather than sensory perception in EDs.
Table 1.
Paradigms assessing Social & Non-Social Stimuli, Facial Emotion, and Emotional Self-Regulation.
| Study and Participants1 | Paradigm and Results |
|---|---|
| Social & Non-Social Stimuli: RDoC Communication and Other | |
| McAdams & Krawczyk (2011) [29]; Adults: recAN (n=17; 26.2 years; BMI 19.7); CW (n=17; 24.7 years; BMI 23.4) |
Paradigm: Decided if shapes either imaged as People were friends or as Bumper Cars had same mass. Results: CW had stronger neural activations in IFG, TPJ, STS, FG than recAN. |
| Schulte-Rüther et al. (2012) [31]; Adolescents: adolAN (n=19; 15.7 years; BMI 15.3); adolCW (n=21; 15.8 years; BMI 22.7) |
Paradigm: Same as [29]. Results: AdolCW with stronger neural activation for social condition in TP than adolAN; activation in MPFC related to clinical outcome. |
| McAdams & Krawczyk (2013) [30]; Adults: recAN (n=18; 26.1 years; BMI 19.8); cBN (n=17; 28.1 years; BMI 22.1); CW (n=18; 24.5 years; BMI 23.2) |
Paradigm: Same as [29]. Results: CW with stronger neural activations for social condition in TPJ than recAN and cBN. |
| Leslie et al. (2020) [33]; Mixed: cAN (n=67; 18.7 years; BMI 16.6); recAN (n=49; 19.7 years; BMI 20.0); CW (n=70; 19.6 years; BMI 22.8) |
Paradigm: Passive viewing of animations moving randomly, with non-social motion, and with social interactions. Results: No differences in the three groups. |
| Ruan et al. (2022) [32]; Adolescents: adolAN (n=18; 15.7 years; BMI 15.8); adolCW-a (n=18; 15.8 years; BMI 22.3); adolBN (n=16; 17.8 years; BMI 20.2); adolCW-b (n=18, 17.5 years; BMI 22.5) |
Paradigm: Same as [29]. Results: adolCW-a with stronger neural activations in TPJ, PreC relative to adolAN at both time points; adolBN with increased neural responses in TP relative to adolCW-b |
| Facial Emotion: RDoC Communication and Other | |
| Ashworth et al. (2011) [39]; Adults: cBN (n=12; 27.4 years; BMI NR); CW (n=16; 24.4 years; BMI NR) |
Paradigm: Emotion matching task with disgust faces, angry faces, or shape orientation. Results: CW with elevated activity in the PreC for both angry and disgust faces and elevated AMY for angry relative to cBN. |
| Fonville et al. (2014) [34]; Adults: cAN (n=31; 23 years; BMI 15.9); CW (n=35; 25 years; BMI 21.9) |
Paradigm: Implicit facial emotion processing task with neutral, slightly happy, or happy faces; participants judged face gender. Results: More FG in cAN than CW; more lingual/cingulate in CW than cAN. |
| Leppanen et al. (2017) [37]; Adults: cAN (n=21; 25 years; BMI 15.8); CW (n=26; 25.5 years; BMI 19.9) |
Paradigm: Viewing happy, sad, neutral faces of infants. Contrasted happy to neutral and sad to neutral. Results: cAN more AMY and DLPFC for happy relative to neutral faces; cAN more left INS than CW for sad relative to neutral faces. |
| Leppanen et al. (2017) [36]; Adults: cAN (n=20; 28.6 years; BMI 15.9); CW (n=20; 25.8 years; BMI 21.1) |
Paradigm: Viewing happy, fearful, and neutral faces. Contrasted happy to neutral and fearful to neutral. Results: cAN more INS for happy relative to neutral than CW; cAN with less AMY & VLPFC for fearful relative to neutral than CW |
| Lulé et al. (2021) [35]; Adolescents: adolAN (n=11; 16.4 years; BMI 17.6); adolCW (n=11; 16.5 years; BMI 21.4) |
Paradigm: Viewing emotional (happiness, sadness, anger, fear, surprise, disgust) faces with variable intensity. Results: adolAN has reduced left MCC for faces in general. Also adolAN with less R DS and R FG for happy faces, and less MPFC and IFG for fearful faces. No differences for disgust, surprise, anger or sadness. |
| Halls et al. (2021) [38]; Mixed: cAN (n=57; 19.4 years; BMI 16.4); recAN (n=60; 18.4 years; BMI 20.4); CW (n=69; 19.4 years; BMI 22.8) |
Paradigm: Same stimuli as [34] and [36]. Results: No whole brain differences across groups. Increased connectivity for recAN relative to CW from occipital face area to frontal regions for happy stimuli |
| Emotional Regulation: RDoC Self | |
| Seidel et al. (2018) [41]; Adolescents: adolAN (n=36; 16.6 years; BMI 14.7); adolCW (n=36; 16.9 years; BMI 20.7) |
Paradigm: Viewing of negative, neutral, positive images with directive to either ‘watch’ or ‘distance’ for positive and negative images. Arousal ratings after images. Results: Differences on passive viewing with more AMY and bilateral DLPFC in cAN for negative pictures; no differences when regulating |
| Pauligk et al. (2021) [42]; Adolescents: adolAN (n=36; 16.5 years; BMI 14.4); adolCW (n=36; 16.7 years; BMI 20.3) |
Paradigm: Passive viewing of negative and neutral images, as well as directed down-regulation of emotional responses to negative images. Task repeated at baseline and in two weeks, and correlated with ecological momentary assessments of psychopathology. Results: Increased reactivity of the bilateral AMY in cAN compared to CW persisted at re-exposure to previously watched negative and neutral pictures. EMA data suggesting that DLPFC at baseline related to increased AMY to negative images at follow-up. |
| Seidel et al. (2022) [44]; Adults: recAN (n=41; 22.1 years; BMI 20.7); CW (n=41; 22.0 years; BMI 21.9) |
Paradigm: Viewing of negative, neutral, positive images with directive to either ‘watch’ or ‘distance’ for positive and negative images. Arousal ratings after images. Results: No differences. |
| Steward et al. (2022) [43]; Adults: cAN (n=21; 22 years; BMI 16.3); CW (n=22; 22 years; BMI 21.0) |
Paradigm: Viewing of negative or neutral pictures with three directives “Look Neutral”, “Look Negative”, “Regulate Negative”. Results: cAN with less DLPFC when regulating emotions; also less connectivity DLPFC to AMY during regulation; DLPFC activity increased with weight. |
| D’Agata et al. (2021) [45]; Adults: cAN (n=25; 22 years; BMI 16.1); cBN (n=19; 22 years; BMI 21.9); CW (n=20; 23 years; BMI 21.5) |
Paradigm: Viewed emotional sentences and asked to imagine one’s own response, and match that with faces showing anger, fear, disgust or neutral. Results: No differences across the three groups. |
Abbreviations: AN – anorexia nervosa; cAN – adults with AN; adolAN – adolescents with AN; recAN – recovered from AN; BN – bulimia nervosa; cBN – adults currently with BN; adolBN – adolescents with BN; CW – comparison women; adolCW – comparison adolescent women. Neural regions: medial prefrontal cortex (MPFC), ventrolateral prefrontal cortex (VLPFC), precuneus (PreC), middle cingulate (MCC), temporoparietal junction (TPJ), superior temporal sulcus (STS), temporal pole (TP), fusiform gyrus (FG), dorsal anterior cingulate (DACC), inferior frontal gyrus (IFG), insula (INS), dorsolateral prefrontal cortex (DLPFC), amygdala (AMY), dorsal striatum (DS).
Interpreting Emotional Stimuli in Faces
Faces also carry social information; interpretation of emotions within faces is particularly crucial for the Communication and Other constructs. Many types of face stimuli have been deployed to evaluate emotional communication in both AN and BN (Table 1, Facial Emotion Tasks). Two studies have suggested reduced cingulate engagement in response to face stimuli, independent of emotion-content, in AN [34, 35]. Two other studies have found altered use of the amygdala (AMY), insula (INS), and dorsolateral prefrontal cortex (DLPFC) in response to emotional content of faces in individuals with AN, although the specific emotions leading to altered activations vary [36, 37]. The largest study considering both ill and recovered AN found no whole-brain activation differences although increased connectivity between the occipital and frontal lobes in response to happy stimuli was observed in the recovered AN cohort [38]. For BN, we identified only one study using face stimuli, reporting reduced AMY (to angry faces) and precuneus (to fearful and angry faces) modulations [39]. Finally, one recent study considered face stimuli in weight-recovered women with AN, finding no differences in neural responses to happy stimuli, suggesting that emotional recognition problems in AN may be state-dependent [40]. More research on facial communication is needed, as most studies utilized different stimuli and contrasts, no studies of facial emotions were found for BED, only one for BN, and only one in adolescents.
Emotional Self-Regulation
Social-emotional regulation tasks typically present a stimulus that activates an emotion, followed by instructions to regulate or experience the stimulated emotion, a process related to the Self construct (Table 1, Emotional Regulation Tasks). Although two studies in adolescents with AN reported elevated AMY responses at baseline to viewing negative images, no differences were found in the ability to regulate this emotion [41, 42]. One study suggested less DLPFC activity and connectivity to the AMY during emotion regulation in AN, and that more DLPFC was associated with more weight gain in subsequent treatment [43]. In adolescents with AN, DLPFC activation at baseline was related to increased AMY as well as self-reported tension during ecological momentary assessments during the two weeks after the scan [42]. A study of recovered AN showed no neural differences [44]. One study considered emotional imagination in both AN and BN, a construct likely similar to emotional regulation, and found no differences in individuals with either disorder [45]. The neural processes engaged during directed regulation of emotions appear to be largely intact in AN and BN.
Self-Other Images
Visual tasks involving participants viewing themselves or others have been conducted in EDs, probing the constructs of Self and Other (Table 2, Self-Other Images). Some paradigms include prompts to compare the image shown to oneself in a specific way. Relative to comparison cohorts, altered activations in MPFC extending to ACC are observed in a variety of self-other body-comparison tasks, including: increased activation in a mixed (adolescent-adult) sample of women with BN when viewing heavy bodies [46], increased activation in women recovered from AN when viewing thin bodies during a self-comparison task [47], reduced activation during physical self-evaluation relative to a physical other-evaluation in women with AN and BN [30, 48], and reduced activation when viewing others engaging in body checking in AN [49]. Elevated activations in the fusiform gyri (FG) are observed in women with AN but not those recovered from AN during passive viewing of self relative to other face-images [50]. Additionally, lower activation in FG is observed in women with BN during a body-image self-other comparison task [51], whereas those with BED have increased activation for body-images relative to other images [52]. Two studies have presented distorted body images to ED participants; they found altered utilization of the AMY and MPFC in AN [53] and altered precuneus, INS, and medial frontal gyrus in BN [54]. Sweitzer et al. [40] examined differences related to the reward of viewing bodies as well as smiling faces in AN, utilizing a region of interest analysis focusing on reward regions (dorsal striatum (DS), ventral striatum (VS), MPFC). Women recovered from AN had decreased DS responses for body images, and that response correlated with course of illness. In sum, altered neural processing for self-other visual stimuli is observed in EDs, with more studies in AN than BN or BED, and has primarily been assessed in adult samples.
Table 2.
Paradigms examining Self-Other Images, Appraisals, and Social Interactions.
| Study and Participants1 | Paradigm and Results |
|---|---|
| Self-Other Images: RDoC Self and Other | |
| Vocks et al. (2010) [79]; Adults: cAN (n=13; 29 years; BMI 15.8); cBN (n=15; 28 years; BMI 21.3); CW (n=27; 27 years; BMI 22.0) |
Paradigm: Viewing one’s own body and other women’s bodies. Results: cAN and cBN with less activations in left IPL and MTG for self-images relative to CW; cAN with more activation in MTG for other-images. |
| Miyake et al. (2010) [53]; Adults: cAN-R (n=11; 22.2 years; BMI 15.3); cAN-BP (n=11; 28 years; BMI 15.9); cBN (n=11; 24.5 years; BMI 20.2); CW (n=11; 27 years; BMI 19.1) |
Paradigm: Viewed one’s own body and other bodies with weight-related distortions. Results: cAN-R, cAN-BP, and CW with increased AMY activation for heavier self-images relative to normal weight self-image but not cBN. cAN-BP and CW with elevated DACC and DLPFC for heavier self-image but not cAN-R or cBN. |
| Mohr et al. (2010) [54]; Adults: cBN (n=15; 24.8 years; BMI 22); CW (n=16; 25.5 years; BMI 21) |
Paradigm: Viewed one’s own body under conditions of weight-related distortions twice; once rating reality and once closeness to idealized size. Results: BN with less activity in MFG, PreC, and PCC to images than CW; altered INS engagement in relation to task condition and group. |
| Spangler & Allen (2012). [46]; Adults2: cBN (n=12; 12-38 years; BMI 19.3-27.8) and CW (n=12; 18-30 years; BMI 18.5-26.9) |
Paradigm: Viewed thin and heavy bodies with comparing one’s own body to those in the images. Results: MPFC activation was greater in cBN when viewing heavy images; no neural differences for thin images. |
| Van den Eynde et al.(2013) [51]; Adults: cBN (n=21; 28 years; BMI 23.4); CW (n=23; 27.3 years; BMI 21.3) |
Paradigm: Viewed calorie-dense food and told to imagine eating food. Viewed images of thin bodies and told to compare to own body. Results: Greater activation of INS and less activation of FG in cBN compared with CW. No differences in viewing food stimuli. |
| Suda et al. (2013) [49]; Adults: cAN (n=20; 27 years; BMI 15.7); CW (n=15; 26 years; BMI 21.9) |
Paradigm: Viewed people engaging in body checking or neutral events. Results: cAN had less activation in MPFC and FG when viewing body checking in comparison to CW. |
| Kodama et al. (2018) [47]; Adults: recAN (n= 15; 33.2 years; BMI 20.7); CW (n= 15; 29.7 years; BMI 21.5) |
Paradigm: Viewed low, normal, heavy bodies and told to either compare to own body or estimate weight of body shown. Results: Greater ACC activation when comparing their own bodies to underweight bodies in cAN than CW; less activation of EBA during weight estimation task in cAN. |
| Sweitzer et al. (2018) [40]; Adults: recAN (n= 20; 22.5 years; BMI 22.5); CW (n=25; 24.5 years; BMI NR) |
Paradigm: Viewed both smiling face images as well as images of thin, average, heavy individuals and rated their attractiveness. Results: ROI Analysis: No differences for faces. Viewing figures decreased activation in right DS for recAN; DS activation negatively correlated with sustained course of AN. Exploratory whole brain analyses related to course, with also reduced activation in DS, VS, MPFC. |
| Press et al. (2022) [52]; Adults: cBED (n=38; 38 years; BMI 29.9); CW (n=22; 38.36 years; BMI 28.7) |
Paradigm: Viewed one’s own body, another person’s body matched for BMI/waist-to-hip ratio/age. Results: cBED had increased activation in left FG when viewing bodies versus control images in comparison to CW. |
| Verbal Appraisals: RDoC Self and Other | |
| Pringle et al. (2011) [58]; Adults: cBN (n=11; 27.4 years; BMI NR); CW (n=16; 24.6 years; BMI NR) |
Paradigm: Rated negative words as “me” or “not me”. Negative words included both ED and depressive descriptors, contrasted both. Results: CW with increased responses to ED descriptors (occipital, PreC, AMY, VS, HPC) as well as depressive descriptors (PreC, occipital) than cBN. |
| McAdams & Krawczyk (2014) [48]; Adults: recAN (n=18; 26.1 years; BMI 19.8); CW (n=18; 24.5 years; BMI 23.2) |
Paradigm: Completed social “nice” or physical “arms are toned” appraisals for three perspectives (Self “I am”, Friend “my friend”, and Reflected “My friend thinks”). Contrasts of perspectives. Results: In social, CW more engagement of PreC relative to recAN for self than other. In physical, CW with more ACC activation than recAN for self than other. |
| McAdams & Krawczyk (2013) [30]; Adults: recAN (n=18; 26.1 years; BMI 19.8); cBN (n=17; 28.1 years; BMI 22.1); CW (n=18; 24.5 years; BMI 23.2) |
Paradigm: Same as [48]. Results: ROI analysis from above regions; cBN differs from CW in PreC and ACC for social task; no differences in physical task. |
| McAdams et al. (2016) [50]; Adults: cAN (n=22; 27.6 years; BMI 17.6); recAN (n=18; 29.6 years; BMI 22.8); CW (n=19; 27.9 years; BMI 22.5) |
Paradigms: Two tasks. Social appraisals with three perspectives (Self “I am”, Friend “my friend”, and Reflected “My friend thinks”). Contrasts for both perspective and agreeing/disagreeing. Self-other face task with passive viewing. Results: For appraisals, CW group engaged MPFC more when agreeing with terms while AN and recAN utilized area more for disagreeing. Mentalization led to elevated IFG and DACC by recAN cohort. For faces, cAN with elevated activity in FG for self relative to other faces. |
| Xu et al. (2017) [59]; Adolescents: adolAN (n=24; 16.4 years; BMI 19.5); adolCW (n=18, 16.1 years; BMI 21.2) |
Paradigm: Social appraisals task as per [50]. Contrasts for both social and in relation to agreeing/disagreeing with terms. Results: No whole-brain differences. ROI analysis with adult ROIs: activity in MPFC and DACC correlate with body-shape and anxiety scores. PreC responses at baseline (self - friend) related to clinical outcomes. |
| McAdams et al. (2018) [80]; Adults: Same cohort as 2016 [50]. |
Paradigm: Same as [50], but whole-brain regressions looking at psychological scale examining social attributions. Results: Activations in left IFG - INS are related to negative self-attributions, across all three cohorts. |
| Mendoza et al. (2022) [60] Mixed: Cohorts from 2016 [50] and 2017 [59]. |
Paradigm: Data from [50, 59]. Valence of the verbal stimuli utilized to further evaluate responses in neural ROIs. Results: Interactions between group and social perspective, with altered use of MPFC, TPJ, PreC in adult cohorts. No differences in negative > positive ROIs: DACC, IFG. |
| Social Interactions: RDoC Affiliation | |
| Via et al. (2015) [61]; Adults; cAN (n=21; 28.4 years; BMI 16.9); CW (n=22; 28.1 years; BMI 21.0) |
Paradigm: See and rate pictures of other people, then told whether they liked your picture or not. Results: In response to acceptance feedback, CW activate VS, INS, and DLPFC but AN do not; AN activate HPC & AMY for acceptance feedback. For rejection feedback, AN activate DS while CW do not. |
| McAdams et al. (2015) [62]; Adults: cAN (n=23; 26.3 years; BMI 18.0); recAN (n=19; 29.6 years; BMI 22.8); CW (n=21; 27.0 years; BMI 22.8) |
Paradigm: Played trustee role in multi-round trust game with neurotypical partner (computational-simulation). Results: Both cAN and recAN with less activations in PreC, TPJ following positive interactions. cAN with less activations in FG after negative interactions. |
| Luo et al. (2021) [63]; Adults: cBN (n=22; 27.6 years; BMI 17.6); CW (n=19; 27.9 years; BMI 22.5) |
Paradigm: Same as above [62]. Results: cBN with more activations in DS, AMY, INS, DACC, PCC, and TPJ after negative interactions. |
Abbreviations: AN - anorexia nervosa; cAN - adults with AN; adolAN - adolescents with AN; recAN - recovered from AN; cAN-R - adults with restricting AN; cAN-BP - adults with binge-purge AN; BN - bulimia nervosa; cBN - adults currently with BN; CW - comparison women; adolCW - comparison adolescent women; BED -binge-eating disorder; cBED - adults with BED. Neural regions: medial prefrontal cortex (MPFC), precuneus (PreC), posterior cingulate (PCC), intraparietal lobule (IPL), temporoparietal junction (TPJ), middle temporal gyrus (MTG), fusiform gyrus (FG), dorsal anterior cingulate (DACC), inferior frontal gyrus (IFG), insula (INS), dorsolateral prefrontal cortex (DLPFC), amygdala (AMY), dorsal striatum (DS), ventral striatum (VS).
Appraisals
Appraisal tasks are amongst the most common stimuli utilized in mapping differences in the neural regions involved during directed, conscious reasoning about people (for review, see [55]). In these tasks, participants conduct in-the-moment evaluations about themself (direct self-appraisal, “I am responsible”) or others (direct other-appraisal, “I believe my best friend is kind”), using conscious decision-making related to the Self and Other constructs. These tasks secure both behavioral evidence of differences in beliefs about oneself and others, and can identify how neural regions respond to self-other as well as positive-negative information [56, 57]. Five verbal appraisal experiments have been conducted in adolescents and adults recovering from AN, with AN, in sustained recovery from AN, and with BN (Table 2, Social Appraisals). Reduced modulation of regions within the default mode network during self-appraisals occurred in all studies of adults with EDs [30, 48, 50, 58]. The single study assessing adolescents recovering from AN found no whole-brain differences, with less activation in the precuneus related to recovery one year later [59]. To more precisely pinpoint the neurocognitive differences related to self-other evaluations in AN, participants (with AN, recovered from AN, and comparison) agreed or disagreed with positive or negative terms presented with three different social perspectives: self, other, reflected (My friend thinks I am..) [60]. Neural regions differentially modulated by valence were identified by contrasting activations to positive [MPFC, bilateral TPJ, precuneus] relative to negative [DACC, left inferior frontal gyrus (IFG)] verbal stimuli across all social perspectives and groups. Activations in these valence-defined neural regions were then compared in relation to clinical group and social perspective. Both adult AN cohorts showed lower responses in the regions more active for positive terms during direct self-appraisals and higher responses in the same regions during direct friend-appraisals while comparison women showed the opposite pattern. No group differences were found for the regions more active for negative terms. Aberrant social processing in AN appears to be related to both stimulus valence and social perspective, with more studies needed in adolescents, BN, and BED.
Social Interactions
Understanding what other people think about us and how they respond to us is critical to form relationships with others, a process most closely related to the Affiliation construct. Via et al. [61] presented face-stimuli while asking if the participant wanted to meet the “individual” presented and receiving feedback about whether the “other person” wanted to meet the participant. In response to receipt of this feedback, women with AN had altered responses in the AMY, DS, VS, INS, DLPFC, and hippocampus (HPC) relative to control women, findings described as an abnormal motivation for social stimuli. The Multi-Round Trust Game simulates building a relationship by investing and receiving benefits from the same investor over multiple trials; this task has been evaluated in both AN and BN. All differences observed in both EDs occurred when the participant found out the other person behaved in a way inconsistent with their own most recent behavior. When the other person was nicer than expected, both ill and recovered women with AN showed reduced neural activations in the PreC and TPJ [62]. When the other person was greedier than expected, women with BN had elevated neurobehavioral responses in both subcortical (AMY, DS, and VS) and cortical (DACC, TPJ, IFG) regions [63]. In considering how these neural responses in the trust game in AN and BN might translate to real-world social interactions, we hypothesize that both groups may internalize a belief that other people are not supportive of them. However, the source of this negative belief about others differs: not recognizing positive interactions in AN while being hypersensitive to negative interactions in BN.
Next Steps
RDoC: Self-Relevance, Valence, and Expectations
Interpreting data in relation to any single paradigm is simpler than creating a model to capture the different types of social processing explored in EDs. Three features related to aberrant processing of social information in EDs emerge across the paradigms: self-relevance, valence, and expectations. The Social Attributions tasks requiring responses show more differences than passive viewing; self-images have more aberrant processing than standardized images. Verbal appraisal differences occur when contrasting self to other and depend on stimulus valence; expectations and valence are critical to observing differences in the interaction paradigms. Importantly, these broad features are also be engaged by paradigms designed for other domains in the RDoC framework. Self-relevance includes motivated experiences and behaviors that impact an individual, and includes two distinct processes: introspection (cognitive and emotional beliefs about oneself) and interoception (sensory and physiological experiences) [16]. Introspective paradigms were reviewed here, falling within the Self construct of the Social Processing Domain but interoceptive paradigms were not, as they are part of the Arousal and Regulatory Domain of RDoC. Interestingly, one recent review of interoception in EDs [64] also identified expectations, operationalized there as prediction errors, as a feature related to aberrant processing in EDs. Similarly, valence has its own two domains (Positive Valence Systems and Negative Valence Systems) in the RDoC framework and self-relevance is common in many of these tasks. Notably, delayed discounting paradigms ask participants about their current desire for a small amount of money now or more money later, a question requiring introspection about one’s current and future needs (Self). A recent review of delayed discounting paradigms in EDs [65] reported altered neural responses in MPFC, DACC, DLPFC, DS and VS – areas also emerging from the social paradigms considered here.
Connecting Social Processes to Clinical Symptoms in EDs
Development of neurocomputational models of EDs then requires integration of data across social and non-social paradigms, as neural regions typically activate for multiple processes. The DACC is active during both social perspective-taking and cognitive control paradigms; value is processed in MPFC for social, monetary, and food rewards. Are the problems in processing self-relevant stimuli in the default mode network regions in AN a result of diminished subcortical responses to rewards in general, or caused by aberrant development of self-identity? Elucidating the mechanisms that underlie the negative self-image characteristic of EDs requires models that integrate across domains, with one promising candidate the identity-value model of social decision-making [66]. This model integrates cognitive control and reward systems with social processing, hypothesizing that value-based choices are part of all self-relevant decisions, such that choice-values congruent with one’s identity (e.g. being kind) receive higher internal values than incongruent choices (e.g. being mean); this computation is proposed to occur in MPFC [67]. As we consider this model for AN, we hypothesize that low self-esteem and a negative self-image may result from diminished identity-value inputs – observable as hypoactivation of MPFC in Self paradigms. The identity-value model also proposes that during adolescence, peer groups impact development of each person’s identity-values, having the ability to promote positive (e.g., do well in school, eat consistently) and negative (e.g., engage in illegal activities, diet) self-relevant decisions [68]. These concepts are consistent with clinical experiences of EDs including their emergence during adolescence in relation to stressors. Longitudinal neuroimaging research in adolescents with EDs are needed to more fully understanding how identity, disordered eating, and environment are related. A large, multi-site study with both adolescent and adult participants completing cognitive control, reward, and social tasks could exponentially accelerate this field towards a precision model of the neuropsychological pathology occurring in EDs.
Psychotherapeutic Implications
One benefit derived from deploying social tasks is that these paradigms may be more readily translated into real-world experiences of patients than typical cognitive control or monetary reward tasks. The appraisal tasks can assess brain function during mentalization, an established therapeutic technique to reduce negative rumination. We conducted a brief, pilot open trial using a group format that combined art experientials with psychoeducation on negative self-concept and mentalization in a mixed cohort of women with AN, BN, and BED [69]. Participants showed improvements in psychopathology with clinical improvements persisting for several months after the brief 4-session intervention [70]. Participants both reported retention of the psychoeducational constructs related to mentalization, and described the social connections established in the group to be motivating in their recovery [69]. Group formats may be especially effective for social processing targets, as discussion about in-the-moment group interactions may promote changes in internalized beliefs essential to improve one’s sense of identity.
Neurofeedback and Negative Self
The data shown suggests altered utilization of neural circuits are related to the persistent negative view of self established in EDs. As this problem can also be part of major depressive disorder, neurofeedback targeting a negative self-view has been explored for depression. Providing neurofeedback to increase AMY responses during recall of positive self-relevant memories improves depressive symptoms, and can alter responses in MPFC, DACC and DLPFC in adults [71]. Cueing adolescents to think about themselves in a positive way while providing real-time feedback about AMY activations increased MPFC activations while decreasing responses in the AMY and DLPFC [72, 73]. These approaches may also be helpful to improve the negative self-esteem observed in individuals with EDs.
Limitations
Many limitations of existing research in the Social Domain were identified. Several common social paradigms have not been conducted in ED cohorts, including autobiographical memory tasks [74] (Self), ultimatum games [75] (Other), and cyberball [76] (Affliation). Neuroimaging paradigms are complex, with task design often limiting interpretations. For example, if a study only shows negative social stimuli, differences may not generalize to positive social stimuli. The total number of studies identified is small, and few studies have been done in BN and BED. Altered brain structure is closely associated with low weight in AN [77, 78], but few social paradigms have been conducted in both underweight and weight-restored cohorts. As neurodevelopmental changes in social processes occur during puberty [20], more studies in adolescents with EDs are needed. Boys and men have not been included in any studies. Finally, few studies include longitudinal components, a critical component to connect social problems with expressed clinical psychopathology and neural differences. A better understanding of how EDs affect neurodevelopment of social processing may lead to earlier intervention and identify age-appropriate treatments for EDs.
Conclusions
Neural differences were observed for all four constructs within the RDoC Social Domain, with differential responsivity in regions within the default mode network, salience network, and subcortical nuclei. Self-relevance, valence, and expectations were key features in the contrasts and task paradigms discerning whole-brain neural differences in EDs. Neural differences were observed in relation to both introspective, belief-based tasks as well as during experiential tasks requiring social interactions. The social processing domain may provide a framework to develop mechanistic neurophysiological models of disordered eating symptoms that can integrate differences observed across cognitive control, reward, and learning circuitry.
Funding
Preparation of this work was supported by the National Institute of Mental Health [R01 MH112927 (C.J.M); R21 MH124016 (C.J.M)].
Footnotes
Disclaimer: The views expressed in this article are those of the authors and do not necessarily reflect the official views of the National Institutes of Health.
Conflict of Interest: The authors declare no competing interests.
References
- 1.Linardon J, Brennan L, de la Piedad Garcia X. Rapid response to eating disorder treatment: A systematic review and meta-analysis. The International journal of eating disorders. 2016;49(10):905–19. doi: 10.1002/eat.22595. [DOI] [PubMed] [Google Scholar]
- 2.Glasofer DR, Muratore AF, Attia E, Wu P, Wang Y, Minkoff H, et al. Predictors of illness course and health maintenance following inpatient treatment among patients with anorexia nervosa. Journal of eating disorders. 2020;8(1):69. doi: 10.1186/s40337-020-00348-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Linardon J, Wade TD. How many individuals achieve symptom abstinence following psychological treatments for bulimia nervosa? A meta-analytic review. The International journal of eating disorders. 2018;51(4):287–94. doi: 10.1002/eat.22838. [DOI] [PubMed] [Google Scholar]
- 4.Eddy KT, Tabri N, Thomas JJ, Murray HB, Keshaviah A, Hastings E, et al. Recovery From Anorexia Nervosa and Bulimia Nervosa at 22-Year Follow-Up. The Journal of clinical psychiatry. 2017;78(2):184–9. doi: 10.4088/JCP.15m10393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Volpe U, Tortorella A, Manchia M, Monteleone AM, Albert U, Monteleone P. Eating disorders: What age at onset? Psychiatry research. 2016;238:225–7. doi: 10.1016/j.psychres.2016.02.048. [DOI] [PubMed] [Google Scholar]
- 6.Raykos BC, McEvoy PM, Fursland A. Socializing problems and low self-esteem enhance interpersonal models of eating disorders: Evidence from a clinical sample. The International journal of eating disorders. 2017;50(9):1075–83. doi: 10.1002/eat.22740. [DOI] [PubMed] [Google Scholar]
- 7.Grilo CM, Pagano ME, Stout RL, Markowitz JC, Ansell EB, Pinto A, et al. Stressful life events predict eating disorder relapse following remission: six-year prospective outcomes. The International journal of eating disorders. 2012;45(2):185–92. doi: 10.1002/eat.20909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lie SO, Ro O, Bang L. Is bullying and teasing associated with eating disorders? A systematic review and meta-analysis. The International journal of eating disorders. 2019;52(5):497–514. doi: 10.1002/eat.23035. [DOI] [PubMed] [Google Scholar]
- 9.Becker AE, Burwell RA, Gilman SE, Herzog DB, Hamburg P. Eating behaviours and attitudes following prolonged exposure to television among ethnic Fijian adolescent girls. The British journal of psychiatry : the journal of mental science. 2002;180:509–14. [DOI] [PubMed] [Google Scholar]
- 10.Becker AE. Television, disordered eating, and young women in Fiji: negotiating body image and identity during rapid social change. Culture, medicine and psychiatry. 2004;28(4):533–59. [DOI] [PubMed] [Google Scholar]
- 11.Fairburn CG, Cooper Z, Shafran R. Cognitive behaviour therapy for eating disorders: a “transdiagnostic” theory and treatment. Behaviour research and therapy. 2003;41(5):509–28. doi: S0005796702000888 [pii]. [DOI] [PubMed] [Google Scholar]
- 12.Jones EJ, Egan SJ, Howell JA, Hoiles KJ, Mazzucchelli TG. An examination of the transdiagnostic cognitive-behavioural model of eating disorders in adolescents. Eating behaviors. 2020;39:101445. doi: 10.1016/j.eatbeh.2020.101445. [DOI] [PubMed] [Google Scholar]
- 13.Treasure J, Schmidt U. The cognitive-interpersonal maintenance model of anorexia nervosa revisited: a summary of the evidence for cognitive, socio-emotional and interpersonal predisposing and perpetuating factors. Journal of eating disorders. 2013;1:13. doi: 10.1186/2050-2974-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Treasure J, Willmott D, Ambwani S, Cardi V, Clark Bryan D, Rowlands K, et al. Cognitive Interpersonal Model for Anorexia Nervosa Revisited: The Perpetuating Factors that Contribute to the Development of the Severe and Enduring Illness. J Clin Med. 2020;9(3). doi: 10.3390/jcm9030630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rieger E, Van Buren DJ, Bishop M, Tanofsky-Kraff M, Welch R, Wilfley DE. An eating disorder-specific model of interpersonal psychotherapy (IPT-ED): causal pathways and treatment implications. Clinical psychology review. 2010;30(4):400–10. doi: 10.1016/j.cpr.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Frewen P, Schroeter ML, Riva G, Cipresso P, Fairfield B, Padulo C, et al. Neuroimaging the consciousness of self: Review, and conceptual-methodological framework. Neuroscience and biobehavioral reviews. 2020;112:164–212. doi: 10.1016/j.neubiorev.2020.01.023. [DOI] [PubMed] [Google Scholar]
- 17.Schurz M, Radua J, Tholen MG, Maliske L, Margulies DS, Mars RB, et al. Toward a hierarchical model of social cognition: A neuroimaging meta-analysis and integrative review of empathy and theory of mind. Psychological bulletin. 2021;147(3):293–327. doi: 10.1037/bul0000303. [DOI] [PubMed] [Google Scholar]
- 18.Molapour T, Hagan CC, Silston B, Wu H, Ramstead M, Friston K, et al. Seven computations of the social brain. Social cognitive and affective neuroscience. 2021;16(8):745–60. doi: 10.1093/scan/nsab024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koban L, Gianaros PJ, Kober H, Wager TD. The self in context: brain systems linking mental and physical health. Nat Rev Neurosci. 2021;22(5):309–22. doi: 10.1038/s41583-021-00446-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pfeifer JH, Allen NB. Puberty Initiates Cascading Relationships Between Neurodevelopmental, Social, and Internalizing Processes Across Adolescence. Biological psychiatry. 2021;89(2):99–108. doi: 10.1016/j.biopsych.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tajfel H, Turner JC. An integrative theory of intergroup conflict. . In: Austin WG, Worchel S, editors. The social psychology of intergroup relations Monterey: Brooks/Cole; 1979. p. 33–47. [Google Scholar]
- 22.Gilbert CD, Li W. Top-down influences on visual processing. Nat Rev Neurosci. 2013;14(5):350–63. doi: 10.1038/nrn3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hensel L, Bzdok D, Muller VI, Zilles K, Eickhoff SB. Neural correlates of explicit social judgments on vocal stimuli. Cerebral cortex. 2015;25(5):1152–62. doi: 10.1093/cercor/bht307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hadders-Algra M Human face and gaze perception is highly context specific and involves bottom-up and top-down neural processing. Neuroscience and biobehavioral reviews. 2022;132:304–23. doi: 10.1016/j.neubiorev.2021.11.042. [DOI] [PubMed] [Google Scholar]
- 25.Uddin LQ, Yeo BTT, Spreng RN. Towards a Universal Taxonomy of Macro-scale Functional Human Brain Networks. Brain Topogr. 2019;32(6):926–42. doi: 10.1007/s10548-019-00744-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yeshurun Y, Nguyen M, Hasson U. The default mode network: where the idiosyncratic self meets the shared social world. Nat Rev Neurosci. 2021;22(3):181–92. doi: 10.1038/s41583-020-00420-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uddin LQ. Salience processing and insular cortical function and dysfunction. Nat Rev Neurosci. 2015;16(1):55–61. doi: 10.1038/nrn3857. [DOI] [PubMed] [Google Scholar]
- 28.Castelli F, Frith C, Happe F, Frith U. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain : a journal of neurology. 2002;125(Pt 8):1839–49. [DOI] [PubMed] [Google Scholar]
- 29.McAdams CJ, Krawczyk DC. Impaired neural processing of social attribution in anorexia nervosa. Psychiatry research. 2011;194(1):54–63. doi: 10.1016/j.pscychresns.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 30.McAdams CJ, Krawczyk DC. Neural Responses during Social and Self-Knowledge Tasks in Bulimia Nervosa. Frontiers in psychiatry. 2013;4:103. doi: 10.3389/fpsyt.2013.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schulte-Ruther M, Mainz V, Fink GR, Herpertz-Dahlmann B, Konrad K. Theory of mind and the brain in anorexia nervosa: relation to treatment outcome. Journal of the American Academy of Child and Adolescent Psychiatry. 2012;51(8):832–41 e11. doi: 10.1016/j.jaac.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 32.Ruan VA, Hartz A, Hueck M, Dahmen B, von Polier G, Herpertz-Dahlmann B, et al. Neural mechanisms underlying social recognition and theory of mind in adolescent patients with bulimia nervosa and transdiagnostic comparison with anorexia nervosa. European eating disorders review : the journal of the Eating Disorders Association. 2022. doi: 10.1002/erv.2911. [DOI] [PubMed] [Google Scholar]
- 33.Leslie M, Halls D, Leppanen J, Sedgewick F, Smith K, Hayward H, et al. Neural Correlates of Theory of Mind Are Preserved in Young Women With Anorexia Nervosa. Frontiers in psychology. 2020;11:568073. doi: 10.3389/fpsyg.2020.568073. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Very large study using passive social/non-social stimuli without neural differences in AN.
- 34.Fonville L, Giampietro V, Surguladze S, Williams S, Tchanturia K. Increased BOLD signal in the fusiform gyrus during implicit emotion processing in anorexia nervosa. NeuroImage Clinical. 2014;4:266–73. doi: 10.1016/j.nicl.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lule D, Muller S, Fladung AK, Uttner I, Schulze UME. Neural substrates of anorexia nervosa patient’s deficits to decode emotional information. Eating and weight disorders : EWD. 2021;26(2):723–8. doi: 10.1007/s40519-020-00900-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leppanen J, Cardi V, Paloyelis Y, Simmons A, Tchanturia K, Treasure J. Blunted neural response to implicit negative facial affect in anorexia nervosa. Biological psychology. 2017;128:105–11. doi: 10.1016/j.biopsycho.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 37.Leppanen J, Cardi V, Paloyelis Y, Simmons A, Tchanturia K, Treasure J. FMRI Study of Neural Responses to Implicit Infant Emotion in Anorexia Nervosa. Frontiers in psychology. 2017;8:780. doi: 10.3389/fpsyg.2017.00780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Halls D, Leslie M, Leppanen J, Sedgewick F, Surguladze S, Fonville L, et al. The emotional face of anorexia nervosa: The neural correlates of emotional processing. Human brain mapping. 2021;42(10):3077–87. doi: 10.1002/hbm.25417. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Large study of emotional face stimuli in AN, no whole-brain neural activation differences but some connectivity differences.
- 39.Ashworth F, Pringle A, Norbury R, Harmer CJ, Cowen PJ, Cooper MJ. Neural response to angry and disgusted facial expressions in bulimia nervosa. Psychological medicine. 2011;41(11):2375–84. doi: 10.1017/S0033291711000626. [DOI] [PubMed] [Google Scholar]
- 40.Sweitzer MM, Watson KK, Erwin SR, Winecoff AA, Datta N, Huettel S, et al. Neurobiology of social reward valuation in adults with a history of anorexia nervosa. PloS one. 2018;13(12):e0205085. doi: 10.1371/journal.pone.0205085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seidel M, King JA, Ritschel F, Boehm I, Geisler D, Bernardoni F, et al. Processing and regulation of negative emotions in anorexia nervosa: An fMRI study. NeuroImage Clinical. 2018;18:1–8. doi: 10.1016/j.nicl.2017.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pauligk S, Seidel M, Furtjes S, King JA, Geisler D, Hellerhoff I, et al. The costs of over-control in anorexia nervosa: evidence from fMRI and ecological momentary assessment. Translational psychiatry. 2021;11(1):304. doi: 10.1038/s41398-021-01405-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steward T, Martinez-Zalacain I, Mestre-Bach G, Sanchez I, Riesco N, Jimenez-Murcia S, et al. Dorsolateral prefrontal cortex and amygdala function during cognitive reappraisal predicts weight restoration and emotion regulation impairment in anorexia nervosa. Psychological medicine. 2022;52(5):844–52. doi: 10.1017/S0033291720002457. [DOI] [PubMed] [Google Scholar]
- 44.Seidel M, Pauligk S, Furtjes S, King JA, Schlief SM, Geisler D, et al. Intact neural and behavioral correlates of emotion processing and regulation in weight-recovered anorexia nervosa: a combined fMRI and EMA study. Translational psychiatry. 2022;12(1):32. doi: 10.1038/s41398-022-01797-1. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Large study of emotional regulation in AN, with no whole-brain neural differences.
- 45.D’Agata F, Caroppo P, Spalatro A, Lavagnino L, Abbate Daga G, Boghi A, et al. Emotional imagination of negative situations: Functional neuroimaging in anorexia and bulimia. PloS one. 2021;16(4):e0231684. doi: 10.1371/journal.pone.0231684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spangler DL, Allen MD. An fMRI investigation of emotional processing of body shape in bulimia nervosa. The International journal of eating disorders. 2012;45(1):17–25. doi: 10.1002/eat.20899. [DOI] [PubMed] [Google Scholar]
- 47.Kodama N, Moriguchi Y, Takeda A, Maeda M, Ando T, Kikuchi H, et al. Neural correlates of body comparison and weight estimation in weight-recovered anorexia nervosa: a functional magnetic resonance imaging study. BioPsychoSocial medicine. 2018;12:15. doi: 10.1186/s13030-018-0134-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McAdams CJ, Krawczyk DC. Who am I? How do I look? Neural differences in self-identity in anorexia nervosa. Social cognitive and affective neuroscience. 2014;9(1):12–21. doi: 10.1093/scan/nss093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suda M, Brooks SJ, Giampietro V, Friederich HC, Uher R, Brammer MJ, et al. Functional neuroanatomy of body checking in people with anorexia nervosa. The International journal of eating disorders. 2013;46(7):653–62. doi: 10.1002/eat.22150. [DOI] [PubMed] [Google Scholar]
- 50.McAdams CJ, Jeon-Slaughter H, Evans S, Lohrenz T, Montague PR, Krawczyk DC. Neural differences in self-perception during illness and after weight-recovery in anorexia nervosa. Social cognitive and affective neuroscience. 2016;11(11):1823–31. doi: 10.1093/scan/nsw092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van den Eynde F, Giampietro V, Simmons A, Uher R, Andrew CM, Harvey PO, et al. Brain responses to body image stimuli but not food are altered in women with bulimia nervosa. BMC psychiatry. 2013;13:302. doi: 10.1186/1471-244X-13-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Press SA, Biehl SC, Vatheuer CC, Domes G, Svaldi J. Neural correlates of body image processing in binge eating disorder. J Psychopathol Clin Sci. 2022;131(4):350–64. doi: 10.1037/abn0000750. [DOI] [PubMed] [Google Scholar]
- 53.Miyake Y, Okamoto Y, Onoda K, Kurosaki M, Shirao N, Yamawaki S. Brain activation during the perception of distorted body images in eating disorders. Psychiatry research. 2010;181(3):183–92. doi: S0925-4927(09)00198-X [pii] 10.1016/j.pscychresns.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 54.Mohr HM, Zimmermann J, Roder C, Lenz C, Overbeck G, Grabhorn R. Separating two components of body image in anorexia nervosa using fMRI. Psychological medicine. 2010;40(9):1519–29. doi: 10.1017/S0033291709991826. [DOI] [PubMed] [Google Scholar]
- 55.Denny BT, Kober H, Wager TD, Ochsner KN. A meta-analysis of functional neuroimaging studies of self- and other judgments reveals a spatial gradient for mentalizing in medial prefrontal cortex. Journal of cognitive neuroscience. 2012;24(8):1742–52. doi: 10.1162/jocn_a_00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang J, Dedovic K, Guan L, Chen Y, Qi M. Self-esteem modulates dorsal medial prefrontal cortical response to self-positivity bias in implicit self-relevant processing. Social cognitive and affective neuroscience. 2014;9(11):1814–8. doi: 10.1093/scan/nst181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Delahoy R, Davey CG, Jamieson AJ, Finlayson-Short L, Savage HS, Steward T, et al. Modulation of the brain’s core-self network by self-appraisal processes. NeuroImage. 2022;251:118980. doi: 10.1016/j.neuroimage.2022.118980. [DOI] [PubMed] [Google Scholar]
- 58.Pringle A, Ashworth F, Harmer CJ, Norbury R, Cooper MJ. Neural correlates of the processing of self-referent emotional information in bulimia nervosa. Neuropsychologia. 2011;49(12):3272–8. doi: 10.1016/j.neuropsychologia.2011.07.032. [DOI] [PubMed] [Google Scholar]
- 59.Xu J, Harper JA, Van Enkevort EA, Latimer K, Kelley U, McAdams CJ. Neural activations are related to body-shape, anxiety, and outcomes in adolescent anorexia nervosa. Journal of psychiatric research. 2017;87:1–7. doi: 10.1016/j.jpsychires.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mendoza CJ, Palka JM, Pelfrey SE, Hunt BJ, Krawczyk DC, McAdams CJ. Neural processes related to negative self-concept in adult and adolescent anorexia nervosa. European eating disorders review : the journal of the Eating Disorders Association. 2022;30(1):23–35. doi: 10.1002/erv.2867. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Analytic design that allows social perspective and positive/negative stimulus valence to be separated, identifying differences related to processing positive social stimuli in different contexts, and includes both adolescent and adults with and recovered from AN.
- 61.Via E, Soriano-Mas C, Sanchez I, Forcano L, Harrison BJ, Davey CG, et al. Abnormal Social Reward Responses in Anorexia Nervosa: An fMRI Study. PloS one. 2015;10(7):e0133539. doi: 10.1371/journal.pone.0133539. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Novel task with social judgment from image, with altered utilization of many subcortical and cortical regions in response to positive and negative feedback in AN.
- 62.McAdams CJ, Lohrenz T, Montague PR. Neural responses to kindness and malevolence differ in illness and recovery in women with anorexia nervosa. Human brain mapping. 2015;36(12):5207–19. doi: 10.1002/hbm.23005. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Neurocomputational experiential task with self-report showing that social expectations (positive personalizing bias) strongly correlated with neural responses in default mode region, and diminished processing of positive social interactions in both ill and recovered AN.
- 63.Luo Y, Mendoza C, Pelfrey S, Lohrenz T, Gu X, Montague PR, et al. Elevated Neurobehavioral Responses to Negative Social Interactions in Women With Bulimia Nervosa. Biol Psychiatry Cogn Neurosci Neuroimaging. 2022. doi: 10.1016/j.bpsc.2021.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Same neurocomputational experiential task but in BN, showing both cortical and subcortical hyperresponsivity to negative social interactions.
- 64.Khalsa SS, Berner LA, Anderson LM. Gastrointestinal Interoception in Eating Disorders: Charting a New Path. Current psychiatry reports. 2022;24(1):47–60. doi: 10.1007/s11920-022-01318-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McClelland J, Dalton B, Kekic M, Bartholdy S, Campbell IC, Schmidt U. A systematic review of temporal discounting in eating disorders and obesity: Behavioural and neuroimaging findings. Neuroscience and biobehavioral reviews. 2016;71:506–28. doi: 10.1016/j.neubiorev.2016.09.024. [DOI] [PubMed] [Google Scholar]
- 66.Berkman ET, Livingston JL, Kahn LE. The identity-value model of self-regulation: Integration, extension, and open questions. Psychol Inq. 2017;28(2–3):157–64. doi: 10.1080/1047840X.2017.1343069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Berkman ET, Livingston JL, Kahn LE. Finding the “self” in self-regulation: The identity-value model. Psychol Inq. 2017;28(2–3):77–98. doi: 10.1080/1047840X.2017.1323463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pfeifer JH, Berkman ET. The Development of Self and Identity in Adolescence: Neural Evidence and Implications for a Value-Based Choice Perspective on Motivated Behavior. Child Dev Perspect. 2018;12(3):158–64. doi: 10.1111/cdep.12279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hagan WS, Mericle S, Hunt BJ, Harper JA, Palka JM, Pelfrey S, et al. Qualitative patient experiences from the Self-Blame and Perspective-Taking Intervention for eating disorders. Journal of eating disorders. 2021;9(1):127. doi: 10.1186/s40337-021-00483-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hunt BJ, Smith Hagan W, Pelfrey S, Mericle S, Harper JA, Palka JM, et al. Pilot data from the Self-Blame and Perspective-Taking Intervention for eating disorders. J Behav Cogn Ther. 2021;31(1):57–66. doi: 10.1016/j.jbct.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Young KD, Zotev V, Phillips R, Misaki M, Drevets WC, Bodurka J. Amygdala real-time functional magnetic resonance imaging neurofeedback for major depressive disorder: A review. Psychiatry and clinical neurosciences. 2018;72(7):466–81. doi: 10.1111/pcn.12665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Quevedo K, Yuan Teoh J, Engstrom M, Wedan R, Santana-Gonzalez C, Zewde B, et al. Amygdala Circuitry During Neurofeedback Training and Symptoms’ Change in Adolescents With Varying Depression. Frontiers in behavioral neuroscience. 2020;14:110. doi: 10.3389/fnbeh.2020.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Quevedo K, Liu G, Teoh JY, Ghosh S, Zeffiro T, Ahrweiler N, et al. Neurofeedback and neuroplasticity of visual self-processing in depressed and healthy adolescents: A preliminary study. Dev Cogn Neurosci. 2019;40:100707. doi: 10.1016/j.dcn.2019.100707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Barry TJ, Chiu CPY, Raes F, Ricarte J, Lau H. The Neurobiology of Reduced Autobiographical Memory Specificity. Trends in cognitive sciences. 2018;22(11):1038–49. doi: 10.1016/j.tics.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 75.Gabay AS, Radua J, Kempton MJ, Mehta MA. The Ultimatum Game and the brain: a meta-analysis of neuroimaging studies. Neuroscience and biobehavioral reviews. 2014;47:549–58. doi: 10.1016/j.neubiorev.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 76.Mwilambwe-Tshilobo L, Spreng RN. Social exclusion reliably engages the default network: A meta-analysis of Cyberball. NeuroImage. 2021;227:117666. doi: 10.1016/j.neuroimage.2020.117666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brodrick BB, Adler-Neal AL, Palka JM, Mishra V, Aslan S, McAdams CJ. Structural brain differences in recovering and weight-recovered adult outpatient women with anorexia nervosa. Journal of eating disorders. 2021;9(1):108. doi: 10.1186/s40337-021-00466-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Walton E, Bernardoni F, Batury V-L, Bahnsen K, Larivière S, Abbate-Daga G, et al. Brain Structure in Acutely Underweight and Partially Weight-Restored Individuals with Anorexia Nervosa - A Coordinated Analysis by the ENIGMA Eating Disorders Working Group. Biological psychiatry. 2022. doi: 10.1016/j.biopsych.2022.04.022. [DOI] [PubMed] [Google Scholar]
- 79.Vocks S, Busch M, Gronemeyer D, Schulte D, Herpertz S, Suchan B. Neural correlates of viewing photographs of one’s own body and another woman’s body in anorexia and bulimia nervosa: an fMRI study. Journal of psychiatry & neuroscience :JPN. 2010;35(3):163–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McAdams CJ, Harper JA, Van Enkevort E. Mentalization and the left inferior frontal gyrus and insula. European eating disorders review : the journal of the Eating Disorders Association. 2018;26(3):265–71. doi: 10.1002/erv.2580. [DOI] [PMC free article] [PubMed] [Google Scholar]


