Abstract
Objective
Depression represents one of the most severe psychiatric disorders, characterized by low mood episodes, as well as loss of interest. Major Depressive Episodes (MDE) treatment relies primarily on monoaminergic prescriptions. However, although the presence of many antidepressant medications, their efficacy is still partial. A promising intervention to improve antidepressant treatment may be the use of adjunctive nutraceuticals. Aim of the present study was to assess the efficacy of a N-Acetyl-cysteine, S-Adenosyl-L-Methionine and Folic acid’s combination for the treatment of depressive symptoms in a sample of MDE patients.
Method
Fifty outpatients with a MDE diagnosis in the context of different psychiatric disorders such as Major Depression, Bipolar Disorder, Anxiety disorders, and Personality disorders were recruited. The sample was divided into different groups based on the nutraceutical administration: a) concurrently with an AD (starter group); b) add-on to an already prescribed treatment; c) single treatment.
Results
A significant reduction of CGI-Severity and Improvement scores from baseline to the end of treatment was found. Moreover, the starter group showed a significantly greater CGI-Improvement score compared to the other groups. Ninety-four percent of patients did not show any side effects.
Conclusions
The present study showed promising results for the use of nutraceuticals in the add-on treatment of MDE. Those compounds may be considered a versatile, tolerable, and effective add-on treatment for the reduction of depressive symptoms impact and for improving the functioning of patients affected by MDE.
Keywords: nutraceutical, adjunctive, major depressive episode
Introduction
Depression is a severe psychiatric disorder, marked by episodes of low mood, as well as loss of interest and enjoyment of everyday activities (APA, 2013). Currently, 4.4% of the global population suffers from depression (IHME, 2018), with a 12-month prevalence of 6% in the world, and a lifetime risk ranging from 15 to 18% (Kessler et al., 2013). In terms of health and functioning, the Global Burden of Disease Group Study ranked Major Depressive Disorder (MDD) as the third cause of burden of disease worldwide (IHME, 2018).
Although some individuals may experience a single depressive episode in their entire life, for most patients depression is a recurrent lifelong condition, characterized by multiple depressive episodes, separated by periods of remission (Otte et al., 2016). Despite Major Depressive Episodes (MDE) represent the main feature of MDD, they are also a characteristic of Bipolar Disorder type I (BD I) and II (BD II; APA, 2013). Moreover, MDE can occur in the context of a comorbidity with other psychiatric disorders, such as Anxiety disorders, Obsessive-Compulsive Disorders, Borderline Personality Disorder, Bulimia and Anorexia (APA, 2013).
MDE treatment is primarily based on monoaminergic prescriptions such as antidepressants (Ads; Hamon et al., 2015). However, regardless of their pharmacological class, ADs show a characteristic delayed response (usually 2-6 weeks) and have comparable modest efficacy (Rush et al., 2006; Cipriani et al., 2012). Results from STAR*D study showed that the response rate was 47%, while the remission rate was 28-33% (Howland, 2003). Furthermore, though the existence of several antidepressant medications, the research found that the efficacy is still partial, even when patients report levels of remission, with subclinical residual symptoms that may hamper the individuals’ functioning and their quality of life (Nierenberg et al., 1999; Fava, 1999; Xiao et al., 2018).
Patients with MDD can show signs of impairment in social and occupational skills even after medications (Fava et al., 2007; Keller, 1998). Residual symptoms that persist even in remitted patients, such as cognitive difficulties, energy shortage, and sleep disturbances, are highly related to functional impairment, involving work performance, home management, social skills, and interpersonal communications (Nil et al., 2016; Kennedy et al., 2007). Furthermore, residual symptoms of MDD could persist also one year later the remission despite the maintenance of antidepressant medications and other therapies, such as psychotherapy and transcranial magnetic stimulation (Xiao et al., 2018). Hence, considering the severe impact of residual symptoms on overall functioning, there is a need to focus on the treatment of residual manifestations.
Therefore, various pharmacological and non-pharmacological strategies have been implemented over the years for the management of the partial response to antidepressant treatment. A promising mode to improve non- or scarce response to antidepressants could be the use of adjunctive nutraceuticals, namely nutrient-based natural products produced via pharmaceutical good manufacturing practice (GMP) smooth and maximized, and in some cases purified or slightly revised (Sarris et al., 2019a). Nutritional psychiatry (NP) has grown rapidly in recent years, with an increasing number of dietary or nutraceutical intervention studies and several preclinical and epidemiological data available. The function of NP is to prevent or treat psychiatric disorders, prescribing dietary modifications and/or selecting the use of nutraceuticals (Sarris et al., 2019b). In addition, macronutrients, diet or supplemental nutrients provide a number of critical cofactors and phytochemicals which can also have important effects that can modify the brain and mental health (Marx et al., 2017). Nonetheless, nutraceuticals differ from dietary complements as their role is not only to supplement the diet but also therapeutic (Kalra, 2003). At present, the growth of safe nutraceutical compounds with enhanced delivery components also owes its success to nanotechnology and biotechnology with liposomes, nanoparticles, and dendrimers (Helal et al., 2019), as well as the use of “food matrix”, a composite set of agents that may improve the bioavailability and benefits from nutraceutical compound (Zou et al., 2015). Moreover, growing attention to remedial proprieties of natural sources as compared with the adverse drug reaction of lost chemically synthesized medications has contributed to nutraceuticals' substantial approval in addition to treatment as usual therapies (Brower, 2005; Gupta et al., 2018; Alvarez-Mon et al., 2021). The pathophysiology of depression is complex and several neurobiological key mechanisms have been considered, such as monoamine impairment, neuroendocrinological changes, reduced neurogenesis, and cytokine alterations consistent with chronic inflammation (Antonijevic, 2006).
Several nutrients are known to have critical involvement in brain function, and some have been reported to determine an array of neurobiological processes that may be involved in depression. By targeting these key neurobiological pathways through specific nutraceuticals, such adjunctive treatments have the potential to augment the response to antidepressants (Belmaker et al., 2008).
Accordingly, several studies showed that nutraceuticals could modulate neurobiological patterns implicated in the development and course of depressive symptoms in mood disorders (Sarris et al., 2015; Malhi & Mann, 2018; Visentin et al., 2020; Sarris et al., 2016); therefore, nutraceutical compounds are frequently used as supplements for MDD treatment (Donohue & Pincus, 2007).
One currently used nutraceutical compound is made of three components: N-Acetyl-cysteine (NAC) 500 mg, S-Adenosyl-L-Methionine (SAMe) 200 mg, Quatrefolic â -5MTFH-Glucosamine 400 mc (Folic acid) (NSF). Concerning components functioning, SAMe may improve depressed mood via enhanced catecholamines methylation and increased turnover of serotonin, reuptake inhibition of noradrenaline, enhanced dopaminergic activity, reduced prolactin secretion, and improved phosphatidylcholine conversion; L-methylfolate involves methylation pathways in the ‘one carbon’ cycle, contributing to the metabolism and synthesis of different monoamines and of SAMe from homocysteine; N-acetylcysteine is a precursor of endogenous antioxidant glutathione and has antioxidant, anti-inflammatory, and neuroprotective properties (Berk et al., 2013).
In 2022, guidelines for the treatment of psychiatric diseases with nutraceutical and phytoceutical compounds were published by the World Federation of Societies of Biological Psychiatry (WFSBP) and Canadian Network for Mood and Anxiety Treatments (CANMAT) Taskforce (Sarris et al., 2022). These guidelines provided an evidence-informed mode to assist clinicians in making decisions regards the use of such agents for major psychiatric diseases, providing details on tolerability and safety, and clinical advice around prescription (Sarris et al., 2022). Concerning the efficacy of nutraceutical compounds on depressive symptoms in MDD and other psychiatric disorders, results were mixed; further research could elucidate their efficacy and safety.
Given these premises, the aim of this study is to assess the efficacy of the NSF for the treatment of symptoms of depression in a sample of patients with a MDE.
Methods
The sample consisted of 50 outpatients of either gender and any age with a diagnosis of MDE in the context of several psychiatric disorders such as, MDD, BD, Anxiety Disorders, and Personality disorders, recruited at the University Department of Mental Health of the Department of Biomedical and Clinical Sciences 'Luigi Sacco', ASST Fatebenefratelli-Sacco, University of Milan. Diagnoses were obtained through the administration of a clinical structured interview based on DSM-5 criteria (APA, 2013). The Clinical Global Impression (CGI) scale was used to measure illness severity and global clinical improvement or change in therapeutic response (Guy, 1976). The CGI scale is a brief and easy to administer scale that assesses symptoms severity both at cross-sectional and follow-up level in the context of multiple psychiatric diagnoses.
Main socio-demographic and clinical characteristics of the patients were collected during a clinical interview. Each patient included in the study received either one or two tablets of NSF per day according to the initial clinical status and the clinical course, for a period of treatment ranging from 4 to 8 weeks.
For the purpose of the study, the sample was divided into three different groups according to the administration scheme of the nutraceutical compound: a) concurrently with an AD (starter group); b) add-on to an already prescribed treatment; c) single treatment. The treatment efficacy was addressed using the Clinical Global Impression- Severity scale (CGI-S) at baseline and at the end of the treatment (4 to 8 weeks from baseline) and the CGI-Improvement scale (CGI-I) at the end of the treatment (4 to 8 weeks from baseline). The CGI scale provides a clinician-determined overview considering all available information, including patient's history, symptoms, behavior, psychosocial circumstances, and the impact of the symptoms on the patient's functioning. Subsequent to a clinical evaluation, the CGI form can be rapid completed by an experienced rater. In addition, the CGI can track clinical progress across time and has been shown to correlate with longer and time-consuming rating instruments across a wide range of psychiatric diagnoses (Busner & Targum, 2007).
The severity of CGI scale was rated as follows: 1=normal, not at all ill; 2=borderline mentally ill; 3=mildly ill; 4=moderately ill; 5=markedly ill; 6=severely ill; 7=among the most extremely ill patients. CGI-improvement scale was rated on a seven-point scale concerning patient’s condition: 1=very much improved since the initiation of treatment; 2=much improved; 3=minimally improved; 4=no change from baseline; 5=minimally worse; 6= much worse; 7=very much worse since the initiation of treatment (Guy, 1976). In addition, in order to evaluate the tolerability profile, side effects were collected both directly from the patient or investigated by the clinician throughout the whole treatment duration.
Inclusion criteria were: (a) diagnosis of current MDE; (b) both gender; (c) age > 18 years at the time of inclusion; (d) written informed consent. Exclusion criteria were: (a) incapacity of giving informed consent; (b) intellectual disability and/or psychiatric disorder due to a medical condition.
Given the non-pharmacological nature of the adjunctive compound studied, the ethical approval was not sought for the present study. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. All patients involved in the study had previously provided a written informed consent for research purposes.
After obtaining written informed consent for using patients’ information for research, socio-demographic and clinical variables were collected and included in a common database.
For the purpose of the present study, selected analyzed variables included: (1) age; (2) gender; (3) educational status; (4) marital status; (5) professional status; (6) age at psychiatric onset; (7) duration of illness (months); (8) duration of untreated illness (months); (9) psychiatric diagnosis; (10) pharmacological therapy (i.e. the pharmacological treatment before the time of inclusion in the study); (11) nutraceutical compounds dosage; (12) duration of current treatment; (13) presence of side effects.
Statistical analyses were performed using Pearson’s chi-squared test for categorical variables and ANOVA test for continuous variables. Paired Samples t-Test for continuous variables was used to compare CGI-I and CGI-S scores at baseline and at the end of treatment for each patient. All analyses were performed using Statistical Package for the Social Sciences (SPSS) 26.0 software for Windows (SPSS Inc, Chicago, IL, USA). Statistical significance was set at p < 0.05.
Results
The sample included 50 patients with a diagnosis of MDE distributed as follows: 42% of the sample was diagnosed with MDD, 4% with BD I, 12% BD II, 16% Personality Disorders, and 26% Anxiety Disorders.
Main socio-demographic and clinical variables of the whole sample were reported in table 1.
Table 1.
Comparison of clinical variables between treatment subgroups
| Variables | Starter n= 10 (20%) | Add-on n= 29 (58%) | Monotherapy n= 11(22%) | Total sample n=50 |
|---|---|---|---|---|
| Age (years) | 52.5±17.5 | 54.9±12.8 | 55.5±16.5 | 54.6±14.4 |
| Gender (M:F) | 3 (30%); 7(70%) | 11(37.9%); 18(62.1%) | 3 (27.3%); 8 (72.7%) | 17(34%); 33(66%) |
| Age at onset (years) | 35.9±16.9 | 34.2±11.8 | 44.6±18.1 | 36.8±14.7 |
| Education | ||||
| Secondary school | 4 (40%) | 7 (24.1%) | 0 (0%) | 11 (22%) |
| High-school | 5 (50%) | 16 (55.2%) | 5 (54%) | 27 (54%) |
| University | 1 (10%) | 6 (20.7%) | 2 (45.5%) | 12 (24%) |
| Marital status | ||||
| Single/Divorced | 4 (40%) | 15 (51.75) | 4 (36.4%) | 23 (46%) |
| Married/Engaged | 6 (60%) | 14 (48.3%) | 7 (63.6%) | 27 (54%) |
| Professional status | ||||
| Unemployed | 2 (20%) | 1 (3.4%) | 2 (18.2%) | 5 (10%) |
| Worker part-time | 1 (14.3%) | 6 (20.7%) | 0 (0%) | 7 (14%) |
| Worker full-time | 3 (30%) | 12 (41.4%) | 6 (54.5%) | 21 (42%) |
| Retired | 4 (40%) | 10 (34.5%) | 3 (27.3%) | 17 (34%) |
| Duration of illness (months) | 199.2±194.3 | 223.8±168.0 | 130.9 ±179.2 | 198.5±176.2 |
| DUI (months) | 12.2±15.2 | 17.2±21.8 | 7.45±8.4 | 14.1±18.6 |
| Diagnosis | ||||
| Major Depressive Disorder | 4 (19%) | 13 (61.9%) | 4 (19%) | 21 (42%) |
| 0 (0%) | 2 (100%) | 0 (0%) | 2 (4%) | |
| Bipolar Disorder 1 | 2 (33%) | 3 (50%) | 1 (16.7%) | 6 (12%) |
| Bipolar Disorder 2 | 2 (25%) | 3 (37.5%) | 3 (37.5%) | 8 (16%) |
| Personality Disorder Anxiety Disorder | 2 (15.4%) | 8 (61.5%) | 3 (23.1%) | 13 (26%) |
| Pharmacological Therapy | 0 (0%) | 0(0%) | 11(100%) | 11 (26%) |
| No therapy | 4 (65.9%) | 20 (69%) | 0 (0%) | 24 (48%) |
| SSRI | 1 (4.3%) | 2 (6.9%) | 0 (0%) | 3 (5%) |
| TCAs | 2 (16.9%) | 2 (6.9%) | 0 (0%) | 4 (5%) |
| SNRI | 1 (4.3%) | 1 (3.4%) | 0 (0%) | 2 (4%) |
| BDZ | 1 (4.3%) | 1 (3.4%) | 0 (0%) | 2 (4%) |
| Lithium | 1 (4.3%) | 3 (10.3%) | 0 (0%) | 4 (8%) |
| Acido valproico | ||||
| NSF | ||||
| 1 cp die | 6 (60%) | 21 (72.4%) | 9 (81.8%) | 36 (72%) |
| 2 cp die | 4 (40%) | 8 (27.6%) | 2 (18.2%) | 14 (28%) |
| Duration of treatment (weeks) | 5.20±1.93 | 6.48±1.97 | 5.09±1.86 | 5.92±2.01 |
| First use of nutraceutical compounds (y/n) | 0 (0%); 10 (100%) | 4 (13.8%); 0 (86.2%) | 0 (0%); 11 (100%) | 4 (8%); 46 (92%) |
| Side effects (y/n) | 1 (10%); 9 (90%) | 1 (3.4%); 28 (96.6%) | 1 (9.1%); 10 (90.9%) | 3(6%), 47(94%) |
Notes: Values for categorical and continuous variables are expressed in percentages and mean ± SD, respectively. DUI: Duration of untreated illness; NSF: NAC, SAMe and Folic Acid compound; SSRI: selective serotonin reuptake inhibitor; TCS: tricyclic antidepressants; SNRI: serotoninnorepinephrine reuptake inhibitor; BDZ: benzodiazepine; CGI-S: Clinical Global Impression-Severity; CGI-I: Clinical Global Impression-Improvement.
The whole sample showed a 66% female rate and a mean age of 54.6 years (s.d.= 14.4). The mean age at psychiatric onset was 36.8 years (s.d.= 14.7) and the mean duration of illness was 198.5 months (s.d = 176.2) with a mean duration of untreated illness (DUI) of 14.1 months (s.d.= 8.6). As regards pharmacological treatment at the time of inclusion in the study, 48% of recruited patients were prescribed selective serotonin reuptake inhibitor (SSRI), 8% valproate, 4% tricyclic antidepressants (TCAs), 4% serotonin-norepinephrine reuptake inhibitor (SNRI), 4% lithium and 2% were assuming benzodiazepines (BDZ).
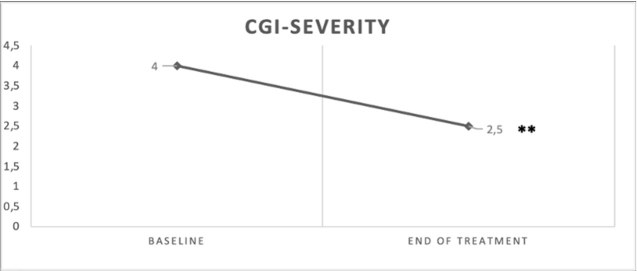
A comparison of CGI-S scores between baseline and the end of treatment was provided in figure 1. Regarding treatment response, the whole sample showed a statistically significant decrease of CGI-S scores between baseline and the end of treatment (m= 4.04, s.d= 0.69 vs. m= 2.44, s.d= 1.24; t= 10.9; p<0.001). Consistently, 86% of the whole sample reported a global improvement after treatment; in particular, 56% showed a minimal improvement, 30% a relevant improvement, and 14% with no changes from baseline. No patients showed a global worsening from baseline to the end of treatment. In terms of tolerability, 94% of the whole sample did not show any side effects after treatment; only 3 (6%) patients reported side effects such as sleep disorders and gastrointestinal symptoms.
Figure 1.
Comparison of CGI-Severity scale between baseline and the end of treatment in the whole sample
**p<0.001
For the purpose of the study, the whole sample was divided into three subgroups of treatment: 10 patients (20%) assumed the nutraceutical compound as a starter; 29 patients (58%) assumed the nutraceutical compound as an add-on strategy and 11 patients (22%) assumed it in monotherapy (see table 1). Globally, 92% of the sample was assuming nutraceutical compounds for the first time.
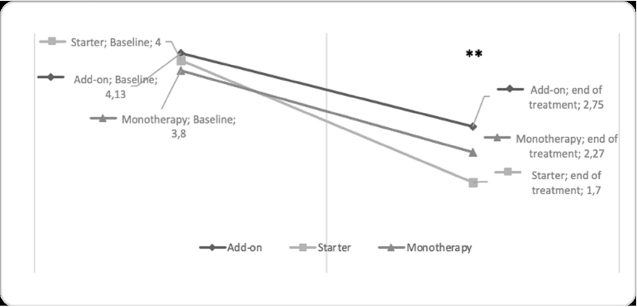
A comparison of CGI scale between treatment group at baseline and the end of treatment was provided in table 2. No differences in terms of CGI-S at baseline between groups emerged (starter group: m=4.0, sd=0.66 vs add-on group: m=4.1, sd=0.63 vs. monotherapy: m=3.8, sd= 0.87; F=0.85, p=0.434). A significant reduction of CGI-S score was reported for each group from baseline to follow-up (add-on group: m= 4.13, sd= 0.63 vs m= 2.75, sd= 1.2; t= 7.58, p<0.001; starter group: m= 4.0, sd= 0.66 vs m= 1.7, sd= 0.94, t= 8.83, p<0.001, and monotherapy group: m= 3.8, sd= 0.87 vs m= 2.27, sd= 1.34; t= 4.54, p<0.001; see figure 2). Moreover, the starter group showed lower scores of CGI-S at the end of treatment compared to add on and monotherapy groups, though not reaching statistical significance (m= 1.7, sd= 0.94 vs add-on group: m= 2.75, sd= 1.2 vs monotherapy group: m= 2.27, sd= 1.34; F= 3.03; p=0.058). Each group showed a clinical global improvement as rated with CGI-I (starter group: m= 1.05, sd= 0.7; add-on group: m= 2.48, sd= 0.98; monotherapy m= 2.1, sd= 1.13). More in detail, a significantly greater CGI-I score emerged in the starter treatment compared to other groups (starter group: m= 1.5, sd= 0.7 vs. add-on group: m= 2.48, sd= 0.98 and monotherapy: m= 2.1, sd= 1.13; F= 3.86, p<0.05).
Table 2.
Comparison of CGI scale between treatment group at baseline and the end of treatment
| Variables | Starter | Add-on | Monotherapy | Total sample |
|---|---|---|---|---|
| n= 10 (20%) | n= 29 (58%) | n= 11(22%) | n=50 | |
| CGI-S baseline | 4.0± 0.66 | 4.13±0.63 | 3.8±0.87 | 4.04±0.69 |
| CGI-S end of treatment | 1.7±0.94 | 2.75±1.2 | 2.27±1.34 | 2.44±1.24 |
| CGI-I end of treatment | 1.5±0.7* | 2.48±0.98 | 2.1±1.13 | 2.2±1.03 |
| Global Improvement (y/n) | 10 (100%), 0 (0%) | 24 (82.8%); 5 (17.2%) | 9 (81.8%); 2 (18.2%) | 43 (86%); 7 (14%) |
| Global Improvement | ||||
| Very much improved | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Much improved | 6 (40%) | 5 (33.3%) | 4 (26.7%) | 15 (30%) |
| Minimally improved | 4 (14.3%) | 19 (67.9%) | 5 (17.3%) | 28 (56%) |
| No change from baseline | 0 (0%) | 5(71.4%) | 2 (28.6%) | 7 (14%) |
| Minimally worse | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Much worse | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Very much worse | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
Notes: Values for categorical and continuous variables are expressed in percentages and mean ± SD, respectively. CGI-S: Clinical Global Impression-Severity; CGI-I: Clinical Global Impression-Improvement. Boldface indicates parameters with statistically significant differences between subgroups. **p<0.001 *p<0.05
Figure 2.
Comparison of CGI-Severity scale between treatment subgroups from baseline to the end of treatment
**A statistically significant difference within the group emerged at p<0.001
Overall, higher rates of “much improved” was found in the starter groups compared to the other group (starter group: 40% vs. add-on group: 33.3% vs. monotherapy: 26.7%). Higher rates of “minimal improvement” and “no change from baseline” were found in the add-on group compared to the others (add-on group: 67.9% vs. monotherapy group: 17.3% vs. starter group: 14.3%; add-on group: 71.4% vs. monotherapy group: 28.6% vs. starter group: 0%, respectively), though not reaching a significant difference. No patients showed a global worsening from baseline to the end of treatment (see table 1). No significant differences emerged in terms of gender, nutraceutical dosage, duration of treatment, psychiatric diagnosis, age, age at onset and duration of illness, and DUI between groups.
Discussion
The present study examined the efficacy and tolerability of NSF in a sample of psychiatric outpatients affected by MDE in the context of different psychiatric diagnoses.
In particular, 42% of patients were affected by MDD, 4% with BD I, 12% BD II, 26%, Anxiety Disorders, and 16% Personality Disorders. Considering psychiatric treatment, one out of two patients was treated with an SSRI. Of note, 26% of patients were currently under no specific psychotropic medication at the time of the first evaluation, while 12% of patients were treated with mood stabilizers (4% Lithium, 8% Valproic Acid). This data underlines the heterogeneity of MDE manifestations, possible diagnoses, and potential treatments (Davidson, 2010). Our results are consistent with other reports in the literature that found high comorbidity rates between MDE and psychiatric disorders such as Anxiety Disorder (Kessler et al., 2015), and Borderline Personality Disorder (Ceresa et al., 2021). MDEs are also a key feature in the depressive phases of BD (APA, 2013), hence the relatively high rate of mood stabilizer treatments reported in our sample could be explained by both the impact of MDEs in BD patients and by the widespread use of long-term lithium pharmacotherapy in order to mitigate the effects on suicidal behaviours among patients with affective disorders and personality disorders (Sarai et al., 2018).
In the total sample, NSF was administered in three different treatment protocols. In particular, in the add-on subgroup, 82.8% of patients were treated with NSF in association with an AD (69% SSRI, 6.9% TCAs, and 6.9% SNRI), and in the starter subgroup, 87.1% were treated with NSF in association with an AD both as starters (65.9% SSRI, 4.3% TCAs and 16.9% SNRI).
Considering efficacy in reducing depressive symptomatology, despite the variety of psychiatric diagnoses and medications in our sample, NSF showed a significant decrease in CGI severity scores both in the total sample and in each specific treatment group. It is important to note that no difference emerged in terms of CGI severity at baseline between groups, therefore the three groups should be considered comparable. Considering CGI-I scores, a significantly greater improvement was found in the starter treatment compared to other groups. In particular, although each group showed a global improvement after treatment, higher rates of "minimal improvement" and "no change from baseline" emerged in the add-on group, and a higher rate of “much improvement” was found in the starter group compared to other groups. Overall, almost 9 out of 10 patients showed a global improvement or no changes, while no patients showed a global worsening from baseline.
Consistently with other authors (Malhi & Mann, 2018; Visentin et al., 2020; Sarris et al., 2016), our results showed that the use of nutraceutical compounds may reduce depressive symptoms and improve global functioning. Particularly, greater efficacy emerged when the compound was combined with a starter drug treatment or with add-on treatments, showing NSF efficacy in enhancing pharmacological response.
It has been largely observed that a wide range of abnormalities such as monoaminergic system disturbance, oxidative stress, inflammation, reduced brain-derived neurotrophic factor (BDNF), and cytokine alterations, may be implicated in the development and progression of depressive symptoms in mood disorders (Sarris et al., 2015; Malhi & Mann, 2018; Visentin et al., 2020). In particular, methylation, or the lack of it, regulates gene expression, potentially contributing to the development of mental disorders such as depression (Dell’Osso et al., 2014; Lande, 2020; Wilson et al., 2019). In this perspective, nutraceutical compounds and the possible combination of different molecules, may be of great use, especially in order to improve the efficacy of other psychotropic medications. In addition, comprehensive data from large‐scale researches showed that mood disorders are related to substantially reduced serum levels of essential nutrients, including zinc, folate, and vitamin D. Since these deficits appear to be connected to treatment response, a nutrient supplementation could improve clinical outcomes (Firth et al., 2019).
NAC’s antidepressant outcome could be in part the result of diminished oxidative stress by increasing glutathione levels and may also have antidepressant effects through its anti-inflammatory action and by increasing extracellular glutamate levels (Ferreira et al., 2008; Porcu et al., 2018). Moreover, the anti-inflammatory effects of NAC may be critical in treatment-resistant depression (Yang et al., 2018).
In this regards, clinical reports have shown mixed results. A large randomized controlled trial (RCT) with 269 participants found that the administration of NAC was associated to a significant improvement in Montgomery Asberg Depression Rating Scale (MADRS) scores at 16 weeks’ follow-up compared to placebo with a greater effect in those with more severe depression (MADRS score > 24; Kishi et al., 2020). Moreover, a significant improvement in the functioning measures was also found.
Consistently with our results, a recent meta-analysis of seven RCTs investigating adjunctive NAC therapy in treating unipolar or bipolar depression reported that NAC significantly improved functioning rated with the CGI-S compared to placebo, although a significant improvement on depression and anxiety rating scales was not found (Berk et al., 2014).
Concerning functioning, SAMe may improve depressed mood via enhanced methylation of catecholamines and increased serotonin turnover, further influencing the expression of key genes in the brain affecting cognition, behavior, learning, and memory (Sudgen, 2006). Another possible explanation for the antidepressant efficacy of these compounds may reside in their anti-inflammatory properties, which are well demonstrated with SAMe (Pfalzer et al., 2014). Two systematic reviews reported SAMe effective as a monotherapy versus placebo in mild to severe MDD (Carpenter, 2011) or versus antidepressants in mild to moderate MDD (De Berardis et al., 2016). According to our results, there is also evidence to support adjunctive SAM-e with antidepressants in mild to moderate MDD (De Berardis et al., 2016; Sarris et al., 2011). Moreover, other studies investigating the use of nutraceuticals in combination with other psychiatric medications showed that adjunctive use of molecules such as SAMe, methylfolate, omega-3, and vitamin D with antidepressants may decrease depressive symptoms (De Berardis et al., 2016; Sarris et al., 2011).
Folate deficiencies may induce depressive symptoms by elevating homocysteine and intracellular one-carbon metabolism (Bottiglieri et al., 2000; Bottiglieri., 2013). The effect of L-methylfolate in subjects with depression has been connected to multiple mechanisms, including monoamine synthesis, neurogenesis, and antioxidant effects (Stahl, 2008).
A recent meta-analysis with nine RCTs described that adjunctive folate was significantly superior to placebo for the improvement of depressive symptoms. In particular, adjunctive folate supplementation was associated with higher remission rates, but not with higher response rates (Zheng et al., 2020).
A double-blind RCT of elderly MMD subjects receiving SSRI treatment in combination with folic acid reported that reduction in the average depression scores on the Beck Depression Inventory II (BDI-II) in the experimental subgroup was statistically significant after six and eight weeks compared to the group that received only SSRI treatment (Sepehrmanesh et al., 2016).
In addition, an interesting retrospective chart review study using folic acid as an antidepressant adjunct in elderly inpatients with treatment-resistance depression reported that patients who received nutraceutical compounds as adjunctive therapy had a significantly reduced days of hospitalization requirement compared to controls (Saxena & Kyomen, 2021). However, other studies showed mixed results (Walker et al., 2012; de Koning et al., 2016; Christensen et al., 2011)
At present, clinician guidelines on nutraceuticals present mixed results and mainly examine compounds singularly. For SAM-e single compound used as adjunctive treatment there are weak recommendation and not as monotherapy. Regarding NAC compounds, guidelines indicate a specialized use across a range of comorbid psychiatric disorders, especially in cases of high oxidative stress. Furthermore, good safety data based on long-standing use in acetaminophen overdose were reported (Sarris et al., 2022).
Finally, regarding folate-based compounds, meta-analytic level results have shown supportive evidence for efficacy in treating MDD (for metabolically active forms of folate), although a large RCT using folic acid, showed null results (Bedson et al., 2014). Fair safety data were reported (Sarris et al., 2022).
Considering safety in our report, only 6% of patients showed side effects, such as referred sleep disturbance and gastrointestinal symptoms. This result is consistent with previous studies showing a good safety profile with few unwanted side effects in nutraceuticals (Augustin & Sanguansri, 2015). Overall, the tolerability of nutraceutical component has been generally wide, being the majority of side effects related to the gastrointestinal trait (Sarris et al., 2022).
No significant differences between subgroups emerged in terms of gender, dosage, duration of treatment, psychiatric diagnosis, age, age at onset and duration of illness, and DUI between groups, suggesting that NSF may be considered a versatile compound in different presentations of depressive symptoms.
Conclusions
Although the presence of many antidepressant treatments, clinical research showed that ADs therapy does not adequately meet the needs of all patients with depressive disorders. In addition, limited treatment lines for MDD patients resistant to conventional treatments exist while residual symptoms may hamper individuals’ functioning and their quality of life.
Accordingly, a potential intervention to improve non-response to antidepressants could be the use of adjunctive nutraceuticals and the combination of different molecules, in order to improve the efficacy of other psychotropic medications. Several studies showed that nutraceuticals could modulate neurobiological patterns involved in the development and progression of depressive symptoms in mood (Antonijevic, 2006; Belmaker et al., 2008; Sarris et al., 2015) and other psychiatric disorders.
Our results showed that the use of nutraceuticals may have a role in reducing depressive symptoms and in the improvement of global functioning in patients with a MDE in the context of a mood disorder or other psychiatric disorders. In particular, greater efficacy emerged when the compound was combined with a starter drug treatment or with add-on treatments, showing NSF efficacy in enhancing pharmacological response. Furthermore, consistently with previous studies (Sarris et al., 2022; Augustin & Sanguansri, 2015), our report showed a good safety profile with few unwanted side effects in nutraceuticals, such as referred sleep disturbance and gastrointestinal symptoms.
In conclusion, our results showed that NSF may be considered an effective, versatile, and tolerable nutraceutical in both treated and untreated patients affected by MDE in MDD and other psychiatric disorders. Future research should be aimed at optimization of nutraceuticals and/or enhancing synergistic effects in combination with psychotropic medications.
The above-mentioned results should be interpreted in light of some methodological limitations. First of all, the sample size was limited, hence limiting the power to detect significant differences between groups. Secondly, the psychiatric diagnoses of the sample were heterogeneous. Therefore, though heterogeneity increased the external validity and the generalizability of the study, conclusions on the influence of different diagnosis on the treatment outcomes were limited. Thirdly, symptoms severity was assessed using the CGI scale only. Other psychometric scales such as Hamilton Depression Rating Scale (Hamilton, 1960) or MADRS (Montgomery & Asberg, 1979) would have improved the quality of the collected clinical data, however, we chose to use CGI given its rapid and easy administration.
References
- Alvarez-Mon, M. A., Ortega, M. A., García-Montero, C., Fraile-Martinez, O., Monserrat, J., Lahera, G., Mora, F., Rodriguez-Quiroga, A., Fernandez-Rojo, S., Quintero, J., & Alvarez-Mon, M. (2021). Exploring the Role of Nutraceuticals in Major Depressive Disorder (MDD): Rationale, State of the Art and Future Prospects. Pharmaceuticals (Basel, Switzerland), 14(8), 821. 10.3390/ph14080821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Arlington, VA. 10.1176/appi.books.9780890425596 [Google Scholar]
- Antonijevic I. A. (2006). Depressive disorders -- is it time to endorse different pathophysiologies?. Psychoneuroendo-crinology, 31(1), 1–15. 10.1016/j.psyneu-en.2005.04.004 [DOI] [PubMed] [Google Scholar]
- Augustin, M. A., & Sanguansri, L. (2015). Challenges and solutions to incorporation of nutraceuticals in foods. Annual review of food science and technology, 6, 463–477. 10.1146/annurev-food-022814-015507 [DOI] [PubMed] [Google Scholar]
- Bedson, E., Bell, D., Carr, D., Carter, B., Hughes, D., Jorgensen, A., Lewis, H., Lloyd, K., McCaddon, A., Moat, S., Pink, J., Pirmohamed, M., Roberts, S., Russell, I., Sylvestre, Y., Tranter, R., Whitaker, R., Wilkinson, C., & Williams, N. (2014). Folate Augmentation of Treatment--Evaluation for Depression (FolATED): randomised trial and economic evaluation. Health technology assessment (Winchester, England), 18(48), vii–159. 10.3310/hta18480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmaker, R. H., & Agam, G. (2008). Major depressive disorder. The New England journal of medicine, 358(1), 55–68. 10.1056/NEJMra073096 [DOI] [PubMed] [Google Scholar]
- Berk, M., Dean, O. M., Cotton, S. M., Jeavons, S., Tanious, M., Kohlmann, K., Hewitt, K., Moss, K., Allwang, C., Schapkaitz, I., Robbins, J., Cobb, H., Ng, F., Dodd, S., Bush, A. I., & Malhi, G. S. (2014). The efficacy of adjunctive N-acetylcysteine in major depressive disorder: a double-blind, randomized, placebo-controlled trial. The Journal of clinical psychiatry, 75(6), 628–636. 10.4088/JCP.13m08454 [DOI] [PubMed] [Google Scholar]
- Berk, M., Williams, L. J., Jacka, F. N., O'Neil, A., Pasco, J. A., Moylan, S., Allen, N. B., Stuart, A. L., Hayley, A. C., Byrne, M. L., & Maes, M. (2013). So depression is an inflammatory disease, but where does the inflammation come from?. BMC medicine, 11, 200. 10.1186/1741-7015-11-200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottiglieri T. (2013). Folate, vitamin B₁₂, and S-adenosylmethionine. The Psychiatric clinics of North America, 36(1), 1–13. 10.1016/j.psc.2012.12.001 [DOI] [PubMed] [Google Scholar]
- Bottiglieri, T., Laundy, M., Crellin, R., Toone, B. K., Carney, M. W., & Reynolds, E. H. (2000). Homocysteine, folate, methylation, and monoamine metabolism in depression. Journal of neurology, neurosurgery, and psychiatry, 69(2), 228–232. 10.1136/jnnp.69.2.228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brower V. (2005). A nutraceutical a day may keep the doctor away. Consumers are turning increasingly to food supplements to improve well-being when pharmaceuticals fail. EMBO reports, 6(8), 708–711. 10.1038/sj.embor.7400498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busner, J., & Targum, S. D. (2007). The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry (Edgmont (Pa: Township), 4(7), 28–37. [PMC free article] [PubMed] [Google Scholar]
- Carpenter D. J. (2011). St. John's wort and S-adenosyl methionine as "natural" alternatives to conventional antidepressants in the era of the suicidality boxed warning: what is the evidence for clinically relevant benefit?. Alternative medicine review : a journal of clinical therapeutic, 16(1), 17–39. [PubMed] [Google Scholar]
- Ceresa, A., Esposito, C. M., & Buoli, M. (2021). How does borderline personality disorder affect management and treatment response of patients with major depressive disorder? A comprehensive review. Journal of affective disorders, 281, 581–589. 10.1016/j.jad.2020.11.111 [DOI] [PubMed] [Google Scholar]
- Christensen, H., Aiken, A., Batterham, P. J., Walker, J., Mackinnon, A. J., Fenech, M., & Hickie, I. B. (2011). No clear potentiation of antidepressant medication effects by folic acid+vitamin B12 in a large community sample. Journal of affective disorders, 130(1-2), 37–45. 10.1016/j.jad.2010.07.029 [DOI] [PubMed] [Google Scholar]
- Cipriani, A., Koesters, M., Furukawa, T. A., Nosè, M., Purgato, M., Omori, I. M., Trespidi, C., & Barbui, C. (2012). Duloxetine versus other anti-depressive agents for depression. The Cochrane database of systematic reviews, 10, CD006533. 10.1002/14651858.CD006533.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson J. R. (2010). Major depressive disorder treatment guidelines in America and Europe. The Journal of clinical psychiatry, 71 Suppl E1, e04. 10.4088/JCP.9058se1c.04gry [DOI] [PubMed] [Google Scholar]
- De Berardis, D., Orsolini, L., Serroni, N., Girinelli, G., Iasevoli, F., Tomasetti, C., de Bartolomeis, A., Mazza, M., Valchera, A., Fornaro, M., Perna, G., Piersanti, M., Di Nicola, M., Cavuto, M., Martinotti, G., & Di Giannantonio, M., (2016). A comprehensive review on the efficacy of S-Adenosyl-L-me-thionine in Major Depressive Disorder. CNS & neurological disorders drug targets, 15(1), 35–44. 10.2174/1871527314666150821103825 [DOI] [PubMed] [Google Scholar]
- de Koning, E. J., van der Zwaluw, N. L., van Wijngaarden, J. P., Sohl, E., Brouwer-Brolsma, E. M., van Marwijk, H. W., Enneman, A. W., Swart, K. M., van Dijk, S. C., Ham, A. C., van der Velde, N., Uitterlinden, A. G., Penninx, B. W., Elders, P. J., Lips, P., Dhonukshe-Rutten, R. A., van Schoor, N. M., & de Groot, L. C. (2016). Effects of Two-Year Vitamin B12 and Folic Acid Supplementation on Depressive Symptoms and Quality of Life in Older Adults with Elevated Homocysteine Concentrations: Additional Results from the B-PROOF Study, an RCT. Nutrients, 8(11), 748. 10.3390/nu8110748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell'Osso, B., D'Addario, C., Carlotta Palazzo, M., Benatti, B., Camuri, G., Galimberti, D., Fenoglio, C., Scarpini, E., Di Francesco, A., Maccarrone, M., & Altamura, A. C. (2014). Epigenetic modulation of BDNF gene: differences in DNA methylation between unipolar and bipolar patients. Journal of afective disorders, 166, 330–333. 10.1016/j.jad.2014.05.020 [DOI] [PubMed] [Google Scholar]
- Donohue, J. M., & Pincus, H. A. (2007). Reducing the societal burden of depression: a review of economic costs, quality of care and efects of treatment. PharmacoEconomics, 25(1), 7–24. 10.2165/00019053-200725010-00003 [DOI] [PubMed] [Google Scholar]
- Fava G. A. (1999). Well-being therapy: conceptual and technical issues. Psychotherapy and psychosomatics, 68(4), 171–179. 10.1159/000012329 [DOI] [PubMed] [Google Scholar]
- Fava, G. A., Ruini, C., & Belaise, C. (2007). The concept of recovery in major depression. Psychological medicine, 37(3), 307–317. 10.1017/S0033291706008981 [DOI] [PubMed] [Google Scholar]
- Ferreira, F. R., Biojone, C., Joca, S. R., & Guimarães, F. S. (2008). Antidepressant-like effects of N-acetyl-L-cysteine in rats. Behavioural pharmacology, 19(7), 747–750. 10.1097/FBP.0b013e3283123c98 [DOI] [PubMed] [Google Scholar]
- Firth, J., Teasdale, S. B., Allott, K., Siskind, D., Marx, W., Cotter, J., Veronese, N., Schuch, F., Smith, L., Solmi, M., Carvalho, A. F., Vancampfort, D., Berk, M., Stubbs, B., & Sarris, J. (2019). The eficacy and safety of nutrient supplements in the treatment of mental disorders: a meta-review of meta-analyses of randomized controlled trials. World psychiatry : oficial journal of the World Psychiatric Association (WPA), 18(3), 308–324. 10.1002/wps.20672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta, R. C., Srivastava, A., & Lall, R. (2018). Toxicity Potential of Nutraceuticals. Methods in molecular biology (Clifton, N.J.), 1800, 367–394. 10.1007/978-1-4939-7899-1_18 [DOI] [PubMed] [Google Scholar]
- Guy, W. (1976). Clinical Global Impressions, ECDEU Assessment Manual for Psychopharmacology, revised (DHEW Publ. No. ADM 76-338). National Institute of Mental Health, Rockville, 218-222. [Google Scholar]
- Hamilton M. (1960). A rating scale for depression. Journal of neurology, neurosurgery, and psychiatry, 23(1), 56–62. 10.1136/jnnp.23.1.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamon, M., & Blier, P. (2013). Monoamine neurocircuitry in depression and strategies for new treatments. Progress in neuro-psychopharmacology & biological psychiatry, 45, 54–63. 10.1016/j.pnpbp.2013.04.009 [DOI] [PubMed] [Google Scholar]
- Helal, N. A., Eassa, H. A., Amer, A. M., Eltokhy, M. A., Edafiogho, I., & Nounou, M. I. (2019). Nutraceuticals' Novel Formulations: The Good, the Bad, the Unknown and Patents Involved. Recent patents on drug delivery & formulation, 13(2), 105–156. 10.2174/1872211313666190503112040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howland, D. The Predicament of Ideas in Culture: Translation and Historiography. (2003). History and Theory, 42(1), 45–60. 10.1111/1468-2303.00229 [Google Scholar]
- Institute for Health Metrics and Evaluation (IHME). (2018). Findings from the Global Burden of Disease Study. Seattle, WA. [Google Scholar]
- Kalra E. K. (2003). Nutraceutical--definition and introduction. AAPS pharmSci, 5(3), E25. 10.1208/ps050325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy, N., Foy, K., Sherazi, R., McDonough, M., & McKeon, P. (2007). Long-term social functioning after depression treated by psychiatrists: a review. Bipolar disorders, 9(1-2), 25–37. 10.1111/j.1399-5618.2007.00326.x [DOI] [PubMed] [Google Scholar]
- Kessler, R. C., & Bromet, E. J. (2013). The epidemiology of depression across cultures. Annual review of public health, 34, 119–138. 10.1146/annurev-publ-health-031912-114409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler, R. C., Sampson, N. A., Berglund, P., Gruber, M. J., Al-Hamzawi, A., Andrade, L., Bunting, B., Demyttenaere, K., Florescu, S., de Girolamo, G., Gureje, O., He, Y., Hu, C., Huang, Y., Karam, E., Kovess-Masfety, V., Lee, S., Levinson, D., Medina Mora, M. E., Moskalewicz, J., … Wilcox, M. A. (2015). Anxious and non-anxious major depressive disorder in the World Health Organization World Mental Health Surveys. Epidemiology and psychiatric sciences, 24(3), 210–226. 10.1017/S2045796015000189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi, T., Miyake, N., Okuya, M., Sakuma, K., & Iwata, N. (2020). N-acetylcysteine as an adjunctive treatment for bipolar depression and major depressive disorder: a systematic review and meta-analysis of double-blind, randomized placebo-controlled trials. Psychopharmacology, 237(11), 3481–3487. 10.1007/s00213-020-05629-2 [DOI] [PubMed] [Google Scholar]
- Lande R. G. (2020). Nutraceutical Augmentation Strategies for Depression: A Narrative Review. The Journal of the American Osteopathic Association, 120(2), 100–106. 10.7556/jaoa.2020.019 [DOI] [PubMed] [Google Scholar]
- Malhi, G. S., & Mann, J. J. (2018). Depression. Lancet (London, England), 392(10161), 2299–2312. 10.1016/S0140-6736(18)31948-2 [DOI] [PubMed] [Google Scholar]
- Marx, W., Moseley, G., Berk, M., & Jacka, F. (2017). Nutritional psychiatry: the present state of the evidence. The Proceedings of the Nutrition Society, 76(4), 427–436. 10.1017/S0029665117002026 [DOI] [PubMed] [Google Scholar]
- Miller, I. W., Keitner, G. I., Schatzberg, A. F., Klein, D. N., Thase, M. E., Rush, A. J., Markowitz, J. C., Schlager, D. S., Kornstein, S. G., Davis, S. M., Harrison, W. M., & Keller, M. B. (1998). The treatment of chronic depression, part 3: psychosocial functioning before and after treatment with sertraline or imipramine. The Journal of clinical psychiatry, 59(11), 608–619. 10.4088/jcp.v59n1108 [DOI] [PubMed] [Google Scholar]
- Montgomery, S. A., & Asberg, M. (1979). A new depression scale designed to be sensitive to change. The British journal of psychiatry: the journal of mental science, 134, 382–389. 10.1192/bjp.134.4.382 [DOI] [PubMed] [Google Scholar]
- Nierenberg, A. A., Keefe, B. R., Leslie, V. C., Alpert, J. E., Pava, J. A., Worthington, J. J., 3rd, Rosenbaum, J. F., & Fava, M. (1999). Residual symptoms in depressed patients who respond acutely to fluoxetine. The Journal of clinical psychiatry, 60(4), 221–225. 10.4088/jcp.v60n0403 [DOI] [PubMed] [Google Scholar]
- Nil, R., Lütolf, S., & Seifritz, E. (2016). Residual symptoms and functionality in depressed outpatients: A one-year observational study in Switzerland with escitalopram. Journal of afective disorders, 197, 245–250. 10.1016/j.jad.2016.02.062 [DOI] [PubMed] [Google Scholar]
- Otte, C., Gold, S. M., Penninx, B. W., Pariante, C. M., Etkin, A., Fava, M., Mohr, D. C., & Schatzberg, A. F. (2016). Major depressive disorder. Nature reviews. Disease primers, 2, 16065. 10.1038/nrdp.2016.65 [DOI] [PubMed] [Google Scholar]
- Pfalzer, A. C., Choi, S. W., Tammen, S. A., Park, L. K., Bottiglieri, T., Parnell, L. D., & Lamon-Fava, S. (2014). S-adenosylmethionine mediates inhibition of inflammatory response and changes in DNA methylation in human macrophages. Physiological genomics, 46(17), 617–623. 10.1152/physiolgenomics.00056.2014 [DOI] [PubMed] [Google Scholar]
- Porcu, M., Urbano, M. R., Verri, W. A. Jr, Barbosa, D. S., Baracat, M., Vargas, H. O., Machado, R. C. B. R., Pescim, R. R., & Nunes, S. O. V. (2018). Efects of adjunctive N-acetylcysteine on depressive symptoms: Modulation by baseline high-sensitivity C-reactive protein. Psychiatry research, 263, 268–274. 10.1016/j.psychres.2018.02.056 [DOI] [PubMed] [Google Scholar]
- Rush, A. J., Trivedi, M. H., Wisniewski, S. R., Nierenberg, A. A., Stewart, J. W., Warden, D., Niederehe, G., Thase, M. E., Lavori, P. W., Lebowitz, B. D., McGrath, P. J., Rosenbaum, J. F., Sackeim, H. A., Kupfer, D. J., Luther, J., & Fava, M. (2006). Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. The American journal of psychiatry, 163(11), 1905–1917. 10.1176/ajp.2006.163.11.1905 [DOI] [PubMed] [Google Scholar]
- Sarai, S. K., Mekala, H. M., & Lippmann, S. (2018). Lithium Suicide Prevention: A Brief Review and Reminder. Innovations in clinical neuroscience, 15(11-12), 30–32. [PMC free article] [PubMed] [Google Scholar]
- Sarris, J., Byrne, G. J., Cribb, L., Oliver, G., Murphy, J., Macdonald, P., Nazareth, S., Karamacoska, D., Galea, S., Short, A., Ee, C., Birling, Y., Menon, R., & Ng, C. H. (2019a). L-theanine in the adjunctive treatment of generalized anxiety disorder: A double-blind, randomised, placebo-controlled trial. Journal of psychiatric research, 110, 31–37. 10.1016/j.jpsychires.2018.12.014 [DOI] [PubMed] [Google Scholar]
- Sarris, J., Byrne, G. J., Stough, C., Bousman, C., Mischoulon, D., Murphy, J., Macdonald, P., Adams, L., Nazareth, S., Oliver, G., Cribb, L., Savage, K., Menon, R., Chamoli, S., Berk, M., & Ng, C. H. (2019b). Nutraceuticals for major depressive disorder- more is not merrier: An 8-week double-blind, randomised, controlled trial. Journal of affective disorders, 245, 1007–1015. 10.1016/j.jad.2018.11.092 [DOI] [PubMed] [Google Scholar]
- Sarris, J., LaPorte, E., & Schweitzer, I. (2011). Kava: a comprehensive review of efficacy, safety, and psychopharmacology. The Australian and New Zealand journal of psychiatry, 45(1), 27–35. 10.3109/00048674.2010.522554 [DOI] [PubMed] [Google Scholar]
- Sarris, J., Logan, A. C., Akbaraly, T. N., Amminger, G. P., Balanzá-Martínez, V., Freeman, M. P., Hibbeln, J., Matsuoka, Y., Mischoulon, D., Mizoue, T., Nanri, A., Nishi, D., Ramsey, D., Rucklidge, J. J., Sanchez-Villegas, A., Scholey, A., Su, K. P., Jacka, F. N., & International Society for Nutritional Psychiatry Research (2015). Nutritional medicine as mainstream in psychiatry. The lancet. Psychiatry, 2(3), 271–274. 10.1016/S2215-0366(14)00051-0 [DOI] [PubMed] [Google Scholar]
- Sarris, J., Murphy, J., Mischoulon, D., Papakostas, G. I., Fava, M., Berk, M., & Ng, C. H. (2016). Adjunctive Nutraceuticals for Depression: A Systematic Review and Meta-Analyses. The American journal of psychiatry, 173(6), 575–587. 10.1176/appi.ajp.2016.15091228 [DOI] [PubMed] [Google Scholar]
- Sarris, J., Ravindran, A., Yatham, L. N., Marx, W., Rucklidge, J. J., McIntyre, R. S., Akhondzadeh, S., Benedetti, F., Caneo, C., Cramer, H., Cribb, L., de Manincor, M., Dean, O., Deslandes, A. C., Freeman, M. P., Gangadhar, B., Harvey, B. H., Kasper, S., Lake, J., Lopresti, A., … Berk, M. (2022). Clinician guidelines for the treatment of psychiatric disorders with nutraceuticals and phytoceuticals: The World Federation of Societies of Biological Psychiatry (WFSBP) and Canadian Network for Mood and Anxiety Treatments (CANMAT) Taskforce. The world journal of biological psychiatry: the official journal of the World Federation of Societies of Biological Psychiatry, 23(6), 424–455. 10.1080/15622975.2021.2013041 [DOI] [PubMed] [Google Scholar]
- Saxena, P. P., & Kyomen, H. (2021). Leucovorin as an Antidepressant Adjunct in Elderly Inpatients With Treatment-resistant Depression. The primary care companion for CNS disorders, 23(2), 20m02767. 10.4088/PCC.20m02767 [DOI] [PubMed] [Google Scholar]
- Sepehrmanesh Z, Omidi A., Gholampoor N.. (2016). Acid folic supplementation in major depressive disorder treatment: a double-blind randomized clinical trial. Red Crescent Med. J., 19(2), 10.5812/ircmj.33243. [Google Scholar]
- Stahl S. M. (2008). L-methylfolate: a vitamin for your monoamines. The Journal of clinical psychiatry, 69(9), 1352–1353. 10.4088/jcp.v69n0901 [DOI] [PubMed] [Google Scholar]
- Sugden C. (2006). One-carbon metabolism in psychiatric illness. Nutrition research reviews, 19(1), 117–136. 10.1079/NRR2006119 [DOI] [PubMed] [Google Scholar]
- Visentin, A. P. V., Colombo, R., Scotton, E., Fracasso, D. S., da Rosa, A. R., Branco, C. S., & Salvador, M. (2020). Targeting Inflammatory-Mitochondrial Response in Major Depression: Current Evidence and Further Challenges. Oxidative medicine and cellular longevity, 2972968. 10.1155/2020/2972968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker, J. G., Batterham, P. J., Mackinnon, A. J., Jorm, A. F., Hickie, I., Fenech, M., Kljakovic, M., Crisp, D., & Christensen, H. (2012). Oral folic acid and vitamin B-12 supplementation to prevent cognitive decline in community-dwelling older adults with depressive symptoms--the Beyond Ageing Project: a randomized controlled trial. The American journal of clinical nutrition, 95(1), 194–203. 10.3945/ajcn.110.007799 [DOI] [PubMed] [Google Scholar]
- Wilson, N., Lee, J. J., & Bei, B. (2019). Postpartum fatigue and depression: A systematic review and meta-analysis. Journal of affective disorders, 246, 224–233. 10.1016/j.jad.2018.12.032 [DOI] [PubMed] [Google Scholar]
- Xiao, L., Feng, L., Zhu, X. Q., Feng, Y., Wu, W. Y., Ungvari, G. S., Ng, C. H., Xiang, Y. T., & Wang, G. (2018). Comparison of residual depressive symptoms and functional impairment between fully and partially remitted patients with major depressive disorder: a multicenter study. Psychiatry research, 261, 547–553. 10.1016/j.psychres.2018.01.020 [DOI] [PubMed] [Google Scholar]
- Yang, C., Bosker, F. J., Li, J., & Schoevers, R. A. (2018). N-acetylcysteine as add-on to antidepressant medication in therapy refractory major depressive disorder patients with increased inflammatory activity: study protocol of a double-blind randomized placebo-controlled trial. BMC psychiatry, 18(1), 279. 10.1186/s12888-018-1845-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, W., Li, W., Qi, H., Xiao, L., Sim, K., Ungvari, G. S., Lu, X. B., Huang, X., Ning, Y. P., & Xiang, Y. T. (2020). Adjunctive folate for major mental disorders: A systematic review. Journal of affective disorders, 267, 123–130. 10.1016/j.jad.2020.01.096 [DOI] [PubMed] [Google Scholar]
- Zou, L., Liu, W., Liu, C., Xiao, H., & McClements, D. J. (2015). Utilizing food matrix effects to enhance nutraceutical bio-availability: increase of curcumin bioaccessibility using excipient emulsions. Journal of agricultural and food chemistry, 63(7), 2052–2062. 10.1021/jf506149f [DOI] [PubMed] [Google Scholar]