Abstract
The feline immunodeficiency virus (FIV) cat model is extensively used to investigate possible vaccination approaches against AIDS in humans. Although consistent levels of protection have been achieved with FIV, as with other model systems, by immunizing with whole inactivated virus or fixed infected cells, the mechanisms responsible for protection are elusive. In previous studies we showed that cats immunized with a vaccine consisting of fixed infected cells were protected or unprotected against cell-free or cell-associated FIV challenge depending on the time interval between completion of vaccination and challenge. In an attempt to define possible humoral immune correlates of protection, selected sera harvested at the times of challenge from such cats were examined for anti-FIV-antibody titers and properties by using binding and functional immunological assays. Binding assays included quantitative Western blotting, enzyme-linked tests for antibodies to FIV glycoproteins and immunodominant linear epitopes, and tests for measuring conformation dependence and avidity of anti-viral-envelope antibodies. Functional assays included virus neutralization performed with two different cell substrates, complement- and antibody-dependent virolysis, blocking of reverse transcriptase, and an assay that measured the ability of sera to prevent FIV growth in cocultures of infected and uninfected cells. Despite the wide spectrum of parameters investigated, no correlation between vaccine-induced protection and the humoral parameters measured was noted.
Although there is general agreement that vaccines against human immunodeficiency virus type 1 (HIV-1) and other lentiviruses should elicit both humoral and cell-mediated immune responses to effectively limit extracellular virus diffusion and clear virus-infected cells, the question of which effector functions are most important for protection is still unresolved. Also unresolved is whether in vitro-measurable indices of protective immunity to HIV-1 exist and can be used to predict vaccine effectiveness in vivo. In fact, convincing evidence has accumulated that certain antilentiviral vaccines, most notably those employing attenuated viruses in the simian immunodeficiency virus (SIV) model, can confer sufficient protective immunity to prevent infection or retard progression to disease. Yet even the most successful vaccination experiments have failed to identify consistently reliable in vitro correlates of vaccine-induced protection (reviewed in references 30, 31, and 39).
In the feline immunodeficiency virus (FIV) model, the attenuated-virus approach has yet to be investigated (19, 77); however, consistent levels of protection have been achieved by immunizing with fixed infected cells or inactivated cell-free virus (6, 32, 35, 47, 48, 77, 80, 81), two types of immunogenic preparations that have provided some satisfactory results in other model systems as well (16, 38). The immune mechanisms responsible for the protection conferred by these vaccines have, however, remained elusive.
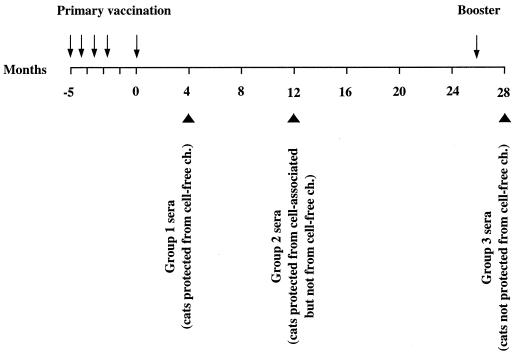
We recently reported that specific-pathogen-free (SPF) cats immunized with a vaccine consisting of fixed infected cells effectively resisted homologous cell-free and cell-associated challenges with a fully virulent, ex vivo-derived FIV. We also found, however, that protection was short-lived and could not be easily boosted. Specifically, vaccinees proved totally protected against cell-free virus when challenged 4 months after completion of the primary vaccination series but not when the same virus was given at 12 or 28 months, despite the fact that 2 months prior to the latter challenge the animals had received a booster vaccine dose. In addition, vaccinees proved to be protected against cell-associated virus at 12 months after completion of primary vaccination but not at 3 years, in spite of a booster given 10 weeks before the latter challenge (47, 48).
Day-of-challenge sera obtained from the vaccinees of the study described above appeared to be ideal for investigating humoral correlates of protection. (i) The vaccinees were homogeneous in every respect except for the time elapsed after immunization and, in some, the administration of a booster. (ii) The vaccine had been prepared with a low-passage isolate that was likely not to present the alterations of the surface properties that can develop during in vitro cultivation and affect induction of protective immunity (62). (iii) The challenge viruses used to probe immunity were obtained directly from infected cats; thus, the FIV approximated closely the viruses these animals are exposed to in nature. (iv) The outcome of protection was clear-cut, since protected animals had apparently cleared challenge virus completely as determined over prolonged periods of follow-up whereas unprotected cats displayed viral loads similar to those displayed by the unvaccinated controls. (v) Immediately prior to challenge the animals had been examined for total serum antibody and helper T-cell-proliferative responses to whole FIV antigen, but no relationship to protection had been observed (47, 48). In the present study, we undertook a detailed analysis of the serum specimens collected during the above-described experiments that appeared more likely to provide useful insights. Despite the use of numerous binding and functional tests, no humoral markers that correlated with protection were detected.
MATERIALS AND METHODS
Cells and viruses.
Feline T-lymphoid MBM cells have been used extensively in our laboratory for FIV isolation and propagation (46). They are routinely grown in RPMI 1640 medium supplemented with 10% fetal bovine serum, 5 μg of concanavalin A per ml, and 20 U of recombinant interleukin-2 per ml. AP32 cells are a line of T-lymphoid origin recently established in our laboratory from the peripheral blood mononuclear cells (PBMC) of an FIV- and feline leukemia virus-negative cat, possess a CD3+, CD4+, and CD8− phenotype, and are also highly susceptible to FIV (49). The medium used for AP32 cell propagation is the same as for MBM cells, except that the interleukin-2 content is 1 U per ml. CrFK cells were propagated either in conventional complete or in low-serum Eagle’s medium depending on the assay in which they were used (75). Primary feline lymphoblasts were derived by stimulating freshly harvested PBMC from four or five healthy SPF cats with concanavalin A for 3 days and cultured by standard methods (47). Normal cat serum (NCS), used when indicated to supplement media, was pooled serum from 10 to 15 healthy SPF cats.
FIV-M2 is a subtype B virus which has been extensively characterized in our laboratory (61). The stocks used for serological assays consisted of supernatant fluids from acutely infected MBM cells. The stocks of FIV-P, the prototype Petaluma strain (59) which belongs to subtype A, were supernatants from acutely infected CrFK cells or chronically infected FL4 cells (a kind gift of J. K. Yamamoto, Gainesville, Fla.). All virus stocks were kept in small aliquots in liquid nitrogen.
Serum specimens.
As mentioned in the introduction, sera from vaccinated cats were from experiments described in detail previously (47, 48). Briefly, vaccination was done as follows. (i) The vaccine was composed of MBM cells infected with a stock of FIV-M2 passaged a limited number of times in vitro and fixed with 1.25% paraformaldehyde for 24 h, a treatment which has repeatedly been shown to consistently inactivate infected-cell infectivity (6, 47, 48, 81). (ii) The primary vaccination schedule consisted of five doses containing 3 × 107 cells given subcutaneously at weeks 0, 3, 6, 9, and 21 in incomplete Freund’s adjuvant and the booster of a single dose administered in the same way. (iii) A mock vaccine composed of uninfected cells, treated and administered as above, was also used. (iv) Protection was tested by injecting the vaccinees with FIV as titered cell-free plasma or PBMC obtained from cats infected with FIV-M2 passaged only in vivo. The three groups of sera selected were harvested at various times after completion of primary vaccination: group 1 at 4 months, when vaccinees proved fully protected against cell-free virus challenge; group 2 at 12 months, when the animals resisted challenge with cell-associated virus but not with cell-free virus; and group 3 at 28 months, when the donors were fully susceptible to cell-free challenge despite a booster given 2 months earlier (Fig. 1). Positive control sera were from SPF cats infected with FIV-M2 7 months earlier, and negative control sera were from mock-vaccinated and unvaccinated cats. All sera were treated at 56°C for 30 min, checked for infectious FIV by standard culture, and kept at −80°C until use.
FIG. 1.
Timing of collection of study sera relative to immunization schedule. Arrows indicate vaccine doses, and large arrowheads indicate the times at which the three groups of vaccinated-cat sera were harvested. The outcomes of the challenges (ch.) performed at serum collection times are also indicated.
Western blot (WB) assay.
Gradient-purified FIV-M2 was lyophilized, resuspended in Laemmli lysis buffer, heated at 100°C for 3 min, and electrophoresed in 12.5% polyacrylamide–sodium dodecyl sulfate. Separated proteins were transferred to nitrocellulose sheets (Bio-Rad, Hercules, Calif.) and processed by standard methods. Strips were exposed to cat sera serially diluted from 1:100 to reach endpoint reactivity and, after overnight incubation at room temperature with gentle shaking, washed thoroughly, and incubated with a biotinylated anti-cat immunoglobulin (IgG) monoclonal antibody (Sigma, St. Louis, Mo.) and then with streptavidin-peroxidase (Pierce, Oud Beijerland, The Netherlands) that was developed with 4-chloro-1-naphthol.
Env gp-specific enzyme-linked immunosorbent assay (ELISA).
Cell-free supernatant fluids from FIV-M2-infected MBM cells or FIV-P-infected CrFK cells were treated with 0.5% Triton X-100 and used as sources of native viral envelope (Env) glycoprotein (gp), while lysates of pellets of the corresponding uninfected cells were used as a source of cellular gp. In the absence of a suitable monoclonal antibody to selectively attach the viral gp to the solid phase, we exploited the ability of FIV gp to adsorb to Galanthus nivalis lectin as described previously (68), with modifications. Briefly, microwells were coated overnight at room temperature with 5 μg of G. nivalis lectin (Sigma) in 100 μl of 0.1 M sodium carbonate (pH 9.6), postcoated with bovine serum albumin, washed extensively, and incubated for 12 h with 100 μl of the appropriate supernatants, with a change of supernatants at 6 h to saturate all the lectin. After a postcoating step with skim milk, twofold dilutions of test sera were added to duplicate wells. IgG bound to the lectin-immobilized viral gp was revealed with a biotinylated mouse anti-cat IgG serum followed by an antibiotin peroxidase conjugate. Absorbance was read at 450 nm.
ELISA for conformation dependence of recognized Env epitopes.
The conformation dependence of the Env gp epitopes recognized by sera was evaluated by comparing their ability to bind native and denatured viral gp, following a previously described method (12). Native gp was as described above for the Env-specific ELISA, while denatured gp was obtained by treatment with denaturing buffer (8 M urea, 0.12 M β-mercaptoethanol, 0.36 M Tris-HCl [pH 8.6], 0.2% EDTA) followed by blocking of the reduced sulfhydryl groups with 0.02 M iodoacetic acid, to irreversibly alter tertiary structures. Native and denatured gp were immobilized to the G. nivalis lectin and then run in parallel in an ELISA identical to the one described above with sera diluted 1:100 in duplicate wells. A conformation index was calculated for each serum by dividing the mean absorbance with native gp by the mean absorbance with denatured gp. Thus, values of >1.0 are indicative of predominant serum reactivity for conformational epitopes, while values of <1.0 connote predominant reactivity with linear epitopes.
ELISA for immunodominant linear epitopes of viral Env.
IgG antibodies to immunodominant linear epitopes of the viral Env were tested as previously described (47) by incubating sera diluted 1:10 with synthetic peptides derived from the V3 region of the surface gp and the apex segment of the transmembrane gp of FIV-P. Titers were expressed as the ratio of the optical density at 450 nm of (OD450) the test sera to the mean OD450 of 10 normal SPF cat sera.
Antibody avidity assays.
Antibody avidity was examined by measuring the stability of gp-antibody and V3 peptide-antibody complexes when treated with urea, essentially as described previously (7). Sera diluted 1:100 or 1:10 were incubated at room temperature for 1 h in quadruplicate microwells coated with G. nivalis lectin-gp or with V3 peptide, respectively. Microwells were then exposed to 8 M urea or diluent alone at 37°C for 5 min, thoroughly washed, and processed as for standard ELISA. The results were expressed as avidity index, calculated as follows: (OD450 without urea/OD450 with urea) × 100.
RT assay.
Except for the neutralization assay, for which a standard radiometric assay was used, reverse transcriptase (RT) activity was measured with the colorimetric method described by Suzuki et al. (73), with minor modifications.
Virus neutralization assays.
Neutralizing antibodies were measured in microplates by two published methods. One is performed in low-serum-adapted CrFK cells and uses the inhibition of syncytium formation by 100 syncytium-forming units of tissue culture-adapted FIV-P as the readout (75). The other is performed in MBM cells and is based on inhibition of replication of 10 50% tissue culture infectious doses of low-passage FIV-M2 (4). Titers were expressed as reciprocals of the highest dilutions of serum that prevented syncytium formation or gave 50% inhibition of RT production, respectively, and were calculated by the method of Reed and Muench (63).
Immune virolysis.
In preliminary experiments, cat complement showed no antibody-independent lytic activity for FIV. The virolytic effect of sera in the presence of complement was determined by quantitating RT activity release in an assay similar to those previously described for HIV-1 (53, 66). To avoid interassay variability, single lots of virus and complement were used throughout. The test virus was FIV-P produced by FL4 cells. Complement was pooled serum obtained from 15 healthy SPF cats and kept in liquid nitrogen, and titers for hemolytic activity were determined by standard methods. Inactivated complement was obtained by heating at 56°C for 30 min. Serum-virus mixtures were incubated in triplicate at 4°C for 1 h and, after addition of active or inactivated complement, for a further 1 h at 37°C. The final dilutions of immune sera and complement were 1:60 and 1:6, respectively. In each test, controls for maximum RT release were included by disrupting virus incubated in medium alone with lysis buffer (0.25% Triton X-100, 12 mM dithiothreitol, 0.5 M Tris-HCl). Virolysis was expressed as percent maximum RT release by using the following formula: [(mean OD in the presence of test serum and active complement − mean OD in the presence of test serum and inactivated complement)/(mean OD of maximum release controls − mean OD in complement alone)] × 100.
RT blocking assay.
The source of RT was a stock of FIV-M2 repeatedly tested for RT content and found to give OD values ranging between 0.5 and 1.0 when diluted 1:32. Prior to each test, RT was released with lysis buffer from virus diluted 1:32 and sera were incubated with 1.25% Triton X-100 at 56°C for 30 min to inactivate any endogenous RT. Test sera and control NCS diluted 1:8, 1:32, 1:128, and 1:512 (dilutions before the addition of RT) in duplicate were used. Serum-RT mixtures were incubated at 37°C for 90 min and then examined for residual RT activity. The percent inhibition of RT activity by test sera was calculated with respect to the corresponding dilution of NCS by using the following formula: [(mean OD in the presence of test serum − mean background OD)/(mean OD in the presence of the corresponding dilution of NCS − mean background OD)] × 100. RT-blocking antibody titers were defined as the reciprocal of the serum dilution required to reduce RT activity by ≥50% and were calculated by the method of Reed and Muench (63).
Assay for inhibition of cell-dependent infection.
Infected cells were produced by exposing primary blasts to FIV-M2 at 37°C for 2 h at low multiplicity (approximately 0.002) and washing three times with abundant medium (the last washing contained no infectious virus, as determined by inoculation into AP32 cells). Preliminary experiments had shown that 104 blasts infected as described above produced a negative RT signal when cultured alone for 8 days; this number of cells was therefore chosen for infecting as the source of cell-dependent infection. Thus, 104 infected blasts in 50 μl of complete RPMI 1640 medium were incubated at 37°C for 2 h with an equal volume of the test sera diluted 1:8, 1:32, 1:128, and 1:512 in triplicate microwells. All the microwells were then seeded with 105 AP32 cells in 100 μl of medium and incubated at 37°C for 8 days (on day 4, half the medium was replaced with fresh medium containing the appropriate concentration of test sera). Wells containing infected blasts and AP32 cells but no sera served as controls for positive cell-dependent infection, while wells with infected blasts alone served to exclude the possibility that these cells produced measurable levels of RT. All media were supplemented with 6% NCS to minimize nonspecific effects of cat sera (14). At the end of incubation, the RT content of wells was measured and percent inhibition was calculated for each serum dilution against the positive infection controls. Because the curves obtained by plotting the percent inhibition were difficult to compare due to irregular shapes and slopes, an inhibition index was calculated for each test serum by measuring the area under each curve as recently proposed by Kostrikis et al. (41) for analyzing HIV-1 neutralization curves. Values of 600 and 0 represented the maximum and minimum extents of inhibition that could be achieved, respectively, while negative values were indicative of infection enhancement.
RESULTS
Antibodies reactive in WB.
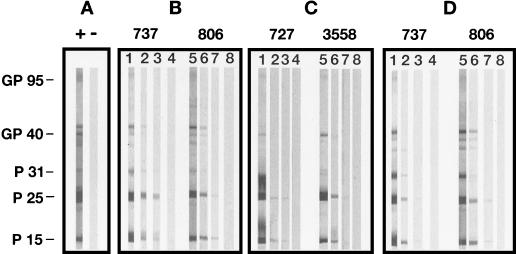
The sera of vaccinated cats under study were first examined for binding to individual FIV proteins by using a quantitative WB. As shown by Fig. 2 and Table 1, the highest antibody titers were detected against the gag products p25 and p15. Other viral antigens consistently detected, albeit at lower titers, included the env products gp95 and gp40 and the p31 integrase. Titers of antibody to gp95 were especially low, since they never exceeded 1:100. The results also showed that average titers were lower in group 2 than in group 1 sera, indicating that humoral immune responses tended to wane rapidly. However, in group 3 sera titers were up again after a booster, reaching levels similar to or higher than those in group 1.
FIG. 2.
Quantitative WB analysis of representative sera from vaccinated cats and control cats. (A) One infected (+) and one negative (−) control serum, each diluted 1:100. (B to D) Two sera each from group 1, group 2, and group 3 sera from vaccinated cats, respectively. Serum dilutions shown are as follows. (B) Lane 1, 1:100; lane 2, 1:2,000; lane 3, 1:32,000; lane 4, 1:50,000; lane 5, 1:100; lane 6, 1:2,000; lane 7, 1:64,000; lane 8, 1:80,000. (C) Lane 1, 1:100; lane 2, 1:2,000; lane 3, 1:200,000; lane 4, 1:400,000; lane 5, 1:100; lane 6, 1:1,000; lane 7, 1:400,000; lane 8, 1:600,000. (D) Lane 1, 1:100; lane 2, 1:26,400; lane 3, 1:102,400; lane 4, 1:140,000; lane 5, 1:100; lane 6, 1:3,200; lane 7, 1:640,000; lane 8, 1:800,000.
TABLE 1.
Antibody to individual FIV proteins, in sera from vaccinated cats, determined by quantitative WB
| Groupa and serum | Titer of antibody to FIV proteinb
|
||||
|---|---|---|---|---|---|
| gp95 | gp40 | p31 | p25 | p15 | |
| 1 | |||||
| 733 | 0.1b | <0.1 | 4 | 64 | 32 |
| 737 | 0.1 | 2 | 4 | 32 | 4 |
| 806 | 0.1 | 4 | 2 | 32 | 32 |
| 3532 | 0.1 | 8 | 8 | 8 | 32 |
| 3535 | 0.1 | 0.1 | 0.1 | 64 | 64 |
| 3585 | <0.1 | 0.1 | 4 | 64 | 64 |
| 2 | |||||
| 727 | <0.1 | 2 | 0.1 | 200 | 5 |
| 759 | 0.1 | 8 | 0.1 | 20 | 20 |
| 824 | <0.1 | <0.1 | <0.1 | 2 | 2 |
| 3558 | 1 | 4 | <0.1 | 400 | 100 |
| 3587 | <0.1 | 8 | <0.1 | 10 | 10 |
| 3607 | <0.1 | 6 | 0.1 | 15 | 15 |
| 3 | |||||
| 733 | <0.1 | 0.1 | 26 | 52 | 51 |
| 737 | 0.1 | 6 | 26 | 102 | 51 |
| 806 | 0.1 | 13 | 3 | 102 | 102 |
| 3532 | 0.1 | 0.1 | 13 | 13 | 13 |
| 3535 | 0.1 | 0.1 | 0.1 | 51 | 51 |
| 3585 | <0.1 | <0.1 | 6 | 13 | 13 |
Group 1, samples collected 4 months postvaccination (animals fully protected against cell-free challenge); group 2, samples collected 12 months postvaccination (animals protected against cell-associated challenge but not against cell-free challenge); group 3, samples collected 28 months postvaccination (animals fully susceptible to cell-free challenge despite a booster given 26 months postvaccination).
Reciprocal (103) of the highest dilution of serum which produced visible bands.
Env binding antibodies.
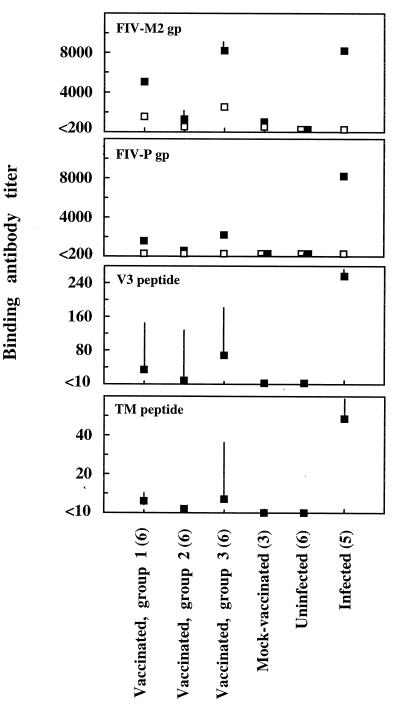
Antibodies to Env were measured in ELISA by using supernatants of infected cells as sources of FIV-M2 and FIV-P gp. A nonionic detergent was used to release the viral gp in order to preserve the secondary structure of the gp as much as possible. The same treatment was used to free control gp from uninfected cell pellets. As shown by Fig. 3, anti-viral gp antibody titers of positive control sera from FIV-infected cats were approximately the same regardless of gp source, whereas titers in sera from vaccinated cats were higher when measured against FIV-M2 gp than when measured against FIV-P gp. This difference most likely reflected the presence in the vaccinees of reactivity to both FIV antigens and the MBM cells used for vaccine preparation and for growing FIV-M2, since vaccinated as well as mock-vaccinated cats had antibodies which reacted with uninfected MBM cell gp but not with CrFK cell gp (Fig. 3). In any case, titers of antibody to both viral cellular gp were relatively high in group 1 sera, markedly reduced in group 2 sera, and highest in group 3 sera.
FIG. 3.
Env binding antibodies detected by ELISA in sera from vaccinated and control cats. Solid squares, geometric mean titers of antibody against the indicated antigens; open squares, geometric mean titers of antibody against gp derived from uninfected substrate cells (MBM cells for FIV-M2 and CrFK cells for FIV-P). Bars represent 95% confidence limits. For squares without bars, the limits lie within the symbols. The numbers of sera tested in each group are shown in parentheses.
Antibodies to immunodominant linear gp epitopes.
FIV-infected cats exhibit a dominant humoral response to two conserved linear epitopes present in the V3 loop of the surface gp and in the apex region of the transmembrane gp (3). As detected by peptide ELISAs, all vaccinated cats had antibodies to these epitopes, albeit at much lower titers than infected cats (Fig. 3). Levels of both antibodies were markedly lower in group 2 than in group 1 sera but were up again in group 3 sera.
Conformation dependence of the viral gp epitopes recognized.
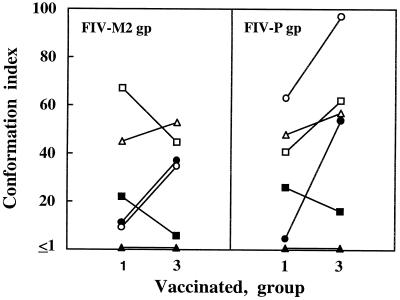
HIV-1 and SIV Env epitopes recognized by infected subjects are predominantly conformational in nature (50, 54). The conformation dependence of the FIV Env epitopes recognized by sera from vaccinated cats was studied by comparing their binding to viral gp, either native or denatured, with a procedure capable of irreversibly disrupting tertiary structure without significantly affecting secondary structure (12). In a series of presumably long-term positive control sera tested against a panel of viral isolates including FIV-M2 (42 virus-serum combinations), we found that conformation index values ranged from 0.54 to 2.24 with a mean ± standard deviation (SD) of 1.15 ± 0.32, suggesting that this parameter may be significantly affected by individual differences (data not shown). We therefore focused on group 1 and group 3 vaccinated-cat sera derived from the same donor animals. Interestingly, the conformation indices of these sera were generally manyfold higher than those of positive control sera, and this was true regardless of whether the assay was conducted with FIV-M2 gp or FIV-P gp. Since the latter gp preparation contained no cellular determinants reactive with sera from vaccinated cats, the results indicated that the vaccine had induced anti-Env antibodies that were overwhelmingly directed to conformational epitopes. Comparison of group 1 and group 3 sera showed that in the group 3 sera the antibody response had often evolved toward a greater conformation dependence, although this was not a constant finding (Fig. 4).
FIG. 4.
Conformation dependence of Env gp epitopes bound by group 1 and group 3 sera from vaccinated cats. Symbols for individual sera are as follows: •, 733; ■, 737; ▴, 806; ○, 3532; □, 3535; ▵, 3585.
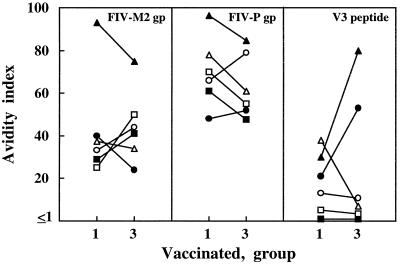
Avidity of binding antibodies.
It has recently been reported that HIV-infected individuals classified as nonprogressors exhibited antiviral antibody of greater avidity than progressors (7, 11). We measured the avidity of anti-Env antibody in sera from vaccinated cats by determining the resistance of gp-antibody complexes to dissociation by a urea wash. The avidity index values of the 42 infected cat serum-FIV combinations described above ranged between 0.9 and 28, with a mean ± SD of 14.4 ± 6.56, suggesting that this parameter also is greatly influenced by individual variability (data not shown). We therefore again compared group 1 and group 3 sera derived from the same donor animals. Env-antibody complexes formed by sera from vaccinated cats had generally higher avidity indices than those formed by positive control sera, and this was especially evident when FIV-P gp was employed as the antigen (Fig. 5), possibly suggesting that the complexes formed with Env epitopes conserved among different FIV subtypes tend to be more stable than those involving strain-specific determinants. Figure 5 also shows that average anti-Env avidity was little different in group 3 than in group 1 sera. The avidity of antibody to the V3 peptide was also measured, but the data were highly dispersed (Fig. 5).
FIG. 5.
Avidity of antibodies binding Env gp and V3 peptide in the indicated group 1 and group 3 sera from vaccinated cats. Symbols for individual sera are as follows: •, 733; ■, 737; ▴, 806; ○, 3532; □, 3535; ▵, 3585.
Neutralizing antibodies.
The FIV neutralizing activity of sera from vaccinated cats was assayed by two methods that have essentially similar formats but evaluate residual virus infectivity in different cell types and detect antibodies of different breadth and potency (4, 58, 75). As expected based on the known performances of the two methods, positive control sera displayed a powerful neutralizing activity in the CrFK cell-based assay and four of five were also positive in the lymphoid MBM cell-based assay, while sera from uninfected cats were uniformly negative in both assays. Conversely, the two assays detected neutralizing activity only sporadically in sera from vaccinated cats, with no apparent correlation to the protection status of the donor animals (Table 2). Since it has been speculated that current methods for measuring lentivirus neutralization in vitro might not adequately reproduce what might happen in vivo (55) and it is debated whether the state of substrate cell activation can affect its measurement (83, 85), we also tested selected sera by using resting PBMC as the substrate. The results were, however, superimposable on those obtained with MBM cells (data not shown).
TABLE 2.
Virus-neutralizing, virolytic, and RT-inhibiting antibodies in sera of vaccinated and control cats
| Group and serum | Titer of neutralizing antibodies
|
Virolysisc | Titer of RT-inhibiting antibodiesd | |
|---|---|---|---|---|
| CrFK cellsa | MBM cellsb | |||
| Vaccinated, group 1 | ||||
| 733 | <16 | <8 | 3.2 | <8 |
| 737 | <16 | <8 | 0 | <8 |
| 806 | 128 | 256 | 0 | <8 |
| 3532 | <16 | <8 | 0 | 11 |
| 3535 | 1,024 | <8 | 0 | <8 |
| 3585 | <16 | <8 | 0 | <8 |
| Vaccinated, group 2 | ||||
| 727 | <8 | <8 | 0 | <8 |
| 759 | <8 | <8 | NDe | <8 |
| 824 | <8 | <8 | 0 | <8 |
| 3558 | 256 | <8 | ND | <8 |
| 3587 | <8 | <8 | ND | <8 |
| 3607 | <8 | <8 | 0 | <8 |
| Vaccinated, group 3 | ||||
| 733 | <8 | <8 | 0 | <8 |
| 737 | <8 | <8 | 0 | <8 |
| 806 | 256 | <8 | 0 | <8 |
| 3532 | <8 | <8 | 0 | <8 |
| 3535 | 32 | <8 | 0 | <8 |
| 3585 | <8 | <8 | 0 | <8 |
| Mock-vaccinated | ||||
| 743 | <16 | <8 | 0 | <8 |
| 3583 | <16 | <8 | 0 | <8 |
| 3591 | <16 | <8 | 0 | <8 |
| Uninfected | ||||
| 753 | <16 | <8 | 0 | <8 |
| 792 | <16 | <8 | 0 | <8 |
| 815 | <16 | <8 | 0 | <8 |
| 3573 | <16 | <8 | 0 | <8 |
| 3588 | <16 | <8 | 0 | <8 |
| 3603 | <16 | <8 | 0 | <8 |
| Infected | ||||
| 753 | >32,000 | 512 | 6 | 45 |
| 792 | 32,000 | <8 | 26 | 11 |
| 3573 | 16,000 | 8 | 14 | 45 |
| 3588 | 16,000 | 256 | 3 | 45 |
| 3603 | >32,000 | 16 | 16 | 181 |
Expressed as reciprocals of the highest serum dilution that prevented syncytium formation by 100 syncytium-forming units of tissue culture-adapted FIV-P.
Expressed as reciprocals of the highest serum dilution that gave 50% inhibition of RT production by 10 50% tissue culture infectious doses of low-passage FIV-M2.
Activity expressed as a percentage of maximum RT release produced by sera diluted 1:60.
Expressed as reciprocals of the highest serum dilution that gave 50% reduction of RT activity.
ND, not done.
Antibody-dependent, complement-mediated virolysis.
Antibodies which effect complement-mediated virolysis have been extensively investigated in HIV-1 infection and are also present in FIV-infected cats (21). Since it was plausible that this effector function of antibodies contributed to vaccine-induced protection, we developed an assay which detected significant levels of complement-mediated FIV virolytic activity in the sera of most FIV-infected cats. However, as shown in Table 2, in the vaccinated groups there was only one serum which effected a marginal level of virolysis.
RT-inhibiting antibodies.
RT-blocking antibodies are found in lentivirus-infected hosts (18, 20, 57) and, although their physiological significance is unclear, have been proposed as markers of HIV-1 progression (27). As shown in Table 2, antibodies which blocked the catalytic activity of RT were detected in five of five positive control sera. In contrast, RT-inhibiting activity was found in only one of the vaccinated-cat sera.
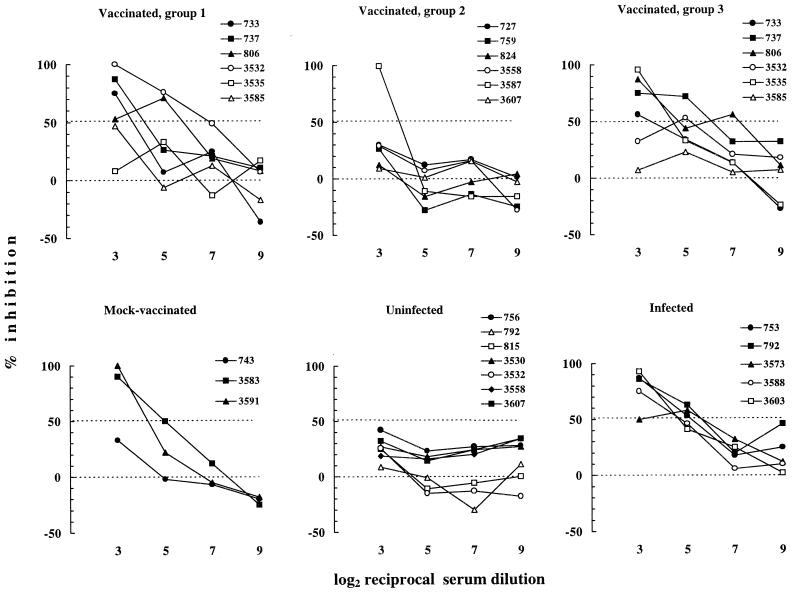
Inhibition of cell-dependent infection.
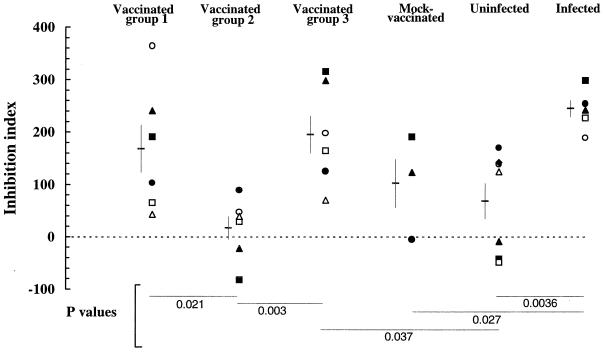
Because the vaccine used to immunize cats had protected against cell-associated FIV possibly better than against cell-free FIV (48), we investigated the ability of sera from vaccinated cats to prevent cell-dependent infection in vitro. In the method used, a number of FIV-exposed primary blasts calibrated in such a way as to give a negative RT signal if cultured alone were preincubated with fourfold dilutions of the sera and then cocultured with cells highly susceptible to FIV in the continuous presence of the test sera. The results are presented in Fig. 6 as percent inhibition of virus production at day 8 of incubation. The assay was carried out in the presence of 6% NCS to minimize nonspecific effects (14), yet certain sera from uninfected cats exhibited some inhibitory activity, although this never exceeded 50%. In contrast, positive control sera were all clearly more inhibitory, especially at low dilutions, providing evidence that the assay was suitable for evaluating the efficiency of cell-dependent FIV infection and its inhibition by immune sera. On the other hand, some group 1 and group 3 sera from vaccinated cats proved as inhibitory as positive-control sera while, with one exception, group 2 sera from vaccinated cats resembled sera from uninfected cats. Of note, two of three sera from mock-vaccinated cats were also inhibitory at 1:8 (the lowest dilution tested), suggesting that anticellular antibodies may have contributed in part to the inhibitory effect of sera from vaccinated cats. Because the inhibition curves had different shapes and were therefore difficult to compare, the data for each serum were also used to calculate an inhibition index that has proven useful for evaluating the HIV-1-neutralizing power of human sera. As pointed out previously (41), the major advantages of this index are that all the data used for generating inhibition curves are taken into account irrespective of the shape of the curve and that both inhibitions and enhancements of infectivity are translated into numerical values susceptible to statistical evaluation. Analysis of the indices thus obtained showed that vaccinated-cat sera in groups 1 and 3 were significantly more inhibitory in this assay than those in group 2 (Fig. 7).
FIG. 6.
Ability of sera from vaccinated and control cats to inhibit the infectivity of cell-associated FIV in cocultures of infected blasts and uninfected cells. Curves were obtained by plotting percent inhibition of virus production in the cocultures by fourfold dilutions of sera.
FIG. 7.
Ability of sera from vaccinated and control cats to inhibit the infectivity of cell-associated FIV in cocultures of infected blasts and uninfected cells. The data in Fig. 5 were used to calculate inhibition indices according to recently proposed criteria (41). Symbols for individual sera are as follows: •, 733; ■, 737; ▴, 806; ○, 3532; □, 3535; ▵, 3585. Horizontal lines with bars represent means ± SDs. Negative values are indicative of the tendency of the serum to produce infection enhancement. Statistical significance was evaluated by Student’s t test.
DISCUSSION
Identifying one or more in vitro predictors of protective immunity to animal lentiviruses would greatly improve our basic understanding of AIDS virus immunobiology and assist in the design and evaluation of candidate immunogens applicable to human vaccination. Here, we have addressed this important issue in the FIV cat model system by performing a comprehensive analysis of sera from vaccinated cats which appeared ideal for the purpose (see the introduction).
Collectively, binding assays showed that vaccination of donor cats elicited a robust humoral immune response to several viral antigens, confirming that vaccines consisting of fixed lentivirus-infected cells are powerful immunogens. The marked predominance in sera from vaccinated cats of antibodies to Gag relative to Env FIV antigens observed by quantitative WB did not simply reflect a low sensitivity of this assay at detecting Env antibodies. Although Env antigens may be depleted during the FIV concentration steps required for WB antigen preparation (36), positive control sera from actively infected animals reacted strongly with Env antigens in our WB, excluding the possibility that this was the cause of the poor reactivity of vaccinated-cat sera with these antigens. It seems more likely that the denaturing conditions used in the assay may have reduced Env antigen reactivity with vaccine-induced antibodies, since these were mainly directed to conformational determinants (see below). Indeed, a lectin-based ELISA using viral gp, released with a nonionic detergent to preserve their tertiary structure as much as possible, detected relatively high antibody titers in sera from vaccinated cats.
No correlation could be found between vaccine-induced protection and titers of binding antibody, irrespective of the type of antigen and assay used for their detection. As an example, group 3 sera from vaccinated cats displayed high levels of binding antibodies specific for viral antigens, indicating that the vaccine booster administered 2 months prior to their collection had effectively restimulated their production by cats even though it had failed to boost protection (48). The lectin ELISA also showed that a substantial fraction of antibodies to the vaccine substrate MBM cells found in vaccinated and mock-vaccinated cats, which we had previously revealed using whole-cell antigen (47, 48), was directed to cellular gp. In some SIV vaccination studies, protection was attributed to immunity to cell-derived antigens incorporated into the Env of the vaccine and challenge viruses (72). However, in the present study, titers of anti-cellular-gp antibody also were unrelated to protection, corroborating previous indications that vaccine protection against FIV is not mediated by host cell antigens (35, 47, 48).
Furthermore, no correlation between protection and other parameters of anti-FIV humoral immune responses studied by using binding assays was found. Among these, the conformation dependence of the epitopes recognized by anti-Env antibodies and the avidity of such antibodies deserve a special comment because these properties of antiviral IgG have been suggested as possible indicators of protective responses to several viruses, including HIV-1 (7, 11, 13, 17, 71). Moreover, to our knowledge, these parameters had never been investigated in FIV infection. Interestingly, the conformation dependence of the Env epitopes recognized was usually much greater for sera from vaccinated cats than for sera from infected cats. One possibility is that vaccination permitted only limited maturation of the immune response compared to infection, due to weaker and less persistent antigenic stimulation. Antibody responses to lentiviruses are known to mature more slowly than responses to other viruses and, with time postinfection, may become increasingly directed either to conformational or to linear epitopes (12, 13, 28, 84). Recently, Cole et al. (13) showed that monkeys infected with attenuated SIV exhibited increased concentrations of antibodies directed to Env linear determinants with increased time postinfection. By contrast, monkeys vaccinated with inactivated SIV vaccines produced immature antibody responses characterized by low conformation dependence and low avidity. The same vaccine also failed to protect against SIV challenge. Indeed, since linear epitopes of FIV Env have been implicated in both virus neutralization in vitro (15, 45) and protection in vivo (65), lack of appropriate maturation of antibody responses may be one reason for the short duration of protective immunity observed in our experiments (48). As an alternative possibility, the vaccinees may have preferentially responded to conformational epitopes of Env because these epitopes were more exposed and possibly stabilized by paraformaldehyde on the surface of the fixed cells used as vaccine. Although the avidity of Env antibodies also was greater in vaccinated than in infected cats, the difference was less striking. This might find an explanation in a recent investigation of humoral responses to the gp120 of HIV-1 showing that avidity assays are strongly biased toward the detection of antibodies to continuous rather than discontinuous epitopes (5).
Relative to binding assays, functional tests detected little anti-FIV activity in the sera of vaccinated cats. Although there is evidence from several viral systems that nonneutralizing antibodies also can be protective in vivo (26, 51, 60, 67), the role of neutralizing antibodies is crucial to any analysis of correlates of protection, especially when the vaccinees resist the establishment of the challenge infection, as was the case in this study (9). Neutralizing antibodies to FIV can be measured by using different cell substrates. Assays with fibroblastoid CrFK cells require tissue culture-adapted strains of FIV and detect broadly reactive antibodies at least partly directed to the variable region V3 (15, 45, 58, 64, 75). In contrast, assays carried out with lymphoid cells reveal isolate-specific neutralization determinants mainly located outside V3 and give much lower antibody titers, especially if the test virus is a recent clinical isolate (4, 14). Previous attempts to correlate neutralizing antibodies, measured with one or both types of assay, with vaccine protection in the FIV model have given conflicting results. In experiments with complex or subunit vaccines, Yamamoto et al. (80, 81) and Hosie and Flynn (35) found a good correlation while Lombardi et al. (45), Bishop et al. (6), Matteucci et al. (47), and Huisman et al. (37) found none. On the other hand, Hohdatsu et al. (32), investigating a dual-subtype fixed-cell vaccine which effectively protected against challenge with both viral strains present in the vaccine, found no neutralizing antibodies against one of the two challenge viruses. Furthermore, Siebelink et al. (69) found that subunit vaccines that induced high titers of neutralizing antibodies paradoxically accelerated infection upon challenge. Here, neutralizing activity was detected in only a few vaccinated cats, and there was no clear relation to their protection status. Thus, there is little hope that neutralizing antibodies will be of practical use for predicting in vivo protection at least against ex vivo-derived FIV. Whether this lack of predictive value is due to the scarce inherent sensitivity of primary FIV isolates to antibody-mediated neutralization (14), to the continuous evolution of the viruses in their hosts which might generate subtle differences in the neutralization epitopes of the viral stocks used in neutralization assays relative to those used as challenges (79, 82), to the concomitant presence in sera of infection enhancing antibodies as recently proposed (37, 69), or to other reasons remains an interesting question for future investigations. Attempts to define the role of virus-neutralizing antibodies in vaccine-induced protection has also been inconclusive in other lentiviral systems (30, 31, 39).
Other antiviral functions of antibodies were also investigated. Confirming previous data (21), immune virolysis was consistently produced by sera from infected cats but was essentially undetectable in the sera of vaccinated cats, excluding the possibility that this effector function played a significant role in vaccine-induced protection. Information on the ability of other experimental antilentivirus vaccines to induce virolytic antibodies is scarce and, in one study, target antigens appeared to be of cell origin (70). HIV-, SIV-, or FIV-infected hosts are known to develop anti-RT antibodies, although it is not known whether they are beneficial, neutral, or detrimental to the host (18, 20, 57). The assay used here detected moderate levels of such antibodies in all the sera of FIV-infected but not vaccinated cats, excluding the possibility that they participated significantly in the generation of protection.
Because the vaccine had induced a longer-lived protection against cell-associated than against cell-free challenge, the efficacy of sera at inhibiting the infectivity of FIV-infected cells in vitro was of special interest. All the positive control sera examined and some group 1 and group 3 sera from vaccinated cats, albeit at low titers, were inhibitory in this assay, even if devoid of neutralizing activity for cell-free virus, suggesting that inhibition of cell-dependent infection was at least partly mediated by other mechanisms. For example, the presence of antiviral antibodies in the extracellular milieu has been seen to restrict virus expression by infected cells (24, 25, 52), and it is possible that the FIV-infected blasts used as a source of cell-associated virus were similarly affected by anti-FIV antibody. In any case, the finding that group 2 sera from vaccinated cats, obtained from animals which effectively resisted cell-associated virus challenge, were completely inactive in the assay rules out the possibility that antibody-mediated inhibition of cell-dependent infection was an important mechanism of vaccine protection against cell-associated challenge.
In conclusion, in spite of the variety of parameters investigated, this study has failed to identify any correlation between the humoral immune status of vaccinees and vaccine-induced protection against FIV. One possibility is that we have not examined crucial antibody-mediated functions such as cell-dependent cytotoxic activity against FIV-infected target cells (1, 76), for which we have so far been unable to develop reliable assays. Also, recently it has been shown that soluble factors different from antibodies including CC and CXC chemokines, such as MIP-1α, MIP-1β, SDF-1α, RANTES, and other less characterized factors, inhibit cell infection by primary HIV-1 isolates not by acting on the virus but by competitively blocking virus coreceptors (reviewed in references 10, 43, 56). Evidence that FIV is sensitive to chemokine-mediated inhibition has also been presented (8, 34, 42, 78). Since most such factors are thermolabile, they would not have been detectable in our assays, which were performed with heat-inactivated serum specimens. Another possibility is that cell-mediated responses rather than humoral factors are critical for defending against FIV. Attempts to transfer protection against lentiviruses by means of passively administered antibodies obtained from vaccinated hosts have provided contradictory results (2, 9, 12, 33, 40, 44) and in one study have even accelerated FIV challenge infection (69). Our prior studies have failed to find a correlation between FIV-specific T-cell-proliferative responses of PBMC from vaccinated cats and protection (47, 48); however, the role of FIV-specific cytotoxic T lymphocytes induced by vaccines is under scrutiny (22, 23, 35, 74). Finally, a possibility that appears most likely is that to achieve protection antibodies must work in concert with other immune and nonimmune effector functions in complex ways that currently available assays cannot reproduce. Fine dissection of the requirements for protecting mice against Friend leukemia virus, a well-characterized model system, has clearly indicated that protection against retroviral infections requires more complex immunological responses than protection against most other viruses (29), a conclusion which is fully borne out by this as well as by other studies.
ACKNOWLEDGMENTS
This work was supported by grants from Ministero della Sanità—Istituto Superiore di Sanità, “Programma per l’AIDS,” and by the Ministero della Università e Ricerca Tecnologica, Rome, Italy.
We are indebted to Janet K. Yamamoto for the kind gift of FL4 cells.
REFERENCES
- 1.Ahmad A, Menezes J. Antibody-dependent cellular cytotoxicity in HIV infections. FASEB J. 1996;10:258–266. doi: 10.1096/fasebj.10.2.8641559. [DOI] [PubMed] [Google Scholar]
- 2.Almond N, Rose J, Sangster R, Silvera P, Stebbings R, Walker B, Stott E J. Mechanisms of protection induced by attenuated simian immunodeficiency virus. I. Protection cannot be transferred with immune serum. J Gen Virol. 1997;78:1919–1922. doi: 10.1099/0022-1317-78-8-1919. [DOI] [PubMed] [Google Scholar]
- 3.Avrameas A, Guillet J-G, Chouchane L, Moraillon A, Sonigo P, Strosberg A D. Localization of three epitopes of the env protein of feline immunodeficiency virus. Mol Immunol. 1992;29:565–572. doi: 10.1016/0161-5890(92)90192-z. [DOI] [PubMed] [Google Scholar]
- 4.Baldinotti F, Matteucci D, Mazzetti P, Giannelli C, Bandecchi P, Tozzini F, Bendinelli M. Serum neutralization of feline immunodeficiency virus is markedly dependent on passage history of the virus and host system. J Virol. 1994;68:4572–4579. doi: 10.1128/jvi.68.7.4572-4579.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Binley J M, Arshad H, Fouts T R, Moore J P. An investigation of the high-avidity antibody response to glycoprotein 120 of human immunodeficiency virus type 1. AIDS Res Hum Retroviruses. 1997;13:1007–1015. doi: 10.1089/aid.1997.13.1007. [DOI] [PubMed] [Google Scholar]
- 6.Bishop S A, Stokes C R, Gruffydd-Jones T J, Whiting C V, Humphries J E, Osborne R, Papanastasopoulou M, Harbour D A. Vaccination with fixed feline immunodeficiency virus (FIV) infected cells: protection, breakthrough and specificity of response. Vaccine. 1996;14:1243–1250. doi: 10.1016/s0264-410x(96)00023-0. [DOI] [PubMed] [Google Scholar]
- 7.Broström C, Sönnerborg A, Sällberg M. Human immunodeficiency virus type 1-infected patients with no disease progression display high-avidity antibody production to autologous V3 sequences. J Infect Dis. 1995;171:509–511. doi: 10.1093/infdis/171.2.509. [DOI] [PubMed] [Google Scholar]
- 8.Bucci J G, English R V, Jordan H L, Childers T A, Tompkins M B, Tompkins W A F. Mucosally transmitted feline immunodeficiency virus induces a CD8+ antiviral response that correlates with reduction of cell-associated virus. J Infect Dis. 1998;177:18–25. doi: 10.1086/513822. [DOI] [PubMed] [Google Scholar]
- 9.Burton D R. A vaccine for HIV type 1: the antibody perspective. Proc Natl Acad Sci USA. 1997;94:10018–10023. doi: 10.1073/pnas.94.19.10018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cairns J S, D’Souza M P. Chemokines and HIV-1 second receptors: the therapeutic connection. Nat Med. 1998;4:563–568. doi: 10.1038/nm0598-563. [DOI] [PubMed] [Google Scholar]
- 11.Chargelegue D, Stanley C M, O’Toole C M, Colvin B T, Steward M W. The absence or loss of antibodies of high affinity to human immunodeficiency virus (HIV) is associated with disease progression in HIV-1 infected patients. J Infect Dis. 1995;172:897. doi: 10.1093/infdis/172.3.897. [DOI] [PubMed] [Google Scholar]
- 12.Clements J E, Montelaro R C, Zink M C, Amedee A M, Miller S, Trichel A M, Jagerski B, Hauer D, Martin L N, Bohm R P, Murphey-Corb M. Cross-protective immune responses induced in rhesus macaques by immunization with attenuated macrophage-tropic simian immunodeficiency virus. J Virol. 1995;69:2737–2744. doi: 10.1128/jvi.69.5.2737-2744.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cole K S, Rowles J L, Jagerski B A, Murphey-Corb M, Unangst T, Clements J E, Robinson J, Wyand M S, Desrosiers R C, Montelaro R C. Evolution of envelope-specific antibody responses in monkeys experimentally infected or immunized with simian immunodeficiency virus and its association with the development of protective immunity. J Virol. 1997;71:5069–5079. doi: 10.1128/jvi.71.7.5069-5079.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Del Mauro D, Matteucci D, Giannecchini S, Maggi F, Pistello M, Bendinelli M. Autologous and heterologous neutralization analyses of primary feline immunodeficiency virus isolates. J Virol. 1998;72:2199–2207. doi: 10.1128/jvi.72.3.2199-2207.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Ronde A J, Stam J G, Boers P, Langedijk H, Meloen R, Hesselink W, Keldermans L C E J M, van Vliet A, Verschoor E J, Horzinek M C, Egberink H F. Antibody response in cats to the envelope proteins of feline immunodeficiency virus: identification of an immunodominant neutralization domain. Virology. 1994;198:257–264. doi: 10.1006/viro.1994.1028. [DOI] [PubMed] [Google Scholar]
- 16.De Thé G. Roundtable: can experience with veterinary retroviral vaccines be applied to the human situation? AIDS Res Hum Retroviruses. 1996;12:365–373. doi: 10.1089/aid.1996.12.365. [DOI] [PubMed] [Google Scholar]
- 17.Devash Y, Calvelli T A, Wood D G, Reagan K J, Rubinstein A. Vertical transmission of human immunodeficiency virus is correlated with absence of high affinity/avidity maternal antibodies to the gp120 principal neutralizing domain. Proc Natl Acad Sci USA. 1990;87:3445–3449. doi: 10.1073/pnas.87.9.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ekstrand D H L, Böttiger D, Andersson H, Gronowitz J S, Clas F R K. Reverse transcriptase and corresponding activity-blocking antibody for monitoring SIVsm infection in macaques. AIDS Res Hum Retroviruses. 1997;13:601–610. doi: 10.1089/aid.1997.13.601. [DOI] [PubMed] [Google Scholar]
- 19.Elyar J S, Tellier M C, Soos J M, Yamamoto J K. Perspectives on FIV vaccine development. Vaccine. 1997;15:1437–1444. doi: 10.1016/s0264-410x(97)00056-x. [DOI] [PubMed] [Google Scholar]
- 20.Fevereiro M, Roneker C, De Noronha F. Antibody response to reverse transcriptase in cats infected with feline immunodeficiency virus. Viral Immunol. 1991;4:225–235. doi: 10.1089/vim.1991.4.225. [DOI] [PubMed] [Google Scholar]
- 21.Fevereiro M, Roneker C, De Noronha F. Enhanced neutralization of feline immunodeficiency virus by complement viral lysis. Vet Immunol Immunopathol. 1993;36:191–206. doi: 10.1016/0165-2427(93)90019-z. [DOI] [PubMed] [Google Scholar]
- 22.Flynn J N, Cannon C A, Neil J C, Jarrett O. Vaccination with a feline immunodeficiency virus multiepitopic peptide induces cell-mediated and humoral immune responses in cats, but does not confer protection. J Virol. 1997;71:7586–7592. doi: 10.1128/jvi.71.10.7586-7592.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flynn J N, Keating P, Hosie M J, Mackett M, Stephens E B, Beatty J A, Neil J C, Jarrett O. Env-specific CTL predominate in cats protected from feline immunodeficiency virus infection by vaccination. J Immunol. 1996;157:3658–3665. [PubMed] [Google Scholar]
- 24.Fujinami R S, Oldstone M B A. Alterations in expression of measles virus polypeptides by antibody: molecular events in antibody-induced antigenic modulation. J Immunol. 1980;125:78–85. [PubMed] [Google Scholar]
- 25.Goldman M B, O’Bryan T A, Buckthal D J, Tetor L M, Goldman J N. Suppression of measles virus expression by noncytolytic antibody in an immortalized macrophage cell line. J Virol. 1995;69:734–740. doi: 10.1128/jvi.69.2.734-740.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Griffin D, Levine B, Tyor W, Ubol S, Despres P. The role of antibody in recovery from alphavirus encephalitis. Immunol Rev. 1997;159:155–161. doi: 10.1111/j.1600-065x.1997.tb01013.x. [DOI] [PubMed] [Google Scholar]
- 27.Grimison B, Laurence J. Immunodominant epitope regions of HIV-1 reverse transcriptase: correlations with HIV-1+ serum IgG inhibitory to polymerase activity and with disease progression. J Acquired Immune Defic Syndr Hum Retrovirol. 1995;9:58–68. [PubMed] [Google Scholar]
- 28.Hammond S A, Cook S J, Lichtenstein D L, Issel C J, Montelaro R C. Maturation of the cellular and humoral immune responses to persistent infection in horses by equine infectious anemia virus is a complex and lengthy process. J Virol. 1997;71:3840–3852. doi: 10.1128/jvi.71.5.3840-3852.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hasenkrug K J, Chesebro B. Immunity to retroviral infection: the Friend virus model. Proc Natl Acad Sci USA. 1997;94:7811–7816. doi: 10.1073/pnas.94.15.7811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haynes B F, Pantaleo G, Fauci A S. Toward an understanding of the correlates of protective immunity to HIV infection. Science. 1996;271:324–328. doi: 10.1126/science.271.5247.324. [DOI] [PubMed] [Google Scholar]
- 31.Heeney J L, Bruck C, Goudsmit J, Montagnier L, Schultz A, Tyrrell D, Zolla-Pazner S. Immune correlates of protection from HIV infection and AIDS. Immunol Today. 1997;18:4–8. doi: 10.1016/s0167-5699(97)80005-9. [DOI] [PubMed] [Google Scholar]
- 32.Hohdatsu T, Okada S, Motokawa K, Aizawa C, Yamamoto J K, Koyama H. Effect of dual-subtype vaccine against feline immunodeficiency virus infection. Vet Microbiol. 1997;58:155–165. doi: 10.1016/S0378-1135(97)00164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hohdatsu T, Pu R, Torres B A, Trujillo S, Gardner M B, Yamamoto J K. Passive antibody protection of cats against feline immunodeficiency virus infection. J Virol. 1993;67:2344–2348. doi: 10.1128/jvi.67.4.2344-2348.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hosie M J, Broere N, Hesselgesser J, Turner J D, Hoxie J A, Neil J C, Willett B J. Modulation of feline immunodeficiency virus infection by stromal cell-derived factor. J Virol. 1998;72:2097–2104. doi: 10.1128/jvi.72.3.2097-2104.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hosie M J, Flynn J N. Feline immunodeficiency virus vaccination: characterization of the immune correlates of protection. J Virol. 1996;70:7561–7568. doi: 10.1128/jvi.70.11.7561-7568.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hosie M J, Jarrett O. Serological responses of cats to feline immunodeficiency virus. AIDS. 1990;4:215–220. doi: 10.1097/00002030-199003000-00006. [DOI] [PubMed] [Google Scholar]
- 37.Huisman W, Karlas J A, Siebelink K H J, Huiman R C, de Ronde A, Francis M J, Rimmelzwaan G F, Osterhaus A D M E. Feline immunodeficiency virus subunit vaccines that induce virus neutralising antibodies but no protection against challenge infection. Vaccine. 1998;16:181–187. doi: 10.1016/s0264-410x(97)00184-9. [DOI] [PubMed] [Google Scholar]
- 38.Israel Z R, Edmonson P F, Maul D H, O’Neil S P, Mossman S P, Thiriart C, Fabry L, Van Opstal O, Bruck C, Bex F, Burny A, Fultz P N, Mullins J I, Hoover E A. Incomplete protection, but suppression of virus burden, elicited by subunit simian immunodeficiency virus vaccines. J Virol. 1994;68:1843–1853. doi: 10.1128/jvi.68.3.1843-1853.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnston M I. HIV/AIDS vaccine development: challenges, progress and future directions. Rev Med Virol. 1996;6:123–140. doi: 10.1002/(SICI)1099-1654(199609)6:3<123::AID-RMV170>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 40.Kent K, Kitchin P, Mills K H G, Page M, Taffs F, Corcoran T, Silvera P, Flanagan B, Powell C, Rose J, Ling C, Aubertin A M, Scott E J. Passive immunization of cynomolgus macaques with immune sera or a pool of neutralizing antibodies failed to protect against challenge with SIVmac251. AIDS Res Hum Retroviruses. 1994;10:189–194. doi: 10.1089/aid.1994.10.189. [DOI] [PubMed] [Google Scholar]
- 41.Kostrikis L G, Cao Y, Ngai H, Moore J P, Ho D D. Quantitative analysis of serum neutralization of human immunodeficiency virus type 1 from subtypes A, B, C, D, E, F, and I: lack of direct correlation between neutralization serotypes and genetic subtypes and evidence for prevalent serum-dependent infectivity enhancement. J Virol. 1996;70:445–458. doi: 10.1128/jvi.70.1.445-458.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leutenegger C M, Hofmann-Lehmann R, Holznagel E, Cuisinier A M, Wolfensberger C, Duquene V, Cronier J, Allenspach K, Aubert A, Ossent P, Lutz H. Partial protection by vaccination with recombinant feline immunodeficiency virus surface glycoproteins. AIDS Res Hum Retroviruses. 1998;14:275–283. doi: 10.1089/aid.1998.14.275. [DOI] [PubMed] [Google Scholar]
- 43.Levy J A. HIV and the pathogenesis of AIDS. 2nd ed. Washington, D.C: ASM Press; 1998. [Google Scholar]
- 44.Lewis M G, Elkins W R, McCutchan F E, Benveniste R E, Lai C Y, Montefiori D C, Burke D S, Eddy G A, Shafferman A. Passively transferred antibodies directed against conserved regions of SIV envelope protect macaques from SIV infection. Vaccine. 1993;11:1347–1355. doi: 10.1016/0264-410x(93)90106-8. [DOI] [PubMed] [Google Scholar]
- 45.Lombardi S, Garzelli C, Pistello M, Massi C, Matteucci D, Baldinotti F, Cammarota G, Da Prato L, Bandecchi P, Tozzini F, Bendinelli M. A neutralizing antibody-inducing peptide of the V3 domain of feline immunodeficiency virus envelope glycoprotein does not induce protective immunity. J Virol. 1994;68:8374–8379. doi: 10.1128/jvi.68.12.8374-8379.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matteucci D, Mazzetti P, Baldinotti F, Zaccaro L, Bendinelli M. The feline lymphoid cell line MBM and its use for feline immunodeficiency virus isolation and quantitation. Vet Immunol Immunopathol. 1995;46:71–82. doi: 10.1016/0165-2427(94)07007-t. [DOI] [PubMed] [Google Scholar]
- 47.Matteucci D, Pistello M, Mazzetti P, Giannecchini S, Del Mauro D, Lonetti I, Zaccaro L, Pollera C, Specter S, Bendinelli M. Studies of AIDS vaccination using an ex vivo feline immunodeficiency virus model: protection conferred by a fixed-cell vaccine against cell-free and cell-associated challenge differs in duration and is not easily boosted. J Virol. 1997;71:8368–8376. doi: 10.1128/jvi.71.11.8368-8376.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matteucci D, Pistello M, Mazzetti P, Giannecchini S, Del Mauro D, Zaccaro L, Bandecchi P, Tozzini F, Bendinelli M. Vaccination protects against in vivo-grown feline immunodeficiency virus even in the absence of detectable neutralizing antibodies. J Virol. 1996;70:617–622. doi: 10.1128/jvi.70.1.617-622.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mazzetti, P., D. Matteucci, and L. Zaccaro. Unpublished data.
- 50.McBride B W, Corthals G, Rud E, Kent K, Webster S, Cook N, Cranage M P. Comparison of serum antibody reactivities to a conformational and to linear antigenic sites in the external envelope glycoprotein of simian immunodeficiency virus (SIVmac) induced by infection and vaccination. J Gen Virol. 1993;74:1033–1041. doi: 10.1099/0022-1317-74-6-1033. [DOI] [PubMed] [Google Scholar]
- 51.McCullough K C, De Simone F, Brocchi E, Capucci L, Crowther J R, Kihm U. Protective immune response against foot-and-mouth disease. J Virol. 1992;66:1835–1840. doi: 10.1128/jvi.66.4.1835-1840.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McEntee M F, Zink M C, Anderson M G, Farzadegan H, Adams R J, Kent K A, Stott E J, Clements J E, Narayan O. Neutralizing antibodies modulate replication of simian immunodeficiency virus SIVmac in primary macaque macrophages. J Virol. 1992;66:6200–6203. doi: 10.1128/jvi.66.10.6200-6203.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Montefiori D C, Cornell R J, Zhou J Y, Zhou J T, Hirsch V M, Johnson P R. Complement control proteins, CD46, CD55, and CD59, as common surface constituents of human and simian immunodeficiency viruses and possible targets for vaccine protection. Virology. 1994;205:82–92. doi: 10.1006/viro.1994.1622. [DOI] [PubMed] [Google Scholar]
- 54.Moore J P, Ho D D. Antibodies to discontinuous or conformationally sensitive epitopes on the gp120 glycoprotein of human immunodeficiency virus type 1 are highly prevalent in sera of infected humans. J Virol. 1993;67:863–875. doi: 10.1128/jvi.67.2.863-875.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moore J P, Ho D D. HIV-1 neutralization: the consequences of viral adaptation to growth on transformed T cells. AIDS. 1995;9:S117–S136. [PubMed] [Google Scholar]
- 56.Moore J P, Trkola A. HIV type 1 coreceptors, neutralization serotypes, and vaccine development. AIDS Res Hum Retroviruses. 1997;13:733–736. doi: 10.1089/aid.1997.13.733. [DOI] [PubMed] [Google Scholar]
- 57.Neumüller M, Karlsson A, Lennerstrand J, Källander C F R, Holmberg V, Långström-Persson U, Thorstensson R, Sandström E, Gronowitz J S. HIV reverse transcriptase inhibiting antibodies detected by a new technique: relation to p24 and gp41 antibodies, HIV antigenemia and clinical variables. J Med Virol. 1991;34:55–63. doi: 10.1002/jmv.1890340110. [DOI] [PubMed] [Google Scholar]
- 58.Osborne R, Rigby M, Siebelink K, Neil J C, Jarrett O. Virus neutralization reveals antigenic variation among feline immunodeficiency virus isolates. J Gen Virol. 1994;75:3641–3645. doi: 10.1099/0022-1317-75-12-3641. [DOI] [PubMed] [Google Scholar]
- 59.Pedersen N C, Ho E W, Brown M L, Yamamoto J K. Isolation of a T-lymphotropic virus from domestic cats with an immunodeficiency-like syndrome. Science. 1987;235:790–793. doi: 10.1126/science.3643650. [DOI] [PubMed] [Google Scholar]
- 60.Pincus S H, Cole R, Ireland R, McAtee F, Fujisawa R, Portis J. Protective efficacy of nonneutralizing monoclonal antibodies in acute infection with murine leukemia virus. J Virol. 1995;69:7152–7158. doi: 10.1128/jvi.69.11.7152-7158.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pistello M, Cammarota G, Nicoletti E, Matteucci D, Curcio M, Del Mauro D, Bendinelli M. Analysis of genetic diversity and phylogenetic relationship of Italian isolates of feline immunodeficiency virus indicates high prevalence and heterogeneity of subtype B. J Gen Virol. 1997;78:2247–2257. doi: 10.1099/0022-1317-78-9-2247. [DOI] [PubMed] [Google Scholar]
- 62.Poignard P, Klasse P J, Sattentau Q J. Antibody neutralization of HIV-1. Immunol Today. 1996;17:239–246. doi: 10.1016/0167-5699(96)10007-4. [DOI] [PubMed] [Google Scholar]
- 63.Reed L J, Muench H A. A simple method for estimating fifty percent end points. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 64.Richardson J, Fossati I, Moraillon A, Castelot S, Sonigo P, Pancino G. Neutralization sensitivity and accessibility of continuous B cell epitopes of the feline immunodeficiency virus envelope. J Gen Virol. 1996;77:759–777. doi: 10.1099/0022-1317-77-4-759. [DOI] [PubMed] [Google Scholar]
- 65.Richardson J, Moraillon A, Crespeau F, Baud S, Sonigo P, Pancino G. Delayed infection after immunization with a peptide from the transmembrane glycoprotein of the feline immunodeficiency virus. J Virol. 1998;72:2406–2415. doi: 10.1128/jvi.72.3.2406-2415.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Saifuddin M, Parker C J, Peeples M E, Gorny M K, Zolla-Pazner S, Ghassemi M, Rooney I A, Atkinson J P, Spear G T. Role of virion-associated glycosylphosphatidylinositol-linked proteins CD55 and CD59 in complement resistance of cell line-derived and primary isolates of HIV-1. J Exp Med. 1995;182:501–509. doi: 10.1084/jem.182.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schmaljohn A L, Johnson E J, Dalrymple J M, Cole G A. Non-neutralising monoclonal antibodies can prevent lethal alphavirus encephalitis. Nature. 1982;297:70–72. doi: 10.1038/297070a0. [DOI] [PubMed] [Google Scholar]
- 68.Sibille P, Avraméas A, Moraillon A, Richardson J, Sonigo P, Pancino G, Strosberg A D. Comparison of serological tests for the diagnosis of feline immunodeficiency virus infection of cats. Vet Microbiol. 1995;45:259–267. doi: 10.1016/0378-1135(94)00128-j. [DOI] [PubMed] [Google Scholar]
- 69.Siebelink K H J, Tijhaar E, Huisman R C, Huisman W, de Ronde A, Darby I H, Francis M J, Rimmelzwaan G F, Osterhaus A D M E. Enhancement of feline immunodeficiency virus infection after immunization with envelope glycoprotein subunit vaccines. J Virol. 1995;69:3704–3711. doi: 10.1128/jvi.69.6.3704-3711.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Spear G T, Takefman D M, Sullivan B L, Landay A L, Jennings M B, Carlson J R. Anti-cellular antibodies in sera from vaccinated macaques can induce complement-mediated virolysis of human immunodeficiency virus and simian immunodeficiency virus. Virology. 1993;195:475–480. doi: 10.1006/viro.1993.1398. [DOI] [PubMed] [Google Scholar]
- 71.Steward M W, Stanley C M, Dimarchi R, Mulcahy G, Doel T R. High-affinity antibody induced by immunization with a synthetic peptide is associated with protection of cattle against foot and mouth disease. Immunology. 1991;72:99–103. [PMC free article] [PubMed] [Google Scholar]
- 72.Stott E J. Anti-cell antibody in macaques. Nature (London) 1991;353:393. doi: 10.1038/353393a0. [DOI] [PubMed] [Google Scholar]
- 73.Suzuki K, Saito T, Kondo M, Osanai M, Watanabe S, Kano T, Kano K, Imai M. Poly A-linked non-isotopic microtiter plate reverse transcriptase assay for sensitive detection of clinical human immunodeficiency virus isolates. J Virol Methods. 1995;55:347–356. doi: 10.1016/0166-0934(95)00073-5. [DOI] [PubMed] [Google Scholar]
- 74.Tellier M C, Soos J, Pu R, Pollock D, Yamamoto J K. Development of FIV-specific cytolytic T-lymphocyte responses in cats upon immunisation with FIV vaccines. Vet Microbiol. 1997;57:1–11. doi: 10.1016/s0378-1135(97)00081-3. [DOI] [PubMed] [Google Scholar]
- 75.Tozzini F, Matteucci D, Bandecchi P, Baldinotti F, Siebelink K, Osterhaus A, Bendinelli M. Neutralizing antibodies in cats infected with feline immunodeficiency virus. J Clin Microbiol. 1993;31:1626–1629. doi: 10.1128/jcm.31.6.1626-1629.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tyler D S, Lyerly H K, Weinhold K J. Minireview: anti-HIV-1 ADCC. AIDS Res Hum Retroviruses. 1989;5:557–563. doi: 10.1089/aid.1989.5.557. [DOI] [PubMed] [Google Scholar]
- 77.Willett B J, Flynn J N, Hosie M J. FIV infection of the domestic cat: an animal model for AIDS. Immunol Today. 1997;18:182–189. doi: 10.1016/s0167-5699(97)84665-8. [DOI] [PubMed] [Google Scholar]
- 78.Willett B J, Picard L, Hosie M J, Turner J D, Adema K, Clapham P R. Shared usage of the chemokine receptor CXCR4 by the feline and human immunodeficiency viruses. J Virol. 1997;71:6407–6415. doi: 10.1128/jvi.71.9.6407-6415.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wrin T, Crawford L, Sawyer L, Weber P, Sheppard H W, Hanson C V. Neutralizing antibody responses to autologous and heterologous isolates of human immunodeficiency virus. J Acquired Immune Defic Syndr. 1994;7:211–219. [PubMed] [Google Scholar]
- 80.Yamamoto J K, Hohdatsu T, Olmsted R A, Pu R, Louie H, Zochlinski H A, Acevedo V, Johnson H M, Soulds G A, Gardner M B. Experimental vaccine protection against homologous and heterologous strains of feline immunodeficiency virus. J Virol. 1993;67:601–605. doi: 10.1128/jvi.67.1.601-605.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yamamoto J K, Okuda T, Ackley C D, Louie H, Pembroke E, Zochlinski H, Munn R J, Gardner M B. Experimental vaccine protection against feline immunodeficiency virus. AIDS Res Hum Retroviruses. 1991;7:911–922. doi: 10.1089/aid.1991.7.911. [DOI] [PubMed] [Google Scholar]
- 82.Zhang L, Diaz R S, Ho D D, Mosley J W, Busch M P, Mayer A. Host-specific driving force in human immunodeficiency virus type 1 evolution in vivo. J Virol. 1997;71:2555–2561. doi: 10.1128/jvi.71.3.2555-2561.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhou J Y, Montefiori D C. Antibody-mediated neutralization of primary isolates of human immunodeficiency virus type 1 in peripheral blood mononuclear cells is not affected by the initial activation state of the cells. J Virol. 1997;71:2512–2517. doi: 10.1128/jvi.71.3.2512-2517.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zinkernagel R M. Immunology taught by viruses. Science. 1996;271:173–178. doi: 10.1126/science.271.5246.173. [DOI] [PubMed] [Google Scholar]
- 85.Zolla-Pazner S, Sharpe S. A resting cell assay for improved detection of antibody-mediated neutralization of HIV type 1 primary isolates. AIDS Res Hum Retroviruses. 1995;11:1449–1457. doi: 10.1089/aid.1995.11.1449. [DOI] [PubMed] [Google Scholar]