Abstract
Background
Atherosclerosis and consequent risk of cardiovascular events or mortality can be accurately assessed by quantifying coronary artery calcium score (CACS) derived from computed tomography. HMG-CoA-reductase inhibitors (statins) are the primary pharmacotherapy used to reduce cardiovascular events, yet there is growing data that support statin use may increase coronary calcification. We set out to determine the likelihood of severe CACS in the context of chronic statin therapy.
Methods
We established a retrospective, case-control study of 1,181 U.S. veterans without coronary artery disease (CAD) from a single site, the Providence VA Medical Center. Duration of statin therapy for primary prevention was divided into 5-year categorical increments. The primary outcome was CACS derived from low-dose lung cancer screening computed tomography (LCSCT), stratified by CACs severity (none = 0; mild = 1–99; moderate = 100–399; and severe ≥400 AU). Statin duration of zero served as the referent control. Ordinal logistic regression analysis determined the association between duration of statin use and CACS categories. Proportional odds assumption was tested using likelihood ratio test. Atherosclerotic cardiovascular disease (ASCVD) risk score, body mass index, and CKD (glomerular filtration rate of <60 ml/min/1.73 m2) were included in the adjustment models.
Results
The mean age of the study population was 64.7±7.2 years, and 706 (60%) patients were prescribed a statin at baseline. Duration of statin therapy was associated with greater odds of having increased CACS (>0–5 years, OR: 1.71 [CI: 1.34–2.18], p<0.001; >5–10 years, OR: 2.80 [CI: 2.01–3.90], p<0.001; >10 years, OR: 5.30 [CI: 3.23–8.70], p<0.001), and the relationship between statin duration and CACS remained significant after multivariate adjustment (>0–5 years, OR: 1.49 [CI: 1.16–1.92], p = 0.002; >5–10 years, OR: 2.38 [CI: 1.7–3.35], p<0.001; >10 years, OR: 4.48 [CI: 2.7–7.43], p<0.001).
Conclusions
Long-term use of statins is associated with increased likelihood of severe CACS in patients with significant smoking history. The use of CACS to interpret cardiovascular event risk may require adjustment in the context of chronic statin therapy.
Introduction
Coronary artery disease (CAD) due to calcific atherosclerosis is a major cause of morbidity and mortality worldwide [1]. Measures of increased atherosclerotic plaque calcification have been predictive of coronary artery disease burden, cardiovascular events, and all-cause mortality [2–3]. Moreover, an increasing rate of progression of the calcification in the coronary vasculature has been associated with worsening prognosis and increased adverse events [5–7]. More recently, there has been evidence supporting the association between increased density of calcification in atherosclerotic plaques and more stable disease [8–13]. Pharmacotherapy with 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors (statins) has been successful at reducing the risk of cardiovascular events such that statins have become the current standard of care in patients at moderate to elevated cardiovascular risk [14–22]. Statins were initially developed as cholesterol-lowering agents, but multiple studies support that statin therapy reduced the risk of myocardial infarction out of proportion to the lipid-lowering effect [18–21]. These pleiotropic effects may be mediated by other biological pathways, including improved endothelial function, reduced oxidative stress, reduced platelet adhesion and thrombogenicity, and alterations in inflammation [23,24].
While statins impact atherosclerotic plaque lipid burden, the exact effect of statin therapy on coronary artery calcification has been less clear [12,25–28]. Some initial reports suggested that statin use either reduced calcification or slowed the progression of atherosclerotic calcification [25,27,29], while others indicated statin use resulted in little to no change in the progression of calcification [26,30]. More recently, a growing number of studies have demonstrated the impact of statin use to be associated with increasing measures of coronary artery calcification [28,31–33]. For example, while coronary lipid atheroma indices have been shown to decline in individuals taking high-intensity statin, this effect was accompanied by a paradoxical increase in coronary calcium indices [28,31]. Additionally, while patients with diabetes mellitus demonstrated increased atherosclerotic calcification, statin use by patients with diabetes mellitus was associated with even more progressive coronary atheroma calcification [34–36].
In experimental atherosclerosis models, we recently identified a potential mechanism of statin-induced atherosclerotic plaque calcification through inhibition of Rac1 protein isoprenylation and consequent dysregulation of the small GTPase, Rac1 [32]. Here we set out to assess the association between statin use and progressive calcification in humans, by studying the total duration of time on statin therapy and its relationship to the consequent degree of coronary artery calcification in a U.S. veteran population with extensive smoking history but without established cardiovascular disease. We took advantage of low dose lung cancer screening CT (LCSCT) for the clinical application of acquiring coronary calcification data without need for additional radiation exposure. Understanding the impact of statin therapy on atherosclerotic calcification may have important implications in our interpretation of cardiovascular event risk associated with elevated CACS derived from CT.
Materials and methods
The Providence Veterans Affairs Medical Center (PVAMC) Institutional Review Board (IRB) approved this study and granted a Health Insurance Portability and Accountability Act (HIPAA) authorization waiver and waiver of consent. This study complies with the Declaration of Helsinki, and all patient data were managed in full compliance of the HIPAA regulations. This study was a retrospective analysis of medical records, and all patient data were fully anonymized for analysis. For the purpose of reproducing the results or replicating the study, the anonymized minimal dataset can be accessed as a (S1 File).
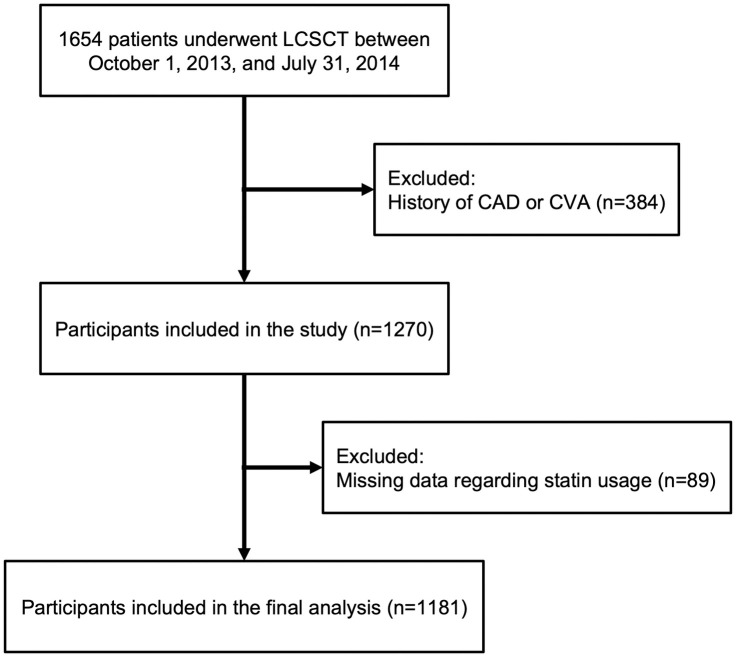
This was a single center, case-control study comprised of 1654 U.S. veterans who underwent U.S. Preventive Services Task Force guideline-recommended (i.e. 55 to 80 years of age, 30-pack-year tobacco smoking history, active or quit < 15 years previously) lung cancer screening CT between October 1, 2013, and July 31, 2014 [37,38]. A total of 384 patients were excluded for prior history of CAD or cerebrovascular accident (CVA). A total of 89 patients were excluded because there were no data regarding statin usage in the U.S. Department of Veterans Affairs (VA) electronic medical record (EMR).
CT examinations did not use electrocardiography gating and were carried out on a 128-slice CT scanner (Siemens Healthcare, Erlangen, Germany), using 120 kV tube voltage and 40 mA tube current, 128×0.6 mm collimation, 0.84 pitch, 0.5-s rotation, 380-mm field of view, 512×512-pixel matrix, and 1.00–1.25 mm image reconstruction thickness. The minimum area required to identify calcification was 0.55 mm2. The Agatston method was employed for quantifying coronary artery calcium score (CACS) via a semi-automated imaging workstation with readers blinded to patient data [39]. The kappa for inter-observer agreement of CACS was 0.92 (0.88–0.96).
The VA EMR was searched for detailed patient demographics and medical history. Information collected on cardiovascular risk included age, body mass index (BMI), diabetes mellitus (DM), hypertension (patients with prior diagnosis and/or use of medications to control blood pressure), hyperlipidemia and/or lipid-lowering medication use, self-reported cigarette smoking status (current or former), family history of premature CAD, and chronic kidney disease (CKD). DM was defined as prior diagnosis of DM or treatment with glucose-lowering medication. BMI was defined as weight in kilograms divided by height in meters squared. CKD was defined as estimated glomerular filtration rate (eGFR) of <60 ml/min/1.73m2 using the Modification of Diet in Renal Disease (MDRD) equation [40]. From the acquired data, atherosclerotic cardiovascular disease (ASCVD) risk scores were calculated [41,42]. Imputations were performed using a mean-value imputation approach for BMI and ASCVD risk score values that were missing at baseline [43].
CAD was defined as having ≥1 of the following: history of myocardial infarction (MI); history of coronary revascularization with either coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI); or abnormalities on cardiac testing (exercise treadmill testing, echocardiography, myocardial perfusion imaging, cardiac computed tomography, or coronary angiography). Cerebrovascular accident (CVA) was defined as a hospital or neurologist report with the diagnosis of either an ischemic stroke or transient ischemic attack (TIA). Each of the included variables in this analysis, except for age, sex, and BMI, were categorized during data processing and management. Race/ethnicity was categorized as Caucasian or non-Caucasian persons.
Through the EMR, data were collected on any statin use before the baseline LCSCT CACS measurements. The duration of time on statin therapy was calculated for each patient using the pharmacy records, which included the total number of days statin was dispensed by the pharmacy to the patient in the time before the baseline LCSCT measurements. The duration of time on statin therapy was expressed in years and modelled as both a continuous and a categorical (0 years, >0–5 years, >5–10 years, and >10 years) variable.
Our primary outcome was CACS. Because CACS did not follow a normal distribution in our study population, this outcome was stratified using previously established categories defined by several large observational cohort studies and current expert consensus statements on CAC scoring (none, 0 AU [reference control]; mild, 1–99 AU; moderate, 100–399 AU; and moderate to severe, ≥400 AU) [44,45].
Stata/SE software package version 17.0 (SataCorp LLC, College Station, Texas) was used to carry out data management and statistical analyses. Results for continuous variables with normal distribution were presented as mean ± standard deviation (SD). Continuous variables without normal distribution were presented as median (interquartile interval). Results involving categorical data were presented as an absolute number (percentage). The t test was used to compare two independent groups that demonstrated normal distribution. If groups did not demonstrate normal distribution, the Mann-Whitney U test was used for comparison between two groups. The Chi-square test was used for comparison of categorical variables. To assess the association between duration of statin use and different CACS categories, ordinal logistic regression models were employed to model the ordinal multi-category CACS outcome (whereby a 2-tailed p-value < 0.05 was considered statistically significant), and all computed confidence intervals (CI) were set at 95% level. The proportional odds assumption was tested using likelihood ratio test and the assumption was not violated (p = 0.8322). In our final model, we adjusted for ASCVD risk score, BMI, and CKD.
Results
We evaluated a total of 1,181 individuals who underwent clinically indicated LCSCT for significant smoking history at PVAMC between October 1, 2013, and July 31, 2014, and met the inclusion/exclusion criteria (Fig 1, Table 1). The mean age of this population was 64.7 (SD: 7.2) years. Indicative of the northeast U.S. veteran population, the vast majority of these patients were Caucasian (94%) and male (95.5%). The mean BMI of the population was 28.9 (SD: 6.1) kg/m2. Twenty-five percent of the patients had DM, 56.5% had hypertension, 69% had dyslipidemia, and 12% had an eGFR of <60 ml/min/1.73m2. Additionally, 42.5%, 40.9%, and 16.6% of patients were classified as having high, intermediate, and low ASCVD risk score, respectively. Sixty percent of patients had a duration of statin use >0. The overall median CACS was 376.6 AU (IQI: 72.9, 1096.8).
Fig 1. Flow chart of the study population.
Table 1. Baseline characteristics among 1,181 veterans by statin use at baseline.
| Total Population (n = 1181) |
No Statin (n = 475) |
Statin >0–5 years (n = 425) |
Statin >5–10 years (n = 188) |
Statin >10 years (n = 93) |
p-value | |
|---|---|---|---|---|---|---|
| Age (years) | 64.7 ± 7.2 | 63.2 ± 7.7 | 64.7 ± 6.3 | 67.2 ± 7.3 | 67.6 ± 6.4 | <0.001 |
| Men | 1128 (95.5) | 450 (94.7) | 411 (96.7) | 180 (95.7) | 87 (93.5) | 0.40 |
| Caucasian | 1107 (94) | 442 (93.2) | 396 (93.6) | 178 (94.7) | 91 (97.8) | 0.37 |
| BMI, kg/m 2 | 28.9 (6.1) | 27.7 (6.3) | 29.7 (6.0) | 29.7 (5.7) | 29.8 (5.5) | <0.001 |
| DM | 291 (24.7) | 50 (10.5) | 129 (30.4) | 76 (40.4) | 36 (38.7) | <0.001 |
| Hypertension | 667 (56.5) | 200 (42.1) | 268 (63.1) | 129 (68.6) | 70 (75.3) | <0.001 |
| Hyperlipidemia | 814 (68.9) | 164 (34.5) | 387 (91.1) | 175 (93.1) | 88 (94.6) | <0.001 |
| Current smoker | 648 (55) | 287 (60.5) | 226 (53.3) | 92 (48.9) | 43 (46.2) | 0.007 |
| Family History of early CAD | 141 (11.9) | 53 (11.2) | 49 (11.5) | 23 (12.2) | 16 (17.2) | 0.42 |
| CKD | 139 (11.8) | 37 (7.8) | 59 (13.9) | 25 (13.3) | 18 (19.4) | 0.002 |
| Atherosclerotic Cardiovascular Disease (ASCVD) risk score | <0.001 | |||||
| High | 324 (42.5) | 93 (32.3) | 140 (46.5) | 57 (48.3) | 34 (60.7) | |
| Intermediate | 312 (40.9) | 118 (41.0) | 127 (42.2) | 47 (39.8) | 20 (35.7) | |
| Low | 127 (16.6) | 77 (26.7) | 34 (11.3) | 14 (11.9) | 2 (3.6) | |
Values are mean ± SD or n (%). BMI = Body mass index; DM = Diabetes Mellitus; CAD = Coronary artery disease; CKD = Chronic kidney disease.
Review of the population stratified by duration of time on statin therapy revealed that patients on a statin for longer periods of time demonstrated a higher prevalence of cardiovascular risk factors (Table 1). Increased duration of statin therapy was associated with slightly older age, increased BMI, and a higher prevalence of DM, hypertension, and hyperlipidemia. Patients with increased duration of statin therapy also had a higher prevalence of CKD and higher ASCVD risk scores. Increased duration of time on statin therapy, divided into 5-year increments, was also associated with increased CACS categories in the unadjusted analyses (Table 2). Further, 2-year increments in duration of statin therapy demonstrated incremental increases in the median CACS of approximately 100–120 Agatston units for every additional 2 years of statin therapy relative to no statin therapy (S1 Fig). Though the majority of participants were Caucasian males, when the sample was stratified by sex and race, there appeared to be no differences in statin use between men and women or white and non-white participants at baseline.
Table 2. CACS among 1,181 veterans at baseline.
| Total Population (n = 1181) |
No Statin (n = 475) |
Statin >0–5 years (n = 425) |
Statin >5–10 years (n = 188) |
Statin >10 years (n = 93) |
p-value | |
|---|---|---|---|---|---|---|
| Total CACS | 377 (73, 1097) |
225 (32, 728) |
410 (81, 1114) |
575 (161, 1383) |
972 (400, 1693) |
<0.001 |
| CACS Categories | <0.001 | |||||
| 0 | 121 (10.25) | 71 (14.9) | 36 (8.5) | 12 (6.4) | 2 (2.2) | |
| 1–99 | 229 (19.39) | 112 (23.6) | 87 (20.5) | 22 (11.7) | 8 (8.6) | |
| 100–399 | 259 (21.93) | 120 (25.3) | 88 (20.7) | 38 (20.2) | 13 (14.0) | |
| ≥400 | 572 (48.43) | 172 (36.2) | 214 (50.4) | 116 (61.7) | 70 (75.3) | |
Values are median (interquartile interval) or n (%). CACS = Coronary artery calcium score.
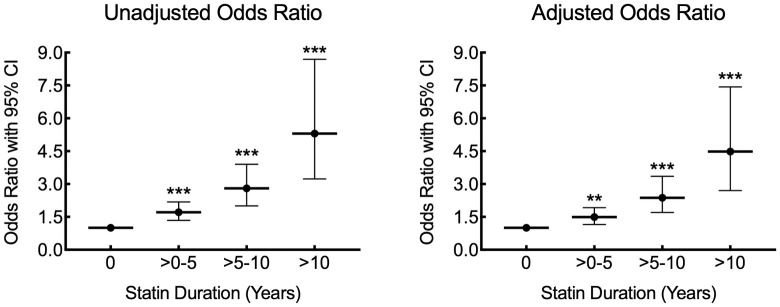
Using duration of statin therapy as a continuous variable in the ordinal logistic regression analysis, increasing duration of statin use (in years) was associated with increasing CACS categories (OR: 1.15 [CI: 1.11–1.18], p<0.001), and this association remained significant after the multivariate adjustment (OR = 1.13; 95% CI = 1.09–1.17; p<0.001). There was also a significant association between increasing duration of statin therapy as a categorical variable and increasing CACS category by ordinal logistic regression with no evidence of a threshold effect (>0–5 years, OR: 1.71 [CI: 1.34–2.18], p<0.001; >5–10 years, OR: 2.80 [CI: 2.01–3.90], p<0.001; >10 years, OR: 5.30 [CI: 3.23–8.70], p<0.001) (Fig 2, Table 3). After adjustment for ASCVD risk score, BMI, and CKD, the association between increasing duration of statin therapy and increasing CACS category remained significant (>0–5 years, OR: 1.49 [CI: 1.16–1.92], p = 0.002; >5–10 years, OR: 2.38 [CI: 1.7–3.35], p<0.001; >10 years, OR: 4.48 [CI: 2.7–7.43], p<0.001).
Fig 2. Association between duration of statin therapy and CACS.
Graphs of unadjusted and adjusted odds ratios relative to no statin therapy (0). Adjustment included ASCVD risk score, BMI, and CKD (**, P = 0.002; ***, P<0.001 relative to control timepoint 0).
Table 3. Relationship between duration of statin therapy as a categorical variable and increasing CACS category.
| Unadjusted | Adjusted | |||||
|---|---|---|---|---|---|---|
| OR | (95% CI) | p-value | OR | (95% CI) | p-value | |
| 0 years | Ref. | — | — | Ref. | — | — |
| >0 to 5 years | 1.71 | 1.34–2.18 | <0.001 | 1.49 | 1.16–1.92 | 0.002 |
| >5 to 10 years | 2.80 | 2.01–3.90 | <0.001 | 2.38 | 1.7–3.35 | <0.001 |
| >10 years | 5.30 | 3.23–8.70 | <0.001 | 4.48 | 2.7–7.43 | <0.001 |
| ASCVD | 1.06 | 1.05–1.07 | <0.001 | 1.05 | 1.04–1.06 | <0.001 |
| BMI | 0.99 | 0.97–1.00 | 0.115 | 0.97 | 0.96–0.99 | 0.003 |
| CKD | 1.43 | 1.02–2.02 | 0.038 | 0.99 | 0.69–1.42 | 0.96 |
ASCVD = Atherosclerotic cardiovascular disease risk score; BMI = body mass index; CKD = chronic kidney disease.
Discussion
Coronary artery calcification is a marker of the overall burden of atherosclerosis and is associated with worsening cardiovascular events and all-cause mortality [2–4]. However, recent studies indicate that the ultrastructural composition of calcium within plaque may confer features of reduced risk and consequent plaque stability in some instances [8–13]. Statins have been tremendously successful at reducing the risk of cardiovascular events in patients, and this risk reduction has been demonstrated to be out of proportion to the lipid-lowering effect, suggesting alternative mechanisms may help to stabilize coronary plaque [14–22]. While there continues to be some disagreement over whether statins can influence the calcium composition of atherosclerotic plaque, growing clinical data support that statins may increase atherosclerotic calcification [25–33]. Here we find a significant association between duration of statin therapy and total CACS. Specifically, long-term duration of statin therapy was significantly associated with a greater risk of having severe CACS. The increasing odds of having severe CACS being associated with higher duration of time on statin therapy remained significant after adjustment for the baseline cardiovascular risk factors known to significantly impact the CACS (ASCVD risk score, BMI, and CKD).
As mentioned, the effect of statin therapy on CAC has remained controversial to date with some studies showing a decrease or no change in calcification [25–27,29,30]. However, there were several concerns with these studies, including open label design, lack of simultaneous comparison between treatment and control groups, small sample size, and short follow-up duration (<1–2 years) [25–27,29,30]. Our findings appear consistent with a number of preclinical and clinical studies supporting the pro-calcific effects of statins [28,31,32]. Recently, a substudy of the prospective PARADIGM registry revealed baseline statin therapy to be associated with higher total CACS at baseline compared to those that were not prescribed statins and that calcified plaques were more likely to increase in the statin treated group [28]. In the cohort of patients already taking statins, CACS was also correlated with calcified plaque volume and density. However, the statin use was treated as a binary in the analysis and duration of time on statin therapy was not taken into account which could have been an important confounder in the analyses. A recent analysis of two clinical trials indicated that 4–6 years of high-intensity statin therapy was associated with progressive CACS [33]. Our data further strengthens the conclusion of a relationship between statin and CACS in about 1200 patients with extensive smoking history and higher cardiovascular risk, incorporating a longer total duration of time (5–10 and >10 years) on statin therapy prior to the CT. Moreover, we studied a higher cardiovascular risk population, taking advantage of low-dose lung cancer screening CT and the application of non-gated image analysis for calcium scoring.
Statin therapy is well-established as the current standard of care for lipid management and the prevention of cardiovascular events [15–17,22,46]. In fact, statins are so effective at reducing events, current guidelines for cholesterol management have taken an aggressive stance on prescribing statins [22]. However, these same guidelines recommend consideration of CACS to refine risk assessment when statin therapy is problematic or there is a question regarding continuation vs. intensification of statin therapy. While CACS independently predicts ASCVD events in a graded fashion, current risk analyses utilizing CACS do not take into account statin use or duration of time on statin therapy. Our study raises questions as to the accuracy of the CACS value for risk assessment in the context of long-term statin use, and our data suggest that modeling of incremental risk associated with CACS may require some degree of adjustment for those patients who are on long-term statin therapy. Though a number of studies suggested that CACS retains predictive value for events [47–49], it is important to note that these studies were carried out in younger populations of patients with much lower CACS, requiring that CACS analysis be treated as binary. Moreover, lipid lowering was either unspecified or also treated as a binary, such that the exact impact of duration of statin therapy was not assessed. More recently, data from the CAC Consortium observational registry suggested that the prognostic significance of CACS may in fact be slightly reduced in statin users, particularly those with higher CACS values, raising important questions about the impact of statin therapy on the components of the Agatston scoring system [50]. Prospective analyses are needed to better quantify this impact of duration statin therapy on the relationship between CACS and outcomes.
We have previously demonstrated a potential mechanistic pathway of statin-induced, Rac1-dependent atherosclerotic vascular calcification [32]. Briefly, we found that statin treatment prevents isoprenylation of Rac1 protein disrupting the complex between Rac1 and its inhibitor, RhoGDI, and leaves Rac1 free to become activated by exchange for guanosine-5’-triphosphate (GTP). In its active form, Rac1 acts by promoting expression of proinflammatory cytokines including IL-1β leading to vascular calcification [32,51–53]. The CANTOS (Canakinumab Anti-Inflammatory Thrombosis Outcome Study) trial demonstrated that inhibition of the potent inflammatory cytokine IL-1β, led to a reduction in secondary cardiovascular events [54]. The overall benefit to all-cause and cardiovascular mortality appeared to be primarily in patients who demonstrated reductions in the systemic inflammation marker high-sensitivity C-reactive protein [55]. The vast majority (93.4%) of patients enrolled in the CANTOS trial were on lipid-lowering therapy, consisting of primarily statins, and thus the trial design is one in which systemic IL-1β inhibition was added to statin therapy. Of note, there have been no data to date regarding CACS or evolution of calcification imaging from CANTOS. It is interesting to speculate whether calcification imaging would be influenced by IL-1β inhibition given the findings in preclinical models.
Limitations to our study include those inherent to the study design as single site, retrospective analysis of primarily Caucasian, male U.S. veterans. Therefore, results are subject to a degree of selection bias and are less generalizable to women and other racial/ethnic groups. Further, our cohort was restricted to veterans who underwent lung cancer screening as recommended by U.S. Preventive Services Task Force guidelines for extensive smoking histories. Key risk factors such as dyslipidemia, hypertension, and diabetes mellitus were captured as binaries in our analyses, and we could not adjust for the degree of control of these conditions in individual patients. We also could not adjust for the original reasons for prescribing statins, and we did not have access to whether low-density lipoprotein cholesterol met target goals with therapy. Moreover, our study makes use of extracted data from the VA Pharmacy’s electronic medical record, and there is a possibility that statin usage outside the Department of Veterans Affairs was not fully captured. Dispensed medication may not fully account for compliance with regards to taking the medication. Finally, the relationship between statin duration, CACS, and cardiovascular events in this cohort was not studied here. Despite these limitations, our findings are consistent with other studies supporting the pro-calcific effect of statin therapy, indicating further study in this area is needed [28,31–33]. These limitations should be addressed in future studies focusing on diverse patient populations, and the future research designs should be expanded to include other factors that can refine the link between statin use, high CACS, and predictive value for outcomes.
Conclusion
In conclusion, long-term duration of time on statin therapy is associated with likelihood of having severe CACS in patients who have sufficient smoking history to qualify for lung cancer screening. These findings highlight an important complexity to the relationship between statin therapy and CACS, indicating that risk from CACS should be interpreted not just in the context of traditional cardiovascular risk factors and serial CACS progression, but also in the context of plaque-altering treatment. The use of CACS to interpret cardiovascular event risk may require adjustment in the context of chronic statin therapy.
Supporting information
Data were plotted as median CACS with interquartile range. (N) indicates the number of patients in each additional 2-year increment in duration of statin therapy. *, P = 0.01; **, P<0.005; ****; P<0.0001, compared to duration of statin “0” using Kuskal-Wallis followed by Dunn’s multiple comparison testing.
(TIF)
(XLS)
Acknowledgments
The authors would like to acknowledge Celia Butler for her outstanding editorial contributions to the manuscript during the revision process.
Data Availability
The data can be found in the fully anonymized minimal data set provided as a supplemental file.
Funding Statement
Research reported in this publication was supported by National Heart, Lung, and Blood Institute of the National Institutes of Health under award numbers R01HL139795 (A.R.M.), R01HL163005 (A.R.M), and R01HL148727 (G.C.). This work was also supported by National Institute of General Medical Sciences of the National Institutes of Health under Institutional Development Award number P20GM103652 (G.C. & A.R.M.). This work was also supported by VA VHA BLR&D IK2BX002527(A.R.M.), VA VHA CSR&D 1I01CX002231 (A.R.M), and VA VHA CSR&D 1I01CX001892 (G.C.). The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the US government. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, et al. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation. 2021;143(8):e254–e743. Epub 2021/01/28. doi: 10.1161/CIR.0000000000000950 . [DOI] [PubMed] [Google Scholar]
- 2.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. The New England journal of medicine. 2008;358(13):1336–45. Epub 2008/03/28. doi: 10.1056/NEJMoa072100 . [DOI] [PubMed] [Google Scholar]
- 3.Polonsky TS, McClelland RL, Jorgensen NW, Bild DE, Burke GL, Guerci AD, et al. Coronary artery calcium score and risk classification for coronary heart disease prediction. JAMA: the journal of the American Medical Association. 2010;303(16):1610–6. doi: 10.1001/jama.2010.461 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raggi P, Gongora MC, Gopal A, Callister TQ, Budoff M, Shaw LJ. Coronary artery calcium to predict all-cause mortality in elderly men and women. Journal of the American College of Cardiology. 2008;52(1):17–23. doi: 10.1016/j.jacc.2008.04.004 . [DOI] [PubMed] [Google Scholar]
- 5.Raggi P, Callister TQ, Shaw LJ. Progression of coronary artery calcium and risk of first myocardial infarction in patients receiving cholesterol-lowering therapy. Arteriosclerosis, thrombosis, and vascular biology. 2004;24(7):1272–7. doi: 10.1161/01.ATV.0000127024.40516.ef . [DOI] [PubMed] [Google Scholar]
- 6.Raggi P, Cooil B, Ratti C, Callister TQ, Budoff M. Progression of coronary artery calcium and occurrence of myocardial infarction in patients with and without diabetes mellitus. Hypertension. 2005;46(1):238–43. doi: 10.1161/01.HYP.0000164575.16609.02 . [DOI] [PubMed] [Google Scholar]
- 7.Raggi P, Cooil B, Shaw LJ, Aboulhson J, Takasu J, Budoff M, et al. Progression of coronary calcium on serial electron beam tomographic scanning is greater in patients with future myocardial infarction. The American journal of cardiology. 2003;92(7):827–9. doi: 10.1016/s0002-9149(03)00892-0 . [DOI] [PubMed] [Google Scholar]
- 8.Rennenberg RJ, Kessels AG, Schurgers LJ, van Engelshoven JM, de Leeuw PW, Kroon AA. Vascular calcifications as a marker of increased cardiovascular risk: a meta-analysis. Vascular health and risk management. 2009;5(1):185–97. Epub 2009/05/14. doi: 10.2147/vhrm.s4822 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Criqui MH, Denenberg JO, Ix JH, McClelland RL, Wassel CL, Rifkin DE, et al. Calcium density of coronary artery plaque and risk of incident cardiovascular events. JAMA: the journal of the American Medical Association. 2014;311(3):271–8. doi: 10.1001/jama.2013.282535 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Motoyama S, Sarai M, Harigaya H, Anno H, Inoue K, Hara T, et al. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. Journal of the American College of Cardiology. 2009;54(1):49–57. doi: 10.1016/j.jacc.2009.02.068 . [DOI] [PubMed] [Google Scholar]
- 11.Puchner SB, Liu T, Mayrhofer T, Truong QA, Lee H, Fleg JL, et al. High-Risk Plaque Detected on Coronary CT Angiography Predicts Acute Coronary Syndromes Independent of Significant Stenosis in Acute Chest Pain: Results From the ROMICAT-II Trial. Journal of the American College of Cardiology. 2014;64(7):684–92. doi: 10.1016/j.jacc.2014.05.039 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Puri R, Nicholls SJ, Shao M, Kataoka Y, Uno K, Kapadia SR, et al. Impact of statins on serial coronary calcification during atheroma progression and regression. Journal of the American College of Cardiology. 2015;65(13):1273–82. Epub 2015/04/04. doi: 10.1016/j.jacc.2015.01.036 . [DOI] [PubMed] [Google Scholar]
- 13.Irkle A, Vesey AT, Lewis DY, Skepper JN, Bird JL, Dweck MR, et al. Identifying active vascular microcalcification by (18)F-sodium fluoride positron emission tomography. Nat Commun. 2015;6:7495. doi: 10.1038/ncomms8495 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease. Circulation. 2019:CIR0000000000000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr., Kastelein JJ, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. The New England journal of medicine. 2008;359(21):2195–207. Epub 2008/11/11. NEJMoa0807646 [pii] doi: 10.1056/NEJMoa0807646 . [DOI] [PubMed] [Google Scholar]
- 16.Taylor F, Huffman MD, Macedo AF, Moore TH, Burke M, Davey Smith G, et al. Statins for the primary prevention of cardiovascular disease. The Cochrane database of systematic reviews. 2013;1:CD004816. Epub 2013/02/27. doi: 10.1002/14651858.CD004816.pub5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Downs JR, Clearfield M, Weis S, Whitney E, Shapiro DR, Beere PA, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA: the journal of the American Medical Association. 1998;279(20):1615–22. Epub 1998/06/05. doi: 10.1001/jama.279.20.1615 . [DOI] [PubMed] [Google Scholar]
- 18.Heart Protection Study Collaborative G. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360(9326):7–22. Epub 2002/07/13. doi: 10.1016/S0140-6736(02)09327-3 . [DOI] [PubMed] [Google Scholar]
- 19.Schwartz GG, Olsson AG, Ezekowitz MD, Ganz P, Oliver MF, Waters D, et al. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA: the journal of the American Medical Association. 2001;285(13):1711–8. Epub 2001/04/13. doi: 10.1001/jama.285.13.1711 . [DOI] [PubMed] [Google Scholar]
- 20.Ridker PM. Inflammatory biomarkers, statins, and the risk of stroke: cracking a clinical conundrum. Circulation. 2002;105(22):2583–5. Epub 2002/06/05. doi: 10.1161/01.cir.0000017822.82512.62 . [DOI] [PubMed] [Google Scholar]
- 21.White HD, Simes RJ, Anderson NE, Hankey GJ, Watson JD, Hunt D, et al. Pravastatin therapy and the risk of stroke. The New England journal of medicine. 2000;343(5):317–26. Epub 2000/08/03. doi: 10.1056/NEJM200008033430502 . [DOI] [PubMed] [Google Scholar]
- 22.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1082–e143. Epub 2018/12/28. doi: 10.1161/CIR.0000000000000625 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oesterle A, Laufs U, Liao JK. Pleiotropic Effects of Statins on the Cardiovascular System. Circulation research. 2017;120(1):229–43. Epub 2017/01/07. doi: 10.1161/CIRCRESAHA.116.308537 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davignon J. Beneficial cardiovascular pleiotropic effects of statins. Circulation. 2004;109(23 Suppl 1):III39–43. Epub 2004/06/17. doi: 10.1161/01.CIR.0000131517.20177.5a . [DOI] [PubMed] [Google Scholar]
- 25.Achenbach S, Ropers D, Pohle K, Leber A, Thilo C, Knez A, et al. Influence of lipid-lowering therapy on the progression of coronary artery calcification: a prospective evaluation. Circulation. 2002;106(9):1077–82. doi: 10.1161/01.cir.0000027567.49283.ff . [DOI] [PubMed] [Google Scholar]
- 26.Arad Y, Spadaro LA, Roth M, Newstein D, Guerci AD. Treatment of asymptomatic adults with elevated coronary calcium scores with atorvastatin, vitamin C, and vitamin E: the St. Francis Heart Study randomized clinical trial. Journal of the American College of Cardiology. 2005;46(1):166–72. Epub 2005/07/05. doi: 10.1016/j.jacc.2005.02.089 . [DOI] [PubMed] [Google Scholar]
- 27.Callister TQ, Raggi P, Cooil B, Lippolis NJ, Russo DJ. Effect of HMG-CoA reductase inhibitors on coronary artery disease as assessed by electron-beam computed tomography. The New England journal of medicine. 1998;339(27):1972–8. doi: 10.1056/NEJM199812313392703 . [DOI] [PubMed] [Google Scholar]
- 28.Lee SE, Sung JM, Andreini D, Budoff MJ, Cademartiri F, Chinnaiyan K, et al. Differential association between the progression of coronary artery calcium score and coronary plaque volume progression according to statins: the Progression of AtheRosclerotic PlAque DetermIned by Computed TomoGraphic Angiography Imaging (PARADIGM) study. Eur Heart J Cardiovasc Imaging. 2019;20(11):1307–14. Epub 2019/02/23. doi: 10.1093/ehjci/jez022 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Budoff MJ, Lane KL, Bakhsheshi H, Mao S, Grassmann BO, Friedman BC, et al. Rates of progression of coronary calcium by electron beam tomography. The American journal of cardiology. 2000;86(1):8–11. doi: 10.1016/s0002-9149(00)00820-1 . [DOI] [PubMed] [Google Scholar]
- 30.Hecht HS, Harman SM. Relation of aggressiveness of lipid-lowering treatment to changes in calcified plaque burden by electron beam tomography. The American journal of cardiology. 2003;92(3):334–6. doi: 10.1016/s0002-9149(03)00642-8 . [DOI] [PubMed] [Google Scholar]
- 31.Puri R, Tuzcu EM, Nissen SE, Nicholls SJ. Exploring coronary atherosclerosis with intravascular imaging. International journal of cardiology. 2013;168(2):670–9. doi: 10.1016/j.ijcard.2013.03.024 . [DOI] [PubMed] [Google Scholar]
- 32.Healy A, Berus JM, Christensen JL, Lee C, Mantsounga C, Dong W, et al. Statins Disrupt Macrophage Rac1 Regulation Leading to Increased Atherosclerotic Plaque Calcification. Arteriosclerosis, thrombosis, and vascular biology. 2020:ATVBAHA119313832. doi: 10.1161/ATVBAHA.119.313832 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Henein M, Granasen G, Wiklund U, Schmermund A, Guerci A, Erbel R, et al. High dose and long-term statin therapy accelerate coronary artery calcification. International journal of cardiology. 2015;184:581–6. Epub 2015/03/15. doi: 10.1016/j.ijcard.2015.02.072 . [DOI] [PubMed] [Google Scholar]
- 34.Saremi A, Bahn G, Reaven PD, Investigators V. Progression of vascular calcification is increased with statin use in the Veterans Affairs Diabetes Trial (VADT). Diabetes care. 2012;35(11):2390–2. Epub 2012/08/10. doi: 10.2337/dc12-0464 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anand DV, Lim E, Darko D, Bassett P, Hopkins D, Lipkin D, et al. Determinants of progression of coronary artery calcification in type 2 diabetes role of glycemic control and inflammatory/vascular calcification markers. Journal of the American College of Cardiology. 2007;50(23):2218–25. Epub 2007/12/07. doi: 10.1016/j.jacc.2007.08.032 . [DOI] [PubMed] [Google Scholar]
- 36.Kronmal RA, McClelland RL, Detrano R, Shea S, Lima JA, Cushman M, et al. Risk factors for the progression of coronary artery calcification in asymptomatic subjects: results from the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation. 2007;115(21):2722–30. doi: 10.1161/CIRCULATIONAHA.106.674143 . [DOI] [PubMed] [Google Scholar]
- 37.Christensen JL, Tan S, Chung HE, Ghosalkar DS, Qureshi R, Chu A, et al. Aortic valve calcification predicts all-cause mortality independent of coronary calcification and severe stenosis. Atherosclerosis. 2020;307:16–20. Epub 2020/07/24. doi: 10.1016/j.atherosclerosis.2020.06.019 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chung HE, Chen J, Ghosalkar D, Christensen JL, Chu AJ, Mantsounga CS, et al. Aortic Valve Calcification Is Associated with Future Cognitive Impairment. J Alzheimers Dis Rep. 2021;5(1):337–43. Epub 2021/06/12. doi: 10.3233/ADR-200253 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr., Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. Journal of the American College of Cardiology. 1990;15(4):827–32. doi: 10.1016/0735-1097(90)90282-t . [DOI] [PubMed] [Google Scholar]
- 40.National Kidney F K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–266. Epub 2002/03/21. . [PubMed] [Google Scholar]
- 41.Goff DC Jr., Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S49–73. doi: 10.1161/01.cir.0000437741.48606.98 . [DOI] [PubMed] [Google Scholar]
- 42.Muntner P, Colantonio LD, Cushman M, Goff DC Jr., Howard G, Howard VJ, et al. Validation of the atherosclerotic cardiovascular disease Pooled Cohort risk equations. JAMA: the journal of the American Medical Association. 2014;311(14):1406–15. Epub 2014/04/01. doi: 10.1001/jama.2014.2630 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Austin PC, White IR, Lee DS, van Buuren S. Missing Data in Clinical Research: A Tutorial on Multiple Imputation. The Canadian journal of cardiology. 2021;37(9):1322–31. Epub 2020/12/05. doi: 10.1016/j.cjca.2020.11.010 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Greenland P, Bonow RO, Brundage BH, Budoff MJ, Eisenberg MJ, Grundy SM, et al. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography) developed in collaboration with the Society of Atherosclerosis Imaging and Prevention and the Society of Cardiovascular Computed Tomography. Journal of the American College of Cardiology. 2007;49(3):378–402. Epub 2007/01/24. doi: 10.1016/j.jacc.2006.10.001 . [DOI] [PubMed] [Google Scholar]
- 45.Hecht H, Blaha MJ, Berman DS, Nasir K, Budoff M, Leipsic J, et al. Clinical indications for coronary artery calcium scoring in asymptomatic patients: Expert consensus statement from the Society of Cardiovascular Computed Tomography. J Cardiovasc Comput Tomogr. 2017;11(2):157–68. Epub 2017/03/12. doi: 10.1016/j.jcct.2017.02.010 . [DOI] [PubMed] [Google Scholar]
- 46.Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, MacFarlane PW, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. The New England journal of medicine. 1995;333(20):1301–7. Epub 1995/11/16. doi: 10.1056/NEJM199511163332001 . [DOI] [PubMed] [Google Scholar]
- 47.Rifai MA, Blaha MJ, Patel J, Xiaoming J, Cainzos-Achirica M, Greenland P, et al. Coronary Artery Calcification, Statin Use and Long-Term Risk of Atherosclerotic Cardiovascular Disease Events (from the Multi-Ethnic Study of Atherosclerosis). The American journal of cardiology. 2020;125(6):835–9. Epub 2020/01/26. doi: 10.1016/j.amjcard.2019.12.031 . [DOI] [PubMed] [Google Scholar]
- 48.Budoff MJ, Shaw LJ, Liu ST, Weinstein SR, Mosler TP, Tseng PH, et al. Long-term prognosis associated with coronary calcification: observations from a registry of 25,253 patients. Journal of the American College of Cardiology. 2007;49(18):1860–70. Epub 2007/05/08. doi: 10.1016/j.jacc.2006.10.079 . [DOI] [PubMed] [Google Scholar]
- 49.McClelland RL, Jorgensen NW, Budoff M, Blaha MJ, Post WS, Kronmal RA, et al. 10-Year Coronary Heart Disease Risk Prediction Using Coronary Artery Calcium and Traditional Risk Factors: Derivation in the MESA (Multi-Ethnic Study of Atherosclerosis) With Validation in the HNR (Heinz Nixdorf Recall) Study and the DHS (Dallas Heart Study). Journal of the American College of Cardiology. 2015;66(15):1643–53. Epub 2015/10/10. doi: 10.1016/j.jacc.2015.08.035 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Osei AD, Mirbolouk M, Berman D, Budoff MJ, Miedema MD, Rozanski A, et al. Prognostic value of coronary artery calcium score, area, and density among individuals on statin therapy vs. non-users: The coronary artery calcium consortium. Atherosclerosis. 2021;316:79–83. Epub 2020/10/31. doi: 10.1016/j.atherosclerosis.2020.10.009 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ceneri N, Zhao L, Young BD, Healy A, Coskun S, Vasavada H, et al. Rac2 Modulates Atherosclerotic Calcification by Regulating Macrophage Interleukin-1beta Production. Arteriosclerosis, thrombosis, and vascular biology. 2017;37(2):328–40. doi: 10.1161/ATVBAHA.116.308507 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hordijk PL. Regulation of NADPH oxidases: the role of Rac proteins. Circulation research. 2006;98(4):453–62. doi: 10.1161/01.RES.0000204727.46710.5e . [DOI] [PubMed] [Google Scholar]
- 53.Khan OM, Ibrahim MX, Jonsson IM, Karlsson C, Liu M, Sjogren AK, et al. Geranylgeranyltransferase type I (GGTase-I) deficiency hyperactivates macrophages and induces erosive arthritis in mice. The Journal of clinical investigation. 2011;121(2):628–39. doi: 10.1172/JCI43758 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ridker PM, MacFadyen JG, Thuren T, Everett BM, Libby P, Glynn RJ, et al. Effect of interleukin-1beta inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet. 2017. doi: 10.1016/S0140-6736(17)32247-X . [DOI] [PubMed] [Google Scholar]
- 55.Ridker PM, MacFadyen JG, Everett BM, Libby P, Thuren T, Glynn RJ, et al. Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: a secondary analysis from the CANTOS randomised controlled trial. Lancet. 2018;391(10118):319–28. doi: 10.1016/S0140-6736(17)32814-3 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data were plotted as median CACS with interquartile range. (N) indicates the number of patients in each additional 2-year increment in duration of statin therapy. *, P = 0.01; **, P<0.005; ****; P<0.0001, compared to duration of statin “0” using Kuskal-Wallis followed by Dunn’s multiple comparison testing.
(TIF)
(XLS)
Data Availability Statement
The data can be found in the fully anonymized minimal data set provided as a supplemental file.