Abstract
Background
Maintenance of cognitive abilities is of critical importance to older adults, yet few effective strategies to slow cognitive decline currently exist. Multivitamin supplementation is used to promote general health; it is unclear whether it favorably affects cognition in older age.
Objectives
To examine the effect of daily multivitamin/multimineral supplementation on memory in older adults.
Methods
The COcoa Supplement and Multivitamin Outcomes Study Web (COSMOS-Web) ancillary study (NCT04582617) included 3562 older adults. Participants were randomly assigned to a daily multivitamin supplement (Centrum Silver) or placebo and evaluated annually with an Internet-based battery of neuropsychological tests for 3 y. The prespecified primary outcome measure was change in episodic memory, operationally defined as immediate recall performance on the ModRey test, after 1 y of intervention. Secondary outcome measures included changes in episodic memory over 3 y of follow-up and changes in performance on neuropsychological tasks of novel object recognition and executive function over 3 y.
Results
Compared with placebo, participants randomly assigned to multivitamin supplementation had significantly better ModRey immediate recall at 1 y, the primary endpoint (t(5889) = 2.25, P = 0.025), as well as across the 3 y of follow-up on average (t(5889) = 2.54, P = 0.011). Multivitamin supplementation had no significant effects on secondary outcomes. Based on cross-sectional analysis of the association between age and performance on the ModRey, we estimated that the effect of the multivitamin intervention improved memory performance above placebo by the equivalent of 3.1 y of age-related memory change.
Conclusions
Daily multivitamin supplementation, compared with placebo, improves memory in older adults. Multivitamin supplementation holds promise as a safe and accessible approach to maintaining cognitive health in older age. This trial was registered at clinicaltrials.gov as NCT04582617.
Keywords: multivitamins, cognition, aging, remote testing, clinical trial, diet
Introduction
The maintenance of cognitive abilities is a top priority for older adults [1]. Healthy dietary patterns are implicated in slowing cognitive aging and may explain some degree of inter-individual differences in cognitive change over time [2]. Many older adults believe that taking vitamins or dietary supplements delays cognitive impairment [3], yet few larger-scale, longer-term randomized trials have rigorously tested this assumption. Although observational data suggest an association between selected blood-derived vitamin concentrations, such as vitamin B12, and cognition [4,5], results from randomized trials of multivitamins and cognitive change in older adults are mixed, with some meta-analytic evidence of improvement to immediate verbal memory [6] and others suggesting a null effect [[7], [8], [9]].
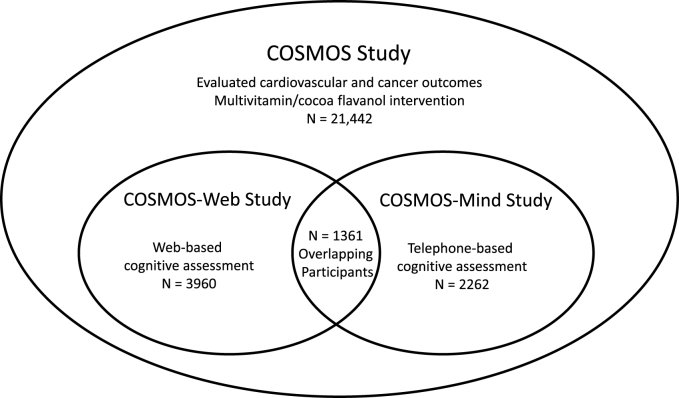
The recently completed COcoa Supplement and Multivitamin Outcomes Study (COSMOS; NCT02422745), a 2 × 2 factorial trial that examined the effect of dietary flavanol and multivitamin/multimineral (hereafter referred to as multivitamin) supplementation compared with placebo on cardiovascular and cancer outcomes in 21,442 older women and men with 3.6 y of follow-up [[10], [11], [12]], included 2 cognitive ancillary studies (Figure 1). In the COSMOS-Mind ancillary study, 2262 COSMOS participants underwent telephone-administered cognitive assessments over 3 y and reported that multivitamin supplementation benefited global cognition compared with placebo, an effect that was more prominent among participants reporting a history of cardiovascular disease (CVD) [13].
FIGURE 1.
The COSMOS study and its ancillary studies. COSMOS, COcoa Supplement and Multivitamin Outcomes Study.
Here, we report results from the COSMOS-Web (NCT04582617) ancillary study, which was designed to examine the effects of dietary flavanol and multivitamin supplementation on hippocampus-mediated cognition in older adults after 1 y of intervention. There is converging work that the hippocampus is particularly susceptible to the effects of normal aging [14], and our previous intervention studies with dietary supplementation showed a positive effect on the hippocampus, indexed both by neuroimaging [15] and neuropsychological assessment [16]. This report focuses on the multivitamin intervention of COSMOS-Web; and the results of the cocoa flavanol intervention are reported separately [17]. In addition to providing the opportunity to replicate multivitamin results from the COSMOS-Mind ancillary study [13], the COSMOS-Web study extends these findings by focusing on outcomes hypothesized to be particularly vulnerable in aging through a novel approach to cognitive outcome assessment. We hypothesized that multivitamin supplementation, similar to our previous observations with cocoa flavanol supplementation, would affect hippocampus-mediated aspects of declarative memory. To test this hypothesis, we designed the COSMOS-Web cognitive battery, which included neuropsychological outcome measures designed to be sensitive to cognitive changes typically seen in older adults and to capture the dynamic range of cognitive function. A unique and innovative design element of the study was that neuropsychological outcome measures were collected remotely via self-administered Web-based assessment tools.
Methods
COSMOS trial
COSMOS-Web (NCT04582617) is an ancillary study to the COSMOS trial (NCT02422745), which examined the effects of daily cocoa extract and multivitamin supplementation for 3 y in a 2 × 2 double-blind, randomized controlled factorial design on cardiovascular and cancer outcomes. The nationwide recruitment of the COSMOS study population (N = 21,442) was described previously [[10], [11], [12],18]. The 2 COSMOS intervention arms included 500 mg/d cocoa flavanol extract (including 80 mg (–)-epicatechin) capsules or a placebo (provided by Mars Edge) and a Centrum Silver daily multivitamin or placebo (provided by Pfizer Consumer Healthcare [now Haleon]; see [18] and Supplemental Data 1 for further details). Randomization occurred between April 2016 and March 2018. A computer-generated permuted block approach, blinded to study investigators, was used to randomly assign equal proportions of eligible participants to 1 of 4 treatment arms, as described previously [18]: 1) active multivitamin + active cocoa extract; 2) active multivitamin + cocoa extract placebo; 3) active cocoa extract + multivitamin placebo; or 4) cocoa extract placebo + multivitamin placebo. Randomization was stratified by gender, age (separate 5-y groups for women and men), and recruitment source, as described previously [18]. Active multivitamin and matching placebo and active cocoa extract and placebo were packaged in blinded monthly calendar packs and regularly sent to participants by US mail. Major exclusion criteria for the main COSMOS trial were age (<65 y for women, <60 y for men), history of myocardial infarction or stroke; diagnosis of invasive cancer within the past 2 y; other serious illnesses precluding participation; unwillingness to stop the use of cocoa extract, multivitamins, high dose vitamin D (>2000 IU/d), or high dose calcium (>1200 mg/d) supplements during the trial; extreme sensitivity to caffeine; <75% compliance to study pills during ≥2-mo placebo run-in period; and inability to communicate in English. Compliance was assessed during the placebo run-in from self-reports on mailed questionnaires returned from participants. After randomization and during follow-up, participants were sent questionnaires via mail or REDCap every 6 mo, on which they self-reported the number of pills missed during a typical month.
COSMOS-Web
COSMOS-Web (NCT04582617) was designed to test the primary hypothesis that supplementation with dietary flavanols improves memory over 1 y [15,16,19]. We separately reported the results for the primary arm of COSMOS-Web that examined the effect of dietary flavanol supplementation on cognitive outcomes [17]. Because the COSMOS-Web ancillary study was embedded in the parent COSMOS trial, we could additionally examine the effect of daily multivitamin supplementation. The COSMOS-Web prespecified primary outcome was performance on the immediate recall trial of the ModRey test [20] following 1 y of intervention. Prespecified secondary outcomes included change in ModRey immediate recall performance after 2 and 3 y, ModRey retention (the ratio of delayed recall to immediate recall), performance on tests of novel object recognition (ModBent; [15]), and executive function (Flanker; [21]). Our decision to assess the primary outcome at the 1-y endpoint was constrained by the design of the parent COSMOS trial and based on our interest in examining a sustained effect of the supplementation when adherence and study pill compliance would be highest to maximize statistical power and reduce potential bias from selective dropouts. Leveraging the 3-y design of the main COSMOS trial, we additionally assessed performance after 2 and 3 y, and averaged over the 3-y follow-up period, as secondary outcomes.
Participants
During the enrollment process, participants in the COSMOS trial were mailed an invitation to enroll in the COSMOS-Web study. The COSMOS-Web enrollment period spanned from August 2016 to August 2017. In addition to inclusion criteria for the COSMOS trial, the COSMOS-Web study required participants to have access to an Internet-connected computer. Instructions on how to access the study online were emailed to the participants who enrolled in COSMOS-Web. Participants received a $15 gift card for attempting each annual assessment, regardless of completion. COSMOS-Web received ethical approval from the Institutional Review Boards at Partners Healthcare/Mass General Brigham and Columbia University.
Participants completed the baseline COSMOS-Web assessment during the placebo run-in period to evaluate compliance and continued eligibility before randomization. Subsequently, they completed follow-up COSMOS-Web assessments annually for 3 y after the baseline assessment. All assessments used the same COSMOS-Web cognitive battery, with counterbalancing on stimulus sets presented across sessions and participants.
COSMOS-Web online platform
Neuropsychological outcome measures were collected via the self-administered COSMOS-Web cognitive battery on home computers. Cognitive tests were administered using the Inquisit-Web platform (Millisecond Software). Following instructions, participants downloaded an Inquisit-Web client and the COSMOS-Web battery on their home computers, where the software was operated locally to avoid latency variability in reaction time measurements. After the completion of the battery, participants’ performance data were uploaded automatically to the Inquisit servers. A phone helpline was provided to aid participants if they had technical difficulties.
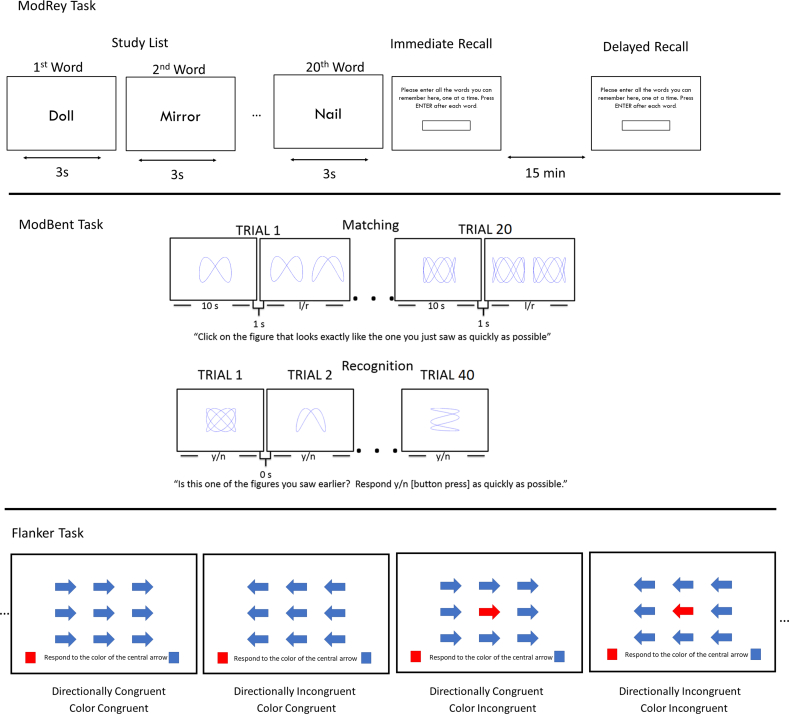
The COSMOS-Web cognitive battery consisted of 3 neuropsychological instruments, the ModRey, the ModBent, and the Flanker test. The ModRey immediate recall trial [20] is the outcome measure designed to be most sensitive and specific to hippocampus function [22] and we, therefore, prespecified it as our primary outcome measure. Although the ModBent task [15,16] was previously proposed as a primary outcome based on its association with dentate gyrus function, we had concerns about its psychometric properties and changed it to a secondary outcome before the completion of the trial and before analyzing any data in the trial. The Flanker test reflects prefrontal cortex function [21] and the retention trial of the ModRey test reflects entorhinal cortex function [23], a cognitive operation not hypothesized to be differentially affected by normal aging. We included the Flanker and ModRey retention trials to test the specificity of our hypothesis regarding hippocampus function.
In addition to using well-established paradigms in the cognitive aging literature and previous literature providing evidence for the validity of these paradigms in the target population [15,16,20,21,[24], [25], [26]], we conducted a small pilot study (unpublished) confirming that in a group of community-dwelling older adults performance assessed with the remote battery was comparable with the performance on the original, in-person, versions of the outcomes.
Cognitive battery
The COSMOS-Web cognitive battery included the following tasks (Figure 2):
-
•
One-trial ModRey, a 20-item word recall task measuring episodic memory [20]. The primary outcome measure was the number of words recalled immediately (immediate recall), which is related to hippocampus function [22]. The secondary outcome measure was the ratio of words recalled after a delay to words recalled immediately (retention), which relates to entorhinal cortex function [23]. The delay trial was administered after administration of the other tests in the battery.
-
•
Short-form ModBent, a test of novel object recognition [15]. The outcome measure was the reaction time to correctly reject the lures during the recognition trials. This task is related to the functioning of the dentate gyrus of the hippocampus [15].
-
•
Color/Directional Flanker, a measure of executive control [21]. The outcome measure was the size of the directional Flanker effect (that is, the difference in reaction time between directionally congruent and incongruent trials). Performance on this task is mediated by the prefrontal cortex [21].
FIGURE 2.
Schematic depiction of the COSMOS-Web Tasks: 1) ModRey list learning task: Participants were presented with a list of 20 words, one at a time, for 3 s each, with the instruction to try to remember the words. Recall was assessed, by having participants type in all the words they remembered, immediately after the word list was presented, and again after ∼15-min delay, following administration of the other tests in the battery. To account for the possibility of typographical errors, responses with ≥80% match to the letters in the original word were scored as correct, unless they spelled out a different common English word. For example, for “river,” “riber” would be counted as correct, but “rider” would not. 2) ModBent object recognition task: Participants first complete 20 matching trials where they are shown a stimulus and are asked to pick it out from an array of two stimuli. Then participants complete 40 forced-choice recognition trials where they indicate if a given stimulus had been studied in the matching trials, and 3) Flanker task: Participants are shown an array of colored arrows, and asked to indicate the color of the central arrow as a test of executive control. Trials vary on directional congruency (that is, whether the direction of the arrow matches the correct response) and color congruency (that is, whether the surrounding arrows are the same color as the central arrow). COSMOS, COcoa Supplement and Multivitamin Outcomes Study.
Statistical analysis
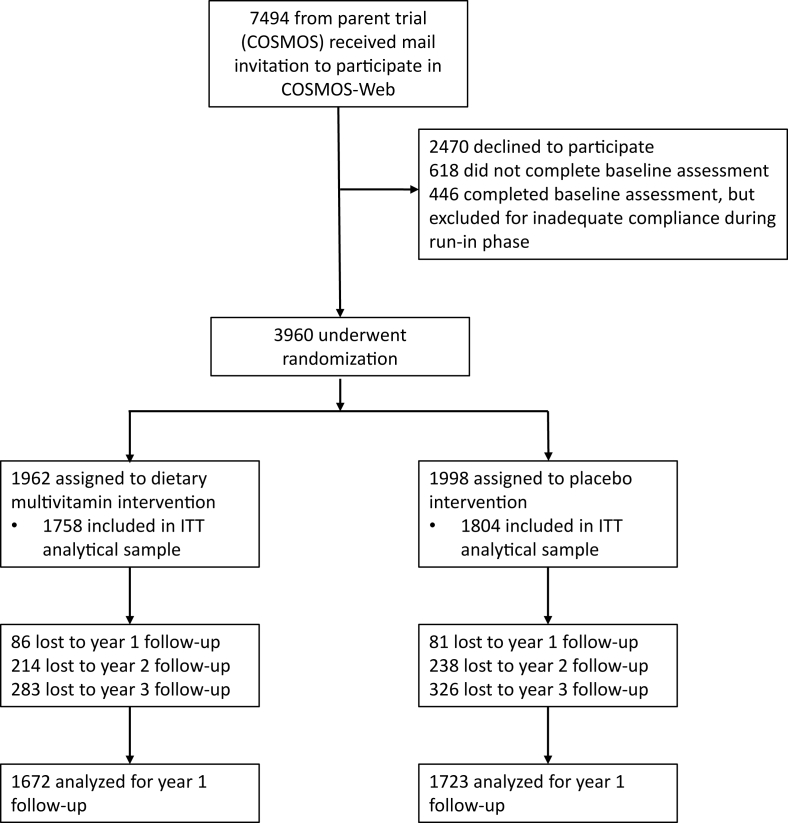
An intention-to-treat (ITT) analysis included randomly assigned participants who had a baseline plus ≥1 follow-up measurement completed (years 1, 2, or 3) regardless of pill compliance [18]; 3562 (90.0%) participants made up the ITT sample (see CONSORT diagram, Figure 3). The proportion of randomly assigned participants included in the ITT analysis was similar between treatment groups [n = 1758 of 1962 (89.6%) multivitamin group; n = 1804 of 1998 (90.3%) placebo group]. Completion of follow-up measurements for the primary outcome, ModRey, at years 1, 2, and 3 among the ITT sample was 95.3%, 87.3%, and 82.9%. Pill compliance (not missing >8 d/mo of study pills) based on self-reported pill counts was high among the ITT sample (6, 12, 18, 24, 30, and 36 mo: 94.4%, 91.8%, 87.0%, 86.6%, 82.7%, and 77.1%, respectively) with no difference between randomly assigned groups at any assessment (all χ2(2) ≤ 4.4, all P ≥ 0.10). We also defined a nonoverlapping sample as the subset of 2475 participants who did not participate in the related COSMOS-Mind study [13].
FIGURE 3.
CONSORT diagram. ITT, intention-to-treat.
A per-protocol sample was defined as the subset of participants who met the definition for pill compliance in the first year. A total of 3189 participants in the ITT sample were included in the per-protocol sample. Task data were set to missing for the years 2 and 3 visits if a participant reported having missed >8 doses/mo at any of the compliance assessments preceding the given visit. Analyses were conducted with SAS 9.4.
Demographic, clinical, and baseline cognitive data were summarized in the ITT sample by treatment group, along with standardized absolute mean differences between groups (values > 0.25 were considered to be nontrivial imbalance due to chance).
Linear mixed-effects models tested for differences between treatment groups in changes in the primary outcome (ModRey immediate recall) from baseline to the 1-y follow-up visit within the ITT sample, the nonoverlapping sample, and the per-protocol sample. Additional linear mixed-effects models were used for secondary outcomes (ModRey immediate recall from baseline to the 2- and 3-y follow-up, and averaged over all 3 y of follow-up, ModRey retention, ModBent correct rejection, and Flanker direction effect from baseline to 1-, 2- and 3-y follow-up visits). Predictors were the baseline value of the respective outcome, dichotomous treatment (multivitamin or placebo), categorical visit, treatment × visit interaction, and a random intercept to account for repeated measures. For all mixed-effects models, missing data for follow-up were accounted for under a missing at-random assumption. Cohen’s d effect sizes were calculated for all estimated treatment differences using baseline standard deviations of each variable across all participants to allow the direct comparison of magnitude. These analyses were repeated with the nonoverlapping and the per-protocol samples. A linear regression model tested the association between age and baseline ModRey immediate recall scores to estimate the expected memory decline for each year of aging. The mean treatment difference was divided by the expected memory decline per year of aging to determine the effect of multivitamin intervention in years of cognitive aging.
Based on results from the COSMOS-Mind study [13], which showed differential effects of multivitamin supplementation by baseline history of CVD, we conducted post hoc analyses that examined the interaction of treatment condition and baseline history of CVD, controlling for baseline performance and the interaction between history of CVD and baseline performance. History of CVD was defined as a dichotomous variable indicating a self-reported history of any of the following at baseline: transient ischemic attack, congestive heart failure, coronary artery bypass graft, percutaneous transluminal coronary angioplasty, or stent (those with a prior history of myocardial infarction or stroke at baseline were excluded from the parent trial).
In COSMOS, safety data were routinely collected via follow-up questionnaires sent to participants every 6 mo for 16 different prespecified self-reported symptoms and conditions. We also collected data on spontaneous reports from participants via phone, e-mail, or other communications. These and other self-reported outcomes collected on follow-up questionnaires were systematically evaluated by an external Data and Safety Monitoring Board (DSMB) to ensure that there were no adverse safety signals that warranted consideration.
Results
Baseline characteristics
Characteristics of the 3562 participants included in the ITT sample are displayed in Table 1. Baseline demographic characteristics, performance on the outcome measures, and alternative Healthy Eating Index (aHEI; [27]) scores were similar between the randomly assigned multivitamin and placebo groups. The nonoverlapping sample of COSMOS-Web participants differed slightly from the overall ITT sample (Supplemental Table 1). Baseline demographic, dietary, and cognitive characteristics were similar between groups in the per-protocol sample and were comparable to the overall COSMOS-Web sample (Supplemental Table 2).
TABLE 1.
Baseline demographic, dietary, and cognitive testing data across intervention groups in COSMOS-Web
| Measure | Total sample (N = 3562) |
Placebo (N = 1804) |
Multivitamin (N = 1758) |
Absolute standardized mean difference1 | |||
|---|---|---|---|---|---|---|---|
| N | % or M (SD) | N | % or M (SD) | N | % or M (SD) | ||
| Demographics | |||||||
| Age | 3562 | 71.0 (4.6) | 1804 | 71.0 (4.6) | 1758 | 70.9 (4.5) | 0.021 |
| Gender | |||||||
| Men | 1180 | 33.1% | 602 | 33.4% | 578 | 32.9% | 0.010 |
| Women | 2382 | 66.9% | 1202 | 66.6% | 1180 | 67.1% | 0.010 |
| Race | |||||||
| White | 3322 | 93.3% | 1686 | 93.5% | 1636 | 93.1% | 0.016 |
| African American | 88 | 2.5% | 47 | 2.6% | 41 | 2.3% | 0.018 |
| Hispanic | 51 | 1.4% | 25 | 1.4% | 26 | 1.5% | 0.008 |
| Other race or multiple | 54 | 1.5% | 22 | 1.2% | 32 | 1.8% | 0.049 |
| Asian or Native Hawaiian | 6 | 0.2% | 2 | 0.1% | 4 | 0.2% | 0.028 |
| American Indian or Alaska Native | 41 | 1.2% | 22 | 1.2% | 19 | 1.1% | 0.013 |
| Education | |||||||
| Missing | 23 | 0.6% | 7 | 0.4% | 16 | 0.9% | 0.065 |
| High school | 173 | 4.9% | 97 | 5.4% | 76 | 4.3% | 0.049 |
| Some college | 1387 | 38.9% | 693 | 38.4% | 694 | 39.5% | 0.022 |
| Completed college or more | 1979 | 55.6% | 1007 | 55.8% | 972 | 55.3% | 0.011 |
| Baseline diet | |||||||
| Alternate Healthy Eating Index | 3344 | 43.3 (10.8) | 1692 | 43.4 (11.0) | 1652 | 43.2 (10.6) | 0.023 |
| Baseline health | |||||||
| History of CVD | |||||||
| No | 3396 | 95.3% | 1725 | 95.6% | 1671 | 95.1% | 0.027 |
| Yes | 166 | 4.7% | 79 | 4.4% | 87 | 4.9% | 0.027 |
| Baseline testing | |||||||
| ModRey immediate recall | 3560 | 7.16 (3.22) | 1803 | 7.21 (3.19) | 1757 | 7.10 (3.26) | 0.034 |
| ModRey retention | 3342 | 0.86 (0.43) | 1707 | 0.84 (0.34) | 1635 | 0.88 (0.51) | 0.082 |
| ModBent correct rejection | 3548 | 2759.56 (1333.59) | 1796 | 2747.93 (1222.19) | 1752 | 2771.47 (1439.12) | 0.018 |
| Flanker direction effect | 3561 | 31.50 (57.93) | 1804 | 33.06 (58.60) | 1757 | 29.89 (57.21) | 0.055 |
Abbreviations: COSMOS, COcoa Supplement and Multivitamin Outcomes Study.
Standardized absolute mean difference is calculated as the difference between treatment groups divided by the overall standard deviation. Values >0.25 are considered to be non-trivial imbalance due to chance.
Multivitamin supplementation improved memory
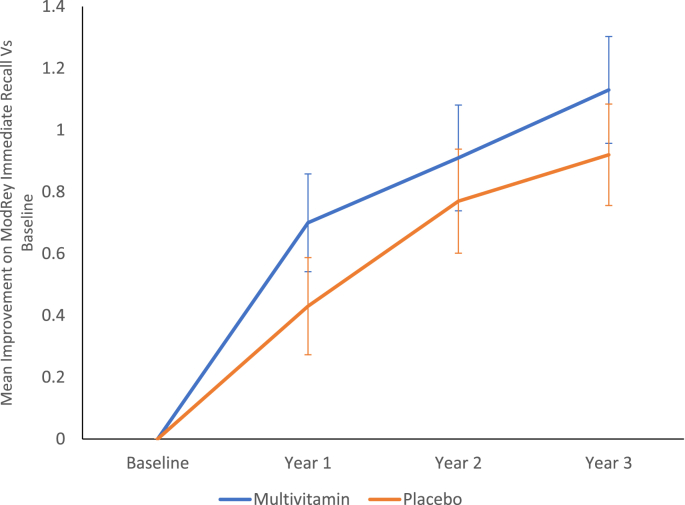
A linear mixed-effects model using the ITT sample showed that, compared with placebo, participants receiving multivitamin supplementation had significantly greater improvement in ModRey immediate recall memory between baseline and year 1 (Table 2 and Figure 4), the primary prespecified outcome for the study. Performance on the ModRey improved from a mean of 7.10 words at baseline to 7.81 words after 1 y in the multivitamin supplementation group and from an average of 7.21 words at baseline to 7.65 words after 1 y in the placebo group. The contrast estimate of the multivitamin compared with placebo effect averaged across all 3 y of follow-up demonstrated a significant effect of the multivitamin intervention, suggesting that the memory improvement is sustained, on average, over ≥3 y postbaseline (Table 2). In contrast, secondary outcomes did not differ significantly between groups in any of the follow-up years. The same effect on the primary outcome remained significant in the nonoverlapping sample that did not include participants co-enrolled in COSMOS-Mind (Supplemental Table 3), and in the per-protocol sample (Supplemental Table 4).
TABLE 2.
Longitudinal mixed effect model results of the randomized multivitamin intervention on primary (ModRey immediate recall) and secondary (ModRey retention, ModBent correct rejection, Flanker direction effect) outcomes (ITT analyses)
| Time point | Placebo |
Multivitamin |
Treatment difference |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Raw mean (SD) | Raw mean change from baseline (SD) | Within group P value | N | Raw mean (SD) | Raw mean change from baseline (SD) | Within group P value | Mean (SE)1 | Cohen’s d | Test statistic | P value | |
| Primary endpoint: ModRey: Immediate Recall | ||||||||||||
| Baseline | 1803 | 7.21 (3.19) | 1757 | 7.10 (3.26) | ||||||||
| Year 1 | 1722 | 7.65 (3.28) | 0.43 (3.33) | <.001 | 1671 | 7.81 (3.37) | 0.70 (3.30) | <.001 | 0.23 (0.10) | 0.070 | t(5889) = 2.25 | 0.025 |
| Secondary endpoints: ModRey: Immediate Recall | ||||||||||||
| Year 2 | 1565 | 8.03 (3.26) | 0.77 (3.40) | <.001 | 1543 | 8.06 (3.39) | 0.91 (3.43) | <.001 | 0.09 (0.10) | 0.028 | t(5889) = 0.86 | 0.391 |
| Year 3 | 1477 | 8.17 (3.39) | 0.92 (3.22) | <.001 | 1475 | 8.28 (3.43) | 1.13 (3.39) | <.001 | 0.16 (0.11) | 0.050 | t(5889) = 1.50 | 0.133 |
| Overall2 | 0.15 (0.06) | 0.048 | t(5889) = 2.54 | 0.011 | ||||||||
| ModRey: Retention | ||||||||||||
| Baseline | 1668 | 0.85 (0.34) | 1593 | 0.88 (0.50) | ||||||||
| Year 1 | 1489 | 0.87 (0.54) | 0.02 (0.62) | 0.233 | 1428 | 0.86 (0.37) | −0.01 (0.60) | 0.793 | −0.02 (0.02) | 0.039 | t(4989) = −1.02 | 0.307 |
| Year 2 | 1394 | 0.85 (0.41) | 0.00 (0.52) | 0.301 | 1348 | 0.87 (0.48) | −0.01 (0.68) | 0.558 | 0.02 (0.02) | 0.045 | t(4989) = 1.14 | 0.254 |
| Year 3 | 1301 | 0.85 (0.39) | 0.01 (0.51) | 0.625 | 1294 | 0.86 (0.43) | −0.02 (0.57) | 0.810 | 0.00 (0.02) | 0.007 | t(4989) = 0.17 | 0.862 |
| ModBent: Correct rejection | ||||||||||||
| Baseline | 1766 | 2747.73 (1219.25) | 1721 | 2774.06 (1446.39) | ||||||||
| Year 1 | 1646 | 2814.83 (1325.91) | 70.89 (1497.58) | 0.047 | 1598 | 2789.45 (1404.38) | 18.09 (1676.45) | 0.439 | −38.07 (45.16) | 0.028 | t(5573) = −0.84 | 0.399 |
| Year 2 | 1509 | 2815.80 (1338.84) | 51.73 (1532.25) | 0.113 | 1482 | 2758.24 (1375.31) | 2.31 (1631.12) | 0.906 | −48.26 (46.84) | 0.036 | t(5573) = −1.03 | 0.303 |
| Year 3 | 1415 | 2813.40 (1286.66) | 71.35 (1500.78) | 0.091 | 1415 | 2803.16 (1428.16) | 53.16 (1565.08) | 0.218 | −15.49 (47.99) | 0.012 | t(5573) = −0.32 | 0.747 |
| Flanker: Direction effect | ||||||||||||
| Baseline | 1773 | 32.59 (57.95) | 1722 | 29.50 (56.82) | ||||||||
| Year 1 | 1654 | 27.62 (56.92) | −4.85 (73.62) | 0.004 | 1598 | 26.24 (51.29) | −3.32 (71.39) | <0.001 | −0.92 (1.86) | 0.016 | t(5581) = −0.50 | 0.619 |
| Year 2 | 1511 | 28.32 (53.49) | −4.95 (69.16) | 0.022 | 1481 | 25.33 (47.16) | −4.02 (70.31) | <0.001 | −2.47 (1.93) | 0.043 | t(5581) = −1.28 | 0.201 |
| Year 3 | 1419 | 24.01 (53.97) | −9.11 (73.11) | <0.001 | 1417 | 23.60 (56.94) | −5.96 (77.59) | <0.001 | 0.21 (1.99) | 0.004 | t(5581) = 0.11 | 0.916 |
Treatment effect controlling for baseline.
Overall is the contrast estimate of the multivitamin compared with placebo effect averaged across all 3 y derived from the longitudinal mixed-effects model.
FIGURE 4.
Mean improvement on ModRey immediate recall between treatment groups. Error bars represent 95% CIs.
Treatment differences in terms of age-related memory differences
Each year of aging was associated with a baseline ModRey immediate recall memory reduction of 0.074 words (B = −0.074; 95% CI: 0.056, 0.092; β = −0.110; P < 0.001). Dividing the mean treatment difference of the multivitamin intervention compared with placebo at the 1-y primary endpoint by this value suggests that the effect of multivitamin supplementation was equivalent to ∼3.1 y of age-related memory change on the ModRey.
Multivitamin supplementation and history of CVD
Individuals with a history of CVD had lower baseline ModRey scores than those without a history of CVD (mean difference = 1.04 words, SE = 0.26 words, t(3558) = 4.05, P < 0.0001). In the ITT sample, the effect of multivitamin supplementation on improvement in ModRey immediate recall memory at the 1-y follow-up was significantly higher than placebo in participants reporting a history of CVD compared with participants with no history of CVD [mean difference in treatment differences = 1.24 words, SE = 0.48 words, t(5885) = 2.60, P = 0.009 (Supplemental Table 5)]. The same effect was observed in the per-protocol sample [mean difference in treatment differences = 1.29 words, SE = 0.51 words, t(4638) = 2.29, P = 0.011 (Supplemental Table 6)], but only trended toward significance in the nonoverlapping sample [mean difference in treatment differences = 1.05 words, SE = 0.60 words, t(4292) = 1.40, P = 0.081 (Supplemental Table 7)].
Safety
Overall in the COSMOS trial, comparing side effects of multivitamin supplementation with placebo included lower rates of stomach pain, diarrhea, skin rash, and bruising, but an increased rate of gastrointestinal bleeding [12].
Discussion
Daily multivitamin supplementation improved immediate recall memory after 1 y among older adults, above practice effects observed in a placebo group. Examining the change from baseline averaged over 3 y, the effect of multivitamin supplementation on memory was sustained over the 3 y of the COSMOS trial. In contrast, we found that multivitamin supplementation did not significantly affect memory retention, executive function, or novel object recognition. Based on cross-sectional analysis of the association between age and performance on the ModRey, we estimated that the effect of the multivitamin intervention improved memory performance above placebo by the equivalent of 3.1 y of age-related memory change. Our study provides evidence that multivitamin supplementation can help maintain or enhance cognitive functioning in later life, a top health concern among older adults [1]. Although our observed effect size is small and may not be noticeable to all individuals receiving multivitamin supplementation, even small effect sizes can result in large health benefits at the population level [28]. Vitamin supplementation is relatively inexpensive, accessible, and has a few adverse effects, and thus might be a potentially useful population health intervention. In addition to its documented impact on nutritional status and selected health outcomes [[29], [30], [31]], our study provides a strong parallel replication of the findings in the COSMOS-Mind ancillary study [13], which found benefits for a multivitamin on a global composite cognitive score and on composite scores for memory and executive function, including among individuals with a history of CVD who might have insufficient or deficient micronutrient concentrations [32]. We found very similar benefits for memory, and we also confirmed that multivitamin supplementation may improve memory after 1 y among nonoverlapping participants between the 2 ancillary studies. Participants in the 2 treatment arms had very similar baseline dietary patterns, as indexed by the aHEI (Table 1), which were consistent with averages from the US population [33]. We therefore do not believe that differences in background dietary quality confounded the results and we did not have a priori or prespecified hypotheses with respect to effect modification by dietary status. A major innovation of our study was the incorporation of novel neuropsychological outcome measures, designed specifically to detect subtle aspects of cognitive aging that were self-administered via a Web-based platform. This approach affords several advantages over traditional methods that rely on in-person testing with traditional neuropsychological instruments. We assessed thousands of participants in ∼15,000 individual testing sessions over 36 mo, which maximized statistical power and geographical representation. A previous trial that tested a multivitamin on cognitive outcomes [9] relied on clinical neuropsychological outcomes that were designed to detect frank cognitive impairment; these scales may result in performance at the ceiling for many participants and lack sensitivity to detect meaningful individual differences among cognitively healthy adults. That study, which included close to 6000 older men, showed that multivitamin supplementation did not affect a global cognition composite score over a period of ≤12 y [9]. Because the baseline cognition assessment was not performed until 2.5 y after randomization, the study could not assess the shorter-term effects of multivitamin supplementation.
We created the COSMOS-Web battery to capture variation in cognitive functioning among healthy older adults by designing tests with varying difficulty, without ceiling effects, and with the ability to capture finely calibrated reaction times. The computerized administration of the test removes systematic biases due to variable interexaminer and site reliability. We also explored whether the multivitamin supplementation differentially affected individuals with a history of CVD. Consistent with the recent report from Baker and colleagues [13], our results show a relatively stronger impact of multivitamin supplementation on memory among those reporting a history of CVD. Participants with a history of CVD had worse baseline memory performance than those without. However, after 1 y of multivitamin supplementation, their performance recovered to a level comparable to those without CVD. The implication here is that multivitamin supplementation might attenuate micronutrient deficits observed in people with CVD [34,35], which might underlie the reduced baseline memory performance.
Our study provides evidence that multivitamin supplementation has cognitive benefits but does not provide information about the underlying mechanisms that mediate this effect, or specific essential nutrients for cognitive aging [36]. We note that the selective effect of the multivitamin intervention on immediate recall memory—a cognitive operation that is particularly vulnerable in normal cognitive aging [37]—suggests that one possible mechanism for the cognitive effects of multivitamin intervention could be through micronutrient receptors in the hippocampus [38]. As the hippocampus is differentially affected by age, the detection of the cognitive effects of multivitamins in older adults might be most obvious with tasks that are hippocampus dependent. However, it is important to note that all cognitive tasks depend on networks of brain regions, and that multivitamin supplementation might also act on any number of other regions of the brain to produce the observed cognitive effects. Low vitamin concentrations, including vitamin B12 and vitamin D, may be linked to cognitive decline [4,5] and dementia [39,40]. We measured selected vitamin concentrations in the blood for a small subset of participants, confirming increases in folate, vitamin B12, and serum 25(OH)D with multivitamin supplementation compared with placebo [12]. Future studies should examine specific nutrients or nutrient combinations that contribute to cognitive health in aging.
The strengths of the study are the large sample size, high compliance with study pills, methodological rigor of the clinical trial, and an innovative and highly scalable cognitive assessment. A key potential limitation of this study, however, is the generalizability of our results. Participants had to meet requirements to have a computer, a degree of computer skills, and internet connectivity and comprised more highly educated, mostly White participants. Therefore, our findings might not generalize to a more educationally and racially/ethnically diverse population. Further, although we could not perform a comprehensive clinical assessment of cognitive status at baseline, the large sample size and randomization scheme provide reassurance that participants with cognitive impairment at baseline would be evenly distributed between treatment groups.
In conclusion, in the COSMOS-Web ancillary study, daily multivitamin supplementation improved verbal memory in older adults at 1 y, an effect that was sustained, over the duration of the trial. Our findings provide support for the cognitive benefits found with multivitamin supplementation in a parallel and independent subcohort in COSMOS [13], warranting further consideration of multivitamin supplementation as a targeted, safe, and accessible approach to slow cognitive decline in older adults.
Acknowledgments
We thank Dr. Scott Small for his contributions to the design of the COSMOS-Web study. We also thank all the COSMOS-Web participants, without whom this work would not have been possible. Finally, thank the following members of the COSMOS research group for their contributions to the COSMOS-Web study: Allison Clar, Georgina Friedenberg, Jimaldy Baez, Alexandra Phillips, Amy Casarella, Christopher Pfeffer, Annalee Wilson, Brielle Salvo, Young Sim, Nicholas Terrell, Leah Hall, Hayara Cardoso, Hanh Huynh, and Connor Rudnicki.
The authors’ responsibilities were as follows—L-KY, RS, HDS, JEM, and AMB designed the research; L-KY, HL-G, TC, and CH conducted the research; DMA and MW analyzed data; L-KY and AMB wrote the article; AMB had primary responsibility for final content. All authors read and approved the final manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajcnut.2023.05.011.
Conflict of interest
HDS, JEM, and AMB received investigator-initiated grant support to their institutions from Mars Edge. Pfizer Consumer Healthcare (now Haleon) provided support through the partial provision of study pills and packaging. HDS received investigator-initiated grants from Pure Encapsulations and Pfizer Inc and honoraria and/or travel for lectures from the Council for Responsible Nutrition, BASF, NIH, and the American Society of Nutrition during the conduct of the study. No funding sources had a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication.
Funding
This work was supported by an investigator-initiated grant from Mars Edge, a segment of Mars Inc dedicated to nutrition research. Pfizer Consumer Healthcare (now Haleon) provided support through the partial provision of study pills and packaging. COSMOS is also supported in part by grants AG050657, AG071611, EY025623, and HL157665 from the National Institutes of Health (NIH), Bethesda, MD. The Women's Health Initiative (WHI) is funded by the National Heart, Lung, and Blood Institute, NIH, US Department of Health and Human Services through contracts 75N92021D00001, 75N92021D00002, 75N92021D00003, 75N92021D00004, and 75N92021D00005. Additional support was provided by NIH grants R61 MH112800, R01 AG058417, and the Nathaniel Wharton Fund.
Data availability
Researchers interested in study data may contact the study principal investigators to request access. Data requests must be submitted in writing for approval by qualified individuals and will be reviewed by the study investigators. External investigators who are granted access to data for analysis must identify a COcoa Supplement and Multivitamin Outcomes Study (COSMOS)-Web investigator, who will participate in analyses and manuscript preparation as a coauthor. Researchers interested in accessing data related to the COSMOS parent trial (NCT02422745) should contact the administrators of that study.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Strout K., Ahmed F., Sporer K., Howard E.P., Sassatelli E., Mcfadden K. What are older adults wellness priorities? A qualitative analysis of priorities within multiple domains of wellness. Heal Aging Res. 2018;7(2) doi: 10.1097/hxr.0000000000000021. [DOI] [Google Scholar]
- 2.Gu Y., Scarmeas N. Dietary patterns in Alzheimer’s disease and cognitive aging. Curr. Alzheimer Res. 2011;8(5):510–519. doi: 10.2174/156720511796391836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedman D.B., Rose I.D., Anderson L.A., Hunter R., Bryant L.L., Wu B., et al. Beliefs and communication practices regarding cognitive functioning among consumers and primary care providers in the United States, 2009. Prev. Chronic Dis. 2013;10(4):120249. doi: 10.5888/pcd10.120249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duthie S.J., Whalley L.J., Collins A.R., Leaper S., Berger K., Deary I.J. Homocysteine, B vitamin status, and cognitive function in the elderly. Am. J. Clin. Nutr. 2002;75(5):908–913. doi: 10.1093/ajcn/75.5.908. [DOI] [PubMed] [Google Scholar]
- 5.Balion C., Griffith L.E., Strifler L., Henderson M., Patterson C., Heckman G., et al. Vitamin D, cognition, and dementia: a systematic review and meta-analysis. Neurology. 2012;79(13):1397–1405. doi: 10.1212/WNL.0b013e31826c197f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grima N.A., Pase M.P., MacPherson H., Pipingas A. The effects of multivitamins on cognitive performance: a systematic review and meta-analysis. J. Alzheimers Dis. 2012;29(3):561–569. doi: 10.3233/JAD-2011-111751. [DOI] [PubMed] [Google Scholar]
- 7.Behrens A., Graessel E., Pendergrass A., Donath C. Vitamin B-can it prevent cognitive decline? A systematic review and meta-analysis. Syst. Rev. 2020;9(1):111. doi: 10.1186/s13643-020-01378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Markun S., Gravestock I., Jäger L., Rosemann T., Pichierri G., Burgstaller J.M. Effects of vitamin B12 supplementation on cognitive function, depressive symptoms, and fatigue: a systematic review, meta-analysis, and meta-regression. Nutrients. 2021;13(3):1–18. doi: 10.3390/nu13030923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grodstein F., O’Brien J., Kang J.H., Dushkes R., Cook N.R., Okereke O., et al. Long-term multivitamin supplementation and cognitive function in men: a randomized trial. Ann. Intern. Med. 2013;159(12):806–814. doi: 10.7326/0003-4819-159-12-201312170-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rautiainen S., Sesso H.D., Manson J.E. Large-scale randomized clinical trials of bioactives and nutrients in relation to human health and disease prevention –lessons from the VITAL and COSMOS trials. Mol. Aspects Med. 2018;61:12–17. doi: 10.1016/j.mam.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sesso H.D., Manson J.E., Aragaki A.K., Rist P.M., Johnson L.G., Friedenberg G., et al. Effect of cocoa flavanol supplementation for the prevention of cardiovascular disease events: the COcoa Supplement and Multivitamin Outcomes Study (COSMOS) randomized clinical trial. Am. J. Clin. Nutr. 2022;115(6):1490–1500. doi: 10.1093/ajcn/nqac055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sesso H.D., Rist P.M., Aragaki A.K., Rautiainen S., Johnson L.G., Friedenberg G., et al. Multivitamins in the prevention of cancer and cardiovascular disease: the COcoa Supplement and Multivitamin Outcomes Study (COSMOS) randomized clinical trial. Am. J. Clin. Nutr. 2022;115(6):1501–1510. doi: 10.1093/ajcn/nqac056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baker L.D., Manson J.E., Rapp S.R., Sesso H.D., Gaussoin S.A., Shumaker S.A., et al. Effects of cocoa extract and a multivitamin on cognitive function: a randomized clinical trial. Alzheimers Dement. 2022;19(4):1308–1319. doi: 10.1002/alz.12767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lister J.P., Barnes C.A. Neurobiological changes in the hippocampus during normative aging. Arch. Neurol. 2009;66(7):829–833. doi: 10.1001/archneurol.2009.125. [DOI] [PubMed] [Google Scholar]
- 15.Brickman A.M., Khan U.A., Provenzano F.A., Yeung L.-K., Suzuki W.A., Schroeter H., et al. Enhancing dentate gyrus function with dietary flavanols improves cognition in older adults. Nat. Neurosci. 2014;17(12):1798–1803. doi: 10.1038/nn.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sloan R.P., Wall M., Yeung L.-K., Feng T., Feng X., Provenzano F., et al. Insights into the role of diet and dietary flavanols in cognitive aging: results of a randomized controlled trial. Sci. Rep. 2021;11(1):3837. doi: 10.1038/s41598-021-83370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brickman A.M., Yeung L.-K., Alschuler D., Ottaviani J.I., Kuhnle G.G., Sloan R.P., et al. Dietary flavanols restore hippocampal-dependent memory in older adults with lower diet quality and habitual flavanol consumption. Proc. Natl. Acad. Sci. U. S. A. 2023;11:3837. doi: 10.1073/pnas.2216932120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rist P.M., Sesso H.D., Johnson L.G., Aragaki A.K., Wang L., Rautiainen S., et al. Design and baseline characteristics of participants in the COcoa Supplement and Multivitamin Outcomes Study (COSMOS), Contemp. Clin. Trials. 2022;116(March):106728. doi: 10.1016/j.cct.2022.106728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Praag H., Lucero M.J., Yeo G.W., Stecker K., Heivand N., Zhao C., et al. Plant-derived flavanol (-)epicatechin enhances angiogenesis and retention of spatial memory in mice. J. Neurosci. 2007;27(22):5869–5878. doi: 10.1523/JNEUROSCI.0914-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hale C., Last B.S., Meier I.B., Yeung L.-K., Budge M., Sloan R.P., et al. The ModRey: an episodic memory test for nonclinical and preclinical populations. Assessment. 2019;26(6):1154–1161. doi: 10.1177/1073191117723113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korsch M., Frühholz S., Herrmann M. Ageing differentially affects neural processing of different conflict types-an fMRI study. Front. Aging Neurosci. 2014;6(April):57. doi: 10.3389/fnagi.2014.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Squire L.R., Stark C.E.L., Clark R.E. The medial temporal lobe. Annu. Rev. Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- 23.Brickman A.M., Stern Y., Small S.A. Hippocampal subregions differentially associate with standardized memory tests. Hippocampus. 2011;21(9):923–928. doi: 10.1002/hipo.20840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pereira A.C., Huddleston D.E., Brickman A.M., Sosunov A.A., Hen R., McKhann G.M., et al. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc. Natl. Acad. Sci. U. S. A. 2007;104(13):5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yeung L.-K., Hale C., Rizvi B., Igwe K., Sloan R.P., Honig L.S., et al. Anterolateral entorhinal cortex volume is associated with memory retention in clinically unimpaired older adults. Neurobiol. Aging. 2021;98:134–145. doi: 10.1016/j.neurobiolaging.2020.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yeung L.-K., Hale C., Last B.S., Andrews H., Sloan R.P., Honig L.S., et al. Cerebrospinal fluid amyloid levels are associated with delayed memory retention in cognitively normal biomarker-negative older adults. Neurobiol. Aging. 2019;84:90–97. doi: 10.1016/j.neurobiolaging.2019.08.010. [DOI] [PubMed] [Google Scholar]
- 27.Chiuve S.E., Fung T.T., Rimm E.B., Hu F.B., McCullough M.L., Wang M., et al. Alternative dietary indices both strongly predict risk of chronic disease. J. Nutr. 2012;142(6):1009–1018. doi: 10.3945/jn.111.157222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matthay E.C., Hagan E., Gottlieb L.M., Tan M.L., Vlahov D., Adler N., et al. Powering population health research: considerations for plausible and actionable effect sizes. SSM Popul. Health. 2021;14:100789. doi: 10.1016/j.ssmph.2021.100789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De-Regil L.M., Peña-Rosas J.P., Fernández-Gaxiola A.C., Rayco-Solon P. Effects and safety of periconceptional oral folate supplementation for preventing birth defects. Cochrane Database Syst. Rev. 2015;2015(12):CD007950. doi: 10.1002/14651858.CD007950.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Webb A.L., Villamor E. Update: effects of antioxidant and non-antioxidant vitamin supplementation on immune function. Nutr. Rev. 2007;65(5):181–217. doi: 10.1111/j.1753-4887.2007.tb00298.x. [DOI] [PubMed] [Google Scholar]
- 31.Gaziano J.M., Sesso H.D., Christen W.G., Bubes V., Smith J.P., MacFadyen J., et al. Multivitamins in the prevention of cancer in men: the physicians’ health study II randomized controlled trial. JAMA. 2012;308(18):1871–1880. doi: 10.1001/jama.2012.14641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Breteler M.M., Claus J.J., Grobbee D.E., Hofman A. Cardiovascular disease and distribution of cognitive function in elderly people: the Rotterdam study. BMJ. 1994;308(6944):1604–1608. doi: 10.1136/bmj.308.6944.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang D.D., Leung C.W., Li Y., Ding E.L., Chiuve S.E., Hu F.B., et al. Trends in dietary quality among adults in the United States, 1999 through 2010. JAMA Intern. Med. 2014;174(10):1587–1595. doi: 10.1001/jamainternmed.2014.3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cvetinovic N., Loncar G., Isakovic A.M., von Haehling S., Doehner W., Lainscak M., et al. Micronutrient depletion in heart failure: common, clinically relevant and treatable. Int. J. Mol. Sci. 2019;20(22):1–14. doi: 10.3390/ijms20225627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boban M., Bulj N., Kolačević Zeljković M., Radeljić V., Krcmar T., Trbusic M., et al. Nutritional considerations of cardiovascular diseases and treatments. Nutr. Metab. Insights. 2019;12 doi: 10.1177/1178638819833705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morris M.C. Nutritional determinants of cognitive aging and dementia. Proc. Nutr. Soc. 2012;71(1):1–13. doi: 10.1017/S0029665111003296. [DOI] [PubMed] [Google Scholar]
- 37.Naveh-Benjamin M., Cowan N., Kilb A., Chen Z. Age-related differences in immediate serial recall: dissociating chunk formation and capacity. Mem. Cognit. 2007;35(4):724–737. doi: 10.3758/bf03193310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Langub M.C., Herman J.P., Malluche H.H., Koszewski N.J. Evidence of functional vitamin D receptors in rat hippocampus. Neuroscience. 2001;104(1):49–56. doi: 10.1016/s0306-4522(01)00049-5. [DOI] [PubMed] [Google Scholar]
- 39.Annweiler C., Llewellyn D.J., Beauchet O. Low serum vitamin D concentrations in Alzheimer’s disease: a systematic review and meta-analysis. J. Alzheimers Dis. 2013;33(3):659–674. doi: 10.3233/JAD-2012-121432. [DOI] [PubMed] [Google Scholar]
- 40.Clarke R., Smith A.D., Jobst K.A., Refsum H., Sutton L., Ueland P.M. Folate, vitamin B12, and serum total homocysteine levels in confirmed Alzheimer disease. Arch. Neurol. 1998;55(11):1449–1455. doi: 10.1001/archneur.55.11.1449. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Researchers interested in study data may contact the study principal investigators to request access. Data requests must be submitted in writing for approval by qualified individuals and will be reviewed by the study investigators. External investigators who are granted access to data for analysis must identify a COcoa Supplement and Multivitamin Outcomes Study (COSMOS)-Web investigator, who will participate in analyses and manuscript preparation as a coauthor. Researchers interested in accessing data related to the COSMOS parent trial (NCT02422745) should contact the administrators of that study.